2020 and retention bonuses. Patent, legal fees, consulting and other professional expenses increased by $970,000 for the year ended December 31, 2020 as compared to December 31, 2019 due to a significant increase in the cost of directors, officers and corporate liability insurance in 2020. Other expenses decreased by $221,000 as compared to the prior year due to lower travel, meals and conference registration expenses as a result of travel restrictions due to the COVID-19 pandemic.
Interest Income
During the year ended December 31, 2020, interest income decreased by $3.0 million, or 86%, compared to the same period in 2019, due to lower average cash balances and lower interest rates on those balances.
Liquidity and Capital Resources
Sources of Liquidity
We have historically financed our operations primarily through public offerings and private placements of our capital stock, including sales agreements with Cowen, and upfront and milestone payments from our license and collaboration agreements. As of December 31, 2020, we had $137.0 million in cash and cash equivalents.
In March 2018, we completed a public offering in which we sold 8,050,000 shares of our common stock at a price to the public of $17.00 per share. We received net proceeds of $128.4 million from this offering, after deducting underwriting discounts, commissions and other offering expenses.
In September 2017, we entered into an at-the-market sales agreement with Cowen, or the 2017 Sales Agreement, under which we could offer and sell, from time to time at our sole discretion, shares of our common stock having an aggregate offering price of up to $100.0 million through Cowen acting as our sales agent. The shares were registered under a shelf registration statement filed with the U.S. Securities and Exchange Commission in September 2017. During the year ended December 31, 2017, we sold an aggregate of 1,600,000 shares of our common stock under the 2017 Sales Agreement for net proceeds of $19.3 million. There were no shares sold under the 2017 Sales Agreement during the years ended December 31, 2018 or 2019. During the year ended December 31, 2020, we sold an additional 4,136,742 shares of common stock under the 2017 Sales Agreement at a weighted average price per share of $3.52, for aggregate net proceeds of $14.1 million, after deducting commissions and offering expenses. The shelf registration statement, under which the shares that could be sold under the 2017 Sales Agreement were registered expired on October 6, 2020.
In October 2020, we filed a prospectus supplement to a new shelf registration statement that we filed in May 2019 and entered into a new at-the-market sales agreement, or the 2020 Sales Agreement, with Cowen. Under the 2020 Sales Agreement, we may sell up to $100.0 million of our common stock registered under the shelf registration statement that we filed in May 2019. The 2020 Sales Agreement replaces the 2017 Sales Agreement between us and Cowen, and the $100.0 million that may be sold under the 2020 Sales Agreement excludes any amounts that were sold under the 2017 Sales Agreement. During the year ended December 31, 2020, we sold 1,024,760 shares of common stock under the 2020 Sales Agreement at a weighted average price of $3.74, for aggregate net proceeds of $3.7 million, after deducting commissions and offering expenses. Subsequent to December 31, 2020 and through February 26, 2021, we sold an additional 2,475,949 shares of common stock under the 2020 Sales Agreement at a weighted average price of $3.92, for aggregate net proceeds of $9.4 million, after deducting commissions and offering expenses.
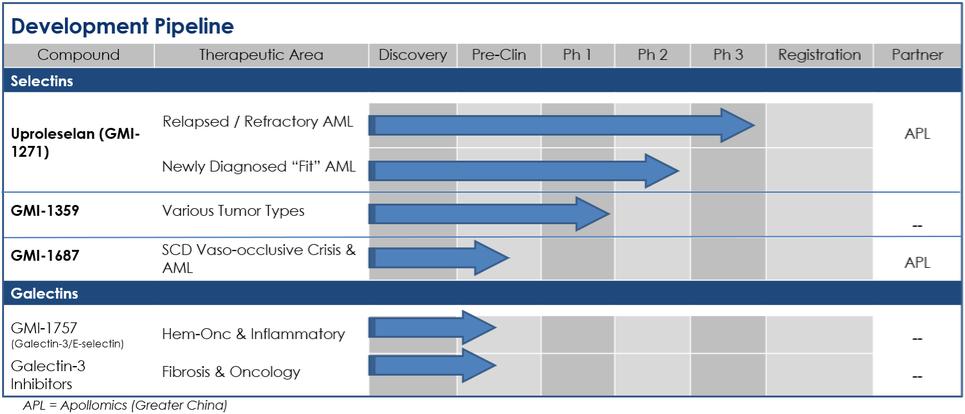
We entered into a collaboration and license agreement with Apollomics in January 2020 and are potentially eligible to earn milestone payments and royalties under that agreement. In January 2020, Apollomics made an upfront payment to us of $9.0 million. We also received a non-refundable payment of $1.0 million in September 2020 as a clinical development milestone payment. Our ability to earn additional milestone payments and potential royalty payments and their timing will be dependent upon the outcome of Apollomics’ activities and is therefore uncertain at this time.
Funding Requirements
Our primary uses of capital are, and we expect will continue to be, compensation and related expenses, third-party clinical research and development services, laboratory and related supplies, clinical costs, legal and other regulatory expenses and general overhead costs.
The successful development of any of our drug candidates is highly uncertain. As such, at this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the remainder