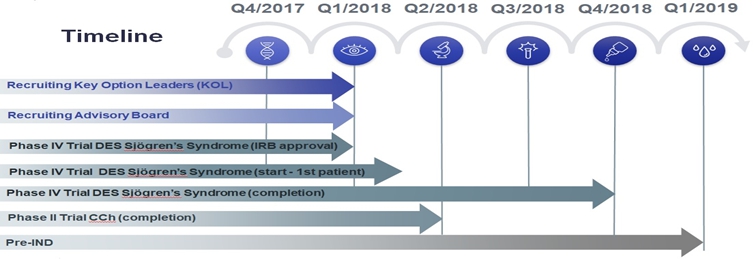
EQUITY OVERVIEW OTCQB: WIZP Share Price (05/03/18): $5.98 Market Cap (05/03/18): ~$29 M Shares Outstanding: ~5 M SUBSTANTIAL MARKET OPPORTUNITY ü $3.7 billion global dry eye market in 2017 to grow to $4.9 billion by 2022 ü 344 million suffer from dry eye syndrome globally ü Large and unaddressed market for dry eye in CCh; up to 1 out of 3 US dry eye patients have CCh ü Unmet need for treating dry eye syndrome in Sjögren's patients, impacting up to 7.7 million people worldwide by 2024 | Investment Highlights LO2A is Approved by Regulatory Authorities In Certain European Countries With 1.53 M Packages Sold by the Licensor in 2017 LO2A is a proven product that has received regulatory approvals and has an established safety and sales history in Germany, Hungary, the Netherlands, and Switzerland by the licensor, who is the product’s inventor. Wize Pharma has the exclusive rights to develop and market LO2A in the US, China, Ukraine, Israel, and may obtain rights in additional territories. Wize benefits from LO2A’s established commercialization and resulting safety and efficacy profile. In addition to being approved to treat dry eye syndrome in four European countries, LO2A is also approved to treat dry eye syndrome and CCh in Hungary and Sjögren's in the Netherlands. $3.7 Billion Global Dry Eye Syndrome Market Opportunity Based on a Market Scope Dry Eye Report, an estimated 344 million people around the world suffer from dry eye syndrome as of 2016 and the market is expected to grow from $3.7 billion in 2017 to $4.9 billion in 2022. According to the Market Scope Report, dry eye syndrome is increasing due to extended viewing of television, computer and Smartphone screens, weather conditions, exposure to air conditioning and increased life expectancy. Wize Sales Expected to Launch in Israel, Ukraine and China in 2018 and 2019 LO2A is already approved for dry eye syndrome in Israel where product launch is expected in 2018. In Ukraine, LO2A is in the approval process for the treatment of dry eye syndrome and CCh, with LO2A product launch expected in 2019. Wize has a framework agreement with a pharmaceutical company in China, which intends to clear LO2A with regulators and launch sales in 2019. Phase II and Phase IV Clinical Trials in Israel and Plan to File IND in US Wize is now conducting a Phase IV study in Sjögren's and has completed patient enrollment in a Phase II study in CCh, both in Israel. After completing these studies, Wize intends to have a pre-IND meeting with the U.S. FDA to present all of the available safety and efficacy clinical trial data that LO2A has established in other markets. Wize plans to seek the FDA’s guidance on the approval path in the US. |