|
Exhibit 99.2 
|
Acquisition of Gentium
Adds Defitelio, an EMA-approved orphan drug
Overview Presentation
December 19, 2013
Forward-Looking Statements
“Safe Harbor” Statement under the Private Securities Litigation Reform Act of 1995
This presentation contains forward-looking statements, including, but not limited to, statements related to the anticipated consummation of the tender offer for Gentium S.p.A. ordinary shares and American Depositary Shares and the timing and benefits thereof, Jazz Pharmaceuticals’ expected financing for the transaction, the plan to launch Defitelio™ and the timing thereof, the potential to develop Defitelio for approval in other conditions, future commercial opportunities, future financial results, anticipated pipeline opportunities and future regulatory matters as well as other statements that are not historical facts. These forward-looking statements are based on the company’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, risks related to Jazz Pharmaceuticals’ ability to complete the acquisition on the proposed terms and schedule, including risks and uncertainties related to the satisfaction of closing conditions and the availability and terms of the financing for the transaction; risks associated with business combination transactions, such as the risk that the acquired business will not be integrated successfully or that such integration may be more difficult, time-consuming or costly than expected; risks related to future opportunities and plans for the combined company, including uncertainty of the expected financial performance and results of the combined company following completion of the proposed acquisition; disruption from the proposed acquisition, making it more difficult to conduct business as usual or maintain relationships with customers, employees or suppliers; the calculations of, and factors that may impact the calculations of, the acquisition price in connection with the proposed transaction and the allocation of such acquisition price to the net assets acquired in accordance with applicable accounting rules and methodologies; and the possibility that if Jazz Pharmaceuticals does not achieve the perceived benefits of the proposed acquisition of Gentium as rapidly or to the extent anticipated by financial analysts or investors, the market price of Jazz Pharmaceuticals’ ordinary shares could decline; as well as other risks related the company’s business, including risks and uncertainties associated with maintaining and increasing sales of and revenue from Xyrem®; effectively commercializing the company’s other marketed products, including Erwinaze® and Prialt®; protecting and expanding the company’s intellectual property rights; obtaining appropriate pricing and reimbursement for the company’s products in an increasingly challenging environment; ongoing regulation and oversight by U.S. and non-U.S. regulatory agencies; dependence on key customers and sole source suppliers, the difficulty and uncertainty of pharmaceutical product development; and those other risks detailed under the caption “Risk Factors” and elsewhere in Jazz Pharmaceuticals plc’s U.S. Securities and Exchange Commission (“SEC”) filings and reports, including in the Quarterly Report on Form 10-Q for the quarter ended September 30, 2013 filed with the SEC and future filings and reports by the company. Jazz Pharmaceuticals undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or changes in its expectations.
2
Strategic Rationale
Executing on Growth Strategy
January 2012
Merger with
Azur Pharma
June 2005
Acquired
Orphan Medical February 2013
Agreement with Concert
Pharmaceuticals
JZP-386
March 2003 February 2007 June 2012 Expect to close 1Q14
Jazz Licensed from Acquired Acquisition of Gentium
Pharmaceuticals Solvay Pharmaceuticals EUSA Pharma
Founded Defitelio™
4
New Addition to our Commercial Portfolio
HEMATOLOGY/
SLEEP PAIN PSYCHIATRY
ONCOLOGY
Defitelio™
(defibrotide)
5
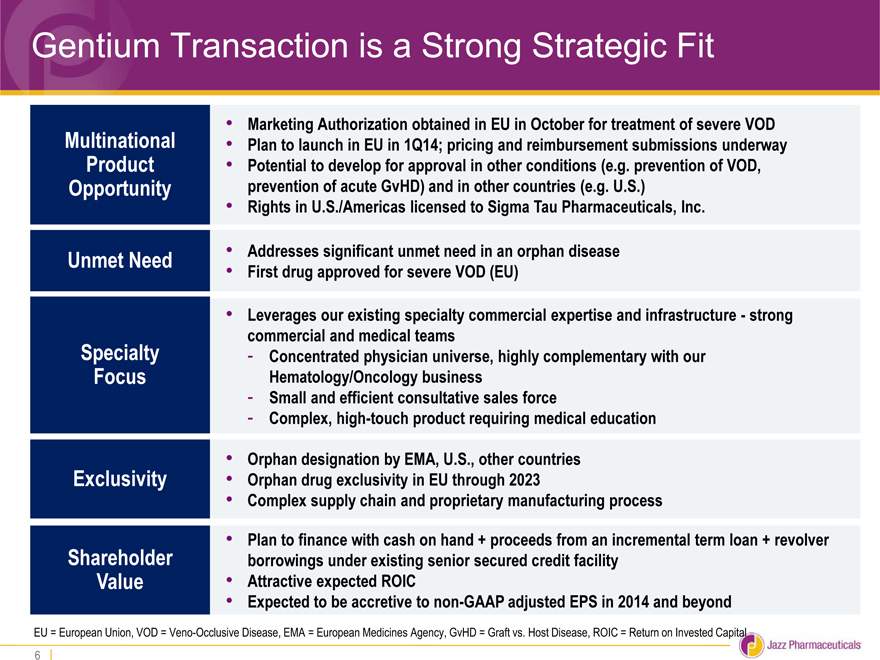
Gentium Transaction is a Strong Strategic Fit
• Marketing Authorization obtained in EU in October for treatment of severe VOD
Multinational • Plan to launch in EU in 1Q14; pricing and reimbursement submissions underway
Product • Potential to develop for approval in other conditions (e.g. prevention of VOD,
Opportunity prevention of acute GvHD) and in other countries (e.g. U.S.)
• Rights in U.S./Americas licensed to Sigma Tau Pharmaceuticals, Inc.
Unmet Need • Addresses significant unmet need in an orphan disease
• First drug approved for severe VOD (EU)
• Leverages our existing specialty commercial expertise and infrastructure—strong
commercial and medical teams
Specialty—Concentrated physician universe, highly complementary with our
Focus Hematology/Oncology business
—Small and efficient consultative sales force
—Complex, high-touch product requiring medical education
• Orphan designation by EMA, U.S., other countries
Exclusivity • Orphan drug exclusivity in EU through 2023
• Complex supply chain and proprietary manufacturing process
• Plan to finance with cash on hand + proceeds from an incremental term loan + revolver
Shareholder borrowings under existing senior secured credit facility
Value • Attractive expected ROIC
• Expected to be accretive to non-GAAP adjusted EPS in 2014 and beyond
EU = European Union, VOD = Veno-Occlusive Disease, EMA = European Medicines Agency, GvHD = Graft vs. Host Disease, ROIC = Return on Invested Capital
6
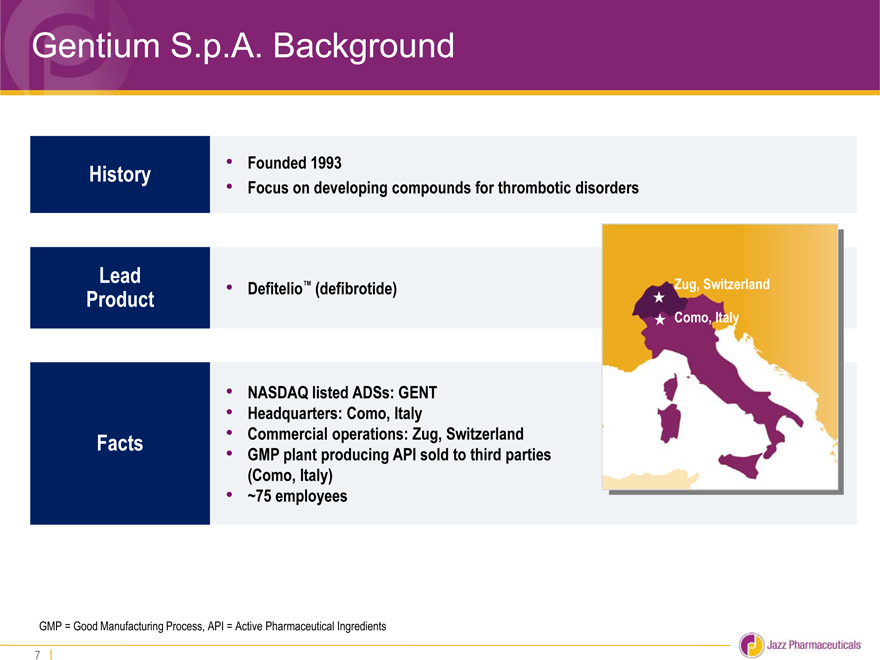
Gentium S.p.A. Background
History • Founded 1993
• Focus on developing compounds for thrombotic disorders
Lead
Product • Defitelio™ (defibrotide) Zug, Switzerland
Como, Italy
• NASDAQ listed ADSs: GENT
• Headquarters: Como, Italy
Facts • Commercial operations: Zug, Switzerland
• GMP plant producing API sold to third parties
(Como, Italy)
• ~75 employees
GMP = Good Manufacturing Process, API = Active Pharmaceutical Ingredients
7
Clinical Overview
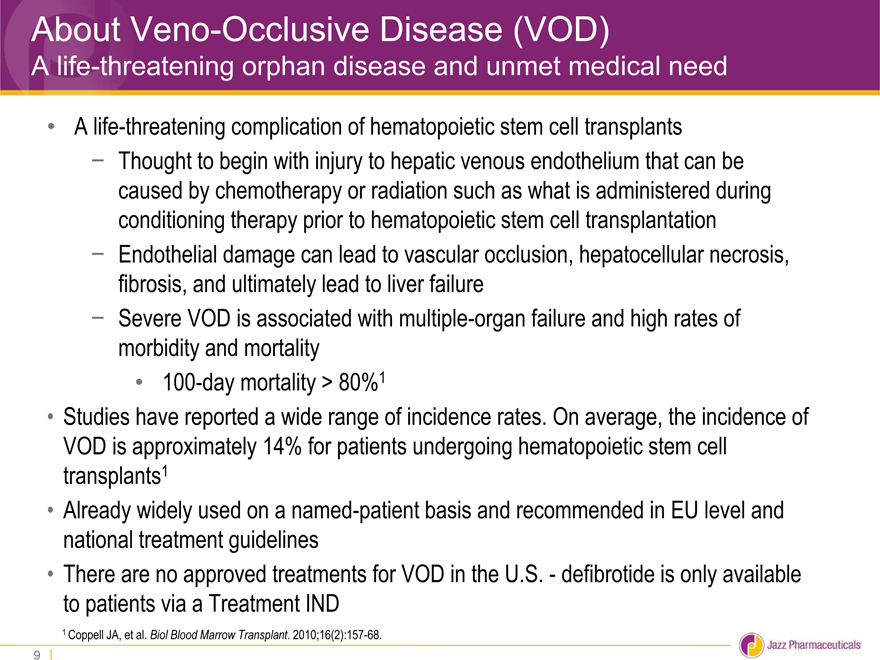
About Veno-Occlusive Disease (VOD)
A life-threatening orphan disease and unmet medical need
A life-threatening complication of hematopoietic stem cell transplants
- Thought to begin with injury to hepatic venous endothelium that can be
caused by chemotherapy or radiation such as what is administered during
conditioning therapy prior to hematopoietic stem cell transplantation
- Endothelial damage can lead to vascular occlusion, hepatocellular necrosis,
fibrosis, and ultimately lead to liver failure
- Severe VOD is associated with multiple-organ failure and high rates of
morbidity and mortality
100-day mortality > 80%1
Studies have reported a wide range of incidence rates. On average, the incidence of
VOD is approximately 14% for patients undergoing hematopoietic stem cell
transplants1
Already widely used on a named-patient basis and recommended in EU level and
national treatment guidelines
There are no approved treatments for VOD in the U.S.—defibrotide is only available
to patients via a Treatment IND
1 Coppell JA, et al. Biol Blood Marrow Transplant. 2010;16(2):157-68.
9
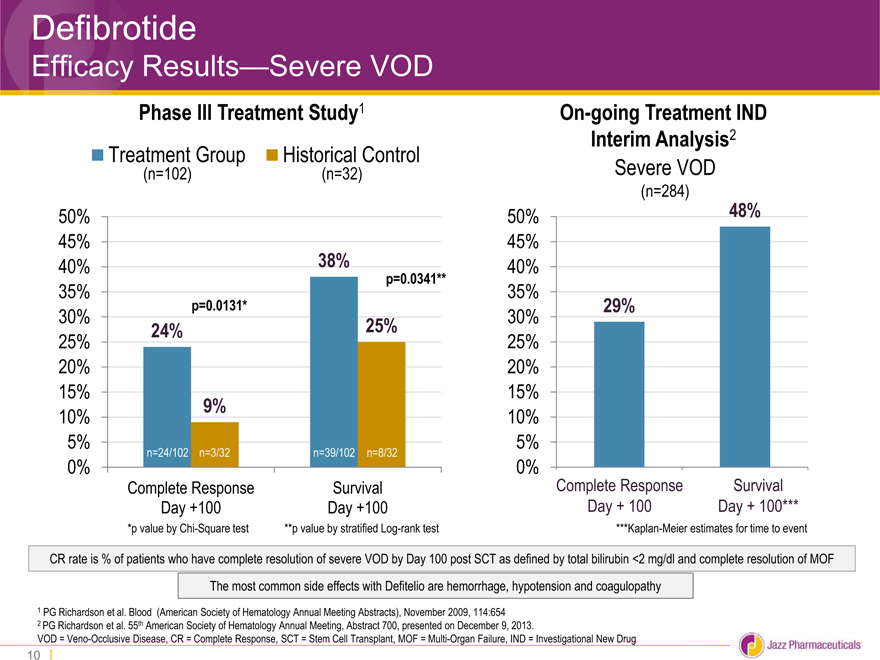
Defibrotide
Efficacy Results—Severe VOD
Phase III Treatment Study1
Treatment Group Historical Control
(n=102)(n=32)
50%
45%
40% 38%
p=0.0341**
35%
p=0.0131*
30% 24% 25%
25%
20%
15%
10% 9%
5% n=24/102 n=3/32 n=39/102 n=8/32
0%
Complete Response Survival
Day +100 Day +100
*p value by Chi-Square test **p value by stratified Log-rank test
On-going Treatment IND
Interim Analysis2
Severe VOD
(n=284)
50% 48%
45%
40%
35%
30% 29%
25%
20%
15%
10%
5%
0%
Complete Response Survival
Day + 100 Day + 100***
***Kaplan-Meier estimates for time to event
CR rate is % of patients who have complete resolution of severe VOD by Day 100 post SCT as defined by total bilirubin <2 mg/dl and complete resolution of MOF The most common side effects with Defitelio are hemorrhage, hypotension and coagulopathy
1 PG Richardson et al. Blood (American Society of Hematology Annual Meeting Abstracts), November 2009, 114:654
2 PG Richardson et al. 55th American Society of Hematology Annual Meeting, Abstract 700, presented on December 9, 2013.
VOD = Veno-Occlusive Disease, CR = Complete Response, SCT = Stem Cell Transplant, MOF = Multi-Organ Failure, IND = Investigational New Drug
10
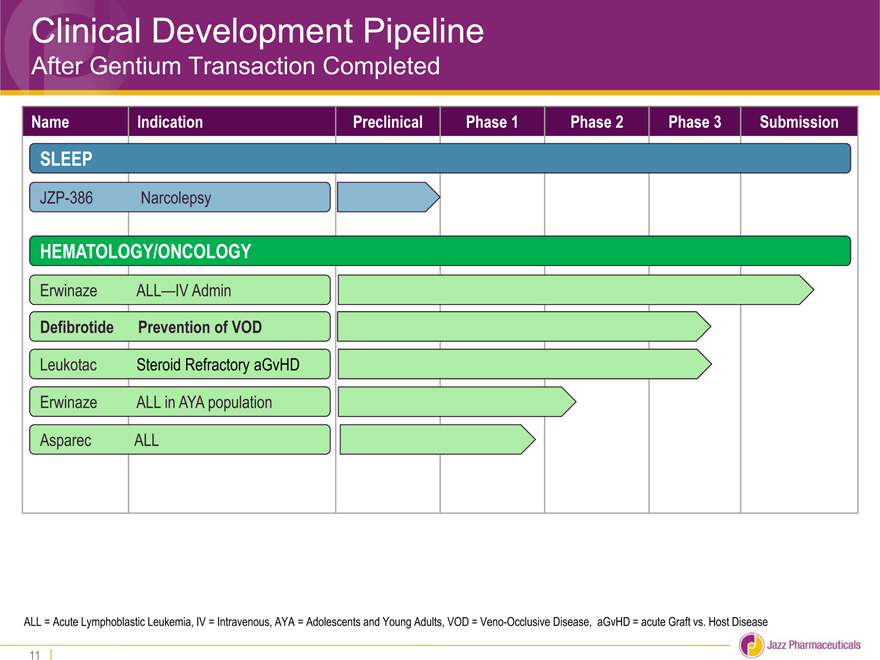
Clinical Development Pipeline
After Gentium Transaction Completed
Name Indication Preclinical Phase 1 Phase 2 Phase 3 Submission
SLEEP
JZP-386 Narcolepsy
HEMATOLOGY/ONCOLOGY
Erwinaze ALL—IV Admin
Defibrotide Prevention of VOD
Leukotac Steroid Refractory aGvHD
Erwinaze ALL in AYA population
Asparec ALL
ALL = Acute Lymphoblastic Leukemia, IV = Intravenous, AYA = Adolescents and Young Adults, VOD = Veno-Occlusive Disease, aGvHD = acute Graft vs. Host Disease
11
Commercial Opportunity
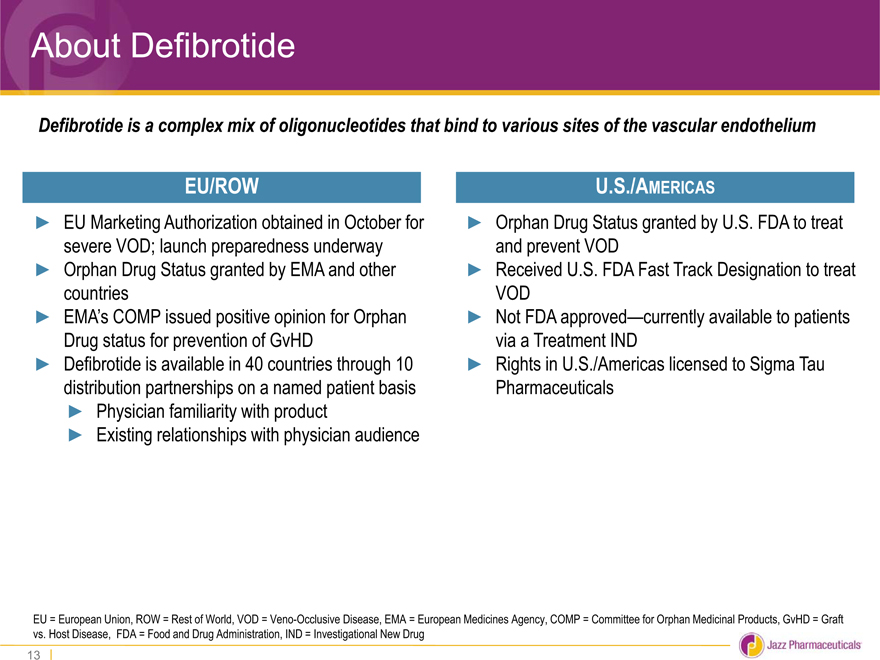
About Defibrotide
Defibrotide is a complex mix of oligonucleotides that bind to various sites of the vascular endothelium
EU/ROW
EU Marketing Authorization obtained in October for
severe VOD; launch preparedness underway
Orphan Drug Status granted by EMA and other
countries
EMA��s COMP issued positive opinion for Orphan
Drug status for prevention of GvHD
Defibrotide is available in 40 countries through 10
distribution partnerships on a named patient basis
Physician familiarity with product
Existing relationships with physician audience
U.S./AMERICAS
Orphan Drug Status granted by U.S. FDA to treat and prevent VOD
Received U.S. FDA Fast Track Designation to treat VOD
Not FDA approved—currently available to patients via a Treatment IND
Rights in U.S./Americas licensed to Sigma Tau Pharmaceuticals
EU = European Union, ROW = Rest of World, VOD = Veno-Occlusive Disease, EMA = European Medicines Agency, COMP = Committee for Orphan Medicinal Products, GvHD = Graft vs. Host Disease, FDA = Food and Drug Administration, IND = Investigational New Drug
13
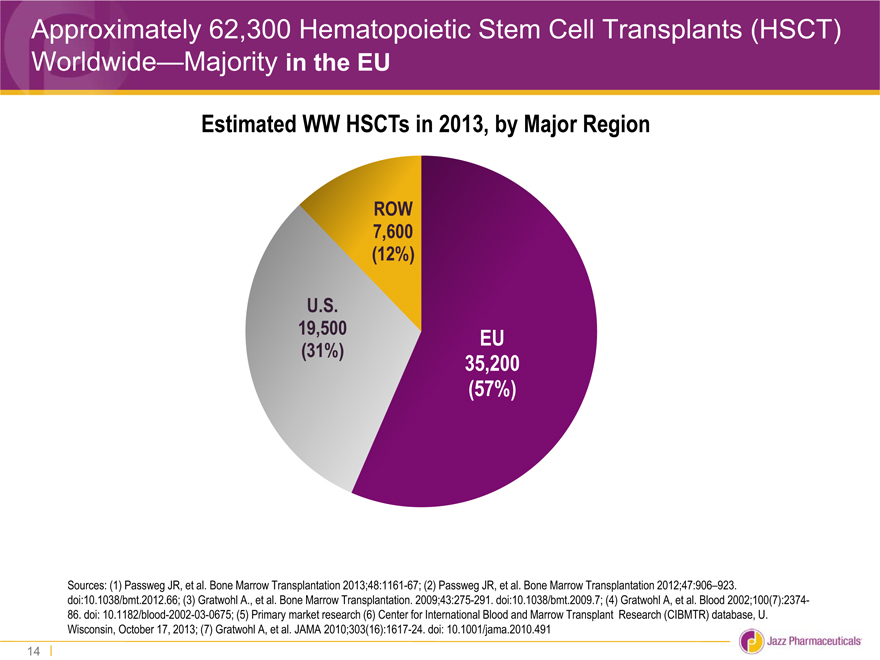
Approximately 62,300 Hematopoietic Stem Cell Transplants (HSCT) Worldwide—Majority in the EU
Estimated WW HSCTs in 2013, by Major Region
ROW
7,600
(12%)
U.S.
19,500 EU
(31%) 35,200
(57%)
Sources: (1) Passweg JR, et al. Bone Marrow Transplantation 2013;48:1161-67; (2) Passweg JR, et al. Bone Marrow Transplantation 2012;47:906–923. doi:10.1038/bmt.2012.66; (3) Gratwohl A., et al. Bone Marrow Transplantation. 2009;43:275-291. doi:10.1038/bmt.2009.7; (4) Gratwohl A, et al. Blood 2002;100(7):2374-86. doi: 10.1182/blood-2002-03-0675; (5) Primary market research (6) Center for International Blood and Marrow Transplant Research (CIBMTR) database, U. Wisconsin, October 17, 2013; (7) Gratwohl A, et al. JAMA 2010;303(16):1617-24. doi: 10.1001/jama.2010.491
14
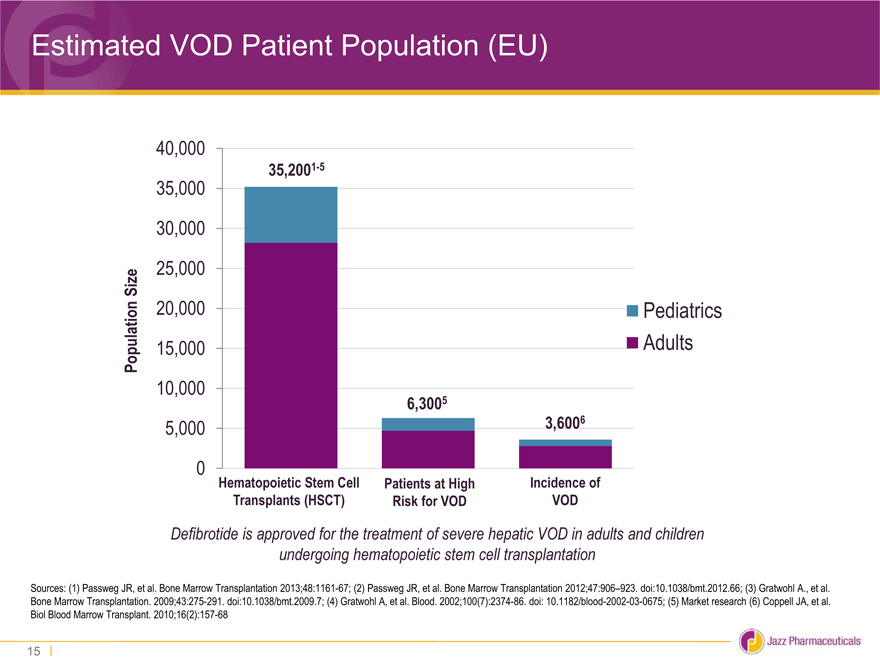
Estimated VOD Patient Population (EU)
40,000
35,2001-5
35,000
30,000
Size 25,000
20,000 Pediatrics
Population 15,000 Adults
10,000
6,3005
5,000 3,6006
0 Hematopoietic Stem Cell Patients at High Incidence of
Transplants (HSCT) Risk for VOD VOD
Defibrotide is approved for the treatment of severe hepatic VOD in adults and children
undergoing hematopoietic stem cell transplantation
Sources: (1) Passweg JR, et al. Bone Marrow Transplantation 2013;48:1161-67; (2) Passweg JR, et al. Bone Marrow Transplantation 2012;47:906–923. doi:10.1038/bmt.2012.66; (3) Gratwohl A., et al. Bone Marrow Transplantation. 2009;43:275-291. doi:10.1038/bmt.2009.7; (4) Gratwohl A, et al. Blood. 2002;100(7):2374-86. doi: 10.1182/blood-2002-03-0675; (5) Market research (6) Coppell JA, et al. Biol Blood Marrow Transplant. 2010;16(2):157-68
15
Financial Review
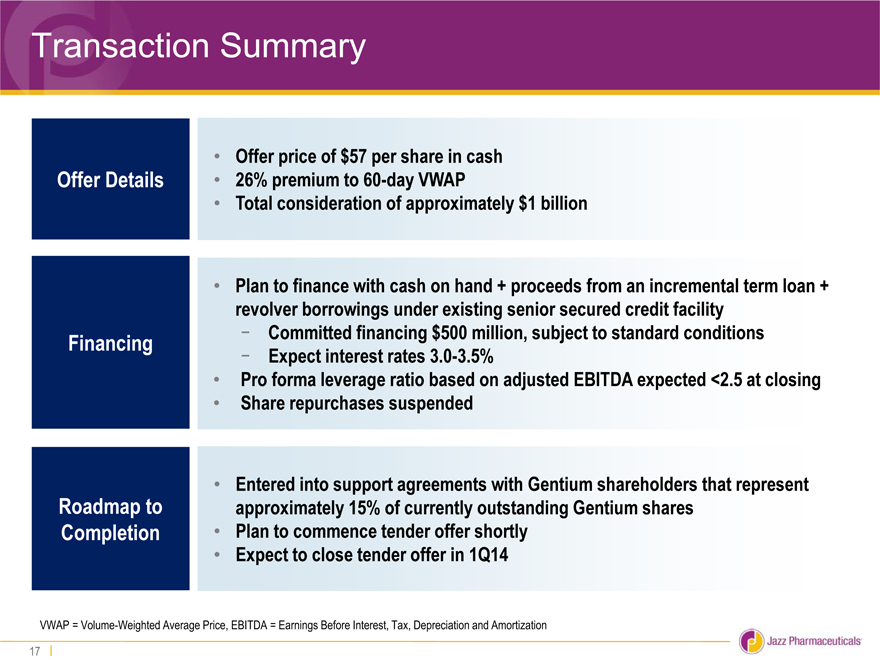
Transaction Summary
• Offer price of $57 per share in cash
Offer Details • 26% premium to 60-day VWAP
• Total consideration of approximately $1 billion
• Plan to finance with cash on hand + proceeds from an incremental term loan +
revolver borrowings under existing senior secured credit facility
Financing—Committed financing $500 million, subject to standard conditions
—Expect interest rates 3.0-3.5%
• Pro forma leverage ratio based on adjusted EBITDA expected <2.5 at closing
• Share repurchases suspended
• Entered into support agreements with Gentium shareholders that represent
Roadmap to approximately 15% of currently outstanding Gentium shares
Completion • Plan to commence tender offer shortly
• Expect to close tender offer in 1Q14
VWAP = Volume-Weighted Average Price, EBITDA = Earnings Before Interest, Tax, Depreciation and Amortization
17
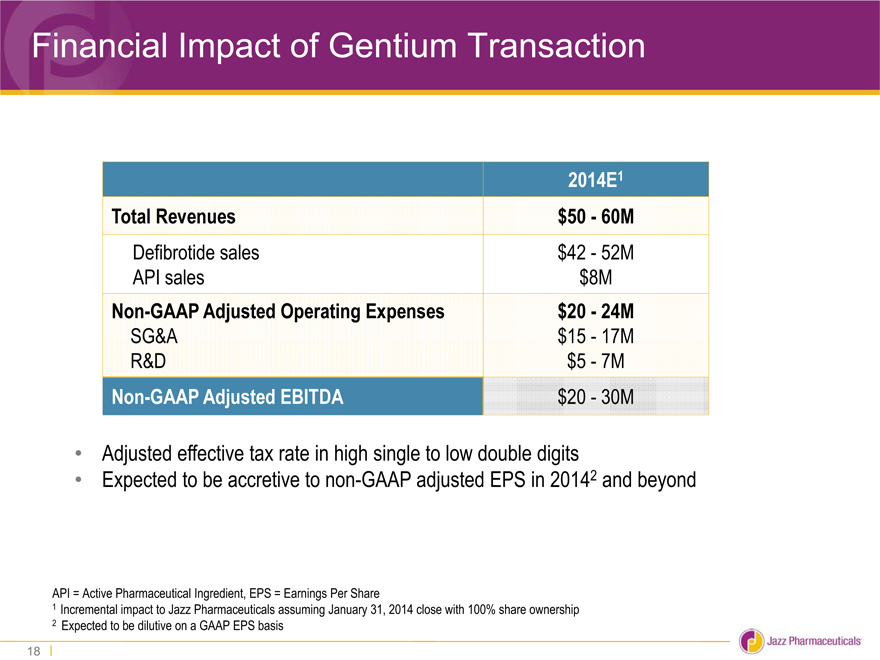
Financial Impact of Gentium Transaction
2014E1
Total Revenues $50—60M
Defibrotide sales $42—52M
API sales $8M
Non-GAAP Adjusted Operating Expenses $20—24M
SG&A $15—17M
R&D $5—7M
Non-GAAP Adjusted EBITDA $20—30M
Adjusted effective tax rate in high single to low double digits
Expected to be accretive to non-GAAP adjusted EPS in 20142 and beyond
API = Active Pharmaceutical Ingredient, EPS = Earnings Per Share
1 Incremental impact to Jazz Pharmaceuticals assuming January 31, 2014 close with 100% share ownership
2 Expected to be dilutive on a GAAP EPS basis
18
Expected Benefits To Jazz
Strategic fit with an attractive financial profile
Synergistic with and enhances our Hematology/Oncology business Leverages our corporate structure Grows and diversifies our revenue base Increases short-term and long-term revenue growth Accretive to non-GAAP EPS – augments earnings growth profile Balance sheet remains strong
19
Additional Information
The tender offer for the outstanding shares of Gentium (including those shares represented by American Depositary Shares) referenced in this presentation has not yet commenced. The statement in this presentation is for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell shares of Gentium, nor is it a substitute for the tender offer materials that Jazz Pharmaceuticals and its acquisition subsidiary will file with the SEC upon commencement of the tender offer. At the time the tender offer is commenced, Jazz Pharmaceuticals and its acquisition subsidiary will file tender offer materials on Schedule TO, and Gentium will file a Solicitation/Recommendation Statement on Schedule 14D-9 with the SEC with respect to the tender offer. The tender offer materials (including an Offer to Purchase, a related Letter of Transmittal and certain other tender offer documents) and the Solicitation/Recommendation Statement will contain important information. Holders of shares of Gentium are urged to read these documents when they become available because they will contain important information that holders of Gentium securities should consider before making any decision regarding tendering their securities. The Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the Solicitation/Recommendation Statement, will be made available to all holders of shares of Gentium at no expense to them. Investors and security holders may obtain free copies of these documents (when they are available) and other related documents filed with the SEC at the SEC’s web site at http://www.sec.gov or by (i) directing a request to Jazz Pharmaceuticals plc, c/o Jazz Pharmaceuticals, Inc., 3180 Porter Drive, Palo Alto, California 94304, U.S.A., Attention: Investor Relations, (ii) calling +353 1 634 7892 (Ireland) or + 1 650 496 2800 (U.S.) or (iii) sending an email to investorinfo@jazzpharma.com. Investors and security holders may also obtain free copies of the documents filed with the SEC on Jazz Pharmaceuticals’ website at www.jazzpharmaceuticals.com under the heading “Investors” and then under the heading “SEC Filings.”
In addition to the Offer to Purchase, the related Letter of Transmittal and certain other tender offer documents, as well as the
Solicitation/Recommendation Statement, Jazz Pharmaceuticals and Gentium file annual, quarterly (except in the case of Gentium) and special reports and other information with the SEC. You may read and copy any reports or other information filed by Jazz Pharmaceuticals or Gentium at the SEC public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the Commission at 1-800-SEC-0330 for further information on the public reference room. Jazz Pharmaceuticals’ and Gentium’s filings with the SEC are also available to the public from commercial document-retrieval services and at the website maintained by the SEC at http://www.sec.gov.
20
Non-GAAP Financial Measures
This presentation contains forward-looking estimates of non-GAAP adjusted EBITDA and non-GAAP adjusted operating expenses (the combination of adjusted SG&A expense and adjusted R&D expense) resulting from the proposed business combination. As used in this presentation, with respect to estimated adjusted EBITDA contribution resulting from the proposed business combination, Jazz Pharmaceuticals defines adjusted EBITDA as GAAP net income before interest, taxes, depreciation and amortization, excluding stock-based compensation, transaction and integration costs and inventory purchase price adjustments associated with the proposed business combination between Jazz Pharmaceuticals and Gentium S.p.A. With respect to estimated adjusted SG&A expense and adjusted R&D expenses, Jazz Pharmaceuticals excludes from GAAP SG&A expense and GAAP R&D expense, respectively, stock-based compensation expense, transaction and integration costs and inventory purchase price adjustments associated with the proposed business combination, as applicable. Reconciliations of estimated adjusted EBITDA contribution, estimated adjusted SG&A expense and estimated R&D expense to their respective comparable GAAP measures are not provided because the comparable GAAP measures from the Gentium S.p.A. operations for the applicable future period are not accessible or estimable at this time. In this regard, for example, Jazz Pharmaceuticals has not yet completed the necessary valuation of the various assets to be acquired in the proposed acquisition, for accounting purposes, or an allocation of the purchase price among the various types of assets. In addition, the final interest and debt expense associated with the transactions contemplated by the commitment letter have not been finalized and are therefore unavailable. Accordingly, the amount of depreciation and amortization and interest and debt expense that will be included in the additional GAAP net income assuming the proposed acquisition is consummated is not accessible or estimable at this time, and is therefore not available without unreasonable effort. The amount of such additional resulting depreciation and amortization and applicable interest and debt expense could be significant, such that actual GAAP net income would vary substantially from the estimated adjusted EBITDA contribution included in this presentation.
21
Jazz Pharmaceuticals
Innovation that performs