Exhibit 99.1
| A better clinical pathway for cancer monitoring Antonius Schuh, Ph.D. | Chief Executive Officer June 2013 |
| Forward-Looking Statements DISCLAIMER Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts are "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission. |
| Our goal: Towards better treatment for cancer INTRODUCTION A better clinical pathway for cancer monitoring Novel clinical utility: non-invasive, near real-time detection of oncogene mutations for any tumor type |
| Welcome to Trovagene, Inc. INTRODUCTION INTRODUCTION Addresses a high unmet need in cancer by enabling easier, more frequent monitoring of emergence of oncogene mutation status, disease progression and recurrence Unique IP around cell-free DNA in urine creates a powerful core diagnostic platform for the non-invasive detection of oncogene mutations, with urine providing the competitive advantages of ease of sampling and large volume Cancer monitoring based on urine testing is now ready for clinical use as improved PCR technologies and next generation sequencing platforms are available at much reduced cost CLIA certified, CAP accredited high complexity diagnostic laboratory to offer diagnostic services Technology development collaborations with leading corporate partners NASDAQ: TROV $111M market cap* Molecular Diagnostic Specialist founded in 1999 NASDAQ listing 2012 $55M invested in R&D and IP Focused on oncology and infectious disease *Correct on 19 June, 2013 |
| TEAM Antonius Schuh PhD CEO CEO Sorrento Therapeutics, AviaraDX, Arcturus Biosciences, Sequenom PhD Pharm. Chem Stephen Zaniboni CFO Mark Erlander PhD CSO Keith McCormick VP, Comm. Ops Michael Terry VP, Corp. Devel. Thomas Adams, PhD (Chair) John P. Brancaccio, CPA Gabriele M. Cerrone, MBA CFO, Awarepoint, XIFIN, Sorrento Therapeutics, AviaraDX, Arcturus, Sequenom CPA, BS Accounting, MBA Boston College CSO, bioTheranostics, AviaraDx, Arcturus Biosciences Research Fellow, J&J Assistant Professor, Scripps Research Institute BS, MS Biochem; Ph.D. Neuroscience Senior Director of Sales Operations, for AviaraDx (now bioTheranostics). BA, MBA President, Ligand DX, EVP Sequenom, EVP and MD of European Operations, Lumenis, Director-level position, GE Healthcare Medical BS Econ & Business, AD Int’l Business Paul Billings, MD, PhD Carlo M. Croce, MD Riccardo Dalla-Favera, MD Brunangelo Falini, MD Kunwar Shailubhai, PhD Gary S. Jacob, PhD Antonius Schuh, PhD Stanley Tennant, MD Chris McGuigan, PhD Scientific Advisors Board of Directors Seasoned Team |
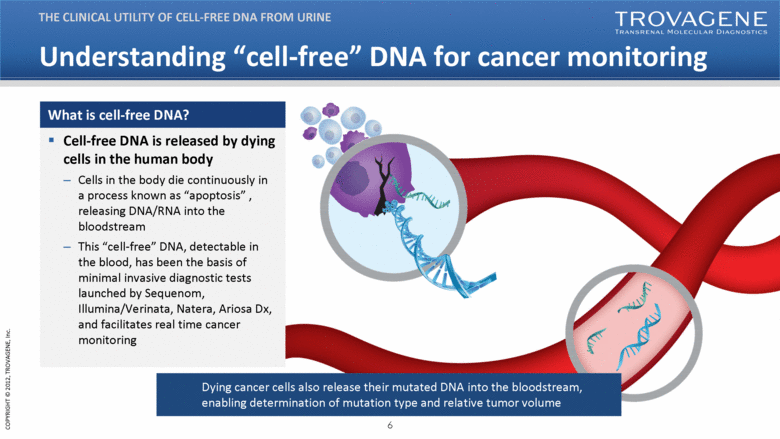
| Understanding “cell-free” DNA for cancer monitoring THE CLINICAL UTILITY OF CELL-FREE DNA FROM URINE Cell-free DNA is released by dying cells in the human body Cells in the body die continuously in a process known as “apoptosis” , releasing DNA/RNA into the bloodstream This “cell-free” DNA, detectable in the blood, has been the basis of minimal invasive diagnostic tests launched by Sequenom, Illumina/Verinata, Natera, Ariosa Dx, and facilitates real time cancer monitoring What is cell-free DNA? Dying cancer cells also release their mutated DNA into the bloodstream, enabling determination of mutation type and relative tumor volume |
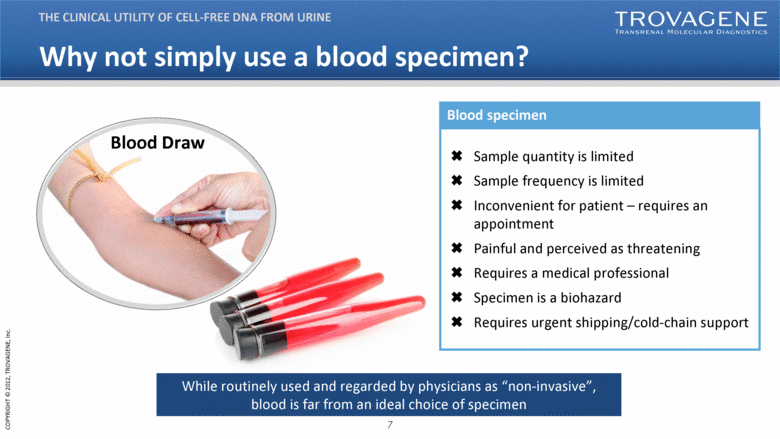
| Why not simply use a blood specimen? THE CLINICAL UTILITY OF CELL-FREE DNA FROM URINE Blood Draw Sample quantity is limited Sample frequency is limited Inconvenient for patient – requires an appointment Painful and perceived as threatening Requires a medical professional Specimen is a biohazard Requires urgent shipping/cold-chain support While routinely used and regarded by physicians as “non-invasive”, blood is far from an ideal choice of specimen Blood specimen |
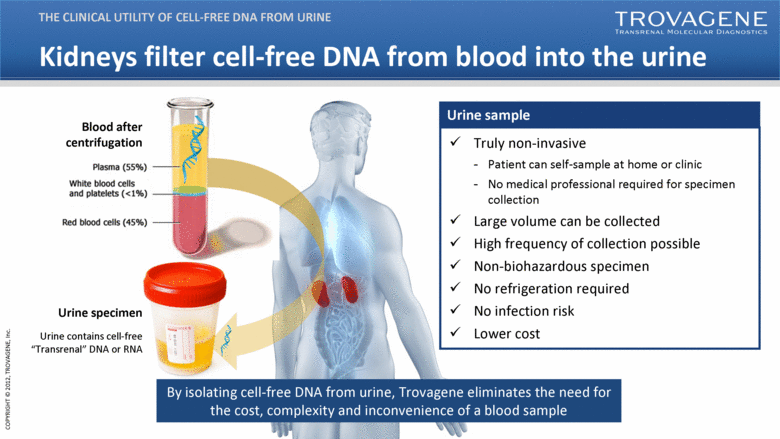
| Kidneys filter cell-free DNA from blood into the urine THE CLINICAL UTILITY OF CELL-FREE DNA FROM URINE By isolating cell-free DNA from urine, Trovagene eliminates the need for the cost, complexity and inconvenience of a blood sample Truly non-invasive Patient can self-sample at home or clinic No medical professional required for specimen collection Large volume can be collected High frequency of collection possible Non-biohazardous specimen No refrigeration required No infection risk Lower cost Urine contains cell-free “Transrenal” DNA or RNA Urine sample Blood after centrifugation Plosmo (55%) White blood cells and platelels (<1%) Red blood cells (45%) Urine specimen |
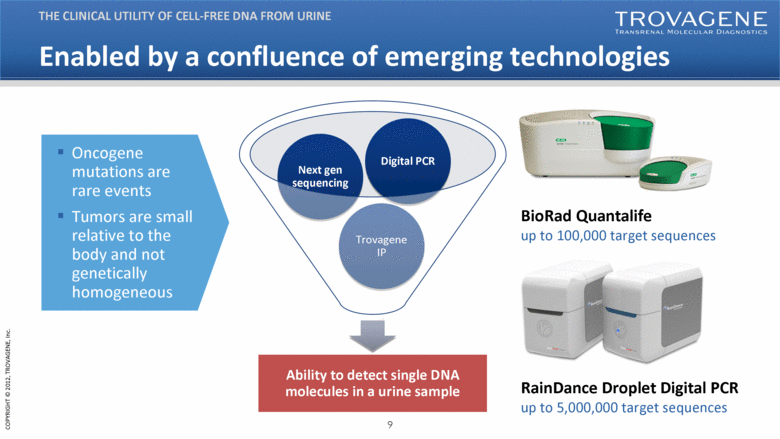
| Enabled by a confluence of emerging technologies Ability to detect single DNA molecules in a urine sample Trovagene IP Next gen sequencing Digital PCR THE CLINICAL UTILITY OF CELL-FREE DNA FROM URINE BioRad Quantalife up to 100,000 target sequences RainDance Droplet Digital PCR up to 5,000,000 target sequences Oncogene mutations are rare events Tumors are small relative to the body and not genetically homogeneous |
| Why Cell-free DNA? Tumors are heterogeneous in their tumor mutational make-up Would need multiple tissue/tumor biopsies to determine heterogeneity and optimal therapy Cell-free DNA from tumors is released into the blood plasma after cell-death Previously established that cell-free DNA increases with cancer stage in plasma Technology now available to detect mutations: Digital droplet PCR and Next Generation Sequencing Platforms THE CLINICAL UTILITY OF CELL-FREE DNA FROM URINE Cell-free DNA gives a global view of tumor heterogeneity for metastatic cancer patients Detection of specific tumor mutations Monitoring of tumor load/response to therapy Detection of emerging tumor resistant mutations/new directed therapy 6-8 weeks treatment cycle Therapeutic Monitor Response Review treatment Options The Need Continual, qualitative and quantitative determination of tumor load and oncogene mutation status Clinical Utility |
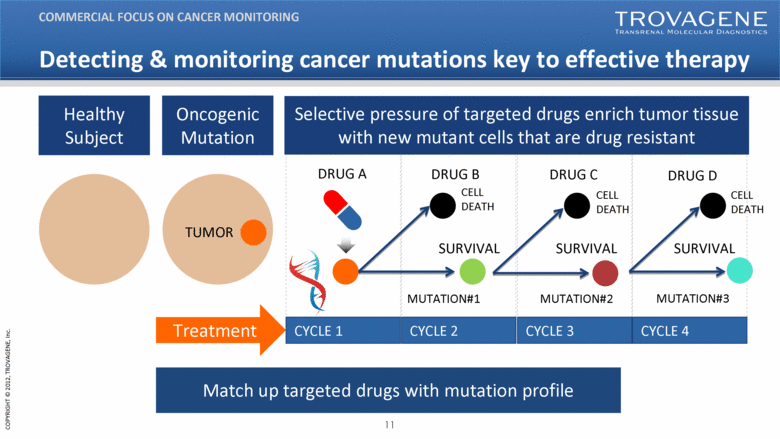
| Healthy Subject Oncogenic Mutation DRUG A CYCLE 1 CYCLE 2 CYCLE 3 CYCLE 4 SURVIVAL TUMOR MUTATION#1 Selective pressure of targeted drugs enrich tumor tissue with new mutant cells that are drug resistant Match up targeted drugs with mutation profile Treatment SURVIVAL DRUG B DRUG C DRUG D SURVIVAL CELL DEATH CELL DEATH CELL DEATH MUTATION#2 MUTATION#3 Detecting & monitoring cancer mutations key to effective therapy COMMERCIAL FOCUS ON CANCER MONITORING |
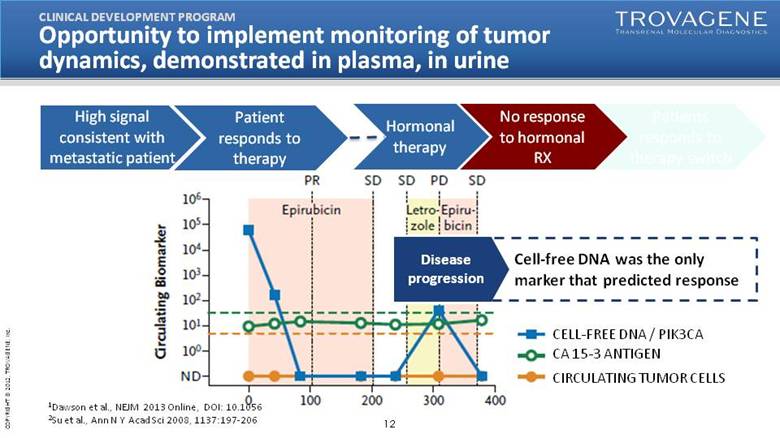
| Opportunity to implement monitoring of tumor dynamics, demonstrated in plasma, in urine High signal consistent with metastatic patient CLINICAL DEVELOPMENT PROGRAM Patient responds to therapy Disease progression Hormonal therapy No response to hormonal RX Circulating Biomarker Epirubicin Letro-zole Epiru-bicin1Dawson et al., NEJM 2013 Online, DOI: 10.1056 2Su et al., Ann N Y Acad Sci 2008, 1137:197-206 Cell-free DNA was the only marker that predicted response CELL-FREE DNA / PIK3CA CA 15-3 ANTIGEN CIRCULATING TUMOR CELLS |
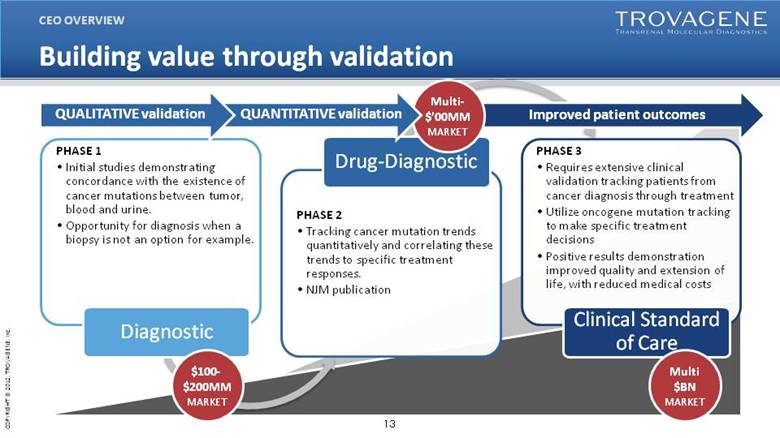
| Building value through validation PHASE 1 Initial studies demonstrating concordance with the existence of cancer mutations between tumor, blood and urine. Opportunity for diagnosis when a biopsy is not an option for example. Diagnostic PHASE 2 Tracking cancer mutation trends quantitatively and correlating these trends to specific treatment responses. NJM publication Drug-Diagnostic PHASE 3 Requires extensive clinical validation tracking patients from cancer diagnosis through treatment Utilize oncogene mutation tracking to make specific treatment decisions Positive results demonstration improved quality and extension of life, with reduced medical costs Clinical Standard of Care $100-$200MM MARKET Multi $BN MARKET Improved patient outcomes Multi-$’00MM MARKET QUANTITATIVE validation QUALITATIVE validation CEO OVERVIEW |
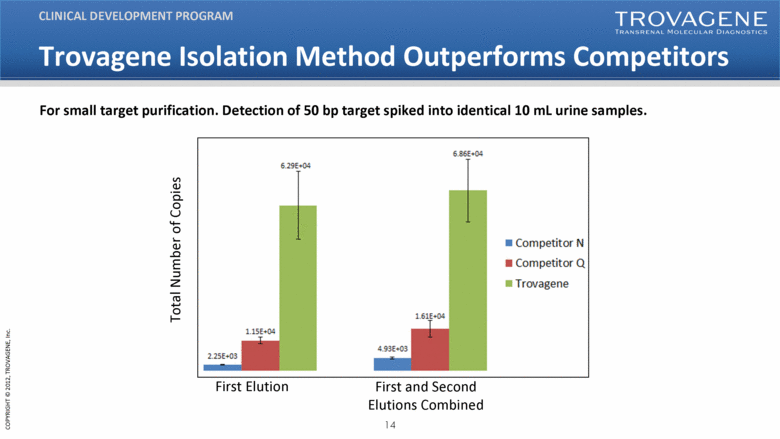
| Trovagene Isolation Method Outperforms Competitors CLINICAL DEVELOPMENT PROGRAM For small target purification. Detection of 50 bp target spiked into identical 10 mL urine samples. Competitor N Competitor Q Travagene First Elution First and Second Elutions Combined Total Number of Copies |
| STEP ONE Pre-amplification with wild-type suppression DNA template with mutation (“M”; Patient DNA) A B STEP TWO Amplification with A,B primers, digital droplet PCR (Raindance) DNA template with no mutation (Wild Type (“WT”; Patient DNA) WT Blocker of A,B primers M WT A B M M TAQMAN Probe Amplification of DNA template with mutation (“M”; Patient DNA) 15 cycles of PCR Two-Step Assay Design for 28-30 bp footprint CLINICAL DEVELOPMENT PROGRAM Solves the challenge of quantitative mutation analysis in very short DNA fragments Small footprint assays |
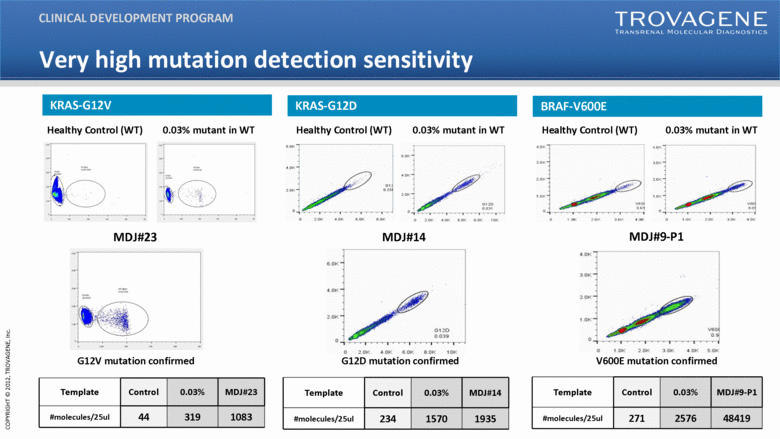
| 0.03% mutant in WT Healthy Control (WT) G12V mutation confirmed MDJ#23 MDJ#9-P1 V600E mutation confirmed MDJ#14 G12D mutation confirmed 0.03% mutant in WT Healthy Control (WT) 0.03% mutant in WT Healthy Control (WT) Template Control 0.03% MDJ#23 #molecules/25ul 44 319 1083 Template Control 0.03% MDJ#14 #molecules/25ul 234 1570 1935 Template Control 0.03% MDJ#9-P1 #molecules/25ul 271 2576 48419 CLINICAL DEVELOPMENT PROGRAM KRAS-G12V KRAS-G12D BRAF-V600E Very high mutation detection sensitivity |
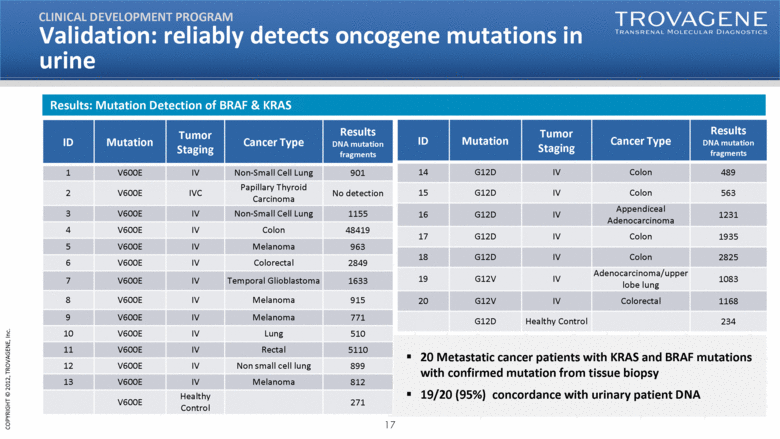
| ID Mutation Tumor Staging Cancer Type Results DNA mutation fragments 14 G12D IV Colon 489 15 G12D IV Colon 563 16 G12D IV Appendiceal Adenocarcinoma 1231 17 G12D IV Colon 1935 18 G12D IV Colon 2825 19 G12V IV Adenocarcinoma/upper lobe lung 1083 20 G12V IV Colorectal 1168 G12D Healthy Control 234 20 Metastatic cancer patients with KRAS and BRAF mutations with confirmed mutation from tissue biopsy 19/20 (95%) concordance with urinary patient DNA ID Mutation Tumor Staging Cancer Type Results DNA mutation fragments 1 V600E IV Non-Small Cell Lung 901 2 V600E IVC Papillary Thyroid Carcinoma No detection 3 V600E IV Non-Small Cell Lung 1155 4 V600E IV Colon 48419 5 V600E IV Melanoma 963 6 V600E IV Colorectal 2849 7 V600E IV Temporal Glioblastoma 1633 8 V600E IV Melanoma 915 9 V600E IV Melanoma 771 10 V600E IV Lung 510 11 V600E IV Rectal 5110 12 V600E IV Non small cell lung 899 13 V600E IV Melanoma 812 V600E Healthy Control 271 Validation: reliably detects oncogene mutations in urine CLINICAL DEVELOPMENT PROGRAM Results: Mutation Detection of BRAF & KRAS |
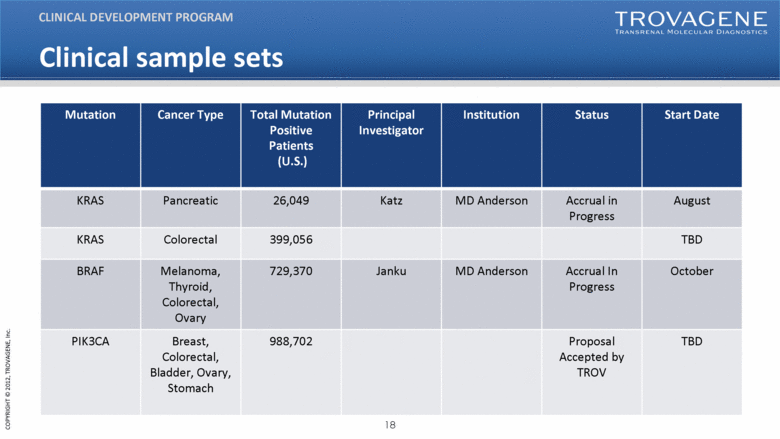
| Clinical sample sets Mutation Cancer Type Total Mutation Positive Patients (U.S.) Principal Investigator Institution Status Start Date KRAS Pancreatic 26,049 Katz MD Anderson Accrual in Progress August KRAS Colorectal 399,056 TBD BRAF Melanoma, Thyroid, Colorectal, Ovary 729,370 Janku MD Anderson Accrual In Progress October PIK3CA Breast, Colorectal, Bladder, Ovary, Stomach 988,702 Proposal Accepted by TROV TBD CLINICAL DEVELOPMENT PROGRAM |
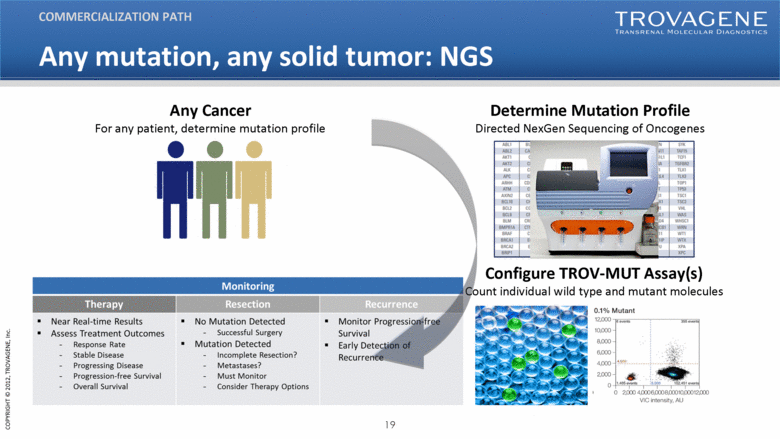
| Any mutation, any solid tumor: NGS Configure TROV-MUT Assay(s) Count individual wild type and mutant molecules Any Cancer For any patient, determine mutation profile Determine Mutation Profile Directed NexGen Sequencing of Oncogenes COMMERCIALIZATION PATH |
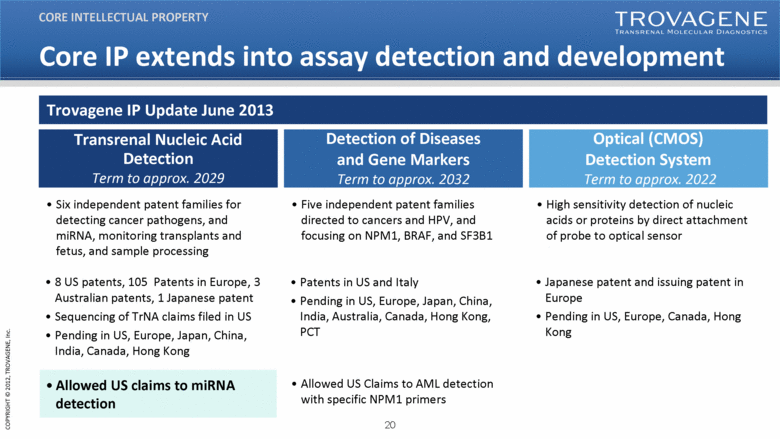
| Core IP extends into assay detection and development Transrenal Nucleic Acid Detection Term to approx. 2029 Allowed US claims to miRNA detection Detection of Diseases and Gene Markers Term to approx. 2032 Allowed US Claims to AML detection with specific NPM1 primers Optical (CMOS) Detection System Term to approx. 2022 Six independent patent families for detecting cancer pathogens, and miRNA, monitoring transplants and fetus, and sample processing Five independent patent families directed to cancers and HPV, and focusing on NPM1, BRAF, and SF3B1 High sensitivity detection of nucleic acids or proteins by direct attachment of probe to optical sensor 8 US patents, 105 Patents in Europe, 3 Australian patents, 1 Japanese patent Sequencing of TrNA claims filed in US Pending in US, Europe, Japan, China, India, Canada, Hong Kong Patents in US and Italy Pending in US, Europe, Japan, China, India, Australia, Canada, Hong Kong, PCT Japanese patent and issuing patent in Europe Pending in US, Europe, Canada, Hong Kong Trovagene IP Update June 2013 CORE INTELLECTUAL PROPERTY |
| Trovagene’s Proprietary HPV Test |
| HPV in Urine: Market Dynamics Market Opportunity Estimated 11-12M HPV tests run annually (US) 1 Represents only about 35% market penetration 8.7 – 14.2% of cases come back as positive2 Repeat HPV testing often performed at 6 and 12 months Advantages Unique primer pair amplifying the E1 region provides Freedom-To-Operate (FTO) for molecular HPV testing E1 region offers novel, proprietary approach to high risk HPV identification Simplicity of testing: only 1 PCR reaction identifies all high-risk HPV subtypes Uses Trovagene’s patented method for DNA isolation HPV 1. McEvoy and Farmer – Licensed Market Research Report – Anatomic Pathology Markets in the US – Cervical Cancer Edition – 2011 – p 305-310 2. Castle et al. Clinical Human Papilloma Virus Detection Forecasts Cervical Cancer Risk in Women Over 18 Years of Follow Up. J Clin Oncol, 30 Jul 2012 |
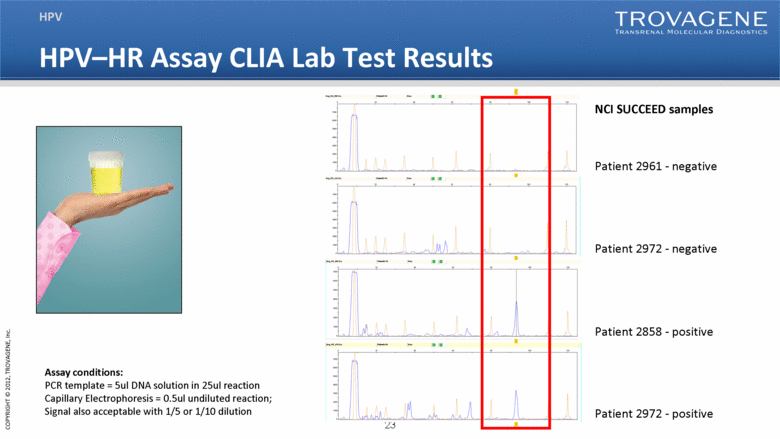
| HPV–HR Assay CLIA Lab Test Results HPV NCI SUCCEED samples Patient 2961 - negative Patient 2972 - negative Patient 2858 - positive Patient 2972 - positive Assay conditions: PCR template = 5ul DNA solution in 25ul reaction Capillary Electrophoresis = 0.5ul undiluted reaction; Signal also acceptable with 1/5 or 1/10 dilution |
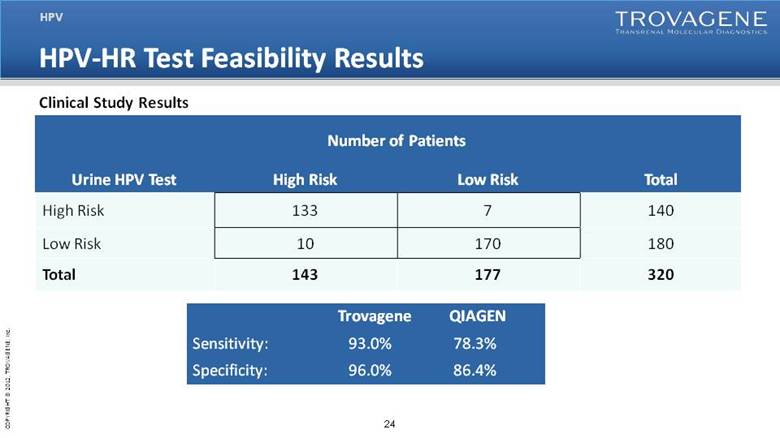
| HPV-HR Test Feasibility Results HPV Urine HPV Test Number of Patients Total High Risk Low Risk High Risk 133 7 140 Low Risk 10 170 180 Total 143 177 320 Trovagene QIAGEN Sensitivity: 93.0% 78.3% Specificity: 96.0% 86.4% Clinical Study Results |
| Validating Analytical Performance of Key Assay Europe (UK) Queen Mary University of London PREDICTORS 4 Study - 500 patient cohort - samples currently being processed HPV Brazil Barretos Cancer Hospital – largest cancer hospital in Brazil 350 patient cohort - enrollment underway India Simbiosys Bioware / Metropolis 320 patient cohort complete Strand Life Science Study ongoing International Validation Studies |
| 2012 Achievements Acquired CLIA Laboratory CAP certified, high complexity molecular diagnostic lab Strengthened Patent Portfolio Issued U.S. Patent for NPM Mutants to Diagnose and Monitor Acute Myeloid Leukemia Issued European Patent for Detection of Pathogenic Infections Improved Liquidity and Access to Capital Completed Public Offering of $10 million and began trading on NASDAQ Added to the Russell Microcap and the MSCI Microcap Index Closed Private Placement of $4.4 million Initiated KRAS Mutation Detection Development Program Commenced Validation Program and Commenced Study at MD Anderson Cancer Center for Transrenal KRAS Mutation Detection in Pancreatic Cancer Expanded of High Risk HPV Carrier Screening Development Program Partnered with Strand Life Sciences to Validate and Offer Urine-based HPV Screening Test in India Partnered with Barretos Cancer Hospital to Evaluate Urine-based HPV Assay in Brazil COMMERCIAL DEVELOPMENT UPDATE |
| 2013 Achievements Initiated R&D Partnership with PerkinElmer Collaborate to develop test to determine liver cancer risk Initiated R&D Partnership with Illumina Collaborate to apply NGS for the analysis of transrenal sequence signals Transferred High Risk HPV Carrier Screening Test into CLIA lab Pilot launch in Southern California in Q1 2013 Extended Oncogene Mutation Detection Development Program Began Second Study at MD Anderson Cancer Center focusing on BRAF and KRAS Mutations in 2013 Initiated HCC development program Expanded Management Team Appointed Mark Erlander, Ph.D., Chief Scientific Officer COMMERCIAL DEVELOPMENT UPDATE |
| PerkinElmer R&D agreement Research & Development agreement with PerkinElmer to develop a test to determine the risk for developing liver cancer TROV will develop the test TROV and PKI will jointly validate the test The Companies will collaborate on potential automation for TROV’s DNA isolation technique PKI has an option to receive an exclusive royalty bearing license in an undisclosed field Financial terms were not disclosed, but include milestone payments. COMMERCIAL DEVELOPMENT UPDATE |
| Illumina Agreement Goal – POC to develop library for NGS (next generation sequencing) from cell-free DNA isolated from urine Strategic imperative to establish TrNAs on NGS NGS will become a platform standard in the industry TROV to gain significant domain knowledge from market leader in NGS Application focus - oncogene mutations and prenatal diagnosis Illumina to provide samples and sequencing Trovagene to provide DNA extraction and purification COMMERCIAL DEVELOPMENT UPDATE Multiphase collaboration signed |
| 2013 Milestones Oncology Mutation Detection Tests Launch KRAS 2Q 2013 Launch BRAF 3Q 2013 Launch PIK3CA 4Q 2013 Launch HCC – HBV and p53 4Q 2013 Expand Clinical Collaboration Program Add Clinical Trial Collaborators 4Q 2013 Execute Commercial Sales and Marketing Plan Initiate HPV Pilot Launch 1Q 2013 Initiate Oncology Pilot Launch 2Q 2013 Expand Sales Force 4Q 2013 COMMERCIAL DEVELOPMENT UPDATE |
| For further information Please contact: Antonius Schuh, CEO aschuh@trovagene.com Stephen Zaniboni, CFO szaniboni@trovagene.com |