EXHIBIT 99.2
 | EndoBarrier®Update |
| | Fall 2019 Newsletter |
To the clinicians who have worked so hard to support EndoBarrier® and who have been so patient with us, I want to say how much we appreciate your efforts over the last few years. We would not be in the positive position we are in now without your constant support and continued progress on your clinical trials, registry, and publication efforts.
Thank you.
I am pleased to update you that we are on our way towards achieving a number of critical milestones at GI Dynamics.
With renewed financial support from our largest shareholder, we have initiated enrollment in the groundbreaking STEP-1 pivotal trial of EndoBarrier in the United States. This study encompasses the traditional type 2 diabetes and obesity endpoints and captures additional data such as cardiovascular risk markers and insulin sensitivity. In addition, this study also will measure liver fat, liver stiffness, and CKD markers- for the first time for a pivotal trial in the United States. The STEP-1 trial will also gather data for future biomarker development and microbiome analysis that we expect will lead to significant future breakthroughs.
Most notably for our clinicians in Europe and the Middle East, we are highly focused on gaining the EndoBarrier CE Mark. We hope to have our CE Mark in the first half of 2020 and appreciate your support as we prepare the necessary documentation and work through the approval process with our notified body.
Finally, we are working closely with Apollo Sugar, which is the joint venture formed by Apollo Hospitals Group and Sanofi for the treatment of diabetes and related comorbidities. Apollo Hospital is the largest and highest-rated private hospital system in India and has been a phenomenal partner as we jointly prepare to initiate the I-STEP pivotal trial in India. This study mirrors many of the endpoints we will gather in the U.S. STEP-1 study and is anticipated to begin enrolling in the fourth quarter of 2019.

STEP-1 Investigator Meeting, April 2019
The team at GI Dynamics is dedicated to helping you treat your patients suffering from type 2 diabetes, obesity, and associated metabolic disorders.
We look forward to updating you on our progress……and again, Thank You!
Scott Schorer
President & CEO
GI Dynamics
GI Dynamics Newsletter: Fall 2019
ENDOBARRIER® UPDATE
| Financial News |  |
On August 21, 2019, GI Dynamics announced up to a US$10 Million financing with Crystal Amber Fund, the company’s largest shareholder. Funds will be used to initiate patient enrollment for STEP-1 (United States) and I-STEP (India), as well as continuing to work towards gaining the EndoBarrier CE Mark.
Launching Clinical Trials
United States: STEP-1
| GI Dynamics has initiated Stage 1 enrollment of theSTEP-1clinical study of EndoBarrier in the United States. Enrollment will occur from Q3 2019 into Q1 2020 at the following five (5) leading sites: |  |
| | 1. | Brigham & Women’s Hospital | Boston, Massachusetts |
| | 2. | University of Michigan | Ann Arbor, Michigan |
| | 3. | Thomas Jefferson University | Philadelphia, Pennsylvania |
| | 4. | Baylor College of Medicine | Houston, Texas |
| | 5. | Surgical Specialists of Louisiana | New Orleans, Louisiana |
TheSTEP-1pivotal trial is led by PI Christopher Thompson, M.D. of Brigham & Women’s Hospital. Stage 1 of the study will consist of 50 EndoBarrier implant procedures and ~ 17 sham control procedures and has started enrolling patients. EndoBarrier implants will be removed twelve months following implant, targeted to be no later than Q1 2021. GI Dynamics will then work with the FDA to gain approval for Stage 2 of theSTEP-1clinical trial.
India: I-STEP
| GI Dynamics has partnered with Apollo Sugar to run the clinical trial of EndoBarrier in India:I-STEP. Apollo Sugar is a division of Apollo Hospitals Group (Apollo) focused on the treatment of metabolic disorders and operates an integrated network of centers of excellence for diabetes, obesity and endocrinology and is a collaboration between Apollo Health & Lifestyle Limited and Sanofi. Apollo is the largest and highest-rated private hospital system in India. Apollo Sugar is currently working to secure Ethics Committee (EC) approval for 5 clinical sites throughout India. |  |
| Patient enrollment is planned to take place between Q4 2019 and Q2 2020. Implant removal is expected to be complete in Q2 2021. |  |
GI Dynamics Newsletter: Fall 2019
ENDOBARRIER® UPDATE
Product Launch Activities
CE Mark Plans
| ● | GI Dynamics has been diligently working towards regaining the EndoBarrier CE Mark to support a relaunch in Europe and the Middle East. |
| | | |
| ● | The team is relying heavily on clinician input and data from multiple investigator initiated clinical trials, registry reports and analyses. |
| | | |
| ● | GI Dynamics is working towards a Q1 2020 goal of receiving the EndoBarrier CE Mark. |
The GI Dynamics team greatly appreciates all the support and time clinicians have provided in support of this line of effort.
Investigator Meeting: STEP-1
In March 2019, GI Dynamics held an Investigator Meeting for all United States clinical sites at the 7thAnnual Flexible Endoscopy Surgery and Bariatric Endoscopy Course (FES).

GI Dynamics Newsletter: Fall 2019
ENDOBARRIER® UPDATE
Product Launch Activities(continued)
Commercialization
United States:GI Dynamics will submit for FDA approval in 2023 with U.S. commercialization slated for 2025.
Europe and Middle East:CE Mark expected in the first half of 2020.
India:India’s commercialization of EndoBarrier with Apollo Sugar JV is scheduled for late 2021 or early 2022.
HOW ENDOBARRIER WORKS
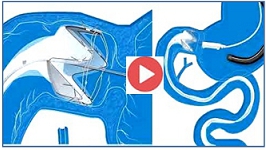
New EndoBarrier Overview Video Released
Milestones to Note
| ● | August 2018:FDA approved GI Dynamics Investigational Device Exemption for the pivotal trial of EndoBarrier conditioned upon IRB approval |
| ● | February 2019:Western IRB provided approval |
| ● | March 2019:UK National Health Service EndoBarrier Service Releases Final Data of One- Year Outcome |
| ● | March 2019: Association of British Clinical Diabetologists Releases Results of EndoBarrier Obstructive Sleep Apnea Study |
Team Additions
GI Dynamics has had the pleasure of growing its Scientific Advisory Board (SAB) and Executive Team in 2019.
| | | Leadership Team | | | Scientific Advisory Board |
| | | | | | |
| |  | Stephen Linhares joins as VP of Clinical and Regulatory Affairs | |  | Allon Friedman M.D. of Indiana University School of Medicine with a focus on chronic kidney disease |
| | | | | | |
| |  | Paul Pelletier joins as Director of Quality Assurance | |  | Judith Korner, M.D. Ph.D., of Columbia University Medical Center with a focuson endocrinology and metabolism |
| | | | | | |
| |  | Charles Carter joins as Chief Financial Officer | |  | Steven Opal, M.D. of Brown University with a focus on liver abscess |
Contact GI Dynamics
| Scott Schorer | Janell Shields |
| President & CEO | Manager, Marketing & IR |
| sschorer@gidynamics.com | jshields@gidynamics.com |
GI Dynamics Newsletter: Fall 2019
4













