UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934
(Amendment No. 3)
| Filed by the Registrant | x |
| Filed by a Party other than the Registrant | ¨ |
Check the appropriate box:
| x | Preliminary Proxy Statement |
| ¨ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| ¨ | Definitive Proxy Statement |
| ¨ | Definitive Additional Materials |
| ¨ | Soliciting Material Pursuant to §240.14a-12 |
| TRANS1 INC. |
| (Name of Registrant as Specified In Its Charter) |
| (Name of Person(s) Filing Proxy Statement if other than the Registrant) |
Payment of Filing Fee (Check the appropriate box):
| ¨ | No fee required. |
| ¨ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| 1) | Title of each class of securities to which transaction applies: |
| Common Stock, par value $0.0001 per share |
| 2) | Aggregate number of securities to which transaction applies: |
| 18,167,910 shares of common stock |
| 3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined): |
| $2.18 per share, based on the average of the high and low prices of the Registrant’s common stock on March 4, 2013, as reported on the NASDAQ Global Market. In accordance with Section 14(g) of the Securities Exchange Act of 1934, as amended, the filing fee was determined by multiplying the transaction value by 0.0001364. |
| 4) | Proposed maximum aggregate value of transaction: |
| $39,606,044 |
| 5) | Total fee paid: |
| $5,403 |
| x | Fee paid previously with preliminary materials. |
| ¨ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| 1) | Amount Previously Paid: | |
| 2) | Form, Schedule or Registration Statement No.: | |
| 3) | Filing Party: | |
| 4) | Date Filed: | |

| NOTICE OF SPECIAL MEETING OF STOCKHOLDERS |
| TO BE HELD[Ÿ], 2013 |
TO OUR STOCKHOLDERS:
You are cordially invited to aSpecial Meeting of Stockholders of TranS1 Inc., aDelawarecorporation. The Special Meeting of Stockholders will be held on[Ÿ], 2013 at[Ÿ], local time, at200 Horizon Drive, Suite 115, Raleigh, North Carolina 27615for the following purposes (as more fully described in theProxy Statementaccompanying this Notice):
1. To approve the issuance of shares of our common stock pursuant to the Agreement and Plan of Merger, dated March 3, 2013, by and among TranS1 Inc., RacerX Acquisition Corp., Baxano, Inc., and Sumeet Jain and David Schulte as Securityholder Representatives thereunder, as amended by the First Amendment to Agreement and Plan of Merger, dated April 10, 2013, by and among the parties;
2. To approve the issuance of shares of our common stock pursuant to the Securities Purchase Agreement, dated March 3, 2013, by and among TranS1 Inc. and certain investors;
3. To approve an amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc.;
4. To grant the proxy holders discretionary authority to vote to adjourn the Special Meeting of Stockholders, if necessary, in order to solicit additional proxies in the event that there are not sufficient affirmative votes present at the Special Meeting of Stockholders to approve Proposal No. 1, Proposal No. 2, and/or Proposal No. 3; and
5. To transact such other business as may properly come before the Special Meeting of Stockholders or any postponement or adjournment thereof.
Pursuant to the terms of the Agreement and Plan of Merger, at the effective time of the merger, RacerX Acquisition Corp. (our wholly-owned subsidiary) will merge with and into Baxano, Inc. with Baxano, Inc. surviving as our wholly-owned subsidiary. The merger consideration to be issued to Baxano, Inc. securityholders will consist of a number of newly issued shares of our common stock calculated as the product of (a) 0.38888889 times (b) the number of issued and outstanding shares of our capital stock (as if converted to common stock) as of the close of business on the last trading day prior to the closing of the merger. This calculation is intended to provide Baxano, Inc. stockholders with ownership of 28% of our post-closing outstanding shares of common stock (excluding the impact of the private placement transaction to be completed pursuant to the Securities Purchase Agreement) prior to certain adjustments to the merger consideration provided for in the Agreement and Plan of Merger.
The transactions contemplated by Proposal Nos. 1 and 2 are, among other conditions described in the accompanying Proxy Statement, conditioned on each other and, therefore, both proposals must be approved in order for the transactions contemplated by such proposals to be consummated. In addition, the amendment contemplated by Proposal No. 3 is contingent upon the approval of Proposal Nos. 1 and 2 and the consummation of the merger contemplated by Proposal No. 1.
Only our stockholders of record at the close of business on[Ÿ], 2013 are entitled to notice of,and to vote at, the Special Meetingof Stockholders.
All stockholders are cordially invited to attend the Special Meeting of Stockholders in person. However, whether or not you plan to attend the Special Meeting of Stockholders, we encourage you to read thisProxy Statementand promptly vote your shares. In order to vote your shares by proxy, please complete, sign, and date the proxy and return it in the envelope provided. Any stockholder attending the Special Meeting of Stockholders may vote in person even if he or she has returned a proxy.
| ON BEHALF OF THE BOARD OF DIRECTORS | |
| [Ÿ] | |
| Ken Reali | |
| President and Chief Executive Officer |
Raleigh, North Carolina
[Ÿ], 2013
| TABLE OF CONTENTS |
| Page No. | |
| General Information | 1 |
| Questions and Answers About the Special Meeting, the Merger, and the Private Placement Transaction | 1 |
| The Special Meeting | 1 |
| The Companies | 5 |
| The Transactions | 5 |
| Risk Factors | 8 |
| Cautionary Note Regarding Forward-Looking Statements | 26 |
| The Special Meeting of Stockholders | 27 |
| Date, Time, and Place | 27 |
| Date of Mailing | 27 |
| Purposes of the Special Meeting; Board Recommendations | 27 |
| Record Date; Voting Securities | 27 |
| Quorum; Required Vote | 27 |
| Abstentions and Broker Non-Votes | 28 |
| Voting at the Special Meeting | 28 |
| Voting of Proxies | 28 |
| Revocability of Proxies | 29 |
| Cost of This Proxy Solicitation | 29 |
| Adjournments and Postponements | 30 |
| Additional Information | 30 |
| Proposal No. 1 – Issuance of Common Stock PURSUANT TO THE MERGER AGREEMENT | 30 |
| The Merger | 31 |
| Background of the Merger | 31 |
| Reasons for the Merger | 33 |
| Opinion of Financial Advisor – Stifel, Nicolaus & Company, Incorporated | 36 |
| The Merger Agreement | 46 |
| Structure and Effective Time of the Merger | 46 |
| Management after the Merger | 46 |
| Merger Consideration | 46 |
| Treatment of Preferred Stock and Common Stock | 47 |
| Treatment of Baxano Notes, Baxano Stock Option Plans, Baxano Stock Options, and Baxano Warrants | 47 |
| Requirements to Receive Merger Consideration | 47 |
| Fractional Shares | 47 |
| Allocation of Merger Consideration | 48 |
| Representations and Warranties | 48 |
| Covenants | 50 |
| Additional Agreements / Covenants | 54 |
| Conditions to the Merger | 59 |
| Indemnification | 61 |
| Termination | 63 |
| Amendment | 64 |
| Securityholder Representatives | 65 |
| Appraisal Rights | 65 |
| Material U.S. Federal Income Tax Consequences of the Merger | 65 |
| Anticipated Accounting Treatment of the Merger | 65 |
| Regulatory Approvals for the Completion of the Merger | 65 |
| Lock-up and Registration of Shares | 66 |
| Stockholder Agreements | 66 |
| Bridge Financing | 66 |
| Proposal No. 2 – Issuance of Common Stock PURSUANT TO THE SECURITIES PURCHASE AGREEMENT | 66 |
| The Private Placement Transaction | 66 |
| Lock-up and Registration of Shares | 67 |
| Stockholder Agreements | 67 |
| INTERESTS OF CERTAIN PERSONS IN THE MERGER AND PRIVATE PLACEMENT TRANSACTION | 68 |
| UnauditedPro Forma Condensed Combined Financial INFORMATION | 71 |
| Information About TranS1 | 78 |
| Business of TranS1 | 78 |
| Properties | 94 |
| Legal Proceedings | 94 |
| Management’s Discussion and Analysis of Financial Condition and Results of Operations | 94 |
| Security Ownership of Certain Beneficial Owners and Management | 104 |
| Market Price of and Dividends on TranS1’s Common Equity and Related Stockholder Matters | 106 |
| Description of Our Common Stock | 107 |
| Information About Baxano | 110 |
| Business of Baxano | 110 |
| Management’s Discussion and Analysis of Financial Condition and Results of Operations | 116 |
| Market Price of and Dividends on Baxano’s Common Equity and Related Stockholder Matters | 122 |
| PROPOSAL NO. 3 –Amendment to Our Amended and Restated Certificate of Incorporation to Change Our Name | 122 |
| Proposal No. 4 – Potential Adjournment of the Special Meeting | 123 |
| Other Matters | 123 |
| Stockholder Proposals | 123 |
| INDEX TO FINANCIAL STATEMENTS | 125 |
Annexes
| Annex A | Agreement and Plan of Merger, dated March 3, 2013, by and among TranS1 Inc., RacerX Acquisition Corp., Baxano, Inc., and Sumeet Jain and David Schulte as Securityholder Representatives, as amended by the First Amendment to Agreement and Plan of Merger, dated April 10, 2013, by and among the parties | |
| Annex B | Securities Purchase Agreement, dated March 3, 2013, by and among TranS1 Inc. and certain investors | |
| Annex C | Opinion of Stifel, Nicolaus & Company, Incorporated |
 |
| PROXY STATEMENT FOR THE |
| SPECIAL MEETING OF STOCKHOLDERS |
| [Ÿ], 2013 |
| ABOUT THE SPECIAL MEETING OF STOCKHOLDERS |
| GENERAL INFORMATION |
The enclosedproxyis solicited on behalf of theBoard of Directors of TranS1 Inc., which we refer to as “TranS1,” “the Company,” “we,” “us,” or “our,” for use at the Company’s Special Meeting of Stockholders, or the “Special Meeting,” to be held on[Ÿ], 2013 at[Ÿ], local time, or at any postponements or adjournments thereof, at200 Horizon Drive, Suite 115, Raleigh, North Carolina 27615. ThisProxy Statementand the accompanying proxy card are being mailed to all stockholdersentitled to vote at the Special Meetingon or about[Ÿ], 2013.
QUESTIONS AND ANSWERS ABOUT
THESPECIAL MEETING, THE MERGER, AND THE PRIVATE PLACEMENT TRANSACTION
The following questions and answers are intended to briefly address potential questions regarding the Special Meeting, the Merger (as defined below), and the Private Placement Transaction (as defined below). They are also intended to provide our stockholders with certain information that is required to be provided under the rules and regulations of the Securities and Exchange Commission, or the SEC. These questions and answers may not address all of the questions that are important to you as a stockholder. You should read this entire document and the other documents referenced in it for a more complete understanding of the Merger, the Private Placement Transaction, and the other transactions contemplated by the Merger Agreement (as defined below) and the Securities Purchase Agreement (as defined below). In particular, you should read the documents accompanying this Proxy Statement, including the Merger Agreement, which is attached as Annex A, the Securities Purchase Agreement, which is attached as Annex B, and the opinion of Stifel, Nicolaus & Company, Incorporated, which is attached as Annex C. If you have additional questions about the Special Meeting, the Merger, or the Private Placement Transaction, please contact our Corporate Secretary using the contact information provided in this Proxy Statement.
The Special Meeting
| Q: | What is the purpose of the Special Meeting? |
| A: | We have entered into an Agreement and Plan of Merger, dated March 3, 2013, by and among us, RacerX Acquisition Corp., or Merger Sub, Baxano, Inc., or Baxano, and Sumeet Jain and David Schulte, as Securityholder Representatives, or the Securityholder Representatives, as amended by the First Amendment to Agreement and Plan of Merger, dated April 10, 2013, by and among the parties, pursuant to which, at the effective time, Merger Sub will merge with and into Baxano with Baxano surviving as a wholly-owned subsidiary of the Company. We refer to this agreement, as amended, as the Merger Agreement and the transaction contemplated in the agreement as the Merger, each of which is described in this Proxy Statement. A copy of the Merger Agreement is attached to this Proxy Statement asAnnex A. |
In addition, we entered into a Securities Purchase Agreement, dated March 3, 2013, with certain investors (including certain of Baxano’s securityholders and certain of our directors and executive officers), or the Investors, pursuant to which we will sell and issue shares of our common stock to the Investors in a private placement transaction concurrent with the closing of the Merger. We refer to this agreement as the Securities Purchase Agreement and the transaction contemplated in the agreement as the Private Placement Transaction, each of which is described in this Proxy Statement. A copy of the Securities Purchase Agreement is attached to this Proxy Statement asAnnex B.
We are also proposing to change our name from TranS1 Inc. to Baxano Surgical, Inc. We were formed to develop and commercialize a novel solution for lumbar fusions at the L5/S1 junction. Because “TranS” was meant to represent the trans-sacral access of this technology, and “S1” was meant to refer to spine anatomy, the name TranS1 will no longer be synonymous with our business activity post-Merger, which will be a broader company focused on lumbar fusion, spinal decompression and other solutions for spine surgeons. The new name leverages the positive brand recognition and past investment in brand development, product packaging, and marketing collateral of Baxano, which will allow us to extend existing relationships with Baxano customers, who are primarily minimally-invasive spine surgeons, in a cost effective manner.
At the Special Meeting, our stockholders will be asked to consider and vote upon the matters described in this Proxy Statement and any other matters that properly come before the Special Meeting.
| Q: | When and where will the Special Meeting be held? |
| A: | You are invited to attend the Special Meeting on[Ÿ], 2013, beginning at[Ÿ], local time. The Special Meeting will be held at200 Horizon Drive, Suite 115, Raleigh, North Carolina 27615. Requests for directions to this location should be directed to our Corporate Secretary, 110 Horizon Drive, Suite 230, Raleigh, NC 27615 (telephone number (866) 256-1206). |
| Q: | Why did I receive these proxy materials? |
| A: | We are providing these proxy materials in connection with the solicitation by the Board of Directors of the Company of proxies to be voted at the Special Meeting, and at any adjournment or postponement thereof. These materials are available on the Internet athttp://ir.trans1.com/sec.cfm. |
You are receiving this Proxy Statement because, in accordance with the rules of the NASDAQ Stock Market LLC, our stockholders must approve the issuance of shares of our common stock pursuant to the Merger Agreement and the Securities Purchase Agreement. In addition, stockholder approval is required under Delaware law in order for us to change the name of our company.
This Proxy Statement contains important information for you to consider when deciding how to vote on the matters brought before the Special Meeting. You are invited to attend the Special Meeting in person to vote on the proposals described in this Proxy Statement. However, you do not need to attend the Special Meeting to vote your shares. Instead, you may vote your shares using one of the other voting methods described in this Proxy Statement. Whether or not you expect to attend the Special Meeting, please vote your shares as soon as possible in order to ensure your representation at the Special Meeting and to minimize the cost of proxy solicitation.
| Q: | What am I being asked to vote upon at the Special Meeting? |
| A: | At the Special Meeting, you will be asked to: |
| · | Vote upon a proposal to approve the issuance of shares of our common stock pursuant to the Merger Agreement (Proposal No. 1); |
| · | Vote upon a proposal to approve the issuance of shares of our common stock pursuant to the Securities Purchase Agreement (Proposal No. 2); |
| · | Vote upon a proposal to approve an amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc. (Proposal No. 3); |
| · | Vote upon a proposal to grant the proxy holders discretionary authority to vote to adjourn the Special Meeting, if necessary, in order to solicit additional proxies in the event that there are not sufficient affirmative votes present at the Special Meeting to approve Proposal No. 1, Proposal No. 2, and/or Proposal No. 3 (Proposal No. 4); and | |
| · | Act upon such other matters as may properly come before the Special Meeting or any postponement or adjournment thereof. |
| Q: | Are any of the proposals conditioned upon the approval of another proposal? |
| A: | Yes. The issuance of shares of common stock pursuant to the Merger Agreement and the issuance of shares of common stock pursuant to the Securities Purchase Agreement are, among other conditions described in this Proxy Statement, conditioned on each other and, therefore, both proposals must be approved in order for the Merger and the Private Placement Transaction to be consummated. In addition, the name change proposal is contingent upon the results of the stockholder votes on the issuance of common stock pursuant to each of the Merger Agreement and the Securities Purchase Agreement and the consummation of the Merger. Accordingly, we will only change our company’s name to Baxano Surgical, Inc. if (1) each of the name change amendment, the proposal to issue common stock pursuant to the Merger Agreement, and the proposal to issue common stock pursuant to the Securities Purchase Agreement are approved by stockholders and (2) the Merger is consummated. |
| Q: | How does the Board of Directors recommend that I vote on the proposals? |
| A: | The Board of Directors, except for Mr. Shapiro, unanimously recommends that you vote each of your shares “FOR” the approval of the issuance of shares of our common stock pursuant to the Merger Agreement, “FOR” the approval of the issuance of shares of our common stock pursuant to the Securities Purchase Agreement, “FOR” the approval of an amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc., and “FOR” the grant to the proxy holders of discretionary authority to vote to adjourn the Special Meeting, if necessary, in order to solicit additional proxies in the event that there are not sufficient affirmative votes present at the Special Meeting to approve Proposal No. 1, Proposal No. 2, and/or Proposal No. 3. Due to Mr. Shapiro’s relationship with Baxano, hedid not participate in discussions and deliberations regarding the Merger or any of the proposals presented in this Proxy Statement. See “Interests of Certain Persons in the Merger and Private Placement Transaction.” |
| Q: | What are the voting requirements to approve the proposals? |
| A: | The voting requirements to approve each of the proposals to be voted upon at the Special Meeting are as follows: |
| · | Issuance of Common Stock Pursuant to the Merger Agreement (Proposal No. 1) — Assuming the existence of a quorum, approval of the issuance of shares of our common stock pursuant to the Merger Agreement will require the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal. |
| 2 |
| · | Issuance of Common Stock Pursuant to the Securities Purchase Agreement (Proposal No. 2) — Assuming the existence of a quorum, approval of the issuance of shares of our common stock pursuant to the Securities Purchase Agreement will require the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal. |
| · | Name Change Amendment (Proposal No. 3) — Assuming the existence of a quorum, approval of the amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc. will require the affirmative vote of a majority of the shares of our common stock outstanding as of the Record Date (as defined below). Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal. |
| · | Potential Adjournment of the Special Meeting (Proposal No. 4) — Assuming the existence of a quorum, approval of the proposal to grant the proxy holders discretionary authority to vote to adjourn the Special Meeting will require the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal. |
| Q: | What happens if I do not vote? |
| A: | The following is a brief explanation of the effect that a decision not to vote shares will have on the proposals at the Special Meeting: |
| · | Abstentions: You may abstain from voting on one or more of the matters to be voted on at the Special Meeting. Shares that a stockholder abstains from voting will be counted for purposes of determining whether a quorum is present at the Special Meeting, and will have the same effect as a vote “AGAINST” each proposal. |
| · | Broker Non-Votes: “Broker non-votes” are shares held by a broker, dealer, bank, or other nominee (each, a “nominee”) that are represented at the Special Meeting, but with respect to which the nominee holding those shares (i) has not received instructions from the beneficial owner of the shares to vote on the particular proposal, and (ii) does not have discretionary voting power with respect to the particular proposal. Whether a nominee has authority to vote shares that it holds is determined by the rules of the New York Stock Exchange that govern most domestic brokerage firms. Nominees holding shares of record for beneficial owners do not have the discretion to vote on any of the proposals without instructions from the beneficial owners of the shares. Accordingly, we anticipate that there will not be any broker non-votes at the Special Meeting in the absence of any additional matter coming before such meeting as to which brokers have discretionary authority. |
| Q: | Who can vote at the Special Meeting? |
| A: | Only our stockholders of record at the close of business on [Ÿ], 2013, which we refer to as the Record Date, will be entitled to vote at the Special Meeting. As of the Record Date, approximately [Ÿ] shares of our common stock were issued and outstanding. Each share of common stock issued and outstanding on the Record Date is entitled to one vote on any matter presented for consideration and action by our stockholders at the Special Meeting. |
| · | Holders of Record: If, on the Record Date, your shares were registered directly in your name with our transfer agent, American Stock Transfer & Trust Company, then you are a “holder of record.” As a “holder of record,” you may vote in person at the Special Meeting or vote by proxy. Whether or not you plan to attend the Special Meeting, we urge you to vote your shares using one of the voting methods described in this Proxy Statement. |
| · | Beneficial Owners: If, on the Record Date, your shares were held in an account with a nominee, then you are the “beneficial owner” of shares held in “street name” and this Proxy Statement is being forwarded to you by that nominee. The nominee holding your account is considered the “holder of record” for purposes of voting at the Special Meeting. As a beneficial owner, you have the right to direct your nominee how to vote the shares in your account. You are also invited to attend the Special Meeting. However, since you are not the “holder of record,” you may not vote your shares in person at the Special Meeting unless you request and obtain a valid proxy from your nominee. Please contact your nominee directly for additional information. |
| Q: | What is the quorum requirement for the Special Meeting? |
| A: | The presence at the Special Meeting, in person or represented by proxy, of holders of a majority of the outstanding shares of our common stock as of the Record Date, will constitute a quorum at the Special Meeting. We will treat shares of common stock represented by a properly voted proxy, including abstentions and broker non-votes, if any, as present at the Special Meeting for the purpose of determining the existence of a quorum. If a quorum is not present, the Special Meeting will be adjourned until a quorum is obtained. |
| 3 |
| Q: | How can I vote my shares? |
| A: | If you are a holder of record, you may vote by mail or in person at the Special Meeting. If you are a beneficial owner of shares held in “street name,” you may vote by mail, in person at the Special Meeting, or over the Internet. The voting methods available for holders of record and beneficial owners are described in greater detail below. Stockholders who execute proxies retain the right to revoke them at any time before they are voted. Ken Reali and Joseph Slattery, the designated proxy holders, are members of the Company’s management. |
| · | Vote by Mail. You can vote by mail pursuant to the instructions provided on the proxy card. In order to be effective, completed proxy cards must be received by 11:59 p.m., Eastern Time, on[Ÿ], 2013. If you choose to vote by mail, simply mark your proxy, date and sign it, and return it in the business reply envelope provided with this Proxy Statement. |
| · | Vote at the Special Meeting. The method you use to vote will not limit your right to vote at the Special Meeting if you decide to attend in person. If you hold shares beneficially in “street name,” you must obtain a proxy, executed in your favor by your nominee to be able to vote at the Special Meeting. All shares that have been properly voted and not revoked will be voted at the Special Meeting. If you sign and return your proxy card but do not give voting instructions, the shares represented by that proxy will be voted as recommended by the Board of Directors. |
| · | Vote by Internet. If you hold shares beneficially in “street name,” you may vote by proxy over the Internet by following the instructions provided in the voting instruction card provided to you by your nominee. Internet voting is available 24 hours a day and will be accessible until 11:59 p.m., Eastern Time, on[Ÿ], 2013 by visiting www.proxyvote.com and following the instructions. Our Internet voting procedures are designed to authenticate stockholders by using individual control numbers, which are located on the voting instruction card. If you vote over the Internet, you do not need to return your proxy card. |
| Q: | How may I attend the Special Meeting? |
| A: | You are entitled to attend the Special Meeting only if you were a stockholder as of the Record Date or hold a valid proxy for the Special Meeting. Since seating is limited, admission to the Special Meeting will be on a first-come, first-served basis. You should be prepared to present government-issued photo identification for admittance, such as a passport or driver’s license. If your shares are held in “street name,” you also will need proof of ownership as of the Record Date to be admitted to the Special Meeting, such as your most recent account statement prior to the Record Date, a copy of the voting instruction card provided by your nominee, or similar evidence of ownership. Please note that for security reasons, you and your bags may be subject to search prior to your admittance to the Special Meeting. If you do not comply with each of the foregoing requirements, you will not be admitted to the Special Meeting. Please let us know if you plan to attend the Special Meeting by marking the appropriate box on the enclosed proxy card. |
| Q: | What can I do if I change my mind after I vote my shares? |
| A: | You may change your vote at any time before your proxy is voted at the Special Meeting. You may change your vote by (i) providing written notice of revocation to the Corporate Secretary of the Company at our corporate headquarters located at 110 Horizon Drive, Suite 230, Raleigh, NC 27615, (ii) by executing a subsequent proxy using one of the voting methods discussed above, or (iii) by attending the Special Meeting and voting in person. However, simply attending the Special Meeting will not, by itself, revoke your proxy. If you have instructed your nominee to vote your shares, you must follow directions received from your nominee to change those instructions. Subject to any such revocation, all shares represented by properly executed proxies will be voted in accordance with the specifications on the enclosed proxy card. |
| Q: | Could other matters be decided at the Special Meeting? |
| A: | At this time, we are unaware of any matters, other than those matters set forth above, that may properly come before the Special Meeting.If any other matters are properly brought before the Special Meeting, the persons named in the enclosed proxy card will have discretion to vote the proxies in accordance with their best judgment to the same extent as the person delivering the proxy would be entitled to vote. |
| 4 |
| Q: | Who is paying for the cost of this proxy solicitation? |
| A: | The cost of soliciting proxies will be borne by us. We expect to reimburse brokerage firms and other persons representing beneficial owners of shares for their expenses in forwarding solicitation materials to such beneficial owners. Proxies may also be solicited by certain of our directors, officers, and regular employees, without additional compensation, personally or by telephone or facsimile. |
| Q: | Where can I find voting results of the Special Meeting? |
| A: | We will announce preliminary voting results with respect to each proposal at the Special Meeting. In accordance with SEC rules, final voting results will be published in a Current Report on Form 8-K within four business days following the Special Meeting, unless final results are not known at that time, in which case preliminary voting results will be published within four business days of the Special Meeting and final voting results will be published once they are known by the Company. |
The COMPANIES
| Q: | Who are the companies involved in the Merger? |
| A: | TranS1 Inc. | |
| 110 Horizon Drive, Suite 230 | ||
| Raleigh, NC 27615 | ||
| We are a medical device company focused on designing, developing, and marketing products to treat degenerative conditions of the spine affecting the lower lumbar region. | ||
| We currently market the AxiaLIF family of products for single and two level lumbar fusion, the VEO lateral access and interbody fusion system, the Vectre™ lumbar posterior fixation system, and Bi-Ostetic™ bone void filler, a biologics product. We also market products that may be used with our AxiaLIF surgical approach, including bowel retractors and additional discectomy tools, as well as a bone graft harvesting system that can be used to extract bone graft in any procedure for which the use of bone graft is appropriate. | ||
| Our company was incorporated in May 2000 and is headquartered in Raleigh, North Carolina. We are publicly traded on the NASDAQ Global Market under the symbol “TSON.” See the section entitled “Information About TranS1—Business of TranS1” for more information about the Company. | ||
| RacerX Acquisition Corp. | ||
| 110 Horizon Drive, Suite 230 | ||
| Raleigh, NC 27615 | ||
| RacerX Acquisition Corp., or Merger Sub, is a wholly-owned subsidiary of our company that was incorporated under the laws of the State of Delaware on February 27, 2013. We formed Merger Sub exclusively for the purpose of completing the Merger, and Merger Sub does not engage in any operations. | ||
| Baxano, Inc. | ||
| 655 River Oaks Parkway | ||
| San Jose, CA 95134 | ||
| Baxano, Inc., based in San Jose, California, is focused on developing minimally invasive tools to restore spine function and preserve healthy tissue. Its commercially available product is the iO-Flex System for both decompression and fusion applications. The minimally invasive approach of the iO-Flex System instruments allows for safe, complete, and precise removal of soft tissue and osseous tissue to achieve thorough direct decompression while preserving the stabilizing structures of the spine. |
The Transactions
| Q: | Why are we proposing the Merger? |
| A: | In determining that the Merger Agreement and the transactions contemplated thereby are advisable and in the best interests of our company and of our stockholders, and in reaching its decision to approve the Merger, our Board of Directors considered a variety of factors that it believed weighted favorably toward the Merger, including the following: |
| · | the potential for significant revenue growth from Baxano’s minimally invasive surgery, or MIS, spine platform including the proprietary iO-Flex® decompression device and the iO-TomeTM facet removal systems; |
| · | the potential for Baxano’s products to expand and complement our MIS solutions including the proprietary AxiaLIF® interbody fusion system and theVEO™ lateral access and interbody fusion system; |
| · | the potential to provide cost savings by combining sales forces and leveraging Baxano’s direct sales channel with built-in relationships, clinical knowledge, and ability to market and sell economic value; |
| · | the potential to enhance our overall growth strategy through accelerated geographic and market expansion into new domestic markets; |
| · | the potential for increased stockholder value by diversifying our product offerings, achieving cost synergies in the near term, and using greater scale to leverage fixed costs; and |
| · | the potential to improve our financial profile by improving our margins and shortening the expected timeline to achieve breakeven cash flow. |
For more information about the factors considered by our Board of Directors in approving the Merger, see “Proposal No. 1 – Issuance of Common Stock Pursuant to the Merger Agreement—The Merger—Reasons for the Merger.”
| Q: | What will happen in the Merger? |
| A: | Before entering into the Merger Agreement, we formed Merger Sub as a wholly-owned subsidiary of the Company. At the effective time of the Merger, Merger Sub will merge with and intoBaxano and cease to exist, and Baxano will continue as the surviving corporation and will be a wholly-owned subsidiary of the Company. |
At the effective time of the Merger, each outstanding share of Baxano’s capital stock will be cancelled and extinguished and converted into the right to receive a portion of the merger consideration in accordance with the Merger Agreement, except for dissenting shares, which will retain the appraisal rights and dissenters’ rights provided for under Delaware law and California law, respectively. The dollar value of the shares of our common stock to be issued in connection with the Merger is expected to be approximately $23.1 million, subject to fluctuations in the price of our common stock on the NASDAQ Global Market, the amount of certain indebtedness held by Baxano at the effective time of the Merger, the amount of Baxano’s transaction expenses that remain unpaid immediately prior to the effective time, and working capital adjustments. Pursuant to the terms of the Merger Agreement, the merger consideration will consist of a number of newly issued shares of common stock of the Company calculated as the product of (a) 0.38888889times (b) the number of issued and outstanding shares of capital stock of the Company (as if converted to common stock) as of the close of business on the last trading day prior to the closing of the Merger. This calculation is intended to provide Baxano stockholders with ownership of 28% of the Company’s post-closing outstanding shares of common stock (excluding the impact of the Private Placement Transaction) prior to certain adjustments to the merger consideration provided for in the Merger Agreement and which are described below. As a result of the Merger, we expect that the current stockholders of Baxano will own approximately 27.6% of the outstanding shares of our common stock (excluding the impact of the Private Placement Transaction), although this percentage will vary due to fluctuations in the price of our common stock on the NASDAQ Global Market, the amount of certain indebtedness held by Baxano at the effective time of the Merger, the amount of Baxano’s transaction expenses that remain unpaid immediately prior to the effective time, and working capital adjustments. See “Proposal No. 1 – Issuance of Common Stock Pursuant to the Merger Agreement.”
| 5 |
| Q: | What will I receive for my shares in the Merger? |
| A: | Our stockholders will not be exchanging or receiving any consideration for their shares in the Merger. Upon completion of the Merger, you will continue to own shares of our common stock. Because we will issue shares of our common stock to the current stockholders of Baxano, however, the percentage ownership interest that your shares represent in the Company will be reduced. |
| Q: | Are there any conditions to the Merger? |
| A: | Yes. The completion of the Merger depends on the satisfaction or waiver of a number of conditions, including but not limited to: |
| · | certain of the representations and warranties in the Merger Agreement made by us and Baxano must have been true and correct as of the date of the Merger Agreement, and must be true and correct as of the closing date as if made on the closing date, except where failure of such representations or warranties to be so true and correct, individually or in the aggregate with all such failures, has not had and would not reasonably be expected to have a Material Adverse Effect (as defined below); |
| · | each of the obligations in the Merger Agreement that we or Baxano are required to perform on or prior to the closing must be performed in all material respects; |
| · | no Material Adverse Effect with respect to Baxano or with respect to the Company and Merger Sub shall have occurred since the date of the Merger Agreement and be continuing; |
| · | Baxano will have obtained the required approval of its stockholders and we will have obtained the required approval of our stockholders; |
| · | other than the filing of the certificate of merger, all authorizations, consents, orders, or approvals of, or declarations or filings with, or expirations of waiting periods imposed by, any Governmental Entity (as defined below) in connection with the Merger and the consummation of the other transactions contemplated by the Merger Agreement, the failure of which to file, obtain, or occur is reasonably expected to have a Material Adverse Effect shall have been filed, been obtained or occurred on terms and conditions that would not reasonably be expected to have a Material Adverse Effect; |
| · | the dissenting shares shall not include shares of preferred stock of Baxano that, absent a demand for appraisal rights, would be entitled to receive in excess of 5% of the shares issued pursuant to the Merger; |
| · | no Governmental Entity of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any order, executive order, stay, decree, judgment, or injunction (preliminary or permanent) or statute, rule, or regulation that is in effect and that has the effect of making the Merger illegal or otherwise prohibiting consummation of the Merger or the other transactions contemplated by the Merger Agreement; |
| · | all conditions precedent to the Private Placement Transaction must have been satisfied or waived (other than delivery of items to be delivered at the closing under the Securities Purchase Agreement and other than satisfaction of those conditions that by their nature are to be satisfied at such closing); and |
| · | we and Baxano must deliver various certificates and other documents to each other. |
See the section entitled “Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—The Merger Agreement—Conditions to the Merger.”
| Q: | Is there a termination fee potentially payable under the Merger Agreement? |
| A: | Yes. The Merger Agreement grants certain termination rights to us and Baxano. In addition, the Merger Agreement provides that we will be required to pay Baxano a termination fee equal to $2,000,000 and to reimburse Baxano for its expenses incurred relating to the transactions contemplated by the Merger Agreement, up to a cap of $750,000, if Baxano or the Company terminates the Merger Agreement under certain conditions. The Merger Agreement also provides that Baxano will be required to pay us a termination fee equal to $1,000,000 and to reimburse us for our expenses incurred relating to the transactions contemplated by the Merger Agreement, up to a cap of $750,000, if we terminate the Merger Agreement under certain conditions. See the section entitled “Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—The Merger Agreement—Termination.” |
| Q: | Has a fairness opinion been issued with respect to the Merger? |
| A: | Yes. Our financial advisor Stifel, Nicolaus & Company, Incorporated, or Stifel, delivered its opinion to our Board of Directors on March 1, 2013 that, as of the date of the opinion and based upon and subject to the factors, assumptions, limitations, and qualifications set forth therein, the aggregate consideration to be paid by us in the Merger pursuant to the Merger Agreement is fair to TranS1, from a financial point of view. The full text of the written opinion of Stifel, dated March 1, 2013, which sets forth the assumptions made, procedures followed, matters considered, and limitations on the review undertaken in connection with the opinion, is attached asAnnex C to this Proxy Statement. Our stockholders should read the opinion in its entirety. Stifel provided its opinion for the information and assistance of our Board of Directors in connection with its consideration of the Merger. Stifel’s opinion is not a recommendation to our Board of Directors as to how it should vote on the Merger or to any TranS1 stockholder or Baxano stockholder as to how such stockholder should vote his, her, or its shares of common stock with respect to the Merger. See the section entitled “ Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—The Merger—Opinion of Financial Advisor – Stifel, Nicolaus & Company, Incorporated.” |
| Q: | Do I have appraisal rights? |
| A: | No. Our stockholders do not have appraisal rights under Delaware law in connection with the Merger. |
| Q: | Why are we proposing the Private Placement Transaction? |
| A: | The Board of Directors determined that it is in our and our stockholders’ best interests to conduct a private placement offering of our common stock on the terms set forth in the Securities Purchase Agreement. We intend to use the net proceeds from the Private Placement Transaction for working capital and general corporate purposes of the Company post-Merger. |
| Q: | What will happen in the Private Placement Transaction? |
| A: | Pursuant to the terms of the Securities Purchase Agreement, we will sell and issue to the Investors, and the Investors will purchase from us, an aggregate of 7,522,009 shares of our common stock, at a purchase price of $2.28 per share, resulting in gross proceeds to us of approximately $17.2 million. We intend to use the net proceeds from the Private Placement Transaction for working capital and general corporate purposes of the Company post-Merger. We have not targeted the net proceeds being raised in the Private Placement Transaction with the intention of achieving a particular percentage ownership in the Company for any of the Investors or any group of Investors following the closing of the Private Placement Transaction. See the section entitled “Proposal No. 2 - Issuance of Common Stock Pursuant to the Securities Purchase Agreement—The Private Placement Transaction.” |
| Q: | What percentage of the Company’s shares will be held by stockholders of Baxano and the Investors after the Merger and the Private Placement Transaction? |
| A: | We anticipate that the aggregate number of shares of our common stock to be issued to the stockholders of Baxano at the closing of the Merger, and to the Investors at the closing of the Private Placement Transaction, will represent up to approximately 39.9% of the outstanding shares of our common stock immediately following the closing of the Merger and the Private Placement Transaction. See “Interests of Certain Persons in the Merger and Private Placement Transaction.” |
| Q: | Are there any registration rights, lock-up provisions, and/or voting agreements related to the Merger and the Private Placement Transaction? |
| A: | Yes. Pursuant to the Securities Purchase Agreement, we agreed to register the resale of the shares of our common stock to be issued pursuant to the Merger Agreement and the Securities Purchase Agreement under a registration statement on Form S-3 (or another appropriate form if Form S-3 is not then available to us). In addition, the Investors and all other holders of shares received pursuant to the Merger Agreement agreed not to sell, transfer, or otherwise dispose of the shares of our common stock issued at the closing of the Merger and the Private Placement Transaction from the period commencing on the closing of the Private Placement Transaction and expiring on the effective date of such registration statement, subject to certain exceptions. See the section entitled “Proposal No. 2 - Issuance of Common Stock Pursuant to the Securities Purchase Agreement—Lock-up and Registration of Shares.” |
In addition, on March 3, 2013 we entered into Stockholder Agreements, or the Stockholder Agreements, with Baxano and certain securityholders of TranS1 identified on the signature pages thereto, or the Stockholders, which include our directors, executive officers, and certain of our affiliates, and the Securityholder Representatives. The Stockholders own in the aggregate approximately 6.6 million shares of our common stock, constituting approximately 24.2% of the outstanding shares of our common stock. Pursuant to the Stockholder Agreements, the Stockholders have agreed to vote all of their shares in favor of the proposals to issue shares of our common stock pursuant to the Merger Agreement and the Securities Purchase Agreement and in favor of the election and re-election of individuals designated to our Board of Directors by the Securityholder Representatives pursuant to the Merger Agreement at our 2013 Annual Meeting of Stockholders and any replacements of such designees until our 2016 Annual Meeting of Stockholders. The Stockholders have also agreed that they will not transfer any shares of our common stock (except to permitted transferees) until the later of the date the registration statement required to be filed under the Securities Purchase Agreement has been declared effective by the SEC or the date directors are elected at TranS1’s 2013 annual meeting of stockholders. We are a party to the Stockholder Agreements solely for purposes of enforcing such transfer restrictions and voting agreements. See the section entitled “Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—Stockholder Agreements.”
| Q: | What will be the composition of the Board of Directors after the Merger and the Private Placement Transaction? |
| A: | The Merger Agreement provides that we will promptly take all actions necessary to fix the number of members of our Board of Directors at nine persons and appoint two directors designated by Baxano to serve as Class III members of our Board of Directors. In addition, we agreed to take all actions necessary to re-elect such directors designated by the Securityholder Representatives to our Board of Directors at our 2013 annual meeting of stockholders such that they would serve as directors at least through our 2016 annual meeting of stockholders. The Merger Agreement also provides that the number of members of our Board of Directors will be reduced to eight persons at the time of our 2013 annual meeting of stockholders. To implement these provisions, one of our existing directors will be required to resign from our Board of Directors at the time of our 2013 annual meeting of stockholders. |
| Q: | Do any of the Company’s directors or executive officers have an interest in the Merger or the Private Placement Transaction beyond the interest of the Company’s stockholders generally? |
| A: | In considering the recommendation of the Board of Directors with respect to the issuance of shares of our common stock pursuant to the Merger Agreement and the Securities Purchase Agreement, you should be aware that James Shapiro, a member of our Board of Directors, has interests in the Merger that are different from, or in addition to, the interests of our stockholders generally. Mr. Shapiro is also a member of Baxano’s board of directors. While he does not own any shares of Baxano’s common stock individually, as a Managing Member of the General Partner of Kearny Venture Partners, L.P. and Kearny Venture Partners Entrepreneurs’ Fund, L.P., Mr. Shapiro has shared voting and dispositive power over, and a partial indirect pecuniary interest in 7,285,290 shares of Baxano’s common stock equivalents. In addition, these funds hold approximately $1,961,571 in aggregate principal amount of the Baxano Notes (as defined below). Therefore, Mr. Shapiro and Kearny Venture Partners will have the right to receive a portion of the merger consideration in accordance with the Merger Agreement. Other than Mr. Shapiro, none of our directors or executive officers has any interests in the Merger that are different from, or in addition to, the interests of our stockholders generally. The Board of Directors was aware of Mr. Shapiro’s potential conflict of interest and Mr. Shapiro did not participate in discussions and deliberations related to the Merger. |
In addition, certain of our directors and executive officers and certain of Baxano’s securityholders are Investors in the Private Placement Transaction. Baxano’s securityholders have agreed to purchase an aggregate of 6,729,909 shares of common stock for a total of $15.3 million in connection with the Private Placement Transaction. The following directors and executive officers have agreed to purchase an aggregate of 123,242 shares of common stock for a total of $281,000 in connection with the Private Placement Transaction: Jeffrey Fischgrund, Ken Reali, David Simpson, Joseph Slattery, and Mark Stautberg.
Because the amount of merger consideration to be paid in connection with the Merger is targeted to provide Baxano stockholders with ownership of 28% of the Company’s post-closing outstanding shares of common stock (excluding the impact of the Private Placement Transaction) prior to certain adjustments, while the shares of common stock to be issued in connection with the Private Placement Transaction have a fixed price, the value of the shares to be issued in connection with the Merger may be different than the value of the shares to be issued in connection with the Private Placement Transaction. This difference in value cannot be quantified until after the Merger is consummated.
The Board of Directors considered Mr. Shapiro’s interests in the Merger and the participation of our directors and executive officers in the Private Placement Transaction, among other matters, in making its recommendation that you approve the issuance of shares of our common stock as described in this Proxy Statement. In addition, certain directors, officers, and employees of Baxano have interests in the Merger that are different from, or in addition to, the interests of Baxano stockholders generally. See the section entitled “Interests of Certain Persons in the Merger and Private Placement Transaction” for a description of the benefits that will accrue to each affiliated person of the Company and Baxano related to the Merger and the Private Placement Transaction.
| 6 |
| Q: | What are the U.S. federal income tax consequences of the Merger to me? |
| A: | We intend for the Merger to qualify for federal income tax purposes as a tax-free reorganization under the provisions of Section 368(a) of the U.S. Internal Revenue Code of 1986, as amended, or the Internal Revenue Code. The Merger is not expected to have any U.S. federal income tax consequences for our current stockholders. |
| Q: | What is the anticipated accounting treatment for the Merger? |
| A: | The Merger will be accounted for as an acquisition of Baxano by us under the purchase method of accounting under accounting principles generally accepted in the United States, or GAAP. See the section entitled “Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—Anticipated Accounting Treatment of the Merger.” |
| Q: | Are there risks I should consider in deciding whether to vote to approve the issuance of shares of common stock pursuant to the Merger Agreement and the Securities Purchase Agreement? |
| A: | Yes. In evaluating the issuance of shares of our common stock pursuant to the Merger Agreement and the Securities Purchase Agreement, you should carefully consider the factors discussed in “Risk Factors” and the other matters discussed in this Proxy Statement. |
In particular, you should be aware that, since its inception in 2005, Baxano has incurred significant losses and, as of December 31, 2012, Baxano had an accumulated deficit of $70.4 million. Baxano has not yet achieved profitability and anticipates that it will continue to incur losses for the foreseeable future. Baxano expects that research and development, sales and marketing, and general and administrative expenses will continue to grow and, as a result, Baxano will need to generate significant revenues to achieve profitability. Baxano’s recurring losses raise substantial doubt about its ability to continue as a going concern. As a result, Baxano’s independent registered public accounting firm included an explanatory paragraph in its report on Baxano’s financial statements as of and for the year ended December 31, 2012 with respect to this uncertainty. At December 31, 2012, Baxano believed that its existing cash and cash equivalents, taken together with cash received from sales of its products, would be sufficient only to meet its cash needs through the first quarter of 2013.
On March 25, 2013, our Board of Directors authorized Company management to provide up to $2,500,000 of bridge financing to Baxano for its working capital needs through the effective time of the Merger. From time to time prior to the effective time of the Merger, Baxano may request that the Company provide bridge financing for specified budget items. If the parties agree that the Company will provide all or any portion of the requested amount, then the Company will loan Baxano such amount and Baxano will issue a promissory note, or a Bridge Note, to the Company to evidence the loan amount. To date, Baxano has issued $2,100,000 aggregate principal amount of Bridge Notes to the Company. For a description of the terms of this bridge financing, see“Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—Bridge Financing.”
| Q: | Where will my shares of common stock be listed after completion of the Merger and the Private Placement Transaction? |
| A: | Our common stock is currently listed on the NASDAQ Global Market under the symbol “TSON” and will continue to be listed on the NASDAQ Global Market after completion of the Merger and the Private Placement Transaction. In connection with our proposal to amend our Amended and Restated Certificate of Incorporation to change our name, we have reserved the NASDAQ stock symbol “BAXS.” If the name change amendment is approved, we intend to request that our common stock be traded on the NASDAQ Global Market under this new stock symbol, rather than under the current “TSON” symbol. |
| Q: | When does the Company expect to complete the Merger and the Private Placement Transaction? |
| A: | We currently expect to complete the Merger and the Private Placement Transaction in the second quarter of 2013. However, because the Merger is subject to stockholder approval and other conditions to closing, we cannot predict exactly when the closing will occur. |
| Q: | Whom should I contact with other questions? |
| A: | If you have additional questions about the Special Meeting, the Merger, or the Private Placement Transaction or would like additional copies of this Proxy Statement, please contact us by either writing to TranS1 Inc. at our principal executive offices located at 110 Horizon Drive, Suite 230, Raleigh, NC 27615, Attention: Corporate Secretary, by calling us at (866) 256-1206, or by emailing us at merger@trans1.com. |
| 7 |
RISK FACTORS
You should carefully consider the following risk factors in evaluating whether to approvethe issuance of shares of our common stock pursuantto the Merger Agreement and theSecurities Purchase Agreement. These factors should be considered in conjunction with the other information included in thisProxy Statement, including the forward-looking statements made herein. The followingrisk factorsdo not include all risks that we will face as a result of theMergerand thePrivate Placement Transaction. Additional risks related to our existing business and markets, which will continue to confront us whether or not theMergerand thePrivate Placement Transactionoccur, are described in this Proxy Statement and in our public filings with the SEC, including ourAnnual ReportsonForm 10-K and Quarterly ReportsonForm10-Q.
Risks Related to the Merger, the Private Placement Transaction, and the Combined Company
Wemay not realize all of the anticipated benefits of the Merger.
The success of theMergerwill depend, in part, on our ability to realize the anticipated growth opportunities and synergies from combining the businesses of our company andBaxano. The integration of the businesses is a complex, time-consuming, and expensive process that, without proper planning and effective and timely implementation, could significantly disrupt our operations following closing. Our ability to realize the benefits of the Merger, and the timing of this realization, depend upon a number of factors, including:
| · | obtaining and maintaining patent rights relating to Baxano’s products; |
| · | effectively consolidating our sales, marketing, research and development, and other operations; |
| · | retaining and attracting key employees; |
| · | integrating and harmonizing financial reporting and information technology systems; |
| · | consolidating corporate and administrative functions; |
| · | preserving our and Baxano’s important business relationships; |
| · | minimizing the diversion of management’s attention from ongoing business concerns; and |
| · | establishing and maintaining appropriate internal controls over financial reporting and disclosure controls and procedures. |
In addition, the actual integration may result in additional and unforeseen expenses, and the anticipated benefits of the integration plan may not be realized. Actual cost synergies, if achieved at all, may be lower than we expect and may take longer to achieve than anticipated. If we are not able to adequately address these challenges, we may be unable to successfully integrate our operations with Baxano’s operations, or to realize the anticipated benefits of the integration following the closing. The anticipated benefits and synergies assume a successful integration and are based on projections, which are inherently uncertain, and other assumptions. Even if integration is successful, anticipated benefits and synergies may not be achieved. An inability to realize the full extent of, or any of, the anticipated benefits of the Merger, as well as any delays encountered in the integration process, could have an adverse effect on our business and results of operations, which may affect the value of the shares of our common stock after the closing.
Completion of theMergerand thePrivate Placement Transactionwill result in immediate dilution of your ownership interest in the Company, and you may not realize a benefit from theMergerand thePrivate Placement Transactioncommensurate with the ownership dilution you will experience.
Issuing shares of our common stockto the stockholders ofBaxanoin theMergerand tothe Investorsin thePrivate Placement Transactionwill dilute the ownership interests of our existing stockholders. If we are unable to realize the strategic, operational, and financial benefits currently anticipated from theMergerand thePrivate Placement Transaction, you may experience dilution of your ownership interest in the Company without receiving any commensurate benefit.
| 8 |
TheMergerand thePrivate Placement Transactionare subject to conditions to closing that could result in such transactions being delayed or not consummated, which could negatively impact our stock price and future business and operations.
TheMergerand thePrivate Placement Transactionare subject to conditions to closing as set forth in the Merger Agreement and the Securities Purchase Agreement, including obtaining the requisite approval of our stockholders ofthe issuance of shares of our common stock pursuantto the Merger Agreement and the Securities Purchase Agreement. In addition, it is a condition to the closing of theMergeror thePrivate Placement Transactionthat both transactions close concurrently. If any of the conditions to the closing of theMergerand thePrivate Placement Transactionare not satisfied or, where permissible, not waived, neither theMergernor thePrivate Placement Transactionwill be consummated. Failure to consummate theMergerand thePrivate Placement Transactioncould negatively impact our stock price, future business and operations, and financial condition. In addition, any delay in the consummation of theMergerand thePrivate Placement Transaction, or any uncertainty about the consummation of theMergerand thePrivate Placement Transaction, may adversely affect our future business, growth, revenue, and results of operations.
The unaudited pro forma financial statementscontained in this Proxy Statement are presented for illustrative purposes only and may not be an indication of ourfinancial condition or results of operationsfollowing theMergerand thePrivate Placement Transaction.
The unaudited pro forma financial statementscontained in this Proxy Statement are presented for illustrative purposes only, are based on various adjustments, assumptions, and preliminary estimates, and are not necessarily indicative of ourfinancial condition or results of operationsfollowing theMergerand thePrivate Placement Transaction. The unaudited pro forma financial statementshave been derived from the historical financial statements of both the Company andBaxanoand certain adjustments and assumptions have been made regarding the Company after giving effect to theMergerand thePrivate Placement Transaction. The information upon which these adjustments and assumptions have been made is preliminary, and such adjustments and assumptions are difficult to make with complete accuracy. Moreover,the unaudited pro forma financial statementsdo not reflect all costs that are expected to be incurred by us in connection with the transactions. As a result, our actualfinancial condition and results of operationsfollowing theMergerand thePrivate Placement Transactionmay differ significantly fromthese unaudited pro forma financial statements.
In addition, the assumptions used in preparingthe unaudited pro forma financial informationmay not prove to be accurate, and other factors may affect ourfinancial condition or results of operationsfollowing theMergerand thePrivate Placement Transaction. Any potential decline in ourfinancial condition or results of operationsmay impact our stock price.
If the former stockholders ofBaxanoand/or the Investors sell a substantial amount of our common stock received in theMerger and the Private Placement Transaction, they could cause our common stock price to decline.
We have agreed to file a registration statement on Form S-1 or FormS-3to registerthe resale of the shares of our common stockto be issued to the stockholders ofBaxanoin theMergerand tothe Investorsin thePrivate Placement Transactionunder the federal securities laws. Pursuant to the Securities Purchase Agreement, the investors in the Private Placement Transaction and all other holders of shares received pursuant to the Merger Agreement agreed not to sell, transfer, or otherwise dispose of the shares of our common stock issued at the closing of the Merger and the Private Placement Transaction from the period commencing on such closing and expiring on the effective date of such registration statement, subject to certain exceptions. Once the registration statement is declared effective, allof the shares of common stockissued pursuant to the Merger Agreement and the Securities Purchase Agreement will be available for resale in the public market. The sale of a substantial number of shares of our common stock by former Baxano stockholders, the Investors, or our other stockholders within a short period of time could cause our stock price to decrease and make it more difficult for us to raise funds through future offerings of common stock.
| 9 |
Wehave incurred and will incur significant costs in connection with the Merger and the Private Placement Transaction, whether or not we complete them.
We have incurred significant costs related to theMergerand thePrivate Placement Transactionand we expect to incur significant additional costs. These costs include our financial advisory, legal, and accounting fees, expenses, and other charges.In addition, the Merger Agreement grants certain termination rights to Baxano under which we would be required to pay Baxano a termination fee equal to $2,000,000 and to reimburse Baxano for its expenses incurred relating to the transactions contemplated by the Merger Agreement, up to a cap of $750,000.We may also incur additional unanticipated costs for any of a number of reasons. Such costs will reduce the assets that we would have if theMergerand thePrivate Placement Transactionare not consummated, or that we would have to operate our business after theMergerand thePrivate Placement Transaction.
After the completion of theMerger, the surviving corporation, which will be a wholly-owned subsidiary of the Company, will possess not only all of the assets, but also all of the liabilities ofBaxano. Discovery of previously undisclosed or unknown liabilities couldhave an adverse effect on our business, operating results, and financial condition.
Acquisitions involve risks, including inaccurate assessment of undisclosed, contingent, or other liabilities or problems. After the completion of theMerger, the surviving corporation, which will be a wholly-owned subsidiary of the Company, will possess not only all of the assets, but also all of the liabilities ofBaxano. Although we conducted a due diligence investigation ofBaxanoand its known and potential liabilities and obligations, it is possible that undisclosed, contingent, or other liabilities or problems may arise after the completion of theMerger, which couldhave an adverse effect on our business, operating results, and financial condition. The amount of such liabilities may be in excess of the value of theMergerconsideration that will be placed into an escrow fund for 12 months following the closing of theMergerto secure our rights to indemnification under the Merger Agreement, or we may not be entitled to indemnification for such liabilities under the Merger Agreement, or such liabilities may not be uncovered until after the escrow fund has been released.
Baxano has incurred losses since inception and expects to continue to incur losses for the foreseeable future. It will need to raise additional capital in order to pursue its business strategy.
Baxano has incurred significant losses since its inception in 2005 and, as of December 31, 2012, had an accumulated deficit of $70.4 million. As a result of Baxano’s operating losses and negative cash flows, the report of its independent registered public accounting firm on the financial statements as of and for the year ended December 31, 2012, included an explanatory paragraph indicating that there is substantial doubt about Baxano’s ability to continue as a going concern. To date, Baxano’s operations have been funded primarily with proceeds from the sale of preferred stock and, most recently, from issuance of convertible promissory notes and term loans. Gross proceeds from preferred stock sales totaled $58.6 million to date, and gross proceeds from Baxano’s March 2012 and October 2012 convertible promissory note issuances were approximately $14.8 million. If the Merger is consummated, we intend to spend substantial sums on sales and marketing initiatives to support the ongoing commercialization of Baxano’s products and on research and development activities, including product development, regulatory and compliance, clinical studies in support of its currently marketed products and future product offerings, and the enhancement and protection of its intellectual property. As a result, we expect to continue to incur operating losses as we support these initiatives. These losses will continue to have an adverse effect on our stockholders’ equity, and the product lines we acquire from Baxano may never achieve or sustain profitability.
At December 31, 2012, Baxano believed that its existing cash and cash equivalents, taken together with cash received from sales of its products, would be sufficient only to meet its cash needs through the first quarter of 2013. On March 25, 2013, our Board of Directors authorized Company management to provide up to $2,500,000 of bridge financing to Baxano for its working capital needs through the effective time of the Merger. To date, Baxano has issued $2,100,000 aggregate principal amount of Bridge Notes to the Company. See “Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—Bridge Financing.” If the Merger and Private Placement Transaction are consummated, we will need to obtain additional financing to pursue our combined business strategy, to respond to new competitive pressures, or to take advantage of opportunities that may arise. Any additional financing may not be available in amounts or on terms acceptable to us. If we are unable to obtain this additional financing, we may be required to reduce the scope of our planned product development and marketing efforts for Baxano’s product lines.
RisksRelated to Our Business
To be commercially successful, spine surgeons must accept that our products are a safe and effective alternative to other surgical treatments of certain spine disorders.
Our revenue is primarily derived from sales of our AxiaLIF and VEO products and related surgical instruments. We expect that sales of our AxiaLIF and VEO products will continue to account for a substantial portion of our revenues for the foreseeable future. We believe spine surgeons may not widely adopt our products unless they determine, based on experience, long-term clinical data, and published peer reviewed journal articles, that our products provide a safe and effective alternative to conventional procedures used to treat certain spine disorders or other non-conventional procedures offered by our competitors. Spine surgeons may be slow to adopt our technology for the following reasons, among others:
| · | lack of long-term clinical data supporting additional patient benefits; |
| · | lack of experience with our products; |
| · | lack of evidence supporting the cost savings, clinical efficacy, or safety of our products and procedure over competitive products and medical technologies; |
| · | perceived liability risks generally associated with the use of new products and procedures; |
| · | training time required to use a new product or procedure; and |
| · | availability of adequate coverage and reimbursement for hospitals and surgeons. |
If we are unable to effectively demonstrate to spine surgeons the benefits of our products as compared to other surgical treatments of spine disorders that are available to them and our products fail to achieve market acceptance, our future revenues will be adversely impacted. In addition, we believe recommendations and support of our products by influential spine surgeons are essential for market acceptance and adoption. If we do not receive support from these spine surgeons or do not have favorable long-term clinical data, spine surgeons may not use our products and our future revenues will be harmed, which could result in a decline in the price of our common stock.
| 10 |
The efficacy of our products is not yet supported by long-term clinical data and may therefore prove to be less effective than initially thought.
We obtained clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or 510(k) clearance, to manufacture, market, and sell all of our currently U.S. marketed products for which U.S. Food and Drug Administration, or FDA, clearance is required. In order to obtain 510(k) clearance, a manufacturer must demonstrate to the FDA’s satisfaction that its device is “substantially equivalent” to other legally marketed devices not requiring a premarket approval, or PMA. In contrast, certain high-risk and/or new devices require submission of a PMA application to the FDA. Such a PMA application must demonstrate that the device is safe and effective for the proposed indication, and must be supported by extensive data from preclinical studies and human clinical trials. The FDA’s 510(k) clearance process is therefore less costly and rigorous than the PMA process and requires less supporting clinical data. Because our devices were cleared for marketing through the 510(k) process or were exempt from the 510(k) process, we currently lack the breadth of published long-term clinical data supporting the efficacy of our AxiaLIF and VEO products and the benefits they offer that might have been generated in connection with the PMA process.
The demand for our products and the prices that customers and patients are willing to pay for our products depend upon the ability of our customers to obtain adequate third-party coverage and reimbursement for their purchases of our products.
Sales of our products depend in part on the availability of adequate coverage and reimbursement from governmental and private payors. In the United States, healthcare providers that purchase our products generally rely on third-party payors, principally Medicare, Medicaid, and private health insurance plans, to pay for all or a portion of the costs and fees associated with our AxiaLIF and VEO procedures.
Physicians generally use billing codes known as Current Procedural Terminology, or CPT, codes to report professional services rendered for reimbursement purposes. The CPT coding system is maintained and updated on an annual basis by the CPT Editorial Panel, or the Panel. In 2008, most of the surgeons performing our AxiaLIF procedure billed third-party payors using existing ALIF procedure codes. Effective January 1, 2009, the American Medical Association, or the AMA, however, created a new emerging technology CPT code — or Category III code — to facilitate data collection on and assessment of new services and procedures such as the AxiaLIF procedure. This change made it more difficult for physicians to obtain reimbursement for our AxiaLIF procedure because many commercial payors view a Category III code as “experimental” or “investigational” and thus will not pre-approve the procedure or will decline to pay for the procedure until they see additional clinical evidence. This uncertainty around the availability and amount of reimbursement has caused some physicians to revert to other fusion surgeries where coverage and reimbursement are more certain. We made a presentation to the Panel at their October 2011 meeting, asking them to consider transitioning the Category III code to a permanent Category I code for our AxiaLIF procedures. On November 8, 2011, we received notice from the AMA that the Panel rejected our request to convert Category III codes 0195T and 0196T, used to bill for the service in which our product is used, to Category I codes. We subsequently presented to the Panel at their February 2012 meeting, again asking them to consider transitioning the Category III code to a permanent Category I code for our AxiaLIF procedures. On March 5, 2012, we announced that the Panel voted to approve an application for a new Category I CPT code, 22586, for L5/S1 spinal fusion utilizing our AxiaLIF implant when performing a pre-sacral interbody fusion. In addition, the Panel voted to establish a new Category III CPT code, 0309T, as an add-on code to the new Category I code for use with 22586 when performing L4/5 spinal fusion. The new CPT codes were announced on the AMA’s website on March 2, 2012 and became effective on January 1, 2013. The Medicare final rule was released in November 2012, which stated a valuation of the Category I CPT code 22586 for pre-sacral interbody single level spinal fusion at L5-S1, and became effective January 1, 2013. This CPT code, which applies to our AxiaLIF Plus 1-Level device, is a bundled lumbar arthrodesis procedure that includes bone graft, posterior instrumentation and fixation.
Third-party payors continue to review their coverage policies carefully for existing and new therapies and can, without notice, deny coverage for treatments that include the use of our products. While we have received several positive coverage and reimbursement decisions from payors for the use of our products during the period from January 2012 through February 2013 and are currently engaged in discussions with other payors, it is difficult to predict the timing of receiving any additional positive coverage decisions and there is no guarantee that payors will make any additional positive coverage decisions. Our business would be negatively impacted to the extent any such coverage decisions reduce our customers’ ability to obtain coverage and reimbursement for the procedures using our products. In addition, our stock price could be negatively affected to the extent that coverage decisions by payors do not reflect market expectations for such decisions.
| 11 |
With respect to coverage and reimbursement outside of the United States, reimbursement systems in international markets vary significantly by country, and by region within some countries, and reimbursement approvals must be obtained on a country-by-country basis and can take up to 18 months, or longer. Many international markets have government-managed healthcare systems that govern reimbursement for new devices and procedures. In most markets, there are private insurance systems as well as government-managed systems. Additionally, some foreign reimbursement systems provide for limited payments in a given period and therefore result in extended payment periods. Reimbursement in international markets may require us to undertake country-specific reimbursement activities, including additional clinical studies, which could be time consuming, expensive and may not yield acceptable reimbursement rates.
Furthermore, healthcare costs have risen significantly over the past decade. There have been and may continue to be proposals by legislators, regulators, and third-party payors to contain these costs. These cost-control methods include prospective payment systems, capitated rates, group purchasing, redesign of benefits, pre-authorizations or second opinions prior to major surgery, encouragement of healthier lifestyles, and exploration of more cost-effective methods of delivering healthcare. Some healthcare providers in the United States have adopted or are considering a managed care system in which the providers contract to provide comprehensive healthcare for a fixed cost per person. Healthcare providers may also attempt to control costs by authorizing fewer elective surgical procedures or by requiring the use of the least expensive devices possible. These cost-control methods also potentially limit the amount that healthcare providers may be willing to pay for certain types of medical procedures.
In addition, in the United States, no uniform policy of coverage and reimbursement for medical technology exists among payors. Therefore, coverage of and reimbursement for medical technology can differ significantly for each payor. The continuing efforts of third-party payors, whether governmental or commercial, whether inside the United States or outside, to contain or reduce these costs, combined with closer scrutiny of such costs, could restrict our customers’ ability to obtain adequate coverage and reimbursement from these third-party payors. The cost containment measures that healthcare providers are instituting both in the United States and internationally could harm our business by adversely affecting the demand for our products or the price at which we can sell our products.
Reforms to the United States healthcare system may adversely affect our business.
The healthcare industry continues to undergo significant changes designed to increase access to medical care, improve safety, and contain costs. In March 2010, U.S. President Barack Obama signed the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Health Care Reform Law, which makes a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other things, the Health Care Reform Law requires medical device manufacturers to pay a sales tax equal to 2.3% of the price for which such manufacturer sells its medical devices, beginning January 1, 2013. Other provisions of this legislation, including Medicare provisions aimed at improving quality and decreasing costs, comparative effectiveness research, an independent payment advisory board, and pilot programs to evaluate alternative payment methodologies, could meaningfully change the way healthcare is developed and delivered, and may adversely affect our business and results of operations. Certain provisions of the legislation will not be effective for a number of years, the details of many programs and requirements have not yet been fully established, and we cannot predict the full impact of the legislation. We anticipate that many of the provisions in the Health Care Reform Law will be subject to further clarification and modification through the rule-making process, the development of agency guidance, and judicial interpretations.
In addition, other legislative and regulatory changes have resulted in limitations on and, in some cases, reductions in levels of payments to healthcare providers for certain services under the Medicare program. On August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee was unable to achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, thereby triggering an automatic reduction in spending programs. These automatic cuts will be made to several government programs and, with respect to Medicare, would include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year. These automatic cuts were scheduled to start on January 2, 2013, but the American Taxpayer Relief Act of 2012 delayed the start date to March 1, 2013. We are unable to predict how these spending reductions will be structured, what other deficit reduction initiatives may be proposed by Congress, or whether Congress will attempt to further suspend or restructure the automatic budget cuts. Additionally, the current economic environment has increased the budgetary pressures on many states, and these budgetary pressures have resulted, and likely will continue to result, in decreased spending, or decreased spending growth, for Medicaid programs in many states.
| 12 |
Further federal and state proposals for healthcare reform are likely. We cannot predict what further reform proposals, if any, will be adopted, when they may be adopted, or what impact they may have on us. However, any changes that lower reimbursements for our products or reduce medical procedure volumes could adversely affect our business and results of operations.
We have incurred losses since inception and we expect to continue to incur losses for the foreseeable future. We may never achieve or sustain profitability.
We have incurred net losses since our inception in May 2000 and we have an accumulated deficit of $138.8 million through December 31, 2012. As a result of our operating losses and negative cash flows, the report of our independent registered public accounting firm on the financial statements as of and for the year ended December 31, 2012, included an explanatory paragraph indicating that there is substantial doubt about our ability to continue as a going concern. To date, we have financed our operations primarily through sales of our equity securities, including the sale of 6,200,000 shares of our common stock for aggregate net proceeds of approximately $18.2 million in September 2011, and have devoted substantially all of our resources to research and development of our products, the commercial launch of our products, and the development of a sales team to market our products. We expect our expenses to increase in connection with our clinical trials and research and development activities, as well as to support our sales and marketing efforts. As a result, we expect to continue to incur operating losses for the foreseeable future. These losses will continue to have an adverse effect on our stockholders’ equity and we may never achieve or sustain profitability.
We are in a highly competitive market segment, which is subject to rapid technological change.
The market for treatment of spine disorders is highly competitive and subject to rapid and profound technological change. Our success depends, in part, upon our ability to maintain a competitive position in the development of technologies and products for use in the treatment of spine disorders. We face competition from both established and development stage companies. Many of these companies have competitive advantages, including:
| · | greater financial and human resources for product development, sales and marketing and patent litigation; |
| · | significantly greater name recognition; |
| · | established relationships with spine surgeons, customers and third-party payors; |
| · | reimbursement codes providing for adequate coverage and reimbursement to hospitals and surgeons; |
| · | additional lines of products, and the ability to offer rebates or bundle products to offer greater discounts or incentives to gain a competitive advantage; |
| · | established sales and marketing, and distribution networks; and |
| · | greater experience in developing new products, conducting clinical trials, preparing regulatory submissions and obtaining regulatory clearance or approval for products and marketing approved products. |
Our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner, receive adequate reimbursement, and demonstrate that our products are safer and less invasive than alternatives available for the same purpose. Because of the size of the potential market, we anticipate that companies will dedicate significant resources to developing competing products. If we cannot successfully introduce new products, adapt to changing technologies, or anticipate changes in our current and potential customers’ requirements, our products may become obsolete and our business could suffer.
Our failure to continue building effective sales and marketing capabilities for our products could significantly impair our ability to increase sales of our products.
We utilize a hybrid model of independent sales agents and direct sales representatives for product sales in the United States and rely on third-party distributors and independent sales agents for international sales. As of December 31, 2012, we employed 26 direct sales representatives, one case coverage specialist, three regional managers and two area vice-presidents in the United States. Effective January 1, 2012, we converted our direct sales representatives in Europe to an independent sales agent, representing Germany, Netherlands, and Belgium. In January 2013, we terminated this sales agent and are transitioning to other distribution channels in those regions. If we are unable to efficiently manage our sales representatives, our sales will suffer.
| 13 |
We also rely on marketing arrangements with independent sales agents in the United States and independent distributors in Europe. We do not control, nor monitor the marketing practices of our independent sales agents or distributors and they may not be successful in implementing our marketing plans or complying with applicable laws regarding marketing practices. Independent distributors and sales agents may terminate their relationship with us, or devote insufficient sales efforts to our products, which could have an adverse effect on our operations.
Our future success depends on our ability to develop, receive regulatory clearance or approval for, and introduce new products or product enhancements that will be accepted by the market in a timely manner.
It is important to our business that we continue to build a more complete product offering for treatment of spine disorders. As such, our success will depend in part on our ability to develop and introduce new products and enhancements to our existing products to keep pace with the rapidly changing spine market. However, we may not be able to successfully develop and obtain regulatory clearance or approval for product enhancements, or new products, or these products may not be accepted by spine surgeons or payors who financially support many of the procedures performed with our products.
The success of any new product offering or enhancement to an existing product will depend on several factors, including our ability to:
| · | properly identify and anticipate spine surgeon and patient needs; |
| · | develop and introduce new products or product enhancements in a timely manner; |
| · | avoid infringing upon the intellectual property rights of third parties; |
| · | demonstrate the safety and efficacy of new products with data from preclinical studies and clinical trials; |
| · | obtain the necessary regulatory clearances or approvals for new products or product enhancements; |
| · | be fully FDA-compliant with marketing of new devices or modified products; |
| · | provide adequate training to potential users of our products; |
| · | receive adequate coverage and reimbursement for procedures performed with our products; and |
| · | develop an effective and FDA-compliant, dedicated marketing and distribution network. |
If we do not develop new products or product enhancements in time to meet market demand or if there is insufficient demand for these products or enhancements, our results of operations will suffer.
If clinical trials of our current or future product candidates do not produce results necessary to support regulatory clearance or approval in the United States or elsewhere, we will be unable to commercialize these products.
Clinical failure can occur at any stage of the testing. Our clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and/or non-clinical testing in addition to those we have planned. Our failure to adequately demonstrate the efficacy and safety of any of our devices would prevent or significantly delay receipt of regulatory clearance or approval and, ultimately, the commercialization of that device.
| 14 |
Our international operations subject us to certain operating risks, which could adversely impact our net revenues, results of operations and financial condition.
Sales of our products outside the United States represented 13.2% of our revenue in 2012. The sale and shipment of our products across international borders, as well as the purchase of components and products from international sources, subject us to extensive U.S. and foreign governmental trade, import and export, and customs regulations and laws. Compliance with these regulations is costly and exposes us to penalties for non-compliance. Other laws and regulations that can significantly impact us include various anti-bribery laws, including the U.S. Foreign Corrupt Practices Act and anti-boycott laws. Any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, civil and administrative penalties, denial of export privileges, seizure of shipments, and restrictions on certain business activities.
Our international operations expose us and our distributors to risks inherent in operating in foreign jurisdictions. These risks include:
| · | the imposition of additional U.S. and foreign governmental controls or regulations; |
| · | the imposition of complicated and costly export licensing requirements; |
| · | the imposition of U.S. or international sanctions against a country, company, person or entity with whom we do business that would restrict or prohibit continued business with the sanctioned country, company, person or entity; |
| · | economic weakness or uncertainty, including the sovereign debt crises affecting several countries in the European Union; |
| · | political instability; |
| · | changes in third-party reimbursement policies that may require some of the patients who receive our products to directly absorb medical costs or that may necessitate the reduction of the selling prices of our products; |
| · | changes in duties and tariffs, license obligations, and other non-tariff barriers to trade; |
| · | the imposition of new trade restrictions; |
| · | the imposition of restrictions on the activities of foreign agents, representatives, and distributors; |
| · | difficulties in maintaining consistency with our internal guidelines; |
| · | complications arising from enforcing agreements and collecting receivables through certain foreign legal systems; and |
| · | uncertainties surrounding the enforcement or defense of our intellectual property rights. |
There can be no assurance that the foregoing factors will not adversely affect our operations in the future or require us to modify our anticipated business practices.
We purchase some of the key components of our products from single suppliers. The loss of these suppliers could prevent or delay shipments of our products or delay our clinical trials or otherwise adversely affect our business.
Due to quality considerations, costs, or constraints resulting from regulatory requirements, some of the key components of our products are currently purchased from only single suppliers with which we do not have long-term contracts. Some of these suppliers may be located outside of the United States, which could make us subject to foreign export laws and U.S. import and customs statutes and regulations, thus complicating and delaying shipments of components. Any of our supplier relationships could be interrupted due to events beyond our control, including natural disasters, or could be terminated. In addition, we may lose a single supplier due to, among other things, the acquisition of such a supplier by a competitor (which may cause the supplier to stop selling its products to us) or the bankruptcy of such a supplier, which may cause the supplier to cease operations. If necessary or desirable, we could source our product components and related services from other suppliers. However, establishing additional or replacement suppliers for these components, and obtaining any necessary regulatory clearances or approvals, could take a substantial amount of time and could result in increased costs and impair our ability to produce our products, which would adversely impact our business, operating results and prospects.
| 15 |
If our independent contract manufacturers fail to deliver to us sufficient quantities of some of our products and components in a timely manner, our operations may be harmed.
Our reliance on independent contract manufacturers to manufacture most of our products and components involves several risks, including:
| · | inadequate capacity of the manufacturer’s facilities; |
| · | financial difficulties experienced by manufacturers due to the current economic weakness and uncertainty; |
| · | interruptions in access to certain process technologies; and |
| · | reduced control over product availability, quality, delivery schedules, manufacturing yields and costs. |
Shortages of raw materials, production capacity or financial constraints, or delays by our contract manufacturers could negatively affect our ability to meet our production obligations and result in increased prices for affected parts. Any such reduction, constraint, or delay may result in delays in shipments of our products or increases in the prices of components, either of which could have a material adverse effect on our business. In addition, we could be forced to secure new or alternative contract manufacturers or suppliers. Securing a replacement contract manufacturer or supplier could be difficult. The introduction of new or alternative manufacturers or suppliers also may require design changes to our devices that are subject to FDA and other regulatory clearances or approvals. We may also be required to assess the new manufacturer’s compliance with all applicable regulations and guidelines, which could further impede our ability to manufacture our products in a timely manner. As a result, we could incur increased production costs, experience delays in deliveries of our products, suffer damage to our reputation, and experience an adverse effect on our business and financial results.
We depend on the specialized knowledge and skills of our officers and other key employees, and if we are unable to retain and motivate them or recruit additional qualified personnel, our business will suffer.
We are highly dependent on our officers and other key employees. Due to the specialized knowledge and skills each of our officers and other key employees possesses with respect to the treatment of spine disorders and our operations, the loss of service of any of our officers and other key employees could delay or prevent the successful completion of our clinical trials, the growth of revenue from existing products, and the commercialization of our new products. This risk may be exacerbated by the fact that our principal offices are located in a smaller metropolitan area, which could make it more difficult for us to retain employees or attract new employees with the required set of skills. Each of our officers and key employees may terminate his or her employment without notice and without cause or good reason.
We will need to raise additional capital in the future and, if we are unable to raise capital, it could force us to delay, reduce, eliminate or abandon our commercialization efforts or product development programs.
We believe that our existing cash and cash equivalent balances and cash receipts generated from sales of our products will be insufficient to meet our anticipated cash requirements through 2013.If the Merger and Private Placement Transaction are consummated as currently planned, however, we believe that we will be able to meet our anticipated cash requirements through mid-2014.Our future funding requirements will depend on many factors, including:
| · | market acceptance of our products and the revenues we are able to generate as a result; |
| · | the availability of adequate coverage and reimbursement for hospitals and surgeons; |
| · | the scope, rate of progress, and cost of our clinical trials; |
| · | the cost of our research and development activities; |
| · | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patent or other intellectual property rights; |
| 16 |
| · | the cost and timing of additional regulatory clearances or approvals; and |
| · | the cost and timing of establishing additional sales, marketing and distribution capabilities. |
As a result of the recent economic recession, and the continuing economic uncertainty, it has been difficult for many companies to obtain equity or debt financing. If we raise additional funds by issuing equity or convertible debt securities, our stockholders may experience dilution and the securities may contain terms that are preferential to the new investors as compared to the holders of our common stock. If we raise additional funds by issuing debt securities, the securities may have rights senior to those associated with our common stock and contain covenants restricting our operations, our ability to pay dividends, the amount of capital expenditures we can make, or our ability to incur additional debt. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders. In addition, if we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us. If we are unable to raise adequate funds, we may have to delay, reduce the scope of or eliminate some or all of our planned product development and marketing efforts. Additionally, if we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support, or other resources devoted to our products or cease operations.
Our independent registered public accounting firm has indicated that our recurring losses from operations raise substantial doubt about our ability to continue as a going concern.
Our audited financial statements for the fiscal year ended December 31, 2012, were prepared on the basis that our business would continue as a going concern in accordance with accounting principles generally accepted in the United States, or GAAP. This basis of presentation assumes that we will continue in operation for the foreseeable future and will be able to realize our assets and discharge our liabilities and commitments in the normal course of business. However, our independent registered public accounting firm has indicated in their audit report on our fiscal 2012 financial statements that our recurring losses from operations raise substantial doubt about our ability to continue as a going concern. We may be forced to delay or limit planned product development, marketing or product commercialization efforts, pursue a plan to license or sell our assets, cease our operations and/or seek bankruptcy protection if we are unable to raise additional funding to meet our working capital needs. However, we cannot guarantee that we will be able to obtain sufficient additional funding when needed or that such funding, if available, will be obtainable on terms satisfactory to us. In the event that our funding plans cannot be effectively realized, there can be no assurance that we will be able to continue as a going concern.
Pricing pressures in the healthcare industry could lead to further demands for price concessions and restricted access to spinal fusions, which could have an adverse effect on our business, financial condition or results of operations.
Because healthcare costs have risen significantly over the past decade, numerous initiatives and reforms initiated by legislators, regulators, and third-party payors to curb these costs have resulted in difficulty in raising or maintaining prices and procedure volumes. As total spine volume growth has begun to subside, many larger companies have cut prices as they struggle to retain market share, which has led to increased pricing pressure. Decreasing healthcare utilization as a result of the economic downturn, high unemployment rates, a reduction in the ranks of the full-time employed and the expiration of COBRA benefits has limited the number of patients that are covered for procedures and patients with coverage are faced with increasing deductibles. Additionally, potential patients are increasingly concerned about an extended absence from work, which would be typical of major surgery like lumbar fusion. The spine market generally, and lumbar fusion market in particular, has seen increasing push back from payors with regard to coverage. Spine surgery is a major cost area for payors, and from 2001 to 2008, lumbar fusions for lower back pain grew more than twice as fast as other lumbar fusions. As a result, private payors have increased the criteria that a patient must meet in order to be pre-authorized for lumbar fusion procedures and have put more effort into the preauthorization process. Payors have specifically stopped covering lumbar spine procedures involving degenerative disc disease patients that have no other symptoms such as leg pain or general spinal instability. In addition, as a result of significant consolidation in the medical technology industry, group purchasing organizations and integrated health delivery networks have served to concentrate purchasing decisions for some customers, which has also placed pricing pressure on medical device suppliers. We expect that market demand, government regulation, third-party reimbursement policies, industry consolidation and societal pressures will continue to exert downward pressure on the prices of our products and may adversely impact our business, financial condition or results of operations.
| 17 |
We face the risk of product liability or other claims and may not be able to obtain sufficient insurance coverage, if at all.
Our business exposes us to the risk of product liability claims that is inherent in the testing, manufacturing, and marketing of implantable medical devices. We may be subject to product liability claims if our products cause, or merely appear to have caused, an injury or death. Claims may be made by patients, consumers, or healthcare providers. Although we have product liability and clinical trial liability insurance that we believe is appropriate for our current level of operations, this insurance is subject to deductibles and coverage limitations. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, these coverages may not be adequate to protect us against any future product liability claims.
Further, we may be subject to claims against us even if the apparent injury is due to the actions of others. For example, we rely on the expertise of spine surgeons, nurses, and other associated medical personnel to perform the medical procedure and related processes for our products. If these medical personnel are not properly trained or are negligent in their provision of care, the therapeutic effect of our products may be diminished or the patient may suffer critical injury, which may subject us to liability. In addition, an injury that is caused by the activities of our suppliers may be the basis for a claim against us.
These liabilities could prevent, delay, or otherwise adversely interfere with our product commercialization efforts, and result in judgments, fines, damages, and other financial liabilities which have adverse effects on our business, operating results, and prospects.
We operate primarily at two locations. Any disruption in these facilities could adversely affect our business and results of operations.
Our main offices are located in Raleigh, North Carolina and Wilmington, North Carolina. Our Raleigh, N.C. location includes a corporate office and training facility.If the Merger and Private Placement Transaction are consummated as currently planned, we will acquire a third facility in San Jose, California.Our facilities may be affected by man-made or natural disasters, such as a hurricane. Our facilities, if damaged or destroyed, could require substantial lead-time to repair or replace. Although we believe we possess adequate insurance for damage to our property and the disruption of our business from casualties, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
Our business and our results of operations could be materially adversely affected as a result of general economic and market conditions, including the current economic environment.
Current economic conditions could adversely affect our business and our results of operations. Among other things, the potential decline in federal and state revenues that may result from such conditions may create additional pressures to contain or reduce reimbursements for our products from Medicare, Medicaid, and other government sponsored programs. Increasing job losses or slow improvement in the unemployment rate in the U.S. as a result of current or recent economic conditions has and may continue to result in a smaller percentage of our patients being covered by an employer group health plan and a larger percentage being covered by lower paying Medicare and Medicaid programs. Employers may also begin to select more restrictive commercial plans with lower reimbursement rates. To the extent that payors are negatively impacted by a decline in the economy, we may experience further pressure on commercial rates, a further slowdown in collections, and a reduction in the amounts we expect to collect. Any or all of these factors, as well as other consequences of the current economic condition that cannot currently be anticipated, could have a material adverse effect on our revenues, earnings, and cash flows and otherwise adversely affect our financial condition.
A security breach or other disruption of our information technology systems could disrupt our business, compromise our information, and expose us to liability, which would cause our business and reputation to suffer.
We rely on information technology systems to process, transmit, and store electronic information in our day-to-day operations. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance, or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost, or stolen. Any such access, disclosure, or other loss of information could result in legal claims or proceedings, disrupt our operations, and damage our reputation, which could adversely affect our revenues and competitive position. While we have policies and procedures designed to prevent or limit the effect of the failure, interruption, or security breach of our information technology systems, there can be no assurance that we can prevent any such failures, interruptions, or security breaches or, if they do occur, that they will be adequately addressed.
| 18 |
Risks Related to the Regulatory Environment
If we fail to maintain regulatory approvals and clearances, or are unable to obtain, or experience significant delays in obtaining, FDA clearances or approvals for our future products or product modifications, our ability to commercially distribute and market our products could suffer.
Our products are subject to rigorous regulation by the FDA and numerous other federal, state and foreign governmental authorities. The process of obtaining regulatory clearances or approvals to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals on a timely basis, if at all. In particular, the FDA permits commercial distribution of most new medical devices only after the device has received clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or is the subject of an approved premarket approval, or PMA. The FDA will clear marketing of a non-exempt lower risk medical device through the 510(k) process if the manufacturer demonstrates that the new product is substantially equivalent to other legally marketed products not requiring PMA approval. Our currently commercialized products that are not exempt have been cleared through the 510(k) process. However, we may need to submit a PMA for future products we develop. The PMA process is more costly, lengthy and uncertain than the 510(k) clearance process. A PMA application must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the device for its intended use.
Some of our new products will require FDA clearance through the 510(k) process. Other products may require the approval of a PMA. In addition some of our new products may require clinical trials to support regulatory approval and we may not successfully complete these clinical trials. The FDA may not approve or clear these products for the indications that are necessary or desirable for successful commercialization. The FDA may refuse our requests for 510(k) clearance or premarket approval of new products. Failure to receive clearance or approval for our new products would have an adverse effect on our ability to expand our business.
Any modification to our currently marketed 510(k)-cleared devices that could significantly affect their safety or efficacy, or that would constitute a change in their intended use, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review the manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. If the FDA requires us to seek 510(k) clearance or a PMA for any modification to a previously cleared product, we may be required to cease marketing and distributing, or to recall the modified product until we obtain such clearance or approval, and we may be subject to significant regulatory fines or penalties. If the FDA disagrees and requires new clearances or approvals for the modifications, we may be required to recall and stop marketing our products as modified, which could require us to redesign our products and harm our operating results. In these circumstances, we may be subject to significant enforcement actions.
Where we determine that modifications to our products require a new 510(k) clearance or premarket approval application, we may not be able to obtain those additional clearances or approvals for the modifications or additional indications in a timely manner, or at all. For those products sold in the European Union, or E.U., we must notify our E.U. Notified Body if significant changes are made to the products or if there are substantial changes to our quality assurance systems affecting those products. Obtaining clearances and approvals can be a time consuming process, and delays in obtaining required future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
| 19 |
The failure by us or one of our suppliers to comply with U.S. federal, state, and foreign governmental regulations could lead to the imposition of injunctions, suspensions or loss of regulatory clearance or approvals, product recalls, termination of distribution, product seizures or civil penalties, among other things. In the most extreme cases, criminal sanctions or closure of our manufacturing facility are possible. To the extent one of our suppliers fails to comply with applicable U.S. federal, state and foreign regulations, it may affect our ability either to obtain components and/or finished products or to have our finished products manufactured. In addition, changes in governmental policies or regulations may impose additional regulatory requirements on us, which could delay our ability to obtain new clearances or approvals and increase the costs of compliance. For instance, the FDA recently clarified the regulatory requirements to obtain 510(k) clearances and PMA approval. These two new guidance documents may make it more difficult for us to apply for and receive 510(k) clearances and PMA approvals for our new devices and to commercialize modifications to our existing devices. Additionally, the Food and Drug Administration Amendments Act of 2007 requires, among other things, that the FDA propose, and ultimately implement, regulations that will require manufacturers to label medical devices with unique identifiers unless a waiver is received from the FDA. Once implemented, compliance with those regulations may require us to take additional steps in the manufacture of our products and labeling. These steps may require additional resources and could be costly.
Further, foreign governmental authorities that regulate the manufacture and sale of medical devices have become increasingly stringent and, to the extent we market and sell our products internationally, we may be subject to rigorous international regulation in the future. In these circumstances, we rely significantly on our foreign independent distributors to comply with the varying regulations, and any failures on their part could result in restrictions on the sale of our products in foreign countries. Approval or clearance by the FDA does not ensure approval by regulatory authorities in other jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign jurisdictions or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval in addition to other risks. In addition, the time required to obtain foreign approval may differ from that required to obtain FDA approval and we may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any foreign market.
Obtaining approval from regulatory authorities may require successful clinical studies. Conducting successful clinical studies may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population, the nature of the trial protocol, the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators and support staff, the proximity of patients to clinical sites, and the ability of those patients to comply with the eligibility and exclusion criteria for participation in the clinical trial and meet study compliance requirements.
Development of sufficient and appropriate clinical protocols and data to demonstrate safety and efficacy are required and we may not adequately develop such protocols to support clearance and approval. Further, the FDA may require us to submit data on a greater number of patients than we originally anticipated and/or for a longer follow-up period or change the data collection requirements or data analysis applicable to our clinical trials. Delays in patient enrollment or failure of patients or clinical investigators to continue to participate in a clinical trial may cause an increase in costs and delays in the clearance or approval and attempted commercialization of our products or result in the failure of the clinical trial. In addition, despite considerable time and expense invested in our clinical trials, the FDA may not consider our data adequate to demonstrate safety and efficacy. Such increased costs and delays or failures could adversely affect our business, operating results and prospects.
Even if our products are approved by regulatory authorities, if we or our suppliers fail to comply with ongoing FDA or other foreign regulatory authority requirements, or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain clearance or approval, and the manufacturing processes, reporting requirements, post-approval clinical data and labeling and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA and other domestic and foreign regulatory bodies. In particular, we and our suppliers are required to comply with Quality System Regulations, or QSR, and Medical Device Directive regulations, which may include International Organization for Standardization, or ISO standards, for the manufacture of our products and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval. Regulatory bodies enforce the QSR and ISO regulations through inspections. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, warning letters, fines and civil penalties, delays in approving products, withdrawal or suspension of the approval of products, product recalls, operating restrictions, or unanticipated expenditures to address or defend regulatory actions. If any of these actions were to occur, it would harm our reputation and cause our product sales and profitability to suffer and may prevent us from generating revenue. Furthermore, our key suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
| 20 |
Even if regulatory clearance or approval of a product is granted, such clearance or approval may be subject to limitations on the intended uses for which the product may be marketed and reduce our potential to successfully commercialize the product and generate revenue from the product. If the FDA determines that our promotional materials, labeling, training, or other marketing or educational activities constitute promotion of an unapproved use, it could request that we cease or modify our training, educational, labeling, or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state, or foreign enforcement authorities might take action if they consider our training, educational, labeling, or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
We are subject to extensive regulation by the FDA. Our failure to meet strict regulatory requirements could require us to pay fines, incur other costs or even close our facilities.
Our facilities and manufacturing techniques generally must conform to standards that are established by the FDA and other government agencies, including those of European and other foreign governments. These regulatory agencies may conduct periodic audits or inspections of our facilities or our processes to monitor our compliance with applicable regulatory standards. If a regulatory agency finds that we have failed to comply with the appropriate regulatory standards, it may impose fines on us, delay or withdraw pre-market clearances or other regulatory approvals, or, if such a regulatory agency determines that our non-compliance is severe, it may close our facilities. Any adverse action by an applicable regulatory agency could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
Our products may in the future be subject to product recalls that could harm our reputation, business and financial results.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA. We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted. Any recall or FDA requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associated with modification of a product, loss of revenue, and potential operating restrictions imposed by the FDA.
| 21 |
If our products, or malfunctions of our products, cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations, medical device manufacturers are required to report to the FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or one of our similar devices were to recur. All manufacturers placing medical devices in the market in the European Union are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the Competent Authority in whose jurisdiction the incident occurred. Were this to happen to us, the relevant Competent Authority would file an initial report, and there would then be a further inspection or assessment if there are particular issues. This would be carried out either by the Competent Authority or it could require that the Notified Body, carry out the inspection or assessment.
Any such adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Adverse events involving our products have been reported to us in the past, and we cannot guarantee that they will not occur in the future. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
We are subject to complex and evolving federal and state healthcare laws, including laws relating to fraud and abuse and health information privacy and security, and could be subjected to governmental investigations into our compliance with such laws and substantial penalties if we are shown to have failed to comply with such laws.
Although we do not provide healthcare services, submit claims for third-party reimbursement, or receive payments directly from Medicare, Medicaid, or other third-party payors for our products or the procedures in which our products are used, we are subject to healthcare fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. These healthcare laws and regulations include, for example:
| · | the federal Anti-kickback Statute, which prohibits, among other things, persons or entities from soliciting, receiving, offering, or providing remuneration, directly or indirectly, in return for or to induce either the referral of an individual for, or the purchase order or recommendation of, any item or services for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs; |
| · | HIPAA, which established federal crimes for knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for healthcare benefits, items, or services; |
| · | federal false claims laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us to the extent that our interactions with customers may affect their billing or coding practices; and |
| · | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers. |
We have adopted policies and procedures designed to comply with the various healthcare laws applicable to our business. However, because of the breadth of these laws and regulations and the sometimes subjective nature of their application, it is possible that some of our business activities could be subject to challenge under one or more of such laws. The risk of being found to have violated such laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. In addition, in recent years, we believe that both federal and state regulators have increased regulation, enforcement, inspections and governmental investigations of the medical device industry, which further increases the risk that our business activities could be subject to challenge under certain laws. The Health Care Reform Law, among other things, also amends the intent requirement of the federal anti-kickback and criminal healthcare fraud laws such that a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it, which further expands the breadth of the applicable laws. Furthermore, it is also possible that the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes.
As discussed in more detail in the risk factor entitled “We are involved in an ongoing governmental investigation, the existence and results of which may adversely impact our business and results of operations,” in October 2011 we received a subpoena issued by the Department of Health and Human Services, Office of Inspector General, or OIG, under the authority of the federal healthcare fraud and false claims laws. We have cooperated with the government’s request and have reached a tentative settlement with the U.S. Department of Justice as of December 24, 2012. We expect to have this settlement finalized in the first half of 2013.
| 22 |
If our past or present operations, or those of our independent sales agents and distributors, are found to be in violation of any federal healthcare fraud and false claims laws, or any other applicable governmental regulations, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from federal healthcare programs and/or the curtailment or restructuring of our operations.
If the healthcare providers or entities with whom we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on us. Any action against us for violation of these laws, even if we successfully defend against them, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
The Health Care Reform Law imposes new reporting and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to prescribers and other healthcare providers, effective March 30, 2013. Such information will be made publicly available in a searchable format beginning September 30, 2013. In addition, device manufacturers will also be required to report and disclose any investment interests held by physicians and their immediate family members during the preceding calendar year. Failure to submit required information may result in civil monetary penalties of up to an aggregate of $150,000 per year (and up to an aggregate of $1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests not reported in an annual submission.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians for marketing. Some states, such as California, Massachusetts and Vermont, mandate implementation of corporate compliance programs, along with the tracking and reporting of gifts, compensation, and other remuneration to physicians. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance and/or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
We are involved in an ongoing governmental investigation, the existence and results of which may adversely impact our business and results of operations.
In October 2011, we received a subpoena issued by the OIG, under the authority of the federal healthcare fraud and false claims laws. The subpoena sought documents for the period January 1, 2008 through October 6, 2011. We have cooperated with the government’s request. On December 24, 2012 we reached a tentative agreement in principle with the U.S. Department of Justice related to the subpoena issued in October 2011. We tentatively agreed with the staff of the Department of Justice to settle its federal investigation for $6 million, subject to completion and approval of written settlement agreements with the Department of Justice and the OIG, which are expected to be finalized in the first half of 2013. We admit no wrongdoing as part of the settlement. We are also unable to predict what impact, if any, the outcome of the matters raised by the OIG will have on the willingness of surgeons to accept our products, our ability to hire or retain sales representatives and other employees, potential future reimbursement decisions by payors or other significant aspects of our business.
The use, misuse, or off-label use of our products may harm our image in the marketplace or result in injuries that lead to product liability suits, which could be costly to our business or result in FDA sanctions if we are deemed to have engaged in such promotion.
Our currently marketed products have been cleared by the FDA’s 510(k) clearance process (or were exempt from the 510(k) process) for use under specific circumstances for the treatment of certain lumbar spine conditions. We cannot, however, prevent a physician from using our products or procedure outside of those indications cleared for use, known as off-label use. There may be increased risk of injury if physicians attempt to use our products off-label. We train our sales force not to promote our products for off-label uses. Furthermore, the use of our products for indications other than those indications for which our products have been cleared by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients. Physicians may also misuse our products or use improper techniques, potentially leading to injury and an increased risk of product liability. If our products are misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management’s attention from our core business, be expensive to defend, and result in sizable damage awards against us that may not be covered by insurance. If we are deemed by the FDA to have engaged in the promotion of any of our products for off-label use, it could request that we modify our training or promotional materials or we could be subject to FDA enforcement action, including significant fines and penalties, and the imposition of these sanctions could also affect our reputation and position within the industry. It is also possible that other federal, state, or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. Any of these events could harm our business and results of operations and cause our stock price to decline.
| 23 |
Risks Related to Our Intellectual Property
Our ability to protect our intellectual property and proprietary technology through patents and other means is uncertain.
The success of our business depends significantly on our ability to protect our proprietary rights to the procedures created with, and the technologies used in, our products. We rely on patent protection, as well as a combination of copyright, trade secret and trademark laws, and nondisclosure, confidentiality and other contractual restrictions to protect our proprietary technology. However, these legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. For example, our pending United States and foreign patent applications may not be approved, may not result in patents in a form that will be advantageous to us, or may result in patents that may be successfully challenged by others and invalidated in the future. In addition, our pending patent applications include claims to material aspects of our products and procedures that are not currently protected by issued patents. Both the patent application process and the process of managing patent disputes can be time consuming and expensive. The patents we own may not be of sufficient scope or strength to provide us with any meaningful protection or commercial advantage, and competitors may be able to design around our patents or develop products that provide outcomes comparable to ours. Although we have taken steps to protect our intellectual property and proprietary technology, including entering into confidentiality agreements and intellectual property assignment agreements with our officers, employees, consultants, and advisors, such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of such agreements. Furthermore, the laws of some foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
We rely on our trademarks, trade names, and brand names to distinguish our products from the products of our competitors, and have registered or applied to register many of these trademarks. However, our trademark applications may not be approved. Third parties may also oppose our trademark applications, or otherwise challenge our use of the trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition, and could require us to devote resources to advertising and marketing new brands. Further, our competitors may infringe our trademarks, or we may not have adequate resources to enforce our trademarks.
Any lawsuit, whether initiated by us to enforce our intellectual property rights or by a third party against us alleging infringement, may cause us to expend significant financial and other resources, and may divert our attention from our business and adversely affect our business, operating results and prospects.
The medical device industry is characterized by the existence of a large number of patents and frequent litigation based on allegations of patent infringement. While we intend to defend against any threats to our intellectual property, intellectual property litigation can involve complex factual and legal questions and its outcome is uncertain. There can be no assurance that litigation will adequately protect our patents or that our competitors will not independently develop products or solutions that are equivalent or superior to ours. Because of the importance of our patent portfolio and unpatented proprietary technology to our business, we may lose market share to our competitors if we fail to protect our patent rights.
Our success will also depend in part on our not infringing patents issued to others, including our competitors and potential competitors. If our products are found to infringe the patents of others, our development, manufacture, and sale of such products could be severely restricted or prohibited. In addition, any claim relating to infringement of patents that is successfully asserted against us may require us to pay substantial damages. Even if we were to prevail, any litigation could be costly and time-consuming and would divert the attention of our management and key personnel from our business operations.
| 24 |
Additionally, if we infringe, or are claimed to infringe, a third party’s intellectual property, we may be forced to obtain licenses to patents or proprietary rights of others in order to continue to commercialize our products. However, even if we, our strategic partners or our licensees are able to obtain rights to the third party’s intellectual property, these rights may be non-exclusive, thereby giving our competitors access to the same intellectual property. Also, there is the possibility that we may not be able to obtain any licenses required under any patents or proprietary rights of third parties on acceptable terms, or at all. Ultimately, we may be unable to commercialize some of our potential products or may have to cease some of our business operations as a result of patent infringement claims, which could severely harm our business.
RisksRelated to Our Common Stock
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
As of December 31, 2012, our officers, directors and principal stockholders collectively held approximately 49.0% of our outstanding common stock. These stockholders, if they act together, could potentially exercise their significant voting power and influence the management and affairs of our company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may not be in the best interests of our other stockholders.
We may be unable to utilize our net operating loss carryforwards to reduce our income taxes.
As of December 31, 2012, we have federal net operating loss, or NOL, carryforwards of approximately $105.6 million. Our ability to utilize NOL carryforwards to reduce future taxable income may be limited under Section 382 of the Internal Revenue Code of 1986 as amended, or the Code, if an “ownership change” in our company occurs during a rolling three-year period. An ownership change could result from, among other things, the offering of stock by us, the purchase or sale of our stock by 5% stockholders, as defined in the Treasury regulations, or the issuance or exercise of rights to acquire our stock. If such ownership changes by 5% stockholders result in aggregate increases that exceed 50 percentage points during the rolling three-year period, then Section 382 imposes an annual limitation on the amount of our taxable income that may be offset by our NOL carryforwards at the time of ownership change. While we do not believe the proposed Merger and Private Placement Transaction will, by itself, constitute an ownership change, we have not yet analyzed whether an ownership change would result if the Merger and Private Placement Transaction are aggregated with our issuances of common stock during the prior three-year period. It is also possible that future transactions could be aggregated with the Merger and Private Placement Transaction to result in an ownership change. If an ownership change were to occur, we may be unable to use a significant portion of our NOL carryforwards to offset taxable income.
The price of our common stock has been, and may continue to be, volatile and our stockholders may not be able to resell shares of our common stock at or above the price paid for such shares.
The price for shares of our common stock on the NASDAQ Global Market has exhibited high levels of volatility with significant price fluctuations, which makes our common stock unsuitable for many investors. For example, for the three years ended December 31, 2012, the closing price of our common stock ranged from a high of $5.35 per share to a low of $1.50 per share. At times, the fluctuations in the price of our common stock may have been unrelated to our operating performance. These broad fluctuations may negatively impact the market price of shares of our common stock.
Trading in our stock has historically been limited, so investors may not be able to sell as much stock as they want at prevailing prices.
The average daily trading volume in our common stock for the year ended December 31, 2012 was approximately 64,000 shares. If limited trading in our stock continues, it may be difficult for investors to sell their shares in the public market at any given time at prevailing prices. Moreover, the market price for shares of our common stock may be made more volatile because of the relatively low volume of trading in our common stock. When trading volume is low, significant price movement can be caused by the trading in a relatively small number of shares. Volatility in our common stock could cause stockholders to incur substantial losses.
| 25 |
Our amended and restated certificate of incorporation, amended and restated bylaws, and Delaware law contain provisions that could have the effect of deterring or delaying changes in incumbent management, proxy contests or changes in control.
Anti-takeover provisions of our amended and restated certificate of incorporation, amended and restated bylaws, and Delaware law may have the effect of deterring or delaying attempts by our stockholders to remove or replace management, engage in proxy contests, and effect changes in control. The provisions of our charter documents include:
| · | a classified board so that only one of the three classes of directors on our Board of Directors is elected each year; |
| · | procedures for advance notification of stockholder director nominations and proposals; |
| · | a prohibition on stockholders being able to call a special meeting of stockholders; |
| · | the ability of our Board of Directors to amend our bylaws without stockholder approval; |
| · | a supermajority stockholder vote requirement for amending certain provisions of our amended and restated certificate of incorporation and our amended and restated bylaws; and |
| · | the ability of our Board of Directors to issue up to 5,000,000 shares of preferred stock without stockholder approval upon the terms and conditions and with the rights, privileges and preferences as our Board of Directors may determine. |
In addition, as a Delaware corporation, we are subject to Delaware law, including Section 203 of the Delaware General Corporation Law, or DGCL. In general, Section 203 prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless certain specific requirements are met as set forth in Section 203. These provisions, alone or together, could have the effect of deterring or delaying changes in incumbent management, proxy contests or changes in control.
CAUTIONARYNOTE REGARDING FORWARD-LOOKING STATEMENTS
ThisProxy Statementcontains “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of theExchange Actthat concern matters that involve risks and uncertainties that could cause actual results to differ materially from those projected in the forward-looking statements. These forward-looking statements are intended to qualify for the safe harbor from liability established by thePrivate Securities Litigation Reform Act of 1995. All statements other than statements of historical fact contained in thisProxy Statement, including statements regarding the expected timing, completion, and benefits of the Merger and Private Placement Transaction, other future events, our future financial performance, our future business strategy, and the plans and objectives of management for future operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” or “will” or the negative of these terms or other comparable terminology. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. Such forward-looking statements are subject to risks, uncertainties, and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by such forward-looking statements. Such risks, uncertainties, and other factors that may cause such differences include, but are not limited to, those discussed in the section entitled “Risk Factors” in thisProxy Statementand in other filings we make with the SEC. Furthermore, such forward-looking statements speak only as of the date of thisProxy Statement. We expressly disclaim any intent or obligation to update any forward-looking statements after the date hereof to conform such statements to actual results or to changes in our opinions or expectations, except as required by applicable law or the rules of The NASDAQ Stock Market LLC.
| 26 |
THESPECIAL MEETING OF STOCKHOLDERS
Date, Time, and Place
The Special Meeting will be held on[Ÿ], 2013 at[Ÿ], local time, at200 Horizon Drive, Suite 115, Raleigh, North Carolina 27615.
Dateof Mailing
The approximate date on which this Proxy Statement and form of proxy card are first being mailed to stockholders is [Ÿ], 2013.
Purposesof the Special Meeting; Board Recommendations
The purposes of the Special Meeting are for our stockholders to consider and vote on the following proposals:
Proposal No. 1 : to approve the issuance of shares of our common stock pursuant to the Merger Agreement;
Proposal No. 2 : to approve the issuance of shares of our common stock pursuant to the Securities Purchase Agreement;
Proposal No. 3 : to approve an amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc.; and
Proposal No. 4 : to grant the proxy holders discretionary authority to vote to adjourn the Special Meeting, if necessary, in order to solicit additional proxies in the event that there are not sufficient affirmative votes present at the Special Meeting to approve Proposal No. 1, Proposal No. 2, and/or Proposal No. 3.
The Special Meeting will also address such other matters as may properly come before the Special Meeting or any postponement or adjournment thereof.
The transactions contemplated by Proposal Nos. 1 and 2 are, among other conditions described in this Proxy Statement, conditioned on each other and, therefore, both proposals must be approved in order for the transactions contemplated by such proposals to be consummated. In addition, the amendment contemplated by Proposal No. 3 is contingent upon the approval of Proposal Nos. 1 and 2 and the consummation of the Merger contemplated by Proposal No. 1.
The Board of Directors, except for Mr. Shapiro, unanimously recommends that you vote each of your shares “FOR” the approval of the issuance of shares of our common stock pursuant to the Merger Agreement, “FOR” the approval of the issuance of shares of our common stock pursuant to the Securities Purchase Agreement, “FOR” the approval of an amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc., and “FOR” the grant to the proxy holders of discretionary authority to vote to adjourn the Special Meeting, if necessary, in order to solicit additional proxies in the event that there are not sufficient affirmative votes present at the Special Meeting to approve Proposal No. 1, Proposal No. 2, and/or Proposal No. 3. Due to Mr. Shapiro’s relationship with Baxano, he did not participate in discussions and deliberations related to the Merger or any of the proposals presented in this Proxy Statement. See “Interests of Certain Persons in the Merger and Private Placement Transaction.”
RecordDate; Voting Securities
Only our stockholders of record at the close of business on[Ÿ], 2013 will beentitled to vote at the Special Meeting. Each share of common stock issued and outstanding on the Record Date is entitled to one vote. As of the Record Date, there were[Ÿ] shares of our common stock entitled to vote at the Special Meeting.
Quorum;RequiredVote
The presence at the Special Meeting, in person or represented by proxy, ofholdersof a majority of theoutstanding shares of our common stockas of the Record Date will constitute a quorum at the Special Meeting. We will treatshares of common stockrepresented by a properly voted proxy, including abstentions andbroker non-votes, if any, as present at the Special Meeting for the purposes of determining the existence of a quorum. If a quorum is not present, the Special Meeting will be adjourned until a quorum is obtained.
| 27 |
The required votes for the proposals are as follows:
Issuance of Common Stock Pursuant to the Merger Agreement (Proposal No. 1). Assuming the existence of a quorum, approval of the issuance of shares of our common stock pursuant to the Merger Agreement will require the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal.
Issuance of Common Stock Pursuant to the Securities Purchase Agreement (Proposal No. 2). Assuming the existence of a quorum, approval of the issuance of shares of our common stock pursuant to the Securities Purchase Agreement will require the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal.
Name Change Amendment (Proposal No. 3). Assuming the existence of a quorum, approval of the amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc. will require the affirmative vote of a majority of the shares of our common stock outstanding as of the Record Date. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal.
Potential Adjournment of the Special Meeting (Proposal No. 4). Assuming the existence of a quorum, approval of the proposal to grant the proxy holders discretionary authority to vote to adjourn the Special Meeting will require the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal.
Our directors and executive officers and their respective affiliates collectively owned approximately 9.0 million shares of our common stock as of March 4, 2013 (inclusive of shares subject to stock options exercisable within 60 days following that date). Such shares represented approximately 31.4% of our outstanding common stock (including shares subject to stock options held by the directors and officers) as of such date. Certain of our directors and executive officers and their respective affiliates collectively holding approximately 24.2% of our outstanding common stock (excluding shares subject to stock options), have entered into the Stockholder Agreements, solely in their capacity as stockholders, pursuant to which they have agreed to vote all of the shares of our common stock held by them in favor of Proposal Nos. 1 and 2. See “Proposal No. 1 - Issuance of Common Stock Pursuant to the Merger Agreement—Stockholder Agreements.”
Abstentions and Broker Non-Votes
You mayabstainfrom voting on one or more of the matters to be voted on at the Special Meeting. Shares that a stockholder abstains from voting will be counted for purposes of determining whether a quorum is present at the Special Meeting, and will have the same effect as a vote against each proposal.
“Broker non-votes” are shares held by a nominee(i.e., a broker, dealer, bank, or other nominee) that are represented at the Special Meeting, but with respect to which the nominee holding those shares (i) has not received instructions from the beneficial owner of the shares to vote on the particular proposal, and (ii) does not have discretionary voting power with respect to the particular proposal. Whether a nominee has authority to vote shares that it holds is determined by the rules of the New York Stock Exchange that govern most domestic brokerage firms. Nominees holding shares of record for beneficial owners do not have the discretion to vote on any of the proposals to be presented at the Special Meeting without instructions from the beneficial owners of the shares. Accordingly, we anticipate that there will not be any broker non-votes at the Special Meeting in the absence of any additional matter coming before such meeting as to which brokers have discretionary authority.
Voting at the Special Meeting
You are entitled to attend the Special Meeting only if you were a stockholder as of the Record Date or hold a valid proxy for the Special Meeting. Whether or not you plan to attend the Special Meeting, we urge you to vote by proxy to ensure your vote is counted. If you plan to attend the Special Meeting and wish to vote in person, you will be given a ballot at the meeting. Please note, however, that if your shares are held in “street name” and youwish to vote at the meeting, you must bring to the meeting a proxy from the record holder of the shares authorizingyou to vote at the meeting.
Many of our stockholders hold their shares through anominee rather than directly in their own names. As summarized below, there are some distinctions between shares held of record and those owned beneficially.
Holders of Record.If, on the Record Date, your shares were registered directly in your name with our transfer agent, American Stock Transfer & Trust Company, then you are a “holder of record.” As a “holder of record,” you may vote in person at the Special Meeting or vote by proxy.
| 28 |
Beneficial Owners. If, on the Record Date, your shares were held in an account with a nominee, then you are the “beneficial owner” of shares held in “street name” and this Proxy Statement is being forwarded to you by that nominee. The nominee holding your account is considered the “holder of record” for purposes of voting at the Special Meeting. As a beneficial owner, you have the right to direct your nominee how to vote the shares in your account. You are also invited to attend the Special Meeting. However, since you are not the “holder of record,” you may not vote your shares in person at the Special Meeting unless you request and obtain a valid proxy from your nominee. Please contact your nominee directly for additional information.
Since seating is limited, admission to the Special Meeting will be on a first-come, first-served basis. You should be prepared to present government-issued photo identification for admittance, such as a passport or driver’s license. If your shares are held in “street name,” you also will need proof of ownership as of the Record Date to be admitted to the Special Meeting, such as your most recent account statement prior to the Record Date, a copy of the voting instruction card provided by your nominee, or similar evidence of ownership. Please note that for security reasons, you and your bags may be subject to search prior to your admittance to the Special Meeting. If you do not comply with each of the foregoing requirements, you will not be admitted to the Special Meeting. Please let us know if you plan to attend the Special Meeting by marking the appropriate box on the enclosed proxy card.
Voting of Proxies
You can vote by mail pursuant to the instructions provided on the proxy card. In order to be effective, completed proxy cards must be received by 11:59 p.m., Eastern Time, on[Ÿ], 2013. If you choose to vote by mail, simply mark your proxy, date and sign it, and return it in the business reply envelope provided with this Proxy Statement.
If you hold shares beneficially in “street name,” you may vote by proxy over the Internet by following the instructions provided in the voting instruction card provided to you by your nominee. Internet voting is available 24 hours a day and will be accessible until 11:59 p.m., Eastern Time, on[Ÿ], 2013 by visiting www.proxyvote.com and following the instructions. Our Internet voting procedures are designed to authenticate stockholders by using individual control numbers, which are located on the voting instruction card. If you vote over the Internet, you do not need to return your proxy card.
All valid, unrevoked proxies will be voted in accordance with their instructions. In the absence of instructions to the contrary, properly executed proxies will be voted “FOR” each of the proposals listed in thisProxy Statement.At this time, we are unaware of any matters, other than those matters set forth above, that may properly come before the special meeting.If any other matters are properly brought before the Special Meeting, Ken Reali and Joseph Slattery, who are members of the Company’s management, will have discretion to vote the proxies in accordance with their best judgment to the same extent as the person delivering the proxy would be entitled to vote.
The enclosed proxy provides that you may voteyour shares of our common stock“FOR,” “AGAINST,” or “ABSTAIN” from voting with respect to each of the proposals.
Revocability of Proxies
You may change your vote at any time before your proxy is voted at the Special Meeting. You may change your vote by (i) providing written notice of revocation to theCorporate Secretaryof the Company at our corporate headquarters located at110 Horizon Drive, Suite 230, Raleigh, NC 27615, (ii) by executing a subsequent proxy using one of the voting methods discussed above, or (iii) by attending the Special Meeting and voting in person. However, simply attending the Special Meeting will not, by itself, revoke your proxy. If you have instructed yournomineeto vote your shares, you must follow directions received from yournomineeto change those instructions. Subject to any such revocation, all shares represented by properly executed proxies will be voted in accordance with the specifications on the enclosed proxy card.
Costof This Proxy Solicitation
The cost of soliciting proxies will be borne by us. We expect to reimburse brokerage firms and other persons representing beneficial owners of shares for their expenses in forwarding solicitation materials to such beneficial owners. Proxies may also be solicited by certain of our directors, officers, and regular employees, without additional compensation, personally or by telephone or facsimile.
| 29 |
Adjournmentsand Postponements
Although it is not currently expected, the Special Meeting may be adjourned for the purpose of soliciting additional proxies if we fail to receive a sufficient number of votes to approve the proposal regarding the issuance of shares of our common stock pursuant to the Merger Agreement, the proposal regarding the issuance of shares of our common stock pursuant to the Securities Purchase Agreement, or the proposal to amend our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc. We are asking our stockholders to vote in favor of granting the proxy holders discretionary authority to vote to adjourn the Special Meeting, if necessary, in order to solicit additional proxies in the event that there are not sufficient affirmative votes present at the Special Meeting to approve any or all of such proposals. See “Proposal No. 4 - Potential Adjournment of the Special Meeting.”
At any time prior to convening the Special Meeting, ourBoard of Directorsmay postpone the Special Meeting for any reason without the approval of our stockholders. If postponed, we will provide notice of the new meeting date as required by law. Similar to adjournments, any postponement of the Special Meeting for the purpose of soliciting additional proxies will allow stockholders who have already sent in their proxies to revoke them at any time prior to their use.
AdditionalInformation
Representatives of PricewaterhouseCoopers LLP are expected to be present at the Special Meeting with the opportunity to make a statement if they desire to do so and are expected to be available to respond to appropriate questions.If you have additional questions about the Special Meeting, theMerger, or thePrivate Placement Transactionor would like additional copies of thisProxy Statement, please contact us by either writing to TranS1 Inc. at our principal executive offices located at110 Horizon Drive, Suite 230, Raleigh, NC 27615, Attention: Corporate Secretary, by calling us at (866) 256-1206, or by emailing us at merger@trans1.com.
PROPOSAL NO. 1
ISSUANCE OF COMMON STOCK PURSUANT TO THE MERGER AGREEMENT
We are submitting a proposal to issue shares of our common stock pursuant to the Merger Agreement in accordance with the listing rules of the NASDAQ Stock Market LLC, on which exchange our common stock is listed. Under these rules, we are required to obtain the approval of our stockholders in connection with the acquisition of the stock or assets of another company or a private placement involving the sale or issuance by us of common stock (or securities convertible into or exercisable for common stock) equal to or in excess of 20% of our common stock or 20% of the voting power outstanding before the issuance. Because completion of the Merger would require us to issue approximately 38.9% of our total outstanding shares of common stock, we are asking our stockholders to approve the issuance at the Special Meeting.
The dollar value of the shares of our common stock to be issued in connection with the Merger is expected to be approximately $23.1 million, subject to fluctuations in the price of our common stock on the NASDAQ Global Market, the amount of certain indebtedness held by Baxano at the effective time of the Merger, the amount of Baxano’s transaction expenses that remain unpaid immediately prior to the effective time, and working capital adjustments. See “—The Merger Agreement—Merger Consideration.”
We anticipate that the maximum aggregate number of shares of our common stock to be issued to the stockholders of Baxano at the closing of the Merger will represent up to approximately 27.6% of the outstanding shares of our common stock immediately following the closing of the Merger. Pursuant to the terms of the Merger Agreement, the merger consideration will consist of a number of newly issued shares of common stock of the Company calculated as the product of (a) 0.38888889times (b) the number of issued and outstanding shares of capital stock of the Company (as if converted to common stock) as of the close of business on the last trading day prior to the closing of the Merger. This calculation is intended to provide Baxano stockholders with ownership of 28% of the Company’s post-closing outstanding shares of common stock (excluding the impact of the Private Placement Transaction) prior to certain adjustments to the merger consideration provided for in the Merger Agreement and which are described below. We anticipate that the maximum aggregate number of shares of our common stock to be issued to the stockholders in connection with the Merger will represent up to approximately 23.4% of the outstanding shares of our common stock immediately following the closing of both the Merger and the Private Placement Transaction.
The shares of our common stock to be issued pursuant to the Merger Agreement will be offered and sold in reliance upon an exemption from the registration requirements of the Securities Act afforded by Regulation D promulgated thereunder. The Merger was a privately negotiated transaction that did not involve general solicitation, and all recipients of shares of our common stock pursuant to the Merger Agreement are “accredited investors” as defined in Regulation D.
| 30 |
The issuance of shares of common stock pursuant to the Merger Agreement described in this Proposal No. 1 and the issuance of shares of common stock pursuant to the Securities Purchase Agreement described in Proposal No. 2 are, among other conditions described in this Proxy Statement, conditioned on each other and, therefore, both proposals must be approved in order for the Merger and the Private Placement Transaction to be consummated. For information about the Private Placement Transaction, see “Proposal No. 2 – Issuance of Common Stock Pursuant to the Securities Purchase Agreement.”
The approval of the issuance of shares of our common stock pursuant to the Merger Agreement requires the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal.
OUR BOARD (EXCEPT FOR MR. SHAPIRO) UNANIMOUSLY RECOMMENDS A VOTE “FOR” THE issuance of shares of our common stock pursuant to the Merger Agreement.
The Merger
Background of the Merger
In the fourth quarter of 2010, the Company became aware of the recently launched iO-Flex technology from Baxano. Ken Reali, then President and Chief Operating Officer of TranS1, contacted Tony Recupero, President and CEO of Baxano, regarding the potential synergy of the two organizations. No transaction was pursued in 2010 as the Baxano products were just being introduced into the market and, while synergy between the two companies was recognized, there was little sales history to establish the merits of a transaction. In addition, the Company was aware that Baxano had recently completed a financing transaction and therefore had adequate financial resources at the time.
In the summer of 2012, the Company’s management and Board of Directors began to consider strategies for addressing the Company’s need to regain revenue growth, scale, new products and how to take advantage of the expected growth of the MIS spine market. This was prompted by the Company’s new category I code for its primary procedure, AxiaLIF, and its strategic intent to gain scale in the MIS spine market. Since 2009, the Board of Directors has periodically evaluated strategic alternatives including selling the Company and merging with another company to achieve scale. It performed a screening process of potential acquisition targets during the summer of 2012, which resulted in the identification of Baxano as the most viable option due to the commonality in the customer base and MIS spine procedures targeted by both Baxano and the Company. Since the MIS spine market accounts for a small percentage of all spine procedures, Baxano is one of only a very small number of companies that target the same MIS spine surgeon customers as the Company. In addition, Baxano’s iO-Flex technology can be used in the same procedures as the Company’s core products, creating further synergy opportunities. Therefore, the Board of Directors believed the acquisition of Baxano would be consistent with the Company’s strategy to continue operating solely in the MIS spine space. In August 2012, the Company’s management began focusing on a potential acquisition of Baxano because of Baxano and the Company’s similar customer base, pre-identified synergies and related proprietary technologies.
On August 15, 2012, Ken Reali, by this time the Company’s President and Chief Executive Officer, contacted James Shapiro, a member of the boards of directors of both Baxano and TranS1, to ask his opinion regarding a potential merger of the two companies. Mr. Shapiro referred Mr. Reali to Russell Hirsch, a member of Baxano’s Board of Directors. Mr. Reali communicated the Company’s strategic interest in writing to Mr. Hirsch on August 23, 2012. On August 22, 2012, Joseph Slattery, the Company’s Executive Vice President and Chief Financial Officer, engaged outside legal counsel Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P., or Smith Anderson, to assist in evaluating a potential acquisition of Baxano.
On September 28, 2012, the Company and Baxano entered into a non-disclosure agreement and began discussions. Between September 28, 2012 and October 18, 2012, the Company and Baxano exchanged preliminary due diligence information concerning their respective businesses. On October 10, 2012, the Company engaged the financial advisory firm Stifel to act as the exclusive financial advisor to the Company in connection with its analysis of a potential acquisition of Baxano. Mr. Shapiro is a Managing Member of Kearny Venture Capital, L.L.C., which serves as a sub-adviser to a venture capital fund that is primarily advised by an affiliate of Stifel and in which an affiliate of Stifel holds a membership interest.
During early October 2012, the Company’s management, with assistance from Smith Anderson and Stifel, began drafting an initial term sheet for consideration by the Company’s Board of Directors. On October 18, 2012, the Board of Directors held a telephonic special meeting to review the draft initial term sheet and materials from Stifel and management evaluating the potential merger. Mr. Shapiro had previously disclosed his interests in any potential merger to Mr. Reali and Paul LaViolette, the Chairman of the Company’s Board of Directors, as described in “Interests of Certain Persons in the Merger and Private Placement Transaction.” At each meeting of the Company’s or Baxano’s boards of directors at which the potential merger was discussed, Mr. Shapiro did not attend, or was recused from the deliberations of the other directors with respect to the transaction. The Company’s management reported that it believed, based on preliminary due diligence, that an acquisition of Baxano by the Company could increase the Company’s presence in the MIS market and that Baxano’s multiple product lines and surgeon training programs would blend well with the Company’s footprint. At the October 18 Board of Directors meeting, the Board of Directors formed a Transaction Committee of the Board of Directors, or the Transaction Committee, consisting of independent members of the Board of Directors, to assist management in evaluating the potential merger and approved distribution of the initial term sheet to Baxano. The members of the Transaction Committee were Paul LaViolette, Jonathan Osgood, and David Simpson. On October 19, 2012, the Company sent the initial draft of the term sheet to Baxano.
| 31 |
On October 24, 2012, Baxano engaged the financial advisory firm of Leerink Swann LLC to act as the exclusive financial advisor to Baxano in connection with its analysis of the potential merger.
From late October through early November 2012, the Company and Baxano continued discussions regarding a proposed merger, and the Company continued to revise the term sheet and the structure of the merger with periodic status updates to the Transaction Committee.
On November 16, 2012, Messrs. Reali and Slattery, together with representatives of Stifel, met with Baxano’s management, board of directors, and certain of Baxano’s major shareholders in San Francisco, California to discuss the business, operations, and finances of both companies and the potential benefits of the merger to both companies.
On December 14, 2012, the Company and Baxano began discussing the need for financing to support the combined companies post-merger. Baxano indicated that certain Baxano stockholders had expressed interest in participating in a potential financing of $15 million. During December 2012 and January 2013, the Company held numerous telephonic meetings with the Transaction Committee to keep it apprised of the negotiations with Baxano.
On January 16, 2013, the Company’s Board of Directors (except for Mr. Shapiro) met with management, Stifel, and Smith Anderson to review the then-current draft of the term sheet and receive reports from management and Stifel regarding potential valuation scenarios. Management described the addition to the term sheet of financing from certain Baxano stockholders of up to $15 million. Stifel and management discussed different valuation possibilities of the combined companies, which were all subject to further financial due diligence. The Company’s Board of Directors approved entering into the non-binding term sheet with Baxano at this meeting.
On January 17, 2013, the Company and Baxano entered into the term sheet, which included an exclusivity obligation by Baxano until February 22, 2013 contingent upon the Company re-affirming its interest in pursuing a transaction by February 8, 2013. The Company and Baxano then commenced full mutual operational, financial, and legal due diligence and began negotiating the key material agreements for the Merger. As a result of the Company’s financial and operational due diligence during late January and early February 2013, the Company determined that the combined entity resulting from the Merger may require additional financing from other third party investors, or the Additional Financing, in addition to the financing of approximately $15 million from certain Baxano stockholders as provided in the term sheet.
On February 7, 2013, the Company and Stifel reported the material results of the Company’s financial, business, and operational due diligence to the Transaction Committee and their determination that the Additional Financing may be needed. Management also reported that the business due diligence had confirmed their belief that the proposed Merger would advance the Company’s strategic goals. The Transaction Committee agreed that the Additional Financing could be an important element to the Merger. On February 8, 2013, the Company sent a letter to Baxano re-affirming its interest in pursuing the Merger. On February 11, 2013, the parties discussed the need for the Additional Financing, agreeing that the Additional Financing could be necessary. The parties also agreed to combine the proposed financing from the Baxano stockholders and the Additional Financing into one financing on the same terms and conditions, or the Private Placement Transaction.
On February 14, 2013, management, Stifel, and Smith Anderson updated the Company’s Board of Directors (except for Mr. Shapiro) regarding the status of the Merger negotiations and the Private Placement Transaction during its regularly scheduled meeting.
During the second half of February through March 3, 2013, the Company and Stifel contacted various third parties to discuss participation in the Private Placement Transaction, receiving commitments for an additional $1.9 million.
| 32 |
On March 1, 2013, the Company’s Board of Directors held a telephonic special meeting to review the proposed Merger and Private Placement Transaction, including fully negotiated documents for the proposed transactions that had been previously distributed for its review. Representatives of Stifel and Smith Anderson participated in the meeting. Messrs. Reali and Slattery updated the Board of Directors on the most recent negotiations and reviewed the strategic rationale for, and risks associated with, the proposed Merger and Private Placement Transaction and the proposed governance and organizational structure of the combined companies. The Transaction Committee reported to the Board of Directors regarding its review of the proposed Merger and Private Placement Transaction. The Board also received an update on the results of the due diligence process relating to the proposed Merger and Private Placement Transaction from management and Smith Anderson. Smith Anderson discussed with the Board of Directors its fiduciary duties in connection with the proposed Merger and Private Placement Transaction and reviewed the material terms of the Merger and Private Placement Transaction. Stifel made a presentation concerning its financial analysis of the Merger and delivered its opinion to the Board of Directors that the Merger was fair, from a financial point of view, to the Company. The Board of Directors then reviewed a draft of resolutions authorizing the Merger and Private Placement Transaction that had been previously distributed for its review. The members of the Board of Directors considered and discussed all of these matters and other information presented at the meeting and at prior meetings and, by unanimous vote (except for Mr. Shapiro) of those members present, determined that the Merger and the Private Placement Transaction were advisable and in the best interests of the Company and its stockholders, approved the Merger Agreement, the Securities Purchase Agreement, and the transactions contemplated thereby and authorized management to execute the transaction documents. After conclusion of the meeting, Mr. Reali notified Mr. Hirsch by telephone that the Company’s Board of Directors (except for Mr. Shapiro) had unanimously approved the Merger and Private Placement Transaction.
On March 2, 2013, Baxano’s Board of Directors held a telephonic special meeting to review the proposed Merger. After careful consideration and deliberation, and based on the analysis described in “—Reasons for the Merger—Baxano’s Reasons for the Merger,” as well as information evaluated at prior meetings, Baxano’s Board of Directors determined that the transaction is advisable, and is fair to and in the best interests of Baxano and its stockholders, and unanimously (except for Mr. Shapiro, who was not present for the vote or deliberations) adopted the Merger Agreement and approved the transactions contemplated thereby. Baxano’s Board of Directors unanimously(except for Mr. Shapiro) recommended that Baxano’s stockholders adopt the Merger Agreement and approve the transactions contemplated thereby.
On March 3, 2013, Baxano and the Company executed the Merger Agreement and related agreements and the Company and the Investors executed the Securities Purchase Agreement. A joint press release announcing the Merger and the Private Placement Transaction was issued on March 4, 2013.
Reasonsfor the Merger
OurReasons for the Merger
Our Board of Directors believes that the Merger will provide substantial benefits to our business and operations. In reaching its determination to approve the Merger Agreement and the transactions contemplated thereby, and to recommend that our stockholders approve the proposal regarding the issuance of shares of our common stock pursuant to the Merger Agreement, our Board of Directors identified several potential benefits for our company and for our stockholders, including:
| · | the potential for significant revenue growth from Baxano’s MIS spine platform including the proprietary iO-Flex decompression device and the iO-Tome facet removal systems; |
| · | the potential for Baxano’s products to expand and complement our MIS solutions including the proprietary AxiaLIF interbody fusion system and theVEO lateral access and interbody fusion system; |
| · | the potential to provide cost savings by combining sales forces and leveraging Baxano’s direct sales channel with built-in relationships, clinical knowledge, and ability to market and sell economic value; |
| · | the potential to enhance our overall growth strategy through accelerated geographic and market expansion into new domestic markets; |
| · | the potential for increased stockholder value by diversifying our product offerings, achieving cost synergies in the near term, and using greater scale to leverage fixed costs; and |
| · | the potential to improve our financial profile by improving our margins and shortening the expected timeline to achieve breakeven cash flow. |
With respect to the last bullet above, we considered two margins in determining that acquiring Baxano may improve our margins and shorten the expected timeline to achieve breakeven cash flow: contribution margin (after selling expenses) and operating margin (after all operating expenses). We expect contribution margin to improve due to the overall increase in scale of the combined business, resulting in a lower impact of fixed costs on contribution margin, as well as the ability to eliminate duplicative costs, such as personnel, travel, sales meetings, trade shows, and consultants. Our operating margin is likewise expected to improve due to the overall increase in scale of the combined business, not only driven by an increase in contribution margin, but also through the ability to eliminate duplicative general and administrative expenses and research and development costs. This improvement in operating margin is the primary contributing factor to our belief that we will be able to achieve breakeven cash flow earlier as a combined business than on a standalone basis.
| 33 |
Our Board of Directors consulted with our management, as well Stifel and legal counsel, in reaching its decision to approve the Merger Agreement and the transactions contemplated thereby. The factors that our Board of Directors considered include:
| · | the potential benefits described above; |
| · | historical information concerning Baxano’s business, financial performance, financial condition, operations, and management and the potential for significant revenue growth and gross margin improvement of the combined company, particularly given the cost synergies expected to be achieved through the Merger, the successful commercialization of Baxano’s products, and the companies’ complementary customer bases; |
| · | our management’s view of the complementary nature and fit of Baxano’s business with ours and its current business prospects; |
| · | our management’s view of the financial condition, results of operations, and business of Baxano and us before and after giving effect to the Merger and information regarding the Merger’s potential effect on our stockholder value; |
| · | reports from our management and legal and financial advisors regarding the results of the due diligence investigation of Baxano; |
| · | the opinion of Stifel as to the fairness to the Company, from a financial point of view, of the Merger; |
| · | our Board of Directors’ belief that the terms of the Merger Agreement are fair and reasonable; |
| · | the terms and conditions of the Merger Agreement; and |
| · | the additional financing to be provided by the Private Placement Transaction, which is contingent on the closing of the Merger. |
Our Board of Directors also identified and considered various potentially negative factors in its deliberations concerning the Merger Agreement and the transactions contemplated thereby, including:
| · | the risk that the Merger might not be consummated in a timely manner or at all, including the risk of being required to pay a substantial termination fee to Baxano under certain circumstances; |
| · | the risks related to the immediate and substantial dilution of the equity interests and voting power of our stockholders upon completion of the Merger; |
| · | the challenges of integrating our businesses, operations, and workforce with those of Baxano and the risks associated with achieving anticipated cost savings and other synergies; |
| · | the potential risk of diverting management’s focus and resources from other strategic opportunities and from operational matters while working to implement the transaction; |
| · | the risk of future competition in Baxano’s business segment; |
| · | the risk that Baxano may be unable to successfully implement its business strategy and growth plan; |
| · | the substantial costs to be incurred in connection with the Merger, including the costs of integrating Baxano’s business with ours and the transaction expenses arising from the transaction; and |
| · | certain of the risks described in the section entitled “Risk Factors.” |
After due consideration, our Board of Directors concluded that the potential benefits of the Merger to our stockholders outweighed the risks associated with the Merger.
| 34 |
Although not exhaustive, this discussion of the information and factors considered by our Board of Directors comprises the material factors considered. In view of the wide variety of factors considered in connection with our Board of Directors’ evaluation of the Merger and related transactions, our Board of Directors did not quantify or otherwise assign relative weights to the factors described. Rather, our Board of Directors made its determination based on the totality of the information it considered and the exercise of its reasoned business judgment as to the best interests of our stockholders. In addition, individual directors may have weighed factors differently. Our Board of Directors cannot assure you that any of the expected results, opportunities, or other benefits described in this section will be achieved as a result of the Merger.
Baxano’s Reasons for the Merger
In considering the transaction with Trans1, Baxano’s Board of Directors consulted with Leerink Swann regarding the financial aspects of the Merger and consulted with representatives of Morrison & Foerster, LLP, outside counsel to Baxano, regarding the fiduciary duties of the members of the board of directors, legal due diligence matters, and the terms of the Merger Agreement and related agreements. Based on these consultations and the factors discussed below, Baxano’s Board of Directors (except for Mr. Shapiro) unanimously determined that the Merger and the Merger Agreement are substantively and procedurally fair to, and in the best interests of, Baxano’s stockholders.
In the course of reaching that determination and recommendation, Baxano’s Board of Directors considered a number of factors supporting the proposed transaction in its deliberations, including the following:
| · | its knowledge of Baxano’s business, financial condition, results of operations, and prospects, competitive position, and its belief that the proposed transaction is more favorable to Baxano’s stockholders than any other strategic alternative reasonably available to Baxano, including remaining as a stand-alone entity; |
| · | its belief that Baxano faces many challenges in its efforts to increase stockholder value as an independent company, including the need to obtain additional financing and the risks associated with obtaining additional financing, Baxano’s inability to attract sufficient committed capital to satisfy the conditions of an agreed upon term sheet with a lead investor for a potential equity financing despite an extensive fundraising process, and the likely terms on which Baxano would be able to obtain financing sufficient to enable the company to attain profitability; |
| · | Baxano’s prospects to develop and commercialize new products, obtain and maintain regulatory approvals, and other execution risks, as well as business and market risks generally; |
| · | Baxano’s financial projections, including the risks related to the achievement of such projections in light of Baxano’s prior history of achieving its projections and current market conditions; |
| · | its view that it was not reasonable to expect that Baxano would be able to solicit or conclude an alternative transaction with another party at a higher price, based on the process Baxano conducted and the results of such process; |
| · | the merger consideration is TranS1 common stock, which provides Baxano’s stockholders the opportunity to participate in the long-term value of Baxano through ownership of TranS1 common stock following the Merger; |
| · | a public offering for Baxano would be very difficult and the Merger will provide Baxano’s stockholders liquidity through merger consideration consisting of shares of TranS1 common stock, which is currently traded on the NASDAQ Global Market; |
| · | Trans1, as a public company, is better positioned than Baxano to raise additional capital; |
| · | the combined company will be led by experienced senior management and board of directors; |
| · | the fact that TranS1 would be required to pay Baxano a $2 million termination fee in connection with termination of the Merger Agreement in certain circumstances; and |
| · | the likelihood that the Merger will be consummated on a timely basis, including the likelihood that the Merger will receive all necessary approvals. |
| 35 |
Baxano’s Board of Directors also considered a number of additional potentially countervailing factors in its deliberations concerning the Merger, including the following:
| · | the price volatility of TranS1’s common stock, which may reduce the value of the common stock that Baxano’s stockholders will receive upon the consummation of the Merger; |
| · | the fact that the proceeds in the transaction will likely be insufficient to provide any merger consideration to the holders of Baxano Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series B Preferred Stock, and Common Stock; |
| · | the fact that the proceeds in the transaction are insufficient to provide consideration to all Baxano stockholders; |
| · | the fact that Baxano would be required to pay TranS1 a $1 million termination fee in connection with termination of the Merger Agreement in certain circumstances; |
| · | the possibility that the Merger might not be completed and the potential adverse effect of the public announcement of the Merger on Baxano’s reputation and ability to obtain financing in the future; |
| · | the risk of diverting management’s attention from other strategic priorities; |
| · | that, under the terms of the Merger Agreement, Baxano agreed that it will carry on its business in the ordinary course of business consistent with past practice and, subject to specified exceptions, that Baxano will not take a number of actions related to the conduct of its business; |
| · | the challenges and costs of combining the operations and the substantial expenses to be incurred in connection with the Merger, including the risks that delays or difficulties in completing the integration and the inability to retain key employees as a result of the management and other changes that will be implemented in integrating the business could adversely affect the combined company’s operating results and preclude the achievement of some benefits anticipated from the Merger; and |
| · | the risk of a delay in the closing of the Merger due to the need for the approval of TranS1’s stockholders and the risk that TranS1’s stockholders will not approve the Merger. |
The foregoing information and factors considered by Baxano’s Board of Directors are not intended to be exhaustive but are believed to include all of the material factors considered by Baxano’s Board of Directors. In view of the wide variety of factors considered in connection with its evaluation of the Merger and the complexity of these matters, Baxano’s Board of Directors did not find it useful, and did not attempt, to quantify, rank, or otherwise assign relative weights to these factors. In considering the factors described above, individual members of Baxano’s Board of Directors may have given different weight to different factors. Baxano’s Board of Directors conducted an overall analysis of the factors described above, including thorough discussions with, and questioning of, Baxano’s management and Baxano’s legal advisors, and unanimously (except for Mr. Shapiro) approved the Merger Agreement and the transaction contemplated thereby, including the Merger.
Opinionof Financial Advisor – Stifel, Nicolaus & Company, Incorporated
The Company retained Stifel on October 10, 2012 to act as the Company’s financial advisor in connection with a possible purchase of Baxano and to provide to the Company’s Board of Directors a fairness opinion in connection with any proposed transaction. On March 1, 2013, Stifel delivered its written opinion, dated March 1, 2013, to the Company’s Board of Directors that, as of the date of the opinion and subject to and based on the assumptions made, procedures followed, matters considered, limitations of the review undertaken, and qualifications contained in such opinion, the aggregate consideration to be paid by the Company in the Merger pursuant to the Merger Agreement is fair to the Company, from a financial point of view.
The Company did not impose any limitations on Stifel with respect to the investigations made or procedures followed in rendering its opinion. In selecting Stifel, the Company’s Board of Directors considered, among other things, the fact that Stifel is a reputable investment banking firm with substantial experience advising companies in the medical technology sector and in providing strategic advisory services in general. Stifel, as part of its investment banking business, is continuously engaged in the evaluation of businesses and their securities in connection with mergers and acquisitions, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements, and valuations for corporate and other purposes. In the ordinary course of its business, Stifel and its affiliates may actively trade and hold the securities of TranS1 for its own account or for the account of its customers and, accordingly, may at any time hold a long or short portion in such securities.
| 36 |
With Stifel’s consent, the full text of the written opinion of Stifel is attached asAnnex C to this Proxy Statement and is incorporated into this document by reference. The summary of Stifel’s opinion set forth in this Proxy Statement has been provided by Stifel and is qualified in its entirety by reference to the full text of the opinion. Stockholders are urged to read the opinion carefully and in its entirety for a discussion of the procedures followed, assumptions made, other matters considered, and limits of the review undertaken by Stifel in connection with such opinion.
Stifel’s opinion was approved by its fairness committee. The opinion was provided for the information of, and directed to, the Company’s Board of Directors for its information and assistance in connection with its consideration of the financial terms of the Merger. The opinion does not constitute a recommendation to the Company’s Board of Directors as to how it should vote on any aspect of the Merger or to any stockholder of TranS1 as to how such stockholder should vote his, her, or its shares of common stock at any stockholders’ meeting at which the Merger is considered, or whether or not any stockholder of TranS1 should enter into a voting or stockholders’, or affiliates’ agreement with respect to the Merger, or exercise any dissenters’ or appraisal rights that may be available to such stockholder. In addition, the opinion does not compare the relative merits of the Merger with any other alternative transaction or business strategies which may have been available to the Company’s Board of Directors or the Company and does not address the underlying business decision of the Company’s Board of Directors or the Company to proceed with or effect the Merger. Moreover, it does not address the fairness of the Merger to, or any consideration to be paid in connection therewith by, the holders of any class of securities, creditors, or other constituencies of the Company; nor does it address the fairness of the amount or nature of any compensation to be paid or payable to any of the Company’s or Baxano’s officers, directors, or employees, or class of such persons, in connection with the Merger, whether relative to the consideration to be paid by the Company or otherwise.
In connection with its opinion, Stifel, among other things:
| · | reviewed the financial terms contained in the draft of the Merger Agreement, dated February 26, 2013, which is the most recent draft made available to Stifel; |
| · | reviewed and analyzed certain internal financial projections concerning Baxano on a standalone basis, prepared by the Company’s management, or the Target Projections; |
| · | reviewed and analyzed certain internal financial projections concerning the operations of the Company and Baxano under the Company’s management following the Merger, including the amounts and timing of benefits of scale, operating efficiencies, and cost savings expected to result from the Merger, or the Expected Synergies, prepared by the Company’s management; |
| · | discussed with certain members of the Company’s management the current status and prospects of Baxano, the Expected Synergies, and such other matters Stifel deemed relevant; |
| · | reviewed and analyzed certain financial terms of the Merger as compared to certain financial information of select publicly traded companies Stifel deemed relevant; |
| · | reviewed and analyzed certain financial terms of the Merger as compared to the financial terms of select business combinations Stifel deemed relevant; |
| · | reviewed and analyzed, based on the Target Projections, the cash flows generated by Baxano on a standalone basis to determine the present value of its discounted cash flows; |
| · | reviewed and analyzed, based on TranS1 and Baxano projections, the cash flows generated by the pro forma company as contemplated to be managed by the Company following the Merger to determine the present value of its discounted cash flows; and |
| 37 |
| · | reviewed and analyzed such other information and financial studies, performed such other analyses and investigations and considered such other factors that Stifel deemed relevant for the purposes of its opinion. |
In addition, Stifel took into account its assessment of general economic, market and financial conditions, and its experience in other transactions, as well as its experience in securities valuations and its general knowledge of the industries in which the Company and Baxano operate.
In connection with its review, Stifel, with the Company’s Board of Directors’ consent, relied upon, and assumed, without independent verification, the accuracy and completeness of all of the financial and other information that was made available, supplied, or otherwise communicated to Stifel by or on behalf of the Company or Baxano or that was otherwise reviewed by Stifel, including, without limitation, publicly available information, and Stifel has not assumed any responsibility for independently verifying any of such information. Stifel further relied upon the assurances of the Company’s management that it was unaware of any facts that would make such information incomplete or misleading.
With respect to estimates, forecasts or projections of the Company’s or Baxano’s future financial performance prepared by or reviewed with the Company’s management (including, without limitation, the Target Projections, the Expected Synergies, and estimates and projections relating to possible future debt or equity financings by the Company on both a standalone basis and on a pro forma combined basis assuming acquisition of Baxano, in each case for the periods reviewed for Stifel’s discounted cash flow analysis) or obtained from public sources, Stifel relied upon the statements of the Company’s management that such estimates, forecasts, and projections (and the assumptions and bases therein) are reasonable and achievable and, with the consent of the Company’s management, assumed that such estimates, forecasts, and projections represent the best available estimates, forecasts, and projections and have been prepared in good faith on assumptions which, in light of the circumstances under which they were made, are reasonable, as has been represented and warranted by the Company or, with respect to estimates, forecasts and projections obtained from public sources, represent reasonable estimates, forecasts and projections and that such estimates, forecasts and projections provided a reasonable basis upon which Stifel could form its opinion. Without limiting the generality of the foregoing, with respect to Baxano, Stifel has, at the direction of the Company’s management, so relied upon and made such assumptions with respect to the Target Projections, which projections the Company’s management has informed Stifel reflect, among other things, the Company’s management’s intent with respect to the operation of Baxano after the closing of the Merger. All such estimates, forecasts, and projections (including the Target Projections) were not prepared with the expectation of public disclosure and are based on numerous variables and assumptions that are inherently uncertain, including, without limitation, factors related to general economic and competitive conditions. Accordingly, actual results could vary significantly from those set forth in such estimates, forecasts and projections. Stifel relied on these estimates, forecasts, and projections without independent verification or analyses and does not in any respect assume any responsibility for the accuracy or completeness thereof. Stifel relied upon, without independent verification, the assessment of the Company’s management as to the existing products and services of the Company and Baxano and viability of, and risks associated with, the future products and services of the Company and Baxano. Stifel expresses no opinion as to the Target Projections or the Company’s projections or any other estimates, forecasts, and projections, or the assumptions on which they were made.
Stifel was not requested to make, and did not make, an independent evaluation, physical inspection, valuation, or appraisal of the properties, facilities, assets, or liabilities (contingent or otherwise) of the Company or Baxano, and was not furnished with any such materials. Stifel assumed, with the consent of the Company’s management, that any material liabilities (contingent or otherwise, known or unknown) of the Company and Baxano are set forth in its financial statements or have been disclosed to it by the Company’s management. Stifel assumed, with the consent of the Company’s management, that there has been no material change in the assets, liabilities, financial condition, results of operations, business, or prospects of the Company and Baxano since the date of the most recent relevant financial statements made available to Stifel. Stifel has not evaluated the solvency or fair value of the Company, Baxano or any other person under any state, federal, or foreign laws relating to bankruptcy, insolvency, or similar matters. With respect to all legal matters relating to the Company, Baxano, and the Merger, Stifel has relied on the advice of legal counsel to the Company.
| 38 |
Stifel’s opinion was limited to whether the aggregate consideration to be paid by the Company in the Merger pursuant to the Merger Agreementis, as of the date of such opinion, fair to the Company, from a financial point of view. Stifel expressed no view as to any other aspect or implication of the Merger, including without limitation the form or structure of the Merger, any financing obtained or utilized or to be obtained or utilized by the Company in connection with the Merger, the sale of shares pursuant to the Securities Purchase Agreement, the allocation of the aggregate consideration, or any other agreement, arrangement, or understanding entered into in connection with the Merger or otherwise. Without limiting the generality of the foregoing, Stifel’s opinion does not address: (i) any strategic alternatives currently (or which have been or may be) contemplated by the Company or the Company’s Board of Directors; (ii) any legal, tax, or accounting matters, including any such consequences of the Merger on the Company or the Company’s stockholders; (iii) the fairness of the amount or nature of any compensation to any officers, directors, or employees of the Company or Baxano, or class of such persons, relative to the compensation to the holders of the securities of the Company or Baxano; (iv) the price or trading range for shares of the common stock of the Company following the announcement or consummation of the Merger or at any other time; (v) any advice or opinions provided by any other advisor to the Company or Baxano or any of their respective affiliates; (vi) the Company’s liquidity, including, without limitation, cash balances following the payment of the aggregate consideration; (vii) the terms of financing (including sale of the shares pursuant to the Securities Purchase Agreement) contemplated in the Merger Agreement; (viii) the availability or terms of any future debt or equity financing contemplated for the Company following the Merger; or (ix) the solvency or financial condition of the Company, Baxano, or any other person.
Stifel’s opinion is necessarily based on economic, market, financial, and other conditions as they exist on, and the information made available to it as of, the date of the opinion. It is understood that subsequent developments may affect the conclusions reached in the opinion and that Stifel does not have any obligation to update, revise or reaffirm the opinion. Further, as the Company’s Board of Directors is aware, the credit, financial and stock markets have been experiencing unusual volatility and Stifel expressed no opinion or view as to any potential effects of such volatility on the Company or the Merger.
For purposes of rendering its opinion Stifel assumed, with the Company’s consent, in all respects material to its analysis, that the representations and warranties of each party contained in the Merger Agreement are true and correct, that each party will perform all of the covenants and agreements required to be performed by it under the Merger Agreement, and that all conditions to the consummation of the Merger will be satisfied without any waiver of material terms or conditions by the Company or any other party and without any anti-dilution or other adjustment to the aggregate consideration. Stifel assumed that the final form of the Merger Agreement will be substantially similar to the last draft reviewed by it. Stifel also assumed that all governmental, regulatory, and other consents and approvals required in connection with the Merger will be obtained and that in the course of obtaining any of those consents and approvals no restrictions will be imposed or waivers made that would have an adverse effect on the contemplated benefits of the Merger. Stifel assumed that the Merger will be consummated in a manner that complies with the applicable provisions of the Securities Act, the Exchange Act, and all other applicable federal and state statutes, rules and regulations. Stifel further assumed that the Company has relied upon the advice of its counsel, independent accountants, and other advisors (other than Stifel) as to all legal, financial reporting, tax, accounting, and regulatory matters with respect to the Company, the Merger, and the Merger Agreement. In rendering its services, Stifel did not and does not provide accounting, legal, or tax advice.
Stifel did not consider any potential legislative or regulatory changes currently being considered or recently enacted by the United States Congress, the SEC, or any other governmental or regulatory bodies, or any changes in accounting methods or generally accepted accounting principles that may be adopted by the SEC or the Financial Accounting Standards Board.
The summary set forth below does not purport to be a complete description of the analyses performed by Stifel, but describes, in summary form, the material elements of the presentation that Stifel made to the Company’s Board of Directors on March 1, 2013, in connection with Stifel’s opinion.
In accordance with customary investment banking practice, Stifel employed generally accepted valuation methods and financial analyses in reaching its opinion. The following is a summary of the material financial analyses performed by Stifel in arriving at its opinion. These summaries of financial analyses alone do not constitute a complete description of the financial analyses Stifel employed in reaching its conclusions. None of the analyses performed by Stifel was assigned a greater significance by Stifel than any other, nor does the order of analyses described represent relative importance or weight given to those analyses by Stifel. The summary text describing each financial analysis does not constitute a complete description of Stifel’s financial analyses, including the methodologies and assumptions underlying the analyses, and if viewed in isolation could create a misleading or incomplete view of the financial analyses performed by Stifel. The summary text set forth below does not represent and should not be viewed by anyone as constituting conclusions reached by Stifel with respect to any of the analyses performed by it in connection with its opinion. Rather, Stifel made its determination as to the fairness to the Company of the aggregate consideration paid by the Company in the Merger pursuant to the Merger Agreement, from a financial point of view, on the basis of its experience and professional judgment after considering the results of all of the analyses performed.
| 39 |
Except as otherwise noted, the information utilized by Stifel in its analyses, to the extent that it is based on market data, is based on market data as it existed on or before February 28, 2013 and is not necessarily indicative of current market conditions. The analyses described below do not purport to be indicative of actual future results, or to reflect the prices at which any securities may trade in the public markets, which may vary depending upon various factors, including changes in interest rates, dividend rates, market conditions, economic conditions, and other factors that influence the price of securities.
In conducting its analysis, Stifel used four primary methodologies: selected publicly-traded comparable companies analysis, selected precedent transactions analysis, discounted cash flow analysis, and contribution analysis. No individual methodology was given a specific weight, nor can any methodology be viewed individually. Additionally, no company or transaction used in any analysis as a comparison is identical to the Company, Baxano, or the Merger, and they all differ in material ways. Accordingly, an analysis of the results described below is not mathematical; rather it involves complex considerations and judgments concerning differences in financial and operating characteristics of the companies and other factors that could affect the public trading value of the selected companies or transactions to which they are being compared. Stifel used these analyses to determine the impact of various operating metrics on the implied equity value of Baxano. Each of these analyses yielded a range of implied equity values, and therefore, such implied equity value ranges developed from these analyses were viewed by Stifel collectively and not individually.
Baxano Financial Analyses
Selected Companies Analysis. Stifel reviewed, analyzed, and compared certain financial information relating to Baxano to corresponding publicly available financial information and market multiples for the following seven publicly-traded, small-capitalization, specialty spine companies with marketed products, a sales force with a spinal surgeon call point, and equity values that Stifel deemed comparable to Baxano: Integra LifeSciences Holdings Corp., Globus Medical Inc., Orthofix International N.V., NuVasive Inc., Alphatec Holdings Inc., TranS1 Inc., and Bacterin International Holdings Inc.
Stifel reviewed, among other things, the range of enterprise values of the selected companies, calculated as equity value based upon closing stock prices on February 28, 2013, plus the book value of debt and minority interests, less cash and cash equivalents, as a multiple of estimated revenue for calendar years 2012, 2013, and 2014, as provided by FactSet Consensus estimates.
The following table sets forth, for the periods indicated, the ranges of enterprise value as a multiple of revenue utilized by Stifel in performing its analysis, which were derived from the selected companies identified above, and the ranges of the enterprise value of Baxano implied by this analysis.
| Enterprise Value to: | Range of Revenue Multiples | Range of Implied Enterprise Value (US$ in Millions) | ||||||
| 2012E Revenue | 0.81x – 2.97x | $7.7 – $28.0 | ||||||
| 2013P Revenue | 0.78x – 2.65x | $12.6 – $42.7 | ||||||
| 2014P Revenue | 0.42x – 2.36x | $11.4 – $63.7 | ||||||
The aggregate consideration represents an enterprise value of Baxano at $25.9 million and a multiple of 2.75x, 1.61x, and 0.96x to Baxano’s estimated or projected revenue of for 2012, 2013, and 2014, respectively.
Stifel selected publicly-traded companies on the basis of various factors, including the size of the public company and the similarity of the lines of business, although, as noted above, no public company used as a comparison is identical to Baxano. Accordingly, these analyses are not purely mathematical, but also involve complex considerations and judgments concerning the differences in financial and operating characteristics of the selected companies and other factors.
Selected Precedent Transactions Analysis.Stifel reviewed and analyzed certain publicly available information for the following six recent business combinations where the target had specialty spine products and where the transaction was announced subsequent to January 1, 2007:
| 40 |
| Date Announced | Target Name | Acquire Name | ||
| August 7, 2012 | Phygen, LLC | Alphatec Spine, Inc. | ||
| May 24, 2011 | SeaSpine, Inc. | Integra LifeSciences Holdings Corporation | ||
| December 17, 2009 | Scient’x Groupe SAS | Alphatec Spine, Inc. | ||
| September 4, 2008 | Abbott Spine (spine business of Abbott Laboratories) | Zimmer Holdings, Inc. | ||
| July 23, 2008 | Theken Spine, LLC, Theken Disc, LLC and Therics, LLC | Integra LifeSciences Holdings Corporation | ||
| December 10, 2007 | Scient’x Groupe S.A. | HealthpointCapital Partners, L.P. |
The following table sets forth, for the periods indicated, the ranges of multiples utilized by Stifel in performing its analysis, which were derived from the selected business combinations described above, and the ranges of the enterprise value of Baxano implied by this analysis.
| Enterprise Value to: | Range of Transaction Multiples | Range of Implied Enterprise Value | ||||||
| LTM Revenue | 1.52x – 3.30x | $14.3 – $31.1 | ||||||
| NTM Revenue | 1.01x – 2.89x | $16.3 – $46.6 | ||||||
| * LTM refers to the last twelve month. NTM refers to the next twelve month. | ||||||||
The aggregate consideration represents an enterprise value of Baxano at $25.9 million and a multiple of 2.75x and 1.61x to Baxano’s estimated 2012 revenue and projected 2013 revenue, respectively.
Because the market conditions, rationale, and circumstances surrounding each of the transactions analyzed were specific to each transaction and because of the inherent differences between Baxano’s businesses, operations, and prospects and those of the acquired companies above, Stifel believed that it was inappropriate to, and therefore did not, rely solely on the quantitative results of the analysis. Accordingly, Stifel also made qualitative judgments concerning the differences between the characteristics of these transactions (including market conditions, rationale, and circumstances surrounding each of the transactions, and the timing, type, and size of each of the transactions) and the Merger that could affect Baxano’s acquisition value.
Discounted Cash Flow Analysis. Stifel used the financial projections and estimates provided by the Company’s management to perform a discounted cash flow analysis. In conducting this analysis, Stifel assumed that Baxano would perform in accordance with these forecasts. Additional information regarding these forecasts is set forth below under the heading “Projected Financial Information.” Stifel performed an analysis of the present value of the unlevered free cash flows that the Company’s management projects Baxano will generate from the second quarter of 2013 through 2017. Stifel discounted both the cash flows projected from the second quarter of 2013 through 2017 and the terminal value to present value using discount rates ranging from 25% to 35% and terminal multiples ranging from 1.5x to 2.5x estimated revenue in 2017 for its valuation reference range. The discount rates were selected based on a weighted-average cost of capital analysis for the selected, publicly-traded, small-capitalization, specialty spine companies identified above and Stifel’s estimates. This analysis resulted in implied enterprise values ranging from negative $7.0 million to $23.2 million.
Merger Financial Analyses
The Company’s management provided to Stifel projections of its expected financial performance as a standalone company and Baxano’s expected financial performance as a standalone company. The Company also provided to Stifel estimated revenue and cost synergies relating to, and estimated for, the combined businesses. Additional information regarding these forecasts is set forth below under the heading “Projected Financial Information.” Upon the Company’s request, Stifel conducted certain analyses of the pro forma combined company. At the Company’s request, Stifel assumed, without independent verification, and relied upon the estimates, projections, and related statements with respect to intent, future plans and synergy assumptions, provided by the Company’s management.
| 41 |
Pro Forma Discounted Cash Flow Analysis. Stifel used the financial projections and estimates provided by the Company’s management to perform a discounted cash flow analysis for the pro forma combined company. In conducting this analysis, Stifel assumed that the combined company would perform in accordance with these forecasts. Additional information regarding these forecasts is set forth below under the heading “Projected Financial Information.” Stifel performed an analysis of the present value of the unlevered free cash flows that the Company’s management projects the combined company will generate from the second quarter of 2013 through 2017, taking into account the effect of financing transactions in connection with the Merger and possible financing transactions during the projected period from the second quarter of 2013 through 2017. Stifel discounted both the cash flows projected from the second quarter of 2013 through 2017 and the terminal value to present value using discount rates ranging from 17.5% to 22.5% and terminal multiples ranging from 2.0x to 3.0x estimated revenue in 2017 for its valuation reference range. The discount rates were selected based on a weighted-average cost of capital analysis for the selected, publicly-traded, small-capitalization, specialty spine companies identified above, and Stifel’s estimates for the combined company. This analysis resulted in implied equity value per share of the combined company ranging from $3.52 to $5.95. In comparison, Stifel performed an analysis of the present value of the unlevered free cash flows that the Company’s management projects the Company will generate from the second quarter of 2013 through 2017 as a standalone company, taking into account the effect of possible financing transactions during the projected period from the second quarter of 2013 through 2017. Stifel discounted both the cash flows projected from the second quarter of 2013 through 2017 and the terminal value to present value using discount rate ranging from 17.5% to 22.5% and terminal multiples ranging from of 1.5x to 2.5x estimated revenue in 2017 for its valuation reference values. This analysis resulted in an implied equity per share of the Company as a standalone company ranging from $2.09 to $3.78.
Contribution Analysis. Stifel analyzed and compared the Company’s and Baxano’s respective contributions to the combined company based upon revenue and gross profit for the years from 2012 through 2017, in each case based upon the financial projections provided by the Company’s management, taking into account the effect of the Expected Synergies. The resulting analysis for net revenue and gross profit is included in the table below:
| Revenue Contribution | Gross Profit Contribution | |||||||||||||||||||||||||||||||
| TranS1 | Baxano | Synergy | Total | TranS1 | Baxano | Synergy | Total | |||||||||||||||||||||||||
| 2012A | 60.7 | % | 39.3 | % | 0.0 | % | 100 | % | 62.4 | % | 37.6 | % | 0.0 | % | 100 | % | ||||||||||||||||
| 2013E | 56.4 | % | 43.6 | % | 0.0 | % | 100 | % | 57.3 | % | 42.7 | % | 0.0 | % | 100 | % | ||||||||||||||||
| 2014P | 53.8 | % | 41.2 | % | 5.0 | % | 100 | % | 54.2 | % | 40.5 | % | 5.4 | % | 100 | % | ||||||||||||||||
| 2015P | 57.8 | % | 37.2 | % | 5.0 | % | 100 | % | 58.6 | % | 36.4 | % | 5.0 | % | 100 | % | ||||||||||||||||
| 2016P | 62.1 | % | 32.9 | % | 5.0 | % | 100 | % | 62.7 | % | 32.3 | % | 5.0 | % | 100 | % | ||||||||||||||||
| 2017P | 64.8 | % | 30.2 | % | 5.0 | % | 100 | % | 65.3 | % | 29.7 | % | 5.0 | % | 100 | % | ||||||||||||||||
Conclusion
Based upon the foregoing analyses and the assumptions and limitations set forth in full in the text of Stifel’s opinion, Stifel was of the opinion that, as of the date of Stifel’s opinion, the aggregate consideration to be paid by the Company in the Merger pursuant to the Merger Agreementwas fair to the Company, from a financial point of view.
The preparation of a fairness opinion is a complex process and is not necessarily susceptible to a partial analysis or summary description. In arriving at its opinion, Stifel considered the results of all of its analyses as a whole and did not attribute any particular weight to any analysis or factor considered by it. Stifel believes that the summary provided and the analyses described above must be considered as a whole and that selecting portions of these analyses, without considering all of them, would create an incomplete view of the process underlying Stifel’s analyses and opinion; therefore, the range of valuations resulting from any particular analysis described above should not be taken to be Stifel’s view of the actual value of Baxano.
Stifel is acting as financial advisor to the Company in connection with the Merger. It received a fee for rendering its opinion which was not contingent upon consummation of the Merger but is creditable against transaction fees payable to Stifel which are contingent upon the completion of the Merger. Stifel will also receive a right of first refusal to act as the Company’s lead left book-running managing underwriter or placement agent for a certain period if the Company retains or otherwise uses an investment bank to pursue a registered, underwritten public offering or private placement of securities. In addition, the Company has agreed to indemnify Stifel for certain liabilities arising out of Stifel’s engagement. In the two years preceding the date of Stifel’s opinion, Stifel has not served as financial advisor to the Company or Baxano. Furthermore, a private equity fund whose general partners are affiliated with Stifel and a private equity fund in whose general partner an affiliate of Stifel holds a membership interest are shareholders of the Company, holding less than 5% of the Company, and a private equity fund in whose general partner is an affiliate of Stifel holds a membership interest is a shareholder of Baxano, holding less than 10% of Baxano. Stifel may seek to provide investment banking services to the Company or its affiliates in the future, for which Stifel would seek customary compensation.
| 42 |
Projected Financial Information
Projections . The Company does not as a matter of course make projections as to future revenues, earnings or other results available to the public due to, among other things, the uncertainty of underlying assumptions and estimates. However, the Company is including certain unaudited prospective financial information that was made available to Stifel in connection with its discounted cash flow analysis of Baxano and of the Merger. The inclusion of this information should not be regarded as an indication that any of the Company, Stifel, Baxano or any other person considered, or now considers, such projections to be predictive of actual future results.
Although presented with numerical specificity, the financial projections are not actual facts and were based on numerous variables and assumptions (including but not limited to assumptions regarding general economic and market conditions, competitive conditions and the existing products and services of the Company and Baxano and viability of, and risks associated with, the future products and services of the Company and Baxano) that are inherently uncertain and, in many cases, are beyond the control of the Company and Baxano. The underlying assumptions may not prove to be accurate. As a result, the projections and estimates included in the unaudited prospective financial information may not be realized and actual results may be significantly higher or lower than estimated. Because the unaudited prospective financial information covers multiple years, that information by its nature becomes less predictive with each successive year. You are encouraged to review the risks and uncertainties described under the headings “Risks Related to the Merger, the Private Placement, and the Combined Company” beginning on page 8, “Risks Related to Our Business” beginning on page 10, “Cautionary Note Regarding Forward-Looking Statements” beginning on page 26 and “Information About Trans1” beginning on page 78.
None of the Company, Baxano, Stifel or their respective affiliates, officers, directors or other representatives can give you any assurance that actual results will not differ materially from the financial projections, and none of them undertakes any obligation to update or otherwise revise or reconcile the financial projections to reflect circumstances existing after the date the financial projections were generated or to reflect the occurrence of subsequent or future events, even in the event that any or all of the assumptions underlying the projections are shown to be in error. None of the Company, Baxano, Stifel or their respective affiliates, officers, directors or other representatives has made or makes any representation to any stockholder or to any other person regarding the Company’s or Baxano’s ultimate performance compared to the information contained in the financial projections or that the projected results will be achieved. The summary of the financial projections included below is not being included to influence your decision whether to vote for the issuance of shares of the Company’s common stock pursuant to the Merger Agreement, but are being provided because the financial projections were provided by the Company to Stifel and considered in connection with the analysis of the merger.
| TranS1 Projections for Baxano for Calendar | ||||||||||||||||||||
| Years Ended December 31, | ||||||||||||||||||||
| 2013E | 2014P | 2015P | 2016P | 2017P | ||||||||||||||||
| ($ in millions) | ||||||||||||||||||||
| Total Revenue | $ | 16.1 | $ | 27.0 | $ | 38.8 | $ | 51.7 | $ | 67.8 | ||||||||||
| Less: Costs of Goods Sold | (4.8 | ) | (7.3 | ) | (9.7 | ) | (12.4 | ) | (15.6 | ) | ||||||||||
| Gross Profit | 11.3 | 19.7 | 29.1 | 39.3 | 52.2 | |||||||||||||||
| Less R&D Expense | (4.7 | ) | (5.0 | ) | (4.3 | ) | (4.8 | ) | (6.2 | ) | ||||||||||
| Less S&M Expense | (18.7 | ) | (23.5 | ) | (29.1 | ) | (35.5 | ) | (42.7 | ) | ||||||||||
| Less G&A Expense | (2.5 | ) | (3.3 | ) | (4.0 | ) | (5.2 | ) | (6.8 | ) | ||||||||||
| Less Other Expense | (0.3 | ) | (0.5 | ) | (0.7 | ) | (0.9 | ) | (1.2 | ) | ||||||||||
| EBIT | (14.9 | ) | (12.6 | ) | (9.0 | ) | (7.0 | ) | (4.6 | ) | ||||||||||
| Plus: Other Income / (Expense) | 0.0 | — | — | — | — | |||||||||||||||
| Pre-tax income | (14.9 | ) | (12.6 | ) | (9.0 | ) | (7.0 | ) | (4.6 | ) | ||||||||||
| Less: Income Tax Expense | — | — | — | — | — | |||||||||||||||
| Net Income | (14.9 | ) | (12.6 | ) | (9.0 | ) | (7.0 | ) | (4.6 | ) | ||||||||||
| EBIT | (14.9 | ) | (12.6 | ) | (9.0 | ) | (7.0 | ) | (4.6 | ) | ||||||||||
| Plus Depreciation & Amortization | 0.4 | 0.5 | 0.5 | 0.7 | 0.9 | |||||||||||||||
| EBITDA | $ | (14.5 | ) | $ | (12.1 | ) | $ | (8.5 | ) | $ | (6.3 | ) | $ | (3.7 | ) | |||||
| 43 |
| TranS1 Projections for TranS1 (Stand-Alone) for Calendar | ||||||||||||||||||||
| Years Ended December 31, | ||||||||||||||||||||
| 2013E | 2014P | 2015P | 2016P | 2017P | ||||||||||||||||
| ($ in millions) | ||||||||||||||||||||
| Total Revenue | $ | 20.8 | (1) | $ | 35.3 | (2) | $ | 60.3 | (3) | $ | 97.4 | (3) | $ | 145.8 | (3) | |||||
| Less: Costs of Goods Sold | (5.7 | ) | (8.9 | ) | (13.5 | ) | (21.3 | ) | (31.2 | ) | ||||||||||
| Gross Profit | 15.1 | 26.4 | 46.8 | 76.2 | 114.6 | |||||||||||||||
| Less R&D Expense | (6.3 | ) | (7.3 | ) | (8.3 | ) | (9.2 | ) | (10.2 | ) | ||||||||||
| Less S&M Expense | (23.4 | ) | (29.1 | ) | (37.3 | ) | (46.7 | ) | (57.3 | ) | ||||||||||
| Less G&A Expense | (6.0 | ) | (7.0 | ) | (8.1 | ) | (9.2 | ) | (10.2 | ) | ||||||||||
| Less Other Expense | (0.3 | ) | (0.6 | ) | (1.2 | ) | (1.6 | ) | (2.4 | ) | ||||||||||
| EBIT | (21.0 | ) | (17.6 | ) | (8.1 | ) | 9.5 | 34.5 | ||||||||||||
| Plus: Other Income / (Expense) | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | |||||||||||||||
| Pre-tax income | (20.9 | ) | (17.6 | ) | (8.1 | ) | 9.5 | 34.5 | ||||||||||||
| Less: Income Tax Expense | — | — | — | — | — | |||||||||||||||
| Net Income | (20.9 | ) | (17.6 | ) | (8.1 | ) | 9.5 | 34.5 | ||||||||||||
| EBIT | (21.0 | ) | (17.6 | ) | (8.1 | ) | 9.5 | 34.5 | ||||||||||||
| Plus Depreciation & Amortization | 1.7 | 2.0 | 2.3 | 3.9 | 6.2 | |||||||||||||||
| EBITDA | $ | (19.2 | ) | $ | (15.6 | ) | $ | (5.8 | ) | $ | 13.4 | $ | 40.6 | |||||||
(1) The Company’s Total Revenue for 2013 assumes growth over the prior year as a result of increased sales of its AxiaLIF products, due primarily to the establishment of a Category I code providing broader reimbursement, as well as increased sales of its VEO direct lateral products, which were fully commercially launched in early 2012.
(2) The Company’s projected Total Revenue for 2014 assumes continuing growth as a result of increased sales of its AxiaLIF products, due primarily to the broader private reimbursement coverage, as well as increased sales of its VEO direct lateral products through increased market share, and the introduction of new products, as well as increases in sales headcounts.
(3) The Company’s projected Total Revenue for the periods 2015-2017 assumes broad reimbursement for the AxiaLIF products, overall increase in market share of all products, as well as increases in sales headcounts.
| Pro Forma Projections (Combined) for Calendar | ||||||||||||||||||||
| Years Ended December 31, | ||||||||||||||||||||
| 2013E | 2014P | 2015P | 2016P | 2017P | ||||||||||||||||
| ($ in millions) | ||||||||||||||||||||
| Total Revenue | $ | 33.4 | $ | 65.6 | $ | 104.3 | $ | 157.0 | $ | 224.9 | ||||||||||
| Less: Costs of Goods Sold | (9.5 | ) | (16.9 | ) | (24.4 | ) | (35.5 | ) | (49.2 | ) | ||||||||||
| Gross Profit | 24.0 | 48.7 | 79.9 | 121.5 | 175.6 | |||||||||||||||
| Less R&D Expense | (9.1 | ) | (11.4 | ) | (12.0 | ) | (11.0 | ) | (15.7 | ) | ||||||||||
| Less S&M Expense | (34.2 | ) | (46.0 | ) | (48.0 | ) | (61.2 | ) | (79.8 | ) | ||||||||||
| Less G&A Expense | (7.9 | ) | (8.4 | ) | (9.7 | ) | (11.0 | ) | (14.6 | ) | ||||||||||
| Less Other Expense | (0.5 | ) | (1.1 | ) | (2.0 | ) | (2.6 | ) | (3.8 | ) | ||||||||||
| EBIT | (27.8 | ) | (18.2 | ) | 8.2 | 35.7 | 61.7 | |||||||||||||
| Plus: Other Income / (Expense) | (0.2 | ) | (0.3 | ) | (0.3 | ) | (0.3 | ) | (0.2 | ) | ||||||||||
| Pre-tax income | (28.0 | ) | (18.4 | ) | 7.9 | 35.4 | 61.4 | |||||||||||||
| Less: Income Tax Expense | — | — | — | — | — | |||||||||||||||
| Net Income | (28.0 | ) | (18.4 | ) | 7.9 | 35.4 | 61.4 | |||||||||||||
| EBIT | (27.8 | ) | (18.2 | ) | 8.2 | 35.7 | 61.7 | |||||||||||||
| Plus Depreciation & Amortization | 2.8 | 3.8 | 4.0 | 6.2 | 9.5 | |||||||||||||||
| EBITDA | $ | (25.0 | ) | $ | (14.4 | ) | $ | 12.2 | $ | 41.9 | $ | 71.2 | ||||||||
| 44 |
Earnings before interest, taxes, depreciation and amortization, which the Company refers to as EBITDA, is calculated as net income plus (i) interest expense, net of interest income, (ii) income tax provision, and (iii) depreciation and amortization. EBITDA is not a calculation provided for under GAAP. EBITDA should not be considered as an alternative to net income or operating income as an indication of operating performance or as an alternative to operating cash flow as a measure of liquidity. EBITDA is not necessarily comparable to similarly titled measures of other companies. EBITDA is presented here because it is a widely used financial indicator used by investors and analysts to measure performance. EBITDA is also used by the Company’s management for internal analysis.
Estimated revenue and cost synergies. In connection with the pro forma (combined) projections set forth above, the Company provided Stifel with estimated forecasts of synergies, which Stifel assumed for purposes of its analysis. Those synergy assumptions were as follows:
| Synergy Assumptions for Calendar | ||||||||||||||||||||
| Years Ended December 31, | ||||||||||||||||||||
| 2013E | 2014P | 2015P | 2016P | 2017P | ||||||||||||||||
| ($ in millions) | ||||||||||||||||||||
| Incremental Revenue (assumes 5% cross-selling opportunity) | -- | 3.3 | 5.2 | 7.8 | 11.2 | |||||||||||||||
| Incremental Cost of Goods Sold (COGS) | -- | (0.8 | ) | (1.2 | ) | (1.7 | ) | (2.4 | ) | |||||||||||
| Incremental COGS synergies | 0.0 | 0.2 | (0.1 | ) | (0.1 | ) | (0.1 | ) | ||||||||||||
| Sales & Marketing (S&M) Cost Synergies | 3.2 | 7.3 | 19.5 | 22.5 | 22.4 | |||||||||||||||
| Incremental S&M | -- | (0.7 | ) | (1.0 | ) | (1.6 | ) | (2.2 | ) | |||||||||||
| Incremental Medical Device Tax | -- | (0.1 | ) | (0.1 | ) | (0.1 | ) | (0.2 | ) | |||||||||||
| General and Administrative (G&A) Cost Synergies | 0.0 | 2.0 | 2.4 | 3.3 | 2.4 | |||||||||||||||
| Research & Development (R&D) Cost Synergies | 0.7 | 0.9 | 0.5 | 3.0 | 0.6 | |||||||||||||||
| Total Synergies | $ | 3.9 | $ | 12.1 | $ | 25.3 | $ | 33.2 | $ | 31.8 | ||||||||||
Projected Cash Flow . In connection with the pro forma (combined) projections set forth above, the Company also provided Stifel with projected cash flow for Baxano, TranS1 on a stand-alone basis and the pro forma (combined) Company and pre-tax synergies underlying the discounted cash flow analysis, which Stifel assumed for purposes of its analysis. Those projections and assumptions were as follows:
| Projected Cash Flow for Baxano for Calendar Years (or, for 2013, 9-Month Period) Ended December 31, | ||||||||||||||||||||
| Calculation of Free Cash Flow | Q2-Q4: 2013E | 2014P | 2015P | 2016P | 2017P | |||||||||||||||
| Revenue | $ | 12.6 | $ | 27.0 | $ | 38.8 | $ | 51.7 | $ | 67.8 | ||||||||||
| EBITDA | $ | (10.4 | ) | $ | (12.1 | ) | $ | (8.5 | ) | $ | (6.3 | ) | $ | (3.7 | ) | |||||
| Less: Depreciation & Amortization | $ | (0.3 | ) | $ | (0.5 | ) | $ | (0.5 | ) | $ | (0.7 | ) | $ | (0.9 | ) | |||||
| EBIT | $ | (10.7 | ) | $ | (12.6 | ) | $ | (9.0 | ) | $ | (7.0 | ) | $ | (4.6 | ) | |||||
| Less: Income Tax Expense | - | - | - | - | - | |||||||||||||||
| Unlevered Net Income | $ | (10.7 | ) | $ | (12.6 | ) | $ | (9.0 | ) | $ | (7.0 | ) | $ | (4.6 | ) | |||||
| Plus: Depreciation & Amortization | $ | 0.3 | $ | 0.5 | $ | 0.5 | $ | 0.7 | $ | 0.9 | ||||||||||
| Plus: Change in Working Capital | $ | (2.0 | ) | $ | (1.4 | ) | $ | (2.5 | ) | $ | (2.8 | ) | $ | (3.6 | ) | |||||
| Plus: Stock Compensation Expense | $ | 0.4 | $ | 0.8 | $ | 1.1 | $ | 1.5 | 2.0 | |||||||||||
| Less: Capital Expenditures | $ | (0.3 | ) | $ | (0.8 | ) | $ | (1.2 | ) | $ | (1.6 | ) | $ | (2.0 | ) | |||||
| Unlevered Free Cash Flow | $ | (12.4 | ) | $ | (13.5 | ) | $ | (11.0 | ) | $ | (9.1 | ) | $ | (7.3 | ) | |||||
| Projected Cash Flow for TranS1 (Stand-Alone) for Calendar Years (or, for 2013, 9-Month Period) Ended December 31, | ||||||||||||||||||||
| Calculation of Free Cash Flow | Q2-Q4: 2013E | 2014P | 2015P | 2016P | 2017P | |||||||||||||||
| Revenue | $ | 16.4 | $ | 35.3 | $ | 60.3 | $ | 97.4 | $ | 145.8 | ||||||||||
| EBITDA | $ | (13.8 | ) | $ | (15.6 | ) | $ | (5.8 | ) | $ | 13.4 | $ | 40.6 | |||||||
| Less: Depreciation & Amortization | $ | (1.4 | ) | $ | (2.0 | ) | $ | (2.3 | ) | $ | (3.9 | ) | $ | (6.2 | ) | |||||
| EBIT | $ | (15.2 | ) | $ | (17.6 | ) | $ | (8.1 | ) | $ | 9.5 | $ | 34.5 | |||||||
| Less: Income Tax Expense | - | - | - | - | - | |||||||||||||||
| Unlevered Net Income | $ | (15.2 | ) | $ | (17.6 | ) | $ | (8.1 | ) | $ | 9.5 | $ | 34.5 | |||||||
| Plus: Depreciation & Amortization | $ | 1.4 | $ | 2.0 | $ | 2.3 | $ | 3.9 | $ | 6.2 | ||||||||||
| Plus: Stock Option Expense | $ | 1.2 | $ | 2.1 | $ | 2.9 | $ | 3.4 | $ | 4.4 | ||||||||||
| Plus: Change in Working Capital | $ | (3.1 | ) | $ | (6.8 | ) | $ | (9.3 | ) | $ | (10.2 | ) | $ | (14.9 | ) | |||||
| Less: Capital Expenditures | $ | (0.9 | ) | $ | (2.8 | ) | $ | (4.5 | ) | $ | (7.3 | ) | $ | (10.9 | ) | |||||
| Unlevered Free Cash Flow | $ | (16.6 | ) | $ | (23.0 | ) | $ | (16.8 | ) | $ | (0.8 | ) | $ | 19.1 | ||||||
| Projected Cash Flow for the Pro Forma (Combined) Company for Calendar Years (or, for 2013, 9-Month Period) Ended December 31, | ||||||||||||||||||||
| Calculation of Free Cash Flow | Q2-Q4: 2013E | 2014P | 2015P | 2016P | 2017P | |||||||||||||||
| Revenue | $ | 29.0 | $ | 65.6 | $ | 104.3 | $ | 157.0 | $ | 224.9 | ||||||||||
| EBITDA | $ | (19.6 | ) | $ | (14.4 | ) | $ | 12.2 | $ | 41.9 | $ | 71.2 | ||||||||
| Less: Existing Depreciation & Amortization | $ | (2.4 | ) | $ | (3.8 | ) | $ | (4.0 | ) | $ | (6.2 | ) | $ | (9.5 | ) | |||||
| EBIT | $ | (22.0 | ) | $ | (18.2 | ) | $ | 8.2 | $ | 35.7 | $ | 61.7 | ||||||||
| Less: Income Tax Expense | - | - | - | - | - | |||||||||||||||
| Unlevered Net Income | $ | (22.0 | ) | $ | (18.2 | ) | $ | 8.2 | $ | 35.7 | $ | 61.7 | ||||||||
| Plus: Existing Depreciation & Amortization | $ | 2.4 | $ | 3.8 | $ | 4.0 | $ | 6.2 | $ | 9.5 | ||||||||||
| Plus: New Depreciation & Amortization | - | - | - | - | - | |||||||||||||||
| Plus: Stock Option Expense | $ | 2.1 | $ | 3.9 | $ | 4.9 | $ | 5.4 | $ | 6.7 | ||||||||||
| Plus: Change in Working Capital | $ | (5.6 | ) | $ | (11.1 | ) | $ | (15.4 | ) | $ | (16.6 | ) | $ | (20.0 | ) | |||||
| Less: Capital Expenditures | $ | (1.6 | ) | $ | (4.6 | ) | $ | (7.0 | ) | $ | (9.9 | ) | $ | (13.5 | ) | |||||
| Unlevered Free Cash Flow | $ | (24.7 | ) | $ | (26.1 | ) | $ | (5.3 | ) | $ | 20.9 | $ | 44.4 | |||||||
| Pre-Tax Synergies for Calendar Years (or, for 2013, 9-Month Period) Ended December 31, | ||||||||||||||||||||
| Q2-Q4: 2013E | 2014P | 2015P | 2016P | 2017P | ||||||||||||||||
| EBIT (Net Synergies) | $ | 3.9 | $ | 12.1 | $ | 25.3 | $ | 33.2 | $ | 31.8 | ||||||||||
| Unlevered Free Cash Flow | $ | 3.9 | $ | 12.1 | $ | 25.3 | $ | 33.2 | $ | 31.8 | ||||||||||
The unaudited financial projections and the estimated forecasts of synergies were not prepared with a view toward public disclosure, nor were they prepared with a view toward compliance with published guidelines of the SEC, the guidelines established by the American Institute of Certified Public Accountants for preparation and presentation of financial projections, or GAAP. In addition, the projections and synergy assumptions were not prepared with the assistance of, or reviewed, compiled or examined by, an independent auditor. Neither the Company’s independent auditors, nor any other independent accountants, have compiled, examined, or performed any procedures with respect to the prospective financial information or synergy assumptions contained herein, nor have they expressed any opinion or any other form of assurance on such information or its achievability, and assume no responsibility for the prospective financial information and synergy assumptions.
| 45 |
No representation is made by the Company or any other person to any stockholder or other person regarding the ultimate performance of the Company, Baxano or the combined company compared to the information included above. The inclusion of unaudited prospective financial information in this proxy statement should not be regarded as an indication that the prospective financial information will be predictive of actual future events.
THE COMPANY DOES NOT INTEND TO UPDATE OR OTHERWISE REVISE THE ABOVE UNAUDITED PROSPECTIVE FINANCIAL INFORMATION TO REFLECT CIRCUMSTANCES EXISTING AFTER THE DATE WHEN MADE OR TO REFLECT THE OCCURRENCE OF FUTURE EVENTS, EVEN IN THE EVENT THAT ANY OR ALL OF THE ASSUMPTIONS UNDERLYING THE PROSPECTIVE FINANCIAL INFORMATION ARE NO LONGER APPROPRIATE, EXCEPT AS MAY BE REQUIRED BY LAW.
The Merger Agreement
The following is a summary of the material terms of the Merger Agreement. A copy of the Merger Agreement is attached asAnnex A to this Proxy Statement and is incorporated by reference into this Proxy Statement. The Merger Agreement has been attached to this Proxy Statement to provide you with information regarding its terms. It is not intended to provide any other factual information about the Company, Baxano, or Merger Sub. The following description does not purport to be complete and is qualified in its entirety by reference to the Merger Agreement. You should refer to the full text of the Merger Agreement for details of the Merger and the terms and conditions of the Merger Agreement.
Structure and Effective Time of the Merger
Under the terms of the Merger Agreement, the Company will acquire Baxano through a merger of Merger Sub, a wholly-owned acquisition subsidiary of the Company formed for the Merger, with and into Baxano. At the effective time of the Merger, or the Effective Time, Merger Sub will cease to exist and Baxano will continue as the surviving corporation (Baxano following the Merger is sometimes referred to herein as the Surviving Corporation). Thereafter, Baxano will be a wholly-owned subsidiary of the Company. Immediately following the Merger, the name of the Surviving Corporation will be changed to “Baxano, Inc.” We currently expect that the closing of the Merger will take place in the second quarter of 2013. However, because the Merger is subject to approval by the Company’s stockholders and other conditions to closing, we cannot predict exactly when the closing will occur.
Management after the Merger
The directors and officers of Merger Sub immediately before the Merger, who are employees of the Company, will become the initial directors and officers, respectively, of the Surviving Corporation after the Merger. Ken Reali, President and Chief Executive Officer of the Company, and Joseph Slattery, Executive Vice President and Chief Financial Officer of the Company, will continue to serve in their respective roles for the combined company. The Company is still considering who will be its other long-term officers and directors following the Merger and, to date, has made only tentative determinations in this regard. For a description of post-Merger changes to our Board of Directors mandated by the Merger Agreement, see “—Additional Agreements / Covenants—Board of Directors of the Company.”
Merger Consideration
Closing Consideration
Pursuant to the terms of the Merger Agreement, the merger consideration will consist of a number of newly issued shares of common stock of the Company calculated as the product of (a) 0.38888889 times (b) the number of issued and outstanding shares of capital stock of the Company (as if converted to common stock) as of the close of business on the last trading day prior to the closing of the Merger. This calculation is intended to provide Baxano stockholders with ownership of 28% of the Company’s post-closing outstanding shares of common stock (excluding the impact of the Private Placement Transaction) prior to certain adjustments to the number of shares the Company will issue described below. For purposes of the adjustments described below to the number of shares the Company will issue, the per share value of the Company common stock to be issued will equal the average of the last reported sales prices of our common stock at 4:00 p.m., Eastern Time, end of regular trading hours on The NASDAQ Stock Market LLC during the ten consecutive trading days ending on the last trading day prior to the closing of the Merger, or the Merger Closing Price. The number of shares comprising the merger consideration will be reduced by a number of shares with a value equal to the following:
| · | Baxano indebtedness in excess of (a) amounts under the promissory notes of Baxano that are convertible into capital stock of Baxano, including all convertible promissory notes issued pursuant to the Note and Warrant Purchase Agreement dated as of March 7, 2012, among Baxano and the investors set forth therein, outstanding at the Effective Time, or the Baxano Notes, and (b) up to $3,000,000 of principal plus accrued interest outstanding under the Loan Documents, as such term is defined in the Loan and Security Agreement dated as of March 15, 2012, among Oxford Finance LLC, Silicon Valley Bank, and Baxano, Inc., or the Oxford/SVB Loan Documents, and such loan, or the Oxford/SVB Loan; |
| · | $250,000 in cash, which the Company has agreed to deposit into an account specified for the purpose of funding the expenses of the representatives, agents, proxies, and attorneys in fact of and for each holder of Baxano Notes and each holder of capital stock of Baxano or warrants exercisable for shares of capital stock of Baxano being converted to common stock of the Company in accordance with the Merger Agreement and each other holder of Baxano warrants, options, or other securities convertible into capital stock of Baxano, or collectively the Securityholders, for purposes of all post-closing matters under the Merger Agreement, or the Securityholder Representatives, which will eventually be distributed to stockholders to the extent such funds are not paid to the Securityholder Representatives; and |
| 46 |
| · | $300,000 in cash, which shall be used to fund a compensation plan, or the Transaction Payment Plan, to be adopted by Baxano prior to closing providing for bonuses to Baxano employees and non-employee directors in the aggregate amount of up to $300,000. |
Escrow Shares and Post-Closing Indebtedness / Working Capital Adjustment
In addition, at the closing of the Merger, we will deliver out of the merger consideration to Branch Banking and Trust Company, as escrow agent, a number of shares equal to 7.5% of the total merger consideration. Such shares will be held in escrow for the benefit of the Company to secure certain indemnification obligations of the Securityholders under the Merger Agreement and to fund any post-closing adjustment payable to the Company based on excess indebtedness or unpaid transaction expenses of Baxano, or to the extent Baxano’s actual closing working capital, as of March 31, 2013, is less than target closing working capital of $2,121,000 (subject to certain limitations). Once the amount of any post-closing adjustment is determined and paid, all remaining escrowed shares in excess of 5% of the merger consideration will be distributed to Securityholders. The remainder of the escrowed shares will be distributed to Securityholders promptly following the first anniversary of the closing date to the extent such funds are not then subject to indemnity claims by the Company or any of its affiliates.
Treatment of Preferred Stock and Common Stock
At the Effective Time, each outstanding share of capital stock of Baxano will be cancelled and extinguished and converted into the right to receive a portion of the merger consideration in accordance with the Merger Agreement, except for dissenting shares, which will retain the appraisal rights and dissenters’ rights provided for under Delaware law and California law, respectively. It is likely that certain of the shares will not be entitled to any merger consideration because of the priorities of the holders of Baxano Notes and senior Baxano Preferred Stock.
Treatment of Baxano Notes, Baxano Stock Option Plans, Baxano Stock Options, and Baxano Warrants
Baxano Notes
Prior to the Effective Time, Baxano must take all actions necessary to cause each Baxano Note to be terminated at the Effective Time and the holder of such note shall be entitled to receive merger consideration in accordance with the Merger Agreement.
Baxano Stock Option Plans, Baxano Stock Options, and Baxano Warrants
Prior to the Effective Time, any and all stock option plans or other stock or equity-related plans of Baxano, together with any employee stock purchase plans shall be terminated. Prior to the Effective Time, Baxano shall use commercially reasonable efforts to cause each outstanding option to purchase common stock of Baxano, whether vested or unvested, and each warrant to acquire capital stock of Baxano to be terminated.
Requirements to Receive Merger Consideration
A letter of transmittal will be provided to each Securityholder as soon as reasonably practical after the Effective Time. Such Securityholder will not be entitled under the Merger Agreement to receive his or her allocated portion of the merger consideration until and unless such former holder completes and returns a completed letter of transmittal to the designated exchange agent in accordance with the instructions set forth in the letter of transmittal. Securityholders must also return the note(s) or stock certificate(s) representing their securities of Baxano. Instructions will be contained in the letter of transmittal for any former holder of a Baxano Note or capital stock of Baxano that is unable to locate the appropriate promissory note(s) or stock certificate(s) or has lost or destroyed such promissory note(s) or certificate(s).
Fractional Shares
The Company will not issue fractional shares of its common stock in exchange for Baxano Notes or shares of Baxano capital stock at the closing of the Merger. In lieu of fractional shares, at closing, each Securityholder will be entitled to receive an amount equal to such fractional share multiplied by the Merger Closing Price for any remaining fractional share.
| 47 |
Allocation of Merger Consideration
The merger consideration will be allocated first to holders of Baxano Notes and then to Baxano stockholders in accordance with Baxano’s certificate of incorporation, with shares of Series C Preferred Stock participating in the merger proceeds before shares of Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series B Preferred Stock, and Common Stock. It is anticipated that no merger consideration will be allocated to the Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series B Preferred Stock, and Common Stock as a result of the Merger Price and the priorities of the Baxano Notes and Preferred Stock. An allocation statement, or the Closing Allocation Statement, will be delivered by Baxano immediately prior to the Effective Time that specifies the amount of consideration to be received by each Securityholder at the Effective Time, as well as the percentage contribution to be paid by each such Securityholder into the escrow fund.
Representations and Warranties
The Merger Agreement contains customary representations and warranties of each of Baxano, the Company, and Merger Sub. These representations are subject, in some cases, to important limitations and qualifications contained in the Merger Agreement or in the disclosure schedules delivered by each party in connection with the Merger Agreement. Some of the representations and warranties are qualified as to “knowledge,” “materiality,” or “Material Adverse Effect.” In addition, certain representations and warranties were qualified in a separate disclosure schedule, made as of a specified date, may be subject to contractual standards of materiality different from those generally applicable to public disclosures to stockholders, or may have been used for the purpose of allocating risk among the parties rather than establishing matters of fact. For the foregoing reasons, you should not read the representations and warranties given by the parties in the Merger Agreement or any description thereof as characterizations of the actual state of facts or condition of Baxano, the Company, or Merger Sub at any time other than the specified date as to which they were made.
Each of Baxano, the Company, and Merger Sub will make various representations and warranties to the other in the Merger Agreement, including representations and warranties related to, among other things:
| · | organization, standing, and power; |
| · | capitalization; |
| · | subsidiaries; |
| · | authority, absence of violations and conflicts, required filings, and consents; |
| · | financial statements; |
| · | absence of undisclosed liabilities; |
| · | absence of certain changes or events; |
| · | taxes; |
| · | real property; |
| · | intellectual property; |
| · | contracts; |
| · | litigation; |
| · | environmental matters; |
| · | employee benefit plans; |
| 48 |
| · | compliance with laws; |
| · | permits and regulatory matters; |
| · | employees; |
| · | insurance; |
| · | brokers – fees and expenses; |
| · | commercial relationships; and |
| · | tangible assets. |
Additional Representations and Warranties of Baxano
Additionally, Baxano will make substantial additional representations and warranties to the Company and Merger Sub in the Merger Agreement, including representations and warranties related to, among other things:
| · | the Closing Allocation Statement; |
| · | absence of existing discussions with any other party with respect to an acquisition proposal; |
| · | certain business relationships with affiliates; |
| · | financial controls and procedures; |
| · | books and records; and |
| · | inventory. |
Many of Baxano’s representations and warranties are qualified by a knowledge standard, a materiality standard, or a “Material Adverse Effect” standard. For the purposes of the Merger Agreement, “Material Adverse Effect” with respect to Baxano means any material adverse change, event, circumstance, or development with respect to, or material adverse effect on, (i) the business, assets, and liabilities (taken together), condition (financial or otherwise), or results of operations of Baxano, or (ii) the ability of Baxano to consummate the transactions contemplated by the Merger Agreement.
However, a Material Adverse Effect with respect to Baxano will not include any change or event caused by or resulting from:
| · | changes in prevailing economic or market conditions in the United States or any other jurisdiction in which Baxano has substantial business operations (except to the extent those changes have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates); |
| · | changes or events, after the date of the Merger Agreement, affecting the industries or markets in which Baxano operates generally (except to the extent those changes or events have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates); |
| · | changes, after the date of the Merger Agreement, in generally accepted accounting principles or requirements applicable to Baxano (except to the extent those changes have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates); |
| · | changes, after the date of the Merger Agreement, in laws, rules, or regulations of general applicability or interpretations thereof by any court, arbitrational tribunal, administrative agency, or commission or other governmental or regulatory authority, agency, or instrumentality, referred to as a Governmental Entity (except to the extent those changes have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates); |
| 49 |
| · | the execution, delivery, and performance of the Merger Agreement or the consummation of the transactions contemplated by the Merger Agreement or the announcement thereof; |
| · | any outbreak of major hostilities in which the United States is involved or any act of terrorism within the United States or directed against its facilities or citizens wherever located; or |
| · | the actions of the Company or Merger Sub (other than any action taken under the Merger Agreement by the Company or Merger Sub in response to an event, circumstance or other development that would otherwise constitute a Material Adverse Effect). |
Additional Representations and Warranties of the Company and Merger Sub
Merger Sub and the Company will make substantial additional representations and warranties to Baxano in the Merger Agreement, including representations and warranties related to, among other things:
| · | SEC filings and the absence of a transaction with any off-balance sheet entity; |
| · | operations of Merger Sub; |
| · | controls and procedures, certifications, and other matters relating to the Sarbanes-Oxley Act of 2002; and |
| · | an ongoing investigation by federal authorities of activities of the Company alleged to violate the False Claims Act. |
Many of the Company’s and Merger Sub’s representations and warranties are qualified by a knowledge standard, a materiality standard, or a “Material Adverse Effect” standard. For the purposes of the Merger Agreement, “Material Adverse Effect” with respect to the Company and Merger Sub means any material adverse change, event, circumstance, or development with respect to, or material adverse effect on, (i) the business, assets, and liabilities (taken together), condition (financial or otherwise), or results of operations of the Company and its subsidiaries, taken as a whole, or (ii) the ability of the Company and its subsidiaries to consummate the transactions contemplated by the Merger Agreement. However, a Material Adverse Effect will not include any change or event caused by or resulting from changes included in the bullet points above under the definition of Material Adverse Effect with respect to Baxano (substituting the Company and its Subsidiaries for Baxano as applicable), as well as the False Claims Act matter described above or a change in the public trading price of the Company’s common stock, by itself.
Covenants
Operation of Baxano’s Business Pending the Merger
During the period between the date of the Merger Agreement and the Effective Time, Baxano agreed that it will:
| · | act and carry on its business in the usual, regular, and ordinary course in substantially the same manner as previously conducted, pay its debts and taxes, and perform its other obligations when due (subject to good faith disputes over such debts, taxes, or obligations); |
| · | comply with applicable laws, rules, and regulations; and |
| · | use commercially reasonable efforts, consistent with past practices, to maintain and preserve in the ordinary course its business organization, assets, and properties. |
During the period between the date of the Merger Agreement and the Effective Time, Baxano agreed that it will not, without our prior written consent:
| 50 |
| · | (i) declare, set aside, or pay any dividends on, or make any other distributions (whether in cash, securities, or other property) in respect of, any of its capital stock; (ii) split, combine, or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of, in lieu of, or in substitution for shares of its capital stock or any of its other securities; or (iii) purchase, redeem, or otherwise acquire any shares of its capital stock or any other of its securities or any rights, warrants, or options to acquire any such shares or other securities, other than, in the case of clause (iii), from former employees, directors, and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of services to Baxano for a repurchase price of the lesser of cost and the then current fair market value; |
| · | except as permitted in the Merger Agreement, issue, deliver, sell, grant, pledge, or otherwise dispose of or encumber any shares of its capital stock, any other voting securities, or any securities convertible into or exchangeable for, or any rights, warrants, or options to acquire, any such shares, voting securities, or convertible or exchangeable securities (other than the issuance of (i) additional Baxano Notes for cash in an amount equal to the face amount of such Baxano Notes, and (ii) shares of Baxano capital stock upon the exercise of Baxano stock options or Baxano warrants outstanding on the date of the Merger Agreement in accordance with their present terms (including cashless exercises); |
| · | amend its certificate of incorporation, bylaws, or other comparable charter or organizational documents, except as expressly provided by the Merger Agreement; |
| · | except for purchases of inventory in the ordinary course of business, acquire (i) any business or any corporation, partnership, joint venture, limited liability company, association, or other business organization or division thereof or (ii) any assets that are material, in the aggregate, to Baxano; |
| · | except in the ordinary course of business, sell, lease, license, pledge, or otherwise dispose of or encumber any properties or assets of Baxano; |
| · | whether or not in the ordinary course of business, sell, dispose of, or otherwise transfer any assets material to Baxano (excluding the sale or license of products and inventory in the ordinary course of business); |
| · | adopt or implement any stockholder rights plan; |
| · | enter into an agreement with respect to any merger, consolidation, liquidation, or business combination, or any acquisition or disposition of all or substantially all of the assets or securities of Baxano; |
| · | (i) incur or suffer to exist any indebtedness (other than with respect to (A) the Oxford/SVB Loan Documents or (B) the Note and Warrant Purchase Agreement dated as of March 7, 2012 among Baxano and the investors set forth therein), (ii) issue, sell, or amend any debt securities or warrants or other rights to acquire any debt securities of Baxano (other than as described in the Merger Agreement), guarantee any debt securities of another person, enter into any “keep well” or other agreement to maintain any financial statement condition of another person or enter into any arrangement having the economic effect of any of the foregoing, (iii) make any loans, advances (other than routine advances to employees of Baxano in the ordinary course of business), or capital contributions to, or investment in, any other person, or (iv) enter into any hedging agreement or other financial agreement or arrangement designed to protect Baxano against fluctuations in commodities prices or exchange rates; |
| · | unless provided for in materials provided to the Company prior to the date of the Merger Agreement, make any capital expenditures or other expenditures with respect to property, plant, or equipment; |
| · | make any changes in accounting methods, principles, or practices, except insofar as may have been required by the SEC or a change in GAAP or, except as so required, change any assumption underlying, or method of calculating, any bad debt, contingency, or other reserve; |
| · | modify, amend, or terminate any material contract or agreement to which Baxano is party, or knowingly waive, release, or assign any material rights or claims (including any write-off or other compromise of any accounts receivable of Baxano), except in the ordinary course of business or, to the extent subject to reserves reflected on Baxano’s balance sheet, in accordance with GAAP; |
| 51 |
| · | (i) except in the ordinary course of business, enter into any material contract or agreement relating to the rendering of services or the distribution, sale, or marketing by third parties of the products of, or products licensed by, Baxano or (ii) license any material intellectual property rights to or from any third party; |
| · | except for the Transaction Payment Plan or as required to comply with applicable law or agreements, plans, or arrangements existing on the date of the Merger Agreement, (i) take any action with respect to, adopt, enter into, terminate, or amend any employment, severance, or similar agreement or benefit plan for the benefit or welfare of any current or former director, officer, employee, or consultant or any collective bargaining agreement, (ii) increase in any material respect the compensation or fringe benefits of, or pay any bonus to, any director, officer, employee, or consultant (except for annual increases of the salaries of non-officer employees in the ordinary course of business), (iii) amend or accelerate the payment, right to payment, or vesting of any compensation or benefits, including any outstanding options or restricted stock awards, (iv) pay any material benefit not provided for as of the date of the Merger Agreement under any benefit plan, (v) grant any awards under any bonus, incentive, performance, or other compensation plan or arrangement or benefit plan (including the grant of stock options, stock appreciation rights, stock based or stock related awards, performance units, or restricted stock, or the removal of existing restrictions in any benefit plans or agreements or awards made thereunder) or (vi) take any action other than in the ordinary course of business to fund or in any other way secure the payment of compensation or benefits under any employee plan, agreement, contract, or arrangement or benefit plan; |
| · | make or rescind any material tax election, settle or compromise any material tax liability, amend any tax return except as required by applicable law, change any accounting method, enter into any closing agreement regarding taxes, or consent to an extension or waiver of the limitation period applicable to any tax claim; |
| · | terminate the employment of any officer or key employee; |
| · | initiate, compromise, or settle any material litigation or arbitration proceeding; |
| · | open or close any facility or office; |
| · | fail to use commercially reasonable efforts to maintain insurance at levels substantially comparable to levels existing as of the date of the Merger Agreement; |
| · | fail to pay accounts payable and other obligations in the ordinary course of business; |
| · | fail to maintain inventory levels in the sales channel to ensure product availability to meet expected patient demand consistent with past practices; |
| · | fail to take any and all actions necessary to maintain and preserve all Baxano rights in and to intellectual property, including the filing of necessary documents with the appropriate Governmental Entities, or |
| · | authorize any of, or commit or agree, in writing or otherwise, to take any of, the foregoing actions or any action that would make any representation or warranty of Baxano in the Merger Agreement untrue or incorrect in any material respect, or would materially impair or prevent the satisfaction of any conditions in the Merger Agreement. |
Operation of the Company’s Business Pending the Merger
During the period between the date of the Merger Agreement and the Effective Time, the Company agreed that it will:
| · | act and carry on its business in the usual, regular, and ordinary course in substantially the same manner as previously conducted, pay its debts and taxes, and perform its other obligations when due (subject to good faith disputes over such debts, taxes, or obligations); |
| 52 |
| · | comply with applicable laws, rules, and regulations; and |
| · | use commercially reasonable efforts, consistent with past practices, to maintain and preserve in the ordinary course its and each of its subsidiaries’ business organization, assets, and properties. |
During the period between the date of the Merger Agreement and the Effective Time, the Company agreed that it will not, without Baxano’s prior written consent:
| · | (i) declare, set aside, or pay any dividends on, or make any other distributions (whether in cash, securities, or other property) in respect of, any of its capital stock (other than dividends and distributions by a direct or indirect wholly owned subsidiary of the Company to its parent); (ii) split, combine, or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of, in lieu of, or in substitution for shares of its capital stock or any of its other securities; or (iii) purchase, redeem, or otherwise acquire any shares of its capital stock or any other of its securities or any rights, warrants, or options to acquire any such shares or other securities, other than, in the case of clause (iii), from former employees, directors, and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of services to the Company or any of its subsidiaries for a repurchase price of the lesser of cost and the then current fair market value; |
| · | except as permitted by the Merger Agreement and the other agreements contemplated therein, issue, deliver, sell, grant, pledge, or otherwise dispose of or encumber any shares of its capital stock, any other voting securities, or any securities convertible into or exchangeable for, or any rights, warrants, or options to acquire, any such shares, voting securities, or convertible or exchangeable securities (other than the issuance of shares of common stock of the Company upon the exercise of the Company stock options outstanding on the date of the Merger Agreement in accordance with their present terms (including cashless exercises) or the Company stock options granted as contemplated by the Merger Agreement); |
| · | amend its certificate of incorporation, bylaws, or other comparable charter or organizational documents, except to the extent necessary to carry into effect the provisions of the Merger Agreement; |
| · | except for purchases of inventory in the ordinary course of business, acquire (i) any business or any corporation, partnership, joint venture, limited liability company, association, or other business organization or division thereof or (ii) any assets that are material, in the aggregate, to the Company and its subsidiaries, taken as a whole; |
| · | except in the ordinary course of business, sell, lease, license, pledge, or otherwise dispose of or encumber any properties or assets of the Company or of any of its subsidiaries; |
| · | whether or not in the ordinary course of business, sell, dispose of, or otherwise transfer any assets material to the Company and its subsidiaries, taken as a whole (excluding the sale or license of products and inventory in the ordinary course of business); |
| · | adopt or implement any stockholder rights plan; |
| · | enter into an agreement with respect to any merger, consolidation, liquidation, or business combination, or any acquisition or disposition of all or substantially all of the assets or securities of the Company or any of its subsidiaries; |
| · | (i) incur or suffer to exist any indebtedness for borrowed money or guarantee any such indebtedness of another person, (ii) issue, sell or amend any debt securities or warrants or other rights to acquire any debt securities of the Company or any of its subsidiaries, guarantee any debt securities of another person, enter into any “keep well” or other agreement to maintain any financial statement condition of another person or enter into any arrangement having the economic effect of any of the foregoing, (iii) make any loans, advances (other than routine advances to employees of the Company in the ordinary course of business), or capital contributions to, or investment in, any other person, other than the Company or any of its direct or indirect wholly owned subsidiaries or (iv) enter into any hedging agreement or other financial agreement or arrangement designed to protect the Company or its subsidiaries against fluctuations in commodities prices or exchange rates; |
| 53 |
| · | make any changes in accounting methods, principles, or practices, except insofar as may have been required by the SEC or a change in GAAP or, except as so required, change any assumption underlying, or method of calculating, any bad debt, contingency, or other reserve; |
| · | modify, amend, or terminate any material contract or agreement to which the Company or any of its subsidiaries is party, or knowingly waive, release, or assign any material rights or claims (including any write-off or other compromise of any accounts receivable of the Company or any of its subsidiaries), except in the ordinary course of business or, to the extent subject to reserves reflected on its balance sheet, in accordance with GAAP; |
| · | (i) except in the ordinary course of business, enter into any material contract or agreement relating to the rendering of services or the distribution, sale, or marketing by third parties of the products of, or products licensed by, the Company or any of its subsidiaries or (ii) license any material intellectual property rights to or from any third party; |
| · | except as required to comply with applicable law or agreements, plans, or arrangements existing on the date of the Merger Agreement, (i) amend or accelerate the payment, right to payment, or vesting of any compensation or benefits, including any outstanding the Company stock options or restricted stock awards or (ii) pay any material benefit not provided for as of the date of the Merger Agreement under any benefit plan; |
| · | make or rescind any material tax election, settle or compromise any material tax liability, amend any tax return except as required by applicable law, change any accounting method, enter into any closing agreement regarding taxes, or consent to an extension or waiver of the limitation period applicable to any tax claim; |
| · | commence any offering of shares of common stock of the Company pursuant to any employee stock purchase plan, permit any employee to enroll in any employee stock purchase plan, or allow any participant in an employee stock purchase plan to increase the current level of such participant’s payroll deductions thereunder; |
| · | fail to use commercially reasonable efforts to maintain insurance at levels substantially comparable to levels existing as of the date of the Merger Agreement; |
| · | fail to pay accounts payable and other obligations in the ordinary course of business; |
| · | fail to maintain inventory levels in the sales channel to ensure product availability to meet expected patient demand consistent with past practices; |
| · | terminate the employment of the current Chief Executive Officer or Chief Financial Officer of the Company; |
| · | fail to take any and all actions necessary to maintain and preserve all the Company rights in and to its intellectual property, including the filing of necessary documents with the appropriate Governmental Entities; or |
| · | authorize any of, or commit or agree, in writing or otherwise, to take any of, the foregoing actions or any action that would make any representation or warranty of the Company in the Merger Agreement untrue or incorrect in any material respect, or would materially impair or prevent the satisfaction of any conditions in the Merger Agreement. |
Additional Agreements / Covenants
No Solicitation
From the date of the Merger Agreement, each of the Company and Baxano agreed to not take or permit any other person on its behalf to take any action to encourage, initiate, or engage in discussions or negotiations with, provide any information to, or enter into any agreement or understanding with, any person (other than the parties to the Merger Agreement) concerning any Acquisition Proposal (as defined below). Each of the Company and Baxano agreed to immediately cease any and all existing activities, discussions, or negotiations related to an Acquisition Proposal conducted prior to the date of the Merger Agreement (other than with parties to the Merger Agreement) and to notify the Company or Baxano, as appropriate, in the event that the Company or Baxano receives any requests for information or proposals relating to any Acquisition Proposal during the period between the date of the Merger Agreement and the Effective Time.
| 54 |
An “Acquisition Proposal” means any inquiry, proposal, or offer from any person (other than the parties to the Merger Agreement) relating to (i) a merger, consolidation, liquidation, recapitalization, share exchange, or other business combination transaction involving the Company or Baxano, respectively, (ii) the issuance or acquisition of shares of capital stock or other equity securities of the Company or Baxano, respectively, other than as permitted by the Merger Agreement, or (iii) the sale, lease, license, exchange, or other disposition of any material portion of the Company’s or Baxano’s properties or assets, respectively, other than in the ordinary course.
Securities Matters
The shares of common stock of the Company issued in accordance with the Merger Agreement will be issued pursuant to an exemption from registration under federal securities regulations. Such shares will be subject to certain transfer restrictions, including applicable holding period requirements. The Company is obligated to register such shares on the terms set forth in and in accordance with the Securities Purchase Agreement. See “Proposal No. 2 – Issuance of Common Stock Pursuant to the Securities Purchase Agreement— Lock-up and Registration of Shares.”
NASDAQ Filings
The Company agreed to make filings required by The NASDAQ Stock Market LLC relating to listing the shares for trading on such exchange.
Access to Information
During the period prior to the Effective Time, the Company and Baxano have agreed to afford each other reasonable access during business hours to the following:
| · | a copy of each report, schedule, registration statement, and other document filed or received by it during such period pursuant to the requirements of federal or state securities laws; and |
| · | all other information concerning its business, properties, assets, and personnel as the other party may reasonably request. |
Stockholder Approval
The Merger and the adoption of the Merger Agreement were approved on March 3, 2013 following the parties’ entry into the Merger Agreement pursuant to a written consent in lieu of a Baxano stockholders’ meeting executed and delivered by the holders of (i) a majority of the votes represented by the outstanding shares of common stock of Baxano, (ii) a majority of the votes represented by the outstanding shares of common stock of Baxano and preferred stock of Baxano voting together, with the holders of the outstanding shares of preferred stock of Baxano entitled to the number of votes equal to the number of shares of common stock of Baxano into which such shares of preferred stock of Baxano could be converted as of the record date for such vote; and (iii) at least 662/3% of the votes represented by the outstanding shares of preferred stock of Baxano.
The Merger and the adoption of the Merger Agreement were approved on March 1, 2013 by the Board of Directors of Merger Sub and on March 3, 2013 by the Company acting as sole stockholder of Merger Sub.
We are obligated under the Merger Agreement to take all actions necessary under applicable law to hold and convene a meeting of our stockholders for the purpose of voting on the issuance of shares of our common stock in connection with the Merger and the Private Placement Transaction and we are required to promptly prepare and file with the SEC a proxy statement relating to such stockholder approval.
| 55 |
Legal Conditions to Merger
Baxano and the Company have agreed to use commercially reasonable efforts to:
| · | take, or cause to be taken, all actions, and do, or cause to be done, and to assist and cooperate with the other parties in doing, all things necessary, proper, or advisable to consummate and make effective the transactions contemplated by the Merger Agreement as promptly as practicable; |
| · | as promptly as practicable, obtain from any Governmental Entity or any other third party any consents, licenses, permits, waivers, approvals, authorizations, or orders required to be obtained or made by Baxano or the Company or any of their subsidiaries in connection with the authorization, execution, and delivery of the Merger Agreement and the consummation of the transactions contemplated by the Merger Agreement; |
| · | as promptly as practicable, make all necessary filings, and thereafter make any other required submissions, with respect to the Merger Agreement and the Merger required under applicable federal or state securities laws and any other applicable law; and |
| · | execute or deliver any additional instruments necessary to consummate the transactions contemplated by, and to fully carry out the purposes of, the Merger Agreement. |
Baxano and the Company have also agreed to use commercially reasonable efforts to obtain any third party consents related to or required in connection with the Merger that are (i) necessary to consummate the transactions contemplated by the Merger Agreement, (ii) disclosed or required to be disclosed in the disclosure schedules, or (iii) required to prevent an event that may have a Material Adverse Effect on Baxano or the Company whether prior to or after the Effective Time.
Public Disclosure
The Company and Baxano have agreed that neither it nor Baxano shall issue any press release or otherwise make any public statement with respect to the Merger or the Merger Agreement without the prior consent of the other, which shall not be unreasonably withheld, conditioned, or delayed.
Section 368(a) Reorganization
Each of the Company, Baxano, and Merger Sub have agreed to use all commercially reasonable efforts to qualify the Merger as a tax-free reorganization under the Internal Revenue Code.
Notification of Certain Matters
During the period between the date of the Merger Agreement and the Effective Time, the Company and Baxano have agreed to give prompt notice to the other party of the occurrence, or failure to occur, of any event, which occurrence or failure to occur would be reasonably expected to cause the non-satisfaction of a closing condition.
Board of Directors of the Company
We agreed to, promptly after the Effective Time, take all actions necessary to fix the number of members of our Board of Directors at nine persons and to appoint two directors designated by Baxano to serve as Class III members of our Board of Directors. In addition, we agreed to take all actions necessary to re-elect such directors designated by the Securityholder Representatives to our Board of Directors at our 2013 annual meeting of stockholders such that they would serve as directors at least through our 2016 annual meeting of stockholders. The Merger Agreement also provides that the number of members of our Board of Directors will be reduced to eight persons at the time of our 2013 annual meeting of stockholders. To implement these provisions, one of our existing directors will be required to resign from our Board of Directors at the time of our 2013 annual meeting of stockholders.
| 56 |
Employee Communications
Baxano has agreed to use commercially reasonable efforts to consult with the Company, and will consider in good faith the Company’s advice, prior to sending any notices or other communication materials to its employees regarding the Merger Agreement, the Merger, or the effects thereof on the employment, compensation, or benefits of its employees.
Additional Indebtedness
Baxano agreed to, prior to March 31, 2013, issue one or more Baxano Notes to one or more of the Baxano noteholders as of the date of the Merger Agreement in an aggregate principal amount of not less than $500,000 in exchange for cash in like amount, and closed such required financing on March 28, 2013 by issuing Baxano Notes to thirteen Baxano noteholders as of the date of the Merger Agreement in an aggregate principal amount of $500,000. The Baxano Notes issued in such financing will be terminated as of the Effective Time and the holders thereof will be entitled to receive merger consideration in full satisfaction of the $500,000 principal amount and accrued interest through the Closing Date under such Baxano Notes. The Merger Agreement does not provide for any adjustment to the overall merger consideration to be issued to Baxano securityholders based upon the issuance of such Baxano Notes. Instead, theadditional merger consideration to be received by holders of such Baxano Notes will result in an equal reduction to the aggregate merger consideration to be received by the holders of shares of Series C Preferred Stock.
Indemnification and Insurance
The Merger Agreement provides that the Company shall cause the Surviving Corporation to indemnify and hold harmless each present and former director and officer of Baxano for a period of six years following the Effective Time. The Surviving Corporation will be obligated to indemnify and hold harmless each such director or officer, to the extent such director or officer is entitled to indemnification by Baxano as of the date of the Merger Agreement pursuant to Baxano’s certificate of incorporation, bylaws, or an indemnification agreement, against and from any costs or expenses (including reasonable attorneys’ fees), judgments, fines, losses, claims, demands, damages, liabilities, and amounts paid in settlement in connection with any claim, action, suit, proceeding, or investigation, whether civil, criminal, administrative, or investigative, to the extent such claim, action, suit, proceeding, or investigation arises out of or pertains to any action or omission or alleged action or omission by such officer or director in his or her capacity as a director, officer, or employee of Baxano (regardless of whether such action or omission, or alleged action or omission, occurred prior to, on, or after the date of the Merger Agreement).
In addition, we agreed, for a period of six years after the Effective Time, to cause the certificate of incorporation and the bylaws of the Surviving Corporation to contain provisions no less favorable with respect to indemnification, advancement of expenses, and exculpation of current or former directors or officers of Baxano than are set forth in Baxano’s current certificate of incorporation and bylaws.
The Merger Agreement also provides that, prior to the Effective Time, Baxano shall obtain and the Company shall pay for “tail” policies of directors’ and officers’ liability insurance with a claims period of not greater than six years from and after the Effective Time with benefits and levels of coverage at least as favorable as the existing policies of Baxano with respect to matters existing or occurring at or prior to the Effective Time (including in connection with the Merger Agreement or the transactions or actions contemplated thereby).
Non-Solicitation
The Company and Baxano have agreed, with limited exceptions, that until the earlier of (i) the first day following the 12-month period from the date of the Merger Agreement if the merger does not close or (ii) the closing of the Merger, except with the prior written consent of the other party, neither party (nor any affiliate of such party) will hire, employ, or otherwise engage or retain in any manner or capacity any person who served as an employee of the other party or any affiliate of such other party within the six month period immediately preceding such proposed hiring, employment, or other engagement or retainer.
Employee Benefits
Following the Effective Time, and to the extent permitted by our employee benefit plans, we will cause any such employee benefit plans that the employees of Baxano as of the Effective Time who continue to remain employed with Baxano are eligible to participate in after the Effective Time to take into account for purposes of eligibility, vesting, and benefit accrual thereunder, service by such employees with Baxano prior to the Effective Time as if such service were with the Company, to the same extent such service was credited under a corresponding benefit plan of Baxano as of the Effective Time. Also, if requested by the Company within 10 days of the signing of the Merger Agreement, Baxano shall use commercially reasonable efforts to terminate any or all Baxano employee benefit plans effective immediately prior to the Effective Time.
| 57 |
Company Transaction Expenses
To the extent unpaid at closing and set forth on the pre-closing statement or conclusive statement (each of which is used to determine the final net working capital calculation in accordance with the Merger Agreement) as transaction expenses, the Company shall pay all fees, costs, and expenses incurred by Baxano in connection with the matters described in this Agreement, including with respect to any brokers, financial advisors, consultants, accountants, attorneys, or other professionals engaged by Baxano in connection with the parties’ due diligence related to, or the structuring, negotiation, or consummation of the transactions contemplated by the Merger Agreement or the Securities Purchase Agreement as and when they become due without delay, in the amounts set forth on such pre-closing statement or conclusive statement.
Post-Closing Operations
If the closing occurs prior to March 31, 2013, the Company shall cause the Surviving Corporation to act and carry on its business in the usual, regular and ordinary course in substantially the same manner as previously conducted and to maintain and preserve in the ordinary course its business organization, assets and properties, each until March 31, 2013. Without limiting the generality of the foregoing, from and after the closing until March 31, 2013, the Company shall cause the Surviving Corporation not to, directly or indirectly, do any of the following without the prior written consent of the Securityholder Representatives:
| · | (i) declare, set aside, or pay any dividends on, or make any other distributions (whether in cash, securities or other property) in respect of, any ofits capital stock; or (ii) purchase, redeem, or otherwise acquire any shares of its capital stock or any other of its securities or any rights, warrants, or options to acquire any such shares or other securities; |
| · | except in the ordinary course of business, sell, lease, license, pledge, or otherwise dispose of or encumber any properties or assets of the Surviving Corporation; |
| · | whether or not in the ordinary course of business, sell, dispose of, or otherwise transfer any assets material to the Surviving Corporation (including any accounts, leases, contracts, or intellectual property, but excluding the sale or license of products and inventory in the ordinary course of business); |
| · | (i) incur or suffer to exist any Indebtedness (other than with respect to the Oxford/SVB Loan Documents), (ii) issue, sell, or amend any debt securities or warrants or other rights to acquire any debt securities of theSurviving Corporation, (iii) make any loans, advances, or capitalcontributions to, or investment in, any otherperson, other than theSurviving Corporationor any of its direct or indirect wholly owned subsidiariesor (iv) enter into any hedgingagreementor other financialagreementor arrangement designed to protect the Surviving Corporation or itssubsidiariesagainst fluctuations in commodities prices or exchange rates; |
| · | make any capital expenditures or other expenditures with respect to property, plant, or equipment; |
| · | make any changes in accounting methods, principles, or practices, except insofar as may have been required by the SEC or a change in GAAP or, except as so required, change any assumption underlying, or method of calculating, any bad debt, contingency, or other reserve. |
Consents of Distributors
Prior to closing, Baxano, in coordination with the Company, has agreed to contact each of the top 15 sales representatives (as measured by sales during 2012) and shall request that each such sales representative consent in writing to the assignment of its independent sales representative agreement with Baxano from Baxano to the Company.
| 58 |
Conditions to the Merger
The Company’s, Merger Sub’s and Baxano’s obligations to effect the Merger are also subject to the satisfaction or waiver by each party at or prior to the Effective Time of the following conditions:
| · | Baxano shall have obtained the required approval of its stockholders and we shall have obtained the required approval of our stockholders; |
| · | other than the filing of the certificate of merger, all authorizations, consents, orders, or approvals of, or declarations or filings with, or expirations of waiting periods imposed by, any Governmental Entity in connection with the Merger and the consummation of the other transactions contemplated by the Merger Agreement, the failure of which to file, obtain, or occur is reasonably expected to have a Material Adverse Effect shall have been filed, been obtained, or occurred on terms and conditions that would not reasonably be expected to have a Material Adverse Effect; and |
| · | no Governmental Entity of competent jurisdiction shall have enacted, issued, promulgated, enforced, or entered any order, executive order, stay, decree, judgment, or injunction (preliminary or permanent) or statute, rule, or regulation which is in effect and which has the effect of making the Merger illegal or otherwise prohibiting consummation of the Merger or the other transactions contemplated by the Merger Agreement. |
Additional Conditions to the Obligations of the Company and Merger Sub
The Company’s and Merger Sub’s obligations to effect the Merger are subject to the satisfaction or waiver by the Company and Merger Sub at or prior to the Effective Time of the following additional conditions:
| · | the representations and warranties of Baxano regarding capitalization and stockholder approval of the Merger shall be true and correct as of the date of the Merger Agreement and as of the closing date, as if made on and as of the closing date (other than such representations and warranties made as of a specific date, which shall have been true and correct as of such specific date), and all other representations and warranties of Baxano set forth in the Merger Agreement, without giving effect to any qualification or limitation as to “materiality” or “Material Adverse Effect,” shall have been true and correct as of the date of the Merger Agreement and shall be true and correct as of the closing date with the same effect as though such representations and warranties were made on and as of the closing date (other than such representations and warranties made as of a specific date, which shall have been true and correct as of such specific date), except where failure of such other representations or warranties to be so true and correct, individually or in the aggregate with all such failures, has not had and would not reasonably be expected to have a Material Adverse Effect; |
| · | Baxano shall have performed in all material respects all obligations required to be performed by it under the Merger Agreement on or prior to the closing date, and we shall have received an officer’s certificate to such effect; |
| · | no Material Adverse Effect on behalf of Baxano shall have occurred since the date of the Merger Agreement and be continuing; |
| · | Baxano shall have obtained certain specified third party consents and approvals and all required consents and approvals of third parties (other than a Governmental Entity) the failure of which to obtain, individually or in the aggregate, would reasonably be expected to have a Material Adverse Effect; |
| · | the dissenting shares shall not include shares of preferred stock of Baxano that, absent a demand for appraisal rights, would be entitled to receive in excess of 5% of the shares issued pursuant to the Merger; |
| · | Baxano shall have delivered (i) copies of the Securities Purchase Agreement, duly executed by individuals and entities purchasing shares in the Private Placement Transaction with an aggregate purchase price of not less than $14,500,000, and (ii) any other closing deliverables required from such individuals and entities pursuant to the Securities Purchase Agreement; |
| 59 |
| · | we shall have received an officers’ certificate duly executed by each of the chief executive officer and chief financial officer of Baxano to the effect that the closing conditions regarding (i) representations and warranties of Baxano in the Merger Agreement, (ii) performance of obligations of Baxano, and (iii) the absence of a Material Adverse Effect have been satisfied; |
| · | certain closing conditions of the Company set forth in the Securities Purchase Agreement shall have been satisfied or waived (other than delivery of items to be delivered at the closing under the Securities Purchase Agreement and other than satisfaction of those conditions that by their nature are to be satisfied at such closing); and |
| · | Baxano shall have delivered (i) a duly executed copy of the Escrow Agreement and (ii) any other closing deliverables required from Baxano pursuant to the Escrow Agreement. |
Additional Conditions to the Obligations of Baxano
Baxano’s obligations to effect the Merger are subject to the satisfaction or waiver by Baxano at or prior to the Effective Time of the following additional conditions:
| · | the representations and warranties of the Company and Merger Sub regarding capitalization and stockholder approval of the Merger shall be true and correct as of the date of the Merger Agreement and as of the closing date, as if made on and as of the closing date (other than such representations and warranties made as of a specific date, which shall have been true and correct as of such specific date), and all other representations and warranties of the Company and Merger Sub set forth in the Merger Agreement, without giving effect to any qualification or limitation as to “materiality” or “Material Adverse Effect,” shall have been true and correct as of the date of the Merger Agreement and shall be true and correct as of the closing date with the same effect as though such representations and warranties were made on and as of the closing date (other than such representations and warranties made as of a specific date, which shall have been true and correct as of such specific date), except where failure of such other representations or warranties to be so true and correct, individually or in the aggregate with all such failures, has not had and would not reasonably be expected to have a Material Adverse Effect; |
| · | the Company and Merger Sub shall have performed in all material respects all obligations required to be performed by them under the Merger Agreement on or prior to the closing date, and Baxano shall have received an officer’s certificate to such effect; |
| · | no Material Adverse Effect on behalf of the Company and Merger Sub shall have occurred since the date of the Merger Agreement and be continuing; |
| · | the Company shall have obtained certain specified third party consents and approvals and all required consents and approvals of third parties (other than a Governmental Entity) the failure of which to obtain, individually or in the aggregate, would reasonably be expected to have a Material Adverse Effect; |
| · | the Company shall have delivered duly executed copies of stockholder agreements from certain stockholders specified in the disclosure schedule to the Merger Agreement; |
| · | the Company shall have delivered (i) a duly executed copy of the Securities Purchase Agreement and (ii) any other closing deliverables required from the Company pursuant to the Securities Purchase Agreement; |
| · | Baxano shall have received an officers’ certificate duly executed by each of the chief executive officer and chief financial officer of the Company to the effect that the closing conditions regarding (i) representations and warranties of the Company and Merger Sub in the Merger Agreement, (ii) performance of obligations of the Company and Merger Sub, and (iii) the absence of a Material Adverse Effect have been satisfied; |
| · | certain closing conditions of the Company set forth in the Securities Purchase Agreement shall have been satisfied or waived (other than delivery of items to be delivered at the closing under the Securities Purchase Agreement and other than satisfaction of those conditions that by their nature are to be satisfied at such closing); and |
| 60 |
| · | the Company shall have delivered (i) a duly executed copy of the Escrow Agreement and (ii) any other closing deliverables required from the Company pursuant to the Escrow Agreement. |
Indemnification
Subject to the limitations discussed below in this section, Securityholders who receive merger consideration, or Escrow Participants, agree to indemnify and hold harmless the Company, its directors, officers, employees, affiliates (including Surviving Corporation), stockholders, agents, attorneys, representatives, successors, and assigns, from and against any and all losses suffered by or asserted against any such indemnified persons arising from or in connection with:
| · | the breach by Baxano of any of its representations or warranties; |
| · | the breach by Baxano of any of its covenants or agreements; |
| · | the failure of any item set forth in the Closing Allocation Statement to be accurate in all respects as of the closing; |
| · | any transaction expenses of Baxano not paid by Baxano prior to closing, except to the extent such expenses are treated as a reduction to the merger consideration or taken into account for purposes of calculating any working capital adjustment; |
| · | the excess, if any, of the aggregate amount ultimately required to be paid to holders of dissenting shares over the aggregate amount such holders would have otherwise received with respect to such dissenting shares pursuant to the terms of the Merger Agreement; and |
| · | claims relating to performance or failure of performance of the Securityholder Representatives of their duties under the Merger Agreement or to actions taken or omitted from being taken by the Company, its affiliates, the escrow agent, agent, or any other person in accordance with or reliance upon any instruction, decision, or action of the Securityholder Representatives. |
Subject to the limitations discussed in this section, we agreed to indemnify and hold harmless the Securityholders and their directors, officers, employees, affiliates, stockholders, agents, attorneys, representatives, successors, and assigns, from and against any and all losses suffered by or asserted against any such indemnified persons arising from or in connection with:
| · | the breach by the Company or Merger Sub of any of their representations or warranties; |
| · | the breach by the Company or Merger Sub of any of their covenants or agreements; and |
| · | the failure of any item set forth in the closing capitalization statement to be delivered by the Company to Baxano immediately prior to the Effective Time to be accurate in all respects as of the closing. |
The Merger Agreement includes the following limitations on indemnification:
| · | no indemnification claims for losses may be asserted unless the aggregate amount of losses exceeds $200,000, and if such losses exceed $200,000, only the excess amount may be recovered; |
| · | no indemnification may be asserted with respect to losses resulting from a single claim or series of related or similar claims which does not exceed $50,000; |
| · | Securityholders who receive merger consideration will not be obligated to indemnify the Company for any loss in excess of (i) their pro rata portion of such loss, and (ii) the value of the shares actually distributed to and received by such person; |
| · | Securityholders who receive merger consideration will not be obligated to indemnify the Company with respect to any loss with respect to which the remedy of the indemnified parties is not limited to the shares paid into escrow, unless such shares have first been exhausted in payment of prior indemnifiable losses; and |
| 61 |
| · | no party shall have liability for losses in the aggregate exceeding the value of the shares in escrow, such escrow shares having a value equal to the average trading price of the such shares in the ten trading days prior to the date of delivery of joint written instructions to the escrow agent to release escrow shares to satisfy a claim for indemnification. |
The above limitations will not apply to losses based upon a breach or inaccuracy of certain specified fundamental representations made by Baxano, fraud, or willful misrepresentation with the intent to deceive in the making of a representation or warranty of Baxano in the Merger Agreement.
The representations and warranties of each party survive for a period of 12 months following the closing date, with the exception of certain specified fundamental representations, which survive for a period of four years. In the event of the assertion or commencement by any person, including any third party, of any claim or legal proceeding with respect to which any person may be entitled to indemnification, prompt written notice shall be given to the Securityholder Representatives or the Company, as applicable.
Each party acknowledges and agrees that the indemnification provisions of the Merger Agreement provide the sole and exclusive right and remedy exercisable by such party with respect to the subject matter of the Merger Agreement (other than claims for injunctive relief, specific performance, or other equitable relief); provided, however, that nothing in the Merger Agreement shall (i) limit any party’s remedies with respect to claims against any individual or legal entity arising from such person’s own fraud or willful misrepresentation with the intent to deceive or (ii) prevent or restrict any person who is a party to any other transaction document, including the Securities Purchase Agreement, from obtaining monetary damages or any other legal or equitable relief in connection with the breach of such agreement.
No party nor any Securityholder of the Company or the Securityholder Representatives, nor any current or former Securityholder, director, officer, employee, affiliate, or advisor of any of the foregoing shall, in any event, be liable to any other person, either in contract, tort, or otherwise, for any special, indirect, exemplary, punitive, or consequential damages other than foreseeable consequential damages, relating to the breach or alleged breach of any provision of the Merger Agreement, whether or not the possibility of such damages has been disclosed to the other party in advance, except in each case to the extent included in a third party claim or arising from a claim based upon fraud, willful misrepresentation with the intent to deceive, or a willful breach of a covenant in the Merger Agreement.
Tax Indemnity
The Merger Agreement provides a separate tax indemnity to indemnify the Company and Merger Sub against any and all losses attributable to, arising from, or related to (i) all taxes (or the nonpayment thereof) of Baxano for all tax periods ending on or before the closing date and the portion through the end of the closing date of any tax period that includes (but does not end on) the closing date, (ii) any breach of, or inaccuracy in, the tax representations, and (iii) any breach of a covenant related to taxes.
The tax representations survive the closing and liability related to indemnification claims arising from tax representations is limited to, in addition to other limitations, the number of shares remaining in escrow.
Escrow
Pursuant to the Merger Agreement, on the closing date, we will deposit a number of shares equal to 7.5% of the total merger consideration into an escrow account pursuant to an Escrow Agreement among the Company, Merger Sub, Baxano, the Securityholder Representatives, and Branch Banking and Trust Company, as escrow agent. The indemnification obligations of Escrow Participants will be satisfied solely by the shares to be held in the escrow account after closing, subject to limited exceptions.
In addition, such shares will be held in escrow to fund any post-closing adjustment payable to the Company based on excess indebtedness or unpaid transaction expenses of Baxano, or to the extent Baxano’s actual closing working capital is less than target closing working capital of $2,121,000 (subject to certain limitations). Once the amount of any post-closing adjustment is determined and paid, all remaining escrowed shares in excess of five percent of the merger consideration will be distributed to Escrow Participants. The remainder of the escrowed shares would be distributed to Securityholders promptly following the first anniversary of the closing date to the extent such funds are not then subject to indemnity claims by the Company or any of its affiliates.
| 62 |
Termination
The Merger Agreement may be terminated by written notice at any time prior to the Effective Time as follows:
| · | by mutual written consent of the Company and Baxano; |
| · | by either the Company or Baxano, subject to limited exceptions, if the Merger shall not have been consummated by May 31, 2013; |
| · | by either the Company or Baxano if a Governmental Entity shall have permanently restrained or otherwise prohibited the Merger; |
| · | by either the Company or Baxano, subject to certain limitations, if the requisite vote of the stockholders of the Company in favor of the Merger shall not have been obtained; |
| · | by Baxano, if: (i) our Board of Directors shall have failed to give its recommendation to the Company’s stockholders to the approval of the issuance of the Company’s common stock pursuant to the Merger Agreement or shall have withdrawn or modified its recommendation of its approval; (ii) the Company shall have breached its obligations with respect to obtaining stockholder approval of the issuance of the Company’s common stock pursuant to the Merger Agreement; or (iii) the Company shall have failed to hold the Special Meeting and submit voting proposals to the Company’s stockholders regarding approval of the issuance of the Company’s common stock pursuant to the Merger Agreement by the date which is three business days prior to May 31, 2013; |
| · | by either the Company or Baxano, if the other party shall have breached its no-shop obligations not to solicit or discuss alternative Acquisition Proposals with third parties; |
| · | by the Company, if there has been a breach of or failure to perform any representation, warranty, covenant, or agreement set forth in the Merger Agreement on the part of Baxano, subject to certain exceptions and limitations, and such failure or breach cannot be cured or, if curable, shall continue unremedied for a period of 30 days after Baxano has received written notice from the Company of the occurrence of such failure or breach; |
| · | by Baxano, if there has been a breach of or failure to perform any representation, warranty, covenant, or agreement set forth in the Merger Agreement on the part of the Company, subject to certain exceptions and limitations, and such failure or breach cannot be cured or, if curable, shall continue unremedied for a period of 30 days after the Company has received written notice from Baxano of the occurrence of such failure or breach; or |
| · | by the Company, if Baxano shall have failed to deliver to the Company by 5:00 p.m., Eastern Time, on the first business day after execution of the Merger Agreement its secretary’s certificate to the effect that its stockholder approval of the Merger Agreement has been obtained; provided, however, that the Company may not terminate the Merger Agreement due to such failure at any time after Baxano shall have delivered such documentation to the Company. |
Fees and Expenses
Each party is responsible for its fees and expenses incurred in connection with the Merger Agreement and the transactions contemplated thereby, whether or not the Merger is consummated, except as follows:
| · | Baxano shall pay a termination fee of $1,000,000, together with all expenses of the Company actually incurred relating to the transactions contemplated by the Merger Agreement prior to termination (including, but not limited to, fees and expenses of the Company’s counsel, accountants, and financial advisors, but excluding any discretionary fees paid to such financial advisors), but not in excess of $750,000, upon the termination of the Merger Agreement by the Company pursuant to a: |
| 63 |
| o | failure of the Merger to close by May 31, 2013 where such failure to close is the result of Baxano’s failure to obtain stockholder approval of the Merger Agreement and deliver a secretary’s certificate to such effect; |
| o | failure of the Merger to close by May 31, 2013 where such failure to close is the result of Baxano’s failure to perform its obligations under the Merger Agreement; |
| o | failure of the Merger to close by May 31, 2013 where such failure to close is the result of Baxano’s failure to deliver the Securities Purchase Agreement; |
| o | breach of Baxano of its no-shop obligations in the Merger Agreement; |
| o | breach of or failure to perform any representation, warranty, covenant, or agreement set forth in the Merger Agreement by Baxano, such that certain closing conditions of the Merger are not satisfied; or |
| o | failure of Baxano to deliver written consents evidencing stockholder approval within one business day of the signing of the Merger Agreement. |
| · | The Company shall pay a termination fee of $2,000,000, together with all expenses of Baxano actually incurred relating to the transactions contemplated by the Merger Agreement prior to termination (including, but not limited to, fees and expenses of Baxano’s counsel, accountants, and financial advisors, but excluding any discretionary fees paid to such financial advisors), but not in excess of $750,000, upon the termination of the Merger Agreement by Baxano (or by either Baxano or the Company with respect to the last bullet point below) pursuant to a: |
| o | failure of the Merger to close by May 31, 2013 where such failure to close is the result of failure of our Board of Directors to recommend the issuance of the Company’s common stock pursuant to the Merger Agreement to its stockholders and convene a meeting of its stockholders to approve such issuance by the date that is three business days prior to April 30, 2013; |
| o | failure of the Merger to close by May 31, 2013 where such failure to close is the result of the Company’s failure to obtain stockholder approval of the issuance of the Company’s common stock pursuant to the Merger Agreement; |
| o | failure of the Merger to close by May 31, 2013 where such failure to close is the result of the Company’s failure to perform its obligations under the Merger Agreement; |
| o | failure by the Company to deliver the Stockholder Agreements; |
| o | breach of the Company of its no-shop obligations in the Merger Agreement; |
| o | breach of or failure to perform any representation, warranty, covenant, or agreement set forth in the Merger Agreement by the Company, such that certain closing conditions of the Merger are not satisfied; or |
| o | failure to obtain the requisite stockholder vote in favor of the issuance of the Company’s common stock pursuant to the Merger Agreement at the special meeting of stockholders. |
Amendment
The Merger Agreement may not be amended except in writing signed by the Company, Merger Sub, Baxano, and the Securityholder Representatives.
| 64 |
Securityholder Representatives
In the Merger Agreement, the Securityholders irrevocably appoint the Securityholder Representatives for all Securityholders for purposes of all post-closing matters under the Agreement, including without limitation the full power and authority on the Securityholders’ behalf to take the following actions:
| · | to consummate the transactions contemplated by the Merger Agreement and the other agreements executed in connection with the Merger Agreement; |
| · | to negotiate and settle disputes arising under, or relating to, the Merger Agreement and the other agreements executed in connection with the Merger Agreement; |
| · | to execute and deliver any amendment or waiver to the Merger Agreement and the other agreements executed in connection with the Merger Agreement (without the prior approval of the Securityholders); |
| · | to retain legal and other advisors in connection with the foregoing, the reimbursement of reasonable fees incurred in connection therewith to be paid from the $250,000 Securityholder Representatives reimbursement fund described above;and |
| · | to take any and all other actions whatsoever to be taken by or on behalf of the Securityholders in connection with the Merger Agreement and the other agreements executed in connection with the Merger Agreement. |
AppraisalRights
Holders of our common stockwill not have appraisal rights under Delaware law in connection with theMergerorthe issuance of shares of our common stock pursuantto the Merger Agreement.
MaterialU.S. Federal Income Tax Consequences of the Merger
We expect theMergerto qualify as a tax-free reorganization under Section 368 of the Internal Revenue Code. We expect that there will be no material federal income tax consequences from theMergerfor us or our stockholders.
AnticipatedAccounting Treatment of the Merger
For accounting purposes, we are considered to be acquiring Baxano in the Merger. TheMergerwill be accounted for as an acquisition ofBaxanoby us under the purchase method of accounting under GAAP. Under the purchase method of accounting, the assets and liabilities of an acquired company (i.e.,Baxano) are, as of completion of the acquisition, recorded at their respective fair values and added to those of the reporting public issuer (i.e., the Company), including an amount for goodwill representing the difference between the purchase price and the fair value of the identifiable net assets. Our financial statements issued after the consummation of theMergerwill reflect only our operations after theMergerand will not be restated retroactively to reflect the historical financial position or results of operations ofBaxano.
Allunaudited pro forma financial informationcontained in this Proxy Statement has been prepared using the purchase method to account for theMerger. The measurement of the purchase price will be performed as of the date theMergeris completed. The final allocation of the purchase price will be determined after theMergeris completed and after completion of an analysis to determine the assigned fair values ofBaxano’s tangible and identifiable intangible assets and liabilities. Accordingly, the final purchase accounting adjustments may be materially different from the unaudited pro forma adjustments.Any decrease in the net fair value of the assets and liabilities of Baxano as compared to the unaudited pro forma information included in this Proxy Statement may have the effect of increasing the amount of the purchase price allocable to goodwill.
RegulatoryApprovals for the Completion of the Merger
As of the date of thisProxy Statement, we are not required to make filings or obtain approvals or clearances from any antitrust regulatory authorities in the United States or other countries to consummate theMerger. In the United States, we must comply with applicable federal and state securities laws and the rules and regulations of theNASDAQ Stock Market LLCin connection withthe issuance of shares of our common stock pursuantto the Merger Agreement and the Securities Purchase Agreement and the filing of thisProxy Statementwith the SEC.
| 65 |
LOCK-UP AND REGISTRATION OF SHARES
See “Proposal No. 2 – Issuance of Common Stock Pursuant to the Securities Purchase Agreement—Lock-up and Registration of Shares” for a description of the lock-up provisions and registration rights applicable to the shares of our common stock to be issued pursuant to the Merger Agreement.
STOCKHOLDERAgreementS
On March 3, 2013, we entered into Stockholder Agreements, or the Stockholder Agreements, with Baxano and certain securityholders of TranS1 identified on the signature pages thereto, or the Stockholders, which include our directors, executive officers, and certain of our affiliates, and the Securityholder Representatives. The Stockholders own in the aggregate approximately 6.6 million shares of our common stock, constituting approximately 24.2% of the outstanding shares of our common stock. Pursuant to the Stockholder Agreements, the Stockholders have agreed to vote all of their shares in favor of the proposal to issue shares of our common stock pursuant to the Merger Agreement and the Securities Purchase Agreement and in favor of the election and re-election of individuals designated to our Board of Directors by the Securityholder Representatives pursuant to the Merger Agreement at our 2013 Annual Meeting of Stockholders and any replacements of such designees until our 2016 Annual Meeting of Stockholders. The Stockholders have also agreed that they will not transfer any shares of our common stock (except to permitted transferees) until the later of the date the registration statement required to be filed under the Securities Purchase Agreement has been declared effective by the SEC or the date directors are elected at TranS1’s 2013 annual meeting of stockholders. We are a party to the Stockholder Agreements solely for purposes of enforcing such transfer restrictions and voting agreements.
BRIDGE FINANCING
On March 25, 2013, our Board of Directors authorized Company management to provide up to $2,500,000 of bridge financing to Baxano for its working capital needs through the Effective Time. From time to time prior to the Effective Time, Baxano may request that the Company provide bridge financing for specified budget items. If the parties agree that the Company will provide all or any portion of the requested amount, then the Company will loan Baxano such amount and Baxano will issue a Bridge Note to the Company to evidence the loan amount. The agreed form of Bridge Note is included in Annex A to this Proxy Statement. To date, Baxano has issued $2,100,000 aggregate principal amount of Bridge Notes to the Company.
Each Bridge Note bears interest at a rate of 6% per annum. Each Bridge Note will be cancelled immediately prior to the Effective Time without consideration, repayment, or any other right of the Company to be repaid or otherwise compensated. All outstanding principal and interest under the Bridge Notes is due and payable on demand by the Company any time on or after September 7, 2013 if the Effective Time has not occurred by such date. Additionally, all principal and accrued interest under the Bridge Notes will be due and payable upon the consummation of any sale by Baxano of its equity securities to venture capital, institutional or private investors, including at least one investor that is not an existing noteholder or stockholder of Baxano, resulting in aggregate cash proceeds to Baxano of at least $15,000,000 (excluding any amount invested by cancellation or conversion of the indebtedness represented by the Baxano Notes).
The Bridge Notes are not be secured by any collateral, are subordinated in right of payment to the Oxford/SVB Loan, and are senior in right of payment to the Baxano Notes.
PROPOSAL NO. 2
ISSUANCE OF COMMON STOCK PURSUANT TO THE SECURITIES PURCHASE AGREEMENT
We are submitting a proposal to issue shares of our common stock pursuant to the Securities Purchase Agreement in accordance with the listing rules of the NASDAQ Stock Market LLC, on which exchange our common stock is listed. Under these rules, we are required to obtain the approval of our stockholders in connection with the acquisition of the stock or assets of another company or a private placement involving the sale or issuance by us of common stock (or securities convertible into or exercisable for common stock) equal to or in excess of 20% of our common stock or 20% of the voting power outstanding before the issuance. Because completion of the Private Placement Transaction would require us to issue approximately 27.5% of our total outstanding shares of common stock, we are asking our stockholders to approve the issuance at the Special Meeting.
Subject to the terms and conditions of the Securities Purchase Agreement, at the closing of the Private Placement Transaction, we will sell and issue an aggregate of 7,522,009 shares of our common stock to the Investors at a purchase price of $2.28 per share. See “—The Private Placement Transaction.”
We anticipate that the maximum aggregate number of shares of our common stock to be issued to the Investors at the closing of the Private Placement Transaction will represent up to approximately 16.5% of the outstanding shares of our common stock immediately following the closing of the Private Placement Transaction. We have not targeted the net proceeds being raised in the Private Placement Transaction with the intention of achieving a particular percentage ownership in the Company for any of the Investors or any group of Investors following the closing of the Private Placement Transaction.
The shares of our common stock to be issued pursuant to the Securities Purchase Agreement will be offered and sold in reliance upon an exemption from the registration requirements of the Securities Act afforded by Regulation D promulgated thereunder. The Private Placement Transaction was a privately negotiated transaction that did not involve general solicitation, and all recipients of shares of our common stock pursuant to the Securities Purchase Agreement are “accredited investors” as defined in Regulation D.
The issuance of shares of common stock pursuant to the Securities Purchase Agreement described in this Proposal No. 2 and the issuance of shares of common stock pursuant to the Merger Agreement described in Proposal No. 1 are, among other conditions described in this Proxy Statement, conditioned on each other and, therefore, both proposals must be approved in order for the Merger and the Private Placement Transaction to be consummated. For information about the Merger, see “Proposal No. 1 – Issuance of Common Stock Pursuant to the Merger Agreement.”
The approval of the issuance of shares of our common stock pursuant to the Securities Purchase Agreement requires the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal.
OUR BOARD (EXCEPT FOR MR. SHAPIRO) UNANIMOUSLY RECOMMENDS A VOTE “FOR” THE issuance of shares of our common stock pursuant to the SECURITES PURCHASE Agreement.
ThePrivate Placement Transaction
Our Board of Directors determined that it was in our best interests and the best interests of our stockholders to enter into a Securities Purchase Agreement with the Investors, pursuant to which we agreed to issue and sell to the Investors, and the Investors agreed to purchase from us, an aggregate of 7,522,009 shares of our common stock, at a purchase price of $2.28 per share, resulting in gross proceeds to us of $17.2 million. The issuance and sale of shares of our common stock pursuant to the Private Placement Transaction will be exempt from the registration requirements under the Securities Act afforded by Regulation D promulgated thereunder. The Company is obligated to register such shares, together with the shares issued pursuant to the Merger Agreement, on the terms set forth in and in accordance with the Securities Purchase Agreement. See “—Lock-up and Registration of Shares.”
The closing of the Private Placement Transaction is conditioned on the closing of the Merger. We intend to use the net proceeds from the Private Placement Transaction for working capital and general corporate purposes of the Company post-Merger. We have not targeted the net proceeds being raised in the Private Placement Transaction with the intention of achieving a particular percentage ownership in the Company for any of the Investors or any group of Investors following the closing of the Private Placement Transaction. For further information regarding the Private Placement Transaction, please see the Securities Purchase Agreement, which is attached to this Proxy Statement asAnnex B.
| 66 |
LOCK-UP AND REGISTRATION OF SHARES
Pursuant to the Securities Purchase Agreement, the Investors and other holders of shares received pursuant to the Merger Agreement agreed not to sell, transfer, or otherwise dispose of the shares of our common stock issued at the closing of the Merger and the Private Placement Transaction from the period commencing on the closing of the Private Placement Transaction and expiring on the effective date of the registration statement described below, subject to certain exceptions.
Pursuant to the Securities Purchase Agreement, we agreed to register the resale of the shares of our common stock to be issued pursuant to the Merger Agreement and the Securities Purchase Agreement under a registration statement on Form S-3 (or another appropriate form if Form S-3 is not then available to us). Subject to certain conditions, we agreed to (i) file the registration statement with the SEC within 30 days following the closing date of the Private Placement Transaction, (ii) use our best efforts, subject to receipt of necessary information from the Investors and other holders of shares received pursuant to the Merger Agreement, to cause the SEC to declare the registration statement effective within 90 days or, if the registration statement is selected for review by the SEC, 120 days after the closing date of the Private Placement Transaction, and (iii) maintain the continual effectiveness of the registration statement until such time as the shares issued pursuant to the Merger Agreement and the Securities Purchase Agreement become eligible for resale by each of the Investors and other holders of shares received pursuant to the Merger Agreement without volume limitations or other restrictions pursuant to Rule 144 promulgated under the Securities Act (or until all such shares have been sold pursuant to the registration statement or Rule 144, if earlier).
We agreed to pay all expenses of registration of the shares of our common stock to be issued pursuant to the Merger Agreement and the Securities Purchase Agreement. In addition, if the registration statement is not declared effective by the SEC by the 90-day or 120-day deadline, as applicable, we must pay each Investor and other holder of shares issued pursuant to the Merger Agreement liquidated damages equal to 1% of the value of their shares (calculated at the closing price of such shares) per 30-day period, up to a maximum of 12%.
STOCKHOLDER AgreementS
See “Proposal No. 1 – Issuance of Common Stock Pursuant to the Merger Agreement—Stockholder Agreements” for a description of the Stockholder Agreements entered into in connection with the Merger and the Private Placement Transaction.
| 67 |
INTERESTS OF CERTAIN PERSONS IN THE MERGER AND PRIVATE PLACEMENT TRANSACTION
In considering the recommendation of the Board of Directors with respect to the issuance of shares of our common stock pursuant to the Merger Agreement and the Securities Purchase Agreement, you should be aware that James Shapiro, a member of our Board of Directors, has interests in the Merger that are different from, or in addition to, the interests of our stockholders generally. Mr. Shapiro is also a member of Baxano’s board of directors. While he does not own any shares of Baxano’s common stock individually, as a Managing Member of the General Partner of Kearny Venture Partners, L.P. and Kearny Venture Partners Entrepreneurs’ Fund, L.P., Mr. Shapiro has shared voting and dispositive power over, and a partial indirect pecuniary interest in 7,285,290 shares of Baxano’s common stock equivalents. In addition, these funds hold approximately $1,961,571 in aggregate principal amount of the Baxano Notes. Therefore, Mr. Shapiro and Kearny Venture Partners will have the right to receive a portion of the merger consideration in accordance with the Merger Agreement. Other than Mr. Shapiro, none of our directors or executive officers has any interests in the Merger that are different from, or in addition to, the interests of our stockholders generally. The Board of Directors was aware of Mr. Shapiro’s potential conflict of interest and Mr. Shapiro did not participate in discussions and deliberations related to the Merger.
In addition, certain of our directors and executive officers and certain of Baxano’s securityholders are Investors in the Private Placement Transaction. Baxano’s securityholders have agreed to purchase an aggregate of 6,729,909 shares of common stock for a total of $15.3 million in connection with the Private Placement Transaction. The following directors and executive officers have agreed to purchase an aggregate of 123,242 shares of common stock for a total of $281,000 in connection with the Private Placement Transaction: Jeffrey Fischgrund, Ken Reali, David Simpson, Joseph Slattery, and Mark Stautberg. The remaining 668,858 shares of common stock are expected to be purchased by a third party investor that will hold less than 5% of the Company’s outstanding common stock post-transaction and a non-executive employee of the Company.
The following table sets forth the approximate percentage holdings of the Company and Baxano held by their respective affiliates before the Merger and the Private Placement Transaction and the expected percentage holdings of such affiliates of the combined company after the proposed consummation thereof.
| Name of Affiliate | Approximate Percentage of Shares Owned Before the Merger and the Private Placement Transaction(1) | Approximate Percentage of Shares Owned After the Merger and the Private Placement Transaction(1) | |||||
| TranS1 Affiliates | |||||||
| Delphi Ventures and Affiliated Entities | 17.18 | % | 10.32 | % | |||
| Advanced Technology Ventures and Affiliated Entities | 11.30 | % | 6.79 | % | |||
| Michael Carusi(2) | 11.30 | % | 6.79 | % | |||
| Jonathan Osgood(3) | 7.31 | % | 4.39 | % | |||
| Joseph Slattery | 0.70 | % | 0.50 | % | |||
| Paul LaViolette | 0.56 | % | 0.34 | % | |||
| David Simpson | 0.22 | % | 0.24 | % | |||
| Rick Feiler | 0.00 | % | 0.00 | % | |||
| Ken Reali | 0.03 | % | 0.03 | % | |||
| Dwayne Montgomery(4) | 0.00 | % | 0.00 | % | |||
| Mark Stautberg | 0.00 | % | 0.04 | % | |||
| Jeffrey Fischgrund | 0.00 | % | 0.02 | % | |||
| Stephen D. Ainsworth | 0.00 | % | 0.00 | % | |||
| Mukesh Ramchandani | 0.00 | % | 0.00 | % | |||
| Stephanie M. Fitts | 0.00 | % | 0.00 | % | |||
| Baxano Affiliates | |||||||
| Prospect Venture Partners III, L.P. | 29.79 | % | 10.97 | % | |||
| Russell Hirsch(5) | 29.79 | % | 10.97 | % | |||
| Rod Young(6) | 21.30 | % | 7.50 | % | |||
| Three Arch Partners and Affiliated Entities | 21.17 | % | 7.50 | % | |||
| CMEA Ventures and Affiliated Entities | 16.19 | % | 8.12 | % | |||
| Sumeet Jain(7) | 16.19 | % | 8.12 | % | |||
| Jeffrey L. Bleich | 7.32 | % | 0.00 | % | |||
| David Schulte(8) | 7.20 | % | 3.61 | % | |||
| Amie Borgstrom | 0.08 | % | 0.00 | % | |||
| Anthony Recupero | 0.02 | % | 0.00 | % | |||
| Frank Phillips | 0.00 | % | 0.00 | % | |||
| George Harter(9) | 0.00 | % | 0.00 | % | |||
| Michael Wallace(9) | 0.00 | % | 0.00 | % | |||
| Christine Mathews | 0.00 | % | 0.00 | % | |||
| Gregory Welsh | 0.00 | % | 0.00 | % | |||
| TranS1 and Baxano Affiliate | |||||||
| James Shapiro | 4.06 | %(10) | 6.39 | % | |||
| 11.26 | %(11) | ||||||
| (1) | This table is based upon ownership information available to us as of March 4, 2013 and shares expected to be issued to affiliates in connection with the Merger and the Private Placement Transaction. For the purposes of this table, we define “affiliates” as the Company’s and Baxano’s directors and executive officers and holders of 10% or more of the Company’s common stock or Baxano’s common stock equivalents. The second column reflects ownership percentages before the consummation of the Merger and the Private Placement of (1) the Company with respect to affiliates of the Company and (2) Baxano with respect to affiliates of Baxano. The third column reflects ownership percentages after the consummation of the Merger and the Private Placement of the combined entity. Applicable percentage ownership is based on 61,017,661 shares of Baxano common stock equivalents outstanding as of March 4, 2013, 27,318,785 shares of TranS1 common stock outstanding as of March 4, 2013, and a maximum of 18,145,981 shares of TranS1 common stock expected to be issued in connection with the Merger and the Private Placement Transaction. In order to determine the allocation of the merger consideration, this table assumes a closing date of March 31, 2013 and an average closing price of TranS1 common stock on the NASDAQ Global Market during the 10 days preceding the closing date of $2.50. |
| (2) | Consists of shares held by Advanced Technology Ventures and affiliated entities. For additional ownership information, see “Information About TranS1—Security Ownership of Certain Beneficial Owners and Management.” |
| (3) | Consists of shares held by Cutlass Capital and affiliated entities. For additional ownership information, see “Information About TranS1—Security Ownership of Certain Beneficial Owners and Management.” |
| (4) | On April 2, 2013, Dwayne Montgomery resigned from his position with the Company, effective as of May 1, 2013, in order to pursue other business opportunities. In connection with his resignation, the Company and Mr. Montgomery executed a General Release and Severance Agreement, under which he is entitled to receive severance in the aggregate amount of $170,186. |
| (5) | Consists of shares held by Prospect Venture Partners III, L.P. |
| 68 |
| (6) | Includes shares held by Three Arch Partners and affiliated entities. |
| (7) | Consists of shares held by CMEA Ventures and affiliated entities. |
| (8) | Consists of shares held by Kaiser Permanente Ventures and affiliated entities. |
| (9) | On April 2, 2013, Messrs. Harter and Wallace were terminated by Baxano at the Company’s request. For information on compensation payable to Messrs. Harter and Wallace related to their termination, see “—Golden Parachute Compensation” below. |
| (10) | Represents Mr. Shapiro’s ownership percentage in the Company. Consists of shares held by Thomas Weisel Healthcare Venture Partners, L.P., Kearny Venture Partners, L.P., and Kearny Venture Partners Entrepreneurs’ Fund, L.P. For additional ownership information, see “Information About TranS1—Security Ownership of Certain Beneficial Owners and Management.” |
| (11) | Represents Mr. Shapiro’s ownership percentage in Baxano. Consists of shares held by Kearny Venture Partners, L.P. and Kearny Venture Partners Entrepreneurs’ Fund, L.P. |
The table above accounts for the conversion of Baxano Notes into shares of our common stock pursuant to the Merger Agreement. The following table describes the affiliations of certain of Baxano’s directors with holders of Baxano Notes and quantifies the total amount of principal under the Baxano Notes that each such director’s affiliated Baxano securityholders will convert into shares of our common stock pursuant to the Merger Agreement.
| Baxano Director’s Name | Principal Converting Under Baxano Notes | |||
| Russell Hirsch(1) | $ | 5,191,013.90 | ||
| Rod Young(2) | $ | 3,689,470.09 | ||
| Sumeet Jain(3) | $ | 2,821,387.68 | ||
| James Shapiro(4) | $ | 1,961,571.23 | ||
| David Schulte(5) | $ | 1,253,949.85 | ||
| (1) | Russell Hirsch is an affiliate of Prospect Venture Partners III, L.P., a Baxano securityholder that holds Baxano Notes. |
| (2) | Rod Young is an affiliate of Three Arch Partners IV, L.P. and Three Arch Associates IV, L.P., each Baxano securityholders that hold Baxano Notes. |
| (3) | Sumeet Jain is an affiliate of CMEA Ventures VII, L.P. and CMEA Ventures VII (Parallel), L.P., each Baxano securityholders that hold Baxano Notes. |
| (4) | James Shapiro is an affiliate of Kearny Venture Partners, L.P. and Kearny Venture Partners Entrepreneurs’ Fund, L.P., each Baxano securityholders that hold of Baxano Notes. |
| (5) | David Schulte is an affiliate of Kaiser Permanente Ventures LLC–Series A, Kaiser Permanente Ventures LLC–Series B, The Permanente Federation LLC–Series I and The Permanente Federation LLC–Series J. Kaiser Permanente Ventures LLC–Series A, Kaiser Permanente Ventures LLC–Series B, The Permanente Federation LLC–Series I and The Permanente Federation LLC–Series J are each Baxano securityholders. Kaiser Permanente Ventures LLC–Series A, Kaiser Permanente Ventures LLC–Series B, and The Permanente Federation LLC–Series J are each holders of Baxano Notes. |
Because the amount of merger consideration to be paid in connection with the Merger is targeted to provide Baxano stockholders with ownership of 28% of the Company’s post-closing outstanding shares of common stock (excluding the impact of the Private Placement Transaction) prior to certain adjustments, while the shares of common stock to be issued in connection with the Private Placement Transaction have a fixed price, the value of the shares to be issued in connection with the Merger may be different than the value of the shares to be issued in connection with the Private Placement Transaction. This difference in value cannot be quantified until after the Merger is consummated.
The Board of Directors considered Mr. Shapiro’s interests in the Merger and the participation of our directors and executive officers in the Private Placement Transaction, among other matters, in making its recommendation that you approve the issuance of shares of our common stock as described in this Proxy Statement.
Golden Parachute Compensation
The following table and the related footnotes present information required by Item 402(t) of Regulation S-K promulgated under the Exchange Act about the amounts of compensation payable by Baxano to its “named executive officers” that is based on or otherwise relates to the Merger.
| Name | Cash ($) | Perquisites/Benefits ($) | Total ($) | |||||||||
| Anthony Recupero | $ | 345,000 | (1) | $ | 19,267 | (2) | $ | 364,267 | ||||
| George Harter(3) | $ | 258,750 | (1) | $ | 19,267 | (2) | $ | 278,017 | ||||
| Michael Wallace(3) | $ | 139,104 | (1) | $ | 9,633 | (2) | $ | 148,737 | ||||
| (1) | Amount relates to a “double trigger” cash severance payment payable pursuant to the executive’s Change of Control Severance Agreement with Baxano in the event, within 12 months following a change of control of Baxano, the executive’s employment is terminated for any reason other than for “cause” or the executive terminates his employment for “good cause.” Amounts are determined based upon current salary level for such executive. |
| (2) | Amount relates to a “double trigger” extension of health, dental, and vision benefits pursuant to the executive’s Change of Control Severance Agreement with Baxano in the event, within 12 months following a change of control of Baxano, the executive’s employment is terminated for any reason other than for “cause” or the executive terminates his employment for “good cause.” Amounts are determined based upon current cost of such benefits for such executive and reflect the maximum benefit payable pursuant to such arrangement with the executive. | |
| (3) | On April 2, 2013, Messrs. Harter and Wallace were terminated by Baxano at the Company’s request. Although their terminations occurred prior to the closing of the Merger, they were treated as if they were terminated without cause following a change of control of Baxano under their Change of Control Severance Agreements. |
The potential payments described in the table above are provided by the Change of Control Severance Agreements between Baxano and its named executive officers, or the Change of Control Agreements. Under the Change of Control Agreements, severance is payable only under “double trigger” circumstances where a change of control of Baxano has occurred and the executive’s employment is terminated for any reason other than for “cause” or the executive terminates his employment for “good cause.” The Change of Control Agreements define “good cause” to include (1) significant diminution in the executive’s position or responsibilities, or the removal of the executive from his position and responsibilities; (2) a substantial reduction in compensation (including benefits) other than as part of a company-wide reduction; and (3) the relocation to a facility or a location more than 30 miles away.
| 69 |
If severance obligations are triggered, the Change of Control Agreements provide for 12 months’ base salary with respect to Messrs. Recupero and Harter and six months’ base salary with respect to Mr. Wallace. The base salary payments are payable in a lump sum within 20 days following the effective date of a release of claims. The Change of Control Agreements also provide for the same level of health (medical, vision, dental) coverage and benefits for the executive. The Change of Control Agreements require Baxano to provide such coverage until the earlier of (i) the date the executive (and his eligible dependents) is no longer eligible to receive continuation coverage pursuant to COBRA, or (ii) the expiration date of such benefits (12 months following termination with respect to Messrs. Recupero and Harter and six months following termination with respect to Mr. Wallace).
As a condition to receiving severance benefits under the Change of Control Agreements, the executive must execute and not revoke a general release of claims that becomes effective within 55 days following the termination of employment.
In addition to the arrangements described above, the Company may enter into employment or consulting arrangements with certain executive officers of Baxano that would become effective following the closing of the Merger on terms to be mutually agreed upon by us and such executive officers. There are no such arrangements as of the date of this Proxy Statement.
| 70 |
UNAUDITEDPRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
On March 3, 2013, TranS1 Inc., or the Company, entered into an agreement and plan of merger, pursuant to which, at the effective time of the merger, or the Effective Time, a wholly-owned subsidiary of the Company, or the Merger Sub, will merge with and into Baxano, Inc., or Baxano, with Baxano surviving as a wholly-owned subsidiary of the Company. The agreement and plan of merger, as amended, is referred to as the Merger Agreement and the transactions contemplated in the Merger Agreement are referred to as the Merger. Pursuant to the terms of the Merger Agreement, the consideration to be transferred by the Company in connection with the Merger will consist of (i) a number of newly issued shares of common stock of the Company calculated as the product of (a) 0.38888889times (b) the number of issued and outstanding shares of common stock of the Company as of the close of business on the last trading day prior to the closing of the Merger, subject to certain downward adjustments as specified in the Merger Agreement, (ii) a cash payment of $250,000 to fund expenses of the Securityholder Representatives appointed under the Merger Agreement, or the Securityholder Representatives, and (iii) a cash payment of up to $300,000 to fund a compensation plan to be adopted by Baxano prior to closing providing for bonuses to Baxano employees and non-employee directors, or the Transaction Payment Plan. The purchase price is subject to further adjustment based on adjustments for working capital, excess indebtedness and unpaid Baxano transaction expenses.
In connection with the entry into the Merger Agreement, the Company also entered into a Securities Purchase Agreement, dated March 3, 2013, with certain investors (including certain of Baxano’s securityholders and certain of the Company’s directors and executive officers), or the Investors. This agreement is referred to as the Securities Purchase Agreement and the transaction contemplated in such agreement is referred to as the Private Placement Transaction. Pursuant to the terms of the Securities Purchase Agreement, the Company will issue and sell to the Investors an aggregate of 7,522,009 shares of common stock at a purchase price of $2.28 per share, resulting in gross proceeds to the Company of approximately $17,150,000.
The following unaudited pro forma condensed combined financial statements, or the pro forma financial statements, combine the historical financial information of the Company and Baxano as of and for the year ended December 31, 2012 and are adjusted on a pro forma basis to give effect to (i) the Merger and (ii) the Private Placement Transaction. The estimated adjustments to reflect the Merger and the Private Placement Transaction are described in the notes to the pro forma financial statements. The unaudited pro forma condensed combined balance sheet, or the pro forma balance sheet, reflects the Merger and the Private Placement Transaction as if they had each been consummated on December 31, 2012, and the unaudited pro forma condensed combined statement of operations, or the pro forma statement of operations, reflects the Merger and Private Placement Transaction as if they had each been consummated on January 1, 2012. The pro forma financial statements have been derived from and should be read in conjunction with the historical consolidated financial statements of each of the Company and Baxano, which are included in this Proxy Statement (see “TranS1 Financial Statements” and “Baxano Financial Statements,” respectively).
The pro forma financial statements are provided for illustrative purposes only and are not intended to represent, and are not necessarily indicative of, what the operating results or financial position of the Company would have been had the Merger and the Private Placement Transaction been completed on the dates indicated, nor are they necessarily indicative of the Company’s future operating results or financial position. The pro forma financial statements do not reflect the impacts of any potential operational efficiencies, asset dispositions, cost savings or economies of scale that the Company may achieve with respect to the combined operations. Additionally, the pro forma statement of operations does not include non-recurring charges or credits which result directly from the transactions.
As of the date of this Proxy Statement, Baxano’s assets and liabilities are presented at their preliminary estimated fair values, with the excess of the purchase price over the sum of these fair values presented as goodwill. The actual adjustments to the Company’s consolidated financial statements on the closing date of the Merger and the Private Placement Transaction and the related allocation of the merger consideration will depend on a number of factors, including additional financial information available at such time, changes in the fair value of the Company’s common stock transferred in connection with the Merger and the Private Placement Transaction, changes in the estimated fair value of Baxano’s assets and liabilities as of the closing date and changes in Baxano’s operating results between the date of preparation of the pro forma financial statements and the closing date. The valuations of acquired assets and liabilities are in process and are not expected to be finalized until later in 2013, as information may become available within the measurement period which indicates a potential change to these valuations, including the value of the net assets on the closing date of the Merger. Accordingly, the final allocations of and the effects on the results of operations may differ materially from the preliminary allocations and unaudited pro forma combined amounts included herein.
| 71 |
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
As of December 31, 2012
(in thousands)
| Pro Forma | ||||||||||||||||||||
| Adjustments | ||||||||||||||||||||
| Pro Forma | (Private | |||||||||||||||||||
| Historical | Adjustments | Placement | Pro Forma | |||||||||||||||||
| TranS1 | Baxano | (Merger) | Transaction) | Combined | ||||||||||||||||
| Assets | ||||||||||||||||||||
| Current assets: | ||||||||||||||||||||
| Cash and cash equivalents | $ | 21,541 | $ | 4,409 | $ (2,144 | )(a) | $ | 16,980 | (g) | $ | 40,786 | |||||||||
| Accounts receivable, net | 3,206 | 1,773 | - | 4,979 | ||||||||||||||||
| Inventory | 5,017 | 1,994 | - | 7,011 | ||||||||||||||||
| Prepaid expenses and other assets | 330 | 377 | - | 707 | ||||||||||||||||
| Total current assets | 30,094 | 8,553 | (2,144 | ) | 16,980 | 53,483 | ||||||||||||||
| Property and equipment, net | 2,166 | 778 | - | 2,944 | ||||||||||||||||
| Intangible assets | - | - | 11,836 | (b) | 11,836 | |||||||||||||||
| Goodwill | - | - | 7,397 | (c) | 7,397 | |||||||||||||||
| Other assets | - | 221 | - | 221 | ||||||||||||||||
| Total assets | $ | 32,260 | $ | 9,552 | $ | 17,089 | $ | 16,980 | $ | 75,881 | ||||||||||
| Liabilities and Stockholders' Equity (Deficit) | ||||||||||||||||||||
| Current liabilities: | ||||||||||||||||||||
| Accounts payable | $ | 2,603 | $ | 708 | $ | - | $ | 3,311 | ||||||||||||
| Accrued expenses related to U.S. Government settlement | 6,792 | - | - | 6,792 | ||||||||||||||||
| Accrued expenses | 1,648 | 1,274 | - | 2,922 | ||||||||||||||||
| Convertible debt | - | 14,768 | (14,768 | )(d) | - | |||||||||||||||
| Notes payable, current portion | - | 3,017 | (2,645 | )(e) | 372 | |||||||||||||||
| Total current liabilities | 11,043 | 19,767 | (17,413 | ) | - | 13,397 | ||||||||||||||
| Noncurrent liabilities: | ||||||||||||||||||||
| Notes payable, net of current portion | - | - | 2,645 | (e) | 2,645 | |||||||||||||||
| Warrant liability | - | 131 | (131 | )(f) | - | |||||||||||||||
| Deferred rent | 78 | 164 | - | 242 | ||||||||||||||||
| Convertible preferred stock | - | 58,339 | (58,339 | )(f) | - | |||||||||||||||
| Stockholders' equity (deficit): | ||||||||||||||||||||
| Common stock | 3 | 7 | (6 | )(f) | 1 | (g) | 5 | |||||||||||||
| Additional paid-in capital | 159,929 | 1,534 | 21,537 | (f) | 16,979 | (g) | 199,979 | |||||||||||||
| Accumulated other comprehensive income (loss) | 14 | - | - | 14 | ||||||||||||||||
| Accumulated deficit | (138,807 | ) | (70,390 | ) | 68,796 | (f) | (140,401 | ) | ||||||||||||
| Total stockholders' equity (deficit) | 21,139 | (68,849 | ) | 90,327 | 16,980 | 59,597 | ||||||||||||||
| Total liabilities and stockholders' equity (deficit) | $ | 32,260 | $ | 9,552 | $ | 17,089 | $ | 16,980 | $ | 75,881 | ||||||||||
See accompanying notes to the pro forma financial statements.
| 72 |
UNAUDITED PRO FORMA CONDENSED COMBINED
STATEMENT OF OPERATIONS
For the year ended December 31, 2012
(in thousands, except per share data)
| Historical | Pro Forma | Pro Forma | ||||||||||||||
| TranS1 | Baxano | Adjustments | Combined | |||||||||||||
| Revenue | $ | 14,570 | $ | 9,417 | $ | - | $ | 23,987 | ||||||||
| Cost of revenue | 4,309 | 3,261 | - | 7,570 | ||||||||||||
| Gross profit | 10,261 | 6,156 | - | 16,417 | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 5,457 | 4,778 | - | 10,235 | ||||||||||||
| Sales and marketing | 20,049 | 17,155 | - | 37,204 | ||||||||||||
| General and administrative | 6,172 | 2,486 | 1,258 | (h) | 9,916 | |||||||||||
| Charges related to U.S. Government settlement | 8,324 | - | - | 8,324 | ||||||||||||
| Total operating expenses | 40,002 | 24,419 | 1,258 | 65,679 | ||||||||||||
| Operating loss | (29,741 | ) | (18,263 | ) | (1,258 | ) | (49,262 | ) | ||||||||
| Interest expense, net | - | (995 | ) | 779 | (i) | (216 | ) | |||||||||
| Other income (expense) | (147 | ) | 415 | - | 268 | |||||||||||
| Net loss | $ | (29,888 | ) | $ | (18,843 | ) | $ | (479 | ) | $ | (49,210 | ) | ||||
| Net loss per common share - basic and diluted | $ | (1.10 | ) | $ | (1.31 | ) | ||||||||||
| Weighted average common shares outstanding - basic and diluted | 27,267 | 10,393 | (j) | 37,660 | ||||||||||||
| Supplemental earnings per share information: | ||||||||||||||||
| Net loss per common share - basic and diluted | $ | (1.10 | ) | $ | (1.09 | ) | ||||||||||
| Weighted average common shares outstanding - basic and diluted | 27,267 | 17,915 | (k) | 45,182 | ||||||||||||
See accompanying notes to the pro forma financial statements.
| 73 |
NOTES TO UNAUDITED PRO FORMA CONDENSED COMBINED CONSOLIDATED
FINANCIAL STATEMENTS
1. Description of Transaction
On March 3, 2013, the Company entered into the Merger Agreement, pursuant to which, at the effective time, Merger Sub, a wholly-owned subsidiary of the Company, will merge with and into Baxano with Baxano surviving as a wholly-owned subsidiary of the Company. Pursuant to the terms of the Merger Agreement, the consideration to be transferred by the Company in connection with the Merger will consist of (i) a number of newly issued shares of common stock of the Company calculated as the product of (a) 0.38888889times (b) the number of issued and outstanding shares of common stock of the Company as of the close of business on the last trading day prior to the closing of the Merger, subject to certain downward adjustments as specified in the Merger Agreement, (ii) a cash payment of $250,000 to fund expenses of the Securityholder Representatives and (iii) cash payments of $300,000 to fund the Transaction Payment Plan. The purchase price is subject to further adjustment based on adjustments for working capital, excess indebtedness and unpaid Baxano transaction expenses.
At the Effective Time, each outstanding share of capital stock of Baxano will be cancelled and extinguished and converted into the right to receive a portion of the merger consideration in accordance with the Merger Agreement, except for dissenting shares, which will retain the appraisal rights and dissenters’ rights provided for under Delaware law and California law, respectively. Prior to the Effective Time, Baxano must take all actions necessary to cause each Baxano Note to be terminated at the Effective Time and the holder of such note shall be entitled to receive merger consideration in accordance with the Merger Agreement. Prior to the Effective Time, any and all stock option plans or other stock or equity-related plans of Baxano, together with any employee stock purchase plans shall be terminated. Prior to the Effective Time, Baxano shall use commercially reasonable efforts to cause each outstanding option to purchase common stock of Baxano, whether vested or unvested, and each warrant to acquire capital stock of Baxano to be terminated.
In connection with the entry into the Merger Agreement, the Company also entered into the Private Placement Transaction, pursuant to which it entered into a Securities Purchase Agreement, dated March 3, 2013, with the Investors. Pursuant to the terms of the Securities Purchase Agreement, the Company will issue and sell to the Investors an aggregate of 7,522,009 shares of common stock at a purchase price of $2.28 per share, resulting in gross proceeds to the Company of approximately $17,150,000. The Private Placement Transaction will close immediately following the closing of the Merger.
2. Basis of Presentation
The pro forma financial statements combine the historical financial information of the Company and Baxano as of and for the year ended December 31, 2012 and are adjusted on a pro forma basis to give effect to (i) the Merger and (ii) the Private Placement Transaction. The estimated adjustments to reflect the Merger and the Private Placement Transaction are described in the notes to the pro forma financial statements. The pro forma balance sheet reflects the Merger and the Private Placement Transaction as if they had each been consummated on December 31, 2012, and the pro forma statement of operations reflects the Merger and Private Placement Transaction as if they had each been consummated on January 1, 2012. The pro forma financial statements have been derived from and should be read in conjunction with the historical consolidated financial statements of each of the Company and Baxano, which are included in this Proxy Statement (see “TranS1 Financial Statements” and “Baxano Financial Statements,” respectively). The pro forma financial statements should be read in connection with the historical consolidated financial statements of the Company and Baxano referred to above.
The pro forma financial statements are provided for illustrative purposes only and are not intended to represent, and are not necessarily indicative of, what the operating results or financial position of the Company would have been had the Merger and the Private Placement Transaction been completed on the dates indicated, nor are they necessarily indicative of the Company’s future operating results or financial position. The pro forma financial statements do not reflect the impacts of any potential operational efficiencies, asset dispositions, cost savings or economies of scale that the Company may achieve with respect to the combined operations. Additionally, the pro forma statement of operations does not include non-recurring charges or credits which result directly from the transactions.
| 74 |
The pro forma financial statements have been prepared using the acquisition method of accounting in accordance with Accounting Standards Codification (“ASC”) 805, “Business Combinations.” ASC 805 requires, among other things, that assets acquired and liabilities assumed be recognized at their fair values, as determined in accordance with ASC 820, “Fair Value Measurements,” as of the acquisition date. For certain assets and liabilities, book value approximates fair value. In addition, ASC 805 establishes that the consideration transferred be measured at the closing date of the asset acquisition at the then-current market price, which may be different than the amount of consideration assumed in the pro forma financial statements. Under ASC 805, acquisition-related transaction costs (i.e., advisory, legal, valuation, other professional fees) and certain acquisition-related restructuring charges impacting the target company are expensed in the period in which the costs are incurred.
As of the date of this Proxy Statement, Baxano’s assets and liabilities are presented at their historical value, and pro forma adjustments have been made to reflect these assets and liabilities at their preliminary estimated fair values, with the excess of the purchase price over the sum of these fair values presented as goodwill. The actual adjustments to the Company’s consolidated financial statements on the closing date of the Merger and the Private Placement Transaction and the related allocation of the merger consideration will depend on a number of factors, including additional financial information available at such time, changes in the fair value of the Company’s common stock transferred in connection with the Merger and the Private Placement Transaction, changes in the estimated fair value of Baxano’s assets and liabilities as of the closing date and changes in Baxano’s operating results between the date of preparation of the pro forma financial statements and the closing date. The valuations of acquired assets and liabilities are in process and are not expected to be finalized until later in 2013, as information may become available within the measurement period which indicates a potential change to these valuations, including the value of the net assets on the closing date of the Merger. Accordingly, the final allocations of and the effects on the results of operations may differ materially from the preliminary allocations and unaudited pro forma combined amounts included herein. The Company intends to engage valuation specialists to assist in the determination of the final allocation of fair value. The final determination of the acquisition consideration allocation is anticipated to be completed as soon as practicable after the completion of the transaction. Any change in the amounts allocated to amortizable identifiable intangible assets will have an impact on amortization expense on the statement of operations.
Under the acquisition method of accounting, the Baxano assets acquired and liabilities assumed will be recorded as of the consummation of the Merger, primarily at their respective fair values, and added to those of the Company. Baxano’s results of operations will be consolidated with the Company’s results of operations beginning on the closing date, and the Company’s results of operations prior to the closing date will not be retroactively restated to reflect Baxano’s results of operations.
3. Accounting Policies
Upon consummation of the Merger, the Company will perform a detailed review of Baxano’s accounting policies. As a result of that review, it may become necessary to conform the combined company’s financial statements to be consistent with those accounting policies that the Company determines are more appropriate for the combined company. To date, the Company has not identified any significant differences in accounting policies.
4. Preliminary Estimated Purchase Consideration
The following table reflects the preliminary estimated merger consideration (in thousands, except percentages and per share amounts):
| Estimated number of shares to be issued (1) | 10,393 | |||
| Estimated fair value per share (2) | $ | 2.22 | ||
| Estimated fair value of shares to be issued (3) | $ | 23,072 | ||
| Cash merger consideration in lieu of shares (4) | 550 | |||
| Total estimated fair value of consideration transferred | $ | 23,622 |
| 75 |
(1) Represents the number of shares of the Company’s common stock outstanding on March 1, 2013, the last trading day prior to the Company’s and Baxano’s entry into the Merger Agreement and the Securities Purchase Agreement, multiplied by 38.888889%, as set forth in the Merger Agreement, as adjusted for cash payments (a) to fund expenses of the Securityholder Representatives and (b) under the Transaction Payment Plan
(2) Represents the closing price of the Company’s common stock on The NASDAQ Stock Market LLC on March 1, 2013
(3) Represents the estimated number of shares to be issued multiplied by the estimated fair value per share
(4) Represents payments of (a) $250,000 to fund expenses of the Securityholder Representatives and (b) $300,000 under the Transaction Payment Plan
The preliminary estimated merger consideration may change based on changes in (i) the number of shares of common stock the Company is required to issue as merger consideration and (ii) the value of those shares. Pursuant to the Merger Agreement, the number of shares of the Company’s common stock the Company is required to issue is based on a fixed percentage of the number of shares of the Company’s common stock outstanding immediately prior to the Effective Time, subject to downward adjustments for the $250,000 Securityholder Representative Fund and $300,000 for the Transaction Payment Plan. The actual amount of these reductions on the number of shares issued will be based on the average of the last reported sales prices of the Company’s common stock at 4:00 p.m., Eastern Time, end of regular trading hours on The NASDAQ Stock Market LLC during the ten consecutive trading days ending on the last trading day prior to the consummation of the Merger. Therefore, the number of shares of common stock the Company is required to issue as merger consideration may change based on changes in (i) the number of shares of the Company’s common stock outstanding immediately prior to the Effective Time and (ii) the closing prices of the Company’s common stock.
The final merger consideration will be based on the fair value of the shares actually issued, which under ASC 805 and ASC 820 will be based on the closing market price of the Company’s common stock on the closing date. Accordingly, a 10% change in the 10-day average from $2.377 per share on March 1, 2013 would have the effect of changing the number of shares issued by 25,709 shares, which would change the fair value of the merger consideration by $57,000 based on the March 1, 2013 closing price of $2.22 per share. In addition, even if there are no changes in estimated number of shares to be issued in connection with the Merger, a 10% change in the closing market price of the Company’s common stock from the $2.22 per share closing price on March 1, 2013 would have the effect of changing the fair value of the merger consideration by $2.3 million, with a corresponding change in the amount of goodwill.
The Merger Agreement also provides that the purchase price is subject to further adjustment based on adjustments for (i) working capital, (ii) excess indebtedness and (iii) unpaid Baxano transaction expenses that are required to be paid by the Company, including transaction expenses related to any brokers, financial advisors, consultants, accountants, attorneys, or other professionals engaged by Baxano in connection with the transactions contemplated by the Merger Agreement or the Securities Purchase Agreement. Such adjustments could result in the issuance of additional shares of common stock or the return of shares of common stock previously issued. For purposes of the pro forma financial statements, the Company has not assumed that there will be any adjustments with respect to these matters. Any such required adjustments would have the effect of changing the fair value of the merger consideration, with a corresponding change in the amount of goodwill.
5. Preliminary Purchase Price Allocation
The following table summarizes the preliminary purchase price allocation based on estimated fair values as if the Merger had been consummated on December 31, 2012 (in thousands):
| Cash | $ | 4,409 | ||
| Accounts receivable, net | 1,773 | |||
| Inventory | 1,994 | |||
| Prepaid expenses and other assets | 377 | |||
| Property and equipment, net | 778 | |||
| Identifiable intangible assets | 11,836 | |||
| Other assets | 221 | |||
| Accounts payable | (708 | ) | ||
| Accrued expenses | (1,274 | ) | ||
| Notes payable | (3,017 | ) | ||
| Deferred rent | (164 | ) | ||
| Total identifiable net assets | 16,225 | |||
| Goodwill | 7,397 | |||
| Total fair value of consideration | $ | 23,622 |
| 76 |
6. Pro Forma Adjustments
Pro forma adjustments reflect those matters that are a direct result of the Merger and Securities Purchase Agreement, which are factually supportable and, for pro forma adjustments to the pro forma statement of operations, are expected to have continuing impact. The pro forma adjustments are based on preliminary estimates that may change as additional information is obtained. Given the historical net loss positions of both companies and the full valuation allowances applied to the deferred tax assets at December 31, 2012, there is no expected impact of these adjustments on the pro forma balance sheet or statement of operations.
Adjustments to the pro forma balance sheet:
| (a) | Represents (i) $250,000 that the Company has agreed to deposit into an account specified for the purpose of funding the expenses of the Securityholder Representatives; (ii) $300,000 that the Company has agreed to fund for the Transaction Payment Plan; (iii) $1.4 million of estimated transaction costs related to the Merger that were not previously reflected in the historical financial statements; and (iv) $150,000 for the commitment fee to be paid to replace the Baxano note payable with a new note payable with the same lender. |
| (b) | Represents the preliminary allocation of the fair value of the consideration transferred to the acquired intangible assets of Baxano based on their estimated fair values. Intangible assets that have been identified in this preliminary allocation include: (i) technology, (ii) customer relationships, (iii) in-process research and development and (iv) trade names. |
| (c) | Represents the difference between the estimated purchase price and the estimated fair values of the identifiable assets acquired and liabilities assumed. |
| (d) | Represents the payoff of Baxano’s convertible debt, including accrued interest, pursuant to the Merger Agreement. |
| (e) | Represents an adjustment to the notes payable to appropriately classify them based on the proposed term sheet from the lender. |
| (f) | The following pro forma adjustments represent the effects of eliminating Baxano’s equity accounts and issuing TranS1 shares pursuant to the Merger Agreement, and consist of: |
| (i) the elimination of Baxano’s warrant liability | $ | (131 | ) | |
| (ii) the elimination of Baxano’s convertible preferred stock | (58,339 | ) | ||
| (iii) the elimination of the par value of Baxano’s common stock | (7 | ) | ||
| the par value of TranS1’s shares to be issued pursuant to the Merger | 1 | |||
| net change in common stock | (6 | ) | ||
| (iv) the elimination of Baxano’s paid-in capital | (1,534 | ) | ||
| paid-in capital for TranS1’s shares to be issued pursuant to the Merger | 23,071 | |||
| 21,537 | ||||
| (v) the elimination of Baxano’s accumulated deficit | 70,390 | |||
| estimated Merger transaction fees to be expensed | (1,444 | ) | ||
| short-term debt commitment fee to be expensed | (150 | ) | ||
| 68,796 |
| (g) | Represents the gross proceeds from the Private Placement Transaction of $17,150,000, less estimated transaction costs of $170,000. |
Adjustments to the pro forma statement of operations:
| (h) | Represents the amortization of intangible assets acquired in the Merger based on their estimated fair values and useful lives. Estimated useful lives of the intangible assets range from one to ten years and amortization expense has been calculated on a straight-line basis. |
| (i) | Represents the reduction of interest expense related to the conversion of Baxano’s debt in connection with the Merger. |
| (j) | Represents the impact of issuance of approximately 10,393,000 shares in connection with the Merger. | |
| (k) | Represents the impact of issuance of approximately 10,393,000 shares in connection with the Merger and 7,522,000 shares in connection with the Private Placement Transaction. |
| 77 |
INFORMATIONABOUT TRANS1
Businessof TranS1
Overview
We are a medical device company focused on designing, developing and marketing products to treat degenerative conditions of the spine affecting the lumbar region. We are committed to delivering minimally invasive surgical technologies that enhance patient clinical care while providing sustained value for our customers. We currently market the AxiaLIF® family of products for single and two level lumbar fusion, the VEOTM lateral access and interbody fusion system, the Vectre™ lumbar posterior fixation system and Bi-OsteticTM bone void filler, a biologics product. Our AxiaLIF products use our pre-sacral approach, through which a surgeon can access discs in the lumbar region of the spine through an incision adjacent to the tailbone and perform an interbody fusion procedure through instrumentation that provides direct access to the intervertebral space. We developed our pre-sacral approach to allow spine surgeons to access and treat intervertebral spaces without compromising important surrounding soft tissue, nerves and bone structures. Our VEO lateral access and interbody fusion system provides for direct visualization of the psoas muscle and unrestricted lateral fluoroscopic views, which has allowed us to take market share in the highly competitive lateral fusion segment. We believe that direct visualization allows a surgeon to have improved visibility of the psoas and the nerves running through this muscle that, when used in conjunction with neuromonitoring, can potentially reduce complications. We also market products that may be used with our AxiaLIF surgical approach, including bowel retractors and additional discectomy tools, as well as a bone graft harvesting system that can be used to extract bone graft in any procedure for which the use of bone graft is appropriate. Our philosophy of continuous improvement is driven by ongoing research and development investment in our core technologies. We support this investment by diligently expanding, maintaining, and protecting our significant patent portfolio.
From our incorporation in 2000 through 2004, we devoted substantially all of our resources to research and development and start-up activities, consisting primarily of product design and development, clinical trials, manufacturing, recruiting qualified personnel and raising capital. We received 510(k) clearance from the FDA for our AxiaLIF Legacy product in the fourth quarter of 2004, and commercially introduced our AxiaLIF Legacy product in the United States in the first quarter of 2005. We received a CE mark to market our AxiaLIF Legacy product in the European market in the first quarter of 2005 and began commercialization in the first quarter of 2006. We received a CE mark for our AxiaLIF 2L product in the third quarter of 2006 and began commercialization in the European market in the fourth quarter of 2006. We received FDA 510(k) clearance for our AxiaLIF 2L product and began marketing this product in the United States in the second quarter of 2008. The AxiaLIF 2L product was discontinued in 2010 after we launched our AxiaLIF Plus 2-Level ™ product in July 2010, for which we had received FDA 510(k) clearance in January 2010. We commercially launched our next generation Vectre facet screw system in April 2010. In the first quarter of 2010, we began distributing Bi-Ostetic bone void filler, a biologics product. We commercially launched our AxiaLIF Plus 1-Level product in September 2011, for which we had received FDA 510(k) clearance in March 2011. In 2010, we received 510(k) clearance for our VEO lateral access and interbody fusion system, which was commercially launched in November 2011, and in July 2012 we received a CE mark for our VEO lateral access and interbody fusion system and began commercialization in the European market. We currently sell our products through a direct sales force, independent sales agents and international distributors.
As of December 31, 2012, over 13,000 fusion procedures have been performed globally using our AxiaLIF products. As of December 31, 2012, we sold our products through 30 direct sales personnel and 33 independent sales agents in the United States and 11 independent distributors and sales agents internationally. For the year ended December 31, 2012, our revenues were $14.6 million and our net loss was $29.9 million.
On March 3, 2013, we entered into an Agreement and Plan of Merger, or the Merger Agreement, with RacerX Acquisition Corp., a wholly-owned subsidiary of the Company, or Merger Sub, Baxano, Inc., or Baxano, and Sumeet Jain and David Schulte solely as Securityholder Representatives, or the Securityholder Representatives. Under the terms of the Merger Agreement, we will acquire Baxano through a merger of Merger Sub with and into Baxano, or the Merger. At the effective time of the Merger, Merger Sub will cease to exist and Baxano will continue as the surviving corporation and as a wholly-owned subsidiary of the Company. The dollar value of the shares of our common stock to be issued in connection with the Merger is expected to be approximately $23.1 million, subject to fluctuations in the price of our common stock on the NASDAQ Global Market and certain adjustments.
| 78 |
Baxano is a medical instrument company focused on designing, developing and marketing innovative tools that restore spine function, preserve healthy tissue, and enable a better quality of life for the patients it serves. Baxano currently markets the patented iO-Flex® system, a proprietary minimally invasive set of flexible instruments allowing surgeons to target lumbar spinal stenosis in all three regions of the spine: central canal, lateral recess, and neural foramen, and has developed the patented iO-TomeTM instrument to rapidly and precisely remove bone, including the facet joints, which is commonly performed in spinal fusion procedures. Baxano was founded in 2005 and is headquartered in San Jose, California. The Merger is expected to close in the second quarter of 2013 and is subject to TranS1 stockholder approval and customary conditions to closing.
Concurrent with entering into the Merger Agreement, we entered into a Securities Purchase Agreement, or the Securities Purchase Agreement, dated March 3, 2013, with certain investors (including certain of Baxano’s securityholders and certain of our directors and executive officers), or the Investors, pursuant to which we will sell to the Investors, and the Investors will purchase from the Company, an aggregate of 7,522,009 shares of our common stock, at a purchase price of $2.28 per share, resulting in gross proceeds to the Company of $17.2 million, or the Private Placement Transaction. Consummation of the Private Placement Transaction is subject to approval by the Company’s stockholders of the issuance of shares of our common stock in connection with the Merger Agreement and the Securities Purchase Agreement, the closing of the Merger, and other customary closing conditions set forth in the Securities Purchase Agreement.
Spine Anatomy
The human spine is the core of the human skeleton and provides important structural support while remaining flexible to allow movement. It consists of 33 separate interlocking bones called vertebrae that are connected by soft tissue and provide stability while facilitating motion. Vertebrae are paired into motion segments that move by means of two facet joints and one disc. The facet joints provide stability and enable the spine to bend and twist while the discs absorb pressures and shocks to the vertebrae. Nerves are contained in the spinal column and run through the foramen openings to the rest of the body.
The vertebrae are categorized into five regions: cervical, thoracic, lumbar, sacral and coccyx. The lumbar region, which is at the bottom of the spine and consists of five vertebrae, is capable of limited movement, and primarily functions as support for the body’s weight. The sacrum consists of five fused vertebrae labeled S1 through S5 directly below the lumbar region and provide attachment for the hipbones as well as protection to organs in the pelvic area. The cervical region is the uppermost portion of the spine (the neck), and consists of seven vertebrae. The thoracic region is the middle portion of the spine below the cervical spine and above the lumbar spine. This area consists of twelve vertebrae and includes the upper body and ribs. The coccyx, also known as the tailbone, is at the end of the spine.
Medical Conditions Affecting the Lumbar Spine and Traditional Treatment Alternatives
Degenerative conditions of the spine consist of indications such as spondylolisthesis, spinal stenosis and degenerative disc disease and are common medical conditions affecting the lumbar spine. These degenerative conditions can pinch the spinal canal and the nerves exiting the spine and result in back pain, leg pain, numbness and loss of motor function. This lower back and leg pain can be overwhelming for patients as it can have significant physical, psychological and financial implications.
Treatment alternatives for lumbar spine conditions range from non-operative conservative therapies to highly invasive surgical interventions. Conservative therapies are typically the initial treatments selected by patients and physicians and they include rest, bracing, physical therapy, chiropractics, electrical stimulation and medication. When conservative therapies fail to provide adequate pain relief, surgical interventions, including fusion procedures, may be used to address the pain.
Fusion procedures attempt to alleviate lower back and leg pain by removing problematic disc material and permanently joining together two or more opposing vertebrae. This is done in a manner that restores the appropriate space between the vertebrae surrounding the degenerative disc and eliminates mobility of the affected vertebrae. By restoring disc height and eliminating motion, fusion attempts to both stabilize the spine and prevent the pinching of the nerves exiting the spine, thereby reducing pain.
| 79 |
Traditional fusion procedures typically involve an incision in the skin, and cutting muscle or moving organs to gain access to the spine. The degenerated disc is then removed, a process referred to as a discectomy, and a rigid implant, such as a bone graft or cage, is inserted to stabilize the diseased vertebrae. This process is referred to as fixation. A combination of the patient’s bone and/or marrow (bone graft) is usually used to fill the voids in the cage. These materials are intended to promote the growth of bone between the vertebrae. Surgeons often also affix supplementary rods and screws along the spine to provide additional stabilization while the vertebrae fuse together during the six to eighteen months following surgery. The primary surgical fusion procedures performed in the lumbar region include: ALIF, PLIF, TLIF and direct lateral interbody fusion.
Anterior Lumbar Interbody Fusion, or ALIF. To perform an ALIF procedure, surgeons access the spine through an incision on the patient’s abdomen which provides them with direct access to the vertebral space for performing a discectomy and inserting bone grafts for fusion. Supporting soft tissue and nerves are manipulated or removed to accommodate the anterior access required by the ALIF procedure. Surgeons commonly perform ALIF procedures in conjunction with a general or vascular surgeon because critical vasculature and organs must be retracted to gain access to the spine. Complications associated with ALIF procedures include vascular damage to the vena cava and aorta.
Posterior Lumbar Interbody Fusion, or PLIF. To perform a PLIF procedure, surgeons access the spine through an incision on the center of the patient’s back. The surgeon then navigates through muscles and nerves to gain access to the spine. Once at the spine, the surgeon removes bone from the lamina to gain access to the affected disc space where a discectomy is performed and a bone graft is placed. When compared to ALIF procedures, PLIF procedures are generally considered easier to perform and can achieve better nerve root decompression in certain cases. However, the anatomy of the spine prevents surgeons from removing the entire degenerated disc and obtaining optimal access for insertion of an implant or bone graft. Complications associated with PLIF procedures include nerve damage, soft tissue damage and implant migration.
Transforaminal Lumbar Interbody Fusion, or TLIF. TLIF procedures are performed in a similar manner to PLIF procedures, except the surgeon accesses the spine through a small incision slightly to the left or right of the center of the patient’s back. After reaching the spine, the surgeon removes a portion of the facet joint and navigates through the foramen which provides better visualization and disc removal capabilities than PLIF. Complications associated with TLIF procedures are similar to those found in PLIF procedures including nerve damage, soft tissue damage and implant migration.
Direct Lateral Interbody Fusion. To perform a direct lateral procedure, the surgeon accesses the spine from the patient’s side through one or two small incisions. The direct lateral procedure is not appropriate for fusions in the L5/S1 segment because the pelvis interferes with access. Complications associated with direct lateral procedures are similar to those found in PLIF and TLIF procedures, including nerve damage and implant migration, and also include bowel injury.
Limitations of Traditional Lumbar Spine Procedures
While traditional fusion procedures, such as ALIF, PLIF and TLIF, can be effective at treating medical conditions affecting the lumbar spine, common drawbacks of these procedures may include:
| · | Disruption to Soft Tissue and Support Structures. Traditional lumbar fusion procedures require the creation of a pathway from the skin to the degenerative disc that is large enough to allow direct visualization and work by the surgeon. It is common to cut through healthy muscle or move critical organs, arteries, nerves and soft tissue, which can lead to bleeding, scarring, nerve damage and bowel disruption. |
| · | Significant Blood Loss. As a result of undergoing current lumbar fusion procedures, patients typically experience significant blood loss of between 100 and 1,400 cc of blood. As a result, it is common for patients to use the hospital’s blood supply or donate units of their own blood before a lumbar fusion procedure to replenish any significant blood loss. |
| · | Lengthy Operative Procedure Times. It is common for current lumbar fusion procedures to take between 90 minutes and 4 hours to complete. With some procedures, a second surgeon may be required. Long procedure times increase the risks of complications and blood loss. Also, hospital and physician resources are consumed for lengthy periods of time, which can reduce productivity and increase costs. |
| 80 |
| · | Lengthy Patient Hospital Stays. Patients remain in the hospital for an average of three days following a lumbar fusion procedure. |
| · | Significant Patient Recovery Time. Patients typically require three to six months to recover and rehabilitate after undergoing a lumbar fusion procedure before resuming normal activities. |
| · | Unresolved Patient Pain. Patients may continue to experience symptoms after undergoing lumbar fusion procedures even though x-rays show successful fusion has been achieved through the growth of new bone. |
Our Solutions
AxiaLIF Products
We have developed what we believe is a less invasive approach for surgeons to perform fusion surgeries in the L4/L5/S1 region without many of the drawbacks associated with other lumbar fusion procedures. We refer to this proprietary approach as our pre-sacral approach. We have developed and are marketing a range of fusion products that are delivered using our pre-sacral approach: AxiaLIF Legacy, AxiaLIF Plus 1-Level, AxiaLIF Plus 2-Level and AxiaLIF Non-Distracting.
To access the spine using our pre-sacral approach, the surgeon creates an incision adjacent to the tailbone while the patient is lying on their stomach. The surgeon then navigates a blunt dissecting tool a short distance along the sacrum using x-ray imaging technologies. As the dissecting tool is advanced, it moves aside soft tissue structures such as fat and loose connective tissue surrounding the anterior sacrum. When the tool reaches an access point near the junction of the S1 and S2 vertebral bodies, a guide pin is inserted through the bone into the disc of the lowest lumbar motion segment (the L5/S1 disc) where the fusion procedure is then performed. A tubular dissector is inserted over the guide pin to create a tissue-protecting working channel between the surgical access site and the L5/S1 disc. This protected working channel provides access to the interior of the disc, where rotating cutters, brushes and rasps are used to remove disc material. This is followed by the introduction of bone graft material with special instrumentation and finally, insertion of our AxiaLIF implants. The implants immediately provide rigid fixation and can provide restoration of disc height based on the clinical pathology of the patient. The AxiaLIF Plus 2-Level procedure uses the same approach to provide access to both the L5/S1 and L4/L5 discs. The AxiaLIF Plus 1-Level and AxiaLIF Plus 2-Level procedures must be supplemented with facet or pedicle screws, which provide fixation for the back of the spine and are supplied by TranS1.
We believe our pre-sacral approach and its associated products provide the following benefits for patients, providers and payors:
| · | Less Invasive Approach Minimizes Complications. Our AxiaLIF products are delivered using our proprietary pre-sacral approach, which we believe is a less invasive solution for delivering fusion products to the L4/L5/S1 region. Procedures performed utilizing our pre-sacral approach have been documented to have favorable clinical safety profiles with low complication rates. In a recently published peer reviewed clinical paper, complication rates from procedures utilizing our pre-sacral approach were shown to be 1.3%. See additional information under the heading “Clinical Experience” below. The most frequent serious complication reported was bowel injury at 0.6% of cases with no reports in this study of permanent injury. |
| · | Spinal Stability. Our approach does not violate or cut through the muscles, ligaments or bones that control the stability of the spine. Thus, the axial approach to the spine does not affect inherent spinal stability, a benefit that cannot be achieved with other approaches to interbody fusion. Moreover, in another recent peer-reviewed retrospective clinical series, the fusion rate associated with procedures utilizing our pre-sacral approach was demonstrated to be 94%. We believe this compares favorably with the fusion rates of 90-95% associated with other fusion procedures. See additional information under the heading “Clinical Experience” below. |
| 81 |
| · | Cost Effectiveness.Increased focus on health care quality and value has brought greater scrutiny than ever on the clinical and economic impact that technologies have on different stakeholders including patient, provider and payor. Various government and commercial programs, including payment bundling, accountable care organizations and value-based purchasing, now incentivize providers to deliver quality care in a cost-effective manner. We believe that pre-sacral interbody fusion employing our AxiaLIF device is well positioned in the current health economic climate based on its evidence of good clinical outcomes combined with a favorable economic profile. In addition to low morbidity, there are procedural efficiencies associated with pre-sacral interbody fusion that can potentially reduce costs borne by the provider and/or payor. A recently submitted analysis by a leading university researcher estimated a cost savings of approximately $4,500 associated with the pre-sacral interbody fusion procedure's short length of stay and reduced complication rate compared to traditional lumbar fusion procedures. Traditionally, surgeon-decision makers weighted a technology's clinical profile more heavily than hospital economic considerations; however with the rapid growth in surgeons seeking employment by hospitals, we believe that economics will be a more important driver of medical technology adoption going forward. We believe that this will provide an opportunity for us to differentiate AxiaLIF from the traditional approaches to lumbar interbody fusion based on quality outcomes combined with positive impact on payor and provider economics. |
VEO Lateral Access and Interbody Fusion System
Our VEO Lateral Access and Interbody Fusion System provides for direct visualization during lateral fusion surgery and is indicated for spinal fusion procedures in skeletally mature patients with degenerative disc disease at one or two contiguous levels from L2-S1. Through a combination of direct visualization of the psoas muscle and unrestricted lateral fluoroscopic views, the VEO Lateral System offers visualization of the operative site. The VEO direct visualization approach was designed to help minimize iatrogenic trauma to the psoas muscle and the nerve plexus to help reduce the risk of post-operative complications.
Our VEO Lateral System includes a radiolucent tubular retractor that was designed to prevent soft tissue intrusion. It facilitates the first of two retraction stages, providing direct visualization of the psoas muscle and associated nerves before dissection of the psoas muscle. This intermediary step allows the surgeon to augment neuromonitoring information with direct visualization and was designed to help avoid the nerves when dissecting through the psoas muscle to the operative site.
The VEO’s retractor system features controlled retraction in the anterior/posterior plane via a muscle-splitting and muscle-sparing approach. The retractor system may reduce muscle disruption and is designed to eliminate the migration of muscle into the surgeon’s working area. The VEO Lateral System offers a comprehensive portfolio of interbody implants in both parallel and lordotic angles to better match each patient’s anatomy. The VEO PEEK interbody implants contain 5 tantalum markers to enable fluoroscopic visualization of placement.
The VEO Lateral System provides a complete discectomy and endplate preparation instrument set. Designed specifically for a lateral approach, these instruments are dimensionally optimized for operative visualization and controlled surgical manipulations.
Our Strategy
Our goal is to become a global leader in minimally invasive treatments for conditions affecting the lumbar region of the spine. To achieve this goal, we are pursuing the following strategies:
| · | Establish our Pre-Sacral Approach as a Standard of Care for Lumbar Spine Surgery. We believe patients commonly avoid spine fusion surgery due to its invasive nature and other drawbacks associated with other surgical treatment options. We expend significant resources promoting our pre-sacral approach as a less invasive approach to back fusion surgery, and we believe the advantages of our technique will enable our AxiaLIF products to become a standard of care for the lumbar region of the spine. We also make substantial research and development expenditures to enhance our AxiaLIF products and develop new products to expand our technological advantage in minimally invasive spine surgeries. |
| · | Drive Reimbursement for the AxiaLIF Procedure.As a result of our efforts over the past two years, a new Category I Medicare reimbursement code was established on January 1, 2013 for pre-sacral access that is applicable for our AxiaLIF procedures. We have also demonstrated success in obtaining reimbursement coverage from private insurance companies through our advocacy efforts. We plan to continue to work with our surgeon customers to generate published, peer reviewed clinical literature that demonstrates our AxiaLIF procedure’s clinical efficacy and safety. The clinical papers discussed below under the heading “Clinical Experience” are examples of our continued efforts towards the execution of this strategy. Working with surgeons that perform AxiaLIF procedures, we will continue our effort to leverage this data with payors to secure positive coverage decisions for the surgeon reimbursement portion of the AxiaLIF procedure (hospital reimbursement for AxiaLIF already exists). |
| 82 |
| · | Focus our Sales and Marketing Infrastructure to Drive Surgeon Adoption of our Products. We intend to continue expending significant resources targeting spine surgeons through our sales and marketing efforts in the United States and internationally in order to drive the adoption of our pre-sacral and direct lateral approaches. |
| · | Opportunistically Pursue Acquisitions of Complementary Businesses and Technologies. In addition to building our internal product development efforts, we intend to selectively license or acquire complementary products and technologies that we believe will enable us to leverage our focus on minimally invasive spine products and our sales and marketing infrastructure. |
| · | Expand Our Presence in the Direct Lateral Interbody Fusion Market. We intend to support the growing adoption of our VEO Lateral System through focused surgeon training programs, expanded sales representative training and marketing promotion. |
Clinical Experience
As of the date of this Proxy Statement, over 13,000 procedures have been performed using our pre-sacral approach by more than 500 surgeons. This amount includes over 11,000 procedures using AxiaLIF Legacy or AxiaLIF Plus 1-Level, with the remainder being implants using AxiaLIF 2L or AxiaLIF Plus 2-Level. Our AxiaLIF Legacy and AxiaLIF Plus 1-Level products have been the subject of a significant number of clinical evaluations and medical publications. We believe that more than 69 articles have now been published about the clinical experience associated with utilizing our AxiaLIF products. The results of three published articles are summarized below:
| · | In September 2011, an article was published in theSAS Journal (“Complications with Axial Presacral Lumbar Interbody Fusion: A Five-Year Postmarket Surveillance Experience,” Gundanna, et al) that discusses the outcome of a retrospective clinical study involving 9,152 patients that had previously undergone procedures using our pre-sacral approach. The publication demonstrated that the complication rate arising from lower lumbar fusion utilizing our pre-sacral approach was approximately 1.3%. This complication rate characterizes early onset complications, and does not include cases of revision or re-operations that may occur months or years later. Reflective of a greater than 90% published fusion rate for AxiaLIF, the overall complications are quite low in comparison to traditional interbody fusions such as ALIF or TLIF, which are in the 5% to well over 15% range in published literature. The most frequent serious complication reported was bowel injury at 0.6% of cases with no reports in this study of permanent injury. |
| · | In September 2011, a separate article was published in Spine (“Minimally-invasive Axial Pre-sacral L5-S1 Interbody Fusion: Two Year Clinical and Radiographic Outcomes,” Tobler, et al) that discusses the outcome of a retrospective clinical series involving 156 patients in four separate medical centers. The publication demonstrated an approximately 63% improvement in patient pain two years after surgery compared to immediately prior to the procedure. In addition, the article discusses that, two years after surgery in patients receiving a fusion procedure utilizing our pre-sacral approach, there was a 54% improvement in the Oswestry Disability Index, or ODI, a commonly used measure for levels of disability in daily living activities. The article also reports that the procedures utilizing our pre-sacral approach demonstrated a 94% fusion rate. We believe this rate compares favorably with the fusion rates of 90-95% associated with other fusion procedures as reported in other studies. The article further concludes that, for the evaluated population, our pre-sacral approach did not result in any vascular or neural injury. In addition, while we have received reports of bowel injury associated with our pre-sacral approach, no bowel injuries were reported in this article. Not all patients have the same experience when treated with AxiaLIF. |
| · | In April 2012, an article was published in theJournal of Spinal Disorders and Techniques (“Axial Presacral Lumbar Interbody Fusion and Percutaneous Posterior Fixation for Stabilization of Lumbosacral Isthmic Spondylolisthesis,” Gerszten, et al) that discusses the outcome of a retrospective case series of 26 patients with Grade 1 or 2 spondylolisthesis. The publication demonstrated a 66% improvement in pain at 2 years post-surgery compared to baseline. Additionally, patients reported 81% excellent/good results based on the Odom’s criteria. The Odom’s criteria is a subjective scale that rates patient outcomes as excellent, good, fair or poor. Lastly, the publication reported a solid fusion rate of 100% for all patients at 2 years post-surgery. The article concludes that the presacral axial interbody fusion and posterior instrumentation technique is a safe and effective treatment for low-grade isthmic spondylolisthesis. |
| 83 |
A literature review is a critical and in-depth evaluation of previous research. In 2011 and 2012 there were two such publications that reviewed the clinical safety and effectiveness of the AxiaLIF system. Additionally, a third review article (which has been submitted for publication) focuses on the health economics of the AxiaLIF system compared to TLIF. These reviews are outlined below:
| · | In August 2011 Rapp, et al in Medical Devices: Evidence and Research (“AxiaLIF System: minimally invasive device for presacral lumbar interbody spinal fusion”) reviewed AxiaLIF data from three separate sites. In his review of 120 patients, there was a reported complication rate of 0-3%, which is comparable to the Gundanna paper (“Complications with Axial Presacral Lumbar Interbody Fusion: A Five-Year Postmarket Surveillance Experience,” SAS Journal, Sept 2011) that reported a complication rate of 1.3% in 9,152 patients. Rapp et al also reported fusion rates of 85-93%. These are comparable to Durrani, et al in Techniques in Orthopaedics (“Presacral Approach for L5-S1 Fusion,” 2011) who noted an overall fusion rate of 88-94% in his review. |
| · | In regards to clinical effectiveness, Durrani’s literature review (Techniques in Orthopaedics) demonstrated significant improvements in pre-operative pain and function levels compared to post-operative levels. The Visual Analog Scale, or VAS, used to denote patient pain, showed a 41-63% reduction in pain post-operatively, and ODI scores, used to calculate a patient’s functional status, improved overall by 50-54%. |
| · | In 2012, a systematic literature review was performed on the health economics of the AxiaLIF system. The findings showed that the axial pre-sacral approach was associated with decreases in blood loss, operative times, length of hospital stay, and resulted in reduced surgical morbidity, all of which are factors that drive healthcare costs. These findings are mirrored in the review conducted by Durrani, et al. Cost estimation suggests that these benefits of minimally invasive fusion can be expected to result in significant cost savings for hospitals and payors. An estimated realization of cost savings averaged approximately $4,500 per patient over open TLIF for single-level fusion for degenerative spine disease. This paper has been submitted toWorld Neurosurgery. |
In August 2012, we completed our first retrospective clinical study comparing AxiaLIF to ALIF. The primary objective of the study was to compare fusion rates and reported complication between the two procedures. There were 48 subjects in each cohort with a minimum of two-year follow-up. The overall fusion was 85% in the AxiaLIF cohort compared to 79% within the ALIF cohort. The complication rate was higher in the ALIF group, who additionally reported one serious adverse event that necessitated significant fluid resuscitation. This paper has been accepted for publication byThe Journal of Spinal Disorders and Techniques and has been accepted for a podium presentation at the North American Spine Society Summer Meeting in July 2013.
Our Products
We currently market the AxiaLIF family of products for single and two level lumbar fusion, the VEO lateral access and interbody fusion system, the Vectre posterior fixation system and Bi-Ostetic bone void filler, a biologics product. We also market products that may be used with our AxiaLIF surgical approach, including bowel retractors and additional discectomy tools, as well as a bone graft harvesting system that can be used to extract bone graft in any procedure for which the use of bone graft is appropriate.
| · | AxiaLIF Lumbar Fusion Implants. Our AxiaLIF products include surgical instruments designed to create a safe and reproducible access route to the L4/L5/S1 vertebral bodies, fusion implants and supplemental stabilization products. We believe our AxiaLIF implants and instruments, combined with facet screws or pedicle screws, provide surgeons with the tools necessary to perform a lumbar fusion in a less invasive manner. We sell these products to our customers in procedure kits that include all the instruments and implants needed to complete a lumbar fusion. |
Our AxiaLIF Plus 1-Level, 2-Level and Non-Distracting implants are threaded titanium anchors, that come in varying lengths to enable one-level L5/S1 fusions and two-level L4/L5/S1 fusions. As they are implanted, our proprietary design can allow for the separation or distraction of the vertebrae to restore disc height based on the clinical pathology of the patient. The increased disc height relieves pressure on the nerve, while the rod itself provides immediate rigid fixation.
| 84 |
Our pre-sacral approach requires the use of a sterile set of surgical instruments that are used to create a working channel and to prepare the disc and vertebrae for our implant. The instrumentation contained in the set includes stainless steel navigation tools and tubular dissectors to create the working channel, as well as nitinol cutters and brushes to cut and remove the degenerated disc material and prepare the disc space for our implant and the bone graft material.
| · | VEO Lateral Access and Interbody Fusion System. In November 2011, we launched our VEO Lateral Access and Interbody Fusion System. This minimally invasive direct lateral system features a two-stage retraction method that focuses on nerve visualization followed by muscle retraction. The VEO Lateral System is designed for direct visualization of the psoas muscles and adjacent nerves prior to muscle dissection, and features a full range of PEEK lateral interbody implants and a variety of ergonomic instruments. |
| · | Vectre Facet Screw System. Our Vectre facet screw system offers a cannulated facet screw inserted over a guidewire to provide stability. The Vectre system features are designed to offer a minimally invasive posterior fixation option in select patients. |
| · | Bi-Ostetic Bone Void Filler.In February 2010, we began selling Bi-Ostetic, a synthetic, osteoconductive material intended to be used to fill voids and gaps that are not intrinsic to the stability of bone structure. These gaps or voids may be located in the extremities, spine, or pelvis. In spine surgery, it can be used to fill a void in the sacrum or as a substitute or adjunct to autograft or allograft during posterolateral fusion. Bi-ostetic is not indicated for use in the interbody space during interbody fusion procedures or in load bearing applications. |
| · | Iliac Crest Bone Graft Harvesting System. Our Iliac Crest Bone Graft Harvesting System was developed to aid surgeons in harvesting iliac crest autograft via a minimally invasive approach. Fusion success is dependent on a proper disc preparation technique, including a thorough discectomy with removal of the cartilaginous endplate and nuclear material. Use of autograft, which is osteogenic, osteoinductive and osteoconductive, further improves the chances of fusion success. Because it is harvested from the patient, it eliminates risk of disease transmission. It provides structural support as well as scaffolding for new bone growth. |
Product Pipeline
Our product development efforts are currently focused on pursuing improvements to enhance our current product lines and to also pursue products that have a short pathway to regulatory clearance and commercialization. We are developing a minimally invasive pedicle screw system that will deliver a simple, complete solution for posterior fixation in the lumbar spine. In 2012, we received FDA 510(k) clearance for a TLIF cage system that we plan to develop in the next two years.
In the future, we expect our product offerings will be expanded to address additional clinical applications in the surgical treatment of conditions affecting the lumbar spine with an emphasis on minimally invasive approaches. Such applications could require FDA 510(k) clearance or a PMA, most likely supported by safety and efficacy data from clinical trials. We also have an active program aimed at developing tools that will improve outcomes and lower complications for our procedures.
Market Opportunity
Our management believes that approximately 240,000 lumbar spinal fusion procedures will be performed in the United States in 2013, of which approximately 190,000, or 79%, are applicable to our AxiaLIF and/or VEO systems. Based on our current product offerings and mix, this represents a $2.5 billion addressable market. With the addition of our planned pedicle screw system, and further penetration of our ancillary products, such as bone void filler and bone graft harvestors, the potential market exceeds $4 billion.
| 85 |
Sales and Marketing
Our sales and marketing effort primarily targets spine surgeons. We also market our products at various industry conferences and through industry organized surgical training courses.
In the United States, we market and sell our products through a combination of direct sales representatives and independent sales agents. As of December 31, 2012, our U.S. sales team included area vice-presidents, regional managers, direct sales representatives, case coverage specialists and independent sales agents covering specific geographic regions. Our sales representatives receive a base salary and a percentage of the net sales that they generate. As of December 31, 2012, we had 26 direct sales representatives, one case coverage specialist, three regional managers and two area vice-presidents. The independent sales agents are compensated based on a percentage of the net sales that they generate. We have agreements with our independent sales agents that provide them with an exclusive right to sell our products in their territories, which are generally terminable upon 90 days’ written notice.
Outside of the United States, we utilize independent sales distributors and sales agents. Through December 31, 2012, the majority of our international sales have been to distributors in Europe. In 2008, we hired direct sales representatives in Germany, and in 2009 we began direct sales through our own sales representatives and agents in Germany, Switzerland, Netherlands and Belgium. Effective January 1, 2012, we replaced our direct sales representatives in Europe with an independent sales agent, comprised of former employees of our direct sales organization, representing Germany, Netherlands, and Belgium. In January 2013, we terminated this sales agent and are transitioning to other distribution channels in these regions. In October 2012, we signed an agreement with Jiade Sunshine to distribute our products in China, and we recorded $1.0 million of revenue in the fourth quarter of 2012 for sales to this distributor. In 2012, 80% of our international revenues were through third-party distributors and 20% were through independent sales agents.
In 2012, no customer accounted for 10% or more of revenues.
Surgeon Training
We devote significant resources to training and educating surgeons on the specialized skills involved in the proper use of our instruments and implants. We believe that the most effective way to introduce and build market demand for our products is by training spine surgeons in the use of our products. We accomplish our training objectives primarily through cadaver and surrogate models and live case observations with surgeons experienced in our pre-sacral approach. We supplement this training with online didactic tutorials. After this training, surgeons are generally able to perform unsupervised surgeries using our pre-sacral approach. As of December 31, 2012, we had trained over 1,500 U.S. spine surgeons and over 300 surgeons outside of the U.S. in the use of our single-level product. In addition, we have trained over 500 U.S. spine surgeons in the use of our AxiaLIF Plus 2-Level and 2L products. Of the U.S. surgeons trained on our pre-sacral approach, approximately 130 have performed a procedure in the 12 months ended December 31, 2012. Through December 31, 2012, we have also trained over 190 U.S. spine surgeons in our VEO lateral access and interbody fusion system and 20 surgeons outside the U.S. Of the U.S. surgeons trained on our VEO lateral access and interbody fusion system, approximately 50 have performed a procedure in the 12 months ended December 31, 2012. We believe we have the necessary capacity to train a sufficient number of surgeons to meet our current goals.
In February 2012, we opened a training facility in Raleigh, N.C., which has a state-of-the art operating room unit with a fully equipped surgical suite. The facility provides cadaveric capabilities to allow hands-on training for spine surgeons and our staff.
Third-Party Reimbursement
In the United States, healthcare providers generally rely on third-party payors, principally private insurers and governmental payors such as Medicare and Medicaid, to cover and reimburse all or part of the cost of a spine fusion surgery in which our medical device is used. Surgeons are reimbursed for performing the surgical procedure, while hospitals are reimbursed for the cost of the device, all patient care related to the fusion procedure and the overhead associated with maintaining the facility.
| 86 |
Most payors follow Medicare's Diagnosis-Related Group, or DRG, based payment system for reimbursing facilities. Under this model, hospitals are paid a set amount to cover the costs associated with a fusion patient, including the cost of the device used in the procedure. The most commonly associated DRGs for spinal fusion are 453/454/455 (“Combined Anterior/Posterior Spinal Fusion with major complications and comorbidities (MCC), with complications and comorbidities (CC) or without MCC/CC”) and 459/460 (“Spinal Fusion Except Cervical with or without MCC”). Private payors typically use Medicare DRGs as a benchmark when setting their own reimbursement rates for facilities but typically pay above Medicare established rates.
Surgeons use the AMA’s CPT system to bill payors for their service in performing the AxiaLIF and VEO procedures. CPT codes describe the services and procedures provided for patients to third-party payors so that physicians may be reimbursed. For CPT coding purposes, our VEO procedure is considered an anterior lumbar interbody fusion, for which a Category I code has existed for some time. Effective January 1, 2009, the AMA implemented a Category III code which describes the work involved in treating AxiaLIF patients. Unlike Category I CPT codes, Category III codes do not have a set value which physicians use as a benchmark for setting their fee. Additionally, some payors view Category III codes as “investigational” or “experimental” and may not reimburse them. However, unlike many new or novel procedures, AxiaLIF is an access variation on the current standard of care (interbody spinal fusion) and has been performed in over 13,000 U.S. procedures. The implementation of a Category III code for our AxiaLIF products, together with a broad reduction in payor approval for spinal fusion procedures beginning in 2009, resulted in a period of persistent decline in our revenues. With the establishment of a Category I code effective January 1, 2013, we believe that we are in a position to transition to a period of sustainable revenue growth.
In March 2012, we announced that the Current Procedural Terminology Editorial Panel, or the Panel, voted to approve an application for a new Category I CPT code, 22586, for L5/S1 spinal fusion utilizing our AxiaLIF implant when performing a pre-sacral interbody fusion. In addition, the Panel voted to establish a new Category III CPT code, 0309T, as an add-on code to the new Category I code for use with 22586 when performing L4/5 spinal fusion. The new CPT codes were announced on the AMA’s website on March 2, 2012, and became effective on January 1, 2013. The Medicare final rule was released in November 2012, which stated a valuation of the Category I CPT code 22586 for pre-sacral interbody single level spinal fusion at L5-S1, and became effective January 1, 2013. This CPT code, which applies to our AxiaLIF Plus 1-Level device, is a bundled lumbar arthrodesis procedure that includes bone graft, posterior instrumentation and fixation.
Internationally, reimbursement and healthcare payment systems vary substantially from country to country and include single-payor, government-managed systems as well as systems in which private payors and government-managed systems exist side-by-side. Our ability to achieve market acceptance or significant sales volume in international markets we enter will depend in large part on the availability of reimbursement for procedures performed using our products under the healthcare payment systems in such markets. There is favorable reimbursement in Germany for the AxiaLIF Legacy procedure. We also received a favorable ruling from the British United Provident Association, the leading provider of private health insurance and healthcare services in the United Kingdom. A small number of countries may require us to gather additional clinical data before recognizing coverage and reimbursement for our products. It is our intent to complete the requisite clinical studies and obtain coverage and reimbursement approval in countries where it makes economic and strategic sense to do so.
We believe that the overall escalating cost of medical products and services has led to, and will continue to lead to, increased pressures on the healthcare industry to reduce the costs of products and services. We cannot assure you that government or other third-party payors will cover and reimburse our procedures in whole or in part in the future or that payment rates will be adequate. In addition, it is possible that future legislation, regulation, or reimbursement policies of third-party payors will adversely affect the demand for our procedures and products or our ability to sell them on a profitable basis. The unavailability or inadequacy of third-party payor coverage or reimbursement could have a material adverse effect on our business, operating results and financial condition.
Competition
The medical device industry is highly competitive, subject to rapid technological change and significantly affected by new product introductions and market activities of other participants. Our currently marketed products are, and any future products we commercialize will be, subject to intense competition. Our competitors include providers of conservative, non-operative therapies for lower lumbar spine conditions, as well as a number of major medical device companies that have developed or plan to develop products for minimally invasive spine surgery in each of our current and future product categories. We believe that the principal competitive factors in our markets include:
| 87 |
| · | improved outcomes for medical conditions affecting the lumbar spine; |
| · | acceptance by spine surgeons; |
| · | ease of use and reliability; |
| · | product price and qualification for reimbursement; |
| · | technical leadership and superiority; |
| · | effective marketing and distribution; and |
| · | speed to market. |
We are aware of several companies that compete or are developing technologies in our current and future product areas. As a result, we expect competition to remain intense. Our most significant competitors are Medtronic Sofamor Danek, Johnson & Johnson DePuy Spine, Stryker Spine, NuVasive, Zimmer Spine, Synthes, Orthofix International, Globus Medical and Alphatec Spine, many of which have substantially greater sales and financial resources than we do. In addition, these companies may have more established distribution networks, entrenched relationships with physicians, and greater experience in launching, marketing, distributing and selling products.
Additional competition also comes from physician-owned spine specific medical device distribution companies, which have expanded in recent years. These companies, which are partially owned or completely owned by spine surgeons, have significant market knowledge and access to the physicians who use our products and the hospitals that purchase our products. These surgeons may have an incentive to direct implant use toward implants that are distributed by these organizations.
Our ability to compete successfully will depend on our ability to develop proprietary products that reach the market in a timely manner, receive adequate reimbursement and demonstrate that they are safer and less invasive than alternatives available for the same purpose. Because of the size of the potential market, we anticipate that companies will dedicate significant resources to developing competing products.
Research and Development
As of December 31, 2012, our research and development team was comprised of 11 employees who have extensive experience in developing products to treat medical conditions affecting the lumbar spine. These employees work closely with our clinical advisors and spine surgeon customers to design and enhance our products and approach. Our research and development spending was $5.5 million, $5.2 million and $4.2 million for the years ending December 31, 2012, 2011 and 2010, respectively. Since inception, we have devoted significant resources to develop and enhance our products and expect to continue to do so in the future.
Manufacturing and Supply
We rely on third parties to manufacture all of our products and their components, except for our nitinol nucleus cutter blades, which we manufacture at our facility in Wilmington, North Carolina. Our outsourcing partners are manufacturers that meet FDA, ISO, and other internal quality standards, where applicable. We believe these manufacturing relationships allow us to work with suppliers who have the best specific competencies while we minimize our capital investment, control costs and shorten cycle times, all of which we believe allows us to compete with larger-volume manufacturers of spine surgery products.
All of our products and components are assembled, packaged, labeled and sterilized, if applicable, at third-party facilities in the United States under our existing contracts requiring compliance with Good Manufacturing Processes, or GMPs. Following receipt of products or components from our third-party manufacturers, we inspect, warehouse and ship the products and components at our facilities in Wilmington or at a third-party distribution facility in Memphis, Tennessee. We reserve the exclusive right to inspect and assure conformance of each product and component to our specifications. In addition, FDA or other regulatory authorities may inspect our facilities and those of our suppliers to ensure compliance with QSR.
| 88 |
The majority of our instruments and implants are produced by third-party manufacturers on precision, high-speed machine shop equipment. However, for a certain number of our products, the components and materials used to manufacture such products and the manufacturing operations are performed or supplied by third-party specialty vendors due to their proprietary or non-conventional nature. For example, the blades for our nucleus cutters are made from a metal called nitinol, which is converted into strip form by three manufacturers in the United States known to us. We have sourced nitinol strip from two of these vendors. The nitinol strip is then further converted for us into cutter blanks by a scalpel blade specialty vendor. Other vendors are available to manufacture the cutter blanks, as we may deem desirable or necessary. We convert the cutter blanks into cutter blades at our facilities. Our tissue extractor product is produced for us by a supplier that specializes in wire forming and coiling specifically for the medical device industry. A limited number of similar vendors exist that could be used to produce the tissue extractor product, and we believe we could replace this supplier on reasonable terms without substantial delay, if necessary.
We are currently working with our third-party manufacturers to plan for our future manufacturing requirements. In most cases, we have redundant manufacturing capability with multiple vendors and enjoy the significant capacity this arrangement provides to us. We may consider manufacturing certain products or product components internally, if and when demand or quality requirements make it appropriate to do so. We believe the manufacturing capacity available to us is sufficient to meet our demands into the foreseeable future.
Patents and Proprietary Technology
We rely on a combination of patent, trademark, copyright, trade secret and other intellectual property laws, nondisclosure agreements and other measures to protect our intellectual property rights. We believe that in order to have a competitive advantage, we must develop and maintain the proprietary aspects of our technologies. We require our employees, consultants and advisors to execute confidentiality agreements in connection with their employment, consulting or advisory relationships with us. We also require our employees, consultants and advisors who we expect to work on our products to agree to disclose and assign to us all inventions conceived during the work day, using our property or which relate to our business. We cannot provide any assurance that employees and consultants will abide by the confidentiality or assignment terms of these agreements. Despite any measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary.
Patents
As of December 31, 2012, we had fifty issued United States patents, nine pending patent applications or provisional patent applications in the United States, eight issued European patents, seven issued Japanese patents and eleven foreign patent application families as counterparts of U.S. cases. The issued and pending patents cover, among other things:
| · | our method for performing trans-sacral procedures in the spine, including diagnostic or therapeutic procedures, and trans-sacral introduction of instrumentation or implants; |
| · | apparatus for conducting these procedures including access, disc preparation and implantation including the current TranS1 instruments individually and in kit form; |
| · | implants for fusion and motion preservation in the spine; and |
| · | a direct lateral access and interbody fusion system. |
Our issued patents begin to expire in 2021 assuming timely payment of all maintenance fees. We have multiple patents covering unique aspects and improvements for many of our methods and products. We do not believe that the expiration of any single patent is likely to significantly affect our business, operating results or prospects.
| 89 |
Trademarks
We own nine trademark registrations in the United States, nine trademark registrations in the European Union and two registered trademarks in Canada. We also own two pending trademark application in the United States and four pending trademark applications in China.
Intellectual Property Licenses
We license certain intellectual property that is used in some of our products, including our VEO Lateral Access and Interbody Fusion System. We pay royalties on sales of licensed intellectual property that has been commercialized. We will continue to look to license products or innovations that enhance our product offerings.
Government Regulation
Our products are medical devices subject to extensive regulation by the FDA and other U.S. federal and state regulatory bodies and comparable authorities in other countries. To ensure that medical products distributed domestically and internationally are safe and effective for their intended use, the FDA and comparable authorities in other countries have imposed regulations that govern, among other things, the following activities that we or our contract manufacturing partners perform and will continue to perform:
| · | product design and development; |
| · | establishment registration and device listing; |
| · | product testing (preclinical and clinical); |
| · | product manufacturing; |
| · | product labeling; |
| · | product storage; |
| · | premarket clearance or approval; |
| · | advertising and promotion; |
| · | product marketing, sales and distribution; and |
| · | post-market surveillance, including reporting deaths or serious injuries related to products and certain product malfunctions. |
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the United States will require either prior 510(k) clearance or prior premarket approval from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risk are placed in either class I or II, which in many cases requires the manufacturer to submit to the FDA a premarket notification requesting permission for commercial distribution. This process is known as requesting 510(k) clearance. Some low risk devices are exempt from this requirement. Devices deemed by the FDA to pose the greatest risk, such as many life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a legally marketable device, are placed in class III, requiring a PMA. Our current commercial products are class II devices marketed under FDA 510(k) premarket clearance or are Class I and exempt from 510(k) or PMA regulation. Premarket clearance applications are subject to the payment of user fees, paid at the time of submission for FDA review.
| 90 |
510(k) Clearance Pathway
To obtain 510(k) clearance, we must submit a premarket notification demonstrating that the proposed device is substantially equivalent to a legally marketable device not requiring a PMA. Although statutorily mandated to respond to a 510(k) premarket notification within 90 days of submission of the application, the FDA’s 510(k) clearance pathway usually takes from three to twelve months, or even longer, depending on the extent of requests for additional information by the FDA. Additional information can include clinical data to make a determination regarding substantial equivalence.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or change in its intended use, will require a new 510(k) clearance or, depending on the modification, require a PMA. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), or a PMA, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or a PMA is obtained. If the FDA requires us to seek 510(k) clearance or a PMA for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties. We have made and plan to continue to make additional enhancements to our products. We use the FDA’s guidance document on changes to approved devices to determine whether a change requires a new submission.
Clinical Trials
Clinical trials are sometimes required for a 510(k) premarket notification. In the U.S., these trials require submission of an application for an investigational device exemption, or IDE, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of patients. Clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the appropriate institutional review boards at the clinical trial sites. Clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy and financial disclosure by clinical investigators. A clinical trial may be suspended by FDA or the investigational review board at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the study. Even if a study is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device, or may be equivocal or otherwise not be sufficient to obtain clearance or approval. Similarly, in Europe the clinical study must be approved by the local ethics committee and in some cases, including studies of high-risk devices, by the Competent Authority in the applicable country.
Extensive and Continuing FDA Regulation
After a device is placed on the market, numerous FDA and other regulatory requirements continue to apply. These include:
| · | quality system regulation, which requires manufacturers, including third-party contract manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance controls, during all aspects of the manufacturing process; |
| · | establishment registration and listing; |
| · | labeling regulations, and FDA prohibitions against the promotion of products for uncleared or unapproved “off-label” uses; |
| · | medical device reporting obligations, which require that manufacturers submit reports to the FDA if information reasonably suggests that their device (i) may have caused or contributed to a death or serious injury, or (ii) malfunctioned and the device or a similar company device would likely cause or contribute to a death or serious injury if the malfunction were to recur; and |
| 91 |
| · | other post-market surveillance requirements, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
We must register with the FDA as a medical device manufacturer and must obtain all necessary state permits or licenses to operate our business. As manufacturers, we are subject to announced and unannounced inspections by the FDA to determine our compliance with quality system regulation and other regulations. We were inspected by the FDA in May 2010 with no Form 483 observations. We believe that we are in substantial compliance with quality system regulation and other regulations.
The manufacturing facilities of our suppliers and contract manufacturer partners are also subject to announced and unannounced inspections by the FDA. Our quality agreements with these partners obligate them to be in substantial compliance with U.S. regulations in this area and none of our critical suppliers is undergoing any FDA enforcement action.
Fraud and Abuse
We may directly or indirectly be subject to various federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback laws. In particular, the federal healthcare program anti-kickback statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service, for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. The anti-kickback statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. In implementing the statute, the OIG has issued a series of regulations, known as the “safe harbors,” which began in July 1991. These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the anti-kickback statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy all requirements of an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG. Penalties for violations of the federal anti-kickback statute include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government. These individuals, sometimes known as “relators” or, more commonly, as “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The number of filings of qui tam actions has increased significantly in recent years, causing more healthcare companies to have to defend a False Claim action. If an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 and $11,000 for each separate false claim. Various states have also enacted similar laws modeled after the federal False Claims Act which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items, or services. A violation of this statute is a felony and may result in fines or imprisonment.
We are also subject to certain state laws that are analogous to each of the federal laws summarized above, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy of certain health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
| 92 |
While we have adopted a comprehensive compliance program to attempt to comply with these laws and regulations, if any of our operations are found to have violated or be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, among them being civil and criminal penalties, damages, fines, exclusion from government healthcare programs, and the curtailment or restructuring of our operations.
In October 2011, we received a subpoena issued by the OIG, under the authority of the federal healthcare fraud and false claims laws. The subpoena sought documents for the period January 1, 2008 through October 6, 2011. We have cooperated with the government’s request. On December 24, 2012 we reached a tentative agreement in principle with the U.S. Department of Justice related to the subpoena issued in October 2011. We tentatively agreed with the staff of the Department of Justice to settle its federal investigation for $6 million, subject to completion and approval of written settlement agreements with the Department of Justice and the OIG, which are expected to be finalized in the second quarter of 2013. We admit no wrongdoing as part of the settlement. We are also unable to predict what impact, if any, the outcome of the matters raised by the OIG will have on the willingness of surgeons to accept our products, our ability to hire or retain sales representatives and other employees, potential future reimbursement decisions by payors or other significant aspects of our business.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ.
The European Union, which consists of 27 of the major countries in Europe, has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking and, accordingly, can be commercially distributed throughout the member states of the European Union, and other countries that comply with or mirror these directives. The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body,” an independent and neutral institution appointed to conduct conformity assessments. This third-party assessment consists of audits of the manufacturer’s quality system for conformance to applicable quality system standards such as the Quality System, or ISO 13485, and compliance of each product with the Medical Device Directive (MDD93/42/EEC as amended by directive 2007/47EC). In August 2004, our quality system was initially certified by Intertek ETL-Semko, a Notified Body, under the European Union Medical Device Directive to be in compliance with the Canadian Medical Device Conformity Assessment System, or CMDCAS, and ISO 13485. A surveillance audit in September of 2012 found no major non-conformities and the system is due for further recertification in September of 2013.
Registration of our products has been completed, or is currently in process, for other countries such as Brazil, China, India, Israel, Japan, Mexico and Russia.
Employees
As of December 31, 2012, we had 89 employees, 88 of whom were full-time employees, with 47 employees in U.S. sales, marketing, professional affairs, customer service and training, 2 employees in international sales and management, 3 employees in operations, 11 employees in research and development, 14 employees in general and administrative and 12 employees in clinical, regulatory and quality assurance. We believe that our future success will depend in part on our continued ability to attract, hire and retain qualified personnel. None of our employees are represented by a labor union, and we believe that we maintain good relations with our employees. Effective January 1, 2012, we replaced our direct sales representatives in Europe with an independent sales agent, comprised of former employees of our direct sales organization, representing Germany, Netherlands, and Belgium. In January 2013, we terminated this sales agent and are transitioning to other distribution channels in these regions.
| 93 |
Properties
In February 2010, we moved into a new facility of approximately 31,000 square feet, located in Wilmington, North Carolina. In September 2012, we subleased out 7,000 square feet of underutilized space leaving us with 24,000 square feet. Of that amount, approximately 5,000 square feet is used for manufacturing and warehousing, 13,000 square feet for office space and 6,000 square feet for research and development activities. This lease expires in December 2014, with an option to extend the lease through December 2019. We believe that our current facility will be sufficient to meet our needs through that time. In October 2011, we entered into a lease for a surgeon training facility of approximately 4,375 square feet, located in Raleigh, North Carolina. Of that amount, approximately 2,200 square feet is used for training activities and 2,175 square feet is used for office space. This lease expires in 2017, with an option to extend the lease through 2022. In October 2012, we entered into a lease for executive office space of approximately 4,350 square feet, located in Raleigh, North Carolina. This office space is adjacent to the surgeon training facility. This lease expires in 2017, with an option to extend the lease through 2023.
Legal Proceedings
We are subject to legal proceedings and claims in the ordinary course of our business. These claims potentially cover a variety of allegations spanning our entire business. The following is a brief discussion of the material pending legal proceedings to which we are a party or to which any of our property is subject. See Note 5 of Notes to Consolidated Financial Statements included at the end of this Proxy Statement for additional discussion of legal matters.
In October 2011, we received a subpoena issued by the OIG under the authority of the federal healthcare fraud and false claims statutes. The subpoena sought documents for the period January 1, 2008 through October 6, 2011. We have cooperated with the government’s request. On December 24, 2012 we reached a tentative agreement in principle with the U.S. Department of Justice related to the subpoena issued in October 2011. We tentatively agreed with the staff of the Department of Justice to settle its federal investigation for $6 million, subject to completion and approval of written settlement agreements with the Department of Justice and the OIG, which are expected to be finalized in the second quarter of 2013. We admit no wrongdoing as part of the settlement.
On January 24, 2012, we received notice that a putative class action lawsuit had been filed in the U.S. District Court for the Eastern District of North Carolina, on behalf of all persons, other than defendants, who purchased TranS1 securities between February 21, 2008 and October 17, 2011. The complaint alleges violations of the Exchange Act based upon purported omissions and/or false and misleading statements concerning TranS1’s financial statements and reimbursement practices. The complaint seeks damages sustained by the putative class, pre- and post-judgment interest, and attorneys’ fees and other costs. On September 7, 2012, we filed a motion to dismiss the complaint for failure to meet the heightened pleading requirements of the Private Securities Litigation Reform Act of 1995, among other grounds; the motion is pending. We are unable to predict what impact, if any, the outcome of this matter might have on our consolidated financial position, results of operations, or cash flows.
Management’s Discussion and Analysis ofFinancial Condition and Results of Operations
The following discussion of our financial condition and results of operations should be read in conjunction with our “Risk Factors” and our consolidated financial statements and the related notes to our consolidated financial statements included in this Proxy Statement. The following discussion contains forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements” on page 31 of this Proxy Statement.
Overview
We are a medical device company focused on designing, developing and marketing products to treat degenerative conditions of the spine affecting the lumbar region. We are committed to delivering minimally invasive surgical technologies that enhance patient clinical care while providing sustained value for our customers. We currently market the AxiaLIF family of products for single and two level lumbar fusion, the VEO lateral access and interbody fusion system, the Vectre lumbar posterior fixation system and Bi-Ostetic bone void filler, a biologics product. Our AxiaLIF products use our pre-sacral approach, through which a surgeon can access discs in the lumbar region of the spine through an incision adjacent to the tailbone and perform an interbody fusion procedure through instrumentation that provides direct access to the intervertebral space. We developed our pre-sacral approach to allow spine surgeons to access and treat intervertebral spaces without compromising important surrounding soft tissue, nerves and bone structures. We also market products that may be used with our AxiaLIF surgical approach, including bowel retractors and additional discectomy tools, as well as a bone graft harvesting system that can be used to extract bone graft in any procedure for which the use of bone graft is appropriate. Our philosophy of continuous improvement is driven by ongoing research and development investment in our core technologies. We support this investment by diligently expanding, maintaining, and protecting our significant patent portfolio.
| 94 |
From our incorporation in 2000 through 2004, we devoted substantially all of our resources to research and development and start-up activities, consisting primarily of product design and development, clinical trials, manufacturing, recruiting qualified personnel and raising capital. We received 510(k) clearance from the FDA for our AxiaLIF Legacy product in the fourth quarter of 2004, and commercially introduced our AxiaLIF Legacy product in the United States in the first quarter of 2005. We received a CE mark to market our AxiaLIF Legacy product in the European market in the first quarter of 2005 and began commercialization in the first quarter of 2006. We received a CE mark for our AxiaLIF 2L product in the third quarter of 2006 and began commercialization in the European market in the fourth quarter of 2006. We received FDA 510(k) clearance for our AxiaLIF 2L product and began marketing this product in the United States in the second quarter of 2008. The AxiaLIF 2L product was discontinued in 2010 after we launched our AxiaLIF Plus 2-Level product in July 2010, for which we had received FDA 510(k) clearance in January 2010. We commercially launched our next generation Vectre facet screw system in April 2010. In the first quarter of 2010, we began distributing Bi-Ostetic bone void filler, a biologics product. We commercially launched our AxiaLIF Plus 1-Level product in September 2011, for which we had received FDA 510(k) clearance in March 2011. In 2010, we received 510(k) clearance for our VEO lateral access and interbody fusion system, which was commercially launched in November 2011, and in July 2012 we received a CE mark for our VEO lateral access and interbody fusion system and began commercialization in the European market. We currently sell our products through a direct sales force, independent sales agents and international distributors.
In March 2012, we announced that the Panel voted to approve an application for a new Category I CPT code, 22586, for L5/S1 spinal fusion utilizing our AxiaLIF implant when performing a pre-sacral interbody fusion. In addition, the Panel voted to establish a new Category III CPT code, 0309T, as an add-on code to the new Category I code for use with 22586 when performing L4/5 spinal fusion. The new CPT codes were announced on the AMA’s website on March 2, 2012, and became effective on January 1, 2013. The Medicare final rule was released in November 2012, which stated a valuation of the Category I CPT code 22586 for pre-sacral interbody single level spinal fusion at L5-S1, and became effective January 1, 2013. This CPT code, which applies to our AxiaLIF Plus 1-Level device, is a bundled lumbar arthrodesis procedure that includes bone graft, posterior instrumentation and fixation. With the establishment of a Category I CPT code effective January 1, 2013, we believe that we are in a position to transition to a period of sustainable revenue growth.
We rely on third parties to manufacture all of our products and their components, except for our nitinol nucleus cutter blades, which we manufacture at our facility in Wilmington, North Carolina. Our outsourcing partners are manufacturers that meet FDA, ISO or other internal quality standards, where applicable. We believe these manufacturing relationships allow us to work with suppliers who have the best specific competencies while we minimize our capital investment, control costs and shorten cycle times.
Since inception, we have been unprofitable. As of December 31, 2012, we had an accumulated deficit of $ 138.8 million.
We expect to continue to invest in sales and marketing infrastructure for our products in order to gain wider acceptance for them. We also expect to continue to invest in research and development and related clinical trials, and increase general and administrative expenses as we grow. As a result, we will need to generate significant revenue in order to achieve profitability.
On March 3, 2013, we entered into the Merger Agreement with Merger Sub, Baxano and the Securityholder Representatives, under which we will acquire Baxano through a merger of Merger Sub with and into Baxano. At the effective time of the Merger, Merger Sub will cease to exist and Baxano will continue as the surviving corporation and as a wholly-owned subsidiary of the Company. The dollar value of the shares of our common stock to be issued in connection with the Merger is expected to be approximately $23.1 million, subject to fluctuations in the price of our common stock on the NASDAQ Global Market and certain adjustments.
Baxano is a medical instrument company focused on designing, developing and marketing innovative tools that restore spine function, preserve healthy tissue, and enable a better quality of life for the patients it serves. Baxano currently markets the patented iO-Flex system, a proprietary minimally invasive set of flexible instruments allowing surgeons to target lumbar spinal stenosis in all three regions of the spine: central canal, lateral recess, and neural foramen, and has developed the patented iO-Tome instrument to rapidly and precisely remove bone, including the facet joints, which is commonly performed in spinal fusion procedures. Baxano was founded in 2005 and is headquartered in San Jose, California. The Merger is expected to close in the second quarter of 2013 and is subject to TranS1 stockholder approval and customary conditions to closing.
| 95 |
Concurrent with entering into the Merger Agreement, we entered into the Securities Purchase Agreement with the Investors, pursuant to which we will sell to the Investors, and the Investors will purchase from the Company, an aggregate of 7,522,009 shares of our common stock, at a purchase price of $2.28 per share, resulting in gross proceeds to the Company of $17.2 million. This Private Placement Transaction is subject to approval by the Company’s stockholders of the issuance of shares of the Company’s common stock in connection with the Merger Agreement and the Securities Purchase Agreement, the closing of the Merger, and other customary closing conditions set forth in the Securities Purchase Agreement.
Results of Operations
| Years Ended December 31, | ||||||||||||||||||||
| (in thousands, except gross margin percentage) | ||||||||||||||||||||
| 2012 | 2011 | % change | 2010 | % change | ||||||||||||||||
| Revenue | $ | 14,570 | $ | 19,153 | (23.9 | )% | $ | 26,154 | (26.8 | )% | ||||||||||
| Cost of revenue | 4,309 | 4,555 | (5.4 | )% | 7,104 | (35.9 | )% | |||||||||||||
| Gross margin % | 70.4 | % | 76.2 | % | (7.6 | )% | 72.8 | % | 4.6 | % | ||||||||||
| Total operating expenses | 40,002 | 32,877 | 21.7 | % | 39,063 | (15.8 | )% | |||||||||||||
| Net loss | (29,888 | ) | (18,273 | ) | (63.6 | )% | (19,527 | ) | 6.4 | % | ||||||||||
Revenue
Revenue is recognized based on the following criteria: (i) persuasive evidence that an arrangement exists with the customer; (ii) delivery of the products and/or services has occurred; (iii) the selling price has been fixed for the products or services delivered; and (iv) collection is reasonably assured. We generate revenue from the sales of our implants and disposable surgical instruments. We have two distinct sales methods. The first method is when implants and/or disposable surgical instruments are sold directly to hospitals or surgical centers for the purpose of conducting a scheduled surgery. Our sales representatives or independent sales agents hand deliver the products to the customer on or before the day of the surgery. The sales representative or independent agent is then responsible for reporting the delivery of the products and the date of the operation to the corporate office for proper revenue recognition. We recognize revenue upon the confirmation that the products have been used in a surgical procedure. The other sales method is for sales to distributors outside the United States. These transactions require the customer to send in a purchase order before shipment will be made and the customer only has the right of return for defective products. We recognize revenue upon the shipment of the product to distributors outside the United States. We expect that a substantial amount of our revenues will continue to be generated in the United States in future periods.
Cost of Revenue
Cost of revenue consists primarily of material and overhead costs related to our products and product royalties. Overhead costs include facilities-related costs, such as rent, utilities and depreciation.
Research and Development
Research and development expenses consist primarily of personnel costs within our product development, regulatory and clinical functions and the costs of clinical studies, product development projects and technology licensing costs. In future periods, we expect research and development expenses to grow as we continue to invest in basic research, clinical trials, product development and intellectual property.
| 96 |
Sales and Marketing
Sales and marketing expenses consist of personnel costs, sales commissions paid to our direct sales representatives, independent sales agents and independent distributors and costs associated with physician training programs, promotional activities and participation in medical and trade conferences. In future periods, we expect sales and marketing expenses to increase as we expand our sales and marketing efforts.
General and Administrative
General and administrative expenses consist of personnel costs related to the executive, finance and human resource functions, as well as professional service fees, legal fees, accounting fees, insurance costs and general corporate expenses. In future periods, we expect general and administrative expenses to increase to support our sales, marketing, research and development efforts.
Other Income (Expense), Net
Other income (expense), net is primarily composed of the gain or loss on disposal of fixed assets, interest earned on our cash, cash equivalents and available-for-sale securities and government grants.
Comparison of the Years Ended December 31, 2012, 2011 and 2010
Revenue. Revenue was $14.6 million in 2012, $19.2 million in 2011 and $26.2 million in 2010. The $4.6 million decrease in revenue from 2011 to 2012 and the $7.0 million decrease in revenue from 2010 to 2011 were primarily a result of a lower number of AxiaLIF cases performed in each subsequent year, which was due primarily to physician reimbursement limitations, as well as an increase, relative to past practice, in insurance denials for lumbar fusion surgery due to the lack of medical necessity. These decreases were partially offset by price increases in April 2012 and April 2011 and increased revenues from the introduction of new or enhanced products, including our VEO lateral access and interbody fusion system and the AxiaLIF Plus 1-Level in 2011. Domestically, sales of our AxiaLIF single level products decreased to $5.4 million in 2012 from $9.4 million in 2011 and $12.9 million in 2010. Sales of our AxiaLIF Plus 2-Level and 2L products were $3.3 million in 2012, $5.1 million in 2011 and $7.8 million in 2010. Sales of our Bi-Ostetic bone void filler, which was introduced in February 2010, decreased to $0.9 million in 2012 from $1.0 million in 2011, which was an increase from $0.6 million in 2010, as a result of the lower overall number of fusion procedures we performed. In 2012, we also generated $1.8 million in revenue from our VEO lateral access and interbody fusion system, which began its limited market release in June 2011 and was commercially launched in November 2011, compared to $0.4 million in 2011. Additionally, during 2012, 2011 and 2010, we generated $0.6 million, $1.0 million and $1.9 million, respectively, in revenues from sales of our facet and Vectre screw systems, which declined primarily as a result of the lower overall number of fusion procedures we performed, and, to a lesser extent, because we introduced a more stringent clinical application guideline for these products to ensure optimal patient outcomes. Sales of our pedicle screw system, which was introduced in the first quarter of 2010, decreased to $0.2 million in 2012 from $0.3 million in 2011 and $0.6 million in 2010. In 2012, average revenue per AxiaLIF case continued to increase from 2011 and 2010, due to a price increase effective April 1, 2012, the release of our new AxiaLIF and VEO products and penetration into existing cases by our other products. In 2012, 2011 and 2010, we recorded 732, 1,298 and 1,985 domestic AxiaLIF cases, respectively, including 220 AxiaLIF Plus 2-Level cases in 2012, 343 AxiaLIF Plus 2-Level cases in 2011 and 555 AxiaLIF Plus 2-Level and 2L cases in 2010. Revenue generated outside the United States increased to $1.9 million in 2012 from $1.7 million in 2011 which was a decrease from $2.4 million in 2010. In 2012, 2011 and 2010, initial stocking shipments to new distributors and shipments of new or enhanced products to existing distributors were $1.2 million, $47,000 and $110,000, respectively. Initial stocking shipments in 2012 included $1.0 million to our new distributor in China. Effective January 1, 2012, we replaced our direct sales representatives in Europe with an independent sales agent, comprised of former employees of our direct sales organization. In January 2013, we terminated this sales agent and are transitioning to other distribution channels. In 2012, 87% of our revenues were generated in the United States compared to 91% in 2011 and 2010.
| 97 |
Cost of Revenue. Cost of revenue decreased to $4.3 million in 2012 from $4.6 million in 2011 and $7.1 million in 2010. The $0.3 million decrease from 2011 to 2012 was primarily related to lower material and overhead costs associated with decreased sales volume for our products and changes in inventory obsolescence reserves. There were inventory obsolescence charges of $0.3 million in 2012 for products that were being replaced, were obsolete or excess, compared to $0.5 million in obsolescence charges taken in 2011. The $2.5 million decrease from 2010 to 2011 was primarily related to inventory obsolescence reserves of $2.0 million taken in 2010 for products that were being replaced, were obsolete or were excess, and lower material and overhead costs associated with decreased sales volume for our products. Gross margin decreased to 70.4% in 2012, from 76.2% in 2011 and 72.8% in 2010. The decrease in gross margin in 2012 was due to a higher percentage of international sales which carry a lower gross margin, a higher percentage of sales being derived from ancillary and distributed products in 2012 as compared to 2011, which have a lower gross margin than our AxiaLIF products, increased depreciation expense on reusable kits, primarily due to building reusable kits as we launched the VEO product, and royalty expense. The increase in gross margin from 2010 to 2011 was primarily related to the additional inventory reserves that were taken in 2010, as discussed above, partially offset by changes in product sales mix and pricing pressures from customers.
Research and Development. Research and development expenses increased to $5.5 million in 2012 from $5.2 million in 2011 and $4.2 million in 2010. The $0.3 million increase in expense from 2011 to 2012 was primarily the result of an increase in personnel-related costs of $0.6 million as we increased our headcount in regulatory and clinical functions, partially offset by a decrease of $0.3 million in product acquisition costs. The $1.0 million increase in expense from 2010 to 2011 was primarily the result of a $0.5 million expenditure in 2011 to acquire the exclusive license of our new VEO lateral access and interbody fusion system and an increase in clinical project-related spending.
Sales and Marketing. Sales and marketing expenses decreased to $20.0 million in 2012 from $21.6 million in 2011 and $26.3 million in 2010. The decrease in expense from 2011 to 2012 of $1.6 million was primarily due to lower personnel-related costs of $1.4 million as we benefited from reductions in our direct sales headcount, lower commissions of $0.9 million as our revenues declined and decreased travel and entertainment expenses of $0.2 million related to the lower headcount, partially offset by increases in consulting costs of $0.4 million related to our reimbursement efforts and $0.4 million related to surgeon training for our new and enhanced products. The decrease in expense from 2010 to 2011 of $4.7 million was primarily due to lower personnel-related costs of $3.6 million, including lower commissions of $1.7 million, as we reduced our direct sales headcount, decreased promotional costs by $0.9 million, decreased travel and entertainment expenses by $0.7 million and decreased surgeon training costs by $0.3 million. These lower expenses were partially offset by an increase in consulting costs of $0.4 million related to our reimbursement efforts and $0.2 million related to the closing costs for our international office.
General and Administrative. General and administrative expenses increased to $6.2 million in 2012 from $5.9 million in 2011, which was a decrease from $8.6 million in 2010. The increase in expenses from 2011 to 2012 of $0.3 million was primarily attributable to the costs of sub-leasing a portion of our Wilmington facility of $0.3 million. The decrease in expenses from 2010 to 2011 of $2.7 million was primarily attributable to a decrease in personnel-related costs related to the management transition that occurred in 2010, including severance, recruiting and other personnel-related expenses of $1.1 million, a decrease in employee and related costs of $0.9 million, decreased legal costs of $0.5 million, a decrease in bad debt expense of $0.2 million and a decrease in consulting expense of $0.1 million, offset by an increase in directors and officers insurance of $0.3 million.
U.S. Government Settlement Charges.In December 2012, we reached a tentative agreement in principle with the U.S. Department of Justice related to the subpoena issued in October 2011. TranS1 and the staff of the Department of Justice tentatively agreed to settle its federal investigation for $6.0 million, subject to completion and approval of written settlement agreements with the Department of Justice and the OIG. We also incurred legal fees related to the investigation of $2.3 million in 2012 and $235,000 in 2011.
Other Income (Expense), Net. Other income (expense), net, decreased to expense of $147,000 in 2012 from $6,000 in income in 2011, and $0.5 million in income in 2010. The decrease of $153,000 from 2011 to 2012 was primarily the result of the loss on the disposal of fixed assets of $90,000 and a decrease in interest income due to lower cash on hand and lower interest rates on investments of $65,000. The decrease of $0.5 million from 2010 to 2011 was primarily the result of a grant we received from the U.S. Treasury under the Qualifying Therapeutic Discovery Program in 2010 for $0.5 million.
| 98 |
Liquidity and Capital Resources
Sources of Liquidity
Since our inception in 2000, we have incurred significant losses and, as of December 31, 2012, we had an accumulated deficit of $138.8 million. We have not yet achieved profitability, and anticipate that we will continue to incur losses in the near term. We expect to continue to fund research and development, sales and marketing and general and administrative expenses at similar to current levels or higher and, as a result, we will need to generate significant revenues to achieve profitability. Our recurring losses raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the year ended December 31, 2012 with respect to this uncertainty. Prior to our October 2007 initial public offering, our operations were funded primarily with the gross proceeds from the sale of preferred stock of $40.5 million. The net proceeds from our October 2007 initial public offering of $86.7 million and the net proceeds of our September 2011 stock offering of $18.2 million have funded our operations since then.
In May 2011, we filed a “universal shelf” Registration Statement on Form S-3 (Filing No. 333-174255), or the Shelf Registration Statement, with the SEC, which became effective on August 1, 2011. Depending on our non-affiliated public equity float during the time period prior to consummating a financing transaction, the Shelf Registration Statement allows us to raise up to an additional $29.85 million through the sale of debt securities, common stock, preferred stock, or warrants, or any combination thereof.
On September 21, 2011, we entered into a purchase agreement with Piper Jaffray & Co. as the lead underwriter, to sell 6,200,000 shares of our common stock in a public offering. The shares were offered and sold pursuant to a prospectus supplement dated September 21, 2011 and an accompanying base prospectus dated August 1, 2011, in connection with a “takedown” offering pursuant to the Shelf Registration Statement. On September 26, 2011, the shares were sold to the public at a price of $3.25 per share. The net proceeds, after deducting underwriting discounts, commissions and offering expenses, were $18.2 million. The timing and terms of any additional financing transactions pursuant to the Shelf Registration Statement or otherwise, have not yet been determined.
As of December 31, 2012, we did not have any significant outstanding debt financing arrangements, we had working capital of $19.1 million and our primary source of liquidity was $21.5 million in cash, cash equivalents and short-term investments. We currently invest our cash and cash equivalents primarily in money market treasury funds and our short-term investments primarily in U.S. agency backed debt instruments.
Cash, cash equivalents and short-term investments decreased from $44.8 million at December 31, 2011 to $21.5 million at December 31, 2012. The decrease of $23.3 million was primarily the result of net cash used in operating activities of $21.4 million and purchases of property and equipment of $2.0 million, partially offset by net cash provided from the issuance of our common stock under our employee stock purchase plan of $0.1 million and upon the exercise of stock options of $0.1 million.
Cash, cash equivalents and short-term investments increased from $42.5 million at December 31, 2010 to $44.8 million at December 31, 2011. The increase of $2.3 million was primarily the result of net cash of $18.2 million provided from the sale of our common stock in the September 2011 public offering, common stock issued under our employee stock purchase plan of $0.2 million and from the exercise of stock options of $0.1 million, offset by net cash used in operating activities of $15.6 million and purchases of property and equipment of $0.7 million.
Cash Flows
Net Cash Used in Operating Activities. Net cash used in operating activities was $21.4 million in 2012, $15.6 million in 2011 and $12.3 million in 2010. For each of these periods, net cash used in operating activities was attributable primarily to net losses after adjustment for non-cash items, such as depreciation, stock-based compensation expense, inventory reserves, bad debt reserves, and the loss on sale of fixed assets, combined with changes in working capital requirements to support the market acceptance of our products. Net cash used in operating activities for the year ended December 31, 2012 also included a $6.6 million increase in accrued expenses related to the OIG settlement.
Net Cash Provided by Investing Activities. Net cash provided by investing activities was $4.0 million in 2012, $11.3 million in 2011 and $7.3 million in 2010. For each of these periods, this amount reflected purchases or sales and maturities of investments and purchases of property and equipment, which were primarily for reusable instrument kits used in the field and information technology needs.
| 99 |
Net Cash Provided by Financing Activities. Net cash provided by financing activities was $0.2 million in 2012, $18.4 million in 2011 and $0.1 million in 2010. For each of these periods, this amount reflected shares issued related to the employee stock purchase plan and shares issued upon the exercise of stock options. Net cash provided by financing activities for the year ended December 31, 2011 also included the net proceeds from the issuance of shares of common stock in the September 2011 public offering of $18.2 million.
Operating Capital and Capital Expenditure Requirements
We believe that our existing cash and cash equivalents and short-term investments, together with cash received from sales of our products, will be insufficient to meet our anticipated cash needs through 2013. We intend to spend substantial amounts on sales and marketing initiatives to support the ongoing commercialization of our products and on research and development activities, including product development, regulatory and compliance, clinical studies in support of our currently marketed products and future product offerings, and the enhancement and protection of our intellectual property. We will need to obtain additional financing to pursue our business strategy, to respond to new competitive pressures or to take advantage of opportunities that may arise. To meet our capital needs, we are considering multiple alternatives, including, but not limited to, additional equity financings, debt financings and other funding transactions. There can be no assurance that we will be able to complete any such transaction on acceptable terms or otherwise. If we are unable to obtain the necessary capital, we will need to pursue a plan to license or sell our assets, cease operations and/or seek bankruptcy protection.
Under the Shelf Registration Statement, we have the ability to issue debt securities, common stock, preferred stock, or warrants, or any combination thereof. The sale of additional equity or convertible debt securities could result in dilution to our stockholders. In addition, any debt securities we issue could have rights senior to those associated with our common stock and could contain covenants that would restrict our operations. Furthermore, any preferred equity securities we issue could have rights senior to those associated with our common stock. Depending on our non-affiliated public equity float during the time period prior to consummating another financing transaction, the Shelf Registration Statement will allow us to raise up to an additional $29.85 million of securities. The timing and terms of any additional financing transactions, whether pursuant to the Shelf Registration Statement or otherwise, have not yet been determined. Any additional financing may not be available in amounts or on terms acceptable to us, if at all. If we are unable to obtain this additional financing, we may be required to reduce the scope of our planned product development and marketing efforts.
In October 2011, we received a subpoena issued by the OIG under the authority of the federal healthcare fraud and false claims statutes. We have cooperated with the government’s request. On December 24, 2012 we reached a tentative agreement in principle with the U.S. Department of Justice related to the subpoena issued in October 2011. We tentatively agreed with the staff of the Department of Justice to settle its federal investigation for $6.0 million, subject to completion and approval of written settlement agreements with the Department of Justice and the OIG, which are expected to be finalized in the second quarter of 2013. We admit no wrongdoing as part of the settlement.
On January 24, 2012, we received notice that a putative class action lawsuit had been filed in the U.S. District Court Eastern District, North Carolina, on behalf of all persons, other than defendants, who purchased TranS1 securities between February 21, 2008 and October 17, 2011. The complaint alleges violations of the Exchange Act, based upon purported omissions and/or false and misleading statements concerning TranS1’s financial statements and reimbursement practices. The complaint seeks damages sustained by the putative class, pre- and post-judgment interest, and attorneys’ fees and other costs. On September 7, 2012, we filed a motion to dismiss the complaint for failure to meet the heightened pleading requirements of the Private Securities Litigation Reform Act of 1995, among other grounds; the motion is pending. We are unable to predict what impact, if any, the outcome of this matter might have on our consolidated financial position, results of operations, or cash flows.
Contractual Obligations and Commitments
The following table discloses information about our contractual obligations by the year in which payments are due as of December 31, 2012:
| 100 |
| Payments Due by Year | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Contractual Obligations and Commitments | Total | Less Than 1 Year | 1-3 Years | 3-5 Years | After 5 Years | |||||||||||||||
| Operating leases(1) | $ | 1,539 | $ | 485 | $ | 680 | $ | 324 | $ | 50 | ||||||||||
(1) We rent office space under an operating lease which expires in 2014, with an option to extend the lease through 2019. We also rent space for a training facility which expires in 2017, with an option to extend the lease through 2022. We signed a lease for executive office space in 2012 which expires in 2017, with an option to extend the lease through 2023. This table does not include obligations for any lease extensions.
U.S. government settlement- The table above does not include our $6.0 million settlement with the U.S. government. Due to the high degree of uncertainty regarding the timing of potential cash flows, we cannot reasonably estimate the settlement periods for these amounts.
Reserves for uncertain tax positions - The table above does not include $1.0 million of total gross unrecognized tax benefits as of December 31, 2012. Due to the high degree of uncertainty regarding the timing of potential cash flows, we cannot reasonably estimate the settlement periods, if any, for these amounts.
Recent Developments
Agreement and Plan of Merger.On March 3, 2013, we entered into the Merger Agreement with Merger Sub, Baxano and the Securityholder Representatives. Under the terms of the Merger Agreement, we will acquire Baxano through a merger of Merger Sub with and into Baxano. At the effective time of the Merger (the “Effective Time”), Merger Sub will cease to exist and Baxano will continue as the surviving corporation and as a wholly-owned subsidiary of the Company.
Pursuant to the terms of the Merger Agreement, the merger consideration will consist of approximately 10.4 million shares. The number of shares comprising the merger consideration will be reduced by a number of shares with a value equal to the following (using for the per share value an average closing price on the NASDAQ Global Market during the 10 days preceding the Effective Time): (1) Baxano’s indebtedness in excess of (a) amounts under promissory notes of Baxano that are convertible into capital stock of Baxano, each a Baxano Note, outstanding at the Effective Time, and (b) up to $3,000,000 of principal as well as interest outstanding under certain long-term indebtedness; (2) $300,000 in cash, which will be used to fund a compensation plan to be adopted by Baxano prior to the closing of the Merger providing for bonuses to Baxano’s employees and certain non-employee directors; and (3) $250,000 in cash, which we have agreed to deposit into an account specified for the purpose of funding the expenses of the Securityholder Representatives under the Merger Agreement.
At the Effective Time, each Baxano Note will be terminated, and the holders of such notes will be entitled to receive merger consideration in accordance with the Merger Agreement. Any and all stock option plans or other stock or equity-related plans of Baxano, together with any employee stock purchase plans, will be terminated prior to the Effective Time. Prior to the Effective Time, Baxano must use commercially reasonable efforts to terminate each outstanding option to purchase common stock of Baxano, whether vested or unvested, and each warrant to acquire capital stock of Baxano.
The Merger Agreement contains customary representations, warranties, and covenants, including covenants related to obtaining the requisite stockholder approvals, appointing two Baxano designees to our Board of Directors, restricting the solicitation of competing acquisition proposals, the lock-up and registration of the shares of our common stock issued in connection with the Merger pursuant to the Securities Purchase Agreement described below, and the Company’s and Baxano’s conduct of their businesses between the date of signing the Merger Agreement and the closing of the Merger.
| 101 |
The stockholders of Baxano approved the Merger and the Merger Agreement pursuant to a written consent in lieu of a stockholders’ meeting on March 3, 2013 following execution of the Merger Agreement. Consummation of the Merger is also subject to approval by our stockholders of the issuance of shares of our common stock in connection with the Merger and the satisfaction or waiver of the closing conditions set forth in the Securities Purchase Agreement and other customary closing conditions set forth in the Merger Agreement. Our directors, officers, and certain affiliates of the Company, who together hold approximately 24.2% of our issued and outstanding common stock, have agreed to vote their shares in favor of the issuance of our common stock in connection with the Merger Agreement and the Securities Purchase Agreement.
The Merger Agreement grants certain termination rights to the Company and Baxano. In addition, the Merger Agreement provides that we will be required to pay Baxano a termination fee equal to $2,000,000 and to reimburse Baxano for its expenses incurred relating to the transactions contemplated by the Merger Agreement, up to a cap of $750,000, if Baxano or the Company terminates the Merger Agreement under certain conditions. The Merger Agreement also provides that Baxano will be required to pay the Company a termination fee equal to $1,000,000 and to reimburse the Company for its expenses incurred relating to the transactions contemplated by the Merger Agreement, up to a cap of $750,000, if the Company terminates the Merger Agreement under certain conditions.
Securities Purchase Agreement. Concurrent with entering into the Merger Agreement, we entered into the Securities Purchase Agreement with the Investors, pursuant to which we will sell to the Investors, and the Investors will purchase from the Company, an aggregate of 7,522,009 shares of our common stock, at a purchase price of $2.28 per share, resulting in gross proceeds to the Company of $17.2 million.
Pursuant to the Securities Purchase Agreement, we agreed to register the resale of the shares of our common stock to be issued pursuant to the Merger Agreement and the Securities Purchase Agreement under a registration statement (the “Registration Statement”) on Form S-3 (or another appropriate form if Form S-3 is not then available to us). In addition, the Investors and all other holders of shares received pursuant to the Merger Agreement agreed not to sell, transfer, or otherwise dispose of the shares of our common stock issued at the closing of the Merger and the Private Placement Transaction from the period commencing on the closing of the Private Placement Transaction and expiring on the effective date of the Registration Statement, subject to certain exceptions.
If the Registration Statement is not declared effective by the SEC by a certain date, we must pay each Investor and other holder of shares issued pursuant to the Merger Agreement liquidated damages equal to 1% of the value of their shares (calculated at the closing price of such shares) per 30-day period, up to a maximum of 12%.
The Securities Purchase Agreement contains customary representations and warranties of the Company and the Investors. Consummation of the Private Placement Transaction is subject to approval by our stockholders of the issuance of shares of our common stock in connection with the Merger Agreement and the Securities Purchase Agreement, the closing of the Merger, and other customary closing conditions set forth in the Securities Purchase Agreement.
Off-Balance Sheet Arrangements
As of December 31, 2012, we did not have any outstanding debt or available debt financing arrangements or off-balance sheet liabilities.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with GAAP. The preparation of financial statements requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, revenue and expenses, and disclosures of contingent assets and liabilities at the date of the financial statements. On an on-going basis, we evaluate our estimates, including those related to revenue recognition, accounts receivable reserves, inventory reserves, accrued expenses, income tax valuations and stock-based compensation. We base our estimates on historical experience and various other assumptions that we believe to be reasonable, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Actual results could differ from those estimates under different assumptions or conditions.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements.
| 102 |
Revenue Recognition.We generate revenue from the sale of our implants and disposable surgical instruments. We have two distinct sales methods. The first method is when implants and/or disposable surgical instruments are sold directly to hospitals or surgical centers for the purpose of conducting a scheduled surgery. Our sales representatives or independent sales agents hand deliver the products to the customer on or before the day of the surgery. The sales representative or independent agent is then responsible for reporting the delivery of the products and the date of the operation for proper revenue recognition. We recognize revenue upon the confirmation that the products have been used in a surgical procedure. The second sales method is for sales to distributors outside the United States. These transactions require the customer to send in a purchase order before shipment and the customer only has the right of return for defective products. We primarily recognize revenue upon the shipment of the product to distributors outside the United States, when risk of loss and title has transferred to the distributor, provided we have no material performance obligations.
Accounts Receivable and Allowances. We monitor collections and payments from our customers and maintain an allowance for doubtful accounts based upon our historical experience and any specific customer collection issues that we have identified. While our credit losses have historically been within our expectations and the allowance established, we may not continue to experience the same credit loss rates that we have in the past. We make estimates on the collectability of customer accounts based primarily on analysis of historical trends and experience and changes in customers’ financial condition. Management uses its best judgment, based on the best available facts and circumstances, and records a reserve against the amounts due to reduce the receivable to the amount that is expected to be collected. These reserves are reevaluated and adjusted as additional information is received that impacts the amount reserved.
Inventory. We state our inventories at the lower of cost or market, computed on a standard cost basis, which approximates actual cost on a first-in, first-out basis and market being determined as the lower of replacement cost or net realizable value. Costs are monitored on an annual basis and updated as necessary to reflect changes in supplier costs and the rate of our overhead absorption is adjusted based on projections of our manufacturing costs and production plan. The components of inventory are reviewed on a periodic basis for excess, obsolete and impaired inventory, and a reserve is recorded for the identified items. An inventory reserve is calculated for estimated excess and obsolete inventory based upon historical turnover and assumptions about future demand for the products and market conditions.
Accounting for Income Taxes. Significant management judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our net deferred tax assets. We account for income taxes using the liability method which requires the recognition of deferred tax assets or liabilities for the temporary differences between financial reporting and tax bases of the Company’s assets and liabilities and for tax carryforwards at enacted statutory tax rates in effect for the years in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in the period that includes the enactment date. In addition, valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. We have recorded a full valuation allowance on our net deferred tax assets as of December 31, 2012 due to uncertainties related to our ability to utilize our deferred tax assets in the foreseeable future.
Stock-Based Compensation.The fair value of stock options is estimated using a Black-Scholes option pricing model. This model requires the input of subjective assumptions, including expected stock price volatility, expected life and estimated forfeitures of each award. All options granted after January 1, 2006 are expensed on a straight-line basis over the vesting period. Due to the limited amount of historical data available to us, particularly with respect to stock-price volatility, employee exercise patterns and forfeitures, actual results could differ materially from our expectations.
Recent Accounting Pronouncements
In May 2011, the Financial Accounting Standards Board, or the FASB, issued new authoritative guidance to provide a consistent definition of fair value and ensure that fair value measurements and disclosure requirements are similar between GAAP and International Financial Reporting Standards. This guidance changes certain fair value measurement principles and enhances the disclosure requirements for fair value measurements. This guidance is effective for interim and annual periods beginning after December 15, 2011 and is applied prospectively. The adoption of this guidance did not have a material impact on our financial statements.
| 103 |
In June 2011, the FASB issued a standard on the presentation of other comprehensive income. This guidance eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity and requires the presentation of other comprehensive income in a single continuous statement, or in two separate, but consecutive, statements. This guidance is effective for fiscal years and interim periods beginning after December 15, 2011. In December 2011, the FASB issued an amendment to indefinitely defer one of the requirements contained in the June 2011 standard. That requirement called for reclassification adjustments from accumulated other comprehensive income to be measured and presented by income statement line item in net income and also in other comprehensive income. The adoption of this standard did not have a material effect on our financial statements.
In February 2013, the FASB issued Accounting Standards Update No. 2013-02, “Reporting of Amounts Reclassified Out of Other Comprehensive Income,” which requires public companies to present information about reclassification adjustments from accumulated other comprehensive income in their annual and interim financial statements in a single note or on the face of the financial statements. This standard is effective prospectively for annual and interim reporting periods beginning after December 15, 2012. This guidance is not expected to have a material effect on our financial condition or results of operations.
SECURITYOWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth the beneficial ownership of our common stock as of April 22, 2013, by (i) each person or entity who is known by us to own beneficially more than 5% of the outstanding shares of common stock, (ii) each of our directors, (iii) each of the named executive officers, and (iv) all of our directors and executive officers as a group.
| Name and Address of Beneficial Owner(1) | Amount and Nature of Beneficial Ownership(2) | Approximate Percentage of Shares Beneficially Owned(2) | ||||||
| 5% Stockholders | ||||||||
| Delphi Ventures and Affiliated Entities(3) | 4,692,695 | 17.2 | % | |||||
| Advanced Technology Ventures and Affiliated Entities(4) | 3,087,906 | 11.3 | % | |||||
| Cutlass Capital and Affiliated Entities(5) | 1,996,360 | 7.3 | % | |||||
| Sapient Capital, L.P.(6) | 1,613,090 | 5.9 | % | |||||
| Named Executive Officers and Directors | ||||||||
| Michael Carusi(7) | 3,133,950 | 11.5 | % | |||||
| Jonathan Osgood(8) | 2,046,360 | 7.5 | % | |||||
| James Shapiro(9) | 1,159,845 | 4.2 | % | |||||
| Joseph Slattery(10) | 441,338 | 1.6 | % | |||||
| Ken Reali(11) | 391,062 | 1.4 | % | |||||
| Paul LaViolette(12) | 231,971 | * | ||||||
| Dwayne Montgomery(13) | 156,664 | * | ||||||
| David Simpson(14) | 102,500 | * | ||||||
| Mark Stautberg | 10,000 | * | ||||||
| Jeffrey Fischgrund(15) | 8,750 | * | ||||||
| All Executive Officers and Directors as a Group (14 persons)(16) | 7,952,023 | 29.1 | % | |||||
| * | Less than 1% |
| (1) | Unless otherwise indicated, the business address of each stockholder is c/o TranS1 Inc., 110 Horizon Drive, Suite 230, Raleigh, NC 27615. |
| (2) | This table is based upon information supplied by our officers and directors, and with respect to principal stockholders, Schedules 13G and 13G/A, as well as Forms 4, filed with the SEC. Beneficial ownership is determined in accordance with the rules of the SEC. Applicable percentage ownership is based on 27,318,785 shares of common stock outstanding as of April 22, 2013. Shares of common stock subject to options currently exercisable or exercisable within 60 days of April 22, 2013, are deemed outstanding for computing the ownership percentage of the person holding such options, but are not deemed outstanding for computing the ownership percentage of any other person. Except as otherwise noted, we believe that each of the stockholders named in the table has sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them, subject to applicable community property laws. |
| 104 |
| (3) | Based on information set forth in a Schedule 13G/A filed with the SEC on September 26, 2011. Consists of (i) 2,236,272 shares held by Delphi Ventures VI, L.P. (“DV VI”), (ii) 22,362 shares held by Delphi BioInvestments VI, L.P. (“DBI VI”), (iii) 2,410,523 shares held by Delphi Ventures VIII, L.P. (“DV VIII”), and (iv) 23,538 shares held by Delphi BioInvestments VIII, L.P. (“DBI VIII”). Delphi Management Partners VI, L.L.C. is the general partner of each of DV VI and DBI VI and may be deemed to have sole voting and dispositive power with respect to the shares owned by DV VI and DBI VI. Delphi Management Partners VIII, L.L.C. is the general partner of each of DV VIII and DBI VIII and may be deemed to have sole voting and dispositive power with respect to the shares owned by DV VIII and DBI VIII. The managing members of each of Delphi Management Partners VI, L.L.C. and Delphi Management Partners VIII, L.L.C. are James J. Bochnowski, David L. Douglass, Douglas A. Roeder, John F. Maroney and Deepika R. Pakianathan (collectively, the “Delphi Managing Members”). The Delphi Managing Members may be deemed to have shared voting and dispositive power with respect to each of the shares listed above. The address for all entities and individuals affiliated with Delphi Ventures is 3000 Sand Hill Road, Building 1, Suite 135, Menlo Park, CA 94025. |
| (4) | Based on information set forth in a Schedule 13G/A filed with the SEC on February 14, 2012. Consists of (i) 2,894,702 shares held by Advanced Technology Ventures VII, L.P. (“ATV VII”), (ii) 116,163 shares held by Advanced Technology Ventures VII (B), L.P. (“ATV VII-B”), (iii) 55,836 shares held by Advanced Technology Ventures VII (C), L.P. (“ATV VII-C”), (iv) 17,249 shares held by ATV Entrepreneurs VII, L.P. (“ATV VII-E” and together with ATV VII, ATV VII-B, and ATV VII-C, collectively referred to as the “ATV VII Entities”), and (v) 3,956 shares held by ATV Alliance 2002, L.P. (“ATV A 2002” and together with the ATV VII Entities, the “ATV Entities”). ATV Associates VII, L.L.C. (“ATV A VII”) is the general partner of each of the ATV VII Entities and ATV Alliance Associates, L.L.C. (“ATV Alliance”) is the general partner of ATV A 2002. Michael Carusi, one of our directors, Steven N. Baloff, Jean M. George, William C. Wiberg, and Robert C. Hower are the five managing directors of ATV A VII and have shared voting and investment power over the shares held by the ATV VII Entities. Jean M. George is the sole manager of ATV Alliance and has sole voting and investment power over the shares held by ATV A 2002. The address for all entities and individuals affiliated with Advanced Technology Ventures is 500 Boylston Street, Suite 1380, Boston, Massachusetts 02116. |
| (5) | Based on information set forth in a Schedule 13G filed with the SEC on February 20, 2013. Consists of (i) 1,755,752 shares held by Cutlass Capital, L.P., (ii) 126,133 shares held by Cutlass Capital Principals Fund, L.L.C., and (iii) 114,475 shares held by Cutlass Capital Affiliates Fund, L.P. Jonathan Osgood, one of our directors, and Edwin D. Hetz are the two managing members of Cutlass Capital Management, L.L.C., which is the general partner of each of Cutlass Capital, L.P. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood and Mr. Hetz are also the two managing members of Cutlass Capital Principals Fund, L.L.C. Mr. Osgood and Mr. Hetz have shared voting and investment power over the shares held by each of Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood and Mr. Hetz disclaim beneficial ownership of the shares held by Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P., except to the extent of their respective pecuniary interest therein. The address for all entities and individuals affiliated with Cutlass Capital is 322 Dunbarton Drive, St. Simons Island, GA 31522. |
| (6) | Based on information set forth in a Schedule 13G filed with the SEC on November 20, 2007. Mitchell Dann, one of our former directors, is the managing member of Sapient Capital Management, L.L.C., which is the general partner of Sapient Capital Management L.P., which is the general partner of Sapient Capital, L.P. Each of Sapient Capital Management, L.L.C., Sapient Capital Management L.P. and Mr. Dann disclaims beneficial ownership of the shares held by Sapient Capital, L.P., except to the extent of their respective proportionate pecuniary interest therein. The address for Sapient Capital is 4020 Lake Creek Drive, P.O. Box 1590, Wilson, WY 83014. |
| (7) | Consists of the shares identified in footnote 4 (excluding the 3,956 shares held by ATV A 2002) and 50,000 shares subject to options exercisable within 60 days of April 22, 2013, which options are held directly by Mr. Carusi. Mr. Carusi is one of our directors and is a managing director of ATV A VII and disclaims beneficial ownership of the shares held by the ATV VII Entities. |
| (8) | Consists of the shares identified in footnote 5 and 50,000 shares subject to options exercisable within 60 days of April 22, 2013, which options are held directly by Mr. Osgood. Mr. Osgood is one of our directors and is one of two managing members of Cutlass Capital Management, L.L.C., which is the general partner of each of Cutlass Capital, L.P. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood is also one of two managing members of Cutlass Capital Principals Fund, L.L.C. Mr. Osgood has shared voting and dispositive power over the shares held by each of Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P. Mr. Osgood disclaims beneficial ownership of the shares held by Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C. and Cutlass Capital Affiliates Fund, L.P., except to the extent of his proportionate pecuniary interest therein. |
| (9) | Consists of (i) 1,029,545 shares held directly by Thomas Weisel Healthcare Venture Partners, L.P. (“TWHVP”), (ii) 78,695 shares held directly by Kearny Venture Partners, L.P. (“KVP”), (iii) 1,605 shares held directly by Kearny Venture Partners Entrepreneurs’ Fund, L.P. (“KVPEF”); and 50,000 shares subject to options exercisable within 60 days of April 22, 2013, which options are held directly by Mr. Shapiro. Ownership of TWHVP, KVP, and KVPEF is based on information set forth in a Schedule 13G/A filed with the SEC on February 14, 2011. Mr. Shapiro is one of our directors and is an affiliate of Thomas Weisel Healthcare Venture Partners L.L.C. (“TWHVP LLC”), the general partner of TWHVP, and has shared voting and dispositive power over the shares held by TWHVP. Mr. Shapiro is also a managing member of Kearny Venture Associates, L.L.C. (“KVA”), which is the general partner of both KVP and KVPEF, and has shared voting and dispositive power with respect to the shares held by KVP and KVPEF. Mr. Shapiro disclaims beneficial ownership of these shares except to the extent of his proportionate pecuniary interest therein. The address for each of the foregoing entities is 88 Kearny Street, 2nd Floor, San Francisco, California 94108. |
| (10) | Includes 249,924 shares subject to options exercisable within 60 days of April 22, 2013. |
| (11) | Includes 384,062 shares subject to options exercisable within 60 days of April 22, 2013. |
| (12) | Includes 78,125 shares subject to options exercisable within 60 days of April 22, 2013. |
| (13) | Includes 156,664 shares subject to options exercisable within 60 days of April 22, 2013. |
| 105 |
| (14) | Includes 42,500 shares subject to options exercisable within 60 days of April 22, 2013. |
| (15) | Includes 8,750 shares subject to options exercisable within 60 days of April 22, 2013. |
| (16) | Includes 1,338,559 shares subject to options exercisable within 60 days of April 22, 2013. |
MarketPrice of and Dividends on trans1’s Common Equity and Related Stockholder Matters
Price of Common Stock
Our common stock is traded on the NASDAQ Global Market under the symbol “TSON.” The following table sets forth the high and low sales prices of our common stock as quoted on the NASDAQ Global Market for the periods indicated.
| Price Range | ||||||||
| High | Low | |||||||
| Fiscal 2012: | ||||||||
| Fourth quarter | $ | 3.40 | $ | 2.37 | ||||
| Third quarter | 3.24 | 2.33 | ||||||
| Second quarter | 3.87 | 2.33 | ||||||
| First quarter | 4.67 | 1.73 | ||||||
| Fiscal 2011: | ||||||||
| Fourth quarter | $ | 3.33 | $ | 1.39 | ||||
| Third quarter | 5.22 | 2.58 | ||||||
| Second quarter | 5.42 | 4.08 | ||||||
| First quarter | 5.20 | 2.00 | ||||||
The closing price for our common stock as reported by the NASDAQ Global Market on March 1, 2013 was $2.22 per share.
As of March 4, 2013, we had approximately 111 stockholders of record.
Dividend Policy
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain future earnings, if any, for development of our business and do not anticipate that we will declare or pay cash dividends on our capital stock in the foreseeable future.
Stock Price Performance Graph
The following graph compares the cumulative total stockholder return on our common stock from December 31, 2007 through December 31, 2012 to that of the cumulative return over such period for (i) The NASDAQ Stock Market Composite Index, and (ii) NASDAQ Medical Equipment Index. Total stockholder return assumes $100.00 invested at the beginning of the period in our common stock and in each of the comparative indices. The graph further assumes that such amount was initially invested in our common stock at the closing market price on December 31, 2007, and that any dividends have been reinvested. We have not paid any dividends on our common stock. The stock price performance on the following graph is not necessarily indicative of future stock price performance.
| 106 |
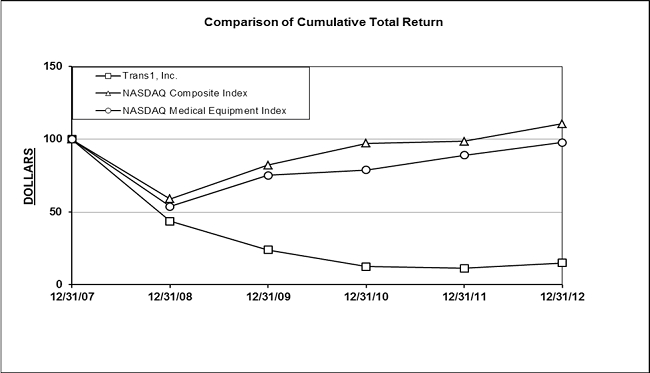
The material in the above performance graph does not constitute soliciting material and should not be deemed filed or incorporated by reference into any other Company filing, whether under the Securities Act or the Exchange Act, whether made on, before or after the date of this annual report and irrespective of any general incorporation language in such filing, except to the extent we specifically incorporate this performance graph by reference therein.
Description of Our Common Stock
General
As ofMarch 4, 2013, there were 27,318,785 shares of common stockoutstanding held of record by approximately 111 stockholders. The following summarizes the rightsof holders of our common stock:
| · | Each holder of common stock is entitled to one vote per share on all matters to be voted upon by the stockholders, including the election of directors; |
| · | The affirmative vote of a majority of the shares present in person or represented and voting at a duly held meeting at which a quorum is present shall be the act of the stockholders; |
| · | Holdersof common stock are not entitled to cumulate votes in the election of directors, which means that the holders of a majority of the shares voted can elect all of the directors then standing for election; |
| · | Subject to preferences that may be applicable to theholdersof outstanding shares of preferred stock, if any, theholdersof common stock are entitled to receive dividends when, as, and if declared by our Board of Directors, out of assets legally available for dividends; |
| · | Upon our liquidation, dissolution, or winding up, after satisfaction of all our liabilities and the payment of any liquidation preference of any outstanding preferred stock, theholders of shares of common stockwill be entitled to receive on a pro rata basis all of our assets remaining for distribution; |
| · | There are no redemption or sinking fund provisions applicable to our common stock; and |
| 107 |
| · | There are no preemptive or conversion rights applicable to our common stock. |
Our common stock is listed on theNASDAQ Global Marketunder the symbol “TSON.” The last reported saleprice of our common stockon March 4, 2013 was $2.20 per share.
The transfer agent and registrar for our common stock isAmerican Stock Transfer & Trust Company. The transfer agent and registrar’s address is 10150Mallard Creek Road,Suite 307, Charlotte,North Carolina 28262.
Anti-Takeover Effects of Certain Provisions of Delaware Law andOur Certificate of Incorporation and Bylaws
We are subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became aninterested stockholder, unless either:
| · | prior to the date at which the person becomes aninterested stockholder, the corporation’s board of directorsapproves such transaction orbusiness combination; |
| · | the stockholder acquires more than 85% of the outstanding voting stock of the corporation (excluding shares held by directors who are officers or held in certain employee stock plans) upon consummation of such transaction; or |
| · | thebusiness combinationis approved by the corporation’s board of directors and by theholdersof two-thirds of the outstanding voting stock of the corporation (excluding shares held by theinterested stockholder) at a meeting of stockholders (and not by written consent). |
For purposes of Section 203, “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years prior, did own) 15% or more of the corporation’s voting stock. A “business combination” includes a merger, asset sale, or other transaction resulting in a financial benefit to suchinterested stockholder.
In addition, certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws may be deemed to have an anti-takeover effect and may delay, defer, or prevent a tender offer or takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the market price for the shares held by our stockholders. Our amended and restated certificate of incorporation provides for our Board of Directors to be divided into three classes, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, our stockholders representing a majority of the shares of common stock outstanding will be able to elect all of our directors. Our amended and restated certificate of incorporation also permits our Board of Directors to issue up to 5,000,000 shares of preferred stock, with any rights, preferences, and privileges as they may designate. Furthermore, our amended and restated certificate of incorporation and bylaws provide that all stockholder action must be effected at a duly called meeting of stockholders and not by a consent in writing, and that only our Board of Directors, chairman of the Board of Directors, and chief executive officer may call a special meeting of stockholders. In addition, our amended and restated certificate of incorporation and bylaws require advance notice for stockholders to nominate directors or to submit proposals for consideration at meetings of stockholders. Our amended and restated certificate of incorporation and bylaws also require a 662/3% stockholder vote and the approval of our Board of Directors for the amendment, repeal, or modification of certain provisions of our amended and restated certificate of incorporation and bylaws relating to the issuance of preferred stock, the absence of cumulative voting, the classification of our Board, the requirement that stockholder actions be effected at a duly called meeting, the requirement of advance notice for stockholders to nominate directors, to submit proposals for consideration at meetings of stockholders, and the designated parties entitled to call a special meeting of the stockholders.
The combination of the classification of ourBoard of Directors, the lack of cumulative voting, the authorization to issue “blank check” preferred stock, and the 662/3% stockholder voting requirements will make it more difficult for our existing stockholders to replace ourBoard of Directorsas well as for another party to obtain control of us by replacing ourBoard of Directors. Since ourBoard of Directorshas the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for ourBoardto issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
| 108 |
These provisions may have the effect of deterring hostile takeovers or delaying changes in our control or management. These provisions are intended to enhance the likelihood of continued stability in the composition of ourBoard of Directorsand in the policies they implement, and to discourage certain types of transactions that may involve an actual or threatened change of our control. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts.
| 109 |
INFORMATION ABOUT BAXANO
Businessof Baxano
Overview
Baxano is a medical device company that designs, develops, and markets minimally invasive tools to restore spine function and preserve healthy tissue.
Baxano currently markets and sells the iO-Flex System. First commercialized in 2009 for both decompression and fusion applications, the minimally invasive approach of the iO-Flex System instruments allows for safe, complete, and precise removal of soft tissue and osseous tissue to achieve thorough direct decompression while preserving the stabilizing structures of the spine.
The iO-Flex System has received FDA clearance with the intended use of “accessing, cutting and biting soft tissue and bone during surgery involving the spinal column.” The iO-Flex system is intended to be used in standalone spinal decompression procedures or during fusion procedures where a direct decompression is still required.
The iO-Tome device received FDA clearance in 2012 with an intended use of “cutting bone for the purpose of removing a facet joint (facetectomy) of the lumbar spinal column.” This device is intended to be used in lumbar fusion procedures where a facetectomy is necessary to complete the procedure.
Baxano generally markets its product in the United States to orthopedic and neurosurgical spinal surgeons. Baxano primarily sells its product through a hybrid sales organization consisting of a direct component including: two Area Directors of Sales, 14 Regional Sales Managers, one Spine Consultant, seven Field Support Specialists, and an indirect organization consisting of 280 independent agents, of which 122 have completed Baxano product and procedure certification. Although Baxano has been granted CE mark and approval through Health Canada, Baxano has been solely focused on the development of the domestic market.
Medical Conditions Affecting the Spine and Traditional Treatment Alternatives
Lumbar spinal stenosis is a degenerative medical condition in which the openings in the lower part of the spine become narrowed, resulting in compression of the spinal nerves. Symptoms can include pain, weakness, numbness, and tingling in the lower back and legs. Decompression surgery (laminectomy/laminotomy) with and without spinal fusion is a common procedure used to treat lumbar spinal stenosis by cutting away overgrown bone and tissue, relieving pressure on the spinal nerves.
Traditional rigid instruments used in decompression surgery are generally limited in their ability to reach lumbar stenosis in all three regions of the spine (central canal, lateral recess, and neural foramen) and frequently must cut through and remove healthy parts of the spine in order to try to access hard to reach areas where overgrown bone and tissue are compressing the nerves. Failure to remove all stenosis during decompression is often recognized as a leading cause of poor surgical outcomes, including continued symptoms and reoperation. In addition, removing anatomy important for spinal stability such as the facet joints and posterior midline structures in an attempt to access all three regions of stenosis may lead to a complication called instability and the possible need for a spinal fusion procedure to help re-stabilize the spine.
Baxano’s iO-Flex System is the first minimally invasive set of flexible instruments allowing surgeons to target lumbar stenosis in all three regions of the spine with minimal disruption to the patient’s healthy anatomy critical for maintaining spinal stability.
Decompression surgery with the iO-Flex system is performed through an open or minimally invasive incision in which the surgeon directly visualizes the thecal sac and then utilizes a fine surgical wire to guide flexible instruments directly to the location of stenosis and remove it from the inside-out, rather than traditional rigid instruments which may cut through healthy tissue to access stenosis.
| 110 |
The key differentiators of the iO-Flex System are:
| · | Economic Benefit |
| o | Less traumatic and effective procedures may result in shorter operating times and hospital stays, lower complications, and improved recovery times compared to traditional methods. |
| · | Minimally Invasive and Thorough Decompression |
| o | System offers improved access to thoroughly target all three areas of lumbar stenosis, which can occur in the lateral recess, foramen and central canal where the spinal nerves exit the spinal canal. |
| o | Flexible instruments can allow surgeons to preserve healthy bone, ligaments, and muscle to help maintain spinal stability. |
| · | Versatility |
| o | Utility in all lumbar decompression procedures with and without spinal fusion. |
| o | Able to fully reach up to two levels of stenotic foraminal openings on each side of the spine through one laminotomy. |
Baxano’s Strategy
Baxano’s goal is to become a global leader in minimally invasive treatments for conditions affecting the spine. To achieve this goal, Baxano is pursuing the following strategies:
| · | offer comprehensive education opportunities to surgeons to help expand customer base; |
| · | leverage sales and marketing infrastructure to drive further surgeon adoption; |
| · | generate published, peer reviewed clinical literature that demonstrates the clinical and economic benefits of decompression surgery utilizing the iO-Flex system in order to help support adoption by all stakeholders; |
| · | utilize direct-to-patient marketing activities to create market pull for iO-Flex technology; and |
| · | expand product offering with complementary technologies that leverage the iO-Flex customer base. |
Baxano’s Products and Product Pipeline
Thus far, Baxano has developed two systems based on its proprietary technology. The flagship product, the iO-Flex System, has been commercially sold in the United States since 2009. The second device, the iO-Tome, received FDA clearance in July 2012. The iO-Tome instrument utilizes the iO-Flex platform to deliver a new MicroBlade Shaver® design configured to rapidly and precisely remove the facet joint, which is commonly performed in spinal fusion applications.
iO-Flex System
Baxano’s iO-Flex System entered the market in 2009 for minimally invasive lumbar procedures with and without spinal fusion and was designed to do a thorough decompression while sparing the facet joints and posterior midline structures. The system is a single use disposable that can be used at multiple levels during the same spinal procedure. The system includes the following components:
iO-Flex® Probedelivers the iO-Flex® Wire for access to the areas of stenosis in the spine.
iO-Flex® Wire is the guide wire over which the other tools will be introduced to the area of stenosis.
iO-Flex® Neuro Check® device helps confirm the location of the nerve to be decompressed when direct visualization of the neural structures is compromised.
| 111 |
iO-Flex® MicroBlade Shaver® instrument is designed to allow surgeons to precisely shave away the targeted bone and tissue that is compressing the nerve.
iO-Flex® Distal Handle attaches to and is used to manipulate the microblade shaver device.
Surgeon Training
Baxano devotes significant resources to educating surgeons and their staffs on the skills involved in the usage of its products. Baxano believes that the most effective way to introduce and build market demand for its products is by offering comprehensive educational opportunities. Baxano accomplishes its surgeon educational objectives primarily through hands-on experience on a cadaveric or surrogate model, supplemented by web-based didactic instruction by surgeons experienced with Baxano’s products. For cadaveric training, Baxano utilizes third-party labs and has a state-of-the-art training facility at its office in San Jose, California.
Research and Development
Baxano’s research and development group develops new technologies and innovative products to address unmet clinical needs in the treatment of degenerative spinal diseases through minimally invasive approaches. Baxano’s research and development spending was $4.8 million and $5.2 millionfor the years ending December 31, 2012 and 2011, respectively. The major focus of the R&D group includes design enhancements to the iO-Flex platform and commercial development of the new Baxano iO-Tome product platform. The R&D team includes three experienced Engineers and a Sr. Technician. Engineering consulting services are utilized on an as needed basis. The current iO-Flex development activities focus on design enhancements to improve foraminal treatment rates, improve product’s ease of use, and reduce costs of goods through material cost reductions and reduced assembly time. iO-Tome development activities include engineering investments to make the iO-Tome a commercial success. Currently the iO-Tome is in beta release as Baxano obtains feedback from select surgeons and prepares for the necessary iterations for the anticipated full launch later in 2013. The engineering team works closely with key surgeons at all phases of development to improve the impact of the project investment. Baxano employs documented design control procedures, instructions, and forms to assure safety and performance of its medical devices throughout their product life-cycle from design, development, manufacturing, and distribution to post-market performance.
Manufacturing
Baxano is located in a 22,000 square-foot facility in San Jose, California and is a vertically integrated manufacturing organization consisting of 7,000 square feet of manufacturing space including a 2,500 square-foot Class 8 controlled environment room. All product manufacturing, including subassemblies, final assemblies, packaging, and product release, are performed at the San Jose facility. Product e-beam sterilization is outsourced and performed by an FDA-registered sterilization supplier who is certified to ISO 13485 and 11137. Baxano manages all supply chain activities and has partnered with primary and secondary suppliers to provide product components in support of iO-Flex and iO-Tome manufacturing. For critical sourced materials respective supplier agreements are in place.
Baxano controls and monitors outsourced component manufacturing through supply chain management (supplier selection, development, and approval process) and process control procedures.
Baxano is in compliance with the following domestic and international Regulations, Directive(s) and Quality Management System (QMS) Standards:
| · | U.S. Food and Drug Administration (FDA) 21 Code of Federal Regulations; |
| · | ISO 13485:2003 Quality systems - Medical devices - Quality management systems -Requirements for regulatory purposes; |
| · | Council Directive 93/42/EEC of 14 June 1993 concerning Medical Devices (MDD) as amended by council directive 2007/47/EEC; and |
| · | Medical Device Regulation (MDR) P.C. 1998-783, Health Canada Parts 1,2 &3 current Revision. |
| 112 |
Intellectual Property Rights
Baxano relies on a combination of patent, copyright, trademark, and other intellectual property laws, confidentiality agreements, and invention assignment agreements to protect its intellectual property rights. As of February 25, 2013, Baxano had 22 issued U.S. patents, eight issued foreign patents, 35 pending U.S. patent applications (including five allowed patent applications), and 31 pending foreign patent applications. The issued patents have expiration dates between 2025 and 2029. Expiration may occur earlier under certain circumstances, such as if Baxano does not continue to pay maintenance fees to the United States Patent and Trademark Office. Most of the issue and pending patents cover the Baxano iO-Flex system and method of use, including individual components of the iO-Flex system such as the MicroBlade Shaver and Neuro Check device. Baxano has not licensed any third-party intellectual property.
Baxano’s patent applications may not result in issued patents, and Baxano cannot give assurances that any patents that issue will provide a competitive advantage. Moreover, any patents issued to Baxano may be challenged by third parties as invalid or parties may independently develop similar or competing technology or design around any of Baxano’s patents. Baxano cannot be certain that the steps it has taken will prevent the misappropriation of its intellectual property, particularly in foreign countries where the laws may not protect its proprietary rights as fully as in the United States.
Baxano has 22 registered and three pending marks in the United States and three registered marks in the European Union. The primary marks include Baxano, iO-Flex, and iO-Tome.
Baxano requires its employees, consultants, and advisors to execute confidentiality agreements in connection with their employment, consulting, or advisory relationships with it. Baxano also requires them to agree to disclose and assign to Baxano all inventions conceived in connection with the relationship. Baxano cannot provide any assurance that employees, consultants, and advisors will abide by the confidentiality or assignment terms of their agreements. Despite measures taken to protect Baxano’s intellectual property, unauthorized parties may copy aspects of its products or obtain and use information that it regards as proprietary.
Competition
Baxano’s industry is subject to significant competition. Baxano competes directly against traditional generic instrumentation such as straight and curved Kerrison ronguers sold by a wide variety of surgical instrument manufacturers. In addition, several companies have entered the branded power instrument category. For example, Misonix Inc. has globally commercialized an ultrasonic bone cutting device called the BoneScalpel, which is designed for bone dissection, bone sculpting, and removal in small joint and spinal surgery. In addition, Baxano competes indirectly against existing and emerging treatment alternatives such as interspinous and interlaminar indirect decompression implants marketed by a variety of companies including VertiFlex and Medtronic. Baxano’s iO-Flex system may compete or be used in conjunction with these devices depending on the surgical objectives of the physician. Some of these devices may be less invasive or more familiar to surgeons than Baxano’s devices. Some of Baxano’s competitors are publicly-traded companies and others have significantly greater operating histories than Baxano does, and many of them may enjoy several competitive advantages, including:
| · | greater name recognition; |
| · | more extensive intellectual property protection; |
| · | established relationships with practitioners and other health care professionals; |
| · | established domestic and international distribution networks; |
| · | additional lines of products or existing treatment systems, and the ability to offer rebates or bundle products to offer higher discounts or incentives; |
| · | greater experience in conducting research and development, manufacturing, clinical trials, obtaining regulatory approval for products, and marketing approved products; and |
| 113 |
| · | greater financial resources for product development, sales and marketing, and patent litigation. |
To compete effectively, Baxano must spend significantly on sales and marketing activities and differentiate its products on the basis of performance, brand name, reputation, and price. Baxano has encountered and expects to continue to encounter potential customers who, due to existing relationships with Baxano’s competitors, are committed to or prefer the products offered by these competitors. Baxano expects that competitive pressures may result in price reductions and reduced margins over time for its products.
Government Regulation
Baxano’s products are medical devices subject to extensive regulation by the FDA and other U.S. federal and state regulatory bodies and comparable authorities in other countries. To ensure that medical products distributed domestically and internationally are safe and effective for their intended use, the FDA and comparable authorities in other countries have imposed regulations that govern, among other things, the following activities that Baxano performs and will continue to perform:
| · | product design and development; |
| · | registration and listing; |
| · | product testing (preclinical and clinical); |
| · | product manufacturing; |
| · | product labeling; |
| · | product storage; |
| · | premarket clearance or approval; |
| · | advertising and promotion; |
| · | product marketing, sales, and distribution; and |
| · | post-market surveillance, including reporting deaths or serious injuries related to products and certain product malfunctions. |
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device Baxano wishes to commercially distribute in the United States will require either prior 510(k) clearance or prior premarket approval from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risk are placed in either class I or II, which in many cases requires the manufacturer to submit to the FDA a premarket notification or 510(k) submission requesting permission for commercial distribution. This process is known as requesting 510(k) clearance. Some low risk devices are exempt from this requirement. Devices deemed by the FDA to pose the greatest risk, such as many life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a legally marketable device, are placed in class III, requiring a PMA. Premarket clearance applications are subject to the payment of user fees, paid at the time of submission for FDA review.
510(k) Clearance Pathway
To obtain 510(k) clearance, Baxano must submit a premarket notification demonstrating that the proposed device is substantially equivalent to a legally marketable device not requiring a PMA. Although statutorily mandated to respond to a 510(k) premarket notification within 90 days of submission of the application, the FDA’s 510(k) clearance pathway usually takes from three to 12 months, or even longer, depending on the extent of requests for additional information by the FDA. Additional information can include clinical data to make a determination regarding substantial equivalence.
| 114 |
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or change in its intended use, will require a new 510(k) clearance or, depending on the modification, require a PMA. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), or a PMA, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or a PMA is obtained. If the FDA requires Baxano to seek 510(k) clearance or a PMA for any modifications to a previously cleared product, Baxano may be required to cease marketing or recall the modified device until it obtains this clearance or approval. Also, in these circumstances, Baxano may be subject to significant regulatory fines or penalties. Baxano has made and plans to continue to make additional product enhancements to its products.
Clinical Trials
Clinical trials are sometimes required for a 510(k) premarket notification. In the U.S., these trials require submission of an application for an IDE unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified number of patients. Clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the appropriate institutional review boards at the clinical trial sites. Clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy and financial disclosure by clinical investigators. A clinical trial may be suspended by the FDA or the investigational review board at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the study. Even if a study is completed, the results of Baxano’s clinical testing may not demonstrate the safety and efficacy of the device, or may be equivocal or otherwise not be sufficient to obtain clearance or approval. Similarly, in Europe the clinical study must be approved by the local ethics committee and in some cases, including studies of high-risk devices, by the Competent Authority in the applicable country.
Extensive and Continuing FDA Regulation
After a device is placed on the market, numerous FDA and other regulatory requirements continue to apply. These include:
| · | quality system regulation, which requires manufacturers, including third-party contract manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance controls, during all aspects of the manufacturing process; |
| · | establishment registration and listing; |
| · | labeling regulations, and FDA prohibitions against the promotion of products for uncleared or unapproved “off-label” uses; |
| · | medical device reporting obligations, which require that manufacturers submit reports to the FDA if information reasonably suggests that their device (i) may have caused or contributed to a death or serious injury, or (ii) malfunctioned and the device or a similar company device would likely cause or contribute to a death or serious injury if the malfunction were to recur; and |
| · | other post-market surveillance requirements, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device. |
Baxano has registered with the FDA as a medical device manufacturer and has obtained a manufacturing license from the California Department of Health Services, or CDHS. Baxano must register and list with the FDA as a medical device manufacturer and must obtain all necessary state permits or licenses to operate its business. As a manufacturer, it is subject to announced and unannounced inspections by the FDA, including the manufacturing facilities of its subcontractors, to determine compliance with quality system regulation and other regulations. Baxano was inspected by the FDA in February 2011 with no Form 483 observations. Baxano believes that it is in substantial compliance with quality system regulation and other regulations.
| 115 |
Baxano’s iO-Flex System and iO-Tome System are regulated as a Class II medical device. Baxano has obtained ten 510(k) clearances to market these two device families and their accessories. Currently there are no pending 510(k) or PMA applications. The iO-Flex system had a 0.8% reportable adverse event rate to the FDA in the fourth quarter of 2012. This adverse event rate is much lower than published adverse event rates for similar decompression procedures. Types of adverse events associated with iO-Flex use included: durotomy, neuropathy, and hematoma.
International
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ.
The European Union, which consists of 27 of the major countries in Europe, has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling, and adverse event reporting for medical devices. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking and, accordingly, can be commercially distributed throughout the member states of the European Union, and other countries that comply with or mirror these directives. The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body,” an independent and neutral institution appointed to conduct conformity assessments. This third-party assessment consists of audits of the manufacturer’s quality system for conformance to applicable quality system standards such as ISO 13485. An assessment by a Notified Body in one country within the European Union is required for each product in order for a manufacturer to commercially distribute the product throughout the European Union. Compliance of the Quality System with ISO 13845 and compliance of each product with the Medical Device Directive (MDD93/42/EEC as amended by directive 2007/47EC) establishes the presumption of conformity with the essential requirements for a CE mark. In August 2004, Baxano’s quality system was initially certified by Intertek ETL-Semko, a Notified Body, under the European Union Medical Device Directive to be in compliance with the Canadian Medical Device Conformity Assessment System, or CMDCAS, and ISO standards 9001:2000 and ISO 13485:2003. The system was recertified in September 2010 to CMDCAS, ISO 9001:2008 and ISO 13485:2003 and is due for further recertification in September of 2013.
Baxano obtained CE mark approval for iO-Flex system which includes the iO-Tome product in February 2012. Also in February 2012, Baxano obtained device license (license # 88060) from Health Canada.
Employees
As of February 26, 2013, Baxano had 74 full-time employees and nopart-time employees.
Facilities
Baxano is headquartered in San Jose, California, where it leases approximately 22,000 square feet under a lease expiring on December 9, 2014.
Management’s Discussion and Analysis ofFinancial Condition and Results of Operations
The following discussion of Baxano’s financial condition and results of operations should be read in conjunction with Baxano’s financial statements and the related notes to Baxano’s financial statements included in this Proxy Statement. The following discussion contains forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements” on page 31 of this Proxy Statement.
| 116 |
Overview
Baxano is a medical device company focused on designing, developing, and marketing products that implement its proprietary minimally invasive surgical instruments in treating stenosis affecting the lumbar region of the spine. Using the iO-Flex system, a surgeon employs small flexible instruments to access, localize, and treat stenosis through targeted direct decompression of soft and osseous tissue. The iO-Flex system allows for decompression of up to four nerve roots through a single point of access. Baxano believes these instruments enable decompression procedures, both with and without fusion, to be performed with minimal disruption to healthy tissue, low complication rates, short procedure times, low blood loss, short hospital stays, fast recovery times, and reduced pain. Baxano has developed and currently markets in the United States the iO-Flex system for direct decompression. In addition, Baxano is planning for initial commercial launch of its new iO-Tome facetectomy device for fusion procedures in the fourth quarter of 2013. All of Baxano’s products leverage its proprietary minimally invasive technology allowing targeted direct decompression and facetectomy from the inside out.
From Baxano’s incorporation in 2005 through 2009, Baxano devoted substantially all of its resources to research and development and start-up activities, consisting primarily of product design and development, clinical trials, manufacturing, recruiting qualified personnel, and raising capital. Baxano received 510(k) clearance for its iO-Flex product in the second quarter of 2007, and commercially introduced the iO-Flex system in the United States in the fourth quarter of 2009. Baxano further received 510(k) clearance for its iO-Tome facetectomy device in the third quarter of 2012, and early commercialization activities are currently underway. Baxano received CE mark and Health Canada approvals in the first quarter of 2012 to market iO-Flex in the European and Canadian markets, respectively. Baxano currently sells its products exclusively in the United States through a direct sales force and national network of independent sales agents.
Baxano currently manufactures its products in its San Jose, California facility and sources components through a variety of third party suppliers. Baxano believes these vendor relationships allow it to work with suppliers that have the best specific competencies while minimizing its capital investment, controlling costs, and shortening cycle times. Since inception, Baxano has been unprofitable. As of December 31, 2012, Baxano had an accumulated deficit of $70.4 million.
Baxano expects to continue to invest in creating a sales and marketing infrastructure for its iO-Flex and iO-Tome products in order to gain wider acceptance for these products. Baxano also expects to continue to invest in research and development and related clinical trials and to increase general and administrative expenses as it grows. As a result, Baxano will need to generate significant revenue in order to achieve profitability.
Financial Operations
Revenue
Baxano generates revenue from the sales of its disposable iO-Flex devices used in minimally invasive decompression for the treatment of lumbar spinal stenosis. Baxano’s revenue is generated by its direct sales force, independent sales agents, and independent distributors. In the United States, Baxano’s procedure kits are shipped from inventories at its facility to its direct sales force or independent sales agents for delivery to the hospital or surgical center. Baxano invoices hospitals and surgical centers and revenue is primarily recognized upon notification of product use and receipt of customer purchase order. For some hospital accounts, Baxano also delivers devices to be inventoried at the hospital for later use in a surgical procedure, recognizing revenue at the time of delivery, which represented 2% of its revenues in 2012. Baxano is focused on commercialization inside the United States and has never generated revenue outside the United States.
Cost of Revenue
Cost of revenue consists primarily of direct material, direct labor, and manufacturing overhead costs related to Baxano’s iO-Flex system instruments. Cost of revenue also includes certain period costs including product sterilization and manufacturing scrap, as well as expenses associated with inventory standard cost revaluation and adjustments for inventory obsolescence, excess, and shortage.
| 117 |
Research and Development
Research and development expenses consist primarily of personnel costs, including stock-based compensation expense, within Baxano’s product development, regulatory, clinical, and quality systems functions and the costs of clinical studies and product development projects. Research and development expenses also include facilities-related costs. In future periods, Baxano expects research and development expenses to remain relatively constant as it maintains current investment levels in product development, clinical trials, and quality systems activities.
Sales and Marketing
Sales and marketing expenses consist of personnel costs, including stock-based compensation expense, sales commissions paid to Baxano’s direct sales representatives and independent sales agents, and costs associated with physician training programs, educational activities, and participation in medical conferences. In future periods, Baxano expects sales and marketing expenses to increase as it expands its sales, marketing, and professional education efforts.
General and Administrative
General and administrative expenses consist of personnel costs, including stock-based compensation, related to the executive, finance, information technology, and human resource functions, as well as professional service fees, legal fees, accounting fees, insurance costs, and general corporate expenses. Baxano expects general and administrative expenses to increase as it grows its business.
Other and Interest Income (Expense)
Interest income is primarily composed of interest earned on Baxano’s cash, cash equivalents, and available-for-sale securities. Interest expense represents Baxano’s cost of employing debt capital, such as convertible promissory notes and term loans. Other expenses include warrant revaluation, reserves for doubtful accounts, and certain tax related expenses.
Results of Operations
Comparison of the Years Ended December 31, 2012 and 2011
Revenue. Revenue increased to $9.4 million in 2012 from $3.9 million in 2011. This increase in revenue was primarily attributable to an increase in the number of products sold, which Baxano believes resulted from the continued market acceptance of its iO-Flex products. Approximately 5% of this annual sales growth was attributable to price increases. Sales of Baxano’s iO-Flex products, which began commercialization in the United States in the fourth quarter of 2009, increased from 1,190 cases in 2011 to 2,700 cases in 2012. Average selling prices in the United States increased from approximately $3,300 in 2011 to approximately $3,500 in 2012.
Cost of Revenue. Cost of revenue increased to $3.3 million in 2012 from $2.0 million in 2011. The $1.3 million increase was primarily the result of higher material, direct labor, and overhead costs associated with increased sales volumes of Baxano’s iO-Flex products. As a percentage of revenue, cost of revenue was 34.6% in 2012 and 51.5% in 2011. The decrease in cost of revenue as a percentage of revenue was primarily attributable to increased efficiencies associated with higher production and sales volumes.
Research and Development. Research and development expenses decreased to $4.8 million in 2012 from $5.2 million in 2011. The $458,000 decrease in expense was primarily the result of decreases in clinical and animal study costs of $320,000, reduced expenses for outside consultants of $115,000, and reduced personnel related costs, including stock-based compensation expense, of $84,000, partially offset by higher inventory withdrawals associated with ongoing development projects.
Sales and Marketing. Sales and marketing expenses increased to $17.2 million in 2012 from $10.6 million in 2011. The increase in expense of $6.6 million was primarily attributable to increased personnel related costs, including commissions and stock-based compensation expense of $3.7 million, higher travel and entertainment expenses of $610,000, increased marketing collateral and communications costs of $650,000, higher consulting costs of $186,000, and increased commissions paid to distributor agents on growing sales volumes of $1.4 million, as Baxano continued to build out its sales and marketing organization in order to continue to drive market acceptance of its iO-Flex products.
General and Administrative. General and administrative expenses increased to $2.5 million in 2012 from $2.3 million in 2011. The increase in expenses of $157,000 was primarily attributable to increased personnel related costs, including stock-based compensation expenses of $125,000, and increased professional fees associated with audit, tax, and legal support of $60,000, partially offset by lower travel, entertainment, and meeting expenses.
| 118 |
Other and Interest Income (Expense). Other and interest expense increased to $580,000 in 2012 from $7,000 in 2011. The increase of $573,000 in other and interest expense was primarily due to higher interest expense on convertible promissory notes and term loans issued during 2012 of $1.0 million, offset partially by other income on the non-cash revaluation of warrants issued with the convertible promissory notes and term debt of $420,000.
Liquidity and Capital Resources
Sources of Liquidity
Since its inception in 2005, Baxano has incurred significant losses and, as of December 31, 2012, Baxano had an accumulated deficit of $70.4 million. Baxano has not yet achieved profitability and anticipates that it will continue to incur losses for the foreseeable future. Baxano expects that research and development, sales and marketing, and general and administrative expenses will continue to grow and, as a result, Baxano will need to generate significant revenues to achieve profitability. Baxano’s recurring losses raise substantial doubt about its ability to continue as a going concern. As a result, Baxano’s independent registered public accounting firm included an explanatory paragraph in its report on Baxano’s financial statements as of and for the year ended December 31, 2012 with respect to this uncertainty. To date, Baxano’s operations have been funded primarily with proceeds from the sale of preferred stock and, most recently, from issuance of convertible promissory notes and term loans. Gross proceeds from preferred stock sales totaled $58.6 million to date, and gross proceeds from Baxano’s March 2012 and October 2012 convertible promissory note issuances were approximately $14.8 million.
As of December 31, 2012, Baxano had $17.8 million in outstanding debt financing arrangements, inclusive of $14.8 million in convertible promissory notes. As of December 31, 2012, Baxano had negative working capital of $11.2 million, and its primary source of liquidity was $4.4 million in cash, cash equivalents, and investments. Baxano currently invests its cash and cash equivalents primarily in money market funds.
Cash and cash equivalents decreased from $6.4 million at December 31, 2011 to $4.4 million at December 31, 2012. The decrease of $2.0 million was primarily the result of cash used in operations of $19.7 million and purchases of property and equipment of $142,000, partially offset by the net proceeds to Baxano from its March and October promissory notes and term loan issuances of approximately $17.8 million.
Cash Flows
Net Cash Used in Operating Activities. Net cash used in operating activities was $19.7 million in 2012 and $16.3 million in 2011. For each of these periods, net cash used in operating activities was attributable primarily to net losses after adjustment for non-cash items, such as depreciation and stock-based compensation expense, and increases in working capital requirements to support the introduction and increased market acceptance of Baxano’s iO-Flex products. The increases in working capital requirements for each of the periods were driven by the growth in inventories to support forecasted demand and the increases in accounts receivable resulting from increasing revenues exceeding cash inflow from customer collections.
Net Cash Used in Investing Activities. Net cash used in investing activities was $142,000 in 2012 and $280,000 in 2011. For each of these periods, net cash used in investing activities reflected the purchases of property and equipment, primarily for research and development, information technology, manufacturing operations, and capital improvements to Baxano’s facilities.
Net Cash Provided by Financing Activities. Net cash provided by financing activities in 2012 was $17.8 million. Net cash used in financing activities in 2011 was $221,000. The $17.8 million in net cash provided by financing activities in 2012 was due primarily to the proceeds to Baxano of its March and October issuances of convertible promissory notes of $14.8 million, a March term loan placement of $2.9 million, and $29,000 in net proceeds from the issuance of shares of common stock upon the exercise of stock options, partially offset by the repurchase of restricted common shares acquired through early exercise of stock options from terminating employees. The $221,000 in net cash used by financing activities in 2011 was due to the final payment of $289,000 on a 2007 term loan partially offset by $68,000 in proceeds from the issuance of shares of common stock upon the exercise of stock options.
| 119 |
Operating Capital and Capital Expenditure Requirements
Baxano believes that its existing cash and cash equivalents, together with cash received from sales of its products, will be sufficient only to meet its cash needs through the first quarter of 2013. Baxano intends to spend substantial sums on sales and marketing initiatives to support the ongoing commercialization of its products and on research and development activities, including product development, regulatory and compliance, clinical studies in support of its currently marketed products and future product offerings, and the enhancement and protection of its intellectual property. Baxano will need to obtain additional financing to pursue its business strategy, to respond to new competitive pressures, or to take advantage of opportunities that may arise. The sale of additional equity or convertible debt securities could result in dilution to its stockholders. If additional funds are raised through the issuance of debt securities, these securities could have rights senior to those associated with Baxano’s common stock and could contain covenants that would restrict its operations. Any additional financing may not be available in amounts or on terms acceptable to Baxano. If Baxano is unable to obtain this additional financing, it may be required to reduce the scope of its planned product development and marketing efforts.
Contractual Obligations
The following table discloses information about Baxano’s contractual obligations by the year in which payments are due as of December 31, 2012:
Payments Due by Year
(in thousands)
| Contractual Obligations | Total | Less Than 1 Year | 1-3 Years | 4-5 Years | After 5 Years | |||||||||||||||
| Operating leases(1) | $ | 507 | $ | 259 | $ | 248 | $ | — | $ | — | ||||||||||
| Convertible Promissory Notes | $ | 16,084 | $ | 16,084 | $ | — | $ | — | $ | — | ||||||||||
| Term Loan | $ | 3,528 | $ | 1,031 | $ | 2,497 | $ | — | $ | — | ||||||||||
(1) Includes separate lease commitments for rental of office space and copiers, both expiring in 2014.
Off-Balance Sheet Arrangements
As of December 31, 2012, Baxano did not have any off-balance sheet liabilities.
Critical Accounting Policies and Estimates
Baxano’s discussion and analysis of its financial condition and results of operations is based upon its financial statements, which have been prepared in accordance with GAAP. The preparation of financial statements requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, revenue and expenses, and disclosures of contingent assets and liabilities at the date of the financial statements. On an on-going basis, Baxano evaluates its estimates, including those related to inventories, revenue recognition, income taxes, and stock-based compensation. Baxano uses authoritative pronouncements, historical experience, and other assumptions as the basis for making estimates. Actual results could differ from those estimates under different assumptions or conditions.
Baxano believes the following critical accounting policies affect its more significant judgments and estimates used in the preparation of its financial statements.
Inventory and Cost of Goods Sold. Inventories are stated at the lower of cost or market value. Cost is determined using standard cost, which approximates actual costs on a first-in, first-out basis. Market value is determined as the lower of replacement cost or net realizable value. Baxano periodically assesses the recoverability of all inventories to determine whether adjustments for impairment are required. Baxano evaluates the remaining shelf life and related commercial mix of inventories and other general obsolescence and impairment criteria in assessing the recoverability of its inventory.
| 120 |
Revenue Recognition. Baxano earns revenue from product sales to hospitals and surgery centers whose physicians perform spinal decompression procedures using Baxano’s device. Baxano generates all of its revenue from sales in the United States. Baxanorecognizes revenues from product sales, in accordance with ASC 605,Revenue Recognition, upon shipment if evidence of an arrangement exists, the fee is fixed and determinable, collection of resulting receivables is reasonably assured, and title and risk of loss has passed. If those criteria are not met, then revenue is not recognized until the criteria are satisfied.
For product revenues generated via direct sales, Baxano uses customer purchase orders to determine the existence of an arrangement. Baxano uses direct delivery by its sales representatives to prove that delivery has occurred. Baxano assesses whether the price is fixed or determinable based on the terms of the agreement associated with the transaction.
Income Taxes. Baxanoaccounts for income taxes under the liability method, whereby deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
Baxano operates in various tax jurisdictions and is subject to audit by various tax authorities. Baxano provides for tax contingencies whenever it is deemed probable that a tax asset has been impaired or a tax liability has been incurred for events such as tax claims or changes in tax laws. Tax contingencies are based upon their technical merits, relative tax law, and the specific facts and circumstances as of each reporting period. Changes in facts and circumstances could result in material changes to the amounts recorded for such tax contingencies.
Baxano records uncertain tax positions in accordance with accounting standards on the basis of a two-step process whereby (1) a determination is made as to whether it is more likely than not that the tax positions will be sustained based on the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold, Baxano recognizes the largest amount of tax benefit that is greater than 50% likely to be realized upon ultimate settlement with the related tax authority.
Accounting for Stock-Based Compensation. Baxanoaccounts for stock-based compensation in accordance with ASC 718,Compensation-Stock Compensation, or ASC 718. ASC 718 requires the recognition of compensation expense, using a fair-value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option pricing model. All option grants valued since inception are expensed on a straight-line basis over the requisite service period.
Baxano accounts for equity instruments issued to nonemployees in accordance with ASC 505-50,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. Equity instruments issued to nonemployees are recorded at their fair value on the measurement date and are subject to periodic adjustment as the underlying equity instruments vest.
Recent Accounting Pronouncements
In June 2011, the FASB issued a new standard on the presentation of comprehensive income that is effective for Baxano’s 2012 financial statements. The new standard eliminated the alternative to report other comprehensive income and its components in the statement of changes in equity. Under the new standard, companies can elect to present items of net income and other comprehensive income in one continuous statement or in two separate, but consecutive statements. The new standard did not have an impact on Baxano’s financial statements because Baxano does not have items of other comprehensive income to report.
In December 2011, the FASB issued ASU No. 2011-11,Balance Sheet (Topic 210). This update provides enhanced disclosure requirements regarding the nature of an entity’s right of offset related to arrangements associated with its financial instruments and derivative instruments. The new guidance requires the disclosure of the gross amounts subject to rights of set-off, the amounts offset in accordance with the accounting standards followed, and the related net exposure. This pronouncement is effective for financial reporting periods beginning on or after January 1, 2013 and full retrospective application is required. Baxano does not expect that the adoption of this accounting standard update will have a material impact on its consolidated financial statements.
No other recently issued, but not yet effective, accounting standards are believed to have a material impact on Baxano.
| 121 |
MarketPrice of and Dividends on Baxano’s Common Equity and Related Stockholder Matters
Baxano has never issued a dividend and its equity has never been publicly traded.
As of March 1, 2013, Baxano had approximately 69 stockholders of record.
PROPOSAL NO. 3
AMENDMENT TO OUR AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION TO CHANGE OUR NAME
On March 7, 2013, our Board of Directors adopted a resolution approving an amendment to our Amended and Restated Certificate of Incorporation to change the name of the Company from TranS1 Inc. to Baxano Surgical, Inc. and recommending that the name change amendment be submitted to our stockholders for approval.
We were formed to develop and commercialize a novel solution for lumbar fusions at the L5/S1 junction. Because “TranS” was meant to represent the trans-sacral access of this technology, and “S1” was meant to refer to spine anatomy, the name TranS1 will no longer be synonymous with our business activity post-Merger, which will be a broader company focused on lumbar fusion, spinal decompression, and other solutions for spine surgeons. The new name leverages the positive brand recognition and past investment in brand development, product packaging, and marketing collateral of Baxano, which will allow us to extend existing relationships with Baxano customers, who are primarily minimally–invasive spine surgeons, in a cost effective manner. Our Board of Directors believes that the name change is advisable and in the best interests of our company and of our stockholders.
If the name change amendment is approved by our stockholders, our name change will be effective when the amendment to the Amended and Restated Certificate of Incorporation is filed with the Secretary of State of the State of Delaware. We have reserved the NASDAQ stock symbol “BAXS.” If the name change amendment is approved, we intend to request that our common stock be traded on the NASDAQ Global Market under this new stock symbol, rather than under the current “TSON” symbol. Article 1 of our Amended and Restated Certificate of Incorporation, as proposed to be amended, will read in its entirety as follows:
ARTICLE 1
The name of the Corporation is Baxano Surgical, Inc.
If the name change becomes effective, the rights of stockholders holding certificated shares under currently outstanding stock certificates and the number of shares represented by those certificates will remain unchanged. The name change will not affect the validity or transferability of any currently outstanding stock certificates nor will it be necessary for stockholders with certificated shares to surrender or exchange any stock certificates they currently hold as a result of the name change. Uncertificated shares currently held in direct registration accounts and any new stock certificates that are issued after the name change becomes effective will bear the name “Baxano Surgical, Inc.”
At the Special Meeting, our stockholders will also be voting on Proposal Nos. 1 and 2, a proposal to issue shares of our common stock pursuant to the Merger Agreement and a proposal to issue shares of our common stock pursuant to the Securities Purchase Agreement, respectively. This name change proposal is contingent upon the results of the stockholder votes on Proposal Nos. 1 and 2. In addition, this name change proposal is contingent upon the consummation of the Merger. Accordingly, our name will be changed to Baxano Surgical, Inc. only if (1) each of the name change amendment, Proposal No. 1, and Proposal No. 2 are approved by our stockholders and (2) the Merger is consummated.
Approval of the amendment to our Amended and Restated Certificate of Incorporation to change our name to Baxano Surgical, Inc. will require the affirmative vote of a majority of the shares of our common stock outstanding as of the Record Date. Abstentions and broker non-votes, if any, will have the same effect as votes against the proposal.
OUR BOARD (EXCEPT FOR MR. SHAPIRO) UNANIMOUSLY RECOMMENDS A VOTE “FOR” APPROVAL OF AN AMENDMENT TO OUR AMENDED AND RESTATED CERTIFICATE OF INCORPORATION TO CHANGE OUR NAME TO BAXANO SURGICAL, INC.
| 122 |
PROPOSAL NO. 4
POTENTIALADJOURNMENT OF THE SPECIAL MEETING
This proposal would give the proxy holders discretionary authority to vote to adjourn the Special Meeting, for a period of not more than 30 days, if there are not sufficient affirmative votes present at the Special Meeting to approve Proposal No. 1, Proposal No. 2, and/or Proposal No. 3. Any adjournment of the Special Meeting may be made without notice, other than by an announcement made at the Special Meeting. Approval of this proposal will allow the Company to solicit additional proxies to approve Proposal No. 1, Proposal No. 2, and/or Proposal No. 3. If the Company is unable to adjourn the Special Meeting to solicit additional proxies, any or all of Proposal No. 1, Proposal No. 2, and/or Proposal No. 3 may fail because there were not sufficient shares represented at the Special Meeting to approve the proposals. We have no reason to believe that an adjournment of the Special Meeting will be necessary at this time.
Approval of the proposal to grantthe proxy holders discretionary authority to vote to adjourn the Special Meetingwill require the affirmative vote of a majority of the shares present or represented by proxy at the Special Meeting and entitled to vote on the matter. Abstentions andbroker non-votes, if any, will have the same effect as votes against the proposal.
OURBOARD (EXCEPT FOR MR. SHAPIRO) UNANIMOUSLY RECOMMENDS A VOTE “FOR” THE potential adjournment of the Special Meeting.
OTHERMATTERS
As of the date of this proxy statement, our Board of Directors knows of no matters other than those referred to in the accompanying notice of special meeting of stockholders which may properly come before the Special Meeting. However, if any other matter should be properly presented for consideration and voting at the Special Meeting or any adjournments or postponements thereof, it is the intention of the named proxy holders to vote the shares represented by all valid proxy cards in accordance with their judgment of what is in our best interest.
STOCKHOLDERPROPOSALS
SEC rules and our bylaws require that a stockholder provide us with timely advance written notice when seeking to nominate a candidate for election as a director or to propose other actions for consideration at an annual meeting of stockholders. To be timely, a stockholder’s notice must comply with the following:
Inclusion of Proposals in our Proxy Statement and Proxy Card under the SEC’s Rules. In order to include information with respect to a stockholder proposal in ourproxy statementand form of proxy for a stockholders’ meeting, stockholders must comply with the requirements of Rule 14a-8 of the Exchange Act and any other applicable rules established by the SEC. Under Rule 14a-8, a stockholder’s proposal must be received at our principal executive offices not less than 120 calendar days prior to the first anniversary of the date ourproxy statementwas released to stockholders in connection with the preceding year’s annual meeting. Thus, in order for a stockholder’s proposal to be considered for inclusion in ourproxy statementand proxy card for the 2013 annual meeting, the proposal must have been received at our corporate offices on orbefore December 31, 2012, unless the date of our 2013 annual meeting is more than 30 days before or after June 5, 2013, in which case the proposal must be received a reasonable time before we begin to print and mail our proxy materials.
| 123 |
Requirements for Stockholder Submission of Nominations and Proposals. A stockholder recommendation for nomination of a person for election to ourBoard of Directorsor a proposal for consideration at our 2013 annual meeting must be submitted in accordance with the advance notice procedures and other requirements set forth in Article II of our bylaws. These requirements are separate from, and in addition to, the requirements discussed above to have the stockholder nomination or other proposal included in ourproxy statementand form of proxy pursuant to the SEC’s rules. Compliance with our bylaw requirements will entitle the proposing stockholder only to present such nominations or proposals before the stockholder meeting, not to have the nominations or proposals included in ourproxy statementor proxy card. Any such nomination or proposal may be made only by persons who are stockholders of record on the date on which such notice is given and on the record date for determination of stockholdersentitled to vote at that meeting. In addition, no such nomination or proposal may be brought before an annual meeting by a stockholder unless that stockholder has given timely written notice in proper form of such nomination or proposal to our Corporate Secretary, as more particularly set forth in Article II of our bylaws. Any stockholder desiring to submit a nomination or proposal for action at our 2013 annual meeting of stockholders should have delivered the proposal to our Corporate Secretary at our corporate office at TranS1 Inc.,110 Horizon Drive, Suite 230, Raleigh, NC 27615, no earlier than February 5, 2013 and no later than March 7, 2013, in order to be presented at the 2013 annual meeting of stockholders. The full text of the bylaw provisions referred to above (which also set forth requirements and limitations as to stockholder nominations or proposals to be considered at any special meeting) may be obtained by contacting our Corporate Secretary.
| 124 |
INDEX TO FINANCIAL STATEMENTS
| TranS1 Financial Statements | |
| Report of Independent Registered Public Accounting Firm | 126 |
| Consolidated Balance Sheets—December 31, 2012 and 2011 | 127 |
| Consolidated Statements of Operations for the years ended December 31, 2012, 2011, and 2010 | 128 |
| Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2012, 2011, and 2010 | 129 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2012, 2011, and 2010 | 130 |
| Notes to Consolidated Financial Statements for the years ended December 31, 2012, 2011, and 2010 | 131 |
| Schedule II—Valuation and Qualifying Accounts | 144 |
| Baxano Financial Statements | |
| Report of Independent Registered Public Accounting Firm | 145 |
| Consolidated Balance Sheets—December 31, 2012 and 2011 | 146 |
| Consolidated Statements of Operations for the years ended December 31, 2012 and 2011 | 147 |
| Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2012 and 2011 | 148 |
| Consolidated Statements of Cash Flows for the years ended December 31, 2012 and 2011 | 149 |
| Notes to Consolidated Financial Statements for the years ended December 31, 2012 and 2011 | 150 |
| 125 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of TranS1 Inc.
In our opinion, the consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of TranS1, Inc. and its subsidiaries at December 31, 2012 and 2011, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2012 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. These financial statements and financial statement schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses and negative cash flows from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regards to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ PricewaterhouseCoopers LLP | |
| Raleigh, NC | |
| March 6, 2013 |
| 126 |
TranS1 Inc.
Consolidated Balance Sheets
(In thousands, except share amounts)
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 21,541 | $ | 38,724 | ||||
| Short-term investments | - | 6,027 | ||||||
| Accounts receivable, net | 3,206 | 2,522 | ||||||
| Inventory | 5,017 | 4,525 | ||||||
| Prepaid expenses and other assets | 330 | 680 | ||||||
| Total current assets | 30,094 | 52,478 | ||||||
| Property and equipment, net | 2,166 | 1,554 | ||||||
| Total assets | $ | 32,260 | $ | 54,032 | ||||
| Liabilities and Stockholders' Equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 2,603 | $ | 3,127 | ||||
| Accrued expenses related to U.S. Government settlement | 6,792 | 180 | ||||||
| Accrued expenses | 1,648 | 1,199 | ||||||
| Total current liabilities | 11,043 | 4,506 | ||||||
| Noncurrent liabilities | 78 | 26 | ||||||
| Commitments and contingencies (Note 5) | ||||||||
| Stockholders' equity: | ||||||||
| Preferred stock, $0.0001 par value; 5,000,000 shares authorized, none issued and outstanding at December 31, 2012 and 2011. | - | - | ||||||
| Common stock, $0.0001 par value; 75,000,000 shares authorized, 27,313,997 and 27,244,059 shares issued and outstanding at December 31, 2012 and 2011, respectively | 3 | 3 | ||||||
| Additional paid-in capital | 159,929 | 158,403 | ||||||
| Accumulated other comprehensive income | 14 | 13 | ||||||
| Accumulated deficit | (138,807 | ) | (108,919 | ) | ||||
| Total stockholders' equity | 21,139 | 49,500 | ||||||
| Total liabilities and stockholders' equity | $ | 32,260 | $ | 54,032 | ||||
The accompanying notes are an integral part of theseconsolidatedfinancial statements.
| 127 |
TranS1 Inc.
Consolidated Statements of Operations
(in thousands, except per share amounts)
| Year Ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| Revenue | $ | 14,570 | $ | 19,153 | $ | 26,154 | ||||||
| Cost of revenue | 4,309 | 4,555 | 7,104 | |||||||||
| Gross profit | 10,261 | 14,598 | 19,050 | |||||||||
| Operating expenses: | ||||||||||||
| Research and development | 5,457 | 5,191 | 4,223 | |||||||||
| Sales and marketing | 20,049 | 21,561 | 26,275 | |||||||||
| General and administrative | 6,172 | 5,890 | 8,565 | |||||||||
| Charges related to U.S. Government settlement | 8,324 | 235 | - | |||||||||
| Total operating expenses | 40,002 | 32,877 | 39,063 | |||||||||
| Operating loss | (29,741 | ) | (18,279 | ) | (20,013 | ) | ||||||
| Other income (expense), net | (147 | ) | 6 | 486 | ||||||||
| Net loss | $ | (29,888 | ) | $ | (18,273 | ) | $ | (19,527 | ) | |||
| Other comprehensive loss: | ||||||||||||
| Foreign currency translation adjustments | 1 | 42 | (24 | ) | ||||||||
| Comprehensive loss | $ | (29,887 | ) | $ | (18,231 | ) | $ | (19,551 | ) | |||
| Net loss per common share – basic and diluted | $ | (1.10 | ) | $ | (0.81 | ) | $ | (0.94 | ) | |||
| Weighted average common shares outstanding – basic and diluted | 27,267 | 22,588 | 20,738 | |||||||||
The accompanying notes are an integral part of theseconsolidatedfinancial statements.
| 128 |
TranS1 Inc.
Consolidated Statements of Stockholders’ Equity
(in thousands, except share data)
| Accumulated | ||||||||||||||||||||||||
| Additional | Other | Total | ||||||||||||||||||||||
| Common Stock | Paid - In | Comprehensive | Accumulated | Stockholders' | ||||||||||||||||||||
| Number | Amount | Capital | Income (Loss) | Deficit | Equity | |||||||||||||||||||
| Balance at December 31, 2009 | 20,648,447 | $ | 2 | $ | 136,402 | $ | (5 | ) | $ | (71,119 | ) | $ | 65,280 | |||||||||||
| Issuance of common stock from exercised options | 228,724 | - | 148 | - | - | 148 | ||||||||||||||||||
| Stock based compensation | - | - | 1,851 | - | - | 1,851 | ||||||||||||||||||
| Other comprehensive loss | - | - | - | (24 | ) | - | (24 | ) | ||||||||||||||||
| Net loss | - | - | - | - | (19,527 | ) | (19,527 | ) | ||||||||||||||||
| Balance at December 31, 2010 | 20,877,171 | 2 | 138,401 | (29 | ) | (90,646 | ) | 47,728 | ||||||||||||||||
| Issuance of common stock from exercised options | 104,498 | - | 111 | - | - | 111 | ||||||||||||||||||
| Issuance of common stock from equity financing | 6,200,000 | 1 | 18,175 | - | - | 18,176 | ||||||||||||||||||
| Issuance of common stock from employee stock purchase plan | 62,390 | - | 158 | - | - | 158 | ||||||||||||||||||
| Stock based compensation | - | - | 1,558 | - | - | 1,558 | ||||||||||||||||||
| Other comprehensive income | - | - | - | 42 | - | 42 | ||||||||||||||||||
| Net loss | - | - | - | - | (18,273 | ) | (18,273 | ) | ||||||||||||||||
| Balance at December 31, 2011 | 27,244,059 | 3 | 158,403 | 13 | (108,919 | ) | 49,500 | |||||||||||||||||
| Issuance of common stock from exercised options | 31,294 | - | 48 | - | - | 48 | ||||||||||||||||||
| Issuance of common stock from employee stock purchase plan | 38,644 | - | 112 | - | - | 112 | ||||||||||||||||||
| Stock based compensation | - | - | 1,366 | - | - | 1,366 | ||||||||||||||||||
| Other comprehensive income | - | - | - | 1 | - | 1 | ||||||||||||||||||
| Net loss | - | - | - | - | (29,888 | ) | (29,888 | ) | ||||||||||||||||
| Balance at December 31, 2012 | 27,313,997 | $ | 3 | $ | 159,929 | $ | 14 | $ | (138,807 | ) | $ | 21,139 | ||||||||||||
The accompanying notes are an integral part of theseconsolidatedfinancial statements.
| 129 |
TranS1 Inc.
Consolidated Statements of Cash Flows
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (29,888 | ) | $ | (18,273 | ) | $ | (19,527 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 1,141 | 662 | 746 | |||||||||
| Stock-based compensation | 1,366 | 1,558 | 1,851 | |||||||||
| Allowance for excess and obsolete inventory | 524 | 521 | 2,004 | |||||||||
| Provision (reversal of provision) for bad debts | (15 | ) | 31 | 226 | ||||||||
| Loss on sale of fixed assets | 261 | 54 | 70 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| (Increase) decrease in accounts receivable | (669 | ) | 1,101 | 46 | ||||||||
| (Increase) decrease in inventory | (1,015 | ) | (1,168 | ) | 1,443 | |||||||
| (Increase) decrease in prepaid expenses | 350 | (291 | ) | 287 | ||||||||
| Increase (decrease) in accounts payable | (524 | ) | 913 | (228 | ) | |||||||
| Increase in accrued expenses related to U.S. Government settlement | 6,612 | 180 | - | |||||||||
| Increase (decrease) in accrued expenses | 500 | (852 | ) | 808 | ||||||||
| Net cash used in operating activities | (21,357 | ) | (15,564 | ) | (12,274 | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Purchases of property and equipment | (2,014 | ) | (708 | ) | (565 | ) | ||||||
| Purchases of investments | - | (16,102 | ) | (18,050 | ) | |||||||
| Sales and maturities of investments | 6,027 | 28,150 | 25,928 | |||||||||
| Net cash provided by investing activities | 4,013 | 11,340 | 7,313 | |||||||||
| Cash flows from financing activities: | ||||||||||||
| Net proceeds from issuance of common stock | 48 | 18,334 | - | |||||||||
| Proceeds from exercise of stock options | 112 | 111 | 148 | |||||||||
| Net cash provided by financing activities | 160 | 18,445 | 148 | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | 1 | 42 | (24 | ) | ||||||||
| Net increase (decrease) in cash and cash equivalents | (17,183 | ) | 14,263 | (4,837 | ) | |||||||
| Cash and cash equivalents, beginning of period | 38,724 | 24,461 | 29,298 | |||||||||
| Cash and cash equivalents, end of period | $ | 21,541 | $ | 38,724 | $ | 24,461 | ||||||
The accompanying notes are an integral part of theseconsolidatedfinancial statements.
| 130 |
TranS1 Inc.
Notes to Consolidated Financial Statements
| 1. | Organization |
TranS1 Inc., a Delaware corporation (the “Company”), was incorporated in May 2000 and, effective March 1, 2013, is headquartered in Raleigh, North Carolina. The Company is a medical device company focused on designing, developing and marketing products to treat degenerative conditions of the spine affecting the lumbar region. The Company operates in one business segment.The Company currently markets the AxiaLIF® family of products for single and two level lumbar fusion,the VEOTM lateral access and interbody fusion system, the Vectre™ lumbar posterior fixation system and Bi-OsteticTM bone void filler, a biologics product.All of the Company’s AxiaLIF products are delivered using its pre-sacral approach.The Company also markets products that may be used with its surgical approach, including bowel retractors and additional discectomy tools, as well as a bone graft harvesting system that can be used to extract bone graft in any procedure for which the use of bone graft is appropriate. The AxiaLIF Legacy product was commercially released in January 2005. The AxiaLIF 2L™ product was commercially released in Europe in the fourth quarter of 2006 and in the United States in the second quarter of 2008. The AxiaLIF 2L product was discontinued in 2010 after the Company launched its AxiaLIFPlus 2-Level™ product in July 2010. The Company commercially launched its next generation Vectre facet screw system in April 2010. In the first quarter of 2010, the Company entered into an agreement to distributeBi-Ostetic bone void filler, a biologics product. The Company commercially launched its AxiaLIF Plus 1-Level product in September 2011, which received FDA 510(k) clearance in March 2011. In 2010, the Company received 510(k) clearance for the VEO lateral access and interbody fusion system, which was commercially launched in November 2011, and in July 2012 the Company received a CE mark for the VEO lateral access and interbody fusion system andbegan commercialization in the European market. The Company sells its products through a direct sales force, independent sales agents and international distributors.
The Company is subject to a number of risks similar to other similarly-sized companies in the medical device industry. These risks include, without limitation, acceptance and continued use of the Company’s products by surgeons, the lack of clinical data about the efficacy of these products, uncertainty of reimbursement from third-party payors, cost pressures in the healthcare industry, competitive pressures from substitute products and larger companies, the historical lack of profitability, the dependence on key employees, regulatory approval and market acceptance for new products, compliance with complex and evolving healthcare laws and regulations, uncertainty surrounding the outcome of the matters relating to the subpoena issued to the Company by theDepartment of Health and Human Services, Office of Inspector General (the “OIG”),stockholder class actionlawsuits, the reliance on a limited number of suppliers to provide these products and their components, changes in economic conditions, the ability to effectively manage a sales force to meet the Company’s objectives and the ability to conduct successful clinical studies.
| 2. | Summary of Significant Accounting Policies |
Basis of Presentation
The Company has prepared the accompanying consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”). The Company’s fiscal year ends on December 31. The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has incurred operating losses and negative cash flows from operations, which raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might result from the outcome of this uncertainty. To meet its capital needs, the Company is considering multiple alternatives, including, but not limited to, additional equity financings, debt financings and other funding transactions. There can be no assurance that the Company will be able to complete any such transaction on acceptable terms or otherwise. If the Company is unable to obtain the necessary capital, it will need to pursue a plan to license or sell its assets, cease operations and/or seek bankruptcy protection.
Reclassification
Certain balances in the prior years’ consolidated financial statements have been reclassified to conform to the December 31, 2012 presentation. These changes consisted of a reclassification on the consolidated statements of operations from general and administrative expense to a separate line item entitled charges related to U.S. Government settlement and reclassifications on the consolidated balance sheets from accounts payable and accrued expenses to a separate line item entitled accrued expenses related to U.S. Government settlement.
Principles of Consolidation
These consolidated financial statements include the accounts of the Company and its subsidiaries. As of December 31, 2012, the Company had one subsidiary in Germany, which is in the process of being dissolved. All intercompany transactions and accounts have been eliminated in consolidation.
| 131 |
For the Company’s international operations, local currencies have been determined to be the functional currencies. The Company translates the financial statements of its international subsidiary to its U.S. dollar equivalent at the end-of-period currency exchange rate for assets and liabilities and at the average exchange rate for revenues and expenses. The Company records these translation adjustments as a component of other comprehensive loss within stockholders’ equity. The Company recognizes transaction gains and losses arising from fluctuations in currency exchange rates on transactions denominated in currencies other than the functional currency in the consolidated income statements. The Company incurred foreign currency exchange gains of $1,000 and $42,000 in the years ended December 2012 and 2011, respectively, and a foreign currency exchange loss of $24,000 in the year ended December 31, 2010.
Use of Estimates
The consolidated financial statements have been prepared in conformity with GAAP. These principles require management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. The principal estimates relate to accounts receivable reserves, inventory reserves, stock-based compensation, accrued expenses and income tax valuations.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents include money market treasury funds. Cash equivalents are carried at fair market value. Related unrealized gains and losses were not material as of December 31, 2012 and 2011.
Investments
All marketable investments are classified as available-for-sale and therefore are carried at fair market value. There were no marketable investments at December 31, 2012. Related unrealized gains and losses were not material as of December 31, 2012 and 2011. Realized gains and losses on the sale of all such investments are reported in earnings and computed using the specific identification cost method and were not material for the year ended December 31, 2011. All of the Company's investments as of December 31, 2011 had maturities of one year or less. Short-term investments at December 31, 2011 consisted ofU.S.agency backed debt instruments.
Fair Value of Financial Instruments
The carrying values of cash equivalents, short-term investments, accounts receivable, and accounts payable at December 31, 2012 and 2011 approximated their fair values due to the short-term nature of these items.
At December 31, 2012, the Company held certain assets that are required to be measured at fair value on a recurring basis. These assets included available for sale securities classified as cash equivalents. Accounting Standards Codification (“ASC”) 820-10 requires the valuation of investments using a three-tiered approach, which requires that fair value measurements be classified and disclosed in one of three tiers. These tiers are: Level 1, defined as quoted prices in active markets for identical assets or liabilities; Level 2, defined as valuations based on observable inputs other than those included in Level 1, such as quoted prices for similar assets and liabilities in active markets, or other inputs that are observable or can be corroborated by observable input data; and Level 3, defined as valuations based on unobservable inputs reflecting the Company’s own assumptions, consistent with reasonably available assumptions made by other market participants.
At December 31, 2012, all available for sale securities are classified as Level 1 assets with a fair value of $21.1 million, which included money market funds of $21.1 million. At December 31, 2011, all available for sale securities were classified as Level 1 assets with a fair value of $44.4 million, which included money market funds of $38.4 million and short-term investments of $6.0 million. The Company had no Level 2 or Level 3 assets or liabilities at December 31, 2012 or 2011.
Cash and available for sale securities classified as Level 1 assets were:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| (In thousands) | ||||||||
| Cash and cash equivalents | $ | 21,116 | $ | 38,340 | ||||
| Short-term investments | - | 6,027 | ||||||
| Total cash and available for sale securities | $ | 21,116 | $ | 44,367 | ||||
| 132 |
Accounts Receivable, Net
Accounts receivable are presented net of an allowance for uncollectible accounts. Estimates on the collectability of customer accounts are based primarily on analysis of historical trends and experience and changes in customers’ financial condition. The following table presents the components of accounts receivable:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| (in thousands) | ||||||||
| Gross accounts receivable | $ | 3,419 | $ | 2,871 | ||||
| Allowance for uncollectible accounts | (213 | ) | (349 | ) | ||||
| Total accounts receivable, net | $ | 3,206 | $ | 2,522 | ||||
Concentration of Credit Risk and Significant Customers
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents.
The Company places cash deposits with a federally insured financial institution, in amounts that at times exceed the federally insured limit, which was $250,000 at December 31, 2012 and 2011. The total amount of deposits in excess of federally insured limits was $175,260 and $133,342 at December 31, 2012 and 2011, respectively.
In 2012, 2011 and 2010 no customer accounted for 10% or more of revenues. As of December 31, 2012, one customer, Beijing Jiade Sunshine in Beijing, China, accounted for 32% of the accounts receivable balance. As of December 31, 2011, no single customer accounted for 10% or more of the accounts receivable balance.
Inventories
Inventories consist of the following:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| (in thousands) | ||||||||
| Finished goods | $ | 2,491 | $ | 1,771 | ||||
| Work-in-process | 2,272 | 2,515 | ||||||
| Raw materials | 254 | 239 | ||||||
| Total inventories, net | $ | 5,017 | $ | 4,525 | ||||
Inventories are stated at the lower of cost or market, computed on a standard cost basis, which approximates actual cost on a first-in, first-out basis and market being determined as the lower of replacement cost or net realizable value. Costs are monitored on an annual basis and updated as necessary to reflect changes in supplier costs and the rate of the Company’s overhead absorption is adjusted based on projections of the Company’s manufacturing costs and production plan. The components of inventory are reviewed on a periodic basis for excess, obsolete and impaired inventory, and a reserve is recorded for the identified items. An inventory reserve is calculated for estimated excess and obsolete inventory based upon historical turnover and assumptions about future demand for the products and market conditions. At December 31, 2012 and 2011, the allowance for excess and obsolete inventory was $2.9 million and $2.6 million, respectively.
Property and Equipment
Property and equipment are recorded at cost and depreciated over their estimated useful lives primarily using the straight-line method. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful life of the related asset. Maintenance and repairs are charged to expense as incurred. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation and amortization are removed from the accounts and any resulting gain or loss is credited or charged to income.
| 133 |
The estimated useful lives are:
| Furniture and fixtures | 5-10 years |
| Equipment | 3-10 years |
| Other depreciable assets | 2-10 years |
| Leasehold improvements | Lesser of estimated useful life or lease term |
Revenue Recognition
Revenue is recognized based on the following criteria: (i) persuasive evidence that an arrangement exists with the customer; (ii) delivery of the products and/or services has occurred; (iii) the selling price has been fixed for the products or services delivered; and (iv) collection is reasonably assured. The Company generates revenue from the sales of its implants and disposable surgical instruments. The Company has two distinct sales methods. The first method is when implants and/or disposable surgical instruments are sold directly to hospitals or surgical centers for the purpose of conducting a scheduled surgery. The Company’s sales representatives or independent sales agents hand deliver the products to the customer on or before the day of the surgery. The sales representative or independent agent is then responsible for reporting the delivery of the products and the date of the operation for proper revenue recognition. The Company recognizes revenue upon the confirmation that the products have been used in a surgical procedure. The second sales method is for sales to distributors outside the United States. These transactions require the customer to send in a purchase order before shipment and the customer only has the right of return for defective products. The Company primarily recognizes revenue upon the shipment of the product to distributors outside the United States, when risk of loss and title has transferred to the distributor, provided the Company has no material performance obligations. The Company may also provide training and marketing materials, at the distributor’s expense, to the distributor and its surgeons, which are not considered revenue.
Cost of Revenue
Cost of revenue consists primarily of material and overhead costs related to the Company’s products, including reusable kit depreciation and product royalties. Overhead costs include facilities-related costs, such as rent and utilities.
Shipping and Handling Costs
Shipping and handling costs in the United States are expensed as incurred and are included in the cost of revenue. These costs are generally not reimbursed by the Company’s customers. Shipping costs relating to sales to distributors outside the United States are either paid directly by the distributor to the freight carrier or charged to the distributor and reimbursed to the Company.
Sales and Marketing Expenses
Sales and marketing expenses consist primarily of personnel costs, sales commissions paid to the Company’s direct sales representatives, independent sales agents and independent distributors and costs associated with physician training programs, promotional activities, and participation in medical and trade conferences. All costs of advertising and promotional activities are expensed as incurred. Advertising expenses were $0.4 million, $0.4 million and $0.7 million for the years ending December 31, 2012, 2011 and 2010, respectively.
Research and Development Expenses
Research and development expenses consist primarily of personnel costs within the Company’s product development, regulatory and clinical functions, and the costs of clinical studies, product development projects and technology licensing costs. Research and development expenses are expensed as incurred.
General and Administrative Expenses
General and administrative expenses consist of personnel costs related to the executive, finance, business development and human resource functions, as well as professional service fees, legal fees, accounting fees, insurance costs and general corporate expenses.
Patent Costs
Costs associated with the submission of a patent application are expensed as incurred given the uncertainty of the patents resulting in probable future economic benefits to the Company.
| 134 |
Other Income (Expense), Net
Other income (expense), net is primarily composed of the gain or loss on disposal of fixed assets, interest earned on cash, cash equivalents and available-for-sale securities and government grants. In 2010, the Company received grants of $0.5 million from the U.S. Treasury under the Qualifying Therapeutic Discovery Program. These grants have been included in other income, net, for the year ended December 31, 2010.
Income Taxes
The Company accounts for income taxes using the liability method which requires the recognition of deferred tax assets or liabilities for the temporary differences between financial reporting and tax bases of the Company’s assets and liabilities and for tax carryforwards at enacted statutory tax rates in effect for the years in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in the period that includes the enactment date. In addition, valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
Stock-Based Compensation
The Company accounts for stock-based compensation under the fair value provisions of ASC 718. ASC 718 requires the recognition of compensation expense, using a fair-value-based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model. All options granted after January 1, 2006 are expensed on a straight-line basis over the vesting period.
The Company accounts for stock-based compensation arrangements with nonemployees in accordance with ASC 505-50. The Company records the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes pricing model. The value of the equity instruments is charged to earnings over the term of the service agreement.
Net Loss Per Common Share
Basic net loss per common share (“Basic EPS”) is computed by dividing net loss by the weighted average number of common shares outstanding. Diluted net loss available to common stockholders per common share (“Diluted EPS”) is computed by dividing net loss by the weighted average number of common shares and dilutive potential common share equivalents then outstanding. The Company’s potential dilutive common shares, which consist of shares issuable upon the exercise of stock options, have not been included in the computation of diluted net loss per share for all periods as the result would be anti-dilutive.
The following table sets forth the potential shares of common stock that are not included in the calculation of diluted net loss per share as the result would be anti-dilutive as of the end of each period presented:
| Year Ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| Weighted average stock options outstanding | 3,227,642 | 2,506,630 | 2,166,657 | |||||||||
Segment and Geographic Reporting
The Company applies ASC 280 which establishes standards for the reporting by business enterprises of information about operating segments, products and services, geographic areas, and major customers. The Company has determined that it did not have any separately reportable segments as of December 31, 2012, 2011 or 2010.
Revenue by geographic area was:
| Year Ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| (in thousands) | ||||||||||||
| United States | $ | 12,640 | $ | 17,466 | $ | 23,798 | ||||||
| Europe | 815 | 1,421 | 1,999 | |||||||||
| Asia | 1,106 | 248 | 328 | |||||||||
| Other | 9 | 18 | 29 | |||||||||
| $ | 14,570 | $ | 19,153 | $ | 26,154 | |||||||
The increase in revenue in Asia was primarily related to a new distributor in China. Long-lived assets are primarily located in the United States.
| 135 |
Recently Issued Accounting Standards
In May 2011, the Financial Accounting Standard Board (“FASB”) issued new authoritative guidance to provide a consistent definition of fair value and ensure that fair value measurements and disclosure requirements are similar between GAAP and International Financial Reporting Standards. This guidance changes certain fair value measurement principles and enhances the disclosure requirements for fair value measurements. This guidance is effective for interim and annual periods beginning after December 15, 2011 and is applied prospectively. The adoption of this guidance did not have a material impact on the Company’s financial statements.
In June 2011, the FASB issued a standard on the presentation of other comprehensive income. This standard eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity and requires the presentation of other comprehensive income in a single continuous statement, or in two separate, but consecutive, statements. This standard is effective for fiscal years and interim periods beginning after December 15, 2011. In December 2011, the FASB issued an amendment to indefinitely defer one of the requirements contained in the June 2011 standard. That requirement called for reclassification adjustments from accumulated other comprehensive income to be measured and presented by income statement line item in net income and also in other comprehensive income. This adoption of this standard did not have a material effect on the Company’s financial statements.
In February 2013, the FASB issued Accounting Standards Update No. 2013-02, “Reporting of Amounts Reclassified Out of Other Comprehensive Income,” which requires public companies to present information about reclassification adjustments from accumulated other comprehensive income in their annual and interim financial statements in a single note or on the face of the financial statements. This standard is effective prospectively for annual and interim reporting periods beginning after December 15, 2012. This guidance is not expected to have a material effect on the Company’s financial condition or results of operations.
| 3. | Property and Equipment |
Property and equipment consist of the following:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| (in thousands) | ||||||||
| Furniture and fixtures | $ | 254 | $ | 245 | ||||
| Equipment | 5,058 | 3,497 | ||||||
| Computer software | 447 | 444 | ||||||
| Leasehold improvements | 599 | 682 | ||||||
| Property held under capital leases | 51 | - | ||||||
| Construction in process | 85 | 55 | ||||||
| 6,494 | 4,923 | |||||||
| Less: accumulated depreciation and amortization | (4,328 | ) | (3,369 | ) | ||||
| $ | 2,166 | $ | 1,554 | |||||
Depreciation and amortization expense for the years ended December 31, 2012, 2011 and 2010 was $1,141,000, $662,000 and $746,000, respectively.
| 4. | Accrued Expenses |
Accrued expenses consist of the following:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| (in thousands) | ||||||||
| Bonus | $ | 630 | $ | 351 | ||||
| Commissions | 333 | 365 | ||||||
| Salaries and benefits | 168 | 33 | ||||||
| Vacation | 160 | 136 | ||||||
| Legal and professional fees | 129 | 171 | ||||||
| Franchise taxes | 47 | 91 | ||||||
| Travel and entertainment | 43 | 23 | ||||||
| Other | 138 | 29 | ||||||
| Total accrued expenses | $ | 1,648 | $ | 1,199 | ||||
| 136 |
| 5. | Commitments and Contingencies |
The Company rents office space under the terms of an operating lease, which expires in 2014, with an option to extend the lease through 2019. The Company also rents space for a training facility which expires in 2017, with an option to extend the lease through 2022. The Company entered into another operating lease agreement in 2012 for executive office space which expires in 2017, with an option to extend the lease through 2023. The Company issued an irrevocable standby letter of credit for $200,000 as security for the training facility lease, which is reduced ratably over the initial lease term.
Future minimum lease payments under operating lease obligations at December 31, 2012 are as follows (in thousands):
| Year ending December 31, | ||||
| 2013 | $ | 485 | ||
| 2014 | 504 | |||
| 2015 | 176 | |||
| 2016 | 181 | |||
| 2017 and after | 193 | |||
| $ | 1,539 |
Rent expense related to operating leases for the years ended December 31, 2012, 2011 and 2010 was $392,000, $405,000 and $401,000, respectively.
In October 2011, the Company received a subpoena issued by OIG under the authority of the federal healthcare fraud and false claims statutes. The subpoena sought documents for the period January 1, 2008 through October 6, 2011. The Company has cooperated with the government’s request.On December 24, 2012 the Company reached a tentative agreement in principle with the U.S. Department of Justice related to the subpoena issued in October 2011. The Company and the staff of the Department of Justice tentatively agreed to settle its federal investigation for $6.0 million, subject to completion and approval of written settlement agreements with the Department of Justice and the OIG, which are expected to be finalized in the first half of 2013. The Company admits no wrongdoing as part of the settlement. In 2012, the Company expensed the $6.0 million related to the tentative settlement and $2.3 million for legal fees related to the investigation. The Company also incurred legal fees of $235,000 in 2011 related to the investigation. The Company had accrued $6.8 million at December 31, 2012 for the settlement and related legal fees.
On January 24, 2012, the Company received notice that a putative class action lawsuit had been filed in the U.S. District Court Eastern District, North Carolina, on behalf of all persons, other than defendants, who purchased the Company’s securities between February 21, 2008 and October 17, 2011. The complaint alleges violations of the Securities Exchange Act of 1934, as amended, based upon purported omissions and/or false and misleading statements concerning TranS1’s financial statements and reimbursement practices. The complaint seeks damages sustained by the putative class, pre- and post-judgment interest, and attorneys’ fees and other costs. On September 7, 2012, TranS1 filed a motion to dismiss the complaint for failure to meet the heightened pleading requirements of the Private Securities Litigation Reform Act of 1995, among other grounds; the motion is pending. The Company is unable to predict what impact, if any, the outcome of this matter might have on the Company’s consolidated financial position, results of operations, or cash flows.
| 6. | Stockholders’ Equity |
At December 31, 2012 and 2011, the Company’s Amended and Restated Certificate of Incorporation, which was adopted in connection with the Company’s initial public offering, authorized up to 80,000,000 shares of capital stock, of which 75,000,000 shares were designated as common stock with a par value of $0.0001 and up to 5,000,000 shares were designated as preferred stock with a par value of $0.0001. At December 31, 2012 and 2011, there were 27,313,997 and 27,244,059 shares of common stock issued and outstanding, respectively, and there were no shares of preferred stock issued and outstanding.
In 2012, the Company issued 31,294 shares of common stock to employees and consultants for $48,000 pursuant to the exercise of stock options and 38,644 shares of common stock under the employee stock purchase plan for $112,000. In 2011, the Company issued 6,200,000 shares of common stock under the September 2011 stock offering for $18.2 million, 104,498 shares of common stock to employees and consultants for $111,000 pursuant to the exercise of stock options, and 62,390 shares of common stock under the employee stock purchase plan for $158,000.
| 137 |
| 7. | Stock Incentive Plans and Stock-Based Compensation |
2007 Employee Stock Purchase Plan
The Company’s board of directors adopted the 2007 Employee Stock Purchase Plan (as amended, the “ESPP”) in July 2007. The ESPP became effective upon the completion of the Company’s initial public offering, with a total of 250,000 shares of common stock available for sale. In addition, the ESPP provides for annual increases in the number of shares available for issuance under the ESPP on the first day of each fiscal year beginning in 2008, equal to the lesser of (i) 2.0% of the outstanding shares of common stock on the first day of such fiscal year or (ii) an amount determined by the administrator of the ESPP. The Company’s Compensation Committee administers the ESPP.
Shares shall be offered pursuant to the ESPP in six-month periods commencing on the first trading day on or after June 1 and December 1 of each year, or on such other date as the administrator may determine.
The ESPP permits participants to purchase common stock through payroll deductions of up to 10% of their eligible compensation, which includes a participant’s base straight time gross earnings, certain commissions, overtime and shift premium, but exclusive of payments for incentive compensation, bonuses and other compensation. A participant may purchase no more than 10,000 shares during a six-month purchase period. Amounts deducted and accumulated by the participant are used to purchase shares of the Company’s common stock at the end of each six-month purchase period. The purchase price of the shares will be 85% of the fair market value of the Company’s common stock on the exercise date. Participants may end their participation at any time during an offering period, and will be paid their accrued payroll deductions that have not yet been used to purchase shares of common stock. Participation ends automatically upon termination of employment with the Company. Pursuant to ASC 718, the Company is required to recognize compensation expense in connection with purchases under the ESPP.
During the fiscal years ended December 31, 2012 and 2011, 38,644 and 62,390 shares were issued to participants under the ESPP respectively.
2000 and 2007 Stock Incentive Plans
The Company established the TranS1 Inc. Stock Incentive Plan in 2000, (as amended, the “2000 Plan”) and the 2007 Stock Incentive Plan (the “2007 Plan”) in October 2007 (collectively, the “Plans”) . Under the 2000 Plan, the Company could have granted options to employees, directors or service providers and contractors for a maximum of 3,159,108 shares of the Company’s common stock. The 2000 Plan remains active, but no new options may be granted. Under the 2007 Plan, the Company may grant options to employees, directors or service providers and contractors for a maximum of 3,600,000 shares of the Company’s common stock. As of December 31, 2012, there were 718,557 shares available for future issuance under the 2007 Plan. Options granted under the Plans may be incentive stock options or non-qualified stock options. Incentive stock options may only be granted to employees. The exercise periods may not exceed ten years for options. However, in the case of an incentive stock option granted to an optionee who, at the time of the option grant owns stock representing more than 10% of the outstanding shares, the term of the option shall be five years from the date of the grant. The exercise price of incentive stock options cannot be less than 100% of the fair market value per share of the Company’s common stock on the grant date. The exercise price of a nonqualified option under the 2000 Plan and the 2007 Plan shall not be less than 85% and 100%, respectively, of the fair market value per share on the date the option is granted. If an optionee owns more than 10% of the outstanding shares, the exercise price cannot be less than 110% of the fair market value of the stock on the date of the grant. Options granted under the Plans generally vest over periods ranging from three to four years.
The following table summarizes the activity of the Company’s 2000 Plan and 2007 Plan, including the number of shares under options and the weighted average exercise price:
| Number | Price | |||||||
| Outstanding as of December 31, 2011 | 2,729,885 | $ | 4.92 | |||||
| Options granted | 1,082,500 | 2.85 | ||||||
| Options exercised | (31,294 | ) | 1.55 | |||||
| Options forfeited | (339,791 | ) | 5.02 | |||||
| Outstanding as of December 31, 2012 | 3,441,300 | $ | 4.29 | |||||
| Options vested or expected to vest as of December 31, 2012 | 3,356,121 | $ | 4.32 | |||||
| 138 |
The following table summarizes information about the Company’s stock options at December 31, 2012:
| Weighted | Weighted | |||||||||||||||
| Range of | Average | Average | Number of | |||||||||||||
| Exercise | Number | Contractual | Exercise | Options | ||||||||||||
| Prices | Outstanding | Life (Years) | Price | Exercisable | ||||||||||||
| $0.11 – $2.00 | 287,445 | 4.9 | $ | 1.28 | 220,357 | |||||||||||
| $2.01 – $3.50 | 1,481,500 | 8.5 | 2.97 | 420,890 | ||||||||||||
| $3.51 – $6.00 | 1,302,355 | 7.4 | 4.18 | 782,750 | ||||||||||||
| $6.01 – $10.00 | 140,000 | 5.9 | 9.04 | 140,000 | ||||||||||||
| $12.00 – $20.00 | 230,000 | 5.2 | 14.30 | 230,000 | ||||||||||||
| 3,441,300 | 7.5 | 4.29 | 1,793,997 | |||||||||||||
The aggregate intrinsic value of outstanding stock options (the amount by which the market price of the stock on the date of exercise exceeded the exercise price of the option) at December 31, 2012 was $0.4 million. At December 31, 2012, exercisable stock options had an aggregate intrinsic value of $0.3 million, a weighted average contractual life of 6.4 years and a weighted average exercise price of $5.31. At December 31, 2012, options vested or expected to vesthad an aggregate intrinsic value of $0.4 million and a weighted average contractual life of 7.4 years.
Stock-Based Compensation for Non-employees (Excluding Non-employee Board Members)
The Company did not issue any options to purchase common stock to consultants and there was no stock-based compensation expense charged to operations on options granted to non-employees for the years ended December 31, 2012, 2011 and 2010. As of December 31, 2012, there was no unrecognized compensation costs related to non-vested stock option awards.
Employee Stock-Based Compensation
Under ASC 718, compensation cost for employee stock-based awards is based on the estimated grant-date fair value and is recognized over the vesting period of the applicable award on a straight-line basis. For the period from January 1, 2006 to December 31, 2012, the Company issued employee stock-based awards in the form of stock options. The Company recorded stock-based compensation expense of $1.4 million, $1.6 million and $1.9 million for the years ended December 31, 2012, 2011 and 2010, respectively. The weighted average grant-date fair value of the employee stock options granted for the years ended December 31, 2012, 2011 and 2010 was $2.85, $3.96 and $3.31 per share, respectively. The aggregate intrinsic value of stock options exercised for the years ended December 31, 2012, 2011 and 2010, was $38,000, $0.3 million, and $0.5 million, respectively.
The Company uses the Black-Scholes pricing model to determine the fair value of stock options. The determination of the fair value of stock-based payment awards on the date of grant is affected by the Company’s stock price as well as assumptions regarding a number of complex and subjective variables. These variables include the Company’s expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rates and expected dividends. The estimated grant-date fair values of the employee stock options were calculated using the Black-Scholes valuation model, based on the following assumptions for the years ended December 31, 2012, 2011 and 2010:
Expected Life. The expected life of six years is based on the “simplified” method described in the SEC Staff Accounting Bulletin No. 110, which provides guidance regarding the application of ASC 718.
Volatility. Prior to 2008, the Company was a private entity with no historical data regarding the volatility of its common stock. Accordingly, the expected volatility was based on a weighted average of the actual volatility of the Company and the volatility of similar entities for prior periods. In evaluating similarity, the Company considered factors such as industry, stage of life cycle and size. The Company utilized an expected volatility range of 70.8% to 75.0% for 2012, 62.0% to 66.7% for 2011 and 59.5% to 62.0% for 2010.
Risk-Free Interest Rate. The risk-free rate is based on U.S. Treasury zero-coupon issues with remaining terms similar to the expected term on the options. The risk-free rates were:
| 139 |
| Option Grant Year | Risk-Free Rate Range | |||
| 2012 | 0.63% to 1.05% | |||
| 2011 | 1.08% to 2.14% | |||
| 2010 | 1.26% to 2.61% |
Dividend Yield. The Company has never declared or paid any cash dividends and does not plan to pay cash dividends in the foreseeable future, and, therefore, used an expected dividend yield of zero in the valuation model.
Forfeitures. ASC 718 also requires the Company to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data to estimate pre-vesting option forfeitures and record stock-based compensation expense only for those awards that are expected to vest. All stock-based payment awards are amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods.
As of December 31, 2012, there was $2.4 million of total unrecognized compensation cost related to non-vested employee stock option awards granted after January 1, 2006, which is expected to be recognized over a weighted-average period of 2.6 years.
| 8. | Income Taxes |
No provision for federal or state income taxes has been recorded as the Company has incurred net operating losses since inception.
Significant components of the Company’s deferred tax assets and liabilities consist of the following:
| 2012 | 2011 | |||||||
| (in thousands) | ||||||||
| Deferred tax assets | ||||||||
| Domestic net operating loss carryforwards | $ | 39,495 | $ | 31,391 | ||||
| Inventory | 1,138 | 1,016 | ||||||
| Fixed assets | 434 | 450 | ||||||
| Other | 2,140 | 1,952 | ||||||
| Research and development credit | 1,153 | 1,219 | ||||||
| Total deferred tax assets | 44,360 | 36,028 | ||||||
| Valuation allowance for deferred assets | (44,360 | ) | (36,028 | ) | ||||
| Deferred tax assets | - | - | ||||||
| Deferred tax liabilities | ||||||||
| Fixed assets | - | - | ||||||
| Total deferred tax liabilities | - | - | ||||||
| Net deferred tax assets (liabilities) | $ | - | $ | - | ||||
The Company provided a full valuation allowance against its net deferred tax assets. A valuation allowance must be established for deferred tax assets when it is more likely than not (a probability level of more than 50 percent) that they will not be realized. The increase in valuation allowance resulted primarily from the additional net operating loss carryforward generated. Included in the Company's deferred tax assets is a foreign net operating loss carryforward of $1.0 million that is fully offset by a valuation allowance. The Germany subsidiary is in the process of being dissolved. In conjunction with this dissolution, it is likely that the foreign net operating losses will not be utilized and may be written off, along with the corresponding valuation allowance. This will have no effect on the Company’s deferred tax assets or income tax expense.
As of December 31, 2012, the Company had federal and state net operating loss carryforwards of approximately $105.6 million and $97.2 million, respectively. These net operating loss carryforwards begin to expire in 2021 and 2016 for federal and state tax purposes, respectively. Additionally, as of December 31, 2012, the Company had research credit carryforwards of $1.2 million for federal tax purposes. These credit carryforwards begin to expire in 2021. The utilization of the federal net operating loss carryforwards may be subject to limitations under the rules regarding a change in stock ownership as determined by the Internal Revenue Code of 1986, as amended.
| 140 |
A reconciliation of differences between the U.S. federal income tax rate and the Company’s effective tax rate for the years ended December 31 is as follows:
| 2012 | 2011 | 2010 | ||||||||||||||||||||||
| % of | % of | % of | ||||||||||||||||||||||
| Amount | net loss | Amount | net loss | Amount | net loss | |||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Tax at statutory rate | $ | (10,461 | ) | 35.0 | % | $ | (6,396 | ) | 35.0 | % | $ | (6,834 | ) | 35.0 | % | |||||||||
| State taxes | (397 | ) | 1.3 | % | (434 | ) | 2.4 | % | (303 | ) | 1.5 | % | ||||||||||||
| Non deductible items | 2,496 | -8.4 | % | 692 | -3.8 | % | 746 | -3.8 | % | |||||||||||||||
| Other | 30 | -0.1 | % | (130 | ) | 0.7 | % | 330 | -1.7 | % | ||||||||||||||
| R&D credits | - | 0.0 | % | (268 | ) | 1.5 | % | (251 | ) | 1.3 | % | |||||||||||||
| Change in valuation allowance | 8,332 | -27.8 | % | 6,536 | -35.8 | % | 6,312 | -32.3 | % | |||||||||||||||
| Total | $ | - | 0.0 | % | $ | - | 0.0 | % | $ | - | 0.0 | % | ||||||||||||
As of January 1, 2007, the Company adopted the provisions of ASC 740, which clarifies the accounting for uncertainty in tax positions. As of that date, the Company had $1.1 million of unrecognized tax benefits related to the adoption of ASC 740. This would be recorded as a component of income tax expense once the valuation allowance is released. For the year ended December 31, 2012, the Company decreased its unrecognized tax benefits by $29,000. The change was recorded as an increase to the respective deferred tax asset which was reflected as an increase in the valuation allowance. As of December 31, 2012, the Company had $1.0 million of unrecognized tax benefits which, if recognized, would be recorded as a component of income tax expense. The Company’s policy is to record estimated interest and penalties related to the underpayment of income taxes as a component of its income tax provision. As of December 31, 2010, 2011 and 2012, the Company had no accrued interest or tax penalties recorded. A reconciliation of the beginning and ending uncertain tax positions is as follows (in thousands):
| Balance at December 31, 2010 | $ | 975 | ||
| Gross increases related to current period tax positions | 6 | |||
| Balance at December 31, 2011 | 981 | |||
| Gross increases related to current period tax positions | 29 | |||
| Balance at December 31, 2012 | $ | 952 |
In many cases, uncertain tax positions are related to tax years that remain subject to examination by the relevant tax authorities. Given the losses accumulated to date, periods open for examination are 2004 to 2012 for the primary taxing jurisdictions of the United States and North Carolina. The Company currently does not expect a significant change in the liability for uncertain tax provisions in the next 12 months.
| 9. | Comprehensive Loss |
The following table presents the components of other comprehensive income (loss):
| Year Ended December 31, | ||||||||||||
| 2012 | 2011 | 2010 | ||||||||||
| (in thousands) | ||||||||||||
| Net loss | $ | (29,888 | ) | $ | (18,273 | ) | $ | (19,527 | ) | |||
| Other comprehensive income (loss): | ||||||||||||
| Translation adjustments | 1 | 42 | (24 | ) | ||||||||
| Total comprehensive loss | $ | (29,887 | ) | $ | (18,231 | ) | $ | (19,551 | ) | |||
| 141 |
| 10. | Quarterly Data (Unaudited) |
The following unaudited quarterly financial data, in the opinion of management, reflects all adjustments, consisting of normal recurring adjustments, necessary to a fair statement of the results for the periods presented (in thousands, except per share data):
| Year Ended December 31, 2012 | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Quarter | Quarter | Quarter | Quarter | |||||||||||||
| Total revenues | $ | 3,782 | $ | 3,460 | $ | 3,198 | $ | 4,130 | ||||||||
| Gross profit | 2,785 | 2,548 | 2,360 | 2,568 | ||||||||||||
| Total operating expenses | 8,513 | 8,839 | 8,111 | 14,539 | ||||||||||||
| Net loss | $ | (5,758 | ) | $ | (6,293 | ) | $ | (5,865 | ) | $ | (11,972 | ) | ||||
| Basic and diluted net loss per common share | $ | (0.21 | ) | $ | (0.23 | ) | $ | (0.22 | ) | $ | (0.44 | ) | ||||
| Year Ended December 31, 2011 | ||||||||||||||||
| First | Second | Third | Fourth | |||||||||||||
| Quarter | Quarter | Quarter | Quarter | |||||||||||||
| Total revenues | $ | 5,130 | $ | 5,337 | $ | 4,696 | $ | 3,990 | ||||||||
| Gross profit | 3,834 | 4,164 | 3,652 | 2,948 | ||||||||||||
| Total operating expenses | 9,578 | 8,493 | 6,947 | 7,859 | ||||||||||||
| Net loss | $ | (5,726 | ) | $ | (4,309 | ) | $ | (3,327 | ) | $ | (4,911 | ) | ||||
| Basic and diluted net loss per common share | $ | (0.27 | ) | $ | (0.21 | ) | $ | (0.16 | ) | $ | (0.18 | ) | ||||
| 11. | Subsequent Events |
Agreement and Plan of Merger
On March 3, 2013, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with RacerX Acquisition Corp., a wholly-owned subsidiary of the Company (“Merger Sub”), Baxano, Inc. (“Baxano”), and Sumeet Jain and David Schulte solely as Securityholder Representatives (the “Securityholder Representatives”). Under the terms of the Merger Agreement, the Company will acquire Baxano through a merger of Merger Sub with and into Baxano (the “Merger”). At the effective time of the Merger (the “Effective Time”), Merger Sub will cease to exist and Baxano will continue as the surviving corporation and as a wholly-owned subsidiary of the Company.
Pursuant to the terms of the Merger Agreement, the merger consideration will consist of approximately 10.4 million shares. The number of shares comprising the merger consideration will be reduced by a number of shares with a value equal to the following (using for the per share value an average closing price on the NASDAQ Global Market during the 10 days preceding the Effective Time): (1) Baxano’s indebtedness in excess of (a) amounts under promissory notes of Baxano that are convertible into capital stock of Baxano (each a “Baxano Note”), outstanding at the Effective Time, and (b) up to $3,000,000 of principal as well as interest outstanding under certain long-term indebtedness; (2) $300,000 in cash, which will be used to fund a compensation plan to be adopted by Baxano prior to the closing of the Merger providing for bonuses to Baxano’s employees and certain non-employee directors; and (3) $250,000 in cash, which the Company has agreed to deposit into an account specified for the purpose of funding the expenses of the Securityholder Representatives under the Merger Agreement.
At the Effective Time, each Baxano Note will be terminated, and the holders of such notes will be entitled to receive merger consideration in accordance with the Merger Agreement. Any and all stock option plans or other stock or equity-related plans of Baxano, together with any employee stock purchase plans, will be terminated prior to the Effective Time. Prior to the Effective Time, Baxano must use commercially reasonable efforts to terminate each outstanding option to purchase common stock of Baxano, whether vested or unvested, and each warrant to acquire capital stock of Baxano.
| 142 |
The Merger Agreement contains customary representations, warranties, and covenants, including covenants related to obtaining the requisite stockholder approvals, appointing two Baxano designees to the Company’s Board of Directors, restricting the solicitation of competing acquisition proposals, the lock-up and registration of the shares of the Company’s common stock issued in connection with the Merger pursuant to the Securities Purchase Agreement described below, and the Company’s and Baxano’s conduct of their businesses between the date of signing the Merger Agreement and the closing of the Merger.
The stockholders of Baxano approved the Merger and the Merger Agreement pursuant to a written consent in lieu of a stockholders’ meeting on March 3, 2013 following execution of the Merger Agreement. Consummation of the Merger is also subject to approval by the Company’s stockholders of the issuance of shares of the Company’s common stock in connection with the Merger and the satisfaction or waiver of the closing conditions set forth in the Securities Purchase Agreement and other customary closing conditions set forth in the Merger Agreement. The Company’s directors, officers, and certain affiliates of the Company, who together hold approximately 24.2% of the issued and outstanding common stock of the Company, have agreed to vote their shares in favor of the issuance of the Company’s common stock in connection with the Merger Agreement and the Securities Purchase Agreement.
The Merger Agreement grants certain termination rights to the Company and Baxano. In addition, the Merger Agreement provides that the Company will be required to pay Baxano a termination fee equal to $2,000,000 and to reimburse Baxano for its expenses incurred relating to the transactions contemplated by the Merger Agreement, up to a cap of $750,000, if Baxano or the Company terminates the Merger Agreement under certain conditions. The Merger Agreement also provides that Baxano will be required to pay the Company a termination fee equal to $1,000,000 and to reimburse the Company for its expenses incurred relating to the transactions contemplated by the Merger Agreement, up to a cap of $750,000, if the Company terminates the Merger Agreement under certain conditions.
Securities Purchase Agreement
Concurrent with entering into the Merger Agreement, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”), dated March 3, 2013, with certain investors identified on the signature pages thereto (the “Investors”), pursuant to which the Company will sell to the Investors, and the Investors will purchase from the Company, an aggregate of 7,522,009 shares of the Company’s common stock, at a purchase price of $2.28 per share, resulting in gross proceeds to the Company of $17.2 million (the “Private Placement Transaction”).
Pursuant to the Securities Purchase Agreement, the Company agreed to register the resale of the shares of the Company’s common stock to be issued pursuant to the Merger Agreement and the Securities Purchase Agreement under a registration statement (the “Registration Statement”) on Form S-3 (or another appropriate form if Form S-3 is not then available to the Company). In addition, the Investors and all other holders of shares received pursuant to the Merger Agreement agreed not to sell, transfer, or otherwise dispose of the shares of the Company’s common stock issued at the closing of the Merger and the Private Placement Transaction from the period commencing on the closing of the Private Placement Transaction and expiring on the effective date of the Registration Statement, subject to certain exceptions.
If the Registration Statement is not declared effective by the Securities and Exchange Commission by a certain date, the Company must pay each Investor and other holder of shares issued pursuant to the Merger Agreement liquidated damages equal to 1% of the value of their shares (calculated at the closing price of such shares) per 30-day period, up to a maximum of 12%.
The Securities Purchase Agreement contains customary representations and warranties of the Company and the Investors. Consummation of the Private Placement Transaction is subject to approval by the Company’s stockholders of the issuance of shares of the Company’s common stock in connection with the Merger Agreement and the Securities Purchase Agreement, the closing of the Merger, and other customary closing conditions set forth in the Securities Purchase Agreement.
| 143 |
TRANS1 INC.
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
FOR THE YEARS ENDED DECEMBER 31, 2012, 2011 AND 2010
(in thousands)
| Balance at Beginning of Period | Additions (1) | Deductions(2) | Balance at End of Period | |||||||||||||
| Accounts Receivable Reserve: | ||||||||||||||||
| Year ended December 31, 2012 | $ | 349 | $ | (15 | ) | $ | 121 | $ | 213 | |||||||
| Year ended December 31, 2011 | 347 | 31 | 29 | 349 | ||||||||||||
| Year ended December 31, 2010 | 193 | 226 | 72 | 347 | ||||||||||||
| Balance at Beginning of Period | Additions (1) | Deductions(3) | Balance at End of Period | |||||||||||||
| Inventory Reserve: | ||||||||||||||||
| Year ended December 31, 2012 | $ | 2,591 | $ | 524 | $ | 215 | $ | 2,900 | ||||||||
| Year ended December 31, 2011 | 2,155 | 521 | 85 | 2,591 | ||||||||||||
| Year ended December 31, 2010 | 581 | 2,004 | 430 | 2,155 | ||||||||||||
| Balance at Beginning of Period | Additions | Deductions | Balance at End of Period | |||||||||||||
| Valuation Allowance for Deferred Tax Assets: | ||||||||||||||||
| Year ended December 31, 2012 | $ | 36,028 | $ | 8,478 | $ | 146 | $ | 44,360 | ||||||||
| Year ended December 31, 2011 | 29,492 | 6,536 | - | 36,028 | ||||||||||||
| Year ended December 31, 2010 | 23,180 | 6,354 | 42 | 29,492 | ||||||||||||
(1) Charged to costs and expenses.
(2) Uncollectible accounts written-off.
(3) Excess and obsolete inventory written-off against reserve.
| 144 |
Report of Independent Auditors
To the Board of Directors and Stockholders of
Baxano, Inc.
We have audited the accompanying financial statements of Baxano, Inc., which comprise the balance sheets as of December 31, 2012 and December 31, 2011, and the related statements of operations, of convertible preferred stock and stockholders’ deficit and of cash flows for the years then ended.
Management's Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of the financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditor's Responsibility
Our responsibility is to express an opinion on the financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on our judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, we consider internal control relevant to the Company's preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Baxano, Inc. at December 31, 2012 and December 31, 2011, and the results of its operations and its cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses and negative cash flows from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ PricewaterhouseCoopers LLP
San Jose, CA
March 5, 2013
| 145 |
| Baxano, Inc. |
| Balance Sheets |
| December 31, 2012 and 2011 |
(in thousands, except share and per share data)
| December 31, | December 31, | |||||||
| 2012 | 2011 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 4,409 | $ | 6,440 | ||||
| Accounts receivable, net | 1,773 | 931 | ||||||
| Inventory | 1,994 | 1,137 | ||||||
| Prepaid expenses and other current assets | 377 | 387 | ||||||
| Total current assets | 8,553 | 8,895 | ||||||
| Property and equipment, net | 778 | 982 | ||||||
| Other assets | 221 | 91 | ||||||
| Total assets | $ | 9,551 | $ | 9,968 | ||||
| Liabilities, Convertible Preferred Stock and Stockholders’ Deficit | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 708 | $ | 743 | ||||
| Accrued liabilities | 1,274 | 926 | ||||||
| Convertible notes payable | 14,768 | - | ||||||
| Notes payable | 3,017 | - | ||||||
| Total current liabilities | 19,767 | 1,669 | ||||||
| Preferred stock warrants liability | 131 | 33 | ||||||
| Deferred rent | 164 | 238 | ||||||
| Total liabilities | 20,062 | 1,940 | ||||||
| Commitments and Contingencies (Note 6) | ||||||||
| Convertible preferred stock, $0.001 par value - 70,673,934 and 49,403,934 shares authorized at December 31, 2012 and 2011, respectively, and 48,721,142 shares issued and outstanding at December 31, 2012 and 2011 | 58,339 | 58,339 | ||||||
| Stockholders’ deficit | ||||||||
| Common stock, $0.001 par value - 96,270,000 and 75,000,000 shares authorized at December 31, 2012 and 2011, 6,741,449 and 6,630,237 shares issued and outstanding at December 31, 2012 and 2011,respectively | 7 | 7 | ||||||
| Additional paid-in capital | 1,534 | 1,228 | ||||||
| Accumulative deficit | (70,390 | ) | (51,546 | ) | ||||
| Total stockholders’ deficit | (68,849 | ) | (50,311 | ) | ||||
| Total liabilities, convertible preferred stock and stockholders’ deficit | $ | 9,551 | $ | 9,968 | ||||
The accompanying notes are an integral part of these financial statements.
| 146 |
| Baxano, Inc. |
| Statements of Operations |
| Year Ended December 31, 2012 and 2011 |
(in thousands)
| December 31, | December 31, | |||||||
| 2012 | 2011 | |||||||
| Revenue | $ | 9,417 | $ | 3,926 | ||||
| Cost of goods sold | (3,261 | ) | (2,020 | ) | ||||
| Gross profit | 6,156 | 1,906 | ||||||
| Operating expenses | ||||||||
| Research and development | 4,778 | 5,236 | ||||||
| General and administrative | 2,486 | 2,329 | ||||||
| Selling and marketing | 17,155 | 10,578 | ||||||
| Total operating expenses | 24,419 | 18,143 | ||||||
| Loss from operations | (18,263 | ) | (16,237 | ) | ||||
| Interest income | 7 | 16 | ||||||
| Interest expense | (1,002 | ) | (10 | ) | ||||
| Other income (expense), net | 415 | (13 | ) | |||||
| Net loss | $ | (18,844 | ) | $ | (16,244 | ) | ||
The accompanying notes are an integral part of these financial statements.
| 147 |
| Baxano, Inc. |
| Statements of Convertible Preferred Stock and Stockholders’ Deficit |
| Year Ended December 31, 2012 and 2011 |
| Additional | ||||||||||||||||||||||||||||
| Convertible Preferred Stock | Common Stock | �� | Paid-In | Accumulated | ||||||||||||||||||||||||
| (in thousands except share data) | Shares | Amount | Shares | Amount | Capital | Deficit | Total | |||||||||||||||||||||
| Balances at December 31, 2010 | 48,721,142 | $ | 58,339 | 6,269,307 | $ | 6 | $ | 845 | $ | (35,302 | ) | $ | (34,451 | ) | ||||||||||||||
| Issuance of common stock upon exercise of options in 2011 | - | - | 360,930 | 1 | 67 | - | 68 | |||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 316 | - | 316 | |||||||||||||||||||||
| Net Loss | - | - | - | - | - | (16,244 | ) | (16,244 | ) | |||||||||||||||||||
| Balances at December 31, 2011 | 48,721,142 | $ | 58,339 | 6,630,237 | $ | 7 | $ | 1,228 | $ | (51,546 | ) | $ | (50,311 | ) | ||||||||||||||
| Issuance of common stock upon exercise of options in 2012 | - | - | 171,630 | - | 42 | - | 42 | |||||||||||||||||||||
| Repurchase of common stock upon exercise of options in 2012 | - | - | (60,418 | ) | - | (13 | ) | - | (13 | ) | ||||||||||||||||||
| Stock-based compensation | - | - | - | - | 277 | - | 277 | |||||||||||||||||||||
| Net Loss | - | - | - | - | - | (18,844 | ) | (18,844 | ) | |||||||||||||||||||
| Balances at December 31, 2012 | 48,721,142 | $ | 58,339 | 6,741,449 | $ | 7 | $ | 1,534 | $ | (70,390 | ) | $ | (68,849 | ) | ||||||||||||||
The accompanying notes are an integral part of these financial statements.
| 148 |
| Baxano, Inc. |
| Statements of Cash Flows |
| Year Ended December 31, 2012 and 2011 |
(in thousands)
| December 31, | December 31, | |||||||
| 2012 | 2011 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (18,844 | ) | $ | (16,244 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities | ||||||||
| Depreciation and amortization | 345 | 322 | ||||||
| Stock-based compensation expense | 277 | 316 | ||||||
| Amortization of debt discount | 265 | 3 | ||||||
| Amortization of debt issuance costs | 86 | - | ||||||
| Accretion of balloon payment related to term loan | 49 | - | ||||||
| Revaluation of preferred stock warrant liability | (420 | ) | (8 | ) | ||||
| Changes in operating assets and liabilities: | ||||||||
| Account receivable | (842 | ) | (776 | ) | ||||
| Inventory | (857 | ) | (601 | ) | ||||
| Prepaid expenses and other current assets | 10 | (75 | ) | |||||
| Other assets | (130 | ) | - | |||||
| Accounts payable | (35 | ) | 352 | |||||
| Accrued and other liabilities | 393 | 388 | ||||||
| Net cash used in operating activities | (19,703 | ) | (16,323 | ) | ||||
| Cash flows from investing activities | ||||||||
| Acquisition of property and equipment | (142 | ) | (280 | ) | ||||
| Net cash used in investing activities | (142 | ) | (280 | ) | ||||
| Cash flows from financing activities | ||||||||
| Proceeds from the issuance of common stock | 42 | 68 | ||||||
| Repurchase of common stock | (13 | ) | - | |||||
| Proceeds from convertible notes payable | 15,000 | - | ||||||
| Proceeds from (repayments of) notes payable | 3,000 | (289 | ) | |||||
| Payments related to debt issuance costs | (216 | ) | - | |||||
| Net cash provided by (used in) financing activities | 17,813 | (221 | ) | |||||
| Net decrease in cash and cash equivalents | (2,031 | ) | (16,824 | ) | ||||
| Cash and cash equivalents at beginning of year | 6,440 | 23,264 | ||||||
| Cash and cash equivalents at end of year | $ | 4,409 | $ | 6,440 | ||||
| Supplemental disclosures of cash flow information | ||||||||
| Cash paid for interest during the period | $ | 144 | $ | 7 | ||||
| Noncash financing activity | ||||||||
| Issuance of preferred stock warrants | $ | 518 | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
| 149 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
| 1. | The Company and Basis of Presentation |
Baxano, Inc. (the “Company”) was incorporated in the state of Delaware on March 24, 2005 to develop and specialize in making tools that restore spine function and preserve healthy tissue. The Company is based in San Jose, California.
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. From the period of March 24, 2005 to December 31, 2010, the Company was engaged primarily in research and development activities, raising capital, and recruiting personnel. The Company exited the development stage in 2011.
On March 3, 2013, the Company entered into a merger agreement to be acquired by TranS1, Inc. ("TranS1") in an all-stock merger whereby the Company would become a wholly owned subsidiary of TranS1 (Note 11).
| 2. | Summary of Significant Accounting Policies |
Liquidity
The accompanying financial statements are prepared on a going concern basis that contemplates the realization of assets and discharge of liabilities in the normal course of business. The Company has incurred net operating losses each year since inception. At December 31, 2012, the Company had an accumulated deficit of $70,390, used cash in operations of $19,703 for the year ended December 31, 2012 and currently does not have financing sufficient for current operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The Company will need to achieve profitability and/or obtain additional financing in order to continue its operations. There can be no assurance, however, that such a financing will be successfully completed or completed on terms acceptable to the Company. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Segment Reporting
The Company operates as one operating segment and uses one measurement of profitability to manage its business. The Company’s only revenue since its inception was from domesticproducts sales to hospitals and surgery centers. All long-lived assets are maintained in the United States.
Significant Risks and Uncertainties
The Company’s future cash flows and results of operations involve a number of risks and uncertainties. Factors that could affect the Company’s future operating results and cause actual results to vary materially from expectations include, but are not limited to, rapid technological change, continued acceptance of the Company’s products, competition from substitute products and larger companies, protection of proprietary technology, strategic relationships and dependence on key individuals. If the Company fails to adhere to ongoing FDA Quality System Regulation, the FDA may withdraw its market clearance or take other action. The Company relies on sole-source suppliers to manufacture some of the components used in its product. The Company’s manufacturers and suppliers may encounter supply interruptions or problems during manufacturing due to a variety of reasons, including failure to comply with applicable regulations, including the FDA’s Quality System Regulation, equipment malfunction and environmental factors, any of which could delay or impede the Company’s ability to meet demand.
| 150 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
Concentration of Credit Risk
Cash and cash equivalents are invested in deposits with one financial institution in the United States. Deposits in this financial institution may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
As of December 31, 2012 and 2011 and the years then ended, no one customer accounted for more than 10% of accounts receivable or revenues.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with original maturities of three months or less from the date of purchase to be cash equivalents. Cash and cash equivalents include money market funds and a deposit account.
Restricted Cash
The Company has $75 invested in Certificates of Deposits held as collateral for the Company’s credit card lines. This amount is included in other current assets on the balance sheet.
Inventory
Inventory is stated at the lower of cost or market value. Cost is determined using standard cost, which approximates actual costs on a first-in, first-out basis. Market value is determined as the lower of replacement cost or net realizable value. The Company periodically assesses the recoverability of the inventory to determine whether adjustments for impairment are required. The Company evaluates the remaining shelf life and related commercial mix of inventory and other general obsolescence and impairment criteria in assessing the recoverability of the Company’s inventory.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Computer equipment and software is depreciated on a straight-line basis over its estimated useful life of three years. Laboratory and other office equipment, and furniture and fixtures are depreciated on a straight-line basis over their estimated useful life of five years. Leasehold improvements are amortized over the lesser of their estimated useful lives or remaining lease term. Upon sale or retirement of assets, the cost and related accumulated depreciation or amortization is removed from the balance sheet and the resulting gain or loss is reflected in the statement of operations. Maintenance and repairs are charged to the statement of operations, as incurred.
Impairment of Long-Lived Assets
The Company reviews property and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by comparison of the carrying amount to the future net cash flows which the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the projected discounted future net cash flows arising from the asset. There have been no such impairments of long-lived assets as of December 31, 2012 and 2011.
Preferred Stock Warrants
The Company accounts for freestanding warrants and other similar instruments related to shares that are redeemable in accordance with ASC 480,Distinguishing Liabilities from Equity (“ASC 480”). Under ASC 480, the outstanding freestanding warrants that are related to the Company’s convertible preferred stock are classified as liabilities on the balance sheet (Note 7). The warrants are subject to re-measurement at each balance sheet date and any change in fair value is recognized as a component of other income (expense), net. The Company will continue to adjust the liability for changes in fair value until the earlier of the exercise or expiration of the warrants, or the completion of a liquidation event (Note 7).
| 151 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
Fair Value of Financial Instruments
For financial instruments consisting of cash equivalents, accounts receivable, accounts payable and accrued liabilities included in the Company’s financial statements, the carrying amounts are reasonable estimates of fair value due to their short maturities. The carrying amount of the preferred stock warrants liability represents their estimated fair values. Based on borrowing rates currently available to the Company for notes payable and convertible notes payable with similar terms, the carrying values of the Company’s notes payable and convertible notes payable approximates fair value using Level 3 inputs.
Revenue Recognition
The Company earns revenue from products sales to hospitals and surgery centers whose physicians perform spinal decompression procedures using the Company's device. The Company generates all its revenue from sales in the United States. The Company recognizes revenues from product sales, in accordance with ASC 605,Revenue Recognition, when evidence of an arrangement exists, delivery has occurred, the fee is fixed and determinable, collection of resulting receivables is reasonably assured and title and risk of loss has passed. If those criteria are not met, then revenue is not recognized until the criteria are satisfied.
The Company uses approved customer purchase orders to determine the existence of an arrangement. The Company ensures collectability by reviewing prior customer orders or credit history. The Company uses direct delivery by its sales representatives to prove that delivery has occurred. The Company assesses whether the price is fixed or determinable based on the terms of the agreement associated with the transaction.
Research and Development
Research and development costs are charged to research and development expense as incurred. Research and development costs include, but are not limited to, payroll and personnel expenses, laboratory supplies, consulting costs and allocated overhead, including rent, equipment, depreciation and utilities.
Income Taxes
The Company accounts for income taxes under the liability method, whereby deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company operates in various tax jurisdictions and is subject to audit by various tax authorities. The Company provides for tax contingencies whenever it is deemed probable that a tax asset has been impaired or a tax liability has been incurred for events such as tax claims or changes in tax laws. Tax contingencies are based upon their technical merits, relative tax law, and the specific facts and circumstances as of each reporting period. Changes in facts and circumstances could result in material changes to the amounts recorded for such tax contingencies.
The Company records uncertain tax positions in accordance with accounting standards on the basis of a two-step process whereby (1) a determination is made as to whether it is more likely than not that the tax positions will be sustained based on the technical merits of the position and (2) for those tax positions that meet the more-likely-than-not recognition threshold the Company recognizes the largest amount of tax benefit that is greater than 50% likely to be realized upon ultimate settlement with the related tax authority.
Accounting for Stock-Based Compensation
The Company accounts for stock-based compensation in accordance with ASC 718,Compensation-Stock Compensation (“ASC 718”). ASC 718 requires the recognition of compensation expense, using a fair-value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option pricing model. All option grants valued since inception are expensed on a straight-line basis over the requisite service period.
| 152 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
The Company accounts for equity instruments issued to nonemployees in accordance with ASC 505-50 Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. Equity instruments issued to nonemployees are recorded at their fair value on the measurement date and are subject to periodic adjustment as the underlying equity instruments vest.
Comprehensive Loss
For all periods presented the comprehensive loss was equal to the net loss; therefore, a separate statement of comprehensive loss is not included in the accompanying financial statements.
Recent Accounting Pronouncements
In June 2011, the FASB issued a new standard on the presentation of comprehensive income that is effective for the Company’s 2012 financial statements. The new standard eliminated the alternative to report other comprehensive income and its components in the statement of changes in equity. Under the new standard, companies can elect to present items of net income and other comprehensive income in one continuous statement or in two separate, but consecutive statements. The new standard did not have an impact on the Company’s financial statements because the Company does not have items of other comprehensive income to report.
In December 2011, FASB issued ASU No. 2011-11,“Balance Sheet (Topic 210,) (“ASU 2011-05”).” This update provides enhanced disclosure requirements regarding the nature of an entity’s right of offset related to arrangements associated with its financial instruments and derivative instruments. The new guidance requires the disclosure of the gross amounts subject to rights of set-off, the amounts offset in accordance with the accounting standards followed, and the related net exposure. This pronouncement is effective for financial reporting period beginning on or after January 1, 2013 and full retrospective application is required. The Company does not expect that the adoption of this accounting standard update will have a material impact on its financial statements.
| 3. | Fair Value Measurements |
In accordance with the ASC 820, the Company discloses and recognizes the fair value of its assets and liabilities using a hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to valuations based upon unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to valuations based upon unobservable inputs that are significant to the valuation (Level 3 measurements). The guidance establishes three levels of the fair value hierarchy as follows:
| Level 1 - | Observable inputs, such as quoted prices in active markets for identical assets or liabilities. |
| Level 2 - | Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the liabilities. |
| Level 3 - | Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The following table sets forth the Company’s financial instruments that were measured at fair value on a recurring basis at December 31, 2012 and 2011 by level within the fair value hierarchy. As required by ASC 820, assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and considers factors specific to the asset or liability:
| 153 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
| December 31, 2012 | ||||||||||||||||
| Quoted Market | ||||||||||||||||
| Prices in | ||||||||||||||||
| Active Markets | Other | Significant | ||||||||||||||
| Identical | Observable | Unobservable | ||||||||||||||
| Assets | Inputs | Inputs | ||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Money market funds | $ | 4,405 | $ | 4,405 | $ | - | $ | - | ||||||||
| Liabilities | ||||||||||||||||
| Preferred stock warrants liability | $ | 131 | $ | - | $ | - | $ | 131 | ||||||||
| December 31, 2011 | ||||||||||||||||
| Quoted Market | ||||||||||||||||
| Prices in | ||||||||||||||||
| Active Markets | Other | Significant | ||||||||||||||
| Identical | Observable | Unobservable | ||||||||||||||
| Assets | Inputs | Inputs | ||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Assets | ||||||||||||||||
| Cash equivalents | ||||||||||||||||
| Money market funds | $ | 6,298 | $ | 6,298 | $ | - | $ | - | ||||||||
| Liabilities | ||||||||||||||||
| Preferred stock warrants liability | $ | 33 | $ | - | $ | - | $ | 33 | ||||||||
The change in the fair value of the preferred stock warrants liability is summarized below:
| Fair value at December 31, 2010 | $ | 41 | ||
| Change in fair value recorded in other (income) expense, net | (8 | ) | ||
| Fair value at December 31, 2011 | 33 | |||
| Recogntion of fair value of preferred stock warrants issued (Note 7) | 518 | |||
| Change in fair value recorded in other (income) expense, net | (420 | ) | ||
| Fair value at December 31, 2012 | $ | 131 |
The valuation of the preferred warrants liability is discussed in Note 7.
The Company has determined that its convertible notes payable and notes payable are classified as a level 3 item in the fair value hierarchy. At December 31, 2012, the fair value of the convertible notes payable and the notes payable approximates the carrying amount of $17.85 million. The estimated fair value of the notes payable was based on the then-current rates available to the Company for debt of similar terms and remaining maturities and also took into consideration default and credit risk. The Company determined the estimated fair value amount by using available market information and commonly accepted valuation methodologies. However, considerable judgment is required in interpreting market data to develop estimates of fair value. The use of different assumptions and/or estimation methodologies may have a material effect on the estimated fair value. For certain financial instruments, including accounts receivable, accounts payable and accrued liabilities, the carrying amounts approximate fair value due to the relatively short maturity of the items.
| 154 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
| 4. | Balance Sheet Components |
| December 31, | December 31, | |||||||
| 2012 | 2011 | |||||||
| Inventory | ||||||||
| Raw materials | $ | 391 | $ | 250 | ||||
| Work in progress | 39 | 94 | ||||||
| Finished goods | 1,564 | 793 | ||||||
| $ | 1,994 | $ | 1,137 | |||||
| Property and Equipment, net | ||||||||
| Laboratory and other office equipment | $ | 1,202 | $ | 1,113 | ||||
| Leasehold improvements | 498 | 498 | ||||||
| Computer equipment and software | 257 | 237 | ||||||
| Furniture and fixtures | 85 | 52 | ||||||
| 2,042 | 1,900 | |||||||
| Less: Accumulated depreciation and amortization | (1,264 | ) | (918 | ) | ||||
| $ | 778 | $ | 982 | |||||
| Accrued Liabilities | ||||||||
| Payroll related expenses | $ | 756 | $ | 889 | ||||
| Accrued interest payable | 469 | - | ||||||
| Other | 49 | 37 | ||||||
| $ | 1,274 | $ | 926 | |||||
| 5. | Borrowings |
Notes Payable
In March 2012, the Company entered into a term loan and security agreement ("Notes Payable") with Silicon Valley Bank and Oxford Finance, LLC for Growth Capital in an aggregate total of $8,000 available in three tranches. Upon satisfaction of additional equity financing requirements, the three tranches of $3,000 (Loan A), $2,000 (Loan B), and $3,000 (Loan C) would become available no later than March 15, 2012, September 15, 2012, and December 31, 2012 respectively. The notes payable accrues interest at a rate equivalent to the three month LIBOR rate at the time of draw plus 6.25%, subject to a LIBOR floor of 0.43%. The notes are payable with 12 month interest only payments followed by 30 months of equal principal and interest payments. The Company is also required to make a final payment of 7% of the total loan amount drawn, due at the earlier of loan maturity or termination. The notes payable are collateralized by all current and future assets of the Company, excluding its intellectual property.
On March 15, 2012 the Company borrowed $3,000 under Loan A. The interest rate was 6.7235% with interest only payments payable monthly to March 1, 2013 and followed by equal payments of principal and interest, maturing on September 1, 2015. In addition, a final payment of $210 is due on maturity. The final payment is being accreted to interest expense using the effective interest method over 42 months.
The Company did not borrow additional amounts under the agreement and the tranches for Loan B and Loan C expired in 2012.
| 155 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
In connection with the notes payable, the Company issued warrants to purchase 164,654 shares of its Series C preferred stock at an exercise price of $0.911 per share. The Company recorded the fair value of the warrants of $41 as a debt discount and warrant liability. The related debt discount will be amortized over the term of the loan at an effective interest rate of 6.7235%. The valuation of the warrants is discussed in Note 7.
The Company is required to maintain compliance with certain customary and routine financial covenants including financial reporting requirements, delivery of audited financial statements, limitations on further indebtedness or investments and limitations on certain corporate transactions. Non-compliance with the covenants could result in the principal of the note becoming due and payable. As of December 31, 2012, the Company was in compliance with the financial covenants as set forth in the notes payable agreement.
The events of default under this notes payable agreement include payment defaults, covenant default, or investor abandonment. In the case of an event of default, the lenders may, declare due all unpaid principal and interest amounts outstanding and exercise other customary remedies subject to the agreement.
At December 31, 2012, future minimum loan payments under the term of the notes payable were as follows:
Year Ending December 31,
| 2013 | $ | 1,031 | ||
| 2014 | 1,307 | |||
| 2015 | 1,190 | |||
| Total | 3,528 | |||
| Less: Interest Expense | 528 | |||
| Less: Unamortized discount | 193 | |||
| Short Term | $ | 3,017 |
Convertible Notes Payable
In March 2012, the Company entered into a subordinated convertible promissory note agreement ("Convertible Notes Payable") for a total of $15,000. The funding occurred in two equal tranches of $7,500 in March 2012 and in October 2012. The notes carry interest of 6% per annum. Unless earlier converted or repaid, the notes, together with any unpaid accrued interest, are repayable on September 7, 2013. If an event of default occurs, all indebtedness under the notes becomes due and payable upon the written election of holders representing 60% of the aggregate outstanding principal (Note 11).
In the event of a qualified financing with proceeds of at least $15,000, excluding the conversion of the notes, the principal and accrued interest automatically convert into the equity securities in that financing, at the price per share paid in the financing. In the event of a sale of the Company or sale of substantially all of its assets, the principal and interest automatically convert into shares of Series C Preferred Stock at a price of $0.911 per share.
In conjunction with convertible notes payable, the Company issued warrants for the Company's Series C preferred stock of 1,646,537 and 1,646,537 for the closings in March and October 2012, respectively. For these warrants issued with the convertible notes payable in March and October 2012, the Company recorded fair values of $415 and $62, respectively, as a debt discount and warrant liability. The debt discount is being amortized over the term of the convertible notes payable. The valuation of the warrants is discussed in Note 7.
In addition, the Company determined that the convertible notes payable may have contingent beneficial conversion features related to the conversion options. Upon the occurrence of the contingent event underlying those conversion options, the Company may recognize a charge based on the difference, if any, between the adjusted conversion price and the fair market value of equity securities at the original issuance date. This charge, if any, will impact net income (loss) attributable to common stockholders.
| 156 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
The events of default under this convertible notes include payment defaults, material adverse change in business operations or conditions, material breaches of covenants, bankruptcy events and the occurrence of any event of default that continues under thatsubordinated convertible promissory note agreementdated as of March 7, 2012. In the case of an event of default, the lenders may, declare due all unpaid principal and interest amounts outstanding and all other obligations that are due and payable subject to the agreement.
Management believes that, based on the current level of operations and anticipated growth, cash and cash equivalents balances, including interest income the Company will earn on these balances, will not be sufficient to meet the anticipated cash requirements beyond one year from the balance sheet. Accordingly, amounts due under the convertible notes payable and notes payable have been classified as current liabilities.
| 6. | Commitments and Contingencies |
During September 2009, the Company executed a noncancelable operating lease agreement, which expires in January 2015, unless extended. Under the lease agreement, payments are required in advance of the following months occupation. Future minimum lease payments at December 31, 2012 under the noncancelable operating lease agreements are as follows:
| Year Ending December 31, | ||||
| 2013 | $ | 258 | ||
| 2014 | 248 | |||
| Total minimum payments | $ | 506 |
Purchase Commitments
As of December 31, 2012, the Company has non-cancelable purchase commitments of $363.
Indemnifications
In the normal course of business, the Company enters into contracts that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations, and accordingly, the Company believes that the estimated fair value of these indemnification obligations is minimal and has not accrued any amounts for these obligations.
Legal Matters
From time to time, the Company is subject to claims and assessments in the ordinary course of business. The Company is not currently a party to any litigation matter that, individually or in the aggregate, is expected to have a material adverse effect on the Company’s business, financial condition, results of operations, or cash flows.
| 7. | Convertible Preferred Stock |
Convertible preferred stock at December 31, 2012 consists of the following:
| 157 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
| Shares | Liquidation | Carrying | ||||||||||||||
| Authorized | Outstanding | Amount | Value | |||||||||||||
| Series A-1 | 4,024,286 | 4,024,286 | $ | 4,024 | $ | 3,969 | ||||||||||
| Series A-2 | 2,911,038 | 2,797,402 | 4,616 | 4,576 | ||||||||||||
| Series B | 8,968,610 | 8,968,610 | 20,000 | 19,946 | ||||||||||||
| Series C | 54,770,000 | 32,930,844 | 30,000 | 29,848 | ||||||||||||
| 70,673,934 | 48,721,142 | $ | 58,640 | $ | 58,339 | |||||||||||
Convertible preferred stock at December 31, 2011 consists of the following:
| Shares | Liquidation | Carrying | ||||||||||||||
| Authorized | Outstanding | Amount | Value | |||||||||||||
| Series A-1 | 4,024,286 | 4,024,286 | $ | 4,024 | $ | 3,969 | ||||||||||
| Series A-2 | 2,911,038 | 2,797,402 | 4,616 | 4,576 | ||||||||||||
| Series B | 8,968,610 | 8,968,610 | 20,000 | 19,946 | ||||||||||||
| Series C | 33,500,000 | 32,930,844 | 30,000 | 29,848 | ||||||||||||
| 49,403,934 | 48,721,142 | $ | 58,640 | $ | 58,339 | |||||||||||
The rights, preferences, privileges and restrictions there of are set forth in the Company’s Amended and Restated Articles of Incorporations, and are summarized as follows:
Dividends
The holders of the Series A-1, Series A-2, Series B and Series C convertible preferred stock are entitled to receive, when and as declared by the Board of Directors and out of funds legally available, noncumulative dividends of $0.0800, $0.1320, $0.1784 and $0.0729, respectively, per annum, payable in preference and priority to any payment of any dividend on common stock. No dividends or other distributions shall be made in respect to the common stock, until all declared dividends on the Series A-1, Series A-2, Series B and Series C convertible preferred stock have been paid. Since March 24, 2005 (date of inception) to December 31, 2012 and 2011, no dividends have been declared or paid by the Company.
Liquidation
In the event of any liquidation, dissolution, or winding up of the Corporation, either voluntary or involuntary, the holders of Series C Preferred Stock shall be entitled to receive, prior and in preference to any distribution of any of the assets of the Company to the holders of Series A-1 Preferred Stock, Series A-2 Preferred Stock, Series B Preferred Stock or Common Stock by reason of their ownership thereof, an amount equal to $0.911 per share, plus any declared but unpaid dividends on such shares (subject to adjustment for Recapitalizations). If upon the occurrence of such event, the assets and funds thus distributed among the holders of the Series C Preferred Stock shall be insufficient to permit the payment to such holders of the full aforesaid preferential amounts, then the entire assets and funds of the Company legally available for distribution to stockholders shall be distributed ratably among the holders of the Series C Preferred Stock in proportion to the full preferential amount each such holder is otherwise entitled to receive.
Upon completion of the distribution of the full amount to the holders of Series C preferred stock, holders of Series A-1, Series A-2, and Series B convertible preferred stock shall be entitled, before any distribution is made to the holders of common stock, to an amount equal to $1.000, $1.650, and $2.230 per share, respectively, plus any declared but unpaid dividends on such shares. In the event that assets of the company are insufficient to permit payment of the above mentioned amounts, Series A-1, Series A-2, and Series B convertible preferred stockholders shall share ratably in any distributions of the remaining assets and funds of the Company. After Series A-1, Series A-2 and Series B receive their liquidation preference, Series C participates with common stock up to three times their liquidation preference of the remaining assets of the Company. Thereafter, all remaining assets available for distribution shall be distributed among the holders of common stock.
| 158 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
These liquidation features cause the convertible preferred stock to be classified as mezzanine capital rather than as a component of stockholders’ deficit.
Mergers
A merger, reorganization, or sale of all or substantially all of the assets of the Company, in which the existing stockholders of the Company prior to the transaction not holding at least 50% of the voting power of the surviving entity (or its parent) immediately after the transaction, shall be deemed to be a liquidation, dissolution or winding up of the Company.
Voting
The holder of each share of preferred stock is entitled to voting rights equal to the number of shares of common stock into which each share of preferred stock could be converted at the record date for a vote or consent of stockholders, except as otherwise required by law, and has voting rights and powers equal to the voting rights and powers of the shares of common stock.
Conversion
Each share of preferred stock, at the option of the holder, is convertible into the number of fully paid and nonassessable shares of common stock which results from dividing the issuance price by the conversion price in effect at that time of conversion. The issuance price for the Series A-1, Series A-2, Series B and Series C preferred is $1.00, $1.65, $2.23 and $0.911, respectively. The conversion price for Series A-1, Series A-2, Series B and Series C preferred is $0.95, $1.24, $1.49 and $0.911, respectively, subject to adjustments as described in the Company’s Amended and Restated Certificate of Incorporation. Conversion is automatic at the then effective conversion rate immediately upon the closing of a firm commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933, as amended, covering the public offering price per share is not less than $6.69 (as adjusted for recapitalizations) and the aggregate proceeds to the Corporation are not less than $30,000,000 or upon receipt of a written request for such conversion from the holders of at least 66.6% of the Preferred Stock then outstanding.
Redeemable
The convertible preferred stock is not redeemable.
Warrants
Preferred stock warrants outstanding as of December 31, 2012 and 2011 were as follows:
| Outstanding | ||||||||||||||||||||
| and | ||||||||||||||||||||
| Exercisable | Fair Value | Fair Value | ||||||||||||||||||
| Exercise | at | at | at | |||||||||||||||||
| Date of | Type of | Price Per | December 31, | December 31, | December 31, | |||||||||||||||
| Issuance | Expiration | Warrant | Share | 2012 | 2012 | 2011 | ||||||||||||||
| March 2007 | March 2017(1) | Series A-2 | $ | 1.650 | 113,636 | $ | - | $ | 33 | |||||||||||
| March 2012 | March 2022(2) | Series C | $ | 0.911 | 164,654 | $ | 7 | $ | - | |||||||||||
| March 2012 | March 2022(3) | Series C | $ | 0.911 | 1,646,537 | $ | 62 | $ | - | |||||||||||
| October 2012 | October 2022(3) | Series C | $ | 0.911 | 1,646,537 | $ | 62 | $ | - | |||||||||||
| $ | 131 | $ | 33 | |||||||||||||||||
| 159 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
(1) The warrants are exercisable until the earlier to occur of (i) the 10th anniversary of the warrant date, (ii) a public acquisition, (iii) five years after the closing of a firm commitment underwritten public offering pursuant to a registration statement under the Securities Act and at the time of such event the effective per share price for the Series A-2 preferred stock is at least five times the exercise price.
(2) The warrants are exercisable in whole or in part at any time before the expiration date and shall be void thereafter.
(3) The warrants are exercisable until the first to occur of (i) the closing of an initial public offering, (ii) the closing of a sale of the Company or substantially all of the assets of the Company, or (iii) the 10th anniversary of the issuance of the warrants. Prior to a qualified financing as defined in the note agreement, the warrants are exercisable for shares of Series C Preferred Stock at a price of $0.911 per share. Following a qualified financing, the warrants are exercisable for shares of the class of stock in that financing, at the price paid in the financing.
In conjunction of the drawdown under the notes payable agreement in March 15, 2012, the Company issued warrants exercisable for 164,654 shares Series C Preferred Stock. The warrants were initially valued at $41. The fair value of warrants in the aggregate was determined by using an income approach and recent market transactions by first estimating the equity value of our company, then allocating the value to our various securities using the Option Pricing Model or ("OPM"). The OPM allocation used the following assumptions: expected term of 2.5 years, volatility of 60%, risk-free rate of 0.37%, and a dividend yield of zero.
In connection with the first and second closing under the convertible notes payable in March and October 2012, the Company issued warrants exercisable for 1,646,537 and 1,646,537 shares Series C Preferred Stock or the class of shares issued in a future qualifying equity financing. The warrants issued with the first closing of the convertible notes payable were initially valued at $415. The fair value of warrants in the aggregate was determined by using an income approach and recent market transactions by first estimating the equity value of our company, then allocating the value to the Company securities using the Option Pricing Model or OPM. The OPM uses the Black-Scholes option-pricing model to price the warrants at the issuance, with the following assumptions: expected term of 2.5 years, volatility of 60%, risk-free rate of 0.37%, and a dividend yield of zero. In addition, the Company valued the 1,646,537 additional warrants issued with the second closing of the convertible notes payable in October 2012 at $62 using the same model and the following assumptions: expected term 1.9 years, volatility 45%, risk-free rate 0.23%, and dividend yield of zero.
As of December 31, 2012, the preferred stock warrants were remeasured based on enterprise value determined by recent market transactions and income approach. This enterprise value was allocated using an OPM with the following assumptions: expected term 1.9 years, volatility 45%, risk-free rate 0.23%, and dividend yield of zero. Based on the Company's remeasurement, the Company's warrants were valued at $131, at December 31, 2012.
| 8. | Common Stock |
The Company increased authorized shares of common stock from 75,000,000 shares as of December 31, 2011 to 96,270,000 shares as of December 31, 2012.
Each share of common stock is entitled to one vote. The holders of common stock are also entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all classes of stock outstanding. Since inception through December 31, 2012 and 2011, no dividends have been declared.
| 9. | Stock Option Plan |
In 2005, the Company established its 2005 Employee, Directors and Consultant Stock Plan (the “Plan”) which provides for granting stock options to employees and consultants of the Company and issuance of restricted shares of common stock. Options granted under the Plan may either have incentive stock options (“ISOs”) or nonqualified stock options (“NSOs”). ISOs may be granted to only Company employees (including officers and board members). NSOs may be granted to Company employees and consultants.
| 160 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
Options under the Plan may be granted for periods of up to ten years. All options issued to date have had a ten year life. The exercise price of an ISO shall not be less than 100% of the estimated fair value of the shares on the date of grant, as determined by the Board of Directors. The exercise price of a NSO shall not be less than 85% of the estimated fair value of the shares on the date of grant as determined by the Board of Directors. The exercise price of an ISO and NSO granted to a 10% shareholder shall not be less than 110% of the estimated fair value of the shares on the date of grant, respectively, as determined by the Board of Directors. Under the Company’s stock option plan, options become vested over variable periods, generally ranging from one to four years, and expire not more than ten years after the date of grant. The plan allows for early exercise of options.
The Company has reserved 11,770,754 shares of its common stock for issuance under the Plan. As of December 31, 2012, the Company had 2,203,120 shares available for future issuance under the Plan.
Activity under the Company's stock option plan is set forth below:
| Outstanding Options | ||||||||||||||||
| Weighted | ||||||||||||||||
| Shares | Average | |||||||||||||||
| Available | Number | Exercise | Aggregate | |||||||||||||
| for Grant | of Shares | Price | Price | |||||||||||||
| Balances at December 31, 2010 | 5,833,715 | 4,820,130 | 0.37 | 1,801 | ||||||||||||
| Options granted | (5,604,626 | ) | 5,604,626 | 0.22 | 1,233 | |||||||||||
| Options exercised | - | (360,930 | ) | 0.19 | (68 | ) | ||||||||||
| Options cancelled | 702,489 | (702,489 | ) | 0.43 | (303 | ) | ||||||||||
| Balances at December 31, 2011 | 931,578 | 9,361,337 | $ | 0.28 | $ | 2,663 | ||||||||||
| Additional shares reserved | 550,000 | - | - | - | ||||||||||||
| Options granted | (1,087,151 | ) | 1,087,151 | 0.22 | 239 | |||||||||||
| Options exercised | - | (171,630 | ) | 0.25 | (43 | ) | ||||||||||
| Options cancelled | 1,748,275 | (1,748,275 | ) | 0.23 | (402 | ) | ||||||||||
| Options repurchased | 60,418 | - | 0.22 | - | ||||||||||||
| Balances, December 31, 2012 | 2,203,120 | 8,528,583 | $ | 0.29 | $ | 2,457 | ||||||||||
Information regarding the Company’s stock option outstanding, stock options vested and expected to vest, and stock option exercisable at December 2012 and 2011 was as follows:
| 161 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
| Weighted | ||||||||||||
| Weighted | Average | |||||||||||
| Average | Remaining | |||||||||||
| Number of | Exercise | Contractual | ||||||||||
| Options | Price | Life (Years) | ||||||||||
| As of December 31, 2012 | ||||||||||||
| Options outstanding | 8,528,583 | $ | 0.29 | 7.15 | ||||||||
| Options vested and expected to vest | 8,528,583 | $ | 0.29 | 7.15 | ||||||||
| Options exercisable | 6,056,539 | $ | 0.31 | 6.56 | ||||||||
| As of December 30, 2011 | ||||||||||||
| Options outstanding | 9,316,337 | $ | 0.28 | 8.00 | ||||||||
| Options vested and expected to vest | 9,316,337 | $ | 0.28 | 8.00 | ||||||||
| Options exercisable | 5,041,463 | $ | 0.31 | 7.30 | ||||||||
At December 31, 2012 and 2011, all shares were exercisable under the Plan, with a repurchase right by the Company, with a weighted average exercise price of $0.29 per share.
Stock-Based Compensation Associated with Awards to Employees
During the years ended December 31, 2012, and 2011, the Company granted stock options to employees to purchase 1,087,151 shares, and 5,353,050 shares of common stock with a weighted average grant date fair value calculated using Black-Scholes of $0.11 per share, and $0.12 per share, respectively. Stock-based compensation expense recognized during the years ended December 31, 2012, and 2011 for stock-based awards granted to employees based on the grant date fair value estimated in accordance with the provisions of ASC 718 was $267, and $288 respectively. As of December 31, 2012, the Company expects to recognize the remaining unamortized stock-based compensation expense of $342 over an estimated remaining weighted average period of 2.22 years.
The Company estimated the fair value of employee stock options using the Black-Scholes valuation model. The fair value of employee stock options is being amortized on a straight-line basis over the requisite service period of the awards. The fair value of employee stock options were estimated using the following weighted-average assumptions for the years ended December 31, 2012 and 2011:
| Year Ended | ||||||||
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Expected volatility | 54 | % | 54 | % | ||||
| Expected term (in years) | 6 | 6 | ||||||
| Risk-free interest rate | 1.4 | % | 2.8 | % | ||||
| Dividend yield | 0 | % | 0 | % | ||||
The expected term of stock options represents the weighted average period the stock options are expected to remain outstanding. The expected term assumption was determined by examining the expected terms for comparable industry peers as the Company did not have sufficient historical information to develop reasonable expectations. The expected stock price volatility assumption was determined by examining the historical volatilities for comparable industry peers, as the Company is still privately owned and does not have trading history for the Company’s common stock. The risk free interest rate assumption is based on the U.S. Treasury instruments whose term was consistent with the expected term of the Company’s stock options. The expected dividend assumption is based on the Company’s history and expectation of dividend payouts.
| 162 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
In addition, ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures were estimated based on historical experience. Prior to adoption of ASC 718, the Company accounted for forfeitures as they occurred.
Nonemployee Stock-Based Compensation
Stock-based compensation expenses related to stock options granted to nonemployees is recognized as the stock options are earned. The Company believes that the estimated fair value of the stock options is more readily measurable than the fair value of the services received. The fair value of the stock options granted to nonemployees is calculated at each grant date and remeasured at each reporting date, using the Black-Scholes option pricing model. During the year ended December 31, 2012, the Company did not grant stock options to nonemployees.
No income tax benefits have been recognized relating to stock-based compensation expenses and no tax benefits have been realized from exercised stock options.
| 10. | Income Taxes |
The tax effects of temporary differences and carryforwards that give rise to significant portions of the deferred tax assets are as follows:
| Year ended | ||||||||
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Deferred tax assets | ||||||||
| Net operating loss carryforwards | $ | 23,890 | $ | 19,546 | ||||
| Credit carryforwards | 1,154 | 1,280 | ||||||
| Other | 539 | 404 | ||||||
| Total deferred tax asset | 25,583 | 21,230 | ||||||
| Valuation allowance | (25,583 | ) | (21,230 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
Based on the available objective evidence, management believes it is more likely than not that the net deferred tax assets will not be fully realizable due to uncertainty surrounding realization of such assets. Accordingly, the Company has provided full valuation allowance against its deferred assets at December 31, 2012 and 2011.
As of December 31, 2012, the Company had net operating loss carryfowards of approximately $67,393, $ 47,075 and $14,598 available to reduce future taxable income, if any, for both Federal, California and other state income tax purposes, respectively. The net operating loss carryforward expire between 2026 and 2016, respectively.
As of December 31, 2012, the Company had research and development ("R&D") credit carryforwards of approximately $808 and $834 available to reduce future taxable income, if any, for both Federal and California state income tax purposes, respectively. The Federal R&D credit carryforwards expire beginning 2026 and California R&D credits carryforward indefinitely.
Utilization of the net operating loss carryforward may be subject to an annual limitation due to ownership percentage change limitations provided by the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of the net operating loss before utilization.
| 163 |
| Baxano, Inc. |
| Notes to Financial Statements |
| December 31, 2012 and 2011 |
| (in thousands, except share and per share data) |
The Company adopted the provisions of FASB Accounting Standards Codification (ASC 740-10), “Accounting for Uncertainty in Income Taxes.” ASC 740-10 prescribes a comprehensive model for the recognition, measurement, presentation and disclosure in financial statements of any uncertain tax positions that have been taken or expected to be taken on a tax return. The cumulative effect of adopting ASC 740-10 resulted in no adjustment to accumulated deficit as of January 1, 2009. As of December 31, 2012, the Company had an unrecognized tax benefit of $246. No liability related to uncertain tax positions is recorded in the financial statements. It is the Company’s policy to include penalties and interest expense related to income taxes as a component of other income (expense), net, as necessary.
The Company's tax years 2005-2012 will remain open for examination by the federal and state authorities for three and four years, respectively, from the date of utilization of any net operating loss credits.
A reconciliation of the beginning and ending balances of the unrecognized tax benefits during the years ended December 31, 2012 is as follows:
| December 31, | ||||
| 2012 | ||||
| Balance at January 1, 2012 | $ | 192 | ||
| Additions based on tax positions related to the current year | 54 | |||
| Balance at December 31, 2012 | $ | 246 | ||
| 11. | Subsequent Events |
On January 13, 2013, the Company and its investors entered into an agreement to amend certain terms associated with the $15,000 of outstanding convertible promissory notes. Under the amendment, holders of at least 60% of the aggregate outstanding principal amount under all notes agreed to modify the conversion feature to allow for full repayment of outstanding principal and unpaid accrued interest without premium or penalty upon consummation of an acquisition where stockholders of the Company prior to such acquisition own less than fifty percent (50%) of the voting interests in the surviving or resulting entity.
On March 3, 2013, the Company entered into a merger agreement to be acquired by TranS1Inc. ("TranS1") in an all-stock merger whereby the Company would become a wholly owned subsidiary of TranS1. TranS1 would issue to the Company’s shareholders a number of newly issued shares of common stock equating to a 28% pro forma equity ownership of the combined entity. The consideration assumes a $3,000 outstanding credit facility and is subject to adjustment based upon negotiated indemnification or escrow provisions, a $3,000 maximum indebtedness ceiling and predefined working capital targets. All of the Company’s outstanding stock options and warrants would be exercised or terminated prior to (and as a condition of) closing and not assumed by TranS1.
The Company has evaluated all transactions and events after the balance sheet date through March 5, 2013, the date which these financial statements were issued.
| 164 |
Annex A
EXECUTION VERSION
AGREEMENT AND PLAN OF MERGER
by and among
TRANS1 INC.,
RACERX ACQUISITION CORP.,
BAXANO, INC.,
and
Sumeet Jain and David Schulte, as Securityholder Representatives
Dated as of March 3, 2013
| A-1 |
TABLE OF CONTENTS
| ARTICLE I THE MERGER | 12 | |
| 1.1 | Effective Time of the Merger | 12 |
| 1.2 | Closing | 12 |
| 1.3 | Effects of the Merger | 12 |
| 1.4 | Certificate of Incorporation; Bylaws | 12 |
| 1.5 | Directors and Officers of Surviving Corporation | 13 |
| ARTICLE II MERGER CONSIDERATION; CONVERSION OF CAPITAL STOCK | 13 | |
| 2.1 | Overview | 13 |
| 2.2 | Calculation of Net Merger Consideration | 14 |
| 2.3 | Allocation of the Net Merger Consideration | 14 |
| 2.4 | Payoff of Baxano Notes | 16 |
| 2.5 | Conversion of Capital Stock | 17 |
| 2.6 | Exchange of Certificates | 18 |
| 2.7 | Treatment of Baxano Stock Option Plans, Baxano Options and Baxano Warrants | 21 |
| 2.8 | Dissenting Shares | 21 |
| 2.9 | Escrow Arrangement | 22 |
| 2.10 | Capitalization and Allocation Statements | 22 |
| 2.11 | Post-Closing Indebtedness and Working Capital Adjustment | 23 |
| ARTICLE III REPRESENTATIONS AND WARRANTIES OF BAXANO | 27 | |
| 3.1 | Organization, Standing and Power | 28 |
| 3.2 | Capitalization and Closing Allocation Statement | 28 |
| 3.3 | Subsidiaries | 31 |
| 3.4 | Authority; No Conflict; Required Filings and Consents | 31 |
| 3.5 | Baxano Financial Statements; Information Provided | 32 |
| 3.6 | No Undisclosed Liabilities | 33 |
| 3.7 | Absence of Certain Changes or Events | 33 |
| 3.8 | Taxes | 33 |
| 3.9 | Owned and Leased Real Properties | 35 |
| 3.10 | Intellectual Property | 35 |
| 3.11 | Contracts | 37 |
| 3.12 | Litigation | 38 |
| 3.13 | Environmental Matters | 39 |
| 3.14 | Employee Benefit Plans | 41 |
| 3.15 | Compliance With Laws | 43 |
| 3.16 | Permits and Regulatory Matters | 44 |
| 3.17 | Employees | 45 |
| 3.18 | Insurance | 45 |
| 3.19 | No Existing Discussions | 45 |
| 3.20 | Brokers; Fees and Expenses | 45 |
| A-2 |
| 3.21 | Financial Controls and Procedures | 45 |
| 3.22 | Certain Business Relationships With Affiliates | 46 |
| 3.23 | Books and Records | 46 |
| 3.24 | Commercial Relationships | 46 |
| 3.25 | Tangible Assets | 46 |
| 3.26 | Inventory | 46 |
| ARTICLE IV REPRESENTATIONS AND WARRANTIES OF TRANS1 AND THE TRANSITORY SUBSIDIARY | 47 | |
| 4.1 | Organization, Standing and Power | 47 |
| 4.2 | Capitalization | 48 |
| 4.3 | Subsidiaries | 50 |
| 4.4 | Authority; No Conflict; Required Filings and Consents | 51 |
| 4.5 | SEC Filings; Financial Statements; Information Provided | 52 |
| 4.6 | No Undisclosed Liabilities | 53 |
| 4.7 | Absence of Certain Changes or Events | 53 |
| 4.8 | Taxes | 54 |
| 4.9 | Owned and Leased Real Properties | 55 |
| 4.10 | Intellectual Property | 55 |
| 4.11 | Contracts | 56 |
| 4.12 | Litigation | 57 |
| 4.13 | Environmental Matters | 57 |
| 4.14 | Employee Benefit Plans | 59 |
| 4.15 | Compliance With Laws | 61 |
| 4.16 | Permits and Regulatory Matters | 61 |
| 4.17 | Employees | 62 |
| 4.18 | Insurance | 62 |
| 4.19 | Brokers; Fees and Expenses | 63 |
| 4.20 | Operations of Transitory Subsidiary | 63 |
| 4.21 | Controls and Procedures, Certifications and Other Matters Relating to the Sarbanes Act | 63 |
| 4.22 | Commercial Relationships | 64 |
| 4.23 | Tangible Assets | 64 |
| 4.24 | False Claims Act Matter | 64 |
| ARTICLE V CONDUCT OF BUSINESS | 64 | |
| 5.1 | Covenants of Baxano | 64 |
| 5.2 | Covenants of TranS1 | 67 |
| 5.3 | Confidentiality | 70 |
| ARTICLE VI ADDITIONAL AGREEMENTS | 70 | |
| 6.1 | No Solicitation | 70 |
| 6.2 | Securities Matters | 71 |
| 6.3 | NASDAQ Filings | 73 |
| A-3 |
| 6.4 | Access to Information | 73 |
| 6.5 | Stockholder Approval | 74 |
| 6.6 | Legal Conditions to Merger | 75 |
| 6.7 | Public Disclosure | 76 |
| 6.8 | Section 368(a) Reorganization | 76 |
| 6.9 | Notification of Certain Matters | 76 |
| 6.10 | Board of Directors of TranS1 | 76 |
| 6.11 | Employee Communications | 77 |
| 6.12 | FIRPTA Tax Certificates | 77 |
| 6.13 | Additional Indebtedness | 78 |
| 6.14 | Indemnification and Insurance | 78 |
| 6.15 | Non-Solicitation | 79 |
| 6.16 | Employee Benefits | 79 |
| 6.17 | Transaction Expenses | 80 |
| 6.18 | Post-Closing Operations | 80 |
| 6.19 | Consents of Distributors | 81 |
| ARTICLE VII CONDITIONS TO MERGER | 81 | |
| 7.1 | Conditions to Each Party’s Obligation To Effect the Merger | 81 |
| 7.2 | Additional Conditions to the Obligations of TranS1 and Transitory Subsidiary | 82 |
| 7.3 | Additional Conditions to the Obligations of Baxano | 83 |
| ARTICLE VIII INDEMNIFICATION | 84 | |
| 8.1 | Survival | 84 |
| 8.2 | Indemnification of TranS1 Indemnified Parties | 85 |
| 8.3 | Indemnification of the Securityholder Indemnified Parties | 87 |
| 8.4 | Indemnification Procedures | 88 |
| 8.5 | Tax Indemnity | 91 |
| 8.6 | Other | 93 |
| 8.7 | Release from Escrow | 95 |
| ARTICLE IX TERMINATION AND AMENDMENT | 96 | |
| 9.1 | Termination | 96 |
| 9.2 | Effect of Termination | 97 |
| 9.3 | Fees and Expenses | 97 |
| ARTICLE X MISCELLANEOUS | 99 | |
| 10.1 | Amendment | 99 |
| 10.2 | Extension; Waiver | 99 |
| 10.3 | Notices | 99 |
| 10.4 | Entire Agreement | 101 |
| 10.5 | No Third Party Beneficiaries | 101 |
| 10.6 | Assignment | 101 |
| 10.7 | Severability | 101 |
| A-4 |
| 10.8 | Counterparts and Signature | 102 |
| 10.9 | Interpretation | 102 |
| 10.10 | Specific Performance | 103 |
| 10.11 | Governing Law | 103 |
| 10.12 | Submission to Jurisdiction | 103 |
| 10.13 | WAIVER OF JURY TRIAL | 103 |
| 10.14 | Securityholder Representatives | 103 |
| Exhibit A | Form of TranS1 Stockholder Agreement |
| Exhibit B | Form of Escrow Agreement |
| Schedule A | TranS1 Key Stockholders |
| A-5 |
TABLE OF DEFINED TERMS
| Terms | Cross Reference in Agreement | |
| Acquisition Proposal | Section 6.1(a) | |
| Adjusted Net Working Capital | Section 2.11(a)(i) | |
| Adjustment Amount | Section 2.11(c) | |
| Adjustment Statement | Section 2.11(c) | |
| Affiliate | Section 3.2(e) | |
| Aggregate Common Stock Merger Consideration | Section 2.3(a)(iv) | |
| Aggregate Junior Preferred Merger Consideration | Section 2.3(a)(iii) | |
| Aggregate Junior Preferred Value | Section 2.3(c)(i) | |
| Aggregate Noteholder Merger Consideration | Section 2.3(a)(i) | |
| Aggregate Series C Merger Consideration | Section 2.3(a)(ii) | |
| Agreement | Preamble | |
| Bankruptcy and Equity Exception | Section 3.4(a) | |
| Basket | Section 8.2(b)(i) | |
| Baxano | Preamble | |
| Baxano Authorizations | Section 3.16(a) | |
| Baxano Balance Sheet | Section 3.5(a) | |
| Baxano Balance Sheet Date | Section 3.5(a) | |
| Baxano Board | Background | |
| Baxano Certificate of Incorporation | Background | |
| Baxano Common Stock | Section 3.2(a) | |
| Baxano Convertible Securities | Section 3.2(d) | |
| Baxano Disclosure Schedule | Article III | |
| Baxano Employee Plans | Section 3.14(a) | |
| Baxano Financial Statements | Section 3.5(a) | |
| Baxano Insurance Policies | Section 3.18 | |
| Baxano Intellectual Property | Section 3.10(b) | |
| Baxano Leases | Section 3.9(b) | |
| Baxano Material Adverse Effect | Section 3.1 | |
| Baxano Note | Section 2.4 | |
| Baxano Preferred Stock | Section 3.2(a) | |
| Baxano Series A-1 Preferred Stock | Section 3.2(a) | |
| Baxano Series A-2 Preferred Stock | Section 3.2(a) | |
| Baxano Series B Preferred Stock | Section 3.2(a) | |
| Baxano Series C Preferred Stock | Section 3.2(a) | |
| Baxano Stock Options | Section 2.7(a) | |
| Baxano Stock Plans | Section 2.7(b) | |
| Baxano Stockholder Approval | Section 3.4(a) | |
| Baxano Third Party Intellectual Property | Section 3.10(b) | |
| Baxano Voting Proposal | Section 3.4(a) | |
| Baxano Warrants | Section 2.7(a) | |
| Cap | Section 8.2(b)(vi) | |
| Cash | Section 2.11(a)(ii) |
| A-6 |
| Terms | Cross Reference in Agreement | |
| CCC | Section 2.5(c) | |
| Certificate of Merger | Section 1.1 | |
| Certificates | Section 2.6(b) | |
| Claim Objection | Section 8.4(c)(i) | |
| Closing | Section 1.2 | |
| Closing Allocation Statement | Section 2.10(b) | |
| Closing Capitalization Statement | Section 2.10(a) | |
| Closing Date | Section 1.2 | |
| Code | Background | |
| Conclusive Adjustment Statement | Section 2.11(e) | |
| Conclusive Statement | Section 2.11(e) | |
| Confidentiality Agreement | Section 5.3 | |
| Continuing Employees | Section 6.16(a) | |
| Current Assets | Section 2.11(a)(iii) | |
| Current Liabilities | Section 2.11(a)(iv) | |
| D&O Indemnitees | Section 6.14(a) | |
| De Minimis Limitation | Section 8.2(b)(ii) | |
| DGCL | Background | |
| Dissenting Shares | Section 2.5(c) | |
| Effective Time | Section 1.1 | |
| Employee Benefit Plan | Section 3.14(l)(i) | |
| Environmental Law | Section 3.13(d) | |
| ERISA | Section 3.14(l)(ii) | |
| ERISA Affiliate | Section 3.14(l)(iii) | |
| Escrow Agent | Section 2.9(a) | |
| Escrow Agreement | Section 2.9(b) | |
| Escrow Funding Percentage | Section 2.3(c)(ii) | |
| Escrow Participant | Section 2.3(c)(iii) | |
| Escrow Participation Percentage | Section 2.3(c)(iv) | |
| Escrow Shares | Section 2.3(a)(i) | |
| Escrow Shares Indemnity Value | Section 8.2(c) | |
| Excess Indebtedness Amount | Section 2.2 | |
| Exchange Act | Section 4.4(c) | |
| Exchange Agent | Section 2.6(a) | |
| Exchange Fund | Section 2.6(a) | |
| FCA Matter | Section 4.1 | |
| FDA | Section 3.16(a) | |
| Final Determination | Section 8.4(c)(iii) | |
| Financing Shares | Background | |
| Fundamental Baxano Representations | Section 8.1(a) | |
| Fundamental TranS1 Representations | Section 8.1(a) | |
| GAAP | Section 3.5(a) | |
| Governmental Entity | Section 3.4(c) |
| A-7 |
| Terms | Cross Reference in Agreement | |
| Hazardous Substance | Section 3.13(e) | |
| Indebtedness | Section 2.11(a)(v) | |
| Indemnified Party | Section 8.4(a) | |
| Indemnified Tax Losses | Section 8.5(a) | |
| Indemnifying Party | Section 8.4(a) | |
| Indemnity Claim Notice | Section 8.4(a) | |
| Intellectual Property | Section 3.10(e)(i) | |
| IRS | Section 3.14(b) | |
| Lien | Section 3.4(b) | |
| Loss | Section 8.2(a) | |
| Losses | Section 8.2(a) | |
| Merger | Background | |
| Merger Closing Price | Section 2.2 | |
| Merger Consideration | Section 2.1 | |
| Merger Shares | Section 2.2 | |
| NASDAQ | Section 2.2 | |
| Net Merger Consideration | Section 2.2 | |
| Neutral Accounting Arbitrator | Section 2.11(e) | |
| Noteholder | Section 2.4 | |
| Objection Period | Section 8.4(c)(i) | |
| Objection Statement | Section 2.11(d) | |
| Ordinary Course of Business | Section 3.6 | |
| Outside Date | Section 9.1(b) | |
| Oxford/SVB Loan Documents | Section 2.2 | |
| Patent Rights | Section 3.10(e)(ii) | |
| Per Noteholder Merger Consideration | Section 2.4 | |
| Permitted Indebtedness | Section 2.2 | |
| Person | Section 3.3 | |
| Post-Closing Statement | Section 2.11(c) | |
| Pre-Closing Statement | Section 2.11(b) | |
| Pre-Closing Tax Period | Section 8.5(a) | |
| Preliminary Allocation Statement | Section 2.10(b) | |
| Preliminary Capitalization Statement | Section 2.10(a) | |
| Proceeding | Section 8.2(a) | |
| Proxy Statement | Section 6.5(b) | |
| Registration Statement | Background | |
| Representative Reimbursement Amount | Section 2.2 | |
| Representatives | Section 6.1(a) | |
| Resolution Period | Section 2.11(d) | |
| Sarbanes Act | Section 4.5(a) | |
| SEC | Section 4.4(c) | |
| Securities Act | Section 3.2(e) | |
| Securities Purchase Agreement | Background |
| A-8 |
| Terms | Cross Reference in Agreement | |
| Securityholder Indemnified Parties | Section 8.3(a) | |
| Securityholder Representatives | Section 10.14(a) | |
| Securityholders | Section 2.4 | |
| Series A-1 Preferred Value | Section 2.3(c)(v) | |
| Series A-2 Preferred Value | Section 2.3(c)(vi) | |
| Series B Preferred Value | Section 2.3(c)(vii) | |
| Series C Preferred Value | Section 2.3(c)(viii) | |
| Shares | Section 6.2(b) | |
| Straddle Period | Section 8.5(b) | |
| Straddle Period Returns | Section 8.5(c) | |
| Subsidiary | Section 2.5(b) | |
| Surviving Corporation | Section 1.3 | |
| Surviving Corporation Bylaws | Section 1.4(b) | |
| Surviving Corporation Certificate of Incorporation | Section 1.4(a) | |
| Target Adjusted Net Working Capital | Section 2.11(a)(vi) | |
| Tax Indemnified Parties | Section 8.5(a) | |
| Tax Representations | Section 8.1(a) | |
| Tax Returns | Section 3.8(a) | |
| Taxes | Section 3.8(a) | |
| Third Party Claim | Section 8.4(b) | |
| Trademarks | Section 3.10(e)(iii) | |
| TranS1 | Preamble | |
| TranS1 Annual Meeting | Section 6.10(c) | |
| TranS1 Authorizations | Section 4.16(a) | |
| TranS1 Balance Sheet | Section 4.5(b) | |
| TranS1 Board | Background | |
| TranS1 Common Stock | Section 4.2(a) | |
| TranS1 Convertible Securities | Section 4.2(c) | |
| TranS1 Disclosure Schedule | Article IV | |
| TranS1 Employee Plans | Section 4.14(a) | |
| TranS1 Form 10-K | Section 4.6 | |
| TranS1 Indemnified Parties | Section 8.2(a) | |
| TranS1 Insurance Policies | Section 4.18 | |
| TranS1 Intellectual Property | Section 4.10(b) | |
| TranS1 Material Adverse Effect | Section 4.1 | |
| TranS1 Material Contracts | Section 4.11(a) | |
| TranS1 Meeting | Section 6.5(b) | |
| TranS1 Preferred Stock | Section 4.2(a) | |
| TranS1 Recent SEC Documents | Section 4.6 | |
| TranS1 SEC Documents | Section 4.5(a) | |
| TranS1 Stock Options | Section 4.2(b) | |
| TranS1 Stock Plans | Section 4.2(b) | |
| TranS1 Stockholder Agreements | Background |
| A-9 |
| Terms | Cross Reference in Agreement | |
| TranS1 Stockholder Approval | Section 6.5(b) | |
| TranS1 Third Party Intellectual Property | Section 4.10(b) | |
| TranS1 Voting Proposal | Section 6.5(b) | |
| Transaction Expenses | Section 2.11(a)(vii) | |
| Transaction Payment Plan | Section 2.2 | |
| Transfer | Section 6.2(a) | |
| Transitory Subsidiary | Preamble | |
| Transitory Subsidiary Board | Background | |
| Written Consents | Section 3.4(d) |
| A-10 |
AGREEMENT AND PLAN OF MERGER
This AGREEMENT AND PLAN OF MERGER (this “Agreement”), dated as of March 3, 2013, is by and among TranS1 Inc., a Delaware corporation (“TranS1”), RacerX Acquisition Corp., a Delaware corporation and a wholly owned subsidiary of TranS1 (“Transitory Subsidiary”), Baxano, Inc., a Delaware corporation (“Baxano”), and Sumeet Jain and David Schulte, solely as the Securityholder Representatives following appointment pursuant to Section 10.14(a).
BACKGROUND
WHEREAS, the Board of Directors of TranS1 (the “TranS1 Board”), the Board of Directors of Transitory Subsidiary (the “Transitory Subsidiary Board”) and the Board of Directors of Baxano (the “Baxano Board”) each deem it advisable and in the best interests of their respective corporation and its stockholders that TranS1 and Baxano combine in order to advance the long-term business interests of TranS1 and Baxano, which shall be effected through a merger (the “Merger”) of Transitory Subsidiary with and into Baxano in accordance with the terms of this Agreement and the General Corporation Law of the State of Delaware (the “DGCL”), as a result of which Baxano will survive and become a wholly owned subsidiary of TranS1;
WHEREAS, the Merger constitutes a “Liquidation Event” under Baxano’s Amended and Restated Certificate of Incorporation, as amended (the “Baxano Certificate of Incorporation”);
WHEREAS, concurrently with the execution and delivery of this Agreement and as a condition and inducement to Baxano’s willingness to enter into this Agreement, the stockholders of TranS1 listed onSchedule A to this Agreement have entered into Stockholder Agreements, dated as of the date of this Agreement, in the form attached hereto asExhibit A (the “TranS1 Stockholder Agreements”), pursuant to which such stockholders have, among other things, agreed (i) to vote all of the shares of capital stock of TranS1 that such stockholders own to approve the issuance of the Merger Shares and the Financing Shares pursuant to applicable NASDAQ rules, (ii) not to transfer or otherwise dispose of any shares of TranS1 Common Stock prior to the registration statement to be filed by TranS1 pursuant to which the Merger Shares and Financing Shares shall be registered under the Securities Act (the “Registration Statement”) being declared effective and (iii) at the TranS1 Annual Meeting, to vote all of the shares of capital stock of TranS1 that such stockholders own in favor of any proposal to re-elect the two (2) persons identified on Section 6.10 of the Baxano Disclosure Schedule (or as otherwise set forth in Section 6.10) to the TranS1 Board for a three year term;
WHEREAS, concurrently with the execution and delivery of this Agreement and as a condition and inducement to the parties’ willingness to enter into this Agreement, certain Baxano stockholders have entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”), pursuant to which such stockholders have agreed to purchase shares of TranS1 Common Stock in a private placement transaction (such shares, the “Financing Shares”) on the terms set forth therein; and
| A-11 |
WHEREAS, for United States federal income tax purposes, it is intended that the Merger shall qualify as a reorganization within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”).
NOW, THEREFORE, in consideration of the foregoing and the respective representations, warranties, covenants and agreements set forth below, TranS1, Transitory Subsidiary and Baxano agree as follows:
ARTICLE I
THE MERGER
1.1 Effective Time of the Merger. Subject to the provisions of this Agreement, prior to the Closing, TranS1 shall prepare (in a form reasonably acceptable to Baxano), and on the Closing Date (as defined in Section 1.2) or as soon as practicable thereafter TranS1 and Baxano shall cause to be filed with the Secretary of State of the State of Delaware, a certificate of merger (the “Certificate of Merger”) in such form as is required by, and executed by Surviving Corporation in accordance with, the relevant provisions of the DGCL and shall make all other filings or recordings required under the DGCL. The Merger shall become effective upon the filing of the Certificate of Merger with the Secretary of State of the State of Delaware or at such later time as is established by TranS1 and Baxano and set forth in the Certificate of Merger (the “Effective Time”). The Certificate of Merger shall provide that the name of Surviving Corporation as of and after the Effective Time shall be “Baxano, Inc.”
1.2 Closing. The closing of the Merger (the “Closing”) will take place at 10:00 a.m., U.S. Eastern time, on a date to be specified by TranS1 and Baxano (the “Closing Date”), which shall be no later than the second business day after satisfaction or waiver of the conditions set forth in ARTICLE VII (other than delivery of items to be delivered at the Closing and other than satisfaction of those conditions that by their nature are to be satisfied at the Closing, it being understood that the occurrence of the Closing shall remain subject to the delivery of such items and the satisfaction or waiver of such conditions at the Closing), at the offices of Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P., 2300 Wells Fargo Capitol Center, Raleigh, NC 27601, unless another date, place or time is agreed to in writing by TranS1 and Baxano. It is the intention of the parties that the Closing shall occur as soon as practicable after the TranS1 Meeting (as defined in Section 6.5(b)).
1.3 Effects of the Merger. At the Effective Time, the separate existence of Transitory Subsidiary shall cease and Transitory Subsidiary shall be merged with and into Baxano (Baxano following the Merger is sometimes referred to herein as “Surviving Corporation”), and the Merger shall have the effects set forth in the DGCL.
1.4 Certificate of Incorporation; Bylaws.
(a) Unless otherwise determined by TranS1 prior to the Effective Time, the certificate of incorporation of Transitory Subsidiary immediately prior to the Effective Time shall be the certificate of incorporation of Surviving Corporation (the “Surviving Corporation Certificate of Incorporation”) until thereafter amended in accordance with the DGCL and as provided the Surviving Corporation Certificate of Incorporation;provided,however, that (i) the Surviving Corporation Certificate of Incorporation shall contain the provisions contemplated by Section 6.14 and (ii) at the Effective Time, the first paragraph of the Surviving Corporation Certificate of Incorporation shall be amended and restated in its entirety to read as follows: “The name of this Corporation is Baxano, Inc.”
| A-12 |
(b) Unless otherwise determined by TranS1 prior to the Effective Time, the bylaws of Transitory Subsidiary immediately prior to the Effective Time shall be the bylaws of Surviving Corporation (the “Surviving Corporation Bylaws”) (other than any express reference to the name of Transitory Subsidiary in such bylaws, which shall be amended to refer to Surviving Corporation) until thereafter amended in accordance with the DGCL and as provided in the Surviving Corporation Certificate of Incorporation and the Surviving Corporation Bylaws;provided,however, that the Surviving Corporation Bylaws shall contain the provisions contemplated by Section 6.14.
1.5 Directors and Officers of Surviving Corporation
(a) The directors of Transitory Subsidiary immediately prior to the Effective Time shall be the initial directors of Surviving Corporation, each to hold office in accordance with the Surviving Corporation Certificate of Incorporation and the Surviving Corporation Bylaws.
(b) The officers of Transitory Subsidiary immediately prior to the Effective Time shall be the initial officers of Surviving Corporation, each to hold office in accordance with the Surviving Corporation Certificate of Incorporation and the Surviving Corporation Bylaws.
ARTICLE II
MERGER CONSIDERATION; CONVERSION OF CAPITAL STOCK
2.1 Overview. The consideration to be paid by TranS1 to acquire all of outstanding shares of the capital stock of Baxano shall be a number of shares of newly issued TranS1 Common Stock (the “Merger Consideration”) calculated as the product of (a) 0.38888889times (b) the number of issued and outstanding shares of capital stock of TranS1 (as if converted to TranS1 Common Stock) as of the close of business on the last trading day prior to the Closing Date (and prior to the issuance of any Financing Shares), and shall be subject to adjustment as described in this ARTICLE II.
| A-13 |
2.2 Calculation of Net Merger Consideration. To the extent that Baxano has any Indebtedness (other than Permitted Indebtedness) that remains unpaid as of the Closing (such amount, the “Excess Indebtedness Amount”), the Merger Consideration shall be reduced by a number of shares equal to the quotient of (a) the Excess Indebtedness Amount,divided by (b) the Merger Closing Price. The Merger Consideration shall be further reduced by a number of shares equal to the quotient of (x) the Representative Reimbursement Amount plus the aggregate amount to be paid by Baxano under the Transaction Payment Plan,divided by (y) the Merger Closing Price. “Permitted Indebtedness” shall mean (I) the principal and interest outstanding under the Baxano Notes and (II) up to $3,000,000 of principal plus accrued interest outstanding under that certain Loan and Security Agreement dated as of March 15, 2012, among Oxford Finance LLC, Silicon Valley Bank, and Baxano, Inc. (collectively, the “Oxford/SVB Loan Documents,”). The “Representative Reimbursement Amount” shall mean a cash amount equal to $250,000. The “Transaction Payment Plan” shall mean a compensation plan to be adopted by Baxano prior to the Closing providing for bonuses in the aggregate amount of up to $300,000. The Merger Consideration, as so reduced in respect of the Excess Indebtedness Amount, the Representative Reimbursement Amount and the Transaction Payment Plan, rounded to the nearest whole share is referred to herein as the “Net Merger Consideration.” The shares of TranS1 Common Stock payable to Securityholders as Net Merger Consideration are referred to herein as the “Merger Shares.” The Merger Shares will be registered with the Securities and Exchange Commission for resale as set forth in the Securities Purchase Agreement. “Merger Closing Price” shall mean the average of the last reported sales prices of TranS1 Common Stock at 4:00 p.m., Eastern time, end of regular trading hours on The NASDAQ Stock Market LLC (“NASDAQ”) during the ten consecutive trading days ending on the last trading day prior to the Closing Date.
2.3 Allocation of the Net Merger Consideration.
(a) Merger Consideration Allocation Priorities. At the Closing, the Merger Shares comprising the Net Merger Consideration shall be allocated to the following funds or for the following purposes in decreasing order of priority until no Merger Shares remain:
(i) first, a number of Merger Shares up to and not exceeding the quotient of (x) the aggregate amount of unpaid principal and accrued interest as of immediately prior to the Effective Time under all Baxano Notes,divided by (y) the Merger Closing Price, shall be allocated for payment of the Per Noteholder Merger Consideration under Section 2.4 (such shares, the “Aggregate Noteholder Merger Consideration”), of which a number of shares equal to the product of (A) the Aggregate Noteholder Merger Considerationmultiplied by (B) the Escrow Funding Percentage (as defined in Section 2.3(c)(ii)) shall be “Escrow Shares” hereunder;
(ii) second, if any Merger Shares remain after the foregoing allocation in this Section 2.3, a number of Merger Shares up to and not exceeding the quotient of (x) the aggregate Series C Preferred Value for all shares of Baxano Series C Preferred Stock issued and outstanding immediately prior to the Effective Time (excluding shares to be cancelled in accordance with Section 2.5(b)),divided by (y) the Merger Closing Price, shall be allocated for distribution in respect of shares of Baxano Series C Preferred Stock under Section 2.5(c)(i) (such shares, if any, the “Aggregate Series C Merger Consideration”), of which a number of shares equal to the product of (A) the Aggregate Series C Merger Considerationmultiplied by (B) the Escrow Funding Percentage shall be “Escrow Shares” hereunder;
(iii) third, if any Merger Shares remain after the foregoing allocations in this Section 2.3, a number of Merger Shares up to and not exceeding the quotient of (x) the Aggregate Junior Preferred Value,divided by (y) the Merger Closing Price, shall be allocated for distribution in respect of shares of Baxano Series A-1 Preferred Stock, Baxano Series A-2 Preferred Stock, and Baxano Series B Preferred Stock under Sections 2.5(c)(ii), 2.5(c)(iii), 2.5(c)(iv) and 2.5(c)(v) (such shares, if any, the “Aggregate Junior Preferred Merger Consideration”), of which a number of shares equal to the product of (A) the Aggregate Junior Preferred Merger Considerationmultiplied by (B) the Escrow Funding Percentage shall be “Escrow Shares” hereunder; and
| A-14 |
(iv) fourth, if any Merger Shares remain after the foregoing allocations in this Section 2.3, all such remaining Merger Shares shall be allocated for distribution in respect of shares of Baxano Series C Preferred Stock and Baxano Common Stock issued and outstanding immediately prior to the Effective Time under Section 2.5(c)(ii) (such shares, if any, the “Aggregate Common Stock Merger Consideration”), of which a number of shares equal to the product of (A) the Aggregate Common Stock Merger Considerationmultiplied by (B) the Escrow Funding Percentage shall be considered “Escrow Shares” hereunder,provided,however, that the value of Merger Shares (calculated using the Merger Closing Price) comprising the Aggregate Series C Merger Consideration plus the portion of the Aggregate Common Stock Merger Consideration attributable to the shares of Baxano Series C Preferred Stock having been deemed converted to Baxano Common Stock under Section 2.5(c)(v) shall not exceed the product of (x) $2.733multiplied by, (y) the aggregate number ofshares of Baxano Series C Preferred Stock issued and outstanding immediately prior to the Effective Time (excluding shares to be cancelled in accordance with Section 2.5(b)).
(b) Escrow Shares. Any release of Escrow Shares or the Representative Reimbursement Amount by the Escrow Agent for payment to Escrow Participants shall be allocated to the Escrow Participants pro rata according to each Escrow Participant’s Escrow Participation Percentage.
(c) Definitions. For purposes of this Agreement, the following terms shall have the following meanings:
(i) “Aggregate Junior Preferred Value” shall mean the sum of (A) the aggregate Series A-1 Preferred Value for all shares of Baxano Series A-1 Preferred Stock issued and outstanding immediately prior to the Effective Time (excluding shares to be cancelled in accordance with Section 2.5(b)),plus (B) the aggregate Series A-2 Preferred Value for all shares of Baxano Series A-2 Preferred Stock issued and outstanding immediately prior to the Effective Time (excluding shares to be cancelled in accordance with Section 2.5(b)),plus (C) the aggregate Series A-2 Preferred Value for all shares of Baxano Series A-2 Preferred Stock exercisable upon exercise of outstanding warrants to purchase Baxano Series A-2 Preferred Stock minus the aggregate exercise price for such shares underlying such warrants,plus (D) the aggregate Series B Preferred Value for all shares of Baxano Series B Preferred Stock issued and outstanding immediately prior to the Effective Time (excluding shares to be cancelled in accordance with Section 2.5(b));
(ii) “Escrow Funding Percentage” shall mean the percentage calculated as (A) 7.5%,times (B) the number of shares comprising the Merger Consideration,divided by (C) the number of shares comprising the Net Merger Consideration.
(iii) “Escrow Participant” means (x) each Securityholder who is entitled to Merger Shares for his, her or its shares of Baxano capital stock pursuant to Section 2.5(c) and (y) each Noteholder who is entitled to Merger Shares for his, her or its Baxano Note pursuant to Section 2.4.
| A-15 |
(iv) “Escrow Participation Percentage” means, with respect to anEscrow Participant, the percentage corresponding to the fraction:(i) having a numerator equal to the aggregate number ofMerger Sharesdistributable to suchEscrow Participantpursuant to Sections2.3,2.4 and 2.5; and (ii) having a denominator equal to the aggregate number ofMerger Sharesdistributable to allEscrow Participantspursuant to Sections2.3,2.4 and 2.5.
(v) “Series A-1 Preferred Value” with respect to each share of Baxano Series A-1 Preferred Stock shall mean the sum of (A)$1.00per share,plus (B) an amount equal to all declared but unpaid dividends on such share immediately prior to the Effective Time;
(vi) “Series A-2 Preferred Value” with respect to each share of Baxano Series A-2 Preferred Stock shall mean the sum of (A)$1.65per share,plus (B) an amount equal to all declared but unpaid dividends on such share immediately prior to the Effective Time;
(vii) “Series B Preferred Value” with respect to each share of Baxano Series B Preferred Stock shall mean the sum of (A)$2.23per share,plus (B) an amount equal to all declared but unpaid dividends on such share immediately prior to the Effective Time; and
(viii) “Series C Preferred Value” with respect to each share of Baxano Series C Preferred Stock shall mean the sum of (A)$0.9110per share,plus (B) an amount equal to all declared but unpaid dividends on such share immediately prior to the Effective Time.
2.4 Payoff of Baxano Notes. Prior to the Effective Time, Baxano shall take all actions necessary to (i) cause each promissory note that is convertible into capital stock of Baxano, including all convertible promissory notes issued pursuant to that certain Note and Warrant Purchase Agreement dated as of March 7, 2012, among Baxano and the investors set forth therein, outstanding at the Effective Time (each, a “Baxano Note”) to be terminated effective as of the Effective Time, and the holder of each such Baxano Note (each a “Noteholder” and together with each holder of shares of capital stock of Baxano or warrants exercisable for shares of capital stock of Baxano being converted as described in Section 2.5(c) and each other holder of Baxano warrants, options or other securities convertible into capital stock of Baxano, collectively, the “Securityholders”) shall, subject to Section 2.6, have the right to receive as of the Effective Time the Per Noteholder Merger Consideration, (ii) cause each Noteholder to agree to irrevocably appoint the Securityholder Representatives as the representatives, agents, proxies and attorneys in fact of and for such Noteholder for all purposes under this Agreement pursuant to Section 10.14 as if such Noteholder were a holder of shares of capital stock of Baxano converted in the Merger and (iii) cause each Noteholder to be bound by this Agreement, the Escrow Agreement and the Securities Purchase Agreement to the same extent as if such Noteholder were a holder of capital stock of Baxano converted in the Merger. “Per Noteholder Merger Consideration” shall mean with respect to each Baxano Note a number of Merger Shares equal to the product of (A) the Aggregate Noteholder Merger Consideration,times (B) the quotient of (I) the amount of unpaid principal and accrued interest as of immediately prior to the Effective Time (or, if earlier, the time such Baxano Note was terminated) under such Baxano Note,divided by (II) the aggregate amount of unpaid principal and accrued interest as of immediately prior to the Effective Time under all Baxano Notes.
| A-16 |
2.5 Conversion of Capital Stock. As of the Effective Time, by virtue of the Merger and without any action on the part of the holder of any shares of the capital stock of Baxano or the holder of any shares of the capital stock of Transitory Subsidiary, the Merger shall have the effects set forth below in this Section 2.5:
(a) Conversion of Capital Stock of Transitory Subsidiary. Each share of the common stock of Transitory Subsidiary issued and outstanding immediately prior to the Effective Time shall be converted into and become one fully paid and nonassessable share of common stock, $0.0001 par value per share, of Surviving Corporation.
(b) Cancellation of Treasury Stock. All shares of Baxano capital stock that are owned by Baxano as treasury stock or by any wholly owned Subsidiary of Baxano and any shares of Baxano capital stock owned by TranS1, Transitory Subsidiary or any other wholly owned Subsidiary of TranS1 immediately prior to the Effective Time shall be cancelled and shall cease to exist and no stock of TranS1 or other consideration shall be delivered in exchange therefor. For purposes of this Agreement, the term “Subsidiary” means, with respect to any party, any Person in which such party (or another Subsidiary of such party) holds stock or other ownership interests representing (i) more that 50% of the voting power of all outstanding stock or ownership interests of such entity or (ii) the right to receive more than 50% of the net assets of such entity available for distribution to the holders of outstanding stock or ownership interests upon a liquidation or dissolution of such entity.
(c) Conversion of Capital Stock of Baxano. Subject to Section 2.6(b)(i), each share of Baxano capital stock issued and outstanding immediately prior to the Effective Time, excluding shares to be cancelled in accordance with Section 2.5(b) and shares of Baxano capital stock held as of the Effective Time that has not been voted in favor of the adoption of this Agreement and with respect to which appraisal or dissent shall have been duly demanded and perfected in accordance with the DGCL and the California Corporations Code (the “CCC”) and not effectively withdrawn or forfeited prior to the Effective Time (the “Dissenting Shares”), shall cease to exist and be converted into and become exchangeable for the right to receive the number of Merger Shares(if any) pursuant to this Section2.5(c), together with anycashin lieu of fractionalsharesofTranS1 Common Stockto be issued or paid in consideration therefor upon the surrender of such certificate in accordance with Section2.6, without interest, equal to:
(i) With respect to each share of Baxano Series C Preferred Stock, the sum of (A) the quotient of (I) the Aggregate Series C Merger Considerationdivided by (II) the aggregate number ofshares of Baxano Series C Preferred Stock issued and outstanding immediately prior to the Effective Time (excluding shares to be cancelled in accordance with Section 2.5(b)),plus (B) the quotient of (I) the portion of the Aggregate Common Stock Merger Consideration attributable to the shares of Baxano Series C Preferred Stockdivided by (II) the aggregate number ofsharesofBaxano Common Stock attributable to the shares of Baxano Series C Preferred Stock issued and outstanding immediately prior to the Effective Time (treating the shares of Baxano Series C Preferred Stock for this purpose as if they had been converted to shares of Baxano Common Stock at the then-effective Conversion Price for such shares under the Baxano Certificate of Incorporation and excluding shares to be cancelled in accordance with Section 2.5(b));
| A-17 |
(ii) With respect to each share of Baxano Series B Preferred Stock, the product of (A) Aggregate Junior Preferred Merger Consideration (if any),times (B) the quotient of (I) the Series B Preferred Value,divided by (II) theAggregate Junior Preferred Value.For the avoidance of doubt, if there are no Merger Shares comprising the Aggregate Junior Preferred Merger Consideration, then no consideration shall be paid for each such share of Baxano Series B Preferred Stock;
(iii) With respect to each share of Baxano Series A-2 Preferred Stock, the product of (A) Aggregate Junior Preferred Merger Consideration (if any),times (B) the quotient of (I) the Series A-2 Preferred Value,divided by (II) theAggregate Junior Preferred Value.For the avoidance of doubt, if there are no Merger Shares comprising the Aggregate Junior Preferred Merger Consideration, then no consideration shall be paid for each such share of Baxano Series A-2 Preferred Stock;
(iv) With respect to each share of Baxano Series A-2 Preferred Stock issuable upon exercise of a warrant to purchase Baxano Series A-2 Preferred Stock, the product of (A) Aggregate Junior Preferred Merger Consideration (if any),times (B) the quotient of (I) (1) the Series A-2 Preferred Valueminus (2) the exercise price for one share ofBaxano Series A-2 Preferred Stockunder such warrant,divided by (II) theAggregate Junior Preferred Value.For the avoidance of doubt, if there are no Merger Shares comprising the Aggregate Junior Preferred Merger Consideration, then no consideration shall be paid for each such share of Baxano Series A-2 Preferred Stock underlying such warrants;
(v) With respect to each share of Baxano Series A-1 Preferred Stock, the product of (A) Aggregate Junior Preferred Merger Consideration (if any),times (B) the quotient of (I) the Series A-1 Preferred Value,divided by (II) theAggregate Junior Preferred Value.For the avoidance of doubt, if there are no Merger Shares comprising the Aggregate Junior Preferred Merger Consideration, then no consideration shall be paid for each such share of Baxano Series A-1 Preferred Stock; and
(vi) With respect to each share of Baxano Common Stock, the quotient of (A) the Aggregate Common Stock Merger Consideration (if any),divided by (B)the number ofshares of Baxano Common Stock issued and outstanding immediately prior to the Effective Time (treating the shares of Baxano Series C Preferred Stock for this purpose as if they had been converted to shares of Baxano Common Stock at the then-effective Conversion Price for such shares under the Baxano Certificate of Incorporation and excluding shares to be cancelled in accordance with Section 2.5(b)).For the avoidance of doubt, if there are no Merger Shares comprising the Aggregate Common Stock Merger Consideration, then no consideration shall be paid for each such share of Baxano Common Stock.
2.6 Exchange of Certificates. The procedures for exchanging Baxano Notes and outstanding shares of Baxano Common Stock for TranS1 Common Stock pursuant to the Merger are as follows:
| A-18 |
(a) Exchange Agent. As of the Effective Time, TranS1 shall deposit with American Stock Transfer & Trust Co. or another bank or trust company designated by TranS1 and reasonably acceptable to Baxano (the “Exchange Agent”), for the benefit of the Securityholders, for exchange in accordance with this Section 2.6, through the Exchange Agent, (i) certificates representing the Merger Shares (such Merger Shares, together with any dividends or distributions with respect thereto with a record date after the Effective Time, being hereinafter referred to as the “Exchange Fund”) issuable pursuant to Sections 2.4 or 2.5 in exchange for Baxano Notes and outstanding shares of Baxano capital stock, (ii) cash in an amount sufficient to make payments for fractional shares required pursuant to Section 2.6(c) and (iii) any dividends or distributions to which Securityholders may be entitled pursuant toSection2.6(d).
(b) Exchange Procedures. As soon as reasonably practicable after the Effective Time, the Exchange Agent shall mail to each Noteholder and each holder of record of a certificate that immediately prior to the Effective Time represented outstanding shares of Baxano capital stock for which Merger Shares are issuable pursuant to Section 2.5(c) (the “Certificates”) whose shares were converted pursuant to Section 2.5 into the right to receive shares of TranS1 Common Stock (i) a letter of transmittal in customary form (which shall specify that delivery shall be effected, and risk of loss and title to the Baxano Notes or the Certificates, as the case may be, shall pass, only upon delivery of the Baxano Notes or the Certificates, as the case may be, to the Exchange Agent) and (ii) instructions for effecting the surrender of theBaxano Notes or the Certificates, as the case may be, in exchange forcertificatesrepresentingsharesofTranS1 Common Stock(pluscashin lieu of fractionalshares, if any, ofTranS1 Common Stockand any dividends or distributions as provided below). Upon surrender of aBaxano Noteor aCertificatefor cancellation to theExchange Agentor to such other agent or agents as may be appointed byTranS1, together with such letter of transmittal, duly executed, and such other documents as may reasonably be required by theExchange Agent, the holder of suchBaxano Noteor Certificate shall be entitled to receive in exchange therefor a certificate representing that number of wholesharesofTranS1 Common Stockwhich such holder has the right to receive pursuant to the provisions of this ARTICLE IIpluscashin lieu of fractionalsharespursuant to Section2.6(c)and any dividends or distributions then payable pursuant to Section2.6(d), and theBaxano Noteor Certificate so surrendered shall immediately be cancelled. In the event of atransferof ownership ofBaxano Common Stockwhich is not registered in thetransferrecords ofBaxano, a certificate representing the proper number ofsharesofTranS1 Common Stockpluscashin lieu of fractionalsharespursuant to Section2.6(c)and any dividends or distributions pursuant to Section2.6(d)may be issued or paid to apersonother than thepersonin whose name the Certificate so surrendered is registered, if such Certificate is presented to theExchange Agent, accompanied by all documents required to evidence and effect suchtransferand by evidence that any applicable stocktransfer taxeshave been paid. Until surrendered as contemplated by this Section2.6, eachBaxano Noteor Certificate shall be deemed at any time after theEffective Timeto represent only the right to receive upon such surrender the certificate representingsharesofTranS1 Common Stockpluscashin lieu of fractionalsharespursuant to Section2.6(c)and any dividends or distributions then payable pursuant to Section2.6(d), as contemplated by this Section2.6.
| A-19 |
(c) No Fractional Shares. No certificate or scrip representing fractional shares of TranS1 Common Stock shall be issued upon the surrender for exchange of Baxano NotesorCertificates, and such fractional share interests shall not entitle the owner thereof to vote or to any other rights of a stockholder of TranS1. Notwithstanding any other provision of this Agreement, each Securityholder who would otherwise have been entitled to receive a fraction of a share of TranS1 Common Stock (after taking into account all Baxano Notes and Certificates delivered by such holder and the aggregate number of shares of Baxano Common Stock represented thereby) shall receive, in lieu thereof, cash (without interest) in an amount equal to such fractional part of a share of TranS1 Common Stock multiplied by the Merger Closing Price.
(d) Distributions with Respect to Unexchanged Baxano Notes and Shares. No dividends or other distributions declared or made after the Effective Time with respect to TranS1 Common Stock with a record date after the Effective Time shall be paid to the holder of any unsurrendered Baxano Note or Certificate until such Baxano Note or Certificate is surrendered as described in Section 2.6(b), subject to Section 2.6(i). Subject to the effect of applicable laws, following surrender of any such Baxano Note or Certificate, there shall be issued and paid to the record holder of the Baxano Note or the Certificate, as the case may be, at the time of such surrender or compliance with Section 2.6(i), the amount of dividends or other distributions with a record date after the Effective Time previously paid with respect to such whole shares of TranS1 Common Stock, without interest, and at theappropriate payment date, the amount of dividends or other distributions having a record date after theEffective Timebut prior to surrender or compliance with Section2.6(i)and a payment date subsequent to surrender or compliance with Section2.6(i)that are payable with respect to such wholesharesofTranS1 Common Stock.
(e) No Further Ownership Rights in Baxano Common Stock. All shares of TranS1 Common Stock issued upon the surrender for exchange of Certificates in accordance with the terms hereof (including any cash or dividends or other distributions paid pursuant to Section 2.6(c) or 2.6(b)) shall be deemed to have been issued (and paid) in full satisfaction of all rights pertaining to such shares of Baxano Common Stock, and from and after the Effective Time there shall be no further registration of transfers on the stock transfer books of Surviving Corporation of the shares of Baxano Common Stock that were outstanding immediately prior to the Effective Time. If, after the Effective Time, Certificates are presented to Surviving Corporation or the Exchange Agent for any reason, they shall be cancelled and exchanged as provided in this ARTICLE II, subject to applicable law in the case of Dissenting Shares.
(f) Termination of Exchange Fund. Any portion of the Exchange Fund that remains undistributed to the Securityholders for 180 days after the Effective Time shall be delivered to TranS1, upon demand, and any Securityholder who has not previously complied with this Section 2.6 shall thereafter look only to TranS1 for payment of its claim for TranS1 Common Stock, any cash in lieu of fractional shares of TranS1 Common Stock and any dividends or distributions with respect to TranS1 Common Stock.
(g) No Liability. To the extent permitted by applicable law, none of TranS1, Transitory Subsidiary, Baxano, Surviving Corporation or the Exchange Agent shall be liable to any Securityholder for such shares (or dividends or distributions with respect thereto) delivered to a public official pursuant to any applicable abandoned property, escheat or similar law.
| A-20 |
(h) Withholding Rights. Each of TranS1 and Surviving Corporation shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any Securityholder and any other recipient of payments hereunder such amounts as it reasonably determines that it is required to deduct and withhold with respect to the making of such payment under the Code, or any other applicable provision of law. To the extent that amounts are so withheld by Surviving Corporation or TranS1, as the case may be, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the Securityholder or other recipient of payments hereunder in respect of which such deduction and withholding was made by Surviving Corporation or TranS1, as the case may be.
(i) Lost Baxano Notes and Certificates. If any Baxano Note or Certificate shall have been lost, stolen or destroyed, upon the making of an affidavit of that fact by the person claiming such Baxano Note or Certificate to be lost, stolen or destroyed and, if required by Surviving Corporation, the posting by such person of a bond in such reasonable amount as Surviving Corporation may direct as indemnity against any claim that may be made against it with respect to such Baxano Note or Certificate, the Exchange Agent shall issue in exchange for such lost, stolen or destroyed Baxano Note or Certificate the shares of TranS1 Common Stock and any cash in lieu of fractional shares, and unpaid dividends and distributions on shares of TranS1 Common Stock deliverable in respect thereof pursuant to this Agreement.
2.7 Treatment of Baxano Stock Option Plans, Baxano Options and Baxano Warrants.
(a) TranS1 shall not assume (1) any outstanding option to purchase Baxano Common Stock (“Baxano Stock Options”), whether vested or unvested or (2) any warrant to acquire capital stock of Baxano (“Baxano Warrants”). As soon as practicable after the date hereof, but no later than the Effective Time, Baxano shall use commercially reasonable efforts to cause each Baxano option to purchase Baxano Common Stock and each warrant to acquire Baxano capital stock, including the options and warrants set forth on Section 3.2 of the Baxano Disclosure Schedule, that is not exercised prior to the Effective Time to be terminated without further action effective immediately prior to the Effective Time.
(b) Baxano shall terminate any and all stock option plans or other stock or equity-related plans of Baxano (the “Baxano Stock Plans”), together with any employee stock purchase plans in accordance with their terms as of or prior to the Effective Time.
2.8 Dissenting Shares.
(a) Dissenting Shares shall not be converted into or represent the right to receive Merger Shares unless the stockholder holding such Dissenting Shares shall have forfeited his, her or its right to appraisal or dissent under the DGCL and the CCC or properly withdrawn his, her or its demand for appraisal or exercise of dissenters rights. If such stockholder has so forfeited or withdrawn his, her or its right to appraisal or dissent of Dissenting Shares, then (i) as of the occurrence of such event, such holder’s Dissenting Shares shall cease to be Dissenting Shares and shall be converted into and represent the right to receive the Merger Shares issuable in respect of such Baxanocapital stock pursuant to Section 2.5(c), if any, and (ii) promptly following the occurrence of such event, TranS1 shall deliver to the Exchange Agent a certificate representing the Merger Shares to which such stockholder is entitled pursuant to Section 2.5(c), if any.
| A-21 |
(b) Baxano shall give TranS1 (i) prompt notice of any written demands for appraisal or exercise of dissenters rights by any holder of Baxano capital stock, withdrawals of such demands and any other instruments that relate to such demands received by Baxano and (ii) the opportunity to direct all negotiations and proceedings with respect to demands for appraisal under the DGCL or CCC. TranS1 shall not, except with the prior written consent of Baxano, make any payment with respect to any demands for appraisal of Baxano capital stock or offer to settle or settle any such demands.
2.9 Escrow Arrangement.
(a) At the Closing, TranS1 shall deposit with Branch Banking and Trust Company (the “Escrow Agent”), (i) the Escrow Shares and (ii) by wire transfer of immediately available funds, a cash amount equal to the fees of the Escrow Agent for providing the services under the Escrow Agreement (which fees shall be borne by TranS1) and the Representative Reimbursement Amount. The Representative Reimbursement Amountshall be deposited in an account segregated from the EscrowSharesand shall be held and released by theEscrow Agentsolely for the purposes of paying for the reasonable costs and expenses of theSecurityholder Representativesin accordance with Section10.14(d)and theEscrow Agreement.
(b) The Escrow Shares shall be held by the Escrow Agent solely for the purposes of providing a fund for the payment of the Escrow Participants’ indemnification obligations hereunder and providing a fund for payment of any adjustment payable to TranS1 pursuant to Section 2.11. The Escrow Shares shall be held by the Escrow Agent in accordance with the terms of this Agreement and an escrow agreement substantially in the form attached hereto asExhibit B (the “Escrow Agreement”), among the Escrow Agent, TranS1 and the Securityholder Representatives. The adoption of this Agreement by the Baxano Stockholder Approval shall constitute approval of the Escrow Agreement and of all of the arrangements relating thereto by the Escrow Participants.
2.10 Capitalization and Allocation Statements.
(a) TranS1 shall prepare and deliver no fewer than three (3) days prior to the Effective Time, a preliminary statement (such statement in final form, the “Preliminary Capitalization Statement”), setting forth the number of outstanding shares of TranS1 capital stock (as if all such shares have converted to TranS1 Common Stock) on the Closing Date (excluding the Merger Shares and Financing Shares) together with appropriate supporting schedules and other documentation to the reasonable satisfaction of Baxano,provided,however, that such Preliminary Capitalization Statement shall be illustrative only and shall not be dispositive. Immediately prior to the Effective Time, TranS1 shall prepare and deliver a statement, certified by TranS1’s Chief Financial Officer, setting forth the number of outstanding shares of TranS1 capital stock (as if all such shares have converted to TranS1 Common Stock) on the Closing Date (excluding the Merger Shares and Financing Shares) (the “Closing Capitalization Statement”).
| A-22 |
(b) Baxano shall prepare and deliver no fewer than three (3) days prior to the Effective Time, a preliminary statement (such statement in final form, the “Preliminary Allocation Statement”), setting forth Baxano’s calculation of each Securityholder’s share (expressed as both a percentage and a share value) using an assumed Merger Closing Price and Net Merger Consideration as of the Effective Time, as well as each Escrow Participant’s Escrow Participation Percentage, together with appropriate supporting schedules and other documentation to the reasonable satisfaction of TranS1,provided,however, that such Preliminary Allocation Statement shall be illustrative only and shall not be dispositive. Immediately prior to the Effective Time, Baxano shall prepare and deliver a statement setting forth Baxano’s definitive calculation of each Securityholder’s share using the Merger Closing Price of the Net Merger Consideration as calculated pursuant to this ARTICLE II, as well as each Escrow Participant’s Escrow Participation Percentage (the “Closing Allocation Statement”). The Closing Allocation Statement shall be the definitive calculation of the matters set forth thereon and shall, without limiting the TranS1 Indemnified Parties’ indemnification rights hereunder, be binding on TranS1, Transitory Subsidiary, Surviving Corporation, Baxano and the Securityholders.
(c) In connection with the delivery of the Closing Allocation Statement, Baxano shall prepare and deliver to TranS1 and the Exchange Agent, at or prior to the Closing, a spreadsheet or spreadsheets in form reasonably acceptable to TranS1 and the Exchange Agent, which spreadsheets shall be dated as of the Closing Date and shall set forth, as of immediately prior to the Effective Time, all factual information relating to Securityholders reasonably requested by TranS1 and the Exchange Agent, including (i) the names of all Securityholders of Baxano and (ii) the number, kind and details (including dates of issuance or grant, vesting schedule of securities, termination date, and repurchase rights) of Baxano securities, including capital stock, warrants, options or other securities convertible into capital stock in Baxano, held by such Persons and, if applicable, the respective certificate numbers.
2.11 Post-Closing Indebtedness and Working Capital Adjustment.
(a) Definitions.
(i) “Adjusted Net Working Capital” shall mean, as of March 31, 2013, Current Assets minus Current Liabilities minus $500,000, subject to Section 6.18.
(ii) “Cash” means all cash, cash equivalents, short term investments and marketable securities of Baxano, as determined in accordance with GAAP.
(iii) “Current Assets” shall mean the aggregate amount of the consolidated current assets of Baxano as of March 31, 2013, calculated in accordance with GAAP in a manner consistent with Baxano’s customary practices, methodologies and manner applied in preparing the Baxano Balance Sheet, plus amounts that Baxano paid as Transaction Expenses on or before March 31, 2013.
(iv) “Current Liabilities” shall mean the aggregate amount of the current liabilities of Baxano as of March 31, 2013, calculated in accordance with GAAP in a manner consistent with Baxano’s customary practices, methodologies and manner applied in preparing the Baxano Balance Sheet, but excluding (A) all Indebtedness, (B) all Transaction Expenses that are unpaid as of March 31, 2013, (C) all expenses incurred by Baxano in connection with retention incentives or bonuses for any of its employees that have been approved in writing by TranS1 as liabilities that will be excluded in calculating Adjusted Net Working Capital, and (D) payments under the Transaction Payment Plan.
| A-23 |
(v) “Indebtedness” of a person shall mean, without duplication, the aggregate amount of principal and accrued and unpaid interest in respect of all liabilities of such Person (A) for money borrowed, whether secured or unsecured, or liabilities issued or incurred in substitution or exchange for any such liabilities for borrowed money or (B) evidenced by notes, debentures, bonds, letters of credit, derivatives or other similar instruments.
(vi) “Target Adjusted Net Working Capital” shall mean $2,121,000.
(vii) “Transaction Expenses” means the sum of all fees, costs and expenses incurred by Baxano in connection with the matters described in this Agreement, including with respect to any brokers, financial advisors (including Leerink Swann LLC), consultants, accountants, attorneys or other professionals engaged by Baxano in connection with the parties’ due diligence related to, or the structuring, negotiation or consummation of the transactions contemplated by this Agreement or the Securities Purchase Agreement.
(b) Pre-Closing Statement. Baxano shall prepare and deliver to TranS1 at least three (3) business days prior to the Closing Date a statement (the “Pre-Closing Statement”), certified by the chief executive or chief financial officer of Baxano, setting forth Baxano’s good faith estimate of (i) the Transaction Expenses of Baxano as of March 31, 2013, including (x) the amount of such Transaction Expenses paid on or before March 31, 2013, (y) the amount that remains unpaid as of March 31, 2013, and (z) the amount that is or will be unpaid immediately prior to the Effective Time, (ii) the Indebtedness of Baxano (and each individual component of Indebtedness of Baxano) as of immediately prior to the Effective Time (excluding amounts under the Baxano Notes), and (iii) the Adjusted Net Working Capital.
(c) Post-Closing Statement and Adjustment Statement. Within thirty (30) calendar days after the Closing Date, TranS1 shall cause Surviving Corporation to prepare and deliver to the Securityholder Representatives a statement (the “Post-Closing Statement”) setting forth (i) the Transaction Expenses of Baxano as of March 31, 2013, including (x) the amount of such Transaction Expenses paid on or before March 31, 2013, (y) the amount that remains unpaid as of March 31, 2013, and (z) the amount that is or will be unpaid immediately prior to the Effective Time, (ii) the Indebtedness of Baxano (and each individual component of Indebtedness of Baxano) and the Excess Indebtedness Amount (if any) as of immediately prior to the Effective Time (excluding amounts under the Baxano Notes), and (iii) the Adjusted Net Working Capital and the components and calculation of the Adjusted Net Working Capital, as well as a statement (the “Adjustment Statement”) setting forth:
-the unpaid Transaction Expenses as of immediately prior to the Effective Time, which shall be a negative number;
-the Adjusted Net Working Capital set forth on the Post-Closing Statement minus the Target Adjusted Net Working Capital;
| A-24 |
-the amount, if any, by which the Excess Indebtedness Amount set forth on the Post-Closing Statement exceeds the Excess Indebtedness Amount calculated as of Closing pursuant to Section 2.2; and
-the aggregate net difference in the foregoingamounts shown on theAdjustment Statement(such net difference, which may be a positive or a negative number, is referred to as the “Adjustment Amount”).
(d) Review of Post-Closing Statement and Adjustment Statement. After receipt of the Post-Closing Statement and the Adjustment Statement, the Securityholder Representatives shall have thirty (30) calendar days to review the Post-Closing Statement and the Adjustment Statement, together with the work papers used in their preparation. TranS1 shall, and shall cause Surviving Corporation to, provide the Securityholder Representatives and their advisors with access upon reasonable notice and at reasonable times to the relevant books and records and employees and independent accountants (subject to execution of a customary independent accountant access letter) of TranS1 and Surviving Corporation in connection with the Securityholder Representatives’ review of the Post-Closing Statement and the Adjustment Statement, together with the work papers used in their preparation and shall furnish the Securityholder Representatives and their advisors with any other information reasonably requested relating to the calculation of the items set forth in the Post-Closing Statement. Unless the Securityholder Representatives deliver to TranS1 written notice setting forth the specific items disputed by the Securityholder Representatives (the “Objection Statement”) on or prior to the thirtieth (30th) calendar day after its receipt of the Post-Closing Statement and the Adjustment Statement, the Securityholder Representatives and Escrow Participants shall be deemed to have accepted and agreed to the Post-Closing Statement and the Adjustment Statement and such agreement shall be final and binding on the parties. Any items on the Post-Closing Statement or Adjustment Statement as to which the Securityholder Representatives have not given notice of their objection on the Objection Statement shall be deemed to have been agreed upon by the parties. If the Securityholder Representatives so notify TranS1 of their objections to any of the Post-Closing Statement or the Adjustment Statement and provides TranS1 with the Objection Statement, TranS1 and the Securityholder Representatives shall, within thirty (30) calendar days following receipt of the Objection Statement, which period may be extended by written agreement of TranS1 and the Securityholder Representatives (such period, as it may be extended, the “Resolution Period”), attempt to resolve their differences. Any resolution by TranS1 and the Securityholder Representatives during the Resolution Period as to any disputed amounts shall be reduced to writing and shall be final, binding and conclusive. For the avoidance of doubt, Escrow Participants shall be deemed to have accepted and agreed to any such resolution reached between TranS1 and the Securityholder Representatives.
| A-25 |
(e) Neutral Accounting Arbitrator. If TranS1 and the Securityholder Representatives do not resolve all disputed items by the end of the Resolution Period, then all items remaining in dispute shall, unless otherwise agreed by the parties in writing, be submitted within ten (10) calendar days after the expiration of the Resolution Period to Ernst & Young LLP or such other independent accounting firm mutually acceptable to TranS1 and the Securityholder Representatives (the “Neutral Accounting Arbitrator”). The Neutral Accounting Arbitrator shall act as an arbitrator to determine only those items in dispute, and for each such item shall determine a value within the range of values submitted therefor by TranS1 and the Securityholder Representatives in the Post-Closing Statement and Objection Statement. All fees and expenses relating to the work, if any, to be performed by the Neutral Accounting Arbitrator shall be allocated between TranS1, on the one hand, and the Securityholder Representatives (on behalf of all of Securityholders), on the other hand, in the same proportion that the aggregate amount of the disputed items so submitted to the Neutral Accounting Arbitrator that is unsuccessfully disputed by such party (as finally determined by the Neutral Accounting Arbitrator) bears to the total amount of such disputed items so submitted. TranS1 and the Securityholder Representatives shall direct the Neutral Accounting Arbitrator to deliver to TranS1 and the Securityholder Representatives a written determination (such determination to include a work sheet setting forth all material calculations used in arriving at such determination and to be based solely on information provided to the Neutral Accounting Arbitrator by the Securityholder Representatives and TranS1) of the disputed items within thirty (30) calendar days of receipt of the disputed items, which determination shall be final, binding and conclusive. The final, binding and conclusive Post-Closing Statement and Adjustment Statement, which either are agreed upon by TranS1 and the Securityholder Representatives (including a deemed agreement under Section 2.11(d)) or are delivered by the Neutral Accounting Arbitrator in accordance with this Section 2.11(e) shall be the “Conclusive Statement” and the “Conclusive Adjustment Statement,” respectively. In the event that either TranS1 or the Securityholder Representatives fails to submit its statement regarding any items remaining in dispute within the time determined by the Neutral Accounting Arbitrator, then the Neutral Accounting Arbitrator shall render a decision based solely on the evidence timely submitted to the Neutral Accounting Arbitrator by TranS1 and the Securityholder Representatives.
(f) Post-Closing True-Up.
(i) If the absolute value of the Adjustment Amount as shown on the Conclusive Adjustment Statement is less than or equal to $250,000, then there shall be no adjustment to the Net Merger Consideration.
(ii) If the Adjustment Amount as shown on the Conclusive Adjustment Statement is a negative number and the absolute value of such number exceeds $250,000, then the Securityholder Representatives and TranS1 shall jointly instruct the Escrow Agent to release to TranS1 from the Escrow Shares (and the Net Merger Consideration shall be decreased by) a number of shares equal to the quotient of (A) the absolute value of such Adjustment Amount,divided by (B) the Merger Closing Price, rounded to the nearest whole share.
(iii) If the Adjustment Amount as shown on the Conclusive Adjustment Statement is a positive number and the absolute value of such number exceeds $250,000, then the Net Merger Consideration shall be increased by a number of shares equal to the quotient of (A) such Adjustment Amount,divided by (B) the Merger Closing Price, rounded to the nearest whole share. Such additional Merger Shares shall be distributed according to the allocation priorities set forth in Section 2.3(a), 2.4 and Section 2.5(c) as if such Merger Shares had been distributed at the Effective Time, taking into account the initial distribution of Merger Shares following the Closing. For clarity, a portion of such Merger Shares shall constitute Escrow Shares to be delivered by TranS1 to the Escrow Agent at such time, as otherwise provided in Section 2.3(a) and the Escrow Agreement.
| A-26 |
(iv) All payments and share distributions to be made pursuant to this Section 2.11(f) shall be made on or before the fifth (5th) business day following the date on which TranS1 and the Securityholder Representatives agree to, or the Neutral Accounting Arbitrator delivers, the Conclusive Statement and the Conclusive Adjustment Statement.
(g) Tax Treatment of True-Up Payments. All payments made in respect of the Adjustment Amount shall be treated as an adjustment to the Merger Consideration for Tax purposes.
(h) Indemnification Rights. Notwithstanding anything to the contrary contained herein, the determination of the Conclusive Statement and the resulting Adjustment Amount shall not preclude TranS1 or the Securityholder Representatives, as the case may be, from pursuing indemnification for the breach of any representation, warranty or covenant pursuant to ARTICLE VIII or any matters otherwise indemnifiable thereunder except to the extent any amount resulting from such breach or matters is or should have been taken into account for purposes of the determination of the Conclusive Statement. Any amounts known as of the date of the Post-Closing Statement or, if applicable, the date of the Objection Notice that may be taken into account for the purposes of the determination of the Conclusive Statement under this Section 2.11 shall be so taken into account.
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF BAXANO
Baxano represents and warrants to TranS1 and Transitory Subsidiary that the statements contained in this ARTICLE III are true and correct, except as expressly set forth herein or in the disclosure schedule delivered by Baxano to TranS1 and Transitory Subsidiary on the date of this Agreement (the “Baxano Disclosure Schedule”). The Baxano Disclosure Schedule shall be arranged in sections corresponding to the numbered and lettered sections contained in this ARTICLE III and the disclosure in any section of the Baxano Disclosure Schedule shall qualify (1) the corresponding section in this ARTICLE III and (2) the other sections in this ARTICLE III only to the extent that it is reasonably apparent from a reading of such disclosure that it also qualifies or applies to such other sections. For purposes hereof, “to the knowledge of Baxano” and similar expressions mean the actual knowledge of the persons identified on the Baxano Disclosure Schedule for this purpose,includingthe knowledge such persons would have in the ordinary performance of their duties toBaxano.
| A-27 |
3.1 Organization, Standing and Power. Baxano is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation, has all requisite corporate power and authority to own, lease and operate its properties and assets and to carry on its business as currently conducted and as currently proposed to be conducted, and is duly qualified to do business and is in good standing as a foreign corporation in each jurisdiction listed on Section 3.1 of the Baxano Disclosure Schedule, which jurisdictions constitute the only jurisdictions in which the character of the properties it owns, operates or leases or the nature of its activities makes such qualification necessary, except for such failures to be so organized, qualified or in good standing, individually or in the aggregate, that have not had, and are not reasonably expected to have, a Baxano Material Adverse Effect. For purposes of this Agreement, the term “Baxano Material Adverse Effect” means any material adverse change, event, circumstance or development with respect to, or material adverse effect on, (i) the business, assets and liabilities (taken together), condition (financial or otherwise), or results of operations of Baxano, or (ii) the ability of Baxano to consummate the transactions contemplated by this Agreement;provided,however, that the following shall not be deemed to be a Baxano Material Adverse Effect: any change or event caused by or resulting from (A) changes in prevailing economic or market conditions in the United States or any other jurisdiction in which Baxano has substantial business operations (except to the extent those changes have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates), (B) changes or events, after the date hereof, affecting the industries or markets in which Baxano operates generally (except to the extent those changes or events have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates), (C) changes, after the date hereof, in generally accepted accounting principles or requirements applicable to Baxano (except to the extent those changes have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates), (D) changes, after the date hereof, in laws, rules or regulations of general applicability or interpretations thereof by any Governmental Entity (except to the extent those changes have a materially disproportionate effect on Baxano as compared to other similarly situated participants in the industries or markets in which Baxano operates), (E) the execution, delivery and performance of this Agreement or the consummation of the transactions contemplated hereby or the announcement thereof, (F) any outbreak of major hostilities in which the United States is involved or any act of terrorism within the United States or directed against its facilities or citizens wherever located, or (G) the actions of TranS1 or Transitory Subsidiary (other than any action taken under this Agreement by TranS1 or Transitory Subsidiary in response to an event, circumstance or other development that would otherwise constitute a Baxano Material Adverse Effect). For the avoidance of doubt, the parties agree that the terms “material,” “materially” and “materiality” as used in thisAgreementwith an initial lower case “m” shall have their respective customary and ordinary meanings, without regard to the meanings ascribed toBaxano Material Adverse Effectin the prior sentence of this paragraph orTranS1 Material Adverse Effectin Section4.1. Baxano has provided or made available to TranS1complete and accurate copies of its Certificate of Incorporation and Bylaws.
3.2 Capitalization and Closing Allocation Statement.
(a) The authorized capital stock of Baxano consists of 96,270,000 shares of Common Stock, par value $0.001 per share (the “Baxano Common Stock”), and 70,673,934 shares of Preferred Stock, $0.001 par value per share (the “Baxano Preferred Stock”), of which 4,024,286 shares are designated Baxano Series A-1 Preferred Stock (the “Baxano Series A-1 Preferred Stock”), 2,911,038 shares are designated Baxano Series A-2 Preferred Stock (the “Baxano Series A-2 Preferred Stock”), 8,968,610 shares are designated Baxano Series B Preferred Stock (the “Baxano Series B Preferred Stock”) and 54,770,000 shares are designated Baxano Series C Preferred Stock (the “Baxano Series C Preferred Stock”). The rights and privileges of each class ofBaxano’s capital stock are as set forth inBaxano’s Certificate of Incorporation. As of the date of thisAgreement, (i) 6,741,449sharesofBaxano Common Stock,4,024,286 shares of Baxano Series A-1 Preferred Stock, 2,797,402 shares of Baxano Series A-2 Preferred Stock, 8,968,610 shares of Baxano Series B Preferred Stock and 32,930,844 shares of Baxano Series C Preferred Stockare issued and outstanding and (ii) nosharesofBaxanocapital stock are held in the treasury ofBaxano.
| A-28 |
(b) Section 3.2(b) of the Baxano Disclosure Schedule sets forth a complete and accurate list,as of the date of thisAgreement, of the holders of Baxano Common Stock, Baxano Series A-1 Preferred Stock, Baxano Series A-2 Preferred Stock, Baxano Series B Preferred Stock and Baxano Series C Preferred Stock showing the number of shares held by each stockholder. Section 3.2(b) of the Baxano Disclosure Schedule also sets forth, as of the date of this Agreement, a complete and accurate list of all issued and outstanding shares of Baxano Common Stock that constitute restricted stock or that are otherwise subject to a repurchase or redemption right or right of first refusal in favor of Baxano, indicating the name of the applicable stockholder, the vesting schedule for any such shares, including the extent to which any such repurchase or redemption right or right of first refusal has lapsed as of the date of this Agreement, whether (and to what extent) the vesting will be accelerated in any way by the transactions contemplated by this Agreement or by termination of employment or change in position following consummation of the Merger, and whether such holder has the sole power to vote and dispose of such shares.
(c) Section 3.2(c) of the Baxano Disclosure Schedule sets forth a complete and accurate list, as of the date of this Agreement, of: (i) all Baxano Stock Plans, indicating for each Baxano Stock Plan, as of the close of business on the business day prior to the date of this Agreement, the number of shares of Baxano Common Stock issued to date under such Plan, the number of shares of Baxano Common Stock subject to outstanding options under such Plan and the number of shares of Baxano Common Stock reserved for future issuance under such Plan; and (ii) all outstanding Baxano Stock Options, indicating with respect to each such Baxano Stock Option the name of the holder thereof, the Baxano Stock Plan under which it was granted, the number of shares of Baxano Common Stocksubject to suchBaxano Stock Option, the exercise price, the date of grant and the vesting schedule,includingwhether (and to what extent) the vesting will be accelerated in any way by the transactions contemplated by thisAgreement.Baxano has provided or made available to TranS1complete and accurate copies of allBaxano Stock Plansand the forms of all stock option agreements used under theBaxano Stock Plans.
(d) Section 3.2(d) of the Baxano Disclosure Schedule sets forth the number of shares of Baxano Common Stock, Baxano Series A-1 Preferred Stock, Baxano Series A-2 Preferred Stock, Baxano Series B Preferred Stock and Baxano Series C Preferred Stock reserved for future issuance pursuant to warrants, convertible promissory notes or other outstanding rights (other than Baxano Stock Options) to purchase shares of Baxano capital stock outstanding as of the date of this Agreement (such outstanding warrants, convertible promissory notes or other rights, the “Baxano Convertible Securities”) and the agreement or other document under which such Baxano Convertible Securities were granted and sets forth a complete and accurate list of all holders of Baxano Convertible Securities indicating the number and type of shares of Baxano Common Stock, Baxano Series A-1 Preferred Stock, Baxano Series A-2 Preferred Stock, Baxano Series B Preferred Stock and Baxano Series C Preferred Stock subject to each of the Baxano Convertible Securities, and the exercise price, the date of grant and the expiration date thereof. Baxano has provided or made available to TranS1 complete and accurate copies of all Baxano Convertible Securities.
| A-29 |
(e) Except (i) as set forth in this Section 3.2 and (ii) as reserved for future grants under Baxano Stock Plans, (A) there are no equity securities of any class ofBaxano, or any security exchangeable into or exercisable for such equity securities, issued, reserved for issuance or outstanding and (B) there are no options, warrants, equity securities, calls, rights, commitments or agreements of any character to whichBaxanois a party or by whichBaxanois bound obligatingBaxanoto issue, exchange,transfer, deliver or sell, or cause to be issued, exchanged, transferred, delivered or sold, additionalsharesof capital stock or other equity interests ofBaxanoor any security or rights convertible into or exchangeable or exercisable for any suchsharesor other equity interests, or obligatingBaxanoto grant, extend, accelerate the vesting of, otherwise modify or amend or enter into any such option, warrant, equity security, call, right, commitment oragreement.Baxanodoes not have any outstanding stock appreciation rights, phantom stock, performance based rights or similar rights or obligations. NeitherBaxanonor,to the knowledge of Baxano,any of itsAffiliatesis a party to or is bound by any, andto the knowledge of Baxano, there are no, agreements or understandings with respect to the voting (includingvoting trusts and proxies) or sale ortransfer(includingagreements imposingtransferrestrictions) of anysharesof capital stock or other equity interests ofBaxano. For purposes of thisAgreement, the term “Affiliate” when used with respect to any party shall mean anypersonwho is an “affiliate” of that party within the meaning ofRule405 promulgated under theSecurities Act of 1933, as amended (the “Securities Act”). Except as contemplated by thisAgreementor described in this Section3.2(e), there are no registration rights, and there is no rightsagreement, “poison pill” anti-takeover plan or otheragreementor understanding to whichBaxanois a party or by which it or they are bound with respect to any equity security of any class ofBaxano.
(f) All outstanding shares of Baxano Common Stock, Baxano Series A-1 Preferred Stock, Baxano Series A-2 Preferred Stock, Baxano Series B Preferred Stock and Baxano Series C Preferred Stock are, and all shares of Baxano Common Stock, Baxano Series A-1 Preferred Stock, Baxano Series A-2 Preferred Stock, Baxano Series B Preferred Stock and Baxano Series C Preferred Stock subject to issuance as specified in Sections 3.2(c) and 3.2(d), upon issuance on the terms and conditions specified in the instruments pursuant to which they are issuable, will be, duly authorized, validly issued, fully paid and nonassessable and not subject to or issued in violation of any purchase option, call option, right of first refusal, preemptive right, subscription right or any similar right under any provision of the DGCL, Baxano’s Certificate of Incorporation or Bylaws or any agreement to which Baxano is a party or is otherwise bound. There are no obligations, contingent or otherwise, of Baxano to repurchase, redeem or otherwise acquire any outstanding shares of Baxano capital stock. All outstanding shares of Baxano capital stock have been offered, issued and sold by Baxano in compliance with all applicable federal and state securities laws.
(g) Except as disclosed in Section 3.2(g) of the Baxano Disclosure Schedule, no consent of the holders of Baxano Stock Options or Baxano Convertible Securities is required in connection with the actions contemplated by Section 2.3.
| A-30 |
(h) The Closing Allocation Statement will be true, complete and correct as of the Effective Time and prepared in accordance with the organizational documents of Baxano and all applicable Law. Upon payment of the Merger Consideration as provided for in this Agreement, the Securityholders will have no further right against Baxano, TranS1, Transitory Subsidiary or Surviving Corporation or any of their respective directors, officers, employees, agents, advisors or other Representatives, for any amount owing to such Securityholders in respect of their Baxano Notes, capital stock, warrants, options or other securities convertible into capital stock in Baxano pursuant to (i) Baxano’s organizational documents or the DGCL, (ii) the Baxano Notes or (iii) relating to or in connection with this Agreement, the Merger or the other transactions contemplated hereby, except, in each case, appraisal rights under the DGCL, dissenters’ rights under the CCC and the rights set forth in this Agreement.
3.3 Subsidiaries. Baxano does not own and has not ever owned, directly or indirectly, of record or beneficially, any outstanding voting securities, or equity interests of any kind or nature (whether controlling or not) in any corporation, limited liability company, partnership, trust, joint venture or other entity (including any interest in any profits, capital or business or any entity) (each a “Person”), and is not a party to any contract to acquire such interest.
3.4 Authority; No Conflict; Required Filings and Consents.
(a) Baxano has all requisite corporate power and authority to enter into this Agreement, subject only to the adoption of this Agreement (the “Baxano Voting Proposal”) by Baxano’s stockholders under the DGCL and the CCC and the Baxano Certificate of Incorporation, requiring the affirmative vote in favor of the Baxano Voting Proposal by the holders of (i) a majority of the votes represented by the outstanding shares of Baxano Common Stock, (ii) a majority of the votes represented by the outstanding shares of Baxano Common Stock and Baxano Preferred Stock voting together, with the holders of the outstanding shares of Baxano Preferred Stock entitled to the number of votes equal to the number of shares of Baxano Common Stock into which such shares of Baxano Preferred Stock could be converted as of the record date; and (iii) sixty-six and two thirds percent (66 2/3%) of the votes represented by the outstanding shares of Baxano Preferred Stock (the “Baxano Stockholder Approval”), to consummate the transactions contemplated by this Agreement. The Baxano Board, at a meeting duly called and held, by the unanimous vote of all directors, (A) determined that the Merger is advisable, fair and in the best interests of Baxano and its stockholders, (B) approved this Agreement and declared its advisability in accordance with the provisions of the DGCL, and (C) directed that this Agreement be submitted to the stockholders of Baxano for their adoption and resolved to recommend that the stockholders of Baxano vote in favor of the adoption of this Agreement. The execution and delivery of this Agreement and the consummation of the transactions contemplated by this Agreement by Baxano have been duly authorized by all necessary corporate action on the part of Baxano, subject only to the required receipt of the Baxano Stockholder Approval. This Agreement has been duly executed and delivered by Baxano and constitutes the valid and binding obligation of Baxano, enforceable in accordance with its terms, subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar laws of general applicability relating to or affecting creditors’ rights and to general equity principles (the “Bankruptcy and Equity Exception”).
| A-31 |
(b) The execution and delivery of this Agreement by Baxano do not, and the consummation by Baxano of the transactions contemplated by this Agreement will not, (i) conflict with, or result in any violation or breach of, any provision of the Certificate of Incorporation or Bylaws of Baxano, (ii) conflict with, or result in any violation or breach of, or constitute (with or without notice or lapse of time, or both) a default (or give rise to a right of termination, cancellation or acceleration of any obligation or loss of any material benefit) under, or require a consent or waiver under, constitute a change in control under, require the payment of a penalty under or result in the imposition of any mortgage, security interest, pledge, lien, charge or encumbrance of any nature (each a “Lien”) on Baxano’s assets under any of the terms, conditions or provisions of any note, bond, mortgage, indenture, lease, license, contract or other agreement, instrument or obligation to which Baxano is a party or by which any of them or any of their properties or assets may be bound, or (iii) subject to obtaining the Baxano Stockholder Approval and compliance with the requirements specified in clauses (i) through (iv) of Section 3.4(c), conflict with or violate any permit, concession, franchise, license, judgment, injunction, order, decree, statute, law, ordinance, rule or regulation applicable to Baxano or any of its or their properties or assets. Section 3.4(b) of the Baxano Disclosure Schedule lists all consents, waivers and approvals under any of Baxano’s agreements, licenses or leases required to be obtained in connection with the consummation of the transactions contemplated by this Agreement, which, if individually or in the aggregate were not obtained, would result in a Baxano Material Adverse Effect.
(c) No consent, approval, license, permit, order or authorization of, or registration, declaration, notice or filing with, any court, arbitrational tribunal, administrative agency or commission or other governmental or regulatory authority, agency or instrumentality (a “Governmental Entity”) is required by or with respect to Baxano in connection with the execution and delivery of this Agreement by Baxano or the consummation by Baxano of the transactions contemplated by this Agreement, except for (i) the filing of the Certificate of Merger with the Delaware Secretary of State and appropriate corresponding documents with the appropriate authorities of other states in which Baxano is qualified as a foreign corporation to transact business, (ii)such consents, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable state securities laws and(iii) such other consents, authorizations, orders, filings, approvals and registrations that, individually or in the aggregate, if not obtained or made, would not be reasonably expected to have a Baxano Material Adverse Effect.
(d) The Baxano Stockholder Approval, which is to be delivered pursuant to written consents of stockholders in lieu of a meeting (collectively, the “Written Consents”), is the only vote of the holders of any class or series of Baxano’s capital stock or other securities necessary to adopt this Agreement and for consummation by Baxano of the other transactions contemplated by this Agreement. There are no bonds, debentures, notes or other indebtedness of Baxano other than the Baxano Notes, having the right to vote (or convertible into, or exchangeable for, securities having the right to vote) on any matters on which stockholders of Baxano may vote.
3.5 Baxano Financial Statements; Information Provided. Baxano has provided to TranS1 the Baxano Financial Statements prior to the date of this Agreement. The Baxano Financial Statements (i) comply as to form in all material respects with applicable accounting requirements, (ii) were prepared in accordance with United States generally accepted accounting principles (“GAAP”) applied on a consistent basis throughout the periods covered thereby (except as may be indicated in the notes to such financial statements) and (iii) fairly present the consolidated financial position of Baxano as of the dates thereof and the consolidated results of its operations and cash flows for the periods indicated. For purposes of this Agreement,“Baxano Financial Statements” means (i) the audited balance sheets and statements of income, changes in stockholders’ equity and cash flows of Baxano as of the end of and for each of the fiscal years ended December 31, 2012 (the “Baxano Balance Sheet Date”) and December 31, 2011 (the audited balance sheet as of the Baxano Balance Sheet Date is referred to herein as the “Baxano Balance Sheet”).
| A-32 |
3.6 No Undisclosed Liabilities. Except as reflected or reserved against on the Baxano Financial Statements (including the notes thereto), and except for normal and recurring liabilities incurred since the Baxano Balance Sheet Date in the ordinary course of business consistent with past practice (the “Ordinary Course of Business”), Baxano does not have any material liabilities (whether known or unknown, whether absolute or contingent, whether liquidated or unliquidated, whether due or to become due, and whether or not required to be reflected in financial statements (including the notes thereto) in accordance with GAAP).
3.7 Absence of Certain Changes or Events. Since the Baxano Balance Sheet Date, Baxano have conducted their respective businesses only in the Ordinary Course of Business and, since such date, except for this Agreement, the Securities Purchase Agreement and the transactions contemplated by such Agreements, there has not been (i) any change, event, circumstance, development or effect that, individually or in the aggregate, has had, or is reasonably expected to have, a Baxano Material Adverse Effect; or (ii) any other action or event that would have required the consent of TranS1 pursuant to Section 5.1 of this Agreement had such action or event occurred after the date of this Agreement.
3.8 Taxes.
(a) Baxano has properly filed on a timely basis all material Tax Returns that it was required to file, and all such Tax Returns were true, correct and complete in all material respects. Baxano has paid on a timely basis all Taxes due and payable. Baxano has never been a member of a group of corporations with which it has filed (or been required to file) consolidated, combined or unitary Tax Returns, other than a group of which the common parent is Baxano. Baxano does not (i) have any actual or potential liability under Treasury Regulations Section 1.1502-6 (or any comparable or similar provision of federal, state, local or foreign law), as a transferee or successor, pursuant to any contractual obligation, or otherwise for any Taxes of any person other than Baxano, and (ii) is not a party to or bound by any Tax indemnity, Tax sharing, Tax allocation or similar agreement. All material Taxes that Baxano was required by law to withhold or collect have been duly withheld or collected and, to the extent required, have been properly paid to the appropriate Governmental Entity. For purposes of this Agreement, (x) “Taxes” shall mean any and all taxes, charges, fees, duties, contributions, levies or other similar assessments or liabilities in the nature of a tax, including, without limitation, income, gross receipts, corporation, ad valorem, premium, value-added, net worth, capital stock, capital gains, documentary, recapture, alternative or add-on minimum, disability, estimated, registration, recording, excise, real property, personal property, sales, use, license, lease, service, service use, transfer, withholding, employment, unemployment, insurance, social security, national insurance, business license, business organization, environmental, workers compensation, payroll, profits, severance, stamp, occupation, windfall profits, customs duties, franchise and other taxes of any kind whatsoever imposed by the United States of America or any state, local or foreign government, or any agency or political subdivision thereof, and any interest, fines, penalties, assessments or additions to tax imposed with respect to such items or any contest or dispute thereof, and (y) “Tax Returns” shall mean any and all reports, returns, declarations, or statements relating to Taxes supplied to a Governmental Entity, including any schedule or attachment thereto and any related or supporting work papers or information with respect to any of the foregoing, including any amendment thereof.
| A-33 |
(b) The unpaid Taxes of Baxano did not, as of the Baxano Balance Sheet Date, exceed the reserve for Tax liability (rather than any reserve for deferred Taxes established to reflect timing differences between book and Tax income) set forth on the face of the Baxano Balance Sheet (rather than in any notes thereto).
(c) Baxano has delivered or made available to TranS1 complete and correct copies of all Tax Returns of Baxano relating to Taxes for all taxable periods for which the applicable statute of limitations has not yet expired. No examination or audit of any Tax Return of Baxano by any Governmental Entity has occurred or is currently in progress or, to the knowledge of Baxano, threatened or contemplated in writing. Baxano has not been informed by any jurisdiction that the jurisdiction believes that Baxano was required to file any Tax Return that was not filed. Baxano has not (x) waived any statute of limitations with respect to Taxes or agreed to extend the period for assessment or collection of any Taxes, (y) requested any extension of time within which to file any Tax Return, which Tax Return has not yet been filed, or (z) executed or filed any power of attorney with any taxing authority.
(d) Baxano has not made any payment, is not obligated to make any payment, and is not a party to any agreement that could obligate it to make any payment that may be treated as an “excess parachute payment” under Section 280G of the Code (without regard to Sections 280G(b)(4) and 280G(b)(5) of the Code).
(e) There are no adjustments under Section 481 of the Code (or any similar adjustments under any provision of the Code or the corresponding foreign, state or local Tax laws) that are required to be taken into account by Baxano in any period ending after the Closing Date by reason of a change in method of accounting in any taxable period ending on or before the Closing Date or as a result of the consummation of the transactions contemplated by this Agreement.
(f) Baxano has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(l)(A)(ii) of the Code.
(g) Baxano has not distributed to its stockholders or security holders stock or securities of a controlled corporation, nor has stock or securities of Baxano been distributed, in a transaction to which Section 355 of the Code applies (i) in the two years prior to the date of this Agreement or (ii) in a distribution that could otherwise constitute part of a “plan” or “series of related transactions” (within the meaning of Section 355(e) of the Code) that includes the transactions contemplated by this Agreement.
| A-34 |
(h) There are no Liens with respect to Taxes upon any of the assets or properties of Baxano, other than with respect to Taxes not yet due and payable or being contested in good faith by appropriate proceedings.
(i) Baxano will not be required to include any item of income in, or exclude any item of deduction from, taxable income for any period (or any portion thereof) ending after the Closing Date as a result of any (i) deferred intercompany gain or any excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding provision of state, local or foreign Tax law), (ii) closing agreementas described in Section 7121 of theCode(or any corresponding or similar provision of state, local or foreignTaxlaw) executed on or prior to theClosing Date, (iii) installment sale or other open transaction disposition made on or prior to the Closing Date, or (iv) prepaid amount received on or prior to the Closing Date.
(j) Baxano has not participated in any “reportable transaction” as defined in section 1.6011-4(b) of the Treasury Regulations or any analogous provision of state or local law.
(k) Since the Baxano Balance Sheet Date, Baxano has not incurred any liability for Taxes arising from extraordinary gains or losses, as that term is used in GAAP, outside the Ordinary Course of Business.
3.9 Owned and Leased Real Properties.
(a) Baxano does not own and has never owned anyreal property.
(b) Section 3.9(b) of the Baxano Disclosure Schedule sets forth a complete and accurate list of all real property formerly or currently leased, subleased or licensed by Baxano, including current contracts for the offsite storage of records (collectively, the “Baxano Leases”), and the location of such premises. Baxanodoes not lease, sublease or license any real property to anyperson.
3.10 Intellectual Property.
(a) To the knowledge of Baxano, Baxano owns, licenses or otherwise possesses legally enforceable rights to use all Intellectual Property used or necessary to conduct the business of Baxano as currently conducted, or that would be used or necessary as such business is currently proposed to be conducted (excluding currently-available, off-the-shelf software programs that are licensed by Baxano pursuant to “shrink wrap” licenses).
(b) The execution and delivery of this Agreement and consummation of the Merger will not result in the breach of, or create on behalf of any third party the right to terminate or modify, (i) any license, sublicense or other agreement relating to any Intellectual Property owned by Baxano that is material to the business of Baxano, including software that is used in the development or manufacture of or forms a part of any product or service sold by or expected to be sold by Baxano (the “Baxano Intellectual Property”) or (ii) any license, sublicense or any other agreement as to which Baxano is a party and pursuant to which Baxano is authorized to use any third party Intellectual Property that is material to the business of Baxano, including software that is used in the development or manufacture of or forms a part of any product or service sold by or expected to be sold by Baxano (the “Baxano Third Party Intellectual Property”). Section 3.10(b) of the Baxano Disclosure Schedule sets forth a complete and accurate list of registered Baxano Intellectual Property (including Baxano Intellectual Property for which registration has been applied) and Section 3.10(b) of the Baxano Disclosure Schedule sets forth a complete and accurate list of all Baxano Third Party Intellectual Property.
| A-35 |
(c) To the knowledge of Baxano, all patents and registrations and applications for Trademarks, service marks and copyrights which are held by Baxano and that are material to the business of Baxano are valid and subsisting. Baxano has taken commercially reasonable measures to protect the proprietary nature of the Baxano Intellectual Property. To the knowledge of Baxano, no other person or entity is infringing, violating or misappropriating any of the Baxano Intellectual Property or Baxano Third Party Intellectual Property.
(d) To the knowledge of Baxano, none of the (i) products previously or currently sold by Baxano or (ii) business or activities previously or currently conducted by Baxanoinfringes, violates or constitutes a misappropriation of, anyIntellectual Propertyof any third party.Baxanohas not received any written complaint, claim or notice alleging any such infringement, violation or misappropriation.
(e) For purposes of this Agreement, the following terms shall have the following meanings:
(i) “Intellectual Property” means the following subsisting throughout the world:
(A) Patent Rights;
(B) Trademarks and all goodwill in the Trademarks;
(C) copyrights, designs, data and database rights and registrations and applications for registration thereof, including moral rights of authors;
(D) mask works and registrations and applications for registration thereof under the laws of any jurisdiction;
(E) inventions, invention disclosures, statutory invention registrations, trade secrets and confidential business information, know-how, manufacturing and product processes and techniques, research and development information, financial, marketing and business data, pricing and cost information, business and marketing plans and customer and supplier lists and information, whether patentable or nonpatentable, whether copyrightable or non-copyrightable and whether or not reduced to practice; and
(F) other proprietary rights relating to any of the foregoing (including remedies against infringement thereof and rights of protection of interest therein under the laws of all jurisdictions).
(ii) “Patent Rights” means all patents, patent applications, utility models, design registrations and certificates of invention and other governmental grants for the protection of inventions or industrial designs (including all related continuations, continuations-in-part, divisionals, reissues and reexaminations).
| A-36 |
(iii) “Trademarks” means all registered trademarks and service marks, logos, Internet domain names, corporate names and doing business designations and all registrations and applications for registration of the foregoing, common law trademarks and service marks and trade dress.
3.11 Contracts.
(a) Section 3.11(a) of the Baxano Disclosure Schedule lists the following agreements (written or oral) to which Baxano is a party as of the date of this Agreement:
(i) any Baxano Lease and any agreement (or group of related agreements) for the lease of personal property from or to third parties providing for lease payments in excess of $50,000 per annum or having a remaining term longer than six months;
(ii) any agreement (or group of related agreements) that is not terminable without cause by Baxano with less than 31 (thirty-one) days’ notice without penalty, including the payment of any termination fee or refund of amounts previously received, and that is for the purchase or sale of products or for the furnishing or receipt of services (A) which calls for performance over a period of more than one year, (B) which would require an aggregate of more than $50,000 in payments following the Closing or (C) in which Baxano has granted manufacturing rights, “most favored nation” pricing provisions or marketing or distribution rights relating to any products or territory or has agreed to purchase a minimum quantity of goods or services or has agreed to purchase goods or services exclusively from a particular party;
(iii) any agreement concerning the establishment or operation of a partnership, joint venture or limited liability company;
(iv) any agreement (or group of related agreements) under which it has created, incurred, assumed or guaranteed (or may create, incur, assume or guarantee) indebtedness (including capitalized lease obligations) or under which it has imposed (or may impose) a Lien on any of its assets, tangible or intangible;
(v) any agreement for the disposition of any significant portion of the assets or business of Baxano (other than sales of products in the Ordinary Course of Business) or any agreement for the acquisition of the assets or business of any other entity (other than purchases of inventory or components in the Ordinary Course of Business);
(vi) any employment agreement that is not terminable at will or that varies in any material respect from the template form of such agreement previously made available to TranS1, and any consulting agreement or sales representative agreement that varies in any material respect from the template form of such agreement previously made available to TranS1;
(vii) any agreement under which Baxano has continuing obligations to any current or former officer, director or stockholder of Baxano or an Affiliate thereof;
| A-37 |
(viii) any agreement which contains any provisions requiring Baxano to indemnify any other party for infringement claims (excluding indemnities contained in agreements for the purchase, sale or license of products entered into in the Ordinary Course of Business);
(ix) any agreement under which Baxano is restricted from selling, licensing or otherwise distributing any of its technology or products, or providing services to, customers or potential customers or any class of customers, in any geographic area, during any period of time or any segment of the market or line of business;
(x) any agreement under which Baxano has licensed any material Intellectual Property to or from any third party (excluding currently-available, off-the-shelf software programs that are licensed by Baxano pursuant to “shrink wrap” licenses);
(xi) any agreement that would entitle any third party to receive a license or any other right to intellectual property of TranS1 or any of TranS1’s Affiliates following the Closing; and
(xii) any other agreement (or group of related agreements) (A) involving more than $100,000 or (B) not entered into in the Ordinary Course of Business.
(b) Baxano has provided or made available to TranS1 a complete and accurate copy of each agreement listed in Section 3.11(a) of the Baxano Disclosure Schedule. With respect to each agreement so listed, except as disclosed in Section 3.11(b) of the Baxano Disclosure Schedule: (i) the agreement is legal, valid, binding and enforceable and in full force and effect; (ii) the agreement will continue to be legal, valid, binding and enforceable and in full force and effect immediately following the Closing in accordance with the terms thereof as in effect immediately prior to the Closing; and (iii) neither Baxano nor, to the knowledge of Baxano, any other party, is in breach or violation of, or default under, any such agreement, and no event has occurred, is pending or, to the knowledge of Baxano, is threatened, which, with or without notice or lapse of time, or both, would constitute a breach, violation or default by Baxano or, to the knowledge of Baxano, any other party under such agreement. Baxano has not received any notice in writing from any other party, and, to the knowledge of Baxano, no party has threatened, to terminate, cancel, fail to renew or otherwise materially modify any such agreements the loss of which, individually or in the aggregate, would reasonably be expected to have a Baxano Material Adverse Effect.
3.12 Litigation. There is no action, suit, proceeding, claim, arbitration or investigation before any Governmental Entity or before any arbitrator that is pending or, to the knowledge of Baxano, has been threatened in writing against Baxano that (a) seeks either damages in excess of $100,000 or equitable relief or (b) in any manner challenges or seeks to prevent, enjoin, alter or delay the transactions contemplated by this Agreement. There are no material judgments, orders or decrees outstanding against Baxano.
| A-38 |
3.13 Environmental Matters.
(a) Except as disclosed in Section 3.13(a) of the Baxano Disclosure Schedule and except for such matters that, individually or in the aggregate, have not had, and are not reasonably expected to have, a Baxano Material Adverse Effect:
(i) Baxano has complied with all applicable Environmental Laws;
(ii) to the actual knowledge of Baxano, and without independent investigation, all real property currently leased by Baxano as disclosed in Section 3.9(b) of the Baxano Disclosure Schedule are in compliance, and since Baxano’s acquisition of an interest in such currently leased real property have been in compliance, in all material respects, and prior to such acquisition were in compliance, with all applicable Environmental Laws;
(iii) to the actual knowledge of Baxano, and without independent investigation, the real properties currently leased or operated by Baxano as disclosed in Section 3.9(b) of the Baxano Disclosure Schedule (including soils, sediments, groundwater, surface water, buildings or other structures) are not contaminated with any Hazardous Substances at levels or in a condition that violate applicable Environmental Laws;
(iv) to the actual knowledge of Baxano, and without independent investigation, the real properties formerly leased or operated by Baxano disclosed in Section 3.9(b) of the Baxano Disclosure Schedule (including soils, sediments, groundwater, surface water, buildings or other structures) were not, during the period of use or operation by Baxano, contaminated with Hazardous Substances at levels or in a condition that violated or would violate applicable Environmental Laws;
(v) Baxano is not subject to liability (whether arising under contract or under Environmental Law) for the impaired environmental condition of, or any Hazardous Substance disposal or contamination at, the real property of any third party;
(vi) Baxano has not released any Hazardous Substance into the environment in amounts or in a manner that, individually or in the aggregate, could reasonably be expected to require notification, investigation, response, abatement or remediation under any Environmental Law;
(vii) Baxano has all Baxano Authorizations and Material Safety Data Sheets for the operation of the business as currently conducted as required under Environmental Laws, copies of all of which have been delivered or made available to TranS1; and has filed all reports required to be filed with any Governmental Entity thereunder or pursuant to any other applicable Environmental Law;
(viii) Baxano has not received any notice, notice of violation, demand, letter, claim or request for information regarding (A) any action instituted or threatened under or pursuant to any Environmental Law, or of any violation of, any Environmental Law applicable to any currently or formerly leased real properties of Baxano as disclosed in Section 3.9(b) of the Baxano Disclosure Schedule, or (B) alleging that Baxano is or may be in violation of, liable or potentially liable under or have outstanding obligations under any Environmental Law, including without limitation, any notice from any Governmental Entity or other person advising that Baxano that it is or is potentially responsible for response, assessment, investigation, abatement, or remediation costs under any Environmental Law with respect to a release or threatened release of any Hazardous Substances;
| A-39 |
(ix) Baxano has not received and is not subject to any judgments, orders, decrees, injunctions or other binding arrangements with any Governmental Entity or is subject to any indemnity or other agreement with any third party relating to liability under any Environmental Law or relating to Hazardous Substances; and
(x) to the actual knowledge of Baxano, there are no circumstances or conditions involving Baxano or any of its currently leased real properties as disclosed in Section 3.9(b) of the Baxano Disclosure Schedule that could reasonably be expected to result in any claims, liabilities, obligations, investigations, costs or restrictions on the ownership, use or transfer of any such real property of Baxano pursuant to any Environmental Law.
(b) Except as set forth in Section 3.13(b) of the Baxano Disclosure Schedule, there are no aboveground or underground storage tank systems, including pumps and lines, on the currently leased real property disclosed in Section 3.9(b) of the Baxano Disclosure Schedule for the storage of Hazardous Substances. Each of the tanks and related equipment and apparatus disclosed on Section 3.13(b) of the Baxano Disclosure Schedule has been upgraded and if required, registered, to meet all applicable requirements under Environmental Laws.
(c) Baxano has delivered to TranS1 true and complete copies and results of any reports, studies, sampling, tests, environmental site assessments or other assessments possessed by or readily available to Baxano pertaining to the environmental or physical condition of any real property (and any buildings, structures, or other improvements thereon) presently or previously leased or used by Baxano.
(d) For purposes of this Agreement, the term “Environmental Law” means any federal, state, local or foreign law, statute, rule, regulation, ordinance, standard, process, order, decree, administrative ruling, permit, authorization, opinion, common law or agency requirement of any jurisdiction relating to: (i) the protection, investigation or restoration of the environment, human or occupational health and safety or natural resources, (ii) the generation, transportation, handling, use, storage, treatment, presence, disposal, release or threatened release of any Hazardous Substance or (iii) land use, building code, noise, odor, wetlands, pollution, contamination or any injury or threat of injury to persons or property.
(e) For purposes of this Agreement, the term “Hazardous Substance” means any substance that is: (i) listed, classified, regulated or which falls within the definition of a “hazardous substance,” “hazardous waste,” “hazardous waste constituent” or “hazardous material” pursuant to any Environmental Law; (ii) any petroleum product, by-product, or constituent; (iii) asbestos-containing material, lead-containing paint or plumbing, polychlorinated biphenyls, radioactive materials or radon; or (iv) any other waste, substance or material, the generation, transportation, handling, use, storage, treatment, presence, disposal or release of which is regulated under or by any Environmental Law or that is the subject of regulatory action by any Governmental Entity pursuant to any Environmental Law.
| A-40 |
3.14 Employee Benefit Plans.
(a) Section 3.14(a) of the Baxano Disclosure Schedule sets forth a complete and accurate list of all Employee Benefit Plans maintained, or contributed to, by Baxano or any of their respective ERISA Affiliates (collectively, the “Baxano Employee Plans”).
(b) With respect to each Baxano Employee Plan, Baxano has provided or made available to TranS1 a complete and accurate copy of (i) such plan (or a written summary of any unwritten plan), (ii) the most recent annual report (Form 5500) filed with the United States Internal Revenue Service (the “IRS”) (including all schedules and financial statements attached to such report), (iii) each trust agreement, group annuity contract and summary plan description, if any, relating to such Baxano Employee Plan, (iv) the most recent financial statements for each Baxano Employee Plan that is funded, (v) all personnel, payroll and employment manuals and policies, (vi) all employee handbooks and (vii) all reports regarding the satisfaction of the nondiscrimination requirements of Sections 410(b), 401(k) and 401(m) of the Code.
(c) Each Baxano Employee Plan has been administered in all material respects in accordance with ERISA, the Code and all other applicable laws and the regulations thereunder and in accordance with its terms, and each of Baxanoand itsERISA Affiliateshas in all material respects met its obligations with respect to suchBaxano Employee Planand has made all required contributions thereto (or reserved such contributions on theBaxano Balance Sheet).Baxanoand each of itsERISA Affiliatesand eachBaxano Employee Planare in compliance in all material respects with the currently applicable provisions ofERISAand theCodeand the regulations thereunder (including, but not limited to, Section 4980B-4980E of theCode, Subtitle K, Chapter 100 of theCodeand Sections 601 through 608 and Section 701 et seq. ofERISA). All filings and reports as to eachBaxano Employee Planrequired to have been submitted to theIRSor to theUnited States Department of Laborhave been timely submitted. With respect toBaxano Employee Plans, no event has occurred, andto the knowledge of Baxano, there exists no condition or set of circumstances in connection with whichBaxanoor any of itsERISA Affiliatescould be subject to any liability that would reasonably be expected,individually or in the aggregate, to have a Baxano Material Adverse EffectunderERISA, theCodeor any other applicable law.
(d) With respect to Baxano Employee Plans, there are no benefit obligations for which contributions have not been made or properly accrued and there are no benefit obligations that have not been accounted for by reserves, or otherwise properly footnoted in accordance with GAAP, on the financial statements of Baxano, which obligations would reasonably be expected, individually or in the aggregate, to have a Baxano Material Adverse Effect. The assets of each Baxano Employee Plan that is funded are reported at their fair market value on the books and records of such Baxano Employee Plan.
| A-41 |
(e) All Baxano Employee Plans that are intended to be qualified under Section 401(a) of the Code have received determination or opinion letters from the IRS to the effect that such Baxano Employee Plans are qualified and the plans and trusts related thereto are exempt from federal income taxes under Sections 401(a) and 501(a), respectively, of the Code, no such determination or opinion letter has been revoked and revocation has not been threatened, and no such Employee Benefit Plan has been amended or operated since the date of its most recent determination letter or application therefor in any respect, and no act or omission has occurred, that would adversely affect its qualification or materially increase its cost. To the knowledge of Baxano, no “prohibited transaction” (within the meaning of Section 4975 of the Code or Sections 406 and 408 of ERISA) has occurred with respect to any such Employee Benefit Plans. Each Baxano Employee Plan that is required to satisfy Section 401(k)(3) or Section 401(m)(2) of the Code has been tested for compliance with, and satisfies the requirements of, Section 401(k)(3) and Section 401(m)(2) of the Code, as the case may be, for each plan year ending prior to the Closing Date.
(f) Neither Baxano nor any of its ERISA Affiliates has (i) ever maintained a Baxano Employee Benefit Plan that was ever subject to Section 412 of the Code or Title IV of ERISA or (ii) ever been obligated to contribute to a “multiemployer plan” (as defined in Section 4001(a)(3) of ERISA). No Baxano Employee Plan is funded by, associated with or related to a “voluntary employees’ beneficiary association” within the meaning of Section 501(c)(9) of the Code. No Baxano Employee Plan holds securities issued by Baxano or any of its ERISA Affiliates.
(g) Each Baxano Employee Plan is amendable and terminable unilaterally by Baxano without additional vesting or acceleration of benefits or any other liability to Baxano as a result thereof (other than for benefits accrued through the date of termination or amendment and reasonable administrative expenses related thereto), and no Baxano Employee Plan, plan documentation or agreement, summary plan description or other written communication distributed generally to employees by its terms prohibits Baxano from amending or terminating any such Baxano Employee Plan.
(h) Except as disclosed in Section 3.14(h) of the Baxano Disclosure Schedule, Baxano is not a party to any oral or written (i) agreement with any stockholders, director, executive officer or other employee of Baxano (A) the benefits of which are contingent, or the terms of which are materially altered, upon the occurrence of a transaction involving Baxano of the nature of any of the transactions contemplated by this Agreement, (B) providing any term of employment or compensation guarantee or (C) providing severance benefits or other benefits after the termination of employment of such director, executive officer or employee; (ii) agreement, plan or arrangement under which any person may receive payments from Baxano that may be subject to the tax imposed by Section 4999 of the Code or included in the determination of such person’s “parachute payment” under Section 280G of the Code, without regard to Section 280G(b)(4); or (iii) agreement or plan binding Baxano, including any stock option plan, stock appreciation right plan, restricted stock plan, stock purchase plan or severance benefit plan, any of the benefits of which shall be increased, or the vesting of the benefits of which shall be accelerated, by the occurrence of any of the transactions contemplated by this Agreement or the value of any of the benefits of which shall be calculated on the basis of any of the transactions contemplated by this Agreement.
(i) None of the Baxano Employee Plans promises or provides retiree medical or other retiree welfare benefits to any person, except as required by applicable law.
| A-42 |
(j) Each Baxano Employee Plan that is a “nonqualified deferred compensation plan” (as defined in Code Section 409A(d)(1)) has been operated since January 1, 2005 in good faith compliance with Code Section 409A and IRS Notice 2005-1. No Baxano Employee Plan that is a “nonqualified deferred compensation plan” has been materially modified (as determined under Notice 2005-1) after October 3, 2004. No event has occurred that would be treated by Code Section 409A(b) as a transfer of property for purposes of Code Section 83. No stock option or equity unit option granted under any Baxano Employee Plan has an exercise price that has been or may be less than the fair market value of the underlying stock or equity units (as the case may be) as of the date such option was granted or has any feature for the deferral of compensation other than the deferral of recognition of income until the later of exercise or disposition of such option.
(k) The Baxano employee handbook, a copy of which has previously been provided or made available to TranS1, sets forth the policy of Baxano with respect to accrued vacation, accrued sick time and earned time off and the amount of such liabilities as of December 31, 2012 are set forth on Section 3.14(k) of the Baxano Disclosure Schedule.
(l) For purposes of this Agreement, the following terms shall have the following meanings:
(i) “Employee Benefit Plan” means any “employee pension benefit plan” (as defined in Section 3(2) of ERISA), any “employee welfare benefit plan” (as defined in Section 3(1) of ERISA) and any other written or oral plan, agreement or arrangement involving direct or indirect compensation or providing for fringe benefits or perquisites, including but not limited to profit-sharing, pension, consulting, retainer, retirement, welfare, insurance coverage, severance, disability, deferred compensation, bonuses, stock options, stock purchase, phantom stock, stock appreciation or other forms of incentive compensation or post-retirement compensation and all unexpired severance agreements, written or otherwise, for the benefit of, or relating to, any current or former employee, officer, director, or agent of the entity in question or any of its Subsidiaries or ERISA Affiliates.
(ii) “ERISA” means the Employee Retirement Income Security Act of 1974, as amended.
(iii) “ERISA Affiliate” means any entity that is, or at any applicable time was, a member of (1) a controlled group of corporations (as defined in Section 414(b) of the Code), (2) a group of trades or businesses under common control (as defined in Section 414(c) of the Code), or (3) an affiliated service group (as defined under Section 414(m) of the Code or the regulations under Section 414(o) of the Code), any of which includes or included the entity in question or any of its Subsidiaries.
3.15 Compliance With Laws. Baxano has materially complied with, is not in material violation of, and has not received any notice from any Governmental Entity alleging any material violation with respect to, any applicable provisions of any statute, law or regulation with respect to the conduct of its business, or the ownership or operation of its properties or assets.
| A-43 |
3.16 Permits and Regulatory Matters.
(a) Baxano has all permits, licenses, registrations, authorizations and franchises from Governmental Entities required to conduct their businesses as currently conducted or as currently proposed to be conducted, including without limitation all such permits, licenses, registrations, authorizations and franchises required by the U.S. Food and Drug Administration (the “FDA”) or any other Governmental Entity exercising comparable authority, except for such permits, licenses, registrations, authorizations and franchises the lack of which, individually or in the aggregate, has not had, and is not reasonably expected to have, a Baxano Material Adverse Effect (the “Baxano Authorizations”). Baxano is in compliance with the terms of the Baxano Authorizations, except where the failure to so comply, individually or in the aggregate, has not had, and is not reasonably expected to have, a Baxano Material Adverse Effect. No Baxano Authorization will cease to be effective as a result of the consummation of the transactions contemplated by this Agreement.
(b) All manufacturing, processing, distribution, labeling, storage, testing, sale or marketing of products performed by or on behalf of Baxano are in compliance with all applicable laws, rules, regulations or orders administered or issued by the FDA or any other Governmental Entity exercising comparable authority, except where the failure to so comply, individually or in the aggregate, has not had, and is not reasonably expected to have, a Baxano Material Adverse Effect. Baxano has not received any notices or correspondence from the FDA or any other Governmental Entity exercising comparable authority, and to the knowledge of Baxano there is no action or proceeding pending or threatened (including any prosecution, injunction, seizure, civil fine, suspension or recall), in each case alleging that Baxano is not currently in compliance with any and all applicable laws, regulations or orders implemented by the FDA or any other Governmental Entity exercising comparable authority.
(c) There are no seizures, recalls, market withdrawals, field notifications or corrective actions, notifications of misbranding, destruction orders, safety alerts or similar actions relating to the safety or efficacy of any products marketed or sold by Baxano being conducted, requested in writing or, to the knowledge of Baxano, threatened by the FDA or any other Governmental Entity exercising comparable authority. Baxano has not, either voluntarily or involuntarily, initiated, conducted or issued or caused to be initiated, conducted or issued any recall, market withdrawal or other similar action by a Governmental Entity.
(d) The studies, tests and preclinical and clinical trials conducted by or on behalf of Baxano were and, if still pending, are being conducted in all material respects in accordance with experimental protocols, procedures and controls pursuant to, where applicable, accepted professional and scientific standards; and Baxano has not received any notices or correspondence from the FDA or any other Governmental Entity exercising comparable authority requiring the termination, suspension or material modification of any studies, tests or preclinical or clinical trials conducted by or on behalf of Baxano.
| A-44 |
3.17 Employees.
(a) Substantially all current or past key employees of Baxano have entered into confidentiality and assignment of inventions agreements with Baxano, a copy or form of which has previously been provided or made available to TranS1. To the knowledge of Baxano, no employee of Baxano is in violation of any term of any patent disclosure agreement, non-competition agreement, or any restrictive covenant to a former employer relating to the right of any such employee to be employed by Baxano because of the nature of the business currently conducted or currently proposed to be conducted by Baxano or to the use of trade secrets or proprietary information of others, the consequences of which, individually or in the aggregate, are reasonably expected to have a Baxano Material Adverse Effect. To the knowledge of Baxano, as of the date of this Agreement, no key employee or group of employees has any plans to terminate employment with Baxano.
(b) Baxano is not a party to or otherwise bound by any collective bargaining agreement, contract or other agreement or understanding with a labor union or labor organization. Baxano is not the subject of any proceeding asserting that Baxano has committed an unfair labor practice or is seeking to compel it to bargain with any labor union or labor organization that, individually or in the aggregate, is reasonably expected to have a Baxano Material Adverse Effect, nor is there pending or, to the knowledge of Baxano, threatened, any labor strike, dispute, walkout, work stoppage, slow-down or lockout involving Baxano.
3.18 Insurance. Section 3.18 of the Baxano Disclosure Schedule sets forth a complete and accurate list as of the date of this Agreement of all insurance policies maintained by Baxano (the “Baxano Insurance Policies”). Each Baxano Insurance Policy is in full force and effect as of the date of this Agreement. As of the date of this Agreement, there is no material claim by Baxano pending under any Baxano Insurance Policy as to which coverage has been questioned, denied or disputed by the underwriters of such policy.
3.19 No Existing Discussions. As of the date of this Agreement, Baxano is not engaged, directly or indirectly, in any discussions or negotiations with any other party with respect to an Acquisition Proposal.
3.20 Brokers; Fees and Expenses. No agent, broker, investment banker, financial advisor or other firm or person is or will be entitled, as a result of any action, agreement or commitment of Baxano or any of its Affiliates, to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with any of the transactions contemplated by this Agreement, except Leerink Swann LLC whose fees and expenses shall be paid by Baxano, subject to Section 2.11 to the extent unpaid as of the Effective Time. Baxano has provided or made available to TranS1 a complete and accurate copy of all agreements pursuant to which Leerink Swann LLC is entitled to any fees and expenses in connection with any of the transactions contemplated by this Agreement.
3.21 Financial Controls and Procedures. Baxano maintains proper and adequate internal control over financial reporting that provides reasonable assurance that (i) transactions are executed with management’s authorization, (ii) transactions are recorded as necessary to permit preparation of the financial statements of Baxano and to maintain accountability for Baxano’s assets, (iii) access to assets of Baxano is permitted only in accordance with management’s authorization, (iv) the reporting of receivables and inventory of Baxano is compared with existing receivables and inventory at regular intervals and (v) accounts, notes and other receivables and inventory were recorded accurately, and proper and adequate procedures are implemented to effect the collection thereof on a current and timely basis.
| A-45 |
3.22 Certain Business Relationships With Affiliates. No Affiliate of Baxano (a) owns any property or right, tangible or intangible, which is used in the business of Baxano, (b) has any claim or cause of action against Baxano or (c) owes any money to, or is owed any money by, Baxano. Section 3.22 of the Baxano Disclosure Schedule describes any commercial transactions or relationships between Baxano and any Affiliate thereof as of the date of this Agreement.
3.23 Books and Records. The minute books and other similar records of Baxano contain complete and accurate records of all actions taken at any meetings of Baxano’s stockholders, Board of Directors or any committee thereof and of all written consents executed in lieu of the holding of any such meeting. The books and records of Baxano accurately reflect in all material respects the assets, liabilities, business, financial condition and results of operations of Baxano and have been maintained in accordance with good business and bookkeeping practices, and as required by applicable law. Section 3.23 of the Baxano Disclosure Schedule sets forth a list of all bank accounts and safe deposit boxes of Baxano and the names of persons having signature authority with respect thereto or access thereto.
3.24 Commercial Relationships. During the past 12 months from the date of this Agreement, none of Baxano’s material suppliers, customers, collaborators, distributors, agents, licensors or licensees has canceled or otherwise terminated its relationship with Baxano or has materially altered its relationship with Baxano. To the knowledge of Baxano, no such person has any plan or intention, and Baxano has not received any written notice from any such person, to terminate, cancel or otherwise materially modify its relationship with Baxano. As of the date of this Agreement, Baxano has not received any written notice (formal or informal) or other communication from any of Baxano’s top ten largest customers (based on fiscal 2012 total revenues) or top ten largest suppliers (based on fiscal 2012 expenditures) that indicates or could reasonably be expected to indicate that any such customer or supplier has any plan or intention not to renew its agreement with Baxano on terms substantially comparable to its current agreement with Baxano.
3.25 Tangible Assets. Baxano owns or leases all machinery, equipment and other tangible assets necessary for the conduct of their business as presently conducted. Each such tangible asset has been maintained in accordance with normal industry practice, is in good operating condition and repair (subject to normal wear and tear) and is suitable for the purposes for which it presently is used. All tangible assets, including all files and records, owned or leased by Baxano are located at real property disclosed in Section 3.9(b) of the Baxano Disclosure Schedule.
3.26 Inventory. Except as set forth on Section 3.26 of the Baxano Disclosure Schedule, the inventories of Baxano are of a quality and quantity useable and saleable in Baxano’s Ordinary Course of Business, subject to appropriate and adequate allowances reflected on the Baxano Balance Sheet for obsolete, excess, slow-moving, lower of cost or market and other reserves required under GAAP, consistently applied. Such allowances have been calculated in accordance with GAAP, consistently applied, and in a manner consistent with the past practices of Baxano. None of the inventory of Baxano is held on consignment, or otherwise, by third parties.
| A-46 |
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF TRANS1 AND THE
TRANSITORY SUBSIDIARY
TranS1 and Transitory Subsidiary represent and warrant to Baxano that the statements contained in this ARTICLE IV are true and correct, except as expressly set forth herein or in the disclosure schedule delivered by TranS1 and Transitory Subsidiary to Baxano on the date of this Agreement (the “TranS1 Disclosure Schedule”). The TranS1 Disclosure Schedule shall be arranged in sections corresponding to the numbered and lettered sections contained in this ARTICLE IV and the disclosure in any section of the TranS1 Disclosure Schedule shall qualify (1) the corresponding section in this ARTICLE IV and (2) the other sections in this ARTICLE IV only to the extent that it is reasonably apparent from a reading of such disclosure that it also qualifies or applies to such other sections. For purposes hereof, “to the knowledge of TranS1” and similar expressions mean the actual knowledge of the persons identified on the TranS1 Disclosure Schedule for this purpose,includingthe knowledge such persons would have in the ordinary performance of their duties toTranS1.
4.1 Organization, Standing and Power. Each of TranS1 and Transitory Subsidiary is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation, has all requisite corporate power and authority to own, lease and operate its properties and assets and to carry on its business as currently conducted and as currently proposed to be conducted, and is duly qualified to do business and is in good standing as a foreign corporation in each jurisdiction listed on Section 4.1 of the TranS1 Disclosure Schedule, which jurisdictions constitute the only jurisdictions in which the character of the properties it owns, operates or leases or the nature of its activities makes such qualification necessary, except for such failures to be so organized, qualified or in good standing, individually or in the aggregate, that have not had, and are not reasonably expected to have, a TranS1 Material Adverse Effect. For purposes of this Agreement, the term “TranS1 Material Adverse Effect” means any material adverse change, event, circumstance or development with respect to, or material adverse effect on, (i) the business, assets and liabilities (taken together), condition (financial or otherwise), or results of operations of TranS1 and its Subsidiaries, taken as a whole, or (ii) the ability of TranS1and itsSubsidiariesto consummate the transactions contemplated by thisAgreement;provided,however, that the following shall not be deemed to be a TranS1 Material Adverse Effect: any change or event caused by or resulting from (A) changes in prevailing economic or market conditions in the United States or any other jurisdiction in which TranS1 has substantial business operations (except to the extent those changes have a materially disproportionate effect on TranS1 and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which TranS1 and its Subsidiaries operate), (B) changes or events, after the date hereof, affecting the industries or markets in which they operate generally (except to the extent those changes or events have a materially disproportionate effect on TranS1 and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which TranS1 and its Subsidiaries operate), (C) changes, after the date hereof, in generally accepted accounting principles or requirements applicable to TranS1 and its Subsidiaries (except to the extent those changes have a materially disproportionate effect on TranS1 and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which TranS1 and its Subsidiaries operate), (D) changes, after the date hereof, in laws, rules or regulations of general applicability or interpretations thereof by any Governmental Entity (except to the extent those changes have a materially disproportionate effect on TranS1 and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which TranS1 and its Subsidiaries operate), (E) the execution, delivery and performance of this Agreement or the consummation of the transactions contemplated hereby or the announcement thereof, (F) any outbreak of major hostilities in which the United States is involved or any act of terrorism within the United States or directed against its facilities or citizens wherever located, (G) the activities under investigation by the U.S. Department of Justice and the Office of Inspector General of the U.S. Department of Health & Human Services and/or alleged by anyqui tamrelator in any False Claims Act complaint giving rise thereto (collectively, the “FCA Matter”), or (H) the actions of Baxano (other than any action taken under this Agreement by Baxano in response to an event, circumstance or other development that would otherwise constitute a TranS1 Material Adverse Effect); andprovided,further, that in no event shall a change in the public trading price of TranS1 Common Stock, by itself, be considered material or constitute a TranS1 Material Adverse Effect, although the underlying cause of any change in the public trading price of TranS1 Common Stock may nonetheless be considered in determining the occurrence of a TranS1 Material Adverse Effect. For the avoidance of doubt, the parties agree that the terms “material,” “materially” and “materiality” as used in thisAgreementwith an initial lower case “m” shall have their respective customary and ordinary meanings, without regard to the meanings ascribed toTranS1 Material Adverse Effectin the prior sentence of this paragraph orBaxano Material Adverse Effectin Section3.1. TranS1 has provided or made available to Baxanocomplete and accurate copies of its Certificate of Incorporation and Bylaws.
| A-47 |
4.2 Capitalization.
(a) The authorized capital stock of TranS1 consists of 75,000,000 shares of TranS1 Common Stock, $0.0001 par value per share (“TranS1 Common Stock”), and 5,000,000 shares of preferred stock, $0.0001 par value pershare (“TranS1 Preferred Stock”). The rights and privileges of each class ofTranS1’s capital stock are as set forth inTranS1’s Certificate of Incorporation. As of the date of thisAgreement, (i)27,318,785 sharesofTranS1 Common Stockare issued and outstanding, and (ii) nosharesofTranS1 Common Stockare held in the treasury ofTranS1or bySubsidiariesofTranS1, and (iii) nosharesofTranS1 Preferred Stockare issued and outstanding.
(b) Section 4.2(b) of the TranS1 Disclosure Schedule sets forth a complete and accurate list of the number of shares of TranS1 Common Stock reserved for future issuance pursuant to stock options granted and outstanding as of the date of this Agreement, the plans under which such options were granted (collectively, “TranS1 Stock Plans”) and the total number of outstanding options to purchase shares of TranS1 Common Stock (such outstanding options, “TranS1 Stock Options”) under TranS1 Stock Plans as of the close of business on the business day prior to the date of this Agreement. TranS1 has provided or made available to Baxano accurate and complete copies of all TranS1 Stock Plans and the forms of all stock option agreements used under the TranS1 Stock Plans.
| A-48 |
(c) Section 4.2(c) of the TranS1 Disclosure Schedule sets forth the number of shares of TranS1 capital stock reserved for future issuance pursuant to warrants, convertible promissory notes or other outstanding rights (other than TranS1 Stock Options) to purchase shares of TranS1 capital stock outstanding as of the date of this Agreement (such outstanding warrants, convertible promissory notes or other rights, the “TranS1 Convertible Securities”) and the agreement or other document under which such TranS1 Convertible Securities were granted and sets forth a complete and accurate list of all holders of TranS1 Convertible Securities indicating the number and type of shares of TranS1 Common Stock subject to each TranS1 warrant, and the exercise price, the date of grant and the expiration date thereof. TranS1 has provided or made available to Baxano complete and accurate copies of the forms of agreements evidencing all TranS1 Convertible Securities.
(d) Except (i) as set forth in this Section 4.2 or in ARTICLE II and (ii) as reserved for future grants under TranS1 Stock Plans, (A) there are no equity securities of any class ofTranS1, or any security exchangeable into or exercisable for such equity securities, issued, reserved for issuance or outstanding and (B) there are no options, warrants, equity securities, calls, rights, commitments or agreements of any character to whichTranS1or any of itsSubsidiariesis a party or by whichTranS1or any of itsSubsidiariesis bound obligatingTranS1or any of itsSubsidiariesto issue, exchange,transfer, deliver or sell, or cause to be issued, exchanged, transferred, delivered or sold, additionalsharesof capital stock or other equity interests ofTranS1or any security or rights convertible into or exchangeable or exercisable for any suchsharesor other equity interests, or obligatingTranS1or any of itsSubsidiariesto grant, extend, accelerate the vesting of, otherwise modify or amend or enter into any such option, warrant, equity security, call, right, commitment oragreement.TranS1does not have any outstanding stock appreciation rights, phantom stock, performance based rights or similar rights or obligations. Other than theTranS1 Stockholder Agreements, neitherTranS1nor any of itsAffiliatesis a party to or is bound by any, andto the knowledge of TranS1, there are no, agreements or understandings with respect to the voting (includingvoting trusts and proxies) or sale ortransfer(includingagreements imposingtransferrestrictions) of anysharesof capital stock or other equity interests ofTranS1. Except as contemplated by thisAgreementor described in this Section4.2(d), there are no registration rights, and there is no rightsagreement, “poison pill” anti-takeover plan or otheragreementor understanding to whichTranS1or any of itsSubsidiariesis a party or by which it or they are bound with respect to any equity security of any class ofTranS1. Stockholders ofTranS1are not entitled to dissenters’ or appraisal rights under applicable state law in connection with theMerger.
(e) All outstanding shares of TranS1 Common Stock are, and all shares of TranS1 Common Stock subject to issuance as specified in Sections 4.2(b) and 4.2(c) or pursuant to ARTICLE II, upon issuance on the terms and conditions specified in the instruments pursuant to which they are issuable, will be, duly authorized, validly issued, fully paid and nonassessable and not subject to or issued in violation of any purchase option, call option, right of first refusal, preemptive right, subscription right or any similar right under any provision oftheDGCL,TranS1’s Certificate of Incorporation or Bylaws or anyagreementto whichTranS1is a party or is otherwise bound.There are no obligations, contingent or otherwise, of TranS1 or any of its Subsidiaries to repurchase, redeem or otherwise acquire any shares of TranS1 Common Stock. All outstanding shares of TranS1 capital stock have been offered, issued and sold by TranS1 in compliance with all applicable federal and state securities laws.
| A-49 |
(f) The Closing Capitalization Statement will be true, complete and accurate in all respects at the Effective Time.
4.3 Subsidiaries.
(a) Section 4.3(a) of the TranS1 Disclosure Schedule sets forth, for each Subsidiary of TranS1: (i) its name; (ii) the number and type of outstanding equity securities and a list of the holders thereof; and (iii) the jurisdiction of organization.
(b) Each Subsidiary of TranS1 is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation, has all requisite corporate power and authority to own, lease and operate its properties and assets and to carry on its business as currently conducted and as currently proposed to be conducted, and is duly qualified to do business and is in good standing as a foreign corporation in each jurisdiction where the character of its properties owned, operated or leased or the nature of its activities makes such qualification necessary, except for such failures to be so organized, qualified or in good standing, individually or in the aggregate, that have not had, and would not reasonably be expected to have, a TranS1 Material Adverse Effect. All of the outstanding shares of capital stock and other equity securities or interests of each Subsidiary of TranS1 are duly authorized, validly issued, fully paid, nonassessable and free of preemptive rights and all such shares are owned, of record and beneficially, by TranS1 or another of its Subsidiaries free and clear of all security interests, liens, claims, pledges, agreements, limitations in TranS1’s voting rights, charges or other encumbrances of any nature. There are no outstanding or authorized options, warrants, rights, agreements or commitments to which TranS1 or any of its Subsidiaries is a party or which are binding on any of them providing for the issuance, disposition or acquisition of any capital stock of any Subsidiary of TranS1. There are no outstanding stock appreciation, phantom stock or similar rights with respect to any Subsidiary of TranS1. There are no voting trusts, proxies or other agreements or understandings with respect to the voting of any capital stock of any Subsidiary of TranS1.
(c) TranS1 has provided or made available to Baxano complete and accurate copies of the charter, bylaws or other organizational documents of each Subsidiary of TranS1.
(d) TranS1 does not control directly or indirectly or have any direct or indirect equity participation or similar interest in any corporation, partnership, limited liability company, joint venture, trust or other business association or entity which is not a Subsidiary of TranS1. There are no obligations, contingent or otherwise, of TranS1 or any of its Subsidiaries to repurchase, redeem or otherwise acquire any shares of capital stock of any Subsidiary of TranS1 or to provide funds to or make any investment (in the form of a loan, capital contribution or otherwise) in any Subsidiary of TranS1 or any other entity, other than guarantees of bank obligations of Subsidiaries of TranS1 entered into in the Ordinary Course of Business.
| A-50 |
4.4 Authority; No Conflict; Required Filings and Consents.
(a) Each of TranS1 and Transitory Subsidiary has all requisite corporate power and authority to enter into this Agreement and, subject only to the TranS1 Stockholder Approval, to consummate the transactions contemplated by this Agreement. Without limiting the generality of the foregoing, each of the TranS1 Board and the Transitory Subsidiary Board, at a meeting duly called and held, by the unanimous vote of all directors (i) determined that the Merger is advisable, fair and in the best interests of TranS1, the Transitory Subsidiary and their respective stockholders and (ii) directed that the TranS1 Voting Proposal be submitted to the stockholders of TranS1 for their approval and resolved to recommend that the stockholders of TranS1 vote in favor of the approval of the TranS1 Voting Proposal. The execution and delivery of this Agreement and the consummation of the transactions contemplated by this Agreement by TranS1 and Transitory Subsidiary have been duly authorized by all necessary corporate action on the part of each of TranS1 and Transitory Subsidiary, subject only to the required receipt of the TranS1 Stockholder Approval and the adoption of this Agreement by TranS1 in its capacity as the sole stockholder of Transitory Subsidiary. TranS1 agrees to take the appropriate action to so adopt this Agreement promptly following the date hereof. This Agreement has been duly executed and delivered by each of TranS1 and Transitory Subsidiary and constitutes the valid and binding obligation of each of TranS1 and Transitory Subsidiary, enforceable in accordance with its terms, subject to the Bankruptcy and Equity Exception.
(b) The execution and delivery of this Agreement by each of TranS1 and Transitory Subsidiary do not, and the consummation by TranS1 and Transitory Subsidiary of the transactions contemplated by this Agreement will not, (i) conflict with, or result in any violation or breach of, any provision of the Certificate of Incorporation or Bylaws of TranS1 or Transitory Subsidiary or of the charter, bylaws or other organizational document of any other Subsidiary of TranS1, (ii) conflict with, or result in any violation or breach of, or constitute (with or without notice or lapse of time, or both) a default (or give rise to a right of termination, cancellation or acceleration of any obligation or loss of any material benefit) under, or require a consent or waiver under, constitute a change in control under, require the payment of a penalty under or result in the imposition of any Lien on TranS1’s or any of its Subsidiaries’ assets under any of the terms, conditions or provisions of any note, bond, mortgage, indenture, lease, license, contract or other agreement, instrument or obligation to which TranS1 or any of its Subsidiaries is a party or by which any of them or any of their properties or assets may be bound, or (iii) subject to obtaining the TranS1 Stockholder Approval and compliance with the requirements specified in clauses (i) through (vii) of Section 4.4(c), conflict with or violate any permit, concession, franchise, license, judgment, injunction, order, decree, statute, law, ordinance, rule or regulation applicable to TranS1 or any of its Subsidiaries or any of its or their properties or assets. Section 4.4(b) of the TranS1 Disclosure Schedule lists all consents, waivers and approvals under any of TranS1’s or anyof itsSubsidiaries’ agreements, licenses or leases required to be obtained in connectionwith the consummation of the transactions contemplated by this Agreement, which, if individually or in the aggregate were not obtained, would result in a materiallossof benefits toTranS1,Baxanoor Surviving Corporation as a result of theMerger.
| A-51 |
(c) No consent, approval, license, permit, order or authorization of, or registration, declaration, notice or filing with, any Governmental Entity or any stock market or stock exchange on which shares of TranS1 Common Stock are listed for trading is required by or with respect to TranS1 or any of its Subsidiaries in connection with the execution and delivery of this Agreement or the consummation by TranS1 or Transitory Subsidiary of the transactions contemplated by this Agreement, except for (i) the filing of the Certificate of Merger with the Delaware Secretary of State, (ii) the filing of the Registration Statement with the U.S. Securities and Exchange Commission (the “SEC”) in accordance with the Securities Act, (iii) the filing of the Proxy Statement with the SEC in accordance with the Securities Exchange Act of 1934, as amended (the “Exchange Act”), (iv) the filing of such reports, schedules or materials under Section 13 of or Rule 14a-12 under the Exchange Act as may be required in connection with this Agreement and the transactions contemplated hereby and thereby, (v) such consents, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable state securities laws and the laws of any foreign country, (vi) the filings with NASDAQ described in Section 6.3, (vii) the filing of a Form D pursuant to SEC Regulation D and (viii) such other consents, authorizations, orders, filings, approvals and registrations that, individually or in the aggregate, if not obtained or made, would not be reasonably expected to have a TranS1 Material Adverse Effect.
(d) The affirmative vote in favor of the TranS1 Voting Proposal by the holders of a majority of the shares of TranS1 Common Stock present or represented by proxy and entitled to vote thereon at the TranS1 Meeting is the only vote of the holders of any class or series of TranS1’s capital stock or other securities necessary to approve the TranS1 Voting Proposal. There are no bonds, debentures, notes or other indebtedness of TranS1 having the right to vote (or convertible into, or exchangeable for, securities having the right to vote) on any matters on which stockholders of TranS1 may vote.
4.5 SEC Filings; Financial Statements; Information Provided.
(a) TranS1 has filed all registration statements, forms, reports, certifications and other documents required to be filed by TranS1 with the SEC since January 1, 2010 and has made available to Baxano copies of all registration statements, forms, reports, certifications and other documents filed by TranS1 with the SEC since January 1, 2010, including all certifications and statements required by (i) Rule 13a-14 or 15d-14 under the Exchange Act or (ii) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes Act”)). All such registration statements, forms, reports, certifications and other documents (including those that TranS1 may file after the date hereof until the Closing) are referred to herein as the “TranS1 SEC Documents.” All TranS1 SEC Documents are publicly available on the SEC’s EDGAR system. TranS1 has made available to Baxano copies of all comment letters received by TranS1 from the staff of the SEC since January 1, 2010 and all responses to such comment letters by or on behalf of TranS1. All TranS1 SEC Documents (A) were or will be filed or deemed filed on a timely basis, (B) at the time filed, were or will be prepared in compliance in allmaterial respects with the applicable requirements of the Securities Act and the Exchange Act, as the case may be, and the rules and regulations of the SEC thereunder applicable to such TranS1 SEC Documents and (C) did not or will not at the time they were or are filed contain any untrue statement of a material fact or omit to state a material fact required to be stated in such TranS1 SEC Documents or necessary in order to make the statements in such TranS1 SEC Documents, in the light of the circumstances under which they were made, not misleading. No Subsidiary of TranS1 is subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act.
| A-52 |
(b) Each of the consolidated financial statements (including, in each case, any related notes and schedules) contained or to be contained in TranS1 SEC Reports at the time filed (i) complied or will comply as to form in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto (including, without limitation, Regulation S-X), (ii) were or will be prepared in accordance with GAAP applied on a consistent basis throughout the periods covered thereby (except as may be indicated in the notes to such financial statements or, in the case of unaudited interim financial statements, as permitted by the SEC on Form 10-Q under the Exchange Act) and (iii) fairly present the consolidated financial position of TranS1 and its Subsidiaries as of the dates thereof and the consolidated results of its operations and cash flows for the periods indicated, consistent with the books and records of TranS1 and its Subsidiaries, except that the unaudited interim financial statements were or are subject to normal and recurring year-end adjustments which were not or will not be material in amount or effect. TranS1’s audited consolidated balance sheet of TranS1 as of December 31, 2011 is referred to herein as the “TranS1 Balance Sheet.”
(c) PricewaterhouseCoopers LLP, TranS1’s current auditors, is and has been at all times since its engagement by TranS1 (i) “independent” with respect to TranS1 within the meaning of Regulation S-X and (ii) in compliance with subsections (g) through (l) of Section 10A of the Exchange Act (to the extent applicable) and the related rules of the SEC and the Public Company Accounting Oversight Board.
(d) There is no transaction, arrangement or other relationship between TranS1 and an unconsolidated or other off-balance sheet entity that is required to be disclosed by TranS1 in its Exchange Act filings and is not so disclosed or that otherwise would be reasonably likely to have a TranS1 Material Adverse Effect. There are no such transactions, arrangements or other relationships with TranS1 that may create contingencies or liabilities that are not otherwise disclosed by TranS1 in its Exchange Act filings.
4.6 No Undisclosed Liabilities. Except as disclosed in TranS1’s Annual Report on Form 10-K for the period ended December 31, 2011 (the “TranS1 Form 10-K”) filed with the SEC or any TranS1 SEC Documents filed after the filing of the TranS1 Form 10-K and prior to the date of this Agreement (together with the TranS1 Form 10-K, the “TranS1 Recent SEC Documents”), and except for normal and recurring liabilities incurred since the date of the TranS1 Balance Sheet in the Ordinary Course of Business, TranS1 and its Subsidiaries do not have any material liabilities (whether known or unknown, whether absolute or contingent, whether liquidated or unliquidated, whether due or to become due, and whether or not required to be reflected in financial statements (including the notes thereto) in accordance with GAAP).
4.7 Absence of Certain Changes or Events. Except as disclosed in the TranS1 Recent SEC Documents, since the date of the TranS1 Balance Sheet, TranS1 and its Subsidiaries have conducted their respective businesses only in the Ordinary Course of Business and, since such date, there has not been (i) any change, event, circumstance, development or effect that, individually or in the aggregate, has had, or is reasonably expected to have, a TranS1 Material Adverse Effect; or (ii) any other action or event that would have required the consent of Baxano pursuant to Section 5.2 of this Agreement had such action or event occurred after the date of this Agreement.
| A-53 |
4.8 Taxes.
(a) Each of TranS1 and its Subsidiaries has properly filed on a timely basis all material Tax Returns that it was required to file, and all such Tax Returns were true, correct and complete in all material respects. Each of TranS1 and its Subsidiaries has paid on a timely basis all Taxes due and payable. Neither TranS1 nor any of its Subsidiaries is or has ever been a member of a group of corporations with which it has filed (or been required to file) consolidated, combined or unitary Tax Returns, other than a group of which the common parent is TranS1. Neither TranS1 nor any of its Subsidiaries (i) has any actual or potential liability under Treasury Regulations Section 1.1502-6 (or any comparable or similar provision of federal, state, local or foreign law), as a transferee or successor, pursuant to any contractual obligation, or otherwise for any Taxes of any person other than TranS1 or any of its Subsidiaries, or (ii) is a party to or bound by any Tax indemnity, Tax sharing, Tax allocation or similar agreement. All material Taxes that TranS1 or any of its Subsidiaries was required by law to withhold or collect have been duly withheld or collected and, to the extent required, have been properly paid to the appropriate Governmental Entity.
(b) The unpaid Taxes of TranS1 did not, as of the most recent fiscal month end, exceed the reserve for Tax liability (rather than any reserve for deferred Taxes established to reflect timing differences between book and Tax income) set forth on the face of the TranS1 Balance Sheet (rather than in any notes thereto).
(c) No examination or audit of any Tax Return of TranS1 or any of its Subsidiaries by any Governmental Entity has occurred or is currently in progress or, to the knowledge of TranS1, threatened or contemplated in writing. Neither TranS1 nor any of its Subsidiaries has been informed by any jurisdiction that the jurisdiction believes that TranS1 or any of its Subsidiaries was required to file any Tax Return that was not filed. Neither TranS1 nor any of its Subsidiaries has (x) waived any statute of limitations with respect to Taxes or agreed to extend the period for assessment or collection of any Taxes, (y) requested any extension of time within which to file any Tax Return, which Tax Return has not yet been filed, or (z) executed or filed any power of attorney with any taxing authority.
(d) Neither TranS1 nor any of its Subsidiaries has made any payment, is obligated to make any payment, or is a party to any agreement that could obligate it to make any payment that may be treated as an “excess parachute payment” under Section 280G of the Code (without regard to Sections 280G(b)(4) and 280G(b)(5) of the Code).
(e) There are no adjustments under Section 481 of the Code (or any similar adjustments under any provision of the Code or the corresponding foreign, state or local Tax laws) that are required to be taken into account by TranS1 or any of its Subsidiaries in any period ending after the Closing Date by reason of a change in method of accounting in any taxable period ending on or before the Closing Date or as a result of the consummation of the transactions contemplated by this Agreement.
(f) Neither TranS1 nor any of its Subsidiaries has been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(l)(A)(ii) of the Code.
(g) Neither TranS1 nor any of its Subsidiaries has distributed to its stockholders or security holders stock or securities of a controlled corporation, nor has stock or securities of TranS1 or any of its Subsidiaries been distributed, in a transaction to which Section 355 of the Code applies (i) in the two years prior to the date of this Agreement or (ii) in a distribution that could otherwise constitute part of a “plan” or “series of related transactions” (within the meaning of Section 355(e) of the Code) that includes the transactions contemplated by this Agreement.
| A-54 |
(h) There are no Liens with respect to Taxes upon any of the assets or properties of TranS1 or any of its Subsidiaries, other than with respect to Taxes not yet due and payable or being contested in good faith by appropriate proceedings.
(i) Neither TranS1 nor any of its Subsidiaries will be required to include any item of income in, or exclude any item of deduction from, taxable income for any period (or any portion thereof) ending after the Closing Date as a result of any (i) deferred intercompany gain or any excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding provision of state, local or foreign Tax law), (ii) closing agreementas described in Section 7121 of theCode(or any corresponding or similar provision of state, local or foreignTaxlaw) executed on or prior to theClosing Date, (iii) installment sale or other open transaction disposition made on or prior to the Closing Date, or (iv) prepaid amount received on or prior to the Closing Date.
(j) Neither TranS1 nor any of its Subsidiaries has participated in any “reportable transaction” as defined in section 1.6011-4(b) of the Treasury Regulations or any analogous provision of state or local law.
(k) Since the date of the TranS1 Balance Sheet, TranS1 has not incurred any liability for Taxes arising from extraordinary gains or losses, as that term is used in GAAP, outside the Ordinary Course of Business.
4.9 Owned and Leased Real Properties. Except as disclosed in the TranS1 Recent SEC Documents,neither TranS1 nor any of its Subsidiaries (a) owns anyreal property or(b)leases, subleases or licenses any real property. NeitherTranS1nor any of itsSubsidiariesleases, subleases or licenses any real property to anypersonother thanTranS1and itsSubsidiaries.
4.10 Intellectual Property.
(a) To the knowledge of TranS1, TranS1 and its Subsidiaries own, license or otherwise possess legally enforceable rights to use all Intellectual Property used or necessary to conduct the business of TranS1 and its Subsidiaries as currently conducted, or that would be used or necessary as such business is currently proposed to be conducted (excluding currently-available, off-the-shelf software programs that are licensed by TranS1 pursuant to “shrink wrap” licenses).
| A-55 |
(b) The execution and delivery of this Agreement and consummation of the Merger will not result in the breach of, or create on behalf of any third party the right to terminate or modify, (i) any license, sublicense or other agreement relating to any Intellectual Property owned by TranS1 or any of its Subsidiaries that is material to the business of TranS1 and its Subsidiaries, taken as a whole, including software that is used in the development or manufacture of or forms a part of any product or service sold by or expected to be sold by TranS1 or any of its Subsidiaries (the “TranS1 Intellectual Property”) or (ii) any license, sublicense or any other agreement as to which TranS1 or any of its Subsidiaries is a party and pursuant to which TranS1 or any of its Subsidiaries is authorized to use any third party Intellectual Property that is material to the business of TranS1 and its Subsidiaries, taken as a whole, including software that is used in the development or manufacture of or forms a part of any product or service sold by or expected to be sold by TranS1 or any of its Subsidiaries (the “TranS1 Third Party Intellectual Property”). Section 4.10(b) of the TranS1 Disclosure Schedule sets forth a complete and accurate list of registered TranS1 Intellectual Property (including TranS1 Intellectual Property for which registration has been applied) and Section 4.10(b) of the TranS1 Disclosure Schedule sets forth a complete and accurate list of all TranS1 Third Party Intellectual Property.
(c) To the knowledge of TranS1, all patents and registrations and applications for Trademarks, service marks and copyrights which are held by TranS1 or any of its Subsidiaries and that are material to the business of TranS1 and its Subsidiaries, taken as a whole, are valid and subsisting. TranS1 and its Subsidiaries have taken commercially reasonable measures to protect the proprietary nature of the TranS1 Intellectual Property. To the knowledge of TranS1, no other person or entity is infringing, violating or misappropriating any of the TranS1 Intellectual Property or TranS1 Third Party Intellectual Property.
(d) To the knowledge of TranS1, none of the (i) products previously or currently sold by TranS1 or any of its Subsidiaries or (ii) business or activities previously or currently conducted by TranS1 orany of itsSubsidiariesinfringes, violates or constitutes a misappropriation of, anyIntellectual Propertyof any third party. Except as disclosed in Section 4.10(d) of the TranS1 Disclosure Schedule, neitherTranS1nor any of itsSubsidiarieshas received any written complaint, claim or notice alleging any such infringement, violation or misappropriation.
4.11 Contracts.
(a) Except for the contracts and agreements identified on the exhibit indices of the TranS1 Recent SEC Documents and as disclosed in Section 4.11(a) of the TranS1 Disclosure Schedule (collectively, the “TranS1 Material Contracts”), there are no material contracts (as such term is defined in Item 601(b)(10) of Regulation S-K) to which TranS1 or its Subsidiaries are a party.
(b) Except as disclosed in the TranS1 Recent SEC Documents, neither TranS1 nor any of its Subsidiaries has entered into any transaction with any Affiliate of TranS1 or any of its Subsidiaries or any transaction that would be subject to proxy statement disclosure pursuant to Item 404 of Regulation S-K.
(c) With respect to each TranS1 Material Contract: (i) the agreement is legal, valid, binding and enforceable and in full force and effect; (ii) the agreement will continue to be legal, valid, binding and enforceable and in full force and effect immediately following the Closing in accordance with the terms thereof as in effect immediately prior to the Closing; and (iii) neither TranS1 nor any of its Subsidiaries nor, to the knowledge of TranS1, any other party, is in breach or violation of, or default under, any such agreement, and no event has occurred, is pending or, to the knowledge of TranS1, is threatened, which, with or without notice or lapse of time, or both, would constitute a breach or default by TranS1 or any of its Subsidiaries or, to the knowledge of TranS1, any other party under such agreement. Neither TranS1 nor any of its Subsidiaries has received any notice in writing from any other party, and, to the knowledge of TranS1, no party has threatened, to terminate, cancel, fail to renew or otherwise materially modify any such agreements the loss of which, individually or in the aggregate, would reasonably be expected to have a TranS1 Material Adverse Effect.
| A-56 |
4.12 Litigation. Except as disclosed in the TranS1 Recent SEC Documents,there is no action, suit, proceeding, claim, arbitration or investigation before any Governmental Entity or before any arbitrator that is pending or, to the knowledge of TranS1, has been threatened in writing against TranS1 or any of its Subsidiaries. There are no material judgments, orders or decrees outstanding against TranS1 or any of its Subsidiaries.
4.13 Environmental Matters.
(a) Except as disclosed in Section 4.13(a) of the TranS1 Disclosure Schedule and except for such matters that, individually or in the aggregate, have not had, and are not reasonably expected to have, a TranS1 Material Adverse Effect:
(i) TranS1 and its Subsidiaries have complied with all applicable Environmental Laws;
(ii) to the actual knowledge of TranS1, and without independent investigation, all real property currently owned or leased by TranS1 or any of its Subsidiaries are in compliance, and since TranS1’s or any of its Subsidiaries’ acquisition of an interest in such currently owned or leased real property have been in compliance, in all material respects, and prior to such acquisition were in compliance, with all applicable Environmental Laws;
(iii) to the actual knowledge of TranS1, and without independent investigation, the real properties currently owned, leased or operated by TranS1 and its Subsidiaries (including soils, sediments, groundwater, surface water, buildings or other structures) are not contaminated with any Hazardous Substances at levels or in a condition that violate applicable Environmental Laws;
(iv) to the actual knowledge of TranS1, and without independent investigation, the real properties formerly owned, leased or operated by TranS1 or any of its Subsidiaries (including soils, sediments, groundwater, surface water, buildings or other structures) were not, during the period of ownership, use or operation by TranS1 or any of its Subsidiaries, contaminated with Hazardous Substances at levels or in a condition that violated or would violate applicable Environmental Laws;
(v) neither TranS1 nor any of its Subsidiaries are subject to liability (whether arising under contract or under Environmental Law) for the impaired environmental condition of, or any Hazardous Substance disposal or contamination at, the real property of any third party;
(vi) neither TranS1 nor any of its Subsidiaries have released any Hazardous Substance into the environment in amounts or in a manner that, individually or in the aggregate, could reasonably be expected to require notification, investigation, response, abatement or remediation under any Environmental Law;
| A-57 |
(vii) TranS1 and its Subsidiaries have all TranS1 Authorizations and Material Safety Data Sheets for the operation of the business as currently conducted as required under Environmental Laws, copies of all of which have been delivered or made available to TranS1; and have filed all reports required to be filed with any Governmental Entity thereunder or pursuant to any other applicable Environmental Law;
(viii) neither TranS1 nor any of its Subsidiaries has received any notice, notice of violation, demand, letter, claim or request for information regarding (A) any action instituted or threatened under or pursuant to any Environmental Law, or of any violation of, any Environmental Law applicable to any currently or formerly owned or leased real properties of TranS1 or its Subsidiaries, or (B) alleging that TranS1 or any of its Subsidiaries is or may be in violation of, liable or potentially liable under or have outstanding obligations under any Environmental Law, including without limitation, any notice from any Governmental Entity or other person advising that TranS1 or its Subsidiaries that it is or is potentially responsible for response, assessment, investigation, abatement, or remediation costs under any Environmental Law with respect to a release or threatened release of any Hazardous Substances;
(ix) neither TranS1 nor any of its Subsidiaries has received or is subject to any judgments, orders, decrees, injunctions or other binding arrangements with any Governmental Entity or is subject to any indemnity or other agreement with any third party relating to liability under any Environmental Law or relating to Hazardous Substances; and
(x) to the actual knowledge of TranS1 and any of its Subsidiaries, there are no circumstances or conditions involving TranS1, any of its Subsidiaries or any of their respective currently owned or leased real properties that could reasonably be expected to result in any claims, liabilities, obligations, investigations, costs or restrictions on the ownership, use or transfer of any such real property of TranS1 or any of its Subsidiaries pursuant to any Environmental Law.
(b) Except as set forth in Section 4.13(b) of the TranS1 Disclosure Schedule, there are no aboveground or underground storage tank systems, including pumps and lines, on the currently owned or leased real property for the storage of Hazardous Substances. Each of the tanks and related equipment and apparatus disclosed on Section 4.13(b) of the TranS1 Disclosure Schedule has been upgraded and if required, registered, to meet all applicable requirements under Environmental Laws.
(c) TranS1 has delivered to TranS1 true and complete copies and results of any reports, studies, sampling, tests, environmental site assessments or other assessments possessed by or readily available to TranS1 pertaining to the environmental or physical condition of any real property (and any buildings, structures, or other improvements thereon) presently or previously owned, leased or used by TranS1 or any of its Subsidiaries.
| A-58 |
4.14 Employee Benefit Plans.
(a) Section 4.14(a) of the TranS1 Disclosure Schedule sets forth a complete and accurate list, as of the date of this Agreement, of all Employee Benefit Plans maintained, or contributed to, by TranS1 or any of its Subsidiaries or any of their respective ERISA Affiliates (collectively, the “TranS1 Employee Plans”).
(b) Each TranS1 Employee Plan has been administered in all material respects in accordance with ERISA, the Code and all other applicable laws and the regulations thereunder and in accordance with its terms, and each of TranS1 and its Subsidiaries and their respective ERISA Affiliates has in all material respects met its obligations with respect to such TranS1 Employee Plan and has made all required contributions thereto (or reserved such contributions on the TranS1 Balance Sheet). TranS1 and its Subsidiaries and each of their respective ERISA Affiliates and each TranS1 Employee Plan are in compliance in all material respects with the currently applicable provisions of ERISA and the Code and the regulations thereunder (including, but not limited to, Section 4980B-4980E of the Code, Subtitle K, Chapter 100 of the Code and Sections 601 through 608 and Section 701 et seq. of ERISA). All filings and reports as to each TranS1 Employee Plan required to have been submitted to the IRS or to the United States Department of Labor have been timely submitted. With respect to TranS1 Employee Plans, no event has occurred, and to the knowledge of TranS1, there exists no condition or set of circumstances in connection with which TranS1 or any of its Subsidiaries or ERISA Affiliates could be subject to any liability that would reasonably be expected, individually or in the aggregate, to have a TranS1 Material Adverse Effect under ERISA, the Code or any other applicable law.
(c) With respect to TranS1 Employee Plans, there are no benefit obligations for which contributions have not been made or properly accrued and there are no benefit obligations that have not been accounted for by reserves, or otherwise properly footnoted in accordance with GAAP, on the financial statements of TranS1, which obligations would reasonably be expected, individually or in the aggregate, to have a TranS1 Material Adverse Effect. The assets of each TranS1 Employee Plan that is funded are reported at their fair market value on the books and records of such TranS1 Employee Plan.
(d) All TranS1 Employee Plans that are intended to be qualified under Section 401(a) of the Code have received determination or opinion letters from the IRS to the effect that such TranS1 Employee Plans are qualified and the plans and trusts related thereto are exempt from federal income taxes under Sections 401(a) and 501(a), respectively, of the Code, no such determination or opinion letter has been revoked and revocation has notbeen threatened, and no suchEmployee Benefit Planhas been amended or operated since the date of its most recent determination letter or application therefor in any respect, and no act or omission has occurred, that would adversely affect its qualification or materially increase its cost. To the knowledge ofTranS1, no “prohibited transaction” (within the meaning of Section 4975 of theCodeor Sections 406 and 408 ofERISA) has occurred with respect to any suchEmployee Benefit Plans. EachTranS1 Employee Planthat is required to satisfy Section 401(k)(3) or Section 401(m)(2) of theCodehas been tested for compliance with, and satisfies the requirements of, Section 401(k)(3) and Section 401(m)(2) of theCode, as the case may be, for each plan year ending prior to theClosing Date.
| A-59 |
(e) Neither TranS1 nor any of its Subsidiaries nor any of their respective ERISA Affiliates has (i) ever maintained a TranS1 Employee Benefit Plan that was ever subject to Section 412 of the Code or Title IV of ERISA or (ii) ever been obligated to contribute to a “multiemployer plan” (as defined in Section 4001(a)(3) of ERISA). No TranS1 Employee Plan is funded by, associated with or related to a “voluntary employees’ beneficiary association” within the meaning of Section 501(c)(9) of the Code. No TranS1 Employee Plan holds securities issued by TranS1 or any of its Subsidiaries or any of their respective ERISA Affiliates.
(f) Each TranS1 Employee Plan is amendable and terminable unilaterally by TranS1 and any of TranS1’s Subsidiaries that are a party thereto or covered thereby at any time without additional vesting or acceleration of benefits or any other liability to TranS1 or any of its Subsidiaries as a result thereof (other than for benefits accrued through the date of termination or amendment and reasonable administrative expenses related thereto), and no TranS1 Employee Plan, plan documentation or agreement, summary plan description or other written communication distributed generally to employees by its terms prohibits TranS1 or any of its Subsidiaries from amending or terminating any such TranS1 Employee Plan.
(g) Except as disclosed in the exhibit index to any TranS1 Recent SEC Document, neither TranS1 nor any of its Subsidiaries is a party to any oral or written (i) agreement with any stockholders, director, executive officer or other employee of TranS1 or any of its Subsidiaries (A) the benefits of which are contingent, or the terms of which are materially altered, upon the occurrence of a transaction involving TranS1 or any of its Subsidiaries of the nature of any of the transactions contemplated by this Agreement, (B) providing any term of employment or compensation guarantee or (C) providing severance benefits or other benefits after the termination of employment of such director, executive officer or employee; (ii) agreement, plan or arrangement under which any person may receive payments from TranS1 or any of its Subsidiaries that may be subject to the tax imposed by Section 4999 of the Code or included in the determination of such person’s “parachute payment” under Section 280G of the Code, without regard to Section 280G(b)(4); or (iii) agreement or plan binding TranS1 or any of its Subsidiaries, including any stock option plan, stock appreciation right plan, restricted stock plan, stock purchase plan or severance benefit plan, any of the benefits of which shall be increased, or the vesting of the benefits of which shall be accelerated, by the occurrence of any of the transactions contemplated by this Agreement or the value of any of the benefits of which shall be calculated on the basis of any of the transactions contemplated by this Agreement.
(h) None of the TranS1 Employee Plans promises or provides retiree medical or other retiree welfare benefits to any person, except as required by applicable law.
(i) Each TranS1 Employee Plan that is a “nonqualified deferred compensation plan” (as defined in Code Section 409A(d)(1)) has been operated since January 1, 2005 in good faith compliance with Code Section 409A and IRS Notice 2005-1. No TranS1 Employee Plan that is a “nonqualified deferred compensation plan” has been materially modified (as determined under Notice 2005-1) after October 3, 2004. No event has occurred that would be treated by Code Section 409A(b) as a transfer of property for purposes of Code Section 83. No stock option or equity unit option granted under any TranS1 Employee Plan has an exercise price that has been or may be less than the fair market value of the underlying stock or equity units (as the case may be) as of the date such option was granted or has any feature for the deferral of compensation other than the deferral of recognition of income until the later of exercise or disposition of such option.
| A-60 |
4.15 Compliance With Laws. TranS1 and each of its Subsidiaries has materially complied with, is not in material violation of, and has not received any notice from any Governmental Entity alleging any material violation with respect to, any applicable provisions of any statute, law or regulation with respect to the conduct of its business, or the ownership or operation of its properties or assets.
4.16 Permits and Regulatory Matters.
(a) TranS1 and each of its Subsidiaries have all permits, licenses, registrations, authorizations and franchises from Governmental Entities required to conduct their businesses as currently conducted or as currently proposed to be conducted, including without limitation all such permits, licenses, registrations, authorizations and franchises required by the FDA or any other Governmental Entity exercising comparable authority, except for such permits, licenses, registrations, authorizations and franchises the lack of which, individually or in the aggregate, has not had, and is not reasonably expected to have, a TranS1 Material Adverse Effect (the “TranS1 Authorizations”). TranS1 and its Subsidiaries are in compliance with the terms of the TranS1 Authorizations, except where the failure to so comply, individually or in the aggregate, has not had, and is not reasonably expected to have, a TranS1 Material Adverse Effect. No TranS1 Authorization will cease to be effective as a result of the consummation of the transactions contemplated by this Agreement.
(b) All manufacturing, processing, distribution, labeling, storage, testing, sale or marketing of products performed by or on behalf of TranS1 or any of its Subsidiaries are in compliance with all applicable laws, rules, regulations or orders administered or issued by the FDA or any other Governmental Entity exercising comparable authority, except where the failure to so comply, individually or in the aggregate, has not had, and is not reasonably expected to have, a TranS1 Material Adverse Effect. Neither TranS1 nor any of its Subsidiaries has received any notices or correspondence from the FDA or any other Governmental Entity exercising comparable authority, and to the knowledge of TranS1 there is no action or proceeding pending or threatened (including any prosecution, injunction, seizure, civil fine, suspension or recall), in each case alleging that TranS1 or any of its Subsidiaries is not currently in compliance with any and all applicable laws, regulations or orders implemented by the FDA or any other Governmental Entity exercising comparable authority.
(c) There are no seizures, recalls, market withdrawals, field notifications or corrective actions, notifications of misbranding, destruction orders, safety alerts or similar actions relating to the safety or efficacy of any products marketed or sold by TranS1 or any of its Subsidiaries being conducted, requested in writing or, to the knowledge of TranS1, threatened by the FDA or any other Governmental Entity exercising comparable authority. TranS1 has not, either voluntarily or involuntarily, initiated, conducted or issued or caused to be initiated, conducted or issued any recall, market withdrawal or other similar action by a Governmental Entity
| A-61 |
(d) The studies, tests and preclinical and clinical trials conducted by or on behalf of TranS1 or any of its Subsidiaries were and, if still pending, are being conducted in all material respects in accordance with experimental protocols, procedures and controls pursuant to, where applicable, accepted professional and scientific standards; and neither TranS1 nor any of its Subsidiaries has received any notices or correspondence from the FDA or any other Governmental Entity exercising comparable authority requiring the termination, suspension or material modification of any studies, tests or preclinical or clinical trials conducted by or on behalf of TranS1 or any of its Subsidiaries.
4.17 Employees.
(a) Substantially all current or past key employees of TranS1 or any of its Subsidiaries have entered into confidentiality and assignment of inventions agreements with TranS1 or such Subsidiary, a copy or form of which has previously been provided or made available to Baxano. To the knowledge of TranS1, no employee of TranS1 or any Subsidiary of TranS1 is in violation of any term of any patent disclosure agreement, non-competition agreement, or any restrictive covenant to a former employer relating to the right of any such employee to be employed by TranS1 or any of its Subsidiaries because of the nature of the business currently conducted or currently proposed to be conducted by TranS1 or any of its Subsidiaries or to the use of trade secrets or proprietary information of others, the consequences of which, individually or in the aggregate, are reasonably expected to have a TranS1 Material Adverse Effect. To the knowledge of TranS1, as of the date of this Agreement, no key employee or group of employees has any plans to terminate employment with TranS1 or its Subsidiaries.
(b) Neither TranS1 nor any of its Subsidiaries is a party to or otherwise bound by any collective bargaining agreement, contract or other agreement or understanding with a labor union or labor organization. Neither TranS1 nor any of its Subsidiaries is the subject of any proceeding asserting that TranS1 or any of its Subsidiaries has committed an unfair labor practice or is seeking to compel it to bargain with any labor union or labor organization that, individually or in the aggregate, is reasonably expected to have a TranS1 Material Adverse Effect, nor is there pending or, to the knowledge of TranS1, threatened, any labor strike, dispute, walkout, work stoppage, slow-down or lockout involving TranS1 or any of its Subsidiaries.
(c) TranS1 has made available to Baxano forms of each severance agreement in effect between TranS1 or its Subsidiaries and any employee of TranS1 or its Subsidiaries.
4.18 Insurance. Section 4.18 of the TranS1 Disclosure Schedule sets forth a complete and accurate list as of the date of this Agreement of all insurance policies maintained by TranS1 or any of its Subsidiaries (the “TranS1 Insurance Policies”). EachTranS1 Insurance Policyis in full force and effect as of the date of thisAgreement. As of the date of thisAgreement, there is no material claim by theTranS1or any of itsSubsidiariespending under anyTranS1 Insurance Policyas to which coverage has been questioned, denied or disputed by the underwriters of such policy.
| A-62 |
4.19 Brokers; Fees and Expenses. No agent, broker, investment banker, financial advisor or other firm or person is or will be entitled, as a result of any action, agreement or commitment of TranS1 or any of its Affiliates, to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with any of the transactions contemplated by this Agreement, except Stifel, whose fees and expenses shall be paid by TranS1. TranS1 has provided or made available to Baxano a complete and accurate copy of all agreements pursuant to which Stifelis entitled to any fees and expenses in connection with any of the transactions contemplated by thisAgreement.
4.20 Operations of Transitory Subsidiary. Transitory Subsidiary was formed solely for the purpose of engaging in the transactions contemplated by this Agreement, has engaged in no other business activities and has conducted its operations only as contemplated by this Agreement.
4.21 Controls and Procedures, Certifications and Other Matters Relating to the Sarbanes Act.
(a) TranS1 and each of its Subsidiaries maintains accurate books and records reflecting its assets and liabilities and maintains proper and adequate internal control over financial reporting that provide reasonable assurance that (i) transactions are executed with management’s authorization, (ii) transactions are recorded as necessary to permit preparation of the consolidated financial statements of TranS1 and to maintain accountability for TranS1’s consolidated assets, (iii) access to assets of TranS1 and its Subsidiaries is permitted only in accordance with management’s authorization, (iv) the reporting of assets of TranS1 and its Subsidiaries is compared with existing assets at regular intervals and (v) accounts, notes and other receivables and inventory were recorded accurately, and proper and adequate procedures are implemented to effect the collection thereof on a current and timely basis.
(b) TranS1 maintains disclosure controls and procedures required by Rules 13a-15 or 15d-15 under the Exchange Act, and such controls and procedures are effective to ensure that all material information concerning TranS1 and its Subsidiaries is made known on a timely basis to the individuals responsible for the preparation of TranS1’s filings with the SEC and other public disclosure documents.
(c) Neither TranS1 nor any of its officers has received notice from any Governmental Entity questioning or challenging the accuracy, completeness or manner of filing or submission of any filing with the SEC, including without limitation any certifications required by Section 906 of the Sarbanes Act.
(d) Neither TranS1 nor any of its Subsidiaries has, since TranS1 became subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act, extended or maintained credit, arranged for the extension of credit, modified or renewed an extension of credit, in the form of a personal loan or otherwise, to or for any director or executive officer of TranS1. Section 4.21 of the TranS1 Disclosure Schedule identifies any loan or extension of credit maintained by TranS1 to which the second sentence of Section 13(k)(1) of the Exchange Act applies.
| A-63 |
4.22 Commercial Relationships. During the past 12 months from the date of this Agreement, none of TranS1’s or any of its Subsidiaries’ material suppliers, customers, collaborators, distributors, agents, licensors or licensees has canceled or otherwise terminated its relationship with TranS1 or any of its Subsidiaries or has materially altered its relationship with TranS1 or any of its Subsidiaries. To the knowledge of TranS1, no such person has any plan or intention, and neither TranS1 nor any of its Subsidiaries has received any written notice from any such person, to terminate, cancel or otherwise materially modify its relationship with TranS1 or any of its Subsidiaries. As of the date of this Agreement, neither TranS1 nor any of its Subsidiaries has received any written notice (formal or informal) or other communication from any of TranS1’s top ten largest customers (based on fiscal 2012 consolidated total revenues) or top ten largest suppliers (based on fiscal 2012 expenditures) that indicates or could reasonably be expected to indicate that any such customer or supplier has any plan or intention not to renew its agreement with TranS1 on terms substantially comparable to its current agreement with TranS1.
4.23 Tangible Assets. TranS1 and its Subsidiaries own or lease all machinery, equipment and other tangible assets necessary for the conduct of their business as presently conducted. Each such tangible asset has been maintained in accordance with normal industry practice, is in good operating condition and repair (subject to normal wear and tear) and is suitable for the purposes for which it presently is used.
4.24 False Claims Act Matter. The aggregate amount of any fines, penalties or other payments by TranS1 to any Governmental Entity in connection with the FCA Matter shall not exceed $6,000,000 (exclusive of additional interest at 1.5% per annum, plus attorney’s fees to thequi tamrelator, which will not exceed $120,000). There are no other claims, actions or proceedings pending, or to TranS1’s knowledge, threatened against TranS1 or its officers, directors, employees, stockholder or agents related to the events giving rise to the FCA Matter except as will be released in the final settlement with the Department of Justice (on behalf of the Office of the Inspector General of the Department of Health and Human Services, the TRICARE Management Activity, the United States Office of Personnel Management, the United States Department of Veteran Affairs, and the Office of Workers’ Compensation Programs of the United States Department of Labor) regarding the FCA Matter. The FCA Matter will not give rise to any exclusion or debarment of TranS1 from participation in any programs funded by the United States government or any state government, the debarment of TranS1 from contracting with any federal or state agency, or to any criminal proceedings against TranS1 or its officer, directors, employees, stockholders or agents.
ARTICLE V
CONDUCT OF BUSINESS
5.1 Covenants of Baxano. Except as disclosed in Section 5.1 of the Baxano Disclosure Schedule hereto or as expressly provided herein or as consented to in writing by TranS1, from and after the date of this Agreement until the earlier of the termination of this Agreement in accordance with its terms and the Effective Time, Baxano shall act and carry on its business in the usual, regular and ordinary course in substantially the same manner as previously conducted, pay its debts and Taxes and perform its other obligations when due (subject to good faith disputes over such debts, Taxes or obligations), comply with applicable laws, rules and regulations, and use commercially reasonable efforts, consistent with past practices, to maintain and preserve in the ordinary course its business organization, assets and properties. Without limiting the generality of the foregoing, from and after the date of this Agreement until the earlier of the termination of this Agreement in accordance with its terms and the Effective Time, Baxano shall not, directly or indirectly, do any of the following without the prior written consent of TranS1:
| A-64 |
(a) (i) declare, set aside or pay any dividends on, or make any other distributions (whether in cash, securities or other property) in respect of, any of its capital stock; (ii) split, combine or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock or any of its other securities; or (iii) purchase, redeem or otherwise acquire any shares of its capital stock or any other of its securities or any rights, warrants or options to acquire any such shares or other securities, other than, in the case of this clause (iii), from former employees, directors and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of services to Baxano for a repurchase price of the lesser of cost and the then current fair market value;
(b) except as permitted by Section 5.1(n), issue, deliver, sell, grant, pledge or otherwise dispose of or encumber any shares of its capital stock, any other voting securities or any securities convertible into or exchangeable for, or any rights, warrants or options to acquire, any such shares, voting securities or convertible or exchangeable securities (other than the issuance of (i) additional Baxano Notes for cash in an amount equal to the face amount of such Baxano Notes, and (ii) shares of Baxano capital stock upon the exercise of Baxano Stock Options or Baxano Warrants outstanding on the date of this Agreement in accordance with their present terms (including cashless exercises));
(c) amend its certificate of incorporation, bylaws or other comparable charter or organizational documents, except as expressly provided by this Agreement;
(d) except for purchases of inventory in the Ordinary Course of Business, acquire (i) by merging or consolidating with, or by purchasing all or a substantial portion of the assets or any stock of, or by any other manner, any business or any corporation, partnership, joint venture, limited liability company, association or other business organization or division thereof or (ii) any assets that are material, in the aggregate, to Baxano;
(e) except in the Ordinary Course of Business, sell, lease, license, pledge, or otherwise dispose of or encumber any properties or assets of Baxano;
(f) whether or not in the Ordinary Course of Business, sell, dispose of or otherwise transfer any assets material to Baxano (including any accounts, leases, contracts or intellectual property, but excluding the sale or license of products and inventory in the Ordinary Course of Business);
(g) adopt or implement any stockholder rights plan;
(h) enter into an agreement with respect to any merger, consolidation, liquidation or business combination, or any acquisition or disposition of all or substantially all of the assets or securities of Baxano;
| A-65 |
(i) (i) incur or suffer to exist any Indebtedness (other than with respect to (A) the Oxford/SVB Loan Documents or (B) the Note and Warrant Purchase Agreement dated as of March 7, 2012 among Baxano and the investors set forth therein), (ii) issue, sell or amend any debt securities or warrants or other rights to acquire any debt securities ofBaxano(other than as described in Section6.13), guarantee any debt securities of anotherperson, enter into any “keep well” or otheragreementto maintain any financial statement condition of anotherpersonor enter into any arrangement having the economic effect of any of the foregoing, (iii) make any loans, advances (other than routine advances to employees ofBaxanoin theOrdinary Course of Business) or capital contributions to, or investment in, any otherperson or(iv) enter into any hedgingagreementor other financialagreementor arrangement designed to protectBaxanoagainst fluctuations in commodities prices or exchange rates;
(j) unless provided for in the Baxano 2013 Operating Plan, a copy of which has previously been provided or made available to TranS1, make any capital expenditures or other expenditures with respect to property, plant or equipment;
(k) make any changes in accounting methods, principles or practices, except insofar as may have been required by the SEC or a change in GAAP or, except as so required, change any assumption underlying, or method of calculating, any bad debt, contingency or other reserve;
(l) modify, amend or terminate any material contract or agreement to which Baxano is party, or knowingly waive, release or assign any material rights or claims (including any write-off or other compromise of any accounts receivable of Baxano), except in the Ordinary Course of Business or, to the extent subject to reserves reflected on the Baxano Balance Sheet, in accordance with GAAP;
(m) (i) except in the Ordinary Course of Business, enter into any material contract or agreement relating to the rendering of services or the distribution, sale or marketing by third parties of the products of, or products licensed by, Baxano or (ii) license any material intellectual property rights to or from any third party;
(n) except for the Transaction Payment Plan or as required to comply with applicable law or agreements, plans or arrangements existing on the date hereof, (i) take any action with respect to, adopt, enter into, terminate or amend any employment, severance or similar agreement or benefit plan for the benefit or welfare of any current or former director, officer, employee or consultant or any collective bargaining agreement, (ii) increase in any material respect the compensation or fringe benefits of, or pay any bonus to, any director, officer, employee or consultant (except for annual increases of the salaries of non-officer employees in the Ordinary Course of Business), (iii) amend or accelerate the payment, right to payment or vesting of any compensation or benefits, including any outstanding options or restricted stock awards, (iv) pay any material benefit not provided for as of the date of this Agreement under any benefit plan, (v) grant any awards under any bonus, incentive, performance or other compensation plan or arrangement or benefit plan (including the grant of stock options, stock appreciation rights, stock based or stock related awards, performance units or restricted stock, or the removal of existing restrictions in any benefit plans or agreements or awards made thereunder) or (vi) take any action other than in theOrdinary Course of Businessto fund or in any other way secure the payment of compensation or benefits under any employee plan,agreement, contract or arrangement or benefit plan;
| A-66 |
(o) make or rescind any material Tax election, settle or compromise any material Tax liability, amend any Tax return except as required by applicable law, change any accounting method, enter into any closing agreement regarding Taxes or consent to an extension or waiver of the limitation period applicable to any Tax claim;
(p) terminate the employment of any officer or key employee;
(q) initiate, compromise or settle any material litigation or arbitration proceeding;
(r) open or close any facility or office;
(s) fail to use commercially reasonable efforts to maintain insurance at levels substantially comparable to levels existing as of the date of this Agreement;
(t) fail to pay accounts payable and other obligations in the Ordinary Course of Business;
(u) fail to maintain inventory levels in the sales channel to ensure product availability to meet expected patient demand consistent with past practices;
(v) fail to take any and all actions necessary to maintain and preserve all Baxano rights in and to Baxano Intellectual Property and Baxano Third Party Intellectual Property, including the filing of necessary documents with the appropriate Governmental Entities, or
(w) authorize any of, or commit or agree, in writing or otherwise, to take any of, the foregoing actions or any action that would make any representation or warranty of Baxano in this Agreement untrue or incorrect in any material respect, or would materially impair or prevent the satisfaction of any conditions in ARTICLE VII hereof.
5.2 Covenants of TranS1. Except as disclosed in Section 5.2 of the TranS1 Disclosure Schedule or as expressly provided herein or as consented to in writing by Baxano, from and after the date of this Agreement until the earlier of the termination of this Agreement in accordance with its terms and the Effective Time, TranS1 shall, and shall cause each of its Subsidiaries to, act and carry on its business in the usual, regular and ordinary course in substantially the same manner as previously conducted, pay its debts and Taxes and perform its other obligations when due (subject to good faith disputes over such debts, Taxes or obligations), comply with applicable laws, rules and regulations, and use commercially reasonable efforts, consistent with past practices, to maintain and preserve in the ordinary course its and each of its Subsidiaries’ business organization, assets and properties. Without limiting the generality of the foregoing, from and after the date of this Agreement until the earlier of the termination of this Agreement in accordance with its terms and the Effective Time, TranS1 shall not, and shall not permit any of its Subsidiaries to, directly or indirectly, do any of the following without the prior written consent of Baxano:
| A-67 |
(a) (i) declare, set aside or pay any dividends on, or make any other distributions (whether in cash, securities or other property) in respect of, any of its capital stock (other than dividends and distributions by a direct or indirect wholly owned Subsidiary of TranS1 to its parent); (ii) split, combine or reclassify any of its capital stock or issue or authorize the issuance of any other securities in respect of, in lieu of or in substitution for shares of its capital stock or any of its other securities; or (iii) purchase, redeem or otherwise acquire any shares of its capital stock or any other of its securities or any rights, warrants or options to acquire any such shares or other securities, other than, in the case of this clause (iii), from former employees, directors and consultants in accordance with agreements providing for the repurchase of shares in connection with any termination of services to TranS1 or any of its Subsidiaries for a repurchase price of the lesser of cost and the then current fair market value;
(b) except as permitted by Section 5.2(m) and as contemplated by the Securities Purchase Agreement, issue, deliver, sell, grant, pledge or otherwise dispose of or encumber any shares of its capital stock, any other voting securities or any securities convertible into or exchangeable for, or any rights, warrants or options to acquire,any suchshares, voting securities or convertible or exchangeable securities (other than the issuance ofsharesofTranS1 Common Stockupon the exercise ofTranS1 Stock Optionsoutstanding on the date of thisAgreementin accordance with their present terms (includingcashless exercises) orTranS1 Stock Optionsgranted as contemplated by Section5.2(m));
(c) amend its certificate of incorporation, bylaws or other comparable charter or organizational documents, except to the extent necessary to carry into effect the provisions of Section 6.10 or as otherwise expressly provided by this Agreement;
(d) except for purchases of inventory in the Ordinary Course of Business, acquire (i) by merging or consolidating with, or by purchasing all or a substantial portion of the assets or any stock of, or by any other manner, any business or any corporation, partnership, joint venture, limited liability company, association or other business organization or division thereof or (ii) any assets that are material, in the aggregate, to TranS1 and its Subsidiaries, taken as a whole;
(e) except in the Ordinary Course of Business, sell, lease, license, pledge, or otherwise dispose of or encumber any properties or assets of TranS1 or of any of its Subsidiaries;
(f) whether or not in the Ordinary Course of Business, sell, dispose of or otherwise transfer any assets material to TranS1 and its Subsidiaries, taken as a whole (including any accounts, leases, contracts or intellectual property or any assets or the stock of any of its Subsidiaries, but excluding the sale or license of products and inventory in the Ordinary Course of Business);
(g) adopt or implement any stockholder rights plan;
(h) enter into an agreement with respect to any merger, consolidation, liquidation or business combination, or any acquisition or disposition of all or substantially all of the assets or securities of TranS1 or any of its Subsidiaries;
| A-68 |
(i) (i) incur or suffer to exist any indebtedness for borrowed money or guarantee any suchindebtednessof anotherperson, (ii) issue, sell or amend any debt securities or warrants or other rights to acquire any debt securities ofTranS1or any of itsSubsidiaries, guarantee any debt securities of anotherperson, enter into any “keep well” or otheragreementto maintain any financial statement condition of anotherpersonor enter into any arrangement having the economic effect of any of the foregoing, (iii) make any loans, advances (other than routine advances to employees ofTranS1in theOrdinary Course of Business) or capital contributions to, or investment in, any otherperson, other thanTranS1or any of its direct or indirect wholly ownedSubsidiariesor (iv) enter into any hedgingagreementor other financialagreementor arrangement designed to protectTranS1or itsSubsidiariesagainst fluctuations in commodities prices or exchange rates;
(j) make any changes in accounting methods, principles or practices, except insofar as may have been required by the SEC or a change in GAAP or, except as so required, change any assumption underlying, or method of calculating, any bad debt, contingency or other reserve;
(k) modify, amend or terminate any material contract or agreement to which TranS1 or any of its Subsidiaries is party, or knowingly waive, release or assign any material rights or claims (including any write-off or other compromise of any accounts receivable of TranS1 or any of its Subsidiaries), except in the Ordinary Course of Business or, to the extent subject to reserves reflected on the TranS1 Balance Sheet, in accordance with GAAP;
(l) (i) except in the Ordinary Course of Business, enter into any material contract or agreement relating to the rendering of services or the distribution, sale or marketingby third parties of the products of, or products licensed by,TranS1or any of itsSubsidiariesor (ii) license any materialintellectual propertyrights to or from any third party;
(m) except as required to comply with applicable law or agreements, plans or arrangements existing on the date hereof, (i) amend or accelerate the payment, right to payment or vesting of any compensation or benefits, including any outstanding TranS1 Stock Options or restricted stock awards or (ii) pay any material benefit not provided for as of the date of this Agreement under any benefit plan;
(n) make or rescind any material Tax election, settle or compromise any material Tax liability, amend any Tax return except as required by applicable law, change any accounting method, enter into any closing agreement regarding Taxes or consent to an extension or waiver of the limitation period applicable to any Tax claim;
(o) commence any offering of shares of TranS1 Common Stock pursuant to any employee stock purchase plan, permit any employee to enroll in any employee stock purchase plan or allow any participant in an employee stock purchase plan to increase the current level of such participant’s payroll deductions thereunder;
(p) fail to use commercially reasonable efforts to maintain insurance at levels substantially comparable to levels existing as of the date of this Agreement;
| A-69 |
(q) fail to pay accounts payable and other obligations in the Ordinary Course of Business;
(r) fail to maintain inventory levels in the sales channel to ensure product availability to meet expected patient demand consistent with past practices;
(s) terminate the employment of the current Chief Executive Officer or Chief Financial Officer of TranS1;
(t) fail to take any and all actions necessary to maintain and preserve all TranS1 rights in and to the TranS1 Intellectual Property and TranS1 Third Party Intellectual Property, including the filing of necessary documents with the appropriate Governmental Entities, or
(u) authorize any of, or commit or agree, in writing or otherwise, to take any of, the foregoing actions or any action that would make any representation or warranty of TranS1 in this Agreement untrue or incorrect in any material respect, or would materially impair or prevent the satisfaction of any conditions in ARTICLE VII hereof.
5.3 Confidentiality. The parties acknowledge that TranS1 and Baxano have previously executed a confidentiality agreement, dated as of September 28, 2012 (the“Confidentiality Agreement”), whichConfidentiality Agreementshall continue in full force and effect in accordance with its terms, except as expressly modified by thisAgreement.
ARTICLE VI
ADDITIONAL AGREEMENTS
6.1 No Solicitation
(a) TranS1 and Baxano shall, and shall cause its Affiliates and their respective directors, officers, employees, investment bankers, financial advisors, attorneys, accountants, agents, consultants and other representatives (collectively, “Representatives”) to immediately cease and cause to be terminated any discussions or negotiations that commenced prior to the date of this Agreement with respect to any inquiry, proposal or offer from any Person (other than the parties hereto) relating to (i) a merger, consolidation liquidation, recapitalization, share exchange or other business combination transaction involving TranS1 or Baxano, respectively, (ii) the issuance or acquisition of shares of capital stock or other equity securities of TranS1 or Baxano, respectively other than as permitted by Section 5.1(b) or 5.2(b), as applicable, or (iii) the sale, lease, license, exchange, or other disposition of any material portion of TranS1’s or Baxano’s properties or assets, respectively other than in the ordinary course (an “Acquisition Proposal”).
(b) Neither the TranS1 Board nor the Baxano Board shall (i) withhold, withdraw or modify, in a manner adverse to the other party hereto, the approval or recommendation by the TranS1 Board or Baxano Board, respectively, with respect to this Agreement, the Merger and the transactions contemplated hereby and thereby, (ii) cause or permit TranS1 or Baxano, respectively, to enter into any letter of intent, memorandum of understanding, agreement in principle, acquisition agreement, merger agreement or similar agreement (whether written or oral) providing for the consummation of a transaction contemplated by any Acquisition Proposal or (iii) adopt, approve or recommend any Acquisition Proposal.
| A-70 |
(c) From the date of this Agreement until the Effective Time or, if earlier, the termination of this Agreement in accordance with its terms, neither TranS1 nor Baxano shall, nor shall either of them authorize or permit any Affiliate or Representative to, directly or indirectly, (i) solicit, initiate, facilitate or knowingly encourage the submission of any Acquisition Proposal, (ii) enter into any agreement, agreement-in-principle or letter of intent providing for or accept any Acquisition Proposal, or (iii) participate or engage in any discussions or negotiations regarding, or furnish to any Person any non-public information for the purpose of encouraging or facilitating, any Acquisition Proposal.
(d) In addition to the other obligations under this Section 6.1, TranS1 and Baxano shall promptly (and in any event within one (1) business day after receipt thereof by TranS1 or Baxano or its Representatives) advise the other party orally and in writing of any Acquisition Proposal, any request for information with respect to any Acquisition Proposal, or any inquiry with respect to or which could reasonably be expected to result in an Acquisition Proposal, the material terms and conditions of such request, Acquisition Proposal or inquiry, and the identity of the Person making the same.
(e) TranS1 and Baxano agree that the rights and remedies for noncompliance with this Section 6.1 shall include having such provision specifically enforced by any court having equity jurisdiction, it being acknowledged and agreed that any such breach or threatened breach would cause irreparable injury to the other party and that money damages would not provide an adequate remedy to the other party.
6.2 Securities Matters.
(a) The parties acknowledge and agree that the Merger Shares will not initially be registered under the Securities Act or the securities laws of any other jurisdiction, and the offer and sale of the Merger Shares is being made in reliance on one or more exemptions for private offerings under Section 4(2) of the Securities Act and other applicable securities Laws. Accordingly, no sale, transfer or other disposition (whether with or without consideration and whether voluntarily or involuntarily or by operation of Law) (“Transfer”) of any of the Merger Shares is permitted, unless such Transfer is registered under the Securities Act and other applicable securities Laws, or an exemption from such registration is available or such registration is otherwise not required. The parties further acknowledge and agree that the Merger Shares constitute “restricted securities” as such term is defined in Rule 144 under the Securities Act.
(b) The parties acknowledge and agree that the Securities Purchase Agreement sets forth additional terms and conditions governing registration of the Merger Shares and the Financing Shares (together, the “Shares”), Transfer restrictions with respect to the Shares, and TranS1’s obligations to facilitate the sale of the Shares pursuant to Rule 144 under the Securities Act. In the event of any conflict between the Securities Purchase Agreement and this Agreement, the Securities Purchase Agreement shall control.
| A-71 |
(c) For purposes of Rule 144(d), the parties intend for the holding period of all of the Merger Shares (including any Merger Shares included in the Escrow Shares), to the extent permitted by applicable law (including applicable interpretations by the SEC), to commence on the Closing Date.
(d) The parties agree that the book-entry notation representing the Merger Shares shall contain legends substantially in the form of the following, as well as any additional legends that may be required by applicable law or as TranS1 may reasonably deem necessary or appropriate from time to time for all shares of TranS1 Common Stock then outstanding (and a stop transfer order may be placed against the transfer of the Merger Shares); provided however, that only Escrow Shares shall bear the first legend identified below:
THESE SECURITIES ARE SUBJECT TO AN ESCROW AGREEMENT WITH THE ISSUER AND THE ESCROW AGENT NAMED THEREIN (THE “ESCROW AGREEMENT”), A COPY OF WHICH IS ON FILE AT THE PRINCIPAL OFFICES OF THE ISSUER AND WHICH, AMONG OTHER MATTERS, PLACES RESTRICTIONS ON THE DISPOSITION OF THE SECURITIES. THESE SECURITIES WILL BE DEPOSITED WITH THE ESCROW AGENT PURSUANT TO THE ESCROW AGREEMENT AND MAY NOT BE OFFERED, EXCHANGED, TRANSFERRED, SOLD, ASSIGNED, PLEDGED, PARTICIPATED, HYPOTHECATED OR OTHERWISE DISPOSED OF FOR SO LONG AS THEY ARE SUBJECT TO THE ESCROW AGREEMENT.
THE SHARES EVIDENCED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”) OR THE SECURITIES LAWS OF ANY STATE OR OTHER JURISDICTION. THE SHARES MAY NOT BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED EXCEPT (1) PURSUANT TO AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OR (2) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT, IN EACH CASE IN ACCORDANCE WITH ALL APPLICABLE STATE SECURITIES LAWS AND THE SECURITIES LAWS OF OTHER JURISDICTIONS, OR IN A TRANSACTION EXEMPT FROM REGISTRATION.
THESE SECURITIES ARE SUBJECT TO CERTAIN RESTRICTIONS SET FORTH IN THE SECURITIES PURCHASE AGREEMENT DATED MARCH 3, 2013 BY AND AMONG THE ISSUER AND CERTAIN OTHER PERSONS, WHICH RESTRICT THE RIGHT TO TRANSFER, SELL OR OTHERWISE DISPOSE OF THESE SECURITIES. A COPY OF SUCH SECURITIES PURCHASE AGREEMENT IS AVAILABLE FOR REVIEW BY THE RECORD HOLDER OF THESE SECURITIES AT THE PRINCIPAL OFFICES OF THE ISSUER.
| A-72 |
(e) TranS1 shall remove (or cause the Escrow Agent to remove) the first legend identified above from the book-entry notation representing any of the Merger Shares (and terminate any related stop-transfer order) upon release of the applicable portion of the Merger Shares from escrow. TranS1, upon the request of any holder of any of the Merger Shares, shall remove (or cause the Escrow Agent to remove) the second legend identified above from the book-entry notation representing any of the Merger Shares (and terminate any related stop-transfer order) if (i) such holder provides TranS1 reasonable assurances that such Merger Shares are eligible for sale, assignment or transfer under Rule 144, including proper documentation in the form of a customary representation letter reasonably sufficient to establish compliance with Rule 144, or (ii) if reasonably requested by TranS1 (provided that any such request shall be deemed to be reasonable if TranS1’s transfer agent requests such an opinion), TranS1 has received a written opinion of counsel reasonably satisfactory to TranS1 that such second legend may be removed from the book-entry notation representing such Merger Shares, or (iii) such Merger Shares have been registered under the Securities Act. TranS1 shall remove (or cause the Escrow Agent to remove) the third legend identified above from the book-entry notation representing any part of the Merger Shares (and terminate any related stop-transfer orders) immediately upon the lapse of the Transfer restrictions under the Securities Purchase Agreement with respect to such Merger Shares.
(f) TranS1 shall register the Merger Shares on the terms set forth in and in accordance with the Securities Purchase Agreement.
6.3 NASDAQ Filings. TranS1 shall file the following with respect to the Merger Shares: (a) a Notification Form: Listing of Additional Shares with NASDAQ not less than fifteen (15) days prior to the Closing Date, and (b) a Notification Form: Change in Shares Outstanding within ten (10) days following the Closing Date.
6.4 Access to Information. Each of TranS1 and Baxano shall (and shall cause each of its Subsidiaries to) afford to the other party’s officers, employees, accountants, counsel and other representatives, reasonable access, during normal business hours during the period prior to the Effective Time, to all its properties, books, contracts, commitments, personnel and records and, during such period, each of TranS1 and Baxano shall (and shall causeeach of itsSubsidiariesto) furnish promptly to the other party(a)a copy of each report, schedule,registration statementand other document filed or received by it during such period pursuant to the requirements of federal or state securities laws and(b)all other information concerning its business, properties, assets and personnel as the other party may reasonably request. Each ofTranS1andBaxanowill hold any such information which is nonpublic in confidence in accordance with theConfidentiality Agreement. No information or knowledge obtained in any investigation pursuant to this Section6.4or otherwise shall affect or be deemed to modify any representation or warranty contained in thisAgreementor the conditions to the obligations of the parties to consummate theMerger.
| A-73 |
6.5 Stockholder Approval.
(a) On or before 5:00 p.m., U.S. Eastern time, on the first business day after execution of this Agreement, Baxano shall seek the Baxano Stockholder Approval by the Written Consents to be executed and delivered by Baxano’sstockholders evidencing the adoption of this Agreement and the approval of the Merger. In connection with the Baxano Stockholder Approval, Baxano shall comply with all disclosure and other obligations to its stockholders under the DGCL and any other applicable laws. Without limiting the generality of the foregoing, Baxano agrees that its obligations under this Section 6.5(a) shall not be affected by the commencement, public proposal, public disclosure or communication to Baxano of any Acquisition Proposal. Within five business days after the date of the Baxano Stockholder Approval, Baxano shall send, pursuant to Sections 228(e) and 262(d) of the DGCL, a written notice to all of its stockholders that did not execute a Written Consent informing them that this Agreement and the Merger were adopted and approved by the stockholders of Baxano and that appraisal rights are available for their shares of Baxano capital stock pursuant to Section 262 of the DGCL, which notice shall include a copy of such Section 262 and shall otherwise be in form and substance reasonably satisfactory to TranS1. Any solicitation or similar disclosure circulated to Baxano’s stockholders shall be in form and substance reasonably satisfactory to TranS1 and, if the Baxano Stockholder Approval has not already been obtained, shall include the recommendation of the Baxano Board that Baxano’s stockholders vote in favor of adoption of this Agreement and approval of the Merger. Notwithstanding the foregoing, nothing herein shall limit a party’s right to terminate this Agreement pursuant to Section 9.1.
(b) The information to be supplied by or on behalf of Baxano for inclusion in the proxy statement (the “Proxy Statement”) to be sent to the stockholders of TranS1 in connection with the meeting of TranS1’s stockholders (the “TranS1 Meeting”) to consider the issuance of shares of TranS1 Common Stock in the Merger (such proposal is referred to herein as the “TranS1 Voting Proposal”) under NASDAQ rules and the DGCL, as applicable (the “TranS1 Stockholder Approval”), shall not, on the date the Proxy Statement is first mailed to stockholders of TranS1, or at the time of the TranS1 Meeting or at the Effective Time, contain any statement that, at such time and in light of the circumstances under which it shall be made, is false or misleading with respect to any material fact, or omit to state any material fact necessary in order to make the statements made in the Proxy Statement not false or misleading; or omit to state any material fact necessary to correct any statement in any earlier communication with respect to the solicitation of proxies for the TranS1 Meeting that has become false or misleading. If at any time prior to the Effective Time any fact or event relating to Baxano or any of its Affiliates which should be set forth in a supplement to the Proxy Statement should be discovered by Baxano or should occur, Baxano shall promptly inform TranS1 in writing of such fact or event.
(c) The information to be supplied by or on behalf of TranS1 for inclusion in the Proxy Statement to be sent to the stockholders of TranS1 in connection with the TranS1 Meeting, shall not, on the date the Proxy Statement is first mailed to stockholders of TranS1, or at the time of the TranS1 Meeting or at the Effective Time, contain any statement that, at such time and in light of the circumstances under which it shall be made, is false or misleading with respect to any material fact, or omit to state any material fact necessary in order to make the statements made in the Proxy Statement not false or misleading; or omit to state any material fact necessary to correct any statement in any earlier communication with respect to the solicitation of proxies for the TranS1 Meeting that has become false or misleading. If at any time prior to the Effective Time any fact or event relating to TranS1 or any of its Affiliates which should be set forth in a supplement to the Proxy Statement should be discovered by TranS1 or should occur, TranS1 shall promptly inform Baxano in writing of such fact or event.
| A-74 |
(d) TranS1, acting through the TranS1 Board, shall take all actions in accordance with applicable law, its Certificate of Incorporation and Bylaws and NASDAQ rules promptly and duly to call, give notice of, convene and hold as promptly as practicable, the TranS1 Meeting for the purpose of considering and voting upon the TranS1 Voting Proposal. The TranS1 Board shall unanimously recommend approval of the TranS1 Voting Proposal by the stockholders of TranS1 and include such recommendation in the Proxy Statement, and neither the TranS1 Board nor any committee thereof shall withdraw or modify, or propose or resolve to withdraw or modify in a manneradverse toBaxano, the recommendation of theTranS1 BoardthatTranS1’s stockholders vote in favor of theTranS1 Voting Proposal.TranS1shall take all action that is both reasonable and lawful to solicit from its stockholders proxies in favor of theTranS1 Voting Proposaland shall take all other action necessary or advisable to secure the vote or consent of the stockholders ofTranS1required byNASDAQrules and theDGCL, as applicable, to obtain such approval. Notwithstanding anything to the contrary contained in thisAgreement,TranS1, after consultation with and with the consent ofBaxano(not to be unreasonably withheld), may adjourn or postpone theTranS1 Meetingto the extent necessary to ensure that any required supplement or amendment to theProxy Statementis provided toTranS1’s stockholders or, if as of the time for which theTranS1 Meetingis originally scheduled (as set forth in theProxy Statement) there are insufficientsharesofTranS1 Common Stockrepresented (either inpersonor by proxy) to constitute a quorum necessary to conduct the business of theTranS1 Meeting.
6.6 Legal Conditions to Merger.
(a) Subject to the terms hereof, including Section 6.6(b), Baxano and TranS1 shall each use commercially reasonable efforts to (i) take, or cause to be taken, all actions, and do, or cause to be done, and to assist and cooperate with the other parties in doing, all things necessary, proper or advisable to consummate and make effective the transactions contemplated hereby as promptly as practicable, (ii) as promptly as practicable, obtain from any Governmental Entity or any other third party any consents, licenses, permits, waivers, approvals, authorizations, or orders required to be obtained or made by Baxano or TranS1 or any of their Subsidiaries in connection with the authorization, execution and delivery of this Agreement and the consummation of the transactions contemplated hereby, (iii) as promptly as practicable, make all necessary filings, and thereafter make any other required submissions, with respect to this Agreement and the Merger required under (A) the Securities Act and the Exchange Act, and any other applicable federal or state securities laws and (B) any other applicable law and (iv) execute or deliver any additional instruments necessary to consummate the transactions contemplated by, and to fully carry out the purposes of, this Agreement. Baxano and TranS1 shall cooperate with each other in connection with the making of all such filings, including providing copies of all such documents to the non-filing party and its advisors prior to filing and, if requested, accepting all reasonable additions, deletions or changes suggested in connection therewith. Baxano and TranS1 shall use their respective commercially reasonableefforts to furnish to each other all information required for any application or other filing to be made pursuant to the rules and regulations of any applicable law (includingall information required to be included in theProxy Statement/Prospectusand theRegistration Statement) in connection with the transactions contemplated by thisAgreement. For the avoidance of doubt,TranS1andBaxanoagree that nothing contained in this Section6.6(a)shall modify or affect their respective rights and responsibilities under Section6.6(b).
| A-75 |
(b) Baxano and TranS1 shall give (or shall cause their respective Subsidiaries to give) any notices to third parties, and use, and cause their respective Subsidiaries to use, their commercially reasonable efforts to obtain any third party consentsrelated to or required in connection with theMergerthat are (i) necessary to consummate the transactions contemplated hereby, (ii) disclosed or required to be disclosed inBaxano Disclosure ScheduleorTranS1 Disclosure Schedule, as the case may be or (iii) required to prevent the occurrence of an event that may, in the case ofBaxano, have aBaxano Material Adverse Effector, in the case ofTranS1, aTranS1 Material Adverse Effectprior to or after theEffective Time.
6.7 Public Disclosure. NeitherTranS1 nor Baxano shall issue any press release or otherwise make any public statement with respect to the Merger or this Agreement without the prior consent of the other, which shall not be unreasonably withheld, conditioned or delayed. Any press release shall be issued only in such form as shall be mutually agreed upon by TranS1 and Baxano.
6.8 Section 368(a) Reorganization. Each of TranS1, Transitory Subsidiary and Baxano shall use commercially reasonable efforts to cause the Merger to qualify, and agree not to take any action which to its knowledge could reasonably be expected to cause the merger to fail to qualify, as a reorganization within the meaning of Section 368(a) of the Code. The parties hereto hereby adopt this Agreement as a plan of reorganization. This Agreement is intended to constitute, and the parties hereto hereby adopt this Agreement as, a “plan of reorganization” within the meaning of Treasury Regulation Sections 1.368- 2(g) and 1.368-3(a). Each of TranS1, Transitory Subsidiary and Baxano shall report the merger as a reorganization within the meaning of Section 368(a) of the Code unless otherwise required pursuant to a “determination” within the meaning of Section 1313(a) of the Code.
6.9 Notification of Certain Matters. TranS1 shall give prompt notice to Baxano of the occurrence, or failure to occur, of any event, which occurrence or failure to occur would be reasonably expected to cause the non-satisfaction of a condition to Closing set forth in Section 7.1 or 7.3.Baxano shall give prompt notice to TranS1 of the occurrence, or failure to occur, of any event, which occurrence or failure to occur would be reasonably expected to cause the non-satisfaction of a condition to Closing set forth in Section 7.1 or 7.2. Notwithstanding the above, the delivery of any notice pursuant to this Section6.9will not limit or otherwise affect the remedies available hereunder to the party receiving such notice or the conditions to such party’s obligation to consummate theMerger.
6.10 Board of Directors of TranS1.
(a) Promptly after the Effective Time, TranS1 shall take all action necessary to cause (i) the number of members of the TranS1 Board to be fixed at nine (9) persons, provided that the number of members of the TranS1 Board shall be fixed at eight(8) persons upon and after the TranS1 2013 annual meeting of stockholders, (ii) one current TranS1 director to resign from the TranS1 Board to create one vacancy in addition to the vacancy created by increasing the board size to nine (9) persons, (iii) one current TranS1 director to resign from the TranS1 Board at the time of the TranS1 2013 annual meeting of stockholders to decrease the total of directors to eight (8) persons at such time, and (iv) the TranS1 Board to appoint the two (2) persons identified on Section 6.10 of the Baxano Disclosure Schedule to fill such vacancies as directors of that class set forth opposite their respective names on Section 6.10 of the Baxano Disclosure Schedule.
| A-76 |
(b) From and after theEffective TimethroughTranS1’s 2016 annual meeting of stockholders,TranS1shall cooperate with theSecurityholder Representativesto ensure that, to the greatest extent possible, theTranS1 Boardconsists of not more than eight directors (or nine (9) directors prior to the 2013 TranS1 annual meeting of stockholders) and that there shall be two directors designated by theSecurityholder Representatives.A majority of the directors shall constitute a quorum for the transaction of business at all meetings of the TranS1 Board and the affirmative vote of not less than a majority of the directors present at any meeting at which there is a quorum shall be the act of the TranS1 Board, in each case without regard to classes.
(c) Subject to compliance with applicable laws and the regulations of any exchange on which the TranS1 Common Stock may from time to time be traded, in connection with TranS1’s 2013 annual meeting of stockholders (the “TranS1 Annual Meeting”),the following director nomination procedures shall be followed:
(i) the Securityholder Representatives shall designate for nomination by the TranS1 nominating committee the two individuals identified in Section 6.10 of the Baxano Disclosure Schedule (or any other nominee(s) in lieu thereof designated by Securityholders holding in the aggregate Escrow Participation Percentages in excess of fifty percent (50%)) for reelection to serve until the2016 annual meeting of stockholders; and
(ii) each individual designated by the Securityholder Representatives for nomination as a director of TranS1 shall be nominated by the TranS1 nominating committee for election as a Director unless, solely in the case of nominees other than those the set forth in Section 6.10 of the Baxano Disclosure Schedule, the TranS1 nominating committee reasonably determines that such individual lacks such business or technical experience, stature and character as is commensurate with service on the board of directors of a publicly held enterprise or if such individual is an officer, director, partner or principal stockholder of any competitor of TranS1 and its subsidiaries. If the TranS1 nominating committee determines that any individual designated by the Securityholder Representatives does not satisfy the criteria set forth in the preceding sentence, the TranS1 nominating committee will promptly notify the Securityholder Representatives of such determination and the Securityholder Representatives will be entitled to designate another individual for nomination.
6.11 Employee Communications. Baxano will use commercially reasonable efforts to consult with TranS1, and will consider in good faith TranS1’s advice, prior to sending any notices or other communication materials to its employees regarding this Agreement, the Merger or the effects thereof on the employment, compensation or benefits of its employees.
6.12 FIRPTA Tax Certificates. On or prior to the Closing, Baxano shall deliver to TranS1 a certificate that the Baxano Common Stock is not a “U.S. real property interest” in accordance with the Treasury Regulations under Sections 897 and 1445 of the Code, together with evidence reasonably satisfactory to TranS1 that Baxano delivered notice to the Internal Revenue Service in accordance with the provisions of Section 1.897-2(h)(2) of the Treasury Regulations. If TranS1 does not receive the certificate described above on or before the Closing Date, TranS1 shall be permitted to withhold from the payments to be made pursuant to this Agreement any required withholding tax under Section 1445 of the Code.
| A-77 |
6.13 Additional Indebtedness. Prior to March 31, 2013, Baxano shall issue to one or more Noteholders as of the date hereof, one or more Baxano Notes in an aggregate principal amount of not less than $500,000 in exchange for cash in like amount.
6.14 Indemnification and Insurance.
(a) From and after the Effective Time, TranS1 shall cause Surviving Corporation to fulfill and honor in all respects the obligations of Baxano pursuant to any agreement of Baxano providing for the indemnification of each present and former director and officer of Baxano (any such person shall be referred to herein together as the “D&O Indemnitees”). Without limiting the effect of the foregoing, during the period commencing on the Closing Date and ending on the sixth anniversary of the Effective Time, TranS1 shall, and shall cause Surviving Corporation to, indemnify and hold harmless each D&O Indemnitee (in each case, to the extent such D&O Indemnitee is entitled to indemnification by Baxano as of the date of this Agreement pursuant to Baxano’s Amended and Restated Certificate of Incorporation, by-laws or an indemnification agreement) against and from any costs or expenses (including reasonable attorneys’ fees), judgments, fines, losses, claims, demands, damages, liabilities and amounts paid in settlement in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, to the extent such claim, action, suit, proceeding or investigation arises out of or pertains to any action or omission or alleged action or omission by such D&O Indemnitee in his or her capacity as a director, officer or employee of Baxano (regardless of whether such action or omission, or alleged action or omission, occurred prior to, on or after the date of this Agreement).
(b) For a period of six (6) years after the Effective Time, TranS1 will cause Surviving Corporation Certificate of Incorporation and Surviving Corporation Bylaws to contain provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of each D&O Indemnitee than are set forth in Baxano’s current certificate of incorporation and bylaws.
(c) Prior to the Effective Time, Baxano shall obtain and TranS1 shall pay for “tail” policies of directors’ and officers’ liability insurance with a claims period of not greater than six (6) years from and after the Effective Time with benefits and levels of coverage at least as favorable as the existing policies of Baxano with respect to matters existing or occurring at or prior to the Effective Time (including in connection with this Agreement or the transactions or actions contemplated hereby).
(d) Each of TranS1, Surviving Corporation and their respective Affiliates (determined after the Effective Time) covenants for itself and its respective successors, assigns, heirs, legatees and personal representatives that it shall not institute any action, suit, claim, litigation, arbitration or proceeding of any nature against any D&O Indemnitee (other than any such action, suit, claim, litigation, arbitration or proceeding arising from or relating to any action, or omission to act, for which such D&O Indemnitee would not otherwise be entitled to indemnification under Applicable Law), in his or her capacity as such, with respect to any Losses or other liabilities, actions or causes of action, judgments, claims and demands of any nature or description arising from or relating to actions occurring on or prior to the Closing. For the avoidance of doubt, this6.14(d) shall not affect any right to indemnification TranS1 may have under ARTICLE VIII.
| A-78 |
(e) If TranS1 or the Surviving Corporation or any of their respective successors or assigns (i) shall consolidate with or merge into any other corporation or entity and shall not be the continuing or surviving corporation or entity of such consolidation or merger or (ii) shall transfer all or substantially all of its properties and assets to any individual, corporation or other entity, then, and in each such case, proper provisions shall be made so that the successors and assigns of TranS1 or Surviving Corporation shall assume all of the obligations set forth in this Section 6.14.
(f) The provisions of this Section 6.14 are intended to be for the benefit of, and shall be enforceable by, each of the D&O Indemnitees, and each of the D&O indemnitees is a third party beneficiary of this Section 6.14.
(g) The rights of the D&O Indemnitees under this Section 6.14 shall be in addition to any rights such D&O Indemnitees may have under any applicable contracts or laws.
6.15 Non-Solicitation. Until the earlier of (i) the first day following the twelve (12) month period from the date hereof or (ii) the Closing, except with the prior written consent of the other party, neither party (nor any of such party’s Affiliates) shall hire, employ or otherwise engage or retain in any manner or capacity any person who served as an employee of the other party or any Affiliate of such other party within the six month period immediately preceding such proposed hiring, employment or other engagement or retainer; provided, however, that this Section 6.15 shall not prohibit or prevent (a) the solicitation or hiring of any employees transferred pursuant to this Agreement or the transactions contemplated thereby in any capacity by TranS1 or any Affiliate of TranS1; (b) general advertisements or solicitations for employment that are not targeted at employees or independent contractors of the other party or its Affiliates; or (c) the hiring by either party or an Affiliate thereof of an employee or exclusive independent contractor of the other party or its Affiliates (i) who is solicited through general advertisements or solicitations for employment that are not targeted at employees or independent contractors of the other party or its Affiliates, or (ii) who have resigned, quit or been terminated by the other party or its Affiliates prior to commencement of communications between such hiring party and such persons.
6.16 Employee Benefits.
(a) To the extent permitted by the TranS1 Employee Plans, TranS1 will cause any such plans which the employees of Baxano as of the Effective Time who continue to remain employed with Baxano (the “Continuing Employees”) are eligible to participate in after the Effective Time to take into account for purposes of eligibility, vesting and benefit accrual thereunder, service by such Continuing Employees with Baxano prior to the Effective Time as if such service were with TranS1, to the same extent such service was credited under a corresponding benefit plan as of the Effective Time. Nothing in this Agreement, express or implied, shall affect the right of TranS1, Surviving Corporation or any Subsidiary to terminate the employment of any employee.
| A-79 |
(b) If requested by TranS1 within ten (10) days of the date hereof, Baxano shall use commercially reasonable efforts to terminate any or all Baxano Employee Plans effective immediately prior to the Effective Time.
6.17 Transaction Expenses. To the extent unpaid at Closing and set forth on the Pre-Closing Statement or Conclusive Statement as Transaction Expenses, TranS1 shall pay all fees, costs and expenses incurred by Baxano in connection with the matters described in this Agreement, including with respect to any brokers, financial advisors (including Leerink Swann LLC), consultants, accountants, attorneys or other professionals engaged by Baxano in connection with the parties’ due diligence related to, or the structuring, negotiation or consummation of the transactions contemplated by this Agreement or the Securities Purchase Agreement as and when they become due without delay, in the amounts set forth on the Pre-Closing Statement or Conclusive Statement.
6.18 Post-Closing Operations. If the Closing occurs prior to March 31, 2013, TranS1 shall cause the Surviving Corporation to act and carry on its business in the usual, regular and ordinary course in substantially the same manner as previously conducted and to maintain and preserve in the ordinary course its business organization, assets and properties, each until March 31, 2013. Without limiting the generality of the foregoing, from and after the Closing until March 31, 2013, TranS1 shall cause the Surviving Corporation not to, directly or indirectly, do any of the following without the prior written consent of the Securityholder Representatives:
(a) (i) declare, set aside or pay any dividends on, or make any other distributions (whether in cash, securities or other property) in respect of, any of its capital stock; or (ii) purchase, redeem or otherwise acquire any shares of its capital stock or any other of its securities or any rights, warrants or options to acquire any such shares or other securities;
(b) except in the Ordinary Course of Business, sell, lease, license, pledge, or otherwise dispose of or encumber any properties or assets of the Surviving Corporation;
(c) whether or not in the Ordinary Course of Business, sell, dispose of or otherwise transfer any assets material to the Surviving Corporation (including any accounts, leases, contracts or intellectual property, but excluding the sale or license of products and inventory in the Ordinary Course of Business);
(d) (i) incur or suffer to exist any Indebtedness (other than with respect to the Oxford/SVB Loan Documents), (ii) issue, sell or amend any debt securities or warrants or other rights to acquire any debt securities of theSurviving Corporation, (iii) make any loans, advances or capital contributions to, or investment in, any otherperson, other than theSurviving Corporationor any of its direct or indirect wholly ownedSubsidiariesor (iv) enter into any hedgingagreementor other financialagreementor arrangement designed to protect the Surviving Corporation or itsSubsidiariesagainst fluctuations in commodities prices or exchange rates;
| A-80 |
(e) make any capital expenditures or other expenditures with respect to property, plant or equipment;
(f) make any changes in accounting methods, principles or practices, except insofar as may have been required by the SEC or a change in GAAP or, except as so required, change any assumption underlying, or method of calculating, any bad debt, contingency or other reserve.
6.19 Consents of Distributors. Prior to the Closing, Baxano, in coordination with TranS1, shall contact each of the top fifteen (15) sales representatives (as measured by sales during 2012) from the list of sales representatives set forth on Section 3.4(b) of the Baxano Disclosure Schedule and shall request that each such sales representative consent in writing to the assignment of its Independent Sales Representative Agreement from Baxano to Trans1.
ARTICLE VII
CONDITIONS TO MERGER
7.1 Conditions to Each Party’s Obligation To Effect the Merger. The respective obligations of each party to this Agreement to effect the Merger shall be subject to the satisfaction prior to the Closing of the following conditions:
(a) Stockholder Approvals. The Baxano Stockholder Approval shall have been obtained. The TranS1 Voting Proposal shall have been approved at the TranS1 Meeting, at which a quorum is present, by the requisite vote of the stockholders of TranS1 under applicable law and stock market regulations.
(b) Governmental Approvals. Other than the filing of the Certificate of Merger, all authorizations, consents, orders or approvals of, or declarations or filings with, or expirations of waiting periods imposed by, any Governmental Entity in connection with the Merger and the consummation of the other transactions contemplated by this Agreement, the failure of which to file, obtain or occur is reasonably expected to have a TranS1 Material Adverse Effect or a Baxano Material Adverse Effect shall have been filed, been obtained or occurred on termsand conditions that would not reasonably be expected to have aTranS1 Material Adverse Effector aBaxano Material Adverse Effect.
(c) No Injunctions. No Governmental Entity of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any order, executive order, stay, decree, judgment or injunction (preliminary or permanent) or statute, rule or regulation which is in effect and which has the effect of making the Merger illegal or otherwise prohibiting consummation of the Merger or the other transactions contemplated by this Agreement.
(d) No Restraints. There shall not be instituted or pending any action or proceeding by any Governmental Entity (i) seeking to restrain, prohibit or otherwise interfere with the ownership or operation by TranS1 or any of its Subsidiaries of all or any portion of the business of Baxano or of TranS1 or any of its Subsidiaries or to compel TranS1 or any of its Subsidiaries to dispose of or hold separate all or any portion of the business or assets of Baxano or of TranS1 or any of its Subsidiaries or (ii) seeking to impose or confirm limitations on the ability of TranS1 or any of its Subsidiaries effectively to exercise full rights of ownership of the shares of Baxano Common Stock (or shares of stock of Surviving Corporation) including the right to vote any such shares on any matters properly presented to stockholders.
| A-81 |
7.2 Additional Conditions to the Obligations of TranS1 and Transitory Subsidiary. The obligations of TranS1 and Transitory Subsidiary to effect the Merger shall be subject to the satisfaction on or prior to the Closing Date of each of the following additional conditions, any of which may be waived in writing exclusively by TranS1 and Transitory Subsidiary:
(a) Representations and Warranties. The representations and warranties of Baxano set forth inSections3.2(a)and 3.4(d) shall be true and correct as of the date of this Agreement and as of the Closing, as if made on and as of the Closing Date (other than such representations and warranties made as of a specific date, which shall have been true and correct as of such specific date), and all other representations and warranties of Baxano set forth in this Agreement, without giving effect to any qualification or limitation as to “materiality” or “Baxano Material Adverse Effect,” shall have been true and correct as of the date of this Agreement and shall be true and correct as of the Closing Date with the same effect as though such representations and warranties were made on and as of the Closing Date (other than such representations and warranties made as of a specific date, which shall have been true and correct as of such specific date), except where failure of such other representations or warranties to be so true and correct, individually or in the aggregate with all such failures, has not had and would not reasonably be expected to have a Baxano Material Adverse Effect.
(b) Performance of Obligations of Baxano. Baxano shall have performed in all material respects all obligations required to be performed by it under this Agreement on or prior to the Closing Date, including under Section 6.13, and TranS1 shall have received a certificate signed on behalf of Baxano by the chief executive officer and the chief financial officer of Baxano to such effect.
(c) No Baxano Material Adverse Effect. No Baxano Material Adverse Effect shall have occurred since the date of this Agreement and be continuing.
(d) Third Party Consents. Baxano shall have obtained (i) all consents and approvals of third parties listed in Section 7.2(d)(i) of the Baxano Disclosure Schedule and (ii) any other required consent or approval of any third party (other than a Governmental Entity) the failure of which to obtain, individually or in the aggregate, would reasonably be expected to have a Baxano Material Adverse Effect (it being understood and agreed that the failure to obtain or effect any or all of the consents and approvals listed in Section 7.2(d)(ii) of the Baxano Disclosure Schedule would not reasonably be expected to have a Baxano Material Adverse Effect).
(e) Dissenting Shares. The Dissenting Shares shall not include shares of Baxano Preferred Stock that, absent a demand for appraisal rights, would be entitled to receive in excess of five percent (5%) of the Merger Shares.
| A-82 |
(f) Escrow Participant Commitments Under Securities Purchase Agreement. Baxano shall have delivered (i) copies of the Securities Purchase Agreement, duly executed by one or more Escrow Participants purchasing Financing Shares with an aggregate purchase price of not less than $14,500,000, and (ii) any other closing deliverables required from such Escrow Participants pursuant to the Securities Purchase Agreement.
(g) Officers’ Certificate. TranS1 shall have received an officers’ certificate duly executed by each of the Chief Executive Officer and Chief Financial Officer of Baxano to the effect that the conditions of Sections 7.2(a), 7.2(b) and 7.2(c) have been satisfied.
(h) Closing Conditions Under the Securities Purchase Agreement. All closing conditions set forth in Section 8.1 of the Securities Purchase Agreement shall have been satisfied or waived (other than delivery of items to be delivered at the closing under the Securities Purchase Agreement and other than satisfaction of those conditions that by their nature are to be satisfied at such closing).
(i) Escrow Agreement. Baxano shall have delivered (i) a duly executed copy of the Escrow Agreement and (ii) any other closing deliverables required from Baxano pursuant to the Escrow Agreement.
7.3 Additional Conditions to the Obligations of Baxano. The obligation of Baxano to effect the Merger shall be subject to the satisfaction on or prior to the Closing Date of each of the following additional conditions, any of which may be waived, in writing, exclusively by Baxano:
(a) Representations and Warranties. The representations and warranties of TranS1 and Transitory Subsidiary set forth inSections4.2(a)and 4.4(d) shall be true and correct in all material respects as of the date of this Agreement and as of the Closing Date, as if made on and as of the Closing Date (other than such representations and warranties made as of a specific date, which shall have been true and correct in all material respects as of such specific date), and all other representations and warranties of TranS1 and Transitory Subsidiary set forth in this Agreement, without giving effect to any qualification or limitation as to “materiality” or “TranS1 Material Adverse Effect,” shall have been true and correct as of the date of this Agreement and shall be true and correct as of the Closing Date with the same effect as though such representations and warranties were made on and as of the Closing Date (other than such representations and warranties made as of a specific date, which shall have been true and correct as of such specific date), except where failure of such other representations or warranties to be so true and correct, individually or in the aggregate with all such failures, has not had and would not reasonably be expected to have a TranS1 Material Adverse Effect.
(b) Performance of Obligations of TranS1 and Sub. TranS1 and Transitory Subsidiary shall have performed in all material respects all obligations required to be performed by them under this Agreement on or prior to the Closing Date, and Baxano shall have received a certificate signed on behalf of TranS1 by the chief executive officer or the chief financial officer of TranS1 to such effect.
(c) No TranS1 Material Adverse Effect. No TranS1 Material Adverse Effect shall have occurred since the date of this Agreement and be continuing.
| A-83 |
(d) Third Party Consents. TranS1 shall have obtained (i) all consents and approvals of third parties listed in Section 7.3(d)(i) of the TranS1 Disclosure Schedule and (ii) any other consent or approval of any third party (other than a Governmental Entity) the failure of which to obtain, individually or in the aggregate, would reasonably be expected to have a TranS1 Material Adverse Effect (it being understood and agreed that the failure to obtain or effect any or all of the consents and approvals listed in Section 7.3(d)(ii) of the TranS1 Disclosure Schedule would not reasonably be expected to have a TranS1 Material Adverse Effect).
(e) TranS1 Stockholder Agreements. TranS1 shall have delivered duly executed copies of the TranS1 Stockholder Agreements from the stockholders of TranS1 listed onSchedule A.
(f) Escrow Participant Commitments Under Securities Purchase Agreement. TranS1 shall have delivered (i) a duly executed copy of the Securities Purchase Agreement and (ii) any other closing deliverables required from TranS1 pursuant to the Securities Purchase Agreement.
(g) Officers’ Certificate. Baxano shall have received an officers’ certificate duly executed by each of the Chief Executive Officer and Chief Financial Officer of TranS1 to the effect that the conditions of Sections 7.3(a), (b) and (c) have been satisfied.
(h) Closing Conditions Under the Securities Purchase Agreement. All closing conditions set forth in Section 8.2 of the Securities Purchase Agreement shall have been satisfied or waived (other than delivery of items to be delivered at the closing under the Securities Purchase Agreement and other than satisfaction of those conditions that by their nature are to be satisfied at such closing).
(i) Escrow Agreement. TranS1 shall have delivered (i) a duly executed copy of the Escrow Agreement and (ii) any other closing deliverables required from TranS1 pursuant to the Escrow Agreement.
ARTICLE VIII
INDEMNIFICATION
8.1 Survival.
(a) Fundamental Representations. The representations and warranties of Baxano (the “Fundamental Baxano Representations”) set forth in Section 3.1 (Organization, Standing and Power), Section 3.2 (Capitalization and Closing Allocation Statement), clause (a) of Section 3.4 (Authority; No Conflict; Required Filings and Consents) and Section 3.20 (Broker; Fees and Expenses) shall survive the Closing for a period of four (4) years after the Closing Date. The representations and warranties of TranS1 and Transitory Subsidiary (the “Fundamental TranS1 Representations”) set forth in Section 4.1 (Organization, Standing and Power), Section 4.2 (Capitalization), clause (a) of Section 4.4 (Authority; No Conflict; Required Filings and Consents) and Section 4.19 (Broker; Fees and Expenses) shall survive the Closing for a period of four (4) years after the Closing Date. The survival of representations and warranties (the “Tax Representations”) set forth in Section 3.8 and Section 4.8 (Tax Matters) are exclusively governed by Section 8.5.
| A-84 |
(b) General Representations. The representations and warranties of the parties contained in this Agreement or any certificate delivered at the Closing pursuant to this Agreement, other than the Fundamental Baxano Representations and the Fundamental TranS1 Representations, shall survive the Closing for a period of twelve (12) months after the Closing Date;provided, that any obligations under Section 8.2 and Section 8.3 shall not terminate with respect to any claim for indemnifiable Losses as to which the person to be indemnified shall have given written notice (stating in reasonable detail the basis of the claim for indemnification) to the Indemnifying Party in accordance with Section 8.4(b)(i) and Section 10.3 before the termination of such survival period.
(c) Survival. The covenants contained in this Agreement that are to be performed at or prior to the Closing shall survive the Closing for a period of twelve (12) months after the Closing Date. The covenants contained in this Agreement that are to be performed following the Closing shall survive unless and until they are otherwise terminated by their respective terms.
8.2 Indemnification of TranS1 Indemnified Parties.
(a) General. If the Closing occurs, each Escrow Participant hereby agrees, subject to the limitations specified in this ARTICLE VIII to indemnify and hold TranS1 and its directors, officers, employees, Affiliates (including Surviving Corporation), stockholders, agents, attorneys, representatives, successors and assigns (collectively, the “TranS1 Indemnified Parties”) harmless from and against any and all losses, liabilities, costs, damages, expenses (including expenses in connection with any action, suit, proceeding or investigation (“Proceeding”), whether involving a Third Party Claim or a claim solely between the parties hereto, including costs of investigation and defense and reasonable fees and expenses of attorneys, accountants and other outside consultants), interest, awards, orders, fines, settlements and other costs of any kind or nature whatsoever, but excluding any consequential, indirect, special and punitive damages (except in each case to the extent included and paid in a Third Party Claim or arising from a claim based upon fraud, willful misrepresentation with the intent to deceive or willful breach of a covenant) (individually, a “Loss” and, collectively, “Losses”), suffered or incurred by such TranS1 Indemnified Party to the extent arising from any:
(i) breach or inaccuracy of any of the representations or warranties made by Baxano in this Agreement or in the certificate delivered pursuant to Section 7.2(h), other than the (A) Fundamental Baxano Representations and (B) the Tax Representations (which are governed by Section 8.5);
(ii) breach of any covenant, obligation or other agreement on the part of Baxano required to be performed at or prior to the Closing under this Agreement, other than a breach of a covenant under Section 5.1(o) (which is governed by Section 8.5);
| A-85 |
(iii) breach or inaccuracy of (A) a Fundamental Baxano Representation and/or (B) the failure of any item set forth in the Closing Allocation Statement to be accurate in all respects as of the Closing;
(iv) Transaction Expenses of Baxano not paid by Baxano prior to Closing, except to the extent treated as a reduction to the Merger Consideration or included in the Conclusive Statement;
(v) the excess, if any, of the aggregate amount ultimately required to be paid to holders of Dissenting Shares with respect thereto by Baxano pursuant to appraisal rights under Section 262 of the DGCL or dissenters’ rights under Chapter 13 of the CCC over the aggregate amount such holders would have otherwise received with respect to such Dissenting Shares pursuant to the terms of this Agreement, plus any reasonable costs incurred by TranS1 or Surviving Corporation arising out of any demands for such appraisal rights or dissenters’ rights, including reasonable fees and expenses of attorneys, accountants and other outside consultants; and
(vi) claims relating to performance or failure of performance of the Securityholder Representatives of their duties under Section 10.14 or to actions taken or omitted from being taken by TranS1, its Affiliates, the Escrow Agent or any other Person in accordance with or reliance upon any instruction, decision, or action of the Securityholder Representatives.
(b) Limitations. Notwithstanding the provisions of Section 8.2(a), Escrow Participants shall not be required to indemnify any TranS1 Indemnified Party, and shall have no liability for any Loss:
(i) under Section 8.2(a)(i), unless the aggregate of all Losses for which Escrow Participants would, but for this clause (i), be liable thereunder exceeds on a cumulative basis an amount equal to $200,000 (the “Basket”), and then only to the extent of any such excess;
(ii) under Section 8.2(a)(i), for any Loss resulting from any single claim or series of related or similar claims which does not exceed $50,000 (the “De Minimis Limitation”);
(iii) under Section 8.2(a), for any Loss in excess of such Escrow Participant’s Escrow Participation Percentage of such Loss;
(iv) under Section 8.2(a), for any Loss in excess of the value of the Merger Shares actually distributed to and received by such Escrow Participant at the Closing (excluding for this purpose any Escrow Shares), based on the Merger Closing Price;
(v) under Section 8.2(a), with respect to any Loss with respect to which the remedy of the TranS1 Indemnified Parties is not limited to the Escrow Shares, unless the Escrow Shares have first been exhausted in payment of prior indemnifiable Losses hereunder; and
| A-86 |
(vi) under Section 8.2(a)(i) or Section 8.5 in the aggregate exceeding the Escrow Shares (the “Cap”).
(c) Source of Recovery; Limitation of Liability. The sole recourse of a TranS1 Indemnified Party pursuant to Section 8.2(a) (other than for claims based upon breach or inaccuracy of a Fundamental Baxano Representation, fraud or willful misrepresentation with the intent to deceive in the making of a representation or warranty of Baxano in this Agreement) is limited to the number of remaining Escrow Shares available for distribution, as calculated using the applicable Escrow Shares Indemnity Value. The “Escrow Shares Indemnity Value” shall mean, for any release of Escrow Shares, the per share value of Escrow Shares on deposit with the Escrow Agent and available for distribution therefrom equal to the average of the last reported sales prices of TranS1 Common Stock at 4:00 p.m., Eastern time, end of regular trading hours on NASDAQ during the ten consecutive trading days ending on the last trading day prior to the date of delivery of joint written instructions to the Escrow Agent to release such Escrow Shares to satisfy a claim made under this ARTICLE VIII. In the event that the Escrow Shares are insufficient to pay any TranS1 Indemnified Party any amounts owed to such TranS1 Indemnified Party pursuant to Section 8.2(a) (other than for claims based upon breach or inaccuracy of a Fundamental Baxano Representation, fraud or willful misrepresentation with the intent to deceive in the making of a representation or warranty of Baxano in this Agreement), TranS1 Indemnified Parties shall not be entitled to collect any remaining amounts not satisfied from the Escrow Shares from Escrow Participants.
(d) Limitations. No Securityholder shall have any liability pursuant to this ARTICLE VIII in excess of the Merger Consideration actually paid to such Securityholder. Nothing contained herein (including Section 8.1, Section 8.2(b), Section 8.2(c), and Section 8.5(g)) shall limit or restrict any TranS1 Indemnified Party's right to recover any amounts which such TranS1 Indemnified Party would otherwise be entitled to receive from any individual or legal entity based upon such person’s own fraud or willful misrepresentation with the intent to deceive.
8.3 Indemnification of the Securityholder Indemnified Parties.
(a) General. If the Closing occurs, TranS1 shall indemnify and hold the Securityholders and their respective directors, officers, employees, Affiliates, equity holders, agents, attorneys, representatives, successors and assigns (collectively, the “Securityholder Indemnified Parties”) harmless from and against any and allLosses suffered or incurred by such Securityholder Indemnified Party to the extent arising from or related to any:
(i) breach or inaccuracy of any of the representations or warranties made by TranS1 or Transitory Subsidiary in this Agreement or in the certificate delivered pursuant to Section 7.3(g) in connection with this Agreement, other than the Fundamental TranS1 Representations;
(ii) breach of any covenant, obligation or other agreement on the part of TranS1 or Transitory Subsidiary required to be performed at or prior to the Closing under this Agreement; and
| A-87 |
(iii) breach or inaccuracy of a Fundamental TranS1 Representation and/or the failure of any item set forth in the Closing Capitalization Statement to be accurate in all respects as of the Closing.
(b) Limitations. TranS1 shall not be required to indemnify any Securityholder Indemnified Party, and shall not have any liability for any Loss:
(i) under Section 8.3(a)(i) unless the aggregate of all Losses for which TranS1 would, but for this clause (i), be liable thereunder exceeds on a cumulative basis the amount of the Basket and, in such event, TranS1 shall only be required to pay such Losses to the extent that such Losses exceed the Basket;
(ii) under Section 8.3(a)(i) for any Loss resulting from any single claim or series of related or similar claims which does not exceed the De Minimis Limitation (other than the Tax Representations); and
(iii) under Section 8.3(a)(i) if the aggregate of all Losses for which TranS1 would, but for this clause (iii), be liable thereunder exceeds an amount equal to the value of the Cap calculated using the Merger Closing Price;provided that the Cap limitation shall not apply to Losses related to any failure by TranS1 to make any payment of Merger Consideration that TranS1 is required to make or cause to be made hereunder.
8.4 Indemnification Procedures.
(a) Indemnity Claim Notices. A claim for indemnification for any matter (whether or not involving a Third Party Claim) shall be asserted by a TranS1 Indemnified Party or Securityholder Indemnified Party (as applicable, the “Indemnified Party”) by prompt written notice (an “Indemnity Claim Notice”) to the Securityholder Representatives (on behalf of the Escrow Participants) or TranS1 (as applicable, the “Indemnifying Party”), and solely in the case of a claim asserted by a TranS1 Indemnified Party, notice to the Escrow Agent;provided,however, that failure to so notify the Indemnifying Party or the Escrow Agent shall not preclude the Indemnified Party from any indemnification which it may claim in accordance with this ARTICLE VIII except to the extent that the Indemnifying Party can demonstrate actual prejudice to its defenses or counterclaims or otherwise, or increased or aggravated Loss as a result of such failure.
(b) Procedures for Third-Party Claims. In the event that any Proceedings shall be instituted or that any claim or demand shall be asserted by any third party in respect of which indemnification may be sought under Section 8.2 or Section 8.3 hereof (a “Third Party Claim”):
(i) The Indemnified Party shall provide the Indemnifying Party an Indemnity Claim Notice in accordance with Section 8.4(a).
| A-88 |
(ii) Subject to the provisions of this Section 8.4, the Indemnifying Party shall have the right, at its sole expense, and at any time upon written notice to the Indemnified Party to assume (with counsel reasonably satisfactory to the Indemnified Party) the defense of, negotiate, settle or otherwise deal with any Third Party Claim which relates to any Losses indemnified against hereunder, and such assumption will conclusively establish for purposes of this Agreement that the claims made in that Third Party Claim are within the scope of and subject to indemnification under this ARTICLE VIII. Notwithstanding the foregoing, the Indemnifying Party shall not be entitled to assume the defense of any Third Party Claim if the Third Party Claim is or relates directly to any criminal Proceeding.
(iii) If the Indemnifying Party shall assume the control of the defense of any Third Party Claim in accordance with the provisions of this Section 8.4(b), the Indemnified Party shall not have any right to settle or compromise such Third Party Claim, and the Indemnifying Party shall obtain the prior written consent of the Indemnified Party (which consent shall not be unreasonably withheld, delayed or conditioned) before entering into any settlement of such Third Party Claim if the settlement does not expressly and unconditionally release the Indemnified Party from all liability for any Loss with respect to such Third Party Claim or the settlement imposes injunctive or other equitable relief against the Indemnified Party.
(iv) If the Indemnifying Party elects not to defend against, negotiate, settle or otherwise deal with any Third Party Claim which relates to any Losses indemnified against hereunder, or fails to notify the Indemnified Party of its election as herein provided, the Indemnified Party shall defend against, negotiate, settle or otherwise deal with such Third Party Claim; provided that the Indemnified Party shall not enter into any settlement of such Third Party Claim that would require payment to such third party without the prior written consent of the Indemnifying Party (which consent shall not be unreasonably withheld, delayed or conditioned), and in no event shall the settlement of any such Third Party Claims be determinative of whether the Indemnifying Party is obligated hereunder to indemnify the Indemnified Party.
(v) If the Indemnifying Party shall assume the defense of any Third Party Claim, the Indemnified Party may participate, at his or its own expense, in (but not control) the defense of such Third Party Claim;provided,however, that such Indemnified Party shall be entitled to participate in any such defense with separate counsel at the expense of the Indemnifying Party if (i) so requested by the Indemnifying Party to participate or (ii) in the reasonable opinion of counsel to the Indemnified Party, a conflict or potential conflict exists between the Indemnified Party and the Indemnifying Party that would make such separate representation advisable;provided,further, that the Indemnifying Party shall not be required to pay for more than one such counsel for all Indemnified Parties in connection with any Third Party Claim. If the Indemnified Party is entitled to participate in such defense with separate counsel at the expense of the Indemnifying Party as set forth in the preceding sentence, then the Indemnifying Party shall promptly reimburse the Indemnified Party for the reasonable costs and expenses (including reasonable attorneys' fees) of such participation in the defense of such Third Party Claim upon submission of periodic bills;provided,however, that if the Indemnifying Party is the Escrow Participants, any such reimbursement shall be made from and limited to the Escrow Shares to the extent the value of the remaining Escrow Shares (based on the applicable Escrow Shares Indemnity Value) then available for distribution is sufficient, and thereafter Indemnified Party shall no longer be entitled to reimbursement (except in the case of Fundamental Baxano Representations, fraud or willful misrepresentation with the intent to deceive in the making of a representation or warranty of Baxano in this Agreement).
| A-89 |
(vi) The parties hereto agree to provide reasonable access to the other parties hereto to such documents, information, personnel and witnesses as may be reasonably requested in connection with the defense, negotiation or settlement of any such Third Party Claim.
(c) Payments.
(i) Within twenty (20) calendar days of receipt of an Indemnity Claim Notice (the “Objection Period”) pursuant to Section 8.4(a), the Indemnifying Party may object (a “Claim Objection”) to any matter, including the basis and amount of such claims, set forth in such Indemnity Claim Notice by delivering to the Indemnified Party written notice setting forth such objection in reasonable detail. If the Indemnified Party does not receive a Claim Objection within the Objection Period, then the Indemnifying Party shall be deemed to have acknowledged and agreed with the correctness of such Indemnity Claim Notice for the full amount set forth therein and shall thereafter be precluded from disputing such amount. If the Indemnifying Party delivers a timely Claim Objection to the Indemnified Party, the Indemnified Party shall not be entitled to payment of such amount of Escrow Shares until a Final Determination has been made with respect to such claim pursuant to Section 8.4(c)(iii).
(ii) If the Indemnified Party is a TranS1 Indemnified Party, TranS1 shall, and shall cause Surviving Corporation to, provide the Securityholder Representatives and their advisors with access upon reasonable notice and at reasonable times to the relevant books and records, materials and employees of TranS1 and the Surviving Corporation in connection with the Securityholder Representatives’ review of the Indemnity Claim Notice, together with the work papers used in their preparation and shall furnish the Securityholder Representatives and their advisors with any other information reasonably requested relating to Indemnity Claim Notice.
(iii) After any final decision, judgment or award shall have been rendered by a Governmental Entity of competent jurisdiction with respect to the matters covered by an Indemnity Claim Notice and the expiration of the time in which to appeal therefrom (or final resolution of any such appeal), or a settlement with respect to the matters covered by an Indemnity Claim Notice shall have been consummated, or the Indemnified Party and the Indemnifying Party shall have arrived at a mutually binding agreement (including a deemed agreement pursuant to Section 8.4(c)(i)) with respect to the matters covered by an Indemnity Claim Notice (any such occurrence, a “Final Determination”), the Indemnified Party shall forward to the Indemnifying Party written notice of all Losses owing by the Indemnifying Party pursuant to this Agreement with respect to such matter as set forth in such Final Determination. If the Indemnified Party entitled to payment is a TranS1 Indemnified Party, then TranS1 and the Securityholder Representatives shall then issue joint written instructions to the Escrow Agent to release within five business days after the date of such notice (or final resolution of the amount then due and owing in the event of any dispute) the number of Escrow Shares (calculated using the applicable Escrow Shares Indemnity Value) equal to the amounts due and owing to the TranS1 Indemnified Party pursuant hereto;provided,however, that if the calculation of the number of Escrow Shares due and payable hereunder (using the applicable Escrow Shares Indemnity Value) results in a fraction, such fractional share shall not be issued and the value of such fractional share will remain in the Escrow Shares. If the Indemnified Party entitled to payment is a Securityholder Indemnified Party, then TranS1 shall pay, within five (5) business days following the occurrence of such Final Determination, such amounts due and owing to the Escrow Participants, which shall be allocated according to each relevant Escrow Participant’s Escrow Participation Percentage.
| A-90 |
8.5 Tax Indemnity. The provisions of this Section 8.5 shall govern the allocation of responsibility between Securityholders and TranS1 for certain Tax matters following the Closing Date.
(a) Tax Indemnification. If the Closing occurs, TranS1 and Surviving Corporation (the “Tax Indemnified Parties”) shall be entitled to be indemnified from the Escrow Shares against any and all Losses (“Indemnified Tax Losses”) attributable to, arising from or related to (i) all Taxes (or the nonpayment thereof) of Baxano for all Tax periods ending on or before the Closing Date and the portion through the end of the Closing Date of any Tax period that includes (but does not end on) the Closing Date (each such Tax period or portion thereof hereinafter is referred to as a “Pre-Closing Tax Period”), (ii) any breach of, or inaccuracy in, the Tax Representations, and (iii) any breach of a covenant under Section 5.1(o);provided,however, that in the case of clause (i) above, the Tax Indemnified Parties shall be entitled to indemnification only to the extent that the Indemnified Tax Losses exceed the amount, if any, reserved for such Taxes on the face of the Conclusive Statement and taken into account in determining Adjusted Net Working Capital.
(b) Straddle Period. In the case of any Tax period that includes (but does not end on) the Closing Date (such Tax period hereinafter is referred to as a “Straddle Period”), the amount of any Taxes based on or measured by income or receipts of Baxano for the Pre-Closing Tax Period shall be determined based on an interim closing of the books as of the close of business on the Closing Date and the amount of other Taxes of Baxano for a Straddle Period that relates to the Pre-Closing Tax Period shall be deemed to be the amount of such Tax for the entire taxable period multiplied by a fraction the numerator of which is the number of days from the beginning of the Straddle Period through and including the Closing Date and the denominator of which is the number of days in such Straddle Period.
(c) Preparation of Tax Returns. TranS1 and Surviving Corporation shall prepare or cause to be prepared and filed on a timely basis all Tax Returns for Baxano for (x) any completed Pre-Closing Tax Period for which Tax Returns have not been filed as of the Closing Date and (y) for any Straddle Period for which Tax Returns are required to be prepared and filed (all such Tax Returns referred to in clause (y) of this Section 8.5(c) being referred to herein as the “Straddle Period Returns”);provided,however, that Baxano shall prepare or cause to be prepared all Tax Returns for Baxano for all Tax periods ending on or prior to December 31, 2012 and shall use commercially reasonable efforts to file same with the IRS before the Closing Date. TranS1 and Surviving Corporation shall prepare or cause to be prepared, consistent with applicable law, all amended Tax Returns of Baxano for all Tax periods ending prior to the Closing Date. TranS1 and Surviving Corporation shall provide to the Securityholder Representatives for their review a draft of each Straddle Period Return and any new or amended Tax Return for any Tax period ending before the Closing Date no later than fifteen (15) days prior to the due date for filing such Tax Return with the appropriate Governmental Entities and shall make such revisions as are reasonably requested by the Securityholder Representatives.
| A-91 |
(d) Cooperation on Tax Matters. After the Closing Date:
(i) TranS1, Surviving Corporation and the Securityholder Representatives shall cooperate fully, as and to the extent reasonably requested by the other party, in connection with the filing of Tax Returns pursuant to Section 8.5(c) and any audits of, or disputes with Governmental Entities regarding, any Taxes or Tax Returns of Baxano or Surviving Corporation and take any actions reasonably requested by the other party in connection therewith;
(ii) TranS1 and Surviving Corporation shall make available to one another and to any Governmental Entity, as reasonably requested in connection with any Tax Return described in this section or any audit, litigation, or other Proceeding with respect to Taxes, all reasonably relevant information relating to any Taxes or Tax Returns of Baxano or Surviving Corporation, and make employees available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder;
(iii) TranS1, Surviving Corporation and the Securityholder Representatives shall furnish one another with copies of all correspondence received from any Governmental Entity in connection with any Tax audit or information request with respect to any Pre-Closing Tax Period;
(iv) TranS1 and Surviving Corporation shall use their respective best efforts to obtain any certificate or other document from any Governmental Entity or any other Person as may be necessary to mitigate, reduce, or eliminate any Tax that could be imposed (including, without limitation, with respect to the transactions contemplated hereby);
(v) TranS1, Surviving Corporation and the Securityholder Representatives shall upon request, provide to each other all information that the other party may be required to report pursuant to Sections 6043 or 6043A of the Code, or Treasury Regulations promulgated thereunder; and
(vi) TranS1 and Surviving Corporation shall retain all books and records with respect to Tax matters pertinent to Baxano or Surviving Corporation relating to any Tax period beginning before the Closing Date until the expiration of the statute of limitations (and, to the extent notified by TranS1 or the Securityholder Representatives, any extensions thereof) of the respective taxable periods, and to abide by all record retention agreements entered into with any Governmental Entity, and to give the other Person reasonable written notice prior to transferring, destroying or discarding any such books and records and, if the other Person so requests, TranS1 or the Securityholder Representatives, as the case may be, shall allow the other Person to take possession of such books and records.
(e) Survival. The Tax Representations and the Tax Indemnified Parties’ right to commence any claim for indemnification for Taxes under Section 8.5(a) shall survive Closing, subject to Section 8.5(g).
| A-92 |
(f) Tax Proceedings. If any Tax Proceeding is initiated by any Governmental Entity that could result in a claim for Indemnified Tax Losses under Section 8.5(a), the Tax Indemnified Party shall promptly, but in no event later than the earlier of (i) ten (10) calendar days after receipt of notice from the Governmental Entity of such claim or (ii) fifteen (15) calendar days prior to the date required for the filing of any response to or protest of such claim, notify the Securityholder Representatives in writing of such fact. Failure to timely provide such notice shall not affect the right of the Tax Indemnified Party’s indemnification hereunder, except to the extent the Securityholder Representatives and Securityholders are prejudiced by such delay or omission. In such event, the parties shall address such Tax Proceeding in the same manner as a Third Party Claim as set forth in Section 8.4.
(g) Source of Recovery. The sole recourse of a Tax Indemnified Party pursuant to this Section 8.5 shall be to the number of remaining Escrow Shares then available for distribution, as calculated using the applicable Escrow Shares Indemnity Value.
8.6 Other.
(a) Knowledge. The right to indemnification, reimbursement or other remedy based on the representations, warranties, covenants and obligations set forth herein will not be affected by any investigation conducted with respect to, or any knowledge acquired (or capable of being acquired) about, the accuracy or inaccuracy of or compliance with any such representation, warranty, covenant or obligation.
(b) Disregard Materiality. For purposes of calculating Losses hereunder (but not for purposes of determining whether any representations or warranties have been breached or are inaccurate), any “in all material respects” or material adverse effect qualifications in the representations and warranties shall be disregarded (but not, for clarification, any dollar thresholds and not for purposes of any of the representations to the extent requiring a listing of material matters).
(c) Adjustments. The amount of any Loss for which indemnification is provided under this ARTICLE VIII shall be net of (i) any amounts actually recovered by the Indemnified Party pursuant to any indemnification by or indemnification agreement with any third party and (ii) any insurance proceeds actually received as an offset against such Loss, in each case, after deducting (but not below zero) any related costs and expenses, including the aggregate cost of pursuing any related insurance claims and any related increases in insurance premiums or other chargebacks. If the amount to be netted hereunder from any payment required under this ARTICLE VIII is determined after payment by the Indemnifying Party of any amount otherwise required to be paid to an Indemnified Party pursuant to this ARTICLE VIII, the Indemnified Party shall repay to the Indemnifying Party, promptly after such determination, any amount that the Indemnifying Party would not have had to pay pursuant to this ARTICLE VIII had such determination been made at the time of such payment. The Indemnifying Party may require, as a condition to the provision of indemnification hereunder, that the Indemnified Party execute an undertaking consistent with its obligations set forth in this Section 8.6(c).
(d) Tax Treatment. Any payments under this ARTICLE VIII shall be treated for Tax purposes (whether foreign or domestic) as a purchase price adjustment, to the extent permitted by applicable Law.
| A-93 |
(e) Insurance. Each Indemnified Party shall consider in good faith the potential availability of insurance coverage under then-current policies (but without any obligation to seek to recover any such insurance proceeds) in connection with making a claim under this ARTICLE VIII. No Indemnifying Party shall be entitled to be subrogated to any rights of an Indemnified Party, except that if and to the extent an Indemnifying Party indemnifies an Indemnified Party for Losses pursuant to this ARTICLE VIII, such Indemnifying Party shall be subrogated to any rights such Indemnified Party may have against any insurer with respect thereto (and, upon the reasonable request of the Indemnifying Party, the Indemnified Party shall take appropriate actions necessary to transfer and assign any such rights to the Indemnifying Party). The indemnification provided for this ARTICLE VIII is not intended and shall not be deemed to limit, condition, reduce or supplant the primary availability of any insurance that would be available in the absence of such indemnification.
(f) Sole and Exclusive Remedy. Subject to Section 10.10, each party acknowledges and agrees that the remedies provided for in this ARTICLE VIII shall be the sole and exclusive right and remedy exercisable by such party with respect to the subject matter of this Agreement (other than claims for injunctive relief, specific performance or other equitable relief);provided,however, that nothing herein shall (i) limit any party’s remedies with respect to claims against any individual or legal entity arising from such person’s own fraud or willful misrepresentation with the intent to deceive or (ii) prevent or restrict any Person who is a party to any other transaction document, including the Securities Purchase Agreement, from obtaining monetary damages or any other legal or equitable relief in connection with the breach of such agreement.
(g) Disclaimers. NOTWITHSTANDING ANYTHING TO THE CONTRARY ELSEWHERE IN THIS AGREEMENT OR PROVIDED FOR UNDER ANY APPLICABLE LAW, NO PARTY NOR ANY SECURITYHOLDER OR SECURITYHOLDER REPRESENTATIVES, NOR ANY CURRENT OR FORMER SECURITYHOLDER, DIRECTOR, OFFICER, EMPLOYEE, AFFILIATE OR ADVISOR OF ANY OF THE FOREGOING SHALL, IN ANY EVENT, BE LIABLE TO ANY OTHER PERSON, EITHER IN CONTRACT, TORT OR OTHERWISE, FOR ANY SPECIAL, INDIRECT, EXEMPLARY, PUNITIVE OR CONSEQUENTIAL DAMAGES OTHER THAN FORESEEABLE CONSEQUENTIAL DAMAGES, RELATING TO THE BREACH OR ALLEGED BREACH OF ANY PROVISION OF THIS AGREEMENT, WHETHER OR NOT THE POSSIBILITY OF SUCH DAMAGES HAS BEEN DISCLOSED TO THE OTHER PARTY IN ADVANCE, EXCEPT IN EACH CASE TO THE EXTENT INCLUDED IN A THIRD PARTY CLAIM OR ARISING FROM A CLAIM BASED UPON FRAUD, WILLFUL MISREPRESENTATION WITH THE INTENT TO DECEIVE OR A WILLFUL BREACH OF A COVENANT IN THIS AGREEMENT.
| A-94 |
(h) No Other Representations by Baxano. TranS1 acknowledges that it is relying and has relied only on the representations and warranties of Baxano expressly and specifically set forth in ARTICLE III and the certificates delivered by Baxano pursuant to this Agreement. TranS1 acknowledges that, except as expressly provided in ARTICLE III and the certificates delivered by Baxano pursuant to this Agreement, TranS1 is not relying and has not relied on any representations or warranties whatsoever regarding the subject matter of this Agreement, express or implied. The representations and warranties of Baxano set forth in ARTICLE III and the certificates delivered to Baxano pursuant to this Agreement, constitute the sole and exclusive representations and warranties to TranS1 in connection with the transactions contemplated by this Agreement, and TranS1 understands, acknowledges and agrees that all other representations and warranties of any kind or nature, express or implied are specifically disclaimed by Baxano. TranS1 acknowledges and agrees that no current or former stockholder, director, officer, employee, Affiliate or advisor of Baxano has made or is making, in such Person’s individual capacity, any representations, warranties or commitments whatsoever regarding the subject matter of this Agreement, express or implied.
(i) No Other Representations by TranS1. Baxano acknowledges that it is relying and has relied only on the representations and warranties of TranS1 expressly and specifically set forth in ARTICLE IV and the certificates delivered by TranS1 pursuant to this Agreement. Baxano acknowledges that, except as expressly provided in ARTICLE IV and the certificates delivered by TranS1 pursuant to this Agreement, Baxano is not relying and has not relied on any representations or warranties whatsoever regarding the subject matter of this Agreement, express or implied. The representations and warranties of TranS1 set forth in ARTICLE IV and the certificates delivered to TranS1 pursuant to this Agreement, constitute the sole and exclusive representations and warranties to Baxano in connection with the transactions contemplated by this Agreement, and Baxano understands, acknowledges and agrees that all other representations and warranties of any kind or nature, express or implied are specifically disclaimed by TranS1. Baxano acknowledges and agrees that no current or former stockholder, director, officer, employee, Affiliate or advisor of TranS1 has made or is making, in such Person’s individual capacity, any representations, warranties or commitments whatsoever regarding the subject matter of this Agreement, express or implied.
8.7 Release from Escrow.
(a) Subject to the Escrow Agreement, promptly following the finalization of the Conclusive Adjustment Statement and any corresponding payment or release of Escrow Shares in accordance with Section 2.11(f) (if any), the Escrow Agent shall release to the Escrow Participants a number of Escrow Shares such that, following such release, no more than two-thirds of the aggregate number of Escrow Shares deposited with the Escrow Agent pursuant to Sections 2.9(a) and 2.11(f)(iii) continue to be held by the Escrow Agent (less any Merger Shares previously released to a TranS1 Indemnified Party in respect of an Indemnity Claim Notice). Such release shall be in accordance with Section 2.3(b).
(b) Subject to the Escrow Agreement, promptly following the twelve (12) month anniversary of the Closing Date, the Escrow Agent shall release to the Escrow Participants any remaining Escrow Shares and Representative Reimbursement Amount, except that the Escrow Agent shall retain in escrow the portion of the Representative Reimbursement Amount so requested by the Securityholder Representatives and a number of Escrow Shares equal in value (calculated using the applicable Escrow Shares Indemnity Value) to the aggregate amount of claims for indemnification under ARTICLE VIII asserted prior to the expiration of the applicable survival period but not yet resolved. The remaining Representative Reimbursement Amount and Escrow Shares retained in respect of any such unresolved claims shall be released to the Escrow Participants by the Escrow Agent (to the extent not utilized to pay TranS1 Indemnified Parties or the Surviving Corporation for any such claims resolved in favor of such Persons) promptly following the resolution of such claims in accordance with this ARTICLE VIII and the terms of the Escrow Agreement. Such release or releases shall be in accordance with Section 2.3(b).
| A-95 |
ARTICLE IX
TERMINATION AND AMENDMENT
9.1 Termination. This Agreement may be terminated at any time prior to the Effective Time (with respect to Sections 9.1(b) through 9.1(i), by written notice by the terminating party to the other party), whether before or after approval of the Merger by the stockholders of Baxano:
(a) by mutual written consent of TranS1 and Baxano;
(b) by either TranS1 or Baxano if the Merger shall not have been consummated by April 30, 2013 (the “Outside Date”) (provided that the right to terminate this Agreement under this Section 9.1(b) shall not be available to any party whose failure to fulfill any obligation under this Agreement has been a principal cause of or resulted in the failure of the Merger to occur on or before the Outside Date);
(c) by either TranS1 or Baxano if a Governmental Entity of competent jurisdiction shall have issued a nonappealable final order, decree or ruling or taken any other nonappealable final action, in each case having the effect of permanently restraining, enjoining or otherwise prohibiting the Merger;
(d) by either TranS1 or Baxano if at the TranS1 Meeting (including any adjournment or postponement permitted by this Agreement), at which a vote on the TranS1 Voting Proposal is taken, the requisite vote of the stockholders of TranS1 in favor of the TranS1 Voting Proposal shall not have been obtained (provided that the right to terminate this Agreement under this Section 9.1(d) shall not be available (i) to any party seeking termination if at such time such party is in breach of or has failed to fulfill its obligations under this Agreement or (ii) to TranS1, if the failure to obtain the requisite vote has been caused by a breach of a TranS1 Stockholder Agreement by any party thereto other than Baxano);
(e) by Baxano, if: (i) the TranS1 Board shall have failed to give its recommendation to the approval of the TranS1 Voting Proposal in the Proxy Statement or shall have withdrawn or modified its recommendation of the TranS1 Voting Proposal; (ii) TranS1 shall have breached its obligations under Section 6.5(b) of this Agreement; or (iii) TranS1 shall have failed to hold the TranS1 Meeting and submit the TranS1 Voting Proposals to TranS1’s stockholders by the date which is three (3) business days prior to the Outside Date;
(f) by TranS1 or Baxano, if the other party shall have breached its obligations under Section 6.1 of this Agreement;
| A-96 |
(g) by TranS1, if there has been a breach of or failure to perform any representation, warranty, covenant or agreement set forth in this Agreement (other than those referred to elsewhere in this Section 9.1) on the part of Baxano, which breach would cause the conditions set forth in Section 7.2(a) or 7.2(b) not to be satisfied, and such failure or breach with respect to any such representation, warranty, covenant or agreement cannot be cured or, if curable, shall continue unremedied for a period of thirty (30) days after Baxano has received written notice from TranS1 of the occurrence of such failure or breach (provided that in no event shall such thirty (30) day period extend beyond the second day preceding the Outside Date), which such written notice must be provided promptly following such time as TranS1 obtains actual knowledge of such failure or breach;
(h) by Baxano, if there has been a breach of or failure to perform any representation, warranty, covenant or agreement set forth in this Agreement (other than those referred to elsewhere in this Section 9.1) on the part of TranS1, which breach would cause the conditions set forth in Section 7.3(a) or 7.3(b) not to be satisfied, and such failure or breach with respect to any such representation, warranty, covenant or agreement cannot be cured or, if curable, shall continue unremedied for a period of thirty (30) days after TranS1 has received written notice from Baxano of the occurrence of such failure or breach (provided that in no event shall such thirty (30) day period extend beyond the second day preceding the Outside Date), which such written notice must be provided promptly following such time as Baxano obtains actual knowledge of such failure or breach;or
(i) by TranS1, if Baxano shall have failed to deliver to TranS1 by 5:00 p.m., U.S. Eastern time, on the first business day after execution of this Agreement its Secretary’s Certificate to the effect that the Baxano Stockholder Approval has been obtained, accompanied by true and complete copies of the executed Written Consents; provided,however, that TranS1 shall not have any right to terminate this Agreement under this Section 9.1(i) at any time after Baxano shall have delivered such documentation to TranS1.
9.2 Effect of Termination. In the event of termination of this Agreement as provided in Section 9.1, this Agreement shall immediately become void and there shall be no liability or obligation on the part of TranS1, Baxano, Transitory Subsidiary or their respective officers, directors, stockholders or Affiliates;provided that (a) any such termination shall not relieve any party from liability for any willful breach of this Agreement (which includes without limitation the making of any representation or warranty by a party in this Agreement that the party knew was not true and accurate when made) and (b) the provisions of Section 5.3 (Confidentiality), Section 9.2 (Effect of Termination), Section 9.3 (Fees and Expenses) and ARTICLE X (Miscellaneous) of this Agreement and the Confidentiality Agreement shall remain in full force and effect and survive any termination of this Agreement.
9.3 Fees and Expenses.
(a) Except as set forth in this Section 9.3 and subject to Section 2.11, all fees and expenses incurred in connection with this Agreement and the transactions contemplated hereby shall be paid by the party incurring such expenses, whether or not the Merger is consummated.
| A-97 |
(b) Baxano shall reimburse all expenses of TranS1 actually incurred relating to the transactions contemplated by this Agreement prior to termination (including, but not limited to, fees and expenses of TranS1’s counsel, accountants and financial advisors, but excluding any discretionary fees paid to such financial advisors), but not in excess of $750,000, upon the termination of this Agreement (i) by TranS1 pursuant to Section 9.1(b) if the failure to satisfy the conditions set forth in the first sentence of Section 7.1(a) or Section 7.2(b) or Section 7.2(f) by the Outside Date shall be the principal cause of the Closing not occurring; or (ii) by TranS1 pursuant to Section 9.1(f), Section 9.1(g) or Section 9.1(i) (provided that TranS1 shall not have provided its notice of termination under such Section on or before the third business day following the date of this Agreement).
(c) Baxano shall pay TranS1 a termination fee of $1,000,000 in the event of the termination of this Agreement:
(i) by TranS1 pursuant to Section 9.1(b) if the failure to satisfy the conditions set forth in the first sentence of Section 7.1(a) or in Section 7.2(b) or Section 7.2(f) by the Outside Date shall have resulted in the Closing not occurring; or
(ii) by TranS1 pursuant to Section 9.1(f), Section 9.1(g) or Section 9.1(i) (provided that TranS1 shall not have provided its notice of termination under such Section on or before the third business day following the date of this Agreement).
(d) TranS1 shall reimburse all expenses of Baxano actually incurred relating to the transactions contemplated by this Agreement prior to termination (including, but not limited to, fees and expenses of Baxano’s counsel, accountants and financial advisors, but excluding any discretionary fees paid to such financial advisors), but not in excess of $750,000, upon the termination of this Agreement (i) by Baxano pursuant to Section 9.1(b) if the failure to satisfy the conditions set forth in the second sentence of Section 7.1(a) or Section 7.3(b) or Section 7.3(e) by the Outside Date shall be the principal cause of the Closing not occurring; (ii) by Baxano or TranS1 pursuant to Section 9.1(d), or (iii) by Baxano pursuant to Section 9.1(e) or Section 9.1(h).
(e) TranS1 shall pay Baxano a termination fee of $2,000,000 in the event of the termination of this Agreement:
(i) by Baxano pursuant to Section 9.1(b) if the failure to satisfy the conditions set forth in the second sentence of Section 7.1(a) or Section 7.3(b) or Section 7.3(e) by the Outside Date shall have resulted in the Closing not occurring;
(ii) by Baxano or TranS1 pursuant to Section 9.1(d); or
(iii) by Baxano pursuant to Section 9.1(e), Section 9.1(f) or Section 9.1(h).
(f) The expenses and fees, if applicable, payable pursuant to Section 9.3(b), 9.3(c), 9.3(d) and 9.3(e) shall be paid by wire transfer of same-day funds within one business day after demand therefor following the first to occur of the events giving rise to the payment obligation described in Section 9.3(b), 9.3(c), 9.3(d) or 9.3(e);provided that in no event shall TranS1 or Baxano, as the case may be, be required to pay the expenses and fees, if applicable, to the other, if, immediately prior to the termination of this Agreement, the party to receive the expenses and fees, if applicable, was in material breach of its obligations under this Agreement. If one party fails to promptly pay to the other any expense reimbursement or fee due hereunder, the defaulting party shall pay the costs and expenses (including legal fees and expenses) in connection with any action, including the filing of any lawsuit or other legal action, taken to collect payment, together with interest on the amount of any unpaid fee at the publicly announced prime rate of Bank of America, N.A. plus five percent per annum, compounded quarterly, from the date such expense reimbursement or fee was required to be paid.
| A-98 |
(g) Payment of any termination fee described in this Section 9.3 shall not be in lieu of damages incurred in the event of a breach of this Agreement described in clause (a) of Section 9.2.
ARTICLE X
MISCELLANEOUS
10.1 Amendment. This Agreement may be amended by the parties hereto by action taken or authorized by their respective Boards of Directors, at any time before or after approval of the matters presented in connection with the Merger by the stockholders of any of the parties, but, after any such approval, no amendment shall be made which by law requires further approval by such stockholders without such further approval. This Agreement may not be amended except by an instrument in writing signed on behalf of each of the parties hereto.
10.2 Extension; Waiver. At any time prior to the Effective Time, the parties hereto by action taken or authorized by their respective Boards of Directors, may, to the extent legally allowed, (a) extend the time for the performance of any of the obligations or other acts of the other parties hereto, (b) waive any inaccuracies in the representations and warranties contained herein or in any document delivered pursuant hereto and (c) waive compliance with any of the agreements or conditions contained herein. Any agreement on the part of a party hereto to any such extension or waiver shall be valid only if set forth in a written instrument signed on behalf of such party. Such extension or waiver shall not be deemed to apply to any time for performance, inaccuracy in any representation or warranty, or noncompliance with any agreement or condition, as the case may be, other than that which is specified in the extension or waiver. The failure of any party to this Agreement to assert any of its rights under this Agreement or otherwise shall not constitute a waiver of such rights.
10.3 Notices. All notices and other communications hereunder shall be in writing and shall be deemed duly delivered (i) four business days after being sent by registered or certified mail, return receipt requested, postage prepaid, or (ii) one business day after being sent for next business day delivery, fees prepaid, via a reputable nationwide overnight courier service, in each case to the intended recipient as set forth below:
(a) if to TranS1 or Transitory Subsidiary, to
TranS1 Inc.
110 Horizon Drive
Suite 230
Raleigh, NC 27615
Attn: Chief Financial Officer
Telephone: (910) 332-1700
| A-99 |
with copies (which shall not constitute notice) to:
Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
150 Fayetteville Street
Suite 2300
Raleigh, NC 27601
Attn: David B. Clement
Email:dclement@smithlaw.com
Telephone: (919) 821-6754
(b) if to Baxano, to
Baxano, Inc.
655 River Oaks Parkway
San Jose, CA 95134
Attn: President
Telephone: (408) 514-2201
with a copy (which shall not constitute notice) to:
Morrison & Foerster, LLP
755 Page Mill Road
Palo Alto, CA 94304
Attn: Stephen B. Thau
Email:sthau@mofo.com
Telephone: (650) 813-5640
(c) if to Securityholder Representatives, to
Sumeet Jain
c/o CMEA Capital
1 Letterman Drive
Building C, Suite CM500
San Francisco, CA 94129
Email:sumeet@cmea.com
Telephone: (415) 962-2550
and
David Schulte
c/o Kaiser Permanente Ventures
One Kaiser Plaza
Oakland, CA 94612
Email:david.schulte@kp.org
Telephone: (510) 271-6383
| A-100 |
with a copy (which shall not constitute notice) to:
Morrison & Foerster, LLP
755 Page Mill Road
Palo Alto, CA 94304
Attn: Stephen B. Thau
Email:sthau@mofo.com
Telephone: (650) 813-5640
Any party to this Agreement may change the address to which notices and other communications hereunder are to be delivered by giving the other parties to this Agreement notice in the manner herein set forth.
10.4 Entire Agreement. This Agreement (including the Schedules and Exhibits hereto and the documents and instruments referred to herein that are to be delivered at the Closing) constitutes the entire agreement among the parties to this Agreement and supersedes any prior understandings, agreements or representations by or among the parties hereto, or any of them, written or oral, with respect to the subject matter hereof;provided that the Confidentiality Agreement shall remain in effect in accordance with its terms.
10.5 No Third Party Beneficiaries. Except as provided in Section 6.2(e), 6.10, 6.14 and ARTICLE VIII, and subject to Section 10.6, this Agreement is not intended, and shall not be deemed, to confer any rights or remedies upon any person other than the parties hereto and their respective successors and permitted assigns, to create any agreement of employment with any person or to otherwise create any third-party beneficiary hereto.
10.6 Assignment. No party may assign any of its rights or delegate any of its performance obligations under this Agreement, in whole or in part, by operation of law or otherwise without the prior written consent of the other parties. Subject to the preceding sentence, this Agreement shall be binding upon, inure to the benefit of, and be enforceable by, the parties hereto and their respective successors and permitted assigns. Any purported assignment of rights or delegation of performance obligations in violation of this Section 10.6 shall be null and void.
10.7 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If the final judgment of a court of competent jurisdiction declares that any term or provision hereof is invalid or unenforceable, the parties hereto agree that the court making such determination shall have the power to limit the term or provision, to delete specific words or phrases, or to replace any invalid or unenforceable term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term.
| A-101 |
10.8 Counterparts and Signature. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original but all of which together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each of the parties hereto and delivered to the other parties, it being understood that all parties need not sign the same counterpart. The exchange of copies of this Agreement or amendments thereto and of signature pages by facsimile transmission or by email transmission in portable document format, or similar format, shall constitute effective execution and delivery of such instrument(s) as to the parties and may be used in lieu of the original Agreement or amendment for all purposes. Signatures of the parties transmitted by facsimile or by email transmission in portable document format, or similar format, shall be deemed to be their original signatures for all purposes.
10.9 Interpretation.
(a) When reference is made in this Agreement to an Article or a Section, such reference shall be to an Article or Section of this Agreement, unless otherwise indicated. The table of contents, table of defined terms and headings contained in this Agreement are for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement. The language used in this Agreement shall be deemed to be the language chosen by the parties hereto to express their mutual intent, and no rule of strict construction shall be applied against any party. Whenever the context may require, any pronouns used in this Agreement shall include the corresponding masculine, feminine or neuter forms, and the singular form of nouns and pronouns shall include the plural, and vice versa. Any reference to any federal, state, local or foreign statute or law shall be deemed also to refer to all rules and regulations promulgated thereunder, unless the context requires otherwise. Whenever the words “include,” “includes” or “including” are used in this Agreement, they shall be deemed to be followed by the words “without limitation.” No summary of this Agreement prepared by any party shall affect the meaning or interpretation of this Agreement.
(b) The information and disclosures in the Baxano Disclosure Schedule or TranS1 Disclosure Schedule, as the case may be, are intended only to qualify and limit the representations and warranties of Baxano, TranS1 or Transitory Subsidiary, as the case may be, contained in the Agreement and shall not be deemed to expand in any way the scope or effect of any of such representations and warranties. The inclusion of any information in any section of the Baxano Disclosure Schedule or TranS1 Disclosure Schedule, as the case may be, shall not be deemed to be an admission or acknowledgment by Baxano, TranS1 or Transitory Subsidiary, as the case may be, that such information is required to be listed in such section or is material to or outside the Ordinary Course of Business of such party, nor shall such information be deemed to establish a standard of materiality (and the actual standard of materiality may be higher or lower than the matters disclosed by such information). In addition, matters reflected in the Baxano Disclosure Schedule or TranS1 Disclosure Schedule, as the case may be, are not necessarily limited to matters required by the Agreement to be reflected in such disclosure schedule. Such additional matters are set forth for informational purposes only and do not necessarily include other matters of a similar nature. The information contained in any such disclosure schedule is disclosed solely for purposes of the Agreement, and no information contained therein shall be deemed to be an admission by any party hereto to any third party of any matter whatsoever (including any violation of applicable law or breach of contract).
| A-102 |
10.10 Specific Performance. The parties hereto agree that irreparable damage would occur in the event that any provision of this Agreement were not performed in accordance with its specific terms or were otherwise breached. It is accordingly agreed that the parties shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement, in addition to any other remedy they may have at law or in equity.
10.11 Governing Law. All matters arising out of or relating to this Agreement and the transactions contemplated hereby (including without limitation its interpretation, construction, performance and enforcement) shall be governed by and construed in accordance with the internal laws of the State of Delaware without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of laws of any jurisdictions other than those of the State of Delaware.
10.12 Submission to Jurisdiction. Each of the parties to this Agreement (a) consents to submit itself to the exclusive personal jurisdiction of any state or federal court sitting in the State of Delaware in any action or proceeding arising out of or relating to this Agreement or any of the transactions contemplated by this Agreement, (b) agrees that all claims in respect of such action or proceeding may be heard and determined in any such court, (c) agrees that it shall not attempt to deny or defeat such personal jurisdiction by motion or other request for leave from any such court and (d) agrees not to bring any action or proceeding arising out of or relating to this Agreement or any of the transaction contemplated by this Agreement in any other court. Each of the parties hereto waives any defense of inconvenient forum to the maintenance of any action or proceeding so brought and waives any bond, surety or other security that might be required of any other party with respect thereto. Any party may make service on another party by sending or delivering a copy of the process to the party to be served at the address and in the manner provided for the giving of notices in Section 10.3. Nothing in this Section 10.13, however, shall affect the right of any party to serve legal process in any other manner permitted by law.
10.13 WAIVER OF JURY TRIAL. EACH OF TRANS1, TRANSITORY SUBSIDIARY AND BAXANO HEREBY IRREVOCABLY WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY ACTION, PROCEEDING OR COUNTERCLAIM (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THE ACTIONS OF TRANS1, TRANSITORY SUBSIDIARY OR BAXANO IN THE NEGOTIATION, ADMINISTRATION, PERFORMANCE AND ENFORCEMENT OF THIS AGREEMENT.
10.14 Securityholder Representatives.
(a) Authority. By the approval of this Agreement pursuant to Delaware Law (or otherwise), the Securityholders hereby irrevocably appoint Sumeet Jain and David Schulte as the representatives, agents, proxies, and attorneys in fact of and for all Securityholders for purposes of all post-Closing matters under this Agreement (the “Securityholder Representatives”), including without limitation the full power and authority on the Securityholders’ behalf to jointly take the actions set forth herein (all of which actions shall be deemed to be facts ascertainable outside of this Agreement and shall be binding on the Securityholders as a matter of contract law), including, without limitation:
| A-103 |
(i) to consummate the transactions contemplated under this Agreement and the other agreements, instruments, and documents contemplated hereby or executed in connection herewith;
(ii) to negotiate and settle disputes arising under, or relating to, this Agreement and the other agreements, instruments, and documents contemplated hereby or executed in connection herewith;
(iii) to execute and deliver any amendment or waiver to this Agreement and the other agreements, instruments, and documents contemplated hereby or executed in connection herewith (without the prior approval of the Securityholders);
(iv) to retain legal and other advisors in connection with the foregoing, the reimbursement of reasonable fees incurred in connection therewith to be considered Representative Reimbursement Amounts hereunder; and
(v) to take any and all other actions whatsoever to be taken by or on behalf of the Securityholders in connection with this Agreement and the other agreements, instruments, and documents contemplated hereby or executed in connection herewith.
(b) Irrevocable Grant. The Securityholders, by approving this Agreement, further agree that such agencies, proxies and powers of attorney are coupled with an interest, are therefore irrevocable without the consent of the Securityholder Representatives, and shall survive the death, incapacity, bankruptcy, dissolution or liquidation of any Securityholder. All decisions and actions by the Securityholder Representatives within the scope of their authority hereunder shall be binding upon all Securityholders, and no Securityholder shall have the right to object, dissent, protest or otherwise contest the same. TranS1 may conclusively rely, without independent verification or investigation, upon any such joint decision or joint action of the Securityholder Representatives as being the binding decision or action of every Securityholder, and TranS1 shall not be liable to any Securityholder or any other Persons for any actions taken or omitted from being taken by them or by TranS1 in accordance with or reliance upon any such joint decision or joint action of the Securityholder Representatives. The Securityholder Representatives shall have no duties or obligations to the Securityholders hereunder, including any fiduciary duties, except those set forth herein, and such duties and obligations shall be determined solely by the express provisions of this Agreement.
(c) Reliance. Any party may rely upon any joint decision, joint consent or joint instruction of the Securityholder Representatives on behalf of itself or, prior to the Effective Time, Baxano in connection with this Agreement and is relieved from any liability to any person for any act performed in accordance with such decision, consent or instruction. Any party may rely, without inquiry, upon any joint written notice from the Securityholder Representatives or any successor Securityholder Representatives, and may deal with the successors with respect to any matter arising under this Agreement.
| A-104 |
(d) Indemnity. By the approval of this Agreement pursuant to Delaware Law (or otherwise), each Escrow Participant hereby severally, for itself only and not jointly, and only to the extent of such Escrow Participant’s Escrow Participation Percentage, agrees to indemnify and hold harmless the Securityholder Representatives and their partners, managers, officers, employees, agents and other representatives against all expenses (including reasonable attorneys’ fees), judgments, fines and amounts incurred by such Persons in connection with any Proceeding to which the Securityholder Representatives or such other Person is made a party by reason of the fact that it is or was acting as a Securityholder Representative pursuant to the terms of this Agreement,provided,however, that (i) the Securityholder Representatives shall be entitled to payment only from the Representative Reimbursement Amount, until such time as the Representative Reimbursement Amount is exhausted or released pursuant to Section 8.7, and (ii) there shall be no such indemnification in the event of gross negligence or willful misconduct by the Securityholder Representatives.
(e) No Liability. Neither the Securityholder Representatives nor any of their members, managers, officers, agents or other representatives shall incur any liability for any Loss to any Securityholder by virtue of the failure or refusal of such Persons for any reason to consummate the transactions contemplated hereby or relating to the performance of their duties hereunder, except for actions or omissions constituting fraud, willful misrepresentation with the intent to deceive or a willful breach of any covenant of this Agreement. The Securityholder Representatives and their members, managers, officers, agents and other representatives shall have no liability for any Loss in respect of any Proceeding brought against such persons by any Securityholder, regardless of the legal theory under which such liability may be sought to be imposed, whether sounding in contract or tort, or whether at law or in equity, or otherwise, if such persons took or omitted taking any action in good faith.
(f) Removal. Following the Closing Date, Securityholders holding in the aggregate Escrow Participation Percentages in excess of 50% may, by written consent, remove either or both Securityholder Representatives and appoint one or two (as applicable) new representatives as their replacements. Notice together with a copy of the written consent appointing such new representative and bearing the signatures of Securityholders of a majority-in-interest of those Securityholders must be delivered to TranS1 not less than ten (10) calendar days prior to such appointment. Such appointment will be effective upon the later of the date indicated in the consent or ten (10) calendar days following the date such consent is received by TranS1.
[signature page follows]
| A-105 |
[Signature Page to Agreement and Plan of Merger]
IN WITNESS WHEREOF, each party hereto has executed or caused this Agreement to be executed on its behalf as of the date first written above.
| TRANS1 INC. | ||
| By: | /s/ Ken Reali | |
| Name: | Ken Reali | |
| Title: | President & CEO | |
| RACERX ACQUISITION CORP. | ||
| By: | /s/ Ken Reali | |
| Name: | Ken Reali | |
| Title: | President & CEO | |
| BAXANO, INC. | ||
| By: | /s/ Anthony J. Recupero | |
| Name: | Anthony J. Recupero | |
| Title: | President and CEO | |
| SECURITYHOLDER REPRESENTATIVES | ||
| By: | /s/ Sumeet Jain | |
| Sumeet Jain | ||
| By: | /s/ David Schulte | |
| David Schulte | ||
| A-106 |
Exhibit A
form of trans1 STOCKHOLDER AGREEMENT
This STOCKHOLDER AGREEMENT (this “Agreement”), dated as of March 3, 2013, is by and among Baxano, Inc., a Delaware corporation (“Baxano”), TranS1 Inc., a Delaware corporation (“TranS1”) (only with respect to Section 2(b) hereof), the undersigned stockholders of TranS1 (each, a “Stockholder” and, together, the “Stockholders”), and Sumeet Jain and David Schulte, solely as Securityholder Representatives (subject to and following appointment by the Securityholders pursuant to Section 10.14(a) of the Merger Agreement (as defined below)).
WHEREAS, concurrently with the execution and delivery of this Agreement, TranS1, RacerX Acquisition Corp.,a Delaware corporation and a wholly owned subsidiary of TranS1 (the “Transitory Subsidiary”), Baxano, and the Securityholder Representatives, have entered into an Agreement and Plan of Merger, dated as of the date hereof (as it may be amended or supplemented from time to time pursuant to the terms thereof, the “Merger Agreement”), which provides for the merger (the “Merger”) of the Transitory Subsidiary into Baxano in accordance with the terms of the Merger Agreement;
WHEREAS, the Stockholders are the beneficial owners (as defined in Rule 13d-3 under the Exchange Act) of such number of shares of each class of capital stock of TranS1 as is indicated on the signature page of this Agreement; and
WHEREAS, in consideration of the execution and delivery of the Merger Agreement by Baxano, each Stockholder desires to agree to certain transfer restrictions and to vote the Shares (as defined herein) over which Stockholder has voting power so as to facilitate the consummation of the Merger;
NOW, THEREFORE, in consideration of the foregoing, intending to be legally bound, the parties hereto hereby agree as follows:
1. Certain Definitions.
(a) Capitalized terms used but not otherwise defined herein shall have the meanings ascribed thereto in the Merger Agreement.
(b) For purposes of this Agreement, the following terms shall have the following meanings:
“Affiliate” means any person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with a person, as such terms are used in and construed under Rule 144 under the Securities Act of 1933, as amended. For clarity, the definition of “Affiliate” for any person that is a partnership shall include without limitation any general partner, managing member, officer or director of such person or any venture capital fund now or hereafter existing that is controlled by one or more general partners or managing members of, or shares the same management company with, such person.
“Constructive Sale” means with respect to any security, a short sale with respect to such security, entering into or acquiring an offsetting derivative contract with respect to such security, entering into or acquiring a futures or forward contract to deliver such security or entering into any other hedging or other derivative transaction that has the effect of either directly or indirectly materially changing the economic benefits or risks of ownership.
| A-107 |
“Registration Statement” means each registration statement required to be filed under Section 8 of that certain Securities Purchase Agreement, dated as of the date hereof, among TranS1 and the investors set forth therein, including (in each case) the Prospectus (as defined therein), amendments and supplements to such registration statement or Prospectus, including pre- and post-effective amendments, all exhibits thereto, and all material incorporated by reference or deemed to be incorporated by reference in such registration statement.
“Shares” means (A) all shares of capital stock of TranS1 owned, beneficially or of record, by each Stockholder as of the date hereof, and (B) all additional shares of capital stock of TranS1 acquired by each Stockholder, beneficially or of record, during the period commencing with the execution and delivery of this Agreement and expiring on the Expiration Date (as such term is defined in Section 8 below).
“Transfer” means, with respect to any security (including the Shares), the direct or indirect assignment, sale, transfer, tender, exchange, pledge, hypothecation, or the grant, creation or suffrage of a lien, security interest or encumbrance in or upon, or the gift, placement in trust, or the Constructive Sale or other disposition of such security (including transfers by testamentary or intestate succession or otherwise by operation of law) or any right, title or interest therein (including, but not limited to, any right or power to vote to which the holder thereof may be entitled, whether such right or power is granted by proxy or otherwise), or the record or beneficial ownership thereof, the offer to make such a sale, transfer, Constructive Sale or other disposition, and each agreement, arrangement or understanding, whether or not in writing, to effect any of the foregoing.
2. Transfer and Voting Restrictions With Respect to the Shares.
(a) Each Stockholder will not Transfer any of the Shares from the date hereof until the date the Registration Statement has been declared effective by the United States Securities and Exchange Commission (the “SEC”). The restrictions in the first sentence of this Section 2(a) shall not apply to (i) Transfers made (1) to an Affiliate of such Stockholder, (2) to such Stockholder’s spouse, lineal descendants, father, mother, brother or sister or any trust for the benefit of any such family member (collectively, “Immediate Family Members”) or (3) to a trust or otherwise for bona fide estate planning purposes if the beneficiaries of such trust consist solely of such Stockholder and/or his or her Immediate Family Members so long as in the case of each of (1), (2) and (3), the transferee agrees to be bound by the restrictions of this Section 2(a) and (ii) Shares or other securities owned by the spouse of such Stockholder or any other Immediate Family Member other than any securities subject to this Section 2(a) that are acquired by a transferee pursuant to the exception in clause (i) above.
(b) Each Stockholder understands and agrees that if Stockholder attempts to Transfer, vote or provide any other person with the authority to vote any of the Shares other than in compliance with this Agreement, TranS1 shall not, and Stockholder hereby unconditionally and irrevocably instructs TranS1 to not, (i) permit any such Transfer on its books and records, (ii) issue a new certificate representing any of the Shares or (iii) record such vote, in each case, unless and until Stockholder shall have complied with the terms of this Agreement.
(c) Except as otherwise permitted by this Agreement or by order of a court of competent jurisdiction, no Stockholder will commit any act that could restrict or affect Stockholder’s legal power, authority and right to vote all of the Shares then owned of record or beneficially by Stockholder or otherwise prevent or disable Stockholder from performing any of his, her or its obligations under this Agreement. Without limiting the generality of the foregoing, except for this Agreement and as otherwise permitted by this Agreement, no Stockholder will enter into any voting agreement with any person or entity with respect to any of the Shares, grant any person or entity any proxy (revocable or irrevocable) or power of attorney with respect to any of the Shares, deposit any of the Shares in a voting trust or otherwise enter into any agreement or arrangement with any person or entity limiting or affecting Stockholder’s legal power, authority or right to vote the Shares in favor of the approval of the Proposed Issuances.
| A-108 |
3. Agreement to Vote Shares Prior to Expiration Date.
(a) Matter One: Issuance of Shares as Net Merger Consideration. Prior to the Expiration Date, at every meeting of the stockholders of TranS1 called, and at every adjournment or postponement thereof, each Stockholder (in Stockholder’s capacity as such) shall appear in person or by proxy at the meeting or otherwise cause the Shares to be present thereat for purposes of establishing a quorum and, to the extent not voted by the persons appointed as proxies pursuant to this Agreement, vote (i) in favor of the issuance of the Merger Shares and the Financing Shares in connection with the transactions contemplated by the Merger Agreement (the “Proposed Issuances”), and (ii) against the approval or adoption of any proposal made in opposition to the Proposed Issuances.
(b) Matter Two: Election of Baxano Director Designees.
| i. | At TranS1’s 2013 annual meeting of stockholders, and at every adjournment or postponement thereof, each Stockholder (in Stockholder’s capacity as such) shall appear in person or by proxy at the meeting or otherwise cause the Shares to be present thereat for purposes of establishing a quorum and, to the extent not voted by the persons appointed as proxies pursuant to this Agreement, vote in favor of the election or re-election as members of the TranS1 Board for a three (3) year term the individuals designated by the Securityholder Representatives and nominated by the TranS1 nominating committee pursuant Section 6.10(c)(ii) of the Merger Agreement (the “Baxano Director Designees”). |
| ii. | From TranS1’s 2013 annual meeting of stockholders through the date immediately preceding the date directors are elected at TranS1’s 2016 annual meeting of stockholders, each Stockholder (in Stockholder’s capacity as such) shall appear in person or by proxy at each meeting of stockholders or otherwise cause the Shares to be present thereat for purposes of establishing a quorum and vote in favor of the election or re-election of any replacements of the Baxano Director Designees nominated by the TranS1 nominating committee pursuant Section 6.10(c)(ii) of the Merger Agreement, or so vote by written consent if votes are solicited by written consent. |
(c) Action by Beneficial Owner with Respect to Voting Shares. If a Stockholder is the beneficial owner, but not the record holder, of the Shares, such Stockholder shall take all actions necessary to cause the record holder and any nominees to vote all of the Shares in accordance with Section 3 hereof.
4. Grant of Irrevocable Proxies.
(a) In the event a Stockholder fails to perform as set forth in Section 3(a) of this Agreement, such Stockholder hereby irrevocably (to the fullest extent permitted by law) grants to, and appoints,Baxano and each of its executive officers and any of them, in their capacities as officers of Baxano(the “Matter One Grantees”), as Stockholder’s proxy and attorney-in-fact (with full power of substitution and re-substitution), for and in the name, place and stead of Stockholder, to vote the Shares, to instruct nominees or record holders to vote the Shares, or grant a consent or approval in respect of such Shares in accordance with Section 3(a) hereof and, in the discretion of the Matter One Grantees with respect to any proposed adjournments or postponements of any meeting of stockholders at which any of the matters described in Section 3(a) hereof is to be considered.
| A-109 |
(b) In the event a Stockholder fails to perform as set forth in Section 3(b) of this Agreement, such Stockholder hereby irrevocably (to the fullest extent permitted by law) grants to, and appoints,the Securityholder Representatives(the “Matter Two Grantee,” and, collectively with the Matter One Grantees, the “Grantees”), as Stockholder’s proxies and attorneys-in-fact (with full power of substitution and re-substitution), for and in the name, place and stead of Stockholder, to vote the Shares, to instruct nominees or record holders to vote the Shares, or grant a consent or approval in respect of such Shares in accordance with Section 3(b) hereof and, in the discretion of the Matter Two Grantee with respect to any proposed adjournments or postponements of any meeting of stockholders at which any of the matters described in Section 3(b) hereof is to be considered.
(c) Each Stockholder represents that any proxies heretofore given in respect of the Shares that may still be in effect are not irrevocable, and such proxies are hereby revoked.
(d) Each Stockholder hereby affirms that the irrevocable proxies set forth in this Section 4 are given in connection with the execution of the Merger Agreement, and that such irrevocable proxies are given to secure the performance of the duties of Stockholder under this Agreement. Each Stockholder hereby further affirms that each of the irrevocable proxies is coupled with an interest and may under no circumstances be revoked. Each Stockholder hereby ratifies and confirms all that such Grantees may lawfully do or cause to be done the actions set forth herein by virtue such irrevocable proxies. Such irrevocable proxies are executed and intended to be irrevocable in accordance with the provisions of Section 212 of the Delaware General Corporation Law.
(e) The Grantees may not exercise any irrevocable proxy set forth in this Section 4 on any other matter except as provided above. Stockholders may vote the Shares on all other matters.
(f) Baxano (prior to the Effective Time) or the Securityholder Representatives (after the Effective Time) may terminate any proxy set forth in this Section 4 with respect to any Stockholder at any time at its sole election by written notice provided to such Stockholder.
5. Action in Stockholder Capacity Only. No Stockholder makes any agreement or understanding herein as a director or officer of TranS1. Each Stockholder signs solely in Stockholder’s capacity as a record holder and beneficial owner, as applicable, of Shares, and nothing herein shall limit or affect any actions taken in Stockholder’s capacity as an officer or director of TranS1.
6. Representations and Warranties of Stockholder.
(a) Each Stockholder hereby represents and warrants to Baxano as follows: (i) Stockholder is the beneficial or record owner of the shares of capital stock of TranS1 indicated on the signature page of this Agreement free and clear of any and all pledges, liens, security interests, mortgage, claims, charges, restrictions, options, title defects or encumbrances; (ii) Stockholder does not beneficially own any securities of TranS1 other than the shares of capital stock and rights to purchase shares of capital stock of TranS1 set forth on the signature page of this Agreement; (iii) Stockholder has full power and authority to make, enter into and perform the terms of this Agreement and to grant the irrevocable proxy as set forth in Section 4; and (iv) this Agreement has been duly and validly executed and delivered by Stockholder and constitutes a valid and binding agreement of Stockholder enforceable against Stockholder in accordance with its terms. Each Stockholder agrees to notify Baxano promptly of any additional shares of capital stock of TranS1 of which Stockholder becomes the beneficial owner after the date of this Agreement.
| A-110 |
(b) As of the date hereof and for so long as this Agreement remains in effect, except for this Agreement or as otherwise permitted by this Agreement, each Stockholder has full legal power, authority and right to vote all of the Shares then owned of record or beneficially by Stockholder, in favor of the approval and authorization of the Proposed Issuances and the election of the Baxano Director Designees without the consent or approval of, or any other action on the part of, any other person or entity (including, without limitation, any governmental entity). Without limiting the generality of the foregoing, no Stockholder has entered into any voting agreement (other than this Agreement) with any person with respect to any of the Shares, granted any person any proxy (revocable or irrevocable) or power of attorney with respect to any of the Shares, deposited any of the Shares in a voting trust or entered into any arrangement or agreement with any person limiting or affecting Stockholder’s legal power, authority or right to vote the Shares on any matter.
(c) The execution and delivery of this Agreement and the performance by each Stockholder of his, her or its agreements and obligations hereunder will not result in any breach or violation of or be in conflict with or constitute a default under any term of any agreement, judgment, injunction, order, decree, law, regulation or arrangement to which Stockholder is a party or by which Stockholder (or any of his, her or its assets) is bound, except for any such breach, violation, conflict or default which, individually or in the aggregate, would not impair or adversely affect Stockholder’s ability to perform his, her or its obligations under this Agreement or render inaccurate any of the representations made by Stockholder herein.
(d) Each Stockholder understands and acknowledges that TranS1, the Transitory Subsidiary and Baxano are entering into the Merger Agreement in reliance upon Stockholder’s execution and delivery of this Agreement and the representations and warranties of Stockholder contained herein.
7. Confidentiality. EachStockholder recognizes that successful consummation of the Proposed Issuances may be dependent upon confidentiality with respect to the matters referred to herein. In this connection, pending public disclosure thereof, and so that TranS1 may rely on the safe harbor provisions of Rule 100(b)(2)(ii) of Regulation FD promulgated under the Exchange Act, eachStockholder hereby agrees not to disclose or discuss such matters with anyone not a party to this Agreement (other than its counsel and advisors, if any) without the prior written consent ofTranS1 and Baxano, except for disclosuresStockholder’s counsel advises are required by applicable law, in which caseStockholder shall give notice of such disclosure toTranS1 and Baxano as promptly as practicable so as to enableTranS1 and Baxano to seek a protective order from a court of competent jurisdiction with respect thereto.
8. Termination. This Agreement shall terminate and be of no further force or effect whatsoever as of the earlier of (a) such date and time as the Merger Agreement shall have been validly terminated pursuant to the terms of Article IX thereof or (b) the later of (i) the date the Registration Statement is declared effective or (ii) the date directors are elected at TranS1’s 2013 annual meeting of Stockholders (the “Expiration Date”). Notwithstanding the foregoing, Section 3(b)(ii) shall survive the termination and Expiration Date with respect to any Shares to the extent owned on a date thereafter and shall only terminate on the date directors are elected at TranS1’s 2016 annual meeting of stockholders.
| A-111 |
9. Miscellaneous Provisions.
(a) Amendments, Modifications and Waivers. This Agreement may not be amended or modified except by an instrument in writing signed on behalf of each of the parties hereto. Any agreement on the part of a party hereto to any waiver of any term or condition hereof shall be valid only if set forth in a written instrument signed on behalf of such party. Such waiver shall not be deemed to apply to any term or condition other than that which is specified in such waiver. The failure of any party to this Agreement to assert any of its rights under this Agreement or otherwise shall not constitute a waiver of such rights.
(b) Entire Agreement. This Agreement constitutes the entire agreement among the parties to this Agreement and supersedes any prior understandings, agreements or representations by or among the parties hereto, or any of them, written or oral, with respect to the subject matter hereof.
(c) Governing Law. All matters arising out of or relating to this Agreement and the transactions contemplated hereby (including without limitation its interpretation, construction, performance and enforcement) shall be governed by and construed in accordance with the internal laws of the State of Delaware without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of laws of any jurisdictions other than those of the State of Delaware.
(d) Submission to Jurisdiction. Each of the parties to this Agreement (i) consents to submit itself to the exclusive personal jurisdiction of the Chancery Court of the State of Delaware in any action or proceeding arising out of or relating to this Agreement or any of the transactions contemplated by this Agreement, (ii) agrees that all claims in respect of such action or proceeding may be heard and determined in such court, (iii) agrees that it shall not attempt to deny or defeat such personal jurisdiction by motion or other request for leave from such court and (iv) agrees not to bring any action or proceeding arising out of or relating to this Agreement or any of the transaction contemplated by this Agreement in any other court. Each of the parties hereto waives any defense of inconvenient forum to the maintenance of any action or proceeding so brought and waives any bond, surety or other security that might be required of any other party with respect thereto. Any party may make service on another party by sending or delivering a copy of the process to the party to be served at the address and in the manner provided for the giving of notices in Section 9(m) hereof. Nothing in this Section 9(d), however, shall affect the right of any party to serve legal process in any other manner permitted by law.
(e) WAIVER OF JURY TRIAL. EACH OF THE PARTIES HERETO HEREBY IRREVOCABLY WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY ACTION, PROCEEDING OR COUNTERCLAIM (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THE ACTIONS OF ANY PARTY IN THE NEGOTIATION, ADMINISTRATION, PERFORMANCE AND ENFORCEMENT OF THIS AGREEMENT.
(f) Attorneys’ Fees. In any action at law or suit in equity to enforce this Agreement or the rights of any of the parties hereunder, the prevailing party in such action or suit shall be entitled to receive its reasonable attorneys’ fees and all other reasonable costs and expenses incurred in such action or suit.
(g) Assignment and Successors. No party may assign any of its rights or delegate any of its performance obligations under this Agreement, in whole or in part, by operation of law or otherwise without the prior written consent of the other parties, except thatthe Securityholder Representatives, without obtaining the consent of any other parties hereto, shall be entitled to assign this Agreement or all or any of its rights or obligations hereunder to any successor Securityholder Representatives. Subject to the foregoing, this Agreement shall be binding upon, inure to the benefit of, and be enforceable by, the parties hereto and their respective successors and permitted assigns, including, without limitation, Stockholder’s estate and heirs upon the death of any Stockholder who is a natural person. Any purported assignment of rights or delegation of performance obligations in violation of this Section 9(g) shall be null and void.
| A-112 |
(h) No Third Party Beneficiaries. This Agreement is not intended, and shall not be deemed, to confer any rights or remedies upon any person other than the parties hereto, the stockholders of Baxano as of immediately prior to the Closing (as defined in the Merger Agreement) and their respective successors and permitted assigns, or to otherwise create any third-party beneficiary hereto.
(i) Cooperation. Each Stockholder agrees to cooperate fully with Baxano and the Securityholder Representatives and to execute and deliver such further documents, certificates, agreements and instruments and to take such other actions as may be reasonably requested by Baxano or the Securityholder Representatives to evidence or reflect the transactions contemplated by this Agreement and to carry out the intent and purpose of this Agreement. Each Stockholder hereby agrees that TranS1 and Baxano may publish and disclose in the Registration Statement (including all documents and schedules filed with the SEC) and the Proxy Statement, each such Stockholder’s identity and ownership of Shares and the nature of such Stockholder’s commitments, arrangements and understandings under this Agreement and may further file this Agreement as an exhibit to the Registration Statement or in any other filing made by TranS1 or Baxano with the SEC relating to the Proposed Issuances.
(j) Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If the final judgment of a court of competent jurisdiction declares that any term or provision hereof is invalid or unenforceable, the parties hereto agree that the court making such determination shall have the power to limit the term or provision, to delete specific words or phrases, or to replace any invalid or unenforceable term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term.
(k) Time of Essence. With regard to all dates and time periods set forth or referred to in this Agreement, time is of the essence.
(l) Specific Performance; Injunctive Relief. The parties hereto acknowledge that TranS1 and Baxano and the Securityholders shall be irreparably harmed and that there shall be no adequate remedy at law for a violation of any of the covenants or agreements of each Stockholder set forth in this Agreement. Each Stockholder accordingly agrees that, in addition to any other remedies that may be available to TranS1 or Baxano or the Stockholder Representative (on behalf of the Securityholders), as applicable, upon any such violation, such party shall have the right to enforce such covenants and agreements by specific performance, injunctive relief or by any other means available to such party at law or in equity without posting any bond or other undertaking.
(m) Notices. All notices and other communications hereunder shall be in writing and shall be deemed duly delivered (i) four business days after being sent by registered or certified mail, return receipt requested, postage prepaid, or (ii) one business day after being sent for next business day delivery, fees prepaid, via a reputable nationwide overnight courier service, in each case to the intended recipient as follows: (A) if to TranS1 or Baxano or to the Securityholder Representatives, to the address provided in the Merger Agreement, including to the persons designated therein to receive copies, and (B) if to any Stockholder, to such Stockholder’s address shown below Stockholder’s signature on the signature page hereof.
| A-113 |
(n) Counterparts and Signature. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original but all of which together shall be considered one and the same agreement and shall become effective when counterparts have been signed by each of the parties hereto and delivered to the other parties, it being understood that all parties need not sign the same counterpart. The exchange of copies of this Agreement or amendments thereto and of signature pages by facsimile transmission or by email transmission in portable document format, or similar format, shall constitute effective execution and delivery of such instrument(s) as to the parties and may be used in lieu of the original Agreement for all purposes. Signatures of the parties transmitted by facsimile or by email transmission in portable document format, or similar format, shall be deemed to be their original signatures for all purposes.
(o) Headings. The headings contained in this Agreement are for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement.
(p) Legal Representation. This Agreement was negotiated by the parties with the benefit of legal representation and any rule of construction or interpretation otherwise requiring this Agreement to be construed or interpreted against any party shall not apply to any construction or interpretation thereof.
[signature page follows]
| A-114 |
[Signature Page to TranS1 Stockholder Agreement]
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
BAXANO, INC.
_______________________________ Name: Title:
SECURITYHOLDER REPRESENTATIVES:
_______________________________ Name: Sumeet Jain
_______________________________ Name: David Schulte
|
| A-115 |
[Signature Page to TranS1 Stockholder Agreement]
IN WITNESS WHEREOF, the undersigned have caused this Agreement to be duly executed as of the date first written above.
STOCKHOLDER:
___________________
_______________________________ Name: Title:
Address: _______________________ _______________________ _______________________
Telephone: (___) _____-________ Facsimile: (___) _____-________ E-Mail Address: ___________________
Shares Beneficially Owned by Stockholder:
___________ shares of TranS1 Common Stock
___________ TranS1 Stock Options
|
| A-116 |
[Signature Page to TranS1 Stockholder Agreement]
With respect to Section 2(b) only:
TRANS1 INC.
_______________________________ Name: Title: |
| A-117 |
exhibit b
form of ESCROW AGREEMENT
This ESCROW AGREEMENT (this “Agreement”) is made and dated as of [March ___], 2013 (the “Closing Date”) by and among TranS1 Inc., a Delaware corporation (“TranS1”), Branch Banking and Trust Company, a North Carolina banking corporation (the “Escrow Agent”), and Sumeet Jain and David Schulte (the “Securityholder Representatives”), acting by virtue of the Merger Agreement (as defined below) as the attorneys-in-fact and representatives of the Securityholders of Baxano, Inc., a Delaware corporation (“Baxano”).
Capitalized terms used but not defined in this Agreement shall have the respective meanings set forth in that certain Agreement and Plan of Merger (as the same may be amended, supplemented or otherwise modified from time to time, the “Merger Agreement”), dated as of March 3, 2013, by and among TranS1, RacerX Acquisition Corp., a Delaware corporation (the “Transitory Subsidiary”), Baxano and the Securityholder Representatives.
RECITALS
TranS1, the Transitory Subsidiary, Baxano and the Securityholder Representatives have entered into the Merger Agreement, which provides for the merger of the Transitory Subsidiary with and into Baxano (the “Merger”), in connection with which the Securityholders of Baxano shall receive as consideration up to the number of shares of Common Stock of TranS1 (“TranS1 Common Stock”) determined, allocated and distributed among the Securityholders in accordance with the Merger Agreement and this Agreement.
Pursuant to the Merger Agreement, the parties thereto have agreed that the Escrow Shares may be applied to fund (i) any adjustment of the Net Merger Consideration payable to TranS1 pursuant to Section 2.11(f) of the Merger Agreement and (ii) any indemnification obligations payable by the Escrow Participants to TranS1 Indemnified Parties pursuant to Article VIII of the Merger Agreement.
Pursuant to the Merger Agreement, the parties thereto have agreed that the Representative Reimbursement Amount shall be applied to fund any indemnification or reimbursement obligations payable by the Securityholders to the Securityholder Representatives pursuant to Section 10.14(d) of the Merger Agreement.
Pursuant to Section 2.9(b) of the Merger Agreement, each Securityholder that is an Escrow Participant is deemed to have approved this Agreement and the escrow arrangements contemplated herein by virtue of the Baxano Stockholder Approval or the actions taken by Baxano pursuant to Section 2.4(iii) of the Merger Agreement.
Pursuant to Section 10.14 of the Merger Agreement, the Securityholder Representatives have been appointed by the Securityholders as their attorneys-in-fact and have been authorized and empowered to act for and on behalf of any or all Securityholders (with full power of substitution in the premises) in connection with this Agreement, including the power and authority on behalf of each Securityholder to do or take any one or more of the actions enumerated in Section 1.3.
| A-118 |
The Escrow Agent is willing to act in the capacity of escrow agent hereunder subject to, and upon the terms and conditions of, this Agreement.
AGREEMENT
NOW, THEREFORE, in consideration of the premises, covenants and agreements set forth in this Agreement and of other good and valuable consideration, the receipt and legal sufficiency of which they hereby acknowledge, and intending to be legally bound, and as an inducement for the consummation of the transactions contemplated by the Merger Agreement, the parties agree as follows:
ARTICLE I.
DESIGNATION OF ESCROW AGENT AND PROPERTY
SUBJECT TO ESCROW
1.1 Designation of Escrow Agent. TranS1 and the Securityholders (by and through the Securityholder Representatives) hereby mutually designate and appoint Branch Banking and Trust Company, a North Carolina banking corporation having an office and place of business located at 223 West Nash Street, Wilson, North Carolina 27893, as escrow agent for the purposes set forth herein, subject to replacement as provided in Section 5.4
. The Escrow Agent hereby accepts such appointment and agrees to act in furtherance of the provisions of the Merger Agreement, but only upon the terms and conditions provided in this Agreement.
1.2 Establishment of Escrow.
(a) In accordance with Section 2.9(a) of the Merger Agreement, at or prior to the Closing of the Merger, TranS1 shall issue and deliver, or cause to be issued and delivered, to the Escrow Agent (i) the Representative Reimbursement Amount, in the amount of $250,000 (the “Cash Escrow”), and (ii) shares of TranS1 Common Stock constituting Escrow Shares, in the number determined pursuant to the Merger Agreement.
(b) In addition, in accordance with Sections 2.11(f)(iii) and 2.3(a) of the Merger Agreement, not more than five (5) Business Days following the finalization of the Conclusive Adjustment Statement, if the Adjustment Amount is a positive number and the absolute value of such number exceeds $250,000, TranS1 shall issue and deliver, or cause to be issued and delivered, to the Escrow Agent, additional shares of TranS1 Common Stock constituting Escrow Shares, in the number determined pursuant to the Merger Agreement.
(c) The Cash Escrow shall be delivered by TranS1 by wire transfer of immediately available funds, and shall be deposited by the Escrow Agent in an account in the name of the Escrow Agent, as escrow agent hereunder. The Escrow Shares shall be delivered by TranS1, at TranS1’s option, in either certificated or book-entry form, and shall be registered in the name of the Escrow Agent, as escrow agent hereunder and as nominee for the Securityholders as beneficial owners of the Escrow Shares. The Escrow Agent shall deposit all Escrow Shares in an account that is segregated from the Cash Escrow. Promptly upon each receipt by the Escrow Agent of the Cash Escrow and Escrow Shares, the Escrow Agent shall execute and deliver a receipt therefor to TranS1 and the Securityholder Representatives. Except as otherwise provided in this Agreement, the Escrow Agent shall also hold under this Agreement any investment proceeds of the Cash Escrow and any cash, securities or other property that may be distributed on account of the Escrow Shares. The Cash Escrow, the Escrow Shares and such investment proceeds and other distributions are collectively referred to as the “Escrow Property.” The Escrow Agent shall hold the Escrow Property in safekeeping and distribute the Escrow Property only in accordance with the terms hereof.
| A-119 |
1.3 Securityholder Representatives.
(a) Pursuant to Section 10.14 of the Merger Agreement, the Securityholders have irrevocably appointed the Securityholder Representatives as their representatives, agents, proxies and attorneys-in-fact with respect to all post-Closing matters arising under the Merger Agreement and all actions to be taken by or on behalf of the Securityholders in connection with this Agreement, including without limitation the full power and authority on the Securityholders’ behalf to jointly take the actions set forth herein (all of which actions shall be deemed to be facts ascertainable outside of this Agreement and shall be binding on the Securityholders as a matter of contract law), including, without limitation:
(i) to consummate the transactions contemplated under this Agreement, the Merger Agreement and the other agreements, instruments, and documents contemplated hereby or thereby or executed in connection herewith or therewith;
(ii) to negotiate and settle disputes arising under, or relating to, this Agreement, the Merger Agreement and the other agreements, instruments, and documents contemplated hereby or thereby or executed in connection herewith or therewith;
(iii) to execute and deliver any amendment or waiver to this Agreement, the Merger Agreement and the other agreements, instruments, and documents contemplated hereby or thereby or executed in connection herewith or therewith (without the prior approval of the Securityholders);
(iv) to retain legal and other advisors in connection with the foregoing, the reimbursement of reasonable fees incurred in connection therewith to be considered Representative Reimbursement Amounts hereunder; and
(v) to take any and all other actions whatsoever to be taken by or on behalf of the Securityholders in connection with this Agreement, the Merger Agreement and the other agreements, instruments, and documents contemplated hereby or thereby or executed in connection herewith or therewith.
(b) Other than the Securityholder Representatives, no Securityholder shall have any right to intervene or take part in, or otherwise delay, any action related to the Escrow Property.
(c) Pursuant to Section 10.14(f) of the Merger Agreement, Securityholders holding in the aggregate Escrow Participation Percentages in excess of 50% may, by written consent, remove either or both of the Securityholder Representatives and appoint new representatives as their replacements. Notice together with a copy of the written consent appointing such new representative and bearing the signatures of Securityholders of a majority-in-interest of those Securityholders must be delivered to TranS1 and the Escrow Agent not less than ten (10) calendar days prior to such appointment. Such appointment will be effective upon the later of the date indicated in the consent or ten (10) calendar days following the date such consent is received by TranS1 and the Escrow Agent.
| A-120 |
ARTICLE II.
DURATION OF ESCROW; TREATMENT OF ACCUMULATIONS
TO ESCROW SHARES; INVESTMENT OF CASH ESCROW
2.1 Duration of Escrow. The Escrow Agent shall hold the Escrow Property as provided in this Agreement until complete distribution thereof in accordance with the applicable provisions of Article III and/or Article IV.
2.2 Additional Property Received on Account of Escrow Property.
(a) At any time after delivery of the Escrow Shares to the Escrow Agent and prior to the complete distribution of the Escrow Shares in accordance with the applicable provisions of Article III and/or Article IV, if the Escrow Agent, as the registered owner of the Escrow Shares under Section 1.2
, shall become entitled to receive or shall receive on account of the Escrow Shares (i) any (A) non-taxable distribution of securities of TranS1 or of any other Person including, without limitation, any certificate in connection with any increase or reduction of capital, reclassification, recapitalization, merger, business combination, consolidation, sale of assets, stock split-up or spin-off; or (B) non-taxable distribution of stock options, warrants or rights, whether as an addition to or in substitution of or exchange for any of the Escrow Shares; or (C) non-taxable stock dividend or other non-taxable distribution payable in securities or property of any description; then, all of the property received upon any such distribution shall be deemed to be Escrow Shares and shall be subject to the terms hereof to the same extent as the original Escrow Shares; (ii) any taxable stock dividends, then the Escrow Agent shall promptly transfer and/or deliver, as applicable, such taxable stock dividends to TranS1 (for furtherdistribution to the Securityholders in accordance with Section 2.3(b) of the Merger Agreement); or (iii) any cash dividends, then the Escrow Agent shall promptly deliver such cash dividends to the Securityholders (in accordance Section 2.3(b) of the Merger Agreement and the Escrow Allocation Percentages attached hereto asExhibit A).
(b) The Escrow Agent is hereby directed to invest the Cash Escrow in a [BB&T Business Managed Money Rate Savings Account] or such other account as determined from time to time by the Securityholder Representatives in writing. Any interest or other income earned thereon shall be deemed to be Cash Escrow and shall be subject to the terms hereof to the same extent as the original Cash Escrow. [The BB&T Business Managed Money Rate Savings Account] will earn a fixed rate of[__]bps for a total of [_______] ([__]) months after the Closing Date. Thereafter, the Cash Escrow will earn the rate then in effect for such account from time to time or the rate otherwise agreed to by the Escrow Agent and the Securityholder Representatives.
| A-121 |
2.3 Retained Voting and Other Rights.
(a) While the Escrow Shares remain in escrow pursuant to this Agreement, the Securityholders will retain and will be able to exercise all incidents of ownership of the Escrow Shares that are not inconsistent with the terms and conditions of this Agreement. The Escrow Shares held pursuant to this Agreement will be shown as issued and outstanding on the books and records of TranS1.
(b) The Escrow Agent shall hold the Escrow Property (including any additional property acquired with respect thereto pursuant to Section 2.2) in safekeeping and dispose thereof only in accordance with the terms of this Agreement. The Escrow Agent may treat the Securityholder Representatives as the duly authorized agents and representatives of the Securityholders with respect to any additional property related to the Escrow Shares. After its receipt thereof, the Escrow Agent shall hold the Escrow Shares in accordance with this Agreement and shall (to the extent legally permissible) vote the Escrow Shares in accordance with the written instructions (if any) provided by the Securityholders. The Escrow Agent shall not vote any Escrow Shares for which the Escrow Agent has not received from a Securityholder written instructions in form and substance reasonably satisfactory to the Escrow Agent.
ARTICLE III.
TERMINATION OF AGREEMENT;
FINAL DISTRIBUTIONS OF ESCROW PROPERTY
3.1 Termination of Agreement. This Agreement shall terminate automatically upon complete distribution of the Escrow Property in accordance with this Agreement, except for provisions hereof that by their terms explicitly survive termination.
3.2 Final Distributions of the Escrow Shares.
(a) In accordance with Section 8.7(b) of the Merger Agreement, promptly after the twelve (12) month anniversary of the Closing Date, the Escrow Agent shall deliver to the Securityholders (in accordance Section 2.3(b) of the Merger Agreement and the Escrow Allocation Percentages attached hereto asExhibit A) that portion of the Escrow Shares not otherwise (i) directed for distribution to TranS1 by joint written instructions received by the Escrow Agent but not yet distributed, if any, or (ii) subject to claims asserted pursuant to Article IV.
(b) Thereafter, any remaining Escrow Shares shall continue to be held by the Escrow Agent in accordance with the terms of this Agreement until distributed to TranS1 (if applicable) and all claims asserted pursuant to Article IV have been resolved or until otherwise directed by joint written instructions received by the Escrow Agent from TranS1 and the Securityholder Representatives. In accordance with Section 8.7(b) of the Merger Agreement, upon such claims resolution or receipt of such instructions, the Escrow Agent shall promptly distribute any remaining Escrow Shares to the Securityholders (in accordance Section 2.3(b) of the Merger Agreement and the Escrow Allocation Percentages attached hereto asExhibit A) or as directed in such instructions, as the case may be, in full discharge of the Escrow Agent's obligations with respect to the Escrow Shares under this Agreement.
| A-122 |
3.3 Final Distribution of the Cash Escrow.
(a) In accordance with Section 8.7(b) of the Merger Agreement, promptly after the twelve (12) month anniversary of the Closing Date, the Escrow Agent shall deliver the balance of the Cash Escrow, if any, to the Securityholders (in accordance Section 2.3(b) of the Merger Agreement and the Escrow Allocation Percentages attached hereto asExhibit A); provided that the Securityholder Representatives may instruct the Escrow Agent to retain such portion of the Cash Escrow as the Securityholder Representatives deem necessary to administer any outstanding claims asserted pursuant to Article IV.
(b) Thereafter, any remaining balance in the Cash Escrow account shall be released to the Securityholders (in accordance Section 2.3(b) of the Merger Agreement and the Escrow Allocation Percentages attached hereto asExhibit A) at the same time as any remaining Escrow Shares are distributed to TranS1 pursuant to Section 3.2(b).
(c) The distribution(s) of the Cash Escrow in accordance with this Section 3.3 shall be in full discharge of the Escrow Agent’s obligationswith respect to the Cash Escrow under this Agreement.
ARTICLE IV.
RELEASE OF ESCROW PROPERTY
4.1 Release of Escrow Shares as Net Merger Consideration Adjustment. In accordance with Section 2.11(f)(ii) of the Merger Agreement, promptly (and in no event more than two (2) Business Days) following the finalization of the Conclusive Adjustment Statement, if the Adjustment Amount is a negative number and the absolute value of such number exceeds $250,000, then TranS1 and the Securityholder Representatives shall issue joint written instructions to the Escrow Agent setting forth the number of Escrow Shares to be delivered to TranS1 in accordance with Section 2.11(f)(ii) of the Merger Agreement. Promptly (and in no event more than three (3) Business Days) following receipt of such instructions, the Escrow Agent shall transfer and/or deliver, as applicable, such number of Escrow Shares to TranS1. “Business Day” shall mean any day except Saturday, Sunday or any other day on which commercial banks located in Wilson, North Carolina are authorized or required by law to be closed for business.
4.2 Distribution of Excess Escrow Shares After Determination of Adjustment Adjustment. In accordance with Section 8.7(a) of the Merger Agreement, promptly following any delivery of additional Escrow Shares by TranS1 pursuant to Section 2.11(f)(iii) of the Merger Agreement or any release of Escrow Shares to TranS1 pursuant to Section 4.1, as applicable, TranS1 and the Securityholder Representatives shall issue joint written instructions to the Escrow Agent setting forth the number of Escrow Shares to be released to the Securityholders (in accordance Section 2.3(b) of the Merger Agreement and the Escrow Allocation Percentages attached hereto asExhibit A), which number shall be determined in accordance with Section 8.7(a) of the Merger Agreement. Promptly (and in no event more than three (3) Business Days) following receipt of such letter, the Escrow Agent shall transfer and deliver such number of Escrow Shares to the Securityholders (in accordance Section 2.3(b) of the Merger Agreement and the Escrow Allocation Percentages attached hereto asExhibit A).
| A-123 |
4.3 Assertion of Claims Against Escrow Shares. If (i) TranS1 (on its own behalf or on behalf of any other TranS1 Indemnified Person) shall timely assert a claim for indemnification pursuant to Article VIII of the Merger Agreement and (ii) the Merger Agreement permits or requires such claim (if valid) to be satisfied from the Escrow Shares, then TranS1 shall submit to the Escrow Agent and the Securityholder Representatives a written claim in good faith signed by an authorized officer of TranS1 stating (a) that a TranS1 Indemnified Party is making such claim and its reasonable estimate of the amount claimed; (b) in reasonable detail, the facts alleged as the basis for such claim and the section or sections of the Merger Agreement alleged as the basis or bases for such claim; and (c) if the related losses have actually been incurred, the number of Escrow Shares (calculated according to the applicable Escrow Shares Indemnity Value) to which such TranS1 Indemnified Party is entitled with respect to such losses. If the claim is for losses based on an event, condition or circumstance occurring on or before the applicable claims deadline but the actual amount of such losses is not yet known with certainty, the written claim of TranS1 shall state the number of Escrow Shares (calculated according to the applicable Escrow Shares Indemnity Value) which such Person reasonably estimates for such losses, in which event a claim shall be deemed to have been asserted against the Escrow Shares in the amount of such losses. No Escrow Shares shall be released by the Escrow Agent until such losses have actually been incurred and TranS1 submits a notice to the Escrow Agent and the Securityholder Representatives in accordance with Section 4.4. Any claim made in accordance with the Merger Agreement and this Section 4.3 at or prior to the applicable deadline established in the Merger Agreement shall continue and shall be subject to final resolution as provided herein, regardless of whether any action or demand has been commenced upon such claim prior to such deadline.
4.4 Resolution of Undisputed Claims Against the Escrow Shares. If, within twenty (20) calendar days after TranS1 gives notice to the Escrow Agent and the Securityholder Representatives of an asserted claim pursuant to Section 4.3, the Escrow Agent and TranS1 do not receive a Claim Objection from the Securityholder Representatives disputing all or a portion of such claim, then the Escrow Agent shall promptly release to TranS1 (for further distribution pursuant to the Merger Agreement) a number of Escrow Shares equal to the amount of the undisputed portion of the asserted claim (calculated according to the applicable Escrow Shares Indemnity Value) if liquidated in amount or, if not liquidated in amount, promptly after such unliquidated claim (or undisputed portion thereof) has become liquidated in amount and TranS1 shall have delivered to the Escrow Agent and the Securityholder Representatives a certificate to that effect stating the number of Escrow Shares (calculated according to the applicable Escrow Shares Indemnity Value) equal to the amount of the undisputed claim (or portion thereof);provided,however, that if, within the twenty (20) calendar day period after notice is given to the Escrow Agent and the Securityholder Representatives that such unliquidated claim (or undisputed portion thereof) has become liquidated in amount, the Securityholder Representatives shall notify the Escrow Agent and TranS1 that the Securityholder Representatives reasonably dispute in good faith any portion of the amount stated in TranS1’s certificate to have become liquidated, then such portion of the claim shall be treated as a disputed claim under Section 4.5. For the avoidance of doubt, if the Securityholder Representatives dispute a portion but not all of an asserted claim, the Escrow Agent promptly shall release to TranS1 (for further distribution pursuant to the Merger Agreement) a number of Escrow Shares equal to the amount (calculated according to the applicable Escrow Shares Indemnity Value) of the undisputed portion of such claim following receipt of the Claim Objection.
| A-124 |
4.5 Resolution of Disputed Claims Against the Escrow Shares. If the Securityholder Representatives shall notify the Escrow Agent and TranS1 that the Securityholder Representatives reasonably dispute in good faith all or a portion of a claim asserted by TranS1 pursuant to Section 4.3, the Escrow Agent shall not release Escrow Shares on account of the disputed portion of such claim unless and until the Escrow Agent shall have received joint written instructions pursuant to Section 8.4(c)(iii) of the Merger Agreement from TranS1 and the Securityholder Representatives directing the Escrow Agent to release a specified number of Escrow Shares (calculated using the Escrow Shares Indemnity Value) equal to the amounts due and owing to the TranS1 Indemnified Party, and the Escrow Agent shall promptly (and in any event within five (5) Business Days) release Escrow Shares in the manner described in Section 4.4.
4.6 No Fractional Shares. Except as set forth in the next sentence, in the event the Escrow Agent would be required to transfer or deliver a fraction of an Escrow Share under any provision of this Agreement, the number of Escrow Shares transferred or delivered shall be rounded down to the next lower whole number of Escrow Shares. In connection with the final distribution of Escrow Shares pursuant to Section 3.2(b), the Escrow Agent shall preliminarily determine the fractional share value (between 0 and 1) to which each Securityholder would be entitled using the Escrow Allocation Percentages attached hereto asExhibit A. Following such calculation, the Escrow Agent shall (i) sum all such fractional shares into a number of whole shares, and (ii) allocate and distribute one whole share to each of the Securityholders with the highest fractional share values, until all such whole shares have been distributed. Upon the Escrow Agent’s request, TranS1 and the Securityholder Representatives shall provide joint written instructions regarding the application of the preceding sentence to the final distribution of Escrow Shares pursuant to Section 3.2(b).
4.7 Release of the Cash Escrow. In accordance with Sections 2.9(a) and 10.14(d) of the Merger Agreement, the Securityholder Representatives shall have full authority to direct the Escrow Agent to disburse all or a portion of the Cash Escrow to the Securityholder Representatives or their designee (for application in accordance with the Merger Agreement) at any time for the reimbursement of (i) reasonable costs and expenses of the Securityholder Representatives in connection with their duties under the Merger Agreement and this Agreement, and (ii) in accordance with Section 10.14(d) of the Merger Agreement, expenses, judgments, fines and amounts incurred by the Securityholder Representatives and the other Persons identified in Section 10.14(d) of the Merger Agreement in connection with a Proceeding to which the Securityholder Representatives or such other Person is made a party by reason of the fact that it is or was acting as a Securityholder Representative pursuant to the terms of the Merger Agreement or this Agreement. Promptly upon receipt of such direction from the Securityholder Representatives, the Escrow Agent shall disburse the Cash Escrow or portion thereof as so directed.
4.8 Redirection of Escrow Property. Notwithstanding anything to the contrary in this Agreement, TranS1 and the Securityholder Representatives may provide the Escrow Agent with joint written instructions (i) specifying the Escrow Property (or any cash dividends or taxable stock dividends received on account of the Escrow Shares) to be transferred and/or delivered, as applicable, directly to another Person (including the transfer agent for TranS1 Common Stock) and (ii) confirming that such transfer and/or delivery is in accordance with the Merger Agreement, and promptly upon receipt of such instructions the Escrow Agent shall effect the distribution of such property to such other Person.
| A-125 |
ARTICLE V.
RIGHTS AND RESPONSIBILITIES OF ESCROW AGENT
5.1 Rights, Duties, Liabilities and Immunities of Escrow Agent. TranS1 and the Securityholder Representatives hereby agree as follows with respect to the rights, duties, liabilities and immunities of the Escrow Agent:
(a) The Escrow Agent shall act as a depository only and shall not be responsible or liable in any manner whatsoever for the sufficiency, correctness, genuineness or validity of the Escrow Property deposited with it, or any part thereof. The Escrow Agent shall hold the Escrow Property (including any additional property acquired with respect thereto pursuant to Section 2.2
) in safekeeping and dispose thereof only in accordance with the terms of this Agreement. The Escrow Agent shall have no implied duties or obligations, and shall not be charged with knowledge or notice of any fact except as specifically provided herein. Without limiting the generality of the foregoing, the Escrow Agent shall not be responsible for or be required to enforce any of the terms or conditions of the Merger Agreement or any other documents contemplated thereby.
(b) The Escrow Agent shall be protected in acting upon any written certificate, notice, request, waiver, consent, receipt or other paper or document furnished to it, not only as to its due execution and the validity and effectiveness of its provisions, but also as to the truth and acceptability of any information therein contained which the Escrow Agent in good faith believes to be genuine and what it purports to be.
(c) The Escrow Agent shall not be liable for any error of judgment, or for any act done or steps taken or made by it in good faith, or for any mistake of fact or law, or for any things which it may do or refrain from doing in connection herewith, except due to the Escrow Agent’s own gross negligence or willful misconduct. In no event shall the Escrow Agent be liable for incidental, indirect, special, consequential or punitive damages, except due to the Escrow Agent’s own gross negligence or willful misconduct.
(d) The Escrow Agent may consult with and obtain advice from legal counsel in the event of any question as to any of the provisions of this Agreement or its duties hereunder, and the Escrow Agent shall incur no liability and shall be fully protected in acting in good faith in accordance with the opinion and instructions of such counsel. Subject to the provisions of Section 5.3 hereof, the reasonable cost of such services shall be added to and shall become a part of the Escrow Agent’s compensation hereunder.
(e) The Escrow Agent shall have no duties except those expressly set forth herein and shall not be bound by any notice of a claim or demand with respect thereto, or any waiver, modification, amendment, termination or rescission of this Agreement, unless in a writing received by it, and, if its duties herein are affected, unless it shall have given its prior written consent thereto.
| A-126 |
(f) The Escrow Agent is not a party to and is not bound by the Merger Agreement, nor is it a party to or bound by or charged with notice of any other agreement (other than this Agreement) to which the Escrow Property may relate.
(g) In the event of any disagreement between any of the parties to this Agreement, or between them or any one of them and any other person, resulting in adverse claims or demands being made in connection with the subject matter of this Agreement, or in the event the Escrow Agent in good faith shall be in doubt as to what action it should take hereunder, the Escrow Agent shall thereupon have the right (i) to refrain from complying with any claims or demands asserted on it as the Escrow Agent or (ii) to refuse to take any other action hereunder, so long as such disagreement continues or exists, and in either such event, the Escrow Agent shall not be or become liable in any way to any person for the Escrow Agent’s failure to act, and the Escrow Agent shall be entitled to continue to refrain from acting, until (x) the rights of all parties shall have been fully and finally adjudicated by a court of competent jurisdiction or (y) all differences shall have been adjusted and all doubts resolved by agreement among all of the interested persons, and the Escrow Agent shall have been notified thereof by a writing signed by all such persons.
(h) From and at all times after the date of this Agreement, TranS1 shall, to the fullest extent permitted by law, indemnify and hold harmless the Escrow Agent and each director, officer, employee, attorney, agent and affiliate of the Escrow Agent (collectively, the “Escrow Indemnified Persons”) against any and all actions, claims (whether or not valid), damages, liabilities, costs and expenses (including without limitation reasonable attorneys’ fees, costs and expenses) (“Escrow Damages”) incurred by or asserted against any of the Escrow Indemnified Persons from and after the date hereof, relating to or arising from or in connection with any claim, demand, suit, action or proceeding by any person arising from or in connection with the negotiation, preparation, execution, performance or failure of performance of this Agreement or any transactions contemplated herein; provided, however, that no Escrow Indemnified Person shall have the right to be indemnified hereunder for any liability finally determined by a court of competent jurisdiction to have resulted from the gross negligence or willful misconduct of such Escrow Indemnified Person. If any such action or claim shall be brought or asserted against any Escrow Indemnified Person, such Escrow Indemnified Person shall promptly notify both TranS1 and the Securityholder Representatives in writing, and TranS1 shall assume the defense thereof, including the employment of counsel and the payment of all expenses. Such Escrow Indemnified Person shall, in its sole discretion, have the right to employ separate counsel in any such action and to participate in the defense thereof, and the reasonable fees and expenses of such counsel shall be paid by such Escrow Indemnified Person, except that TranS1 shall be required to pay such reasonable fees and expenses if (i) TranS1 agrees to pay such fees and expenses, (ii) TranS1 does not assume the defense of such action or proceeding or does not, in the reasonable discretion of such Escrow Indemnified Person, employ counsel reasonably satisfactory to the Escrow Indemnified Person in any such action or proceeding, (iii) TranS1 is the plaintiff in any such action or proceeding or (iv) the named parties to any such action or proceeding (including any impleaded parties) include both such Escrow Indemnified Person and TranS1, and the Escrow Indemnified Person shall have been advised by counsel that there may be one or more legal defenses available to it which are different from or additional to those available to TranS1. The obligations of TranS1 under this Section 5.1(h) shall survive any termination of this Agreement and the resignation or removal of the Escrow Agent. The Securityholders (by and through the Securityholder Representatives) shall reimburse TranS1 for its expenses incurred pursuant to this Section5.1(h)as, if and to the extent required by Section6.8. The amount of such reimbursement shall be treated as an undisputed, liquidated claim against the Escrow Shares pursuant to Section4.4
| A-127 |
.
(i) The Escrow Agent is authorized, in its discretion, to comply with orders issued or process entered by any court with respect to the Escrow Property, without determination by the Escrow Agent of such court’s jurisdiction in the matter.
(j) If, at any time, there shall exist any dispute with respect to the holding or disposition of any portion of the Escrow Property or any other obligations of the Escrow Agent hereunder, or if at any time the Escrow Agent is unable to determine, to the Escrow Agent’s reasonable satisfaction, the proper disposition of any portion of the Escrow Property or the Escrow Agent’s proper actions with respect to its obligations hereunder, or if TranS1 and the Securityholder Representatives have not within thirty (30) days of the furnishing by the Escrow Agent of a notice of resignation pursuant to Section 5.4 hereof appointed a successor escrow agent to act hereunder, then the Escrow Agent may, in its sole discretion, take either or both of the following actions upon written notice to Buyer and the Securityholder Representatives:
(i) Hold and decline to make further disbursements from the Escrow Property that the Escrow Agent would otherwise be obligated to make hereunder until such dispute or uncertainty shall be resolved to the reasonable satisfaction of the Escrow Agent or until a successor escrow agent shall have been appointed (as the case may be); or
(ii) Petition (by means of an interpleader action or any other appropriate method) the Superior Court for Wake County, North Carolina, or if that court should be without subject matter jurisdiction or should decline to exercise jurisdiction, any other state or federal court of competent jurisdiction in North Carolina, for instructions with respect to such dispute or uncertainty, and pay into such court all of the Escrow Property for holding and disposition in accordance with the instructions of such court.
The Escrow Agent shall have no liability to TranS1, the Securityholders, the Securityholder Representatives or any other person with respect to any such actions taken pursuant to this Section 5.1(j), specifically including any liability or claimed liability that may arise, or be alleged to have arisen, out of or as a result of any delay in the disbursement from the Escrow Property or any delay in or with respect to any other action required or requested of the Escrow Agent, except for any Escrow Damages resulting from the gross negligence or willful misconduct of the Escrow Agent.
5.2 Copies of Certifications, Notices and Other Documentation. Promptly after receipt by the Escrow Agent from the Securityholder Representatives or TranS1, as the case may be, of any written certificate, notice, request, waiver, consent, receipt or other document, the Escrow Agent shall furnish a copy of such item to TranS1 or the Securityholder Representatives, as the case may be.
5.3 Compensation. The Escrow Agent shall receive a fee of $[____] per year for its services hereunder. The first year’s fee shall be payable upon the delivery of the Escrow Property to the Escrow Agent, and such fee shall not be subject to proration in the event that the escrow arrangement terminates before the end of a year. The Escrow Agent shall also be entitled to reimbursement for all reasonable expenses, disbursements and advances (including reasonable attorneys’ fees) incurred or made by the Escrow Agent in accordance with any of the provisions of this Agreement, exclusive of any such expense, disbursement or advance that may arise from its own gross negligence or willful misconduct. Any such compensation and reimbursement of the Escrow Agent under the provisions of this Section 5.3 shall be paid by TranS1, subject to Section6.8. The annual fee indicated above shall be waived if the Cash Escrow is invested in a [BB&T Business Managed Money Rate Savings Account].
| A-128 |
5.4 Successor Escrow Agent. The Escrow Agent or any successor to it hereafter appointed may at any time resign by giving notice in writing to the Securityholder Representatives and TranS1, and such resignation shall become effective and the Escrow Agent shall be discharged from its prospective duties hereunder upon the appointment of a successor escrow agent as hereinafter provided. In the event of any such resignation, a successor escrow agent shall be appointed by written consent of the Securityholder Representatives and TranS1 and shall be a bank or trust company organized under the laws of the United States of America having (or if such bank or trust company is a member of a bank company, its bank holding company has) a combined capital and surplus of not less than $50,000,000. Any successor escrow agent shall deliver to the Securityholder Representatives and TranS1 a written instrument accepting the appointment hereunder, and thereupon it shall succeed to all the rights and duties of the Escrow Agent hereunder and shall be entitled to receive all assets then held by the predecessor escrow agent hereunder.
ARTICLE VI.
MISCELLANEOUS
6.1 Successors and Assigns. This Agreement shall be binding upon and shall inure to the benefit of the Securityholder Representatives (in their individual capacities and as the attorneys-in-fact and representatives of the Securityholders), TranS1 and the Escrow Agent, and their respective successors, heirs and permitted assigns.
6.2 Waiver of Consent. No failure or delay on the part of any party hereto in exercising any power or right hereunder shall operate as a waiver thereof, nor shall any single or partial exercise of any such right or power, or any abandonment or discontinuance of steps to enforce such a right or power, preclude any other or further exercise thereof or the exercise of any other right or power. The rights and remedies of the parties hereunder are cumulative and not exclusive of any rights or remedies which they would otherwise have. No modification or waiver of any provision of this Agreement, nor consent to any departure by any party therefrom, shall in any event be effective unless the same shall be in writing, and then such waiver or consent shall be effective only in the specific instance and for the purpose for which given. No notice to or demand on any party in any case shall entitle such party to any other or further notice or demand in similar or other circumstances.
6.3 Interpretation. The Article and Section captions used herein are for reference purposes only and shall not in any way affect the meaning or interpretation of this Agreement. Unless otherwise specified, references in this Agreement to a Section or an Article shall be to a Section or an Article of this Agreement. Whenever the context may require, any pronouns used in this Agreement shall include the corresponding masculine, feminine or neuter forms, and the singular form of nouns and pronouns shall include the plural, and vice versa. Whenever the words “include,” “includes” or “including” are used in this Agreement, they shall be deemed to be followed by the words “without limitation.”
| A-129 |
6.4 Notices. Notice or other communications required or permitted hereunder shall be in writing and shall be deemed to have been sufficiently given (a) four (4) Business Days following deposit in the mails if sent by registered or certified mail, postage prepaid; (b) when delivered, if delivered personally to the intended recipient; and (c) one (1) Business Day following deposit with an overnight courier service, in each case addressed as follows:
If to TranS1, to:
TranS1 Inc.
110 Horizon Drive
Suite 230
Raleigh, North Carolina 27615
Attention: Chief Financial Officer
Telephone: (910) 332-1700
with a copy to,
Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
150 Fayetteville Street
Suit 2300
Raleigh, North Carolina 27601
Attention: David B. Clement
Telephone: (919) 821-6754
E-mail:dclement@smithlaw.com
If to the Escrow Agent, to:
[Branch Banking and Trust Company
Corporate Trust Services
223 West Nash Street
Wilson, NC 27893
Facsimile: (252) 246-4303
Attention: Marsha Hart, CTTS]
If to the Securityholder Representatives, to:
Sumeet Jain
c/o CMEA Capital
| A-130 |
1 Letterman Drive
Building C, Suite CM500
San Francisco, CA 94129
Email:sumeet@cmea.com
Telephone: (415) 962-2550
and
David Schulte
c/o Kaiser Permanente Ventures
One Kaiser Plaza
Oakland, CA 94612
Email:david.schulte@kp.org
Telephone: (510) 271-6383
with a copy (which shall not constitute notice) to:
Morrison & Foerster, LLP
755 Page Mill Road
Palo Alto, CA 94304
Attn: Stephen B. Thau
Email:sthau@mofo.com
Telephone: (650) 813-5640
or to such other address or number as shall be furnished in writing by any such party in such manner, and such notice or communication shall be deemed to have been given as of the date so delivered, sent by telecopier, e-mail, pdf format or mailed.
6.5 Taxes.
(a) Ownership for Tax Purposes. TranS1 and the Securityholder Representatives (on behalf of the Securityholders) agree that, for purposes of United States federal and other taxes based on income, the Securityholders shall be treated as the owners of the Escrow Property and that the Securityholders shall report the income, if any, that is earned on, or derived from, the Escrow Property as their income, in the taxable year or years in which such income is properly includible, and pay any taxes attributable thereto.
(b) Withholding. The Escrow Agent shall be entitled to deduct and withhold from any amount distributed or released from the Cash Escrow all taxes which may be required to be deducted or withheld under any provision of applicable tax law. All such withheld amounts shall be treated as having been delivered to the party entitled to the amount distributed or released in respect of which such tax has been deducted or withheld.
(c) Tax Reporting. The Escrow Agent does not provide tax reporting services and will not file any tax reports on any payments made pursuant to this Agreement, other than IRS Form 1099 INT/DIV (as the case may be) for the Securityholder Representatives.
| A-131 |
(d) “Know Your Customer” Requirements. In order for the Escrow Agent to comply with its internal policies and with the requirements of the Uniting and Strengthening of America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (commonly known as the USA PATRIOT Act), all parties to this Agreement shall have provided to the Escrow Agent prior to the execution of this Agreement a fully executed Form W-9 (in such form as is currently available from the United States Internal Revenue Service).
6.6 Assignment. This Agreement may not be transferred, assigned, pledged or hypothecated by any party hereto without the other parties’ prior written consent; except that TranS1 may assign without consent its rights and obligations hereunder to any affiliate of TranS1 or any Person that succeeds to all or substantially all of TranS1’s business; provided, however, that any such assignee must comply with the Escrow Agent’s “Know Your Customer” requirements then in effect. This Agreement shall be binding upon and shall inure to the benefit of the parties hereto and their respective successors and permitted assigns.
6.7 Counterparts. This Agreement may be executed in two or more counterparts, all of which taken together shall constitute one instrument. Signatures delivered by facsimile, electronic mail or in pdf shall be binding for all purposes hereof.
6.8 Governing Law; Certain Costs and Expenses. All matters arising out of or relating to this Agreement and the transactions contemplated hereby (including without limitation its interpretation, construction, performance and enforcement) shall be governed by and construed in accordance with the internal laws of the State of Delaware without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of laws of any jurisdictions other than those of the State of Delaware. If in connection with any dispute arising out of or in connection with this Agreement, TranS1 or the Escrow Agent is the substantially prevailing party between TranS1 or the Escrow Agent on the one hand, and the Securityholder Representatives on the other hand, the Securityholder Representatives (for and on behalf of the Securityholders) shall reimburse TranS1 for any and all amounts paid by TranS1 to the Escrow Agent in respect of the costs and expenses incurred by the Escrow Agent in connection with such dispute. Any such amount due from the Securityholder Representatives under this Section shall be treated as an undisputed, liquidated claim against the Escrow Shares pursuant to Section 4.4.
6.9 Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction or other authority to be invalid, void, unenforceable or against its regulatory policy, the remainder of the terms, provisions, covenants and restrictions of this Agreement shall remain in full force and effect and shall in no way be affected, impaired or invalidated.
[signatures appear on next page]
| A-132 |
[Signature Page to Escrow Agreement]
IN WITNESS WHEREOF, the parties have caused their respective names to be hereunto subscribed individually or by their respective officers thereunto duly authorized, as the case may be, all as of the day and year first above written.
| TRANS1: |
TRANS1 INC.
By: ___________________________________ | |
| Name: | |
| Title: |
| ESCROW AGENT: |
BRANCH BANKING AND TRUST COMPANY
By: ___________________________________ | |
| Name: | |
| Title: |
SECURITYHOLDER REPRESENTATIVES:
_____________________________________
Name: Sumeet Jain
______________________________________
Name: David Schulte
| A-133 |
Schedule A
TranS1 Key Stockholders
Directors
| · | David Simpson |
| · | Paul LaViolette |
| · | Jeff Fischgrund |
| · | Mark Stautberg |
| · | Jonathan Osgood |
| · | James Shapiro |
| · | Michael Carusi |
| · | Ken Reali |
Executive Officers
| · | Joseph Slattery |
| · | Stephanie Fitts |
| · | Frederic Feiler, Jr. |
| · | Stephen Ainsworth |
| · | Dwayne Montgomery |
| · | Mukesh Ramchandani |
5% Shareholders
| · | Advanced Technology Ventures and Affiliated Entities: Advanced Technology Ventures VII, L.P., Advanced Technology Ventures VII (B), L.P., Advanced Technology Ventures VII (C), L.P., ATV Entrepreneurs VII, L.P., and ATV Alliance 2002, L.P. | |
| · | Cutlass Capital and Affiliated Entities: Cutlass Capital, L.P., Cutlass Capital Principals Fund, L.L.C., and Cutlass Capital Affiliates Fund, L.P. |
Other Stockholders That Own Less Than 5% of TranS1 Common Stock Affiliated with Director James Shapiro
| · | Thomas Weisel Healthcare Venture Partners, L.P., Kearny Venture Partners, L.P., Kearny Venture Partners Entrepreneurs’ Fund, L.P. |
| A-134 |
EXECUTION VERSION
FIRST AMENDMENT TO
AGREEMENT AND PLAN OF MERGER
This First Amendment (this “First Amendment”) to Agreement and Plan of Merger (the “Merger Agreement”), dated as of March 3, 2013, by and among TranS1 Inc., a Delaware corporation (“TranS1”), RacerX Acquisition Corp., a Delaware corporation and a wholly owned subsidiary of TranS1 (“Transitory Subsidiary”), Baxano, Inc., a Delaware corporation (“Baxano”), and Sumeet Jain and David Schulte, solely as the Securityholder Representatives following appointment pursuant to Section 10.14(a) of the Merger Agreement, shall be effective April 10, 2013 (the “Effective Date”). Terms that are used herein with initial capital letters and that are not otherwise defined shall have the meanings given to them in the Merger Agreement.
BACKGROUND
TranS1, Transitory Subsidiary, Baxano, and the Securityholder Representatives (collectively, the “Parties”) previously entered into the Merger Agreement on March 3, 2013.
The Parties desire to amend the Merger Agreement as described herein.
AGREEMENT
NOW THEREFORE, in consideration of the mutual covenants contained herein, the Parties agree as follows:
1.Extension of Outside Date.Section 9.1(b) of the Merger Agreement is hereby deleted in its entirety and replaced with a new Section 9.1(b) that reads as follows:
by either TranS1 or Baxano if the Merger shall not have been consummated by May 31, 2013 (the “Outside Date”) (provided that the right to terminate this Agreement under this Section 9.1(b) shall not be available to any party whose failure to fulfill any obligation under this Agreement has been a principal cause of or resulted in the failure of the Merger to occur on or before the Outside Date);
2.Bridge Financing.
(a) The Parties acknowledge and agree that each of the Baxano Board and the TranS1 Board have approved an arrangement whereby TranS1 may provideup to $2,500,000 of bridge financing to Baxano for its working capital needs through the Effective Time. Pursuant to the arrangement, from time to time prior to the Effective Time, Baxano may request that TranS1 provide bridge financing for specified budget items.TranS1 may agree in its reasonable discretion to fund all or any portion or none of such requested amount, and the Company may in its discretion make appropriate adjustments to its budget as requested by TranS1.
| A-135 |
(b) TranS1 hereby confirms that, subject to theaggregate $2,500,000 limit approved by the TranS1 Board, and further subject to paragraph 2(c) below, and provided further that neither Baxano nor TranS1 has delivered any notice of termination under Section 9.1 of the Merger Agreement,if Baxano and TranS1 agree that TranS1 will provide the requested amount to fund the items included in the budget included with Baxano’s request, including any agreed adjustments, then TranS1 will loan Baxano such agreed amount and the closing will take place as soon as practicable thereafter or on such other date as TranS1 and Baxano may mutually agree. At the closing ofsuch loan, Baxano will issue a promissory note (each, a “Bridge Note”) to TranS1 to evidence the loan amount in the form attached hereto asAttachment A.
(c) Prior to the first closing of any such bridge financing, (i) TranS1 and Baxano shall execute a subordination agreement with Oxford Finance LLC substantially identical to the form of subordination agreement previously executed by the Noteholders and Baxano with Oxford Finance LLC providing that the Bridge Notes held by TranS1 shall be subordinate in right of payment to amounts due under the Oxford/SVB Loan Documents, and (ii) TranS1, Baxano and the Noteholders shall execute a subordination agreement substantially identical to the form agreed by the parties.
3.The terms and conditions of the Merger Agreement shall continue in full force and effect except as modified by this First Amendment.
[signature page follows]
| A-136 |
[Signature Page to First Amendment to Agreement and Plan of Merger]
IN WITNESS WHEREOF, each Party hereto has executed or caused this First Amendment to be executed on its behalf as of the date first written above.
TRANS1 INC.
By: /s/ Ken Reali
Name: Ken Reali
Title: President and Chief Executive Officer
RACERX ACQUISITION CORP.
By: /s/ Ken Reali
Name: Ken Reali
Title: President and Chief Executive Officer
BAXANO, INC.
By: /s/ Anthony Recupero
Name: Anthony Recupero
Title: President and Chief Executive Officer
SECURITYHOLDER REPRESENTATIVES
By: /s/ Sumeet Jain
Sumeet Jain
By: /s/ David Schulte
David Schulte
| A-137 |
CONFIDENTIAL
ATTACHMENT A
Form of Bridge Note
THIS NOTE IS SUBJECT TO THAT CERTAIN SUBORDINATION AGREEMENT, DATED [__] 2013, AMONG TRANS1 INC. AND OXFORD FINANCE LLC, THE TERMS OF WHICH WERE APPROVED BY THE COMPANY.
PROMISSORY NOTE
PN-__
| $_________________ | [__], 2013 |
| San Jose, California |
FOR VALUE RECEIVED,BAXANO, INC., a Delaware corporation (the “Company”), promises to pay to the order of TranS1 Inc., a Delaware corporation having a business address at 110 Horizon Drive, Suite 230, Raleigh, North Carolina 27615, or its assigns (the “Holder”), the principal sum of _____________ ($______________) with interest on the outstanding principal amount at the rate of 6.00% per annum (computed on the basis of actual calendar days elapsed and a year of 365 days) compounded annually or at the highest rate of interest then permitted under applicable law, if less. Interest shall commence with the date hereof and shall continue on the outstanding principal until paid in accordance with the provisions hereof. In the event that any interest is paid on this Promissory Note (this “Note”) which is deemed to be in excess of the then legal maximum rate, then that portion of the interest payment representing an amount in excess of the then legal maximum rate shall be deemed a payment of principal and applied against the principal of this Note.
1. Definitions. For purposes of this Note, the following terms shall have the following meanings:
“Agreement” shall mean that certain Note and Warrant Purchase Agreement (the “Agreement”) dated as of March 7, 2012, by and among the Company and the investors set forth in the Schedule of Investors attached thereto asExhibit A.”
“Equity Securities” shall mean any securities evidencing an ownership interest in the Company, or any securities convertible into or exercisable or exchangeable for any shares of the foregoing, or any agreement or commitment to issue any of the foregoing.
“Investor Notes” shall mean the convertible promissory notes issued pursuant to the terms of the Agreement.
“Qualified Financing” shall mean the sale by the Company of shares of Equity Securities in one or more transactions to venture capital, institutional or private investors, including at least one such investor that is not an existing noteholder or stockholder of the Company, for aggregate cash proceeds to the Company of not less than $15,000,000 (excluding any amount invested by cancellation or conversion of the indebtedness represented by the Investor Notes).
| A-138 |
“Qualified Financing Securities” shall mean the securities issued in a Qualified Financing.
2. Maturity Date. Unless sooner paid in accordance with the terms hereof, the entire unpaid principal amount and all unpaid accrued interest shall become fully due and payable on demand by the Holder any time on or after September 7, 2013 (the “Maturity Date”).
3. Payments.
(a) Form of Payment. All payments of interest and principal shall be in lawful money of the United States of America to Holder, at the address specified in the first paragraph of this Note, or at such other address as may be specified from time to time by Holder in a written notice delivered to the Company. All payments shall be applied first to accrued interest, and thereafter to principal.
(b) Prepayment. No prepayments shall be permitted without the written consent of the Holder.
4. Acceleration of Note.
(a) Acceleration Upon Qualified Financing. All principal and accrued interest under this Note and any other amounts payable hereunder, shall be due and payable upon the consummation of a Qualified Financing.
(b) Treatment Upon Acquisition.
(i) Except as set forth in Section 5, in the event that the Company sells, conveys or otherwise disposes of all or substantially all of its assets or is acquired by way of a merger, consolidation, reorganization or other transaction or series of transactions pursuant to which stockholders of the Company prior to such acquisition own less than fifty percent (50%) of the voting interests in the surviving or resulting entity (an “Acquisition”) and the Investor Notes are canceled, terminated, paid off or converted in connection with such Acquisition, then this Note shall, at the consummation of such Acquisition, be cancelled and exchanged for the right to receive consideration, in cash or securities of the acquirer (with a value calculated as set forth in the definitive Acquisition agreement), in the amount of the then outstanding principal and unpaid accrued interest under this Note (less any amount agreed by the Company to be withheld in escrow to satisfy potential indemnification claims or to fund any post-closing adjustments under the definitive Acquisition agreement). Holder hereby agrees to irrevocably appoint a representative determined by the stockholders of the Company as the representative, agent, proxy and attorney in fact of and for Holder for all purposes under the definitive Acquisition agreement as if Holder were a stockholder of the Company. Holder hereby also agrees, in exchange for the consideration to be paid as set forth above, to be bound by the definitive Acquisition agreement to the same extent as if Holder were a stockholder of the Company.
(ii) In the event of an Acquisition in connection with which the Investor Notes remain outstanding and are not canceled, terminated, paid off or converted as described in Section 4(b)(i), then all principal and accrued interest under this Note and any other amounts payable hereunder, shall be due and payable upon consummation of such Acquisition.
| A-139 |
5. Cancellation of Note. The Note shall be cancelled without consideration, repayment, or any other right of Holder to be repaid or otherwise compensated hereunder, immediately prior to the effective time of the merger contemplated by that certain Agreement and Plan of Merger among the Holder, the Company, RacerX Acquisition Corp., and Sumeet Jain and David Schulte as the Securityholder Representatives, dated as of March 3, 2013 (the “Merger Agreement”).
6. Use of Proceeds. The proceeds of the Note shall be used solely as set forth in the budget attached hereto asExhibit A (the “Budget”), with such exceptions as may be approved in writing by the Chief Financial Officer of the Holder. None of the proceeds of this Note shall be used for Transaction Expenses (as defined in the Merger Agreement) except as may be expressly approved in writing by the Chief Financial Officer of the Holder. If any portion of the proceeds of this Note is used to pay Transaction Expenses, then such Transaction Expenses nevertheless shall be deemed to be unpaid immediately prior to the effective time of the merger for purposes of Section 2.11 of the Merger Agreement.
7. Accounting Reports. The Company shall provide a weekly (or more frequently as reasonably requested by the Holder) accounting, in form and substance reasonably acceptable to the Holder, to the Holder’s Chief Financial Officer regarding the Company’s current cash position and demonstrating the Company’s use of proceeds of the Note consistent with the Budget or as approved in writing by the Chief Financial Officer of the Holder.
8. Events of Default.
(a) Definition. For purposes of this Note, an “Event of Default” shall be deemed to have occurred if:
(i) any indebtedness under this Note is not paid when and as the same shall become due and payable, whether at maturity, by acceleration or otherwise;
(ii) any Event of Default (as such term is defined under the Investor Notes) has occurred under the Investor Notes and the Majority Holders (as such term is defined under the Agreement) elect in writing to declare all indebtedness under the Investor Notes due and payable; or
(iii) the Company shall (A) apply for or consent to the appointment of a receiver, trustee, custodian or liquidator of itself or any part of its property, (B) become subject to the appointment of a receiver, trustee, custodian or liquidator for itself or any part of its property if such appointment is not terminated or dismissed within thirty (30) days, (C) make an assignment for the benefit of creditors, (D) be adjudicated as bankrupt or insolvent, (E) institute any proceedings under the United States Bankruptcy Code or any other federal or state bankruptcy, reorganization, receivership, insolvency or other similar law affecting the rights of creditors generally, or file a petition or answer seeking reorganization or an arrangement with creditors to take advantage of any insolvency law, or file an answer admitting the material allegations of a bankruptcy, reorganization or insolvency petition filed against it, or (F) become subject to any proceedings under the United States Bankruptcy Code or any other federal or state bankruptcy, reorganization, receivership, insolvency or other similar law affecting the rights of creditors generally, which proceeding is not dismissed within thirty (30) days of filing, or have an order for relief entered against it in any proceeding under the United States Bankruptcy Code.
| A-140 |
(b) Consequences of Events of Default.
(i) If an Event of Default occurs, all indebtedness under this Note shall become due and payable, and upon such election the Company shall immediately pay to Holder all such amounts. The Company agrees to pay Holder all reasonable out-of-pocket costs and expenses incurred by Holder in any effort to collect indebtedness under this Note, including reasonable attorney fees, and to pay interest at the highest rate permitted by applicable law on such costs and expenses to the extent not paid when demanded.
(ii) Holder shall also have any other rights which Holder may have been afforded under any contract or agreement at any time and any other rights which Holder may have pursuant to applicable law.
9. Lost, Stolen, Destroyed or Mutilated Notes. In case any Note shall be mutilated, lost, stolen or destroyed, the Company shall issue a new Note of like date, tenor and denomination and deliver the same in exchange and substitution for and upon surrender and cancellation of any mutilated Note, or in lieu of any Note lost, stolen or destroyed, upon receipt of evidence reasonably satisfactory to the Company of the loss, theft or destruction of such Note.
10. Governing Law. This Note is to be construed in accordance with and governed by the internal laws of the State of Delaware without giving effect to any choice of law rule that would cause the application of the laws of any jurisdiction other than the internal laws of the State of Delaware to the rights and duties of the parties. All disputes and controversies arising out of or in connection with this Note shall be resolved exclusively as described in Sections 10.12 and 10.13 of the Merger Agreement.
11. Amendment. Any term of this Note may be amended and the observance of any term of this Note may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and the Holder.
12. Notices. Except as may be otherwise provided herein, all notices, requests, waivers and other communications made pursuant to this Note shall be made in accordance with Section 10.3 of the Merger Agreement.
13. Severability. If one or more provisions of this Note are held to be unenforceable under applicable law, such provision shall be excluded from this Note and the balance of the Note shall be interpreted as if such provision were so excluded and shall be enforceable in accordance with its terms.
[signature page follows]
| A-141 |
[Signature Page to Promissory Note]
IN WITNESS WHEREOF, the Company has caused this Note to be duly executed by its officers, thereunto duly authorized as of the date first above written.
| BAXANO, INC. | ||
| By: | ||
| Anthony Recupero President and Chief Executive Officer | ||
Acknowledged and Agreed:
HOLDER
TRANS1 INC.
| By: | |
| Ken Reali | |
| President and Chief Executive Officer |
| A-142 |
Exhibit A
Budget
[To be inserted]
| A-143 |
Annex B
EXECUTION VERSION
SECURITIES PURCHASE AGREEMENT
This SECURITIES PURCHASE AGREEMENT (this “Agreement”) is made as of the 3rd day of March, 2013, by and between TranS1 Inc. (the “Company”), a Delaware corporation, with its principal offices at 301 Government Center Drive, Wilmington, NC 28403 and each of the Investors (as defined below).
IN CONSIDERATION of the mutual covenants contained in this Agreement, the Company and the Investors agree as follows:
SECTION 1.Definitions. As used in this Agreement, the following terms have the meanings indicated:
“Affiliate” means any person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with a person, as such terms are used in and construed under Rule 144 under the Securities Act. For clarity, the definition of “Affiliate” for any person that is a partnership shall include without limitation any general partner, managing member, officer or director of such person or any venture capital fund now or hereafter existing that is controlled by one or more general partners or managing members of, or shares the same management company with, such person.
“Agreement” has the meaning set forth in the Preamble.
“Balance Sheet” has the meaning set forth in Section 4.4(b).
“Business Day” means any day other thanSaturday,Sunday, any day which shall be a federal legal holiday in theUnited Statesor any day on which banking institutions in TheState of New Yorkare authorized or required by law or other governmental action to close.
“Closing” means the closing of the purchase and sale of theSharespursuant to Section2.
“Closing Date” has the meaning set forth in Section 3.
“Code” has the meaning set forth in the Merger Agreement.
“Common Stock” has the meaning set forth in Section 2.
“Company” has the meaning set forth in the Preamble.
“Company Authorizations” has the meaning set forth in Section 4.15(a).
“Company Form 10-K” has the meaning set forth in Section 4.5.
“Company Intellectual Property” has the meaning set forth in Section 4.9(b).
“Company Material Adverse Effect” has the meaning set forth in the Section 4.1.
| B-1 |
“Controlling Person” has the meaning set forth in Section 8.3.
“Convertible Securities” has the meaning set forth in Section 4.2(c).
“DGCL” means theGeneral CorporationLaw of the State of Delaware.
“Disclosure Schedule” has the meaning set forth in Section 4.
“Effective Deadline” has the meaning set forth in Section 8.1(b).
“Employee Benefit Plan” has the meaning set forth in the Merger Agreement.
“Employee Plans” has the meaning set forth in Section 4.13(a).
“Environmental Law” has the meaning set forth in the Merger Agreement.
“ERISA” has the meaning set forth in the Merger Agreement.
“ERISA Affiliate” has the meaning set forth in the Merger Agreement.
“Exchange Act” means theSecurities Exchange Act of 1934, as amended.
“FCA Matter” has the meaning set forth in Section 4.1.
“FDA” has the meaning set forth in the Merger Agreement.
“GAAP” means the United States generally accepted accounting principles.
“Governmental Entity” has the meaning set forth in the Merger Agreement.
“Hazardous Substance” has the meaning set forth in the Merger Agreement.
“Immediate Family Members” has the meaning set forth in Section 5.6.
“Insurance Policies” has the meaning set forth in Section 4.17.
“Intellectual Property” has the meaning set forth in the Merger Agreement.
“Investor” means(i) each investor identified on the signature pages hereto as signing with respect to all Sections hereof and (ii) solely with respect to Sections 5.6, 5.7, 5.9, 8.1, 8.2, 8.3(a), 8.3(c), 8.3(d), 8.4, 8.6, and 10-23, inclusive hereof, eachholder of MergerShareswho is not also purchasingSharesunder Section2hereof and identified on the signature pages hereto as signing in such capacity. All such persons in clauses(i)and(ii)are referred to collectively in such Sections, as applicable, asthe “Investors.”
“Investor Affiliate” has the meaning set forth in Section 8.3.
“IRS” has the meaning set forth in the Merger Agreement.
| B-2 |
“Lien” has the meaning set forth in the Merger Agreement.
“Material Contracts” has the meaning set forth in 4.10(a).
“Merger” has the meaning set forth in the Merger Agreement.
“Merger Agreement” means that certainAgreement and Plan of Merger, dated as of March3, 2013, by and among theCompany, RacerX Acquisition Corp., a Delaware corporation and a wholly owned subsidiary of theCompany, Baxano, Inc., a Delaware corporation, andSumeet Jain and David Schulteas theSecurityholder Representativesthereunder.
“Merger Closing Price” has the meaning set forth in the Merger Agreement.
“Merger Shares” has the meaning set forth in the Merger Agreement.
“NASDAQ” means TheNASDAQ Stock Market LLC.
“Ordinary Course of Business” has the meaning set forth in the Merger Agreement.
“Preferred Stock” has the meaning set forth in Section 4.2(a).
“Prospectus” means the prospectus included in theRegistration Statement(including, without limitation, a prospectus that includes any information previously omitted from a prospectus filed as part of an effectiveregistration statementin reliance upon Rule 430A promulgated under theSecurities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the offering of any portion of theSharescovered by theRegistration Statement, and all other amendments and supplements to theProspectusincluding post-effective amendments, and all material incorporated by reference or deemed to be incorporated by reference in suchProspectus.
“Recent SEC Documents” has the meaning set forth in Section 4.5.
“Registrable Securities” means theSharesissued pursuant to thisAgreementand the MergerShares, together with any securities issued or issuable upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
“Registration Statement” means each registration statement required to be filed under Section8, including (in each case) theProspectus, amendments and supplements to such registration statement orProspectus, including pre- and post-effective amendments, all exhibits thereto, and all material incorporated by reference or deemed to be incorporated by reference in such registration statement.
“Rule 144” and “Rule 415” meansRule 144 and Rule 415, respectively, promulgated by theSECpursuant to theSecurities Act, as such Rules may be amendedfrom time to time, or any similar rule or regulation hereafter adopted by theSEChaving substantially the same effect as such Rule.
| B-3 |
“Sarbanes Act” has the meaning set forth in Section 4.4(a).
“SEC”means theUnited States Securities and Exchange Commission.
“SEC Documents” has the meaning set forth in Section 4.4(a).
“Securities Act” means theSecurities Act of 1933, as amended.
“Shares” has the meaning set forth in Section 2.
“Stock Options” has the meaning set forth in Section 4.2(b).
“Stock Plans” has the meaning set forth in Section 4.2(b).
“Subsidiaries” or “Subsidiary” has the meaning set forth in the Merger Agreement.
“Suspension” has the meaning set forth in Section 5.7.
“Tax Returns” has the meaning set forth in the Merger Agreement.
“Taxes” has the meaning set forth in the Merger Agreement.
“Third Party Intellectual Property” has the meaning set forth in Section 4.9(b).
“Trademarks” has the meaning set forth in the Merger Agreement.
“Trading Day” means (i) a day on which theCommon Stockis traded on aTrading Market(other than the OTC Bulletin Board), or (ii) if theCommon Stockis not listed or quoted on aTrading Market(other than the OTC Bulletin Board), a day on which theCommon Stockis traded in the over-the-counter market, as reported by the OTC Bulletin Board, or (iii) if theCommon Stockis not listed or quoted on anyTrading Market, a day on which theCommon Stockis quoted in the over-the-counter market as reported by thePink Sheets LLC(or any similar organization or agency succeeding to its functions of reporting prices); provided, that in the event that theCommon Stockis not listed or quoted as set forth in (i), (ii) and (iii) hereof, thenTrading Dayshall mean aBusiness Day.
“Trading Market” means whichever of the New York Stock Exchange, the American Stock Exchange,NASDAQor OTC Bulletin Board on which theCommon Stockis listed or quoted for trading on the date in question.
“TranS1 Stockholder Agreements” has the meaning set forth in the Merger Agreement.
“TranS1 Stockholder Approval” has the meaning set forth in the Merger Agreement.
| B-4 |
“Transfer Agent” meansAmerican Stock Transfer & Trust Company,10150 Mallard Creek Road,Suite 307, Charlotte,NC 28262 or any successor transfer agent for theCompany.
SECTION 2.Agreement to Sell and Purchase the Shares.EachInvestor, severally and not jointly, wishes to purchase, and theCompanywishes to sell, upon the terms and subject to the conditions stated in thisAgreement,(i)that aggregate number ofsharesof common stock, par value $0.0001 per share (“Common Stock”), of theCompanyset forth on suchInvestor’s signature page to thisAgreementfor a price per share equal to $2.28(which aggregate amount for allInvestorstogether shall be7,543,938shares ofCommon Stockand shall collectively be referred to herein as the “Shares,” for an aggregate purchase price of $17,200,192.52).
SECTION 3.The Closing. The Closing shall occur at the offices of Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P., 150 Fayetteville Street, Suite 2300, Raleigh, NC 27601 as soon as practicable and as agreed to by the parties hereto, but not prior to the date that the conditions for Closing set forth below have been satisfied or waived by the appropriate party (the “Closing Date”).
3.1 At the Closing, each Investor shall deliver to the Company in immediately available funds, the full amount of the purchase price for the number of Shares being purchased by such Investor hereunder by wire transfer to an account designated by the Company.
3.2 At the Closing, the Company shall deliver to the Investor:
(a) one or more stock certificates registered in the name of each Investor, or in such nominee name(s) as designated by the Investor in writing, representing the number of Shares set forthon suchInvestor’s signature page to thisAgreement and bearing an appropriate legend referring to the fact that the Shares were sold in reliance upon the exemption from registration under the Securities Act, provided by Section 4(a)(2) thereof and Rule 506 thereunder. The Company will promptly substitute one or more replacement certificates without the legend at such time as the Registration Statement becomes effective. The name(s) in which the stock certificates are to be registered are set forth in the Stock Certificate Questionnaire attached hereto as part ofExhibit A;
(b) a legal opinion of Company counsel, in the form ofExhibit B, executed by such counsel and delivered to the Investors;
(c) a certificate of the Secretary of the Company, dated as of the Closing Date, (i) certifying the resolutions adopted by the Board of Directors of the Company approving the transactions contemplated by this Agreement and the issuance of the Shares, (ii) certifying the current versions of the Certificate of Incorporation and Bylaws of the Company and (iii) certifying as to the signatures and authority of persons signing this Agreement and related documents on behalf of the Company; and
(d) an officers’ certificate duly executed by each of the Chief Executive Officer and Chief Financial Officer of the Company to the effect that the conditions of Sections 7.1(b) and (c) have been satisfied.
| B-5 |
SECTION 4.Representations, Warranties and Covenants of the Company. The Company represents and warrants to the Investor that the statements contained in this SECTION 4 are true and correct, except as expressly set forth herein or in the disclosure schedule delivered by the Company to the Investor on the date of this Agreement (the “Disclosure Schedule”). The Disclosure Schedule shall be arranged in sections corresponding to the numbered and lettered sections contained in this Section 4 and the disclosure in any section of the Disclosure Schedule shall qualify (1) the corresponding section in this Section 4 and (2) the other sections in this Section 4 only to the extent that it is reasonably apparent from a reading of such disclosure that it also qualifies or applies to such other sections. For purposes hereof, “to the knowledge of the Company” and similar expressions mean the actual knowledge of the persons identified on the Disclosure Schedule for this purpose,includingthe knowledge such persons would have in the ordinary performance of their duties tothe Company.
4.1Organization, Standing and Power. The Company is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation, has all requisite corporate power and authority to own, lease and operate its properties and assets and to carry on its business as currently conducted and as currently proposed to be conducted, and is duly qualified to do business and is in good standing as a foreign corporation in each jurisdiction listed on Section 4.1 of the Disclosure Schedule, which jurisdictions constitute the only jurisdictions in which the character of the properties it owns, operates or leases or the nature of its activities makes such qualification necessary, except for such failures to be so organized, qualified or in good standing, individually or in the aggregate, that have not had, and are not reasonably expected to have, a Company Material Adverse Effect. For purposes of this Agreement, the term “Company Material Adverse Effect” means any material adverse change, event, circumstance or development with respect to, or material adverse effect on, (i) the business, assets and liabilities (taken together), condition (financial or otherwise), or results of operations of the Company and its Subsidiaries, taken as a whole, or (ii) the ability of the Companyand itsSubsidiariesto consummate the transactions contemplated by thisAgreement;provided,however, that the following shall not be deemed to be a Company Material Adverse Effect: any change or event caused by or resulting from (A) changes in prevailing economic or market conditions in the United States or any other jurisdiction in which the Company has substantial business operations (except to the extent those changes have a materially disproportionate effect on the Company and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which the Company and its Subsidiaries operate), (B) changes or events, after the date hereof, affecting the industries or markets in which they operate generally (except to the extent those changes or events have a materially disproportionate effect on the Company and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which the Company and its Subsidiaries operate), (C) changes, after the date hereof, in generally accepted accounting principles or requirements applicable to the Company and its Subsidiaries (except to the extent those changes have a materially disproportionate effect on the Company and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which the Company and its Subsidiaries operate), (D) changes, after the date hereof, in laws, rules or regulations of general applicability or interpretations thereof by any Governmental Entity (except to the extent those changes have a materially disproportionate effect on the Company and its Subsidiaries as compared to other similarly situated participants in the industries or markets in which the Company and its Subsidiaries operate), (E) the execution, delivery and performance of this Agreement or the consummation of the transactions contemplated hereby or the announcement thereof, (F) any outbreak of major hostilities in which the United States is involved or any act of terrorism within the United States or directed against its facilities or citizens wherever located, (G) the activities under investigation by the U.S. Department of Justice and the Office of Inspector General of the U.S. Department of Health & Human Services and/or alleged by anyqui tamrelator in any False Claims Act complaint giving rise thereto (collectively, the “FCA Matter”), or (H) the actions of the Investor (other than any action taken under this Agreement by the Investor in response to an event, circumstance or other development that would otherwise constitute a Company Material Adverse Effect); andprovided,further, that in no event shall a change in the public trading price of the Company’s Common Stock, by itself, be considered material or constitute a Company Material Adverse Effect, although the underlying cause of any change in the public trading price of the Company’s Common Stock may nonetheless be considered in determining the occurrence of a Company Material Adverse Effect. For the avoidance of doubt, the parties agree that the terms “material,” “materially” and “materiality” as used in thisAgreementwith an initial lower case “m” shall have their respective customary and ordinary meanings, without regard to the meanings ascribed toCompany Material Adverse Effectin the prior sentence of this paragraph.The Company has provided or made available to the Investorcomplete and accurate copies of its Certificate of Incorporation and Bylaws.
| B-6 |
4.2Capitalization.
(a) The authorized capital stock of the Company consists of 75,000,000 shares of Common Stock, $0.0001 par value per share, and 5,000,000 shares of preferred stock, $0.0001 par value pershare (“Preferred Stock”). The rights and privileges of each class ofthe Company’s capital stock are as set forth inthe Company’s Certificate of Incorporation. As of the date of thisAgreement, (i)27,318,785 sharesofCommon Stockare issued and outstanding, and (ii) nosharesofCommon Stockare held in the treasury ofthe Companyor bySubsidiariesofthe Company,and (iii) nosharesofPreferred Stockare issued and outstanding.
(b) Section 4.2(b) of the Disclosure Schedule sets forth a complete and accurate list of the number of shares of Common Stock reserved for future issuance pursuant to stock options granted and outstanding as of the date of this Agreement, the plans under which such options were granted (collectively, “Stock Plans”) and the total number of outstanding options to purchase shares of Common Stock (such outstanding options, “Stock Options”) under Stock Plans as of the close of business on the Business Day prior to the date of this Agreement. The Company has provided or made available to the Investor accurate and complete copies of all Stock Plans and the forms of all stock option agreements used under the Stock Plans.
(c) Section 4.2(c) of the Disclosure Schedule sets forth the number of shares of the Company’s capital stock reserved for future issuance pursuant to warrants, convertible promissory notes or other outstanding rights (other than Stock Options) to purchase shares of the Company’s capital stock outstanding as of the date of this Agreement (such outstanding warrants, convertible promissory notes or other rights, the “Convertible Securities”) and the agreement or other document under which such Convertible Securities were granted and sets forth a complete and accurate list of all holders of Convertible Securities indicating the number and type of shares of Common Stock subject to each Company warrant, and the exercise price, the date of grant and the expiration date thereof. The Company has provided or made available to the Investor complete and accurate copies of the forms of agreements evidencing all Convertible Securities.
| B-7 |
(d) Except (i) as set forth in this Section 4.2 or in Article II of the Merger Agreement and (ii) as reserved for future grants under Stock Plans, (A) there are no equity securities of any class ofthe Company, or any security exchangeable into or exercisable for such equity securities, issued, reserved for issuance or outstanding and (B) there are no options, warrants, equity securities, calls, rights, commitments or agreements of any character to whichthe Companyor any of itsSubsidiariesis a party or by whichthe Companyor any of itsSubsidiariesis bound obligatingthe Companyor any of itsSubsidiariesto issue, exchange,transfer, deliver or sell, or cause to be issued, exchanged, transferred, delivered or sold, additionalsharesof capital stock or other equity interests ofthe Companyor any security or rights convertible into or exchangeable or exercisable for any suchsharesor other equity interests, or obligatingthe Companyor any of itsSubsidiariesto grant, extend, accelerate the vesting of, otherwise modify or amend or enter into any such option, warrant, equity security, call, right, commitment oragreement.The Companydoes not have any outstanding stock appreciation rights, phantom stock, performance based rights or similar rights or obligations. Other than theTranS1 Stockholder Agreements, neitherthe Companynor any of itsAffiliatesis a party to or is bound by any, andto the knowledge of the Company, there are no, agreements or understandings with respect to the voting (includingvoting trusts and proxies) or sale ortransfer(includingagreements imposingtransferrestrictions) of anysharesof capital stock or other equity interests ofthe Company. Except as contemplated by thisAgreement, theMerger Agreementor described in this Section4.2(d), there are no registration rights, and there is no rightsagreement, “poison pill” anti-takeover plan or otheragreementor understanding to whichthe Companyor any of itsSubsidiariesis a party or by which it or they are bound with respect to any equity security of any class ofthe Company. Stockholders ofthe Companyare not entitled to dissenters’ or appraisal rights under applicable state law in connection with theMerger.
(e) All outstanding shares of Common Stock are, and all shares of Common Stock subject to issuance as specified in Sections 4.2(b) and 4.2(c), upon issuance on the terms and conditions specified in the instruments pursuant to which they are issuable, will be, duly authorized, validly issued, fully paid and nonassessable and not subject to or issued in violation of any purchase option, call option, right of first refusal, preemptive right, subscription right or any similar right under any provision oftheDGCL,the Company’s Certificate of Incorporation or Bylaws or anyagreementto whichthe Companyis a party or is otherwise bound.There are no obligations, contingent or otherwise, of the Company or any of its Subsidiaries to repurchase, redeem or otherwise acquire any shares of Common Stock. All outstanding shares of the Company’s capital stock have been offered, issued and sold by the Company in compliance with all applicable federal and state securities laws.
| B-8 |
4.3Subsidiaries.
(a) Section 4.3(a) of the Disclosure Schedule sets forth, for each Subsidiary of the Company: (i) its name; (ii) the number and type of outstanding equity securities and a list of the holders thereof; and (iii) the jurisdiction of organization.
(b) Each Subsidiary of the Company is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation, has all requisite corporate power and authority to own, lease and operate its properties and assets and to carry on its business as currently conducted and as currently proposed to be conducted, and is duly qualified to do business and is in good standing as a foreign corporation in each jurisdiction where the character of its properties owned, operated or leased or the nature of its activities makes such qualification necessary, except for such failures to be so organized, qualified or in good standing, individually or in the aggregate, that have not had, and would not reasonably be expected to have, a Company Material Adverse Effect. All of the outstanding shares of capital stock and other equity securities or interests of each Subsidiary of the Company are duly authorized, validly issued, fully paid, nonassessable and free of preemptive rights and all such shares are owned, of record and beneficially, by the Company or another of its Subsidiaries free and clear of all security interests, liens, claims, pledges, agreements, limitations in the Company’s voting rights, charges or other encumbrances of any nature. There are no outstanding or authorized options, warrants, rights, agreements or commitments to which the Company or any of its Subsidiaries is a party or which are binding on any of them providing for the issuance, disposition or acquisition of any capital stock of any Subsidiary of the Company. There are no outstanding stock appreciation, phantom stock or similar rights with respect to any Subsidiary of the Company. There are no voting trusts, proxies or other agreements or understandings with respect to the voting of any capital stock of any Subsidiary of the Company.
(c) The Company has provided or made available to the Investor complete and accurate copies of the charter, bylaws or other organizational documents of each Subsidiary of the Company.
(d) The Company does not control directly or indirectly or have any direct or indirect equity participation or similar interest in any corporation, partnership, limited liability company, joint venture, trust or other business association or entity which is not a Subsidiary of the Company. There are no obligations, contingent or otherwise, of the Company or any of its Subsidiaries to repurchase, redeem or otherwise acquire any shares of capital stock of any Subsidiary of the Company or to provide funds to or make any investment (in the form of a loan, capital contribution or otherwise) in any Subsidiary of the Company or any other entity, other than guarantees of bank obligations of Subsidiaries of the Company entered into in the Ordinary Course of Business.
4.4SEC Filings; Financial Statements; Information Provided.
(a) The Company has filed all registration statements, forms, reports, certifications and other documents required to be filed by the Company with the SEC since January 1, 2010 and has made available to the Investor copies of all registration statements, forms, reports, certifications and other documents filed by the Company with the SEC since January 1, 2010, including all certifications and statements required by (i) Rule 13a-14 or 15d-14 under the Exchange Act or (ii) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes Act”)). All such registration statements, forms, reports, certifications and other documents (including those that the Company may file after the date hereof until the Closing) are referred to herein as the “SEC Documents.” All SEC Documents are publicly available on the SEC’s EDGAR system. The Company has made available to the Investor copies of all comment letters received by the Company from the staff of the SEC since January 1, 2010 and all responses to such comment letters by or on behalf of the Company. All SEC Documents (A) were or will be filed or deemed filed on a timely basis, (B) at the time filed, were or will be prepared in compliance in allmaterial respects with the applicable requirements of the Securities Act and the Exchange Act, as the case may be, and the rules and regulations of the SEC thereunder applicable to such SEC Documents and (C) did not or will not at the time they were or are filed contain any untrue statement of a material fact or omit to state a material fact required to be stated in such SEC Documents or necessary in order to make the statements in such SEC Documents, in the light of the circumstances under which they were made, not misleading. No Subsidiary of the Company is subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act.
| B-9 |
(b) Each of the consolidated financial statements (including, in each case, any related notes and schedules) contained or to be contained in SEC Documents at the time filed (i) complied or will comply as to form in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto (including, without limitation, Regulation S-X), (ii) were or will be prepared in accordance with GAAP applied on a consistent basis throughout the periods covered thereby (except as may be indicated in the notes to such financial statements or, in the case of unaudited interim financial statements, as permitted by the SEC on Form 10-Q under the Exchange Act) and (iii) fairly present the consolidated financial position of the Company and its Subsidiaries as of the dates thereof and the consolidated results of its operations and cash flows for the periods indicated, consistent with the books and records of the Company and its Subsidiaries, except that the unaudited interim financial statements were or are subject to normal and recurring year-end adjustments which were not or will not be material in amount or effect. The Company’s audited consolidated balance sheet of the Company as of December 31, 2011 is referred to herein as the “Balance Sheet.”
(c) PricewaterhouseCoopers LLP, the Company’s current auditors, is and has been at all times since its engagement by the Company (i) “independent” with respect to the Company within the meaning of Regulation S-X and (ii) in compliance with subsections (g) through (l) of Section 10A of the Exchange Act (to the extent applicable) and the related rules of the SEC and the Public Company Accounting Oversight Board.
(d) There is no transaction, arrangement or other relationship between the Company and an unconsolidated or other off-balance sheet entity that is required to be disclosed by the Company in its Exchange Act filings and is not so disclosed or that otherwise would be reasonably likely to have a Company Material Adverse Effect. There are no such transactions, arrangements or other relationships with the Company that may create contingencies or liabilities that are not otherwise disclosed by the Company in its Exchange Act filings.
| B-10 |
4.5No Undisclosed Liabilities. Except as disclosed in the Company’s Annual Report on Form 10-K for the period ended December 31, 2011 (the “Company Form 10-K”) filed with the SEC or any SEC Documents filed after the filing of the Company Form 10-K and prior to the date of this Agreement (together with the Company Form 10-K, the “Recent SEC Documents”), and except for normal and recurring liabilities incurred since the date of the Balance Sheet in the Ordinary Course of Business, the Company and its Subsidiaries do not have any material liabilities (whether known or unknown, whether absolute or contingent, whether liquidated or unliquidated, whether due or to become due, and whether or not required to be reflected in financial statements (including the notes thereto) in accordance with GAAP).
4.6Absence of Certain Changes or Events. Except as disclosed in the Recent SEC Documents, since the date of the Balance Sheet, the Company and its Subsidiaries have conducted their respective businesses only in the Ordinary Course of Business and, since such date, there has not been any change, event, circumstance, development or effect that, individually or in the aggregate, has had, or is reasonably expected to have, a Company Material Adverse Effect.
4.7Taxes.
(a) Each of the Company and its Subsidiaries has properly filed on a timely basis all material Tax Returns that it was required to file, and all such Tax Returns were true, correct and complete in all material respects. Each of the Company and its Subsidiaries has paid on a timely basis all Taxes due and payable. Neither the Company nor any of its Subsidiaries is or has ever been a member of a group of corporations with which it has filed (or been required to file) consolidated, combined or unitary Tax Returns, other than a group of which the common parent is the Company. Neither the Company nor any of its Subsidiaries (i) has any actual or potential liability under Treasury Regulations Section 1.1502-6 (or any comparable or similar provision of federal, state, local or foreign law), as a transferee or successor, pursuant to any contractual obligation, or otherwise for any Taxes of any person other than the Company or any of its Subsidiaries, or (ii) is a party to or bound by any Tax indemnity, Tax sharing, Tax allocation or similar agreement. All material Taxes that the Company or any of its Subsidiaries was required by law to withhold or collect have been duly withheld or collected and, to the extent required, have been properly paid to the appropriate Governmental Entity.
(b) The unpaid Taxes of the Company did not, as of the most recent fiscal month end, exceed the reserve for Tax liability (rather than any reserve for deferred Taxes established to reflect timing differences between book and Tax income) set forth on the face of the Balance Sheet (rather than in any notes thereto).
(c) No examination or audit of any Tax Return of the Company or any of its Subsidiaries by any Governmental Entity has occurred or is currently in progress or, to the knowledge of the Company, threatened or contemplated in writing. Neither the Company nor any of its Subsidiaries has been informed by any jurisdiction that the jurisdiction believes that the Company or any of its Subsidiaries was required to file any Tax Return that was not filed. Neither the Company nor any of its Subsidiaries has (x) waived any statute of limitations with respect to Taxes or agreed to extend the period for assessment or collection of any Taxes, (y) requested any extension of time within which to file any Tax Return, which Tax Return has not yet been filed, or (z) executed or filed any power of attorney with any taxing authority.
| B-11 |
(d) Neither the Company nor any of its Subsidiaries has made any payment, is obligated to make any payment, or is a party to any agreement that could obligate it to make any payment that may be treated as an “excess parachute payment” under Section 280G of the Code (without regard to Sections 280G(b)(4) and 280G(b)(5) of the Code).
(e) There are no adjustments under Section 481 of the Code (or any similar adjustments under any provision of the Code or the corresponding foreign, state or local Tax laws) that are required to be taken into account by the Company or any of its Subsidiaries in any period ending after the Closing Date by reason of a change in method of accounting in any taxable period ending on or before the Closing Date or as a result of the consummation of the transactions contemplated by this Agreement.
(f) Neither the Company nor any of its Subsidiaries has been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(l)(A)(ii) of the Code.
(g) Neither the Company nor any of its Subsidiaries has distributed to its stockholders or security holders stock or securities of a controlled corporation, nor has stock or securities of the Company or any of its Subsidiaries been distributed, in a transaction to which Section 355 of the Code applies (i) in the two years prior to the date of this Agreement or (ii) in a distribution that could otherwise constitute part of a “plan” or “series of related transactions” (within the meaning of Section 355(e) of the Code) that includes the transactions contemplated by this Agreement.
(h) There are no Liens with respect to Taxes upon any of the assets or properties of the Company or any of its Subsidiaries, other than with respect to Taxes not yet due and payable or being contested in good faith by appropriate proceedings.
(i) Neither the Company nor any of its Subsidiaries will be required to include any item of income in, or exclude any item of deduction from, taxable income for any period (or any portion thereof) ending after the Closing Date as a result of any (i) deferred intercompany gain or any excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding provision of state, local or foreign Tax law), (ii) closing agreementas described in Section 7121 of theCode(or any corresponding or similar provision of state, local or foreignTaxlaw) executed on or prior to theClosing Date, (iii) installment sale or other open transaction disposition made on or prior to the Closing Date, or (iv) prepaid amount received on or prior to the Closing Date.
(j) Neither the Company nor any of its Subsidiaries has participated in any “reportable transaction” as defined in section 1.6011-4(b) of the Treasury Regulations or any analogous provision of state or local law.
| B-12 |
(k) Since the date of the Balance Sheet, the Company has not incurred any liability for Taxes arising from extraordinary gains or losses, as that term is used in GAAP, outside the Ordinary Course of Business.
4.8Owned and Leased Real Properties. Except as disclosed in the Recent SEC Documents,neither the Company nor any of its Subsidiaries (a) owns anyreal property or(b)leases, subleases or licenses any real property. Neitherthe Companynor any of itsSubsidiariesleases, subleases or licenses any real property to anypersonother thanthe Companyand itsSubsidiaries.
4.9Intellectual Property.
(a) To the knowledge of the Company, the Company and its Subsidiaries own, license or otherwise possess legally enforceable rights to use all Intellectual Property used or necessary to conduct the business of the Company and its Subsidiaries as currently conducted, or that would be used or necessary as such business is currently proposed to be conducted (excluding currently-available, off-the-shelf software programs that are licensed by the Company pursuant to “shrink wrap” licenses).
(b) The execution and delivery of this Agreement and consummation of the Merger will not result in the breach of, or create on behalf of any third party the right to terminate or modify, (i) any license, sublicense or other agreement relating to any Intellectual Property owned by the Company or any of its Subsidiaries that is material to the business of the Company and its Subsidiaries, taken as a whole, including software that is used in the development or manufacture of or forms a part of any product or service sold by or expected to be sold by the Company or any of its Subsidiaries (the “Company Intellectual Property”) or (ii) any license, sublicense or any other agreement as to which the Company or any of its Subsidiaries is a party and pursuant to which the Company or any of its Subsidiaries is authorized to use any third party Intellectual Property that is material to the business of the Company and its Subsidiaries, taken as a whole, including software that is used in the development or manufacture of or forms a part of any product or service sold by or expected to be sold by the Company or any of its Subsidiaries (the “Third Party Intellectual Property”). Section 4.9(b) of the Disclosure Schedule sets forth a complete and accurate list of registered Company Intellectual Property (including Company Intellectual Property for which registration has been applied) and Section 4.9(b) of the Disclosure Schedule sets forth a complete and accurate list of all Third Party Intellectual Property.
(c) To the knowledge of the Company, all patents and registrations and applications for Trademarks, service marks and copyrights which are held by the Company or any of its Subsidiaries and that are material to the business of the Company and its Subsidiaries, taken as a whole, are valid and subsisting. The Company and its Subsidiaries have taken commercially reasonable measures to protect the proprietary nature of the Company Intellectual Property. To the knowledge of the Company, no other person or entity is infringing, violating or misappropriating any of the Company Intellectual Property or Third Party Intellectual Property.
| B-13 |
(d) To the knowledge of the Company, none of the (i) products previously or currently sold by the Company or any of its Subsidiaries or (ii) business or activities previously or currently conducted by the Company orany of itsSubsidiariesinfringes, violates or constitutes a misappropriation of, anyIntellectual Propertyof any third party. Except as disclosed in Section 4.9(d) of the Disclosure Schedule, neither the Companynor any of itsSubsidiarieshas received any written complaint, claim or notice alleging any such infringement, violation or misappropriation.
4.10Contracts.
(a) Except for the contracts and agreements identified on the exhibit indices of the Recent SEC Documents and as disclosed in Section 4.10(a) of the Disclosure Schedule (collectively, the “Material Contracts”), there are no material contracts (as such term is defined in Item 601(b)(10) of Regulation S-K) to which the Company or its Subsidiaries are a party.
(b) Except as disclosed in the Recent SEC Documents, neither the Company nor any of its Subsidiaries has entered into any transaction with any Affiliate of the Company or any of its Subsidiaries or any transaction that would be subject to proxy statement disclosure pursuant to Item 404 of Regulation S-K.
(c) With respect to each Material Contract: (i) the agreement is legal, valid, binding and enforceable and in full force and effect; (ii) the agreement will continue to be legal, valid, binding and enforceable and in full force and effect immediately following the Closing in accordance with the terms thereof as in effect immediately prior to the Closing; and (iii) neither the Company nor any of its Subsidiaries nor, to the knowledge of the Company, any other party, is in breach or violation of, or default under, any such agreement, and no event has occurred, is pending or, to the knowledge of the Company, is threatened, which, with or without notice or lapse of time, or both, would constitute a breach or default by the Company or any of its Subsidiaries or, to the knowledge of the Company, any other party under such agreement. Neither the Company nor any of its Subsidiaries has received any notice in writing from any other party, and, to the knowledge of the Company, no party has threatened, to terminate, cancel, fail to renew or otherwise materially modify any such agreements the loss of which, individually or in the aggregate, would reasonably be expected to have a Company Material Adverse Effect.
4.11Litigation. Except as disclosed in the Recent SEC Documents,there is no action, suit, proceeding, claim, arbitration or investigation before any Governmental Entity or before any arbitrator that is pending or, to the knowledge of the Company, has been threatened in writing against the Company or any of its Subsidiaries. There are no material judgments, orders or decrees outstanding against the Company or any of its Subsidiaries.
4.12Environmental Matters. (a) Except as disclosed in Section 4.12(a) of the Disclosure Schedule and except for such matters that, individually or in the aggregate, have not had, and are not reasonably expected to have, a Company Material Adverse Effect:
| B-14 |
(i) The Company and its Subsidiaries have complied with all applicable Environmental Laws;
(ii) to the actual knowledge of the Company, and without independent investigation, all real property currently owned or leased by the Company or any of its Subsidiaries are in compliance, and since the Company’s or any of its Subsidiaries’ acquisition of an interest in such currently owned or leased real property have been in compliance, in all material respects, and prior to such acquisition were in compliance, with all applicable Environmental Laws;
(iii) to the actual knowledge of the Company, and without independent investigation, the real properties currently owned, leased or operated by the Company and its Subsidiaries (including soils, sediments, groundwater, surface water, buildings or other structures) are not contaminated with any Hazardous Substances at levels or in a condition that violate applicable Environmental Laws;
(iv) to the actual knowledge of the Company, and without independent investigation, the real properties formerly owned, leased or operated by the Company or any of its Subsidiaries (including soils, sediments, groundwater, surface water, buildings or other structures) were not, during the period of ownership, use or operation by the Company or any of its Subsidiaries, contaminated with Hazardous Substances at levels or in a condition that violated or would violate applicable Environmental Laws;
(v) neither the Company nor any of its Subsidiaries are subject to liability (whether arising under contract or under Environmental Law) for the impaired environmental condition of, or any Hazardous Substance disposal or contamination at, the real property of any third party;
(vi) neither the Company nor any of its Subsidiaries have released any Hazardous Substance into the environment in amounts or in a manner that, individually or in the aggregate, could reasonably be expected to require notification, investigation, response, abatement or remediation under any Environmental Law;
(vii) The Company and its Subsidiaries have all Company Authorizations and Material Safety Data Sheets for the operation of the business as currently conducted as required under Environmental Laws, copies of all of which have been delivered or made available to the Investor; and have filed all reports required to be filed with any Governmental Entity thereunder or pursuant to any other applicable Environmental Law;
(viii) neither the Company nor any of its Subsidiaries has received any notice, notice of violation, demand, letter, claim or request for information regarding (A) any action instituted or threatened under or pursuant to any Environmental Law, or of any violation of, any Environmental Law applicable to any currently or formerly owned or leased real properties of the Company or its Subsidiaries, or (B) alleging that the Company or any of its Subsidiaries is or may be in violation of, liable or potentially liable under or have outstanding obligations under any Environmental Law, including without limitation, any notice from any Governmental Entity or other person advising that the Company or its Subsidiaries that it is or is potentially responsible for response, assessment, investigation, abatement, or remediation costs under any Environmental Law with respect to a release or threatened release of any Hazardous Substances;
| B-15 |
(ix) neither the Company nor any of its Subsidiaries has received or is subject to any judgments, orders, decrees, injunctions or other binding arrangements with any Governmental Entity or is subject to any indemnity or other agreement with any third party relating to liability under any Environmental Law or relating to Hazardous Substances; and
(x) to the actual knowledge of the Company and any of its Subsidiaries, there are no circumstances or conditions involving the Company, any of its Subsidiaries or any of their respective currently owned or leased real properties that could reasonably be expected to result in any claims, liabilities, obligations, investigations, costs or restrictions on the ownership, use or transfer of any such real property of the Company or any of its Subsidiaries pursuant to any Environmental Law.
(b) Except as set forth in Section 4.12(b) of the Disclosure Schedule, there are no aboveground or underground storage tank systems, including pumps and lines, on the currently owned or leased real property for the storage of Hazardous Substances. Each of the tanks and related equipment and apparatus disclosed on Section 4.12(b) of the Disclosure Schedule has been upgraded and if required, registered, to meet all applicable requirements under Environmental Laws.
(c) The Company has delivered to the Investor true and complete copies and results of any reports, studies, sampling, tests, environmental site assessments or other assessments possessed by or readily available to the Company pertaining to the environmental or physical condition of any real property (and any buildings, structures, or other improvements thereon) presently or previously owned, leased or used by the Company or any of its Subsidiaries.
4.13Employee Benefit Plans.
(a) Section 4.13(a) of the Disclosure Schedule sets forth a complete and accurate list, as of the date of this Agreement, of all Employee Benefit Plans maintained, or contributed to, by the Company or any of its Subsidiaries or any of their respective ERISA Affiliates (collectively, the “Employee Plans”).
(b) Each Employee Plan has been administered in all material respects in accordance with ERISA, the Code and all other applicable laws and the regulations thereunder and in accordance with its terms, and each of the Company and its Subsidiaries and their respective ERISA Affiliates has in all material respects met its obligations with respect to such Employee Plan and has made all required contributions thereto (or reserved such contributions on the Balance Sheet). The Company and its Subsidiaries and each of their respective ERISA Affiliates and each Employee Plan are in compliance in all material respects with the currently applicable provisions of ERISA and the Code and the regulations thereunder (including, but not limited to, Section 4980B-4980E of the Code, Subtitle K, Chapter 100 of the Code and Sections 601 through 608 and Section 701 et seq. of ERISA). All filings and reports as to each Employee Plan required to have been submitted to the IRS or to the United States Department of Labor have been timely submitted. With respect to Employee Plans, no event has occurred, and to the knowledge of the Company, there exists no condition or set of circumstances in connection with which the Company or any of its Subsidiaries or ERISA Affiliates could be subject to any liability that would reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect under ERISA, the Code or any other applicable law.
| B-16 |
(c) With respect to Employee Plans, there are no benefit obligations for which contributions have not been made or properly accrued and there are no benefit obligations that have not been accounted for by reserves, or otherwise properly footnoted in accordance with GAAP, on the financial statements of the Company, which obligations would reasonably be expected, individually or in the aggregate, to have a Company Material Adverse Effect. The assets of each Employee Plan that is funded are reported at their fair market value on the books and records of such Employee Plan.
(d) All Employee Plans that are intended to be qualified under Section 401(a) of the Code have received determination or opinion letters from the IRS to the effect that such Employee Plans are qualified and the plans and trusts related thereto are exempt from federal income taxes under Sections 401(a) and 501(a), respectively, of the Code, no such determination or opinion letter has been revoked and revocation has notbeen threatened, and no suchEmployee Benefit Planhas been amended or operated since the date of its most recent determination letter or application therefor in any respect, and no act or omission has occurred, that would adversely affect its qualification or materially increase its cost. To the knowledge ofthe Company, no “prohibited transaction” (within the meaning of Section 4975 of theCodeor Sections 406 and 408 ofERISA) has occurred with respect to any suchEmployee Benefit Plans. EachEmployee Planthat is required to satisfy Section 401(k)(3) or Section 401(m)(2) of theCodehas been tested for compliance with, and satisfies the requirements of, Section 401(k)(3) and Section 401(m)(2) of theCode, as the case may be, for each plan year ending prior to theClosing Date.
(e) Neither the Company nor any of its Subsidiaries nor any of their respective ERISA Affiliates has (i) ever maintained a Employee Benefit Plan that was ever subject to Section 412 of the Code or Title IV of ERISA or (ii) ever been obligated to contribute to a “multiemployer plan” (as defined in Section 4001(a)(3) of ERISA). No Employee Plan is funded by, associated with or related to a “voluntary employees’ beneficiary association” within the meaning of Section 501(c)(9) of the Code. No Employee Plan holds securities issued by the Company or any of its Subsidiaries or any of their respective ERISA Affiliates.
(f) Each Employee Plan is amendable and terminable unilaterally by the Company and any of the Company’s Subsidiaries that are a party thereto or covered thereby at any time without additional vesting or acceleration of benefits or any other liability to the Company or any of its Subsidiaries as a result thereof (other than for benefits accrued through the date of termination or amendment and reasonable administrative expenses related thereto), and no Employee Plan, plan documentation or agreement, summary plan description or other written communication distributed generally to employees by its terms prohibits the Company or any of its Subsidiaries from amending or terminating any such Employee Plan.
| B-17 |
(g) Except as disclosed in the exhibit index to any Recent SEC Document, neither the Company nor any of its Subsidiaries is a party to any oral or written (i) agreement with any stockholders, director, executive officer or other employee of the Company or any of its Subsidiaries (A) the benefits of which are contingent, or the terms of which are materially altered, upon the occurrence of a transaction involving the Company or any of its Subsidiaries of the nature of any of the transactions contemplated by this Agreement, (B) providing any term of employment or compensation guarantee or (C) providing severance benefits or other benefits after the termination of employment of such director, executive officer or employee; (ii) agreement, plan or arrangement under which any person may receive payments from the Company or any of its Subsidiaries that may be subject to the tax imposed by Section 4999 of the Code or included in the determination of such person’s “parachute payment” under Section 280G of the Code, without regard to Section 280G(b)(4); or (iii) agreement or plan binding the Company or any of its Subsidiaries, including any stock option plan, stock appreciation right plan, restricted stock plan, stock purchase plan or severance benefit plan, any of the benefits of which shall be increased, or the vesting of the benefits of which shall be accelerated, by the occurrence of any of the transactions contemplated by this Agreement or the value of any of the benefits of which shall be calculated on the basis of any of the transactions contemplated by this Agreement.
(h) None of the Employee Plans promises or provides retiree medical or other retiree welfare benefits to any person, except as required by applicable law.
(i) Each Employee Plan that is a “nonqualified deferred compensation plan” (as defined in Code Section 409A(d)(1)) has been operated since January 1, 2005 in good faith compliance with Code Section 409A and IRS Notice 2005-1. No Employee Plan that is a “nonqualified deferred compensation plan” has been materially modified (as determined under Notice 2005-1) after October 3, 2004. No event has occurred that would be treated by Code Section 409A(b) as a transfer of property for purposes of Code Section 83. No stock option or equity unit option granted under any Employee Plan has an exercise price that has been or may be less than the fair market value of the underlying stock or equity units (as the case may be) as of the date such option was granted or has any feature for the deferral of compensation other than the deferral of recognition of income until the later of exercise or disposition of such option.
4.14Compliance With Laws. The Company and each of its Subsidiaries has materially complied with, is not in material violation of, and has not received any notice from any Governmental Entity alleging any material violation with respect to, any applicable provisions of any statute, law or regulation with respect to the conduct of its business, or the ownership or operation of its properties or assets.
4.15Permits and Regulatory Matters.
(a) The Company and each of its Subsidiaries have all permits, licenses, registrations, authorizations and franchises from Governmental Entities required to conduct their businesses as currently conducted or as currently proposed to be conducted, including without limitation all such permits, licenses, registrations, authorizations and franchises required by the FDA or any other Governmental Entity exercising comparable authority, except for such permits, licenses, registrations, authorizations and franchises the lack of which, individually or in the aggregate, has not had, and is not reasonably expected to have, a Company Material Adverse Effect (the “Company Authorizations”). The Company and its Subsidiaries are in compliance with the terms of the Company Authorizations, except where the failure to so comply, individually or in the aggregate, has not had, and is not reasonably expected to have, a Company Material Adverse Effect. No Company Authorization will cease to be effective as a result of the consummation of the transactions contemplated by this Agreement.
| B-18 |
(b) All manufacturing, processing, distribution, labeling, storage, testing, sale or marketing of products performed by or on behalf of the Company or any of its Subsidiaries are in compliance with all applicable laws, rules, regulations or orders administered or issued by the FDA or any other Governmental Entity exercising comparable authority, except where the failure to so comply, individually or in the aggregate, has not had, and is not reasonably expected to have, a Company Material Adverse Effect. Neither the Company nor any of its Subsidiaries has received any notices or correspondence from the FDA or any other Governmental Entity exercising comparable authority, and to the knowledge of the Company there is no action or proceeding pending or threatened (including any prosecution, injunction, seizure, civil fine, suspension or recall), in each case alleging that the Company or any of its Subsidiaries is not currently in compliance with any and all applicable laws, regulations or orders implemented by the FDA or any other Governmental Entity exercising comparable authority.
(c) There are no seizures, recalls, market withdrawals, field notifications or corrective actions, notifications of misbranding, destruction orders, safety alerts or similar actions relating to the safety or efficacy of any products marketed or sold by the Company or any of its Subsidiaries being conducted, requested in writing or, to the knowledge of the Company, threatened by the FDA or any other Governmental Entity exercising comparable authority. The Company has not, either voluntarily or involuntarily, initiated, conducted or issued or caused to be initiated, conducted or issued any recall, market withdrawal or other similar action by a Governmental Entity
(d) The studies, tests and preclinical and clinical trials conducted by or on behalf of the Company or any of its Subsidiaries were and, if still pending, are being conducted in all material respects in accordance with experimental protocols, procedures and controls pursuant to, where applicable, accepted professional and scientific standards; and neither the Company nor any of its Subsidiaries has received any notices or correspondence from the FDA or any other Governmental Entity exercising comparable authority requiring the termination, suspension or material modification of any studies, tests or preclinical or clinical trials conducted by or on behalf of the Company or any of its Subsidiaries.
| B-19 |
4.16Employees.
(a) Substantially all current or past key employees of the Company or any of its Subsidiaries have entered into confidentiality and assignment of inventions agreements with the Company or such Subsidiary, a copy or form of which has previously been provided or made available to the Investor. To the knowledge of the Company, no employee of the Company or any Subsidiary of the Company is in violation of any term of any patent disclosure agreement, non-competition agreement, or any restrictive covenant to a former employer relating to the right of any such employee to be employed by the Company or any of its Subsidiaries because of the nature of the business currently conducted or currently proposed to be conducted by the Company or any of its Subsidiaries or to the use of trade secrets or proprietary information of others, the consequences of which, individually or in the aggregate, are reasonably expected to have a Company Material Adverse Effect. To the knowledge of the Company, as of the date of this Agreement, no key employee or group of employees has any plans to terminate employment with the Company or its Subsidiaries.
(b) Neither the Company nor any of its Subsidiaries is a party to or otherwise bound by any collective bargaining agreement, contract or other agreement or understanding with a labor union or labor organization. Neither the Company nor any of its Subsidiaries is the subject of any proceeding asserting that the Company or any of its Subsidiaries has committed an unfair labor practice or is seeking to compel it to bargain with any labor union or labor organization that, individually or in the aggregate, is reasonably expected to have a Company Material Adverse Effect, nor is there pending or, to the knowledge of the Company, threatened, any labor strike, dispute, walkout, work stoppage, slow-down or lockout involving the Company or any of its Subsidiaries.
(c) The Company has made available to the Investor forms of each severance agreement in effect between the Company or its Subsidiaries and any employee of the Company or its Subsidiaries.
4.17Insurance. Section 4.17 of the Disclosure Schedule sets forth a complete and accurate list as of the date of this Agreement of all insurance policies maintained by the Company or any of its Subsidiaries (the “Insurance Policies”). EachInsurance Policyis in full force and effect as of the date of thisAgreement. As of the date of thisAgreement, there is no material claim by theCompanyor any of itsSubsidiariespending under anyInsurance Policyas to which coverage has been questioned, denied or disputed by the underwriters of such policy.
4.18Controls and Procedures, Certifications and Other Matters Relating to the Sarbanes Act.
(a) The Company and each of its Subsidiaries maintains accurate books and records reflecting its assets and liabilities and maintains proper and adequate internal control over financial reporting that provide reasonable assurance that (i) transactions are executed with management’s authorization, (ii) transactions are recorded as necessary to permit preparation of the consolidated financial statements of the Company and to maintain accountability for the Company’s consolidated assets, (iii) access to assets of the Company and its Subsidiaries is permitted only in accordance with management’s authorization, (iv) the reporting of assets of the Company and its Subsidiaries is compared with existing assets at regular intervals and (v) accounts, notes and other receivables and inventory were recorded accurately, and proper and adequate procedures are implemented to effect the collection thereof on a current and timely basis.
| B-20 |
(b) The Company maintains disclosure controls and procedures required by Rules 13a-15 or 15d-15 under the Exchange Act, and such controls and procedures are effective to ensure that all material information concerning the Company and its Subsidiaries is made known on a timely basis to the individuals responsible for the preparation of the Company’s filings with the SEC and other public disclosure documents.
(c) Neither the Company nor any of its officers has received notice from any Governmental Entity questioning or challenging the accuracy, completeness or manner of filing or submission of any filing with the SEC, including without limitation any certifications required by Section 906 of the Sarbanes Act.
(d) Neither the Company nor any of its Subsidiaries has, since the Company became subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act, extended or maintained credit, arranged for the extension of credit, modified or renewed an extension of credit, in the form of a personal loan or otherwise, to or for any director or executive officer of the Company. Section 4.18 of the Disclosure Schedule identifies any loan or extension of credit maintained by the Company to which the second sentence of Section 13(k)(1) of the Exchange Act applies.
4.19Commercial Relationships. During the past 12 months from the date of this Agreement, none of the Company’s or any of its Subsidiaries’ material suppliers, customers, collaborators, distributors, agents, licensors or licensees has canceled or otherwise terminated its relationship with the Company or any of its Subsidiaries or has materially altered its relationship with the Company or any of its Subsidiaries. To the knowledge of the Company, no such person has any plan or intention, and neither the Company nor any of its Subsidiaries has received any written notice from any such person, to terminate, cancel or otherwise materially modify its relationship with the Company or any of its Subsidiaries. As of the date of this Agreement, neither the Company nor any of its Subsidiaries has received any written notice (formal or informal) or other communication from any of the Company’s top ten largest customers (based on fiscal 2012 consolidated total revenues) or top ten largest suppliers (based on fiscal 2012 expenditures) that indicates or could reasonably be expected to indicate that any such customer or supplier has any plan or intention not to renew its agreement with the Company on terms substantially comparable to its current agreement with the Company.
4.20Tangible Assets. The Company and its Subsidiaries own or lease all machinery, equipment and other tangible assets necessary for the conduct of their business as presently conducted. Each such tangible asset has been maintained in accordance with normal industry practice, is in good operating condition and repair (subject to normal wear and tear) and is suitable for the purposes for which it presently is used.
| B-21 |
4.21False Claims Act Matter. The aggregate amount of any fines, penalties or other payments by the Company to any Governmental Entity in connection with the FCA Matter shall not exceed $6,000,000 (exclusive of additional interest at 1.5% per annum, plus attorney’s fees to thequi tamrelator, which will not exceed $120,000). There are no other claims, actions or proceedings pending, or to the Company’s knowledge, threatened against the Company or its officers, directors, employees, stockholder or agents related to the events giving rise to the FCA Matter except as will be released in the final settlement with the Department of Justice (on behalf of the Office of the Inspector General of the Department of Health and Human Services, the TRICARE Management Activity, the United States Office of Personnel Management, the United States Department of Veteran Affairs, and the Office of Workers’ Compensation Programs of the United States Department of Labor) regarding the FCA Matter. The FCA Matter will not give rise to any exclusion or debarment of the Company from participation in any programs funded by the United States government or any state government, the debarment of the Company from contracting with any federal or state agency, or to any criminal proceedings against the Company or its officer, directors, employees, stockholders or agents.
4.22Reporting Company; Form S-3. The Company is not an “ineligible issuer” (as defined in Rule 405 promulgated under the Securities Act) and is eligible to register the Registrable Securities for resale by the Investors on a registration statement on Form S-3 under the Securities Act. The Company is subject to the reporting requirements of the Exchange Act, and has filed all reports required thereby. To the Company’s knowledge, there exist no facts or circumstances (including without limitation any required approvals or waivers or any circumstances that may delay or prevent the obtaining of accountant’s consents) that reasonably would be expected to prohibit or delay the preparation and filing of a registration statement on Form S-3 that will be available for the resale of the Registrable Securities by the Investors.
4.23Issuance, Sale and Delivery of the Shares. The Shares have been duly authorized and, when issued, delivered and paid for in the manner set forth in this Agreement, will be validly issued, fully paid and nonassessable. No preemptive rights or other rights to subscribe for or purchase any shares of Common Stock of the Company exist with respect to the issuance and sale of the Shares by the Company pursuant to this Agreement. No stockholder of the Company has any right (which has not been waived or has not expired by reason of lapse of time following notification of the Company’s intention to file the Registration Statement (as hereinafter defined)) to require the Company to register the sale of any capital stock owned by such stockholder under the Registration Statement. No further approval or authority of the stockholders (other than the TranS1 Stockholder Approval) or the Board of Directors of the Company will be required for the issuance and sale of the Shares to be sold by the Company as contemplated herein.
4.24Due Execution, Delivery and Performance of the Agreement. The Company has full legal right, corporate power and authority to enter into this Agreement and, subject only to the TranS1 Stockholder Approval, perform the transactions contemplated hereby. This Agreement has been duly authorized, executed and delivered by the Company. This Agreement constitutes a legal, valid and binding agreement of the Company, enforceable against the Company in accordance with its terms, except as enforceability may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws of general application relating to or affecting the enforcement of creditors’ rights and the application of equitable principles relating to the availability of remedies, and except as rights to indemnity or contribution, including but not limited to, indemnification provisions set forth in Section 8.3 of this Agreement may be limited by federal or state securities law or the public policy underlying such laws. The execution and performance of this Agreement by the Company and the consummation of the transactions herein contemplated will not violate any provision of the Certificate of Incorporation or Bylaws of the Company or the organizational documents of any Subsidiary and will not result in the creation of any lien, charge, security interest or encumbrance upon any assets of the Company or any Subsidiary pursuant to the terms or provisions of, or will not conflict with, result in the breach or violation of, or constitute, either by itself or upon notice or the passage of time or both, a default under any agreement, mortgage, deed of trust, lease, franchise, license, indenture, permit or other instrument to which any of the Company or any Subsidiary is a party or by which any of the Company or any Subsidiary or their respective properties may be bound or affected or any statute or any authorization, judgment, decree, order, rule or regulation of any court or any regulatory body, administrative agency or other governmental agency or body applicable to the Company or any Subsidiary or any of their respective properties. No consent, approval, authorization or other order of any court, regulatory body, administrative agency or other governmental agency or body is required for the execution and delivery of this Agreement or the consummation of the transactions contemplated by this Agreement, except for the TranS1 Stockholder Approval and compliance with the Blue Sky laws and federal securities laws applicable to the offering of the Shares.
| B-22 |
4.25Investment Company. The Company is not an “investment company” or an “affiliated person” of, or “promoter” or “principal underwriter” for an investment company, within the meaning of the Investment Company Act of 1940, as amended, and the rules and regulations of the SEC promulgated thereunder.
4.26Offering Materials. Each of the Company, its directors and officers has not distributed and will not distribute prior to the Closing Date any offering material, including any “free writing prospectus” (as defined in Rule 405 promulgated under the Securities Act), in connection with the offering and sale of the Shares. The Company has not in the past nor will it hereafter take any action to sell, offer for sale or solicit offers to buy any securities of the Company that could result in the initial sale of the Shares not being exempt from the registration requirements of Section 5 of the Securities Act.
4.27Price of Common Stock. The Company has not taken, and will not take, directly or indirectly, any action designed to cause or result in, or that has constituted or that might reasonably be expected to constitute, the stabilization or manipulation of the price of the shares of the Common Stock to facilitate the sale or resale of the Shares.
4.28Use of Proceeds. The Company shall use the proceeds from the sale of the Sharesfor working capital and general corporate purposes.
4.29Related Party Transactions. No transaction has occurred between or among the Company, on the one hand, and its Affiliates, officers or directors on the other hand, that is required to have been described under applicable securities laws in its Exchange Act filings and is not so described in such filings.
| B-23 |
4.30Listing Compliance. The Company is in compliance with the requirements of NASDAQ for continued listing of the Common Stock thereon. The Company has taken no action designed to, or likely to have the effect of, terminating the registration of the Common Stock under the Exchange Act or the listing of the Common Stock on NASDAQ, nor has the Company received any notification that the SEC or NASDAQ is contemplating terminating such registration or listing. The transactions contemplated by this Agreement will not contravene the rules and regulations of NASDAQ. The Company will comply with all requirements of NASDAQ with respect to the issuance of the Shares and shall cause the Registrable Securities to be listed on NASDAQ and listed on any other exchange on which the Company’s Common Stock is listed on or before the Closing Date.
4.31Integration; Other Issuances of Shares. Neither the Company nor its Subsidiaries nor any Affiliates, nor any person acting on its or their behalf, has issued any shares of Common Stock or shares of any series of preferred stock or other securities or instruments convertible into, exchangeable for or otherwise entitling the holder thereof to acquire shares of Common Stock under circumstances that would cause such issuance to be integrated with the sale of the Shares to the Investors for purposes of the Securities Act or of any applicable stockholder approval provisions, including, without limitation, under the rules and regulations of the Trading Market, such that the sale of the Shares would not be exempt from registration under the Securities Act or would require stockholder approval (other than the TranS1 Stockholder Approval) under the rules and regulations of the Trading Market. Assuming the accuracy of the representations and warranties of Investors, the offer and sale of the Shares by the Company to the Investors pursuant to this Agreement will be exempt from the registration requirements of the Securities Act.
SECTION 5.Representations, Warranties and Covenants of the Investors. Each Investor, severally and not jointly, represents and warrants to, and covenants with, the Company that:
5.1Experience. (a) The Investor is knowledgeable, sophisticated and experienced in financial and business matters, in making, and is qualified to make, decisions with respect to investments in shares representing an investment decision like that involved in the purchase of the Shares, including investments in securities issued by the Company and comparable entities, has the ability to bear the economic risks of an investment in the Shares and has requested, received, reviewed and considered all information it deems relevant in making an informed decision to purchase the Shares; (b) the Investor is acquiring the number of Shares set forth on suchInvestor’s signature page to thisAgreement above in the ordinary course of its business and for its own account for investment only and with no present intention of distributing any of such Shares or any arrangement or understanding with any other persons regarding the distribution of such Shares (this representation and warranty not limiting the Investor’s right to sell pursuant to the Registration Statement or in compliance with the Securities Act and the rules and regulations promulgated thereunder, or, other than with respect to any claims arising out of a breach of this representation and warranty, the Investor’s right to indemnification under Section 8.3); (c) the Investor will not, directly or indirectly, offer, sell, pledge, transfer or otherwise dispose of (or solicit any offers to buy, purchase or otherwise acquire or take a pledge of) any of the Shares, except in compliance with the Securities Act and the rules and regulations promulgated thereunder and any applicable state securities laws; (d) the Investor has completed or caused to be completed the Registration Statement Questionnaire attached hereto as part ofExhibit A, for use in preparation of the Registration Statement, and the answers thereto are true and correct as of the date hereof and will be true and correct as of the effective date of the Registration Statement and the Investor will notify the Company immediately of any material change in any such information provided in the Registration Statement Questionnaire until such time as the Investor has sold all of its Shares or until the Company is no longer required to keep the Registration Statement effective; (e) the Investor has had an opportunity to discuss this investment with representatives of the Company and ask questions of them and (f) the Investor is an “accredited investor” within the meaning of Rule 501(a) of Regulation D promulgated under the Securities Act.
| B-24 |
5.2Reliance on Exemptions. The Investor understands that the Shares are being offered and sold to it in reliance upon specific exemptions from the registration requirements of the Securities Act, the rules and regulations promulgated thereunder and state securities laws and that the Company is relying upon the truth and accuracy of, and the Investor’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of the Investor set forth herein in order to determine the availability of such exemptions and the eligibility of the Investor to acquire the Shares.
5.3Investment Decision. The Investor understands that nothing in the Agreement or any other materials presented to the Investor in connection with the purchase and sale of the Shares constitutes legal, tax or investment advice. The Investor has consulted such legal, tax and investment advisors as it, in its sole discretion, has deemed necessary or appropriate in connection with its purchase of the Shares.
5.4Legends. The Investor understands that, until such time as the Registration Statement has been declared effective (with respect to both legends identified below) or the Registrable Securities may be sold pursuant to Rule 144 under the Securities Act without any restriction as to the number of securities as of a particular date that can then be immediately sold (with respect to the first legend identified below), the Registrable Securities will bear restrictive legends in substantially the following form:
THE SHARES EVIDENCED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”) OR THE SECURITIES LAWS OF ANY STATE OR OTHER JURISDICTION. THE SHARES MAY NOT BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED EXCEPT (1) PURSUANT TO AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OR (2) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT, IN EACH CASE IN ACCORDANCE WITH ALL APPLICABLE STATE SECURITIES LAWS AND THE SECURITIES LAWS OF OTHER JURISDICTIONS, OR IN A TRANSACTION EXEMPT FROM REGISTRATION.
| B-25 |
THESE SECURITIES ARE SUBJECT TO CERTAIN RESTRICTIONS SET FORTH IN THE SECURITIES PURCHASE AGREEMENT DATED MARCH 3, 2013 BY AND AMONG THE ISSUER AND CERTAIN OTHER PERSONS, WHICH RESTRICT THE RIGHT TO TRANSFER, SELL OR OTHERWISE DISPOSE OF THESE SECURITIES. A COPY OF SUCH SECURITIES PURCHASE AGREEMENT IS AVAILABLE FOR REVIEW BY THE RECORD HOLDER OF THESE SECURITIES AT THE PRINCIPAL OFFICES OF THE ISSUER.
5.5Residency. The Investor’s principal executive offices are in the jurisdiction set forth immediately below the Investor’s name on the signature pages hereto.
5.6Lock-up. The Investor hereby agrees with the Company that the Investor will not offer, sell, contract to sell, assign, transfer, hypothecate, pledge or grant a security interest in, or otherwise dispose of, or enter into any transaction which is designed to, or might reasonably be expected to, result in the disposition of (whether by actual disposition or effective economic disposition due to cash settlement or otherwise by the Company or any Affiliate of the Company or any person in privity with the Company or any Affiliate of the Company), directly or indirectly, any of the Registrable Securities from the period commencing on the Closing and expiring on the effective date of the Registration Statement. The restrictions in the first sentence of this Section 5.6 shall not apply to (a) shares of Common Stock or other securities acquired in open market transactions or otherwise after the Closing, (b) transfers made (1) to an Affiliate of the Investor, (2) to Investor’s spouse, lineal descendants, father, mother, brother or sister or any trust for the benefit of any such family member (collectively, “Immediate Family Members”) or (3) to a trust or otherwise for bona fide estate planning purposes if the beneficiaries of such trust consist solely of the Investor and/or his Immediate Family Members so long as in the case of each of (1), (2) and (3), the transferee agrees to be bound by the restrictions of this Section 5.6 and (c) shares of Common Stock or other securities owned by the spouse of the Investor or any other Immediate Family Member other than any securities subject to this Section 5.6 that are acquired by a transferee pursuant to the exception in (b) above.
5.7Public Sale or Distribution. (a) Each Investor agrees that it will comply with the prospectus delivery requirements of the Securities Act as applicable to it in connection with sales of Registrable Securities pursuant to the Registration Statement and shall sell its Registrable Securities in accordance with the plan of distribution set forth in the Prospectus. The Investor acknowledges that there may occasionally be times when the Company must suspend the use of the Prospectus (a “Suspension”) until such time as an amendment to the Registration Statement has been filed by the Company and declared effective by the SEC, or until such time as the Company has filed an appropriate report with the SEC pursuant to the Exchange Act. Each Investor further agrees that, upon receipt of a notice from the Company of the occurrence of a Suspension, such Investor will discontinue disposition of such Registrable Securities under the Registration Statement until such Investor is advised in writing by the Company that the use of the Prospectus, or amended or supplemented Prospectus, as applicable, may be resumed. The Company may provide appropriate stop orders to enforce the provisions of this paragraph. Each Investor, severally and not jointly with the other Investors, agrees that the removal of the restrictive legend from certificates representing Registrable Securities is predicated upon the Company’s reliance that the Investor will comply with the provisions of this subsection. Both the Company and the Transfer Agent, and their respective directors, officers, employees and agents, may rely on this subsection. Without the Company’s prior written consent, which consent shall not unreasonably be withheld or delayed, the Investor shall not use any written materials to offer the Registrable Securities for resale other than the Prospectus, including any “free writing prospectus” as defined in Rule 405 under the Securities Act. The Investor covenants that it will not sell any Registrable Securities pursuant to said Prospectus during the period commencing at the time when the Company gives the Investor written notice of the Suspension and ending at the time when the Company gives the Investor written notice that the Investor may thereafter effect sales pursuant to said Prospectus. Notwithstanding the foregoing, the Company agrees that no Suspension shall be for a period of longer than 60 consecutive days, and no Suspension shall be for a period longer than 90 days in the aggregate in any 365-day period.
| B-26 |
(b) At any time that the Investor is an Affiliate of the Company, any resale of the Registrable Securities that purports to be effected under Rule 144 shall comply with all of the requirements of such rule, including the “manner of sale” requirements set forth in Rule 144(f).
5.8Organization; Validity; Enforcements. The Investor further represents and warrants to, and covenants with, the Company that (i) the Investor has full right, power, authority and capacity to enter into this Agreement and to consummate the transactions contemplated hereby and has taken all necessary action to authorize the execution, delivery and performance of this Agreement, (ii) the making and performance of this Agreement by the Investor and the consummation of the transactions herein contemplated will not violate any provision of the organizational documents of the Investor or conflict with, result in the breach or violation of, or constitute, either by itself or upon notice or the passage of time or both, a default under any material agreement, mortgage, deed of trust, lease, franchise, license, indenture, permit or other instrument to which the Investor is a party or, any statute or any authorization, judgment, decree, order, rule or regulation of any court or any regulatory body, administrative agency or other governmental agency or body applicable to the Investor, (iii) no consent, approval, authorization or other order of any court, regulatory body, administrative agency or other governmental agency or body is required on the part of the Investor for the execution and delivery of this Agreement or the consummation of the transactions contemplated by this Agreement, (iv) upon the execution and delivery of this Agreement, this Agreement shall constitute a legal, valid and binding obligation of the Investor, enforceable in accordance with its terms, except as such enforceability may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws of general application relating to or the enforcement of creditor’s rights and the application of equitable principles relating to the availability of remedies, and except as rights to indemnity or contribution, including, but not limited to, the indemnification provisions set forth in Section 8.3 of this Agreement, may be limited by federal or state securities laws or the public policy underlying such laws and (v) there is not in effect any order enjoining or restraining the Investor from entering into or engaging in any of the transactions contemplated by this Agreement.
| B-27 |
5.9Short Sales. Prior to the date hereof, the Investor has not taken, and prior to the public announcement of the transaction after the Closing the Investor shall not take, any action that has caused or will cause the Investor to have, directly or indirectly, sold or agreed to sell any shares of Common Stock, effected any short sale, whether or not against the box, established any “put equivalent position” (as defined in Rule 16a-1(h) under the Exchange Act) with respect to the Common Stock, granted any other right (including, without limitation, any put or call option) with respect to the Common Stock or with respect to any security that includes, relates to or derives any significant part of its value from the Common Stock.
SECTION 6.Survival. Notwithstanding any investigation made by any party to this Agreement, all covenants and agreements made by the Company and the Investor herein and in the certificates for the Shares delivered pursuant hereto shall survive the execution of this Agreement, the delivery to the Investor of the Shares being purchased and the payment therefor.
SECTION 7.Conditions.
7.1Conditions Precedent to the Obligations of theInvestors. The obligation of eachInvestorto purchaseSharesat theClosingis subject to the satisfaction or waiver by suchInvestor,at or before theClosing, of each of the following conditions:
(a)Stockholder Approval.Prior to the Closing, the Company shall have received the TranS1 Stockholder Approval.
(b)Representations and Warranties. The representations and warranties of theCompanycontained herein shall be true and correct in all material respects as of the date when made and as of theClosingas though made on and as of such date, other than with respect to representations and warranties of the Company which are qualified by materiality or by Company Material Adverse Effect, which shall be true and correct in all respects (provided, that any representation made “as of the date hereof” shall be deemed, for purposes of this section, to be made as of theClosing Date).
(c)Performance. The Company shall have performed, satisfied and complied in all material respects with all covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by it at or prior to the Closing, other than with respect to covenants, agreements and conditions of the Company which are qualified by materiality or by Company Material Adverse Effect, which shall be complied with in all respects.
(d)Consents. The Company shall have obtained in a timely fashion any and all consents, permits, approvals, registrations and waivers necessary for consummation of the purchase and sale of the Shares at the Closing, all of which shall be and remain so long as necessary in full force and effect.
(e)No Suspensions of Trading in Common Stock; Listing. Trading in the Common Stock shall not have been suspended by the SEC or any Trading Market (except for any suspensions of trading of not more than one Trading Day solely to permit dissemination of material information regarding the Company) at any time since the date of execution of this Agreement, and the Common Stock shall have been at all times since such date listed for trading on a Trading Market.
| B-28 |
(f)Absence of Litigation. No action, suit or proceeding by or before any court or any governmental body or authority, against the Company or any Subsidiary or pertaining to the transactions contemplated by this Agreement or their consummation, shall have been instituted on or before the Closing Date, which action, suit or proceeding would, if determined adversely, reasonably be expected to have a Company Material Adverse Effect.
(g)Company Deliverables. The Company shall have delivered the Company deliverables in accordance with Section 3.2.
(h)Closing of the Merger. All closing conditions under the Merger Agreement shall have been satisfied or waived (other than satisfaction of those conditions that by their nature are to be satisfied at such closing), including, without limitation, the filing of the Certificate of Merger (as defined in the Merger Agreement) with the Delaware Secretary of State.
7.2Conditions Precedent to the Obligations of theCompany. The obligation of theCompanyto sell theSharesat theClosingis subject to the satisfaction or waiver by theCompany, at or before theClosing, of each of the following conditions:
(a)Stockholder Approval. Prior to theClosing, theCompanyshall have received theTranS1 Stockholder Approval.
(b)Representations and Warranties. The representations and warranties of theInvestorscontained herein shall be true and correct in all material respects as of the date when made and as of theClosing Dateas though made on and as of such date(provided, that any representation made “as of the date hereof” shall be deemed, for purposes of this section, to be made as of theClosing Date).
(c)Performance. The Investors shall have performed, satisfied and complied in all material respects with all covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by the Investors at or prior to the Closing, other than with respect to covenants, agreements and conditions of the Investors which are qualified by materiality, which shall be complied with in all respects.
(d)Investor Deliverables. Each Investor shall have delivered its share of the aggregate purchase price in accordance with Section 3.1.
(e)Closing of the Merger. All closing conditions under the Merger Agreement shall have been satisfied or waived (other than satisfaction of those conditions that by their nature are to be satisfied at such closing).
| B-29 |
SECTION 8.Registration of the Registrable Securities; Compliance with the Securities Act; Indemnification.
8.1Registration Procedures and Expenses.The Company shall:
(a) as soon as practicable, but in no event later than 30 days following the Closing Date, prepare and file with the SEC the Registration Statement on Form S-3(unless theCompanyis not then eligible to register for resale theRegistrable Securitieson Form S-3, in which case such registration shall be on another appropriate form in accordance with theSecurities Actand theExchange Act) relating to the resale of the Registrable Securities by the Investors on a continuous basis pursuant to Rule 415 on NASDAQ or the facilities of any national securities exchange on which the Common Stock is then traded;
(b) use its best efforts, subject to receipt of necessary information from the Investors, to cause the SEC to declare the Registration Statement effective within 90 days or, if the Registration Statement is selected for review by the SEC, 120 days after the Closing Date (as applicable, the “Effective Deadline”);
(c) promptly prepare and file with the SEC such amendments and supplements to the Registration Statement and the Prospectus as may be necessary to keep the Registration Statement effective untilsuch time as theRegistrable Securitiesbecome eligible for resale by each of theInvestorswithout any volume limitations or other restrictions pursuant toRule 144 or any other rule of similar effect; provided that, for the avoidance of doubt, in no event shall the Company have any obligation to keep the Registration Statement effective after such time as all of the Registrable Securities have been sold pursuant to the Registration Statement or Rule 144;
(d) furnish to each Investor with respect to the Registrable Securities registered under the Registration Statement (and to each underwriter, if any, of such Registrable Securities) such number of copies of the Prospectus and such other documents as the Investor may reasonably request, in order to facilitate the public sale or other disposition of all or any of the Registrable Securities held by the Investor;
(e) file documents required of the Company for normal Blue Sky clearance in states specified in writing by each Investor; provided, however, that the Company shall not be required to qualify to do business or consent to service of process in any jurisdiction in which it is not now so qualified or has not so consented;
(f) (i) bear all expenses in connection with the procedures in paragraphs (a) through (e) of this Section 8.1 and the registration of the Registrable Securities pursuant to the Registration Statement and (ii) fees and expenses of one counsel to the Investors in connection with this Agreement and the transactions contemplated hereby, up to a maximum of $30,000;
(g) file a Form D with respect to the Registrable Securities as required under Regulation D of the Securities Act and to provide a copy thereof to each Investor promptly after filing;
| B-30 |
(h) issue a press release describing the transactions contemplated by this Agreement no later than one Business Day following the Closing Date;
(i) in order to enable the Investors to sell the Registrable Securities under Rule 144, for a period of one year from Closing,use its commercially reasonable efforts to comply with the requirements of Rule 144, including without limitation, use its commercially reasonable efforts to comply with the requirements of Rule 144(c)(1) with respect to public information about the Company and to timely file all reports required to be filed by the Company under the Exchange Act; and
(j) not include any securities of the Company in the Registration Statement other than the Registrable Securities.
The Company understands that each Investor disclaims being an underwriter, but any Investor being deemed an underwriter shall not relieve the Company of any obligations it has hereunder. A draft of the proposed form of the questionnaire related to the Registration Statement to be completed by the Investor is attached hereto as part ofExhibit A.
8.2Transfer of Registrable Securities After Registration. Each Investor agrees that it will not effect any disposition of the Registrable Securities or its right to purchase the Shares that would constitute a sale within the meaning of the Securities Act or pursuant to any applicable state securities laws, except as contemplated in the Registration Statement or as otherwise permitted by law, and that it will promptly notify the Company of any changes in the information set forth in the Registration Statement regarding the Investor or its plan of distribution.
8.3Indemnification. For the purpose of this Section 8.3:
(i) the term “Investor Affiliate” shall mean any Affiliate of an Investor, including a transferee who is an Affiliate of an Investor;
(ii) the term “Registration Statement” shall include any preliminary prospectus, final prospectus, “issuer free writing prospectus” as defined in Rule 433(h)(1) of the Securities Act, exhibit, supplement or amendment included in or relating to, and any document incorporated by reference in, the Registration Statement referred to in Section 8.1; and
(iii) the term “Controlling Person” shall mean each person, if any, who controls the Company within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act.
(a) The Company agrees to indemnify and hold harmless each Investor and its Investor Affiliates, against any losses, claims, damages, liabilities or expenses, joint or several, to which the Investor or Investor Affiliates may become subject, under the Securities Act, the Exchange Act, or any other federal or state statutory law or regulation, or at common law or otherwise (including in settlement of any litigation, if such settlement is effected with the prior written consent of the Company), insofar as such losses, claims, damages, liabilities or expenses (or actions in respect thereof as contemplated below) arise out of or are based upon any untrue statement or alleged untrue statement of any material fact contained in the Registration Statement at the time of effectiveness or at the time of any amendment or supplement thereto, or arise out of or are based upon the omission or alleged omission to state in the Registration Statement a material fact required to be stated therein or necessary to make the statements in the Registration Statement not misleading in light of the circumstances under which they were made, or arise out of or are based in whole or in part on any inaccuracy in the representations or warranties of the Company contained in this Agreement, or any failure of the Company to perform its obligations hereunder, and will promptly reimburse each Investor and each Investor Affiliate for any legal and other expenses as such expenses are reasonably incurred by such Investor or such Investor Affiliate in connection with investigating, defending or preparing to defend, settling, compromising or paying any such loss, claim, damage, liability, expense or action; provided, however, that the Company will not be liable for amounts paid in settlement of any such loss, claim, damage, liability or action if such settlement is effected without the prior consent of the Company, which consent shall not be unreasonably withheld, and the Company will not be liable in any such case to the extent that any such loss, claim, damage, liability or expense arises out of or is based upon (i) an untrue statement or alleged untrue statement or omission or alleged omission made in the Registration Statement in reliance upon and in conformity with written information furnished to the Company by or on behalf of the Investor or an Investor Affiliate expressly for use therein, or (ii) the failure of such Investor, or Investor Affiliate who is a transferee of such Investor, to comply with the covenants and agreements contained in Sections 5.6 and 8.2 hereof, or (iii) the inaccuracy of any representation or warranty made by such Investor herein or (iv) any statement or omission in any Prospectus that is corrected in any subsequent Prospectus that was delivered to the Investor prior to the pertinent sale or sales by the Investor.
| B-31 |
(b) Each Investor will severally, but not jointly, indemnify and hold harmless the Company, each of its directors, each of its officers who signed the Registration Statement and each Controlling Person against any losses, claims, damages, liabilities or expenses to which the Company, each of its directors, each of its officers who signed the Registration Statement or Controlling Persons may become subject, under the Securities Act, the Exchange Act, or any other federal or state statutory law or regulation, or at common law or otherwise (including in settlement of any litigation, but only if such settlement is effected with the prior written consent of such Investor) insofar as such losses, claims, damages, liabilities or expenses (or actions in respect thereof as contemplated below) arise out of or are based upon (i) any failure to comply with the covenants and agreements contained in this Agreement or (ii) the inaccuracy of any representation or warranty made by such Investor herein or (iii) any untrue or alleged untrue statement of any material fact contained in the Registration Statement at the time of effectiveness or at the time of any amendment or supplement thereto, or arise out of or are based upon the omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements in the Registration Statement not misleading in the light of the circumstances under which they were made, in each case to the extent, but only to the extent, that such untrue statement or alleged untrue statement or omission or alleged omission was made in the Registration Statement in reliance upon and in conformity with written information furnished to the Company by or on behalf of any Investor or its Investor Affiliate expressly for use therein; and will reimburse the Company, each of its directors, each of its officers who signed the Registration Statement or Controlling Persons for any legal and other expense reasonably incurred by the Company, each of its directors, each of its officers who signed the Registration Statement or Controlling Persons in connection with investigating, defending, settling, compromising or paying any such loss, claim, damage, liability, expense or action; provided, however, that each Investor’s aggregate liability under this Section 8.3 shall not exceed the amount of proceeds received by such Investor or its Investor Affiliate on the sale of the Shares pursuant to the Registration Statement.
| B-32 |
(c) Promptly after receipt by an indemnified party under this Section 8.3 of notice of the threat or commencement of any action, such indemnified party will, if a claim in respect thereof is to be made against an indemnifying party under this Section 8.3, promptly notify the indemnifying party in writing thereof, but the omission to notify the indemnifying party will not relieve it from any liability that it may have to any indemnified party for contribution or otherwise under the indemnity agreement contained in this Section 8.3 to the extent it is not prejudiced as a result of such failure. In case any such action is brought against any indemnified party and such indemnified party seeks or intends to seek indemnity from an indemnifying party, the indemnifying party will be entitled to participate in, and, to the extent that it may wish, jointly with all other indemnifying parties similarly notified, to assume the defense thereof with counsel reasonably satisfactory to such indemnified party; provided, however, if the defendants in any such action include both the indemnified party and the indemnifying party, and the indemnified party shall have reasonably concluded that there may be a conflict of interest between the positions of the indemnifying party and the indemnified party in conducting the defense of any such action or that there may be legal defenses available to it and/or other indemnified parties that are different from or additional to those available to the indemnifying party, the indemnified party or parties shall have the right to select separate counsel to assume such legal defenses and to otherwise participate in the defense of such action on behalf of such indemnified party or parties. Upon receipt of notice from the indemnifying party to such indemnified party of its election to assume the defense of such action and approval by the indemnified party of counsel, the indemnifying party will not be liable to such indemnified party under this Section 8.3 for any legal or other expenses subsequently incurred by such indemnified party in connection with the defense thereof unless (i) the indemnified party shall have employed such counsel in connection with the assumption of legal defenses in accordance with the proviso to the preceding sentence or (ii) the indemnifying party shall not have employed counsel reasonably satisfactory to the indemnified party to represent the indemnified party within a reasonable time after notice of commencement of action, in each of which cases the reasonable fees and expenses of counsel shall be at the expense of the indemnifying party. The indemnifying party shall not be liable for any settlement of any action without its prior written consent. In no event shall any indemnifying party be liable in respect of any amounts paid in settlement of any action unless the indemnifying party shall have approved in writing the terms of such settlement; provided that such consent shall not be unreasonably withheld. No indemnifying party shall, without the prior written consent of the indemnified party, effect any settlement of any pending or threatened proceeding in respect of which any indemnified party is or could have been a party and indemnification could have been sought hereunder by such indemnified party from all liability on claims that are the subject matter of such proceeding.
| B-33 |
(d) If the indemnification provided for in this Section 8.3 is required by its terms but is for any reason held to be unavailable to or otherwise insufficient to hold harmless an indemnified party under paragraphs (a), (b) or (c) of this Section 8.3 in respect to any losses, claims, damages, liabilities or expenses referred to herein, then each applicable indemnifying party shall contribute to the amount paid or payable by such indemnified party as a result of any losses, claims, damages, liabilities or expenses referred to herein (i) in such proportion as is appropriate to reflect the relative benefits received by the Company and each Investor from the private placement of Common Stock hereunder or (ii) if the allocation provided by clause (i) above is not permitted by applicable law, in such proportion as is appropriate to reflect not only the relative benefits referred to in clause (i) above but the relative fault of the Company and each Investor in connection with the statements or omissions or inaccuracies in the representations and warranties in this Agreement and/or the Registration Statement that resulted in such losses, claims, damages, liabilities or expenses, as well as any other relevant equitable considerations. The relative fault of the Company on the one hand and each Investor on the other shall be determined by reference to, among other things, whether the untrue or alleged statement of a material fact or the omission or alleged omission to state a material fact or the inaccurate or the alleged inaccurate representation and/or warranty relates to information supplied by the Company or by such Investor and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent such statement or omission. The amount paid or payable by a party as a result of the losses, claims, damages, liabilities and expenses referred to above shall be deemed to include, subject to the limitations set forth in paragraph (c) of this Section 8.3, any legal or other fees or expenses reasonably incurred by such party in connection with investigating or defending any action or claim. The provisions set forth in paragraph (c) of this Section 8.3 with respect to the notice of the threat or commencement of any threat or action shall apply if a claim for contribution is to be made under this paragraph (d); provided, however, that no additional notice shall be required with respect to any threat or action for which notice has been given under paragraph (c) for purposes of indemnification. The Company and each Investor agree that it would not be just and equitable if contribution pursuant to this Section 8.3 were determined solely by pro rata allocation (even if the Investors were treated as one entity for such purpose) or by any other method of allocation which does not take account of the equitable considerations referred to in this paragraph. Notwithstanding the provisions of this Section 8.3, no Investor shall be required to contribute any amount in excess of the amount by which (x) the difference between the amount such Investor paid for its Shares that were sold pursuant to the Registration Statement and the net amount received by such Investor from such sale exceeds (y) the amount of any damages that such Investor has otherwise been required to pay by reason of such untrue or alleged untrue statement or omission or alleged omission. No person guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution from any person who was not guilty of such fraudulent misrepresentation. The Investors’ obligations to contribute pursuant to this Section 8.3 are several and not joint.
8.4Termination of Conditions and Obligations. The restrictions imposed by Section 8.2 upon the transferability of the Registrable Securities shall cease and terminate as to any particular number of the Registrable Securities upon the earlier of (i) the passage of two years from the effective date of the Registration Statement covering such Registrable Securities and (ii) such time as an opinion of counsel satisfactory in form and substance to the Company shall have been rendered to the effect that such conditions are not necessary in order to comply with the Securities Act.
| B-34 |
8.5Information Available. The Company, upon the reasonable request of an Investor, shall make available for inspection by such Investor, any underwriter participating in any disposition pursuant to the Registration Statement and any attorney, accountant or other agent retained by the Investor or any such underwriter, all financial and other records, pertinent corporate documents and properties of the Company, and cause the Company’s officers, employees and independent accountants to supply all information reasonably requested by the Investor or any such underwriter, attorney, accountant or agent in connection with the Registration Statement.
8.6Delay in Effectiveness of Registration Statement. If the Registration Statement is not declared effective by the SEC by the Effective Deadline, then for each day following the Effective Deadline, until but excluding the date the SEC declares the Registration Statement effective, the Company shall, for each such day, pay each Investor with respect to any such failure, as liquidated damages and not as a penalty, an amount per 30-day period equal to 1.0% of the total of (x) the per-share purchase price paid by such Investor for its Shares pursuant to this Agreementmultiplied by the number of Shares then held by such Investor plus (y) the number of Merger Shares then held by such Investormultiplied by the Merger Closing Price; and for any such 30-day period, such payment shall be made no later than three Business Days following such 30-day period. If the Investor shall be prohibited from selling Registrable Securities under the Registration Statement as a result of a Suspension of more than 60 days or Suspensions on more than two occasions of more than 90 days each in any 12-month period, then for each day on which a Suspension is in effect that exceeds the maximum allowed period for a Suspension or Suspensions, but not including any day on which a Suspension is lifted, the Company shall pay each Investor, as liquidated damages and not as a penalty, an amount per 30-day period equal to 1.0% of the total of (x) the per-share purchase price paid by such Investor for its Shares pursuant to this Agreementmultiplied by the number of Shares then held by such Investor plus (y) the number of Merger Shares then held by such Investormultiplied by the Merger Closing Price, and such payment shall be made no later than the first Business Day of the calendar month next succeeding the month in which such day occurs. For purposes of this Section 8.6, a Suspension shall be deemed lifted on the date that notice that the Suspension has been lifted is delivered to the Investor pursuant to Section 5.7 of this Agreement. Any payments made pursuant to this Section 8.6 shall not constitute the Investor’s exclusive remedy for such events. Notwithstanding the foregoing provisions, in no event shall the Company be obligated to pay any liquidated damages pursuant to this Section 8.6 (i) to more than one Investor in respect of the same Registrable Securities for the same period of time or (ii) to any Investor in an aggregate amount that exceeds 12% of the sum of the purchase price paid by such Investor for the Shares pursuant to this Agreement plus the number of Merger Shares acquired in the Merger by such Investormultiplied by the Merger Closing Price. Such payments shall be made to the Investors in cash.
SECTION 9.Broker’s or Finder’s Fee. Each party represents that it neither is nor will be obligated for any finder’s or broker’s fee or commission in connection with this transaction. Each Investor agrees to indemnify and to hold harmless the Company from any liability for any commission or compensation in the nature of a finder’s or broker’s fee (and the costs and expenses of defending against such liability or asserted liability) for which such Investor or any of its officers, partners, employees or representatives is responsible. The Company agrees to indemnify and hold harmless each Investor from any liability for any commission or compensation in the nature of a finder’s or broker’s fee (and the costs and expenses of defending against such liability or asserted liability) for which the Company or any of its officers, employees or representatives is responsible.
| B-35 |
SECTION 10.Independent Nature of Investors’ Obligations and Rights. The obligations of each Investor under this Agreement are several and not joint with the obligations of any other Investor, and no Investor shall be responsible in any way for the performance of the obligations of any other Investor under the Agreement. The decision of each Investor to purchase the Shares pursuant to this Agreement has been made by such Investor independently of any other Investor. Nothing contained in this Agreement, and no action taken by any Investor pursuant thereto, shall be deemed to constitute the Investors as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Investors are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by this Agreement. Each Investor acknowledges that no other Investor has acted as agent for such Investor in connection with making its investment hereunder and that no other Investor will be acting as agent of such Investor in connection with monitoring its investment in the Shares or enforcing its rights under this Agreement. Each Investor shall be entitled to independently protect and enforce its rights, including without limitation the rights arising out of this Agreement, and it shall not be necessary for any other Investor to be joined as an additional party in any proceeding for such purpose.
SECTION 11.Notices. All notices, requests, consents and other communications hereunder shall be in writing, shall be mailed by first-class registered or certified airmail, e-mail, confirmed facsimile or nationally recognized overnight express courier postage prepaid, and shall be deemed given when so mailed and shall be delivered as addressed as follows:
(a) if to the Company, to:
TranS1 Inc. 110 Horizon Drive, Suite 230 Raleigh, NC 27615 Attn: Chief Financial Officer Email: joe.slattery@trans1.com Telephone: (919) 825-0868
|
| with copies (which shall not constitute notice) to: |
| B-36 |
Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P. Suite 2300 Raleigh, NC 27601 Attn: David B. Clement Email: dclement@smithlaw.com Telephone: (919) 821-6754 |
or to such other person at such other place as the Company shall designate to the Investor in writing; and
(b) if to the Investor, at its address as set forth at the end of this Agreement, or at such other address or addresses as may have been furnished to the Company in writing.
SECTION 12.Amendments; Waivers. No provision of thisAgreementmay be waived or amended except in a written instrument signed, in the case of an amendment, by theCompanyand each of theInvestorsor, in the case of a waiver, by the party against whom enforcement of any such waiver is sought. No waiver of any default with respect to any provision, condition or requirement of thisAgreementshall be deemed to be a continuing waiver in the future or a waiver of any subsequent default or a waiver of any other provision, condition or requirement hereof, nor shall any delay or omission of any party to exercise any right hereunder in any manner impair the exercise of any such right. Notwithstanding the foregoing, a waiver or consent to depart from the provisions hereof with respect to a matter that relates exclusively to the rights ofInvestorsunder Section8.1may be given byInvestorsholding at least a majority of theRegistrable Securitiesto which such waiver or consent relates.
SECTION 13.Headings. The headings of the various sections of this Agreement have been inserted for convenience of reference only and shall not be deemed to be part of this Agreement.
SECTION 14.Severability. In case any provision contained in this Agreement should be invalid, illegal or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions contained herein shall not in any way be affected or impaired thereby.
SECTION 15.Governing Law. All matters arising out of or relating to this Agreement and the transactions contemplated hereby (including without limitation its interpretation, construction, performance and enforcement) shall be governed by and construed in accordance with the internal laws of the State of Delaware without giving effect to any choice or conflict of law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of laws of any jurisdictions other than those of the State of Delaware.
SECTION 16.Submission to Jurisdiction. Each of the parties to this Agreement (a) consents to submit itself to the exclusive personal jurisdiction of any state or federal court sitting in the State of Delaware in any action or proceeding arising out of or relating to this Agreement or any of the transactions contemplated by this Agreement, (b) agrees that all claims in respect of such action or proceeding may be heard and determined in any such court, (c) agrees that it shall not attempt to deny or defeat such personal jurisdiction by motion or other request for leave from any such court and (d) agrees not to bring any action or proceeding arising out of or relating to this Agreement or any of the transactions contemplated by this Agreement in any other court. Each of the parties hereto waives any defense of inconvenient forum to the maintenance of any action or proceeding so brought and waives any bond, surety or other security that might be required of any other party with respect thereto. Any party may make service on another party by sending or delivering a copy of the process to the party to be served at the address and in the manner provided for the giving of notices in Section 11. Nothing in this Section 16, however, shall affect the right of any party to serve legal process in any other manner permitted by law.
| B-37 |
SECTION 17.WAIVER OF JURY TRIAL. EACH OF THE COMPANY AND EACH INVESTOR IRREVOCABLY WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY ACTION, PROCEEDING OR COUNTERCLAIM (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THE ACTIONS OF THE COMPANY OR ANY INVESTOR IN THE NEGOTIATION, ADMINISTRATION, PERFORMANCE AND ENFORCEMENT OF THIS AGREEMENT.
SECTION 18.Counterparts. This Agreement may be executed in counterparts, each of which shall constitute an original, but all of which, when taken together, shall constitute but one instrument, and shall become effective when one or more counterparts have been signed by each party hereto and delivered to the other parties. Facsimile signatures shall be deemed original signatures
SECTION 19.Subsequent Joinder of Investors Holding Only Merger Shares. From time to time following the date hereof, a holder of Merger Shares who is not also purchasing Shares under Section 2 hereof and who was not a party to this Agreement on the date hereof may execute a signature page, and upon delivery of the same to the Company shall be deemed to be an Investor and bound by and subject to Sections 5.6, 5.7, 5.9, 8.1, 8.2, 8.3(a), 8.3(c), 8.3(d), 8.4, 8.6, and 10-23 of this Agreement with the same force and effect as if such Investor were originally a party hereto.
SECTION 20.Entire Agreement. This Agreement and the instruments referenced herein contain the entire understanding of the parties with respect to the matters covered herein and therein and, except as specifically set forth herein or therein, neither the Company nor any Investor makes any representation, warranty, covenant or undertaking with respect to such matters. Each party expressly represents and warrants that it is not relying on any oral or written representations, warranties, covenants or agreements outside of this Agreement.
SECTION 21.Fees and Expenses. Except as set forth herein, each of the Company and the Investor shall pay its respective fees and expenses related to the transactions contemplated by this Agreement.
SECTION 22.Parties. This Agreement is made solely for the benefit of and is binding upon the Investors and the Company and, to the extent provided in Section 8.3, any Controlling Person of the Company or Investor Affiliate, the officers and directors of the Company, and their respective executors, administrators, successors and assigns and subject to the provisions of Section 8.3, no other person shall acquire or have any right under or by virtue of this Agreement.
| B-38 |
SECTION 23.Further Assurances. Each party agrees to cooperate fully with the other parties and to execute such further instruments, documents and agreements and to give such further written assurance as may be reasonably requested by any other party to evidence and reflect the transactions described herein and contemplated hereby and to carry into effect the intents and purposes of this Agreement.
[Remainder of Page Left Intentionally Blank]
| B-39 |
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their duly authorized representatives as of the day and year first above written.
| TRANS1 INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
[Signature Page to Securities Purchase Agreement]
| B-40 |
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their duly authorized representatives as of the day and year first above written.
The undersigned, with respect to all Sections of this Agreement:
Print or Type:
| Number of Shares Purchased by Investor | Name of Investor (Individual or Institution) | |
Jurisdiction of Investor’s Executive Offices
| ||
Name of Individual representing
| ||
| Title of Individual representing Investor (if an Institution) |
Signature by:
| Individual Investor or Individual representing Investor: |
| _________________________________ |
| Address: ___________________________ |
| Telephone: ___________________________ |
| Facsimile: ___________________________ |
| E-mail: ___________________________ |
[Signature Page to Securities Purchase Agreement]
| B-41 |
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their duly authorized representatives as of the day and year first above written.
The undersigned, as a holder of Merger Shares only, solely with respect to Sections 5.6, 5.7, 5.9, 8.1, 8.2, 8.3(a), 8.3(c), 8.3(d), 8.4, 8.6, and 10-23 of the Agreement:
Print or Type:
| Number of Shares Held by Investor | Name of Investor (Individual or Institution) | |
Jurisdiction of Investor’s Executive Offices
| ||
Name of Individual representing
| ||
| Title of Individual representing Investor (if an Institution) |
Signature by:
| Individual Investor or Individual representing Investor: |
| _________________________________ |
| Address: ___________________________ |
| Telephone: ___________________________ |
| Facsimile: ___________________________ |
| E-mail: ___________________________ |
[Signature Page to Securities Purchase Agreement –Merger Shares Only]
| B-42 |
EXHIBIT A
INSTRUCTION SHEET FOR INVESTOR
(to be read in conjunction with the entire Securities Purchase Agreement)
| A. | Complete the following items in the Securities Purchase Agreement: |
| 1. | Complete and execute theInvestor Signature Page. TheAgreementmust be executed by an individual authorized to bind theInvestor. |
| 2. | Exhibit A-1 - Stock Certificate Questionnaire: |
Provide the information requested by the Stock Certificate Questionnaire;
| 3. | Exhibit A-2 - Registration Statement Questionnaire: |
Provide the information requested by the Registration Statement Questionnaire.
| 4. | Exhibit A-3 - Investor Certificate: |
Provide the information requested by the Investor Certificate.
| 5. | Return, via e-mail, the signed Securities Purchase Agreement including the properly completed Exhibits A-1 through A-3, to: |
Name:David B. Clement
E-mail:dclement@smithlaw.com
Telephone: (919) 821-6754
| 6. | After completing instruction number five (5) above, deliver the original signed Securities Purchase Agreement including the properly completed Exhibits A-1 through A-3 to: |
Address:Smith, Anderson, Blount, Dorsett,
Mitchell & Jernigan, L.L.P.
150 Fayetteville Street
Suite 2300
Raleigh, NC 27601
Attn: David B. Clement
| B. | Instructions regarding the wire transfer of funds for the purchase of the Shares will be provided to the Investor by the Company at a later date. |
| B-43 |
EXHIBITA-1
TRANS1 INC.
STOCK CERTIFICATE QUESTIONNAIRE
| Please provide us with the following information: | ||
| 1. | The exact name that the Shares are to be registered in (this is the name that will appear on the stock certificate(s)). You may use a nominee name if appropriate: | |
| 2. | The relationship between the Investor of the Shares and the registered holder listed in response to item 1 above: | |
| 3. | The mailing address, telephone number and e-mail address of the registered holder listed in response to item 1 above: | |
| 4. | The tax identification number of the registered holder listed in response to item 1 above: |
| B-44 |
EXHIBITA-2
TRANS1 INC.
REGISTRATION STATEMENT QUESTIONNAIRE
In connection with theRegistration Statement, please provide us with the following information regarding theInvestor.
| 1. | Please state your organization’s name exactly as it should appear in theRegistration Statement: |
Except as set forth below, your organization does not hold any equity securities of theCompanyon behalf of another person or entity.
State any exceptions here:
If the Investor is not a natural person, please identify the natural person or persons who will have voting and investment control over the Registrable Securities owned by the Investor:
| 2. | Address of your organization: |
______________________________________________________
______________________________________________________
Telephone:______________________________
E-mail:_________________________________
Contact Person:__________________________
| B-45 |
3. Have you or your organization had any position, office or other material relationship within the past three years with theCompanyor its affiliates? (Include any relationships involving you or any of your affiliates, officers, directors, or principal equity holders (5% or more) that has held any position or office or has had any other material relationship with theCompany(or its predecessors or affiliates) during the past three years.)
_______Yes _______No
If yes, please indicate the nature of any such relationship below:
4. Are you the beneficial owner of any other securities of theCompany? (Include any equity securities that you beneficially own or have a right to acquire within sixty (60) days after the date hereof, and as to which you have sole voting power, shared voting power, sole investment power or shared investment power.)
_______Yes _______No
If yes, please describe the nature and amount of such ownership as of a recent date.
5. Except as set forth below, you wish that all of your beneficially owned shares of theCompany’s common stock acquired pursuant to the Securities Purchase Agreement and theMerger Agreementbe offered for your account in theRegistration Statement. (Please note that any shares of theCompany’s common stock not acquired pursuant to the Securities Purchase Agreement and theMerger Agreementwill not be offered for your account in theRegistration Statement.)
State any exceptions here:
| B-46 |
6. Have you made or are you aware of any arrangements relating to the distribution of the shares of theCompanypursuant to theRegistration Statement?
_______Yes _______No
If yes, please describe the nature and amount of such arrangements.
7. FINRA Matters
(a)State below whether(i)you or anyassociate oraffiliate of yours are amember of FINRA, acontrolling shareholder of a FINRAmember, aperson associated with a member, a direct or indirectaffiliate of amember, or anunderwriter or related person with respect to the proposed offering;(ii)you or anyassociate oraffiliate of yours owns any stock or other securities of any FINRAmember not purchased in the open market; or(iii)you or anyassociate oraffiliate of yours has made any outstanding subordinated loans to any FINRAmember. If you are a general or limited partnership, a “no” answer asserts that no such relationship exists for you as well as for each of your general or limited partners. Italicized terms are defined below.
| Yes: __________ | No: __________ |
If “yes,” please identify the FINRAmember and describe your relationship, including, in the case of a general or limited partner, the name of the partner:
If you answer “no” toQuestion7(a), you need not respond toQuestion7(b).
(b) State below whether you or anyassociate oraffiliate of yours has been an underwriter, or acontrolling person ormember of any investment banking or brokerage firm which has been or might be an underwriter for securities of theCompanyor anyaffiliate thereof including, but not limited to, the common stock now being registered.
| Yes: __________ | No: __________ |
| B-47 |
If “yes,” please identify the FINRAmember and describe your relationship, including, in the case of a general or limited partner, the name of the partner.
For purposes of this Question 7:
Anaffiliate of any person (including a sole proprietorship, partnership, limited liability company, corporation or other legal entity such as a trust or estate) is a person thatcontrols, iscontrolled by or is under commoncontrol with such person. Officers, directors, partners, sole proprietors and branch managers, or persons of a similar status or performing similar functions, of a person should be presumed to be anaffiliate of such person.
Anassociated person of a member orperson associated with a member includes, among others, (1) a natural person who is registered or has applied for registration under the rules of FINRA and (2) a sole proprietor, partner, officer, director, or branch manager of amember, or other natural person occupying a similar status or performing similar functions, or a natural person engaged in the investment banking or securities business who is directly or indirectlycontrolling orcontrolled by amember, whether or not any such person is registered or exempt from registration with FINRA under FINRA’s By-Laws or the FINRA Rules.
The termcontrol means the following:
| (i) | beneficial ownership of 10% or more of the outstanding common equity of any entity, including any right to receive such securities within 60 days of themember’sparticipation in the public offering; |
| (ii) | the right to 10% or more of the distributable profits or losses of an entity that is a partnership, including any right to receive an interest in such distributable profits or losses within 60 days of themember’s participation in the public offering; |
| (iii) | beneficial ownership of 10% or more of the outstanding subordinated debt of an entity, including any right to receive such subordinated debt within 60 days of themember’s participation in the public offering; |
| (iv) | beneficial ownership of 10% or more of the outstanding preferred equity of an entity, including any right to receive such preferred equity within 60 days of themember’s participation in the public offering; or |
| (v) | the power to direct or cause the direction of the management or policies of an entity. |
The termimmediate family means the parents, mother-in-law, father-in-law, spouse, brother or sister, brother-in-law or sister-in-law, son-in-law or daughter-in-law, and children of an employee orassociated personof amember, except any person other than the spouse and children who does not live in the same household as, have a business relationship with, provide material support to, or receive material support from, the employee orassociated personof a member. In addition,immediate family includes any other person who either lives in the same household as, provides material support to, or receives material support from, an employee orassociated personof amember.
| B-48 |
The termmember means any broker or dealer admitted to membership in FINRA.
The termparticipating member means any FINRAmember that is participating in a public offering, anyassociated personof themember, any members of theirimmediate family and anyaffiliate of themember.
The termunderwriter or related person includes, with respect to the proposed offering, any underwriters and such underwriters’ counsel, financial consultants and advisors, finders,participating members, and any other persons related to anyparticipating member.
| B-49 |
ACKNOWLEDGEMENT
The undersigned hereby agrees to notify the Company promptly of any changes in the foregoing information which should be made as a result of any developments, including the passage of time. The undersigned also agrees to provide the Company and the Company’s counsel any and all such further information regarding the undersigned promptly upon request in connection with the preparation, filing, amending, and supplementing of the Registration Statement (or any prospectus contained therein). The undersigned hereby consents to the use of all such information in the Registration Statement.
The undersigned understands and acknowledges that the Company will rely on the information set forth herein for purposes of the preparation and filing of the Registration Statement.
The undersigned understands that the undersigned may be subject to serious civil and criminal liabilities if the Registration Statement, when it becomes effective, either contains an untrue statement of a material fact or omits to state a material fact required to be stated in the Registration Statement or necessary to make the statements in the Registration Statement not misleading. The undersigned represents and warrants that all information it provides to the Company and its counsel is currently accurate and complete and will be accurate and complete at the time the Registration Statement becomes effective and at all times subsequent thereto, and agrees, during the effectiveness period and any additional period in which the undersigned is making sales of Registrable Securities under and pursuant to the Registration Statement, to notify the Company immediately of any misstatement of a material fact in the Registration Statement or the omission of any material fact necessary to make the statements contained therein not misleading.
Dated: __________
______________________________
Name
______________________________
Signature
______________________________
Name and Title of Signatory
| B-50 |
EXHIBITA-3
TRANS1 INC.
CERTIFICATE FOR CORPORATE, PARTNERSHIP, LIMITED LIABILITY COMPANY,
TRUST, FOUNDATION AND JOINT INVESTORS
If theInvestoris a corporation, partnership, limited liability company, trust, pension plan, foundation, jointInvestor(other than a married couple) or other entity, an authorized officer, partner, or trustee must complete, date and sign this Certificate.
CERTIFICATE
The undersigned certifies that the representations and responses below are true and accurate:
(a)TheInvestorhas been duly formed and is validly existing and has full power and authority to invest in theCompany. The person signing on behalf of the undersigned has the authority to execute and deliver the Securities Purchase Agreement on behalf of theInvestorand to take other actions with respect thereto.
(b)Indicate the form of entity of the undersigned:
____Limited Partnership
____General Partnership
____Limited Liability Company
____Corporation
____ Revocable Trust(identify each grantor and indicate under what circumstances the trust is revocable by the grantor):
(Continue on a separate piece of paper, if necessary.)
____ Othertype of Trust (indicate type of trust and, for trusts other than pension trusts, name the grantors and beneficiaries):
(Continue on a separate piece of paper, if necessary.)
____ Otherform of organization (indicate form of organization
(______________________________________________________________________________________________________).
(c)Indicate the approximate date the undersigned entity was formed:___________________________________________________.
| B-51 |
(d)In order for theCompanyto offer and sell theRegistrable Securitiesin conformance with state and federal securities laws, the following information must be obtained regarding your investor status. Pleaseinitial each category applicable to you as anInvestorin theCompany.
___1. A bank as defined in Section 3(a)(2) of theSecurities Act, or any savings and loan association or other institution as defined in Section 3(a)(5)(A) of the Securities Act whether acting in its individual or fiduciary capacity;
___2. A broker or dealer registered pursuant to Section 15 of the Securities Exchange Act of 1934;
___3. An insurance company as defined in Section 2(13) of theSecurities Act;
___4. An investment company registered under the Investment CompanyActof 1940 or a business development company as defined in Section 2(a)(48) of thatAct;
___5. ASmall Business Investment Companylicensed by theU.S. Small Business Administrationunder Section 301(c) or (d) of theSmall Business Investment Act of 1958;
___6. A plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions, for the benefit of its employees, if such plan has total assets in excess of $5,000,000;
___7. An employee benefit plan within the meaning of theEmployee Retirement Income Security Act of 1974, if the investment decision is made by a plan fiduciary, as defined in Section 3(21) of suchAct, which is either a bank, savings and loan association, insurance company, or registered investment advisor, or if the employee benefit plan has total assets in excess of $5,000,000 or, if a self-directed plan, with investment decisions made solely by persons that qualify under any of investor categories 1 through 11;
___8. A private business development company as defined in Section 202(a)(22) of theInvestment Advisers Act of 1940;
___9. Any partnership or corporation or any organization described in Section 501(c)(3) of theInternal Revenue Codeor similar business trust, not formed for the specific purpose of acquiring theShares, with total assets in excess of $5,000,000;
___10. A trust, with total assets in excess of $5,000,000, not formed for the specific purpose of acquiring theShares, whose purchase is directed by a sophisticated person as described in Rule 506(b)(2)(ii) of theSecurities Act;
| B-52 |
___11. An entity in which all of the equity owners qualify under any of the above subparagraphs. If the undersigned belongs to this investor category only, list the equity owners of the undersigned, and the investor category which each such equity owner satisfies:
(Continue on a separate piece of paper, if necessary.)
Please set forth in the space provided below the(i) states, if any, in theU.S.in which you maintained your principal office during the past two years and the dates during which you maintained your office in each state,(ii) state(s), if any, in which you are incorporated or otherwise organized and(iii) state(s), if any, in which you pay income taxes.
Dated:__________________________, 2013
____________________________________
Print Name ofInvestor
____________________________________
Name:
Title:
(Signature and title of authorized officer, partner or trustee)
| B-53 |
EXHIBIT B
OPINION OF COMPANY COUNSEL
1. The Company is a corporation duly incorporated, validly existing and in good standing under the laws of the State of Delaware, and has the corporate power and authority to conduct its business as presently conducted. The Company is duly qualified to do business as a foreign corporation in the State of North Carolina.
2. The Company has the corporate power and authority to execute and deliver, and to perform its obligations under, the Agreement.
3. The Agreement has been duly authorized, executed and delivered by the Company. The Agreement constitutes the valid and binding obligation of the Company, enforceable against the Company in accordance with its terms.
4. The Registrable Securities have been duly authorized, and upon payment and delivery in accordance with the Agreement or Merger Agreement, as applicable, and upon either (i) the countersigning of the certificates representing the Registrable Securities by a duly authorized signatory of the registrar for the Registrable Securities, or (ii) the book entry of the Registrable Securities by the transfer agent for the Registrable Securities, will be validly issued, and fully paid and nonassessable.
5. No person is entitled to any pre-emptive right or right of first refusal with respect to the issuance of the Registrable Securities pursuant to (i) the terms of the Certificate of Incorporation or Bylaws (ii) the provisions of the DGCL or theNorth Carolina Business Corporation Act or (iii) any agreement, contract or other arrangement identified, pursuant to Item 601(b)(10) of Regulation S-K, as a material agreement of the Company in the Exhibit Index of the Company’s Annual Report on Form 10-K for the year ended December 31, 2012 filed by the Company with the Securities and Exchange Commission (each a “Material Agreement”).
6. The execution, delivery and performance of the Agreement by the Company will not violate or result in a breach of any of the terms of or constitute a default under any Material Agreement.
7. The execution, delivery and performance of the Agreement by the Company (i) is not in violation of the Certificate of Incorporation or Bylaws, (ii) does notviolate the DGCL, the North Carolina Business Corporation Act, or any federal law of the United States and (iii)does notviolate anyjudgment, injunction, order or decree disclosed in the Company’s Annual Report on Form 10-K for the year ended December 31, 2011 filed by the Company with the Securities and Exchange Commission.
8. No authorization, approval or consent of any court or governmental authority or agency of the United States, the State of Delaware (pursuant to the DGCL) or the State of North Carolina is required in connection with the transactions contemplated by the Agreement, except such as may be required under federal and state securities or blue sky laws in connection with the offer and sale of the Registrable Securities by the Company.
| B-54 |
9. Assuming the accuracy of the representations and warranties of the Investors and the Company set forth in the Agreement (including the questionnaires attached to the Agreement and completed by each of the Investors) and compliance by the Investors and the Company with their respective obligations, and subject to the timely filing by the Company of a Form D pursuant to Regulation D promulgated under the Securities Act of 1933, as amended (the “Act”), the offer, sale and delivery of the Shares, in the manner contemplated by the Agreement, are not required to be registered under the Act, it being understood that no opinion is expressed as to when or under what circumstances any Shares may be reoffered or resold.
10. The Company is not an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
| B-55 |
Annex C
March 1, 2013
PROPRIETARY AND CONFIDENTIAL
Board of Directors
TranS1 Inc.
301 Government Center Dr.
Wilmington, NC 28403
Members of the Board:
Stifel, Nicolaus & Company, Incorporated (“Stifel” or “we”) understands that TranS1 Inc., a Delaware corporation (the “Company”) proposes to enter into an Agreement and Plan of Merger (the “Agreement”) with Baxano Acquisition Corp., a wholly owned subsidiary of the Company and a Delaware corporation (“Merger Sub”), and Baxano, Inc., a Delaware corporation (the “Target”), pursuant to which Merger Sub will be merged with and into the Target (the “Merger”) with the Target surviving the Merger and becoming a wholly owned subsidiary of the Company. In the Merger, as more fully described in the Agreement, the Company will pay anumber of shares of newly issued common stock of the Company (the “Merger Consideration Shares”) as the purchase price for the Target, which shares will amount to 28.0% of the pro forma outstanding shares of the combined company. In addition, the Company will assume or repay the Target’s indebtedness in an amount up to $3,000,000 of principal plus accrued interest outstanding under the Oxford/SVB Loan Documents (the “Assumed Debt,” collectively with the Merger Consideration Shares, the “Aggregate Consideration”).Capitalized terms used but not defined herein shall have the meanings set forth in the Agreement.
You have requested Stifel’s opinion, as financial advisor, as to the fairness, from a financial point of view, as of the date hereof, to the Company of the Aggregate Consideration to be paid by the Company in the Merger pursuant to the Agreement (the “Opinion”).
In connection with rendering our Opinion, we have, among other things:
| i) | reviewed the financial terms contained in the draft of the Agreement dated February 26, 2013 which is the most recent draft made available to Stifel; |
| ii) | reviewed and analyzed certain internal financial projections concerning the Target on a standalone basis, prepared by the Company’s management (the “Target Projections”); |
| iii) | reviewed and analyzed certain internal financial projections concerning the operations of the Company and the Target under the Company’s management following the Merger, including the amounts and timing of benefits of scale, operating efficiencies and cost savings expected to result from the Merger (the “Expected Synergies”), prepared by the Company’s management; |
| iv) | discussed with certain members of the management of the Company the current status and prospects of the Target, the Expected Synergies and such other matters Stifel deemed relevant; |
| v) | reviewed and analyzed certain financial terms of the Merger as compared to certain financial information of select publicly traded companies Stifel deemed relevant; |
| vi) | reviewed and analyzed certain financial terms of the Merger as compared to the financial terms of select business combinations Stifel deemed relevant; |
| vii) | reviewed and analyzed, based on the Target Projections, the cash flows generated by the Target on a standalone basis to determine the present value of its discounted cash flows; |
| viii) | reviewed and analyzed, based on the Company and the Target projections, the cash flows generated by the pro forma company as contemplated to be managed by the Company following the Merger to determine the present value of its discounted cash flows; and |
| ix) | reviewed and analyzed such other information and financial studies, performed such other analyses and investigations and considered such other factors that Stifel deemed relevant for the purposes of its Opinion. In addition, Stifel took into account its assessment of general economic, market and financial conditions and its experience in other transactions, as well as its experience in securities valuations and its general knowledge of the industries in which the Company and the Target operates. |
| C-1 |
In conducting our review and rendering our Opinion, we have, with your consent, relied upon and assumed, without independent verification, the accuracy and completeness of all of the financial and other information that was made available, supplied, or otherwise communicated to Stifel by or on behalf of the Company or the Target, or that was otherwise reviewed by Stifel, including, without limitation, publicly available information, and have not assumed any responsibility for independently verifying any of such information.We will not in any respect be responsible for the accuracy or completeness of any of the foregoing kinds of information.We have further relied upon the assurances of the management of the Company that they are unaware of any facts that would make such information incomplete or misleading. To the extent such information includes estimates, forecasts or projections of future financial performance, prepared by or reviewed with management of the Company (including, without limitation, the Target Projections, the Expected Synergies and estimates and projections relating to possible future debt or equity financings by the Company on both a stand-alone basis and on a pro forma combined basis assuming acquisition of the Target, in each case for the periods reviewed for Stifel’s discounted cash flow analysis) or obtained from public sources, Stifel has relied upon the statements of the Company’s management that such estimates, forecasts and projections (and the assumptions and bases therein) are reasonable and achievable and, with the consent of the Company’s management, has assumed that such estimates, forecasts and projections represent the best available estimates, forecasts and projections and have been prepared in good faith on assumptions which, in light of the circumstances under which they were made, are reasonable, as has been represented and warranted by the Company or, with respect to estimates, forecasts and projections obtained from public sources, represent reasonable estimates, forecasts and projections and that such estimates, forecasts and projections provided a reasonable basis upon which Stifel could form its Opinion. Without limiting the generality of the foregoing, with respect to the Target we have, at the direction of Company management, so relied upon and made such assumptions with respect to the Target Projections, which projections Company management has informed us reflect, among other things, the Company management’s intent with respect to the operation of the Target after the closing of the Merger. All such estimates, forecasts and projections (including the Target Projections) were not prepared with the expectation of public disclosure and are based on numerous variables and assumptions that are inherently uncertain, including, without limitation, factors related to general economic and competitive conditions. Accordingly, actual results could vary significantly from those set forth in such estimates, forecasts and projections. We have relied on these estimates, forecasts and projections without independent verification or analyses and do not in any respect assume any responsibility for the accuracy or completeness thereof. We have relied upon, without independent verification, the assessment of the Company management as to the existing products and services of the Company and the Target and viability of, and risks associated with, the future products and services of the Company and the Target. We express no opinion as to the Target Projections, the Company’s projections or the Expected Synergies or any other estimates, forecasts and projections, or the assumptions on which they were made. For purposes of rendering our Opinion we have assumed, with the Company’s consent, that there will be at least $500,000 cash on the Target’s balance sheet immediately prior to the Closing and no adjustment of the Aggregate Consideration will be required in respect of the Target’s working capital.
We have not been requested to make, nor have we made, any independent evaluations, physical inspections, valuations or appraisals of the assets or liabilities of the Company or the Target, nor have we been furnished with such materials. Stifel assumed, with the consent of the Company’s management, that any material liabilities (contingent or otherwise, known or unknown) of the Company and the Target are set forth in its financial statements or have been disclosed to us by the Company’s management. Stifel has assumed, with the consent of the Company’s management, that there has been no material change in the assets, liabilities, financial condition, results of operations, business or prospects of the Company and the Target since the date of the most recent relevant financial statements made available to Stifel. In addition, we have not evaluated the solvency or fair value of the Company, the Target or any other person under any state, federal or foreign laws relating to bankruptcy, insolvency or similar matters. With respect to all legal matters relating to the Company, the Target, and the Merger, we have relied on the advice of legal counsel to the Company.
Our Opinion is limited to whether the Aggregate Consideration to be paid by the Company in the Merger pursuant to the Agreement is, as of the date hereof, fair to the Company, from a financial point of view. We express no view as to any other aspect or implication of the Merger, including without limitation the form or structure of the Merger, any financing obtained or utilized or to be obtained or utilized by the Company in connection with the Merger, the sale of Financing Shares pursuant to the Securities Purchase Agreement, the allocation of the Aggregate Consideration, or any other agreement, arrangement or understanding entered into in connection with the Merger or otherwise. Without limiting the generality of the foregoing, our Opinion does not address:(i) any strategic alternatives currently (or which have been or may be) contemplated by the Company or its board of directors (the “Board”); (ii) any legal, tax or accounting matters, including any such consequences of the Merger on the Company or its shareholders;(iii) the fairness of the amount or nature of any compensation to any officers, directors or employees of the Company or the Target, or class of such persons, relative to the compensation to the holders of the securities of the Company or the Target; (iv) the price or trading range for shares of the common stock of the Company following the announcement or consummation of the Merger or at any other time; (v) any advice or opinions provided by any other advisor to the Company or the Target or any of their respective affiliates; (vi) the Company’s liquidity, including, without limitation, cash balances following the payment of the Aggregate Consideration; (vii) the terms of any financing (including sale of Financing Shares pursuant to the Securities Purchase Agreement) contemplated by the Agreement; (viii) the availability or terms of any future debt or equity financing contemplated for the Company following the Merger; or (ix) the solvency or financial condition of the Company or the Target or any other person.
| C-2 |
Our Opinion is necessarily based on economic, market, financial and other conditions as they exist on, and the information made available to us as of, the date of this letter. It is understood that subsequent developments may affect the conclusions reached in this Opinion and that Stifel does not have any obligation to update, revise or reaffirm this Opinion.Further, as the Board is aware, the credit, financial and stock markets have been experiencing unusual volatility and we express no opinion or view as to any potential effects of such volatility on the Company or the Merger.Our Opinion is solely for the information of, and directed to, the Board in connection with its consideration of the financial terms of the Merger and is not to be relied upon by any shareholder of the Company or the Target or any other person. Our Opinion does not constitute a recommendation to the Board as to how to vote on or otherwise act with respect to the Merger or any other matteror to any stockholder of TranS1 or the Target as to how such stockholder should vote his, her or its shares of common stock at any stockholders’ meeting at which the Merger or any other matter is considered, or whether or not any stockholder of TranS1 or the Target should enter into a voting or stockholders’, or affiliates’ agreement with respect to the Merger, or exercise any dissenters’ or appraisal rights that may be available to such stockholder. Our Opinion is not for the benefit of, and does not convey any rights or remedies to, any holder of securities of the Company or any other person other than as described above. In addition, our Opinion does not compare the relative merits of the Merger with any other alternative transaction or business strategy which may be or may have been available to the Board or the Company and does not address the underlying business decision of the Board or the Company to proceed with or effect the Merger.
For purposes of rendering our Opinion we have assumed, with your consent, in all respects material to our analysis, that the representations and warranties of each party contained in the Agreement are true and correct, that each party will perform all of the covenants and agreements required to be performed by it under the Agreement and that all conditions to the consummation of the Merger will be satisfied withoutany waiver of material terms or conditions by the Company or any other party and without any anti-dilution or other adjustment to the Aggregate Consideration. We have assumed that the final form of the Agreement will be substantially similar to the last draft reviewed by us. We have also assumed that all governmental, regulatory and other consents and approvals required in connection with the Merger will be obtained and that in the course of obtaining any of those consents and approvals no restrictions will be imposed or waivers made that would have an adverse effect on the contemplated benefits of the Merger.We have assumed that the Merger will be consummated in a manner that complies with the applicable provisions of the Securities Act of 1933, as amended, the Securities Exchange Act of 1934, as amended, and all other applicable federal and state statutes, rules and regulations. We have further assumed that the Company has relied upon the advice of its counsel, independent accountants and other advisors (other than Stifel) as to all legal, financial reporting, tax, accounting and regulatory matters with respect to the Company, the Merger and the Agreement. In rendering services hereunder, Stifel does not provide accounting, legal or tax advice.
Stifel has not considered any potential legislative or regulatory changes currently being considered by the United States Congress, the Securities and Exchange Commission (the “SEC”), or any other governmental or regulatory bodies, or any changes in accounting methods or generally accepted accounting principles that may be adopted by the SEC or the Financial Accounting Standards Board.
We have acted as financial advisor to the Company in connection with the Merger. We will receive a fee upon the delivery of this Opinion. We also will receive a fee upon the consummation of the Merger, against which the fee paid upon delivery of this Opinion, as described in the preceding sentence, shall be credited. We will not receive any other significant payment or compensation contingent upon the successful consummation of the Merger. Stifel will also receive a right of first refusal to act as the Company’s lead left book-running managing underwriter or placement agent for a certain period if the Company retains or otherwise uses an investment bank to pursue a registered, underwritten public offering or private placement of securities. In addition, the Company has agreed to reimburse a portion our expenses and indemnify us for certain liabilities arising out of our engagement. In the two years preceding the date of this Opinion, we have not served as financial advisor to the Company or the Target. Furthermore, a private equity fund whose general partners are affiliated with Stifel and a private equity fund in whose general partner an affiliate of Stifel holds a membership interest are shareholders of the Company, holding less than 5% of the Company, and a private equity fund in whose general partner an affiliate of Stifel holds a membership interest is a shareholder of the Target, holding less than 10% of the Target. We may seek to provide other investment banking services to the Company or the Target or their respective affiliates in the future, for which we would seek customary compensation.
Stifel, as a part of its investment banking business, is continually engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements and valuations for corporate and other purposes. In the ordinary course of our business, we and our affiliates may actively trade and hold the securities of the Company for our own account and for the accounts of our customers and, accordingly, may at any time hold a long or short position in such securities.
Stifel’ Fairness Opinion Committee has approved the issuance of this Opinion. Our Opinion is confidential and may not be published, quoted or otherwise used or referred to, in whole or in part, nor shall any reference to Stifel or this Opinion be made, in any registration statement, prospectus or proxy statement or in any other document used in connection with the Merger or otherwise, nor shall this Opinion be used for any other purpose, without the prior written consent of Stifel.
| C-3 |
Signature page follows
| C-4 |
Based upon and subject to the foregoing, including the various assumptions and limitations set forth herein, we are of the opinion that, as of the date hereof, the Aggregate Consideration to be paid by the Company in the Mergerpursuant to the Agreement is fair to the Company, from a financial point of view.
Very truly yours,
/s/ STIFEL, NICOLAUS & COMPANY, INCORPORATED
STIFEL, NICOLAUS & COMPANY, INCORPORATED
| C-5 |
PROXY
TRANS1 INC.
Special Meeting of Stockholders
to be held on
[Ÿ], 2013
[Ÿ] Eastern Time
THIS PROXY IS SOLICITED ON BEHALF OF THE BOARD OF DIRECTORS
FOR THE SPECIAL MEETING TO BE HELD[Ÿ], 2013
The undersigned hereby nominates, constitutes and appoints Ken Reali and Joseph Slattery, and each of them individually, the attorney, agent and proxy of the undersigned, with full power of substitution, to vote all common stock of TranS1 Inc. (“TranS1”), which the undersigned is entitled to represent and vote at the Special Meeting of Stockholders of TranS1 to be held at 200 Horizon Drive, Suite 115, Raleigh, North Carolina 27615 on [Ÿ], 2013, at [Ÿ] Eastern Time, and at any and all adjournments or postponements thereof, and to vote all shares of the common stock of TranS1 standing in the name of the undersigned with all the powers the undersigned would possess if personally present at such meeting. This Proxy may be revoked by the undersigned at any time prior to its use. The undersigned directs that this Proxy be voted as follows:
THE DIRECTORS RECOMMEND A VOTE “FOR” ITEMS 1, 2, 3, AND 4
| 1. | Approval of the issuance of shares of TranS1 common stock pursuant to the Agreement and Plan of Merger, dated March 3, 2013, by and among TranS1, RacerX Acquisition Corp., Baxano, Inc., and Sumeet Jain and David Schulte as Securityholder Representatives, as amended by the First Amendment to Agreement and Plan of Merger, dated April 10, 2013, by and among the parties: |
| ¨FOR | ¨AGAINST | ¨ABSTAIN |
| 2. | Approval of the issuance of shares of TranS1 common stock pursuant to the Securities Purchase Agreement, dated March 3, 2013, by and among TranS1 and certain investors: |
| £FOR | £AGAINST | £ABSTAIN |
| 3. | Approval of the amendment to TranS1’s Amended and Restated Certificate of Incorporation to change its name to Baxano Surgical, Inc.: |
| ¨FOR | ¨AGAINST | ¨ABSTAIN |
| 4. | Approval of the proposal to grant the proxy holders discretionary authority to vote to adjourn the Special Meeting of Stockholders, if necessary, in order to solicit additional proxies in the event that there are not sufficient affirmative votes present at the Special Meeting of Stockholders to approve Item 1, Item 2, and/or Item 3. |
| ¨FOR | ¨AGAINST | ¨ABSTAIN |
| 5. | In the proxy holders’ discretion, on such other business as may properly come before the Special Meeting of Stockholders of TranS1 or any adjournment thereof. |
The transactions contemplated by Items 1 and 2 are, among other conditions described in the accompanying Proxy Statement, conditioned on each other and, therefore, both Items must be approved in order for the transactions contemplated by such Items to be consummated. In addition, the amendment contemplated by Item 3 is contingent upon the approval of Items 1 and 2 and the consummation of the merger contemplated by Item 1.
THE SHARES REPRESENTED BY THIS PROXY WILL BE VOTED AS DIRECTED BY THESTOCKHOLDER. WHERE NO DIRECTION IS GIVEN, SUCH SHARES WILL BE VOTED “FOR” THE APPROVAL OF THE ISSUANCE OF SHARES OF TRANS1 COMMON STOCK PURSUANT TO THE AGREEMENT AND PLAN OF MERGER, “FOR” THE APPROVAL OF THE ISSUANCE OF SHARES OF TRANS1 COMMON STOCK PURSUANT TO THE SECURITIES PURCHASE AGREEMENT, “FOR” THE APPROVAL OF THE AMENDMENT TO TRANS1’S AMENDED AND RESTATED CERTIFICATE OF INCORPORATION, AND “FOR” THE GRANT OF DISCRETIONARY AUTHORITY TO THE PROXY HOLDERS.
| Date _________________________________________, 2013 | |
| (Signature of stockholder) | |
| Please sign your name exactly as it appears hereon. Executors, administrators, guardians, officers of corporations, and others signing in a fiduciary capacity should state their full titles as such. |
VOTE BY MAIL
Mark, sign and date your proxy card and return it in the enclosed postage-paid envelope.
Please detach here