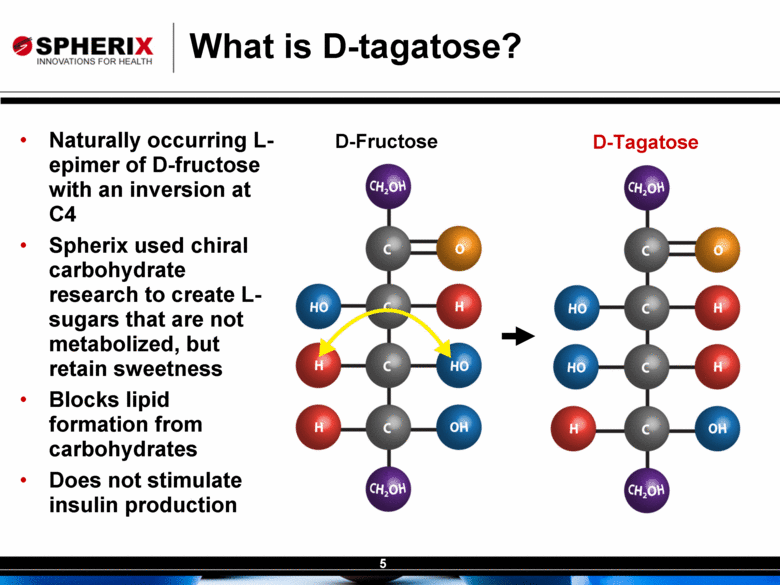
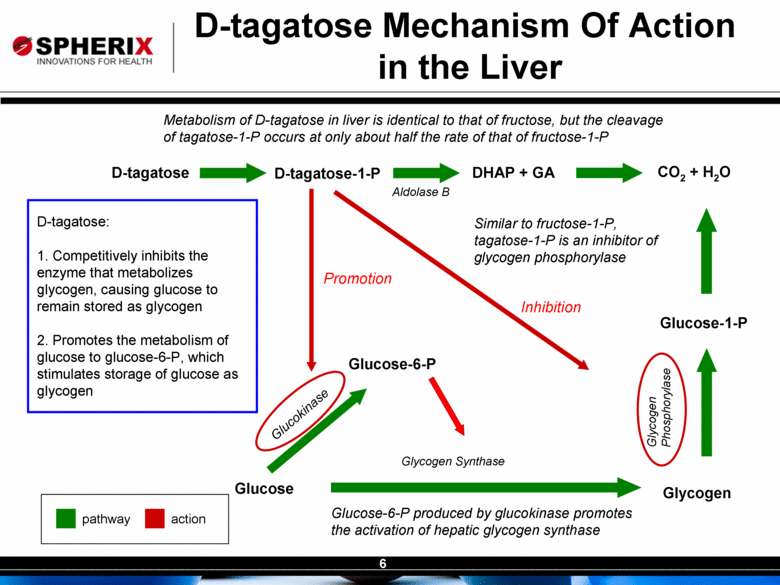
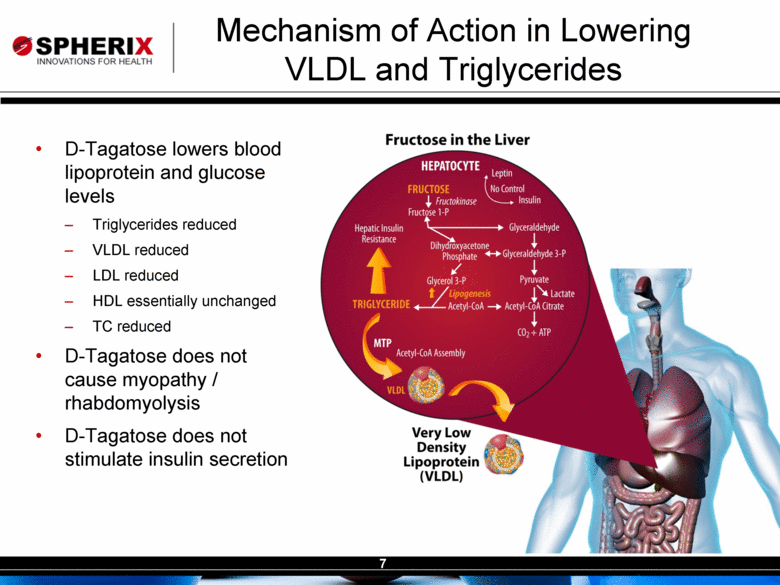
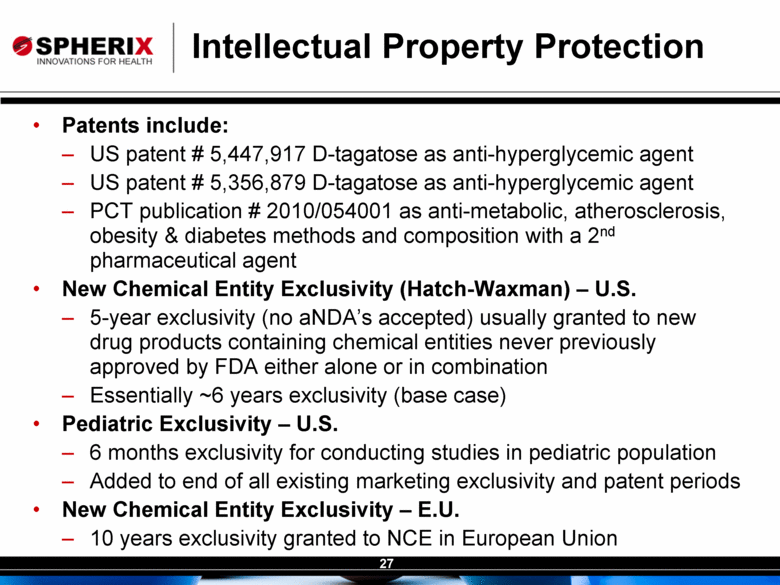
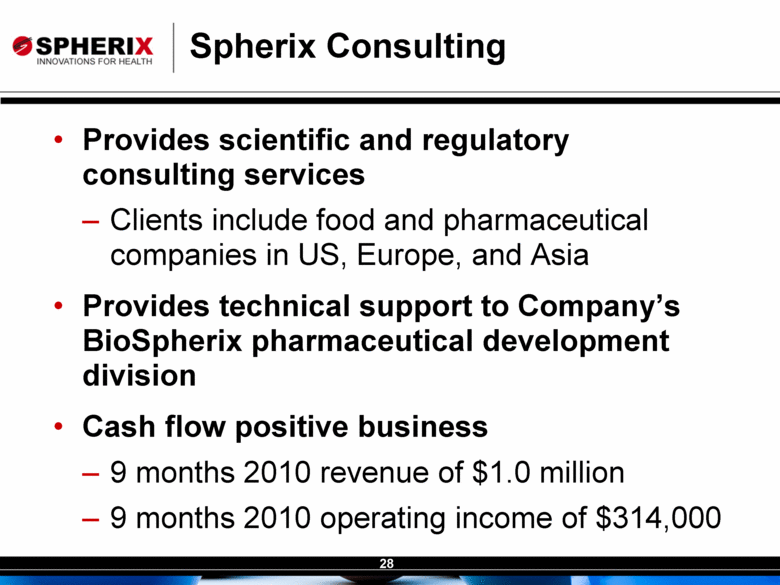
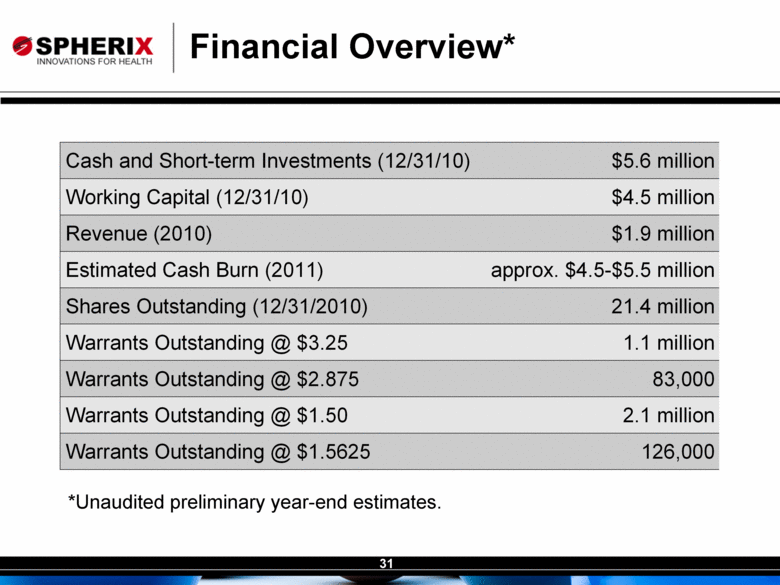
| Contracted Staff Highly Experienced in Drug Development Leisa Dennehy, Commercial and Corporate Development Advisor With over 20 years in a variety of commercial roles including new product planning, product launches, and pharma partnering at Glaxo/GW, Protez and Affinium Pharmaceuticals, Leisa guides development of commercial strategy and business development for Spherix Vicky Hines, Ph.D, Regulatory Affairs Advisor Formerly with small phama companies such as Chiron and Celtic Pharma, Dr Hines’ experience, ranging from discovery research to commercialized products, brings an investor-oriented mindset to help small companies navigate the complexities of Regulatory strategy with the FDA and EMA Nick Livington, Ph.D., Pre-Clinical Advisor With decades of senior roles in pre-clinical development at Bayer and GlaxoSmithKline for various metabolic products, Dr Livingston is ideally suited to guide Spherix in developing robust proof of principle and MoA studies in animals & cell lines which meet pharma companies diligence criteria John Amatruda, M.D., Clinical Advisor Previously head of Clinical for Januvia and Janumet at Merck, and Precose at Bayer , Dr Amatruda brings more than 20 years of experience in strategizing and conducting clinical studies in Type 2 Diabetes. Dr Amatruda brings the experience few consultants have in diabetes drug development Jonathan Tobert, M.B., B. Chir (MD) Ph.D, Clinical Advisor Dr Tobert has more experience in lipid drug development than almost anyone, with 30 years of clinical experience at Merck developing statins such as Mevacor and Zetia Dr Tobert supports clinical trial strategy and design for triglycerides |