Use these links to rapidly review the document
TABLE OF CONTENTS
VASO ACTIVE PHARMACEUTICALS, INC. INDEX TO FINANCIAL STATEMENTS
As filed with the Securities and Exchange Commission on November 13, 2003
Registration No. 333-106785
| | | | |
OMB APPROVAL
|
|
|
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 3
TO
FORM SB-2 |
|
OMB Number: 3235-0418
Expires: February 28, 2006
Estimated average burden
hours per response: 137.0
|
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
VASO ACTIVE PHARMACEUTICALS, INC.
DELAWARE
(State of Other Jurisdiction
of Incorporation or Organization) | | 2834
(Primary Standard Industrial Classification
Identification "SIC" Number) | | 02-0670926
(IRS Employment
or "EIN" Number) |
VASO ACTIVE PHARMACEUTICALS, INC.
99 ROSEWOOD DRIVE, SUITE 260
DANVERS, MASSACHUSETTS 01923
(978) 750-0090
(Address and telephone number of principal executive offices)
99 ROSEWOOD DRIVE, SUITE 260
DANVERS, MASSACHUSETTS 01923
(Address of principal place of business or intended principal place of business)
JOHN J. MASIZ
PRESIDENT AND CHIEF EXECUTIVE OFFICER
99 ROSEWOOD DRIVE, SUITE 260
DANVERS, MASSACHUSETTS 01923
(978) 750-0090
(Name, address and telephone number of agent for service)
APPROXIMATE DATE OF PROPOSED SALE TO THE PUBLIC:As soon as practicable after the effective date of this Registration Statement.
Copies to:
Gregory Sichenzia, Esq.
Thomas A. Rose, Esq.
Sichenzia Ross Friedman Ference LLP
1065 Avenue of the Americas
New York, NY 10018
(212) 930-9700 | | David A. Garbus, Esq.
J. Michael Wirvin, Esq.
Robinson & Cole LLP
One Boston Place
Boston, Massachusetts 02108
(617) 557-5900
|
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434, check the following box. o
CALCULATION OF REGISTRATION FEE
|
Title of each class of
securities to be registered
| | Dollar amount to
be registered
| | Proposed maximum
offering price per unit
| | Proposed maximum
aggregate
offering price
| | Amount of
Registration Fee
|
|---|
|
| Class A common stock, $.0001 par value per share(1) | | $7,475,000 | | $5.00 | | $7,475,000 | | $610.71 |
|
| Representative's Warrants to purchase Class A common stock, $.0001 par value per share(2) | | 130,000 | | $.0001 | | $13.00 | | $— |
|
| Class A common stock, $.0001 par value per share issuable upon exercise of Representative's Warrants | | $780,000 | | $6.00 | | $780,000 | | $63.72 |
|
| Total | | | | | | | | $674.43(3) |
|
- (1)
- Includes 195,000 shares of Class A common stock which the underwriters have the option to purchase to cover over-allotments, if any.
- (2)
- In connection with the Registrant's sale of the Securities offered hereby, the Registrant is granting to the Representative warrants to purchase 130,000 shares of Class A common stock.
- (3)
- Includes $622.55 previously paid.
No market currently exists for the Registrant's Class A common stock. The proposed maximum offering price per share is estimated solely for the purposes of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any state in which the offer or sale is not permitted.
Subject to completion, dated November 13, 2003
PROSPECTUS
1,300,000 Shares

VASO ACTIVE PHARMACEUTICALS, INC.
1,300,000 Shares Class A Common Stock
This is the initial public offering of Vaso Active Pharmaceuticals, Inc., and we are offering 1,300,000 shares of our Class A common stock. We have applied for listing on the Nasdaq Small Cap Market under the symbol "VAHP" and on the Boston Stock Exchange under the symbol • . No public market currently exists for our shares.
We have two classes of common stock, Class A common stock and Class B common stock. The Class A common stock is substantially identical to the Class B common stock, except with respect to voting power. With respect to all matters on which stockholders are entitled to vote, holders of the Class A common stock are entitled to one vote per share of Class A common stock held. Holders of the Class B common stock are entitled to three votes per share of Class B common stock held.
Investing in our Class A common stock involves risks. See "Risk Factors" beginning on page 7.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| | Price to Public
| | Underwriting Discounts
and Commissions
| | Proceeds Before Expenses
|
|---|
| Per Share | | $ | 5.00 | | $ | .50 | | $ | 4.50 |
| Total | | $ | 6,500,000 | | $ | 650,000 | | $ | 5,850,000 |
- *
- As additional compensation to the underwriters, we have granted to the representative of the underwriters five year warrants to purchase 130,000 shares of our Class A common stock at an exercise price equal to 120% of the initial offering price to the public.
We have granted the underwriters the right to purchase up to an additional 195,000 shares of Class A common stock to cover over-allotments.
KASHNER DAVIDSON
SECURITIES CORP.
THE DATE OF THIS PROSPECTUS IS , 2003
TABLE OF CONTENTS
In this prospectus, the terms "Vaso Active", "we", "our" and "us" refer to Vaso Active Pharmaceuticals, Inc. unless otherwise specified. You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from the information contained in this prospectus. We are offering to sell shares of Class A common stock, and seeking offers to buy shares of Class A common stock, only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of when this is delivered or when any sale of our Class A common stock occurs.
2
PROSPECTUS SUMMARY
You should read the following summary together with the more detailed information regarding our company and the Class A common stock being sold in this offering, including the "Risk Factors" section and our financial statements and accompanying notes, which are included elsewhere in this prospectus.
Our Business
We are an early-stage company focused on commercializing, marketing and selling over-the-counter, or OTC, pharmaceutical products, with a particular focus on drugs that incorporate the vaso active lipid encapsulated, or VALE, transdermal delivery technology. We began our operations as a division of BioChemics, Inc., a biopharmaceutical company focused on the development of transdermal drug delivery systems, in January 2001. In January 2003, we were formed as an independent subsidiary of BioChemics to further commercialize our OTC products and further develop our OTC product candidates, although BioChemics continues to provide us with administrative support services, manufacturing and development services and technology licenses.
BioChemics currently holds patents for the Vaso Active Lipid Encapsulated transdermal delivery system. We refer to this as VALE technology. VALE technology can be thought of as a "liquid needle", given its ability to introduce drugs into the bloodstream in an efficient and highly effective manner. We believe that the VALE technology provides an efficient, predictable and reliable transdermal drug delivery system that eliminates the need for a patch and allows for the efficient and effective delivery of a myriad of drugs that could not otherwise be effectively delivered using existing transdermal delivery technology.
We have an exclusive, worldwide license to use and practice the VALE technology in the development, marketing and sale of products in the OTC pharmaceutical market. We do not currently, nor do we intend to, participate in the manufacturing of, nor conduct any research and development with respect to, any of our products or product candidates. Prior to the consummation of this offering, we will enter into an agreement with BioChemics with respect to the ongoing manufacturing and development of our products and product candidates. Under this agreement, BioChemics will research, develop and manufacture products for us pursuant to specific purchase orders submitted by us from time to time. BioChemics will charge us a development and manufacturing fee at a rate of cost plus 10%. We will then market, commercialize, distribute and sell these products.
Before we were established, BioChemics initiated the marketing and commercialization of the following products on a limited basis:
- •
- deFEET®, for treatment of athlete's foot fungal infections;
- •
- Athlete's Relief, for treatment of pain associated with athletic activity; and
- •
- Osteon®, for treatment of arthritis pain.
Currently, we market Athlete's Relief and Osteon in the United States. We have begun negotiating agreements with third parties for the marketing of Osteon and Athlete's Relief in the United States and internationally. We plan to rebrand the deFEET athlete's foot anti-fungal medication product as Termin8 and/or Xtinguish, depending on targeted markets in the United States. In accordance with this plan, BioChemics has begun to remove all deFEET branded product currently in circulation. We have applied for trademark protection in the United States for the marks Termin8 and Xtinguish.
In addition to expanding our launch of these three products, we have identified and are currently developing, in collaboration with BioChemics, six additional OTC products utilizing the VALE technology. Our current OTC product candidates according to their medical or clinical indication are:
- •
- Analgesic;
- •
- Toenail fungus treatment;
- •
- Acne treatment;
3
- •
- First aid treatment;
- •
- Hand and body lotion; and
- •
- Psoriasis treatment.
Technology Behind Our Products and Product Candidates
Transdermal drug delivery is the process of delivering drugs into or through the skin without requiring the use of an invasive instrument such as a needle. There are many different approaches to accomplishing transdermal drug delivery and numerous technologies exist to carry it out. The most common technologies employed use either (i) liposomes, which are applied topically, (ii) patches that adhere to the skin, holding the drug in place while it is administered, or (iii) an outside energy source producing electricity (iontophoresis) or sound (sonophoresis) to help move the drug through the skin layers.
In order to efficiently and effectively move drugs through the skin and into the blood stream, a drug delivery system must penetrate three skin barriers. Competitive technologies using patch and/or liposome technologies are able to penetrate the first and second barriers but, except for a few limited instances where more complicated and costly technologies such as iontophoresis and sonotophoresis are used, they are generally unable to penetrate the capillaries and enter the bloodstream. Unlike competing technologies, the VALE process "unlocks" this final barrier and promotes the efficient and complete transportation of drugs from the skin's second barrier, or dermis, into the bloodstream. We believe that the VALE delivery system is the only transdermal system that penetrates all three skin barriers in a timely and efficient fashion.
BioChemics has been issued three U.S. patents relating to the VALE technology. In addition, foreign patents have been issued to BioChemics in 17 foreign countries and are pending in seven others. The first of the U.S. patents expires in 2013. BioChemics has filed for second generation patents on a number of its existing products.
Our Commercialization Strategy
We intend to utilize a portion of the offering proceeds to implement our product rollout strategy. Our plan is focused on the systematic rollout of our products into major retail drug store chains, select independent pharmacies, and nontraditional channels, including websites and catalogues.
The initial phase of our OTC marketing and sales strategy is to expand the launch of Osteon and Athlete's Relief into the retail marketplace and initiate the commercialization, marketing and distribution of our Termin8 and Xtinguish brands. This will be accomplished through our own marketing efforts or through licensing agreements with third parties.
Organization and Offices
We organized as a Delaware corporation on January 13, 2003. Our principal executive offices are located at 99 Rosewood Drive, Suite 260, Danvers, MA 01923. Our telephone number is (978) 750-0090 and our website is located at www.vasoactive.us.
"Vaso Active" and our logo are trademarks of Vaso Active. Osteon® and PENtoCORE® are registered trademarks of BioChemics. deFEET® is a registered trademark of a third party, De Feet International, Inc., which has been licensed to BioChemics. We license from BioChemics the Osteon and PENtoCORE trademarks under our license agreement, as amended, with BioChemics. In addition, we have applied for U.S. trademark protection for the marks "Termin8" and "Xtinguish" for our athlete's foot preparations. Any reference in this prospectus relating to the functionality, formulation or performance of deFEET shall apply in all respects to Termin8 and Xtinguish. This prospectus also contains trademarks and tradenames of other parties.
4
THE OFFERING
| Class A common stock offered by us in this offering | | 1,300,000 shares |
| Class A common stock outstanding after this offering | | 1,514,000 shares |
| Class B common stock outstanding after this offering | | 1,500,000 shares |
| Class A and Class B common stock taken together and outstanding after this offering | | 3,014,000 shares |
| Use of proceeds | | The proceeds of this offering will be used for continued development, commercialization and sale of our products and product candidates; to implement our strategic marketing and commercialization plan; to recruit and hire individuals for key senior management positions; to repay BioChemics for management fees and other charges paid by BioChemics on our behalf; and for working capital and general corporate purposes. |
| Nasdaq Small Cap Market Symbol | | "VAPH" |
| Boston Stock Exchange Symbol | | • |
Unless otherwise noted, this prospectus:
- •
- assumes that no shares of Class A common stock were issued and outstanding as of September 30, 2003;
- •
- excludes 450,000 shares of Class A common stock issuable upon exercise of options to be granted pursuant to our 2003 stock incentive plan and 300,000 shares of Class A common stock issuable upon exercise of options to be granted pursuant to our 2003 non-employee director compensation plan;
- •
- assumes the issuance, upon the consummation of this offering, of 214,000 shares of Class A common stock issuable upon conversion of our 10% convertible subordinated pay-in-kind promissory notes outstanding as of September 30, 2003, including accrued pay-in-kind interest;
- •
- assumes no exercise of the underwriter's over-allotment option; and
- •
- assumes an initial public offering price of $5.00 per share of Class A common stock.
In addition to our Class A common stock, 1,500,000 shares of our Class B common stock are issued and outstanding as of September 30, 2003. Each share of Class B common stock is convertible at any time into Class A common stock on a share-for-share basis. With respect to all matters on which stockholders are entitled to vote, holders of the Class A common stock will be entitled to one vote per share of Class A common stock held. Holders of the Class B common stock will be entitled to three votes per share of Class B common stock held.
5
SUMMARY FINANCIAL DATA
Statement of Operations Data:
| | Years Ended
December 31,
| | Nine Months Ended
September 30,
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| | (Unaudited)
| |
|---|
| Revenues | | $ | 100,209 | | $ | 91,957 | | $ | 76,499 | | $ | 51,876 | |
| Cost of sales | | | 40,791 | | | 40,811 | | | 32,779 | | | 31,279 | |
| | |
| |
| |
| |
| |
| Gross Profit | | | 59,418 | | | 51,146 | | | 43,720 | | | 20,597 | |
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Marketing, advertising and promotion | | | 556,614 | | | 38,962 | | | 11,565 | | | 87,125 | |
| | Selling, general and administrative | | | 545,748 | | | 464,832 | | | 340,526 | | | 296,538 | |
| | |
| |
| |
| |
| |
| Total operating expenses | | | 1,102,362 | | | 503,794 | | | 352,091 | | | 383,663 | |
| | |
| |
| |
| |
| |
| Loss from operations | | | (1,042,944 | ) | | (452,648 | ) | | (308,371 | ) | | (363,066 | ) |
| | |
| |
| |
| |
| |
| Other (income) expenses | | | (2,549 | ) | | (5,786 | ) | | (6,621 | ) | | (13,115 | ) |
| | |
| |
| |
| |
| |
| Net loss | | $ | (1,040,395 | ) | $ | (446,862 | ) | $ | (301,750 | ) | $ | (349,951 | ) |
| | |
| |
| |
| |
| |
| | Basic and diluted loss per share | | $ | — | | $ | — | | $ | — | | $ | (0.64 | ) |
| | |
| |
| |
| |
| |
| Shares used in computing basic and diluted loss per share amounts | | | — | | | — | | | — | | | 549,451 | |
| | |
| |
| |
| |
| |
No shares were issued as of any of the periods presented except for the period ended September 30, 2003, during which period we issued to BioChemics 1,500,000 shares of Class B common stock pursuant to our license agreement dated as of February 1, 2003.
Shares used in the computation of basic and diluted loss per share represent the weighted average shares outstanding over the six-month period ended September 30, 2003, which includes the issuance of 1,500,000 shares of Class B common stock to BioChemics on June 20, 2003.
Balance Sheet Data:
| | As of September 30, 2003
|
|---|
| | Actual
| | Pro Forma
| | Pro Forma
as Adjusted
|
|---|
| | (in thousands)
|
|---|
| Cash and cash equivalents | | $ | 159 | | $ | 159 | | $ | 5,179 |
| Working capital | | | (350 | ) | | 144 | | | 5,227 |
| Total assets | | | 821 | | | 787 | | | 5,227 |
| Total stockholders' (deficit) equity | | | (350 | ) | | 144 | | | 5,227 |
The preceding table presents a summary of our unaudited balance sheet data as of September 30, 2003:
- •
- on an actual basis;
- •
- on a pro forma basis to give effect to the automatic conversion of $500,000 principal amount of our 10% pay-in-kind promissory notes, including accrued pay-in-kind interest, into 214,000 shares of our Class A common stock upon the consummation of this offering; and
- •
- on a pro forma as adjusted basis to give effect to the issuance and the sale of 1,300,000 shares of Class A common stock offered by this prospectus at an assumed initial public offering price of $5.00 per share, after deducting the underwriting discounts and commissions and estimated offering expenses, and our receipt of the net proceeds from that sale.
6
RISK FACTORS
AN INVESTMENT IN OUR STOCK INVOLVES A HIGH DEGREE OF RISK. YOU SHOULD CAREFULLY CONSIDER THE FOLLOWING RISK FACTORS AND UNCERTAINTIES AND ALL OTHER INFORMATION CONTAINED IN THIS PROSPECTUS BEFORE MAKING AN INVESTMENT DECISION. THIS SECTION SETS FORTH WHAT WE BELIEVE ARE ALL OF THE MATERIAL RISKS TO AN INVESTMENT IN VASO ACTIVE. IF ANY OF THE FOLLOWING RISKS AND UNCERTAINTIES OCCURS, OUR BUSINESS, FINANCIAL CONDITION OR RESULTS OF OPERATIONS COULD BE MATERIALLY ADVERSELY AFFECTED.
Risks Related to Our Business
We are an early stage company with a history of losses and may never achieve or sustain profitability.
We have never been profitable and we may not achieve profitability in the foreseeable future, if at all. Our ability to generate profits in the future will depend on a number of factors, including:
- •
- start-up costs relating to the commercialization, sale and marketing of our products;
- •
- costs of acquiring product candidates;
- •
- general and administrative costs relating to our operations;
- •
- increases in our research and development costs; and
- •
- charges related to purchases of technology or other assets.
At September 30, 2003, we had an accumulated deficit of approximately $350,000 and a working capital deficiency in the same approximate amount.
If we are not successful in completing this offering or obtaining other sources of funding through product sales or otherwise, we may not be able to fund our operations or support our capital needs and business strategy. Accordingly our ability to continue as a going concern is in question. We believe that the proceeds from this offering, together with our current cash and investment position, will be sufficient to fund our operations and capital expenditures at least through the next 12 months.
We are an early stage company that has a limited operating history.
We are an early stage company focused on commercializing, marketing and selling over-the-counter, or OTC, pharmaceutical products. We began our operations as a division of BioChemics, Inc. in January 2001. We have only operated as an entity independent of BioChemics since January 2003. Our operating history is therefore limited. The deFEET, Athlete's Relief and Osteon products are in the early stages of commercialization. Other of our product candidates are only in the early stages of development. With the exception of the introduction of deFEET to the marketplace by BioChemics while we were still a division of BioChemics, we have not yet commercialized, marketed or sold any products or recognized significant revenue from product sales. You should evaluate the likelihood of financial and operational success in light of the uncertainties and complexities present in an early-stage company, many of which are beyond our control, including:
- •
- our potential inability to distribute, sell and market our products; and
- •
- the significant investment to achieve our commercialization, marketing and sales objectives.
Our operations have been limited to organizing and staffing our company, acquiring our license, developing and testing our revenue distribution models and test marketing our products. These
7
operations provide a limited basis for you to assess our ability to commercialize our products and product candidates and the advisability of investing in us.
We depend on BioChemics to provide us with certain support and services. The loss of such support and services would have a material adverse effect on our business.
We were originally formed as a division of BioChemics and the viability and financial strength of BioChemics is critical to our success. Throughout our development, we have relied on services and financing provided to us by BioChemics. When we became an independent operating entity, we entered into a license agreement and, prior to the consummation of this offering, will enter into a manufacturing and development agreement, with BioChemics. We presently maintain our executive offices on premises that we share with BioChemics. We do not have a lease agreement with BioChemics. We believe that we can obtain suitable alternative space without any material disruption of our business and that such space will be available to us in the future on commercially reasonable terms. In addition, BioChemics provides us with back office support and management services. Allocations of BioChemics' expenses for centralized accounting, data processing, utilities, office space rental, supplies, telephone and other corporate services and infrastructure are charged back to us as a management fee. These amounts approximated $135,600 and $114,150 for the years ended December 31, 2002 and 2001 respectively, and approximated $99,000 for the nine months ended September 30, 2003. The loss of the services provided by BioChemics or the loss of the license of the VALE transdermal delivery technology under the license agreement would have a material adverse effect on our business, financial condition and results of operations.
In this regard, potential investors should be aware that BioChemics has never been profitable and most likely will not achieve profitability in the near future, if ever. Although BioChemics was founded in 1989, and incorporated in 1991, it is still a development stage company. It has generated significant losses through December 31, 2002, has limited revenue, and is likely to sustain operating losses in the foreseeable future. BioChemics' operations are subject to all of the risks inherent in the establishment of a business enterprise. Through December 31, 2002, BioChemics had an accumulated deficit of approximately $9.9 million. BioChemics anticipates that it will continue to incur net losses and be unprofitable for the foreseeable future. There can be no assurance that BioChemics will ever operate at a profit even if its or our products are commercialized.
In addition, it is expected that BioChemics will encounter significant marketing difficulties and will also face significant regulatory hurdles. The likelihood of success of BioChemics must be considered in light of the problems, expenses, difficulties, complications and delays frequently encountered in connection with any non-profitable business enterprise, including but not limited to the identification and development of new products, difficulties with corporate partners, vendors, and a very competitive environment. Additionally, BioChemics itself requires additional capital and/or revenues to continue its operations and there is no guarantee that it will be able to fund its own operations or those of Vaso Active.
We depend on a small number of customers for the majority of our gross revenue. The loss any one of these customers, or a substantial reduction of the product purchased and sold by them, would have a material adverse affect on our business.
Since our inception, substantially all of our gross revenue has been generated as a result of sales to a small number of customers, the largest being the Walgreens drug store chain. Sales to Walgreens accounted for approximately 66% of our 2002 gross revenues. Walgreens and Eckerd drug stores accounted for approximately 98% of 2001 gross revenues. Accounts receivable from these customers were $53,437 in 2002 and $0 in 2001. In the nine months ended September 30, 2003, Walgreens
8
accounted for approximately 96% of our net revenues. At September 30, 2003, we had no accounts receivable.
In accordance with our plan to rebrand the deFEET product as Termin8 and/or Xtinguish, BioChemics has begun to remove deFeet products from the Walgreens drug stores. Since we are in this transition period, there can be no assurance that Walgreens will continue as one of our customers. Although we plan to market our products to other customers, the loss of Walgreens as a customer could result in a substantial reduction in our gross revenue and have a materially adverse impact on our business and on our ability to continue to operate.
The development of additional products and product candidates will be subject to a number of risk factors and may not be successful.
The development of our product candidates by BioChemics is subject to the risks of failure inherent in the development of new pharmaceutical products and product candidates based on new technologies. These risks include:
- •
- delays in product development or manufacturing;
- •
- unplanned expenditures for product development or manufacturing;
- •
- failure of our product candidates to have the desired effect or an acceptable safety profile;
- •
- failure to receive required regulatory approvals;
- •
- emergence of superior or equivalent products;
- •
- inability to manufacture on our own, or through others, product candidates on a commercial scale;
- •
- inability to market products due to third-party proprietary rights; and
- •
- failure by our collaborative partners to successfully develop products.
Because of these risks, our research and development efforts or those of our collaborative partners may not result in any commercially viable products. If a significant portion of these development efforts is not successfully completed, required regulatory approvals are not obtained, or any approved products are not commercially successful, we are not likely to generate significant revenues or become profitable.
We depend on BioChemics and third parties to develop and manufacture our products and product candidates and our commercialization of our products could be stopped, delayed or made less profitable if BioChemics or those third parties fail to provide us with sufficient quantities at acceptable prices.
We currently have limited product development capability. As a result, we depend on collaborations with third parties, such as BioChemics, for development of our product candidates. In addition, we have no manufacturing capability. As a result, we will depend on BioChemics, which in turn will rely upon third parties to manufacture our products. Although our strategy is based on leveraging BioChemics' ability to develop and manufacture our products for commercialization in the OTC marketplace, we will be dependent on BioChemics' collaborations with drug development and manufacturing collaborators. If we and BioChemics are not able to maintain existing collaborative arrangements or establish new arrangements on commercially acceptable terms, we would be required to undertake product manufacturing and development activities at our own expense, which would increase our capital requirements or require us to limit the scope of our development activities. Moreover, we have limited or no experience in conducting full scale bioequivalence studies, preparing and submitting regulatory applications and manufacturing and marketing controlled release products. There can be no assurance that we will be successful in performing these activities and any failure to
9
perform such activities could have a material adverse effect on our business, financial condition and results of our operations.
If any of our developmental collaborators, especially BioChemics, breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities in a timely manner, the preclinical and/or clinical development and/or commercialization of our product candidates will be delayed, and we would be required to devote additional resources to product development and commercialization or terminate certain development programs. Also, these relationships generally may be terminated at the discretion of our collaborators, in some cases with only limited notice to us. The termination of collaborative arrangements could have a material adverse effect on our business, financial condition and results of operations. There also can be no assurance that disputes will not arise with respect to the ownership of rights to any technology developed with third parties. These and other possible disagreements with collaborators could lead to delays in the development or commercialization of our product candidates or could result in litigation or arbitration, which could be time consuming and expensive and could have a material adverse effect on our business, financial condition and results of operations.
If we or BioChemics fail to adequately protect or enforce our intellectual property rights, we may be unable to operate effectively.
BioChemics owns proprietary technology developed in connection with its three U.S. patents. In addition, foreign patents have been issued to BioChemics in 17 foreign countries and are pending in seven others. BioChemics also owns fifteen registered trademarks, including Osteon and PENtoCORE. Our license agreement, as amended, with BioChemics permits us to commercialize, market and sell our products and product candidates using these patents and the Osteon and PENtoCORE trademarks. Our success and ability to compete are substantially dependent on the patents and trademarks. Although both we and BioChemics believe that the patents and associated trademarks and licenses are valid, there can be no assurance that they will not be challenged and subsequently invalidated and/or canceled. The invalidation or cancellation of any one or all of the patents or trademarks would significantly damage our commercial prospects. Further, BioChemics may find it necessary to legally challenge parties infringing its patents or trademarks or licensed trademarks to enforce its rights thereto. There can be no assurance that any of the patents would ultimately be held valid or that efforts to defend any of the patents, trade secrets, know-how or other intellectual property rights would be successful.
If we infringe on the intellectual property rights of others, our business and profitability may be adversely affected.
Our commercial success will also depend, in part, on us and BioChemics not infringing on the patents or proprietary rights of others. There can be no assurance that the technologies and products used or developed by BioChemics and marketed and sold by Vaso Active will not infringe such rights. If such infringement occurs and neither we nor BioChemics is able to obtain a license from the relevant third party, we will not be able to continue the development, manufacture, use, or sale of any such infringing technology or product. There can be no assurance that necessary licenses to third-party technology will be available at all, or on commercially reasonable terms. In some cases, litigation or other proceedings may be necessary to defend against or assert claims of infringement or to determine the scope and validity of the proprietary rights of third parties. Any potential litigation could result in substantial costs to, and diversion of, our resources and could have a material and adverse impact on us. An adverse outcome in any such litigation or proceeding could subject us and/or BioChemics to significant liabilities, require us to cease using the subject technology or require us and/or BioChemics to license the subject technology from the third party, all of which could have a material adverse effect on our business.
10
In addition, BioChemics is party to a settlement agreement and license with De Feet International, Inc. dated April 20, 2002, pursuant to which BioChemics licenses the trademark "deFEET" from De Feet International, Inc. Pursuant to the settlement agreement and license, BioChemics was granted a non-exclusive, non-transferable and non-assignable license to use the "deFEET" trademark throughout the United States in connection with the manufacturing, advertisement, promotion, distribution and sale of athlete's foot preparations. In the event that BioChemics breaches the terms of the settlement and licensing agreement with De Feet International, Inc., BioChemics could lose its right to use the "deFEET" trademark in connection with its athlete's foot preparation product and could be prevented from selling its athlete's foot preparation product until such product had been rebranded. In that event, we would not realize any revenue from Biochemics' sale of its athlete's foot preparation product which could have a material adverse affect on our business and results of operations.
If we lose the services of John J. Masiz or other key personnel, our business will suffer.
We are highly dependent on the principal members of our scientific and management staff, particularly John J. Masiz, our chief executive officer. Mr. Masiz is also the chief executive officer of BioChemics. Although Mr. Masiz intends to initially devote approximately 70% of his business time to our affairs, as BioChemics and our respective operations continue to grow, there can be no assurance that he will be able to continue to do so. Our chief scientific officer, Dr. Stephen Carter, will devote approximately 30% of his business time to our affairs. For us to pursue our product development, marketing and commercialization plans, we will need to hire personnel with experience in clinical testing, government regulation, manufacturing, marketing and finance. We may not be able to attract and retain personnel on acceptable terms given the intense competition for such personnel among high technology enterprises, including biotechnology, pharmaceutical and healthcare companies, universities and non-profit research institutions. If we lose any of these persons, or are unable to attract and retain qualified personnel, our business, financial condition and results of operations may be materially and adversely affected.
We operate in a competitive environment and there can be no assurances that competing technologies would not harm our business development.
We are engaged in a rapidly evolving field. Competition from numerous pharmaceutical companies including Pfizer, Bristol-Myers Squibb, Schering-Plough, and biotechnology companies including, Alza, Cygnus and Elan, as well as research and academic institutions, is intense and expected to increase. The market for transdermal drug delivery systems is large and growing rapidly and is likely to attract new entrants. Numerous biotechnology and biopharmaceutical companies have focused on developing new drug delivery systems and most, if not all of these companies, have greater financial and other resources and development capabilities than we do. They also have greater collective experience in undertaking pre-clinical and the clinical testing of products; obtaining regulatory approvals; and manufacturing and marketing OTC and pharmaceutical products. Accordingly, certain of our competitors may succeed in obtaining approval for products more rapidly than us. In addition to competing with universities and other research institutions in the development of products, technologies and processes, we may compete with other companies in acquiring rights to products or technologies from universities. There can be no assurance that our products, existing or to be developed, will be more effective or achieve greater market acceptance than competitive products, or that our competitors will not succeed in developing products and technologies that are more effective than those being developed by us or that would render our products and technologies less competitive or obsolete.
11
Technological advancement by our competitors could result in the obsolescence of some or all of our products and may harm business development.
The areas in which we are commercializing, distributing, and/or selling products involve rapidly developing technology. There can be no assurance that we will be able to establish ourselves in such fields, or, if established, that we will be able to maintain our position. There can be no assurance that the development by others of new or improved products will not make our products and product candidates, if any, superfluous or our products and product candidates obsolete.
Should product liability claims be brought successfully against us exceeding the product liability coverage we currently have in place there can be no assurances that such events would not materially impact our performance and viability.
The sale of our products may expose us to potential liability resulting from the sale and use of such products. Liability might result from claims made directly by consumers or by pharmaceutical companies or by others selling such items. We currently maintain $5.0 million of product liability insurance. There can be no assurance that we will be able to renew our current insurance, renew it at a rate comparable to what we now pay, or that the coverage will be adequate to protect us against liability. If we were held liable for a claim or claims exceeding the limits of our current or future insurance coverage, or if coverage was discontinued for any reason, it could have a materially adverse effect on our business and our financial condition.
Our limited sales and marketing experience may adversely impact our ability to successfully commercialize and sell our products.
We have limited sales and marketing experience, particularly with respect to marketing and selling products in commercial quantities. If we are unable to expand our sales and marketing capabilities we may not be able to effectively commercialize our products and product candidates.
Our principal stockholder is also the principal stockholder of BioChemics and will have substantial control over our affairs, possibly to the detriment of other holders of our Class A common stock.
Our principal stockholder, BioChemics, owns 1,500,000 shares of our Class B common stock, which will represent approximately 75% of our voting interests after giving effect to this offering. Mr. Masiz, as President, Chief Executive Officer and Chairman of both us and BioChemics and, as the principal stockholder in BioChemics, will be able to control the outcome of stockholder votes, including votes concerning the election of our directors, the adoption or amendment to provisions in our certificate of incorporation or by-laws, the approval of mergers and/or acquisitions, decisions affecting our capital structure and other significant corporate transactions. This concentration of ownership may delay, deter or prevent transactions that would result in a change of control, which in turn could reduce the value of our common stock.
In the event of a conflict of interest between BioChemics and us, our stockholders could be negatively affected.
There are likely to be situations where our best interests and those of BioChemics will conflict. To the extent that decisions are made that will enhance the value to Biochemics versus the value to us, our stockholders could be negatively affected. Buyers of our Class A common stock should be aware of these potential conflicts.
12
Risks Associated with this Offering
Our stock price could be volatile, and your investment could suffer a decline in value.
The trading price of our stock is likely to be highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
- •
- changes in, or failure to achieve, financial estimates by ourselves and others such as investors and securities analysts;
- •
- new products or services introduced or announced by us or our competitors;
- •
- announcements of technological innovations by us or our competitors;
- •
- actual or anticipated variations in quarterly operating results;
- •
- conditions or trends in the biotechnology and pharmaceutical industries;
- •
- announcements by us of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
- •
- additions or departures of key personnel; and
- •
- sales of our common stock.
In addition, the stock market in general, and the Nasdaq Small Cap Market in particular, has experienced significant price and volume fluctuations. Volatility in the market price for particular companies has often been unrelated or disproportionate to the operating performance of those companies. Further, there has been particular volatility in the market prices of securities of biotechnology and pharmaceutical companies. These broad market and industry factors may seriously affect the market price of our Class A common stock, regardless of our operating performance.
There may not be an active, liquid trading market for our Class A common stock.
Prior to this offering, there has been no public market for our Class A common stock. An active trading market for our Class A common stock may not develop or be sustained following this offering. You may not be able to sell your shares quickly or at the market price if trading in our stock is not active. The initial public offering price will be determined by negotiations between us and representatives of the underwriters based upon a number of factors. The initial public offering price may not be indicative of prices that will prevail in the trading market.
Future sales of our Class A common stock may depress our stock price.
The market price of our Class A common stock could decline as a result of sales of substantial amounts of our common stock in the public market, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of common stock. There will be an aggregate of 1,500,000 shares of Class B common stock outstanding before the consummation of this offering, each share convertible into one share of Class A common stock, and 1,514,000 shares of Class A common stock outstanding immediately after this offering, after giving effect to conversion of our 10% convertible pay-in-kind promissory notes upon the consummation of this offering, and an aggregate of 1,709,000 shares of Class A common stock outstanding if the underwriters exercise their over-allotment option in full. All of the shares of Class A common stock sold in the offering will be freely transferable without restriction or further registration under the Securities Act, except for any shares purchased by our "affiliates," as defined in Rule 144 of the Securities Act. The remaining shares of our Class A common stock outstanding will be "restricted securities" as defined in Rule 144. These shares may be sold in the future without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act.
13
You will experience immediate and substantial dilution.
The initial public offering price of our Class A common stock is expected to be substantially higher than the pro forma net tangible book value per share of our common stock. Therefore, if you purchase shares of our Class A common stock in this offering, you will incur immediate dilution of approximately $3.27 in the pro forma net tangible book value per share of common stock from the price per share that you pay for the Class A common stock. Accordingly, if you purchase Class A common stock in this offering, you will incur immediate and substantial dilution of your investment.
We do not intend to pay cash dividends on our common stock.
We intend to retain any future earnings to finance the growth and development of our business and we do not plan to pay cash dividends on our common stock in the foreseeable future.
14
STATEMENTS CONCERNING FUTURE PERFORMANCE
This prospectus contains forward-looking statements. The forward-looking statements are principally contained in the sections entitled "Prospectus Summary," "Business" and "Management's Discussion and Analysis of Financial Condition and Results of Operations." These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to differ, perhaps materially, from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
- •
- our marketing, sales and commercialization activities and projected expenditures;
- •
- our manufacturing and development partnerships with BioChemics and third parties and our ability to maintain and increase those and other strategic partnerships;
- •
- the use of the proceeds of this offering;
- •
- our capital requirements;
- •
- implementation of our corporate strategy; and
- •
- our financial forecast and performance.
In some cases, you can identify forward-looking statements by terms such as "may," "should," "expect," "plan," "anticipate," believe," "estimate," "project," "predict," "intend," "potential," and similar expressions intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss many of these risks in greater detail under the heading "Risk Factors." Also, these forward-looking statements represent our estimates and assumptions only as of the date of this prospectus.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is part, completely and with the understanding that our actual future results may be materially different from what we expect. We may not update these forward-looking statements, even though our situation may change in the future, unless we have obligations under the federal securities laws to update and disclose material developments related to previously disclosed information. We qualify all of our forward-looking statements by these cautionary statements.
15
USE OF PROCEEDS
We estimate that the net proceeds from the sale of the shares of Class A common stock we are offering will be approximately $5.08 million, or approximately $5.96 million if the underwriter's over-allotment option is exercised in full, assuming an initial public offering price of $5.00 per share and after deducting the estimated underwriting discounts and commissions and estimated offering expenses. Based on an initial public offering price of $5.00 per share, the gross proceeds from the sale of Class A common stock we are offering will be $6.5 million. If the underwriter's over-allotment option is exercised in full, the gross proceeds from the offering will be $7.5 million. Underwriting discounts and commissions will be $650,000, or $747,500 if the underwriter's over-allotment option is exercised in full. We estimate the offering expenses to be approximately $770,000.
We anticipate using the net proceeds from the sale of our Class A common stock offered hereby as follows:
- •
- to fund our continued development, commercialization and sale of our products and product candidates, which we estimate will require approximately $1.1 million (21.7% of net proceeds);
- •
- to implement our strategic marketing and commercialization plan, which we estimate will require approximately $1.5 million (29.5% of net proceeds), of which approximately $1.2 million is allocated to fund our radio advertising campaign;
- •
- to recruit and hire individuals for key senior management positions, which we estimate will require approximately $200,000 (3.9% of net proceeds);
- •
- to cover the cost of providing sales incentives, products samples and discounts, which we estimate will require approximately $350,000 (6.9% of net proceeds);
- •
- to repay, without interest, BioChemics approximately $99,000 (1.9% of net proceeds) for management fees charged back to us since we became a stand-alone entity upon our incorporation for centralized accounting, data processing, utilities, office space rental, supplies, telephone and other BioChemics corporate services and infrastructure costs, and approximately $347,000 (6.8% of net proceeds) for other operational charges paid by BioChemics to third parties on our behalf since that time; and
- •
- to provide working capital and for general corporate purposes, which we estimate will require approximately $1.46 million (28.7% of net proceeds).
We anticipate, based on our current plans and assumptions relating to our operations, that the net proceeds of this offering, together with net cash from operations, should be sufficient to satisfy our cash requirements for at least twelve months after the date of this prospectus.
Although we have no current plans, agreements or commitments with respect to any acquisition, we may, if the opportunity arises, use an unspecified portion of the net proceeds to acquire or invest in products, technologies or companies.
Until we use the net proceeds of this offering for the above purposes, we intend to invest the funds in short-term, investment grade, interest-bearing securities. We cannot predict whether the proceeds invested will yield a favorable return.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our capital stock. We anticipate that we will retain any earnings to support operations and to finance the growth and development of our business. Therefore, we do not expect to pay cash dividends in the foreseeable future. Any future determination relating to our dividend policy will be made at the discretion of our board and will depend on a number of factors, including future earnings, capital requirements, financial conditions and future prospects and other factors the board may deem relevant.
16
CAPITALIZATION
The following table sets forth our capitalization as of September 30, 2003:
- •
- on an actual basis;
- •
- on a pro forma basis to give effect to the automatic conversion of $500,000 principal amount of our 10% pay-in-kind promissory notes, including accrued pay-in-kind interest, into 214,000 shares of our Class A common stock upon the consummation of this offering; and
- •
- on a pro forma as adjusted basis to give effect to the issuance and the sale of 1,300,000 shares of Class A common stock offered by this prospectus at an assumed initial public offering price of $5.00 per share, after deducting the underwriting discounts and commissions and estimated offering expenses, and our receipt of the net proceeds from that sale.
| | September 30, 2003 (Unaudited)
| |
|---|
| | Actual
| | Pro Forma
| | Pro Forma
As Adjusted
| |
|---|
| Total current liabilities | | $ | 1,170,755 | | $ | 642,672 | | $ | — | |
Stockholders' equity (deficit): |
|
|
|
|
|
|
|
|
|
|
| Undesignated preferred stock—$.0001 par value; authorized 10,000,000 shares; issued and outstanding, none | | | — | | | — | | | — | |
Class A common stock—$.0001 par value; authorized 10,000,000 shares; no shares issued; 214,000 shares issued and outstanding pro forma; 1,514,000 shares issued and outstanding, pro forma as adjusted |
|
|
— |
|
|
21 |
|
|
151 |
|
Class B common stock—$.0001 par value; authorized 5,000,000 shares; 1,500,000 issued and outstanding; 1,500,000 shares issued and outstanding pro forma; 1,500,000 shares issued and outstanding, pro forma as adjusted |
|
|
150 |
|
|
150 |
|
|
150 |
|
Additional paid-in capital |
|
|
— |
|
|
1,035,778 |
|
|
6,118,742 |
|
Accumulated deficit |
|
|
(349,951 |
) |
|
(891,617 |
) |
|
(891,617 |
) |
Total stockholders' equity (deficit) |
|
|
(349,801 |
) |
|
144,332 |
|
|
5,227,426 |
|
| | |
| |
| |
| |
Total capitalization |
|
$ |
820,954 |
|
$ |
787,004 |
|
$ |
5,227,426 |
|
| | |
| |
| |
| |
17
DILUTION
Our pro forma net tangible book value as of September 30, 2003 was $144,332 or $0.08 per share, based on the pro forma number of shares of common stock outstanding of 1,714,000 as of September 30, 2003, calculated after giving effect to the automatic conversion of $500,000 principal amount and accrued pay-in-kind interest of our 10% pay-in-kind promissory notes into an aggregate of 214,000 shares of our Class A common stock upon the consummation of this offering.
Dilution in pro forma net tangible book value per share represents the difference between the amount per share paid by purchasers of shares of our common stock in this offering and the pro forma net tangible book value per share of our common stock immediately afterwards, after giving effect to the sale of 1,300,000 shares in this offering at an assumed initial offering price of $5.00 per share and after deducting underwriting discounts and commissions and offering expenses. This represents an immediate increase in pro forma net tangible book value of $1.65 per share to existing stockholders and an immediate dilution in pro forma net tangible book value of $3.27 per share to new investors.
The following table illustrates this per share dilution:
| Initial public offering price per share | | | | | $ | 5.00 |
| Pro forma net tangible book value per share as of September 30, 2003 | | $ | 0.08 | | | |
| Increase per share attributable to new investors | | $ | 1.65 | | | |
| Pro forma as adjusted net tangible book value per share after this offering | | | | | $ | 1.73 |
| Dilution per share to new investors | | | | | $ | 3.27 |
In percentage terms, the dilution per share to new investors will be approximately 65% of the initial public offering price of $5.00.
The following table summarizes, on a pro forma basis as of September 30, 2003, after giving effect to this offering, the total number of shares of Class A common stock purchased from us and the total consideration and the average price per share paid by existing stockholders and by new investors:
| | Shares Issued(1)
| | Total Consideration
| |
|
|---|
| | Average Price
Per Share
|
|---|
| | Number
| | Percent
| | Amount
| | Percent
|
|---|
| Existing Stockholders(2) | | 1,714,000 | | 57 | % | $ | 533,000 | | 8 | % | $ | 0.31 |
| New Investors | | 1,300,000 | | 43 | % | | 6,500,000 | | 92 | % | | 5.00 |
| | |
| |
| |
| |
| |
|
| Total | | 3,014,000 | | 100 | % | $ | 7,033,000 | | 100 | % | | |
- (1)
- The Class A common stock is entitled to one vote per share, while the Class B common stock is entitled to three votes per share, with the Class A and Class B common stock voting together as a class on substantially all matters including the appointment of directors. After giving effect to the sale of the Class A common stock offered hereby, the outstanding shares of the Class A common stock held by new investors will represent approximately 21.6% of the total combined voting power of both classes of common stock outstanding, while the outstanding shares of Class A common stock and Class B common stock held by existing stockholders will represent approximately 3.6% and 74.8%, respectively, or 78.4% taken together, of the total combined voting power of both classes of common stock outstanding.
- (2)
- Includes 1,500,000 shares of Class B common stock issued and outstanding as of September 30, 2003 and which are convertible on a share-for-share basis into shares of Class A common stock, and 214,000 shares of Class A common stock to be issued upon conversion of our 10% pay-in-kind promissory notes upon the consummation of this offering.
18
SELECTED FINANCIAL DATA
You should read the following selected financial data in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the financial statements and related notes included elsewhere in this prospectus. The selected balance sheet data set forth below, as of December 31, 2001 and 2002 and the selected statements of operations data for the years ended December 31, 2001 and 2002 are derived from our financial statements, which are included elsewhere in this prospectus and which have been audited by Stowe & Degon, independent certified public accountants. The selected financial data presented below as of and for the nine months ended September 30, 2002 and September 30, 2003 have been derived from our unaudited financial statements, which in the opinion of management include all adjustments necessary for a fair presentation of the data for interim periods. The results of operations for the nine months ended September 30, 2003 are not necessarily indicative of the results that may be expected for the full fiscal year.
Statement of Operations Data:
| | Years Ended December 31,
| | Nine Months Ended
September 30,
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| | (Unaudited)
| |
|---|
| Revenues | | $ | 100,209 | | $ | 91,957 | | $ | 76,499 | | $ | 51,876 | |
| Cost of Sales | | | 40,791 | | | 40,811 | | | 32,779 | | | 31,279 | |
| | |
| |
| |
| |
| |
| Gross profit | | | 59,418 | | | 51,146 | | | 43,720 | | | 20,597 | |
Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Marketing, advertising and promotion | | | 556,614 | | | 38,962 | | | 11,565 | | | 87,125 | |
| | Selling, general and administrative | | | 545,748 | | | 464,832 | | | 340,526 | | | 296,538 | |
| | |
| |
| |
| |
| |
| Total operating expenses | | | 1,102,362 | | | 503,794 | | | 352,091 | | | 383,663 | |
| | |
| |
| |
| |
| |
| Loss from operations | | | (1,042,944 | ) | | (452,648 | ) | | (308,371 | ) | | (363,066 | ) |
| | |
| |
| |
| |
| |
| Other (income) expenses | | | (2,549 | ) | | (5,786 | ) | | (6,621 | ) | | (13,115 | ) |
| | |
| |
| |
| |
| |
| Net loss | | $ | (1,040,395 | ) | $ | (446,862 | ) | $ | (301,750 | ) | $ | (349,951 | ) |
| | |
| |
| |
| |
| |
| Basic and diluted loss per share | | $ | — | | $ | — | | $ | — | | $ | (0.64 | ) |
| | |
| |
| |
| |
| |
| Shares used in computing basic and diluted loss per share amounts | | | — | | | — | | | — | | | 549,451 | |
| |
Balance Sheet Data:
| | As of December 31,
| | As of September 30,
| |
|---|
| | 2001
| | 2002
| | 2003
| |
|---|
| |
| |
| | (Unaudited)
| |
|---|
| Cash and cash equivalents | | $ | — | | $ | — | | $ | 158,576 | |
| Working capital | | | — | | | — | | | (349,801 | ) |
| Total assets | | | 25,983 | | | 72,243 | | | 820,954 | |
| Accumulated (deficit) | | | (1,040,395 | ) | | (1,487,257 | ) | | (349,951 | ) |
19
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion in conjunction with our financial statements, the related notes and other financial information appearing elsewhere in this prospectus. This discussion may contain forward-looking statements based upon current beliefs of management and expectations that involve risks and uncertainties. Our actual results, performance or achievements and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of many factors, including those set forth under "Risk Factors" and elsewhere in this prospectus.
Overview
We are an early-stage company focused on commercializing, marketing and selling over-the-counter, or OTC, pharmaceutical products. We began our operations as a division of BioChemics in January 2001. In January 2003, we were formed as an independent subsidiary of BioChemics to further commercialize our OTC products and further develop our OTC product candidates, although BioChemics continues to provide us with administrative support services, manufacturing and development services and technology licenses. By becoming a subsidiary of BioChemics, as opposed to continuing as a BioChemics division, we have facilitated our ability to obtain independent financing for our operations and reduced our exposure to the potential liabilities inherent in the development and manufacturing of pharmaceutical products.
Our parent, BioChemics, is a biopharmaceutical company focused on the development of transdermal drug delivery systems and is based in Danvers, Massachusetts. BioChemics was founded by John J. Masiz in 1989 and incorporated in Delaware in 1991. BioChemics is controlled by Mr. Masiz, its President and Chief Executive Officer. Biochemics began developing the VALE technology in 1989. BioChemics has subsequently been issued three patents on this technology by the U.S. Patent Office. In addition, foreign patents have been issued to BioChemics in 17 foreign countries and are pending in 7 others. Prior to being established as a subsidiary of BioChemics, we operated as a division of BioChemics, responsible for the commercialization, distribution, marketing and sales of BioChemics' OTC products. BioChemics also manufactures and develops prescription drugs through its Xevex Pharmaceuticals division as well as veterinary drugs through its MediVet Pharmaceuticals division.
Although BioChemics was founded in 1989, and incorporated in 1991, it is still a development stage company. BioChemics has never been profitable. Through December 31, 2002, BioChemics had an accumulated deficit of approximately $9.9 million. BioChemics anticipates that it will continue to incur net losses and be unprofitable for the foreseeable future.
Upon our formation, we agreed to issue to BioChemics 1,500,000 shares of our Class B common stock in return for the exclusive worldwide rights to the vaso active lipid encapsulation, or VALE, technology as it relates to the OTC marketplace as well as to the existing BioChemics portfolio of OTC products. Our board of directors authorized issuance of these shares on June 20, 2003. For accounting purposes, the value of the issued shares of our Class B common stock and the related rights to the license agreement were calculated in accordance with Staff Accounting Bulletin No. 48, "Transfers of Nonmonetary Assets by Promoters or Shareholders," and, as a result, were reported using BioChemics' historical cost basis which is zero.
We have incurred net losses since our inception and have generated limited net revenue from product sales. We incurred net losses of approximately $1.04 million in 2001, $447,000 in 2002 and $350,000 in the nine months ended September 30, 2003. Since we commenced operations, we have incurred an accumulated net loss attributable to our Class A common stockholders of approximately $1.84 million through September 30, 2003. During 2001 and 2002, BioChemics made contributions to our capital to cover our deficits. These contributions totaled approximately $1.04 million in 2001 and $447,000 in 2002. Historically, BioChemics has also provided us with office space, industry expertise,
20
financial support and certain administrative assistance. Pursuant to an administrative services agreement effective September 1, 2003, BioChemics will continue to provide us, at our request, with administrative support services such as secretarial support, accounting and tax services, data processing services, utilities, designated office, warehouse and storage space, office supplies, telephone and computer services, for which BioChemics will charge us an administrative services fee at a rate of cost plus 10%. In addition, as we continue to grow our operations as a stand-alone entity and segregate our operations from those of BioChemics, we anticipate that our operating expenses will increase. For example, compensation expenses for senior personnel and other employees, marketing, advertising and promotional expenses relating to the commercialization, marketing and distribution of our products, expenses, including royalty payments related to deFEET and legal and accounting expenses will increase our over-all expenses as we operate as a stand-alone entity. We expect that our operating expenses will begin to increase as we begin to implement our roll out strategy following consummation of, and the availability of the net proceeds from, this offering. We expect that we will not begin to offset these increasing costs until we realize increasing revenues from the commercialization of our products. We expect this to occur during the second and third quarters of 2004.
Our financial statements up to and including the year ended December 31, 2002, are based on operations of the OTC pharmaceutical division of BioChemics, our predecessor. The financial statements have been prepared using BioChemics' historical book value in the assets and liabilities and historical results of operation of this division. BioChemics' net investment in us is reflected as additional paid-in capital. Transactions were processed through an inter-company account whose balance represents the net obligation from BioChemics to us. Additionally, these financial statements are included in BioChemics's consolidated financial statements using the consolidated method.
The results of operations of this division include allocations of certain BioChemics expenses, such as centralized accounting, data processing, utilities, office space rental, supplies, telephone and other BioChemics corporate services and infrastructure costs. These expenses have been charged back to us as a management fee. These amounts approximated $135,600 and $114,150 for the years ended December 31, 2002 and 2001 respectively. For the nine months ended September 30, 2003, we incurred management fees of approximately $99,000 and other operational charges of approximately $347,000 that had been paid by BioChemics to third parties on our behalf. We will repay these amounts from the net proceeds of this offering. The expense allocations have been determined on the basis that we and BioChemics consider to be reasonable reflections of the utilization of services provided for the benefit received by us. The financial information included herein may not reflect our financial position, operating results, changes in stockholder's deficiency and cash flows in the future or what they would have been had we been a separate stand-alone entity during the periods presented. However, we believe that these results would not be significantly different.
Critical Accounting Policies
Going Concern Assumption. Our financial statements do not include any adjustments relating to the recoverability and classification of assets or the amounts and classification liabilities that might be necessary should we be unable to continue as a going concern. If the financial statements were prepared on a liquidation basis, the carrying value of our assets and liabilities would be adjusted to net realizable amounts. In addition, the classification of the assets and liabilities would be adjusted to reflect the liquidation basis of accounting.
Revenue Recognition. The Company recognizes revenue from product sales in accordance with generally accepted accounting principles in the United States, including the guidance in Staff Accounting Bulletin 101. Revenue from product sales is recognized when there is persuasive evidence of an arrangement, delivery has occurred, the price is fixed and determinable, and collectibility is reasonably assured. However, because our products are sold with limited rights of return, our recognition of revenue from product sales is also subject to Statement of Financial Accounting
21
Standards No. 48 (SFAS 48) "Revenue Recognition When Right of Return Exists." Under SFAS 48, revenue is recognized when the price to the buyer is fixed, the buyer is obligated to pay us and the obligation to pay is not contingent on resale of the product, the buyer has economic substance apart from the us, we have no obligation to bring about the sale of the product and the amount of returns can be reasonably estimated.
The Company records allowances for product returns, rebates and discounts, and reports revenue net of such allowances. We must make judgments and estimates in preparing the allowances that could require adjustments in the future. For instance, our customers have the right to return any product that is held past the labeled expiration date. We base our estimates on historic patterns of returns and on the expiration dates of product currently being shipped, or as a result of an actual event that may give rise to a significant return amount such as the discontinuance of a product.
We do not recognize revenue unless collectibility is reasonably assured. We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. If the financial condition of our customers were to deteriorate and result in an impairment of their ability to make payments, additional allowances may be required.
Expense Allocations. BioChemics provides us certain administrative, marketing and management services, as well as our facilities and general corporate infrastructure. Our statement of operations includes allocations of these costs that we and BioChemics considered to be reasonable.
Deferred Income Taxes. Prior to 2003, we were a division of BioChemics and were not subject to federal or state income tax reporting requirements. Effective 2003, we will begin preparing our income taxes on a stand-alone basis. We account for income taxes and deferred tax assets and liabilities in accordance with Statement of Financial Accounting Standards (SFAS) No. 109, "Accounting for Income Taxes." Because we project future operating losses in the near term, we will provide a full valuation allowance against the deferred tax assets created by these losses.
Stock-based Compensation. As part of our compensation programs offered to our employees, we grant stock options. We grant stock options to employees based on their value at the grant date. We account for stock-based compensation in accordance with SFAS No. 123, "Accounting for Stock-Based Compensation."
Operating Plan
Net Revenues. During the year ended December 31, 2002, we were engaged in ongoing negotiations with a large pharmaceutical company regarding a proposed private label strategic alliance that would have permitted that company to distribute and market our pain relief products under its private label. A private label strategic alliance is a licensing arrangement in which a third party is given all the rights to package, market and distribute a company's products under the third party's own name label in return for a distributor's fee or royalty fee payable to the licensing company on a per unit basis. As part of our negotiations with the large pharmaceutical company, that company advised that it would be seeking exclusive rights with respect to distribution and marketing. No formal agreement was executed, but we reduced our efforts to market, distribute or replenish our retail supply of our pain relief products during the negotiation period so as to prevent the excessive cost of recalling these products in the event that the exclusivity rights that this company was seeking were granted. Near the end of the year ended December 31, 2002, we determined that we did not wish to grant exclusivity rights to this company with respect to our pain relief products and the negotiations ended.
Effective January 2003, we began the commercialization of two of these pain relief products, Osteon and Athlete's Relief. In addition, BioChemics continued to directly commercialize, market and distribute deFEET. BioChemics assigned its revenues from deFEET to us pursuant to an amendment to our license agreement with BioChemics. Under this amendment, we agreed to be responsible for all
22
expenses related to these revenues, including the royalty payments payable by BioChemics to De Feet International, Inc.
Commencing with the next scheduled shipment of our athlete's foot preparation, we will begin marketing that product under our new brand name, Termin8 and/or Xtinguish. We are rebranding and remarketing deFEET primarily because the current license of the deFEET trademark to BioChemics by De Feet International, Inc., pursuant to a settlement and license agreement, dated as of April 20, 2002, is limited. Pursuant to the terms of this agreement, the license from De Feet International to BioChemics to use the DeFEET trademark is exclusive to BioChemics and is non-transferable to any third party, including us. The formulation and functionality of Termin8 and Xtinguish will be identical to that of deFEET. In accordance with our plan to rebrand the deFEET product as Termin8 and/or Xtinguish, BioChemics has ceased to replenish the shelves with deFEET product.
The majority of our net revenues to date have been generated as a result of test marketing projects. Our test marketing projects consist of arrangements we have established with national drug store chains, namely Walgreens, to strategically place products in certain target branches of these chains and then apply various market strategies to these select target markets. For example, in the fourth quarter of 2002, BioChemics began marketing deFEET in the Walgreens pharmacy retail chain. During this time, deFEET was not marketed or distributed in any other drug store chains. The strategies used include the evaluation of advertising medium, pricing and distribution models, the results of which are to be used in future marketing initiatives as we officially launch our products on a large scale basis. The distribution of products in these situations is limited because of its very nature as a test, but results have enhanced and will enhance in the future, our ability to more accurately predict optimum pricing and seasonality of product demand. This will, in turn, allow us to direct our advertising strategy toward a national launch of the brand.
In light of the fact that most of our product shipments to date have been of the test market variety and given that labeled product expiration dates are generally 12 to 24 months past the product shipment date, return activity has not been significant except in the case of the discontinuance of deFEET and its removal from the Walgreens shelves due to our rebranding of our athlete's foot preparation product to Termin8 in September, 2003. We have estimated a return amount of approximately $30,000 which we believe represents the quantity of the deFEET-branded product at retail value residing on shelves at Walgreens that would subsequently be shipped back to the Company. This amount is reflected as a contra revenue amount on our statement of operations for the nine-month period ended September 30, 2003. As of October 13, 2003, we believe all product that will ultimately be returned has been returned.
Cost of Sales. Our costs have consisted of manufacturing costs charged by outside contractors. We expect to continue to use outside contract manufacturers for the production of our products. As a result, all of our inventory balances are in the form of finished goods.
Gross Profit. We anticipate that our gross profit margins will be subject to variations due to the multiple revenue distribution models that are available to us. For example, our gross profit margins on products sold directly through major retail drug store chains and selected pharmacies will likely vary from those realized from sales through joint ventures and licensing relationships with third party companies that have substantial pharmaceutical distribution networks. Accordingly, we expect our overall gross margin ratios to decline as our private label, joint venture, and other strategic licensing alliances grow. However we expect total gross profit to increase as a direct result of this growth.
Expenses. Historically, our marketing, advertising and promotion expenses have varied due to the multiple revenue distribution models that we have used in connection with our test marketing projects. We expect this variation to continue as we implement such multiple revenue distribution models. In addition, marketing, advertising and promotional expenses include expenses related to the
23
commercialization, marketing and distribution of deFEET, the expenses of which we agreed to asume pursuant to an amendment to our license agreement with BioChemics. Our selling, general and administrative expenses consist primarily of selling costs, wages, and general operating costs such as office rent, phone, insurance and utilities. We expect to increase our operating expenses significantly as we implement our business strategy and expand our operating efforts. In particular, we plan on hiring senior personnel to manage our operations, on undertaking advertising and direct mail programs and on entering into marketing and distribution arrangements with third parties in the United States and abroad.
Taxes. Our operating results have historically been included in BioChemics' federal and state income tax returns as part of a division of BioChemics. Effective January 1, 2003, we will be preparing and filing federal and state income tax returns on a stand-alone basis. As a result, we will no longer be included as part of the BioChemics consolidated tax return as a wholly owned subsidiary. We will account for income tax transactions in accordance with Statement of Financial Accounting Standards (SFAS) No. 109,Accounting for Income Taxes, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities, using enacted tax rates in effect for the year in which the differences are expected to be recovered or settled. Under SFAS No. 109, the effect of a change in tax rates on deferred tax assets and liabilities is recognized into income (expense) in the period of the rate change.
Nine Months Ended September 30, 2003 and 2002
Net Revenues. Net revenues decreased 32.2% to $51,876 for the nine-month period ended September 30, 2003 from $76,499 for the nine-month period ended September 30, 2002. This decrease was a direct result of rebranding our athlete's foot product deFEET to Termin8. This strategic development caused Walgreens to return most of the deFEET product that it had in its possession at the time of the decision. This caused a consequential determination that an allowance for returns in the amount of approximately $30,000 would be required and posted as a charge against net revenue. This was a unique one-time occurrence due to the discontinuance of deFEET. The total amount of net revenue prior to this decision was approximately 7% higher during the nine month period ended September 30, 2003 when compared to the nine month period ended September 30, 2002. This was primarily attributable to our test marketing of deFEET in the Walgreens drug store chain. DeFEET represented approximately 96% of the total net revenues for the nine-month period ended September 30, 2003. Although we expected a greater net revenue increase during this nine-month period, the cost of sales per unit as a percentage of net revenue on deFEET were substantially higher during this period due to a 33% pricing discount we gave us to the Walgreens pharmacy retail chain as part of a promotional pricing program to test the effect of a lower retail price for deFEET. Although unit sales of deFEET did increase substantially during this period, the overall net revenue from such sales was not as substantial because of this pricing program. We subsequently determined that a lower retail price did have a positive effect on retail sales of deFEET and we changed the packaging of this product (from individual packets to a single bottle) in order to reduce packaging costs and thus lower the overall price of the product. As a result, the promotional pricing program was ended. It is not anticipated that a similar program will be required in the future with our Termin8 product.
In addition, the reduction in marketing, distribution and sales of our pain relief products during the year ended December 31, 2002 while we were conducting our private label strategic alliance negotiations with a large pharmaceutical company had a negative impact on net revenue from sales of these products in the nine months ended September 30, 2003. Although we do intend to pursue similar private label strategic alliances with other third parties in the future, we do not anticipate that such alliances will be exclusive. Thus, while third parties may distribute and market our products under
24
private labels pursuant to individual distribution agreements, we do not intend to bind ourselves by exclusivity provisions in these agreements. This will allow us to continue to pursue the distribution and marketing of our products on our own or through other strategic alliance partners.
Cost of Sales. All manufacturing costs are outsourced. As a result, cost of sales is limited to those costs incurred and billed by outside contractors. Cost of sales decreased 4.6% to $31,279 for the nine-month period ended September 30, 2003 from $32,779 for the nine-month period ended September 30, 2002. This decrease was primarily due to the decrease in overall net revenue as previously discussed.
Gross Profit. Gross profit as a percentage of net revenues decreased 17.5% to approximately 39.7% for the nine-month period ended September 30, 2003 from a gross profit of 57.2% for the nine-month period ended September 30, 2002. The decline in gross profit was due to a larger portion of our net revenue being made up of deFEET which underwent a promotional price discount of 33% to the Walgreens drug store chain in connection with BioChemics' test marketing of deFEET.
Marketing, Advertising and Promotion Expenses. Marketing, advertising and promotion expenses increased approximately 653% to $87,125 for the nine-month period ended September 30, 2003 from $11,565 for the nine-month period ended September 30, 2002. This was primarily due to increased advertising and marketing as BioChemics ramped up its test marketing of deFEET.
Selling General and Administrative Expenses. Selling general and administrative expenses decreased 13% to $296,538 for the nine-month period ended September 30, 2003 from $340,526 for the nine-month period ended September 30, 2002. The decrease was primarily due to the resignation of certain personnel effective March 31, 2003 who have yet to be replaced.
Years Ended December 31, 2002 and 2001
Net Revenues. We generated no net revenues during the years ended December 31, 2002 and 2001 other than those derived from sales of our pain relief product and athlete's foot product made to retailers and end users through test markets.
Net revenues decreased 8.2% to $91,957 in the year ended December 31, 2002 from $100,209 during the year ended December 31, 2001. The decrease in revenue was primarily due to a shift in focus with respect to our revenue distribution model. More specifically during the year ending December 31, 2002, we actively pursued private label strategic alliances for our pain relief products. A private label strategic alliance is a licensing arrangement in which a third party is given all the rights to package, market and distribute a company's products under the third party's own name label in return for a distributor's fee or royalty fee payable to the licensing company on a per unit basis. During the year ended December 31, 2002, we were engaged in ongoing negotiations with a large pharmaceutical company regarding a proposed private label strategic alliance that would permit that company to distribute and market our pain relief products under its private label. As part of its negotiations with us, that company advised that it would be seeking exclusive rights with respect to distribution and marketing. No formal agreement was executed, but we reduced our efforts to market, distribute or replenish our retail supply of our pain relief products during the negotiation period so as to prevent the excessive cost of recalling these products in the event that the exclusivity rights that company was seeking were granted. Near the end of the year ended December 31, 2002, we determined that we did not wish to grant exclusivity rights to that company with respect to our pain relief products and the negotiations with that company ended. Our marketing and distribution of these products resumed in January 2003. No product recalls occurred as a result of our efforts to pursue these strategic alliances because no private lable strategic alliances were consummated during this period.
25
Cost of Sales. All manufacturing costs are outsourced. As a result, cost of sales is limited to those costs incurred and billed by the outside contractor. Cost of sales increased less than one percent to $40,811 for the year ended December 31, 2002 from $40,791 for the year ended December 31, 2001.
Gross Profit. Gross profit as a percentage of net revenues decreased by 3.7% to 55.6% for the year ended December 31, 2002 from a gross profit of 59.3% for the year ended December 31, 2001. The decline in gross profit was due to a larger portion of the net revenue being made up of the athlete's foot product which only carried a per unit gross profit of 35.5% during the year ended December 31, 2002. This gross profit reduction resulted from a promotional price discount of 33% to Walgreen's pharmacy retail chain that is currently test marketing this product.
Marketing, Advertising and Promotion Expenses. Marketing, advertising and promotion expenses decreased 93% to $38,962 for the year ended December 31, 2002 from $556,614 for the year ended December 31, 2001. The decrease was due to a shift in focus with respect to our revenue distribution model as we pursued private label strategic alliances.
Selling, General and Administrative Expenses. Selling general and administrative expenses decreased 14.8% to $464,832 for the year ended December 31, 2002 from $545,748 for the year ended December 31, 2001. This decrease was due to reductions in travel, meals, sales-commission and outside sales contractors. We also shifted our focus with respect to our revenue distribution model which allowed us to reduce these expenses as we pursued private label strategic alliances.
Liquidity and Capital Resources
Historically, BioChemics has managed cash on a centralized basis. Cash receipts associated with our business have been transferred to BioChemics directly upon receipt and in turn BioChemics has provided funds to cover our disbursements. Accordingly, we had reported no cash or cash equivalents at December 31, 2001 or December 31, 2002, or at September 30, 2002. In accordance with our plan to obtain independent financing as a stand-alone entity, we initiated a private placement of our 10% convertible subordinated pay-in-kind promissory notes in the aggregate principal amount of $500,000. At September 30, 2003, we reported cash of $158,576 received as part of our private placement of $500,000 principal amount 10% convertible pay-in-kind promissory notes. The proceeds from the sale of these notes have been used for funding the expenses related to this offering discussed further below. At the time of this public offering, it is anticipated that the Company will incur a non-cash interest charge of $533,333 as a result of the beneficial conversion feature associated with these convertible notes.
The convertible pay-in-kind promissory notes bear interest at 10% and the principal and accumulated interest are convertible into shares of Class A common stock either voluntarily on or before the maturity date of the notes or mandatorily upon consummation of this offering. These notes are convertible at the lower rate of 50% of the public offering price or $2.50, up to a maximum of 220,000 shares of Class A common stock. Upon the consummation of this offering, these notes will be converted into an aggregate of 214,000 shares of Class A common stock.
On June 20, 2003, our board of directors authorized Vaso Active to make an initial public offering of 1,000,000 shares of its Class A common stock, plus an additional 150,000 shares of Class A common stock to cover over-allotments, if any, at a price per share of $5.00 to a group of underwriters. On August 22, 2003, the board authorized the increase of our initial public offering from 1,000,000 to 1,200,000 shares of our Class A common stock, plus an additional 180,000 shares to cover over-allotments, if any. On October 27, 2003, our board authorized the increase of the offering from 1,200,000 shares of our Class A common stock plus an additional 180,000 shares to cover over-allotments, to 1,300,000 shares of our Class A common stock plus an additional 195,000 to cover over-allotments, if any.
26
During 2001 and 2002, BioChemics funded all of our working capital needs. Accordingly, BioChemics made cash contributions to our capital in 2001 and 2002 to cover our deficits incurred during those years. These contributions totaled $1,040,395 in 2001 and $446,862 in 2002. For the nine months ended September 30, 2003, BioChemics provided financing to us in the amount of $445,822 to fund our operating losses incurred during that nine-month period. We do not anticipate receiving any further financing from BioChemics after the consummation of this offering.
Our independent auditors stated in their "Independent Auditors' Report" on our financial statements as of and for the years ended December 31, 2002 and 2001 that we may be unable to continue as a going concern. If we are not successful in completing this initial public offering or obtaining other sources of funding through product sales or otherwise, we may not be able to fund our operations or support our capital needs and business strategy beyond September 30, 2004. Accordingly our ability to continue as a going concern is in question.
Based on our operating plans, we believe that the proceeds from this offering, together with our cash flow from future operations, will be sufficient to fund our operations and capital expenditures at least through the next 12 months.
Quantitative and Qualitative Disclosures Regarding Market Risk
Interest Rate Sensitivity
As of September 30, 2003, we had cash and cash equivalents of $158,576 that consisted of highly liquid money market instruments with maturities less than 90 days. Because of the short maturities of these instruments, a sudden change in market interest rates would not have a material impact on the fair value of the portfolio. Accordingly we do not expect our operating results or cash flows to be affected to any significant degree by the effect of a sudden change in market interest rates on our portfolio.
Equity Security Price Risk
We do not own any equity investments. Therefore, we do not currently have any direct equity price risk.
Market/Price Risk
We are exposed to market risk in the normal course of business operations. We face competition and must offer our products and services at prices that the market will bear. We believe that we are well positioned with our products such that price risk will not affect our operating results to any significant degree. However, should the prices of our products decline, this could adversely affect our future profitability and competitiveness.
27
BUSINESS
Overview
We are an early-stage company focused on commercializing, marketing and selling over-the-counter, or OTC, pharmaceutical products, with a particular focus on drugs that incorporate the vaso active lipid encapsulated, or VALE, transdermal delivery technology. We began our operations as a division of BioChemics, Inc., a biopharmaceutical company focused on the development of transdermal drug delivery systems, in January 2001. In January 2003, we were formed as an independent subsidiary of BioChemics to further commercialize our OTC products and further develop our OTC product candidates, although BioChemics continues to provide us with administrative support services, manufacturing and development services and technology licenses. By becoming a subsidiary of BioChemics, as opposed to continuing as a BioChemics division, we have facilitated our ability to obtain independent financing for our operations and reduced our exposure to the potential liabilities inherent in the development and manufacturing of pharmaceutical products.
Our parent, BioChemics, is a biopharmaceutical company focused on the development of transdermal drug delivery systems and is based in Danvers, Massachusetts. BioChemics was founded by John J. Masiz in 1989 and incorporated in Delaware in 1991. BioChemics is controlled by Mr. Masiz, its President and Chief Executive Officer. Biochemics began developing the VALE technology in 1989. BioChemics has subsequently been issued three patents on this technology by the U.S. Patent Office. In addition, foreign patents have been issued to BioChemics in 17 foreign countries and are pending in 7 others. Prior to being established as a subsidiary of BioChemics, we operated as a division of BioChemics, responsible for the commercialization, distribution, marketing and sales of BioChemics' OTC products. BioChemics also manufactures and develops prescription drugs through its Xevex Pharmaceuticals division as well as veterinary drugs through its MediVet Pharmaceuticals division.
Although BioChemics was founded in 1989, and incorporated in 1991, it is still a development stage company. BioChemics has never been profitable. Through December 31, 2002, BioChemics had an accumulated deficit of approximately $9.9 million. BioChemics anticipates that it will continue to incur net losses and be unprofitable for the foreseeable future.
BioChemics currently holds patents for the Vaso Active Lipid Encapsulated transdermal drug delivery system. We refer to this as VALE technology. VALE technology can be thought of as a "liquid needle", given its ability to introduce drugs into the bloodstream in an efficient and highly effective manner. We believe that the VALE technology provides an efficient, predictable and reliable transdermal drug delivery system that eliminates the need for a patch and allows for the efficient and effective delivery of a myriad of drugs that could not otherwise be effectively delivered using existing transdermal delivery technology.
We have an exclusive, worldwide license to use and practice the VALE technology in the commercialization, distribution, marketing and sale of products in the OTC pharmaceutical market. We do not currently, nor do we intend to, participate in the manufacturing of, nor conduct any research and development with respect to, any of our products or product candidates. In August 2003, we entered into an agreement with BioChemics with respect to the ongoing manufacturing and development of our products and product candidates. Under this agreement, BioChemics will research, develop and manufacture products for us pursuant to specific purchase orders submitted by us from time to time. BioChemics will charge us a development and manufacturing fee at a rate of cost plus 10%. We will then market, commercialize, distribute and sell these products.
Before we were established, BioChemics initiated the marketing and commercialization of the following products on a limited basis:
- •
- deFEET®, for treatment of athlete's foot fungal infections;
28
- •
- Athlete's Relief, for treatment of pain associated with athletic activity; and
- •
- Osteon®, for treatment of arthritis pain.
Currently, we market Athlete's Relief and Osteon in the United States. We have begun negotiating agreements with third parties for the marketing of Osteon and Athlete's Relief in the United States and internationally. We plan to rebrand the deFEET athlete's foot anti-fungal medication product as Termin8 and/or Xtinguish, depending on targeted markets in the United States. In accordance with this plan, BioChemics has begun to remove all deFEET branded product currently in circulation. We have applied for trademark protection in the United States for the marks Termin8 and Xtinguish. We are rebranding and remarketing deFEET primarily because the current license of the deFEET trademark to BioChemics by De Feet International, Inc., pursuant to a settlement and license agreement, dated as of April 20, 2002, is limited. Pursuant to the terms of this agreement, the license from De Feet International to BioChemics to use the DeFEET trademark is exclusive to BioChemics and is non-transferable to any third party, including us. The formulation and functionality of Termin8 and Xtinguish will be identical to that of deFEET. During this transitional period, we do not expect that we will realize significant revenues from sales of our products.
In addition to expanding our launch of these three products, we have identified and are currently developing, in collaboration with BioChemics, six additional OTC products utilizing the VALE technology. Our current OTC product candidates according to their medical or clinical indication are:
- •
- Analgesic;
- •
- Toenail fungus treatment;
- •
- Acne treatment;
- •
- First aid treatment;
- •
- Hand and body lotion; and
- •
- Psoriasis treatment.
Traditional Transdermal Drug Delivery Technologies
Transdermal drug delivery is any process of delivering drugs into or through the skin without requiring the use of an invasive instrument such as a needle. Transdermal drug delivery offers many advantages over most other commonly accepted modes of drug delivery for the treatment of many diseases and medical conditions, including, the following:
- •
- Reduction/Elimination of Route-of-Administration Related Drug Side Effects. Some orally administered medicines can cause significant side effects, often leading to discontinuation of the medication. For example, long-term use of non-steroidal anti-inflammatory drugs, or NSAIDS, can cause bleeding and ulceration of the stomach lining. These side effects are responsible for many deaths and hospitalizations of patients annually. A transdermal application of these same medications will significantly reduce the incidence of gastrointestinal side effects. There are also side effects associated with the delivery of a drug through the nasal lining, the lungs and the skin. These include bleeding, irritation to the linings of the airways, and scar tissue formation at the sites of administration. A broad-based transdermal delivery system, such as the VALE transdermal delivery system, can reduce or eliminate these problems.
- •
- Improved Drug Performance. Transdermal medications have the potential to improve the effectiveness of a drug by avoiding the stomach and the first-pass metabolism associated with oral delivery of drugs. Transdermal drug delivery has the potential to create a higher bioavailability index for drugs, allowing for a higher concentration of the drug molecule in the bloodstream from a smaller dose of drug applied to the patient, as compared to oral drug
29
Limitations of Traditional Transdermal Drug Delivery Technologies
There are many different technologies used to deliver drugs transdermally. The most common technologies employed use either (i) patches that adhere to the skin, holding a drug in place while it is administered over time (ii) liposomes, or artificially prepared cell-like structures, which are applied topically and absorbed or (iii) an outside energy source producing electricity, otherwise known as iontophoresis, or sound, otherwise known as sonophoresis, to help move the drug through the various skin layers. These traditional technologies tend to be of more limited utility, are not very effective and, in the case of ionophoresis and sonophoresis, expensive:
Table 1: Performance Evaluation Summary of Transdermal Drug
Delivery Technologies including VALE
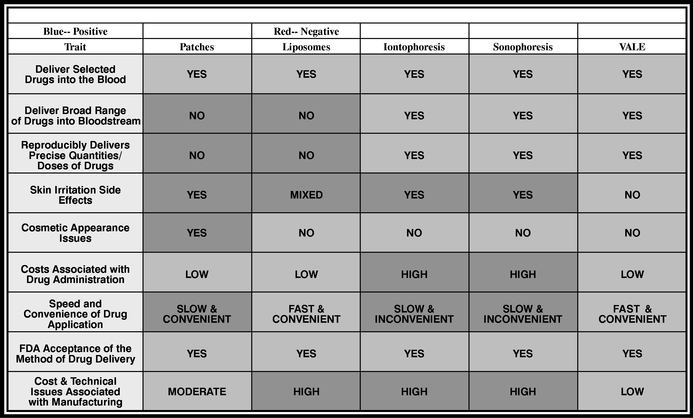
Limitations of Traditional Patch Technology
Patch technology works by holding a drug in constant contact with the outer skin to deliver a specific drug dosage. Over substantial periods of time, these drug molecules will be passively absorbed through the various skin layers and eventually migrate into the bloodstream. Patch technology has been used for the delivery of nicotine, in an effort to assist smokers in quitting; the delivery of nitroglycerine
30
to angina sufferers; the delivery of replacement hormones in post-menopausal women; and to treat fewer than 10 other medical disorders.
Traditional patch technology has several limitations:
- •
- Because of their mass, structure and solubility, the skin poses a significant barrier to most active drug molecules. Since patches are a passive system, overcoming this barrier is difficult and often inefficient. Most drug molecules used in patient treatment are too large to be effectively delivered using patch technology.
- •
- Real world conditions such as the patient's physical size, metabolism and circulatory efficiency can prevent drug delivery from occurring through the use of only a patch.
- •
- Due to the slow flow rate of drug molecules that are delivered using a patch, such technology can be used only for treatments that permit long treatment periods.
- •
- Patch adhesion to the skin can cause extensive skin trauma as well as cosmetic problems. Most of the adhesives that are currently used in patches tend to aggressively adhere to the skin so that their removal may cause irritation and trauma. Subsequent patches used by the same individual are often applied to a different area of the skin in order to minimize such irritation and trauma.
Our Solution
The goal behind the development of the VALE system was to develop an active transdermal drug system that addresses the deficiencies present in traditional technology and to create a system that efficiently and effectively delivers drugs through the skin and into the blood supply without the need of a patch. In order to efficiently and effectively deliver a drug transdermally, the delivery system must be able to overcome three skin barriers.
- •
- the skin's outer layer, or epidermis;
- •
- the second layer, the dermis, which is composed of connective tissue, glands and hair; and
- •
- the walls of individual capillaries, tiny, thin-walled blood vessels that transport blood to the larger veins and arteries of the body.
The VALE system is a patchless, lipid-based delivery system, which uses an active, as opposed to a passive, process to deliver drugs into the bloodstream. It has been developed around the unique concept of incorporating chemical vasodilators into the drug delivery vehicle. These chemical compounds act on the network of blood vessels located near the surface of the skin and induce dilation of the blood vessels in the immediate area. This, in turn, has the immediate and natural effect of increasing the blood flow to the area. As blood flow increases and the blood vessels dilate (up to 300% above normal state) the active drug molecules incorporated into the delivery system are transported actively and efficiently into the bloodstream, as demonstrated in Fig. 1.
We believe this chemical vasodilator is the operative key to a broadly applicable transdermal drug delivery system. This process "unlocks" this final barrier and promotes the efficient and complete transportation of drugs from the dermis into the blood. It provides an efficient, predictable and reliable transdermal drug delivery system that eliminates the need for a patch and allows for the efficient and effective delivery a myriad of drugs that can not be effectively delivered transdermally using prior transdermal drug delivery technology.
31
Fig. 1: BioChemics' VALE Delivery System
(VALE's Operation through a Skin Cross Section)
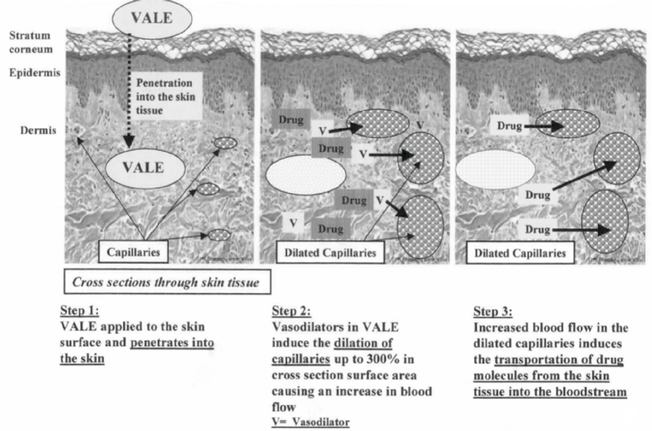
Strategic Agreements
License Agreement
Our parent, BioChemics, demonstrated that vasodilation is a critical physiological element that enables transdermal drug delivery to be an effective and efficient process. Following the development of this general concept and the distillation of the concept to practice, John Masiz submitted a series of patent applications to legally establish him as the inventor of the process. Subsequently, Mr. Masiz was issued three U.S. patents relating to the VALE transdermal drug delivery system: Patent Nos. 5,460,821 (October 24, 1995), 5,645,854 (July 8, 1997), and 5,853,751 (December 29, 1998), each under the title, Molecular Transdermal Transport System.
Mr. Masiz assigned these patents to BioChemics in March 2002. The first of these patents expires in 2013. Internationally, BioChemics has been issued foreign patents in 17 foreign countries related to this intellectual property and has patents pending in seven others.
We have entered into a license agreement with BioChemics under which we have a worldwide, exclusive right to use and practice BioChemics' patents to sell, market and commercialize all products that (i) utilize and incorporate BioChemics' patents and know-how; (ii) are classified by the FDA as "over-the-counter products"; and (iii) require less than $1.0 million of clinical development. Specifically excluded from the license are certain diabetic neuropathy products developed by BioChemics which are the subject of a clinical study being conducted at the Jocelyn Diabetes Center in Boston. All rights to use and practice BioChemics' patents for the purposes of manufacturing and developing OTC Products are held by BioChemics pursuant to the license agreement. In exchange for the granting of the license, we have agreed to issue an aggregate of 1,500,000 shares of our Class B common stock to BioChemics as a license fee. This is a one-time license fee and there are no royalties payable to BioChemics under the license agreement. These shares will be issued and outstanding before the consummation of this
32
offering. Pursuant to the terms of the license agreement, BioChemics retains all rights to use and practice the patents for all purposes with respect to non-OTC products, including pharmaceuticals and pet products. Any improvements to the patents remain the property of BioChemics, but to the extent that any such improvements relate to our products and product candidates, the improvements are licensed to us on the same terms as the initial license under the license agreement. Maintenance of the patent filings remains the responsibility of BioChemics, although we maintain the right under the license agreement to make any required filings at BioChemics' expense in the event that such filings are not made on a timely basis by BioChemics. The license agreement is subject to termination on the date of the last to expire patent subject to the agreement.
BioChemics is also party to a settlement agreement and license with De Feet International, Inc., dated April 20, 2002, whereby De Feet International, Inc. granted a non-exclusive, non-transferable and non-assignable license to BioChemics to use the "deFEET" trademark throughout the United States in connection with the manufacture, advertisement, promotion, distribution and sale of athlete's foot preparations.
Pursuant to an amendment to license agreement, dated July 2, 2003, the trademarks Osteon and PENtoCORE, together with the goodwill associated with each mark, were licensed to us by BioChemics. In addition, pursuant to the amendment, BioChemics assigned to us its ownership of any and all revenue associated with the sale, commercialization and marketing of deFEET by BioChemics and we agreed to be responsible for all expenses related to these revenues, including the royalty payments payable by BioChemics to De Feet International, Inc. Pursuant to the terms of this amendment to license agreement, the nature and quality of all services rendered in connection with the Osteon and PENtoCORE marks remains under the control of BioChemics.
Development and Manufacturing Agreement
In August 2003, we entered into a development and manufacturing agreement with BioChemics with respect to the ongoing manufacturing and development of our products and product candidates. Under this agreement, BioChemics will research, develop and manufacture products for us pursuant to specific purchase orders submitted by us from time to time. BioChemics will charge us a development and manufacturing fee at a rate of cost plus 10%. The development and manufacturing agreement permits BioChemics to use third party contractors to manufacture and produce the products that BioChemics provides to us thereunder. Currently, BioChemics uses a privately held third party company as its sole contract manufacturer. We do not currently, nor do we intend to, engage in the manufacturing of, nor conduct any research and development with respect to, any of our products or product candidates.
Our Products and Product Candidates
Current Products
Before entering into the license agreement with us, BioChemics initiated a marketing campaign on a limited basis for the three OTC products deFEET, Athlete's Relief and Osteon. The following table summarizes information regarding these products, including a description of the medical condition that
33
each product was developed to treat, as well as the total annual size of the market in which that respective product competes or will compete.
Drug
| | Medical Condition
| | Total Annual
Market Size*
|
|---|
| deFEET | | Treatment of athlete's foot fungal infections | | $310 million** |
| Athlete's Relief | | Treatment of pain associated with athletic activity | | $278 million*** |
| Osteon | | Treatment of arthritis pain | | $278 million*** |
- *
- Based on 2002 MMR/Information Resources Inc. Health and Beauty Aids Report, these total annual market size estimates represent the approximate total annual market size for treatment of these medical conditions in the U.S.
- **
- Total estimated market size of foot care medication market, which would include deFEET.
- ***
- Total estimated market size of external analgesic market, which would include both Athlete's Relief and Osteon.
Osteon and Athlete's Relief will now be marketed, distributed, commercialized and sold by us in the United States. During the course of the 12 month period immediately following this offering, all deFEET branded product currently in circulation will either be sold or removed from circulation and replaced with an identical product which will be commercialized, marketed and sold by us under the name Termin8 and/or Xtinguish. The formulation and functionality of Termin8 and Xtinguish will be identical to that of deFEET.
All three of these products have been through the research and development, pre-clinical study and clinical trial stages and have received FDA approval. They are either currently being marketed and commercialized or will enter the marketing and commercialization phase upon the consummation of this offering.
deFEET is a topically-applied, transdermal athlete's foot anti-fungal medication designed to eliminate athlete's foot infection in less than 10 days. It employs the VALE drug delivery system to effectively and efficiently deliver a mild antifungal agent called Tolnaftate into the skin surrounding the affected area. In a pilot clinical trial, supervised by independent physicians and analyzed by the New England Medical Center in Boston, MA, 20 severely infected athlete's foot patients were treated and studied over a 42-day period. There were two groups in the study, one treated with deFEET and the other with Schering-Plough's Tinactin®. In this study, deFEET eliminated the infection in 90% of the test group in 7 days and 100% of its patient population in 10 days. Tinactin®, which also uses Tolnaftate in the same concentration as deFEET, required 42 days to cure its first patient. These results demonstrate the ability of the VALE technology to deliver Tolnaftate much more effectively than a product not utilizing VALE technology (see Table 1).
34
Table 1—Comparison of Clinical Effectiveness of
deFEET and Tinactin
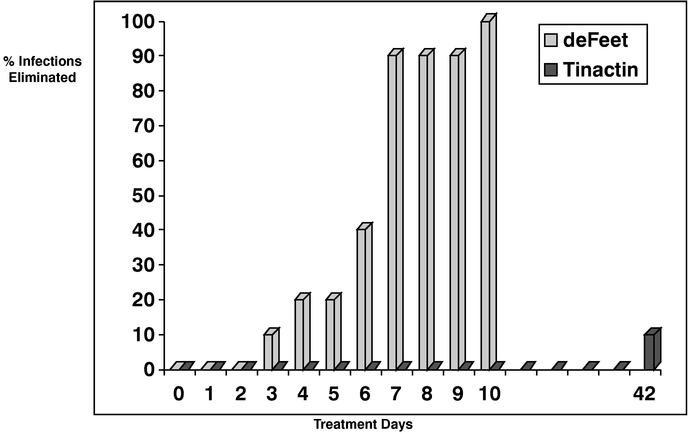
The foot care medication market is large and well established. Approximately $310 million is spent annually on OTC athlete's foot treatments. The market is dominated primarily by three products - Schering-Plough's Lotramin AF and Tinactin, which collectively account for approximately $95 million in sales annually; and Novartis' Lamisil AT, which accounts for approximately $44 million in annual sales. The next 7 most popular foot care medication products average about $11 million each in annual sales. We believe that the superior performance of our athlete's foot medication, as demonstrated in Table 1 above, will allow us to penetrate this market and build a high degree of product loyalty, which we do not believe the current market leaders enjoy. This belief is based on our discussions with members of the podiatric profession. We intend to position our product as a "super-powered" alternative to Lotramin AF and Tinactin. This will enable us to capitalize on the brand recognition of the market leaders, while at the same time differentiating our product through its superior performance.
Osteon is a topically-applied, transdermal arthritis pain reliever designed to relieve pain within 10-15 minutes of application and last for up to 6 hours. We have received favorable consumer and physician comments that Osteon compares favorably to prescription NSAIDS in relieving muscle and joint pain, particularly arthritis pain, without the drug-induced side effects. The annual market for OTC pain relievers and analgesics in the United States is approximately $3.1 billion, while the OTC external analgesic market is approximately $278 million. We believe that the population of 45-64 year olds will grow significantly in the next decade. As the "graying" of the baby-boomer demographic continues, we believe that the market for arthritis pain relief products will continue to grow and that we will be able to position Osteon as a competitive product in this market.
Athlete's Relief is a topically-applied, transdermal muscle and joint pain treatment designed to relieve the pain associated with athletics and physical exertion. Competitive products in this category rely on a chemical induction of the sensation of intense heat or cold on the skin surface. This chemical induction is designed to mask the sensation of pain. In contrast, Athlete's Relief uses the VALE transdermal drug delivery technology to deliver a natural chemical substance that inhibits the release of
35
specific neurotransmitters that are responsible for transmitting the pain signals to the brain. The result is a true blockage of the pain sensation. The difference between our products and others on the market is our ability to use the VALE technology and thus gain access to the deep tissue nerve endings in order to block their activity. The annual OTC external analgesic market, of which sports injury pain medication is a component, is approximately $278 million in the United States. We believe that our Athlete's Relief will provide an alternative treatment for pain associated with athletics and physical exertion.
Although Osteon and Athlete's Relief both occupy the external analgesic market, they each target different and specific segments of that market. Osteon is marketed to arthritis sufferers—primarily 45-64 year olds—while Athlete's Relief targets a younger demographic who tend to use analgesics to treat muscle and joint pain attributable more to athletics and physical activity than to chronic conditions such as arthritis. For these reasons, we believe that these two products do not and will not adversely effect each other's sales.
Product Candidates
Although all of our product candidates will be entering established markets, they will be distinct and unique among existing products in those markets in that they will all incorporate the VALE technology. We intend to market these product candidates using this distinction in much the same way as our current products have been marketed. Since these product candidates are in the early development stage, we are not able to predict when these product candidates will be ready for distribution and sale, if ever.
Our current product candidates include:
Product Classification
| | Product Description
| | Total Annual Market
Size*
| | Development
Stage
|
|---|
| Analgesic | | Topically applied analgesic | | $3.1 billion | | Formulation being finalized |
Toenail fungus treatment |
|
Termin8/Xtinguish derivative topically applied to treat toenail fungus |
|
$800 million |
|
Formula finalized |
Acne treatment |
|
Topically applied, anti-bacterial, anti-inflammatory Transdermally delivered Hydrocortisone |
|
$325 million |
|
In development |
First aid treatment |
|
Transdermally delivered anti-bacterial and Hydrocortisone |
|
$395 million |
|
Formula finalized |
Hand and body lotion |
|
OTC skin hydration |
|
$1.06 billion |
|
Formula finalized |
Psoriasis |
|
Transdermal anti-inflammatory, anti-itch and skin hydration |
|
— |
|
Formula in development |
- *
- Based on the 2002 MMR/Information Resources Inc. Health and Beauty Aids Report, these total annual market size estimates reflect U.S. retail sales by product classification.
Our Strategy
We believe that we can commercialize a broad range of products by applying the VALE technology to a significant array of currently off-patent drugs and by leveraging BioChemics' ability to manufacture and develop these drugs for commercialization in the OTC marketplace.
We will expand the marketing and commercialization of Osteon and Athlete's Relief, initiate our marketing and commercialization of Termin8 and Xtinguish and initiate roll-outs for our existing and future product candidates using the strategic marketing and commercialization plan described below.
36
We intend to utilize a portion of the offering proceeds to implement this plan, which is focused on the systematic rollout of the products into major retail drug store chains, select independent pharmacies, and nontraditional channels, including multilevel marketing, direct marketing, infomercials, websites and catalogues. The methodical and multi-phased launch for each product will allow us to maintain control over the required costs associated with these launches, and will maximize the return on investment for the advertising and marketing expenditures.
Our Global Commercialization and Marketing Strategy
We intend to undertake an aggressive marketing and commercialization strategy that will focus on obtaining rapid market penetration and establishing consumer brand recognition and loyalty with manageable capital expenditures. This strategy will require us to utilize a significant portion of the offering proceeds. These proceeds will be used to fund our advertising and direct mail programs and to enter into marketing and distribution and joint venture agreements with third parties in the United States and abroad.
Our global marketing strategy consists of dividing the world markets into four primary territories:
- •
- North America;
- •
- Asia;
- •
- Europe; and
- •
- South America.
In North America, our marketing strategy is bifurcated. In many instances, we will seek joint venture partners to participate with us in the sales and distribution of our products. This will most likely occur with respect to products that are entering an already crowded market and thus need substantial distribution and marketing might to achieve significant market penetration. For instance, the topical arthritis and muscle and joint pain markets are very crowded. In this instance, we feel that substantial marketing and commercialization, beyond what we would be able to accomplish alone, must be carried out in order to provide our products with greater visibility in the marketplace. In situations where our products will be entering markets that are more open, or where such products already have some market presence, our strategy will be to retain all distribution and marketing rights and market the products ourselves without the assistance of third parties.
In the non-North American markets we intend to establish joint venture and licensing relationships with partners that have substantial pharmaceutical distribution networks already established in those regions.
We currently market, distribute and sell products only in North America. We are currently engaged in negotiating marketing and distribution arrangements for our products and product candidates in Europe and Asia and we are seeking similar arrangements in the rest of the world. These discussions have not yet been finalized. As a result, we do not know at this time when we will be ready to introduce our products into these additional markets.
Overview of Roll-Out Strategy
Our roll-out strategy is two tiered:
The first phase is to place our products in a critical mass of stores on a national basis. Once a sufficient number of stores have the product on their shelves, the consumer marketing phase can begin. BioChemics has secured placement of the deFEET product in the Walgreens pharmacy chain. Walgreens has 4,000 stores nationwide and we believe that deFEET has been placed throughout the entire chain. For the nine months ended September 30, 2003, sales of deFEET amounted to
37
approximately $50,000, or 96% of our total revenues for that period. In accordance with our plan to rebrand the deFEET product as Termin8 and/or Xtinguish, BioChemics has begun to remove deFEET product from the Walgreens stores.
The second phase of our strategy consists primarily of a mix of print and radio advertising. Termin8's and Xtinguish's print campaigns will be placed in print media targeting males between the ages of 25 and 55. For radio advertising, we intend to purchase radio spots primarily from large radio station owners such as Infinity Radio which appeal to sports markets. We plan to spend approximately $1.2 million from the net proceeds of this offering to fund our radio advertising campaign.
In addition to print and radio advertising, we intend to launch a promotion campaign to podiatrists and dermatologists that would allow these specialists to sell our Termin8 and/or Xtinguish brands directly to their patients. We plan to build a team of sales representatives to launch this initiative.
We have also created a Sports Division which focuses on telemarketing to professional and collegiate sports teams, health clubs, and golf and tennis clubs. As a result of these efforts, many National Football League and National Hockey League teams, and many major college athletics programs are currently using deFEET. In addition, we have designed promotional campaigns targeted toward running, cycling, and ski clubs.
We believe that this is an effective commercialization and marketing strategy for our products and that it can be applied, followed and, if required, modified on a going forward basis as we roll out additional product candidates.
Government Regulation
Our business is focused on, and limited to, the commercialization, marketing, distribution and sale of OTC products and product candidates. Accordingly, we are required to conform to the standard FDA labeling monographs with respect to the ingredients, or combinations of ingredients, that are used in these products and follow the monograph specifications established by the FDA with respect to marketing. Because we are not involved in the development and manufacturing of these products, we are not, and will not be, subject to the more extensive regulations imposed by the FDA and other regulatory authorities in the United States and other countries regarding research and development, pre-clinical studies and clinical trials and ultimately manufacturing of OTC products. BioChemics is subject to these regulations and is required to conform to them at all times pursuant to the terms and conditions of its manufacturing and development agreement with us.
Competition
We are engaged in a rapidly evolving field. Competition from numerous pharmaceutical companies including, but not limited to Pfizer, Bristol-Myers Squibb, Schering-Plough, and biotechnology companies including, but not limited to, Alza, Cygnus and Elan, as well as research and academic institutions, is intense and expected to increase. The market for transdermal drug delivery systems is large and growing rapidly and is likely to attract new entrants. Numerous biotechnology and biopharmaceutical companies have focused on developing new drug delivery systems and most, if not all of these companies, have significant financial resources and development capabilities.
It is our opinion that competing transdermal drug delivery technologies using patches, liposomes, and equipment assisted deliveries such as iontophoresis and sonophoresis have some utility with a small group of select drugs but are thus far not generally useful for most drugs. Our success will depend on our ability to leverage the VALE transdermal delivery system to achieve market share at the expense of our existing and future competitors who cannot offer a delivery system of comparable effectiveness and efficiency.
38
Our current competitive position is not substantial. However, we intend to compete in these large markets and against the current market leaders by using advanced technology to produce more effective and faster acting products. Footcare products such as Lotramin AF, Tinactin and Lamisil AT do not use transdermal drug delivery technology and are thus, in our opinion, less efficient and effective than our athlete's foot anti-fungal medication products. Competing transdermal drug delivery technologies using patches, liposomes and equipment assisted deliveries such as ionophoresis and sonophoresis, while having some utility with a small group of select drugs, are generally not considered useful for the delivery of most drugs. Our competitive success will depend on our ability to leverage the VALE technology to achieve market share at the expense of our existing and future competitors who cannot offer a drug delivery system of comparable effectiveness and efficiency.
Employees
As of September 30, 2003, we employed seven full-time persons. We also employed three part-time persons, who also devote a portion of their time to Biochemics.
Facilities
Currently, we share office space with BioChemics who, in addition to office space, also maintains laboratory and manufacturing facilities in Danvers, Massachusetts. We currently have no lease agreement with BioChemics. We intend to obtain independent office space, likely at the same location, in the near future and will expand our facilities as the need arises.
Legal Proceedings
We are not a party to any legal proceedings.
39
MANAGEMENT
Executive Officers and Directors
Set forth below is the name, age position and a brief account of the business experience of each of our executive officers and directors:
NAME
| | AGE
| | POSITION
|
|---|
| John J. Masiz | | 44 | | Chief Executive Officer, President, and Chairman of the Board |
| Kevin J. Seifert | | 43 | | Chief Operating Officer |
| Stephen G. Carter, Ph.D. | | 50 | | Chief Scientific Officer, Director |
| Joseph Frattaroli | | 41 | | Chief Financial Officer |
| Bruce A. Shear | | 48 | | Director |
| Brian Strasnick, Ph.D. | | 50 | | Director |
| Robert Anderson | | 47 | | Director |
| Gary Fromm, Ph.D. | | 69 | | Director |
| William P. Adams, M.D. | | 57 | | Director |
John J. Masiz. Mr. Masiz has served as our Chief Executive Officer, President and Chairman of our board of directors since our inception. In addition, Mr. Masiz has served as the Chief Executive Officer and Chairman of BioChemics since 1989. From 1987 to 1990, Mr. Masiz served as the Chief Operating Officer of Linsco Financial Group (now named LPL Financial), currently one of the largest private brokerage firms in the United States. From 1985 to1987, Mr. Masiz was with the strategic consulting group of Coopers & Lybrand. Before joining Coopers & Lybrand, Mr. Masiz served as a financial analyst with Hannaford Bros., Inc., including its Shop & Save and Welby Drug groups. Mr. Masiz is a cum laude graduate of Colby College, with distinction in two majors. He received a Juris Doctorate degree from Suffolk University Law School and is a member of the Massachusetts Bar Association.
Kevin J. Seifert. Mr. Seifert joined the Company as our Chief Operating Officer on November 1, 2003. Mr. Seifert was employed with Becton, Dickinson and Company, a medical technology and manufacturing company, from 1997 to October 31, 2003 in various management positions. From July 2000 until his resignation, Mr. Seifert acted as President of Becton, Dickinson's Consumer Healthcare division. As President, Mr. Seifert oversaw global consumer product production and sales of from Becton, Dickinson's core business including the diabetes franchise. From 1996 to 1997, Mr. Seifert acted as Founder and Executive Vice President of Telergy, Inc., a broadband telecommunications service provider. From 1990 to 1996, Mr. Seifert was the Vice President of Marketing and Sales and Investor Relations for Bio-Plexus, Inc., a medical device manufacturer. From 1989 to 1990 Mr. Seifert acted as Vice President of Advanced Pulmonary Technology, Inc., a manufacturer of life support ventilators. From 1985 to 1989, Mr. Seifert was a Product Development Manager for Sage Products, Inc., a manufacturer and developer of medical and healthcare products. Mr. Seifert holds 4 medical device patents. He received his Bachelors of Science degree in Marketing from New Hampshire College in May 1984.
Stephen G. Carter. Ph.D. Dr. Carter has served as our Chief Scientific Officer since June 2003. He has served as a member of our board of directors since June 2003. From September 1999 to the present, Dr. Carter has also served as Chief Scientific Officer of BioChemics. From April 1999 to September 1999, Dr. Carter was a self-employed consultant in the pharmaceuticals industry. From January 1996 to April 1999, Dr. Carter served as the Dean for Research at Harvard Medical School
40
with responsibilities for the funding of all HMS research activities and for initiation and implementation of several new Harvard-wide research programs. From 1988 to 1996, Dr. Carter served in various executive positions with Glaxo/Glaxo Wellcome, including Head of U.S. Virology Research with a responsibility for biotechnology and academic alliances in drug delivery and gene therapy/genetics/genomics. From 1986 to 1988, Dr. Carter led a group of scientists and conducted research at the National Cancer Institute-Frederick Cancer Research Facility. From 1985 to 1986, Dr. Carter also served as a Research Faculty member at the Yale University School of Medicine in the Department of Molecular Biophysics and Biochemistry and as a NIH postdoctoral fellow in Molecular Virology with the Department of Human Genetics at Yale University. Dr. Carter received his Ph.D. from Iowa State University, Department of Biochemistry & Biophysics.
Joseph Frattaroli. Mr. Frattaroli has served as our Chief Financial Officer since June 2003. From January 2002 through April 2003, Mr. Frattaroli served as an accounting and financial consultant to Biochemics. From April 2000 through December 2001, Mr. Frattaroli was chief financial officer of Getov Machine Incorporated, a machined products manufacturer. From January 1998 through April 2000, Mr. Frattaroli served as managing principal for the EPI group, a consulting firm that provided per diem chief financial officer services as well as tax preparation and compliance services. From March 1993 through January 1998, Mr. Frattaroli served as both an audit manager and tax director for Tucci and Roselli, Certified Public Accountants. From April 1989 through March 1993, Mr. Frattaroli served as tax department manager for Lockheed Martin Corporation. From May 1987 through April 1989, Mr. Frattaroli served as tax department senior for Ernst and Young, certified public accountants. From October 1985 through May 1987, Mr. Frattaroli served as a program field Revenue Agent for the Internal Revenue Service. Mr. Frattaroli holds a Bachelor of Science Degree in Business Administration from Salem State College and is a certified public accountant licensed in New Hampshire.
Bruce A. Shear. Mr. Shear has served as a member of our board of directors since June 2003. Mr. Shear is the co-founder, President and chief executive officer of PHC, Inc., a publicly traded nationally recognized behavioral health company. Mr. Shear has served PHC since 1979. Prior to his current position, Mr. Shear served as vice president of financial affairs. Mr. Shear has served on the Board of Governors of the Federation of American Health Systems for over 15 years. In addition, Mr. Shear previously served on the Board of Trustees for the National Association of Psychiatric health systems, which represents the nation's behavioral health hospitals and organizations. Mr. Shear received an MBA from Suffolk University in 1980 and a B.S. in Accounting and Finance from Marquette University in 1976.
Brian J. Strasnick, Ph.D. Dr. Strasnick has served as a member of our board of directors since June 2003. Dr. Strasnick is the founder, chairman, president and chief executive officer of Willow Laboratories, one of New England's leading drug testing facilities. Dr. Strasnick has served that company since September 1995. Dr. Strasnick has provided behavioral healthcare and pharmacological services as a practitioner for over 18 years. His educational background is in pharmacology and psychology with an emphasis in substance abuse treatment. A nationally recognized expert, Dr. Strasnick lectures throughout the country as a National Visiting Professor for a national pharmaceutical company. He has extensive experience in pharmacology and metabolism of prescription medications and their effects on drug testing. Dr. Strasnick is a diplomat of the American College of Forensic Examiners as well as a fellow of the College of Pharmacological and Apothecary Sciences. Dr. Strasnick also serves on the Board for the Drug and Alcohol Testing Industry Association (DATIA).
Robert E. Anderson. Mr. Andersen has served as a member of our board of directors since June 2003. From January 1998 to the present, Mr. Anderson has been a self-employed consultant and investor. From January 1995 to December 1997, he served as a senior managing director-financial at
41
Furman Selz LLC a financial services firm. From March 1989 to November 1994, Mr. Anderson was a senior vice president with Lehman Brothers. From September 1982 to March 1989, he was a vice president with Goldman Sachs. Mr. Anderson received his A.B. in Economics from Brown University in 1978 and an MBA with Distinction from Harvard Business School in 1982.
Gary Fromm, Ph.D. Dr. Fromm has been a member of our board of directors since June 2003. Dr. Fromm now serves as chairman and has served as a director of IDC Financial Publishing, a leading analyst of government reporting financial institutions, since 1983. He has served as a director to Sky Venture Capital, Inc., a financial services firm, and Sky Capital Holdings Limited, also a financial services firm, since April 2003. He has served as Chairman of Sky Capital UK Limited since June 2003. Dr. Fromm has also served as a director of Neoterik Health Technologies, Inc. since 1984. Dr. Fromm is also co-founder and president of Investment Intelligence Systems Corporation and co-founder of Data Resources, both of which are market data distribution companies. Dr. Fromm holds an A.M. and a Ph.D in Economics from Harvard University, an M.S. in Industrial Management from M.I.T. and a B.M.E. in Mechanical Engineering from Cornell University.
William P. Adams, M.D., F.A.C.S. Dr. Adams has been a member of our board of directors since June 2003. Dr. Adams has served as the president and a director of the Adams Center for Aesthetic Surgery, in Boston, Massachusetts for more than five years. Dr. Adams is a Diplomate of the American Board of Plastic Surgery and a Fellow of the American College of Surgeons. He is also a member of the American Society of Plastic and Reconstructive Surgeons, the American Society for Aesthetic Plastic Surgery and serves as a Clinical Associate Instructor in Surgery at Massachusetts General Hospital and Harvard Medical School. Dr. Adams is a graduate of Dartmouth College and Harvard Medical School.
John J. Masiz and Dr. Stephen Carter are also directors and executive officers of BioChemics. Robert Anderson is also a director of BioChemics. BioChemics has no other directors. We expect that Mr. Masiz will spend approximately 70% of his business time on behalf of Vaso Active and that Dr. Carter will spend approximately 30% of his business time on behalf of Vaso Active. We expect that Mr. Masiz and Dr. Carter will spend the balance of their respective business time on behalf of BioChemics. As of January 2004, Kevin Seifert will act as Chief Operating Officer of BioChemics, in addition to acting in his capacity as Chief Operating Officer of Vaso Active. We expect that Mr. Seifert will spend approximately 70% of his business time on behalf of Vaso Active and the balance of his business time on behalf of BioChemics.
Board Composition
Our charter provides that candidates for directors may be nominated only by the board or by a stockholder who gives written notice to us in the manner prescribed by our bylaws. The number of directors may be fixed by resolution of the board from time to time. The board currently consists of seven members—John J. Masiz, Dr. Stephen Carter, Bruce A. Shear, Dr. Brian Strasnick, Dr. Gary Fromm, Dr. William Adams and Robert Anderson. The board may appoint new directors to fill vacancies or newly created directorships between stockholder meetings. Our charter does not provide for cumulative voting at stockholder meetings for election of directors. A director may be removed from office with cause by the affirmative vote of at least 75% of all eligible votes present in person or by proxy at a meeting of stockholders at which a quorum is present or without cause by the affirmative vote of 75% of all eligible votes present in person or by proxy at a meeting of stockholders at which a quorum is present, provided that removal without cause is recommended to the stockholders by the board pursuant to a vote of not less than 75% of the directors then in office. Any action required or permitted to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by written consent.
42
Board Committees
Audit Committee. We have established an audit committee to be responsible for monitoring and overseeing our accounting and internal financial controls, appointing, compensating and overseeing the work of our independent auditors, meeting with our officers regarding our financial controls, acting upon recommendations of our auditors and taking further action as our audit committee deems necessary to complete an audit of our books and accounts. Our audit committee also performs other duties as may from time to time be determined such as set forth in our code of ethics, including review of related party-transactions. Our audit committee is composed of two independent directors, each with experience in acting as directors of public companies. Our audit committee currently consists of Mr. Shear and Dr. Strasnick. The board has determined that Mr. Shear qualifies as an "audit committee financial expert" as that term is defined in relevant Securities and Exchange Commission rules.
Compensation Committee. We have also established a compensation committee that reviews and approves the compensation and benefits of our executive officers, administers our stock incentive plan, makes recommendations to the board of directors regarding these matters and performs other duties as may from time to time be determined by the board. Our compensation committee currently consists of Mr. Shear and Dr. Strasnick.
Compensation Committee Interlocks and Insider Participation. None of our executive officers serves on our compensation committee. None of our executive officers serves on the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board or our compensation committee.
Director Compensation
Following the consummation of this offering, our board members will receive annual compensation in the amount of $5,000 for service as a board member at each subsequent annual meeting of our stockholders. In addition, board members will receive the sum of $500 for each board meeting that they attend and will be reimbursed for all reasonable expenses incurred by them in relation to attending the board meeting. Our non-employee directors will also receive compensation in the form of stock options issued under our 2003 non-employee director compensation plan. Under this plan, each non-employee director is entitled to receive an option to purchase 20,000 shares of our Class A common stock. Upon consummation of this offering, Bruce A. Shear, Brian Strasnick, Robert Anderson, Dr. Gary Fromm and Dr. William P. Adams, each as non-employee directors, will receive an option to purchase 20,000 shares of our Class A common stock at an exercise price equal to the initial public offering price, or $5.00 per share. These options have terms of ten years and will vest 25% immediately and 25% on each subsequent annual anniversary date of the date of grant.
Executive Compensation
The following table shows the estimated annual compensation to be paid by us for the year ending December 31, 2003 to our Chief Executive Officer and our other executive officers, collectively referred to as the Named Executive Officers.
43
Summary Compensation Table
Name and Principal Position
| | Year
| | Salary
| | Bonus
| | Other Annual Compensation
| | Securities Under Option
|
|---|
John J. Masiz*
Chief Executive Officer and President | | 2003 | | $ | 94,792 | | | — | | — | | 100,000 |
Kevin J. Seifert**
Chief Operating Officer | | 2003 | | $ | 16,667 | | $ | 150,000 | | — | | 75,000 |
Dr. Stephen G. Carter*
Chief Scientific Officer | | 2003 | | $ | 75,833 | | | — | | — | | 75,000 |
Joseph Frattaroli
Chief Financial Officer | | 2003 | | $ | — | | | — | | — | | 30,000 |
- *
- Represents the compensation payable by the Company for the period of June 16, 2003 (the effective date of Mr. Masiz's and Dr. Carter's respective employment agreements with the Company) through December 31, 2003. In 2004, Mr. Masiz will receive an annual salary of $175,000 and Dr. Carter will receive an annual salary of $140,000.
- **
- On November 1, 2003 Mr. Seifert commenced employment as our chief operating officer on a transitional and "as-needed" basis and will gradually transition to full-time employment with Vaso Active. Mr. Seifert's annual salary will be $200,000. Payment of his salary will commence on a pro rated basis once Mr. Seifert commences full time employment with Vaso Active, which we anticipate will be December 1, 2003. On this basis, we estimate paying the sum of $16,666 to Mr. Seifert as salary in 2003. As of January 2004, Mr. Seifert will also act as chief operating officer of BioChemics, but his annual salary will remain the same. We expect that Mr. Seifert will spend approximately 70% of his business time on behalf of Vaso Active and the balance of his business time on behalf of BioChemics as of January 2004.
Stock Option Grants
The following table provides information about the number of stock options that we anticipate granting during the year ending December 31, 2003. As our Class A common stock is not publicly traded, a readily ascertainable market value is not available:
Name
| | Number of Securities Underlying Options to be Granted
| | % of Total Options to be Granted to Employees in Fiscal Year
| | Exercise Price Per Share
| | Expected Expiration Date
|
|---|
| John J. Masiz* | | 100,000 | | 31.8% | | 5.00 | | June 30, 2013 |
| Kevin J. Seifert* | | 75,000 | | 23.8% | | 5.00 | | November 1, 2013 |
| Dr. Stephen G. Carter* | | 75,000 | | 23.8% | | 5.00 | | June 30, 2013 |
| Joseph Frattaroli* | | 30,000 | | 9.5% | | 5.00 | | June 30, 2013 |
- *
- Following at least 60 days after the closing of this offering, we expect that we will grant to certain of our executives and other key employees options to purchase an aggregate of 314,500 shares of our Class A common stock under our 2003 stock incentive plan. Of these options, Mr. Masiz, Dr. Carter, Mr. Seifert and Mr. Frattaroli will receive options to purchase 100,000 shares, 75,000 shares, 75,000 shares and 30,000 shares, respectively, of our Class A common stock at an exercise price equal to the initial public offering price, or $5.00, per share. 50% of the options granted to Mr. Masiz and Dr. Carter will vest immediately after 60 days from the date of grant and 25% will vest on each of the first and second anniversary dates of the grant. One-third of the options granted to Mr. Frattaroli will vest on each of the first, second and third anniversary dates of the
44
grant. One quarter of the options granted to Mr. Seifert will vest immediately after 60 days from the date of the grant and 25% will vest on each of the first, second and third anniversary dates of the grant.
Employment Agreements
We have entered into an employment agreement with Mr. Masiz, effective as of June 16, 2003, which provides for his employment as President and Chief Executive Officer through June 30, 2008. The agreement is automatically renewed for successive two year terms thereafter unless terminated earlier by either party in accordance with the terms and conditions of the employment agreement. The agreement provides for an annual salary of $175,000 per year, subject to yearly merit and performance-based bonuses to be granted in such amounts as may be determined by the board in the board's discretion. If we terminate Mr. Masiz without cause, he is entitled to his base salary for 12 months following the time of termination. If we terminate Mr. Masiz following a change of control, we are obligated to pay him a lump sum equal to 200% of his salary as in effect at the time of such termination plus a pro-rata portion of any performance-based bonus, assuming that all 100% of the applicable performance targets for the relevant fiscal year had been achieved. For the purposes of his agreement, a change of control occurs if one person or entity acquires control of 30% or more of our voting common stock, there is a change of control in the board, we sell all or substantially all of our assets, or we are the non-surviving party in a merger.
We have entered into an employment agreement with Dr. Carter, effective as of June 16, 2003, which provides for his employment as Chief Scientific Officer through June 30, 2008. The agreement is automatically renewed for successive two year terms thereafter unless terminated earlier by either party in accordance with the terms and conditions of the employment agreement. The agreement provides for an annual salary of $140,000 per year, subject to yearly merit and performance-based bonuses to be granted in such amounts as may be determined by the board in the board's discretion. If we terminate Dr. Carter without cause, he is entitled to his base salary for 12 months following the time of termination. If we terminate Dr. Carter following a change of control, we are obligated to pay him a lump sum equal to 200% of his salary as in effect at the time of such termination plus a pro-rata portion of any performance-based bonus, assuming that all 100% of the applicable performance targets for the relevant fiscal year had been achieved. For the purposes of his agreement, a change of control occurs if one person or entity acquires control of 30% or more of our voting common stock, there is a change of control in the board, we sell all or substantially all of our assets, or we are the non-surviving party in a merger.
Employee Benefit Plans
2003 Stock Incentive Plan. Our 2003 stock incentive plan was adopted by our board of directors on June 20, 2003 and approved by our stockholders on August 22, 2003.
We have reserved 450,000 shares of our Class A common stock for issuance under our 2003 stock incentive plan. The number of shares of Class A common stock available for issuance under our stock incentive plan will automatically increase the first trading day in January of each calendar year, beginning in calendar year 2004, by an amount equal to 5% of the total number of shares our Class A common stock and Class B common stock, taken together, and outstanding on the last trading day in December of the preceding calendar year, but in no event will any annual increase exceed 200,000 shares of Class A common stock.
The individuals eligible to participate in our stock incentive plan include our present and future executives and other key employees, our directors (other than our non-employee directors), members of our scientific advisory board and any consultants we hire. Our stock incentive plan provides for the grant of incentives in any one or a combination of restricted stock grants, incentive stock options,
45
non-qualified stock options and stock appreciation rights or SARs. Any of the incentive grants to participants may be made subject to vesting, in one or more installments, upon the happening of certain specified events, upon the passage of a specified period of time, upon the fulfillment of certain conditions or upon the achievement by the Company or any subsidiary, division, affiliate or joint venture of the Company of certain performance goals and continued employment.
Restricted Stock Grants. Our compensation committee may award shares under a restricted stock grant. Restricted stock grants may be subject to forfeiture by the holder (or repurchase by us, upon termination of employment) or other specified events. The restricted stock grants include the grant or sale of shares of our Class A common stock at a fixed price but not less than the then par value of a share of our Class A common stock. For example, the grant could set forth a "vesting" or restriction period during which the grantee must remain in the employ of the Company in order to retain the shares under grant. If the grantee's employment terminates during the vesting period, the grant would terminate and the grantee would be required to return the shares to the Company for repurchase. However, our compensation committee may provide complete or partial exceptions to this requirement as it deems equitable. For example, our compensation committee could make such exception in the case where employment is terminated at the time of a sale of the Company or within a specified period of time thereafter.
Stock Options. Our compensation committee may grant options qualifying as incentive stock options under the Internal Revenue Code of 1986, as amended from time to time, and nonqualified stock options. The term of a stock option may be fixed by the compensation committee, but shall not exceed ten years. Stock option grants may be made subject to vesting, in one or more installments, typically based on continued employment with the Company. The option price will be fixed on the date of the grant and will not be less than the fair market value of our Class A Common stock on the date of the grant.
Stock Appreciation Rights. The compensation committee may grant SARs either alone or in combination with an underlying stock option under the stock incentive plan. SARs provide for a payment by the Company equal to the increase in the fair market value per share of our Class A common stock from a price specified on the date of grant. The dollar amount of this appreciation may be made in cash or in shares of our Class A common stock or combination of both, at the discretion of the committee. The term of a SAR may be fixed by the compensation committee. If an SAR grant in combination with an underlying stock option is exercised, the right under the underlying option to purchase shares would terminate.
Administration. The stock incentive plan is administered by our compensation committee. Within the limits of the stock incentive plan, the compensation committee has discretion to determine which eligible individuals are to receive restricted stock grants, stock options, SARs or a combination of such awards, the time or times when such awards are to be made, the number of shares of our Class A common stock subject to each such award, the status of any granted stock option as either an incentive stock option or a nonqualified stock option under the federal tax laws, the vesting schedule and related forfeiture and repurchase provisions to be in effect for the award and the maximum term for which any award is to remain outstanding.
Subject to certain limitations, our compensation committee may delegate to one or more of our executive officers the power to make awards to individuals who are not reporting persons or "covered employees" within the meaning of Section 162(m) of the Internal Revenue Code of 1986, as amended from time to time. "Reporting persons" are our executive officers and directors who are subject to the reporting and short-swing profit recovering provisions of Section 16 of the Exchange Act.
Our compensation committee may interpret the stock incentive plan, amend and rescind administrative guidelines, make such determinations and interpretations with respect to the stock
46
incentive plan or awards made thereunder and include such further terms and conditions and awards granted under the stock incentive plan as it deems advisable. The decisions of the compensation committee under the stock incentive plan are conclusive and binding upon the participants and the Company.
The board of directors may amend or modify the stock incentive plan at any time, subject to any required stockholder approval. The stock incentive plan will terminate no later than June 30, 2013.
2003 Non-Employee Director Compensation Plan. Our 2003 non-employee director compensation plan was adopted by our board of directors on August 22, 2003 and approved by our stockholders on August 22, 2003. We have reserved 300,000 shares of our Class A common stock for issuance under our 2003 non-employee director compensation plan. The options will be granted upon initial election or appointment and will become fully vested over three years, with 25% of the options vesting immediately, and 25% vesting on each subsequent annual anniversary dates of the grant. The options have a term of ten years and an exercise price equal to the fair market value on the date of grant. After we become a public company, the exercise price of these options will be equal to the closing price of our Class A common stock as reported by the Nasdaq Small Cap Market on the date of grant.
Limitation of Liability and Indemnification of Directors and Officers
Our certificate of incorporation and our by-laws provide that our directors and officers will be indemnified by us to the fullest extent authorized by Delaware law, as it now exists or may in the future be amended, against all expenses and liabilities reasonably incurred in connection with their service for or on our behalf. In addition, our certificate of incorporation provides that our directors will not be personally liable for monetary damages to us for breaches of their fiduciary duty as directors, unless they violated their duty of loyalty to us or our stockholders, acted in bad faith, knowingly or intentionally violated the law, authorized illegal dividends or redemptions or derived an improper personal benefit from their action as directors.
We intend to obtain insurance which insures our directors and officers against certain losses and which insures us against our obligations to indemnify the directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than a payment by us of expenses incurred or paid by a director, officer or controlling person of Vaso Active in the defense of any action, suit or proceeding) is asserted by such officer, director or controlling person in connection with the securities being registered, we will, unless in the opinion of our legal counsel that matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Code of Ethics
We have established and adopted a code of ethics in accordance with Section 406 of the Sarbanes-Oxley Act of 2002 rules established by the SEC. The code of ethics establishes guidelines to be followed by our principal executive officer, who is our chief executive officer, and our senior financial officers. Currently our chief financial officer is our only senior financial officer under the code of ethics. In the event that we appoint a treasurer, controller, or other officer who is principally responsible for our accounting, such employees will automatically be deemed as senior financial officers. In addition, our audit committee may, from time to time, change the officers designated as senior financial officers. Compliance is mandatory for those employees subject to our code of ethics.
47
Our code of ethics requires that any actual or perceived conflicts of interest be disclosed to our audit committee. The audit committee has been authorized by our board of directors to review all such matters and to grant or deny waivers. Our code of ethics also requires that all related-party transactions be reviewed and approved by our audit committee. The issue of the dual role played by John Masiz, as president and chief executive officer of both Vaso Active and BioChemics, has been disclosed and reviewed by our audit committee. In addition, our license agreement, manufacturing and development agreement, administrative services agreement, registration rights agreement and other transactions with BioChemics since we became a stand-alone entity have been reviewed by our audit committee for compliance with corporate policy. A majority of the disinterested independent directors subsequently confirmed these agreements. The audit committee also concluded that the relationship between Mr. Masiz, Vaso Active and BioChemics, the potential conflicts that may rise as a result of this relationship and our related-party agreements with BioChemics are being handled honestly and ethically. To ensure that these potential conflicts and related-party transactions are handled ethically in the future, our audit committee has reserved the right to review all transactions between Vaso Active and BioChemics and demand additional information on how various decisions affect BioChemics as an entity and Mr. Masiz personally.
48
RELATED PARTY TRANSACTIONS
In contemplation of this offering, the Company has adopted a policy to the effect that any future transactions between it and its officers, directors, principal stockholders and affiliates be on terms no less favorable to the Company than could reasonably be obtained in arms' length transactions with independent third parties, and that any such transaction also be approved by a majority of our outside independent directors disinterested in the transaction. In addition, under our code of ethics, all related-party transactions must be reviewed and approved by our audit committee.
Private Placement of Securities
Through a private placement transaction that closed in April 2003, we issued subordinated 10% convertible pay-in-kind promissory notes in the aggregate principal amount of $500,000 to accredited investors. These notes are convertible into our Class A common stock. The maturity date of the notes is March 31, 2005 and the conversion price of the notes is the lower of 50% of the public offering price (as defined in the notes) or $2.50, subject to certain adjustments. Conversion may occur on either a mandatory or voluntary basis. Each note may be voluntarily converted in whole or in part at any time prior to the maturity date by the note's holder or on a mandatory basis upon the consummation of this offering. In this private placement transaction, we issued notes in the principal amount of $50,000 to Dr. Brian J. Strasnick, one of our directors.
Immediately upon consummation of this offering, we expect to issue an aggregate of 214,000 shares of our Class A common stock on mandatory conversion of all of the notes in accordance with their terms. As a result, we expect to issue an aggregate of approximately 21,300 shares of our Class A common stock to Dr. Strasnick upon conversion of his notes.
License Agreement
Certain of the BioChemics' patents were issued initially in the name of John Masiz. In March 2002, Mr. Masiz assigned these patents to BioChemics, free and clear of any encumbrances. We have entered into a license agreement with BioChemics under which we have a worldwide, exclusive right to use and practice these patents to sell, market and commercialize all products that (i) utilize and incorporate the BioChemics' patents and know-how; (ii) are classified by the FDA as "over-the-counter products", and (iii) require less than $1.0 million of clinical development. Specifically excluded from the license are certain diabetic neuropathy products developed by BioChemics which are the subject of a clinical study being conducted at the Joslin Diabetes Center in Boston. In exchange for the granting of the license, we agreed to issue 1,500,000 shares of our Class B common stock to BioChemics as a license fee with a deemed value equal to the par value of such shares or $150. This is a one-time license fee and there are no royalties payable to BioChemics under the license agreement. These shares were issued and outstanding during the period ended September 30, 2003. Pursuant to the terms of the license agreement, BioChemics retains all rights to use and practice the patents for all purposes with respect to non-OTC products, including pharmaceuticals and pet products. Any improvements to the patents remain the property of BioChemics, but to the extent that any such improvements relate to products and product candidates, the improvements are licensed to us on the same terms as the initial license under the license agreement. Maintenance of the patent filings remains the responsibility of BioChemics, although we maintain the right under the license agreement to make any required filings at BioChemics' expense in the event that such filings are not made on a timely basis by BioChemics.
BioChemics is also party to a settlement agreement and license with De Feet International, Inc., dated April 20, 2002, whereby De Feet International, Inc. granted a non-exclusive, non-transferable and non-assignable license to BioChemics to use the "deFEET" trademark throughout the United States in connection with the manufacture, advertisement, promotion, distribution and sale of athlete's foot
49
preparations. Pursuant to the settlement agreement and license with De Feet International, Inc., BioChemics is required to remit to De Feet International, Inc. a royalty equal to 5% of all gross sales by BioChemics of products using the deFEET trademark. The royalty is capped at an annual maximum amount of $50,000. Effective January 1, 2003, all sales of deFEET-branded product have been made by BioChemics. BioChemics makes these royalty payments to De Feet International, Inc. in connection with such sales, and in accordance with the settlement agreement and license. As a result of the rebranding of the deFEET product, we expect royalties payable under this settlement agreement to be insignificant in future periods.
Pursuant to an amendment to license agreement, dated July 2, 2003, the trademarks Osteon and PENtoCORE, together with the goodwill associated with each mark, were licensed to us by BioChemics. In addition, BioChemics assigned to us its ownership of any and all revenue associated with the sale, commercialization and marketing of deFEET by BioChemics and we agreed to be responsible for all expenses related to these revenues, including the royalty payments payable by BioChemics to DeFeet International, Inc. Pursuant to the terms of the amendment to license agreement, the nature and quality of all services rendered in connection with the Osteon and PENtoCORE marks remain under the control of BioChemics.
Manufacturing and Development Agreement
In August 2003, we entered into a manufacturing and development agreement with BioChemics with respect to the ongoing research, manufacturing and development of our products and product candidates. Under this agreement, BioChemics will research, develop and manufacture products for us pursuant to specific purchase orders submitted by us from time to time. BioChemics will charge us a development and manufacturing fee at a rate of cost plus 10%. This agreement has an initial term of 5 years and is automatically renewable for an additional period of 12 months.
Office Space, Administrative Services and Financial Support
We presently maintain our executive offices on premises that we share with BioChemics. We do not have a lease agreement with BioChemics. We believe that we can obtain suitable alternative space without any material disruption of our business and that such space will be available to us in the future on commercially reasonable terms. In addition, BioChemics provides us with back office support and corporate management services. Allocations of BioChemic's expenses for centralized accounting, data processing, utilities, office space rental, supplies, telephone and other corporate services and infrastructure are charged back to us as a management fee. These amounts approximated $135,600 and $114,150 for the years ended December 31, 2002 and 2001 respectively.
Effective of September 1, 2003, we entered into an administrative services agreement with BioChemics. Under this agreement, BioChemics will provide to us, at our request, administrative support services including secretarial support, accounting and tax services, data processing services, utilities, designated office space, designated warehouse and storage space, office supplies, telephone and computer services and equipment and such other office and corporate support services as we may reasonably require from time to time. BioChemics will charge us an administrative services fee at a rate of cost plus 10%. This agreement will be in effect for an initial term of five years and will be automatically renewable on each anniversary date for an additional period of twelve months unless sooner terminated (i) for a material breach by us, not cured within three months, and upon written notice (ii) upon 30 days written notice by us, (iii) upon 45 days written notice by either party if BioChemics ceases to own beneficially shares of our capital stock to which are attached at least 49% of the votes that may be cast to elect our directors, or (iv) upon written notice by either party in the event that we shall have disposed of all or substantially all of our assets.
50
Registration Rights Agreement
We have entered into a registration rights agreement with BioChemics under which we have granted to BioChemics, or its permitted transferees, as holders of shares of Class A common stock issuable upon conversion of the Class B common stock held by BioChemics, rights with respect to registration of these shares under the Securities Act. Subject to limitations set forth in the registration rights agreement, holders of the securities may require us, at our expense, to file one registration statement under the Securities Act with respect to the public resale of the securities. With the exception of this offering, if we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, BioChemics or its permitted transferees are entitled to notice of the registration and are entitled, subject to certain conditions and limitations, to include, at our expense, their shares in the registration. Notwithstanding the foregoing, BioChemics has agreed with Kashner Davidson Securities Corp. on behalf of the underwriters not to exercise its registration rights for a period of two years from the date of this prospectus without the consent of Kashner Davidson Securities Corp. All registration expenses, as specified in the registration rights agreement, must be borne by us and all expenses relating to the sale of the securities registered must be borne by BioChemics or the other holder of the securities being registered.
Other Transactions with BioChemics
BioChemics made cash contributions to our capital in 2001 and 2002 to cover our deficits incurred during those two years. These contributions totaled $1,040,395 in 2001 and $446,862 in 2002. For the nine months ended September 30, 2003, BioChemics provided financing to us in the amount of $445,822 to fund our operating losses incurred during that nine month period. After the consummation of this offering, we do not anticipate receiving further financing from BioChemics.
In the nine-month period ended September 30, 2003, Vaso Active became a stand-alone entity effective upon its incorporation in January 2003. During this period of time, we incurred management fees of approximately $99,000 of BioChemics and other operational charges of approximately $347,000 that had been paid by BioChemics to third parties on our behalf. These amounts which aggregate approximately $446,000 will be repaid from the net proceeds of this public offering.
51
PRINCIPAL STOCKHOLDERS
The following table sets forth information we know with respect to the beneficial ownership of our common stock as of November 1, 2003, for each person or group of affiliated persons, whom we know to beneficially own more than 5% of our common stock. The table also sets forth such information for our directors and executive officers, individually and as a group.
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission, or SEC. Except as indicated by footnote, to our knowledge, the persons named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially owned by them. Options to purchase shares of common stock that are exercisable within 60 days of November 1, 2003 are deemed to be beneficially owned by the person holding such options for the purpose of computing ownership of such person, but are not treated as outstanding for the purpose of computing the ownership of any other person. 1,500,000 shares of our Class B common stock BioChemics are deemed to be beneficially owned by BioChemics and John J. Masiz as set forth in the following table, but are not treated as outstanding for the purpose of computing the ownership of any other person. Applicable percentage of beneficial ownership is based on no shares of Class A common stock outstanding as of November 1, 2003. Unless otherwise indicated in the footnotes, the address of each listed stockholder is: c/o Vaso Active Pharmaceuticals, Inc., 99 Rosewood Drive, Suite 260, Danvers, MA 01923.
Class A common stock
| |
| | Percent of Class Outstanding
| |
|---|
Name of Beneficial Owner(1)
| | Number of Shares
Beneficially Owned(1)
| | Before the
Offering
| | After the Offering
| |
|---|
| John J. Masiz(2)(3) | | 1,500,000 | | 100 | % | 49.8 | % |
| BioChemics(2)(4) | | 1,500,000 | | 100 | % | 49.8 | % |
| Kevin J. Siefert | | — | | — | | — | |
| Stephen G. Carter, Ph.D | | — | | — | | — | |
| Bruce A. Shear(5) | | 5,000 | | * | | * | |
| Brian Strasnick, Ph.D(5)(6) | | 26,300 | | * | | 1.7 | % |
| Robert Anderson(5) | | 5,000 | | * | | * | |
| Gary Fromm, Ph.D(5) | | 5,000 | | * | | * | |
| William P. Adams, M.D.(5) | | 5,000 | | * | | * | |
All directors and executive officers as a group
(eight persons)(2)(3)(4)(5)(6) | | 1,546,300 | | 100 | % | 51.3 | % |
Class B common stock
| |
| | Percent of Class Outstanding
| |
|---|
Name of Beneficial Owner(1)
| | Number of Shares
Beneficially Owned(2)
| | Before the
Offering
| | After the Offering
| |
|---|
| John J. Masiz(3) | | 1,500,000 | | 100 | % | 100 | % |
| BioChemics(4) | | 1,500,000 | | 100 | % | 100 | % |
All directors and executive officers as a group
(seven persons)(3)(4) | | 1,500,000 | | 100 | % | 100 | % |
- *
- Less than 1% of the outstanding Class A common stock.
52
- (1)
- The persons listed in the tables own of record the shares of our Class A common stock and Class B common stock listed opposite their respective names, and have sole voting and investment power with respect to such shares of common stock, unless otherwise indicated.
- (2)
- Includes shares of Class B common stock which are convertible at any time into shares of Class A common stock on a share-for-share basis.
- (3)
- All the aggregate 1,500,000 shares of Class B common stock deemed beneficially owned by Mr. Masiz are held of record by BioChemics. Mr. Masiz has sole voting and investment power with respect to approximately 63% of the issued and outstanding capital stock of BioChemics and has the voting power to elect all of its directors. Mr. Masiz disclaims beneficial ownership of all shares listed as beneficially owned by BioChemics except to the extent of his pecuniary interest therein. Of the aggregate 1,500,000 shares of Class B common stock deemed beneficially owned Mr. Masiz, all of such shares are also deemed beneficially owned by BioChemics. As a result of the Class B common stock having three votes per share, Mr. Masiz beneficially owns shares representing approximately 75% of the combined voting power of the outstanding common stock of the Company.
- (4)
- BioChemics has sole voting and investment power with respect to the 1,500,000 shares of Class B common stock held of record by BioChemics. All the aggregate 1,500,000 shares of Class B common stock held of record and beneficially owned by BioChemics are also deemed beneficially owned by Mr. Masiz. As a result of the Class B common stock having three votes per share, BioChemics beneficially owns shares representing approximately 75% of the combined voting power of the outstanding common stock of the Company.
- (5)
- Includes the following shares of Class A common stock, subject to currently exercisable options: Mr. Shear—5,000 shares; Dr. Strasnick—5,000 shares; Mr. Anderson—5,000 shares; Dr. Fromm—5,000 shares; Dr. Adams—5,000 shares; and all directors and executive officers as a group—an aggregate of 25,000 shares.
- (6)
- Includes 21,300 shares of Class A common stock issueable to Dr. Strasnick upon mandatory conversion of $50,000 principal amount, including accrued pay-in-kind interest, of our subordinated 10% convertible pay-in-kind promissory notes held by him upon consummation of this offering.
The following table sets forth information we know with respect to the beneficial ownership of the common stock of BioChemics as of November 1, 2003 by our directors and executive officers, individually and as a group. Options to purchase shares of BioChemics common stock that are exercisable within sixty days of November 1, 2003 are deemed to be beneficially owned by the person holding such options for the purpose of computing ownership of such person, but are not treated as outstanding for the purpose of computing the ownership of any other person. None of our directors or executive officers is the beneficial owner of any other class of equity securities of BioChemics.
53
BioChemics Common Stock
Name of Beneficial Owner(1)
| | Number of Shares
Beneficially Owned(1)
| | Percent of Class Outstanding
| |
|---|
| John J. Masiz | | 4,500,000 | | 63 | % |
| Kevin J. Seifert | | — | | — | |
| Stephen G. Carter, Ph.D.(2) | | 275,000 | | 3.7 | % |
| Bruce A. Shear | | — | | — | |
| Brian Strasnick, Ph.D. | | — | | — | |
| Robert Anderson | | — | | — | |
| Gary Fromm, Ph.D. | | — | | — | |
| William P. Adams, MD. | | — | | — | |
| All directors and executive officers as a group (eight persons)(2) | | 4,775,000 | | 64.6 | % |
| | |
| |
| |
- (1)
- The persons listed in the tables own of record the shares of BioChemics common stock listed opposite their respective names, and have sole voting and investment power with respect to such shares of common stock, unless otherwise indicated.
- (2)
- Includes 275,000 shares of BioChemics common stock subject to currently exercisable options.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no market for our common stock, and a liquid trading market for our Class A common stock may not develop or be sustained after this offering. Future sales of substantial amounts of Class A common stock, including shares issued upon exercise of outstanding options and conversion of any shares of our Class B common stock, in the public market after this offering or the anticipation of those sales could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of our equity securities.
Upon the consummation of this offering, we will have outstanding 1,514,000 shares of Class A common stock, assuming no exercise of the underwriters' over-allotment option and no exercise of outstanding options or conversion of any shares of our Class B common stock. Of these shares, the shares sold in this offering will be freely tradable without restriction under the Securities Act unless purchased by our "affiliates" as that term is defined in Rule 144 under the Securities Act. The remaining 214,000 shares of Class A common stock outstanding will be restricted shares held by existing stockholders. Upon the consummation of this offering, we will also have outstanding 1,500,000 shares of Class B common stock.
Lock-Up Agreements
All of our directors, officers, employees and the holders of the remaining 214,000 shares of our Class A common stock and the holders of our Class B common stock have entered into lock-up agreements in connection with this offering. These lock-up agreements provide that, with limited exceptions, the stockholder will not offer, sell, contact to sell, grant an option to purchase, effect a short sale or otherwise dispose of or engage in any hedging or other transaction that is designed or reasonably expected to lead to a disposition of shares of Class A common stock or any option to purchase Class A common stock or any securities exchangeable for or convertible into Class A common stock for a period of one year (two years in the case of BioChemics) after the date of this prospectus without the prior written consent of Kashner Davidson Securities Corp. on behalf of the underwriters. Though these shares may be eligible for earlier sale under the provisions of the Securities Act, none of
54
these shares will be saleable until one year after the date of this prospectus as a result of these lock-up agreements.
Rule 144
In general, under Rule 144 as currently in effect, after the expiration of the lock-up agreements, a person, or persons whose shares are aggregated, who has beneficially owned restricted shares for at least one year is entitled to sell within any three-month period up to that number of shares that does not exceed the greater of:
- •
- 1% of the number of shares of common stock then outstanding, which immediately following this offering is expected to be approximately 30,140 shares of common stock, or
- •
- the average weekly trading volume of the common stock during the four calendar weeks preceding the fling of a Form 144 with respect to the sale.
Sales under Rule 144 are also subject to certain "manner of sale" provisions and notice requirements and to the requirement that current public information about itself be available.
Beginning one year after the date of this prospectus, 214,000 restricted shares of our Class A common stock will be eligible for sale in the United States public market, subject to volume and other limitations.
Rule 144(k)
Under Rule 144(k), a person who is not deemed to have been our affiliate at any time during the three months preceding a sale, and who has beneficially owned the shares proposed to be sold for at least two years, including the holding period of any prior owner except an affiliate, is entitled to sell those shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144.
Rule 701
Rule 701 permits our directors, officers, employees or consultants who purchase shares pursuant to a written compensatory plan or contract to resell such shares in reliance upon Rule 144, but without compliance with certain restrictions. Rule 701 provides that affiliates may sell their Rule 701 shares under Rule 144 90 days after effectiveness of the registration statement of which this prospectus forms a part without complying with the holding period requirement and that non-affiliates may sell such shares in reliance on Rule 144 90 days after the effectiveness of such registration statement without complying with the holding period, public information, volume limitation or notice requirements of Rule 144.
Registration Rights
Upon the closing of this offering, BioChemics will be entitled to rights with respect to the registration of its shares under the Securities Act. In addition, we have agreed to certain registration rights for the securities underlying the representative's warrants that we have agreed to issue to Kashner Davidson Securities Corp. on behalf of the underwriters. These warrants entitle the holder to purchase 130,000 shares of our Class A common stock. These warrants are not exercisable until one year from the date of effectiveness of the registration statement, of which this prospectus forms a part. Registration of these shares under the Securities Act would result in these shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates, immediately upon the effectiveness of such registration. BioChemics has agreed with Kashner Davidson Securities Corp. not to exercise its registration rights for a period of two years from the date of this prospectus without the consent of Kashner Davidson Securities Corp.
55
DESCRIPTION OF CAPITAL STOCK
Our authorized capital stock consists of 10,000,000 shares of Class A common stock, par value $0.0001 per share, 5,000,000 shares of Class B common stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share. The following description of our capital stock is a summary only. It does not purport to be complete and is subject to and qualified in its entirety by the provisions of our charter, which is incorporated by reference as an exhibit to the registration statement of which this prospectus is a part, and by applicable law.
Common Stock
The holders of Class A common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders and holders of shares of Class B common stock are entitled to three votes per share on all matters to be voted on by stockholders, with holders of both such classes of common stock voting as a single class (except for class votes as required by law). After the sale of the Class A common stock offered by this prospectus, the outstanding shares of the Class A common stock will represent approximately 50% of the total number of shares of common stock and approximately 25% of the total combined voting power, respectively, of both classes of common stock outstanding. As a result, the holders of Class B common stock will be able to elect all of our directors. The voting power of the Class B common stock may discourage or preclude certain transactions, whether or not beneficial to holders of the Class A common stock, including transactions in which stockholders might receive a substantial premium for their shares of Class A common stock over the then current prices. As a result, stockholders who might desire to participate in such a transaction may not have an opportunity to do so. In addition, the voting power of Class B common stock could discourage certain types of transactions that involve an actual or threatened acquisition or change of control of our Company. The holders of common stock do not have cumulative voting rights.
Holders of Class A common stock and holders of Class B common stock are entitled to receive dividends ratably, if any, as may be declared from time to time by our board out of legally available funds, subject to any preferential dividend rights applicable to any outstanding preferred stock that may be issued in the future. Dividends and distributions payable in shares of Class A common stock may be paid only on shares of Class A common stock and dividends and distributions payable in shares of Class B common stock may be paid only on shares of Class B common stock. If a dividend or distribution payable in Class A common stock is made on the Class A common stock, we must also make a simultaneous dividend or distribution on the Class B common stock. If a dividend or distribution payable in Class B common stock is made on the Class B common stock, we must make a simultaneous dividend or distribution on the Class A common stock. We have not declared or paid cash dividends on our capital stock. We currently intend to retain any future earnings to fund our operations and, therefore, do not anticipate paying any cash dividends in the foreseeable future. See "Dividend Policy."
In the event of liquidation, dissolution or winding up of Vaso Active, the holders of common stock are entitled to share ratably in all of our assets remaining after the payment of all debts and other liabilities, subject to the prior distribution rights of outstanding shares of preferred stock. There are no preemptive, subscription or conversion rights applicable to the common stock, except each share of Class B common stock may be converted at any time into one share of Class A common stock at the election of the holder. In addition, each share of Class B common stock automatically converts into a share of Class A common stock at the time it is sold, transferred or otherwise disposed of to any person or entity, other than when such share is (i) transfered to BioChemics, to John J. Masiz or to a person "controlled" by or under common control with either of them or the transferor of such share; and (ii) under certain other circumstances, such as bankruptcy or attachment or any judgment whereby the holder is obligated to transfer such share of Class B common stock.
56
The rights, preferences and privileges of holders of common stock are subject to the rights of the holders of shares of any class or series of preferred stock that we may designate and issue in the future.
Preferred Stock
The board has the authority, without action by the stockholders, to designate and issue up to 10,000,000 shares of preferred stock in one or more series and to designate the rights, preferences and limitations of all series, any or all of which may be superior to the rights of the common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock upon the rights of the holders of common stock until the board determines the specific rights of the holders of preferred stock. However, effects of the issuance of preferred stock include restricting dividends on common stock, diluting the voting power of the common stock (other than Class B common stock), impairing the liquidation rights of the common stock and making it more difficult for a third party to acquire us, which could have the effect of discouraging a third party from acquiring, or deterring a third party from paying a premium to acquire a majority of our outstanding voting stock. We have no present plans to issue any shares of preferred stock.
Registration Rights
BioChemics or its permitted transferees, as holders of shares of Class A common stock issuable upon conversion of the Class B common stock to be issued upon consummation of this offering, are entitled to rights with respect to the registration of these shares under the Securities Act. These registration rights are provided under the terms of agreements between us and BioChemics. BioChemics has agreed with Kashner Davidson Securities Corp. on behalf of the underwriters not to exercise its registration rights for a period of two years from the date of this prospectus without the consent of Kashner Davidson Securities Corp. Subject to limitations set forth in the agreement, holders of these securities may require us, at our expense, to file one registration statement under the Securities Act with respect to the public resale of these securities. With the exception of this offering, if we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, the holders of the securities are entitled to notice of the registration and are entitled, subject to certain conditions and limitations, to include, at our expense, their shares in the registration. The shares of these holders are not included in the registration statement of which this prospectus forms a part. All registration expenses, as specified in the agreements, must be borne by us and all expenses relating to the sale of the securities registered must be borne by the holder of the securities being registered.
In addition, we have agreed to certain registration rights for the securities underlying the representative's warrants that we have agreed to issue to Kashner Davidson Securities Corp. on behalf of the underwriters. These warrants entitle the holder to purchase 130,000 shares of our Class A common stock. These warrants are not exercisable until one year from the date of effectiveness of the registration statement, of which this prospectus forms a part.
Delaware Law and Provisions of Our Charter
We are subject to the provisions of Section 203 of the Delaware General Corporation Law which, subject to some exceptions, prohibits us from engaging in specified business combinations with any interested stockholder for a period of three years following the time that such stockholder became an interested stockholder, unless the business combination or the transaction in which the stockholder became an interested stockholder is approved in a prescribed manner. Generally, a "business combination" includes a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. For purposes of Section 203, an "interested stockholder" is a person who, together with affiliates and associates, owns or within three years prior to the determination of
57
interested stockholder status, did own, 15% or more of the corporation's voting stock. The application of Section 203 could have the effect of delaying or preventing a change of control of Vaso Active.
Our charter provides that candidates for directors may be nominated only by the board or by a stockholder who gives written notice to us in the manner prescribed by the bylaws. The number of directors may be fixed by resolution of the board but in any event shall not be less than three. The board currently consists of four (4) members and the board may appoint new directors to fill vacancies or newly created directorships between stockholder meetings. Our charter does not provide for cumulative voting at stockholder meetings for election of directors. A director may be removed from office with cause by the affirmative vote of at least 75% of all eligible votes present in person or by proxy at a meeting of stockholders at which a quorum is present or without cause by the affirmative vote of 75% of all eligible votes present in person or by proxy at a meeting of stockholders at which a quorum is present, provided that removal without cause is recommended to the stockholders by the board pursuant to a vote of not less than 75% of the directors then in office. Any action required or permitted to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders. Our charter and other provisions of the Delaware General Corporation Law may have the effect of delaying, deterring or preventing a change in control of Vaso Active, may discourage bids for common stock at a premium over the market price and may adversely affect the market price, and the voting and other rights of the holders, of our common stock.
Transfer Agent
The transfer agent for our common stock is EquiServe Trust Company, N.A. Its telephone number is (800) 633-4236.
58
UNDERWRITING
Under the terms and subject to the conditions contained in an underwriting agreement dated the date of this prospectus, the underwriters named below, for whom Kashner Davidson Securities Corp. is acting as representative, have severally agreed to purchase, and we have agreed to sell to them, severally, the number of shares indicated below:
Name
| | Number of Shares
|
|---|
| Kashner Davidson Securities Corp. | | |
| | | |
| | | |
| | | |
| | |
|
| Total | | 1,300,000 |
| | |
|
The underwriters are offering the shares of our Class A common stock subject to their acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the shares of our Class A common stock offered by this prospectus are subject to the approval of legal matters by their counsel and some other conditions. The underwriters are obligated to take and pay for all of the shares of Class A common stock offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares covered by the underwriters' over-allotment option described below.
The underwriters initially propose to offer part of the shares of our Class A common stock directly to the public at the public offering price listed on the cover page of this prospectus and part to a limited number of dealers at a price that represents a concession not in excess of $ a share under the public offering price. The underwriters have informed us that they do not expect sales to discretionary accounts by the underwriters to exceed five percent of the shares offered by us.
We have granted to the underwriters an option, exercisable for 60 days from the date of this prospectus, to purchase up to an aggregate of 195,000 additional shares of our Class A common stock at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with the offering of the shares of our Class A common stock offered by this prospectus. To the extent the option is exercised, each underwriter will become obligated, subject to specified conditions, to purchase about the same percentage of the additional shares of our Class A common stock as the number listed next to the underwriter's name in the preceding table bears to the total number of shares of our Class A common stock listed next to the names of all underwriters in the preceding table.
The underwriting fee is equal to the public offering price per share of Class A common stock less the amount paid by the underwriters to us per share of Class A common stock. The underwriting fee is 10% of the initial public offering price. We have agreed to pay the underwriters the following fees, assuming either no exercise or full exercise by the underwriters of the underwriters' over allotment option:
| |
| | Total Underwriting Fees
|
|---|
| | Fees per Share
| | Without Exercise of Over-Allotment Option
| | With Full Exercise of Over-Allotment Option
|
|---|
| Fees paid by Vaso Active | | $ | 0.50 | | $ | 650,000 | | $ | 745,000 |
59
In addition, we estimate that our share of the total expenses of this offering, excluding underwriting discounts and commissions, will be approximately $1,420,000.
We have also agreed to issue to the representative five year warrants, at a price of $0.001 per warrant, to purchase 130,000 shares of our Class A common stock at an exercise price equal to 120% of the initial offering price of the Class A common stock. The exercise price and the number of underlying securities are subject to adjustment upon certain terms. These warrants are exercisable during the four year period beginning one year from the date of effectiveness of the registration statement, of which this prospectus forms a part. These warrants, and the securities underlying them, are not transferable for one year following the effective date, except to other underwriters (or members of the selling group and/or their officers or partners), an individual who is an officer or partner of an underwriter, by will or by the laws of descent and distribution.
The representative's warrants are not redeemable by us. In addition, we have agreed to certain "demand" and "piggyback" registration rights for the securities underlying the representative's warrants. The holder of the representative's warrants can demand, on one occasion, at anytime until five years from the consummation of this offering that we register the shares and warrants for resale under the Securities Act. In addition, the warrants provide for one piggyback registration during the seven years from the consummation of this offering.
The holders of the representative's warrants will have, in that capacity, no voting, dividend or other stockholder rights. Any profit realized by the representative on the sale of the securities issuable upon exercise of the representative's warrants may be deemed to be additional underwriting compensation. The securities underlying the representative's warrants are being registered on the registration statement. During the term of the representative's warrants, the holders thereof are given the opportunity to profit from a rise in the market price of our Class A common stock. We may find it more difficult to raise additional equity capital while the representative's warrants are outstanding. At any time at which the representative's warrants are likely to be exercised, we may be able to obtain additional equity capital on more favorable terms.
Pursuant to an advisory and investment banking agreement between ourselves and Kashner Davidson Securities Corp., we have agreed to engage Kashner Davidson Securities Corp. to render consulting advice to us with respect to certain financial matters. The term of this agreement is two years, commencing on the date that this offering is consummated.
During the term of the agreement, we will pay Kashner Davidson Securities Corp. a consulting fee of $5,000 per month, payable in its entirety upon the closing of this offering. In consideration of this fee, Kashner Davidson Securities Corp. will seek out potential sales and/or acquisition transactions on our behalf and furnish advice to us in connection with these transactions. In the event that any such transaction is consummated during the term of the agreement or one year thereafter, we will pay Kashner Davidson Securities Corp. an additional fee ranging from 1% to 5% of the consideration received by us in connection with the transaction. We will also reimburse Kashner Davidson Securities Corp. for all actual fees and disbursements paid by it in connection with its provision of services under the agreement. The agreement does not require Kashner Davidson Securities Corp. to provide any minimum number of hours of consulting services or to originate any minimum number of transactions.
We have also paid to the representative $10,000 on account of the representative's expenses in connection with this offering to be applied to the non-accountable expense allowance equal to 3% of the gross proceeds of the offering (including proceeds from the sale, if any, of the over-allotment option securities).
60
We have agreed that, without the prior written consent of Kashner Davidson Securities Corp. on behalf of the underwriters, we will not, during the period ending one year after the date of this prospectus:
- •
- offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock; or
- •
- enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our common stock.
whether any transaction described above is to be settled by delivery of our common stock or other securities, in cash or otherwise.
The restrictions described in the above paragraph do not apply to:
- •
- the sale of shares to the underwriters,
- •
- the issuance by us of shares of common stock upon the exercise of options or warrants or the conversion of securities outstanding on the date hereof, or the registration of the resale of those shares of common stock, of which the underwriters have been advised in writing,
- •
- grants of employee or director stock options or issuances of shares of our common stock to employees or directors, in each case pursuant to the terms of a plan in effect on the date hereof, or
- •
- the issuance of our common stock, any options, rights or warrants with respect to shares of our common stock, or any other securities convertible into or exchangeable for our common stock to a collaborative partner or licensee in connection with any collaborative or licensing arrangement, provided that prior to such issuance the collaborative partner or licensee enters into a lock-up agreement with the underwriters with respect to these securities during the 90 days following the date of the prospectus.
Our officers and directors and certain stockholders have agreed that they will not, without, in each case, the prior written consent of Kashner Davidson Securities Corp., for a period of one year (two years in the case of BioChemics) after the date of this prospectus:
- •
- offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of directly or indirectly, any shares of our common stock or any securities convertible into or exercisable or exchangeable for our common stock, or
- •
- enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our common stock.
whether any transaction described above is to be settled by delivery of our common stock or other securities, in cash or otherwise.
The restrictions described in the above paragraphs relating to our officers, directors and certain stockholders do not apply:
- •
- to any shares of our common stock acquired in the open market, or
- •
- the transfer of any shares of our common stock to a family member, trust or as a bona fide gift, provided that the transferee agrees to be bound in writing by these restrictions prior to such transfer.
61
In order to facilitate the offering of the Class A common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our Class A common stock in accordance with Regulation M under the Securities Exchange Act of 1934.
- •
- Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum.
- •
- Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares which they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any short position by either exercising their over-allotment option and/or purchasing shares in the open market.
- •
- Syndicate covering transactions involve purchase of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option—a naked short position—that position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering.
- •
- Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
- •
- In passive market making, market makers in the Class A common stock who are underwriters or prospective underwriters may, subject to limitations, make bids for or purchases of the Class A common stock until the time, if any, at which a stabilizing bid is made.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of the Class A common stock or preventing or retarding a decline in the market price of the Class A common stock. As a result, the price of the Class A common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the Nasdaq Small Cap Market or otherwise and, if commenced, may be discontinued at any time.
At our request, the underwriters have reserved for sale, at the initial public offering price, up to 100,000 shares for our vendors, employees, family members of employees, customers, and other third parties. The number of shares of our Class A common stock available for sale to the general public will be reduced to the extent these reserved shares are purchased. Any reserved shares that are not purchased by these persons will be offered by the underwriters to the general public on the same basis as the other shares in this offering.
The underwriters have advised us that they will not be offering our securities over the Internet and that the prospectus will not be made available in electronic format on any Internet web sites.
We have agreed with the underwriters to indemnify each other against certain civil liabilities, including liabilities under the Securities Act of 1933, as amended. We will also indemnify the underwriters against liabilities arising from breaches of our representations and warranties in the
62
underwriting agreement. If we are unable to provide our indemnifications, we will contribute to payments that the underwriters may be required to make in respect of these liabilities.
We have been advised that in the opinion of the Securities and Exchange Commission, indemnification against liabilities under the Securities Act of 1933, as amended, is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Pricing of this Offering
Prior to this offering, there had been no public market for our common stock. Consequently, the initial public offering price for our Class A Common stock has been determined by negotiation among us and the representative of the underwriters. Among the primary factors considered in determining the public offering price were:
- •
- Prevailing market conditions;
- •
- Our results of operation in recent periods;
- •
- The present stage of our development;
- •
- The market capitalization and stage of development of other companies that we and the representative of the underwriters believe to be comparable to our business; and
- •
- Estimates of our business potential.
LEGAL MATTERS
The validity of the shares of common stock offered will be passed upon for us by Robinson & Cole LLP, One Boston Place, Boston, Massachusetts 02108. Certain legal matters in connection with this offering will be passed upon for the underwriters by Sichenzia Ross Friedman Ference LLP, 1065 Avenue of the Americas, New York, NY 10018 (212) 930-9700.
EXPERTS
The financial statements of Vaso Active Pharmaceuticals, Inc. as of December 31, 2001 and 2002 and for each of the years in the two-year period ended December 31, 2002 have been included and incorporated by reference herein and in the registration statement in reliance upon the report of Stowe & Degon, independent certified public accountants, included and incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form SB-2 with the SEC in connection with this offering. You may read and copy this information at the following locations of the SEC:
Public Reference Room
450 Fifth Street, N.W.
Room 1024
Washington, D.C. 20549 |
You may also obtain copies of this information by mail from the Public Reference Section of the SEC, 450 Fifth Street, N.W., Room 1024, Washington, DC 20549, at prescribed rates. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
The SEC also maintains a Vaso Active file electronically with the SEC. The address of that site is www.sec.gov.
63
This prospectus is part of the registration statement and does not contain all of the information included in the registration statement. Whenever a reference is made in this prospectus to any contract or other document of Vaso Active, the reference may not be complete and you should refer to the exhibits that are a part of the registration statement for a copy of the contract or document.
64
VASO ACTIVE PHARMACEUTICALS, INC.
INDEX TO FINANCIAL STATEMENTS
F-1
INDEPENDENT AUDITORS' REPORT
To the Stockholders and Board of Directors
Vaso Active Pharmaceuticals, Inc.
Danvers, Massachusetts
We have audited the accompanying balance sheets of Vaso Active Pharmaceuticals, Inc. "the Company" as of December 31, 2002 and 2001 and the related statements of operations, stockholders' deficiency, and cash flows for each of the years in the two-year period ended December 31, 2002. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2002 and 2001 and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2002 in conformity with accounting principles generally accepted in the United States of America.
As discussed in note 2, the accompanying financial statements have been prepared on the going concern assumption that the Company will be able to realize its assets and discharge its liabilities in the normal course of business. Certain conditions such as the Company's inability to generate sufficient cash from operations and obtain debt or equity financing to meet its future obligations raise substantial doubt about the Company's ability to continue as a going concern. Management's plan to overcome doubts about the Company's ability to continue as a going concern are contained also in note 2. The accompanying financial statements do not include any adjustments reflecting the possible future effects on the recoverability of assets or the amount and classification of liabilities that may result from the outcome of these uncertainties.
February 22, 2003 (Except October 27, 2003 as to notes 1, 2 and 5)
Worcester, Massachusetts
F-2
VASO ACTIVE PHARMACEUTICALS, INC.
BALANCE SHEETS
| | December 31,
| | September 30
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| | (Unaudited)
| |
|---|
| ASSETS | | | | | | | | | | | | | |
| Current: | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | — | | $ | — | | $ | — | | $ | 158,576 | |
| Inventory | | | 17,359 | | | 14,502 | | | 19,523 | | | 48,438 | |
| Accounts receivable—trade | | | 380 | | | 53,437 | | | 57,092 | | | — | |
| Prepaid expenses | | | 2,484 | | | 4,304 | | | — | | | 613,940 | |
| Due from parent company | | | 5,760 | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| Total current assets | | | 25,983 | | | 72,243 | | | 76,615 | | | 820,954 | |
| | |
| |
| |
| |
| |
TOTAL ASSETS |
|
$ |
25,983 |
|
$ |
72,243 |
|
$ |
76,615 |
|
$ |
820,954 |
|
| | |
| |
| |
| |
| |
LIABILITIES AND STOCKHOLDERS' DEFICIENCY |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Current: | | | | | | | | | | | | | |
| Accounts payable—trade | | $ | 22,640 | | $ | 49,300 | | $ | — | | $ | 196,850 | |
| Accrued interest | | | — | | | — | | | — | | | 28,083 | |
| Accrued salaries, wages and taxes | | | 3,343 | | | 604 | | | — | | | — | |
| Due to parent | | | — | | | 22,339 | | | 76,615 | | | 445,822 | |
| Convertible promissory notes payable | | | — | | | — | | | — | | | 500,000 | |
| | |
| |
| |
| |
| |
| Total Current Liabilities | | | 25,983 | | | 72,243 | | | 76,615 | | | 1,170,755 | |
| | |
| |
| |
| |
| |
| Commitments and contingencies (see note 6) | | | | | | | | | | | | | |
| TOTAL LIABILITIES | | | 25,983 | | | 72,243 | | | 76,615 | | | 1,170,755 | |
| | |
| |
| |
| |
| |
STOCKHOLDERS' DEFICIENCY |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Preferred stock—$0.0001 par value; authorized 25,000 shares; issued and outstanding, none | | | — | | | — | | | — | | | — | |
| Common stock—$0.0001 par value; authorized 5,000,000 shares; issued and outstanding at December 31, 2001 and 2002 and September 30, 2002, none; 1,500,000 shares issued and outstanding at September 30, 2003 | | | — | | | — | | | — | | | 150 | |
| Additional paid-in capital | | | 1,040,395 | | | 1,487,257 | | | 1,342,145 | | | — | |
| Accumulated deficit | | | (1,040,395 | ) | | (1,487,257 | ) | | (1,342,145 | ) | | (349,951 | ) |
| | |
| |
| |
| |
| |
| TOTAL STOCKHOLDERS' DEFICIENCY | | | — | | | — | | | — | | | (349,801 | ) |
| | |
| |
| |
| |
| |
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIENCY | | $ | 25,983 | | $ | 72,243 | | $ | 76,615 | | $ | 820,954 | |
| | |
| |
| |
| |
| |
See notes to financial statements.
F-3
VASO ACTIVE PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
| | Years Ended December 31,
| | Nine Months Ended September 30
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| | (Unaudited)
| |
|---|
| NET REVENUES | | $ | 100,209 | | $ | 91,957 | | $ | 76,499 | | $ | 51,876 | |
| COST OF SALES | | | 40,791 | | | 40,811 | | | 32,779 | | | 31,279 | |
| | |
| |
| |
| |
| |
| GROSS PROFIT | | | 59,418 | | | 51,146 | | | 43,720 | | | 20,597 | |
| | |
| |
| |
| |
| |
| EXPENSES | | | | | | | | | | | | | |
| Marketing, advertising and promotion | | | 556,614 | | | 38,962 | | | 11,565 | | | 87,125 | |
| Selling, general and administrative | | | 545,748 | | | 464,832 | | | 340,526 | | | 296,538 | |
| | |
| |
| |
| |
| |
| | | | 1,102,362 | | | 503,794 | | | 352,091 | | | 383,663 | |
| | |
| |
| |
| |
| |
| LOSS FROM OPERATIONS | | | (1,042,944 | ) | | (452,648 | ) | | (308,371 | ) | | (363,066 | ) |
| | |
| |
| |
| |
| |
| OTHER (INCOME) EXPENSES | | | (2,549 | ) | | (5,786 | ) | | (6,621 | ) | | (13,115 | ) |
| | |
| |
| |
| |
| |
| NET LOSS | | $ | (1,040,395 | ) | $ | (446,862 | ) | $ | (301,750 | ) | $ | (349,951 | ) |
| | |
| |
| |
| |
| |
Basic and diluted loss per share |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
(0.64 |
) |
| | |
| |
| |
| |
| |
| Shares used in computing basic and diluted loss per share amounts | | | — | | | — | | | — | | | 549,451 | |
| | |
| |
| |
| |
| |
See notes to financial statements.
F-4
VASO ACTIVE PHARMACEUTICALS, INC.
STATEMENTS OF STOCKHOLDERS' DEFICIENCY
| | Years Ended December 31, 2001 and 2002
| |
|---|
| | Preferred stock
number of shares
| | $0.0001
Par value
| | Common
stock
number of
shares
| | $.0001
Par Value
| | Additional
Paid-in
Capital
| | Total Stockholders'
Deficiency
| |
|---|
| Balance January 1, 2001 | | — | | $ | — | | — | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
| |
| |
| |
| Loss from operations | | — | | | — | | — | | | — | | | — | | | (1,040,395 | ) |
| Contributed by parent | | — | | | — | | — | | | — | | | 1,040,395 | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| Balance December 31, 2001 | | — | | | — | | — | | | — | | | 1,040,395 | | | (1,040,395 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Loss from operations | | — | | | — | | — | | | — | | | — | | | (446,862 | ) |
| Contributed by parent | | — | | | — | | — | | | — | | | 446,862 | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| Balance December 31, 2002 | | — | | $ | — | | — | | $ | — | | $ | 1,487,257 | | $ | (1,487,257 | ) |
| | |
| |
| |
| |
| |
| |
| |
| | Nine Months Ended September 30, 2002 (Unaudited)
| |
|---|
| | Preferred stock
number of shares
| | $0.0001
Par value
| | Common stock
number of shares
| | $.0001
Par Value
| | Additional
Paid-in
Capital
| | Total Stockholders'
Deficiency
| |
|---|
| Balance January 1, 2002 | | — | | $ | — | | — | | $ | — | | $ | 1,040,395 | | $ | (1,040,395 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Loss from operations | | — | | | — | | — | | | — | | | — | | | (301,750 | ) |
| Contributed by parent | | — | | | — | | — | | | — | | | 301,750 | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| Balance September 30, 2002 (Unaudited) | | — | | $ | — | | — | | $ | — | | $ | 1,342,145 | | $ | (1,342,145 | ) |
| | |
| |
| |
| |
| |
| |
| |
| | Nine Months Ended September 30, 2003 (Unaudited)
| |
|---|
| | Preferred stock
number of shares
| | $0.0001
Par value
| | Common stock
number of shares
| | $.0001
Par Value
| | Additional
Paid-in
Capital
| | Total Stockholders'
Deficiency
| |
|---|
| Balance January 1, 2003 | | — | | $ | — | | — | | $ | — | | $ | 1,487,257 | | $ | (1,487,257 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Loss from operations | | — | | | — | | — | | | — | | | — | | | (349,951 | ) |
| Issuance of common stock to parent | | — | | | — | | 1,500,000 | | | 150 | | | — | | | — | |
| Effect of incorporation | | — | | | — | | — | | | — | | | (1,487,257 | ) | | 1,487,257 | |
| | |
| |
| |
| |
| |
| |
| |
Balance September 30, 2003
(Unaudited) | | — | | $ | — | | 1,500,000 | | $ | 150 | | $ | — | | $ | (349,951 | ) |
| | |
| |
| |
| |
| |
| |
| |
See notes to financial statements.
F-5
VASO ACTIVE PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
| | Years Ended December 31,
| | Nine Months Ended September 30,
| |
|---|
| | 2001
| | 2002
| | 2002
| | 2003
| |
|---|
| |
| |
| | (Unaudited)
| |
|---|
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | | | | | |
| Net loss from operations | | $ | (1,040,395 | ) | $ | (446,862 | ) | $ | (301,750 | ) | $ | (349,951 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | |
| Inventory | | | (17,359 | ) | | 2,857 | | | (2,164 | ) | | (33,936 | ) |
| Accounts receivable—trade | | | (380 | ) | | (53,057 | ) | | (56,712 | ) | | 53,437 | |
| Prepaid expenses | | | (2,484 | ) | | (1,820 | ) | | 2,484 | | | 4,304 | |
| Accounts payable—trade | | | 22,640 | | | 26,660 | | | (22,640 | ) | | 147,550 | |
| Accrued interest | | | — | | | — | | | — | | | 28,083 | |
| Accrued salaries, wages and taxes | | | 3,343 | | | (2,739 | ) | | (3,343 | ) | | (604 | ) |
| | |
| |
| |
| |
| |
| Cash used in operating activities | | | (1,034,635 | ) | | (474,961 | ) | | (384,125 | ) | | (151,117 | ) |
| | |
| |
| |
| |
| |
CASH FLOWS FROM FINANCING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Investment by parent | | | — | | | — | | | — | | | 150 | |
| Contributions from parent | | | 1,040,395 | | | 446,862 | | | 301,750 | | | — | |
| Payments from parent company (net) | | | — | | | 28,099 | | | — | | | — | |
| Issuance of convertible promissory notes | | | — | | | — | | | — | | | 500,000 | |
| Obligation from parent | | | (5,760 | ) | | — | | | 82,375 | | | 423,483 | |
| Prepaid public offering costs | | | — | | | — | | | — | | | (613,940 | ) |
| | |
| |
| |
| |
| |
| Cash provided by financing activities | | | 1,034,635 | | | 474,961 | | | 384,125 | | | 309,693 | |
| | |
| |
| |
| |
| |
| INCREASE IN CASH AND CASH EQUIVALENTS | | | — | | | — | | | — | | | 158,576 | |
| CASH AND CASH EQUIVALENTS, Beginning of Year | | | — | | | — | | | — | | | — | |
| | |
| |
| |
| |
| |
| CASH AND CASH EQUIVALENTS, End of Year | | $ | — | | $ | — | | $ | — | | $ | 158,576 | |
| | |
| |
| |
| |
| |
| SUPPLEMENTAL DISCLOSURES: | | | | | | | | | | | | | |
| Interest paid | | $ | — | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
| |
See notes to financial statements.
F-6
VASO ACTIVE PHARMACEUTICALS INC.
NOTES TO FINANCIAL STATEMENTS
For the Years Ended December 31, 2002 and 2001 (audited) and the
Nine Months Ended September 30, 2003 and 2002 (unaudited)
1. BACKGROUND AND BASIS OF PRESENTATION
In January 2003, BioChemics, Inc. ("BioChemics") created a wholly-owned subsidiary, Vaso Active Pharmaceuticals, Inc. ("the Company") comprised of BioChemics' over-the-counter pharmaceutical division. As discussed in Note 5, BioChemics received 1,500,000 shares of the subsidiary's Class B common stock in return for the exclusive worldwide rights to BioChemics' patented vaso active lipid encapsulated "VALE" technology for over-the-counter pharmaceutical products. The terms of this exchange are contained under a license agreement executed between BioChemics and the Company. These shares of Class B common stock were issued pursuant to authorization of the Board of Directors on June 20, 2003 (see note 5).
The financial statements of the Company reflect the historical results of operations and cash flows of the over-the-counter pharmaceutical division of BioChemics during each respective period. The financial statements have been prepared using Biochemic's historical bases in the assets and liabilities and historical results of operations of this division. BioChemics' net investment in the Company is reflected as additional paid-in capital. Transactions are processed through an inter-company account, of which the balance represents the net obligation from BioChemics to the Company. Additionally, these financial statements are included in BioChemics' consolidated financial statements on a consolidated basis.
The statements of operations include allocations of certain BioChemics expenses, such as centralized accounting, data processing, utilities, office space rental, supplies, telephone and other BioChemics corporate services and infrastructure costs. The expense allocations have been determined on the bases that BioChemics and the Company considered to be reasonable reflections of the utilization of services provided for the benefit received by the Company. In addition, the Company's marketing, advertising and promotion expenses include expenses related to the commercialization, marketing and distribution of the BioChemics athlete's foot preparation product, the revenues of which are realized by the Company through an assignment of BioChemics ownership interests in such revenues. The financial information included herein may not reflect the financial position, operating results, changes in stockholder's deficiency and cash flows of the Company in the future or what they would have been had the Company been a separate stand-alone entity during the periods presented. However, it is probable that the actual historic results would not be significantly different from those presented.
In accordance with generally accepted accounting principles with respect to the allocation of corporate management services, the financial statements of a carved out public registrant should appropriately reflect these expenses to fairly present its operating results with an offset to additional paid-in capital. In addition, it is required that upon incorporation of a carved out division into a stand-alone entity, the accumulated deficiency balance should be eliminated against additional paid-in capital. During January 2003, upon incorporation of the Company, the existing accumulated deficiency of $1,487,257 was eliminated using such treatment.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies is presented to assist in understanding the financial statements of the Company. The financial statements and notes are representations of the Company's
F-7
management who are responsible for their fair presentation, integrity and objectivity. These accounting policies conform with accounting principles generally accepted in the United States of America.
Interim Financial Information
The financial information presented as of September 30, 2003 and 2002 and for the nine months then ended is unaudited and includes all adjustments, consisting only of normal recurring accruals, that management considers necessary for a fair presentation of its financial position, operating results and cash flows. Results for the nine months ended September 30, 2003 are not necessarily indicative of results to be expected for the full fiscal year ended December 31, 2003 or for any future period.
Business Description
The Company is a transdermal drug delivery company engaged in developing, licensing, marketing and distributing a broad range of over-the-counter pharmaceutical products. The Company has incurred operating losses since inception. The Company's principal risks are the ability to obtain adequate financing, successful development and marketing of products, competition from substitute products and larger companies, dependence on key individuals and continued assistance from BioChemics. BioChemics is a company that has experienced significant operating deficits since its inception in 1989, and it anticipates on-going significant losses as it evolves through its own development process. These financial statements have been prepared on the going concern assumption that the Company will be able to realize its assets and discharge its liabilities in the normal course of business. This assumption is presently in question and contingent upon the Company's ability to raise additional funds through equity or debt financing and successfully utilize these funds to commercialize products.
Management has identified various fund-raising strategies that it is currently implementing. These include the issuance of $500,000 in convertible promissory notes, the proceeds of which the Company had received in full as of April 21, 2003, which notes will be converted into shares of Class A common stock mandatorily upon consummation of a qualified public offering (see note 5) and an authorization by the Company's Board of Directors on June 20, 2003 to issue 1,000,000 shares of its Class A common stock, plus up to an additional 150,000 shares of Class A common stock to cover over-allotments, if any, at a price per share of $5.00 in an initial public offering to a group of underwriters (see note 5).
On August 22, 2003, the Company's Board of Directors authorized an increase in the initial public offering to 1,200,000 shares of Class A common stock, plus up to an additional 180,000 shares to cover over-allotments, if any, at the same price per share. Further on October 27, 2003, the Company's Board of Directors authorized an increase in the initial public offering to 1,300,000 of Class A common stock, plus up to an additional 195,000 shares to cover over-allotments if any, at the same price per share.
Accounting Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that effect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities, and the reported amounts of revenue and expenses. Actual results could differ from those estimates.
F-8
Cash and Cash Equivalents
Cash and cash equivalents include cash in banks, money market funds, securities of the US Treasury, and certificates of deposit with original maturities of 90 days or less. Historically, BioChemics has managed cash and cash equivalents on a centralized basis. Cash receipts associated with the Company's business have been transferred to BioChemics on a periodic basis and BioChemics has funded the Company's disbursements.
Accounts Receivable
Accounts receivable consists primarily of trade receivables from the sale of over-the-counter pharmaceutical products. The allowance for doubtful accounts is based on the Company's assessment of the collectibility of specific customer accounts and an assessment of economic risk as well as the aging of the accounts receivable. The Company's policy is to write-off uncollectible trade receivables after significant measures have failed to result in their collection. An allowance for doubtful accounts is established to represent the estimated uncollectible trade receivables. The Company expects to collect in full all outstanding trade receivables at December 31, 2002. As of December 31, 2002 and September 30, 2003, the Company held trade receivables that are over ninety days past due of approximately $43,000 and $0 respectively. The Company expects to collect in full all outstanding trade receivables for all periods presented and, therefore no allowance for doubtful accounts provision has been established.
Inventory
Inventory is valued at lower of cost or market on a first-in first-out basis. The Company uses outside contract manufacturers for the production of its products, therefore all of the inventory balance is in the form of finished goods.
Prepaid Expenses
Disbursements made in the current period that are actual charges of future periods are classified as prepaid expenses. The September 30, 2003 amounts in prepaid expenses represent charges incurred through this date that relate to the public offering described in note 5.
Due to/from Parent Company
The Company's transactions are processed through an inter-company account, due to/from parent, the balance of which represents the net obligation from the Company to BioChemics or vice-versa. BioChemics funded the Company during the period of time in which the Company was a division. BioChemics provided the Company $1,040,395, $446,862 and $301,750 during the years ended December 31, 2002 and 2001 and the nine-month period ended September 30, 2002, respectively. These amounts are not required to be paid back by the Company. Due to the fact that certain over-the-counter product contracts are with BioChemics, the Company also records on its financial statements over-the-counter activities the net impact of which has resulted in the recognition of certain receivables and liabilities of BioChemics on the Company's financial statements. The net impact of recording these accounts on the Company's financial statements has necessitated the need to reflect a
F-9
receivable from BioChemics for the residual obligation incurred only during the time the Company was a division of BioChemics. In the nine-month period ended September 30, 2003, the Company became a stand-alone entity effective upon its incorporation in January 2003. During this period of time, the Company has incurred management fees to BioChemics of approximately $99,000 and other operational charges of approximately $347,000 that have been paid by BioChemics on the Company's behalf. These advances will be required to be repaid upon the consummation of an initial public offering (see note 5). The obligation approximated $446,000 as of September 30, 2003.
Revenue Recognition
The Company recognizes revenue from product sales in accordance with generally accepted accounting principles in the United States, including the guidance in Staff Accounting Bulletin 101. Revenue from product sales is recognized when there is persuasive evidence of an arrangement, delivery has occurred, the price is fixed and determinable, and collectibility is reasonably assured. However, because our products are sold with limited rights of return, our recognition of revenue from product sales is also subject to Statement of Financial Accounting Standards No. 48 (SFAS 48) "Revenue Recognition When Right of Return Exists." Under SFAS 48, revenue is recognized when the price to the buyer is fixed, the buyer is obligated to pay us and the obligation to pay is not contingent on resale of the product, the buyer has economic substance apart from the us, we have no obligation to bring about the sale of the product and the amount of returns can be reasonably estimated.
The Company records allowances for product returns, rebates and discounts, and reports revenue net of such allowances. We must make judgments and estimates in preparing the allowances that could require adjustments in the future. For instance, our customers have the right to return any product that is held past the labeled expiration date. We base our estimates on historic patterns of returns and on the expiration dates of product currently being shipped.
We do not recognize revenue unless collectibility is reasonably assured. We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. If the financial condition of our customers were to deteriorate and result in an impairment of their ability to make payments, additional allowances may be required.
Fair Values
Unless otherwise noted, deposits, accounts payable, accrued expenses and other liabilities have all been stated at values that approximate fair value.
Income Taxes
The Company's operating results have historically been included in BioChemics' federal and state income tax returns. Effective January 1, 2003, the Company will be preparing and filing federal and state income returns on a stand-alone basis. As a result, the Company will no longer be included as part of BioChemics' consolidated tax return as a wholly-owned subsidiary. As such, the Company will account for income taxes separately and in accordance with Statement of Financial Accounting
F-10
Standards (SFAS) No. 109,Accounting for Income Taxes, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities, using enacted tax rates in effect for the year in which the differences are expected to be recovered or settled. Under SFAS No. 109, the effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
Historical Earnings (Loss) Per Share
The historical capital structure of the Company is not representative of the future capital structure. Accordingly, the historical net loss per share and weighted average number of common shares outstanding are not shown for any periods presented prior to January 1, 2003. See note 7 for required pro-forma calculations. Shares used in the computation of basic and diluted loss per share represent the weighted average shares outstanding during the period.
Advertising Costs
Costs incurred to advertise and promote the Company are expensed as incurred. Advertising costs were approximately $39,000 and $557,000 for the years ended December 31, 2002 and 2001, respectively, and approximately $87,000 and $11,500 for the nine-month periods ended September 30, 2003 and 2002 respectively.
Sales Incentives
Sales incentives when incurred are netted against revenue. These are made up primarily of slotting fees, coupons and rebates. These approximated $5,000 and $24,300 for the years ended December 31, 2002 and 2001, respectively and $5,000 for each of the nine-month periods ended September 30, 2003 and 2002, respectively.
Research and Development Costs
There were no research and development costs incurred for any of the periods presented on the financial statements.
Management Fees
Allocations of BioChemics' expenses such as centralized accounting, data processing, utilities, office space rental, supplies, telephone and other BioChemics' corporate services and infrastructure are charged back to the Company as a management fee. These amounts approximated $135,600 and $114,150 for the years ended December 31, 2002 and 2001, respectively and approximately $99,000 and $89,000 for the nine-month periods ended September 30, 2003 and 2002, respectively.
F-11
3. INCOME TAXES
There are no income taxes on a pro-forma basis for the years ended December 31, 2002 and 2001 or the nine-month period ended September 30, 2002. There are also no income taxes for the nine-month period ended September 30, 2003. The components of the Company's deferred tax asset are as follows:
| | September 30, 2003
(pro forma)
| | September 30, 2002
(pro-forma)
| | December 31, 2002
(pro forma)
| | December 31, 2001
(pro forma)
| |
|---|
| |
| | (Unaudited)
| |
| |
| |
|---|
| Deferred tax assets: | | | | | | | | | | | | | |
| | Net operating loss | | $ | 139,980 | | $ | 536,858 | | $ | 594,903 | | $ | 416,158 | |
| | Less: Valuation allowance | | | (139,980 | ) | | (536,858 | ) | | (594,903 | ) | | (416,158 | ) |
| | |
| |
| |
| |
| |
| Net deferred tax asset | | $ | — | | $ | — | | $ | — | | $ | — | |
| | |
| |
| |
| |
| |
As of September 30, 2003, the Company has net operating loss carryforwards for federal and state income taxes of approximately $139,980 that are available to offset future taxable income and expire in 2023.
4. CONCENTRATIONS OF CREDIT RISK
Concentrations of credit risk associated with gross revenues arose as a result of sales to significant customers. One customer accounted for 66% of gross revenues for the year ended December 31, 2002. Two customers accounted for 98% of gross revenues for the year ended December 31, 2001. Accounts receivable from these customers were $53,437 in 2002 and $0 in 2001. One customer accounted for 96% and 77% of gross revenues for the nine-month periods ended September 30, 2003 and 2002, respectively. Accounts receivable from this customer were $0 and $57,092 as of September 30, 2003 and 2002, respectively.
5. CAPITALIZATION/SUBSEQUENT EVENTS
As of December 31, 2002 and 2001, the Company was still a division of BioChemics and accordingly maintained no classes of stock. Upon incorporation in January 2003, the Company maintained two classes of stock; common stock and preferred stock. The Company had 5,000,000 shares of common authorized with none issued or outstanding. There were 25,000 shares of preferred stock authorized with none issued or outstanding. The Company also had reserved up to 300,000 shares of common stock that it expected to issue pursuant to a future stock option plan.
Subsequent to incorporation, the Company initiated a private placement to sell up to $500,000, 10% convertible subordinated pay-in-kind promissory notes of the Company at par. In April 2003, the Company completed the private placement. All notes are convertible into common stock of the Company either voluntarily on or before the maturity date of the note or mandatorily upon the consummation of a qualified public offering. The Notes will be converted at the lesser of 50% of the Qualified Public Offering Price or $2.50 up to a maximum of 220,000 shares of Class A common stock.
F-12
The convertible notes have beneficial conversion features as discussed in note 6 that will result in a charge to income upon the consummation of a public offering.
On June 20, 2003, the Board of Directors of the Company took the following actions: (i) resolved to amend the Company's Certificate of Incorporation to (a) increase the number of shares of preferred stock that the Company shall have authority to issue from 25,000 shares to 10,000,000 shares, (b) increase the number of shares of common stock that the Company shall have authority to issue from 5,000,000 shares without designation to 10,000,000 shares of Class A common stock and 5,000,000 shares of Class B common stock; (ii) authorized the adoption of the Company's 2003 Stock Incentive Plan, (iii) authorized the reservation of 450,000 shares of the Company's Class A common stock for issuance upon the exercise or grant of Incentives under the 2003 Stock Incentive Plan; (iv) adopted and ratified the license agreement between the Company and BioChemics executed as of February 1, 2003; (v) authorized the Company to issue 1,500,000 of its Class B common stock to BioChemics pursuant to the license agreement in exchange for the license granted to the Company by BioChemics thereunder, and (vi) authorized the Company to make an initial public offering of 1,000,000 shares of its Class A common stock, plus up to an additional 150,000 shares of Class A common stock to cover overallotments, if any, at a price per share of $5.00 to a group of underwriters. Class A common stock will have one vote per share. Class B common stock will have three votes per share and will be convertible into shares of Class A common stock on a one-for-one basis.
On August 22, 2002, the Board of Directors of the Company authorized an increase in the number of shares of Class A common stock in the initial public offering from 1,000,000 to 1,200,000 and authorized an increase in the number of shares of Class A common stock to cover over-allotments, if any, from 150,000 to 180,000, at the same price per share in each case.
On October 27, 2003, the Company's Board of Directors authorized an increase in the initial public offering to 1,300,000 of Class A common stock, plus up to an additional 195,000 shares to cover over-allotments if any, at the same price per share. Pursuant to the underwriters agreement to be executed in connection with the initial public offering, the Company will issue to the representative of the underwriters 130,000 warrants to purchase an equivalent number of shares of Class A common stock at $6.00 per share.
6. COMMITMENTS AND CONTINGENCIES
The Company may become involved in litigation or other claims in the ordinary course of business. Management is not aware of any claims that will have a material adverse effect on the financial condition of the Company.
Upon completion of an initial public offering (see note 5), the Company will incur a non-cash interest charge of approximately $533,000 as a result of the beneficial conversion feature associated with the Company's convertible promissory notes discussed in note 5.
F-13
7. REQUIRED PRO FORMA INFORMATION (UNAUDITED)
Required pro forma financial information is identical in content to that information presented on the financial statements with the exception of required loss per share data. The pro forma loss per share calculations below are presented as if the 1,500,000 shares of Class B common stock had been issued to BioChemics as of January 1, 2001 and as if the $500,000, 10% convertible promissory notes had been converted to Class A common stock as of January 1, 2001. Accordingly, pro forma loss per share for the years ended December 31, 2002 and 2001 and the nine-month periods ended September 30, 2003 and 2002 are $0.26, $0.61, $0.20 and $0.18 respectively.
F-14
1,300,000 Shares

Class A Common Stock
PROSPECTUS
Kashner Davidson
Securities Corp.
November , 2003
No underwriter, dealer, salesperson or any other person has been authorized to give any information or to make any representations other than those contained in this prospectus and, if given or made, such information or representations must not be relied upon as having been authorized by us or any underwriter. Neither the delivery of this prospectus nor any sale made hereunder shall, under any circumstances, create any implication that there has been no change in our business or affairs since the date hereof or that the information contained in this prospectus is correct as of any time subsequent to the date hereof. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered hereby by anyone in any jurisdiction in which such offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
Until 2003, all dealers that effect transactions in these shares of common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II—INFORMATION NOT REQUIRED IN PROSPECTUS
Item 24. Indemnification of Directors and Officers.
Section 145 of the DGCL empowers a corporation to indemnify its directors and officers and to purchase insurance with respect to liability arising out of their capacity or status as directors and officers, provided that this provision shall not eliminate or limit the liability of a director: (i) for any breach of the director's duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) arising under Section 174 of the DGCL, or (iv) for any transaction from which the director derived an improper personal benefit. The DGCL provides further that the indemnification permitted thereunder shall not be deemed exclusive of any other rights to which the directors and officers may be entitled under the corporation's bylaws, any agreement, a vote of stockholders or otherwise.
The Registrant's certificate of incorporation and our by-laws provide that its directors and officers will be indemnified by us to the fullest extent authorized by Delaware law, as it now exists or may in the future be amended, against all expenses and liabilities reasonably incurred in connection with their service for or on our behalf. In addition, the Registrant's certificate of incorporation provides that its directors will not be personally liable for monetary damages for breaches of their fiduciary duty as directors, unless they violated their duty of loyalty to the Registrant or the Registrant's stockholders, acted in bad faith, knowingly or intentionally violated the law, authorized illegal dividends or redemptions or derived an improper personal benefit from their action as directors.
The Registrant will be obtaining liability insurance coverage for its officers and directors.
The underwriting agreement provides that the underwriters are obligated, under certain circumstances, to indemnify directors, officers and controlling persons of Vaso Active against certain liabilities, including liabilities under the Securities Act. Reference is made to the form of underwriting agreement filed as Exhibit 1.1 hereto.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons issuer pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than a payment by us of expenses incurred or paid by a director, officer or controlling person of Vaso Active in the defense of any action, suit or proceeding) is asserted by such officer, director or controlling person in connection with the securities being registered, we will, unless in the opinion of our legal counsel that matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-1
Item 25. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses payable by Vaso Active Pharmaceuticals, Inc. in connection with the sale of the securities being registered. All amounts are estimates except the SEC registration fee:
| SEC registration fee | | $ | 675 |
| Boston Stock Exchange filing fee | | $ | 3,000 |
| NASD Filing Fee | | $ | 1,075 |
| Blue Sky fees and expenses (including legal fees) | | $ | 25,000 |
| Printing and EDGAR Conversion expenses* | | $ | 60,000 |
| Accounting fees and expenses* | | $ | 50,000 |
| Legal fees and expenses* | | $ | 350,000 |
| Transfer agent's fees and expenses* | | $ | 2,500 |
| Miscellaneous* | | $ | 157,750 |
| | |
|
| Total | | $ | 650,000 |
| | |
|
- *
- Estimated
Item 26. Recent Sales of Unregistered Securities.
Set forth below is information regarding the issuance and sales of the Registrant's common stock without registration during the last three years. Other than as set forth below, no such sales involved the use of an underwriter and no commissions were paid in connection with the sale of any securities. All of the issuances were made in arm's length transactions which did not involve a public offering:
1. In April 2003, the Registrant raised approximately $500,000 by issuing subordinated 10% convertible pay-in-kind promissory notes to a small group of private accredited investors, including one of our directors, in reliance on the exemption from registration set forth in Section 4(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder and on similar exemptions in states where the securities were issued (the "Private Placement"). These notes are convertible into the Registrant's Class A common stock. The maturity date of the notes is March 31, 2005 and the conversion price of the notes is the lower of 50% of the public offering price (as defined in the notes) or $2.50, subject to certain adjustments. Conversion may occur on either a mandatory or voluntary basis. Each note may be voluntarily converted in whole or in part at any time prior to the maturity date by the note's holder or on a mandatory basis upon the consummation of a qualified public offering. The proceeds from the placement of these notes have been used for funding the expenses related to this offering. Upon the consummation of this offering, these notes will be converted into an aggregate of 214,000 shares of the Registrant's Class A common stock.
2. In June, 2003, the Registrant issued 1,500,000 shares of its Class B common stock to BioChemics as full consideration for the granting of a license by BioChemics to the Registrant of the right to use and practice the BioChemic's patents to sell, market and commercialize all products that (i) utilize and incorporate BioChemics' patents and know-how; (ii) are classified by the FDA as "over-the-counter products", and (iii) require less than one million dollars ($1,000,000) of clinical development. The Registrant relied on Section 4(2) of the Securities Act and the rules and regulations promulgated thereunder in issuing these securities without registration under the Securities Act.
II-2
Item 27. Exhibits.
Exhibit Number
| | Description
|
|---|
| 1.1 | | Amended Form of Underwriting Agreement* |
3.1 |
|
Amended and Restated Certificate of Incorporation** |
3.2 |
|
Amended and Restated By-laws** |
4.1 |
|
Specimen Certificate for Class A common stock** |
4.2 |
|
Form of 10% convertible pay-in-kind promissory note for investors in the Private Placement** |
4.3. |
|
Amended Form of Representative's Warrant Agreement* |
5 |
|
Opinion of Robinson & Cole LLP* |
10.1 |
|
Employment Agreement, effective June 16, 2003 by and between Vaso Active Pharmaceuticals, Inc. and John J. Masiz** |
10.2 |
|
Employment Agreement, effective June 16, 2003 by and between Vaso Active Pharmaceuticals, Inc. and Stephen G. Carter, Ph.D** |
10.3 |
|
2003 Stock Incentive Plan** |
10.4 |
|
Form of 2003 Non-Employee Director Compensation Plan.** |
10.5 |
|
Registration Rights Agreement by and between Vaso Active Pharmaceuticals, Inc. and BioChemics, Inc.** |
10.6 |
|
License Agreement, dated as of February 1, 2003, between BioChemics, Inc. and Vaso Active Pharmaceuticals, Inc., as amended by an amendment agreement, dated as of July 2, 2003, between Vaso Active Pharmaceuticals, Inc. and BioChemics, Inc.** |
10.7 |
|
Manufacturing and Development Agreement, dated as of February 1, 2003, between BioChemics, Inc. and Vaso Active Pharmaceuticals, Inc.** |
10.8 |
|
Administrative Services Agreement, dated as of September 1, 2003, between BioChemics, Inc. and Vaso Active Pharmaceuticals, Inc.** |
10.9 |
|
Form of Advisory and Investment Banking Agreement between Kashner Davidson Securities Corp. and Vaso Active Pharmaceuticals, Inc.* |
23.1 |
|
Amended Consent of Stowe & Degon* |
23.2 |
|
Consent of Robinson & Cole LLP (see Exhibit 5)* |
- *
- Filed herewith
- **
- Previously filed
Item 28. Undertakings.
The Registrant hereby undertakes:
(1) To file, during any period in which it offers or sells securities, a post-effective amendment to this registration statement to:
II-3
(ii) Reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) (§230.424(b) of this chapter) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement; and
(iii) Include any additional or changed material information on the plan of distribution.
(2) For determining liability under the Securities Act, treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering.
(3) File a post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering.
(4) To provide to the underwriter at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
(5) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
(6) In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Amendment No. 3 to the Registration Statement on Form SB-2 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Boston, State of Massachusetts, on November 13, 2003.
| | | VASO ACTIVE PHARMACEUTICALS, INC. |
|
|
By: |
|
/s/ JOHN J. MASIZ
John J. Masiz
President, Chief Executive Officer and Director |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints John J. Masiz and Joseph Frattaroli and each of them individually, with full power of substitution and resubstitution, his or her true and lawful attorney-in-fact and agent, with full powers to each of them to sign for us, in our names and in the capacities indicated below, the Registration Statement on Form SB-2 filed with the Securities and Exchange Commission, and any and all amendments to said Registration Statement, (including post-effective amendments) filed under the Securities Act of 1933, as amended, in connection with the registration under the Securities Act of 1933, as amended, of equity securities of the Registrant, and to file or cause to be filed the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each of them might or could do in person, and hereby ratifying and confirming all that said attorneys, and each of them, or their substitute or substitutes, shall do or cause to be done by virtue of this Power of Attorney. This Power of Attorney may be executed in counterparts and all capacities to sign any and all documents.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment No. 3 to the Registration Statement has been signed below by the following persons in the capacities and on the dates indicated.
Name
| | Title
| | Date
|
|---|
|
|
|
|
|
/s/ JOHN J. MASIZ
John J. Masiz | | President, Chief Executive Officer and Director (Principal Executive Officer) | | November 13, 2003 |
/s/ JOSEPH FRATTAROLI
Joseph Frattaroli |
|
Chief Financial Officer and Secretary (Principal Financial and Principal Accounting Officer) |
|
November 13, 2003 |
/s/ STEPHEN G. CARTER, PH.D
Stephen G. Carter, Ph.D. |
|
Chief Scientific Officer and Director |
|
November 13, 2003 |
| | | | | |
II-5
/s/ BRUCE A. SHEAR
Bruce A. Shear |
|
Director |
|
November 13, 2003 |
/s/ BRIAN J. STRASNICK, PH.D
Brian J. Strasnick, Ph.D. |
|
Director |
|
November 13, 2003 |
/s/ ROBERT E. ANDERSON
Robert E. Anderson |
|
Director |
|
November 13, 2003 |
/s/ GARY FROMM, PH.D
Gary Fromm, Ph.D. |
|
Director |
|
November 13, 2003 |
/s/ WILLIAM P. ADAMS, M.D.
William P. Adams, M.D. |
|
Director |
|
November 13, 2003 |
II-6



