Exhibit 99.2

BLU - 5937 for Refractory Chronic Cough: Positive FDA End of Phase 2 Meeting and its CALM Phase 3 Program Design JULY 12, 2022

Forward Looking Statements 1 Certain statements contained in this presentation, other than statements of fact that are independently verifiable at the dat e h ereof, may constitute "forward - looking statements" within the meaning of Canadian securities legislation and regulations, the U.S. Private Securities Litigation Reform Act of 1995, as ame nde d, and other applicable securities laws. Forward - looking statements are frequently, but not always, identified by words such as “expects,” “anticipates,” “believes,” “intends,” “esti mat es,” “potential,” “possible,” “projects,” “plans,” and similar expressions. Such statements, based as they are on the current expectations of management, inherently involve numerous import ant risks, uncertainties and assumptions, known and unknown, many of which are beyond BELLUS Health Inc.'s (“BELLUS Health”) control. Such statements include, but are not limite d t o, the potential of BLU - 5937 to successfully treat refractory chronic cough (“RCC”) and other hypersensitization - related disorders and benefit such patients, BELLUS Health's expec tations related to its preclinical studies and clinical trials, including the timing of initiation of and the design of its Phase 3 clinical trials of BLU - 5937 in RCC, the timing and o utcome of interactions with regulatory agencies, the potential activity and tolerability profile, selectivity, potency and other characteristics of BLU - 5937, including as compared to other co mpetitor candidates, especially where head - to - head studies have not been conducted and cross - trial comparisons may not be directly comparable due to differences in study protocols, condit ions and patient populations, the commercial potential of BLU - 5937, including with respect to patient population, pricing and labeling, BELLUS Health's financial position and sufficie ncy of cash resources to bring through topline results with CALM - 1, and the potential applicability of BLU - 5937 and BELLUS Health's P2X3 platform to treat other disorders. Risk factors tha t may affect BELLUS Health's future results include but are not limited to: the benefits and impact on label of its enrichment strategy, estimates and projections regarding the size an d opportunity of the addressable RCC market for BLU - 5937, the ability to expand and develop its project pipeline, the ability to obtain adequate financing, the ability of BELLUS Healt h t o maintain its rights to intellectual property and obtain adequate protection of future products through such intellectual property, the impact of general economic conditions, general co nditions in the pharmaceutical industry, the impact of the ongoing COVID - 19 pandemic on BELLUS Health's operations, plans and prospects, including to the initiation and completion of clin ical trials in a timely manner or at all, changes in the regulatory environment in the jurisdictions in which BELLUS Health does business, supply chain impacts, stock market volatili ty, fluctuations in costs, changes to the competitive environment due to consolidation, achievement of forecasted burn rate, achievement of forecasted preclinical study and clinic al trial milestones, reliance on third parties to conduct preclinical studies and clinical trials for BLU - 5937 and that actual results may differ from topline results once the final and quality - controlled verification of data and analyses has been completed. In addition, the length of BELLUS Health's product candidate's development process and its market size and commerc ial value are dependent upon a number of factors. Moreover, BELLUS Health's growth and future prospects are mainly dependent on the successful development, patient tolerability, regulatory approval, commercialization and market acceptance of its product candidate BLU - 5937 and other products. Consequently, actual future results and events may differ mater ially from the anticipated results and events expressed in the forward - looking statements. BELLUS Health believes that expectations represented by forward - looking statements are reason able, yet there can be no assurance that such expectations will prove to be correct. The reader should not place undue reliance, if any, on any forward - looking statements inc luded in this presentation. These forward - looking statements speak only as of the date made, and BELLUS Health is under no obligation and disavows any intention to update publ icl y or revise such statements as a result of any new information, future event, circumstances or otherwise, unless required by applicable legislation or regulation. Please see BELLUS Health's public filings with the Canadian securities regulatory authorities, including, but not limited to, its Annual Information Form, and the United States Securities and Exch ang e Commission, including, but not limited to, its Annual Report on Form 40 - F, for further risk factors that might affect BELLUS Health and its business.

Positive FDA End - of - Phase 2 Meeting and Announcing CALM Phase 3 Program • CALM Phase 3 Program designed to support approval of BLU - 5937 for treatment of refractory chronic cough (RCC): x Two pivotal Phase 3 trials, CALM - 1 and CALM - 2, with 3 expected arms: 25mg, 50mg and placebo BID x Primary efficacy endpoint of 24H cough frequency at 12 weeks in CALM - 1 and 24 weeks months in CALM - 2 » FDA alignment on using primary efficacy endpoint in population enriched for baseline cough frequency, similar to successful SOOTHE Phase 2b trial x Safety database supported by randomized extension of CALM - 1 and open label extension of CALM - 2 x First patient enrollment in CALM - 1 expected in Q4 2022 and pivotal topline data from CALM - 1 expected in 2H 2024 x Use of VitaloJAK cough monitoring system in CALM Phase 3 program; preliminary validation (n=30) study results using historical SOOTHE Ph2b data demonstrated 98.4% sensitivity • Well financed for Phase 3 following positive FDA End of Phase 2 meeting x $234M as of March 31 st provides cash runway to Q4 2024 and through top - line data from CALM - 1 Positive FDA End - of - Phase 2 meeting with clear path forward for BLU - 5937’s CALM Phase 3 program in refractory chronic cough 3
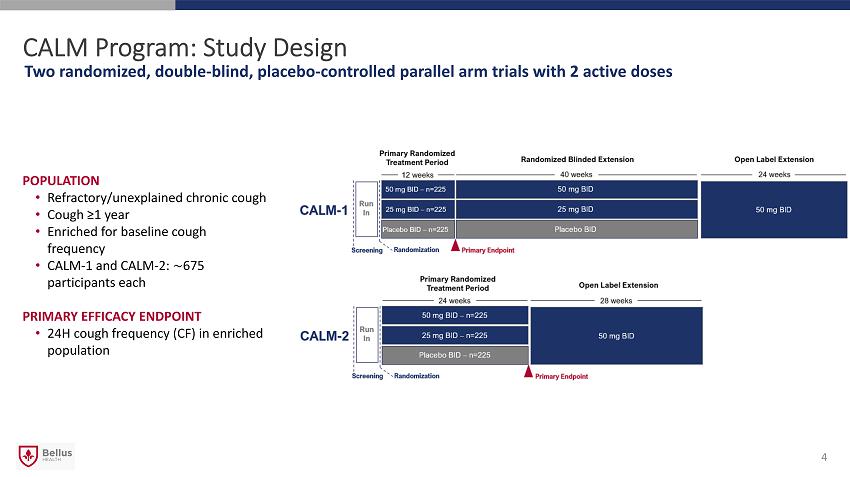
CALM Program: Study Design Two randomized, double - blind, placebo - controlled parallel arm trials with 2 active doses POPULATION • Refractory/unexplained chronic cough • Cough ≥1 year • Enriched for baseline cough frequency • CALM - 1 and CALM - 2: ∼ 675 participants each PRIMARY EFFICACY ENDPOINT • 24H cough frequency (CF) in enriched population 4
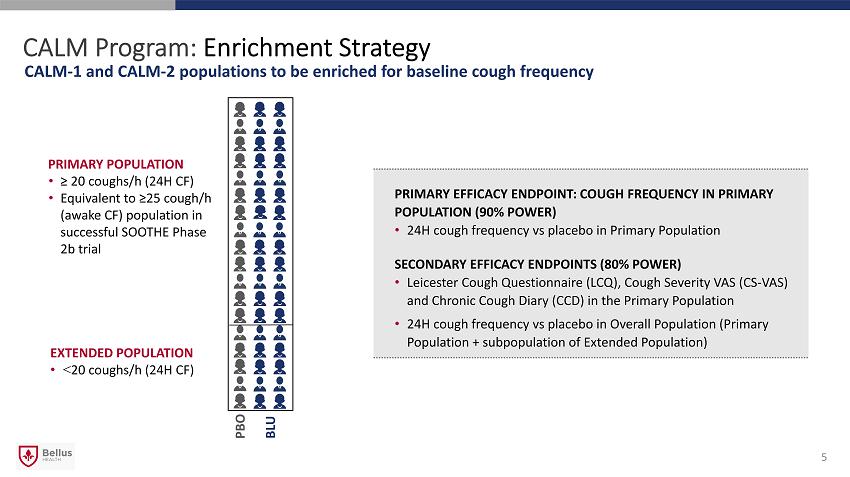
CALM Program: Enrichment Strategy CALM - 1 and CALM - 2 populations to be enriched for baseline cough frequency PRIMARY EFFICACY ENDPOINT: COUGH FREQUENCY IN PRIMARY POPULATION (90% POWER) • 24H cough frequency vs placebo in Primary Population SECONDARY EFFICACY ENDPOINTS (80% POWER) • Leicester Cough Questionnaire (LCQ), Cough Severity VAS (CS - VAS) and Chronic Cough Diary (CCD) in the Primary Population • 24H cough frequency vs placebo in Overall Population (Primary Population + subpopulation of Extended Population) PRIMARY POPULATION • ≥ 20 coughs/h (24H CF) • Equivalent to ≥25 cough/h (awake CF) population in successful SOOTHE Phase 2b trial EXTENDED POPULATION • < 20 coughs/h (24H CF) PBO BLU 5
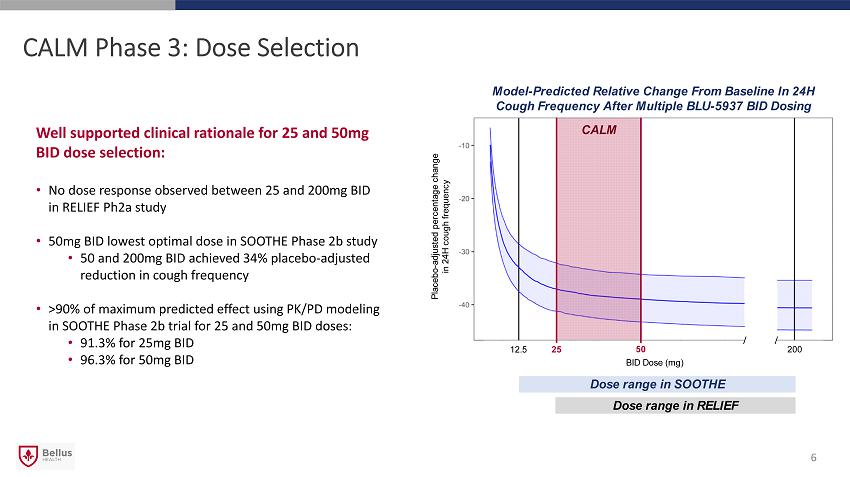
CALM Phase 3: Dose Selection Well supported clinical rationale for 25 and 50mg BID dose selection: • No dose response observed between 25 and 200mg BID in RELIEF Ph2a study • 50mg BID lowest optimal dose in SOOTHE Phase 2b study • 50 and 200mg BID achieved 34% placebo - adjusted reduction in cough frequency • >90% of maximum predicted effect using PK/PD modeling in SOOTHE Phase 2b trial for 25 and 50mg BID doses: • 91.3% for 25mg BID • 96.3% for 50mg BID 6 Model - Predicted Relative Change From Baseline In 24H Cough Frequency After Multiple BLU - 5937 BID Dosing Placebo - adjusted percentage change in 24H cough frequency Dose range in SOOTHE Dose range in RELIEF CALM 25 50 12.5 200 BID Dose (mg)

• Statistical consultants recommended and FDA was aligned with using the negative binomial (NB) statistical method to analyze cough count data in CALM Phase 3 Program • NB expected to have a better statistical fit for count data like cough frequency • Previous method of log - transformation of cough data does not provide optimal statistical fit • Well documented use 1 - 7 in trials with analysis of count data • In BELLUS clinical datasets, NB provides better statistical fit: • Better manages variability and increases power • Minimal impact of extreme outliers CALM Phase 3: Statistical Methodology 7 In CALM Phase 3, use of Negative Binomial is expected to provide better power to detect treatment benefit and reduce risk of outliers skewing results 1. Ortega et al. (2014) N Engl J Med 371(13):1198 - 207. 2. Castro et al ( 2015) Lancet Resp Med 3(5):355 - 66. 3. Pavord et al. ( 2017) N Engl J Med 377(17):1613 - 1629. 4. Criner et al. (2019) N Engl J Med 381(11):1023 - 1034. 5. Smith et al. (CHEST) 2020 157(1):111 - 118. 6. Miller (2020) JAMA Neurol. 77(5):613 - 621. 7. Thiele (2021) JAMA Neurol 2021 Mar 1;78(3):285 - 292. Dose Δ p-value Δ p-value 12.5 mg -28% 0.0013 -21% 0.098 50 mg -33% 0.0005 -34% 0.0033 200 mg -32% 0.0021 -34% 0.0047 Dose Δ p-value Δ p-value 200 mg -23% 0.012 -20% 0.078 SOOTHE Main Population: ≥25 awake c/h SOOTHE Exploratory Population: 10-<25 awake c/h Negative Binomial Log Transformation SOOTHE Primary Efficacy Endpoint (Main Population): Placebo- adjusted 24H cough frequency at Day 28 SOOTHE Secondary Endpoint (Main + Exploratory Population): Placebo-adjusted 24H cough frequency at Day 28 Negative Binomial Log Transformation
• VitaloJAK is the cough recording and counting system used to capture the 24H cough frequency data in most cough trials • Used in BLU - 5937 and gefapixant RCC trials • Will be used in the CALM Phase 3 program • The Company and FDA aligned on validation of VitaloJAK cough frequency measurement to support the potential BLU - 5937 New Drug Application • Validation study comparing compressed vs non - compressed recordings in SOOTHE Phase 2b trial participants is on - going • Preliminary results from the first 30 recordings demonstrated a sensitivity of 98.4% with no systematic errors present • Complete results are expected in Q4 2022/Q1 2023 • Validation work has no impact on start of Phase 3 CALM Phase 3: VitaloJAK Cough Monitoring System 8 VitaloJAK Cough Monitoring Device

CALM Phase 3: Clinical Development Milestones and Expected Next Steps Topline results from CALM - 1 Phase 3 trial expected in 2H 2024 9 December 13 , 2021 SOOTHE Phase 2b Topline Results 2H 2021 2022 PH 2B June 2022 End - of - Phase 2 Meeting with FDA 4Q 2022 Expected First Patient Enrolled in Phase 3 CALM - 1 Trial 3Q 2022 Expected Final Protocols (IND Submission) EMA Feedback PH 3
10 Potential BLU - 5937 best - in - class profile for the treatment of refractory chronic cough, a large and growing market with limited competition BELLUS Today Potential for building pipeline targeting cough hypersensitivity in additional cough indications World - class team focused on delivering value to patients and shareholders 100% economics and global rights to BLU - 5937 intellectual property Positive FDA End - of - Phase 2 meeting with clear path forward for BLU - 5937’s Phase 3 CALM program in RCC









