Unless the context otherwise requires, we use the terms “GlycoMimetics,” the “Company,” “we,” “us” and “our” in this Current Report on Form 8-K, or this Report, to refer to GlycoMimetics, Inc., a Delaware corporation. ‘‘GlycoMimetics,’’ the GlycoMimetics logo and other trademarks or service marks of GlycoMimetics, Inc. appearing in this Report are the property of GlycoMimetics, Inc. The other trademarks, trade names and service marks appearing in this Report are the property of their respective owners.
Company Overview
We are a clinical-stage biotechnology company focused on the discovery and development of novel glycomimetic drugs to address unmet medical needs resulting from diseases in which carbohydrate biology plays a key role. Glycomimetics are molecules that mimic the structure of carbohydrates involved in important biological processes. Using our expertise in carbohydrate chemistry and knowledge of carbohydrate biology, we are developing a pipeline of proprietary glycomimetics that inhibit disease-related functions of carbohydrates, such as the roles they play in inflammation, cancer and infection. We believe this represents an innovative approach to drug discovery to treat a wide range of diseases.
We are focusing our initial efforts on drug candidates for rare diseases that we believe will qualify for orphan drug designation. Our first drug candidate, rivipansel, is being developed for the treatment of vaso-occlusive crisis, or VOC, a debilitating and painful condition that occurs periodically throughout the life of a person with sickle cell disease. We have entered into a collaboration with Pfizer Inc. for the further development and potential commercialization of rivipansel worldwide. Rivipansel has received fast track designation from the U.S. Food and Drug Administration, or FDA, as well as orphan drug designation from the FDA in the United States and from the European Medicines Agency in the European Union. We believe the clinical progress of rivipansel provides evidence of the significant potential of our lead program and our proprietary glycomimetics platform. Building on our experience with rivipansel, we are developing our second glycomimetic drug candidate, GMI-1271, to be used in combination with chemotherapy to treat either acute myeloid leukemia, or AML, or multiple myeloma, or MM, both of which are life-threatening hematologic cancers, and potentially other hematologic cancers as well. We have retained the worldwide development and commercialization rights to all of our drug candidates other than rivipansel.
Our proprietary glycomimetics platform is based on our expertise in carbohydrate chemistry and our understanding of the role carbohydrates play in key biological processes. Most human proteins are modified by the addition of complex carbohydrates to the surface of the proteins. The addition of these carbohydrate structures affects the functions of these proteins and their interactions with other molecules. Our initial research and development efforts have focused on drug candidates targeting selectins, which are proteins that serve as adhesion molecules and bind to carbohydrates that are involved in the inflammatory component and progression of a wide range of diseases, including hematologic disorders, cancer and cardiovascular disease. For example, we believe that members of the selectin family play a key role in the onset and progression of VOC. Inhibiting specific carbohydrates from binding to selectins has long been viewed as a potentially attractive approach for therapeutic intervention. The ability to successfully develop drug-like compounds that inhibit binding with selectins, known as selectin antagonists, has been limited by the complexities of carbohydrate chemistry. We believe our expertise in carbohydrate chemistry and our understanding of carbohydrate-protein binding interactions enable us to design selectin antagonists and other glycomimetics that inhibit the disease-related functions of certain carbohydrates. We believe this expertise and knowledge enable us to develop novel drug candidates to address unmet medical needs.
Our intellectual property portfolio includes ownership of, or exclusive rights to, issued patents and pending patent applications claiming fundamental features of glycomimetic therapeutics, as well as those claiming methods of use for and chemical modifications of our drug candidates. Given the importance of our intellectual property portfolio to our business operations, we intend to vigorously enforce our rights and defend against challenges that have arisen or may arise in this area. Our issued patents directed to rivipansel and methods of use are expected to expire between 2023 and 2030. We have a U.S. patent covering GMI-1271 that is expected to expire in 2032. In addition, we have several pending patent applications covering GMI-1271 and methods of using it, the last expiring of which, if issued, is predicted to expire in 2034.
1
Our Drug Candidates
We have discovered our drug candidates internally through a rational drug design approach that couples our expertise in carbohydrate chemistry with our knowledge of carbohydrate biology. We are actively developing glycomimetic drug candidates based on this expertise.
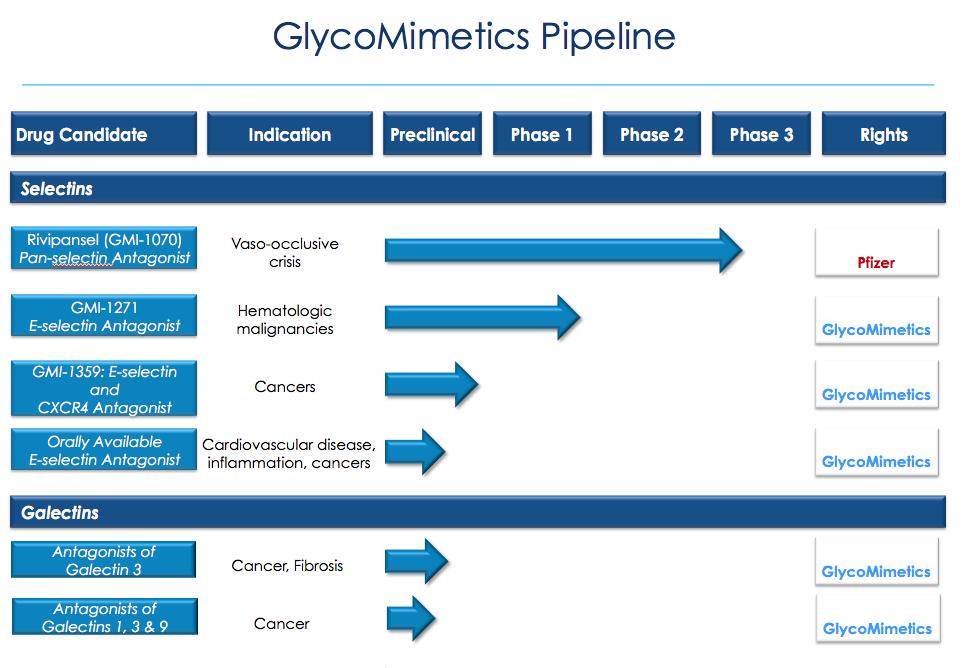
Rivipansel – Targeting Selectins to Treat VOC
We are developing rivipansel to treat VOC, with the goal of reducing duration of VOC episodes, length of hospital stay and use of opioid analgesics for pain management. In our completed Phase 2 clinical trial, patients treated with rivipansel plus the standard of care achieved improvement in these endpoints, in each case as compared to patients receiving placebo plus the standard of care.
Sickle cell disease is a genetic disease that, according to the U.S. Centers for Disease Control and Prevention, or CDC, affects millions of people throughout the world, including an estimated 90,000 to 100,000 people in the United States. VOC is one of the most severe complications of sickle cell disease. VOC episodes are typically characterized by excruciating musculoskeletal pain, visceral pain and pain in other locations, and occur periodically throughout the life of a person with sickle cell disease. The CDC estimates that VOC resulted in approximately 73,000 hospitalizations in the United States in 2010. According to the National Hospital Discharge Survey conducted by the National Center for Health Statistics, these hospitalizations have an average duration of approximately six days. The standard of care in the United States for people experiencing VOC is to manage its symptoms, which typically includes hospitalization, narcotic pain management and hydration. There are no approved therapies that interrupt VOC once it has started or that treat the underlying cause of the pain.
Among both adults and children with sickle cell disease, VOC is the most common reason for seeking medical attention resulting in hospitalization. VOC affects multiple organ systems and may result in significant clinical complications. Most sickle cell disease-related deaths occur during acute VOC and are due to infection, acute chest syndrome, stroke or multi-organ failure. We believe that rivipansel, if approved, would be the first drug to interrupt
2
the underlying cause of VOC, thereby potentially reducing the use of narcotics for pain management and enabling patients to leave the hospital more quickly.
We have completed four clinical trials of rivipansel involving a total of 163 subjects. In April 2013, we completed a Phase 2 clinical trial in which 76 patients hospitalized for VOC, ranging from 12 to 60 years old, were treated with the standard of care plus either rivipansel or placebo. In this trial, patients treated with rivipansel experienced reductions in the time to reach resolution of VOC, length of hospital stay and use of opioid analgesics for pain management, in each case as compared to patients receiving placebo. This improvement was seen in both adult and pediatric patients. Adverse event rates and severity were comparable between those treated with rivipansel and those receiving placebo.
We entered into a license agreement in October 2011 with Pfizer, under which Pfizer has rights to develop and commercialize rivipansel for all indications worldwide. Following the completion of our Phase 2 clinical trial, Pfizer is now responsible for the further clinical development, regulatory approval and potential commercialization of rivipansel. Under the Pfizer agreement, we received an upfront payment of $22.5 million from Pfizer. We are also eligible to receive payments of up to $115.0 million upon the achievement of specified development milestones, including the dosing of the first patients in Phase 3 clinical trials for up to two indications and the first commercial sale of a licensed product in the United States and selected European countries for up to two indications, up to $70.0 million upon the achievement of specified regulatory milestones, including the acceptance of our filings for review by regulatory authorities in the United States and Europe for up to two indications, and up to $135.0 million upon the achievement of specified levels of annual net sales of licensed products. We are also eligible to receive tiered royalties, with percentages ranging from the low double digits to the low teens, based on net sales of rivipansel worldwide, subject to reductions in specified circumstances.
The first potential milestone payment under the Pfizer agreement was $35.0 million upon the initiation of dosing of the first patient in a Phase 3 trial of rivipansel by Pfizer. Under the collaboration, Pfizer made a $15.0 million non-refundable milestone payment to us in May 2014, and the dosing of the first patient in the Phase 3 clinical trial triggered the remaining $20.0 million milestone payment to us, which we received in August 2015.
Under a separate research agreement with the University of Basel, we have agreed to pay 10% of any future milestone payments and royalties we may receive from Pfizer with respect to rivipansel.
GMI-1271—Targeting the Bone Marrow Microenvironment to Treat Hematologic Cancers
We are developing a pipeline of other drug candidates based on our expertise in carbohydrate chemistry, including compounds that are designed to be specific to particular selectins. We are developing GMI-1271, a specific E-selectin inhibitor, to be used in combination with chemotherapy to treat patients with AML, MM and potentially other hematologic cancers.
E-selectin plays a critical role in binding cancer cells within vascular niches in the bone marrow, which prevents the cells from entering circulation where they can be more readily killed by chemotherapy. In animal studies, GMI-1271 mobilized AML and MM cancer cells out of the bone marrow, making them more sensitive to chemotherapy. In both the AML and MM studies, the combination of GMI-1271 with chemotherapy resulted in improved survival rates for the treated animals, compared to chemotherapy alone. In other animal studies, GMI-1271 appeared to also protect normal cells from some of the side effects of chemotherapy. Common side effects of chemotherapy include bone marrow toxicity resulting in neutropenia, which is an abnormally low number of neutrophils, the white blood cells that serve as the primary defense against infection, and mucositis, which is the inflammation and sloughing of the mucous membranes lining the digestive tract. Animals treated with GMI-1271 and chemotherapy had less severe neutropenia and mucositis and lower bone marrow toxicity as compared to animals treated with chemotherapy alone. We believe that treatment with GMI-1271 results in lower bone marrow toxicity due to its inhibition of E-selectin, which makes stem cells in the bone marrow divide less frequently, thereby protecting them from chemotherapy agents that target rapidly dividing cells.
Acute Myeloid Leukemia
AML is a cancer of the blood and bone marrow. The accumulation of cancer cells in the bone marrow potentially inhibits the production of mature blood cells, such as red blood cells, white blood cells and platelets. It is the most common form of acute leukemia among adults and accounts for the largest number of annual deaths from leukemias in the United States. The National Cancer Institute estimates that in the United States in 2016 there will
3
be approximately 20,000 new cases of AML diagnosed and approximately 10,000 deaths resulting from the disease. Approximately 300,000 patients in the world are diagnosed with AML annually.
AML is more commonly present in elderly patients, with a median age at diagnosis of 67 years. In a review published in the Journal of Clinical Oncology, the median overall survival of patients 60 years old or older was 8.7 months. The overall five-year relative survival rate for all AML patients is approximately 26%, and only 5% for patients over 65 years old at diagnosis.
A number of published studies indicate that only some AML patients who receive chemotherapy achieve a complete remission, which is defined as the disappearance of all signs of AML, and that most of those with a complete remission will eventually relapse. Patients who do not enter remission are referred to as refractory, meaning that they are resistant to the chemotherapy treatment.
In August 2014, we completed a Phase 1 trial of GMI-1271 in healthy volunteers. The single-site Phase 1 trial was a randomized, double-blind, placebo-controlled, single ascending intravenous dose trial. In the trial, we evaluated the safety, tolerability and pharmacokinetics of GMI-1271. Twenty-eight healthy adult subjects were enrolled in cohorts to receive study drug at three dose levels. In the trial, we observed that the subjects tolerated GMI-1271 well, and that the pharmacokinetics for GMI-1271 were as predicted based on preclinical data.
Following the completion of the Phase 1 trial, in May 2015 we commenced a multinational, Phase 1/2, open-label trial of GMI-1271 as an adjunct to standard chemotherapy in patients with AML. This trial in males and females with AML is being conducted at a number of academic institutions in the United States, Ireland and Australia.
The trial consists of two parts. In the Phase 1 portion, which we recently completed, escalation testing was performed to determine a recommended GMI-1271 dose in combination with standard chemotherapy. The primary objective of this portion of the trial was to evaluate the safety of GMI-1271 in combination with chemotherapy. Secondary objectives were to characterize pharmacokinetics, or PK, pharmacodynamics, or PD, and to observe anti-leukemic activity. There were a total of 19 patients enrolled and dosed with a single cycle of treatment with GMI-1271 and chemotherapy in the Phase 1 portion of the trial. The patients ranged from 26 to 77 years of age, with a median of 51 years. Patients had relapsed or refractory AML and other risk factors indicating poor prognosis.
In the Phase 1 portion of the trial, the combination of GMI-1271 and chemotherapy was well-tolerated, with no dose-limiting toxicities observed and no mortality reported during the treatment phase of 44 days. Serious adverse events, including sepsis, pneumonia, device-related infection, enterocolitis, hypernatremia and adjustment disorder, were observed in five patients, with all such events resolving during the treatment phase. Fourteen of the 19 patients were reported to have no mucositis after chemotherapy was completed, which often develops following treatment with this intensive therapy.
In terms of efficacy, eight of the 19 patients achieved complete remission, with a full bone marrow response, or CR, and full blood count recovery. One additional patient achieved CR but with an incomplete blood count recovery prior to receiving a hematopoietic stem cell transplant, or HSCT, a response referred to as CRi. The total of nine patients achieving remission represents an overall response rate of 47%. Standard chemotherapy regimens for relapsed/refractory AML patients typically have remission rates of between 25-30%. One additional patient achieved a status known as morphologic leukemia-free state. Five patients proceeded to receiving an HSCT.
PK data showed a dose-dependent increase in plasma concentrations of GMI-1271 that were above levels associated with anti-leukemic activity in animal models of AML. In addition, biomarker analysis showed a biological effect of GMI-1271 at all dose levels.
With an optimal dose of 10 mg/kg having been determined, in June 2016 we dosed the first patient in the Phase 2 portion of the trial. In this portion of the trial, dose-expansion testing will be conducted to obtain additional safety and efficacy data in defined sub-populations of AML, including patients over 60 years of age with newly diagnosed AML. We expect to enroll a total of approximately 50 patients in the Phase 2 portion of the trial. Unlike in the Phase 1 portion, some of the patients in the Phase 2 portion may be treated with multiple cycles of GMI-1271.
4
Multiple Myeloma
MM is a cancer of the blood and bone marrow that affects a type of white blood cell that normally helps a person fight infections by making antibodies that recognize and attack germs. MM causes cancer cells to accumulate in the bone marrow, where they crowd out healthy blood cells. MM is the most frequent tumor that occurs primarily in bone and is the second most common hematologic cancer in the United States and Europe. MM accounts for 10% to 15% of hematologic cancers and 20% of deaths from these cancers. The National Cancer Institute estimates that in the United States in 2016 there will be approximately 30,000 new cases of MM diagnosed and over 12,000 deaths resulting from the disease. In the European Union, in 2012, 39,000 new cases of MM were diagnosed and 24,000 deaths occurred. MM is rare in individuals younger than 40 years old and the median age at diagnosis is approximately 70 years. More than 35% of patients are over 70 years of age, making treatment with chemotherapy more complicated due to fewer treatment options being available, patients being ineligible for transplant, and decreased ability to tolerate sustained chemotherapy due to poor general health.
Despite the fact that recent treatment options for MM have led to improved response rates and increased short-term survival, responses are transient and most patients with MM will ultimately relapse and succumb to their cancer. MM is not considered curable with current approaches. The 5-year overall survival rate for all patients in the United States is less than 50%. Although second and later remissions can be achieved with additional treatment, tumors typically recur more aggressively after each relapse, leading to decreased duration of response and ultimately culminating in the development of treatment-refractory disease. Median survival for treatment-refractory disease typically ranges from five months for event-free survival and to nine months for overall survival and responses to treatment are characteristically short, most likely due to resistant disease. This loss of response complicates therapy of patients in later-line treatment, shortens survival and results in high mortality rates.
In December 2015, at the annual meeting of the American Society of Hematology, or ASH, we presented preclinical data suggesting that GMI-1271 could reverse resistance of certain chemotherapies seen in MM. We have initiated preparations for a Phase 1 multiple dose-escalation clinical trial in defined populations of patients with MM. In this trial we plan to evaluate GMI-1271 as an adjunct to bortezomib, another therapy approved to treat MM. We have received approval for a Clinical Trial Application from the Health Products Regulatory Authority in Ireland and plan to initiate a trial in patients with MM in the second half of 2016.
Other Indications
In December 2013, researchers at the University of Michigan received a grant from the National Heart Lung and Blood Institute to evaluate GMI-1271 as a potential treatment for venous thromboembolic disease, or VTE, a serious blood clotting disorder. VTE, which can occur after a major operation or severe illness, such as a heart attack, stroke or some cancers, refers to both pulmonary embolism and deep vein thrombosis, or DVT, which is the formation of blood clots in large veins, primarily in the legs. The clots become dangerous when they break loose and can affect blood flow to the heart and lungs. Because GMI-1271, as an E-selectin antagonist, also inhibits the activation of processes leading to thrombosis, we believe that it has therapeutic potential to decrease thrombosis and its inflammatory effects.
The University of Michigan began dosing healthy volunteers in a Phase 1 randomized, partially blinded, active placebo-controlled trial in December 2014. The primary objective of the trial was to evaluate the safety and pharmacokinetic profile of GMI-1271 in a single ascending dose in healthy volunteers. The secondary objectives included evaluation of the incidence of bleeding and other adverse events and evaluation of the effects of GMI-1271 on biomarkers of coagulation, cell adhesion and leukocyte and platelet activation. The single ascending dose-escalation clinical trial in 24 healthy volunteers was completed in October 2015 and the trial has been extended to include a multiple ascending dose-escalation in eight healthy volunteers. We expect that results from this trial will be available in the third quarter of 2016. We also plan to initiate a Phase 2 clinical trial in patients with DVT in the second half of 2016.
Other Drug Candidates
GMI-1359 – Targeting E-Selectin and CXCR4
The chemokine CXCR4 has emerged as an important pro-inflammatory cytokine that is involved in cell migration throughout the body. Like E-selectin, tumor cells may also use the CXCR4 cellular pathway, contributing to chemoresistance, metastatic disease and ultimately decreased survival.
5
We have designed a family of small molecule drug candidates that simultaneously inhibit both E-selectin and CXCR4. We have selected one of these compounds, GMI-1359, to be developed further as part of an IND-enabling nonclinical program. Since E-selectin and CXCR4 are both adhesion molecules that keep cancer cells in the bone marrow, we believe that targeting both E-selectin and CXCR4 with a single compound could improve efficacy in the treatment of cancers that affect the bone marrow such as AML and MM, as compared to targeting CXCR4 alone. GMI-1359 is currently undergoing testing in preclinical models from which we intend to select a target clinical indication, likely in oncology. We received pre-IND guidance from the FDA in October 2015 and we plan to submit an Investigational New Drug application, or IND, for GMI-1359 for the treatment of hematologic malignancies in the third quarter of 2016. We also plan to initiate a Phase 1 single-dose escalation trial in healthy volunteers in the fourth quarter of 2016. In December 2015 at the ASH annual meeting, we presented preclinical data suggesting that GMI-1359 enhanced the ability of chemotherapy to target and improve survival from a high-risk form of mutated AML.
Galectin Inhibitors
Galectin-3 and galectin-9 are proteins that are known to play critical roles in many pathological processes, including checkpoints in T-cell exhaustion during cancer immunotherapy, chemotherapy resistance, fibrosis and cardiovascular disease. Using our glycomimetics platform, we have designed galectin-3 and galectin-9 inhibitors that specifically block the binding of galectin-3 and galectin-9 to carbohydrate structures. We plan to optimize these compounds and conduct preclinical experiments in 2016 to further characterize the effects of galectin-3 and galectin-9 inhibitors on immune processes and anti-fibrotic activity.
Forward-Looking Statements
This Report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and we intend that such forward-looking statements be subject to the safe harbors created thereby. All statements, other than statements of historical fact, contained in this Report, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
The forward-looking statements in this Report include, among other things, statements about:
· | our plans to develop and commercialize our glycomimetic drug candidates; |
· | our ongoing and planned clinical trials for our drug candidates GMI-1271 and GMI-1359, including the timing of initiation of and enrollment in the trials, the timing of availability of data from the trials and the anticipated results of the trials; |
· | our ability to achieve anticipated milestones under our collaboration with Pfizer for our drug candidate rivipansel; |
· | the timing of and our ability to obtain and maintain regulatory approvals for our drug candidates; |
· | the clinical utility of our drug candidates; |
· | our commercialization, marketing and manufacturing capabilities and strategy; |
· | our intellectual property position; |
· | our ability to identify additional drug candidates with significant commercial potential that are consistent with our commercial objectives; and |
· | our estimates regarding future revenues, expenses and needs for additional financing. |
The “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2015, as well as other filings we make with the SEC from time to time, contain a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements in this Report. As a result of these factors, we cannot assure you that the forward-looking statements in this Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. We qualify all of our forward-looking statements by these cautionary statements.
6