Exhibit 99.1
2019 © All Rights Reserved Planned Move Into Non - Viral Gene Therapy for Inherited Retinal Disorders September 11 , 2019

Cautionary Note Regarding Forward - Looking Statements . This presentation (both written and oral) contains forward - looking statements about our expectations, beliefs or intentions regarding, among other things, our product development efforts, business, financial condition, results of operations, strateg ies or prospects. In addition, from time to time, we or our representatives have made or may make forward - looking statements, orally or in writing. Forward - looking statements can be identi fied by the use of forward - looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. These forward - looking statements may be included in, but are not limited t o, this presentation, various filings made by us with the SEC, press releases or oral statements made by or with the approval of one of our authorized executive officers. Forward - looking stat ements relate to anticipated or expected events, activities, trends or results as of the date they are made. For example, when we discuss the potential benefits of the techno log y licensed from Copernicus or its market potential, we are using a forward - looking statement. Because forward - looking statements relate to matters that have not yet occurred, these statem ents are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward - looking sta tements. Many factors could cause Wize’s actual activities or results to differ materially from the activities and results anticipated in forward - looking statements, including, but not li mited to, the following: the possibility that we will not consummate the transaction with Copernicus or that we shall not meet certain financing conditions under our agreement with Co per nicus, allowing Copernicus to terminate its agreement with us; the possibility that we will not be able to successfully operate our joint venture with Cannabics Pharmace uti cals, Inc.; risks related to the substantial debt that we have incurred; our needs for additional financing; our dependence on a single compound, LO 2 A and on the continuation of our license to commercialize LO 2 A (or, if we consummate the transaction with Copernicus, our dependence on LO 2 A and WP - REP 1 ); our inability to expand our rights under our license of LO 2 A; the initiation, timing, progress and results of our trials and product candidate development efforts; our ability to advance LO 2 A (or, if we consummate the transaction with Copernicus, also WP - REP 1 ) into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for LO 2 A, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of LO 2 A (or, if we consummate the transaction with Copernicus, also WP - REP 1 ); our ability to establish and maintain corporate collaborations; the implementation of our business model and strategic plans for our business and product candidate s; the scope of protection we are able to establish and maintain for intellectual property rights covering LO 2 A (or, if we consummate the transaction with Copernicus, also WP - REP 1 ) and our ability to operate our business without infringing the intellectual property rights of others; estimates of our expenses, future revenues, and capital requirements; competitive co mpanies, technologies and our industry; and statements as to the impact of the political and security situation in Israel on our business. More detailed information about the risks an d uncertainties affecting Wize is included in filings Wize has made and may make with the SEC in the future. These statements are only current predictions and are subject to known and unkn own risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from t hos e anticipated by the forward - looking statements. Given these uncertainties, you should not rely upon forward - looking statements as predictions of future events. All forward - looking st atements attributable to us or persons acting on our behalf included in, but not limited to, this presentation speak only as of the date hereof and are expressly qualified in the ir entirety by the foregoing. Except as required by applicable law, we undertake no obligations to update or revise forward - looking statements to reflect events or circumstances that arise af ter the date made or to reflect the occurrence of unanticipated events. In evaluating forward - looking statements, you should consider these risks and uncertainties. Industry Data . Unless otherwise indicated, information contained in this presentation concerning our industry and the targeted markets, in clu ding for the Copernicus licensed technology, is based on information from our own management estimates and research, as well as from industry and general publ ica tions and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and ass ump tions based on such information and knowledge, which we believe to be reasonable. Our management estimates have not been verified by any independent source, and we have not in dependently verified any third - party information. Additional Information and Where to Find It. This presentation (both written and oral) is not intended to provide you with a complete summary of Wize’s business or financ i al results. For further information about us, you should read our reports and filings with the SEC. Our SEC filings are available to the pub lic through the SEC’s web site at www.sec.gov. and on our website at www.wizepharma.com CAUTIONARY Note
Wize Pharma, Inc. (OTCQB: WIZP) is a clinical - stage ophthalmology company expanding into treatment of rare retinal disorders through gene therapies. HQ: Israel Founded: 2015 Gene Therapy: 2019 Main shareholders: Ridge Valley Corp: ~ 10 % Other large investors: ~ 24 % CORPORATE Overview
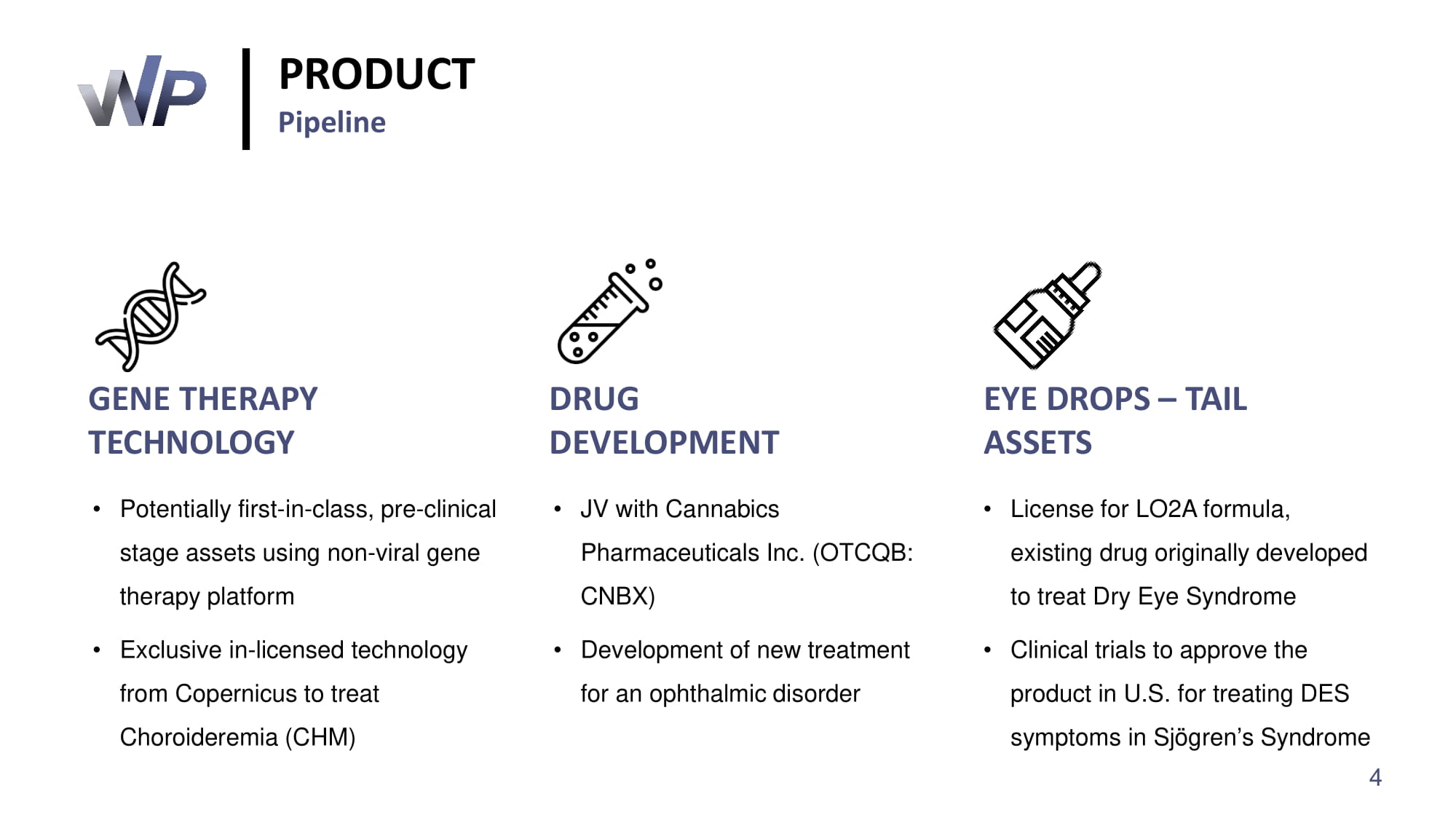
• Potentially first - in - class, pre - clinical stage assets using non - viral gene therapy platform • Exclusive in - licensed technology from Copernicus to treat Choroideremia (CHM) • JV with Cannabics Pharmaceuticals Inc. (OTCQB: CNBX) • Development of new treatment for an ophthalmic disorder • License for LO 2 A formula, existing drug originally developed to treat Dry Eye Syndrome • Clinical trials to approve the product in U.S. for treating DES symptoms in Sjögren’s Syndrome GENE THERAPY TECHNOLOGY DRUG DEVELOPMENT EYE DROPS – TAIL ASSETS PRODUCT Pipeline

• Gene therapy has been successful in eye diseases • First FDA - approved gene therapy for a genetic disease is for an inherited retinal disorder • The eye represents an ideal target organ for gene therapy: - easy visibility and accessibility - highly compartmentalized - ample research on genetic basis of eye conditions • Recent gene therapy acquisitions: Novartis acquires AveXis for $ 8.7 billion & Roche acquiring Spark Therapeutics for $ 4.3 billion • Other recent $ 800 million acquisition in the ocular gene therapy space • Global gene therapy market is expected to reach $ 5.5 billion by 2026 Significant unmet medical need in ophthalmic conditions with no approved treatments and potential for efficient rare - disease commercial model Ophthalmic Non - Viral Gene Therapy Technology EXCLUSIVE License Agreement

DEAL TERMS & Financial details • Wize agreed to license WP - REP 1 from Copernicus Therapeutics • Closing of transaction is contingent on meeting certain closing conditions • Expect to complete exclusive license in Q 4 2019 • Expected return on invested capital to exceed our weighted average cost of capital in a reasonable time period • Wize and Copernicus may also discuss potential future extensions to their collaboration into other gene therapy ophthalmic indications
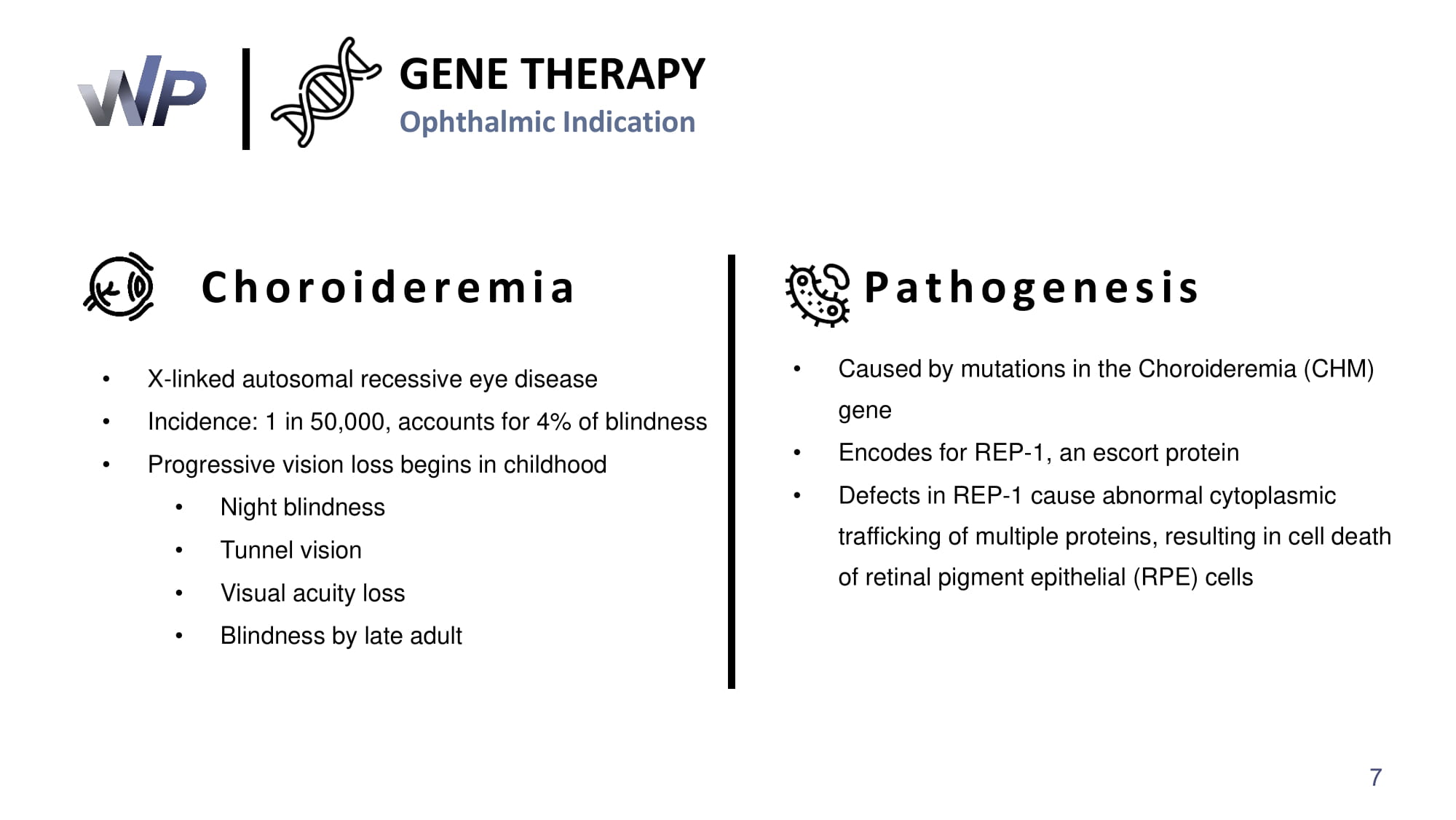
GENE THERAPY Ophthalmic Indication Choroideremia • X - linked autosomal recessive eye disease • Incidence: 1 in 50,000 , accounts for 4 % of blindness • Progressive vision loss begins in childhood • Night blindness • Tunnel vision • Visual acuity loss • Blindness by late adult • Caused by mutations in the Choroideremia (CHM) gene • Encodes for REP - 1 , an escort protein • Defects in REP - 1 cause abnormal cytoplasmic trafficking of multiple proteins, resulting in cell death of retinal pigment epithelial (RPE) cells Pathogenesis
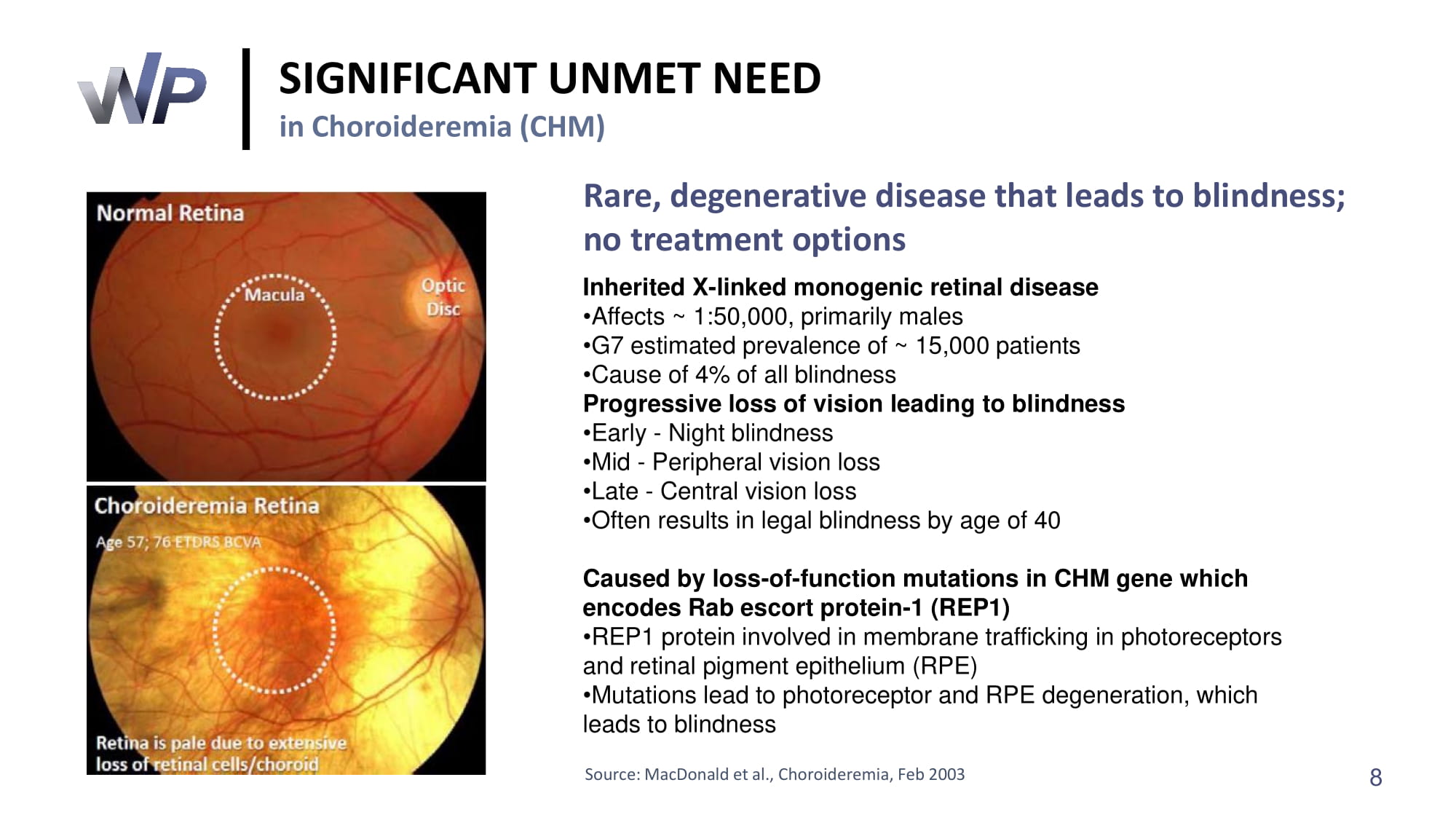
SIGNIFICANT UNMET NEED in Choroideremia (CHM) Rare, degenerative disease that leads to blindness; no treatment options Inherited X - linked monogenic retinal disease • Affects ~ 1:50,000 , primarily males • G 7 estimated prevalence of ~ 15,000 patients • Cause of 4 % of all blindness Progressive loss of vision leading to blindness • Early - Night blindness • Mid - Peripheral vision loss • Late - Central vision loss • Often results in legal blindness by age of 40 Caused by loss - of - function mutations in CHM gene which encodes Rab escort protein - 1 (REP 1 ) • REP 1 protein involved in membrane trafficking in photoreceptors and retinal pigment epithelium (RPE) • Mutations lead to photoreceptor and RPE degeneration, which leads to blindness Source: MacDonald et al., Choroideremia, Feb 2003
• DNA nanoparticle (NP)s encode a normal CHM gene • Ocular administration of CHM DNA NPs will express normal CHM in RPE and photoreceptor retinal cells • Visual deterioration halted, and potential improvements in vision anticipated Strong scientific rationale for intraocular delivery of gene therapy and encouraging early data suggests potentially meaningful benefit by slowing, arresting, or even potentially partially reversing versus natural progression of the disease. DNA NANOPARTICLE TREATMENT of Choroideremia
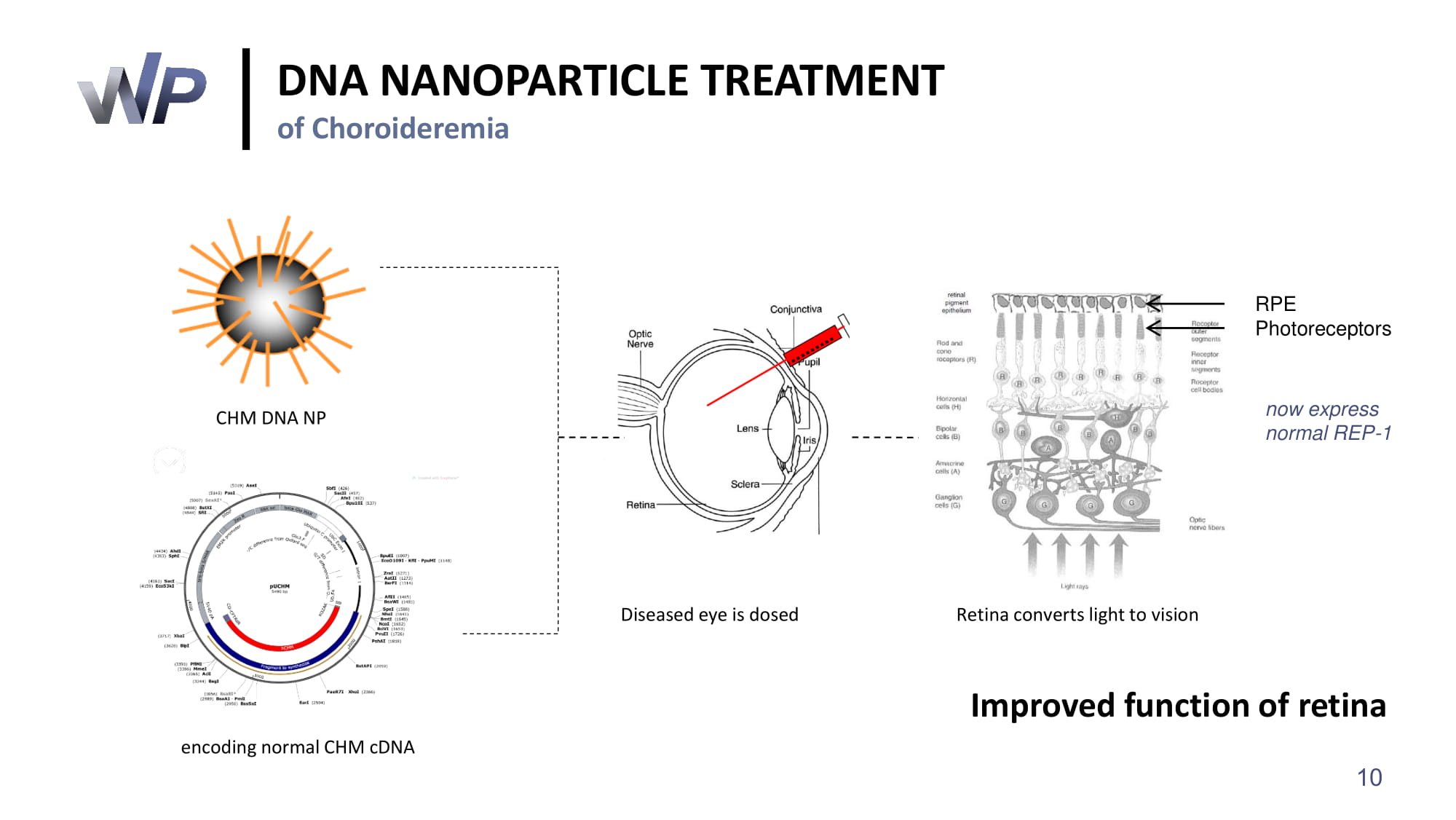
DNA NANOPARTICLE TREATMENT of Choroideremia CHM DNA NP encoding normal CHM cDNA Diseased eye is dosed Retina converts light to vision RPE Photoreceptors Improved function of retina now express normal REP - 1

Visual Acuity • Routinely tested in clinical practice • Direct functional measure of vision loss/gain • Realtime imaging of entire macula • Automated eye tracking • Stimulation of same retinal loci across tests/visits • Higher sensitivity than visual acuity Microperimetry CLEAR ENDPOINTS IN RETINAL DISEASE Clinical Trials All Rights Reserved Source: Maia Macular Integrity Assessment Counterview SpA. Ophthal Retina 2018

WP - REP 1 for Choroideremia • DNA nanoparticle Proof - of - Concept (non - viral gene therapy) • Potential to maintain and perhaps reverse vision loss • Approvals do not require large clinical trials • FDA Regenerative Medicine Advanced Therapy Designation (RMAT) • FDA and EMA Orphan Drug Designation • Eligible for rare pediatric disease priority review voucher POTENTIAL FOR PRODUCT LAUNCH

Maybe we don’t need viral vectors 1 ? The purpose of a viral vector is to deliver DNA. But what if DNA and thus genes, could be packaged in nanoparticles and delivered to any cell type with an injection or an aerosol? Copernicus Therapeutics (private) has developed a non - viral approach for taking a gene and condensing it into a nanoparticle. The condensing process mirrors our own chromosomes, which themselves are packaged, condensed DNA, thus the process is natural. 1 Source: Gene Therapy and Immune Oncology: It's Just the Beginning Could Regenerative Medicine Emerge Next? COPERNICUS The Technology
RETHINKING GENE THERAPY LONG TERM TREATMENTS Utilizing Copernicus proprietary compacted nanoparticle delivery system to treat diseases without some of the drawbacks of AAV therapies. AAV therapies have limits when it comes to repeat administration. Non - immunogenic technology allows repeat dosing. MORE THAN ONCE Copernicus technology is optimizing gene expression for stability over the long term, so that means less trips to the doctor and more time enjoying life. COPERNICUS The Technology
WE ARE DIFFERENT Not toxic Repeat dosing possible (virus vectors produce immune responses) Drug does NOT alter person’s DNA (no insertion) Efficient and consistent (≥AAV) delivery to cells, Both dividing AND non - dividing cells Magnitude and duration of expression ≥AAV vectors Commercializable formulation Copernicus does final assembly (raw materials are commodities)

OVERCOMING The Delivery Hurdle In collaboration with D. Kube and P. Davis, CWRU tight binding to cell surface nucleolin complex non - degradative, active trafficking pathway into nucleus Nanoparticle nucleolus nucleolin nuclear pore Released DNA

Robert C. Moen MD, PhD Dr . Moen has served as Copernicus‘ President and CEO since 1998 . He has been involved in over three dozen cellular and gene therapy clinical trials (mostly involving viral - based systems) as VP of Clinical and Regulatory Affairs of the Gene Therapy Unit at Baxter Healthcare ; as VP of Clinical Development at Geneic Sciences ; and as a co - founder and Director of Clinical and Regulatory Affairs (including QA) at Genetic Therapy, Inc . where he also served as Director of Cell Engineering, and also was in charge of the initial manufacturing and QC efforts . Genetic Therapy, Inc . was acquired by Sandoz (now Novartis) in 1995 for over $ 300 M . He also served at the National Institutes of Health (NIH) where he assisted in the development of the gene therapy program . Prior to his service at the NIH, Dr . Moen was on the faculty of the University of Wisconsin . He is Board Certified in Pediatrics (Stanford University) and Allergy, Immunology (University of Wisconsin) . He received his M . D . and Ph . D . in Biochemistry from the University of Washington – Seattle, and his B . A . in Biochemistry from Harvard . Mark J. Cooper MD As one of Copernicus’ academic founders and the current Senior VP of Science and Medical Affairs, Dr . Cooper is actively involved in the clinical development of our nanoparticle gene therapy technology . He joined Copernicus from Case Western Reserve University School of Medicine where he was an Associate Professor of Medicine, Division of Hematology and Oncology . He is Board Certified in Medical Oncology (National Cancer Institute, NIH) and Internal Medicine (Johns Hopkins Hospital) . He received his M . D . degree from Johns Hopkins University School of Medicine and an AB degree in chemistry and biochemistry from Cornell University . Dr . Cooper has authored 64 publications and is the inventor on seven issued U . S . patents and one patent pending . Dr . Cooper has over 15 years of direct experience in the field of non - viral gene therapy and has successfully managed R&D as well as clinical development programs . COPERNICUS Leading Development Team
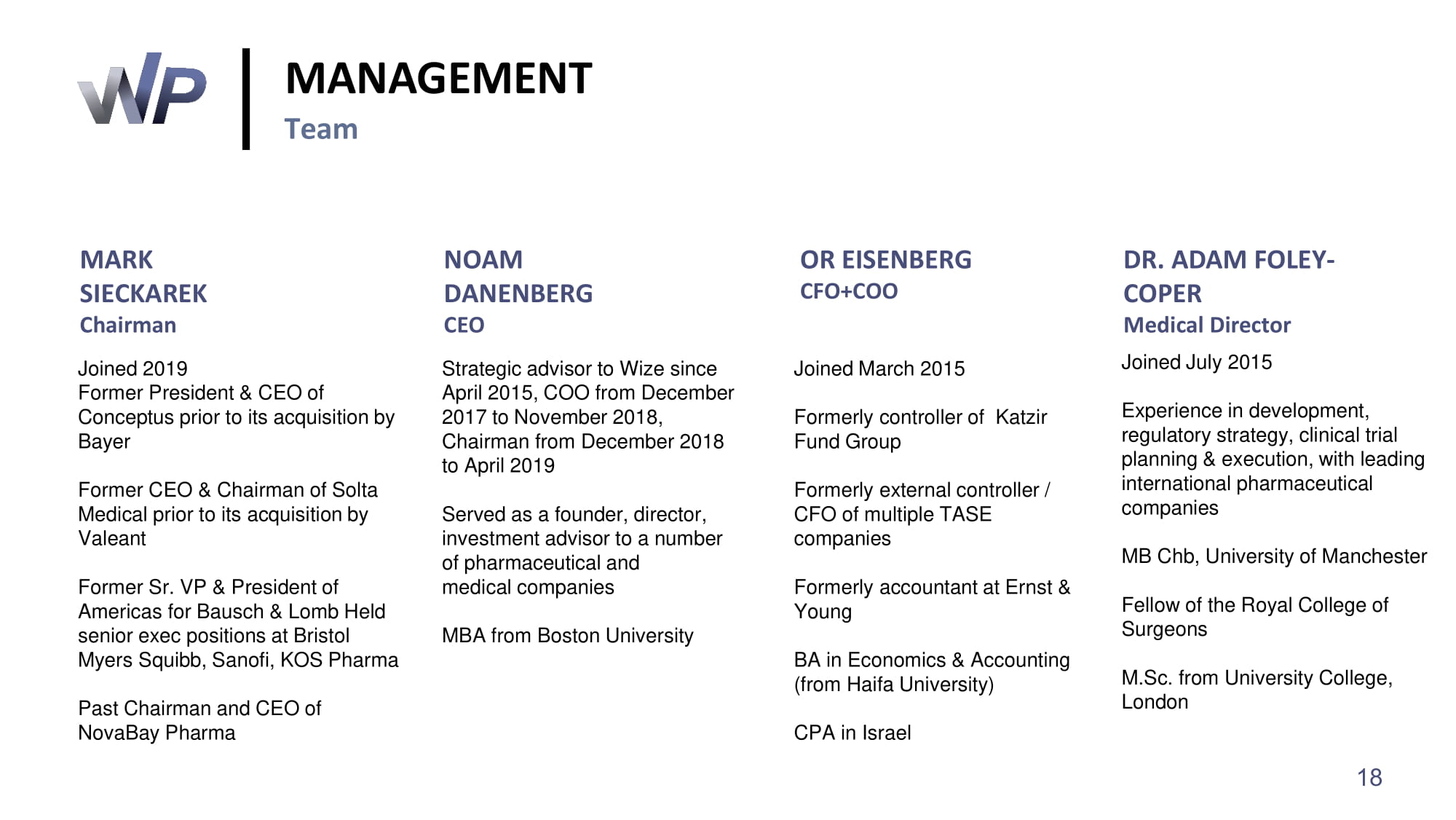
MARK SIECKAREK Chairman Joined 2019 Former President & CEO of Conceptus prior to its acquisition by Bayer Former CEO & Chairman of Solta Medical prior to its acquisition by Valeant Former Sr. VP & President of Americas for Bausch & Lomb Held senior exec positions at Bristol Myers Squibb, Sanofi, KOS Pharma Past Chairman and CEO of NovaBay Pharma MANAGEMENT Team NOAM DANENBERG CEO Strategic advisor to Wize since April 2015 , COO from December 2017 to November 2018 , Chairman from December 2018 to April 2019 Served as a founder, director, investment advisor to a number of pharmaceutical and medical companies MBA from Boston University OR EISENBERG CFO+COO Joined March 2015 Formerly controller of Katzir Fund Group Formerly external controller / CFO of multiple TASE companies Formerly accountant at Ernst & Young BA in Economics & Accounting (from Haifa University) CPA in Israel DR. ADAM FOLEY - COPER Medical Director Joined July 2015 Experience in development, regulatory strategy, clinical trial planning & execution, with leading international pharmaceutical companies MB Chb, University of Manchester Fellow of the Royal College of Surgeons M.Sc. from University College, London

DR. PENNY ASBELL KOL in the treatment of DES PI in clinical studies that led to development of pharmaceuticals for pivotal treatment of ocular conditions including DES Prof. of Ophthalmology at Icahn School of Medicine, Mount Sinai Established Mount Sinai's Lowenstein Foundation Sjogren's Center & Founded Ocular Inflammatory Biomarker Laboratory Authored 100 s of articles, 25 book chapters and presented over 200 lectures and courses One of the world’s leading experts in the field of ocular surface diseases, including DES and meibomitis management PI in dozens of clinical programs including two FDA approved medications for DES Wrote 5 book chapters and over 60 articles in ophthalmology medical journals Received doctorate from Harvard Medical School Founder of Tauber Eye Center in Kansas City, Missouri SCIENTIFIC ADVISORY Board DR. JOSEPH TAUBER

THANK YOU Visit wizepharma.com or email info@wizepharma.com 24 Hanagar St. Hod Hasharon, Israel Tel: + 972 72 2600536 Fax: + 972 72 2600537



















