Exhibit 99.3

The tender offer described in this material has not yet commenced. This material is for informational purposes only, and it is neither an offer to purchase nor a solicitation of an offer to sell shares of Chelsea Therapeutics International, Ltd.’s common stock. At the time any such tender offer is commenced, H. Lundbeck A/S will cause a new indirect wholly-owned subsidiary, Charlie Acquisition Corp., to file a Tender Offer Statement, containing an offer to purchase, a form of letter of transmittal and other related tender offer documents, with the United States Securities and Exchange Commission (the “SEC”). Chelsea Therapeutics’ stockholders are strongly advised to read these tender offer materials carefully and in their entirety when they become available, as they may be amended from time to time, because they will contain important information about such tender offer that Chelsea Therapeutics’ stockholders should consider prior to making any decisions with respect to such tender offer. Once filed, stockholders of Chelsea Therapeutics will be able to obtain a free copy of these documents at the website maintained by the SEC at www.sec.gov, or by directing a request to H. Lundbeck A/S; Ottiliavej 9; 2500 Valby, Denmark.

LUNDBECK TO ACQUIRE CHELSEA THERAPEUTICS (CHTP) May 2014

Company disclaimer This presentation contains forward-looking statements that provide our expectations or forecasts of future events such as new product introductions, product approvals and financial performance. Such forward-looking statements are subject to risks, uncertainties and inaccurate assumptions. This may cause actual results to differ materially from expectations and it may cause any or all of our forward-looking statements here or in other publications to be wrong. Factors that may affect future results include interest rate and currency exchange rate fluctuations, delay or failure of development projects, production problems, unexpected contract breaches or terminations, government-mandated or market-driven price decreases for Lundbeck’s products, introduction of competing products, Lundbeck’s ability to successfully market both new and existing products, exposure to product liability and other lawsuits, changes in reimbursement rules and governmental laws and related interpretation thereof, unexpected growth in costs and expenses, the possibility that the transaction may not be consummated or that the expected benefits of the transaction may not materialize as expected, Lundbeck’s ability to timely complete the transaction, if at all, or to, prior to the completion of the transaction, if at all, satisfy all closing conditions, and the possibility that the merger agreement may be terminated. Lundbeck undertakes no duty to update forward-looking statements. Certain assumptions made by Lundbeck are required by Danish Securities Law for full disclosure of material corporate information. Some assumptions, including assumptions relating to sales associated with product that is prescribed for unapproved uses, are made taking into account past performances of other similar drugs for similar disease states or past performance of the same drug in other regions where the product is currently marketed. It is important to note that although physicians may, as part of their freedom to practice medicine in the US, prescribe approved drugs for any use they deem appropriate, including unapproved uses, at Lundbeck, promotion of unapproved uses is strictly prohibited.

Chelsea acquisition rationale Northera is an orphan neuro opportunity with excellent commercial and strategic fit to our US neurology franchise Chelsea Therapeutics Northera, approved by the FDA in February 2014, represents a low risk opportunity Northera (droxidopa) Capsules 100mg 200mg 300mg Northera allows us to continue to strengthen our capabilities for future opportunities with Lu AE58054 and desmoteplase in the US Clinical practice, as supported by the data from Japan, supports longer-term utilization of the product
Creating a unique neurology portfolio Xenazine (tetrabenazine) 12.5 and 25 mg Tablets Sabril vigabatrin 500 mg tablet 500 mg powder for oral solution Onfi (clobazam) 5, 10 and 20 mg Tablets Northera (droxidopa) Capsules 100mg 200mg 300mg Desmoteplase Lu AE58054

Chelsea business overview A US biotech company based in Charlotte, North Carolina, founded in 2002 Chelsea acquired the global rights to droxidopa in 2006 from Dainippon Sumitomo, excluding Japan, Korea, China and Taiwan Net loss of USD 16.4m for 2013 compared to a net loss of USD 31.7m in 2012 Cash and cash equivalents totaled USD 45.3m as of 31 December 2013 18 employees Research Report Chelsea Therapeutics Droxidopa in Patients with Neurogenic Orthostatic Hypotension Associated with Parkinson’s Disease (NOH306A)
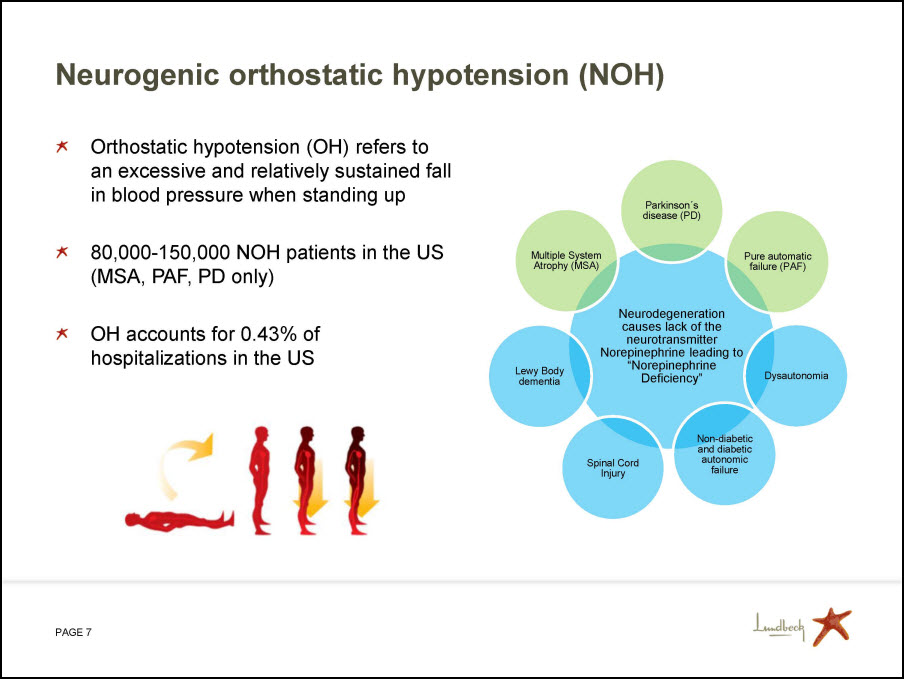
Neurogenic orthostatic hypotension (NOH) Orthostatic hypotension (OH) refers to an excessive and relatively sustained fall in blood pressure when standing up 80,000-150,000 NOH patients in the US (MSA, PAF, PD only) OH accounts for 0.43% of hospitalizations in the US Parkinson’s disease (PD) Pure automatic failure (PAF) Dysautonomia Non-diabetic and diabetic autonomic failure Spinal Cord Injury Lewy Body dementia Multiple System Atrophy (MSA) Neurodegeneration causes lack of the neurotransmitter Norepinephrine leading to “Norepinephrine Deficiency”
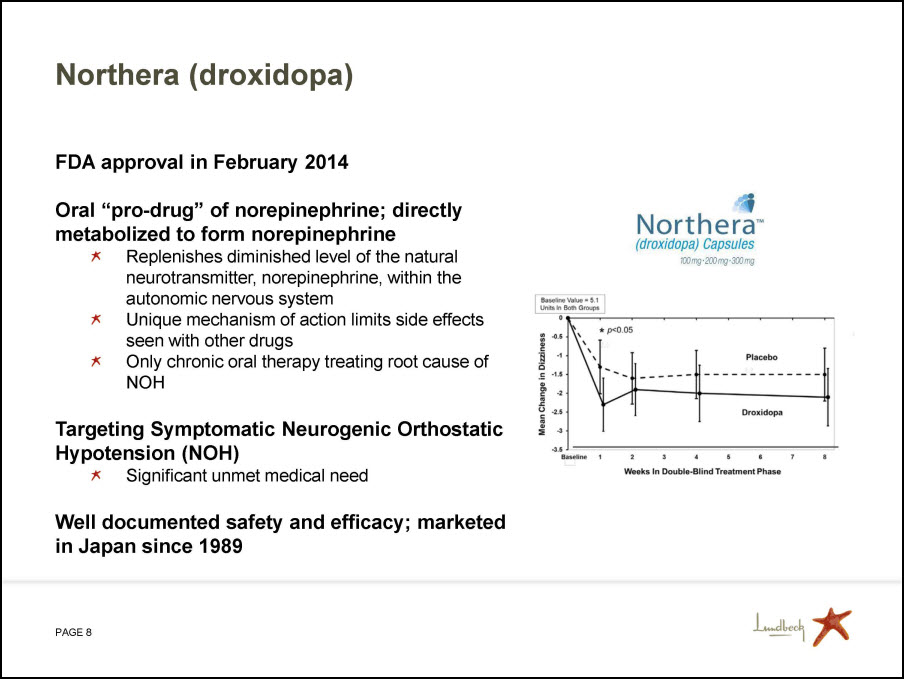
Northera (droxidopa) FDA approval in February 2014 Oral “pro-drug” of norepinephrine; directly metabolized to form norepinephrine Replenishes diminished level of the natural neurotransmitter, norepinephrine, within the autonomic nervous system Unique mechanism of action limits side effects seen with other drugs Only chronic oral therapy treating root cause of NOH Targeting Symptomatic Neurogenic Orthostatic Hypotension (NOH) Significant unmet medical need Well documented safety and efficacy; marketed in Japan since 1989 Northera (droxidopa) Capsules 100mg 200mg 300mg Weeks in Double-Blind Treatment Phase Mean Change in Dizziness Baseline 1 2 3 4 5 6 7 8 Placebo Droxidopa Baseline Value = 5.1 Units in Both Groups * p<0.05 0 -0.5 -1 -1.5 -2 -2.5 -3 -3.5
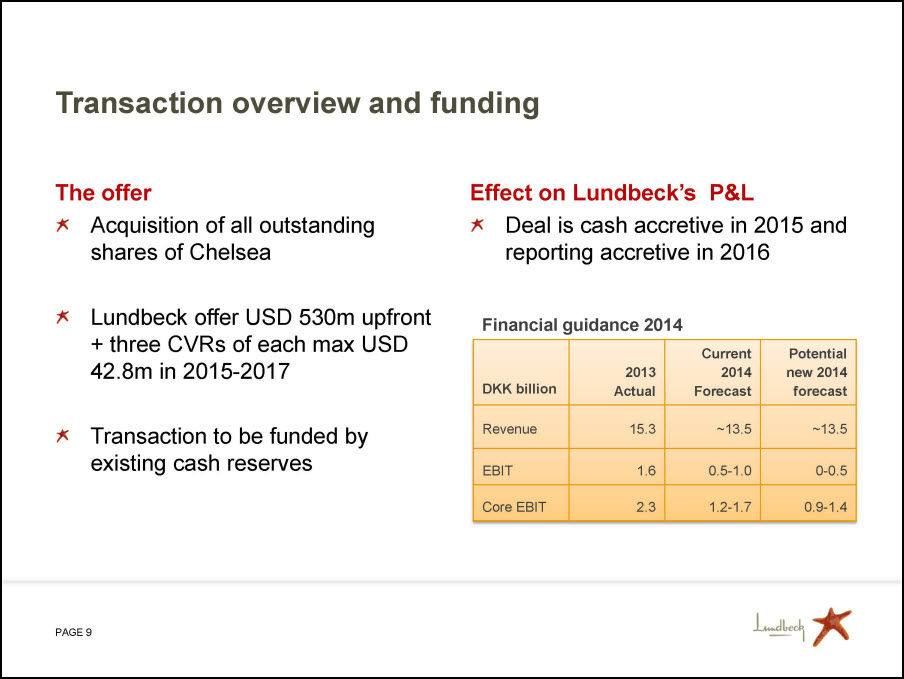
Transaction overview and funding The offer Acquisition of all outstanding shares of Chelsea Lundbeck offer USD 530m upfront + three CVRs of each max USD 42.8m in 2015-2017 Transaction to be funded by existing cash reserves Effect on Lundbeck’s P&L Deal is cash accretive in 2015 and reporting accretive in 2016 Financial guidance 2014 DKK billion 2013 Actual Current 2014 Forecast Potential new 2014 forecast Revenue 15.3 ~13.5 ~13.5 EBIT 1.6 0.5-1.0 0-0.5 Core EBIT 2.3 1.2-1.7 0.9-1.4
SIGNIFICANTLY ENHANCE LUNDBECK US’ PLATFORM FOR LONG-TERM GROWTH Continue our position as an innovative neurology company Significant overlap to Xenazine target group Relatively low risk
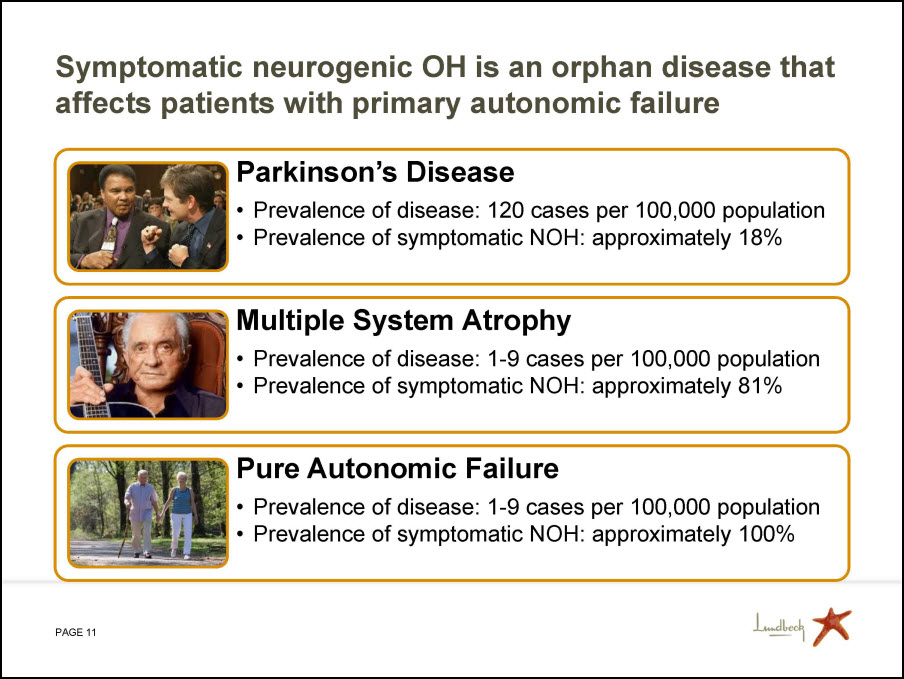
Symptomatic neurogenic OH is an orphan disease that affects patients with primary autonomic failure Parkinson’s Disease Prevalence of disease: 120 cases per 100,000 population Prevalence of symptomatic NOH: approximately 18% Multiple System Atrophy Prevalence of disease: 1-9 cases per 100,000 population Prevalence of symptomatic NOH: approximately 81% Pure Autonomic Failure Prevalence of disease: 1-9 cases per 100,000 population Prevalence of symptomatic NOH: approximately 100%