Exhibit 99.1
| Investor Presentation November 2009 Robert A. Lodder, Ph.D. President |
| Forward-Looking Statement This presentation contains forward-looking statements that involve risks and uncertainties, including those described in the Company’s Annual Report for the year ended December 31, 2008 filed on Form 10-K, and most recent Quarterly Report filed on Form 10-Q. |
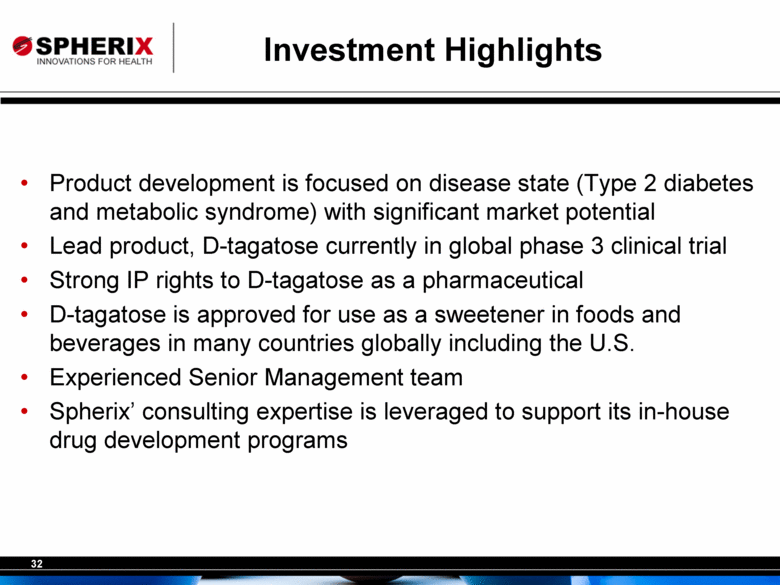
| Investment Highlights Product development is focused on disease state (Type 2 diabetes and metabolic syndrome) with significant market potential Lead product, D-tagatose currently in global phase 3 clinical trial Strong IP rights to D-tagatose as a pharmaceutical D-tagatose is approved for use as a sweetener in foods and beverages in many countries globally including the U.S. Experienced Senior Management team Spherix’ consulting expertise is leveraged to support its in-house drug development programs |
| Senior Management Team Claire L. Kruger, Ph.D., DABT, CEO Toxicologist with 20 years of consulting experience. Primary area of expertise is in pharmaceuticals, consumer products and foods, where she provides scientific, regulatory, and strategic support to clients in both the U.S. and international regulatory arenas. Robert A. Lodder, Ph.D. President Founder of InfraReDx, Inc. and Prescient Medical, Inc. Professor of Pharmaceutical Sciences at the College of Pharmacy, University of Kentucky Medical Center, he currently holds joint appointments in the Department of Electrical and Computer Engineering, and the Department of Chemistry at Kentucky. Robert L. Clayton, CPA, CFO 16 years of experience in finance and accounting. Previously served as Director of Finance and Controller. Randy Brown, BS, Chief of Operations 20 years in the pharmaceutical industry. Primary area of expertise is in large global clinical trials. Previous positions at Pfizer, Eli Lilly, and Genentech. Experience managing global trials in roughly 30 different countries including new emerging areas such as India. Ram Nimmagudda, Ph.D., Director of New Business Development 15 years of experience in business development, nutrition and nutraceutical industries, with particular expertise in novel food ingredients. Previous positions of Director of New Business Development for South Asia at DSM Functional Foods, Director and Sr. Principal Technologist, External Technologies at Wm. Wrigley Jr. Co. and Vice President of Technology Growth at Char. Hansen, Inc. |
| Preclinical Phase 1 Phase 2 Phase 3 Marketed Pharmaceutical Pipeline Compounds Formulated with D-Tagatose SPX 8522876 dyslipidemias Dose Ranging Study (D-Tagatose) Type-2 diabetes Phase 2 Ongoing SPX 7233801 atherosclerosis SPX 10624258 metabolic syndrome SPX 8818309 obesity SPX 8818440 diabetes NEET Study (D-Tagatose) 1 Type-2 diabetes Phase 3 Ongoing 1. Proceeded directly from phase 1 to 3 due to FDA GRAS approval |
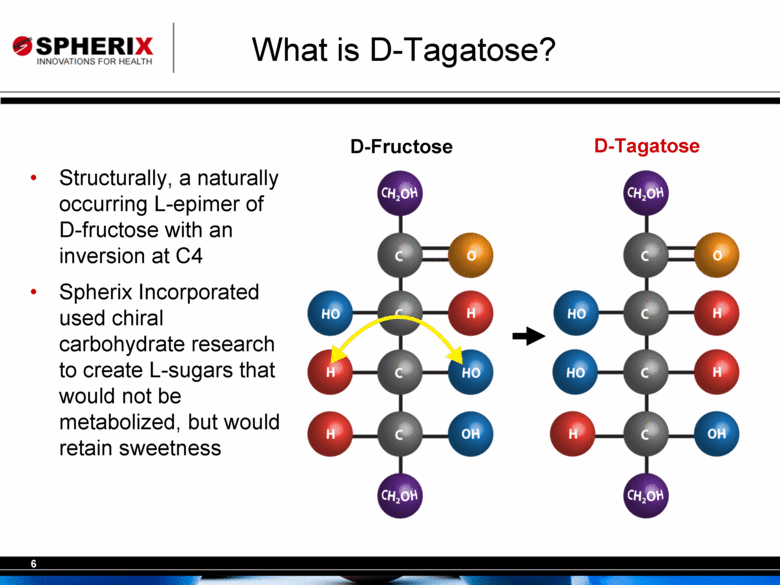
| What is D-Tagatose? Structurally, a naturally occurring L-epimer of D-fructose with an inversion at C4 Spherix Incorporated used chiral carbohydrate research to create L-sugars that would not be metabolized, but would retain sweetness D-Tagatose D-Fructose |
| Overview of D-Tagatose Being evaluated in phase 2 and phase 3 clinical trials as a first-in-class natural oral diabetes treatment Originally developed as a reduced calorie sugar substitute 92% as sweet as sucrose with only 38% of the calories (1.5 kcal/g, compared to 4 kcal/g ) D-tagatose is safe for use in food US FDA (GRAS) since 2001 WHO JECFA since June 2004 South Korea 2003 Australia/New Zealand since 2004 South Africa and Brazil since 2005 (novel food ingredient) EU since 2006 Approved for use as a low calorie sweetener in foods, and in the U.S. as an excipient in non-chronic OTC drugs (throat lozenges, cough syrup), toothpaste, mouthwash and cosmetics |
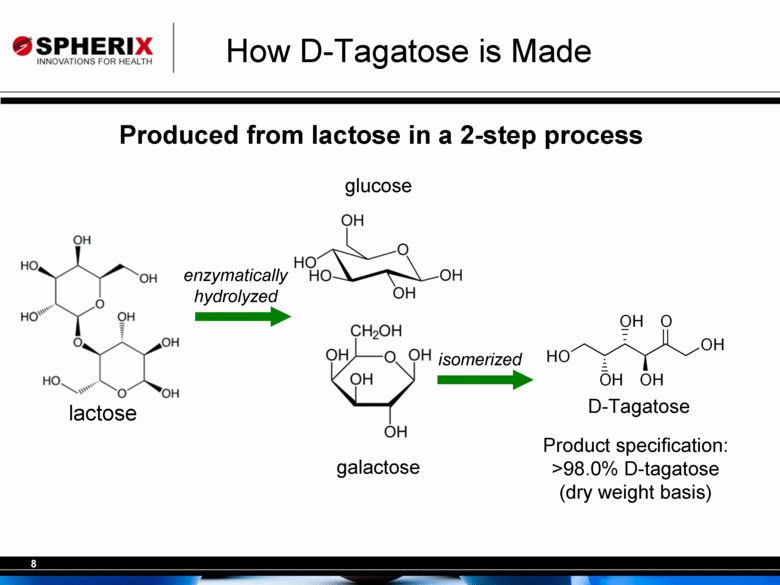
| How D-Tagatose is Made Produced from lactose in a 2-step process lactose galactose glucose D-Tagatose isomerized enzymatically hydrolyzed Product specification: >98.0% D-tagatose (dry weight basis) |
| What Makes D-Tagatose Different? Approved for use in food; established safety profile Chemically, it is an epimer of fructose with an inversion at C4 Behaves like and contains the identical constituent elements as L-tagatose but the human body lacks the enzymes to efficiently metabolize its structure D-Tagatose is poorly absorbed in the small intestine (25%), prevents the stimulation of insulin secretion (no beta cell exhaustion) and naturally lowers blood glucose levels Absorbed D-tagatose is metabolized to CO2 and H20, at a slower rate compared to related sugars (e.g., fructose and glucose) Passes to lower intestine, where it is fermented by bacteria to produce short-chain fatty acids and carbon dioxide |
| Established Safety Profile of D-Tagatose Preclinical studies (rodents) Acute: LD50 > 10 g/kg Subchronic : 28 day, 90 day, 13-Week: NOAEL at 5% in diet 2 Years: NOAEL at 5% in diet No maternal toxicity, embryotoxicity, or teratogenicity up to 20 g/kg/day (gavage) Not genotoxic Clinical studies Over 20 studies in healthy, diabetic, hyperuricemic or gouty individuals, at doses up to 25 g per eating occasion and 75 g per day reported gastrointestinal symptoms that were mild and transient; no adverse effects on clinical chemistries, hematological parameters or urinary endpoints. Types and severity of gastrointestinal symptoms similar to other incompletely absorbed carbohydrates or polyols. |
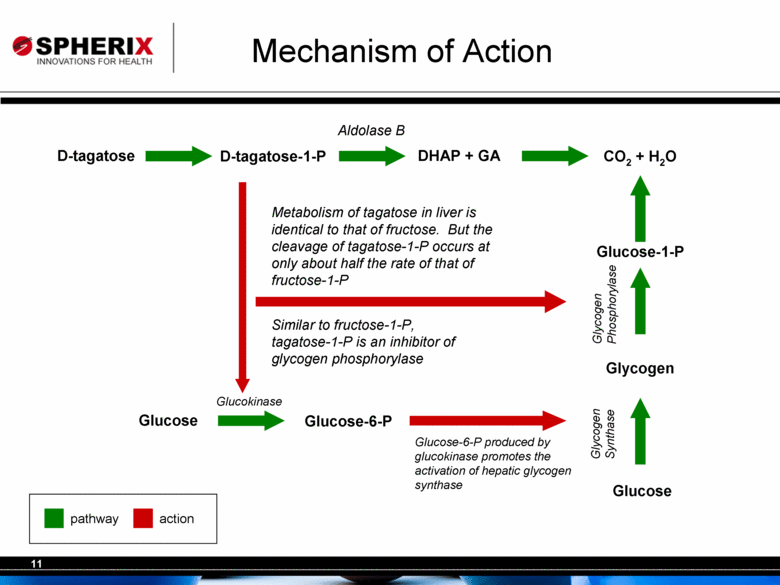
| Mechanism of Action D-tagatose D-tagatose-1-P DHAP + GA CO2 + H2O Glucose Glucose-6-P Glucose Glycogen Aldolase B Glucokinase Metabolism of tagatose in liver is identical to that of fructose. But the cleavage of tagatose-1-P occurs at only about half the rate of that of fructose-1-P Glycogen Synthase Glucose-6-P produced by glucokinase promotes the activation of hepatic glycogen synthase Glucose-1-P Glycogen Phosphorylase Similar to fructose-1-P, tagatose-1-P is an inhibitor of glycogen phosphorylase pathway action |
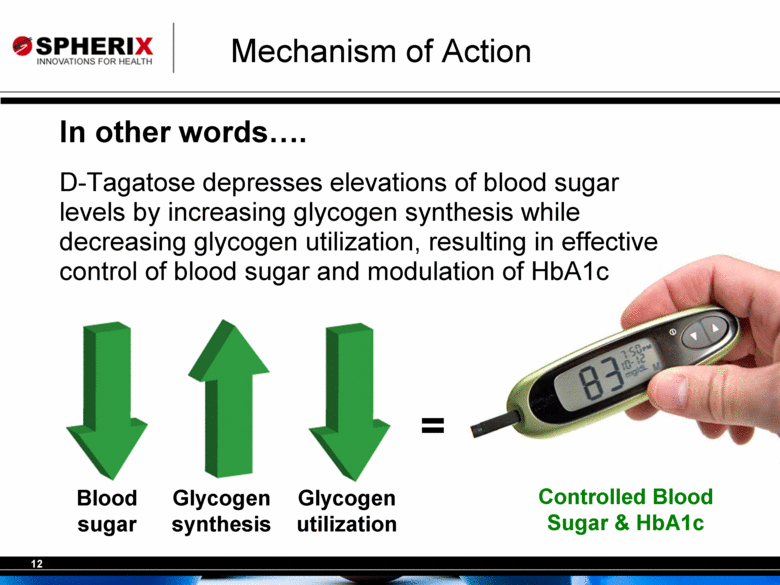
| Mechanism of Action In other words.... D-Tagatose depresses elevations of blood sugar levels by increasing glycogen synthesis while decreasing glycogen utilization, resulting in effective control of blood sugar and modulation of HbA1c Blood sugar Glycogen synthesis Glycogen utilization = Controlled Blood Sugar & HbA1c |
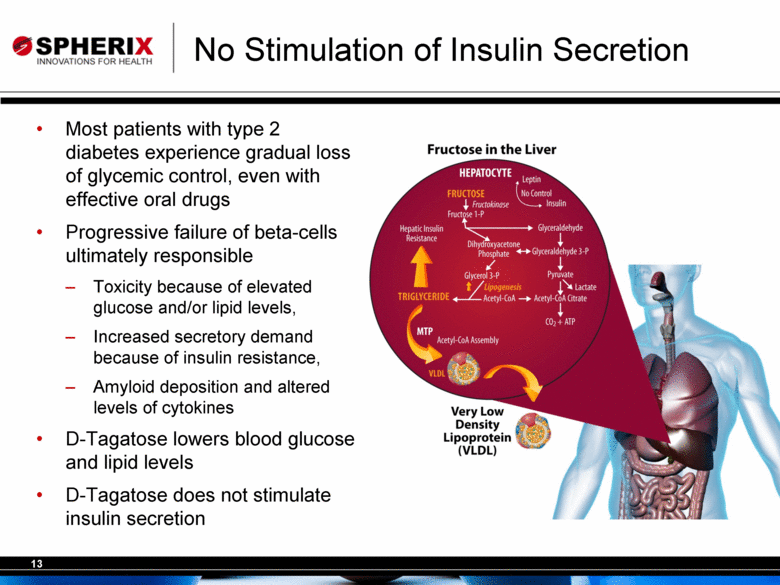
| No Stimulation of Insulin Secretion Most patients with type 2 diabetes experience gradual loss of glycemic control, even with effective oral drugs Progressive failure of beta-cells ultimately responsible Toxicity because of elevated glucose and/or lipid levels, Increased secretory demand because of insulin resistance, Amyloid deposition and altered levels of cytokines D-Tagatose lowers blood glucose and lipid levels D-Tagatose does not stimulate insulin secretion |
| Pharmaceutical: Phase 3 Trial D-Tagatose NEET Study (Protocol 70971-004) Double-blind phase 3 clinical trial 443 patients randomized 30 Sites in the USA, 32 Sites in India Powered to detect 0.5% HbA1c reduction Endpoints Primary: HbA1c Secondary: glucose, insulin, and lipid profiles, changes in body weight Expect interim data in Q4 2009 Anticipate NDA filing in 2H 2010 |
| Pharmaceutical: Phase 2 Trial D-Tagatose (Protocol 70971-005) To evaluate the dose-response effect of minimal doses of D-tagatose on glycemic control in subjects with type 2 diabetes under diet control and exercise Phase 2 Six-month Multi-center Single-blinded Randomized Parallel clinical study |
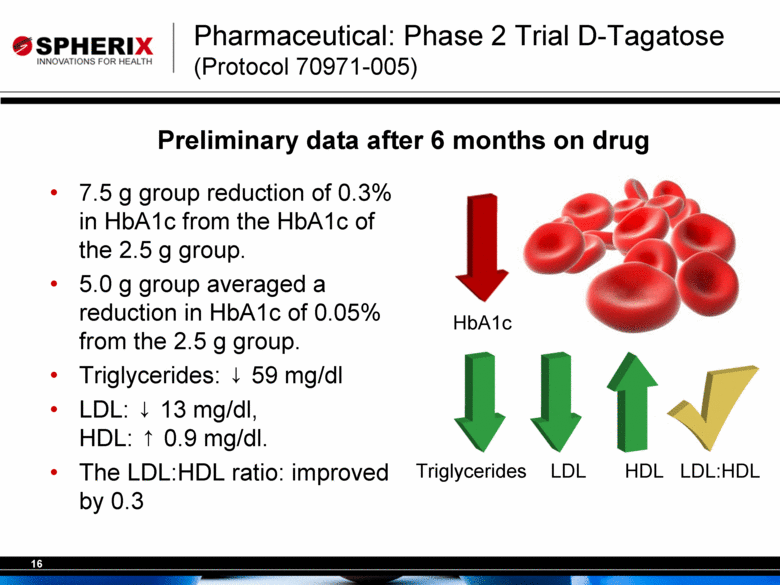
| Pharmaceutical: Phase 2 Trial D-Tagatose (Protocol 70971-005) 7.5 g group reduction of 0.3% in HbA1c from the HbA1c of the 2.5 g group. 5.0 g group averaged a reduction in HbA1c of 0.05% from the 2.5 g group. Triglycerides: 59 mg/dl LDL: 13 mg/dl, HDL: 0.9 mg/dl. The LDL:HDL ratio: improved by 0.3 Triglycerides LDL HDL LDL:HDL Preliminary data after 6 months on drug HbA1c |
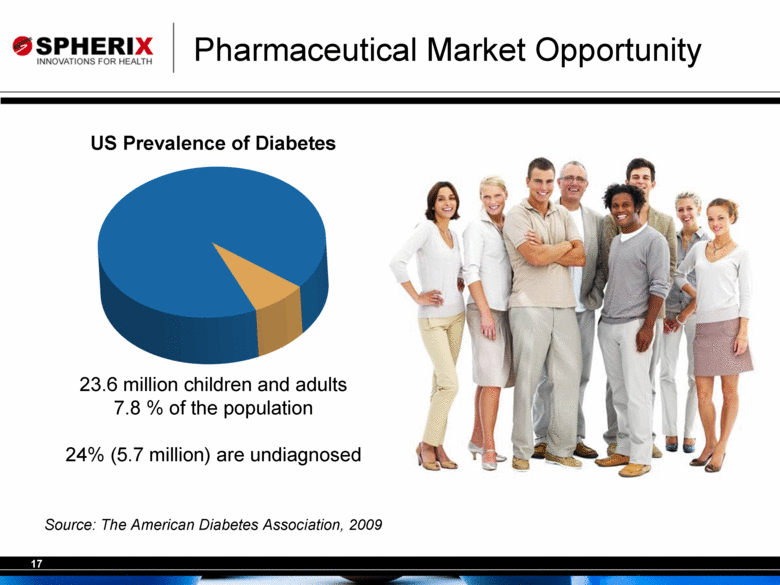
| Pharmaceutical Market Opportunity Source: The American Diabetes Association, 2009 23.6 million children and adults 7.8 % of the population 24% (5.7 million) are undiagnosed US Prevalence of Diabetes |
| Pharmaceutical Market Opportunity Type 2 Diabetes Results from insulin resistance, a condition in which cells fail to use insulin properly, sometimes combined with relative insulin deficiency Most Americans who are diagnosed with diabetes have type 2 diabetes Many people destined to develop type 2 diabetes spend many years in a state of Pre-diabetes "America's largest healthcare epidemic” Occurs when a person's blood glucose levels are higher than normal but not high enough for a diagnosis of type 2 diabetes As of 2009 there are 57 million Americans who have pre-diabetes |
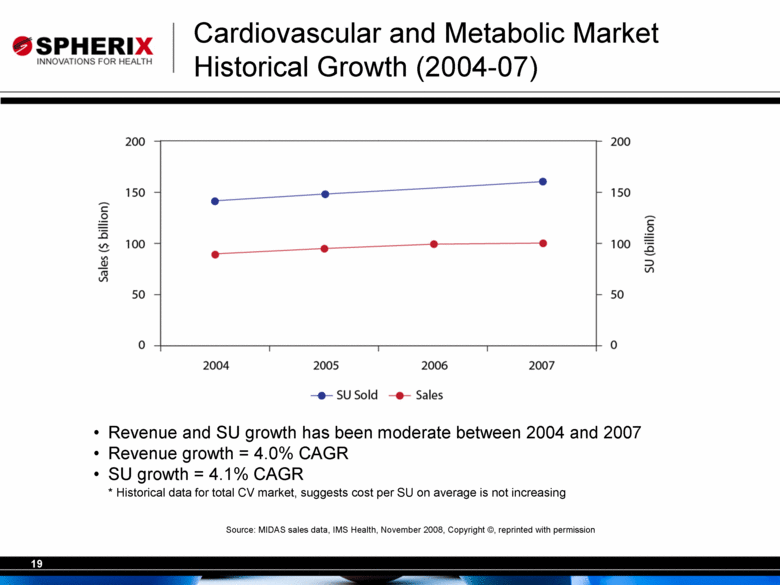
| Revenue and SU growth has been moderate between 2004 and 2007 Revenue growth = 4.0% CAGR SU growth = 4.1% CAGR * Historical data for total CV market, suggests cost per SU on average is not increasing Source: MIDAS sales data, IMS Health, November 2008, Copyright ©, reprinted with permission Cardiovascular and Metabolic Market Historical Growth (2004-07) |
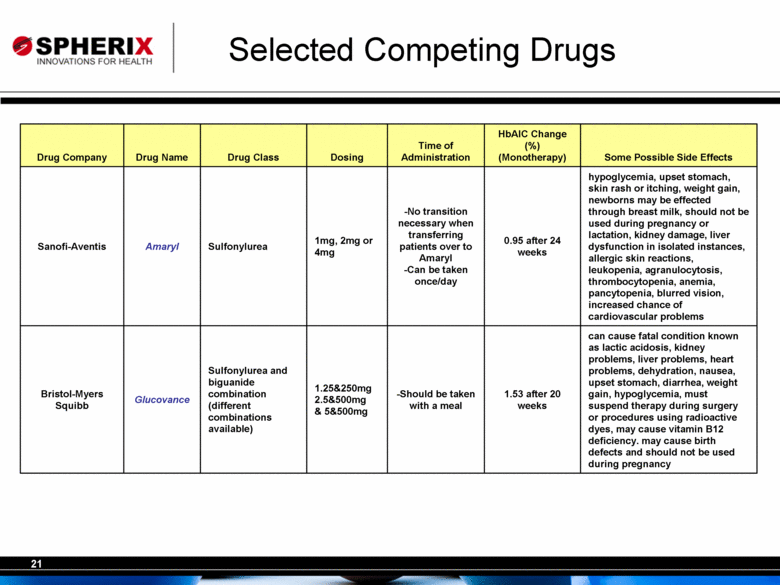
| Selected Competing Drugs alcohol consumption can increase chances of lactic acidosis (a fatal condition for ~1/2 of those diagnosed), see side effects of Avandia above, diarrhea, nausea, upset stomach, increased risk of cardiovascular problems, liver problems, and kidney disease 0.8 after 26 weeks (Add-on therapy) -Can be taken with or without food 500mg or 1000mg Thiazolidinedione and biguanide combination (Various levels of metformin are offered) Avandamet GlaxoSmithKline fluid retention leading to heart failure or swelling causing kidney problems, can react with medications to cause high blood sugar and low blood sugar, may take 2 weeks to start working and 2-3 months to show full effects on blood sugar, liver problems, weight gain, anemia, ovulation, headache, cold-like symptoms, increased blood cholesterol 0.7 after 26 weeks -Can be taken with or without food -Can be taken once/day or twice/day 2mg, 4mg, or 8mg Thiazolidinedione Avandia GlaxoSmithKline sore throat, swelling, bloating, rapid weight gain, chest pain, shortness of breath, fatigue, stomach pain, loss of appetite, unusual thirst, dark urine, liver problems, anemia, less effective birth control pills 1.9 after 26 weeks -Can be taken with or without a meal -Will not cause hypoglycemia 15mg, 30mg, or 45mg Thiazolidinedione Actos Takeda Pharmaceuticals Some Possible Side Effects HbAlC Change (%) (Monotherapy) Time of Administration Dosing Drug Class Drug Name Drug Company Diabetes Drug Competitors of Tagatose |
| Selected Competing Drugs can cause fatal condition known as lactic acidosis, kidney problems, liver problems, heart problems, dehydration, nausea, upset stomach, diarrhea, weight gain, hypoglycemia, must suspend therapy during surgery or procedures using radioactive dyes, may cause vitamin B12 deficiency. may cause birth defects and should not be used during pregnancy 1.53 after 20 weeks -Should be taken with a meal 1.25&250mg 2.5&500mg & 5&500mg Sulfonylurea and biguanide combination (different combinations available) Glucovance Bristol-Myers Squibb hypoglycemia, upset stomach, skin rash or itching, weight gain, newborns may be effected through breast milk, should not be used during pregnancy or lactation, kidney damage, liver dysfunction in isolated instances, allergic skin reactions, leukopenia, agranulocytosis, thrombocytopenia, anemia, pancytopenia, blurred vision, increased chance of cardiovascular problems 0.95 after 24 weeks -No transition necessary when transferring patients over to Amaryl -Can be taken once/day 1mg, 2mg or 4mg Sulfonylurea Amaryl Sanofi-Aventis Some Possible Side Effects HbAlC Change (%) (Monotherapy) Time of Administration Dosing Drug Class Drug Name Drug Company |
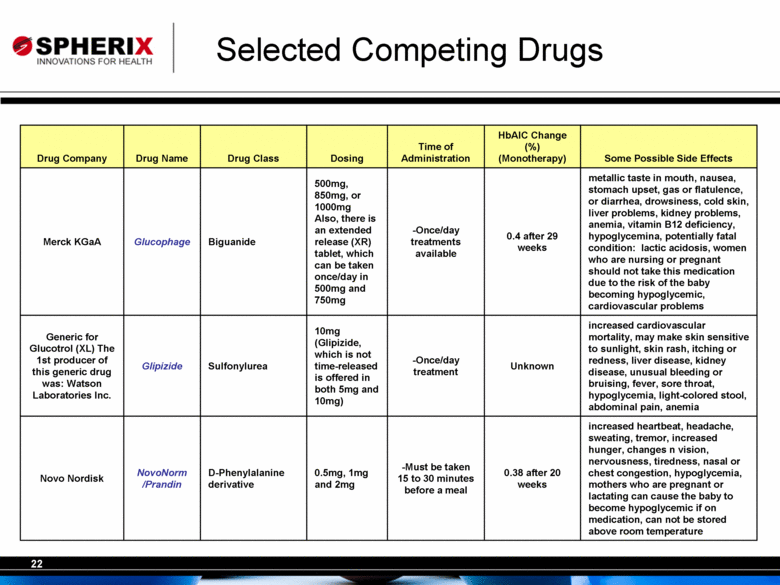
| Selected Competing Drugs increased heartbeat, headache, sweating, tremor, increased hunger, changes n vision, nervousness, tiredness, nasal or chest congestion, hypoglycemia, mothers who are pregnant or lactating can cause the baby to become hypoglycemic if on medication, can not be stored above room temperature 0.38 after 20 weeks -Must be taken 15 to 30 minutes before a meal 0.5mg, 1mg and 2mg D-Phenylalanine derivative NovoNorm /Prandin Novo Nordisk increased cardiovascular mortality, may make skin sensitive to sunlight, skin rash, itching or redness, liver disease, kidney disease, unusual bleeding or bruising, fever, sore throat, hypoglycemia, light-colored stool, abdominal pain, anemia Unknown -Once/day treatment 10mg (Glipizide, which is not time-released is offered in both 5mg and 10mg) Sulfonylurea Glipizide Generic for Glucotrol (XL) The 1st producer of this generic drug was: Watson Laboratories Inc. metallic taste in mouth, nausea, stomach upset, gas or flatulence, or diarrhea, drowsiness, cold skin, liver problems, kidney problems, anemia, vitamin B12 deficiency, hypoglycemina, potentially fatal condition: lactic acidosis, women who are nursing or pregnant should not take this medication due to the risk of the baby becoming hypoglycemic, cardiovascular problems 0.4 after 29 weeks -Once/day treatments available 500mg, 850mg, or 1000mg Also, there is an extended release (XR) tablet, which can be taken once/day in 500mg and 750mg Biguanide Glucophage Merck KGaA Some Possible Side Effects HbAlC Change (%) (Monotherapy) Time of Administration Dosing Drug Class Drug Name Drug Company |
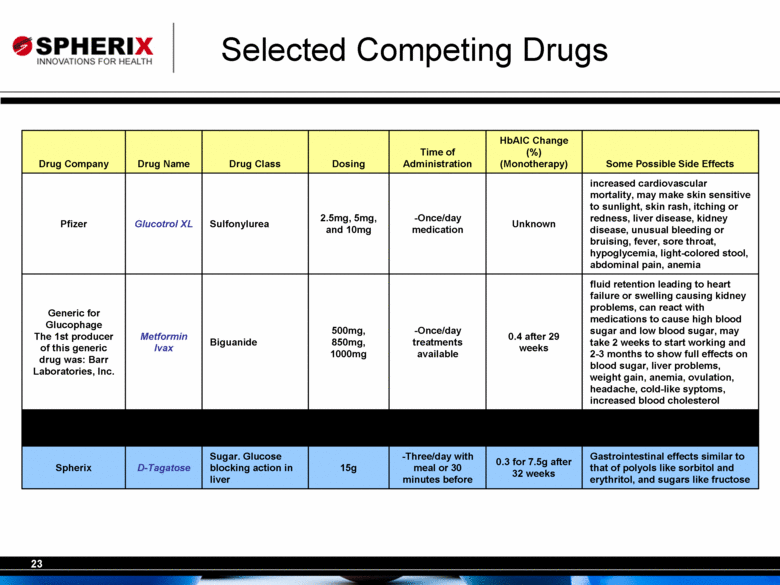
| Selected Competing Drugs Gastrointestinal effects similar to that of polyols like sorbitol and erythritol, and sugars like fructose 0.3 for 7.5g after 32 weeks -Three/day with meal or 30 minutes before 15g Sugar. Glucose blocking action in liver D-Tagatose Spherix fluid retention leading to heart failure or swelling causing kidney problems, can react with medications to cause high blood sugar and low blood sugar, may take 2 weeks to start working and 2-3 months to show full effects on blood sugar, liver problems, weight gain, anemia, ovulation, headache, cold-like syptoms, increased blood cholesterol 0.4 after 29 weeks -Once/day treatments available 500mg, 850mg, 1000mg Biguanide Metformin Ivax Generic for Glucophage The 1st producer of this generic drug was: Barr Laboratories, Inc. increased cardiovascular mortality, may make skin sensitive to sunlight, skin rash, itching or redness, liver disease, kidney disease, unusual bleeding or bruising, fever, sore throat, hypoglycemia, light-colored stool, abdominal pain, anemia Unknown -Once/day medication 2.5mg, 5mg, and 10mg Sulfonylurea Glucotrol XL Pfizer Some Possible Side Effects HbAlC Change (%) (Monotherapy) Time of Administration Dosing Drug Class Drug Name Drug Company |
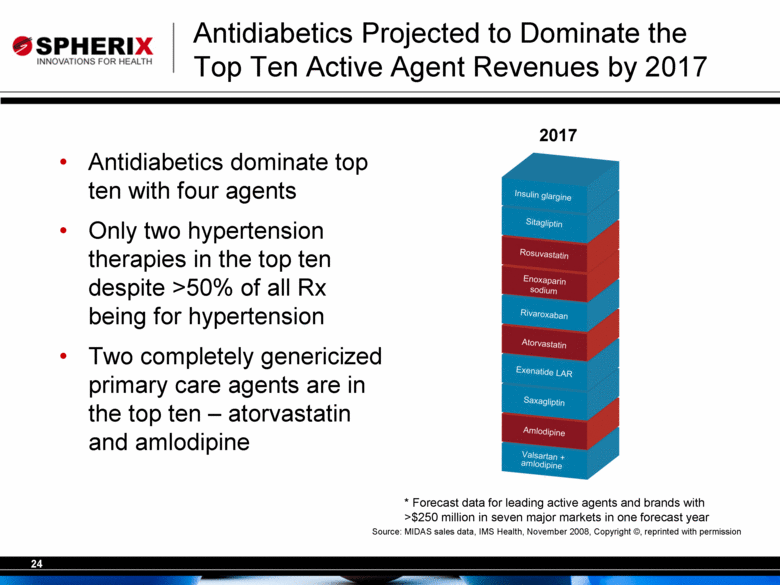
| Antidiabetics Projected to Dominate the Top Ten Active Agent Revenues by 2017 Antidiabetics dominate top ten with four agents Only two hypertension therapies in the top ten despite >50% of all Rx being for hypertension Two completely genericized primary care agents are in the top ten – atorvastatin and amlodipine Source: MIDAS sales data, IMS Health, November 2008, Copyright ©, reprinted with permission * Forecast data for leading active agents and brands with >$250 million in seven major markets in one forecast year 2017 |
| Manufacturing at Full Scale Inalco S.p.A. of Italy DMF 22715 and LoA One metric ton batches In use in phase 3 trial |
| IP Rights to D-Tagatose 5,447,917 D-tagatose as anti-hyperglycemic agent 5,356,879 D-tagatose as anti-hyperglycemic agent One patent pending for pipeline |
| Extension of Exclusivity New Chemical Entity Exclusivity (Hatch-Waxman) 5-year period of exclusivity is granted to new drug products containing chemical entities never previously approved by FDA either alone or in combination |
| Extension of Exclusivity Patent Term Extension Up to 5 years of patent extension for creating innovative products that benefit the public. Includes most products regulated by FDA Must be the first commercial marketing under the provision of the law which such regulatory review occurred FDA assists patent trademark office (PTO) in determining a products eligibility for patent extension |
| Extension of Exclusivity Pediatric Exclusivity Six months exclusivity as an incentive to sponsors to conduct more studies of the use of drug in pediatric populations Attaches to the END of all existing marketing exclusivity and patent periods. (Hatch/Waxman exclusivity and patent periods run concurrently) |
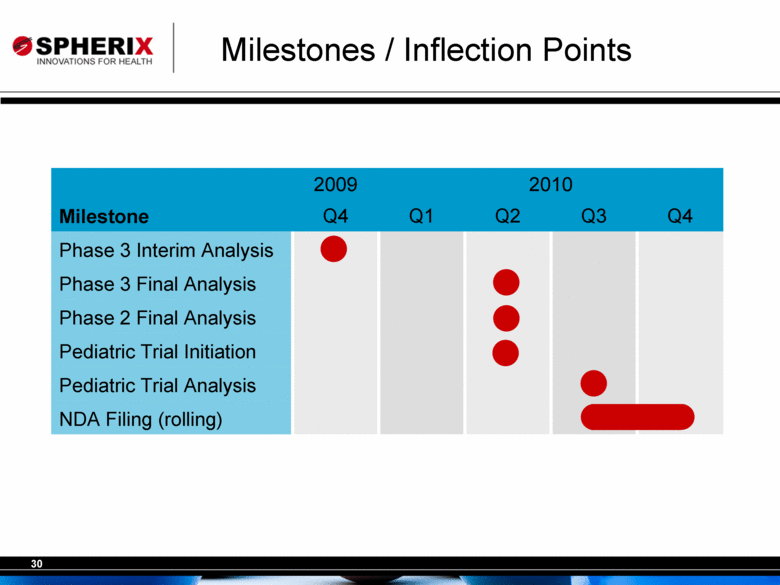
| Milestones / Inflection Points 2009 2010 Milestone Q4 Q1 Q2 Q3 Q4 Phase 3 Interim Analysis • Phase 3 Final Analysis • Phase 2 Final Analysis • Pediatric Trial Initiation Pediatric Trial Analysis NDA Filing (rolling) • |
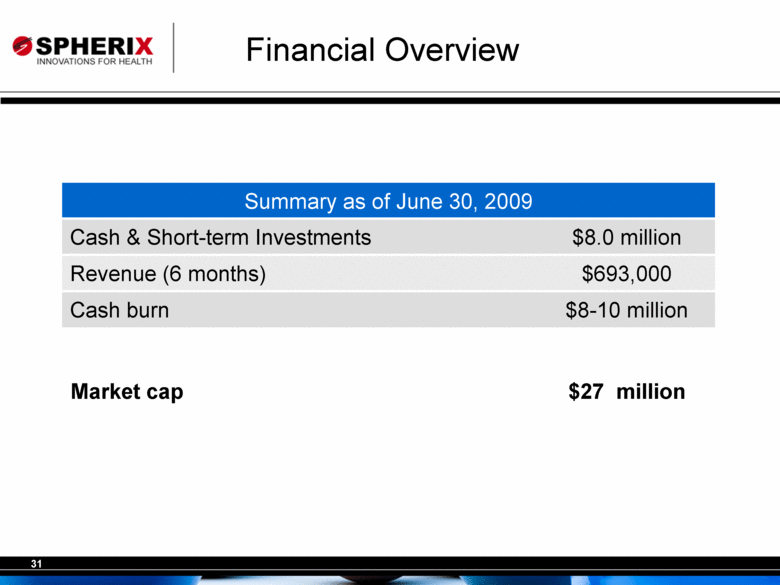
| Financial Overview Summary as of June 30, 2009 Cash & Short-term Investments $8.0 million Revenue (6 months) $693,000 Cash burn $8-10 million Market cap $27 million |
| Investment Highlights Product development is focused on disease state (Type 2 diabetes and metabolic syndrome) with significant market potential Lead product, D-tagatose currently in global phase 3 clinical trial Strong IP rights to D-tagatose as a pharmaceutical D-tagatose is approved for use as a sweetener in foods and beverages in many countries globally including the U.S. Experienced Senior Management team Spherix’ consulting expertise is leveraged to support its in-house drug development programs |