Ocuphire Pharma, Inc.
Form 10-K
PART I | 6
|
| |
|
| | ITEM 1. | | 6
|
| | ITEM 1A. | | 61
|
| | ITEM 1B. | | 103
|
| | ITEM 2. | | 103
|
| | ITEM 3. | | 103
|
| | ITEM 4. | | 103
|
| | | |
|
PART II | 104
|
| |
|
| | ITEM 5. | | 104
|
| | ITEM 6. | | 104
|
| | ITEM 7. | | 105
|
| | ITEM 7A. | | 119
|
| | ITEM 8. | | 119
|
| | ITEM 9. | | 119
|
| | ITEM 9A. | | 119
|
| | ITEM 9B. | | 120
|
| | ITEM 9C. | | 120
|
| |
|
PART III | 121
|
| |
|
| | ITEM 10. | | 121
|
| | ITEM 11. | | 121
|
| | ITEM 12. | | 121
|
| | ITEM 13. | | 121
|
| | ITEM 14. | | 121
|
| | | |
|
PART IV | 122
|
| | | |
|
| | ITEM 15. | | 122
|
| | ITEM 16. | |
|
| | 125
|
| 154
|
In this Annual Report on Form 10-K, unless otherwise specified, references to “we,” “us,” “our,” “Ocuphire” or “the Company” mean Ocuphire Pharma, Inc. Our financial statements are prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These forward-looking statements relate to us, our business prospects and our results of operations and are subject to certain risks and uncertainties posed by many factors and events that could cause our actual business, prospects and results of operations to differ materially from those anticipated by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those described under the heading “Risk Factors” included in this Annual Report on Form 10-K. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. In some cases, you can identify forward-looking statements by the following words: “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. We undertake no obligation to revise any forward-looking statements in order to reflect events or circumstances that might subsequently arise. Readers are urged to carefully review and consider the various disclosures made by us in this report and in our other reports filed with the U.S. Securities and Exchange Commission (the “SEC”) that advise interested parties of the risks and factors that may affect our business.
SUMMARY RISK FACTORS
Our business is subject to a number of risks, as fully described in “Item 1A. Risk Factors” in this Annual Report. The principal factors and uncertainties include, among others:
| • | We currently depend entirely on the success of Nyxol and APX3330, our only product candidates. We may never complete clinical development of, receive marketing approval for, or successfully commercialize, Nyxol alone or as adjunctive therapy with low dose pilocarpine (LDP), APX3330, or other product candidates we may pursue in the future for any indication. |
| • | Viatris has exclusive global rights to commercialize our Nyxol products in key global markets. Viatris’ failure to timely develop or commercialize these products would have a material adverse effect on our business and operating results. |
| • | The results of previous clinical trials may not be predictive of future results, and the results of our current and planned clinical trials may not satisfy the requirements of the FDA or non-U.S. regulatory authorities. |
| • | Changes in regulatory requirements or FDA guidance, or unanticipated events during our clinical trials, may result in changes to clinical trial protocols or additional clinical trial requirements, which could result in increased costs to us or delays in its development timeline. |
| • | We expect to incur losses for the foreseeable future and may never achieve or maintain profitability. |
| • | Adverse global economic conditions could have a negative effect on our business results of operations and financial condition and liquidity. |
| • | Adverse developments affecting the financial services industry could negatively affect our current and projected business operations, financial condition and results of operations. |
| • | Raising additional capital may cause dilution to our stockholders, restrict our operations, or require us to relinquish rights to our technologies or product candidates. |
| • | Even if we receive marketing approval for our product candidates in the United States, we may never receive regulatory approval to market such product candidates outside of the United States. |
| • | Our employees or our representatives may engage in misconduct or other improper activities, including violating applicable regulatory standards and requirements or engaging in insider trading, which could significantly harm our business. |
| • | We face substantial competition, which may result in others discovering, developing, or commercializing products before or more successfully than we do. |
| • | We lack experience in commercializing products, which may have an adverse effect on our business. |
| • | If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell, market, and distribute APX3330, if approved, we may not be successful in commercializing APX3330 if and when it is approved. |
| • | Product liability lawsuits against us, or our suppliers and manufacturers, could cause us to incur substantial liabilities and could limit commercialization of any product candidate that we may develop. |
| • | We are unable to control all aspects of our clinical trials due to our reliance on clinical research organizations (“CROs”), contract development and manufacturing organizations (“CDMOs”) and other third parties that assist us in conducting clinical trials. |
| • | We are unable to control the supply, manufacture and testing of bulk drug substances and the formulation, testing and packaging of preclinical and clinical drug supplies of our product candidates, and will be unable to control these elements at the commercial stage, due to our reliance on third party manufacturers and analytical facilities. |
| • | If we are not able to establish new collaborations for APX3330 on commercially reasonable terms, we may have to alter our development, manufacturing, and commercialization plans. |
| • | If we are unable to obtain and maintain sufficient patent protection for our product candidates, our competitors could develop and commercialize products or technology similar or identical to those of us, which would adversely affect our ability to successfully commercialize any product candidates we may develop, our business, results of operations, financial condition and prospects. |
| • | If we do not obtain protection under the Hatch-Waxman Act and similar foreign legislation by extending the patent terms and obtaining data exclusivity for our product candidate, our business may be materially harmed. |
| • | We may not be able to protect or practice our intellectual property rights throughout the world. |
| • | Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by governmental agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements. |
| • | We depend on intellectual property sublicensed from Apexian Pharmaceuticals, Inc. (“Apexian”) for our APX3330 product candidate under development and our additional pipeline candidates, and the termination of, or reduction or loss of rights under, this sublicense would harm our business. |
| • | We are dependent on our key personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy. |
| • | We will need to develop and expand our company and may encounter difficulties in managing this development and expansion, which could disrupt our operations. |
| • | Our insurance policies are expensive and protect only from some business risk, which leaves us exposed to significant uninsured liabilities. |
| • | Environmental, social, and governance matter and any related reporting obligations may impact our business. |
| • | If we fail to comply with the continued listing standards of the Nasdaq Capital Market, our common stock could be delisted. If it is delisted, our common stock and the liquidity of our common stock would be impacted. |
| • | The market price of our common stock may fluctuate significantly. |
| • | We may be subject to securities litigation, which is expensive and could divert management attention. |
INDUSTRY AND MARKET DATA
In this Annual Report, we reference information, statistics and estimates regarding the medical devices and healthcare industries. We have obtained this information from various third-party sources, including industry and general publications, reports by market research firms and other sources. This information involves a number of assumptions and limitations, and we have not independently verified the accuracy or completeness of this information. Some data and other information are also based on the good faith estimates of management, which are derived from our research, review of internal surveys, general information discussed in the industry, and third-party sources. We believe that these external sources and estimates are reliable but have not independently verified them. The industries in which we operate are subject to a high degree of uncertainty, change, and risk due to a variety of factors, including those described in “Item 1A. Risk Factors.” These and other factors could cause results to differ materially from those expressed in this Annual Report and other publications.
PART I
Overview
Ocuphire is a late clinical-stage ophthalmic biopharmaceutical company focused on developing and commercializing therapies for the treatment of refractive and retinal eye disorders. Ocuphire’s pipeline currently includes two small molecule product candidates targeting several of such indications.
Nyxol
In November 2022, Ocuphire signed a license and collaboration agreement (the “Nyxol License Agreement”) with FamyGen Life Sciences, Inc. (acquired by Viatris, Inc. (“Viatris”) in January 2023) pursuant to which Ocuphire has granted to Viatris an exclusive license to develop, manufacture, import, export and commercialize Ocuphire’s product candidate phentolamine ophthalmic solution 0.75% (Nyxol® Eye Drops or “Nyxol”) in all three indications (RM, presbyopia and DLD) in key territories. Under the terms of the Nyxol License Agreement, Ocuphire is conducting development of Nyxol in the United States with its partner Viatris, and Viatris is responsible for developing Nyxol in countries and jurisdictions outside of the United States. Viatris will reimburse Ocuphire for budgeted costs related to the development of Nyxol through U.S. Food and Drug Administration (“FDA”) approval as long as Ocuphire continues to conduct such development activities. Viatris or its affiliates will commercialize Nyxol in the Territory for each indication that receives regulatory approval.
Pursuant to the Nyxol License Agreement, Ocuphire received an upfront cash payment of $35 million. In addition Ocuphire is eligible to receive potential additional payments of up to $130 million, in the aggregate, upon achieving certain specified regulatory or net sales milestones, with the first potential payment of $10 million to be made following approval by the FDA of Nyxol for the reversal of pharmacologically-induced mydriasis (“RM”) (dilation of the pupil). Ocuphire will also receive tiered royalties, starting at low double-digit royalties up to low twenty percent royalties, based on the aggregate annual net sales of Nyxol in the United States, and will receive low double-digit royalties based on all annual net sales outside of the United States. Nyxol is a once-daily eye drop formulation of phentolamine mesylate designed to reduce pupil diameter and improve visual acuity. Nyxol can potentially be used across multiple indications such as treatment of RM, presbyopia (age-related blurry near vision) and dim light or night vision disturbances (“DLD”) (halos, glares and starbursts). Ocuphire’s management believes these multiple indications potentially represent a significant market opportunity. Nyxol has been studied in a total of 12 clinical trials (3 Phase 1, 5 Phase 2 and 4 Phase 3) in a total of over 650 patients (with over 400 Nyxol-treated) and has demonstrated promising clinical data across the three targeted refractive indications.
Ocuphire reported positive top-line data from Phase 3 trials in RM: MIRA-2 in March 2021, MIRA-3 in March 2022 and MIRA-4 in April 2022. Ocuphire also reported positive top-line data from a Phase 2 trial of Nyxol for treatment of presbyopia, both alone and with low-dose pilocarpine (pilocarpine hydrochloride ophthalmic solution 0.4%, “LDP”) as adjunctive therapy (VEGA-1). Ocuphire reported top-line data from a Phase 3 trial in DLD in May 2022 (LYNX-1). Ocuphire submitted a new drug application (“NDA”) to the U.S. Food and Drug Administration (“FDA”) in November 2022 under the 505(b)(2) pathway for Nyxol for RM with a Prescription Drug User Fee Act (PDUFA) goal date of September 28, 2023. The first phase 3 registration trial of Nyxol for the treatment of presbyopia (VEGA-2), both alone and with LDP as adjunctive therapy, was started in late December 2022, and topline results from this trial are expected in late 2023. Future trials are planned to start in 2023 including the second Phase 3 registration trials for presbyopia (VEGA-3) and DLD (LYNX-2), and supportive long-term safety trial for both chronic indications (LYRA-1).
APX3330
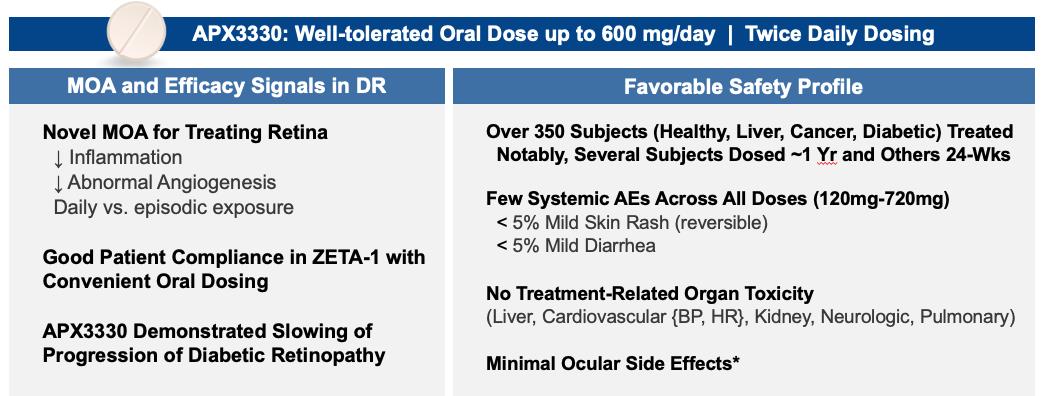
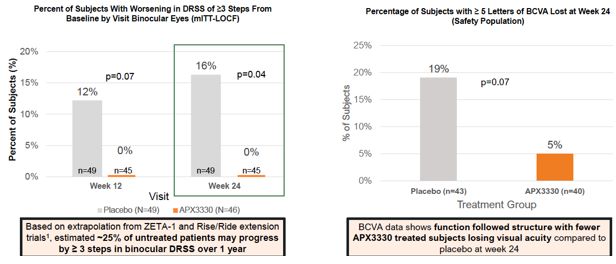
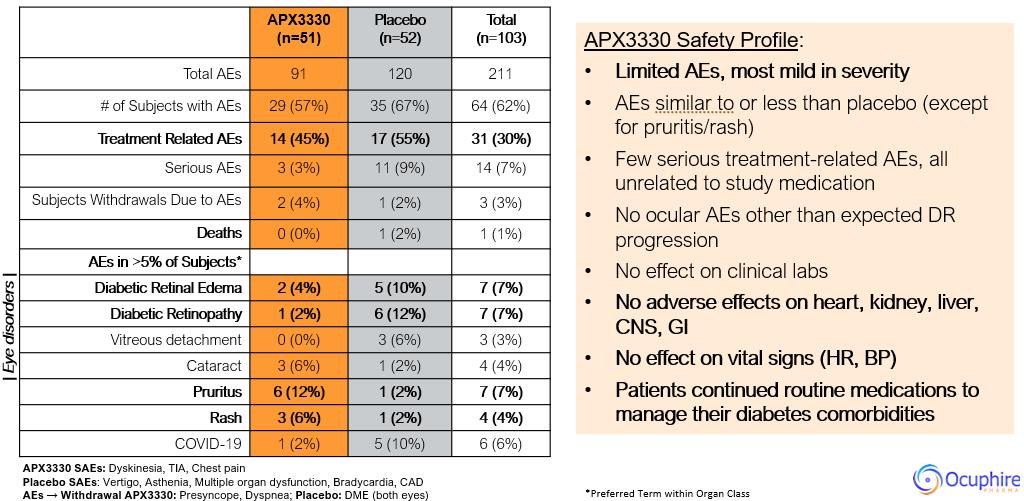
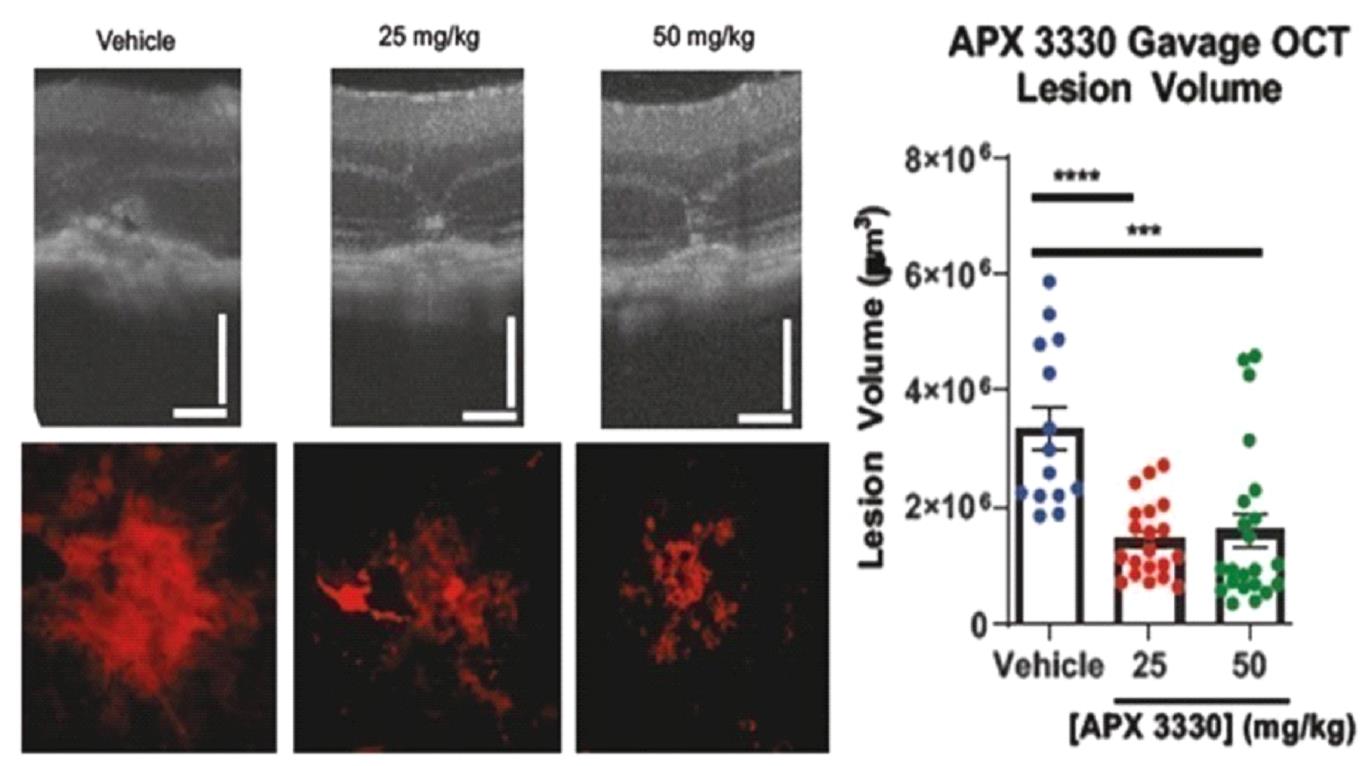
Ocuphire’s product candidate, APX3330, is a twice-a-day oral tablet designed to target multiple pathways relevant to retinal and choroidal (the vascular layer of the eye) diseases such as diabetic retinopathy (“DR”) and diabetic macular edema (“DME”) which, if left untreated, can result in permanent visual acuity loss and eventual blindness. DR is a disease resulting from diabetes in which chronically elevated blood sugar levels cause progressive damage to blood vessels in the retina. DME is a severe form of DR which involves leakage of protein and fluid into the macula, the central portion of the retina, causing swelling and vascular damage. Prior to Ocuphire’s in-licensing this product candidate, APX3330 had been studied by other sponsors in a total of 11 clinical trials (6 Phase 1 and 5 Phase 2) in a total of over 420 healthy volunteers or patients (with over 340 APX3330-treated) for inflammatory (hepatic) and oncology indications, and had demonstrated evidence of target engagement, pharmacokinetics, durability, and favorable safety and tolerability. Ocuphire has also in-licensed APX2009 and APX2014, which are second-generation product candidates and analogs of APX3330. In January 2023, Ocuphire reported top-line efficacy and safety results from the ZETA-1 Phase 2 trial conducted in 103 subjects (51 treated with 600 mg daily dose of APX3330) in DR, including moderately severe non-proliferative DR (“NPDR”) and mild proliferative DR (“PDR”), as well as patients with DME without loss of central vision. Although the ZETA-1 clinical trial did not meet the primary endpoint of % of patients with a ≥ 2-step improvement in Early Treatment of Diabetic Retinopathy Study (ETDRS) diabetic retinopathy severity scale (DRSS) at week 24 in the study eye, statistical significance was achieved on a key pre-specified secondary endpoint of preventing clinically meaningful progression of diabetic retinopathy (defined by binocular 3 or more steps worsening on the DRSS scale, calculated as the sum of changes in each eye) after 24 weeks of treatment. Given the oral systemic delivery of APX3330, an endpoint that evaluates the effects on both eyes is the planned Phase 3 primary endpoint for future registration trials; this will be confirmed at an End-of-Phase 2 (EOP2) meeting with the FDA in second half of 2023. APX3330 demonstrated favorable safety and tolerability in the ZETA-1 trial, consistent with the safety data from the prior 11 clinical trials. Treatment-related adverse events were uncommon, and most were mild in severity. There were no treatment-related serious adverse events. No changes were observed in liver, kidney, or heart function as well as complete blood count and comprehensive metabolic panel.
As part of its strategy, Ocuphire will continue to explore opportunities to acquire additional ophthalmic assets and seek strategic partners for late-stage development, regulatory preparation, and commercialization of drugs in key global markets.
Corporate History
In February 2018, Ocuphire Pharma, Inc. (“Private Ocuphire”) was founded and subsequently merged in April 2018 with Ocularis Pharma, LLC, (the original innovator of phentolamine mesylate ophthalmic solution to treat DLD), and in January 2020 obtained from Apexian Pharmaceuticals, Inc. certain rights to its Ref-1inhibitor program, including APX3330 (see “Apexian Sublicense Agreement”). Many of Ocuphire’s employees, directors, advisors and consultants have been involved in the development of Nyxol and other ophthalmic drugs including approved products such as LUMIFY®, Zirgan®, Durezol®, Upneeq®, Rhopressa®, Roclatan®, Vyzulta®, Xiidra®, Cequa®, and Dextenza®. The management team, led by CEO and founder Mina Sooch, collectively has significant experience in operating pharmaceutical companies and discovering, developing, and commercializing treatments in multiple therapeutic areas. Ocuphire also has a world-class medical advisory board of over 20 key opinion leaders including retina specialists, refractive surgeons, and optometrists.
In November 2020, Private Ocuphire completed a reverse merger (the "Merger”) into Rexahn Pharmaceuticals, Inc. (“Rexahn”), a publicly-traded company that had ceased its business of drug development activities, and simultaneously raised $20 million through an offering of common shares and warrants to purchase common shares. In connection with the Merger, Rexahn changed its name to Ocuphire Pharma, Inc. and has since conducted as a public company the business previously conducted by Private Ocuphire.
In November 2022, Ocuphire entered into the Nyxol License Agreement, licensing its product Nyxol to Viatris.
Strategy
Ocuphire’s goal is to build a leading ophthalmic biopharmaceutical company that discovers, develops, commercializes and/or out-licenses best-in-class therapies for patients and provides attractive solutions for physicians and payers. The key elements of Ocuphire’s strategy to achieve its goal are the following:
| • | Advance the clinical development of Nyxol and APX3330. Ocuphire entered into the Nyxol License Agreement in November 2022, pursuant to which Viatris has exclusive rights to develop and commercialize Nyxol. Pursuant to the Nyxol License Agreement, Ocuphire continues to conduct development activities in the United States in partnership with Viatris, and is reimbursed by Viatris for such budgeted development activities. Ocuphire submitted a United States NDA for Nyxol for RM with a PDUFA goal date of September 28, 2023, and is advancing Phase 3 trials for presbyopia and DLD. Viatris has exclusive rights to pursue development and undertake commercialization efforts for Nyxol outside of the United States. Ocuphire plans an EOP2 meeting with the FDA to advance Phase 3 trials for APX3330 in DR. |
| • | Target Nyxol and APX3330 for large ophthalmic indications. Ocuphire believes Nyxol has therapeutic potential to improve vision performance in RM, presbyopia and DLD. Ocuphire also believes AXP3330 has potential to prevent or delay the progression of disease in patients with DR, DME, and other retinal diseases, while potentially reducing the burden of intravitreal injections. |
| • | Maintain and expand its intellectual property portfolio. Ocuphire has out-licensed the global patent rights to Nyxol with respect to its formulation, combinations, and use in multiple indications to Viatris. Ocuphire owns an exclusive worldwide sublicense for the Ref-1 Inhibitor program, including its product candidate APX3330, for all its ophthalmic and diabetic indications, and compositions and methods of use for Ref-1 pipeline candidates, including APX2009 and APX2014. Ocuphire continues to explore additional opportunities to expand and extend this intellectual property protection, both in the U.S. and in other jurisdictions. |
| • | Maximize the global commercial value of Nyxol and APX3330. If cleared to market by the FDA, Nyxol will be commercialized by the Viatris Eye Care Division in the U.S. and major non-U.S. markets pursuant to the Nyxol License Agreement. Ocuphire plans to seek one or more partners to commercialize APX3330 both in and outside of the United States. |
| • | Evaluate in-licensing and acquisition opportunities. Ocuphire’s team is well qualified to identify and in-license or acquire clinical-stage ophthalmological assets and continually evaluates opportunities to expand and diversify its pipeline. |
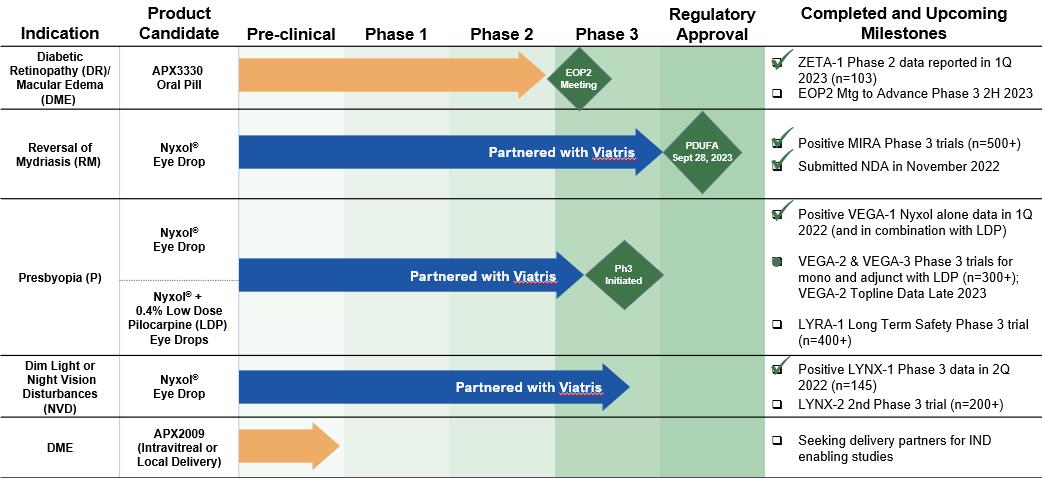
Ocuphire is developing Nyxol with its partner Viatris for multiple indications, and Ocuphire is continuing to develop APX3330 for multiple indications with the goal to eventually seek a commercial partner for APX3330. Ocuphire believes the two programs present similar potential advantages: (1) promising clinical data to date; (2) both small-molecule clinical candidates; (3) convenient dosing route and schedule; (4) potential for first-line or adjunctive therapy; and (5) significant commercial potential. TABLE 1 below summarizes Ocuphire’s current development pipeline of product candidates and their target indications and anticipated milestones:
TABLE 1: Ocuphire Pipeline: Product Candidates and Indications Pipeline
Note: 0.75% POS (Phentolamine Ophthalmic Solution) is the same as 1% PMOS (Phentolamine Mesylate Ophthalmic Solution). References to Nyxol with both designations appear throughout this document, there is no difference in formulation between the two designations.
Ocuphire submitted its first NDA to the FDA for Nyxol for RM in November 2022 utilizing the 505(b)(2) pathway of the U.S. Federal Food, Drug, and Cosmetic Act (“FDCA”). The FDA notified Ocuphire in February 2023 that it accepted the application for filing and that it has set a PDUFA goal date of September 28, 2023 for approval. Ocuphire anticipates submitting supplemental NDAs for Nyxol for presbyopia and DLD and is advancing APX3330 towards an NDA in the future.
Overview of Eye Disease Market
Anterior (Front of the Eye) Segment Disease Market
There are approximately 100 million eye dilations in the United States and this number is expected to go up with the increasing aging and diabetic population that requires more frequent eye exams and procedures. Millions of Americans also suffer from various refractive errors. Presbyopia, one such refractive error, is common in patients over the age of 40 years which results in decreased ability to see objects at a near distance. This condition affects over 120 million Americans and usually requires reading glasses and/or contact lenses for focusing on near objects. Further according to GlobalData, approximately 38 million patients in the U.S suffer from dim light or night vision disturbances caused by LASIK, night myopia, keratoconus, eye surgery, or the natural aging process. There is also a global trend in vision disturbances in younger individuals due to the overuse of smartphone screens. Nyxol, which was out-licensed in 2022, is currently in late-stage clinical development for reversal of mydriasis (eye dilation), presbyopia and night vision disturbances, and has the potential to address an unmet need for millions of patients in the U.S.
Retinal (Back of the Eye) Disease Market
Retinal damage is one of the leading causes of blindness and continues to grow with aging and larger diabetic populations around the world. Diabetes is the leading cause of blindness among adults aged 20 – 74. According to the National Eye Institute, in the United States alone, over 7 million patients suffer from diabetic retinopathy (DR), a complication of diabetes in which chronically elevated blood sugar levels cause damage to blood vessels in the retina. An additional 750,000 patients suffer from diabetic macular edema (DME), one of the most common complications of diabetic retinopathy where the macula swells from fluid leaked from damaged blood vessels. The disease progression of both DR and DME involves abnormal vessel proliferation via VEGF signaling and inflammation. Ocuphire’s APX3330 oral tablet recently completed a Phase 2 clinical trial for DR and has the potential to address this large DR and DME market with a novel, dual mechanism of action of inhibiting VEGF and inflammation. In addition, over 1 million patients in the United States suffer from wAMD. These retinal and choroidal vascular diseases, which cause damage to the macula, are leading causes of severe, permanent vision loss. Currently, there are several drugs on the market indicated for anti-VEGF therapy, including Lucentis® (ranibizumab), a monoclonal antibody marketed by Genentech, and EYLEA® (aflibercept), a recombinant fusion protein marketed by Regeneron Pharmaceuticals, Inc., that have become the standard of care for treating severe forms of DME and wAMD amongst other retinal conditions. Avastin® (bevacizumab), a monoclonal antibody marketed by Genentech, is also used off-label to treat these same indications as it is more cost-effective than the other branded drugs. These three injectable drugs are biologics with treatment administered in an ophthalmologist’s office. Annual worldwide sales of Lucentis and EYLEA for all indications totaled over $13 billion in 2020 ($3.5 billion for Lucentis and over $10 billion for EYLEA).
Summary of Nyxol and APX3330
Nyxol (phentolamine 0.75% ophthalmic solution)
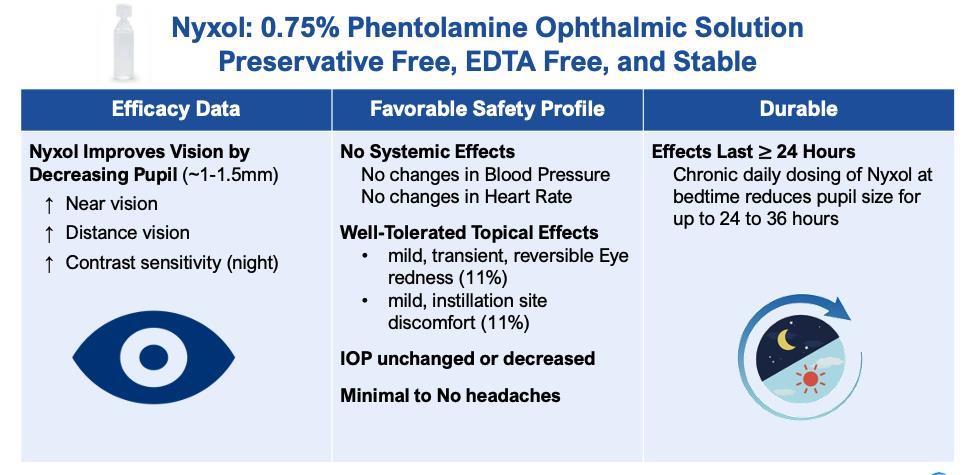
Nyxol, out-licensed to Viatris in 2022, is a once-daily, sterile, preservative-free eye drop formulation containing phentolamine mesylate, a reversible, non-selective alpha-1 and alpha-2 adrenergic antagonist that acts on the adrenergic nervous system and inhibits contraction of smooth muscle. Ocuphire submitted an NDA to the FDA in November 2022 under the 505(b)(2) pathway for Nyxol for RM. The submission was accepted for filing by FDA with a PDUFA goal date of September 28, 2023. Phentolamine mesylate, the drug substance and active component of Nyxol, is the active pharmaceutical ingredient (API) in two FDA-approved drugs, Regitine® and OraVerse®. Regitine, an injectable approved in 1952, is used mainly to treat pre- or intra-operative hypertensive episodes in patients with pheochromocytoma. OraVerse, approved in 2007, is an intraoral submucosal injection used to reverse anesthesia after oral surgery. The FDA has stated that it would be acceptable for the Nyxol application to reference the FDA’s previous review of safety and efficacy for Regitine® (Phentolamine Mesylate Injection, NDA 008278) and Oraverse® (Phentolamine Mesylate Injection, NDA 22159), pursuant to section 505(b)(2) of the U.S. Federal Food, Drug, and Cosmetic Act (“FDCA”). In multiple clinical trials, Nyxol has been shown to reduce pupil size, improve near and distance visual acuity in light and dark conditions, and improve low contrast visual acuity. Ocuphire and Viatris are pursuing multiple indications for Nyxol, including RM, presbyopia, and DLD. For treatment of presbyopia, Ocuphire is evaluating the efficacy of Nyxol both as a single-agent eye drop and as adjunctive therapy with LDP.
Key attributes of Nyxol include the following:
| • | Reduction in pupil diameter with durable effects. In multiple Phase 2 and Phase 3 trials Nyxol reduced pupil diameter by approximately 1 – 1.5 mm in both mesopic (dim) and photopic (bright) conditions, with such reductions sustained over 24 hours. |
| • | Improvement in distance corrected near visual acuity. When studied in patients with presbyopia in Phase 2 trials, Nyxol alone and in combination with LDP showed statistically significant improvement in distance-corrected near visual acuity with ≥3 lines gain from baseline. Nyxol provides an optimal pupil size of 2 mm – 3 mm. |
| • | Improvement in low contrast visual acuity. When studied in patients with DLD in multiple Phase 2 trials, Nyxol showed statistically significant improvement in low contrast mesopic best-corrected distance visual acuity at ≥1 and ≥2 lines, with a trend at ≥3 lines on a standard visual chart. |
| • | Favorable tolerability profile. To date, Nyxol has been observed to be well-tolerated, with unchanged or decreased intraocular pressure in the 12 completed Phase 1, Phase 2 and Phase 3 clinical trials conducted. Nyxol produces a transient, mild hyperemia effect that disappears within several hours or immediately upon application of anti-redness eye drops. Nyxol is also observed to have no systemic effects such as changes in blood pressure or heart rate. |
| • | Designed to be a convenient, once-daily eye drop or tunable combination option. Nyxol is being evaluated for chronic use as a once-daily administration before bedtime. Nyxol has been shown in multiple Phase 2 trials and Phase 3 trials to have a durable effect of over 24 hours, which could encourage patient compliance. Use of LDP eye drops as an adjunct to Nyxol may offer the benefit of tunability to presbyopia patients based on their vision and lifestyle needs. |
| • | Stable, cost-effective ophthalmic formulation. Nyxol is a single-use, preservative-free, proprietary eye drop formulation with stability suitable to support potential commercialization. Its active pharmaceutical ingredient, phentolamine mesylate USP grade, is a small molecule with advantages of standardized, scalable, and lower-cost manufacturing processes. |
Ocuphire and Viatris are initially pursuing Nyxol for the following three indications under the Nyxol License Agreement as a first-line therapy (in the case of presbyopia, both as a single agent and with low-dose pilocarpine as an adjunctive drop):
| • | RM, the reversal of pharmacologically induced dilation of the pupils, where dilation leads to increased sensitivity to light and an inability to focus, making it difficult to read, work, and drive. RM is a single-use indication for which no approved therapy is commercially available at present. |
| • | Presbyopia, a condition in which the eye’s lens loses elasticity, affecting its ability to focus on near objects. Presbyopia typically occurs after age 40 and most patients use reading glasses in order to read or see objects close to them. VuityTM, approved in October 2021, is the only eye drop currently marketed for the treatment of presbyopia. |
| • | DLD, a condition in which peripheral imperfections (aberrations) of the cornea scatter light when the pupil opens wide in dim light. Patients with DLD experience glare, halos, starbursts, and decreased contrast sensitivity. DLD is a new indication with no approved therapies. |
APX3330
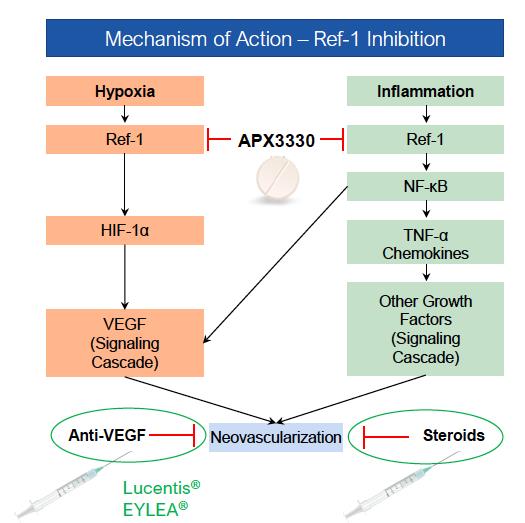
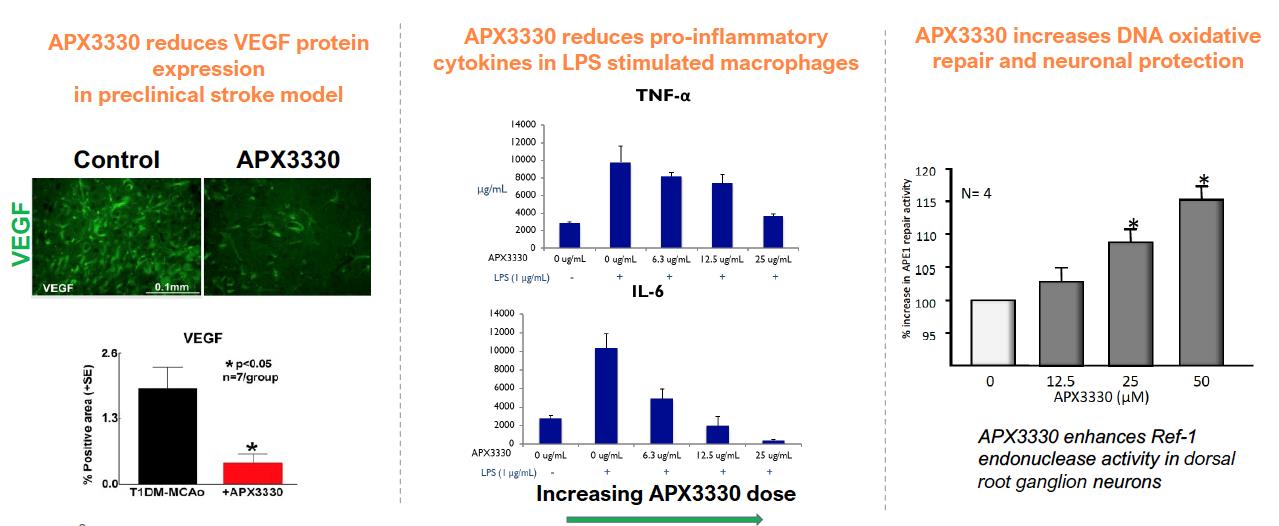
APX3330 (E3330), originally developed by Eisai Co., Ltd. and Apexian Pharmaceuticals, Inc., is a small molecule that specifically targets Apurinic/Apyrimidinic Endonuclease 1/Redox Factor-1 (APE-1/Ref-1, referred to as Ref-1), a dual function protein involved in the regulation of transcription factors critical to cell signaling. Ref-1 regulates inflammation, angiogenesis (blood vessel formation), and reduction-oxidation (redox) signaling, as well as DNA repair that is critical to normal function of neurons. By inhibiting redox activity and not DNA repair, APX3330 has been shown in preclinical studies to reduce angiogenesis and inflammation via modulation of several important proangiogenic and proinflammatory transcription factors such as NF-κB and HIF-1a and its downstream target, VEGF (Vascular Endothelial Growth Factor). These transcription factors are implicated in multiple pathways relevant to the pathophysiology of retinal and choroidal vascular diseases, including diabetic retinopathy (DR), diabetic macular edema (DME), wet age-related macular degeneration (wAMD) and geographic atrophy (GA). Moreover, data from these preclinical studies suggest that APX3330 is a promising candidate for clinical evaluation of the efficacy and safety of an oral systemic therapy to treat these important diseases.
Key attributes of Ocuphire’s product candidate APX3330 include the following:
| • | Potential to be the first oral therapy. Compared to frequent intravitreal anti-VEGF injections, associated with ocular complications, once or twice a day oral administration of APX3330 could be a convenient, new preventative therapeutic option or adjunctive treatment option for large number of patients with retinal diseases, if approved. |
| • | Upstream target implicated in two validated pathways. APX3330 is designed to lead to inhibition of two validated cell signaling pathways (angiogenesis and inflammation) known to cause various retinal diseases. Moreover, the APX3330 mechanism of action is distinct in working upstream of the current anti-VEGF therapies, suggesting that it could complement anti-VEGF therapies and potentially reduce frequency of doctor visits and intravitreal injections. |
| • | Favorable tolerability profile. In 12 completed Phase 1 and Phase 2 clinical trials, APX3330 was well-tolerated. The AEs were mostly infrequent and mild with transient pruritis being the most common. No systemic effects such as changes in blood pressure or heart rate were seen, and no toxicities related to neurological, cardiovascular, renal, pulmonary, or gastrointestinal organs were observed. |
| • | Potential benefit of systemic administration. As a systemic agent, APX3330 can be expected to treat bilateral binocular (both eyes) retinal vascular disease. |
| • | Stable, cost-effective oral tablet. APX3330 is formulated as an oral tablet with favorable stability characteristics, and its active pharmaceutical ingredient is a small molecule with the advantages of standardized, scalable, and lower-cost manufacturing processes. |
Ocuphire is initially pursuing APX3330 for the DR indication as first-line therapy and may explore opportunities for clinical benefit as adjunctive therapy for other retinal indications such as DME, wAMD, and GA:
| • | DR, the leading cause of vision loss in adults aged 20–74 years, which results from chronic elevations of glucose in the blood that leads to cell damage in the retina. Retinal key opinion leaders’ feedback suggests that slowing of DR progression with an oral agent would be a useful treatment in patients with background DR and good visual function. |
| • | DME, one of the most common complications of DR, in which vascular leakage causes swelling of the retinal macula and a loss of visual acuity. |
| • | wAMD, a chronic eye disorder that causes visual distortions in the central part of one’s vision, in which abnormal blood vessels leak fluid or blood into the macula, the part of the eye that is critical for central and color vision. |
| • | GA, an advanced form of age-related macular degeneration (AMD) that leads to progressive and irreversible vision loss. |
Nyxol’s Target Indications
RM (Nyxol)
Mydriasis Overview
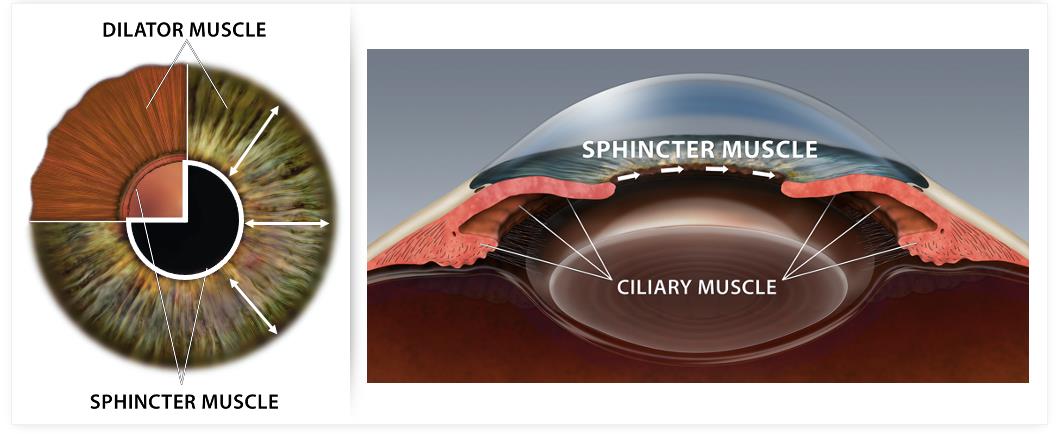
Every year in the U.S., over 100 million eye exams or procedures are performed that require dilation of the pupil (mydriasis) to examine the back of the eye either for routine check-ups, disease monitoring or surgical procedures. The mydriasis is achieved either by stimulating the iris dilator muscle with the use of alpha agonists (e.g., phenylephrine), or by blocking the iris sphincter muscle with the use of muscarinic antagonists (e.g., tropicamide) or a combination of both mydriatic agents. Typically, pharmacologically induced mydriasis dilates the pupil to 7 mm to 8 mm, a size suitable for ophthalmic examination of the retina and other structures of the interior of the eye. Such pharmacologically induced mydriasis can last from a few hours (typically 6 hours) up to 24 hours, depending on the pigmentation of the iris, one’s age, and other factors. Side effects of mydriasis include sensitivity to light and blurred vision, which make it difficult to read, work, or drive. Many dilating drops also cause cycloplegia, the temporary paralysis of the muscle which allows the eye to focus on near objects. For this reason, many patients may request to avoid dilation, thus limiting the eye care provider’s ability to conduct a comprehensive annual eye exam.
Limitations of Existing Treatments for Reversal of Mydriasis
There is no approved product presently on the market for reversal of mydriasis and Ocuphire is not aware of any others in development. In 1990, the FDA approved the selective alpha-1 antagonist dapiprazole, marketed as Rev-Eyes®, to reverse mydriasis induced by adrenergic or anticholinergic agents. Rev-Eyes was eventually withdrawn from the market for reasons unrelated to safety or efficacy, according to the FDA.
Nyxol Opportunity in RM
Nyxol has been shown in clinical studies to expedite the reversal of mydriasis compared to the eye’s natural process. According to GlobalData market research, over 65% of patients report a moderate to severe negative impact of a dilated exam, underscoring the potential value of Nyxol’s role in improving comfort and daily function after pupil dilation. Additionally, an estimated 45% of patients responded that they would be very likely to request a dilation reversal drop, and more than 40% of eye care providers would be likely to use a reversal drop if such a treatment were commercially available. Ocuphire believes that many people who undergo pupil dilation would benefit from a reversal treatment that has the potential to get patients back to their normal routines faster and avoid the subjective discomfort of dilation. Ocuphire also believes that if providers can offer a reversal drop there could potentially be more compliance with annual dilated eye exams.
Presbyopia (Nyxol)
Presbyopia Overview
Presbyopia is an age-related condition with onset most common in people over 40 years old. As the eye ages, the lens becomes stiffer, which limits the eye’s ability to adjust its focus for reading or for other tasks that require clear vision at near distances. Presbyopia patients experience blurred near vision, difficulty seeing in dim light, and eye strain. In young healthy eyes, lenses are able to focus light from objects at different distances by a process called accommodation. During accommodation, muscles surrounding the lens contract, causing the lens to change shape and increasing the focusing power of the eye. This allows dynamic, clear vision at both near and far distances. With increasing age, the lens becomes stiffer as the structural crystallin proteins become misfolded. This increased lens stiffness limits the eye’s ability to adjust its focus for reading or for other tasks that require clear vision at near distances. Because of the ubiquity of the condition, presbyopia represents a large market both in the United States and abroad totaling over 2 billion presbyopia patients. It is estimated that 120 million Americans have presbyopia, and this number is expected to grow as the population above the age of 45 increases.
Existing Treatments for Presbyopia
The U.S FDA approved VuityTM (1.25% pilocarpine) eye drop for the treatment of presbyopia in October 2021. Vuity was launched in December 2021 and is marketed by Allergan, an AbbVie company. Additional available treatments for presbyopia include reading glasses, bifocals, gradients, bifocal contact lenses, and multifocal intraocular lenses. Reading glasses can be inconvenient and must be taken off and put on frequently throughout the day to see objects at far and near distances, respectively. Many patients express frustration with losing or forgetting their glasses. Additionally, some patients find glasses unflattering. Contact lenses for presbyopia also have drawbacks. They can only be used monocularly, where one eye is fitted with a presbyopic lens while the other is used for distance vision, which often leads to eye strain. Cholinergic agonist (pilocarpine, carbachol, aceclidine) eyedrops have potential negative side effects such as headache, brow ache and retinal detachment.
A small portion of patients elect surgical intervention, including laser treatment to achieve monovision and insertion of KAMRA Inlays, a plastic implant into the cornea of the non-dominant eye to increase its depth of field. The risks of such interventions are those associated with all ocular surgeries, such as a potential decrease in contrast sensitivity and the creation or worsening of dim light or night vision disturbances.
Nyxol Opportunity in Presbyopia
Pupil diameter management is a promising strategy for the pharmacological treatment of presbyopia. Recent research suggests that an optimal pupil size of 2 mm to 3 mm diameter will lead to significant improvement in presbyopia symptoms by increasing depth of focus without compromising distance vision in photopic or mesopic lighting conditions. Ocuphire has been evaluating Nyxol as both a single-agent eye drop and with LDP as an adjunctive eye drop to achieve optimal pupil size and improve near vision. Nyxol has shown in several Phase 2 trials the ability to reduce pupil diameter size by 1-1.5 mm alone and by 2-2.5 mm when Nyxol is used with LDP. Nyxol alone provides durable near vision efficacy gain of up to 18 hours, and the Nyxol + LDP combination allows additional efficacy gains of up to least 6 hours.
With respect to the treatment of presbyopia, Ocuphire believes that tolerability, convenience, and preservation of distance vision quality are of the utmost importance. Presbyopia is considered a “benign” condition, in that there is no risk of death or complete vision loss. Thus, any therapies without robust tolerability will not be suitable alternatives to reading glasses or contact lenses. Nyxol is being developed to be applied once daily before bed, with potential resolution of any mild transient hyperemia by morning. Nyxol’s unique mechanism of action on the iris dilator muscle has no to low risk of retinal detachment. According to GlobalData market research, 69% of patients would consider an eye drop alternative. Ocuphire believes that many presbyopes who are unsatisfied with their reading glasses or monocular contact lenses, and who would prefer a less invasive alternative than surgical intervention, would find Nyxol single-agent eye drop or the Nyxol + LDP drops a promising option, if approved.
DLD (Nyxol)
DLD Overview
Vision at night or in dim light conditions is different from daytime vision in several important ways. Most notably, at night, the pupils dilate to allow more light into the eye. Diminished night vision is a natural part of aging as well as a common side effect of several conditions and procedures. DLD is caused by peripheral imperfections (aberrations) of the cornea which scatter light when the pupil dilates in dim light conditions. These imperfections can be naturally occurring, especially with age, or surgically induced from refractive procedures such as LASIK. As the pupil dilates in response to mesopic conditions, light passes through the periphery of the cornea and lens, unlike during photopic conditions. Any imperfections or aberrations present on the periphery cause light to reach the retina in a non-focused and scattered way, creating glare, halos, starbursts, ghosting, and a loss of contrast sensitivity (“CS”). These visual disturbances can be debilitating to a variety of everyday activities, especially driving. The light emitted by traffic lights and other cars scatters and obscures most of the visual field, making driving in dim light conditions hazardous. Glare, in particular, can be dangerous while driving. In one study of 297 drivers given vision tests that correlate with accidents, 45% of the drivers who reported difficulty driving at night were unable to perform any of the tests with glare.
The effects of DLD can be reduced or eliminated by reducing the pupil size to a smaller diameter that prevents the scattering effect without impeding the ability to see at night. DLD can occur naturally (night myopia) and is commonly caused by ocular surgery (“LASIK”). One significant cause of night myopia is keratoconus, an orphan disease that starts at a young age with progressive thinning of the cornea usually due to genetic and environmental causes. Ocuphire estimates there are about 38 million individuals in the U.S. that suffer from DLD, with an estimated 16 million having moderate-to-severe DLD that may be directly addressable with a pupil management approach. Market research conducted by GlobalData of patients who self-report DLD showed 25% completely avoid driving at night. Furthermore, 67% who report moderate or severe DLD would be willing to try an eye drop treatment option. These patients can be segmented by the origins of their vision disturbance. Approximately 44% of DLD are the result of night myopia, followed by approximately 30% from cortical cataracts, 15% from post-intraocular lens (“IOL”) implants, and 10% following LASIK surgery. These conditions span an age range of late teenagers to those 80 years and older.
Limitations of Existing Treatments for DLD
The biggest challenge for the treatment of DLD is the lack of safe, tolerable, convenient, and effective treatments. Despite a large number of addressable patients with moderate-to-severe DLD, there is no FDA-approved treatment on the market for DLD. Some commonly used tools such as tinted glasses are not effective, and in fact, may worsen patients’ vision at night. Off-label use of approved miotic agents, such as regular-strength pilocarpine, are unsuitable for the treatment of DLD because they reduce pupil size to a degree that may impede safe night vision and may cause loss of accommodation.
Nyxol Opportunity in DLD
Ocuphire believes it may have a new DLD treatment option that could improve patients’ ability to see in dim lighting and significantly improve their quality of life. Nyxol is currently the only product candidate in development for DLD and could become the first pharmacological treatment option if approved. In addition to a potential first-mover advantage, Nyxol is being developed to be administered via convenient, once-daily dosing before bedtime and has been shown in multiple Phase 2 clinical trials to improve low contrast visual acuity in mesopic (dim) conditions on the standard visual chart. Nyxol has also been shown to be well-tolerated in these trials.
APX3330’s Target Indications
Diabetic Retinopathy (APX3330)
Diabetic Retinopathy Overview
Diabetic Retinopathy (“DR”) is an eye disease resulting from diabetes, affecting over 7 million patients in the U.S., in which chronically elevated blood sugar levels cause damage to blood vessels in the retina. It is the leading cause of vision loss in adults aged 20–74 years. There are two major types of DR:
| • | Non-proliferative DR, or NPDR. NPDR is an earlier, more typical stage of DR and can progress into more severe forms of DR over time if untreated and if exposure to elevated blood sugar levels persists. |
| • | Proliferative DR, or PDR. PDR is a more advanced stage of DR than NPDR. It is characterized by retinal neovascularization and, if left untreated, leads to permanent damage and blindness. |
Therapies for NPDR and PDR are distinct. For NPDR, treatment is usually directed at observation, lifestyle changes, and control of elevated blood sugars that led to progression of NPDR in the first place. Additionally, the current treatment paradigm is for physicians to wait and monitor early-stage DR patients, with anti-VEGF or steroid injectable therapy or laser treatment reserved for patients who advance to proliferative DR or DME. In the Protocol S trial by the Diabetic Retinopathy Clinical Research Network, Lucentis was found to be noninferior to laser therapy in patients with PDR. Moreover, in 2018, from Regeneron’s PANORAMA trial, EYLEA® reversed disease progression in patients with moderately severe to severe NPDR.
Diabetic Macular Edema (APX3330)
Diabetic Macular Edema Overview
Diabetic Macular Edema (“DME”) is a common complication of DR where the macula swells with fluid leaked from damaged blood vessels as a result of worsening diabetic retinopathy. It is one of the most common reasons for blindness in diabetics, affecting approximately 750,000 patients. DME may cause blurriness in the center of vision, the appearance of straight lines as wavy, colors that look dull or washed out, or blind spots. The pathogenesis of DME involves vascular leakage, retinal ischemia, and release of vaso-proliferative growth factors and inflammatory mediators.
In DME, corticosteroids and anti-VEGF agents are used to treat vascular leakage, inflammation and hypoxia/angiogenesis. In patients whose disease has progressed to DR with DME, anti-VEGF agents are first line therapy followed by corticosteroids. Lucentis was approved for treatment of DME with a dosing regimen of a 0.3 mg injection approximately every four weeks. Similarly, EYLEA® was approved with a dosing regimen of a 2.0 mg injection approximately every four weeks.
Limitations of Existing Treatments for DR and DME
In DR (especially NPDR), despite the approvals of anti-VEGF therapeutics in recent years, the use of injectables is not adopted in practice as preferred treatment as the disease is asymptomatic and patients are reluctant to undergo injections or laser therapy.
In DME and late-stage DR, intravitreal VEGF inhibitors are approved globally, however these therapies rarely provide a complete solution to the underlying vascular problem associated with DR and DME. Although these therapeutic agents have been successful for some patients, significant proportions of patients are resistant and refractory. Moreover, serious side effects including hemorrhage and intraocular infections are possible with intravitreal injections. Both Lucentis and EYLEA are also associated with increased risks of blood clots in the arteries. In addition, intravitreal injections require frequent visits to the ophthalmologist, usually on the order of every 4 weeks with a few anti-VEGF therapies in development that are working on increasing the time between injections (8 – 12 weeks).
Furthermore, retinal diseases are initially or over time bilateral, and thus treatments that only treat one eye, leave the other eye to remain untreated.
APX3330 Opportunity in DR and DME
In addition to being characterized by abnormal increases in VEGF levels, recent scientific literature reports indicate that diabetic eye disease has an inflammatory component, unrelated to VEGF. Because inflammation and hypoxic signaling (VEGF production) play crucial roles in both vascular leakage and neovascularization of DR and DME, treatments that impinge upon both pro-inflammatory and hypoxic signaling offer a promising therapeutic strategy. APX3330’s target of Ref-1 (a protein associated with inflammation and immune response) may leverage this dual mechanism of action (MOA) to reduce the production and hence the quantity of VEGF while also preventing inflammatory damage. The MOA of APX3330 is differentiated from traditional anti-VEGF treatments in that it does not neutralize the elevated levels of VEGF, but rather brings VEGF levels to normal homeostatic levels, thereby making it an ideal treatment option to prevent progression or worsening in earlier stages of diabetic eye disease.
This potentially allows for improved response to DR treatment and may extend the duration between invasive treatments for late-stage retinal diseases (DME, wAMD). Moreover, as a potential first-in-class, orally administered product candidate twice a day, it has the potential to be a more convenient option at an earlier stage of disease especially for DR than intravitreal anti-VEGF injections, which are burdensome to patients and have a significant side effect profile including cataract formation, increased intraocular pressure, intraocular infections, and retinal detachments. Furthermore, as a systemic therapy, APX3330 offers the potential to treat both eyes while maintaining a favorable safety profile.
Other Indications:
wAMD
Age-Related Macular Degeneration (“AMD”) is a common eye condition affecting 11 million individuals in the U.S. and 196 million globally, mostly over the age of 55 years. It is a progressive disease affecting the central portion of the retina, known as the macula, which is the region of the eye responsible for sharpness, central vision and color perception. wAMD is an advanced form of AMD characterized by neovascularization and fluid leakage under the retina. It is the leading cause of severe vision loss in patients over the age of 50 in the United States and EU. While wAMD represents only 10% of the number of cases of AMD overall, it is responsible for 90% of AMD-related severe vision loss. Untreated or undertreated wAMD results in further blood vessel leakage, fluid in the macula, and ultimately scar tissue formation, which can lead to permanent vision loss or even blindness as a result of the scarring and retinal deformation that occur during periods of non-treatment or undertreatment. Similar to severe DR and DME, current therapy for wAMD consists of intravitreal injections, mainly of Lucentis and EYLEA. The limitations of these therapies are described in the section above titled, “Limitations of Existing Treatment for DR and DME”. Based on APX3330 targeting Ref-1 with a MOA of reducing overexpression of VEGF and inflammation, it has potential use in wAMD. Further, to enter the wAMD injectable market, Ocuphire is considering the utility of an intravitreal or sustained delivery formulation of APX3330 and its second-generation analogs, APX2009 and APX2014. APX2009 and APX2014 data suggest improved efficacy against the Ref-1 target compared to APX3330 (as published in the Journal of Pharmacology and Experimental Therapeutics).
GA
Geographic atrophy (GA) is an advanced form of age-related macular degeneration (AMD) that leads to progressive and irreversible vision loss. AMD is the leading cause of permanent vision loss in people over the age of 65 in developed countries, and the risk of developing AMD increases with age. Based on published studies, approximately 1 million people have GA in the United States, and 5 million people have GA globally. In people with GA, photoreceptors, which are light sensitive cells, deteriorate in the macula, a central portion of the retina responsible for central vision and color perception. This damage starts as small spots that grow into larger patches. As the cells in the macula die, the person starts to lose vision. A person with early AMD may notice problems with reading or night vision. Eventually, if the disease progresses to advanced stages, permanent blind spots (scotomas) in the center of the visual field will develop. The cause of GA is thought to be multifactorial including inflammation, with numerous environmental and genetic risk factors. The dysregulation of the complement cascade, an important part of the body’s immune system, plays a pivotal role. Excessive activation of the complement cascade results in destruction of healthy cells, which can lead to the onset or progression of many diseases including GA. SYFOVRE® is the only FDA approved treatment for GA and requires monthly or every other month intravitreal injections. Based on APX3330 targeting Ref-1 with a MOA of anti-VEGF and anti-inflammatory MOA, it has potential use in GA.
Product Candidates-Nyxol
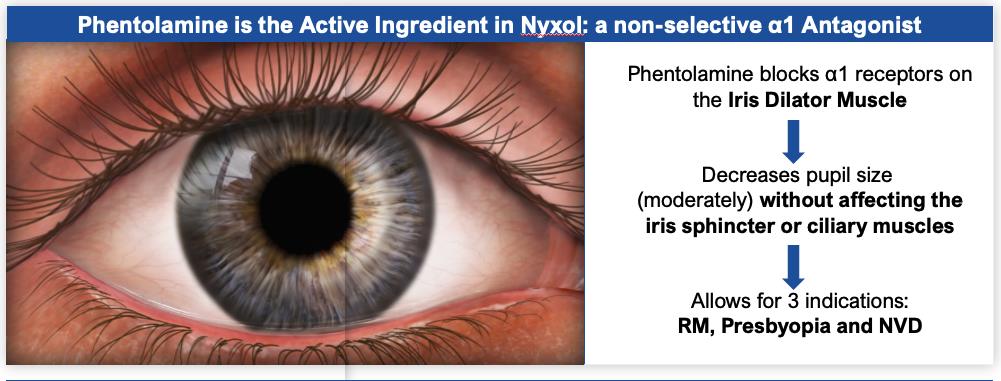
Nyxol Mechanism of Action
Nyxol is a once-daily sterile eye drop formulation of phentolamine mesylate designed to reduce pupil diameter and improve visual acuity. The active pharmaceutical ingredient of Nyxol, phentolamine mesylate, is a non-selective alpha-1 and alpha-2 adrenergic antagonist that inhibits activation of the smooth muscle of the iris, reducing pupil diameter. Unlike phentolamine, cholinergic agonists (such as pilocarpine, carbachol and aceclidine) work on the iris sphincter muscle to reduce the pupil diameter, and are associated with side effects given the engagement of the cilliary muscle such as headaches, brow aches and retinal detachments and have limited use in patients with high myopia. Nyxol shares many of the attributes of existing ophthalmic eye drops, including a convenient route of administration and cost-effective manufacturing process, with the potential advantage of once-daily dosing (FIGURE 1).
FIGURE 1: Nyxol Product Candidate Profile
Phentolamine is a nonselective alpha-1 & alpha-2 adrenergic antagonist. Dilation of the pupil is controlled by the radial iris dilator muscles surrounding the pupil which are activated by the alpha-1 receptors of the adrenergic nervous system. Alpha-1 antagonists bind to the receptors to inhibit the pupillary response and reduce dilation (FIGURE 2). Phentolamine mesylate is the active ingredient in two injectable FDA-approved drugs, Regitine and OraVerse, as described previously.
For the RM indication, pharmacologically induced mydriasis is achieved either by stimulating the iris dilator muscle with the use of alpha agonists (e.g., phenylephrine), or by blocking the iris sphincter muscle with the use of muscarinic antagonists (e.g., tropicamide). Nyxol, either by directly antagonizing the alpha-1 agonist or by indirectly antagonizing the pupil dilation effect of muscarinic blocking, may expedite the reversal of mydriasis prior to natural reversal.
For presbyopic patients, to overcome the lens’ inability to change shape (accommodation) and focus light from near objects, pupil diameter reduction to a small size will allow light to come in the eye only in a near straight direction and increase the depth of focus (the “pinhole effect”). Ocuphire believes that it is possible to reach a target 2 mm to 3 mm optimal pupil diameter by relaxing the dilator iris muscle with Nyxol and contracting the iris sphincter muscle with a muscarinic agonist such as a low dose pilocarpine. This could result in an optimal depth of focus and near vision clarity without the assistance of lenticular accommodation.
Lastly, for the DLD indication, it is proposed that a moderate miotic effect by application of Nyxol might mitigate night vision difficulties, a large portion of which are caused by imperfections or aberrations present on the periphery of the cornea. Therefore, the effects of these imperfections can be reduced or eliminated by reducing the pupil size to a smaller diameter, knowing that a smaller pupil blocks what would be unfocused, aberrant rays of light on the retina.
FIGURE 2: Nyxol’s Mechanism of Action
Nyxol Clinical Experience Summary
Nyxol has been assessed in twelve investigator-initiated and company-sponsored Phase 1, Phase 2 and Phase 3 clinical trials. Across all trials, over 400 adult patients have been exposed to at least one dose of phentolamine ophthalmic solution. Phase 2 and Phase 3 trials have been accepted for poster or oral presentation at the annual American Academy of Ophthalmology (AAO), Association for Research in Vision and Ophthalmology (ARVO), or American Society of Cataract and Refractive Surgery (ASCRS) meetings.
Ocuphire believes that results from Nyxol’s Phase 1, Phase 2 and Phase 3 trials support its current development plan focused on RM, presbyopia and DLD patients. Specifically, patients treated with Nyxol were observed to have statistically significant decreases in pupil diameter and improved visual acuity. Nyxol has shown consistent ability to decrease pupil diameter at the selected dose of 0.75% Phentolamine Ophthalmic Solution (POS) by approximately 20-25% (~1 – 1.5 mm) in both mesopic and photopic conditions.
A summary of Ocuphire’s completed clinical trials is shown below (TABLE 2). Note that Nyxol in its current proprietary formulation of phentolamine mesylate ophthalmic solution was first introduced in the NYX-01a2 trial, and prior to that, a formulation of phentolamine mesylate in artificial tears solution was used. In subsequent sections, completed Phase 3 trials for RM (MIRA-2, MIRA-3, and MIRA-4), a Phase 2 trial for Presbyopia (VEGA-1) and a Phase 3 trial for DLD (LYNX-1) will be highlighted. Additionally, an ongoing Phase 3 trial for Presbyopia (VEGA-2) and preclinical animal studies will also be presented.
TABLE 2: Summary of Completed Clinical Trials with Nyxol
Trial Name (IND Number) | | Patient / Indication | | Phase | | Trial Objectives | | Doses | | Number of Patients^ | | Dosing | | Key Endpoints |
NYX-001 (67-288) | | Healthy Volunteers | | 1 | | Double-masked, randomized, single dose, 3-arm controlled, parallel trial to determine the efficacy and safety of phentolamine mesylate | | 0.2% PMOS | | Nyxol*=15, Visine=15, Visine + Nyxol*=15 Total = 45 | | Single-dose | | Safety and Efficacy (PD) |
NYX-002^ (67-288) | | Healthy Volunteers | | 1 | | Double-masked, randomized, placebo-controlled, single-dose, incomplete block, 3-period crossover, dose escalation trial evaluating the tolerability and efficacy of phentolamine mesylate | | 0.2%, 0.4%, 0.8% PMOS | | Nyxol*=16 Placebo=12 Total = 16 | | Single-dose | | Safety and Efficacy (PD, VA) |
OP-NYX-004^ (73-987) | | Night Vision Disturbances Patients | | 1 / 2 | | Double-masked, randomized, placebo-controlled, single-dose, incomplete block 3-period crossover, dose escalation trial to determine the efficacy and safety of phentolamine mesylate | | 0.2%, 0.4%, 0.8% PMOS | | Nyxol*=16 Placebo=12 Total = 16 | | Single-dose | | Safety and Efficacy |
OP-NYX-SNV (70-736) | | Severe Night Vision Disturbances Patients | | 2 | | Double-masked, randomized, placebo-controlled, single-dose trial to assess the efficacy and safety of phentolamine mesylate ophthalmic solution | | 1.0% PMOS | | Nyxol*=16, Placebo=8 Total = 24 | | Single-dose | | Safety and Efficacy (PD, LCVA, CS, WA) |
OP-NYX-01a2 (70-499) | | Severe Night Vision Disturbances Patients | | 2 | | Double-masked, randomized, placebo-controlled, single-dose, 3-arm trial to assess the efficacy and safety of Nyxol | | 0.5%, 1.0% PMOS | | Nyxol=40 Placebo=20 Total = 60 | | Multiple doses (15-28 days) | | Safety and Efficacy (PD, LCVA, CS) |
OPI-NYXG-201 (ORION-1) (70-499) | | Glaucoma and Ocular Hypertension, Elderly Patients | | 2b | | Double-masked, randomized, placebo-controlled, multiple-dose, multi-center trial to assess the efficacy and safety of Nyxol | | 1.0% PMOS | | Nyxol=19 Placebo=20 Total = 39 | | Multiple doses (14 days) | | Safety and Efficacy (IOP, PD, near VA, VA) |
OPI- NYXRM-201 (MIRA-1) (70-499) | | Healthy Patients/ Reversal of Mydriasis | | 2b | | Double-masked, randomized, placebo-controlled, crossover, single-dose, multi-center trial to assess the efficacy and safety of Nyxol in reducing pharmacologically induced mydriasis | | 1.0% PMOS | | Nyxol=31 Placebo=32 Total = 32 | | Single-dose | | Safety and Efficacy (PD, Accommodation, VA) |
OPI- NYXRM-301 (MIRA-2) (70-499) | | Healthy Patients/ Reversal of Mydriasis (including 12–17 years-old) | | 3 | | Double-masked, randomized, placebo-controlled, single-dose, multi-center trial to assess the efficacy and safety of Nyxol in reducing pharmacologically induced mydriasis | | 0.75% POS | | Nyxol=94 Placebo=91 Total = 185 | | Single-dose | | Safety and Efficacy (PD, Accommodation, VA) |
OPI-NYXRM-302 (MIRA-3) (70-499) | | Healthy Patients/ Reversal of Mydriasis (including 12-17-years old) | | | | Double-masked, randomized, placebo-controlled, single-dose, multi-center trial to assess the efficacy and safety of Nyxol in reducing pharmacologically induced mydriasis | | 0.75% POS | | POS=214 Placebo=124 Total = 368 | | Single-dose | | Safety and Efficacy (PD, Accommodation, VA) |
OPI-NYXRMP-303 (MIRA-4) (70-499) | | Healthy Patients/ Reversal of Mydriasis (between ages of 3-and 11) | | | | Randomized, Parallel-Arm, Double-Masked, Placebo-Controlled Study of the Safety and Efficacy of Nyxol (0.75% Phentolamine Ophthalmic Solution) to Reverse Pharmacologically Induced Mydriasis in Healthy Pediatric Subjects | | 0.75% POS | | POS=11 Placebo-12 Total=23 | | Single-dose | | Safety and Efficacy (PD, VA) |
OPI-NYXP-201 (VEGA-1) (70-499) | | Presbyopia patients (ages of 40 and 64) | | 2 | | Randomized, Placebo-Controlled, Double-Masked Study of the Safety and Efficacy of Nyxol (0.75% Phentolamine Ophthalmic Solution) with Low-Dose (0.4%) Pilocarpine Eye Drops in Subjects with Presbyopia | | 0.75% POS | | Nyxol +LDP = 44 Placebo alone = 45 Nyxol alone = 30 Placebo +LDP = 31 | | Multiple doses (4-5 days), Single dose of LDP | | Safety and Efficacy (DCNVA, VA, PD) |
OPI-NYXDLD-301 (LYNX-1) (70-499) | | Night Vision Disturbances in adults | | | | Randomized, Placebo-Controlled, Double-Masked Study of the Safety and Efficacy of Nyxol (0.75% Phentolamine Ophthalmic Solution) in Subjects with Dim Light Vision Disturbances | | 0.75% POS | | POS=72 Placebo=73 Total =145 | | Multiple doses (14 days) | | Safety and Efficacy (mLCVA, VA, PD) |
Note: Nyxol = phentolamine mesylate in proprietary formulation, Nyxol* = phentolamine mesylate in commercial artificial tears solution. ^ Total patient numbers will not equal to the sum of the subgroups in crossover studies (NYX-002, NYX-004, and NYXRM-201). 0.75% POS (Phentolamine Ophthalmic Solution) is the same as 1% PMOS (Phentolamine Mesylate Ophthalmic Solution). References to Nyxol with both designations appear throughout this document, and there is no difference in formulation between the two designations.
CS, contrast sensitivity; DCNVA, distance-corrected near visual acuity; DLD, dim light vision disturbances; IND, Investigational New Drug application; IOP, intraocular pressure; LCVA, low-contrast visual acuity; LDP, low-dose pilocarpine; OHT, ocular hypertension; PD, pupil diameter; POS, Phentolamine Ophthalmic Solution or Nyxol; PMOS, Phentolamine Mesylate Ophthalmic Solution or Nyxol; * represents phentolamine mesylate in commercial artificial tears solution; RM, reversal of mydriasis; VA, visual acuity; WA, wavefront aberrometry.
Total subject numbers will not equal the sum of the subgroups in crossover studies (NYX-002, NYX-004, and NYXRM-201).
MIRA PROGRAM – Reversal of Mydriasis Indication for Nyxol
Nyxol RM: MIRA-3 and MIRA-2 Phase 3 Registration Trials (Completed)
MIRA-2 and MIRA-3 were double-masked, randomized, placebo-controlled, multi-center trials of Nyxol compared with vehicle (placebo) in normal healthy subjects. In MIRA-2, a total of 185 subjects (including 14 pediatric subjects aged 12–17 years) were randomized 1:1 to receive Nyxol or placebo treatment 1 hour following dilation with 1 of 3 mydriatic agents (2.5% phenylephrine, 1% tropicamide, or Paremyd 3:1:1, respectively). Stratification by light or dark irides was 1:1. Measurements were taken at 0 minutes, 30 minutes, 1 hour, 90 minutes, 2 hours, 3 hours, 4 hours, and 6 hours following administration of study medication. In MIRA-3, treatment randomization was 2:1 (Nyxol or vehicle [placebo], respectively) and stratification by light or dark irides was 1:1. As in MIRA-2, the mydriatic agent randomization was 3:1:1 (2.5% phenylephrine, 1% tropicamide, or Paremyd), respectively, and the randomized subjects received 1 drop of mydriatic agent 1 hour before treatment. In MIRA-3, a total of 368 subjects (including 31 pediatric subjects aged 12–17 years) were randomized to treatment.
In MIRA-2 and MIRA-3, treatment (Nyxol or placebo) was administered OU, with the study eye defined as the right eye (OD) and the fellow eye defined as the left eye (OS). Subjects had 2 drops of treatment administered 5 minutes apart in the study eye (OD) and 1 drop of treatment administered in the fellow eye (OS) 1 hour after mydriatic drug administration. Pediatric subjects (aged 12–17 years) in MIRA-2 received only 1 drop of treatment OU.
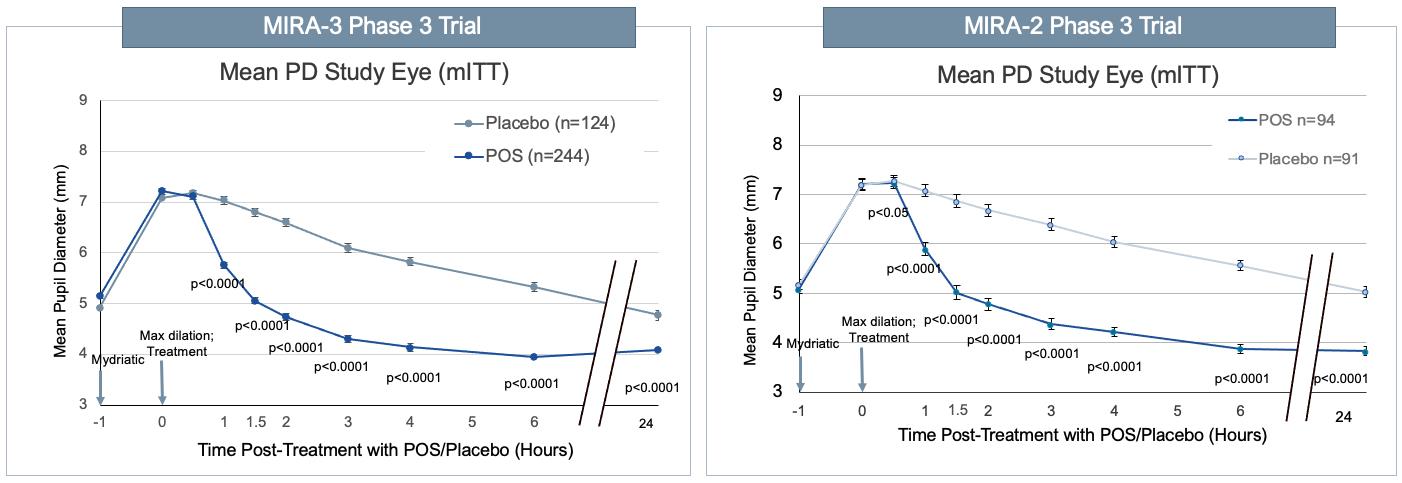
In both of the 2 Phase 3 registration studies, Nyxol met the primary endpoint (FIGURE 3A). In the mITT Population, a statistically significant and clinically meaningful greater percent of subjects treated with Nyxol had study eyes that showed reversal of mydriasis 90 minutes post-dose, using a PD threshold of ≤ 0.2 mm from baseline compared with the placebo treatment (49% vs 7%, respectively, in MIRA-2 and 58% vs 6%, respectively, in MIRA-3; all p<0.0001). Importantly, similar statistically significant results were observed 60 minutes post-dose (28% vs 2%, respectively, in MIRA-2; p<0.0001 and 42% vs 2%, respectively, in MIRA-3; p<0.0001). The statistically significant benefit was also seen at all other time points and persisted through 24 hours. In the 2 studies 80% and 79% of Nyxol-treated subjects had their pupils return to pre-dilation pupil size within 3 hours compared with only 18% and 14% of subjects treated with placebo for the MIRA-2 and MIRA-3 studies, respectively.
In each of the studies, Nyxol effect on mean PD further supported the results of the primary endpoint analysis. A statistically significant reduction in mean PD from maximum PD (0 minutes) with Nyxol versus placebo treatment was observed as early as 60 minutes post-dose, increasing in magnitude through 90 minutes. The significant reduction in PD compared to placebo persisted through 24 hours. Results were consistent between the 2 trials (FIGURE 3B). In both studies, the mean PD of Nyxol-treated study eyes returned from maximum PD to predilation baseline values (> 1.8 mm decrease) at 90 minutes post-dose. In contrast, in study eyes treated with placebo, the mean PD decreased by < 0.5 mm at 90 minutes post-treatment and the mean PD remained greater than baseline through 6 hours (7 hours after dosing of the mydriatic) in both studies. Overall, Nyxol reduced PD from baseline by 20 to 30% (1 to 1.5 mm)
FIGURE 3: Study OPI-NYXRM-302 (MIRA-3) and OPI-NYXRM-301 (MIRA-2): Percent of patients returning to ≤0.2 mm of baseline pupil diameter in MIRA-3 (left panel) and MIRA-2 (right panel)
1.
B.
Note: POS = Nyxol
Similar efficacy results were observed regardless of whether eyes were treated with 1 drop or 2 drops. Efficacy was demonstrated across all 3 mydriatic agents and in light and dark irides.
Treatment with Nyxol was generally safe and well tolerated. The most common adverse reactions (> 5.0%) that have been reported are conjunctival hyperemia, instillation site discomfort, and dysgeusia; all were transient and primarily mild. Most TEAEs were ocular, as expected, and the vast majority were mild in intensity and resolved within hours of study medication instillation. There were no deaths, serious AEs or other significant AEs. Results from MIRA-2 and MIRA-3 studies were presented at several medical conferences in 2022.
Nyxol RM: MIRA-4 Pediatric Trial (Completed)
MIRA-4 was a 1-day, double-masked, randomized, placebo-controlled, single-dose, multi‑center study with 23 pediatric subjects aged 3 to 11 years, healthy or with ocular conditions. A total of 23 randomized pediatric subjects were evaluated for the safety and efficacy of Nyxol in reversal of pharmacologically induced mydriasis. Each subject was randomized to unmasked mydriatic agent and masked treatment. Treatment randomization was 1:1 (Nyxol or placebo [vehicle], respectively) and stratification by subject age group was 1:1, 3 to 5 years of age or 6 to 11 years of age. The mydriatic agent randomization was 3:1:1 (2.5% phenylephrine, 1% tropicamide, or Paremyd, respectively).
In MIRA-4, study treated with Nyxol, 64% had PD returned to ≤ 0.2 mm from baseline PD at 90 minutes compared to 25% of study eyes treated with placebo eyes in the mITT Population. This effect is similar to those seen in the adult registration studies (study eye: 49% vs 7%, respectively, in MIRA-2; p<0.0001 and 58% vs 6%, respectively, in MIRA-3; p<0.0001; fellow eye: 49% vs 6%, respectively, in MIRA-2; p<0.0001 and 52% vs 6%, respectively, in MIRA-3; p<0.0001). Among subjects aged 3 to 5 years (n=11), Nyxol showed numerically greater reversal of mydriasis in the study eye compared with placebo at 90 minutes (60% vs 17%, respectively), 3 hours (80% vs 50%, respectively), and 24 hours (100% vs 50%, respectively). Among subjects aged 6 to 11 years (n=12), Nyxol showed numerically greater reversal of mydriasis in the study eye compared with placebo at 90 minutes (67% vs 33%, respectively), 3 hours (83% vs 17%, respectively), and 24 hours (83% vs 50%, respectively).
The efficacy of Nyxol in pediatric subjects showed a similar magnitude of reversal of mydriasis to the efficacy of Nyxol in adults overall and across mydriatic agents. In a pooled analysis of 45 pediatric subjects (aged 12-17 years) in MIRA-2 and MIRA-3, 44% of Nyxol-treated study eyes (vs 6% placebo; p=0.0286) at 60 minutes and 63% (vs 0% placebo; p=0.0067) at 90 minutes returned to baseline PD. Results with the individual agents were also similar to those observed in adults.
No overall differences in safety have been observed between pediatric subjects aged 3 to 17 years or the elderly when compared to adult subjects. Results from MIRA-4 study were presented at the annual American Academy of Optometry 2022 meeting in San Diego.
VEGA PROGRAM – Presbyopia Indication for Nyxol and Nyxol+LDP
Nyxol Presbyopia: Phase 2 VEGA-1 Trial (Completed)
VEGA-1 (NYXP-201) was a double-masked, randomized, placebo-controlled, multi-center trial of Nyxol and LDP compared with vehicle (placebo) ophthalmic solution in presbyopic patients. A total of 150 patients were randomized 3:2:2:3 to receive Nyxol + LDP, Nyxol alone, LDP alone, or placebo, respectively. Nyxol or placebo was dosed for 3 or 4 consecutive evenings prior to binocular and monocular testing under photopic and mesopic lighting conditions. Measurements were made between 0 and 6 hours following administration of Treatment 2 (LDP or No Treatment). The primary efficacy endpoint for this study was the percent of patients who improved by ≥ 15 letters in DCNVA at 90 minutes post-treatment. The data from this study was presented at several medical conferences in 2021 and 2022.
Nyxol as a Single Agent
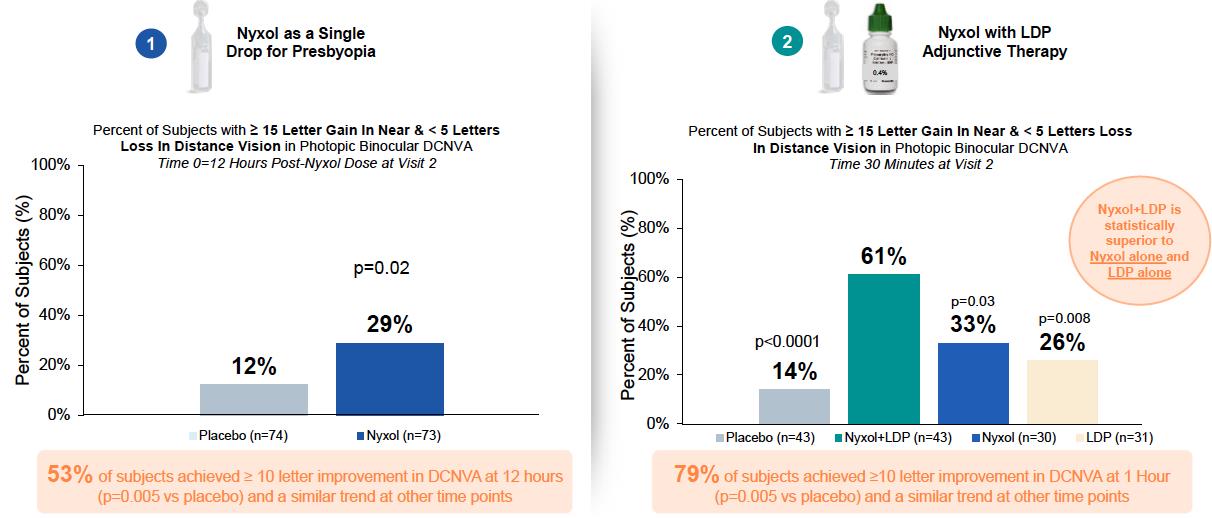
Following 3 to 4 days of treatment with Nyxol or placebo, 29% of Nyxol-treated subjects had ≥ 15 letters improvement in photopic binocular DCNVA compared with 12% of placebo-treated subjects 12 hours after the last dose (p=0.02). This benefit was durable and remained stable over the next 6 hours (or 18 hours), with a mean of 37% of Nyxol-treated subjects improving by ≥ 15 letters DCNVA 18 hours post-dose. Using a clinically meaningful criterion of a 10-letter improvement in DCNVA, 53% of subjects responded to Nyxol at the 12-hour time point compared to 28% of placebo-treated subjects (p=0.0046) (FIGURE 4).
Nyxol + LDP as Adjunctive Therapy
A statistically significant greater number of subjects in the PP Population treated with Nyxol + LDP had ≥ 15 letters improvement in photopic binocular DCNVA at 1-hour post-LDP treatment (primary endpoint) compared with placebo alone (61% vs 14%, respectively; p<0.0001). In addition, the Nyxol + LDP arm had significantly more subjects with ≥ 15 letters improvement in photopic binocular DCNVA compared with placebo alone at all time points from 30 minutes through 4 hours post-LDP treatment (p≤0.0166). This benefit was retained for all time points between 30 minutes and 4 hours when requiring that subjects with ≥ 15 letters improvement in photopic binocular DCNVA also had to have < 5 letters loss in photopic binocular BCDVA, the accepted registration endpoint. The percentage of Nyxol + LDP responders was also significantly better than placebo at 30 minutes (77% vs 51%, respectively; p=0.0146) and all subsequent timepoints through 3 hours (p≤0.0312) when using a clinically meaningful criterion of a 10-letter improvement in DCNVA (FIGURE 4).
FIGURE 4: Study OPI-NYXP-201 (VEGA-1): Percent of Patients Gaining ≥ 15 Letters in Binocular Photopic DCNVA by Treatment Arm and Timepoints
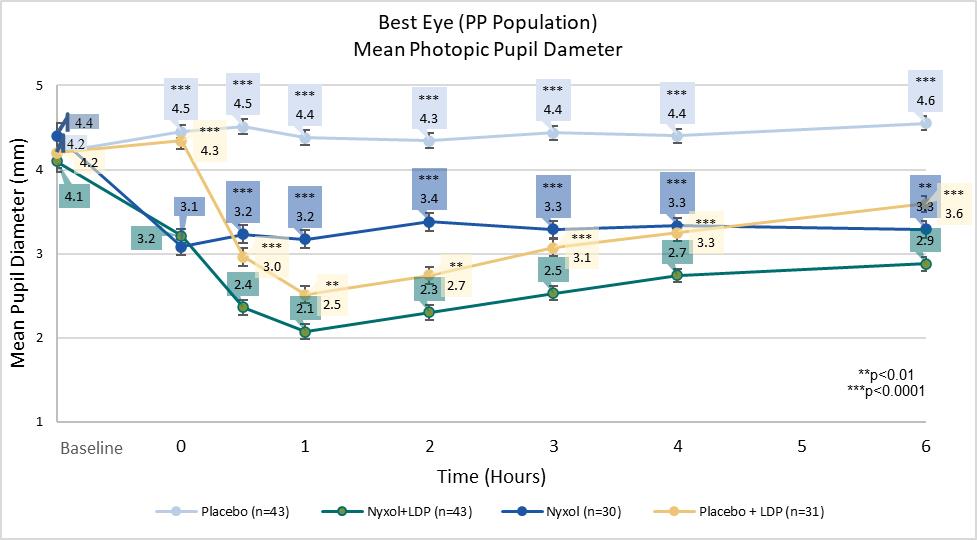
Nyxol and Nyxol+LDP provided durable optimal pupil diameter of ~ 2 mm to 3 mm, offering improvement in near vision without the loss of distance vision (FIGURE 5). Nyxol and Nyxol+LDP maintained a dynamic pupillary response when transitioning between photopic and mesopic lighting conditions.
FIGURE 5: Study OPI-NYXP-201 (VEGA-1): Mean pupil diameter
Nyxol alone as well as Nyxol +LDP were both well-tolerated with a favorable safety profile. Instillation site discomfort and conjunctival hyperemia were the only adverse events (AEs) that occurred in ˃5% patients, and 95% of the AEs were mild and none were severe. Visual acuity was not adversely affected. There were no deaths, no systemic AEs, no serious AEs or withdrawals due to AEs in patients receiving Nyxol only. No headaches, brow aches or blurry vision AEs were observed.
Nyxol Presbyopia: Phase 3 VEGA-2 Trial (Ongoing)
VEGA-2 (NYXP-301) is a double-masked, randomized, placebo-controlled multi-center trial of Nyxol as a single agent compared to placebo and with adjunctive LDP compared with vehicle (placebo) in presbyopic patients. Approximately 320 subjects are randomized to one of 4 treatment groups in 2 stages.
The first stage consists of 2 treatment groups (Nyxol or Placebo [i.e., Nyxol vehicle]), with approximately 160 subjects in each group. Stage 2 consists of 4 treatment groups (Nyxol + LDP, Nyxol + LDP vehicle, placebo + LDP, and placebo + LDP vehicle), with approximately 80 subjects per treatment group. Randomization will be stratified by light/dark irides. The first subject was enrolled into the study in late December 2022. Patients are treated with Nyxol or Placebo for 7 days followed by a washout period of 7 to 14 days. During the washout period, patients will return on Day 10 to assess the resolution of drug treatments effects on PD.
In the second stage, Nyxol + LDP will be evaluated following 1 day and 7 days of dosing with both treatment regimes. For both stages of the trial, Nyxol or Placebo is dosed once daily in each eye in the evening and for stage 2, LDP or LDP vehicle is dosed once daily in each eye in the morning.
The primary efficacy endpoint is the percent of subjects with ≥ 15 letters of improvement in photopic binocular DCNVA and with < 5 letters of loss in photopic binocular BCDVA from Baseline comparing Nyxol-treated subjects to placebo-treated subjects at 12 hours post-dose at Day 8 during stage 1. The key secondary endpoint to evaluate LDP adjunctive to Nyxol in stage 2 is the percent of subjects with ≥ 15 letters of improvement in photopic binocular DCNVA and with < 5 letters of loss in photopic binocular BCDVA at 30 minutes post-LDP/vehicle comparing LDP as adjunctive therapy to Nyxol to placebo, Nyxol alone, and LDP alone following 8 days of dosing. This trial is ongoing with topline results expected in late 2023.
LYNX PROGRAM – Dim Light Vision Disturbances Indication for Nyxol
Nyxol DLD: Phase 3 LYNX-1 Trial (Completed)
LYNX-1 (NYXDLD-301) was a Phase 3 double-masked, randomized, placebo-controlled, multi-center study of POS compared with placebo ophthalmic solution in patients with dim light vision disturbances (DLD) at multiple sites in the U.S. In this trial, 145 patients who experienced vision impairment under dim light conditions were randomized to receive either Nyxol or placebo. Each subject was randomized 1:1 to treatment with Nyxol or placebo ophthalmic solution and stratified by iris color (light/dark irides). Treatment was self-administered in each eye QD at or near bedtime for 14 days. Efficacy was measured by assessment of mLCVA, photopic LCVA (pLCVA), best-corrected distance visual acuity (BCDVA), mesopic high-contrast BCDVA (mHCVA), distance-corrected near visual acuity (DCNVA), mesopic high-contrast DCNVA, mesopic PD, photopic PD, subject questionnaire, and wavefront aberrometry. Safety assessments included conjunctival hyperemia, slit lamp biomicroscopy, ophthalmoscopy, IOP, physical exams, HR, and BP. Data from LYNX-1 may support the Nyxol-single agent differentiation in presbyopia.
At Day 8 (primary endpoint), a statistically significantly greater percent of subjects treated with Nyxol in the mITT Population had study eyes with ≥ 15 letters improvement from baseline in mLCVA compared with placebo treatment (13% vs 3%, respectively; p=0.0459). A significantly greater percentage of subjects treated with Nyxol also had study eyes with ≥ 15 letters improvement from baseline in mLCVA compared with placebo treatment at Day 15 (21% vs 3%, respectively; p=0.0042) (FIGURE 6).
FIGURE 6: Percent of Subjects With ≥ 15 Letters Improvement in Mesopic Low-Contrast Best-Corrected Distance Visual Acuity in the Study Eye by Visit (mITT Population)
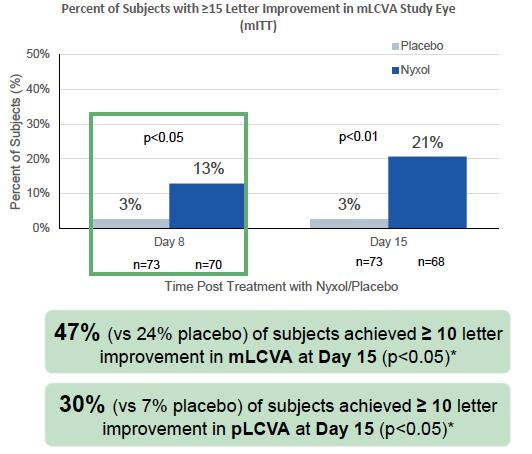
Nyxol was safe and well tolerated in this study. Adverse events occurring in ≥ 5% of subjects were instillation site erythema, instillation site pain, conjunctival hyperemia, and dysgeusia. No subjects in the Nyxol group experienced AEs of vitreous floaters, retinal tears, or retinal detachment. No subjects had any serious TEAEs or any TEAEs leading to withdrawal from the study, and no subjects died during the study. One subject in each treatment group had TEAEs leading to study medication discontinuation (instillation site erythema and instillation site pain in the Nyxol group and instillation site irritation and lacrimation increased in the placebo group. A total of 66 TEAEs were reported in 23 subjects (32%) treated with Nyxol and 22 TEAEs were reported in 12 subjects (16%) treated with placebo. All TEAEs were mild or moderate in intensity, except for 1 TEAE experienced by a subject in the Nyxol group, which was severe in intensity (instillation site pain), which was considered definitely related per the Investigator).
Nyxol Nonclinical Toxicology Studies
As part of a comprehensive nonclinical toxicity program, Ocuphire conducted 3 exploratory and 2 GLP single- and repeated-dose toxicity studies of phentolamine mesylate drug substance in rabbits and beagle dogs. There were no Nyxol-related histopathologic ocular pathology findings.
Nonclinical information (pharmacological properties, general and reproductive toxicology) for phentolamine mesylate is described in the literature in connection with other approved phentolamine drug products and formulations, and was reviewed by the FDA in the approval process of the market applications for Oraverse and Regitine.
For chronic administration of Nyxol, a 6-month repeated-dose toxicity study with Phentolamine Ophthalmic Solution 0.75% in Dutch-belted rabbits showed that topical administration of the drug product is safe and well tolerated. The study was reported to the FDA and no adverse comments were received. This 6-month toxicology study supports the long-term safety exposure trial which will be the basis of the marketing authorization for long-term treatment and chronic indications of presbyopia and DLD for Nyxol.
In support of administration of Pilocarpine Hydrochloride Ophthalmic Solution 0.4% (LDP) as adjuctive therapy to Nyxol for long term treatment, Ocuphire has performed a 99-day nonclinical toxicology study with Nyxol and LDP doses in Dutch belted rabbits. The study showed that the combination was well tolerated, with findings similar across all nonclinical studies performed with Nyxol by Ocuphire. The findings of this study support any future chronic indication for such combination.
Product Candidates-APX3330
APX3330 (E3330) is an oral formulation administered twice a day and is designed to target multiple pathways relevant to retinal and choroidal vascular diseases, such as diabetic retinopathy (DR) and diabetic macular edema (DME), which, if left untreated, may progress to permanent visual acuity loss and eventual blindness. Mechanistic studies and prior clinical experience suggest that APX3330 is a promising candidate for clinical evaluation of its efficacy and safety in the treatment of these diseases, beginning with DR and DME. Ocuphire believes APX3330 shares desirable attributes for back of the eye therapies, including broad therapeutic applications, a convenient route of administration and cost-effective manufacturing process, without the need for uncomfortable intravitreal injections (FIGURE 7).
In preclinical pharmacology studies in animal models or in vitro, APX3330 has demonstrated the ability to decrease angiogenesis and inflammation in the retina whether delivered orally, systemically, or directly into the eye via intravitreal injections. In humans, APX3330 was shown to be clinically well-tolerated in multiple Phase 1 and 2 trials with fewer than 10% of the patients experiencing mild, self-limiting side effects, such as nausea or diarrhea. In addition, it was shown that significant amounts of oral APX3330 reach the bloodstream concentrations in humans and higher than the levels in mice which showed effects in the retina.
Ocuphire is initially pursuing a moderate-to-severe non-proliferative retinopathy (NPDR) or mild proliferative retinopathy (PDR) indication, as well as patients with DME without loss of central vision. Additionally, Ocuphire may explore opportunities for clinical benefit as adjunctive therapy for other retinal indications such as DME, wAMD, and GA. Second-generation candidate, APX2009, may also be considered for intravitreal or sustained injections.
FIGURE 7: APX3330 Product Candidate Profile
*1 subject had vision blur thought to be related by investigator in ZETA-1 trial
APX3330 Mechanism of Action
APX3330 is a highly selective small molecule that acts on the dual-functioning Apurinic/Apyrimidinic Endonuclease 1/Redox Effector Factor-1 (APE1/Ref-1) protein, referred to as Ref-1. This protein is implicated in both redox signaling and DNA repair. Because APX3330 selectively inhibits the redox function without affecting the molecule’s ability to carry out DNA repair, normal cell function is left intact. Moreover, interference of Ref-1 activity with APX3330 blocks angiogenesis and inflammation by simultaneously decreasing the activity of several important transcription factors such as HIF-1α and NF-κB (FIGURE 8). HIF-1α regulates the expression of VEGF, a protein that is paramount for angiogenesis, and NF-κB is an upstream regulator of proteins involved in inflammatory processes such as TNFα and chemokines.
The development of DR/DME involves leakage from retinal vessels, lack of blood flow to the retina, and release of angiogenic growth factors and inflammatory mediators. The downstream targets of HIF-1α and NF-κB serve as key mediators of these disease features and are targets of current therapy for diabetic eye disease and wAMD. Rather than inhibiting the action of VEGF protein, APX3330 has been shown in preclinical models to inhibit its formation; this is a key potential distinction of APX3330 from the drugs currently approved or under development for DR/DME such as Lucentis and EYLEA. APX3330’s potential ability to inhibit the activity of these two transcription factors may mitigate the need for frequent intravitreal anti-VEGF or steroid injections.
APX3330 has a dual mechanism that decreases both abnormal angiogenesis and inflammation. APX3330 blocks pathways downstream of Ref-1. Blocking HIF-1α reduces VEGF signaling, and blocking NF-kB modulates VEGF, TNF-α and other inflammatory cytokine production. In contrast, anti-VEGF agents solely inhibit the actions of VEGF (FIGURE 8).
FIGURE 8: APX3330 Dual Mechanism of Action in Validated Disease Pathways
Note: Eylea® is registered trademark of Regeneron and Lucentis® is registered trademark of Roche/Genentech
APX3330 Clinical Experience Summary
APX3330 has been studied in over 375 (of over 470 total) healthy volunteers or patients with hepatitis or cancer or diabetic retinopathy patients, over 270 of whom were given the product candidate for an average of 75 days or more. In these 12 Phase 1 and 2 non-ocular and ocular clinical trials, clinical data on safety and efficacy, effect upon the Ref-1 molecular target, and pharmacodynamic characteristics were collected.
A summary of the 12 completed trials including the Phase 2 ZETA-1 trial can be found below (TABLE 3). In subsequent sections, a Phase 2b trial for DR/DME (ZETA-1), a Phase 1 trial for oncology (APX CLN 0011), Phase 1 and 2 trials for chronic hepatitis disease (APX CLN 0001-0010), and nonclinical studies will be highlighted.
TABLE 3: Summary of APX3330 Completed Clinical Trials
Trial Number / Name | | Patient / Indication | | Phase | | Trial Objectives | | Doses | | Number of Patients^ | | APX3330 Total Exposure Days | | Dosing | | Key Endpoints |
APX_CLN_0001 (Eisai) Completed | | Healthy Volunteers | | 1 | | Single-dose placebo-controlled trial of APX3330 to investigate safety and pharmacokinetics | | 10 mg 30 mg 60 mg 120 mg 180 mg 240 mg | | APX3330 = 12 Placebo = 6 | | 36 days | | Single dose | | Plasma Concentration of total quinone forms, safety |
APX_CLN_0002 (Eisai) Completed | | Healthy Volunteers | | 1 | | Repeat-dose placebo-controlled trial to investigate safety and pharmacokinetics | | 120 mg QD 120 mg BID | | APX3330 = 12 Placebo = 6 | | 96 days | | 8 days | | Plasma Concentration of APX3330, safety |
APX_CLN_0003 (Eisai) Completed | | Healthy Volunteers | | 1 | | Repeat-dose trial to determine effects of food on pharmacokinetics | | 240 mg | | APX3330 = 6 | | 84 days | | 1 week | | Plasma Concentration of APX3330, safety |
APX_CLN_0004 (Eisai) Completed | | Healthy Volunteers | | 1 | | Single-dose trial to determine the effects of meals on pharmacokinetics | | 120 mg | | APX3330 = 6 | | 6 days | | Single dose | | Plasma Concentration of APX3330, Safety |
APX_CLN_0005 (Eisai) Completed | | Chronic Hepatitis B Patients | | 2 | | Dose-escalation trial to investigate safety, efficacy and tolerability | | 20 mg 60 mg 120 mg 240 mg | | APX3330 = 40 | | 3360 days | | 12 weeks | | Safety |
APX_CLN_0006 (Eisai) Completed | | Chronic Hepatitis C Patients | | 2 | | Dose-escalation trial to investigate safety, efficacy and tolerability | | 20 mg 60 mg 120 mg 240 mg | | APX3330 = 51 | | 4284 days | | 12 weeks | | Safety |
APX_CLN_0007 (Eisai) Completed | | Chronic Hepatitis C Patients | | 2 | | Double-masked, placebo-controlled trial to investigate safety, efficacy and tolerability | | 120 mg 240 mg | | APX3330 = 128 Placebo = 68 | | 10,752 days | | Placebo = 82 days APX3330 120 mg = 79 days 240 mg = 78 days | | Rate of change in GPT level, improvement in liver function, general performance |
APX_CLN_0008^ (Eisai) Completed | | Healthy Patients | | 1 | | Single-blind, single-dose, 3-step trial to investigate safety and pharmacokinetics of higher doses | | 300 mg 420 mg 600 mg | | APX3330 = 18 | | 54 days | | Single dose | | Plasma Concentration of APX3330, safety |
APX_CLN_0009 (Eisai) Completed | | Advanced Liver Cirrhosis Patients | | 2 | | Repeated-dose trial to investigate safety, efficacy and tolerability | | 120 mg | | APX3330 = 30 | | 420 days | | 2 weeks | | Liver function, patient functional status, tolerability |
APX_CLN_0010 (Eisai) Completed | | Advanced Liver Cirrhosis Patients | | 2 | | Repeated-dose trial to investigate safety, efficacy and tolerability | | 120 mg | | APX3330 = 18 | | 504 days | | 4 weeks | | Liver function, patient functional status, tolerability |
APX_CLN_0011 (Apexian) Completed | | Advanced Solid Tumor Patients | | 1 | | Multicenter, open-label, dose-escalation to investigate safety, efficacy, pharmacokinetics, and recommended Phase 2 dose | | 240 mg 360 mg 480 mg 600 mg 720 mg | | APX3330 = 19 | | 2354 days | | 21-day cycles until disease progression or study withdrawal | | Tumor response, safety, PK, target engagement |
ZETA-1 (Ocuphire) Completed | | Diabetic Retinopathy and DME | | 2 | | Double-masked, randomized, placebo-controlled, multi-center trial | | 600mg | | APX3330 = 51 Placebo =52 | | >7,900 | | 600mg daily for 24 weeks | | ≥ 2 step improvement on the DRSS score at week 24, binocular ≥3 step worsening or improvement and safety |
^ Total patient numbers will not equal to the sum of the subgroups in crossover studies
APX3330 Phase 2b Trial in DR/DME Patients (ZETA-1) (Completed)
In August 2022 Ocuphire completed ZETA-1, a Phase 2b double-masked, randomized, placebo-controlled, multi-center trial in patients with DR and DME, which started in April 2021. 103 DR subjects were enrolled with 90% having NPDR (baseline DRSS of 47 or 53); mean baseline CST was 270 µm. This study evaluated the effect of 600 mg daily dose of APX3330 in treating patients with DR, including moderately severe NPDR to mild PDR, as well as patients with DME without loss of central vision. The primary endpoint was percent of patients with a ≥2 step improvement in Early Treatment of Diabetic Retinopathy Study (ETDRS) diabetic retinopathy severity scale (DRSS) at week 24 in the study eye. Key secondary endpoints at multiple time points included change in monocular and binocular DRSS, CST, and BCVA. Patient safety was assessed by AE monitoring, clinical laboratory evaluations, IOP, and vital sign assessments.
The ZETA-1 trial did not meet the primary endpoint in the study eye, however, the trial achieved statistical significance on a key pre-specified secondary endpoint of preventing clinically meaningful progression of diabetic retinopathy when evaluating both eyes. In contrast to intravitreal injections which treat a single eye, systemic drugs treat both eyes, and thus the response of both eyes needs to be considered. Furthermore, APX3330 inhibits Ref-1 which reduces VEGF and inflammatory cytokines to normal physiological levels supporting the prevention of worsening. In ZETA-1, binocular 3 or more steps worsening on the DRSS scale, calculated as the sum of changes in each eye, after 24 weeks of treatment showed no (0%) APX3330-treated patients had a binocular ≥ 3-step worsening of DRSS from baseline compared with 16% for placebo-treated patients (p=0.04) (Figure 11). This is the planned primary endpoint for Phase 3 registration trials that will be confirmed at the EOP2 meeting with the FDA in the second half of 2023. Additional efficacy endpoints were directionally favorable to support the effect of APX3330 in slowing the progression of DR and preserving vision. Visual acuity was stable with APX3330 and a trend was seen with fewer APX3330 treated patients losing 5 or more letters of distance vision compared to placebo patients (5% vs 19%, p=0.07) (FIGURE 9).
FIGURE 9: Percentage of Subjects with Binocular ≥3-Step Worsening in DRSS and ≥5 Letters of BCVA
APX3330 showed a favorable safety profile. 14 treatment-emergent serious AEs were considered unrelated to study medication, 11 in the placebo group and 3 in the APX3330 group. Two subjects in each group withdrew due to an AE, where one placebo subject had OU DME worsening considered treatment related. Overall, there were 211 AEs (91 APX3330, 120 placebo) in 64 subjects (29 APX3330, 35 placebo). Only 31 of these AEs were considered drug-related (14 APX3330, 17 placebo). All treatment related AEs were mild or moderate in severity. There were no adverse treatment effects on any other characteristics of the ophthalmic examination or on any assessments of systemic safety (FIGURE 10).
FIGURE 10: ZETA-1 Safety Overview
APX3330 Phase 1 Oncology Trial - Apexian (APX CLN 0011)
Clinical development of APX3330 by Eisai Co., Ltd. in Japan was suspended with the in-licensing of anti-viral and biological agents for hepatitis C and rheumatoid arthritis. Later, while doing research on the Ref-1 protein, Dr. Mark Kelley from Indiana University and others identified that the molecular target of APX3330 was the Ref-1 protein. The elucidation of the mechanism of action with which APX3330 modulated the Ref-1 protein, and the concurrent advancement in understanding the role played by Ref-1 as a critical “gate-keeper” for controlling a variety of pro-inflammatory transcription factors, led to the establishment of Apexian in order to determine the utility of using APX3330 as a modulator of the Ref-1 protein in the treatment of inflammatory diseases. The clinical trial, APX_CLN_0011 under IND 125360 with the FDA Division of Oncology, was initiated by Apexian in order to identify the highest dose of APX3330 that could be safely administered in a chronic manner and to confirm molecular engagement of APX3330 with the Ref-1 protein by obtaining tumor biopsy samples and circulating tumor cell samples.
APX_CLN_0011 was a multi-center, open-label, dose-escalation Phase 1 oncology trial in patients with advanced solid tumors. Patients received daily oral doses of APX3330 each day of repeated 21-day cycles until disease progression or trial withdrawal. Nineteen patients received APX3330 in escalating doses from 240 mg/d dose to 720 mg/day in increments of 120mg/day. The highest dose tested (720 mg/day) produced a self-limiting, diffuse macular rash and was confirmed as the dose-limiting toxicity. The dose of 600 mg/day was then confirmed as a dose tolerable for chronic administration and for further clinical development as a modulator of Ref-1 activity in inflammatory diseases. Biopsy analyses of patients participating in the trial confirmed that APX3330 directly targets the Ref-1 protein and that the targeting produces subsequent regulation of transcription factors such as NF-κB and HIF-1α, regulators of VEGF and other inflammatory molecules. This mechanism of action provides significant rationale for testing APX3330 in diseases in which inflammation and neo-vascular development play a critical pathogenic role. APX3330 was also well-tolerated in cancer patients who were treated daily for up to 400 days (4% diarrhea/soft stool and 4% rash).
Overall, APX3330 demonstrated a favorable safety profile and was well tolerated. No effects of APX3330 on vital signs on laboratory measures have been observed. Across 11 Phase 1 and Phase 2 trials, the rate of adverse events was similar in APX3330 treated patients (N=346) and placebo treated patients, including a <3% difference between APX3330 and placebo in rates of diarrhea/soft stool and rash/pruritis. Among healthy patients and hepatitis patients (N=327), any adverse event occurred in 14% of patients receiving APX3330 and 14% of patients receiving placebo.
APX3330 – Ten Phase 1 and 2 Trials - Eisai (APX CLN 0001-0010)
Under the sponsorship of Eisai Co., Ltd., 10 clinical trials were conducted involving healthy volunteers as well as patients with chronic hepatitis diseases (i.e., Type C, B, alcohol-induced) in Japan with the intent of developing a TNF-α blocking agent. At the time of their clinical trials, the molecular target of APX3330 had not been confirmed and was not known to be the Ref-1 protein.
Across these 10 trials, it was found that APX3330 exhibits predictable pharmacokinetics that were consistent with the pharmacokinetic data obtained in non-clinical studies. In addition, there was a lack of significant acute toxicity at doses up to 600 mg/day. APX3330 has been demonstrated to be well-tolerated. Moreover, in two studies it was found that meals have no impact on the product candidate’s pharmacokinetics. Safety tolerability measures showed no changes in vital signs and no changes in clinical laboratory values. Only adverse events included diarrhea and rash, which each occurred in <5% of patients and were mild. Liver function tests were used primarily to evaluate efficacy in Phase 2 studies of hepatitis patients and the overall assessment of liver function over time suggests that APX3330 had a minor positive, and no negative, effect. Additionally, there was a lack of acute neurologic, cardiovascular, hepatic, or pulmonary toxicity. Only a single ocular adverse event has been reported in APX3330 clinical trials; mild orbital region discomfort at 60 mg/day in CLN_0006. No mild, no moderate, and no severe rashes were observed in healthy patients dosed with APX3330.
APX3330 Nonclinical Studies
Preclinical Studies with APX3330 and Next Generation APX Small Molecules
In animal studies, APX3330 delivered orally, intraperitoneally or intravitreally (directly into the eye), and APX2009 and APX2014 delivered by intraperitoneal injection reduced neovascularization in mouse models that recapitulate features of retinal neovascularization (seen in PDR and wAMD) called the L-CNV model. Although in humans intravitreal injection is the habitual delivery route of the standard-of-care anti-VEGF biologics, to ensure the delivery of the drug to the affected area, such administration is labor-intensive, causes patient discomfort, and incurs a risk of potentially vision-threatening intraocular infections. As a result, systemic administration (intraperitoneal injections) of Ref-1 inhibitors were explored for similar effects as those seen with anti-VEGF biologics in mouse models. Treatment with APX3330 (10 mg/kg) via oral gavage in rats with type 1 diabetes and induced stroke (conditions that promote neovascularization) shows a significant decrease (~55%) of VEGF signaling (or lesion volume) (FIGURE 11). As seen in the first panel in FIGURE 11, expression of VEGF was lower in APX3330-treated cells compared to control cells in a stroke model. As seen in the second panel, APX3330 also demonstrated anti-inflammatory effects by reducing cytokines in lipopolysaccharide (LPS)- stimulated macrophages. The third panel demonstrates APX3330 increased DNA oxidative repair and neuronal protection by enhancing endonuclease activity.
FIGURE 11: APX3330 Reduces VEGF Levels and Inflammatory Cytokines and Provides Neuronal Protection (in-vitro)
While numerous published studies using APX3330 through intravitreal or systemic intraperitoneal administration have shown successful neovascularization reduction in vitro or animal models, additional studies with oral administration of 2 doses of APX3330 (25 mg/kg and 50 mg/kg per day) resulted in a more robust correction of the lesion volume in the L-CNV mouse model. Animals treated with APX3330 displayed a significant reduction (~55%) in the volume of the neovascular lesion (red staining) (FIGURE 12).
FIGURE 12: Lesion Size and Corresponding Fluorescent Stains in L-CNV Models Treated with APX3330
L-CNV mice treated with APX3330 at either 25 mg/kg or 50 mg/kg resulted in a decreased volume of neovascularization (lesion volume).
Pharmacokinetics/Metabolism
Pharmacokinetic (PK) studies were conducted in rats and dogs to understand the absorption, distribution, and elimination of APX3330. APX3330 is well absorbed orally with a bioavailability of ≥ 60%. In a protein binding study in rats, it was shown that ≥ 90% of the product candidate is bound to protein. Half-life after intravenous administration of APX3330 was 8 hours in rats, 7.8 to 8.7 hours in dogs, and 25.5 hours in monkeys. Excretion occurred mainly in bile, as a monoglucuronide or other conjugates. In rats and beagles, a part of the administered APX3330 is excreted in stool as the unchanged compound.
Toxicology
APX3330 has been studied extensively in over 20 in vitro and animal studies and demonstrated a favorable safety profile in each. APX3330 was not genotoxic and had no toxicologically significant effects in developmental studies performed to date. Over 15 single- and repeat- dose toxicology studies in rats and dogs up to 3 months duration have been conducted. Also, PK, ADME and safety pharmacology studies along with over 5 developmental, genotoxicity, and antigenicity studies have been completed. The FDA did not request any further toxicology studies to support the initiation of the 24-week ZETA-1 clinical trial.
Ocuphire Clinical Development Plan
Ocuphire is developing Nyxol in partnership with Viatris. For Nyxol, the investigational new drug (IND) application was submitted to the FDA Division of Ophthalmology in July 2011 and is in effect (IND 70499). In November 2022, Ocuphire submitted an NDA for Nyxol for RM and in February 2023 received notification of FDA acceptance to file and a PDUFA goal date of September 28, 2023. Nyxol has completed 12 trials (3 Phase 1 trials, 5 Phase 2 trials and 4 Phase 3 trials), mostly in young and older healthy volunteers and in presbyopia, DLD and glaucoma patients. In May 2020, Ocuphire completed an EOP2 meeting with the FDA, which included a discussion and agreement around the design and scope of future registration trials for Nyxol across its indications. In June 2021, Ocuphire completed a Type C meeting with the FDA on CMC for Nyxol. In February 2022, Ocuphire completed a Type C meeting with the FDA which included discussion and guidance around the design of VEGA Phase 3 trials and registration package for Presbyopia for Nyxol alone and Nyxol with LDP as adjunctive therapy. In June 2022, Ocuphire completed a Type B (Pre-NDA) meeting with the FDA to obtain FDA scientific and regulatory input to guide the NDA submission for RM. Ocuphire plans a Type C meeting in the first half of 2023 to obtain further guidance on the presbyopia and DLD registration programs. Ocuphire anticipates engaging in similar discussions with other foreign regulatory authorities in the future.
For APX3330, the IND application for APX3330 to pursue retinal choroidal vascular diseases was submitted to the FDA Division of Ophthalmology in December 2018 and is in effect (IND 142152). APX3330 also has an IND with the FDA Division of Oncology for the treatment of pancreatic cancer (IND 125360). APX3330 has completed 12 trials (6 Phase 1 and 6 Phase 2 trials), mostly related to patients with liver disease, patients with solid tumors and diabetic retinopathy. Ocuphire plans an EOP2 meeting in the second half of 2023 to obtain guidance on the registration program including confirmation of the primary endpoint for registration of a systemic agent for DR.
Current and Planned Nyxol Trials:
Nyxol Presbyopia: Phase 3 Trials
The VEGA-2 (NYXP-301) a double-masked, randomized, placebo-controlled, multicenter trial in approximately 320 patients with presbyopia was initiated in December 2022. This registration trial is to evaluate efficacy and safety to achieve both labels - single agent Nyxol and LDP as adjunctive therapy to Nyxol. Key endpoints such as distance corrected near visual acuity, pupil diameter, and best corrected distance acuity will be measured at multiple timepoints after the first dose and multiple doses to assess the onset and duration of efficacy. The primary endpoint to establish the efficacy will be the percentage of patients who gain 15 or more letters of DCNVA without loss of 5 or more letters of BCDVA. Key secondary endpoints such as distance corrected near visual acuity, pupil diameter, and best corrected distance acuity will be measured at multiple timepoints after the first dose and multiple doses to assess the onset and duration of efficacy. A hierarchical analysis will be used to allow both single agent (compared to placebo) and combination (compared to individual components) endpoints to be evaluated with appropriate statistical significance.
The VEGA-3 (NYXP-302) trial is planned as a double-masked, randomized, placebo-controlled, multicenter trial in approximately 400 patients with presbyopia. This second registration trial will evaluate efficacy and safety and be similarly designed to VEGA-2 and include similar primary and key secondary endpoints and analysis. If the VEGA Phase 3 program is successful, Ocuphire expects to file a sNDA for Nyxol to treat presbyopia in 2024 and to follow later with a NDA for Nyxol + LDP.
Nyxol DLD: LYNX-2 2nd Phase 3 Trial
Based on the positive results of the first Phase 3 trial, LYNX-1, Ocuphire and its partner, Viatris, are planning a similar second registration trial for Nyxol in up to 200 subjects for the treatment of DLD. If the LYNX Phase 3 program is successful, Ocuphire expects to file a sNDA for Nyxol to treat DLD in 2024.
Nyxol Chronic Safety Trial for Nyxol as a single agent and with adjunctive low dose pilocarpine (“LDP”): LYRA-1 Phase 3 Trial
For an NDA for Nyxol chronic indications (Presbyopia and DLD), Ocuphire and its partner, Viatris, plan to conduct a chronic safety trial LYRA-1 with approximately 425 subjects, which is expected to initiate after the FDA review of the 6-month repeated-dose toxicity study. The planned LYRA-1 Phase 3 trial evaluating chronic safety exposure is targeting 300 patients for 6 months, followed by 100 of the patients continuing for an additional 6 months in a double-masked, placebo-controlled design. Long-term endothelial cell count (ECC) clinical data will also be collected.
Potential Clinical Plans for APX3330:
Based on the Phase 2 safety, tolerability and efficacy results of APX3330 in patients with DR/DME reported in January 2023, Ocuphire expects to request an EOP2 meeting with the FDA in the second half of 2023 to confirm registration endpoints to finalize the design of the Phase 3 registration trials for APX3330 as first-line therapy in DR/DME in addition to defining the chronic safety exposure trial and any further animal toxicology studies necessary prior to an NDA submission. Ocuphire may explore opportunities for clinical benefit as adjunctive therapy for other retinal indications such as DME, wAMD, and GA.
Future In-Licensing and Acquisition Opportunities
Ocuphire continually evaluates product candidates based on scientific merit, patent protection, regulatory pathways, and commercial opportunity. Its focus is on product candidates in the ophthalmology space.
Sales and Marketing
Ocuphire has entered into a licensing agreement with Viatris for the development and commercialization of all Nyxol indications in the U.S. and ex-U.S. markets (excluding certain countries in Asia). The company maintains discussions with a range of ophthalmic drug companies regarding development and commercialization of APX3330, including co-development, distribution, license, or mergers and acquisitions. As part of the pre-commercialization planning, Ocuphire has started market development activities which include engaging with Key Opinion Leaders as well as increased visibility and presence at retina, ophthalmology and optometry medical and industry conferences. There are several global pharmaceuticals with major ophthalmic drug businesses as well as numerous other smaller global or regional companies that could provide significant reach in specific markets such as Europe or Asia.
Manufacturing
For Nyxol, APX3330, and for other product candidates that will be developed in the future, Ocuphire’s contract manufacturers are currently producing, and will produce, its bulk drug substances and drug products for use in Ocuphire’s preclinical studies and clinical trials, utilizing reliable and reproducible synthetic processes and common manufacturing techniques. Pursuant to the Nyxol License Agreement, Ocuphire intends to transfer commercial manufacturing responsibilities for Nyxol to Viatris. Ocuphire does not have any long-term agreements but Ocuphire or its development partners intend to secure such arrangements for drug substances or drug products as appropriate, and currently uses purchase orders with multiple manufacturers. Ocuphire is qualifying its selected manufacturers to provide bulk drug substances and drug products in conjunction with the NDA regulatory submission to the FDA. Ocuphire plans to continue to rely upon contract manufacturers and, potentially, collaboration partners to manufacture commercial quantities of its drug substances and drug products, if approved for marketing by the applicable regulatory authorities. Ocuphire does not own or operate, and currently has no plans to establish, any manufacturing facilities.
Nyxol
The proprietary formulation of Nyxol is a sterile, preservative-free, isotonic, buffered aqueous solution containing phentolamine mesylate, mannitol, and sodium acetate. The drug substance phentolamine mesylate USP is a small molecule that can be manufactured by reliable and reproducible synthetic processes from readily available starting materials. Ocuphire currently obtains the active pharmaceutical ingredient for Nyxol from a single supplier in Italy and is presently taking steps to develop a second source, but is working with Viatris to determine the long-term commercial manufacturing strategy. All lots of drug substance phentolamine mesylate and Nyxol drug product used in clinical trials are manufactured under current good manufacturing practices (cGMP), a quality-system regulating manufacturing, with processes registered by the supplier with FDA in an active Drug Master File (DMF). Nyxol was previously packaged in a single-use bottle with cap served as the container closure system for Phase 1 and 2 clinical trials. Ocuphire transitioned the container closure system to an industry standard, single-use preservative-free blow-fill-seal (“BFS”) container for Nyxol, which is being formulated and filled by a leading U.S. manufacturer. Nyxol eye drops in the BFS container are classified by FDA as a drug-device combination product. The current manufacturing process has been scaled to a commercial capacity. Nyxol has demonstrated stability at 5°C refrigerated for a minimum of two years. Ocuphire is performing additional stability studies on lots of both the drug substance phentolamine mesylate and the drug product of Nyxol in order to establish expiry dating and to support regulatory submissions and commercial manufacturing. To supply eventual global markets and to avoid reliance on a single facility, Ocuphire and its partner Viatris are evaluating the establishment of second-source manufacturing facilities for drug substance and drug product.
Pilocarpine
Pilocarpine Hydrochloride Ophthalmic Solution 0.4% or Low Dose Pilocarpine (LDP) is a proprietary formulation of pilocarpine hydrochloride. It is a preserved, aqueous sterile solution comprised of the active ingredient pilocarpine hydrochloride, benzalkonium chloride, boric acid, Hypromellose 2910, sodium citrate, and sodium chloride, in sterile water for injection. Similarly to Nyxol, the drug substance for LDP, pilocarpine hydrochloride USP is a small molecule that can be manufactured by reliable and reproducible processes as a plant extract. Ocuphire obtains the active pharmaceutical ingredient for LDP from a single supplier in Brazil, and intends to secure a long-term supply contract.
APX3330
APX3330 is an oral formulation of a small molecule drug substance that is synthesized from readily available raw materials and using conventional chemical processes. The APX3330 drug substance is currently obtained from a single supplier in India, although alternative manufacturing sources are available. The APX3330 drug product is manufactured in the U.S.. Process and analytical development of APX3330 drug product have been completed, and its production has been scaled-up under cGMP regulatory requirements. Previously, the APX3330 drug product manufacturer has performed pharmaceutical development to support the cGMP manufacturing campaign for tablets of 60 mg and 120 mg dose strengths, the latter being used in ongoing clinical trials. Under this tablet size, long-term ICH-stability studies of various strengths (60 and 120 mg tablet) have been conducted and have demonstrated a 3-year shelf life when stored at 25°C/60% relative humidity. Ocuphire is evaluating 300 mg tablets for convenient twice a day dosing. Ocuphire is also planning additional stability studies for future lots of both the drug substance and drug product of APX3330 in order to establish expiry and to support regulatory approval and commercial stage.
Apexian Sublicense Agreement
On January 21, 2020, Ocuphire entered into a sublicense agreement with Apexian pursuant to which it in-licensed patents and other intellectual property relating to the APX3330 product candidate and second-generation product candidates owned by Apexian, and intellectual property that Apexian in-licensed from Eisai, including certain study reports, manufacturing and analytical records, data, know-how, technical and other proprietary information relating to APX3330. This intellectual property constitutes a Ref-1 Inhibitor program focused on developing therapeutic applications to treat disorders related to ophthalmic and diabetes mellitus conditions. The lead compound in the Ref-1 Inhibitor program is APX3330, which Ocuphire intends to develop as an oral tablet therapeutic to treat DR and DME, and potentially wAMD. See “Ocuphire Management’s Discussion and Analysis of Financial Condition and Results of Operations—Contractual Obligations and Commitments—Apexian Sublicense Agreement” for more details regarding the Apexian Sublicense Agreement.
Intellectual Property
Nyxol
Ocuphire’s patent estate includes patents and patent applications to forms of phentolamine mesylate, formulations containing phentolamine mesylate, methods of using phentolamine mesylate, and methods of manufacturing phentolamine mesylate. Ocuphire primarily protects its intellectual property through a combination of patents and patent applications on inventions, trademark protection on Ocuphire’s product name, and trade secret protection as Ocuphire deems appropriate. Ocuphire owns all of the worldwide rights to Nyxol for all indications, but has out-licensed certain rights to Nyxol pursuant to the Nyxol License Agreement.
As of February 10, 2023, Ocuphire’s patent estate relating to Nyxol contains ten U.S. patents, eight pending U.S. non-provisional patent applications, as well as issued patents in Australia, Canada, Europe, Japan, and Mexico and pending patent applications in Australia, Canada, Europe, Japan, China, and other foreign countries.
Ocuphire’s U.S. Patents 9,795,560; 10,278,918; 10,772,829 and 11,090,261 and counterpart Australian Canadian, European, and Japanese patents each contain composition of matter claims to aqueous phentolamine mesylate formulations and are scheduled to expire in year 2034. In the same patent family, Ocuphire also has 1 pending U.S. patent application with additional claims to aqueous phentolamine mesylate formulations, whereby a patent, if granted, would expire in year 2034. The patents and patent applications cover the current clinical formulation for the Nyxol product.
Ocuphire’s U.S. Patent Nos. 9,089,560; 9,789,088 and 11,000,509 contain claims directed to methods of improving visual performance using, for example, phentolamine mesylate and are scheduled to expire in year 2034. Counterpart patents have issued in Australia, Canada, Europe, and Japan, which are scheduled to expire in year 2034. In the same patent family, Ocuphire also has 1 pending U.S. patent application with additional claims to methods of improving visual performance using, for example, phentolamine mesylate, whereby a patent, if granted, based on this pending application would expire in year 2034. The patents and patent applications cover uses of the current clinical formulation for the Nyxol product.
Ocuphire has patent applications pending in the U.S., Australia, Canada, China, Europe, and Japan directed to treating glaucoma and other medical disorders using phentolamine mesylate. Patents, if granted, based on these pending applications would expire in year 2039.
Ocuphire’s U.S. Patent 10,993,932 contains claims directed to methods of treating presbyopia using phentolamine mesylate with adjunctive pilocarpine and is scheduled to expire in year 2039. Ocuphire’s U.S. Patent 11,400,077 contains claims directed to methods of treating mydriasis using phentolamine mesylate and is scheduled to expire in year 2039. In the same patent family as U.S. Patent Nos. 10,993,932 and 11,400,077, Ocuphire has four pending U.S. patent applications, two of which have claims to treating presbyopia and the other two U.S. applications have claims to treating mydriasis. Counterpart patent applications are pending in Australia, Canada, China, Europe, Japan, and other foreign countries, whereby a patent, if granted, based on these pending U.S. and foreign patent applications would expire in year 2039.
Ocuphire’s U.S. Patent 11,566,005 contains claims directed to phentolamine mesylate composition of matter (the active pharmaceutical component in Nyxol), process to make phentolamine mesylate from phentolamine and methods for inhibiting pupil dilation, improving visual contrast sensitivity or visual acuity, and for treating dim light or night vision disturbance, reversal of mydriasis and presbyopia, with the above-claimed phentolamine mesylate. In the same patent family, there is a pending U.S. patent application, and a pending international patent application directed to methods of making high-purity phentolamine mesylate and compositions resulting from such methods. This U.S. patent and other patents, if granted, based on the foregoing patent applications are scheduled to expire in year 2042. Ocuphire also has one pending patent application in China directed to methods of making high-purity phentolamine mesylate and compositions resulting from such methods, whereby any patents, if granted, based on this patent application in China would expire in year 2041. Ocuphire also has a pending international patent application directed to additional methods for treating mydriasis and glaucoma, whereby any U.S. or foreign patents, if granted, from on a patent application filed based on this international patent application would expire in year 2042.
Ocuphire also owns an issued patent in Mexico that is scheduled to expire in year 2025 and has claims to ophthalmic formulations.
Ocuphire has registered trademark protection in the United States for the NYXOL® .
APX3330
As of February 10, 2023, the patent estate that Ocuphire has in-licensed for APX3330 and related compounds contains seven U.S. patents and two pending U.S. non-provisional patent applications, as well as issued patents in Europe, Japan, Canada, and Australia, and pending patent applications in Europe, Japan, Canada, China, South Korea and Australia. The license is for the use and commercialization of APX3330 and related composition of matter compounds covered by the subject patents and patent applications in the field of human health uses for ophthalmic and diabetes mellitus indications.
In-licensed U.S. patent 9,040,505 has claims to methods of treating diabetic retinopathy and other diseases using, for example, APX3330 and is scheduled to expire in year 2030. Counterpart patents have issued in Europe, Japan, Australia, and Canada, which are scheduled to expire in year 2028, and there is a related pending U.S. patent application with method of treatment claims that, if issued as a patent, would expire in year 2028. Pending U.S. application 16,968,009 and pending applications in Europe, Japan, Canada, China, South Korea and Australia have claims to methods of treating wAMD and other diseases using, for example, APX3330, along with other formulations such as APX2009 and APX2014. These patents, if granted, would expire in year 2039. The U.S. and certain foreign countries permit extension of patent term for up to five years to compensate for patent term lost during the government regulatory review process for a new medicine. If U.S. patent 9,040,505 qualifies for the full five years of patent term extension, the expiration of U.S. patent 9,040,505 would be in year 2035. Whether U.S. patent 9,040,505 qualifies for the full five years of patent term extension depends in part on the date of FDA approval for the new medicine.
In-licensed patent applications directed to a combination therapy composition comprising an APE1/REF-1 inhibitor, such as APX3330, and a second therapeutic agent, and methods of using such combination therapy to treat retinal diseases and/or treat other indications are pending in the U.S. and Canada. Patents, if granted, would expire in year 2038. In-licensed patent applications directed to use of an APE1/REF-1 inhibitor, such as APX3330, in monotherapy or combination therapy to reduce neuronal sensitivity and/or treat other indications are pending in Europe, Japan, and Canada, whereby patents, if granted based on these applications, would expire in year 2038. This same patent family includes one in-licensed U.S. patent directed to methods using APX3330 to treat inflammation and pain as part of a combination therapy.
U.S. patents that Ocuphire has in-licensed to derivatives of APX3330 include U.S. patents 9,089,605; 9,193,700; 9,877,936; 10,154,973; and 11,160,770. These U.S. patents are schedule to expire from 2029 to 2032. Foreign patents that Ocuphire has in-licensed to derivatives of APX3330 include patents in Europe, Japan, China, and Canada that are scheduled to expire between the years 2028 to 2032.
In addition to patents and patent applications that Ocuphire has in-licensed, as of February 10, 2023, Ocuphire owns one pending international patent application directed to methods of treating diabetic retinal diseases using APX3330. Patents, if granted, from an application filed based on this pending international patent application would expire in year 2042. Additionally, Ocuphire owns one pending U.S. provisional patent application directed to certain salt forms of APX3330 and methods of use, whereby any patents, if granted, from on an application filed based on this provisional patent application would expire in year 2043, and Ocuphire owns one pending U.S. provisional patent application directed to additional therapeutic methods using APX3330 in patients with diabetic retinal disease, whereby any patents, if granted, from on an application filed based on this provisional patent application would expire in year 2044.
Additional Background
As background, the patent term is typically 20 years from the date of filing a non-provisional application. In the United States, a patent’s term may be lengthened several ways. First, patent term adjustment (PTA) compensates a patentee for administrative delays by the USPTO in granting a patent. Second, in certain instances, a patent term extension (PTE) can be granted to recapture a portion of the term effectively lost as a result of the FDA regulatory review period, as provided under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. This restoration period cannot be longer than 5 years for approval of a drug compound, and the total patent term, including the restoration period, must not exceed 14 years following FDA approval. Only patent(s) applicable to an approved drug is eligible for the PTE and the application for the extension must be submitted prior to the expiration of the patent and within 60 days from market approval. Independent of patent protection, in the United States, the Hatch-Waxman Act provides a 5-year period of non-patent data exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity (NCE). Under this provision, APX3330 may be eligible for up to 5 years of data and market exclusivity under the Hatch-Waxman Act, because it is considered an NCE as the FDA has not previously approved any other drug containing the active ingredient of APX3330.
In Europe, under the Data Exclusivity Directive, pharmaceutical companies may receive up to 11 years to market their product without risk of competition. In Japan, under the Pharmaceuticals Act of Japan, the market authorization holder, based on the length of a required study period reexamination, may have up to 10 years before a generic can enter the market. Further, the expiration date of certain patents may be extended for up to a maximum of 5 additional years to accommodate for time spent seeking government approval to market a new medicine, in those countries that permit extension of patent term to accommodate for time spent seeking government approval to market a new medicine.
Ocuphire also protects its proprietary information through written agreements. Ocuphire’s employees, consultants, contractors, partners and other advisors are required to execute nondisclosure and assignment of invention agreements upon commencement of employment or engagement. In addition, Ocuphire protects its proprietary information through written confidentiality agreements with outside parties who may come into possession of Ocuphire’s confidential information.
Competition
There is intense competition within the pharmaceutical industry. While Ocuphire believes that its product candidates, APX3330 and Nyxol, are well positioned for development in each indication, Ocuphire or its development partners will face competition from both branded and generic pharmaceutical companies as well as products that are currently in development. Many of these companies have significantly greater financial and human resources and experience in drug development, R&D, and commercialization. These competitors compete with Ocuphire in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient enrollment for clinical trials as well as acquiring products, product candidates or other technologies complementary to Ocuphire program. Smaller and other early-stage companies may also prove to be significant competitors if they choose to partner with large, established companies.
APX3330
The key competitive factors affecting the success of APX3330, assuming APX3330 is approved, are likely to be its oral dosage form, tolerability, durability, price, and the availability of coverage and reimbursement from government and other third-party payors.
Competition in Diabetic Retinopathy / Diabetic Macular Edema / wAMD
Ocuphire believes that APX3330, if approved, could have a competitive advantage in the DR/DME/wAMD markets because it is an oral tablet with a dual mechanism and potential to address multiple indications. However, Ocuphire may face potential competition from both existing therapies and those in development. Current therapies for these retinal diseases rely on suppressing the activity of vascular endothelial growth factors (VEGF) via intravitreal injection or by mitigating the inflammation via intravitreal corticosteroid-releasing implants including:
| • | Lucentis® (ranibizumab) and Avastin® (bevacizumab), which are anti-VEGF monoclonal antibody intravitreal injections, developed by Genentech, Inc and Roche AG. |
| • | EYLEA® (aflibercept), a VEGF inhibitor intravitreal injection, developed by Regeneron Pharmaceuticals. |
| • | Vabysmo® (Faricimab), a bispecific monoclonal antibody targeting VEGF-A and Ang-Tie2 pathway developed by Genentech, Inc and Roche AG. |
| • | Beovu® (Brolucizumab), an anti-VEGF monoclonal antibody intravitreal injection, developed by Novartis AG. |
| • | MACUGEN® (pegaptanib sodium injection), a selective inhibitor of VEGF-165, developed by Bausch + Lomb. |
| • | Ozurdex® (dexamethasone), a corticosteroid IVT implant, developed by Allergan plc. |
| • | Iluvien (fluocinolone acetonide), a corticosteroid IVT implant, developed by Alimera Sciences, Inc. |
There are also several pharmacological therapies in development, including:
| • | Abicipar, an anti-VEGF intravitreal injection with a long duration of action, developed by Allergan plc and Molecular Partners. |
| • | KSI-301, an anti-VEGF antibody intravitreal injection coupled with a biopolymer that is intended to increase the time between injections, developed by Kodiak Sciences. |
| • | OPT-302, an intravitreal injection which binds to multiple types of VEGF receptors that could be used with other anti-VEGF agents, developed by Opthea Limited. |
| • | ALG-1001, an integrin peptide therapy intravitreal injection that is being evaluated as a sequential or in-combination therapy with bevacizumab in patients with DME, developed by Allegro Ophthalmics, LLC. |
| • | RG-7774, an orally administered selective CB2 (Cannabinoid 2) receptor agonist that is being evaluated in patients with moderately severe to severe non-proliferative diabetic retinopathy, developed by Hoffmann-LA Roche, AG. |
| • | RZ402, an oral small molecule selective and potent plasma kallikrein inhibitor (PKI) for the chronic treatment of diabetic macular edema (DME), developed by Rezolute, Inc. |
| • | Xiflam™, an oral small molecule drug for the treatment of dry form of Age-Related Macular Degeneration (AMD), Geographic Atrophy (GA), Diabetic Retinopathy (DR) manifesting Diabetic Macular Edema (DME), developed by InflammX. |
| • | AKST4290, an oral small molecule CCR3 Eotaxin inhibitor for the treatment of diabetic retinopathy (terminated in 2022) and wet AMD by Alkahest. |
| • | BAY1101042, an oral guanylate cycles activator for the treatment of diabetic retinopathy developed by Bayer. |
| • | OPL-0401, an oral ROCK 1/2 inhibitor for the treatment of diabetic retinopathy, developed by Valo Health. |
| • | BI 1467335, an oral AOC3 inhibitor for the treatment of diabetic retinopathy, developed by Boehringer Ingelheim. |
| • | LY333531, an oral Protein Kinase C inhibitor for the treatment of diabetic retinopathy was terminated by Eli Lilly in 2006. |
Nyxol will be commercialized by Viatris pursuant to the Nyxol License Agreement, and Viatris may face competition as well.
The key competitive factors affecting the success of Nyxol, assuming Nyxol is approved, are likely to be the combination of efficacy, durability, tolerability, convenience, price (private pay), and stable, preservative-free formulation that will potentially allow it to compete effectively in these markets.
Competition in RM
There are currently no approved and commercially available drug treatments for RM, and Ocuphire is not aware of any in development. Rev-Eyes® (dapiprazole), an alpha-1 antagonist, was approved by the FDA in 1990 to reverse mydriasis induced by adrenergic or anticholinergic agents. Rev-Eyes was withdrawn in the past from the market for reasons unrelated to safety or efficacy, according to the FDA.
Competition in Presbyopia
The FDA approved VuityTM eye drop for the treatment of presbyopia in October 2021. Vuity was launched in December 2021 and is marketed by Allergan, an AbbVie company. The competition also includes reading glasses, multifocal contact lenses, and monovision contact lenses (e.g., where one eye wears a near vision lens and the other eye wears a distance vision lens). Nyxol will also compete against several pharmacological therapies in development for the temporary treatment of presbyopia, many of which are cholinergic agonist-based pupil management therapies, including:
| • | CSF-1, with low dose pilocarpine and a secondary agent (lubricant), developed by Orasis Pharmaceuticals Ltd. (PDUFA date is October 22, 2023) |
| • | LNZ100 and LNZ101, with aceclidine (another miotic agent) and brimonidine, developed by Lenz Therapeutics. |
| • | MicroLine®, which is a micro-dose delivery of pilocarpine using proprietary device developed by Eyenovia, Inc. |
| • | KT-101, which uses pilocarpine in the AcuStream delivery system, developed by Kedalion Therapeutics, Inc. |
| • | BrimocholTM, with brimonidine and carbachol (both are miotic agents), developed by Visus Therapeutics, Inc. |
| • | UNR844, which uses a mechanism that involves softening the lens to increase near visual acuity, developed by Novartis AG (originally Encore Vision, Inc.). This development program has been terminated. |
There are a few approved devices for presbyopia. One of these is the KAMRA Inlay, developed by AcuFocus, Inc. and marketed by SightLife Surgical, Inc. Another is the eyelike NoanPinhole, developed by Koryo Eyetech, the first commercially available pinhole soft contact lens. Nyxol would not directly compete against these devices, but rather would be a non-invasive alternative for presbyopes who are averse to surgical intervention.
Competition in DLD
DLD is a new indication in which Nyxol could be the first approved drug. There are currently no FDA-approved therapies for DLD nor is Ocuphire aware of any in development. Existing miotic agents are rarely used off-label given their limitations of tachyphylaxis (brimonidine, a generic drug, marketed by various pharmaceutical companies) and warning in the label of difficulties while driving at night or performing hazardous activities in poor illuminations (attributable to pilocarpine, a generic molecule marketed by various pharmaceutical companies at common doses of 1%, 2%, and 3%).
Government Regulation and Product Approvals
Government authorities in the United States, at the federal, state and local level, and in other countries and jurisdictions, including the European Union (EU), extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
The EMA is a decentralized agency governed by an independent management board responsible for the evaluation, supervision, and safety monitoring of medicines in the EU. The Medicines and Healthcare products Regulatory Agency (MHRA) regulates medicines, medical devices, and blood components in the United Kingdom (UK) and serves as a similar function to the EMA in the EU, following the exit of the UK from the EU in Brexit. The Japanese Pharmaceuticals and Medical Devices Agency serves a similar function to the FDA in the United States and is an independent administrative institution. The National Medical Products Administration (NMPA) is the Chinese agency for regulating drugs and medical devices (formerly the China Food and Drug Administration or CFDA).
Review and Approval of Drugs in the United States
In the United States, the FDA regulates drug products under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. The failure to comply with applicable requirements under the FDCA and other applicable laws at any time during the product development process, approval process or after approval may subject an applicant and/or sponsor to a variety of administrative or judicial sanctions, including refusal by the FDA to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by the FDA and the Department of Justice or other governmental entities.
An applicant seeking approval to market and distribute a new drug product in the United States must typically undertake the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies in compliance, as applicable, with the Animal Welfare Act and FDA’s good laboratory practice, or GLP, regulations; |
| • | submission to the FDA of an IND, which must take effect before human clinical trials may begin; |
| • | approval by an independent institutional review board, or IRB, representing each clinical site before each clinical trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, and other applicable regulations to establish the safety and efficacy of the proposed drug product for each proposed indication; |
| • | manufacturing, packaging, labelling, and distribution of drug substances and drug products consistent with the FDA’s Good Manufacturing Practice (GMP) regulations which are utilized in the GLP non-clinical and GCP clinical studies to investigate the drug candidate; |
| • | development of product label, package inserts, and prescriber information that is intended to be used and included with the commercial product; |
| • | preparation and submission to the FDA of an NDA; |
| • | review of the product by an FDA advisory committee, where appropriate or if applicable; |
| • | satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with current Good Manufacturing Practices, or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
| • | satisfactory completion of FDA audits of clinical trial sites to assure compliance with GCPs and the integrity of the clinical data; |
| • | payment of user fees and securing FDA approval of the NDA; and |
| • | compliance with any post-approval requirements, including Risk Evaluation and Mitigation Strategies, or REMS, and post-approval studies required by the FDA. |
Preclinical Studies
Preclinical studies include laboratory evaluation of the purity and stability of the manufactured drug substance or active pharmaceutical ingredient and the formulated drug or drug product, as well as in vitro and in vivo animal studies to assess the safety and activity of the drug for initial testing in humans and to establish a rationale for therapeutic use. The conduct of preclinical studies is subject to federal regulations and requirements, including GLP regulations. The results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical trials, among other things, are submitted to the FDA as part of an IND. Some long-term preclinical testing, such as 6-month toxicology studies, may continue after the IND is submitted.
Companies usually must complete some long-term preclinical testing, such as 6-month or longer toxicology studies, and must also develop additional information about the chemistry and physical characteristics of the investigational product and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the candidate product and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the candidate product does not undergo unacceptable deterioration over its shelf life.
The IND and IRB Processes
An IND is an exemption from the FDCA that allows an unapproved drug to be shipped in interstate commerce for use in an investigational clinical trial. In support of a request for an IND, applicants must submit a protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, the results of preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and plans for clinical trials, among other things, are submitted to the FDA as part of an IND. An IND goes into effect 30-days after its filing, unless during this 30-day period the FDA raises concerns or questions and imposes a clinical hold.
A clinical hold is an order issued by the FDA to the sponsor to delay a proposed clinical investigation or to suspend an ongoing investigation. A partial clinical hold is a delay or suspension of only part of the clinical work requested under the IND. For example, a specific protocol or part of a protocol is not allowed to proceed, while other protocols may do so. No more than 30 days after imposition of a clinical hold or partial clinical hold, the FDA will provide the sponsor a written explanation of the basis for the hold. Following issuance of a clinical hold or partial clinical hold, an investigation may only resume after the FDA has notified the sponsor that the investigation may proceed. The FDA will base that determination on information provided by the sponsor correcting the deficiencies previously cited or otherwise satisfying the FDA that the investigation can proceed. The FDA may also place a clinical hold or partial clinical hold on a trial after a clinical trial has begun.
A sponsor may choose, but is not required, to conduct a foreign clinical trial under an IND. When a foreign clinical trial is conducted under an IND, all FDA IND requirements must be met unless waived. When the foreign clinical trial is not conducted under an IND, the sponsor must ensure that the trial complies with certain FDA regulatory requirements in order to use the trial as support for an IND or application for marketing approval, including that such trials must be conducted in accordance with GCP, including review and approval by an independent ethics committee, or IEC, and obtaining informed consent from patients. The GCP requirements in the final rule encompass both ethical and data integrity standards for clinical studies. The FDA’s regulations are intended to help ensure the protection of human patients enrolled in non-IND foreign clinical studies, as well as the quality and integrity of the resulting data. They further help ensure that non-IND foreign studies are conducted in a manner comparable to that required for IND studies.
In addition, an IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must exercise continuing supervision over the trial. The IRB must review and approve, among other things, the trial protocol and informed consent information to be provided to trial patients. An IRB must operate in compliance with FDA regulations. An IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the product candidate has been associated with unexpected serious harm to patients.
Additionally, some trials are overseen by an independent group of qualified experts organized by the trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated check points based on access that only the group maintains to available data from the trial. Suspension or termination of development during any phase of clinical trials can occur if it is determined that the participants or patients are being exposed to an unacceptable health risk. Other reasons for suspension or termination may be made by Ocuphire based on evolving business objectives and/or competitive climate.
Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on its ClinicalTrials.gov website.
Human Clinical Trials in Support of an NDA
Clinical trials involve the administration of the investigational product to human patients under the supervision of qualified investigators in accordance with GCP requirements, which include, among other things, the requirement that all research patients provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written trial protocols detailing, among other things, the inclusion and exclusion criteria, the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated.
Human clinical trials are typically conducted in 3 sequential phases, but the phases may overlap.
| • | Phase 1. The drug is initially introduced into healthy human patients or, in certain indications such as cancer, patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage. |
| • | Phase 2. The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3. The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product and to provide adequate information for the labeling of the product. |
Reports detailing activities under, and the status of, an IND must be submitted at least annually to the FDA. In addition, IND safety reports must be submitted to the FDA for any of the following: serious and unexpected suspected adverse reactions; findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the drug; and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. The FDA will typically inspect one or more clinical sites to assure compliance with GCP and the integrity of the clinical data submitted.
Concurrent with clinical trials, companies often complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and, among other things, must develop methods for testing the identity, strength, quality, purity, and potency of the final drug. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
Submission of an NDA to the FDA
Assuming successful completion of required clinical testing and other requirements, the results of the preclinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the drug product for one or more indications. Under federal law, the submission of most NDAs is subject to an application user fee (up to a maximum to 5), which for federal fiscal year 2022 is $3,117,281 for an application requiring clinical data. The sponsor of an approved NDA is also subject to an annual prescription drug program fee, which for fiscal year 2022 is $369,413. Certain exceptions and waivers are available for some of these fees, such as an exception from the application fee for drugs with orphan designation and a waiver for certain small businesses. The FDA conducts a preliminary review of an NDA within 60 days of its receipt and informs the sponsor by the 74th day after the FDA’s receipt of the submission to determine whether the application is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing, and the sponsor receives a Refuse to File Notice. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA has agreed to certain performance goals in the review process of NDAs. The goal for review of most standard applications is within 10 months from the date of filing, and for “priority review” products the review goal is within 6 months of filing. The review process may be extended by the FDA for 3 additional months to consider new information or clarification provided by the applicant to address an outstanding deficiency identified by the FDA following the original submission.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is or will be manufactured. These pre-approval inspections (PAIs) may cover all facilities associated with an NDA submission, including drug component manufacturing (such as active pharmaceutical ingredients), finished drug product manufacturing, and control testing laboratories. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications at the commercial scale. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
In addition, as a condition of approval, the FDA may require an applicant to develop a Risk Evaluation and Mitigation Strategies (REMS). REMS uses risk minimization strategies to ensure that the benefits of the product outweigh the potential risks. REMS can include medication guides, physician communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU may include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The FDA may require a REMS at the time of approval or post-approval if it becomes aware of a serious risk associated with use of the product. The requirement for a REMS can materially affect the potential market and profitability of a product.
The FDA may refer an application for a novel drug to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Fast Track, Breakthrough Therapy, and Priority Review Designations
The FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs are referred to as fast-track designation, breakthrough therapy designation, and priority review designation.
Specifically, the FDA may designate a product for Fast Track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For Fast Track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a Fast Track product’s application before the application is complete. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA’s time period goal for reviewing a Fast Track application does not begin until the last section of the application is submitted. In addition, the Fast Track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Second, a product may be designated as a Breakthrough Therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to Breakthrough Therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
Third, the FDA may designate a product for Priority Review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case- by-case basis, whether the proposed product represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting product reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from 10 months to 6 months.
The FDA’s Decision on an NDA
On the basis of the FDA’s evaluation of the NDA and accompanying information, including the results of the inspection of the manufacturing facilities, the FDA may issue an approval letter, or a complete response letter (CRL). An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA may issue an approval letter. The FDA has committed to reviewing such resubmissions in 2 or 6 months depending on the type of information included. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
If the FDA approves a product, it may limit the approved indications for use for the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess the drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution restrictions or other risk management mechanisms, including REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting (such as annual reports and quarterly safety reports for the first 3 years), product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing user fee requirements for any marketed products, as well as new application fees for supplemental applications with clinical data.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or holds on post-approval clinical trials; |
| • | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; or |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. All promotional materials must be submitted to FDA prior to the time of their first use. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act, or PDMA, which regulates the distribution of drug samples at the federal level and sets minimum standards for the registration and regulation of drug sample distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution.
Section 505(b)(2) NDAs
NDAs for most new drug products are based on 2 adequate and well-controlled clinical trials which must contain substantial evidence of the safety and efficacy of the proposed new product. These applications are submitted under Section 505(b)(1) of the FDCA. The FDA is, however, authorized to approve an alternative type of NDA under Section 505(b)(2) of the FDCA. This type of application allows the applicant to rely, in part, on the FDA’s previous findings of safety and efficacy for a similar product, or published literature. Specifically, Section 505(b)(2) applies to an NDA for a drug for which the investigations to show whether the drug is safe and effective and relied upon by the applicant for approval of the application “were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use from the person by or for whom the investigations were conducted.”
Thus, Section 505(b)(2) authorizes the FDA to approve an NDA based in part on safety and effectiveness data that were not developed by the applicant. Section 505(b)(2) may provide an alternate and potentially more expeditious pathway to FDA approval for new or improved formulations or new uses of previously approved products. If the Section 505(b)(2) applicant can establish that reliance on the FDA’s previous approval is scientifically appropriate, the applicant may eliminate the need to conduct certain preclinical studies or clinical trials of the new product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new drug candidate for all or some of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant.
Abbreviated New Drug Applications for Generic Drugs
In 1984, with passage of the Hatch-Waxman Amendments to the FDCA, Congress authorized the FDA to approve generic drugs that are the same as drugs previously approved by the FDA under the NDA provisions of the statute. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application, or ANDA, to the agency. In support of such applications, a generic manufacturer may rely on the preclinical and clinical testing previously conducted for a drug product previously approved under an NDA, known as the reference-listed drug, or RLD.
Specifically, in order for an ANDA to be approved, the FDA generally must find that the generic version is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form, and the strength of the drug. The FDA must also determine that the generic drug is “bioequivalent” to the innovator drug. Under the statute, a generic drug is bioequivalent to an RLD if “the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug.”
Upon approval of an ANDA, the FDA indicates whether the generic product is “therapeutically equivalent” to the RLD in its publication Approved Drug Products with Therapeutic Equivalence Evaluations, also referred to as the Orange Book. Clinicians and pharmacists often consider a therapeutic equivalent generic drug to be fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, the FDA’s designation of therapeutic equivalence often results in substitution of the generic drug without the knowledge or consent of either the prescribing clinicians or patient.
Under the Hatch-Waxman Amendments, the FDA may not approve an ANDA until any applicable period of non-patent exclusivity for the RLD has expired. The FDCA provides a period of five years of non-patent data exclusivity for a new drug containing a new chemical entity. For the purposes of this provision, a new chemical entity, or NCE, is a drug that contains no active moiety that has previously been approved by the FDA in any other NDA. An active moiety is the molecule or ion responsible for the physiological or pharmacological action of the drug substance. In cases where such NCE exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval.
The FDCA also provides for a period of three years of exclusivity if the NDA includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted by or for the applicant and are essential to the approval of the application. This three-year exclusivity period applies to the condition(s) of use for which the new clinical investigation was conducted, and often protects changes to a previously approved drug product, such as a new dosage form, route of administration, combination or indication. Three-year exclusivity would be available for a drug product that contains a previously approved active moiety, provided the statutory requirement for a new clinical investigation is satisfied. Unlike five-year NCE exclusivity, an award of three-year exclusivity does not block the FDA from accepting ANDAs seeking approval for generic versions of the drug as of the date of approval of the original drug product.
Hatch-Waxman Patent Certification and the 30-Month Stay
Upon approval of an NDA or a supplement thereto, NDA sponsors are required to list with the FDA each patent with claims that cover the applicant’s product or an approved method of using the product. Each of the patents listed by the NDA sponsor is published in the Orange Book. When an ANDA applicant files its application with the FDA, the applicant is required to certify to the FDA concerning any patents listed for the reference product in the Orange Book, except for patents covering methods of use for which the ANDA applicant is not seeking approval. To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would.
Specifically, the applicant must certify with respect to each patent that: (1) the required patent information has not been filed, (2) the listed patent has expired, (3) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (4) the listed patent is invalid, unenforceable or will not be infringed by the new product.
A certification that the new product will not infringe the already approved product’s listed patents or that such patents are invalid or unenforceable is called a Paragraph IV certification. If the applicant does not challenge the listed patents or indicates that it is not seeking approval of a patented method of use, the ANDA or 505(b)(2) application will not be approved until all the listed patents claiming the referenced product have expired (other than method of use patents involving indications for which the applicant is not seeking approval).
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days after the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months after the receipt of the Paragraph IV notice, expiration of the patent, or a decision in the infringement case that is favorable to the ANDA applicant.
To the extent that the Section 505(b)(2) applicant is relying on studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. As a result, approval of a Section 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
505(b)(2) and NCE Data Exclusivity in U.S.
In the United States, the Hatch-Waxman Act provides a 3-year period of non-patent data exclusivity within the United States to the first applicant to gain approval through a 505(b)(2) application seeking regulatory approval of, for example, a new indication, dosage, or strength of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigation and does not prohibit the FDA from approving an ANDA for drugs containing the original active agent. Under this provision, Nyxol for use in treating presbyopia, mydriasis, or DLD may be eligible for 3 years of data exclusivity under the Hatch-Waxman Act.
In the United States, the Hatch-Waxman Act provides period of 5-years of non-patent data exclusivity for a new drug containing a new chemical entity. For the purposes of this provision, a new chemical entity, or NCE, is a drug that contains no active moiety that has previously been approved by the FDA in any other NDA. An active moiety is the molecule or ion responsible for the physiological or pharmacological action of the drug substance. In cases where such NCE exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval.
Pediatric Studies and Exclusivity
Under the Pediatric Research Equity Act of 2003, an NDA or supplement thereto must contain data that are adequate to assess the safety and effectiveness of the drug product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. With enactment of the Food and Drug Administration Safety and Innovation Act or FDASIA, in 2012, sponsors must also submit pediatric trial plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric trial or studies the applicant plans to conduct, including trial objectives and design, any deferral or waiver requests, and other information required by regulation. The applicant, the FDA, and the FDA’s internal review committee must then review the information submitted, consult with each other, and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in FDASIA. Unless otherwise required by regulation, the pediatric data requirements do not apply to products with orphan designation.
Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional 6 months to the term of any patent or regulatory exclusivity, including orphan exclusivity. This 6-month exclusivity may be granted if an NDA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, the latest statutory or regulatory period of exclusivity or patent covering the product is extended by 6 months. This is not a patent term extension, but it effectively extends the regulatory period during which the FDA cannot approve another application.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may designate a drug product as an “orphan drug” if it is intended to treat a rare disease or condition (generally meaning that it affects fewer than 200,000 individuals in the United States, or more in cases in which there is no reasonable expectation that the cost of developing and making a drug product available in the United States for treatment of the disease or condition will be recovered from sales of the product). A company must request orphan product designation before submitting an NDA. If the request is granted, the FDA will disclose the identity of the therapeutic agent and its potential use. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product with orphan status receives the first FDA approval for the disease or condition for which it has such designation or for a select indication or use within the rare disease or condition for which it was designated, the product generally will receive orphan drug exclusivity. Orphan drug exclusivity means that the FDA may not approve any other applications for the same product for the same indication for 7 years, except in certain limited circumstances. Competitors may receive approval of different products for the indication for which the orphan product has exclusivity and may obtain approval for the same product but for a different indication. If a drug or drug product designated as an orphan product ultimately receives marketing approval for an indication broader than what was designated in its orphan product application, it may not be entitled to exclusivity.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Act, which permits a patent restoration of up to 5 years for patent term lost during product development and the FDA regulatory review. The restoration period granted is typically one-half the time between the effective date of an IND and the submission date of an NDA, plus the time between the submission date of an NDA and the ultimate approval date. Patent term restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the product’s approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple drugs for which approval is sought can only be extended in connection with one of the approvals. The U.S. Patent and Trademark Office reviews and approves the application for any patent term extension or restoration in consultation with the FDA. Ocuphire cannot provide any assurance that any patent term extension with respect to any U.S. patent will be obtained and, if obtained, the duration of such extension, in connection with any of its product candidates.
Review and Approval of Drug Products in the European Union
In order to market any product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not it obtains FDA approval for a product, Ocuphire would need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Procedures Governing Approval of Drug Products in the European Union
Pursuant to the European Clinical Trials Directive, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of a European Union member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent ethics committee has issued a favorable opinion. Clinical trial application must be accompanied by an investigational medicinal product dossier with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member states and further detailed in applicable guidance documents.
To obtain marketing approval of a product under European Union regulatory systems, an applicant must submit a marketing authorization application, or MAA, either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union member states. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products and products with a new active substance indicated for the treatment of certain diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional.
Under the centralized procedure, the Committee for Medicinal Products for Human Use, or the CHMP, established at the European Medicines Agency, or EMA, is responsible for conducting the initial assessment of a product. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing marketing authorization. Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops, when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated evaluation might be granted by the CHMP in exceptional cases when a medicinal product is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation. In this circumstance, the EMA ensures that the opinion of the CHMP is given within 150 days.
The decentralized procedure is available to applicants who wish to market a product in various European Union member states where such product has not received marketing approval in any European Union member states before. The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one-member state designated by the applicant, known as the reference member state. Under this procedure, an applicant submits an application based on identical dossiers and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference member state and concerned member states. The reference member state prepares a draft assessment report and drafts of the related materials within 210 days after receipt of a valid application. Within 90 days of receiving the reference member state’s assessment report and related materials, each concerned member state must decide whether to approve the assessment report and related materials.
If a member state cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points are subject to a dispute resolution mechanism and may eventually be referred to the European Commission, whose decision is binding on all member states.
Within this framework, manufacturers may seek approval of hybrid medicinal products under Article 10(3) of Directive 2001/83/EC. Hybrid applications rely, in part, on information and data from a reference product and new data from appropriate pre-clinical tests and clinical trials. Such applications are necessary when the proposed product does not meet the strict definition of a generic medicinal product, or bioavailability studies cannot be used to demonstrate bioequivalence, or there are changes in the active substance(s), therapeutic indications, strength, pharmaceutical form or route of administration of the generic product compared to the reference medicinal product. In such cases the results of tests and trials must be consistent with the data content standards required in the Annex to the Directive 2001/83/EC, as amended by Directive 2003/63/EC.
Hybrid medicinal product applications have automatic access to the centralized procedure when the reference product was authorized for marketing via that procedure. Where the reference product was authorized via the decentralized procedure, a hybrid application may be accepted for consideration under the centralized procedure if the applicant shows that the medicinal product constitutes a significant therapeutic, scientific or technical innovation, or the granting of a community authorization for the medicinal product is in the interest of patients at the community level.
Clinical Trial Approval in the European Union
Requirements for the conduct of clinical trials in the European Union including Good Clinical Practice, or GCP, are set forth in the Clinical Trials Directive 2001/20/EC and the GCP Directive 2005/28/EC. Pursuant to Directive 2001/20/EC and Directive 2005/28/EC, as amended, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the E.U. member states. Under this system, approval must be obtained from the competent national authority of each E.U. member state in which a trial is planned to be conducted. To this end, a CTA is submitted, which must be supported by an investigational medicinal product dossier, or IMPD, and further supporting information prescribed by Directive 2001/20/EC and Directive 2005/28/EC and other applicable guidance documents. Furthermore, a clinical trial may only be started after a competent ethics committee has issued a favorable opinion on the clinical trial application in that country.
In April 2014, the E.U. passed the new Clinical Trials Regulation (EU) No 536/2014. The new Clinical Trials Regulation, which will replace the Clinical Trials Directive, introduces a complete overhaul of the existing regulation of clinical trials for medicinal products in the E.U., including a new coordinated procedure for authorization of clinical trials that is reminiscent of the mutual recognition procedure for marketing authorization of medicinal products, and increased obligations on sponsors to publish clinical trial results. The entry into application of the Clinical Trials Regulation has been delayed. The Clinical Trials Directive may be replaced with the new Clinical Trials Regulation in late 2022. The new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials in the European Union. The main characteristics of the regulation include: a streamlined application procedure via a single entry point, the E.U. portal; a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures that will spare sponsors from submitting broadly identical information separately to various bodies and different member states; a harmonized procedure for the assessment of applications for clinical trials, which is divided into two parts (Part I is assessed jointly by all member states concerned, and Part II is assessed separately by each member state concerned); strictly defined deadlines for the assessment of clinical trial applications; and the involvement of the ethics committees in the assessment procedure in accordance with the national law of the member state concerned but within the overall timelines defined by the Clinical Trials Regulation.
Periods of Authorization and Renewals
Marketing authorization is valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the European Union market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid (the so-called sunset clause).
Data and Market Exclusivity in the European Union
In the European Union, new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic (abbreviated) application for eight years, after which generic marketing authorization can be submitted, and the innovator’s data may be referenced, but not approved for two years. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the sponsor is able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the product if such company can complete a full MAA with a complete database of pharmaceutical test, preclinical tests and clinical trials and obtain marketing approval of its product.
Orphan Drug Designation and Exclusivity
Regulation 141/2000 provides that a drug shall be designated as an orphan drug if its sponsor can establish: that the product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the European Community when the application is made, or that the product is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the European Community and that without incentives it is unlikely that the marketing of the drug in the European Community would generate sufficient return to justify the necessary investment. For either of these conditions, the applicant must demonstrate that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the European Community or, if such method exists, the drug will be of significant benefit to those affected by that condition.
Regulation 847/2000 sets out criteria and procedures governing designation of orphan drugs in the European Union. Specifically, an application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. Marketing authorization for an orphan drug leads to a ten-year period of market exclusivity. This period may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan drug designation, for example because the product is sufficiently profitable not to justify market exclusivity. Market exclusivity can be revoked only in very selected cases, such as consent from the marketing authorization holder, inability to supply sufficient quantities of the product, demonstration of “clinically relevant superiority” by a similar medicinal product, or, after a review by the Committee for Orphan Medicinal Products, requested by a member state in the fifth year of the marketing exclusivity period (if the designation criteria are believed to no longer apply). Medicinal products designated as orphan drugs pursuant to Regulation 141/2000 shall be eligible for incentives made available by the European Community and by the member states to support research into, and the development and availability of, orphan drugs.
Regulatory Requirements after Marketing Authorization
As in the United States, both marketing authorization holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA and the competent authorities of the individual EU Member States both before and after grant of the manufacturing and marketing authorizations. The holder of an EU marketing authorization for a medicinal product must, for example, comply with EU pharmacovigilance legislation and its related regulations and guidelines which entail many requirements for conducting pharmacovigilance, or the assessment and monitoring of the safety of medicinal products. The manufacturing process for medicinal products in the European Union is also highly regulated and regulators may shut down manufacturing facilities that they believe do not comply with regulations. Manufacturing requires a manufacturing authorization, and the manufacturing authorization holder must comply with various requirements set out in the applicable EU laws, including compliance with EU cGMP standards when manufacturing medicinal products and active pharmaceutical ingredients.
In the European Union, the advertising and promotion of approved products are subject to EU Member States’ laws governing promotion of medicinal products, interactions with clinicians, misleading and comparative advertising and unfair commercial practices. In addition, other legislation adopted by individual EU Member States may apply to the advertising and promotion of medicinal products. These laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics, or SmPC, as approved by the competent authorities. Promotion of a medicinal product that does not comply with the SmPC is considered to constitute off-label promotion, which is prohibited in the European Union.
Healthcare Reform
The healthcare industry in the United States, including the research-based pharmaceutical sector, is highly regulated and subject to frequent substantial changes. Any significant efforts from the federal or state governments to change how healthcare is provided or funded within the United States could have a material impact on Ocuphire’s business. Currently, the United States Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act (the “ACA”) is the seminal legislation that has had, and continues to have, the most substantial impact on the healthcare industry. The ACA is intended to expand coverage for uninsured individuals and contain the overall cost of healthcare services. Some provisions of the ACA have yet to be fully implemented, while certain other provisions have been subject to reform through legislation, Executive Orders of the President, and to judicial challenges. With regard to the research-based pharmaceutical sector and pharmaceutical products, provisions in the ACA impacting Ocuphire’s potential drug candidates include:
| • | A special, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications; |
| • | Expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability; |
| • | Expanded manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate for both branded and generic drugs and revising the definition of “average manufacturer price,” or AMP, for calculating and reporting Medicaid drug rebates on outpatient prescription drug prices and extending rebate liability to prescriptions for individuals enrolled in Medicare Advantage plans; addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; |
| • | Expanded the types of entities eligible for the 340B drug discount program; |
| • | Established the Medicare Part D coverage gap discount program by requiring manufacturers to provide a 50% point-of-sale-discount off the negotiated price of applicable brand drugs to eligible beneficiaries during their coverage gap period as a condition for the manufacturers’ outpatient drugs to be covered under Medicare Part D; |
| • | A new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and |
| • | Established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. |
There may be additional legislative changes, including potential repeal and replacement of certain provisions of the ACA. While the timing and scope of any potential future legislation to repeal and replace ACA provisions is highly uncertain in many respects, it is also possible that some of the ACA provisions that generally are not favorable for the research-based pharmaceutical sector could also be repealed along with ACA coverage expansion provisions.
Healthcare Laws and Regulations
There are other healthcare-related laws and regulations that extensively govern how pharmaceutical companies, like Ocuphire, are operated and regulate activities related to pharmaceutical products. These laws and regulations may require administrative guidance to implement. Failure to comply could subject the company to legal and/or administrative actions, which may include substantial fines and/or penalties; orders to stop non-compliant activities; criminal charges; warning letters; product recalls or seizures; delays in product approvals; exclusion from participation in government reimbursement programs or contracts as well as limitations on conducting business in applicable jurisdictions.
Applicable federal and state healthcare laws and regulations include:
| • | The federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
| • | The federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | The federal Physician Payments Sunshine Act (part of the ACA), commonly known as the Sunshine Act or Open Payments, requires certain manufacturers of drugs, devices, biologics and medical supplies to disclose certain payments and financial relationships with healthcare providers directly to the Centers for Medicare & Medicaid Services (CMS). Failure to comply with these laws can result in significant civil monetary penalties; |
| • | The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (collectively, "HIPAA"). HIPAA mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common health care transactions as well as standards relating to the privacy and security of individually identifiable health information. These standards require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent than HIPAA. Failure to comply with these laws can result in the imposition of significant civil and criminal penalties; |
| • | Other analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to healthcare related items or services that are reimbursed by non-governmental third-party payors, including private insurers. |
Healthcare Reimbursement
Healthcare providers and third-party payors play a primary role in recommending and prescribing pharmaceutical products that are granted regulatory approval. If our potential drug candidates receive regulatory approval, successful commercialization of the products may depend, in part, on obtaining and maintaining adequate reimbursement levels with third-party payors, including commercial health insurers, managed care organizations, and government health programs in the United States such as Medicare and Medicaid. However, a growing trend in the United States healthcare industry and elsewhere is cost containment.
Recently, there has been greater scrutiny from governmental bodies over how pharmaceutical manufacturers and distributors set prices for their products. This has resulted in congressional inquiries as well as other proposed and enacted legislation designed to (i) bring more transparency to product pricing, (ii) limit coverage and reimbursement for drugs and other medical products, and (iii) reform government health program reimbursement within the healthcare system as a whole. While proposed reform measures will require the U.S. Congress to pass legislation to become effective, President Biden‘s administration and the U.S. Congress have each indicated that they will continue to seek new legislative and/or administrative measures to control drug costs.
For example, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap for single source and innovator multiple source drugs, beginning January 1, 2024. Further, on July 9, 2021, President Biden signed an executive order to promote competition in the U.S. economy that included several initiatives addressing prescription drugs. Among other provisions, the executive order directed the Secretary of HHS to issue a report to the White House that includes a plan to, among other things, reduce prices for prescription drugs, including prices paid by the federal government for such drugs.
The report, issued on September 9, 2021 entitled a Comprehensive Plan for Addressing High Drug Prices: A Report in Response to the Executive Order on Competition in the American Economy, identified potential legislative policies and administrative tools that Congress and the agency can pursue in order to (i) make drug prices more affordable and equitable, (ii) improve and promote competition throughout the prescription drug industry, and (iii) foster scientific innovation. In addition, individual states in the United States have also become increasingly active in passing laws and implementing regulations designed to control pharmaceutical product pricing, including reimbursement constraints, discounts, restrictions on certain product access, marketing cost disclosure and transparency measures and, in some cases, mechanisms to encourage importation from other countries and bulk purchasing.
Furthermore, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022 (the “IRA”) into law. The IRA includes several provisions that may potentially impact our business, including provisions that (i) create a $2,000 cap on out-of-pocket expenses for Medicare Part D beneficiaries, (ii) impose new manufacturer financial liability on all drugs in Medicare Part D, (iii) allow the U.S. government to negotiate Medicare Part B and Part D pricing for certain high-cost drugs and biologics without generic or biosimilar competition, (iv) require companies to pay rebates to Medicare for drug prices that increase faster than inflation, and (v) delay the rebate rule that would require pass-through of pharmacy benefit manager rebates to beneficiaries. The effect of IRA on our business and the healthcare industry in general is not yet known.
Human Capital Resources
As of December 31, 2022, Ocuphire had nine full-time employees, with the following assignments: two engaged in clinical research and development activities, one of whom holds a Ph.D. degree, four engaged in research and development activities and also business development and finance, and three engaged in finance, human resources, and administrative support. Ocuphire plans to continue to utilize expert consultants and contract organizations to support execution of the day-to-day operations. None of Ocuphire’s employees are represented by labor unions or covered by collective bargaining agreements. Ocuphire believes that it maintains good relations with its employees.
An investment in our securities has a high degree of risk. Before you invest you should carefully consider the risks and uncertainties described below and the other information in this Annual Report. Any of the risks and uncertainties set forth herein could materially and adversely affect our business, results of operations and financial condition, which in turn could materially and adversely affect the trading price or value of our securities. Additional risks not currently known to us or which we consider immaterial based on information currently available to us may also materially adversely affect us. As a result, you could lose all or part of your investment.
Risks Related to Development of Our Product Candidates
Viatris has exclusive global rights to commercialize our Nyxol products in key global markets. Viatris’ failure to timely develop or commercialize these products would have a material adverse effect on our business and operating results.
We granted Viatris an exclusive right to commercialize our Nyxol products in key global markets. Additionally, we granted Viatris the exclusive right and license to develop Nyxol outside of the United States. The collaboration with Viatris may not be successful due to several factors, including the following:
| • | Viatris may not be able to obtain from us or manufacture our products in a timely or cost-effective manner; |
| • | Viatris may not timely perform its obligations under the Nyxol License Agreement; |
| • | Viatris may fail to effectively commercialize our products;
|
| • | Viatris may not be able to sublicense Nyxol to one or more suitable parties outside the United States; or |
| • | Contractual disputes or other disagreements between us and Viatris, including those regarding the development, manufacture, sub licensure and commercialization of our products, interpretation of the Nyxol License Agreement, and ownership of proprietary rights. Viatris may select a new development partner for Nyxol in the U.S. upon 90 days’ notice to Ocuphire. |
Any of the foregoing could adversely impact the likelihood and timing of any payments we are eligible to receive under the Nyxol License Agreement. The Company will be reliant on Viatris to drive the commercialization and sales of our products. If Viatris does not perform its obligations under the Nyxol License Agreement, this could result in a material adverse effect on our business, results of operations and prospects and would likely cause our stock price to decline.
We currently depend entirely on the success of Nyxol and APX3330, our only product candidates.
We currently have only two product candidates, Nyxol and APX3330, in clinical development, and our business depends on their successful clinical development, regulatory approval and commercialization (by us our by our strategic partner). The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of a drug product are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, where regulations may differ. We are not permitted to market our product candidates in the United States until we receive approval of an NDA from the FDA or in any foreign countries until we receive the requisite approval from such countries. We have submitted an NDA to the FDA for Nyxol for the treatment of RM. The application was accepted for filing by the FDA with a PDUFA date of September 28, 2023. For other indications, and for APX3330, before obtaining regulatory approval for the commercial sale of our product candidates for a particular indication, we must demonstrate through preclinical testing and clinical trials that the applicable product candidate is safe and effective for use in that target indication. This process can take many years and may be followed by post-marketing studies and surveillance together which will require the expenditure of substantial resources beyond the proceeds raised in our equity and debt financings to date. Of the large number of drugs in development in the United States, only a small percentage of drugs successfully complete the FDA regulatory approval process and are commercialized. Accordingly, even if we are able to complete development of our product candidates, we cannot assure you that our product candidates will be approved or commercialized.
Obtaining approval of an NDA is an extensive, lengthy, expensive and uncertain process, and the FDA may delay, limit or deny approval of our product candidates for many reasons, including:
| • | the data collected from preclinical studies and clinical trials of our product candidates may not be sufficient to support the submission or acceptance of an NDA for one or more indications; |
| • | we may not be able to demonstrate to the satisfaction of the FDA that our product candidates are safe and effective for any indication; |
| • | the results of clinical trials may not meet the level of statistical significance or clinical significance required by the FDA for approval; |
| • | the FDA may disagree with the number, design, size, conduct, or implementation of our clinical trials; |
| • | the FDA may not find the data from preclinical studies and clinical trials sufficient to demonstrate that our product candidates’ clinical and other benefits outweigh the safety risks; |
| • | the FDA may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | the FDA may not accept data generated at our clinical trial sites; |
| • | the FDA may have difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| • | the FDA may require development of a Risk Evaluation and Mitigation Strategy (REMS) as a condition of approval; |
| • | the FDA may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we entered or enter into agreements for clinical and commercial supplies; or |
| • | the FDA may change its approval policies or adopt new regulations. |
The results of previous clinical trials may not be predictive of future results, and the results of our current and planned clinical trials may not satisfy the requirements of the FDA or non-U.S. regulatory authorities.
The results from the prior preclinical studies and clinical trials for Nyxol and APX3330 discussed elsewhere in this Annual Report may not necessarily be predictive of the results of future preclinical studies or clinical trials. Even if we are able to complete our planned clinical trials of our product candidates according to our current development timeline, the results from our prior clinical trials of our product candidates may not be replicated in these future trials. Many companies in the pharmaceutical and biotechnology industries (including those with greater resources and experience than us) have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in clinical trials, including previously unreported adverse events (“AEs”). Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless have failed to obtain FDA approval. If we fail to produce positive results in our clinical trials of any of our product candidates, the development timelines, regulatory approvals, and commercialization prospects for our product candidates, as well as Ocuphire’s business and financial prospects, would be adversely affected. Further, Ocuphire’s product candidates may not be approved even if they achieve their respective primary endpoints in additional Phase 3 registration trials. The FDA or non-U.S. regulatory authorities may disagree with our trial designs or our interpretation of data from preclinical studies and clinical trials. In addition, any of these regulatory authorities may change requirements for the approval of a product candidate even after reviewing and providing comments or advice on a protocol for a clinical registration trial that has the potential to result in approval by the FDA or another regulatory authority. Furthermore, any of these regulatory authorities may also approve our product candidates for fewer or more limited indications than we request or may grant approval contingent on the performance of costly post-marketing clinical trials.
Before obtaining regulatory approvals for the commercial sale of any product candidate for any target indication, we must demonstrate with substantial evidence gathered in preclinical studies and adequate and well-controlled clinical studies, and, with respect to approval in the United States, to the satisfaction of the FDA, that the product candidate is safe and effective for use for that target indication. We cannot assure you that the FDA or non-U.S. regulatory authorities would consider our planned clinical trials to be sufficient to serve as the basis for approval of our product candidates for any indication. The FDA and non-U.S. regulatory authorities retain broad discretion in evaluating the results of our clinical trials and in determining whether the results demonstrate that our product candidates are safe and effective. If we are required to conduct clinical trials of our product candidates in addition to those we have planned prior to approval, we may need substantial additional funds, and cannot assure you that the results of any such outcomes trial or other clinical trials will be sufficient for approval.
Additional data and/or time may be required to obtain U.S. regulatory approval for any of our product candidates that are deemed to be a drug/device combination product candidate.
Our eye drop product candidates are now considered combination products with both drug and device components. The FDA requires both the drug and device components of combination product candidates to be reviewed as part of an NDA submission. The FDA’s application of the regulations is evolving for drug/device combination products including single-use and multi-dose eye droppers. We may experience requests for additional data and/or delays in the development and commercialization of our drug-led combination product candidates, due to regulatory uncertainties in the product development and approval process.
If clinical trials of APX3330 fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of such product candidates.
Before obtaining marketing approval from regulatory authorities for the sale of APX3330, we need to conduct further animal toxicology studies and additional clinical trials before obtaining marketing approval from regulatory authorities. Clinical testing is expensive, difficult to design and implement, can take many years to complete, and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of development.
We may experience delays in manufacturing and our clinical trials, and we, or our future collaborators, may experience numerous unforeseen events during, or as a result of, clinical trials that could result in increased development costs and delay, and could limit or prevent our, or our future collaborators’, ability to receive marketing approval or commercialize our product candidates, including:
| • | regulators or IRBs may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site including due to the ongoing COVID-19 pandemic or other public health emergency; |
| • | government or regulatory delays and changes in regulatory requirements, policy and guidelines may require us to perform additional clinical trials or use substantial additional resources to obtain regulatory approval; |
| • | we may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites; |
| • | clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require it, to conduct additional clinical trials or abandon product development programs; |
| • | the number of patients required for clinical trials may be larger, enrollment in these clinical trials may be slower or participants may drop out of these clinical trials at a higher rate than we anticipate; |
| • | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
| • | our patients or medical investigators may be unwilling to follow our clinical trial protocols; |
| • | we might have to suspend or terminate clinical trials for various reasons, including a finding that the participants are being exposed to unacceptable health risks; |
| • | the cost of clinical trials may be greater than we anticipate; |
| • | the supply or quality of any product candidate or other materials necessary to conduct clinical trials may be insufficient or inadequate; |
| • | the product candidate may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators or IRBs to suspend or terminate the trials; |
| • | clinical trials may be delayed or terminated ; and |
| • | federal agencies may, due to reduced manpower or diverted resources, require more time to review clinical trial protocols and INDs. |
If we experience delays or difficulties in the enrollment of patients in clinical trials, our ability to conduct and complete those clinical trials, and our ability to seek and receive necessary regulatory approvals, could be delayed or prevented.
We or our future collaborators may not be able to initiate or continue clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or analogous regulatory authorities outside the United States. Patient enrollment can be affected by many factors, including:
| • | severity of the disease under investigation; |
| • | availability and efficacy of medications already approved for the disease under investigation; |
| • | eligibility criteria and visit schedule for the trial in question; |
| • | competition for eligible patients with other companies conducting clinical trials for product candidates seeking to treat the same indication or patient population; |
| • | our payments for conducting clinical trials; |
| • | perceived risks and benefits of the product candidate under study; |
| • | efforts to facilitate timely enrollment in clinical trials; |
| • | patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after treatment; |
| • | proximity and availability of clinical trial sites for prospective patients; and |
the ability of patients to safely participate in clinical trials during any public health emergencies such as the COVID-19 pandemic. Our inability to enroll a sufficient number of patients for our clinical trials or retain sufficient enrollment through the completion of our trials would result in significant delays or may require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our product candidates and cause our stock price to decline.
We or others could discover that our product candidates lack sufficient efficacy, or sufficient efficacy compared to competitor products or that they cause undesirable side effects that were not previously identified, which could delay or prevent regulatory approval or commercialization.
Because both Nyxol and APX3330 have been tested in relatively small patient populations, at a limited range of daily doses up to 0.75% Phentolamine Ophthalmic Solution (which is the same as 1.0% Phentolamine Mesylate Ophthalmic Solution) and up to 720 mg respectively, and for limited durations to date, it is possible that our clinical trials have or will indicate an apparent positive effect of Nyxol or APX3330 that is greater than the actual positive effect, if any, or that additional and unforeseen side effects may be observed as its development progresses. The discovery that either Nyxol (alone or with adjunctive LDP) or APX3330 lacks sufficient efficacy, or that they cause undesirable side effects (including side effects not previously identified in our completed clinical trials), could cause us or regulatory authorities to interrupt, delay, or discontinue clinical trials, and could result in the denial of regulatory approval by the FDA or other non-U.S. regulatory authorities for any or all targeted indications.
The discovery that our product candidates lack sufficient efficacy or that they cause undesirable side effects that were not previously identified could prevent us from commercializing such product candidates and generating revenues from sales. In addition, if we receive marketing approval for our product candidates and we or others later discover that they are less effective, or identify undesirable side effects caused by our product candidates:
| • | regulatory authorities may withdraw their approval of the product; |
| • | we may be required to recall the product, change the way this product is administered, conduct additional clinical trials, or change the labeling or distribution of the product (including REMS); |
| • | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the product; |
| • | we may be subject to fines, injunctions, or the imposition of civil or criminal penalties; |
| • | we could be sued and held liable for harm caused to patients; |
| • | the product may be rendered less competitive and sales may decrease; or |
| • | our reputation may suffer generally among both clinicians and patients. |
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate or could substantially increase the costs and expenses of commercializing the product candidate, which in turn could delay or prevent us from generating significant, or any, revenues from the sale of the product candidate.
Changes in regulatory requirements or FDA guidance, or unanticipated events during our clinical trials, may result in changes to clinical trial protocols or additional clinical trial requirements, which could result in increased costs to us or delays in development timelines.
Changes in regulatory requirements or FDA guidance, or unanticipated events during our clinical trials, may require us to amend clinical trial protocols or the FDA may impose additional clinical trial requirements. Amendments to our clinical trial protocols would require resubmission to the FDA and IRBs for review and approval, and may adversely impact the cost, timing or successful completion of a clinical trial. If we experience delays completing, or if we terminate, any Phase 2 or Phase 3 trials, or if we are required to conduct additional clinical trials, the commercial prospects for our product candidates may be harmed and our ability to generate product revenues will be delayed.
If we fail to receive regulatory approval for any of our planned indications for our product candidates or fail to develop additional product candidates, our commercial opportunity will be limited.
We are initially focused on the development of our product candidates for our target indications, the reversal of pharmacologically-induced mydriasis, treatment of presbyopia, DLD, DR and DME. However, we cannot assure you that we will be able to obtain regulatory approval of our product candidates for any indication, or successfully commercialize our product candidates, if approved. If we do not receive regulatory approval for, or successfully commercialize, our product candidates for one or more of our targeted or other indications, our commercial opportunity will be limited.
We may pursue clinical development of additional acquired or in-licensing product candidates. Developing, obtaining regulatory approval for and commercializing additional product candidates will require substantial additional funding beyond the net proceeds of our completed equity and debt financings, and are prone to the risks of failure inherent in drug product development. We cannot assure you that we will be able to successfully advance any additional product candidates through the development process.
Even if we obtain FDA approval to market additional product candidates, we cannot assure you that any such product candidates will be successfully commercialized, widely accepted in the marketplace, or more effective than other commercially available alternatives. If we are unable to successfully develop and commercialize additional product candidates, our commercial opportunity will be limited.
We have limited drug research and discovery capabilities and may need to acquire or license product candidates from third parties to expand our product candidate pipeline.
We currently have limited drug research and discovery capabilities. Accordingly, if we are to expand our product candidate pipeline beyond Nyxol and APX3330 and our pipeline candidates, we may need to acquire or license product candidates from third parties. We would face significant competition in seeking to acquire or license promising product candidates. Many of our competitors for such promising product candidates may have significantly greater financial resources and more extensive experience in preclinical testing and clinical trials, obtaining regulatory approvals, and manufacturing and marketing pharmaceutical products, and thus, may be a more attractive option to a potential licensor than us. If we are unable to acquire or license additional promising product candidates, we may not be able to expand our product candidate pipeline.
If we are able to acquire or license other product candidates, such license agreements will likely impose various obligations upon us, and our licensors may have the right to terminate the license thereunder in the event of a material breach or, in some cases, at will. A termination of a future license could result in our loss of the right to use the licensed intellectual property, which could adversely affect our ability to develop and commercialize a future product candidate, if approved, as well as harm our competitive business position and our business prospects.
We may expend our limited resources to pursue a particular indication and fail to capitalize on indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we are currently focusing only on development programs that we identify for specific indications for our product candidates. As a result, we may forego or delay pursuit of opportunities for other indications, or with other potential product candidates that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for specific indications or future product candidates may not yield any commercially viable product. If we do not accurately evaluate the commercial potential or target market for our product candidates, we may not gain approval or achieve market acceptance of that candidate, and our business and financial results will be harmed.
Risks Related to Our Financial Position and Need for Additional Capital
We expect to incur losses for the foreseeable future and may never achieve or maintain profitability.
As of December 31, 2022, we had an accumulated deficit of $71.5 million. We have funded our operations primarily through issuance of promissory notes and convertible notes in private placements, and then common stock and warrants after becoming a publicly-traded company, and more recently, through fees received under the Nyxol License Agreement. We have devoted substantially all of our financial resources and efforts to the clinical development of our product candidates. Even assuming we obtain regulatory approval for one or more of our product candidates, we expect it to be at least a year before we potentially receive any royalty payments under the Nyxol License Agreement, and several years before APX3330 is potentially ready for commercialization.
To become and remain profitable from our product candidates, we must develop and eventually commercialize a product with market potential. This will require us to be successful in a range of challenging activities, including completing preclinical testing and clinical trials, obtaining regulatory approval for a product candidate, manufacturing, marketing, and selling any drug for which it may obtain regulatory approval and satisfying any post-marketing requirements. We are in the early stages of most of these activities. We may never succeed in these activities and, even if we does, we may never generate revenues that are significant or large enough to achieve profitability.
If we do achieve profitability from our product candidates, we may not be able to sustain or increase profitability on an annual basis. Our failure to become or remain profitable from our product candidates may decrease our value and could impair our ability to raise capital, maintain our research and development efforts, expand our business, or continue our operations.
We have not generated any revenue from sales of any products and may never be profitable.
We have no products approved for commercial sale, and do not anticipate generating any product revenue, unless and until APX3330 or another product candidate receives the regulatory approvals necessary for commercialization in one or more jurisdictions. Our ability to generate revenue from APX3330 depends on a number of factors, including our ability to:
| • | obtain favorable results from and complete the clinical development of APX3330 for their planned indications, including successful completion of additional clinical trials for these indications; |
| • | submit applications to regulatory authorities for both product candidates and receive timely marketing approvals in the United States and foreign countries; |
| • | establish and maintain commercially viable supply and manufacturing relationships with third parties that can provide adequate, in both amount and quality, products and services to support clinical development and meet the market demand for product candidates that we develop, if approved; |
| • | establish sales and marketing capabilities to effectively market and sell our product candidates in the United States or other markets, either alone or with a pharmaceutical partner; |
| • | address any competing products and technological and market developments; |
| • | obtain coverage and adequate reimbursement for customers and patients from government and third-party payors for product candidates that we develop; and |
| • | achieve market acceptance of our product candidates. |
Even if APX3330 is approved for commercial sale in one or all of the initial indications that we are pursuing, it may not gain market acceptance or achieve commercial success. In addition, we anticipate incurring significant costs associated with commercializing our product candidates. We may not achieve profitability soon after generating product revenue, if ever, and may be unable to continue operations without continued funding.
Our relatively short operating history may make it difficult for investors to evaluate the success of our business to date and to assess our future viability.
We are a clinical-stage company, and our operations to date have been limited to organizing and staffing our company, business planning, raising capital, and developing our product candidates. We have not yet demonstrated our ability to successfully obtain regulatory approval, manufacture a product at commercial scale, or conduct sales and marketing activities necessary for successful product commercialization.
Additionally, there is no operating history on which investors may evaluate our business and our prospects. Investment in a start-up company such as ours is inherently subject to many risks. These risks and difficulties include challenges in accurate financial planning as a result of: (a) accumulated losses; (b) uncertainties resulting from a relatively limited time period in which to develop and evaluate business strategies as compared to companies with longer operating histories; (c) compliance with regulations required to commence sales on future products; (d) reliance on third parties for clinical, manufacturing, analytical laboratory work, preclinical, regulatory, commercialization or other activities; (e) financing the business; and (f) meeting the challenges of the other risk factors described herein. We have no operating history upon which investors may base an evaluation of our performance; therefore, we are subject to all risks incident to the creation and development of a new business. There can be no assurance that we can realize our plans on our projected timetable in order to reach sustainable or profitable operations.
Adverse developments affecting the financial services industry could negatively affect our current and projected business operations and our financial condition and results of operations.
Although we assess our banking relationships as we believe necessary or appropriate, our access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations could be significantly impaired by factors that affect us, the financial institutions with which we have arrangements directly, or the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit or liquidity agreements or arrangements, disruptions or instability in the financial services industry or financial markets, or concerns or negative expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions or financial services industry companies with which we have financial or business relationships, but could also include factors involving financial markets or the financial services industry generally.
The results of events or concerns that involve one or more of these factors could include a variety of material and adverse impacts on our current and projected business operations and our financial condition and results of operations. These could include, but may not be limited to, the following:
| • | Delayed access to deposits or other financial assets or the uninsured loss of deposits or other financial assets; |
| • | Loss of access to revolving existing credit facilities or other working capital sources and/or the inability to refund, roll over or extend the maturity of, or enter into new credit facilities or other working capital resources; |
| • | Potential or actual breach of contractual obligations that require us to maintain letters or credit or other credit support arrangements; or |
| • | Termination of cash management arrangements and/or delays in accessing or actual loss of funds subject to cash management arrangements. |
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses, financial obligations or fulfill our other obligations, result in breaches of our financial and/or contractual obligations or result in violations of federal or state wage and hour laws. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
In addition, any further deterioration in the macroeconomic economy or financial services industry could lead to losses or defaults by parties with whom we conduct business, which in turn, could have a material adverse effect on our current and/or projected business operations and results of operations and financial condition. For example, a party with whom we conduct business may fail to make payments when due, default under their agreements with us, become insolvent or declare bankruptcy. Any bankruptcy or insolvency, or the failure to make payments when due, of any counterparty of ours, or the loss of any significant relationships, could result in material losses to us and may material adverse impacts on our business.
We will need substantial additional capital in the future. If additional capital is not available, we will have to delay, reduce or cease operations.
We will need to raise additional capital to continue to fund the further development of our product candidates and operations. Our future capital requirements may be substantial and will depend on many factors including:
| • | the scope, size, rate of progress, results, and costs of researching and developing our product candidates, and initiating and completing our preclinical studies and clinical trials; |
| • | the cost, timing and outcome of our efforts to obtain marketing approval for our product candidates in the United States and other countries, including to fund the preparation and filing of NDAs with the FDA for our product candidates and to satisfy related FDA requirements and regulatory requirements in other countries; |
| • | the number and characteristics of any additional product candidates we develop or acquire, if any; |
| • | our ability to establish and maintain collaborations on favorable terms, if at all; |
| • | the amount of revenue, if any, from commercial sales, should our product candidates receive marketing approval; |
| • | the costs associated with commercializing our product candidates, if we receive marketing approval, including the cost and timing of developing sales and marketing capabilities or entering into strategic collaborations to market and sell our product candidates; |
| • | the cost of manufacturing our product candidates or products we successfully commercialize; and |
| • | the costs associated with general corporate activities, such as the cost of filing, prosecuting and enforcing patent claims and making regulatory filings. |
Changing circumstances may cause us to consume capital significantly faster than we currently anticipate. Because the outcome of any clinical trial is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development, regulatory approval and commercialization of our product candidates. Additional financing may not be available when we need it, or may not be available on terms that are favorable to us. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. If adequate funds are unavailable to us on a timely basis, or at all, we may not be able to continue the development of our product candidates, or commercialize our product candidates, if approved, unless we find a strategic partner.
Worldwide economic and social instability could adversely affect our revenue, financial condition, or results of operations.
The health of the global economy, and the equity and credit markets in particular, as well as the stability of the social fabric of our society, affects our business and operating results. For example, the equity and credit markets may be adversely affected by the current conflict between Russia and Ukraine and measures taken in response thereto. If the equity and credit markets are not favorable, we may be unable to raise additional financing when needed or on favorable terms. Our vendors and development partners may experience financial difficulties or be unable to borrow money to fund their operations, which may adversely impact their ability to purchase our products or to pay for our products on a timely basis, if at all. In addition, adverse economic conditions, such as recent supply chain disruptions and labor shortages and persistent inflation, have affected, and may continue to adversely affect our suppliers’ ability to provide our manufacturers with materials and components, which may negatively impact our business. These economic conditions make it more difficult for us to accurately forecast and plan our future business activities.
Adverse global economic conditions could have a negative effect on our business, results of operations and financial condition and Adverse global economic conditions could have a negative effect on our business, results of operations and financial condition and liquidity.
A general slowdown in the global economy, including a recession, or in a particular region or industry, an increase in trade tensions with U.S. trading partners, inflation or a tightening of the credit markets could negatively impact our business, financial condition and liquidity. Adverse global economic conditions have from time to time caused or exacerbated significant slowdowns in the industries and markets in which we operate, which have adversely affected our business and results of operations. Macroeconomic weakness and uncertainty also make it more difficult for us to accurately forecast revenue, gross margin and expenses, and may make it more difficult to raise or refinance debt.
Raising additional capital may cause dilution to our stockholders, restrict our operations, or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity and debt financings as well as potential strategic collaborations and licensing arrangements. We do not have any committed external source of funds. Debt financing or preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise funds through strategic collaborations or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. This may reduce the value of our common stock.
Risks Related to Government Regulation
The FDA requires the completion of a toxicology study of similar duration before trials longer than six months can be conducted such as Phase 3 safety exposure trials for chronic indications or efficacy trials with such six-month endpoints. This may lead to a significant delay in the commencement of long-term clinical trials by us or the failure of our product candidates to obtain marketing approval.
At this time, we can run long-term trials for chronic indications using Nyxol based on our completed 6-month toxicology study using phentolamine mesylate in a ocular-relevant rodent species (rabbit). This 6-month study validates the duration of the registration studies and their safety extensions: a planned 1-year Phase 3 safety exposure trial to support chronic indications of presbyopia and DLD. For APX3330, the drug has already been dosed for more than a year in humans and completed over 15 single- and repeat-dose toxicology studies in rats and dogs (including 2 studies up to 3 months in duration); with this data we initiated our 24-week clinical trial for APX3330 without further toxicology studies being requested by the FDA. We expect to complete further toxicology studies in support of future clinical trials, per FDA’s guidelines, prior to any marketing approval from regulatory authorities for the sale of APX3330. Clinical trials may be delayed due to these regulatory restrictions and additional oversight by the FDA. In addition, the findings in the toxicology studies could affect the outcome of NDA reviews, and, if approved, labels and uses of our product candidates.
Even if we receive marketing approval for our product candidates in the United States, we may never receive regulatory approval to market such product candidates outside of the United States.
In addition to the United States, we intend to seek regulatory approval to market our product candidates in Europe, Japan, Canada, and Australia, and potentially other markets. If we pursue additional product candidates in the future, we may seek regulatory approval of such product candidates outside the United States. In order to market any product outside of the United States, however, we must establish and comply with the numerous and varying safety, efficacy and other regulatory requirements of these other countries. Approval procedures vary among countries and can involve additional product candidate testing and additional administrative review periods. The time required to obtain approvals in other countries might differ from that required to obtain FDA approval. The marketing approval processes in other countries may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. In particular, in many countries outside of the United States, products must receive pricing and reimbursement approval before the product can be commercialized. Obtaining this approval can result in substantial delays in bringing products to market in such countries. Marketing approval in one country does not ensure marketing approval in another, but a failure or delay in obtaining marketing approval in one country may have a negative effect on the regulatory process in others. Failure to obtain marketing approval in other countries or any delay or other setback in obtaining such approval would impair our ability to market our product candidates in such foreign markets. Any such impairment would reduce the size of our potential market, which could have an adverse impact on our business, results of operations and prospects.
Even if we obtain marketing approval for our product candidates, such product candidates could be subject to post-marketing, obligations, restrictions or withdrawal from the market, and we may be subject to substantial penalties if we fail to comply with regulatory requirements or experience unanticipated problems with a product following approval.
Any product candidate for which we, or our future collaborators, obtain marketing approval in the future, as well as the manufacturing processes, post-approval studies and measures, labeling, advertising, and promotional activities for such drug, among other things, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the drug may be marketed or to the conditions of approval, including the requirement to implement a REMS, which could include requirements for a restricted distribution system.
The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of a product candidate. The FDA and other agencies, including the Department of Justice, closely regulate and monitor the post-approval marketing and promotion of drugs to ensure that they are manufactured, marketed, and distributed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we, or any future collaborator, does not market a product candidate for which it receives marketing approval for only its approved indications, we, or the collaborator, may be subject to warnings or enforcement action for off-label promotion. Violation of the Federal Food, Drug, and Cosmetic Act (“FDC Act”) and other statutes, including the False Claims Act, relating to the promotion and advertising of prescription drugs, may lead to investigations or allegations of violations of federal or state healthcare fraud and abuse laws and state consumer protection laws.
In addition, later discovery of previously unknown AEs or other problems with our product candidates or our manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
| • | litigation involving patients taking our drugs; |
| • | restrictions on such drugs, manufacturers, or manufacturing processes; |
| • | restrictions on the labeling or marketing of a drug; |
| • | restrictions on drug distribution or use; |
| • | requirements to conduct post-marketing studies or clinical trials; |
| • | warning letters or untitled letters; |
| • | withdrawal of the drugs from the market; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | product recall or public notification or medical product safety alerts to healthcare professionals; |
| • | fines, restitution, or disgorgement of profits or revenues; |
| • | suspension or withdrawal of marketing approvals; |
| • | damage to relationships with any potential collaborators; |
| • | unfavorable press coverage and damage to our reputation; |
| • | refusal to permit the import or export of drugs; |
| • | injunctions or the imposition of civil or criminal penalties. |
We may seek to avail ourselves of mechanisms to expedite the development or approval for product candidates we may pursue in the future, such as fast track or breakthrough designation, but such mechanisms may not actually lead to a faster development or regulatory review or approval process.
We may seek fast track designation, breakthrough designation, orphan drug designation, priority review, or accelerated approval for product candidates we may pursue in the future. For example, if a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply for FDA fast track designation. However, the FDA has broad discretion with regard to these mechanisms, and even if we believe a particular product candidate is eligible for any such mechanism, we cannot guarantee that the FDA would decide to grant it. Even if we obtain fast track or priority review designation or pursue an accelerated approval pathway, we may not experience a faster development process, review, or approval compared to conventional FDA procedures. The FDA may withdraw a particular designation if it believes that the designation is no longer supported by data from our clinical development program.
A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if we believe a product candidate meets the criteria for designation as a breakthrough therapy, the FDA may disagree and instead determine not to make such designation. We cannot be sure that our evaluation of a product candidate as qualifying for breakthrough therapy designation will meet the FDA’s requirements. In any event, the receipt of a breakthrough therapy designation for a product candidate may not result in a faster development process, review, or approval compared to conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more product candidates qualifies as a breakthrough therapy, the FDA may later decide that the product candidate no longer meets the conditions for qualification or may decide that the time period for FDA review or approval will not be shortened.
Recently enacted and future legislation may increase the difficulty and cost for us and our future collaborators to obtain marketing approval of our product candidates and affect their pricing.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of a product candidate, restrict or regulate post-approval activities and affect our ability, or the ability of our future collaborators, to profitably sell any drug for which we, or they, obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and cause downward pressure on the price that we, or our future collaborators, may charge for any approved drug.
For example, in March 2010, the United States Congress enacted the Patient Protection and Affordable Care Act (“ACA”), and the Health Care and Education Reconciliation Act, or the Healthcare Reform Act, which expanded health care coverage through Medicaid expansion and the implementation of the individual mandate for health insurance coverage and which included changes to the coverage and reimbursement of drug products under government healthcare programs.
There have also been efforts by federal and state government officials or legislators to implement measures to regulate prices or payment for pharmaceutical products, including legislation on drug importation. Recently, there has been considerable public and government scrutiny of pharmaceutical pricing and proposals to address the perceived high cost of pharmaceuticals. There have also been recent state legislative efforts to address drug costs, which generally have focused on increasing transparency around drug costs or limiting drug prices.
General legislative cost control measures may also affect reimbursement for our product candidates. The Budget Control Act, as amended, resulted in the imposition of 2% reductions in Medicare (but not Medicaid) payments to providers in 2013 and will remain in effect through 2027 unless additional Congressional action is taken. Any significant spending reductions affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented and/or any significant taxes or fees that may be imposed on us could have an adverse impact on results of operations.
Adoption of new legislation at the federal or state level could affect demand for, or pricing of, our current or future products if approved for sale. We cannot, however, predict the ultimate content, timing or effect of any changes to the Healthcare Reform Act or other federal and state reform efforts. There is no assurance that federal or state health care reform will not adversely affect our future business and financial results.
There have been judicial and congressional challenges and amendments to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future, as well as efforts to repeal and replace it. In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These new laws have resulted in additional reductions in Medicare and other healthcare funding and otherwise may affect the prices we may obtain for any product candidate for which marketing approval is obtained. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payors. Moreover, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our drugs. Further, on March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap for single source and innovator multiple source drugs, beginning January 1, 2024. In addition, Congress is considering additional health reform measures, such as capping the costs for prescription drugs covered by Medicare Part D and by setting the annual out-of-pocket limit at $2,000 beginning in 2024, as part of other health reform initiatives.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance, or interpretations will be changed, or what the impact of such changes on the marketing approvals of a product candidate, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval or subject us or our future collaborators to more stringent drug labeling and post-marketing testing and other requirements.
Governments outside of the United States tend to impose strict price controls, which may adversely affect our revenue from the sales of a drug, if any.
In some countries, particularly in the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a drug. To obtain reimbursement or pricing approval in some countries, we, or our future collaborators, may be required to conduct a clinical trial that compares the cost-effectiveness of our products to other available therapies. If reimbursement of our drugs is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be harmed.
Our relationships with healthcare providers and third-party payors will be subject to applicable fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, and diminished profits and future earnings, among other penalties and consequences.
Healthcare providers and third-party payors will play a primary role in the recommendation and prescription of any product candidate for which we obtain marketing approval. Our current and future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell, and distribute product candidates for which we obtain marketing approval. Restrictions and obligations under applicable federal and state healthcare laws and regulations include the following:
| • | the federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid; |
| • | the federal false claims and civil monetary penalties laws, including the civil False Claims Act, impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government; |
| • | HIPAA imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and its implementing regulations, also imposes obligations, including mandatory contractual terms, on certain people and entities with respect to safeguarding the privacy, security, and transmission of individually identifiable health information; |
| • | the federal Physician Payments Sunshine Act under the Affordable Care Act requires certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to report specially to the Centers for Medicare & Medicaid Services within the U.S. Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and |
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. Certain state and foreign laws also govern the privacy and security of health information in ways that differ from each other and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our current and future business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations, or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal, and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, disgorgement, individual imprisonment, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil, and administrative sanctions, including exclusions from government funded healthcare programs. Defending against any such actions can be costly, time-consuming, and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We could face criminal liability and other serious consequences for violations which could harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other partners from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties for clinical trials outside of the United States, to sell our products abroad once we enter a commercialization phase, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other partners, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
Our employees or representatives may engage in misconduct or other improper activities, including violating applicable regulatory standards and requirements or engaging in insider trading, which could significantly harm our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to:
| • | comply with the regulations of the FDA and applicable non-U.S. regulators; |
| • | provide accurate information to the FDA and applicable non-U.S. regulators; |
| • | comply with healthcare fraud and abuse laws and regulations in the United States and abroad; |
| • | report financial information or data accurately; or |
| • | disclose unauthorized activities to us. |
In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Employee misconduct could also involve the improper use of, including trading on, information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may be ineffective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal, and administrative penalties, damages, fines, exclusion from government funded healthcare programs such as Medicare and Medicaid, disgorgement, individual imprisonment, contractual damages, reputational harm, diminished profits and future earnings, and the curtailment or restructuring of our operations.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. If found to have improperly promoted off-label uses, we may become subject to significant liability.
The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. If we receive marketing approval for our product candidates for a certain indication, physicians may nevertheless prescribe such products to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If we cannot successfully manage the promotion of our product candidates, if approved, we could become subject to significant liability, which would adversely affect our business and financial condition.
Changes to U.S. tax laws may adversely affect our financial condition or results of operations and create the risk that we may need to adjust our accounting for these changes.
The accounting treatment of these tax law changes is complex, and some of the changes may affect both current and future periods. Consistent with guidance from the SEC, our consolidated financial statements reflect our estimates of the tax effects of the current tax laws and regulation.
Risks Related to Commercialization of Our Product Candidates
We face substantial competition, which may result in others discovering, developing, or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We expect to face competition with respect to our product candidates, if approved, and will face competition with respect to any future product candidates that we may seek to develop or commercialize from major pharmaceutical companies, specialty pharmaceutical companies, biotechnology companies, universities and other research institutions, and government agencies worldwide. The ophthalmic therapies market is highly competitive and dynamic. Our success will depend, in part, on our ability to obtain a share of the market for our planned indications.
APX3330
We are developing APX3330 for use in two different indications initially: the treatment of DR and DME, and potentially later the treatment of wAMD. In addition to currently approved therapies, any product that is developed for either of the three indications could directly compete with APX3330. Such a product could reduce the overall market opportunity for APX3330. Other pharmaceutical companies may develop therapies for the same indications that would compete with APX3330, if approved, and that would not infringe the claims of our in-licensed patents, pending patent applications, or other proprietary rights, which could adversely affect our business and results of operations.
Competition in Diabetic Retinopathy / Diabetic Macular Edema / wAMD
We may face potential competition from both existing therapies and those in development. Current therapies for these retinal diseases rely on suppressing VEGF activity via intravitreal injection or by mitigating the inflammation via intravitreal corticosteroid-releasing implants including:
| • | Lucentis® (ranibizumab) and Avastin® (bevacizumab), which are anti-VEGF monoclonal antibody intravitreal injections, developed by Genentech, Inc and Roche AG. |
| • | EYLEA® (aflibercept), a VEGF inhibitor intravitreal injection, developed by Regeneron Pharmaceuticals. |
| • | Vabysmo® (Faricimab), a bispecific monoclonal antibody targeting VEGF-A and Ang-Tie2 pathway developed by Genentech, Inc and Roche AG. |
| • | Beovu® (Brolucizumab), an anti-VEGF monoclonal antibody intravitreal injection, developed by Novartis AG. |
| • | MACUGEN® (pegaptanib sodium injection), a selective inhibitor of VEGF-165, developed by Bausch + Lomb. |
| • | Ozurdex® (dexamethasone), a corticosteroid IVT implant, developed by Allergan plc. |
| • | Iluvien (fluocinolone acetonide), a corticosteroid IVT implant, developed by Alimera Sciences, Inc |
There are also several pharmacological therapies in development, including:
| • | Abicipar, an anti-VEGF intravitreal injection with a long duration of action, developed by Allergan plc and Molecular Partners. |
| • | KSI-301, an anti-VEGF antibody intravitreal injection coupled with a biopolymer that is intended to increase the time between injections, developed by Kodiak Sciences. |
| • | OPT-302, an intravitreal injection which binds to multiple types of VEGF receptors that could be used with other anti-VEGF agents, developed by Opthea Limited. |
| • | ALG-1001, an integrin peptide therapy intravitreal injection that is being evaluated as a sequential or in-combination therapy with bevacizumab in patients with DME, developed by Allegro Ophthalmics, LLC. |
| • | RG-7774, an orally administered selective CB2 (Cannabinoid 2) receptor agonist that is being evaluated in patients with moderately severe to severe non-proliferative diabetic retinopathy, developed by Hoffmann-LA Roche, AG. |
| • | RZ402, a small molecule selective and potent plasma kallikrein inhibitor (PKI) for the chronic treatment of diabetic macular edema (DME), developed by Rezolute, Inc. |
| • | Xiflam™, an oral small molecule drug for the treatment of dry form of Age-Related Macular Degeneration (AMD), Geographic Atrophy (GA), Diabetic Retinopathy (DR) manifesting Diabetic Macular Edema (DME), developed by InflammX. |
| • | AKST4290, an oral small molecule CCR3 Eotaxin inhibitor for the treatment of diabetic retinopathy and wet AMD. |
| • | BAY1101042, an oral guanylate cycles activator for the treatment of diabetic retinopathy. |
Nyxol
Ocuphire is developing Nyxol, with our partner Viatris, for use in three different indications: the reversal of pharmacologically induced mydriasis (“RM”), the treatment of presbyopia and the treatment of NVD. In addition to currently approved therapies, any product that is developed for any of the three indications could compete with Nyxol. Such a product could reduce the overall market opportunity for Nyxol. Other pharmaceutical companies may develop therapies for the same indications that would compete with Nyxol, if approved, and that would not infringe the claims of Ocuphire’s patents, pending patent applications, or other proprietary rights, which could adversely affect its business and results of operations.
RM
Currently, there are no available and approved pharmacological therapies for NVD or RM and Ocuphire is not aware of any in development. Rev-Eyes® (dapiprazole), an alpha-1 antagonist, was approved by the FDA in 1990 to reverse mydriasis induced by adrenergic or anticholinergic agents. Rev-Eyes was withdrawn in the past from the market for reasons unrelated to safety or efficacy, according to the FDA.
Presbyopia
The FDA approved VuityTM eye drop for the treatment of presbyopia in October 2021. Vuity was launched in December 2021 and is marketed by Allergan, an AbbVie company. The competition also includes reading glasses, multifocal contact lenses, and monovision contact lenses (e.g., where one eye wears a near vision lens and the other eye wears a distance vision lens). Ocuphire will also compete against several pharmacological therapies in development for the temporary treatment of presbyopia, many of which are cholinergic agonist-based pupil management therapies, including:
| • | CSF-1, with low dose pilocarpine and a secondary agent (lubricant), developed by Orasis Pharmaceuticals Ltd. |
| • | LNZ100 and LNZ101, with aceclidine (another miotic agent), developed by Lenz Therapeutics. |
| • | MicroLine®, which is a micro-dose delivery of pilocarpine using proprietary device developed by Eyenovia, Inc. |
| • | KT-101, which uses pilocarpine in the AcuStream delivery system, developed by Kedalion Therapeutics, Inc. |
| • | BrimocholTM, with brimonidine and carbachol (both are miotic agents), developed by Visus Therapeutics, Inc. |
| • | UNR844, which uses a mechanism that involves softening the lens to increase near visual acuity, developed by Novartis AG (originally Encore Vision, Inc.). |
There are approved devices for presbyopia. One of these is the KAMRA Inlay, developed by AcuFocus, Inc. and marketed by SightLife Surgical, Inc. Another is the eyelike NoanPinhole, developed by Koryo Eyetech, the first commercially available pinhole soft contact lens. Nyxol would not directly compete against these devices, but rather would be a non-invasive alternative for presbyopes who are averse to surgical intervention.
Our competitors may develop products that are more effective, safer, more convenient, or less costly than any that we are developing, or that would render our product candidates obsolete or non-competitive. Our competitors may also render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process. Our competitors may also obtain marketing approval from the FDA or other regulatory authorities for their products more rapidly than we obtain approval for our products, which could result in our competitors establishing a strong market position before we are able to enter the market.
Many of our competitors have significantly greater name recognition, financial resources, and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These companies compete with us in recruiting, hiring, and retaining qualified scientific and management personnel, engaging contract service providers, manufacturers and consultants, establishing clinical trial sites, recruiting patients for clinical trials, and entering into strategic transactions, as well as in acquiring technologies complementary to, or necessary for, our programs.
We lack experience in commercializing products, which may have an adverse effect on our business.
If APX3330 receives marketing approval, we will need to transition from a company with a development focus to a company capable of supporting commercial activities, and we may not be successful in making that transition. We have not yet demonstrated the ability to obtain marketing approval for, or to commercialize, any product candidate. As a result, our clinical development and regulatory approval activities, and our ability to successfully commercialize any approved products, may involve more inherent risk, take longer, and cost more than would be the case if we were a company with experience obtaining marketing approval for and commercializing a product candidate.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell, market, and distribute APX3330, if approved, we may not be successful in commercializing APX3330 if and when it is approved.
We do not have any sales or marketing infrastructure and have no capabilities in place at the present time for the sale, marketing, or distribution of pharmaceutical products. To achieve commercial success for any approved product for which we retain sales and marketing responsibilities, we must either develop a sales and marketing organization or outsource part or all of these functions to other third parties.
There are risks involved with us both establishing our own sales and marketing capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time-consuming, which could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
| • | the inability to recruit and retain adequate numbers of effective sales and marketing personnel or enter into distribution agreements with third parties; |
| • | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe our product candidate; |
| • | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; |
| • | unforeseen costs and expenses associated with creating an independent sales and marketing organization; and |
| • | the inability to obtain sufficient coverage and reimbursement from third-party payors and governmental agencies. |
| • | If we enter into arrangements with third parties to perform sales, marketing, and distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower than if we were to market and sell a product that we developed ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market any product candidate or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market a drug effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates. |
Our future commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among physicians, patients, third-party payors, and others in the medical community.
Even if our product candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, healthcare payors, or others in the medical community. If such product candidates do not achieve an adequate level of acceptance, we may not generate significant product revenues and may not become profitable. The degree of market acceptance of a product candidate, if approved for commercial sale, will depend on a number of factors, including:
| • | efficacy and potential advantages compared to alternative treatments; |
| • | the ability to offer our product for sale at competitive prices; |
| • | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| • | any restrictions on the use of our product together with other medications; |
| • | interactions of our product with other medicines patients are taking; |
| • | inability of certain types of patients to take our product; |
| • | demonstrated ability to treat patients and, if required by any applicable regulatory authority in connection with the approval for target indications as compared with other available therapies; |
| • | the relative convenience and ease of administration as compared with other treatments available for approved indications; |
| • | the prevalence and severity of any adverse side effects; |
| • | limitations or warnings contained in the labeling approved by the FDA; |
| • | availability of alternative treatments already approved or expected to be commercially launched in the near future; |
| • | the effectiveness of our sales and marketing strategies; |
| • | our ability to increase awareness through marketing efforts; |
| • | guidelines and recommendations of organizations involved in research, treatment and prevention of various diseases that may advocate for alternative therapies; |
| • | our ability to obtain sufficient third-party coverage and adequate reimbursement; |
| • | the willingness of patients to pay out-of-pocket in the absence of third-party coverage; and |
| • | physicians or patients may be reluctant to switch from existing therapies even if potentially more effective, safe or convenient. |
We have not yet sold any of our products. We cannot assure investors that there is a sufficient market demand for our products. Achieving market acceptance for our products will require substantial marketing efforts and expenditure of funds to create awareness and demand by participants in the industry. We have not conducted any independent market research to determine the extent of any demand that exists for the products to be provided by us and there is no guarantee that a sufficient interest in the market will exist for the products and services being produced by, or for, us. Any lack of sufficient demand for the products contemplated to be provided by us will have a material adverse effect on us.
If the FDA or a comparable foreign regulatory authority approves generic versions of our product candidates that receive marketing approval, or if such authorities do not grant our product candidates appropriate periods of exclusivity before approving generic versions of our products, the sales of our products could be adversely affected.
Once an NDA is approved, the product covered thereby becomes a “reference listed drug” in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluations.” Manufacturers may seek approval of generic versions of reference listed drugs through submission of abbreviated new drug applications (“ANDAs”) in the United States. In support of an ANDA, a generic manufacturer need not conduct clinical studies. Rather, the applicant generally must show that its product has the same active ingredient(s), dosage form, strength, route of administration, and conditions of use or labeling as the reference listed drug (“RLD”) and that the generic version is bioequivalent to the RLD, meaning it is absorbed in the body at the same rate and to the same extent. Generic products may be significantly less costly to bring to market than the RLD, and companies that produce generic products are generally able to offer them at lower prices. Thus, following the introduction of a generic drug, a significant percentage of the sales of any branded product or RLD may be lost to the generic product.
The FDA may not approve an ANDA for a generic product until any applicable period of non-patent exclusivity for the reference listed drug has expired. The FDC Act provides a period of five years of non-patent exclusivity for a new drug containing a new chemical entity (“NCE”). Specifically, in cases where such exclusivity has been granted, an ANDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification that a patent covering the reference listed drug is either invalid or will not be infringed by the generic product, in which case the applicant may submit its application four years after approval of the RLD. It is unclear whether the FDA will treat the active ingredients in its product candidates as NCEs and, therefore, afford them five years of NCE exclusivity if they are approved. If any product we develop does not receive five years of NCE exclusivity, we may nonetheless be eligible for three years of exclusivity, which means that the FDA may approve generic versions of such product three years after its date of approval. Manufacturers may seek to launch these generic products following the expiration of the applicable marketing exclusivity period, even if we still have patent protection for our product.
Competition that our product candidates would face from generic versions could materially and adversely impact our future revenue, profitability, and cash flows and substantially limit our ability to obtain a return on the investments we have made in any such product candidate.
Even if we are able to commercialize APX3330, our profitability will likely depend in significant part on third-party reimbursement practices, which, if unfavorable, would harm our business.
Our ability to commercialize APX3330 successfully will depend in part on the extent to which coverage and adequate reimbursement will be available from government health administration authorities, private health insurers and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. Government authorities and third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for certain medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that coverage will be available for any product candidate that we commercialize and, if coverage is available, whether the level of reimbursement will be adequate. Assuming we obtain coverage for our product candidates, if approved, by a third-party payor, the resulting reimbursement payment rates may not be adequate or may require co-payments that patients find unacceptably high. Patients who are prescribed medications for the treatment of their conditions, and their prescribing physicians, generally rely on third-party payors to reimburse all or some of the costs associated with their prescription drugs. Patients are unlikely to use a product candidate, if approved, unless coverage is provided and reimbursement is adequate to cover all or a significant portion of the cost of its products. Therefore, coverage and adequate reimbursement are critical to new product acceptance. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval. Furthermore, drug pricing and access policies in the United States and internationally may change and negatively impact our product candidates’ commercial viability. Proposed policy changes, including the potential for Medicare to negotiate with drug manufacturers, may limit our ability to competitively price our product candidates, if approved.
There may be significant delays in obtaining reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which a product candidate is approved by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale, and distribution. Interim reimbursement levels for a new product, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the product and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost medicines, and may be incorporated into existing payments for other services. Net prices for products may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of medicines from countries where they may be sold at lower prices than in the United States. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. However, there is no uniform policy requirement for coverage and reimbursement for drug products among third-party payors in the United States. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often time-consuming and costly, and it will require us to provide scientific and clinical support for the use of our products to each payor separately. There is no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance.
Any inability to promptly obtain coverage and profitable payment rates from government-funded or private payors for any approved products that we develop could have an adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our overall financial condition.
Product liability lawsuits against us, or our suppliers and manufacturers, could cause us to incur substantial liabilities and could limit commercialization of any product candidate that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. Product liability claims might be brought against us by patients, healthcare providers, or others selling or otherwise coming into contact with our product candidates during product testing, manufacturing, marketing, or sale. For example, we may be sued under allegations that a product candidate caused injury or that the product was otherwise unsuitable. Any such product liability claims may include allegations of manufacturing or design defects, failure to warn of dangers inherent in the product, such as interactions with alcohol or other drugs, negligence, or breach of warranty. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against claims that our product candidate caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for any product candidate that we are developing; |
| • | injury to our reputation and significant negative media attention; |
| • | withdrawal of clinical trial participants; |
| • | increased FDA warnings on product labels; |
| • | significant costs to defend the related litigation; |
| • | substantial monetary awards to trial participants or patients; |
| • | distraction of management’s attention from our primary business; |
| • | the inability to commercialize any product candidate that we may develop. |
Our product liability and/or clinical trial insurance coverage may not be adequate to cover all liabilities that we may incur. We may need to increase our insurance coverage as we expand clinical trials and if we successfully commercialize our product candidates. Insurance coverage is increasingly expensive, and we may not be able to obtain product liability insurance on commercially reasonable terms or for a sufficient amount to satisfy liabilities that may arise.
Similarly, we may be a party to, or may be otherwise responsible for, pending or threatened lawsuits or other claims related to products purchased from our manufacturers and suppliers. Although we intend to require our providers to have product liability insurance, the ability to obtain such coverage and the sufficiency thereof is uncertain. Such cases and claims may raise difficult and complex factual and legal issues and may be subject to many uncertainties and complexities, including, but not limited to, the facts and circumstances of each particular case or claim, the jurisdiction in which each suit is brought, and differences in applicable law. Such litigation could result in additional expense and exposure in excess of our anticipated reserves, especially if such matters are not covered by insurance. Upon resolution of any pending legal matters or other claims, we may incur charges in excess of established reserves. Product liability lawsuits and claims, safety alerts or product recalls in the future, regardless of their ultimate outcome, could have a material adverse effect on the business and reputation and on our ability to attract and retain customers and strategic partners. The business, profitability and growth prospects could suffer if we face such negative publicity.
If we or our third-party manufacturers fail to comply with environmental or health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have an adverse effect on the success of our business.
Our research and development activities involve the controlled use of potentially hazardous substances, including chemical and biological materials, by ourselves and our third-party manufacturers. Our manufacturers are subject to federal, state, and local laws and regulations in the United States and abroad governing laboratory procedures and the use, manufacture, storage, handling, and disposal of medical and hazardous materials. Although we believe that our manufacturers’ procedures for using, handling, storing, and disposing of these materials comply with legally prescribed standards, we cannot eliminate the risk of contamination or injury resulting from medical or hazardous materials. As a result of any such contamination or injury, we may incur liability, or federal, state, city, or local authorities may curtail our use of these materials and interrupt our business operations. In the event of an accident, we could be held liable for damages or fined, and such liability or fines could exceed our resources. We do not have insurance for liabilities arising from medical or hazardous materials. Although we maintain workers’ compensation insurance for costs and expenses that we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. Compliance with applicable environmental and health and safety laws and regulations is expensive, and current or future environmental regulations may impair our research, development, and production efforts, which could harm our business, prospects, financial condition, or results of operations.
Federal legislation and actions by state and local governments could permit reimportation of drugs from foreign countries into the United States, which could adversely affect our operating results when the drugs are sold at lower prices in foreign countries than in the United States.
We may face competition for our product candidates, if approved, from other therapies sourced from foreign countries that have price controls on pharmaceutical products. The Medicare Modernization Act contains provisions that may change U.S. reimportation laws and expand pharmacists’ and wholesalers’ ability to import cheaper versions of approved drugs or competing products from Canada, where there are government price controls. These changes to U.S. importation laws would not take effect unless and until the Secretary of Health and Human Services certifies that the changes would pose no additional risk to the public’s health and safety and would result in a significant reduction in the cost of products to consumers. The Secretary of Health and Human Services has so far declined to approve a reimportation plan. Proponents of drug reimportation may attempt to pass legislation that would directly allow reimportation under certain circumstances. Legislation or regulations allowing the reimportation of drugs, if enacted, could decrease the price we receive for any product we may develop and adversely affect our future revenues and prospects for profitability.
Risks Related to Our Reliance on Third Parties
We will be unable to control all aspects of our non-clinical studies and our clinical trials due to our reliance on CROs and other third parties that assist us in conducting non-clinical studies and clinical trials.
We rely on third-party CROs and other third parties to assist in managing, monitoring, and otherwise carrying out our non-clinical studies and clinical trials. We expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions, and clinical investigators, to conduct our non-clinical studies and clinical trials in the future, including our Phase 3 development program for Nyxol. We compete with many other companies for the resources of these third parties.
As a result, we will have limited control over the conduct, timing, and completion of these non-clinical studies and clinical trials and the management of data developed through the non-clinical studies and clinical trials. We have experienced in the past, and may experience in the future, schedule disruptions due to events affecting the performance of third parties on which we rely. Communicating with outside parties can also be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Additionally, other unexpected natural events and disruptions in the supply chain and operations may affect the ability of third parties to fulfill their obligations to us. Outside parties may:
| • | have staffing difficulties; |
| • | fail to comply with contractual obligations; |
| • | experience regulatory compliance issues; |
| • | undergo changes in ownership or management; |
| • | undergo changes in priorities or become financially distressed; or |
| • | form relationships with other entities, some of which may be our competitors. |
These factors may adversely affect the willingness or ability of third parties to conduct our clinical trials and may subject us to unexpected cost increases that are beyond our control.
While our reliance on these third parties for research and development activities will reduce our control over these activities, it will not relieve us of our responsibilities and requirements. For example, the FDA requires us to comply with standards, commonly referred to as good clinical practices (“GCP”), for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of clinical trial participants are protected.
Problems with the timeliness or quality of the work of any CRO may lead us to seek to terminate our relationship with any such CRO and use an alternative service provider. Making this change may be costly or delay our clinical trials, and contractual restrictions may make such a change difficult or impossible. If we must replace any CRO that is conducting our clinical trials, our clinical trials may have to be suspended until we find another CRO that offers comparable services. The time that it would take us to find alternative organizations may cause a delay in the commercialization of our product candidates, or it may cause us to incur significant expenses to replicate any lost data. Although we do not believe that any CRO on which we would rely would offer services that are not available elsewhere, we may be difficult to find a replacement organization that can conduct our clinical trials in an acceptable manner and at an acceptable cost. Any delay in or inability to complete our clinical trials could significantly compromise our ability to secure regulatory approval for our product candidates and preclude our ability to commercialize our product candidates, thereby limiting or preventing our ability to generate sales revenue.
We rely completely on third parties to supply and manufacture bulk drug substances and to formulate and package preclinical and clinical drug supplies of our product candidates as well as to conduct analytical testing of drug substances and products in the manufacturing processes and intend to rely on third parties to produce and test commercial supplies of our current and any future product candidates.
We do not currently have, nor do we plan to acquire, the infrastructure or capability to internally manufacture our clinical drug supply of product candidates for use in the conduct of our preclinical studies and clinical trials. We lack the internal resources and the capability to manufacture any product candidates on a clinical or commercial scale. The process of manufacturing drug products is complex, highly regulated, and subject to several risks. For example, the facilities used by our contract manufacturers to manufacture and conduct analytical testing of the active pharmaceutical ingredient (or drug substance) and final drug product for product candidates must be inspected by the FDA and other comparable foreign regulatory agencies in connection with our submission of an NDA or relevant foreign regulatory submission to the applicable regulatory agency. In addition, the manufacturing of drug substance or product is susceptible to product loss due to contamination, equipment failure, improper installation or operation of equipment, or vendor or operator error. Moreover, the manufacturing facilities in which product candidates are made could be adversely affected by equipment failures, labor shortages, natural disasters, power failures, or other factors. Manufacturing timelines may be negatively affected by material shortages, construction delays and supply chain challenges due to, among other factors, global supply chain shortages .
We do not control the manufacturing and testing processes of our contract manufacturers and analytical labs, and are completely dependent on them to comply with current good manufacturing practices (“cGMP”) for manufacture and good lab practices (“GLP”) of both active drug substances and finished drug products. If our contract manufacturers and analytical labs cannot successfully manufacture and test materials that conform to our specifications and the strict regulatory requirements of the FDA or applicable foreign regulatory agencies, we will not be able to secure and/or maintain regulatory approval for our products. In addition, we have no control over our contract manufacturers’ and analytical labs’ ability to maintain adequate quality control, quality assurance, and qualified personnel. Failure to satisfy the regulatory requirements for the production and testing of those materials and products may affect the regulatory clearance of our contract manufacturers’ and analytical labs’ facilities generally. If the FDA or a comparable foreign regulatory agency does not approve these facilities for the manufacture and testing of product candidates, or if it withdraws its approval in the future, we may need to find alternative manufacturing and testing facilities, which would adversely impact our ability to develop, obtain regulatory approval for, or market product candidates. Furthermore, all of our contract manufacturers and analytical labs are engaged with other companies to supply and/or manufacture and/or test materials or products for such companies, which exposes our manufacturers to regulatory and sourcing risks for the production of such materials and products. To the extent practicable, our attempts to identify more than one supplier. However, some raw materials are available only from a single source or only one supplier has been identified, even in instances where multiple sources exist.
We have relied and will rely upon third-party manufacturers and testing labs in the United States and overseas for the manufacture and testing of Nyxol and APX3330 for preclinical and clinical testing purposes and intend to continue to do so in the future for Nyxol, APX3330, Nyxol with adjunctive low-dose pilocarpine, and any other product candidates, including for commercial purposes. If our third-party manufacturers and analytical labs are unable to supply or test drug substance and/or drug product on a commercial basis, we may not be able to successfully produce and market product candidates, if approved, or we could be delayed in doing so. For instance, we presently rely on one supplier in Italy for the drug substance for Nyxol, and one manufacturer in India for APX3330 drug substance. If there is any delay or problem with the manufacture of these drug substances or if there is a delay in producing finished drug product from these drug substances, the development and possible approval of our product candidates and potential commercial launch may be delayed or otherwise adversely affected. We will rely on comparison of product specifications (identity, strength, quality, and potency) to demonstrate equivalence of the current drug substance and/or drug product to the drug substance and/or drug product used in previously completed preclinical and clinical testing. If we are unable to demonstrate such equivalence, we may be required to conduct additional preclinical and/or clinical testing of our product candidates. The formulation of the low-dose pilocarpine as adjunctive product candidate with Nyxol is still in development. We have already experienced a few interruptions in our manufacturing, supply chain, research and development operations, regulatory and financial position, including, for example, the shipment of active pharmaceutical ingredient supply from overseas.
Due to these and other potential problems, we are exploring the possibility of establishing additional sources of supply, with U.S. manufacturers, for the active pharmaceutical ingredients of both Nyxol and APX3330. Establishing these additional sources, including qualifying their manufacturing processes and demonstrating the equivalence of their products, may be costly, time-consuming, and difficult to effectuate, and may delay our research and development activities. If we must replace any manufacturer, our research and development activities may have to be suspended until we find another manufacturer that offers comparable services. The time that it takes us to find alternative organizations may cause a delay in the development and commercialization of product candidates.
We have entered into the Nyxol License Agreement and may form or seek additional strategic alliances or enter into licensing arrangements in the future, and may not realize benefits from such alliances or licensing arrangements.
We have entered into the Nyxol License Agreement, and may form or seek additional strategic alliances, create joint ventures or collaborations or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to product candidates. Any of these relationships may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, or issue securities that dilute our existing stockholders, which may disrupt our management and business. Our likely collaborators include large, mid-size, regional, or national pharmaceutical companies and biotechnology companies. If we enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of product candidates. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. We cannot be certain that, following a strategic transaction or license, we will achieve the revenue or specific net income that justifies such transaction. Collaborations involving product candidates pose the following risks to us:
| • | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| • | collaborators may not perform their obligations as expected; |
| • | collaborators may not pursue development and commercialization or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| • | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials, or require a new formulation of a product candidate for clinical testing; |
| • | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidate if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more attractive than ours; |
| • | a collaborator with marketing and distribution rights to one or more product candidates may not commit sufficient resources to the marketing or distribution of any such product candidate; |
| • | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to litigation; |
| • | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| • | disputes may arise between us and collaborators that result in the delay or termination of research, development, or commercialization of our product candidates, or in litigation or arbitration that diverts management attention and resources; |
| • | we may lose certain valuable rights under circumstances identified in our collaborations, including if we undergo a change of control; |
| • | collaborations may be terminated and such terminations may create a need for additional capital to pursue further development or commercialization of the applicable product candidates; |
| • | collaborators may learn about our discoveries and use this knowledge to compete with us in the future; |
| • | the results of collaborators’ preclinical or clinical studies could harm or impair other development programs; |
| • | there may be conflicts between different collaborators that could negatively affect those collaborations and potentially others; |
| • | the number and nature of our collaborations could adversely affect our attractiveness to potential future collaborators or acquirers; |
| • | collaboration agreements may not lead to development or commercialization of our product candidate in the most efficient manner or at all. If a present or future collaborator of us were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program under such collaboration could be delayed, diminished, or terminated; and |
| • | collaborators may be unable to obtain the necessary marketing approvals. |
If future collaboration partners fail to develop or effectively commercialize product candidates for any of these reasons, such product candidates may not be approved for sale and our sales of such product candidates, if approved, may be limited, which would have an adverse effect on our operating results and financial condition.
If we are not able to establish new collaborations for APX3330 on commercially reasonable terms, we may have to alter our development, manufacturing, and commercialization plans.
We face significant competition in attracting collaborators for development, manufacturing or commercialization plans. Whether we reach a definitive agreement for collaboration for APX3330 will depend, among other things, upon our assessment of the proposed collaborator’s resources, expertise, and evaluation of a number of factors related to the associated product candidate, as well as the terms and conditions of the proposed collaboration. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which may exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available for collaborations and whether such a collaboration could be more attractive than one with us. We may not be able to enter into these agreements on commercially reasonable terms, or at all.
Much of the potential revenue from future commercial collaborations may consist of contingent payments, such as payments for achieving regulatory milestones or royalties payable on sales of our product candidate, if approved. The milestone and royalty revenue that we may receive under these collaborations would depend upon our collaborators’ ability to successfully develop, introduce, market and sell our product candidate, if approved. In addition, collaborators may decide to enter into arrangements with third parties to commercialize products developed under collaborations related to our product candidates, which could reduce the milestone and royalty revenue received, if any.
We may also be restricted under existing collaboration agreements from entering into future agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaborations on a timely basis and on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of the product candidate for which we are seeking to collaborate, reduce or delay our development program or that of one or more of our other development programs, delay our potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidate or bring us to market and generate product revenue.
If we engage in acquisitions, in-licensing or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities and subject us to other risks.
We may engage in various acquisitions and strategic partnerships, including licensing or acquiring complementary products, intellectual property rights, technologies, or businesses. Any acquisition or strategic partnership may entail numerous risks, including:
| • | increased operating expenses and cash requirements; |
| • | the assumption of indebtedness or contingent liabilities; |
| • | the issuance of our equity securities which would result in dilution to our stockholders; |
| • | assimilation of operations, intellectual property, products and product candidates of an acquired company, including difficulties associated with integrating new personnel; |
| • | the diversion of management’s attention from our existing product candidates and initiatives in pursuing such an acquisition or strategic partnership; |
| • | retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships; |
| • | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals; and |
| • | our inability to generate revenue from acquired intellectual property, technology and/or products sufficient to meet our objectives or even to offset the associated transaction and maintenance costs. |
In addition, if we undertake such a transaction, we may incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain sufficient patent protection for our product candidates, our competitors could develop and commercialize products or technology similar or identical to those of us, which would adversely affect our ability to successfully commercialize any product candidates we may develop, our business, results of operations, financial condition and prospects.
We primarily protect our intellectual property through a combination of patents and patent applications on inventions, trademark protection on our product name, and trade secret protection as we deem appropriate.
As of February 10, 2023, our patent estate relating to the Nyxol contains ten U.S. patents, eight pending U.S. non-provisional patent applications, as well as issued patents in Australia, Canada, Europe, Japan, and Mexico and pending patent applications in Australia, Canada, Europe, Japan, China, and other foreign countries, all of which are owned by us.
Our U.S. Patents 9,795,560; 10,278,918; 10,772,829, 11,090,261, 11,566,005 and counterpart Australian, Canadian, European, and Japanese patents each contain composition of matter claims to aqueous phentolamine mesylate formulations and are scheduled to expire in year 2034. In the same patent family, we also have 1 pending U.S. patent application with additional claims to aqueous phentolamine mesylate formulations, whereby a patent, if granted, based on this patent application would expire in year 2034. The patents and patent applications cover the current clinical formulation for the Nyxol product.
Our U.S. Patent Nos. 9,089,560; 9,789,088; and 11,000,509 contain claims directed to methods of improving visual performance using, for example, phentolamine mesylate and are scheduled to expire in year 2034. Counterpart patents have issued in Australia, Canada, Europe and Japan, which are scheduled to expire in year 2034. In the same patent family, we also have 1 pending U.S. patent application with additional claims to methods of improving visual performance using, for example, phentolamine mesylate, whereby a patent, if granted from this pending patent application, would expire in year 2034. The patents and patent applications cover uses of the current clinical formulation for the Nyxol product.
We have patent applications pending in the U.S., Australia, Canada, China, Europe, and Japan directed to treating glaucoma and other medical disorders using phentolamine mesylate. Patents, if granted, based on these pending applications would expire in year 2039.
Our U.S. Patent 10,993,932 contains claims directed to methods of treating presbyopia using phentolamine mesylate in combination with pilocarpine and is scheduled to expire in year 2039. Our U.S. Patent 11,400,077 contains claims directed to methods of treating mydriasis using phentolamine mesylate and is scheduled to expire in year 2039. In the same patent family as U.S. Patent Nos. 10,993,932 and 11,400,077 , we have four pending U.S. patent applications, two of which have claims to treating presbyopia and the other two U.S. application have claims to treating mydriasis. Counterpart patent applications are pending in Australia, Canada, China, Europe, Japan, and other foreign countries, whereby a patent, if granted, based on these pending U.S. and foreign patent applications would expire in year 2039.
We have one U.S. Patent 11,566,005, a pending U.S. patent application, and a pending international patent application directed to phentolamine mesylate composition of matter and methods of making high-purity phentolamine mesylate, and compositions with claimed phentolamine mesylate for the treatment of presbyopia, dim light or night vision disturbances and others. Our U.S. patent 11,566,005 claims include phentolamine mesylate composition of matter, topical ophthalmic composition containing 1% of the claimed phentolamine mesylate composition of matter and methods of use for the claimed composition in presbyopia, dim light and night vision disturbances and treatment of pharmacologically induced mydriasis. This patent and other patents based on the foregoing patent applications in its class if granted, are scheduled to expire in year 2042. We also have one pending patent application in China directed to methods of making high-purity phentolamine mesylate and compositions resulting from such methods, whereby any patents, if granted, based on this patent application in China would expire in year 2041. We also have a pending international patent application directed to additional methods for treating mydriasis and glaucoma, whereby any U.S. or foreign patents, if granted, from on a patent application filed based on this international patent application would expire in year 2042
We also own an issued patent in Mexico that is scheduled to expire in year 2025 and has claims to ophthalmic formulations.
We have in-licensed a patent estate directed to APX3330 and related compounds that, as of February 10, 2023, contains seven U.S. patents, two pending U.S. non-provisional patent applications, as well as issued patents in Europe, Japan, Canada, and Australia, and pending patent applications in Europe, Japan, Canada, China, South Korea and Australia. Our in-licensed U.S. patent 9,040,505 has claims to methods of treating diabetic retinopathy and other diseases using, for example, APX3330 and is scheduled to expire in year 2030. Counterpart patents have issued in Europe, Japan, Australia, and Canada, which are scheduled to expire in year 2028, and there is a related pending U.S. patent application with method of treatment claims that, if issued as a patent, would expire in year 2028. Our in-licensed pending U.S. patent application 16,968,009 and pending applications in Europe, Japan, Canada, South Korea and Australia have claims to methods of treating wAMD and other diseases using, for example, APX3330, whereby patents, if granted based on these pending patent applications, would expire in year 2039. Our in-licensed patent applications directed to a combination therapy composition comprising an APE1/REF-1 inhibitor, such as APX3330, and a second therapeutic agent, and are pending in the U.S. and Canada, whereby patents, if granted based on these pending patent applications, would expire in year 2038. In-licensed patent applications directed to use of an APE1/REF-1 inhibitor, such as APX3330, in monotherapy or combination therapy to reduce neuronal sensitivity and/or treat other indications are pending in Europe, Japan, and Canada, whereby patents, if granted based on these applications, would expire in year 2038. This same patent family includes one in-licensed U.S. patent directed to methods using APX3330 to treat inflammation and pain as part of a combination therapy. Patents to derivatives of APX3330 have been issued in the U.S., Europe, and other countries that are scheduled to expire from year 2028 to 2032.
In addition to patents and patent applications that we have in-licensed, as of February 10, 2023, we own one pending international patent application directed to methods of treating diabetic retinal diseases using APX3330. Patents, if granted, from an application filed based on this pending international patent applications would expire in year 2042. Additionally, we own one pending U.S. provisional patent application directed to certain salt forms of APX3330 and methods of use, whereby any patents, if granted, from an application filed based on this provisional patent application would expire in year 2043, and we own one pending U.S. provisional patent application directed to additional therapeutic methods using APX3330 in patients with diabetic retinal disease, whereby any patents, if granted, from an application filed based on this provisional patent application would expire in year 2044.
The patent prosecution process is expensive and time-consuming, and we and our future licensors, licensees, or collaboration partners may not be able to prepare, file, and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we or any future licensors, licensees, or collaboration partners may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. We and our licensors’ patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent is issued from such applications, and then only to the extent the issued claims cover the technology.
We cannot assure you that any of our patents have matured, or that any of our pending patent applications will mature, into issued patents that will include, claims with a scope sufficient to protect our product candidates. Others have developed technologies that may be related or competitive to our approach, and may have filed or may file patent applications and may have received or may receive patents that overlap or conflict with our patent applications, for example by claiming the same compounds, methods or formulations or by claiming subject matter that could dominate the patents that we owns or in-licenses. The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, the issuance, scope, validity, and enforceability of any patent claims that we may obtain cannot be predicted with certainty. Patents, if issued, may be challenged, deemed unenforceable, invalidated, or circumvented. U.S. patents and patent applications may also be subject to interference proceedings, ex parte reexamination, or inter partes review proceedings, supplemental examination and challenges in district court. Patents may be subjected to opposition, post-grant review, or comparable proceedings in various national and regional patent offices. These proceedings could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such interference, re-examination, opposition, post-grant review, inter partes review, supplemental examination, or revocation proceedings may be costly or time-consuming. Thus, any patents that we may own or exclusively license may not provide any protection against competitors. Furthermore, an adverse decision in an interference proceeding can result in a third party receiving the patent right sought by us, which in turn could affect our ability to develop, market or otherwise commercialize our product candidates.
Furthermore, the issuance of a patent, while presumed valid, is not conclusive as to its validity or its enforceability and it may not provide us with adequate proprietary protection or competitive advantages against competitors with similar products. Competitors may also be able to design around our patents. Other parties may develop and obtain patent protection for more effective technologies, designs, or methods. We may not be able to prevent the unauthorized disclosure or use of any technical knowledge or trade secrets by consultants, vendors, former employees, or current employees. The laws of some foreign countries do not protect proprietary rights to the same extent as do the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries. If these developments were to occur, they could have a material adverse effect on our sales.
Our ability to enforce our patent rights depends on our ability to detect infringement. It is difficult to detect infringers who do not advertise the components that are used in their products. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product. Any litigation to enforce or defend our patent rights, if any, even if we were to prevail, could be costly and time-consuming and would divert the attention of management and key personnel from our business operations. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded if we were to prevail may not be commercially meaningful.
In addition, proceedings to enforce or defend our patents could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly. Such proceedings could also provoke third parties to assert claims against us, including that some or all of the claims in one or more of our patents are invalid or otherwise unenforceable. If, in any proceeding, a court invalidated or found unenforceable our patents covering our product candidates, our financial position and results of operations would be adversely impacted. In addition, if a court found that valid, enforceable patents held by third parties covered our product candidates, our financial position and results of operations would also be adversely impacted.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| • | any of our patents, or any of our pending patent applications, if issued, will include claims having a scope sufficient to protect our product candidates; |
| • | any of our pending patent applications will result in issued patents; |
| • | we will be able to successfully commercialize our product candidates, if approved, before our relevant patents expire; |
| • | we were the first to make the inventions covered by each of our patents and pending patent applications; |
| • | we were the first to file patent applications for these inventions; |
| • | others will not develop similar or alternative technologies that do not infringe our patents; |
| • | any of our patents will be valid and enforceable; |
| • | any patents issued to us will provide a basis for an exclusive market for our commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
| • | we will develop additional proprietary technologies or product candidates that are separately patentable; or |
| • | our commercial activities or products will not infringe upon the patents of others. |
Patents have a limited lifespan. The natural expiration of a patent is generally 20 years after its effective filing date. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Given the extensive period of time between patent filing and regulatory approval for a product candidate, the time during which we can market a product candidate under patent protection is limited, and our patent may expire before we obtain such approval. Without patent protection for our product candidates, we may be vulnerable to competition from generic versions of our product candidates, which may affect the profitability of our product candidates.
If we do not obtain protection under the Hatch-Waxman Act and similar foreign legislation by extending the patent terms and obtaining data exclusivity for our product candidate, our business may be materially harmed.
Depending upon the timing, duration of regulatory review, and date of FDA marketing approval of our APX3330 or other product candidates, if any, one of such U.S. patents may be eligible for patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. The Hatch-Waxman Act provides for a patent restoration term, or patent term extension, of up to five years as compensation for the time the product is under FDA regulatory review. The duration of patent term extension is calculated based on the time spent in the regulatory review process. In the future, we may plan to seek patent term extension for one or more of our patents related to our APX3330 or other product candidates. However, we may not be granted an extension because of, for example, failing to apply within the applicable deadline, expiration of relevant patents prior to obtaining approval, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be shorter or less than what we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, our revenue could be reduced, possibly materially.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
In 2011, the United States enacted wide-ranging patent reform legislation with the America Invents Act (“AIA”).
An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before we could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application, but circumstances could prevent us from promptly filing patent applications on our inventions.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. The AIA and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Additionally, the U.S. Supreme Court’s holdings in several patent cases in recent years, such as Association for Molecular Pathology v. Myriad Genetics, Inc. (Myriad I), Mayo Collaborative Services v. Prometheus Laboratories, Inc., and Alice Corporation Pty. Ltd. v. CLS Bank International, have narrowed the scope of patent protection available in certain circumstances or weakened the rights of patent owners in certain situations. In addition to increasing uncertainty about our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may not be able to protect or practice our intellectual property rights throughout the world.
In jurisdictions where we have not obtained patent protection, competitors may use our intellectual property to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where it is more difficult to enforce a patent as compared to the United States. Competitor products may compete with our product candidates in jurisdictions where we do not have issued or granted patents or where our issued or granted patent claims or other intellectual property rights are not sufficient to prevent competitor activities in these jurisdictions. The legal systems of certain countries, particularly certain developing countries, make it difficult to enforce patents and such countries may not recognize other types of intellectual property protection, particularly that relating to pharmaceuticals. This could make it difficult for us to prevent the infringement of our patents or marketing of competing products in violation of our proprietary rights generally in certain jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the United States, and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we, or any future licensor, encounters difficulties in protecting, or is otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions. Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we, or any licensor, is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position in the relevant jurisdiction may be impaired and our business and results of operations may be adversely affected.
We may become involved in lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe on our patents, the patents of our licensing partners, or other intellectual property rights. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time consuming. In addition, in an infringement proceeding, a court may decide that our patent is invalid or unenforceable, or may refuse to stop the other party from using the technology on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are concluded.
Litigation proceedings may fail and, even if successful, may be costly and a distraction to our management and other employees. We may not be able to prevent, alone or with our collaborators, misappropriation of our proprietary rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, we could have a substantial adverse effect on the price of our common stock.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have an adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the proprietary rights and intellectual property of third parties. The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights. We may in the future become party to, or threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our medicines and technology, including interference or derivation proceedings, post-grant reviews, inter partes reviews, or other procedures before the USPTO or other similar procedures in foreign jurisdictions. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our medicines and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, we could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us. We could be forced, including by court order, to cease developing and commercializing the infringing technology or medicine. In addition, we could be held liable for substantial monetary damages, potentially including treble damages and attorneys’ fees, if found to have willfully infringed. A finding of infringement could prevent us from commercializing a product candidate or force us to cease some of our business operations, which could harm our business. Alternatively, we may need to redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
The cost to us of any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial and may result in substantial costs and distraction to our management and other employees. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could delay our research and development efforts and limit our ability to continue our operations.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Our employees and consultants have been previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we are not aware of any claims currently pending against us, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information or intellectual property of the former employers of our employees. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying money claims, we may lose valuable intellectual property rights or personnel. A loss of key personnel or their work product could detract from our ability to develop or commercialize our product candidates.
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of any product we may pursue could be significantly diminished.
We may rely upon trade secrets, know-how, and continuing technological innovation to develop and maintain our competitive position. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, contract manufacturers, vendors, and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, we cannot guarantee that we have executed these agreements with each party that may have or has had access to trade secrets.
If a party breaches an agreement and discloses our proprietary information, including our trade secrets, we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time consuming, and the outcome is unpredictable. In addition, some courts in and outside of the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they disclose such trade secrets, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to, or independently developed by, a competitor or other third party, our competitive position would be harmed.
Obtaining and maintaining our trademark protection depends on approval from the USPTO and other foreign government agencies, and third parties may challenge, infringe, or otherwise weaken our trademark rights.
We have obtained registration of the “Nyxol” trademark in the United States. We have not yet registered trademarks for any other product candidates in any jurisdiction. If we do not secure and maintain registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would, which could affect our business. When we file trademark applications for a product candidate, those applications may not be allowed for registration, and registered trademarks may not be obtained, maintained, or enforced. During trademark registration proceedings in the United States and foreign jurisdictions, we may receive rejections. We are given an opportunity to respond to those rejections, but may not be able to overcome such rejections. In addition, the USPTO and comparable agencies in many foreign jurisdictions allow third parties opportunities to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks and our trademarks may not survive such proceedings.
In addition, any proprietary name we propose to use with a future product candidate in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed drug names, including an evaluation of potential for confusion with other drug names. If the FDA objects to any proposed proprietary drug name for any product candidate, we may be required to expend significant additional resources in an effort to identify a suitable substitute proprietary drug name that would qualify under applicable trademark laws, not infringe the existing rights of third parties, and be acceptable to the FDA.
If we register any of our trademarks, our trademarks or trade names may be challenged, infringed, circumvented, declared generic, or determined to infringe on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively, and our business may be adversely affected.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by governmental agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment or other provisions during the patent application process. In addition, periodic maintenance and annuity fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market, which would have an adverse effect on our business.
We depend on intellectual property sublicensed from Apexian Pharmaceuticals, Inc. (“Apexian”) for our APX3330 product candidate under development and our additional pipeline candidates, and the termination of, or reduction or loss of rights under, this sublicense would harm our business.
We entered into a sublicense agreement with Apexian (as amended, the “Apexian Sublicense Agreement”) to in-license patents and other intellectual property relating to the APX3330 product candidate and second-generation product candidates owned by Apexian, and intellectual property that Apexian in-licensed from Eisai Co., Ltd. (“Eisai”) including certain study reports, manufacturing and analytical records, data, know-how, technical and other proprietary information relating to APX3330. The rights granted under the Apexian Sublicense Agreement are subject to various milestone payment, royalty, insurance or other obligations on us, and may be revocable under certain circumstances including if we cease to do business, fail to make the payments due thereunder, commit a material breach of the agreement that is not cured within a certain time period after receiving written notice or fail to meet certain specified development and commercial timelines. Termination of the Apexian Sublicense Agreement may result in us having to negotiate a new or reinstated agreement, which may not be available to us on equally favorable terms, or at all, which may mean we are unable to develop or commercialize APX3330 and second-generation assets.
We do not have total control over the preparation, filing, prosecution and maintenance of patents and patent applications covering the technology that we license under the Apexian Sublicense Agreement. Under the Sublicense Agreement, Indiana University Research and Technology Corp. (“IURTC”), the owner of the patents licensed to Apexian and sublicensed to us, maintains the right to control all prosecution and maintenance of such patents. Therefore, we cannot always be certain that these patents and patent applications will be prepared, filed, prosecuted and maintained in a manner consistent with the best interests of our business. Although we have a right to have our comments considered in connection with, and have agreed to bear the costs of, the prosecution and maintenance of the licensed patents, if IURTC fails to prosecute and maintain such patents, or loses rights to those patents or patent applications as a result of its control of the prosecution activities, the rights we have licensed may be reduced or eliminated, and our right to develop and commercialize any of our product candidates that are the subject of such licensed rights could be adversely affected.
Further, if Apexian breaches its license agreement with IURTC and fails to cure such breach within a 60-day cure period, IURTC may terminate such license agreement with Apexian, in which case, our license shall also terminate and we will lose all rights under the license agreement with Apexian. While the Apexian Sublicense Agreement provides that Apexian must cooperate with us to remedy and cure Apexian’s breach of the license agreement with IURTC in order to prevent the termination of such license agreement, we cannot guarantee that such efforts will be successful in preventing the termination of the license agreement between Apexian and IURTC. Similarly, if Apexian breaches its license agreement with Eisai and fails to cure such breach within a 60-day cure period, Eisai may terminate such license agreement with Apexian, in which case, our sublicense rights under such license shall also terminate. While we do not have any material obligations under the license agreement between Eisai and Apexian, Apexian has certain confidentiality and payment obligations that, if not met, could result in breach of the Eisai license agreement.
Under Apexian’s license agreement with IURTC, any act or omission by us that would be a breach of the license agreement with IURTC if imputed to Apexian is deemed to be a breach by Apexian of such license agreement and cause for termination, including, in particular, any breach by us of our payment, reporting, audit, and indemnification obligations.
The Apexian Sublicense Agreement obligates us to make certain milestone payments.
We are obligated to pay certain milestone payments to Apexian pursuant to the Apexian Sublicense Agreement. These milestone payments include (i) payments for specified developmental and regulatory milestones totaling up to $11 million in the aggregate and (ii) payments for specified sales milestones of up to $20 million in the aggregate.
Because certain milestone payments payable by us are due upon certain events related to the development and regulatory approval of our product candidates, we may be required to make such payments prior to the time at which we are able to generate revenue, if any, from sales any of our product candidates, if approved. There can be no assurance that we will have the funds necessary to make such payments, or be able to raise such funds when needed, on terms acceptable to us, or at all. Furthermore, if we are forced to raise additional funds, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts, or grant rights to develop and market product candidates that we would otherwise develop and market themselves. If we are unable to raise additional funds or maintain sufficient liquidity to make our payment obligations if and when they become due, we may be in material breach of our license and acquisition agreements and our counterparties may seek legal action or remedies against us, which would harm our business, financial condition, results of operations and prospects.
We may enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances or partnerships with third parties that may not result in the development of commercially viable products or the generation of significant future revenues.
We may enter into certain license or other collaboration agreements in the future. Such agreements may impose various diligence, milestone payment, royalty, insurance or other obligations on us. If we fail to comply with such obligations, our licensor or collaboration partners may have the right to terminate the relevant agreement, in which event we would not be able to develop or market the products covered by such licensed intellectual property. Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights under our collaborative development relationships; |
| • | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| • | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property; and |
| • | the priority of invention of patented technology. |
In addition, the agreements under which intellectual property or technology is licensed from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations, and prospects.
In addition, we cannot be certain that the preparation, filing, prosecution and maintenance activities by any future licensors have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights.
Risks Related to Our Employee Matters and Managing Growth
We are dependent on our key personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
We are highly dependent on our management, scientific, and medical personnel, including Mina Sooch, our President, Chief Executive Officer and Board Vice Chair. We have entered into employment agreements with our executive officers, but any employee may terminate his or her employment with us. The loss of the services of any of our executive officers, other key employees or consultants, or other scientific and medical advisors in the foreseeable future might impede the achievement of our research, development, and commercialization objectives. If we fail to retain key personnel and are unable to hire highly qualified replacements, we may not be able to meet key objectives, such as meeting financial goals, and maintaining or expanding our business. We rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. Recruiting and retaining qualified scientific personnel and business and commercial personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. Failure to succeed in clinical trials may also make it more challenging to recruit and retain qualified scientific personnel.
We will need to develop and expand our company and may encounter difficulties in managing this development and expansion, which could disrupt our operations.
As of March 1, 2023, we had ten full-time employees, and we expect to increase our number of employees and the scope of our operations as we further the clinical development of our product candidates. To manage our anticipated development and expansion, we must continue to implement and improve our managerial, operational, and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Also, our management may need to divert a disproportionate amount of our attention away from our day-to-day activities and devote a substantial amount of time to managing these development activities. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, and give rise to operational mistakes, loss of business opportunities, loss of employees, or reduced productivity among remaining employees. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of product candidates. If our management is unable to effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize product candidates, if approved, and compete effectively will depend, in part, on our ability to effectively manage our future development and expansion.
A variety of risks associated with operating internationally for us and our collaborators could adversely affect our business.
In addition to our U.S. operations, we may pursue international operations in the future and would face risks associated with such global operations, including possible unfavorable regulatory, pricing and reimbursement, legal, political, tax, and labor conditions, which could harm our business. We plan to conduct clinical trials outside of the United States. We are subject to numerous risks associated with international business activities, including:
| • | compliance with differing or unexpected regulatory requirements for our product candidates; |
| • | different medical practices and customs affecting acceptance of our product candidates, if approved, or any other approved product in the marketplace; |
| • | the interpretation of contractual provisions governed by foreign law in the event of a contract dispute; |
| • | difficulties in staffing and managing foreign operations, and an inability to control commercial or other activities where it is relying on third parties; |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| • | potential liability under the Foreign Corrupt Practice Act of 1977 or comparable foreign regulations; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capability abroad; |
| • | foreign government taxes, regulations, and permit requirements; |
| • | U.S. and foreign government tariffs, trade restrictions, price and exchange controls, and other regulatory requirements; |
| • | economic weakness, including inflation, natural disasters, war, events of terrorism, or political instability in particular foreign countries; |
| • | fluctuations in currency exchange rates, which could result in increased operating expenses and reduced revenues; |
| • | compliance with tax, employment, immigration, and labor laws, regulations, and restrictions for employees living or traveling abroad; |
| • | changes in diplomatic and trade relationships; and |
| • | challenges in enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the United States. |
Our business and operations would suffer in the event of system failures or unplanned events.
Despite the implementation of security measures, our internal computer systems and those of our current and future contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war, and telecommunications and electrical failures. In March 2021, we were the victim of a business email compromise. This fraud did not cause any losses to us. If another such event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed. We may be required to expend significant resources, fundamentally change our business activities and practices, or modify our operations, including our clinical trial activities, or information technology in an effort to protect against security breaches and to mitigate, detect and remediate actual or potential vulnerabilities.
Furthermore, any unplanned event, such as flood, fire, explosion, tornadoes, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunications failure, other natural or manmade accidents or incidents, or pandemics, that result in us being unable to fully utilize the facilities, may have an adverse effect on our ability to operate our business, particularly on a daily basis, and have significant negative consequences on its financial and operating conditions. Loss of access to these facilities may result in increased costs, delays in the development of our product candidates, or interruption of our business operations.
Our insurance policies are expensive and protect only from some business risk, which leaves us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risks that our business may encounter, and insurance coverage is becoming increasingly expensive. We do not know if we will be able to maintain existing insurance with adequate levels of coverage, and any liability insurance coverage we acquire in the future may not be sufficient to reimburse the company for any expenses or losses we may suffer. If we obtain marketing approval for any product candidates that we may develop, we intend to acquire insurance coverage to include the sale of commercial products, but we may be unable to obtain such insurance on commercially reasonable terms or in adequate amounts. Required coverage limits for such insurances are difficult to predict and may not be sufficient. If potential losses exceed our insurance coverage, our financial condition would be adversely affected. In the event of contamination or injury, we could be held liable for damages or be penalized with fines in an amount exceeding our resources. Clinical trials or regulatory approvals for any of our product candidates could be suspended, which could adversely affect our results of operations and business, including by preventing or limiting the development and commercialization of any product candidates that the company or our collaborators may develop.
In addition, as a public company, it may be more difficult or more costly for us to obtain certain types of insurance, including directors’ and officers’ liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified personnel to serve on our board of directors, our board committees or as executive officers.
Environmental, social and governance matters and any related reporting obligations may impact our businesses.
U.S. and international regulators, investors and other stakeholders are increasingly focused on environmental, social, and governance (ESG) matters. For example, new domestic and international laws and regulations relating to ESG matters, including human capital, diversity, sustainability, climate change and cybersecurity, are under consideration or being adopted, which may include specific, target-driven disclosure requirements or obligations. Our response will require additional investments and implementation of new practices and reporting processes, all entailing additional compliance risk. Our aspirations and disclosures related to environmental, social, and governance (“ESG”) matters expose us to risks that could adversely affect our reputation and performance.
Risks Related to Ownership of Our Common Stock
We do not anticipate paying any cash dividends in the foreseeable future.
The current expectation is that we will retain our future earnings, if any, to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be investors’ sole source of gain, if any, for the foreseeable future.
If we fail to comply with the continued listing standards of the Nasdaq Capital Market, our common stock could be delisted. If it is delisted, the liquidity of our common stock would be impacted.
The continued listing of our common stock on Nasdaq is contingent on our continued compliance with a number of listing standards. There is no assurance that we will remain in compliance with these standards. Delisting from Nasdaq would adversely affect our ability to raise additional financing through the public or private sale of equity securities, significantly affect the ability of investors to trade our securities and negatively affect the value and liquidity of our common stock. Delisting also could limit our strategic alternatives and attractiveness to potential counterparties and have other negative results, including the potential loss of employee confidence, the loss of institutional investors or interest in business development opportunities.
In addition, if our common stock is delisted from the Nasdaq Capital Market and the trading price remains below $5.00 per share, trading in our common stock might also become subject to the requirements of certain rules promulgated under the Exchange Act, which require additional disclosure by broker-dealers in connection with any trade involving a stock defined as a “penny stock” (generally, any equity security not listed on a national securities exchange or quoted on Nasdaq that has a market price of less than $5.00 per share, subject to certain exceptions).
The market price of our common stock may fluctuate significantly.
The market price of our common stock may fluctuate significantly in response to factors, some of which are beyond our control, such as:
| • | the announcement of new products or product enhancements by us or our competitors; |
| • | changes in our relationships with our licensors or other strategic partners; |
| • | developments concerning intellectual property rights and regulatory approvals; |
| • | variations in ours and our competitors’ results of operations; |
| • | substantial sales of shares of our common stock due to the release of lock-up agreements; |
| • | the announcement of clinical trial results; |
| • | the announcement of potentially dilutive financings; |
| • | changes in earnings estimates or recommendations by securities analysts; |
| • | changes in the structure of healthcare payment systems; and |
| • | developments and market conditions in the pharmaceutical and biotechnology industries, including due to the COVID-19 pandemic. |
Further, the stock market, in general, and the market for biotechnology companies, in particular, have experienced extreme price and volume fluctuations. Continued market fluctuations could result in extreme volatility in the price of our common stock, which may be unrelated or disproportionate to our operating performance and which could cause a decline in the value of our common stock.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our common stock may be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and direct our management’s attention from other business concerns, which could seriously harm our business.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
Our headquarters is currently located in Farmington Hills, Michigan, and consists of approximately 1,600 square feet of leased office space under a lease that expires on December 31, 2023. We may extend our current space or require additional space and facilities as our business expands, and we believe that suitable additional and alternative spaces will be available in the future on commercially reasonable terms.
From time to time, we are subject to litigation and claims arising in the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, as of the date of this filing, we do not believe we are party to any claim or litigation, the outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business or financial condition. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our shares of common stock trade on the Nasdaq Capital Market under the symbol “OCUP”.
Holders
As of March 27, 2023, there were approximately 83 holders of record of our common stock. The number of holders of record is based on the actual number of holders registered on the books of our transfer agent and does not reflect holders of shares in “street name” or persons, partnerships, associations, corporations, or other entities identified in security position listings maintained by depository trust companies.
Dividend Policy
We have not paid any cash dividends on our common stock since our inception and do not anticipate paying any cash dividends in the foreseeable future. We plan to retain our earnings, if any, to provide funds for the expansion of our business.
Recent Sales of Unregistered Securities
None.
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and the related notes included elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this Annual Report, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a late clinical-stage ophthalmic biopharmaceutical company focused on developing and commercializing therapies for the treatment of refractive and retinal eye disorders. Our pipeline currently includes two small molecule product candidates targeting several of such indications.
Nyxol
In November 2022, we entered into a license and collaboration agreement (the “Nyxol License Agreement”) with FamyGen Life Sciences, Inc. (acquired by Viatris, Inc. (“Viatris”) in January 2023) pursuant to which we granted Viatris an exclusive license to develop, manufacture, import, export and commercialize our product candidate phentolamine ophthalmic solution 0.75% (Nyxol® Eye Drops or “Nyxol”).
Nyxol is a once-daily eye drop formulation of phentolamine mesylate designed to reduce pupil diameter and improve visual acuity. Nyxol can potentially be used across multiple indications such as treatment of pharmacologically-induced mydriasis (“RM”) (dilation of the pupil), presbyopia (age-related blurry near vision) and dim light or night vision disturbances (“DLD”) (halos, glares and starbursts). Our management believes these multiple indications potentially represent a significant market opportunity. Nyxol has been studied in a total of 12 clinical trials (3 Phase 1, 5 Phase 2 and 4 Phase 3) in a total of over 650 patients (with over 400 Nyxol-treated) and has demonstrated promising clinical data across the three targeted refractive indications.
We reported positive top-line data from Phase 3 trials in RM: MIRA-2 in March 2021, MIRA-3 in March 2022 and MIRA-4 in April 2022. We also reported positive top-line data from a Phase 2 trial of Nyxol for treatment of presbyopia, both alone and with low-dose pilocarpine (pilocarpine hydrochloride ophthalmic solution 0.4%, “LDP”) as adjunctive therapy (VEGA-1). We reported top-line data from a Phase 3 trial in DLD in May 2022 (LYNX-1). We submitted a new drug application (“NDA”) to the U.S. Food and Drug Administration (“FDA”) in November 2022 under the 505(b)(2) pathway for Nyxol for RM with a Prescription Drug User Fee Act (PDUFA) goal date of September 28, 2023. The first phase 3 registration trial of Nyxol for the treatment of presbyopia (VEGA-2), both alone and with LDP as adjunctive therapy, was started in late December 2022, and topline results from this trial are expected in late 2023. Future trials are planned to start in 2023 including the second registration trials for presbyopia (VEGA-3) and DLD (LYNX-2), and supportive long-term safety trial for both chronic indications (LYRA-1).
APX3330
Our product candidate, APX3330, is a twice-a-day oral tablet designed to target multiple pathways relevant to retinal and choroidal (the vascular layer of the eye) diseases such as diabetic retinopathy (“DR”) and diabetic macular edema (“DME”) which, if left untreated, can result in permanent visual acuity loss and eventual blindness. DR is a disease resulting from diabetes in which chronically elevated blood sugar levels cause progressive damage to blood vessels in the retina. DME is a severe form of DR which involves leakage of protein and fluid into the macula, the central portion of the retina, causing swelling and vascular damage. Prior to our in-licensing this product candidate, APX3330 had been studied by other sponsors in a total of 11 clinical trials (6 Phase 1 and 5 Phase 2) in a total of over 420 healthy volunteers or patients (with over 340 APX3330-treated) for inflammatory (hepatic) and oncology indications, and had demonstrated evidence of target engagement, pharmacokinetics, durability, and favorable safety and tolerability. We also in-licensed APX2009 and APX2014, which are second-generation product candidates and analogs of APX3330. In January 2023, we reported top-line efficacy and safety results from the ZETA-1 Phase 2 trial conducted in 103 subjects (51 treated with 600 mg daily dose of APX3330) in DR, including moderately severe non-proliferative DR (“NPDR��) and mild proliferative DR (“PDR”), as well as patients with DME without loss of central vision. Although the ZETA-1 clinical trial did not meet the primary endpoint of % of patients with a ≥ 2-step improvement in Early Treatment of Diabetic Retinopathy Study (ETDRS) diabetic retinopathy severity scale (DRSS) at week 24 in the study eye, statistical significance was achieved on a key pre-specified secondary endpoint of preventing clinically meaningful progression of diabetic retinopathy (defined by binocular 3 or more steps worsening on the DRSS scale, calculated as the sum of changes in each eye) after 24 weeks of treatment. Given the oral systemic delivery of APX3330, an endpoint that evaluates the effects on both eyes is the planned Phase 3 primary endpoint for future registration trials; this will be confirmed at an End-of-Phase 2 (EOP2) meeting with the FDA in second half of 2023. APX3330 demonstrated favorable safety and tolerability in the ZETA-1 trial, consistent with the safety data from the prior 11 clinical trials. Treatment-related adverse events were uncommon, and most were mild in severity. There were no treatment-related serious adverse events. No changes were observed in liver, kidney, or heart function as well as complete blood count and comprehensive metabolic panel.
As part of its our strategy, we will continue to explore opportunities to acquire additional ophthalmic assets and seek strategic partners for late-stage development, regulatory preparation, and commercialization of drugs in key global markets.
In November 2022, we entered into the Nyxol License Agreement. As part of our strategy, we will continue to explore opportunities to acquire additional ophthalmic assets and to seek strategic partners for late-stage development, regulatory preparation and commercialization of APX3330 in key global markets. To date, our primary activities have been conducting research and development activities, planning clinical trials, performing business and financial planning, recruiting personnel and raising capital. We do not have any products approved for sale, and we do not expect to consistently generate significant revenues, other than license and collaborations revenue, until, and unless, the FDA or other regulatory authorities approve Nyxol or APX3330 and we successfully commercialize our product candidates. Until such time, if ever, as we can consistently generate substantial product revenue, we expect to finance our cash needs through a combination of equity and debt financings as well as through collaborations, strategic alliances and licensing arrangements.
Through December 31, 2022, we have funded our operations primarily through equity financings that totaled $54.1 million in gross proceeds, of which $21.15 million was received in connection with the merger (“Merger”) with Rexahn Pharmaceuticals, Inc. (“Rexahn”) and through the issuance of convertible notes in private placements that totaled $8.5 million in gross proceeds net cash. In addition, we recently received a one-time non-refundable licensee fee payment in connection with the Nyxol License Agreement of $35.0 million.
Our net income was $17.9 million for the year ended December 31, 2022 as compared to a net loss of $56.7 million for the year ended December 31, 2021. As of December 31, 2022, we had an accumulated deficit of $71.5 million. As noted above, our net income for the year ended December 31, 2022 was primarily due to a one-time non-refundable payment made to us pursuant to the Nyxol License Agreement. Furthermore, we anticipate that our expenses will increase as we:
| | • | continue clinical trials for Nyxol, APX3330 and for any other product candidate in our future pipeline; |
| | • | continue preclinical studies for Nyxol, APX3330 and for any other product candidate in our future pipeline; |
| | • | develop additional product candidates that we identify, in-license or acquire; |
| | • | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| | • | contract to manufacture our product candidates; |
| | • | maintain, expand and protect our intellectual property portfolio; |
| | • | hire additional staff, including clinical, scientific, operational and financial personnel, to execute our business plan; |
| | • | add operational, financial and management information systems and personnel, including personnel to support our product development and potential future commercialization efforts; |
| | • | continue to operate as a public company; and |
| | • | establish on our own or with partners, a sales, marketing and distribution infrastructure to commercialize any products for which we may obtain regulatory approval; |
Our net income (loss) will likely continue to fluctuate significantly from quarter to quarter and year to year, depending on the timing of our preclinical studies, clinical trials, expenditures on other research and development activities (and reimbursement thereof), and from potential milestone payments received from and revenue earned under the Nyxol License Agreement or any other license and collaboration agreements that we enter into, and potential payments we that may become payable from time to time under the Apexian Sublicense Agreement.
Recent Developments
License and Collaboration Agreement with Viatris
In November 2022, we entered into a license and collaboration agreement (the “Nyxol License Agreement”) with FamyGen Life Sciences, Inc. (acquired by Viatris, Inc. (“Viatris”) in January 2023) pursuant to which we granted Viatris an exclusive license to develop, manufacture, import, export and commercialize our product candidate phentolamine ophthalmic solution 0.75% (Nyxol® Eye Drops or “Nyxol”).
Clinical Milestones
APX3330
In January 2023, we announced topline efficacy and safety results from ZETA-1, a Phase 2b trial of APX3330 in diabetic retinopathy patients. Although the primary endpoint (a precedented endpoint for local administration of anti-VEGF intravitreal injections) was not met, the ZETA-1 results on a key pre-specified endpoint demonstrated statistical significance on a potential binocular DR worsening registration endpoint with a favorable systemic and ocular safety profile that support our plans to move forward to an End-of-Phase 2 meeting with the FDA.
Nyxol
In January 2023, we announced the initiation of the VEGA-2 Phase 3 pivotal trial with the first patient enrolled in late December 2022. This is the first of two Phase 3 registration trials intended to support a presbyopia indication for Nyxol alone and Nyxol with LDP. In addition, the VEGA-3 trial (the second Phase 3), the LYNX-2 trial (the second Phase 3), and LYRA-1 trial (1-year safety) are planned to begin in 2023. If successful, we plan to file a supplemental NDA for Nyxol as a single-agent for presbyopia and DLD and a new NDA for Nyxol + LDP thereafter.
Non-clinical Update
In support of conducting clinical trials using Nyxol for chronic indications such as presbyopia and DLD, we have successfully completed a 6-month rabbit ocular toxicology study. The findings from the 6-month study provide support for the conduct of the planned 1-year Phase 3 safety trial LYRA-1. The submission of the final was completed November 2022 to the FDA.
In support of the combination of Nyxol and LDP treatment in presbyopia, a 99-day nonclinical ocular toxicology study with Nyxol and LDP in Dutch-belted rabbits has been completed. The final report is being finalized with a subsequent submission to the FDA in 2Q 2023.
Regulatory Update
In November 2022, we announced the submission of an NDA to the FDA for Nyxol for the reversal of pharmacologically-induced mydriasis produced by adrenergic agonist (e.g., phenylephrine) or parasympatholytic (e.g., tropicamide) agents, or a combination thereof.
In January 2023, we announced that the FDA has accepted the NDA for Nyxol for the treatment of pharmacologically-induced mydriasis. The FDA assigned a Prescription Drug User Fee Act (PDUFA) date of September 28, 2023.
Presentations, Publications and Conferences
Our management team and medical advisors have participated by invitation at over 35 medical, scientific, industry and investment conferences in 2022, at which over 20 papers, posters and panel talks were presented. We have been engaging with many key opinion leaders to expand awareness of the Nyxol and APX3330 development programs.
Global Economic Conditions
Generally, worldwide economic conditions remain uncertain, particularly due to the effects of the recent instability in the financial services industry, the COVID-19 pandemic and increased inflation. The general economic and capital market conditions both in the U.S. and worldwide, have been volatile in the past and at times have adversely affected our access to capital and increased the cost of capital. The capital and credit markets may not be available to support future capital raising activity on favorable terms. If economic conditions decline, our future cost of equity or debt capital and access to the capital markets could be adversely affected.
The COVID-19 pandemic that began in late 2019 introduced significant volatility to the global economy, disrupted supply chains and had a widespread adverse effect on the financial markets. As a result of the COVID-19 pandemic, we have experienced, and may continue to experience, delays and disruptions in our clinical trials, as well as interruptions in our manufacturing, supply chain, shipping and research and development operations. Testing and clinical trials, manufacturing, component supply, shipping and research and development operations may be further impacted by the continuing effects of COVID-19.
Additionally, our operating results could be materially impacted by changes in the overall macroeconomic environment and other economic factors. Changes in economic conditions, supply chain constraints, logistics challenges, labor shortages, the conflict in Ukraine, and steps taken by governments and central banks, particularly in response to the COVID-19 pandemic as well as other stimulus and spending programs, have led to higher inflation, which has led to an increase in costs and has caused changes in fiscal and monetary policy, including increased interest rates.
Financial Operations Overview
License and Collaborations Revenue
License and collaborations revenue to date was derived from a one-time non-refundable payment and reimbursement of expenses earned under the Nyxol License Agreement, and to a much lesser degree, from license agreements with BioSense Global LLC (“BioSense”) and Processa Pharmaceuticals, Inc. (“Processa”) in connection with the Rexahn RX-3117 drug compound. We anticipate that we will recognize revenue as we earn reimbursement for research and development services in connection with the Nyxol License Agreement and we may earn additional revenues from potential milestone and royalty payments from the agreements with Viatris, BioSense, Processa, or from other license agreements entered into the future; however, the attainment of milestones or level of sales required to earn royalty payments is highly uncertain for the reasons explained below.
To date, outside of the license and collaborations revenue referenced above, we do not expect to generate significant revenue unless or until regulatory approval is obtained and commercialization begins for Nyxol or APX3330. If we fail to complete the development of Nyxol, APX3330, or any other product candidate we may pursue in the future, in a timely manner, or fail to obtain regulatory approval, our ability to generate significant revenue would be compromised.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel-related costs, including salaries, benefits and stock-based compensation costs, for personnel in functions not directly associated with research and administrative activities. Other significant costs include insurance coverage for directors and officers and other property and liability exposures, legal fees relating to intellectual property and corporate matters, professional fees for accounting and tax services, other services provided by business consultants and legal settlements.
Research and Development Expenses
To date, our research and development expenses have related primarily to the clinical stage development of Nyxol and APX3330. Research and development expenses consist of costs incurred in performing research and development activities, including compensation and benefits for research and development employees and costs for consultants, costs associated with preclinical studies and clinical trials, regulatory activities, manufacturing activities to support clinical activities, license fees, nonlegal patent costs, fees paid to external service providers that conduct certain research and development, and an allocation of overhead expenses.
Pursuant to the Nyxol License Agreement, our budgeted research and development expenses related to the development of Nyxol are fully reimbursed by Viatris. However, all research and development costs, including those related to Nyxol, are expensed as incurred and costs incurred by third parties are expensed as the contracted work is performed. We accrue for costs incurred as the services are being provided by monitoring the status of the study or project, and the invoices are received from our external service providers. We adjust our accrual as actual costs become known. Research and development activities are central to our business model.
We expect that Nyxol and APX3330 will have higher development costs during the later stages of clinical development, as compared to costs incurred during their earlier stages of development, primarily due to the increased size and duration of the later-stage clinical trials. We expect our research and development expenses to increase over the next several years. However, it is difficult for us to determine with certainty the duration, costs and timing to complete our current or future preclinical programs and clinical trials of Nyxol, APX3330, and other product candidates. The duration, costs and timing of clinical trials and development of Nyxol, APX3330 and other product candidates will depend on a variety of factors that include, but are not limited to, the following:
| • | per patient trial costs; |
| • | the number of patients that participate in the trials; |
| • | the number of sites included in the trials; |
| • | the countries in which the trials are conducted; |
| • | the length of time required to enroll eligible patients; |
| • | the number of doses that patients receive; |
| • | the drop-out or discontinuation rates of patients; |
| • | potential additional safety monitoring or other studies requested by regulatory agencies; |
| • | the duration of patient follow-up; |
| • | the phase of development of the product candidate; |
| • | arrangements with contract research organizations and other service providers; and |
| • | the efficacy and safety profile of the product candidates. |
Interest Expense
Interest expense consists of interest costs on principal related to a short-term loan (related to financing an insurance policy) during the period it was outstanding. The short-term loan had an annual interest rate of 5.5%. The short-term loan was fully repaid in May 2022.
Fair Value Change in Warrant Liabilities
The fair value change in warrant liabilities consists of the change in the fair value of the warrant liabilities during the period they are outstanding. The applicable warrants were reclassified to equity in the first quarter of 2021, and as a result, are no longer subject to fair value remeasurement.
Other Expense, net
Other expense, net reflected in this line item includes payments made by us in connection with the Contingent Value Rights Agreement (the “CVR Agreement”) with former Rexahn shareholders. In addition, other expense, net includes interest earned from cash and cash equivalent investments, realized and unrealized gains (losses) from equity investments and reimbursements in connection with grants and other sources when they occur.
Provision for Income Taxes
Provision for income taxes consists of federal and state income taxes in the United States, as well as deferred income taxes and changes in related valuation allowance reflecting the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Currently, a full valuation allowance has been provided on the net deferred tax assets as of December 31, 2022 and 2021 given the uncertainty of future taxable income and other related factors impacting the realizability or our remaining net deferred tax assets.
Results of Operations
The following table summarizes our operating results for the periods indicated (in thousands):
| | | For the Year Ended December 31, | |
| | | 2022 | | | 2021 | | | Change | |
| | | | | | | | | | |
| License and collaborations revenue | | $ | 39,850 | | | $ | 589 | | | $ | 39,261 | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| General and administrative | | | 7,269 | | | | 8,121 | | | | (852 | ) |
| Research and development | | | 14,355 | | | | 15,173 | | | | (818 | ) |
| Total operating expenses | | | 21,624 | | | | 23,294 | | | | (1,670 | ) |
| Income (loss) from operations | | | 18,226 | | | | (22,705 | ) | | | 40,931 | |
| Interest expense | | | (9 | ) | | | (2 | ) | | | (7 | ) |
| Fair value change in warrant liabilities | | | — | | | | (33,829 | ) | | | 33,829 | |
| Other expense, net | | | (14 | ) | | | (157 | ) | | | 143 | |
| Income (loss) before income taxes | | | 18,203 | | | | (56,693 | ) | | | 74,896 | |
| Provision for income taxes | | | (315 | ) | | | — | | | | (315 | ) |
| Net income (loss) | | $ | 17,888 | | | $ | (56,693 | ) | | $ | 74,581 | |
Comparison of Years Ended December 31, 2022 and 2021
License and Collaborations Revenue
License and collaborations revenue was $39.8 million for the year ended December 31, 2022 compared to $0.6 million for the year ended December 31, 2021. Revenue during 2022 was derived from the Nyxol License Agreement in the fourth quarter associated largely with the transfer of a perpetual, sub-licensable license to develop, manufacture, import, export and commercialize the Nyxol Products, and to a lesser extent, from the reimbursement of research and development services. Revenue during the year ended December 31, 2021 was derived from the collaboration and license agreements with Processa and BioSense related to certain technology transfers in connection with the Rexahn RX-3117 drug compound, a legacy asset not under development by Ocuphire.
General and Administrative
General and administrative expenses for the year ended December 31, 2022 were $7.3 million compared to $8.1 million for the year ended December 31, 2021. The $0.9 million decrease was primarily attributable to a non-cash settlement cost of $1.6 million in the prior year period. Other expense decreases from the prior year were attributed to stock-based compensation of $0.1 million and other operating expenses of $0.2 million. Partially offsetting the expense decreases from the prior year were increases in administrative employee headcount costs in the amount of $0.4 million, legal fees of $0.4 million and professional service costs in the amount of $0.2 million. General and administrative expenses included $1,060,000 and $1,116,000 in stock-based compensation expense during the years ended December 31, 2022, and 2021, respectively.
Research and Development
Research and development expenses for the year ended December 31, 2022 were $14.4 million compared to $15.2 million for the year ended December 31, 2021. The $0.8 million decrease was primarily attributable to a decrease in contract research organization expense of $1.5 million as a result of the completion of clinical trials along with an associated decrease in manufacturing activities to support clinical advancement of $0.9 million, offset in part by cost increases attributable to staff headcount in the amount of $0.5 million, consulting services of $0.8 million as well as increases attributable to regulatory and other research and development efforts of $0.3 million on a net basis. Research and development expenses also included $0.7 million and $0.8 million in stock-based compensation expense during the years ended December 31, 2022 and 2021, respectively.
Interest Expense
Interest expense for the years ended December 31, 2022 and 2021 was $9,000 and $2,000, respectively, and was attributable to a short-term loan (related to financing an insurance policy).
Fair Value Change in Warrant Liabilities
The fair value change in warrant liabilities was a non-cash expense of $33.8 million for the year ended December 31, 2021 and was due primarily to the issuance of the Series A warrants in connection with the Pre-Merger Financing and to the fluctuations in Ocuphire’s common stock fair value and the number of potential shares of common stock issuable upon conversion of the underlying Ocuphire warrant liabilities that were outstanding during that period. Upon the execution of the Waiver Agreements in the first quarter of 2021 described in Note 3 — Pre-Merger Financing included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report, the Series A Warrants were reclassified to equity, and as a result, were no longer subject to fair value remeasurement.
The fair value change in warrant liabilities during the year ended December 31, 2022 was de minimis in connection with the Rexahn warrants.
Other Expense, net
During the year ended December 31, 2022, we had other expense, net of $14,000 stemming from net unrealized losses attributed to our short-term investments of $170,000 and realized currency losses of approximately $3,000, offset largely by interest income of $159,000 related to cash and cash equivalents.
During the year ended December 31, 2021, we had other expense, net of $91,000 stemming from payments due in connection with the CVR Agreement and $70,000 stemming principally from net unrealized losses from our short-term investments, offset in part by interest earnings of $4,000 on our cash and cash equivalent investments.
Provision for Income Taxes
Provision for income taxes consisted of federal and state income taxes in the United States in the amount of $315,000 for the year ended December 31, 2022 resulting from our net taxable income after the application of net operating loss carryforwards and research credits. There was no tax provision during the prior year period.
Liquidity and Capital Resources
Capital Resources
As of December 31, 2022, our principal sources of liquidity consisted of cash and cash equivalents of $42.6 million. We believe that our cash on hand at the end of 2022 will be sufficient to fund our operations for at least twelve months beyond the date of this filing. As of December 31, 2022, our cash and cash equivalents were invested primarily in cash deposits and cash equivalent investments at two large financial institutions.
Historical Capital Resources
Our primary source of cash to fund our operations has been various equity offerings in the amount of $54.1 million and the issuance of convertible notes in the amount of $8.5 million, inclusive of the promissory notes exchanged for Ocuphire convertible notes. In addition, during the fourth quarter of 2022,we received a one-time non-refundable cash payment of $35.0 million in connection with the Nyxol License Agreement.
At-The-Market Program
On February 4, 2021, we filed a Form S-3 shelf registration under the Securities Act which was declared effective by the SEC on February 12, 2021 (the “2021 Shelf”) under which the Company may offer and sell, from time to time in our sole discretion, securities having an aggregate offering price of up to $125 million. In connection with the 2021 Shelf, on March 11, 2021, we entered into a sales agreement with JonesTrading Institutional Services LLC (“JonesTrading”) under which we may offer and sell, from time to time at our sole discretion, to or through JonesTrading, acting as agent and/or principal, shares of our common stock having an aggregate offering price of up to $40 million (the “ATM”). A total of 4,627,870 shares of common stock were sold under the ATM for net proceeds through September 2022 in the amount of $17.3 million. No shares of common stock were sold under the ATM during the fourth quarter of 2022.
Registered Direct Offering
On June 4, 2021, we entered into a placement agency agreement with A.G.P./Alliance Global Partners (“AGP”). Pursuant to the terms of the placement agency agreement, AGP on June 8, 2021, sold an aggregate of 3,076,923 shares of our common stock and warrants to purchase 1,538,461 shares of our common stock (the “RDO Warrants”) at an offering price of $4.875 per share and 0.50 RDO Warrants, for gross proceeds of $15.0 million, before deducting AGP’s fees and related offering expenses in the amount of $1.1 million. The purchase agreement contains customary representations, warranties and agreements by the Ocuphire, customary conditions to closing, indemnification obligations of Ocuphire, other obligations of the parties and termination provisions.
The RDO Warrants have an exercise price of $6.09 per share, are exercisable upon the initial issuance date of June 8, 2021, and will expire five years following the initial exercise date. Subject to limited exceptions, a holder of a RDO Warrant will not have the right to exercise any portion of its RDO Warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of a holder prior to the date of issuance, 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise; provided, however, that upon prior notice to us, the holder may increase or decrease the beneficial ownership limitation, provided further that in no event shall the beneficial ownership limitation exceed 9.99%. As of December 31, 2022, 1,538,461 RDO Warrants were still outstanding. The offering of the securities was made pursuant to our effective shelf registration statement on Form S-3.
Pre-Merger Financing
Securities Purchase Agreement
On June 17, 2020, Ocuphire, Rexahn and certain investors entered into a Securities Purchase Agreement, which was amended and restated in its entirety on June 29, 2020 (as amended and restated, the “Securities Purchase Agreement”). Pursuant to the Securities Purchase Agreement, the investors invested a total of $21.15 million in cash, including $300,000 invested by directors of Ocuphire Pharma, Inc. prior to the Merger, and one director of Rexahn, upon closing of the Merger (the “Pre-Merger Financing”). Pursuant to the Pre-Merger Financing, (i) Ocuphire issued and sold to the investors shares of common stock of Ocuphire Pharma, Inc. prior to the Merger (the “Initial Shares”) which converted pursuant to the exchange ratio in the Merger into an aggregate of 1,249,996 shares (the “Converted Initial Shares”) of common stock, (ii) Ocuphire deposited into escrow, for the benefit of the Investors, additional shares of common stock of Ocuphire Pharma, Inc. prior to the Merger (the “Additional Shares”) which converted pursuant to the exchange ratio in the Merger into an aggregate of 3,749,992 shares of common stock (the “Converted Additional Shares”), which Converted Additional Shares were delivered (or became deliverable) to the investors on November 19, 2020, and (iii) we agreed to issue to each investor on the tenth trading day following the consummation of the Merger (x) Series A Warrants representing the right to acquire shares of common stock equal to the sum of (A) the Converted Initial Shares purchased by the investor, (B) the Converted Additional Shares delivered or deliverable to the investor, without giving effect to any limitation on delivery contained in the Securities Purchase Agreement and (C) the initial number of shares of common stock, if any, underlying the Series B Warrants issued to the Investor and (y) additional warrants to purchase shares of common stock.
Waiver Agreements
Effective February 3, 2021, each investor that invested in the Pre-Merger Financing (each, a “Holder”) entered into a Waiver Agreement with the Company (collectively, the “Waiver Agreements”). Pursuant to the Waiver Agreements, the Holders and Ocuphire agreed to waive certain rights, finalize the exercise price and number of Series A Warrants and Series B Warrants, eliminate certain financing restrictions, extend the term of certain leak-out agreements, and, in the case of certain Holders, grant certain registration rights for the shares underlying the warrants.
The Waiver Agreements provide for the permanent waiver of the full ratchet anti-dilution provisions, contained in the Series A Warrants (as certain of the anti-dilution provisions had previously caused liability accounting treatment for the Series A Warrants). Upon the effective date of the Waiver Agreement, the Series A Warrants were reclassified to equity.
Pursuant to the Waiver Agreements, the number of shares underlying all of the Series B Warrants was fixed to 1,708,335 in the aggregate with respect to all Holders.
Series A Warrants
The Series A Warrants were issued on November 19, 2020 at an initial exercise price of $4.4795 per share, were immediately exercisable upon issuance and have a term of five years from the date of issuance. The Series A Warrants are exercisable for 5,665,838 shares of common stock in the aggregate (without giving effect to any limitation on exercise contained therein). As of December 31, 2022, 5,665,838 Series A Warrants were still outstanding.
At issuance, the Series A Warrants contained certain provisions that could have resulted in a downward adjustment of the initial exercise price and an upward adjustment in the number of shares underlying the warrants if Ocuphire were to have issued or sold, or made an agreement to issue or sell, any shares of common stock for a price lower than the exercise price then in effect. Pursuant to the terms of the Waiver Agreements, these provisions are no longer in effect.
Series B Warrants
The Series B Warrants have an exercise price of $0.0001, were exercisable upon issuance and will expire on the day following the later to occur of (i) the Reservation Date (as defined therein), and (ii) the date on which the investor’s Series B Warrants have been exercised in full (without giving effect to any limitation on exercise contained therein) and no shares remain issuable thereunder. The Series B Warrants were initially exercisable for 665,836 shares of common stock in the aggregate (without giving effect to any limitation on exercise contained therein) and ultimately became exercisable for 1,708,335 shares of common stock upon execution of the Waiver Agreements. As of December 31, 2022, 17,869 Series B Warrants were still outstanding.
At issuance, the Series B Warrants contained certain provisions that could have resulted in the issuance of additional Series B Warrants depending on the dollar volume-weighted average prices of a share of Common Stock during a 45-trading day Reset Period. Pursuant to the terms of the Waiver Agreements, those provisions are no longer in effect.
Ocuphire Convertible Notes
From May 2018 through March 2020, we issued convertible notes (the “Ocuphire convertible notes”) for aggregate gross proceeds of $8.5 million, inclusive of the promissory notes exchanged for Ocuphire convertible notes. The final closing of the Ocuphire convertible notes occurred on March 10, 2020. The Ocuphire convertible notes had an interest rate of 8% per annum. On November 4, 2020, all of Ocuphire’s outstanding notes were converted into 977,128 shares of Ocuphire common stock in connection with the completion of the Merger.
Cash Flows
The following table summarizes our cash flows for the periods indicated (in thousands):
| | | For the Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| | | | |
| Net cash provided by (used in) operating activities | | $ | 14,314 | | | $ | (19,370 | ) |
| Net cash used in investing activities | | | — | | | | (100 | ) |
| Net cash provided by financing activities | | | 3,786 | | | | 27,605 | |
| Net increase in cash and cash equivalents | | $ | 18,100 | | | $ | 8,135 | |
Cash Flow from Operating Activities
For the year ended December 31, 2022, cash provided by operating activities of $14.3 million was attributable to net income of $17.9 million, coupled with $2.0 million in non-cash operating expenses and offset by a net cash use of approximately $5.6 million resulting from the change in Ocuphire’s operating assets and liabilities. The non-cash expenses consisted largely of stock-based compensation of $1.8 million and a net unrealized loss in our short-term investments of $0.2 million. The change in operating assets and liabilities was primarily attributable to our increase in our accounts receivable and contract asset associated with the Nyxol License Agreement of $8.3 million and to a lesser extent from the decrease in our accounts payable and accrued expenses and increases in our prepaid expenses associated with the fluctuations of Ocuphire’s operating expenses.
For the year ended December 31, 2021, cash used in operating activities of $19.4 million was attributable to a net loss of $56.7 million, partially offset by $37.1 million in non-cash operating expenses and a net change of $0.2 million in Ocuphire’s net operating assets and liabilities. The non-cash expenses consisted largely of stock-based compensation of $1.9 million, fair value change in warrant liabilities in the amount of $33.8 million, a share settlement with certain investors in the amount of $1.6 million and non-cash impact from the receipt of common stock stemming from the fulfillment of revenue milestones ($0.2) million. The change in operating assets and liabilities was primarily attributable to an overall net increase in our accounts payable offset in part by both an increase in prepaid expenses and a decrease in accrued expenses associated with the fluctuations of our operating expenses.
Cash Flow from Investing Activities
During the year ended December 31, 2021, net cash used in investing activities was $0.1 million and were attributed to the payment of the remaining transaction costs associated with the Merger. There were no investing activities during the current year period.
Cash Flow from Financing Activities
Net cash provided by financing activities during the year ended December 31, 2022 was $3.8 million that consisted principally of proceeds received from the ATM, net of issuance costs, in the amount of $4.3 million, offset in part by payments made on the short-term loan of $0.5 million.
Net cash provided by financing activities during the year ended December 31, 2021 was $27.6 million relating principally to proceeds received in connection with both the Registered Direct Offering and ATM, net of issuance costs, in the amount of $27.0 million. Proceeds, net of payments, received in connection with a short-term loan in the amount of $0.5 million and the exercise of stock options in the amount of $0.1 million comprised the balance of financing activities during the period.
Liquidity and Capital Resource Requirements
As of December 31, 2022, we had cash and cash equivalents of $42.6 million. License and collaborations revenue to date was derived from a one-time non-refundable payment of $35 million and expected reimbursement of expenses earned under the Nyxol License Agreement, and to a much lesser degree, from license agreements with BioSense Global LLC (“BioSense”) and Processa Pharmaceuticals, Inc. (“Processa”) in connection with the Rexahn RX-3117 drug compound. We anticipate that we will recognize revenue as we earn reimbursement for research and development services in connection with the Nyxol License Agreement and we may earn additional revenues from future potential milestone and royalty payments from the agreements with Viatris, BioSense, Processa, or from other license agreements entered into the future; however, the attainment of milestones or level of sales required to earn royalty payments is highly uncertain for the reasons explained below.
To date, outside of the license and collaborations revenue referenced above, we do not expect to generate significant revenue unless or until regulatory approval is obtained and commercialization begins for Nyxol or APX3330. If we fail to complete the development of Nyxol, APX3330, or any other product candidate we may pursue in the future, in a timely manner, or fail to obtain regulatory approval, our ability to generate significant revenue would be compromised.
To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation, warrants, or other preferences that adversely affect your rights as a common stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through future collaborations, strategic alliances or licensing arrangements with pharmaceutical partners, we may have to relinquish valuable rights to our technologies, future revenue streams or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or through collaborations, strategic alliances or licensing arrangements when needed, we may be required to delay, limit, reduce or terminate our product development, future commercialization efforts, or grant rights to develop and market our product candidates that we would otherwise prefer to develop and market ourselves.
Future Capital Requirements
The development of APX3330 is subject to numerous uncertainties, and we have based these estimates on assumptions that may prove to be substantially different than what we currently anticipate and could result in cash resources being used sooner than what we currently expect. Additionally, the process of advancing early-stage product candidates and testing product candidates in clinical trials is costly, and the timing of progress in these clinical trials is uncertain. Our ability to successfully transition to profitability will be dependent upon achieving a level of product sales adequate to support our cost structure. We cannot give any assurance that we will ever be profitable or generate positive cash flow from operating activities.
Contractual Obligations and Commitments
Facility Lease
We lease a facility under a non-cancellable operating lease that expires on December 31, 2023, as amended, for a base rent in the amount of $3,000 per month.
Apexian Sublicense Agreement
On January 21, 2020, we entered into the Apexian Sublicense Agreement, pursuant to which we obtained exclusive worldwide patent and other intellectual property rights that constitute a Ref-1 Inhibitor program relating to therapeutic applications to treat disorders related to ophthalmic and diabetes mellitus conditions. The lead compound in the Ref-1 Inhibitor program is APX3330, which we intend to develop as an oral tablet therapeutic to treat DR and DME, and potentially wAMD.
In connection with the Apexian Sublicense Agreement, we issued 843,751 shares of our common stock to Apexian and certain of Apexian’s affiliates.
We agreed to make one-time milestone payments under the Apexian Sublicense Agreement for each of the first ophthalmic indication and the first diabetes mellitus indication. These milestone payments include (i) payments for specified developmental and regulatory milestones (including completion of the first Phase 2 trial (if such trial meets a primary endpoint) and the first Phase 3 pivotal trial in the United States, and filing and achieving regulatory approval from the FDA for the first New Drug Application for a compound) totaling up to $11 million in the aggregate and (ii) payments for specified sales milestones of up to $20 million in the aggregate, each of which net sales milestone payments is payable once, upon the first achievement of such milestone.
Lastly, we also agreed to make royalty payments equal to a single-digit percentage of our net sales of products covered by the patents under the Apexian Sublicense Agreement. None of the milestone or royalty payments were triggered as of the date of this Annual Report.
In the course of normal operations, we entered into cancellable purchase commitments with our suppliers for various key research, clinical and manufacturing services. The purchase commitments covered by these arrangements are subject to change based on our research and development efforts.
Other Funding Requirements
As noted above, certain of our cash requirements relate to the funding of our ongoing research and development of APX3330, inclusive of any potential milestone and royalty obligations under our intellectual property licenses. See “Part I, Item 1— Business—APX3330 Clinical Experience Summary —Ocuphire Clinical Development Plan —Potential Clinical Plans for APX3330—Future In-Licensing and Acquisition Opportunities—Manufacturing—Apexian Sublicense Agreement— Review and Approval of Drugs in the United States” in this Annual Report for a discussion of design, development, pre-clinical and clinical activities that we may conduct in the future, including expected cash expenditures required for some of those activities, to the extent we are able to estimate such costs.
Our other cash requirements within the next twelve months include accounts payable, accrued expenses, purchase commitments and other current liabilities. Our other cash requirements greater than twelve months from various contractual obligations and commitments may include operating leases and contractual agreements with third-party service providers for clinical research, product development, manufacturing, commercialization, supplies, payroll, equipment maintenance, and audits for periods into calendar year 2024. Refer to Note 4 – Commitments and Contingencies included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report for further detail of our lease obligation and license agreements with regard to the timing of expected future payments.
We expect to satisfy our short-term and long-term obligations through cash on hand, from future equity and debt financings, and from reimbursement payments, potential milestone and royalty payments under the Nyxol License Agreement and any future collaborations and license agreements, until we generate an adequate level of revenue from commercial sales to cover expenses, if ever.
Critical Accounting Policies and Estimates
Our financial statements are prepared in accordance with U.S. GAAP. These accounting principles require us to make estimates and judgments that can affect the reported amounts of assets and liabilities as of the date of the financial statements as well as the reported amounts of revenue and expense during the periods presented. We believe that the estimates and judgments upon which we rely are reasonably based upon information available to us at the time that we make these estimates and judgments. To the extent that there are material differences between these estimates and actual results, our financial results will be affected. The accounting policies that reflect our more significant estimates and judgments and which we believe are the most critical to aid in fully understanding and evaluating our reported financial results are described below.
Our significant accounting policies are discussed in Note 1 — Company Description and Summary of Significant Accounting Policies, included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report. We believe that the following accounting policies and estimates are the most critical to aid in fully understanding and evaluating our reported financial results. These estimates require our most difficult, subjective, or complex judgments because they relate to matters that are inherently uncertain. We have reviewed these critical accounting policies and estimates and related disclosures with the Audit Committee of our Board of Directors. We have not made any material changes to date, nor do we believe there is a reasonable likelihood of a material future change to the accounting methodologies for the areas described below.
License and Collaborations Revenue
We account for license and collaborations revenue in accordance with the provisions of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers. The guidance provides a unified model to determine how revenue is recognized. We have entered into license and collaboration agreements which have revenue recognition implications. We recognize license and collaborations revenue by first allocating the transaction price of a contract to each performance obligation under the contract based on its stand-alone price. The stand-alone price of each performance obligation is based on its fair value utilizing a discounted cash flow approach, taking into consideration assumptions, including projected worldwide net profit for each of the respective programs based on probability assessments, projections based on internal forecasts, industry data, and information from other guideline companies within the same industry and other relevant factors. To date, we have not had, nor do we expect to have in the future, significant variable consideration adjustments related to our existing license and collaborations revenue recognized. For discussion about the determination of license and collaborations revenue, see Note 10 — License and Collaboration Agreements included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report.
Warrant Liabilities
Following the Merger, we issued the Series A Warrants in connection with the Pre-Merger Financing, and assumed Rexahn warrants issued prior to the Merger. We account for these warrants as a liability at fair value as long as certain provisions precluding equity accounting treatment are present. Upon the execution of the Waiver Agreements described in Note 9 — Stockholders’ Equity included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report, the Series A Warrants were no longer subject to cash settlement or indexation provisions precluding equity classification, and as a result, not subject to fair value remeasurement. We will continue to adjust the Rexahn warrant liability for changes in fair value until the earlier of the exercise, expiration, or until such time that cash settlement or indexation provisions are no longer in effect for the Rexahn warrants. We do not expect that the fluctuations in fair value attributed to the Rexahn warrant liability will be significant.
Stock-based Compensation
We account for stock-based compensation in accordance with the provisions of ASC 718, Compensation — Stock Compensation. Accordingly, compensation costs related to equity instruments granted are recognized at the grant date fair value based on a Black-Scholes model which is not subject to remeasurement. We record equity instrument forfeitures when they occur. For discussions about the application of grant date fair value associated with our stock-based compensation, see Note 7 — Stock-based Compensation included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report.
Income Tax Assets and Liabilities
A full valuation allowance has been provided on our net deferred tax assets given the uncertainty of future taxable income and other related factors impacting the realizability of our remaining net deferred tax assets. For additional information, see Note 12 — Income Taxes included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report.
We are subject to numerous contingencies arising in the ordinary course of business, including obligations related to certain license agreements. For additional information, see Note 4 — Commitments and Contingencies included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report.
Recent Accounting Pronouncements
From time to time the FASB, or other standard-setting bodies, issue new accounting pronouncements. Where applicable, we adopt these new standards according to the specified effective dates. Unless otherwise disclosed in the notes to the financial statements appearing in this Annual Report, we believe that the impact of any recently issued standard(s) that are not yet effective will not have a material impact on our financial position or results of operations upon adoption. See Note 1, “Company Description and Summary of Significant Accounting Policies,” included in “Part II, Item 8 – Financial Statements and Supplementary Data” of this Annual Report for a more in-depth discussion of recently issued accounting standard(s).
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Not applicable.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The information required by this item is included in this Annual Report beginning on page F-1 and is incorporated herein by reference.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
As required by Rule 13a-15(b) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, under the direction of the Chief Executive Officer and the principal financial officer, we have evaluated our disclosure controls and procedures as defined in Rule 13a-15(e) or 15d-15(e) as of the end of the period covered by this Annual Report. Based on this evaluation, our Chief Executive Officer and principal financial officer have concluded that our disclosure controls and procedures were effective as of the end of the period covered by this Annual Report.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of our assets; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and Board; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Our management, including our Chief Executive Officer and principal financial officer, recognizes that our internal control over financial reporting cannot prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
Management, with the participation of the Chief Executive Officer and principal financial officer, assessed our internal control over financial reporting as of December 31, 2022, the end of our fiscal year. Management based its assessment on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on that evaluation, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2022.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d‑15(f) under the Exchange Act) that occurred during the quarter ended December 31, 2022 which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
PART III
We will file a definitive Proxy Statement for our 2023 Annual Meeting of Stockholders (the “2023 Proxy Statement”) with the SEC, pursuant to Regulation 14A, not later than 120 days after the end of our fiscal year. Accordingly, certain information required by Part III has been omitted under General Instruction G(3) to Form 10-K. Only those sections of the 2023 Proxy Statement that specifically address the items set forth herein are incorporated by reference.
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by Item 10 is hereby incorporated by reference to the sections of the 2023 Proxy Statement under the captions “Board and Committee Information”, “Delinquent Section 16(a) Reports”, and “Proposal No. 1 – Election of Directors,” “Executive Officers”.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by Item 11 is hereby incorporated by reference to the sections of the 2023 Proxy Statement under the captions “Executive Compensation” and “Proposal No. 1 – Election of Directors – Non-Employee Director Compensation.”
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by Item 12 is hereby incorporated by reference to the sections of the 2023 Proxy Statement under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation – Securities Authorized for Issuance under Equity Compensation Plans.”
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by Item 13 is hereby incorporated by reference to the sections of the 2023 Proxy Statement under the captions “Certain Relationships and Related-Party Transactions” and “Board and Committee Information.”
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by Item 14 is hereby incorporated by reference to the sections of the 2023 Proxy Statement under the caption “Proposal No. 2 – Ratification of Independent Registered Public Accounting Firm.”
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
The following documents are filed as a part of this Annual Report on Form 10-K:
| | (a) | Financial Statements: The financial statements filed as part of this report are listed in Part II, Item 8. |
| | (b) | Financial Statement Schedules: The schedules are either not applicable or the required information is presented in the consolidated financial statements or notes thereto. |
| | (c) | Exhibits: The following exhibits are incorporated by reference or filed as part of this Annual Report on Form 10-K: |
EXHIBIT NUMBER | | DESCRIPTION OF DOCUMENT |
| | | |
| | Agreement and Plan of Merger, dated as of June 17, 2020, by and among the Registrant, Razor Merger Sub, Inc. and Ocuphire Pharma, Inc. (incorporated by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K, filed on June 19, 2020). |
| | First Amendment to Agreement and Plan of Merger and Reorganization, dated as of June 29, 2020, by and among Rexahn, Merger Sub and Ocuphire (incorporated by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K , filed on July 1, 2020). |
| | Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Appendix G to the Registrant’s Definitive Proxy Statement on Schedule 14A, filed on April 29, 2005). |
| | Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on May 5, 2017). |
| | Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on August 30, 2018). |
| | Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on April 12, 2019). |
| | Certificate of Amendment of Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on November 6, 2020). |
| | Certificate of Amendment of Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.2 to the Registrant’s Current Report on Form 8-K, filed on November 6, 2020). |
| | Second Amended and Restated Bylaws of the Registrant (incorporated by reference to Exhibit 3.3 to the Registrant’s Current Report on Form 8-K, filed on November 6, 2020). |
| | Amendment to Second Amended and Restated Bylaws of Ocuphire Pharma, Inc. (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on June 10, 2022). |
| | Second Amendment to Second Amended and Restated Bylaws of Ocuphire Pharma, Inc. (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on June 17, 2022). |
| | Specimen Certificate for the Registrant’s Common Stock, par value $.0001 per share (incorporated by reference to Exhibit 4.3 to the Registrant’s Registration Statement on Form S-8 (File No. 333-129294), filed on October 28, 2005). |
| | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on October 13, 2017). |
| | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on October 19, 2018). |
| | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on January 25, 2019). |
| | Form of Series A/B Warrants (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on July 1, 2020). |
| | Description of Securities (incorporated by reference to Exhibit 4.11 to the Registrant’s Annual Report on Form 10-K, filed on March 11, 2021). |
| | Form of Warrant to purchase shares of common stock (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K/A, filed on June 7, 2021). |
| | Amended and Restated Employment Agreement by and among the Company and Mina Sooch, effective as of November 5, 2020 (incorporated by reference to Exhibit 10.27 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | First Amendment to the Amended and Restated Employment Agreement by and among the Company and Mina Sooch, effective as of March 26, 2023. |
| | Amended and Restated Employment Agreement by and among the Company and Bernhard Hoffmann, effective as of November 5, 2020 (incorporated by reference to Exhibit 10.29 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | First Amendment to the Amended and Restated Employment Agreement by and among the Company and Bernhard Hoffmann, effective as of March 26, 2023. |
| | Form of Indemnification Agreement (incorporated by reference to Exhibit 10.30 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | Sublicense Agreement, dated as of January 21, 2020, by and between Ocuphire Pharma, Inc. and Apexian Pharmaceuticals, Inc (incorporated by reference to Exhibit 10.31 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | First Amendment to Sublicense Agreement, dated as of June 4, 2020, by and between Apexian Pharmaceuticals, Inc. and Ocuphire Pharma, Inc (incorporated by reference to Exhibit 10.32 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | Lease Agreement, dated as of May 19, 2019, by and between Ocuphire Pharma, Inc. and Duke & Duke, LP (incorporated by reference to Exhibit 10.33 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | First Amendment to Lease Agreement, dated as of October 29, 2019, by and between Ocuphire Pharma, Inc. and Duke & Duke, LP (incorporated by reference to Exhibit 10.34 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | Second Lease Amendment, dated as of November 17, 2020, by and between the Company and Duke & Duke (incorporated by reference to Exhibit 10.43 to the Registrant’s Annual Report on Form 10-K, filed on March 11, 2021). |
| | Third Lease Amendment, dated as of September 9, 2021, by and between the Company and Duke & Duke (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q, filed on November 12, 2021). |
| | Fourth Lease Amendment, dated as of October 17, 2022, by and between the Company and Duke & Duke (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q, filed on November 4, 2022). |
| | Ocuphire Pharma, Inc. 2018 Equity Incentive Plan, dated as of April 9, 2018 (incorporated by reference to Exhibit 10.35 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | First Amendment to 2018 Equity Incentive Plan, dated as of December 23, 2019 (incorporated by reference to Exhibit 10.36 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | Form of Option Agreement issuable under the Ocuphire Pharma, Inc. 2018 Equity Incentive Plan (incorporated by reference to Exhibit 10.37 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | Ocuphire Pharma, Inc. 2020 Equity Incentive Plan (incorporated by reference to Exhibit 10.38 to the Registrant’s Registration Statement on Form S-4 (File No. 333-239702), filed on September 30, 2020). |
| | Form of Restricted Stock Unit Grant Notice issued under the Ocuphire Pharma, Inc. 2020 Equity Incentive Plan. |
| | Form of Stock Option Grant Notice issued under the Ocuphire Pharma, Inc. 2020 Equity Incentive Plan. |
| | Contingent Value Rights Agreement, dated as of November 5, 2020, by and among the Company, Shareholder Representative Services LLC and the Olde Monmouth Stock Transfer Co., Inc. (incorporated by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K, filed on November 6, 2020). |
| | Ocuphire Pharma, Inc. 2021 Inducement Plan (incorporated by reference to Exhibit 10.41 to the Registrant’s Annual Report on Form 10-K, filed on March 11, 2021) |
| | Form of Stock Option Grant Notice issued under the Ocuphire Pharma, Inc. 2021 Inducement Plan |
| | Employment Agreement dated November 11, 2020, by and between the Company and Amy Rabourn (incorporated by reference to Exhibit 10.42 to the Registrant’s Annual Report on Form 10-K, filed on March 11, 2021) |
| | First Amendment to the Employment Agreement by and among the Company and Amy Rabourn, effective as of March 26, 2023. |
| | Capital on Demand™ Sales Agreement, dated March 11, 2021 between the Company and JonesTrading Institutional Services LLC (incorporated by reference to Exhibit 1.1 to the Registrant’s Current Report on Form 8-K, filed on March 11, 2021). |
| | Form of Securities Purchase Agreement, dated as of June 4, 2021, by and among Ocuphire Pharma, Inc. and the purchasers identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K/A, filed on June 7, 2021). |
| | Processa License Agreement dated June 16, 2021 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on June 23, 2021). |
| | Famy License and Collaboration Agreement dated November 6, 2022 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on November 7, 2022). |
| | Consulting Agreement dated April 8, 2022, by and between the Company and Jay Pepose (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q, filed on May 13, 2022). |
| | First Amendment to the Consulting Agreement dated September 19, 2022, by and between the Company and Jay Pepose. |
| | Second Amendment to the Consulting Agreement dated December 1, 2022, by and between the Company and Jay Pepose. |
| | Amended and Restated Non-Employee Director Compensation Policy dated July 1, 2022 (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q, filed on August 12, 2022). |
| | Subsidiaries of the Registrant. |
| | Consent of Ernst & Young, LLP. |
| | Certification of Principal Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | Certification of Principal Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities Exchange Act of 1934, as adopted pursuant to section 302 of the Sarbanes-Oxley Act of 2002. |
| | Certification of Principal Executive Officer and Principal Financial Officer pursuant to Rules 13a-14(b) and 15d-14(b) promulgated under the Securities Exchange Act of 1934 and 18 U.S.C. Section 1350, as adopted pursuant to section 906 of The Sarbanes-Oxley Act of 2002. |
| 101.INS | | Inline XBRL Instance Document (the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document). |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| 104 | | Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
| * | Indicates management contract or compensatory plan. |
| + | Certain schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request. |
++ Portions of this exhibit have been omitted in compliance with Item 601 of Regulation S-K.
| ITEM 16. | FORM 10-K SUMMARY |
None