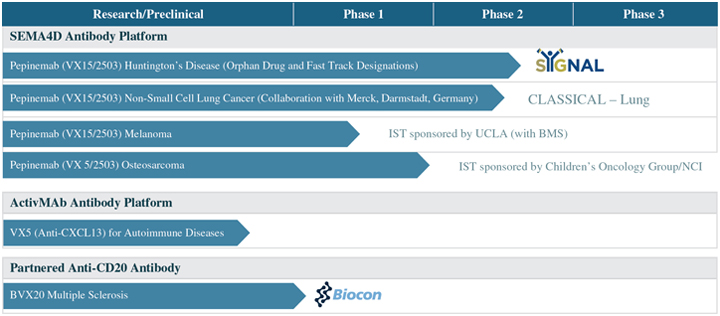
October 2014. Pepinemab was well tolerated in this clinical trial. In October 2017 in collaboration with Merck KGaA, we initiated the CLASSICAL–Lung clinical trial, a Phase 1b/2 clinical trial of pepinemab in combination with avelumab, an inhibitor of thePD-1/PD-L1 checkpoint pathway, in patients with NSCLC who have not previously been treated with immunotherapy. In July 2018, an additional cohort was added to the CLASSICAL – Lung study to include patients who failed prior immunotherapy. The CLASSICAL-Lung clinical trial has completed enrollment. We anticipate topline data for this trial in the first half of 2020. In February 2018, The Children’s Oncology Group with financial support of the National Cancer Institute, initiated a Phase 1/2 clinical trial of pepinemab as a single agent in pediatric patients with recurrent, relapsed, or refractory solid tumors, including osteosarcoma. In June 2018, a Phase 1 IST of pepinemab in combination withYervoy® or withOpdivo® began at the UCLA Jonsson Comprehensive Cancer Center in patients with advanced melanoma who have progressed on prioranti-PD-1/PD-L1 based therapies.
Huntington’s Disease
We are studying pepinemab as a treatment for Huntington’s disease, which is a neurodegenerative genetic disorder that typically manifests inmid-adult life. Our study of pepinemab in Huntington’s disease is based on our prior research of neurodegenerative disease mechanisms, where we demonstrated in preclinical models that SEMA4D triggers activation of both microglia and astrocytes, the innate inflammatory cells of the central nervous system. The chronic activation of microglia and astrocytes has been implicated as an important disease mechanism in Huntington’s disease, progressive MS, and other neurodegenerative disorders. We initiated the SIGNAL study, a Phase 2 clinical trial, in July 2015 in early manifest and late prodromal Huntington’s disease patients. This clinical trial builds upon preclinical studies in an animal model of Huntington’s disease and safety data from a Phase 1 dose-escalation clinical trial of pepinemab in MS patients that we completed in November 2014. We anticipate topline data from the SIGNAL trial in the second half of 2020. The U.S. Food and Drug Administration, or the FDA, has granted both Orphan Drug designation and Fast Track designation to pepinemab for Huntington’s disease.
Our Corporate Information
We were incorporated under the laws of the State of Delaware in April 2001. Our principal executive offices are located at 1895 Mount Hope Avenue, Rochester, New York 14620, and our telephone number is (585)271-2700. Our website address is www.vaccinex.com. Our website and the information contained on, or that can be accessed through, the website will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus. You should not rely on any such information in making your decision to purchase our common stock.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Private Placement
On January 21, 2020, we entered into a stock purchase agreement, or the Stock Purchase Agreement, with certain investors pursuant to which we sold an aggregate of 1,468,563 shares, or the Shares, of our common stock in a private placement, or the Private Placement, at a purchase price of $5.09 per share, with aggregate gross proceeds totaling $7,474,986.67. In connection with the Private Placement, we entered into a Registration Rights Agreement with the investors in the Private Placement, or the Registration Rights Agreement, pursuant to which we agreed, among other things, to file with the SEC a registration statement covering the resale of the Shares.