Exhibit 99.2

Phase 2b SOOTHE Trial in Refractory Chronic Cough Topline Results and P2X3 Pipeline Update DECEMBER 13 , 2021

Forward Looking Statements 2 Certain statements contained in this presentation, other than statements of fact that are independently verifiable at the date hereof, may constitute “forward - looking statements” within the meaning of Canadian securities legislation and regulations and other applicable securities laws . Forward - looking statements are frequently, but not always, identified by words such as “expects,” “anticipates,” “believes,” “intends,” “estimates,” “potential,” “possible,” “projects,” “plans,” and similar expressions . Such statements, based as they are on the current expectations of management, inherently involve numerous important risks, uncertainties and assumptions, known and unknown, many of which are beyond the control of BELLUS Health Inc . ("BELLUS Health," "BELLUS," the "Company," "we" or "us") . Such statements include, but are not limited to, the potential of BLU - 5937 to successfully treat chronic cough, chronic pruritus associated with AD and other hypersensitization - related disorders, BELLUS Health’s expectations related to its preclinical studies and clinical trials, including the design, timing and results of its Phase 2 b clinical trial of BLU - 5937 in refractory chronic cough and its Phase 2 a clinical trial of BLU - 5937 in chronic pruritus associated with AD , including the timing and outcome of interactions with regulatory agencies, including with the FDA regarding the SOOTHE Phase 2 b trial design, the potential activity and tolerability profile, selectivity, potency and other characteristics of BLU - 5937 , including as compared to other competitor candidates, the commercial potential of BLU - 5937 , including with respect to patient population, pricing and labeling, BELLUS Health’s financial position, and the potential applicability of BLU - 5937 and BELLUS Health’s P 2 X 3 platform to treat other disorders . Risk factors that may affect BELLUS Health’s future results include but are not limited to : the benefits and impact on label of its enrichment strategy, estimates and projections regarding the size and opportunity of the addressable refractory chronic cough market for BLU - 5937 , the ability to expand and develop its project pipeline, the ability to obtain adequate financing, the ability of BELLUS Health to maintain its rights to intellectual property and obtain adequate protection of future products through such intellectual property, the impact of general economic conditions, general conditions in the pharmaceutical industry, the impact of the ongoing COVID - 19 pandemic on BELLUS Health’s operations, plans and prospects, including to the initiation and completion of clinical trials in a timely manner or at all, changes in the regulatory environment in the jurisdictions in which BELLUS Health does business, supply chain impacts, stock market volatility, fluctuations in costs, changes to the competitive environment due to consolidation, achievement of forecasted burn rate, potential payments/outcomes in relation to indemnity agreements and contingent value rights , achievement of forecasted preclinical study and clinical trial milestones, reliance on third parties to conduct preclinical studies and clinical trials for BLU - 5937 , the ability of the Company’s interim analysis of the Phase 2 b SOOTHE trial to predict the final results of the trial and the interpretability thereof, and that actual results may differ from topline results once the final and quality - controlled verification of data and analyses has been completed . In addition, the length of BELLUS Health’s product candidate’s development process and its market size and commercial value are dependent upon a number of factors . Moreover, BELLUS Health’s growth and future prospects are mainly dependent on the successful development, patient tolerability, regulatory approval, commercialization and market acceptance of its product candidate BLU - 5937 and other products . Consequently, actual future results and events may differ materially from the anticipated results and events expressed in the forward - looking statements . BELLUS Health believes that expectations represented by forward - looking statements are reasonable, yet there can be no assurance that such expectations will prove to be correct . The reader should not place undue reliance, if any, on any forward - looking statements included in this presentation . These forward - looking statements speak only as of the date made, and BELLUS Health is under no obligation and disavows any intention to update publicly or revise such statements as a result of any new information, future event, circumstances or otherwise, unless required by applicable legislation or regulation . Please see BELLUS Health’s public filings with the Canadian securities regulatory authorities, including, but not limited to, its Annual Information Form, and the United States Securities and Exchange Commission, including, but not limited to, its Annual Report on Form 40 - F, for further risk factors that might affect BELLUS Health and its business .
• Positive SOOTHE trial with primary efficacy endpoint statistically significant at 50 & 200 mg BID doses (p≤ 0.005) Efficacy x 34% placebo - adjusted reduction in 24 - hour cough frequency at 50 and 200 mg BID doses (p≤ 0.005) x 53% reduction from baseline in 24 - hour cough frequency at 50 and 200 mg BID doses x Dose response between 12.5 and 50 mg BID doses x Statistically significant and clinically meaningful improvement in key secondary endpoints Safety & Tolerability x Well - tolerated with adverse event profile comparable to placebo x Low incidence (≤ 6.5%) of taste - related adverse events at all doses • Pursuing Phase 3 in RCC with regulatory interactions expected in Q2 2022 • BLU - 5937 pipeline to focus on cough hypersensitivity Phase 2a BLUEPRINT trial in chronic pruritus did not achieve statistical significance on primary efficacy endpoint x Success of SOOTHE supports potential evaluation of BLU - 5937 in other cough hypersensitivity indications Compelling efficacy and tolerability results from SOOTHE position BLU - 5937 as potential best - in - class P2X3 antagonist Positive SOOTHE Phase 2b trial in Refractory Chronic Cough (RCC) 3

SOOTHE Phase 2b Topline Data
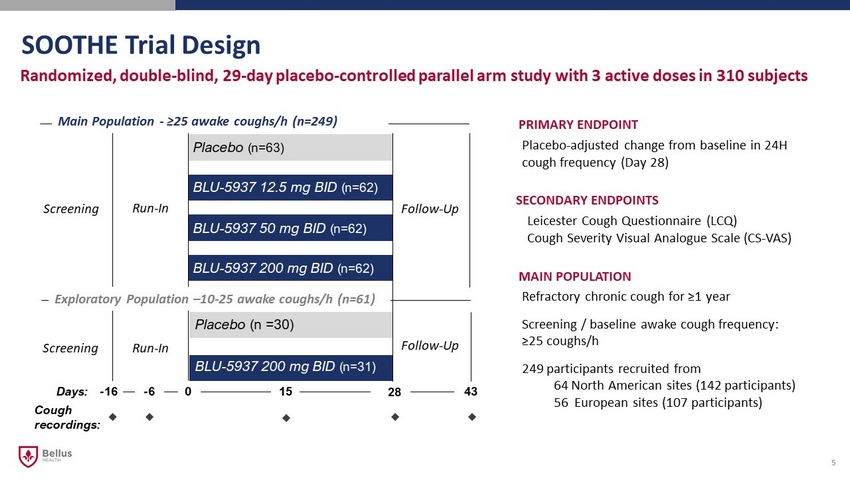
SOOTHE Trial Design 5 Randomized, double - blind, 29 - day placebo - controlled parallel arm study with 3 active doses in 310 subjects PRIMARY ENDPOINT Placebo - adjusted change from baseline in 24H cough frequency (Day 28) MAIN POPULATION Refractory chronic cough for ≥1 year Screening / baseline awake cough frequency: ≥25 coughs/h 249 participants recruited from 64 North American sites ( 142 participants) 56 European sites (107 participants) Run - In Placebo (n= 63) BLU - 5937 12.5 mg BID (n= 62) BLU - 5937 50 mg BID (n= 62) BLU - 5937 200 mg BID (n= 62) Follow - Up Main Population - ≥25 awake coughs/h (n=249) BLU - 5937 200 mg BID (n= 31) Placebo (n = 30) Exploratory Population – 10 - 25 awake coughs/h (n=61) Follow - Up Run - In Screening Screening 43 Days: - 16 0 28 Cough recordings: - 6 15 SECONDARY ENDPOINTS Leicester Cough Questionnaire (LCQ) Cough Severity Visual Analogue Scale (CS - VAS)

• Population randomized in SOOTHE was representative of RCC • Demographics and clinical characteristics were generally well - balanced across arms SOOTHE Baseline Characteristics 6 Main population Placebo BLU - 5937 (BID) 12.5 mg 50 mg 200 mg Number of subjects , n 63 62 62 62 Female , n (%) 49 (78%) 48 (77%) 52 (84%) 55 (89%) Age (years) , mean (SD) 61.4 (11.3) 60.7 (10.1) 61.6 (9.6) 59.7 (11.4) BMI (kg/m 2 ) , mean (SD) 27.9 (5.6) 28.1 (5.3) 28.6 (7.3) 27.9 (5.7) Race n (%) White 62 (98%) 58 (94%) 60 (97%) 60 (97%) Asian 1 (2%) 3 (5%) 0 0 Black 0 0 1 (2%) 2 (3%) American Indian/ Alaska Native 0 1 (2%) 1 (2%) 0 24H cough frequency (coughs/h) , mean geo 39.6 41.3 39.9 35.2
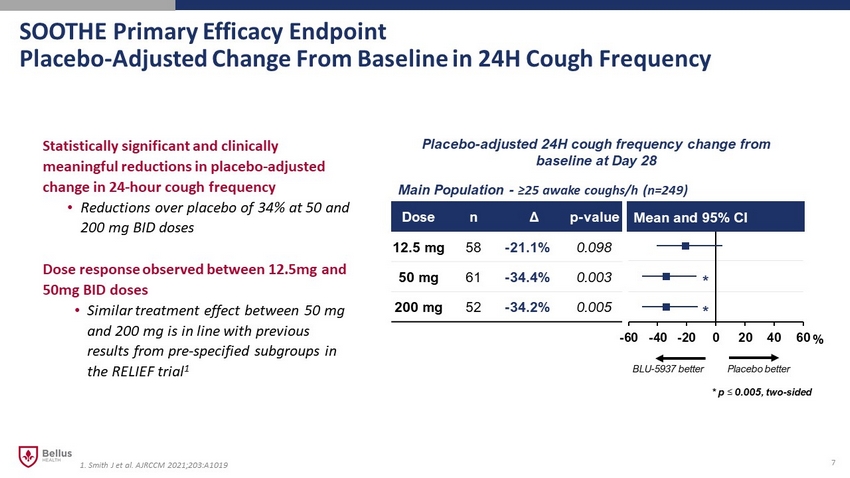
SOOTHE Primary Efficacy Endpoint Placebo - Adjusted Change From Baseline in 24H Cough Frequency 7 Statistically significant and clinically meaningful reductions in placebo - adjusted change in 24 - hour cough frequency • Reductions over placebo of 34% at 50 and 200 mg BID doses Dose response observed between 12.5mg and 50mg BID doses • Similar treatment effect between 50 mg and 200 mg is in line with previous results from pre - specified subgroups in the RELIEF trial 1 -60 -40 -20 0 20 40 60 Dose n Δ p - value 12.5 mg 58 - 21.1% 0.098 50 mg 61 - 34.4% 0.003 200 mg 52 - 34.2% 0.005 Mean and 95% CI Main Population - ≥25 awake coughs/h (n=249) Placebo - adjusted 24H cough frequency change from baseline at Day 28 * * * p ≤ 0.005, two - sided BLU - 5937 better Placebo better % 1. Smith J et al. AJRCCM 2021;203:A1019
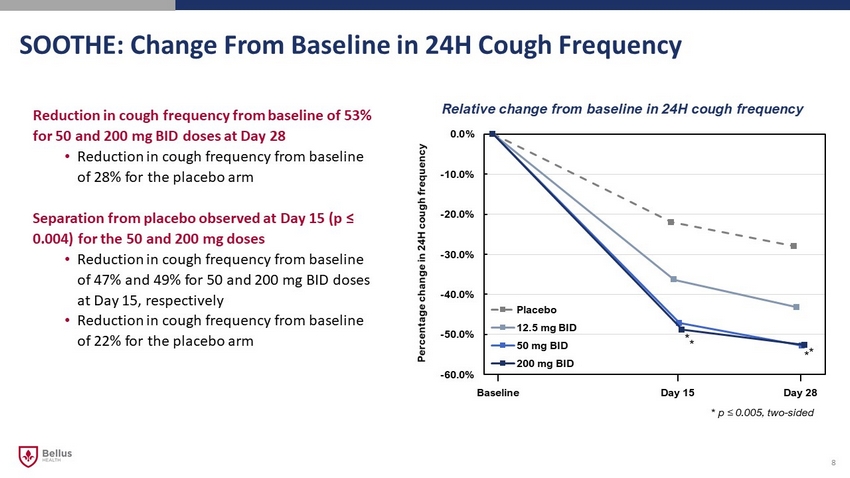
-60.0% -50.0% -40.0% -30.0% -20.0% -10.0% 0.0% Placebo 12.5 mg BID 50 mg BID 200 mg BID Relative change from baseline in 24H cough frequency Baseline Day 15 Day 28 SOOTHE: Change From Baseline in 24H Cough Frequency 8 Percentage change in 24H cough frequency Reduction in cough frequency from baseline of 53% for 50 and 200 mg BID doses at Day 28 • Reduction in cough frequency from baseline of 28% for the placebo arm Separation from placebo observed at Day 15 (p ≤ 0.004) for the 50 and 200 mg doses • Reduction in cough frequency from baseline of 47% and 49% for 50 and 200 mg BID doses at Day 15, respectively • Reduction in cough frequency from baseline of 22% for the placebo arm * p ≤ 0.005, two - sided * * * *
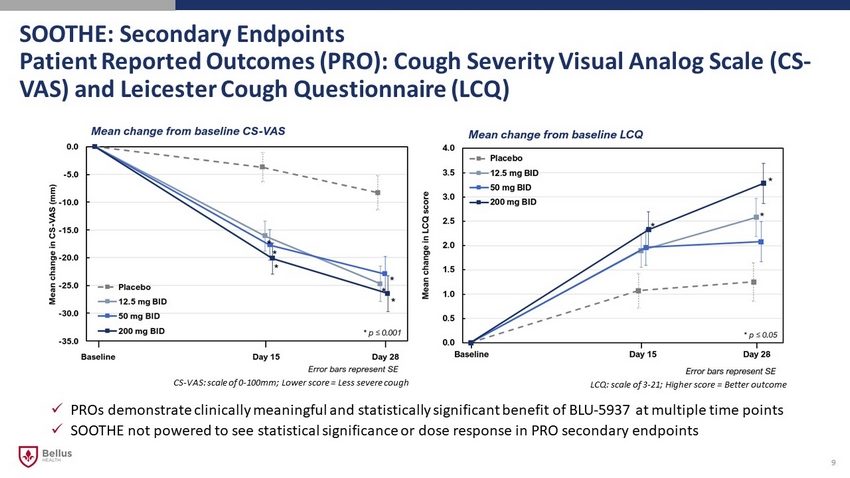
SOOTHE: Secondary Endpoints Patient Reported Outcomes (PRO): Cough Severity Visual Analog Scale (CS - VAS) and Leicester Cough Questionnaire (LCQ) 9 x PROs demonstrate clinically meaningful and statistically significant benefit of BLU - 5937 at multiple time points x SOOTHE not powered to see statistical significance or dose response in PRO secondary endpoints LCQ: scale of 3 - 21; Higher score = Better outcome CS - VAS: scale of 0 - 100mm; Lower score = Less severe cough
SOOTHE: Exploratory Population 10 Exploratory analysis to generate additional data in a population with baseline cough frequency between 10 and 25 coughs/h • Not powered to observe a statistically significant response over placebo • 200 mg BID dose only Results in exploratory population: • No treatment effect of BLU - 5937 (p=0.560) • Large placebo response and high variability • Statistical trend of interaction between baseline cough frequency and treatment effect (p= 0.077) Further analyses will inform cough frequency enrichment strategy for future trials BLU - 5937 200 mg BID (n= 31) Placebo (n = 30) Exploratory Population – 10 - 25 awake coughs/h (n=61) Follow - Up Run - In Screening 43 Days: - 16 0 28 Cough recordings: - 6 15
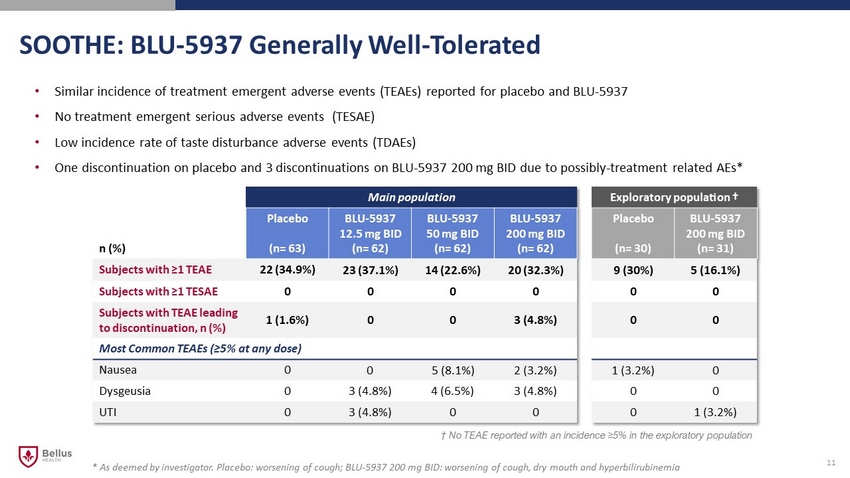
SOOTHE: BLU - 5937 Generally Well - Tolerated 11 • Similar incidence of treatment emergent adverse events (TEAEs) reported for placebo and BLU - 5937 • No treatment emergent serious adverse events (TESAE) • Low incidence rate of taste disturbance adverse events (TDAEs) • One discontinuation on placebo and 3 discontinuations on BLU - 5937 200 mg BID due to possibly - treatment related AEs* Main population Exploratory population † n (%) Placebo (n= 63) BLU - 5937 12.5 mg BID (n= 62) BLU - 5937 50 mg BID (n= 62) BLU - 5937 200 mg BID (n= 62) Placebo (n= 30) BLU - 5937 200 mg BID (n= 31) Subjects with ≥1 TEAE 22 (34.9%) 23 (37.1%) 14 (22.6%) 20 (32.3%) 9 (30%) 5 (16.1%) Subjects with ≥1 TE SAE 0 0 0 0 0 0 Subjects with TEAE leading to discontinuation, n (%) 1 (1.6%) 0 0 3 (4.8%) 0 0 Most Common TEAEs (≥5% at any dose) Nausea 0 0 5 (8.1%) 2 (3.2%) 1 (3.2%) 0 Dysgeusia 0 3 (4.8%) 4 (6.5%) 3 (4.8%) 0 0 UTI 0 3 (4.8%) 0 0 0 1 (3.2%) † No TEAE reported with an incidence ≥ 5% in the exploratory population * As deemed by investigator. Placebo: worsening of cough; BLU - 5937 200 mg BID: worsening of cough, dry mouth and hyperbilirubinemia
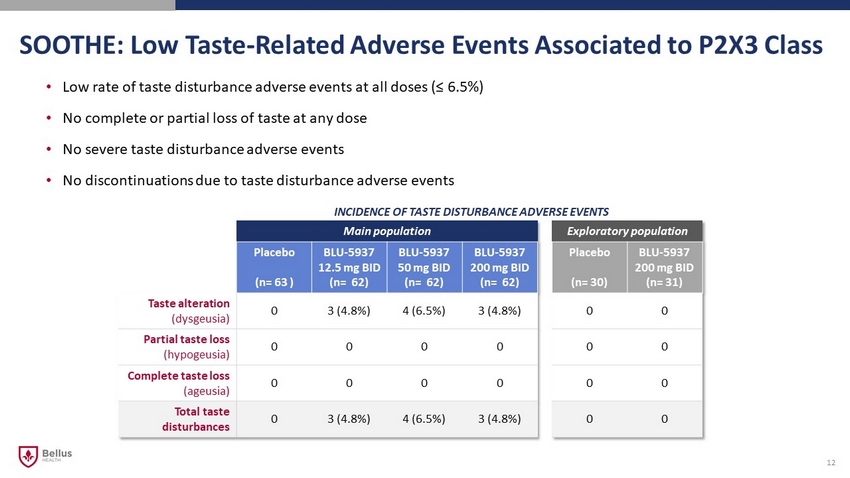
SOOTHE: Low Taste - Related Adverse Events Associated to P2X3 Class 12 • Low rate of taste disturbance adverse events at all doses (≤ 6.5%) • No complete or partial loss of taste at any dose • No severe taste disturbance adverse events • No discontinuations due to taste disturbance adverse events INCIDENCE OF TASTE DISTURBANCE ADVERSE EVENTS Main population Exploratory population Placebo (n= 63 ) BLU - 5937 12.5 mg BID (n= 62) BLU - 5937 50 mg BID (n= 62) BLU - 5937 200 mg BID (n= 62) Placebo (n= 30) BLU - 5937 200 mg BID (n= 31) Taste alteration (dysgeusia) 0 3 (4.8%) 4 (6.5%) 3 (4.8%) 0 0 Partial taste loss (hypogeusia) 0 0 0 0 0 0 Complete taste loss (ageusia) 0 0 0 0 0 0 Total taste disturbances 0 3 (4.8%) 4 (6.5%) 3 (4.8%) 0 0
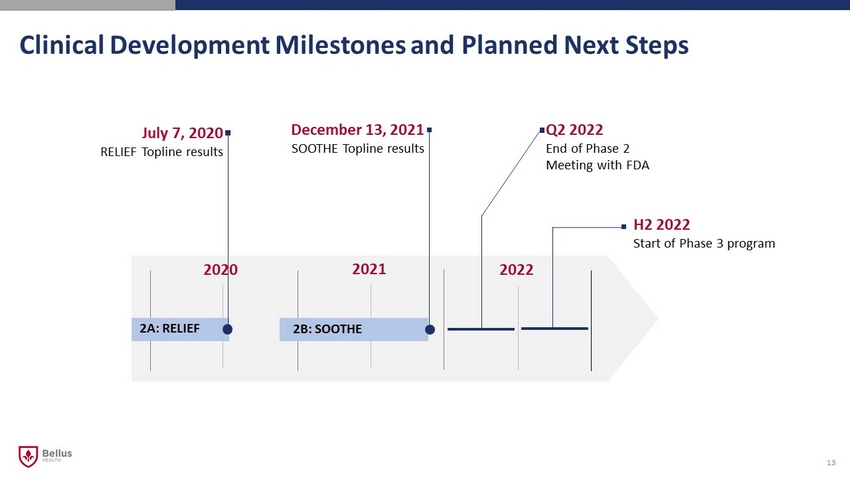
December 13 , 2021 SOOTHE Topline results Clinical Development Milestones and Planned Next Steps 13 2020 2021 2022 2A: RELIEF 2B: SOOTHE Q2 2022 End of Phase 2 Meeting with FDA H2 2022 Start of Phase 3 program July 7, 2020 RELIEF Topline results

Preparation for Phase 3 14 * Total across all human trials; over 450 subjects exposed to BLU - 5937 to - date SOOTHE final data to inform ongoing Phase 3 planning: • Dose selection • Population enrichment strategy • Key secondary endpoints • Mitigation of placebo effect Other Phase 3 Design Considerations: • 2 randomized, placebo - controlled trials • Primary endpoint: Placebo - adjusted change in 24H cough frequency • ICH guidelines recommend: - 12 - month safety data in at least 100 subjects and 6 - month safety data in at least 300 subjects - Minimum total of 1500 subjects exposed *

Refractory Chronic Cough 15 Cough lasting ≥ 8 weeks that does not respond to treatment for underlying cause or is unexplained 1 No approved treatment , current options are inadequate and non - specific 3 Significant impact on patients ’ quality of life including impact on social, physical and psychosocial well - being 2 Large patient population 4 - up to ~ 9 M refractory chronic cough patients in the U . S ., ~ 9 M in Europe Top - 5 and ~7M in China 1. Irwin RS et al, (2018) CHEST 153 (1): 196 - 209. 2. Kuzniar et al. (2007) Mayo Clin. Proc. 82(1) 56 - 60. 3. Ryan NM, (2018) Expert Opin Pharmacother 19(7): 687 - 711. 4. Company sponsored market research.
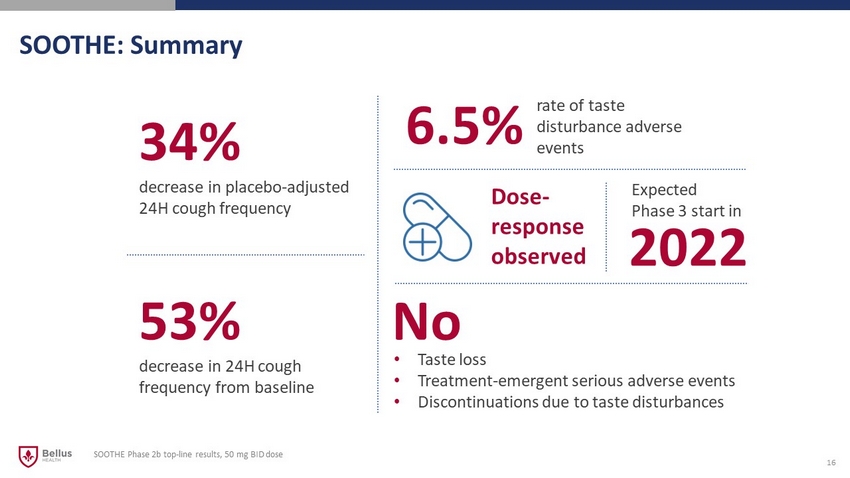
SOOTHE: Summary 16 SOOTHE Phase 2b top - line results, 50 mg BID dose Expected Phase 3 start in 34% decrease in placebo - adjusted 24H cough frequency 53% decrease in 24H cough frequency from baseline 6.5% No Dose - response observed rate of taste disturbance adverse events • Taste loss • Treatment - emergent serious adverse events • Discontinuations due to taste disturbances 2022

P2X3 Pipeline Update
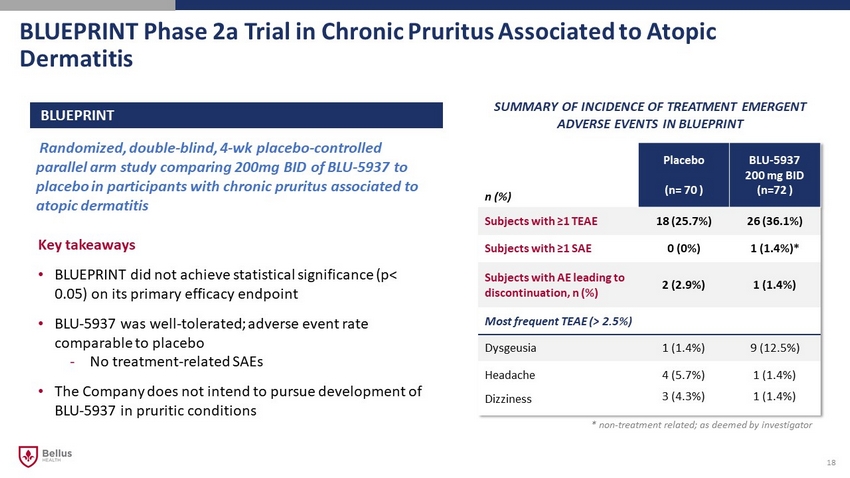
BLUEPRINT Phase 2a Trial in Chronic Pruritus Associated to Atopic Dermatitis 18 Randomized, double - blind, 4 - wk placebo - controlled parallel arm study comparing 200mg BID of BLU - 5937 to placebo in participants with chronic pruritus associated to atopic dermatitis BLUEPRINT Key takeaways • BLUEPRINT did not achieve statistical significance (p< 0.05) on its primary efficacy endpoint • BLU - 5937 was well - tolerated; adverse event rate comparable to placebo - No treatment - related SAEs • The Company does not intend to pursue development of BLU - 5937 in pruritic conditions SUMMARY OF INCIDENCE OF TREATMENT EMERGENT ADVERSE EVENTS IN BLUEPRINT n (%) Placebo (n= 70 ) BLU - 5937 200 mg BID (n=72 ) Subjects with ≥1 TEAE 18 (25.7%) 26 (36.1%) Subjects with ≥1 SAE 0 (0%) 1 (1.4%)* Subjects with AE leading to discontinuation, n (%) 2 (2.9%) 1 (1.4%) Most frequent TEAE (> 2.5%) Dysgeusia 1 (1.4%) 9 (12.5%) Headache 4 (5.7%) 1 (1.4%) Dizziness 3 (4.3%) 1 (1.4%) * non - treatment related; as deemed by investigator CB0 DG1
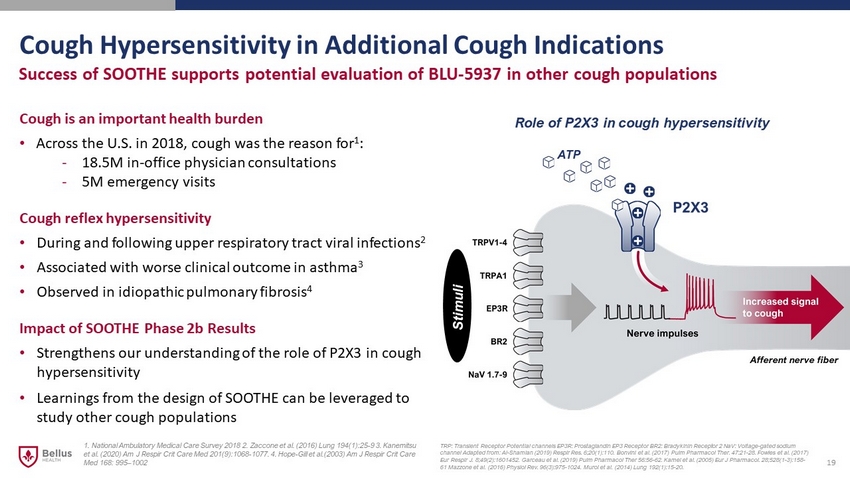
Cough Hypersensitivity in Additional Cough Indications 19 Success of SOOTHE supports potential evaluation of BLU - 5937 in other cough populations Cough is an important health burden • Across the U.S. in 2018, cough was the reason for 1 : - 18.5M in - office physician consultations - 5M emergency visits Cough reflex hypersensitivity • During and following upper respiratory tract viral infections 2 • Associated with worse clinical outcome in asthma 3 • Observed in idiopathic pulmonary fibrosis 4 Impact of SOOTHE Phase 2b Results • Strengthens our understanding of the role of P2X3 in cough hypersensitivity • Learnings from the design of SOOTHE can be leveraged to study other cough populations TRP: Transient Receptor Potential channels EP3R: Prostaglandin EP3 Receptor BR2: Bradykinin Receptor 2 NaV : Voltage - gated sodium channel Adapted from: Al - Shamlan (2019) Respir Res . 6;20(1):110. Bonvini et al. (2017) Pulm Pharmacol Ther . 47:21 - 28. Fowles et al. (2017) Eur Respir J. 8;49(2):1601452. Garceau et al. (2019) Pulm Pharmacol Ther 56:56 - 62 . Kamei et al. (2005) Eur J Pharmacol . 28;528(1 - 3):158 - 61 Mazzone et al. (2016) Physiol Rev. 96(3):975 - 1024 . Muroi et al. (2014) Lung 192(1):15 - 20. ATP Stimuli Afferent nerve fiber Role of P2X3 in cough hypersensitivity 1. National Ambulatory Medical Care Survey 2018 2. Zaccone et al. (2016) Lung 194(1):25 - 9 3. Kanemitsu et al. (2020) Am J Respir Crit Care Med 201(9):1068 - 1077. 4. Hope - Gill et al.(2003) Am J Respir Crit Care Med 168: 995 – 1002

Conclusion
BELLUS Today 21 Compelling SOOTHE Phase 2b topline data support potential best in class profile for BLU - 5937 and move into Phase 3 in refractory chronic cough Success of SOOTHE Phase 2b opens avenues for treating cough hypersensitivity in additional cough indications World class team focused on delivering value to patients and shareholders 100% economics and global rights to BLU - 5937 intellectual property; Composition of Matter IP to 2034

Closing Q&A Session 22 Prof. Jacky A. Smith, MB, ChB, FRCP, PhD University of Manchester, Principal Investigator of Phase 2b SOOTHE trial Roberto Bellini President & Chief Executive Officer Dr. Catherine Bonuccelli , MD Chief Medical Officer Dr. Denis Garceau Chief Scientific Officer Management Team Participating KOLs Ramzi Benamar Chief Financial OfficerRB0

Appendix
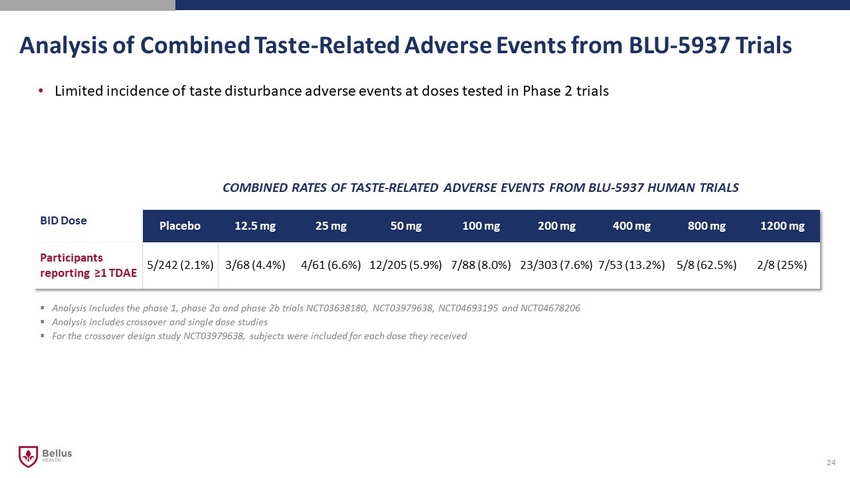
Analysis of Combined Taste - Related Adverse Events from BLU - 5937 Trials 24 BID Dose Placebo 12.5 mg 25 mg 50 mg 100 mg 200 mg 400 mg 800 mg 1200 mg Participants reporting ≥1 TDAE 5/242 (2.1%) 3/68 (4.4%) 4/61 (6.6%) 12/205 (5.9%) 7/88 (8.0%) 23/303 (7.6%) 7/53 (13.2%) 5/8 (62.5%) 2/8 (25%) COMBINED RATES OF TASTE - RELATED ADVERSE EVENTS FROM BLU - 5937 HUMAN TRIALS ▪ Analysis includes the phase 1, phase 2a and phase 2b trials NCT03638180, NCT03979638, NCT04693195 and NCT04678206 ▪ Analysis includes crossover and single dose studies ▪ For the crossover design study NCT03979638, subjects were included for each dose they received • Limited incidence of taste disturbance adverse events at doses tested in Phase 2 trials























