
Exhibit 99.1 Q1’15 Earnings & Strategic Review Conference Call Gi Dynamics DIABETES OBESITY endobarrier

Important Notice Currency References Financial amounts in this presentation are expressed in US Dollars, except where specifically noted. Forward-Looking Statements This presentation contains forward-looking statements concerning: our development and commercialization plans; our potential revenues and revenue growth, costs, excess inventory, profitability and financial performance; our ability to obtain reimbursement for our products; our clinical trials, and associated regulatory submissions and approvals; the number and location of commercial centers offering the EndoBarrier®; and our intellectual property position. These forward-looking statements are based on the current estimates and expectations of future events by the management of GI Dynamics, Inc. as of the date of this presentation and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those indicated in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: risks associated with the possibility that clinical trials will not be successful or confirm earlier results; risks associated with obtaining funding from third parties; risks relating to the timing and costs of clinical trials, the timing of regulatory submissions, the timing, receipt and maintenance of regulatory approvals, the timing and amount of other expenses, and the timing and extent of third-party reimbursement; risks associated with commercial product sales, including product performance; competition; risks related to market acceptance of products; intellectual property risks; risks related to excess inventory; risks related to assumptions regarding the size of the available market, benefits of our products, product pricing, timing of product launches, future financial results and other factors including those described in our filings with the U.S. Securities and Exchange Commission. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law. Disclaimer This presentation and any supplemental materials have been prepared by GI Dynamics, Inc. based on available information. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness, or correctness of such information and opinions and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of GI Dynamics, Inc., or any of its members, directors, officers, employees, or agents or advisors, nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of GI Dynamics, Inc. or any of its directors, officers, employees or agents. Gi Dynamics DIABETES OBESITY endobarrier

Conference Call Agenda • High-level summary of Q1’15 financial and operating performance • Outcomes from strategic review • Update on ENDO Trial • Financial guidance for 2015 & operating priorities Gi Dynamics DIABETES OBESITY endobarrier

Q1’15 Summary • Revenue: – $0.6 million, down $0.3 million y/y • Operating expenses: – $9.6 million, down $0.5 million y/y • Net loss: – $10.3 million, up $0.6 million y/y • Cash and cash equivalents: – US$40.0 million at 31 March 2015, compared to US$51.2 million at 31 December 2014, a decrease of US$11.2 million. • Organization-wide focus on regulatory activities in Q1 • Commercial execution impacted by efforts to manage regulatory requirements and organization realignment as part of strategic review • ENDO Trial enrollment hold has and will likely continue to adversely affect commercial operations Gi Dynamics DIABETES OBESITY endobarrier

Strategic Review Introduction & Summary Gi Dynamics DIABETES OBESITY endobarrier

Strategic Review: Overview • Standard planning process – Emphasis on situation analysis and understanding strengths, weaknesses, opportunities, and threats – Clarification of value proposition and related gaps versus vision Necessary goals and strategy for the future • Participants – All GID employees – Board of Directors – Experienced EndoBarrier users • Duration – Q4’14 to Q1’15 Gi Dynamics DIABETES OBESITY endobarrier
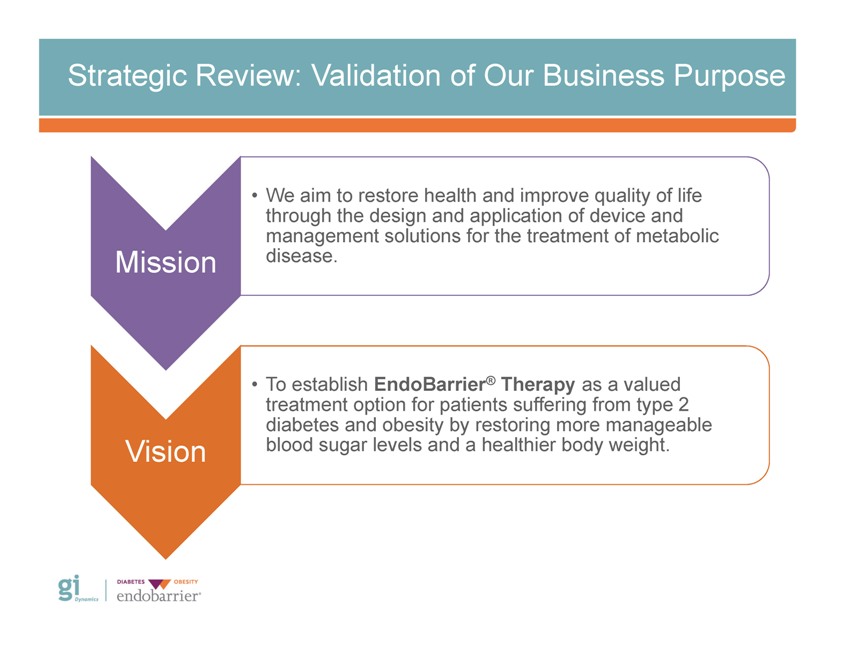
Strategic Review: Validation of Our Business Purpose Mission Vision • We aim to restore health and improve quality of life through the design and application of device and management solutions for the treatment of metabolic disease. To establish EndoBarrier® Therapy as a valued treatment option for patients suffering from type 2 diabetes and obesity by restoring more manageable blood sugar levels and a healthier body weight. Gi Dynamics DIABETES OBESITY endobarrier

Outcomes of Strategic Review Validation of EndoBarrier Therapy Opportunity Gi Dynamics DIABETES OBESITY endobarrier
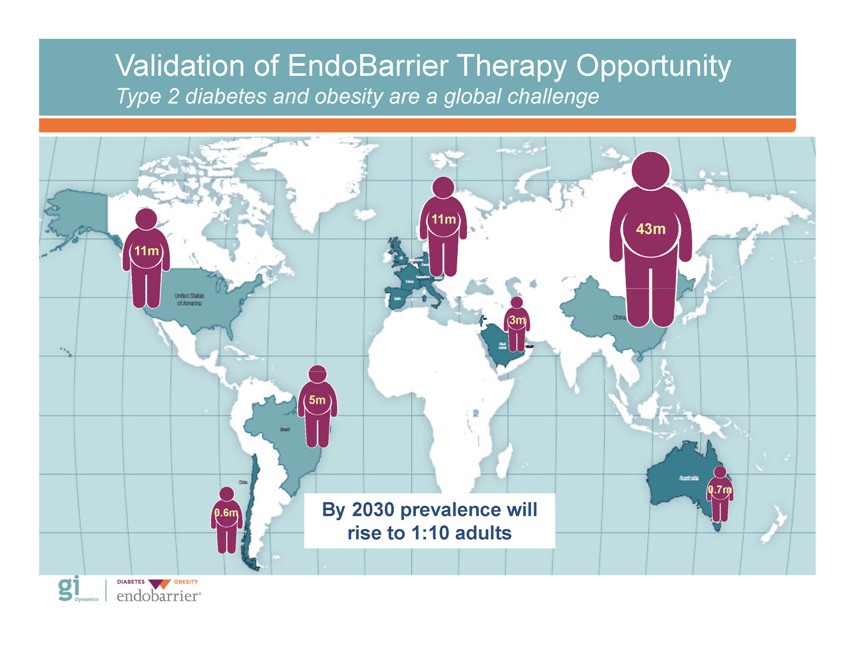
Validation of EndoBarrier Therapy Opportunity Type 2 diabetes and obesity are a global challenge By 2030 prevalence will rise to 1:10 adults Gi Dynamics DIABETES OBESITY endobarrier
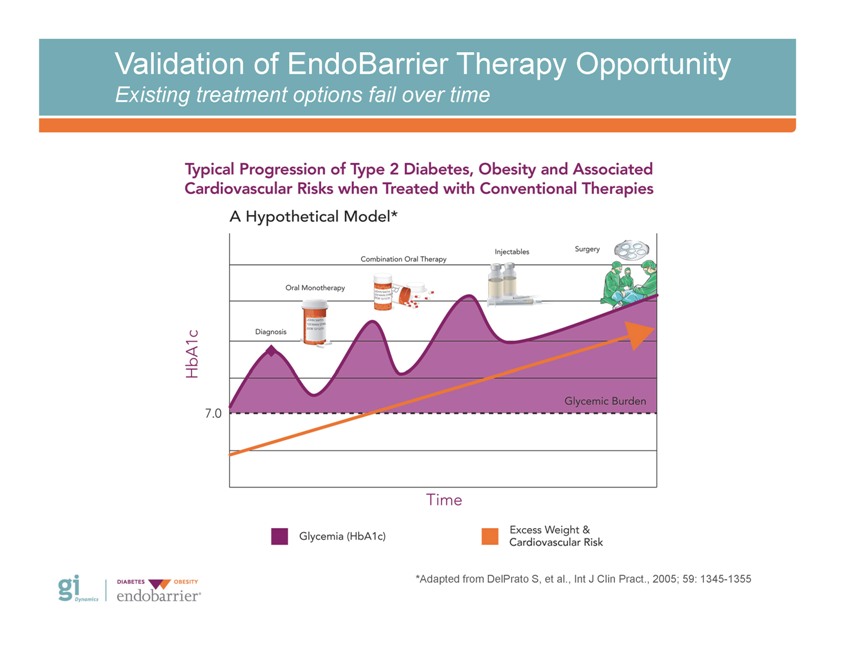
Validation of EndoBarrier Therapy Opportunity Existing treatment options fail over time *Adapted from DelPrato S, et al., Int J Clin Pract., 2005; 59: 1345-1355 Gi Dynamics DIABETES OBESITY endobarrier
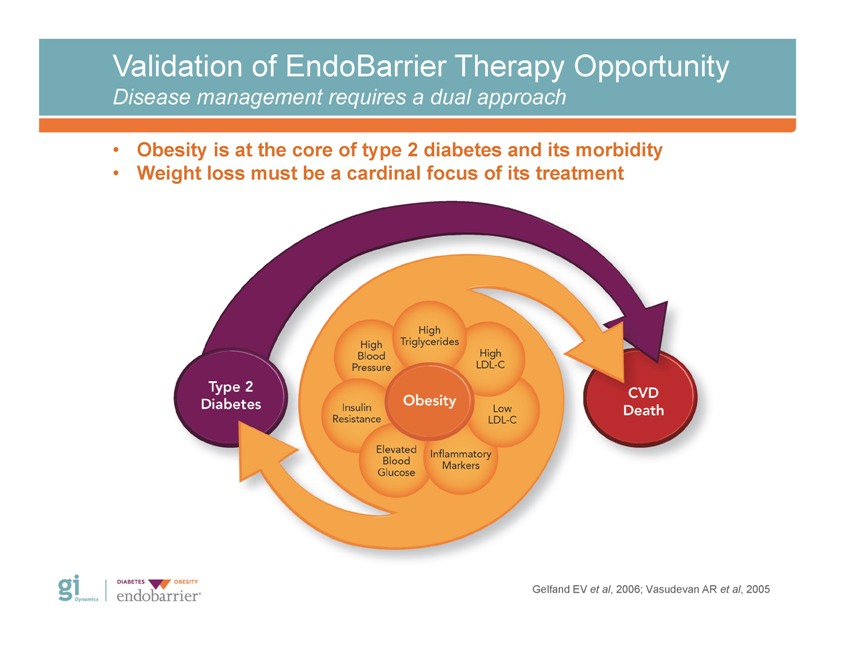
Validation of EndoBarrier Therapy Opportunity Disease management requires a dual approach • Obesity is at the core of type 2 diabetes and its morbidity • Weight loss must be a cardinal focus of its treatment Gelfand EV et al, 2006; Vasudevan AR et al, 2005
Gi Dynamics DIABETES OBESITY
endobarrier
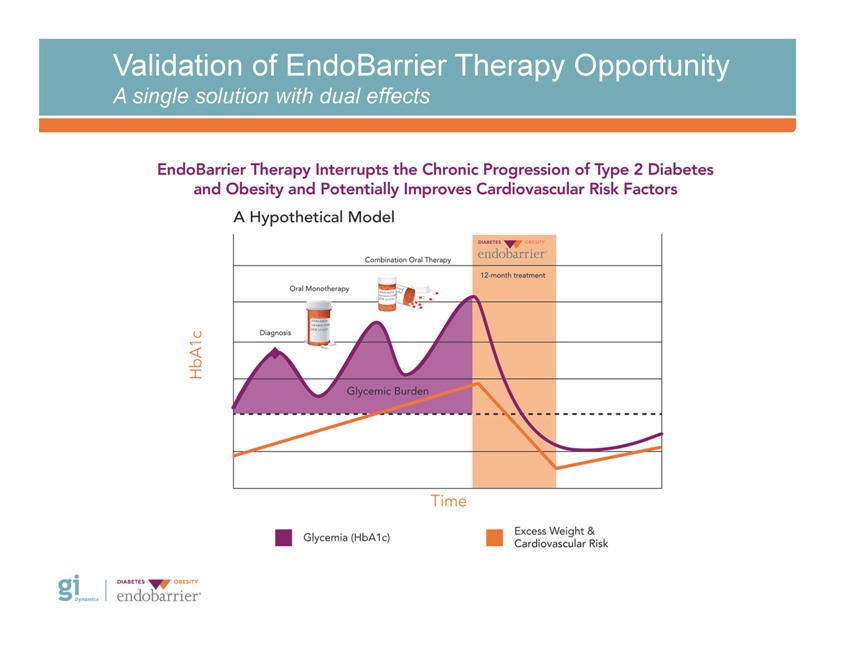
Validation of EndoBarrier Therapy Opportunity A single solution with dual effects
Gi Dynamics DIABETES OBESITY
endobarrier

EndoBarrier Therapy: Customer Value Proposition EndoBarrier is a duodenal-jejunal bypass liner that provides patients living with type 2 diabetes and obesity with the only incision-free, endoscopic solution for immediate glycemic control and weight reduction, giving them the opportunity to change the course of their disease and improve their quality of life.
Gi Dynamics DIABETES OBESITY
endobarrier

EndoBarrier Therapy: Unique Advantages • EndoBarrier is the only incision-free, non-anatomy-altering solution designed to mimic the duodenal-jejunal exclusion that gastric bypass surgery creates. • EndoBarrier is the only endoscopic procedure indicated to treat . • Because EndoBarrier is a passive device, its functional benefits are obtained independently of patient adherence to complex nutritional changes or intensive follow-up care.
Gi Dynamics DIABETES OBESITY
endobarrier
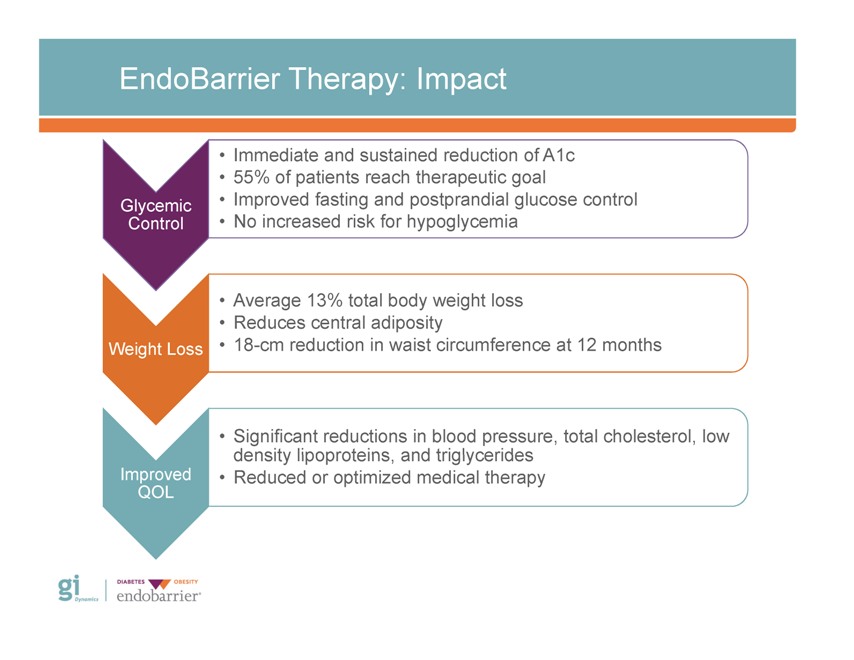
EndoBarrier Therapy: Impact Glycemic Control Weight Loss Improved QOL • Immediate and sustained reduction of A1c • 55% of patients reach therapeutic goal • Improved fasting and postprandial glucose control • No increased risk for hypoglycemia • Average 13% total body weight loss • Reduces central adiposity • 18-cm reduction in waist circumference at 12 months • Significant reductions in blood pressure, total cholesterol, low density lipoproteins, and triglycerides • Reduced or optimized medical therapy
Gi Dynamics DIABETES OBESITY
endobarrier

Outcomes of Strategic Review Validation of the primary challenges to broad commercial adoption
Gi Dynamics DIABETES OBESITY
endobarrier

Outcomes of Strategic Review Primary challenges to broad commercialization Primary issue(s) identified: – Addressing multiple customer audiences – Lack of reimbursement (coding, coverage & payment) for novel medical technology GID’s distribution strategy – Awareness of EndoBarrier risk:benefit profile – Product enhancement and platform expansion – Lack of randomized control trial evidence
Gi Dynamics DIABETES OBESITY
endobarrier
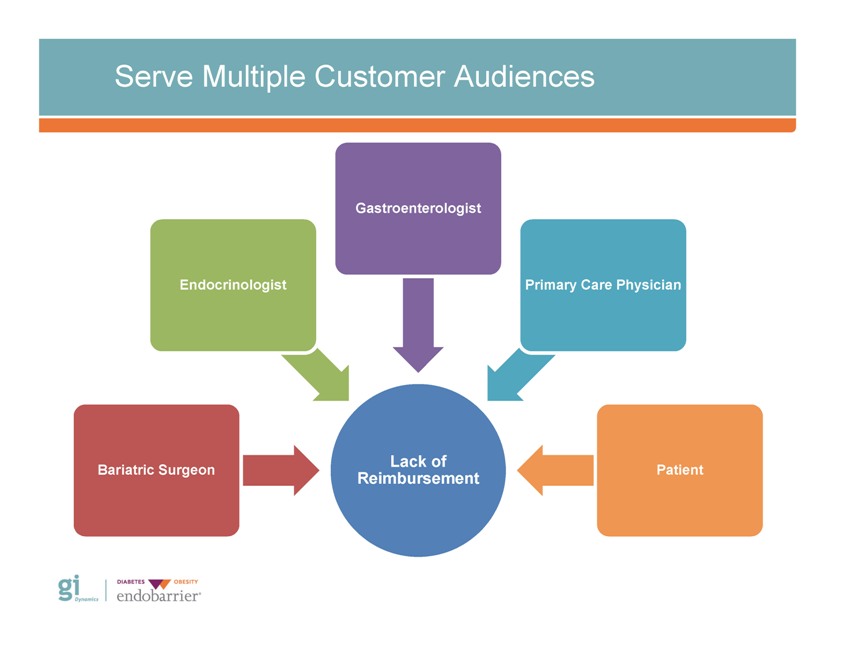
Serve Multiple Customer Audiences Gastroenterologist Endocrinologist Primary Care Physician Patient Lack of Reimbursement Bariatric Surgeon
Gi Dynamics DIABETES OBESITY
endobarrier
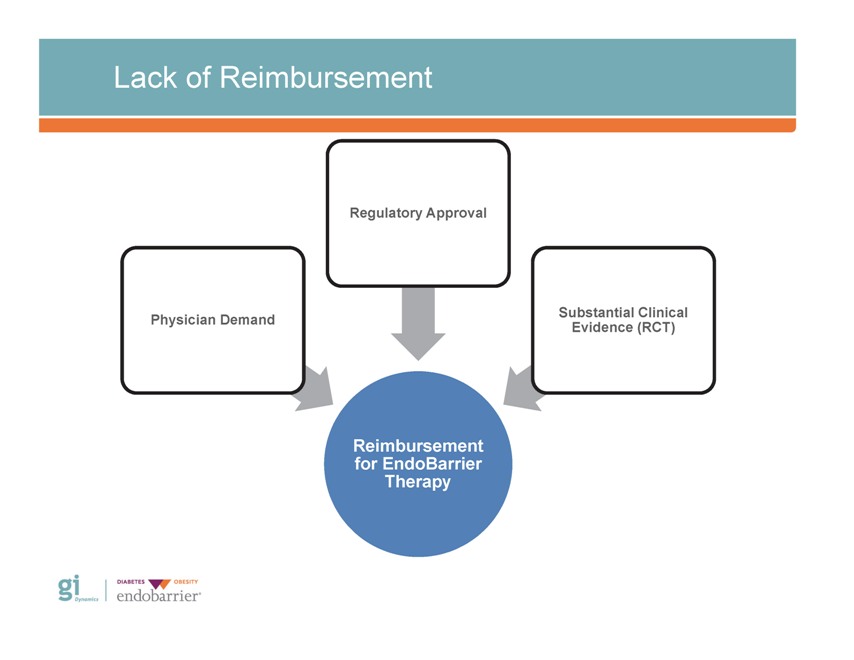
Lack of Reimbursement Regulatory Approval Substantial Clinical Evidence (RCT) Physician Demand Reimbursement for EndoBarrier Therapy
Gi Dynamics DIABETES OBESITY
endobarrier

GID’s Distribution Strategy Primary issue(s) identified: – EndoBarrier Therapy is a novel technology that requires development of multidisciplinary care pathways for optimal patient management. – Because of its novelty, formal reimbursement does not exist for the device, nor for the services associated for therapy delivery. – Market development efforts to establish payment for EndoBarrier and physician training require significant investment. – GID distribution models need to be adapted to encourage traditional partnering and cost sharing, and to establish clear lines of responsibility and leverage local market knowledge
Gi Dynamics DIABETES OBESITY
endobarrier
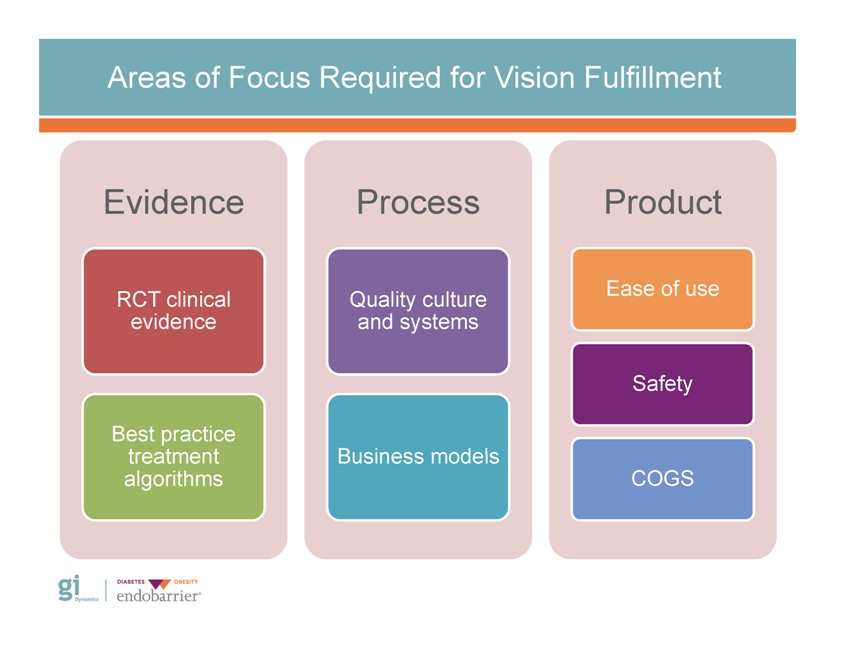
Areas of Focus Required for Vision Fulfillment Evidence RCT clinical evidence Best practice treatment algorithms Process Quality culture and systems Business models Product Ease of use Safety COGS Gi Dynamics DIABETES OBESITY endobarrier
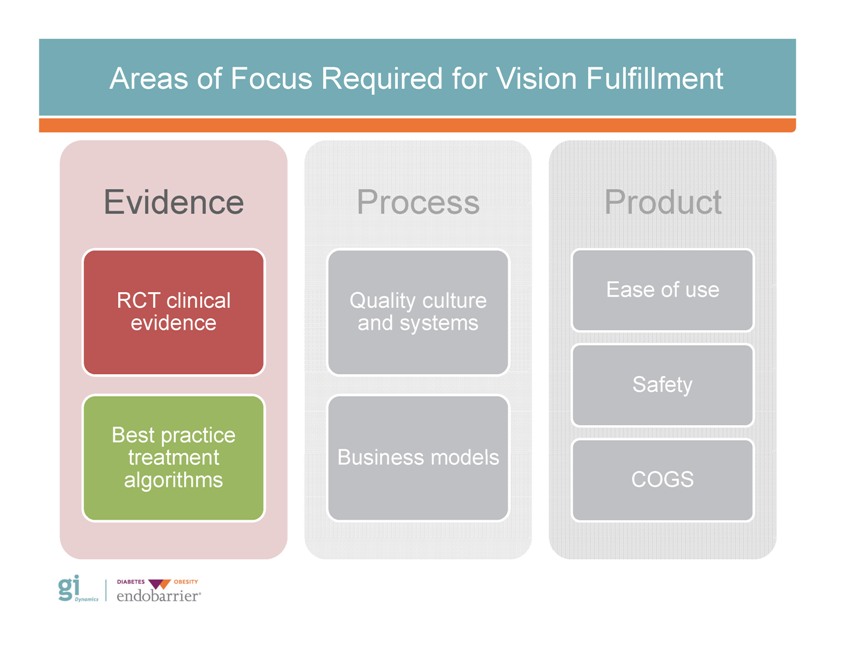
Areas of Focus Required for Vision Fulfillment Evidence RCT clinical evidence Best practice treatment algorithms Process Quality culture and systems Business models Product Safety COGS Gi Dynamics DIABETES OBESITY endobarrier
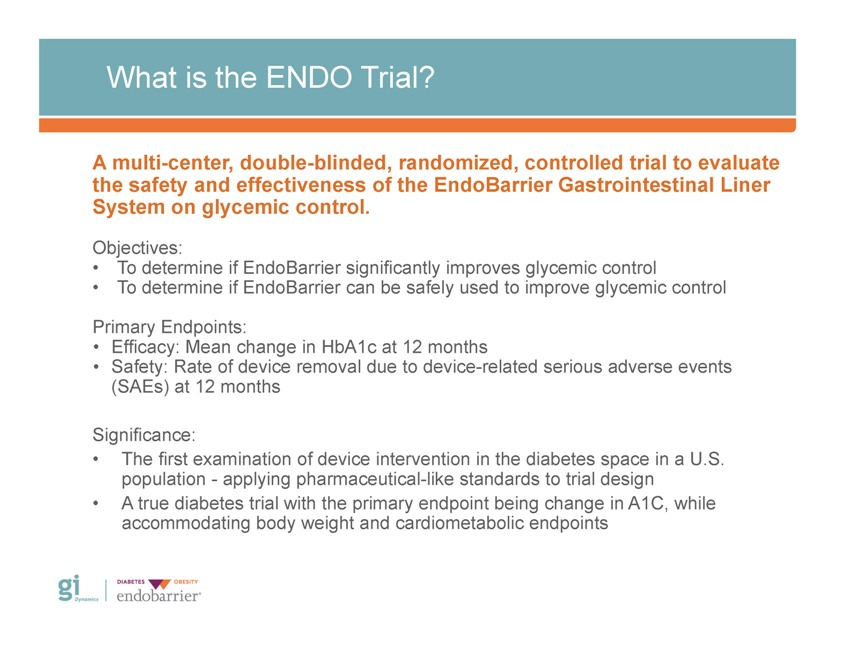
What is the ENDO Trial? A multi-center, double-blinded, randomized, controlled trial to evaluate the safety and effectiveness of the EndoBarrier Gastrointestinal Liner System on glycemic control. Objectives: • To determine if EndoBarrier significantly improves glycemic control • To determine if EndoBarrier can be safely used to improve glycemic control Primary Endpoints: • Efficacy: Mean change in HbA1c at 12 months • Safety: Rate of device removal due to device-related serious adverse events (SAEs) at 12 months Significance: • The first examination of device intervention in the diabetes space in a U.S. population—applying pharmaceutical-like standards to trial design • A true diabetes trial with the primary endpoint being change in A1C, while accommodating body weight and cardiometabolic endpoints Gi Dynamics DIABETES OBESITY endobarrier
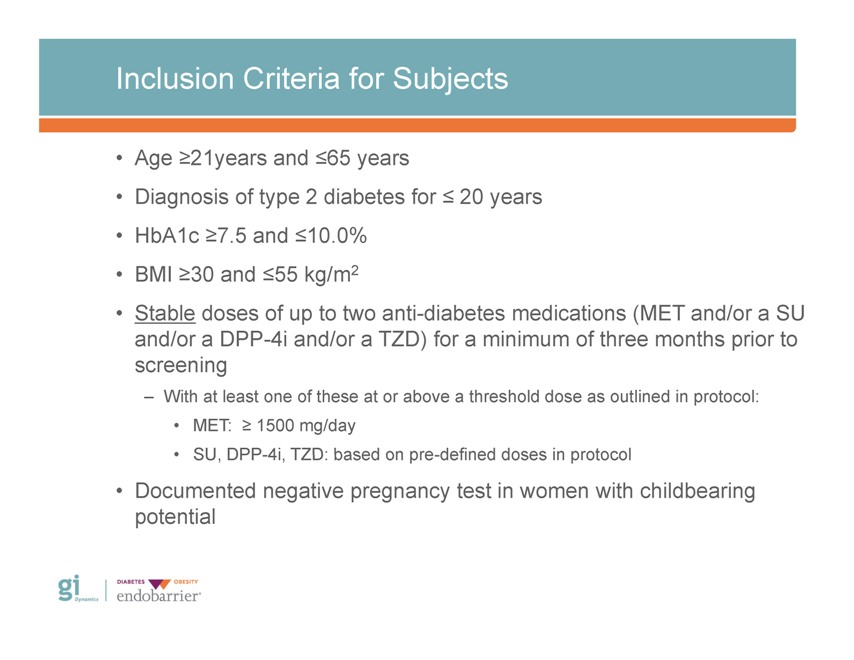
Inclusion Criteria for Subjects • Age ?21years and ?65 years • Diagnosis of type 2 diabetes for ? 20 years • HbA1c ?7.5 and ?10.0% • BMI ?30 and ?55 kg/m2 • Stable doses of up to two anti-diabetes medications (MET and/or a SU and/or a DPP-4i and/or a TZD) for a minimum of three months prior to screening – With at least one of these at or above a threshold dose as outlined in protocol: • MET: ? 1500 mg/day • SU, DPP-4i, TZD: based on pre-defined doses in protocol • Documented negative pregnancy test in women with childbearing potential Gi Dynamics DIABETES OBESITY endobarrier
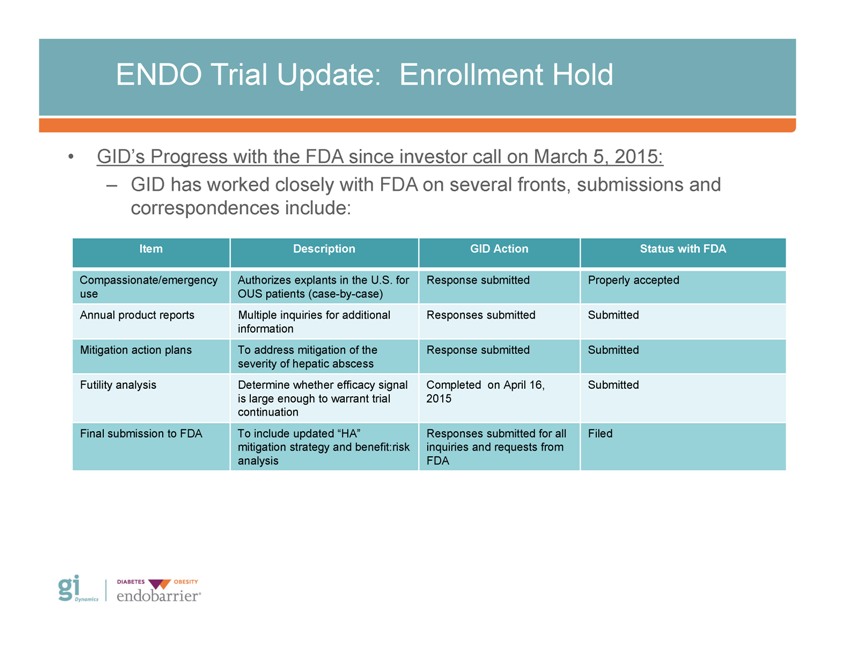
ENDO Trial Update: Enrollment Hold • GID’s Progress with the FDA since investor call on March 5, 2015: – GID has worked closely with FDA on several fronts, submissions and correspondences include: Item Description GID Action Status with FDA Compassionate/emergency use Annual product reports Mitigation action plans Futility analysis Final submission to FDA Authorizes explants in the U.S. for OUS patients (case-by-case) Multiple inquiries for additional information To address mitigation of the severity of hepatic abscess Determine whether efficacy signal is large enough to warrant trial continuation To include updated “HA” mitigation strategy and benefit:risk analysis Response submitted Responses submitted Response submitted Completed on April 16, 2015 Responses submitted for all inquiries and requests from FDA Properly accepted Submitted Submitted Submitted Filed Gi Dynamics DIABETES OBESITY endobarrier
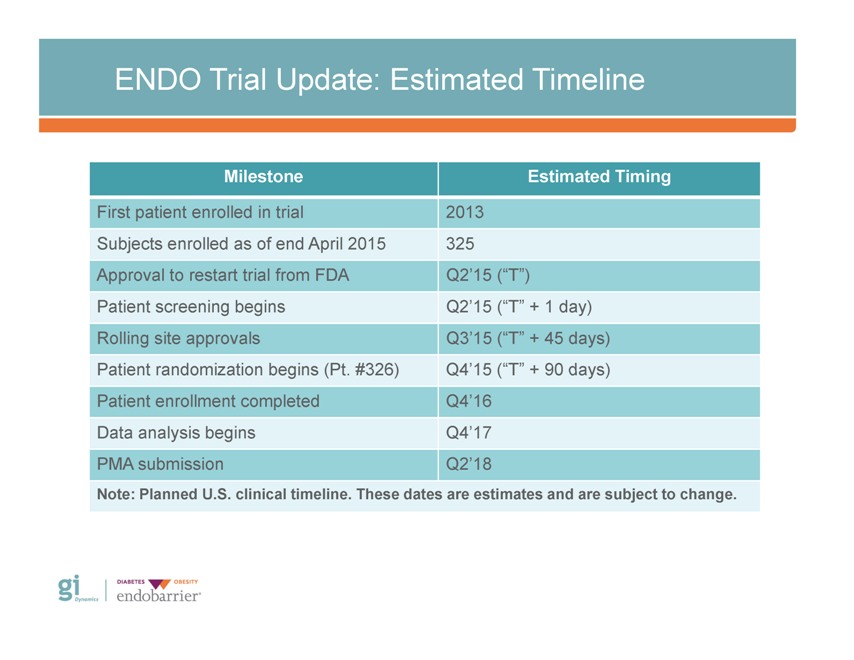
ENDO Trial Update: Estimated Timeline Milestone Estimated Timing First patient enrolled in trial 2013 Subjects enrolled as of end April 2015 325 Approval to restart trial from FDA Q2’15 (“T”) Patient screening begins Q2’15 (“T” + 1 day) Rolling site approvals Q3’15 (“T” + 45 days) Patient randomization begins (Pt. #326) Q4’15 (“T” + 90 days) Patient enrollment completed Q4’16 Data analysis begins Q4’17 PMA submission Q2’18 Note: Planned U.S. clinical timeline. These dates are estimates and are subject to change. Gi Dynamics DIABETES OBESITY endobarrier
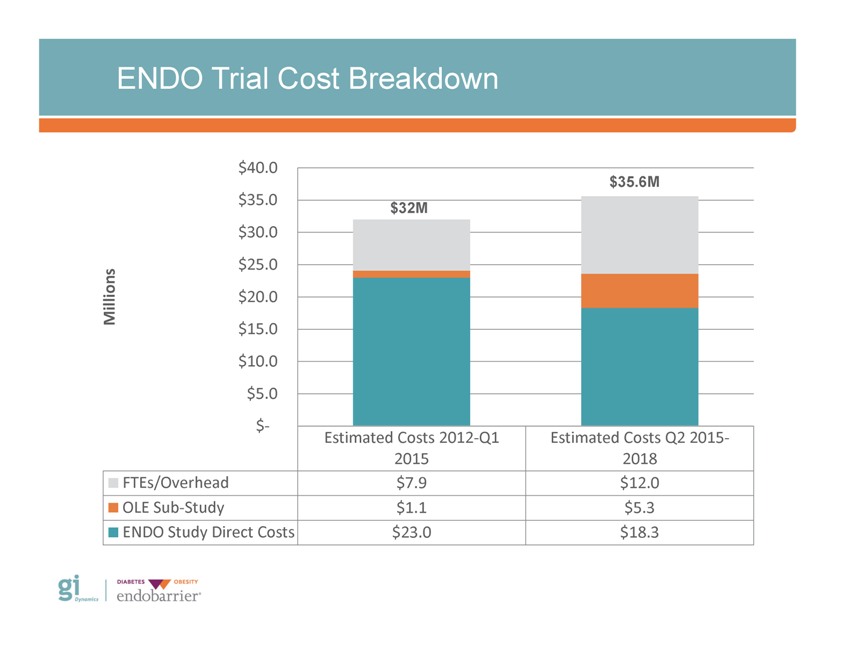
ENDO Trial Cost Breakdown $40.0 $35.6M $35.0 $32M $30.0 $25.0 ns Millio $20.0 $15.0 $10.0 $5.0 $? Estimated Costs 2012?Q1 Estimated Costs Q2 2015?2015 2018 FTEs/Overhead $7.9 $12.0 OLE Sub?Study $1.1 $5.3 ENDO Study Direct Costs $23.0 $18.3 Gi Dynamics DIABETES OBESITY endobarrier
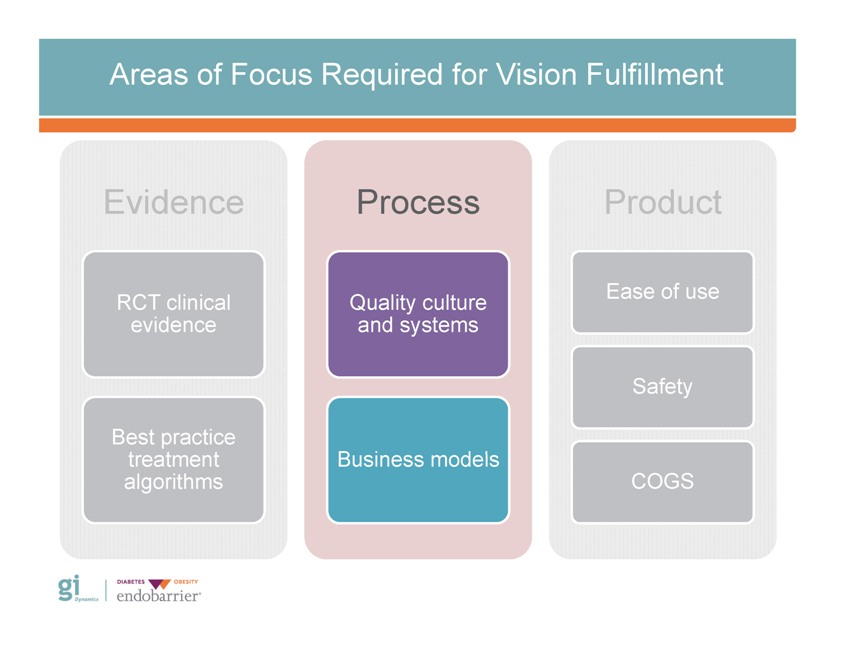
Areas of Focus Required for Vision Fulfillment Evidence Process Product Ease of use RCT clinical Quality culture evidence and systems Safety Best practice treatment Business models algorithms COGS Gi Dynamics DIABETES OBESITY endobarrier

GID’s Process Quality systems & regulatory affairs update Progress since Q4’14:—Continue effort to build and simplify quality system (QS) – surveillance audits will serve to validate these efforts in 2015.—GID has addressed all previous CARs from notified body—GID shipment hold lifted in Dec 1, 2014 – resulted in a modification to the Indication For Use, which since then has been aligned to be the same in all markets.—GID has closed FSN/FSCA with all 85+ customers and 10 competent authorities in EU + AUS + Chile + Israel WW.—Continue to build credibility and relationship with all competent authorities—Continued demonstration of compliant vigilance reporting system Ongoing objectives:—Continue QS enhancements—Continue to build quality culture and organization to support business needs—Resolve UADE correspondence with FDA to re-start ENDO Trial Gi Dynamics DIABETES OBESITY endobarrier

GID’s Distribution Strategy Prudent business models • Direct investment will be limited to Germany and Australia – German operations are dedicated to care pathway development and reimbursement development. • Six direct employees • German EndoBarrier registry – Australia operations are dedicated to support of experienced EndoBarrier user base. We believe the self-pay market can support break-even operations within two years. Reimbursement in Australia will require market registry data and RCT data. • Two direct employees • Australian EndoBarrier registry • TGA-approved consumer advertisement • Beginning in Q2 2015, all prior distribution arrangements will be cancelled and, where common interests align, will be served by traditional, independent distributor arrangements • Based on ability to support market development – Customer training – Self-payment or country-specific reimbursement development – Quality control Gi Dynamics DIABETES OBESITY endobarrier
Areas of Focus Required for Vision Fulfillment Evidence Process Product Ease of use RCT clinical Quality culture evidence and systems Safety Best practice treatment Business models algorithms COGS
Gi Dynamics DIABETES OBESITY
endobarrier
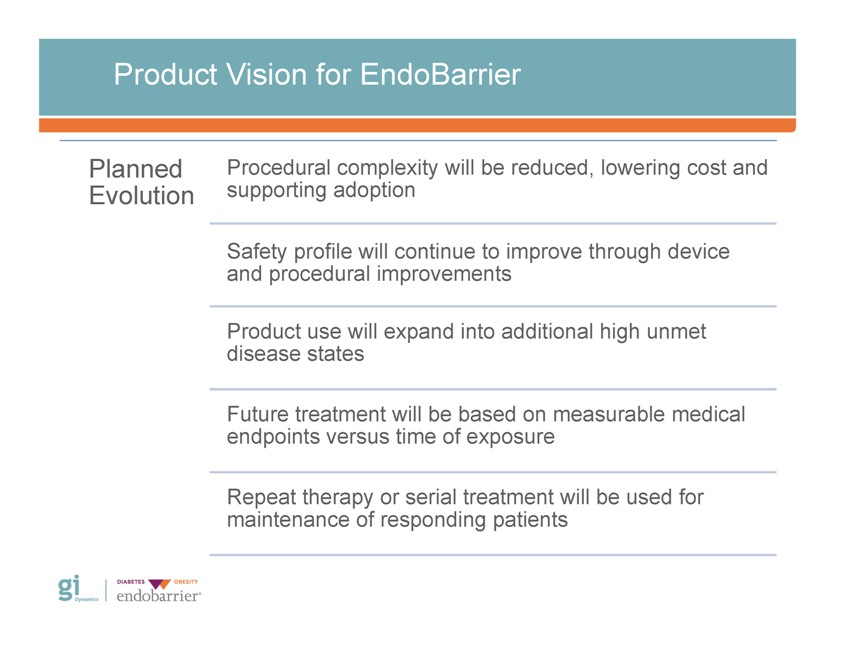
Product Vision for EndoBarrier Planned Procedural complexity will be reduced, lowering cost and Evolution supporting adoption Safety profile will continue to improve through device and procedural improvements Product use will expand into additional high unmet disease states Future treatment will be based on measurable medical endpoints versus time of exposure Repeat therapy or serial treatment will be used for maintenance of responding patients
Gi Dynamics DIABETES OBESITY
endobarrier

Product Enhancement and Platform Expansion Opportunities to improve and leverage our technology across existing and expanded indications within the obese and/or T2DM population • EndoBarrier Liner with EndoBarrier Restrictor This device combines our EndoBarrier liner with the EndoBarrier Restrictor, a combination intended to treat diabetes and create greater weight loss than either device alone. • EndoBarrier Restrictor This device is targeted for short-term weight loss or weight loss in a patient who is not obese. The device employs the same anchoring technology as EndoBarrier, but has a cover with a small hole to slow emptying of the patient’s stomach and create a feeling of satiety. • Next Generation EndoBarrier Product enhancements for greater safety and ease of use Accommodates wider indications for use Allows for serial use of EndoBarrier Therapy based on anchoring technique and ease of use. • Bariatric Surgery Solutions Fistula repair—uses a variation of EndoBarrier to repair leaks after bariatric procedures, such as sleeve gastrectomy. Present treatment includes stenting, distal-enteral feeding, antibiotics and drainage. Hospital stay can be weeks.
Gi Dynamics DIABETES OBESITY
endobarrier
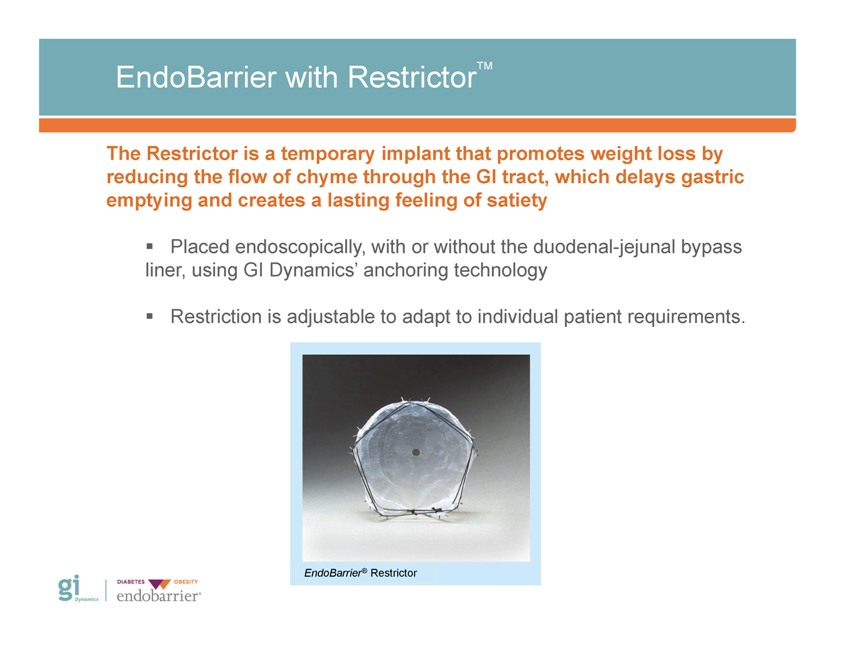
EndoBarrier with RestrictorTM The Restrictor is a temporary implant that promotes weight loss by reducing the flow of chyme through the GI tract, which delays gastric emptying and creates a lasting feeling of satiety ? Placed endoscopically, with or without the duodenal-jejunal bypass liner, using GI Dynamics’ anchoring technology ? Restriction is adjustable to adapt to individual patient requirements. EndoBarrier® Restrictor Gi Dynamics DIABETES OBESITY
endobarrier
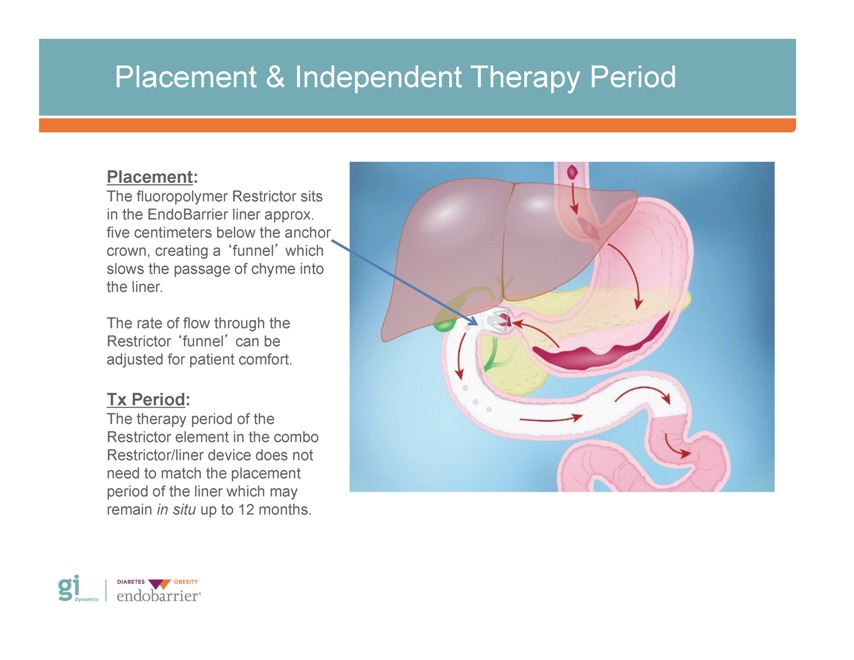
Placement & Independent Therapy Period Placement: The fluoropolymer Restrictor sits in the EndoBarrier liner approx. five centimeters below the anchor crown, creating a ‘funnel’ which slows the passage of chyme into the liner. The rate of flow through the Restrictor ‘funnel’ can be adjusted for patient comfort. Tx Period: The therapy period of the Restrictor element in the combo Restrictor/liner device does not need to match the placement period of the liner which may remain in situ up to 12 months.
Gi Dynamics DIABETES OBESITY
endobarrier
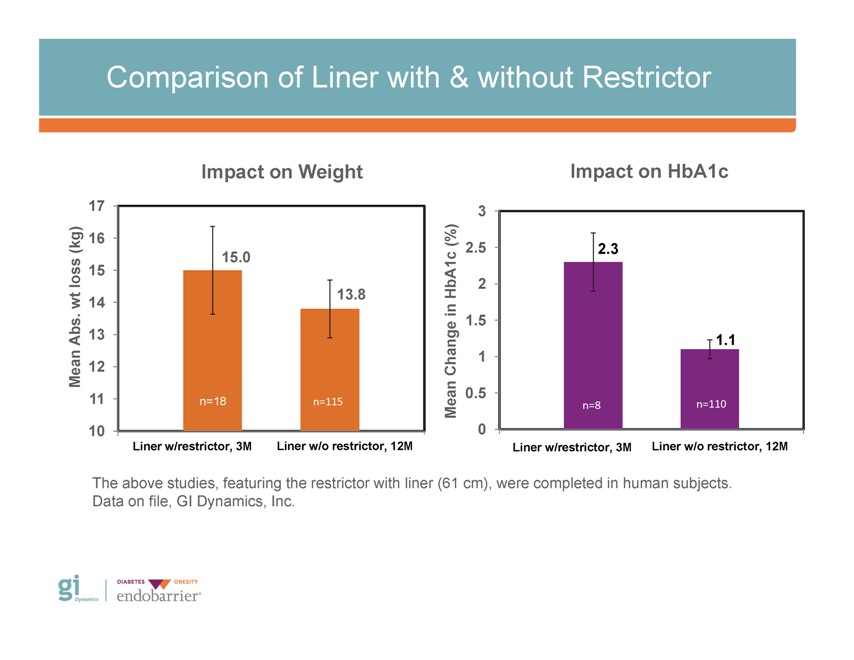
Comparison of Liner with & without Restrictor Impact on Weight Impact on HbA1c 17 3 16 (%) (kg) 2.5 2.3 15.0 loss 15 bA1c 2 13.8 H wt 14 . in 1.5 Abs 13 1.1 an 1 12 Change M 11 0.5 n=18 n=115 Mean n=8 n=110 10 0 Liner w/restrictor, 3M Liner w/o restrictor, 12M Liner w/restrictor, 3M Liner w/o restrictor, 12M The above studies, featuring the restrictor with liner (61 cm), were completed in human subjects. Data on file, GI Dynamics, Inc.
Gi Dynamics DIABETES OBESITY
endobarrier
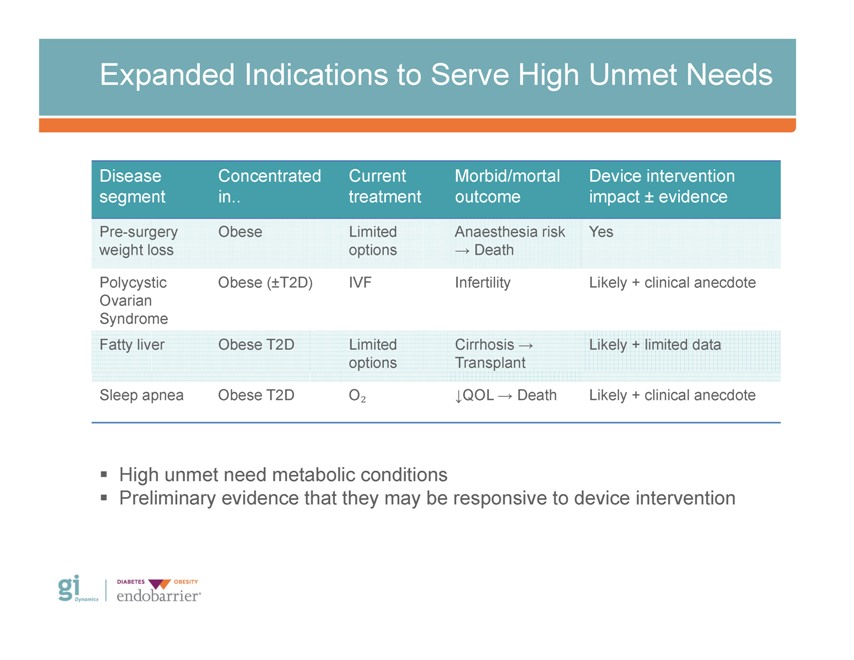
Expanded Indications to Serve High Unmet Needs Disease Concentrated Current Morbid/mortal Device intervention segment in. treatment outcome impact ± evidence Pre-surgery Obese Limited Anaesthesia risk Yes weight loss options ? Death Polycystic Obese (±T2D) IVF Infertility Likely + clinical anecdote Ovarian Syndrome Fatty liver Obese T2D Limited Cirrhosis ? Likely + limited data options Transplant Sleep apnea Obese T2D O? ?QOL ? Death Likely + clinical anecdote ? High unmet need metabolic conditions ? Preliminary evidence that they may be responsive to device intervention
Gi Dynamics DIABETES OBESITY
endobarrier
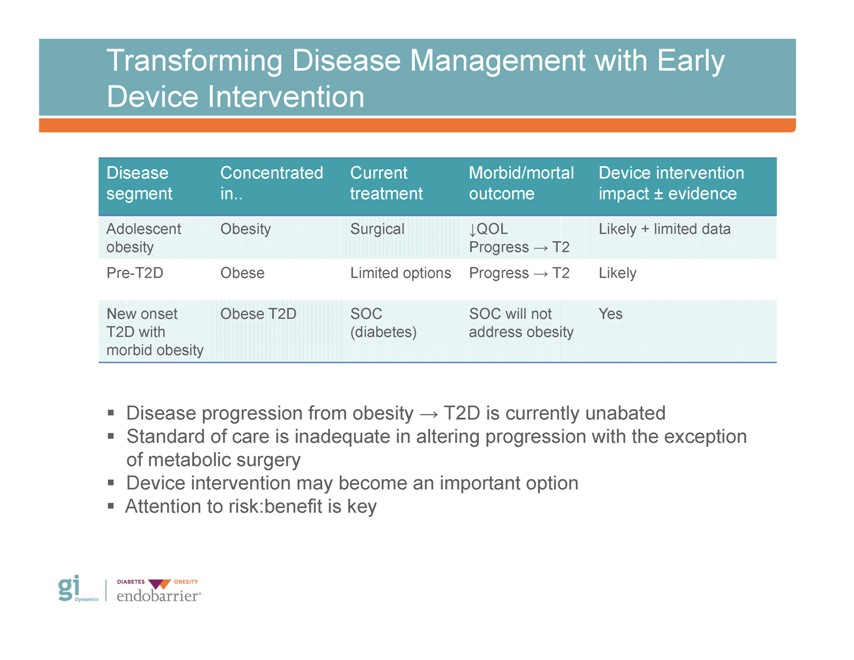
Transforming Disease Management with Early Device Intervention Disease Concentrated Current Morbid/mortal Device intervention segment in. treatment outcome impact ± evidence Adolescent Obesity Surgical ?QOL Likely + limited data obesity Progress ? T2 Pre-T2D Obese Limited options Progress ? T2 Likely New onset Obese T2D SOC SOC will not Yes T2D with (diabetes) address obesity morbid obesity ? Disease progression from obesity ? T2D is currently unabated ? Standard of care is inadequate in altering progression with the exception of metabolic surgery? Device intervention may become an important option? Attention to risk:benefit is key
Gi Dynamics DIABETES OBESITY
endobarrier

Financial Guidance and Operating Goals
Gi Dynamics DIABETES OBESITY
endobarrier

Financial Guidance for 2015 • Revenue – Assuming enrollment restarts October 1, 2015 and includes related overhang impacting the business in 1H’15, we now expect revenue in the range of $2.0 million to $2.5 million • Balance sheet & cash flow: – At March 31, 2015, the Company had approximately $40.0 million in cash and cash equivalents, which it believes is sufficient to fund its operations through March 2016. – Due to the nature and timing of the cash flows related to our ENDO Trial, we do not believe that the enrollment hold had a significant effect on our cash flows from operations for the three month’s ended March 31, 2015. – The enrollment hold’s impact on the costs of the ENDO Trial is uncertain at this time. It is possible that in the short-term we may realize savings in patient recruiting expenses; however, the long-term costs associated with the risk mitigation strategies we implement are unknown at this time.
Gi Dynamics DIABETES OBESITY
endobarrier

Operating Goals Through 2016 • Achieve awareness of our mission, vision and value proposition among 100% of our stakeholders. • Establish a quality organization, culture and systems that exceed global regulatory requirements. • Develop and implement business models for market development by geography that are reproducible, scalable and based on return on investment. • Generate clinical evidence in support of our value proposition with clinical trials, publications and geographically-specific patient registries. • Restart and complete ENDO Trial in a manner that ensures the best possible chance of clinical and regulatory success. • Pursue research and development focused on improving and expanding the EndoBarrier platform. • Obtain a NASDAQ company listing in 2015.
Gi Dynamics DIABETES OBESITY
endobarrier








































