
NOVEMBER 2, 2023 Q3 2023 Financial Results and Business Update

2 Forward-looking statements CERTAIN STATEMENTS IN THIS PRESENTATION ARE FORWARD-LOOKING within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern our expectations, strategy, plans or intentions. These forward-looking statements are based on our current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, our need for additional financing; our ability to continue as a going concern; clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidates; our clinical trials may encounter delays in initiation or enrollment that impact the cost and timing of the trial readout; risks related to business interruptions, including the outbreak of COVID-19 coronavirus, which could seriously harm our financial condition and increase our costs and expenses; uncertainties of government or third-party payer reimbursement; dependence on key personnel; limited experience in marketing and sales; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; regulatory, and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. There are no guarantees that any of our technology or products will be utilized or prove to be commercially successful. Additionally, there are no guarantees that future clinical trials will be completed or successful or that any precision medicine therapeutics will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in our Form 10-K for the year ended December 31, 2022, and other periodic reports filed with the Securities and Exchange Commission. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward- looking statements. Forward-looking statements included herein are made as of the date hereof, and we do not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.

3 Q3 2023 announcements were transformational for Cardiff Oncology • Data from 2nd line Phase 1b/2 trial in KRAS-mutated mCRC • Conclusions from Type C meeting with FDA • Expansion of Pfizer relationship August data release • Metastatic pancreatic ductal adenocarcinoma (mPDAC) • Small cell lung cancer (SCLC) September data release

4 Cardiff Oncology has several core strengths to create value 1. Onvansertib’s clinical signal of efficacy and tolerability 2. Onvansertib’s novel approach to PLK1 inhibition 3. Onvansertib can combine with 1st / 2nd line SoC regimens that target large populations 4. Third party support and validation including FDA, Pfizer 5. Strong financial position with cash runway into 2025

5 Cardiff Oncology is focused on three objectives to create value 1. Lead program: RAS-mut mCRC Generate data from the 1st line RAS-mutated mCRC CRDF-004 trial in mid-2024 2. Earlier-stage programs: Pancreatic, SCLC, TNBC 3. Pipeline expansion: Preclinical programs Develop through investigator-initiated trials Conduct preclinical studies of new combinations and indications
6 Cardiff Oncology is focused on three objectives to create value 1. Lead program: RAS-mut mCRC Generate data from the 1st line RAS-mutated mCRC CRDF-004 trial in mid-2024 • Phase 1b/2 data from 2nd line KRAS-mut mCRC – 73% ORR in bev naïve patients vs. ~25% for historical controls – 15-month median PFS in bev naïve patients vs. ~7-8 months for historical controls • Preclinical program – Onvansertib and bevacizumab have independent MOAs reducing tumor vasculature – Data from mCRC study in 135 patients identified resistance mechanisms • Agreed 1st line clinical path from Type C meeting with FDA – CRDF-004: provides randomized efficacy data, dose confirmation – CRDF-005: provides registrational path for accelerated and full approval • Pfizer Ignite responsible for clinical execution of CRDF-004 • CRDF-004 interim data readout expected mid-2024
7 Cardiff Oncology is focused on three objectives to create value 1. Lead program: RAS-mut mCRC Generate data from the 1st line RAS-mutated mCRC CRDF-004 trial in mid-2024 2. Earlier-stage programs: Pancreatic, SCLC, TNBC 3. Pipeline expansion: Preclinical programs Develop through investigator-initiated trials Conduct preclinical studies of new combinations and indications
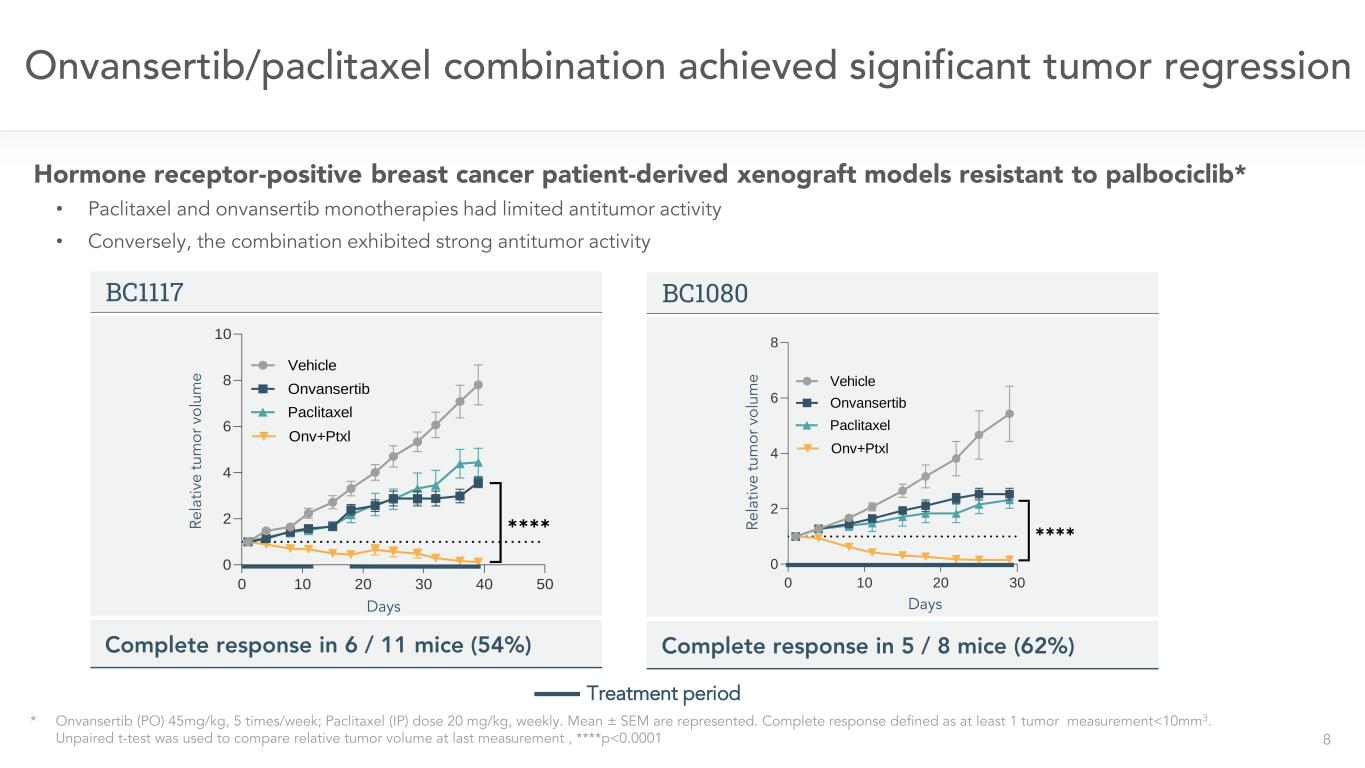
8 Onvansertib/paclitaxel combination achieved significant tumor regression * Onvansertib (PO) 45mg/kg, 5 times/week; Paclitaxel (IP) dose 20 mg/kg, weekly. Mean ± SEM are represented. Complete response defined as at least 1 tumor measurement<10mm3. Unpaired t-test was used to compare relative tumor volume at last measurement , ****p<0.0001 Treatment period Hormone receptor-positive breast cancer patient-derived xenograft models resistant to palbociclib* • Paclitaxel and onvansertib monotherapies had limited antitumor activity • Conversely, the combination exhibited strong antitumor activity BC1117 Complete response in 6 / 11 mice (54%) 0 10 20 30 40 50 0 2 4 6 8 10 Days R e la ti v e T u m o r V o lu m e Vehicle Onvansertib BC1117 (acquired) Paclitaxel Onv+Ptxl ✱✱✱✱Re la tiv e tu m or v ol um e Days BC1080 Complete response in 5 / 8 mice (62%) 0 10 20 30 0 2 4 6 8 Days R e la ti v e T u m o r V o lu m e BC1080 (acquired) Vehicle Onvansertib Paclitaxel Onv+Ptxl ✱✱✱✱Re la tiv e tu m or v ol um e Days

9 Our financial position is strong as of Q3 2023 We expect clinical data from our 1st line RAS-mutated mCRC trial in mid-2024 September 30, 2023 cash and investments* $81.4M Q3 2023 net cash used in Operating Activities* $8.0M Runway with current cash extends into 2025 * Financial information above is derived from our unaudited financials in Form 10Q filed on 11/2/23. Summary financial information as of September 30, 2023

10 Cardiff Oncology has several core strengths to create value 1. Onvansertib’s clinical signal of efficacy and tolerability 2. Onvansertib’s novel approach to PLK1 inhibition 3. Onvansertib can combine with 1st / 2nd line SoC regimens that target large populations 4. Third party support and validation including FDA, Pfizer 5. Strong financial position with cash runway into 2025









