Table of Contents
As filed with the Securities and Exchange Commission on October 9, 2007
RegistrationNo. 333-144802
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
TRANS1 INC.
(Exact name of registrant as specified in its charter)
| Delaware | 3841 | 33-0909022 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
411 Landmark Drive
Wilmington, NC28412-6303
(910) 332-1700
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Richard Randall
President and Chief Executive Officer
TranS1 Inc.
411 Landmark Drive
Wilmington, NC28412-6303
(910) 332-1700
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Bruce Feuchter, Esq. Michael A. Hedge, Esq. Stradling Yocca Carlson & Rauth 660 Newport Center Drive, Suite 1600 Newport Beach, California 92660 (949) 725-4000 | Marc D. Jaffe, Esq. Latham & Watkins LLP 885 Third Avenue New York, New York 10022 (212) 906-1200 | |
| B. Shayne Kennedy, Esq. Latham & Watkins LLP 650 Town Center Drive, 20th Floor Costa Mesa, California 92626 (714) 540-1235 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
| The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the SEC is effective. This prospectus is not an offer to sell and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted. |
Subject to Completion, dated October 9, 2007
5,500,000 shares

Common Stock
This is the initial public offering of shares of common stock by TranS1 Inc. We are offering 5,500,000 shares of our common stock. Prior to this offering, there has been no public market for our common stock. The initial public offering price of our common stock is expected to be between $12.00 and $14.00 per share. We have applied to have our common stock approved for quotation on The Nasdaq Global Market under the symbol “TSON.”
This investment involves a high degree of risk. See “Risk Factors” beginning
on page 8 of this prospectus.
| Per Share | Total | |||||||
| Public Offering Price | $ | $ | ||||||
| Underwriting Discount | $ | $ | ||||||
| Proceeds, Before Expenses, to TranS1 Inc. | $ | $ | ||||||
We have granted the underwriters a30-day option to purchase up to an additional 825,000 shares from us on the same terms and conditions as set forth above to the extent that the underwriters sell more than 5,500,000 shares of common stock in this offering. The underwriters expect to deliver the shares of common stock to investors on or about , 2007.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Lehman Brothers | Piper Jaffray |
| Cowen and Company | Wachovia Securities |
The date of this prospectus is , 2007
Table of Contents
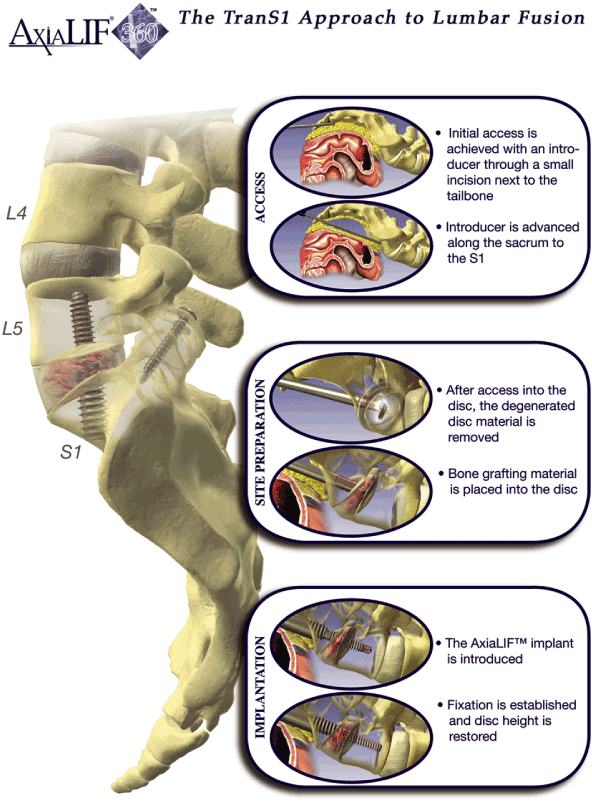
TABLE OF CONTENTS
| 1 | ||||||||
| 8 | ||||||||
| 28 | ||||||||
| 29 | ||||||||
| 29 | ||||||||
| 30 | ||||||||
| 32 | ||||||||
| 34 | ||||||||
| 36 | ||||||||
| 47 | ||||||||
| 65 | ||||||||
| 70 | ||||||||
| 84 | ||||||||
| 86 | ||||||||
| 89 | ||||||||
| 93 | ||||||||
| 95 | ||||||||
| 98 | ||||||||
| 103 | ||||||||
| 103 | ||||||||
| 103 | ||||||||
| F-1 | ||||||||
| EXHIBIT 3.2 | ||||||||
| EXHIBIT 5.1 | ||||||||
| EXHIBIT 23.2 | ||||||||
You should rely only on the information contained in this prospectus and in any free writing prospectus authorized by us. We have not, and the underwriters have not, authorized anyone to provide you with different information. We are not making offers to sell or seeking offers to buy these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information contained in this prospectus is accurate as of the date on the front of this prospectus only, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, operating results and prospects may have changed since that date.
Market and Industry Data
Unless otherwise indicated, information contained in this prospectus concerning the medical device industry, the spinal surgery market, including our general expectations and market position, market opportunity and market share, is based on information from independent industry analysts and third-party sources and management estimates. Management estimates are derived from publicly available information released by independent industry analysts and third-party sources, as well as our own management’s experience in the industry, and are based on assumptions made by us based on such data and our knowledge of such industry and markets, which we believe to be reasonable. Other than Millennium Research Group, which prepared a report at our request and expense for general marketing purposes, none of the sources cited in this prospectus has consented to the inclusion of any data from its reports, nor have we sought their consent. Our estimates have not been verified by any independent source, and we have not independently verified any third-party information. In addition, while we believe our market position, market opportunity and market share information included in this prospectus is generally reliable, such information is inherently imprecise. Such data involves risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors.”
Table of Contents
The items in the following summary are described in more detail later in this prospectus. This summary does not contain all the information you should consider before investing in our common stock. You should carefully read the more detailed information set out in this prospectus, especially the risks of investing in our common stock that we discuss under the “Risk Factors” section, as well as the financial statements and the related notes to those statements included elsewhere in this prospectus. References in this prospectus to “we,” “us,” “our,” “company” and “TranS1” refer to TranS1 Inc. unless the context requires otherwise.
Our Business
We are a medical device company focused on designing, developing and marketing products that implement our proprietary minimally invasive surgical approach to treat degenerative disc disease affecting the lower lumbar region of the spine. Using this TranS1 approach, a surgeon can access discs in the lower lumbar region of the spine through a 1.5 cm incision adjacent to the tailbone and can perform an entire fusion procedure through a small tube that provides direct access to the degenerative disc. We developed our TranS1 approach to allow spine surgeons to access and treat degenerative lumbar discs without compromising important surrounding soft tissue. We believe this approach enables fusion procedures to be performed with low complication rates, short procedure times, low blood loss, short hospital stays, fast recovery times and reduced pain. We have developed and currently market in the United States and Europe two single-level fusion products, AxiaLIF and AxiaLIF 360°. In addition, we have developed and currently market in Europe a two-level fusion product, AxiaLIF 2L, which has not yet been cleared in the United States. All of our products are delivered using our TranS1 approach.
Our AxiaLIF product received 510(k) clearance in December 2004 and a CE mark in March 2005 and was commercially launched in the United States in January 2005. Our AxiaLIF 360° product received 510(k) clearance in September 2005 and a CE mark in March 2006 and was commercially launched in the United States in July 2006. We received a CE mark for our AxiaLIF 2L product in the third quarter of 2006 and began commercialization in the European market in the fourth quarter of 2006. We intend to commercialize our AxiaLIF 2L product in the United States after receipt of 510(k) clearance. We are currently conducting additional testing requested by the U.S. Food and Drug Administration, or FDA, in support of our 510(k) clearance for our AxiaLIF 2L product. We expect to obtain FDA 510(k) clearance for, and commercially launch, our AxiaLIF 2L product in the United States in 2008. As of August 31, 2007, over 2,000 fusion procedures have been performed globally using our AxiaLIF products. We currently sell our AxiaLIF products through 23 direct sales personnel and 21 independent sales agents in the United States and seven independent distributors internationally. By mid-2008, we expect to have increased the number of our direct sales representatives to 50. Our revenues were $5.8 million for the year ended December 31, 2006 and $7.2 million for the six months ended June 30, 2007. Our net losses were $9.5 million for the year ended December 31, 2006 and $4.1 million for the six months ended June 30, 2007.
We also have two additional products under development that implement our TranS1 approach: our Percutaneous Nucleus Replacement, or PNR, and Partial Disc Replacement, or PDR, devices, both of which we expect to commercialize in the United States and Europe. We expect these devices will be used earlier in the treatment of degenerative disc disease than a fusion, as they are designed to preserve motion in the lumbar spine while providing stabilization. Our PNR implant is currently in early-stage human clinical trials outside the United States, and we expect to commercialize this product in Europe in the second half of 2008. We expect to file an investigational device exemption, or IDE, with the FDA in 2008 for our PNR implant in support of U.S. commercialization. We anticipate commencing human clinical trials of our PDR implant outside the United States in 2008.
Market Opportunity
Degenerative disc disease is a common medical condition affecting the lower lumbar spine and refers to the degeneration of the disc from aging and repetitive stresses resulting in a loss of flexibility, elasticity and shock-absorbing properties. As degenerative disc disease progresses, the space between the vertebrae narrows, which can pinch the nerves exiting the spine resulting in back pain, leg pain, numbness and loss of motor
1
Table of Contents
function. This lower back pain can be overwhelming for patients as the resulting pain can have significant physical, psychological and financial implications.
Patients suffering from lower back pain are typically treated initially with conservative therapies, which include rest, bracing, physical therapy, chiropractics, electrical stimulation and drugs. When conservative therapies fail to provide adequate pain relief, surgical interventions, including fusion procedures, may be used to address the pain. Fusion attempts to alleviate lower back pain by removing problematic disc material and permanently joining together two or more opposing vertebrae. This is done in a manner that restores the appropriate space between the vertebrae surrounding the degenerative disc and eliminates mobility of the affected vertebrae. By restoring disc height and eliminating motion, fusion attempts to prevent the pinching of the nerves exiting the spine thereby reducing pain.
Lower back pain affects over six million people annually in the United States and is a leading cause of healthcare expenditures globally. Our currently marketed products address the lower lumbar spine fusion market, which Millennium Research Group valued at over $1.4 billion in the United States in 2006 and estimated to grow to $1.8 billion by 2011. We believe the introduction of minimally invasive spine procedures, such as ours, will attract more back pain patients, and attract them earlier, to a definitive surgical solution, thereby increasing the rate at which the lower lumbar spine fusion market grows.
Limitations of Current Lumbar Surgical Procedures
The primary surgical fusion procedures performed in the lower lumbar region of the spine are generally referred to by their approach to the spine and include anterior, posterior, transforaminal and extreme lateral interbody fusion. Typically, a spine surgeon performs an additional surgical procedure following the fusion procedure to implant screws and rods in the back to provide additional support while the vertebrae fuse together. These two procedures together are referred to as a 360° fusion, which is generally considered the most structurally rigid fusion. Most of the current fusion treatment alternatives available for lower lumbar spine conditions are available in minimally invasive approaches. While current fusion alternatives can be effective at reducing lower back pain, common drawbacks of these procedures, traditional and minimally invasive, include:
| • | disruption to soft tissue and support structures; | |
| • | significant blood loss between 100 and 1,400 cc; | |
| • | lengthy operative procedure times between 90 minutes and 4 hours; | |
| • | lengthy patient hospital stays averaging three days; | |
| • | significant patient recovery time requiring three to six months recovery and rehabilitation; and | |
| • | unresolved patient pain related to muscle dissection, implant irritation and scar tissue. |
We believe that while current fusion procedures for lower lumbar spine procedures can provide effective therapy for patients, there is a significant opportunity for improvement in efficacy, efficiency and cost due to the limitations described above.
Our Solution
We believe we have developed what may be the least invasive approach for spine surgeons to perform fusion and motion preserving surgeries in the lower lumbar, or L4/L5/S1, region of the spine, which can reduce the drawbacks associated with current lumbar fusion procedures. We believe our TranS1 approach and associated products provide the following benefits for patients, providers and payors:
| • | Least Invasive Approach Minimizes Complications. All of our products are delivered using our TranS1 approach. Procedures performed utilizing our TranS1 approach have been documented to have favorable clinical safety profiles with complication rates of approximately 1%. |
2
Table of Contents
| • | Ease of Use and Short Procedure Times. We have received feedback from practicing spine surgeons that they can perform our AxiaLIF procedure utilizing our TranS1 approach within 45 to 60 minutes after just two to three surgeries. We also believe that these short procedure times can result in reduced patient recovery times, decreased costs for the hospitals and increased productivity for the spine surgeons. | |
| • | Low Blood Loss. We believe the minimally invasive nature of our TranS1 approach results in the average patient losing approximately 25 to 125 cc of blood during an AxiaLIF or AxiaLIF 360° procedure, which we believe correlates to reduced pain and faster recoveries. Given the associated low blood loss, patients generally do not need to donate blood prior to undergoing procedures that utilize our TranS1 approach. | |
| • | Short Patient Hospital Stays. Patients typically stay only one night in the hospital after receiving a procedure performed using our TranS1 approach. In a small percentage of cases, patients are able to return home the same day. | |
| • | Reduction in Patient Pain. We believe our TranS1 approach is effective at reducing lower back pain because surrounding soft tissue is not compromised, which prevents the creation of scar tissue, a leading cause of pain. |
Our Strategy
Our goal is to become a global leader in the treatment of conditions affecting the L4/L5/S1 region of the lumbar spine utilizing our TranS1 approach and associated products. To achieve this goal, we are pursuing the following strategies:
| • | establish our TranS1 approach as a standard of care for lower lumbar spine surgery; | |
| • | expand our sales and marketing infrastructure to drive surgeon adoption; | |
| • | broaden the clinical applications of our TranS1 approach; and | |
| • | opportunistically pursue acquisitions of complementary businesses and technologies. |
Risks Associated with Our Business
Our business is subject to numerous risks, as discussed more fully in the section entitled “Risk Factors” following this prospectus summary. Some of these risks include:
| • | The potential reluctance or failure altogether of spine surgeons to adopt our products as safe and effective alternatives to existing surgical treatments of certain spine disorders given that the efficacy of our products is not yet supported by long-term clinical data. | |
| • | Our limited operating history. We have incurred net losses since our inception and, through June 30, 2007, we had an accumulated deficit of $26.4 million. We may never achieve profitability. | |
| • | Our ability to develop, receive approval for, and introduce new products or product enhancements. Some new products may require lengthy clinical trials to support regulatory clearance or approval and we may not successfully complete these clinical trials. | |
| • | Our ability to effectively maintain and prosecute adequate intellectual property protection, obtain and maintain regulatory clearances or approvals for our products, continue building effective sales and marketing capabilities and the ability of our customers to obtain adequate third-party coverage and reimbursement for their purchase of our products. | |
| • | Our ability to compete with large international companies who have greater resources in sales and marketing, manufacturing and research and development than we do. |
3
Table of Contents
Corporate Information
We were incorporated in Delaware in May 2000 under the name “aXiaMed, Inc.” and changed our name to “TranS1 Inc.” in February 2003. Our principal executive office is located at 411 Landmark Drive, Wilmington, North Carolina28412-6303 and our telephone number is(910) 332-1700. Our website is located at www.trans1.com. The information on, or that can be accessed through, our website is not incorporated by reference into this prospectus and should not be considered to be a part of this prospectus.
We own trademark registrations for the marks TranS1® and AxiaLIF® in the United States and the European Union. We own six pending United States trademark applications in the United States for the following marks: 3D Axial RodTM, TranS1 StructsureTM, AxiaLIF 2LTM, AxiaLIF 360°TM, PNRTM and TranS1 PDRTM, and two pending trademark applications in the European Union for the marks AxiaLIF 2L and AxiaLIF 360°.
4
Table of Contents
The Offering
| Common stock offered by us | 5,500,000 shares | |
| Common stock to be outstanding after this offering | 18,776,502 shares | |
| Initial public offering price | $ per share | |
| Use of proceeds | We intend to use the net proceeds of this offering to support the commercialization of our existing and future products and to support our research and development activities, clinical trials, regulatory approvals and for capital expenditures, working capital and other general corporate purposes. See “Use of Proceeds.” | |
| Proposed Nasdaq Global Market symbol | TSON | |
| Risk factors | Investing in our common stock involves certain risks. See the risk factors described under the heading “Risk Factors” beginning on page 8 of this prospectus and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. |
The number of shares of our common stock that will be outstanding after this offering is based on 13,276,502 shares of our common stock outstanding as of June 30, 2007, and excludes:
| • | 2,339,663 shares of our common stock issuable upon exercise of outstanding options under our existing Amended and Restated 2000 Stock Incentive Plan, at a weighted average exercise price of $1.74 per share; | |
| • | 1,400,000 shares of our common stock available for future issuance under our 2007 Stock Incentive Plan, which is currently effective, and 250,000 shares of our common stock available for future grant under our 2007 Employee Stock Purchase Plan, which has been adopted and will become effective upon the completion of this offering; and | |
| • | automatic annual increases in the number of shares of common stock reserved for issuance under our 2007 Employee Stock Purchase Plan. |
Except as otherwise noted, all information in this prospectus assumes:
| • | a 0.9-for-1 reverse stock split of our common stock and preferred stock on or before the closing of this offering; | |
| • | no exercise of the underwriters’ option to purchase additional shares; | |
| • | the adoption of our amended and restated certificate of incorporation and amended and restated bylaws effective immediately upon the closing of this offering; and | |
| • | the conversion of all our outstanding preferred stock into 10,793,182 shares of our common stock immediately upon the closing of this offering. |
5
Table of Contents
Summary Financial Data
The following tables summarize our financial data for the periods presented. The statements of operations data for the years ended December 31, 2004, 2005 and 2006 have been derived from our audited financial statements, included elsewhere in this prospectus. The statement of operations data for the year ended December 31, 2003 has been derived from our audited financial statements not included in this prospectus. The statement of operations data for the year ended December 31, 2002 has been derived from our unaudited financial statements not included in this prospectus. The balance sheet data as of June 30, 2007 and the statements of operations data for the six months ended June 30, 2006 and 2007 have been derived from our unaudited financial statements, included elsewhere in this prospectus. We have prepared the unaudited financial statements on the same basis as our audited financial statements and, in our opinion, have included all adjustments, which include only normal recurring adjustments, necessary to present fairly our financial position and results of our operations for each of the periods mentioned. The historical results are not necessarily indicative of the results to be expected for any future periods and the results for the six months ended June 30, 2007 should not be considered indicative of results expected for the full fiscal year. You should read the following financial information together with the information under “Selected Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| Six Months Ended | ||||||||||||||||||||||||||||
| Year Ended December 31, | June 30, | |||||||||||||||||||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||||||
| (In thousands, except share data) | ||||||||||||||||||||||||||||
Statements of Operations Data: | ||||||||||||||||||||||||||||
| Revenue | $ | — | $ | — | $ | — | $ | 1,469 | $ | 5,812 | $ | 2,161 | $ | 7,187 | ||||||||||||||
| Cost of revenue | — | — | — | 386 | 1,580 | 618 | 1,459 | |||||||||||||||||||||
| Gross profit | — | — | — | 1,083 | 4,232 | 1,543 | 5,728 | |||||||||||||||||||||
| Operating expenses | ||||||||||||||||||||||||||||
| Research and development | 483 | 1,620 | 1,758 | 2,444 | 4,246 | 2,094 | 2,278 | |||||||||||||||||||||
| Sales and marketing | — | 163 | 746 | 2,635 | 9,288 | 3,950 | 6,837 | |||||||||||||||||||||
| General and administrative | 561 | 927 | 1,117 | 1,293 | 1,166 | 625 | 1,083 | |||||||||||||||||||||
| Total operating expenses | 1,044 | 2,710 | 3,621 | 6,372 | 14,700 | 6,669 | 10,198 | |||||||||||||||||||||
| Operating loss | (1,044 | ) | (2,710 | ) | (3,621 | ) | (5,289 | ) | (10,468 | ) | (5,126 | ) | (4,470 | ) | ||||||||||||||
| Interest income | 19 | 86 | 150 | 264 | 858 | 528 | 343 | |||||||||||||||||||||
| Other income (expense) | — | — | — | (17 | ) | 131 | — | — | ||||||||||||||||||||
| Net loss | $ | (1,025 | ) | $ | (2,624 | ) | $ | (3,471 | ) | $ | (5,042 | ) | $ | (9,479 | ) | $ | (4,598 | ) | $ | (4,127 | ) | |||||||
Net loss per common share — basic and diluted(1) | $ | (0.63 | ) | $ | (1.28 | ) | $ | (1.63 | ) | $ | (2.25 | ) | $ | (3.91 | ) | $ | (1.90 | ) | $ | (1.67 | ) | |||||||
Weighted average common shares outstanding — basic and diluted(1) | 1,637,719 | 2,046,753 | 2,127,856 | 2,243,019 | 2,423,226 | 2,418,154 | 2,467,550 | |||||||||||||||||||||
Pro forma net loss per common share — basic and diluted (unaudited)(1) | $ | (0.72 | ) | $ | (0.31 | ) | ||||||||||||||||||||||
Pro forma weighted average shares outstanding — basic and diluted (unaudited)(1) | 13,216,407 | 13,260,732 | ||||||||||||||||||||||||||
| (1) | See note 2 of the notes to our financial statements included elsewhere in this prospectus for an explanation of the determination of the number of shares used in computing per share data. |
6
Table of Contents
| June 30, 2007 | ||||||||||||
| Pro Forma | ||||||||||||
| Actual | Pro Forma(1) | As Adjusted(2) | ||||||||||
| (Unaudited) | ||||||||||||
| (In thousands) | ||||||||||||
Balance Sheet Data: | ||||||||||||
| Cash, cash equivalents and short-term investments | $ | 10,172 | $ | 10,172 | $ | 74,667 | ||||||
| Working capital | 14,306 | 14,306 | 78,801 | |||||||||
| Total assets | 18,193 | 18,193 | 82,688 | |||||||||
| Preferred stock | 40,089 | — | — | |||||||||
| Common stock | — | 1 | 2 | |||||||||
| Additional paid in capital | 1,889 | 41,977 | 106,471 | |||||||||
| Total stockholders’ equity (deficit) | (24,549 | ) | 15,540 | 80,035 | ||||||||
| (1) | The pro forma column gives effect to the conversion of all of our outstanding preferred stock into 10,793,182 shares of our common stock immediately upon the closing of this offering. | |
| (2) | The pro forma as adjusted column gives further effect to the sale of shares of our common stock in this offering at an assumed offering price of $13.00 per share, the midpoint of the range on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering costs payable by us. A $1.00 increase or decrease in the assumed public offering price of $13.00 per share would increase or decrease, respectively, each line item in the balance sheet data above by approximately $5.1 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions. |
7
Table of Contents
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors, as well as the other information in this prospectus, before deciding whether to invest in shares of our common stock. If any of the following risks actually occur, our business, financial condition, operating results and prospects would suffer. In that case, the trading price of our common stock would likely decline and you might lose all or part of your investment in our common stock. The risks described below are not the only ones we face. Additional risks that we currently do not know about or that we currently believe to be immaterial may also impair our operations and business results.
Risks Related to Our Business
To be commercially successful, spine surgeons must accept that our products are a safe and effective alternative to existing surgical treatments of certain spine disorders.
Our revenue is derived entirely from sales of our AxiaLIF products and related surgical instruments. We expect that sales of our AxiaLIF products will continue to account for substantially all of our revenues for the foreseeable future. We believe spine surgeons may not widely adopt our products unless they determine, based on experience, long-term clinical data and published peer reviewed journal articles, that our products provide a safe and effective alternative to conventional procedures used to treat certain spine disorders. Spine surgeons may be slow to adopt our technology for the following reasons, among others:
| • | lack of long-term clinical data supporting additional patient benefits; | |
| • | lack of experience with our products; | |
| • | lack of evidence supporting cost savings of our procedure over existing surgical alternatives; | |
| • | perceived liability risks generally associated with the use of new products and procedures; | |
| • | training time required to use a new product; and | |
| • | availability of adequate coverage and reimbursement for hospitals and surgeons. |
If we are unable to effectively demonstrate to spine surgeons the benefits of our products as compared to existing surgical treatments of spine disorders and our products fail to achieve market acceptance, our future revenues will be adversely impacted. In addition, we believe recommendations and support of our products by influential spine surgeons are essential for market acceptance and adoption. If we do not receive support from these spine surgeons or have favorable long-term clinical data, spine surgeons may not use our products and our future revenues will be harmed and our stock price would likely decline.
The efficacy of our products is not yet supported by long-term clinical data and may therefore prove to be less effective than initially thought.
We obtained 510(k) clearance to manufacture, market and sell all of our currently marketed products from the FDA. The FDA’s 510(k) clearance process is less costly and rigorous than the premarket approval, or PMA, process and requires less supporting clinical data. As a result, we currently lack the breadth of published long-term clinical data supporting the efficacy of our AxiaLIF and AxiaLIF 360° products and the benefits they offer that might have been generated in connection with the PMA process. In addition, we may determine from post-market experience that certain patient characteristics, such as age or preexisting medical conditions, could affect fusion rates, which could lead to misleading or contradictory data on the efficacy of our products. For these reasons, spine surgeons may be slow to adopt our products. Also, we may not be able to generate the comparative data that our competitors have or are generating and we may be subject to greater regulatory and product liability risks. Further, any long-term safety or efficacy data we generate may not be consistent with our existing data and may demonstrate less favorable safety or efficacy. These results could reduce demand for our products, significantly reduce our ability to achieve expected revenues and could prevent us from becoming profitable. Moreover, if future results and experience indicate that our products cause unexpected or
8
Table of Contents
serious complications or other unforeseen negative effects, we could be subject to significant legal and regulatory liability and harm to our business reputation.
Our future growth depends on increasing physician awareness of our TranS1 approach and our related products for appropriate treatment, intervention and referral.
We target our sales and education efforts to spine surgeons. However, the initial point of contact for many patients may be primary care physicians who commonly treat patients experiencing lower lumbar spine pain. We believe that we must educate physicians to change their screening and referral practices. If we do not educate referring physicians about lower lumbar spine conditions in general, and the existence of the TranS1 approach and our related products in particular, they may not refer patients who are candidates for the procedures utilizing our TranS1 approach to spine surgeons, and those patients may go untreated or receive conservative, non-operative therapies. If we are not successful in educating physicians about screening for lower lumbar spine conditions or about referral opportunities, our ability to increase our revenue may be impaired.
We have a limited operating history and have incurred losses since inception and we expect to incur increasing losses for the foreseeable future. We may never achieve or sustain profitability.
We were incorporated in May 2000 and began commercial sales of our products in early 2005. We have incurred net losses since our inception and through June 30, 2007, we had an accumulated deficit of $26.4 million. To date, we have financed our operations primarily through private placements of our equity securities and have devoted substantially all of our resources to research and development of our products and the commercial launch of our AxiaLIF products. We expect our expenses to increase significantly in connection with our additional clinical trials and research and development activities, as well as to support the expansion of our sales and marketing efforts. Additionally, following this offering, our general and administrative expense will increase due to the additional operational and reporting costs associated with being a public company. As a result, we expect to continue to incur significant operating losses for the foreseeable future. These losses will continue to have an adverse effect on our stockholders’ equity and we may never achieve or sustain profitability.
We are in a highly competitive market segment, which is subject to rapid technological change. If our competitors are better able to develop and market products that are safer, more effective, less costly or otherwise more attractive than any products that we may develop, our ability to generate revenue will be reduced or eliminated.
The market for treatment of spine disorders is highly competitive and subject to rapid and profound technological change. Our success depends, in part, upon our ability to maintain a competitive position in the development of technologies and products for use in the treatment of spine disorders. We face competition from both established and development stage companies. Many of the companies developing or marketing competing products are publicly traded or are divisions of publicly-traded companies, and these companies enjoy several competitive advantages, including:
| • | greater financial and human resources for product development, sales and marketing and patent litigation; | |
| • | significantly greater name recognition; | |
| • | established relationships with spine surgeons, customers and third-party payors; | |
| • | additional lines of products, and the ability to offer rebates or bundle products to offer greater discounts or incentives to gain a competitive advantage; | |
| • | established sales and marketing, and distribution networks; and |
9
Table of Contents
| • | greater experience in conducting research and development, manufacturing, clinical trials, preparing regulatory submissions and obtaining regulatory clearance or approval for products and marketing approved products. |
Our competitors may develop and patent processes or products earlier than us, obtain regulatory clearance or approvals for competing products more rapidly than us, and develop more effective or less expensive products or technologies that render our technology or products obsolete or non-competitive. We also compete with our competitors in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient enrollment in clinical trials, as well as in acquiring technologies and technology licenses complementary to our products or advantageous to our business. If our competitors are more successful than us in these matters, our business may be harmed.
Our failure to continue building effective sales and marketing capabilities for our products could significantly impair our ability to increase sales of our products.
We commercially launched our AxiaLIF product in 2005 and our AxiaLIF 360° product in 2006 and have limited experience marketing and selling our products. We utilize a hybrid model of independent sales agents and direct sales representatives for product sales in the United States and rely solely on third-party distributors for international sales. We currently employ 23 direct sales representatives and expect that we will need to increase that number significantly to continue to grow our business. We have limited experience managing a direct sales force, which can be an expensive and time consuming process. If we are unable to sufficiently increase the number of direct sales representatives and efficiently manage those individuals, our sales will suffer. We also rely on marketing arrangements with independent sales agents in the United States and independent distributors in Europe, in particular their sales and service expertise and relationships with the customers in the marketplace. We do not control, nor monitor the marketing practices of, our independent sales agents or distributors and they may not be successful in implementing our marketing plans or complying with applicable laws regarding marketing practices. Independent distributors and sales agents may terminate their relationship with us, or devote insufficient sales efforts to our products. Our failure to maintain our existing relationships with our independent sales agents or distributors, or our failure to recruit and retain additional skilled independent sales distributors and sales agents or directly-employed sales professionals, could have an adverse effect on our operations.
The demand for our products and the prices which customers and patients are willing to pay for our products depend upon the ability of our customers to obtain adequate third-party coverage and reimbursement for their purchases of our products.
Sales of our products depend in part on the availability of adequate coverage and reimbursement from governmental and private payors. In the United States, healthcare providers that purchase our products generally rely on third-party payors, principally Medicare, Medicaid and private health insurance plans, to pay for all or a portion of the costs and fees associated with the AxiaLIF procedure. While our currently marketed products are eligible for reimbursement in the United States, if surgical procedures utilizing our products are performed on an outpatient basis, it is possible that private payors may no longer provide reimbursement for our products without further supporting data on our procedure. Any delays in obtaining, or an inability to obtain, adequate coverage or reimbursement for procedures using our products could significantly affect the acceptance of our products and have a material adverse effect on our business. Additionally, third-party payors continue to review their coverage policies carefully for existing and new therapies and can, without notice, deny coverage for treatments that include the use of our products. Our business would be negatively impacted to the extent any such changes reduce reimbursement for our products.
With respect to coverage and reimbursement outside of the United States, reimbursement systems in international markets vary significantly by country, and by region within some countries, and reimbursement approvals must be obtained on acountry-by-country basis and can take up to 18 months, or longer. Many international markets have government-managed healthcare systems that govern reimbursement for new devices and procedures. In most markets, there are private insurance systems as well as government-managed systems. Additionally, some foreign reimbursement systems provide for limited payments in a given period
10
Table of Contents
and therefore result in extended payment periods. Reimbursement in international markets may require us to undertake country-specific reimbursement activities, including additional clinical studies, which could be time consuming, expensive and may not yield acceptable reimbursement rates.
Furthermore, healthcare costs have risen significantly over the past decade. There have been and may continue to be proposals by legislators, regulators and third-party payors to contain these costs. These cost-control methods include prospective payment systems, capitated rates, group purchasing, redesign of benefits, requiring pre-authorizations or second opinions prior to major surgery, encouragement of healthier lifestyles and exploration of more cost-effective methods of delivering healthcare. Some healthcare providers in the United States have adopted or are considering a managed care system in which the providers contract to provide comprehensive healthcare for a fixed cost per person. Healthcare providers may also attempt to control costs by authorizing fewer elective surgical procedures or by requiring the use of the least expensive devices possible. These cost-control methods also potentially limit the amount which healthcare providers may be willing to pay for medical devices. In addition, in the United States, no uniform policy of coverage and reimbursement for medical technology exists among all these payors. Therefore, coverage of and reimbursement for medical technology can differ significantly from payor to payor. The continuing efforts of third-party payors, whether governmental or commercial, whether inside the United States or outside, to contain or reduce these costs, combined with closer scrutiny of such costs, could restrict our customers’ ability to obtain adequate coverage and reimbursement from these third-party payors. The cost containment measures that healthcare providers are instituting both in the United States and internationally could harm our business by adversely affecting the demand for our products or the price at which we can sell our products.
Our future success depends on our ability to timely develop, receive regulatory clearance or approval, and introduce new products or product enhancements that will be accepted by the market.
It is important to our business that we continue to build a more complete product offering for treatment of spine disorders. As such, our success will depend in part on our ability to develop and introduce new products and enhancements to our existing products to keep pace with the rapidly changing spine market. However, we may not be able to successfully develop and obtain regulatory clearance or approval for product enhancements, or new products or our future products, or these products may not be accepted by spine surgeons or the payors who financially support many of the procedures performed with our products.
The success of any new product offering or enhancement to an existing product will depend on several factors, including our ability to:
| • | properly identify and anticipate spine surgeon and patient needs; | |
| • | develop and introduce new products or product enhancements in a timely manner; | |
| • | avoid infringing upon the intellectual property rights of third parties; | |
| • | demonstrate, if required, the safety and efficacy of new products with data from preclinical studies and clinical trials; | |
| • | obtain the necessary regulatory clearances or approvals for new products or product enhancements; | |
| • | be fullyFDA-compliant with marketing of new devices or modified products; | |
| • | provide adequate training to potential users of our products; | |
| • | receive adequate coverage and reimbursement for procedures performed with our products; and | |
| • | develop an effective andFDA-compliant, dedicated marketing and distribution network. |
If we do not develop new products or product enhancements in time to meet market demand or if there is insufficient demand for these products or enhancements, our results of operations will suffer.
11
Table of Contents
If clinical trials of our current or future product candidates do not produce results necessary to support regulatory clearance or approval in the United States or elsewhere, we will be unable to commercialize these products.
We have several product candidates in our development pipeline, including our Percutaneous Nucleus Replacement, or PNR, and Partial Disc Replacement, or PDR, devices which we expect will require premarket approval, or PMA, from the FDA. In addition, the FDA has indicated that our two-level AxiaLIF 2L implant, which we anticipate will require 510(k) clearance, will require additional safety and efficacy data beyond the typical 510(k) requirements prior to clearance. A PMA application must be supported by extensive information including, technical data, preclinical and clinical trial data, and manufacturing and labeling information to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. As a result, to receive regulatory approval for our products requiring PMA approval, we must conduct, at our own expense, adequate and well controlled clinical trials to demonstrate efficacy and safety in humans for their intended uses. Clinical testing is expensive, typically takes many years and has an uncertain outcome. The initiation and completion of any of these studies may be prevented, delayed or halted for numerous reasons, including, but not limited to, the following:
| • | the FDA, institutional review boards or other regulatory authorities do not approve a clinical study protocol, force us to modify a previously approved protocol, or place a clinical study on hold; | |
| • | patients do not enroll in, or enroll at the expected rate, or complete a clinical study; | |
| • | patients or investigators do not comply with study protocols; | |
| • | patients do not return for post-treatmentfollow-up at the expected rate; | |
| • | patients experience serious or unexpected adverse side effects for a variety of reasons that may or may not be related to our products such as the advanced stage of co-morbidities that may exist at the time of treatment, causing a clinical study to be put on hold; | |
| • | sites participating in an ongoing clinical study may withdraw, requiring us to engage new sites; | |
| • | difficulties or delays associated with bringing additional clinical sites on-line; | |
| • | third-party clinical investigators decline to participate in our clinical studies, do not perform the clinical studies on the anticipated schedule or consistent with the investigator agreement, clinical study protocol, good clinical practices, and other FDA and Institutional Review Board requirements; | |
| • | third-party organizations do not perform data collection and analysis in a timely or accurate manner; | |
| • | regulatory inspections of our clinical studies require us to undertake corrective action or suspend or terminate our clinical studies; | |
| • | changes in U.S. federal, state, or foreign governmental statutes, regulations or policies; | |
| • | interim results are inconclusive or unfavorable as to immediate and long-term safety or efficacy; or | |
| • | the study design is inadequate to demonstrate safety and efficacy. |
Clinical failure can occur at any stage of the testing. Our clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinicaland/or non-clinical testing in addition to those we have planned. Our failure to adequately demonstrate the efficacy and safety of any of our devices would prevent receipt of regulatory clearance or approval and, ultimately, the commercialization of that device.
Our international operations subject us to certain operating risks, which could adversely impact our net sales, results of operations and financial condition.
Sales of our products outside the United States represented 6.2% of our revenue in 2006. To date, we have sold our products in the following countries outside of the United States: United Kingdom, Spain, Sweden, Austria, Germany, Switzerland, Turkey, the Netherlands, Belgium and Israel. The sale and shipment
12
Table of Contents
of our products across international borders, as well as the purchase of components and products from international sources, subject us to extensive U.S. and foreign governmental trade, import and export, and custom regulations and laws. Compliance with these regulations is costly and exposes us to penalties for non-compliance. Other laws and regulations that can significantly impact us include various anti-bribery laws, including the U.S. Foreign Corrupt Practices Act and anti-boycott laws. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments, restrictions on certain business activities, and exclusion or debarment from government contracting. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our shipping and sales activities.
In addition, many of the countries in which we sell our products are, to some degree, subject to political, economic or social instability. Our international operations expose us and our distributors to risks inherent in operating in foreign jurisdictions. These risks include:
| • | the imposition of additional U.S. and foreign governmental controls or regulations; | |
| • | the imposition of costly and lengthy new export licensing requirements; | |
| • | the imposition of U.S. or international sanctions against a country, company, person or entity with whom we do business that would restrict or prohibit continued business with the sanctioned country, company, person or entity; | |
| • | economic instability; | |
| • | a shortage of high-quality sales people and distributors; | |
| • | changes in third-party reimbursement policies that may require some of the patients who receive our products to directly absorb medical costs or that may necessitate the reduction of the selling prices of our products; | |
| • | changes in duties and tariffs, license obligations and other non-tariff barriers to trade; | |
| • | the imposition of new trade restrictions; | |
| • | the imposition of restrictions on the activities of foreign agents, representatives and distributors; | |
| • | scrutiny of foreign tax authorities which could result in significant fines, penalties and additional taxes being imposed on us; | |
| • | pricing pressure that we may experience internationally; | |
| • | laws and business practices favoring local companies; | |
| • | longer payment cycles; | |
| • | difficulties in maintaining consistency with our internal guidelines; | |
| • | difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; and | |
| • | difficulties in enforcing or defending intellectual property rights. |
Any of these factors may adversely impact our operations. Our international sales are predominately in Europe. In Europe, healthcare regulation and reimbursement for medical devices vary significantly from country to country. This changing environment could adversely affect our ability to sell our products in some European countries, which could negatively affect our results of operations.
13
Table of Contents
The use, misuse or off-label use of our products may harm our image in the marketplace or result in injuries that lead to product liability suits, which could be costly to our business or result in FDA sanctions if we are deemed to have engaged in such promotion.
Our currently marketed products have been cleared by the FDA’s 510(k) clearance process for use under specific circumstances for the treatment of certain lower lumbar spine conditions. We cannot, however, prevent a physician from using our products or procedure outside of those indications cleared for use, known as off-label use. There may be increased risk of injury if physicians attempt to use our products off-label. We train our sales force not to promote our products for off-label uses, and our instructions for use in all markets specify that our products are not intended for use outside of those indications cleared for use. Furthermore, the use of our products for indications other than those indications for which our products have been cleared by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients. Physicians may also misuse our products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If our products are misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us that may not be covered by insurance. If we are deemed by FDA to have engaged in the promotion of any our products for off-label use, we could be subject to FDA prohibitions on the sale or marketing of our products or significant fines and penalties, and the imposition of these sanctions could also affect our reputation and position within the industry. Any of these events could harm our business and results of operations and cause our stock to decline.
We purchase some of the key components of our products from single suppliers. The loss of these suppliers could prevent or delay shipments of our products or delay our clinical trials or otherwise adversely affect our business.
Some of the key components of our products and related services are currently purchased from only single suppliers. We do not have long-term contracts with the third-party suppliers of our product components. If necessary or desirable, we could source our product components and related services from other suppliers. However, establishing additional or replacement suppliers for these components, and obtaining any additional regulatory clearances or approvals, if necessary, that may result from adding or replacing suppliers, will take a substantial amount of time and could result in increased costs and impair our ability to produce our products, which would adversely impact our business, operating results and prospects. In addition, some of our products, which we acquire from third parties, are highly technical and are required to meet exacting specifications, and any quality control problems that we experience with respect to the products supplied by third-party vendors could adversely and materially affect our reputation, our attempts to complete our clinical trials or commercialization of our products. We may also have difficulty obtaining similar components from other suppliers that are acceptable to the FDA or foreign regulatory authorities, and the failure of our suppliers to comply with strictly enforced regulatory requirements could expose us to regulatory action including, warning letters, product recalls, termination of distribution, product seizures or civil penalties, among others. Furthermore, since some of these suppliers are located outside of the United States, we are subject to foreign export laws and U.S. import and customs statutes and regulations, which complicate and could delay shipments of components to us.
If we experience any delay or deficiency in the quality of products supplied to us by third-party suppliers, or if we have to switch to replacement suppliers, we may face additional regulatory delays and the manufacture and delivery of our products would be interrupted for an extended period of time, which would adversely affect our business, operating results and prospects. In addition, we may be required to obtain prior regulatory clearance or approval from the FDA or foreign regulatory authorities to use different suppliers or components. As a result, regulatory clearance or approval of our products may not be received on a timely basis, or at all, and our business, operating results and prospects would be harmed.
14
Table of Contents
We depend on our officers and other key employees, and if we are not able to retain and motivate them or recruit additional qualified personnel, our business will suffer.
We are highly dependent on our officers and other key employees. Due to the specialized knowledge each of our officers and other key employees possesses with respect to the treatment of spine disorders and our operations, the loss of service of any of our officers and other key employees could delay or prevent the successful completion of our clinical trials, the growth of revenue from existing products and the commercialization of our new products. Each of our officers and key employees may terminate his or her employment without notice and without cause or good reason.
If we fail to properly manage our anticipated growth, our business could suffer.
The rapid growth of our business has placed a significant strain on our managerial, operational and financial resources and systems. To execute our anticipated growth successfully, we must attract and retain qualified personnel and manage and train them effectively. We must also upgrade our internal business processes and capabilities to create the scalability that a growing business demands. We will be dependent on our personnel and third parties to accomplish this, as well as to effectively market our products to an increasing number of spine surgeons. We will also depend on our personnel to develop next generation technologies.
Further, our anticipated growth will place additional strain on our suppliers and manufacturers, resulting in increased need for us to carefully monitor quality assurance. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our development and commercialization goals.
We expect to rapidly expand our operations and grow our research and development, product development, clinical, regulatory, operations, sales and marketing and administrative functions. Our growth will require hiring a significant number of qualified clinical, scientific, regulatory, quality, commercial and administrative personnel. Recruiting, motivating and retaining such personnel will be critical to our success. There is intense competition from other companies and research and academic institutions for qualified personnel in the areas of our activities. In addition, our operations are located in a geographic region which historically does not have a large number of medical device companies and it may be difficult to convince qualified personnel to relocate to our area. If we fail to identify, attract, retain and motivate these highly skilled personnel, we may be unable to continue our development and commercialization activities.
We may need substantial additional funding beyond the proceeds of this offering and may be unable to raise capital when needed, which would force us to delay, reduce, eliminate or abandon our commercialization efforts or product development programs.
We may need to raise substantial additional capital to:
| • | expand the commercialization of our products; | |
| • | fund our operations and clinical trials; | |
| • | continue our research and development; | |
| • | defend, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; | |
| • | address FDA or other governmental, legal/enforcement actions and remediate underlying problems | |
| • | commercialize our new products, if any such products receive regulatory clearance or approval for commercial sale; and | |
| • | acquire companies and in-license products or intellectual property. |
We believe that the net proceeds from this offering, together with our existing cash and cash equivalent balances and cash receipts generated from sales of our products, will be sufficient to meet our anticipated cash
15
Table of Contents
requirements for at least the next two years. However, our future funding requirements will depend on many factors, including:
| • | market acceptance of our products; | |
| • | the scope, rate of progress and cost of our clinical trials; | |
| • | the cost of our research and development activities; | |
| • | the cost of filing and prosecuting patent applications and defending and enforcing our patent and other intellectual property rights; | |
| • | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patent or other intellectual property rights; | |
| • | the cost and timing of additional regulatory clearances or approvals; | |
| • | the cost and timing of establishing additional sales, marketing and distribution capabilities; | |
| • | the effect of competing technological and market developments; and | |
| • | the extent to which we acquire or invest in businesses, products and technologies, although we currently have no commitments or agreements relating to any of these types of transactions. |
If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Debt financing, if available, may involve covenants restricting our operations or our ability to incur additional debt. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. If we are unable to raise adequate funds, we may have to liquidate some or all of our assets, or delay, reduce the scope of or eliminate some or all of our development programs.
If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support or other resources devoted to our products or cease operations. Any of these factors could harm our operating results.
If we choose to acquire new businesses, products or technologies, we may experience difficulty in the identification or integration of any such acquisition, and our business may suffer.
Our success depends on our ability to continually enhance and broaden our product offerings in response to changing customer demands, competitive pressures and technologies. Accordingly, we may in the future pursue the acquisition of complementary businesses, products or technologies instead of developing them ourselves. We have no current commitments with respect to any acquisition or investment. We do not know if we will be able to identify or complete any acquisitions, or whether we will be able to successfully integrate any acquired business, product or technology or retain key employees. Integrating any business, product or technology we acquire could be expensive and time consuming, and could disrupt our ongoing business and distract our management. If we are unable to integrate any acquired businesses, products or technologies effectively, our business will suffer. In addition, any amortization or charges resulting from acquisitions could harm our operating results.
Consolidation in the healthcare industry could lead to demands for price concessions or to the exclusion of some suppliers from certain of our markets, which could have an adverse effect on our business, financial condition or results of operations.
Because healthcare costs have risen significantly over the past decade, numerous initiatives and reforms initiated by legislators, regulators and third-party payors to curb these costs have resulted in a consolidation trend in the healthcare industry to create new companies with greater market power, including hospitals. As
16
Table of Contents
the healthcare industry consolidates, competition to provide products and services to industry participants has become and will continue to become more intense. This in turn has resulted and will likely continue to result in greater pricing pressures and the exclusion of certain suppliers from important market segments as group purchasing organizations, independent delivery networks and large single accounts continue to use their market power to consolidate purchasing decisions for some of our customers. We expect that market demand, government regulation, third-party reimbursement policies and societal pressures will continue to change the worldwide healthcare industry, resulting in further business consolidations and alliances among our customers, which may reduce competition, exert further downward pressure on the prices of our products and may adversely impact our business, financial condition or results of operations.
We face the risk of product liability or other claims and may not be able to obtain sufficient insurance coverage, if at all.
Our business exposes us to the risk of product liability claims that is inherent in the testing, manufacturing and marketing of implantable medical devices. We may be subject to product liability claims if our products cause, or merely appear to have caused, an injury or death. Claims may be made by patients, consumers or healthcare providers. Although we have product liability and clinical trial liability insurance that we believe is appropriate for our current level of operations, this insurance is subject to deductibles and coverage limitations. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, the coverages may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at acceptable cost or on acceptable terms with adequate coverage or otherwise protect against potential product liability claims, we could be exposed to significant financial and other liabilities, which may harm our business. A product liability claim, product recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, operating results and prospects.
We may be subject to claims against us even if the apparent injury is due to the actions of others. For example, we rely on the expertise of spine surgeons, nurses and other associated medical personnel to perform the medical procedure and related processes for our product. If these medical personnel are not properly trained or are negligent in their provision of care, the therapeutic effect of our products may be diminished or the patient may suffer critical injury, which may subject us to liability. In addition, an injury that is caused by the activities of our suppliers may be the basis for a claim against us.
In addition, medical malpractice carriers are withdrawing coverage in certain regions or substantially increasing premiums. In the event we become a defendant in a product liability suit in which the treating surgeon or hospital does not have adequate malpractice insurance, the likelihood of liability being imposed on us could increase.
These liabilities could prevent, delay or otherwise adversely interfere with our product commercialization efforts, and result in judgments, fines, damages and other financial liabilities which have adverse effects on our business, operating results and prospects. Defending a suit, regardless of merit, could be costly, could divert management’s attention from our business and might result in adverse publicity, which could result in the withdrawal of, or inability to recruit, clinical trial patient participants or result in reduced acceptance of our products in the market. In addition to adversely impacting our business and prospects, such adverse publicity could materially adversely affect our stock price.
If our independent contract manufacturers fail to timely deliver to us sufficient quantities of some of our products and components in a timely manner, our operations may be harmed.
Our reliance on independent contract manufacturers to manufacture most of our products and components involves several risks, including:
| • | inadequate capacity of the manufacturer’s facilities; | |
| • | interruptions in access to certain process technologies; and | |
| • | reduced control over product availability, quality, delivery schedules, manufacturing yields and costs. |
17
Table of Contents
Shortages of raw materials, production capacity constraints or delays by our contract manufacturers could negatively affect our ability to meet our production obligations and result in increased prices for affected parts. Any such reduction, constraint or delay may result in delays in shipments of our products or increases in the prices of components, either of which could have a material adverse effect on our business.
We do not have supply agreements with all of our current contract manufacturers and we often utilize purchase orders, which are subject to acceptance by the supplier. Failure to accept purchase orders could result in an inability to obtain adequate supply of our product or components in a timely manner or on commercially reasonable terms.
An unanticipated loss of any of our contract manufacturers could cause delays in our ability to deliver our products while we identify and qualify a replacement manufacturer, which delays could negatively impact our revenues.
We operate at a single location. Any disruption in this facility or any inability to ship a sufficient number of our products to meet demand could adversely affect our business and results of operations.
We operate at a single location in Wilmington, North Carolina. Our facility may be affected by man-made or natural disasters, such as a hurricane. While we currently rely on third parties to manufacture, assemble, package, label and sterilize our products and components, we might also be forced to rely on third parties to inspect, warehouse or ship our products and components in the event our facilities were affected by a disaster. Our facility, if damaged or destroyed, could be difficult to replace and could require substantial lead-time to repair or replace. In the case of a device with a PMA approval, we might be required to obtain prior FDA, or notified body, approval of an alternate facility, which could delay or prevent our marketing of the affected product until this supplemental approval is obtained. Although we believe we possess adequate insurance for damage to our property and the disruption of our business from casualties, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
We have no experience operating as a public company. Compliance with public company requirements will increase our costs and require additional management resources, and we still may fail to comply.
We have operated as a private company and have not been subject to many of the requirements applicable to public companies. Recently enacted and proposed changes in the laws and regulations affecting public companies, including the provisions of the Sarbanes-Oxley Act of 2002 and the rules related to corporate governance and other matters subsequently adopted by the Securities and Exchange Commission, or SEC, and the Nasdaq Global Market, will result in increased administrative, legal and accounting costs. The impact of these events and heightened corporate governance standards could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. As directed by Section 404 of the Sarbanes-Oxley Act of 2002, the SEC adopted rules requiring public companies to include a report of management on the company’s internal controls over financial reporting in their annual reports onForm 10-K. In addition, the independent registered public accounting firm auditing a company’s financial statements must attest to and report on management’s assessment of the effectiveness of a company’s internal control over financial reporting. We may be unable to comply with these requirements by the applicable deadlines, beginning with ourForm 10-K for the period ending December 31, 2008. Both we and our independent registered public accounting firm will be testing our internal controls over financial reporting in connection with Section 404 requirements and could, as part of that documentation and testing, identify material weaknesses, significant deficiencies or other areas requiring further attention or improvement, which could cause investors to lose confidence in the accuracy and completeness of our financial reports, which would have an adverse effect on our stock price.
Changes to existing accounting pronouncements regarding stock-based compensation may affect how we conduct our business and affect our reported results of operations.
Effective January 1, 2006, we adopted Statement of Financial Accounting Standards No. 123(R), or FAS No. 123(R), “Share-Based Payment,” which requires that stock options be expensed. As of December 31,
18
Table of Contents
2006, total compensation cost related to non-vested stock options was $2.5 million, which is expected to be recognized over the vesting period of the options. We rely heavily on stock options to motivate current employees and to attract new employees. As a result of the requirement to expense stock options, we may choose to reduce our reliance on stock options as a motivational tool. If we reduce our use of stock options, it may be more difficult for us to attract and retain qualified employees. However, if we do not reduce our reliance on stock options, our reported net losses may increase, which may have an adverse effect on our stock price.
Risks Related to Regulatory Environment
If we fail to maintain regulatory approvals and clearances, or are unable to obtain, or experience significant delays in obtaining, FDA clearances or approvals for our future products or product modifications, our ability to commercially distribute and market these products could suffer.
Our products are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. The process of obtaining regulatory clearances or approvals to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals on a timely basis, if at all. In particular, the FDA permits commercial distribution of most new medical devices only after the device has received clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or is the subject of an approved PMA. The FDA will clear marketing of a non-exempt lower risk medical device through the 510(k) process if the manufacturer demonstrates that the new product is substantially equivalent to other legally marketed products not requiring PMA approval. High risk devices deemed to pose the greatest risk, such as life-sustaining, life-supporting, or implantable devices, or devices not deemed substantially equivalent to a legally marketed device, require a PMA. The PMA process is more costly, lengthy and uncertain than the 510(k) clearance process. A PMA application must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the device for its intended use. Our currently commercialized products have been cleared through the 510(k) process. However, we expect to submit a PMA for each of our PNR and PDR devices currently under development. In addition, the FDA has indicated that our two-level AxiaLIF 2L implant, which we anticipate will require 510(k) clearance, will require additional safety and efficacy data beyond that typically required for a 510(k) clearance.
Our failure to comply with U.S. federal, state and foreign governmental regulations could lead to the imposition of injunctions, suspensions or loss of regulatory clearance or approvals, product recalls, termination of distribution, product seizures or civil penalties, among other things. In the most extreme cases, criminal sanctions or closure of our manufacturing facility are possible.
Foreign governmental authorities that regulate the manufacture and sale of medical devices have become increasingly stringent and, to the extent we market and sell our products internationally, we may be subject to rigorous international regulation in the future. In these circumstances, we would rely significantly on our foreign independent distributors to comply with the varying regulations, and any failures on their part could result in restrictions on the sale of our products in foreign countries.
Modifications to our marketed products may require new 510(k) clearances or PMA approvals, or may require us to cease marketing or recall the modified products until clearances or approvals are obtained.
Any modification to our currently marketed 510(k)-cleared device that could significantly affect its safety or efficacy, or that would constitute a change in its intended use, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review the manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. If the FDA requires us to seek 510(k) clearance or a PMA for any modification to a previously cleared product, we may be required to cease marketing and distributing, or to recall the modified product until we obtain such clearance or approval, and we may be subject to significant regulatory fines or penalties. Further, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective because they are in violation of the FDCA. Any recall or
19
Table of Contents
FDA requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
Clinical trials necessary to support a PMA application will be expensive and will require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Delays or failures in our clinical trials will prevent us from commercializing any modified or new products and will adversely affect our business, operating results and prospects.
Initiating and completing clinical trials necessary to support a PMA application for our PNR and PDR devices, and additional safety and efficacy data beyond that typically required for a 510(k) clearance for our two-level AxiaLIF 2L implant, as well as other possible future product candidates, will be time consuming and expensive and the outcome uncertain. Moreover, the results of early clinical trials are not necessarily predictive of future results, and any product we advance into clinical trials may not have favorable results in later clinical trials.
Conducting successful clinical studies will require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation andfollow-up depends on many factors, including the size of the patient population, the nature of the trial protocol, the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators, support staff, and proximity of patients to clinical sites. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures orfollow-up to assess the safety and effectiveness of our products or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competitive products. In addition, patients participating in clinical trials may die before completion of the trial or suffer adverse medical events unrelated to investigational products.
Development of sufficient and appropriate clinical protocols and data to demonstrate safety and efficacy are required and we may not adequately develop such protocols to support clearance and approval. Further, the FDA may require us to submit data on a greater number of patients than we originally anticipatedand/or for a longerfollow-up period or change the data collection requirements or data analysis applicable to our clinical trials. Delays in patient enrollment or failure of patients to continue to participate in a clinical trial may cause an increase in costs and delays in the clearance or approval and attempted commercialization of our products or result in the failure of the clinical trial. In addition, despite considerable time and expense invested in our clinical trials, FDA may not consider our data adequate to demonstrate safety and efficacy. Such increased costs and delays or failures could adversely affect our business, operating results and prospects.
If the third parties on which we rely to conduct our clinical trials and to assist us with pre-clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval for or commercialize our products.
We do not have the ability to independently conduct our pre-clinical and clinical trials for our products and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct such trials. If these third parties do not successfully perform their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance or approval for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
20
Table of Contents
Even if our products are approved by regulatory authorities, if we or our suppliers fail to comply with ongoing FDA or other foreign regulatory authority requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain clearance or approval, and the manufacturing processes, reporting requirements, post-approval clinical data and labeling and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA and other domestic and foreign regulatory bodies. In particular, we and our suppliers are required to comply with the Quality System Regulations, or QSR, and International Standards Organization, or ISO, regulations for the manufacture of our products and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval. Regulatory bodies enforce the QSR and ISO regulations through inspections. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, any of the following enforcement actions:
| • | warning letters or untitled letters; | |
| • | fines and civil penalties; | |
| • | unanticipated expenditures to address or defend such actions; | |
| • | delays in clearing or approving, or refusal to clear or approve, our products; | |
| • | withdrawal or suspension of approval of our products or those of our third-party suppliers by the FDA or other regulatory bodies; | |
| • | product recall or seizure; | |
| • | orders for physician notification or device repair, replacement or refund; | |
| • | interruption of production; | |
| • | operating restrictions; | |
| • | injunctions; and | |
| • | criminal prosecution. |
If any of these actions were to occur it would harm our reputation and cause our product sales to suffer and may prevent us from generating revenue. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Even if regulatory clearance or approval of a product is granted, such clearance or approval may be subject to limitations on the intended uses for which the product may be marketed and reduce our potential to successfully commercialize the product and generate revenue from the product. If the FDA determines that our promotional materials, labeling, training or other marketing or educational activities constitute promotion of an unapproved use, it could request that we cease or modify our training educational, labeling or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training educational, labeling or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with medical device reporting requirements, including the reporting of adverse events and certain malfunctions related to our products. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements
21
Table of Contents
such as the QSR or GMP, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
We may be subject to or otherwise affected by federal and state healthcare laws, including fraud and abuse and health information privacy and security laws, and could face substantial penalties if we are unable to fully comply with such laws.
Although we do not provide healthcare services, submit claims for third-party reimbursement, or receive payments directly from Medicare, Medicaid, or other third-party payors for our products or the procedures in which our products are used, healthcare regulation by federal and state governments could significantly impact our business. Healthcare fraud and abuse and health information privacy and security laws potentially applicable to our operations include:
| • | the federal Anti-Kickback Law, which constrains our marketing practices and those of our independent sales agents and distributors, educational programs, pricing policies, and relationships with healthcare providers, by prohibiting, among other things, soliciting, receiving, offering or providing remuneration, intended to induce the purchase or recommendation of an item or service reimbursable under a federal healthcare program (such as the Medicare or Medicaid programs); | |
| • | federal false claims laws which prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; | |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, and its implementing regulations, which created federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of individually identifiable health information; and | |
| • | state laws analogous to each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy of certain health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
If our past or present operations, or those of our independent sales agents and distributors, are found to be in violation of any of such laws or any other governmental regulations that may apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from federal healthcare programsand/or the curtailment or restructuring of our operations. Similarly, if the healthcare providers or entities with whom we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on us. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Risks Related to Our Intellectual Property
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain.
Our success depends significantly on our ability to protect our proprietary rights to the procedures created with, and the technologies used in, our products. We rely on patent protection, as well as a combination of
22
Table of Contents
copyright, trade secret and trademark laws, and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. For example, our pending United States and foreign patent applications may not be approved, may not issue as patents in a form that will be advantageous to us, or may issue and be subsequently successfully challenged by others and invalidated. In addition, our pending patent applications include claims to material aspects of our products and procedures that are not currently protected by issued patents. Both the patent application process and the process of managing patent disputes can be time consuming and expensive. The patents we own may not be of sufficient scope or strength to provide us with any meaningful protection or commercial advantage, and competitors may be able to design around our patents or develop products which provide outcomes which are comparable to ours. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with our officers, employees, consultants and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of such agreements. Furthermore, the laws of some foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
We rely on our trademarks, trade names, and brand names to distinguish our products from the products of our competitors, and have registered or applied to register many of these trademarks. However, our trademark applications may not be approved. Third parties may also oppose our trademark applications, or otherwise challenge our use of the trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition, and could require us to devote resources to advertising and marketing new brands. Further, our competitors may infringe our trademarks, or we may not have adequate resources to enforce our trademarks.
In the event a competitor infringes upon our patent or other intellectual property rights, enforcing those rights may be costly, difficult and time consuming. Even if successful, litigation to enforce our intellectual property rights or to defend our patents against challenge could be expensive and time consuming and could divert our management’s attention. We may not have sufficient resources to enforce our intellectual property rights or to defend our patents or other intellectual property rights against a challenge.
Any lawsuit, whether initiated by us to enforce our intellectual property rights or by a third party against us alleging infringement, may cause us to expend significant financial and other resources, and may divert our attention from our business and adversely affect our business, operating results and prospects.
The medical device industry is characterized by the existence of a large number of patents and frequent litigation based on allegations of patent infringement. Patent litigation can involve complex factual and legal questions and its outcome is uncertain. Any claim relating to infringement of patents that is successfully asserted against us may require us to pay substantial damages. Even if we were to prevail, any litigation could be costly and time-consuming and would divert the attention of our management and key personnel from our business operations. Our success will also depend in part on our not infringing patents issued to others, including our competitors and potential competitors. If our products are found to infringe the patents of others, our development, manufacture and sale of such products could be severely restricted or prohibited. In addition, our competitors may independently develop similar technologies. Because of the importance of our patent portfolio and unpatented proprietary technology to our business, we may lose market share to our competitors if we fail to protect our patent rights.
As the number of entrants into our market increases, the possibility of a patent infringement claim against us grows. Our products and methods may be covered by patents held by our competitors. Some of our competitors have considerable resources available to them to engage in this type of litigation. We, on the other hand, are an early stage company with comparatively few resources available to us to engage in costly and protracted litigation. Because some patent applications are maintained in secrecy for a period of time after they are filed, there is a risk that we could adopt a technology without knowledge of a pending patent application, which technology would infringe a third-party patent once that patent is issued. In addition, our competitors may assert that future products we may market infringe their patents.
23
Table of Contents
A patent infringement suit or other infringement or misappropriation claim brought against us or any of our strategic partners or licensees may force us or any of our strategic partners or licensees to stop or delay developing, manufacturing or selling potential products that are claimed to infringe a third party’s intellectual property, unless that party grants us or any strategic partners or licensees rights to use its intellectual property. In such cases, we may be required to obtain licenses to patents or proprietary rights of others in order to continue to commercialize our products. However, we may not be able to obtain any licenses required under any patents or proprietary rights of third parties on acceptable terms, or at all. Even if our strategic partners or licensees or we were able to obtain rights to the third party’s intellectual property, these rights may be non-exclusive, thereby giving our competitors access to the same intellectual property. Ultimately, we may be unable to commercialize some of our potential products or may have to cease some of our business operations as a result of patent infringement claims, which could severely harm our business.
In any infringement lawsuit, a third party could seek to enjoin, or prevent, us from commercializing our existing or future products,and/or may seek damages from us, and any such lawsuit would likely be expensive for us to defend against. A court may determine that patents held by third parties are valid and infringed by us and we may be required to:
| • | pay damages, including, but not limited to, treble damages and attorneys’ fees, which may be substantial; | |
| • | cease the development, manufacture, use and sale of products that infringe the patent rights of others, through a court-imposed sanction called an injunction; | |
| • | expend significant resources to redesign our technology so that it does not infringe others’ patent rights, or develop or acquire non-infringing intellectual property, which may not be possible; | |
| • | discontinue manufacturing or other processes incorporating infringing technology; or | |
| • | obtain licenses to the infringed intellectual property, which may not be available to us on acceptable terms, or at all. |
Any development or acquisition of non-infringing products or technology or licenses could require the expenditure of substantial time and other resources and could have a material adverse effect on our business and financial results. If we are required to, but cannot, obtain a license to valid patent rights held by a third party, we would likely be prevented from commercializing the relevant product. We believe that it is unlikely that we would be able to obtain a license to any necessary patent rights controlled by companies against which we would, directly or indirectly, compete. If we need to redesign products to avoid third-party patents, we may suffer significant regulatory delays associated with conducting additional studies or submitting technical, manufacturing or other information related to the redesigned product and, ultimately, in obtaining regulatory approval.
Risks Related to this Offering
Our common stock has not been publicly traded and an active trading market may not develop, and we expect that the price of our common stock will fluctuate substantially.
Before this offering, there has been no public market for our common stock. An active public trading market may not develop after completion of this offering or, if developed, may not be sustained. Accordingly, you may not be able to sell your shares quickly or at the market price if trading in our stock is not active. We and representatives of the underwriters will determine the initial public offering price of our common stock through negotiation. The price of the common stock sold in this offering will not necessarily reflect the market price of our common stock after this offering. In addition, the trading price of our common stock following this offering is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include those discussed in the “Risk Factors” section of this prospectus and others such as:
| • | announcements relating to litigation or our commencement of, or involvement in, litigation; | |
| • | any major change in our board of directors or management; |
24
Table of Contents
| • | the announcement of new products, acquisitions, commercial relationships, or service enhancements by us or our competitors; | |
| • | failure to achieve certain milestones within certain timeframes, if at all; | |
| • | quarterly variations in our or our competitors’ operating results; | |
| • | changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earning estimates; and | |
| • | general economic and market conditions and other factors unrelated to our operating performance or the operating performance of our competitors. |
These and other factors may materially and adversely affect the market price of our common stock.
New investors in our common stock will experience immediate and substantial dilution after this offering.
The initial public offering price of our common stock is substantially higher than the book value per share of our common stock. Purchasers of shares of our common stock in this offering will incur immediate dilution of $8.74 in net tangible book value per share of common stock, based on an assumed initial public offering price of $13.00 per share, the midpoint of the range on the front cover of this prospectus. Following this offering, purchasers in this offering will have contributed 63.8% of a total consideration paid by stockholders to us for the purchase of shares of our common stock. Investors will incur additional dilution upon the exercise of stock options.
A sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our stockholders sell substantial amounts of our common stock in the public market after this offering, including shares issued upon the exercise of options, the market price of our common stock could decline. Based on shares outstanding as of June 30, 2007, we will have outstanding a total of 18,776,502 shares of our common stock upon the completion of this offering, an increase of 41% from the number of shares outstanding prior to the offering. Of these shares, only the 5,500,000 shares of our common stock sold in this offering will be freely tradable, without restriction, in the public market. Our underwriters may, in their sole discretion, permit our officers, directors and other current stockholders who are subject to contractuallock-ups to sell shares prior to the expiration of theirlock-up agreements. Thus, there will be 13,276,502 shares of our common stock eligible for sale beginning 180 days after the date of this prospectus upon the expiration oflock-up arrangements between our stockholders and underwriters, although theselock-up agreements may be extended for up to an additional 34 days under certain circumstances. After thelock-up agreements expire, these shares will be eligible for sale in the public market, of which 9,840,035 shares are held by directors, executive officers and other affiliates and are subject to volume limitations under Rule 144 under the Securities Act. In addition, the 2,339,663 shares of our common stock that are subject to outstanding options as of June 30, 2007 will be eligible for sale in the public market to the extent permitted by the provisions of the various vesting agreements, thelock-up agreements and Rules 144 and 701 under the Securities Act. If these additional shares are sold, or it is perceived they will be sold, the trading price of our common stock could decline. These sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
After this offering, our officers, directors and principal stockholders, each holding more than 5% of our common stock, will collectively control approximately 42.1% of our outstanding common stock. As a result, these stockholders, if they act together, will be able to control the management and affairs of our company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change
25
Table of Contents
in control and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of our other stockholders.
If securities or industry analysts do not publish research or reports about our business, if they change their recommendations regarding our stock adversely or if our operating results do not meet their expectations, our stock price and trading volume could decline.
The trading market for our stock will likely be influenced by the research and reports that industry or securities analysts publish about us or our business. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline. Moreover, if one or more of the analysts who cover us downgrade our stock or if our operating results do not meet their expectations, our stock price could decline.
We have broad discretion in the use of proceeds of this offering for working capital and general corporate purposes.
The net proceeds of this offering will be allocated to expanding our sales and marketing efforts for our products, clinical trials, research and development projects, and infrastructure, and working capital and general corporate purposes, as well as to potential acquisitions of complementary businesses, products or technologies. Our management will have broad discretion over the use and investment of the net proceeds of this offering within those categories, and, accordingly, investors in this offering will need to rely upon the judgment of our management with respect to the use of proceeds, with only limited information concerning management’s specific intentions.
Volatility in the stock price of other companies may contribute to volatility in our stock price.
The Nasdaq Global Market, particularly in recent years, has experienced significant volatility with respect to medical technology, pharmaceutical, biotechnology and other life science company stocks. The volatility of medical technology, pharmaceutical, biotechnology and other life science company stocks often does not relate to the operating performance of the companies represented by the stock. Further, there has been particular volatility in the market price of securities of early stage life science companies. These broad market and industry factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. A securities class action suit against us could result in substantial costs, potential liabilities and the diversion of management’s attention and resources.
Our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law contain provisions that could discourage a takeover.
Anti-takeover provisions of our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law may have the effect of deterring or delaying attempts by our stockholders to remove or replace management, engage in proxy contests and effect changes in control. The provisions of our charter documents include:
| • | a classified board so that only one of the three classes of directors on our board of directors is elected each year; | |
| • | procedures for advance notification of stockholder director nominations and proposals; | |
| • | the ability of our board of directors to amend our bylaws without stockholder approval; | |
| • | a supermajority stockholder vote requirement for amending certain provisions of our amended and restated certificate of incorporation and our amended and restated bylaws; and | |
| • | the ability of our board of directors to issue up to 5,000,000 shares of preferred stock without stockholder approval upon the terms and conditions and with the rights, privileges and preferences as our board of directors may determine. |
26
Table of Contents
In addition, as a Delaware corporation, we are subject to Delaware law, including Section 203 of the Delaware General Corporation Law, or DGCL. In general, Section 203 prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless certain specific requirements are met as set forth in Section 203. These provisions, alone or together, could have the effect of deterring or delaying changes in incumbent management, proxy contests or changes in control. See the section entitled “Description of Capital Stock” in this prospectus.
We have not paid dividends in the past and do not expect to pay dividends in the future, and any return on investment may be limited to the value of our stock.
We have never paid or declared any cash dividends on our common stock. We currently intend to retain any future earnings to finance the growth and development of our business and we do not expect to pay any cash dividends on our common stock in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in current or future financing instruments and other factors our board of directors deems relevant. If we do not pay dividends, our stock may be less valuable to you because a return on your investment will only occur if our stock price appreciates.
27
Table of Contents
This prospectus contains forward-looking statements that involve risks and uncertainties, principally in the sections entitled “Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Use of Proceeds” and “Business.” All statements other than statements of historical fact contained in this prospectus, including statements regarding future events, our future financial performance, business strategy and plans and objectives of management for future operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should” or “will” or the negative of these terms or other comparable terminology. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks outlined under “Risk Factors” or elsewhere in this prospectus, which may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time and it is not possible for us to predict all risk factors, nor can we address the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual results to differ materially from those contained in any forward-looking statements.
You should not place undue reliance on any forward-looking statement, each of which applies only as of the date of this prospectus. Before you invest in our common stock, you should be aware that the occurrence of the events described in the section entitled “Risk Factors” and elsewhere in this prospectus could negatively affect our business, operating results, financial condition and stock price. Except as required by law, we undertake no obligation to update or revise publicly any of the forward-looking statements after the date of this prospectus to conform our statements to actual results or changed expectations.
28
Table of Contents
Assuming a public offering price of $13.00, the midpoint of the range on the front cover of this prospectus, we estimate the net proceeds to us from the sale of 5,500,000 shares of common stock that we are selling in this offering will be approximately $64.5 million, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 increase or decrease in the assumed public offering price of $13.00 per share would increase or decrease, respectively, the net proceeds to us by approximately $5.1 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions. If the underwriters’ option to purchase additional shares is exercised in full, we estimate we will receive net proceeds of approximately $74.5 million.
Of the net proceeds from this offering, we expect to use approximately:
| • | $30.0 million for sales and marketing initiatives to support the commercialization of our existing and any future products; and | |
| • | $10.0 million to support our research and development activities, clinical trials and obtaining necessary regulatory approvals. |
We intend to use the remainder of the net proceeds from this offering for capital expenditures, working capital and general corporate purposes. The amounts actually spent for these purposes may vary significantly and will depend on a number of factors, including our operating costs and other factors described under “Risk Factors.” While we have no present understandings, commitments or agreements to enter into any potential acquisitions, we may also use a portion of the net proceeds for the acquisition of, or investment in, technologies or products that complement our business. Accordingly, management will retain broad discretion as to the allocation of the net proceeds of this offering.
Pending the uses described above, we will invest the net proceeds of this offering in short-term, interest-bearing, investment-grade securities. We cannot predict whether the proceeds will yield a favorable return.
We have never paid or declared any cash dividends on our common stock. We currently intend to retain any future earnings to finance the growth and development of our business and we do not expect to pay any cash dividends on our common stock in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our board of directors and will depend on our financial condition, results of operations, capital requirements, restrictions contained in current or future financing instruments and other factors our board of directors deems relevant.
29
Table of Contents
You should read this capitalization table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
The following table sets forth our capitalization as of June 30, 2007 on:
| • | an actual basis; | |
| • | A pro forma basis to give effect to the conversion of all of our outstanding preferred stock into 10,793,182 shares of our common stock immediately upon the closing of this offering; and | |
| • | a pro forma as adjusted basis to give further effect to the sale of 5,500,000 shares of our common stock in this offering at an assumed offering price of $13.00 per share, the midpoint of the range on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering costs payable by us. |
| As of June 30, 2007 | ||||||||||||
| Pro Forma | ||||||||||||
| Actual | Pro Forma | As Adjusted(1) | ||||||||||
| (Unaudited) | ||||||||||||
| (In thousands, except per share | ||||||||||||
| and par value data) | ||||||||||||
| Preferred Stock: | ||||||||||||
| Series A preferred stock; $0.0001 par value; 1,125,000 shares authorized, 1,125,000 shares issued and outstanding, actual; no shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted | $ | 956 | $ | — | $ | — | ||||||
| Series AA preferred stock; $0.0001 par value; 1,260,000 shares authorized, 1,260,000 shares issued and outstanding, actual; no shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted | 1,694 | — | — | |||||||||
| Series B preferred stock; $0.0001 par value; 4,909,091 shares authorized, 4,909,091 shares issued and outstanding, actual; no shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted | 11,894 | — | — | |||||||||
| Series C preferred stock; $0.0001 par value; 3,499,091 shares authorized, 3,499,091 shares issued and outstanding, actual; no shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted | 25,545 | — | — | |||||||||
| Stockholders’ equity (deficit): | ||||||||||||
| Preferred stock, $0.0001 par value, no shares authorized, no shares issued or outstanding, actual; 5,000,000 shares authorized, no shares issued or outstanding, pro forma as adjusted | — | — | — | |||||||||
| Common stock; $0.0001 par value, 17,100,000 shares authorized, 2,483,320 issued and outstanding, actual and 13,276,502 shares issued and outstanding, pro forma; 75,000,000 shares authorized, 18,776,502 issued and outstanding, pro forma as adjusted | — | 1 | 2 | |||||||||
| Additional paid-in capital | 1,889 | 41,977 | 106,471 | |||||||||
| Accumulated deficit | (26,438 | ) | (26,438 | ) | (26,438 | ) | ||||||
| Total stockholders’ equity (deficit) | (24,549 | ) | 15,540 | 80,035 | ||||||||
| Total capitalization | $ | 15,540 | $ | 15,540 | $ | 80,035 | ||||||
30
Table of Contents
| (1) | A $1.00 increase or decrease in the assumed public offering price of $13.00 per share would increase or decrease, respectively, each of additional paid-in capital, total stockholders’ equity and total capitalization by approximately $5.1 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions. |
The information in the table above excludes, as of June 30, 2007:
| • | 2,339,663 shares of our common stock issuable upon exercise of outstanding options under our existing Amended and Restated 2000 Stock Incentive Plan, at a weighted average exercise price of $1.74 per share; | |
| • | 1,400,000 shares of our common stock available for future issuance under our 2007 Stock Incentive Plan, which is currently effective, and 250,000 shares of our common stock available for future grant under our 2007 Employee Stock Purchase Plan, which has been adopted and will become effective upon the completion of this offering; and | |
| • | automatic annual increases in the number of shares of common stock reserved for issuance under our 2007 Employee Stock Purchase Plan. |
31
Table of Contents
If you invest in our common stock, your ownership interest will be diluted to the extent of the difference between the offering price per share of our common stock and the pro forma net as adjusted tangible book value per share of our common stock after this offering. Net tangible book value per share represents the amount of our total tangible assets less total liabilities, divided by the number of shares of common stock outstanding. Dilution in pro forma net tangible book value per share represents the difference between the amount per share paid by purchasers of our common stock in this offering and the pro forma as adjusted net tangible book value per share of common stock immediately after the completion of this offering.
Our historical net tangible book value as of June 30, 2007 was $(24.5) million, or $(9.89) per share of common stock, not taking into account the conversion of our outstanding preferred stock. Our pro forma net tangible book value as of June 30, 2007 was approximately $15.5 million, or $1.17 per share of common stock, after giving effect to the conversion of all outstanding shares of our preferred stock into shares of our common stock.
After giving effect to the conversion of all of our preferred stock and the sale of the 5,500,000 shares of our common stock in this offering at an assumed offering price of $13.00 per share, the midpoint of the range on the cover page of this prospectus, less underwriting discounts and commissions and estimated offering costs payable by us, our pro forma as adjusted net tangible book value as of June 30, 2007 would have been approximately $80.0 million, or approximately $4.26 per share. This represents an immediate increase in net tangible book value of $3.09 per share to existing stockholders and an immediate dilution in net tangible book value of $8.74 per share to new investors of common stock in this offering. If the offering price is higher or lower, the dilution to the new investors will be greater or less. The following table illustrates this per share dilution:
| Assumed public offering price per share | $ | 13.00 | ||||||
| Historical net tangible book value per share as of June 30, 2007 | $ | (9.89 | ) | |||||
| Pro forma increase in net tangible book value per share attributable to conversion of preferred stock | 11.06 | |||||||
| Pro forma net tangible book value per share as of June 30, 2007 | 1.17 | |||||||
| Increase in pro forma net tangible book value per share attributable to this offering | 3.09 | |||||||
| Pro forma as adjusted net tangible book value per share after this offering | 4.26 | |||||||
| Dilution per share to new investors | $ | 8.74 | ||||||
If the underwriters exercise their option to purchase up to 825,000 additional shares in this offering, our pro forma as adjusted net tangible book value per share as of June 30, 2007 will be $90.0 million representing an immediate increase in pro forma net tangible book value per share attributable to this offering of $3.42 to our existing investors and an immediate dilution per share to new investors in this offering of $8.41.
The following table sets forth, on a pro forma as adjusted basis, as of June 30, 2007, the differences between the number of shares of common stock purchased from us, the total consideration paid, and the weighted average price per share paid by existing stockholders and new investors purchasing shares of our common stock in this offering, before deducting underwriting discounts and commissions and estimated expenses payable by us at an assumed initial public offering price of $13.00 per share, the midpoint of the range on the front cover of this prospectus.
| Weighted | ||||||||||||||||||||
| Average | ||||||||||||||||||||
| Shares Purchased | Total Consideration | Price | ||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | ||||||||||||||||
| Existing stockholders | 13,276,502 | 70.7 | % | $ | 40,565,000 | 36.2 | % | $ | 3.06 | |||||||||||
| New investors | 5,500,000 | 29.3 | 71,500,000 | 63.8 | $ | 13.00 | ||||||||||||||
| Total | 18,776,502 | 100.0 | % | $ | 112,065,000 | 100.0 | % | |||||||||||||
32
Table of Contents
A $1.00 increase or decrease in the assumed public offering price of $13.00 per share would increase or decrease, respectively, total consideration paid by new investors and total consideration paid by all stockholders by approximately $5.1 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions.
If the underwriters exercise their option to purchase additional shares in full, our existing stockholders would own approximately 67.7% and our new investors would own approximately 32.3% of the total number of shares of our common stock outstanding after this offering.
The preceding discussion and tables above assume the conversion of all our outstanding shares of preferred stock into 10,793,182 shares of common stock immediately upon the closing of this offering, and excludes, as of June 30, 2007:
| • | 2,339,663 shares issuable upon exercise of outstanding options under our existing Amended and Restated 2000 Stock Incentive Plan, at a weighted average exercise price of $1.74 per share; | |
| • | 1,400,000 shares available for future issuance under our 2007 Stock Incentive Plan, which is currently effective, and 250,000 shares available for future grant under our 2007 Employee Stock Purchase Plan, which has been adopted and will become effective upon the completion of this offering; and | |
| • | automatic annual increases in the number of shares of common stock reserved for issuance under our 2007 Employee Stock Purchase Plan. |
Because the exercise prices of the outstanding options are significantly below the assumed offering price of $13.00 per share, the midpoint of the offering range on the cover page of this prospectus, investors purchasing common stock in this offering will suffer additional dilution when and if these options are exercised. Assuming the exercise in full of the 2,339,663 outstanding options, pro forma net tangible book value before this offering at June 30, 2007 would be $1.26 per share, representing an immediate increase of $0.09 per share to our existing stockholders, and, after giving effect to the sale of shares of common stock in this offering, there would be an immediate dilution of $9.02 per share to new investors in this offering.
Assuming all outstanding options are fully exercised, our existing stockholders would, after this offering, before deducting underwriting discounts and commissions and estimated expenses payable by us at an assumed initial public offering price of $13.00 per share, own approximately 74% of the total number of outstanding shares of our common stock while contributing 38.4% of the total consideration for all shares, and our new investors would own approximately 26% of the total number of outstanding shares of our common stock while contributing 61.6% of the total consideration for all shares, all as depicted in the table below.
| Weighted | ||||||||||||||||||||
| Average | ||||||||||||||||||||
| Shares Purchased | Total Consideration | Price | ||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | ||||||||||||||||
| Existing stockholders | 15,616,165 | 74.0 | % | $ | 44,632,394 | 38.4 | % | $ | 2.86 | |||||||||||
| New investors | 5,500,000 | 26.0 | 71,500,000 | 61.6 | $ | 13.00 | ||||||||||||||
| Total | 21,116,165 | 100.0 | % | $ | 116,132,394 | 100.0 | % | |||||||||||||
In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. If additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
33
Table of Contents
The statements of operations data for the years ended December 31, 2004, 2005 and 2006, and the balance sheet data as of December 31, 2005 and 2006 have been derived from our audited financial statements included elsewhere in this prospectus. The statement of operations data for the year ended December 31, 2003, and the balance sheet data as of December 31, 2003 and 2004, have been derived from our audited financial statements that do not appear in this prospectus. The statement of operations data for the year ended, and the balance sheet data as of, December 31, 2002, have been derived from our unaudited financial statements not included in this prospectus. The statements of operations data for the six months ended June 30, 2006 and 2007, and the balance sheet data as of June 30, 2006 and 2007, have been derived from our unaudited financial statements included elsewhere in this prospectus. This unaudited interim financial information has been prepared on the same basis as our audited annual financial statements and, in our opinion, reflects all adjustments, consisting only of normal recurring adjustments, that we consider necessary for a fair statement of our financial position as of June 30, 2007 and operating results for the periods ended June 30, 2007 and 2006. The historical results are not necessarily indicative of the results to be expected for any future periods and the results for the six months ended June 30, 2007 should not be considered indicative of results expected for the fiscal year 2007. You should read the following financial information together with the information under “Management’s Discussions and Analysis of Financial Condition and Results of Operations” and our financial statements and the notes included elsewhere in this prospectus.
| Year Ended December 31, | Six Months Ended June 30, | |||||||||||||||||||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||||||
| (In thousands, except share data) | ||||||||||||||||||||||||||||
Statements of Operations Data: | ||||||||||||||||||||||||||||
| Revenue | $ | — | $ | — | $ | — | $ | 1,469 | $ | 5,812 | $ | 2,161 | $ | 7,187 | ||||||||||||||
| Cost of revenue | — | — | — | 386 | 1,580 | 618 | 1,459 | |||||||||||||||||||||
| Gross profit | — | — | — | 1,083 | 4,232 | 1,543 | 5,728 | |||||||||||||||||||||
| Operating expenses | ||||||||||||||||||||||||||||
| Research and development | 483 | 1,620 | 1,758 | 2,444 | 4,246 | 2,094 | 2,278 | |||||||||||||||||||||
| Sales and marketing | — | 163 | 746 | 2,635 | 9,288 | 3,950 | 6,837 | |||||||||||||||||||||
| General and administrative | 561 | 927 | 1,117 | 1,293 | 1,166 | 625 | 1,083 | |||||||||||||||||||||
| Total operating expenses | 1,044 | 2,710 | 3,621 | 6,372 | 14,700 | 6,669 | 10,198 | |||||||||||||||||||||
| Operating loss | (1,044 | ) | (2,710 | ) | (3,621 | ) | (5,289 | ) | (10,468 | ) | (5,126 | ) | (4,470 | ) | ||||||||||||||
| Interest income | 19 | 86 | 150 | 264 | 858 | 528 | 343 | |||||||||||||||||||||
| Other income (expense) | — | — | — | (17 | ) | 131 | — | — | ||||||||||||||||||||
| Net loss | $ | (1,025 | ) | $ | (2,624 | ) | $ | (3,471 | ) | $ | (5,042 | ) | $ | (9,479 | ) | $ | (4,598 | ) | $ | (4,127 | ) | |||||||
Net loss per common share — basic and diluted(1) | $ | (0.63 | ) | $ | (1.28 | ) | $ | (1.63 | ) | $ | (2.25 | ) | $ | (3.91 | ) | $ | (1.90 | ) | $ | (1.67 | ) | |||||||
Weighted average common shares outstanding — basic and diluted(1) | 1,637,719 | 2,046,753 | 2,127,856 | 2,243,019 | 2,423,226 | 2,418,154 | 2,467,550 | |||||||||||||||||||||
Pro forma net loss per common share — basic and diluted (unaudited)(1) | $ | (0.72 | ) | $ | (0.31 | ) | ||||||||||||||||||||||
Pro forma weighted average shares outstanding — basic and diluted (unaudited)(1) | 13,216,407 | 13,260,732 | ||||||||||||||||||||||||||
| (1) | See note 2 of the notes to our financial statements included elsewhere in this prospectus for an explanation of the determination of the number of shares used in computing per share data. |
34
Table of Contents
| June 30, 2007 | ||||||||||||||||||||||||||||||||
| December 31, | Pro Forma | |||||||||||||||||||||||||||||||
| 2002 | 2003 | 2004 | 2005 | 2006 | Actual | Pro Forma(1) | As Adjusted(2) | |||||||||||||||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||||||
Balance Sheet Data: | ||||||||||||||||||||||||||||||||
| Cash, cash equivalents and short-term investments | $ | 1,119 | $ | 10,283 | $ | 6,708 | $ | 25,994 | $ | 14,962 | $ | 10,172 | $ | 10,172 | $ | 74,667 | ||||||||||||||||
| Working capital | 1,013 | 10,193 | 6,674 | 26,695 | 17,448 | 14,306 | 14,306 | 78,801 | ||||||||||||||||||||||||
| Total assets | 1,144 | 10,461 | 7,139 | 28,013 | 20,004 | 18,193 | 18,193 | 82,688 | ||||||||||||||||||||||||
| Preferred stock | 2,650 | 14,544 | 14,544 | 40,089 | 40,089 | 40,089 | — | — | ||||||||||||||||||||||||
| Common stock | — | — | — | — | — | — | 1 | 2 | ||||||||||||||||||||||||
| Additional paid in capital | 83 | 141 | 147 | 219 | 820 | 1,889 | 41,977 | 106,471 | ||||||||||||||||||||||||
| Total stockholders’ deficit | (1,613 | ) | (4,215 | ) | (7,680 | ) | (12,654 | ) | (21,529 | ) | (24,549 | ) | 15,540 | 80,035 | ||||||||||||||||||
| (1) | The pro forma column gives effect to the conversion of all of our outstanding preferred stock into 10,793,182 shares of our common stock immediately upon the closing of this offering. | |
| (2) | The pro forma as adjusted column gives further effect to the sale of shares of our common stock in this offering at an assumed offering price of $13.00 per share, the midpoint of the range on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering costs payable by us. A $1.00 increase or decrease in the assumed public offering price of $13.00 per share would increase or decrease, respectively, each line item in the balance sheet data above by approximately $5.1 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions. |
35
Table of Contents
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes to our financial statements included elsewhere in this prospectus. In addition to historical financial information, the following discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results and timing of selected events may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a medical device company focused on designing, developing and marketing products that implement our proprietary minimally invasive surgical approach to treat degenerative disc disease affecting the lower lumbar region of the spine. Using this TranS1 approach, a surgeon can access discs in the lower lumbar region of the spine through a 1.5 cm incision adjacent to the tailbone and can perform an entire fusion procedure through a small tube that provides direct access to the degenerative disc. We developed our TranS1 approach to allow spine surgeons to access and treat degenerative lumbar discs without compromising important surrounding soft tissue. We believe this approach enables fusion procedures to be performed with low complication rates, short procedure times, low blood loss, short hospital stays, fast recovery times and reduced pain. We have developed and currently market in the United States and Europe two single-level fusion products, AxiaLIF and AxiaLIF 360°. In addition, we have developed and currently market in Europe a two-level fusion product, AxiaLIF 2L, which has not yet been cleared in the United States. All of our products are delivered using our TranS1 approach.
From our incorporation in 2000 through 2004, we devoted substantially all of our resources to research and development andstart-up activities, consisting primarily of product design and development, clinical trials, manufacturing, recruiting qualified personnel and raising capital. We received FDA 510(k) clearance for our AxiaLIF product in the fourth quarter of 2004, and commercially introduced our AxiaLIF product in the United States in the first quarter of 2005. We further received FDA 510(k) clearance for our AxiaLIF 360° product in the United States in the third quarter of 2005 and began commercialization in the United States in the third quarter of 2006. We received a CE mark to market AxiaLIF in the European market in the first quarter of 2005 and began commercialization in the first quarter of 2006. For AxiaLIF 360°, we received a CE mark in the first quarter of 2006. We received a CE mark for our AxiaLIF 2L product in the third quarter of 2006 and began commercialization in the European market in the fourth quarter of 2006. We currently sell our AxiaLIF and AxiaLIF 360° products through a direct sales force and independent sales agents in the United States and independent distributors in Europe. Our revenues were $1.5 million in 2005, $5.8 million in 2006 and $7.2 million for the six months ended June 30, 2007. From our inception to June 30, 2007, we generated $14.5 million in revenue.
We rely on third parties to manufacture most of our products and their components. We believe these manufacturing relationships allow us to work with suppliers who have the best specific competencies while we minimize our capital investment, control costs and shorten cycle times, all of which allows us to compete with larger volume manufacturers of spine surgery products.
Since inception, we have been unprofitable. We incurred losses of $3.5 million in 2004, $5.0 million in 2005, $9.5 million in 2006 and $4.1 million for the six months ended June 30, 2007. As of June 30, 2007, we had an accumulated deficit of $26.4 million.
We expect to continue to invest in creating a sales and marketing infrastructure for our AxiaLIF and AxiaLIF 360° products in order to gain wider acceptance for these products. We also expect to continue to invest in research and development and related clinical trials. As a result, we will need to generate significant revenue in order to achieve profitability.
36
Table of Contents
Financial Operations
Revenue
We generate revenue from the sales of our procedure kits and implants used in our AxiaLIF fusion procedure for the treatment of degenerative disc disease. Our revenue is generated by our direct sales force, independent sales agents and independent distributors. In the United States, our procedure kits and implants are shipped from inventories at our facility to our direct sales force or independent sales agents for delivery to the hospital or surgical center. We invoice hospitals and surgical centers and revenue is recognized upon notification of product use or implantation. In Europe, we determine revenue recognition on a case by case basis dependent upon the terms and conditions of each individual distributor agreement. Under the distributor agreements currently in place, a distributor only has the right of return for defective products and accordingly revenue is recognized upon shipment of our products to our independent distributors. Although we intend to continue to expand our international sales and marketing efforts, we expect that a substantial amount of our revenues will be generated in the United States in future periods.
Cost of Revenue
Cost of revenue consists primarily of material and overhead costs related to our AxiaLIF and AxiaLIF 360° instruments and implants. Cost of revenue also includes facilities-related costs, such as rent, utilities and depreciation.
Research and Development
Research and development expenses consist primarily of personnel costs, including stock-based compensation expense in 2006 and in the six months ended June 30, 2007, within our product development, regulatory and clinical functions and the costs of clinical studies and product development projects. Research and development expense also includes legal expenses related to the development and protection of our intellectual property portfolio and facilities-related costs. In future periods, we expect research and development expenses to grow as we continue to invest in basic research, clinical trials, product development and in our intellectual property.
Sales and Marketing
Sales and marketing expenses consist of personnel costs, including stock-based compensation expense in 2006 and in the six months ended June 30, 2007, sales commissions paid to our direct sales representatives and independent sales agents, and costs associated with physician training programs, promotional activities, and participation in medical conferences. In future periods, we expect sales and marketing expenses to increase as we expand our sales and marketing efforts.
General and Administrative
General and administrative expenses consist of personnel costs, including stock-based compensation in 2006 and in the six months ended June 30, 2007, related to the executive, finance, information technology and human resource functions, as well as professional service fees, legal fees, accounting fees, insurance costs and general corporate expenses. We expect general and administrative expenses to increase as we grow our business and as we incur additional professional fees and increased insurance costs related to operating as a public company.
Other and Interest Income (Expense)
Other and interest income (expense) is primarily composed of interest earned on our cash, cash equivalents and available-for-sale securities.
37
Table of Contents
Results of Operations
Comparison of Six Months Ended June 30, 2006 and 2007 (Unaudited)
Revenue. Revenue increased from $2.2 million in the six months ended June 30, 2006 to $7.2 million in the six months ended June 30, 2007. The $5.0 million increase in revenue was primarily attributable to an increase in the number of products sold, which we believe resulted from continued market acceptance of our AxiaLIF and AxiaLIF 360° products. None of this increase was attributable to price increases. Sales of our AxiaLIF 360° product, which began commercialization in the United States in the third quarter of 2006, increased from zero in the six months ended June 30, 2006 to $2.8 million in the six months ended June 30, 2007. As a result, average selling prices in the United States increased from approximately $8,000 in the six months ended June 30, 2006 to approximately $9,200 in the six months ended June 30, 2007. In the six months ended June 30, 2007, we recorded 719 domestic AxiaLIF cases, including 281 AxiaLIF 360° cases, as compared to 268 domestic cases in the six months ended June 30, 2006. Our AxiaLIF 360° product has not yet begun commercialization in Europe and although our AxiaLIF 2L began commercialization in Europe in the fourth quarter of 2006, we did not generate significant revenues from the AxiaLIF 2L in the six months ended June 30, 2007. Revenue generated in Europe increased from $18,000 in the six months ended June 30, 2006 to $546,000 in the six months ended June 30, 2007. In the six months ended June 30, 2006 and 2007, 99% and 92%, respectively, of revenue was generated in the United States.
Cost of Revenue. Cost of revenue increased from $618,000 in the six months ended June 30, 2006 to $1.5 million in the six months ended June 30, 2007. The $841,000 increase in cost of revenue resulted primarily from higher material and overhead costs associated with increased sales volume for our AxiaLIF and AxiaLIF 360° products. As a percentage of revenue, cost of revenue decreased from 28.6% in the six months ended June 30, 2006, to 20.3% in the six months ended June 30, 2007. This decrease was primarily attributable to increased efficiencies associated with higher production and sales volumes, partially offset by an increase in the mix of lower margin sales outside the United States.
Research and Development. Research and development expenses increased from $2.1 million in the six months ended June 30, 2006, to $2.3 million in the six months ended June 30, 2007. The increase was primarily the result of an increase in personnel related costs, including stock based compensation, of $179,000 and an increase in legal costs of $350,000 related to the enhancement and protection of our intellectual property portfolio, partially offset by a reduction in spending on project related research and development and clinical trials of $288,000.
Sales and Marketing. Sales and marketing expenses increased from $4.0 million in the six months ended June 30, 2006, to $6.8 million in the six months ended June 30, 2007. The increase of $2.8 million was primarily the result of increased personnel related costs, including sales commissions and stock-based compensation expenses, of $2.5 million, as we continued to grow our sales and marketing organization in conjunction with increased product sales, increased training costs of $58,000 and increased cost for trade shows, advertising and promotions of $184,000.
General and Administrative. General and administrative expenses increased from $625,000 in the six months ended June 30, 2006, to $1.1 million in the six months ended June 30, 2007. The $458,000 increase was primarily attributable to increased personnel related costs, including stock based compensation expenses, of $160,000, increased professional fees related to planning and preparation for our initial public offering of $173,000 and increased occupancy costs of $98,000.
Other and Interest Income (Expense). Other and interest income decreased from $528,000 in the six months ended June 30, 2006, to $343,000 in the six months ended June 30, 2007. The $185,000 decrease in interest income was primarily the result of lower cash and short term investment balances earning interest because we continue to operate in a net loss position and to be cash flow negative as we grow and invest in our business.
38
Table of Contents
Comparison of the Years Ended December 31, 2004, 2005 and 2006
Revenue. Revenue increased from zero in 2004, to $1.5 million in 2005 and to $5.8 million in 2006. The $1.5 million increase in revenue from 2004 to 2005 was primarily attributable to the commercial introduction of AxiaLIF in the United States during the first quarter of 2005. The $4.3 million increase in revenue from 2005 to 2006 was primarily attributable to an increase in the number of AxiaLIF procedures performed in the United States, the introduction of AxiaLIF in Europe in the first quarter of 2006 and the commercialization of our AxiaLIF 360° product in the United States in the third quarter of 2006. None of this increase was attributable to price increases. In 2006, our AxiaLIF 360° product generated $530,000 of revenue, which contributed to the improvement in average selling prices in the United States from approximately $7,500 in 2005 to approximately $8,200 in 2006. In 2004, 2005 and 2006, we recorded zero, 197 and 661 domestic AxiaLIF cases, respectively, including 53 AxiaLIF 360° cases in 2006. Revenue generated in Europe increased from zero in 2004 and 2005 to $359,000 in 2006. In 2006, 93.8% of our revenue was generated in the United States and 6.2% was generated in Europe.
Cost of Revenue. Cost of revenue increased from zero in 2004, to $386,000 in 2005 and to $1.6 million in 2006. The $386,000 increase in cost of revenue from 2004 to 2005 was primarily attributable to the initial commercialization of the AxiaLIF product in 2005. The $1.2 million increase from 2005 to 2006 was primarily attributable to the increased number of AxiaLIF products sold in 2006 as compared to 2005 in the United States and Europe. As a percentage of revenue, cost of revenue was 26.3% in 2005 and 27.2% in 2006. The increase in cost of revenue as a percentage of revenue from 2005 to 2006 was primarily attributable to an increase in the mix of lower margin sales outside the United States, partially offset by increased efficiencies associated with higher production and sales volumes.
Research and Development. Research and development expenses increased from $1.8 million in 2004, to $2.4 million in 2005 and to $4.2 million in 2006. The $686,000 increase in expense in 2005 compared to 2004 was primarily the result of increases in personnel related costs of $284,000 as we continued to build our organization, increased project related research and development expenses and clinical trial costs of $293,000, and increased costs of $77,000 related to the enhancement of our intellectual property portfolio. The $1.8 million increase in expense in 2006 compared to 2005 was primarily the result of increases in personnel related costs, including stock-based compensation expense, of $616,000, increases in project related research and development and clinical trial costs of $891,000, increases in occupancy costs of $191,000 and increased cost of $86,000 related to the enhancement of our intellectual property portfolio.
Sales and Marketing. Sales and marketing expenses increased from $746,000 in 2004, to $2.6 million in 2005 and to $9.3 million in 2006. The increase in expenses from 2004 to 2005 was primarily the result of increased personnel related costs, including commissions, of $1.2 million, as we started to market and sell the AxiaLIF product in the United States, increased training costs of $390,000 and increased promotional activities of $86,000. The increase in expenses from 2005 to 2006 was primarily driven by further increases in personnel related costs, including commissions and stock-based compensation expense, of $4.9 million, as we continued to grow our sales and marketing organization in conjunction with increased product sales and our entry into the European marketplace, increased training of $1.1 million and increased promotional activities of $416,000.
General and Administrative. General and administrative expenses increased from $1.1 million in 2004, to $1.3 million in 2005 and decreased to $1.2 million in 2006. The increase in general and administrative expenses in 2004 compared to 2005 was primarily driven by increased employee related expenses of $109,000, and increased professional fees of $33,000. The decrease in expenses from 2005 to 2006 was primarily due to increased absorption of overhead costs, as we ramped production, of $383,000. These costs had previously been expensed as period costs resulting from excess capacity. We also saw an increase in personnel related costs, including stock-based compensation expense, of $287,000 and a decrease in professional fees of $99,000.
Other and Interest Income (Expense). Other and interest income was $150,000 in 2004, $247,000 in 2005 and increased to $989,000 in 2006 primarily due to interest income from $25.5 million raised as a result of the sale of series C preferred stock in September 2005.
39
Table of Contents
Liquidity and Capital Resources
Sources of Liquidity
Since our inception in 2000, we have incurred significant losses and, as of June 30, 2007, we had an accumulated deficit of $26.4 million. We have not yet achieved profitability, and anticipate that we will continue to incur losses in the near term. We expect that research and development, sales and marketing and general and administrative expenses will continue to grow and, as a result, we will need to generate significant revenues to achieve profitability. To date, our operations have been funded primarily with proceeds from the sale of preferred stock. Gross proceeds from our preferred stock sales total $40.5 million to date.
As of June 30, 2007, we did not have any outstanding debt financing arrangements, we had working capital of $14.3 million and our primary source of liquidity was $10.2 million in cash, cash equivalents and investments. We currently invest our cash and cash equivalents primarily in money market funds and high grade commercial paper. We currently place our short-term investments primarily in U.S. agency backed debt instruments and high grade corporate bonds and commercial paper.
Cash, cash equivalents and short-term investments decreased from $15.0 million at December 31, 2006 to $10.2 million at June 30, 2007. The decrease of $4.8 million was primarily driven by cash used in operations of $4.4 million and purchases of property and equipment of $390,000.
Cash, cash equivalents and short-term investments decreased from $26.0 million at December 31, 2005 to $15.0 million at December 31, 2006. This decrease of $11.0 million was primarily the result of net cash used in operating activities of $10.4 million and purchases of property and equipment of $609,000.
Cash, cash equivalents and short-term investments increased from $6.7 million at December 31, 2004 to $26.0 million at December 31, 2005. This increase of $19.3 million was primarily the result of $25.5 million in net proceeds from the sale of series C preferred stock and $68,000 in proceeds from the sale of common stock, offset by net cash used in operating activities of $5.7 million and purchases of property and equipment of $672,000.
Cash Flows
Net Cash Used in Operating Activities. Net cash used in operating activities was $4.4 million in the six months ended June 30, 2007. Net cash used in operating activities was $3.5 million in 2004, $5.7 million in 2005 and $10.4 million in 2006. For each of these periods, net cash used in operating activities was attributable primarily to net losses after adjustment for non-cash items, such as depreciation and stock-based compensation expense, and increases in net working capital requirements to support the introduction of our AxiaLIF product. The increases in working capital requirements for each of the periods were driven by the growth in inventories to support forecasted demand and the increases in accounts receivable resulting from increasing revenues exceeding cash inflow from customer collections.
Net Cash Provided by (Used in) Investing Activities. Net cash provided by investing activities in the six months ended June 30, 2007 was $4.9 million. Net cash used in investing activities was $106,000 in 2004, $6.1 million in 2005 and $5.1 million in 2006.
For each of these periods, net cash provided by or used in investing activities reflected the purchases of property and equipment, primarily for research and development, information technology, manufacturing operations and capital improvements to our facilities, and purchases or sales and maturities of short-term investments.
Net Cash Provided by Financing Activities. There was no significant net cash provided by financing activities in the six months ended June 30, 2007, in 2004 or in 2006. Net cash provided by financing activities in 2005 was $25.6 million. The $25.6 million in net cash provided by financing activities in 2005 was due to the net proceeds from the sale of $25.5 million in shares of our series C preferred stock and $68,000 in proceeds from the issuance of shares of our common stock.
40
Table of Contents
Operating Capital and Capital Expenditure Requirements
We believe that our existing cash and cash equivalents, together with the net proceeds of this offering and interest that can be earned on these balances, will be sufficient to meet our cash needs for at least the next two years. We intend to spend substantial sums on sales and marketing initiatives to support the ongoing commercialization of our products and on research and development activities, including product development, regulatory and compliance, clinical studies in support of our currently marketed products and future product offerings, and the enhancement and protection of our intellectual property. We may need to obtain additional financing to pursue our business strategy, to respond to new competitive pressures or to take advantage of opportunities that may arise. The sale of additional equity or convertible debt securities could result in dilution to our stockholders. If additional funds are raised through the issuance of debt securities, these securities could have rights senior to those associated with our common stock and could contain covenants that would restrict our operations. Any additional financing may not be available in amounts or on terms acceptable to us. If we are unable to obtain this additional financing, we may be required to reduce the scope of our planned product development and marketing efforts.
Contractual Obligations
The following table discloses information about our contractual obligations by the year in which payments are due as of December 31, 2006:
| Payments Due by Year | ||||||||||||||||||||
| Less than | After | |||||||||||||||||||
Contractual Obligations | Total | 1 Year | 1-3 Years | 4-5 Years | 5 Years | |||||||||||||||
Operating leases(1) | $ | 563,000 | $ | 152,000 | $ | 318,000 | $ | 93,000 | — | |||||||||||
| (1) | We rent office space under an operating lease which expires in 2010. |
There have been no material changes to our contractual obligations in the six months ended June 30, 2007.
Related Party Transactions
For a description of our related party transactions, see the “Related Party Transactions” section of this prospectus.
Off-Balance Sheet Arrangements
As of June 30, 2007, we did not have any outstanding debt or available debt financing arrangements or off-balance sheet liabilities.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of financial statements requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, revenue and expenses, and disclosures of contingent assets and liabilities at the date of the financial statements. On an on-going basis, we evaluate our estimates, including those related to revenue recognition, accounts receivable, inventories, income taxes and stock-based compensation. We use authoritative pronouncements, historical experience and other assumptions as the basis for making estimates. Actual results could differ from those estimates under different assumptions or conditions.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
41
Table of Contents
Revenue recognition. We recognize revenue in accordance with SEC Staff Accounting Bulletin, or SAB, No. 104, “Revenue Recognition.” SAB No. 104 requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) title has transferred; (3) the fee is fixed or determinable; and (4) collectibility is reasonably assured. We generally use customer purchase orders to determine the existence of an arrangement. We use documents from our sales agents to verify that product has been delivered and title has transferred. In the United States, we deem title to transfer upon implantation of our products. In Europe, we assess title transfer on a case by case basis dependent upon the terms and conditions of each individual distributor agreement. Under the distributor agreements currently in place, a distributor only has right of return for defective products and accordingly title is deemed to transfer upon shipment of our products to our independent distributors. We assess whether the fee is fixed or determinable based on the terms of the agreement associated with the transaction. In order to determine whether collection is reasonably assured we assess a number of factors, including past transaction history with the customer. If we determine that collection is not reasonably assured, we defer the recognition of revenue until collection becomes reasonably assured, which is generally upon receipt of payment.
Accounts receivable and allowances. We monitor collections and payments from our customers and maintain an allowance for doubtful accounts based upon our historical experience and any specific customer collection issues that we have identified. While our credit losses have historically been within our expectations and the allowance established, we may not continue to experience the same credit loss rates that we have in the past. We make estimates on the collectibility of customer accounts based primarily on analysis of historical trends and experience and changes in customers’ financial condition. Management uses its best judgment, based on the best available facts and circumstances, and records a reserve against the amounts due to reduce the receivable to the amount that is expected to be collected. These reserves are reevaluated and adjusted as additional information is received that impacts the amount reserved.
Inventory. We state our inventories at the lower of cost or market, computed on a standard cost basis, which approximates actual cost on afirst-in, first-out basis and market being determined as the lower of replacement cost or net realizable value. Costs are monitored on an annual basis and updated as necessary to reflect changes in supplier costs and the rate of our overhead absorption is adjusted based on projections of our manufacturing department costs and production plan. Inventory reserves are established when conditions indicate that the selling price could be less than cost due to obsolescence, usage, or we deem we hold excessive levels of inventory based on market demand.
Accounting for Income Taxes. Significant management judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We have recorded a full valuation allowance on our net deferred tax assets as of December 31, 2006 due to uncertainties related to our ability to utilize our deferred tax assets in the foreseeable future.
Stock-Based Compensation
Through December 31, 2005, we accounted for employee stock options using the intrinsic-value method in accordance with Accounting Principles Board Opinion No. 25, Accounting for Stock Issued to Employees, or APB No. 25, Financial Accounting Standards Board, and related interpretations. In accordance with APB No. 25, stock-based compensation was calculated using the intrinsic value method and represented the difference between the deemed per share market price of our common stock and the per share exercise price of the stock option. Based on this method, we did not incur any compensation expense under APB No. 25. For periods prior to January 1, 2006, we have complied with the disclosure-only provisions of SFAS 123. Effective January 1, 2006, we adopted SFAS 123(R) using the prospective transition method, which requires the measurement and recognition of compensation expense for all share-based payment awards granted, modified and settled to our employees and directors after January 1, 2006. The fair value of stock options was estimated using a Black-Scholes option pricing model. This model requires the input of subjective assumptions in implementing SFAS 123(R), including expected stock price volatility, expected life and estimated forfeitures of each award. The fair value of equity-based awards is amortized over the vesting period of the award, and we have elected to use the straight-line method of amortization. Due to the limited amount of historical data
42
Table of Contents
available to us, particularly with respect to stock-price volatility, employee exercise patterns and forfeitures, actual results could differ materially from our expectations.
We have granted stock options with exercise prices ranging from $1.11 to $6.67 per share during the18-month period ended June 30, 2007. In determining the fair value of the common stock at the time of the stock option grants our board of directors, with the assistance of management, performed contemporaneous fair value analyses for the value of our common stock at the time of such stock option grants. Determining the fair value of our common stock required us to make complex and subjective judgments, assumptions and estimates, which involved inherent uncertainty. Given the absence of an active market for our common stock, uncertainty of the results of long-term data from our AxiaLIF procedures and uncertainty prior to the second quarter of 2007 as to whether we would pursue an initial public offering, our board of directors, with input from management, determined the estimated fair value of our common stock on the date of grant based on several factors, including:
| • | important developments in our operations; | |
| • | our performance and the status of our research and development efforts; | |
| • | the likelihood of achieving a liquidity event for the shares of common stock, such as an initial public offering or an acquisition of the company, given prevailing market conditions; | |
| • | the lack of control of the minority interest granted under the stock options to influence the occurrence of a liquidity event; | |
| • | the options to acquire shares of our common stock were subject to vesting, generally vesting over a four-year period; | |
| • | the prices at which we issued preferred stock in September 2005 and the rights and privileges associated with those preferred stock issuances; and | |
| • | the illiquidity of our common stock as a private company. |
The fair value of common stock for options granted from January 1, 2006 through June 30, 2007 was determined contemporaneously by our board of directors, with input from management.
One of the methodologies we used to value our common stock for stock option grants occurring between January 1, 2006 and June 30, 2007 was to first estimate our future expected equity value in an initial public offering based upon a multiple of the estimated twelve months forward revenues, using the forward multiples of public, high growth medical device and orthopedic companies. Second, we deducted the estimated initial public offering proceeds to arrive at an estimated future value which was discounted back to present, given the time involved and the risks related to timing, the stock market in general and the risks inherent in our ability to execute our operating plans. Third, we further discounted the value to account for the preferences of our preferred stockholders, relating primarily to their liquidation rights, and for the option holders’ minority interests. In late January 2007, based upon improved stock market and initial public offering conditions, we began assessing with investment bankers the potential for an initial public offering of our common stock. Subsequently, we received estimated valuations from several investments bankers, along with a recommendation to commence preparing for an initial public offering. These initial valuations from the investment bankers assumed the favorable outcome of the twelve month clinical data that we were then awaiting. We did not receive such twelve month data until May 2007, at which point the board determined that a further increase in the fair market value of our common stock was warranted.
We granted no stock options during the six month period ending June 30, 2006, we granted 735,374 stock options in November 2006 with an exercise price of $1.11 per share, 67,388 stock options with an exercise price of $2.00 per share in January 2007, 29,251 stock options with an exercise price of $2.38 per share in March 2007, 418,770 stock options with an exercise price of $5.56 per share in May 2007 and 72,000 stock options with an exercise price of $6.67 per share in June 2007. The exercise prices for all of these options were set at a price which the board believed represented the fair value of our common stock at the time of
43
Table of Contents
grant. Certain of the reasons for the increase in the estimated fair value from $1.11 to $6.67 per share are as follows:
| • | During 2006 we were still implementing and attempting to prove our business model and increasing the number of spine surgeons trained to use our products and perform our procedures in an effort to achieve a market share that we believed could allow us to achieve profitable operations in the future. | |
| • | At the time of our option grants in November 2006, while we continued to prove our business model, we were awaiting the preliminary twelve month clinical results from our prospective registry study. As we awaited this data, we did not believe it was appropriate to increase the revenue estimates for the twelve-month period ending December 31, 2007 that we used in implementing our valuation methodology. Moreover, we recognized that we continued to operate at a loss and expected to do so for the foreseeable future. We also took into account the aggregate liquidation preference of $40 million on our preferred stock outstanding and that any sale of the company would need to exceed that amount before holders of common stock received any benefit in such sale. Finally, we believed that an initial public offering of our common stock was unlikely for at least the next 18 months. As a result of these considerations, the board determined that the fair value of our common stock was $1.11 per share. | |
| • | At the time of our option grants in January 2007, we continued to prove our business model and await the results of our twelve month clinical data regarding the use of our AxiaLIF products. At the time of such stock option grants, we had completed interviews with investment bankers which provided us with valuations that assumed the positive outcome of such clinical data. Other than such interviews and the increase in the number of spine surgeons who had been trained to perform our surgery, there was no new information to support changes to the fair valuation of our stock from November 2006. For these reasons, the board determined that the fair value of our common stock was $2.00 per share. |
At the time of our stock option grants in March 2007, we continued to prove our business model and await the results of our twelve month clinical data regarding the use of our AxiaLIF products. The increase in the number of spine surgeons trained to perform our AxiaLIF procedure and our results of operations caused us to update our estimated revenues for the twelve-month period used in our common stock valuation methodology. This increase in forecasted revenue caused us to increase the estimated fair value of our common stock. At this time, without definitive twelve month clinical data, we continued to believe that a public offering of our common stock was likely to be at least one year away. While the growth in the number of surgeons trained to perform our AxiaLIF procedure and the initial results from our clinical data were encouraging, the data was still early. As a result of these considerations, the board believed that the fair value of our common stock had increased to $2.38 per share.
At the time of our stock option grants in May 2007, we were receiving preliminary twelve month clinical data outcomes on our AxiaLIF procedure. Such clinical data was encouraging and generally met our expectations. We also continued to increase the number of surgeons trained to perform our AxiaLIF procedure. In addition, our results of operations for the first quarter of 2007 showed continued growth in revenue. The outcome from the clinical data convinced the board that our AxiaLIF procedure, implants and tools could succeed in the marketplace. The board also believed that while it was likely that we would continue to operate at a loss for the foreseeable future, we could increase revenue enough in the future to achieve profitable operating results and positive cash flow.
| • | Additionally, during this period, investment bankers advised us that general market conditions and the initial public offering market conditions had improved significantly, such that an offering of our common stock could be completed late in 2007 or early in 2008. Consequently, we engaged investment bankers to initiate the process of an initial public offering and began drafting our registration statement. In the light of all of these considerations, the board believed the fair market value of our common stock had increased to $5.56 per share. | |
| • | At the time of the stock option grants in June 2007, we continued to train surgeons to perform the AxiaLIF procedure and we also continued to receive positive results from our twelve month clinical data. In addition, our results of operations reinforced our belief that our products and procedures had |
44
Table of Contents
| become recognized by surgeons as a viable alternative for lower back pain surgery. Using our valuation methodology, and without changing the forecasted revenues we relied on for our valuation in May 2007, we found that the valuation was stable. On the other hand, using a valuation methodology of discounting from the date of a projected public offering of our common stock, the board believed that the fair value of our common stock had further increased to $6.67 per share. |
We believe that at the time of the grant of stock options as described above, the estimated fair value set by our board of directors was appropriate given the information available to our board and management. Although we believe these determinations accurately reflected the historical value of our common stock, in connection with the preparation of the financial statements necessary for the filing of the registration statement in connection with our initial public offering of common stock, we retrospectively analyzed the fair value of our common stock at option grant dates from January 1, 2006 to June 30, 2007. As part of our retrospective analysis, we considered the status and progress of a number of our specific business and financial conditions and milestones during 2006 and the first six months of 2007, including our results of operations, research and development activities, regulatory milestones, product and operational milestones, the lack of liquidity in our common stock, the increasing likelihood we would pursue an initial public offering, preliminary pricing indications in connection with this offering and industry trends in the market for medical device issuers. In addition, we further analyzed the discounts we applied to our valuation as they applied to our common stock and eliminated the discount for minority interest. We also considered the rights and preferences of our preferred stock in relation to our common stock. With the benefit of hindsight and actual knowledge of how the various factors noted above have progressed over time and the uncertainties existing at the time of the option grants were resolved, we reassessed the deemed fair value of our common stock for financial reporting purposes for all stock options granted since January 1, 2006.
The following table summarizes information for all stock options granted by us during the 18 months ended June 30, 2007, including the exercise price and estimated reassessed fair value of our common stock based on our retrospective reassessment described above, as well as the intrinsic value or difference between the reassessed fair value and the exercise price per share:
| Estimated | ||||||||||||||||
| Reassessed Fair | Intrinsic Value Per | |||||||||||||||
Date of Grant | Number | Exercise Price | Value | Share | ||||||||||||
| Nov. 11, 2006 | 735,374 | $ | 1.11 | $ | 5.20 | $ | 4.09 | |||||||||
| Jan. 18, 2007 | 67,388 | $ | 2.00 | $ | 5.94 | $ | 3.94 | |||||||||
| Mar. 15, 2007 | 29,251 | $ | 2.38 | $ | 5.94 | $ | 3.56 | |||||||||
| May 16, 2007 | 418,770 | $ | 5.56 | $ | 10.00 | $ | 4.44 | |||||||||
| June 18, 2007 | 72,000 | $ | 6.67 | $ | 10.00 | $ | 3.33 | |||||||||
The difference between the weighted average exercise per share of all of our outstanding stock options and the assumed offering price of $13.00 per share, the midpoint of the range on the cover page of this prospectus, is $11.26.
We recorded no stock-based compensation charges in 2004 or in 2005, and we recorded charges of $522,000 in 2006 and $1,058,000 in the six months ended June 30, 2007. At June 30, 2007, we had a total of $5.6 million remaining to be amortized over the vesting period of the stock options. We expect the total unamortized deferred stock compensation recorded for all option grants through June 30, 2007 will be amortized as follows: $990,000 during the remainder of 2007, $1,683,000 during 2008, $1,489,000 during 2009, $893,000 during 2010, and $578,000 during 2011. As of June 30, 2007, 169,470 stock options were subject to periodic remeasurement.
Although it is reasonable to expect that the completion of the initial public offering will add value to our shares because they will have increased liquidity and marketability, the amount of the additional value cannot be measured with precision or certainty. Determining the reassessed fair value of our common stock required our board of directors and management to make complex and subjective judgments, assumptions and estimates, which involved inherent uncertainty. Had our board of directors used different assumptions and estimates, the
45
Table of Contents
resulting fair value of our common stock and the resulting stock-based compensation expense could have been different.
Recent Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157, “Fair Value Measurements”, or SFAS 157. SFAS 157 defines fair value, establishes a framework for measuring fair value in accordance with generally accepted accounting principles and expands disclosures about fair value measurements. The provisions of SFAS 157 are effective for fiscal years beginning after November 15, 2007 and are to be applied prospectively. We are currently evaluating the impact, if any, of the adoption of SFAS 157 will have on our financial reporting.
In February 2007, the FASB issued FAS No. 159, “Fair Value Option For Financial Assets and Financial Liabilities”, or FAS 159. FAS 159 provides companies with an option to report selected financial assets and liabilities at fair value. FAS 159 requires that the fair value of the assets and liabilities that the company has chosen to report at fair value be shown on the face of the balance sheet. FAS 159 also requires companies to provide additional information to enable users of the financial statements to understand the company’s reasons for electing the fair value option and how changes in the fair values affect earnings for the period. FAS 159 also establishes presentation and disclosure requirements designed to facilitate comparisons between companies that choose different measurement attributes for similar types of assets and liabilities. FAS 159 is effective for fiscal years beginning on or before November 15, 2007. We are currently evaluating the potential impact FAS 159 may have on our financial position and operating results.
Effective January 1, 2007, we adopted the provisions of FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes: An Interpretation of FASB Statement No. 109, or FIN 48. This interpretation requires that we recognize in our financial statements the impact of a tax position, if that position is more likely than not of being sustained on audit, based on the technical merits of the position. At the adoption date of January 1, 2007, we had $1,043,000 of unrecognized deferred tax benefits, all of which was subject to a full valuation allowance, which effectively reduced the deferred tax benefits to $0, since realization of these benefits could not be reasonably assured. Accordingly, there is no change recorded to the beginning retained earnings as a result of the adoption of FIN 48.
Quantitative and Qualitative Disclosures About Market Risk
Our exposure to interest rate risk at June 30, 2007 is related to our investment portfolio. We invest our excess cash primarily in money market funds, debt instruments of the U.S. government and its agencies and in high quality corporate bonds and commercial paper. Due to the short-term nature of these investments, we have assessed that there is no material exposure to interest rate risk arising from our investments.
Although substantially all of our sales and purchases are denominated in U.S. dollars, future fluctuations in the value of the U.S. dollar may affect the competitiveness of our products outside the United States. We do not believe, however, that we currently have significant direct foreign currency exchange rate risk and have not hedged exposures denominated in foreign currencies.
Income Taxes
Realization of deferred tax assets is dependent upon the uncertainty of the timing and amount of future earnings, if any. Accordingly, full deferred tax asset valuation allowances have been established at December 31, 2004, 2005, 2006 and at June 30, 2007 to reflect these uncertainties.
As of December 31, 2006, we had federal net operating loss carryforwards of $19.6 million, and federal research and development credit carryforwards of $624,000. The federal net operating loss and research and development credit carryfowards will begin to expire in 2021 unless previously utilized. Utilization of the net operating loss and tax credit carryforwards may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, or the Code. This annual limitation may result in the expiration of net operating loss and tax credit carryforwards before utilization.
46
Table of Contents
Overview
We are a medical device company focused on designing, developing and marketing products that implement our proprietary minimally invasive surgical approach to treat degenerative disc disease affecting the lower lumbar region of the spine. Using this TranS1 approach, a surgeon can access discs in the lower lumbar region of the spine through a 1.5 cm incision adjacent to the tailbone and can perform an entire fusion procedure through a small tube that provides direct access to the degenerative disc. We developed our TranS1 approach to allow spine surgeons to access and treat degenerative lumbar discs without compromising important surrounding soft tissue. We believe this approach enables fusion procedures to be performed with low complication rates, short procedure times, low blood loss, short hospital stays, fast recovery times and reduced pain. We have developed and currently market in the United States and Europe two single-level fusion products, AxiaLIF and AxiaLIF 360°. In addition, we have developed and currently market in Europe a two-level fusion product, AxiaLIF 2L, which has not yet been cleared in the United States. All of our products are delivered using our TranS1 approach.
Currently, our single-level AxiaLIF product for L5/S1 fusions is commercially available in the United States and Europe, while our two-level AxiaLIF product for L4/L5/S1 fusions is available in Europe, but has not yet been approved in the United States. Our AxiaLIF product received FDA 510(k) clearance in December 2004 and a CE mark in March 2005 and was commercially launched in the United States in January 2005. Our AxiaLIF 360° product received FDA 510(k) clearance in September 2005 and a CE mark in March 2006 and was commercially launched in the United States in July 2006. We received a CE mark for our AxiaLIF 2L product in the third quarter of 2006 and began commercialization in the European market in the fourth quarter of 2006. We intend to commercialize our AxiaLIF 2L product in the United States after receipt of 510(k) clearance. We are currently conducting additional testing required by the FDA in support of our 510(k) clearance for our AxiaLIF 2L product. We expect to obtain FDA 510(k) clearance for, and commercially launch, our AxiaLIF 2L product in the United States in 2008. As of August 31, 2007, over 2,000 fusion procedures have been performed globally using our AxiaLIF products. We currently sell the AxiaLIF and AxiaLIF 360° products through 23 direct sales personnel and 21 independent sales agents in the United States and seven independent distributors internationally. By mid-2008, we expect to have increased the number of our direct sales representatives to 50. For the year ended December 31, 2006 our revenues were $5.8 million and $7.2 million for the six months ended June 30, 2007. Our net losses were $9.5 million for the year ended December 31, 2006 and $4.1 million for the six months ended June 30, 2007.
Lower back pain affects over six million people annually in the United States and is a leading cause of healthcare expenditures globally. Our currently marketed products address the lower lumbar spine fusion market, which Millennium Research Group valued at over $1.4 billion in the United States in 2006 and estimated to grow to $1.8 billion by 2011. We believe the introduction of minimally invasive spine procedures, such as ours, will attract more back pain patients, and attract them earlier, to a definitive surgical solution, thereby increasing the rate at which the market grows.
We also have two additional products under development that implement our TranS1 approach: our Percutaneous Nucleus Replacement, or PNR, and Partial Disc Replacement, or PDR, devices. We expect these devices would be used earlier in the treatment of degenerative disc disease than a fusion, as they are designed to preserve motion in the lumbar spine while providing stabilization. Our PNR device is currently in early-stage human clinical trials outside the United States, and we expect to commercialize this product in Europe in the second half of 2008. We expect to file an investigational device exemption, or IDE, with the FDA in 2008 for our PNR implant in support of U.S. commercialization. We anticipate commencing human clinical trials of our PDR implant outside the United States in 2008.
Spine Anatomy
The human spine is the core of the human skeleton and provides important structural support while remaining flexible to allow movement. It consists of 33 separate interlocking bones called vertebrae that are
47
Table of Contents
connected by soft tissue and provide stability while facilitating motion. Vertebrae are paired into motion segments that move by means of two facet joints and one disc. The facet joints provide stability and enable the spine to bend and twist while the discs absorb pressures and shocks to the vertebrae. Nerves are contained in the spinal column and run through the foramen openings to the rest of the body.
The vertebrae are categorized into five regions: cervical, thoracic, lumbar, sacral and coccyx. The lumbar region, which is at the bottom of the spine and consists of five vertebrae, is capable of limited movement, and primarily functions as support for the body’s weight. The sacrum consists of five fused vertebrae labeled S1 through S5 directly below the lumbar region and that provide attachment for the hipbones as well as protection to organs in the pelvic area. The coccyx, also known as the tailbone, is at the end of the spine.
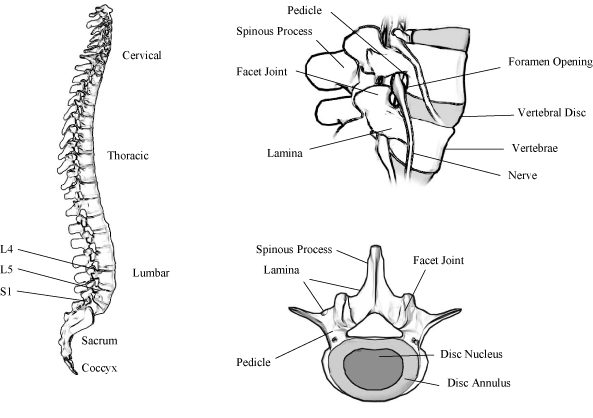
Medical Conditions Affecting the Lower Lumbar Spine and Traditional Treatment Alternatives
Degenerative disc disease is a common medical condition affecting the lower lumbar spine and refers to the degeneration of the disc from aging and repetitive stresses resulting in a loss of flexibility, elasticity and shock-absorbing properties. As degenerative disc disease progresses, the space between the vertebrae narrows, which can pinch the nerves exiting the spine and result in back pain, leg pain, numbness and loss of motor function. This lower back pain can be overwhelming for patients as the resulting pain can have significant physical, psychological and financial implications.
Treatment alternatives for lower lumbar spine conditions range from non-operative conservative therapies to highly invasive surgical interventions. Conservative therapies are typically the initial treatments selected by patients and physicians and they include rest, bracing, physical therapy, chiropractics, electrical stimulation and drugs. When conservative therapies fail to provide adequate pain relief, surgical interventions, including fusion procedures, may be used to address the pain. If a patient’s disc degeneration has not progressed to a stage requiring fusion, but has progressed beyond the stage where conservative therapies provide pain relief, physicians may use non-fusion surgical procedures. Non-fusion surgical procedures utilize implants that are
48
Table of Contents
designed to restore disc height and allow limited movement of the vertebrae similar to a healthy spine in order to avoid increased pressures on adjacent vertebrae. These procedures and implants include dynamic stabilization devices, artificial discs and prosthetic disc nucleuses.
Fusion procedures attempt to alleviate lower back pain by removing problematic disc material and permanently joining together two or more opposing vertebrae. This is done in a manner that restores the appropriate space between the vertebrae surrounding the degenerative disc and eliminates mobility of the affected vertebrae. By restoring disc height and eliminating motion, fusion attempts to prevent the pinching of the nerves exiting the spine and thereby reducing pain.
Traditional fusion procedures typically involve an incision in the skin, and cutting muscle or moving organs to gain access to the spine. The degenerated disc is then removed, referred to as a discectomy, and a rigid implant is inserted, such as a bone graft or cage, to stabilize the diseased vertebrae. This process is referred to as fixation. The bone graft or cage promotes the growth of bone between the vertebrae. Surgeons often also affix supplementary rods and screws along the spine to provide additional stabilization while the vertebrae fuse together during the six to eighteen months following surgery. The primary surgical fusion procedures performed in the lower lumbar region include: ALIF, PLIF, TLIF and XLIF.
Anterior Lumbar Interbody Fusion, or ALIF. To perform an ALIF procedure, surgeons access the spine through an incision on the patient’s abdomen which provides them with optimal access to the vertebral space for performing a discectomy and inserting bone grafts for fusion. Supporting soft tissue and nerves are manipulated or removed to accommodate the anterior access required by the ALIF procedure. Surgeons commonly perform ALIF procedures in conjunction with a general or vascular surgeon because critical vasculature and organs must be retracted to gain access to the spine. The assistance of a second surgeon can reduce the economics for the spine surgeon and increase the difficulty in scheduling the surgery. Complications associated with ALIF procedures include vascular damage to the vena cava and aorta.
Posterior Lumbar Interbody Fusion, or PLIF. To perform a PLIF procedure, surgeons access the spine through an incision on the center of the patient’s back. The surgeon then navigates through muscles and nerves to gain access to the spine. Once at the spine, the surgeon removes bone from the lamina to gain access to the affected disc space where a discectomy is performed and a bone graft is placed. When compared to ALIF procedures, PLIF procedures are generally considered easier to perform and can achieve better nerve root decompression in certain cases. However, the anatomy of the spine prevents surgeons from removing the entire degenerated disc and obtaining optimal access for insertion of an implant or bone graft. Complications associated with PLIF procedures include nerve damage, soft tissue damage and implant migration.
Transforaminal Lumbar Interbody Fusion, or TLIF. TLIF procedures are performed in a similar manner to PLIF procedures except the surgeon accesses the spine through a small incision slightly to the left or right of the center of the patient’s back. After reaching the spine, the surgeon removes a portion of the facet joint and navigates through the foramen which provides better visualization and disc removal capabilities than PLIF. Complications associated with TLIF procedures are similar to those found in PLIF procedures including nerve damage, soft tissue damage and implant migration.
Extreme Lateral Interbody Fusion, or XLIF. To perform an XLIF procedure, the surgeon accesses the spine from the patient’s side through one or two small incisions. The XLIF procedure is not appropriate for fusions in the L5/S1 segment because the pelvis interferes with access. While the XLIF procedure damages less patient tissue than other fusion procedures, we believe that there is a significant physician learning curve.
360° Lumbar Fusion Procedure. Currently, the most common and structurally rigid lumbar fusion surgery is referred to as a 360° fusion and requires a second surgical procedure immediately following an ALIF, PLIF, TLIF or XLIF procedure. The additional procedure involves the permanent placement of screws and rods in the back to provide additional support while the vertebrae fuse together during the six to eighteen months following surgery.
49
Table of Contents
Limitations of Traditional Lower Lumbar Spine Procedures
While traditional and minimally invasive fusion procedures can be effective at treating lower back pain, common drawbacks of these procedures include:
| • | Disruption to Soft Tissue and Support Structures. Current lumbar fusion procedures require creating a pathway from the skin to the degenerative disc that is large enough to allow direct visualization and work by the surgeon. It is common to cut through healthy muscle or move critical organs, arteries, nerves and soft tissue, which can lead to bleeding, scarring, nerve damage and bowel disruption. | |
| • | Significant Blood Loss. As a result of undergoing current lumbar fusion procedures, patients typically experience significant blood loss of between 100 and 1,400 cc of blood. As a result, it is common for patients to use the hospital’s blood supply or donate units of their own blood before a lumbar fusion procedure to replenish any significant blood loss. | |
| • | Lengthy Operative Procedure Times. We believe it is common for current lumbar fusion procedures to take between 90 minutes and 4 hours to complete. With some procedures a second surgeon may be required. Long procedure times increase the risks of complications and blood loss. Also, hospital and physician resources are consumed for lengthy periods of time, which can reduce productivity and increase costs. | |
| • | Lengthy Patient Hospital Stays. Patients remain in the hospital for an average of three days following a lumbar fusion procedure which consumes hospital and physician resources. | |
| • | Significant Patient Recovery Time. Patients require three to six months to recover and rehabilitate after undergoing a lumbar fusion procedure before resuming normal activities. | |
| • | Unresolved Patient Pain. Patients may continue to experience lower back pain after undergoing lumbar fusion procedures even though x-rays show successful fusion has been achieved through the growth of new bone. We believe this may be caused by muscle dissection, implant irritation and scar tissue that develops around the site of the surgery. |
Market Opportunity
Lower back pain affects over six million people annually in the United States and is a leading cause of healthcare expenditures globally. According to Millennium Research Group, in 2006, 233,300 instrumented lumbar fusion procedures were performed in the United States, of which 46,460 were single-level L5/S1, 40,430 were two-level L4/L5/S1 and 51,170 were more than two level procedures involving the L4/L5/S1 level. These single-level, two-level and multi-level procedures represent an estimated market opportunity of $1.4 billion in the United States that is anticipated to exceed $1.8 billion by 2011.
In recent years, the trend in surgical procedures for the spine has been toward more minimally invasive alternatives. Historically, meaningful technology improvements to conventional therapies have resulted in surgical procedures being performed earlier in the patient continuum of care, such as minimally invasive knee arthroscopy in the sports medicine market and coronary angioplasty in the cardiovascular surgery markets, resulting in larger market opportunities. Certain minimally invasive spine surgery procedures could have the same impact on lower back surgery.
Developments are being made in non-fusion technologies including dynamic stabilization devices, artificial discs, nuclear disc prosthetics, annulus repair and facet arthroplasty devices. The 2006 non-fusion market was valued at $128 million and is forecasted to be valued at $1.8 billion by 2011. In 2006, dynamic lumbar stabilization devices and artificial lumbar discs were the only non-fusion technologies available in the United States and accounted for $104 million and $24 million, respectively. In 2011, the non-fusion market is anticipated to be comprised of dynamic stabilization devices totaling $815 million, artificial lumbar discs totaling $397 million, artificial cervical discs totaling $450 million, partial disc replacement totaling $92 million, annulus repair totaling $29 million and facet arthroplasty totaling $22 million.
50
Table of Contents
Our Solution
We believe we have developed the least invasive approach for surgeons to perform fusion and motion preserving surgeries in the L4/L5/S1 region without the drawbacks associated with current lumbar fusion procedures. We refer to this proprietary approach as our TranS1 approach. We have developed and are marketing two fusion products that are delivered using our TranS1 approach: AxiaLIF and AxiaLIF 360°. In addition, we are developing two motion preserving devices that are delivered using our TranS1 approach: Percutaneous Nucleus Replacement, or PNR, and Partial Disc Replacement, or PDR.
To access the spine using our TranS1 approach, the surgeon creates a 1.5 cm incision adjacent to the tailbone while the patient is lying on their stomach. The surgeon then navigates a guidepin introducer a short distance along the sacrum using imaging technologies to a spine access point near the junction of the S1/S2 vertebral bodies. As the guidepin is advanced it moves soft tissue structures including the bowel to the side. From this access point, the guidepin is inserted through the bone into the disc of the lowest lumbar motion segment known as the L5/S1 disc. A cannula, or tube, is inserted over the guidepin to create a tissue-protecting working channel between the surgical access site and the L5/S1 disc where the entire fusion operation is then performed. This protected working channel provides access to the interior of the disc for rotating cutters and brushes to remove disc material, the introduction of graft material and the insertion of our implant. The implant immediately provides fixation and restores disc height. In order to perform our AxiaLIF 360° procedure, a second incision is made approximately eight inches above the first incision, and two facet screws are implanted. The 360° fusion is widely regarded as the most stable lumbar fusion procedure available. The entire AxiaLIF procedure takes approximately 45 to 60 minutes to perform, and the AxiaLIF 360° requires an additional 20 to 30 minutes.
51
Table of Contents
The diagrams below depict the principal steps involved in a stand-alone AxiaLIF procedure.
 |  | |
| 1. Initial access is achieved with an introducer through a small incision next to the tailbone | 2. Introducer is advanced along the sacrum to the S1 | |
 |  | |
| 3. After access into the disc, the degenerated disc material is removed | 4. Bone grafting material is placed into the disc | |
 |  | |
| 5. The AxiaLIF implant is introduced | 6. Fixation is established and disc height is restored |
We believe our TranS1 approach and associated products provide the following benefits for patients, providers and payors:
| • | Least Invasive Approach Minimizes Complications. Our Axia LIF products are delivered using our TranS1 approach, which we believe is the least invasive approach to deliver fusion and motion preserving products to the L4/L5/S1 region. Procedures performed utilizing our TranS1 approach have been documented to have favorable clinical safety profiles with complication rates of approximately 1%. | |
| • | Ease of Use and Short Procedure Times. We have received feedback from practicing surgeons that they can perform AxiaLIF procedures utilizing our TranS1 approach within 45 to 60 minutes after they have performed just two to three surgeries. We believe that these short procedure times can result in reduced patient recovery times, decreased costs for the hospitals and increased productivity for the surgeons. We also believe the ease of use of our TranS1 approach enables reduced physician training as compared to alternative lower lumbar spine procedures. |
52
Table of Contents
| • | Low Blood Loss. We believe the least invasive nature of our TranS1 approach results in the average patient losing approximately 25 to 125 cc of blood during an AxiaLIF or AxiaLIF 360° procedure, which correlates to reduced pain and faster recoveries. Given the associated low blood loss, patients generally do not need to donate blood prior to undergoing procedures that utilize our TranS1 approach. |
| • | Short Patient Hospital Stays. Patients typically stay only one night in the hospital after receiving a procedure performed using our TranS1 approach. In a small percentage of cases, patients are able to return home the same day. |
| • | Reduction in Patient Pain. We believe our TranS1 approach is effective at reducing lower back pain because surrounding soft tissue is not violated which prevents the creation of scar tissue, a leading cause of pain. |
Our Strategy
Our goal is to become a global leader in the treatment of conditions affecting the L4/L5/S1 region of the lumbar spine utilizing our TranS1 approach and associated products. To achieve this goal, we are pursuing the following strategies:
| • | Establish our TranS1 Approach as a Standard of Care for Lower Lumbar Spine Surgery. We believe patients commonly avoid back surgery due to its invasive nature and other drawbacks associated with current surgical treatment options. We expend significant resources promoting our TranS1 approach as the least invasive approach to lower back surgery and we believe the advantages of our TranS1 approach will enable our AxiaLIF products to become a standard of care for the lower lumbar region of the spine. | |
| • | Expand our Sales and Marketing Infrastructure to Drive Surgeon Adoption. We intend to continue expending significant resources targeting spine surgeons through our sales and marketing efforts in the United States and internationally in order to drive the adoption of our TranS1 approach. We believe the ease of use, short procedure times, short hospital stays and reduction in patient pain will be compelling reasons for surgeon adoption of our technologies. | |
| • | Broaden the Applications of the TranS1 Approach. We believe that the applications for our TranS1 approach can be expanded beyond AxiaLIF and AxiaLIF 360° to include multi-level fusion and multi-level motion preservation technologies. We are currently undertaking research and development activities with respect to these applications. | |
| • | Opportunistically Pursue Acquisitions of Complementary Businesses and Technologies. In addition to building our internal product development efforts, we intend to selectively license or acquire complementary products and technologies that we believe will enable us to leverage our growing distribution platform. |
Products
Our products include surgical instruments for creating a safe and reproducible access route to the L4/L5/S1 vertebrae, fusion implants, as well as supplemental stabilization products. We believe our AxiaLIF implants and instruments, combined with facet screws, provide surgeons with the tools necessary to perform a 360° lumbar fusion in what may be the least invasive manner available. We sell these products to our customers in procedure kits that include all the instruments and implants needed to complete a lumbar fusion.
AxiaLIF Lumbar Fusion Implants. Our AxiaLIF implant is a threaded titanium rod, called our 3D Axial Rod, that comes in varying lengths to enable one-level L5/S1 fusions and two-level L4/L5/S1 fusions. As it is implanted, the proprietary thread design on our rod separates the vertebrae, restoring disc height, which relieves pressure on the nerve, and provides immediate rigid fixation. Currently, our single-level AxiaLIF product for L5/S1 fusions is commercially available in the United States and Europe, while our two-level AxiaLIF 2L product for L4/L5/S1 fusions is available in Europe.
53
Table of Contents
AxiaLIF 360° Implants. Our proprietary AxiaLIF 360° implants consist of our 3D Axial Rod plus our titanium facet screws for supplemental posterior fixation. Our AxiaLIF 360° facet screws are implanted using our proprietary delivery system, and require a second 1.5 cm incision in the patient’s back, through which the facet screws are delivered. Currently, our AxiaLIF 360° implants are commercially available in the United States and Europe.
TranS1 Access and Disc Preparation Instruments. Our TranS1 approach requires the use of a sterile set of surgical instruments that are used to create a safe and reproducible working channel and to prepare the disc and vertebrae for our implant. The instrumentation contained in the set includes stainless steel navigation tools and access cannulae to create the working channel, and nitinol cutters and brushes to cut and remove the degenerated disc material and prepare the disc space for our implant. Surgeons must have access to imaging technology to navigate the tools.
Product Pipeline
We are focused on developing novel percutaneous implants that will utilize and leverage our TranS1 approach to the lumbar spine. We currently have two products in development that are intended to be used as a surgical therapy earlier in the progression of degenerative disc disease than fusion, as they are designed to preserve motion in the lumbar spine while providing stabilization. Our motion preservation product development efforts include our PNR and PDR implants. Our modular product designs enable physicians to revise the implant to address disease progression. For example, our PNR implant is designed to be used for patients in the early stage of degenerative disc disease and to be capable of conversion to our PDR implant and ultimately our AxiaLIF fusion products if the patient’s condition warrants. We believe these features may encourage more patients and physicians to elect surgical intervention, as well as encourage them to elect surgical intervention earlier in the progression of their disease.
Percutaneous Nucleus Replacement. Our proprietary PNR implant incorporates a soft polymer that facilitates normal range of motion and restores disc height to simulate the performance of a healthy disc. This design is intended for patients suffering from earlier stage disc degeneration prior to requiring disc replacement or fusion. By utilizing our TranS1 approach, the PNR implant enables surgeons to replace the disc nucleus while preserving other native anatomy that provides stabilization, such as the annulus. We believe all other nucleus replacement technologies currently in development require access to the nucleus through the annulus. Our PNR implant is currently in early-stage human clinical trials outside the United States, and in the United States we anticipate filing an investigational device exemption, or IDE, in 2008 to begin the process of obtaining a PMA.
Partial Disc Replacement. Our proprietary PDR implant is our PNR implant with the addition of a cobalt chrome rod incorporating aball-and-socket design intended to provide additional stability while preserving normal range of motion. This design is intended for patients suffering from mid-stage disc degeneration, or PNR patients whose disease has progressed but does not yet require fusion. The PDR implantation procedure is identical to that of the PNR, with the addition of the insertion of the cobalt chrome rod. If the patient already has a PNR, converting to our PDR only requires the insertion of the cobalt chrome rod through the existing PNR implant. Our PDR implant is currently in late-stage development and we anticipate commencing human clinical trials outside the United States in 2008.
In the future, we believe our product offerings will be expanded to address additional clinical applications in the surgical treatment of conditions affecting the lower lumbar spine, such as neuro stimulation and biologics. Such applications would require FDA 510(k) clearance or PMA approval, most likely supported by safety and efficacy data from clinical trials.
AxiaLIF Clinical Data Summary
To date, we have completed several pre-clinical studies and clinical trials related to our TranS1 approach and AxiaLIF products. The results of these trials were sufficient to allow us to receive 510(k) clearance of our AxiaLIF and AxiaLF 360° products in 2004. Since that time, we are aware of three clinical studies, consisting of two retrospective studies and one company-sponsored prospective study, that are being conducted to further
54
Table of Contents
validate the safety, reproducibility and efficacy of the AxiaLIF procedure. We believe these are the only clinical studies involving our TranS1 approach and AxiaLIF products that include verifiable data and have been submitted for publication or presented at conferences since our initial 510(k) clearances.
Each of these studies defines safety as the ability to access the lumbar spine in a reproducible manner with minimal or no access-related complications. Efficacy is evaluated based on quantifiable pain reduction, fusion rates, blood loss, length of patient stay and length of procedure. In order to determine pain reduction, patients were evaluated before and after surgery utilizing the visual analogue scale, or VAS, and Oswestry disability index, or ODI. VAS compares a patient’s level of pain prior to the procedure to the patient’s level of pain at various intervals following the procedure. This is indicated by the patient on a horizontal line measuring from one, which represents no pain, to 100, which represents the worst pain. ODI measures a patient’s disability before and at various intervals after the procedure. The index is calculated based on the patient’s response to a questionnaire and is expressed as a percentage of disability, with 100% suggesting complete disability. Fusion rates are determined by the percentage of patients that experience less than 3 mm of movement in the space between the fused vertebrae while bending forward and backward or by bone growth across the disc space.
One of these studies was a retrospective, multicenter study conducted by lead investigator, Christopher Ames, M.D. This study enrolled 35 patients, consisting of 15 men and 20 women. Averagefollow-up was 14.5 months. Participating patients had back pain and degenerative disc disease, degenerative lumbar scoliosis or lytic spondylolisthesis. Twenty-one patients underwent the AxiaLIF procedure followed by pedicle screw fixation. Two patients underwent AxiaLIF followed by XLIF of the L4/L5 verterbrae. Ten patients received only the AxiaLIF procedure. Two patients received the AxiaLIF procedure as part of a larger procedure which included open fusion procedures elsewhere in the spine.
For patients in this study, the average blood loss during the procedure was 30 cc and the average length of the procedure was 42 minutes, in each case as measured only during the AxiaLIF portion of the procedure. Average length of hospital stay varied depending on the type of procedure and ranged from 21 hours for a standalone AxiaLIF procedure, to 2.2 days for an AxiaLIF procedure followed by pedicle screw fixation, to 3.5 days for AxiaLIF followed by an XLIF. One patient in the study suffered local infection at the site of the incision that was treated with oral antibiotics and resolved. The following table summarizes the12-month results from the registry.
| VAS | ODI | |||||||||||||
| Improvement | Improvement | |||||||||||||
| 12-Months | 12-Months | |||||||||||||
| Complication | Before | Post- | Point | Before | Post- | Point | ||||||||
Fusion Rate | Rate | Procedure | Procedure | Change | Procedure | Procedure | Change | |||||||
91% | 3% | 75 | 31 | 44 | 42% | 22% | 20 | |||||||
There were three patients that did not show evidence of fusion, two were among the standalone group and one received AxiaLIF and XLIF. Of these patients, one requested to have the AxiaLIF removed, which was done and replaced with a bone graft. The patient showed evidence of fusion at six months following that procedure. Unfavorable anatomy precluded access to the L5/S1 disc space during open lumbar interbody fusion in 2 patients who subsequently underwent AxiaLIF at this level as part of a large construct. These two patients experienced a mean hospital stay of eight days.
The combined study data is pending publication with the Journal of Minimally Invasive Neurosurgery. After the surgeries for this study were completed, we entered into a consulting agreement with the lead investigator of this study for training other surgeons and providing clinical feedback to us. In connection with the consulting agreement, we granted the lead investigator an option to purchase shares of our common stock.
Another study was a retrospective, single-center study conducted by lead investigator, W. Daniel Bradley, M.D. This study involved the first 20 patients, consisting of 13 women and 7 men, who underwent an AxiaLIF procedure at the study center. All patients were treated for symptomatic disc degeneration at the L5/S1 level that was unresponsive to conservative therapies. Fifteen patients underwent the AxiaLIF procedure followed by pedicle screw fixation, and five patients received only the AxiaLIF procedure.
55
Table of Contents
For patients in this study, the mean blood loss was 38.8 cc. The average length of the procedure was 80 minutes for a standalone AxiaLIF procedure and 152 minutes for an AxiaLIF procedure together with pedicle screw fixation. The average length of hospital stay, including both types of procedure, was 1.2 days. No device or procedure related complications were observed among the patients. Given that none of the patients in this study have reached a12-monthfollow-up, fusion rates for the this study have not yet been reported. However, we are aware of one patient that experienced an unrelated traumatic event three months after the procedure that is expected to prevent fusion. The following table summarizes the average VAS and ODI scores for all 20 patients measured as of their most recent follow-up visit, which could be either three weeks, three months or six months:
| VAS | ODI | |||||||||||
| Improvement | Improvement | |||||||||||
| Complication | Before | Post- | Point | Before | Post- | Point | ||||||
| Rate | Procedure | Procedure | Change | Procedure | Procedure | Change | ||||||
0% | 73 | 29 | 44 | 43% | 26% | 17 | ||||||
This study data was presented at the International Meeting of Advanced Spinal Techniques in July 2007 by its authors. After the surgeries for this study were completed, we entered into a consulting agreement with the lead investigator of this study for training other surgeons and providing clinical feedback to us. In connection with the consulting agreement, we granted the lead investigator an option to purchase shares of our common stock.
We are conducting a single-center, non-randomized, 24-month study of 26 patients, consisting of 15 men and 11 women, which we refer to as our AxiaLIF Data Registry. Study enrollment began in July 2005 and clinical information has been collected from the medical records of enrolled patients who will be tracked at three weeks, and 3-, 6-, 12-,24-month intervals following surgery by their surgeon investigators. All subjects have completed their12-monthfollow-up and we expect to complete the registry by April 2008.
For patients enrolled in the registry the average blood loss during the procedure was 88.5 cc, the average length of stay in the hospital was 2.6 days and the average length of the procedure was 214 minutes. For purposes of the registry, patients also received posterior pedicle screws in conjunction with the AxiaLIF to further augment the stability of the spine. The reported blood loss, length of hospital stay and length of procedure all include the effects of the additional procedure to implant pedicle screws. There were six reports of serious adverse events in three patients none of which were considered to be related to the AxiaLIF procedure. The following table summarizes the12-month results from the registry.
| VAS | ODI | |||||||||||||||||||||||||||||
| Improvement | Improvement | |||||||||||||||||||||||||||||
| 12-Months | 12-Months | |||||||||||||||||||||||||||||
| Complication | Before | Post- | Point | Before | Post- | Point | ||||||||||||||||||||||||
Fusion Rate | Rate | Procedure | Procedure | Change | Procedure | Procedure | Change | |||||||||||||||||||||||
| 88% | 0% | 67 | 41 | 26 | 49% | 33% | 16 | |||||||||||||||||||||||
This study data was presented at the Annual Meeting of the American Association of Neurological Surgeons in April 2007. After the surgeries for this study were completed, we entered into a consulting agreement with the lead investigator of this study for training other surgeons and providing clinical feedback to us. In connection with the consulting agreement, we granted the lead investigator an option to purchase shares of our common stock.
Sales and Marketing
Our sales and marketing effort primarily targets industry leaders and high volume spine surgeons. We also market our products at various industry conferences and through industry organized surgical training courses. In addition, we intend to develop and implement marketing programs targeted at potential patients, which we believe will accelerate the demand for our products.
In 2005, two customers, Christ Hospital in Cincinnati, Ohio, and Faith Regional Health Services in Norfolk, Nebraska, accounted for 16% and 11%, respectively, of our revenues. In 2006, one customer, Christ Hospital, accounted for 11% of revenues.
56
Table of Contents
In the United States, we market and sell our products through a combination of direct sales representatives and independent sales agencies that have allocated representatives to solicit our products. Our U.S. sales and marketing team is currently comprised of our vice president of sales and marketing, 5 regional sales managers, 1 director of training and professional development, 23 direct sales representatives and 21 independent sales agents covering specific geographic regions. Bymid-2008, we expect to have increased the number of our direct sales representatives to 50. We select our sales representatives and independent sales agents based on their expertise in spine surgery medical device sales, reputation within the surgeon community and sales coverage. We compensate our sales representatives and independent sales agents based on a percentage of the net sales that they generate. We have agreements with our independent sales agents that provide them with an exclusive right to sell our products in their territories, which are generally terminable upon 90 days’ written notice.
Outside of the United States, we utilize third-party distributors, with support from our U.S. sales and marketing staff, to support the commercialization of our products. To date, the majority of our international sales have been in Europe. We intend to continue to hire sales and marketing personnel as appropriate to enable us to support the commercialization of our products.
Surgeon Training
We devote significant resources to training and educating surgeons on the specialized skills involved in the proper use of our instruments and implants. We believe that the most effective way to introduce and build market demand for our products is by training spine surgeons in the use of our products. We accomplish our training objectives primarily through cadaver and surrogate models and live case observations with surgeons experienced in our TranS1 approach. After this training, surgeons are generally able to perform unsupervised surgeries using our TranS1 approach. We supplement our training with online didactic tutorials. As of June 30, 2007, we had trained approximately 500 U.S. spine surgeons and 54 surgeons outside of the U.S. in the use of our products. Of the surgeons trained on our TranS1 approach, approximately 190 have performed a procedure in the 12 months ended June 30, 2007 using our TranS1 approach. We believe we have the necessary capacity to train a sufficient number of surgeons to meet our current goals.
Third-Party Reimbursement
In the United States, healthcare providers generally rely on third-party payors, principally private insurers and governmental payors such as Medicare and Medicaid, to cover and reimburse all or part of the cost of a spine fusion surgery in which our medical device is used. The cost of a spine fusion surgery in which our medical device is used is currently covered and reimbursed by such third-party payors under Diagnosis-Related Groups 496 (“Combined Anterior/Posterior Spinal Fusion”), 497 (“Spinal Fusion Except Cervical with CC”) or 498 (“Spinal Fusion Except Cervical without CC”). We expect that sales volumes and prices of our products will depend in large part on the continued availability of reimbursement from such third-party payors. These third-party payors may deny reimbursement if they determine that a device used in a procedure was not used in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication. Particularly in the United States, third-party payors continue to carefully review, and increasingly challenge, the prices charged for procedures and medical products.
Medicare coverage and reimbursement policies are developed by the Centers for Medicare and Medicaid Services, or CMS, the federal agency responsible for administering the Medicare program, and its contractors. CMS establishes Medicare coverage and reimbursement policies for medical products and procedures and such policies are periodically reviewed and updated. While private payors vary in their coverage and payment policies, the Medicare program is viewed as a benchmark. Medicare reimbursement rates for the same or similar procedures vary due to geographic location, nature of the facility in which the procedure is performed (i.e., teaching or community hospital) and other factors. We cannot assure you that government or private third-party payors will cover and reimburse the procedures using our products in whole or in part in the future or that payment rates will be adequate.
In addition, a large percentage of insured individuals receive their medical care through managed care programs, which monitor and often require pre-approval of the services that a member will receive. Many
57
Table of Contents
managed care programs are paying their providers on a capitated basis, which puts the providers at financial risk for the services provided to their patients by paying them a predetermined payment per member per month. The percentage of individuals covered by managed care programs continues to grow in the United States.
Internationally, reimbursement and healthcare payment systems vary substantially from country to country and include single-payor, government-managed systems as well as systems in which private payors and government-managed systems existside-by-side. Our ability to achieve market acceptance or significant sales volume in international markets we enter will be dependent in large part on the availability of reimbursement for procedures performed using our products under the healthcare payment systems in such markets. A small number of countries may require us to gather additional clinical data before recognizing coverage and reimbursement for our products. It is our intent to complete the requisite clinical studies and obtain coverage and reimbursement approval in countries where it makes economic sense to do so.
We believe that the overall escalating cost of medical products and services has led to, and will continue to lead to, increased pressures on the healthcare industry to reduce the costs of products and services. We cannot assure you that government or private third-party payors will cover and reimburse the procedures using our products in whole or in part in the future or that payment rates will be adequate. In addition, it is possible that future legislation, regulation, or reimbursement policies of third-party payors will adversely affect the demand for our procedures and products or our ability to sell them on a profitable basis. The unavailability or inadequacy of third-party payor coverage or reimbursement could have a material adverse effect on our business, operating results and financial condition.
Competition
The medical device industry is highly competitive, subject to rapid technological change and significantly affected by new product introductions and market activities of other participants. Our currently marketed products are, and any future products we commercialize will be, subject to intense competition. Our competitors include providers of conservative, non-operative therapies for lower lumbar spine conditions, as well as a number of major medical device companies that have developed or plan to develop products for minimally invasive spine surgery in each of our current and future product categories. We believe that the principal competitive factors in our markets include:
| • | improved outcomes for medical conditions affecting the lower lumbar spine; | |
| • | acceptance by spine surgeons; | |
| • | ease of use and reliability; | |
| • | product price and qualification for reimbursement; | |
| • | technical leadership and superiority; | |
| • | effective marketing and distribution; and | |
| • | speed to market. |
We are aware of several companies that compete or are developing technologies in our current and future product areas. As a result, we expect competition to remain intense. We believe that our most significant competitors are Medtronic Sofamor Danek, Johnson & Johnson DePuy Spine, Stryker, NuVasive, Kyphon, Zimmer, Synthes, Abbott, Orthofix, Globus, Alphatec and others, many of which have substantially greater sales and financial resources than we do. In addition, these companies may have more established distribution networks, entrenched relationships with physicians, and greater experience in launching, marketing, distributing and selling products.
Our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner, receive adequate reimbursement and are safer, less invasive and less expensive than alternatives available for the same purpose. Because of the size of the potential market, we anticipate that companies will dedicate significant resources to developing competing products.
58
Table of Contents
Research and Development
As of June 30, 2007, our research and development team was comprised of nine employees and consultants who have extensive experience in developing products to treat medical conditions affecting the lower lumbar spine. These employees and consultants work closely with our clinical advisors and spine surgeon customers to design and enhance our products and approach.
Since inception, we have devoted significant resources to develop and enhance our AxiaLIF product kits utilizing the TranS1 approach. We expect our research and development expenditures to increase as we continue to devote significant resources to developing our PNR and PDR products and completing the clinical trials necessary to support submissions to gain regulatory approvals.
Manufacturing and Supply
We rely on third parties to manufacture all of our products and their components, except for our nitinol nucleus cutter blades and nucleus cutter sheaths, which are manufactured by us at our facilities in Wilmington, North Carolina. Our outsourcing partners are manufacturers that meet FDA, International Organization for Standardization, or ISO, and other quality standards. We believe these manufacturing relationships allow us to work with suppliers who have the best specific competencies while we minimize our capital investment, control costs and shorten cycle times, all of which we believe allows us to compete with larger-volume manufacturers of spine surgery products.
All of our products and components are assembled, packaged, labeled and sterilized at third-party facilities in the United States under our existing contracts requiring compliance with Good Manufacturing Processes, or GMPs. Following receipt of products or components from our third-party manufacturers, we inspect, warehouse and ship the products and components at our facilities in Wilmington. We reserve the exclusive right to inspect and assure conformance of each product and component to our specifications. In addition, FDA or other regulatory authorities may inspect our facilities and those of our suppliers to ensure compliance with quality system regulations.
The majority of our instruments and implants are produced by third-party manufacturers on precision, high-speed machine shop equipment. However, certain of our products, components, materials used to manufacture such products and components, and manufacturing operations are produced, performed or supplied by third-party specialty vendors due to their proprietary or non-conventional nature. For example, the blades for our nucleus cutter are made from a metal called nitinol, which is converted into strip form by three manufacturers in the United States known to us. We have sourced nitinol strip from two of these vendors. The nitinol strip is then further converted for us into cutter blanks by a scalpel blade specialty vendor. Other vendors are available to manufacture the cutter blanks, as we may deem desirable or necessary. We convert the cutter blanks into cutter blades at our facilities. Our tissue extractor product is produced for us by a supplier that specializes in wire forming and coiling specifically for the medical device industry. A limited number of similar vendors exist that could be used to produce the tissue extractor product, and we believe we could replace this supplier on reasonable terms without substantial delay, if necessary.
We are currently working with our third-party manufacturers to plan for our manufacturing requirements as we increase our commercialization efforts. In most cases, we have redundant manufacturing capability with multiple vendors and enjoy the significant capacity this arrangement provides to us. We may consider manufacturing certain products or product components internally, if and when demand or quality requirements make it appropriate to do so. We believe the manufacturing capacity available to us is sufficient to meet our demands into the foreseeable future.
Patents and Proprietary Technology
We rely on a combination of patent, trademark, copyright, trade secret and other intellectual property laws, nondisclosure agreements and other measures to protect our intellectual property rights. We believe that in order to have a competitive advantage, we must develop and maintain the proprietary aspects of our technologies. We require our employees, consultants and advisors to execute confidentiality agreements in
59
Table of Contents
connection with their employment, consulting or advisory relationships with us. We also require our employees, consultants and advisors who we expect to work on our products to agree to disclose and assign to us all inventions conceived during the work day, using our property or which relate to our business. We cannot provide any assurance that employees and consultants will abide by the confidentiality or assignment terms of these agreements. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary.
Patents
As of June 30, 2007, we had 9 issued United States patents, 33 pending patent applications in the United States, and 48 foreign patent applications as counterparts of U.S. cases. The issued and pending patents cover, among other things:
| • | our method for performing trans-sacral procedures in the spine, including diagnostic or therapeutic procedures, and trans-sacral introduction of instrumentation or implants; | |
| • | apparatus for conducting these procedures including access, disc preparation and implantation including the current TranS1 instruments individually and in kit form; and | |
| • | implants for fusion and motion preservation in the spine. |
Our issued patents begin to expire in 2021 assuming timely payment of all maintenance fees. We have multiple patents covering unique aspects and improvements for many of our methods and products. We do not believe that the expiration of any single patent is likely to significantly affect our business, operating results or prospects.
Our pending patent applications may not result in issued patents, and we cannot assure you that any patents that have issued or might issue will protect our intellectual property rights. The medical device industry is characterized by the existence of a large number of patents and frequent litigation based on allegations of patent infringement. Any patents issued to us may be challenged by third parties as being invalid. Any claim relating to infringement of patents that is successfully asserted against us may require us to pay substantial damages. Even if we were to prevail, any litigation could be costly and time-consuming and would divert the attention of our management and key personnel from our business operations. Our success will also depend in part on our ability to not infringe patents issued to others, including our competitors and potential competitors. If our products are found to infringe the patents of others, our development, manufacture and sale of such potential products could be severely restricted or prohibited. In addition, our competitors may independently develop similar technologies that avoid our patents. In addition, we cannot be certain that the steps we have taken will prevent the misappropriation of our intellectual property. Because of the importance of our patent portfolio to our business, we may lose market share to our competitors if we fail to protect our intellectual property rights.
Trademarks
We own trademark registrations for the marks TranS1 and AxiaLIF in the United States and the European Union. We own six trademark applications pending in the United States for the following marks: 3D Axial Rod, TranS1 Structsure, AxiaLIF 2L, AxiaLIF 360°, PNR and TranS1 PDR. We also own pending trademark applications for the marks AxiaLIF 2L and AxiaLIF 360° in the European Union.
Government Regulation
Our products are medical devices subject to extensive regulation by the FDA and other U.S. federal and state regulatory bodies and comparable authorities in other countries. To ensure that medical products distributed domestically and internationally are safe and effective for their intended use, FDA and comparable authorities in other countries have imposed regulations that govern, among other things, the following activities that we or our partners perform and will continue to perform:
| • | product design and development; |
60
Table of Contents
| • | product testing; | |
| • | product manufacturing; | |
| • | product labeling; | |
| • | product storage; | |
| • | premarket clearance or approval; | |
| • | advertising and promotion; | |
| • | product marketing, sales and distribution; and | |
| • | post-market surveillance, including reporting deaths or serious injuries related to products and certain product malfunctions. |
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require either prior 510(k) clearance or prior premarket approval from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risk are placed in either class I or II, which in many cases requires the manufacturer to submit to the FDA a premarket notification or 510(k) submission requesting permission for commercial distribution. This process is known as requesting 510(k) clearance. Some low risk devices are exempt from this requirement. Devices deemed by the FDA to pose the greatest risk, such as many life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a legally marketable device, are placed in class III, requiring premarket approval. Our current commercial products are class II devices marketed under FDA 510(k) premarket clearance. Both premarket clearance and premarket approval applications are subject to the payment of user fees, paid at the time of submission for FDA review.
510(k) Clearance Pathway
To obtain 510(k) clearance, we must submit a premarket notification demonstrating that the proposed device is substantially equivalent to a legally marketable device not requiring PMA approval. Although statutorily mandated to clear or deny a 510(k) premarket notification within 90 days of submission of the application, FDA’s 510(k) clearance pathway usually takes from three to twelve months, based on requests for additional information by FDA, but it can take significantly longer. Additional information can include clinical data to make a determination regarding substantial equivalence.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, require premarket approval. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), or a premarket approval, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketingand/or recall the modified device until 510(k) clearance or premarket approval is obtained. If the FDA requires us to seek 510(k) clearance or premarket approval for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties. We have made and plan to continue to make additional product enhancements to our AxiaLIF and AxiaLIF 360° products that we believe do not require new 510(k) clearances.
Premarket Approval Pathway
A premarket approval application must be submitted if the device cannot be cleared through the 510(k) process. The premarket approval application process is generally more costly and time consuming than the 510(k) process. A premarket approval application must be supported by extensive data including, but not
61
Table of Contents
limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use.
After a premarket approval application is sufficiently complete, the FDA will accept the application and begin an in-depth review of the submitted information. By statute, the FDA has 180 days to review the “accepted application”, although, generally, review of the application can take between one and three years, but it may take significantly longer. During this review period, the FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulations. New premarket approval applications or premarket approval application supplements are required prior to marketing for product modifications that affect the safety and efficacy of the device. Premarket approval supplements often require submission of the same type of information as a premarket approval application, except that the supplement is limited to information needed to support any changes from the device covered by the original premarket approval application, and may not require as extensive clinical data or the convening of an advisory panel. None of our products are currently approved under a premarket approval but devices in development may require it.
Clinical Trials
Clinical trials are almost always required to support a premarket approval application and are sometimes required for a 510(k) premarket notification. In the U.S., these trials often require submission of an application for an investigational device exemption, or IDE. The investigational device exemption application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The investigational device exemption application must be approved in advance by the FDA for a specified number of patients, unless the product is deemed a non-significant risk device and eligible for more abbreviated investigational device exemption requirements. Clinical trials for a significant risk device may begin once the investigational device exemption application is approved by the FDA and the appropriate institutional review boards at the clinical trial sites. Future clinical trials of our motion preservation designs will require that we obtain an investigational device exemption from the FDA prior to commencing clinical trials and that the trial be conducted under the oversight of an institutional review board at the clinical trial site. Our clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy and financial disclosure by clinical investigators. A clinical trial may be suspended by FDA or the investigational review board at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the study. Even if a study is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device, or may be equivocal or otherwise not be sufficient to obtain clearance or approval of one of our products. Similarly, in Europe the clinical study must be approved by the local ethics committee and in some cases, including studies of high-risk devices, by the Ministry of Health in the applicable country.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, numerous FDA and other regulatory requirements continue to apply. These include:
| • | quality system regulation, which requires manufacturers, including third-party contract manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance controls, during all aspects of the manufacturing process; | |
| • | labeling regulations, and FDA prohibitions against the promotion of products for uncleared or unapproved “off-label” uses; | |
| • | medical device reporting obligations, which require that manufacturers submit reports to the FDA if information reasonably suggests their device (i) may have caused or contributed to a death or serious injury, or (ii) malfunctioned and the device or a similar company device would likely cause or contribute to a death or serious injury if the malfunction were to recur; and |
62
Table of Contents
| • | other post-market surveillance requirements, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
We and our third-party manufacturers must register with FDA as medical device manufacturers and must obtain all necessary state permits or licenses to operate our business. As manufacturers, we and our third-party manufacturers are subject to announced and unannounced inspections by FDA to determine our compliance with quality system regulation and other regulations. We have not yet been inspected by the FDA. We believe that we are in substantial compliance with quality system regulation and other regulations.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include, among other things, any of the following sanctions:
| • | warning letters, fines, injunctions, consent decrees and civil penalties; | |
| • | repair, replacement, refunds, recall or seizure of our products; | |
| • | operating restrictions, partial suspension or total shutdown of production; | |
| • | refusing our request for 510(k) clearance or premarket approval of new products, new intended uses or other modifications to existing products; | |
| • | withdrawing or suspending premarket approvals that are already granted; and | |
| • | criminal prosecution. |
We are subject to announced and unannounced inspections by the FDA and these inspections may include the manufacturing facilities of our subcontractors.
Fraud and Abuse
We may directly or indirectly be subject to various federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws. In particular, the federal healthcare program anti-kickback statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service, for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. The anti-kickback statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. In implementing the statute, the Office of Inspector General, or OIG, has issued a series of regulations, known as the “safe harbors,” which began in July 1991. These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the anti-kickback statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy all requirements of an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. Penalties for violations of the federal anti-kickback statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government. These individuals, sometimes known as “relators” or, more commonly, as “whistleblowers”, may share in any amounts paid by the entity to the government in fines or settlement. The number of filings of qui tam actions has increased significantly in recent years, causing more healthcare companies to have to defend a False Claim action. If an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 to $11,000 for each separate false claim. Various states have also enacted similar laws modeled after the federal False Claims Act which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
HIPAA created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in
63
Table of Contents
fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. A violation of this statute is a felony and may result in fines or imprisonment.
If any of our operations are found to have violated or be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, among them being civil and criminal penalties, damages, fines, exclusion from government healthcare programs, and the curtailment or restructuring of our operations.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ.
The European Union, which consists of 25 of the major countries in Europe, has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking and, accordingly, can be commercially distributed throughout the member states of the European Union, and other countries that comply with or mirror these directives. The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body,” an independent and neutral institution appointed to conduct conformity assessment. This third-party assessment consists of an audit of the manufacturer’s quality system and technical review and testing of the manufacturer’s product. An assessment by a Notified Body in one country within the European Union is required in order for a manufacturer to commercially distribute the product throughout the European Union. In addition, compliance with voluntary harmonizing standards ISO 9001 and ISO 13845 issued by the International Organization for Standards establishes the presumption of conformity with the essential requirements for a CE mark. In August 2004, we were certified by Semko, a Notified Body, under the European Union Medical Device Directive allowing the CE conformity marking to be applied.
Employees
As of June 30, 2007, we had 63 employees, 61 of whom were full-time employees, with 34 employees in sales and marketing, 9 employees in manufacturing, 8 employees in research and development, including 1 part-time employee, 7 employees in general and administrative, including 1 part-time employee, and 5 employees in clinical, regulatory and quality assurance. We believe that our future success will depend in part on our continued ability to attract, hire and retain qualified personnel. None of our employees are represented by a labor union, and we believe our employee relations are good.
Facilities
We lease approximately 30,000 square feet of space in a single-user building located in an industrial park in Wilmington, North Carolina. Of that amount, approximately 12,200 square feet are used for manufacturing and warehousing, approximately 5,700 square feet are used for office space, approximately 500 square feet are used for research and development activities, and approximately 11,600 square feet are available as expansion space. This lease expires in June 2010. We believe our current facilities will be sufficient to meet our needs through at least that time.
Litigation
We are not currently party to any material legal proceedings. We may be subject to various claims and legal actions arising in the ordinary course of business from time to time.
64
Table of Contents
Executive Officers and Directors
The following table sets forth certain information with respect to our executive officers and directors as of June 30, 2007:
Name | Age | Position | ||||
| Richard Randall | 55 | President, Chief Executive Officer and Director | ||||
| Michael Luetkemeyer | 57 | Chief Financial Officer | ||||
| Rick Simmons | 45 | Vice President, Marketing and Sales | ||||
| Robert Assell | 54 | Vice President, Product Development | ||||
| William Jackson | 56 | Vice President, Regulatory, Clinical and Quality | ||||
| Robert Martin | 42 | Vice President, International Sales | ||||
| Michael Carusi | 42 | Director | ||||
| Mitchell Dann | 47 | Director | ||||
| Jonathan Osgood | 58 | Director | ||||
| James Shapiro | 49 | Director | ||||
Richard Randallhas been our President, Chief Executive Officer and a member of our board of directors since June 2002. From June 2000 to June 2002, Mr. Randall served as President and Chief Executive Officer of Incumed, Inc., a privately-held medical device incubator company. He was President, Chief Executive Officer and a director of Innovasive Devices, Inc., a developer, manufacturer and marketer of arthroscopic surgical products which was acquired by the Ethicon Division of Johnson & Johnson in 2000, from January 1994 to February 2000. Mr. Randall served as President and Chief Executive Officer of Conceptus, Inc. from December 1992 to July 1993 and Chief Financial Officer from December 1992 to January 1995. He served as President and Chief Executive Officer of Target Therapeutics, Inc., an interventional neurovascular medical device company which was acquired by Boston Scientific Corporation in 1997, from June 1989 to May 1993 and was a director of Target Therapeutics from June 1989 to April 1997. Mr. Randall received a B.S. degree in Biology and Science Education from State University College of New York at Buffalo.
Michael Luetkemeyerhas been our Chief Financial Officer since April 2007. Prior to that, Mr. Luetkemeyer held various positions with Micromuse, Inc., a network management software provider that was acquired by IBM in 2006, including Chief Financial Officer from October 2001 to January 2005, interim Chief Executive Officer from January 2003 to August 2003, and Senior Vice President from February 2005 to March 2006. Mr. Luetkemeyer also served as a member of the board of directors of Micromuse from January 2003 to February 2005. Prior to joining Micromuse, he served as Chief Financial Officer of Aprisma Management Technologies, a network management software provider, from 2000 until October 2001, and held a variety of senior finance positions at GE Aerospace, GE Semiconductor and GE Plastics for more than ten years. Mr. Luetkemeyer received a B.S. degree in Finance from Southwest Missouri State, an M.A. degree in Economics from the University of Missouri and a B.S. degree in Accounting from Rollins College.
Rick Simmonshas been our Vice President, Marketing and Sales since November 2003. From 2000 to 2003 Mr. Simmons served as Vice President of Sales and Marketing at Nuvasive, Inc., a publicly traded minimally invasive spinal platform technology and implant company. From 1997 to 2000, he served as Vice President, Global Marketing and Sales of Innovasive Devices, Inc., a developer, manufacturer and marketer of arthroscopic surgical products which was acquired by the Ethicon Division of Johnson & Johnson in 2000. From 1995 to 1997, Mr. Simmons served as Director of Marketing, Managed Care and Business Development of Genzyme Tissue Repair, Inc., a human tissue engineering technology company serving the orthopaedic surgical sports medicine market and publicly traded division within Genzyme Corporation. Mr. Simmons received a B.A. degree in Exercise Physiology from the California State University, Northridge.
Robert Assellhas been our Vice President, Product Development since February 2003. From February 2002 to February 2003, Mr. Assell served as our consultant with respect to research and development activities. From January 1997 to February 2002, Mr. Assell served as Vice President, Operations for Bioamide, Inc., an early stage technology company focusing on tissue engineered hair replacement, which is now Aderans Research. During the
65
Table of Contents
period from November 1999 to February 2002, he also served as the interim President of Closys, Inc., a development stage medical device company focusing on development of a vascular access closure device. From October 1998 to September 1999, Mr. Assell served as the Vice President, Research and Development for Vascular Solutions, Inc., a publicly traded company concentrating on the vascular sealing segment of the interventional cardiology market. Prior to that, he served as the Vice President of Research and Development and Operations for RayMedica, Inc., a development stage medical device company focusing on developing spinal implants and treating degenerative disc disease. Mr. Assell received a B.S. degree in Mechanical Engineering from the University of Minnesota and an M.B.A. degree from the University of St. Thomas.
William Jacksonhas been our Vice President, Regulatory, Clinical and Quality since May 2007. Mr. Jackson has over 29 years of regulatory experience, including responsibility for regulatory and clinical affairs at St. Jude Medical, Inc., and holding senior positions in regulatory and clinical affairs at Genetic Laboratories, Neuromed and Intermedics, Inc. Mr. Jackson also founded W.F. Jackson Associates, Ltd., a regulatory affairs consulting business for medical device companies, in 1991 and has served as its President since that time. Mr. Jackson received a B.A. degree in Zoology and Chemistry from Concordia College and an M.B.A. degree from the University of Minnesota (Moorhead).
Robert Martinhas been our Vice President of International Sales since July 2007. From 2000 to June 2007, Mr. Martin served as Vice President of Global Sales and Marketing and, most recently, as President and CEO at Ascension Orthopedics Inc., a privately held extremity orthopedics company. From 1999 to 2000, he served as European Sales Manager for Innovasive Devices, Inc. a developer, manufacturer, and marketer of arthroscopic surgical products which was acquired by the Ethicon Division of Johnson & Johnson in 2000. From 1996 to 1999, Mr. Martin served as Sales and Marketing Manager for 3 divisions of Stryker Inc. (Canada), a publicly traded global orthopedic company. Mr. Martin received a B.A. degree in Physical Education from the University of Western Ontario.
Michael Carusihas served as a member of our board of directors since April 2003. He has served as a General Partner at Advanced Technology Ventures, a venture capital firm, since October 1998. Advanced Technology Ventures beneficially owns approximately 19.5% of the shares of our common stock prior to the completion of this offering, and will beneficially own approximately 13.8% after the completion of this offering. Mr. Carusi serves on the board of directors of XTENT, Inc., a publicly traded medical device company, as well as the boards of several privately-held life sciences and medical device companies. Mr. Carusi received a B.S. degree in Mechanical Engineering from Lehigh University and an M.B.A. degree from the Amos Tuck School of Business at Dartmouth College.
Mitchell Dannhas served as a member of our board of directors since September 2000. Mr. Dann is the founder and managing member of Sapient Capital Management, LLC, the general partner of the general partner of Sapient Capital, L.P., a venture capital firm specializing in the medical device industry. Sapient Capital beneficially owns approximately 12.0% of the shares of our common stock prior to the completion of this offering, and will beneficially own approximately 8.5% after the completion of this offering. Previously, Mr. Dann was President of M. Dann & Co., Inc., a venture capital advisory firm, and was a co-founder of Urologix, Inc., a publicly traded medical device company, where he served as a director from 1991 until 2005, including as Chairman of the Board from 1993 until 2003. Mr. Dann also serves as a member of the board of directors of several privately-held medical device companies. Mr. Dann received a B.S. degree in Engineering; Management from the University of Vermont.
Jonathan Osgoodhas served as a member of our board of directors since March 2002. In 2001, Mr. Osgood co-founded, and is the managing member of, Cutlass Capital, L.L.C., a venture capital firm that invests exclusively in the healthcare industry. Cutlass Capital beneficially owns approximately 15.0% of the shares of our common stock prior to the completion of this offering, and will beneficially own approximately 10.6% after the completion of this offering. Mr. Osgood also serves as a member of the board of directors of several privately-held medical device companies. Mr. Osgood is a Certified Financial Analyst and received a B.A. degree in Economics from Dartmouth College and an M.B.A. degree from the Amos Tuck School of Business at Dartmouth College.
James Shapirohas served as a member of our board of directors since September 2005. Mr. Shapiro has served as a General Partner of Kearny Venture Partners, a venture capital firm, and its predecessor, Thomas
66
Table of Contents
Weisel Healthcare Venture Partners, since March 2003. Since January 2000, Mr. Shapiro has also been a General Partner of ABS Healthcare Ventures. Mr. Shapiro serves on the board of directors of Hansen Medical, Inc., a publicly traded medical device company, as well as on the boards of several privately-held medical device companies. Mr. Shapiro received a B.A. degree from Princeton University and an M.B.A degree from the Stanford University Graduate School of Business.
Board Composition
Our board of directors currently consists of five members and two vacancies. Our amended and restated investors’ rights agreement confers upon certain classes of our stockholders the right to elect a specified number of directors to our board of directors, with such directors being designated by certain of our investors pursuant to their contractual right. These rights terminate upon the closing of this offering. Our current directors were designated and elected as follows:
| • | Mr. Mitchell Dann was elected by the holders of our series A preferred stock, voting together as a single class, and was designated by Sapient Capital, L.P. pursuant to its contractual right. | |
| • | Mr. Jonathan Osgood was elected by the holders of our series AA preferred stock, voting together as a single class, and was designated by Cutlass Capital, L.P. pursuant to its contractual right. | |
| • | Mr. Michael Carusi was elected by the holders of our series B preferred stock, voting together as a single class, and was designated by Advanced Technology Ventures pursuant to its contractual right. | |
| • | Mr. James Shapiro was elected by the holders of our series C preferred stock, voting together as a single class, and was designated by Thomas Weisel Healthcare Venture Partners pursuant to its contractual right. | |
| • | Mr. Richard Randall was elected by the holders of our common stock and preferred stock, voting together as a combined class, and was designated by such holders in his capacity as our chief executive officer. |
Our board of directors has determined that four of our five directors are independent directors, as defined by Rule 4200(a)(15) of the Nasdaq Marketplace Rules. The four independent directors are Messrs. Dann, Osgood, Carusi and Shapiro.
Upon completion of this offering, our amended and restated certificate of incorporation will provide that our board of directors will be divided into three classes, each with staggered three-year terms. As a result, only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Mr. Shapiro has been designated as a Class I director, whose term will expire at the 2008 annual meeting of stockholders. Messrs. Carusi and Osgood have been designated as Class II directors, whose terms will expire at the 2009 annual meeting of stockholders. Messrs. Randall and Dann have been designated as Class III directors, whose terms will expire at the 2010 annual meeting of stockholders. This classification of the board of directors may delay or prevent a change in control of our company or our management. See “Description of Capital Stock — Anti-Takeover Effects of Provisions of the Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws.”
Committees of the Board of Directors
Upon completion of this offering, our board of directors will have three standing committees: an audit committee, a compensation committee and a nominating and corporate governance committee. At that time, each of those committees will have the composition and responsibilities set forth below.
Audit Committee
Our audit committee is a standing committee of, and operates under a written charter adopted by, our board of directors. The functions of our audit committee include appointing and determining the compensation for our independent auditors, establishing procedures for the receipt, retention and treatment of complaints regarding internal accounting controls and reviewing and overseeing our independent registered public accounting firm. The chairman of the audit committee is Mr. Osgood and the other current member is Mr. Carusi. All members of the audit committee are non-employee directors and satisfy the current standards with respect to independence, financial expertise and experience established by Nasdaq and SEC rules. Our board of directors has determined
67
Table of Contents
that Mr. Osgood meets the SEC’s current definition of “audit committee financial expert.” Prior to the completion of this offering, we expect to appoint another member to the audit committee to satisfy the listing standards established by Nasdaq, and we expect that such new member will also satisfy the current standards with respect to independence, financial expertise and experience established by Nasdaq and SEC rules.
Compensation Committee
Our compensation committee is a standing committee of, and operates under a written charter adopted by, our board of directors. The compensation committee reviews and recommends to our board of directors the salaries and benefits for our executive officers and recommends overall employee compensation policies. The compensation committee also administers our equity compensation plans. The chairman of the compensation committee is Mr. Dann and the other current member is Mr. Shapiro. All members of the compensation committee are non-employee directors and satisfy the current independence standards established by Nasdaq and SEC rules.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee is a standing committee of, and operates under a written charter adopted by, our board of directors. The nominating and corporate governance committee identifies individuals qualified to serve as members of our board of directors, recommends to our board nominees for our annual meetings of stockholders, evaluates our board’s performance, develops and recommends to our board corporate governance guidelines and provides oversight with respect to corporate governance and ethical conduct. The chairman of the nominating and corporate governance committee is Mr. Carusi and the other current members are Messrs. Osgood and Dann. All members of the nominating and corporate governance committee are non-employee directors and satisfy the current independence standards established by Nasdaq and SEC rules.
Other Committees
Our board of directors may establish other committees as it deems necessary or appropriate from time to time.
Non-Employee Director Compensation for 2006
We did not pay any compensation to our non-employee directors in 2006.
Effective upon the closing of this offering, each of our non-employee directors will receive an annual cash retainer equal to $18,000 and each non-employee director who serves as a member of our audit committee will receive an annual retainer equal to $2,000, while non-employee directors who serve as members of our compensation or nominating and governance committees receive an annual retainer equal to $1,000. In addition to the annual retainers, non-employee directors will receive $2,500 for each board meeting attended in person, $750 for each board meeting attended telephonically and $750 for each committee meeting attended in person or telephonically.
Each non-employee director who serves as the chairperson of our audit committee, compensation committee or nominating and governance committee will also receive, for services performed in such capacity, an additional annual retainer of $12,000, $5,000 and $2,500, respectively. We reimburse each non-employee member of our board of directors for out-of-pocket expenses incurred in connection with attending our board and committee meetings. None of our non-employee directors held options to purchase common stock as of December 31, 2006. Each non-employee director first appointed to the board after the completion of this offering will automatically receive an initial option to purchase 30,000 shares of common stock upon such appointment, which will vest in four equal annual installments. In addition, beginning in 2008, at each annual meeting, non-employee directors who were non-employee directors for at least the preceding six months will automatically receive an option to purchase 10,000 shares of common stock, which will be immediately vested and fully exercisable.
Compensation Committee Interlocks and Insider Participation in Compensation Decisions
Our compensation committee consists of Messrs. Dann and Shapiro. None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation
68
Table of Contents
committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Messrs. Dann and Shapiro are affiliated with Sapient Capital, L.P. and Thomas Weisel Healthcare Venture Partners, L.P., respectively, and each of Sapient Capital and Thomas Weisel Healthcare participated in our series C preferred stock financing in September 2005, the details of which are disclosed under the heading “Certain Relationships and Related Party Transactions — Preferred Stock Financings.”
Family Relationships
There are no family relationships between any of our directors or executive officers.
69
Table of Contents
General
The following discussion and analysis of compensation arrangements of our named executive officers for 2006 should be read together with the compensation tables and related disclosures set forth below.
Our Compensation Objectives
The primary objective of our executive compensation program is to attract and retain talented executives to lead our company and create value for our stockholders. Executive compensation generally consists of a base salary, an annual short-term incentive payment based upon achievement of personal or corporate objectives and long-term equity-based incentive awards, which to date have generally been in the form of stock options. The equity component of our compensation is designed to align executive officers compensation with the goal of creation of long-term value for our stockholders. The goal of our compensation program is to be competitive with other companies in the medical device industry.
Role of Compensation Committee
Our compensation committee was appointed by our board of directors, and consists entirely of directors who are independent directors under applicable Nasdaq regulations, “outside directors” for purposes of Section 162(m) of the Code, and “non-employee directors” for purposes ofRule 16b-3 under the Securities Exchange Act of 1934, as amended, or Exchange Act. Our compensation committee reviews and recommends to our board of directors our executive compensation and benefit policies. Our compensation committee is responsible for, among other things, analyzing individual and corporate achievements and recommending to our board of directors appropriate compensation packages for our named executive officers. In addition, the compensation committee administers our equity-based compensation plans.
Our compensation committee solicits input from Richard Randall, our chief executive officer, in determining executive compensation, in particular with respect to salary and option grant awards to our executive officers other than Mr. Randall. While Mr. Randall discusses his recommendations with the compensation committee, he does not participate in deliberation or determination of his own compensation. In 2006, the compensation committee accepted Mr. Randall’s recommendations for executive officer compensation without change. None of our other executive officers participate in the compensation committee’s discussions regarding executive compensation. The compensation committee does not delegate any of its functions to others in determining executive compensation and has not previously engaged consultants with respect to executive compensation matters, although the compensation committee expects it may engage a compensation consultant in the future. The compensation of our executive officers is ultimately approved by our board of directors, upon recommendation of the compensation committee. Mr. Randall abstains from voting with respect to his compensation. In 2006, the board of directors approved the compensation committee’s recommendations for executive officer compensation without change.
We believe that in order to attract and retain sufficient executive talent, it is appropriate to compensate our executive officers at a level at least comparable to the compensation amounts provided to executives at comparable medical device companies, subject to the individual’s experience and expected contribution to the company. Historically, our compensation committee relied on their experience with other medical device companies and publicly available data relating to compensation of executives at other medical device companies to establish compensation for our executive officers. The group of companies that our compensation committee has used to compare our executive officers’ compensation has varied, based on our stage of development, from venture capital funded companies to publicly traded medical device companies in similar stages of development, and is set forth below under “Compensation Components — Base Salary.”
We believe the components of our current compensation program, which include cash salary, short-term cash incentive payments and long term equity awards are generally consistent with the compensation components of other comparable medical device companies, identified on page 71 under “Compensation
70
Table of Contents
Components — Base Salary,” although we generally do not benchmark the type of compensation awards with those of other companies.
The compensation committee has not established any formal policies or guidelines for allocating compensation between short-term and long-term incentive compensation, or between cash and non-cash compensation. In determining the amount and mix of compensation elements and whether each element provides the correct incentives and rewards for performance consistent with our short and long-term goals and objectives, our compensation committee relies on its judgment about each individual’s performance in a rapidly changing business environment rather than adopting a formulaic approach to compensatory decisions that are too narrowly responsive to short-term changes in business performance. As our company has matured from an early-stage start-up venture to a revenue producing commercial company, we have decreased the amount of our equity compensation and expect to continue to do so for the foreseeable future.
Compensation Components
Executive compensation currently consists of the following components:
Base Salary
We determine our executive officers’ salaries based on job responsibilities, individual experience and expected level of contribution, and we intend to benchmark our base salaries against those of comparable companies within the medical device industry. Our compensation committee generally reviews the salaries of our executives annually at the beginning of each calendar year and recommends to our board of directors changes in salaries based primarily on comparative market data, significant changes in responsibilities during the prior calendar year and, to a lesser extent, individual performance.
As a private company, the compensation committee relied on the experience of our directors, including Mr. Randall, and compensation surveys for venture capital funded companies in benchmarking our executive officers’ base salary. Beginning in 2007, when it became more likely that we would undertake an initial public offering of our common stock, we felt it was more appropriate to compare our executive officers’ base salaries to executive officers at other publicly traded medical device companies in similar development stages, including Nuvasive, Inc., Kyphon, Inc., St. Francis Medical, Inc., Hansen Medical, Inc., Xtent, Inc., Northstar Neuroscience, Inc. and NxStage Medical, Inc. These companies were used for comparative purposes for each of our executive officers, other than Mr. Randall. In establishing or evaluating our base salaries relative to these companies, we generally target the median base salary for these companies, while taking into consideration other factors such as the executive officer’s experience level and the cost of living differences between the areas where our executives are located and the locations of the peer companies, as well as our desire to attract and retain high quality individuals. For example, if our executive officers have greater experience than their counterparts who are in the median salary range at our peer companies, then we would expect to increase the base salary relative to the median salary for our peer companies. However, any increase could be somewhat offset by lower cost of living standards. Generally, our executive officers have had extensive executive level operational experience with numerous medical device companies prior to joining our company, which results in their base salary generally being at least in the median range of base salaries of our peer companies.
Mr. Randall’s base salary was established when he joined the company in 2002 and remained at substantially the same level through 2006. Although we believe his salary was fair based on our stage of development and the equity position afforded to Mr. Randall at the time he joined us, by 2007 the compensation committee determined that Mr. Randall’s salary was significantly below market for executives at other publicly traded medical device companies with similar experience levels based on an analysis of numerous companies. The companies included Restore Medical, Inc., Alphatec Holdings, Inc., Xtent, Inc., Atricure, Inc., Northstar Neuroscience, Inc., NxStage Medical, Inc., Micrus Endovascular Corporation, Sonic Innovations, Inc., SenoRx, Inc., VNUS Medical Technologies, Inc., Neurometrix, Inc., St. Francis Medical, Inc., Angiodynamics, Inc., Stereotaxis, Inc., Dexcom, Inc., ATS Medical, Inc., Conceptus, Inc., NMT Medical, Inc., Possis Medical, Inc., Thermage, Inc. and Nuvasive, Inc. An analysis of these companies indicated that the
71
Table of Contents
median salary for the chief executive officer was $319,000. In May 2007, upon recommendation of our compensation committee as part of its annual review of executive officers’ base salary originally intended to be performed in January 2007, our board of directors approved an increase to Mr. Randall’s annual base salary from $215,000 to $300,000, which increase was retroactive to January 1, 2007 as a result of the delayed review. In establishing Mr. Randall’s new salary at slightly below the median peer companies, the compensation committee took into account that Mr. Randall had a higher equity ownership percentage in our company than the average equity ownership of chief executive officers earning the median base salary level of the peer companies.
Short-Term Incentive Program
Our compensation committee believes that cash-based annual incentive payments for achievement of defined objectives that create value in our company align the executive’s compensation with the interests of our stockholders.
Our executive officers are eligible to receive cash-based incentive payments based upon the achievement of certain personal and corporate objectives. In the case of Mr. Randall, his incentive payment is based wholly on the achievement of corporate objectives, while our other executive officers’ incentive payments are based on a combination of achievement of personal objectives, which are generally part of the corporate objectives, and a subjective review of the executive officer’s total contribution to our company. At the beginning of each year, Mr. Randall recommends the corporate objectives to our compensation committee, which are then submitted to the board of directors for review. In 2006, the compensation committee modified Mr. Randall’s proposed corporate objectives by generally increasing the difficulty to attain certain objectives. In addition, the compensation committee included as part of the corporate objectives additional financial metrics which it believed to be important to the evaluation of our operations. The compensation committee and Mr. Randall discuss and assign weights to the corporate objectives based on their level of importance to our operating plan and corporate development. As we shifted from a research and development company to a commercialization company, greater weight has been assigned to our revenue and financial objectives. In 2006, financial objectives were 70% of the total objectives while product development and other operational objectives represented the remaining 30% of the total objectives. After the corporate objectives are approved by the compensation committee and reviewed by the board of directors, Mr. Randall establishes the personal objectives for each executive officer for that calendar year. The personal objectives are generally the same as the corporate objectives which relate to that part of our business for which the executive officer has primary responsibility. In 2006, our corporate objectives for the first six months of the year and the second six months of the year, along with their relative weight and achievement level, were as follows:
72
Table of Contents
| Weight | Achievement | |||||||
2006 First Half Objectives | ||||||||
| Achieve six month revenue target of $2.77 million | 45 | % | 50 | % | ||||
| Gross margin of 75% | 15 | % | 100 | % | ||||
| Net cash used in operating activities of $6.6 million for first six months of 2006 | 10 | % | 100 | % | ||||
| AxiaLIF 360° commercial launch in United States | 10 | % | 100 | % | ||||
| AxiaLIF commercial launch in European Union | 5 | % | 100 | % | ||||
| Completion of PNR pilot study and pre-IDE meeting | 15 | % | 0 | % | ||||
2006 Second Half Objectives | ||||||||
| Achieve twelve month revenue target of $9.66 million | 45 | % | 35 | % | ||||
| Gross margin of 76% | 15 | % | 100 | % | ||||
| Net cash used in operating activities of $6.8 million for second six months of 2006 | 10 | % | 100 | % | ||||
| Submit IDE for PNR implant | 10 | % | 0 | % | ||||
| AxiaLIF 2L commercial launch in European Union | 10 | % | 100 | % | ||||
| Miscellaneous operational objectives | 10 | % | 100 | % | ||||
If all of the corporate objectives were achieved, the executive officers would be entitled to 100% of their target bonus. However, in establishing the corporate objectives, the compensation committee believed that many of the corporate objectives, particularly with respect to financial metrics, would be difficult to achieve.
In 2007, our corporate objectives consist of achieving certain targets for revenues, gross margin, operating loss and average product sales price, clinical milestones for our PNR and PDR implants, hiring key personnel in finance and sales and marketing, development of AxiaLIF product enhancements, sales and marketing objectives, completion of initial public offering and miscellaneous operating objectives. The compensation committee has not assigned any particular weight to any of these objectives, but may do so at a later time, as discussed further below. Mr. Randall recommended these objectives to our compensation committee. The compensation committee accepted Mr. Randall’s recommendations without change and added two additional objectives for intellectual property management and surgeon training. The board of directors approved the compensation committee’s recommendations without changes. In establishing the corporate and personal objectives for each of our executives, particularly the financial objectives, the compensation committee believed that each of these objectives would be challenging to achieve.
Mr. Randall will not be eligible to receive a bonus for fiscal year 2007 if the corporate objective relating to revenue is not achieved. If the revenue target is achieved, the amount of Mr. Randall’s bonus will be based upon the relative achievement of all of the other 2007 corporate objectives. The compensation committee has not yet determined, and thus has not yet assigned, any particular weight to these corporate objectives primarily because the objectives will not be evaluated for purposes of determining Mr. Randall’s bonus award if the revenue target is not achieved. However, if the revenue target is achieved, the compensation committee will determine how to evaluate, and may assign weights to, the corporate objectives for purposes of determining Mr. Randall’s potential bonus award. The 2007 personal objectives for each of our other executive officers, along with their relative weight are as follows:
Mr. Simmons
| Weight | ||||
Objective | ||||
| Achieve revenue target | 40 | % | ||
| Hire product manager, 4 regional sales managers and 6 direct sales representatives | 20 | % | ||
| Train 200 surgeons | 15 | % | ||
| Average product sales price target | 10 | % | ||
| Miscellaneous operating objectives | 15 | % | ||
73
Table of Contents
Mr. Luetkemeyer
| Weight | ||||
Objective | ||||
| Complete initial public offering in 2007 | 50 | % | ||
| Timely report financial results | 25 | % | ||
| Hire operations director, SEC reporting manager and investor relations firm | 20 | % | ||
| Reduce days sales outstanding | 5 | % | ||
Mr. Assell
| Weight | ||||
Objective | ||||
| Complete PNR product design and testing | 30 | % | ||
| Development of AxiaLIF product enhancements | 25 | % | ||
| Operations milestones, including ERP system conversion, no product recalls and achieving a target gross margin | 25 | % | ||
| Obtain intellectual property opinion letters on all commercial products | 10 | % | ||
| Miscellaneous product milestones | 10 | % | ||
Mr. Jackson
| Weight | ||||
Objective | ||||
| Collect AxiaLIF 2L fusion data | 25 | % | ||
| Complete AxiaLIF data registry | 20 | % | ||
| Successfully complete ISO or FDA internal audit | 20 | % | ||
| Pre-IDE meeting with FDA for PNR | 15 | % | ||
| Miscellaneous regulatory milestones | 20 | % | ||
Historically, and in 2006, the performance of Mr. Randall was reviewed by our compensation committee against the established corporate objectives on a semi-annual basis and the performance of our other executive officers was reviewed by Mr. Randall and our compensation committee against the established objectives annually at the end of the year. The compensation committee’s subjective determination on the degree of achievement of the corporate objectives determines the amount of the cash incentive payment to be made to Mr. Randall. Mr. Randall evaluates the degree of achievement of the other executive officer’s personal objectives, as well as a subjective determination of the officer’s overall contribution to our company, and then recommends a cash bonus payment for the other executive officers. In 2006, the compensation committee approved Mr. Randall’s recommendations without change and the board of directors approved the compensation committee’s recommendations without change.
Our compensation committee, based upon recommendations from Mr. Randall, determines the target level of cash bonuses for our executive officers, which targets are based on a percentage of annual salary. In 2006, the target level of cash bonuses for Messrs. Randall, Assell and Simmons was 25%, 20% and 20%, respectively, of their annual salary. In 2007, the target level of cash bonuses for Messrs. Randall, Assell, Simmons, Luetkemeyer and Jackson is 35%, 20%, 20%, 20% and 20%, respectively, of their annual salary. In 2006 and 2007, the compensation committee approved Mr. Randall’s recommendations without change. In addition to his cash target bonus, Mr. Randall was entitled to receive a fixed number offully-vested stock options as determined by the compensation committee asshort-term incentive compensation. The compensation committee does not generally
74
Table of Contents
benchmark short-term incentive compensation to that of our peer companies primarily because of the uncertainty associated with the subjective nature of determining achievement of objectives.
The following table sets forth Mr. Simmons’ 2006 personal objectives, their relative weight and achievement level:
Objective | Weight | Achievement | ||||||
| Achieve twelve month revenue of $9.66 million | 40% | 60% | ||||||
| Comply with budgeted sales and marketing expenses | 20% | 100% | ||||||
| Hire director of marketing, product manager, regional manager and 8 direct sales representatives | 20% | 100% | ||||||
| Train 65 surgeons | 15% | 100% | ||||||
| Miscellaneous operational objectives | 5% | 100% | ||||||
The following table sets forth Mr. Assell’s 2006 personal objectives, their relative weight and achievement level:
Objective | Weight | Achievement | ||||||
| Design PNR pilot study and submit IDE for PNR | 45% | 44% | ||||||
| AxiaLIF 360° commercial launch in United States | 20% | 100% | ||||||
| Gross margin target of 76% | 15% | 100% | ||||||
| No product recalls | 15% | 100% | ||||||
| Hire AxiaLIF engineer and production manager | 5% | 100% | ||||||
Based upon the evaluation of the degree of achievement of the corporate and personal objectives, the board of directors approved, based upon the recommendation of the compensation committee, cash incentive payments for Messrs. Assell and Simmons equal to 75% of their respective target bonus amounts. Mr. Randall’s cash incentive payments were 62% and 61% of his target bonus amounts for the first six months of 2006 and the second six months of 2006, respectively.
Mr. Randall is the only executive officer who was entitled to receive fully-vested stock options as part of his short term incentive compensation. This arrangement, which is subject to compensation committee approval was originally approved when Mr. Randall joined the company in 2002 in an effort to compensate Mr. Randall commensurate with his experience level, while conserving cash for anearly-stage company without revenue. In connection with the increase of Mr. Randall’s base salary described above, Mr. Randall’s target bonus was set at 35% of his annual salary and he is no longer entitled to receive any portion of his short-term incentive compensation in the form of fully-vested options. In the future, our compensation committee expects to review Mr. Randall’s performance for purposes of determining short-term incentive awards on an annual basis rather than a semi-annual basis. Our compensation committee will continue to assess the goals and mechanics of our cash-based incentive program as a means of adding specific incentives towards achievement of specific company and departmental goals that could be key factors in our success.
Long-Term Equity-Based Incentive Awards
We believe that equity ownership in our company is important to provide our executive officers with long-term incentives to build value for our stockholders. Long-term equity can be awarded to executives by the board of directors in the form of stock options or restricted stock. Equity grants or awards are made to our executives by our board of directors at regularly scheduled meetings. The exercise price of our stock options is the fair market value of our stock as established based upon the good faith determination of our board of directors. In the future, we expect the exercise price of our options to be set at the closing price of our common stock on the date of the individual’s commencing employment or the date of grant. Each executive officer was provided with an option grant when they joined our company based upon their position with us, expected level of contribution and relevant prior experience. These initial grants typically vest over four years, and no shares vest before the one year anniversary of the option grant. We spread the vesting of our options
75
Table of Contents
over four years to compensate executives for their contribution over a period of time and to more properly align the executive’s interest with our stockholders’ interests.
In addition to the initial option grants, our board of directors has granted additional options to retain our executives and combine the achievement of corporate goals with strong individual performance. Options are granted based on a combination of individual contributions to our company and on general corporate achievements and expectations. Additional option grants are not communicated to executives in advance and generally vest over a period of four years. While our compensation committee may benchmark our executive officers’ equity ownership against our peer companies in establishing equity grants for new hires, it does not generally do so in approving additional equity grants for existing executives. In 2006, we granted additional stock options to our named executive officers in an effort to retain and continue to provide incentive to the executives with unvested equity awards as most of the executives’ existing awards had become vested. The number of options granted to the executive officers in 2006 was based on a subjective determination by the compensation committee and Mr. Randall of the number of unvested options they deemed reasonable to provide the sufficient incentive for the executive officers’ continued employment with us.
On an annual basis, our compensation committee intends to assess the contribution of the individual and the expectation for future performance by this executive and provide additional awards in the form of stock options or restricted stock based upon this assessment. We expect that we will continue to provide new employees with initial stock option grants in 2007 to provide long-term compensation incentives and will continue to rely on retention stock option grants in 2007 to provide additional incentives for current employees.
Other Benefits
We have a 401(k) plan for the benefit of all of our eligible employees, including the named executive officers. We do not provide for matching contributions under the 401(k) plan. For a more detailed description of the 401(k) plan, see below under “— 401(k) Plan.” We also provide health, dental, vision and life insurance and other customary employee assistance plans to all full-time employees, including the named executive officers.
Accounting and Tax Considerations
Effective January 1, 2006, we adopted the fair value provisions of Financial Accounting Standards Board Statement No. 123(R) (revised 2004), “Share-Based Payment,” or SFAS 123(R). Under SFAS 123(R), we are required to estimate and record an expense for each award of equity compensation (including stock options) over the vesting period of the award.
Section 162(m) of the Code limits the amount that we may deduct for compensation paid to our chief executive officer and to each of our four most highly compensated officers to $1,000,000 per person, unless certain exemption requirements are met. Exemptions to this deductibility limit may be made for various forms of “performance-based compensation.” In the past, annual cash compensation to our executive officers has not exceeded $1,000,000 per person, so the compensation has been deductible. In addition to salary and bonus compensation, upon the exercise of stock options that are not treated as incentive stock options, the excess of the current market price over the option price, or option spread, is treated as compensation and accordingly, in any year, such exercise may cause an officer’s total compensation to exceed $1,000,000.
Summary Compensation Table
The following table sets forth summary compensation information for the year ended December 31, 2006 for our chief executive officer, chief financial officer and each of our other three most highly compensated
76
Table of Contents
executive officers as of the end of the last fiscal year whose total compensation exceeded $100,000. We refer to these persons as our named executive officers elsewhere in this prospectus.
| Non-Equity | ||||||||||||||||||||
| Incentive Plan | ||||||||||||||||||||
| Salary | Option | Compensation | ||||||||||||||||||
Name and Principal Position | ($) | Awards(1) | ($) | Total | ||||||||||||||||
| Richard Randall | $ | 215,000 | $ | 36,351 | $ | 33,057 | (2) | $ | 284,408 | |||||||||||
| President, Chief Executive Officer and Director | James Barrile(3) | 158,333 | — | — | 158,333 | |||||||||||||||
| Chief Financial Officer | ||||||||||||||||||||
| Rick Simmons | 199,385 | 19,487 | 30,000 | 248,872 | ||||||||||||||||
| Vice President, Marketing and Sales | Robert Assell | 197,543 | 19,487 | 30,000 | 247,030 | |||||||||||||||
| Vice President, Product Development | ||||||||||||||||||||
Robert Sheridan(4) | 100,000 | — | — | 100,000 | ||||||||||||||||
| Vice President, Regulatory and Clinical Affairs | ||||||||||||||||||||
| (1) | Amounts represent the fair value of stock options expensed in 2006 under SFAS 123(R) as discussed in note 2 to our financial statements included elsewhere in this prospectus. | |
| (2) | In addition to his cash incentive payment, Mr. Randall received 2,251 options to purchase common stock at $2.38 per share and 8,273 options to purchase common stock at $1.11 per share, which options were fully vested and immediately exercisable. These options were granted as part of Mr. Randall’s annual incentive compensation. | |
| (3) | Mr. Barrile terminated his employment with us in October 2006. Prior to his resignation, Mr. Barrile’s annual base salary was $200,000. Mr. Randall, our president and chief executive officer, served as our interim chief financial officer from October 2006 to April 2007. Michael Luetkemeyer was appointed chief financial officer in April 2007. Mr. Luetkemeyer’s annual base salary for fiscal 2007 is $225,000. | |
| (4) | Mr. Sheridan terminated his employment with us in June 2007, but remains engaged with us as a clinical affairs consultant. Prior to his resignation, Mr. Sheridan worked on a half-time basis and his annual base salary was $100,000. William Jackson was appointed Vice President, Regulatory, Clinical and Quality in May 2007. Mr. Jackson works on a full-time basis and his annual base salary for fiscal 2007 is $200,000. |
Grants of Plan-Based Awards in 2006
The following table lists grants of plan-based awards made to our named executive officers in 2006 and related total fair value compensation for 2006.
| All Other | ||||||||||||||||||||||||
| Awards: | Grant Date | |||||||||||||||||||||||
| Possible | Number of | Exercise or | Fair Value of | |||||||||||||||||||||
| Future Payouts | Securities | Base Price of | Stock and | |||||||||||||||||||||
| Under Non-Equity | Underlying | Option | Option | |||||||||||||||||||||
Name | Grant Date | Incentive Plan Awards | Options | Awards | Awards(1) | |||||||||||||||||||
| Richard Randall | 11/08/2006 | $ | 53,750 | 8,273 | $ | 1.11 | $ | 36,351 | ||||||||||||||||
| James Barrile | — | — | — | — | — | |||||||||||||||||||
| Rick Simmons | 11/08/2006 | 40,000 | 18,000 | 1.11 | 79,093 | |||||||||||||||||||
| Robert Assell | 11/08/2006 | 40,000 | 18,000 | 1.11 | 79,093 | |||||||||||||||||||
| Robert Sheridan | — | — | — | — | — | |||||||||||||||||||
| (1) | Amounts represent the estimated total fair value of stock options granted in 2006 under SFAS 123(R) as discussed in note 2 to our financial statements included elsewhere in this prospectus. |
77
Table of Contents
Outstanding Equity Awards at Fiscal Year-End for 2006
The following table lists the outstanding equity incentive awards held by our named executive officers as of December 31, 2006.
| Option Awards | ||||||||||||||||
| Number of | Number of | |||||||||||||||
| Securities | Securities | |||||||||||||||
| Underlying | Underlying | |||||||||||||||
| Unexercised | Unexercised | Option | ||||||||||||||
| Options | Options | Option | Expiration | |||||||||||||
Name | Exercisable | Unexercisable(1) | Exercise Price | Date(1) | ||||||||||||
| Richard Randall | 122,907 | — | $ | 0.28 | 5/22/2013 | |||||||||||
| 8,964 | — | 0.28 | 1/06/2015 | |||||||||||||
| 4,410 | — | 0.78 | 9/22/2015 | |||||||||||||
| 8,273 | — | 1.11 | 11/08/2016 | |||||||||||||
| James Barrile | — | — | — | — | ||||||||||||
| Rick Simmons | 57,375 | 19,125 | 0.28 | 11/13/2013 | ||||||||||||
| 26,250 | 5,250 | 0.28 | 7/29/2013 | |||||||||||||
| 3,562 | 5,438 | 0.28 | 5/17/2015 | |||||||||||||
| 4,155 | 13,845 | (2) | 1.11 | 11/08/2016 | ||||||||||||
| Robert Assell | 18,000 | — | 0.11 | 9/27/2012 | ||||||||||||
| 54,000 | — | 0.11 | 3/20/2013 | |||||||||||||
| 54,000 | — | 0.28 | 5/22/2013 | |||||||||||||
| 3,937 | 5,063 | 0.28 | 3/22/2015 | |||||||||||||
| 5,344 | 8,156 | 0.28 | 5/17/2015 | |||||||||||||
| 4,155 | 13,845 | (2) | 1.11 | 11/08/2016 | ||||||||||||
| Robert Sheridan | 25,875 | 28,125 | 0.28 | 1/06/2015 | ||||||||||||
| (1) | Except where otherwise noted, all options expire ten years from the date of grant and option shares vest at the rate of 25% on the first anniversary of the option grant and 1/48 per month thereafter, such that options are fully vested upon the fourth anniversary of the option grant date. In addition, the vesting of options may accelerate upon a change in control of the company. | |
| (2) | Option shares vest at the rate of 21% on the date of grant and 1/48 per month thereafter, such that options are fully vested upon the fourth anniversary of the option grant date. |
Option Exercises for 2006
There were no options exercised by our named executive officers in 2006.
Employment and Severance Agreements and Employee Benefit Plans
Employment Agreements
We have not entered into any employment agreements with our employees.
Severance Agreements
We have not entered into any severance agreements with our employees.
78
Table of Contents
Employee Benefit Plans
Amended and Restated 2000 Stock Incentive Plan
The Amended and Restated 2000 Stock Incentive Plan, or 2000 Plan, was adopted by our board of directors and stockholders on June 14, 2000, and has been subsequently amended and restated from time to time. The 2000 Plan provides for the grant of incentive stock options, as defined under Section 422 of the Code, to employees, and for the grant of non-statutory stock options and restricted stock to employees, consultants, and directors. A total of 3,510,120 shares of our common stock have been authorized and reserved for issuance under the 2000 Plan. As of June 30, 2007, options to purchase a total of 2,339,663 shares of common stock, with a weighted exercise price of $1.74 per share, were outstanding under the 2000 Plan.
The exercise price of all incentive and non-statutory stock options granted under the 2000 Plan must be equal to at least 100% of the fair market value of the common stock on the date of grant. With respect to any optionee who owns stock possessing more than 10% of the voting power of all our classes of stock (including stock of any parent or subsidiary or ours), the exercise price of any incentive stock option granted must equal at least 110% of the fair market value on the grant date. The 2000 Plan provides for an option term of up to 10 years, but not to exceed five years for incentive stock options granted to 10% stockholders. The purchase price of restricted stock issued under the 2000 Plan shall be determined by the board as set forth in each individual stock purchase agreement.
Options granted under the 2000 Plan are non-transferable except by will, the laws of descent and distribution or other applicable laws, and, during the life of the optionee, may be exercisable only by such optionee. In addition, shares of restricted stock acquired under the 2000 Plan may not be sold, assigned, transferred, pledged or otherwise encumbered or disposed of except as specifically provided in the stock purchase agreement.
Unless otherwise determined by our board of directors, upon a change in control event, defined as a merger, sale of a majority of our voting stock, sale of all or substantially all of our assets, or liquidation or dissolution of the company, the outstanding unvested options will become fully vested and exercisable immediately prior to the consummation of the change in control, and in the case of shares of our common stock subject to a repurchase right that lapses over time, the such repurchase right shall, immediately prior to the consummation of the change in control, cease to apply.
The 2000 Plan will continue to be administered by our board of directors or a committee thereof. Our board of directors may amend or terminate the 2000 Plan as desired without further action by our stockholders except as set forth therein, as required by applicable law and to the extent the amendment or termination, as the case may be, will not substantially affect or impair the rights of any participant under an outstanding option agreement or stock purchase agreement without such participant’s consent. The 2000 Plan will continue in effect until terminated by our board of directors or for a term of ten years from its original adoption date, whichever is earlier.
2007 Stock Incentive Plan
Our board of directors adopted our 2007 Stock Incentive Plan, or 2007 Plan, in July 2007. The 2007 Plan became effective upon its adoption by our board of directors and our stockholders. The 2007 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Code to our employees and for the grant of nonstatutory stock options, restricted stock, restricted stock units and stock appreciation rights to our employees, directors and consultants.
A total of 1,400,000 shares of our common stock are reserved for issuance pursuant to the 2007 Plan, 6,750 of which are issued and outstanding.
The compensation committee of our board of directors administers the 2007 Plan. Our compensation committee consists of at least two or more “outside directors” within the meaning of Section 162(m) of the Code so that options granted under the 2007 Plan qualify as “performance based compensation.” Under Section 162(m) of the Code, the annual compensation paid to our named executive officers will only be
79
Table of Contents
deductible to the extent it does not exceed $1,000,000. However, we can preserve our deduction with respect to income recognized pursuant to options if the conditions for performance based compensation under Section 162(m) are met, which requires, among other things, that options be granted by a committee consisting of at least two “outside directors.” Subject to the provisions of the 2007 Plan, the compensation committee has the power to determine the terms of the awards, including the exercise price, the number of shares subject to each such award, the exercisability of the awards and the form of consideration, if any, payable upon exercise.
The exercise price of options granted under the 2007 Plan must at least be equal to the fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed ten years, except that with respect to any participant who owns 10% of the voting power of all classes of our outstanding stock, the term must not exceed five years and the exercise price must equal at least 110% of the fair market value on the grant date. Subject to the provisions of the 2007 Plan, the compensation committee determines the term of all other options.
After the termination of service of an employee, director or consultant, he or she may exercise his or her option for the period of time stated in his or her option agreement. Generally, if termination is due to death or disability, the option will remain exercisable for 12 months. In all other cases, the option will generally remain exercisable for three months following the termination of service. However, in no event may an option be exercised later than the expiration of its term.
Stock appreciation rights may be granted under the 2007 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. Subject to the provisions of the 2007 Plan, the compensation committee determines the terms of stock appreciation rights, including when such rights become exercisable and whether to pay any increased appreciation in cash or with shares of our common stock, or a combination thereof, except that the per share exercise price for the shares to be issued pursuant to the exercise of a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant.
Restricted stock may also be granted under the 2007 Plan. Restricted stock awards are grants of shares of our common stock that vest in accordance with terms and conditions established by the compensation committee. The compensation committee will determine the number of shares of restricted stock granted to any employee, director or consultant. The compensation committee may impose whatever conditions to vesting it determines to be appropriate (for example, the compensation committee may set restrictions based on the achievement of specific performance goals or continued service to us); provided, however, that the compensation committee, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture.
Restricted stock units may also be granted under the 2007 Plan. Restricted stock units are bookkeeping entries representing an amount equal to the fair market value of one share of our common stock. The compensation committee determines the terms and conditions of restricted stock units including the vesting criteria (which may include accomplishing specified performance criteria or continued service to us) and the form and timing of payment. Notwithstanding the foregoing, the administrator, in its sole discretion may accelerate the time at which any restrictions will lapse or be removed.
Performance units and performance shares may also be granted under the 2007 Plan. Performance units and performance shares are awards that will result in a payment to a participant only if performance goals established by the compensation committee are achieved or the awards otherwise vest. The compensation committee will establish organizational or individual performance goals in its discretion, which, depending on the extent to which they are met, will determine the numberand/or the value of performance units and performance shares to be paid out to participants. After the grant of a performance unit or performance share, the compensation committee, in its sole discretion, may reduce or waive any performance objectives or other vesting provisions for such performance units or performance shares. Performance units shall have an initial dollar value established by the compensation committee prior to the grant date. Performance shares shall have an initial value equal to the fair market value of our common stock on the grant date. The compensation committee, in its sole discretion, may pay earned performance units or performance shares in the form of cash, in shares or in some combination thereof.
80
Table of Contents
The 2007 Plan provides that all non-employee directors will be eligible to receive all types of awards (except for incentive stock options) under the 2007 Plan, including discretionary awards. Each non-employee director first appointed to the board of directors after the completion of this offering, except for those directors who become non-employee directors by ceasing to be employee directors, will receive an automatic initial nonstatutory stock option to purchase 30,000 shares of common stock upon such appointment. In addition, beginning in 2008, non-employee directors who have been directors for at least the preceding six months will receive a subsequent nonstatutory stock option to purchase 10,000 shares of common stock immediately following each annual meeting of our stockholders. All options granted under the automatic grant provisions will have a term of ten years and an exercise price equal to fair market value on the date of grant. Each 30,000 share option grant to non-employee directors will become exercisable as to one-fourth of the shares subject to the option on each anniversary of its date of grant, provided the non-employee director remains a director on such dates. Each 10,000 share option grant will be immediately vested and fully exercisable.
The 2007 Plan provides that in the event of a merger or “change in control,” as defined in the 2007 Plan, each outstanding award will be treated as the compensation committee determines, including that the successor corporation or its parent or subsidiary will assume or substitute an equivalent award for each outstanding award. The compensation committee is not required to treat all awards similarly. If there is no assumption or substitution of outstanding awards, the awards will fully vest, all restrictions will lapse, all performance goals or other vesting criteria will be deemed achieved at 100% of target levels and the awards will become fully exercisable. In addition, if the outstanding awards are assumed or substituted by an acquiring entity and the holder of the options is terminated without cause, as defined in the 2007 Plan, within twelve months after the change of control transaction, their awards will fully vest, all restrictions will lapse, all performance goals or other vesting criteria will be deemed achieved at 100% of target levels and the awards will become fully exercisable. The compensation committee will provide notice to the recipient that he or she has the right to exercise the option and stock appreciation right as to all of the shares subject to the award, all restrictions on restricted stock will lapse, and all performance goals or other vesting requirements for performance shares and units will be deemed achieved, and all other terms and conditions deemed met. The award will terminate upon the expiration of the period of time the compensation committee provides in the notice.
Our 2007 Plan will automatically terminate in 2017, unless we terminate it sooner. In addition, our board of directors has the authority to amend, suspend or terminate the 2007 Plan provided such action does not impair the rights of any participant.
2007 Employee Stock Purchase Plan
Our executive officers and all of our other employees will be allowed to participate in our 2007 Employee Stock Purchase Plan, which will be offered effective upon the completion of our public offering. We believe that providing them with the ability to participate in the 2007 Employee Stock Purchase Plan provides them with a further incentive towards ensuring our success and accomplishing our corporate goals.
Our board of directors adopted our 2007 Employee Stock Purchase Plan in July 2007. The 2007 Employee Stock Purchase Plan will become effective soon after the completion of this offering. A total of 250,000 shares of our common stock will be made available for sale. In addition, our 2007 Employee Stock Purchase Plan provides for annual increases in the number of shares available for issuance under the 2007 Employee Stock Purchase Plan on the first day of each fiscal year beginning in 2008, equal to the least of (i) 2.0% of the outstanding shares of our common stock on the first day of such fiscal year or (ii) an amount determined by the administrator of the 2007 Employee Stock Purchase Plan.
Our compensation committee administers the 2007 Employee Stock Purchase Plan and has full and exclusive authority to interpret the terms of the 2007 Employee Stock Purchase Plan and determine eligibility to participate subject to the conditions of our 2007 Employee Stock Purchase Plan as described below.
81
Table of Contents
All of our employees are eligible to participate if they are employed by us, or any participating subsidiary, for at least 20 hours per week and more than five months in any calendar year. However, an employee may not be granted an option to purchase stock under the 2007 Employee Stock Purchase Plan if such employee:
| • | immediately after the grant would own stock possessing 5% or more of the total combined voting power or value of all classes of our capital stock; or | |
| • | whose rights to purchase stock under all of our employee stock purchase plans accrues at a rate that exceeds $25,000 worth of stock for each calendar year. |
Our 2007 Employee Stock Purchase Plan is intended to qualify under Section 423 of the Code. Each offering period includes purchase periods, which will be approximately the six-month period commencing with one exercise date and ending with the next exercise date. The offering periods are scheduled to start on the first trading day on or after June 1 and December 1 of each year, except for the first such offering period, which will commence on the first trading day on or after completion of this offering and will end on the first trading day on or after the earlier of (i) May 31, 2008 or (ii) 12 months from the beginning of the first offering period.
Our 2007 Employee Stock Purchase Plan permits participants to purchase common stock through payroll deductions of up to 10% of their eligible compensation, which includes a participant’s base straight time gross earnings, certain commissions, overtime and shift premium, but exclusive of payments for incentive compensation, bonuses and other compensation. A participant may purchase a maximum of 2,500 shares during a six-month purchase period.
Amounts deducted and accumulated by the participant are used to purchase shares of our common stock at the end of each six-month purchase period. The purchase price of the shares will be 95% of the fair market value of our common stock on the exercise date. Participants may end their participation at any time during an offering period, and will be paid their accrued payroll deductions that have not yet been used to purchase shares of common stock. Participation ends automatically upon termination of employment with us.
A participant may not transfer rights granted under the 2007 Employee Stock Purchase Plan other than by will, the laws of descent and distribution, or as otherwise provided under the 2007 Employee Stock Purchase Plan.
In the event of our merger or change in control, as defined under the 2007 Employee Stock Purchase Plan, a successor corporation may assume or substitute for each outstanding option. If the successor corporation refuses to assume or substitute for the option, the offering period then in progress will be shortened, and a new exercise date will be set. The administrator will notify each participant that the exercise date has been changed and that the participant’s option will be exercised automatically on the new exercise date unless prior to such date the participant has withdrawn from the offering period.
Our 2007 Employee Stock Purchase Plan will automatically terminate in 2027, unless we terminate it sooner. Our board of directors has the authority to amend, suspend or terminate our 2007 Employee Stock Purchase Plan, except that, subject to certain exceptions described in the 2007 Employee Stock Purchase Plan, no such action may adversely affect any outstanding rights to purchase stock under our 2007 Employee Stock Purchase Plan.
401(k) Plan
We maintain a retirement savings plan, or a 401(k) plan, for the benefit of our eligible employees. Employees eligible to participate in our 401(k) plan will be those employees who have attained the age of 21. Employees may elect to defer their compensation up to the statutorily prescribed limit. An employee’s interests in his or her deferrals will be 100% vested when contributed. The 401(k) plan will be intended to qualify under Sections 401(a) and 501(a) of the Code. As such, contributions to the 401(k) plan and earnings on those contributions will not be taxable to the employees until distributed from the 401(k) plan.
82
Table of Contents
Nonqualified Deferred Compensation
None of our named executive officers participate in non-qualified defined contribution plans or other deferred compensation plans maintained by us. Our compensation committee, which is comprised solely of “outside directors” as defined for purposes of Section 162(m) of the Code, may elect to provide our officers and other employees with non-qualified defined contribution or deferred compensation benefits if the compensation committee determines that doing so is in our best interests.
Potential Payments Following a Change in Control
We have not entered into any agreements with our executive officers providing for payments payable to them upon termination of their employment following a change in control.
Limitation of Liability and Indemnification Matters
On completion of this offering, our amended and restated certificate of incorporation will contain provisions that eliminate the personal liability of directors and executive officers to the fullest extent permitted by the DGCL and provides that we may fully indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such person is or was our director or executive officer or is or was serving at our request as a director or officer of another corporation, partnership, joint venture, trust, employee benefit plan or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding.
Section 145 of the DGCL empowers a corporation to indemnify its directors and officers and to purchase insurance with respect to liability arising out of their capacity or status as directors and officers, provided that this provision shall not eliminate or limit the personal liability of a director for monetary damages:
| • | for any breach of the director’s duty of loyalty to the corporation or its stockholders; | |
| • | for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; | |
| • | arising under Section 174 of the DGCL; or | |
| • | for any transaction from which the director derived an improper personal benefit. |
We have entered into agreements to indemnify our directors and officers, in addition to the indemnification provided for in our bylaws. We believe that these provisions and agreements are necessary to attract and retain qualified directors and officers. Our bylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions, regardless of whether the DGCL would permit indemnification. On completion of this offering we intend to obtain directors’ and officers’ liability insurance. We are not currently aware of any pending litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification will be required or permitted. Moreover, we are not currently aware of any threatened litigation or proceeding that might result in a claim for such indemnification.
83
Table of Contents
We describe below transactions and series of similar transactions that have occurred this year or during our last three fiscal years to which we were a party or will be a party in which:
| • | the amounts involved exceeded or will exceed $120,000; and | |
| • | a director, executive officer, holder of more than 5% of our common stock or any member of their immediate family had or will have a direct or indirect material interest. |
Preferred Stock Financings
In September 2005, we issued and sold an aggregate of 3,499,091 shares of our Series C preferred stock to certain private investors, including certain of executive officers and 5% stockholders and persons and entities associated with them, at a price of $7.33 per share, for an aggregate purchase price of approximately $25,660,001. Each share of series C preferred stock will automatically convert into one share of common stock immediately upon the closing of this offering.
The table below sets forth the participation in the series C preferred stock financing by certain of our officers and 5% stockholders and persons and entities associated with them, as applicable.
| Number of | ||||
| Shares of | ||||
| Series C | ||||
Investor | Preferred Stock | |||
Officers | ||||
| Rick Simmons | 27,270 | |||
5% Stockholders | ||||
Advanced Technology Ventures and Affiliated Entities(1) | 954,545 | |||
Delphi Ventures and Affiliated Entities(2) | 681,818 | |||
Cutlass Capital and Affiliated Entities(3) | 409,091 | |||
Sapient Capital, L.P.(4) | 245,250 | |||
Thomas Weisel Healthcare Venture Partners, L.P.(5) | 954,545 | |||
| (1) | Consists of (i) 895,969 shares held by Advanced Technology Ventures VII, L.P., (ii) 35,955 shares held by Advanced Technology Ventures VII (B), L.P., (iii) 17,283 shares held by Advanced Technology Ventures VII (C), L.P., and (iv) 5,339 shares held by ATV Entrepreneurs VII, L.P. Michael Carusi, one of our directors, is a managing director of ATV Associates VII, L.L.C., the general partner of the funds affiliated with Advanced Technology Ventures which hold these shares. | |
| (2) | Consists of (i) 675,068 shares held by Delphi Ventures VI, L.P., and (ii) 6,751 shares held by Delphi BioInvestments VI, L.P. | |
| (3) | Consists of (i) 359,786 shares held by Cutlass Capital, L.P., (ii) 25,847 shares held by Cutlass Capital Principals Fund, L.L.C., and (iii) 23,459 shares held by Cutlass Capital Affiliates Fund, L.P. Jonathan Osgood, one of our directors, is one of two managing members of Cutlass Capital Management, L.L.C., which is the general partner of each of Cutlass Capital, L.P. and Cutlass Capital Affiliates Fund, L.P. | |
| (4) | Mitchell Dann, one of our directors, is the managing member of Sapient Capital Management, L.L.C., which is the general partner of Sapient Capital Management L.P., which is the general partner of Sapient Capital, L.P. | |
| (5) | James Shapiro, one of our directors, is a general partner of Kearny Venture Partners, the successor of Thomas Weisel Healthcare Venture Partners LLC, the general partner of Thomas Weisel Healthcare Venture Partners L.P. |
84
Table of Contents
Registration Rights
We have entered into an agreement with holders of our preferred stock, including entities affiliated with some of our directors and entities that hold more than 5% of our outstanding common stock on an as-converted basis, pursuant to which we granted them registration rights with respect to their shares of common stock issuable upon conversion of their preferred stock. For more information regarding registration rights, see “Description of Capital Stock — Registration Rights.”
Indemnification of Directors and Executive Officers
We have entered into an indemnification agreement with each of our directors and certain of our executive officers. These indemnification agreements and our amended and restated certificate of incorporation and bylaws indemnify each of our directors and certain of our officers to the fullest extent permitted by the DGCL. For information regarding these indemnification arrangements, please refer to the section entitled “Management — Limitation of Liability and Indemnification Matters.”
Review, Approval or Ratification of Transactions with Related Persons
As provided by our audit committee charter, all related party transactions are to be reviewed and pre-approved by our audit committee. A “related party transaction” is defined to include any transaction or series of transactions exceeding $120,000 in which we are a participant and any related person has a material interest. Related persons would include our directors, executive officers (and immediate family members of our directors and executive officers), and persons controlling over five percent of our outstanding common stock. In determining whether to approve a related party transaction, the audit committee will generally evaluate the transaction in terms of: (i) the benefits to us; (ii) the impact on a director’s independence in the event the related person is a director, an immediate family member of a director or an entity in which a director is a partner, shareholder or executive officer; (iii) the availability of other sources for comparable products or services; (iv) the terms and conditions of the transaction; and (v) the terms available to unrelated third parties or to employees generally. The audit committee will then document its findings and conclusions in written minutes. In the event a transaction relates to a member of our audit committee, that member will not participate in the audit committee’s deliberations.
85
Table of Contents
Set forth below is certain information as of June 30, 2007 regarding the beneficial ownership of our common stock by:
| • | any person (or group of affiliated persons) who was known by us to own more than 5% of our voting securities; | |
| • | each of our directors; | |
| • | each of our named executive officers; and | |
| • | all current directors and executive officers as a group. |
Applicable percentage ownership in the following table is based on the number of shares of common stock outstanding as of June 30, 2007, as adjusted to reflect the conversion of all our outstanding preferred stock into 10,793,182 shares of common stock immediately upon the closing of this offering. The percentage of shares beneficially owned after this offering excludes the shares that are subject to the underwriters’ option to purchase additional shares.
| Number of | Percentage of Shares | |||||||||||
| Shares | Beneficially Owned | |||||||||||
| Beneficially | Before | After | ||||||||||
Beneficial Owners(1) | Owned(2) | Offering | Offering | |||||||||
5% Stockholders | ||||||||||||
Advanced Technology Ventures and Affiliated Entities(3) | 2,590,909 | 19.5 | % | 13.8 | % | |||||||
Delphi Ventures and Affiliated Entities(4) | 2,158,637 | 16.3 | % | 11.5 | % | |||||||
Cutlass Capital and Affiliated Entities(5) | 1,996,364 | 15.0 | % | 10.6 | % | |||||||
Sapient Capital, L.P.(6) | 1,588,091 | 12.0 | % | 8.5 | % | |||||||
Thomas Weisel Healthcare Venture Partners, L.P.(7) | 954,545 | 7.2 | % | 5.1 | % | |||||||
Andrew Cragg, M.D.(8) | 1,126,239 | 8.5 | % | 6.0 | % | |||||||
George Wallace(9) | 794,250 | 6.0 | % | 4.2 | % | |||||||
Named Executive Officers and Directors | ||||||||||||
Richard Randall(10) | 671,024 | 5.0 | % | 3.5 | % | |||||||
| Michael Luetkemeyer | — | — | — | |||||||||
Rick Simmons(11) | 142,699 | 1.1 | % | * | ||||||||
Robert Assell(12) | 128,179 | * | * | |||||||||
| William Jackson | — | — | — | |||||||||
| Robert Martin | — | — | — | |||||||||
Michael Carusi(13) | 2,590,909 | 19.5 | % | 13.8 | % | |||||||
Mitchell Dann(14) | 1,588,091 | 12.0 | % | 8.5 | % | |||||||
Jonathan Osgood(15) | 1,996,364 | 15.0 | % | 10.6 | % | |||||||
James Shapiro(16) | 954,545 | 7.2 | % | 5.1 | % | |||||||
All executive officers and directors as a group (9 persons)(17) | 8,071,810 | 59.1 | % | 42.1 | % | |||||||
| * | Represents beneficial ownership of less than 1%. | |
| (1) | Unless otherwise indicated, the business address of each holder is:c/o TranS1 Inc., 411 Landmark Drive, Wilmington, North Carolina28412-6303. | |
| (2) | Beneficial ownership is based on information furnished by the individuals or entities and is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock subject to options currently exercisable, or exercisable within 60 days of June 30, 2007 are deemed outstanding for computing the percentage of the person holding such options but are not deemed outstanding for computing the percentage of any other person. As of June 30, |
86
Table of Contents
| 2007, we had a total of 13,276,502 shares of common stock issued and outstanding, including the shares of common stock issuable upon conversion of our preferred stock. Except as indicated by footnote and subject to community property laws where applicable, to our knowledge, the companies and persons named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them. | ||
| (3) | Consists of (i) 2,427,307 shares held by Advanced Technology Ventures VII, L.P. (“ATV VII”), (ii) 97,407 shares held by Advanced Technology Ventures VII (B), L.P. (“ATV VII-B”), (iii) 46,821 shares held by Advanced Technology Ventures VII (C), L.P. (“ATV VII-C”), (iv) 14,464 shares held by ATV Entrepreneurs VII, L.P. (“ATV VII-E” and together with ATV VII, ATV VII-B, and ATV VII-C, collectively referred to as the “ATV VII Entities”), and (v) 4,910 shares held by ATV Alliance 2002, L.P. (“ATV A 2002” and together with the ATV VII Entities, the “ATV Entities”). ATV Associates VII, L.L.C. (“ATV A VII”) is the general partner of each of the ATV VII Entities and exercises voting and dispositive authority over the shares held by the ATV VII Entities. ATV Alliance Associates, L.L.C. (“ATV Alliance”) is the sole general partner of ATV A 2002 and exercises voting and dispositive authority over the shares held by ATV A 2002. Voting and dispositive decisions of ATV A VII are made by a board of six managing directors (the “ATV Managing Directors”), including Michael Carusi, one of our directors. Each of the ATV Managing Directors disclaims beneficial ownership of the shares held by the ATV VII Entities. Voting and dispositive decisions of ATV Alliance are made by its sole manager, Jean George, who disclaims beneficial ownership of the shares held by ATV A 2002. Each of ATV A VII and ATV Alliance disclaims beneficial ownership of any shares held by any of the ATV Entities. The address for Advanced Technology Ventures is 1000 Winter Street, Suite 3700, Waltham, MA 02451. | |
| (4) | Consists of (i) 2,137,264 shares held by Delphi Ventures VI, L.P., and (ii) 21,373 shares held by Delphi BioInvestments VI, L.P. (together, the “Delphi Funds”). Delphi Management Partners VI, LLC is the general partner of each of the Delphi Funds. The managing members of Delphi Management Partners VI, LLC are James J. Bochnowski, David L. Douglass, Douglas A. Roeder, John F. Maroney and Deepika R. Pakianathan. Delphi Management Partners VI, LLC and each of the foregoing managing members may be deemed a beneficial owner of the reported shares but each disclaims beneficial ownership except to the extent of any indirect pecuniary interest therein. The address for all entities and individuals affiliated with Delphi Ventures is 3000 Sand Hill Road, Building 1, Suite 135, Menlo Park, CA 94025. | |
| (5) | Consists of (i) 1,755,754 shares held by Cutlass Capital, L.P., (ii) 126,134 shares held by Cutlass Capital Principals Fund, L.L.C., and (iii) 114,476 shares held by Cutlass Capital Affiliates Fund, L.P. Jonathan Osgood, one of our directors, is one of two managing members of Cutlass Capital Management, L.L.C., which is the general partner of each of Cutlass Capital, L.P. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood is also one of two managing members of Cutlass Capital Principals Fund, L.L.C. Mr. Osgood has shared voting and investment power over the shares held by each of Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood disclaims beneficial ownership of the shares held by Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P., except to the extent of his proportionate pecuniary interest in them. The address for Cutlass Capital is 84 State Street, Suite 1040, Boston, MA 02109. | |
| (6) | Mitchell Dann, one of our directors, is the managing member of Sapient Capital Management, L.L.C., which is the general partner of Sapient Capital Management L.P., which is the general partner of Sapient Capital, L.P. Mr. Dann has sole voting and investment power over the shares held by Sapient Capital, L.P. Mr. Dann disclaims beneficial ownership of the shares held by Sapient Capital, L.P., except to the extent of his proportionate pecuniary interest in them. The address for Sapient Capital is 4020 Lake Creek Drive, P.O. Box 1590, Wilson, WY 83014. | |
| (7) | James Shapiro, one of our directors, is a general partner of Kearny Venture Partners, the successor of Thomas Weisel Healthcare Venture Partners LLC, the general partner of Thomas Weisel Healthcare Venture Partners L.P., and has shared voting and investment power over the 954,545 shares held by Thomas Weisel Healthcare Venture Partners L.P. The address for Thomas Weisel Healthcare Venture Partners is One Montgomery St., San Francisco, CA 94104. |
87
Table of Contents
| (8) | Includes 27,000 shares subject to options exercisable within 60 days of June 30, 2007. The address for Mr. Cragg is 5024 Bruce Ave., Edina, MN 55424. | |
| (9) | Consists of (i) 648,000 shares held by Wallace Partners, L.P., of which Mr. Wallace is the general partner, and (ii) 146,250 shares held by George B. Wallace and Jane F. Wallace, as Co-Trustees of the Wallace Family Trust, U/D/T March 26, 2002. The address for Mr. Wallace is 26429 Rancho Parkway South, #140, Lake Forest, CA 92679. | |
| (10) | Includes 146,804 shares subject to options exercisable within 60 days of June 30, 2007. | |
| (11) | Includes 115,429 shares subject to options exercisable within 60 days of June 30, 2007. | |
| (12) | Comprised of shares subject to options exercisable within 60 days of June 30, 2007. | |
| (13) | Consists solely of shares identified in footnote 3. Mr. Carusi, one of our directors, is a managing director of ATV Associates VII, LLC. Mr. Carusi disclaims beneficial ownership of the shares held by the ATV Entities except to the extent of his proportionate pecuniary interest in them. | |
| (14) | Consists solely of the shares identified in footnote 6. Mitchell Dann, one of our directors, is the managing member of Sapient Capital Management, L.L.C., which is the general partner of Sapient Capital Management L.P., which is the general partner of Sapient Capital, L.P. Mr. Dann has sole voting and investment power over the shares held by Sapient Capital, L.P. Mr. Dann disclaims beneficial ownership of the shares held by Sapient Capital, L.P., except to the extent of his proportionate pecuniary interest in them. | |
| (15) | Consists solely of the shares identified in footnote 5, which include (i) 1,755,754 shares held by Cutlass Capital, L.P., (ii) 126,134 shares held by Cutlass Capital Principals Fund, L.L.C., and (iii) 114,476 shares held by Cutlass Capital Affiliates Fund, L.P. Jonathan Osgood is one of two managing members of Cutlass Capital Management, L.L.C., which is the general partner of each of Cutlass Capital, L.P. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood is also one of two managing members of Cutlass Capital Principals Fund, L.L.C. Mr. Osgood has shared voting and investment power over the shares held by each of Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood disclaims beneficial ownership of the shares held by Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P., except to the extent of his proportionate pecuniary interest in them. | |
| (16) | Consists solely of shares identified in footnote 7. Mr. Shapiro, one of our directors, is a general partner of Kearny Venture Partners, the successor of Thomas Weisel Healthcare Venture Partners LLC, the general partner of Thomas Weisel Healthcare Venture Partners L.P., and has shared voting and investment power over the shares held by Thomas Weisel Healthcare Venture Partners L.P. Mr. Shapiro disclaims beneficial ownership of these shares except to the extent of his proportionate pecuniary interest in them. | |
| (17) | Includes 390,412 shares subject to options exercisable within 60 days of June 30, 2007. |
88
Table of Contents
Upon completion of this offering, our authorized capital stock, after giving effect to the conversion of all our outstanding preferred stock into common stock and the filing of our amended and restated certificate of incorporation, will consist of 75,000,000 shares of common stock, $0.0001 par value per share, and 5,000,000 shares of preferred stock, $0.0001 par value per share. The following description summarizes the terms of our capital stock. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description you should refer to our amended and restated certificate of incorporation and amended and restated bylaws, effective upon completion of this offering, copies of which have been filed as exhibits to the registration statement of which this prospectus is a part, and to the applicable provisions of the DGCL.
Common Stock
As of June 30, 2007, there were 2,483,320 shares of common stock outstanding held by 20 stockholders of record. An additional 10,793,182 shares of our common stock will be issued upon the conversion of all of our outstanding preferred stock immediately upon the closing of this offering. There will be 18,776,502 shares of common stock outstanding after giving effect to the conversion of all our outstanding preferred stock into common stock, and the sale of the shares of our common stock in this offering. All outstanding shares of common stock are, and the common stock to be issued in this offering will be, fully paid and nonassessable.
The following summarizes the rights of holders of our common stock:
| • | each holder of common stock is entitled to one vote per share on all matters to be voted upon by the stockholders, including the election of directors; | |
| • | the affirmative vote of a majority of the shares present in person or represented and voting at a duly held meeting at which a quorum is present shall be the act of the stockholders; | |
| • | holders of common stock are not entitled to cumulate votes in the election of directors, which means that the holders of a majority of the shares voted can elect all of the directors then standing for election; | |
| • | subject to preferences that may be applicable to the holders of outstanding shares of preferred stock, if any, the holders of common stock are entitled to receive dividends when, as and if declared by our board of directors out of assets legally available for dividends; | |
| • | upon our liquidation, dissolution or winding up, after satisfaction of all our liabilities and the payment of any liquidation preference of any outstanding preferred stock, the holders of shares of common stock will be entitled to receive on a pro rata basis all of our assets remaining for distribution; | |
| • | there are no redemption or sinking fund provisions applicable to our common stock; and | |
| • | there are no preemptive or conversion rights applicable to our common stock. |
Preferred Stock
Effective upon completion of this offering, there will be no shares of preferred stock outstanding because all our issued and outstanding preferred stock will have been be converted into an aggregate of 10,793,182 shares of common stock immediately upon the closing of this offering. However, our amended and restated certificate of incorporation will authorize our board of directors, without further action by the stockholders, to create and issue one or more series of preferred stock and to fix the rights, preferences and privileges thereof. Among other rights, our board of directors may determine, without further vote or action by our stockholders:
| • | the number of shares constituting the series and the distinctive designation of the series; |
89
Table of Contents
| • | the dividend rate on the shares of the series, whether dividends will be cumulative, and if so, from which date or dates, and the relative rights of priority, if any, of payment of dividends on shares of the series; | |
| • | whether the series will have voting rights in addition to the voting rights provided by law and, if so, the terms of the voting rights; | |
| • | whether the series will have conversion privileges and, if so, the terms and conditions of conversion; | |
| • | whether or not the shares of the series will be redeemable or exchangeable, and, if so, the dates, terms and conditions of redemption or exchange, as the case may be; | |
| • | whether the series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of the sinking fund; and | |
| • | the rights of the shares of the series in the event of our voluntary or involuntary liquidation, dissolution or winding up and the relative rights or priority, if any, of payment of shares of the series. |
Although we presently have no plans to issue any shares of preferred stock, any future issuance of shares of preferred stock, or the issuance of rights to purchase preferred shares, may delay, defer or prevent a change of control in our company or an unsolicited acquisition proposal and make removal of management more difficult. The issuance of preferred stock also could decrease the amount of earnings and assets available for distribution to the holders of common stock or could adversely affect the rights and powers, including voting rights, of the holders of the common stock. The issuance of preferred stock may have the effect of decreasing the market price of our common stock, and may adversely affect the voting and other rights of the holders of our common stock.
Registration Rights
The holders of our series A, series AA, series B and series C preferred stock, which will convert into an aggregate of 10,793,182 shares of our common stock immediately upon the closing of this offering, have the right to require us to register their shares with the SEC, or to include their shares in any registration statement we file, so that the shares may be publicly resold. These registration rights are summarized below.
Demand registration rights
Beginning six months after the completion of this offering, the holders of a majority of the shares issuable upon conversion of the series A, series AA, series B and series C preferred stock have the right to demand, on not more than two occasions (subject to limited exceptions), that we file a registration statement onForm S-1 under the Securities Act, having an aggregate offering price to the public of not less than $5,000,000, in order to register the shares registrable under such registration rights. Further, at any time after we become eligible to file a registration statement onForm S-3, the holders of the shares subject to these registration rights may require us to file up to two registrations statements onForm S-3 per year with respect to shares of common stock having an aggregate offering price to the public of at least $500,000, subject to certain exceptions.
Piggyback registration rights
If we register any shares of our capital stock for public sale, holders of the shares of common stock issued on conversion of our preferred stock will have the right to include their shares in the registration. The underwriters of any underwritten public offering will have the right to limit the number of shares to be included in the registration, provided that the holders of the registrable shares shall not be reduced to less than 20% of the aggregate shares offered.
90
Table of Contents
Postponement of Registration
We may postpone the filing of a registration statement for a period of 90 days in response to the exercise of the registration rights set forth above if we determine that the filing would be significantly detrimental to us. We may only exercise such deferral rights once within any 12 month period.
Expenses of Registration
We will pay all expenses relating to any demand or piggyback registration (except for underwriting discounts, commissions and stock transfer taxes) and the reasonable expenses and fees of one special counsel for the holders of registration rights. However, we will not pay for the expenses of any demand registration if the request is subsequently withdrawn by the holders of a majority of the shares having registration rights, subject to limited exceptions.
Indemnification
We are generally required to indemnify the holders participating in an offering against civil liabilities resulting from any registration under these provisions.
Termination of registration rights
The demand,Form S-3 and piggyback registration rights described above will terminate on the earlier of (i) the date after the closing of this offering on which all shares subject to such registration rights may immediately be sold under Rule 144 during any90-day period, or (ii) the fourth anniversary of the closing of this offering.
Options
As of June 30, 2007, options to purchase a total of 2,339,663 shares of our common stock were outstanding with a weighted average exercise price of $1.74 per share. These options typically vest over a four-year period. All outstanding options provide for anti-dilution adjustments in the event of reorganizations, recapitalizations, stock dividends, stock splits or other changes in our capital structure. For a discussion of the provisions applicable to our stock options, see the section entitled “Management — Employee Benefit Plans” in this prospectus.
Anti-Takeover Effects of Certain Provisions of Delaware Law and Our Certificate of Incorporation and Bylaws
Application of Interested Director Provisions of Delaware Law. We are subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless either:
| • | prior to the date at which the person becomes an interested stockholder, the board of directors approves such transaction or business combination; | |
| • | the stockholder acquires more than 85% of the outstanding voting stock of the corporation (excluding shares held by directors who are officers or held in certain employee stock plans) upon consummation of such transaction; or | |
| • | the business combination is approved by the board of directors and by the holders of two-thirds of the outstanding voting stock of the corporation (excluding shares held by the interested stockholder) at a meeting of stockholders (and not by written consent). |
For purposes of Section 203, “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years prior, did own) 15% or more of the corporation’s voting stock. A “business combination” includes a merger, asset sale or other transaction resulting in a financial benefit to such interested stockholder.
91
Table of Contents
In addition, certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws may be deemed to have an anti-takeover effect and may delay, defer or prevent a tender offer or takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares held by our stockholders. Our amended and restated certificate of incorporation to be effective upon completion of this offering will provide for our board of directors to be divided into three classes, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, our stockholders representing a majority of the shares of common stock outstanding will be able to elect all of our directors. Our amended and restated certificate of incorporation and bylaws to be effective upon completion of this offering will provide that all stockholder action must be effected at a duly called meeting of stockholders and not by a consent in writing, and that only our board of directors, chairman of the board and chief executive officer may call a special meeting of stockholders. In addition, our amended and restated certificate of incorporation and bylaws will require advance notice for stockholders to nominate directors or to submit proposals for consideration at meetings of stockholders. Our amended and restated certificate of incorporation and bylaws will require a 662/3% stockholder vote and the approval of our board of directors for the amendment, repeal or modification of certain provisions of our amended and restated certificate of incorporation and bylaws relating to the issuance of preferred stock, the absence of cumulative voting, the classification of our board of directors, the requirement that stockholder actions be effected at a duly called meeting, the requirement of advance notice for stockholders to nominate director to submit proposals for consideration at meetings of stockholders and the designated parties entitled to call a special meeting of the stockholders.
The combination of the classification of our board of directors, the lack of cumulative voting and the 662/3% stockholder voting requirements will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Since our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
These provisions may have the effect of deterring hostile takeovers or delaying changes in our control or management. These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and in the policies they implement, and to discourage certain types of transactions that may involve an actual or threatened change of our control. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in our management.
Transfer Agent and Registrar
American Stock Transfer and Trust Company will be the transfer agent and registrar for our common stock.
Nasdaq Global Market Listing
We have applied to list our common stock on the Nasdaq Global Market under the symbol “TSON.”
92
Table of Contents
Prior to this offering, there has been no market for our common stock. Future sales of substantial amounts of our common stock in the public market could adversely affect prevailing market prices and adversely affect our ability to raise additional capital in the capital markets at a time and price favorable to us.
Upon completion of this offering, assuming no exercise of outstanding options after June 30, 2007, and assuming the conversion of all our outstanding preferred stock into shares of our common stock, we will have 18,776,502 shares of common stock outstanding (19,601,502 shares if the underwriters’ option to purchase additional shares is exercised in full). Of these shares, all of the shares of common stock sold in this offering, plus any shares sold as a result of the underwriter’s exercise of their option to purchase additional shares, will be freely tradable without restriction under the Securities Act, unless purchased by our affiliates, as that term is defined in Rule 144 under the Securities Act.
The remaining 13,276,502 shares of outstanding common stock as of June 30, 2007, held by existing stockholders are “restricted securities” under the Securities Act and are subject to“lock-up” agreements. Of this amount, all of the shares will be eligible for resale pursuant to Rule 144 as of June 30, 2007, and all such shares will be subject to“lock-up” agreements as described below. “Restricted securities” as defined under Rule 144 were issued and sold by us in reliance on exemptions from the registration requirements of the Securities Act. These shares may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 144(k) under the Securities Act.
Registration Rights
Upon completion of this offering, holders of 10,793,182 shares of our common stock will be entitled to certain rights with respect to the registration of their shares under the Securities Act. See the section entitled “Description of Capital Stock — Registration Rights” in this prospectus. Registration of their shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act immediately upon effectiveness of the registration. All of these stockholders have agreed not to exercise their registration rights for at least 180 days following this offering.
Lock-Up Agreements
We, our directors and executive officers and all of our existing stockholders and option holders have entered intolock-up agreements with the underwriters. Under these agreements, subject to customary exceptions, we may not issue any new shares of common stock, and those holders of stock and options may not, directly or indirectly, sell, offer, contract or grant any option to sell, pledge, transfer, establish a put equivalent position or otherwise dispose of any shares of common stock or options to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock beneficially owned by them, or publicly announce the intention to do any of the foregoing, without the prior written consent of Lehman Brothers Inc. and Piper Jaffray & Co., for a period of 180 days from the date of this prospectus. This consent may be given at any time without public notice. Notwithstanding the foregoing, if, (1) during the last 17 days of the180-daylock-up period, we release earnings results or material news or a material event relating to us occurs; or (2) prior to the expiration of the180-daylock-up period, we announce that we will release earnings results or become aware that material news or a material event relating to us will occur during the16-day period beginning on the last day of the180-daylock-up period, the180-daylock-up period will be extended until 18 days following the issuance of the earnings release or the occurrence of the material news or material event, as applicable, unless Lehman Brothers Inc. and Piper Jaffray & Co. waive, in writing, such extension.
Rule 144
Under Rule 144 as currently in effect, beginning 90 days after the date of this prospectus, a person who has beneficially owned restricted securities for at least one year, including persons who may be deemed our affiliates, would be entitled to sell within any three-month period a number of shares that does not exceed the greater of the following:
| • | one percent of the number of shares of common stock then outstanding (equal to approximately 187,765 shares upon completion of this offering), assuming no exercise of the underwriters’ option to purchase additional shares; or |
93
Table of Contents
| • | the average weekly trading volume of the common stock as reported through The Nasdaq Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. Sales under Rule 144 are also subject to certain manner of sale provisions, and notice requirements and the availability of current public information about us. |
Rule 144(k)
Under Rule 144(k), a person who is not and is not deemed to have been our affiliate at any time during the 90 days preceding a sale, and who has beneficially owned the shares proposed to be sold for at least two years, is entitled to sell such shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144.
As a result, 3,097,366 shares of our common stock will qualify for resale under Rule 144(k) beginning on the date of this prospectus. However, such shares will be subject to thelock-up agreements described above.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been our affiliate during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation, or notice provisions of Rule 144. Rule 701 also permits our affiliates to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701.
As of June 30, 2007, 339,100 shares of our outstanding common stock had been issued in reliance on Rule 701 as a result of exercises of stock options. All of these shares, however, are subject tolock-up agreements as discussed above. As a result, unless otherwise extended, these shares will only become eligible for sale at the earlier of the expiration of the180-day lockup period or upon obtaining the prior written consent of Lehman Brothers Inc. and Piper Jaffray & Co. to release all or any portion of the shares subject to lockup agreements.
Registration of Shares Issuable Under Benefit Plans
We intend to file aForm S-8 registration statement under the Securities Act after the completion of this offering to register 3,770,715 shares of common stock issuable under the Amended and Restated 2000 Stock Incentive Plan, the 2007 Stock Incentive Plan and the 2007 Employee Stock Purchase Plan, which is all the shares issuable pursuant to such plans. Such registration statement will become effective immediately upon filing, and shares covered by those registration statements will thereupon be eligible for sale in the public markets, subject to anylock-up agreements and Rule 144 limitations applicable to affiliates. For a more complete discussion of our stock option plans, see the sections entitled “Management — Employee Benefit Plans” and “Description of Capital Stock — Options” in this prospectus.
94
Table of Contents
The following summary discusses material United States federal income tax consequences, and certain United States federal estate tax consequences, of the purchase, ownership and disposition of our common stock by aNon-U.S. Holder, as defined below. This summary deals only with common stock held as a capital asset within the meaning of Section 1221 of the Code, and is applicable only toNon-U.S. Holders who purchase common stock pursuant to this offering. This summary does not address specific tax consequences that may be relevant to you if you are aNon-U.S. Holder subject to special tax treatment (including pass-through entities, banks and insurance companies, dealers in securities, persons holding our common stock as part of a “straddle,” “hedge,” “conversion transaction” or other risk-reduction transaction, controlled foreign corporations, passive foreign investment companies, companies that accumulate earnings to avoid United States federal income tax, foreign tax-exempt organizations, former U.S. citizens or residents and persons who hold or receive common stock as compensation or pursuant to the exercise of compensatory options), and does not address alternative minimum tax consequences, if any, or any state, local, or foreign tax consequences.
This summary is based upon the provisions of the Code and United States Treasury regulations, rulings and judicial decisions as of the date hereof, all of which are subject to change, possibly with retroactive effect.
If you are considering the purchase of common stock, you should consult your own tax advisors regarding the United States federal income tax consequences to you of the purchase, ownership, and disposition of common stock, as well as any consequences arising under the laws of any other taxing jurisdiction.
For purposes of this summary, you are aNon-U.S. Holder if you are a beneficial owner of common stock who is not a U.S. person or a partnership (or other entity treated as a partnership) for United States federal income tax purposes. A U.S. person is (i) a citizen or resident alien individual of the United States; (ii) a corporation, or other entity treated as a corporation for United States federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; (iii) an estate, the income of which is subject to United States federal income taxation regardless of its source; or (iv) a trust (a) whose administration is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (b) if it has a valid election in effect under applicable United States Treasury regulations to be treated as a U.S. person.
U.S. Trade or Business Income
For purposes of this discussion, dividend income and gain on the sale or other taxable disposition of our common stock will be considered to be “U.S. trade or business income” if such income or gain is (i) effectively connected with the conduct by aNon-U.S. Holder of a trade or business within the United States, or (ii) in the case of aNon-U.S. Holder that is eligible for the benefits of an income tax treaty with the United States, attributable to a permanent establishment (or, for an individual, a fixed base) maintained by theNon-U.S. Holder in the United States. Generally, U.S. trade or business income is not subject to United States federal withholding tax (provided theNon-U.S. Holder complies with applicable certification and disclosure requirements). Instead, U.S. trade or business income is subject to United States federal income tax on a net income basis at regular United States federal income tax rates in the same manner as a U.S. person, unless an applicable income tax treaty provides otherwise. Any U.S. trade or business income received by a corporateNon-U.S. Holder may be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
Distributions
Distributions of cash or property that we pay will generally constitute dividends for United States federal income tax purposes to the extent paid from our current or accumulated earnings and profits (as determined under United States federal income tax principles). ANon-U.S. Holder generally will be subject to withholding of United States federal income tax at a 30% rate on any dividends received in respect of our stock, or at a lower rate provided by an applicable income tax treaty. If the amount of a distribution exceeds our current and accumulated earnings and profits, such excess first will be treated as a tax-free return of capital to the extent
95
Table of Contents
of theNon-U.S. Holder’s tax basis in our common stock (with a corresponding reduction in suchNon-U.S. Holder’s tax basis in our common stock), and thereafter will be treated as gain realized on the sale or other disposition of the common stock and will be treated as described under “— Sale or Other Disposition of Our Common Stock” below. In order to obtain a reduced rate of United States federal withholding tax under an applicable income tax treaty, aNon-U.S. Holder, who is otherwise entitled to benefits under an income tax treaty, will be required to provide a properly executed IRSForm W-8BEN certifying under penalties of perjury its entitlement to benefits under the treaty. Special certification requirements and other requirements apply to certainNon-U.S. Holders that are entities rather than individuals.
If you are eligible for a reduced rate of United States withholding tax pursuant to an applicable income tax treaty, you may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim for refund with the United States Internal Revenue Service, or IRS. ANon-U.S. Holder should consult its own tax advisor regarding its possible entitlement to benefits under an income tax treaty and the filing of a United States tax return for claiming a refund of United States federal withholding tax.
The United States federal withholding tax does not apply to dividends that are U.S. trade or business income, as defined above, of aNon-U.S. Holder who provides a properly executed IRSForm W-8ECI, certifying that the dividends are effectively connected with theNon-U.S. Holder’s conduct of a trade or business within the United States.
Sale or Other Disposition of Our Common Stock
Any gain that aNon-U.S. Holder realizes upon the sale or other disposition of a share of common stock generally will not be subject to United States federal income or withholding tax unless:
| • | The gain is U.S. trade or business income, as defined and discussed above; | |
| • | TheNon-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of the disposition, and certain other conditions are met; or | |
| • | Our common stock constitutes a “United States Real Property interest” by reason of our status as a United States real property holding corporation, or a USRPHC, under Section 897 of the Code at any time during the shorter of the five-year period ending on the date of disposition and theNon-U.S. Holder’s holding period for our common stock. |
In general, a corporation is a USRPHC if the fair market value of its “United States real property interests” (as defined in the Code and applicable United States Treasury regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests and its other assets used or held for use in a trade or business. If we are determined to be a USRPHC, the United States federal income and withholding taxes relating to interests in USRPHCs nevertheless will not apply to gains derived from the sale or other disposition of our common stock by aNon-U.S. Holder whose shareholdings, actual and constructive, at all times during the applicable period, amount to 5% or less of our common stock, provided that our common stock is regularly traded on an established securities market. We do not believe we currently are, and do not anticipate becoming, a USRPHC. However, no assurance can be given that we will not be a USRPHC, or that our common stock will be considered regularly traded, when aNon-U.S. Holder sells its shares of our common stock.
Gain described in the second bullet point above will be subject to United States federal income tax at a flat 30% rate (or such lower rate as may be specified by an applicable income tax treaty), but may be offset by United States source capital losses (even though the individual is not considered a resident of the United States).
U.S. Federal Estate Tax
If you are an individualNon-U.S. Holder, common stock that you hold at the time of death will be included in your gross estate for United States federal estate tax purposes, and may be subject to United States federal estate tax, unless an applicable estate tax treaty provides otherwise.
96
Table of Contents
Information Reporting and Backup Withholding
We must report annually to the IRS and to you the amount of dividends paid to you on your shares of common stock and the tax withheld with respect to such dividends, regardless of whether withholding was required. Copies of the information returns reporting such dividends and withholding may also be made available to the tax authorities in the country in which you reside under the provisions of an applicable income tax treaty or exchange of information treaty.
The United States imposes a backup withholding tax on dividends and certain other types of payments to U.S. persons. The backup withholding rate is currently 28%. You will not be subject to backup withholding on dividends you receive on your shares of common stock if you provide proper certification (usually on an IRSForm W-8BEN) of your status of aNon-U.S. Holder or otherwise establishes an exemption, provided that the payor does not have actual knowledge or reason to know that you are a U.S. person or that the conditions of any other exemption are not, in fact, satisfied.
Information reporting and, depending on the circumstances, backup withholding, generally will apply to the proceeds of a sale of common stock within the United States or conducted through the United States office of any broker, United States or foreign, unless you certify under penalties of perjury that you are aNon-U.S. Holder or otherwise establish an exemption, provided that the broker does not have actual knowledge or reason to know that you are a U.S. person or that the conditions of any other exemption are not, in fact, satisfied. The payment of the proceeds from the disposition of our common stock to or through anon-U.S. office of anon-U.S. broker generally will not be subject to information reporting or backup withholding unless thenon-U.S. broker has certain types of relationships with the United States, or a U.S. related person. In the case of the payment of the proceeds from the disposition of our common stock to or through anon-U.S. office of a broker that is either a U.S. person or a U.S. related person, the United States Treasury regulations generally require information reporting (but not backup withholding) on the payment unless the broker has documentary evidence that the holder is aNon-U.S. Holder and the broker has no knowledge to the contrary.Non-U.S. Holders should consult their own tax advisors on the application of information reporting and backup withholding to them in their particular circumstances (including upon their disposition of our common stock).
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to aNon-U.S. Holder may be allowed as a refund or a credit against your United States federal income tax liability, if any, provided that you furnish the required information to the IRS in a timely manner.
97
Table of Contents
Lehman Brothers Inc. and Piper Jaffray & Co. are acting as representatives of the underwriters and joint book-running managers of this offering. Under the terms of an underwriting agreement, which will be filed as an exhibit to the registration statement, each of the underwriters named below has severally agreed to purchase from us the respective number of shares of common stock shown opposite its name:
| Number of | ||||
Underwriters | Shares | |||
| Lehman Brothers Inc. | ||||
| Piper Jaffray & Co. | ||||
| Cowen and Company, LLC | ||||
| Wachovia Capital Markets, LLC | ||||
| Total | 5,500,000 | |||
The underwriting agreement provides that the underwriters’ obligation to purchase shares of common stock depends on the satisfaction of the conditions contained in the underwriting agreement including:
| • | the obligation to purchase all of the shares of common stock offered hereby other than those shares of common stock covered by their option to purchase additional shares as described below, if any of the shares are purchased; | |
| • | the representations and warranties made by us to the underwriters are true; | |
| • | there is no material change in our business or the financial markets; and | |
| • | we deliver customary closing documents to the underwriters. |
Commissions and Expenses
The following table summarizes the underwriting discounts and commissions we will pay to the underwriters. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares. The underwriting fee is the difference between the initial price to the public and the amount the underwriters pay to us for the shares.
| No Exercise | Full Exercise | |||||||
| Per Share | $ | $ | ||||||
| Total | $ | $ | ||||||
The representatives of the underwriters have advised us that the underwriters propose to offer the shares of common stock directly to the public at the public offering price on the cover of this prospectus and to selected dealers, which may include the underwriters, at such offering price less a selling concession not in excess of $ per share. After the offering, the representatives may change the offering price and other selling terms.
The expenses of the offering that are payable by us are estimated to be $2,000,000, excluding underwriting discounts and commissions.
Option to Purchase Additional Shares
We have granted the underwriters an option exercisable for 30 days after the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of 825,000 shares at the public offering price less underwriting discounts and commissions. This option may be exercised to the extent that the underwriters sell more than 5,500,000 shares in connection with this offering. To the extent that this option is exercised, each underwriter will be obligated, subject to certain conditions, to purchase its pro rata portion of these additional shares based on the underwriter’s underwriting commitment in the offering as indicated in the table at the beginning of this Underwriting Section.
98
Table of Contents
Lock-Up Agreements
We, all of our directors and executive officers and substantially all of the holders of our outstanding stock have agreed that, subject to customary exceptions, without the prior written consent of each of Lehman Brothers Inc. and Piper Jaffray & Co., we will not directly or indirectly, (1) offer for sale, sell, pledge, or otherwise dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the disposition by any person at any time in the future of) any shares of common stock (including, without limitation, shares of common stock that may be deemed to be beneficially owned by us or them in accordance with the rules and regulations of the Securities and Exchange Commission and shares of common stock that may be issued upon exercise of any options or warrants) or securities convertible into or exercisable or exchangeable for common stock, (2) enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock, (3) make any demand for or exercise any right or file or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible, exercisable or exchangeable into common stock or any of our other securities, or (4) publicly disclose the intention to do any of the foregoing for a period of 180 days after the date of this prospectus.
The180-day restricted period described in the preceding paragraph will be extended if:
| • | during the last 17 days of the180-day restricted period we issue an earnings release or material news or a material event relating to us occurs; or | |
| • | prior to the expiration of the180-day restricted period, we announce that we will release earnings results during the16-day period beginning on the last day of the180-day period, |
in which case the restrictions described in the preceding paragraph will continue to apply until the expiration of the18-day period beginning on the issuance of the earnings release or the announcement of the material news or occurrence of a material event, unless such extension is waived in writing by Lehman Brothers Inc. and Piper Jaffray & Co.
Lehman Brothers Inc. and Piper Jaffray & Co., in their sole discretion, may release the common stock and other securities subject to thelock-up agreements described above in whole or in part at any time with or without notice. When determining whether or not to release common stock and other securities fromlock-up agreements, Lehman Brothers Inc. and Piper Jaffray & Co. will consider, among other factors, the holder’s reasons for requesting the release, the number of shares of common stock and other securities for which the release is being requested and market conditions at the time.
Offering Price Determination
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be negotiated between the underwriters and us. In determining the initial public offering price of our common stock, we and the underwriters will consider:
| • | the history and prospects for the industry in which we compete; | |
| • | our financial information; | |
| • | the ability of our management and our business potential and earning prospects; | |
| • | the prevailing securities markets at the time of this offering; and | |
| • | the recent market prices of, and the demand for, publicly traded shares of generally comparable companies. |
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
99
Table of Contents
Stabilization, Short Positions and Penalty Bids
The representatives may engage in stabilizing transactions, short sales and purchases to cover positions created by short sales, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act:
| • | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. | |
| • | A short position involves a sale by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase in the offering, which creates the syndicate short position. This short position may be either a covered short position or a naked short position. In a covered short position, the number of shares involved in the sales made by the underwriters in excess of the number of shares they are obligated to purchase is not greater than the number of shares that they may purchase by exercising their option to purchase additional shares. In a naked short position, the number of shares involved is greater than the number of shares in their option to purchase additional shares. The underwriters may close out any short position by either exercising their option to purchase additional sharesand/or purchasing shares in the open market. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through their option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. | |
| • | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. | |
| • | Penalty bids permit the representatives to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on The Nasdaq Global Market or otherwise and, if commenced, may be discontinued at any time.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the common stock. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Electronic Distribution
A prospectus in electronic format may be made available on the Internet sites or through other online services maintained by one or more of the underwritersand/or selling group members participating in this offering, or by their affiliates. In those cases, prospective investors may view offering terms online and, depending upon the particular underwriter or selling group member, prospective investors may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares for sale to online brokerage account holders.
Any such allocation for online distributions will be made by the representatives on the same basis as other allocations.
Other than the prospectus in electronic format, the information on any underwriter’s or selling group member’s web site and any information contained in any other web site maintained by an underwriter or selling group member is not part of the prospectus or the registration statement of which this prospectus forms
100
Table of Contents
a part, has not been approvedand/or endorsed by us or any underwriter or selling group member in its capacity as underwriter or selling group member and should not be relied upon by investors.
Nasdaq Global Market
We have applied to list our shares of common stock for quotation on The Nasdaq Global Market under the symbol “TSON”.
Discretionary Sales
The underwriters have informed us that they do not intend to confirm sales to discretionary accounts that exceed 5% of the total number of shares offered by them.
Stamp Taxes
If you purchase shares of common stock offered in this prospectus, you may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase, in addition to the offering price listed on the cover page of this prospectus.
Relationships
From time to time in the ordinary course of their respective businesses, certain of the underwriters and their affiliates may in the future engage in commercial banking or investment banking transactions with us and our affiliates.
Notice to Prospective Investors in the European Economic Area
In relation to each member state of the European Economic Area that has implemented the Prospectus Directive (each, a relevant member state), with effect from and including the date on which the Prospectus Directive is implemented in that relevant member state (the relevant implementation date), an offer of the shares of common stock described in this prospectus may not be made to the public in that relevant member state prior to the publication of a prospectus in relation to the shares of common stock that has been approved by the competent authority in that relevant member state or, where appropriate, approved in another relevant member state and notified to the competent authority in that relevant member state, all in accordance with the Prospectus Directive, except that, with effect from and including the relevant implementation date, an offer of securities may be offered to the public in that relevant member state at any time:
| • | to any legal entity that is authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; or | |
| • | to any legal entity that has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; or | |
| • | in any other circumstances that do not require the publication of a prospectus pursuant to Article 3 of the Prospectus Directive. |
Each purchaser of the shares of common stock described in this prospectus located within a relevant member state will be deemed to have represented, acknowledged and agreed that it is a “qualified investor” within the meaning of Article 2(1)(e) of the Prospectus Directive.
For purposes of this provision, the expression an “offer to the public” in any relevant member state means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the expression may be varied in that member state by any measure implementing the Prospectus Directive in that member state, and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each relevant member state.
101
Table of Contents
The sellers of the shares of common stock have not authorized and do not authorize the making of any offer of the shares of common stock through any financial intermediary on their behalf, other than offers made by the underwriter with a view to the final placement of the shares of common stock as contemplated in this prospectus. Accordingly, no purchaser of the shares of common stock, other than the underwriter, is authorized to make any further offer of the shares of common stock on behalf of the sellers or the underwriter.
Notice to Prospective Investors in the United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive (“Qualified Investors”) that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (ii) high net worth entities, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a relevant person should not act or rely on this document or any of its contents.
102
Table of Contents
The validity of the issuance of the shares of common stock offered hereby will be passed upon for us by Stradling Yocca Carlson & Rauth, a Professional Corporation, Newport Beach, California. As of the date of this prospectus, certain attorneys of Stradling Yocca Carlson & Rauth hold an aggregate of 248,113 shares of our common stock, assuming the conversion of preferred stock to common stock immediately upon the closing of this offering. The underwriters are being represented by Latham & Watkins LLP, New York, New York and Costa Mesa, California.
The financial statements as of December 31, 2006 and 2005 and for each of the three years in the period ended December 31, 2006 included in this Prospectus have been so included in reliance on the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given the authority of said firm as experts in auditing and accounting.
We have filed a registration statement onForm S-1 under the Securities Act with the SEC with respect to the common stock offered by this prospectus. This prospectus does not include all of the information contained in the registration statement or the exhibits and schedules filed therewith. You should refer to the registration statement and its exhibits for additional information. Whenever we make reference in this prospectus to any of our contracts, agreements or other documents, the references are not necessarily complete and you should refer to the exhibits attached to the registration statement for copies of the actual contract, agreement or other document. When we complete this offering, we will also be required to file annual, quarterly and special reports, proxy statements and other information with the SEC.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website atwww.sec.gov. You can also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, NE, Washington, DC 20549. Please call the SEC at(800) SEC-0330 for further information on the operations of the public reference facilities.
Upon the completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act and, accordingly, will file annual reports containing financial statements audited by an independent public accounting firm, quarterly reports containing unaudited financial data, current reports, proxy statements and other information with the SEC. You will be able to inspect and copy such periodic reports, proxy statements, and other information at the SEC’s public reference room, and the website of the SEC referred to above.
103
| Page | ||
| F-2 | ||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 | ||
| F-7 |
F-1
Table of Contents
To the Board of Directors and Shareholders of TranS1 Inc.:
In our opinion, the accompanying balance sheets and the related statements of operations, of stockholder’s deficit, and of cash flows, present fairly, in all material respects, the financial position of TranS1 Inc. at December 31, 2006 and 2005, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2006 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2 and Note 7 to the financial statements, the Company changed the manner in which it accounts for share-based compensation in 2006.
/s/ PricewaterhouseCoopers LLP
Raleigh, NC
July 23, 2007, except for Note 9, as to which the date is
October 5, 2007
October 5, 2007
F-2
Table of Contents
TranS1 Inc.
| Pro Forma | ||||||||||||||||
| December 31, | June 30, | June 30, | ||||||||||||||
| 2005 | 2006 | 2007 | 2007 | |||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| (In thousands, except share amounts) | ||||||||||||||||
ASSETS | ||||||||||||||||
| Current assets: | ||||||||||||||||
| Cash and cash equivalents | $ | 20,544 | $ | 5,034 | $ | 5,537 | $ | 5,537 | ||||||||
| Short-term investments | 5,450 | 9,928 | 4,635 | 4,635 | ||||||||||||
| Accounts receivable | 564 | 1,620 | 3,248 | 3,248 | ||||||||||||
| Inventory | 584 | 2,080 | 3,055 | 3,055 | ||||||||||||
| Prepaid expenses and other assets | 131 | 230 | 484 | 484 | ||||||||||||
| Total current assets | 27,273 | 18,892 | 16,959 | 16,959 | ||||||||||||
| Property and equipment, net | 740 | 1,112 | 1,234 | 1,234 | ||||||||||||
| Total assets | $ | 28,013 | $ | 20,004 | $ | 18,193 | $ | 18,193 | ||||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||||||||||
| Current liabilities: | ||||||||||||||||
| Accounts payable | $ | 335 | $ | 843 | $ | 1,023 | $ | 1,023 | ||||||||
| Accrued expenses | 243 | 601 | 1,630 | 1,630 | ||||||||||||
| Total current liabilities | 578 | 1,444 | 2,653 | 2,653 | ||||||||||||
| Preferred Stock: | ||||||||||||||||
| Series A convertible preferred stock, $0.0001 par value; 1,125,000 shares authorized, issued and outstanding at December 31, 2005 and 2006 and June 30, 2007 (unaudited) | 956 | 956 | 956 | — | ||||||||||||
| Series AA convertible preferred stock, $0.0001 par value; 1,260,000 shares authorized, issued and outstanding at December 31, 2005 and 2006 and June 30, 2007 (unaudited) | 1,694 | 1,694 | 1,694 | — | ||||||||||||
| Series B convertible preferred stock, $0.0001 par value; 4,909,091 shares authorized, issued and outstanding at December 31, 2005 and 2006 and June 30, 2007 (unaudited) | 11,894 | 11,894 | 11,894 | — | ||||||||||||
| Series C convertible preferred stock, $0.0001 par value; 3,499,091 shares authorized, issued and outstanding at December 31, 2005 and 2006 and June 30, 2007 (unaudited) | 25,545 | 25,545 | 25,545 | — | ||||||||||||
| Commitments (Note 5) | ||||||||||||||||
| Stockholders’ equity (deficit): | ||||||||||||||||
| Common stock, $0.0001 par value; 13,500,000, 17,100,000 and 17,100,000 shares authorized at December 31, 2005 and 2006 and June 30, 2007 (unaudited), respectively; 2,411,775, 2,435,486 and 2,483,320 shares issued and outstanding at December 31, 2005 and 2006 and June 30, 2007 (unaudited), respectively; 13,276,502 shares issued and outstanding, pro forma at June 30, 2007 (unaudited) | — | — | — | 1 | ||||||||||||
| Additional paid-in capital | 219 | 820 | 1,889 | 41,977 | ||||||||||||
| Notes receivable for common stock | (41 | ) | (38 | ) | — | — | ||||||||||
| Accumulated deficit | (12,832 | ) | (22,311 | ) | (26,438 | ) | (26,438 | ) | ||||||||
| Total stockholders’ equity (deficit) | (12,654 | ) | (21,529 | ) | (24,549 | ) | 15,540 | |||||||||
| Total liabilities and stockholders’ equity (deficit) | $ | 28,013 | $ | 20,004 | $ | 18,193 | $ | 18,193 | ||||||||
The accompanying notes are an integral part of these financial statements.
F-3
Table of Contents
TranS1 Inc.
| Six Months Ended | ||||||||||||||||||||
| Years Ended December 31, | June 30, | |||||||||||||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||
| (In thousands, except share amounts) | ||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Revenue | $ | — | $ | 1,469 | $ | 5,812 | $ | 2,161 | $ | 7,187 | ||||||||||
| Cost of revenue | — | 386 | 1,580 | 618 | 1,459 | |||||||||||||||
| Gross profit | — | 1,083 | 4,232 | 1,543 | 5,728 | |||||||||||||||
Operating expenses | ||||||||||||||||||||
| Research and development | 1,758 | 2,444 | 4,246 | 2,094 | 2,278 | |||||||||||||||
| Sales and marketing | 746 | 2,635 | 9,288 | 3,950 | 6,837 | |||||||||||||||
| General and administrative | 1,117 | 1,293 | 1,166 | 625 | 1,083 | |||||||||||||||
| Total operating expenses | 3,621 | 6,372 | 14,700 | 6,669 | 10,198 | |||||||||||||||
| Operating loss | (3,621 | ) | (5,289 | ) | (10,468 | ) | (5,126 | ) | (4,470 | ) | ||||||||||
| Interest income | 150 | 264 | 858 | 528 | 343 | |||||||||||||||
| Other income (expense) | — | (17 | ) | 131 | — | — | ||||||||||||||
| Net loss | $ | (3,471 | ) | $ | (5,042 | ) | $ | (9,479 | ) | $ | (4,598 | ) | $ | (4,127 | ) | |||||
| Net loss per common share — basic and diluted | $ | (1.63 | ) | $ | (2.25 | ) | $ | (3.91 | ) | $ | (1.90 | ) | $ | (1.67 | ) | |||||
| Weighted average common shares outstanding — basic and diluted | 2,127,856 | 2,243,019 | 2,423,226 | 2,418,154 | 2,467,550 | |||||||||||||||
| Pro forma net loss per common share — basic and diluted (unaudited) | $ | (0.72 | ) | $ | (0.31 | ) | ||||||||||||||
| Pro forma weighted average shares outstanding — basic and diluted (unaudited) | 13,216,407 | 13,260,732 | ||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
TranS1 Inc.
| Additional | Total | |||||||||||||||||||||||
| Common Stock | Paid - In | Notes | Accumulated | Stockholders’ | ||||||||||||||||||||
| Number | Amount | Capital | Receivable | Deficit | Deficit | |||||||||||||||||||
| (In thousands, except share amounts) | ||||||||||||||||||||||||
Balance at December 31, 2003 | 2,090,435 | $ | — | $ | 142 | $ | (38 | ) | $ | (4,319 | ) | $ | (4,215 | ) | ||||||||||
| Issuance of common stock from exercised options | 57,600 | — | 6 | 6 | ||||||||||||||||||||
| Net loss | (3,471 | ) | (3,471 | ) | ||||||||||||||||||||
Balance at December 31, 2004 | 2,148,035 | — | 148 | (38 | ) | (7,790 | ) | (7,680 | ) | |||||||||||||||
| Issuance of common stock from exercised options | 263,740 | — | 71 | (3 | ) | 68 | ||||||||||||||||||
| Net loss | (5,042 | ) | (5,042 | ) | ||||||||||||||||||||
Balance at December 31, 2005 | 2,411,775 | — | 219 | (41 | ) | (12,832 | ) | (12,654 | ) | |||||||||||||||
| Issuance of common stock from exercised options | 23,711 | — | 2 | 3 | 5 | |||||||||||||||||||
| Stock based compensation | 599 | 599 | ||||||||||||||||||||||
| Net loss | (9,479 | ) | (9,479 | ) | ||||||||||||||||||||
Balance at December 31, 2006 | 2,435,486 | — | 820 | (38 | ) | (22,311 | ) | (21,529 | ) | |||||||||||||||
| Issuance of common stock from exercised options (unaudited) | 47,834 | 11 | 11 | |||||||||||||||||||||
| Stock based compensation (unaudited) | 1,058 | 1,058 | ||||||||||||||||||||||
| Repayment of note receivable (unaudited) | 38 | 38 | ||||||||||||||||||||||
| Net loss (unaudited) | (4,127 | ) | (4,127 | ) | ||||||||||||||||||||
Balance at June 30, 2007 (unaudited) | 2,483,320 | $ | — | $ | 1,889 | $ | — | $ | (26,438 | ) | $ | (24,549 | ) | |||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
Table of Contents
TranS1 Inc.
| Six Months Ended | ||||||||||||||||||||
| Years Ended December 31, | June 30, | |||||||||||||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
Cash flows from operating activities: | ||||||||||||||||||||
| Net loss | $ | (3,471 | ) | $ | (5,042 | ) | $ | (9,479 | ) | $ | (4,598 | ) | $ | (4,127 | ) | |||||
| Adjustments to reconcile net loss to net cash used in operating activities | ||||||||||||||||||||
| Depreciation | 52 | 106 | 237 | 83 | 268 | |||||||||||||||
| Stock-based compensation | — | — | 599 | — | 1,058 | |||||||||||||||
| Disposal of fixed assets | — | 17 | — | — | — | |||||||||||||||
| Changes in operating assets and liabilities | ||||||||||||||||||||
| Increase in accounts receivable | — | (564 | ) | (1,056 | ) | (444 | ) | (1,628 | ) | |||||||||||
| (Increase) decrease in prepaid expenses | (13 | ) | (76 | ) | (99 | ) | 42 | (254 | ) | |||||||||||
| Increase in inventory | (185 | ) | (400 | ) | (1,496 | ) | (848 | ) | (975 | ) | ||||||||||
| Increase (decrease) in accounts payable | 40 | 280 | 508 | 160 | 180 | |||||||||||||||
| Increase in accrued liabilities | 102 | 23 | 358 | 228 | 1,030 | |||||||||||||||
| Net cash used in operating activities | (3,475 | ) | (5,656 | ) | (10,428 | ) | (5,377 | ) | (4,448 | ) | ||||||||||
Cash flows from investing activities: | ||||||||||||||||||||
| Purchase of property and equipment | (106 | ) | (672 | ) | (609 | ) | (273 | ) | (390 | ) | ||||||||||
| Purchases of short-term investments | — | (5,449 | ) | (13,925 | ) | (41,128 | ) | (10,716 | ) | |||||||||||
| Sales of short-term investments | — | — | 9,447 | 41,347 | 16,009 | |||||||||||||||
| Net cash provided by (used in) investing activities | (106 | ) | (6,121 | ) | (5,087 | ) | (54 | ) | 4,903 | |||||||||||
Cash flows from financing activities: | ||||||||||||||||||||
| Proceeds from issuance of convertible preferred stock, net | — | 25,545 | — | — | — | |||||||||||||||
| Proceeds from issuance of common stock | 6 | 68 | 5 | — | 48 | |||||||||||||||
| Net cash provided by financing activities | 6 | 25,613 | 5 | — | 48 | |||||||||||||||
| Net increase (decrease) in cash and cash equivalents | (3,575 | ) | 13,836 | (15,510 | ) | (5,431 | ) | 503 | ||||||||||||
| Cash and cash equivalents, beginning of period | 10,283 | 6,708 | 20,544 | 20,544 | 5,034 | |||||||||||||||
| Cash and cash equivalents, end of period | $ | 6,708 | $ | 20,544 | $ | 5,034 | $ | 15,113 | $ | 5,537 | ||||||||||
The accompanying notes are an integral part of these financial statements.
F-6
Table of Contents
TranS1 Inc.
| 1. | Organization |
TranS1 Inc., a Delaware corporation (the “Company”), was incorporated on June 28, 2000 and is headquartered in Wilmington, North Carolina. The Company is a medical device company focused on designing, developing and marketing products that implement its minimally invasive surgical approach to treat degenerative disc disease affecting the lower lumbar region of the spine. The Company has developed and currently markets in the United States and Europe two single-level fusion products, AxiaLIF and AxiaLIF 360°. In addition, the Company has developed and currently markets in Europe a two-level fusion product, AxiaLIF 2L, which has not yet been approved in the United States. All of the Company’s products are delivered using its TranS1 approach. The AxiaLIF product was commercially released in January 2005 the AxiaLIF 360° product was commercially released in July 2006 and the AxiaLIF 2L product was commercially released in Europe in the fourth quarter of 2006. The Company generates revenue from the sale of implants and procedure kits. The Company sells its products directly to hospitals and surgical centers in the United States and independent distributors in Europe.
The Company is subject to a number of risks similar to other companies in the medical device industry. These risks include rapid technological change, uncertainty of market acceptance of our products, uncertainty of regulatory approval, competition from substitute products and larger companies, the need to obtain additional financing, compliance with government regulation, protection of proprietary technology, product liability, and the dependence on key individuals.
| 2. | Summary of Significant Accounting Policies |
Unaudited Interim Financial Information
The consolidated financial statements as of June 30, 2007 and for the six-month periods ended June 30, 2006 and 2007 are unaudited and reflect all adjustments (consisting of normal recurring adjustments) which are, in the opinion of the Company’s management, necessary for a fair presentation of financial position, results of operations and cash flows. All financial statement disclosures related to the six-month periods ended June 30, 2006 and 2007 are unaudited.
Basis of Presentation
The Company has prepared the accompanying financial statements in conformity with accounting principles generally accepted in the United States of America. The Company’s fiscal year ends on December 31.
Pro Forma Balance Sheet (Unaudited)
The Company’s pro forma balance sheet as of June 30, 2007 gives effect to the automatic conversion of all Series A, AA, B and C convertible preferred stock into an aggregate of 10,793,182 shares of common stock upon consummation of the Company’s initial public offering.
Use of Estimates
The financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. These principles require management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. The principal estimates relate specifically to inventory reserves, stock-based compensation and accrued expenses.
F-7
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents.
Fair Value of Financial Instruments
The carrying values of cash and cash equivalents, short-term investments, accounts receivable, and accounts payable at December 31, 2006 and 2005 and June 30, 2007 approximated their fair values due to the short-term nature of these items.
Accounts Receivable
Accounts receivable are presented net of an allowance for uncollectible accounts which is not significant at December 31, 2005 and 2006 and June 30, 2006 and 2007. Estimates on the collectibility of customer accounts are based primarily on analysis of historical trends and experience and changes in customers’ financial condition.
Concentration of Credit Risk and Significant Customers
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents.
The Company places cash deposits with a federally insured financial institution, in amounts which at times exceed the federally insured limit of $100,000. The total amount of deposits in excess of federally insured limits was $14,862,000 and $25,894,000 at December 31, 2006 and 2005, respectively.
In 2005, two customers, Christ Hospital in Cincinnati, Ohio, and Faith Regional Health Services in Norfolk, Nebraska, accounted for 16% and 11%, respectively, of revenues. In 2006, one customer, Christ Hospital, accounted for 11% of revenues. During the six months ended June 30, 2006, one customer, Christ Hospital, accounted for 14%, of revenues. As of December 31, 2005, two customers, Faith Regional Health Services and Christ Hospital, accounted for 26% and 15%, respectively, of the accounts receivable balance. As of December 31, 2006, one customer, University Medical Center in Las Vegas, Nevada, accounted for 12% of the accounts receivable balance. As of June 30, 2007, two customers, University Medical Center and Christ Hospital, accounted for 14% and 12%, respectively, of the accounts receivable balance.
Inventories
Inventories consist of the following (in thousands):
| December 31, | June 30, | |||||||||||
| 2005 | 2006 | 2007 | ||||||||||
| (Unaudited) | ||||||||||||
| Raw materials | $ | 361 | $ | 1,261 | $ | 1,877 | ||||||
| Finished goods | 223 | 819 | 1,178 | |||||||||
| $ | 584 | $ | 2,080 | $ | 3,055 | |||||||
Inventories are stated at the lower of cost or market, computed on a standard cost basis, which approximates actual cost on afirst-in, first-out basis and market being determined as the lower of replacement cost or net realizable value. Costs are monitored on an annual basis and updated as necessary to reflect changes in supplier costs and the rate of overhead absorption is adjusted based on projections of manufacturing department costs and production plan. Inventory reserves are established when conditions indicate that the
F-8
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
selling price could be less than cost due to obsolescence, usage, or the Company determines that it holds excessive levels of inventory based on market demand.
Property and Equipment
Property and equipment are recorded at cost and depreciated over their estimated useful lives using the straight-line method. Property and equipment held under capital leases and leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the related asset. Maintenance and repairs, which neither materially add to the value of the property nor appreciably prolong its life, are charged to expense as incurred. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation and amortization are removed from the accounts and any resulting gain or loss is credited or charged to income.
The estimated useful lives are as follows:
| Furniture and fixtures | 5-10 years | |||
| Equipment | 3-5 years | |||
| Other depreciable assets | 2-10 years | |||
| Leasehold improvements | Lesser of estimated useful life or lease term |
Revenue Recognition
Revenue is recognized in accordance with Staff Accounting Bulletin (“SAB”) No. 101 as amended by SAB No. 104. The Company recognizes revenue based on the following criteria: (i) evidence that an arrangement exists with the customer; (ii) the delivery of the productsand/or services has occurred; (iii) the selling price has been established for the products or services delivered; and (iv) the collection is reasonably assured based on these criteria or historical practices. Revenue is generated from the sale of implants and procedure kits, which consists of disposable instruments. The Company has two distinct sale methods. The first method is when procedure kits are sold directly to hospitals or surgical centers. The Company’s sales representatives or independent sales agents hand deliver the procedure kit to the customer on the day of the surgery or several days prior to the surgery. The sales representative or independent agent is then responsible for reporting the delivery of the procedure kit, and the date of the operation to the corporate office for proper revenue recognition. The Company recognizes revenue upon the confirmation that the procedure kit has been used in a surgical procedure. The other sales method is for sales to European distributors. These distributors order multiple procedure kits at one time to have on hand. These transactions require the customer to send in a purchase order before shipment will be made to the customer. The Company determines revenue recognition on a case by case basis dependent upon the terms and conditions of each individual distributor agreement. Under the distributor agreements currently in place, a distributor only has the right of return for defective products and, accordingly, revenue is recognized upon shipment.
During 2006, the Company had $5,453,000 and $359,000 of domestic and foreign sales, respectively. During the six months ended June 30, 2006, the Company had $2,143,000 and $18,000 of domestic and foreign sales, respectively. During the six months ended June 30, 2007, the Company had $6,641,000 and $546,000 of domestic and foreign sales, respectively. There were no foreign sales during 2005 or 2004.
Shipping and Handing Costs
Shipping and handling costs are expensed as incurred and are included in the cost of revenue. These costs are not reimbursed by the Company’s customers.
F-9
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
Sales and Marketing Expenses
Sales and marketing expenses consist of personnel costs, including stock-based compensation expense in 2006 and 2007, sales commissions paid to the Company’s direct sales representatives and independent sales agents, and costs associated with physician training programs, promotional activities, and participation in medical conferences. All costs of advertising and promotional activities are expensed as incurred.
Research and Development Expenses
Research and development expenses consist primarily of personnel costs, including stock-based compensation expense in 2006 and 2007, within the Company’s product development, regulatory and clinical functions and the costs of clinical studies and product development projects. Research and development expenses also include legal expenses related to the development and protection of the Company’s intellectual property portfolio and facilities-related costs. Research and development expenses are expensed as incurred.
Patent Costs
Costs associated with the submission of a patent application are expensed as incurred given the uncertainty of the patents resulting in probable future economic benefits to the Company.
Income Taxes
The Company accounts for income taxes using the liability method which requires the recognition of deferred tax assets or liabilities for the temporary differences between financial reporting and tax bases of the Company’s assets and liabilities and for tax carryforwards at enacted statutory tax rates in effect for the years in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in the period that includes the enactment date. In addition, valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
Stock-Based Compensation
Prior to January 1, 2006, the Company accounted for stock-based employee compensation arrangements in accordance with the provisions of Accounting Principles Board Opinion No. 25 (APB 25),Accounting for Stock Issued to Employees, and related interpretations, and followed the disclosure provisions of Statement of Financial Accounting Standards No. 123 (SFAS 123),Accounting for Stock-Based Compensation. Under APB 25, compensation expense is based on the difference, if any, on the date of the grant, between the fair value of the Company’s stock and the exercise price. Employee stock-based compensation determined under APB 25 is recognized over the option vesting period.
The weighted-average fair value of options granted to employees was $0.18 and $0.17 for the years ended December 31, 2005 and 2004.
Effective January 1, 2006, the Company adopted the fair value provisions of Statement of Financial Accounting Standards No. 123R (SFAS 123R),Share-Based Payment, which supersedes its previous accounting under APB 25. SFAS 123R requires the recognition of compensation expense, using a fair-value-based method, for costs related to all share-based payments including stock options. SFAS 123R requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. The Company adopted SFAS 123R using the prospective transition method, which requires that for nonpublic entities that used the minimum value method for either pro forma or financial statements recognition purposes, SFAS 123R shall be applied to options grants or modifications to existing options after the required effective date. For options granted prior to the new SFAS 123R effective date and for which the requisite service period has not been performed as of January 1, 2006, the Company will continue to apply the intrinsic value
F-10
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
provisions of APB 25 on the remaining unvested awards. All option grants valued after January 1, 2006 will be expensed on a straight-line basis over the vesting period.
The Company accounts for stock-based compensation arrangements with nonemployees in accordance with the Emerging Issues Task Force Abstract No.96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling Goods or Services. The Company records the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes pricing model. The value of the equity instruments is charged to earnings over the term of the service agreement.
Net Loss Per Common Share
Historical
Basic net loss per common share (“Basic EPS”) is computed by dividing net loss available to common stockholders by the weighted average number of common shares outstanding. Diluted net loss available to common stockholders per common share (“Diluted EPS”) is computed by dividing net loss available to common stockholders by the weighted average number of common shares and dilutive potential common share equivalents then outstanding. The Company’s potential dilutive common shares, which consist of shares issuable upon the exercise of stock options and conversion of convertible preferred stock, have not been included in the computation of diluted net loss per share for all periods as the result would be anti-dilutive.
The following table sets forth the potential shares of common stock that are not included in the calculation of diluted net loss per share as the result would be anti-dilutive as of the end of each period presented:
| December 31, | June 30, | |||||||||||||||||||
| 2004 | 2005 | 2006 | 2006 | 2007 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| Convertible preferred stock | 7,294,091 | 10,793,182 | 10,793,182 | 10,793,182 | 10,793,182 | |||||||||||||||
| Stock options outstanding | 1,033,226 | 1,199,381 | 1,841,675 | 1,190,381 | 2,339,663 | |||||||||||||||
Pro Forma (Unaudited)
Pro forma net loss per common share is calculated assuming the conversion of all outstanding shares of Series A preferred stock, Series AA preferred stock, Series B preferred stock and Series C preferred stock into common stock upon the effectiveness of the Company’s initial public offering (see Note 6).
Segment Reporting
The Company has adopted SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information”. SFAS No. 131 establishes standards for the reporting by business enterprises of information about operating segments, products and services, geographic areas, and major customers. The Company has determined that it did not have any separately reportable segments as of December 31, 2005 or 2006 or June 30, 2007.
Recently Issued Accounting Standards
In July 2006, the Financial Accounting Standards Board (FASB) issued FASB Interpretation No. 48 (FIN 48),Accounting for Uncertainty in Income Taxes — an interpretation of FASB Statement No. 109, which clarifies the accounting for uncertainty in tax positions. FIN 48 requires that the Company recognize in its financial statements the impact of a tax position if that position is more likely than not of being sustained on audit, based on the technical merits of the position. The provisions of FIN 48 are effective as of the beginning of the Company’s 2007 fiscal year, with the cumulative effect, if any, of the change in accounting principle
F-11
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
recorded as an adjustment to opening retained earnings. The adoption of FIN 48 had no impact on the Company’s financial statements. However, at the adoption date of January 1, 2007, the Company had $1,043,000 of unrecognized deferred tax benefits, all of which was subject to a full valuation allowance, which effectively reduced the deferred tax benefits to $0, since realization of these benefits could not be reasonably assured.
In September 2006, the FASB issued Statement of Financial Accounting Standards No. 157 (SFAS 157),Fair Value Measurements, which defines fair value, establishes guidelines for measuring fair value and expands disclosures regarding fair value measurements. SFAS 157 does not require any new fair value measurements but rather eliminates inconsistencies in guidance found in various prior accounting pronouncements. SFAS 157 is effective for fiscal years beginning after November 15, 2007. Earlier adoption is permitted, provided the company has not yet issued financial statements, including for interim periods, for that fiscal year. The Company is currently evaluating the impact of SFAS 157.
In September 2006, the United Stated Securities and Exchange Commission issued Staff Accounting Bulletin No. 108 (SAB 108),Considering the Effects of Prior Year Misstatements When Quantifying Misstatements in Current Year Financial Statements, which provides interpretive guidance on how registrants should quantify financial statement misstatements. Under SAB 108, registrants are required to consider both a “rollover” method which focuses primarily on the income statement impact of misstatements and the “iron curtain” method which focuses primarily on the balance sheet impact of misstatements. SAB 108 was effective for 2006 and had no impact on the Company’s financial statements.
In February 2007, the FASB issued Statement of Financial Accounting Standards No. 159 (SFAS 159),The Fair Value Option for Financial Assets and Financial Liabilities — Including an amendment of FASB Statement No. 115. SFAS 159 permits entities to choose to measure many financial instruments and certain other items at fair value. The amendment to SFAS 115 applies to all entities with investments in available-for-sale or trading securities. The statement is effective for fiscal years beginning after November 15, 2007. The Company has not yet determined the effect SFAS 159 will have on its financial statements.
| 3. | Property and Equipment |
Property and equipment consist of the following:
| December 31, | June 30, | |||||||||||
| 2005 | 2006 | 2007 | ||||||||||
| (In thousands) | ||||||||||||
| (unaudited) | ||||||||||||
| Furniture and fixtures | $ | 110 | $ | 115 | $ | 115 | ||||||
| Equipment | 349 | 682 | 1,037 | |||||||||
| Computer software | 45 | 158 | 276 | |||||||||
| Leasehold improvements | 299 | 314 | 316 | |||||||||
| Tools | 73 | 73 | 73 | |||||||||
| Construction in process | — | 143 | 58 | |||||||||
| 876 | 1,485 | 1,875 | ||||||||||
| Less: accumulated depreciation and amortization | (136 | ) | (373 | ) | (641 | ) | ||||||
| $ | 740 | $ | 1,112 | $ | 1,234 | |||||||
Depreciation and amortization expense for the years ended December 31, 2006, 2005 and 2004 and the period ended June 30, 2007 (unaudited) was $237,000, $106,000, $52,000 and $268,000, respectively.
F-12
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
| 4. | Accrued Expenses |
Accrued expense consisted of the following:
| December 31, | June 30, | |||||||||||
| 2005 | 2006 | 2007 | ||||||||||
| (In thousands) | ||||||||||||
| (Unaudited) | ||||||||||||
| Bonus | $ | 71 | $ | 167 | $ | 250 | ||||||
| Commissions | 72 | 209 | 356 | |||||||||
| Vacation | 48 | 73 | 73 | |||||||||
| Legal and IPO related | — | 50 | 637 | |||||||||
| Clinical trial expenses | — | 69 | 50 | |||||||||
| Travel | 20 | — | 89 | |||||||||
| Other | 32 | 33 | 175 | |||||||||
| $ | 243 | $ | 601 | $ | 1,630 | |||||||
| 5. | Lease Obligations |
The Company rents office space under the terms of an operating lease, which expires in 2010.
Future minimum lease payments under operating lease obligations at December 31, 2006 are as follows:
Year ending December 31, | ||||
| (In thousands) | ||||
| 2007 | $ | 152 | ||
| 2008 | 157 | |||
| 2009 | 161 | |||
| 2010 | 93 | |||
| 2011 | — | |||
| $ | 563 | |||
Rent expense related to operating leases for the years ended December 31, 2006, 2005 and 2004 was $148,000, $87,000 and $50,000, respectively.
| 6. | Capital Stock |
At December 31, 2006, the Company has authorized 27,893,181 shares of capital stock of which 17,100,000 shares are designated common stock with a par value of $0.0001 and 10,793,181 shares are designated preferred stock with a par value of $0.0001, as amended on September 20, 2005. Of the 10,793,181 shares of preferred stock (“Preferred Stock”), 1,125,000 shares have been designated Series A Convertible Preferred Stock (“Series A”), 1,260,000 shares have been designated Series AA Convertible Preferred Stock (“Series AA”), 4,909,091 shares have been designated Series B Convertible Preferred Stock (“Series B”), and 3,499,091 shares have been designated Series C Convertible Preferred Stock (“Series C”).
Convertible Preferred Stock
On September 15, 2000, the Company issued 1,125,000 shares of Series A for proceeds of $956,000, net of issuance costs of $44,000. On March 14, 2002, the Company issued 1,260,000 shares of Series AA for proceeds of $1,694,000, net of issuance costs of $56,000. On April 17, 2003, the Company issued 4,909,091 shares of Series B for proceeds of $11,894,000, net of issuance costs of $105,000. On September 20, 2005, the Company issued 3,499,091 shares of Series C for proceeds of $25,545,000, net of issuance costs of $115,000.
F-13
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
The following is a summary of the rights, preferences and terms of the Company’s Series A, Series AA, Series B and Series C:
Voting
The holders of Preferred Stock are entitled to vote, together with the holders of common stock, on all matters submitted to stockholders for a vote. Each preferred stockholder is entitled to the number of votes equal to the number of shares of common stock into which each preferred share is convertible at the time of such vote.
Dividends
Dividends on the Preferred Stock are payable prior and in preference to any declaration or payment of any dividend on the common stock of the Company. The Preferred Stock earns a 6% per share noncumulative dividend on the original issuance price per annum, payable when, as and if declared by the Board of Directors. Further, the Preferred Stock is to receive dividends at least equal to any dividend rate to be paid to common stockholders, times the number of common shares into which each share of preferred stock is convertible. In 2006 and 2005, no dividends have been declared or paid by the Company.
Liquidation
In the event of liquidation, dissolution, or winding up of the Company, holders of Preferred Stock (Series A, Series AA, Series B and Series C) shall be entitled to receive in preference to the holders of common stock, an amount equal to the original purchase price plus declared but unpaid dividends (the “Liquidation Preference”). After the payment of the Liquidation Preference to the holders of Series A, Series AA, Series B and Series C Preferred, the remaining assets shall be distributed to the holders of the common stock. The Preferred Stock contains provisions stating that a change in control is considered to be a deemed liquidation event. The inclusion of this potential triggering event requires the Preferred Stock to be classified outside of permanent equity, and is presented separately in the balance sheets.
If the assets of the Company available for distribution upon liquidation are not sufficient to pay the Liquidation Preference, the assets will be distributed pro rata to each holder of Series A, Series AA, Series B and Series C Preferred Stock.
A merger or sale of substantially all of the assets or intellectual property of the Company in which the stockholders of the Company do not own a majority of the outstanding shares of the surviving corporation, or a sale or issuance of shares with the power to elect a majority of the Board, shall be deemed to be a liquidation.
The Company may not sell or dispose of all or substantially all of its property or business or merge into another corporation or series of related transactions in which the control of all or substantially all of the Company (the “Exit Event”) without the vote of the Preferred Stock holders, unless the consideration received from the transaction is in the form of cash or publicly traded securities equal to at least $18.33 per share with an aggregate offering price of not less than $40,000,000.
Conversion
Each share of Series A, Series AA, Series B, and Series C Preferred Stock shall be convertible at any time, at the option of the holder, into shares of Common Stock of the Company at an initial conversion price equal to the original purchase price. The Series A, Series AA, Series B and Series C will be automatically converted into Common Stock, at the then applicable conversion price, upon the election of a majority of the combined holders of the then outstanding Preferred Stock. All of the Preferred Stock will be automatically converted upon the vote of 80% of the holders of the Series C. On August 31, 2007, the Company amended its certificate of incorporation to provide for the automatic conversion of all of its Preferred Stock upon (A) the closing of the Company’s sale of its Common Stock in a firm commitment underwritten public offering pursuant to an effective registration statement on Form S-1 or SB-2 under the Securities Act of 1933, as
F-14
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
amended (the Securities Act), the public offering price of which was not less than $7.33 per share (as adjusted for any stock dividends, contributions or splits with respect to such shares); provided that such closing occurs prior to December 31, 2007, or (B) on or after January 1, 2008, the closing of the Company’s sale of its Common Stock in a firm commitment underwritten public offering pursuant to an effective registration statement on Form S-1 or SB-2 under the Securities Act, the public offering price of which was not less than $18.33 per share (as adjusted for any stock dividends, contributions or splits with respect to such shares) and results in gross proceeds to the Company of at least $40,000,000 (a Qualified Offering).
The conversion rate for all Preferred Stock may be adjusted if additional stock is issued for an amount less than the applicable initial conversation prices.
Protective Rights
The Preferred Stock has certain protective rights regarding the authorization, alteration, redemption or sale of any class of stock; the declaration of any dividends; or changes in the size of the Board of Directors. Such actions must be approved by holders of Preferred Stock, as specified in the amended certificate of incorporation.
Common Stock
The following is a summary of the rights, preferences and terms of the Company’s common stock:
Dividends
The holders of common stock are entitled to receive dividends from time to time as may be declared by the Board of Directors. No cash dividend may be declared or paid to common stockholders until paid on each series of outstanding preferred stock in accordance with its terms.
Voting
The holders of shares of common stock are entitled to one vote for each share held with respect to all matters voted on by the stockholders of the Company.
Liquidation
After payment to the preferred stockholders, holders of common stock shall be entitled, together with holders of preferred stock, to share ratably in all remaining assets of the Company.
During the six months ended June 30, 2007, the Company issued 47,833 shares of common stock to three employees for $11,200 upon the exercise the stock options. In 2006, the Company issued 23,711 shares of common stock to three employees and two consultants for $4,400 upon the exercise of stock options. In 2005, the Company issued 263,740 shares of the common stock to three employees and one consultant for $71,000 upon the exercise of stock options.
| 7. | Stock Options |
The Company established the TranS1 Inc. Stock Incentive Plan in 2000, and amended it in 2005 (as amended, the “Plan”). Under the Plan, the Company may grant options to employees, directors or service providers and contractors for a maximum of 3,159,108 shares of the Company’s common stock. Options granted under the Plan may be incentive stock options or non-qualified stock options. Non-qualified stock options may be granted to service providers and incentive stock options may be granted only to employees. The exercise periods may not exceed ten years for options. However, in the case of an incentive stock option granted to an optionee who, at the time of the option grant owns stock representing more than 10% of the
F-15
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
outstanding shares, the term of the option shall be five years from the date of the grant. The exercise price of incentive stock options cannot be less than 100% of the fair market value per share of the Company’s common stock on the grant date. The exercise price of a nonqualified option shall not be less than 85% of the fair market value per share on the date the option is granted. If an optionee owns more than 10% of the outstanding shares, the exercise price cannot be less than 110% of the fair market value of the stock on the date of the grant. Options granted under the Plan generally vest over periods ranging from three to four years.
The following table summarizes the activity of the Company’s Plan including the number of shares under options (“Number”) and weighted average exercise price (“Price”):
| Number | Price | |||||||
Outstanding as of December 31, 2003 | 949,504 | $ | 0.13 | |||||
| Options granted | 159,322 | 0.28 | ||||||
| Options exercised | (57,600 | ) | 0.11 | |||||
| Options forfeited | (18,000 | ) | 0.11 | |||||
Outstanding as of December 31, 2004 | 1,033,226 | 0.27 | ||||||
| Options granted | 416,574 | 0.29 | ||||||
| Options exercised | (263,739 | ) | 0.28 | |||||
| Options forfeited | — | — | ||||||
Outstanding as of December 31, 2005 | 1,186,061 | 0.27 | ||||||
| Options granted | 735,374 | 1.11 | ||||||
| Options exercised | (30,274 | ) | 0.21 | |||||
| Options forfeited | (49,486 | ) | 0.28 | |||||
Outstanding as of December 31, 2006 | 1,841,675 | 0.59 | ||||||
| Options granted (unaudited) | 587,408 | 5.12 | ||||||
| Options exercised (unaudited) | (47,833 | ) | 0.23 | |||||
| Options forfeited (unaudited) | (41,587 | ) | 0.28 | |||||
Outstanding as of June 30, 2007 (unaudited) | 2,339,663 | $ | 1.74 | |||||
The following table summarizes information about the Company’s stock options at June 30, 2007 (unaudited):
| Weighted | Weighted | |||||||||||||||
| Average | Average | Number of | ||||||||||||||
| Number | Contractual | Exercise | Options | |||||||||||||
Exercise Price | Outstanding | Life (Years) | Price | Exercisable | ||||||||||||
| $0.11 | 271,125 | 4.6 | $ | 0.11 | 271,125 | |||||||||||
| $0.28 | 741,346 | 6.9 | 0.28 | 595,002 | ||||||||||||
| $0.78 | 4,410 | 8.2 | 0.78 | 4,410 | ||||||||||||
| $1.11 | 735,374 | 9.4 | 1.11 | 241,456 | ||||||||||||
| $2.00 | 67,387 | 9.6 | 2.00 | 5,188 | ||||||||||||
| $2.38 | 29,251 | 9.7 | 2.38 | 3,001 | ||||||||||||
| $5.56 | 418,770 | 9.9 | 5.56 | 5,395 | ||||||||||||
| $6.67 | 72,000 | 9.9 | 6.67 | — | ||||||||||||
| 2,339,663 | 8.1 | $ | 1.74 | 1,125,577 | ||||||||||||
F-16
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
The following table summarizes information about the Company’s stock options at December 31, 2006:
| Weighted | Weighted | |||||||||||||||
| Average | Average | Number of | ||||||||||||||
| Number | Contractual | Exercise | Options | |||||||||||||
Exercise Price | Outstanding | Life (Years) | Price | Exercisable | ||||||||||||
| $0.11 | 283,725 | 5.1 | $ | 0.11 | 217,125 | |||||||||||
| $0.28 | 818,166 | 7.5 | 0.28 | 524,647 | ||||||||||||
| $0.78 | 4,410 | 8.7 | 0.78 | 4,410 | ||||||||||||
| $1.11 | 735,374 | 9.9 | 1.11 | 87,736 | ||||||||||||
| 1,841,675 | 8.1 | $ | 0.59 | 833,918 | ||||||||||||
Stock Based Compensation for Non-employees
During 2006, the Company issued options to purchase 51,651 shares of common stock with an exercise price of $1.00 per share to consultants. These options vest over a range of zero to three years and expire 10 years from the date of issuance. During 2005, the Company issued options to purchase 93,150 shares of common stock with an exercise price of $0.28 per share to consultants. These options vest over a three year period and expire 10 years from the date of issuance. The Company determined the estimated fair value of the options issued to the consultants using the Black-Scholes pricing model. The Company used the following assumptions in the Black-Scholes pricing model for 2005 grants: 100% volatility, dividend yield-0%, 4.33% risk-free rate and a 10 year expected legal life. The Company used the following assumptions in the Black-Scholes pricing model for 2006 grants: 45% volatility, 0% dividend yield, 4.58% risk-free rate and 6 year expected life.
Stock-based compensation expense charged to operations on options granted to non-employees for the six months ended June 30, 2007 (unaudited) and for years ended December 31, 2006 and 2005 was $420,000, $77,000 and $9,400, respectively. As of June 30, 2007 (unaudited) and December 31, 2006, there were $311,000 and $150,000, respectively, of total unrecognized compensation costs related to non-vested stock option awards granted after January 1, 2006, which are expected to be recognized over a weighted-average period of 1.98 years and 2.42 years, respectively.
Employee Stock-Based Compensation on or Subsequent to January 1, 2006
As a result of adopting SFAS 123R on January 1, 2006, the Company’s net loss for the six months ended June 30, 2007 (unaudited) and for the year ended December 31, 2006 was $638,000 and $522,000, respectively, higher than if it had continued to account for employee stock-based compensation under APB 25.
Under SFAS 123R, compensation cost for employee stock-based awards is based on the estimated grant-date fair value and is recognized over the vesting period of the applicable award on a straight-line basis. For the period from January 1, 2006 to June 30, 2007, the Company issued employee stock-based awards in the form of stock options. The weighted average estimated fair value of the employee stock options granted for the six months ended June 30, 2007 (unaudited) and for the year ended December 31, 2006 was $5.13 and $4.18 per share, respectively. The aggregate intrinsic value of stock options (the amount by which the market price of the stock on the date of exercise exceeded the exercise price of the option) exercised during the six months ended June 30, 2007 (unaudited) and for the years ended December 31, 2006, was $273,000 and $114,000, respectively.
The Company uses the Black-Scholes pricing model to determine the fair value of stock options. The determination of the fair value of stock-based payment awards on the date of grant is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include our expected stock price volatility over the term of the awards, actual and projected employee stock option
F-17
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
exercise behaviors, risk-free interest rates and expected dividends. The estimated grant-date fair values of the employee stock options were calculated using the Black-Scholes valuation model, based on the following assumptions for the six months ended June 30, 2007 (unaudited) and for the year ended December 31, 2006:
Expected Life. The expected life of six years is based on the “simplified” method described in the SEC Staff Accounting Bulletin, Topic 14:Share-Based Payment.
Volatility. Since the Company is a private entity with no historical data regarding the volatility of its common stock, the expected volatility used for 2006 and 2007 is based on volatility of similar entities, referred to as “guideline” companies. In evaluating similarity, the Company considered factors such as industry, stage of life cycle and size. The Company utilized an expected volatility of 45% for 2006 and 2007.
Risk-Free Interest Rate. The risk-free rate is based on U.S. Treasury zero-coupon issues with remaining terms similar to the expected term on the options. The risk-free rate was 4.58% and 4.48% for 2006 and the six month period ended June 30, 2007, respectively.
Dividend Yield. The Company has never declared or paid any cash dividends and does not plan to pay cash dividends in the foreseeable future, and, therefore, used an expected dividend yield of zero in the valuation model.
Forfeitures. SFAS 123R also requires the Company to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those awards that are expected to vest. All stock-based payment awards are amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods. If the Company’s actual forfeiture rate is materially different from its estimate, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
As of June 30, 2007 (unaudited) and December 31, 2006, there were $5,322,000 and $2,483,000, respectively, of total unrecognized compensation costs related to non-vested stock option awards granted after January 1, 2006, which are expected to be recognized over a weighted-average period of 3.34 years and 2.96 years, respectively.
| 8. | Income Taxes |
No provision for federal or state income taxes has been recorded as the Company has incurred net operating losses since inception.
F-18
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
Significant components of the Company’s deferred tax assets and liabilities consist of the following:
| December 31, | ||||||||
| 2005 | 2006 | |||||||
Deferred tax assets | ||||||||
| Domestic net operating loss carryforwards | $ | 4,761 | $ | 7,584 | ||||
| Other | 3 | 3 | ||||||
| Research and development credit | 402 | 624 | ||||||
| Total deferred tax assets | 5,166 | 8,211 | ||||||
| Valuation allowance for deferred assets | (5,137 | ) | (8,121 | ) | ||||
| Deferred tax assets | 29 | 90 | ||||||
Deferred tax liabilities | ||||||||
| Fixed assets | 29 | 90 | ||||||
| Total deferred tax liabilities | 29 | 90 | ||||||
| Net deferred tax assets (liabilities) | $ | — | $ | — | ||||
At December 31, 2006 and 2005, the Company provided a full valuation allowance against its net deferred tax assets since realization of these benefits could not be reasonably assured. The increase in valuation allowance resulted primarily from the additional net operating loss carryforward generated.
As of December 31, 2006, the Company had federal and state net operating loss carryforwards of approximately $19,563,000 and $16,425,000, respectively. These net operating loss carryforwards begin to expire in 2021 and 2016 for federal and state tax purposes, respectively. The utilization of the federal net operating loss carryforwards may be subject to limitations under the rules regarding a change in stock ownership as determined by the Internal Revenue Code.
Additionally, as of December 31, 2006, the Company had research and development credit carryforwards of $624,000 for federal tax purposes. These credit carryforwards begin to expire in 2021.
A reconciliation of differences between the U.S. federal income tax rate and the Company’s effective tax rate for the years ended December 31 is as follows:
| 2004 | 2005 | 2006 | ||||||||||||||||||||||
| % of | % of | % of | ||||||||||||||||||||||
| Amount | Net loss | Amount | Net loss | Amount | Net loss | |||||||||||||||||||
| Tax at statutory rate | $ | (1,215 | ) | 35.0 | % | $ | (1,765 | ) | 35.0 | % | $ | (3,318 | ) | 35.0 | % | |||||||||
| State taxes | (157 | ) | 4.5 | % | (226 | ) | 4.5 | % | (213 | ) | 2.2 | % | ||||||||||||
| Non deductible items | 25 | (0.7 | )% | 71 | (1.4 | )% | 270 | (2.8 | )% | |||||||||||||||
| Other | 421 | (4.3 | )% | |||||||||||||||||||||
| R&D credits | (62 | ) | 1.8 | % | (97 | ) | 1.9 | % | (144 | ) | 1.5 | % | ||||||||||||
| Change in valuation allowance | 1,409 | (40.6 | )% | 2,017 | (40.0 | )% | 2,984 | (31.5 | )% | |||||||||||||||
| Total | $ | — | 0.0 | % | $ | — | 0.0 | % | $ | — | 0.0 | % | ||||||||||||
| 9. | Subsequent Event |
On October 5, 2007, the Company’s Board of Directors approved an amendment to the Company’s existing certificate of incorporation effecting a0.9-for-1 reverse stock split and reducing the authorized number of shares of common stock and preferred stock to 17,100,000 and 10,793,182, respectively. All share
F-19
Table of Contents
TranS1 Inc.
Notes to Financial Statements — (Continued)
and per share information in the accompanying financial statements and notes to the financial statements has been retroactively restated to reflect the effect of the stock split.
F-20
Table of Contents

Table of Contents
5,500,000 shares

Common Stock
PROSPECTUS
| Lehman Brothers | Piper Jaffray |
| Cowen and Company | Wachovia Securities |
Until , 2007, all dealers that effect transactions in these securities may be required to deliver a prospectus, regardless of whether they are participating in this offering. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
, 2007
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The following table sets forth all costs and expenses, other than underwriting discounts and commissions, to be paid in connection with the sale of the common stock being registered hereunder, all of which will be paid by us. All of the amounts shown are estimates except for the Securities and Exchange Commission registration fee, The Nasdaq Global Market application fee and the NASD filing fee.
| SEC registration fee | $ | 2,719 | ||
| NASD filing fee | 9,355 | |||
| Nasdaq Global Market listing application fee | 100,000 | |||
| Printing expenses | 200,000 | |||
| Legal fees and expenses | 1,000,000 | |||
| Accounting fees and expenses | 600,000 | |||
| Blue sky fees and expenses | 15,000 | |||
| Transfer Agent fees | 15,500 | |||
| Miscellaneous expenses | 57,426 | |||
| Total | $ | 2,000,000 | ||
| Item 14. | Indemnification of Directors and Officers |
Our amended and restated certificate of incorporation limits, to the maximum extent permitted by the DGCL, the personal liability of directors for monetary damages for breach of their fiduciary duties as a director. Our amended and restated certificate of incorporation and amended and restated bylaws provide that we shall indemnify our officers and directors and may indemnify our employees and other agents to the fullest extent permitted by the DGCL.
Section 145 of the DGCL provides that a corporation may indemnify any person made a party to an action (other than an action by or in the right of the corporation) by reason of the fact that he or she was a director, officer, employee or agent of the corporation or was serving at the request of the corporation against expenses (including attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action (other than an action by or in the right of the corporation), has no reasonable cause to believe his or her conduct was unlawful. As permitted by our bylaws, we have entered into indemnification agreements with our officers and directors.
Our directors and officers are covered by insurance policies indemnifying against certain liabilities, including certain liabilities arising under the Securities Act, which might be incurred by them in such capacities and against which they cannot be indemnified by us.
Reference is made to the indemnification and contribution provisions of the underwriting agreement to be filed by amendment as Exhibit 1.1 to this registration statement.
| Item 15. | Recent Sales of Unregistered Securities |
The following is a summary of transactions by us from March 1, 2004 through the date of this registration statement involving sales of our securities that were not registered under the Securities Act. Each offer and sale was made in reliance on Regulation D promulgated under Section 4(2) of the Securities Act or Rule 701 promulgated under Section 3(b) of the Securities Act, as transactions by an issuer not involving a public offering or transactions pursuant to compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The purchasers were “accredited investors,” officers, directors or employees of the
II-1
Table of Contents
registrant or known to the registrant and its management through pre-existing business relationships, friends and employees. All purchasers were provided access to all material information which they requested, and all information necessary to verify such information and was afforded access to management of the registrant in connection with their purchases. All holders of the unregistered securities acquired such securities for investment and not with a view toward distribution, acknowledging such intent to the registrant. All certificates or agreements representing such securities that were issued contained restrictive legends, prohibiting further transfer of the certificates or agreements representing such securities, without such securities either being first registered or otherwise exempt from registration under the Securities Act, in any further resale or disposition.
1. In September 2005, we issued and sold an aggregate of 3,499,091 shares of our Series C preferred stock to certain private investors, including our directors, executive officers and 5% stockholders and persons and entities associated with them, at a price of $7.33 per share, for an aggregate purchase price of approximately $25,660,001.
2. From March 1, 2004 through the date of this registration statement, we granted stock options and stock purchase rights to acquire an aggregate of 1,887,314 shares of our common stock at prices ranging from $0.11 to $7.78 per share to employees, consultants and directors pursuant to our Amended and Restated 2000 Stock Incentive Plan, as amended. Of the 1,887,314 shares granted, 1,605,784 shares remain outstanding, 149,742 have been exercised and 131,788 shares have been canceled and returned to our Amended and Restated 2000 Stock Incentive Plan.
We used the proceeds of the foregoing sales of securities for working capital and other general corporate purposes. We did not employ any underwriters, brokers or finders in connection with any of the transactions set forth above.
| Item 16. | Exhibits and Financial Statement Schedules |
(a) Exhibits
| Exhibit | ||||
No. | Description | |||
| 1 | .1** | Form of Underwriting Agreement | ||
| 3 | .1** | Fourth Amended and Restated Certificate of Incorporation, as currently in effect | ||
| 3 | .2 | Form of Amended and Restated Certificate of Incorporation (to be filed in connection with the closing of this offering) | ||
| 3 | .3** | Amended and Restated Bylaws, as currently in effect | ||
| 3 | .4** | Form of Amended and Restated Bylaws (to be effective upon the closing of this offering) | ||
| 4 | .1** | Specimen common stock certificate | ||
| 4 | .2** | Third Amended and Restated Investor Rights Agreement dated September 20, 2005, among TranS1 Inc. and certain of its stockholders | ||
| 5 | .1 | Opinion of Stradling Yocca Carlson & Rauth, a Professional Corporation | ||
| 10 | .1** | Amended and Restated 2000 Stock Incentive Plan† | ||
| 10 | .2** | Form of Stock Option Agreement under Amended and Restated 2000 Stock Incentive Plan† | ||
| 10 | .3** | 2007 Stock Incentive Plan† | ||
| 10 | .4** | Form of Stock Option Agreement under 2007 Stock Incentive Plan† | ||
| 10 | .5** | 2007 Employee Stock Purchase Plan† | ||
| 10 | .6** | Lease, dated March 16, 2005, between TranS1 Inc. and Ellmore Enterprises | ||
| 10 | .7** | Form of Indemnification Agreement | ||
| 10 | .7.1** | Schedule of Parties to Indemnification Agreement | ||
| 10 | .8** | Form of Series C Preferred Stock Purchase Agreement dated September 20, 2005, among TranS1 Inc. and the investors listed therein | ||
| 23 | .1 | Consent of Stradling Yocca Carlson & Rauth, a Professional Corporation (included in Exhibit 5.1) | ||
II-2
Table of Contents
| Exhibit | ||||
No. | Description | |||
| 23 | .2 | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm | ||
| 23 | .3** | Consent of W. Daniel Bradley, M.D. | ||
| 23 | .4** | Consent of Christopher Ames, M.D. | ||
| 24 | .1** | Power of Attorney (included on the signature page to this Registration Statement) | ||
| * | To be filed by amendment. | |
| ** | Previously filed. | |
| † | Indicates management contract or compensatory plan or arrangement. |
(b) Financial Statement Schedules
All schedules have been omitted because the information required to be presented in them is not applicable or is shown in the financial statements or related notes.
| Item 17. | Undertakings. |
The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
1. For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
2. For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
3. For purposes of determining liability under the Securities Act of 1933 to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use,
II-3
Table of Contents
supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
4. That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
II-4
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Amendment No. 3 to the Registration Statement onForm S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Wilmington, State of North Carolina, on the 9th day of October, 2007.
TRANS1 INC.
| By: | /s/ Richard Randall |
Richard Randall
President and Chief Executive Officer
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment No. 3 to the Registration Statement onForm S-1 has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||||
/s/ Richard Randall Richard Randall | President, Chief Executive Officer and Director (Principal Executive Officer) | October 9, 2007 | ||||
/s/ Michael Luetkemeyer Michael Luetkemeyer | Chief Financial Officer (Principal Financial and Accounting Officer) | October 9, 2007 | ||||
/s/ Michael Carusi* Michael Carusi | Director | October 9, 2007 | ||||
/s/ Mitchell Dann* Mitchell Dann | Director | October 9, 2007 | ||||
/s/ James Shapiro* James Shapiro | Director | October 9, 2007 | ||||
/s/ Jonathan Osgood* Jonathan Osgood | Director | October 9, 2007 | ||||
| * | /s/ Richard Randall Richard Randall Attorney-in-Fact | |||||
II-5
Table of Contents
EXHIBIT INDEX
| Exhibit | ||||
No. | Description | |||
| 1 | .1** | Form of Underwriting Agreement | ||
| 3 | .1** | Fourth Amended and Restated Certificate of Incorporation, as currently in effect | ||
| 3 | .2 | Form of Amended and Restated Certificate of Incorporation (to be filed in connection with the closing of this offering) | ||
| 3 | .3** | Amended and Restated Bylaws, as currently in effect | ||
| 3 | .4** | Form of Amended and Restated Bylaws (to be effective upon the closing of this offering) | ||
| 4 | .1** | Specimen common stock certificate | ||
| 4 | .2** | Third Amended and Restated Investor Rights Agreement dated September 20, 2005, among TranS1 Inc. and certain of its stockholders | ||
| 5 | .1 | Opinion of Stradling Yocca Carlson & Rauth, a Professional Corporation | ||
| 10 | .1** | Amended and Restated 2000 Stock Incentive Plan† | ||
| 10 | .2** | Form of Stock Option Agreement under Amended and Restated 2000 Stock Incentive Plan† | ||
| 10 | .3** | 2007 Stock Incentive Plan† | ||
| 10 | .4** | Form of Stock Option Agreement under 2007 Stock Incentive Plan† | ||
| 10 | .5** | 2007 Employee Stock Purchase Plan† | ||
| 10 | .6** | Lease, dated March 16, 2005, between TranS1 Inc. and Ellmore Enterprises | ||
| 10 | .7** | Form of Indemnification Agreement | ||
| 10 | .7.1** | Schedule of Parties to Indemnification Agreement | ||
| 10 | .8** | Form of Series C Preferred Stock Purchase Agreement dated September 20, 2005, among TranS1 Inc. and the investors listed therein | ||
| 23 | .1 | Consent of Stradling Yocca Carlson & Rauth, a Professional Corporation (included in Exhibit 5.1) | ||
| 23 | .2 | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm | ||
| 23 | .3** | Consent of W. Daniel Bradley, M.D. | ||
| 23 | .4** | Consent of Christopher Ames, M.D. | ||
| 24 | .1** | Power of Attorney (included on the signature page to this Registration Statement) | ||
| * | To be filed by amendment. | |
| ** | Previously filed. | |
| † | Indicates management contract or compensatory plan or arrangement. |
II-6