- ONCT Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
424B3 Filing
Oncternal Therapeutics (ONCT) 424B3Prospectus supplement
Filed: 8 May 19, 12:00am
File pursuant to Rule 424(b)(3)
Registration No. 333-230758
 |  |
PROPOSED MERGER
YOUR VOTE IS VERY IMPORTANT
To the Stockholders of GTx, Inc. and Oncternal Therapeutics, Inc.:
GTx, Inc. (“GTx”) and Oncternal Therapeutics, Inc. (“Oncternal”) have entered into an Agreement and Plan of Merger and Reorganization as amended by Amendment No. 1 to Agreement and Plan of Merger and Reorganization dated April 30, 2019, (the “Merger Agreement”) pursuant to which a wholly-owned subsidiary of GTx will merge with and into Oncternal, with Oncternal surviving as a wholly-owned subsidiary of GTx (the “merger”). Oncternal and GTx believe that the merger will result in a clinical-stage biopharmaceutical company focused on developingfirst-in-class product candidates for cancers with critical unmet medical need.
At the effective time of the merger (the “Effective Time”), each share of common stock of Oncternal, $0.0001 par value (“Oncternal common stock”) will be converted into the right to receive approximately 0.5137 shares of GTx’s common stock, subject to adjustment for the reverse stock split of GTx’s common stock to be implemented prior to the consummation of the merger as discussed in this proxy statement/prospectus/information statement. This exchange ratio is an estimate only as of the date hereof and the final exchange ratio will be determined pursuant to a formula described in more detail in the Merger Agreement and in the attached proxy statement/prospectus/information statement. Prior to the Effective Time each share of preferred stock, $0.0001 par value, of Oncternal (“Oncternal preferred stock” and, together with the Oncternal common stock, “Oncternal capital stock”), will be converted into one share of Oncternal common stock in accordance with the applicable provisions of Oncternal’s certificate of incorporation. GTx will assume outstanding and unexercised warrants and options to purchase shares of Oncternal capital stock, and in connection with the merger they will be converted into warrants and options, as applicable, to purchase shares of GTx’s common stock. At the Effective Time, GTx’s stockholders will continue to own and hold their existing shares of GTx’s common stock, and all outstanding and unexercised options to purchase shares of GTx’s common stock and outstanding and unexercised warrants to purchase shares of GTx’s common stock will remain in effect pursuant to their terms, except that the vesting of such options will be accelerated in full effective as of immediately prior to the Effective Time. Immediately after the merger, assuming an exchange ratio of 0.5137, Oncternal’s stockholders as of immediately prior to the Effective Time will own approximately 77.5% of the outstanding capital stock of GTx, with GTx’s stockholders as of immediately prior to the Effective Time owning approximately 22.5% of the outstanding capital stock of GTx. The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants. These estimates are subject to adjustment prior to closing of the merger.
Shares of GTx’s common stock are currently listed on the Nasdaq Capital Market (“Nasdaq”) under the symbol “GTXI.” GTx has filed an initial listing application with Nasdaq pursuant to Nasdaq’s “reverse merger” rules. After completion of the merger, GTx will be renamed Oncternal Therapeutics, Inc. and expects to trade on Nasdaq under the symbol “ONCT.” On May 6, 2019, the last trading day before the date of this proxy statement/prospectus/information statement, the closing sale price of GTx’s common stock on Nasdaq was $1.16 per share.
GTx is holding a special meeting of its stockholders (the “GTx special meeting”) in order to obtain the stockholder approvals necessary to complete the merger and related matters. At the GTx special meeting, which will be held at 9:00 a.m., Central time, on June 5, 2019 at 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103, unless postponed or adjourned to a later date, GTx will ask its stockholders to, among other things:
| 1. | approve the Merger Agreement, and the transactions contemplated thereby, including the merger, the issuance of shares of GTx’s common stock to Oncternal’s stockholders pursuant to the terms of the Merger Agreement and the change of control resulting from the merger; |
| 2. | approve a series of alternative amendments to the restated certificate of incorporation of GTx to effect a reverse stock split of GTx’s common stock, within a range, as determined by GTx’s board of directors, of one new share for every six to eight (or any number in between) shares outstanding; |
| 3. | approve an amendment to the restated certificate of incorporation of GTx to change the corporate name of GTx from “GTx, Inc.” to “Oncternal Therapeutics, Inc.”; |
| 4. | approve the adoption of the GTx, Inc. 2019 Incentive Award Plan; |
| 5. | approve, on a nonbinding, advisory basis, the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger; |
| 6. | consider and vote upon an adjournment of the GTx special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1 or 2; and |
| 7. | transact such other business as may properly come before the GTx special meeting or any adjournment or postponement thereof. |
As described in the accompanying proxy statement/prospectus/information statement, certain of Oncternal’s stockholders who in the aggregate own approximately 44% of the outstanding shares of Oncternal capital stock on an as converted to common stock basis, and certain of GTx’s stockholders who in the aggregate own approximately 45% of the outstanding shares of GTx’s common stock, are parties to voting agreements with GTx and Oncternal, whereby such stockholders have agreed to vote their shares, in favor of the adoption or approval, among other things, of the Merger Agreement and the approval of the transactions contemplated therein, including the merger, the issuance of shares of GTx’s common stock to Oncternal’s stockholders and the change of control resulting from the merger, subject to the terms of the voting agreements.
In addition, following the registration statement on FormS-4, of which this proxy statement/prospectus/information statement is a part, being declared effective by the U.S. Securities and Exchange Commission (the “SEC”) and pursuant to the conditions of the Merger Agreement and the voting agreements, Oncternal’s stockholders who are party to the voting agreements will each execute an action by written consent of Oncternal’s stockholders, referred to as the written consent, adopting the Merger Agreement, thereby approving the transactions contemplated therein, including the merger. No meeting of Oncternal’s stockholders to adopt the Merger Agreement and approve the merger and related transactions will be held; all of Oncternal’s stockholders will have the opportunity to elect to adopt the Merger Agreement, thereby approving the merger and related transactions, by signing and returning to Oncternal a written consent.
After careful consideration, GTx’s board of directors (the “GTx Board”) has (i) determined that the merger and all related transactions contemplated by the Merger Agreement are fair to, advisable and in the best interests of GTx and its stockholders, (ii) approved and declared advisable the Merger Agreement and the transactions contemplated therein and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the Merger Agreement, that its stockholders vote to approve the Merger Agreement and the transactions contemplated thereby. The GTx Board recommends that GTx’s stockholders vote “FOR” Proposal Nos. 1, 2, 3, 4, 5 and 6.
After careful consideration, Oncternal’s board of directors (the “Oncternal Board”) has (i) determined that the merger and all related transactions contemplated by the Merger Agreement are fair to, advisable and in the best interests of Oncternal and its stockholders, (ii) approved and declared advisable the Merger Agreement and the transactions contemplated therein and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the Merger Agreement, that its stockholders vote to approve the Merger Agreement and the transactions contemplated thereby. The Oncternal Board recommends that Oncternal’s stockholders sign and return the written consent, indicating their (i) adoption and approval of the Merger Agreement and the transactions contemplated thereby, (ii) acknowledgement that the approval given is irrevocable and that such stockholder is aware of its rights to demand appraisal for its shares pursuant to Section 262 of the General Corporation Law of the State of Delaware (“DGCL”), and that such stockholder has received and read a copy of Section 262 of the DGCL, (iii) acknowledgement that by its approval of the Merger it is not entitled to appraisal rights with respect to its shares in connection with the Merger and thereby waives any rights to receive payment of the fair value of its capital stock under the DGCL, and (iv) approval of the conversion of Oncternal’s outstanding preferred stock into Oncternal’s common stock immediately prior to the Effective Time (collectively, the “Required Oncternal Stockholder Approval”).
More information about GTx, Oncternal and the proposed transaction is contained in this proxy statement/prospectus/information statement. GTx and Oncternal urge you to read the accompanying proxy statement/prospectus/information statement carefully and in its entirety. IN PARTICULAR, YOU SHOULD CAREFULLY CONSIDER THE MATTERS DISCUSSED UNDER “RISK FACTORS” BEGINNING ON PAGE 28.
GTx and Oncternal are excited about the opportunities the merger brings to both GTx’s and Oncternal’s stockholders, and thank you for your consideration and continued support.
| Marc S. Hanover | James B. Breitmeyer, M.D., Ph.D. | |
| Chief Executive Officer | President & Chief Executive Officer | |
| GTx, Inc. | Oncternal Therapeutics, Inc. |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this proxy statement/prospectus/information statement. Any representation to the contrary is a criminal offense.
The accompanying proxy statement/prospectus/information statement is dated May 7, 2019, and is first being mailed to GTx’s and Oncternal’s stockholders on or about May 10, 2019.
GTX, INC.
17 W Pontotoc Ave., Suite 100
Memphis, Tennessee 38103
(901)523-9700
NOTICE OF SPECIAL MEETING OF STOCKHOLDERS
TO BE HELD ON JUNE 5, 2019
Dear Stockholders of GTx:
On behalf of the board of directors of GTx, Inc., a Delaware corporation (“GTx”), we are pleased to deliver this proxy statement/prospectus/information statement for the 2019 special meeting of stockholders of GTx and for the proposed merger between GTx and Oncternal Therapeutics, Inc., a Delaware corporation (“Oncternal”), pursuant to which Grizzly Merger Sub, Inc., a Delaware corporation and a wholly-owned subsidiary of GTx (“Merger Sub”), will merge with and into Oncternal, with Oncternal surviving as a wholly-owned subsidiary of GTx. The special meeting of stockholders of GTx will be held on June 5, 2019 at 9:00 a.m., Central time, at 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103, for the following purposes:
| 1. | To consider and vote upon a proposal to approve the Agreement and Plan of Merger and Reorganization, dated as of March 6, 2019, by and among GTx, Merger Sub, and Oncternal, a copy of which is attached asAnnex A to this proxy statement/prospectus/information statement as amended by Amendment No. 1 to Agreement and Plan of Merger and Reorganization dated April 30, 2019, (the “Merger Agreement”), and the transactions contemplated thereby, including the merger, the issuance of shares of GTx’s common stock to Oncternal’s stockholders pursuant to the terms of the Merger Agreement and the change of control resulting from the merger. |
| 2. | To approve a series of alternative amendments to the restated certificate of incorporation of GTx to effect a reverse stock split of GTx’s common stock, within a range, as determined by GTx’s board of directors, of one new share for every six to eight (or any number in between) shares outstanding, in the form attached asAnnex Dto this proxy statement/prospectus/information statement. |
| 3. | To approve an amendment to the restated certificate of incorporation of GTx to change the corporate name of GTx from “GTx, Inc.” to “Oncternal Therapeutics, Inc.” in the form attached asAnnex E to this proxy statement/prospectus/information statement. |
| 4. | To approve the adoption of the GTx, Inc. 2019 Incentive Award Plan in the form attached asAnnex F to this proxy statement/prospectus/information statement. |
| 5. | To approve, on a nonbinding, advisory basis, the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger. |
| 6. | To consider and vote upon an adjournment of the GTx special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1 or 2. |
| 7. | To transact such other business as may properly come before the GTx special meeting or any adjournment or postponement thereof. |
The GTx Board has fixed April 15, 2019, as the record date for the determination of stockholders entitled to notice of, and to vote at, the GTx special meeting and any adjournment or postponement thereof. Only holders of record of shares of GTx’s common stock at the close of business on the record date are entitled to notice of, and to vote at, the GTx special meeting. At the close of business on the record date, GTx had 24,051,844 shares of common stock outstanding and entitled to vote.
Your vote is important. The affirmative vote of the holders of a majority of the shares of GTx’s common stock entitled to vote and present in person or represented by proxy at the GTx special meeting is required for approval of Proposal Nos. 1, 4, 5 and 6. The affirmative vote of the holders of a majority of shares of GTx’s common stock having voting power outstanding on the record date for the GTx special meeting is required for approval of Proposal Nos. 2 and 3. Each of Proposal Nos. 1 and 2 are conditioned upon each other. Therefore, the merger cannot be consummated without the approval of Proposal Nos. 1 and 2.
Proposal Nos. 3 and 4 are conditioned upon the consummation of the merger. If the merger is not completed or the stockholders do not approve Proposal No. 3, GTx will not change its name to “Oncternal Therapeutics, Inc.” If the merger is not completed or the stockholders do not approve Proposal No. 4, the GTx, Inc. 2019 Incentive Award Plan will not become effective. Proposal Nos. 1 and 2 are not conditioned on Proposal No. 3 or Proposal No. 4 being approved.
Even if you plan to attend the GTx special meeting in person, GTx requests that you sign and return the enclosed proxy to ensure that your shares will be represented at the GTx special meeting if you are unable to attend.
THE GTX BOARD HAS DETERMINED AND BELIEVES THAT EACH OF THE PROPOSALS OUTLINED ABOVE IS ADVISABLE TO, AND IN THE BEST INTERESTS OF, GTX AND ITS STOCKHOLDERS AND HAS APPROVED EACH SUCH PROPOSAL. THE GTX BOARD RECOMMENDS THAT GTX’S STOCKHOLDERS VOTE “FOR” EACH SUCH PROPOSAL.
By Order of the GTx Board of Directors,
Marc S. Hanover
Chief Executive Officer
Memphis, Tennessee
May 7, 2019
REFERENCES TO ADDITIONAL INFORMATION
This proxy statement/prospectus/information statement incorporates important business and financial information about GTx that is not included in or delivered with this document. You may obtain this information without charge upon your written or oral request by contacting the Chief Legal Officer of GTx, Inc., 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103 or by calling (901)523-9700.
To ensure timely delivery of these documents, any request should be made no later than May 20, 2019 to receive them before the special meeting.
For additional details about where you can find information about GTx, please see the section entitled “Where You Can Find More Information” in this proxy statement/prospectus/information statement.
i
ii
QUESTIONS AND ANSWERS ABOUT THE MERGER
Except where specifically noted, the following information and all other information contained in this proxy statement/prospectus/information statement does not give effect to the proposed reverse stock split within a range, as determined by GTx’s board of directors, of one new share for every six to eight (or any number in between) shares outstanding, as described in Proposal No. 2 beginning on page 206 in this proxy statement/prospectus/information statement (the “GTx Reverse Stock Split”).
The following section provides answers to frequently asked questions about the merger. This section, however, provides only summary information. For a more complete response to these questions and for additional information, please refer to the cross-referenced sections.
| Q: | What is the merger? |
| A: | GTx, Merger Sub and Oncternal entered into the Agreement and Plan of Merger and Reorganization on March 6, 2019 (the “Original Merger Agreement”). On April 30, 2019, the parties entered into Amendment No. 1 to Agreement and Plan of Merger (the “Merger Agreement Amendment,” and together with the Original Merger Agreement, the “Merger Agreement”). The Merger Agreement contains the terms and conditions of the proposed merger of GTx and Oncternal. Under the Merger Agreement, Merger Sub will merge with and into Oncternal, with Oncternal surviving as a wholly-owned subsidiary of GTx. This transaction is referred to as “the merger.” |
At the effective time of the merger (the “Effective Time”), each share of Oncternal’s common stock immediately prior to the Effective Time (excluding certain shares to be canceled pursuant to the Merger Agreement, and shares held by stockholders who have exercised and perfected appraisal rights as more fully described in the section entitled “The Merger—Appraisal Rights” below) will be converted into the right to receive approximately 0.5137 shares of GTx’s common stock, subject to adjustment for the GTx Reverse Stock Split (the “exchange ratio”). Prior to the Effective Time, all outstanding shares of Oncternal’s preferred stock will convert into shares of Oncternal’s common stock. This exchange ratio is an estimate only and the final exchange ratio will be determined pursuant to a formula described in more detail in the Merger Agreement and in the attached proxy statement/prospectus/information statement.
As a result of the merger, based on the estimated exchange ratio of 0.5137, current holders of Oncternal’s capital stock are expected to own in the aggregate approximately 77.5% of the outstanding capital stock of GTx, with GTx’s current stockholders owning approximately 22.5% of the outstanding capital stock of GTx. The ownership percentage to be held by GTx’s stockholders is subject to adjustment prior to closing of the merger, including a downward adjustment to the extent that GTx’s “Parent Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement, which adjusts based on the date of closing (and as a result, GTx stockholders could own less, and Oncternal stockholders could own more, of the combined company), or an upward adjustment to the extent that Oncternal’s “Company Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement (and as a result, GTx stockholders could own more, and Oncternal stockholders could own less, of the combined company). The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants. GTx will assume outstanding and unexercised warrants and options to purchase shares of Oncternal capital stock, and such securities will be converted into warrants and options, as applicable, to purchase shares of GTx’s common stock.
At the Effective Time, GTx’s stockholders will continue to own and hold their existing shares of GTx’s common stock, and all outstanding and unexercised options to purchase shares of GTx’s common stock and outstanding and unexercised warrants to purchase shares of GTx’s common stock will remain in effect pursuant to their terms, except that the vesting of such options will be accelerated in full effective as of immediately prior to the Effective Time. After the completion of the merger, GTx will change its corporate name to “Oncternal Therapeutics, Inc.” as required by the Merger Agreement (the “GTx Name Change”).
1
| Q: What | will happen to GTx if, for any reason, the merger does not close? |
| A: | If, for any reason, the merger does not close, the GTx Board may elect to, among other things, attempt to complete another strategic transaction like the merger, attempt to sell or otherwise dispose of the various assets of GTx, resume its research and development activities and continue to operate the business of GTx or dissolve and liquidate its assets. If GTx decides to dissolve and liquidate its assets, GTx would be required to pay all of its debts and contractual obligations, and to set aside certain reserves for potential future claims. There can be no assurances as to the amount or timing of available cash left to distribute to stockholders after paying the debts and other obligations of GTx and setting aside funds for reserves. |
If GTx were to continue its business, it would need to hire scientific personnel necessary to resume research and development activities. To conserve its cash resources, GTx has substantially reduced its workforce since November 2018 and has ceased its selective androgen receptor modulators (“SARM”) development activities and all other operations except forday-to-day business operations, completing ongoing mechanistic selective androgen receptor degrader (“SARD”) preclinical studies and those activities necessary to complete the merger. As of March 31, 2019, GTx had 13 full-time employees. If the merger is not completed and GTx is able to raise sufficient additional funds necessary to pursue the continued development of its SARD program, GTx will need to hire experienced personnel to continue to develop its SARD program and to develop and commercialize any potential future product candidates, and GTx will need to expand the number of its managerial, operational, financial and other employees to support that growth.
| Q: Why | are the two companies proposing to merge? |
| A: | Oncternal and GTx believe that the merger will result in a clinical-stage biopharmaceutical company focused on developingfirst-in-class product candidates for cancers with critical unmet medical need. For a discussion of GTx’s and Oncternal’s reasons for the merger, please see the section entitled “The Merger—GTx Reasons for the Merger” and “The Merger—Oncternal Reasons for the Merger” in this proxy statement/prospectus/information statement. |
| Q: Why | am I receiving this proxy statement/prospectus/information statement? |
| A: | You are receiving this proxy statement/prospectus/information statement because you have been identified as a stockholder of GTx as of the record date, or a stockholder of Oncternal eligible to execute the Oncternal written consent. If you are a stockholder of GTx, you are entitled to vote at GTx’s annual stockholder meeting (referred to herein as the “GTx special meeting”) to approve Proposal Nos. 1, 2, 3, 4, 5 and 6. If you are a stockholder of Oncternal, you are being requested to sign and return the Oncternal written consent to adopt the Merger Agreement and approve the transactions contemplated thereby, including the merger. |
This document serves as:
| • | a proxy statement of GTx used to solicit proxies for the GTx special meeting; |
| • | a prospectus of GTx used to offer shares of GTx’s common stock in exchange for shares of Oncternal’s capital stock in the merger and issuable upon exercise of Oncternal’s warrants and options, as applicable; and |
| • | an information statement of Oncternal used to solicit the written consent of its stockholders for the adoption of the Merger Agreement and the approval of the merger and related transactions. |
| Q: What | is required to consummate the merger? |
| A: | To consummate the merger, GTx’s stockholders must approve Proposal Nos. 1 and 2. |
Proposal No. 1, the approval of the merger and the issuance of GTx’s common stock pursuant to the Merger Agreement by GTx’s stockholders and the change of control resulting from the merger, requires the
2
affirmative vote of the holders of a majority of the shares of GTx’s outstanding common stock entitled to vote and present in person or represented by proxy at the GTx special meeting. Proposal Nos. 2 and 3, the approval of the amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split and the GTx Name Change, each requires the affirmative vote of the holders of a majority of the shares of GTx’s common stock having voting power outstanding on the record date for the GTx special meeting. Each of Proposal Nos. 1 and 2 are conditioned upon each other. Therefore, the merger cannot be consummated without the approval of Proposal Nos. 1 and 2. Proposal Nos. 3 and 4 are conditioned upon the consummation of the merger. If the merger is not completed or the stockholders do not approve Proposal No. 3, GTx will not change its name to “Oncternal Therapeutics, Inc.” If the merger is not completed or the stockholders do not approve Proposal No. 4, the GTx, Inc. 2019 Incentive Award Plan will not become effective. Proposal Nos. 1 and 2 are not conditioned on Proposal No. 3 or Proposal No. 4 being approved.
The (i) adoption and approval of the Merger Agreement and the transactions contemplated thereby, and (ii) the conversion of Oncternal’s outstanding preferred stock into Oncternal’s common stock immediately prior to the Effective Time, requires the written consent of the following, in each case, outstanding as of the record date for the written consent:
| • | the holders of a majority of the shares of Oncternal’s common stock and Oncternal’s preferred stock, voting as a single class; |
| • | the holders of at least 60% of the shares of Oncternal’s preferred stock, voting together as a single class; |
| • | the holders of at least a majority of the outstanding shares of Oncternal’s Series A preferred stock, voting as a single class; |
| • | the holders of at least a majority of the outstanding shares of Oncternal’s Series B preferred stock and Oncternal’s SeriesB-2 preferred stock, voting together as a single class; and |
| • | the holders of at least 70% of the shares of Oncternal’s Series C preferred stock, voting as a single class. |
Certain of Oncternal’s stockholders who in the aggregate own approximately 44% of the outstanding shares of Oncternal’s capital stock on an as converted to common stock basis, and certain of GTx’s stockholders who in the aggregate own approximately 45% of the outstanding shares of GTx’s common stock, are parties to voting agreements with GTx and Oncternal, whereby such stockholders have agreed, subject to the terms of the voting agreements, to vote their shares in favor of the adoption or approval, among other things, of the Merger Agreement and the transactions contemplated therein, including the merger and the issuance of GTx’s common stock to Oncternal’s stockholders pursuant to the Merger Agreement. In addition, following the registration statement on FormS-4, of which this proxy statement/prospectus/information statement is a part, being declared effective by the SEC and pursuant to the conditions of the Merger Agreement, Oncternal’s stockholders who are party to the voting agreements will each execute written consents approving the merger and related transactions. Stockholders of Oncternal, including those who are parties to voting agreements, are being requested to execute written consents providing such approvals. Oncternal’s largest stockholder prior to the merger, Shanghai Pharmaceutical (USA) Inc. (“SPH USA”), which holds 100% of the outstanding Series C preferred stock and which represents approximately 20.9% of the outstanding shares of Oncternal capital stock on as converted common stock basis, has not executed a voting agreement. Although Oncternal expects to receive stockholder approval from SPH USA approximately two months after the date of the Merger Agreement, there can be no assurance that all of the necessary stockholder approvals will be obtained.
In addition to the requirement of obtaining the stockholder approvals described above and appropriate regulatory approvals, each of the other closing conditions set forth in the Merger Agreement must be satisfied or waived. For a more complete description of the closing conditions under the Merger Agreement, we urge you to read the section entitled “The Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement.
3
| Q: What | will Oncternal’s stockholders, warrant holders and option holders receive in the merger? |
| A: | As a result of the merger, assuming an estimated exchange ratio of 0.5137, Oncternal’s stockholders will become entitled to receive shares of GTx’s common stock equal to, in the aggregate, approximately 77.5% of the outstanding capital stock of GTx. The ownership percentage to be held by GTx’s stockholders is subject to adjustment prior to closing of the merger, including a downward adjustment to the extent that GTx’s “Parent Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement, which adjusts based on the date of closing (and as a result, GTx stockholders could own less, and Oncternal stockholders could own more, of the combined organization), or an upward adjustment to the extent that Oncternal’s “Company Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement (and as a result, GTx stockholders could own more, and Oncternal stockholders could own less, of the combined organization). |
GTx will assume outstanding and unexercised warrants and options to purchase shares of Oncternal capital stock, and in connection with the merger they will be converted into warrants and options, as applicable, to purchase shares of GTx’s common stock, with the number of GTx shares subject to such warrant or option, and the exercise price, being appropriately adjusted to reflect the exchange ratio between GTx’s common stock and Oncternal capital stock determined in accordance with the Merger Agreement.
For a more complete description of what Oncternal’s stockholders, warrant holders and option holders will receive in the merger, please see the sections entitled and “The Merger Agreement—Merger Consideration” in this proxy statement/prospectus/information statement.
| Q: What | will GTx’s stockholders, warrant holders and option holders receive in the merger? |
| A: | At the Effective Time, GTx’s stockholders will continue to own and hold their existing shares of GTx’s common stock, and all outstanding and unexercised options to purchase shares of GTx’s common stock and outstanding and unexercised warrants to purchase shares of GTx’s common stock will remain in effect pursuant to their terms, except that the vesting of such options will be accelerated in full effective as of immediately prior to the Effective Time. |
In addition, GTx stockholders as of immediately prior to the Effective Time will receive one contingent value right (“CVR”) for each share of GTx common stock held of record as of immediately prior to the Effective Time. Each CVR will represent the right to receive payments based on GTx’s SARD or SARM technology. In particular, CVR holders will be entitled to 75% of the aggregate amount of any net proceeds received by the combined company during the15-year period after the closing of the merger from the grant, sale or transfer of rights to GTx’s SARD or SARM technology that occurs during the10-year period after the closing (or in the 11th year if based on a term sheet approved during the initial10-year period) and, if applicable, to receive royalties on the sale of any SARD or SARM products by the combined company during the15-year period after the closing. The CVRs will be issued pursuant to a Contingent Value Rights Agreement the original form of which was agreed upon on March 6, 2019 (the “Original Form of CVR Agreement”) and was subsequently revised in connection with the Merger Agreement Amendment (“Amended Form of CVR Agreement”, and together with the Original Form of CVR Agreement, the “CVR Agreement”) and Marc Hanover will act as the representative of holders of the CVRs.
As further discussed in the section titled “The Merger–Background of the Merger,” GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with castration-resistant prostate cancer (“CRPC”) would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2)in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent
4
suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. In connection with the receipt of the new preclinical data, in addition to amending the Merger Agreement, GTx and Oncternal amended the Original Form CVR Agreement to, among other things: (i) increase from 50% to 75% the portion of the net proceeds the CVR holders will be entitled to receive under the CVR Agreement, and (ii) provide that Oncternal (as successor in interest to GTx) will be obligated to use commercially reasonable efforts to either develop or divest the SARD technology, as the Oncternal Board shall determine in its sole discretion, and to divest its SARM technology, subject to certain limitations. Accordingly, Oncternal may decide, in its sole discretion, to abandon the development of the SARD technology following the merger and would then be obligated only to use commercially reasonable efforts to divest the SARD technology, subject to certain limitations. Likewise, Oncternal is obligated only to use commercially reasonable efforts to divest the SARM technology, subject to certain limitations, and in light of the results of the ASTRID trial, Oncternal has no current intent to develop the SARM technology.
| Q: Who | will be the directors of GTx following the merger? |
| A: | In connection with the merger, the GTx Board will be expanded to include a total of nine directors. Pursuant to the terms of the Merger Agreement, two of such directors will be designated by GTx and two of such directors will be designated by SPH USA, Oncternal’s largest stockholder prior to the merger. Four of the remaining five directors are expected to be current directors of Oncternal, including one such director who will be the Chairman of the combined organization and one such director who will be the Chief Executive Officer of the combined organization. It is anticipated that, following the closing of the merger, the GTx Board will be constituted as follows: |
Name | Age | Current Principal Affiliation | ||||
David F. Hale | 70 | Oncternal Therapeutics, Inc., Chairman | ||||
James B. Breitmeyer, M.D., Ph.D. | 65 | Oncternal Therapeutics, Inc., President, Chief Executive Officer and Director | ||||
Michael G. Carter, M.D., Ch.B., F.R.C.P. | 81 | GTx, Inc., Director | ||||
Daniel L. Kisner, M.D. | 71 | Oncternal Therapeutics, Inc., Director Designee | ||||
William R. LaRue | 68 | Oncternal Therapeutics, Inc., Director | ||||
Yanjun Liu, Ph.D. | 54 | Oncternal Therapeutics, Inc., Director | ||||
Xin Nakanishi, Ph.D. | 56 | Oncternal Therapeutics, Inc., Director | ||||
Charles P. Theuer, M.D., Ph.D. | 55 | Oncternal Therapeutics, Inc., Director | ||||
Robert J. Wills, Ph.D. | 65 | GTx, Inc., Executive Chairman | ||||
| Q: Who | will be the executive officers of GTx immediately following the merger? |
| A: | Immediately following the consummation of the merger, the executive management team of GTx is expected to be composed solely of the members of the Oncternal executive management team prior to the merger: |
Name | Title | |
| James B. Breitmeyer, M.D. Ph.D. | President and Chief Executive Officer | |
| Richard G. Vincent | Chief Financial Officer | |
| Hazel M. Aker | General Counsel |
5
| Q: As | a stockholder of GTx, how does the GTx Board recommend that I vote? |
| A: | After careful consideration, the GTx Board recommends that GTx’s stockholders vote: |
| • | “FOR” Proposal No. 1 to approve the Merger Agreement and the transactions contemplated thereby, including the merger, the issuance of shares of GTx’s common stock to Oncternal’s stockholders in the merger and the change of control resulting from the merger; |
| • | “FOR” Proposal No. 2 to approve a series of alternative amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split; |
| • | “FOR” Proposal No. 3 to approve an amendment to the restated certificate of incorporation of GTx to effect the GTx Name Change; |
| • | “FOR” Proposal No. 4 to approve the adoption of the GTx, Inc., 2019 Incentive Award Plan; |
| • | “FOR” Proposal No. 5 to approve, on a nonbinding, advisory basis, the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger; and |
| • | “FOR” Proposal No. 6 to adjourn the special meeting, if necessary, if a quorum is present, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1 or 2. |
| Q: As | a stockholder of Oncternal, how does the Oncternal Board recommend that I vote? |
| A: | After careful consideration, the Oncternal Board recommends that Oncternal’s stockholders execute the written consent indicating their vote in favor of the adoption of the Merger Agreement and the approval of the merger and the transactions contemplated by the Merger Agreement. |
| Q: | What risks should I consider in deciding whether to vote in favor of the merger or to execute and return the written consent, as applicable? |
| A: | You should carefully review the section of this proxy statement/prospectus/information statement entitled “Risk Factors,” which sets forth certain risks and uncertainties related to the merger, risks and uncertainties to which the combined company’s business will be subject, and risks and uncertainties to which each of GTx and Oncternal, as an independent company, is subject. |
| Q: When | do you expect the merger to be consummated? |
| A: | We anticipate that the merger will occur during the second quarter of 2019, soon after the GTx special meeting to be held on June 5, 2019 but we cannot predict the exact timing. For more information, please see the section entitled “The Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement. |
| Q: | What are the material U.S. federal income tax consequences of the merger to U.S. Holders of Oncternal shares? |
| A: | It is a condition to GTx’s obligation to consummate the merger that GTx receive an opinion from Cooley LLP, dated as of the closing date, to the effect that the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”). It is a condition to Oncternal’s obligation to consummate the merger that Oncternal receive an opinion from Latham & Watkins LLP, dated as of the closing date, to the effect that the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. Subject to the tax opinion representations and assumptions (as defined on page 178), in the opinions of Cooley LLP and Latham & Watkins LLP, the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. Accordingly, a U.S. Holder (as defined on page 177) of Oncternal’s common stock will not recognize any gain or loss for |
6
| U.S. federal income tax purposes on the exchange of shares of Oncternal common stock for shares of GTx common stock in the merger, except with respect to cash received by a U.S. Holder of Oncternal common stock in lieu of a fractional share of GTx common stock. If any of the tax opinion representations and assumptions is incorrect, incomplete or inaccurate or is violated, the accuracy of the opinions described above may be affected and the U.S. federal income tax consequences of the merger could differ from those described in this proxy statement/prospectus/information statement. |
Please review the information in the section entitled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” for a more complete description of the material U.S. federal income tax consequences of the merger to U.S. Holders of Oncternal common stock. The tax consequences to you of the merger will depend on your particular facts and circumstances. Please consult your tax advisors as to the specific tax consequences to you of the merger.
| Q: | What are the material U.S. federal income tax consequences of the receipt of CVRs and the GTx Reverse Stock Split to GTx U.S. Holders? |
| A: | GTx intends to report the issuance of the CVRs, to be received by GTx stockholders pursuant to the Merger Agreement, to GTx U.S. Holders (as defined on page 202) as a distribution of property with respect to its stock. Please review the information in the section entitled “Agreements Related to the Merger—CVR Agreement—Material U.S. Federal Income Tax Consequences of the Receipt of CVRs” for a more complete description of the material U.S. federal income tax consequences of the receipt of CVRs to GTx U.S. Holders, including possible alternative treatments. A GTx U.S. Holder generally should not recognize gain or loss upon the GTx Reverse Stock Split, except to the extent a GTx U.S. Holder receives cash in lieu of a fractional share of GTx common stock. Please review the information in the section entitled “Proposal No. 2: Approval of the GTx Reverse Stock Split—Material U.S. Federal Income Tax Consequences of the GTx Reverse Stock Split” for a more complete description of the material U.S. federal income tax consequences of the GTx Reverse Stock Split to GTx U.S. Holders. |
The tax consequences to you of the receipt of CVRs and the GTx Reverse Stock Split will depend on your particular facts and circumstances. Please consult your tax advisors as to the specific tax consequences to you.
| Q: | What do I need to do now? |
| A: | GTx and Oncternal urge you to read this proxy statement/prospectus/information statement carefully, including its annexes, and to consider how the merger affects you. |
If you are a stockholder of GTx, you may provide your proxy instructions in one of four different ways. First, you can mail your signed proxy card in the enclosed return envelope. Second, you may provide your proxy instructions via phone by following the instructions on your proxy card or voting instruction form. Third, you may provide your proxy instructions via the Internet by following the instructions on your proxy card or voting instruction form. Finally, you may vote in person at the GTx special meeting, as described below. Please provide your proxy instructions only once, unless you are revoking a previously delivered proxy instruction, and as soon as possible so that your shares can be voted at the GTx special meeting.
If you are a stockholder of Oncternal, you may execute and return your written consent to Oncternal in accordance with the instructions provided by Oncternal.
| Q: What | happens if I do not return a proxy card or otherwise provide proxy instructions, as applicable? |
| A: | If you are a stockholder of GTx, the failure to return your proxy card or otherwise provide proxy instructions (a) will reduce the aggregate number of votes required to approve Proposal Nos. 1, 4, 5 and 6, (b) will have the same effect as voting against Proposal Nos. 2 and 3 and (c) your shares will not be counted for purposes of determining whether a quorum is present at the GTx special meeting. |
7
| Q: May | I vote in person at the special meeting of stockholders of GTx? |
| A: | If your shares of GTx’s common stock are registered directly in your name with GTx’s transfer agent, you are considered to be the stockholder of record with respect to those shares, and the proxy materials and proxy card are being sent directly to you by GTx. If you are a stockholder of GTx of record, you may attend the GTx special meeting and vote your shares in person. Even if you plan to attend the GTx special meeting in person, GTx requests that you sign and return the enclosed proxy to ensure that your shares will be represented at the GTx special meeting if you become unable to attend. If your shares of GTx’s common stock are held in a brokerage account or by another nominee, you are considered the beneficial owner of shares held in “street name,” and the proxy materials are being forwarded to you by your broker or other nominee together with a voting instruction card. As the beneficial owner, you are also invited to attend the GTx special meeting. Because a beneficial owner is not the stockholder of record, you may not vote these shares in person at the GTx special meeting unless you obtain a proxy from the broker, trustee or nominee that holds your shares, giving you the right to vote the shares at the GTx special meeting. |
| Q: When | and where is the special meeting of GTx’s stockholders? |
| A: | The GTx special meeting will be held at 9:00 a.m., Central time, on June 5, 2019 at 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103, unless postponed or adjourned to a later date. Subject to space availability, all of GTx’s stockholders as of the record date, or their duly appointed proxies, may attend the GTx special meeting. |
| Q: If | my GTx shares are held in “street name” by my broker, will my broker vote my shares for me? |
| A: | Unless your broker has discretionary authority to vote on certain matters, your broker will not be able to vote your shares of GTx’s common stock without instructions from you. Brokers are not expected to have discretionary authority to vote for any of the Proposals. To make sure that your vote is counted, you should instruct your broker to vote your shares, following the procedures provided by your broker. |
| Q: May | I change my vote after I have submitted a proxy or provided proxy instructions? |
| A: | GTx’s stockholders of record, other than those of GTx’s stockholders who are parties to voting agreements, may change their vote at any time before their proxy is voted at the GTx special meeting in one of three ways. First, a stockholder of record of GTx can send a written notice to the Secretary of GTx stating that it would like to revoke its proxy. Second, a stockholder of record of GTx can submit new proxy instructions either on a new proxy card or via the Internet. Third, a stockholder of record of GTx can attend the GTx special meeting and vote in person. Attendance alone will not revoke a proxy. If a stockholder of GTx of record or a stockholder who owns GTx shares in “street name” has instructed a broker to vote its shares of GTx’s common stock, the stockholder must follow directions received from its broker to change those instructions. |
| Q: Who | is paying for this proxy solicitation? |
| A: | GTx and Oncternal will share equally the cost of printing and filing of this proxy statement/prospectus/information statement and the proxy card. Arrangements will also be made with brokerage firms and other custodians, nominees and fiduciaries who are record holders of GTx’s common stock for the forwarding of solicitation materials to the beneficial owners of GTx’s common stock. GTx will reimburse these brokers, custodians, nominees and fiduciaries for the reasonableout-of-pocket expenses they incur in connection with the forwarding of solicitation materials. |
8
| Q: Who | can help answer my questions? |
| A: | If you are a stockholder of GTx and would like additional copies, without charge, of this proxy statement/prospectus/information statement or if you have questions about the merger, including the procedures for voting your shares, you should contact: |
GTx, Inc.
17 W Pontotoc Ave., Suite 100
Memphis, TN 38103
Tel: (901)523-9700
Attn: Henry Doggrell
If you are a stockholder of Oncternal, and would like additional copies, without charge, of this proxy statement/prospectus/information statement or if you have questions about the merger, including the procedures for voting your shares, you should contact:
Oncternal Therapeutics, Inc.
12230 El Camino Real, Ste 300
San Diego, California 92130
Tel: (858)434-1113
Attn: Richard G. Vincent
Email: info@oncternal.com
9
This summary highlights selected information from this proxy statement/prospectus/information statement and may not contain all of the information that is important to you. To better understand the merger, the proposals being considered at the GTx special meeting and Oncternal’s stockholder actions that are the subject of the written consent, you should read this entire proxy statement/prospectus/information statement carefully, including the Merger Agreement attached as Annex A, the opinion of Aquilo Partners, L.P. attached asAnnex B-2 and the other annexes to which you are referred herein. For more information, please see the section entitled “Where You Can Find More Information” in this proxy statement/prospectus/information statement.
The Companies
GTx, Inc.
17 W Pontotoc Ave., Suite 100
Memphis, Tennessee 38103
(901)523-9700
Attn: Marc S. Hanover
GTx is a biopharmaceutical company dedicated to the discovery, development and commercialization of medicines to treat serious and/or significant unmet medical conditions. Under an exclusive worldwide license agreement with the University of Tennessee Research Foundation (“UTRF”), GTx is developing UTRF’s proprietary selective androgen receptor degrader (“SARD”) technology, which GTx believes may have the potential to provide compounds that can degrade or antagonize multiple forms of androgen receptor thereby potentially inhibiting tumor growth in patients with progressive CRPC, including those patients who do not respond to or are resistant to current androgen targeted therapies. GTx has been conducting preclinical studies to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an investigational new drug application (“IND”) to enable the initiation of a first-in-human clinical trial, if any. However, GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2)in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. Accordingly, additional preclinical research would be required in order to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies.
Oncternal Therapeutics, Inc.
12230 El Camino Real, Ste 300
San Diego, California 92130
Tel: (858)434-1113
Attn: James B. Breitmeyer, M.D., Ph.D.
10
Oncternal is a clinical-stage biopharmaceutical company focused on developing potentialfirst-in-class therapies for cancers in which there is critical unmet medical need. The company’s drug development efforts are focused on promising, yet untreated biological pathways implicated in cancer genesis and progression. Oncternal’s pipeline includes several key programs.
| • | Cirmtuzumab, Oncternal’s lead product candidate, is an investigational, potentiallyfirst-in-class humanized monoclonal antibody that is designed to bind with high affinity to Receptor-tyrosine kinase-like Orphan Receptor 1 (“ROR1”), a protein that is selectively expressed in many forms of cancer including hematological malignancies as well as many solid tumors. Cirmtuzumab is being developed in collaboration with the University of California San Diego (“UC San Diego”) and in collaboration and with funding support from the California Institute for Regenerative Medicine, (“CIRM”). Early preclinical and clinical results suggest that ROR1 is a target with broad potential in oncology. Cirmtuzumab is being studied in a Phase 1/2 clinical trial in combination with ibrutinib in patients with chronic lymphocytic leukemia (“CLL”) and mantle cell lymphoma (“MCL”), and in combination with paclitaxel for the treatment of women with metastatic breast cancer. |
| • | TK216 is an investigational, potentiallyfirst-in-class small molecule that is designed to inhibit the biological activity of E26 transformation-specific (“ETS”), transcription factor oncoproteins including fusion proteins. TK216 is being evaluated alone and in combination with vincristine in a Phase 1 clinical trial in patients with relapsed or refractory Ewing sarcoma, a rare pediatric cancer that has historically been very challenging to treat effectively. |
| • | ROR1CAR-T—Oncternal is also developing aCAR-T program targeting ROR1 in collaboration with UC San Diego, who has received funding support directly from CIRM. This program is currently in preclinical development as a potential treatment for both hematologic malignancies and solid tumors. |
Grizzly Merger Sub, Inc.
Merger Sub is a wholly-owned subsidiary of GTx, and was formed solely for the purposes of carrying out the merger.
The Merger (see page 124)
If the merger is completed, Merger Sub will merge with and into Oncternal, with Oncternal surviving as a wholly-owned subsidiary of GTx.
Prior to the Effective Time, each share of Oncternal preferred stock will be converted into one share of Oncternal common stock. At the Effective Time, each share of Oncternal common stock outstanding immediately prior to the Effective Time (excluding certain shares to be canceled pursuant to the Merger Agreement, and shares held by stockholders who have exercised and perfected appraisal rights as more fully described in the section entitled “The Merger—Appraisal Rights” below) will be converted into the right to receive approximately 0.5137 shares of GTx’s common stock, subject to adjustment for the GTx Reverse Stock Split. This exchange ratio is an estimate only and the final exchange ratio will be determined pursuant to a formula described in more detail in the Merger Agreement and in this proxy statement/prospectus/information statement. Immediately after the merger, assuming an exchange ratio of 0.5137, Oncternal’s stockholders as of immediately prior to the Effective Time will own approximately 77.5% of the outstanding capital stock of GTx, and GTx’s stockholders as of immediately prior to the Effective Time will own approximately 22.5% of the outstanding capital stock of GTx. The ownership percentage to be held by GTx’s stockholders is subject to adjustment prior to closing of the merger, including a downward adjustment to the extent that GTx’s “Parent Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement, which adjusts based on the date of closing (and as a result, GTx stockholders could own less, and Oncternal stockholders could own more, of the combined company), or an upward adjustment to the extent that Oncternal’s
11
“Company Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement (and as a result, GTx stockholders could own more, and Oncternal stockholders could own less, of the combined company). The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants. GTx will assume outstanding and unexercised options and warrants to purchase Oncternal capital stock, and each such option or warrant will be converted into options or warrants, as applicable, to purchase GTx’s common stock.
For a more complete description of the merger exchange ratio please see the section entitled “The Merger Agreement” in this proxy statement/prospectus/information statement.
The closing of the merger will occur no later than the second business day after the last of the conditions to the merger has been satisfied or waived (other than those conditions that by their nature are to be satisfied at the closing, but subject to the satisfaction or waiver of each such conditions), or at such other time as GTx and Oncternal agree. GTx and Oncternal anticipate that the consummation of the merger will occur in the second quarter of the fiscal year. However, because the merger is subject to a number of conditions, neither GTx nor Oncternal can predict exactly when the closing will occur or if it will occur at all. After completion of the merger, assuming that GTx receives the required stockholder approval of Proposal No. 3, GTx will be renamed “Oncternal Therapeutics, Inc.”
Reasons for the Merger (see page 140)
The merger will produce a clinical-stage biopharmaceutical company focused on developingfirst-in-class product candidates for cancers with critical unmet medical need. GTx and Oncternal believe that the combined company will have the following characteristics found in successful biotech companies:
| • | Diverse Clinical Stage Pipeline. The combined company will focus on developing potentiallyfirst-in-class product candidates for cancers with critical unmet medical need and will have two clinical stage product candidates, cirmtuzumab and TK216, in clinical trials for CLL, MCL and Ewing sarcoma. Additional indications are under consideration for future clinical trials. |
| • | Novel Preclinical Programs: The combined company will develop aCAR-T program targeting ROR1 as a potential treatment for both hematologic malignancies and solid tumors, and a selective androgen receptor degrader, or SARD, as a potential treatment for patients with castration-resistant prostate cancer. |
| • | Management Team. The combined company will be led by the experienced senior management from Oncternal and a board of directors with representation from each of Oncternal and GTx. |
| • | Cash Resources. The combined company is expected to have approximately $26.0 million in cash and cash equivalents at the closing of the merger, which GTx and Oncternal believe is sufficient to enable Oncternal to implement its near-term business plans. |
Each of GTx’s and Oncternal’s respective board of directors also considered other reasons for the merger, as described herein. For example, the GTx Board considered, among other things:
| • | the strategic alternatives of GTx to the merger, including the discussions that GTx senior management and the GTx Board previously conducted with other potential merger partners; |
| • | the failure to demonstrate the effectiveness of enobosarm as a potential treatment for stress urinary incontinence (“SUI”), and the unlikelihood that such circumstances would change for the benefit of GTx’s stockholders in the foreseeable future; |
| • | the risks of developing a product candidate out of the SARD program, including the costs of contracting with third parties to complete the necessary preclinical development work to select a lead compound, submitting an IND, and developing a product candidate through further preclinical studies and potentially clinical trials; |
12
| • | the risk associated with, and uncertain value and costs to stockholders of, winding down operations of GTx; |
| • | the risks of continuing to operate GTx on a stand-alone basis, including developing its SARD program and the need to raise additional funding and expend significant resources to advance this portfolio and to rebuild its infrastructure and management to continue its operations; and |
| • | the opportunity as a result of the merger for GTx’s stockholders to participate in the potential value of the Oncternal product candidate portfolio as well as the potential value derived from the sale or licensing of its SARD or SARM programs pursuant to the CVR Agreement. |
In addition, the Oncternal Board approved the merger based on a number of factors, including the:
| • | potential increased access to sources of capital and a broader range of investors to support the clinical development of its products than it could otherwise obtain if it continued to operate as a privately held company; |
| • | potential to provide its current stockholders with greater liquidity by owning stock in a public company; |
| • | Oncternal Board’s belief that no alternatives to the merger were reasonably likely to create greater value for Oncternal’s stockholders, or enable accelerated investment in Oncternal’s portfolio, after reviewing the various strategic options to enhance stockholder value that were considered by the Oncternal Board; |
| • | cash resources of the combined organization expected to be available at the closing of the merger; and |
| • | expectation that the merger will be treated as a reorganization for U.S. federal income tax purposes. |
Opinion of the GTx Financial Advisor (see page 146)
The GTx Board engaged Aquilo Partners, L.P. (“Aquilo”) to provide financial advisory services and to consider and evaluate potential strategic transactions on its behalf. GTx ultimately requested that Aquilo deliver a fairness opinion with respect to the merger with Oncternal. On March 6, 2019, Aquilo delivered its oral opinion, subsequently confirmed in writing, to the GTx Board to the effect that, as of the date of its opinion and based upon and subject to the qualifications, limitations and assumptions set forth therein, the consideration is fair, from a financial point of view, to GTx’s stockholders. On April 29, 2019, at the request of the GTx Board and in light of the Merger Agreement Amendment, Aquilo subsequently rendered a revised oral opinion, subsequently confirmed in writing to the GTx Board, to the GTx Board to the effect that, as of the date of its opinion and based upon and subject to the qualifications, limitations and assumptions set forth therein, the consideration is fair, from a financial point of view, to GTx’s stockholders.
The full text of Aquilo’s written opinions, which set forth the procedures followed, assumptions made, matters considered, and limitations and qualifications of the review undertaken in connection with the opinions, are attached asAnnexes B-1 and B-2 and are incorporated by reference in their entirety. You are urged to, and should, read the most recent written opinions of Aquilo carefully and in its entirety. Aquilo’s opinions were intended for the use and benefit of the GTx Board (in its capacity as such) in connection with its evaluation of the merger. Aquilo’s opinions were not intended to be used for any other purpose without Aquilo’s prior written consent in each instance, except as GTx’s counsel advises is required by law. Aquilo has consented to the inclusion of Aquilo’s opinions in this proxy statement. Aquilo’s opinions do not address GTx’s underlying business decision to enter into the Merger Agreement or CVR Agreement, the relative merits of the merger compared to any alternative transactions or strategies that were or may be available to GTx. Aquilo’s opinions did not constitute a recommendation to the GTx Board as to how to act or to any GTx stockholder or any other person as to how to vote with respect to the merger with Oncternal or any other matter.
13
Material U.S. Federal Income Tax Consequences of the Merger (see page 219)
It is a condition to GTx’s obligation to consummate the merger that GTx receive an opinion from Cooley LLP, dated as of the closing date, to the effect that the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. It is a condition to Oncternal’s obligation to consummate the merger that Oncternal receive an opinion from Latham & Watkins LLP, dated as of the closing date, to the effect that the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. Subject to the tax opinion representations and assumptions (as defined on page 178), in the opinions of Cooley LLP and Latham & Watkins LLP, the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. Accordingly, a U.S. Holder (as defined on page 177) of Oncternal common stock will not recognize any gain or loss for U.S. federal income tax purposes on the exchange of shares of Oncternal common stock for shares of GTx common stock in the merger, except with respect to cash received by a U.S. Holder of Oncternal common stock in lieu of a fractional share of GTx common stock. If any of the tax opinion representations and assumptions is incorrect, incomplete or inaccurate or is violated, the accuracy of the opinions described above may be affected and the U.S. federal income tax consequences of the merger could differ from those described in this proxy statement/prospectus/information statement.
Please review the information in the section entitled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” for a more complete description of the material U.S. federal income tax consequences of the merger to U.S. Holders of Oncternal common stock. The tax consequences to you of the merger will depend on your particular facts and circumstances. Please consult your tax advisors as to the specific tax consequences to you of the merger.
Material U.S. Federal Income Tax Consequences of the Receipt of CVRs and the GTx Reverse Stock Split (see pages 200 and 209)
GTx intends to report the issuance of CVRs to GTx U.S. Holders (as defined on page 184) as a distribution of property with respect to its stock. Please review the information in the section entitled “Agreements Related to the Merger—CVR Agreement—Material U.S. Federal Income Tax Consequences of the Receipt of CVRs” for a more complete description of the material U.S. federal income tax consequences of the receipt of CVRs to GTx U.S. Holders, including possible alternative treatments.
A GTx U.S. Holder generally should not recognize gain or loss upon the GTx Reverse Stock Split, except to the extent a GTx U.S. Holder receives cash in lieu of a fractional share of GTx common stock. Please review the information in the section entitled “Proposal No. 2: Approval of the GTx Reverse Stock Split—Material U.S. Federal Income Tax Consequences of the GTx Reverse Stock Split” for a more complete description of the material U.S. federal income tax consequences of the GTx Reverse Stock Split to GTx U.S. Holders.
The tax consequences to you of the receipt of CVRs and the GTx Reverse Stock Split will depend on your particular facts and circumstances. Please consult your tax advisors as to the specific tax consequences to you.
Overview of the Merger Agreement
Merger Consideration (see page 185)
Prior to the Effective Time, each share of outstanding Oncternal preferred stock will be converted into one share of Oncternal common stock. At the Effective Time, each share of Oncternal’s common stock outstanding immediately prior to the Effective Time (excluding shares of Oncternal’s common stock held as treasury stock or held by Oncternal, Merger Sub or any subsidiary of Oncternal) will automatically be converted into the right to receive a number of shares of GTx’s common stock equal to approximately 0.5137. This exchange ratio is an estimate only and the final exchange ratio will be determined pursuant to a formula described in more detail in the Merger Agreement and in this proxy statement/prospectus/information statement.
14
Immediately after the merger, assuming an exchange ratio of 0.5137, Oncternal’s stockholders as of immediately prior to the Effective Time will own approximately 77.5% of the outstanding capital stock of GTx and GTx stockholders as of immediately prior to the Effective Time will own approximately 22.5% of the outstanding capital stock of GTx. The ownership percentage to be held by GTx’s stockholders is subject to adjustment prior to closing of the merger, including a downward adjustment to the extent that GTx’s “Parent Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement, which adjusts based on the date of closing (and as a result, GTx stockholders could own less, and Oncternal stockholders could own more, of the combined organization), or an upward adjustment to the extent that Oncternal’s “Company Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement (and as a result, GTx stockholders could own more, and Oncternal stockholders could own less, of the combined organization). The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants.
The Merger Agreement does not include a price-based termination right, and there will be no adjustment to the total number of shares of GTx’s common stock that Oncternal’s stockholders will be entitled to receive for changes in the market price of GTx’s common stock after the date the Merger Agreement was signed. Accordingly, the market value of the shares of GTx’s common stock issued pursuant to the merger will depend on the market value of the shares of GTx’s common stock at the time the merger closes, and could vary significantly from the market value on the date of this proxy statement/prospectus/information statement.
Treatment of GTx’s Stock Awards and Warrants (see page 186)
Prior to the closing of the merger, the GTx Board will adopt appropriate resolutions and take all other actions necessary and appropriate to provide that the vesting of each unexpired and unexercised option to purchase GTx’s common stock will be accelerated in full effective as of immediately prior to the Effective Time. The number of shares of common stock underlying each option and warrant and the exercise price for such options and warrants will be adjusted to account for the GTx Reverse Stock Split. The terms governing options and warrants to purchase GTx’s common stock will otherwise remain in full force and effect following the closing of the merger.
Under the Merger Agreement, as of immediately prior to the closing of the merger (but in no event more than 30 days prior to the Effective Time), GTx shall take all actions necessary to cause the termination and liquidation of the GTx 2018 Amended and Restated Directors’ Deferred Compensation Plan (the “GTx Director Deferred Compensation Plan”), and all deferred stock rights thereunder, effective immediately prior to the closing of the merger, subject to the consummation of the merger (the “GTx Deferred Stock Rights”). GTx shall also ensure that any deferrals under the GTx Director Deferred Compensation Plan on or after January 3, 2019, shall be settled only in cash and that the maximum number of shares of common stock of GTx issuable upon settlement of the GTx Deferred Stock Rights shall be limited to the number of GTx Deferred Stock Rights outstanding as of the date of the Merger Agreement.
Treatment of Oncternal’s Stock Awards and Warrants (see page 187)
Pursuant to the Merger Agreement, at the Effective Time:
| • | each option to purchase shares of Oncternal’s capital stock that is outstanding and unexercised immediately prior to the Effective Time granted under the Oncternal Therapeutics 2015 Equity Incentive Plan, whether or not vested, will be assumed by GTx and will become an option to purchase that number of shares of GTx’s common stock equal to the product obtained by multiplying (i) the number of shares of Oncternal’s common stock that were subject to such option immediately prior to the Effective Time by (ii) the exchange ratio, rounded down to the nearest whole share. The per share |
15
exercise price for shares of GTx’s common stock issuable upon exercise of each Oncternal option assumed by GTx shall be determined by dividing (a) the per share exercise price of Oncternal’s common stock subject to such Oncternal option, as in effect immediately prior to the Effective Time, by (b) the exchange ratio, rounded up to the nearest whole cent. Any restriction on the exercise of any Oncternal option assumed by GTx will continue in full force and effect and the term, exercisability, vesting schedule and other provisions of such Oncternal option shall otherwise remain unchanged; and |
| • | each warrant to purchase shares of Oncternal capital stock outstanding and unexercised immediately prior to the Effective Time will be assumed by GTx and will become a warrant to purchase that number of shares of GTx’s common stock equal to the product obtained by multiplying (i) the number of shares of Oncternal’s common stock, or the number of shares of Oncternal’s common stock issuable upon conversion of the shares of Oncternal’s preferred stock issuable upon exercise of the Oncternal warrant, as applicable, that were subject to such warrant immediately prior to the Effective Time by (ii) the exchange ratio, rounded down to the nearest whole share. The per share exercise price for shares of GTx’s common stock issuable upon exercise of each Oncternal warrant assumed by GTx shall be determined by dividing (a) the per share exercise price of Oncternal’s capital stock subject to such Oncternal warrant, as in effect immediately prior to the Effective Time, by (b) the exchange ratio rounded up to the nearest whole cent. Any restriction on any Oncternal warrant assumed by GTx shall continue in full force and effect and the terms and other provisions of such Oncternal warrant shall otherwise remain unchanged. |
In addition, pursuant to the Merger Agreement, at the Effective Time, each restricted share of Oncternal common stock that is outstanding will be converted into a share of GTx on the same basis as other shares of Oncternal common stock. Any restrictions on such restricted shares will continue in full force and effect and the vesting schedule and other provisions of such Oncternal restricted shares shall otherwise remain unchanged.
Conditions to the Completion of the Merger (see page 188)
To consummate the merger, GTx’s stockholders must approve Proposal Nos. 1 and 2. Additionally, Oncternal’s stockholders must (i) adopt and approve the Merger Agreement and the transactions contemplated thereby, (ii) acknowledge that the approval given is irrevocable and that such stockholders are aware of their rights to demand appraisal for its shares pursuant to Section 262 of the DGCL, and that such stockholders have received and read a copy of Section 262 of the DGCL, which is included asAnnex C in this proxy statement/prospectus/information statement, (iii) acknowledge that by their approval of the merger the approving stockholders are not entitled to appraisal rights with respect to their shares in connection with the merger and thereby waive any rights to receive payment of the fair value of their capital stock under the DGCL, and (iv) approve the conversion of Oncternal’s outstanding preferred stock into Oncternal’s common stock immediately prior to the Effective Time.
In addition to obtaining such stockholder approvals and appropriate regulatory approvals, each of the other closing conditions set forth in the Merger Agreement, as described under the section entitled “The Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement must be satisfied or waived.
No Solicitation (see page 192)
Each of GTx and Oncternal agreed that, except as described below, from the date of the Merger Agreement until the earlier of the consummation of the merger or the termination of the Merger Agreement in accordance with its terms, GTx and Oncternal and any of their respective subsidiaries will not, nor will either party or any of its subsidiaries authorize any of the directors, officers, employees, agents, attorneys, accountants, investment bankers, advisors or representatives retained by it or any of its subsidiaries to, directly or indirectly:
| • | solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of, any “acquisition proposal” (as defined in the section entitled “The Merger |
16
Agreement—No Solicitation” below), or “acquisition inquiry” (as defined in the section entitled “The Merger Agreement—No Solicitation” below); |
| • | furnish anynon-public information with respect to it to any person in connection with or in response to an acquisition proposal or acquisition inquiry; |
| • | engage in discussions or negotiations with any person with respect to any acquisition proposal or acquisition inquiry; |
| • | approve, endorse or recommend an acquisition proposal; |
| • | execute or enter into any letter of intent or similar document or any contract contemplating or otherwise relating to an acquisition transaction (other than a confidentiality agreement as permitted by the Merger Agreement) (as defined in the section entitled “The Merger Agreement—No Solicitation” below); or |
| • | publicly propose to do any of the above. |
Termination of the Merger Agreement (see page 197)
Either GTx or Oncternal can terminate the Merger Agreement under certain circumstances, which would prevent the merger from being consummated.
Termination Fee (see page 199)
If the Merger Agreement is terminated under certain circumstances, GTx or Oncternal will be required to pay the other party a termination fee of up to $2.0 million.
CVR Agreement (see page 200)
At the closing of the merger, GTx, Marc Hanover, as representative of holders of the CVRs, and a rights agent will enter into the CVR Agreement. Pursuant to the CVR Agreement, GTx stockholders will receive one CVR for each share of GTx common stock held as of immediately prior to the Effective Time. Each CVR will represent the right to receive payments based on net proceeds derived from GTx’s SARD or SARM technology during the term of the CVR. In particular, CVR holders will be entitled to 75% of the aggregate amount of any net proceeds received by the combined company during the15-year period after the closing of the merger from the grant, sale or transfer of rights to GTx’s SARD or SARM technology that occurs during the10-year period after the closing (or in the 11th year if based on a term sheet approved during the initial10-year period) and, if applicable, to receive royalties on the sale of any SARD or SARM products by the combined company during the15-year period after the closing. As further discussed in the section titled “The Merger—Background of the Merger,” GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2)in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro
17
andin vivo findings. In connection with the receipt of the new preclinical data, in addition to amending the Merger Agreement, GTx and Oncternal amended the form of CVR Agreement to, among other things: (i) increase from 50% to 75% the portion of the net proceeds the CVR holders will be entitled to under the CVR Agreement, and (ii) provide that Oncternal (as successor in interest to GTx) will be obligated to use commercially reasonable efforts to either develop or divest GTx’s SARD technology, as the Oncternal Board shall determine in its sole discretion, and to divest its SARM technology, subject to certain limitations. Accordingly, Oncternal may decide, in its sole discretion, to abandon the development of GTx’s SARD technology following the merger and would then be obligated only to use commercially reasonable efforts to divest the SARD technology, subject to certain limitations. Likewise, Oncternal is obligated only to use commercially reasonable efforts to divest the SARM technology, subject to certain limitations, and in light of the results of the ASTRID trial, Oncternal has no current intent to develop the SARM technology.
Voting Agreements and Written Consents (see page 204)
In order to induce GTx to enter into the Merger Agreement, certain stockholders of Oncternal are parties to a voting agreement with Oncternal and GTx pursuant to which, among other things, each stockholder has agreed, solely in its capacity as a stockholder of Oncternal, to vote all of its shares of Oncternal’s capital stock in favor of (i) the adoption and approval of the Merger Agreement and the transactions contemplated thereby, (ii) acknowledgement that the approval given for the Merger Agreement is irrevocable and that the stockholder is aware of its appraisal rights under the DGCL, (iii) acknowledgement that the stockholder is not entitled to appraisal rights by voting in favor of the transaction and waiving appraisal rights under the DGCL, and (iv) the conversion of each share of Oncternal preferred stock into Oncternal common stock. Additionally, each stockholder has agreed, solely in its capacity as a stockholder of Oncternal, to vote against any competing acquisition proposal and any action, proposal or transaction that would reasonably be expected to result in a material breach of the voting agreement. These stockholders of Oncternal have also granted an irrevocable proxy to Oncternal and its designee to vote their respective Oncternal’s capital stock in accordance with the voting agreements. Oncternal’s stockholders may vote their shares of Oncternal capital stock on all other matters not referred to in such proxy.
The Oncternal stockholders who are parties to these voting agreements include all directors, executive officers and certain stockholders, including entities related to MagnaSci Ventures, which represents approximately 10.4% of the outstanding shares of Oncternal capital stock on as converted common stock basis. SPH USA, which holds 100% of the outstanding Series C preferred stock and which represents approximately 20.9% of the outstanding shares of Oncternal capital stock on as converted common stock basis, has not executed a voting agreement. Although Oncternal expects to receive stockholder approval from SPH USA approximately two months after the date of the Merger Agreement, there can be no assurance that all of the necessary stockholder approvals will be obtained.
The Oncternal stockholders who are party to a voting agreement held, as of March 31, 2019:
| • | an aggregate of 32,059,203 shares of Oncternal’s common stock and 38,883,369 shares of Oncternal preferred stock, representing approximately 44% of the outstanding shares of Oncternal capital stock on an as converted to common stock basis; |
| • | an aggregate of 38,883,369 shares of Oncternal’s preferred stock, representing approximately 35.0% of the outstanding Oncternal preferred stock, considered as a single class; |
| • | an aggregate of 5,960,000 shares of Oncternal’s Series A preferred stock, representing approximately 44.0% of the outstanding Series A preferred stock; and |
| • | an aggregate of 32,923,369 shares of Oncternal’s Series B preferred stock and SeriesB-2 preferred stock, representing approximately 51.9% of the outstanding Series B preferred stock and SeriesB-2 preferred stock, considered as a single class. |
18
Following the effectiveness of the registration statement of which this proxy statement/prospectus/information statement is a part and pursuant to the Merger Agreement, these stockholders will execute a written consent providing for such adoption and approval.
Under these voting agreements, subject to certain exceptions, such stockholders have also agreed not to sell or transfer shares of Oncternal’s capital stock and securities held by them, or any voting rights with respect thereto, until the earlier of the termination of the Merger Agreement or the completion of the merger. To the extent that any such sale or transfer is permitted pursuant to the exceptions included in the voting agreement, each person to which any shares of Oncternal’s capital stock or securities are so sold or transferred must agree in writing to be bound by the terms and provisions of the voting agreement, subject to certain further exceptions.
In addition, in order to induce Oncternal to enter into the Merger Agreement, certain of GTx’s stockholders have entered into voting agreements with GTx and Oncternal pursuant to which, among other things, each such stockholder has agreed, solely in his, her or its capacity as a stockholder of GTx, to vote all of his, her or its shares of GTx’s common stock in favor of Proposal Nos. 1, 2, 3, 4 and 5. Additionally, each such stockholder has agreed, solely in his, her or its capacity as a stockholder of GTx, to vote against any competing acquisition proposal and any action, proposal or transaction that would reasonably be expected to result in a material breach of the voting agreement. These stockholders of GTx have also granted GTx and its designee an irrevocable proxy to vote their respective shares in accordance with the voting agreements. GTx’s stockholders may vote their shares of GTx’s common stock on all other matters not referred to in such proxy.
The GTx stockholders who are parties to these voting agreements are:
| • | Robert J. Wills, Ph.D. |
| • | Marc S. Hanover |
| • | J.R. Hyde, III |
| • | Michael G. Carter, M.D., Ch.B., F.R.C.P. |
| • | J. Kenneth Glass |
| • | Garry A. Neil, M.D. |
| • | Kenneth S. Robinson, M.D., M.Div. |
| • | Henry P. Doggrell |
| • | Jason Shackelford |
| • | Pyramid Peak Foundation |
As of March 31, 2019, the stockholders of GTx who are party to a voting agreement (including any affiliated entities) owned an aggregate of 10,938,824 shares of GTx’s common stock representing approximately 45% of the outstanding shares of GTx’s common stock.
Under these voting agreements, subject to certain exceptions, such stockholders also have agreed not to sell or transfer their shares of GTx’s common stock and securities held by them until the earlier of the termination of the Merger Agreement or the completion of the merger. To the extent that any such sale or transfer is permitted pursuant to the exceptions included in the voting agreements, each person to whom any shares of GTx’s common stock or securities are so sold or transferred must agree in writing to be bound by the terms and provisions of the voting agreement, subject to certain further exceptions.
19
Lock-up Agreements (see page 205)
As a condition to the closing of the merger, certain stockholders of each of GTx and Oncternal and their affiliates, have entered intolock-up agreements, pursuant to which such parties have agreed not to, except in limited circumstances, offer, pledge, sell, contract to sell, transfer or dispose of, directly or indirectly, engage in swap or similar transactions with respect to, or make any demand for or exercise any right with respect to, any shares of GTx’s common stock or any security convertible into or exercisable or exchangeable for GTx’s common stock, including, as applicable, shares received in the merger and issuable upon exercise of certain warrants and options, during the period commencing at the Effective Time and continuing until the date that is 180 days from the Effective Time.
Each of the stockholders who is party to a GTx voting agreement is a party to alock-up agreement. As of March 31, 2019, GTx’s stockholders who have executedlock-up agreements owned in the aggregate approximately 45% of the outstanding common stock of GTx.
Each of the stockholders who is party to an Oncternal voting agreement is a party to alock-up agreement. Oncternal’s stockholders who have executedlock-up agreements, as of March 31, 2019, beneficially owned in the aggregate approximately 44% of the outstanding shares of Oncternal’s capital stock on an as converted to common stock basis. SPH USA, which holds 100% of the outstanding Series C preferred stock and which represents approximately 20.9% of the outstanding shares of Oncternal capital stock on as converted common stock basis, but Oncternal expects it to execute alock-up agreement prior to the closing of the merger, which is a condition to closing.
Management Following the Merger (see page 334)
Effective as of the closing of the merger, GTx’s executive officers are expected to include:
Name | Title | |
| James B. Breitmeyer, M.D., Ph.D. | President and Chief Executive Officer | |
| Richard G. Vincent | Chief Financial Officer | |
| Hazel M. Aker | General Counsel |
Interests of Certain Directors, Officers and Affiliates of GTx and Oncternal (see pages 370 and 372)
In considering the recommendation of the GTx Board with respect to the issuance of common stock of GTx pursuant to the Merger Agreement and the other matters to be acted upon by GTx’s stockholders at the GTx special meeting, GTx’s stockholders should be aware that certain members of the GTx Board and executive officers of GTx have interests in the merger that may be different from, or in addition to, interests they have as GTx’s stockholders. For example, GTx has entered into employment agreements with its executive officers that may result in the receipt by such executive officers of cash severance payments and other benefits upon an eligible termination of employment of each executive officer’s employment.
As of March 31, 2019, GTx’s directors and executive officers beneficially owned, in the aggregate approximately 39.2% of the outstanding shares of common stock of GTx. As of March 31, 2019, GTx’s directors and officers beneficially owned, in the aggregate, 1,035,549 options to purchase GTx common stock, all of which will become vested immediately prior to the closing of the merger and will be entitled to an extension of the post-termination exercise period of stock options upon an eligible termination of service following the merger or upon their retirement in accordance with the applicable equity plan.
Under the Merger Agreement, as of immediately prior to the closing of the merger (but in no event more than 30 days prior to the Effective Time), GTx shall take all actions necessary to cause the termination and liquidation of
20
the GTx Deferred Stock Rights. GTx shall also ensure that any deferrals under the GTx Director Deferred Compensation Plan on or after January 3, 2019 shall be settled only in cash and that the maximum number of shares of common stock of GTx issuable upon settlement of the GTx Deferred Stock Rights shall be limited to the number of GTx Deferred Stock Rights outstanding as of the date of the Merger Agreement. As of March 31, 2019, five of GTx’s directors held Deferred Stock Rights and an aggregate of 155,426 shares of GTx common stock were issuable pursuant to the GTx Deferred Stock Rights.
In addition, Dr. Carter and Dr. Wills, each of whom is currently a director of GTx, are expected to continue as directors of the combined organization after the Effective Time.
The compensation arrangements with GTx’s officers and directors are discussed in greater detail in the section entitled “The Merger—Interests of GTx Directors and Executive Officers in the Merger” in this proxy statement/prospectus/information statement.
In considering the recommendation of the Oncternal Board with respect to approving the merger and related transactions by written consent, Oncternal’s stockholders should be aware that employees of Oncternal, including Oncternal’s executive officers, are expected to become employees and/or executive officers of GTx upon the closing of the Merger. David F. Hale is expected to be appointed to the board as Chairman of the board of directors and James B. Breitmeyer, M.D., Ph.D. is expected to be appointed to the board pursuant to his role as Chief Executive Officer. It is anticipated that Yanjun Liu, Ph.D. and Xin Nakanishi, Ph.D. will be appointed as the designees of SPH USA and that Charles P. Theuer, M.D., Ph.D., William R. LaRue and Daniel L. Kisner, M.D. will be appointed to the remaining three director positions. It is anticipated that GTx’s executive officers upon the closing of the merger will be Dr. Breitmeyer, President and Chief Executive Officer, Richard G. Vincent, Chief Financial Officer and Hazel M. Aker, General Counsel. In addition, Oncternal’s directors and executive officers and will be entitled to certain indemnification and liability insurance coverage pursuant to the terms of the Merger Agreement. Following completion of the merger, it is expected that the combined organization will provide compensation tonon-employee directors. GTx’s current director compensation program will be suspended at the time of the closing of the merger and the director compensation policies for the combined organization following the merger will bere-evaluated by the compensation committee and board of directors of the combined organization following completion of the merger and may be subject to change.Non-employee directors of the combined organization are, however, expected to receive annual cash retainers and equity compensation, although the amount of such compensation has not yet been determined.
As of March 31, 2019, Oncternal’s directors and executive officers beneficially owned: (i) approximately 43% of the outstanding shares of common stock of Oncternal, (ii) approximately 19% of the outstanding shares of preferred stock of Oncternal, (iii) warrants to purchase 1,910,604 shares of Oncternal SeriesB-2 preferred stock, all of which will be converted into warrants to purchase GTx common stock in connection with the closing of the merger pursuant to the merger agreement, and (iv) options to purchase 4,920,000 shares of Oncternal common stock, all of which will be converted into options to purchase GTx common stock in connection with the closing of the merger pursuant to the Merger Agreement.
The compensation arrangements with Oncternal’s officers and directors are discussed in greater detail in the section entitled “Agreements Related to the Merger—Interests of Oncternal Directors and Executive Officers in the Merger” in this proxy statement/prospectus/information statement.
Certain of Oncternal’s and GTx’s executive officers and directors have also entered into voting agreements, pursuant to which certain directors, officers and stockholders of Oncternal and GTx, respectively, have agreed, solely in their capacity as stockholders of Oncternal and GTx, respectively, to vote all of their shares of Oncternal capital stock or GTx’s common stock in favor of the adoption or approval, respectively, of the Merger Agreement and the transactions contemplated therein in connection with the merger. The voting agreements are
21
discussed in greater detail in the section entitled “Agreements Related to the Merger—Voting Agreements and Written Consent” in this proxy statement/prospectus/information statement.
Risk Factors (see page 28)
Both GTx and Oncternal are subject to various risks associated with their businesses and respective assets. In addition, the merger poses a number of risks to each company and its respective stockholders, including the possibility that the merger may not be completed and the following risks:
| • | the exchange ratio is not adjustable based on the market price of GTx’s common stock, so the merger consideration at the closing may have a greater or lesser value than at the time the Merger Agreement was signed; |
| • | failure to complete the merger may result in either GTx or Oncternal paying a termination fee or expenses to the other and could harm the price of GTx’s common stock and the future business and operations of each company; |
| • | the merger is subject to approval by the GTx stockholders and Oncternal stockholders, including Oncternal’s largest stockholder, SPH USA, which has not delivered a voting agreement; |
| • | the merger may be completed even though material adverse changes may result solely from the announcement of the merger, changes in the operations of GTx and Oncternal operate that apply to all companies generally and other causes; |
| • | some of GTx’s and Oncternal’s respective officers and directors have interests that are different from or in addition to those considered by other stockholders of Oncternal and GTx and which may influence them to support or approve the merger; |
| • | the market price of the combined organization’s common stock may decline as a result of the merger; |
| • | GTx’s and Oncternal’s stockholders may not realize a benefit from the merger commensurate with the ownership dilution they will experience in connection with the merger; |
| • | during the pendency of the merger, GTx and Oncternal may not be able to enter into a business combination with another party under certain circumstances because of restrictions in the Merger Agreement, which could adversely affect their respective businesses; |
| • | certain provisions of the Merger Agreement may discourage third parties from submitting alternative takeover proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement; |
| • | because the lack of a public market for shares of Oncternal’s capital stock makes it difficult to evaluate the fairness of the merger, Oncternal’s stockholders may receive consideration in the merger that is less than the fair market value of the shares of Oncternal’s capital stock and/or GTx may pay more than the fair market value of the shares of Oncternal’s capital stock; and |
| • | if the conditions to the merger are not met, the merger will not occur. |
These risks and other risks are discussed in greater detail under the section entitled “Risk Factors” in this proxy statement/prospectus/information statement. GTx and Oncternal both encourage you to read and consider all of these risks carefully.
Regulatory Approvals (see page 176)
In the United States, GTx must comply with applicable federal and state securities laws and the rules and regulations of the Nasdaq Capital Market (“Nasdaq”) in connection with the issuance of shares of GTx’s
22
common stock and the filing of this proxy statement/prospectus/information statement with the SEC. As of the date hereof, the registration statement of which this proxy statement/prospectus/information statement is a part has not become effective.
Nasdaq Stock Market Listing (see page 180)
GTx has filed an initial listing application with Nasdaq pursuant to Nasdaq Stock Market LLC “reverse merger” rules. If such application is accepted, GTx anticipates that GTx’s common stock will be listed on Nasdaq following the closing of the merger under the trading symbol “ONCT.”
Anticipated Accounting Treatment (see page 180)
The merger will be recorded by GTx using the reverse asset acquisition method of accounting. For accounting purposes, Oncternal is considered to be acquiring GTx in the merger.
Appraisal Rights (see page 180)
Holders of GTx’s common stock are not entitled to appraisal rights in connection with the merger. Oncternal’s stockholders are entitled to appraisal rights in connection with the merger under Delaware law. For more information about such rights, see the provisions of Section 262 of the DGCL attached hereto asAnnex C, and the section entitled “The Merger—Appraisal Rights” in this proxy statement/prospectus/information statement.
Litigation Related to the Merger (see page 183)
Between April 10, 2019 and May 1, 2019, five purported stockholder class action lawsuits were filed, naming as defendants GTx, the GTx Board and, in certain cases, Oncternal and Merger Sub. Collectively, these lawsuits allege, among other things, violations of sections 14(a) and 20(a) of the Exchange Act, as well asRule 14a-9 promulgated thereunder, in connection with GTx’s filing of the registration statement of which this proxy statement/prospectus/information statement is a part with the U.S. Securities and Exchange Commission. As relief, these lawsuits each separately seek an order, among other things, enjoining the defendants from closing the proposed transaction or taking any steps to consummate the merger and/or awarding rescissory damages. GTx and the GTx Board believe that the above-described claims are without merit and intend to vigorously defend these actions. GTx cannot predict the outcome of or estimate the possible loss or range of loss from any of these matters. It is possible that additional, similar complaints may be filed or the complaints described above will be amended. If this occurs GTx does not intend to announce the filing of each additional, similar complaint or any amended complaint unless it contains allegations that are substantially distinct from those made in the pending actions described above.
Comparison of Stockholder Rights (see page 358)
Both GTx and Oncternal are incorporated under the laws of the State of Delaware and, accordingly, the rights of the stockholders of each are currently, and will continue to be, governed by the DGCL. If the merger is completed, Oncternal’s stockholders will become stockholders of GTx, and their rights will be governed by the DGCL, GTx’s amended and restated bylaws and, GTx’s restated certificate of incorporation, as amended by the amendments set forth inAnnex D andAnnex E, assuming Proposal Nos. 2 and 3 are approved. The rights of GTx’s stockholders contained in GTx’s restated certificate of incorporation and GTx’s amended and restated bylaws differ from the rights of Oncternal’s stockholders under Oncternal’s amended and restated certificate of incorporation and Oncternal’s bylaws, as more fully described under the section entitled “Comparison of Rights of Holders of GTx Stock and Oncternal Stock” in this proxy statement/prospectus/information statement.
23
SELECTED HISTORICAL AND UNAUDITED PRO FORMA CONDENSED
COMBINED FINANCIAL DATA
The following tables present summary historical financial data for GTx and Oncternal, summary unaudited pro forma condensed financial data for GTx and Oncternal, and comparative historical and unaudited pro forma per share data for GTx and Oncternal.
Selected Historical Financial Data of GTx
The selected financial data as of December 31, 2018 and 2017 and for the years ended December 31, 2018 and 2017 are derived from the GTx audited financial statements prepared using accounting principles generally accepted in the United States (“U.S. GAAP”), which are included in this proxy statement/prospectus/information statement. The financial data should be read in conjunction with “GTx Management’s Discussion and Analysis of Financial Condition and Results of Operations” and GTx’s financial statements and related notes appearing elsewhere in this proxy statement/prospectus/information statement. GTx’s historical results are not necessarily indicative of results to be expected in any future period.
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
Statements of Operations Data (in thousands, except per share data): | ||||||||
Operating expenses: | ||||||||
Research and development | $ | 29,669 | $ | 21,467 | ||||
General and administrative | 9,390 | 9,188 | ||||||
|
|
|
| |||||
Total operating expenses | 39,059 | 30,655 | ||||||
|
|
|
| |||||
Loss from operations | (39,059 | ) | (30,655 | ) | ||||
Other income, net | 641 | 216 | ||||||
|
|
|
| |||||
Net loss | $ | (38,418 | ) | $ | (30,439 | ) | ||
|
|
|
| |||||
Net loss per share, basic and diluted | $ | (1.65 | ) | $ | (1.75 | ) | ||
|
|
|
| |||||
Weighted-average shares of common stock outstanding, basic and diluted | 23,346,231 | 17,441,280 | ||||||
|
|
|
| |||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Balance Sheet Data (in thousands): | ||||||||
Cash, cash equivalents and short-term investments (a) | $ | 28,458 | $ | 43,899 | ||||
Working capital (b) | 25,998 | 38,102 | ||||||
Total assets | 31,321 | 46,236 | ||||||
Accumulated deficit | (600,055 | ) | (561,637 | ) | ||||
Total stockholders’ equity | 26,111 | 38,261 | ||||||
| (a) | Cash, cash equivalents and short-term investments for the year ended December 31, 2018 includes the net proceeds of $24.5 million received from the sale of common stock under ourAt-the-Market Equity Offering SM Sales Agreement with Stifel, Nicolaus & Company, Incorporated, in May 2018. Cash, cash equivalents and short-term investments for the year ended December 31, 2017 includes the net proceeds of $45.6 million received from the private placement of common stock and warrants completed in September 2017. |
| (b) | Working capital is defined as current assets less current liabilities. |
Selected Historical Consolidated Financial Data of Oncternal
The selected consolidated financial data as of December 31, 2018 and 2017 and for the years ended December 31, 2018 and 2017 are derived from Oncternal’s audited consolidated financial statements prepared
24
using U.S. GAAP, which are included in this proxy statement/prospectus/information statement. These historical results are not necessarily indicative of results to be expected in any future period. The selected consolidated financial data should be read in conjunction with Oncternal’s consolidated financial statements and the related notes to those statements included in this proxy statement/prospectus/information statement and “Oncternal Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
Selected Consolidated Statements of Operations Data (in thousands, except per share data): | ||||||||
Grant revenue | $ | 2,521 | $ | 1,674 | ||||
Operating expenses: | ||||||||
Research and development | 8,287 | 9,363 | ||||||
General and administrative | 1,820 | 2,871 | ||||||
|
|
|
| |||||
Total operating expenses | 10,107 | 12,234 | ||||||
|
|
|
| |||||
Loss from operations | (7,586 | ) | (10,560 | ) | ||||
Other income (expense): | ||||||||
Change in fair value of warrant liability | 713 | 124 | ||||||
Other income | 216 | — | ||||||
Interest income | 79 | 10 | ||||||
Interest expense | (1 | ) | (10 | ) | ||||
|
|
|
| |||||
Total other income (expense) | 1,007 | 124 | ||||||
|
|
|
| |||||
Net loss | $ | (6,579 | ) | $ | (10,436 | ) | ||
|
|
|
| |||||
Net loss per share, basic and diluted | $ | (0.13 | ) | $ | (0.23 | ) | ||
|
|
|
| |||||
Weighted average shares of common stock outstanding, basic and diluted | 48,930,354 | 45,914,263 | ||||||
|
|
|
| |||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Selected Consolidated Balance Sheet Data (in thousands): | ||||||||
Cash and cash equivalents | $ | 20,645 | $ | 10,188 | ||||
Working capital (a) | 16,879 | 6,558 | ||||||
Total assets | 21,962 | 11,069 | ||||||
Warrant liability | 674 | 1,387 | ||||||
Convertible preferred stock | 46,588 | 28,715 | ||||||
Total stockholders’ deficit | (29,631 | ) | (23,278 | ) | ||||
| (a) | Working capital is defined as current assets less current liabilities. |
Selected Unaudited Pro Forma Condensed Combined Financial Data of GTx and Oncternal
The following information does not give effect to the GTx Reverse Stock Split described in Proposal No. 2 discussed in this proxy statement/prospectus/information statement.
The following selected unaudited pro forma condensed combined financial data was prepared using the reverse asset acquisition method of accounting under U.S. GAAP. For accounting purposes, Oncternal is considered to be acquiring GTx and the merger is expected to be accounted for as an asset acquisition as the fair value of the acquired preclinical assets is deemed to be substantially concentrated in a group of similar assets that do not meet the definition of a business. The GTx and Oncternal unaudited pro forma combined balance sheet data assume
25
that the merger took place on December 31, 2018, and combines the GTx and Oncternal historical balance sheets at December 31, 2018. The GTx and Oncternal unaudited pro forma condensed combined statements of operations data assume that the merger took place as of January 1, 2018, and combines the historical results of GTx and Oncternal for the year ended December 31, 2018.
The selected unaudited pro forma condensed combined financial data are presented for illustrative purposes only and are not necessarily indicative of the combined financial position or results of operations of future periods or the results that actually would have been realized had the entities been a single entity during these periods. The selected unaudited pro forma condensed combined financial data as of and for the year ended December 31, 2018 are derived from the unaudited pro forma condensed combined financial information and should be read in conjunction with that information. For more information, please see the section entitled “Unaudited Pro Forma Condensed Combined Financial Information” in this proxy statement/prospectus/information statement.
The unaudited pro forma condensed combined financial information assumes that, at the Effective Time, each share of Oncternal common stock will be converted into the right to receive shares of GTx common stock such that, immediately following the Effective Time, GTx’s stockholders as of immediately prior to the Effective Time are expected to own approximately 22.5% of the outstanding common stock of GTx, and Oncternal’s stockholders as of immediately prior to the Effective Time are expected to own approximately 77.5% of the outstanding common stock of GTx, and is subject to adjustment to account for the occurrence of certain events discussed elsewhere in this proxy statement/prospectus/information statement. The ownership percentage to be held by GTx’s stockholders is subject to adjustment prior to closing of the merger, including a downward adjustment to the extent that GTx’s “Parent Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement, which adjusts based on the date of closing (and as a result, GTx stockholders could own less, and Oncternal stockholders could own more, of the combined organization), or an upward adjustment to the extent that Oncternal’s “Company Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement (and as a result, GTx stockholders could own more, and Oncternal stockholders could own less, of the combined organization). The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants.
| Year Ended December 31, 2018 | ||||
Selected Unaudited Pro Forma Condensed Combined Statement of Operations (in thousands, except per share data) | ||||
Grant revenue | $ | 2,521 | ||
Total operating expenses | 48,948 | |||
Net loss | (45,492 | ) | ||
Net loss per share, basic and diluted | (0.51 | ) | ||
| As of December 31, 2018 | ||||
Selected Unaudited Pro Forma Condensed Combined Balance Sheet Data (in thousands) | ||||
Cash, cash equivalents and short-term investments | $ | 39,320 | ||
Total assets | 43,500 | |||
Total liabilities | 9,323 | |||
Stockholders’ equity | 34,177 | |||
26
Comparative Historical and Unaudited Pro Forma Per Share Data
The information below reflects the historical net loss and book value per share of GTx common stock and the historical net loss and book value per share of Oncternal common stock in comparison with the unaudited pro forma net loss and book value per share after giving effect to the proposed merger of GTx with Oncternal on a pro forma basis. The unaudited pro forma net loss and book value per share does not give effect to the GTx Reverse Stock Split.
You should read the tables below in conjunction with the audited financial statements of GTx included in this proxy statement/prospectus/information statement and the audited financial statements of Oncternal included in this proxy statement/prospectus/information statement and the related notes and the unaudited pro forma condensed combined financial information and notes related to such financial statements included elsewhere in this proxy statement/prospectus/information statement.
| Year Ended December 31, 2018 | ||||
GTx Historical Per Share Data | ||||
Net loss per share, basic and diluted | $ | (1.65 | ) | |
Book value per share | $ | 1.12 | ||
Oncternal Historical Per Share Data | ||||
Net loss per share, basic and diluted | $ | (0.13 | ) | |
Book value per share | $ | (0.61 | ) | |
Combined Organization Per Share Data | ||||
Net loss per share, basic and diluted | $ | (0.51 | ) | |
Book value per share | $ | 0.38 | ||
27
The combined organization will be faced with a market environment that cannot be predicted and that involves significant risks, many of which will be beyond its control. In addition to the other information contained in this proxy statement/prospectus/information statement, you should carefully consider the material risks described below before deciding how to vote your shares of stock. In addition, you should read and consider the risks associated with GTx’s business because these risks may also affect the combined organization — these risks can be found under the heading “Risk Factors—Risks Related to GTx” in this proxy statement/prospectus/information statement and in GTx’s Annual Report on Form10-K, as updated by subsequent Quarterly Reports on Form10-Q, and other documents GTx has filed with the SEC and incorporated by reference into this proxy statement/prospectus/information statement. You should also read and consider the other information in this proxy statement/prospectus/information statement and the other documents incorporated by reference into this proxy statement/prospectus/information statement. Please see the section entitled “Where You Can Find More Information” in this proxy statement/prospectus/information statement.
Risks Related to the Merger
The exchange ratio set forth in the Merger Agreement is not adjustable based on the market price of GTx common stock, so the merger consideration at the closing of the merger may have a greater or lesser value than at the time the Merger Agreement was signed.
The Merger Agreement has set the exchange ratio for the Oncternal capital stock, and the exchange ratio is based on the outstanding capital stock of Oncternal and the outstanding common stock of GTx, in each case immediately prior to the closing of the merger as described under the heading “The Merger—Merger Consideration.” Applying the exchange ratio formula in the Merger Agreement, the former Oncternal stockholders immediately before the merger are expected to own approximately 77.5% of the outstanding capital stock of GTx immediately following the merger, and the stockholders of GTx immediately before the merger are expected to own approximately 22.5% of the outstanding capital stock of GTx immediately following the merger, subject to certain assumptions. Under certain circumstances further described in the Merger Agreement, however, these ownership percentages may be adjusted upward or downward based on cash levels of the respective companies at the closing of the merger, and as a result, either GTx’s stockholders or the Oncternal stockholders could own less of the combined company than expected.
Any changes in the market price of GTx’s common stock before the completion of the merger will not affect the number of shares of GTx’s common stock issuable to Oncternal’s stockholders pursuant to the Merger Agreement. Therefore, if before the completion of the merger the market price of GTx’s common stock declines from the market price on the date of the Merger Agreement, then Oncternal’s stockholders could receive merger consideration with substantially lower value than the value of such merger consideration on the date of the Merger Agreement. Similarly, if before the completion of the merger the market price of GTx’s common stock increases from the market price of GTx’s common stock on the date of the Merger Agreement, then Oncternal’s stockholders could receive merger consideration with substantially greater value than the value of such merger consideration on the date of the Merger Agreement. The Merger Agreement does not include a price-based termination right. Because the exchange ratio does not adjust as a result of changes in the market price of GTx’s common stock, for each one percentage point change in the market price of GTx’s common stock, there is a corresponding one percentage point rise or decline, respectively, in the value of the total merger consideration payable to Oncternal’s stockholders pursuant to the Merger Agreement.
Failure to complete the merger may result in either GTx or Oncternal paying a termination fee to the other party and could significantly harm the market price of GTx’s common stock and negatively affect the future business and operations of each company.
If the merger is not completed and the Merger Agreement is terminated under certain circumstances, GTx or Oncternal may be required to pay the other party a termination fee of up to $2.0 million. Even if a termination fee
28
is not payable in connection with a termination of the Merger Agreement, each of GTx and Oncternal will have incurred significant fees and expenses, which must be paid whether or not the merger is completed. Further, if the merger is not completed, it could significantly harm the market price of GTx’s common stock.
In addition, if the Merger Agreement is terminated and the board of directors of GTx or Oncternal determines to seek another business combination, there can be no assurance that either GTx or Oncternal will be able to find a partner and close an alternative transaction on terms that are as favorable or more favorable than the terms set forth in the Merger Agreement.
The merger is subject to approval of the Merger Agreement by GTx’s stockholders and the Oncternal stockholders. Failure to obtain these approvals would prevent the closing of the merger.
Before the merger can be completed, the stockholders of each of GTx and Oncternal must approve the Merger Agreement. Additionally, the Merger Agreement must be approved by multiple classes of Oncternal preferred stockholders, one class of which is held by a sole stockholder, SPH USA, which has not executed a voting agreement and has not otherwise agreed to vote in favor of the Merger Agreement. Although Oncternal expects to receive stockholder approval from SPH USA approximately two months after the date of the Merger Agreement, there can be no assurance that all of the necessary stockholder approvals will be obtained. Failure to obtain the required stockholder approvals, including as a result of SPH USA refusing to approve the transactions contemplated by the Merger Agreement, may result in a material delay in, or the abandonment of, the merger. Any delay in completing the merger may materially adversely affect the timing and benefits that are expected to be achieved from the merger.
The merger may be completed even though certain events occur prior to the closing that materially and adversely affect GTx or Oncternal.
The Merger Agreement provides that either GTx or Oncternal can refuse to complete the merger if there is a material adverse change affecting the other party between March 6, 2019, the date of the Original Merger Agreement, and the closing of the merger. However, certain types of changes do not permit either party to refuse to complete the merger, even if such change could be said to have a material adverse effect on GTx or Oncternal, including:
| • | general business, economic or political conditions or conditions generally affecting the industries in which Oncternal or GTx, as applicable, operates; |
| • | any natural disaster or any acts of war, armed hostilities or terrorism; |
| • | any changes in financial, banking or securities markets; |
| • | with respect to GTx, any change in the stock price or trading volume of GTx excluding any underlying effect that may have caused such change; |
| • | with respect to GTx, failure to meet internal or analysts’ expectations or projects or the results of operations; |
| • | any clinical trial programs or studies, including any adverse data, event or outcome arising out of or related to any such programs or studies; |
| • | any change in accounting requirements or principles or any change in applicable laws, rules, or regulations or the interpretation thereof; |
| • | any effect resulting from the announcement or pendency of the merger or any related transactions; and |
| • | the taking of any action, or the failure to take any action, by either GTx or Oncternal required to comply with the terms of the Merger Agreement. |
If adverse changes occur and GTx and Oncternal still complete the merger, the market price of the combined organization’s common stock may suffer. This in turn may reduce the value of the merger to the stockholders of GTx, Oncternal or both.
29
Some GTx and Oncternal officers and directors have interests in the merger that are different from the respective stockholders of GTx and Oncternal and that may influence them to support or approve the merger without regard to the interests of the respective stockholders of GTx and Oncternal.
Certain officers and directors of GTx and Oncternal participate in arrangements that provide them with interests in the merger that are different from the interests of the respective stockholders of GTx and Oncternal, including, among others, the continued service as an officer or director of the combined organization, severance benefits, the acceleration of stock option vesting, continued indemnification and the potential ability to sell an increased number of shares of common stock of the combined organization in accordance with Rule 144 under the Securities Act of 1933, as amended.
For example, GTx has entered into employment agreements with its executive officers that may result in the receipt by such executive officers of cash severance payments and other benefits in the event of a covered termination of employment of each executive officer’s employment. For more information concerning the treatment of GTx’s stock options in connection with the merger, see the section entitled “The Merger Agreement—Treatment of GTx’s Stock Awards and Warrants” in this proxy statement/prospectus/information statement. The closing of the merger will also result in the acceleration of vesting of options to purchase shares of GTx’s common stock held by GTx’s executive officers and directors, whether or not there is a covered termination of such officer’s employment. In addition, and for example, certain of Oncternal’s directors and executive officers have options, subject to vesting, to purchase shares of Oncternal’s common stock which, at the closing of the merger, shall be converted into and become options to purchase shares of GTx’s common stock, certain of Oncternal’s directors and executive officers are expected to become directors and executive officers of GTx upon the closing of the merger, and all of Oncternal’s directors and executive officers are entitled to certain indemnification and liability insurance coverage pursuant to the terms of the Merger Agreement. These interests, among others, may influence the officers and directors of GTx and Oncternal to support or approve the merger. For more information concerning the interests of GTx’s and Oncternal’s executive officers and directors, see the sections entitled “The Merger— Interests of GTx Directors and Executive Officers in the Merger” and “The Merger—Interests of Oncternal Directors and Executive Officers in the Merger.”
The market price of GTx’s common stock following the merger may decline as a result of the merger.
The market price of GTx’s common stock may decline as a result of the merger for a number of reasons including if:
| • | investors react negatively to the prospects of the combined organization’s product candidates, business and financial condition following the merger; |
| • | the effect of the merger on the combined organization’s business and prospects is not consistent with the expectations of financial or industry analysts; or |
| • | the combined organization does not achieve the perceived benefits of the merger as rapidly or to the extent anticipated by financial or industry analysts. |
GTx and Oncternal securityholders will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the combined organization following the closing of the merger as compared to their current ownership and voting interest in the respective companies.
After the completion of the merger, the current securityholders of GTx and Oncternal will own a smaller percentage of the combined organization than their ownership in their respective companies prior to the merger. Immediately after the merger, it is currently estimated that Oncternal securityholders will own approximately 77.5% of the common stock of the combined organization, and GTx securityholders, whose shares of GTx common stock will remain outstanding after the merger, will own approximately 22.5% of the common stock of the combined organization. These estimates are based on the anticipated exchange ratio and are subject to adjustment as provided in the Merger Agreement. See also the risk factor above titled, “The exchange ratio set
30
forth in the Merger Agreement is notadjustable based on the market price of GTx common stock, so the merger consideration at the closing of the merger may have a greater or lesser value than at the time the Merger Agreement was signed.”
In addition, the nine member board of directors of the company will initially include six individuals with prior affiliations with Oncternal and two individuals with prior affiliations with GTx. Consequently, securityholders of GTx and Oncternal will be able to exercise less influence over the management and policies of the combined organization following the closing of the merger than they currently exercise over the management and policies of their respective companies.
GTx and Oncternal stockholders may not realize a benefit from the merger commensurate with the ownership dilution they will experience in connection with the merger.
If the combined organization is unable to realize the strategic and financial benefits currently anticipated from the merger, GTx’s and Oncternal’s stockholders will have experienced substantial dilution of their ownership interests in their respective companies without receiving the expected commensurate benefit, or only receiving part of the commensurate benefit to the extent the combined organization is able to realize only part of the expected strategic and financial benefits currently anticipated from the merger.
The combined company will need to raise additional capital by issuing securities or debt or through licensing or other strategic arrangements, which may cause dilution to the combined company’s stockholders or restrict the combined company’s operations or impact its proprietary rights.
The combined company may be required to raise additional funds sooner than currently planned. In this regard, while the exchange ratio may be impacted by cash levels of the respective companies at the closing of the Merger, the Merger Agreement does not condition the completion of the merger upon either company holding a minimum amount of cash at the Effective Time. If either or both of GTx or Oncternal hold less cash at the time of the closing merger than the parties currently expect, the combined company will need to raise additional capital sooner than expected. Additional financing may not be available to the combined company when it needs it or may not be available on favorable terms. To the extent that the combined company raises additional capital by issuing equity securities, such an issuance may cause significant dilution to the combined company’s stockholders’ ownership and the terms of any new equity securities may have preferences over the combined company’s common stock. Any debt financing the combined company enters into may involve covenants that restrict its operations. These restrictive covenants may include limitations on additional borrowing and specific restrictions on the use of the combined company’s assets, as well as prohibitions on its ability to create liens, pay dividends, redeem its stock or make investments. In addition, if the combined company raises additional funds through licensing, partnering or other strategic arrangements, it may be necessary to relinquish rights to some of the combined company’s technologies or product candidates and proprietary rights, or grant licenses on terms that are not favorable to the combined company.
During the pendency of the merger, GTx and Oncternal may not be able to enter into a business combination with another party at a favorable price because of restrictions in the Merger Agreement, which could adversely affect their respective businesses.
Covenants in the Merger Agreement impede the ability of GTx and Oncternal to make acquisitions, subject to certain exceptions relating to fiduciary duties, as set forth below, or to complete other transactions that are not in the ordinary course of business pending completion of the merger. As a result, if the merger is not completed, the parties may be at a disadvantage to their competitors during such period. In addition, while the Merger Agreement is in effect, each party is generally prohibited from soliciting, initiating, encouraging or entering into certain extraordinary transactions, such as a merger, sale of assets, or other business combination outside the ordinary course of business with any third-party, subject to certain exceptions relating to fiduciary duties. Any such transactions could be favorable to such party’s stockholders.
31
Certain provisions of the Merger Agreement may discourage third parties from submitting alternative takeover proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement.
The terms of the Merger Agreement prohibit each of GTx and Oncternal from soliciting alternative takeover proposals or cooperating with persons making unsolicited takeover proposals, except in limited circumstances when such party’s board of directors determines in good faith that an unsolicited alternative takeover proposal is or is reasonably likely to lead to a superior takeover proposal and that failure to cooperate with the proponent of the proposal would be reasonably likely to be inconsistent with the applicable board’s fiduciary duties.
Because the lack of a public market for Oncternal’s capital stock makes it difficult to evaluate the value of Oncternal’s capital stock, the stockholders of Oncternal may receive shares of GTx’s common stock in the merger that have a value that is less than, or greater than, the fair market value of Oncternal’s capital stock.
The outstanding capital stock of Oncternal is privately held and is not traded in any public market. The lack of a public market makes it extremely difficult to determine the fair market value of Oncternal. Because the percentage of GTx’s common stock to be issued to Oncternal’s stockholders was determined based on negotiations between the parties, it is possible that the value of GTx’s common stock to be received by Oncternal’s stockholders will be less than the fair market value of Oncternal, or GTx may pay more than the aggregate fair market value for Oncternal.
If the conditions to the merger are not met, the merger will not occur.
Even if the merger is approved by the stockholders of GTx and Oncternal, specified conditions must be satisfied or waived to complete the merger. These conditions are set forth in the Merger Agreement and described in the section entitled “The Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement. GTx cannot assure you that all of the conditions will be satisfied or waived. If the conditions are not satisfied or waived, the merger will not occur or will be delayed, and GTx and Oncternal each may lose some or all of the intended benefits of the merger.
Five class action lawsuits have been filed and additional lawsuits may be filed against GTx, the GTx Board, Oncternal, and/or Merger Sub relating to the merger. An adverse ruling in any such lawsuit may prevent the merger from being consummated.
Between April 10, 2019 and May 1, 2019, five purported stockholder class action lawsuits were filed, naming as defendants GTx, the GTx Board and, in certain cases, Oncternal and Merger Sub. Collectively, these lawsuits allege, among other things, violations of sections 14(a) and 20(a) of the Exchange Act, as well as Rule 14a-9 promulgated thereunder, in connection with GTx’s filing of the registration statement of which this proxy statement/prospectus/information statement is a part with the U.S. Securities and Exchange Commission. As relief, these lawsuits each separately seek an order, among other things, enjoining the defendants from closing the proposed transaction or taking any steps to consummate the merger and/or awarding rescissory damages. GTx and the GTx Board believe that the above-described claims are without merit and intend to vigorously defend these actions. GTx cannot predict the outcome of or estimate the possible loss or range of loss from any of these matters. It is possible that additional, similar complaints may be filed or the complaints described above will be amended. If this occurs GTx does not intend to announce the filing of each additional, similar complaint or any amended complaint unless it contains allegations that are substantially distinct from those made in the pending actions described above. It is possible that these complaints will be further amended to make additional claims and/or that additional lawsuits making similar or additional claims relating to the merger will be brought.
One of the conditions to completion of the merger is the absence of any order being in effect that prohibits the consummation of the merger. Accordingly, if any of these plaintiffs or any future plaintiff is successful in obtaining an order enjoining consummation of the merger, then such order may prevent the merger from being completed, or from being completed within the expected time frame. See “The Merger—Litigation Related to the Merger” for more information about the lawsuits related to the merger that have been filed.
32
Risks Related to GTx
Risks Related to GTx’s Financial Condition and GTx’s Need for Additional Financing, and Additional Risks Related to the Merger
There is no assurance that the merger will be completed in a timely manner or at all. If the merger is not consummated, GTx’s business could suffer materially and GTx’s stock price could decline.
The closing of the merger is subject to the satisfaction or waiver of a number of closing conditions, as described above, including the required approvals by GTx and Oncternal stockholders (including stockholder approval from one of Oncternal’s significant stockholders, SPH USA, which holds all of the outstanding shares of one series of Oncternal’s preferred stock that must approve the transactions contemplated by the Merger Agreement) and other customary closing conditions. See the risk factors above titled, “The merger is subject to approval of the Merger Agreement by GTx’s stockholders and the Oncternal stockholders. Failure to obtain these approvals would prevent the closing of the merger” and “If the conditions to the merger are not met, the merger will not occur.” If the conditions are not satisfied or waived, including as a result of SPH USA refusing to approve the transactions contemplated by the Merger Agreement, the merger may be materially delayed or abandoned. If the merger is not consummated, GTx’s ongoing business may be adversely affected and, without realizing any of the benefits of having consummated the merger, GTx will be subject to a number of risks, including the following:
| • | GTx has incurred and expects to continue to incur significant expenses related to the merger even if the merger is not consummated; |
| • | GTx could be obligated to pay Oncternal a termination fee of up to $2.0 million under certain circumstances set forth in the Merger Agreement; |
| • | the market price of GTx’s common stock may decline to the extent that the current market price reflects a market assumption that the merger will be completed; and |
| • | matters relating to the merger have required and will continue to require substantial commitments of time and resources by GTx’s remaining management and employees, which could otherwise have been devoted to other opportunities that may have been beneficial to us. |
GTx also could be subject to further litigation related to any failure to consummate the merger or to perform its obligations under the Merger Agreement. If the merger is not consummated, these risks may materialize and may adversely affect its business, financial condition and the market price of GTx’s common stock.
If the merger is not completed, GTx may be unsuccessful in completing an alternative transaction on terms that are as favorable as the terms of the merger with Oncternal, or at all, and GTx may otherwise be unable to continue to operate its business. The GTx Board may decide to pursue a dissolution and liquidation of GTx. In such an event, the amount of cash available for distribution to its stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and contingent liabilities.
GTx’s assets currently consist primarily of cash, cash equivalents and short-term investments, GTx’s SARD and SARM assets, the remaining value, if any, of GTx’s deferred tax assets, GTx’s listing on the Nasdaq Capital Market and the Merger Agreement with Oncternal. While GTx has entered into the Merger Agreement with Oncternal, the closing of the merger may be delayed or may not occur at all and there can be no assurance that the merger will deliver the anticipated benefits GTx expects or enhance stockholder value. If GTx is unable to consummate the merger, the GTx Board may elect to pursue an alternative strategy, one of which may be a strategic transaction similar to the merger. Attempting to complete an alternative transaction like the merger will be costly and time consuming, and GTx can make no assurances that such an alternative transaction would occur at all. Alternatively, the GTx Board may elect to continue its operations to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an IND to enable the initiation of a first-in-human clinical trial, if any. However, GTx’s existing
33
capital resources may not be adequate to enable it to conduct and complete any IND-enabling studies of a SARD compound, particularly in light of the conflicting preclinical SARD data it has received to date and the additional preclinical research that will be required to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete any IND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. The GTx Board may also resume its efforts to seek potential collaborative, partnering or other strategic arrangements for GTx’s SARM or SARD assets, including a sale or other divestiture of one or both of these assets. The GTx Board could instead decide to abandon these efforts, including any further SARD development, and pursue a dissolution and liquidation of GTx’s company. In such an event, the amount of cash available for distribution to GTx’s stockholders will depend heavily on the timing of such decision, as with the passage of time the amount of cash available for distribution will be reduced as GTx continues to fund GTx’s operations, including its SARD preclinical development efforts. In addition, if the GTx Board were to approve and recommend, and GTx’s stockholders were to approve, a dissolution and liquidation of GTx’s company, GTx would be required under Delaware corporate law to pay GTx’s outstanding obligations, as well as to make reasonable provision for contingent and unknown obligations, prior to making any distributions in liquidation to GTx’s stockholders. GTx’s commitments and contingent liabilities may include severance obligations, regulatory and preclinical obligations, and fees and expenses related to the merger. As a result of this requirement, a portion of GTx’s assets may need to be reserved pending the resolution of such obligations. In addition, GTx may be subject to litigation or other claims related to a dissolution and liquidation. If a dissolution and liquidation were pursued, the GTx Board, in consultation with its advisors, would need to evaluate these matters and make a determination about a reasonable amount to reserve. Accordingly, holders of GTx’s common stock could lose all or a significant portion of their investment in the event of a liquidation, dissolution or winding up of the company.
The issuance of shares of GTx’s common stock to Oncternal stockholders in the merger will substantially dilute the voting power of GTx’s current stockholders.
If the merger is completed, each outstanding share of Oncternal common stock will be converted into the right to receive a number of shares of GTx’s common stock equal to the exchange ratio determined pursuant to the Merger Agreement. Immediately following the merger, the former Oncternal stockholders immediately before the merger are expected to own approximately 77.5% of GTx’s outstanding capital stock, and GTx’s stockholders immediately before the merger are expected to own approximately 22.5% of GTx’s outstanding capital stock, subject to certain assumptions. Accordingly, the issuance of shares of GTx’s common stock to Oncternal stockholders in the merger will reduce significantly the relative voting power of each share of GTx common stock held by GTx’s current stockholders. Consequently, GTx’s stockholders as a group will have significantly less influence over the management and policies of the combined company after the merger than prior to the merger. These estimates are based on the anticipated exchange ratio and are subject to adjustment as provided in the Merger Agreement. See also the risk factor above titled, “The exchange ratio set forth in the Merger Agreement is not adjustable based on the market price of GTx common stock, so the merger consideration at the closing of the merger may have a greater or lesser value than at the time the Merger Agreement was signed.”
GTx stockholders may not receive any payment on the CVRs and the CVRs may otherwise expire valueless.
If the merger is completed, GTx and certain other parties will enter into the CVR Agreement pursuant to which, for each share of GTx common stock held, GTx stockholders of record as of immediately prior to the Effective Time will receive one CVR entitling such holders to receive in the aggregate 75% of any net proceeds received during the15-year period after the closing of the merger from the grant, sale or transfer of rights to GTx’s SARD or SARM technology that occurs during the10-year period after the closing of the merger (or in the 11th year if based on a term sheet approved during the initial10-year period) and, if applicable, to receive royalties on the
34
sale of any SARD products or SARM products by the combined company during the15-year period after the closing of the merger. As further discussed in the section titled “The Merger–Background of the Merger,” GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2) in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. In connection with the receipt of the new preclinical data, and the Merger Agreement Amendment, GTx and Oncternal agreed upon the Amended Form of CVR Agreement to, among other things: (i) increase from 50% to 75% the portion of the net proceeds the CVR holders will be entitled to under the CVR Agreement, and (ii) provide that Oncternal (as successor in interest to GTx) will be obligated to use commercially reasonable efforts to either develop or divest GTx’s SARD technology, as the Oncternal Board shall determine in its sole discretion, and to divest its SARM technology, subject to certain limitations. Accordingly, Oncternal may decide, in its sole discretion, to abandon the development of GTx’s SARD technology following the merger and would then be obligated only to use commercially reasonable efforts to divest the SARD technology, subject to certain limitations. Likewise, Oncternal is obligated only to use commercially reasonable efforts to divest the SARM technology, subject to certain limitations, and in light of the results of the ASTRID trial, Oncternal has no current intent to develop the SARM technology. In addition, the CVRs will not be transferable, will not have any voting or dividend rights, and interest will not accrue on any amounts potentially payable on the CVRs. Accordingly, the right of any GTx stockholder to receive any future payment on or derive any value form the CVRs will be contingent solely upon the achievement of the foregoing events within the time periods specified in the CVR Agreement and if these events are not achieved for any reason within the time periods specified in the CVR Agreement, no payments will be made under the CVRs, and the CVRs will expire valueless. In addition, as set forth above, Oncternal (as successor in interest to GTx) has agreed only to use commercially reasonable efforts to either develop or divest the SARD technology, as the Oncternal Board shall determine in its sole discretion, and to divest its SARM technology, subject to certain limitations, which allows for the consideration of a variety of factors in determining the efforts that the combined company is required to use to develop or divest (in Oncternal’s sole discretion) GTx’s SARD technology and to divest GTx’s SARM technology, and it does not require the combined company to take all possible actions to continue efforts to develop or divest the SARD technology and to divest the SARM technology. Accordingly, under certain circumstances, the combined company may not be required to continue efforts to develop or divest the SARD technology or to divest the SARM technology, which would have an adverse effect on the value, if any, of the CVRs. Furthermore, the CVRs will be unsecured obligations of the combined company and all payments under the CVRs, all other obligations under the CVR Agreement and the CVRs and any rights or claims relating thereto will be subordinated in right of payment to the prior payment in full of all current or future senior obligations of the combined company. Finally, the U.S. federal income tax treatment of the CVRs is unclear. There is no legal authority directly addressing the U.S. federal income tax treatment of the receipt of, and payments on, the CVRs, and there can be no assurance that the Internal Revenue Service (the “IRS”), would not assert, or that a court would not sustain, a position that could result in adverse U.S. federal income tax consequences to holders of the CVRs.
35
GTx has incurred losses since inception, and GTx anticipates that it will incur continued losses for the foreseeable future.
As of December 31, 2018, GTx had an accumulated deficit of $600.1 million. GTx’s net loss for the year ended December 31, 2018 was $38.4 million and it expects to incur significant operating losses for the foreseeable future depending on the extent of its preclinical and any clinical development activities and, if any such development activities are successful, potentially seeking regulatory approval of any potential future product candidates. These losses, among other things, have had and will continue to have an adverse effect on GTx’s stockholders’ equity and working capital.
A substantial portion of GTx’s recent efforts and expenditures have been devoted to, and its prospects were substantially dependent upon, the development of enobosarm for the treatment of postmenopausal women with SUI. However, in September 2018, GTx announced that its placebo-controlled Phase 2 clinical trial of enobosarm to evaluate the change in frequency of daily SUI episodes following 12 weeks of treatment (the “ASTRID trial”), failed to achieve statistical significance on the primary endpoint of the proportion of patients with a greater than 50% reduction in incontinence episodes per day compared to placebo. The failure of the ASTRID trial to achieve its primary endpoint has significantly depressed GTx’s stock price and has severely harmed GTx’s ability to raise additional capital and to secure potential collaborative, partnering or other strategic arrangements for its SARM assets, and consequently, GTx’s prospects to continue as a going concern have been severely diminished. Following GTx’s review of the full data sets from the ASTRID trial, it determined to discontinue further development of enobosarm to treat SUI and to otherwise discontinue any further development of its SARM technology generally. GTx continues its efforts to seek potential collaborative, partnering or other strategic arrangements for its SARM assets, including a sale or other divestiture of its SARM assets. GTx has for many years actively pursued, but has been unable to successfully enter into, potential collaborative, partnering or other strategic arrangements for its SARM assets. If GTx is unable to ultimately enter into any such arrangements for its SARM assets, it will not receive any return on its investment in enobosarm and its other SARMs.
As a result of GTx’s decision to discontinue its SARM development efforts, GTx’s development activities have been focused solely on conducting preclinical studies to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an IND to enable the initiation ofa first-in-human clinical trial, if any. However, as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity, along with the resultant uncertainty with respect to the overall preclinical data for SARDs to date, additional preclinical research will be required in order to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies. GTx’s existing capital resources, however, may not be adequate to enable it to conduct and complete anyIND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete anyIND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. Accordingly, if, for any reason, the merger is not consummated, GTx may resume its efforts to seek additional funds through potential collaborative, partnering or other strategic arrangements to provide it with the necessary resources for the development of its SARD program. In addition, the preclinical evaluation of GTx’s SARD technology is at a very early stage and is subject to the substantial risk and probability of failure inherent in the development of early-stage programs.
Because of the numerous risks and uncertainties associated with developing and commercializing small molecule drugs, GTx is unable to predict the extent of any future losses or when GTx will become profitable, if at all. GTx has funded its operations primarily through public offerings and private placements of its securities, as well as
36
payments from its former collaborators. GTx also previously recognized product revenue from the sale of FARESTON, the rights to which it sold to a third-party in the third quarter of 2012. Currently, GTx has no ongoing collaborations for the development and commercialization of its product candidates, and as a result of the sale of its rights and certain assets related to FARESTON, GTx also currently has no sources of revenue.
If the merger is not completed and GTx is unable to raise sufficient additional funds for the development of its SARD program, whether through potential collaborative, partnering or other strategic arrangements or otherwise, or if GTx otherwise determines to discontinue the development of its SARD program, whether as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity or otherwise, GTx will likely determine to cease operations. Even if GTx is able to successfully complete additional preclinical research to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies and to raise additional funds to permit the continued development of its SARD program, if GTx and/or any potential collaborators are unable to develop and commercialize its SARDs or SARM technology, if development is further delayed or is eliminated, or if sales revenue from any SARD or partnered SARM products upon receiving marketing approval, if ever, is insufficient, GTx may never become profitable and it will not be successful.
If GTx does not successfully complete the merger, it will need to raise substantial additional capital and may be unable to raise the capital necessary to permit the continued development of its SARD program, which would force GTx to delay, reduce or eliminate its SARD program and would likely cause it to cease operations.
At December 31, 2018, GTx had cash, cash equivalents and short-term investments of $28.5 million. If the merger is not completed, based on GTx’s current business plan and spending assumptions as a standalone company, GTx estimates that its current cash, cash equivalents and short-term investments, together with interest thereon, will be sufficient to meet its projected operating requirements for at least the next 12 months. GTx has based its cash sufficiency estimates on its current business plan and its assumptions that may prove to be wrong. GTx could utilize its available capital resources sooner than it currently expects, and it could need additional funding sooner than currently anticipated.
GTx’s existing capital resources may not be adequate to enable it to conduct and complete any IND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete any IND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. If GTx is unable to raise sufficient additional funds for the development of its SARD program, whether through potential collaborative, partnering or other strategic arrangements or otherwise, or if GTx otherwise determines to discontinue the development of its SARD program, whether as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity or otherwise, GTx will likely determine to cease operations.
GTx’s future funding requirements will depend on many factors, including:
| • | its ability to successfully complete the merger; |
| • | the scope, rate of progress and cost of its preclinical and potential future clinical development programs; |
| • | the terms and timing of any potential collaborative, partnering and other strategic arrangements that GTx may establish; |
| • | the amount and timing of any licensing fees, milestone payments and royalty payments from potential collaborators, if any; |
37
| • | potential future preclinical studies and clinical trial results; |
| • | the cost and timing of regulatory filings and/or approvals to commercialize any potential future product candidates and any related restrictions, limitations, and/or warnings in the label of an approved product candidate; |
| • | the effect of competing technological and market developments; and |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights, and the cost of defending any other litigation claims. |
While GTx has been able to fund its operations to date, GTx has no ongoing collaborations for the development and commercialization of any product candidates and no source of revenue, nor does it expect to generate product revenue for the foreseeable future. GTx does not have any commitments for future external funding. In addition, although GTx has entered into anAt-the-Market Equity Offering SM Sales Agreement with Stifel, Nicolaus & Company, Incorporated (the “ATM Sales Agreement”), under which approximately $25.0 million of shares of its common stock remained available for sale at December 31, 2018, it is unlikely GTx could raise sufficient funds under the ATM Sales Agreement to permit it to initiate and complete any initial human clinical trials of a SARD compound, and given its currently-depressed stock price, the ATM Sales Agreement is not otherwise expected to be a practical source of liquidity for GTx at this time. Further, given GTx’s currently-depressed stock price, it is significantly limited in its ability to sell shares of common stock under the ATM Sales Agreement since the issuance and sale of GTx’s common stock under the ATM Sales Agreement, if it occurs, would be effected under a registration statement onForm S-3 that it filed with the Securities and Exchange Commission, and in accordance with the rules governing those registration statements, GTx generally can only sell shares of its common stock under that registration statement in an amount not to exceedone-third of its public float, which limitation for all practical purposes precludes its ability to obtain any meaningful funding through the ATM Sales Agreement at this time.
Until GTx can generate a sufficient amount of product revenue, which it may never do, it will need to finance future cash needs through potential collaborative, partnering or other strategic arrangements, as well as through public or private equity offerings or debt financings or a combination of the foregoing. If GTx is unable to raise additional funds, it will need to continue to reduce its expenditures in order to preserve its cash. Further cost-cutting measures that GTx may take may not be sufficient to enable it to meet its cash requirements, and they may negatively affect GTx’s business and its ability to derive any value from its SARD program. In any event, in order to further the development of its SARD program, if at all, GTx will need to raise substantial additional capital. GTx’s failure to do so would likely result in it determining to cease operations.
To the extent that GTx raises additional funds through potential collaborations, partnering or other strategic arrangements, it may be necessary to relinquish rights to some of its technologies or product candidates and intellectual property rights thereof, or grant licenses on terms that are not favorable to it, any of which could result in GTx’s stockholders having little or no continuing interest in its SARD program and/or SARM assets as stockholders or otherwise. To the extent GTx raises additional funds by issuing equity securities, GTx’s stockholders may experience significant dilution, particularly given its currently-depressed stock price, and debt financing, if available, may involve restrictive covenants. For example, GTx completed substantially dilutive private placements of its common stock and warrants in March 2014, November 2014 and September 2017, in addition to a registered direct offering of its common stock that it completed in October 2016 and the sale of GTx’s common stock pursuant to the ATM Sales Agreement. GTx’s stockholders will experience additional, perhaps substantial, dilution should GTx again raise additional funds by issuing equity securities. Any additional debt or equity financing that GTx raises may contain terms that are not favorable to it or its stockholders. GTx’s ability to raise additional funds and the terms upon which it is able to raise such funds have been severely harmed by the failure of the ASTRID trial to meet its primary endpoint and the resulting significant uncertainty regarding GTx’s prospects to continue as a going concern. If GTx is unable to complete the merger, its ability to raise additional funds and the terms upon which it is able to raise such funds may also be adversely affected by the
38
uncertainties regarding its financial condition, uncertainties with respect to the prospects for its early-stage SARD program as an effective treatment of men with CRPC, particularly in light of GTx’s recent receipt of new preclinical data that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity, the sufficiency of its capital resources, potential future management turnover, and volatility and instability in the global financial markets. As a result of these and other factors, there is no guarantee that sufficient additional funding will be available to GTx on acceptable terms, or at all.
GTx is substantially dependent on its remaining employees to facilitate the consummation of the merger.
GTx has substantially reduced its workforce since November 2018 and as of March 31, 2019, it had only 13 full-time employees. GTx’s ability to successfully complete the merger depends in large part on its ability to retain its remaining personnel. Despite GTx’s efforts to retain these employees, one or more may terminate their employment with GTx on short notice. The loss of the services of any of these employees could potentially harm GTx’s ability to consummate the merger, to run itsday-to-day business operations, as well as to fulfill its reporting obligations as a public company.
The pendency of the merger could have an adverse effect on the trading price of GTx’s common stock and its business, financial condition and prospects.
While there have been no significant adverse effects to date, the pendency of the merger could disrupt GTx’s business in many ways, including:
| • | the attention of its remaining management and employees may be directed toward the completion of the merger and related matters and may be diverted from GTx’sday-to-day business operations; and |
| • | third parties may seek to terminate or renegotiate their relationships with GTx as a result of the merger, whether pursuant to the terms of their existing agreements with GTx or otherwise. |
Should they occur, any of these matters could adversely affect the trading price of GTx’s common stock or harm its business, financial condition and prospects.
Risks Related to GTx’s Development Activities
GTx was substantially dependent on the success of enobosarm, and the recent failure of the ASTRID trial to meet its primary endpoint has severely diminished enobosarm’s prospects and GTx’s prospects to continue as a going concern. As GTx is now focused solely on its SARD program, its failure to obtain funding for and to advance the development of its SARD program would likely require it to cease operations.
A substantial portion of GTx’s recent efforts and expenditures has been devoted to, and its prospects were substantially dependent upon, the development of enobosarm for the treatment of postmenopausal women with SUI. However, in September 2018, GTx announced that the ASTRID trial failed to achieve statistical significance on the primary endpoint of a greater than 50% reduction in incontinence episodes per day compared to placebo. The failure of the ASTRID trial to achieve its primary endpoint has significantly depressed GTx’s stock price and has severely harmed its ability to raise additional capital and to secure potential collaborative, partnering or other strategic arrangements for its SARM assets, and consequently, GTx’s prospects to continue as a going concern have been severely diminished. Following GTx’s review of the full data sets from the ASTRID trial, GTx determined to discontinue further development of enobosarm to treat SUI and to otherwise discontinue any further development of its SARM technology generally. GTx continues its efforts to seek potential collaborative, partnering or other strategic arrangements for its SARM assets, including a sale or other divestiture of its SARM assets. GTx has for many years actively pursued, but has been unable to successfully enter into, potential collaborative, partnering or other strategic arrangements for its SARM assets. If GTx is unable to ultimately enter into any such arrangements for its SARM assets, it will not receive any return on its investment in enobosarm and its other SARMs.
39
As a result of GTx’s decision to discontinue its SARM development efforts, GTx’s development activities have been focused solely on conducting preclinical studies to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an IND to enable the initiation of a first-in-human clinical trial, if any. However, GTx recently received new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitrodata showing either partial agonist activity or no partial agonist activity, (2)in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. Accordingly, additional preclinical research would be required in order to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies. GTx’s existing capital resources, however, may not be adequate to enable it to conduct and complete any IND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete any IND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. In addition, the preclinical evaluation of GTx’s SARD technology is at a very early stage and is subject to the substantial risk and probability of failure inherent in the development of early-stage programs.
In any event, if the merger is not completed and GTx is unable to raise sufficient additional funds for the development of its SARD program, whether through potential collaborative, partnering or other strategic arrangements or otherwise, or if GTx otherwise determines to discontinue the development of its SARD program, whether as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity or otherwise, GTx will likely determine to cease operations.
GTx and any potential collaborators will not be able to commercialize any SARD or SARM product candidates if its preclinical studies do not produce successful results or if GTx or its SARD or SARM clinical trials do not adequately demonstrate safety and efficacy in humans.
Significant additional clinical development, financial resources and personnel would be required to obtain necessary regulatory approvals for any potential future SARD or SARM product candidates and to develop them into commercially viable products. Preclinical and clinical testing is expensive, can take many years to complete and has an uncertain outcome. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, andtop-line or interim results of a clinical trial do not necessarily predict final results. In this regard, from time to time, GTx has and may in the future publish or reporttop-line, interim or other preliminary data from its clinical trials, which data is based on a preliminary analysis of then-available efficacy and safety data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. GTx also makes assumptions, estimations, calculations and conclusions as part of its analyses of data, and it may not have received or had the opportunity to fully and carefully evaluate all data from the applicable trial. As a result, thetop-line results that GTx reports may differ from future results of the same studies, or different conclusions or considerations may
40
qualify such results, once additional data have been received and fully evaluated. Similarly, interim or other preliminary data from clinical trials that GTx may conduct may not be indicative of the final results of the trial and are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues and more patient data become available.Top-line, interim and other preliminary data also remain subject to audit and verification procedures that may result in the final data being materially different from suchtop-line, interim or other preliminary data GTx previously published. As a result,top-line, interim or preliminary data should be viewed with caution until the final data are available.
Typically, the failure rate for development candidates is high. If a product candidate fails at any stage of development, GTx will not have the anticipated revenues from that product candidate to fund its operations, and GTx will not receive any return on its investment in that product candidate. For example, in September 2018, GTx announced that the ASTRID trial failed to achieve statistical significance on the primary endpoint of the proportion of patients with a greater than 50% reduction in incontinence episodes per day compared to placebo. The failure of the ASTRID trial to achieve its primary endpoint has significantly depressed its stock price and has severely harmed GTx’s ability to raise additional capital and to secure potential collaborative, partnering or other strategic arrangements for its SARM assets, and consequently, GTx’s prospects to continue as a going concern have been severely diminished. Likewise, during the third quarter of 2017, GTx determined that there were insufficient patients achieving clinical benefit from enobosarm treatment to continue its Phase 2proof-of-concept clinical trial evaluating enobosarm in patients with advanced AR positive triple-negative breast cancer. Additionally, in the third quarter of 2017, GTx decided not to pursue additional clinical development of enobosarm to treat women with ER positive, AR positive advanced breast cancer after evaluating the breast cancer environment where the treatment paradigms are shifting to immunotherapies and/or combination therapies, along with the time and cost of conducting the necessary clinical trials for potential approval, even though GTx announced that its Phase 2 clinical trial of enobosarm in this indication achieved its primary endpoint in both the 9 mg and 18 mg cohorts of the clinical trial. Following GTx’s review of the full data sets from the ASTRID trial, GTx determined to discontinue further development of enobosarm to treat SUI and to otherwise discontinue any further development of its SARM technology generally. GTx continues its efforts to seek potential collaborative, partnering or other strategic arrangements for its SARM assets, including a sale or other divestiture of its SARM assets. GTx has for many years actively pursued, but has been unable to successfully enter into, potential collaborative, partnering or other strategic arrangements for its SARM assets. If GTx is unable to ultimately enter into any such arrangements for its SARM assets, GTx will not receive any return on its investment in enobosarm and its other SARMs.
In the first quarter of 2015, GTx entered into an exclusive worldwide license agreement with UTRF to develop its proprietary SARD technology and GTx is currently focused solely on the further development of its SARD program. GTx’s preclinical evaluation of its SARD technology is at an early stage and is subject to the substantial risk and probability of failure inherent in the development of early-stage programs. GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. If GTx’s research and preclinical development of its SARD program is unsuccessful, is discontinued and/or GTx is not able to obtain sufficient funding to advance the development of its SARD program, GTx will likely cease operations.
Significant delays in preclinical studies and clinical testing could materially impact GTx’s product development costs. For example, as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity along with the resultant uncertainty with respect to the overall preclinical data for SARDs to date, additional preclinical research would be required in order to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies. Such additional preclinical research will increase GTx’s costs, and GTx cannot be certain that its existing capital resources will be adequate to enable it to conduct and complete any IND-enabling studies of a SARD compound. In any event, GTx does not know whether its potential future preclinical studies and clinical trials will need to be modified or will be completed on schedule, if at all. GTx or any potential collaborators may experience numerous unforeseen
41
and/or adverse events during, or as a result of, preclinical testing and the clinical trial process that could delay or prevent GTx or its potential collaborators’ ability to commercialize any product candidates, including:
| • | regulators or institutional review boards may not authorize GTx or any potential collaborators to commence a clinical trial or conduct a clinical trial at a prospective trial site, or GTx or any potential collaborators may experience substantial delays in obtaining these authorizations; |
| • | GTx or any potential collaborators may be delayed in reaching, or may fail to reach, agreement on acceptable terms with prospective clinical research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | additional preclinical studies or clinical trials may produce negative, inconclusive or further conflicting results, which may require GTx or any potential collaborators to conduct additional preclinical or clinical testing, such as the additional preclinical research that will be required to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies, or to abandon projects that GTx expected to be promising; |
| • | even if preclinical or clinical trial results are positive, the United States Food and Drug Administration (the “FDA”), or foreign regulatory authorities could nonetheless require GTx to conduct unanticipated additional preclinical development or clinical trials; |
| • | patient registration or enrollment in clinical trials may be slower than GTx anticipates resulting in significant delays, additional costs and/or study terminations; |
| • | GTx or any potential collaborators may suspend or terminate clinical trials if the participating patients are being exposed to unacceptable health risks; |
| • | regulators or institutional review boards may suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; |
| • | GTx’s product candidates may not have the desired effects or may include undesirable side effects; and |
| • | changes in regulatory requirements, policies and guidelines. |
If any of these events were to continue to occur in the future and, as a result, GTx or any potential collaborators have significant delays in or termination of potential future clinical trials, GTx’s costs could increase and its ability to generate revenue could be impaired, which would materially and adversely impact its business, financial condition and growth prospects.
If GTx or any potential collaborators observe serious or other adverse events during the time any potential future product candidates are in development or after GTx’s products are approved and on the market, GTx or any potential collaborators may be required to perform lengthy additional clinical trials, may be required to cease further development of such product candidates, may be denied regulatory approval of such products, may be forced to change the labeling of such products or may be required to withdraw any such products from the market, any of which would hinder or preclude GTx’s ability to generate revenues.
In GTx’s Phase 2 clinical trials for enobosarm for the treatment of muscle wasting in patients with cancer and healthy older males and postmenopausal females, GTx observed mild elevations of hepatic enzymes, which in certain circumstances may lead to liver failure, in a few patients in both the placebo and enobosarm treated groups. Reductions in high-density lipoproteins (“HDL”), have also been observed in subjects treated with enobosarm. Lower levels of HDL could lead to increased risk of adverse cardiovascular events. Mild transient elevations in liver enzymes that were within normal limits were observed in GTx’s Phase 2proof-of-concept clinical trial of enobosarm to treat postmenopausal women with SUI, except for one patient with levels greater than 1.5 times the upper limit of normal which returned to normal following her12-week treatment period. Reductions in total cholesterol,low-density lipoproteins (“LDL”), HDL and triglycerides were also observed.
42
Results of the ASTRID trial in postmenopausal women with SUI indicated that enobosarm was generally safe and well tolerated, and reported adverse events were generally mild to moderate in intensity and similar across all treatment groups. Mild transient elevations in hepatic enzymes and changes in lipid profile were dose dependent, and consistent with results seen in previous trials. In addition, in GTx’s Phase 2proof-of-concept clinical trial evaluating enobosarm in a 9 mg daily dose for the treatment of patients with ER positive and AR positive metastatic breast cancer, bone pain of the chest cage, a serious adverse event (“SAE”), was assessed as possibly related to enobosarm. Although doses up to 30 mg have been evaluated in short duration studies, the 3 mg dose that was the subject of the ASTRID trial and higher enobosarm doses that may potentially be tested by potential future collaborators in later stage longer duration trials, if any, may increase the risk or incidence of known potential side effects of SARMs, including elevations in hepatic enzymes and further reductions in HDL, in addition to the emergence of side effects that have not been seen to date.
If the incidence of serious or other adverse events related to enobosarm or any other SARD or SARM product candidates increases in number or severity, if a regulatory authority believes that these or other events constitute an adverse effect caused by the drug, or if other effects are identified during clinical trials that GTx or any potential collaborators may conduct in the future or after any potential future product candidates are approved and marketed:
| • | GTx or any potential collaborators may be required to conduct additional preclinical or clinical trials, make changes in the labeling of any such approved products, reformulate any such products, or implement changes to or obtain new approvals of its contractors’ manufacturing facilities; |
| • | regulatory authorities may be unwilling to approve GTx’s product candidates or may withdraw approval of its products; |
| • | GTx may experience a significant drop in the sales of the affected products; |
| • | GTx’s reputation in the marketplace may suffer; and |
| • | GTx may become the target of lawsuits, including class action suits. |
Any of these events could prevent approval or harm adoption and sales of the affected product candidates or products, or could substantially increase the costs and expenses of commercializing and marketing any such products.
Risks Related to GTx’s Dependence on Third Parties
If the merger is not completed and GTx does not establish collaborative, partnering or other strategic arrangements for its SARD program and SARM assets or otherwise raise substantial additional capital, GTx will likely determine to cease operations.
GTx’s current strategy is dependent on its ability to secure potential collaborative, partnering or other strategic arrangements with other pharmaceutical and biotechnology companies to assist GTx in furthering development and potential commercialization of any SARD and SARM product candidates, and to otherwise obtain funding for such activities. For example, GTx is currently focused solely on the further development of its SARD program; however, GTx’s existing capital resources may not be adequate to enable it to conduct and complete any IND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete any IND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. Accordingly, if, for any reason, the merger is not consummated, GTx may resume its efforts to seek additional funds through potential collaborative, partnering or other strategic
43
arrangements to provide it with the necessary resources for the development of its SARD program. GTx faces significant competition in seeking such arrangements, and such arrangements are complex and time consuming to negotiate and document. In any event, GTx may not be successful in entering into new collaborative, partnering or other strategic arrangements with third parties for the further development of its SARD program (or GTx’s SARD assets) on acceptable terms, or at all. In this regard, GTx has for many years actively pursued, but has been unable to successfully enter into, potential collaborative, partnering or other strategic arrangements for its SARM assets and GTx likewise has not been successful to date in entering into potential collaborative, partnering or other strategic arrangements for its SARD program. Moreover, as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity, which data conflicts with certain other preclinical data previously received by GTx, this new preclinical data along with the resultant uncertainty with respect to the overall preclinical data for SARDs to date may negatively impact or preclude altogether GTx’s prospects for entering into potential collaborative, partnering or other strategic arrangements for its SARD program. In addition, GTx is unable to predict when, if ever, it will enter into any potential collaborative, partnering or other such strategic arrangements because of the numerous risks and uncertainties associated with establishing such arrangements, and GTx has otherwise been unsuccessful, for many years, in its efforts to establish such arrangements. In any event, if the merger is not completed and GTx is unable to raise sufficient additional funds for the development of its SARD program, whether through potential collaborative, partnering or other strategic arrangements or otherwise, or if GTx otherwise determines to discontinue the development of GTx’s SARD program, it will likely determine to cease operations. In addition, because GTx has discontinued its SARM development efforts, if it is unable to ultimately enter into any potential collaborative, partnering or other such strategic arrangements for its SARM assets, GTx will not receive any return on its investment in enobosarm and its other SARMs.
Any collaborative arrangements that GTx establishes in the future may not be successful or GTx may otherwise not realize the anticipated benefits from these collaborations. In addition, any future collaborative arrangements may place the development and commercialization of GTx’s product candidates outside its control, may require GTx to relinquish important rights or may otherwise be on terms unfavorable to GTx.
GTx has in the past established, and, if the merger is not completed, GTx intends to continue to seek to establish, partnering, collaborative and similar strategic arrangements with third parties to develop and commercialize any potential future product candidates, and these collaborations may not be successful or GTx may otherwise not realize the anticipated benefits from these collaborations. For example, in March 2011, GTx and Ipsen Biopharm Limited, or Ipsen, mutually agreed to terminate the collaboration for the development and commercialization of GTx’s toremifene-based product candidate. As of the date of this report, GTx has no ongoing collaborations for the development and commercialization of any product candidate. GTx may not be able to locate third-party collaborators to develop and market any product candidates, and GTx lacks the necessary financial resources to develop any product candidates alone.
Dependence on collaborative arrangements subjects GTx to a number of risks, including:
| • | GTx may not be able to control the amount and timing of resources that its potential collaborators may devote to GTx’s product candidates; |
| • | potential collaborations may experience financial difficulties or changes in business focus; |
| • | GTx may be required to relinquish important rights such as marketing and distribution rights; |
| • | should a collaborator fail to develop or commercialize one of GTx’s compounds or product candidates, GTx may not receive any future milestone payments and will not receive any royalties for the compound or product candidate; |
| • | business combinations or significant changes in a collaborator’s business strategy may also adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement; |
44
| • | under certain circumstances, a collaborator could move forward with a competing product candidate developed either independently or in collaboration with others, including GTx’s competitors; and |
| • | collaborative arrangements are often terminated or allowed to expire, which could delay the development and may increase the cost of developing GTx’s product candidates. |
If third parties do not manufacture GTx’s clinical and commercial drug supplies in sufficient quantities, in the required timeframe, at an acceptable cost, and with appropriate quality control, clinical development and commercialization of any potential future product candidates would be delayed.
GTx does not currently own or operate manufacturing facilities, and it relies, and expects to continue to rely, on third parties for the production of clinical and commercial quantities of any product candidates. GTx’s current and anticipated future dependence upon others for the manufacture of its product candidates may adversely affect GTx’s future profit margins, if any, and GTx’s ability to develop product candidates and commercialize any product candidates on a timely and competitive basis.
GTx relies and expects to continue to rely on third-party vendors for drug substance and drug product manufacturing, including drug substance for SARDs used in its current and potential future preclinical studies. If the contract manufacturers that GTx is currently utilizing to meet its supply needs for SARD compounds or any potential future SARD product candidates prove incapable or unwilling to continue to meet its supply needs, GTx could experience a delay in conducting any additional preclinical or clinical trials of SARD compounds or any potential future SARD product candidates. GTx may not be able to maintain or renew its existing or any other third-party manufacturing arrangements on acceptable terms, if at all. If GTx’s suppliers fail to meet its requirements for its product candidates for any reason, GTx would be required to obtain alternate suppliers. Any inability to obtain alternate suppliers, including an inability to obtain approval from the FDA of an alternate supplier, would delay or prevent the clinical development and commercialization of any potential future product candidates.
Use of third-party manufacturers may increase the risk that GTx will not have adequate drug supplies for preclinical, clinical and commercial use.
Reliance on third-party manufacturers entails risks, to which GTx would not be subject if GTx manufactured its product candidates itself, including:
| • | reliance on the third-party for regulatory compliance and quality assurance; |
| • | the possible breach of the manufacturing agreement by the third-party because of factors beyond GTx’s control; |
| • | the possible termination ornon-renewal of the agreement by the third-party, based on its own business priorities, at a time that is costly or inconvenient for GTx; and |
| • | drug product supplies not meeting the requisite requirements for clinical trial use. |
If GTx is not able to obtain adequate drug supplies, including SARD compounds, it will be more difficult for GTx to develop any product candidates and compete effectively. GTx’s potential future product candidates and any products that GTx and/or its potential collaborators may develop may compete with other product candidates and products for access to manufacturing facilities.
GTx’s present or future manufacturing partners may not be able to comply withFDA-mandated current Good Manufacturing Practice regulations, other FDA regulatory requirements or similar regulatory requirements outside the United States. Failure of GTx’s third-party manufacturers or GTx to comply with applicable regulations could result in sanctions being imposed on GTx, including fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of its product candidates, delays, suspension or withdrawal
45
of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of GTx’s product candidates.
If third parties on whom GTx rely do not perform as contractually required or expected, GTx may not be able to obtain regulatory approval for or successfully commercialize any potential future product candidates.
GTx does not have the ability to independently conduct clinical trials for its product candidates, and GTx must rely on third parties, such as CROs, medical institutions, clinical investigators and contract laboratories to conduct its clinical trials. In addition, GTx relies on third parties to assist with its preclinical development of product candidates. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to GTx’s clinical protocols or regulatory requirements or for other reasons, GTx’s preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and GTx may not be able to obtain regulatory approval for or successfully commercialize any potential future product candidates.
Risks Related to GTx’s Intellectual Property
If GTx loses its licenses from UTRF, GTx may be unable to continue its business and, if the merger is completed, the CVR holders may not receive any proceeds from GTx’s SARD or SARM technology.
GTx has licensed intellectual property rights and technology from UTRF used in substantially all of its business. GTx’s license agreements with UTRF, under which GTx was granted rights to enobosarm and other SARM compounds, and to SARD compounds and, for both, to methods of use thereof, may be terminated by UTRF if GTx is in breach of its obligations under, or fails to perform any terms of, the relevant agreement and fails to cure that breach. If one or both of these agreements are terminated, then GTx may lose its rights to utilize enobosarm and other SARM compounds and/or SARD compounds and the intellectual property covered by those agreements to market, distribute and sell licensed products, which may prevent GTx from continuing its business and would likely cause GTx to cease operations altogether.
In addition, if the merger is completed and the combined company breaches its obligations under one or both license agreements, resulting in a termination of the relevant agreement, then the combined company may not be able to develop or divest the SARD technology or divest the SARM technology. As a result, the combined company may not receive proceeds from the transfer of rights to the applicable technologies or the sale of SARD or SARM technology. If the combined company does not receive any such proceeds, then the CVR holders would not receive any payments on the CVRs.
If some or all of GTx’s or GTx’s licensor’s patents expire or are invalidated or are found to be unenforceable, or if some or all of GTx’s patent applications do not result in issued patents or result in patents with narrow, overbroad, or unenforceable claims, or claims that are not supported in regard to written description or enablement by the specification, or if GTx is prevented from asserting that the claims of an issued patent cover a product of a third-party, GTx may be subject to competition from third parties with products in the same class of products as GTx’s product candidates or products with the same active pharmaceutical ingredients as GTx’s product candidates, including in those jurisdictions in which GTx has no patent protection.
GTx’s commercial success, if any, will depend in part on obtaining and maintaining patent and trade secret protection for any product candidates that it may develop, as well as the methods for treating patients in the product indications using these product candidates. GTx will be able to protect any potential future product candidates and the methods for treating patients in the product indications using these product candidates from unauthorized use by third parties only to the extent that GTx or its exclusive licensor owns or controls such valid and enforceable patents or trade secrets.
46
Even if any potential future product candidates and/or the methods for treating patients for prescribed indications using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. GTx’s and GTx’s licensor’s ability to obtain patents can be highly uncertain and involve complex and in some cases unsettled legal issues and factual questions. Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries provide different degrees of protection against the use of a patented invention by others. Therefore, if the issuance to GTx or GTx’s licensor, in a given country, of a patent covering an invention is not followed by the issuance, in other countries, of patents covering the same invention, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, GTx’s ability to protect its intellectual property in those countries may be limited. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may materially diminish the value of GTx’s intellectual property or narrow the scope of its patent protection.
GTx may be subject to competition from third parties with products in the same class of products as its product candidates or products with the same active pharmaceutical ingredients as GTx’s product candidates in those jurisdictions in which GTx has no patent protection. Even if patents are issued to GTx or its licensor regarding its product candidates or methods of using them, those patents can be challenged by GTx’s competitors who can argue such patents are invalid or unenforceable, lack of utility, lack sufficient written description or enablement, or that the claims of the issued patents should be limited or narrowly construed. Patents also will not protect GTx’s product candidates if competitors devise ways of making or using these product candidates without legally infringing GTx’s patents. The Federal Food, Drug, and Cosmetic Act and FDA regulations and policies create a regulatory environment that encourages companies to challenge branded drug patents or to createnon-infringing versions of a patented product in order to facilitate the approval of abbreviated new drug applications for generic substitutes. These same types of incentives encourage competitors to submit new drug applications that rely on literature and clinical data not prepared for or by the drug sponsor, providing another less burdensome pathway to approval.
GTx also relies on trade secrets to protect its technology, especially where GTx does not believe that patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. GTx’s employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose GTx’s confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Enforcing a claim that a third-party illegally obtained and is using its trade secrets is expensive and time-consuming, and the outcome is unpredictable. Moreover, GTx’s competitors may independently develop equivalent knowledge, methods andknow-how. Failure to obtain or maintain trade secret protection could adversely affect GTx’s competitive business position.
If GTx infringes intellectual property rights of third parties, it may increase GTx’s costs or prevent it from being able to commercialize its product candidates.
There is a risk that GTx is infringing the proprietary rights of third parties because numerous United States and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields that are the focus of GTx’s development and manufacturing efforts. Others might have been the first to make the inventions covered by each of GTx’s or its licensor’s pending patent applications and issued patents and/or might have been the first to file patent applications for these inventions. In addition, because patent applications take many months to publish and patent applications can take many years to issue, there may be currently pending applications, unknown to GTx or its licensor, which may later result in issued patents that cover the production, manufacture, synthesis, commercialization, formulation or use of GTx’s product candidates. In addition, the production, manufacture, synthesis, commercialization, formulation or use of GTx’s product candidates may infringe existing patents of which GTx is not aware. Defending itself against third-party claims, including
47
litigation in particular, would be costly and time consuming and would divert management’s attention from GTx’s business, which could lead to delays in its development or commercialization efforts. If third parties are successful in their claims, GTx might have to pay substantial damages or take other actions that are adverse to its business.
As a result of intellectual property infringement claims, or to avoid potential claims, GTx might:
| • | be prohibited from selling or licensing any product that GTx and/or any potential collaborators may develop unless the patent holder licenses the patent to GTx, which the patent holder is not required to do; |
| • | be required to pay substantial royalties or other amounts, or grant a cross license to GTx’s patents to another patent holder; or |
| • | be required to redesign the formulation of a product candidate so that it does not infringe, which may not be possible or could require substantial funds and time. |
Risks Related to Regulatory Approval
If GTx or any potential collaborators are not able to obtain required regulatory approvals, GTx or such collaborators will not be able to commercialize its product candidates, and GTx’s ability to generate revenue will be materially impaired.
The activities associated with the development and commercialization of product candidates are subject to comprehensive regulation by the FDA, other regulatory agencies in the United States and by comparable authorities in other countries, including the European Medicines Agency (“EMA”). Failure to obtain regulatory approval for a product candidate will prevent GTx or any potential collaborator from commercializing the product candidate. GTx has not received regulatory approval to market any product candidate in any jurisdiction, and it does not expect to obtain FDA, EMA or any other regulatory approvals to market any potential future product candidates for the foreseeable future, if at all. The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon the type, complexity and novelty of the product candidates involved.
Changes in the regulatory approval policy during the development period, changes in or the enactment of additional regulations or statutes, or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an application. Even if the FDA or the EMA approves a product candidate, the approval may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, marketing and/or production of such product, and may impose ongoing requirements for post-approval studies, including additional research and development and clinical trials. Any FDA approval may also impose Risk Evaluation Mitigation Strategy, or REMS, on a product if the FDA believes there is a reason to monitor the safety of the drug in the market place. REMS may include requirements for additional training for health care professionals, safety communication efforts and limits on channels of distribution, among other things. The sponsor would be required to evaluate and monitor the various REMS activities and adjust them if need be. The FDA and EMA also may impose various civil or criminal sanctions for failure to comply with regulatory requirements, including withdrawal of product approval.
Furthermore, the approval procedure and the time required to obtain approval varies among countries and can involve additional testing beyond that required by the FDA. Approval by one regulatory authority does not ensure approval by regulatory authorities in other jurisdictions. Failure to obtain approval in one jurisdiction may negatively impact GTx’s ability to obtain approval elsewhere.
The FDA, the EMA and other foreign regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that GTx’s data is insufficient for approval and require additional preclinical, clinical or other studies, including Phase 4 clinical studies. For example, in October 2009,
48
GTx received a Complete Response Letter from the FDA regarding its new drug application, or NDA, for toremifene 80 mg to reduce fractures in men with prostate cancer on androgen deprivation therapy notifying GTx that the FDA would not approve its NDA as a result of certain clinical deficiencies identified in the Complete Response Letter. GTx has since discontinued its toremifene 80 mg development program, as well as other toremifene-based products. Although GTx evaluated the potential submission of a marketing authorization application (“MAA”), to the EMA seeking marketing approval of enobosarm 3 mg in the European Union, or EU, for the prevention and treatment of muscle wasting in patients with advanced NSCLC, based on input from the Medicines and Healthcare Products Regulatory Agency (“MHRA”), GTx determined that the data from the POWER trials was not sufficient to support the filing and approval of a MAA without confirmatory data from another Phase 3 clinical trial of enobosarm 3 mg. As a result of this input, GTx elected not to submit a MAA in the absence of such confirmatory data. In addition, since data from the two POWER trials failed to meet the primary statistical criterionpre-specified for theco-primary endpoints of lean body mass and physical function, GTx was unable to file with the FDA a NDA for enobosarm 3 mg for the prevention and treatment of muscle wasting in patients with advanced NSCLC.
In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit, or prevent regulatory approval of a product candidate. Even if GTx submits an application to the FDA, the EMA and other foreign regulatory authorities for marketing approval of a product candidate, it may not result in any marketing approvals.
GTx does not expect to receive regulatory approval for the commercial sale of any product candidates for the foreseeable future, if at all. The inability to obtain approval from the FDA, the EMA and other foreign regulatory authorities for its product candidates would prevent GTx or any potential collaborators from commercializing these product candidates in the United States, the EU, or other countries. See the section entitled “GTx Business—Government Regulation” of this proxy statement/prospectus/information statement for additional information regarding risks associated with marketing approval, as well as risks related to potential post-approval requirements.
Risks Related to Commercialization
The commercial success of any products that GTx and/or any potential collaborators may develop and for which GTx may obtain regulatory approval will depend upon the market and the degree of market acceptance among physicians, patients, health care payors and the medical community.
Any products that GTx and/or any potential collaborators may develop may not gain market acceptance for its stated indication among physicians, patients, health care payors and the medical community despite regulatory approval. If these products do not achieve an adequate level of acceptance, GTx may not generate material product revenues or receive royalties to the extent GTx currently anticipates, and GTx may not become profitable. The degree of market acceptance of its product candidates, if approved for commercial sale, will depend on a number of factors, including:
| • | efficacy and safety results in clinical trials; |
| • | the prevalence and severity of any side effects; |
| • | potential advantages over alternative treatments; |
| • | whether the products GTx commercializes become and/or remain a preferred course of treatment; |
| • | the ability to offer GTx’s product candidates for sale at competitive prices; |
| • | relative convenience and ease of administration compared to alternative treatment; |
| • | the strength of marketing and distribution support; and |
| • | sufficient third-party coverage or reimbursement. |
49
If GTx is unable to establish sales and marketing capabilities or establish and maintain agreements with third parties to market and sell its product candidates, GTx may be unable to generate product revenue from such candidates.
GTx has limited experience as a company in the sales, marketing and distribution of pharmaceutical products. In the event one of GTx’s potential future product candidates is approved, GTx will need to establish sales and marketing capabilities or establish and maintain agreements with third parties to market and sell any such product candidates. Either of these options would be expensive and time-consuming. GTx may be unable to build its own sales and marketing capabilities, and there are risks involved with entering into arrangements with third parties to perform these services, which could delay the commercialization of any of its product candidates if approved for commercial sale. In addition, to the extent that GTx enters into arrangements with third parties to perform sales, marketing and distribution services, its product revenues are likely to be lower than if GTx markets and sells any products that it develop itself.
If GTx and/or any potential collaborators are unable to obtain reimbursement or experience a reduction in reimbursement from third-party payors for products GTx sells, its revenues and prospects for profitability will suffer.
Sales of products developed by GTx and/or any potential collaborators are dependent on the availability and extent of reimbursement from third-party payors, both governmental and private. Changes in the coverage and/or reimbursement policies of these third-party payors that reduce reimbursements for any products that GTx and/or any potential collaborators may develop and sell could negatively impact its future operating and financial results.
Medicare coverage and reimbursement of prescription drugs exists under Medicare Part D for oral drug products capable of self-administration by patients. GTx’s oral drug product candidates would likely be covered by Medicare Part D (if covered by Medicare at all). In March 2010, the United States Congress enacted the Healthcare Reform Act, which, among other initiatives, implemented cost containment and other measures that could adversely affect revenues from sales of product candidates, including an increase in the drug rebates that manufacturers must pay under Medicaid for brand name prescription drugs and extension of these rebates to Medicaid managed care and a requirement that manufacturers provide a 50% discount on the negotiated price of Medicare Part D brand name drugs utilized by Medicare Part D beneficiaries during the coverage gap (theso-called “donut hole”) (which discount has subsequently been increased to 70% in 2019).
The provisions of the Healthcare Reform Act have been subject to judicial and Congressional challenges, as well as efforts by the Trump administration to modify certain requirements of the Healthcare Reform Act by executive branch order. For example, on January 20, 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the Healthcare Reform Act to waive, defer, grant exemptions from, or delay the implementation of any provision of the Healthcare Reform Act that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. On October 12, 2017, President Trump signed another Executive Order directing certain federal agencies to propose regulations or guidelines to provide small businesses with greater opportunities to form association health plans, expand the availability of short-term, limited duration insurance, and allow employees to make use of certain employer-paid health benefits, called health reimbursement arrangements, to pay for health insurance that does not meet all Healthcare Reform Act requirements. In addition, citing legal guidance from the U.S. Department of Justice, the U.S. Department of Health and Human Services (“HHS”), concluded that cost-sharing reduction, or CSR, payments to insurance companies required under the Healthcare Reform Act had not received necessary appropriations from Congress. President Trump subsequently discontinued these payments. The loss of the CSR payments is expected to increase premiums on certain policies issued by qualified health plans under the Healthcare Reform Act. Certain administrative actions have been subject to judicial challenge. In Congress, there have been a number of legislative initiatives to modify, repeal and/or replace portions of the Healthcare Reform Act. Tax reform legislation enacted at the end of
50
2017 eliminated the tax penalty for individuals who do not maintain sufficient health insurance coverage beginning in 2019. The Bipartisan Budget Act of 2018 contained various provisions that affect coverage and reimbursement of drugs, including an increase in the discount that manufacturers of Medicare Part D brand name drugs must provide to Medicare Part D beneficiaries during the coverage gap from 50% to 70% starting in 2019. Congress may consider other legislation to modify, repeal and/or replace certain elements of the Healthcare Reform Act. In December 2018, a federal district court judge, in a challenge brought by a number of state attorneys general, found the Healthcare Reform Act unconstitutional in its entirety because, once Congress repealed the individual mandate provision, there was no longer a basis to rely on Congressional taxing authority to support enactment of the law. Pending appeals, which could take some time, the Healthcare Reform Act is still operational in all respects. GTx continues to evaluate the effect that the Healthcare Reform Act and its possible repeal, replacement or modification may have on GTx’s business. Such legislation and other healthcare reform measures that may be adopted in the future could have a material adverse effect on GTx’s industry generally and on its ability to successfully commercialize its product candidates, if approved.
Economic pressure on state budgets may result in states increasingly seeking to achieve budget savings through mechanisms that limit coverage or payment for drugs. State Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization for use of drugs where supplemental rebates are not provided. Private health insurers and managed care plans are likely to continue challenging the prices charged for medical products and services, and many of these third-party payors may limit reimbursement for newly-approved health care products. In particular, third-party payors may limit the indications for which they will reimburse patients who use any products that GTx and/or any potential collaborators may develop or sell. These cost-control initiatives could decrease the price GTx might establish for products that it or any potential collaborators may develop or sell, which would result in lower product revenues or royalties payable to GTx.
Similar cost containment initiatives exist in countries outside of the United States, particularly in the countries of the EU, where the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can extend well beyond the receipt of regulatory marketing approval for a product and may require GTx or any potential collaborators to conduct a clinical trial that compares the cost effectiveness of GTx’s product candidates or products to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in GTx’s or a potential collaborators’ commercialization efforts. Third-party payors are challenging the prices charged for medical products and services, and many third-party payors limit reimbursement for newly-approved health care products. Recently budgetary pressures in many EU countries are also causing governments to consider or implement various cost-containment measures, such as price freezes, increased price cuts and rebates. If budget pressures continue, governments may implement additional cost containment measures. Cost-control initiatives could decrease the price GTx might establish for products that GTx or any potential collaborators may develop or sell, which would result in lower product revenues or royalties payable to it.
Another development that could affect the pricing of drugs would be if the Secretary of HHS allowed drug reimportation into the United States. The Medicare Prescription Drug, Improvement and Modernization Act of 2003 gives discretion to the Secretary of Health and Human Services to allow drug reimportation into the United States under some circumstances from foreign countries, including from countries where the drugs are sold at a lower price than in the United States. If the circumstances were met and the Secretary exercised the discretion to allow for the direct reimportation of drugs, it could decrease the price GTx or any potential collaborators receive for any products that GTx and/or any potential collaborators may develop, negatively affecting GTx’s revenues and prospects for profitability.
Health care reform measures could hinder or prevent GTx’s product candidates’ commercial success.
Among policy makers and payors in the United States and elsewhere, there is significant interest in health care reform, as evidenced by the initial enactment of, as well as the efforts to repeal, replace and/or modify the
51
Healthcare Reform Act in the United States. Federal and state legislatures within the United States and foreign governments will likely continue to consider other changes to existing health care legislation. These changes adopted by governments may adversely impact GTx’s business by lowering the price of health care products in the United States and elsewhere. For example, there has been increasing administrative, legislative and enforcement interest in the United States with respect to drug pricing practices. There have been several U.S. Congressional inquiries and legislative and administrative initiatives at the federal and state levels intended to, among other things, bring more transparency to drug pricing and modify government program reimbursement for drugs. GTx cannot predict what health care reform initiatives may be adopted in the future. Further federal, state and foreign legislative and regulatory developments are likely, and GTx expects ongoing initiatives to increase pressure on drug pricing, which could decrease the price it might establish for products that it or any potential collaborators may develop or sell, which would result in lower product revenues or royalties payable to GTx.
GTx operates in a highly regulated industry and new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to health care availability, method of delivery or payment for health care products and services, or sales, marketing and pricing practices could negatively impact its business, operations and financial condition.
If product liability lawsuits are brought against GTx, GTx may incur substantial liabilities and may be required to limit commercialization of any products that it may develop.
GTx faces an inherent risk of product liability exposure related to its prior commercial sales of FARESTON and the testing of its product candidates in human clinical trials, and GTx will face an even greater risk if GTx commercially sells any product that it may develop. If GTx cannot successfully defend itself against claims that its product candidates or products caused injuries, GTx will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for any product candidates or products; |
| • | injury to GTx’s reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs to defend the related litigation; |
| • | substantial monetary awards to trial participants or patients; |
| • | loss of revenue; and |
| • | the inability to commercialize any products for which GTx obtains or holds marketing approvals. |
GTx has product liability insurance that covers its clinical trials and any commercial products up to a $25 million annual aggregate limit. Insurance coverage is increasingly expensive. GTx may not be able to maintain insurance coverage at a reasonable cost, and GTx may not be able to obtain insurance coverage that will be adequate to satisfy any liability that may arise.
If GTx’s competitors are better able to develop and market products than any products that GTx and/or any potential collaborators may develop, GTx’s commercial opportunity will be reduced or eliminated.
GTx faces competition from commercial pharmaceutical and biotechnology enterprises, as well as from academic institutions, government agencies and private and public research institutions. GTx’s commercial opportunities will be reduced or eliminated if its competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that GTx and/or any potential collaborators may develop. Competition could result in reduced sales and pricing pressure on its product candidates, if approved, which in turn would reduce GTx’s ability to generate meaningful revenue and have a negative impact on its results of operations. In addition, significant delays in the development of GTx’s product candidates could allow its competitors to bring products to market before GTx and impair any ability to commercialize any potential future product candidates.
52
Various products are currently marketed or usedoff-label for some of the diseases and conditions that GTx are targeting in its pipeline, and a number of companies are or may be developing new treatments. These product uses, as well as promotional efforts by competitors and/or clinical trial results of competitive products, could significantly diminish any ability to market and sell any products that GTx and/or any potential collaborators may develop.
GTx believes SARDs may have the potential to provide compounds that can degrade or antagonize multiple forms of the AR thereby inhibiting tumor growth in patients with CRPC, including those patients who do not respond or are resistant to current therapies. Drugs in development having potentially similar approaches to removing the AR by degradation include Arvinas Inc.’sARV-110, which is a chimera with an AR binding moiety on one end and an E3 ligase recruiting element on the other that has recently entered Phase 1 development for the treatment of advanced prostate cancer, and Androscience Corporation’s androgen receptor degrader enhancer,ASC-J9, which is currently in development for acne and alopecia with the potential for development as a treatment for prostate cancer. Additionally, Essa Pharma Inc. recently completed a Phase 1 study withEPI-506, an AR antagonist that targets theN-terminal domain of the AR, and has plans to develop a second generation agent. C4 Therapeutics, Inc. is developing degronimids as means to degrade the AR through the ligand binding domain associated degradation. CellCentric is developing therapies that target the histone methyltransferase enzyme to lower AR levels, and recently initiated a clinical trial with CCS1477 in prostate cancer. Oric Pharmaceuticals is targeting the glucocorticoid receptor as a means to impact men that have CRPC, and has a lead candidateORIC-101 in preclinical testing. In addition to this specific potential mechanistic competition, there are various products approved or under clinical development in the broader space of treating men with advanced prostate cancer who have metastatic CRPC which may compete with GTx’s proposed initial clinical objective for its SARD compounds. Pfizer and Astellas Pharma market XTANDI® (enzalutamide), an oral androgen receptor antagonist, for the treatment of metastatic CRPC in men previously treated with docetaxel as well as those that have not yet received chemotherapy. XTANDI® received FDA approval in July 2018 for the treatment of men withnon-metastatic CRPC. Zytiga®, sold by Johnson & Johnson, has been approved for the treatment of metastatic CRPC and metastatic high-risk castration-sensitive prostate cancer. Johnson & Johnson also received FDA approval for a second generation anti-androgen ERLEADA (apalutamide) for the treatment of men withnon-metastatic castrate-resistant prostate cancer. Bayer HealthCare and Orion Corporation recently announced that the primary endpoint of increased metastatic free survival was met in a Phase 3 study of darolutamide(ODM-201) in men with CRPC without metastases and with a rising PSA. Another target in prostate cancer that is being pursued by several companies is bromodomain inhibition. Zenith Epigenetics, Gilead Sciences Inc., CellCentric, Incyte Corporation and GlaxoSmithKline are among the companies that are evaluating BET inhibitors inPhase 1-2 trials.
With respect to SARMs, there are other SARM product candidates in development that may compete with enobosarm and any future SARM product candidates, if approved for commercial sale. For example, Viking Therapeutic’s VK5211 recently reported positive results from a Phase 2 study for patients recovering fromnon-elective hip fracture surgery. Radius Health Inc.’s RAD140 is currently being evaluated in a Phase 1 study in postmenopausal women with hormone-receptor positive locally advanced or metastatic breast cancer. GlaxoSmithKline is conducting a Phase 1 study to assess the effect of GSK2881078 on physical strength and function after 13 weeks of treatment in patients with chronic obstructive pulmonary disease (“COPD”), and muscle weakness. OPKO Health’s OPK88004 is enrolling in a dose ranging study to improve symptoms of benign prostatic hyperplasia (“BPH”) by reducing prostate size and, on the basis of data from a previous trial in 350 men, increase muscle mass and bone strength and decrease body fat.
Many of GTx’s competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than GTx does. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with GTx in recruiting and retaining qualified scientific and management personnel, establishing
53
clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to GTx’s programs or advantageous to its business.
Risks Related to Employees, Growth and Other Aspects of GTx’s Operations
GTx’s internal computer and information technology systems, or those of its CROs or other contractors or consultants, may fail or suffer security breaches, or could otherwise face serious disruptions, which could result in a material disruption of GTx’s product development efforts and could result in significant financial, legal, regulatory, business and reputational harm to GTx.
Despite the implementation of security measures, GTx’s internal computer and information technology systems and those of its CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, and telecommunication and electrical failures. Such events could cause interruptions of its operations. For instance, the loss of preclinical data or data from potential future clinical trials involving its product candidates, if any, could result in delays in GTx’s development and regulatory filing efforts and significantly increase its costs. In addition, while all information technology operations are inherently vulnerable to inadvertent or intentional security breaches, incidents, attacks and exposures, the size, complexity, accessibility and distributed nature of GTx’s information technology systems, and the large amounts of sensitive information stored on those systems, make such systems potentially vulnerable to unintentional or malicious, internal and external attacks on GTx’s technology environment. Potential vulnerabilities can be exploited from inadvertent or intentional actions of its employees, third-party vendors, business partners, or by malicious third parties. Attacks of this nature are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives (including, but not limited to, industrial espionage) and expertise, including organized criminal groups, “hacktivists,” nation states and others. To the extent that any disruption or security breach or incident were to result in a loss of, or damage to, GTx’s data, or inappropriate disclosure of confidential, proprietary or protected health information, GTx could be subject to significant legal, financial and regulatory exposure and suffer reputational harm, and the development of its product candidates could be delayed. In addition, security breaches and other inappropriate access events can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above. Moreover, the prevalent use of mobile devices to access confidential information increases the risk of security breaches. While GTx has implemented security measures to protect its information technology systems and infrastructure, there can be no assurance that such measures will prevent service interruptions or security breaches that could adversely affect its business. In addition, GTx’s information technology and other internal infrastructure systems, including corporate firewalls, servers, leased lines and connection to the Internet, face the risk of systemic failure that could disrupt its operations. A significant disruption in the availability of its information technology and other internal infrastructure systems could cause delays in its research and development work and could otherwise adversely affect GTx’s business. In addition, failure to maintain effective internal accounting controls related to security breaches and cybersecurity in general could impact GTx’s ability to produce timely and accurate financial statements and subject GTx to regulatory scrutiny.
If GTx fails to keep senior management and personnel, GTx may be unable to continue its business operations.
GTx’s success depends on its continued ability to retain and motivate highly qualified management and personnel. Significant competition exists for qualified personnel in the biotechnology field. GTx may incur greater costs than anticipated, or may not be successful, in retaining or motivating its existing personnel. If GTx is not able to keep senior management and personnel, its ability to continue its business operations could be impaired, and the value of stockholders’ investment would be adversely impacted. All of GTx’s employees areat-will employees and can terminate their employment at any time.
To conserve its cash resources, GTx has substantially reduced its workforce since November 2018 and has ceased its SARM development activities and all other operations except forday-to-day business operations,
54
completing ongoing SARD preclinical studies and those activities necessary to complete the merger. As of March 31, 2019, GTx had only 13 full-time employees. Accordingly, GTx has been and is continuing operating with a shortage of resources and may not be able to effectively conduct its operations with this limited number of employees. In addition, GTx’s ability to successfully complete the merger depends in large part on its ability to retain its remaining personnel. Despite its efforts to retain these employees, one or more may terminate their employment with GTx on short notice. The loss of the services of any of these employees could potentially harm GTx’s ability to consummate the merger, to run itsday-to-day business operations, as well as to fulfill its reporting obligations as a public company.
If the merger is not completed and GTx is able to raise sufficient additional funds necessary to pursue the continued development of its SARD program, GTx will need to hire a substantial number of additional employees. Any inability to manage future growth could harm GTx’s ability to develop and commercialize any potential future product candidates, increase its costs and adversely impact its ability to compete effectively.
As of March 31, 2019, GTx had only 13 full-time employees. If the merger is not completed and GTx is able to raise sufficient additional funds necessary to pursue the continued development of its SARD program, GTx will need to hire experienced personnel to continue to develop its SARD program and to develop and commercialize any potential future product candidates, and GTx will need to expand the number of its managerial, operational, financial and other employees to support that growth. Significant competition exists for qualified Future growth, if any, will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. GTx’s future financial performance and its ability to develop and commercialize any potential future product candidates and to compete effectively will depend, in part, on its ability to manage any future growth effectively.
Management transition creates uncertainties and could harm GTx’s business.
GTx has in the past, and may again in the future, experience significant changes in executive leadership. Changes to company strategy, which can often times occur with the appointment of new executives, can create uncertainty, may negatively impact GTx’s ability to execute quickly and effectively, and may ultimately be unsuccessful. In addition, executive leadership transition periods are often difficult as the new executives gain detailed knowledge of GTx’s operations, and friction can result from changes in strategy and management style. Management transition inherently causes some loss of institutional knowledge, which can negatively affect strategy and execution. Until GTx integrates new personnel, and unless they are able to succeed in their positions, GTx may be unable to successfully manage and grow its business, and its results of operations and financial condition could suffer as a result. In any event, changes in GTx’s organization as a result of executive management transition may have a disruptive impact on its ability to implement its strategy and could have a material adverse effect on its business, financial condition and results of operations.
Risks Related to GTx’s Common Stock
The market price of GTx’s common stock has been volatile and may continue to be volatile in the future. This volatility may cause GTx’s stock price and the value of stockholders’ investment to decline.
The market prices for securities of biotechnology companies, including those of GTx, have been highly volatile and may continue to be so in the future. In this regard, the market price for GTx’s common stock has varied between a high of $25.60 on September 13, 2018, and a low of $0.74 on December 24, 2018, in the12-month period ended December 31, 2018. The market price of GTx’s common stock is likely to continue to be volatile and subject to significant price and volume fluctuations. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of GTx’s common stock:
| • | GTx’s ability to consummate the transactions contemplated by the Merger Agreement, including the merger; |
55
| • | GTx’s ability to execute on its SARD development program, including its ability to conduct and completeIND-enabling studies and potentially advance one of its SARD compounds into afirst-in-human clinical trial; |
| • | GTx’s ability to raise sufficient additional funds necessary for the continued development of its SARD program, whether through potential collaborative, partnering or other strategic arrangements or otherwise; |
| • | GTx’s ability to realize any value from its SARM assets, particularly in light of its decision to discontinue the development of enobosarm and its SARM technology generally; |
| • | the terms and timing of any future collaborative, licensing or other strategic arrangements that GTx may establish; |
| • | uncertainties created by GTx’s potential future management turnover; |
| • | GTx’s inability to comply with the minimum listing requirements of the Nasdaq Stock Market LLC; |
| • | the timing of achievement of, or failure to achieve, GTx’s and any potential collaborators’ clinical, regulatory and other milestones, such as the commencement of clinical development, the completion of a clinical trial or the receipt of regulatory approval; |
| • | reports of unacceptable incidences of adverse events observed in any future clinical trials of any product candidates that GTx and/or any potential collaborators may develop; |
| • | announcement of FDA approval ornon-approval of any potential future product candidates or delays in or adverse events during the FDA review process; |
| • | actions taken by regulatory agencies with respect to any potential future product candidates or GTx’s potential future clinical trials, if any, including regulatory actions requiring or leading to a delay or stoppage of any clinical trials; |
| • | introductions or announcements of technological innovations or new products by GTx, its potential collaborators, or its competitors, and the timing of these introductions or announcements; |
| • | the commercial success of any product approved by the FDA or its foreign counterparts; |
| • | market conditions for equity investments in general, or the biotechnology or pharmaceutical industries in particular; |
| • | regulatory developments in the United States and foreign countries; |
| • | changes in the structure or reimbursement policies of health care payment systems; |
| • | if GTx’s patents covering its products candidates expire or are invalidated or are found to be unenforceable, or if some or all of its patent applications do not result in issued patents or result in patents with narrow, overbroad, or unenforceable claims; |
| • | competition from third parties with products in the same class of products as any potential future product candidates or products with the same active pharmaceutical ingredients as those product candidates; |
| • | any intellectual property infringement lawsuit involving GTx; |
| • | actual or anticipated fluctuations in GTx’s results of operations; |
| • | changes in financial estimates or recommendations by securities analysts; |
| • | hedging or arbitrage trading activity that may develop regarding GTx’s common stock; |
| • | sales of GTx common stock and other securities by it; |
| • | sales of GTx common stock by its executive officers, directors and significant stockholders; |
56
| • | the low trading volume of GTx common stock; |
| • | changes in accounting principles; and |
| • | additional losses of any of GTx’s key management personnel. |
In addition, the stock markets in general, and the markets for biotechnology and pharmaceutical stocks in particular, have experienced significant volatility that has often been unrelated to the operating performance of particular companies. For example, negative publicity regarding drug pricing and price increases by pharmaceutical companies has negatively impacted, and may continue to negatively impact, the markets for biotechnology and pharmaceutical stocks. Likewise, as a result of significant changes in U.S. social, political, regulatory and economic conditions or in laws and policies governing foreign trade and health care spending and delivery, including the possible repeal and/or replacement of all or portions of the Healthcare Reform Act or changes in tariffs and other restrictions on free trade stemming from the Trump Administration and foreign government policies, the financial markets could experience significant volatility that could also negatively impact the markets for biotechnology and pharmaceutical stocks. These broad market fluctuations may adversely affect the trading price of GTx’s common stock.
In the past, class action litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. Any such litigation brought against GTx could result in substantial costs, which would hurt its financial condition and results of operations and divert management’s attention and resources, which could result in delays of GTx’s development efforts.
If GTx fails to meet continued listing standards of the Nasdaq Stock Market LLC, its common stock may be delisted. Delisting could adversely affect the liquidity of GTx’s common stock and the market price of its common stock could decrease, and GTx’s ability to obtain sufficient additional capital to fund its operations would be substantially impaired.
GTx’s common stock is currently listed on the Nasdaq Capital Market. The Nasdaq Stock Market LLC, or Nasdaq, has minimum requirements that a company must meet in order to remain listed on the Nasdaq Capital Market. These requirements include maintaining a minimum closing bid price of $1.00 per share (the “Bid Price Requirement”), and the closing bid price of GTx’s common stock has in the past been well below $1.00 per share. In this regard, on December 5, 2016, GTx effectedone-for-ten reverse stock split of its outstanding common stock (the “2016 Reverse Stock Split”), the primary purpose of which was to enable GTx to regain compliance with the Bid Price Requirement, which compliance was regained on December 20, 2016. However, the closing bid price of GTx’s common stock has recently been well below $1.00 per share, and there can be no assurance that GTx will meet the Bid Price Requirement, or any other Nasdaq continued listing requirement, in the future. If GTx fails to meet these requirements, including the Bid Price Requirement and requirements to maintain minimum levels of stockholders’ equity or market values of its common stock, Nasdaq may notify GTx that it has failed to meet the minimum listing requirements and initiate the delisting process.
If GTx’s common stock is delisted, GTx would expect its common stock to be traded in theover-the-counter market, which could adversely affect the liquidity of its common stock. Additionally, GTx could face significant material adverse consequences, including:
| • | a limited availability of market quotations for its common stock; |
| • | a reduced amount of news and analyst coverage for GTx; |
| • | a decreased ability to issue additional securities and a concomitant substantial impairment in GTx’s ability to obtain sufficient additional capital to fund its operations and to continue as a going concern; |
| • | reduced liquidity for its stockholders; |
| • | potential loss of confidence by employees and potential future partners or collaborators; and |
| • | loss of institutional investor interest and fewer business development opportunities. |
57
GTx’s executive officers, directors and largest stockholders have the ability to control all matters submitted to stockholders for approval.
Based solely on the most recent Schedules 13G and 13D filed with the SEC and reports filed with the SEC under Section 16 of the Exchange Act, GTx’s executive officers, directors and holders of 5% or more of its outstanding common stock, including their affiliated or associated entities, held approximately 53.5% of GTx’s outstanding common stock, and GTx’s executive officers and directors alone, including their affiliated or associated entities, held approximately 30.0% of GTx’s outstanding common stock as well as warrants to purchase up to an additional 3.2 million shares of common stock. As a result, these stockholders, acting together, have the ability to control all matters requiring approval by its stockholders, including the election of directors, the approval of the issuance of shares of GTx’s common stock pursuant to the Merger Agreement, and the approval of potential alternative mergers or other business combination transactions. The interests of this group of stockholders may not always coincide with GTx’s interests or the interests of other stockholders.
GTx’s ability to use its net operating loss carryforwards and certain other tax attributes may be limited.
GTx has a significant amount of federal and state net operating loss (“NOL”) carryforwards. In this regard, as of December 31, 2018, GTx had net federal operating loss carryforwards of approximately $472.1 million. The federal operating loss carryforwards originating prior to 2018 will expire from 2019 to 2037 if not utilized, and state operating loss carryforwards of approximately $411.4 million will expire from 2019 to 2038 if not utilized. GTx’s ability to use its federal and state NOL carryforwards to offset potential future taxable income and related income taxes that would otherwise be due is dependent upon its generation of future taxable income before the expiration dates of the NOL carryforwards, and GTx cannot predict with certainty when, or whether, it will generate sufficient taxable income to use all of its NOL carryforwards. On December 22, 2017, President Trump signed into law U.S. federal income tax legislation, informally titled the Tax Cuts and Jobs Act (the “Tax Act”). Under the Tax Act, federal NOLs incurred in taxable years ending after December 31, 2017 may be carried forward indefinitely, but the deductibility of NOLs generated in taxable years beginning after December 31, 2017 is limited. It is uncertain if and to what extent various states will conform to the Tax Act. In addition, under Sections 382 and 383 of the Code, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use itspre-change NOL carryforwards and otherpre-change tax attributes (such as research tax credits) to offset its post-change taxable income or taxes may be limited. GTx completed a study through December 31, 2016 to determine whether any Section 382 limitations exist and, as a result of this study and GTx’s analysis of subsequent ownership changes, GTx does not believe that any Section 382 limitations exist through December 31, 2018, though GTx has not yet conducted anin-depth analysis since the last study. Section 382 of the Code is an extremely complex provision with respect to which there are many uncertainties, however and GTx has not established whether the IRS agrees with its determination. In any event, GTx’s 2016 and 2017 equity offerings, its past and potential future issuances of common stock pursuant to the ATM Sales Agreement, other future equity offerings and/or changes in its stock ownership, some of which are outside of its control, could in the future result in an ownership change and an accompanying Section 382 limitation. In addition, the merger, if consummated, will constitute an ownership change (within the meaning Section 382 of the Code) which could eliminate or otherwise substantially limit GTx’s federal and state NOL carryforwards. Therefore, utilization of a portion of GTx’s domestic NOL and tax credit carryforwards will likely be limited in future periods and a portion of the carryforwards could expire before being available to reduce future income tax liabilities.
Anti-takeover provisions in GTx’s charter documents and under Delaware law could make an acquisition of GTx, which may be beneficial to its stockholders, more difficult and may prevent attempts by its stockholders to replace or remove GTx’s current management.
Provisions in GTx’s certificate of incorporation and its bylaws may delay or prevent an acquisition of GTx or a change in its management. In addition, these provisions may frustrate or prevent any attempts by its stockholders
58
to replace or remove its current management by making it more difficult for stockholders to replace members of its Board of Directors. Because the GTx Board is responsible for appointing the members of the management team, these provisions could in turn affect any attempt by GTx’s stockholders to replace current members of its management team. These provisions include:
| • | a classified Board of Directors; |
| • | a prohibition on actions by its stockholders by written consent; |
| • | the ability of the GTx Board to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by the GTx Board; and |
| • | limitations on the removal of directors. |
Moreover, because GTx is incorporated in Delaware, it is governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns 15% or more of its outstanding voting stock from merging or combining with GTx for a period of three years after the date of the transaction in which the person acquired 15% or more of GTx’s outstanding voting stock, unless the merger or combination is approved in a prescribed manner. Finally, these provisions establish advance notice requirements for nominations for election to the GTx Board or for proposing matters that can be acted upon at stockholder meetings. These provisions would apply even if the offer may be considered beneficial by some stockholders.
GTx’s amended and restated bylaws provide that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between GTx and its stockholders, which could limit GTx’s stockholders’ ability to obtain a favorable judicial forum for disputes with GTx or its directors, officers or employees.
GTx’s amended and restated bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on behalf of GTx, for any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, other employee or stockholder of GTx to GTx or to its stockholders, for any action asserting a claim arising pursuant to any provision of the DGCL, GTx’s restated certificate of incorporation or its amended and restated bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware, or for any action asserting a claim governed by the internal affairs doctrine. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with GTx or its directors, officers or other employees, which may discourage such lawsuits against GTx and its directors, officers and other employees. If a court were to find the choice of forum provision contained in GTx’s amended and restated bylaws to be inapplicable or unenforceable in an action, GTx may incur additional costs associated with resolving such action in other jurisdictions, which could harm its financial condition.
If there are substantial sales of GTx’s common stock, the market price of its common stock could drop substantially, even if its business is doing well.
For the12-month period ended December 31, 2018, the average daily trading volume of GTx’s common stock on the Nasdaq Capital Market was only 705,027 shares. As a result, future sales of a substantial number of shares of its common stock in the public market, or the perception that such sales may occur, could adversely affect the then-prevailing market price of GTx’s common stock. As of December 31, 2018, GTx had 24,051,844 shares of common stock outstanding. In addition, as a result of the low trading volume of its common stock, which was exacerbated by the 2016 Reverse Stock Split, the trading of relatively small quantities of shares by its stockholders may disproportionately influence the market price of its common stock in either direction. The price for GTx shares could, for example, decline significantly in the event that a large number of its common shares are sold on the market without commensurate demand, as compared to an issuer with a higher trading volume that could better absorb those sales without an adverse impact on its stock price. In addition, due to the
59
limitations of its market, the volatility in the market price of GTx common stock and its currently-depressed stock price, stockholders may face difficulties in selling shares at attractive prices when they want to sell.
In September 2017, GTx completed a private placement of 5.5 million shares of its common stock and warrants to purchase 3.3 million shares of its common stock. In November 2014, GTx completed a private placement of 6.4 million shares of its common stock and warrants to purchase 6.4 million shares of its common stock (as adjusted to give effect to the 2016 Reverse Stock Split). Similarly, in March 2014 GTx completed a private placement of 1.2 million shares of its common stock and warrants to purchase 1.0 million shares of its common stock (as adjusted to give effect to the 2016 Reverse Stock Split). Pursuant to the terms of the registration rights or securities purchase agreements GTx entered into in connection with these private placements, GTx has filed registration statements under the Securities Act registering the resale of an aggregate of approximately 23.8 million shares of common stock that GTx issued to, or are issuable upon the exercise of warrants that GTx issued to, the investors in these private placements, which investors include its largest stockholders. Moreover, J.R. Hyde, III and certain of his affiliates, have rights under a separate registration rights agreement with GTx to require GTx to file resale registration statements covering an additional 785,000 shares of common stock held in the aggregate or to include these shares in registration statements that GTx may file for itself or other stockholders. If Mr. Hyde or his affiliates or any of GTx’s other significant stockholders, including the other investors in GTx’s private placements, were to sell large blocks of shares in a short period of time, the market price of GTx’s common stock could drop substantially.
The comprehensive U.S. tax reform bill passed in 2017 could adversely affect GTx’s business and financial condition.
On December 22, 2017, President Trump signed the Tax Act into law, which significantly revised the Code. The Tax Act, among other things, contained significant changes to corporate taxation, including reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, limitation of the tax deduction for interest expense to 30% of adjusted taxable income (except for certain small businesses), limitation of the deduction for NOLs generated in tax years beginning after December 31, 2017 to 80% of current year taxable income and elimination of carrybacks of NOLs arising in taxable years ending after December 31, 2017,one-time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifying or repealing many business deductions and credits (including reducing the business tax credit for certain clinical testing expenses incurred in the testing of certain drugs for rare diseases or conditions). Notwithstanding the reduction in the corporate income tax rate, the overall impact of the Tax Act could adversely affect GTx. In addition, it is uncertain if and to what extent various states will conform to the Tax Act. The impact of the Tax Act on holders of GTx’s common stock is also uncertain and could be adverse. GTx urges its stockholders to consult with their legal and tax advisors with respect to this legislation and the potential tax consequences of investing in or holding GTx common stock.
Risks Related to Oncternal
Risks Related to Oncternal’s Limited Operating History, Financial Position and Capital Requirements
Oncternal has a limited operating history, has incurred significant operating losses since its inception and expects to incur significant losses for the foreseeable future. Oncternal may never generate any revenue or become profitable or, if Oncternal achieves profitability, it may not be able to sustain it.
Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. Oncternal is a clinical-stage biopharmaceutical company with a limited operating history upon which you can evaluate Oncternal’s business and prospects. Oncternal commenced operations in 2013, and to date, Oncternal has focused primarily on organizing and staffing its company, business planning, raising capital, identifying, acquiringand in-licensing Oncternal’s product candidates and conducting preclinical studies and early-stage clinical trials. Cirmtuzumab and TK216 are in clinical development, while Oncternal’s other
60
development programs, including its ROR1CAR-T program, remain in the preclinical stage. Oncternal has not yet demonstrated an ability to successfully obtain regulatory approvals, manufacture a commercial scale product, or arrange for a third-party to do so on Oncternal’s behalf, or embark on sales and marketing activities necessary for successful post regulatory approval product commercialization, and has not developed any companion diagnostic test for its product candidates. Consequently, any predictions made about Oncternal’s future success or viability may not be as accurate as they could be if Oncternal had a history of successfully developing and commercializing biopharmaceutical products.
Oncternal has incurred significant operating losses since its inception. If Oncternal’s product candidates are not successfully developed and approved, it may never generate any revenue. Oncternal’s net losses were $6.6 million and $10.4 million for the years ended December 31, 2018, and December 31, 2017, respectively. As of December 31, 2018, Oncternal had an accumulated deficit of $31.4 million. Substantially all of Oncternal’s losses have resulted from expenses incurred in connection with its research and development programs and from general and administrative costs associated with Oncternal’s operations. All of Oncternal’s product candidates will require substantial additional development time and resources before Oncternal would be able to apply for or receive regulatory approvals and begin generating revenue from product sales. Oncternal expects to continue to incur losses for the foreseeable future, and anticipates these losses will increase substantially as Oncternal continues to develop, seek regulatory approval for and potentially commercialize any of Oncternal’s product candidates, and seeks to identify, assess,acquire, in-license or develop additional product candidates.
To become and remain profitable, Oncternal must succeed in developing and eventually commercializing products that generate significant revenue. This will require Oncternal to be successful in a range of challenging activities, including completing clinical trials and preclinical studies of its product candidates, obtaining regulatory approval for these product candidates and manufacturing, marketing and selling any products for which Oncternal may obtain regulatory approval. Oncternal is only in the preliminary stages of most of these activities. Oncternal may never succeed in these activities and, even if it does, may never generate revenues that are significant enough to achieve profitability. In addition, Oncternal has not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical industry. Because of the numerous risks and uncertainties associated with biopharmaceutical product development, Oncternal is unable to accurately predict the timing or amount of increased expenses or when, or if, Oncternal will be able to achieve profitability. Even if Oncternal does achieve profitability, it may not be able to sustain or increase profitability on a quarterly or annual basis. Oncternal’s failure to become and remain profitable would depress the value of Oncternal and could impair its ability to raise capital, expand its business, maintain its research and development efforts, diversify its product candidates or even continue its operations. A decline in the value of Oncternal could also cause stockholders to lose all or part of their investment.
Oncternal will require substantial additional financing to achieve its goals, and a failure to obtain this necessary capital when needed and on acceptable terms, or at all, could force Oncternal to delay, limit, reduce or terminate its product development programs, commercialization efforts or other operations.
The development of biopharmaceutical product candidates is capital-intensive. Oncternal expects its expenses to increase in connection with its ongoing activities, particularly as Oncternal conducts its ongoing and planned clinical trials of cirmtuzumab and TK216, continues research and development and initiates clinical trials of Oncternal’s other development programs and seeks regulatory approval for its current product candidates and any future product candidates Oncternal may develop. In addition, as Oncternal’s product candidates progress through development and toward commercialization, Oncternal will need to make milestone payments to the licensors and other third parties from whom Oncternalhas in-licensed or acquired its product candidates, including cirmtuzumab, TK216 andCAR-T. If Oncternal obtains regulatory approval for any of its product candidates, Oncternal also expects to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. Because the outcome of any clinical trial or preclinical study is highly uncertain, Oncternal cannot reasonably estimate the actual amounts necessary to successfully complete the
61
development and commercialization of its product candidates. Furthermore, following the completion of the merger, Oncternal will incur the additional costs associated with operating as a public company. Accordingly, Oncternal will need to obtain substantial additional funding in connection with its continuing operations. If Oncternal is unable to raise capital when needed or on attractive terms, Oncternal could be forced to delay, reduce or eliminate its research and development programs or any future commercialization efforts.
Oncternal has based its estimates on assumptions that may prove to be wrong, and Oncternal could use its capital resources sooner than it currently expects. Oncternal’s operating plans and other demands on its cash resources may change as a result of many factors currently unknown to Oncternal, and Oncternal may need to seek additional funds sooner than planned, through public or private equity or debt financings or other capital sources, including potentially government funding, collaborations, licenses and other similar arrangements. In addition, Oncternal may seek additional capital due to favorable market conditions or strategic considerations even if Oncternal believes it has sufficient funds for its current or future operating plans. Attempting to secure additional financing may divert Oncternal’s management fromits day-to-day activities, which may adversely affect Oncternal’s ability to develop its product candidates.
Oncternal’s future capital requirements will depend on many factors, including:
| • | the type, number, scope, progress, expansions, results, costs and timing of, its clinical trials and preclinical studies of product candidates that Oncternal is pursuing or may choose to pursue in the future; |
| • | Oncternal’s efforts to evaluate, develop or partner the GTx product candidates, including the SARD assets; |
| • | the costs and timing of manufacturing for Oncternal’s product candidates, including commercial manufacturing if any product candidate is approved; |
| • | the costs, timing and outcome of regulatory review of Oncternal’s product candidates; |
| • | the costs of obtaining, maintaining and enforcing Oncternal’s patents and other intellectual property rights; |
| • | Oncternal’s efforts to enhance operational systems and hire additional personnel to satisfy its obligations as a public company, including enhanced internal controls over financial reporting; |
| • | the costs associated with hiring additional personnel and consultants as Oncternal’s clinical and other development activities increase; |
| • | the timing and amount of the milestone or other payments Oncternal must make to the licensors and other third parties from whom Oncternalhas in-licensed or acquired its product candidates or technology; |
| • | the costs and timing of establishing or securing sales and marketing capabilities if any product candidate is approved; |
| • | Oncternal’s ability to achieve sufficient market acceptance, coverage and adequate reimbursement from third-party payors and adequate market share and revenue for any approved products; |
| • | the terms and timing of establishing and maintaining collaborations, licenses and other similar arrangements; and |
| • | costs associated with any products or technologies that Oncternalmay in-license or acquire. |
Conducting clinical trials and preclinical studies is a time consuming, expensive and uncertain process that takes years to complete, and Oncternal may never generate the necessary data or results required to obtain regulatory approval and achieve product sales. In addition, Oncternal’s product candidates, if approved, may not achieve commercial success. Oncternal’s commercial revenues, if any, will be derived from sales of products that Oncternal does not expect to be commercially available for many years, if at all.
62
Accordingly, Oncternal will need to continue to rely on additional financing to achieve its business objectives. Adequate additional financing may not be available to Oncternal on acceptable terms, or at all. In addition, Oncternal may seek additional capital due to favorable market conditions or strategic considerations, even if Oncternal believes it has sufficient funds for its current or future operating plans.
Raising additional capital may cause dilution to Oncternal’s stockholders, restrict Oncternal’s operations or require Oncternal to relinquish rights to its technologies or product candidates.
Until such time, if ever, as Oncternal can generate substantial product revenues, Oncternal expects to finance its cash needs through equity offerings, debt financings or other capital sources, including potentially government funding, collaborations, licenses and other similar arrangements. To the extent that Oncternal raises additional capital through the sale of equity or convertible debt securities, existing stockholders’ ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect stockholders’ rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting Oncternal’s ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If Oncternal raises funds through future collaborations, licenses and other similar arrangements, Oncternal may have to relinquish valuable rights to its future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to Oncternal and/or that may reduce the value of Oncternal’s common stock.
Risks Related to the Discovery, Development and Regulatory Approval of Oncternal’s Product Candidates
Oncternal depends heavily on the success of cirmtuzumab and TK216, which are in Phase 1 or Phase 2 clinical trials, as well as its ROR1CAR-T program, which is in preclinical development. If Oncternal is unable to advance its product candidates in clinical development, obtain regulatory approval and ultimately commercialize its product candidates, or experiences significant delays in doing so, Oncternal’s business will be materially harmed.
Oncternal’s two clinical-stage product candidates are in Phase 1 or Phase 2 clinical development. In May 2018, Oncternal commenced a Phase 1b/2 clinical trial evaluating cirmtuzumab in combination with ibrutinib in patients with MCL and CLL. In addition, TK216 is currently being evaluated in a Phase 1 clinical trial in patients with relapsed or refractory Ewing sarcoma. Oncternal plans to initiate a Phase 1 clinical trial of TK216 in AML, and to commenceIND-enabling preclinical studies for TK216 for the treatment of patients with prostate cancer. Additionally, Oncternal’s ROR1CAR-T program will need further preclinical development andIND-enabling studies prior to commencing clinical development. None of Oncternal’s product candidates have advanced into a pivotal or registrational study for the indications for which Oncternal is studying them. Oncternal’s ability to generate product revenues, which Oncternal does not expect will occur for many years, if ever, will depend heavily on the successful development and eventual commercialization of its product candidates. The success of Oncternal’s product candidates will depend on various factors, including the following:
| • | successful completion of preclinical and clinical studies with favorable results; |
| • | acceptance of INDs by the FDA or similar regulatory filing by comparable foreign regulatory authorities for the conduct of clinical trials of Oncternal’s product candidates and its proposed designs for future clinical trials; |
| • | demonstrating safety and efficacy of Oncternal’s product candidates to the satisfaction of applicable regulatory authorities; |
| • | receiving marketing approvals from applicable regulatory authorities, including Biologics License Applications (“BLAs”), or new drug applications (“NDAs”), from the FDA and maintaining such approvals; |
63
| • | making arrangements with Oncternal’s third-party manufacturers for commercial manufacturing capabilities for Oncternal’s product candidates; |
| • | establishing sales, marketing and distribution capabilities and launching commercial sales of Oncternal’s product candidates, if and when approved, whether alone or in collaboration with others; |
| • | establishing and maintaining patent and trade secret protection or regulatory exclusivity for Oncternal’s product candidates; |
| • | the demonstration of an acceptable safety profile of Oncternal’s products following approval, if any; |
| • | developing,in-licensing or acquiring companion diagnostics to Oncternal’s product candidates; and |
| • | maintaining and growing an organization for people who can develop Oncternal’s product candidates and technology. |
The success of Oncternal’s business, including its ability to finance the company and generate any revenue in the future, will primarily depend on the successful development, regulatory approval and commercialization of Oncternal’s product candidates, which may never occur. Oncternal has not yet succeeded and may not succeed in demonstrating efficacy and safety for any of its product candidates in clinical trials or in obtaining marketing approval thereafter. Given Oncternal’s early stage of development, it may be several years, if at all, before Oncternal has demonstrated the safety and efficacy of a product candidate sufficient to warrant approval for commercialization. If Oncternal is unable to develop, or obtain regulatory approval for, or, if approved, successfully commercialize its product candidates, Oncternal may not be able to generate sufficient revenue to continue its business.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome, and the results of preclinical studies and early clinical trials are not necessarily predictive of future results. Oncternal’s product candidates may not have favorable results in clinical trials or receive regulatory approval on a timely basis, if at all.
Clinical drug development is expensive and can take many years to complete, and its outcome is inherently uncertain. Oncternal cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all, and failure can occur at any time during the preclinical study or clinical trial process. Despite promising preclinical or clinical results, any product candidate can unexpectedly fail at any stage of preclinical or clinical development. The historical failure rate for product candidates in Oncternal’s industry is high.
The results from preclinical studies or clinical trials of a product candidate may not predict the results of later clinical trials of the product candidate, and interim results of a clinical trial are not necessarily indicative of final results. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy characteristics despite having progressed through preclinical studies and initial clinical trials. In particular, while cirmtuzumab was well tolerated and was shown to inhibit ROR1 signaling in patients with CLL in early clinical trials, we do not know how cirmtuzumab will perform in the Phase 1b/2 clinical trial in combination with ibrutinib or any other future clinical trials, including as a result of any differences in the target population, drug interactions or other differences in our trial design. It is not uncommon to observe results in clinical trials that are unexpected based on preclinical studies and early clinical trials, and many product candidates fail in clinical trials despite very promising early results. Under the License and Development Agreement (the “SPH USA License Agreement”) by and between Oncternal and SPH USA, SPH USA has the right to manufacture, develop, market, distribute and sell Oncternal’s cirmtuzumab, ROR1CAR-T, and TK216 product candidates in the People’s Republic of China, Hong Kong, Macau and Taiwan (“Greater China”), and the obligation to perform all preclinical and clinical development activities required to obtain regulatory approvals for such product candidates in Greater China. In the event that SPH USA’s preclinical studies or clinical trials of Oncternal’s product candidates raise new safety or efficacy concerns, the prospects for obtaining regulatory approval of Oncternal’s product candidates in the United States and other countries, and Oncternal’s business, could be adversely impacted.
64
Moreover, this and any future preclinical and clinical data may be susceptible to varying interpretations and analyses. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies. Furthermore, we cannot assure you that we will be able to successfully progress our preclinical programs from candidate identification to Phase 1 clinical development.
For the foregoing reasons, Oncternal cannot be certain that its ongoing and planned clinical trials and preclinical studies will be successful. Any safety concerns observed in any one of Oncternal’s clinical trials in its targeted indications could limit the prospects for regulatory approval of Oncternal’s product candidates in those and other indications, which could have a material adverse effect on Oncternal’s business, financial condition and results of operations.
Any difficulties or delays in the commencement or completion, or termination or suspension, of Oncternal’s current or planned clinical trials could result in increased costs to Oncternal, delay or limit its ability to generate revenue, and adversely affect its commercial prospects.
Before obtaining marketing approval from regulatory authorities for the sale of Oncternal’s product candidates, Oncternal must conduct extensive clinical studies to demonstrate the safety and efficacy of the product candidates in humans. Oncternal is currently enrolling a Phase 1b/2a trial of cirmtuzumab in combination with ibrutinib in patients with CLL and MCL and conducting a dose-escalation Phase 1 trial of TK216 in patients with relapsed or refractory Ewing sarcoma. Oncternal will have to follow the same procedure for its other preclinical product candidates that Oncternal plans to advance to clinical development, and would also be required to submit regulatory filings to foreign regulatory authorities if Oncternal decides to initiate clinical trials outside of the United States.
Oncternal does not know whether its planned trials will begin on time or be completed on schedule, if at all. The commencement and completion of clinical trials can be delayed for a number of reasons, including delays related to:
| • | subjects failing to enroll or remain in Oncternal’s trial at the rate Oncternal expects, or failing to return forpost-treatment follow-up; |
| • | subjects choosing an alternative treatment for the indication for which Oncternal is developing its product candidates, or participating in competing clinical trials; |
| • | the FDA or comparable foreign regulatory authorities disagreeing as to the design or implementation of Oncternal’s clinical studies; |
| • | difficulties in obtaining regulatory authorizations to commence a trial or reaching a consensus with regulatory authorities on trial design; |
| • | difficulties in recruiting clinical trial investigators with the appropriate competencies and experience; |
| • | failure or delay in reaching an agreement with contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | delays in obtaining approval from one or more institutional review boards, or IRBs; |
| • | IRBs refusing to approve, suspending or terminating the trial at an investigational site, precluding enrollment of additional subjects, or withdrawing their approval of the trial; |
| • | changes to clinical trial protocols; |
| • | clinical sites deviating from trial protocols or dropping out of a trial; |
| • | challenges in manufacturing sufficient quantities of product candidates or obtaining sufficient quantities of combination therapies for use in clinical trials; |
65
| • | lack of adequate funding to continue clinical trials; |
| • | subjects experiencing severe or unexpected drug-related adverse effects; |
| • | occurrence of serious adverse events in clinical trials of the same class of agents conducted by other companies; |
| • | selection of clinical endpoints that require prolonged periods of clinical observation or analysis of the resulting data; |
| • | a facility manufacturing Oncternal’s product candidates or any of their components being ordered by the FDA or comparable foreign regulatory authorities to temporarily or permanently shut down due to violations of current Good Manufacturing Practices (“cGMP”) regulations or other applicable requirements, or infections or cross-contaminations of product candidates in the manufacturing process; |
| • | any changes to Oncternal’s manufacturing process that may be necessary or desired; |
| • | third-party clinical investigators losing the licenses or permits necessary to perform Oncternal’s clinical trials, not performing Oncternal’s clinical trials in a timely manner or consistent with applicable clinical trial protocols, good clinical practices (“GCP”), or other regulatory requirements; third-party contractors not performing data collection or analysis in a timely or accurate manner; or |
| • | third-party contractors becoming debarred or suspended or otherwise penalized by the FDA or other government or regulatory authorities for violations of regulatory requirements, in which case Oncternal may need to find a substitute contractor, and Oncternal may not be able to use some or all of the data produced by such contractors in support of Oncternal’s marketing applications. |
Oncternal could also encounter delays if its clinical trials are suspended or terminated by Oncternal, by the IRBs of the institutions in which such trials are being conducted, by a Data Safety Monitoring Board for such trial, or by the FDA or comparable foreign regulatory authorities. Regulatory authorities may suspend or terminate clinical trials due to a number of factors, including failure to conduct clinical trials in accordance with regulatory requirements or the applicable clinical protocols, inspection of the clinical trial operations or trial site by the FDA or comparable foreign regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. In addition, changes in regulatory requirements and policies may occur, and Oncternal may need to amend clinical trial protocols to comply with these changes. Amendments may require Oncternal to resubmit its clinical trial protocols to IRBs for reexamination, which may impact the costs, timing or successful completion of a clinical trial.
Further, if Oncternal decides to conduct clinical trials of its product candidates in foreign countries additional risks may arise that may delay completion of those clinical trials. These risks include the failure of enrolled patients in other countries to adhere to clinical protocol as a result of differences in healthcare practices or cultural customs, managing additional administrative burdens associated with the regulatory schemes of other countries, as well as political and economic risks relevant to other countries. Under Oncternal’s license and development agreement with SPH USA, SPH USA has the right to manufacture, develop, market, distribute and sell Oncternal’s cirmtuzumab, ROR1CAR-T, and TK216 product candidates in the People’s Republic of China, Hong Kong, Macau and Taiwan, or Greater China, and the obligation to perform all preclinical and clinical development activities required to obtain regulatory approvals for such product candidates in Greater China. In the event that SPH USA’s preclinical studies or clinical trials of Oncternal’s product candidates raise new safety or efficacy concerns, the prospects for obtaining regulatory approval of Oncternal’s product candidates in the United States and other countries, and Oncternal’s business, could be adversely impacted.
Moreover, principal investigators for Oncternal’s clinical trials may serve as scientific advisors or consultants to Oncternal from time to time and receive compensation in connection with such services. Under certain
66
circumstances, Oncternal may be required to report some of these relationships to the FDA or comparable foreign regulatory authorities. The FDA or comparable foreign regulatory authority may conclude that a financial relationship between Oncternal and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. The FDA or comparable foreign regulatory authority may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of Oncternal’s marketing applications by the FDA or comparable foreign regulatory authority, as the case may be, and may ultimately lead to the denial of marketing approval of one or more of Oncternal’s product candidates.
If Oncternal experiences delays in the completion of, or termination of, clinical trials of its product candidates, the commercial prospects of such product candidates may be harmed, and its ability to generate product revenues from such product candidates may be delayed. Moreover, delays in completing Oncternal’s clinical trials may increase its costs, slow down its product candidate development and approval process and jeopardize its ability to commence product sales and generate revenues.
In addition, many of the factors that cause, or lead to, the termination, suspension or delay in the commencement or completion of, clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate. If Oncternal makes formulation or manufacturing changes to its product candidates, it may be required to conduct additional preclinical or clinical studies to bridge its modified product candidates to earlier versions. The need to conduct additional preclinical or clinical studies could result in delays in the approval or commercialization of Oncternal’s product candidates, which could shorten any period during which Oncternal may have the exclusive right to commercialize its product candidates and enable Oncternal’s competitors to bring products to market before Oncternal does. In such an event, the commercial viability of Oncternal’s product candidates could be significantly reduced. Any of these occurrences may harm Oncternal’s business, financial condition and prospects significantly.
Oncternal may find it difficult to enroll patients in its clinical trials. If Oncternal encounters difficulties enrolling subjects in its clinical trials, its clinical development activities could be delayed or otherwise adversely affected.
Oncternal may not be able to initiate or continue clinical trials for its product candidates if Oncternal is unable to identify and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. Subject enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of patients to clinical sites, the eligibility and exclusion criteria for the trial, the design of the clinical trial, the availability of competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages and risks of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications Oncternal is investigating as well as any drugs under development. Oncternal will be required to identify and enroll a sufficient number of subjects for each of its clinical trials. Potential subjects for any planned clinical trials may not be adequately diagnosed or identified with the diseases which Oncternal is targeting or may not meet the entry criteria for such trials. . For example, a limited number of patients are affected by CLL, MCL and particularly Ewing sarcoma, which are Oncternal’s initial target indications for cirmtuzumab and TK216. Oncternal also may encounter difficulties in identifying and enrolling subjects with a stage of disease appropriate for Oncternal’s planned clinical trials. Oncternal may not be able to initiate or continue clinical trials if Oncternal is unable to locate a sufficient number of eligible subjects to participate in the clinical trials required by the FDA or comparable foreign regulatory authorities. In addition, the process of finding and diagnosing subjects may prove costly.
The timing of Oncternal’s clinical trials depends, in part, on the speed at which Oncternal can recruit patients to participate in its trials, as well as completion ofrequired follow-up periods. For certain of Oncternal’s product candidates, including cirmtuzumab and TK216, the conditions which Oncternal currently plans to evaluate are orphan or rare diseases with limited patient pools from which to draw for clinical trials. The eligibility criteria of
67
Oncternal’s clinical trials will further limit the pool of available trial participants. If patients are unwilling to participate in Oncternal’s trials for any reason, including the existence of concurrent clinical trials for similar patient populations or the availability of approved therapies, or Oncternal otherwise has difficulty enrolling a sufficient number of patients, the timeline for recruiting subjects, conducting studies and obtaining regulatory approval of Oncternal’s product candidates may be delayed. Oncternal’s inability to enroll a sufficient number of subjects for any of its clinical trials would result in significant delays or may require Oncternal to abandon one or more clinical trials altogether. In addition, Oncternal expects to rely on CROs and clinical trial sites to ensure proper and timely conduct of its future clinical trials and, while Oncternal intends to enter into agreements governing their services, Oncternal will have limited influence over their actual performance.
Oncternal cannot assure stockholders that its assumptions used in determining expected clinical trial timelines are correct or that Oncternal will not experience delays in enrollment, which would result in the delay of completion of such trials beyond Oncternal’s expected timelines.
Use of Oncternal’s product candidates could be associated with side effects, adverse events or other properties or safety risks, which could delay or preclude approval, cause Oncternal to suspend or discontinue clinical trials, abandon a product candidate, limit the commercial profile of the label for an approved product candidate, or result in other significant negative consequences that could severely harm Oncternal’s business, prospects, operating results and financial condition.
As is the case with oncology drugs generally, it is likely that there may be side effects and adverse events associated with the use of Oncternal’s product candidates. Results of Oncternal’s clinical trials could reveal a high and unacceptable severity and prevalence, or unexpected characteristics of side effects. Undesirable side effects caused by Oncternal’s product candidates could cause Oncternal or regulatory authorities to interrupt, delay or halt clinical trials, result in a more restrictive label for the product candidate, or delay or cause the denial of regulatory approval of the product candidate by the FDA or comparable foreign regulatory authorities. The drug-related side effects could also affect patient recruitment for Oncternal’s clinical trials, or the ability of enrolled patients to complete the trials, or result in potential product liability claims. Any of these occurrences may harm Oncternal’s business, financial condition and prospects significantly.
Moreover, if Oncternal’s product candidates are associated with undesirable side effects in clinical trials or have characteristics that are unexpected, Oncternal may elect to abandon their development or limit their development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective, which may limit the commercial prospects for the product candidate if approved. Oncternal may also be required to modify its plans for future studies based on findings in Oncternal’s ongoing clinical trials. Many compounds that initially showed promise in early-stage testing have later been found to cause side effects that prevented further development of the compound. In addition, regulatory authorities may draw different conclusions or require additional testing to confirm these determinations.
It is possible that as Oncternal tests its product candidates in larger, longer and more extensive clinical trials, or as the use of these product candidates becomes more widespread if they receive regulatory approval, illnesses, injuries, discomforts and other adverse events that were observed in earlier trials, as well as conditions that did not occur or went undetected in previous trials, will be reported by subjects. If such side effects become known later in development or upon approval, if any, such findings may harm Oncternal’s business, financial condition and prospects significantly. In addition, our ongoing clinical trials of cirmtuzumab in combination with ibrutinib and TK216 in combination with vincristine, and the ongoing investigator-initiated clinical trial of cirmtuzumab in combination with paclitaxel, may reveal adverse events based on the combination therapy that may negatively impact the reported safety profile in such clinical trial.
68
In addition, if one or more of Oncternal’s product candidates receives marketing approval, and Oncternal or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may withdraw, suspend or limit approvals of such product; |
| • | Oncternal may be required to recall a product or change the way such product is administered to patients; |
| • | regulatory authorities may require additional warnings on the label, such as a “black box” warning or a contraindication; |
| • | Oncternal may be required to implement a Risk Evaluation and Mitigation Strategy, or REMS, or create a medication guide outlining the risks of such side effects for distribution to patients; |
| • | Oncternal may be required to change the way a product is distributed or administered, conduct additional clinical trials or change the labeling of a product or be required to conduct additional post-marketing studies or surveillance; |
| • | Oncternal could be sued and held liable for harm caused to patients; |
| • | sales of the product may decrease significantly or the product could become less competitive; and |
| • | Oncternal’s reputation could suffer. |
Any of these events could prevent Oncternal from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm Oncternal’s business, results of operations and prospects.
The regulatory landscape that will apply to development of gene therapy or cell-based therapeutic product candidates by Oncternal or its collaborators is rigorous, complex, uncertain and subject to change, which could result in delays or termination of development of such product candidates or unexpected costs in obtaining regulatory approvals.
Regulatory requirements governing products involving gene therapy treatment have changed frequently and will likely continue to change in the future. Approvals by one regulatory agency may not be indicative of what any other regulatory agency may require for approval, and there is substantial, and sometimes uncoordinated, overlap in those responsible for regulation of gene therapy products, cell therapy products and other products created with genome editing technology. For example, in addition to the submission of an investigational new drug application, or IND, to the FDA, before initiation of a clinical trial in the United States, certain human clinical trials for cell therapy products and gene therapy had historically been subject to review by the Recombinant DNA Advisory Committee (the “RAC”), of the National Institutes of Health (“NIH”), Office of Biotechnology Activities (“OBA”), pursuant to the NIH Guidelines for Research Involving Recombinant DNA Molecules (“NIH Guidelines”). Following an initial review, RAC members would make a recommendation as to whether the protocol raises important scientific, safety, medical, ethical or social issues that warrantin-depth discussion at the RAC’s quarterly meetings. Even though the FDA decides whether individual cell therapy or gene therapy protocols may proceed under an IND, the RAC’s recommendations were shared with the FDA and the RAC public review process, if undertaken, could delay the initiation of a clinical trial, even if the FDA had reviewed the trial design and details and has not objected to its initiation or has notified the sponsor that the study may begin. Conversely, the FDA could have put an IND on clinical hold even if the RAC provided a favorable review or had recommended against anin-depth, public review. On August 17, 2018, the NIH issued a notice in the Federal Register and issued a public statement proposing changes to the oversight framework for gene therapy trials, including changes to the applicable NIH Guidelines to modify the roles and responsibilities of the RAC with respect to human clinical trials of gene therapy products, and requesting public comment on its proposed modifications. The NIH announced that during the public comment period, which closed October 16, 2018, it would no longer accept new human gene transfer protocols for review as part of the protocol registration process
69
under the existing NIH Guidelines or convene the RAC to review individual clinical protocols. These trials remain subject to the FDA’s oversight and other clinical trial regulations, and oversight at the local level will continue as otherwise set forth in the NIH Guidelines. Specifically, under the NIH Guidelines, supervision of human gene transfer trials includes evaluation and assessment by an institutional biosafety committee, or IBC, a local institutional committee that reviews and oversees research utilizing recombinant or synthetic nucleic acid molecules at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment, and such review may result in some delay before initiation of a clinical trial. While the NIH Guidelines are not mandatory unless the research in question is being conducted at or sponsored by institutions receiving NIH funding of recombinant or synthetic nucleic acid molecule research, many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them. Even though Oncternal may not be required to submit a protocol for its gene therapy product candidates such as a ROR1 targetedCAR-T through the NIH for RAC review, Oncternal will still be subject to significant regulatory oversight by the FDA, and in addition to the government regulators, the applicable IBC and institutional review board, or IRB, of each institution at which Oncternal or its collaborators conduct clinical trials of its product candidates, or a central IRB if appropriate, would need to review and approve the proposed clinical trial.
The same applies in the European Union. The European Medicines Agency (the “EMA”), has a Committee for Advanced Therapies, or CAT, that is responsible for assessing the quality, safety and efficacy of advanced therapy medicinal products. Advanced-therapy medical products include gene therapy medicine, somatic-cell therapy medicines and tissue-engineered medicines. The role of the CAT is to prepare a draft opinion on an application for marketing authorization for a gene therapy medicinal candidate that is submitted to the EMA. In the European Union, the development and evaluation of a gene therapy medicinal product must be considered in the context of the relevant European Union guidelines. The EMA may issue new guidelines concerning the development and marketing authorization for gene therapy medicinal products and require that Oncternal complies with these new guidelines. Similarly complex regulatory environments exist in other jurisdictions in which Oncternal might consider seeking regulatory approvals for Oncternal’s product candidates, further complicating the regulatory landscape. As a result, the procedures and standards applied to gene therapy products and cell therapy products may be applied to any of Oncternal’s gene therapy product candidates such asCAR-T, but that remains uncertain at this point.
The clinical trial requirements of the FDA, the EMA and other regulatory authorities and the criteria these regulators use to evaluate the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of the potential products. The regulatory approval process for product candidates involving gene therapy can be more lengthy, rigorous and expensive than the process for other better known or more extensively studied product candidates and technologies. Since Oncternal is developing novel treatments for diseases in which there is little clinical experience with new endpoints and methodologies, there is heightened risk that the FDA, the EMA or comparable regulatory bodies may not consider the clinical trial endpoints to provide clinically meaningful results, and the resulting clinical data and results may be more difficult to analyze. This may be a particularly significant risk for many of the genetically defined diseases for which Oncternal may develop product candidates alone or with collaborators due to small patient populations for those diseases, and designing and executing a rigorous clinical trial with appropriate statistical power is more difficult than with diseases that have larger patient populations. Regulatory agencies administering existing or future regulations or legislation may not allow production and marketing of products utilizing gene therapy in a timely manner or under technically or commercially feasible conditions. Even if Oncternal’s product candidates obtain required regulatory approvals, such approvals may later be withdrawn as a result of changes in regulations or the interpretation of regulations by applicable regulatory agencies.
Changes in applicable regulatory guidelines may lengthen the regulatory review process for Oncternal’s product candidates, require additional studies or trials, increase development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of such product candidates, or lead to significant post-approval limitations or restrictions. Additionally, adverse developments in clinical trials of gene therapy products conducted by others, may cause the FDA, the EMA and other regulatory bodies to
70
revise the requirements for approval of any product candidates Oncternal may develop or limit the use of products utilizing gene therapy, either of which could materially harm Oncternal’s business. Furthermore, regulatory action or private litigation could result in increased expenses, delays or other impediments to Oncternal’s research programs or the development or commercialization of current or future product candidates.
As Oncternal advances its product candidates alone or with collaborators, Oncternal will be required to consult with these regulatory and advisory groups and comply with all applicable guidelines, rules and regulations. If Oncternal fails to do so, it or its collaborators may be required to delay or terminate development of such product candidates. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a product candidate to market could decrease Oncternal’s ability to generate sufficient product revenue to maintain its business.
As an organization, Oncternal has limited experience in the process of enrolling patients in its clinical trials, has never conducted later-stage clinical trials or submitted a BLA or an NDA, and may be unable to do so for any of Oncternal’s product candidates.
Oncternal is early in its development efforts for its product candidates, and will need to successfully complete later-stage and pivotal clinical trials in order to obtain FDA or comparable foreign regulatory approval to market cirmtuzumab, TK216, ROR1CAR-T, or any future product candidates. Carrying out later-stage clinical trials and submitting a successful BLA or NDA is a complicated process. As an organization, Oncternal is in the process of conducting a Phase 1b/2 clinical trial for cirmtuzumab in combination with ibrutinib and a Phase 1 clinical trial for TK216, alone and in combination with vincristine. Oncternal has not yet conducted any clinical trials for its other product candidates. Oncternal has not previously conducted any later stage or pivotal clinical trials, has limited experience as a company in preparing, submitting and prosecuting regulatory filings and has not previously submitted a BLA, an NDA or other comparable foreign regulatory submission for any product candidate. In addition, Oncternal has had limited interactions with the FDA and cannot be certain how many additional clinical trials of cirmtuzumab, TK216 or any other product candidates will be required or how such trials should be designed. Oncternal may be unable to successfully and efficiently execute and complete necessary clinical trials in a way that leads to regulatory submission and approval of any of Oncternal’s product candidates. Oncternal may require more time and incur greater costs than its competitors and may not succeed in obtaining regulatory approvals of product candidates that it develops. Failure to commence or complete, or delays in Oncternal’s planned clinical trials could delay or prevent Oncternal from submitting BLAs or NDAs for, and commercializing, its product candidates.
Oncternal’s product candidates are subject to extensive regulation and compliance, which is costly and time consuming, and such regulation may cause unanticipated delays or prevent the receipt of the required approvals to commercialize Oncternal’s product candidates.
The clinical development, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing and distribution of Oncternal’s product candidates are subject to extensive regulation by the FDA in the United States and by comparable foreign regulatory authorities in foreign markets. In the United States, Oncternal is not permitted to market its product candidates until it receives regulatory approval from the FDA. The process of obtaining regulatory approval is expensive, often takes many years following the commencement of clinical trials and can vary substantially based upon the type, complexity and novelty of the product candidates involved, as well as the target indications and patient population. Approval policies or regulations may change, and the FDA has substantial discretion in the drug approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. Despite the time and expense invested in clinical development of product candidates, regulatory approval is never guaranteed. Oncternal is not permitted to market any of its product candidates in the United States until it receives approval of a BLA or an NDA from the FDA.
Prior to obtaining approval to commercialize a product candidate in the United States or abroad, Oncternal must demonstrate with substantial evidence from adequate and well-controlled clinical trials, and to the satisfaction of
71
the FDA or comparable foreign regulatory authorities, that such product candidates are safe and effective for their intended uses, and in the case of biological products, that such product candidates are safe, pure and potent. Results from nonclinical studies and clinical trials can be interpreted in different ways. Even if Oncternal believes the nonclinical or clinical data for Oncternal’s product candidates are promising, such data may not be sufficient to support approval by the FDA and comparable foreign regulatory authorities. The FDA or comparable foreign regulatory authorities, as the case may be, may also require Oncternal to conduct additional preclinical studies or clinical trials for Oncternal’s product candidates either prior to or post-approval, or may object to elements of Oncternal’s clinical development program.
The FDA or comparable foreign regulatory authorities can delay, limit or deny approval of a product candidate for many reasons, including:
| • | such authorities may disagree with the design or execution of Oncternal’s clinical trials; |
| • | negative or ambiguous results from Oncternal’s clinical trials or results may not meet the level of statistical significance required by the FDA or comparable foreign regulatory agencies for approval; |
| • | serious and unexpected drug-related side effects may be experienced by participants in Oncternal’s clinical trials or by individuals using drugs similar to Oncternal’s product candidates; |
| • | the population studied in the clinical trial may not be sufficiently broad or representative to assure safety in the full population for which Oncternal seeks approval; |
| • | such authorities may not accept clinical data from trials that are conducted at clinical facilities or in countries where the standard of care is potentially different from that of their own country; |
| • | Oncternal may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | such authorities may disagree with Oncternal’s interpretation of data from preclinical studies or clinical trials; |
| • | such authorities may not agree that the data collected from clinical trials of Oncternal’s product candidates are acceptable or sufficient to support the submission of a BLA, NDA or other submission or to obtain regulatory approval in the United States or elsewhere, and such authorities may impose requirements for additional preclinical studies or clinical trials; |
| • | such authorities may disagree with Oncternal regarding the formulation, labeling and/or the product specifications of Oncternal’s product candidates; |
| • | approval may be granted only for indications that are significantly more limited than those sought by Oncternal, and/or may include significant restrictions on distribution and use; |
| • | such authorities may find deficiencies in the manufacturing processes or facilities of the third-party manufacturers with which Oncternal contracts for clinical and commercial supplies; or |
| • | such authorities may not accept a submission due to, among other reasons, the content or formatting of the submission. |
With respect to foreign markets, approval procedures vary among countries and, in addition to the foregoing risks, may involve additional product testing, administrative review periods and agreements with pricing authorities. In addition, events raising questions about the safety of certain marketed pharmaceuticals may result in increased cautiousness by the FDA and comparable foreign regulatory authorities in reviewing new drugs based on safety, efficacy or other regulatory considerations and may result in significant delays in obtaining regulatory approvals. Any delay in obtaining, or inability to obtain, applicable regulatory approvals would prevent Oncternal or any of Oncternal’s potential future collaborators from commercializing Oncternal’s product candidates.
72
Of the large number of drugs in development, only a small percentage successfully complete the FDA or foreign regulatory approval processes and are commercialized. The lengthy approval process as well as the unpredictability of future clinical trial results may result in Oncternal’s failure to obtain regulatory approval to market its product candidates, which would significantly harm Oncternal’s business, financial condition, results of operations and prospects.
Even if Oncternal eventually completes clinical trials and receives approval of a BLA, NDA or comparable foreign marketing application for Oncternal’s product candidates, the FDA or comparable foreign regulatory authority may grant approval contingent on the performance of costly additional clinical trials, including Phase 4 clinical trials, and/or the implementation of a REMS, which may be required because the FDA believes it is necessary to ensure safe use of the drug after approval. The FDA or the comparable foreign regulatory authority also may approve a product candidate for a more limited indication or patient population than Oncternal originally requested, and the FDA or comparable foreign regulatory authority may not approve the labeling that Oncternal believes is necessary or desirable for the successful commercialization of a product. Any delay in obtaining, or inability to obtain, applicable regulatory approval would delay or prevent commercialization of that product candidate and would materially adversely impact Oncternal’s business and prospects.
Oncternal may expend its limited resources to pursue a particular product candidate and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because Oncternal has limited financial and managerial resources, it is focused on specific product candidates, indications and development programs. As a result, Oncternal may forgo or delay the pursuit of opportunities with other indications or other product candidates that could have greater commercial potential. Oncternal’s resource allocation decisions may cause Oncternal to fail to capitalize on viable commercial products or profitable market opportunities. Oncternal’s spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If Oncternal does not accurately evaluate the commercial potential for a particular product candidate, it could relinquish valuable rights to that product candidate through collaborations, licenses and other similar arrangements, when it might be more advantageous for Oncternal to retain sole development and commercialization rights to such product candidate.
Fast Track designation by the FDA for TK216 or Oncternal’s other product candidates may not actually lead to a faster development or regulatory review or approval process.
Oncternal has been granted a Fast Track designation for TK216 in the United States for the treatment of Ewing sarcoma and may seek Fast Track designation for cirmtuzumab or its other product candidates. The Fast Track program is intended to expedite or facilitate the process for reviewing new product candidates that meet certain criteria. Specifically, new drugs are eligible for Fast Track designation if they are intended, alone or in combination with one or more drugs, to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Fast Track designation applies to the combination of the product candidate and the specific indication for which it is being studied. With a Fast Track product candidate, the FDA may consider for review sections of the NDA or BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA or BLA, the FDA agrees to accept sections of the NDA or BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA or BLA.
Obtaining a Fast Track designation does not change the standards for product approval, but may expedite the development or approval process. Even though the FDA has granted such designation for TK216, it may not actually result in faster clinical development or regulatory review or approval. Furthermore, such a designation does not increase the likelihood that TK216 or any other product candidate that may be granted Fast Track designation will receive marketing approval in the United States.
73
Oncternal may not be able to obtain or maintain orphan drug designations for certain of its product candidates, and may be unable to maintain the benefits associated with orphan drug designation, including the potential for market exclusivity.
Regulatory authorities in some jurisdictions, including the United States and Europe, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act of 1983, the FDA may designate a product as an orphan product if it is intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States, or a patient population of greater than 200,000 individuals in the United States, but for which there is no reasonable expectation that the cost of developing the drug will be recovered from sales in the United States. In the European Union, the EMA’s, Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 persons in the European Union. Oncternal has received orphan drug designation in the United Statements for TK216 for patients with Ewing sarcoma and it may seek orphan drug designation in the European Union for TK216 for patients with Ewing sarcoma, as well as seek orphan drug designation for certain of our other product candidates. There can be no assurance that the FDA or the EMA’s Committee for Orphan Medicinal Products will grant orphan designation for any indication for which Oncternal applies, or that Oncternal will be able to maintain such designation.
In the United States, orphan designation entitles a party to financial incentives such as opportunities for grant funding for clinical trial costs, tax advantagesand user-fee waivers. In addition, if a product candidate that has orphan designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications, including an NDA or BLA, to market the same drug for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or where the manufacturer is unable to assure sufficient product quantity. The applicable exclusivity period is ten years in Europe, but such exclusivity period can be reduced to six years if a product no longer meets the criteria for orphan designation or if the product is sufficiently profitable so that market exclusivity is no longer justified.
Even if Oncternal obtains orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA or comparable foreign regulatory authority can subsequently approve the same drug for the same condition if such regulatory authority concludes that the later drug is clinically superior if it is shown to be safer, more effective or makes a major contribution to patient care. Orphan drug designation neither shortens the development time or regulatory review time of a drug nor gives the drug any advantage in the regulatory review or approval process.
Oncternal may conduct certain of or portions of its clinical trials for its product candidates outside of the United States and the FDA may not accept data from such trials, in which case Oncternal’s development plans will be delayed, which could materially harm its business.
Oncternal may in the future choose to conduct one or more of its clinical trials or a portion of its clinical trials for its product candidates outside the United States. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of this data is subject to certain conditions imposed by the FDA. For example, the clinical trial must be well designed and conducted and performed by qualified investigators in accordance with GCP requirements, and, and FDA must be able to validate the data from the study through an onsite inspection, if required. In general, the patient population for any clinical studies conducted outside of the United States must be representative of the population for whom Oncternal intends to label the product in the United States. In addition, while these clinical trials are subject to the applicable local laws, FDA acceptance of the data will be dependent upon its determination that the studies also complied with all applicable U.S. laws and regulations. There can be no assurance the FDA will accept data from trial conducted outside of the United
74
States. If the FDA does not accept the data from Oncternal’s clinical trials of its product candidates, it would likely result in the need for additional trials, which would be costly and time consuming and delay or permanently halt Oncternal’s development of its product candidates.
Interim, topline and preliminary data from Oncternal’s clinical trials that Oncternal announces or publishes from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data.
From time to time, Oncternal may publicly disclose preliminary or topline data from Oncternal’s clinical studies, which are based on preliminary analyses of then-available data. Such preliminary results and related findings and conclusions are subject to change following more comprehensive reviews of the data related to the particular study or trial. Oncternal also makes assumptions, estimations, calculations and conclusions as part of its analyses of data, and Oncternal may not have received or had the opportunity to fully and carefully evaluate all data. As a result, the topline results that Oncternal reports may differ from future results of the same studies, or different conclusions or considerations may qualify such results once additional data have been received and fully evaluated. Topline data also remain subject to audit and verification procedures that may result in the final data being materially different from the preliminary data Oncternal previously published. As a result, topline data should be viewed with caution until the final data are available. From time to time, Oncternal may also disclose interim data from its clinical studies. Interim data from clinical trials that Oncternal may complete are subject to the risk that one or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data, and as more patient data become available. Adverse differences between preliminary or interim data and final data could significantly harm Oncternal’s business prospects.
Further, others, including regulatory agencies, may not accept or agree with Oncternal’s assumptions, estimates, calculations, conclusions or analyses of data from preclinical studies or clinical trials of its product candidates, or may interpret or weigh the importance of data differently, which could impact the value of the particular product candidate, the approvability or prospects for commercialization of the product candidate, or Oncternal, as a company, in general. In addition, the information Oncternal chooses to publicly disclose regarding a particular study or clinical trial is based on what is typically extensive information, and stockholders and others may not agree with what Oncternal determines is the material or otherwise appropriate information to include in its disclosure. Information that Oncternal decides not to disclose may ultimately be deemed significant with respect to future decisions, conclusions, views, activities or otherwise regarding a particular product, product candidate or Oncternal’s business. If the interim, topline or preliminary data that Oncternal discloses differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached by Oncternal based on its analyses of such data, Oncternal’s ability to obtain approval for, and commercialize its product candidates may be harmed, which could harm Oncternal’s business, operating results, prospects or financial condition.
Any breakthrough therapy designation that Oncternal may receive from the FDA for its product candidates may not lead to a faster development or regulatory review or approval process, and it does not increase the likelihood that its product candidates will receive marketing approval.
Oncternal may seek breakthrough therapy designation for some of its product candidates, including cirmtuzumab and TK216. A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. For drugs that have been designated as breakthrough therapies, interaction and communication between the FDA and the sponsor of the trial can help to identify the most efficient path for clinical development while minimizing the number of patients placed in ineffective control regimens. Drugs designated as breakthrough therapies by the FDA are also eligible for accelerated approval. Designation as a breakthrough therapy is within the discretion of the FDA. Accordingly, even if Oncternal believes one of its product candidates meets the criteria for designation as a breakthrough therapy, the FDA may disagree and
75
instead determine not to make such designation. The availability of breakthrough therapy designation was established with the passage of the Food and Drug Administration Safety and Innovation Act of 2012. Oncternal cannot be sure that any evaluation it may make of its product candidates as qualifying for breakthrough therapy designation will meet the FDA’s expectations. In any event, the receipt of a breakthrough therapy designation for a product candidate may not result in a faster development process, review or approval compared to drugs considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of Oncternal’s product candidates qualify as breakthrough therapies, the FDA may later decide that such product candidates no longer meet the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Risks Related to Oncternal’s Reliance on Third Parties
Oncternal relies on third parties to conduct many of its preclinical studies and clinical trials. Any failure by a third-party to conduct the clinical trials according to GLPs, GCPs and other requirements and in a timely manner may delay or prevent Oncternal’s ability to seek or obtain regulatory approval for or commercialize its product candidates.
Oncternal is dependent on third parties to conduct its clinical trials and preclinical studies, including Oncternal’s ongoing clinical trials for cirmtuzumab and TK216 and preclinical studies for ROR1CAR-T and Oncternal’s other development programs. Specifically, Oncternal has used and relied on, and intends to continue to use and rely on, medical institutions, clinical investigators, CROs and consultants to conduct Oncternal’s clinical trials in accordance with Oncternal’s clinical protocols and applicable regulatory requirements. These CROs, investigators and other third parties play a significant role in the conduct and timing of these trials and subsequent collection and analysis of data. While Oncternal has agreements governing the activities of its third-party contractors, Oncternal has limited influence over their actual performance. Nevertheless, Oncternal is responsible for ensuring that each of its clinical trials is conducted in accordance with the applicable protocol and legal, regulatory and scientific standards, and Oncternal’s reliance on the CROs and other third parties does not relieve Oncternal of its regulatory responsibilities. Oncternal and its CROs are required to comply with GCP requirements, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for all of Oncternal’s product candidates in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If Oncternal or any of its CROs or trial sites fail to comply with applicable GCPs, the clinical data generated in Oncternal’s clinical trials may be deemed unreliable, and the FDA or comparable foreign regulatory authorities may require Oncternal to perform additional clinical trials before approving Oncternal’s marketing applications. In addition, Oncternal’s clinical trials must be conducted with product produced under cGMP regulations. Oncternal’s failure to comply with these regulations may require Oncternal to repeat clinical trials, which would delay the regulatory approval process.
There is no guarantee that any such CROs, investigators or other third parties will devote adequate time and resources to such trials or perform as contractually required. If any of these third parties fail to meet expected deadlines, adhere to Oncternal’s clinical protocols or meet regulatory requirements, or otherwise performs in a substandard manner, Oncternal’s clinical trials may be extended, delayed or terminated. In addition, many of the third parties with whom Oncternal contracts may also have relationships with other commercial entities, including Oncternal’s competitors, for whom they may also be conducting clinical trials or other drug development activities that could harm Oncternal’s competitive position. In addition, principal investigators for Oncternal’s clinical trials may serve as scientific advisors or consultants to Oncternal from time to time and may receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, or the FDA concludes that the financial relationship may have affected the interpretation of the study, the integrity of the data generated at the applicable clinical trial site may be questioned and the utility of the clinical trial itself may be jeopardized, which could result in the delay or rejection of any BLA or NDA Oncternal submits to the FDA. Any such delay or rejection could prevent Oncternal from commercializing its product candidates.
76
If any of Oncternal’s relationships with these third parties terminate, Oncternal may not be able to enter into arrangements with alternative third parties or do so on commercially reasonable terms. Switching or adding additional CROs, investigators and other third parties involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays may occur, which can materially impact Oncternal’s ability to meet its desired clinical development timelines. Though Oncternal carefully manages its relationships with its CROs, investigators and other third parties, there can be no assurance that Oncternal will not encounter challenges or delays in the future or that these delays or challenges will not have a material adverse impact on Oncternal’s business, financial condition and prospects.
Oncternal relies on third parties for the manufacture of its product candidates for clinical and preclinical development and expects to continue to do so for the foreseeable future. This reliance on third parties increases the risk that Oncternal will not have sufficient quantities of its product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair Oncternal’s development or commercialization efforts.
Oncternal does not own or operate manufacturing facilities and has no plans to build its own clinical or commercial scale manufacturing capabilities. Oncternal relies, and expects to continue to rely, on third parties for the manufacture of its product candidates and related raw materials for clinical and preclinical development, as well as for commercial manufacture if any of Oncternal’s product candidates receive marketing approval. The facilities used by third-party manufacturers to manufacture Oncternal’s product candidates must be approved by the FDA or other regulatory agencies pursuant to inspections that will be conducted after Oncternal submits a BLA or an NDA to the FDA or their equivalent to other regulatory agencies. Oncternal does not control the manufacturing process of, and is completely dependent on, third-party manufacturers for compliance with cGMP requirements for manufacture of its drug products. If these third-party manufacturers cannot successfully manufacture material that conforms to Oncternal’s specifications and the strict regulatory requirements of the FDA or others, including requirements related to the manufacturing of high potency and pure compounds or other products, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. In addition, Oncternal has no control over the ability of third-party manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of Oncternal’s product candidates, or if regulatory authorities withdraw any such approval in the future, Oncternal may need to find alternative manufacturing facilities, which would significantly impact Oncternal’s ability to develop, obtain regulatory approval for or market its product candidates, if approved. Oncternal’s failure, or the failure of its third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on Oncternal, including clinical holds, fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of Oncternal’s products.
Oncternal’s or a third-party’s failure to execute on Oncternal’s manufacturing requirements, to do so on commercially reasonable terms, or to comply with cGMP could adversely affect Oncternal’s business in a number of ways, including:
| • | an inability to initiate or continue clinical trials of cirmtuzumab, TK216 or any future product candidates under development; |
| • | delay in submitting regulatory applications, or receiving marketing approvals, for Oncternal’s product candidates; |
| • | subjecting third-party manufacturing facilities to additional inspections by regulatory authorities; |
| • | requirements to cease development or to recall batches of Oncternal’s product candidates; and |
| • | in the event of approval to market and commercialize Oncternal’s product candidates, an inability to meet commercial demands for Oncternal’s product candidates. |
77
In addition, Oncternal may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms. Even if Oncternal is able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| • | failure of third-party manufacturers to comply with regulatory requirements and maintain quality assurance; |
| • | breach of the manufacturing agreement by the third-party; |
| • | failure to manufacture Oncternal’s product according to Oncternal’s specifications; |
| • | failure to manufacture Oncternal’s product according to Oncternal’s schedule, or at all; |
| • | misappropriation of Oncternal’s proprietary information, including Oncternal’s trade secretsand know-how; and |
| • | termination or nonrenewal of the agreement by the third-party at a time that is costly or inconvenient for Oncternal. |
Oncternal’s product candidates and any products that Oncternal may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for Oncternal.
Any performance failure on the part of Oncternal’s existing or future manufacturers could delay clinical development or marketing approval, and any related remedial measures may be costly or time consuming to implement. Oncternal does not currently have arrangements in place for redundant supply or a second source for all required raw materials used in the manufacture of Oncternal’s product candidates. If Oncternal’s current third-party manufacturers cannot perform as agreed, Oncternal may be required to replace such manufacturers and Oncternal may be unable to replace them on a timely basis or at all.
Oncternal’s current and anticipated future dependence upon others for the manufacture of Oncternal’s product candidates or products may adversely affect Oncternal’s future profit margins and Oncternal’s ability to commercialize any products that receive marketing approval on a timely and competitive basis.
Oncternal relies on a third party for the supply of ibrutinib in connection with its ongoing Phase 1b/2 clinical trial. If there are any delays in obtaining sufficient quantities of ibrutinib or if the costs of supplying ibrutinib materially increase, Oncternal’s Phase 1b/2 clinical trial could be delayed.
Oncternal relies on a third party for the supply of ibrutinib in connection with its ongoing Phase 1b/2 clinical trial. In April 2018, Oncternal entered into a clinical trial and supply agreement in support of a clinical trial to evaluate the combination of cirmtuzumab with ibrutinib, an inhibitor of Bruton’s tyrosine kinase (“BTK”), a key component of cell signaling inB-cells. Oncternal initiated a Phase 1b/2 clinical trial in May 2018 to assess cirmtuzumab in combination with ibrutinib in patients with CLL and MCL. Pursuant to the agreement, the third party has supplied ibrutinib up to a maximum aggregate amount for part 1 (a dose-finding arm) and part 2 (dose expansion arm) of the ongoing Phase 1b/2 clinical trial evaluating cirmtuzumab in combination with ibrutinib. Under the clinical trial and supply agreement, Oncternal is required to provide periodic reports, including safety data reports, and collaborate with the clinical supplier in relation to any interactions with regulatory authorities regarding ibrutinib, but the agreement includes no upfront costs, milestone or royalty payment commitments. In the event the agreement is terminated, Oncternal would likely incur substantial additional costs in order to obtain and purchase ibrutinib from a source other than Oncertnal’s current supplier and the Phase 1b/2 clinical trial may be delayed.
Oncternal’s reliance on third parties requires Oncternal to share its trade secrets, which increases the possibility that Oncternal’s trade secrets will be misappropriated or disclosed.
Because Oncternal currently relies on third parties to manufacture its product candidates and to perform quality testing, Oncternal must, at times, share its proprietary technology and confidential information, including trade
78
secrets, with them. Oncternal seeks to protect its proprietary technology, in part, by entering into confidentiality agreements, consulting agreements or other similar agreements with its advisors, employees, consultants and contractors prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose Oncternal’s confidential information. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by Oncternal’s competitors, are intentionally or inadvertently incorporated into the technology of others or are disclosed or used in violation of these agreements. Given that Oncternal’s proprietary position is based, in part, onOncternal’s know-how and trade secrets and despite Oncternal’s efforts to protect its trade secrets, a competitor’s discovery of Oncternal’s proprietary technology and confidential information or other unauthorized use or disclosure would impair Oncternal’s competitive position and may have a material adverse effect on Oncternal’s business, financial condition, results of operations and prospects.
Oncternal may seek to enter into collaborations, licenses and other similar arrangements and may not be successful in doing so, and even if Oncternal is, it may not realize the benefits of such relationships.
Oncternal may seek to enter into collaborations, joint ventures, licenses and other similar arrangements for the development or commercialization of Oncternal’s product candidates, due to capital costs required to develop or commercialize the product candidate or manufacturing constraints, in addition to our collaboration with SPH and SPH USA. Oncternal may not be successful in its efforts to establish such collaborations for Oncternal’s product candidates because its research and development pipeline may be insufficient, its product candidates may be deemed to be at too early of a stage of development for collaborative effort or third parties may not view Oncternal’s product candidates as having the requisite potential to demonstrate safety and efficacy or significant commercial opportunity. In addition, Oncternal faces significant competition in seeking appropriate strategic partners, and the negotiation process can be time consuming and complex. Further, any future collaboration agreements may restrict Oncternal from entering into additional agreements with potential collaborators. Oncternal cannot be certain that, following a strategic transaction or license, Oncternal will achieve an economic benefit that justifies such transaction.
Even if Oncternal is successful in its efforts to establish such collaborations, the terms that Oncternal agrees upon may not be favorable to Oncternal, and Oncternal may not be able to maintain such collaborations if, for example, development or approval of a product candidate is delayed, the safety of a product candidate is questioned or sales of an approved product candidate are unsatisfactory.
In addition, any potential future collaborations may be terminable by Oncternal’s strategic partners, and Oncternal may not be able to adequately protect its rights under these agreements. Furthermore, strategic partners may negotiate for certain rights to control decisions regarding the development and commercialization of Oncternal’s product candidates, if approved, and may not conduct those activities in the same manner as Oncternal would. Any termination of collaborations Oncternal enters into in the future, or any delay in entering into collaborations related to Oncternal’s product candidates, could delay the development and commercialization of Oncternal’s product candidates and reduce their competitiveness if they reach the market, which could have a material adverse effect on Oncternal’s business, financial condition and results of operations.
Risks Related to Commercialization of Oncternal’s Product Candidates
Even if Oncternal receives regulatory approval for any product candidate, Oncternal will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense. Additionally, Oncternal’s product candidates, if approved, could be subject to labeling and other restrictions on marketing or withdrawal from the market, and Oncternal may be subject to penalties if Oncternal fails to comply with regulatory requirements or if Oncternal experiences unanticipated problems with its product candidates, when and if any of them are approved.
Following potential approval of any of Oncternal’s product candidates, the FDA may impose significant restrictions on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly and
79
time consuming post-approval studies, post-market surveillance or clinical trials to monitor the safety and efficacy of the product. The FDA may also require a REMS as a condition of approval of Oncternal’s product candidates, which could include requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. In addition, if the FDA or a comparable foreign regulatory authority approves Oncternal’s product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping for Oncternal’s products will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and GCP requirements for any clinical trials that Oncternal conducts post-approval. Later discovery of previously unknown problems with Oncternal’s products, including adverse events of unanticipated type, severity or frequency, or with Oncternal’s third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of Oncternal’s products, withdrawal of the product from the market or voluntary or mandatory product recalls; |
| • | restrictions on product distribution or use, or requirements to conduct post-marketing studies or clinical trials; |
| • | fines, restitutions, disgorgement of profits or revenues, warning letters, untitled letters or holds on clinical trials; |
| • | refusal by the FDA to approve pending applications or supplements to approved applications filed by Oncternal or suspension or revocation of approvals; |
| • | product seizure or detention, or refusal to permit the import or export of Oncternal’s products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The occurrence of any event or penalty described above may inhibit Oncternal’s ability to commercialize its product candidates and generate revenue and could require Oncternal to expend significant time and resources in response and could generate negative publicity.
In addition, if any of Oncternal’s product candidates is approved, Oncternal’s product labeling, advertising and promotion will be subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about drug products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling. If Oncternal receives marketing approval for a product candidate, physicians may nevertheless prescribe it to their patients in a manner that is inconsistent with the approved label. If Oncternal is found to have promotedsuch off-label uses, Oncternal may become subject to significant liability. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotionof off-label uses, and a company that is found to have improperlypromoted off-label uses may be subject to significant sanctions. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engagingin off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed.
The FDA and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of Oncternal’s product candidates. If Oncternal is slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if Oncternal is not able to maintain regulatory compliance, Oncternal may lose any marketing approval that Oncternal may have obtained and Oncternal may not achieve or sustain profitability.
Oncternal also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad. For example, certain
80
policies of the current U.S. administration may impact Oncternal’s business and industry. Namely, the current U.S. administration has taken several executive actions, including the issuance of a number of Executive Orders, that could impose significant burdens on, or otherwise materially delay, the FDA’s ability to engage in routine regulatory and oversight activities such as implementing statutes through rulemaking, issuance of guidance, and review and approval of marketing applications. It is difficult to predict how these executive actions, including any Executive Orders, will be implemented, and the extent to which they will impact the FDA’s ability to exercise its regulatory authority. If these executive actions impose constraints on the FDA’s ability to engage in oversight and implementation activities in the normal course, Oncternal’s business may be negatively impacted.
If Oncternal is slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if Oncternal is not able to maintain regulatory compliance, Oncternal may lose any marketing approval that Oncternal may have obtained and Oncternal may not achieve or sustain profitability, which would adversely affect Oncternal’s business, prospects, financial condition and results of operations.
Changes in funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new products and services from being developed or commercialized in a timely manner, which could negatively impact Oncternal’s business.
The ability of the FDA and other regulatory agencies to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs and biologics to be reviewed and/or approved by necessary government agencies, which would adversely affect Oncternal’s business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process Oncternal’s regulatory submissions, which could have a material adverse effect on its business.
The commercial success of Oncternal’s product candidates will depend upon the degree of market acceptance of such product candidates by physicians, patients, healthcare payors and others in the medical community.
Oncternal’s product candidates may not be commercially successful. Even if any of Oncternal’s product candidates receive regulatory approval, they may not gain market acceptance among physicians, patients, healthcare payors or the medical community. The commercial success of any of Oncternal’s current or future product candidates will depend significantly on the broad adoption and use of the resulting product by physicians and patients for approved indications. The degree of market acceptance of Oncternal’s products will depend on a number of factors, including:
| • | demonstration of clinical efficacy and safety compared to other more-established products; |
| • | the indications for which Oncternal’s product candidates are approved; |
| • | the limitation of Oncternal’s targeted patient population and other limitations or warnings contained inany FDA-approved labeling; |
| • | acceptance of a new drug for the relevant indication by healthcare providers and their patients; |
| • | the pricing and cost-effectiveness of Oncternal’s products, as well as the cost of treatment with Oncternal’s products in relation to alternative treatments and therapies; |
81
| • | Oncternal’s ability to obtain and maintain sufficient third-party coverage and adequate reimbursement from government healthcare programs, including Medicare and Medicaid, private health insurers and other third-party payors; |
| • | the willingness of patients to pay all, or a portionof, out-of-pocket costs associated with Oncternal’s products in the absence of sufficient third-party coverage and adequate reimbursement; |
| • | any restrictions on the use of Oncternal’s products, and the prevalence and severity of any adverse effects; |
| • | potential product liability claims; |
| • | the timing of market introduction of Oncternal’s products as well as competitive drugs; |
| • | the effectiveness of Oncternal’s or any of its potential future collaborators’ sales and marketing strategies; and |
| • | unfavorable publicity relating to the product. |
If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, hospitals, healthcare payors or patients, Oncternal may not generate sufficient revenue from that product and may not become or remain profitable. Oncternal’s efforts to educate the medical community and third-party payors regarding the benefits of Oncternal’s products may require significant resources and may never be successful.
The market opportunities for Oncternal’s product candidates may be limited to patients who are ineligible for or have failed prior treatments and may be small or different from Oncternal’s estimates.
Cancer therapies are sometimes characterized as first line, second line or third line, and the FDA often approves new therapies initially only for third line use. When cancer is detected early enough, first line therapy is sometimes adequate to cure the cancer or prolong life without a cure. Whenever first line therapy, including targeted therapy, immunotherapy, chemotherapy, hormone therapy, surgery or a combination of these, proves unsuccessful, second line therapy may be administered. Second line therapies often consist of more chemotherapy, radiation, antibody drugs, tumor targeted small molecules or a combination of these. Third line therapies can include bone marrow transplantation, antibody and small molecule targeted therapies, more invasive forms of surgery and new technologies. In markets with approved therapies, there is no guarantee that Oncternal’s product candidates, even if approved, would be approved for second line or first line therapy. This could limit Oncternal’s potential market opportunity. In addition, Oncternal may have to conduct additional clinical trials prior to gaining approval for second line or first line therapy.
Oncternal’s projections of both the number of people who have the cancers it is targeting, as well as the subset of people with these cancers in a position to receive later stage therapy and who have the potential to benefit from treatment with its product candidates, are based on Oncternal’s beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations or market research and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these cancers. The number of patients may turn out to be lower than expected. In addition, the potentially addressable patient population for Oncternal’s product candidates may be limited or may not be amenable to treatment with its product candidates. Even if Oncternal obtains significant market share for its product candidates, it may never achieve profitability without obtaining regulatory approval for additional indications, including use as a first or second line therapy.
Any product candidates for which Oncternal intends to seek approval as biologic products may face competition sooner than anticipated.
The Affordable Care Act includes a subtitle called the Biologics Price Competition and Innovation Act of 2009 (“BPCIA”), which created an abbreviated approval pathway for biological products that are biosimilar to or
82
interchangeable with anFDA-licensed reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of its product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning are subject to uncertainty, and any processes adopted by the FDA to implement the BPCIA could have a material adverse effect on the future commercial prospects for Oncternal’s biological products.
Oncternal believes that any of its future product candidates approved as a biological product under a BLA should qualify for the12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to Congressional action or otherwise, or that the FDA will not consider Oncternal’s product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, could be substituted for any one of our reference products in a way that is similar to traditional generic substitution fornon-biological products will depend on a number of marketplace and regulatory factors that are still developing.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotionof off-label uses. If Oncternal is found or alleged to have improperlypromoted off-label uses, Oncternal may become subject to significant liability.
The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, as Oncternal’s product candidates would be, if approved. In particular, a product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. If Oncternal is found to have promotedsuch off-label uses, it may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engagingin off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. If Oncternal cannot successfully manage the promotion and avoidoff-label promotion of its product candidates, if approved, it could become subject to significant liability, which would materially adversely affect Oncternal’s business and financial condition.
The successful commercialization of Oncternal’s product candidates, if approved, will depend in part on the extent to which governmental authorities and health insurers establish coverage, adequate reimbursement levels and favorable pricing policies. Failure to obtain or maintain coverage and adequate reimbursement for Oncternal’s products could limit its ability to market those products and decrease its ability to generate revenue.
The availability of coverage and the adequacy of reimbursement by governmental healthcare programs such as Medicare and Medicaid, private health insurers and other third-party payors are essential for most patients to be able to afford prescription medications such as Oncternal’s product candidates, if approved. Oncternal’s ability to achieve coverage and acceptable levels of reimbursement for Oncternal’s products by third-party payors will have an effect on Oncternal’s ability to successfully commercialize those products. Even if Oncternal obtains coverage for a given product by a third-party payor, the resulting reimbursement payment rates may not be adequate or mayrequire co-payments that patients find unacceptably high. Oncternal cannot be sure that coverage and reimbursement in the United States, the European Union or elsewhere will be available for any product that Oncternal may develop, and any reimbursement that may become available may be decreased or eliminated in the future.
83
Third-party payors increasingly are challenging prices charged for pharmaceutical products and services, and many third-party payors may refuse to provide coverage and reimbursement for particular drugs when an equivalent generic drug or a less expensive therapy is available. It is possible that a third-party payor may consider Oncternal’s products as substitutable and only offer to reimburse patients for the less expensive product. Even if Oncternal is successful in demonstrating improved efficacy or improved convenience of administration with Oncternal’s products, pricing of existing drugs may limit the amount Oncternal will be able to charge for its products. These payors may deny or revoke the reimbursement status of a given product or establish prices for new or existing marketed products at levels that are too low to enable Oncternal to realize an appropriate return on its investment in product development. If reimbursement is not available or is available only at limited levels, Oncternal may not be able to successfully commercialize its products and may not be able to obtain a satisfactory financial return on products that Oncternal may develop.
There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved products. In the United States, third-party payors, including private and governmental payors, such as the Medicare and Medicaid programs, play an important role in determining the extent to which new drugs will be covered. Some third-party payors mayrequire pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for Oncternal’s products.
Obtaining and maintaining reimbursement status is time consuming, costly and uncertain. The Medicare and Medicaid programs increasingly are used as models for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs. However, no uniform policy for coverage and reimbursement for products exists among third-party payors in the United States. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time consuming and costly process that will require Oncternal to provide scientific and clinical support for the use of Oncternal’s products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and Oncternal believes that changes in these rules and regulations are likely.
Additionally, Oncternal or its collaborators may develop companion diagnostic tests for use with its product candidates as Oncternal is targeting certain defined populations for its treatments. Oncternal, or its collaborators, will be required to obtain coverage and reimbursement for these tests separate and apart from the coverage and reimbursement sought for its product candidates, once approved. While Oncternal, or its collaborators, has not yet developed any companion diagnostic test for its product candidates, if it does, there is significant uncertainty regarding Oncternal’s ability to obtain approval, coverage and adequate reimbursement for the same reasons applicable to its product candidates.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and Oncternal believes the increasing emphasis on cost-containment initiatives in Europe and other countries has and will continue to put pressure on the pricing and usage of Oncternal’s products. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that Oncternal is able to charge for its products. Accordingly, in markets outside the United States, the reimbursement for Oncternal’s products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for Oncternal’s
84
products. Oncternal expects to experience pricing pressures in connection with the sale of any of its products due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products.
Oncternal faces significant competition, and if its competitors develop technologies or product candidates more rapidly than Oncternal does or their technologies are more effective, Oncternal’s ability to develop and successfully commercialize products may be adversely affected.
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary and novel products and product candidates. Oncternal’s competitors have developed, are developing or may develop products, product candidates and processes competitive with Oncternal’s product candidates. Any product candidates that Oncternal successfully develops and commercializes will compete with existing therapies and new therapies that may become available in the future. Oncternal believes that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which Oncternal may attempt to develop product candidates. In particular, there is intense competition in the fields of immunology, inflammation and oncology. Oncternal’s competitors include larger and better funded pharmaceutical, biopharmaceutical, biotechnological and therapeutics companies. Moreover, Oncternal may also compete with universities and other research institutions who may be active in the indications Oncternal is targeting and could be in direct competition with Oncternal. Oncternal also competes with these organizations to recruit management, scientists and clinical development personnel, which could negatively affect its level of expertise and its ability to execute its business plan. Oncternal will also face competition in establishing clinical trial sites, enrolling subjects for clinical trials and in identifyingand in-licensing new product candidates. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
If any of our product candidates are approved in oncology indications such as CLL or MCL, they will compete with small molecule therapies, biologics, cell-based therapies and vaccines, either approved or under development, that are intended to treat the same cancers that Oncternal is targeting, including through approaches that may prove to be more effective, have fewer side effects, be less costly to manufacture, be more convenient to administer or have other advantages over any product candidates Oncternal develops. In addition to competing with other therapies targeting similar indications, there are numerous other companies and academic institutions focused on similar targets as our product candidates and/or different scientific approaches to treating the same indications. Oncternal faces competition from such companies in seeking any future potential collaborations to partner our product candidates, as well as potentially competing commercially for any approved products.
CLL has traditionally been treated with standard cytotoxic agents such as fludarabine, chlorambucil, cyclophosphamide, and bendamustine. Rituximab, marketed as Rituxan by Genentech, which is a monoclonal antibody that specifically recognizes CD20, an antigen onB-cells from which the tumor cells in CLL arise, was approved for use in CLL in 2010, but was previously widely prescribedoff-label. Rituximab, which is typically used to treat patients with CLL in combination with cytotoxic agents, remains a treatment option for younger patients who can tolerate the side effects of the associated chemotherapy. Regulatory authorities have also approved other monoclonal antibody products that target CD20, as well as antibodies targeting another surface protein found on CLL tumor cells known as CD52, and three classes of small molecules: ibrutinib, venetoclax, an inhibitor of the proteinB-celllymphoma-2(“Bcl-2”), which is marketed as Venclexta and Venclyxto by AbbVie and Roche/Genentech, and idelalisib, an inhibitor of Phosphoinositide3-kinase (“PI3K”), which is marketed as Zydelig by Gilead Sciences. These agents are approved for use as single agents, but are being investigated in combination with each other and with various monoclonal antibody products. Additionally, clinicians are investigating their potential in earlier stage disease in multiple clinical trials.
85
There are several therapeutic options available to treat MCL. Newly diagnosed patients are typically treated with rituximab combined with a chemotherapy regimen known as CHOP, comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone. Alternative chemotherapy regimens include bortezomib or bendamustine. Patients with clinical responses to chemotherapy may become candidates for another therapeutic approach, autologous stem cell transplantation, a procedure in which radiation and/or chemotherapy is used to eliminate the patient’s immune cells, including residual MCL cells. Recently, ibrutinib was granted accelerated approval by the FDA for the treatment of relapsed MCL.
The current standard therapy for patients with localized Ewing sarcoma in the U.S. is a combination of chemotherapy agents, including vincristine, doxorubicin and cyclophosphamide, with alternating cycles of ifosfamide and etoposide, which is a therapy known as VDC/IE. This may also be supplemented by local radiation therapy or systemic radiation followed by autologous hematopoietic stem cell transplant.
Many of Oncternal’s competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources or experience than Oncternal does. If Oncternal successfully obtains approval for any product candidate, Oncternal will face competition based on many different factors, including the safety and effectiveness of Oncternal’s products, the ease with which Oncternal’s products can be administered and the extent to which patients accept relatively new routes of administration, the timing and scope of regulatory approvals for these products, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position. Competing products could present superior treatment alternatives, including by being more effective, safer, more convenient, less expensive or marketed and sold more effectively than any products Oncternal may develop. Competitive products may make any products Oncternal develops obsolete or noncompetitive before Oncternal recovers the expense of developing and commercializing Oncternal’s product candidates. If Oncternal is unable to compete effectively, Oncternal’s opportunity to generate revenue from the sale of its products it may develop, if approved, could be adversely affected.
If the market opportunities for Oncternal’s products are smaller than Oncternal believes they are, Oncternal’s revenue may be adversely affected, and its business may suffer.
The precise incidence and prevalence for all the conditions Oncternal aims to address with its product candidates are unknown. Oncternal’s projections of both the number of people who have these diseases, the number who have the specific indicated stage or treatment history Oncternal believes will be the approved indication, as well as the subset of people with these diseases who have the potential to benefit from treatment with Oncternal’s product candidates, are based on Oncternal’s beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, surveys of clinics, patient foundations or market research, and may prove to be incorrect. Further, new trials may change the estimated incidence or prevalence of these diseases. The total addressable market across all of Oncternal’s product candidates will ultimately depend upon, among other things, the indication approved by regulatory agencies and the diagnostic criteria included in the final label for each of Oncternal’s product candidates approved for sale for these indications, the availability of alternative treatments and the safety, convenience, cost and efficacy of Oncternal’s product candidates relative to such alternative treatments, acceptance by the medical community and patient access, drug pricing and reimbursement. The number of patients in the United States and other major markets and elsewhere may turn out to be lower than expected, patients may not be otherwise amenable to treatment with Oncternal’s products or new patients may become increasingly difficult to identify or gain access to, all of which would adversely affect Oncternal’s results of operations and its business. Further, even if Oncternal obtains significant market share for its product candidates, because some of Oncternal’s potential target populations are very small, Oncternal may never achieve profitability despite obtaining such significant market share.
86
Oncternal currently has no marketing and sales organization and has no experience as a company in commercializing products, and Oncternal may have to invest significant resources to develop these capabilities. If Oncternal is unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell its products, Oncternal may not be able to generate product revenue.
Oncternal has no internal sales, marketing or distribution capabilities, nor has it commercialized a product. If any of Oncternal’s product candidates ultimately receives regulatory approval, Oncternal must build a marketing and sales organization with technical expertise and supporting distribution capabilities to commercialize each such product in major markets, which will be expensive and time consuming, or collaborate with third parties that have sales forces and established distribution systems, either to augment Oncternal’s own sales force and distribution systems or in lieu of Oncternal’s own sales force and distribution systems. Oncternal has no prior experience as a company in the marketing, sale and distribution of biopharmaceutical products and there are significant risks involved in building and managing a sales organization, including Oncternal’s ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of Oncternal’s internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products. Oncternal may not be able to enter into collaborations or hire consultants or external service providers to assist Oncternal in sales, marketing and distribution functions on acceptable financial terms, or at all. In addition, Oncternal’s product revenues and its profitability, if any, may be lower if Oncternal relies on third parties for these functions than if Oncternal were to market, sell and distribute any products that Oncternal develops itself. Oncternal likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market Oncternal’s products effectively. If Oncternal is not successful in commercializing its products, either on its own or through arrangements with one or more third parties, Oncternal may not be able to generate any future product revenue and Oncternal would incur significant additional losses.
Oncternal’s future growth may depend, in part, on its ability to operate in foreign markets, where Oncternal would be subject to additional regulatory burdens and other risks and uncertainties.
Oncternal’s future growth may depend, in part, on its ability to develop and commercialize its product candidates in foreign markets. Oncternal is not permitted to market or promote any of its product candidates before it receives regulatory approval from applicable regulatory authorities in foreign markets, and Oncternal may never receive such regulatory approvals for any of its product candidates. To obtain separate regulatory approval in most other countries Oncternal must comply with numerous and varying regulatory requirements regarding safety and efficacy and governing, among other things, clinical trials, commercial sales, manufacturing, pricing and distribution of Oncternal’s product candidates. If Oncternal receives regulatory approval of its product candidates and ultimately commercialize its products in foreign markets, Oncternal would be subject to additional risks and uncertainties, including:
| • | different regulatory requirements for approval of drugs in foreign countries; |
| • | reduced protection for intellectual property rights; |
| • | the existence of additional third-party patent rights of potential relevance to Oncternal’s business; |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; |
| • | foreign reimbursement, pricing and insurance regimes; |
| • | workforce uncertainty in countries where labor unrest is common; |
87
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
Risks Related to Oncternal’s Business Operations and Industry
Oncternal’s operating results may fluctuate significantly, which makes Oncternal’s future operating results difficult to predict and could cause Oncternal’s operating results to fall below expectations or any guidance it may provide.
Oncternal’s quarterly and annual operating results may fluctuate significantly, which makes it difficult for Oncternal to predict its future operating results. These fluctuations may occur due to a variety of factors, many of which are outside of Oncternal’s control, including, but not limited to:
| • | the timing and cost of, and level of investment in, research, development, regulatory approval and commercialization activities relating to Oncternal’s product candidates, which may change from time to time; |
| • | coverage and reimbursement policies with respect to Oncternal’s product candidates, if approved, and potential future drugs that compete with Oncternal’s products; |
| • | the cost of manufacturing Oncternal’s product candidates, which may vary depending on the quantity of production and any manufacturing issues or challenges requiring additional manufacturing activities, and the terms of Oncternal’s agreements with third-party manufacturers; |
| • | the timing and amount of any milestone or other payments Oncternal must make to the licensors and other third parties from whom Oncternalhas in-licensed or acquired its product candidates; |
| • | expenditures that Oncternal may incur to acquire, develop or commercialize additional product candidates and technologies; |
| • | the level of demand for any approved products, which may vary significantly; |
| • | future accounting pronouncements or changes in Oncternal’s accounting policies; and |
| • | the timing and success or failure of preclinical studies or clinical trials for Oncternal’s product candidates or competing product candidates, or any other change in the competitive landscape of Oncternal’s industry, including consolidation among Oncternal’s competitors or partners. |
The cumulative effects of these factors could result in large fluctuations and unpredictability in Oncternal’s quarterly and annual operating results. As a result, comparing Oncternal’s operating results ona period-to-period basis may not be meaningful. Investors should not rely on Oncternal’s past results as an indication of its future performance.
This variability and unpredictability could also result in Oncternal’s failing to meet the expectations of industry or financial analysts or investors for any period. If Oncternal’s revenue or operating results fall below the expectations of analysts or investors or below any forecasts Oncternal may provide to the market, or if the forecasts Oncternal provides to the market are below the expectations of analysts or investors, the price of Oncternal’s common stock could decline substantially. Such a stock price decline could occur even when Oncternal has met any previously publicly stated revenue or earnings guidance Oncternal may provide.
Oncternal is dependent on the services of its management and if it is not able to retain these individuals or recruit additional management or other key personnel, Oncternal’s business will suffer.
Oncternal’s success depends in part on its continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel. Oncternal is highly dependent upon its senior management,
88
particularly its Chief Executive Officer, as well as other members of its senior management team. The loss of services of any of these individuals could delay or prevent the successful development of Oncternal’s product pipeline, initiation or completion of Oncternal’s planned operations, planned clinical trials or the commercialization of Oncternal’s product candidates. Although Oncternal has executed employment agreements or offer letters with each member of its senior management team, these agreements are terminable at will with or without notice and, therefore, Oncternal may not be able to retain their services as expected. Oncternal does not currently maintain “key person” life insurance on the lives of any of its employees. This lack of insurance means that Oncternal may not have adequate compensation for the loss of the services of these individuals.
Oncternal will need to expand and effectively manage its managerial, operational, financial and other resources in order to successfully pursue its clinical development and commercialization efforts. Oncternal may not be successful in maintaining its unique company culture and continuing to attract or retain qualified management and scientific and clinical personnel in the future due to the intense competition for qualified personnel among pharmaceutical, biotechnology and other businesses, particularly in the San Diego area. Oncternal’s industry has experienced a high rate of turnover of management personnel in recent years. If Oncternal is not able to attract, integrate, retain and motivate necessary personnel to accomplish its business objectives, Oncternal may experience constraints that will significantly impede the achievement of its development objectives, its ability to raise additional capital and its ability to implement its business strategy.
Oncternal may encounter difficulties in managing its growth and expanding its operations successfully.
As of March 31, 2019, Oncternal had five full-time employees and three part-time employees. As Oncternal continues research and development activities and pursues the potential commercialization of its product candidates, as well as function as a public company, Oncternal will need to expand its financial, research, development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for the company. As Oncternal’s operations expand, it expects that it will need to manage additional relationships with various strategic partners, suppliers and other third parties. Oncternal’s future financial performance and its ability to develop and commercialize its product candidates and to compete effectively will depend, in part, on its ability to manage any future growth effectively.
Oncternal is subject to various foreign, federal, and state healthcare and privacy laws and regulations, and Oncternal’s failure to comply with these laws and regulations could harm its results of operations and financial condition.
Oncternal’s business operations and current and future arrangements with investigators, healthcare professionals, consultants, third-party payors and customers expose Oncternal to broadly applicable foreign, federal and state fraud and abuse and other healthcare and privacy laws and regulations. These laws may constrain the business or financial arrangements and relationships through which Oncternal conducts its operations, including how Oncternal researches, markets, sells and distributes any products for which it obtains marketing approval. Such laws include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or providing any remuneration (including any kickback, bribe or certain rebates), directly or indirectly, overtly or covertly, in cash or in kind, in return for, either the referral of an individual or the purchase, lease, or order, or arranging for or recommending the purchase, lease, or order of any good, facility, item or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the federal Anti- Kickback Statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act; |
| • | the federal false claims and civil monetary penalties laws, including the civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be |
89
presented, to the federal government, claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or from knowingly making or causing to be made a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”), and their implementing regulations, also impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information without appropriate authorization by covered entities subject to the rule, such as health plans, healthcare clearinghouses and certain healthcare providers as well as their business associates that perform certain services for or on their behalf involving the use or disclosure of individually identifiable health information; |
| • | the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare and Medicaid Services (“CMS”), information related to payments and other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members; |
| • | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and |
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to Oncternal’s business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursedby non- governmental third-party payors, including private insurers, or by the patients themselves; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information or which require tracking gifts and other remuneration and items of value provided to physicians, other healthcare providers and entities; state and local laws that require the registration of pharmaceutical sales representatives; state and foreign laws governing the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA; state and foreign governments that have enacted or proposed requirements regarding the collection, distribution, use, security, and storage of personally identifiable information and other data relating to individuals (including the EU General Data Protection Regulation 2016/679 (“GDPR”), and the California Consumer Protection Act (“CCPA”)), and federal and state consumer protection laws are being applied to enforce regulations related to the online collection, use, and dissemination of data, thus complicating compliance efforts. |
As of May 25, 2018, the GDPR replaced the Data Protection Directive with respect to the processing of personal data in the European Union. The GDPR imposes many requirements for controllers and processors of personal data, including, for example, higher standards for obtaining consent from individuals to process their personal
90
data, more robust disclosures to individuals and a strengthened individual data rights regime, shortened timelines for data breach notifications, limitations on retention and secondary use of information, increased requirements pertaining to health data and pseudonymised (i.e.,key-coded) data and additional obligations when Oncternal contracts third-party processors in connection with the processing of the personal data. The GDPR allows EU member states to make additional laws and regulations further limiting the processing of genetic, biometric or health data. Failure to comply with the requirements of GDPR and the applicable national data protection laws of the EU member states may result in fines of up to €20 million or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, and other administrative penalties.
Ensuring that Oncternal’s internal operations and business arrangements with third parties comply with applicable healthcare laws and regulations could involve substantial costs. It is possible that governmental authorities will conclude that Oncternal’s business practices, including its consulting arrangements with physicians and other healthcare providers, some of whom received stock options as compensation for services provided, do not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. If Oncternal’s operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that may apply to Oncternal, Oncternal may be subject to significant penalties, including civil, criminal and administrative penalties, damages, fines, exclusion from U.S. government funded healthcare programs, such as Medicare and Medicaid, or similar programs in other countries or jurisdictions, disgorgement, individual imprisonment, contractual damages, reputational harm, additional reporting requirements and oversight if Oncternal becomes subject to a corporate integrity agreement or similar agreement to resolve allegationsof non-compliance with these laws, diminished profits and the curtailment or restructuring of Oncternal’s operations. Further, defending against any such actions can be costly, time consuming and may require significant financial and personnel resources. Therefore, even if Oncternal is successful in defending against any such actions that may be brought against Oncternal, its business may be impaired. If any of the physicians or other providers or entities with whom Oncternal expects to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusion from government funded healthcare programs and imprisonment. If any of the above occur, it could adversely affect Oncternal’s ability to operate its business and its results of operations.
Recently enacted legislation, future legislation and healthcare reform measures may increase the difficulty and cost for Oncternal to obtain marketing approval for and commercialize its product candidates and may affect the prices Oncternal may set.
In the United States and some foreign jurisdictions, there have been, and Oncternal expects there will continue to be, a number of legislative and regulatory changes to the healthcare system, including cost-containment measures that may reduce or limit coverage and reimbursement for newly approved drugs and affect Oncternal’s ability to profitably sell any product candidates for which Oncternal obtains marketing approval. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare.
For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act, was enacted in the United States. Among the provisions of the Affordable Care Act of importance to Oncternal’s potential product candidates, the Affordable Care Act: establishes an annual, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents; extends manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations; expands eligibility criteria for Medicaid programs; expands the entities eligible for discounts under the Public Health program; increases the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; creates a new Medicare Part D coverage gap discount program; establishes a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in and conduct comparative clinical effectiveness research, along with funding for such research; and establishes a Center for Medicare Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending.
91
At this time, Oncternal is unsure of the full impact that the Affordable Care Act will have on its business. There have been judicial and political challenges to certain aspects of the Affordable Care Act. For example, since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements of the Affordable Care Act. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the Affordable Care Act. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the Affordable Care Act have been signed into law. The Tax Act includes a provision repealing, effective January 1, 2019,the tax-based shared responsibility payment imposed by the Affordable Care Act on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain AffordableCare Act-mandated fees, includingthe so-called ”Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share. The Bipartisan Budget Act of 2018 (the “BBA”), among other things, amends the Affordable Care Act, effective January 1, 2019, to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole,” by increasing from 50 percent to 70 percentthe point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D. In July 2018, CMS published a final rule permitting further collections and payments to and from certain Affordable Care Act qualified health plans and health insurance issuers under the Affordable Care Act risk adjustment program in response to the outcome of federal district court litigation regarding the method CMS uses to determine this risk adjustment. On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, or Texas District Court Judge, ruled that the individual mandate is a critical and inseverable feature of the ACA, and therefore, because it was repealed as part of the Tax Act, the remaining provisions of the ACA are invalid as well. While the Texas District Court Judge, as well as the Trump Administration and CMS, have stated that the ruling will have no immediate effect, it is unclear how this decision, subsequent appeals, and other efforts to repeal and replace the ACA will impact the ACA and Oncternal’s business.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, resulted in reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, including the BBA, will remain in effect through 2027 unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Further, there has been heightened governmental scrutiny in the United States of pharmaceutical pricing practices in light of the rising cost of prescription drugs. Such scrutiny has resulted in several recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products.
At the federal level, the Trump administration’s budget proposal for fiscal year 2019 contains further drug price control measures that could be enacted during the 2019 budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid and to eliminate cost sharing for generic drugsfor low-income patients. Additionally, the Trump administration released a “Blueprint” to lower drug prices through proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. The U.S. Department of Health and Human Services has begun the process of soliciting feedback on some of these measures and, at the same time, is implementing others under its existing authority. Although some of these, and other, proposals will require authorization through additional
92
legislation to become effective, Congress and the Trump administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm Oncternal’s business, results of operations, financial condition and prospects. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. This could reduce the ultimate demand for Oncternal’s product candidates, if approved, or put pressure on Oncternal’s product pricing, which could negatively affect its business, results of operations, financial condition and prospects.
Additionally, on May 30, 2018, the Trickett Wendler, Frank Mongiello, Jordan McLinn, and Matthew Bellina Right to Try Act of 2017 (“Right to Try Act”), was signed into law. The law, among other things, provides a federal framework for certain patients with life-threatening diseases or conditions to access certain investigational new drug products that have completed a Phase 1 clinical trial. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without obtaining FDA approval under the FDA expanded access program. There is no obligation for a drug manufacturer to make its drug products available to eligible patients as a result of the Right to Try Act.
Oncternal expects that the Affordable Care Act, these new laws and other healthcare reform measures that may be adopted in the future may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and additional downward pressure on the price that Oncternal receives for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent Oncternal from being able to generate revenue, attain profitability or commercialize its product candidates, if approved.
Oncternal and any of its third-party manufacturers or suppliers may use potent chemical agents and hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly.
Oncternal and any of its third-party manufacturers or suppliers will use biological materials, potent chemical agents and may use hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety of the environment. Oncternal’s historical operations and the operations of its third-party manufacturers and suppliers also produce hazardous waste products. Federal, state and local laws and regulations govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair Oncternal’s product development efforts. In addition, Oncternal cannot eliminate the risk of accidental injury or contamination from these materials or wastes. Oncternal does not carry specific biological or hazardous waste insurance coverage, and Oncternal’s property, casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. In the event of contamination or injury, Oncternal could be held liable for damages or be penalized with fines in an amount exceeding its resources, and its clinical trials or regulatory approvals could be suspended.
Although Oncternal maintains workers’ compensation insurance for certain costs and expenses it may incur due to injuries to Oncternal’s employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. Oncternal does not maintain insurance for toxic tort claims that may be asserted against Oncternal in connection with its storage or disposal of biologic, hazardous or radioactive materials.
93
In addition, Oncternal may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations, which have tended to become more stringent over time. These current or future laws and regulations may impair Oncternal’s research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions or liabilities, which could materially adversely affect Oncternal’s business, financial condition, results of operations and prospects.
If product liability lawsuits are brought against Oncternal, Oncternal may incur substantial liabilities and may be required to limit commercialization of its products.
Oncternal faces an inherent risk of product liability as a result of the clinical trials of Oncternal’s product candidates and will face an even greater risk if Oncternal commercializes its product candidates. For example, Oncternal may be sued if its product candidates allegedly cause injury or are found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product candidate, negligence, strict liability and a breach of warranties. Claims may be brought against Oncternal by clinical trial participants, patients or others using, administering or selling products that may be approved in the future. Claims could also be asserted under state consumer protection acts.
If Oncternal cannot successfully defend itself against product liability claims, Oncternal may incur substantial liabilities or be required to limit or cease the commercialization of its products. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | decreased demand for Oncternal’s products; |
| • | injury to Oncternal’s reputation and significant negative media attention; |
| • | withdrawal of clinical trial participants; |
| • | costs to defend the related litigation; |
| • | a diversion of management’s time and Oncternal’s resources; |
| • | substantial monetary awards to trial participants or patients; |
| • | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| • | significant negative financial impact; |
| • | the inability to commercialize Oncternal’s product candidates; and |
| • | a decline in Oncternal’s stock price. |
Oncternal currently holds approximately $10.0 million in product liability insurance coverage in the aggregate. Oncternal may need to increase its insurance coverage as it expands its clinical trials or if it commences commercialization of its product candidates. Insurance coverage is increasingly expensive. Oncternal’s inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of Oncternal’s product candidates. Although Oncternal maintains such insurance, any claim that may be brought against it could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by Oncternal’s insurance or that is in excess of the limits of its insurance coverage. Oncternal’s insurance policies will also have various exclusions, and Oncternal may be subject to a product liability claim for which it has no coverage. Oncternal may have to pay any amounts awarded by a court or negotiated in a settlement that exceed its coverage limitations or that are not covered by its insurance, and Oncternal may not have, or be able to obtain, sufficient capital to pay such amounts.
94
Oncternal and any of its potential future collaborators will be required to report to regulatory authorities if any of Oncternal’s approved products cause or contribute to adverse medical events, and any failure to do so would result in sanctions that would materially harm Oncternal’s business.
If Oncternal and any of its potential future collaborators are successful in commercializing Oncternal’s products, the FDA and foreign regulatory authorities would require that Oncternal and any of its potential future collaborators report certain information about adverse medical events if those products may have caused or contributed to those adverse events. The timing of Oncternal’s obligation to report would be triggered by the date Oncternal becomes aware of the adverse event as well as the nature of the event. Oncternal and any of its potential future collaborators or CROs may fail to report adverse events within the prescribed timeframe. If Oncternal or any of its potential future collaborators or CROs fail to comply with such reporting obligations, the FDA or a foreign regulatory authority could take action, including criminal prosecution, the imposition of civil monetary penalties, seizure of Oncternal’s products or delay in approval or clearance of future products.
Oncternal’s internal computer systems, or those of any of its CROs, manufacturers, other contractors or consultants or potential future collaborators, may fail or suffer security breaches, which could result in a material disruption of Oncternal’s product development programs.
The United States federal and various state and foreign governments have adopted or proposed requirements regarding the collection, distribution, use, security, and storage of personally identifiable information and other data relating to individuals, and federal and state consumer protection laws are being applied to enforce regulations related to the online collection, use, and dissemination of data. Despite the implementation of security measures, Oncternal’s internal computer systems and those of its current and any future CROs and other contractors, consultants and collaborators are vulnerable to damage from computer viruses, cybersecurity threats, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If such an event were to occur and cause interruptions in Oncternal’s operations or result in the unauthorized disclosure of or access to personally identifiable information or individually identifiable health information (violating certain privacy laws such as GDPR), it could result in a material disruption of Oncternal’s development programs and its business operations, whether due to a loss of Oncternal’s trade secrets or other similar disruptions. Some of the federal, state and foreign government requirements include obligations of companies to notify individuals of security breaches involving particular personally identifiable information, which could result from breaches experienced by Oncternal or by its vendors, contractors, or organizations with which Oncternal has formed strategic relationships. Even though Oncternal may have contractual protections with such vendors, contractors, or other organizations, notificationsand follow-up actions related to a security breach could impact Oncternal’s reputation, cause Oncternal to incur significant costs, including legal expenses, harm customer confidence, hurt Oncternal’s expansion into new markets, cause Oncternal to incur remediation costs, or cause Oncternal to lose existing customers. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in Oncternal’s regulatory approval efforts and significantly increase Oncternal’s costs to recover or reproduce the data. Oncternal also relies on third parties to manufacture its product candidates, and similar events relating to their computer systems could also have a material adverse effect on Oncternal’s business. To the extent that any disruption or security breach were to result in a loss of, or damage to, Oncternal’s data or applications, or inappropriate disclosure of confidential or proprietary information, Oncternal could incur liability, the further development and commercialization of Oncternal’s product candidates could be delayed, and Oncternal could be subject to significant fines, penalties or liabilities for any noncompliance to certain privacy and security laws.
Business disruptions could seriously harm Oncternal’s future revenue and financial condition and increase its costs and expenses.
Oncternal’s operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions, for which Oncternal is predominantly self-insured. Oncternal
95
relies on third- party manufacturers to produce Oncternal’s product candidates. Oncternal’s ability to obtain clinical supplies of its product candidates could be disrupted if the operations of these suppliers were affected bya man-made or natural disaster or other business interruption. In addition, Oncternal’s corporate headquarters is located in San Diego, California near major earthquake faults and fire zones, and the ultimate impact on Oncternal of being located near major earthquake faults and fire zones and being consolidated in a certain geographical area is unknown. The occurrence of any of these business disruptions could seriously harm Oncternal’s operations and financial condition and increase its costs and expenses.
Oncternal’s employees and independent contractors, including principal investigators, CROs, consultants and vendors, may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
Oncternal is exposed to the risk that its employees and independent contractors, including principal investigators, CROs, consultants and vendors may engage in misconduct or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to Oncternal that violate: (1) the laws and regulations of the FDA and other similar regulatory requirements, including those laws that require the reporting of true, complete and accurate information to such authorities, (2) manufacturing standards, including cGMP requirements, (3) federal and state data privacy, security, fraud and abuse and other healthcare laws and regulations in the United States and abroad or (4) laws that require the true, complete and accurate reporting of financial information or data. Activities subject to these laws also involve the improper use or misrepresentation of information obtained in the course of clinical trials, the creation of fraudulent data in Oncternal’s preclinical studies or clinical trials, or illegal misappropriation of drug product, which could result in regulatory sanctions and cause serious harm to Oncternal’s reputation. It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions Oncternal takes to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting Oncternal from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. In addition, Oncternal is subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against Oncternal, and Oncternal is not successful in defending itself or asserting its rights, those actions could have a significant impact on Oncternal’s business and financial results, including, without limitation, the imposition of significant civil, criminal and administrative penalties, damages, monetary fines, disgorgements, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, individual imprisonment, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements and oversight if Oncternal becomes subject to a corporate integrity agreement or similar agreement to resolve allegationsof non-compliance with these laws, and curtailment of Oncternal’s operations, any of which could adversely affect Oncternal’s ability to operate its business and its results of operations.
Oncternal is subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair Oncternal’s ability to compete in domestic and international markets. Oncternal could face criminal liability and other serious consequences for violations, which could harm its business.
Oncternal is subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, and various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, and anti-corruption and anti-money laundering laws and regulations, including the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which Oncternal conducts activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, clinical research organizations, contractors and other collaborators and partners from authorizing, promising, offering, providing, soliciting or receiving, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. Oncternal may engage third parties for clinical trials outside of the United States,
96
to sell its products abroad once Oncternal enters a commercialization phase, and/or to obtain necessary permits, licenses, patent registrations and other regulatory approvals. Oncternal has direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities and other organizations. Oncternal can be held liable for the corrupt or other illegal activities of its employees, agents, clinical research organizations, contractors and other collaborators and partners, even if Oncternal does not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm and other consequences.
Oncternal may engage in strategic transactions that could impact its liquidity, increase its expenses and present significant distractions to Oncternal’s management.
From time to time, Oncternal may consider strategic transactions, such as acquisitions of companies, asset purchasesand out-licensing or in-licensing of intellectual property, products or technologies, similar to Oncternal’s approachin in-licensing and acquiring its current product candidates. Any future transactions could increase Oncternal’s near and long-term expenditures, result in potentially dilutive issuances of Oncternal’s equity securities, including its common stock, or the incurrence of debt, contingent liabilities, amortization expenses oracquired in-process research and development expenses, any of which could affect Oncternal’s financial condition, liquidity and results of operations. Additional potential transactions that Oncternal may consider in the future include a variety of business arrangements, including spin-offs, strategic partnerships, joint ventures, restructurings, divestitures, business combinations and investments. Future acquisitions may also require Oncternal to obtain additional financing, which may not be available on favorable terms or at all. These transactions may never be successful and may require significant time and attention of management. In addition, the integration of any business that Oncternal may acquire in the future may disrupt Oncternal’s existing business and may be a complex, risky and costly endeavor for which Oncternal may never realize the full benefits of the acquisition. Accordingly, although there can be no assurance that Oncternal will undertake or successfully complete any additional transactions of the nature described above, any additional transactions that Oncternal does complete could have a material adverse effect on Oncternal’s business, results of operations, financial condition and prospects.
Risks Related to Oncternal’s Intellectual Property
Oncternal’s success depends on its ability to protect its intellectual property and its proprietary technologies.
Oncternal’s commercial success depends in part on its ability to obtain and maintain patent protection and trade secret protection for its product candidates, proprietary technologies and their uses as well as its ability to operate without infringing upon the proprietary rights of others. If Oncternal is unable to protect its intellectual property rights or if its intellectual property rights are inadequate for its technology or its product candidates, Oncternal’s competitive position could be harmed. Oncternal generally seeks to protect its proprietary position by licensing or filing patent applications in the United States and abroad related to its product candidates, proprietary technologies and their uses that are important to Oncternal’s business. Oncternal’s or its licensor’s patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless, and until, patents issue from such applications, and then only to the extent the issued claims cover the technology. There can be no assurance that Oncternal’s or its licensor’s patent applications will result in patents being issued or that issued patents will afford sufficient protection against competitors with similar technology, nor can there be any assurance that the patents if issued will not be infringed, designed around or invalidated by third parties. Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for Oncternal’s proprietary rights is uncertain. Only limited protection may be available and may not adequately protect Oncternal’s rights or permit it to gain or keep any competitive advantage. These uncertainties and/or limitations in Oncternal’s ability to properly protect the intellectual property rights relating to Oncternal’s
97
product candidates could have a material adverse effect on Oncternal’s financial condition and results of operations.
Although Oncternal owns and licenses issued patents in the United States and foreign countries, Oncternal cannot be certain that the claims in Oncternal’s or its licensor’s other U.S. pending patent applications, corresponding international patent applications and patent applications in certain foreign countries will be considered patentable by the United States Patent and Trademark Office (“USPTO”), courts in the United States or by the patent offices and courts in foreign countries, nor can Oncternal be certain that the claims in its or its licensor’s issued patents will not be found invalid or unenforceable if challenged.
The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that Oncternal, its licensors or any of its potential future collaborators will be successful in protecting Oncternal’s product candidates by obtaining and defending patents. These risks and uncertainties include the following:
| • | the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process, the noncompliance with which can result in abandonment or lapse of a patent or patent application, and partial or complete loss of patent rights in the relevant jurisdiction; |
| • | patent applications may not result in any patents being issued; |
| • | patents may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable or otherwise may not provide any competitive advantage; |
| • | Oncternal’s competitors, many of whom have substantially greater resources than Oncternal does and many of whom have made significant investments in competing technologies, may seek or may have already obtained patents that will limit, interfere with or block Oncternal’s ability to make, use and sell Oncternal’s product candidates; |
| • | there may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns; and |
| • | countries other than the United States may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop and market competing products. |
The patent prosecution process is also expensive and time consuming, and Oncternal and its licensors may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner or in all jurisdictions where protection may be commercially advantageous. It is also possible that Oncternal or its licensors will fail to identify patentable aspects of its research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, Oncternal does not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, directed to technology that Oncternal licenses from third parties. Oncternal may also require the cooperation of its licensor in order to enforce the licensed patent rights, and such cooperation may not be provided. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of Oncternal’s business. Oncternal cannot be certain that patent prosecution and maintenance activities by its licensors have been or will be conducted in compliance with applicable laws and regulations, which may affect the validity and enforceability of such patents or any patents that may issue from such applications. If they fail to do so, this could cause Oncternal to lose rights in any applicable intellectual property thatit in-licenses, and as a result Oncternal’s ability to develop and commercialize products or product candidates may be adversely affected and it may be unable to prevent competitors from making, using and selling competing products.
In addition, although Oncternal entersinto non-disclosure and confidentiality agreements with parties who have access to patentable aspects of its research and development output, such as Oncternal’s employees, outside
98
scientific collaborators, CROs, third-party manufacturers, consultants, advisors, licensees, collaboration partners, and other third parties, any of these parties may breach such agreements and disclose such output before a patent application is filed, thereby jeopardizing Oncternal’s ability to seek patent protection.
If Oncternal fails to comply with its obligations in the agreements under which it licenses intellectual property rights from third parties, including with respect to cirmtuzumab and TK216, or otherwise experiences disruptions in its business relationships with its licensors, Oncternal could lose license rights that are important to its business.
Oncternal is a party to several license agreements under which it is granted rights to intellectual property that are important to its business and Oncternal may enter into additional license agreements in the future. For example, in March 2014, Oncternal entered into an exclusive license agreement with Georgetown University, or Georgetown, to obtain an exclusive license to certain intellectual property rights to develop and commercialize compounds targetingEWS-FLI1. In March 2016, Oncternal entered into an exclusive license agreement with the Regents of the University of California (the “Regents”), to obtain an exclusive license to certain intellectual property rights to develop and commercialize cirmtuzumab and other ROR1 related naked antibodies.
These license agreements impose, and Oncternal expects that any future license agreements whereOncternal in-licenses intellectual property, will impose on Oncternal, various development, regulatory and/or commercial diligence obligations, payment of milestones and/or royalties and other obligations. If Oncternal fails to comply with its obligations under these agreements, or Oncternal is subject to bankruptcy-related proceedings, the licensor may have the right to terminate the license, in which event Oncternal would not be able to market products covered by the license.
Oncternal may need to obtain licenses from third parties to advance its research or allow commercialization of its product candidates, and Oncternal cannot provide any assurances that third-party patents do not exist which might be enforced against Oncternal’s product candidates in the absence of such a license. Oncternal may fail to obtain any of these licenses on commercially reasonable terms, if at all. Even if Oncternal is able to obtain a license, it maybe non-exclusive, thereby giving Oncternal’s competitors access to the same technologies licensed to Oncternal. In that event, Oncternal may be required to expend significant time and resources to develop or license replacement technology. If Oncternal is unable to do so, Oncternal may be unable to develop or commercialize the affected product candidates, which could materially harm Oncternal’s business and the third parties owning such intellectual property rights could seek either an injunction prohibiting Oncternal’s sales, or, with respect to Oncternal’s sales, an obligation on Oncternal’s part to pay royalties and/or other forms of compensation. Licensing of intellectual property is of critical importance to Oncternal’s business and involves complex legal, business and scientific issues. Disputes may arise between Oncternal and its licensors regarding intellectual property subject to a license agreement, including:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | whether and the extent to which Oncternal’s technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | Oncternal’s right to sublicense patents and other rights to third parties; |
| • | Oncternal’s diligence obligations with respect to the use of the licensed technology in relation to its development and commercialization of Oncternal’s product candidates, and what activities satisfy those diligence obligations; |
| • | Oncternal’s right to transfer or assign the license; and |
| • | the ownership of inventionsand know-how resulting from the joint creation or use of intellectual property by Oncternal’s licensors and Oncternal and its partners. |
99
If disputes over intellectual property that Oncternal has licensed prevent or impair Oncternal’s ability to maintain its current licensing arrangements on acceptable terms, Oncternal may not be able to successfully develop and commercialize the affected product candidates, which would have a material adverse effect on Oncternal’s business.
If the scope of any patent protection Oncternal obtains is not sufficiently broad, or if it loses any of its patent protection, Oncternal’s ability to prevent its competitors from commercializing similar or identical product candidates would be adversely affected.
The patent position of biopharmaceutical companies generally is highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. As a result, the issuance, scope, validity, enforceability and commercial value of Oncternal’s and its licensor’s patent rights are highly uncertain. Oncternal’s and its licensor’s pending and future patent applications may not result in patents being issued which protect Oncternal’s product candidates or which effectively prevent others from commercializing competitive product candidates.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if patent applications Oncternal owns or licenses currently or in the future issue as patents, they may not issue in a form that will provide Oncternal with any meaningful protection, prevent competitors or other third parties from competing with Oncternal, or otherwise provide Oncternal with any competitive advantage. Any patents that Oncternal owns or licenses may be challenged or circumvented by third parties or may be narrowed or invalidated as a result of challenges by third parties. Consequently, Oncternal does not know whether its product candidates will be protectable or remain protected by valid and enforceable patents. Oncternal’s competitors or other third parties may be able to circumvent Oncternal’s or its licensor’s patents by developing similar or alternative technologies or products ina non-infringing manner which could materially adversely affect Oncternal’s business, financial condition, results of operations and prospects.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and Oncternal’s and it licensor’s patents may not cover its product candidates or may be challenged in the courts or patent offices in the United States and abroad. Oncternal’s and its licensor’s patents may be subject to athird-party pre-issuance submission of prior art to the USPTO, or become involved in opposition, derivation, revocation, reexamination, post-grant review, or PGR, and inter partes review (“IPR”), or other similar proceedings in the USPTO or foreign patent offices challenging Oncternal’s or its licensor’s patent rights. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, Oncternal cannot be certain that there is no invalidating prior art, of which Oncternal or its predecessors or its licensor and the patent examiner were unaware during prosecution. There is no assurance that all potentially relevant prior art relating to Oncternal’s patents and patent applications or those of Oncternal’s licensors has been found. There is also no assurance that there is not prior art of which Oncternal, its predecessors or licensors are aware, but which Oncternal does not believe affects the validity or enforceability of a claim in Oncternal’s patents and patent applications or those of its licensors, which may, nonetheless, ultimately be found to affect the validity or enforceability of a claim. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, Oncternal’s or its licensor’s patent rights, allow third parties to commercialize Oncternal’s product candidates and compete directly with Oncternal, without payment to Oncternal. Such loss of patent rights, loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable could limit Oncternal’s ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of Oncternal’s product candidates. Such proceedings also may result in substantial cost and require significant time from Oncternal’s scientists and management, even if the eventual outcome is favorable to Oncternal. In addition, if the breadth or strength of protection provided by Oncternal’s or its licensor’s patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with Oncternal to license, develop or commercialize current or future product candidates.
100
The patent protection and patent prosecution for some of Oncternal product candidates may be dependent on third parties.
Oncternal or its licensors may fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, Oncternal or its licensors may miss potential opportunities to strengthen its patent position. It is possible that defects of form in the preparation or filing of Oncternal’s or its licensor’s patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, or requests for patent term adjustments. If there are material defects in the form, preparation, prosecution, or enforcement of Oncternal’s or its licensor’s patents or patent applications, such patents may be invalid and/or unenforceable, and such applications may never result in valid, enforceable patents. If Oncternal or its licensors, whether current or future, fail to establish, maintain or protect its patents and other intellectual property rights, such rights may be reduced or eliminated. If Oncternal’s licensors are not fully cooperative or disagree with Oncternal as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. Any of these outcomes could impair Oncternal’s ability to prevent competition from third parties, which may have an adverse impact on Oncternal’s business.
As a licensee of third parties, Oncternal relies on third parties to file and prosecute patent applications and maintain patents and otherwise protect the licensed intellectual property under some of Oncternal’s license agreements. Oncternal has not had and does not have primary control over these activities for certain of Oncternal’s patents or patent applications and other intellectual property rights. Oncternal cannot be certain that such activities by third parties have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents or other intellectual property rights. Pursuant to the terms of the license agreements with some of Oncternal’s licensors, the licensors may have the right to control enforcement of Oncternal’s licensed patents or defense of any claims asserting the invalidity of these patents and even if Oncternal is permitted to pursue such enforcement or defense, Oncternal will require the cooperation of its licensors. Oncternal cannot be certain that its licensors will allocate sufficient resources or prioritize their or Oncternal’s enforcement of such patents or defense of such claims to protect Oncternal’s interests in the licensed patents. Even if Oncternal is not a party to these legal actions, an adverse outcome could harm Oncternal’s business because it might prevent Oncternal from continuing to license intellectual property that Oncternal may need to operate its business. If any of Oncternal’s licensors or any of its future licensors or future collaborators fail to appropriately prosecute and maintain patent protection for patents covering any of Oncternal’s product candidates, Oncternal’s ability to develop and commercialize those product candidates may be adversely affected and Oncternal may not be able to prevent competitors from making, using and selling competing products.
In addition, even where Oncternal has the right to control patent prosecution of patents and patent applications Oncternal has acquired or licensed from third parties, Oncternal may still be adversely affected or prejudiced by actions or inactions of its predecessors or licensors and their counsel that took place prior to Oncternal assuming control over patent prosecution.
Oncternal’s technology acquired or licensed from various third parties may be subject to retained rights. Oncternal’s predecessors or licensors often retain certain rights under their agreements with Oncternal, including the right to use the underlying technology for noncommercial academic and research use, to publish general scientific findings from research related to the technology, and to make customary scientific and scholarly disclosures of information relating to the technology. It is difficult to monitor whether Oncternal’s predecessors or licensors limit their use of the technology to these uses, and Oncternal could incur substantial expenses to enforce Oncternal’s rights to its licensed technology in the event of misuse.
If Oncternal is limited in its ability to utilize acquired or licensed technologies, or if Oncternal loses its rights tocritical in-licensed technology, Oncternal may be unable to successfullydevelop, out-license, market and sell its products, which could prevent or delay new product introductions. Oncternal’s business strategy depends on the successful development of licensed and acquired technologies into commercial products. Therefore, any
101
limitations on Oncternal’s ability to utilize these technologies may impair Oncternal’s ability todevelop, out-license or market and sell its product candidate.
Some of Oncternal’s intellectual property has been discovered through government funded programs and thus may be subject to federal regulations suchas ”march-in” rights, certain reporting requirements and a preference for U.S.-based companies. Compliance with such regulations may limit Oncternal’s exclusive rights, and limit Oncternal’s ability to contractwith non-U.S. manufacturers.
Some of the intellectual property rights Oncternal has acquired or licensed or may acquire or license in the future may have been generated through the use of U.S. government funding and may therefore be subject to certain federal regulations. For example, some of the research and development work on cirmtuzumab and TK216 was funded by government research grants. As a result, the U.S. government may have certain rights to intellectual property embodied in Oncternal’s product candidates pursuant to the Bayh-Dole Act of 1980, or Bayh-Dole Act. These U.S. government rights includea non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right, under certain limited circumstances, to require Oncternal to grant exclusive, partially exclusive,or non-exclusive licenses to any of these inventions to a third-party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred toas ”march-in rights”). The U.S. government also has the right to take title to these inventions if the grant recipient fails to disclose the invention to the government or fails to file an application to register the intellectual property within specified time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require Oncternal to expend substantial resources. In addition, the U.S. government requires that any products embodying any of these inventions or produced through the use of any of these inventions be manufactured substantially in the United States. This preference for U.S. industry may be waived by the federal agency that provided the funding if the owner or assignee of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. industry may limit Oncternal’s ability to contractwith non-U.S. product manufacturers for products covered by such intellectual property. To the extent any of Oncternal’s future intellectual property is also generated through the use of U.S. government funding, the provisions of the Bayh-Dole Act may similarly apply.
Intellectual property rights do not necessarily address all potential threats to Oncternal’s competitive advantage.
The degree of future protection afforded by Oncternal’s intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect Oncternal’s business or permit Oncternal to maintain its competitive advantage. For example:
| • | others may be able to develop products that are similar to Oncternal’s product candidates but that are not covered by the claims of the patents that Oncternal owns or licenses; |
| • | Oncternal or its licensors or predecessors might not have been the first to make the inventions covered by the issued patents or patent applications that Oncternal owns or licenses; |
| • | Oncternal or its licensors or predecessors might not have been the first to file patent applications covering certain of Oncternal’s inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of Oncternal’s technologies without infringing Oncternal’s intellectual property rights; |
| • | it is possible that Oncternal’s or its licensor’s pending patent applications will not lead to issued patents; |
102
| • | issued patents that Oncternal owns or licenses may be held invalid or unenforceable, as a result of legal challenges by Oncternal’s competitors; |
| • | Oncternal’s competitors might conduct research and development activities in countries where Oncternal does not have patent rights and then use the information learned from such activities to develop competitive products for sale in Oncternal’s major commercial markets; |
| • | Oncternal may not develop additional proprietary technologies that are patentable; and |
| • | the patents of others may have an adverse effect on Oncternal’s business. |
Should any of these events occur, it could significantly harm Oncternal’s business, results of operations and prospects.
Oncternal relies on licensee relationships, and any disputes or litigation with our partners or termination or breach of any of the related agreements could reduce the financial resources available to us, including milestone payments and future royalty revenues.
Oncternal’s existing collaborations may not continue or be successful, and Oncternal may be unable to enter into future collaborative arrangements to develop and commercialize its unpartnered assets. If any of Oncternal’s collaborative partners breach or terminate their agreements with Oncternal or otherwise fail to conduct their collaborative activities successfully, Oncternal’s product development under these agreements will be delayed or terminated. Disputes or litigation may also arise with our collaborators (with us and/or with one or more third parties), including those over ownership rights to intellectual property,know-how or technologies developed with our collaborators. Such disputes or litigation could adversely affect our rights to one or more of our product candidates and could delay, interrupt or terminate the collaborative research, development and commercialization of certain potential products, create uncertainty as to ownership rights of intellectual property, or could result in litigation or arbitration. In addition, a significant downturn or deterioration in the business or financial condition of our collaborators or partners could result in a loss of expected revenue and our expected returns on investment. The occurrence of any of these problems could be time-consuming and expensive and could adversely affect our business.
Oncternal’s commercial success depends significantly on its ability to operate without infringing the patents and other proprietary rights of third parties. Claims by third parties that Oncternal infringes their proprietary rights may result in liability for damages or prevent or delay Oncternal’s developmental and commercialization efforts.
Oncternal’s commercial success depends in part on avoiding infringement of the patents and proprietary rights of third parties. However, Oncternal’s or its licensee’s research, development and commercialization activities may be subject to claims that Oncternal or its licensee infringes or otherwise violates patents or other intellectual property rights owned or controlled by third parties. Other entities may have or obtain patents or proprietary rights that could limit Oncternal’s or its licensee’s ability to make, use, sell, offer for sale or import Oncternal’s product candidates and products that may be approved in the future, or impair Oncternal’s competitive position. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biopharmaceutical industry, including patent infringement lawsuits, oppositions, reexaminations, IPR proceedings and PGR proceedings before the USPTO and/or foreign patent offices. Numerous third-party U.S. and foreign issued patents and pending patent applications exist in the fields in which Oncternal is developing product candidates. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of Oncternal’s product candidates.
As the biopharmaceutical industry expands and more patents are issued, the risk increases that Oncternal’s product candidates may be subject to claims of infringement of the patent rights of third parties. Because patent
103
applications are maintained as confidential for a certain period of time, until the relevant application is published Oncternal may be unaware of third-party patents that may be infringed by commercialization of any of Oncternal’s product candidates, and Oncternal cannot be certain that Oncternal was the first to file a patent application related to a product candidate or technology. Moreover, because patent applications can take many years to issue, there may be currently-pending patent applications that may later result in issued patents that Oncternal’s product candidates may infringe. In addition, identification of third-party patent rights that may be relevant to Oncternal’s technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. In addition, third parties may obtain patents in the future and claim that use of Oncternal’s technologies infringes upon these patents. Any claims of patent infringement asserted by third parties would be time consuming and could:
| • | result in costly litigation that may cause negative publicity; |
| • | divert the time and attention of Oncternal’s technical personnel and management; |
| • | cause development delays; |
| • | subject Oncternal to an injunction preventing Oncternal from making, using, selling, offering for sale, or importing Oncternal products; |
| • | prevent Oncternal from commercializing any of its product candidates until the asserted patent expires or is held finally invalid or not infringed in a court of law; |
| • | require Oncternal todevelop non-infringing technology, which may not be possible on a cost-effective basis; |
| • | subject Oncternal to significant liability to third parties; or |
| • | require Oncternal to enter into royalty or licensing agreements, which may not be available on commercially reasonable terms, or at all, or which mightbe non-exclusive, which could result in Oncternal’s competitors gaining access to the same technology. |
Although no third-party has asserted a claim of patent infringement against Oncternal as of the date of this prospectus, others may hold proprietary rights that could prevent Oncternal’s product candidates from being marketed. Any patent-related legal action against Oncternal claiming damages and seeking to enjoin activities relating to Oncternal’s product candidates or processes could subject Oncternal to potential liability for damages, including treble damages if Oncternal was determined to willfully infringe, and require Oncternal to obtain a license to manufacture or develop its product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from Oncternal’s business. Oncternal cannot predict whether it would prevail in any such actions or that any license required under any of these patents would be made available on commercially reasonable terms, if at all. Moreover, even if Oncternal or its future strategic partners were able to obtain a license, the rights may be nonexclusive, which could result in Oncternal’s competitors gaining access to the same intellectual property. In addition, Oncternal cannot be certain that it could redesign its product candidates or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent Oncternal from developing and commercializing its product candidates, which could harm Oncternal’s business, financial condition and operating results.
Parties making claims against Oncternal may be able to sustain the costs of complex patent litigation more effectively than Oncternal can because they have substantially greater resources. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or administrative proceedings, there is a risk that some of Oncternal’s confidential information could be compromised by disclosure. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on Oncternal’s ability to raise additional funds or otherwise have a material adverse effect on Oncternal’s business, results of operations, financial condition and prospects.
104
Oncternal may be involved in lawsuits to protect or enforce Oncternal’s patents or the patents of its licensors, which could be expensive, time consuming and unsuccessful. Further, Oncternal’s issued patents could be found invalid or unenforceable if challenged in court.
Competitors may infringe Oncternal’s intellectual property rights or those of its licensors. To prevent infringement or unauthorized use, Oncternal and/or its licensors may be required to file infringement claims, which can be expensive and time consuming. In addition, in a patent infringement proceeding, a court may decide that a patent Oncternal owns or licenses is not valid, is unenforceable and/or is not infringed. If Oncternal or any of its licensors or potential future collaborators were to initiate legal proceedings against a third-party to enforce a patent directed at one of Oncternal’s product candidates, the defendant could counterclaim that Oncternal’s or its licensor’s patent is invalid and/or unenforceable in whole or in part. In patent litigation, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, lack of written descriptionor non-enablement. Grounds for an unenforceability assertion could include an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution.
If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, Oncternal would lose at least part, and perhaps all, of the patent protection on such product candidate. In addition, if the breadth or strength of protection provided by Oncternal’s patents and patent applications or those of its licensors is threatened, it could dissuade companies from collaborating with Oncternal to license, develop or commercialize current or future product candidates. Such a loss of patent protection would have a material adverse impact on Oncternal’s business.
Even if resolved in Oncternal’s favor, litigation or other legal proceedings relating to Oncternal’s or its licensor’s intellectual property rights may cause Oncternal to incur significant expenses, and could distract Oncternal’s technical and management personnel from their normal responsibilities. Such litigation or proceedings could substantially increase Oncternal’s operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. Oncternal or its licensor may not have sufficient financial or other resources to conduct or participate in such litigation or proceedings adequately. Some of Oncternal’s competitors may be able to sustain the costs of such litigation or proceedings more effectively than Oncternal or its licensor can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise Oncternal’s ability to compete in the marketplace.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or other legal proceedings relating to Oncternal’s intellectual property rights, there is a risk that some of Oncternal’s confidential information could be compromised by disclosure during this type of litigation or other proceedings.
Intellectual property litigation may lead to unfavorable publicity that harms Oncternal’s reputation and causes the market price of Oncternal’s common shares to decline.
During the course of any intellectual property litigation, there could be public announcements of the initiation of the litigation as well as results of hearings, rulings on motions, and other interim proceedings in the litigation. If securities analysts or investors regard these announcements as negative, the perceived value of Oncternal’s existing products, programs or intellectual property could be diminished. Accordingly, the market price of shares of Oncternal’s common stock may decline. Such announcements could also harm Oncternal’s reputation or the market for Oncternal’s future products, which could have a material adverse effect on Oncternal’s business.
105
Derivation or interference proceedings may be necessary to determine priority of inventions, and an unfavorable outcome may require Oncternal to cease using the related technology or to attempt to license rights from the prevailing party.
Derivation or interference proceedings provoked by third parties or brought by Oncternal or its licensors or declared by the USPTO or similar proceedings in foreign patent offices may be necessary to determine the priority of inventions with respect to Oncternal’s or its licensor’s patents or patent applications. An unfavorable outcome could require Oncternal to cease using the related technology or to attempt to license rights to it from the prevailing party. Oncternal’s business could be harmed if the prevailing party does not offer Oncternal a license on commercially reasonable terms. Oncternal’s or its licensor’s defense of such proceedings may fail and, even if successful, may result in substantial costs and distract Oncternal’s management and other employees. In addition, the uncertainties associated with such proceedings could have a material adverse effect on Oncternal’s ability to raise the funds necessary to continue its clinical trials, continue its research programs, license necessary technology from third parties or enter into development or manufacturing partnerships that would help Oncternal bring its product candidates to market.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of Oncternal’s patent applications and the enforcement or defense of Oncternal’s issued patents.
On September 16, 2011, the Leahy-Smith America Invents Act, or Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first inventor to file” system in which, assuming that other requirements of patentability are met, the first inventor to file a patent application will be entitled to the patent regardless of whether a third-party was first to invent the claimed invention. A third-party that files a patent application in the USPTO after March 2013 but before Oncternal could therefore be awarded a patent covering an invention of Oncternal’s even if Oncternal had made the invention before it was made by such third-party. This will require Oncternal to be cognizant going forward of the time from invention to filing of a patent application. Furthermore, Oncternal’s ability to obtain and maintain valid and enforceable patents depends on whether the differences between Oncternal’s technology and the prior art allow Oncternal’s technology to be patentable over the prior art. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, Oncternal cannot be certain that it or its licensor was the first to either (1) file any patent application related to Oncternal’s product candidates or (2) invent any of the inventions claimed in Oncternal’s or its licensor’s patents or patent applications.
The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including PGR, IPR, and derivation proceedings. An adverse determination in any such submission or proceeding could reduce the scope or enforceability of, or invalidate, Oncternal’s patent rights, which could adversely affect Oncternal’s competitive position.
Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third-party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third-party may attempt to use the USPTO procedures to invalidate Oncternal’s or its licensor’s patent claims that would not have been invalidated if first challenged by the third-party as a defendant in a district court action. Thus, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of Oncternal’s or its licensor’s patent applications and the enforcement or defense of Oncternal’s or its licensor’s issued patents, all of which could have a material adverse effect on Oncternal’s business, financial condition, results of operations and prospects.
106
Changes in U.S. patent law, or laws in other countries, could diminish the value of patents in general, thereby impairing Oncternal’s ability to protect its product candidates.
As is the case with other biopharmaceutical companies, Oncternal’s success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve a high degree of technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time consuming and inherently uncertain. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of Oncternal’s intellectual property rights and may increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Oncternal cannot predict the breadth of claims that may be allowed or enforced in Oncternal’s or its licensor’s patents or in third-party patents. In addition, Congress or other foreign legislative bodies may pass patent reform legislation that is unfavorable to Oncternal.
For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to Oncternal’s and its licensor’s ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the U.S. federal courts, the USPTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that would weaken Oncternal’s or its licensor’s ability to obtain new patents or to enforce its existing patents and patents it might obtain in the future.
Oncternal may be subject to claims challenging the inventorship or ownership of Oncternal’s patents and other intellectual property.
Oncternal may also be subject to claims that former employees or other third parties have an ownership interest in Oncternal’s patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If Oncternal fails in defending any such claims, in addition to paying monetary damages, Oncternal may lose valuable intellectual property rights. Such an outcome could have a material adverse effect on Oncternal’s business. Even if Oncternal is successful in defending against such claims, litigation could result in substantial costs and distraction to management and other employees.
Patent terms may be inadequate to protect Oncternal’s competitive position on its product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliestU.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering Oncternal’s product candidates are obtained, once the patent life has expired, Oncternal may be open to competition from competitive products. Given the amount of time required for the development, testing and regulatory review of product candidates, patents protecting Oncternal’s product candidates might expire before or shortly after such candidates are commercialized. As a result, Oncternal’s patent portfolio may not provide it with sufficient rights to exclude others from commercializing products similar or identical to Oncternal’s.
If Oncternal does not obtain patent term extension for its product candidates, its business may be materially harmed.
Depending upon the timing, duration and specifics of FDA marketing approval of Oncternal’s product candidates, one or more of its or its licensor’s U.S. patents may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984 (the “Hatch-Waxman Amendments”). The Hatch- Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. A
107
maximum of one patent may be extended per FDA approved product as compensation for the patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only those claims covering such approved drug product, a method for using it or a method for manufacturing it may be extended. Patent term extension may also be available in certain foreign countries upon regulatory approval of Oncternal’s product candidates. However, Oncternal may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than it requests. If Oncternal is unable to obtain patent term extension or restoration or the term of any such extension is less than Oncternal requests, its competitors may obtain approval of competing products following Oncternal’s or its licensor’s patent expiration, and Oncternal’s revenue could be reduced, possibly materially. Further, if this occurs, Oncternal’s competitors may take advantage of its investment in development and trials by referencing Oncternal’s clinical and preclinical data and launch their product earlier than might otherwise be the case.
Oncternal may not be able to protect its intellectual property rights throughout the world.
Although Oncternal and its licensors have issued patents and pending patent applications in the United States and certain other countries, filing, prosecuting and defending patents in all countries throughout the world would be prohibitively expensive, and Oncternal’s intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, Oncternal may not be able to prevent third parties from practicing Oncternal’s inventions in all countries outside the United States or from selling or importing products made using Oncternal’s inventions in and into the United States or other jurisdictions. Competitors may use Oncternal’s technologies in jurisdictions where Oncternal or its licensor has not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where Oncternal has patent protection but enforcement is not as strong as that in the United States. These products may compete with Oncternal’s product candidates, and Oncternal’s and its licensor’s patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of many foreign countries do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for Oncternal to stop the infringement of its patents or marketing of competing products in violation of Oncternal’s proprietary rights. Proceedings to enforce Oncternal’s patent rights in foreign jurisdictions could result in substantial costs and divert Oncternal’s efforts and attention from other aspects of its business, could put its patents at risk of being invalidated or interpreted narrowly and its patent applications at risk of not issuing and could provoke third parties to assert claims against Oncternal. Oncternal or its licensor may not prevail in any lawsuits that it initiates, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, Oncternal’s or its licensor’s efforts to enforce its intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that Oncternal develops or licenses.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If Oncternal or its licensor is forced to grant a license to third parties with respect to any patents relevant to Oncternal’s business, Oncternal’s competitive position may be impaired, and its business, financial condition, results of operations and prospects may be adversely affected.
108
Obtaining and maintaining Oncternal’s patent protection depends on compliance with various procedural, documentary, fee payment and other requirements imposed by regulations and governmental patent agencies, and Oncternal’s patent protection could be reduced or eliminatedfor non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to the USPTO and various foreign patent offices at various points over the lifetime of Oncternal’s and its licensors’ patents and/or applications. Oncternal has systems in place to remind it to pay these fees, and Oncternal relies on third parties to pay these fees when due. Additionally, the USPTO and various foreign patent offices require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. Oncternal employs reputable law firms and other professionals to help it comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with rules applicable to the particular jurisdiction. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If such an event were to occur, it could have a material adverse effect on Oncternal’s business.
If Oncternal is unable to protect the confidentiality of its trade secrets, its business and competitive position would be harmed.
In addition, Oncternal relies on the protection of its trade secrets, includingunpatented know-how, technology and other proprietary information to maintain Oncternal’s competitive position. Although Oncternal has taken steps to protect its trade secrets andunpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with employees, consultants and advisors, Oncternal cannot provide any assurances that all such agreements have been duly executed, and any of these parties may breach the agreements and disclose Oncternal’s proprietary information, including its trade secrets, and Oncternal may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets.
Moreover, third parties may still obtain this information or may come upon this or similar information independently, and Oncternal would have no right to prevent them from using that technology or information to compete with Oncternal. If any of these events occurs or if Oncternal otherwise loses protection for its trade secrets, the value of this information may be greatly reduced and Oncternal’s competitive position would be harmed. If Oncternal does not apply for patent protection prior to such publication or if Oncternal cannot otherwise maintain the confidentiality of its proprietary technology and other confidential information, then Oncternal’s ability to obtain patent protection or to protect its trade secret information may be jeopardized.
Oncternal may be subject to claims that it has wrongfully hired an employee from a competitor or that Oncternal or its employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is common in the biopharmaceutical industry, in addition to Oncternal’s employees, Oncternal engages the services of consultants to assist it in the development of its product candidates. Many of these consultants, and many of Oncternal’s employees, were previously employed at, or may have previously provided or may be currently providing consulting services to, other biopharmaceutical companies including Oncternal’s competitors or potential competitors. Oncternal may become subject to claims that Oncternal, its employees or a consultant inadvertently or otherwise used or disclosed trade secrets or other information proprietary to their former employers or their former or current clients. Litigation may be necessary to defend against these claims. If Oncternal fails in defending any such claims, in addition to paying monetary damages, it may lose valuable
109
intellectual property rights or personnel, which could adversely affect Oncternal’s business. Even if Oncternal is successful in defending against these claims, litigation could result in substantial costs and be a distraction to Oncternal’s management team and other employees.
Risks Related to Oncternal’s Common Stock
An active, liquid and orderly market for the combined company’s common stock may not develop, and you may not be able to resell your common stock at or above the purchase price.
There has been no public market for Oncternal’s common stock. Although GTx’s common stock is listed on the Nasdaq Capital Market, or Nasdaq, and Oncternal and GTx have applied to have the combined company’s common stock listed on Nasdaq, an active trading market for the combined company’s common stock may never develop or be sustained following the merger. Oncternal, GTx and their financial advisors will set the final reverse split ratio to target a trading price to provide for sufficient liquidity. The price that the combined company trades at immediately after the merger may not necessarily reflect the price at which investors in the market will be willing to buy and sell the shares on a sustained basis. In addition, an active trading market may not develop following the consummation of the merger or, if it is developed, may not be sustained. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. An inactive market may also impair the combined company’s ability to raise capital by selling shares and may impair the combined company’s ability to acquire other businesses or technologies using the combined company’s shares as consideration, which, in turn, could materially adversely affect the combined company’s business.
The trading price of the shares of the combined company’s common stock could be highly volatile, and purchasers of the combined company’s common stock after the merger could incur substantial losses.
The combined company’s stock price is likely to be volatile. The stock market in general and the market for stock of biopharmaceutical companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above their purchase price. The market price for the combined company’s common stock may be influenced by those factors discussed in this “Risk Factors” section and many others, including:
| • | the combined company’s ability to enroll subjects in its ongoing and planned clinical trials; |
| • | results of the combined company’s clinical trials and preclinical studies, and the results of trials of the combined company’s competitors or those of other companies in the combined company’s market sector; |
| • | regulatory approval of the combined company’s product candidates, or limitations to specific label indications or patient populations for its use, or changes or delays in the regulatory review process; |
| • | regulatory developments in the United States and foreign countries; |
| • | changes in the structure of healthcare payment systems, especially in light of current reforms to the U.S. healthcare system; |
| • | the success or failure of the combined company’s efforts to acquire, license or develop additional product candidates; |
| • | innovations or new products developed by the combined company’s or its competitors; |
| • | announcements by the combined company or its competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | manufacturing, supply or distribution delays or shortages; |
| • | any changes to the combined company’s relationship with any manufacturers, suppliers, licensors, future collaborators or other strategic partners; |
110
| • | achievement of expected product sales and profitability; |
| • | variations in the combined company’s financial results or those of companies that are perceived to be similar to the combined company; |
| • | market conditions in the biopharmaceutical sector and issuance of securities analysts’ reports or recommendations; |
| • | trading volume of the combined company’s common stock; |
| • | an inability to obtain additional funding; |
| • | sales of the combined company’s stock by insiders and stockholders; |
| • | general economic, industry and market conditions other events or factors, many of which are beyond the combined company’s control; |
| • | additions or departures of key personnel; and |
| • | intellectual property, product liability or other litigation against the combined company. |
In addition, in the past, stockholders have initiated class action lawsuits against biopharmaceutical companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against the combined company, could cause Oncternal to incur substantial costs and divert management’s attention and resources, which could have a material adverse effect on the combined company’s business, financial condition and results of operations.
The combined company’s failure to meet the continued listing requirements of the Nasdaq could result in a delisting of the combined company’s common stock.
If, after listing, the combined company fails to satisfy the continued listing requirements of the Nasdaq, such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to delist the combined company’s common stock. Such a delisting would likely have a negative effect on the price of the combined company’s common stock and would impair your ability to sell or purchase the combined company’s common stock when you wish to do so. In the event of a delisting, the combined company can provide no assurance that any action taken by the combined company to restore compliance with listing requirements would allow the combined company’s common stock to become listed again, stabilize the market price or improve the liquidity of the combined company’s common stock, prevent the combined company’s common stock from dropping below the Nasdaq minimum bid price requirement or preventfuture non-compliance with Nasdaq’s listing requirements.
After the merger, the combined company’s executive officers, directors and principal stockholders, if they choose to act together, will continue to control or significantly influence all matters submitted to stockholders for approval. Furthermore, two of the combined company’s anticipated directors will be appointed by one of Oncternal’s principal stockholders.
Following the completion of the merger, the combined company’s executive officers, directors and greater than 5% stockholders, in the aggregate, will own approximately 38% of Oncternal’s outstanding common stock (assuming no exercise of outstanding options). Furthermore, two of the combined company’s anticipated directors will be appointed by the combined company’s largest stockholder, SPH USA. As a result, such persons or their appointees to the combined company’s board of directors, acting together, will have the ability to control or significantly influence all matters submitted to the combined company’s board of directors or stockholders for approval, including the appointment of the combined company’s management, the election and removal of directors and approval of any significant transaction, as well as the combined company’s management and business affairs. This concentration of ownership may have the effect of delaying, deferring or preventing a change in control, impeding a merger, consolidation, takeover or other business combination involving the combined company, or discouraging a potential acquiror from making a tender offer or otherwise attempting to obtain control of the combined company’s business, even if such a transaction would benefit other stockholders.
111
Oncternal does not currently intend to pay dividends on the combined company’s common stock, and, consequently, your ability to achieve a return on your investment will depend on appreciation, if any, in the price of the combined company’s common stock.
Oncternal has never declared or paid any cash dividend on Oncternal’s common stock. Oncternal currently anticipates that it will retain future earnings for the development, operation and expansion of the combined company’s business and does not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, the terms of any future debt agreements may preclude the combined company from paying dividends. Any return to stockholders will therefore be limited to the appreciation of their stock. There is no guarantee that shares of the combined company’s common stock will appreciate in value or even maintain the price at which stockholders have purchased their shares.
Sales of a substantial number of shares of the combined company’s common stock by the combined company’s stockholders in the public market could cause the combined company’s stock price to fall.
Sales of a substantial number of shares of the combined company’s common stock in the public market or the perception that these sales might occur could significantly reduce the market price of the combined company’s common stock and impair the combined company’s ability to raise adequate capital through the sale of additional equity securities.
Based on shares of GTx’s common stock outstanding and issuable under the GTx Director Deferred Compensation Plan as of March 31, 2019 and assuming an exchange ratio of 0.5137, upon the closing of the merger, the combined company will have outstanding a total of 107,587,866 shares of common stock after the merger, assuming no exercise of outstanding options. Of these shares, only 60,206,643 shares of common stock will be freely tradable, without restriction, in the public market immediately following the merger, unless they are purchased by one of the combined company’s affiliates.
Oncternal’s directors and executive officers and holders of approximately 43.7% of Oncternal’s outstanding securities have enteredinto lock-up agreements with GTx pursuant to which they may not, with limited exceptions, for a period of 180 days from the date of the Effective Time, offer, sell or otherwise transfer or dispose of any of the GTx’s securities, without the prior written consent of GTx, subject to certain exceptions. Sales of these shares, or perceptions that they will be sold, could cause the trading price of the combined company’s common stock to decline. Afterthe lock-up agreements expire, up to an additional 47,381,223 shares of common stock will be eligible for sale in the public market.
In addition, as of March 31, 2019, up to 4,514,683 shares of common stock that are either subject to outstanding options or reserved for future issuance under GTx’s equity incentive plans will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules,the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the trading price of the combined company’s common stock could decline.
After the merger, the holders of approximately 0.8 million shares of GTx’s outstanding common stock (prior to adjustment for the GTx Reverse Stock Split), or approximately 3.3% of GTx’s total outstanding common stock as of March 31, 2019, will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to vesting and the180-daylock-up agreements described above. See “Description of GTx’s Capital Stock—Registration Rights.” Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by affiliates, as defined in Rule 144 under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of the combined company’s common stock.
112
Oncternal will incur significant increased costs as a result of operating as a public company, and its management will be required to devote substantial time to new compliance initiatives.
As a public company, Oncternal will incur significant legal, accounting and other expenses that Oncternal did not incur as a private company. Oncternal will be subject to the reporting requirements of the Exchange Act, which will require, among other things, that Oncternal files with the U.S. Securities and Exchange Commission, or SEC, annual, quarterly and current reports with respect to Oncternal’s business and financial condition. In addition, Sarbanes-Oxley, as well as rules subsequently adopted by the SEC and Nasdaq to implement provisions of Sarbanes-Oxley, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, the SEC has adopted additional rules and regulations in these areas, such as mandatory “say on pay” voting requirements that will apply to Oncternal when it ceases to be an emerging growth company. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which Oncternal operates its business in ways Oncternal cannot currently anticipate.
Oncternal expects the rules and regulations applicable to public companies to substantially increase Oncternal’s legal and financial compliance costs and to make some activities more time consuming and costly. If these requirements divert the attention of Oncternal’s management and personnel from other business concerns, they could have a material adverse effect on Oncternal’s business, financial condition and results of operations. The increased costs will increase Oncternal’s net loss, and may require Oncternal to reduce costs in other areas of its business or increase the prices of its products or services. For example, Oncternal expects these rules and regulations to make it more difficult and more expensive for Oncternal to obtain director and officer liability insurance, and Oncternal may be required to incur substantial costs to maintain the same or similar coverage. Oncternal cannot predict or estimate the amount or timing of additional costs Oncternal may incur to respond to these requirements. The impact of these requirements could also make it more difficult for Oncternal to attract and retain qualified persons to serve on its board of directors, its board committees or as executive officers.
If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about the combined company’s business, the combined company’s stock price and trading volume could decline.
The trading market for the combined company’s common stock will depend in part on the research and reports that securities or industry analysts publish about the combined company, its business, its market or its competitors. Oncternal does not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of the combined company, the trading price for the combined company’s stock would be negatively impacted. In the event the combined company obtains securities or industry analyst coverage, if one or more of the analysts who covers the combined company downgrades its stock, the combined company’s stock price would likely decline. If one or more of these analysts ceases to cover the combined company or fails to regularly publish reports on the combined company, interest in the combined company’s stock could decrease, which could cause the combined company’s stock price or trading volume to decline.
If the combined company fails to maintain proper and effective internal control over financial reporting, Oncternal’s ability to produce accurate and timely financial statements could be impaired, investors may lose confidence in the combined company’s financial reporting and the trading price of the combined company’s common stock may decline.
Pursuant to Section 404 of Sarbanes-Oxley, the combined company’s management will be required to report upon the effectiveness of the combined company’s internal control over financial reporting beginning with the annual report for the combined company’s fiscal year ending December 31, 2019. Additionally, if the combined
113
company reaches an accelerated filer threshold, the combined company’s independent registered public accounting firm will be required to attest to the effectiveness of the combined company’s internal control over financial reporting. The rules governing the standards that must be met for management to assess the combined company’s internal control over financial reporting are complex and require significant documentation, testing and possible remediation. To comply with the requirements of being a reporting company under the Exchange Act, the combined company will need to upgrade its information technology systems; implement additional financial and management controls, reporting systems and procedures; and hire additional accounting and finance staff. If the combined company or, if required, its auditors are unable to conclude that the combined company’s internal control over financial reporting is effective, investors may lose confidence in the combined company’s financial reporting and the trading price of the combined company’s common stock may decline.
The combined company cannot assure you that there will not be material weaknesses or significant deficiencies in the combined company’s internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit the combined company’s ability to accurately report its financial condition, results of operations or cash flows. If the combined company is unable to conclude that its internal control over financial reporting is effective, or if the combined company’s independent registered public accounting firm determines the combined company has a material weakness or significant deficiency in the combined company’s internal control over financial reporting once that firm begin its Section 404 reviews, investors may lose confidence in the accuracy and completeness of the combined company’s financial reports, the market price of the combined company’s common stock could decline, and the combined company could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities. Failure to remedy any material weakness in the combined company’s internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict the combined company’s future access to the capital markets.
Provisions in the combined company’s charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable and may lead to entrenchment of management.
The anticipated amended and restated certificate of incorporation and amended and restated bylaws of the combined company that will be in effect immediately after consummation of the merger will contain provisions that could significantly reduce the value of the combined company’s shares to a potential acquiror or delay or prevent changes in control or changes in the combined company’s management without the consent of the combined company’s board of directors. The provisions in the combined company’s charter documents are expected to include the following:
| • | a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of the combined company’s board of directors; |
| • | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| • | the exclusive right of the combined company’s board of directors, unless the board of directors grants such right to the stockholders, to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on the combined company’s board of directors; |
| • | the prohibition on removal of directors without cause due to the classified board of directors; |
| • | the ability of the combined company’s board of directors to authorize the issuance of shares of preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquiror; |
| • | the ability of the combined company’s board of directors to alter Oncternal’s amended and restated bylaws without obtaining stockholder approval; |
114
| • | the required approval of atleast 66-2/3% of the shares entitled to vote to adopt, amend or repeal the combined company’s amended and restated bylaws or repeal certain provisions of the combined company’s amended and restated certificate of incorporation; |
| • | a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of Oncternal’s stockholders; |
| • | an exclusive forum provision providing that the Court of Chancery of the State of Delaware will be the exclusive forum for certain actions and proceedings; |
| • | the requirement that a special meeting of stockholders may be called only by the chairman of the board of directors, the chief executive officer or the board of directors, which may delay the ability of the combined company’s stockholders to force consideration of a proposal or to take action, including the removal of directors; and |
| • | advance notice procedures that stockholders must comply with in order to nominate candidates to the combined company’s board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of the combined company. |
The combined company is also subject to the anti-takeover provisions contained in Section 203 of the Delaware General Corporation Law. Under Section 203, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other exceptions, the board of directors has approved the transaction.
The combined company’s amended and restated bylaws will provide that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between the combined company and its stockholders, which could limit the combined company’s stockholders’ ability to obtain a favorable judicial forum for disputes with the combined company or its directors, officers or employees.
The combined company’s amended and restated bylaws will provide that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on the combined company’s behalf, any action asserting a breach of fiduciary duty, any action asserting a claim against the combined company arising pursuant to the Delaware General Corporation Law, the combined company’s amended and restated certificate of incorporation or the combined company’s amended and restated bylaws, or any action asserting a claim against the combined company that is governed by the internal affairs doctrine. These choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with the combined company or its directors, officers or other employees, which may discourage such lawsuits against the combined company and its directors, officers and other employees. By agreeing to this provision, however, stockholders will not be deemed to have waived the combined company’s compliance with the federal securities laws and the rules and regulations thereunder. Furthermore, the enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. If a court were to find the choice of forum provisions in the combined company’s amended and restated bylaws to be inapplicable or unenforceable in an action, the combined company may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect the combined company’s business and financial condition.
If the merger does not qualify as a “reorganization” for U.S. federal income tax purposes, U.S. Holders of Oncternal common stock will be required to recognize gain or loss for U.S. federal income tax purposes upon the exchange of their Oncternal common stock for GTx common stock in the merger.
The U.S. federal income tax consequences of the merger to U.S. Holders (as defined under the heading “The Merger—Material U.S. Federal Income Tax Consequences of the Merger”) will depend on whether the merger
115
qualifies as a “reorganization” for U.S. federal income tax purposes. GTx’s and Oncternal’s obligations to effect the merger are subject to the satisfaction, or waiver, at or prior to the effective time of the merger, of the condition that each company receive an opinion of counsel, dated as of the closing date of the merger, to the effect that the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. If, contrary to the opinions from counsel, the merger fails to qualify as a reorganization within the meaning of Section 368(a) of the Code, a U.S. Holder of Oncternal common stock would recognize gain or loss for U.S. federal income tax purposes on each share of Oncternal common stock surrendered in the merger for GTx common stock and any cash received in lieu of a fractional share. For a more complete discussion of the material U.S. federal income tax consequences of the merger, please carefully review the information set forth in the section entitled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger.”
Oncternal’s ability to use net operating loss carryforwards and other tax attributes may be limited in connection with the merger and other ownership changes.
Oncternal has incurred substantial losses during its history and does not expect to become profitable in the near future, and Oncternal may never achieve profitability. To the extent that Oncternal continues to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire (if at all). At December 31, 2018, Oncternal had federal and state NOL carryforwards of approximately $29.7 million. Such federal and state NOL carryforwards will begin to expire in 2033, unless previously utilized. At December 31, 2018, Oncternal had federal and state research and development credit carryforwards of approximately $0.9 million and $0.5 million, respectively. The federal research and development credit carryforwards will begin expiring in 2034, unless previously utilized. The state research and development credits do not expire.
Under the Tax Act, federal NOLs generated in taxable years ending after December 31, 2017, may be carried forward indefinitely but federal NOLs generated in taxable years beginning after December 31, 2017 may only be used to offset 80% of Oncternal’s taxable income annually. Oncternal’s NOL carryforwards are subject to review and possible adjustment by the IRS and state tax authorities. Under Sections 382 and 383 of the Code, Oncternal’s federal NOL and research and development tax credit carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant stockholders over a three-year period in excess of 50 percentage points. Oncternal’s ability to utilize its NOL carryforwards and other tax attributes to offset future taxable income or tax liabilities may be limited as a result of ownership changes, including in connection with the merger. Similar rules may apply under state tax laws. Oncternal has not yet determined the amount of the cumulative change in its ownership resulting from the merger or other transactions, or any resulting limitations on its ability to utilize its NOL carryforwards and other tax attributes. If Oncternal earns taxable income, such limitations could result in increased future tax liability to Oncternal and its future cash flows could be adversely affected. Oncternal has recorded a full valuation allowance related to its NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future benefits of those assets.
U.S. tax legislation may materially adversely affect Oncternal’s financial condition, results of operations and cash flows.
The Tax Act has significantly changed the U.S. federal income taxation of U.S. corporations, including by reducing the U.S. corporate income tax rate and revising the rules governing NOLs. Many of these changes became effective beginning in 2018, without any transition periods or grandfathering for existing transactions. The legislation is unclear in many respects and could be subject to potential amendments and technical corrections, as well as interpretations and implementing regulations by the U.S. Treasury Department and the IRS, any of which could lessen or increase certain adverse impacts of the legislation. In addition, it is unclear how these U.S. federal income tax changes will affect state and local taxation, which often uses federal taxable income as a starting point for computing state and local tax liabilities. As a result of the rate reduction from the Tax Act, Oncternal has reduced its deferred tax asset balance as of December 31, 2017 by $2.8 million. However,
116
due to Oncternal’s full valuation allowance position, there was no net impact on Oncternal’s income tax provision at December 31, 2017, as the reduction in the deferred tax asset balance was fully offset by a corresponding decrease in the valuation allowance.
There may be other material adverse effects resulting from the legislation that Oncternal has not yet identified. While some of the changes made by the tax legislation may adversely affect Oncternal in one or more reporting periods and prospectively, other changes may be beneficial on a going forward basis. Oncternal continues to work with its tax advisors to determine the full impact that the recent tax legislation as a whole will have on Oncternal. Oncternal urges its investors to consult with their legal and tax advisors with respect to such legislation.
The combined company could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for the combined company, because biotechnology and pharmaceutical companies have experienced significant stock price volatility in recent years. If the combined company faces such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm the combined company’s business.
117
This proxy statement/prospectus/information statement and the documents incorporated by reference into this proxy statement/prospectus/information statement contain forward-looking statements (including within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Section 27A of the United States Securities Act of 1933, as amended (the “Securities Act”)) concerning GTx, Oncternal, the merger and other matters. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the management of GTx, as well as assumptions made by, and information currently available to, management. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “plan,” “believe,” “intend,” “look forward,” and other similar expressions among others. Statements that are not historical facts are forward-looking statements. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation: (i) the risk that the conditions to the closing of the merger are not satisfied, including the failure to timely obtain stockholder approval for the transaction, if at all; (ii) uncertainties as to the timing of the consummation of the merger and the ability of each of GTx and Oncternal to consummate the merger; (iii) risks related to GTx’s ability to manage its operating expenses and its expenses associated with the merger pending closing; (iv) risks related to the failure or delay in obtaining required approvals from any governmental or quasi-governmental entity necessary to consummate the merger; (v) the risk that as a result of adjustments to the exchange ratio, GTx stockholders and Oncternal stockholders could own more or less of the combined company than is currently anticipated; (vi) risks related to the market price of GTx’s common stock relative to the exchange ratio; (vii) unexpected costs, charges or expenses resulting from the transaction; (viii) potential adverse reactions or changes to business relationships resulting from the announcement or completion of the merger; (ix) the uncertainties associated with the clinical development and regulatory approval of product candidates such as cirmtuzumab and TK216, including potential delays in the commencement, enrollment and completion of clinical trials; (x) risks related to the inability of the combined company to obtain sufficient additional capital to continue to advance these product candidates and its preclinical programs, including Oncternal’sCAR-T program and, depending on the determination of the combined company’s board of directors, potentially the SARD program; (xi) uncertainties in obtaining compelling or successful preclinical and clinical results for product candidates and unexpected costs that may result therefrom; (xii) risks related to the failure to realize any value from product candidates and preclinical programs being developed and anticipated to be developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; (xiii) the risk that the conditions to payment under the CVRs will be not be met and that the CVRs may otherwise never deliver any value to GTx stockholders, including in connection with the potential determination of the combined company’s board of directors to discontinue any SARD development efforts and to discontinue any divestment efforts with respect to the SARD technology or SARM technology; (xiv) risks associated with the possible failure to realize certain anticipated benefits of the merger, including with respect to future financial and operating results; and (xv) risks related to the impact of the workforce reduction reported herein on GTx’s business and unanticipated charges not currently contemplated that may occur as a result of the workforce reduction, including that the workforce reduction charges, costs and expenditures may be greater than currently anticipated. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. Except as required by applicable law, GTx undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
For a discussion of the factors that may cause GTx, Oncternal or the combined organization’s actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied in such forward-looking statements, or for a discussion of risk associated with the ability of
118
GTx and Oncternal to complete the merger and the effect of the merger on the business of GTx, Oncternal and the combined organization, see the section entitled “Risk Factors” beginning on page 26.
Additional factors that could cause actual results to differ materially from those expressed in the forward-looking statements are discussed in reports filed with the SEC by GTx including GTx’s most recent Annual Report on Form10-K, Form 10-K/A and Current Reports on Form8-K filed with the SEC. See the section entitled “Where You Can Find More Information” beginning on page 358.
If any of these risks or uncertainties materialize or any of these assumptions prove incorrect, the results of GTx, Oncternal or the combined organization could differ materially from the forward-looking statements. All forward-looking statements in this proxy statement/prospectus/information statement are current only as of the date on which the statements were made. GTx and Oncternal do not undertake any obligation to publicly update any forward-looking statement to reflect events or circumstances after the date on which any statement is made or to reflect the occurrence of unanticipated events.
119
THE SPECIAL MEETING OF GTX’S STOCKHOLDERS
Date, Time and Place
The GTx special meeting will be held on June 5, 2019, at 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103 commencing at 9:00 a.m. Central time. GTx is sending this proxy statement/prospectus/information statement to its stockholders in connection with the solicitation of proxies by the GTx Board for use at the GTx special meeting and any adjournments or postponements of the GTx special meeting. This proxy statement/prospectus/information statement is first being furnished to GTx’s stockholders on or about , 2019.
Purpose of the GTx Special Meeting
The purpose of the GTx special meeting is:
1. To approve the Merger Agreement, and the transactions contemplated thereby, including the merger, the issuance of GTx’s common stock to Oncternal’s stockholders in accordance with the Merger Agreement and the change of control resulting from the merger.
2. To approve a series of alternative amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split, in the form attached asAnnex D to this proxy statement/prospectus/information statement.
3. To approve the amendment to the restated certificate of incorporation of GTx to effect the GTx Name Change in the form attached asAnnex E to this proxy statement/prospectus/information statement.
4. To approve the adoption of the GTx, Inc. 2019 Incentive Award Plan in the form attached asAnnex F to this proxy statement/prospectus/information statement.
5. To approve, on a nonbinding, advisory basis, the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger.
6. To consider and vote upon an adjournment of the GTx special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1 and 2.
7. To transact such other business as may properly come before the GTx special meeting or any adjournment or postponement thereof.
Recommendation of The GTx Board
| • | The GTx Board has determined that the transactions contemplated by the Merger Agreement, including the merger, the issuance of shares of GTx’s common stock to Oncternal’s stockholders pursuant to the Merger Agreement and the change of control resulting from the merger are fair to, advisable and in the best interest of GTx and its stockholders and has approved and declared advisable the Merger Agreement and such transactions. The GTx Board recommends that GTx’s stockholders vote “FOR” Proposal No. 1 to approve the Merger Agreement and the transactions contemplated thereby, including the merger, the issuance of shares of GTx’s common stock to Oncternal’s stockholders and the change of control resulting from the merger. |
| • | The GTx Board has determined that the GTx Reverse Stock Split is fair to, advisable and in the best interest of GTx and its stockholders and has approved and declared advisable the GTx Reverse Stock Split. The GTx Board recommends that GTx’s stockholders vote “FOR” Proposal No. 2 to approve a series of alternative amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split. |
120
| • | The GTx Board has determined that the GTx Name Change is fair to, advisable and in the best interest of GTx and its stockholders and has approved and declared advisable the GTx Name Change. The GTx Board recommends that GTx’s stockholders vote “FOR” Proposal No. 3 to approve an amendment to the restated certificate of incorporation of GTx effecting the GTx Name Change. |
| • | The GTx Board has determined that the adoption of the GTx, Inc. 2019 Incentive Award Plan (the “GTx 2019 Plan”) is fair to, advisable and in the best interests of GTx and its stockholders and has approved and declared advisable the GTx 2019 Plan. The GTx Board recommends that GTx’s stockholders vote “FOR” Proposal No. 4 to approve the GTx 2019 Plan. |
| • | The GTx Board has determined that the approval of the nonbinding, advisory vote on the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger is advisable and in the best interests of GTx and its stockholders and has approved such nonbinding advisory vote. The GTx Board recommends that GTx’s stockholders vote “FOR” Proposal No. 5 to approve, on a nonbinding, advisory basis, the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger. |
| • | The GTx Board has determined and believes that adjourning the GTx special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1 or 2 is advisable to, and in the best interests of, GTx and its stockholders and has approved and adopted the proposal. The GTx Board recommends that GTx’s stockholders vote “FOR” Proposal No. 6 to adjourn the GTx special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1 or 2. |
Record Date and Voting Power
Only holders of record of GTx’s common stock at the close of business on the record date, April 15, 2019, are entitled to notice of, and to vote at, the GTx special meeting. There were approximately 67 holders of record of GTx’s common stock at the close of business on the record date. At the close of business on the record date, 24,051,844 shares of GTx’s common stock were issued and outstanding. Each share of GTx’s common stock entitles the holder thereof to one vote on each matter submitted for stockholder approval. See the section entitled “Principal Stockholders of GTx” in this proxy statement/prospectus/information statement for information regarding persons known to GTx’s management to be the beneficial owners of more than 5% of the outstanding shares of GTx’s common stock.
Voting and Revocation of Proxies
The proxy accompanying this proxy statement/prospectus/information statement is solicited on behalf of the GTx Board for use at the GTx special meeting.
If you are a stockholder of record of GTx as of the record date referred to above, you may vote in person at the GTx special meeting or vote by proxy using the enclosed proxy card. Whether or not you plan to attend the GTx special meeting, GTx urges you to vote by proxy to ensure your vote is counted. You may still attend the GTx special meeting and vote in person if you have already voted by proxy. As a stockholder of record you may vote in any of the following ways:
| • | to vote in person, attend the GTx special meeting and GTx will provide you a ballot when you arrive. |
| • | to vote using the proxy card, simply mark, sign and date your proxy card and return it promptly in the postage-paid envelope provided. If you return your signed proxy card to GTx before the GTx special meeting, GTx will vote your shares as you direct on the proxy card. |
| • | to vote by telephone or on the Internet, dial the number on the proxy card or voting instruction form or visit the website on the proxy card or voting instruction form to complete an electronic proxy card. You will be asked to provide GTx’s number and control number from the enclosed proxy card. Your vote must be received by 11:59 p.m., Central time on June 4, 2019 to be counted. |
121
If your shares of GTx’s common stock are held by your broker as your nominee, that is, in “street name,” the enclosed voting instruction card is sent by the institution that holds your shares. Please follow the instructions included on that proxy card regarding how to instruct your broker to vote your shares of GTx’s common stock. If you do not give instructions to your broker, your broker can vote your shares of GTx’s common stock with respect to “discretionary” items but not with respect to“non-discretionary” items. Discretionary items are proposals considered routine under certain rules applicable to brokers on which your broker may vote shares held in “street name” in the absence of your voting instructions. Onnon-discretionary items for which you do not give your broker instructions, your shares of GTx’s common stock will be treated as brokernon-votes. It is anticipated that all proposals will benon-discretionary items.
All properly executed proxies that are not revoked will be voted at the GTx special meeting and at any adjournments or postponements of the GTx special meeting in accordance with the instructions contained in the proxy. If a holder of GTx’s common stock executes and returns a proxy and does not specify otherwise, the shares represented by that proxy will be voted “FOR” Proposal No. 1 to approve the Merger Agreement and the transactions contemplated thereby, including the merger, the issuance of shares of GTx’s common stock to Oncternal’s stockholders pursuant to the Merger Agreement and the change of control resulting from the merger; “FOR” Proposal No. 2 to approve a series of alternative amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split; “FOR” Proposal No. 3 to approve an amendment to the restated certificate of incorporation of GTx to effect the GTx Name Change; “FOR” Proposal No. 4 to approve the adoption of the GTx 2019 Plan; FOR” Proposal No. 5 to approve, on a nonbinding, advisory basis, the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger; and “FOR” Proposal No. 6 to approve the adjournment of the GTx special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1 or 2 in accordance with the recommendation of the GTx Board.
GTx’s stockholders of record, other than those GTx’s stockholders who have executed voting agreements, may change their vote at any time before their proxy is voted at the GTx special meeting in one of three ways. First, a stockholder of record of GTx can send a written notice to the Secretary of GTx stating that the stockholder would like to revoke its proxy. Second, a stockholder of record of GTx can submit new proxy instructions either on a new proxy card or by telephone or via the Internet. Third, a stockholder of record of GTx can attend the GTx special meeting and vote in person. Attendance alone will not revoke a proxy. If a stockholder of GTx of record or a stockholder who owns shares of GTx’s common stock in “street name” has instructed a broker to vote its shares of GTx’s common stock, the stockholder must follow directions received from its broker to change those instructions.
Required Vote
The presence, in person or represented by proxy, at the GTx special meeting of the holders of a majority of the shares of GTx’s common stock outstanding and entitled to vote at the GTx special meeting is necessary to constitute a quorum at the meeting. Abstentions and brokernon-votes will be counted towards a quorum. Approval of Proposal Nos. 1, 4, 5 and 6 requires the affirmative vote of the holders of a majority of the shares of GTx’s common stock entitled to vote and present in person or represented by proxy at the GTx special meeting. Approval of Proposal Nos. 2 and 3 requires the affirmative vote of holders of a majority of GTx’s common stock having voting power outstanding on the record date for the GTx special meeting.
Votes will be counted by the inspector of election appointed for the GTx special meeting, who will separately count “FOR” and “AGAINST” votes, abstentions, brokernon-votes, and in the case of the election of directors, “WITHHOLD” votes. Abstentions will be counted towards the vote total and will have the same effect as “AGAINST” votes for Proposal Nos. 1, 2, 3, 4, 5 and 6. Brokernon-votes will have the same effect as “AGAINST” votes for Proposal Nos. 2 and 3. For Proposal Nos. 1, 4, 5 and 6, brokernon-votes will have no effect and will not be counted towards the vote total, but will be used to determine whether a quorum is present at the GTx special meeting.
122
Each of Proposal Nos. 1 and 2 are conditioned upon each other. Therefore, the merger cannot be consummated without the approval of Proposal Nos. 1 and 2. Proposal Nos. 3 and 4 are conditioned upon the consummation of the merger. If the merger is not completed or the stockholders do not approve Proposal No. 3, GTx will not change its name to “Oncternal Therapeutics, Inc.” If the merger is not completed or the stockholders do not approve Proposal No. 4, the GTx 2019 Plan will not become effective. Proposal Nos. 1 and 2 are not conditioned on Proposal No. 3 or Proposal No. 4 being approved.
As of March 31, 2019 the directors and executive officers of GTx and other stockholders who signed voting agreements beneficially owned approximately 45% of the outstanding shares of GTx’s common stock entitled to vote at the GTx special meeting. Pursuant to the voting agreements, each such director, executive officer and other signatory stockholder has agreed to be present (in person or by proxy) at the GTx special meeting to vote all shares of GTx’s common stock owned by him, her or it as of the record date in favor of Proposals Nos. 1, 2, 3, 4 and 5. Additionally, each such stockholder has agreed, solely in his, her or its capacity as a stockholder of GTx, to vote against any competing acquisition proposal and any action, proposal or transaction that would reasonably be expected to result in a material breach of the voting agreement. As of March 31, 2019 GTx is not aware of any affiliate of Oncternal owning any shares of GTx’s common stock entitled to vote at the GTx special meeting.
Solicitation of Proxies
In addition to solicitation by mail, the directors, officers, employees and agents of GTx may solicit proxies from GTx’s stockholders by personal interview, telephone, telegram or otherwise. GTx and Oncternal will share equally the costs of printing and filing this proxy statement/prospectus/information statement and proxy card. Arrangements will also be made with brokerage firms and other custodians, nominees and fiduciaries who are record holders of GTx’s common stock for the forwarding of solicitation materials to the beneficial owners of GTx’s common stock. GTx will reimburse these brokers, custodians, nominees and fiduciaries for the reasonableout-of-pocket expenses they incur in connection with the forwarding of solicitation materials. GTx has not retained a proxy solicitor with respect to the GTx special meeting.
Other Matters
As of the date of this proxy statement/prospectus/information statement, the GTx Board does not know of any business to be presented at the GTx special meeting other than as set forth in the notice accompanying this proxy statement/prospectus/information statement. If any other matters should properly come before the GTx special meeting, it is intended that the shares represented by proxies will be voted with respect to such matters in accordance with the judgment of the persons voting the proxies.
123
This section and the section entitled “The Merger Agreement” in this proxy statement/prospectus/information statement describe the material aspects of the merger, including the Merger Agreement. While GTx and Oncternal believe that this description covers the material terms of the merger and the Merger Agreement, it may not contain all of the information that is important to you. You should read carefully this entire proxy statement/prospectus/information statement for a more complete understanding of the merger and the Merger Agreement, including the Merger Agreement attached as Annex A, the opinion of Aquilo attached asAnnex B-2, and the other documents to which you are referred herein. See the section entitled “Where You Can Find More Information” in this proxy statement/prospectus/information statement.
Background of the Merger
Historical Background for GTx
GTx is a biopharmaceutical company dedicated to the discovery and development of medicines to treat serious and/or significant unmet medical conditions. For the past several years, GTx has focused its development efforts on its SARM and SARD programs, two technologies licensed from UTRF.
In September 2017, GTx initiated a randomized, placebo-controlled Phase 2 clinical trial, or the ASTRID Trial, of its lead SARM product candidate enobosarm (also known as Ostarine orGTx-024) with both 3 mg and 1 mg doses to assess the safety and efficacy of the drug candidate compared to placebo.
In early August 2018, Company A reached out to GTx seeking an update on SARDs.
Following receipt of data on September 20, 2018, indicating that the ASTRID Trial had failed to achieve statistical significance on the trial’s primary endpoint, a special meeting of the GTx Board was held, with GTx senior management attending. The purpose of the meeting was for GTx senior management to discuss the data with the GTx Board and to share details of the press release GTx prepared for immediate release. GTx senior management informed the GTx Board that it was halting its financing plans and would undertake a more thorough assessment of data from the clinical trial to ascertain whether there were problems with the trial that caused the unexpected results or whether the data suggests that certain subsets of patients might potentially benefit from treatment versus the universe of stress urinary incontinence (“SUI”) patients included in the clinical trial. SUI is the involuntary leakage of urine during activities such as coughing, laughing, sneezing, exercising or other movements that increase intra-abdominal pressure and thus increase pressure on the bladder. In the interim, GTx senior management would assess whether it could realistically expedite the preclinical studies already underway for SARDs, and the GTx Board authorized GTx senior management to reach out to third parties who might have an interest in collaborating on SARD research and development or acquiring GTx to access the SARD technology.
On September 21, 2018, GTx announced that the ASTRID Trial failed to achieve statistical significance on the primary endpoint of the proportion of patients with a greater than 50% reduction in incontinence episodes per day compared to placebo. The percentage of patients with a greater than 50% reduction after 12 weeks of enobosarm treatment was 58.9% for 3 mg, 57.7% for 1 mg and 52.7% for placebo. Enobosarm was generally safe and well tolerated, and reported adverse events were minimal and similar across all treatment groups. After completing its review of the full data sets from the clinical trial and discussing the data with clinical experts, GTx determined that there is not a sufficient path forward to warrant additional clinical development of enobosarm to treat SUI. It has discontinued further development of enobosarm to treat SUI, including discontinuing the related durability and open-label safety extension studies which were initiated before GTx received topline data from the ASTRID Trial.
Remembering that he had received an inquiry in early August 2018 from pharmaceutical Company A seeking to get an update on SARDs, Mr. Hanover contacted the representative for Company A on September 24, 2018
124
suggesting that GTx senior management review with Company A GTx’s current SARD data. It was noted that the parties had previously entered into a confidentiality agreement in December 2016 regarding prior SARDs discussions which was subsequently amended to address SARMs and extend the term to December 2018, and the parties agreed to assemble appropriate scientific personnel from both sides to review and discuss GTx’s SARD program.
On September 25, 2018, Dr. Wills received a call from a hedge fund representative with whom he and Mr. Hanover knew from previous interactions, indicating that his fund was an investor in Company B, which might be interested in considering a merger with GTx.
On September 28, 2018, Company C contacted Dr. Wills expressing an interest in SARDs and learning more about the technology.
On October 1, 2018, Mr. Hanover was introduced by telephone to the chief executive officer of Company D, a holding company for various subsidiaries, including a subsidiary developing selective estrogen receptor degraders (“SERD”) compounds. The chief executive officer of Company D expressed an interest in merging his SERD program with GTx’s SARD technology and suggested that the companies enter into a mutual confidentiality agreement for the exchange of information, which was done on October 2, 2018.
On October 2, 2018, Dr. Wills received a call from two senior executives from Company E stating an interest in better understanding GTx’s SARD technology, and the parties entered into a confidentiality agreement on October 3, 2018.
On October 4, 2018, GTx entered into a confidentiality agreement with Company C and the parties agreed to hold initial diligence discussions on October 10, 2018.
On October 10, 2018, GTx entered into a confidentiality agreement with Company F, which contacted Dr. Wills expressing an interest in a potential combination with GTx. Dr. Wills had a conversation and initial scientific discussion with Company F personnel on October 12, 2018 to assess their interest in moving forward with a broader discussion.
In early October 2018, Mr. Hanover inquired of pharmaceutical Company H whether it would have an interest in learning more about GTx’s SARD program. A business development executive from Company H indicated Company H was interested in learning more about GTx’s SARD program as well as its SARM technology, including enobosarm as a potential treatment for breast cancer. On October 10, 2018, GTx and Company H entered into a confidentiality agreement, and information about GTx and its SARD and SARM programs was sent to Company H for its review. Since SARD and SARMs comprised substantially all of the assets of GTx, Mr. Hanover told the Company H executive that it should analyze the opportunity as an acquisition of GTx.
On October 11, 2018, the chief executive officer of Company D visited GTx at GTx’s headquarters, and met with GTx’s senior management and GTx’s largest stockholder, Mr. Hyde. The chief executive officer of Company D also met with GTx personnel and indicated his willingness to provide employment to several clinical and financial personnel should a transaction between Company D and GTx come to fruition.
Also, on October 12, 2018, GTx senior management held a teleconference with executives and scientists representing Company C to discuss more specifically GTx’s SARD program.
GTx had previously sent a confidential slide deck about its SARD program to Company A on October 1, 2018, and on October 16, 2018, GTx senior management and scientists reviewed the information with various Company A personnel during a prearranged teleconference. At the end of the call, Company A stated that it would assemble its team and decide soon whether it wanted to make a business proposal to GTx. Also, the parties agreed to amend their existing confidentiality agreement to extend the term to December 2019.
On October 16, 2018, Company B notified Dr. Wills that it wanted to undertake some preclinical assays of GTx’s SARD compounds to determine if the information GTx provided Company B could be reproduced by Company
125
B personnel and to determine if the SARD technology was sufficiently developed for Company B to make a proposal to GTx. A material transfer agreement (“MTA”) was executed between the companies on October 18, 2018.
Similarly, following a discussion between Company C executives and GTx senior management, an MTA was executed with Company C on October 23, 2018 for Company C to assess GTx’s SARD compounds. Under both MTAs, Company B and Company C were given a short period to conduct their assays and they were required to report their findings to GTx senior management.
On October 22, 2018, GTx senior management held a teleconference with Company F personnel to review GTx’s SARD technology.
On October 14, 2018, Dr. Wills reached back out to Company G based on discussions regarding a potential collaboration for the development of SARDs between Dr. Wills and Company G from several years earlier. Dr. Wills let Company G’s executives know that the SARD data it reviewed several years ago was not current and GTx had made strides in further developing the technology, which may be of interest to Company G. As a result of that conversation, the parties entered into a new confidentiality agreement on October 23, 2018, so that GTx could share current SARD data with Company G.
On October 23 and 24, 2018, Dr. Wills, Mr. Hanover and Dr. Johnston visited the headquarters of Company D to learn more about Company D’s organization and corporate structure and its ongoing pharmaceutical programs, including its SERD program. Following that meeting, per the direction received from the GTx Board in September, GTx senior management decided to continue discussions with Company D and move toward Company D making a proposal for a merger with GTx.
On October 30, 2018, Company A informed Mr. Hanover and Dr. Wills that Company A had decided not to proceed with an offer at this time until GTx has been able to better understand more about how SARDs produce the outcomes seen in the various preclinical assays conducted by GTx. Company A stated that it remained interested in SARDs and would welcome additional data once the technology was better understood.
Throughout October, GTx senior management provided the GTx Board with interim updates of its ongoing conversations with potential acquirers.
During the first week of November 2018, Company D proposed that it be combined with GTx’s SARD program in a combined company that would be a subsidiary of Company D. GTx and Company D discussed the possibility of a potential reverse merger between the parties, but Company D was not willing to consider a reverse merger transaction structure given its future plans for its company. Company D also proposed an equity split for the combined company stockholders that GTx senior management believed to be inadequate.
On November 1, 2018, a discussion between Company H scientists and GTx personal was held to review both programs.
Also on November 1, 2018, GTx and Company F had afollow-up discussion regarding SARDs.
On November 5, 2018, GTx senior management team and its largest stockholder and Mr. Hyde metin-person with Company D’s chief executive officer and other personnel at Company D’s corporate headquarters to learn more about Company D’s pharmaceutical programs and its proposal for a business combination with GTx. Company D continued to propose a corporate structure for a business combination that would be difficult for a public company like GTx to accomplish and an equity split for the combined company stockholders that GTx senior management believed to be inadequate. Nevertheless, GTx senior management and Mr. Hyde stated an intention to have further discussion with the full GTx Board at the upcoming quarterly meeting on November 7, 2018.
126
On November 5, 2018, Company I contacted Dr. Wills inquiring about whether GTx would be interested in discussing a merger with Company I. Although Company I’s pharmaceutical programs appeared to be in areas outside of any fields of expertise pertaining to SARDs or SARMs, Dr. Wills agreed that GTx senior management would entertain discussions with Company I. GTx entered into a confidentiality agreement with Company I later that day and confidential information was exchanged between the parties.
On November 6, 2018, Dr. Johnston received an inquiry from Company J about GTx’s SARM program and stated an interest in exploring an acquisition of that asset. GTx and Company J entered into a confidentiality agreement on November 14, 2018, and information regarding enobosarm, including efficacy and safety data from prior clinical studies, was made available to Company J through GTx’s electronic data room.
On November 7, 2018, the GTx board held its regularly scheduled quarterly meeting at the company headquarters in Memphis, Tennessee. At the meeting, Dr. Wills reported that GTx senior management had not as yet seen any clear path forward for the continued development of enobosarm to treat SUI, but it was continuing to review all data from the ASTRID Trial and discuss the data with GTx’s key opinion leaders and experts. Since the announcement of data from the enobosarm study, GTx senior management had engaged with several companies expressing interest in SARDs, and a few companies potentially interested in an acquisition of GTx. He reported that GTx senior management had discussions ongoing with three private pharmaceutical companies with either preclinical and/or androgen receptor expertise which would be helpful in furthering GTx’s SARD development efforts, but any merger with a private company presented difficult corporate structuring issues for a public company and the proposals that had been suggested so far would result in significant dilution for GTx’s stockholders. Also, he noted that while GTx had sufficient cash to undertake SARD development on its own without the need to raise additional funds until sometime in 2020, GTx would face the risk of having all of its value resting on a single preclinical technology. The best case for GTx would be to find a merger partner with expertise that would be helpful for continued SARD development and assets of its own to spread the risk for a combined company as the development programs progress. He noted that the companies expressing interest in GTx appeared to have little or no real cash of their own or were without sufficient expertise to help with SARD development. Mr. Hanover reported that GTx senior management also had been in discussions with at least three large pharmaceutical companies interested in SARDs, and while one has recently decided not to pursue the opportunity absent receiving additional data, two companies remained interested. During this meeting, the GTx Board also discussed potentially engaging a financial advisor to assist with the process and authorized GTx senior management to begin discussions with potential financial advisors. The GTx Board agreed that it would meet again on November 19, 2018 to review GTx senior management’s progress in its ongoing discussions.
On November 7, 2018, Company K contacted Mr. Hanover regarding the potential of licensing enobosarm. Mr. Hanover informed Company K that other parties were interested in acquiring enobosarm and a license of the asset was something that GTx senior management could not recommend to the GTx Board. However, should Company K be interested in making a proposal to acquire the asset, GTx senior management would be interested in receiving it.
On November 8, 2018, Company H informed Mr. Hanover that after evaluating the data for GTx’s SARD and SARM program, it would not be making a proposal to acquire GTx.
On November 9, 2018, Company B called Dr. Wills and told him they had completed their assays for the SARD compounds sent to them under the MTA, and while their data was confirmatory to the data GTx previously provided them, SARDs were too early stage for them and they preferred to focus only on their own pharmaceutical programs. On November 30, 2018, Company C provided the company with a report on the assays it conducted on certain of the company’s SARDs and indicated that it had decided not to pursue a merger with GTx. Under both MTAs, Company B and Company C ended their research work on SARDs and either returned excess SARD compound material to GTx or destroyed the compounds in accordance with GTx’s instructions.
127
On November 8, 2018, Company L contacted Dr. Wills regarding the possibility of a merger transaction with GTx. After entering into a confidentiality agreement on November 16, 2018, information about both companies were exchanged between the parties.
On November 16, 2018, Company J and GTx discussed the enobosarm data.
Between November 16, 2018 and December 4, 2018, Company J and its scientific personnel continued its evaluation of enobosarm as a potential treatment for SUI.
On November 19, 2018, the GTx Board held a special meeting, with GTx senior management attending, for the purpose of receiving an update from GTx senior management of its ongoing discussions with various parties interested in discussing a transaction with GTx. Dr. Wills reported that one pharmaceutical company continued to express its interest in collaborating with GTx in the its ongoing SARD research but was not likely to be interested in discussing either a licensing of SARDs or an acquisition of GTx or its asset until such time as the preclinical research of SARDs had been completed and there was a compound identified as an IND candidate for clinical studies. Mr. Hanover reported that GTx senior management was continuing to discuss with Company D its interest in merging one of its subsidiary companies with and into GTx, but given both the continued complexity of accomplishing what Company D was suggesting and the inadequacy of its proposed equity split, Mr. Hanover believed Company D was not going to remain a viable merger prospect unless it significantly changed its proposal. Mr. Hanover reported on GTx senior management discussions with Company L and stated that it was too early in the process to know if a reasonable merger proposal could be negotiated, and GTx senior management had just started work on understanding Company L’s pharmaceutical programs and cash position.
Dr. Wills reported to the GTx Board that a few other pharmaceutical companies have determined not to continue discussions with GTx senior management about a corporate transaction and Company B and Company C had decided they would not pursue a merger with GTx. Mr. Hanover noted that Dr. Johnston had received a call from Company J expressing an interest in GTx’s SARM assets, including enobosarm, and he had been contacted by Company K also requesting GTx consider licensing enobosarm.
Mr. Doggrell updated the GTx Board on GTx senior management’s efforts to engage a financial advisor, noting that it had been difficult to find interest from financial advisors given GTx’s position unless there was a significant advisory fee. Mr. Doggrell explained to the GTx Board that it had discussed an engagement with Aquilo and had reached agreement on the terms, subject to approval by the GTx Board. Mr. Doggrell reviewed with the GTx Board an engagement letter from Aquilo, to provide financial advisory services for GTx and the GTx Board.
Between November 20, 2018 and December 11, 2018, GTx continued to engage in discussions with Company D, but the parties did not agree to revised terms.
On November 25, 2018, a GTx Board member, Dr. Carter, was contacted by David F. Hale, an Oncternal Board member, who had seen GTx’s recent press releases and whom Dr. Carter knew through other board memberships. Mr. Hale indicated he would be interested in discussing a merger of Oncternal with GTx.
On November 26, 2018, Dr. Wills discussed Oncternal’s business and a potential transaction with Mr. Hale and Dr. James B. Breitmeyer, Oncternal’s Chief Executive Officer.
On November 30, 2018, GTx and Oncternal entered into a confidentiality agreement and the parties begin exchanging information.
Between November 2018 and early February, 2019, Dr. Wills continued to have discussions with Company G about a possible collaboration whereby Company G would undertake preclinical research and development of GTx’s SARDs in collaboration with GTx and GTx’s third party contractors and consultants. At the direction of
128
the GTx Board on November 7, 2018, Dr. Wills was exploring with Company G representatives whether it would consider making an offer to acquire either the company or its SARD assets or, alternatively, entering into a collaboration agreement to develop SARDs with an option for Company G to acquire GTx or the SARD assets should it be willing to do so when an IND had been filed and a SARD product candidate was ready to enter the clinic. Before committing to undertake any development efforts, Company G suggested entering into an MTA to allow it to conduct some initial experiments on GTx’s existing lead SARD compounds. A draft MTA was sent to Company G at the end of January 2019, to which Company G responded, but the finalization and execution of the MTA was placed on hold by GTx senior management as it was concluding discussions with Oncternal on a letter of intent for GTx senior management to present to the GTx Board.
During this same time period, Company O made a proposal to Dr. Wills about selling or licensing enobosarm to it. As drafted, the proposal would provide for a minimal upfront payment with milestones payable only upon the occurrence of certain events subsequent to the transaction. GTx decided to delay responding to the proposal from Company O with the expectation that the combined company of Oncternal and GTx would be able to assess whether a counter offer was appropriate or some other strategic alternative for enobosarm and the company’s SARM portfolio may be more appropriate.
On December 4, 2018, Company J called Mr. Hanover to communicate that it had decided not to proceed with a bid to acquire enobosarm.
On December 7, 2018, Oncternal and GTx had discussions regarding GTx’s SARD technology.
On December 12, 2018, the GTx Board held a special meeting, with GTx senior management attending, for the purpose of receiving an update from Dr. Wills and Mr. Hanover on their discussions with various parties interested in some form of a potential business transaction with GTx. Mr. Hanover reported that discussions with Company D were ongoing over the last several weeks but were becoming more protracted and complex, and he was unsure whether GTx senior management would be making a favorable recommendation to the GTx Board about pursuing that transaction. Mr. Hanover explained that the complexity of trying to merge GTx’s SARD technology with Company D’s SERD program under the umbrella of a holding company controlled by Company D would make it difficult for GTx shareholders to have any liquidity in this investment and the equity split being suggested for GTx shareholders was inadequate. Dr. Wills reported that discussions with Company L continued to be positive but their proposed equity split for a combined company was, in his opinion, insufficient and there was little synergy in Company L’s technology and what GTx would be bringing to the combined company for development. Dr. Wills reported that while discussions with Oncternal were at an early stage, he, Mr. Hanover and Mr. Hyde had good discussions with Oncternal and there seemed to be a willingness to structure a transaction that may be more beneficial to GTx and its stockholders than other companies have been willing to offer. He noted that Oncternal was an oncology company and the synergies between the two companies were good, and that Oncternal has asked for and had been given access to GTx’s data room so it and its advisors could undertake more extensive due diligence of GTx and its technologies. Mr. Hanover reported that Company J had decided not to pursue the acquisition of SARMs, including enobosarm, although he had received some interest in the asset from Company K and would explore with Company K whether it was in a position to make a meaningful proposal for GTx’s SARMs. He also stated that GTx senior management would be exploring with Oncternal whether it wanted GTx to retain SARMs as an asset should Oncternal and GTx decide to combine.
Mr. Shackelford reviewed with the GTx Board financial projections for 2019, assuming GTx agreed to accept one of the merger proposals then being discussed on terms which GTx senior management believed may be possible to negotiate, versus remaining independent and continuing its ongoing preclinical development of SARDs. Mr. Doggrell reported on his most recent discussions with Aquilo and reviewed with the GTx Board Aquilo’s engagement letter, which the GTx Board then approved and authorized GTx senior management to sign.
In addition, the GTx Board approved and authorized GTx senior management to enter into an agreement with Aquilo which was executed on December 12, 2018.
129
On December 18, 2018, Dr. Wills and Mr. Hanover metin-person with Company L’s executives and scientists at Company L’s headquarters, and the principal executive officer thereafter informed Dr. Wills that Company L’s board of directors had authorized him and his team to negotiate anon-binding merger proposal to bring back to its board for discussion. He reiterated his proposal from a telephone conversation with Dr. Wills on November 18, 2018, and stated that was the proposal his Board was willing to accept, subject to the completion of diligence by both companies.
Also on December 18, 2018, a due diligence meeting was held in San Diego, California between Dr. Wills, Mr. Hanover and members of the Oncternal management.
Thereafter, on December 19, 2018, Dr. Wills telephoned Company L with a counter proposal that GTx senior management believed might be acceptable to bring to its Board for further discussion. Later in the day, this counter proposal was rejected by Company L, which added a proposal that the reverse merger be completed in a structure that would make the combined companies a subsidiary of Company L. However, the senior executive of Company L reiterated his desire to pursue the proposed reverse merger with GTx and suggested the parties meet again for a more protracted discussion about merging the two companies.
On December 19, 2018, Dr. Wills received a call from Company M expressing an interest in SARDs and suggesting a possible reverse merger with GTx. Dr. Wills explained that GTx had discussion underway with several other companies but would remain open to undertake discussions with Company M if it could move quickly through its review of GTx’s assets following execution of a confidentiality agreement.
On December 21, 2018, GTx and Company M entered into a confidentiality agreement. Given the focus of Company M, a combination between it and GTx seemed an unlikely fit, but information was exchanged to determine if there was reason to accelerate these discussions.
On December 21, 2018, the GTx Board held a special meeting, with GTx senior management attending, for the purpose of receiving an update on GTx senior management’s discussions with various interested parties. Representatives from each of Aquilo and GTx’s outside legal counsel, Cooley LLP (“Cooley”) participated in the meeting. Dr. Wills summarized ongoing discussions with both Company L and Oncternal, both of which are private companies interested in a reverse merger with GTx. He noted that Company L had proposed an unacceptable equity split for the combined company and seemed to now be suggesting that the combined company become a subsidiary of Company L, which raised additional issues about whether GTx’s stockholders could hope to effectively participate in any exit strategy that Company L may have longer term. He stated that GTx senior management had countered Company L’s proposal but their counter proposal was rejected. On the other hand, Dr. Wills reported that he was having good conversation with and feedback from the executive team at Oncternal and believed a transaction may be possible with Oncternal if neither Oncternal nor GTx identify any concerns during the diligence process. Dr. Wills also noted that he had just received a call from a senior executive at Company M expressing an interest in a reverse merger with GTx, and he would see if there was any reason to actively pursue that opportunity.
Mr. Hanover reported to the GTx Board that little has changed in GTx senior management’s discussions with Company D throughout December, and he believed it unlikely a deal will come together that the GTx Board would support. Mr. Hanover subsequently communicated to the chief executive officer of Company D that unless he was willing to consider a proposal that would include merging his SERD technology into GTx through a reverse merger, with an equity split more favorable than he been proposing, the GTx Board was not interested in GTx senior management continuing discussions with Company D. The GTx Board indicated to GTx senior management that it should continue to pursue the opportunity with Company L or Oncternal, failing which there remained the option for GTx to remain independent and continue its preclinical development of SARDs through the calendar year 2019. Discussions with Company D about a merger of SARDs and SERDs ceased after December 2018.
130
Given the main concern expressed by the pharmaceutical companies that initially indicated an interest in GTx’s SARD technology was the lack of a definitive mechanism of action, GTx’s senior management contacted a leading academic researcher in late December 2018 to get the researcher’s recommendation regarding additional preclinical studies that should be undertaken to better understand SARDs’ mechanism of action. The academic researcher engaged by GTx suggested a colleague at the same institution who assisted GTx in putting in place the confidentiality and other agreements and research plan necessary to execute the work intended to delineate the mechanism of action. This additional preclinical research was initiated in January 2019.
In late December 2018, Mr. Hanover received a call from Company O stating that it had a potential interest in acquiring GTx’s SARM, enobosarm, if its proprietary diagnostic technology indicated that certain patients in GTx’s prior clinical studies would more likely benefit from enobosarm treatment for certain indications. Company O believed its technology could quickly determine if enobosarm could be used in particular ways that could enhance its effectiveness in a variety of indications. Company O expressed a desire to acquire the asset for minimal upfront costs, followed by larger milestone payments if it achieved certain criteria to be more particularly set forth in an acquisition agreement.
During the remainder of December 2018 and during the first several days of January 2019, representatives from companies Company L, Company M and Oncternal continued their respective diligence of GTx, and GTx senior management began its review of those respective companies and their assets. Meetings were scheduled between GTx senior management and executives of both Company M and Oncternal during the JP Morgan conference in San Francisco on January 8, 2019.
On December 26 and 27, 2018, Mr. Hale and Dr. Wills discussed potential terms and conditions for a reverse merger between GTx and Oncternal, including the potential for entering into a CVR Agreement, with respect to GTx’s SARD and SARM technology.
On December 29, 2018, GTx entered into a confidentiality agreement with Company K and enobosarm data was made available to Company K in GTx’s electronic data room throughout January.
On December 31, 2018, the GTx Board held a special meeting, with GTx senior management attending, for the purpose of receiving an update from GTx senior management regarding its ongoing discussions with potential acquirers. Dr. Wills reported that Company L was holding firm on its initial proposal for an equity split for the combined company, and it wanted the combined company to become a subsidiary of Company L. The GTx Board indicated that it had no interest in GTx senior management continuing to pursue discussions with Company L, which Dr. Wills subsequently communicated to Company L during the first week of January 2019. Similarly, Mr. Hanover reported that Company D had not altered its proposal, and he did not believe there was a realistic opportunity for GTx to continue to pursue discussions with Company D, and the GTx Board agreed. Dr. Wills noted that discussions with Oncternal continued to progress and the terms now being discussed were more favorable for GTx stockholders. The GTx Board directed GTx senior management to continue to pursue that opportunity.
Also on December 31, 2018, Dr. Wills indicated to Mr. Hale that GTx’s Board held a meeting to discuss the potential merger between GTx and Oncternal, and expressed the GTx board’s interest in moving forward with diligence on a potential transaction.
On January 2, 2019, GTx and Company K had a discussion regarding a potential transaction between the parties.
On January 7, 2019, Dr. Wills had a discussion with Company M regarding a potential reverse merger transaction with GTx.
On January 8, 2019, Dr. Wills, Mr. Hyde, Mr. Doggrell, Mr. Hanover and Mr. Shackelford met with representatives of Oncternal to discuss the business of Oncternal and the proposed transaction.
131
On January 9, 2019, GTx entered into a confidentiality agreement with Company O and Company O was provided access to GTx’s data room to review enobosarm and other SARM data.
On January 9, 2019, Dr. Wills and representatives from Aquilo met with representatives of Oncternal to review the business of Oncternal.
On January 9, 2019, representatives from Aquilo met with representatives of Company M to review the business of Company M.
On January 11, 2019, the GTx Board held a special meeting, with GTx senior management attending, for the purpose of receiving an update from GTx senior management on its ongoing strategic discussions. Representatives from each of Aquilo and Cooley attended the meeting and participated in the discussions. Dr. Wills reported that he met with the senior executive representing Company M and learned that he and his team had a real interest in trying to reach an acceptable reverse Merger Agreement with GTx. He made a proposal for Dr. Wills’ consideration and indicated a willingness to consider improving the proposal if his team’s diligence did not signal any concerns on their part. Dr. Wills arranged for Mr. Hyde, himself and Mr. Hanover to meet with Company M’s senior executive the following day. Dr. Wills continued to have concerns about whether Company M’s lack of funds was one of its primary drivers of its discussion with GTx, and the dissimilarities in each company’s respective technologies made Company M less appealing from a synergistic standpoint. Lastly, Dr. Wills stated that Oncternal’s management team continued to be committed to reaching an agreement and they were currently negotiating a draft letter of intent to bring to the GTx Board for its review and approval.
During January, GTx determined to not pursue business combinations with Company E, Company F, or Company I given that such parties did not continue contact with GTx after initial discussions.
On January 15, 2019, GTx received an initial draft of the proposednon-binding letter of intent from Oncternal, which included a unilateral exclusivity agreement of GTx.
On January 17, 2019, Cooley sent Latham & Watkins LLP (“Latham”), outside legal counsel to Oncternal, a revised draft of the letter of intent, which among other things, included a mutual exclusivity agreement binding the parties rather than only a unilateral exclusivity agreement proposed by Oncternal.
On January 18, 2019, Latham sent Cooley a revised draft of the letter of intent.
On January 22, 2019, GTx and Company O discussed additional information regarding Company O’s proposal.
On January 24, 2019, Latham sent Cooley and GTx a revised draft of the letter of intent from Oncternal.
On January 26, 2019, Cooley sent Latham and Oncternal a revised draft of the letter of intent from GTx.
On January 27, 2019, Latham sent Cooley and GTx a revised draft of the letter of intent.
On January 28, 2019, the GTx Board held a special meeting, with GTx senior management attending, for the purpose of reviewing and considering Oncternal’s proposed letter of intent. Representatives from Aquilo and Cooley attended the meeting. Dr. Wills reviewed the ongoing discussions GTx senior management had had with both Oncternal and Company M and noted that discussions with Oncternal had progressed much more quickly. Although Company M had not made a made a proposal as detailed as what the GTx Board was considering from Oncternal, Dr. Wills believed the proposed equity split being offered by both companies was similar, but Oncternal was the only company then discussing the additional contingent value right for GTx shareholders as a potential value enhancement. Dr. Wills told the GTx Board that he believed that Oncternal offered a better fit as a merger counterparty for GTx, given its oncology focus and expertise, and noted that Oncternal had a preferable financial profile than Company M. Aquilo noted to the GTx Board that it had met with representatives of both
132
companies. Dr. Wills summarized the terms of the proposed letter of intent with the GTx Board, including the equity split for Oncternal and GTx stockholders and the CVR being offered to GTx’s stockholders from proceeds derived from the potential development and subsequent sale, licensing or commercialization of SARDs and SARMs by the combined company. He also told the GTx Board that he had received a proposal from Company O to license or acquire enobosarm, but Oncternal has asked that those discussions await conclusion of the proposed merger to allow Oncternal’s senior management time to assess the most appropriate next steps forward for enobosarm.
The GTx Board agreed that the proposal set forth in the letter of intent was an attractive offer for GTx’s stockholders and represented a fair transaction for its stockholders. The Aquilo representative stated that he saw no reason why it would not be able to issue a fairness opinion for the proposed merger. Cooley reviewed with the GTx Board the mutual exclusivity provision in the letter of intent that would prevent either party from considering alternative transactions during the exclusivity period. The GTx Board also considered the request for mutual exclusivity included in the letter of intent and determined that it was acceptable given the process undertaken by the GTx Board in identifying a potential acquirer.
On January 28, 2019, Cooley sent Latham and Oncternal a revised draft of the letter of intent.
On January 28, 2019, Cooley corresponded with Latham regarding the revised draft of the letter of intent.
On January 29, 2019, a letter of intent with Oncternal was executed by both companies following the approval of each of the GTx Board and the Oncternal Board on the preceding day. Since there was an exclusivity provision contained in the letter of intent, there was no further conversation with Company M about its proposal following GTx’s execution of the letter of intent.
On January 31, 2019, Cooley sent Latham an initial draft of the Original Merger Agreement, which among other things, contemplated the execution of voting agreements andlock-up agreements by stockholders of GTx and Oncternal, as contemplated by the letter of intent. Also on January 31, 2019, GTx sent Oncternal a summary of terms for a proposed CVR Agreement (the “CVR Term Sheet”).
On February 1, 2019 Dr. Wills had a subsequent email exchange with a Company K’s executive, which also had expressed an interest in GTx’s SARMs, and told him GTx was in discussions with other parties and could not consider Company K’s less attractive offer for GTx’s SARMs.
On February 6, 2019, Cooley sent Latham an initial draft of the proposedlock-up agreement to be signed by certain stockholders of each of Oncternal and GTx. Also on February 6, 2019, Latham sent Cooley a revised draft of the CVR Term Sheet.
On February 7, 2019, Latham sent Cooley a revised draft of the Original Merger Agreement.
On February 7, Cooley sent Latham an initial draft of the proposed voting agreement to be signed by certain stockholders of each of Oncternal and GTx. Around this same time period, both companies began more in depth diligence of each other’s IP, financial and corporate records and began formulating and sharing diligence information to be exhibited to a definitive Merger Agreement.
On February 8, 2019, Dr. Wills received another inquiry from Company G asking whether he or others at GTx had any additional comments on Company G’s proposed MTA to further assess GTx’s SARD compounds. Dr. Wills subsequently responded that GTx was still reviewing the proposed MTA and had been focusing on other matters but would be back in touch with Company G soon. It was determined in discussions with Oncternal management that following the execution and announcement of a Original Merger Agreement between Oncternal and GTx, Dr. Wills would be freer to explore with Company G whether an MTA would still be something both Company G and the combined companies of GTx and Oncternal wished to undertake.
133
Later in the evening on February 8, 2019, Cooley sent Latham an initial draft of GTx’s disclosure schedules.
On February 9, 2019, Oncternal sent GTx a revised draft of the CVR Term Sheet following a discussion between the Oncternal and GTx management teams.
On February 10, 2019, Company K contacted Dr. Wills again inquiring whether he was interested in responding to the proposal of Company K to acquire enobosarm. Since it was apparent to GTx senior management that the proposal initially received from Company O was likely a superior offer, assuming Oncternal decided that is was preferable to sell or license enobosarm and the rest of the SARM technology to a third party, Dr. Wills responded that GTx was in discussions with other interested parties but it would continue to evaluate Company K’s proposal and respond accordingly.
On February 13, 2019, Cooley sent Latham a revised draft of the Original Merger Agreement and an initial draft of the Original Form of CVR Agreement.
On February 14, 2019, Latham sent Cooley revised drafts of the voting agreements.
On February 15, 2019, Latham sent Cooley a revised draft of the Original Merger Agreement and GTx’s disclosure schedules.
On February 15, 2019, Company O inquired of Dr. Wills whether he and GTx senior management would be responding soon to its proposal to acquire enobosarm. Since GTx was subject to the exclusivity provisions of the letter of intent with Oncternal, and the proposed merger was not yet public, Dr. Wills was only able to respond that he and GTx senior management was continuing to evaluate the proposal and would be responding soon.
On February 17, 2019, Lathan sent Cooley an initial draft of Oncternal’s disclosure schedules.
On February 18, 2019, GTx received a proposal from the University of Tennessee to continue the contract work by University of Tennessee scientists on SARDs after its contract expires on March 31, 2019. This proposal was transmitted to Oncternal for its evaluation and input with a recommendation that the current contract be extended in accordance with University of Tennessee’s new contract proposal.
On February 18, 2019, Latham sent Cooley a revised draft of thelock-up agreement.
On February 20, 2019, Latham sent Cooley a revised draft of the Original Form of CVR Agreement.
On February 20, 2019, Latham and Cooley had a discussion regarding Oncternal’s disclosure schedules.
On February 20, 2019, representatives from each of Oncternal, GTx, Latham, and Cooley had a discussion regarding the approvals required from SPH USA in connection with the transaction and the potential impact on the anticipated announcement of the transaction.
On February 21, 2019, Dr. Wills responded to the proposal from Company O regarding a potential transaction between the parties for the acquisition of enobosarm. GTx and Oncternal agreed that if a transaction between Company O and GTx was agreed upon in writing before the proposed merger between GTx and Oncternal closed, then any upfront cash paid by Company O to GTx for the acquisition of enobosarm would be reflected as additional cash on GTx’s balance sheet for purposes of determining whether it meets its cash target at closing of the merger, even if the transaction with Company O was closed after the closing of the merger. It was further agreed between GTx and Oncternal that any milestone payments to be paid pursuant to such agreement between GTx and Company O would be split between the combined company and GTx’s stockholders in accordance with the Original Form of CVR Agreement to be executed between Oncternal and GTx at closing.
On February 22, 2019, Cooley sent Latham a revised draft of GTx’s disclosure schedules.
134
On February 26, 2019, Cooley sent Latham a revised draft of the Original Merger Agreement.
On February 27, 2019, Cooley sent Latham a revised draft of the Original Form of CVR Agreement.
On February 28, 2019, Latham sent Cooley and GTx a revised draft of the Original Merger Agreement.
Late in the evening on February 28, 2019, Cooley and Latham had a discussion regarding the transaction documentation and progress towards signing and announcing the transaction. Latham also sent Cooley a revised draft of GTx’s disclosure schedules later that day.
On March 1, 2019, Latham sent Cooley a further revised version of the Original Merger Agreement, a revised draft of the Original Form of CVR Agreement, and a revised version of Oncternal’s disclosure schedules. Over the following several days, Cooley and Latham held a number of meetings to finalize these drafts.
On March 2, 2019, Oncternal and GTx had a discussion regarding certain terms of the Original Form of CVR Agreement. Later in the day on March 2, 2019, Cooley sent Latham and Oncternal a revised draft of the Original Form of CVR Agreement.
Between March 2, 2019 and March 4, 2019, Oncternal and GTx had discussions regarding an increased termination fee that would be payable if either party is unable to deliver its required stockholder vote given that SPH USA was unable to deliver a voting agreement concurrent with signing the Original Merger Agreement.
On March 3, 2019, Oncternal and GTx had a discussion regarding certain terms of the Original Form of CVR Agreement. Later in the day on March 3, 2019, Latham sent Cooley and GTx a revised draft of the Original Form of CVR Agreement.
On March 4, 2019, Cooley had a discussion with Latham, Oncternal, and GTx regarding certain terms of the Original Form of CVR Agreement.
On March 4, 2019, Mr. Hyde had discussions with Oncternal to express his concerns about whether GTx should continue pursuing a transaction for which SPH USA may not deliver a consent that was necessary to complete the transaction.
Later in the evening on March 4, 2019, Cooley sent Latham and Oncternal a revised draft of the Original Form of CVR Agreement. Cooley also sent Latham and Oncternal a revised draft of the Original Merger Agreement, which among other things, included a termination fee of $2.0 million payable by either GTx or Oncternal under certain circumstances, including in the event that such party does not obtain its required stockholder vote within the time period specified in the Original Merger Agreement.
On March 5, 2019, Latham sent Cooley a revised version of GTx’s disclosure schedules and Latham and Cooley exchanged multiple drafts of the Original Form of CVR Agreement and Original Merger Agreement. Later that evening, Latham and Cooley had a discussion regarding certain issues in the Original Merger Agreement.
On March 6, 2019, GTx was informed by Oncternal senior management that the Oncternal Board had unanimously approved entering into the Original Merger Agreement with GTx, including the form of the Original Form of CVR agreement attached thereto to be executed between the parties at closing.
On March 6, 2019, the GTx Board held a special meeting, with GTx senior management and representatives of each of Aquilo and Cooley attending. GTx senior management updated the GTx Board on the status of the transaction and the planned timing of the announcement of the transaction and other related communications. Aquilo then reviewed with the GTx Board its financial analysis of the transaction and rendered an oral opinion, subsequently confirmed in writing by delivery of a written opinion, dated as of March 6, 2019, to the effect that as of the date of such opinion and based upon and subject to the various assumptions made, procedures followed, matters considered and qualifications and limitations on the scope of review undertaken by Aquilo as set forth in
135
the written opinion, the exchange ratio and CVR pursuant to the Original Merger Agreement was fair, from a financial point of view, to the holders of GTx common stock, Cooley reviewed in detail the material terms of the substantially final draft of the Original Merger Agreement, which had been provided to the GTx Board prior to the meeting, including the treatment of equity awards, conditions to closing, the reciprocalnon-solicitation clauses subject to certain fiduciary exceptions, circumstances under which the GTx Board and Oncternal Board could change their respective recommendations, the definition of superior proposal, termination rights, the amount of termination fees and the conditions under which the termination fees become payable, the stockholder approval requirements for GTx and Oncternal and the related shares subject to voting agreements andlock-up agreements. Cooley also reviewed the certain material terms of the substantially final drafts of the Original Form of CVR Agreement, the voting agreements, and thelock-up agreements, each of which had been provided to the GTx Board prior to the meeting. After discussions, the GTx Board unanimously (i) determined that the Original Merger Agreement and the transactions contemplated thereby including the merger, are advisable and in the best interests of GTx and its stockholders, (ii) approved the Original Merger Agreement and the merger, the execution of the Merger Agreement and the consummation of the transactions contemplated thereby, (iii) declared advisable and recommended that GTx’s stockholders adopt the Original Merger Agreement and (iv) authorized and approved certain other matters in connection with the execution and performance of the Original Merger Agreement, including certain regulatory filings.
Later in the day on March 6, 2019, the parties finalized, executed and delivered the Original Merger Agreement (including the Original Form of CVR Agreement), the voting agreements, and thelock-up agreements.
The following morning, on March 7, 2019, Oncternal and GTx issued a joint press release announcing the execution of the Original Merger Agreement. An investor conference call was held later that morning to explain the transaction and provide an overview of the oncology products the combined company would be developing and the expected timing of certain ongoing development efforts.
On April 1, 2019, a report dated March 29, 2019 (the “SARD Report”), was sent to GTx senior management, including Dr. Wills, regarding the findings of the independent laboratory of the academic researcher engaged by GTx in January 2019 to assist GTx with a better understanding of the SARDs’ mechanism of action. The SARD Report summarized findings from the independent laboratory that, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if this translates to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included, (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2)in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to evaluate the conflictingin vitro andin vivo findings.
On April 3, 2019, Mr. Hanover and Dr. Wills held a discussion with the academic researcher to seek his input regarding the SARD Report. The academic researcher reviewed the findings in the SARD Report and expressed his concern that additional pre-clinical research was needed to understand these new findings given the data previously received by GTx.
On April 8, 2019, Dr. Wills and Dr. Breitmeyer discussed developments with the SARD program, including the SARD Report. After their discussion, Dr. Wills instructed that the SARD Report be received by Dr. Breitmeyer.
On April 9, 2019, representatives from Oncternal requested a summary of the conflicting information regarding the SARD compounds, and a meeting to discuss the SARD Report. Representatives of GTx asked Dr. Ramesh
136
Narayanan, Associate Professor, Department of Medicine, University of Tennessee Health Science Center, who is the Principal Investigator of a Sponsored Research agreement between GTx and the University of Tennessee Research Foundation and one of the principal inventors of the SARD technology, to provide the requested information to Oncternal and its consultant, which he did between April 9 and April 10, 2019.
On April 11, 2019, representatives from Oncternal, Oncternal’s consultant, Dr. Wills, Mr. Hanover and Dr. Narayanan held a telephonic discussion to review the requested SARD data and the SARD Report.
On April 12, 2019, representatives from Latham informed representatives from Cooley that Oncternal had received and was evaluating the SARD Report. Cooley contacted Mr. Doggrell following the call and informed him that based on Oncternal’s review of the SARD Report, Oncternal might want to renegotiate the Original Merger Agreement because the value Oncternal thought it would be receiving from GTx’s preclinical SARD program might be diminished from the value it had anticipated at the time the Original Merger Agreement was signed on March 6, 2019.
On April 14, 2019, Dr. Wills had a conversation with Mr. Hale regarding Oncternal’s concern about the value of the SARD program and whether Oncternal was seeking to renegotiate the Merger Agreement. Mr. Hale indicated Oncternal would discuss the matter further internally and get back with Dr. Wills.
On April 15, 2019, a discussion between representatives from Latham, Cooley, Oncternal and GTx was held. During such discussion, Oncternal expressed concerns about moving forward with the transaction based on the existing terms of the Original Merger Agreement and Original Form of CVR agreement given the recent SARD Report, Oncternal’s evaluation of the recent SARD Report and discussions held with its consultants. Oncternal also expressed concern that it might not be able to recommend the transaction to the Oncternal Board and stockholders based on the existing terms of the Original Merger Agreement and Original Form of CVR agreement. Oncternal did not propose revised terms of the transaction during the discussion, but did reiterate that the valuation it had attributed to SARDs had been diminished. GTx acknowledged that the findings in the SARD Report were in conflict with some of the preclinical data it had previously received and shared with Oncternal. Mr. Doggrell communicated this information to other members of senior management and to Mr. Hyde.
On April 16, 2019, a special meeting of the GTx Board was held, with GTx senior management and representatives from Cooley and Aquilo in attendance. The purpose of the meeting was to share with the GTx Board the details of the SARD Report and the concern that Oncternal had expressed with moving forward with the transaction without an adjustment in the transaction terms to reflect the diminished value of the SARD assets. Mr. Doggrell explained that if the parties were unable to reach an agreement on revised terms, the merger may not be approved by Oncternal’s stockholders, and the transaction would not be able to close. The GTx Board discussed that it was desirable to GTx’s stockholders to reach an agreement to proceed with closing the transaction. The GTx Board stated a willingness to discuss a reasonable solution to Oncternal’s concerns and directed GTx senior management to undertake discussions with Oncternal. The GTx Board further instructed GTx senior management that it would be willing to move forward with the transaction if the revised terms would encompass a minimal reduction in GTx’s stockholders’ post-closing ownership of the combined equity through revisions to the exchange ratio.
On April 16, 2019, following the GTx Board meeting, discussions were undertaken between Dr. Wills and Mr. Hale regarding adjustments that may be required to accommodate Oncternal’s concern about the potential loss of value attributable to the SARD assets.
Between April 16, 2019 and April 22, 2019, representatives from Oncternal and GTx held multiple discussions regarding proposed revised terms of the Original Merger Agreement and Original Form of CVR agreement.
On April 22, 2019, the parties agreed to revise the terms of the Original Merger Agreement such that the exchange ratio would reflect that the GTx allocation would be 22.5% instead of 25% and the Oncternal allocation
137
would be 77.5% instead of 75%, in each case, subject to a downward adjustment for the gross cash and cash equivalent balances of each company as of the Closing. The parties also agreed to revise the associated calculations of the cash balance of GTx to be based on the gross cash and cash equivalents balance and to exclude deductions for its current liabilities, transaction expenses and severance costs that are incurred in connection with the transaction. Additionally, the parties agreed that the Original Form of CVR agreement would be amended to provide that GTx’s share of net proceeds for any SARD and/or SARM transaction would be increased from 50% to 75%.
On April 23, 2019, Mr. Doggrell provided an update to the GTx Board with the proposed revised terms of the Original Merger Agreement and Original Form of CVR agreement and scheduled a special meeting of the GTx Board to consider and discuss such terms.
On April 23, 2019, Oncternal sent GTx an initial draft of the Amended Form of CVR Agreement and GTx sent Oncternal comments to such agreement later that day.
Subsequently on April 23, 2019, Latham sent Cooley a draft of the Merger Agreement Amendment.
On April 24, 2019, Oncternal sent GTx a revised draft of the Amended Form of CVR Agreement. Also on April 24, 2019, Latham and Cooley discussed revisions necessary to the Merger Agreement Amendment to reflect the agreement reached between the parties. Later on April 24, 2019, GTx sent to Oncternal a revised draft of the Amended Form of CVR Agreement.
On April 25, 2019, Oncternal sent GTx a revised draft of the Amended Form of CVR Agreement.
On April 26, 2019, Cooley sent Latham a revised draft of the Merger Agreement Amendment.
On April 28, 2019, Latham sent Cooley a revised draft of the Merger Agreement Amendment and a revised draft of the Amended Form of CVR Agreement.
On April 29, 2019, the GTx Board held a special meeting, with GTx senior management and representatives of each of Aquilo and Cooley attending. GTx senior management updated the GTx Board on the status of the transaction timeline. Aquilo then reviewed with the GTx Board its financial analysis of the transaction given the revised terms of the Original Merger Agreement and CVR agreement, and rendered an oral opinion, subsequently confirmed in writing by delivery of a written opinion, dated as of April 29, 2019, to the effect that as of the date of such opinion and based upon and subject to the various assumptions made, procedures followed, matters considered and qualifications and limitations on the scope of review undertaken by Aquilo as set forth in the written opinion, the exchange ratio and CVR pursuant to the Merger Agreement was fair, from a financial point of view, to the holders of GTx common stock, as more fully described in the section entitled “The Merger—Opinion of the GTx Financial Advisor.” Cooley reviewed in detail the material terms of the final draft of the Merger Agreement Amendment and the amended form of the CVR Agreement, which had been provided to the GTx Board prior to the meeting. After discussions, the GTx Board unanimously (i) determined that Merger and all related transactions set forth in and contemplated by the Merger Agreement continue to be fair to, advisable and in the best interests of GTx and its stockholders, (ii) approved and declared advisable the Merger Agreement and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the Merger Agreement, that the stockholders of GTx vote to approve the Merger and adopt the Merger Agreement.
On April 30, 2019, the parties executed the Merger Agreement Amendment, and GTx subsequently announced the Merger Agreement Amendment through a Form 8-K filing that same day.
Historical Background for Oncternal
The Oncternal Board and management regularly review its operating and strategic plans in an effort to enhance stockholder value. These reviews involve, among other things, discussions regarding alternatives for raising the additional financing required to advance Oncternal’s product development programs, including consideration of strategic alternatives that would allow the company greater access to capital markets.
138
During a meeting on November 25, 2018, David F. Hale, an Oncternal Board member, and GTx Board member Dr. Michael G. Carter discussed GTx’s September 21, 2018, public announcement that GTx’s lead product candidate had failed to achieve statistical significance on the primary endpoint of a Phase 2 clinical trial. Dr. Carter indicated that, as a result, the GTx Board was considering strategic options for the company. Mr. Hale and Dr. Carter agreed to discuss with other members of their respective boards of directors the possibility of a merger between GTx and Oncternal.
On November 26, 2018, Oncternal sent GTx anon-confidential presentation detailing Oncternal’s business and product development programs and the parties discussed Oncternal’s business.
On November 30, 2018, Oncternal and GTx executed a bilateral confidentiality agreement.
On December 7, 2018, Oncternal CEO Dr. James B. Breitmeyer had discussions with GTx management regarding GTx’s SARD technology.
A due diligence meeting was held in San Diego, California, on December 18, 2018, between Dr. Robert J. Wills, a GTx Board member, Mr. Marc S. Hanover, CEO of GTx, Mr. Hale and Dr. Breitmeyer.
Following internal discussions with members of the Oncternal Board and legal advisors, on December 26 and 27, Mr. Hale and Dr. Wills discussed potential terms and conditions for a reverse merger between GTx and Oncternal, including the potential for entering into a CVR Agreement, with respect to GTx’s SARD and SARM technology.
On December 31, 2018, Dr. Wills indicated to Mr. Hale that the GTx Board held a meeting to discuss the potential merger between GTx and Oncternal, and expressed the GTx board’s interest in moving forward with diligence on a potential transaction. From this date until the execution of the definitive Merger Agreement on March 6, 2019, each of Oncternal and GTx and their respective advisors performed extensive due diligence on the other company and on the potential merger transaction.
On January 8, 2019, Mr. Hale and Dr. Breitmeyer met with members of the GTx Board and GTx management team to review Oncternal’s business.
On January 9, 2019, Mr. Hale, Dr. Breitmeyer and Mr. Vincent met with representatives of Aquilo Partners, LP, GTx’s investment bank, as well as a member of the GTx Board to review Oncternal’s business.
On January 15, 2019, Dr. Breitmeyer submitted to representatives of GTx a letter of intent for a potential reverse merger transaction between Oncternal and GTx. From this date forward, Oncternal and GTx negotiated the terms and conditions of the merger, including exchanging numerous calls, messages and drafts of the Merger Agreement, CVR Agreement, and related documents.
On January 29, 2019, following several formal and informal discussions between representatives of Oncternal and GTx, with the support of the Oncternal Board, Oncternal and GTx executed a letter of intent containing certain limited exclusivity provisions to allow the parties to conduct further due diligence and negotiate a definitive agreement related to the merger.
On March 5, 2019, Oncternal’s management team and its legal counsel reviewed with members of Oncternal’s Board the terms and conditions of the merger and discussed the Board’s fiduciary duties in the context of the consideration and approval of the merger. Following such discussion, the Oncternal Board approved resolutions (i) determining that the merger was in the best interests of Oncternal and its stockholders and that the terms of the merger were fair, (ii) authorizing the entry by Oncternal into the Merger Agreement and CVR Agreement and related merger documents, and (iii) approving certain other related matters.
139
On March 6, 2019, Dr. Breitmeyer and the chief executive officer of GTx executed the Merger Agreement, and on March 7, 2019, Oncternal and GTx issued a joint press release and held a conference call announcing the execution of the Merger Agreement.
On April 8, 2019, Dr. Wills and Dr. Breitmeyer discussed developments with the SARD program. After their discussion, the SARD Report was sent to Oncternal.
On April 9, 2019, Dr. Breitmeyer requested a summary of the conflicting information regarding the SARD compounds, and a meeting to discuss the SARD Report. Oncternal received a summary of information from Dr. Narayanan on April 10, 2019.
On April 11, 2019, Dr. Breitmeyer, Mr. Hale, Mr. Vincent, Oncternal’s consultant, and representatives of GTx held a telephonic discussion to review the requested SARD data and the SARD Report.
Between April 12, 2019 and April 19, 2019, representatives from Oncternal and its legal counsel had multiple discussions with representatives from GTx and its legal counsel regarding the SARD Report and potentially renegotiating the terms of the Merger Agreement and CVR Agreement in light of the SARD Report.
On April 13, 2019 Dr. Breitmeyer and Oncternal’s consultant held a discussion with one of the academic researchers involved with the SARD Report to review and discuss the SARD Report.
On April 19, 2019, a meeting of the Oncternal Board was held, during which Oncternal’s management team and its legal counsel reviewed with members of Oncternal’s Board the SARD Report and Oncternal’s management team’s understanding that the valuation it had attributed to SARDs had been diminished by the data in the SARD Report. After further discussion the Oncternal Board instructed Oncternal’s management team to renegotiate the terms of the Merger Agreement and CVR Agreement in light of the SARD Report.
Between April 19, 2019 and April 29, 2019, Oncternal and GTx Board members and management negotiated the terms of an amendment to the Merger Agreement (the “Merger Agreement Amendment”) in which the GTx allocation of the combined company would be 22.5% instead of 25% and the Oncternal allocation would be 77.5% instead of 75%, in each case, subject to a downward adjustment for the cash balances of each company as of the Closing. Additionally, the Merger Agreement Amendment provided that GTx’s share of net proceeds for any SARD and/or SARM transaction would be increased from 50% to 75%.
On April 30, 2019, Oncternal’s management team and its legal counsel reviewed with members of Oncternal’s Board the terms and conditions of the Merger Agreement Amendment. Following such discussion, the Oncternal Board approved resolutions (i) determining that the Merger Agreement Amendment was in the best interests of Oncternal and its stockholders and that the terms of the Merger Agreement Amendment were fair, (ii) authorizing the entry by Oncternal into the Merger Agreement Amendment, and (iii) approving certain other related matters.
On April 30, 2019, the parties executed the Merger Agreement Amendment.
GTx Reasons for the Merger
At a special meeting held on March 6, 2019, among other things, the GTx Board unanimously (i) determined that the Original Merger Agreement and the transactions contemplated thereby, including the merger are fair to, advisable and in the best interests of GTx and its stockholders, (ii) approved and declared advisable the Original Merger Agreement and the merger, including the issuance of shares of GTx common stock to the stockholders of Oncternal pursuant to the terms of the Original Merger Agreement, and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the Original Merger Agreement, that the stockholders of GTx vote to approve the amendment of GTx’s certificate of incorporation to effect the GTx Reverse Stock Split, the Original Merger Agreement, the change of control of GTx resulting from the merger pursuant to the Nasdaq Rules, and the 2019 Equity Incentive Plan.
140
In the course of its evaluation of the Original Merger Agreement and merger with Oncternal, the GTx Board held numerous meetings, consulted with GTx senior management, GTx’s outside legal counsel and GTx’s financial advisor, and reviewed and assessed a significant amount of information, and considered a number of factors, including the following:
| • | the GTx Board’s belief that GTx’s business, operational and financial prospects, including its cash position, the substantially diminished price of its common stock following the results from the ASTRID trial, the early developmental stage of its SARD technology, and the limited time frame and expertise available to GTx to potentially enhance the value of its SARD program by conducting and completing the preclinical studies needed to potentially file an IND to initiate clinical trials for a SARD compound, a go it alone scenario, was possible but not without significant risk; |
| • | the GTx Board’s belief, given the risks associated with deriving value from an early-stage preclinical technology and based in part on the judgement, advice and analysis of GTx senior management with respect to the potential strategic, financial and operational benefits of the merger (which judgement was informed in part by the business, technical, financial and legal due diligence investigation performed by GTx with respect to Oncternal), that Oncternal’s proprietary oncology-based technology platform, as well as its product pipeline, including clinical stage candidates, along with the demonstrated expertise of its management and other personnel in areas central to the development of GTx’s SARDs, would create more value for GTx’s stockholders in the long term than GTx may potentially create as an independent stand-alone company; |
| • | the GTx Board’s review of the current development plans of Oncternal to confirm the likelihood that the combined company would possess sufficient resources, or have access to sufficient resources, to allow Oncternal senior management to focus on its plans for the continued development of Oncternal’s product pipeline, as well as the continued development of SARDs, including concluding those preclinical studies needed to identify a lead SARD compound for which an IND can be filed to initiate clinical studies; |
| • | the GTx Board’s consideration that while both GTx and Oncternal should have at the closing of the merger sufficient cash for the combined company to sustain its operations into calendar year 2020, the benefit of combining GTx’s public company structure with Oncternal’s business will continue to provide the combined company with access to the public market to raise additional funds in the future; |
| • | the GTx Board’s consideration of the valuation and business prospects of all the potential strategic transaction candidates, and its collective view that Oncternal was the most attractive candidate for GTx because of the synergies afforded from allying GTx’s SARD program, as a potential treatment for men with castration resistant prostate cancer, with Oncternal’s oncology programs to create a broader based oncology focused public company, the demonstrated expertise Oncternal can bring to the development of SARDs, and the recognition that, unlike many of the other potential strategic prospects then under consideration by GTx, Oncternal’s ability to bring to the combined company its own financial resources to create a more robust company that could await potential value increasing events before having to access the public markets for additional financial resources; |
| • | the GTx Board’s conclusion that the merger provides existing GTx stockholders a significant opportunity to participate in the potential growth of the combined company following the merger, while potentially sharing in 50% of any net proceeds derived from the sale or licensing of GTx’s SARD or SARM technologies or in royalties derived from the commercialization of SARD products, in both cases on account of the CVR Agreement to be executed between GTx and Oncternal at the closing of the merger; |
| • | the GTx Board’s consideration that the combined company will be led by an experienced senior management team from Oncternal and a board of directors with representation from each of the current boards of directors of GTx and Oncternal; and |
| • | the GTx Board’s consideration of the financial analysis of Aquilo and the opinion of Aquilo delivered to the GTx Board on March 6, 2019, to the effect that, as of the date of such opinion, and based upon |
141
and subject to the various assumptions made, procedures followed, matters considered and limitations and qualifications on the scope of the review undertaken by Aquilo, as set forth in its written opinion, the merger consideration to be paid by GTx to Oncternal stockholders in the merger agreement was fair to GTx, from a financial point of view. |
The GTx Board also considered the recent results of operations and financial conditions of GTx, including:
| • | the perceived value of GTx reflected in the diminished price of its common stock following the failure of the ASTRID trial to demonstrate the effectiveness of enobosarm as a potential treatment for SUI, and the limited value given by the marketplace to SARDs as an early-stage preclinical asset; |
| • | the development risks associated with using GTx’s remaining cash to fund operations for at least through calendar year 2019 as GTx attempts to complete its ongoing preclinical studies and undertake those additional preclinical studies needed to move a SARD compound to the IND stage to initiate clinical studies; |
| • | the risk that even if GTx were to be able to file an IND to initiate Phase 1 clinical trials for a SARD compound, the value of the asset would not then be sufficiently demonstrated to either (i) attract a potential acquirer willing to pay a reasonable price for the technology or GTx or (ii) raise additional funds in the public markets to fund the continued development of SARDs at a valuation that would not lead to further substantial dilution for existing stockholders; |
| • | the loss of certain operational capabilities of GTx, and risks associated with continuing to operate GTx on a stand-alone basis, including limiting the number of employees to only those personnel essential to running a public company and overseeing SARD preclinical development and relying on outside consultants and third-party contractors for the necessary preclinical SARD development work; |
| • | the results of substantial efforts made over a four-month period following GTx’s announcement of its disappointing results from its enobosarm ASTRID trial to solicit strategic alternatives for GTx to the merger, including the discussions that GTx senior management and Aquilo had during this period with other strategic transaction candidates; |
| • | the current financial market conditions and historical market prices, volatility and trading information with respect to GTx common stock; |
| • | the risks, costs and timing and limited amount, if any, that would be distributed to GTx stockholders associated with a potential liquidation of GTx if it appeared to the GTx Board, from GTx’s ongoing preclinical development of SARDs, that SARDs may not be sufficiently developed to identify a likely SARD candidate for an IND filing by the end of calendar year 2019; and |
| • | the fact that the GTx Board determined that at the end of 2019 there may be only approximately $7 million remaining for continued operations and if GTx was unable to acquire additional proceeds from a sale of equity or through a collaboration or licensing of SARDs, there would be limited funds available for distribution to stockholders if the GTx Board decided to dissolve GTx. |
The GTx Board also reviewed the terms of the Original Merger Agreement, the Original Form of CVR agreement and associated transactions, including:
| • | the fact that the exchange ratio, which is expected to give GTx stockholders approximately 25% of the combined company’s outstanding stock, immediately following the merger, is financially attractive in light of GTx’s standalone value, GTx’s recent stock price, GTx’s strategic alternatives, and the potential value of Oncternal following the merger; |
| • | the number and nature of the conditions to Oncternal’s obligations to consummate the merger, including the requirement that a significant minority shareholder of Oncternal will have to obtain the approval of the merger from its parent corporate owner, the failure of which will preclude Oncternal from completing the merger; |
142
| • | the rights of, and limitation on, GTx under the Original Merger Agreement to consider certain unsolicited acquisition proposals under the certain circumstances, should GTx receive a “superior offer”; |
| • | the GTx Board’s belief that the terms of the Original Merger Agreement, including the parties’ representations, warranties and covenants, deal protection provisions and the conditions are reasonable for a transaction of this nature; and |
| • | the GTx Board’s belief that the CVR Agreement providing up to 50% of net proceeds to GTx stockholders of record as of the closing of the merger, whether or not they continue to hold GTx shares subsequent to the merger, is reasonable and fair under the circumstances. |
The GTx Board also considered a variety of risks and other countervailing factors related to the merger, including:
| • | the fact that the exchange ratio may be adjusted downward if GTx’s cash at the closing does not meet the applicable cash target set forth in the Original Merger Agreement; |
| • | the up to $2 million termination fee payable by GTx to Oncternal upon the occurrence of certain events and the potential effect of such termination fee in deterring other potential acquirers from proposing an alternative transaction that may be more advantageous to GTx stockholders; |
| • | the up to $2 million termination fee payable by Oncternal to GTx upon the occurrence of certain events, including the failure of Oncternal to obtain the approval of the merger from Oncternal’s largest stockholder, SPH USA, and the likelihood the receipt of the termination fee from Oncternal will only offset a portion of expenses incurred by GTx in connection with the merger; |
| • | the substantial expenses to be incurred by GTx in connection with the merger; |
| • | the possible volatility of the trading price of the GTx common stock resulting from the announcement of the merger; |
| • | the risks that the merger might not be consummated in a timely manner or at all and the potential effect of the public announcement of the merger or failure to complete the merger on the reputation of GTx; |
| • | the risks to GTx’s business, operations and financial results in the event that the merger is not consummated; |
| • | the strategic direction of the combined company following the closing of the merger, which will be determined by a combination of individuals from Oncternal senior management and the Oncternal Board composed in the majority of members of Oncternal’s existing board of directors, including their ability to determine whether there have been sufficient efforts undertaken by the combined company to develop SARDs or sell or license SARMs before deciding to discontinue such efforts; and |
| • | various other risks associated with the combined company and the merger, including those described in the sections titled “Risk Factors” beginning on page 26 and “Forward-Looking Statements” beginning on page 114. |
In addition, the GTx Board considered the interests that certain of its directors and executive officers may have with respect to the merger that are different from or in addition to their interests as stockholders of GTx, generally and specifically with respect to the fact that Mr. Hyde, a director of GTx, independently and through Pittco Associates III, L.P. and Pittco Investments, L.P., and each of its related entities, is a substantial securityholder of GTx, as more fully described under “The Merger—Interests of GTx Directors and Executive Officers in the Merger.” The GTx Board concluded that the risks, uncertainties, restrictions and potentially negative factors associated with the merger were outweighed by the potential benefits of the merger.
At a special meeting held on April 29, 2019, the GTx Board unanimously (i) determined that the Merger Agreement and the transactions contemplated thereby, including the merger are fair to, advisable and
143
in the best interests of GTx and its stockholders, (ii) approved and declared advisable the Merger Agreement, as amended and reaffirmed that the merger, including the issuance of shares of GTx common stock to the stockholders of Oncternal pursuant to the terms of the Merger Agreement, and (iii) determined to recommend, upon the terms and subject to the conditions set forth in the Merger Agreement, as amended, that the stockholders of GTx vote to approve the Merger Agreement, as amended.
In the course of its evaluation of the Merger Agreement, and merger with Oncternal, the GTx Board held multiple meetings, consulted with GTx senior management, GTx’s outside legal counsel and GTx’s financial advisor, and reviewed and assessed a significant amount of information, and considered a number of factors, including the following:
| • | the GTx Board’s consideration that the potential receipt of a termination fee from Oncternal for termination of the Merger Agreement would not provide GTx’s stockholders with the benefit of combining GTx’s public company structure with Oncternal’s business and the opportunity to participate in the potential future growth of the combined company; |
| • | the GTx Board’s conclusion that on the amended terms of the Merger Agreement, the merger continued to provide existing GTx stockholders an opportunity to participate in the potential growth of the combined company following the merger, while potentially sharing in 75% of any net proceeds derived from the sale or licensing of GTx’s SARD or SARM technologies or in royalties derived from the commercialization of SARD products, in both cases on account of the CVR Agreement to be executed at the closing of the merger; |
| • | the GTx Board’s consideration that the Merger Agreement is a more attractive alternative than terminating the Merger Agreement given that there are no other interested acquirers and that GTx would likely be required to wind-down operations if the merger with Oncternal is not consummated; and |
| • | the GTx Board’s consideration of the updated financial analysis of Aquilo and the oral opinion of Aquilo delivered to the GTx Board on April 29, 2019, subsequently confirmed in writing, to the effect that, based upon and subject to the various assumptions made, procedures followed, matters considered and limitations and qualifications on the scope of the review undertaken by Aquilo, as set forth in its written opinion, the revised merger consideration to be paid by GTx to Oncternal stockholders in the Merger Agreement was fair to GTx, from a financial point of view, as more fully described in the section entitled “The Merger—Opinion of the GTx Financial Advisor.” |
The GTx Board also reviewed the amended terms of the Merger Agreement, the CVR Agreement and associated transactions, including:
| • | the fact that the exchange ratio, which is expected to give GTx stockholders approximately 22.5% of the combined company’s outstanding stock, immediately following the merger, remained financially attractive in light of GTx’s standalone value, GTx’s recent stock price, GTx’s strategic alternatives, and the potential value of Oncternal following the merger; |
| • | the number and nature of the conditions to Oncternal’s obligations to consummate the merger, including the outstanding requirement that a significant minority shareholder of Oncternal will have to approve, the failure of which will preclude Oncternal from completing the merger; and |
| • | the GTx Board’s belief that the CVR Agreement providing up to 75% of net proceeds to GTx stockholders of record as of the closing of the merger, whether or not they continue to hold GTx shares subsequent to the merger, continued to be reasonable and fair under the circumstances. |
The foregoing information and factors considered by the GTx Board are not intended to be exhaustive but are believed to include all of the material factors considered by the GTx Board. In view of the wide variety of factors considered in connection with its evaluation of the merger and the complexity of these matters, the GTx Board
144
did not find it useful, and did not attempt, to quantify, rank or assign relative weights to these factors. In considering the factors described above, individual members of the GTx Board may have given weight to different factors. The GTx Board conducted an overall analysis of the factors discussed above, including thorough discussions with, and questioning of, GTx senior management and the legal and financial advisors of GTx, and considered the factors overall to be favorable to, and to support, its determination.
Oncternal Reasons for the Merger
In the course of reaching its decision to approve the merger, including the Merger Agreement Amendment the Oncternal Board consulted with Oncternal’s senior management, financial and tax advisors and legal counsel, reviewed a significant amount of information and considered a number of factors, including, among others:
| • | the potential increased access to sources of capital and a broader range of investors to support the clinical development of its product candidates following consummation of the transaction compared to if Oncternal continued to operate as a privately held company; |
| • | the potential to provide its current stockholders with greater liquidity by owning stock in a public company; |
| • | the board’s belief that no alternatives to the merger were reasonably likely to create greater value for Oncternal’s stockholders, after reviewing the various financing and other strategic options to enhance stockholder value that were considered by the Oncternal Board; |
| • | the cash resources of the combined organization, which are expected to be approximately $26.0 million at the closing of the merger; |
| • | the business, history and credibility of GTx and its affiliates, and its financial resources; |
| • | the availability of appraisal rights under the DGCL to holders of Oncternal’s capital stock who comply with the required procedures under the DGCL, which allow such holders to seek appraisal of the fair value of their shares of Oncternal capital stock as determined by the Delaware Court of Chancery; |
| • | the expectation that the merger with GTx would be a more time- and cost-effective means to access capital than other options considered by the Oncternal Board, including additional private financings or an initial public offering; |
| • | the terms and conditions of the Merger Agreement, including, without limitation, the following: |
| • | the determination that the expected relative percentage ownership of GTx’s stockholders and Oncternal’s stockholders in the combined organization was appropriate based, in the judgment of the Oncternal Board, on the board of directors’ assessment of the approximate valuations of GTx (including the potential value of the SARD program and the value of the net cash GTx is expected to provide to the combined organization) and Oncternal (including the value of the net cash Oncternal is expected to provide to the combined organization); |
| • | the expectation that the merger will be treated as a reorganization for U.S. federal income tax purposes; |
| • | the limited number and nature of the conditions of the obligation of GTx to consummate the merger; |
| • | the rights of Oncternal under the Merger Agreement to consider certain unsolicited acquisition proposals under certain circumstances should Oncternal receive a superior proposal; |
| • | the conclusion of the Oncternal Board that the potential termination fee of up to $2 million, payable by GTx or Oncternal to the other party, and the circumstances when such fee may be payable, were reasonable; and |
145
| • | the belief that the other terms of the Merger Agreement, including the parties’ representations, warranties and covenants, and the conditions to their respective obligations, were reasonable in light of the entire transaction; |
| • | the shares of GTx’s common stock issued to Oncternal’s stockholders will be registered on a FormS-4 registration statement and will become freely tradable for Oncternal’s stockholders who are not affiliates of Oncternal and who are not parties tolock-up agreements; |
| • | the voting agreements, pursuant to which certain directors, officers and stockholders of Oncternal and GTx, respectively, have agreed, solely in their capacity as stockholders of Oncternal and GTx, respectively, to vote all of their shares of Oncternal capital stock or GTx common stock in favor of the adoption or approval, respectively, of the Merger Agreement; |
| • | the ability to obtain a Nasdaq listing and the change of the combined organization’s name to Oncternal Therapeutics, Inc. upon the closing of the merger; |
| • | the merger may enable certain stockholders of GTx and Oncternal to increase the value of their current shareholding; and |
| • | the likelihood that the merger will be consummated on a timely basis. |
The Oncternal Board also considered a number of uncertainties and risks in its deliberations concerning the merger and the other transactions contemplated by the Merger Agreement, including the following:
| • | the possibility that the merger might not be completed and the potential adverse effect of the public announcement of the merger on the reputation of Oncternal and the ability of Oncternal to obtain financing in the future in the event the merger is not completed; |
| • | the exchange ratio used to establish the number of shares of GTx’s common stock to be issued to Oncternal’s stockholders in the merger is fixed, except for adjustments due to the parties’ cash balances at closing, and thus the relative percentage ownership of GTx’s stockholders and Oncternal’s stockholders in the combined organization immediately following the completion of the merger is similarly fixed; |
| • | the termination fee of up to $2.0 million, payable by Oncternal to GTx upon the occurrence of certain events, and the potential effect of such termination fee in deterring other potential acquirers from proposing an alternative transaction that may be more advantageous to Oncternal’s stockholders; |
| • | the risk that the merger might not be consummated in a timely manner or at all; |
| • | the expenses to be incurred in connection with the merger and related administrative challenges associated with combining the companies; |
| • | the additional expenses and obligations to which Oncternal’s business will be subject following the merger that Oncternal has not previously been subject to, and the operational changes to Oncternal’s business, in each case that may result from being a public company; |
| • | the fact that the representations and warranties in the Merger Agreement do not survive the closing of the merger and the potential risk of liabilities that may arise post-closing; and |
| • | various other risks associated with the combined organization and the merger, including the risks described in the section entitled “Risk Factors” in this proxy statement/prospectus/information statement. |
Opinion of the GTx Financial Advisor as of March 6, 2019
The GTx Board requested that Aquilo evaluate the fairness, from a financial point of view, to GTx’s stockholders, of the exchange ratio set forth in the Original Merger Agreement and the right of GTx’s stockholders to receive contingent cash payments pursuant to the Original Form CVR Agreement, together, the
146
“Original Consideration”. On March 6, 2019, Aquilo delivered its initial oral opinion, subsequently confirmed in writing, to the GTx Board to the effect that, as of the date of its initial opinion and based upon and subject to the qualifications, limitations and assumptions set forth therein, the Original Consideration is fair, from a financial point of view, to GTx’s stockholders.
The summary of the initial written opinion of Aquilo in this proxy statement is qualified in its entirety by reference to the full text of the initial written opinion of Aquilo, dated March 6, 2019 (the “March Opinion), attached to this proxy statement asAnnexB-1. Further, in connection with the Merger Agreement Amendment and the revised form of the CVR Agreement, the GTx Board requested and Aquilo delivered an additional opinion. See “Opinion of the GTx Financial Advisory as of April 29, 2019.”
The March Opinion of Aquilo addresses only the fairness, from a financial point of view, to GTx’s stockholders of the Original Consideration and does not address any other aspect or implication of the merger or any other agreement, arrangement or understanding entered into in connection with the merger or otherwise. The March Opinion relies and is based only on the information available as of March 6, 2019. Aquilo was not requested to opine as to, and its March Opinion does not in any manner address, GTx’s underlying business decision to proceed with or effect the merger, or any other aspect of GTx’s business or any of its other assets.
In arriving at its March Opinion, Aquilo reviewed and analyzed certain information available as of March 6, 2019, among other things:
| • | the Original Merger Agreement and the Original Form CVR Agreement; |
| • | certain publicly available business and financial information relating to GTx and Oncternal; |
| • | publicly available financial terms of certain sale transactions involving companies Aquilo deemed relevant and the consideration paid for such companies and comparisons of these terms with the proposed financial terms of the Original Merger Agreement and Original Form CVR Agreement; |
| • | publicly available financial and business information concerning certain other companies Aquilo deemed relevant and comparisons of this financial and business information to that of GTx and Oncternal; |
| • | certainnon-public information relating to GTx that was prepared and provided to Aquilo by GTx, including certain operating and financial information relating to GTx’s business, including GTx’s unaudited financial statements for the year ended December 31, 2018 and financial and business forecasts and projections prepared by management of GTx relating to GTx’s prospects; |
| • | certainnon-public information relating to Oncternal that was prepared and provided to Aquilo by Oncternal, including certain operating and financial information relating to Oncternal’s business, including Oncternal’s unaudited financial statements for the year ended December 31, 2018 and financial and business forecasts and projections prepared by management of Oncternal relating to Oncternal’s prospects; and |
| • | such other information that Aquilo considered appropriate to opine as to the fairness of the Original Consideration. |
In addition, Aquilo discussed with management of GTx and management of Oncternal, the business, operations, financial condition and prospects of each of GTx and Oncternal, as of March 6, 2019, respectively, and as a combined company.
In connection with its review, Aquilo did not assume any responsibility for independent verification of any of the foregoing information and, with GTx’s consent, relied on such information being complete and accurate. With respect to the financial forecasts for GTx, the management of GTx advised Aquilo, and Aquilo assumed with GTx’s consent, that such forecasts were reasonably prepared on bases reflecting the best currently available estimates and judgments of GTx’s management as to the future financial performance of GTx. With respect to
147
the financial forecasts for Oncternal, the management of Oncternal advised Aquilo, and Aquilo assumed with GTx’s consent, that such forecasts were reasonably prepared on bases reflecting the best currently available estimates and judgments of the management of Oncternal as to the future financial performance of Oncternal.
Aquilo relied upon, without independent verification, the assessment of each of GTx’s management and Oncternal’s management as to the viability of, and risks associated with, the current and future products of the combined company following the merger, including without limitation, the development, testing and marketing of such products, the receipt of all necessary governmental and other regulatory approvals for the development, testing and marketing thereof, and the life and enforceability of all relevant patents and other intellectual and other property rights associated with such products. Aquilo assumed that combined company will not materially breach its obligations under the Original Form CVR Agreement and will use commercially reasonable efforts, as provided in the Original Form CVR Agreement, to develop one or more SARD Compounds in accordance with the development plan and monetize the SARM Technology and SARM Products following the closing of the merger, but expressed no view as to whether the SARD Compounds, SARM Technology or SARM Products will ultimately be developed or monetized. Aquilo also assumed, with GTx’s consent, that, in the course of obtaining any regulatory or third-party consents, approvals or agreements in connection with the merger, no delay, limitation, restriction or condition will be imposed that would have an adverse effect on GTx, Oncternal or the combined company, or the contemplated benefits of the merger, and that the merger will be consummated in accordance with the terms of the Original Merger Agreement without waiver, modification or amendment of any material term, condition or agreement thereof or any waiver, modification or amendment of any material term, condition or agreement of the Original Form CVR Agreement.
In preparing its March Opinion, Aquilo performed a number of financial and comparative analyses based on data available as of March 6, 2019. The order in which the analyses are described below does not represent the relative importance or weight given to the analyses by Aquilo. The preparation of a fairness opinion is a complex process and is not necessarily susceptible to partial analysis or summary description. Aquilo believes that its analyses must be considered as a whole and that selecting portions of its analyses and of the factors considered by it, without considering all analyses and factors, could create a misleading view of the processes underlying its opinion. No company or transaction used in the analyses performed by Aquilo as a comparison is identical to GTx or Oncternal. In addition, Aquilo may have given some analyses more or less weight than other analyses, and may have deemed various assumptions more or less probable than other assumptions, so the range of valuation resulting from any particular analysis described below should not be taken to be Aquilo’s view of the actual Original Consideration. The analyses performed by Aquilo are not necessarily indicative of actual values or actual future results, which may be significantly more or less favorable than suggested by such analyses. In addition, analyses relating to the value of businesses or assets do not purport to be appraisals or to necessarily reflect the prices at which businesses or assets may actually be sold. The analyses performed were prepared solely as part of Aquilo’s analysis of the fairness, from a financial point of view, of the Original Consideration to the holders of GTx common stock set forth in the Original Merger Agreement and Original Form CVR Agreement and do not address any other aspect or implication of the merger, including any other agreement, arrangement or understanding entered into in connection with the merger or otherwise.
At a meeting of the GTx Board held on March 6, 2019, Aquilo presented certain financial analyses in connection with the delivery of its initial oral opinion, subsequently confirmed in writing. The following is a summary of the material financial analyses performed by Aquilo in arriving at its March Opinion. Certain of the following summaries of financial analyses include information presented in tabular format. In order to understand fully the material financial analyses that were performed by Aquilo, the tables should be read together with the text of each summary. The tables alone do not constitute a complete description of the material financial analyses.
Exchange Ratio and Pro Forma Ownership as of the Date of the March Opinion.Based on the initial estimated exchange ratio, GTx’s stockholders as of immediately prior to the Effective Time would own approximately 25% of the outstanding common stock of GTx, and Oncternal’s stockholders as of immediately prior to the Effective Time would own approximately 75% of the outstanding common stock of GTx, which is subject to adjustment
148
for each company’s cash balance at closing in accordance with the Original Merger Agreement. The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants. Based on each of GTx’s and Oncternal’s outstanding capital stock as of March 5, 2019 and assuming no adjustment for cash levels and excluding the issuance of shares related to the exercise of any options, restricted stock awards, warrants or rights to receive such shares, and any shares of stock reserved for issuance, other than shares of GTx common stock reserved for issuance pursuant to the GTx Deferred Stock Rights, Aquilo determined that the initial exchange ratio would be 0.4475.
Value of GTx Shares Issued to Oncternal Stockholders as of the Date of the March Opinion.Aquilo analyzed the value of the shares to be issued to Oncternal’s stockholders based on a 0.4475 exchange ratio and the most recent closing price of GTx’s common stock prior to the delivery of its March Opinion. Aquilo noted the value of the outstanding shares was approximately $72.4 million, that none of GTx’s outstanding options or warrants werein-the-money, and the value of Oncternal’s outstanding options using the treasury stock method was approximately $2.7 million and the value of Oncternal’s outstanding warrants using the Black-Scholes method was approximately $1.9 million, resulting in a value of the shares of GTx’s common stock to be issued to Oncternal’s stockholders to be approximately $76.9 million.
Oncternal Valuation as of the Date of the March Opinion
Comparable Public Company Analysis as of the Date of the March Opinion. Aquilo reviewed, analyzed and compared Oncternal to corresponding publicly available financial information for 12 publicly-traded biotechnology companies that had a lead product candidate in oncology, and in which the lead product candidate’s stage was no earlier than an ongoing Phase 1 clinical trial and no later than an ongoing Phase 2 clinical trial. The following list sets forth the comparable companies selected by Aquilo and their respective enterprise values.
Company | Enterprise Value ($ millions, rounded) | |||
Aduro Biotech, Inc. | 57.2 | |||
Affimed N.V. | 103.7 | |||
Arcus Biosciences, Inc. | 276.5 | |||
Calithera Biosciences, Inc. | 71.3 | |||
Compugen Ltd. | 156.2 | |||
Constellation Pharmaceuticals, Inc. | 108.7 | |||
Forty Seven, Inc. | 389.5 | |||
Marker Therapeutics, Inc. | 274.7 | |||
Merus N.V. | 79.2 | |||
miRagen Therapeutics, Inc. | 19.6 | |||
Replimmune Group, Inc. | 280.0 | |||
ZIOPHARM Oncology, Inc. | 413.6 | |||
Source: SEC filings
Aquilo reviewed the enterprise values of the selected companies, which ranged from $19.6 million to $413.6 million. The result of the analysis implied a mean and median implied enterprise value for these comparable companies of $185.9 million and $132.5 million, respectively. Aquilo compared these ranges to the approximately $76.9 million in value of shares of GTx’s common stock to be issued to Oncternal’s stockholders.
No company used in any analysis as a comparison had a lead product candidate identical to Cirmtuzumab and they all differ in material ways. Accordingly, an analysis of the results described above is not mathematical; rather it involves complex considerations and judgments concerning differences in financial and operating characteristics of the companies and other factors that could affect the public trading value of the selected companies to which they are being compared. This analysis yielded a range of enterprise values, and therefore,
149
such implied enterprise value ranges developed from these analyses were viewed by Aquilo collectively and not individually.
Comparable Initial Public Offering Analysis as of the Date of the March Opinion. Aquilo reviewed, analyzed and compared Oncternal to corresponding publicly available financial information for 12 initial public offerings of biotechnology companies since January 2015 that had a lead product candidate in oncology and in which the lead product candidate’s stage was no earlier than an ongoing Phase 1 clinical trial and no later than an ongoing Phase 2 clinical trial. The following list sets forth the initial public offerings selected by Aquilo, including the date priced and thepre-money enterprise value.
Company | Date Priced | Pre-Money Enterprise Value ($ millions, rounded) | ||||
Arcus Biosciences, Inc. | March 14, 2018 | 360.8 | ||||
Constellation Pharmaceuticals, Inc. | July 18, 2018 | 249.5 | ||||
Corvus Pharmaceuticals, Inc. | March 22, 2016 | 240.0 | ||||
Deciphera Pharmaceuticals, Inc. | September 27, 2017 | 378.6 | ||||
Forty Seven, Inc. | June 27, 2018 | 321.9 | ||||
Jounce Therapeutics, Inc. | January 26, 2017 | 177.1 | ||||
Merus N.V. | May 18, 2016 | 65.6 | ||||
Mirna Therapeutics, Inc. | October 1, 2015 | 39.5 | ||||
Nucana plc | September 27, 2017 | 415.7 | ||||
Replimmune Group, Inc. | July 19, 2018 | 338.4 | ||||
TRACON Pharmaceuticals, Inc. | January 29, 2015 | 52.4 | ||||
Zymeworks Inc. | April 27, 2017 | 234.3 | ||||
Source: Company press releases, SEC filings, Capital IQ
Aquilo reviewed the enterprise values of the selected initial public offerings, which ranged from $39.5 million to $415.7 million. The result of the analysis implied a mean and median impliedpre-money enterprise value for these comparable companies of $239.5 million and $244.8 million, respectively. Aquilo compared these ranges to the approximately $76.9 million in value of shares of GTx common stock to be issued to Oncternal’s stockholders.
Although the initial public offerings were used for comparison purposes, none of these initial public offerings is directly comparable to the merger, and none of the companies in those initial public offerings is directly comparable to Oncternal, and none had a lead product candidate directly comparable to Cirmtuzumab. Accordingly, an analysis of the results of such a comparison is not purely mathematical, but instead involves complex considerations and judgments concerning differences in historical financial and operating characteristics of the companies involved and other factors that could affect the acquisition value of such companies or the company to which they are being compared.
Comparable Transaction Analysis as of the Date of the March Opinion.Aquilo reviewed, analyzed and compared Oncternal to corresponding publicly available financial information for 12 business combinations of biotechnology companies since January 2015 where the acquired company had a lead product candidate in oncology, and in which the lead product candidate’s stage was no earlier than an ongoing Phase 1 clinical trial and no later than an ongoing Phase 2 clinical trial. The following list sets forth the business combinations
150
selected by Aquilo, including the phase of the target’s lead product candidate, the upfront equity consideration, any milestone consideration and total deal value.
Acquirer | Target | Date Announced | Phase of Target’s Lead Product Candidate | Upfront Equity Consideration ($ millions, rounded) | Milestone Consideration ($ millions, rounded) | Total Deal Value ($ millions, rounded) | ||||||
Eli Lilly and Company | AurKa Pharma Inc. | May 14, 2018 | Phase 1 | 110.0 | 465.0 | 575.0 | ||||||
Merck & Co., Inc. | Viralytics Limited | February 21, 2018 | Phase 2 | 394.0 | 0 | 394.0 | ||||||
Seattle Genetics, Inc. | Cascadian Therapeutics, Inc. | January 31, 2018 | Phase 2 | 614.0 | 0 | 614.0 | ||||||
Merck & Co., Inc. | Rigontec GmbH | September 6, 2017 | Phase 1/2 | 131.5 | 399.2 | 530.7 | ||||||
NantCell, Inc. | Altor BioScience Corporation | June 27, 2017 | Phase 2 | 96.7 | 193.3 | 290.0 | ||||||
Debiopharm International S.A. | ImmunoGen, Inc. | May 23, 2017 | Phase 2 | 25.0 | 30.0 | 55.0 | ||||||
Celldex Therapeutics, Inc. | Kolltan Pharmaceuticals, Inc. | November 1, 2016 | Phase 1b | 62.5 | 172.5 | 235.0 | ||||||
Bristol-Myers Squibb Company | Cormorant Pharmaceuticals AB | July 1, 2016 | Phase 1/2 | 95.0 | 425.0 | 520.0 | ||||||
Roche Holding AG | Tensha Therapeutics, Inc. | January 7, 2016 | Phase 1b | 115.0 | 420.0 | 535.0 | ||||||
Agenus Inc. | PhosImmune, Inc. | December 21, 2015 | Phase 1 | 9.9 | 35.0 | 44.9 | ||||||
Novartis International AG | Admune Therapeutics LLC | October 21, 2015 | Phase 1 | 140.0 | 120.0 | 260.0 | ||||||
Merck & Co., Inc. | cCAM Biotherapeutics Ltd | July 27, 2015 | Phase 1 | 95.0 | 510.0 | 605.0 |
Source: Company press releases, SEC filings
Aquilo reviewed the range of upfront equity considerations paid to the targets within the comparable transaction set, which ranged from $9.9 million to $614.0 million. The result of the analysis implied a mean and median upfront equity value for the comparable transactions of $157.4 million and $103.3 million, respectively.
Aquilo also considered an adjusted milestone consideration payable to the target or its stockholders within the comparable transaction set. Aquilo applied an adjustment factor, based on the phase of the target company’s lead product candidate, to the total milestone consideration associated with each transaction, and added the upfront equity value to the adjusted milestone consideration for each transaction. The total milestone consideration payable to targets with a lead product candidate in a Phase 1 clinical trial were adjusted by 5.1% and targets with a lead product candidate in a Phase 2 clinical trial were adjusted by 8.1%. The adjustment factors are the probability of success that oncology assets at these stages would achieve FDA approval, as reported by BIO in their “Clinical Development Success Rates 2006-2015” report.
The total deal values, including the upfront payment plus any adjusted milestone consideration, ranged from $11.7 million to $614.0 million. The result of this analysis implied a mean and median total adjusted deal value for the comparable transactions of $172.2 million and $131.6 million, respectively. Aquilo compared these ranges to the approximately $76.9 million in value of shares of GTx’s common stock to be issued to Oncternal’s stockholders.
Although the transactions were used for comparison purposes, none of these transactions is directly comparable to the merger, and none of the companies in those transactions is directly comparable to Oncternal, and none had a lead product candidate directly comparable to Cirmtuzumab. Accordingly, an analysis of the results of such a
151
comparison is not purely mathematical, but instead involves complex considerations and judgments concerning differences in historical financial and operating characteristics of the companies involved and other factors that could affect the acquisition value of such companies or the company to which they are being compared.
Recent Oncternal Series C Private Financing Valuation as of the Date of the March Opinion.On September 22, 2018, Oncternal issued shares of its Series C preferred stock to Shanghai at an implied fully-diluted post-money valuation of $87.7 million. Aquilo noted that Shanghai also received certain rights to Oncternal products in China and certain selected other territories in connection with its purchase of Oncternal’s Series C preferred stock. Aquilo compared the $87.7 million post-money valuation of Oncternal to the approximately $81.4 million in value of shares of GTx’s common stock to be issued to Oncternal’s stockholders.
GTx Valuation as of the Date of the March Opinion
Comparable Public Company Analysis as of the Date of the March Opinion. Aquilo reviewed, analyzed and compared GTx to corresponding publicly available financial information for 10 publicly-traded biotechnology companies in which the company’s lead product candidate failed in late-stage clinical trials, across all therapeutics areas. The following list sets forth the comparable companies selected by Aquilo and their respective net cash multiples.
Company | Net Cash Multiple | |||
Aevi Genomic Medicine, Inc. | 0.7x | |||
Aquinox Pharmaceuticals, Inc. | 0.7x | |||
Arsanis, Inc. | 1.5x | |||
Edge Therapeutics, Inc. | 0.4x | |||
Gemphire Therapeutics Inc. | 1.4x | |||
Histogenics Corporation | 1.5x | |||
Merrimack Pharmaceuticals, Inc. | 1.1x | |||
Realm Therapeutics Plc | 0.6x | |||
Vical Incorporated | 0.5x | |||
Vital Therapies, Inc. | 0.5x | |||
Source: SEC filings
Aquilo multiplied the median and mean net cash multiples, determined by the quotient of the equity market capitalization divided by the net cash of the selected companies, by GTx’s estimated net cash as of December 31, 2018. The net cash multiples of these comparable public companies ranged from 0.4x to 1.5x, and the mean and median net cash multiples were 0.9x and 0.7x, respectively. The analysis implied a mean and median equity value for GTx of $25.4 million and $20.1 million, respectively.
No company used in any analysis as a comparison is identical to GTx and they all differ in material ways. Accordingly, an analysis of the results described below is not mathematical; rather it involves complex considerations and judgments concerning differences in financial and operating characteristics of the companies and other factors that could affect the public trading value of the selected companies to which they are being compared. This analysis yielded a range of enterprise values, and therefore, such implied enterprise value ranges developed from these analyses were viewed by Aquilo collectively and not individually.
Summary of Valuation Analyses as of the Date of the March Opinion
This summary of the valuation methodologies presented in this March Opinion refer to the Oncternal equity valuation range, which is calculated from the means of the low and high valuations implied by the means and
152
medians (as the case may be) from each of the valuation analyses described above, and set forth below:
| • | Comparable Public Company Analysis as of the Date of the March Opinion; |
| • | Comparable Initial Public Offering Analysis as of the Date of the March Opinion; |
| • | Comparable Biotechnology Transaction Analysis as of the Date of the March Opinion – Upfront Equity; |
| • | Comparable Biotechnology Transaction Analysis as of the Date of the March Opinion – Upfront Equity plus Adjusted Milestone; and |
| • | Recent Oncternal Series C Private Financing Valuation as of the Date of the March Opinion. |
Value of GTx Shares Issued Relative to Oncternal Implied Valuation as of the Date of the March Opinion.Aquilo compared the value of GTx’s common stock issued to Oncternal’s stockholders based on the most recent closing price of GTx common stock prior to delivery of this March Opinion, and as determined by an assumed exchange ratio of 0.4475, and including:
| • | Oncternal’s outstanding shares of capital stock; |
| • | the number of shares of Oncternal’s common stock issuable upon the exercise of options to purchase Oncternal’s common stock, as calculated by the Treasury Method; and |
| • | Value attributable to Oncternal’s warrants to purchase shares of SeriesB-2 preferred stock of Oncternal as determined by a Black-Scholes analysis, to the Oncternal equity valuation range. |
($ millions) | Low | High | ||||||
Oncternal equity valuation range | $ | 155.4 | $ | 186.3 | ||||
Value of GTx common stock issued to Oncternal | $ | 76.9 | ||||||
Implied Value of Outstanding GTx’s Common Stock Based on Implied Value of the Combined Company as of the Date of the March Opinion. Aquilo calculated the implied value of the combined company by grossing up the Oncternal equity valuation range to 100% when assuming Oncternal contributed 75% of the value to the combined company as of the closing. Aquilo also looked separately at the implied value of the combined company based solely on Oncternal’s post-money valuation following the issuance of Oncternal’s Series C preferred stock.
Aquilo then calculated the implied equity valuation attributable to the holders of GTx’s common stock, assuming 25% ownership in the combined company by the holders of GTx common stock as of the closing.
($ millions) | Low | High | Series C Private Financing Valuation | |||||||||
Oncternal equity valuation range | $ | 155.4 | $ | 186.3 | $ | 87.7 | ||||||
Implied valuation of the combined company | $ | 207.2 | $ | 248.4 | $ | 116.9 | ||||||
Value attributable to holders of GTx common stock based on 25% ownership: | $ | 51.8 | $ | 62.1 | $ | 29.2 | ||||||
153
Aquilo then reviewed the premium of the value attributable to holders of GTx common stock in this analysis as compared to GTx’s most recent equity market capitalization prior to the delivery of its March Opinion and the value of GTx as determined by the GTx comparable public company analysis.
($ millions) | Low | High | Series C Private Financing Valuation | |||||||||
Value attributable to holders of GTx common stock based on 25% ownership: | $ | 51.8 | $ | 62.1 | $ | 29.2 | ||||||
Premium to: | ||||||||||||
GTx market capitalization as of March 5, 2019 | 115 | % | 157 | % | 21 | % | ||||||
GTx comparable public company analysis | 104 | % | 145 | % | 15 | % | ||||||
Aquilo also noted that this analysis does not ascribe any value to any consideration that may be paid to the holders of GTx common stock related to the Original Form CVR Agreement.
Miscellaneous
Aquilo’s March Opinion and presentation to the GTx Board was one of many factors taken into consideration by the GTx Board in deciding to enter into the transactions contemplated by the Original Merger Agreement. Consequently, the analyses described above should not be viewed as determinative of the GTx Board’s opinion, or that of GTx senior management, with respect to whether the board would have been willing to agree to different Consideration in the merger.
Opinion of the GTx Financial Advisor as of April 29, 2019
As stated above, in light of the changes proposed to the Original Merger Agreement as set forth in the Merger Agreement Amendment, and the changes proposed to the Original Form CVR Agreement as set forth in the Amended Form CVR Agreement, the GTx Board requested that Aquilo evaluate the fairness, from a financial point of view, to GTx’s stockholders, of the exchange ratio set forth in the Merger Agreement and the right of GTx’s stockholders to receive contingent cash payments pursuant to the CVR Agreement, together, the “Consideration”. On April 29, 2019, Aquilo delivered its oral opinion, subsequently confirmed in writing, to the GTx Board to the effect that, as of the date of its opinion and based upon and subject to the qualifications, limitations and assumptions set forth therein, the Consideration is fair, from a financial point of view, to GTx’s stockholders.
The summary of the written opinion of Aquilo in this proxy statement is qualified in its entirety by reference to the full text of the written opinion of Aquilo, dated April 29, 2019 (the “April Opinion”), attached to this proxy statement asAnnexB-2. You are urged to, and should, read the written April Opinion of Aquilo carefully and in its entirety.
The April Opinion of Aquilo addresses only the fairness, from a financial point of view, to GTx’s stockholders of the Consideration and does not address any other aspect or implication of the merger or any other agreement, arrangement or understanding entered into in connection with the merger or otherwise. Aquilo was not requested to opine as to, and its April Opinion does not in any manner address, GTx’s underlying business decision to proceed with or effect the merger, or any other aspect of GTx’s business or any of its other assets.
154
In arriving at its April Opinion, Aquilo reviewed and analyzed, among other things:
| • | the Merger Agreement (including the Merger Agreement Amendment); |
| • | the CVR Agreement and the Amended Form CVR Agreement; |
| • | certain publicly available business and financial information relating to GTx and Oncternal, including GTx’s and Oncternal’s respective audited financial statements for the year ended December 31, 2018; |
| • | publicly available financial terms of certain sale transactions involving companies Aquilo deemed relevant and the consideration paid for such companies and comparisons of these terms with the proposed financial terms of the Merger Agreement and the Amended Form CVR Agreement; |
| • | publicly available financial and business information concerning certain other companies Aquilo deemed relevant and comparisons of this financial and business information to that of GTx and Oncternal; |
| • | certainnon-public information relating to GTx that was prepared and provided to Aquilo by GTx, including certain operating and financial information relating to GTx’s business, and financial and business forecasts and projections prepared by management of GTx relating to GTx’s prospects; |
| • | certainnon-public information relating to Oncternal that was prepared and provided to Aquilo by Oncternal, including certain operating and financial information relating to Oncternal’s business, and financial and business forecasts and projections prepared by management of Oncternal relating to Oncternal’s prospects; and |
| • | such other information that Aquilo considered appropriate to opine as to the fairness of the Consideration. |
In addition, Aquilo discussed with management of GTx and management of Oncternal, the business, operations, financial condition and prospects of each of GTx and Oncternal, respectively, and as a combined company.
In connection with its review, Aquilo did not assume any responsibility for independent verification of any of the foregoing information and, with GTx’s consent, relied on such information being complete and accurate. With respect to the financial forecasts for GTx, the management of GTx advised Aquilo, and Aquilo assumed with GTx’s consent, that such forecasts were reasonably prepared on bases reflecting the best currently available estimates and judgments of GTx’s management as to the future financial performance of GTx. With respect to the financial forecasts for Oncternal, the management of Oncternal advised Aquilo, and Aquilo assumed with GTx’s consent, that such forecasts were reasonably prepared on bases reflecting the best currently available estimates and judgments of the management of Oncternal as to the future financial performance of Oncternal.
Aquilo relied upon, without independent verification, the assessment of each of GTx’s management and Oncternal’s management as to the viability of, and risks associated with, the current and future products of the combined company following the merger, including without limitation, the development, testing and marketing of such products, the receipt of all necessary governmental and other regulatory approvals for the development, testing and marketing thereof, and the life and enforceability of all relevant patents and other intellectual and other property rights associated with such products. Aquilo assumed that combined company will not materially breach its obligations under the CVR Agreement and expressed no view as to whether the SARD Technology, SARD Compounds, SARD Products, SARM Technology, SARM Compounds or SARM Products will ultimately be developed or monetized. Aquilo also assumed, with GTx’s consent, that, in the course of obtaining any regulatory or third-party consents, approvals or agreements in connection with the merger, no delay, limitation, restriction or condition will be imposed that would have an adverse effect on GTx, Oncternal or the combined company, or the contemplated benefits of the merger, and that the merger will be consummated in accordance with the terms of the Merger Agreement without waiver, modification or amendment of any material term, condition or agreement thereof or any waiver, modification or amendment of any material term, condition or agreement of the CVR Agreement.
155
In preparing its April Opinion, Aquilo performed a number of financial and comparative analyses. The order in which the analyses are described below does not represent the relative importance or weight given to the analyses by Aquilo. The preparation of a fairness opinion is a complex process and is not necessarily susceptible to partial analysis or summary description. Aquilo believes that its analyses must be considered as a whole and that selecting portions of its analyses and of the factors considered by it, without considering all analyses and factors, could create a misleading view of the processes underlying its opinion. No company or transaction used in the analyses performed by Aquilo as a comparison is identical to GTx or Oncternal. In addition, Aquilo may have given some analyses more or less weight than other analyses, and may have deemed various assumptions more or less probable than other assumptions, so the range of valuation resulting from any particular analysis described below should not be taken to be Aquilo’s view of the actual Consideration. The analyses performed by Aquilo are not necessarily indicative of actual values or actual future results, which may be significantly more or less favorable than suggested by such analyses. In addition, analyses relating to the value of businesses or assets do not purport to be appraisals or to necessarily reflect the prices at which businesses or assets may actually be sold. The analyses performed were prepared solely as part of Aquilo’s analysis of the fairness, from a financial point of view, of the Consideration to the holders of GTx common stock set forth in the Merger Agreement and Amended Form CVR Agreement and do not address any other aspect or implication of the merger, including any other agreement, arrangement or understanding entered into in connection with the merger or otherwise.
At a meeting of the GTx Board held on April 29, 2019, Aquilo presented certain financial analyses in connection with the delivery of its oral opinion, subsequently confirmed in writing. The following is a summary of the material financial analyses performed by Aquilo in arriving at its April Opinion. Certain of the following summaries of financial analyses include information presented in tabular format. In order to understand fully the material financial analyses that were performed by Aquilo, the tables should be read together with the text of each summary. The tables alone do not constitute a complete description of the material financial analyses.
Exchange Ratio and Pro Forma Ownership as of the Date of the April Opinion.Based on the estimated exchange ratio, GTx’s stockholders as of immediately prior to the Effective Time are expected to own approximately 22.5% of the outstanding common stock of GTx, and Oncternal’s stockholders as of immediately prior to the Effective Time are expected to own approximately 77.5% of the outstanding common stock of GTx, which is subject to adjustment for each company’s cash balance at closing in accordance with the Merger Agreement. The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants. Based on each of GTx’s and Oncternal’s outstanding capital stock as of April 29, 2019 and assuming no adjustment for cash levels and excluding the issuance of shares related to the exercise of any options, restricted stock awards, warrants or rights to receive such shares, and any shares of stock reserved for issuance, other than shares of GTx common stock reserved for issuance pursuant to the GTx Deferred Stock Rights, Aquilo determined that the exchange ratio would be 0.5137.
Value of GTx Shares Issued to Oncternal Stockholders as of the Date of the April Opinion.Aquilo analyzed the value of the shares to be issued to Oncternal’s stockholders based on a 0.5137 exchange ratio and the closing price of GTx’s common stock on March 6, 2019, the day prior to the announcement of the merger. Aquilo noted the value of the outstanding shares was approximately $76.6 million, that none of GTx’s outstanding options or warrants werein-the-money, and the value of Oncternal’s outstanding options using the treasury stock method was approximately $2.8 million and the value of Oncternal’s outstanding warrants using the Black-Scholes method was approximately $2.0 million, resulting in a value of the shares of GTx’s common stock to be issued to Oncternal’s stockholders to be approximately $81.4 million. Using the most recent closing price of GTx’s common stock prior to the delivery of its April Opinion, and the same methodology described above, Aquilo noted that the value of the shares of GTx’s common stock to be issued to Oncternal’s stockholders to be approximately $101.6 million.
Oncternal Valuation as of the Date of the April Opinion
Comparable Public Company Analysis as of the Date of the April Opinion. Aquilo reviewed, analyzed and compared Oncternal to corresponding publicly available financial information for 13 publicly-traded
156
biotechnology companies that had a lead product candidate in oncology, and in which the lead product candidate’s stage was no earlier than an ongoing Phase 1 clinical trial and no later than an ongoing Phase 2 clinical trial. Forty Seven, Inc., which was not included in the Comparable Public Company Analysis as of the Date of the March Opinion because such company had not yet gone public, was added to the analysis for the April Opinion. The following list sets forth the comparable companies selected by Aquilo and their respective enterprise values.
Company | Enterprise Value ($ millions, rounded) | |||
Aduro Biotech, Inc. | 30.6 | |||
Affimed N.V. | 109.8 | |||
Arcus Biosciences, Inc. | 227.8 | |||
Calithera Biosciences, Inc. | 102.3 | |||
Compugen Ltd. | 165.2 | |||
Constellation Pharmaceuticals, Inc. | 193.8 | |||
Forty Seven, Inc. | 448.7 | |||
Marker Therapeutics, Inc. | 176.5 | |||
Merus N.V. | 163.3 | |||
miRagen Therapeutics, Inc. | 39.3 | |||
Replimmune Group, Inc. | 361.0 | |||
Turning Point Therapeutics, Inc. | 870.0 | |||
ZIOPHARM Oncology, Inc. | 670.4 | |||
Source: SEC filings
Aquilo reviewed the enterprise values of the selected companies, which ranged from $30.6 million to $870.0 million. The result of the analysis implied a mean and median implied enterprise value for these comparable companies of $273.7 million and $176.5 million, respectively. Aquilo compared these ranges to the approximately $81.4 million in value of shares of GTx’s common stock to be issued to Oncternal’s stockholders.
No company used in any analysis as a comparison had a lead product candidate identical to Cirmtuzumab and they all differ in material ways. Accordingly, an analysis of the results described above is not mathematical; rather it involves complex considerations and judgments concerning differences in financial and operating characteristics of the companies and other factors that could affect the public trading value of the selected companies to which they are being compared. This analysis yielded a range of enterprise values, and therefore, such implied enterprise value ranges developed from these analyses were viewed by Aquilo collectively and not individually.
Comparable Initial Public Offering Analysis as of the Date of the April Opinion. Aquilo reviewed, analyzed and compared Oncternal to corresponding publicly available financial information for 13 initial public offerings of biotechnology companies since January 2015 that had a lead product candidate in oncology and in which the lead product candidate’s stage was no earlier than an ongoing Phase 1 clinical trial and no later than an ongoing Phase 2 clinical trial. Forty Seven, Inc., which was not included in the Comparable Initial Public Offering Analysis as of the Date of the March Opinion because such company had not yet gone public, was added to the
157
analysis for the April Opinion. The following list sets forth the initial public offerings selected by Aquilo, including the date priced and thepre-money enterprise value.
Company | Date Priced | Pre-Money Enterprise Value ($ millions, rounded) | ||||
Turning Point Therapeutics, Inc. | April 17, 2019 | 323.7 | ||||
Arcus Biosciences, Inc. | March 14, 2018 | 360.8 | ||||
Constellation Pharmaceuticals, Inc. | July 18, 2018 | 249.5 | ||||
Corvus Pharmaceuticals, Inc. | March 22, 2016 | 240.0 | ||||
Deciphera Pharmaceuticals, Inc. | September 27, 2017 | 378.6 | ||||
Forty Seven, Inc. | June 27, 2018 | 321.9 | ||||
Jounce Therapeutics, Inc. | January 26, 2017 | 177.1 | ||||
Merus N.V. | May 18, 2016 | 65.6 | ||||
Mirna Therapeutics, Inc. | October 1, 2015 | 39.5 | ||||
Nucana plc | September 27, 2017 | 415.7 | ||||
Replimmune Group, Inc. | July 19, 2018 | 338.4 | ||||
TRACON Pharmaceuticals, Inc. | January 29, 2015 | 52.4 | ||||
Zymeworks Inc. | April 27, 2017 | 234.3 | ||||
Source: Company press releases, SEC filings, Capital IQ
Aquilo reviewed the enterprise values of the selected initial public offerings, which ranged from $39.5 million to $415.7 million. The result of the analysis implied a mean and median impliedpre-money enterprise value for these comparable companies of $245.9 million and $249.5 million, respectively. Aquilo compared these ranges to the approximately $81.4 million in value of shares of GTx common stock to be issued to Oncternal’s stockholders.
Although the initial public offerings were used for comparison purposes, none of these initial public offerings is directly comparable to the merger, and none of the companies in those initial public offerings is directly comparable to Oncternal, and none had a lead product candidate directly comparable to Cirmtuzumab. Accordingly, an analysis of the results of such a comparison is not purely mathematical, but instead involves complex considerations and judgments concerning differences in historical financial and operating characteristics of the companies involved and other factors that could affect the acquisition value of such companies or the company to which they are being compared.
Comparable Transaction Analysis as of the Date of the April Opinion.Aquilo reviewed, analyzed and compared Oncternal to corresponding publicly available financial information for 13 business combinations of biotechnology companies since January 2015 where the acquired company had a lead product candidate in oncology, and in which the lead product candidate’s stage was no earlier than an ongoing Phase 1 clinical trial and no later than an ongoing Phase 2 clinical trial. Kiadis Pharma N.V.’s acquisition of Cyto-Sen Therapeutics, Inc., which was not included in the Comparable Transaction Analysis as of the Date of the March Opinion because such transaction had not yet announced, was added to the analysis for the April Opinion. The following
158
list sets forth the business combinations selected by Aquilo, including the phase of the target’s lead product candidate, the upfront equity consideration, any milestone consideration and total deal value.
Acquirer | Target | Date Announced | Phase of Target’s Lead Product Candidate | Upfront Equity Consideration ($ millions, rounded) | Milestone Consideration ($ millions, rounded) | Total Deal Value ($ millions, rounded) | ||||||||||||||
Kiadis Pharma N.V. | Cyto-Sen Therapeutics, Inc. | April 17, 2019 | Phase 1 | 21.9 | 65.6 | 87.5 | ||||||||||||||
Eli Lilly and Company | AurKa Pharma Inc. | May 14, 2018 | Phase 1 | 110.0 | 465.0 | 575.0 | ||||||||||||||
Merck & Co., Inc. | Viralytics Limited | February 21, 2018 | Phase 2 | 394.0 | 0 | 394.0 | ||||||||||||||
Seattle Genetics, Inc. | Cascadian Therapeutics, Inc. | January 31, 2018 | Phase 2 | 614.0 | 0 | 614.0 | ||||||||||||||
Merck & Co., Inc. | Rigontec GmbH | September 6, 2017 | Phase 1/2 | 131.5 | 399.2 | 530.7 | ||||||||||||||
NantCell, Inc. | Altor BioScience Corporation | June 27, 2017 | Phase 2 | 96.7 | 193.3 | 290.0 | ||||||||||||||
Debiopharm International S.A. | ImmunoGen, Inc. | May 23, 2017 | Phase 2 | 25.0 | 30.0 | 55.0 | ||||||||||||||
Celldex Therapeutics, Inc. | Kolltan Pharmaceuticals, Inc. | November 1, 2016 | Phase 1b | 62.5 | 172.5 | 235.0 | ||||||||||||||
Bristol-Myers Squibb Company | Cormorant Pharmaceuticals AB | July 1, 2016 | Phase 1/2 | 95.0 | 425.0 | 520.0 | ||||||||||||||
Roche Holding AG | Tensha Therapeutics, Inc. | January 7, 2016 | Phase 1b | 115.0 | 420.0 | 535.0 | ||||||||||||||
Agenus Inc. | PhosImmune, Inc. | December 21, 2015 | Phase 1 | 9.9 | 35.0 | 44.9 | ||||||||||||||
Novartis International AG | Admune Therapeutics LLC | October 21, 2015 | Phase 1 | 140.0 | 120.0 | 260.0 | ||||||||||||||
Merck & Co., Inc. | cCAM Biotherapeutics Ltd | July 27, 2015 | Phase 1 | 95.0 | 510.0 | 605.0 | ||||||||||||||
Source: Company press releases, SEC filings
Aquilo reviewed the range of upfront equity considerations paid to the targets within the comparable transaction set, which ranged from $9.9 million to $614.0 million. The result of the analysis implied a mean and median upfront equity value for the comparable transactions of $147.0 million and $96.7 million, respectively.
Aquilo also considered an adjusted milestone consideration payable to the target or its stockholders within the comparable transaction set. Aquilo applied an adjustment factor, based on the phase of the target company’s lead product candidate, to the total milestone consideration associated with each transaction, and added the upfront equity value to the adjusted milestone consideration for each transaction. The total milestone consideration payable to targets with a lead product candidate in a Phase 1 clinical trial were adjusted by 5.1% and targets with a lead product candidate in a Phase 2 clinical trial were adjusted by 8.1%. The adjustment factors are the probability of success that oncology assets at these stages would achieve FDA approval, as reported by BIO in their “Clinical Development Success Rates 2006-2015” report.
The total deal values, including the upfront payment plus any adjusted milestone consideration, ranged from $11.7 million to $614.0 million. The result of this analysis implied a mean and median total adjusted deal value
159
for the comparable transactions of $160.9 million and $129.4 million, respectively. Aquilo compared these ranges to the approximately $81.4 million in value of shares of GTx’s common stock to be issued to Oncternal’s stockholders.
Although the transactions were used for comparison purposes, none of these transactions is directly comparable to the merger, and none of the companies in those transactions is directly comparable to Oncternal, and none had a lead product candidate directly comparable to Cirmtuzumab. Accordingly, an analysis of the results of such a comparison is not purely mathematical, but instead involves complex considerations and judgments concerning differences in historical financial and operating characteristics of the companies involved and other factors that could affect the acquisition value of such companies or the company to which they are being compared.
Recent Oncternal Series C Private Financing Valuation as of the Date of the April Opinion.On September 22, 2018, Oncternal issued shares of its Series C preferred stock to Shanghai at an implied fully-diluted post-money valuation of $87.7 million. Aquilo noted that Shanghai also received certain rights to Oncternal products in China and certain selected other territories in connection with its purchase of Oncternal’s Series C preferred stock. Aquilo compared the $87.7 million post-money valuation of Oncternal to the approximately $76.9 million in value of shares of GTx’s common stock to be issued to Oncternal’s stockholders.
GTx Valuation as of the Date of the April Opinion
Comparable Public Company Analysis as of the Date of the April Opinion. Aquilo reviewed, analyzed and compared GTx to corresponding publicly available financial information for 8 publicly-traded biotechnology companies in which the company’s lead product candidate failed in late-stage clinical trials, across all therapeutics areas. Arsanis, Inc., Edge Therapeutics, Inc., Vital Therapies, Inc., and Histogenics Corporation, which were included in GTx’s Comparable Public Company Analysis as of the Date of the March Opinion, were not included in the analysis for the April Opinion, as they have since announced or completed mergers. Conatus Pharmaceuticals Inc. and Proteon Therapeutics, Inc., which were not included in GTx’s Comparable Public Company Analysis as of the Date of the March Opinion, were included in the analysis for the April Opinion, as they have since announced lead product candidate failures. The following list sets forth the comparable companies selected by Aquilo and their respective net cash multiples.
Company | Net Cash Multiple | |||
Aevi Genomic Medicine, Inc. | 1.2x | |||
Aquinox Pharmaceuticals, Inc. | 0.8x | |||
Conatus Pharmaceuticals Inc. | 0.6x | |||
Gemphire Therapeutics Inc. | 2.0x | |||
Merrimack Pharmaceuticals, Inc. | 1.5x | |||
Proteon Therapeutics, Inc. | 0.4x | |||
Realm Therapeutics Plc | 0.9x | |||
Vical Incorporated | 0.5x | |||
Source: SEC filings
Aquilo multiplied the median and mean net cash multiples, determined by the quotient of the equity market capitalization divided by the net cash of the selected companies, by GTx’s estimated net cash as of March 31, 2019. The net cash multiples of these comparable public companies ranged from 0.4x to 2.0x, and the mean and median net cash multiples were 1.0x and 0.9x, respectively. The analysis implied a mean and median equity value for GTx of $21.0 million and $18.4 million, respectively.
No company used in any analysis as a comparison is identical to GTx and they all differ in material ways. Accordingly, an analysis of the results described below is not mathematical; rather it involves complex
160
considerations and judgments concerning differences in financial and operating characteristics of the companies and other factors that could affect the public trading value of the selected companies to which they are being compared. This analysis yielded a range of enterprise values, and therefore, such implied enterprise value ranges developed from these analyses were viewed by Aquilo collectively and not individually.
Summary of Valuation Analyses as of the Date of the April Opinion
This summary of the valuation methodologies presented in this April Opinion refer to the Oncternal equity valuation range, which is calculated from the means of the low and high valuations implied by the means and medians (as the case may be) from each of the valuation analyses described above, and set forth below:
| • | Comparable Public Company Analysis as of the Date of the April Opinion; |
| • | Comparable Initial Public Offering Analysis as of the Date of the April Opinion; |
| • | Comparable Biotechnology Transaction Analysis as of the Date of the April Opinion – Upfront Equity; |
| • | Comparable Biotechnology Transaction Analysis as of the Date of the April Opinion – Upfront Equity plus Adjusted Milestone; and |
| • | Recent Oncternal Series C Private Financing Valuation as of the Date of the April Opinion. |
Value of GTx Shares Issued Relative to Oncternal Implied Valuation as of the Date of the April Opinion.Aquilo compared the value of GTx’s common stock issued to Oncternal’s stockholders based on the closing price of GTx common stock prior to the announcement of the merger, and as determined by an assumed exchange ratio of 0.5137, and including:
| • | Oncternal’s outstanding shares of capital stock; |
| • | the number of shares of Oncternal’s common stock issuable upon the exercise of options to purchase Oncternal’s common stock, as calculated by the Treasury Method; and |
| • | Value attributable to Oncternal’s warrants to purchase shares of SeriesB-2 preferred stock of Oncternal as determined by a Black-Scholes analysis, to the Oncternal equity valuation range. |
($ millions) | Low | High | ||||||
Oncternal equity valuation range | $ | 162.8 | $ | 199.6 | ||||
Value of GTx common stock issued to Oncternal | $ | 81.4 | ||||||
Implied Value of Outstanding GTx’s Common Stock Based on Implied Value of the Combined Company as of the Date of the April Opinion. Aquilo calculated the implied value of the combined company by grossing up the Oncternal equity valuation range to 100% when assuming Oncternal contributed 77.5% of the value to the combined company as of the closing. Aquilo also looked separately at the implied value of the combined company based solely on Oncternal’s post-money valuation following the issuance of Oncternal’s Series C preferred stock.
Aquilo then calculated the implied equity valuation attributable to the holders of GTx’s common stock, assuming 22.5% ownership in the combined company by the holders of GTx common stock as of the closing.
($ millions) | Low | High | Series C Private Financing Valuation | |||||||||
Oncternal equity valuation range | $ | 162.8 | $ | 199.6 | $ | 87.7 | ||||||
Implied valuation of the combined company | $ | 210.1 | $ | 257.5 | $ | 113.2 | ||||||
Value attributable to holders of GTx common stock based on 22.5% ownership: | $ | 47.3 | $ | 57.9 | $ | 25.5 | ||||||
161
Aquilo then reviewed the premium of the value attributable to holders of GTx common stock in this analysis as compared to GTx’s most recent equity market capitalization prior to the delivery of its April Opinion and the value of GTx as determined by the GTx comparable public company analysis.
($ millions) | Low | High | Series C Private Financing Valuation | |||||||||
Value attributable to holders of GTx common stock based on 22.5% ownership: | $ | 47.3 | $ | 57.9 | $ | 25.5 | ||||||
Premium to: | ||||||||||||
GTx market capitalization as of March 6, 2019 | 113 | % | 161 | % | 15 | % | ||||||
GTx comparable public company analysis | 125 | % | 176 | % | 21 | % | ||||||
Aquilo also noted that this analysis does not ascribe any value to any consideration that may be paid to the holders of GTx common stock related to the CVR Agreement.
Miscellaneous
Aquilo’s April Opinion and presentation to the GTx Board was one of many factors taken into consideration by the GTx Board in deciding to enter into the transactions contemplated by the Merger Agreement. Consequently, the analyses described above should not be viewed as determinative of the GTx Board’s opinion, or that of GTx senior management, with respect to whether the board would have been willing to agree to different Consideration in the merger.
Pursuant to the terms of the engagement letter and an addendum to the engagement letter dated as of December 12, 2018, GTx paid Aquilo a retainer fee of $200,000 and $400,000 upon the delivery of its March Opinion. GTx has also agreed to pay Aquilo an additional $300,000 for the delivery of the April Opinion and $900,000 upon completion of the merger. In addition, GTx has agreed to indemnify Aquilo for certain liabilities and expenses arising out of or in conjunction with its rendering of services under its engagement, including liabilities arising under the federal securities laws.
Pursuant to an engagement letter dated as of December 12, 2018, the GTx Board engaged Aquilo to provide financial advisory services to GTx in connection with exploring and evaluating opportunities for GTx to, among other things, combine with or be acquired by another company including, if requested, rendering its opinion to the GTx Board. Aquilo was selected by GTx based on Aquilo’s qualifications, expertise and reputation. Aquilo, as part of its investment banking business, is continuously engaged in the valuation of businesses and securities in connection with mergers and acquisitions, private placements and valuations for corporate and other purposes.
The terms of the merger were determined through arm’s length negotiations between GTx and Oncternal and were approved by the GTx Board. Although Aquilo provided advice to the GTx Board during the course of these negotiations, the decision to enter into the merger was solely that of the GTx Board. Aquilo did not recommend any specific consideration to GTx or the GTx Board, or that any specific amount or type of consideration constituted the only appropriate consideration for the merger. As described above, the opinion of Aquilo and its presentation to the GTx Board were among a number of factors taken into consideration by the GTx Board in making its determination to approve the Merger Agreement and the transactions contemplated by such agreement.
Aquilo had not been engaged by GTx prior to this engagement, nor has Aquilo previously been engaged by Oncternal.
162
Interests of GTx Directors and Executive Officers in the Merger
In considering the recommendation of the GTx Board with respect to issuing shares of GTx’s common stock as contemplated by the Merger Agreement and the other matters to be acted upon by GTx’s stockholders at the GTx special meeting, GTx’s stockholders should be aware that certain members of the GTx Board and certain of GTx’s executive officers have interests in the merger that may be different from, or in addition to, the interests of GTx’s stockholders. These interests may present them with actual or potential conflicts of interest, and these interests, to the extent material, are described below.
Each of the GTx Board and the Oncternal Board was aware of these potential conflicts of interest and considered them, among other matters, in reaching their respective decisions to approve the Merger Agreement and the merger, and to recommend, as applicable, that GTx’s stockholders approve the proposals to be presented to GTx’s stockholders for consideration at the GTx special meeting as contemplated by this proxy statement/prospectus/information statement, and that Oncternal’s stockholders sign and return the written consent as contemplated by this proxy statement/prospectus/information statement.
Ownership Interests
As of March 31, 2019, GTx’s directors and executive officers beneficially owned, in the aggregate, 30% of the shares of common stock of GTx, which for purposes of this subsection excludes any GTx shares issuable upon exercise or settlement of GTx stock options, warrants or GTx Deferred Stock Rights held by such individual. The affirmative vote of the holders of a majority of the total outstanding shares of common stock of GTx is required for approval of Proposal Nos. 2 and 3. Approval of Proposal Nos. 1 and 4 require the affirmative vote of the holders of a majority of the shares of GTx’s common stock entitled to vote and present in person or represented by proxy at the GTx special meeting. Abstentions will have the same effect as votes “AGAINST” Proposal Nos. 1, 2, 3, 4, 5 and 6.
The table below sets forth information regarding the ownership of GTx’s common stock as of March 31, 2019 by GTx’s directors and named executive officers.
| Directors and Named Executive Officers | Number of Shares of Common Stock as of March 31, 2019 | |||
Marc S. Hanover | 172,049(1) | |||
Robert J. Wills, Ph.D. | 137,344(2) | |||
Henry P. Doggrell | 47,995(3) | |||
Michael G. Carter, M.D., Ch.B., F.R.C.P. | — (4) | |||
J. Kenneth Glass | 24,226(5) | |||
J. R. Hyde, III | 6,807,338(6) | |||
Garry A. Neil, M.D. | — (7) | |||
Kenneth S. Robinson, M.D., M.Div. | — (8) | |||
| (1) | Includes 35,287 shares held by Equity Partners XII, LLC, an entity controlled by Mr. Hanover and 12,400 shares held by trusts of which Mr. Hanover is the trustee. Excludes 22,726 shares issuable upon exercise of a warrant and 305,000 shares of common stock issuable upon the exercise of options held by Mr. Hanover |
| (2) | Excludes 200,000 shares of common stock issuable upon the exercise of options held by Dr. Wills. |
| (3) | Includes 934 shares held by trusts with respect to which Mr. Doggrell may be deemed to have beneficial ownership and 400 shares of common stock held by Mr. Doggrell through an individual retirement account. Also includes 664 shares held by Mr. Doggrell’s wife and 2,547 shares of common stock held by Mr. Doggrell’s wife through an individual retirement account. Excludes 195,999 shares of common stock issuable upon the exercise of options held by Mr. Doggrell and 11,435 shares held by a trust of which Mr. Doggrell is theco-trustee and are included in the shares reported below by J.R. Hyde, III. |
163
| (4) | Excludes 46,500 shares of common stock issuable upon the exercise of options held by Dr. Carter, and 3,631 shares issuable to Dr. Carter pursuant to the GTx Directors Deferred Compensation Plan. |
| (5) | Includes 2,450 shares of common stock held by Mr. Glass’ wife through an individual retirement account. Excludes 46,500 shares of common stock issuable upon the exercise of options held by Mr. Glass, and 655 shares issuable to Mr. Glass pursuant to GTx’s Directors Deferred Compensation Plan. |
| (6) | Includes 14,535 shares held by Pittco Associates III, L.P. and 391,571 shares held by Pittco Investments, L.P., entities controlled by Mr. Hyde. Also includes 21,646 shares held by Mr. Hyde’s spouse and 184,480 shares held by trusts for the benefit of Mr. Hyde’s children. Excludes 2,454,483 shares issuable upon exercise of a warrant issued to Mr. Hyde in November 2014, 678,349 shares issuable upon exercise of a warrant issued to Mr. Hyde in September 2017, and 70,276 shares issuable to Mr. Hyde pursuant to the GTx Directors Deferred Compensation Plan. |
| (7) | Excludes 28,750 shares of common stock issuable upon the exercise of options held by Dr. Neil, and 36,759 shares issuable to Dr. Neil pursuant to the GTx Directors Deferred Compensation Plan. |
| (8) | Excludes 46,500 shares of common stock issuable upon the exercise of options held by Dr. Robinson, and 44,105 shares issuable to Dr. Robinson pursuant to the GTx Directors Deferred Compensation Plan. |
Effect of Merger on GTx Stock Awards
Under the Merger Agreement, as of immediately prior to the Effective Time, the vesting of all outstanding options to purchase shares of common stock of GTx, including those held by GTx’s executive officers and directors, will accelerate in full. The number of shares of common stock of GTx underlying such options and the exercise price of such options will be adjusted appropriately to reflect the GTx Reverse Stock Split.
Based on a per share GTx stock price of $1.40, and the other assumptions set forth in footnote 2 of the table under the section entitled “- GTx Named Executive Officers Golden Parachute Payments” of this proxy statement/prospectus/information statement, none of the executive officers or directors would receive any amount, net of exercise price, if such individual exercised his or her unvested options that will vest at the time of closing and immediately sold the common stock of GTx acquired upon exercise.
The table below sets forth information regarding the GTx stock options held by each of GTx’s executive officers and directors as of March 31, 2019. The number of shares of common stock of GTx underlying such options will be adjusted appropriately to reflect the GTx Reverse Stock Split.
| Name | Number of Vested Company Stock Options Held | Number of Unvested Company Stock Options Held | ||||||
Executive Officers | ||||||||
Marc Hanover | 100,001 | 204,999 | ||||||
Robert Wills | 13,334 | 186,666 | ||||||
Henry Doggrell | 47,667 | 148,332 | ||||||
Jason Shackelford | 33,934 | 132,366 | ||||||
Non-Employee Directors | ||||||||
J.R. Hyde, III | — | — | ||||||
Michael G. Carter, M.D., Ch.B., F.R.C.P | 27,334 | 19,166 | ||||||
J. Kenneth Glass | 27,334 | 19,166 | ||||||
Garry A. Neil, M.D. | 10,417 | 18,333 | ||||||
Kenneth S. Robinson, M.D., M.Div. | 27,334 | 19,166 | ||||||
Director Deferred Compensation Plan
Under the Merger Agreement, as of immediately prior to the Effective Time (but in no event more than 30 days prior to the Effective Time), GTx shall take all actions necessary to cause the termination and liquidation of the
164
GTx Deferred Stock Rights. As a result, the outstanding GTx Deferred Stock Rights will be settled at the closing in shares, to the extent shares have been credited tonon-employee director stock accounts under the plan. GTx shall also ensure that any deferrals under the GTx Director Deferred Compensation Plan on or after January 3, 2019 shall be settled only in cash and that the maximum number of shares of common stock of GTx issuable upon settlement of the GTx Deferred Stock Rights shall be limited to the number of GTx Deferred Stock Rights outstanding as of the date of the Merger Agreement.
The table below sets forth information regarding the shares credited to individualnon-employee director stock accounts as of March 31, 2019 under the GTx Director Deferred Compensation Plan and the value of the shares issuable upon settlement of the corresponding GTx Deferred Stock Rights based on a per share GTx stock price of $1.40, and the other assumptions set forth in footnote 2 of the table under the section entitled “- GTx Named Executive Officers Golden Parachute Payments” of this proxy statement/prospectus/information statement. As of March 31, 2019, five of GTx’snon-employee directors held Deferred Stock Rights and an aggregate of 155,426 shares of GTx common stock were issuable pursuant to the GTx Deferred Stock Rights. In addition, as of March 31, 2019, two of GTx’snon-employee directors had elected to defer compensation under the GTx Director Deferred Compensation Plan after January 3, 2019, which deferrals will be paid to thenon-employee directors at the closing in cash. The table below also includes, as of March 31, 2019, the aggregate deferrals under the GTx Director Deferred Compensation Plan that were accrued as of such date and will be settled in cash.
| Name | Number of Shares Subject to GTx Deferred Stock Rights | Value of Shares Subject to GTx Deferred Stock Rights | ||||||
J.R. Hyde, III | 70,276 | 98,386 | ||||||
Michael G. Carter, M.D., Ch.B., F.R.C.P | 3,631 | 5,083 | ||||||
J. Kenneth Glass | 655 | 917 | ||||||
Garry A. Neil, M.D. | 36,759 | 51,463 | ||||||
Kenneth S. Robinson, M.D., M.Div. | 44,105 | 61,747 | ||||||
Director Positions Following the Merger
Dr. Carter and Dr. Wills are currently directors of GTx and will continue as directors of the combined organization after the Effective Time. For a description of GTx’s director compensation, see “Director Compensation” below.
Director Compensation
Cash Retainers
The GTx Board has approved the GTXNon-Employee Director Compensation Policy (the “GTx Director Compensation Policy”), pursuant to which the following cash compensation payments are made quarterly to the GTx Board and committee members:
| • | a $35,000 annual retainer for service as a member of the GTx Board of Directors; |
| • | a supplemental annual retainer for the Lead Director of the GTx Board and for the Chairs of each GTx Board committee in the following amounts: $15,000 for the Lead Director of the GTx Board; $17,500 for Chair of the GTx Audit Committee; $10,000 for Chair of the GTx Compensation Committee; and $8,500 for Chair of the GTx Nominating and Corporate Governance Committee; and |
| • | a supplemental annual retainer for each member of the following committees other than the Chairs, in the following amounts: $10,000 for members of the GTx Audit Committee; $7,500 for members of the GTx Compensation Committee; $5,000 for members of the GTx Nominating & Corporate Governance Committee; and $10,000 for members of the GTx Scientific and Development Committee. |
165
No directors currently receive consulting fees from GTx. GTx Directors who are also employees receive no additional compensation for service on the GTx Board.
Equity Compensation
Pursuant to the GTx Director Compensation Policy, eachnon-employee director of GTx (who does not own more than ten percent of the combined voting power of GTx’s then outstanding securities) is eligible for certain initial and annual stock awards, which grants are currently made pursuant to GTx’s 2013Non-Employee Director Equity Incentive Plan (the “GTx Directors’ Plan”). Under the GTx Director Compensation Policy, any individual who first becomes anon-employee director is eligible for a stock award in such form and in such amount that the Board deems necessary to attract such individual to join the Board. In addition, under the GTx Director Compensation Policy, any individual who is serving as anon-employee director on the day following an annual meeting of GTx’s stockholders automatically will be granted an option to purchase shares of common stock on that date;provided,however, that if the individual has not been serving as anon-employee director for the entire period since the preceding annual meeting, the number of shares subject to such individual’s annual grant will be reduced pro rata for each full month prior to the date of grant during which such individual did not serve as anon-employee director. The shares subject to each initial grant and each annual grant vest in a series of three successive equal annual installments measured from the date of grant, so that each initial grant and each annual grant will be fully vested three years after the date of grant. The exercise price per share for the options granted under the 2013 Directors’ Plan is not less than the fair market value of the stock on the date of grant.
In March 2018, the GTx Board, upon the recommendations of its Nominating and Corporate Governance Committee and the Compensation Committee, determined that the number of shares subject to the automatic annual grants occurring on the date following the 2018 annual meeting would be 7,500 shares of GTx common stock. GTx’s current director compensation program will be suspended at the time of the closing of the merger and the director compensation policies for the combined organization following the merger will bere-evaluated by the compensation committee and board of directors of the combined organization following completion of the merger and may be subject to change.Non-employee directors of the combined organization are, however, expected to receive annual cash retainers and equity compensation, although the amount of such compensation has not yet been determined.
Pursuant to the Merger Agreement, all outstanding unvested options held by GTx’snon-employee directors will vest in full upon the closing of the merger.
Employment Agreements
GTx has entered into employment agreements with each of its executive officers. These agreements set forth the individual’s base salary, annual incentive opportunities, equity compensation and other employee benefits. All employment agreements provide for“at-will” employment, meaning that either party can terminate the employment relationship at any time, although GTx’s agreements with its named executive officers provide that they would be eligible for severance benefits in certain circumstances following an involuntary or constructive termination, including an involuntary or constructive termination following a change of control. For purposes of these agreements, the merger, if consummated, will constitute a change of control transaction.
Termination Without “Cause” or for “Good Reason” after a Change of Control
The employment agreements with GTx’s executive officers generally provide for cash post-termination change of control payments equal to one year’s base salary and monthly premium payments to continue the executive officer’s health insurance coverage for up to 12 months following his or her termination. These change of control salary continuation and health insurance coverage benefits are structured on a “double-trigger” basis, meaning that before an executive officer is eligible to receive such change of control benefits, (1) a change of control must occur and (2) within 12 months after such change of control, the named executive officer’s employment must be
166
terminated without “cause” or the named executive officer must resign for “good reason.” GTx’s obligation to make the salary continuation payments and health insurance premium payments under the employment agreements is conditioned upon the former named executive officer’s compliance with the confidentiality provisions of the employment agreement and the provisions of thenon-competition provisions of the employment agreement for a period of one year following termination. In addition, GTx’s obligation to make the salary continuation payments and health insurance premium payments is conditioned upon GTx’s receipt of an effective general release of claims executed by the named executive officer. The post-termination salary continuation payments will be generally made over theone-year period following termination on GTx’s regular payroll dates rather than in a lump sum, except that the timing of these payments may be deferred for up to six months if these payments would constitute deferred compensation under Section 409A of the Code (in which case, the deferred payment would be made in a lump sum following the end of the deferral period, with the balance being paid thereafter on GTx’s regular payroll dates).
A change of control generally means the following:
| • | the sale or other disposition of all or substantially all of GTx’s assets (including a liquidation or dissolution of GTx); |
| • | if any person or group acquires beneficial ownership of 50% or more of GTx’s voting securities (subject to certain exceptions); |
| • | a merger or consolidation of GTx with or into any other entity, if immediately after the transaction more than 50% of the voting stock of the surviving entity is held by persons who were not holders of at least 50% of GTx’s voting stock as of the effective date of the named executive officer’s employment agreement; or |
| • | a majority of GTx’s Board becomes comprised of individuals whose nomination, appointment, or election was not approved by a majority of the Board members or their approved successors. |
“Cause” is generally defined as the named executive officer’s:
| • | conviction for a felony; |
| • | theft, embezzlement, misappropriation of or intentional infliction of material damage to GTx’s property or business opportunities; |
| • | breach of his or her confidentiality ornon-competition obligations, as applicable, under his or her employment agreement; or |
| • | ongoing willful neglect of or failure to perform his or her duties, or his or her ongoing willful failure or refusal to follow any reasonable, unambiguous duly adopted written direction that is not inconsistent with the description of such named executive officer’s duties, provided that such willful neglect or failure is materially damaging or materially detrimental to the business and operations of GTx, and after 30 days’ notice and the opportunity to cure. |
“Good reason” is generally defined as the following actions taken without the consent of the executive officer after a change of control (in each case where the executive officer has provided written notice within 30 days of the action, such action is not remedied by GTx within 30 days following such notice, and the executive officer’s resignation is effective not later than 60 days after the expiration of such30-day cure):
| • | an adverse change in the executive officer’s authority, duties or responsibilities (including reporting responsibilities) which, without the executive officer’s consent, represents a material reduction in or a material demotion of the named executive officer’s authority, duties or responsibilities as in effect immediately prior to the change of control, or the assignment to the executive officer of any duties or responsibilities that are materially inconsistent with and materially adverse to such authority, duties or responsibilities; |
167
| • | a material reduction in the then current base salary of the executive officer; |
| • | the relocation of the executive officer’s principal office to a location that increases hisone-way commute by more than 20 miles (or, in the case of Dr. Wills, a relocation outside of New Jersey); |
| • | the failure of GTx to obtain an agreement reasonably satisfactory to the executive officer from any successor entity upon the change of control to assume and agree to perform his or her employment agreement in all material respects; or |
| • | a material breach by GTx of any provision of the executive officer’s employment agreement or any other then-effective agreement with the named executive officer. |
Termination Without “Cause” or For “Good Reason” Prior to or Not in Connection with a Change of Control
GTx’s employment agreement with Dr. Wills provides for post-termination cash payments equal to one year’s base salary (generally to be made over theone-year period following termination on GTx’s regular payroll dates) and monthly premium payments to continue his health insurance coverage for up to 12 months following his termination, should his employment be terminated without “cause” or should he resign for “good reason”, in each case irrespective of whether such termination is within 12 months after (or otherwise in connection with) a change of control.
Other Termination Scenarios
If GTx terminates an executive officer’s employment for “cause,” or if an executive officer voluntarily terminates his or her employment without “good reason,” or upon the death of an executive officer, the executive officer would generally have no right to receive any compensation or benefits under his or her employment agreement on or after the effective date of termination, other than any accrued and unpaid salary and expense reimbursement. However, under GTx’s employment agreement with Dr. Wills, Dr. Wills would nonetheless be entitled to any earned but unpaid annual bonus with respect to any completed calendar year immediately preceding his termination date. Likewise, except as described above under “ —Termination Without “Cause” or For “Good Reason” Prior to or Not in Connection with a Change of Control” with respect to Dr. Wills, if GTx terminates an executive officer’s employment without “cause,” or if an executive officer voluntarily terminates his or her employment with “good reason,” in each case not within 12 months following a change of control, the executive officer would have no right to receive any compensation or benefits under his employment agreement on or after the effective date of termination, other than any accrued and unpaid salary and expense reimbursement and, solely in the case of Dr. Wills, subject to GTx’s obligation under his employment agreement to pay any accrued but unpaid annual bonus with respect to any completed calendar year immediately preceding his termination date.
Other Employment Agreement Benefits
Except as set forth above, under the employment agreements with GTx’s executive officers, GTx’s executive officers would not be entitled to any other benefits following termination of service, including the continuation of general employee benefits, life insurance coverage and long term disability coverage, except as otherwise required by applicable law.
Extended Post-Termination Option Exercise Period for GTx Options
As a general matter, the terms of the options GTx has granted to its executive officers and directors provided that the vested portion of these options will expire three months after the executive officer’s or director’s termination of service. GTx refers to the period following termination of service during which an executive officer or director can continue to exercise his or her vested stock options as the post-termination exercise period. However, in connection with the adoption of a retention bonus program by the Compensation Committee in September 2013,
168
the options held by GTx’s executive officers and outstanding on or prior to September 27, 2013 were modified to generally provide for a six month post-termination exercise period. In addition, a retention stock option granted to Mr. Doggrell in 2013 generally provides for a six month post-termination exercise period. All such post-termination exercise periods are limited by, and will not exceed, the original expiration date of the option. The terms of the retention benefit agreements with GTx’s executive officers will, however, be less favorable than the terms for an extension of the post-termination exercise period provided under the terms of our equity plans. Such more favorable terms will apply under the circumstances described below.
Under GTx’s 2004 Equity Incentive Plan (the “GTx 2004 Plan”), and the form of stock option agreement under GTx’s 2004 Plan, the post-termination exercise period will generally be one year following termination if the termination of service is a result of an involuntary termination without cause or a constructive termination within 12 months after a change of control. Under GTx’s 2013 Equity Incentive Plan (the “GTx 2013 Plan”), and the form of stock option agreement under GTx’s 2013 Plan, the post-termination exercise period will generally be one year following termination if the termination of service occurs either as a condition of a change of control or upon the effectiveness of a change of control, unless the stock option is not assumed, continued or replaced by the successor or acquiring entity. If the termination is a retirement, the exercise period will be two years under each of the GTx 2004 Plan and GTx 2013 Plan. Currently, Messrs. Hanover and Doggrell are retirement-eligible.
The standard form of stock option agreement under the 2004 Plan generally defines “cause” as the grant recipient:
| • | committing an act that materially injures the business of GTx; |
| • | refusing or failing to follow the lawful and reasonable directions of the Board or the appropriate individual to whom he or she reports, after 15 days’ notice and the opportunity to cure; |
| • | willfully or habitually neglecting his or her duties with GTx, after 15 days’ notice and the opportunity to cure; |
| • | being convicted of a felony that is likely to inflict or has inflicted material injury on the business of GTx; or |
| • | committing a material fraud, misappropriation, embezzlement or other act of gross dishonesty that resulted in material loss, damage or injury to GTx. |
The standard form of stock option agreement under the 2004 Plan generally defines a “constructive termination” as a voluntary termination within 12 months after a change of control after any of the following actions are taken without the consent of the grant recipient:
| • | the assignment to the grant recipient of any duties or responsibilities which results in a significant reduction in his or her function as in effect immediately prior to the change of control; |
| • | a material reduction in the grant recipient’s salary, as in effect on the effective date of the change of control; |
| • | the failure to continue in effect any benefit plan or program in which the grant recipient was participating immediately prior to the effective date of the change of control, or the taking of any action that would adversely affect his or her participation in (or reduce his or her benefits under) any such benefit plan or program (but either circumstance will only be grounds for a “constructive termination” if the range of benefit plans and programs offered by the acquirer is not comparable to the benefit plans previously offered by GTx, when considered as a whole); |
| • | a relocation of the grant recipient’s principal office to a location more than 50 miles from the location at which he or she performed his or her duties as of the effective date of the change of control; or |
| • | a material breach by GTx of any provision of the grant recipient’s stock option agreement under the 2004 Plan. |
169
GTx Named Executive Officer Golden Parachute Compensation
This section sets forth the information required by Item 402(t) of RegulationS-K regarding the compensation of each of GTx’s named executive officers that is based on or otherwise relates to the merger. The consummation of the merger will constitute a change of control of GTx under the terms of the employment agreements between GTx and its named executive officers and for purposes of their equity awards. The table below describes the estimated potential payments to each of GTx’s named executive officers under the terms of their employment agreements and their GTx equity awards. The benefits shown reflect only the additional payments or benefits that the individual would have received upon the occurrence of a change in control or an involuntary termination within 12 months following a change of control. The amounts shown do not include the value of payments or benefits that would have been earned absent the closing of the change of control or such a qualifying termination.
Please note the amounts shown in the table are estimates only and are based on assumptions regarding events that may or may not actually occur, including assumptions described in this proxy statement/prospectus/information statement and in the notes to the table below, which may or may not actually occur or may occur at times different than the time assumed. Some of these assumptions are based on information currently available and, as a result, the actual amounts, if any, that may become payable to a named executive officer may materially differ from the amounts set forth below. Furthermore, for purposes of calculating these amounts, GTx has assumed:
| • | the Effective Time occurred on March 31, 2019; |
| • | a price per share of GTx common stock of $1.40, which represents the average closing trading price of GTx common stock over the first five business days following the first public announcement of the transaction; |
| • | the employment of each of Messrs. Hannover and Doggrell and Dr. Wills will be terminated on such date in a manner that entitles the named executive officer to receive the severance payments and benefits under the terms of the employment agreements between GTx and such named executive officer (as described in above under the heading “Employment Agreements”). The employment of each of named executive officer is expected to be terminated effective as of the closing of the merger; |
| • | the named executive officers’ base salaries are those in place as of March 31, 2019; and |
| • | no named executive officer enters into new agreements or is otherwise legally entitled to, prior to the Effective Time, additional compensation or benefits. |
Name | Cash(1) | Option Acceleration and Extension(2) | Benefits(3) | Total(4) | ||||||||||||
Marc S. Hanover | $ | 445,628 | $ | — | $ | 28,281 | $ | 473,909 | ||||||||
Robert J. Wills, Ph.D. | $ | 226,600 | $ | — | $ | — | $ | 226,600 | ||||||||
Henry P. Doggrell | $ | 389,463 | $ | — | $ | 40,266 | $ | 429,729 | ||||||||
| (1) | With respect to Messrs. Hanover and Doggrell and Dr. Wills, under the employment agreements, cash severance would be payable following termination of the named executive officer’s employment by GTx other than for cause (and other than due to death or disability) or the named executive officer’s resignation for good reason, in either case, within 12 months following a change of control, subject to the named executive officer’s execution of a release of claims. In either such event, pursuant to the employment agreements, the named executive officer will receive (1) severance payments equal to one year’s base salary and (2) 12 months’ continued health coverage at company expense. Any amounts payable in connection with the termination of an executive’s employment are subject to applicable withholdings and are payable over theone-year period following the effective date of the named executive officer’s release, except that the timing of these payments may be deferred for up to six months if these payments would constitute deferred compensation under Section 409A of the Code (in which case, the deferred payment would be made in a lump sum following the end of the deferral period, with the balance being paid thereafter on GTx’s regular payroll dates). These severance benefits are double-trigger benefits in that they will be paid |
170
| only if the named executive officer experiences a qualifying termination of employment during the period described above, in accordance with the employment agreements. |
| (2) | With respect to the named executive officers, under the Merger Agreement, effective as of immediately prior to the Effective Time, each GTx stock option will fully vest. The accelerated vesting is a single-trigger (closing of the merger) benefit that will be received solely because of the merger and regardless of whether a named executive officer’s employment is terminated. As noted above in the section entitled, “Extended Post-Termination Option Exercise Period for GTx Options,” an extended exercise period will be provided upon certain qualifying terminations of employment. |
Based on a per share GTx stock price of $1.40, and the other assumptions set forth above, none of the executive officers or directors would receive any amount, net of exercise price, if such individual exercised his or her unvested options that will vest at the time of closing and immediately sold the common stock of GTx acquired upon exercise. As a result, there are no amounts reported in this column.
Name | Number of Unvested GTx Stock Options Subject to Acceleration | |||
Marc S. Hanover | 204,999 | |||
Robert J. Wills, Ph.D. | 186,666 | |||
Henry P. Doggrell | 148,332 | |||
| (3) | Consists of COBRA coverage for a period of 12 months following the date of termination. The value is based upon the type of insurance coverage GTx carried for each named executive officer as of March 31, 2019 and is valued at the premiums in effect on such date. These benefits are double-trigger benefits in that they will be paid only if the executive officer experiences a qualifying termination of employment following the Effective Time in accordance with the employment agreements and does not otherwise receive other healthcare coverage from a new employer prior to the end of the 12 month period. |
| (4) | The severance benefits prescribed by the employment agreements are subject to a Section 280Gbetter-off cutback provision, which provides that, in the event that the benefits provided to the named executive officer pursuant to the employment agreements or otherwise constitute parachute payments with the meaning of Section 280G of the Code, the severance benefits will either be delivered in full or reduced to the extent necessary to avoid an excise tax under Section 4999 of the Code, whichever would result in the named executive officer receiving the largest amount of severance benefits on anafter-tax basis. The amounts reported in this table do not reflect any such reductions as a result of the limit under Section 280G of the Code. |
Interests of Oncternal Directors and Executive Officers in the Merger
In considering the recommendation of the Oncternal Board with respect to adopting the Merger Agreement, Oncternal’s stockholders should be aware that certain members of the Oncternal Board and certain executive officers of Oncternal may have interests in the merger that may be different from, or in addition to, the interests of Oncternal’s stockholders. Each of the GTx Board and the Oncternal Board was aware of these potential conflicts of interest and considered them, among other matters, in reaching their respective decisions to approve the Merger Agreement and the merger, and to recommend, as applicable, that GTx’s stockholders approve the proposals to be presented to GTx’s stockholders for consideration at the GTx special meeting as contemplated by this proxy statement/prospectus/information statement, and that Oncternal’s stockholders sign and return the written consent as contemplated by this proxy statement/prospectus/information statement.
Ownership Interests
Certain of Oncternal’s directors and executive officers or entities affiliated with them currently hold shares of Oncternal’s capital stock, which such shares of capital stock will be converted into shares of GTx’s common stock at the Effective Time. The table below sets forth the ownership of Oncternal’s capital stock as of March 31, 2019 by Oncternal’s directors and executive officers and their anticipated ownership of Oncternal common stock immediately prior to the closing of the merger.
171
Directors and Named Executive Officers | Number of Shares of Capital Stock as of March 31, 2019 | Number of Shares of Capital Stock Immediately Prior to the Closing of the Merger | ||||||
Executive Officers | ||||||||
James B. Breitmeyer, M.D., Ph.D.(1) | 3,786,433 | 3,786,433 | ||||||
Richard G. Vincent(2) | 692,574 | 692,574 | ||||||
Hazel M. Aker(3) | 126,719 | 126,719 | ||||||
Non-Employee Directors | ||||||||
David F. Hale(4) | 9,780,554 | 9,780,554 | ||||||
Cooper Collins(5) | 16,888,889 | 16,888,889 | ||||||
Cam Gallagher(6) | 3,545,159 | 3,545,159 | ||||||
Scott Glenn(7) | 8,028,793 | 8,028,793 | ||||||
Yanjun Liu, M.D., Ph.D. | — | — | ||||||
Xin Nakanishi, Ph.D. | — | — | ||||||
William R. LaRue(8) | 356,677 | 356,677 | ||||||
Charles P. Theuer, M.D., Ph.D. | 200,000 | 200,000 | ||||||
| (1) | Consists of (i) 3,482,856 shares of common stock held directly by Dr. Breitmeyer, (ii) 293,577 shares of common stock held by a family trust (the “Breitmeyer Trust”) and (iii) 10,000 shares of common stock held by Dr. Breitmeyer as custodian for his child. Dr. Breitmeyer and Ms. Breitmeyer are the trustees of the Breitmeyer Trust, and in such capacity have joint power to vote and dispose of the shares held by the Breitmeyer Trust. |
| (2) | Consists of (i) 555,897 shares of common stock held directly by Mr. Vincent, including 315,512 shares subject to repurchase by Oncternal and (ii) 136,677 shares of common stock held by a family trust (the “Vincent Trust”). Mr. Vincent and his wife, Stacy Vincent, are the trustees of the Vincent Trust, and in such capacity have joint power to vote and dispose of the shares held by the Vincent Trust. |
| (3) | Consists of (i) 58,381 shares of common stock held directly by Ms. Aker and (ii) 68,338 shares of common stock held by a family trust (the “Aker Trust”). Ms. Aker and her husband, Larry Aker, are the trustees of the Aker Trust, and in such capacity have joint power to vote and dispose of the shares held by the Aker Trust. |
| (4) | Consists of (i) 9,530,554 shares of common stock held by Hale BioPharma Ventures, LLC and (ii) 250,000 shares of common stock held by Hale Trading Company. Mr. Hale is the Chairman and Chief Executive Officer of Hale BioPharma Ventures and the Managing Director of Hale Trading Company, and as such has voting and investment control over the shares held by Hale BioPharma Ventures and Hale Trading Company. |
| (5) | Consists of (i) 9,455,556 shares of common stock held by MagnaSci Fund, L.P., (ii) 2,444,445 shares of common stock held by MagnaSci Fund II, L.P. and (iii) 4,988,888 shares of common stock held by MagnaSciCo-Investments, L.L.C. MagnaSci GP, L.L.C. is the sole general partner of MagnaSci Fund and MagnaSci Fund II. Cooper Collins is a Manager of MagnaSci GP and MagnaSciCo-Investments, and has voting and investment power over the shares held by MagnaSci Fund, MagnaSci Fund II and MagnaSciCo-Investments. |
| (6) | Consists of (i) 3,345,159 shares of common stock, including 263,021 shares subject to repurchase by Oncternal, held directly by Mr. Gallagher and (ii) 200,000 shares of common stock held by Mr. Gallagher as custodian for his child. |
| (7) | Consists of 8,028,793 shares of common stock held by Glenn Holdings, L.P. Mr. Glenn is the General Partner of Glenn Holdings, and as such has voting and investment control over the shares held by Glenn Holdings. |
| (8) | Consists of (i) 220,000 shares of common stock held directly by Mr. LaRue, including 151,250 shares subject to repurchase by Oncternal, and (ii) 136,677 shares of common stock held by a family trust (the “LaRue Trust”). Mr. LaRue and his wife, Joyce LaRue, are the trustees of the LaRue Trust, and in such capacity have joint power to vote and dispose of the shares held by the LaRue Trust. |
172
Treatment of Oncternal Options and Warrants
Under the Merger Agreement, at the Effective Time, each outstanding and unexercised option or warrant to purchase shares of Oncternal’s capital stock as of immediately prior to the Effective Time, whether or not vested, shall be converted into and become an option or warrant, as applicable, to purchase shares of GTx’s common stock, in accordance with the terms and conditions of such Oncternal option or warrant, as applicable, immediately prior to the Effective Time. Certain of Oncternal’s directors and executive officers currently hold options, subject to vesting, to purchase shares of Oncternal’s common stock. The table below sets forth certain information with respect to such options.
Option holder Name | Grant Date | Expiration Date | Exercise Price ($) | Number of Shares of Common Stock Underlying Option as of March 31, 2019 | Number of Vested Shares of Common Stock Underlying Option as of March 31, 2019 | |||||||||||||||
James B. Breitmeyer | 9/1/2015 | 8/31/2025 | 0.05 | 1,600,000 | 1,300,000 | |||||||||||||||
| 11/14/2018 | 11/14/2028 | 0.06 | 2,300,000 | — | ||||||||||||||||
Richard G. Vincent | 11/14/2018 | 11/14/2028 | 0.06 | 1,000,000 | — | |||||||||||||||
Management Prior to and Following the Merger
As described elsewhere in this proxy statement/prospectus/information statement, including in the section captioned “Management Prior to and Following the Merger,” certain of Oncternal’s directors and executive officers are expected to become the directors and executive officers of GTx upon the closing of the merger.
Indemnification and Insurance
Under the Merger Agreement, from the Effective Time through the sixth anniversary of the date on which the Effective Time occurs, GTx and Oncternal, as the surviving corporation in the merger, shall indemnify and hold harmless each person who is or has served as a director or officer of Oncternal against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees and disbursements, incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that such person is or was a director or officer of Oncternal, to the fullest extent permitted under the DGCL for directors or officers of Delaware corporations. In addition, each such director and officer, or former director and officer, is entitled to advancement of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation.
Under the Merger Agreement, the provisions of GTx’s restated certificate of incorporation and amended and restated bylaws with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers of GTx shall not be amended, modified or repealed for a period of six years from the Effective Time in a manner that would adversely affect the rights thereunder of individuals who, at or prior to the Effective Time, were officers or directors of GTx. The certificate of incorporation and bylaws of Oncternal, as the surviving corporation in the merger, shall contain provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of former and present directors and officers that are presently set forth in the certificate of incorporation and bylaws of GTx.
The Merger Agreement also provides that GTx shall maintain directors’ and officers’ liability insurance policies commencing at the closing time of the merger, on commercially available terms and conditions with coverage limits customary for U.S. public companies similar situated to GTx.
173
Limitations of Liability and Indemnification
In addition to the indemnification obligations required by the restated certificate of incorporation and amended and restated bylaws of GTx, GTx has entered into indemnification agreements with each of its directors and officers. These agreements provide for the indemnification of GTx’s directors and executive officers for all reasonable expenses and liabilities incurred in connection with any action or proceeding brought against them by reason of the fact that they are or were agents of GTx. GTx believes that these restated certificate of incorporation provisions, amended and restated bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
Oncternal Stock Options and Warrants
As of March 31, 2019, an aggregate of 6,868,251 shares of Oncternal common stock were issuable upon the exercise of outstanding stock options under the Oncternal Therapeutics 2015 Equity Incentive Plan at a weighted-average exercise price of $0.06 per share. At the Effective Time, each Oncternal option that is outstanding and unexercised immediately prior to the Effective Time under the Oncternal Therapeutics 2015 Equity Incentive Plan, whether or not vested, will be converted into and become an option to purchase shares of GTx’s common stock, and GTx will assume the Oncternal Therapeutics 2015 Equity Incentive Plan and each such Oncternal option in accordance with the terms of the Oncternal Therapeutics 2015 Equity Incentive Plan and the terms of the stock option agreement by which such Oncternal option is evidenced.
As of March 31, 2019, an aggregate of 5,064,712 shares of Oncternal’s preferred stock were issuable upon the exercise of outstanding warrants at an exercise price of $0.45 per share. At the Effective Time, each Oncternal warrant that is outstanding and unexercised will become a warrant to purchase shares of GTx’s common stock and GTx will assume each Oncternal warrant in accordance with its terms.
Form of the Merger
The Merger Agreement provides that at the Effective Time, Merger Sub will be merged with and into Oncternal. Upon the consummation of the merger, Oncternal will continue as the surviving corporation and will be a wholly-owned subsidiary of GTx.
After completion of the merger, assuming Proposal No. 3 is approved by GTx’s stockholders at the GTx special meeting, GTx will be renamed “Oncternal Therapeutics, Inc.” and expects to trade on Nasdaq under the symbol “ONCT.”
Merger Consideration
At the Effective Time:
| • | each share of Oncternal common stock outstanding immediately prior to the Effective Time will automatically be converted into the right to receive a number of shares of GTx’s common stock equal to the exchange ratio, subject to adjustment to account for the GTx Reverse Stock Split (prior to the Effective Time, each share of Oncternal preferred stock will be converted into one share of Oncternal common stock); |
| • | each option to purchase shares of Oncternal’s common stock outstanding and unexercised immediately prior to the Effective Time will be assumed by GTx and will become an option, subject to vesting, to purchase shares of GTx’s common stock with the number of shares of GTx’s common stock underlying such options and the exercise prices for such options adjusted to reflect the exchange ratio and the GTx Reverse Stock Split; and |
| • | each warrant to purchase shares of Oncternal’s capital stock outstanding and not terminated or exercised as of immediately prior to the Effective Time will be assumed by GTx and will become a warrant to purchase shares of GTx’s common stock with the number of shares of GTx’s common stock |
174
underlying such warrants and the exercise prices for such warrants adjusted to reflect the exchange ratio and the GTx Reverse Stock Split. |
Immediately after the merger, based on the estimated exchange ratio, it is expected that Oncternal’s existing stockholders will own approximately 77.5% of the outstanding capital stock of GTx with GTx’s existing stockholders owning approximately 22.5% of the outstanding capital stock of GTx. The ownership percentage to be held by GTx’s stockholders is subject to adjustment prior to closing of the merger, including a downward adjustment to the extent that GTx’s “Parent Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement, which adjusts based on the date of closing (and as a result, GTx stockholders could own less, and Oncternal stockholders could own more, of the combined organization), or an upward adjustment to the extent that Oncternal’s “Company Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than $12,500,000 (and as a result, GTx stockholders could own more, and Oncternal stockholders could own less, of the combined organization). The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants.
The Merger Agreement does not include a price-based termination right, and there will be no adjustment to the total number of shares of GTx’s common stock that Oncternal’s stockholders will be entitled to receive for changes in the market price of GTx’s common stock. Accordingly, the market value of the shares of GTx’s common stock issued pursuant to the merger will depend on the market value of the shares of GTx’s common stock at the time the merger closes, and could vary significantly from the market value on the date of this proxy statement/prospectus/information statement.
No fractional shares of GTx’s common stock will be issuable to Oncternal’s stockholders pursuant to the merger. Instead, each stockholder of Oncternal who would otherwise be entitled to receive a fraction of a share of GTx’s common stock, after aggregating all fractional shares of GTx’s common stock issuable to such stockholder, will be entitled to receive in cash the dollar amount, rounded to the nearest whole cent, without interest, determined by multiplying such fraction by the volume weighted-average closing trading price of a share of GTx’s common stock on Nasdaq for the five consecutive trading days ending five trading days immediately prior to the date upon which the merger becomes effective.
The Merger Agreement provides that, at the Effective Time, GTx will deposit with an exchange agent acceptable to GTx and Oncternal certificates or evidence of book-entry shares representing the shares of GTx’s common stock issuable to Oncternal’s stockholders and a sufficient amount of cash to make payments in lieu of fractional shares.
The Merger Agreement provides that, promptly after the Effective Time, the exchange agent will mail to each record holder of Oncternal capital stock immediately prior to the Effective Time a letter of transmittal and instructions for surrendering and exchanging Oncternal stock certificates held by such record holder in exchange for certificates or book-entry shares of GTx’s common stock. Upon surrender of an Oncternal stock certificate for exchange to the exchange agent, together with a duly signed letter of transmittal and such other documents as the exchange agent or GTx may reasonably require, the Oncternal stock certificate surrendered will be cancelled and the holder of such Oncternal stock certificate will be entitled to receive the following:
| • | a certificate or certificates or book-entry shares representing the number of whole shares of GTx’s common stock that such holder has the right to receive pursuant to the provisions of the Merger Agreement, and |
| • | cash in lieu of any fractional share of GTx’s common stock. |
From and after the Effective Time, until it is surrendered, each certificate that previously evidenced shares of Oncternal common stock or shares of Oncternal’s preferred stock will be deemed to represent only the right to receive shares of GTx’s common stock, and cash in lieu of any fractional share of GTx’s common stock.
175
If any Oncternal stock certificate has been lost, stolen or destroyed, GTx may, in its discretion, and as a condition precedent to the delivery of any book-entry shares of GTx’s common stock, require the owner of such lost, stolen or destroyed certificate to provide an affidavit claiming such certificate has been lost, stolen or destroyed and that includes an obligation of such owner to indemnify GTx against any claim suffered by GTx related to the lost, stolen or destroyed Oncternal stock certificate as GTx may reasonably request.
GTx will not pay dividends or other distributions on any shares of GTx’s common stock to be issued in exchange for shares of Oncternal’s capital stock represented by any unsurrendered Oncternal stock certificate until such Oncternal stock certificate is surrendered as provided in the Merger Agreement.
Effective Time of the Merger
The Merger Agreement requires the parties to consummate the merger as promptly as practicable (and in any event within two business days) after all of the conditions to the consummation of the merger contained in the Merger Agreement are satisfied or waived. The merger will become effective upon the filing of a certificate of merger with the Secretary of State of the State of Delaware or at such later time as is agreed by GTx and Oncternal and specified in the certificate of merger. Neither GTx nor Oncternal can predict the exact timing of the consummation of the merger.
Regulatory Approvals
In the United States, GTx must comply with applicable federal and state securities laws and the rules and regulations of the Nasdaq Capital Market in connection with the issuance of shares of GTx’s common stock and the filing of this proxy statement/prospectus/information statement with the SEC.
Tax Treatment of the Merger
GTx and Oncternal intend the merger to qualify as a “reorganization” within the meaning of Section 368(a) of the Code. GTx and Oncternal have agreed to use their reasonable best efforts to cause the merger to qualify as a reorganization under Section 368(a) of the Code, and to not take any actions that are reasonably expected to cause the merger to fail to so qualify. For a description of certain of the considerations regarding U.S. federal tax consequences of the merger, see the section entitled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger” below.
Material U.S. Federal Income Tax Consequences of the Merger
The following discussion is a summary of the material U.S. federal income tax consequences of the merger to U.S. Holders (as defined below) who exchange their Oncternal common stock for GTx common stock in the merger, but does not purport to be a complete analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local ornon-U.S. tax laws are not discussed. This discussion is based on the Code, Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the IRS, in each case in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a U.S. Holder. Neither GTx nor Oncternal has sought or intend to seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a position regarding the tax consequences of the merger contrary to that discussed below. This discussion assumes that the merger will be consummated in accordance with the Merger Agreement and as described in this proxy statement/prospectus/information statement.
This discussion is limited to U.S. Holders that hold Oncternal common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a U.S. Holder’s particular circumstances, including the
176
impact of the alternative minimum tax or the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to U.S. Holders subject to special rules, including, without limitation:
| • | U.S. expatriates and former citizens or long-term residents of the United States; |
| • | U.S. Holders whose functional currency is not the U.S. dollar; |
| • | persons holding Oncternal common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| • | banks, insurance companies, and other financial institutions; |
| • | real estate investment trusts or regulated investment companies; |
| • | brokers, dealers or traders in securities; |
| • | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | S corporations, partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | persons for whom Oncternal common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code; |
| • | tax-exempt organizations or governmental organizations; |
| • | persons subject to special tax accounting rules as a result of any item of gross income with respect to Oncternal common stock being taken into account in an “applicable financial statement” (as defined in the Code); |
| • | persons deemed to sell Oncternal common stock under the constructive sale provisions of the Code; |
| • | persons who hold or received Oncternal common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
| • | tax-qualified retirement plans. |
If an entity treated as a partnership for U.S. federal income tax purposes holds Oncternal common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding Oncternal common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
THIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT TAX ADVICE. HOLDERS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE MERGER ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL ORNON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
For purposes of this discussion, a U.S. Holder is a beneficial owner of Oncternal common stock that, for U.S. federal income tax purposes, is or is treated as:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
177
| • | a trust that (i) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code) over all of its substantial decisions or (ii) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
U.S. Federal Income Tax Consequences of the Merger to U.S. Holders of Oncternal Common Stock
It is a condition to GTx’s obligation to consummate the merger that GTx receive an opinion from Cooley LLP, dated as of the closing date, to the effect that the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. It is a condition to Oncternal’s obligation to consummate the merger that Oncternal receive an opinion from Latham & Watkins LLP, dated as of the closing date, to the effect that the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code. Subject to the representations, assumptions and exclusions in such tax opinions, in the opinions of Cooley LLP and Latham & Watkins LLP, the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code.
These opinions will be based on customary assumptions and representations from GTx and Oncternal, as well as certain warranties, covenants and undertakings by GTx, Oncternal and Merger Sub (collectively, the “tax opinion representations and assumptions”). If any of the tax opinion representations and assumptions is incorrect, incomplete or inaccurate, or is violated, the validity of the opinions described above may be affected and the tax consequences of the merger could differ from those described in this proxy statement/prospectus/information statement.
An opinion of counsel represents counsel’s best legal judgment but is not binding on the IRS or any court, and there can be no certainty that the IRS will not challenge the conclusions reflected in the opinions or that a court would not sustain such a challenge. Neither GTx nor Oncternal intends to obtain a ruling from the IRS with respect to the tax consequences of the merger. If the IRS were to successfully challenge the “reorganization” status of the merger, the tax consequences would differ materially from those described in this proxy statement/prospectus/information statement.
Accordingly, on the basis of the opinions described above:
| • | a U.S. Holder of shares of Oncternal common stock generally will not recognize any gain or loss upon the exchange of shares of Oncternal common stock for shares of GTx common stock in the merger, except with respect to cash received in lieu of fractional shares (as discussed below); |
| • | a U.S. Holder of shares of Oncternal common stock will have a tax basis in the shares of GTx common stock received in the merger (including fractional shares deemed received and redeemed as described below) equal to the tax basis of the shares of Oncternal common stock surrendered in exchange therefor; |
| • | a U.S. Holder of shares of Oncternal common stock will have a holding period for the shares of GTx common stock received in the merger (including fractional shares deemed received and redeemed as described below) that includes its holding period for its shares of Oncternal common stock surrendered in exchange therefor; and |
| • | if a U.S. Holder of shares of Oncternal common stock acquired different blocks of shares of Oncternal common stock at different times or at different prices, the shares of GTx common stock received in the merger (including fractional shares deemed received and redeemed as described below) will be allocated pro rata to each block of shares of Oncternal common stock, and the basis and holding period of such shares of GTx common stock will be determined on ablock-for-block approach depending on the basis and holding period of each block of shares of Oncternal common stock exchanged for such shares of GTx common stock. |
178
Cash in Lieu of Fractional Shares
A U.S. Holder that receives cash in lieu of a fractional share of GTx common stock generally will be treated as having received such fractional share and then as having received such cash in redemption of the fractional share. Gain or loss generally will be recognized based on the difference between the amount of cash received in lieu of the fractional share of GTx common stock and the portion of the U.S. Holder’s aggregate adjusted tax basis in the shares of Oncternal common stock surrendered which is allocable to the fractional share of GTx common stock deemed received. Such gain or loss generally will be long-term capital gain or loss if the U.S. Holder’s holding period for its shares of Oncternal common stock exceeds one year at the Effective Time.
Tax Consequences if the Merger Fails to Qualify as a Reorganization
If the merger does not qualify as a “reorganization” within the meaning of Section 368(a) of the Code, a U.S. Holder of Oncternal common stock generally would recognize gain or loss for U.S. federal income tax purposes on each share of Oncternal common stock surrendered in the merger in an amount equal to the difference between the fair market value, at the time of the merger, of the GTx common stock received in the merger (including any cash received in lieu of a fractional share) and such U.S. Holder’s tax basis in the Oncternal common stock surrendered in the merger. Gain or loss must be calculated separately for each block of Oncternal common stock exchanged by such U.S. Holder if such blocks were acquired at different times or for different prices. Any gain or loss recognized generally would be capital gain or loss, and generally would be long-term capital gain or loss if the U.S. Holder’s holding period in a particular block of Oncternal common stock exceeds one year at the effective time of the merger. Long-term capital gain ofnon-corporate U.S. Holders (including individuals) generally is taxed at reduced U.S. federal income tax rates. The deductibility of capital losses is subject to limitations. A U.S. Holder’s tax basis in shares of GTx common stock received in the merger would be equal to the fair market value thereof as of the effective time of the merger, and such U.S. Holder’s holding period in such shares would begin on the day following the merger.
Information Reporting and Backup Withholding
If the merger qualifies as a “reorganization” under Section 368(a) of the Code, current Treasury Regulations require certain U.S. Holders who are “significant holders” of Oncternal common stock (generally, a U.S. Holder that owns at least 1% of the outstanding Oncternal common stock or has a basis in Oncternalnon-stock securities of at least $1,000,000 immediately before the merger) to comply with certain reporting requirements. Significant holders generally will be required to file a statement with their U.S. federal income tax returns for the taxable year in which the merger occurs setting forth certain information with respect to the transaction. U.S. Holders should consult their tax advisors to determine whether they are significant holders required to provide the foregoing statement. In addition, a U.S. Holder may be subject to information reporting and backup withholding when such holder receives cash in lieu of fractional shares of GTx common stock in the merger. Certain U.S. Holders are exempt from backup withholding, including corporations and certaintax-exempt organizations. A U.S. Holder will be subject to backup withholding if such holder is not otherwise exempt and:
| • | the holder fails to furnish the holder’s taxpayer identification number, which for an individual is ordinarily his or her social security number; |
| • | the holder furnishes an incorrect taxpayer identification number; |
| • | the applicable withholding agent is notified by the IRS that the holder previously failed to properly report payments of interest or dividends; or |
| • | the holder fails to certify under penalties of perjury that the holder has furnished a correct taxpayer identification number and that the IRS has not notified the holder that the holder is subject to backup withholding. |
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability, provided the required
179
information is timely furnished to the IRS. U.S. Holders should consult their tax advisors regarding their qualification for an exemption from backup withholding and the procedures for obtaining such an exemption.
Nasdaq Stock Market Listing
GTx’s common stock currently is listed on Nasdaq under the symbol “GTXI.” GTx has agreed to use commercially reasonable efforts to maintain its existing listing on Nasdaq, to obtain approval for listing on Nasdaq of the shares of GTx’s common stock that Oncternal’s stockholders will be entitled to receive pursuant to the merger and to obtain approval to have the combined company’s common stock listed on Nasdaq. In addition, under the Merger Agreement, each party’s obligation to complete the merger is subject to the satisfaction or waiver by each of the parties, at or prior to the merger, of various conditions, including that the existing shares of GTx’s common stock must have been continually listed on Nasdaq, and GTx must have caused the shares of GTx’s common stock to be issued in the merger to be approved for listing on Nasdaq as of the closing of the merger.
GTx has filed an initial listing application with Nasdaq pursuant to Nasdaq “reverse merger” rules. If such application is accepted, GTx anticipates that the shares of GTx’s common stock will be listed on Nasdaq following the closing of the merger under the trading symbol “ONCT.”
Anticipated Accounting Treatment
The merger will be recorded by GTx as a reverse asset acquisition in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). For accounting purposes, Oncternal is considered to be acquiring GTx in this transaction. The transaction is expected to be accounted for as a reverse asset acquisition as the fair value of the acquired preclinical assets is deemed to be substantially concentrated in a group of similar assets that do not meet the definition of a business under existing U.S. GAAP, which are subject to change and interpretation. Under the reverse asset acquisition method of accounting, management of GTx and Oncternal have made a preliminary estimated purchase price calculated as described in Note 2 to the Notes to the Unaudited Pro Forma Condensed Combined Financial Information. The net tangible and intangible assets acquired and liabilities assumed in connection with the transaction are at their estimated acquisition date fair values. The reverse asset acquisition method of accounting is dependent upon certain valuations and other studies that have yet to commence or progress to a stage where there is sufficient information for a definitive measurement. A final determination of these estimated fair values, which cannot be made prior to the completion of the transaction, will be based on the actual net tangible and intangible assets of GTx that exist as of the date of completion of the transaction.
Appraisal Rights
Delaware Law
If the merger is completed, Oncternal’s stockholders who do not deliver a written consent approving the merger are entitled to appraisal rights under Section 262 of the DGCL (“Section 262”),provided that they comply with the conditions established by Section 262. Holders of GTx common stock are not entitled to dissenter’s rights under Delaware law or other appraisal rights in connection with the merger.
The discussion below is not a complete summary regarding the appraisal rights of Oncternal’s stockholders under Delaware law and is qualified in its entirety by reference to the text of the relevant provisions of Delaware law, which are attached to this proxy statement/prospectus/information statement asAnnex C. Stockholders intending to exercise appraisal rights should carefully reviewAnnex Cof this proxy statement/prospectus/information statement. Failure to follow precisely any of the statutory procedures set forth inAnnex Cof this proxy statement/prospectus/information statement may result in a termination or waiver of these rights. This summary does not constitute legal or other advice, nor does it constitute a recommendation that Oncternal’s stockholders exercise their appraisal rights under Delaware law.
180
Under Section 262, where a merger is adopted by stockholders by written consent in lieu of a meeting of stockholders pursuant to Section 228 of the DGCL, either the constituent corporation, before the effective date of the merger, or the surviving corporation, within 10 days after the effective date of the merger, must notify each stockholder of the constituent corporation entitled to appraisal rights, if any, of the approval of the merger, the effective date of the merger and that appraisal rights are available.
If the merger is completed, within 10 days after the effective date of the merger Oncternal will notify its stockholders that the merger has been approved, the effective date of the merger and that appraisal rights are available to any stockholder who has not approved the merger, if any. Holders of shares of Oncternal capital stock who desire to exercise their appraisal rights must deliver a written demand for appraisal to Oncternal within 20 days after the date of mailing of that notice, and the stockholder must not have delivered a written consent approving the merger. A demand for appraisal must reasonably inform Oncternal of the identity of the stockholder and that such stockholder intends thereby to demand appraisal of the shares of Oncternal capital stock held by such stockholder. Failure to deliver a written consent approving the merger will not in and of itself constitute a written demand for appraisal satisfying the requirements of Section 262. All demands for appraisal should be addressed to Oncternal Therapeutics, Inc., 12230 El Camino Real, Ste 300, San Diego, California 92130, Attention: General Counsel, and should be executed by, or on behalf of, the record holder of shares of Oncternal capital stock. ALL DEMANDS MUST BE RECEIVED BY ONCTERNAL WITHIN TWENTY (20) DAYS AFTER THE DATE ONCTERNAL MAILS A NOTICE TO ITS STOCKHOLDERS NOTIFYING THEM THAT THE MERGER HAS BEEN APPROVED, THE EFFECTIVE DATE OF THE MERGER AND THAT APPRAISAL RIGHTS ARE AVAILABLE TO ANY STOCKHOLDER WHO HAS NOT APPROVED THE MERGER, IF ANY.
If a holder of shares of Oncternal’s capital stock fails to deliver a written demand for appraisal within the time period specified above, such holder will be entitled to receive the merger consideration for such holder’s shares of Oncternal capital stock as provided for in the Merger Agreement, but will have no appraisal rights with respect to his, her or its shares of Oncternal’s capital stock.
To be effective, a demand for appraisal by a holder of shares of Oncternal’s capital stock must be made by, or in the name of, the registered stockholder, fully and correctly, as the stockholder’s name appears on the stockholder’s stock certificate(s). Beneficial owners who do not also hold the shares of record may not directly make appraisal demands to Oncternal. The beneficial owner must, in these cases, have the registered owner, such as a broker, bank or other custodian, submit the required demand in respect of those shares. If shares are owned of record in a fiduciary capacity, such as by a trustee, guardian or custodian, execution of a demand for appraisal should be made by or for the fiduciary; and if the shares are owned of record by more than one person, as in a joint tenancy or tenancy in common, the demand should be executed by or for all joint owners. An authorized agent, including an authorized agent for two or more joint owners, may execute the demand for appraisal for a stockholder of record; however, the agent must identify the record owner or owners and expressly disclose the fact that, in executing the demand, he or she is acting as agent for the record owner. A record owner, such as a broker, who holds shares as a custodian for others, may exercise the record owner’s right of appraisal with respect to the shares held for one or more beneficial owners, while not exercising this right for other beneficial owners. In that case, the written demand should state the number of shares as to which appraisal is sought. Where no number of shares is expressly mentioned, the demand will be presumed to cover all shares held in the name of the record owner. In addition, the stockholder must continuously hold the shares of record from the date of making the demand through the Effective Time.
If a holder of shares of Oncternal’s capital stock holds shares of Oncternal’s capital stock in a brokerage account or in other custodian form and such holder wishes to exercise appraisal rights, such holder should consult with such holder’s bank, broker or other custodian to determine the appropriate procedures for the making of a demand for appraisal by the custodian.
181
At any time within 60 days after the Effective Time, any stockholder who has demanded an appraisal, but has neither commenced an appraisal proceeding or joined an appraisal proceeding as a named party, has the right to withdraw such stockholder’s demand and accept the terms of the merger by delivering a written withdrawal to Oncternal. If, following a demand for appraisal, a holder of shares of Oncternal’s capital stock who has demanded an appraisal has withdrawn such holder’s demand for appraisal in accordance with Section 262, such holder will have the right to receive the merger consideration for such holder’s shares of Oncternal capital stock.
Within 120 days after the Effective Time, any stockholder who has delivered a demand for appraisal in accordance with Section 262 will, upon written request to the surviving corporation, be entitled to receive a written statement setting forth the aggregate number of shares not voted in favor of the Merger Agreement and with respect to which demands for appraisal rights have been received and the aggregate number of holders of such shares. This written statement will be mailed to the requesting stockholder within ten days after the stockholder’s written request is received by the surviving corporation or within ten days after expiration of the period for delivery of demands for appraisal, whichever is later. Within 120 days after the Effective Time, either the surviving corporation or any stockholder who has delivered a demand for appraisal in accordance with Section 262 may file a petition in the Delaware Court of Chancery demanding a determination of the fair value of the shares held by all such stockholders. Upon the filing of the petition by a stockholder, service of a copy of the petition must be made upon the surviving corporation. The surviving corporation has no obligation to file a petition in the Delaware Court of Chancery in the event there are dissenting stockholders, and Oncternal, which is expected to be the surviving corporation, has no present intent to file a petition in the Delaware Court of Chancery. Accordingly, the failure of a stockholder to file a petition within the period specified could nullify the stockholder’s previously written demand for appraisal.
If a petition for appraisal is duly filed by a stockholder and a copy of the petition is delivered to the surviving corporation, the surviving corporation will then be obligated, within 20 days after receiving service of a copy of the petition, to provide the Delaware Court of Chancery with a duly verified list containing the names and addresses of all stockholders who have demanded an appraisal of their shares and with whom agreements as to the value of their shares have not been reached by the surviving corporation. After notice to dissenting stockholders who demanded appraisal of their shares, if any, the Delaware Court of Chancery is empowered to conduct a hearing upon the petition, and to determine those stockholders who have complied with Section 262 and who have become entitled to the appraisal rights provided thereby. The Delaware Court of Chancery may require the stockholders who have demanded appraisal for their shares to submit their stock certificates to the Register in Chancery for notation thereon of the pendency of the appraisal proceedings; and if any stockholder fails to comply with that direction, the Delaware Court of Chancery may dismiss the proceedings as to that stockholder. If immediately before the merger the shares of the class or series of stock as to which appraisal rights are available were listed on a national securities exchange, the Delaware Court of Chancery will dismiss the proceedings as to all holders of such shares who are otherwise entitled to appraisal rights unless (1) the total number of shares entitled to appraisal exceeds 1% of the outstanding shares of the class or series eligible for appraisal, (2) the value of the consideration provided in the merger for such total number of shares exceeds $1.0 million or (3) the merger was approved pursuant to Sections 253 or 267 of the DGCL.
After determination of the stockholders entitled to appraisal of their shares, the Delaware Court of Chancery will appraise the “fair value” of the shares owned by those stockholders. This value will be exclusive of any element of value arising from the accomplishment or expectation of the merger, but may include a fair rate of interest, if any, upon the amount determined to be the fair value. At any time before the entry of judgment in the proceedings, the surviving corporation may pay to each shareowner entitled to appraisal an amount in cash, in which case interest shall accrue thereafter only upon the sum of (1) the difference, if any, between the amount paid and the fair value of the shares as determined by the Delaware Court of Chancery, and (2) interest theretofore accrued, unless paid at that time. When the value is determined, the Delaware Court of Chancery will direct the payment of the value, with interest thereon accrued during the pendency of the proceeding, if the Delaware Court of Chancery so determines, to the stockholders entitled to receive the same, upon surrender by the holders of the certificates representing those shares.
182
In determining fair value, and, if applicable, a fair rate of interest, the Delaware Court of Chancery is required to take into account all relevant factors. InWeinberger v. UOP, Inc., the Delaware Supreme Court discussed the factors that could be considered in determining fair value in an appraisal proceeding, stating that “proof of value by any techniques or methods which are generally considered acceptable in the financial community and otherwise admissible in court” should be considered, and that “fair price obviously requires consideration of all relevant factors involving the value of a company.”
Section 262 provides that fair value is to be “exclusive of any element of value arising from the accomplishment or expectation of the merger.” InCede & Co. v. Technicolor, Inc., the Delaware Supreme Court stated that this exclusion is a “narrow exclusion [that] does not encompass known elements of value,” but which rather applies only to the speculative elements of value arising from such accomplishment or expectation. InWeinberger, the Delaware Supreme Court construed Section 262 to mean that “elements of future value, including the nature of the enterprise, which are known or susceptible of proof as of the date of the merger and not the product of speculation, may be considered.”
Holders of shares of Oncternal’s capital stock should be aware that the fair value of such holder’s shares as determined under Section 262 could be more than, the same as, or less than the value that such holder is entitled to receive under the terms of the Merger Agreement.
Costs of the appraisal proceeding may be imposed upon the surviving corporation and the stockholders participating in the appraisal proceeding by the Delaware Court of Chancery as the Court deems equitable in the circumstances. Upon the application of a stockholder, the Delaware Court of Chancery may order all or a portion of the expenses incurred by any stockholder in connection with the appraisal proceeding, including, without limitation, reasonable attorneys’ fees and the fees and expenses of experts, to be charged pro rata against the value of all shares entitled to appraisal. In the absence of such a determination of assessment, each party bears its own expenses. Any stockholder who had demanded appraisal rights will not, after the Effective Time, be entitled to vote shares subject to that demand for any purpose or to receive payments of dividends or any other distribution with respect to those shares, other than with respect to payment as of a record date prior to the Effective Time; however, if no petition for appraisal is filed within 120 days after the Effective Time, or if the stockholder delivers a written withdrawal of his or her demand for appraisal and an acceptance of the terms of the merger within 60 days after the Effective Time, then the right of that stockholder to appraisal will cease and that stockholder will be entitled to receive the merger consideration for shares of his or her Oncternal capital stock pursuant to the Merger Agreement. Any withdrawal of a demand for appraisal made more than 60 days after the Effective Time may only be made with the written approval of the surviving corporation. No appraisal proceeding in the Delaware Court of Chancery will be dismissed as to any stockholder without the approval of the court.
Failure to follow the steps required by Section 262 for perfecting appraisal rights may result in the loss of appraisal rights. In view of the complexity of Section 262, stockholders who may wish to dissent from the merger and pursue appraisal rights should consult their legal advisors.
Litigation Related to the Merger
On April 10, 2019, a purported stockholder of GTx commenced a putative class action lawsuit captionedWhebyv. GTx, Inc. et al. in the U.S. District Court for the District of Delaware, naming as defendants GTx, Michael G. Carter, J. Kenneth Glass, Marc S. Hanover, J. R. Hyde, III, Garry A. Neil, Kenneth S. Robinson, and Robert J. Wills, collectively comprising the members of the GTx Board, its CEO (who is also a director), and the Chairman of the Board, Oncternal, and Merger Sub (the “Wheby Action”).
On April 11, 2019, a purported stockholder of GTx commenced a putative class action lawsuit captionedMiller v. GTx, Inc. et al. in the U.S. District Court for the District of Delaware, naming as defendants GTx, the GTx Board, Oncternal, and Merger Sub (the “Miller Action”).
183
On April 11, 2019, a purported stockholder of GTx commenced a putative class action lawsuit captionedKopanic v. GTx, Inc. et al. in the U.S. District Court for the Southern District of New York, naming as defendants GTx and the GTx Board (the “Kopanic Action”).
On April 23, 2019, a purported stockholder of GTx commenced a putative class action lawsuit captionedTabb v. GTx, Inc. et al. in the U.S. District Court for the District of Delaware, naming as defendants GTx and the GTx Board (the “Tabb Action”).
On May 1, 2019, a purported stockholder of GTx commenced a putative class action lawsuit captionedLiving Seas LLC v. GTx, Inc. et al. in the U.S. District Court for the District of Delaware, naming as defendants GTx and the GTx Board (the “Living Seas Action,” together with the Tabb Action, the Kopanic Action, the Miller Action, and the Wheby Action, the “Recent Actions”).
Collectively, the Recent Actions allege violations of sections 14(a) and 20(a) of the Exchange Act, as well as Rule 14a-9 promulgated thereunder, in connection with GTx’s filing of the registration statement of which this proxy statement/prospectus/information statement is a part with the U.S. Securities and Exchange Commission.
The Living Seas, Wheby, Kopanic and Miller actions each separately assert that the registration statement is materially deficient and misleading because it failed to disclose information regarding (i) GTx’s and Oncternal’s financial projections, (ii) communications and purported conflicts of interest between Oncternal and members of the GTx Board and management regarding their future employment with the combined successor company, and (iii) the confidentiality agreements entered into between GTx and other potential strategic partners during the process leading up the signing of the Merger Agreement. The Tabb Action asserts that the registration statement is materially deficient and misleading on the basis of assertions (ii) and (iii), as contained in the preceding sentence.
As relief, the Recent Actions each separately seek an order, among other things, enjoining the defendants from closing the proposed transaction or taking any steps to consummate the merger and/or awarding rescissory damages.
GTx and the GTx Board believe that the above-described claims are without merit and intend to vigorously defend these actions. GTx cannot predict the outcome of or estimate the possible loss or range of loss from any of these matters. It is possible that additional, similar complaints may be filed or the complaints described above will be amended. If this occurs GTx does not intend to announce the filing of each additional, similar complaint or any amended complaint unless it contains allegations that are substantially distinct from those made in the pending actions described above.
184
The following is a summary of the material terms of the Merger Agreement. A copy of the Merger Agreement is attached as Annex A to this proxy statement/prospectus/information statement and is incorporated by reference into this proxy statement/prospectus/information statement. The Merger Agreement has been attached to this proxy statement/prospectus/information statement to provide you with information regarding its terms. It is not intended to provide any other factual information about GTx, Oncternal or Merger Sub. The following description does not purport to be complete and is qualified in its entirety by reference to the Merger Agreement. You should refer to the full text of the Merger Agreement for details of the merger and the terms and conditions of the Merger Agreement.
The Merger Agreement contains representations and warranties that GTx and Merger Sub, on the one hand, and Oncternal, on the other hand, have made to one another as of specific dates. These representations and warranties have been made for the benefit of the other parties to the Merger Agreement and may be intended not as statements of fact but rather as a way of allocating the risk to one of the parties if those statements prove to be incorrect. In addition, the assertions embodied in the representations and warranties are qualified by information in confidential disclosure schedules exchanged by the parties in connection with signing the Merger Agreement. While GTx and Oncternal do not believe that these disclosure schedules contain information required to be publicly disclosed under the applicable securities laws, other than information that has already been so disclosed, the disclosure schedules do contain information that modifies, qualifies and creates exceptions to the representations and warranties set forth in the attached Merger Agreement. Accordingly, you should not rely on the representations and warranties as current characterizations of factual information about GTx or Oncternal, because they were made as of specific dates, may be intended merely as a risk allocation mechanism between GTx, Merger Sub and Oncternal and are modified by the disclosure schedules.
General
Under the Merger Agreement, at the Effective Time, Merger Sub will merge with and into Oncternal, with Oncternal surviving as a wholly-owned subsidiary of GTx.
Merger Consideration
Prior to the Effective Time, each share of Oncternal’s preferred stock will be converted into one share of Oncternal common stock. At the Effective Time, each share of Oncternal’s common stock outstanding immediately prior to the Effective Time (excluding shares of Oncternal’s capital stock held as treasury stock or held by Oncternal, Merger Sub or any subsidiary of Oncternal, and shares held by Oncternal stockholders who have exercised and perfected appraisal rights) will automatically be converted into the right to receive a number of shares of GTx’s common stock equal to the exchange ratio.
The Merger Agreement does not include a price-based termination right and there will be no adjustment to the total number of shares of GTx’s common stock that Oncternal’s stockholders, optionholders and warrantholders will be entitled to receive for changes in the market price of GTx’s common stock. Accordingly, the market value of the shares of GTx’s common stock issued pursuant to the merger will depend on the market value of the shares of GTx’s common stock at the time the merger closes, and could vary significantly from the market value on the date of this proxy statement/prospectus/information statement.
No fractional shares of GTx’s common stock will be issuable to Oncternal’s stockholders pursuant to the Merger Agreement. Instead, each stockholder of Oncternal who would otherwise be entitled to receive a fraction of a share of GTx’s common stock, after aggregating all fractional shares of GTx’s common stock issuable to such stockholder, will be entitled to receive in cash the dollar amount, rounded to the nearest whole cent, without interest, determined by multiplying such fraction by volume weighted-average closing trading price of a share of GTx’s common stock on Nasdaq for the five consecutive trading days ending five trading days immediately prior to the date upon which the merger becomes effective.
185
The Merger Agreement provides that, at the Effective Time, GTx will deposit with an exchange agent acceptable to GTx and Oncternal certificates and evidence of book-entry shares representing GTx’s common stock issuable to Oncternal’s stockholders and a sufficient amount of cash to make payments in lieu of fractional shares.
The Merger Agreement provides that, promptly after the Effective Time, the exchange agent will mail to each record holder of Oncternal’s capital stock immediately prior to the Effective Time a letter of transmittal and instructions for surrendering and exchanging stock certificates representing shares of Oncternal’s capital stock held by such record holder in exchange for book-entry shares of GTx’s common stock. Upon surrender of a stock certificate representing shares of Oncternal’s capital stock for exchange to the exchange agent, together with a duly signed letter of transmittal and such other documents as the exchange agent or GTx may reasonably require, the stock certificate surrendered will be cancelled and the holder of such stock certificate will be entitled to receive the following:
| • | a certificate or certificates or book-entry shares representing the number of whole shares of GTx’s common stock that such holder has the right to receive pursuant to the provisions of the Merger Agreement; and |
| • | cash in lieu of any fractional share of GTx’s common stock. |
At the Effective Time, all holders of certificates representing shares of Oncternal’s capital stock that were outstanding immediately prior to the Effective Time will cease to have any rights as stockholders of Oncternal. In addition, no transfer of Oncternal’s capital stock after the Effective Time will be registered on the stock transfer books of Oncternal.
If any stock certificate representing shares of Oncternal’s capital stock has been lost, stolen or destroyed, GTx may, in its discretion, and as a condition to the delivery of any book-entry shares of GTx’s common stock, require the owner of such lost, stolen or destroyed certificate to deliver an affidavit claiming such certificate has been lost, stolen or destroyed and indemnify GTx against any claim suffered by GTx related to the lost, stolen or destroyed certificate or any of GTx’s common stock issued in exchange for such certificate as GTx may reasonably request.
From and after the Effective Time, until it is surrendered, each certificate that previously evidenced shares of Oncternal’s capital stock will be deemed to represent only the right to receive book-entry shares of GTx’s common stock and cash in lieu of any fractional share of GTx’s common stock. GTx will not pay dividends or other distributions on any shares of GTx’s common stock to be issued in exchange for any unsurrendered stock certificate representing shares of Oncternal until the stock certificate is surrendered as provided in the Merger Agreement.
Treatment of GTx’s Stock Awards and Warrants
Prior to the closing of the merger, the GTx Board will adopt appropriate resolutions and take all other actions necessary and appropriate to provide that the vesting of each unexpired and unexercised option to purchase shares of GTx’s common stock will be accelerated in full effective as of immediately prior to the Effective Time. The number of shares of GTx’s common stock underlying such options and the exercise prices for such options will be appropriately adjusted to reflect the GTx Reverse Stock Split.
Warrants to purchase shares of GTx’s common stock will remain outstanding according to their terms. The number of shares of GTx’s common stock underlying warrants and the exercise prices for such warrants will be appropriately adjusted to reflect the GTx Reverse Stock Split.
Under the Merger Agreement, as of immediately prior to the closing of the merger (but in no event more than 30 days prior to the Effective Time), GTx shall take all actions necessary to cause the termination and liquidation of the GTx Director Deferred Compensation Plan, and all deferred stock rights thereunder, effective immediately
186
prior to the closing of the merger, subject to the consummation of the merger (the “GTx Deferred Stock Rights”). GTx shall also ensure that any deferrals under the GTx Director Deferred Compensation Plan on or after January 3, 2019 shall be settled only in cash and that the maximum number of shares of common stock of GTx issuable upon settlement of the GTx Deferred Stock Rights shall be limited to the number of GTx Deferred Stock Rights outstanding as of the date of the Merger Agreement.
Treatment of Oncternal’s Awards Options and Warrants
At the Effective Time:
| • | each option to purchase shares of Oncternal’s capital stock outstanding and unexercised immediately prior to the Effective Time under the Oncternal Therapeutics 2015 Equity Incentive Plan, whether or not vested, will be converted into an option to purchase shares of GTx’s common stock. GTx will assume the Oncternal Therapeutics 2015 Equity Incentive Plan. From and after the Effective Time, each Oncternal option assumed by GTx may be exercised for such number of shares of GTx’s common stock as is determined by multiplying the number of shares of Oncternal’s common stock subject to the option by the exchange ratio and rounding that result down to the nearest whole number of shares of GTx’s common stock. The per share exercise price of the converted option will be determined by dividing the existing exercise price of the option by the exchange ratio and rounding that result up to the nearest whole cent. Any restrictions on the exercise of any Oncternal option assumed by GTx will continue following the conversion and the term, exercisability, vesting schedules and other provisions of assumed Oncternal options will generally remain unchanged; provided, that any Oncternal options assumed by GTx may be subject to adjustment to reflect changes in GTx’s capitalization after the Effective Time and that the GTx Board will succeed to the authority of the Oncternal Board with respect to each assumed Oncternal option; and |
| • | each warrant to purchase shares of Oncternal capital stock outstanding and unexercised immediately prior to the Effective Time will be assumed by GTx and will become a warrant to purchase that number of shares of GTx’s common stock equal to the product obtained by multiplying (i) the number of shares of Oncternal’s common stock, or the number of shares of Oncternal’s common stock issuable upon conversion of the shares of Oncternal’s preferred stock issuable upon exercise of the Oncternal warrant, as applicable, that were subject to such warrant immediately prior to the Effective Time by (ii) the exchange ratio and rounding that result down to the nearest whole share. The per share exercise price for GTx’s common stock issuable upon exercise of each Oncternal warrant assumed by GTx shall be determined by dividing (a) the per share exercise price of the Oncternal preferred stock subject to such Oncternal warrant, as in effect immediately prior to the Effective Time, by (b) the exchange ratio and rounding that result up to the nearest whole cent. Any restriction on any Oncternal warrant assumed by GTx shall continue in full force and effect and the terms and other provisions of such Oncternal warrant shall otherwise remain unchanged. |
In addition, pursuant to the Merger Agreement, at the Effective Time, each restricted share of Oncternal common stock that is outstanding will be converted into a share of GTx on the same basis as other shares of Oncternal common stock. Any restrictions on such restricted shares will continue in full force and effect and the vesting schedule and other provisions of such Oncternal restricted shares shall otherwise remain unchanged.
Directors and Officers of GTx Following the Merger
Pursuant to the Merger Agreement, each of the directors and officers of GTx who will not continue as directors or officers of GTx or the combined organization following the consummation of the merger, shall resign effective upon the closing of the merger. In connection with the merger, the GTx Board will be expanded to include a total nine directors. Pursuant to the terms of the Merger Agreement, two of such directors will be designated by GTx, two of such directors will be designated by SPH USA, Oncternal’s largest stockholder prior to the merger, one of such directors will be the Chairman of the combined organization, one of such directors
187
will be the Chief Executive Officer of the combined organization and the remaining three such directors as indicated in the Merger Agreement. It is anticipated that Michael G. Carter, M.D., Ch.B., F.R.C.P. and Robert J. Wills, Ph.D. will remain as directors of GTx following the closing of the merger, with Dr. Carter remaining among Class III directors, and that all other GTx directors will resign as of the Effective Time. Drs. Carter and Wills shall appoint the remaining directors to the GTx Board to fill the resulting vacancies. David F. Hale is expected to be appointed to the board as Chairman of the board of directors and James B. Breitmeyer, M.D., Ph.D. is expected to be appointed to the board pursuant to his role as Chief Executive Officer. It is anticipated that Yanjun Liu, Ph.D. and Xin Nakanishi, Ph.D. will be appointed as the designees of SPH USA and that Charles P. Theuer, M.D., Ph.D., William R. LaRue and Daniel L. Kisner, M.D. will be appointed to the remaining three director positions. It is anticipated that GTx’s executive officers upon the closing of the merger will be Dr. Breitmeyer, President and Chief Executive Officer, Richard G. Vincent, Chief Financial Officer and Hazel M. Aker, General Counsel.
Amendment to the Restated Certificate of Incorporation of GTx
Stockholders of record of GTx’s common stock on the record date for the GTx special meeting will also be asked to approve Proposal Nos. 2 and 3, which include a series of alternative amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split and the GTx Name Change, in each case, upon consummation of the merger, each of which requires the affirmative vote of holders of shares representing a majority of all shares of GTx’s common stock outstanding on the record date for the GTx special meeting.
Conditions to the Completion of the Merger
Each party’s obligation to complete the merger is subject to the satisfaction or waiver by each of the parties, at or prior to the merger, of various conditions, which include the following:
| • | the registration statement on FormS-4, of which this proxy statement/prospectus/information statement is a part, must have been declared effective by the SEC in accordance with the Securities Act and must not be subject to any stop order or proceeding, or any proceeding threatened by the SEC, seeking a stop order that has not been withdrawn; |
| • | there must not have been issued, and remain in effect, any temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the merger or any of the other transactions contemplated by the Merger Agreement by any court of competent jurisdiction or other governmental entity of competent jurisdiction, and no law, statute, rule, regulation, ruling or decree shall be in effect which has the effect of making the consummation of the merger or any of the other transactions contemplated by the Merger Agreement illegal; |
| • | the holders of a (i) a majority of the outstanding shares of Oncternal’s common stock and preferred stock, voting together as one class, (ii) at least 60% of the outstanding shares of Oncternal’s preferred stock, voting together as a single class, (iii) at least a majority of the outstanding shares of Oncternal’s Series A preferred stock, voting as a separate class, (iv) a majority of the outstanding shares of Oncternal’s Series B preferred stock and SeriesB-2 preferred stock, voting together as a single class, and (v) at least 70% of the shares of Oncternal’s Series C preferred stock, voting as a separate class, must have adopted and approved the merger; |
| • | the holders of a majority of the outstanding shares of GTx’s common stock having voting power present in person or represented by proxy at the GTx special meeting must have approved Proposal No. 1, the approval of the Merger Agreement and the transactions contemplated thereby, including the merger and the issuance of GTx’s common stock in the merger; |
| • | the existing shares of GTx’s common stock must have been continually listed on Nasdaq through the closing of the merger, and GTx must have caused the shares of GTx’s common stock to be issued in the merger to be approved for listing on Nasdaq (subject to official notice of issuance) as of the closing of the merger; and |
188
| • | all applicable waiting periods (and any extension thereof) applicable to the merger under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 shall must have expired or early termination of such waiting periods must have been granted and all applicable foreign antitrust approvals must have been obtained. |
In addition, each party’s obligation to complete the merger is subject to the satisfaction or waiver by that party of the following additional conditions:
| • | the representations and warranties regarding certain matters related to organization, authority, vote required, capitalization and financial advisors of the other party in the Merger Agreement must be true and correct in all material respects on the date of the Merger Agreement and on the closing date of the merger with the same force and effect as if made on the date on which the merger is to be completed or, if such representations and warranties address matters as of a particular date, then as of that particular date; |
| • | the remaining representations and warranties of the other party in the Merger Agreement must be true and correct on the date of the Merger Agreement and on the closing date of the merger with the same force and effect as if made on the date on which the merger is to be completed or, if such representations and warranties address matters as of a particular date, then as of that particular date, except in each case, or in the aggregate, where the failure to be so true and correct would not reasonably be expected to have a Company Material Adverse Effect or Parent Material Adverse Effect (each as defined in the Merger Agreement), as applicable (without giving effect to any references therein to any Company Material Adverse Effect or Parent Material Adverse Effect, as applicable, or other materiality qualifications); |
| • | the other party to the Merger Agreement must have performed or complied with in all material respects all of such party’s agreements and covenants required to be performed or complied with by it under the Merger Agreement at or prior to the Effective Time; |
| • | the other party must have delivered certain certificates and other documents required under the Merger Agreement for the closing of the merger; |
| • | the party must have received from the other partylock-up agreements executed by certain stockholders of such party (including any stockholder of Oncternal expected to own more than 10% of the outstanding common stock of the combined organization after the merger) and each person who shall be elected or appointed as an executive officer or director of such party immediately following the closing; |
| • | the party must have received the opinion of its legal counsel, dated as of the closing date of the merger, to the effect that the merger will be treated, for U.S. federal income tax purposes, as a reorganization within the meaning of Section 368(a) of the Code; and |
| • | the party must have received a copy of the opinion of the other party’s legal counsel, and dated as of the closing date of the merger, to the effect that the merger will be treated, for U.S. federal income tax purposes, as a reorganization within the meaning of Section 368(a) of the Code. |
In addition, the obligation of GTx and Merger Sub to complete the merger is further subject to the satisfaction or waiver of the following conditions:
| • | there shall have been no effect, change, event, circumstance, or development that (considered together with all other effects, changes, events, circumstances, or developments that have occurred prior to the applicable date of determination) has or would reasonably be expected to have a material adverse effect on the business, financial condition, assets, liabilities or results of operations of Oncternal or its subsidiaries, taken as a whole (a “Company Material Adverse Effect”); provided that effects, changes, |
189
events, circumstances or developments resulting from the following shall not be taken into account for purposes of determining whether a Company Material Adverse Effect shall have occurred: |
| • | any general business, economic or political conditions affecting the industry in which Oncternal or its subsidiaries operate; |
| • | any natural disaster or any acts of war, armed hostilities or terrorism; |
| • | any changes in financial, banking or securities markets; |
| • | any failure of Oncternal to meet internal or analysts’ expectations or projections or the results of Oncternal; |
| • | any clinical trial programs or studies, including any adverse data, event or outcome arising out of or relating to any such programs or studies; |
| • | any change in, or any compliance with or action taken for the purpose of complying with any law or U.S. GAAP; |
| • | resulting from the announcement of the Merger Agreement or the pendency of the transactions contemplated by the Merger Agreement; or |
| • | resulting from the taking of any action, or the failure to take any action, by Oncternal that is required to be taken pursuant to the Merger Agreement. |
| • | GTx shall have received (i) an original signed statement from Oncternal that Oncternal is not, and has not been at any time during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code, a “United States real property holding corporation,” as defined in Section 897(c)(2) of the Code, conforming to the requirements of Treasury RegulationsSection 1.1445-2(c)(3) and1.897-2(h), and (ii) an original signed notice to be delivered to the IRS in accordance with the provisions of Treasury RegulationsSection 1.897-2(h)(2), together with written authorization for GTx to deliver such notice to the IRS on behalf of Oncternal following the closing of the merger, each dated as of the closing date of the merger, duly executed by an authorized officer of Oncternal, and in form and substance reasonably acceptable to GTx; |
| • | certain agreements between Oncternal and its stockholders must have been terminated; and |
| • | all Oncternal preferred stock must have been converted to Oncternal common stock. |
In addition, the obligation of Oncternal to complete the merger is further subject to the satisfaction or waiver of the following conditions:
| • | there shall have been no effect, change, event, circumstance, or development that (considered together with all other effects, changes, circumstances, or developments that have occurred prior to the applicable date of determination) has or would reasonably be expected to have a material adverse effect on the business, financial condition, assets, liabilities or results of operations of GTx and its subsidiaries, taken as a whole (a “Parent Material Adverse Effect”); provided, that effects, changes, events, circumstances or developments resulting from the following shall not be taken into account for purposes of determining whether a Parent Material Adverse Effect shall have occurred: |
| • | any general business, economic or political conditions affecting the industry in which GTx operates; |
| • | any natural disaster or any acts of war, armed hostilities or terrorism; |
| • | any changes in financial, banking or securities markets; |
| • | any change in the stock price or trading volume of GTx common stock (it being understood, however, that any effects, changes, events, circumstances or developments causing or contributing to any change in stock price or trading volume of GTx common stock may be taken into account in determining whether a Parent Material Adverse Effect has occurred, unless such effects, changes, events, circumstances or developments or otherwise are specifically excepted); |
190
| • | any failure of GTx to meet internal or analysts’ expectations or projections or the results of GTx; |
| • | any clinical trial programs or studies, including any adverse data, event or outcome arising out of or relating to any such programs or studies; |
| • | any change in, or any compliance with or action taken for the purpose of complying with any law or U.S. GAAP; |
| • | resulting from the announcement of the Merger Agreement or the pendency of the transactions contemplated by the Merger Agreement; or |
| • | resulting from the taking of any action, or the failure to take any action, by GTx that is required to be taken pursuant to the Merger Agreement. |
| • | Oncternal must have received the resignations of each of the officers and directors of GTx who are not to continue as officers and directors of the combined organization after the merger; and |
| • | GTx must have caused the GTx board of directors to be constituted as required by the Merger Agreement. |
Representations and Warranties
The Merger Agreement contains customary representations and warranties of GTx and Oncternal for a transaction of this type relating to, among other things:
| • | corporate organization and power, and similar corporate matters; |
| • | subsidiaries; |
| • | authority to enter into the Merger Agreement and the related agreements; |
| • | votes required for completion of the merger and approval of the proposals that will come before the GTx special meeting and that will be the subject of Oncternal’s stockholder written consent; |
| • | except as otherwise specifically disclosed pursuant to in the Merger Agreement, the fact that the consummation of the merger would not contravene or require the consent of any third-party; |
| • | capitalization; |
��
| • | financial statements and with respect to GTx, documents filed with the SEC and the accuracy of information contained in those documents; |
| • | material changes or events; |
| • | liabilities; |
| • | title to assets; |
| • | real property and leaseholds; |
| • | intellectual property; |
| • | the validity of material contracts to which the parties or their subsidiaries are a party and any violation, default or breach to such contracts; |
| • | regulatory compliance, permits and restrictions; |
| • | legal proceedings and orders; |
| • | tax matters; |
| • | employee and labor matters and benefit plans; |
| • | environmental matters; |
191
| • | insurance; |
| • | any brokerage or finder’s fee or other fee or commission in connection with the merger; |
| • | transactions with affiliates; |
| • | anti-bribery laws; and |
| • | with respect to GTx, the valid issuance in the merger of GTx’s common stock and the opinion of Aquilo. |
The representations and warranties are, in many respects, qualified by materiality and knowledge, and will not survive the merger, but their accuracy forms the basis of one of the conditions to the obligations of GTx and Oncternal to complete the merger.
No Solicitation
Each of GTx and Oncternal agreed that during the period commencing on the date of the Merger Agreement and ending on the earlier of the consummation of the merger or the termination of the Merger Agreement, except as described below, GTx and Oncternal and any of their respective subsidiaries will not, nor will either party or any of its subsidiaries authorize any of the directors, officers, employees, agents, attorneys, accountants, investment bankers, advisors or representatives retained by it or any of its subsidiaries to, directly or indirectly:
| • | solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of, any “acquisition proposal” or “acquisition inquiry” or take any action that could reasonably be expected to lead to an acquisition proposal or acquisition inquiry; |
| • | furnish anynon-public information with respect to it to any person in connection with or in response to an acquisition proposal or acquisition inquiry; |
| • | engage in discussions or negotiations with any person with respect to any acquisition proposal or acquisition inquiry; |
| • | approve, endorse or recommend an acquisition proposal; |
| • | execute or enter into any letter of intent or similar document or any contract contemplating or otherwise relating to any acquisition transaction (other than a confidentiality agreement permitted by the Merger Agreement); or |
| • | publicly propose to do any of the above. |
An “acquisition inquiry” means an inquiry, indication of interest or request for information (other than an inquiry, indication of interest or request for information made or submitted by Oncternal, on the one hand, or GTx, on the other hand, to the other party) that would reasonably be expected to lead to an acquisition proposal.
An “acquisition proposal” means any offer or proposal, whether written or oral (other than an offer or proposal made or submitted by or on behalf of Oncternal or any of its affiliates, on the one hand, or by or on behalf of GTx or any of its affiliates, on the other hand, to the other party) contemplating or otherwise relating to any “acquisition transaction.”
An “acquisition transaction” means any transaction or series of related transactions involving:
| • | any merger, consolidation, amalgamation, share exchange, business combination, issuance or acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or similar transaction: (i) in which GTx, Oncternal or Merger Sub is a constituent entity, (ii) in which any individual, entity, governmental entity, or “group,” as defined under applicable securities laws, directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the |
192
outstanding securities of any class of voting securities of GTx, Oncternal or Merger Sub or any of their respective subsidiaries or (iii) in which GTx, Oncternal or Merger Sub or any of their respective subsidiaries issues securities representing more than 20% of the outstanding securities of any class of voting securities of such party or any of its subsidiaries; or |
| • | any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for 20% or more of the consolidated book value or the fair market value of the assets of GTx, Oncternal or Merger Sub and their respective subsidiaries, as applicable, taken as a whole. |
Notwithstanding the foregoing, before obtaining the applicable approvals of the stockholders of GTx or Oncternal required to consummate the merger, as applicable, each party may furnishnon-public information regarding such party and its subsidiaries to, and may enter into discussions or negotiations with, any third-party in response to a bona fide acquisition proposal made or received after the date of the Merger Agreement, which such party’s board of directors determines in good faith, after consultation with such party’s outside financial advisors or outside legal counsel, constitutes or is reasonably likely to result in a “superior offer,” as defined below, if:
| • | neither such party nor any representative of such party has materially breached the solicitation provisions of the Merger Agreement described above; |
| • | such party’s board of directors concludes in good faith, based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to be inconsistent with the fiduciary duties of such board of directors under applicable legal requirements; |
| • | such party gives the other party at least two business days’ prior written notice of the identity of the third-party and of that party’s intention to furnish information to, or enter into discussions with, such third-party before furnishing any information or entering into discussions with such third-party; |
| • | such party receives from the third-party an executed confidentiality agreement containing provisions at least as favorable to such party as those contained in the confidentiality agreement between GTx and Oncternal; and |
| • | at least two business days prior to the furnishing of anynon-public information to a third-party, such party furnishes the samenon-public information to the other party to the extent not previously furnished. |
A “superior offer” means an unsolicited, bona fide written acquisition proposal (with all references to 20% in the definition of acquisition transaction being treated as references to greater than 80% for these purposes) that (a) was not obtained or made as a direct or indirect result of a breach, or violation, of the Merger Agreement, and (b) is on terms and conditions that the board of directors of the party receiving the offer determines in good faith, based on such matters that it deems relevant (including the likelihood of consummation of the transaction), as well as any written offer by the other party to the Merger Agreement to amend the terms of the Merger Agreement, and following consultation with outside legal counsel and outside financial advisors, if any, are more favorable, from a financial point of view, to that party’s stockholders than the terms of the merger. An acquisition proposal will not be considered a superior offer if any financing required to consummate the transaction contemplated by such acquisition proposal is not reasonably capable of being obtained by such third-party.
The Merger Agreement also provides that each party will promptly advise the other of the status and terms of, and keep the other party reasonably informed with respect to, any acquisition proposal or any inquiry, indication of interest or request for information that would reasonably be expected to lead to an acquisition proposal or any material change or proposed material change to that acquisition proposal or inquiry, indication of interest or request for information that would reasonably be expected to lead to an acquisition proposal.
193
Meetings of Stockholders
GTx is obligated under the Merger Agreement to call, give notice of and hold the GTx special meeting for the purposes of considering the approval of the Merger Agreement and the transactions contemplated thereby, including the merger and the issuance of shares of GTx’s common stock to Oncternal’s stockholders in the merger.
Oncternal is obligated under the Merger Agreement to obtain written consents of its stockholders sufficient to adopt the Merger Agreement thereby approving the merger and related transactions within ten business days following the registration statement on FormS-4, of which this proxy statement/prospectus/information statement is a part, being declared effective by the SEC.
Covenants; Conduct of Business Pending the Merger
GTx has agreed that, except as permitted by the Merger Agreement, as required by law, or unless Oncternal shall have provided written consent, during the period commencing on the date of the Merger Agreement and continuing until the earlier to occur of the closing of the merger and the termination of the Merger Agreement, GTx will conduct its business and operations in the ordinary course consistent with past practices and in compliance with all applicable laws, regulations and certain contracts, and to take other agreed-upon actions. GTx has also agreed that, subject to certain limited exceptions, without the consent of Oncternal, it will not, during the period commencing on the date of the Merger Agreement and continuing until the earlier to occur of the closing of the merger and the termination of the Merger Agreement:
| • | declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock or repurchase, redeem or otherwise reacquire any shares of capital stock or other securities (except in connection with the payment of withholding taxes incurred upon the exercise, settlement or vesting of any award granted under a GTx employee benefit plan in accordance with the terms of such award in effect on the date of the Merger Agreement); |
| • | sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: any capital stock or other security (except for GTx’s common stock issued upon the valid exercise of outstanding options or warrants to purchase shares of GTx’s common stock); any option, warrant or right to acquire any capital stock or any other security; or any instrument convertible into or exchangeable for any capital stock or other security of GTx; |
| • | except as required to give effect to anything in contemplation of the closing of the merger, amend the certificate of incorporation, bylaws or other charter or organizational documents of GTx, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except as related to the proposed transactions under the Merger Agreement; |
| • | form any subsidiary or acquire any equity interest or other interest in any other entity or enter into any joint venture with any other entity; |
| • | lend money to any person; incur or guarantee any indebtedness for borrowed money; guarantee any debt securities of others; or make any capital expenditure or commitment in excess of the amounts set forth in GTx’s operating budget delivered to Oncternal concurrently with the Merger Agreement; |
| • | other than as required by law or the terms of a GTx employee plan in effect as of the date of the Merger Agreement, adopt, terminate, establish or enter into any GTx employee plan; cause or permit any GTx employee plan to be amended in any material respect, other than approval of the GTx 2019 Plan; pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its employees, officers or directors; increase the severance, retention or change of control benefits offered to any current or former or new employees, directors or consultants; hire or retain any new officer, employees or consultants; or terminate or give notice of termination to any officer or employee, other than termination for cause; |
194
| • | recognize any labor union, labor organization, or similar entity except as otherwise required by law and after advance notice to Oncternal; |
| • | enter into any transaction other than in the ordinary course of business; |
| • | enter into any transaction with respect to the SARD Compound or SARM Compound (each, as defined in the CVR Agreement); |
| • | acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any encumbrance with respect to such assets or properties; |
| • | make, change or revoke any material tax election, fail to pay any income or other material tax as such tax becomes due and payable, file any amendment making any material change to any tax return, settle or compromise any income or other material tax liability, enter into any tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the ordinary course of business the principal subject matter of which is not taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material taxes (other than pursuant to an extension of time to file any tax return granted in the ordinary course of business of not more than six months), or adopt or change any material accounting method in respect of taxes; |
| • | enter into, materially amend or terminate certain material contracts; |
| • | except as otherwise set forth in the GTx operating budget delivered to Oncternal concurrently with the execution of the Merger Agreement (and other than incurrence or payment of GTx transaction expenses up to an aggregate of $100,000 in excess of the amount budgeted for the aggregate GTx transaction expenses in the GTx operating budget provided to Oncternal), make any expenditures, incur any liabilities or discharge or satisfy any liabilities, in each case, in amounts that exceed the aggregate amount of the GTx operating budget; |
| • | other than as required by law or U.S. GAAP, take any action to change accounting policies or procedures; |
| • | initiate or settle any legal proceeding; or |
| • | agree, resolve or commit to do any of the foregoing. |
Oncternal has agreed that, except as permitted by the Merger Agreement, as required by law, or unless GTx shall have provided written consent, during the period commencing on the date of the Merger Agreement and continuing until the earlier to occur of the closing of the merger and the termination of the Merger Agreement, Oncternal will conduct its business and operations in the ordinary course consistent with past practices and in compliance with all applicable laws, regulations and certain contracts, and to take other agreed-upon actions. Oncternal has also agreed that, subject to certain limited exceptions, without the consent of GTx, it will not, during the period commencing on the date of the Merger Agreement and continuing until the earlier to occur of the closing of the merger and the termination of the Merger Agreement:
| • | declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock of Oncternal or repurchase, redeem or otherwise reacquire any shares of capital stock or other securities (except for shares of Oncternal common stock from terminated employees, directors or consultants of Oncternal); |
| • | except as required to give effect to anything in contemplation of the closing of the merger, amend the certificate of incorporation, bylaws or other charter or organizational documents of Oncternal or its subsidiaries, or effect or become a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except as related to the proposed transactions under the Merger Agreement; |
| • | sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing actions with respect to: any capital stock or other security of Oncternal or any of its subsidiaries (except for |
195
shares of Oncternal common stock issued upon the valid exercise of Oncternal options or warrants); any option, warrant or right to acquire any capital stock or any other security; or any other instrument convertible into or exchangeable for any capital stock or any other security of Oncternal or its subsidiaries; |
| • | except as required to give effect to anything in contemplation of the closing of the merger, amend the certificate of incorporation, bylaws or other charter or organizational documents of Oncternal or its subsidiaries, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except as related to the proposed transactions under the Merger Agreement; |
| • | form any subsidiary or acquire any equity interest or other interest in any other entity or enter into a joint venture with any other entity; |
| • | lend money to any person; incur or guarantee any indebtedness for borrowed money; guarantee any debt securities of others; or make any capital expenditure or commitment in excess of $500,000; |
| • | other than as required by applicable law or the terms of any Oncternal employee benefit plan: adopt, terminate, establish or enter into any employee plan; cause or permit any employee plan to be amended in any material respect; pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees; increase the severance or change of control benefits offered to any current or new employees, directors or consultants; or terminate or give notice of termination to any officer or any employee whose annual base salary is expected to be more than $125,000 per year, other than any termination for cause; |
| • | recognize any labor union, labor organization or similar entity, except as otherwise required by law and after advance notice to GTx; |
| • | enter into any transaction other than in the ordinary course of business; |
| • | acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any encumbrance with respect to such assets or properties; |
| • | sell, assign, transfer, license, sublicense or otherwise dispose of any material Oncternal intellectual property rights (other than pursuant tonon-exclusive licenses in the ordinary course of business); |
| • | make, change or revoke any material tax election, fail to pay any income or other material tax as such tax becomes due and payable, file any amendment making any material change to any tax return, settle or compromise any income or other material tax liability, enter into any tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the ordinary course of business the principal subject matter of which is not taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material taxes (other than pursuant to an extension of time to file any tax return granted in the ordinary course of business of not more than six months), or adopt or change any material accounting method in respect of taxes; |
| • | enter into, materially amend or terminate certain material contracts; |
| • | other than incurrence or payment of any Oncternal transaction expenses, make any expenditures, incur any liabilities or discharge or satisfy any liabilities, in each case, in amounts that exceed $500,000 in the aggregate; |
| • | other than as required by law or U.S. GAAP, take any action to change accounting policies or procedures; |
| • | initiate or settle any legal proceeding; or |
| • | agree, resolve or commit to do any of the foregoing. |
196
Other Agreements
Each of GTx and Oncternal has agreed to use its commercially reasonable efforts to cause to be taken all actions necessary to consummate the merger and the other transactions contemplated by the Merger Agreement. In connection therewith, each party has agreed to: file or otherwise submit all applications and notices required to be filed in connection with the merger and the other transactions contemplated by the Merger Agreement;
| • | use commercially reasonable efforts to obtain each consent reasonably required to be obtained in connection with the merger and the other transactions contemplated by the Merger Agreement; |
| • | use commercially reasonable efforts to lift any injunction prohibiting, or any other legal bar to, the merger or the other transactions contemplated by the Merger Agreement; and |
| • | use commercially reasonable efforts to satisfy the conditions precedent to the consummation of the transactions contemplated by the Merger Agreement. |
Pursuant to the Merger Agreement, GTx and Oncternal have further agreed that:
| • | GTx will use its commercially reasonable efforts to (i) maintain the listing of its common stock on Nasdaq until the closing of the merger and to obtain approval for listing of the combined organization on Nasdaq and (ii) to the extent required by the rules and regulations of Nasdaq, to prepare and submit to Nasdaq a notification form for the listing of the shares of GTx common stock to be issued in connection with the merger and to cause such shares to be approved for listing (subject to official notice of issuance); (iii) to effect the GTx Reverse Stock Split; and (iv) to the extent required by Nasdaq Marketplace Rule 5110, to file an initial listing application for GTx’s common stock on Nasdaq and to cause such listing application to be conditionally approved prior to the Effective Time; |
| • | for a period of six years after the closing of the merger, GTx will indemnify each of the directors and officers of GTx and Oncternal to the fullest extent permitted under the DGCL and will maintain directors’ and officers’ liability insurance for the directors and officers of GTx and Oncternal; and |
| • | GTx shall maintain directors’ and officers’ liability insurance policies commencing at the closing of the merger, on commercially reasonable terms and conditions and with coverage limits customary for U.S. public companies similarly situated to GTx. |
Termination
The Merger Agreement may be terminated at any time before the completion of the merger, whether before or after the required stockholder approvals to complete the merger have been obtained, as set forth below:
| • | by mutual written consent of GTx and Oncternal; |
| • | by either GTx or Oncternal if the merger shall not have been consummated by August 6, 2019 (the “End Date”); provided, however, that this right to terminate the Merger Agreement will not be available to any party whose action or failure to act has been a principal cause of the failure of the merger to occur on or before the End Date and such action or failure to act constitutes a breach of the Merger Agreement; and provided, further, that the End Date shall be extended by 60 days upon request of either party if a request for additional information has been made by any government authority, or in the event that the SEC has not declared effective the registration statement on FormS-4, of which this proxy statement/prospectus/information statement is a part, by such date; |
| • | by either GTx or Oncternal if a court of competent jurisdiction or governmental entity has issued a final and nonappealable order, decree or ruling or taken any other action that has the effect of permanently restraining, enjoining or otherwise prohibiting the merger or any of the other transactions contemplated by the Merger Agreement; |
| • | by GTx if the Required Oncternal Stockholder Approval has not been obtained within the later of (i) 15 business days of the registration statement on FormS-4, of which this proxy statement/prospectus/ |
197
information statement is a part, becoming effective or (ii) the date on which GTx stockholders have approved Proposal Nos. 1 and 2; provided that this right to terminate the Merger Agreement will not be available to GTx once Oncternal obtains such stockholder approval; |
| • | by either GTx or Oncternal if the GTx special meeting shall have been held and completed and GTx’s stockholders shall have taken a final vote and shall not have approved Proposal Nos. 1 and 2; provided, that GTx may not terminate the Merger Agreement pursuant to this provision if the failure to obtain the approval of GTx’s stockholders was caused by the action or failure to act of GTx or Merger Sub and such action or failure to act constitutes a material breach by GTx or Merger Sub of the Merger Agreement; |
| • | by Oncternal, at any time prior to the approval by GTx’s stockholders of the proposals to be considered at the GTx special meeting, if any of the following circumstances shall occur (each of the following, a “GTx triggering event”): |
| • | The GTx Board fails to recommend that the stockholders of GTx vote to approve Proposal Nos. 1 and 2 or withdraws or modifies its recommendation in a manner adverse to Oncternal; |
| • | GTx fails to include in this proxy statement/prospectus/information statement such recommendation; |
| • | The GTx Board, or any committee thereof, publicly approves, endorses or recommends any acquisition proposal; |
| • | GTx enters into any letter of intent or similar document or any contract relating to any acquisition proposal, other than a confidentiality agreement permitted pursuant to the Merger Agreement; or |
| • | GTx or any director, officer or agent of GTx willfully and intentionally breaches the no solicitation provisions or the provisions regarding the GTx special meeting set forth in the Merger Agreement; |
| • | by GTx, at any time prior to the adoption of the Merger Agreement by Oncternal’s stockholders, if any of the following circumstances shall occur (each an “Oncternal triggering event”): |
| • | The Oncternal Board fails to recommend that Oncternal’s stockholders vote to adopt the Merger Agreement, thereby approving the merger, or withdraws or modifies its recommendation in a manner adverse to GTx; |
| • | The Oncternal Board, or any committee thereof, publicly approves, endorses or recommends any acquisition proposal; |
| • | Oncternal enters into any letter of intent or similar document or any contract relating to any acquisition proposal; or |
| • | Oncternal or any director, officer or agent of Oncternal willfully and intentionally breaches the no solicitation provisions set forth in the Merger Agreement; or |
| • | by GTx or Oncternal if the other party has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of the other party has become inaccurate, in either case such that the conditions to the closing of the merger would not be satisfied as of time of such breach or inaccuracy, but if such breach or inaccuracy is curable, then the Merger Agreement will not terminate pursuant to this provision as a result of a particular breach or inaccuracy until the expiration of a15-day period after delivery of written notice of such breach. |
198
Termination Fee
Fee payable by GTx
GTx must pay Oncternal a termination fee of $2.0 million if:
| • | the Merger Agreement is terminated by either GTx or Oncternal if the GTx special meeting shall have been held and completed, and GTx’s stockholders shall have not approved Proposal Nos. 1 and 2; or |
| • | the Merger Agreement is terminated by GTx after the End Date and GTx’s stockholders have not approved Proposal Nos. 1 and 2. |
GTx must pay Oncternal a termination fee of $1.0 million if the Merger Agreement is terminated by Oncternal if (i) prior to the GTx stockholder approval of Proposal Nos. 1 and 2, a GTx triggering event shall have occurred, (ii) at any time after the date of Merger Agreement and before the termination of the Merger Agreement, an acquisition proposal with respect to GTx was publicly announced, disclosed or otherwise communicated to the board of directors of GTx, and (iii) within 12 months after the date of such termination, GTx enters into a definitive agreement for or consummates an acquisition transaction.
GTx must pay Oncternal a termination fee of $500,000 if the Merger Agreement is terminated by Oncternal because GTx or Merger Sub has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of GTx or Merger Sub has become inaccurate, in either case such that the conditions to the closing of the merger would not be satisfied as of the time of such breach or inaccuracy, subject to a15-day cure period.
Fee payable by Oncternal
Oncternal must pay GTx a termination fee of $2.0 million if:
| • | the Merger Agreement is terminated by GTx if the Required Oncternal Stockholder Approval has not been obtained within the later of (i) 15 business days of the registration statement on FormS-4, of which this proxy statement/prospectus/information statement is a part, becoming effective or (ii) the date on which GTx stockholders have approved Proposal Nos. 1 and 2; or |
| • | the Merger Agreement is terminated by Oncternal after the End Date and Oncternal has not obtained the Required Oncternal Stockholder Approval at the time of such termination. |
Oncternal must pay GTx a termination fee of $1.0 million if the Merger Agreement is terminated by Oncternal if (i) prior to obtaining the Required Oncternal Stockholder Approval, an Oncternal triggering event shall have occurred, (ii) at any time after the date of Merger Agreement and before the termination of the Merger Agreement, an acquisition proposal with respect to Oncternal was publicly announced, disclosed or otherwise communicated to the board of directors of Oncternal, and (iii) within 12 months after the date of such termination, Oncternal enters into a definitive agreement for or consummates an acquisition transaction.
Oncternal must pay GTx a termination fee of $500,000 if the Merger Agreement is terminated by GTx because Oncternal has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of Oncternal has become inaccurate, in either case such that the conditions to the closing of the merger would not be satisfied as of the time of such breach or inaccuracy, subject to a15-day cure period.
Amendment
The Merger Agreement may be amended by the parties at any time if such amendment is in writing, is approved by the boards of directors of each party to the Merger Agreement and is signed by each party to the Merger Agreement, except that after the Merger Agreement has been adopted and approved by the stockholders of GTx or Oncternal, no amendment which by law requires further approval by the stockholders of GTx or Oncternal, as the case may be, shall be made without such further approval.
199
AGREEMENTS RELATED TO THE MERGER
CVR Agreement
Prior to the closing of the merger, GTx, Marc Hanover, as representative of holders of the CVRs, and a rights agent will enter into the CVR Agreement. Pursuant to the CVR Agreement, GTx stockholders will receive one CVR for each share of GTx common stock held of record immediately prior to the Effective Time, after giving effect to the GTx Reverse Stock Split. Each CVR will represent the right to receive payments based on GTx’s SARD or SARM technology. In particular, CVR holders will be entitled to, in the aggregate, 75% of any net proceeds received during the15-year period after the Closing from the grant, sale or transfer of rights to GTx’s SARD or SARM technology that occurs during the10-year period after the Closing (or in the 11th year if based on a term sheet approved during the initial10-year period) and, if applicable, to receive royalties on the sale of any SARD or SARM products by the combined company during the15-year period after the Closing. In order to be eligible for the CVR, a GTx stockholder must be a holder of record as of immediately prior to the Effective Time.
As further discussed in the section titled “The Merger–Background of the Merger,” GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2) in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. In connection with the receipt of the new preclinical data, in addition to amending the Merger Agreement, GTx and Oncternal amended the form of CVR Agreement to, among other things: (i) increase from 50% to 75% the portion of the net proceeds the CVR holders will be entitled to under the CVR Agreement, and (ii) provide that Oncternal (as successor in interest to GTx) will be obligated to use commercially reasonable efforts to either develop or divest GTx’s SARD technology, as the Oncternal Board shall determine in its sole discretion, and to divest its SARM technology, subject to certain limitations. Accordingly, Oncternal may decide, in its sole discretion, to abandon the development of GTx’s SARD technology following the merger and would then be obligated only to use commercially reasonable efforts to divest the SARD technology, subject to certain limitations. Likewise, Oncternal is obligated only to use commercially reasonable efforts to divest the SARM technology, subject to certain limitations, and in light of the results of the ASTRID trial, Oncternal has no current intent to develop the SARM technology.
The sole right of the holders of CVRs is to receive cash from GTx, if any, through the rights agent in accordance with the CVR Agreement. The CVRs will not have any voting or dividend rights, will not represent any equity or ownership interest in GTx or its subsidiaries, and interest will not accrue on any amounts payable on the CVRs. The CVRs will not be transferable, except in certain limited circumstances, will not be certificated or evidenced by any instrument and will not be registered with the SEC or any state and will not listed for trading on any exchange.
Material U.S. Federal Income Tax Consequences of the Receipt of CVRs
The following discussion is a summary of the material U.S. federal income tax consequences of the receipt of CVRs to GTx U.S. Holders (as defined below) who receive CVRs with respect to GTx common stock, but this
200
discussion does not purport to be a complete analysis of all potential tax consequences that may be relevant to a GTx U.S. Holder. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local ornon-U.S. tax laws are not discussed. This discussion is based on the Code, Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the IRS, in each case in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a GTx U.S. Holder. GTx has not sought and does not intend to seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a position contrary to that discussed below regarding the tax consequences of the receipt of CVRs.
This discussion is limited to GTx U.S. Holders that hold GTx common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a GTx U.S. Holder’s particular circumstances, including the impact of the alternative minimum tax or the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to GTx U.S. Holders subject to special rules, including, without limitation:
| • | U.S. expatriates and former citizens or long-term residents of the United States; |
| • | GTx U.S. Holders whose functional currency is not the U.S. dollar; |
| • | persons holding GTx common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| • | banks, insurance companies, and other financial institutions; |
| • | real estate investment trusts or regulated investment companies; |
| • | brokers, dealers or traders in securities; |
| • | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | S corporations, partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | persons for whom GTx common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code; |
| • | tax-exempt organizations or governmental organizations; |
| • | persons subject to special tax accounting rules as a result of any item of gross income with respect to GTx common stock being taken into account in an “applicable financial statement” (as defined in the Code); |
| • | persons deemed to sell GTx common stock under the constructive sale provisions of the Code; |
| • | persons who hold or received GTx common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
| • | tax-qualified retirement plans. |
If an entity treated as a partnership for U.S. federal income tax purposes holds GTx common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding GTx common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
201
THIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT TAX ADVICE. HOLDERS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE RECEIPT OF CVRs ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL ORNON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
For purposes of this discussion, a GTx U.S. Holder is a beneficial owner of GTx common stock that, for U.S. federal income tax purposes, is or is treated as:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust that (i) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code) over all of its substantial decisions or (ii) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
Receipt of CVRs by GTx U.S. Holders
Although the matter is not free from doubt, GTx intends to treat the receipt of CVRs and the GTx Reverse Stock Split as separate transactions for U.S. federal income tax purposes, and the following discussion assumes this treatment will be respected.
There is no authority directly addressing whether contingent value rights with characteristics similar to the CVRs should be treated as a distribution of property with respect to the corporation’s stock, a distribution of equity, a “debt instrument” or an “open transaction” for U.S. federal income tax purposes. Under applicable U.S. tax principles such questions are inherently factual in nature. Based on the specific characteristics of the CVRs, GTx intends to report the issuance of the CVRs as a distribution of property with respect to its stock. GTx U.S. Holders are urged to consult their tax advisors regarding the tax consequences to them of the receipt of CVRs.
Specifically, GTx intends to report the issuance of the CVRs to GTx U.S. Holders as a distribution of property with respect to its stock, because the CVRs will be issued to all holders of GTx common stock prior to completion of the merger. Each GTx U.S. Holder will be treated as receiving a distribution in an amount equal to the fair market value of the CVRs issued to such GTx U.S. Holder on the date of the issuance. This distribution generally should be treated first as a taxable dividend to the extent of the GTx U.S. Holder’s pro rata share of GTx’s current or accumulated earnings and profits (as determined for U.S. federal income tax purposes), then as anon-taxable return of capital to the extent of the GTx U.S. Holder’s basis in its GTx common stock, and finally as capital gain from the sale or exchange of GTx common stock with respect to any remaining value. GTx currently has negative accumulated earnings and profits and expects no or a small amount of current earnings and profits for the relevant taxable year. Thus, GTx expects most or all of this distribution to be treated as other than a dividend for U.S. federal income tax purposes. GTx U.S. Holders will receive a Form1099-DIV notifying them of the portion of the CVR value that is treated as a dividend for U.S. federal income tax purposes. A GTx U.S. Holder’s initial tax basis in such holder’s CVRs should equal the fair market value of such CVRs on the date of their issuance. The holding period of such CVRs should begin on the day after the date of issuance.
As a result of the above treatment, future payments received by a GTx U.S. Holder on a CVR would likely be treated as anon-taxable return of such GTx U.S. Holder’s adjusted tax basis in the CVR to the extent thereof, and payments in excess of such amount would likely be treated as ordinary income.
202
However, the treatment of such future payments is uncertain and alternative treatments are possible, although not expected. One such possible treatment is that the CVRs could be treated as one or more “debt instruments.” If that were to be the case, then payments received with respect to the CVRs generally would likely treated as payments in retirement of a “debt instrument,” except to the extent interest is imputed under the Code. If those rules were to apply, interest generally should be imputed under complex rules. In such a case, a GTx U.S. Holder would be required to include any such interest in income on an annual basis, whether or not currently paid.
It is possible, although GTx believes unlikely, that the issuance of the CVRs could be treated as a distribution of equity for U.S. federal income tax purposes, in which case GTx U.S. Holders should not recognize gain or loss as a result of the issuance of the CVRs. Depending on the fair market value of the CVRs on the date of their issuance, each GTx U.S. Holder’s tax basis in such holder’s GTx common stock would be allocated between such holder’s GTx common stock and such holder’s CVRs. The holding period of such CVRs should include the GTx U.S. Holder’s holding period of such holder’s GTx common stock. Future payments on a CVR received by a GTx U.S. Holder would likely be treated as dividends to the extent of the GTx U.S. Holder’s pro rata share of GTx’s current or accumulated earnings and profits (as determined for U.S. federal income tax purposes), then as anon-taxable return of capital to the extent of the GTx U.S. Holder’s basis in the CVR, and finally as capital gain from the sale or exchange of the CVR with respect to any remaining value. As discussed above, GTx does not intend to report the issuance of the CVRs as a distribution of equity and any GTx U.S. Holder reporting the CVR issuance as a distribution of equity likely faces an increased chance of being audited by the IRS with respect to such reporting.
It is possible, although again GTx believes unlikely, that the issuance of the CVRs could be treated as subject to the “open transaction” doctrine if the value of the CVRs on the closing date cannot be “reasonably ascertained.” If the receipt of CVRs were treated as an “open transaction” for U.S. federal income tax purposes, each GTx U.S. Holder should not immediately take the CVRs into account in determining whether such holder must recognize gain, if any, on the receipt of the CVRs and such holder would take no tax basis in the CVRs. Rather, the GTx U.S. Holder’s U.S. federal income tax consequences would be determined in line with the discussion above based on whether the CVRs are treated as a distribution of property or of equity at the time the payments with respect to the CVRs are received or deemed received in accordance with the GTx U.S. Holder’s regular method of accounting. As discussed above, GTx does not intend to report the issuance of the CVRs as an open transaction and any GTx U.S. Holder reporting the CVR issuance as an open transaction likely faces an increased chance of being audited by the IRS with respect to such reporting.
The CVRs should generally be treated as capital assets for U.S. federal income tax purposes once issued.
Alternative Treatment of the Receipt of CVRs and the GTx Reverse Stock Split as a Single Recapitalization
Notwithstanding GTx’s position that the receipt of CVRs and the GTx Reverse Stock Split are appropriately treated as separate transactions, it is possible that the IRS or a court could determine that the receipt of the CVRs and the GTx Reverse Stock Split constitute a single “recapitalization” for U.S. federal income tax purposes. In such case, the tax consequences of the receipt of CVRs and the GTx Reverse Stock Split would differ from those described above and would depend in part on many of the same considerations described above, including whether the CVRs should be treated as property, equity or debt instruments or should be subject to the “open transaction” doctrine. In general, if the CVRs are treated as property and are not subject to the “open transaction” doctrine, then a GTx U.S. Holder should recognize gain (but not loss) equal to the lesser of (i) the fair market value of the CVRs received, and (ii) the excess (if any) of (A) the sum of (1) the fair market value of the CVRs received and (2) the fair market value of the GTx shares received in the GTx Reverse Stock Split (treating fractional shares as received for this purpose), over (B) the GTx U.S. Holder’s adjusted tax basis in the GTx common stock surrendered in the GTx Reverse Stock Split.
PLEASE CONSULT YOUR TAX ADVISOR WITH RESPECT TO THE PROPER CHARACTERIZATION OF THE RECEIPT OF THE CVRs.
203
Voting Agreements and Written Consent
In order to induce GTx to enter into the Merger Agreement, certain stockholders of Oncternal are parties to a voting agreement with Oncternal and GTx pursuant to which, among other things, each stockholder has agreed, solely in its capacity as a stockholder of Oncternal, to vote all of its shares of Oncternal’s capital stock in favor of (1) the adoption and approval of the Merger Agreement and the transactions contemplated thereby, (2) acknowledgement that the approval given for the Merger Agreement and is irrevocable and that the stockholder is aware of its appraisal rights under the DGCL, (3) acknowledgement that the stockholder is not entitled to appraisal rights by voting in favor of the transaction and waiving appraisal rights under the DGCL, and (3) the conversion of each share of Oncternal preferred stock into Oncternal common stock. Additionally, each stockholder has agreed, solely in its capacity as a stockholder of Oncternal, to vote against any competing acquisition proposal and any action, proposal or transaction that would reasonably be expected to result in a material breach of the voting agreement. These stockholders of Oncternal have also granted an irrevocable proxy to Oncternal and its designee to vote their respective Oncternal’s capital stock in accordance with the voting agreements. Oncternal’s stockholders may vote their shares of Oncternal capital stock on all other matters not referred to in such proxy.
The Oncternal stockholders who are parties to these voting agreements include all directors, executive officers and certain stockholders, including entities related to MagnaSci Ventures, which represents 10.4% of the outstanding shares of Oncternal capital stock on as converted common stock basis. SPH USA which holds 100% of the outstanding Series C preferred stock and which represents 20.9% of the outstanding shares of Oncternal capital stock on as converted common stock basis, has not executed a voting agreement. Although Oncternal expects to receive stockholder approval from SPH USA approximately two months after the date of the Merger Agreement, there can be no assurance that all of the necessary stockholder approvals will be obtained
The stockholders of Oncternal that are party to a voting agreement with GTx held, as of March 31, 2019:
| • | an aggregate of 32,059,203 shares of Oncternal’s common stock and 38,883,369 shares of Oncternal preferred stock, representing approximately 43.7% of the outstanding shares of Oncternal capital stock on an as converted to common stock basis; |
| • | an aggregate of 38,883,369 shares of Oncternal’s preferred stock, representing approximately 35.0% of the outstanding Oncternal preferred stock, considered as a single class; |
| • | an aggregate of 5,960,000 shares of Oncternal’s Series A Preferred Stock, representing approximately 44.0% of the outstanding Series A Preferred Stock; and |
| • | an aggregate of 32,923,369 shares of Oncternal’s Series B Preferred Stock and SeriesB-2 Preferred Stock, representing approximately 51.9% of the outstanding Series B Preferred Stock and SeriesB-2 Preferred Stock, considered as a single class. |
Following the effectiveness of the registration statement of which this proxy statement/prospectus/information statement is a part and pursuant to the Merger Agreement, these stockholders will execute written consents providing for such adoption and approval.
Under these voting agreements, subject to certain exceptions, such stockholders have also agreed not to sell or transfer shares of Oncternal’s capital stock and securities held by them, or any voting rights with respect thereto, until the earlier of the termination of the Merger Agreement or the completion of the merger. To the extent that any such sale or transfer is permitted pursuant to the exceptions included in the voting agreement, each person to which any shares of Oncternal’s capital stock or securities are so sold or transferred must agree in writing to be bound by the terms and provisions of the voting agreement, subject to certain further exceptions.
In addition, in order to induce Oncternal to enter into the Merger Agreement, certain of GTx’s stockholders have entered into voting agreements with GTx and Oncternal pursuant to which, among other things, each such stockholder has agreed, solely in his, her or its capacity as a stockholder of GTx, to vote all of his, her or its shares of GTx’s common stock in favor of Proposal Nos. 1, 2, 3, 4 and 5. Additionally, each such stockholder has
204
agreed, solely in his, her or its capacity as a stockholder of GTx, to vote against any competing acquisition proposal and any action, proposal or transaction that would reasonably be expected to result in a material breach of the voting agreement. These stockholders of GTx have also granted GTx and its designee an irrevocable proxy to vote their respective shares in accordance with the voting agreements. GTx’s stockholders may vote their shares of GTx’s common stock on all other matters not referred to in such proxy.
The GTx stockholders who are parties to these voting agreements are:
| • | Robert J. Wills, Ph.D. |
| • | Marc S. Hanover |
| • | J.R. Hyde, III |
| • | Michael G. Carter, M.D., Ch.B., F.R.C.P |
| • | J. Kenneth Glass |
| • | Garry A. Neil, M.D. |
| • | Kenneth S. Robinson, M.D., M.Div. |
| • | Henry P. Doggrell |
| • | Jason Shackelford |
| • | Pyramid Peak Foundation |
As of March 31, 2019, the stockholders of GTx that are party to a voting agreement (including affiliated entities) owned an aggregate of 10,938,824 shares of GTx’s common stock representing approximately 45% of the outstanding shares of GTx’s common stock.
Under these voting agreements, subject to certain exceptions, such stockholders also have agreed not to sell or transfer their shares of GTx’s common stock and securities held by them until the earlier of the termination of the Merger Agreement or the completion of the merger. To the extent that any such sale or transfer is permitted pursuant to the exceptions included in the voting agreements, each person to which any shares of GTx’s common stock or securities are so sold or transferred must agree in writing to be bound by the terms and provisions of the voting agreement, subject to certain further exceptions.
Lock-up Agreements
As a condition to the closing of the merger, certain stockholders of each of GTx and Oncternal and their affiliates, have entered intolock-up agreements, pursuant to which such parties have agreed not to, except in limited circumstances, offer, pledge, sell, contract to sell, transfer or dispose of, directly or indirectly, engage in swap or similar transactions with respect to, or make any demand for or exercise any right with respect to, any shares of GTx’s common stock or any security convertible into or exercisable or exchangeable for GTx’s common stock, including, as applicable, shares received in the merger and issuable upon exercise of certain warrants and options, during the period commencing at the Effective Time and continuing until the date that is 180 days from the Effective Time.
Each of the stockholders who is party to a GTx voting agreement, as identified above, is a party to alock-up agreement. As of March 31, 2019, GTx’s stockholders who have executedlock-up agreements beneficially owned in the aggregate approximately 45% of the outstanding common stock of GTx.
Each of the stockholders who is party to an Oncternal voting agreement is a party to alock-up agreement. Oncternal’s stockholders who have executedlock-up agreements, as of March 31, 2019, beneficially owned in the aggregate approximately 44% of the outstanding shares of Oncternal’s capital stock on an as converted to common stock basis. SPH USA, the holder of the largest amount of Oncternal capital stock, has not executed alock-up agreement, but Oncternal expects it to execute alock-up agreement prior to the closing of the merger, which is a condition to closing.
205
MATTERS BEING SUBMITTED TO A VOTE OF GTX’S STOCKHOLDERS
Proposal No. 1: Approval of the Merger Agreement, the Merger, the Issuance of Common Stock in the Merger and the Change of Control Resulting from the Merger
At the GTx special meeting, GTx’s stockholders will be asked to approve the Merger Agreement and the transactions contemplated thereby, including the merger, the issuance of GTx’s common stock to Oncternal’s stockholders pursuant to the Merger Agreement and the change of control resulting from the merger. Immediately following the merger, it is expected that Oncternal’s current stockholders will own approximately 77.5% of the outstanding common stock of GTx and current GTx stockholders with GTx’s current stockholders will own approximately 22.5% of the outstanding common stock of GTx. The ownership percentage to be held by GTx’s stockholders is subject to adjustment prior to closing of the merger, including a downward adjustment to the extent that GTx’s “Parent Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than the threshold provided in the Merger Agreement, which adjusts based on the date of closing (and as a result, GTx stockholders could own less, and Oncternal stockholders could own more, of the combined organization), or an upward adjustment to the extent that Oncternal’s “Company Cash Amount” (as defined in the Merger Agreement) at the Effective Time is less than $12,500,000 (and as a result, GTx stockholders could own more, and Oncternal stockholders could own less, of the combined organization).
The terms of, reasons for and other aspects of the Merger Agreement, the merger, the issuance of GTx’s common stock pursuant to the Merger Agreement and the change of control resulting from the merger are described in detail in the other sections in this proxy statement/prospectus/information statement.
Required Vote
The affirmative vote of the holders of a majority of the shares of GTx’s common stock entitled to vote and present in person or represented by proxy at the GTx special meeting is required for approval of Proposal No. 1. Abstentions will have the same effect as votes “AGAINST” this Proposal.
THE GTX BOARD RECOMMENDS THAT GTX’S STOCKHOLDERS VOTE “FOR” PROPOSAL NO. 1 TO APPROVE THE MERGER AGREEMENT AND THE TRANSACTIONS CONTEMPLATED THEREBY, INCLUDING THE MERGER, THE ISSUANCE OF GTX’S COMMON STOCK PURSUANT TO THE MERGER AGREEMENT AND THE CHANGE OF CONTROL RESULTING FROM THE MERGER. EACH OF PROPOSAL NOS. 1 AND 2 ARE CONDITIONED UPON EACH OTHER AND THE APPROVAL OF EACH SUCH PROPOSAL IS REQUIRED TO CONSUMMATE THE MERGER.
Proposal No. 2: Approval of a Series of Alternative Amendments to the Restated Certificate of Incorporation of GTx Effecting the GTx Reverse Stock Split
General
At the GTx special meeting, GTx’s stockholders will be asked to approve a series of alternative amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split. Upon the effectiveness of the amendment to the restated certificate of incorporation of GTx effecting the GTx Reverse Stock Split, or the split effective time, the issued shares of GTx’s common stock immediately prior to the split effective time will be reclassified into a smaller number of shares within a range, as determined by the GTx Board, such that a stockholder of GTx will own one new share of GTx’s common stock for every six to eight (or any number in between) shares of issued common stock held by that stockholder immediately prior to the split effective time.
If Proposal No. 2 is approved, the GTx Reverse Stock Split would become effective in connection with the closing of the merger. The GTx Board may effect only one reverse stock split in connection with this Proposal No. 2. The GTx Board’s decision will be based on a number of factors, including market conditions, existing and expected trading prices for GTx’s common stock and the listing requirements of Nasdaq.
206
The form of the amendment to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split, as more fully described below, will effect the GTx Reverse Stock Split but will not change the number of authorized shares of common stock or preferred stock, or the par value of GTx’s common stock or preferred stock.
Purpose
The GTx Board approved the proposal approving the series of alternative amendments to the restated certificate of incorporation of GTx effecting the GTx Reverse Stock Split for the following reasons:
| • | the GTx Board believes effecting the GTx Reverse Stock Split may be an effective means of avoiding a delisting of GTx’s common stock from Nasdaq in the future; |
| • | the GTx Board believes that the GTx Reverse Stock Split will result in a number of authorized but unissued shares of GTx’s common stock sufficient for the issuance of shares of GTx’s common stock to Oncternal’s stockholders pursuant to the Merger Agreement; and |
| • | the GTx Board believes a higher stock price may help generate investor interest in GTx and help GTx attract and retain employees. |
If the GTx Reverse Stock Split successfully increases the per share price of GTx’s common stock, the GTx Board believes this increase may increase trading volume in GTx’s common stock and facilitate future financings by GTx.
Nasdaq Requirements for Listing on Nasdaq
GTx’s common stock is quoted on Nasdaq under the symbol “GTXI.” GTx has filed an initial listing application with Nasdaq to seek listing on Nasdaq upon the closing of the merger.
According to Nasdaq rules, an issuer must, in a case such as this, apply for initial inclusion following a transaction whereby the issuer combines with anon-Nasdaq entity, resulting in a change of control of the issuer and potentially allowing thenon-Nasdaq entity to obtain a Nasdaq listing. Accordingly, the listing standards of Nasdaq will require GTx to have, among other things, a $4.00 per share minimum bid price upon the closing of the merger. Therefore, the GTx Reverse Stock Split may be necessary in order to consummate the merger.
One of the effects of the GTx Reverse Stock Split will be to effectively increase the proportion of authorized shares which are unissued relative to those which are issued. This could result in GTx’s management being able to issue more shares without further stockholder approval. For example, before the GTx Reverse Stock Split, GTx’s authorized but unissued shares immediately prior to the closing of the merger would be approximately 35.9 million compared to shares issued of approximately 24.1 million. If GTx effects the GTx Reverse Stock Split using a 1:7 ratio (the midpoint of the range of the GTx Reverse Stock Split), its authorized but unissued shares immediately prior to the closing of the merger would be approximately 56.6 million compared to shares issued of approximately 3.4 million. GTx currently has no plans to issue shares, other than in connection with the merger, and to satisfy obligations under the GTx warrants and employee stock options from time to time as these warrants and options are exercised. The GTx Reverse Stock Split will not affect the number of authorized shares of GTx’s common stock which will continue to be authorized pursuant to the certificate of incorporation of GTx.
Potential Increased Investor Interest
On May 6, 2019, GTx’s common stock closed at $1.16 per share. An investment in GTx’s common stock may not appeal to brokerage firms that are reluctant to recommend lower priced securities to their clients. Investors may also be dissuaded from purchasing lower priced stocks because the brokerage commissions, as a percentage of the total transaction, tend to be higher for such stocks. Moreover, the analysts at many brokerage firms do not monitor the trading activity or otherwise provide coverage of lower priced stocks. Also, the GTx Board believes that most investment funds are reluctant to invest in lower priced stocks.
207
There are risks associated with the GTx Reverse Stock Split, including that the GTx Reverse Stock Split may not result in an increase in the per share price of GTx’s common stock.
GTx cannot predict whether the GTx Reverse Stock Split will increase the market price for GTx’s common stock. The history of similar stock split combinations for companies in like circumstances is varied. There is no assurance that:
| • | the market price per share of GTx’s common stock after the GTx Reverse Stock Split will rise in proportion to the reduction in the number of shares of GTx’s common stock outstanding before the GTx Reverse Stock Split; |
| • | the GTx Reverse Stock Split will result in a per share price that will attract brokers and investors who do not trade in lower priced stocks; |
| • | the GTx Reverse Stock Split will result in a per share price that will increase the ability of GTx to attract and retain employees; or |
| • | the market price per share will either exceed or remain in excess of the $1.00 minimum bid price as required by the Nasdaq Stock Market LLC for continued listing, or that GTx will otherwise meet the requirements of the Nasdaq Stock Market LLC for inclusion for trading on Nasdaq, including the $4.00 minimum bid price upon the closing of the merger. |
The market price of GTx’s common stock will also be based on performance of GTx and other factors, some of which are unrelated to the number of shares outstanding. If the GTx Reverse Stock Split is effected and the market price of GTx’s common stock declines, the percentage decline as an absolute number and as a percentage of the overall market capitalization of GTx may be greater than would occur in the absence of a reverse stock split. Furthermore, the liquidity of GTx’s common stock could be adversely affected by the reduced number of shares that would be outstanding after the GTx Reverse Stock Split.
Principal Effects of the GTx Reverse Stock Split
The amendment to the restated certificate of incorporation of GTx effecting the GTx Reverse Stock Split is set forth inAnnexD to this proxy statement/prospectus/information statement.
The GTx Reverse Stock Split will be effected simultaneously for all outstanding shares of GTx’s common stock. The GTx Reverse Stock Split will affect all of GTx’s stockholders uniformly and will not affect any stockholder’s percentage ownership interest in GTx, except to the extent that the GTx Reverse Stock Split results in any of GTx’s stockholders owning a fractional share. Shares of GTx’s common stock issued pursuant to the GTx Reverse Stock Split will remain fully paid and nonassessable. The GTx Reverse Stock Split does not affect the total proportionate ownership of GTx following the merger. The GTx Reverse Stock Split will not affect GTx continuing to be subject to the periodic reporting requirements of the Exchange Act.
Procedure for Effecting the GTx Reverse Stock Split and Exchange of Stock Certificates
If GTx’s stockholders approve the series of alternative amendments to the restated certificate of incorporation of GTx effecting the GTx Reverse Stock Split, and if the GTx Board still believes that the GTx Reverse Stock Split is in the best interests of GTx and its stockholders, GTx will file the amendment to the restated certificate of incorporation with the Secretary of State of the State of Delaware at such time as the GTx Board has determined to be the appropriate split effective time within the range approved. The GTx Board may delay effecting the GTx Reverse Stock Split without resoliciting stockholder approval. Beginning at the split effective time, each certificate representingpre-split shares will be deemed for all corporate purposes to evidence ownership of post-split shares.
As soon as practicable after the split effective time, GTx’s stockholders will be notified that the GTx Reverse Stock Split has been effected. GTx expects that the GTx transfer agent will act as exchange agent for purposes of
208
implementing the exchange of stock certificates. Holders ofpre-split shares will be asked to surrender to the exchange agent certificates representingpre-split shares held in certificated form in exchange for certificates representing post-split shares in accordance with the procedures to be set forth in a letter of transmittal to be sent by GTx. In the event that the GTx Name Change under Proposal No. 3 is approved by GTx’s stockholders, the certificates reflecting the post-split shares will also reflect the GTx Name Change. No new certificates will be issued to a stockholder until such stockholder has surrendered such stockholder’s outstanding certificate(s) together with the properly completed and executed letter of transmittal to the exchange agent. Anypre-split shares submitted for transfer, whether pursuant to a sale or other disposition, or otherwise, will automatically be exchanged for post-split shares.Stockholders should not destroy any stock certificate(s) and should not submit any certificate(s) unless and until requested to do so.
Fractional Shares
No fractional shares will be issued in connection with the GTx Reverse Stock Split. Stockholders of record who otherwise would be entitled to receive fractional shares because they hold a number ofpre-split shares not evenly divisible by the number ofpre-split shares for which each post-split share is to be reclassified, will be entitled, upon surrender to the exchange agent of certificates representing such shares, to a cash payment in lieu thereof at a price equal to the fraction to which the stockholder would otherwise be entitled multiplied by the closing price of the common stock on Nasdaq on the date immediately preceding the split effective time. The ownership of a fractional interest will not give the holder thereof any voting, dividend, or other rights except to receive payment therefor as described herein.
By approving the series of alternative amendments to the restated certificate of incorporation of GTx effecting the GTx Reverse Stock Split, stockholders will be approving the combination of six to eight shares of GTx’s common stock, as determined by the GTx Board, into one share of GTx’s common stock.
Stockholders should be aware that, under the escheat laws of the various jurisdictions where stockholders reside, where GTx is domiciled, and where the funds will be deposited, sums due for fractional interests that are not timely claimed after the effective date of the split may be required to be paid to the designated agent for each such jurisdiction, unless correspondence has been received by GTx or the exchange agent concerning ownership of such funds within the time permitted in such jurisdiction. Thereafter, stockholders otherwise entitled to receive such funds will have to seek to obtain them directly from the state to which they were paid.
Potential Anti-Takeover Effect
Although the increased proportion of unissued authorized shares to issued shares could, under certain circumstances, have an anti-takeover effect, for example, by permitting issuances that would dilute the stock ownership of a person seeking to effect a change in the composition of The GTx Board or contemplating a tender offer or other transaction for the combination of GTx with another company, the GTx Reverse Stock Split proposal is not being proposed in response to any effort of which GTx is aware to accumulate shares of GTx’s common stock or obtain control of GTx, other than in connection with the merger, nor is it part of a plan by management to recommend a series of similar amendments to the GTx Board and stockholders. Other than the proposals being submitted to GTx’s stockholders for their consideration at the GTx special meeting, the GTx Board does not currently contemplate recommending the adoption of any other actions that could be construed to affect the ability of third parties to take over or change control of GTx. For more information, please see the section entitled “Risk Factors—Risks Related to the Common Stock of GTx”, and “Description of GTx’s Capital Stock—Anti-Takeover Effects of Provisions of GTx Charter Documents” and “—Anti-Takeover Effects of Delaware Law.”
Material U.S. Federal Income Tax Consequences of the GTx Reverse Stock Split
The following discussion is a summary of the material U.S. federal income tax consequences of the GTx Reverse Stock Split to GTx U.S. Holders (which, for purposes of this discussion, has the same meaning as in
209
“Agreements Related to the Merger—CVR Agreement—Material U.S. Federal Income Tax Consequences of the Receipt of CVRs”), but does not purport to be a complete analysis of all potential tax consequences that may be relevant to GTx U.S. Holders. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local ornon-U.S. tax laws are not discussed. This discussion is based on the Code, Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the IRS, in each case in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a GTx U.S. Holder. GTx has not sought and does not intend to seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a position contrary to that discussed below regarding the tax consequences of the GTx Reverse Stock Split.
This discussion is limited to GTx U.S. Holders that hold GTx common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences that may be relevant to a GTx U.S. Holder’s particular circumstances, including the impact of the alternative minimum tax or the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to GTx U.S. Holders subject to special rules, including, without limitation:
| • | U.S. expatriates and former citizens or long-term residents of the United States; |
| • | GTx U.S. Holders whose functional currency is not the U.S. dollar; |
| • | persons holding GTx common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| • | banks, insurance companies, and other financial institutions; |
| • | real estate investment trusts or regulated investment companies; |
| • | brokers, dealers or traders in securities; |
| • | persons for whom GTx common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code; |
| • | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | S corporations, partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | tax-exempt organizations or governmental organizations; |
| • | persons subject to special tax accounting rules as a result of any item of gross income with respect to GTx common stock being taken into account in an “applicable financial statement” (as defined in the Code); |
| • | persons deemed to sell GTx common stock under the constructive sale provisions of the Code; |
| • | persons who hold or received GTx common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
| • | tax-qualified retirement plans. |
If an entity treated as a partnership for U.S. federal income tax purposes holds GTx common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding GTx common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
210
THIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT TAX ADVICE. HOLDERS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE GTX REVERSE STOCK SPLIT ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL ORNON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
GTx Reverse Stock Split
The GTx Reverse Stock Split should constitute a “recapitalization” for U.S. federal income tax purposes. As a result, a GTx U.S. Holder generally should not recognize gain or loss upon the GTx Reverse Stock Split, except with respect to cash received in lieu of a fractional share of GTx common stock, as discussed below. A GTx U.S. Holder’s aggregate tax basis in the shares of GTx common stock received pursuant to the GTx Reverse Stock Split should equal the aggregate tax basis of the shares of GTx common stock surrendered (excluding any portion of such basis that is allocated to any fractional share of GTx common stock), and such GTx U.S. Holder’s holding period in the shares of GTx common stock received should include the holding period in the shares of GTx common stock surrendered. Treasury Regulations provide detailed rules for allocating the tax basis and holding period of the shares of GTx common stock surrendered to the shares of GTx common stock received pursuant to the GTx Reverse Stock Split. Holders of shares of GTx common stock acquired on different dates and at different prices should consult their tax advisors regarding the allocation of the tax basis and holding period of such shares.
A GTx U.S. Holder that receives cash in lieu of a fractional share of GTx common stock pursuant to the GTx Reverse Stock Split should recognize capital gain or loss in an amount equal to the difference between the amount of cash received and the GTx U.S. Holder’s tax basis in the shares of GTx common stock surrendered that is allocated to such fractional share of our common stock. Such capital gain or loss should be long-term capital gain or loss if the GTx U.S. Holder’s holding period for GTx common stock surrendered exceeded one year at the effective time of the GTx Reverse Stock Split.
Although GTx intends to treat the GTx Reverse Stock Split and the receipt of CVRs as separate transactions, it is possible that the IRS or a court could determine that the GTx Reverse Stock Split and the receipt of CVRs constitute a single “recapitalization” for U.S. federal income tax purposes. For a discussion of such treatment, please see the section entitled “Agreements Related to the Merger—CVR Agreement—Material U.S. Federal Income Tax Consequences of the Receipt of CVRs—Alternative Treatment of the Receipt of CVRs and the GTx Reverse Stock Split as a Single Recapitalization.”
Information Reporting and Backup Withholding
A GTx U.S. Holder may be subject to information reporting and backup withholding when such holder receives cash in lieu of fractional shares of GTx common stock in the GTx Reverse Stock Split. Certain GTx U.S. Holders are exempt from backup withholding, including corporations and certaintax-exempt organizations. A GTx U.S. Holder will be subject to backup withholding if such holder is not otherwise exempt and:
| • | the holder fails to furnish the holder’s taxpayer identification number, which for an individual is ordinarily his or her social security number; |
| • | the holder furnishes an incorrect taxpayer identification number; |
| • | the applicable withholding agent is notified by the IRS that the holder previously failed to properly report payments of interest or dividends; or |
| • | the holder fails to certify under penalties of perjury that the holder has furnished a correct taxpayer identification number and that the IRS has not notified the holder that the holder is subject to backup withholding. |
211
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a GTx U.S. Holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS. GTx U.S. Holders should consult their tax advisors regarding their qualification for an exemption from backup withholding and the procedures for obtaining such an exemption.
Vote Required; Recommendation of Board of Directors
The affirmative vote of holders of a majority of the shares of GTx’s common stock having voting power outstanding on the record date for the GTx special meeting is required to approve the series of alternative amendments to the restated certificate of incorporation of GTx to effect the GTx Reverse Stock Split. Abstentions and brokernon-votes will have the same effect as votes “AGAINST” this Proposal.
THE GTX BOARD RECOMMENDS THAT GTX’S STOCKHOLDERS VOTE “FOR” PROPOSAL NO. 2 TO APPROVE THE SERIES OF ALTERNATIVE AMENDMENTS TO THE RESTATED CERTIFICATE OF INCORPORATION OF GTX TO EFFECT THE GTX REVERSE STOCK SPLIT. EACH OF PROPOSAL NOS. 1 AND 2 ARE CONDITIONED UPON EACH OTHER AND THE APPROVAL OF EACH SUCH PROPOSAL IS REQUIRED TO CONSUMMATE THE MERGER.
Proposal No. 3: Approval of GTx Name Change
At the GTx special meeting, GTx’s stockholders will be asked to approve the amendment to the restated certificate of incorporation of GTx to effect the GTx Name Change. The primary reason for the corporate name change is that management believes this will allow for brand recognition of Oncternal’s products and programs following the consummation of the merger. GTx’s management believes that the current name will no longer accurately reflect the business of GTx and the mission of GTx subsequent to the consummation of the merger.
The affirmative vote of holders of a majority of the shares of GTx’s common stock having voting power outstanding on the record date for the GTx special meeting is required to approve the amendment to the restated certificate of incorporation to effect the GTx Name Change. Abstentions and brokernon-votes will have the same effect as votes “AGAINST” this Proposal.
THE GTX BOARD RECOMMENDS THAT GTX’S STOCKHOLDERS VOTE “FOR” PROPOSAL NO. 3 TO APPROVE THE GTX NAME CHANGE. PROPOSAL NO. 3 IS CONDITIONED UPON THE APPROVAL OF EACH OF PROPOSAL NOS. 1 AND 2.
Proposal No. 4: Approval of the Adoption of the GTx, Inc. 2019 Incentive Award Plan
Overview
In this Proposal No. 4, GTx is requesting GTx stockholders to approve and adopt the GTx, Inc. 2019 Incentive Award Plan (the “GTx 2019 Plan”) and the material terms thereunder. The GTx Board approved the GTx 2019 Plan prior to the GTx special meeting, subject to stockholder approval at the GTx special meeting. The GTx 2019 Plan will become effective on the day prior to the closing date of the merger, subject to consummation of the merger, provided stockholder approval has been obtained prior to such date.
The GTx 2019 Plan is described in more detail below. A copy of the GTx 2019 Plan is attached to this proxy statement asAnnex F.
All share numbers in this Proposal 4 do not reflect the GTx Reverse Stock Split which will be applied to the share numbers in the GTx 2019 Plan.
212
The GTx 2019 Plan
The purpose of the GTx 2019 Plan is to enhance GTx’s ability to attract, retain and motivate persons who make (or are expected to make) important contributions to GTx by providing these individuals with equity ownership opportunities. GTx believes that the GTx 2019 Plan is essential to its success. Equity awards are intended to motivate high levels of performance and align the interests of GTx’s directors, employees and consultants with those of GTx’s stockholders by giving directors, employees and consultants the perspective of an owner with an equity stake in GTx and providing a means of recognizing their contributions to the success of GTx. The GTx Board and management believe that equity awards are necessary to remain competitive in its industry and are essential to recruiting and retaining the highly qualified employees who help GTx meet its goals.
213
Equity Incentive Awards Are Critical to Long-Term Stockholder Value Creation
The table below presents information about the number of shares that were subject to outstanding equity awards under GTx’s equity incentive plans and the shares remaining available for issuance under the such plan, each at March 31, 2019, and the proposed share reserve under the GTx 2019 Plan. The GTx, Inc. 2013 Equity Incentive Plan (as amended) (the “GTx 2013 Plan”) and the GTx, Inc. 2001 Stock Option Plan, the GTx, Inc. 2002 Stock Option Plan, the GTx, Inc. 2004 Equity Incentive Plan, the GTx, Inc. Amended and Restated 2004Non-Employee Directors’ Stock Option Plan, the GTx, Inc. 2013Non-Employee Director Equity Incentive Plan (the “GTx Directors Plan”), 2018 Amended and Restated Directors’ Deferred Compensation Plan (the “GTx Director Deferred Compensation Plan”) are the only equity incentive plans GTx currently has in place and awards may only be granted pursuant to the GTx 2013 Plan, the GTx Directors Plan and the GTx Director Deferred Compensation Plan. None of the following share numbers give effect to the GTx Reverse Stock Split or the merger.
| Number of Shares # | As of a % of Shares Outstanding(1) | Dollar Value $(2) | ||||||||||
GTx 2001 Stock Option Plan | ||||||||||||
Options outstanding | 450 | 0.002 | % | $ | 540.00 | |||||||
Weighted-average exercise price of outstanding options | $ | 42.00 | ||||||||||
Weighted-average remaining term of outstanding options | 0.75 years | |||||||||||
Shares remaining available for grant under the GTx 2001 Stock Option Plan | — | — | % | $ | — | |||||||
GTx 2002 Stock Option Plan | ||||||||||||
Options outstanding | 3,118 | 0.013 | % | $ | 3,741.60 | |||||||
Weighted-average exercise price of outstanding options | $35.80 | |||||||||||
Weighted-average remaining term of outstanding options | 2.23 years | |||||||||||
Shares remaining available for grant under the GTx 2002 Stock Option Plan | — | — | % | $ | — | |||||||
GTx 2004 Equity Incentive Plan | ||||||||||||
Options outstanding | 198,429 | 0.825 | % | $ | 238,114.80 | |||||||
Weighted-average exercise price of outstanding options | $ | 36.98 | ||||||||||
Weighted-average remaining term of outstanding options | 1.68 years | |||||||||||
Shares remaining available for grant under the GTx 2004 Equity Incentive Plan | — | — | % | $ | — | |||||||
GTx Amended and Restated 2004Non-Employee Directors’ Stock Option Plan | ||||||||||||
Options outstanding | 15,000 | 0.062 | % | $ | 18,000.00 | |||||||
Weighted-average exercise price of outstanding options | $ | 50.88 | ||||||||||
Weighted-average remaining term of outstanding options | 1.79 years | |||||||||||
Shares remaining available for grant under the GTx Amended and Restated 2004Non-Employee Directors’ Stock Option Plan | — | — | % | $ | — | |||||||
GTx 2013 Plan | ||||||||||||
Options outstanding | 1,948,400 | 8.101 | % | $ | 2,338,080.00 | |||||||
Weighted-average exercise price of outstanding options | $ | 8.76 | ||||||||||
Weighted-average remaining term of outstanding options | 5.90 years | |||||||||||
Shares remaining available for grant under the GTx 2013 Plan | 1,979,921 | 8.232 | % | $ | 2,375,905.20 | |||||||
GTx Director Deferred Compensation Plan | ||||||||||||
Deferred stock rights outstanding | 155,426 | 0.646 | % | $ | 186,511.20 | |||||||
Shares remaining available for grant under the GTx Director Deferred Compensation Plan | — | — | % | $ | — | |||||||
GTx Directors Plan | ||||||||||||
Options outstanding | 153,250 | 0.637 | % | $ | 1,8390.00 | |||||||
Weighted-average exercise price of outstanding options | $ | 9.96 | ||||||||||
Weighted-average remaining term of outstanding options | 7.49 years | |||||||||||
Shares remaining available for grant under the GTx Directors Plan | 216,115 | 0.899 | % | $ | 259,338.00 | |||||||
GTx 2019 Plan | ||||||||||||
Proposed shares available for issuance under the GTx 2019 Plan(3) | 11,750,000 | 48.853 | % | $ | 14,100,000.00 | |||||||
214
| (1) | Based on 24,051,844 shares of GTx common stock outstanding as of March 31, 2019. |
| (2) | Based on the closing price of GTx common stock on March 29, 2019, of $1.20 per share. |
| (3) | Does not include (a) possible future increases to the share reserve under the evergreen provision of the GTx 2019 Plan. Pursuant to the evergreen provision, the GTx 2019 Plan will be subject to an annual increase on the first day of each calendar year beginning January 1, 2020 and ending on and including January 1, 2029, equal to the lesser of (i) 5% of the aggregate number of shares outstanding on the final day of the immediately preceding calendar year and (ii) such smaller number of shares as is determined by the board of GTx, or (b) any shares subject to awards under the GTx 2013 Plan as of the effective date of the GTx 2019 Plan that become available for issuance under the GTx 2019 Plan (which number is added to the overall share limit under the GTx 2019 Plan). |
In determining whether to approve the GTx 2019 Plan, including the proposed share reserve under the GTx 2019 Plan, the GTx Board considered, among other things, the following:
| • | The purpose of the share reserve under the GTx 2019 Plan is to provide the combined organization with appropriate capacity to issue equity compensation following the closing of the merger. Assuming the GTx 2019 Plan is approved, and after giving effect to the GTx Reverse Stock Split and the merger, the requested increase to the share reserve is expected to represent approximately 12% of the outstanding GTx common stock immediately following the merger. |
| • | In determining the size of the share reserve under the GTx 2019 Plan, the GTx Board considered the substantial changes to the capitalization structure of GTx that will occur as a result of the GTx Reverse Stock Split and the merger, which will have the effect of significantly diminishing the remaining share reserve under GTx’s existing equity plans. In calendar years 2018, 2017 and 2016, GTx’s annual equity burn rates (calculated by dividing the number of shares subject to equity awards granted during the year by the weighted-average number of shares outstanding during the applicable year) under GTx’s equity plans were 2.0%, 5.6% and 2.6%, respectively. |
| • | GTx expects the proposed aggregate share reserve under the GTx 2019 Plan to provide GTx with enough shares for awards for at least five years, assuming GTx continues to grant awards consistent with GTx’s current practices and historical usage, as reflected in its historical burn rate, assuming GTx receives the maximum annual evergreen increases under the GTx 2019 Plan during itsten-year term, and further dependent on the price of GTx shares and hiring activity during the next few years, forfeitures of outstanding awards, and noting that the consummation of the merger and future circumstances may require GTx to change its current equity grant practices. GTx cannot predict its future equity grant practices, the future price of GTx shares or future hiring activity with any degree of certainty at this time, and the share reserve under the GTx 2019 Plan could last for a shorter or longer time. |
| • | In fiscal years 2018, 2017 and 2016, GTx’s end of year overhang rate (calculated by dividing (1) the sum of the number of shares subject to equity awards outstanding at the end of the calendar year plus shares remaining available for issuance for future awards at the end of the calendar year by (2) the number of shares outstanding at the end of the calendar year) was 14.6%, 13.6% and 14.9%, respectively. |
| • | If the GTx 2019 Plan is approved, the merger is consummated and the GTx Reverse Stock Split is implemented, GTx expects the combined organization’s overhang at the end of 2019 will be approximately 14%. |
| • | Following the closing of the merger, the GTx 2019 Plan will be the only plan under which GTx will be able to grant new equity awards. |
In light of the factors described above, and the fact that the ability to continue to grant equity compensation is vital to GTx’s ability to continue to attract and retain employees in the extremely competitive labor markets in which it competes, the GTx Board has approved a share reserve under the GTx 2019 Plan that is reasonable
215
and appropriate at this time. The GTx Board will not create a subcommittee to evaluate the risk and benefits for issuing shares under the GTx 2019 Plan.
Summary of the GTx 2019 Plan
This section summarizes certain principal features of the GTx 2019 Plan. The summary is qualified in its entirety by reference to the complete text of the GTx 2019 Plan, which is attached to this proxy statement asAnnex F.
Eligibility and Administration
GTx’s employees, consultants and directors, and employees and consultants of GTx’s subsidiaries, will be eligible to receive awards under the GTx 2019 Plan. As of March 31, 2019, GTx had 13 employees and fivenon-employee directors. Following the closing of the merger, the combined company is expected to have approximately 10 employees, eightnon-employee directors and 18 other service providers who will be eligible to receive awards under the GTx 2019 Plan.
The GTx 2019 Plan will be administered by the GTx Board, which may delegate its duties and responsibilities to one or more committees of GTx’s directors and/or officers (referred to collectively as the plan administrator), subject to the limitations imposed under the GTx 2019 Plan, Section 16 of the Exchange Act, stock exchange rules and other applicable laws. The plan administrator will have the authority to take all actions and make all determinations under the GTx 2019 Plan, to interpret the GTx 2019 Plan and award agreements and to adopt, amend and repeal rules for the administration of the GTx 2019 Plan as it deems advisable. The plan administrator will also have the authority to determine which eligible service providers receive awards, grant awards and set the terms and conditions of all awards under the GTx 2019 Plan, including any vesting and vesting acceleration provisions, subject to the conditions and limitations in the GTx 2019 Plan.
Shares Available for Awards
The sum of (a) 11,750,000 shares of common stock of GTx; (b) any shares of common stock of GTx which are subject to awards under the GTx 2013 Plan as of the effective date of the GTx 2019 Plan which become available for issuance under the GTx 2019 Plan (which number added to the overall share limit pursuant to this clause (b) shall not exceed 1,948,400 shares of common stock of GTx); and (c) an annual increase on the first day of each calendar year beginning January 1, 2020 and ending on and including January 1, 2029, equal to the lesser of (i) 5% of the aggregate number of Shares outstanding on the final day of the immediately preceding calendar year and (ii) such smaller number of shares of common stock of GTx as is determined by the GTx Board, will be available for issuance under the GTx 2019 Plan. Shares issued under the GTx 2019 Plan may be authorized but unissued shares, shares purchased on the open market or treasury shares. Notwithstanding anything to the contrary in the GTx 2019 Plan, no more than 50,000,000 shares of common stock of GTx may be issued pursuant to the exercise of incentive stock options (“ISOs”) under the GTx 2019 Plan. Upon the effectiveness of the GTx 2019 Plan, no further awards will be granted under the GTx 2013 Plan or the GTx Directors Plan. In addition, the GTx Director Deferred Compensation Plan will be terminated at the time of the closing of the merger.
If an award under the GTx 2019 Plan or the GTx 2013 Plan expires, lapses or is terminated, exchanged for cash, surrendered, repurchased, canceled without having been fully exercised or forfeited, any unused shares subject to the award will again be available for new grants under the GTx 2019 Plan. Further, shares delivered to satisfy the purchase price or tax withholding obligation for any award or award under the GTx 2013 Plan will again be available for new grants under the GTx 2019 Plan.
Awards granted under the GTx 2019 Plan in substitution for any options or other stock or stock-based awards granted by an entity before the entity’s merger or consolidation with GTx (or any of GTx’s subsidiaries) or GTx’s (or any of GTx’s subsidiary’s) acquisition of the entity’s property or stock will not reduce the shares available for grant under the GTx 2019 Plan, but will count against the maximum number of shares that may be issued upon the exercise of incentive stock options.
216
Awards
The GTx 2019 Plan provides for the grant of stock options, including ISOs and nonqualified stock options (“NSOs”), stock appreciation rights (“SARs”), restricted stock, dividend equivalents, restricted stock units (“RSUs”) and other stock or cash based awards. Certain awards under the GTx 2019 Plan may constitute or provide for payment of “nonqualified deferred compensation” under Section 409A of the Code. All awards under the GTx 2019 Plan will be set forth in award agreements, which will detail the terms and conditions of awards, including any applicable vesting and payment terms and post-termination exercise limitations. A brief description of each award type follows.
| • | Stock Options and SARs. Stock options provide for the purchase of shares of common stock of GTx in the future at an exercise price set on the grant date. ISOs, in contrast to NSOs, may provide tax deferral beyond exercise and favorable capital gains tax treatment to their holders if certain holding period and other requirements of the Code are satisfied. SARs entitle their holder, upon exercise, to receive from us an amount equal to the appreciation of the shares subject to the award between the grant date and the exercise date. The plan administrator will determine the number of shares covered by each option and SAR, the exercise price of each option and SAR and the conditions and limitations applicable to the exercise of each option and SAR. The exercise price of a stock option or SAR will not be less than 100% of the fair market value of the underlying share on the grant date (or 110% in the case of ISOs granted to certain significant stockholders), except with respect to certain substitute awards granted in connection with a corporate transaction. The term of a stock option or SAR may not be longer than ten years (or five years in the case of ISOs granted to certain significant stockholders). The closing share price per share of GTx common stock on Nasdaq on March 29, 2019 was $1.20. |
| • | Restricted Stock. Restricted stock is an award of nontransferable shares of common stock of GTx that remain forfeitable unless and until specified conditions are met and which may be subject to a purchase price. Upon issuance of restricted stock, recipients generally have the rights of a stockholder with respect to such shares, which generally include the right to receive dividends and other distributions in relation to the award. The terms and conditions applicable to restricted stock will be determined by the plan administrator, subject to the conditions and limitations contained in the GTx 2019 Plan. |
| • | RSUs.RSUs are contractual promises to deliver shares of common stock of GTx in the future, which may also remain forfeitable unless and until specified conditions are met and may be accompanied by the right to receive the equivalent value of dividends paid on shares of common stock of GTx prior to the delivery of the underlying shares (i.e., dividend equivalent rights). The plan administrator may provide that the delivery of the shares underlying RSUs will be deferred on a mandatory basis or at the election of the participant. The terms and conditions applicable to RSUs will be determined by the plan administrator, subject to the conditions and limitations contained in the GTx 2019 Plan. |
| • | Other Stock or Cash Based Awards. Other stock or cash based awards are awards of cash, fully vested shares of common stock of GTx and other awards valued wholly or partially by referring to, or otherwise based on, shares of common stock of GTx or other property. Other stock or cash based awards may be granted to participants and may also be available as a payment form in the settlement of other awards, as standalone payments and as payment in lieu of compensation to which a participant is otherwise entitled. The plan administrator will determine the terms and conditions of other stock or cash based awards, which may include any purchase price, performance goal, transfer restrictions and vesting conditions. |
Certain Transactions
In connection with certain corporate transactions and events affecting the common stock of GTx, including a change in control, or change in any applicable laws or accounting principles, the plan administrator has broad discretion to take action under the GTx 2019 Plan to prevent the dilution or enlargement of intended benefits, facilitate the transaction or event or give effect to the change in applicable laws or accounting principles. This includes canceling awards for cash or property, accelerating the vesting of awards, providing for the assumption
217
or substitution of awards by a successor entity, adjusting the number and type of shares subject to outstanding awards and/or with respect to which awards may be granted under the GTx 2019 Plan and replacing or terminating awards under the GTx 2019 Plan. In addition, in the event of certainnon-reciprocal transactions with GTx’s stockholders, the plan administrator will make equitable adjustments to the GTx 2019 Plan and outstanding awards as it deems appropriate to reflect the transaction. In the event of change in control, if awards are not continued, converted, assumed, or replaced with a substantially similar award by GTx or a successor entity or its parent or subsidiary, then, immediately prior to the change in control, and contingent on a participant’s then-current employment with GTx, such awards shall become fully vested, exercisable and/or payable, as applicable, and all forfeiture, repurchase and other restrictions on such awards shall lapse, in which case, such awards shall be canceled upon the consummation of the change in control in exchange for the right to receive the change in control consideration payable to other holders of common stock.
Provisions of the GTx 2019 Plan Relating to Director Compensation
The GTx 2019 Plan provides that the plan administrator may establish compensation fornon-employee directors from time to time subject to the GTx 2019 Plan’s limitations. The plan administrator will from time to time determine the terms, conditions and amounts of allnon-employee director compensation in its discretion and pursuant to the exercise of its business judgment, taking into account such factors, circumstances and considerations as it shall deem relevant from time to time, provided that, the sum of any cash compensation or other compensation and the grant date fair value of any equity awards granted under the GTx 2019 Plan as compensation for services as anon-employee director during any fiscal year may not exceed $0.75 million (increased to $1.0 million in the fiscal year of anon-employee director’s initial service as anon-employee director). The plan administrator may make exceptions to this limit for individualnon-employee directors in extraordinary circumstances, as the plan administrator may determine in its discretion, subject to the limitations in the GTx 2019 Plan.
Plan Amendment and Termination
The GTx Board may amend or terminate the GTx 2019 Plan at any time; however, no amendment, other than an amendment that increases the number of shares available under the GTx 2019 Plan, may materially and adversely affect an award outstanding under the GTx 2019 Plan without the consent of the affected participant and stockholder approval will be obtained for any amendment to the extent necessary to comply with applicable laws. The GTx 2019 Plan will remain in effect until the tenth anniversary of the date the GTx Board adopted the GTx 2019 Plan, which was April 16, 2019, unless earlier terminated by the GTx Board. No awards may be granted under the GTx 2019 Plan after its termination. Under the GTx 2019 Plan, the plan administrator may, without the approval of our stockholders, authorize the repricing of any outstanding option or SAR to reduce its price per share, or cancel any option or SAR in exchange for cash or another award when the price per share exceeds the Fair Market Value (as that term is defined in the GTx 2019 Plan) of the underlying shares.
Foreign Participants, Claw-back Provisions, Transferability and Participant Payments
The plan administrator may modify awards granted to participants who are foreign nationals or employed outside the United States or establish subplans or procedures to address differences in laws, rules, regulations or customs of such foreign jurisdictions. All awards will be subject to any company claw-back policy as set forth in such claw-back policy or the applicable award agreement. Except as the plan administrator may determine or provide in an award agreement, awards under the GTx 2019 Plan are generallynon-transferrable, except by will or the laws of descent and distribution, or, subject to the plan administrator’s consent, pursuant to a domestic relations order, and are generally exercisable only by the participant. With regard to tax withholding obligations arising in connection with awards under the GTx 2019 Plan, and exercise price obligations arising in connection with the exercise of stock options under the GTx 2019 Plan, the plan administrator may, in its discretion, accept cash, wire transfer or check, shares of common stock of GTx that meet specified conditions, a promissory note, a “market sell order,” such other consideration as the plan administrator deems suitable or any combination of the foregoing.
218
Securities Laws
The GTx 2019 Plan is intended to conform to all provisions of the Securities Act of 1933, as amended, and the Exchange Act, and any and all regulations and rules promulgated by the Securities and Exchange Commission thereunder, including without limitation Rule16b-3. The GTx 2019 Plan will be administered, and options will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations.
Material U.S. Federal Income Tax Consequences
The following summary is based on an analysis of the Code as currently in effect, existing laws, judicial decisions, administrative rulings, regulations and proposed regulations, all of which are subject to change. Moreover, the following is only a summary of United States federal income tax consequences. Actual tax consequences to participants may be either more or less favorable than those described below depending on the participant’s particular circumstances.
ISO. No income will be recognized by a participant for federal income tax purposes upon the grant or exercise of an ISO. The basis of shares transferred to a participant upon exercise of an ISO is the price paid for the shares. If the participant holds the shares for at least one year after the transfer of the shares to the participant and two years after the grant of the option, the participant will recognize capital gain or loss upon sale of the shares received upon exercise equal to the difference between the amount realized on the sale and the basis of the stock. Generally, if the shares are not held for that period, the participant will recognize ordinary income upon disposition in an amount equal to the excess of the fair market value of the shares on the date of exercise over the amount paid for the shares, or if less, the gain on disposition. Any additional gain realized by the participant upon the disposition will be a capital gain. The excess of the fair market value of shares received upon the exercise of an ISO over the option price for the shares is generally an item of adjustment for the participant for purposes of the alternative minimum tax. Therefore, although no income is recognized upon exercise of an ISO, a participant may be subject to alternative minimum tax as a result of the exercise.
NSOs. No income is expected to be recognized by a participant for federal income tax purposes upon the grant of an NSO. Upon exercise of an NSO, the participant will recognize ordinary income in an amount equal to the excess of the fair market value of the shares on the date of exercise over the amount paid for the shares. Income recognized upon the exercise of an NSO will be considered compensation subject to withholding at the time the income is recognized, and, therefore, the participant’s employer must make the necessary arrangements with the participant to ensure that the amount of the tax required to be withheld is available for payment. NSOs are designed to provide the employer with a deduction equal to the amount of ordinary income recognized by the participant at the time of the recognition by the participant, subject to the deduction limitations described below.
SARs. There is expected to be no federal income tax consequences to either the participant or the employer upon the grant of SARs. Generally, the participant will recognize ordinary income subject to withholding upon the receipt of payment pursuant to SARs in an amount equal to the aggregate amount of cash and the fair market value of any common stock received. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Restricted Stock. If the restrictions on an award of shares of restricted stock are of a nature that the shares are both subject to a substantial risk of forfeiture and are not freely transferable (within the meaning of Section 83 of the Code), the participant will not recognize income for federal income tax purposes at the time of the award unless the participant affirmatively elects to include the fair market value of the shares of restricted stock on the date of the award, less any amount paid for the shares, in gross income for the year of the award pursuant to Section 83(b) of the Code. In the absence of this election, the participant will be required to include in income for federal income tax purposes on the date the shares either become freely transferable or are no longer subject to a substantial risk of forfeiture (within the meaning of Section 83 of the Code), the fair market value of the shares of restricted stock on such date, less any amount paid for the shares. The employer will be entitled to a deduction at
219
the time of income recognition to the participant in an amount equal to the amount the participant is required to include in income with respect to the shares, subject to the deduction limitations described below. If a Section 83(b) election is made within 30 days after the date the restricted stock is received, the participant will recognize ordinary income at the time of the receipt of the restricted stock, and the employer will be entitled to a corresponding deduction, equal to the fair market value of the shares at the time, less the amount paid, if any, by the participant for the restricted stock. If a Section 83(b) election is made, no additional income will be recognized by the participant upon the lapse of restrictions on the restricted stock, but, if the restricted stock is subsequently forfeited, the participant may not deduct the income that was recognized pursuant to the Section 83(b) election at the time of the receipt of the restricted stock.
Dividends paid to a participant holding restricted stock before the expiration of the restriction period will be additional compensation taxable as ordinary income to the participant subject to withholding, unless the participant made an election under Section 83(b). Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the dividends includible in the participant’s income as compensation. If the participant has made a Section 83(b) election, the dividends will be dividend income, rather than additional compensation, to the participant.
If the restrictions on an award of restricted stock are not of a nature that the shares are both subject to a substantial risk of forfeiture and not freely transferable, within the meaning of Section 83 of the Code, the participant will recognize ordinary income for federal income tax purposes at the time of the transfer of the shares in an amount equal to the fair market value of the shares of restricted stock on the date of the transfer, less any amount paid therefore. The employer will be entitled to a deduction at that time in an amount equal to the amount the participant is required to include in income with respect to the shares, subject to the deduction limitations described below.
RSUs. There will be no federal income tax consequences to either the participant or the employer upon the grant of RSUs. Generally, the participant will recognize ordinary income subject to withholding upon the receipt of cash and/or transfer of shares of common stock in payment of the RSUs in an amount equal to the aggregate of the cash received and the fair market value of the common stock so transferred. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Generally, a participant will recognize ordinary income subject to withholding upon the payment of any dividend equivalents paid with respect to an award in an amount equal to the cash the participant receives. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Excess Parachute Payments. Section 280G of the Code limits the deduction that the employer may take for otherwise deductible compensation payable to certain individuals if the compensation constitutes an “excess parachute payment.” Excess parachute payments arise from payments made to disqualified individuals that are in the nature of compensation and are contingent on changes in ownership or control of the employer or certain affiliates. Accelerated vesting or payment of awards under the GTx 2019 Plan upon a change in ownership or control of the employer or its affiliates could result in excess parachute payments. In addition to the deduction limitation applicable to the employer, a disqualified individual receiving an excess parachute payment is subject to a 20% excise tax on the amount thereof.
Application of Section 409A of the Code. Section 409A of the Code imposes an additional 20% tax and interest on an individual receivingnon-qualified deferred compensation under a plan that fails to satisfy certain requirements. For purposes of Section 409A,“non-qualified deferred compensation” includes equity-based incentive programs, including some stock options, SARs and RSU programs. Generally speaking, Section 409A does not apply to ISOs,non-discounted NSOs and appreciation rights if no deferral is provided beyond exercise, or restricted stock.
220
The awards made pursuant to the GTx 2019 Plan are expected to be designed in a manner intended to comply with the requirements of Section 409A of the Code to the extent the awards granted under the GTx 2019 Plan are not exempt from coverage. However, if the GTx 2019 Plan fails to comply with Section 409A in operation, a participant could be subject to the additional taxes and interest.
State and local tax consequences may in some cases differ from the federal tax consequences. The foregoing summary of the income tax consequences in respect of the GTx 2019 Plan is for general information only. Interested parties should consult their own advisors as to specific tax consequences of their awards. The GTx 2019 Plan is not subject to the Employee Retirement Income Security Act of 1974, as amended, and is not intended to be qualified under Section 401(a) of the Code.
Plan Benefits
The benefits or amounts that may be received or allocated to participants under the GTx 2019 Plan will be determined at the discretion of the plan administrator and are not currently determinable. GTx expects to continue to make automatic equity awards under the GTx 2019 Plan to GTx’snon-employee directors. GTx’s current director compensation program will be suspended at the time of the closing of the merger and the director compensation policies for the combined organization following the merger will bere-evaluated by the compensation committee and board of directors of the combined organization following completion of the merger and may be subject to change.Non-employee directors of the combined organization are, however, expected to receive annual cash retainers and equity compensation, although the amount of such compensation has not yet been determined.
Vote Required for Approval
The affirmative vote of holders of a majority of the shares of GTx’s common stock having voting power outstanding on the record date for the GTx special meeting is required to approve the GTx 2019 Plan Proposal. Abstentions will have the same effect as votes “AGAINST” this proposal.
THE GTX BOARD RECOMMENDS THAT GTX’S STOCKHOLDERS VOTE “FOR” PROPOSAL NO. 4 TO APPROVE THE GTX 2019 PLAN PROPOSAL. PROPOSAL NO. 4 IS CONDITIONED UPON THE APPROVAL OF EACH OF PROPOSAL NOS. 1 AND 2.
Proposal No. 5: Advisory Vote on Merger Related Compensation
Section 14A of the Exchange Act and Rule14a-21(c) under the Exchange Act require that GTx seek a nonbinding advisory vote from its stockholders to approve the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger. For further information, see the section entitled “The Merger—Interests of GTx Directors and Executive Officers in the Merger—GTx Named Executive Officer Golden Parachute Compensation” beginning on page 153 of this proxy statement/prospectus/information statement. As required by these provisions, GTx is asking its stockholders to vote on the adoption of the following resolution:
“RESOLVED, that the compensation that will be paid or may become payable to GTx’s named executive officers in connection with the merger, as disclosed in the table entitled “ GTx Named Executive Officer Golden Parachute Compensation” pursuant to Item 402(t) of RegulationS-K, including the associated narrative discussion, and the agreements or understandings pursuant to which such compensation will be paid or may become payable, are hereby APPROVED.”
As this vote is advisory, it will not be binding upon The GTx Board or compensation committee and neither the board of directors nor the compensation committee will be required to take any action as a result of the outcome of this vote. Approval of this proposal is not a condition to completion of the merger. The vote with respect to this proposal is an advisory vote and will not be binding on GTx or Oncternal. Therefore, regardless of whether
221
GTx stockholders approve this proposal, if the merger is approved by the stockholders and completed, the merger-related compensation will still be paid to such named executive officers to the extent payable in accordance with the terms of such compensation contracts and arrangements.
Required Vote
The affirmative vote of the holders of a majority of the shares of GTx’s common stock having entitled to vote and present in person or represented by proxy at the GTx special meeting is required to approve the proposal to approve, on anon-binding, advisory basis, the “GTx named Executive Officer Golden Parachute Compensation.” Abstentions will have the same effect as votes “AGAINST” this Proposal.
THE GTX BOARD RECOMMENDS A VOTE “FOR” THIS PROPOSAL NO. 5 TO APPROVE, ON ANON-BINDING, ADVISORY BASIS, THE “GTX NAMED EXECUTIVE OFFICER GOLDEN PARACHUTE COMPENSATION.”
Proposal No. 6: Approval of Possible Adjournment of the GTx special meeting
If GTx fails to receive a sufficient number of votes to approve Proposal Nos. 1 or 2, GTx may propose to adjourn the GTx special meeting, for a period of not more than 30 days, for the purpose of soliciting additional proxies to approve Proposal Nos. 1 or 2. GTx currently does not intend to propose adjournment at the GTx special meeting if there are sufficient votes to approve Proposal Nos. 1 or 2. The affirmative vote of the holders of a majority of the shares of GTx’s common stock having entitled to vote and present in person or represented by proxy at the GTx special meeting is required to approve the adjournment of the GTx special meeting for the purpose of soliciting additional proxies to approve Proposal Nos. 1 or 2. Abstentions will have the same effect as votes “AGAINST” this Proposal.
THE GTX BOARD RECOMMENDS THAT GTX’S STOCKHOLDERS VOTE “FOR” PROPOSAL NO. 6 TO ADJOURN THE GTX SPECIAL MEETING, IF NECESSARY, TO SOLICIT ADDITIONAL PROXIES IF THERE ARE NOT SUFFICIENT VOTES IN FAVOR OF PROPOSAL NOS. 1 OR 2. EACH OF PROPOSAL 1 AND 2 ARE CONDITIONED UPON EACH OTHER AND THE APPROVAL OF EACH SUCH PROPOSAL IS REQUIRED TO CONSUMMATE THE MERGER.
222
Overview
GTx is a biopharmaceutical company dedicated to the discovery, development and commercialization of medicines to treat serious and/or significant unmet medical conditions. Under an exclusive worldwide license agreement with the University of Tennessee Research Foundation, or UTRF, GTx is developing UTRF’s proprietary potential selective androgen receptor degrader, or SARD, technology, which it believes may have the potential to provide compounds that can degrade or antagonize multiple forms of androgen receptor, or AR, thereby potentially inhibiting tumor growth in patients with progressive castration-resistant prostate cancer, or CRPC, including those patients who do not respond to or are resistant to current androgen targeted therapies. GTx has been conducting preclinical studies to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an investigational new drug application (“IND”), to enable the initiation ofa first-in-human clinical trial, if any. However, GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2) in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. Accordingly, additional preclinical research would be required in order to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies.
GTx had been developing selective androgen receptor modulators, or SARMs. GTx’s SARM product candidate, enobosarm(GTx-024), was most recently evaluated in post-menopausal women with stress urinary incontinence, or SUI. During the third quarter of 2018, GTx announced that the ASTRID trial, evaluating the change in the mean number of daily SUI episodes following 12 weeks of enobosarm treatment failed to achieve statistical significance on the primary endpoint of the proportion of patients with a greater than 50% reduction in incontinence episodes per day compared to placebo. GTx has completed the ASTRID trial, including its review of the full data sets from the clinical trial, and has determined that there is not a sufficient path forward to warrant additional clinical development of enobosarm to treat SUI. GTx has therefore discontinued further development of enobosarm to treat SUI, including discontinuing the related durability and open-label safety extension studies GTx initiated before it received topline data from the ASTRID trial. GTx has also discontinued any further development of its SARM program generally.
Following the announcement of the ASTRID trial results, the GTx Board commenced a process of evaluating strategic alternatives to maximize stockholder value. To assist with this process, the GTx Board engaged a financial advisory firm to help explore its available strategic alternatives, including possible mergers and business combinations, a sale of part or all of its assets, and collaboration and licensing arrangements as further discussed in the section titled “The Merger–Background of the Merger.” On March 6, 2019, GTx and Oncternal announced the signing of the Original Merger Agreement and, as further discussed in the section titled “The Merger–Background of the Merger,” GTx and Oncternal amended the Original Merger Agreement on April 30, 2019 Merger Agreement by entering into the Merger Agreement Amendment. Although GTx has entered into the Merger Agreement and intends to consummate the merger, there is no assurance that it will be able to successfully consummate the merger on a timely basis, or at all. If, for any reason, the merger is not completed, GTx will reconsider its strategic alternatives and could pursue one or more of the following courses of action:
| • | Continue development of GTx’s SARD program. As set forth above, GTx has conducted preclinical studies in order to determine if it can identify an appropriate SARD compound to move forward into |
223
additional preclinical studies required for the potential submission of an IND to enable the initiation ofa first-in-human clinical trial, if any. Accordingly, if, for any reason, the merger is not consummated, GTx may determine to move forward with additional preclinical research and studies of its SARD compounds. However, GTx’s existing capital resources may not be adequate to enable it to conduct and complete anyIND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete anyIND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. |
As a result, GTx may also resume its efforts to seek additional funds through potential collaborative, partnering or other strategic arrangements to provide it with the necessary resources for the development of GTx’s SARD program.
| • | Pursue potential collaborative, partnering or other strategic arrangements for GTx’s SARM assets, including a sale or other divestiture of its SARM assets. GTx has discontinued further development of its SARM technology, including enobosarm, and does not currently have any plans to resume development of its SARM technology. GTx continues its efforts to seek potential collaborative, partnering or other strategic arrangements for its SARM assets, including a sale or other divestiture of its SARM assets. |
| • | Pursue another strategic transaction like the merger. The GTx Board may elect to pursue an alternative strategy, one of which may be a strategic transaction similar to the merger. |
| • | Dissolve and liquidate GTx’s assets. If, for any reason, the merger is not consummated and GTx is unable to identify and complete an alternative strategic transaction like the merger or potential collaborative, partnering or other strategic arrangements for its SARM assets, or to continue to operate GTx’s business due to its inability to identify an appropriate SARD compound to move forward into potential IND-enabling studies or to raise additional funding for the development of its SARD program or otherwise, GTx may be required to dissolve and liquidate its assets. In such case, GTx would be required to pay all of its debts and contractual obligations, and to set aside certain reserves for potential future claims, and there can be no assurances as to the amount or timing of available cash left to distribute to its stockholders after paying its debts and other obligations and setting aside funds for reserves. |
GTx’s SARD Program
SARDs for the Potential Treatment of Castration Resistant Prostate Cancer
Scientific Overview. SARDs are a potentially novel class of drugs. The AR is a major driver of prostate tumor cell proliferation, and blocking its activity is a therapeutic target. Despite the use of therapies designed to inhibit the AR pathway in men with advanced prostate cancer, a significant number of men have tumors that do not respond to such therapeutic approaches and/or become resistant to them. This lack of response may be due to the presence of forms of the AR (splice variants and mutations) for which these therapies are not effective. SARDs are being designed to not only bind to androgen receptors, but also induce androgen receptor antagonism or potential degradation to inhibit tumor cell growth. Selective AR antagonism or degradation which targets theN-terminus may be an effective therapeutic strategy where a variant or mutated AR can be antagonized or degraded by a potential SARD. This ability to circumvent common drug resistance in prostate cancer patients, if confirmed, could provide an important tool for effective new treatments.
224
GTx believes SARDs might potentially have the potential to treat prostate cancer, as well as other diseases such as benign prostatic hyperplasia and Kennedy’s disease. GTx envisions initially developing SARDs as a potentially novel treatment of men with CRPC, including those who do not respond or are resistant to currently approved therapies. Although current therapies have improved overall survival in men with CRPC, approximatelyone-third of the CRPC patients do not respond to these therapies, due in part to the presence of splice variants, includingAR-V7, as well as the presence of mutations in the androgen receptor. Splice variants of the androgen receptor have been identified in which the ligand binding domain, the binding site for androgens and necessary for the action of many of the current therapies, is lost. In addition, most patients who initially respond to available treatments eventually progress due to the emergence of resistance to these therapies. It is believed that CRPC growth remains highly dependent on androgen receptor activity, although the mechanisms which underlie this resistance are not fully understood. GTx believes a therapeutic agent that would safely antagonize or degrade multiple forms of the androgen receptor, including those without the ligand binding domain, could be uniquely positioned to address this patient population.
Potential Market. In the United States alone, GTx believes there are approximately 80,000 men who have developed resistance to luteinizing hormone-releasing hormone (“LHRH”), therapies and therefore have CRPC but who have not received chemotherapy. GTx believes there are approximately 36,000 men diagnosed each year with metastatic hormone sensitive prostate cancer. Zytiga® and XTANDI® are currently the only drugs approved for the treatment of metastatic CRPC in patients who have not yet received chemotherapy, although several other drugs are in clinical development for this indication. GTx believes new hormonal therapies in development, if approved, will be used prior to chemotherapy as physicians and patients look for treatment options capable of delaying cancer progression and possibly prolonging survival prior to chemotherapy.
Preclinical Development. GTx has been conducting preclinical studies to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an IND to enable the initiation of a first-in-human clinical trial, if any. However, GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2)in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. Accordingly, additional preclinical research would be required in order to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies. However, GTx’s existing capital resources may not be adequate to enable it to conduct and complete any IND-enabling studies of a SARD compound, particularly in light of the conflicting preclinical SARD data it has received to date and the additional preclinical research that will be required to determine whether an appropriate SARD compound can potentially be advanced into any IND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete any IND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. Accordingly, if, for any reason, the merger is not consummated, GTx may resume its efforts to seek additional funds through potential collaborative, partnering or other strategic arrangements to provide it with the necessary resources for the development of the SARD program.
225
SARMs
Evaluation of Enobosarm for the Treatment of Postmenopausal Women with SUI. In the third quarter of 2017, GTx initiated the ASTRID trial at over 60 clinical trial centers in the United States to evaluate the change in the mean number of daily SUI episodes following 12 weeks of enobosarm treatment. The ASTRID trial evaluated the safety and efficacy of enobosarm (1 mg and 3 mg) compared with placebo in post-menopausal women who have demonstrated SUI symptoms for more than six months, with an average of 3 to 15 reported SUI episodes per day over athree-day period, and a positive bladder stress test. The primary endpoint for the ASTRID trial was the percentage of patients with at least a 50 percent reduction in mean leaks per day at week 12, compared to baseline. During the third quarter of 2018, GTx announced that the ASTRID trial failed to achieve statistical significance on the primary endpoint of the proportion of patients with a greater than 50% reduction in incontinence episodes per day compared to placebo. The percentage of patients with a greater than 50% reduction after 12 weeks of enobosarm treatment was 58.9% for 3 mg, 57.7% for 1 mg and 52.7% for placebo. Enobosarm was generally safe and well tolerated, and reported adverse events were minimal and similar across all treatment groups. GTx has completed the ASTRID trial, including GTx’s review of the full data sets from the clinical trial, and has determined that there is not a sufficient path forward to warrant additional clinical development of enobosarm to treat SUI. GTx has therefore discontinued further development of enobosarm to treat SUI, including discontinuing the related durability and open-label safety extension studies GTx initiated before it received topline data from the ASTRID trial.
Evaluation of Enobosarm for the Treatment of Breast Cancer. GTx has previously evaluated enobosarm in a Phase 2 clinical trial designed to evaluate the efficacy and safety of a 9 mg and 18 mg dose of enobosarm in patients whose advanced breast cancer is both estrogen receptor (“ER”), positive and AR positive. GTx announced in November 2016 that enobosarm achieved thepre-specified primary efficacy endpoint in the 9 mg dose cohort with 9 patients achieving a clinical benefit response (“CBR”), defined as a complete response, partial response, or stable disease, among the first 22 evaluable patients in that cohort. In November 2017, GTX announced that in the 9 mg cohort, a total of 14 patients achieved a CBR following 24 weeks of treatment. GTx also announced in November of 2017 that the 18 mg cohort achieved thepre-specified primary efficacy endpoint as 12 patients achieved a CBR at 24 weeks. Although both the 9 mg and 18 mg cohorts met the primary efficacy endpoint in the Phase 2 clinical trial, after evaluating the breast cancer environment where the treatment paradigms are shifting to immunotherapies and/or combination therapies, GTx decided in the third quarter of 2017 that the time and cost of conducting the necessary clinical trials for potential approval in this indication does not warrant further development of enobosarm in this indication. In 2015, GTx also commenced enrollment in a Phase 2proof-of-concept clinical trial designed to evaluate the efficacy and safety of an 18 mg dose of enobosarm in patients with advanced AR positive triple-negative breast cancer (“TNBC”). This clinical trial was conducted utilizing a Simon’stwo-stage trial design whereby if at least 2 of the first 21 patients achieved clinical benefit, the trial was designed to enroll the second stage, which would result in enrolling 41 evaluable patients in the clinical trial. During the third quarter of 2017, GTx completed its review of the data from the first stage of the clinical trial. While GTx’s review of the data did not raise any safety concerns, it did confirm that there were insufficient patients achieving clinical benefit from enobosarm treatment to continue this clinical trial and it closed the clinical trial down.
Discontinuation of SARM Development Efforts. Following GTx’s review of the full data sets from the ASTRID trial, GTx discontinued further development of enobosarm to treat SUI and otherwise discontinued any further development of its SARM technology. GTx continues its efforts to seek potential collaborative, partnering or other strategic arrangements for its SARM assets, including a sale or other divestiture of GTx’s SARM assets. If the merger is completed, any net proceeds derived from the disposition or licensing of its SARM assets following completion of the merger will be made available to its stockholders in accordance with the CVR Agreement. GTx has for many years actively pursued, but has been unable to successfully enter into, potential collaborative, partnering or other strategic arrangements for its SARM assets. If it is unable to ultimately enter into any such arrangements for its SARM assets, GTx will not receive any return on its investment in enobosarm and its other SARMs.
226
Licenses and Collaborative Relationships
GTx has in the past established and, if the merger is not completed, it may continue to pursue,in-licenses and partnering, and collaborative or other strategic relationships with academic institutions and with other pharmaceutical and biotechnology companies.
In March 2015, GTx and UTRF entered into a license agreement (the “SARD License Agreement”), pursuant to which GTx was granted exclusive worldwide rights in all existing SARD technologies owned or controlled by UTRF, including all improvements thereto. Under the SARD License Agreement, GTx is obligated to employ active, diligent efforts to conduct preclinical research and development activities for the SARD program to advance one or more lead compounds into clinical development. GTx is also obligated to pay UTRF annual license maintenance fees, low single-digit royalties on net sales of products and additional royalties on sublicense revenues, depending on the state of development of a clinical product candidate at the time it is sublicensed. Unless terminated earlier, the term of the SARD License Agreement will continue, on acountry-by-country basis, until the expiration of the last valid claim of any licensed patent in the particular country in which a licensed patent is granted. UTRF may terminate the SARD License Agreement for GTx’s uncured breach or upon its bankruptcy.
In July 2007, GTx and UTRF also previously entered into a consolidated, amended and restated license agreement (the “SARM License Agreement”), to consolidate and replace GTx’s two previously existing SARM license agreements with UTRF and to modify and expand certain rights and obligations of each of the parties under both license agreements. Pursuant to the SARM License Agreement, GTx was granted exclusive worldwide rights in all existing SARM technologies owned or controlled by UTRF, including enobosarm, and certain improvements thereto, and exclusive rights to certain future SARM technology that may be developed by certain scientists at the University of Tennessee or subsequently licensed to UTRF under certain existing inter-institutional agreements with The Ohio State University. Unless terminated earlier, the term of the SARM License Agreement will continue, on acountry-by-country basis, for the longer of 20 years or until the expiration of the last valid claim of any licensed patent in the particular country in which a licensed product is being sold. UTRF may terminate the SARM License Agreement for GTx’s uncured breach or upon its bankruptcy.
Under the SARM License Agreement, GTx paid UTRF aone-time, upfront fee of $290,000 as consideration for entering into the SARM License Agreement. GTx is also obligated to pay UTRF annual license maintenance fees, low single-digit royalties on net sales of products andmid-single-digit royalties on sublicense revenues. GTx also agreed to pay all expenses to file, prosecute and maintain the patents relating to the licensed SARM technologies, and is obligated to use commercially reasonable efforts to develop and commercialize products based on the licensed SARM technologies. While GTx currently has ceased development efforts for SARMs, it continues to seek potential collaborative, partnering or other strategic arrangements for its SARM assets, including a sale or other divestiture of its SARM assets. In December 2008, GTx and UTRF amended the SARM License Agreement (the “SARM License Amendment”), to, among other things, clarify the treatment of certain payments that GTx may receive from its current and future sublicensees for purposes of determining sublicense fees payable to UTRF, including the treatment of payments made to GTx in exchange for the sale of its securities in connection with sublicensing arrangements. In consideration for the execution of the SARM License Amendment, GTx paid UTRF $494,000.
Manufacturing
GTx does not currently own or operate manufacturing facilities, and it relies, and expects to continue to rely, on third parties for the production of clinical and commercial quantities of any product candidates.
There are no complicated chemistries or unusual equipment required in the manufacturing process for either SARMs or SARDs. GTx relies and expects to continue to rely on third-party vendors for drug substance and drug product manufacturing, including drug substance for SARDs used in its current and potential future preclinical studies.
227
Competition
The biotechnology and biopharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. GTx faces competition from many different sources, including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions.
Many of GTx’s competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than GTx does. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. GTx’s commercial opportunities will be reduced or eliminated if its competitors develop and commercialize similar products that are safer, more effective, have fewer side effects or are less expensive than any products that it and/or its collaborators may develop.
SARDs for the Potential Treatment of CRPC
GTx has entered into an exclusive worldwide license agreement with UTRF to develop its proprietary SARD technology which GTx believes may have the potential to provide compounds that can degrade or antagonize multiple forms of the AR thereby inhibiting tumor growth in patients with CRPC, including those patients who do not respond or are resistant to current therapies. Drugs in development having potentially similar approaches to removing the AR by degradation include Arvinas Inc.’sARV-110, which is a chimera with an AR binding moiety on one end and an E3 ligase recruiting element on the other that has recently entered Phase 1 development for the treatment of advanced prostate cancer, and Androscience Corporation’s androgen receptor degrader enhancer,ASC-J9, which is currently in development for acne and alopecia with the potential for development as a treatment for prostate cancer. Additionally, Essa Pharma Inc. recently completed a Phase 1 study withEPI-506, an AR antagonist that targets theN-terminal domain of the AR, and has plans to develop a second generation agent. C4 Therapeutics, Inc. is developing degronimids as means to degrade the AR through the ligand binding domain associated degradation. CellCentric is developing therapies that target the histone methyltransferase enzyme to lower AR levels, and recently initiated a clinical trial with CCS1477 in prostate cancer. Oric Pharmaceuticals is targeting the glucocorticoid receptor as a means to impact men that have CRPC, and has a lead candidateORIC-101 in preclinical testing. In addition to this specific potential mechanistic competition, there are various products approved or under clinical development in the broader space of treating men with advanced prostate cancer who have metastatic CRPC which may compete with GTx’s proposed initial clinical objective for GTx’s SARD compounds. Pfizer and Astellas Pharma market XTANDI® (enzalutamide), an oral androgen receptor antagonist, for the treatment of metastatic CRPC in men previously treated with docetaxel as well as those that have not yet received chemotherapy. XTANDI® received FDA approval in July 2018 for the treatment of men withnon-metastatic CRPC. Zytiga®, sold by Johnson & Johnson, has been approved for the treatment of metastatic CRPC and metastatic high-risk castration-sensitive prostate cancer. Johnson & Johnson also received FDA approval for a second generation anti-androgen ERLEADA (apalutamide) for the treatment of men withnon-metastatic castrate-resistant prostate cancer. Bayer HealthCare and Orion Corporation recently announced that the primary endpoint of increased metastatic free survival was met in a Phase 3 study of darolutamide(ODM-201) in men with CRPC without metastases and with a rising PSA. Another target in prostate cancer that is being pursued by several companies is bromodomain inhibition. Zenith Epigenetics, Gilead Sciences Inc., CellCentric, Incyte Corporation and GlaxoSmithKline are among the companies that are evaluating BET inhibitors inPhase 1-2 trials.
SARMs
With respect to SARMs, there are other SARM product candidates in development that may compete with enobosarm and any future SARM product candidates, if approved for commercial sale. For example, Viking Therapeutic’s VK5211 recently reported positive results from a Phase 2 study for patients recovering from
228
non-elective hip fracture surgery. Radius Health Inc.’s RAD140 is currently being evaluated in a Phase 1 study in postmenopausal women with hormone-receptor positive locally advanced or metastatic breast cancer. GlaxoSmithKline is conducting a Phase 1 study to assess the effect of GSK2881078 on physical strength and function after 13 weeks of treatment in patients with chronic obstructive pulmonary disease, or COPD, and muscle weakness. OPKO Health’s OPK88004 is enrolling in a dose ranging study to improve symptoms of benign prostatic hyperplasia (BPH) by reducing prostate size and, on the basis of data from a previous trial in 350 men, increase muscle mass and bone strength and decrease body fat.
Intellectual Property
GTx will be able to protect its technology from unauthorized use by third parties only to the extent it is covered by valid and enforceable patents or is effectively maintained as trade secrets. Patents and other proprietary rights are an essential element of its business.
For its SARD compounds and methods of use thereof, GTx has filed certain patent applications in the United States, Canada, Mexico, Australia, Japan, China, and other countries in Asia and before the European Patent Office and is the exclusive licensee of worldwide rights for the SARD technology under a license agreement with UTRF executed in 2015. Thus far GTx has six issued patents and one is allowed, all in the United States. The patents and patent applications (if are issued) will expire between 2036 and 2039.
For enobosarm and its other SARM compounds, GTx has an exclusive license from UTRF under its issued patents and pending patent applications in the United States, Canada, Australia, Japan, China and other countries in Asia, before the European Patent Office designating Germany, Great Britain, Spain, France, Italy, and other European Union countries, as well as in certain other countries outside those regions, covering the composition of matter of the active pharmaceutical ingredient for pharmaceutical products, pharmaceutical compositions and methods of synthesizing the active pharmaceutical ingredients. GTx has also exclusively licensed from UTRF issued and pending patent applications in the United States, Canada, Australia, Japan, China and other countries in Asia, before the European Patent Office designating Germany, Great Britain, Spain, France, Italy and other European Union countries, as well as in certain other countries outside those regions, related to methods for treating muscle wasting disorders, including Duchenne Muscular Dystrophy (“DMD”), and cancer cachexia, and for treating conditions such as SUI and fecal incontinence, as well as sarcopenia, and increasing muscle performance, muscle size and muscle strength and increasing the strength of or mass of a bone and for treating bone related disorders, including bone frailty and osteoporosis. Issued patents for enobosarm composition of matter that GTx licensed from UTRF and issued in the United States expire in 2024. Issued patents for composition of matter for its other SARM compounds in the United States will expire from 2021-2029, depending on the specific SARM compound. The issued patents outside of the United States for enobosarm expire in 2025, and with respect to other SARM compounds, expire in 2023 and 2027, depending on the specific SARM compound. GTx has pending patent applications directed to composition of matter and methods of use for its other SARM compounds that, if issued, would expire in the United States and in countries outside the United States in 2027. GTx has issued patents in the United States, and issued patents and pending applications in countries outside the United States for enobosarm and certain other SARM compounds as a feed composition for animals. The patents in the United States will expire in 2025. Issued patents outside the United States, and patent applications, if issued, which are pending outside the United States, will expire in 2027 or 2031 depending on the country. Patent applications which are pending in the United States and outside the United States using SARMs for SUI and pelvic floor disorders will expire in 2035, if the patents are issued. GTx’s issued patent in the United States using enobosarm for DMD will expire in 2021. GTx’s issued patent in the United States using other SARMs for DMD will expire in 2024. Patent applications, if issued, which are pending in the United States, using other SARMs for DMD will expire in 2024 or 2027 depending on the SARM.
GTx has its own issued patents and pending patent applications in the United States, Canada, Australia, Europe, Japan, China and other countries in Asia, as well as in certain other countries outside those regions, related to solid forms of enobosarm. Issued patents covering solid forms of enobosarm in the United States will expire in
229
2029. Issued patents and pending patent applications, if issued, in countries outside of the United States will expire in 2028. GTx has its own pending patent applications and issued patents in the United States and in Europe, Canada, Australia, Japan, China and other countries in Asia related to methods of treating breast cancer using its SARM compounds. Such patents and patent applications, if issued, would expire in 2033 in the United States and outside of the United States. GTx has issued patents in the United States directed to androgen receptor positive breast cancer in general, various categories of estrogen receptor and androgen receptor positive breast cancer, as well as triple negative breast cancer.
GTx cannot be certain that any of its pending patent applications, or those of UTRF, will result in issued patents. In addition, because the patent positions of biopharmaceutical companies are highly uncertain and involve complex legal and factual questions, the patents it owns and licenses, or any further patents it may own or license, may not prevent other companies from developing similar or therapeutically equivalent products. Patents also will not protect GTx’s product candidates if competitors devise ways of making or using these product candidates without legally infringing its patents. In recent years, several companies have been extremely aggressive in challenging patents covering pharmaceutical products, and the challenges have often been successful. GTx cannot be assured that its patents will not be challenged by third parties or that it will be successful in any defense it undertakes. Failure to successfully defend a patent challenge could materially and adversely affect its business.
In addition, changes in patent laws, rules or regulations or in their interpretations in the United States and other countries by the courts may materially diminish the value of GTx’s intellectual property or narrow the scope of its patent protection, which could have a material adverse effect on its business and financial condition.
GTx also relies on trade secrets, technicalknow-how and continuing innovation to develop and maintain its competitive position. GTx seeks to protect its proprietary information by requiring its employees, consultants, contractors, outside scientific collaborators and other advisors to executenon-disclosure and confidentiality agreements and its employees to execute assignment of invention agreements to it on commencement of their employment. Agreements with its employees also prevent them from bringing any proprietary rights of third parties to GTx. GTx also requires confidentiality or material transfer agreements from third parties that receive its confidential data or materials.
Government Regulation
New Drug Development and Approval Process
Numerous governmental authorities in the United States and other countries extensively regulate the testing, clinical development, manufacturing and marketing of pharmaceutical products and ongoing research and development activities. In the United States, the FDA rigorously reviews pharmaceutical products under the Federal Food, Drug, and Cosmetic Act and applicable regulations.Non-compliance with FDA regulations can result in administrative and judicial sanctions, including warning or untitled letters, clinical holds, fines, recall or seizure of products, injunctions, total or partial suspension of production, refusal of the government to approve marketing applications or allow entry into supply contracts, refusal to permit import or export of products, civil penalties, criminal prosecution and other actions affecting a company and its products. The FDA also has the authority to revoke previously granted marketing authorizations.
To secure FDA approval, an applicant must submit extensive preclinical and clinical data, as well as information about product manufacturing processes and facilities and other supporting information to the FDA for each indication to establish a product candidate’s safety and efficacy. The development and approval process takes many years, requires the expenditure of substantial resources and may be subject to delays or limitations of approval or rejection of an applicant’s new drug application (“NDA”). Even if the FDA approves a product, the approval is subject to post-marketing surveillance, adverse drug experience and other recordkeeping and reporting obligations, and may involve ongoing requirements for post-marketing studies. The FDA also has
230
authority to place conditions on any approvals that could restrict the commercial applications, advertising, promotion or distribution of these products. Product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
Preclinical and Clinical Testing
Preclinical studies involve laboratory evaluation of product characteristics and animal studies to assess the biological activity and safety of the product. In some cases, long-term preclinical studies are conducted while clinical studies are ongoing. The FDA, under its Good Laboratory Practices regulations, regulates preclinical studies. Violations of these regulations can, in some cases, lead to invalidation of the studies, requiring these studies to be replicated. When the preclinical testing is considered adequate by the sponsor to demonstrate the safety and scientific rationale for initial human studies, the results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND submission. The IND becomes effective, if not rejected by the FDA, within 30 days after the FDA receives the IND. The FDA may, either during the30-day period after filing of an IND or at any future time, impose a clinical hold on proposed or ongoing clinical trials on various grounds, including that the study subjects are or would be exposed to an unreasonable and significant health risk. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA authorization and then only under terms authorized by the FDA.
Clinical trials involve the administration of the investigational product candidates to humans under the supervision of a qualified principal investigator. Clinical trials must be conducted in accordance with Good Clinical Practices under protocols submitted to the FDA as part of the IND. In addition, each clinical trial must be approved and conducted under the auspices of an Investigational Review Board (“IRB”), and with patient informed consent. The IRB typically considers, among other things, ethical factors and the safety of human subjects.
Clinical trials are conducted in three sequential phases, but the phases may overlap. Phase 1 clinical trials usually involve healthy human subjects. The goal of a Phase I clinical trial is to establish initial data about the safety, tolerability and pharmacokinetic properties of the product candidates in humans. In Phase 2 clinical trials, controlled studies are conducted on an expanded population of patients with the targeted disease. The primary purpose of these tests is to evaluate the initial effectiveness of the product candidate on the intended target and to determine if there are any side effects or other risks associated with the drug and to determine the optimal dose of the drug from the safety and efficacy profile developed from the clinical study. Phase 3 trials involve even larger patient populations, often with several hundred or even several thousand patients, depending on the use for which the drug is being studied. Phase 3 trials are intended to establish the overall risk-benefit ratio of the drug and provide, if appropriate, an adequate basis for product labeling. During all clinical trials, physicians monitor the patients to determine effectiveness and to observe and report any reactions or other safety risks that may result from use of the product candidate.
Product Formulation and Manufacture
Concurrent with clinical trials and preclinical studies, companies must develop information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product. In addition, manufacturers, including contract manufacturers, are required to comply with current applicable FDA Good Manufacturing Practice, or cGMP, regulations. The cGMP regulations include requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation. The manufacturing process must be capable of consistently producing quality batches of the product and the manufacturer must develop methods for testing the quality, purity and potency of the final drugs. Additionally, appropriate packaging must be selected and tested and chemistry stability studies must be conducted to demonstrate that the product does not undergo unacceptable deterioration over its shelf-life.
231
Compliance with cGMP regulations also is a condition of new drug application approval. The FDA must approve manufacturing facilities before they can be used in the commercial manufacture of drug products. In addition, manufacturing establishments are subject topre-approval inspections and unannounced periodic inspections.
New Drug Application Process
After the completion of the clinical trial phases of development, if the sponsor concludes that there is substantial evidence that the product candidate is safe and effective for its intended use, the sponsor may submit a NDA to the FDA. The application must contain all of the information on the product candidate gathered to that date, including data from the clinical trials, and be accompanied by a user fee.
Under the Prescription Drug User Fee Act (“PDUFA”), submission of a NDA with clinical data requires payment of a fee, with some exceptions. In return, the FDA assigns a goal of six or ten months from filing of the application to return of a first “complete response,” in which the FDA may approve the product or request additional information. There can be no assurance that an application will be approved within the performance goal timeframe established under PDUFA. The FDA initially determines whether a NDA as submitted is acceptable for filing. The FDA may refuse to file an application, in which case the FDA retainsone-half of the user fees. If the submission is accepted for filing, the FDA begins anin-depth review of the application. As part of this review, the FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians, for review, evaluation and a recommendation. The FDA is not bound by the recommendation of an advisory committee.
If the FDA evaluations of the NDA and the manufacturing facilities are favorable, the FDA may issue an approval letter authorizing commercial marketing of the product candidate for specified indications. The FDA could also issue a “complete response” letter at the end of the review period. A “complete response” letter will be issued to let a company know that the review period for a drug is complete and that the application is not yet ready for approval. The letter will describe specific deficiencies and, when possible, will outline recommended actions the applicant might take to get the application ready for approval, including calling for additional clinical trial data.
Marketing Approval and Post-Marketing Obligations
If the FDA approves an application, the drug becomes available for physicians to prescribe. Periodic reports must be submitted to the FDA, including descriptions of any adverse reactions reported. The FDA may require post-marketing studies, also known as Phase IV studies, as a condition of approval. In addition to studies required by the FDA after approval, trials and studies are often conducted to explore new indications for the drug. The purpose of these trials and studies and related publications is to develop data to support additional indications for the drug, which must be approved by the FDA, and to increase its acceptance in the medical community. In addition, some post-marketing studies are done at the request of the FDA to develop additional information regarding the safety of a product.
The FDA may impose risk evaluation mitigation strategies (“REMS”), on a product if the FDA believes there is a reason to monitor the safety of the drug in the marketplace. REMS could add training requirements for healthcare professionals, safety communications efforts, and limits on channels of distribution, among other things. The sponsor would be required to evaluate and monitor the various REMS activities and adjust them if need be. Whether a REMS would be imposed on a product and any resulting financial impact is uncertain at this time.
Any products manufactured or distributed pursuant to FDA approvals are subject to continuing regulation by the FDA, including record keeping requirements, reporting of adverse experiences with the drug, drug sampling and distribution requirements, notifying the FDA and gaining its approval of certain manufacturing or labeling changes, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. Drug manufacturers and their subcontractors are required to register
232
their establishments and are subject to periodic unannounced inspections for compliance with cGMP requirements. Also, newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, or even in some instances revocation or withdrawal of the product’s approval.
Approval Outside of the United States
In order to market any product outside of the United States, GTx must comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales and distribution of GTx’s products, which broadly reflect the issues addressed by the FDA above. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from and be longer than that required to obtain FDA approval. Marketing approval in one country does not ensure marketing approval in another, but a failure or delay in obtaining marketing approval in one country may negatively impact the regulatory process in other countries.
As in the United States, the marketing approval process in Europe and in other countries is a lengthy, challenging and inherently uncertain process. If GTx fails to comply with applicable foreign regulatory requirements, it may be subject to fines, suspension or withdrawal of marketing approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. Generally the development and approval procedures are harmonized throughout the European Union: however, there is limited harmonization in relation to national pricing and reimbursement practices.
Under European Union regulatory systems, a company may not market a medicinal product without marketing authorization. There are three procedures for submitting a MAA in the EU: (1) the mutual recognition procedure (“MRP”); (2) the decentralized procedure (“DCP”) and (3) the centralized procedure (“CP”). The submission strategy for a given product will depend on the nature of the product, the target indication(s), the history of the product, and the marketing plan. The centralized procedure is compulsory for medicinal products which are produced by biotechnology processes, advanced therapy medicinal products and orphan drugs. Besides the products falling under the mandatory scope, the centralized procedure is also open for other innovative products that are new active substances or other medicinal products that constitute a significant therapeutic, scientific or technical innovation.
The centralized procedure leads to approval of the product in all 27 EU member states and in Norway, Iceland and Liechtenstein. Submission of one MAA thus leads to one assessment process and one authorization that allows access to all applicable markets within the entire EU. The process of the centralized procedure is triggered when the applicant sends the letter announcing the intent to submit a MAA (letter of intent). The letter of intent also initiates the assignment of the Rapporteur andCo-Rapporteur, who are the two appointed members of the Committee for Human Medicinal Products (“CHMP”), representing two EU member states. However, in light of the United Kingdom’s vote in 2016 to leave the European Union, theso-called Brexit vote, there may be changes forthcoming in the scope of the centralized approval procedure as the terms of that exit are negotiated between the UK and the European Union.
When using the MRP or DCP, the applicant must select which and how many EU member states in which to seek approval. In the case of an MRP, the applicant must initially receive national approval in one EU member state. This will be theso-called reference member state (“RMS”) for the MRP. Then, the applicant seeks approval for the product in other EU member states, theso-called concerned member states (“CMS”) in a second step: the mutual recognition process. For the DCP, the applicant will approach all chosen member states at the same time. To do so, the applicant will identify the RMS that will assess the submitted MAA and provide the other selected member states with the conclusions and results of the assessment.
233
When the application for marketing authorization is made, the competent authority responsible for granting a marketing authorization must verify whether the application complies with the relevant requirements, including compliance with the agreed pediatric investigational plan (“PIP”). Assuming it does, the marketing authorization may be granted and the relevant results are included in the summary of product characteristics (“SmPC”) for the product, along with a statement indicating compliance with the agreed PIP. It is not necessary for the product actually to be indicated for use in the pediatric population (for example, if the results show that that would not be appropriate).
Drug Price Competition and Patent Term Restoration Act of 1984
Under the Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Act, a portion of a product’s patent term that was lost during clinical development and application review by the FDA may be restored. The Hatch-Waxman Act also provides for a statutory protection, known as exclusivity, against the FDA’s acceptance or approval of certain competitor applications. The Hatch-Waxman Act also provides the legal basis for the approval of abbreviated new drug applications (“ANDAs”).
Patent term extension can compensate for time lost during product development and the regulatory review process by returning up to five years of patent life for a patent that covers a new product or its use. This period is generallyone-half the time between the effective date of an IND and the submission date of a NDA, plus the time between the submission date of a NDA and the approval of that application. Patent term extensions, however, are subject to a maximum extension of five years, and the patent term extension cannot extend the remaining term of a patent beyond a total of 14 years.
The application for patent term extension is subject to approval by the United States Patent and Trademark Office in conjunction with the FDA. It generally takes at least six months to obtain approval of the application for patent term extension.
The Hatch-Waxman Act also provides for a period of statutory protection for new drugs that receive NDA approval from the FDA. If a new drug receives NDA approval as a new chemical entity, meaning that the FDA has not previously approved any other new drug containing the same active entity, then the Hatch-Waxman Act prohibits an ANDA or a NDA submitted pursuant to section 505(b)(2) of the Federal Food, Drug, and Cosmetics Act, where the applicant does not own or have a legal right of reference to all of the data required for approval to be submitted by another company for a generic version of such drug (505(b)(2) NDA), with some exceptions, for a period of five years from the date of approval of the NDA. The statutory protection provided pursuant to the Hatch-Waxman Act will not prevent the filing or approval of a full NDA, as opposed to an ANDA or 505(b)(2) NDA, for any drug, including, for example, a drug with the same active ingredient, dosage form, route of administration, strength and conditions of use. In order to obtain a NDA, however, a competitor would be required to conduct its own clinical trials, and any use of the drug for which marketing approval is sought could not violate another NDA holder’s patent claims.
If NDA approval is received for a new drug containing an active ingredient that was previously approved by the FDA but the NDA is for a drug that includes an innovation over the previously approved drug, for example, a NDA approval for a new indication or formulation of the drug with the same active ingredient, and if such NDA approval was dependent upon the submission to the FDA of new clinical investigations, other than bioavailability studies, then the Hatch-Waxman Act prohibits the FDA from making effective the approval of an ANDA or 505(b)(2) NDA for a generic version of such drug for a period of three years from the date of the NDA approval. This three year exclusivity, however, only covers the innovation associated with the NDA to which it attaches. Thus, the three year exclusivity does not prohibit the FDA, with limited exceptions, from approving ANDAs or 505(b)(2) NDAs for drugs containing the same active ingredient but without the new innovation.
While the Hatch-Waxman Act provides certain patent restoration and exclusivity protections to innovator drug manufacturers, it also permits the FDA to approve ANDAs for generic versions of their drugs assuming the
234
approval would not violate another NDA holder’s patent claims. The ANDA process permits competitor companies to obtain marketing approval for a drug with the same active ingredient for the same uses but does not require the conduct and submission of clinical studies demonstrating safety and effectiveness for that product. Instead of safety and effectiveness data, an ANDA applicant needs only to submit data demonstrating that its product is bioequivalent to the innovator product as well as relevant chemistry, manufacturing and product data. The Hatch-Waxman Act also instituted a third type of drug application that requires the same information as a NDA, including full reports of clinical and preclinical studies, except that some of the information from the reports required for marketing approval comes from studies which the applicant does not own or have a legal right of reference. This type of application, a 505(b)(2) NDA, permits a manufacturer to obtain marketing approval for a drug without needing to conduct or obtain a right of reference for all of the required studies.
If a competitor submits an ANDA or 505(b)(2) NDA for a compound or use of any compound covered by another NDA holder’s patent claims, the Hatch-Waxman Act requires, in some circumstances, the applicant to notify the patent owner and the holder of the approved NDA of the factual and legal basis of the applicant’s opinion that the patent is not valid or will not be infringed. Upon receipt of this notice, the patent owner and the NDA holder have 45 days to bring a patent infringement suit in federal district court and obtain a30-month stay against the company seeking to reference the NDA. The NDA holder could still file a patent suit after the 45 days, but if they miss the45-day deadline, they would not have the benefit of the30-month stay. Alternatively, after this45-day period, the applicant may file a declaratory judgment action, seeking a determination that the patent is invalid or will not be infringed. Depending on the circumstances, however, the applicant may not be able to demonstrate a controversy sufficient to confer jurisdiction on the court. The discovery, trial and appeals process in such suits can take several years. If such a suit is commenced, the Hatch-Waxman Act provides a30-month stay on the approval of the competitor’s ANDA or 505(b)(2) NDA. If the litigation is resolved in favor of the competitor or the challenged patent expires during the30-month period, unless otherwise extended by court order, the stay is lifted and the FDA may approve the application. Under regulations issued by the FDA, and essentially codified under the Medicare prescription drug legislation, the patent owner and the NDA holder have the opportunity to trigger only a single 30-month stay per ANDA or 505(b)(2) NDA. Once the applicant of the ANDA or 505(b)(2) NDA has notified the patent owner and the NDA holder of the infringement, the applicant cannot be subjected to another30-month stay, even if the applicant becomes aware of additional patents that may be infringed by its product.
Pharmaceutical Pricing and Reimbursement
GTx currently has no marketed products. In both domestic and foreign markets, sales of any products for which GTx receives regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors. Third-party payors include government authorities or programs, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. GTx may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of its products. GTx’s product candidates may not be considered cost-effective. Adequate third-party reimbursement may not be available to enable it to maintain price levels sufficient to realize an appropriate return on GTx’s investment in product development. Third-party payors may also control access to, or manage utilization of, its products with various utilization management techniques, such as requiring prior authorization for coverage of its products.
Within the United States, if GTx obtains appropriate approval in the future to market any of its oral drug product candidates, those products could potentially be covered by various government health benefit programs as well as purchased by government agencies. The participation in such programs or the sale of products to such agencies is subject to regulation. The marketability of any products for which GTx receives regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement.
235
Medicaid is a joint federal and state program that is administered by the states for low income and disabled beneficiaries. Under the Medicaid Drug Rebate Program, participating manufacturers are required to pay a rebate for each unit of product reimbursed by the state Medicaid programs. The amount of the rebate for each product is set by law and may be subject to an additional discount if certain pricing increases more than inflation.
Medicare is a federal program that is administered by the federal government that covers individuals age 65 and over as well as those with certain disabilities. Oral drugs may be covered under Medicare Part D. Medicare Part D provides coverage to enrolled Medicare patients for self-administered drugs (i.e., drugs that do not need to be injected or otherwise administered by a physician). Medicare Part D is administered by private prescription drug plans approved by the U.S. government and each drug plan establishes its own Medicare Part D formulary for prescription drug coverage and pricing, which the drug plan may modify fromtime-to-time. The prescription drug plans negotiate pricing with manufacturers and may condition formulary placement on the availability of manufacturer discounts. Since 2011, manufacturers with marketed brand name drugs have been required to provide a 50% discount the negotiated price for on brand name prescription drugs utilized by Medicare Part D beneficiaries when those beneficiaries reach the coverage gap in their drug benefits, and, beginning in 2019, that discount increased to 70%.
Drug products are subject to discounted pricing when purchased by federal agencies via the Federal Supply Schedule (“FSS”). FSS participation is required for a drug product to be covered and reimbursed by certain federal agencies and for coverage under Medicaid, Medicare Part B and the Public Health Service (“PHS”) pharmaceutical pricing program. FSS pricing is negotiated periodically with the Department of Veterans Affairs. FSS pricing is intended not to exceed the price that a manufacturer charges its most-favorednon-federal customer for its product. In addition, prices for drugs purchased by the Veterans Administration, Department of Defense (including drugs purchased by military personnel and dependents through the TRICARE retail pharmacy program), Coast Guard, and PHS are subject to a cap on pricing (known as the “federal ceiling price”) and may be subject to an additional discount if pricing increases more than the rate of inflation.
To maintain coverage of drugs under the Medicaid Drug Rebate Program, manufacturers are required to extend discounts to certain purchasers under the PHS pharmaceutical pricing program. Purchasers eligible for discounts include hospitals that serve a disproportionate share of financially needy patients, community health clinics and other entities that receive health services grants from the PHS.
The United States and state governments continue to propose and pass legislation designed to reform delivery of, or payment for, health care, which include initiatives to reduce the cost of healthcare. For example, in March 2010, the United States Congress enacted the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act (“Healthcare Reform Act”) which includes changes to the coverage and reimbursement of drug products under government health care programs. Under the Trump administration, there have been ongoing efforts to modify or repeal all or certain provisions of the Healthcare Reform Act. For example, tax reform legislation was enacted at the end of 2017 that eliminates the tax penalty for individuals who do not maintain sufficient health insurance coverage beginning in 2019 (theso-called “individual mandate”). In a May 2018 report, the Congressional Budget Office estimated that, compared to 2018, the number of uninsured will increase by 3 million in 2019 and 6 million in 2028, in part due to the elimination of the individual mandate. The Healthcare Reform Act has also been subject to judicial challenge. In December 2018, a federal district court judge, in a challenge brought by a number of state attorneys general, found the Healthcare Reform Act unconstitutional in its entirety because, once Congress repealed the individual mandate provision, there was no longer a basis to rely on Congressional taxing authority to support enactment of the law. Pending appeals, which could take some time, the Healthcare Reform Act is still operational in all respects.
There have also been other reform initiatives under the Trump Administration, including initiatives focused on drug pricing. For example, in May of 2018, President Trump and the Secretary of the Department of Health and Human Services released a “blueprint” to lower prescription drug prices andout-of-pocket costs. Certain proposals in the blueprint, and related drug pricing measures proposed since the blueprint, could cause significant
236
operational and reimbursement changes for the pharmaceutical industry. As another example, in November of 2018, CMS issued an advance notice of proposed rulemaking that proposed revisions to Medicare Part D to support health plans’ negotiation of lower drug prices with manufacturers and reduce health plan members’out-of-pocket costs. The HHS Office of Inspector General also issued a proposed rule in February of 2019 that would revise the federal anti-kickback statute to limit protection for discounts offered by pharmaceutical manufacturers to pharmacy benefit managers (“PBMs”), Medicare Part D plans, and Medicaid managed care plans that are not reflected in the price charged to the patient at the pharmacy counter and to provide protection only for certain types of service fees paid by pharmaceutical manufacturers to PBMs.
Recently, there has been considerable public and government scrutiny in the U.S. of pharmaceutical pricing and proposals to address the perceived high cost of pharmaceuticals. There have also been several recent state legislative efforts to address drug costs, which generally have focused on increasing transparency around drug costs or limiting drug prices or price increases. Adoption of new legislation at the federal or state level could affect demand for, or pricing of, GTx’s product candidates if approved for sale.
GTx cannot predict the ultimate content, timing or effect of any changes to the Healthcare Reform Act or other federal and state reform efforts. There is no assurance that federal or state health care reform will not adversely affect its future business and financial results.
Although GTx currently has no products approved for commercial sale, GTx marketed FARESTON through September 30, 2012 and the product was covered under various government health benefit programs as well as purchased by federal agencies. GTx could be subject to liability under federal laws regulating GTx’s participation in such programs or the sale of GTx’s product to such agencies if it failed to comply with applicable requirements, including reporting prices for its products or offering products for sale at certain prices.
Regulations Pertaining to Sales and Marketing
Although GTx currently has no products approved for commercial sale, GTx may be subject to various federal and state laws pertaining to health care “fraud and abuse,” including anti-kickback laws and false claims laws for activities related to its previous sales of FARESTON, which GTx sold to a third-party in 2012, or to future sales of any of its product candidates that may in the future receive regulatory and marketing approval. Anti-kickback laws generally prohibit a prescription drug manufacturer from soliciting, offering, receiving, or paying any remuneration to generate business, including the purchase or prescription of a particular drug. Although the specific provisions of these laws vary, their scope is generally broad and there may not be regulations, guidance or court decisions that apply the laws to particular industry practices. There is therefore a possibility that GTx’s practices might be challenged under such anti-kickback laws. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented, any claims for payment for reimbursed drugs or services to third-party payors (including Medicare and Medicaid) that are false or fraudulent. Violations of fraud and abuse laws may be punishable by criminal or civil sanctions, including fines and civil monetary penalties, and/or exclusion from federal health care programs (including Medicare and Medicaid).
Laws and regulations have been enacted by the federal government and various states to regulate the sales and marketing practices of pharmaceutical manufacturers with marketed products. The laws and regulations generally limit financial interactions between manufacturers and health care providers and/or require disclosure to the government and public of such interactions. Many of these laws and regulations contain ambiguous requirements or require administrative guidance for implementation. Given the lack of clarity in laws and their implementation, GTx’s prior activities (when it marketed FARESTON) or any future activities (if it obtains approval and/or reimbursement from federal healthcare programs for its product candidates) could be subject to the penalty provisions of the pertinent laws and regulations.
237
Employees
As of March 31, 2019, GTx had 13 employees, three of whom were M.D.s, Pharm.D.s and/or Ph.D.s. None of GTx’s employees are subject to a collective bargaining agreement. GTx believes that it has good relations with its employees.
Available Information
GTx was originally incorporated under the name Genotherapeutics, Inc. in Tennessee in September 1997. GTx changed its name to GTx, Inc. in 2001, and it reincorporated in Delaware in 2003. GTx’s principal executive office is located at 17 W Pontotoc Ave., Suite 100, Memphis, TN 38103, and its telephone number is(901) 523-9700.
GTx files electronically with the U.S. Securities and Exchange Commission, or SEC, its annual reports onForm 10-K, quarterly reports onForm 10-Q, current reports onForm 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934.
238
Overview
Oncternal Therapeutics, Inc. is a clinical-stage biopharmaceutical company focused on developing a diverse pipeline of product candidates for cancers with critical unmet medical need. The company’s development efforts are focused on promising, yet untreated biological pathways implicated in cancer generation or progression. Receptor tyrosine kinase-like Orphan Receptor 1 (“ROR1”), is a growth factor receptor that is widely expressed on many tumors and whose overexpression has been correlated with poor prognosis, which activates pathways that lead to increased tumor proliferation, invasiveness and drug resistance. Oncternal’s lead product candidate is cirmtuzumab, a monoclonal antibody that is designed to inhibit the ROR1 receptor, which is being evaluated in a Phase 1/2 clinical trial in combination with ibrutinib for the treatment of chronic lymphocytic leukemia (“CLL”), and mantle cell lymphoma (“MCL”), and in a Phase 1b clinical trial in combination with paclitaxel for women with metastatic breast cancer. Oncternal is also developing TK216, a small molecule that is designed to inhibit ETS, or E26 Transformation Specific, family oncoproteins, which alter gene transcription and RNA processing and lead to increased cell proliferation and invasion. TK216 is being evaluated in a Phase 1 clinical trial, alone and in combination with vincristine, in patients with relapsed or refractory Ewing sarcoma, a rare pediatric cancer. In addition, Oncternal is developing aCAR-T product candidate that targets ROR1, which is currently in preclinical development as a potential treatment for solid tumors and hematologic cancers including AML.
Cirmtuzumab targets ROR1, a receptor that is widely expressed on many tumors, but one that has not been successfully targeted by other therapies. Researchers at UC San Diego discovered that targeting a critical epitope on ROR1 was the key to specifically targeting ROR1 expressing tumors and this finding led to the discovery of the potent and highly selective activity of cirmtuzumab observed in preclinical studies. ROR1 activates pathways that lead to increased cancer cell proliferation, invasiveness and drug resistance. Oncternal believes ROR1 is an attractive target for cancer therapy because it is an oncofetal antigen – a protein not normally expressed in adults. Overexpression of ROR1 in tumors results in cancer cells becoming less differentiated, increasing their ability to self-renew and metastasize by increasing cell migration and the ability to initiate new tumors. Patients with tumors that overexpress ROR1 have poor prognoses, consistent with the increased cell migration, tumor initiation, and chemotherapy resistance observed in preclinical models. Oncternalin-licensed cirmtuzumab from UC San Diego and is developing it in collaboration with UC San Diego and CIRM.
Cirmtuzumab is a potentialfirst-in-class antibody product candidate directed at ROR1, and the first to enter clinical trials. Oncternal believes that preclinical results suggest the potential to reverse the self-renewing stem-like properties of cancer cells, which may lead to anti-tumor effects including sensitizing tumors to other therapies. Interim results of the company’s Phase 1/2 clinical trial of cirmtuzumab in combination with ibrutinib, an approved inhibitor of Bruton’s tyrosine kinase for the treatment of patients with CLL or MCL, indicate that several patients have achieved complete responses, which are uncommon with ibrutinib alone. Cirmtuzumab is also in a Phase 1b investigator-initiated clinical trial for the treatment of women with advanced breast cancer where it is being dosed in combination with paclitaxel, a standard of care chemotherapy agent for this indication. Based on the high levels of overexpression in multiple tumors and the importance of ROR1 for tumor proliferation and metastases, Oncternal believes that cirmtuzumab has potential in other solid tumors with high unmet medical need including lung and prostate cancers.
TK216 was the product of a novel approach based on developing small molecule inhibitors of a critical protein-protein interaction linked to the ETS family of transcription factors. Tumorigenic gene fusions involving ETS factors are frequently found in tumors such as Ewing sarcoma and prostate cancer and ETS factors are often overexpressed in other tumors such as AML. Despite the importance of these factors in the oncogenic process, inhibitors of their function had not previously been identified. Researchers at Georgetown University identified the precursor to TK216 by using a chemical screening assay that they developed based on a deep understanding of the underlying biological mechanism of ETS factors. In preclinical models, TK216 has been shown to inhibit the interaction between ETS family members and RNA helicase A (“RHA”), and by doing so, effectively shuts
239
down excessive cell proliferation. Oncternal is currently enrolling patients with relapsed or refractory Ewing sarcoma, an aggressive, rare pediatric cancer, in a Phase 1 clinical trial of TK216. A dose-finding arm of this study is nearing completion and the company expects to begin enrolling patients in an expansion cohort to evaluate the clinical response of treatment with TK216 in combination with vincristine, an approved chemotherapy agent that is commonly used in combination programs to treat Ewing sarcoma. Oncternalin-licensed TK216 from Georgetown University.
Oncternal is also developing a ROR1 targeted chimeric antigen receptor T cell, orCAR-T,product candidate based on the binding domain of cirmtuzumab as a potential treatment for patients with aggressive hematological malignancies or solid tumors that may require the increased potency of aCAR-T therapy may justify the potential increased toxicity that has been seen with otherCAR-T therapies. The company believes that the selective expression of ROR1 on tumor cells and its absence on normal cells make it an ideal target for aCAR-T approach. In addition, the company believes that resistance to ROR1CAR-T therapy may be less likely to develop because ROR1 stimulates a survival and fitness pathway in cancer cells, and mutations that inactivated or suppressed ROR1 would potentially diminish the cancer cells’ stem cell-like properties, limiting their ability to metastasize or establish new tumors. Oncternal’s ROR1 targetedCAR-T product candidate is in preclinical development at UC San Diego, with funding from the CIRM.
Oncternal’s scientific founders and management team have significant experience in successfully developing and commercializing medicines for endocrine and orphan diseases. Oncternal’s CEO, James Breitmeyer, played important roles in the development and approval of a number of drugs, including Fertinex, Geref,Gonal-F, Ofirmev, Rebif, Saizen, Serostim, and Zohydro. David Hale, chairman of Oncternal, is an industry veteran and investor, involved in numerous successful private and public biotech companies, including Micromet, CancerVax, Gensia, Viagene, Santarus and Hybritech.
Oncternal’s strategy
Oncternal’s mission is to build a leading oncology company that creates distinct and transformative treatments for a wide range of oncology indications in which there is significant unmet medical need. Oncternal’s product development strategy is based on the belief that, despite the long history of pharmaceutical development in oncology, there still exists a wide array of therapeutic targets and targeting mechanisms that have yet to be vigorously pursued by pharmaceutical companies. The company’s current pipeline is derived from its ability to identify potential assets that have generated promising, late-stage preclinical results or early clinical data, andin-license them for further clinical development. Oncternal is particularly focused on assets for which there is a genetic or protein marker that can be used to identify populations of patients most likely to respond. The company prioritizes targets that it believes have the potential to transform the treatment ofdifficult-to-treat cancers with either a single agent or as combination therapy. As is the case for many oncology products, Oncternal believes that potential efficacy in one indication suggests the potential for application in other indications that carry the same target.
Key elements of Oncternal’s strategy are as follows:
| • | Rapidly advance the company’s lead product candidate, cirmtuzumab, through clinical development, initially in CLL, MCL and breast cancer; |
| • | Generate clinical proof of concept data with TK216 in Ewing sarcoma, an orphan pediatric cancer and AML; |
| • | Evaluate cirmtuzumab in additional ROR1-positive tumors such as lung, ovarian and prostate cancers; and |
| • | Advance to clinical testing of ROR1-targetingCAR-T technology. |
240
Pipeline
The following figure summarizes our current programs:
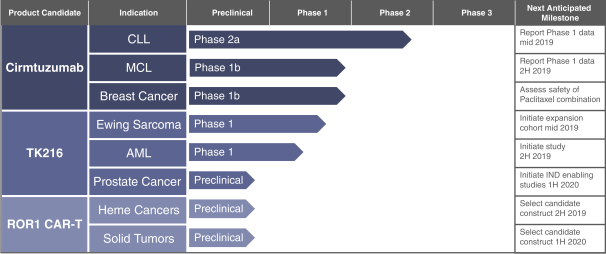
Cirmtuzumab
Oncternal’s lead product candidate, cirmtuzumab, is an investigational, humanized monoclonal antibody that was designed to bind to a specific epitope of ROR1, a protein expressed on many tumors, but not to bind to normal adult tissues. Cirmtuzumab was developed in the laboratory of one of the company’s scientific advisor, Thomas Kipps, M.D., Ph.D., Professor of Medicine and Evelyn and Edwin Tasch Chair in Cancer Research at UC San Diego with support from CIRM. The company exclusivelyin-licensed cirmtuzumab for therapeutic use from UC San Diego. The company is studying cirmtuzumab in CLL, MCL, and advanced breast cancer.
Cirmtuzumab has completed a Phase 1a dose-finding trial in patients with CLL and is currently enrolling a Phase 1b/2 trial of cirmtuzumab in combination with ibrutinib in patients with CLL and MCL.
Scientific Background
Cirmtuzumab’s discovery was based on the characteristics of autoantibodies that developed in patients enrolled in an investigator initiated clinical trial that was designed to elicit an immune response to CLL cells. CLL cells, modified using gene therapy to become more immunogenic, were used as autologous vaccines to immunize patients against their own tumors. Of the autoantibodies developed, antibodies against ROR1 were identified as having selective antitumor activity against CLL cells from both the vaccinated patient themselves and against CLL cells from other patients. Cirmtuzumab was designed to bind to the epitope on ROR1 that is associated with this activity. Unlike ROR1 antibodies that bind to other epitopes of ROR1, cirmtuzumab has not been observed to bind to normal adult tissues such as adipose tissue or pancreatic islet cells.
ROR1 is a protein that is preferentially expressed on multiple cancers and is essential for their survival, migration and proliferation. ROR1 is a member of the receptor tyrosine kinase (“RTK”), family of proteins, a group of proteins that have been shown to be effective targets for cancer therapies. Examples of approvedRTK-targeted therapies include trastuzumab which targets the human epidermal growth factor receptor 2 (“HER2”), protein, and is marketed as Herceptin by Genentech for the treatment of advanced breast cancer and HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinoma; and cetuximab which targets the epidermal growth factor receptor, or EGFR, and is marketed as Erbitux by Eli Lilly for the treatment of head and neck cancer and colorectal cancer. Approved therapeutic agents that target RTKs are thought to have been
241
effective, not only because of selective RTK expression, but because these RTKs have a vital role in promoting the growth and survival of malignant cells.
Oncternal believes that ROR1 represents an attractive target for cancer therapy for several reasons, beginning with its pattern of expression. During fetal development, ROR1 is broadly expressed and is essential for normal fetal development. After birth, the ROR1 gene is suppressed, and ROR1 expression on adult cells is greatly reduced. The known cases when ROR1 is switched back on are limited to cancer cells. The switching on of ROR1 is consistent with the typical pattern in cancer in which normal cells lose their highly differentiated functions and abilities and return to a more primal state in which they exhibit a greatly increased capacity for proliferation. This dedifferentiation activates a number of genes normally restricted to fetal development, one of which is ROR1. Cancer cells with the highest potential for self-renewal, which are sometimes referred to as tumor-initiating cells or cancer stem cells, are capable of invading other tissues or metastasizing to disseminate tumors to distant sites in the body. These tumor-initiating cells are also the cells that have been found to be the most resistant to current therapies including chemotherapy and radiation therapy. Expression of ROR1 in ovarian cancer, for example, appears highest in a subpopulation of tumor cells that also have other markers of cancer stem cells. Cells that overexpress ROR1 show increased survival, migration, and resistance to chemotherapy.
In adults, ROR1 expression is very limited on normal cells, but ROR1 is overexpressed on CLL cells and other tumor cells. CLL patients with high levels of ROR1 have more aggressive disease that requires treatment earlier than those with lower levels. These patients also have a significant reduction survival: CLL patients having high ROR1 expression have an approximately 50% survival rate at twenty years compared to an 80% survival rate for those with low ROR1 expression.
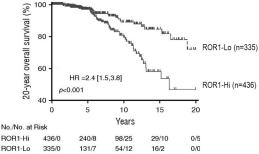
Figure 1. CLL patients with high levels of ROR1 expression have lower overall survival than those with low levels of ROR1
ROR1 is also expressed in a variety of other cancers, particularly those that are less differentiated, and is associated with early relapse after therapy or metastasis. Histological staining of over 350 human tumor samples identified that a majority expressed ROR1, including 90% or more of uterine cancers, lymphomas, and prostate cancers.
Cancer type | ROR1 Expressed (%) | Cancer type | ROR1 Expressed (%) | |||
| Uterus | 96% | Lung | 77% | |||
| Lymphoma | 90% | Breast | 75% | |||
| Prostate | 90% | Testicular | 73% | |||
| Skin | 89% | Colon | 57% | |||
| Pancreas | 83% | Ovarian | 54% | |||
| Adrenal | 83% | Bladder | 43% |
242
ROR1 expression is substantially higher in tumors that are more advanced and that contain poorly differentiated cells. Whereas Grade 1 or 2 ovarian tumors have been found to be 21% positive for ROR1, Grade 3 or 4 tumors have been found to be 62% positive. Similar increases in the percent of ROR1 positive tumors were seen in pancreatic cancers, with 54% of Grade 1 or 2 tumors and 100% of Grade 3 or 4 tumors testing positive for ROR1.
The ligand for ROR1 in hematologic malignancies is Wnt5a, a secreted glycoprotein that has a critical role in embryonic and fetal development. During development, Wnt5a controls the ability of stem cells to self-renew as well as regulating cell migration and adhesion. Cancer patients whose tumors have high levels of Wnt5a have a lower probability of long-term survival than patients with low Wnt5a levels, analogous to the situation for patients whose tumors express ROR1. In tumor models derived from primary human tumors, such as glioblastoma, overexpression of Wnt5a has been observed to lead to tumors with more rapid growth that have increased invasiveness into other tissues. Similarly, cells from human melanoma engineered to overexpress Wnt5a have shown increased motility and invasiveness.
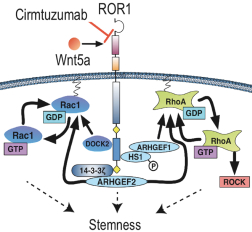
Figure 2. Cirmtuzumab blocks activation of ROR1 by Wnt5a preventing a cascade of intracellular signaling events that lead to expression of genes associated with dedifferentiated stem cells
Studies in mice have shown that ROR1 accelerated the development and progression of leukemia in models of CLL and that Wnt5a enhanced CLL cell viability, migration and proliferation in a ROR1-dependent manner. Inhibition or silencing of ROR1 signaling in multiple cancer models, including breast cancer, ovarian cancer, and glioblastoma suppressed the expression of genes related to tumor initiating cells and repressed cancer migration and metastasis. In this model, ROR1 levels were selectively reduced using a genetic construct that delivers a short-hairpin RNA (“shRNA”), that is intended to prevent ROR1 protein from being produced.
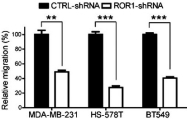
243
Figure 3. Suppression of ROR1 reduced migration in multiple cell lines
In summary, Oncternal believes that ROR1 is an attractive therapeutic target in oncology for several reasons:
| • | ROR1 is widely expressed on many tumors, including hematological malignancies and solid tumors |
| • | Tumors with high levels of ROR1 expression result in more rapid progression and shorter overall survival |
| • | ROR1 expression leads to dedifferentiation of cells, increasing their self-renewal and survival potential |
| • | ROR1 expression is increased in cells resistant to chemotherapy |
| • | When stimulated by Wnt5a, ROR1 leads to increased tumor cell migration and invasiveness |
CLL disease overview
CLL is the most common form of leukemia in adults, accounting for25-30% of all leukemias in the United States. There are an estimated 21,000 new cases of CLL each year with a prevalence of 130,000 in the United States. CLL is primarily a disease of older adults. The median age of diagnosis is about 70 years of age. Most patients are diagnosed through the result of routine blood work when elevated levels of lymphocytes are detected.
Until about 2005, CLL was traditionally treated with standard cytotoxic agents such as fludarabine, chlorambucil, cyclophosphamide, and bendamustine. The landscape for CLL therapy then began to change dramatically based on the introduction of rituximab, marketed as Rituxan by Genentech, into the standard CLL treatment paradigm. Rituximab is a monoclonal antibody that specifically recognizes CD20, an antigen onB-cells from which the tumor cells in CLL arise. Rituximab, which was approved for use in CLL only in 2010 but was previously widely prescribedoff-label, has typically been used in combination with cytotoxic agents, resulting in significant improvements in progression-free survival of later-stage patients. This drug combination is still a treatment option for younger patients who can tolerate the side effects of the associated chemotherapy.
Over the past few years, regulatory agencies have approved additional monoclonal antibody products that target CD20, as well as antibodies targeting another surface protein found on CLL tumor cells known as CD52, and three classes of small molecules: ibrutinib, an inhibitor of Bruton’s tyrosine kinase or BTK, a key component of cell signaling inB-cells, which is marketed as Imbruvica by AbbVie and Johnson & Johnson; venetoclax, an inhibitor of the proteinB-celllymphoma-2, orBcl-2, which is marketed as Venclexta and Venclyxto by AbbVie and Roche/Genentech; and idelalisib, an inhibitor of Phosphoinositide3-kinase, or PI3K, which is marketed as Zydelig by Gilead Sciences. These agents are approved for use as single agents but are being investigated in combination with each other and with various monoclonal antibody products. Clinicians are investigating their potential in earlier stage disease in multiple clinical trials. Combinations of these new small molecules with the monoclonal antibodies are defining a new standard of care in CLL. The most recently reported five-year survival rate for patients with newly diagnosed CLL is 84.2% which, although it does not reflect all of the potential benefits derived from the more recently approved therapies, represents an improvement from the approximately 65% survival rate from the 1970’s.
While these new therapies extend survival of patients with CLL, only a limited number of patients achieve a complete response, or CR, which is defined by three factors: the normalization of blood counts, an observed normalization in lymph node and/or spleen size, and normalization of the histological appearance of the bone marrow. The proportion of patients with relapsed or refractory CLL who achieve a CR when treated with single-agent ibrutinib is consistently below 10%. In one study, approximately 26% oftreatment-naïve patients treated with ibrutinib monotherapy achieve a CR with a median duration of response of 14.7 months. Subsequent trials have not shown a significant improvement in the rate of CR when ibrutinib is used in combination with rituximab.
The market for CLL therapies in the United States, France, Germany, Italy, Spain, the UK, and Canada is estimated to be over $7 billion, with the majority of sales associated with recently-approved therapies, including
244
ibrutinib, venetoclax and idelalisib. Oncternal believes that CLL represents an attractive clinical and commercial opportunity for cirmtuzumab.
MCL disease overview
MCL is an aggressive form ofnon-Hodgkin’s lymphoma. There are approximately 4,200 new cases of MCL each year in the United States, with the average age at diagnosis in themid-60s. Most patients with MCL have advanced stage disease at diagnosis characterized by swollen lymph nodes as outward signs of disease that has spread to other organs in the body. MCL has a poor prognosis with a median survival time of individuals with MCL of about two to five years. The10-year survival rate is only approximately5%-10%.
While there are several therapeutic options available to treat MCL, none of these options offers long-term benefit, with most patients relapsing in less than 18 months. Similar to CLL, MCL is a cancer of B cells and is treated with some of the same therapies utilized to treat CLL. Newly diagnosed patients are typically treated with rituximab combined with a chemotherapy regimen known as CHOP, comprised of cyclophosphamide, doxorubicin, vincristine, and prednisone. Alternative chemotherapy regimens include bortezomib or bendamustine. Patients with clinical responses to chemotherapy may become candidates for another therapeutic approach, autologous stem cell transplantation, a procedure in which radiation and/or chemotherapy is used to eliminate the patient’s immune cells, including residual MCL cells. Recently, ibrutinib was granted accelerated approval by the FDA for the treatment of relapsed MCL on the basis of overall response rates of 72% and a CR rate of 19%. All of these therapies, however, are associated with significant toxicity and, given that the majority of patients with MCL are advanced in age, the company believes that less aggressive and more effective therapies are needed.
Despite continuous therapy with ibrutinib, remissions are not durable for most patients, and prognosis is poor for patients who discontinue the drug, characterized by rapid relapse where patients experience more aggressive disease and overall survival as short as three months. Moreover, the proportion of patients electing to discontinue therapy with ibrutinib appears to be higher in community practice than reported in clinical trials, possibly due to intolerance for evenlow-grade toxicity as well as drug-related costs incurred by patients, who face the prospect of life-long therapy.
Breast cancer disease overview
Breast cancer is the most common type of invasive cancer among women and the second leading cause of cancer deaths among women. There are approximately 266,000 new diagnoses and 41,000 breast cancer deaths in the United States each year, and 12.4% of women will develop breast cancer in their lifetime. The Centers for Disease Control and Prevention, or CDC, estimates that there are approximately one million women in the United States living with breast cancer that has been diagnosed within the past five years.
Breast cancers can be segregated into subtypes based upon the presence of three protein receptors:
| • | estrogen receptor, or ER |
| • | progesterone receptor, or PR |
| • | human epidermal growth factor receptor 2, or HER2 |
Therapies have been developed that target tumors containing one or more of these receptors. Approximately 15% to 20% of breast cancers, however, do not express any of these three receptors and are referred to as triple-negative breast cancers (“TNBC”). These tumors have a more aggressive phenotype and a poorer prognosis due to the high propensity for metastatic progression and absence of specific targeted treatments. The only approved targeted therapy for TNBC is olaparib, marketed as Lynparza by AstraZeneca, for the small minority of patients with mutations in the BRCA1 or BRCA2 genes. The five-year survival fornon-TNBC has been reported to be 80.8% but only 62.1% for TNBC.
245
One hypothesis for the high rate of metastasis and poor response to chemotherapy with TNBC is that these tumors contain a high number of tumor-initiating cells, or cancer stem cells, that are highly migratory and insensitive to standard chemotherapy.
Clinical Development Program
Cirmtuzumab Phase 1a clinical trial for potential treatment of CLL
A Phase 1a dose escalation trial of cirmtuzumab funded jointly by CIRM and Oncternal was conducted in 26 patients with actively progressing CLL who had relapsed or refractory disease. All patients had previously received treatment with any of several potential anti-CD20 monoclonal antibodies. Analysis of blood samples from these patients showed significantly higher plasma levels of Wnt5a compared to healthy matched controls and they also had higher levels of expression of ROR1 on their CLL cells.
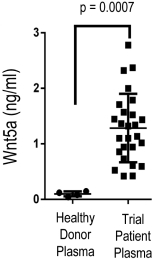
Figure 4. Wnt5a was overexpressed in all CLL patients enrolled in the cirmtuzumab Phase 1a clinical trial
Patients were treated with four doses of cirmtuzumab administered every two weeks, in cohorts of three receiving escalating doses from 0.16 to 20 mg/kg/dose. Patients receiving doses of cirmtuzumab of 2 mg/kg or greater had a 33% reduction in ROR1 expression relative to baseline. To assess the levels of activation of ROR1, CLL cells from patients in the 16 mg/kg cohort were analyzed for levels of phosphorylated hematopoietic-lineage-cell-specific protein 1 (“HS1”), a direct target of activated ROR1. The ratio of phosphorylated HS1 to unphosphorylated HS1 dropped within 24 hours of dosing and remained low for several months, at which time the levels of cirmtuzumab in the bloodstream had become undetectable. These results provide evidence that the higher doses of cirmtuzumab administered to patients were sufficient to block the endogenous Wnt5a signaling and ROR1 activation in their CLL cells.
246
An analysis of genes expressed in CLL cells from treated patients was found to negatively correlate with gene signatures associated with stem cells and oncogenic dedifferentiation. In this analysis, called a gene set enrichment analysis (“GSEA”), changes in gene expression of thousands of genes are compared to those from reference cells with particular phenotypes. Individual genes are ranked by how strongly their expression correlates with the phenotype. When compared to baseline, cells from cirmtuzumab treated patients showed a reversal in the enrichment for genes that were identified as being the most highly correlated with stem cells and oncogenic differentiation.
|
|
Figure 5. Reversal of GSEA signature for stemness observed in CLL cells from Cirmtuzumab treated patients
These results are consistent with preclinical observations that cirmtuzumab and ROR1 inhibition drive cells away from a stem-cell like profile. Cancer cells with stem-cell like profiles have the potential for self-renewal and are more likely to result in disease progression and poor prognosis due to resistance to therapy. Oncternal believes that the potential of cirmtuzumab to reverse these stem cell properties may lead to anti-tumor effects, including sensitizing tumors to other therapies.
In the clinical trial, seventeen patients had stable disease and five had progressive disease. Three of the patients with progressive disease had received the lowest dose of cirmtuzumab. Most patients experienced reductions in their leukemic lymphocyte counts and were able to delay initiation of further treatments for an average of 262 days, at which point plasma levels of cirmtuzumab were undetectable.
Cirmtuzumab infusions were generally well tolerated. Pharmacokinetic analyses indicated a long half-life of cirmtuzumab, over 30 days. There were no dose-limiting toxicities, no serious adverse events, and no discontinuations related to adverse events. The main laboratory findings included anemia, thrombocytopenia and neutropenia which were primarily attributed to the underlying CLL. Three patients enrolled in an extension arm of this trial continued to receive cirmtuzumab every two weeks for additional 3, 7 and 16 doses with no additional adverse events.
Cirmtuzumab in combination with ibrutinib for potential treatment of CLL and MCL
Oncternal and UC San Diego are conducting a Phase 1b/2 trial of cirmtuzumab in combination with ibrutinib in CLL patients and previously treated MCL patients who have not previously received ibrutinib or other forms of BTK therapy. Part 1 of this trial, which has been completed, was a dose-finding arm designed to determine the recommended dose of cirmtuzumab to be used in combination with ibrutinib. Part 2 of this trial, which is now enrolling, is a dose expansion trial where approximately 12 patients will be treated with cirmtuzumab administered every two weeks for the first month, then every four weeks afterwards, while ibrutinib is
247
administered daily. In Part 3 of this trial, approximately 90 additional patients will be randomized to receive cirmtuzumab plus ibrutinib or ibrutinib as monotherapy. The primary endpoint of Part 3 of this trial is to determine the CR rate. The data which are emerging from this trial will be used to determine the company’s regulatory strategy, including whether the company will seek regulatory approval through standard review or an accelerated approval pathway. This trial isco-sponsored by UC San Diego with support from CIRM.
The rationale for this trial istwo-fold. First, ibrutinib is emerging as a leading therapy for both CLL and MCL. Despite its efficacy in extending progression-free survival, ibrutinib does not provide the majority of patients with a CR even after prolonged dosing. Therefore, the company believes there is an opportunity for improving efficacy by dosing ibrutinib in combination with another agent. Secondly,in vivo studies conducted in mouse CLL models have shown that ibrutinib and cirmtuzumab exerted their antitumor activities through independent pathways; that is, inhibition of BTK by ibrutinib did not alter ROR1 signaling nor did it impair the rate at which cirmtuzumab blocked ROR1 signaling. The combination of both drugs reduced the size of the spleen, the primary site of leukemic disease in these mice, as well as the number of CLL cells in these spleens.
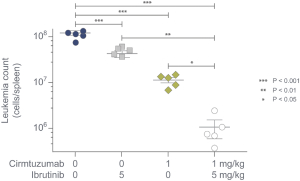
Figure 6. Combined administration of cirmtuzumab and ibrutinib reduced leukemic cell count in the spleen in a mouse model of CLL.
The ongoing Phase 1b/2 is an open-label trial and as of March 2019 Oncternal has already observed several clinical responses with one confirmed CR in a patient with MCL and one confirmed CR in a patient with CLL. In one case, a patient with MCL who had relapsed following high-dose chemotherapy and allogeneic stem cell transplant was treated with the combination of cirmtuzumab plus ibrutinib. The primary tumor in this patient, which was measured at 9 cm x 6.7 cm at baseline, rapidly shrank and was undetectable at three months of treatment with the cirmtuzumab/ibrutinib combination. The CR was confirmed at seven months and 10 months of combination treatment. In a CLL patient who enrolled in the trial after relapsing from chemoimmunotherapy, the combination of cirmtuzumab and ibrutinib led to normalization of lymph node size and lymphocyte counts by 10 months of combination treatment, and there was no evidence of CLL upon histologic examination of the bone marrow. As of March 2019, CLL patients in this trial have obtained an 80% average reduction in lymph node size in 24 weeks.
Cirmtuzumab in combination with paclitaxel for potential treatment of metastatic breast cancer
A single arm, open-label, Phase 1b trial of cirmtuzumab in combination with paclitaxel has been initiated by an investigator at UC San Diego. The Phase 1b trial will enroll up to 20 patients with Her2 negative, metastatic or locally advanced, unresectable breast cancer. Patients in this trial will receive a fixed dose of cirmtuzumab every other week for two doses then once every four weeks. The patients will also receive standard of care paclitaxel dosed weekly starting on day 1.
248
Approximately 75% of breast tumors express ROR1, and TNBC patients with high levels of ROR1 have a substantially significantly reduced survival rate compared to those with low levels.
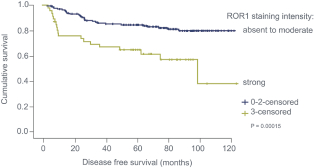
Figure 7. TNBC patients with high levels of ROR1 expression had lower disease-free survival
A retrospective, long-term analysis of 582 breast cancer patients who had their tumors removed showed that those with tumors expressing high levels of ROR1 were at a statistically significantly higher risk of developing metastases within the first several years. Over 60% of patients with high ROR1 developed metastases compared to only 35% of patients with the lowest levels of ROR1.
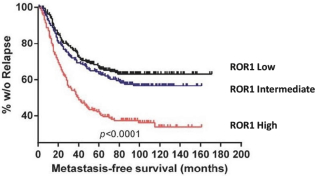
Figure 8. High levels of ROR1 in breast cancer was associated with shorter metastasis-free survival
Preclinical experiments have shown that treatment of breast tumors with paclitaxel increased the percentage of cells with high levels of ROR1. In these experiments, immunodeficient mice were implanted with primary human breast tumors then treated with paclitaxel. While paclitaxel either slowed tumor growth or reduced the size of tumors in these mice, the surviving cells were enriched for expression of ROR1.
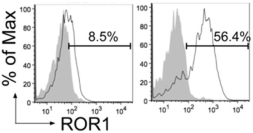
249
Figure 9. Breast tumors treated with paclitaxel showed elevated levels of ROR1 expression
This increased expression of ROR1 was also associated with a shift in the properties of cells from these tumors towards a more metastatic and more tumorigenic phenotype. Cells from tumors that had been treated with paclitaxel were more likely to form spheroids in tissue culture, and were enriched for cells with the ability to form new tumors when transplanted, both properties that are correlated with tumor aggressiveness.
Together, these clinical and preclinical data are consistent with a model of the natural disease progression in TNBC centered on the critical role played by tumor-initiating cells or stem-like cancer cells that express high levels of ROR1.
| • | TNBC is initially responsive to chemotherapy such as paclitaxel, because chemotherapy kills the majority of cancer cells, leaving cells with stem-like properties that express ROR1. |
| • | TNBC returns more often than other types of breast cancer in part because the initial chemotherapy enriches for cells with a higher propensity to form tumors. |
| • | The site of recurrence is often at another place in the body than the original tumor because cells with stem cell-like properties are able to metastasize. |
| • | The recurring tumor may be resistant to therapy because it contains a high percentage of cells with stem cell-like properties. |
Preclinical experiments in anMDA-MD-231 TNBC model in mice provided evidence that reductions in ROR1 can limit metastases and improve overall survival. In this model, ROR1 levels were selectively reduced using a genetic construct that delivers a short-hairpin RNA, or shRNA, that is designed to prevent ROR1 protein from being produced. Inhibition of ROR1 production resulted in significantly fewer cancer cells that have metastasized to the lungs.
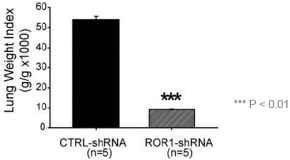
250
Figure 10. Suppression of ROR1 led to fewer metastases to the lungs in anMDA-MD-231 model TNBC model
Inhibition of ROR1 production in these mice also improved overall survival to a mean of approximately 43 days compared to 30 days for mice containing control shRNA.
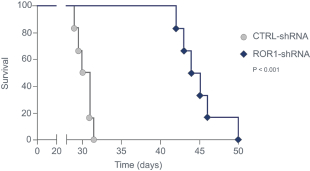
Figure 11. Inhibition of ROR1 expression led to improved survival in anMDA-MD-231 TNBC model.
Cirmtuzumab reduced the growth rate of primary human breast cancers in immunodeficient mice and led to complete suppression of tumor growth for twenty days when used in combination with paclitaxel. Even after tumors did eventually grow, they lacked the ability to form new tumors. All tumor samples isolated from control mice and most of the tumor samples from cirmtuzumab-treated or paclitaxel-treated mice were able to establish new tumors when transplanted into other mice. No tumors, however, were formed when equal numbers of tumor cells from mice treated with the combination of cirmtuzumab and paclitaxel were introduced into other mice.
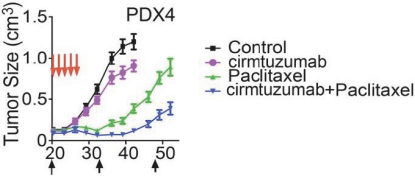
Figure 12. Combination of cirmtuzumab and paclitaxel suppressed growth of primary human breast tumors in a mouse model
Additional clinical opportunities
ROR1 is expressed by 75% of lung cancers and by 93% of lung adenocarcinomas. In adenocarcinoma of the lung, patients at advanced stages and those with positive lymph node metastasis expressed higher level of ROR1, which was correlated to stage and lymph node metastasis. Kaplan-Meier survival analysis indicated an association of high ROR1 expression with worse overall survival in lung adenocarcinoma patients that was independent of lymph node status. ROR1 expression has been shown to be correlated with the presence of other negative prognostic factors such as phosphorylated AKT(“p-AKT”), or phosphorylated CREB(“p-CREB”) Inhibition of ROR1 in lung cancer cell lines induced apoptosis and cell cycle arrest and led to a reduction in levels ofp-CREB andp-AKT.
251
ROR1 is expressed by 90% of prostate cancers and the Wnt5a signaling pathway is activated in patients with advanced prostate cancer that is progressing while on treatment with an androgen receptor, or AR, inhibitor. Treatment of prostate cancer cell lines with an AR inhibitor was found to increase the expression of Wnt5a, and the addition of Wnt5a attenuated the antiproliferative effect of AR inhibition. The expression of Wnt5a in patients with metastatic castrate resistant prostate cancer, or mCRPC, has been associated with poor overall survival. Oncternal is collaborating with UC San Diego to investigate the potential effects of cirmtuzumab on this disease.
TK216
TK216 is an investigational, potentiallyfirst-in-class small molecule that is designed to inhibit the biological activity of E26 transformation-specific (“ETS”), transcription factor oncoproteins. TK216 is being evaluated alone and in combination with vincristine in a Phase 1 clinical trial in patients with relapsed or refractory Ewing sarcoma, a rare pediatric cancer that has historically been very challenging to treat effectively.
Scientific Background
TK216 is an investigational, potentiallyfirst-in-class small molecule inhibitor of a set of cancer-related proteins known to be associated with both solid tumors and hematological malignancies. In some tumors, the target for TK216 is a fusion protein that arises from chromosomal translocation. Fusion proteins are a well-known category of targets for small molecule cancer therapy that have been cited in the scientific literature as providing a number of diagnostic and therapeutic advantages because of their tumor-specific expression.
TK216 targets the ETS family of oncoproteins. The association of these transcription factors with cancer is related both to their fusion with other proteins to create potent oncogenes and to their overexpression in cancer cells, either of which may drive overabundant or aberrant cell growth. Fused or overexpressed transcription factors in this family have been identified in multiple tumors including solid tumors such as Ewing sarcoma and prostate cancer, as well as in hematological malignancies such as AML and diffuse large B cell lymphoma (“DLBCL”). Fusions of ETS family members have been shown to be critical to the onset and development of cancer.
TK216 has been observed to inhibit the interaction between ETS family members and RNA helicase A or RHA, a critical component of the human transcriptional complex, and by doing so, shut down excessive cell proliferation in preclinical tumor models.
ETS transcription factors and oncogenesis
In normal development and physiology, ETS transcription factors govern processes such as cell cycle control, differentiation, proliferation, apoptosis, tissue remodeling and angiogenesis. These processes play a key role in normal cell functioning, and when mutation-driven disruptions in the functions of ETS factors develop, they have been shown to lead to tumor initiation, progression, and metastasis.
Fusion proteins involving ETS factors have been implicated in various solid tumors, including Ewing sarcoma and prostate cancer. For example, approximately 85% of Ewing sarcomas contain a genomic rearrangement between chromosomes 11 and 22. DNA is exchanged between these chromosomes in a pathological manner, and this exchange results in a fusion of two genes: the FLI1 gene, an ETS family member, and the EWSR1 gene, an unrelated transcription factor. This gene fusion, known as EWS/FLI, functions as a transcription activator that is no longer controlled by the relevant regulatory machinery in the cell. In addition to escaping regulation, the dysregulated function of the EWS/FLI fusion causes a series of abnormalities in RNA processing including aberrant mRNA splicing, where it leads to defects in the synthesis of proteins such as BRCA1, a DNA repair protein. EWS/FLI fusions also cause the formation of abnormal and potentially deleterious DNA and RNA structures known asR-loops that are associated with replication and transcriptional blocks as well as being prone to increased DNA damage.
252
Multiple other tumors contain gene fusions of other ETS factors. For example, over 50% of metastatic prostate cancers carry theTMPRSS2-ETS fusion. Other tumors have genetic changes that result in overexpression of ETS factors.
ETS Fusion Proteins
|
ETS Overexpression
| |
• Ewing sarcoma • EWS-FLI1 | • AML • FLI1, ERG, ETV5, ETS2 | |
• Prostate cancer • TMPRSS2-ERG | • DLBCL • ETV1, FLI1, ETV4, SPIB | |
• AML • ETV6-various (20+) | • Prostate cancer • ERG, ETV1, ETV4, ETV6 | |
• ALL • ETV6-RUNX1 | • Lung cancer • ETV5, ETV1, FLI1, ETS1 | |
• Secretory breast cancer • ETV6-NTRK3 | • Breast cancer • ETV6, ETV4, SPIB, ETV5 |
The ETS family member ERG is overexpressed in many cancers, such as AML, with no obvious correlation between the levels of ERG and the presence of other known tumorigenic mutations. In a retrospective analysis of patients with breast cancer followed for up to 20 years following diagnosis, the quartile of patients with the highest levels of ERG expression had a significantly higher rate of relapse and poorer overall survival than patients with lower levels of ERG expression. Those with the highest levels of ERG had a five-year survival rate of 20% while those with lower levels had a survival rate of approximately 50%. Similarly, AML patients with high levels of ETS2, another ETS family member, had a significantly lower five-year survival rate of approximately 15% compared to 40% for patients with lower levels.
|
|
Figure 13. Survival of the quartile of AML patients with the highest ERG (left) or ETS2 (right) expression was significantly lower than those with lower expression.
Despite the genetic associations between ETS factors and tumorigenesis and the strong correlation between high levels of ETS factor expression and survival, there are currently no therapeutics available that target these factors. It had been widely considered that transcription factors are difficult to target due to theirnon-enzymatic mechanism of action, so the company believe the approach of inhibiting protein-protein interactions is novel. Oncternal believes that a product candidate targeting ETS factors could fill an important gap in the treatment landscape for both solid tumors and hematological malignancies.
Ewing sarcoma disease overview
Ewing sarcoma is the second most common bone tumor of children that occurs most often in adolescents and accounts for approximately 2% of all childhood cancer diagnoses. Ewing sarcoma is part of a spectrum of tumors known as Ewing sarcoma family of tumors which also includes peripheral primitive neuroectodermal tumor. Approximately750-950 people are diagnosed with Ewing sarcoma each year in the United States.
Ewing sarcoma typically develops in the pelvis, femur, and bones of the head and trunk, but its diagnosis often takes months as other causes fornon-specific symptoms such as localized pain, fever, fatigue, weight loss, or
253
anemia are ruled out. The five-year survival of patients who are diagnosed withnon-metastatic disease is between 50% and 70%. Patients diagnosed with metastatic disease have five-year survival between 18% and 30%.
Ewing sarcoma is usually treated systemically due to the fact that local treatments, even in patients without overt metastases, have an 80% to 90% relapse rate. The current standard therapy for patients with localized Ewing sarcoma in the United States is a combination of chemotherapy agents, including vincristine, doxorubicin and cyclophosphamide, with alternating cycles of ifosfamide and etoposide – a therapy known as VDC/IE. Patients that respond to this therapy may be candidates for tumor resection and continued treatment for a total of 14 to 17 cycles. This therapeutic regimen, however, is associated with significant toxicities. Patients with metastatic disease are often treated with VDC/IE or variations of this therapy with higher or more compressed dosing. This may also be supplemented by local radiation therapy or systemic radiation followed by autologous hematopoietic stem cell transplant. Oncternal believes that more effective therapies are needed for this rare pediatric disease.
AML disease overview
AML is a hematologic malignancy characterized by dysregulated maturation of myeloid or blood stem cells and failure of the bone marrow to properly function. Myeloid cells normally differentiate into mature red blood cells, white blood cells, and platelets, however in AML, this maturation process does not progress normally. As a result, an overabundance of immature leukemia cells accumulates in the blood, replacing healthy mature cells, leaving patients with anemia and immune deficiency, and at high risk of infections and bleeding.
AML is the most common type of acute leukemia in adults. Approximately 21,450 new AML cases and 10,920 AML associated deaths occur annually in the United States. The average age of an AML patient is 68 years. The National Cancer Institute estimated in 2018 that the five-year survival rate for adult patients with AML was approximately 27%.
First-line therapy for AML patients is high dose chemotherapy usually consisting of cytarabine plus an anthracycline such as daunorubicin, a therapeutic regimen that has changed little in the past 40 years. This intensive chemotherapy treatment is associated with a treatment related mortality rate of approximately 10%. Patients who are older or in poor health are treated with cocktails of various other chemotherapy agents at doses they can tolerate. Two therapies have recently been approved by the FDA for the treatment of AML in elderly patients who are not candidates for intensive chemotherapy due to their health condition. Glasdegib, marketed as Daurismo by Pfizer, is an inhibitor of the hedgehog signaling pathway that was shown to increase overall survival to 8.3 months when given with low dose cytarabine compared to 4.3 months for low dose cytarabine alone. Venetoclax, marketed as Venclexta by Abbott, was approved based on the ability to induce CR in a subset of patients that lasted for five to six months. Other therapies such as ivosidenib, marketed as Tibsovo by Agios, enasidenib, marketed as Idhifa by Celgene, and gilteritibin, marketed as Xospata by Astellas, have been approved for subsets of AML patients with specific genetic mutations.
Between 20% and 30% of young adult patients and 50% of older patients are refractory to initial treatment and many others who initially respond suffer relapses of their disease. Options for second-line therapy for these patients are limited. The only potential curative therapy for AML is allogeneic hematopoietic stem cell transplantation where the patient’s AML and entire immune system are eliminated by high dose chemotherapy and/or radiation and replaced with stem cells isolated from a compatible healthy donor. However, up to 55% of these patients’ relapse, with median survival after relapse of approximately five months. The averagetwo-year survival after relapse is 20%. The rigorous conditioning regimen required for stem cell transplants limit this option to younger and healthier patients. Therefore, there is a clear need for more effective and less toxic therapies for AML.
Preclinical data
TK216 is a more potent derivative of a research compound,YK-4-279, that was identified through screening for molecules that bind to the EWS/FLI fusion protein and prevent its interaction with RNA Helicase A, or RHA.
254
Previous studies had shown that the interaction between EWS/FLI and RHA was essential forin vitroproliferation and formation of cell colonies in an EWS/FLI dependent cell line.
EWS/FLI and other ETS family members function as transcriptional regulators. They function by directing the assembly of a complex of other proteins on DNA templates which carry out the process of transcription of the DNA sequence into mRNA transcripts. These complexes include RNA polymerase II, cyclic AMP response element-binding protein, and RHA. Disruption of this complex inhibits transcription, leading to inhibition of the oncogenic activity of EWS/FLI.
When added to cells,YK-4-279 inhibited proliferation of EWS/FLI dependent cells but had no effect on the proliferation of control cell lines. When dosed in aCHP-100 xenograft model of Ewing sarcoma,YK-4-279 resulted in marked inhibition of tumor growth. Subsequent studies have identified anti-tumor activity in a variety of tumors including neuroblastoma, prostate cancer, and AML. TK216 is a structural analog ofYK-4-279 that has shown increased potency in biochemical, cellular and xenograft tumor models.
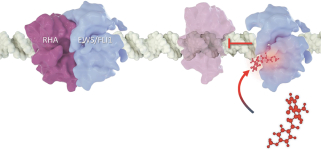
Figure 14. Model depicting the inhibition of the interaction of EWS/FLI1 and RHA
TK216 was designed to inhibit the physical interaction between RHA and EWS/FLI in cells. In preclinical studies, RHA was found to be physically associated with EWS/FLI in Ewing sarcoma cells, but the two proteins were no longer bound together if the cells had been incubated with TK216.
Treatmentin vitro with TK216 led to dose-dependent inhibition of transcription from a luciferase reporter assay in COS7 cells. TK216 also inhibited proliferation of Ewing sarcoma cell line A4573.
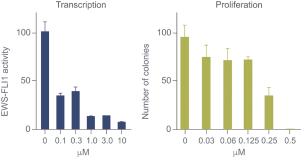
Figure 15. TK216 inhibited transcription of a reporter gene dependent on EWS/FLI (left). TK216 inhibited proliferation of a Ewing sarcoma cell line A4573 (right)
Treatment of mice bearing xenografts of theTMD-8 DLBCL cell line with 100 mg/kg of TK216 led to a 77% reduction in tumor growth over thirteen days of treatment. Histology of tumor samples isolated after treatment showed reductions in Ki67, a cellular marker of proliferation, and increases in the level of cleaved caspase 3, a marker of apoptosis.
255
TK216 has inhibited proliferation of multiple cell lines containing EWS/FLI fusions, as well as other cell lines containing other ETS translocations or overexpressing ETS factors. These results suggest that TK216 bound to a site that is commonly used by multiple ETS family members to interact with other factors such as RHA and therefore the company believes that TK216 has potential beyond targeting the EWS/FLI fusion that is commonly found in Ewing sarcoma.
Treatment of aggressive tumors such as Ewing sarcoma typically requires a combination of agents. A systematic analysis combining approved agents tested in combination withYK-4-279, an analogue of TK216, was conducted using Ewing sarcoma cell lines.YK-4-279 led to synergistic cytotoxicity with 28% of the agents tested including antimetabolites, nucleic acid synthesis inhibitors, immunosuppressive or immunomodulating agents and microtubule inhibitors. One of these agents was vincristine, a mainstay of treatment for Ewing sarcoma, a tumor where 85% of patients have an EWS/FLI fusion protein.In vivo activity in an A4573 xenograft model of Ewing sarcoma showed tumor shrinkage and increased survival whenYK-4-279 was combined with vincristine.
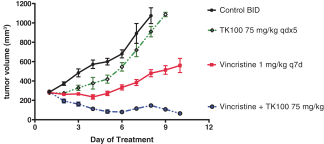
Figure 16. Combination ofYK-4-279 and vincristine resulted in tumor shrinkage and prolonged survival in an A4573 model of Ewing sarcoma
Clinical Development Program
TK216 in combination with vincristine for potential treatment for Ewing sarcoma
Oncternal is conducting an open-label dose escalation Phase 1 trial of TK216 in Ewing sarcoma patients that have relapsed or are refractory to current therapies. In the first part of this trial, TK216 is being is administered for seven days as a continuous infusion repeated every three weeks. Twenty-nine patients have been treated, and data collected as of March 2019 have identified dose-limiting neutropenia, or a low level of white blood cells, at a dose of 288 mg/m2/day, and 220 mg/m2/day has been determined as the maximum tolerated dose. Additional cohorts are now being recruited that extend the dosing period for TK216 from seven days to ten and fourteen days. Patients in these cohorts may also receive vincristine. Once a recommended Phase 2 dose is identified, a cohort of patients will receive the recommended Phase 2 dose of TK216 combined with vincristine.
TK216 as a potential treatment for AML
In partnership with M.D. Anderson Cancer Center, Oncternal is planning to initiate a Phase 1 trial in relapsed and refractory AML patients, a patient population known to express in certain cases fusion proteins involving ETV6, and to have overexpression of ETS family members including FLI1, ERG, ETS2, and ETV5.
ROR1CAR-T
Oncternal is developing a chimeric antigen receptor T cell, orCAR-Ttherapy based on the ROR1 binding domain of cirmtuzumab to treat patients with aggressive hematological malignancies or solid tumors. The company believes that the selective expression of ROR1 on tumor cells and its absence on normal cells make it an ideal target for aCAR-T approach. In addition, the company believes that the survival benefit imparted on
256
cancer cells expressing ROR1 will limit the development of ROR1-negative resistant tumors, and that tumors that generate mutations that escape an ROR1CAR-T therapeutic by inactivating or suppressing ROR1 would lose their stem cell-like properties, limiting their ability to metastasize or establish new tumors. Oncternal’s ROR1 targetedCAR-T therapy is in preclinical development at UC San Diego, with funding from the CIRM.
Scientific Background
CAR-T cell therapy overview
Immuno-oncology describes the concept of using the patient’s own immune system to attack cancer. It has been widely recognized that this approach, by specifically killing cancer cells without harming healthy cells and tissues, can overcome some of the most undesirable side effects of standard cancer therapies such as chemotherapy and radiation.
Immuno-oncology redirects one of the pillars of the immune system, the adaptive immune system, so that it specifically and efficaciously recognizes not only pathogens and other threats to the body but also cancerous cells that might previously have escaped immune recognition. A key element in the adaptive immune response is the T cell. T cells are white blood cells that can recognize and kill infected and abnormal cells. T cells also act to signal other immune cells to respond to threats. T cells recognize their targets because they are created in a way that allows them to specifically recognize foreign antigens on the surface of other cells.
T cells are ideally suited for immuno-oncology applications based on several characteristics. They are created to be exquisitely specific and avid killers. One T cell can eliminate numerous target cells. T cells are extremely specific, able to recognize an infected cell and kill it while ignoring an almost identical yet uninfected healthy cell. T cells are thought to be active all the time, eliminating cancer cells from the body before they can form tumors. However, tumor cells sometimes evolve to escape killing by T cells by activating a number of pathways that suppress T cell function. Taking this concept one step further by modifying the T cell to kill cancer cells selectively despite thesebuilt-in defenses represents the basic ideabehind CAR-T therapies.
CAR-T therapeutics are created by isolating T cells from patients and modifying them to recognize specific antigens on tumors. T cells have potent cell killing activity that is directed to target cells that are recognized by specific T cell receptors (“TCRs”), that are expressed on the surface of these T cells. While some T cells have TCRs that can recognize cancer cells leading to their killing, potent T cells do not develop to all targets. In some cases, the potential cancer cell target is also a protein that has an essential role in other tissues or at other stages of development and TCRs that recognize these targets are eliminated during the normal T cell development.
CAR-T therapy has emerged as a way to engineer T cells to recognize specific targets, such as those that are selectively expressed on cancer cells. A gene encoding a chimeric protein is constructed that contains a single antigen-binding domain of an antibody that recognizes the target coupled to a T cell costimulatory domain and a portion of the T cell receptor.
257
CAR-T therapies are typically produced from a patient’s own T cells which are isolated by leukapheresis. These cells are then genetically modified with the chimeric antigen gene construct which can be delivered by various mechanisms such as lentiviral gene delivery vectors. Transduced cells are then expanded and undergo quality testing before being reintroduced into the same patient.
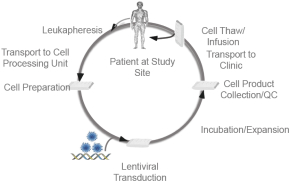
Figure 17.CAR-T production and patient treatment
TwoCAR-T cell therapies, Yescarta, developed by Kite Pharma, and Kymriah, developed by Novartis, have been approved by the FDA. Both of these therapies target the CD19 protein, a protein expressed on the surface of the majority of B cells, including B cell tumorigenic cells. Yescarta has been approved for the treatment of relapsed or refractory largeB-cell lymphoma and Kymriah for the treatment of relapsed or refractoryB-cell precursor acute lymphoblastic leukemia. These therapies have received breakthrough designations from the FDA and have shown high response rates with prolonged treatment effects for a subset of patients. NoCAR-T therapies have been approved for use in patients with solid tumors. Despite the high response rates and prolonged treatment effects observed for a subset of patients, Oncternal believes that novelCAR-T approaches have the potential to improve efficacy, duration of response as well as safety.
ROR1CAR-T
Oncternal’sCAR-T program is based on a collaboration with UC San Diego, with support from CIRM. Genetic constructs are being designed and tested that incorporate the following elements: (1) a single chain variable region (“ScFv”) based on the cirmtuzumab antibody and with high affinity for ROR1, (2) a hinge and spacer region derived from CD4 or CD8, (3) a transmembrane domain, (4) an intracellular costimulatory domain comprised of CD28 and/or4-1BB, and (5) the CD3z. activating domain. This construct is designed to be delivered into T cells using a lentivirus transduction system.
Various permutations of the five elements of the ROR1 targeting construct have been tested, and the design of the construct is nearly finalized. Batches of lentivirus can be produced that carry the construct, and T cells can be transfected and shown to express the construct. Inin vitro assays, ROR1 targetingCAR-T cells killed tumor target cells expressing while relatively sparing target cells not expressing ROR1.
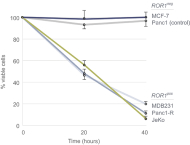
258
Figure 18. Killing of tumor cells from cell lines derived from breast and pancreatic cancers in anin vitro assay with ROR1CAR-T was dependent on ROR1 expression
Oncternal also plans to collaborate with its strategic partner, Shanghai Pharmaceuticals Holding Co., Ltd. (“SPH”), for itsCAR-T program. Through its US subsidiary SPH USA, SPH has entered into the SPH USA License Agreement with Oncternal to develop ROR1 targetedCAR-T products in greater China. Oncternal plans to collaborate with SPH to develop processes to produce and manufacture lentiviruses carrying the ROR1 construct. This represents a potential advantage for the OncternalCAR-T program, because viral manufacturing capacity is constrained in the US and EU, and someCAR-T developers are experiencing delays in theirCAR-T programs caused by the limited manufacturing capacity. Oncternal and SPH also intend to collaborate by conducting one or more clinical trials of its potentialCAR-T product candidate in China at hospitals in China that are known to SPH and that have substantial experience processing cellular immunotherapy materials, and substantial experience conductingCAR-T clinical trials. Initial clinical trials of the OncternalCAR-T program may occur in the United States at UC San Diego and at sites in China.
Licenses and Collaborative Relationships
UC San Diego
In March 2016, Oncternal entered into a license agreement with the Regents, represented by UC San Diego, which was amended and restated in August 2018, for the development, manufacturing and distribution rights to naked antibodies, including cirmtuzumab and genetically engineered cellular therapy products, includingCAR-T products that are covered by licensed patents for all human therapeutic, diagnostic and preventive applications in all indications. Under the license agreement with UC San Diego (the “UC San Diego License Agreement”), the company paid an upfront license fee of $500,000 and issued 1,459,524 shares of common stock shares of common stock. Commencing in 2017, Oncternal also pays UC San Diego an annual license maintenance fee and reimburses to UC San Diego its annual patent costs for the licensed patents. The UC San Diego License Agreement also requires the payment of certain development and regulatory milestones, aggregating from $10.0 million to $12.5 million, on a per product basis, certain worldwide sales milestones based on achievement of tiered revenue levels aggregating $75.0 million, low single-digit royalties including potential future minimum annual royalties on net sales of each product, and requires certain minimum diligence efforts to advance the licensed assets, including spending at least $1.0 million in development annually through 2023. Unless terminated earlier, the UC San Diego License Agreement will expire upon the later of the expiration date of the longest-lived patent rights or the 15th anniversary of the first commercial sale of a licensed product. UC San Diego may terminate the UC San Diego License Agreement if a material breach by Oncternal is not cured within a reasonable time, the company files a claim asserting the licensed patent rights are invalid or unenforceable, or the company files for bankruptcy. Oncternal may terminate the agreement at any time upon at least 90 days’ written notice. In July 2016, Oncternal entered into a research agreement with UC San Diego (the “UC San Diego Research Agreement”), for further research on the ROR1 therapeutic development program. Under this five-year agreement, UC San Diego will have an aggregate budget of $3.6 million, with $125,000 payable quarterly. The costs paid to UC San Diego under the UC San Diego Research Agreement are included as part of the Company’s annual diligence obligations under the UC San Diego license agreement.
CIRM
In August 2017, CIRM awarded an $18.3 million grant to researchers at UC San Diego to advance Oncternal’s Phase 1b/2 clinical trial evaluating cirmtuzumab in combination with ibrutinib for the treatment of patients withB-cell lymphoid malignancies, including MCL and CLL. The trial is being conducted in collaboration between Oncternal and UC San Diego, with Oncternal responsible for study conduct and data management. Oncternal estimates it will receive $16.1 million in development milestones under research subaward agreements throughout the award project period, which runs through March 31, 2022. Under the CIRM research subaward agreements, the company is committed to certainco-funding requirements, including providing UC San Diego with progress and financial update reports. The CIRM subaward does not bear a royalty payment commitment.
259
CIRM may suspend or permanently cease disbursements of funds under the research subaward agreements, or pursue other remedies as allowed by law, if CIRM determines that UC San Diego has not complied with the terms and conditions of the award, or if there are unexpected, substantial manufacturing failure leading to delayed enrollment in the clinical trial, failure to enroll the trial, or if FDA issues a clinical hold order with respect to the clinical trial.
Georgetown University
In March 2014, the Company entered into an exclusive license agreement (the “Georgetown License Agreement”), with Georgetown University, or Georgetown, pursuant to which Oncternal licensed the exclusive worldwide right to patents and technologies for the development and commercialization of certain product candidates targetingEWS-FLI1 as an anti-tumor therapy for therapeutic, diagnostics, or research tool purposes. Under the Georgetown License Agreement, the company is solely responsible for all development and commercialization activities and costs in its respective territories, and is also responsible for all costs related to the filing, prosecution and maintenance of the licensed patent rights. Commencing in 2015, the Company is obligated to pay Georgetown an annual license maintenance fee until the first commercial sale occurs, make up to $200,000 in aggregate milestone payments upon the achievement of certain regulatory milestones, and will be required to pay low single digit royalties based on annual net product sales. The term of the Georgetown License Agreement continues until the expiration of the last valid claim within the patent rights covering the product, but may be terminated by either party upon material breach, or by Oncternal as to one or more countries with 90 days written notice of termination. Additionally, Georgetown may terminate the agreement in the event the company fails to pay any amount and fails to cure such failure within 30 days after receipt of notice, defaults in its obligation to obtain and maintain insurance and fails to remedy such breach within 60 days after receipt of notice, or declares insolvency or bankruptcy. Oncternal may terminate the agreement at any time upon at least 60 days’ written notice.
Shanghai Pharmaceutical (USA) Inc. (“SPH USA”)
In November 2018, Oncternal entered into the SPH USA License Agreement, with SPH USA under which Oncternal granted exclusive rights to SPH USA to manufacture, develop, market, distribute and sell in the People’s Republic of China, Hong Kong, Macau, and Taiwan (the “SPH USA Territory”), the company’s product candidates under the Georgetown License Agreement and the UC San Diego License Agreement. Under the SPH USA License Agreement, SPH USA is solely responsible for allpre-clinical and clinical development activities specific to obtaining regulatory approval for such product candidates in the SPH USA Territory, any third-party license milestone or royalty payments owed under the Georgetown License Agreement and the UC San Diego License Agreement, and paying Oncternal a low single digit royalty on net sales of licensed products in the SPH USA Territory. The SPH USA License Agreement will expire on a licensedproduct-by-licensed product andcountry/region-by-country/region basis on the later of ten years from the date of first commercial sale or when there is no longer a valid patent claim covering such licensed product in such country/region. The Agreement may be terminated by SPH USA, on acountry/region-by-country/region orproduct-by-product basis with 180 days written notice following the first anniversary of the effective date of the agreement or at any time on aproduct-by-product basis for a safety concern with respect to such product. Either party may terminate the Agreement in its entirety or on a licensedproduct-by-licensed product basis upon material breach that is not cured within 90 days, or in its entirety the event the other party becomes insolvent or enters into bankruptcy proceedings. Oncternal may terminate the agreement with 60 days written notice if SPH USA or its affiliates or sublicensees commence an action challenging the validity or enforceability of any licensed patent, or with 10 days written notice if SPH USA fails to own at least 20% of the voting securities of any assignee of the SPH USA License Agreement. Upon termination of the agreement for any reason all rights and licenses granted to SPH USA under the agreement will terminate, and in the event of termination for reasons other than Oncternal’s material breach, SPH USA would grant Oncternal non- exclusive, royalty-free, worldwide license to any intellectual property rights controlled by SPH USA or its affiliates to exploit the terminated program in the SPH USA Territory.
260
Selexis S.A.
In May 2014, ROAR Therapeutics, Inc., a predecessor company of Oncternal, entered into a commercial license agreement (the “Selexis License Agreement”), with Selexis, S.A., a Swiss company, pursuant to which Oncternal obtained a world-wide,non-exclusive license under certain of Selexis’ patents and technology rights to use a recombinant cell line produced using the Selexis technology to produce cirmtuzumab. Under the terms of the Selexis License Agreement, Oncternal will pay Selexis milestone payments totaling, in the aggregate, CHF 1,235,000, and a royalty in the low single digits on net sales of cirmtuzumab to third parties. The Selexis License Agreement remains in effect until the last to expire of the licensed Selexis patents, but may be terminated by either party if the other party materially breaches the agreement and fails to cure the breach within sixty days after receipt of a notice of default from the other party, or in the event the other party becomes insolvent or enters into bankruptcy proceedings. Additionally, Oncternal may terminate the Selexis License Agreement and the license granted therein at any time upon sixty days prior written notice to Selexis. In May 2015, Selexis’ rights to receive future milestone payments and royalties under the Selexis License Agreement were assigned to Ligand Pharmaceuticals, Inc.
Manufacturing
Oncternal has adopted a manufacturing strategy of contracting with third parties in accordance with cGMP for the manufacture of drug substance and product, and additional manufacturers are used to label, package and distribute investigational drug products. This strategy allows Oncternal to maintain a more flexible infrastructure while focusing its expertise on developing its products.
Oncternal expects to continue to rely on third parties for the production of clinical and commercial quantities of any product candidates.
There are no complicated biochemistries or unusual equipment required in the manufacturing process for either cirmtuzumab or TK216.
Oncternal has established a quality control and quality assurance program, which includes a set of standard operating procedures and specifications designed to ensure that our products are manufactured in accordance with cGMPs, and other applicable domestic and foreign regulations.
Intellectual Property
Oncternal strives to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to its business, including seeking, maintaining, and defending patent rights, whether developed internally or acquired or licensed from third parties. Oncternal’s policy is to seek to protect its proprietary position by, among other methods, filing patent applications in the United States and in jurisdictions outside of the United States related to its proprietary technology, inventions, and improvements that are important to the development and implementation of its business. Oncternal also relies on trade secrets andknow-how relating to its proprietary technology, continuing innovation, and acquisition andin-licensing opportunities to develop, strengthen, and maintain our proprietary position in the field of cancer therapeutics.
Oncternal’s commercial success may depend in part on its ability to obtain and maintain patent and other proprietary protection for its technology, inventions, and improvements; to preserve the confidentiality of its trade secrets; to defend and enforce its proprietary rights, including its patents; and to operate without infringing on the valid and enforceable patents and other proprietary rights of third parties.
Oncternal has licensed and acquired numerous patents and patent applications and it possesses substantialknow-how and trade secrets relating to the development and commercialization of healthcare products and services. As of March 31, 2019, Oncternal owned andin-licensed patent portfolio consisted of approximately 18
261
issued U.S. patents and eight pending U.S. patent applications related to certain of our proprietary technology, inventions, and improvements, and 24 issued patents and 86 pending patent applications in jurisdictions outside of the United States.
ROR1 Program
Oncternal has an exclusive, commercial, worldwide, transferrable license to a portfolio of patents and patent applications with rights to make, use, sell, offer for sale, and import ROR1 antibodies andCAR-T therapies for all therapeutic indications. This portfolio is licensed from the Regents. Oncternal hasknow-how and trade secrets related to compositions of matter for treating cancers, methods for treating cancer, and methods of screening for additional compositions of matter useful for treating cancer, as well as to additional antibodies and molecules that modulate ROR1 signaling.
As of March 31, 2019, Oncternal’s licensed patent portfolio included patents related to its clinical candidate currently in phase 1/2 clinical trials, cirmtuzumab. Cirmtuzumab is a humanized monoclonal antibody that specifically binds to the ROR1 receptor. Oncternal has two issued U.S. patents directed to the cirmtuzumab composition of matter: U.S. Pat. No. 9,217,040, with a patent term not due to expire before 2032; and U.S. Patent No. 9,758,591, with a patent term not due to expire before March 2033. Oncternal has one patent application pending in the U.S. in this family related to methods of using cirmtuzumab to treat cancer. Oncternal also has patents issued in Australia, China, Europe, Japan, Korea and Mexico directed to the cirmtuzumab compositions of matter. Oncternal has approximately 14 pending applications in foreign jurisdictions related to cirmtuzumab composition of matter and methods of use in treating cancer, including Australia, Canada, China, Europe, Japan, Korea, Malaysia, Mexico, Philippines, and Thailand. Patents that issue from these pending foreign applications would not be due to expire before 2032.
As of March 31, 2019, Oncternal had patent applications pending in the U.S. and in 15 jurisdictions outside the U.S. related to methods of treating cancer using a combination of cirmtuzumab and small-molecule chemotherapeutics. Patents that issue from these pending applications would not be due to expire before 2037.
As of March 31, 2019, Oncternal had patents and patent applications related to additional ROR1 binding antibodies and chimeric antigen receptor T cells specific for ROR1. Oncternal had four issued U.S. patents directed tonon-cirmtuzumab antibodies: U.S. Pat. No. 8,212,009, with a patent term not due to expire before November, 2026; U.S. Patent No. 9,242,014, with a patent term not due to expire before June 2031; U.S. Patent No. 9,938,350, with a patent term not due to expire before June 2031; and U.S. Patent No. 9,217,040, with a patent term not due to expire before January 2032. Oncternal had two patent applications pending in the U.S. related to additionalnon-cirmtuzumab ROR1 binding antibodies andnon-cirmtuzumab chimeric antigen receptor T cells specific for ROR1. Oncternal also had patents issued in Europe and Canada directed to additional ROR1 binding antibodies. Oncternal had two patent applications pending in Europe and Canada related to additional ROR1 binding antibodies and chimeric antigen receptor T cells specific for ROR1. Patents that issue from these pending foreign applications would not be due to expire before 2031.
As of March 31, 2019, Oncternal had intellectual property related to methods of screening for antibodies that specifically bind to ROR1. Oncternal had two issued U.S. patents, U.S. Pat. Nos. 9,523,695, and 9,933,434, with patent terms not due to expire before January 2032, directed to methods of screening for antibodies that specifically bind to ROR1. Oncternal additionally has patent applications pending directed to methods of screening for modulators of ROR1 signaling in jurisdictions including the U.S., Australia, Canada, China, Hong Kong, Japan, and Europe.
TK216 Program
Oncternal has exclusive worldwide rights to a portfolio of patents and patent applications related to small molecules, including TK216, targetingEWS-FLI1 for use in therapeutics and companion diagnostics. TK216
262
targets the EWS/FLI1 fusion protein, inhibits tumor cell proliferation and induces apoptosis in Ewing sarcoma cells. This portfolio, the Georgetown Licensed Portfolio, is licensed from Georgetown University. In addition to the portfolio licensed from Georgetown University, Oncternal holds a portfolio of patents and patent applications, the Oncternal Portfolio, related to TK216, analogs thereof, and uses thereof.
As of March 31, 2019, the Georgetown Licensed Portfolio consisted of approximately six U.S. issued patents, as well as 14 patents and 13 pending patent applications in jurisdictions outside of the United States. As of March 31, 2019, Oncternal had two U.S. patents directed to TK216: U.S. Pat. No. 9,604,927, with a patent term not due to expire before October 2035, and U.S. Pat. No. 9,987,251, with a patent term not due to expire before October 2035. Oncternal also had claims covering methods of inhibiting growth of or killing neoplastic cells: U.S. Pat. No. 9,895,352, with a patent term not due to expire before October 2035. Oncternal had approximately one pending U.S. application and 15 patents or pending applications in jurisdictions outside the U.S., including Australia, Argentina, Canada, China, Eurasia, Europe, Hong Kong, Israel, Japan, Korea, Mexico, New Zealand, Pakistan, and Taiwan. Patents issuing from these applications would not be due to expire before October 2035. Oncternal also had claims covering compositions of TK216 in combination with venetoclax and methods of inducing apoptosis in cells in AML and DLBCL: U.S. Pat. No. 10,159,660, with a patent term not due to expire before July 2037. Oncternal had approximately one pending U.S. application and 13 pending applications filed in jurisdictions outside the U.S., including Argentina, Canada, China, Europe, Indonesia, Japan, Korea, Mexico, Malaysia, Philippines, Singapore, Taiwan, and Vietnam. Patents issuing from these applications would not be due to expire before July 2037.
As of March 31, 2019, the Georgetown Licensed Portfolio contained patents directed to other compounds that function as, e.g.,EWS-FLI1 inhibitors. Oncternal had three U.S. patents directed to compounds and methods for treating Ewing sarcoma or pancreatic cancer: U.S. Pat. No. 8,232,301, with a patent term not due to expire before November 2028, U.S. Pat. No. 9,045,415, with a patent term not due to expire before August 2028, and U.S. Pat. No. 9,758,481, with a patent term not due to expire before December 2027. Oncternal had four issued patents in jurisdictions outside the U.S., including Australia, Canada, Europe, and Hong Kong. These patents are not due to expire before December 2027.
As of March 31, 2019, the Georgetown Licensed Portfolio contained additional patents and pending applications related to other compounds that targetEWS-FLI1. Oncternal had two issued U.S. patents directed to compounds and methods for treating pancreatic cancer or Ewing sarcoma: U.S. Pat. No. 9,290,449, with a patent term not due to expire before April 2033, and U.S. Pat. No. 9,714,222, with a patent term not due to expire before April 2033. There were patents or pending applications outside the U.S. in Australia, Canada, China, Europe, Hong Kong, Israel, India, Japan, Korea, Mexico, and New Zealand. These patents have a patent term not due to expire before April 2033, and patents issuing from these applications would not be due to expire before April 2033.
As of March 31, 2019, the Georgetown Licensed Portfolio contained additional patents and pending applications related to methods of treating cancers. Oncternal had one issued U.S. patent directed to methods of treating lung cancer or glioblastoma multiforme: U.S. Pat. No. 9,511,050, with a patent term not due to expire before October 2034. There were patents or pending applications outside the U.S. in Australia, Canada, China, Japan, Korea, Mexico and New Zealand. These patents have a patent term not due to expire before October 2034, and patents issuing from these applications would not be due to expire before October 2034.
As of March 31, 2019, the Oncternal Portfolio further contained additional patents and pending applications related to indoline derivative compounds, which are analogs of TK216, that act asEWS-FLI1 inhibitors. Oncternal had one issued U.S. patent directed to compounds and methods of inhibiting proliferation of a cell expressing an ETS gene or comprising an ETS fusion gene: U.S. Pat. No. 9,822,122, with a patent term not due to expire before March 2037. There was one pending U.S. application and approximately eight applications pending outside the U.S. in Argentina, Pakistan, Taiwan, China, Europe, Japan, Korea, and Malaysia. Patents issuing from these applications would not be due to expire before March 2037.
263
Individual patents extend for varying periods of time, depending upon the date of filing of the patent application, the date of patent issuance, and the legal term of patents in the countries in which they are obtained. Generally, patents issued for applications filed in the United States are effective for 20 years from the earliest effective filing date. The patent term may be adjusted to compensate for delayed patent issuance, when such delays are caused by the patent office or successful appeals against patent office actions. There is no limit on this patent term adjustment. In addition, in certain instances, a patent term can be extended to recapture a portion of the term effectively lost as a result of the FDA regulatory review period. The restoration period cannot be longer than five years and the total patent term, including the restoration period, must not exceed 14 years following the date of FDA approval of the applicable drug product. The duration of patents outside of the United States varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. Our issued patents are due to expire on dates ranging from 2026-2037. If patents are issued on our pending patent applications, the resulting patents would be due to expire on dates ranging from 2026-2037. However, the actual protection afforded by a patent varies on aproduct-by-product basis, fromcountry-to-country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country, and the validity and enforceability of the patent.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage,record-keeping, promotion, advertising, distribution, marketing and export and import of products such as those Oncternal is developing. A new drug must be approved by the FDA through the NDA process and a new biologic must be approved by the FDA through the BLA process before it may be legally marketed in the United States.
United States Drug Development Process
In the United States, the FDA regulates drugs under the federal Food, Drug, and Cosmetic Act (“FDCA”), and in the case of biologics, also under the Public Health Service Act (“PHSA”), and their implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on Oncternal.
The process required by the FDA before a drug or biologic may be marketed in the United States generally involves the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies in accordance with GLP regulations and other applicable regulations; |
| • | submission to the FDA of an Investigational New Drug Application, or IND, which must become effective before human clinical trials may begin; |
| • | performance of adequate andwell-controlled human clinical trials in accordance with Good Clinical Practice (“GCP”), requirements to establish the safety and efficacy of the proposed drug for its intended use; |
| • | submission to the FDA of an NDA or BLA; |
264
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP requirements to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA or BLA. |
The clinical study sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA places the clinical study on a clinical hold within that30-day time period. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical study can begin. The FDA may also impose clinical holds on a biological product candidate at any time before or during clinical trials due to safety concerns ornon-compliance. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA.
In addition to the submission of an IND to the FDA before initiation of a clinical trial in the United States, certain human clinical trials involving recombinant or synthetic nucleic acid molecules had historically been subject to review by the Recombinant DNA Advisory Committee (“RAC”), of the NIH Office of Biotechnology Activities, or OBA, pursuant to the NIH Guideline. On August 17, 2018, the NIH issued a notice in the Federal Register and issued a public statement proposing changes to the oversight framework for gene therapy trials, including changes to the applicable NIH Guidelines to modify the roles and responsibilities of the RAC with respect to human clinical trials of gene therapy products, and requesting public comment on its proposed modifications. During the public comment period, which closed on October 16, 2018, the NIH announced that it will no longer accept new human gene transfer protocols for review as a part of the protocol registration process or convene the RAC to review individual clinical protocols. These trials will remain subject to the FDA’s oversight and other clinical trial regulations, and oversight at the local level as set forth in the applicable NIH Guidelines. Specifically, under the NIH Guidelines, supervision of human gene transfer trials includes evaluation and assessment by an IBC, a local institutional committee that reviews and oversees research utilizing recombinant or synthetic nucleic acid molecules at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment, and such review may result in some delay before initiation of a clinical trial. While the NIH Guidelines are not mandatory unless the research in question is being conducted at or sponsored by institutions receiving NIH funding of recombinant or synthetic nucleic acid molecule research, many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them.
Clinical trials involve the administration of a product candidate to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the study sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical study, dosing procedures, patient selection and exclusion criteria, and the parameters to be used to monitor patient safety, including stopping rules that assure a clinical study will be stopped if certain adverse events should occur. Each protocol and any amendments to the protocol must be submitted to the FDA as part of the IND. Clinical trials must be conducted and monitored in accordance with the FDA’s regulations including GCP requirements, including the requirement that all research patients provide informed consent. Further, each clinical study must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical study will be conducted. Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| • | Phase 1: The product candidate is initially administered to healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe orlife-threatening diseases, such as cancer, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
265
| • | Phase 2: This phase involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine the appropriate dosage for further clinical trials. |
| • | Phase 3: Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These clinical trials are intended to establish the safety and efficacy of the product and the overallrisk-benefit ratio of the product candidate and provide, if appropriate, an adequate basis for product labeling and commercial use of the product. |
Post-approval trials, sometimes referred to as Phase 4 studies, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA or BLA.
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. In addition, some clinical trials are overseen by an independent group of qualified experts organized by the sponsor, known as a data safety monitoring board or committee. Depending on its charter, this group may determine whether a trial may move forward at designated check points based on access to certain data from the trial.
During the development of a new drug or biologic, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA or BLA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach agreement on the next phase of development. Sponsors typically use the meetings at the end of the Phase 2 trial to discuss Phase 2 clinical results and present plans for the pivotal Phase 3 clinical trial that they believe will support approval of the new drug or biologic.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life. While the IND is active and before approval, progress reports summarizing the results of the clinical trials and nonclinical studies performed since the last progress report must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs, findings from animal orin vitro testing suggesting a significant risk to humans, and any clinically important increased incidence of a serious suspected adverse reaction compared to that listed in the protocol or investigator brochure.
There are also requirements governing the reporting of ongoing clinical trials and completed trial results to public registries. Sponsors of certain clinical trials ofFDA-regulated products are required to register and disclose specified clinical trial information, which is publicly available at www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, trial sites and investigators and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved.
266
United States Review and Approval Process
The results of product development, preclinical and othernon-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances.
The FDA reviews all NDAs and BLAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA or BLA for filing. In this event, the NDA or BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
Once the submission is accepted for filing, the FDA begins anin-depth substantive review. The FDA may refer the NDA or BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The approval process is lengthy and often difficult, and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information are submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing iscGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. The FDA reviews a BLA to determine, among other things whether the product is safe, pure and potent and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency. Before approving an NDA or BLA, the FDA will inspect the facility or facilities where the product is manufactured.
After the FDA evaluates an NDA or BLA, it will issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application will not be approved in its present form. A Complete Response Letter usually describes the specific deficiencies in the NDA or BLA identified by the FDA and may require additional clinical data, such as an additional pivotal Phase 3 trial or other significant andtime-consuming requirements related to clinical trials, nonclinical studies or manufacturing. If a Complete Response Letter is issued, the sponsor must resubmit the NDA or BLA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA or BLA does not satisfy the criteria for approval. If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require a sponsor to conduct Phase 4 testing, which involves clinical trials designed to further assess a drug’s safety and effectiveness after NDA or BLA approval, and may require testing and surveillance programs to monitor the safety of approved products which have been commercialized. The FDA may also place other conditions on approval including the requirement for a risk evaluation and mitigation strategy (“REMS”) to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS. The FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Marketing approval may be withdrawn fornon-compliance with regulatory requirements or if problems occur following initial marketing.
The Food and Drug Administration Safety and Innovation Act (“FDASIA”), made permanent the Pediatric Research Equity Act (“PREA”), which requires a sponsor to conduct pediatric clinical trials for most drugs and
267
biologics, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs, BLAs and supplements thereto must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must evaluate the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric clinical trials for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug or biologic is ready for approval for use in adults before pediatric clinical trials are complete or that additional safety or effectiveness data needs to be collected before the pediatric clinical trials begin. The FDA must send anon-compliance letter to any sponsor that fails to submit the required assessment, keep a deferral current or fails to submit a request for approval of a pediatric formulation.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the United States or, if it affects more than 200,000 individuals in the United States, there is no reasonable expectation that the cost of developing and making a drug or biologic product available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan designation must be requested before submitting an NDA or BLA. After the FDA grants orphan designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity or inability to manufacture the product in sufficient quantities. The designation of such drug or biologic also entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages anduser-fee waivers. However, competitors, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan exclusivity also could block the approval of one of Oncternal’s product candidates for seven years if a competitor obtains approval of the same drug or biologic as defined by the FDA or if Oncternal’s product candidate is determined to be contained within the competitor’s product for the same indication or disease. If an orphan designated product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan exclusivity. Orphan drug status in the European Union has similar but not identical benefits in that jurisdiction.
Although Oncternal has not sought or obtained orphan designation for any of its product candidates, the company may pursue such designation in the future if it determines that its proposed indications meet the qualifying criteria for such designation.
Expedited Development and Review Programs
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drug products that meet certain criteria. Specifically, new drugs are eligible for Fast Track designation if they are intended to treat a serious orlife-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. Unique to a Fast Track product, the FDA may consider for review sections of the NDA or BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA or BLA, the FDA agrees to accept sections of the NDA or BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA or BLA.
268
Any product submitted to the FDA for approval, including a product with a Fast Track designation, may also be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. A product is eligible for priority review if it has the potential to provide a significant improvement in the treatment, diagnosis or prevention of a serious disease or condition compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug designated for priority review in an effort to facilitate the review. The FDA endeavors to review applications with priority review designations within six months of the filing date as compared to ten months for review of original BLAs and new molecular entity NDAs under its standard review goals.
In addition, a product may be eligible for accelerated approval. Drug and biologic products intended to treat serious orlife-threatening diseases or conditions may be eligible for accelerated approval upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate andwell-controlledpost-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approvalpre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Fast Track designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
FDASIA established a category of drugs and biologics referred to as “breakthrough therapies” that may be eligible to receive Breakthrough Therapy Designation. A sponsor may seek FDA designation of a drug or biologic candidate as a “breakthrough therapy” if the product is intended, alone or in combination with one or more other products, to treat a serious orlife-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes all of the Fast Track program features, as well as more intensive FDA interaction and guidance. The Breakthrough Therapy Designation is a distinct status from both accelerated approval and priority review, which can also be granted to the same drug if relevant criteria are met. If a product is designated as breakthrough therapy, the FDA will expedite the development and review of such drug. All requests for breakthrough therapy designation will be reviewed within 60 days of receipt, and the FDA will either grant or deny the request.
Post-approval requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. After approval, some types of changes to the approved product, such as adding new indications, certain manufacturing changes and additional labeling claims, are subject to further FDA review and approval. Drug and biologics manufacturers and other entities involved in the manufacture and distribution of approved drugs and biologics are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP regulations and other laws and regulations.
Any drug products manufactured or distributed by us or Oncternal’s partners pursuant to FDA approvals will be subject to continuing regulation by the FDA, including, among other things,record-keeping requirements, reporting of adverse experiences with the drug, providing the FDA with updated safety and efficacy information, drug sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. The FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market and
269
imposes requirements and restrictions on drug and biologics manufacturers, such as those related todirect-to-consumer advertising, the prohibition on promoting products for uses or in patient populations that are not described in the product’s approved labeling (known as“off-label use”),industry-sponsored scientific and educational activities, and promotional activities involving the internet. Discovery of previously unknown problems or the failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant or manufacturer to administrative or judicial civil or criminal sanctions and adverse publicity. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, clinical hold, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, mandated corrective advertising or communications with doctors, debarment, restitution, disgorgement of profits, or civil or criminal penalties.
Biosimilars and Exclusivity
The Affordable Care Act includes a subtitle called the Biologics Price Competition and Innovation Act of 2009 (“BPCIA”), which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with anFDA-licensed reference biological product. To date, few biosimilars have been licensed under the BPCIA, although numerous biosimilars have been approved in Europe. The FDA has issued several guidance documents outlining an approach to review and approval of biosimilars.
Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structures of biological products, as well as the processes by which such products are manufactured, pose significant hurdles to implementation of the abbreviated approval pathway that are still being addressed by the FDA.
Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own preclinical data and data from adequate andwell-controlled clinical trials to demonstrate the safety, purity and potency of their product. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products deemed “interchangeable” by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law.
The BPCIA is complex and continues to be interpreted and implemented by the FDA. In addition, recent government proposals have sought to reduce the12-year reference product exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. As a result, the ultimate impact, implementation and meaning of the BPCIA is subject to significant uncertainty.
270
Approval Process Outside of the United States
In addition to regulations in the United States, the company will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical studies and any commercial sales and distribution of Oncternal’s product candidates.
Whether or not Oncternal obtains FDA approval for a product candidate, the company must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical studies or marketing of the product candidates in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical study application much like the IND prior to the commencement of human clinical studies. In the European Union, for example, a clinical trial authorization (“CTA”) must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical study development may proceed.
To obtain regulatory approval of an investigational biological product under European Union regulatory systems, Oncternal must submit a marketing authorization application. The application used to file the BLA in the United States is similar to that required in the European Union, with the exception of, among other things,country-specific document requirements. The European Union also provides opportunities for market exclusivity. For example, in the European Union, upon receiving marketing authorization, new chemical entities generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic application. During the additionaltwo-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic product can be marketed until the expiration of the market exclusivity. However, there is no guarantee that a product will be considered by the European Union’s regulatory authorities to be a new chemical entity, and products may not qualify for data exclusivity. Products receiving orphan designation in the European Union can receive ten years of market exclusivity, during which time no similar medicinal product for the same indication may be placed on the market. An orphan product can also obtain an additional two years of market exclusivity in the European Union for pediatric studies. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications.
The criteria for designating an “orphan medicinal product” in the European Union are similar in principle to those in the United States. Under Article 3 of Regulation (EC) 141/2000, a medicinal product may be designated as orphan if (1) it is intended for the diagnosis, prevention or treatment of alife-threatening or chronically debilitating condition; (2) either (a) such condition affects no more than five in 10,000 persons in the European Union when the application is made, or (b) the product, without the benefits derived from orphan status, would not generate sufficient return in the European Union to justify investment; and (3) there exists no satisfactory method of diagnosis, prevention or treatment of such condition authorized for marketing in the European Union, or if such a method exists, the product will be of significant benefit to those affected by the condition, as defined in Regulation (EC) 847/2000. Orphan medicinal products are eligible for financial incentives such as reduction of fees or fee waivers and are, upon grant of a marketing authorization, entitled to ten years of market exclusivity for the approved therapeutic indication. The application for orphan drug designation must be submitted before the application for marketing authorization. The applicant will receive a fee reduction for the marketing authorization application if the orphan drug designation has been granted, but not if the designation is still pending at the time the marketing authorization is submitted. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
271
The10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. In addition, marketing authorization may be granted to a similar product for the same indication at any time if:
| • | the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; |
| • | the applicant consents to a second orphan medicinal product application; or |
| • | the applicant cannot supply enough orphan medicinal product. |
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical studies, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical studies are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If Oncternal fails to comply with applicable foreign regulatory requirements, Oncternal may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Other Healthcare Laws and Compliance Requirements
Pharmaceutical companies are subject to additional healthcare regulation and enforcement by the U.S. federal and state governments and by authorities in the foreign jurisdictions in which they conduct their business. At the federal level, such laws include, without limitation: the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, to induce, or in return for, either the referral of an individual, or the purchase or recommendation of an item or service for which payment may be made under any federal healthcare program; federal civil and criminal false claims laws and civil monetary penalty laws, including the civil False Claims Act, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment to the federal government, including federal healthcare programs, that are false or fraudulent; the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which prohibits, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; and the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to annually report to the federal government, information related to payments or other transfers of value made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members.
Pharmaceutical companies are also subject to U.S. state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed bynon-governmental third-party payors, including private insurers, or that apply regardless of payor; laws which require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, laws which require pharmaceutical companies to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and information related to drug pricing, and laws requiring the registration of pharmaceutical sales and medical representatives. Violation of these laws or other governmental regulations may result in penalties, including, without limitation, significant civil, criminal and administrative penalties, damages, fines, exclusion from government-funded healthcare programs, such as Medicare and Medicaid or similar programs in other countries or jurisdictions, integrity oversight and reporting obligations to resolve allegations ofnon-compliance, disgorgement, imprisonment, contractual damages, reputational harm, diminished profits and the curtailment or restructuring of operations.
272
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any pharmaceutical or biological product for which we obtain regulatory approval. Sales of any product depend, in part, on the extent to which such product will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations, and the level of reimbursement for such product by third-party payors. Decisions regarding the extent of coverage and amount of reimbursement to be provided are made on aplan-by-plan basis. Further, no uniform policy for coverage and reimbursement exists in the United States, and coverage and reimbursement can differ significantly from payor to payor. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement rates, but also have their own methods and approval process apart from Medicare determinations. As a result, the coverage determination process is often a time-consuming and costly process that may require companies to provide scientific and clinical support for the use of a product to each payor separately. For products administered under the supervision of a physician, obtaining coverage and adequate reimbursement may be particularly difficult because of the higher prices often associated with such drugs. Additionally, separate reimbursement for the product itself or the treatment or procedure in which the product is used may not be available, which may impact physician utilization. Lastly, companion diagnostic tests require coverage and reimbursement separate and apart from the coverage and reimbursement for their companion pharmaceutical or biological products. Similar challenges to obtaining coverage and reimbursement, applicable to pharmaceutical or biological products, will apply to companion diagnostics.
In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Third-party payors are increasingly challenging the prices charged for medical products and services, examining the medical necessity and reviewing the cost effectiveness of pharmaceutical or biological products, medical devices and medical services, in addition to questioning safety and efficacy. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of any product. Decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage and patient demand for the product.
Healthcare Reform
The United States and some foreign jurisdictions are considering or have enacted a number of reform proposals to change the healthcare system. There is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by federal and state legislative initiatives, including those designed to limit the pricing, coverage, and reimbursement of pharmaceutical and biopharmaceutical products, especially under government-funded health care programs, and increased governmental control of drug pricing.
In March 2010, the ACA was signed into law, which substantially changed the way healthcare is financed by both governmental and private insurers in the United States, and significantly affected the pharmaceutical industry. The ACA contains a number of provisions of particular import to the pharmaceutical and biotechnology industries, including, but not limited to, those governing enrollment in federal healthcare programs, a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. Since its enactment, there have been judicial, Congressional, and executive branch challenges to certain aspects of the ACA. For example, the Tax Act, was enacted on December 22, 2017, which includes a provision repealing, effective January 1, 2019, thetax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health insurance for all or part of a year that is commonly referred to as the “individual mandate.” Additionally, on
273
December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, ruled that the individual mandate is a critical and inseverable feature of the ACA, and therefore, because it was repealed as part of the Tax Act, the remaining provisions of the ACA are invalid as well. While the Trump Administration and CMS have both stated that the ruling will have no immediate effect, it is unclear how this decision, subsequent appeals, if any, and other efforts to repeal and replace the ACA will impact the law.
Other legislative changes have been proposed and adopted since the ACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level, the Trump administration’s budget proposal for fiscal year 2019 contains further drug price control measures that could be enacted during the 2019 budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs forlow-income patients. Additionally, the Trump administration released a “Blueprint” to lower drug prices and reduce the out of pocket costs of prescription drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. The Department of Health and Human Services, or HHS, has already started the process of soliciting feedback on some of these measures and, at the same time, is immediately implementing others under its existing authority. For example, in September 2018, CMS announced that it will allow Medicare Advantage Plans the option to use step therapy for Part B drugs beginning January 1, 2019, and in October 2018, CMS proposed a new rule that would requiredirect-to-consumer television advertisements of prescription drugs and biological products, for which payment is available through or under Medicare or Medicaid, to include in the advertisement the Wholesale Acquisition Cost, or list price, of that drug or biological product. On January 31, 2019, the HHS Office of Inspector General proposed modifications to U.S. federal Anti-Kickback Statute safe harbors which, among other things, will affect rebates paid by manufacturers to Medicare Part D plans, the purpose of which is to further reduce the cost of drug products to consumers. Although some of these, and other, proposals will require authorization through additional legislation to become effective, Congress and the Trump administration have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Data Privacy and Security Laws
Pharmaceutical companies may be subject to U.S. federal and state health information privacy, security and data breach notification laws, which may govern the collection, use, disclosure and protection of health-related and other personal information. State laws may be more stringent, broader in scope or offer greater individual rights with respect to protected health information, or PHI, than HIPAA, and state laws may differ from each other, which may complicate compliance efforts. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAAnon-compliance.
As of May 25, 2018, Regulation 2016/676, known as the General Data Protection Regulation (“GDPR”), replaced the Data Protection Directive with respect to the processing of personal data in the European Union. The
274
GDPR imposes many requirements for controllers and processors of personal data, including, for example, higher standards for obtaining consent from individuals to process their personal data, more robust disclosures to individuals and a strengthened individual data rights regime, shortened timelines for data breach notifications, limitations on retention and secondary use of information, increased requirements pertaining to health data and pseudonymized (i.e.,key-coded) data and additional obligations when we contract third-party processors in connection with the processing of the personal data. The GDPR allows EU member states to make additional laws and regulations further limiting the processing of genetic, biometric or health data. Failure to comply with the requirements of GDPR and the applicable national data protection laws of the EU member states may result in fines of up to €20 million or up to 4% of the total worldwide annual turnover of the preceding financial year, whichever is higher, and other administrative penalties.
Employees
As of March 31, 2019, Oncternal had five full-time employees, three part-time employees, and a number of consultants, most of whom are engaged in research and development activities. None of Oncternal’s employees are represented by labor unions or covered by collective bargaining agreements. Oncternal considers its relationship with its employees to be good.
Facilities
Oncternal’s corporate headquarters are located in San Diego, California, where it currently subleases approximately 4,700 square feet of office space used primarily for corporate, research, development, clinical, regulatory, manufacturing and quality functions. Oncternal’s sublease for this facility expires in March 2021.
Legal Proceedings
Oncternal is not currently subject to any material legal proceedings except as otherwise described in the section titled “The Merger—Litigation Related to the Merger” beginning on page 167 of the proxy statement/prospectus/information statement. From time to time, Oncternal may be involved in legal proceedings or subject to claims incident to the ordinary course of business. Regardless of the outcome, such proceedings or claims can have an adverse impact on Oncternal because of defense and settlement costs, diversion of resources and other factors, and there can be no assurances that favorable outcomes will be obtained.
275
GTX MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with GTx’s financial statements and related notes included elsewhere in this proxy statement/prospectus/information statement. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. GTx’s actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under Part I, Item 1A “Risk Factors” and elsewhere in this proxy statement/prospectus/information statement. See “Special Note Regarding Forward-Looking Statements” in this proxy statement/prospectus/information statement.
Overview
Business Overview and Highlights
GTx is a biopharmaceutical company dedicated to the discovery, development and commercialization of medicines to treat serious and/or significant unmet medical conditions. Under an exclusive worldwide license agreement with the University of Tennessee Research Foundation, or UTRF, GTx is developing UTRF’s proprietary potential selective androgen receptor degrader, or SARD, technology, which it believes may have the potential to provide compounds that can degrade or antagonize multiple forms of androgen receptor, or AR, thereby potentially inhibiting tumor growth in patients with progressive castration-resistant prostate cancer, or CRPC, including those patients who do not respond to or are resistant to current androgen targeted therapies. GTx has been conducting preclinical studies to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an IND to enable the initiation ofa first-in-human clinical trial, if any. However, GTx recently received and evaluated new preclinical data from an independent laboratory of an academic researcher engaged by GTx, which, among other things, showed that at higher dose concentrations, the SARD compounds tested by the independent laboratory demonstrated partial androgen receptor agonist activity. The academic researcher pointed out that if these results translate to the clinical setting where there is little or no dose separation between antagonist activity and agonist activity, the future of the SARD program as an effective treatment of men with CRPC would likely not be viable. This information was in conflict with other independent laboratory preclinical data previously received by GTx senior management and with internal preclinical data generated by GTx, that included: (1) conflictingin vitro data showing either partial agonist activity or no partial agonist activity, (2) in vivo data showing no evidence of agonist activity, and (3) data from another independent laboratory showing the dose-dependent suppression of enzalutamide-resistant prostate cancer tumors in a rat xenograft model. Considering this conflicting information, it was concluded that additional preclinical studies were required to better understand SARDs and their mechanism of action, and to reconcile the conflictingin vitro andin vivo findings. Accordingly, additional preclinical research would be required in order to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies.
GTx had been developing selective androgen receptor modulators, or SARMs. GTx’s SARM product candidate, enobosarm(GTx-024), was most recently evaluated in post-menopausal women with stress urinary incontinence, or SUI. During the third quarter of 2018, GTx announced that the ASTRID trial, evaluating the change in the mean number of daily SUI episodes following 12 weeks of enobosarm treatment failed to achieve statistical significance on the primary endpoint of the proportion of patients with a greater than 50% reduction in incontinence episodes per day compared to placebo. GTx has completed the ASTRID trial, including its review of the full data sets from the clinical trial, and has determined that there is not a sufficient path forward to warrant additional clinical development of enobosarm to treat SUI. GTx has therefore discontinued further development of enobosarm to treat SUI, including discontinuing the related durability and open-label safety extension studies it initiated before GTx received topline data from the ASTRID trial. GTx has also discontinued any further development of its SARM technology generally.
276
Following the announcement of the ASTRID trial results, the GTx Board commenced a process of evaluating strategic alternatives to maximize stockholder value. To assist with this process, the GTx Board engaged a financial advisory firm to help explore its available strategic alternatives, including possible mergers and business combinations, a sale of part or all of its assets, and collaboration and licensing arrangements. On March 6, 2019, GTx and Oncternal announced the signing of the Original Merger Agreement and, as further discussed in the section titled “The Merger—Background of the Merger,” GTx and Oncternal amended the Merger Agreement on April 30, 2019 by entering into the Merger Agreement Amendment.
Although GTx has entered into the Merger Agreement and intends to consummate the merger, there is no assurance that it will be able to successfully consummate the merger on a timely basis, or at all. If, for any reason, the merger is not completed, GTx will reconsider its strategic alternatives and could pursue one or more of the following courses of action:
| • | Continue development of GTx’s SARD program. As set forth above, GTx has been conducting preclinical studies in order to determine if it can identify an appropriate SARD compound to move forward into additional preclinical studies required for the potential submission of an IND to enable the initiation ofa first-in-human clinical trial, if any. Accordingly, if, for any reason, the merger is not consummated, GTx may determine to move forward with additional preclinical research and studies of its SARD compounds. However, GTx’s existing capital resources may not be adequate to enable it to conduct and complete anyIND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete anyIND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. As a result, GTx may also resume its efforts to seek additional funds through potential collaborative, partnering or other strategic arrangements to provide it with the necessary resources for the development of the SARD program. |
| • | Pursue potential collaborative, partnering or other strategic arrangements for GTx’s SARM assets, including a sale or other divestiture of its SARM assets. GTx has discontinued further development of its SARM technology, including enobosarm, and does not currently have any plans to resume development of its SARM technology. GTx continues its efforts to seek potential collaborative, partnering or other strategic arrangements for its SARM assets, including a sale or other divestiture of its SARM assets. |
| • | Pursue another strategic transaction like the merger. The GTx Board may elect to pursue an alternative strategy, one of which may be a strategic transaction similar to the merger. |
| • | Dissolve and liquidate GTx’s assets. If, for any reason, the merger is not consummated and GTx is unable to identify and complete an alternative strategic transaction like the merger or potential collaborative, partnering or other strategic arrangements for its SARM assets, or to continue to operate GTx’s business due to its inability to identify an appropriate SARD compound to move forward into potential IND-enabling studies or to raise additional funding for the development of its SARD program or otherwise, GTx may be required to dissolve and liquidate its assets. In such case, GTx would be required to pay all of its debts and contractual obligations, and to set aside certain reserves for potential future claims, and there can be no assurances as to the amount or timing of available cash left to distribute to its stockholders after paying its debts and other obligations and setting aside funds for reserves. |
277
Financial Highlights
GTx’s net loss for the year ended December 31, 2018 was $38.4 million. GTx expects to incur significant operating losses for the foreseeable future depending on the extent of its preclinical and any clinical development activities and, if any such development activities are successful, potentially seeking regulatory approval of any potential future product candidates. GTx has funded its operations primarily through the sale of equity securities, collaboration and license agreements, and prior to September 2012, product revenue from sales of FARESTON, the rights to which GTx sold to a third-party in the third quarter of 2012. GTx does not expect to receive regulatory approval for the commercial sale of any product candidates for the foreseeable future, if at all.
At December 31, 2018, GTx had cash, cash equivalents and short-term investments of $28.5 million compared to $43.9 million at December 31, 2017. In May 2018, GTx sold 1.5 million shares of common stock under itsAt-the-Market Equity Offering SM Sales Agreement (the “ATM Sales Agreement”), with Stifel, Nicolaus & Company, Incorporated, or Stifel, and raised net proceeds of $24.5 million.
To conserve its cash resources, GTx has substantially reduced its workforce since November 2018 and has ceased its SARM development activities and all other operations except forday-to-day business operations, completing ongoing SARD preclinical studies and those activities necessary to complete the merger. In the first quarter of 2019, due to the entry into the Merger Agreement with Oncternal, its board of directors committed to reducing its workforce down to a total of eleven full-time employees, who will remain with GTx until the closing of the transaction to assist with itsday-to-day business operations, including continuing its ongoing SARD preclinical studies, and those activities necessary to complete the merger. All employees affected by the workforce reduction will be eligible to receive, among other things, specified severance payments based on the applicable employee’s level and years of service with GTx and the continuation of group health insurance coverage. In addition, the affected employees will also be eligible for full vesting acceleration of their outstanding stock options as well as an extension of the post-termination exercise period for their outstanding stock options. As a result of the workforce reduction and prior termination of three employees earlier in the first quarter of 2019, GTx estimates that it will incur total severance-related charges for these employees of approximately $1.0 million in the first quarter of 2019 and up to an additional $500,000 contingent upon the closing of the merger. GTx does not expect to record anon-cash charge related to the modification of outstanding stock options in connection with the workforce reduction.
If the merger is not completed, based on its current business plan and spending assumptions as a standalone company, GTx estimates that its current cash, cash equivalents and short-term investments, together with interest thereon, will be sufficient to meet its projected operating requirements for at least the next 12 months. GTx has based its cash sufficiency estimates on its current business plan and its assumptions that may prove to be wrong. GTx could utilize its available capital resources sooner than it currently expects, and it could need additional funding sooner than currently anticipated.
GTx’s existing capital resources may not be adequate to enable it to conduct and complete anyIND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete anyIND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. If GTx is unable to raise sufficient additional funds for the development of its SARD program, whether through potential collaborative, partnering or other strategic arrangements or otherwise, or if GTx otherwise determines to discontinue the development of its SARD program, whether as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity or otherwise, GTx will likely determine to cease operations.
278
While GTx has been able to fund its operations to date, GTx has no ongoing collaborations for the development and commercialization of any product candidates and no source of revenue, nor does it expect to generate product revenue for the foreseeable future. GTx does not have any commitments for future external funding. In addition, although GTx has entered into anAt-the-Market Equity Offering SM Sales Agreement with Stifel, Nicolaus & Company, Incorporated (the “ATM Sales Agreement”), under which approximately $25.0 million of shares of its common stock remained available for sale at December 31, 2018, it is unlikely it could raise sufficient funds under the ATM Sales Agreement to permit it to initiate and complete any initial human clinical trials of a SARD compound, and given its currently-depressed stock price, the ATM Sales Agreement is not otherwise expected to be a practical source of liquidity for it at this time. Further, given its currently-depressed stock price, GTx is significantly limited in its ability to sell shares of common stock under the ATM Sales Agreement since the issuance and sale of its common stock under the ATM Sales Agreement, if it occurs, would be effected under a registration statement onForm S-3 that it filed with the Securities and Exchange Commission, and in accordance with the rules governing those registration statements, it generally can only sell shares of its common stock under that registration statement in an amount not to exceedone-third of its public float, which limitation for all practical purposes precludes its ability to obtain any meaningful funding through the ATM Sales Agreement at this time.
Until GTx can generate a sufficient amount of product revenue, which it may never do, it will need to finance future cash needs through potential collaborative, partnering or other strategic arrangements, as well as through public or private equity offerings or debt financings or a combination of the foregoing. If GTx is unable to raise additional funds, it will need to continue to reduce its expenditures in order to preserve its cash. Further cost-cutting measures that GTx may take may not be sufficient to enable it to meet its cash requirements, and they may negatively affect its business and its ability to derive any value from its SARD program. In any event, in order to further the development of its SARD program, if at all, GTx will need to raise substantial additional capital. GTx’s failure to do so would likely result in it determining to cease operations.
Research and Development
Since its inception in 1997, GTx has been focused on drug discovery and development programs. Research and development expenses include, but are not limited to, its expenses for personnel and supplies associated with its research activities, screening and identification of product candidates, formulation and synthesis activities, manufacturing, preclinical studies, toxicology studies, clinical trials, regulatory and medical affairs activities, quality assurance activities and license fees. GTx expects that its research and development expenses for fiscal year 2019 to be significantly less than fiscal year 2018 primarily due to the completion of the ASTRID trial and termination of the related extension studies and due to the reductions in headcount during the fourth quarter of 2018 and the first quarter of 2019.
There is a substantial risk that any development program may not produce revenue. Moreover, because of uncertainties inherent in drug development, including those factors described in the “Risk Factors” section of this proxy statement/prospectus/information statement, GTx and/or potential future collaborators may not be able to successfully develop and commercialize any of its product candidates.
The successful development and commercialization of GTx’s product candidates is highly uncertain. GTx cannot reasonably estimate or know the nature, timing and estimated costs of the efforts necessary to complete the development and commercialization of, or the period in which material net cash inflows are expected to commence from, any of its product candidates due to the numerous risks and uncertainties associated with developing and commercializing drugs, including the uncertainty of:
| • | the scope, rate of progress and cost of GTx’s preclinical and potential future clinical development programs; |
| • | the terms and timing of any potential collaborative, partnering and other strategic arrangements that GTx may establish; |
279
| • | the amount and timing of any licensing fees, milestone payments and royalty payments from potential collaborators, if any; |
| • | potential future clinical trial results; |
| • | the cost and timing of regulatory filings and/or approvals to commercialize any potential future product candidates and any related restrictions, limitations, and/or warnings in the label of an approved product candidate; |
| • | the effect of competing technological and market developments; and |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights, and the cost of defending any other litigation claims. |
Any failure to complete the development of any potential future product candidates in a timely manner could have a material adverse effect on GTx’s operations, financial position and liquidity. A discussion of the risks and uncertainties associated with completing GTx’s development efforts on schedule, or at all, and some consequences of failing to do so, are set forth under the “Risk Factors” section of this proxy statement/prospectus/information statement.
General and Administrative Expenses
GTx’s general and administrative expenses consist primarily of salaries and other related costs for personnel serving executive, finance, legal, human resources, information technology, and investor relations functions. General and administrative expenses also include facility costs, insurance costs, and professional fees for legal, accounting, and public relations services. GTx expects its general and administrative expenses for fiscal year 2019 to decrease in comparison to fiscal year 2018 due to the reductions in headcount during the fourth quarter of 2018 and the first quarter of 2019.
Critical Accounting Policies and Significant Judgments and Estimates
GTx’s management’s discussion and analysis of its financial condition and results of operations is based on its financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires GTx to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, GTx evaluates its estimates and judgments related to revenue recognition, income taxes, intangible assets, long-term service contracts, share-based compensation, and other contingencies. GTx bases its estimates on historical experience and on various other factors that it believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While GTx’s significant accounting policies are more fully described in Note 2 to its financial statements included in this proxy statement/prospectus/information statement, GTx believes that the following accounting policies are most critical to aid you in fully understanding and evaluating its reported financial results.
Research and Development Expenses
Research and development expenses include, but are not limited to, its expenses for personnel and supplies associated with its research activities, screening and identification of product candidates, formulation and synthesis activities, manufacturing, preclinical studies, toxicology studies, clinical trials, regulatory and medical affairs activities, quality assurance activities and license fees. GTx expenses these costs in the period in which they are incurred. GTx estimates its liabilities for research and development expenses in order to match the
280
recognition of expenses to the period in which the actual services are received. As such, accrued liabilities related to third-party research and development activities are recognized based upon GTx’s estimate of services received and degree of completion of the services in accordance with the specific third-party contract.
Share-Based Compensation
GTx has stock option and equity incentive plans that provide for the purchase or acquisition of its common stock by certain of its employees andnon-employees. GTx measures compensation expense for its share-based payments based on the fair value of the awards on the grant date and recognize the expense over the period during which an employee ornon-employee director is required to provide service in exchange for the award.
The determination of the fair value of stock options on the date of grant include the expected life of the award, the expected stock price volatility over the expected life of the awards, and risk-free interest rate. GTx estimates the expected life of options by calculating the average of the vesting term and contractual term of the options. GTx estimates the expected stock price volatility based on the historical volatility of its common stock. The risk-free interest rate is determined using U.S. Treasury rates where the term is consistent with the expected life of the stock options. Expected dividend yield is not considered as GTx has not made any dividend payments and has no plans of doing so in the foreseeable future. The fair value of each stock option is amortized into compensation expense on a straight-line basis between the grant date for the award and each vesting date. During the first quarter of 2017, GTx adopted the Financial Accounting Standards Board Accounting Standards Update2016-09, Improvements to Employee Share Based Payment Accounting. This guidance addresses the income tax effects of stock-based payments and eliminates the windfall pool concept, as all of the tax effects related to stock-based payments are now being recorded at settlement (or expiration) through the income statement. The new guidance also permits entities to make an accounting policy election for the impact of forfeitures on the recognition of expense for stock-based payment awards, allowing for forfeitures to be estimated or recognized when they occur. GTx elected to prospectively adopt the policy that forfeitures be recorded when they occur. The adoption of this guidance did not have a material impact on its financial position or results of operations.
The following table summarizes share-based compensation expense included within the statements of operations for the years ended December 31, 2018, 2017 and 2016:
| Years ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| (in thousands) | ||||||||||||
Research and development expenses | $ | 807 | $ | 1,171 | $ | 1,260 | ||||||
General and administrative expenses | 1,556 | 2,146 | 1,829 | |||||||||
|
|
|
|
|
| |||||||
Total share-based compensation | $ | 2,363 | $ | 3,317 | $ | 3,089 | ||||||
|
|
|
|
|
| |||||||
Share-based compensation expense recorded in the statement of operations as general and administrative expense for the years ended December 31, 2018, 2017 and 2016 included share-based compensation expense related to deferred compensation arrangements for GTx’snon-employee directors of $166,000, $166,000 and $132,000, respectively. At December 31, 2018, the total compensation cost related tonon-vested stock options not yet recognized was approximately $7.7 million with a weighted-average expense recognition period of 2.95 years.
Income Taxes
GTx accounts for deferred taxes by recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. Accordingly, at December 31, 2018 and 2017, net of the valuation allowance, the net deferred tax assets were reduced to zero.
281
Results of Operations
Research and Development Expenses
The following table identifies the research and development expenses for GTx’s SARD program and its discontinued SARM program, as well as research and development expenses pertaining to its other research and development efforts, for each of the periods presented. Research and development spending for past periods is not indicative of spending in future periods.
| Years Ended December 31, | ||||||||||||||||
Proposed Candidate / Proposed Indication | Program | 2018 | 2017 | 2016 | ||||||||||||
Enobosarm | ||||||||||||||||
Treatment of postmenopausal women with SUI (1 mg and 3 mg) | SARM | $ | 25,576 | $ | 11,279 | $ | 1,286 | |||||||||
Enobosarm | ||||||||||||||||
Treatment of women with ER positive and AR positive advanced breast cancer (9 mg and 18 mg) | SARM | 1,957 | 5,541 | 7,316 | ||||||||||||
SARDs | ||||||||||||||||
Treatment of castration resistant prostate cancer | SARD | 1,052 | 1,772 | 2,157 | ||||||||||||
Enobosarm | ||||||||||||||||
Treatment of women with advanced AR positive TNBC (18 mg) | SARM | 801 | 2,348 | 4,853 | ||||||||||||
Other research and development | 283 | 527 | 1,616 | |||||||||||||
|
|
|
|
|
| |||||||||||
Total research and development expenses | $ | 29,669 | $ | 21,467 | $ | 17,228 | ||||||||||
|
|
|
|
|
| |||||||||||
Research and development expenses increased 38% to $29.7 million for the year ended December 31, 2018 from $21.5 million for the year ended December 31, 2017. Research and development expenses increased 25% to $21.5 million for the year ended December 31, 2017 from $17.2 million for the year ended December 31, 2016.
Research and development expenses for enobosarm for the treatment of postmenopausal women with SUI substantially increased from the years ended December 31, 2017 and 2016 due to the initiation of the ASTRID trial, which opened for enrollment in the third quarter of 2017 and completed enrollment in the second quarter of 2018, and due to the related durability and open-label safety extension studies, which were initiated in the second quarter of 2018. During the third quarter of 2018, GTx announced that the ASTRID trial failed to achieve statistical significance on the primary endpoint of the proportion of patients with a greater than 50% reduction in incontinence episodes per day compared to placebo. The years ended December 31, 2016 and 2017 also included expenses related to the Phase 2 open-label,non-placebo controlled,proof-of-concept clinical trial of enobosarm to treat postmenopausal women with SUI that initiated enrollment in the first quarter of 2016.
Research and development expenses for enobosarm for the treatment of women with ER positive and AR positive advanced breast cancer decreased from the years ended December 31, 2017 and 2016 due primarily to the timing and nature of activities related to conducting the Phase 2 clinical trial evaluating enobosarm 9 mg and enobosarm 18 mg in this indication. The clinical trial commenced enrollment during the third quarter of 2015 and completed enrollment in the first quarter of 2017.
Research and development expenses for the SARD program for the year ended December 31, 2018 decreased from the prior years due to fewer drug formulation and preclinical research expenses being incurred during 2018 than in the comparable periods. If the merger is not completed, GTx expects increased research and development expenses for the SARD program in 2019 as it plans to complete ongoing preclinical studies, and to select the most appropriate SARD compounds to move forward withIND-enabling preclinical studies.
Research and development expenses for enobosarm for the treatment of women with AR positive TNBC decreased from the years ended December 31, 2017 and 2016 due to the timing and nature of activities related to conducting the first stage of the Phase 2 clinical trial, which commenced enrollment during the fourth quarter of
282
2015. During the third quarter of 2017, GTx determined that there were insufficient patients achieving clinical benefit from enobosarm treatment to continue this clinical trial.
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2018 of $9.4 million remained relatively consistent with the year ended December 31, 2017 of $9.2 million. General and administrative expenses increased 6% to $9.2 million for the year ended December 31, 2017 from $8.7 million for the year ended December 31, 2016. The increase during the year ended December 31, 2017 from the prior year was due primarily to an increase in share-based compensation expense.
Other Income (Expense), Net
Other income, net for the years ended December 31, 2018, 2017, and 2016 was $641,000, $216,000 and $46,000, respectively, and consisted of interest earned on GTx’s cash, cash equivalents and short-term investments, foreign currency transaction gains and losses, and othernon-operating income or expense. The increase in other income, net for each year over year was primarily due to interest earned on the net proceeds received from issuances of common stock by the Company.
Liquidity and Capital Resources
GTx has financed its operations to date primarily through public offerings and private placements of its securities, as well as payments from its former collaborators. GTx has incurred significant losses since its inception in 1997 as GTx has devoted substantially all of its resources to research and development, including its clinical trials. As of December 31, 2018, GTx had an accumulated deficit of $600.1 million, which resulted primarily from:
| • | its research and development activities associated with: |
| • | the preclinical development of its SARD program; |
| • | the preclinical and clinical development of its SARM compounds, including enobosarm; |
| • | the preclinical and clinical development of its discontinuedGTx-758 product candidate for the treatment of advanced prostate cancer; |
| • | the development of its discontinued toremifene 80 mg product candidate to reduce fractures and treat other estrogen deficiency side effects of androgen deprivation therapy in men with prostate cancer, including two Phase 2 clinical trials, a Phase 3 clinical trial, and the preparation and submission of a NDA to the FDA; |
| • | the development of its discontinued toremifene 20 mg product candidate for the prevention of prostate cancer in high risk men with high grade prostatic intraepithelial neoplasia, including a Phase 2b clinical trial and a Phase 3 clinical trial; |
| • | the preclinical development of other product candidates; and |
| • | general and administrative expenses. |
GTx expects to incur significant operating losses for the foreseeable future depending on the extent of its preclinical and any clinical development activities and, if any such development activities are successful, potentially seeking regulatory approval of any potential future product candidates. These losses, among other things, have had and will continue to have an adverse effect on its stockholders’ equity and working capital. GTx does not expect to receive regulatory approval for the commercial sale of any product candidates for the foreseeable future, if at all.
At December 31, 2018, GTx had cash, cash equivalents and short-term investments of $28.5 million, compared to $43.9 million at December 31, 2017 and $21.9 million at December 31, 2016.
283
In February 2018, GTx entered into the ATM Sales Agreement, pursuant to which it may offer and sell, from time to time, through Stifel, shares of its common stock having an aggregate offering price of up to $50 million. GTx is not obligated to sell any shares under the ATM Sales Agreement. Subject to the terms and conditions of the sales agreement, Stifel will use commercially reasonable efforts, consistent with its normal trading and sales practices, applicable state and federal law, rules and regulations and the rules of the Nasdaq Capital Market, to sell shares from time to time based upon GTx’s instructions, including any price, time or size limits specified by it. Under the ATM Sales Agreement, Stifel may sell shares by any method deemed to be an“at-the-market” offering as defined in Rule 415 under the Securities Act of 1933, as amended, or any other method permitted by law, including in privately negotiated transactions. GTx will pay Stifel a commission of up to 3.0% of the aggregate gross proceeds from each sale of shares. In May 2018, GTx sold 1.5 million shares of common stock under the ATM Sales Agreement for net proceeds of $24.5 million. As of December 31, 2018, GTx had approximately $25.0 million of common stock remaining available to be sold under the ATM Sales Agreement. However, it is unlikely it could raise sufficient funds under the ATM Sales Agreement to permit it to initiate and complete any initial human clinical trials of a SARD compound, and given its currently-depressed stock price, the ATM Sales Agreement is not otherwise expected to be a practical source of liquidity for GTx at this time. Further, given its currently-depressed stock price, GTx is significantly limited in its ability to sell shares of common stock under the ATM Sales Agreement since the issuance and sale of its common stock under the ATM Sales Agreement, if it occurs, would be effected under a registration statement onForm S-3 that it filed with the Securities and Exchange Commission, and in accordance with the rules governing those registration statements, GTx generally can only sell shares of its common stock under that registration statement in an amount not to exceedone-third of its public float, which limitation for all practical purposes precludes its ability to obtain any meaningful funding through the ATM Sales Agreement at this time.
On September 29, 2017, GTx completed a private placement of units consisting of an aggregate of 5.5 million shares of common stock and warrants to purchase an aggregate of 3.3 million shares of its common stock for net proceeds to it of approximately $45.6 million. The purchasers in the registered direct offering consisted solely of accredited investors that included certain institutional and existing stockholders, including a member of its board of directors.
On October 14, 2016, GTx completed a registered direct offering of its common stock consisting of 1.7 million shares of its common stock for net proceeds of approximately $13.7 million. The purchasers in the registered direct offering consisted of certain existing GTx stockholders and certain members of the GTx management team and board of directors.
The following table shows a summary of GTx’s cash flows for the periods indicated:
| Years Ending December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| (in thousands) | ||||||||||||
Net cash used in operating activities | $ | (39,346 | ) | $ | (23,460 | ) | $ | (20,778 | ) | |||
Net cash provided by (used in) investing activities | 27,883 | (15,126 | ) | 2,151 | ||||||||
Net cash provided by financing activities | 23,905 | 45,492 | 13,481 | |||||||||
|
|
|
|
|
| |||||||
Net increase (decrease) in cash and cash equivalents | $ | 12,442 | $ | 6,906 | $ | (5,146 | ) | |||||
|
|
|
|
|
| |||||||
Net cash used in operating activities in all periods resulted primarily from funding its operations.
Net cash provided by investing activities was $27.9 million for the year ended December 31, 2018 and resulted primarily from maturities of short-term investments of $72.0 million offset by the purchase of short-term investments of $44.2 million. Net cash used in investing activities for the year ended December 31, 2017 primarily resulted from the purchase of short-term investments of $39.3 million offset by the maturities of short-term investments of $24.2 million. Net cash provided by investing activities for the year ended December 31, 2016 primarily resulted from the maturities of short-term investments of $37.6 million offset by the purchase of short-term investments of $35.4 million.
284
Net cash provided by financing activities for the year ended December 31, 2018 of $23.9 million resulted from the sale of common stock under the ATM Sales Agreement with Stifel and proceeds from the exercise of stock options of $103,000, offset slightly by $672,000 of tax payments related to shares withheld for vested restricted stock units. Net cash provided by financing activities for the year ended December 31, 2017 reflected net proceeds of $45.6 million from the issuance of common stock and warrants related to the September 2017 private placement, partially offset by $156,000 of employee withholding tax payments related to vested RSUs. Net cash provided by financing activities for the year ended December 31, 2016 reflected net proceeds of $13.7 million from the issuance of common stock related to the October 2016 registered direct offering, partially offset by $208,000 of employee withholding tax payments related to vested RSUs.
To conserve its cash resources, GTx has substantially reduced its workforce since November 2018 and has ceased its SARM development activities and all other operations except forday-to-day business operations, completing ongoing SARD preclinical studies and those activities necessary to complete the merger. If the merger is not completed, based on its current business plan and spending assumptions as a standalone company, GTx estimates that its current cash, cash equivalents and short-term investments, together with interest thereon, will be sufficient to meet its projected operating requirements for at least the next 12 months. GTx has based its cash sufficiency estimates on its current business plan and its assumptions that may prove to be wrong. GTx could utilize its available capital resources sooner than it currently expects, and it could need additional funding sooner than currently anticipated.
GTx’s existing capital resources may not be adequate to enable it to conduct and complete anyIND-enabling studies of a SARD compound, particularly in light of the additional preclinical research that would be required in order to reconcile the conflicting preclinical SARD data GTx has received to date and to determine whether an appropriate SARD compound can potentially be advanced into anyIND-enabling preclinical studies in a timely manner, if at all. Even if it is able to successfully complete such additional preclinical research and to conduct and complete anyIND-enabling studies of a SARD compound, which it may not be able to do with its existing capital resources, GTx will in any event require significant additional financial resources in order to initiate and complete initial human clinical trials of a SARD compound and to otherwise further the development of its SARD program. If GTx is unable to raise sufficient additional funds for the development of its SARD program, whether through potential collaborative, partnering or other strategic arrangements or otherwise, or if GTx otherwise determines to discontinue the development of its SARD program, whether as a result of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity or otherwise, GTx will likely determine to cease operations.
GTx’s estimate of the period of time or events through which its financial resources will be adequate to support its projected operating requirements is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed under the “Risk Factors” section of this proxy statement/prospectus/information statement. Because of the numerous risks and uncertainties associated with the development and potential commercialization of its product candidates and other research and development activities, including risks and uncertainties that could impact the rate of progress of its development activities, GTx is unable to estimate with certainty the amounts of increased capital outlays and operating expenditures associated with the future development of potential future product candidates, if any. GTx’s future funding requirements will depend on many factors, including:
| • | its ability to successfully complete the merger; |
| • | the scope, rate of progress and cost of its preclinical and potential future clinical development programs; |
| • | the terms and timing of any potential collaborative, partnering and other strategic arrangements that it may establish; |
| • | the amount and timing of any licensing fees, milestone payments and royalty payments from potential collaborators, if any; |
285
| • | potential future clinical trial results; |
| • | the cost and timing of regulatory filings and/or approvals to commercialize any potential future product candidates and any related restrictions, limitations, and/or warnings in the label of an approved product candidate; |
| • | the effect of competing technological and market developments; and |
| • | the cost of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights, and the cost of defending any other litigation claims. |
While GTx has been able to fund its operations to date, GTx has no ongoing collaborations for the development and commercialization of any product candidates and no source of revenue, nor does it expect to generate product revenue for the foreseeable future. GTx does not have any commitments for future external funding.
Until GTx can generate a sufficient amount of product revenue, which it may never do, it will need to finance future cash needs through potential collaborative, partnering or other strategic arrangements, as well as through public or private equity offerings or debt financings or a combination of the foregoing. If GTx is unable to raise additional funds, it will need to continue to reduce its expenditures in order to preserve its cash. Further cost-cutting measures that it may take may not be sufficient to enable it to meet its cash requirements, and they may negatively affect its business and GTx’s ability to derive any value from its SARD program. In any event, in order to further the development of its SARD program, if at all, GTx will need to raise substantial additional capital. GTx’s failure to do so would likely result in it determining to cease operations.
To the extent that GTx raises additional funds through potential collaborations, partnering or other strategic arrangements, it may be necessary to relinquish rights to some of its technologies or product candidates and intellectual property rights thereof, or grant licenses on terms that are not favorable to it, any of which could result in GTx’s stockholders having little or no continuing interest in its SARD program and/or SARM assets as stockholders or otherwise. To the extent GTx raises additional funds by issuing equity securities, its stockholders may experience significant dilution, particularly given its currently-depressed stock price, and debt financing, if available, may involve restrictive covenants. For example, GTx completed substantially dilutive private placements of its common stock and warrants in March 2014, November 2014 and September 2017, in addition to a registered direct offering of its common stock that GTx completed in October 2016 and the sale of its common stock pursuant to the ATM Sales Agreement. GTx’s stockholders will experience additional, perhaps substantial, dilution should it again raise additional funds by issuing equity securities. Any additional debt or equity financing that it raises may contain terms that are not favorable to it or its stockholders. GTx’s ability to raise additional funds and the terms upon which GTx is able to raise such funds have been severely harmed by the failure of the ASTRID trial to meet its primary endpoint and the resulting significant uncertainty regarding its prospects to continue as a going concern. If GTx is unable to complete the merger, its ability to raise additional funds and the terms upon which GTx is able to raise such funds may also be adversely affected by the uncertainties regarding its financial condition, uncertainties with respect to the prospects for its early-stage SARD program as an effective treatment of men with CRPC, particularly in light of GTx’s recent receipt of new preclinical data from an independent laboratory that showed that at higher dose concentrations the tested SARD compounds demonstrated partial androgen receptor agonist activity, the sufficiency of its capital resources, potential future management turnover, and volatility and instability in the global financial markets. As a result of these and other factors, there is no guarantee that sufficient additional funding will be available to GTx on acceptable terms, or at all.
286
Contractual Obligations
At December 31, 2018, GTx had contractual obligations as follows:
| Payment Due by Period (in thousands) | ||||||||||||||||||||
Contractual Obligations (1) | Total | Less than 1 year | 1-3 years | 4-5 years | More than 5 years | |||||||||||||||
Operating lease obligations(2) | $ | 162 | $ | 162 | $ | — | $ | — | $ | — | ||||||||||
| (1) | This table does not include any royalty obligations under GTx’s SARM and SARD license agreements with UTRF as the timing and likelihood of such payments are not known. In addition to the minimum payments due under its SARM and SARD license agreements, GTx may be required to pay royalties on any net sales of product if GTx receives regulatory approval for a SARM, including enobosarm, or SARD product candidate and successfully market the product. Additionally, if GTx sublicenses rights under its SARM or SARD license agreements, it is also obligated to pay a sublicense royalty on any licensing fee or milestone payments it may receive from a sublicensee. |
| (2) | GTx’s operating lease obligations consist of payments relating to a lease for office space at 175 Toyota Plaza, Memphis, Tennessee, which expires on April 30, 2019. |
Off-Balance Sheet Arrangements
GTx has not engaged in anyoff-balance sheet arrangements, including the use of standard finance, special purpose entities or variable interest entities.
287
ONCTERNAL MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of Oncternal’s financial condition and results of operations together with Oncternal’s consolidated financial statements and related notes appearing in this proxy statement/prospectus/information statement. Some of the information contained in this discussion and analysis is set forth elsewhere in this prospectus, including information with respect to Oncternal’s plans and strategy for Oncternal’s business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk factors” section of this registration statement/prospectus/information statement, Oncternal’s actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
Oncternal is a clinical-stage biopharmaceutical company focused on developingfirst-in-class product candidates for cancers with critical unmet medical need. The company’s development efforts are focused on promising, yet untreated biological pathways implicated in cancer generation or progression. ROR1 is a growth factor receptor that is widely expressed on many tumors and whose overexpression has been correlated with poor prognosis, which activates pathways that lead to increased tumor proliferation, invasiveness and drug resistance. Oncternal’s lead product candidate is cirmtuzumab, a monoclonal antibody that is designed to inhibit the ROR1 receptor, which is being evaluated in a Phase 1b/2 clinical trial in combination with ibrutinib for the treatment of CLL and MCL, and in a Phase 1b clinical trial in combination with paclitaxel for women with metastatic breast cancer. Oncternal is also developing TK216, a small molecule that is designed to inhibit ETS, or E26 Transformation Specific, family oncoproteins, which alter gene transcription and RNA processing and lead to increased cell proliferation and invasion. TK216 is being evaluated in a Phase 1 clinical trial, alone and in combination with vincristine, in patients with relapsed or refractory Ewing sarcoma, a rare pediatric cancer. In addition, Oncternal is developing aCAR-T product candidate that targets ROR1, which is currently in preclinical development as a potential treatment for solid tumors and hematologic cancers including AML.
Since Oncternal’s inception in 2013, it has devoted most of its resources to organizing and staffing, business planning, raising capital, acquiring product candidates and securing related intellectual property rights and advancing its cirmtuzumab and TK216 clinical development programs. Under research subaward agreements between Oncternal and UC San Diego, Oncternal is eligible to receive up to $16.1 million in development milestones throughout the award project period, estimated to be from October 1, 2017 to March 31, 2022. Through December 31, 2018, Oncternal has funded its operations primarily through: (i) gross proceeds of $49.0 million from the issuance of convertible preferred stock, and (ii) receipt of $4.0 million in subaward grant payments received from UC San Diego. As of December 31, 2018, Oncternal had cash and cash equivalents of $20.6 million.
Oncternal has incurred net losses in each year since inception. Oncternal’s ability to generate product revenue sufficient to achieve profitability will depend heavily on the successful development and eventual commercialization of one or more of Oncternal’s current or future product candidates. Oncternal’s net losses were $6.6 million and $10.4 million for the years ended December 31, 2018 and 2017, respectively. As of December 31, 2018, Oncternal had an accumulated deficit of $31.4 million. Substantially all of Oncternal’s net losses have resulted from costs incurred in connection with advancing Oncternal’s research and development programs and from general and administrative costs associated with Oncternal’s operations. Oncternal expects to continue to incur significant and increasing operating losses for at least the next several years. Oncternal expects that its expenses and capital funding requirements will increase substantially in connection with its ongoing activities, particularly if and as Oncternal:
| • | conducts its ongoing Phase 1b/2 clinical trial of cirmtuzumab and any additional clinical trials for its product candidates; |
288
| • | continues to develop additional product candidates; |
| • | advances preclinical studies for itsCAR-T program; |
| • | acquires orin-licenses other product candidates and technologies; |
| • | maintains, expands and protects its intellectual property portfolio; |
| • | establishes a commercial manufacturing source and secures supply chain capacity sufficient to provide commercial quantities of any product candidates for which it may obtain regulatory approval; |
| • | seeks regulatory approvals for any product candidates that successfully complete clinical trials; |
| • | establishes a sales, marketing and distribution infrastructure to commercialize any products for which it may obtain regulatory approval; and |
| • | adds operational, financial and management information systems and personnel, including personnel to support its planned product development and future commercialization efforts, as well as to support its transition to a public reporting company. |
Oncternal will not generate revenue from product sales unless and until Oncternal successfully completes clinical development and obtains regulatory approval for its product candidates. If Oncternal obtains regulatory approval for any of its product candidates and does not enter into a commercialization partnership, Oncternal expects to incur significant expenses related to developing Oncternal’s internal commercialization capability to support product sales, marketing and distribution. Further, in the event the merger, as described below, occurs, Oncternal expects to incur additional costs associated with operating as a public company.
As a result, Oncternal believes it will need substantial additional funding to support its continuing operations and pursue its business strategy. Until such time as Oncternal can generate significant revenue from product sales, if ever, Oncternal expects to finance its operations through a combination of equity offerings, debt financings or other sources, including potentially government funding, collaborations, licenses and other similar arrangements. Oncternal may not be able to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If Oncternal fails to raise capital or enter into such agreements as and when needed, Oncternal may have to significantly delay, reduce or eliminate the development and commercialization of one or more of its product candidates or delay its pursuit of potentialin-licenses or acquisitions.
Because of the numerous risks and uncertainties associated with product development, Oncternal is unable to predict the timing or amount of increased expenses or when or if it will be able to achieve or maintain profitability. Even if Oncternal is able to generate product sales, Oncternal may not become profitable. If Oncternal fails to become profitable or is unable to sustain profitability on a continuing basis, then Oncternal may be unable to continue its operations at planned levels and be forced to reduce or terminate its operations.
Oncternal expects that its existing cash and cash equivalents will be sufficient to fund its operating expenses and capital expenditure requirements into the first quarter of 2020. Oncternal has based this estimate on assumptions that may prove to be wrong, and Oncternal could exhaust its available capital resources sooner than it expects. See “Liquidity and Going Concern.” Beyond that point, Oncternal will need to raise additional capital to finance its operations, which cannot be assured. Oncternal has concluded that this circumstance raises substantial doubt about its ability to continue as a going concern within one year after the April 5, 2019 issuance date of its annual consolidated financial statements for the year ended December 31, 2018. See Note 1 of Oncternal’s consolidated financial statements included elsewhere in this proxy statement/prospectus/information statement for additional information on its assessment.
Similarly, in its report on Oncternal’s financial statements for the year ended December 31, 2018, Oncternal’s independent registered public accounting firm included an explanatory paragraph stating that Oncternal’s recurring losses from operations and required additional funding to finance Oncternal’s operations raise substantial doubt about Oncternal’s ability to continue as a going concern.
289
Proposed Merger with GTx
On March 6, 2019, GTx, Merger Sub and Oncternal entered into the Original Merger Agreement and amended the Original Merger Agreement on April 30, 2019, pursuant to which Merger Sub, a wholly-owned subsidiary of GTx, will merge with and into Oncternal, with Oncternal continuing as a wholly-owned subsidiary of GTx and the surviving corporation of the merger. The merger is intended to qualify as atax-free reorganization for U.S. federal income tax purposes. Under the exchange ratio formula in the Merger Agreement, the former Oncternal stockholders immediately before the merger are expected to own approximately 77.5% of the outstanding capital stock of GTx, and the stockholders of GTx immediately before the merger are expected to own approximately 22.5% of the outstanding capital stock of GTx, subject to certain assumptions and adjustments.
The merger is expected to be accounted for as a reverse asset acquisition in accordance with U.S. GAAP. Oncternal will be deemed to be the accounting acquirer for financial reporting purposes. This determination is supported based on the expectations that, immediately following the merger: (i) Oncternal stockholders will own a substantial majority of the voting rights of the combined organization; (ii) Oncternal will designate a majority (seven of nine) of the initial members of the board of directors of the combined organization; and (iii) Oncternal’s senior management will hold all key positions in senior management of the combined organization and no employees will be retained from GTx. The transaction is expected to be accounted for as a reverse asset acquisition as the fair value of the acquired preclinical assets is deemed to be substantially concentrated in a group of similar assets that do not meet the definition of a business. Accordingly, for accounting purposes: (i) the merger will be treated as the equivalent of Oncternal issuing stock to acquire the net assets of GTx, (ii) the net assets of GTx will be recorded based upon the fair values in the financial statements at the time of closing and (iii) the reported historical operating results of the combined company prior to the merger will be those of Oncternal.
Components of Results of Operations
Grant Revenue
Oncternal has not generated any product revenue from product sales, and does not expect to generate any product revenue from the sale of products in the foreseeable future. If Oncternal’s development efforts for its product candidates are successful and result in regulatory approval, Oncternal may generate revenue in the future from product sales. Oncternal cannot predict if, when, or to what extent it will generate revenue from the commercialization and sale of its product candidates. Oncternal may never succeed in obtaining regulatory approval for any of its product candidates. Oncternal’s total revenue to date has been derived from a CIRM grant subaward with UC San Diego.
In August 2017, CIRM awarded an $18.3 million grant to researchers at UC San Diego, to advance Oncternal’s Phase 1b/2 clinical trial evaluating cirmtuzumab in combination with ibrutinib for the treatment of patients withB-cell lymphoid malignancies, including MCL and CLL. Oncternal is conducting this study in collaboration with UC San Diego and estimates it will receive $16.1 million in development milestones under research subaward agreements throughout the award project period, estimated to be from October 1, 2017 to March 31, 2022. In addition, Oncternal is committed to certainco-funding requirements and is required to provide UC San Diego progress and financial update reports throughout the award project period. Oncternal received subaward payments of $0.5 million and $3.6 million in 2018 and 2017, respectively. As of December 31, 2018, Oncternal believes it has met its obligations under the CIRM award and UC San Diego subawards.
Operating Expenses
Research and Development
Research and development expenses consist primarily of costs incurred for the preclinical and clinical development of Oncternal’s lead product candidate cirmtuzumab as well as TK216, which include:
| • | expenses under agreements with third-party contract organizations, investigative clinical trial sites that conduct research and development activities on Oncternal’s behalf, and consultants; |
290
| • | costs related to develop and manufacture preclinical study and clinical trial material; |
| • | salaries and employee-related costs, including stock-based compensation; |
| • | costs incurred under Oncternal’s collaboration and third-party licensing agreements; and |
| • | laboratory and vendor expenses related to the execution of preclinical and clinical trials. |
Oncternal accrues all research and development costs in the period for which they are incurred. Costs for certain development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by Oncternal’s vendors, collaborators and third-party service providers. Advance payments for goods or services to be received in future periods for use in research and development activities are deferred and then expensed as the related goods are delivered and as services are performed.
Oncternal expects its research and development expenses to increase substantially for the foreseeable future as it continues to invest in developing Oncternal’s product candidates, as Oncternal’s product candidates advance into later stages of development, and as Oncternal begins to conduct larger clinical trials. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials.
Oncternal’s direct research and development expenses are tracked by product candidate and consist primarily of external costs, such as fees paid under third-party license agreements and to outside consultants, CROs, CMOs and research laboratories in connection with its preclinical development, process development, manufacturing and clinical development activities. Oncternal does not allocate employee costs and costs associated with its discovery efforts, laboratory supplies and facilities, including other indirect costs, to specific product candidates because these costs are deployed across multiple programs and, as such, are not separately classified. Oncternal uses internal resources primarily to conduct its research as well as for managing its preclinical development, process development, manufacturing and clinical development activities. These employees work across multiple programs and, therefore, Oncternal does not track its costs by product candidate unless such costs are includable as subaward costs.
Oncternal cannot determine with certainty the timing of initiation, the duration or the completion costs of current or future preclinical studies and clinical trials of its product candidates due to the inherently unpredictable nature of preclinical and clinical development. Clinical and preclinical development timelines, the probability of success and development costs can differ materially from expectations. Oncternal anticipates that it will make determinations as to which product candidates to pursue and how much funding to direct to each product candidate on an ongoing basis in response to the results of ongoing and future preclinical studies and clinical trials, regulatory developments and Oncternal’s ongoing assessments as to each product candidate’s commercial potential. Oncternal will need to raise substantial additional capital in the future. In addition, Oncternal cannot forecast which product candidates may be subject to future collaborations, when such arrangements will be secured, if at all, and to what degree such arrangements would affect its development plans and capital requirements.
General and Administrative
General and administrative expenses consist primarily of personnel-related costs, and professional fees for legal, patent, consulting, investor and public relations, accounting and audit services. Personnel-related costs consist of salaries, benefits and stock-based compensation.
Other Income (Expense), Net
Change in Fair Value of Preferred Stock Warrant Liability
In connection with Oncternal’s Series B and SeriesB-2 preferred stock financings in 2017, Oncternal issued warrants to purchase shares of its preferred stock. Oncternal classifies these warrants as a liability on its
291
consolidated balance sheets and remeasures to fair value at each reporting date, and Oncternal recognizes changes in the fair value of the warrant liability as a component of other income (expense), net in its consolidated statements of operations. Oncternal will continue to recognize changes in the fair value of each warrant comprising the warrant liability until each respective warrant is exercised, expires or qualifies for equity classification.
Upon the closing of the merger, all of Oncternal’s outstanding preferred stock warrants will become exercisable for GTx common stock and are expected to qualify for equity classification. As a result, the preferred stock warrants will no longer require liability accounting and the fair value of the warrant liability upon the closing of the merger will be reclassified to additionalpaid-in capital.
Interest Income
Interest income consists of interest earned on Oncternal’s cash equivalents, which consist of money market funds. Oncternal’s interest income has not been significant due to low interest rates earned on invested balances.
Results of Operations
Comparison of Years Ended December 31, 2018 and 2017
The following table summarizes Oncternal’s results of operations for the years ended December 31, 2018 and 2017:
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | Change | ||||||||||
| (in thousands) | ||||||||||||
Grant revenues | $ | 2,521 | $ | 1,674 | $ | 847 | ||||||
Operating expenses: | ||||||||||||
Research and development | 8,287 | 9,363 | (1,076 | ) | ||||||||
General and administrative | 1,820 | 2,871 | (1,051 | ) | ||||||||
|
|
|
|
|
| |||||||
Total operating expenses | 10,107 | 12,234 | (2,127 | ) | ||||||||
|
|
|
|
|
| |||||||
Loss from operations | (7,586 | ) | (10,560 | ) | 2,974 | |||||||
Other income (expense): | ||||||||||||
Change in fair value of warrant liability | 713 | 124 | 589 | |||||||||
Other income | 216 | — | 216 | |||||||||
Interest income | 79 | 10 | 69 | |||||||||
Interest expense | (1 | ) | (10 | ) | 9 | |||||||
|
|
|
|
|
| |||||||
Total other income (expense) | 1,007 | 124 | 883 | |||||||||
|
|
|
|
|
| |||||||
Net loss | $ | (6,579 | ) | $ | (10,436 | ) | $ | 3,857 | ||||
|
|
|
|
|
| |||||||
Grant Revenue
Grant revenue for the year ended December 31, 2018 was $2.5 million, compared to $1.7 million for the year ended December 31, 2017. The increase was driven by higher qualifying subaward costs in 2018 over 2017.
292
Research and Development Expenses
The following table summarizes Oncternal’s research and development expenses for the periods indicated:
| Years Ended December 31, | Increase/ (Decrease) | |||||||||||
| 2018 | 2017 | |||||||||||
| (in thousands) | ||||||||||||
Cirmtuzumab | $ | 5,561 | $ | 6,143 | $ | (582 | ) | |||||
TK216 | 1,465 | 1,601 | (136 | ) | ||||||||
Unallocated research and development expenses | 1,261 | 1,619 | (358 | ) | ||||||||
|
|
|
|
|
| |||||||
Total research and development expenses | $ | 8,287 | $ | 9,363 | $ | (1,076 | ) | |||||
|
|
|
|
|
| |||||||
Research and development expenses for the 12 months ended December 31, 2018 and 2017 were $8.3 million and $9.4 million, respectively, a decrease of $1.1 million. The decrease was due to a $0.7 million decrease in direct product candidate costs and a $0.4 million decrease in unallocated research and development expenses.
Direct expenses of the cirmtuzumab product candidate decreased $0.6 million for the year ended December 31, 2018, compared to the year ended December 31, 2017, due primarily to the following partially offsetting factors: (i) a $1.3 million decrease in preclinical development expenses as Oncternal amended its Regents research agreement in August 2018 and reduced its preclinical program activities as Oncternal transitioned into clinical trial activities, (ii) a $2.1 million increase in clinical trial activities related to Oncternal’s Phase 1/2 clinical trial of cirmtuzumab, in combination with ibrutinib for the treatment of patients withB-cell lymphoid malignancies, including MCL and CLL, that commenced in the latter part of 2017 and continued through 2018, (iii) a $0.9 million decrease in manufacturing costs, primarily due to anon-recurring cell-line optimization costs incurred in 2017, and (iv) a $0.5 million decrease in consultant costs as Oncternal reduced its use of consultants to support its preclinical and clinical activities.
Direct expenses of the TK216 product candidates decreased $0.1 million for the year ended December 31, 2018, compared to the year ended December 31, 2017, due primarily to the following partially offsetting factors: (i) a $0.1 million decrease in preclinical development expenses as Oncternal generally reduced its preclinical program activities, (ii) a $0.5 million increase in clinical trial activities related to Oncternal’s continuing Phase 1 clinical trial of TK216 in refractory Ewing sarcoma, and (iii) a $0.5 million decrease in manufacturing costs, primarily due to anon-recurring clinical trial material batch purchased in 2017.
Unallocated research and development expenses decreased of $0.4 million for the years ended December 31, 2018 and 2017 primarily due to lower personnel costs resulting from entering into the VelosBio transition services agreement, as further described in the “Contractual Obligations”sectionbelow.
General and Administrative Expenses
General and administrative expenses for the years ended December 31, 2018 and 2017 were $1.8 million and $2.9 million, respectively, a decrease of $1.1 million. The decrease is primarily due to legal expenses decreasing $1.0 million resulting from Oncternal’s efforts to significantly expand its intellectual property portfolio on the cirmtuzumab platform and product candidates in 2017.
Other Income (Expense), Net
Other income, net was $1.0 million for the year ended December 31, 2018, compared to $0.1 million for the year ended December 31, 2017. The increase in other income, net of $0.9 million was primarily due to: (i) a $0.6 million decrease in the fair value of the preferred stock warrant liability, and (ii) a gain of $0.2 million related to the VelosBio asset purchase agreement, as further described in the “Contractual Obligations”sectionbelow.
293
Liquidity and Going Concern
From its inception through December 31, 2018, Oncternal has devoted substantially all of its efforts to organizational activities including raising capital, building infrastructure, acquiring assets, developing intellectual property, and conducting preclinical studies, clinical trials and product development activities. Oncternal has a limited operating history and the sales and income potential of Oncternal’s business and market are unproven. Oncternal has experienced recurring net losses and negative cash flows from operating activities. At December 31, 2018, Oncternal had an accumulated deficit of $31.4 million and had cash and cash equivalents of $20.6 million. Oncternal will need to continue to raise a substantial amount of funds until it is able to generate revenues to fund its development and operating activities.
Oncternal expects to continue to incur net losses into the foreseeable future. Successful transition to attaining profitable operations is dependent upon achieving a level of revenues adequate to support Oncternal’s cost structure. Oncternal has incurred net losses since inception and has relied on its ability to fund its operations through debt and equity financings and grant funding. These conditions raise substantial doubt about Oncternal’s ability to continue as a going concern. The accompanying consolidated financial statements have been prepared assuming that Oncternal will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty. This basis of accounting contemplates the recovery of Oncternal’s assets and the satisfaction of liabilities in the normal course of business.
Oncternal plans to continue to fund its losses from operations and capital funding needs through a combination of equity offerings, debt financings, government funding, or other sources, including potentially collaborations, licenses and other similar arrangements. There can be no assurance that Oncternal will be able to obtain any sources of financing on acceptable terms, or at all. To the extent that Oncternal can raise additional funds by issuing equity securities, Oncternal’s stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact Oncternal’s ability to conduct its business.
Cash Flows
The following table summarizes Oncternal’s sources and uses of cash for each of the periods presented:
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| (in thousands) | ||||||||
Net cash provided by (used in): | ||||||||
Operating activities | $ | (7,417 | ) | $ | (9,135 | ) | ||
Financing activities | 17,874 | 11,405 | ||||||
|
|
|
| |||||
Net increase in cash and cash equivalents | $ | 10,457 | $ | 2,270 | ||||
|
|
|
| |||||
Operating activities
During the year ended December 31, 2018, operating activities used $7.4 million of cash, resulting primarily from Oncternal’s net loss of $6.6 million,non-cash change in fair value of warrant liability of $0.7 million andnon-cash other income of $0.2 million, a $0.1 million change in operating assets and liabilities, partially offset by stock-based compensation charges of $0.2 million. The $0.1 million change in operating assets and liabilities primarily consisted of a $0.4 million increase in prepaid expenses and other assets, a $1.9 million decrease in deferred revenue, offset by a $2.2 million increase in accounts payable and accrued expenses.
During the year ended December 31, 2017, operating activities used $9.1 million of cash, resulting from Oncternal’s net loss of $10.4 million,non-cash change in fair value of warrant liability of $0.1 million, partially offset by a $1.1 million change in operating assets and liabilities, and stock-based compensation charges of $0.3 million. The $1.1 million change in operating assets and liabilities for the year ended December 31, 2017 primarily consisted of a $0.6 million increase in prepaid expenses and other assets, a $1.9 million increase in deferred revenue, and a $0.2 million decrease in accounts payable and accrued expenses.
294
Financing activities
Net cash provided by financing activities was $17.9 million for the year ended December 31, 2018 consisting of net proceeds of $16.8 million from Oncternal’s sale of Series C convertible preferred stock in November 2018 and the collection of $1.1 million of SeriesB-2 convertible preferred stock subscriptions receivable. Net cash provided by financing activities was $11.4 million for the year ended December 31, 2017 consisting of net proceeds from Oncternal’s sale of Series B and SeriesB-2 convertible preferred stock.
Oncternal believes that its existing cash and cash equivalents will be sufficient to meet its anticipated cash requirements into the first quarter of 2020. However, Oncternal’s forecast of the period of time through which its financial resources will be adequate to support its operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially. Oncternal has based this estimate on assumptions that may prove to be wrong, and it could use its capital resources sooner than we expect. Additionally, the process of testing product candidates in clinical trials is costly, and the timing of progress and expenses in these trials is uncertain.
Oncternal’s future capital requirements will depend on many factors, including:
| • | the type, number, scope, progress, expansions, results, costs and timing of, its preclinical studies and clinical trials of its product candidates which it is pursuing or may choose to pursue in the future; |
| • | the costs and timing of manufacturing for its product candidates, including commercial manufacturing if any product candidate is approved; |
| • | the costs, timing and outcome of regulatory review of its product candidates; |
| • | the costs of obtaining, maintaining and enforcing its patents and other intellectual property rights; |
| • | its efforts to enhance operational systems and hire additional personnel to satisfy its obligations as a public company, including enhanced internal controls over financial reporting; |
| • | the costs associated with hiring additional personnel and consultants as its preclinical and clinical activities increase; |
| • | the costs and timing of establishing or securing sales and marketing capabilities if any product candidate is approved; |
| • | its ability to achieve sufficient market acceptance, adequate coverage and reimbursement from third-party payors and adequate market share and revenue for any approved products; |
| • | the terms and timing of establishing and maintaining collaborations, licenses and other similar arrangements; and |
| • | costs associated with any products or technologies that itmay in-license or acquire. |
Until such time, if ever, as Oncternal can generate substantial product revenues to support its cost structure, it expects to finance its losses from operations and capital funding needs through a combination of equity offerings debt financings or other sources, including potentially government funding, collaborations, licenses and other similar arrangements. To the extent that Oncternal raises additional capital through the sale of equity or debt securities, the ownership interest of its stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of its common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting Oncternal’s ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If Oncternal raises funds through collaborations, licenses and other similar arrangements with third parties, it may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to it and/or may reduce the value of its common stock. If Oncternal is unable to raise additional funds through equity or debt
295
financings when needed, it may be required to delay, limit, reduce or terminate its product development or future commercialization efforts or grant rights to develop and market its product candidates even if it would otherwise prefer to develop and market such product candidates by itself. There can be no assurance that Oncternal will be able to obtain any sources of financing on acceptable terms, or at all.
Contractual Obligations and Commitments
Georgetown University (“Georgetown”)
In March 2014, Oncternal entered into an Exclusive License Agreement (the “Georgetown License Agreement”) with Georgetown, pursuant to which Oncternal: (i) licensed the exclusive worldwide right to patents and technologies for the development and commercialization of certain product candidates targetingEWS-FLI1 as an anti-tumor therapy for therapeutic, diagnostics, or research tool purposes, (ii) is solely responsible for all development and commercialization activities and costs in its respective territories, and (iii) is also responsible for all costs related to the filing, prosecution and maintenance of the licensed patent rights.
Under the terms of the Georgetown License Agreement, commencing in 2015, Oncternal: (i) shall pay and has paid an annual license maintenance fee of $10,000 until the first commercial sale occurs, (ii) is required to make up to $200,000 in aggregate milestone payments upon the achievement of certain regulatory milestones, and (iii) will be required to pay low single digit royalties based on annual net product sales. Oncternal accounted for the licensed technology as an asset acquisition because it did not meet the definition of a business. All milestone payments under the Georgetown License Agreement will be recognized as research and development expense upon completion of the required events, as the triggering events are not considered to be probable until they are achieved. As of December 31, 2018, Oncternal had not triggered or made any milestone payments under the Georgetown License Agreement.
The Georgetown License Agreement may be terminated by either party upon material breach or may be terminated by Oncternal as to one or more countries with 90 days written notice of termination. The term of the Georgetown License Agreement will continue until the expiration of the last valid claim within the patent rights covering the product. Georgetown may terminate the agreement in the event (i) Oncternal fails to pay any amount and fails to cure such failure within 30 days after receipt of notice, (ii) Oncternal defaults in its obligation to obtain and maintain insurance and fails to remedy such breach within 60 days after receipt of notice, or (iii) Oncternal declares insolvency or bankruptcy. Oncternal may terminate the agreement at any time upon at least 60 days’ written notice.
In 2017, Oncternal entered into a research agreement with Georgetown for up to $150,000. For the years ended December 31, 2018 and 2017, Oncternal recorded research and development expenses of $53,000 and $75,000, respectively.
The University of Texas MD Anderson Cancer Center (“MD Anderson”)
In December 2014, Oncternal entered into a collaboration agreement (the “MD Anderson Collaboration”) with MD Anderson, which, as amended, provides for the conduct of preclinical and clinical research on TK216 in exchange for certain program payments. If MD Anderson successfully completes all the requirements of the MD Anderson Collaboration in full and the program is successfully commercialized, Oncternal will be required to pay aggregate milestone payments of $1.0 million based on net product sales. For the years ended December 31, 2018 and 2017, Oncternal recorded $330,000 and $0, respectively, of research and development expenses earned by MD Anderson under the MD Anderson Collaboration agreement.
Agreements with the Regents of the University of California (the “Regents”)
In March 2016, Oncternal entered into a license agreement with the Regents, which was amended and restated in August 2018, for the development, manufacturing and distribution rights to naked antibodies, including
296
cirmtuzumab and genetically engineered cellular therapy products, includingCAR-T products that are covered by licensed patents for all human therapeutic, diagnostic and preventive applications in all indications.
The Regents License Agreement provides for the following: (i) in May 2016, an upfront license fee of $0.5 million was paid and 1,459,524 shares of Oncternal common stock were issued, (ii) $25,000 in annual license maintenance fees commencing in 2017, (iii) reimbursement of up to $30,000 in annual patent costs, (iv) certain development and regulatory milestones aggregating from $10.0 million to $12.5 million, on a per product basis, (v) certain worldwide sales milestones based on achievement of tiered revenue levels aggregating $75.0 million, (vi) low single-digit royalties, including potential future minimum annual royalties, on net sales of each target, and (vii) minimum diligence to advance licensed assets consisting of at least $1.0 million in development spend annually through 2021. Under the Regents License Agreement in 2018 and 2017, Oncternal recorded: (i) $25,000 in annual license maintenance fees recorded as research and development expense, and (ii) $0.1 million and $0.2 million in patent costs recorded as general and administrative expense for the years ended December 31, 2018 and 2017, respectively. As of December 31, 2018, Oncternal believes it has met its obligations under the Regents License Agreement.
In July 2016, and as modified by the amended and restated Regents License Agreement in August 2018, Oncternal entered into a Research Agreement (the “Research Agreement”) with the Regents for further research on a ROR1 therapeutic development program. Under this five-year agreement, the Regents will have an aggregate budget of $2.5 million, with $125,000 payable quarterly. For the years ended December 31, 2018 and 2017, Oncternal recorded $0.5 million and $1.0 million, respectively, in research and development costs under this Research Agreement. Such costs are includable as part of Oncternal’s annual diligence obligations under the Regents License Agreement.
The Regents License Agreement will expire upon the later of the expiration date of the longest-lived patent rights or the 15th anniversary of the first commercial sale of a licensed product. The Regents may terminate the Regents License Agreement if: (i) a material breach by Oncternal is not cured within a reasonable time, (ii) Oncternal files a claim asserting the Regents licensed patent rights are invalid or unenforceable, and (iii) Oncternal files for bankruptcy. Oncternal may terminate the agreement at any time upon at least 60 days’ written notice.
In September 2016, Oncternal entered into an Investigator-Initiated Clinical Trial Agreement with the Regents to provide partial support for a Phase I clinical study to determine the safety and tolerability of cirmtuzumab for the treatment of patients with relapsed or refractory CLL. Under this agreement that was concluded in 2017, Oncternal recorded $0.2 million in research and development expenses for the year ended December 31, 2017.
Velos Biopharma Holdings, LLC (“VBH”) and VelosBio, Inc. (“VelosBio”)Spin-off Transactions
In November and December 2017, Oncternal formed VBH and made anin-kindtax-free distribution of 100% of its interest in VBH to Oncternal’s stockholders, option holders and warrant holders of record. On February 6, 2018, Oncternal licensed and assigned its rights to two preclinical product candidates, previously under the Regents License Agreement, to VBH. In consideration for the license, Oncternal: (i) received a promissory note receivable from VBH of $0.1 million, with an annual interest rate of 2.64% and a due date of 10 years, and (ii) made a partial assignment of its March 2016 Regents License Agreement. Pursuant to the partial assignment, VBH assumed certain obligations related to the licensed Products under the Regents License Agreement as follows: (i) reimbursement of certain historical and future patent costs related to the Products, (ii) certain development and sales milestones for advancing licensed Products targets, (iii) low single-digit royalties, including potential future minimum annual royalties, on net sales of each licensed Product target are to be allocated between Oncternal and VBH, (iv) certain third-party agreements and related obligations specifically related to the licensed Products, (v) minimum diligence requirements to advance licensed assets consisting of a minimum of $0.5 million in development spend annually through 2021, and (vi) Research Agreement obligations equal to $0.5 million annually commencing January 1, 2018. Due to the high uncertainty of the success of VBH
297
ever repaying the note and associated interest, Oncternal has provided a full valuation allowance for these amounts as of December 31, 2018.
In December 2017, VelosBio was incorporated with VBH being its sole stockholder with VelosBio common shares only. On February 6, 2018, VBH sublicensed and assigned its intellectual property rights to its two preclinical product candidates to VelosBio. In consideration for the license, VelosBio agreed to use commercially reasonable efforts to develop the licensed products as well as the following payment obligations: (i) the assumption of each of the VBH assumed obligations under the partial assignment between Oncternal and VBH as outlined above, and (ii) certain tiered development milestone and royalty payments to VBH. In August 2018, Oncternal entered into the amended and restated Regents License Agreement and VelosBio entered into their own license agreement directly with the Regents. In 2018, VelosBio secured substantially independent preferred stock financings for its programs and there is no common control overlap between the companies.
Also on February 6, 2018, Oncternal and VelosBio entered into: (i) an asset purchase agreement whereby VelosBio purchased Oncternal’s right, title and interest in Oncternal’s nominal assets related to the two preclinical product candidates and assumed Oncternal’s $0.2 million convertible note payable and related $16,000 of accrued interest which has been recorded as other income in Oncternal’s consolidated financial statements, and (ii) a transition services agreement whereby Oncternal agreed to provide VelosBio with certain transition services, which expired as of December 31, 2018, as follows: (i) access to certain common laboratory equipment at Oncternal’s lab facility, (ii) certain named employees were to devote up to 80% of their time supporting VelosBio related activities, (iii) cirmtuzumab manufacturing, process optimization and ancillary activities until VelosBio was able to establish their own, and (iv) agreement to cost share the purchase of certain antibody materials with VelosBio. Such services were to be provided at cost or cost plus. During 2018, Oncternal incurred $3.0 million of costs on behalf of VelosBio that were substantially reimbursed and recorded on a net basis within operating expenses. As of December 31, 2018, there are no ongoing rights or commitments under the asset purchase or transition services agreements.
SPH USA License Agreement
In November 2018, Oncternal entered into the SPH USA License Agreement with SPH USA for: (i) the territory of Greater China, and (ii) rights to manufacture, develop, market, distribute and sell all of Oncternal’s product candidates under the Georgetown License Agreement and the Regents License Agreement (exclusive to Greater China only). Under the SPH USA License Agreement, SPH USA is solely responsible for: (a) all preclinical and clinical development activities specific to obtaining regulatory approval in Greater China for such product candidates, (b) any third-party license milestone or royalty payments owed under the License Agreement and the Regents License Agreement, and (c) paying Oncternal a low single digit royalty on net sales in the territory.
Government Contracts, Grant Agreements and Incentive Programs
The California Institute for Regenerative Medicine (“CIRM”) Award
In August 2017, CIRM awarded an $18.3 million grant to researchers at UC San Diego, to advance Oncternal’s Phase 1b/2 clinical trial evaluating cirmtuzumab in combination with ibrutinib for the treatment of patients withB-cell lymphoid malignancies, including MCL and CLL. Oncternal: (i) is conducting this study in collaboration with UC San Diego, (ii) estimates it will receive $16.1 million in development milestones under research subaward agreements throughout the award project period, estimated to be from October 1, 2017 to March 31, 2022, (iii) is committed to certainco-funding requirements, (iv) received subaward payments of $0.5 million and $3.6 million in December 2018 and 2017, respectively, and (v) is required to provide UC San Diego progress and financial update reports throughout the award project period. The subaward does not bear a royalty payment commitment, nor is the subaward otherwise refundable. For the years ended December 31, 2018 and 2017, Oncternal recorded revenue of $2.5 million and $1.7 million, respectively. Related qualifying subaward costs during the years ending December 31, 2018 and 2017 were $5.7 million and $3.1 million, respectively. As of December 31, 2018, Oncternal believes it has met its obligations under the CIRM award and UC San Diego subawards to date.
298
Critical Accounting Policies
Oncternal management’s discussion and analysis of Oncternal’s financial condition and results of operations are based on its consolidated financial statements, which have been prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”). The preparation of the financial statements requires Oncternal to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Oncternal estimates are based on its historical trends and other factors that it believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Oncternal’s significant accounting policies are described in more detail in Note 1, “Description of Business, Basis of Presentation and Summary of Significant Accounting Policies,” in the notes to its consolidated financial statements as of December 31, 2018 and 2017 and for each of the years ended December 31, 2018 and 2017, appearing elsewhere in this proxy statement/prospectus/information statement. However, Oncternal believes that the following accounting policies are the most critical for fully understanding and evaluating our financial condition and results of operations.
Research and Development Expenses and Accruals
Research and development expenses consist of costs incurred for Oncternal’s own and for sponsored and collaborative research and development activities. Research and development costs are expensed as incurred and include manufacturing drug product, costs associated with preclinical studies and clinical trials, regulatory and medical affairs activities, quality assurance activities, salaries and benefits, including stock-based compensation, fees paid to third-party consultants, license fees and overhead.
Oncternal has entered into various research and development contracts with research institutions, clinical research organizations, clinical manufacturing organizations and other companies. Payments for these activities are based on the terms of the individual agreements, which may differ from the pattern of costs incurred, and payments made in advance of performance are reflected in the consolidated balance sheets as prepaid expenses and other assets or accrued liabilities. Oncternal records accruals for estimated costs incurred for ongoing research and development activities. When evaluating the adequacy of the accrued liabilities, Oncternal analyzes progress of the services, including the phase or completion of events, invoices received and contracted costs. Significant judgments and estimates may be made in determining the prepaid or accrued balances at the end of any reporting period. Actual results could differ from Oncternal’s estimates.
Valuation of Warrants to Purchase Convertible Preferred Stock
Oncternal has classified warrants to purchase shares of its SeriesB-2 convertible preferred stock as a liability on its consolidated balance sheets as these warrants were free-standing financial instruments exercisable into contingently redeemable shares. The warrants were initially recorded at fair value on the date of grant, and were subsequently remeasured to fair value at each balance sheet date while the instrument was outstanding. Changes in fair value of these warrants were recognized as a component of other income (expense), net in Oncternal’s consolidated statements of operations.
Oncternal used the Black-Scholes option-pricing model, which incorporates assumptions and estimates, to value the preferred stock warrants. Oncternal assessed these assumptions and estimates on a quarterly basis as additional information impacting the assumptions was obtained. Estimates and assumptions impacting the fair value measurement included the fair value per share of the underlying convertible preferred stock, the remaining contractual term of the warrant, risk-free interest rate, expected dividend yield and expected volatility of the price of the underlying preferred stock. Oncternal determined the fair value per share of the underlying preferred stock
299
by taking into consideration Oncternal’s most recent sales of its convertible preferred stock, results obtained from third-party valuations and additional factors that Oncternal deemed relevant. During the period that these instruments were outstanding, Oncternal had historically been a private company and lacked company-specific historical and implied volatility information of its stock. Therefore, Oncternal estimated expected stock volatility based on the historical volatility of publicly traded peer companies for a term equal to the remaining contractual term of the warrants. The risk-free interest rate was determined by reference to the U.S. Treasury yield curve for time periods approximately equal to the remaining contractual term of the warrants. Oncternal estimated a 0% dividend yield based on the expected dividend yield and the fact that it has never paid or declared cash dividends. Significant changes to the fair value of the underlying stock would have resulted in a significant change in the fair value measurements.
Revenue Recognition
Oncternal currently generates revenue from a research subaward agreement with UC San Diego, which provides Oncternal with payments for certain types of expenditures in return for research and development activities over a contractually defined period. Revenue from such subaward is recognized in the period during which the related qualifying costs are incurred and services are rendered, provided that the applicable conditions under the subaward agreement have been met.
300
MANAGEMENT PRIOR TO AND FOLLOWING THE MERGER
Executive Officers and Directors of GTx Prior to the Merger
Directors of GTx Prior to the Merger
Michael G. Carter, M.D., Ch.B., F.R.C. P
Dr. Carter, age 81, was appointed as a GTx director in May 2006 and currently serves as Chair of the GTx Compensation Committee and as a member of both the GTx Audit Committee and the GTx Scientific and Development Committee. Dr. Carter was anon-executive director of Santarus, Inc. from 2004 to 2013, served as anon-executive director of Micromet AG from 2001 to 2005 and of MICROMET, Inc. from 2006 to March 2012, and served as anon-executive director of Fulcrum Pharma, PLC from 2005 to 2010. Dr. Carter was a member of the Advisory Board of Paul Capital Royalty Fund from 2005 to 2008, and was a venture partner with SV Life Sciences Advisors, LLP from 1998 to 2016. He has served as a member of the strategic advisory board of Healthcare Royalty Partners (HCRP) since September 2009 and a member of the HCRP Investment Committee since 2015. Dr. Carter was thenon-executive chairman of Metris Therapeutics, Ltd., a biotechnology firm specializing in women’s healthcare from 1999 to 2008. He was also anon-executive director of ONCOETHIX from June 2013 until its sale to Merck & Co., in December 2014. Dr. Carter served on the Pharmaceutical Board of I.C.I. Zeneca Pharmaceuticals, a predecessor company of AstraZeneca, and held various positions with I.C.I. Zeneca from 1984 to 1998, including International Medical Director and International Marketing Director. From 1985 to 1995, Dr. Carter served as a member of the U.K. Government’s Medicines Commission. Dr. Carter is an Elected Fellow of the Royal Pharmaceutical Society, Faculty of Pharmaceutical Medicine, and of the Royal College of Physicians of Edinburgh. Dr. Carter holds a degree in pharmacy from London University (U.K.) and a medical degree from Sheffield University Medical School (U.K.). Dr. Carter brings to the GTx Board specific expertise in the development and commercialization of pharmaceutical products by both large pharmaceutical companies and small specialty biotech companies.
J. Kenneth Glass
Mr. Glass, age 72, has served as a GTx director since March 2004, and currently serves as the Chair of the GTx Audit Committee and also currently serves on the GTx Compensation Committee. Mr. Glass retired as Chairman of the Board, President and Chief Executive Officer of First Horizon National Corporation (NYSE: FHN), or First Horizon, as of January 29, 2007. Mr. Glass was named President and Chief Executive Officer of First Horizon in July 2002, and he also became First Horizon’s Chairman of the Board in January 2004. From 2003 through 2007, Mr. Glass served as a director of FedEx Corporation (NYSE: FDX). From July 2001 through July 2002, Mr. Glass was President and Chief Operating Officer of First Horizon. From 1993 to 2001, Mr. Glass was Business Unit President of First Tennessee Bank. Mr. Glass received his B.A. in Accounting from Harding University and graduated from Harvard Business School’s Advanced Management Program. With his background in accounting and as a Chief Executive Officer, Mr. Glass serves in the role of a financial expert for our Audit Committee, and his years of experience leading a publicly-owned bank holding company has provided him with the organizational skills, risk management expertise and leadership he currently brings to the GTx Board and the Audit Committee.
Marc S. Hanover
Mr. Hanover, age 56, aco-founder of GTx, served as GTx’s President and Chief Operating Officer from its inception in September 1997 until his appointment as GTx’s permanent Chief Executive Officer in February 2015, and served as its acting Principal Financial Officer from December 31, 2013 until his appointment as its interim Chief Executive Officer on April 3, 2014. He also previously served as a member of the GTx Board from September 1997 to August 2011. Prior to joining GTx, Mr. Hanover was a founder of Equity Partners International, Inc., a private equity firm in Memphis, Tennessee, and participated as a founder and investor in three healthcare companies. From 1985 to 1997, Mr. Hanover was a Senior Vice President and a member of the
302
Executive Management Committee of National Bank of Commerce in Memphis, Tennessee. Mr. Hanover holds a B.S. in Biology from the University of Memphis and an MBA in Finance from the University of Memphis. Mr. Hanover serves as the GTx Chief Executive Officer and he is responsible for overseeing all aspects of our business, including product development and business strategies. Accordingly, the GTx Nominating and Corporate Governance Committee and the GTx Board has determined that Mr. Hanover should serve as a member of tthe GTx Board since he is best able to impart to the GTx Board the business and financial acumen essential for a complete understanding by the GTx Board’s operations, strategies and developmental plans.
J. R. Hyde, III
Mr. Hyde, age 76, has served as a GTx director since November 2000, and currently serves as a member of the GTx Compensation Committee and the GTx Nominating and Corporate Governance Committee. From November 2000 to March 2015, Mr. Hyde served asnon-executive Chairman of the GTx Board. In connection with Dr. Wills’ assumption of duties as the GTx Executive Chairman in March 2015, Mr. Hyde was appointed as GTx’s Lead Director. Since 1989, Mr. Hyde has been the sole stockholder and President of Pittco Holdings, Inc., a private institutional investment company. Since 1996, when Mr. Hyde made a substantial contribution to support the research of GTx’s prior CEO, Mr. Hyde has been instrumental in forming and financing GTx and is GTx’s largest stockholder. Mr. Hyde was the Chairman of the Board of Directors of AutoZone, Inc. (NYSE: AZO) from 1986 to 1997 and the Chief Executive Officer of AutoZone from 1986 to 1996. From March 2005 to June 2007, Mr. Hyde served as thenon-executive chairman of the Board of Directors of AutoZone, Inc. He was also Chairman and Chief Executive Officer of Malone & Hyde, Inc., AutoZone’s former parent company, from 1972 until 1988. Mr. Hyde also served as a director of FedEx Corporation (NYSE: FDX) from 1977 to 2011. As the largest stockholder of GTx and with a long history of serving as both Chairman and Chief Executive Officer of a large publicly-traded company and a member of the board of directors of other public companies, Mr. Hyde has continued to serve as a principal architect of the GTx public company governance structure, and continues to be a primary advisor to senior management on all matters of strategic importance. The GTx Board believes that Mr. Hyde’s leadership role and public company experience, as well as his significant ownership interest in the company, qualifies him to serve as the Lead Director of the GTx Board.
Garry A. Neil, M.D.
Dr. Neil, age 65, has served as a GTx director since August 2016 and currently serves as a member of the GTx Nominating and Corporate Governance Committee and the GTx Board’s Scientific and Development Committee. Dr. Neil joined as CSO of Aevi Genomic Medicine in September 2013. Prior to joining Aevi Genomic Medicine, Dr. Neil held a number of senior positions in the pharmaceutical industry, academia and venture capital. These include Corporate Vice President of Science & Technology at Johnson & Johnson and Group President at Johnson & Johnson Pharmaceutical Research and Development, Vice President of Research and Development at Merck KGaA/EMD Pharmaceuticals, Vice President of Clinical Research at Astra Zeneca and Astra Merck. Under his leadership a number of important new medicines for the treatment of cancer, anemia, infections, central nervous system and psychiatric disorders, pain, and genitourinary and gastrointestinal diseases gained initial or expanded approvals. Dr. Neil holds a B.S. from the University of Saskatchewan and an M.D. from the University of Saskatchewan College of Medicine. He completed postdoctoral clinical training in internal medicine and gastroenterology at the University of Toronto. Dr. Neil also completed a postdoctoral research fellowship at the Research Institute of Scripps Clinic. He is the Founding Chairman of the Pharmaceutical Industry R&D Consortium, TransCelerate Biopharmaceuticals Inc., and remains on the Board. He also serves on the Boards of Reagan Udall Foundation, Arena Pharmaceuticals (Nasdaq: ARNA) and is a past member of the Board of Foundation for the National Institutes of Health (FNIH), and the Science Management Review Board of the NIH. He is past Chairman of the Pharmaceutical Research and Manufacturers Association (PhRMA) Science and Regulatory Executive Committee and the PhRMA Foundation Board. The GTx Nominating and Corporate Governance Committee and the GTx Board finds Dr. Neil’s experience and background in drug development and regulatory interactions helpful on the GTx Board.
303
Kenneth S. Robinson, M.D., M.Div.
Dr. Robinson, age 64, has served as a GTx director since May 2008 and currently serves as Chair of the GTx Nominating and Corporate Governance Committee and as a member of the GTx Audit Committee. From 2003 through 2007, Dr. Robinson served in the cabinet of Tennessee Governor Phil Bredesen as Commissioner of Health, and in April 2009, Dr. Robinson accepted an appointment to provide executive-level public health leadership and consultation as the Health Officer of Shelby County, Tennessee, the county in which GTx is located. In February 2011, Dr. Robinson was appointed as Public Health Policy Advisor for Shelby County, Tennessee. From 1982 through 1991, Dr. Robinson taught and practiced internal medicine at Vanderbilt University School of Medicine, and from 1991 through 2003, he was an Assistant Dean at the University of Tennessee College of Medicine. Since 2015, he has served as President and CEO of United Way of theMid-South. Dr. Robinson holds a B.A., cum laude, from Harvard University, a M.D. from Harvard Medical School, and a Master of Divinity from Vanderbilt Divinity School. As a Harvard-trained physician who has experience in overseeing the complexities of federal and state agencies’ provision of healthcare to elderly and indigent patients, Dr. Robinson brings to the GTx Board expertise in governance, governmental reimbursement related issues, population health data and priorities, and the role of government in the development and delivery of healthcare services. Dr. Robinson, an African-American, adds an element of racial balance to the GTx Board and also provides a voice for GTx with state and local officials.
Robert J. Wills, Ph.D.
Dr. Wills, age 65, has over three decades of experience as a leader in the pharmaceutical and biotechnology industry. Dr. Wills joined GTx as the Executive Chairman of the Board of Directors and as Chairman of the GTx Board’s Scientific and Development Committee on March 2, 2015. He also serves as Chairman of the Board of CymaBay Therapeutics, as board member at Parion Sciences, Inc., as board member at Go Therapeutics and as a member of the Emerging Companies Section Governing Board of Biotechnology Innovation Organization (BIO). Prior to these roles, Dr. Wills spent over 25 years at Johnson & Johnson. Most recently he was Vice President, Alliance Management, Janssen Pharmaceutical Companies of Johnson & Johnson. He also served as Senior Vice President Global Development, where he was responsible for the R&D pipeline and a member of the R&D Board of Directors. In addition he served on several of the commercial Operating Company Boards and key pharmaceutical group decision-making committees. Dr. Wills began his career at Hoffmann-LaRoche where he spent 10 years in several roles of scientific responsibility. He holds a BS in Biochemistry and an MS in Pharmaceutics from the University of Wisconsin and a PhD in Pharmaceutics from the University of Texas.
Executive Officers of GTx Prior to the Merger
The following table sets forth information about GTx’s executive officers as of March 31, 2019.
Name | Age | Position(s) | ||
Executive Officers | ||||
| Marc S. Hanover | 56 | Chief Executive Officer | ||
| Robert J. Wills, Ph.D. | 65 | Executive Chairman | ||
| Henry P. Doggrell | 70 | Vice President, Chief Legal Officer and Secretary | ||
| Jason T. Shackelford | 43 | Vice President, Finance and Accounting, and Principal Financial and Accounting Officer |
The biographies of Marc S. Hanover and Robert J. Wills, Ph.D. are provided above in the subsection “Directors of GTx Prior to the Merger.”
Henry P. Doggrell currently serves as GTx’s Vice President, Chief Legal Officer and Secretary, after joining GTx in October 2001 as General Counsel and Secretary. From April 1998 to August 2001, Mr. Doggrell was Senior Vice President, Corporate Affairs at Buckeye Technologies, Inc., a specialty cellulose company,
304
where he was responsible for matters including corporate finance, investor relations, mergers and acquisitions, intellectual property and licensing and strategic development. From 1996 to 1998, Mr. Doggrell served as General Counsel and Secretary of Buckeye Technologies. Prior to joining Buckeye Technologies, Mr. Doggrell was a partner of the Baker, Donelson, Bearman, Caldwell and Berkowitz law firm from 1988 to 1996, where he served as a member of the law firm management committee and Chair of the firm’s Corporate Securities department. Mr. Doggrell holds a B.S. in Commerce from the University of Virginia and a JD from Vanderbilt University.
Jason T. Shackelford currently serves as GTx’s Vice President, Finance and Accounting, after joining GTx in July 2007 as Director, Accounting and Corporate Controller, and has served as our principal accounting officer since December 31, 2013 and as our principal financial and accounting officer since April 3, 2014. Prior to joining GTx, Mr. Shackelford was a Senior Audit Manager at KPMG LLP. Mr. Shackelford is a Certified Public Accountant and holds a Bachelor of Business Administration and Master of Accountancy from the University of Mississippi.
Executive Officers and Directors Following the Merger
Resignation of Current Executive Officers of GTx
Pursuant to the Merger Agreement, all of the current executive officers of GTx will resign immediately prior to the completion of the merger.
Executive Officers and Directors of the Combined Organization Following the Merger
The GTx Board is currently composed of seven directors. Following the merger, the GTx Board will be increased to nine directors. Pursuant to the Merger Agreement, all of the current directors of GTx, other than two designees selected by GTx to remain on the GTx Board, Drs. Carter and Wills, shall resign from the GTx Board at or prior to the Effective Time. Drs. Carter and Wills will then appoint, effective as of the Effective Time, (a) the two directors designated by SPH USA, (b) the one director who will serve as Chairman of the combined organization, (c) the one director who will serve as Chief Executive Officer of the combined organization and (d) the remaining three directors as designated in the Merger Agreement. It is anticipated that, following the closing of the merger, the GTx Board will be constituted as follows:
Name | Age | Current Principal Affiliation | ||
| David F. Hale | 70 | Oncternal Therapeutics, Inc., Chairman | ||
| James B. Breitmeyer, M.D., Ph.D. | 65 | Oncternal Therapeutics, Inc., President, Chief Executive Officer and Director | ||
| Michael G. Carter, M.D., Ch.B., F.R.C.P. | 81 | GTx, Inc., Director | ||
| Daniel L. Kisner, M.D. | 71 | Oncternal Therapeutics, Inc., Director Nominee | ||
| William R. LaRue | 68 | Oncternal Therapeutics, Inc., Director | ||
| Yanjun Liu, Ph.D. | 54 | Oncternal Therapeutics, Inc., Director | ||
| Xin Nakanishi, Ph.D. | 56 | Oncternal Therapeutics, Inc., Director | ||
| Charles P. Theuer, M.D., Ph.D. | 55 | Oncternal Therapeutics, Inc., Director | ||
| Robert J. Wills, Ph.D. | 65 | GTx, Inc., Executive Chairman |
Following the merger, the management team of GTx is expected to be composed of the current management team of Oncternal. The following table lists the names, ages and positions of the individuals who are expected to serve as executive officers GTx upon completion of the merger:
Name | Age | Position(s) | ||
| James B. Breitmeyer, M.D. Ph.D. | 65 | President and Chief Executive Officer | ||
| Richard G. Vincent | 56 | Chief Financial Officer | ||
| Hazel M. Aker | 63 | General Counsel |
305
Executive Officers
James B. Breitmeyer, M.D., Ph.D.
President, Chief Executive Officer, Director
Since September 2015, Dr. Breitmeyer has served as President, Chief Executive Officer and director of Oncternal Therapeutics, Inc. formally Tokalas, Inc. Dr. Breitmeyer is a veteran biotech executive with experience successfully starting and growing biotechnology organizations. He has been responsible for both the development and implementation of both operational and drug development strategies, as well as supervising and managing both large organizations and emerging biotechnology companies. Dr. Breitmeyer served as President of Bavarian Nordic, Inc. and Executive Vice President of Bavarian Nordic A/S, a multinational corporation headquartered in Denmark, from February 2013 to July 2015 where he oversaw business operations and development strategy both for Bavarian Nordic, Inc. and Bavarian Nordic A/S. He has been a director of Zogenix, Inc., a public pharmaceutical company, since March 2014, and was their acting Chief Medical Officer from August 2012 to February 2013 where he was responsible for clinical development and regulatory strategy. He previously served as the Executive Vice President of Development and Chief Medical Officer of Cadence Pharmaceuticals Inc., a public pharmaceutical company, from August 2006 to August 2012, and the Chief Medical Officer of Applied Molecular Evolution Inc., a wholly- owned subsidiary of Eli Lilly and Co., a global pharmaceutical company, from December 2001 to August 2006. Dr. Breitmeyer was also the founder, President and Chief Executive Officer of the Harvard Clinical Research Institute, and Chief Medical Officer and Head of Research & Development for North America at Serono Laboratories Inc., an international biopharmaceutical company. Dr. Breitmeyer served as a founding collaborator and scientific advisor to Immunogen Inc., a biotechnology company, and held clinical and teaching positions at the Dana Farber Cancer Institute and Harvard Medical School. Currently, Dr. Breitmeyer serves as a director on two public boards, Zogenix, Inc. (ZGNX) where he is also a member of the compensation committee and Otonomy, Inc. (OTIC) where he is a director and member of the compensation and audit committees. Dr. Breitmeyer earned his B.A. in Chemistry from the University of California, Santa Cruz and his M.D. and Ph.D. from Washington University School of Medicine and is Board Certified in Internal Medicine and Oncology. He holds an active California medical license.
The GTx Board and the Oncternal Board believe that Dr. Breitmeyer’s perspective and experience as Oncternal’s President and CEO, as well as his depth of operating and senior management experience in the pharmaceutical industry in both private and public organizations and educational background, provide him with the qualifications and abilities to serve as President and CEO of the combined company.
Richard G. Vincent
Chief Financial Officer
Mr. Vincent has served as Oncternal’s Chief Financial Officer since April 2017. From 2012 to the present, Mr. Vincent has worked as an independent Chief Financial Officer, and was Chief Financial Officer and Secretary of Sorrento Therapeutics from January 2011 through February 2015. From 2008 to January 2011, Mr. Vincent served as an independent Chief Financial Officer to several pharmaceutical, biotech and medical device companies, including Avalyn Pharma(co-founder), Meritage Pharma, and Elevation Pharmaceuticals. Mr. Vincent served as Chief Financial Officer for Verus Pharmaceuticals from 2004 to 2008, and Women First Healthcare from 2003 to 2005. Mr. Vincent’s areas of responsibility have spanned all areas of finance, treasury, investor and public relations, human resources, information technology, facilities and project management. From 1987 to 1995, Mr. Vincent held a number of positions with Deloitte & Touche LLP, the last of which was senior manager, where he specialized in emerging growth and publicly-reporting companies. Mr. Vincent became a Certified Public Accountant in California in 1989 and holds a B.S. degree in business with an emphasis in accounting from San Diego State University.
306
Hazel M. Aker
General Counsel
Hazel Aker has served as General Counsel to Oncternal since February 2019. Prior to Oncternal, Ms. Aker worked as an independent legal consultant from 2014 to the present, and was Senior Vice President, General Counsel and Secretary of Cadence Pharmaceuticals, Inc., from April 2007 through its acquisition by Mallinckrodt plc in March 2014. Previously, Ms. Aker served as General Counsel for several pharmaceutical, biotech and medical device companies. Ms. Aker is a member of the State Bar of California and holds a J.D. from the University of San Diego School of Law, and a B.A. from the University of California, San Diego.
Non-Employee Directors
David F. Hale
Chairman of the Board
David F. Hale is aco-founder and has served as a member of the Oncternal Board since 2013 and as chairman of the Oncternal Board since December 2018. Since May 2006, Mr. Hale has served as Chairman & CEO of Hale Biopharma Ventures, LLC. He is a serial entrepreneur who has been involved in the formation and development of numerous life sciences companies. He was previously President and CEO of CancerVax Corporation, a cancer therapeutic company from October 2000 through May 2006 when CancerVax merged with Micromet, Inc. He became Chairman of Micromet, Inc. until the sale of the company to Amgen Inc. in 2012. After joining Hybritech, Inc., in 1982, he was President & Chief Operating Officer and became CEO in 1986, when Hybritech was acquired by Eli Lilly and Co. From 1987 to 1997 he was Chairman, President and CEO of Gensia, Inc. He was aco-founder and Chairman of Viagene, Inc. from 1987 to 1995. He was President and CEO of Women First HealthCare, Inc. from January 1998 to June 2000. Prior to joining Hybritech in 1982, Mr. Hale was Vice President and General Manager of BBL Microbiology Systems, a division of Becton, Dickinson & Co. and from 1971 to 1980, held various marketing and sales management positions with Ortho Pharmaceutical Corporation, a division of Johnson & Johnson, Inc. Mr. Hale also serves as Chairman of Biocept, Inc and Conatus Pharmaceuticals Inc. Mr. Hale previously served as Chairman of Santarus, Inc., Somaxon, Inc., SkinMedica, Inc., CRISIMed, Inc. and Agility Clinical, Inc. He also serves as Chairman of a number of privately held companies, including MDR Aesthetics Inc., Recros Medica, Inc., Clarify Medical, Inc., Neurana Pharmaceuticals, Inc. and Adigica Health, Inc., and as a Director of Neurelis, Inc. Mr. Hale also is aco-founder and serves on the Board of Directors of BIOCOM, is a former member of the board of Biotechnology Industry Organization, or BIO, and the Biotechnology Institute. Mr. Hale also serves as a member of the board of directors of the San Diego Economic Development Corporation, as a board trustee of Rady Children’s Hospital of San Diego, Chairman of the board of Rady Children’s Institute of Pediatric Genomics and a trustee of the Salk Institute. He is aco-founder of the CONNECT Program in Technology and Entrepreneurship. Mr. Hale holds a B.A. in Biology and Chemistry from Jacksonville State University.
The GTx Board and the Oncternal Board believe Mr. Hale is qualified to serve as chairman of the combined company’s board of directors because of his extensive knowledge of Oncternal’s business and history, experience as a board member of multiple publicly-traded and privately-held companies, and expertise in developing, financing and providing strong executive leadership to numerous biopharmaceutical companies.
Michael G. Carter, M.D., Ch.B., F.R.C.P
The biography of Michael G. Carter is provided above in the subsection “Directors of GTx Prior to the Merger.”
Daniel L. Kisner, M.D.
Daniel L. Kisner, M.D. currently serves as an independent consultant in the life science industry. He was a partner at Aberdare Ventures from 2003 to 2011. Dr. Kisner served as Chairman of the Board of Directors of Caliper Life Sciences from 2002 to 2008, and as President and CEO of its predecessor company, Caliper
307
Technologies, from 1999 to 2002. He held positions of increasing responsibility at Isis Pharmaceuticals, Inc., from 1991 to 1999, most recently as President and COO. Dr. Kisner previously served in pharmaceutical research and development executive positions at Abbott Laboratories from 1988 to 1991 and at SmithKline Beckman Laboratories from 1985 to 1988. He held a tenured faculty position in the Division of Medical Oncology at the University of Texas, San Antonio School of Medicine until 1985 after a five-year advancement through the Cancer Treatment Evaluation Program of the National Cancer Institute. Dr. Kisner is board certified in internal medicine and medical oncology. Dr. Kisner holds a B.A. from Rutgers University and an M.D. from Georgetown University. Dr. Kisner currently serves as a director at Conatus Pharmaceuticals Inc., Zynerba Pharmaceuticals and Dynavax Technologies Corporation, and has extensive prior private and public company board experience, including serving as Chairman of the Board of Directors at Tekmira Pharmaceuticals. Dr. Kisner’s extensive leadership experience in the biotechnology and biopharmaceutical industries and as a venture capital investor contributed to the board of directors’ conclusion that he should serve as a director of the combined company.
William R. LaRue
William R. LaRue has served as a member of the Oncternal Board since December 2017. Mr. LaRue currently serves as an independent board member for multiple public and private companies in the life science industry. He served as Senior Vice President and Chief Financial Officer at Cadence Pharmaceuticals, Inc., a biopharmaceutical company, starting in June 2006, and expanded his role to serve as Assistant Secretary at Cadence in April 2007, serving in both capacities until the company’s acquisition by Mallinckrodt plc in March 2014. At Cadence, Mr. LaRue was a member of the Executive Committee with direct responsibility for the company’s financial leadership including corporate financing, investor relations, financial planning and reporting, SEC reporting, accounting, treasury, risk management, tax and information technology. During his tenure, Cadence raised over $375 million in public and private equity and senior debt, including an IPO in October 2006 as the company transitioned from a development stage to a commercial stage company. Prior to joining Cadence, Mr. LaRue served as the Senior Vice President and Chief Financial Officer of CancerVax Corporation, a biotechnology company, from 2001 until its merger with Micromet, Inc. in May 2006. Mr. LaRue currently serves as a member of the board of directors and chair of the Audit Committee of Tracon Pharmaceuticals, Inc., Conatus Pharmaceuticals, Inc. and Alastin Skincare, Inc. He previously served on the boards of directors of Applied Proteomics, Inc., Neurelis, Inc. and Cadence Pharmaceuticals, Inc. Mr. LaRue received a B.S. in business administration and an M.B.A. from the University of Southern California. Mr. LaRue’s extensive financial experience and leadership in both private and public companies contributed to the board of directors’ conclusion that he should serve as a director of the combined company.
Yanjun Liu, M.D., Ph.D.
Yanjun Liu, M.D., Ph.D., has served as a member of the Oncternal Board since November, 2018. He has been Vice President of SPH and holds the position of President of the Central Research Institute, a division of SPH, since 2013. Dr. Liu serves as Chairman of the Board of Shanghai Jiaolian Medicine Research and Development Co., Ltd, a wholly-owned subsidiary of SPH. From 2001 to 2012, Dr. Liu served as a Vice General Manager in Shanghai Fudan-ZhangjiangBio-Pharmaceutical Co. Dr. Liu holds a M.D. and a Ph.D. from Second Military Medical University in Shanghai, China. Dr. Liu’s extensive experience as a chairman, director, and senior management of international healthcare companies contributed to the board of directors’ conclusion that he should serve as a director of the combined company.
Xin Nakanishi, Ph.D.
Xin Nakanishi, Ph.D. has served as a member of the Oncternal Board since November 2018. She has served as the Chief Executive Officer of Shanghai Pharma Biotherapeutics USA Inc., a subsidiary of SPH USA, since July 2018. Dr. Nakanishi previously served as a venture partner at Yuansheng bioVENTURE from 2017-2018, and was CEO and founder of Sunvita Therapeutics, LLC from 2009- 2018 a company that provided cross border business development for various U.S. and Chinese biopharmaceutical companies. She was also the Director of
308
Biology at Phenomix Inc., a senior scientist at Pfizer, and a group leader at Immusol Inc. Dr. Nakanishi holds a B.A. in Virology from Wuhan University and a Ph.D. in Biochemistry from the University of Kansas. Dr. Nakanishi’s extensive experience in the life science and pharmaceutical industries contributed to the board of directors’ conclusion that she should serve as a director of the combined company.
Charles P. Theuer, M.D., Ph.D.
Since March of 2018, Dr. Theuer has served as a member of the Oncternal Board. He has been President, Chief Executive Officer and a member of the board of TRACON Pharmaceuticals, Inc. since July 2006. From 2004 to 2006, Dr. Theuer was the Chief Medical Officer at TargeGen, Inc., a biotechnology company. Prior to joining TargeGen, Inc., Dr. Theuer was Director of Clinical Oncology at Pfizer, Inc., a pharmaceutical corporation, from 2003 to 2004. Dr. Theuer has also held senior positions at IDEC Pharmaceuticals Corp. from 2002 to 2003 and at the National Cancer Institute from 1991 to 1993. In addition, he has held academic positions at the University of California, Irvine, where he was Assistant Professor in the Division of Surgical Oncology and Department of Medicine. Dr. Theuer currently serves as a director at 4D Molecular Therapeutics, a position he has held since January 2016. Dr. Theuer received a B.S. from the Massachusetts Institute of Technology, an M.D. from the University of California, San Francisco, and a Ph.D. from the University of California, Irvine. He completed a general surgery residency program at Harbor-UCLA Medical Center and was board certified in general surgery in 1997. Dr. Theuer’s extensive clinical development experience and service as a director or officer of healthcare companies contributed to the board of directors’ conclusion that he should serve as a director of the combined company.
Robert J. Wills, Ph.D.
The biography of Robert J. Wills is provided above in the subsection “Directors of GTx Prior to the Merger.”
Composition of the Board of Directors Prior to and Following the Merger
The GTx Board is currently comprised of seven directors divided into three staggered classes, each class serving three-year terms. The staggered structure of the GTx Board will remain in place following completion of the merger. At the most recent annual meeting of GTx’s stockholders held in 2018, Class II directors were elected. As a result, the term of the Class II directors of the combined organization will expire upon the election and qualification of successor directors at the annual meeting of stockholders in 2021, with the terms of the Class III directors and Class I directors expiring upon the election and qualification of successor directors at the annual meetings of stockholders to be held in 2019 and 2020, respectively.
The director classes for GTx are currently as follows:
| • | Class I directors: Marc S. Hanover, Gary A. Neil and Kenneth S. Robinson; |
| • | Class II directors: J. Kenneth Glass and Robert J. Wills; and |
| • | Class III directors: Michael G. Carter and J.R. Hyde, III. |
Pursuant to the Merger Agreement, each of the directors and officers of GTx who will not continue as directors or officers of GTx or the surviving corporation following the consummation of the merger shall resign immediately prior to the Effective Time. In connection with the merger, the GTx Board will be expanded to include a total of nine directors. Pursuant to the Merger Agreement, two such directors shall be designated by GTx, two such directors shall be designated by SPH USA, one director shall be the Chairman of the combined organization, one director shall be the Chief Executive Officer of the combined organization and the remaining three directors shall be as designated in the Merger Agreement. Effective as of the Effective Time, it is anticipated that Drs. Carter and Wills will remain on the GTx Board and that the board of directors will increase the size of the board to nine. Then, Dr. Carter and Dr. Wills will elect Mr. Hale, Dr. Breitmeyer, Dr. Kisner, Mr. LaRue, Dr. Liu, Dr. Nakanishi and Dr. Theuer, to the GTx Board. It is anticipated that these directors will be appointed to the three staggered director classes of the combined organization’s board of directors as follows:
| • | Class I directors (expiring in 2020): Daniel L. Kisner, Xin Nakanishi and Charles P. Theuer; |
309
| • | Class II directors (expiring in 2021): William R. LaRue, Yanjun Liu and Robert J. Willis; and |
| • | Class III directors (expiring in 2022): James B. Breitmeyer, Michael G. Carter and David F. Hale. |
The division of the GTx Board into three classes with staggered three-year terms may delay or prevent a change of management or a change of control of GTx, or, following the completion of the merger, the combined organization.
GTx’s Nominating and Governance Committee is responsible for reviewing the board of directors, on an annual basis. In evaluating the suitability of individual candidates (both new candidates and current members), the Nominating and Corporate Governance Committee and the board of directors of the combined organization may take into account many factors, including the following:
| • | diversity of personal and professional background, perspective, experience, age, gender, ethnicity and country of citizenship; |
| • | personal and professional integrity and ethical values; |
| • | experience in one or more fields of business, professional, governmental, scientific or educational endeavors, and a general appreciation of major issues facing public companies similar in scope and size to GTx; |
| • | experience relevant to GTx’s industry or social policy concerns; |
| • | relevant academic expertise or other proficiency in an area of GTx’s operations; |
| • | objective and mature business judgment and expertise; and |
| • | any other relevant qualifications, attributes or skills. |
There are no family relationships among any of GTx’s current directors and executive officers, and there are no family relationships among any of the combined organization’s proposed directors and executive officers.
Director Independence
As required under the Nasdaq listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by the board of directors. The GTx Board has determined that after the completion of the merger, seven of the combined company’s nine directors are expected to be independent members of the combined company’s board of directors within the meaning of the applicable Nasdaq listing standards: Mr. Hale, Dr. Carter, Dr. Kisner, Mr. LaRue, Dr. Liu, Dr. Nakanishi and Dr. Theuer. Dr. Wills is not expected to be “independent” within the meaning of the Nasdaq listing standards because he will have served as executive chairman of GTx immediately prior to the Effective Time. Dr. Breitmeyer is not expected to be “independent” within the meaning of the Nasdaq listing standards because he will have served as an executive officer of Oncternal immediately prior to the Effective Time and is expected to serve as Chief Executive Officer of the combined organization.
Committees of the Board of Directors Prior to and Following the Merger
The GTx Board currently has four standing committees: the Audit Committee, the Compensation Committee, the Nominating and Corporate Governance Committee and the Scientific and Development Committee. The charters for the Audit Committee, the Compensation Committee, the Nominating and Corporate Governance Committee and the Scientific and Development Committee are available on GTx’s website (www.gtxinc.com) under “Investors” at “Corporate Governance.” The current membership and anticipated membership after the merger of each of the Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee are shown below. Information about the duties and responsibilities of each of the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee are provided below. After the merger, each of these committees are expected to retain these duties and responsibilities. The purpose of the Scientific and Development Committee is to assist the GTx Board by reviewing and evaluating GTx’s research strategy, as well as its research, development and clinical programs.
310
After the merger, the Compensation Committee and the Nominating and Corporate Governance Committee of the combined company’s board of directors are expected to be comprised entirely of directors who are independent within the meaning of the Nasdaq listing standards, and the members of the Audit Committee are expected to be independent under applicable Nasdaq listing standards and SEC rules. In addition, the GTx Board has determined that William R. LaRue, qualifies as an “audit committee financial expert” within the meaning of the SEC rules.
Audit Committee
Number of Meetings held in 2018: Four
| Current Members | Anticipated Members After Merger | Current and Anticipated Committee Functions | ||
| J. Kenneth Glass (Chair) Michael G. Carter Kenneth S. Robinson | William R. LaRue (Chair) David F. Hale Daniel L. Kisner | • Oversees financial and operational matters involving accounting, corporate finance, auditing, internal control over financial reporting, compliance, and business ethics.
• Oversees other financial audit and compliance functions as assigned by the board of directors.
• Oversees those functions which may pose material financial risk to GTx.
• Has the sole authority to select, evaluate, replace and oversee GTx’s independent registered public accounting firm.
• Has the sole authority to approvenon-audit and audit services to be performed by the independent registered public accounting firm.
• Monitors the independence and performance of the independent registered public accounting firm.
• Provides an avenue of communications among the independent registered public accounting firm, management and the board of directors.
• Reviews, approves and provides oversight of “related party transactions.”
• Has the specific responsibilities and authority necessary to comply with the Nasdaq listing standards applicable to audit committees.
• Reviews, approves and provides oversight of “related party transactions.”
• Has the specific responsibilities and authority necessary to comply with the Nasdaq listing standards applicable to audit committees. |
311
Compensation Committee
Number of Meetings held in 2018: One
| Current Members | Anticipated Members After Merger | Committee Functions | ||
| Michael G. Carter (Chair) J. Kenneth Glass J.R. Hyde, III | David F. Hale (Chair) William R. LaRue Daniel L. Kisner | • Reviews the performance of GTx officers and establishes overall executive compensation policies and programs.
• Reviews and approves compensation elements such as base salary, bonus awards, stock option grants and other forms of long-term incentives for GTx officers.
• Has the authority, in its sole discretion, to retain (or obtain the advice of) any compensation consultant, legal counsel or other adviser to assist it in the performance of its duties.
• Evaluates the independence of GTx’s compensation advisers.
• Has the direct responsibility for the appointment, compensation and oversight of the work of any advisers retained or engaged by the Compensation Committee.
• Reviews board of directors compensation.
• Has the specific responsibilities and authority necessary to comply with the Nasdaq listing standards applicable to compensation committees. |
Nominating and Corporate Governance Committee
Number of Meetings held in 2018: One
| Current Members | Anticipated Members After Merger | Committee Functions | ||
Kenneth S. Robinson (Chair) J.R. Hyde, III | Michael G. Carter (Chair) David F. Hale Charles P. Theuer | • Evaluates governance standards for GTx to ensure that appropriate governance policies and procedures have been established and are being followed.
• Develops criteria to determine the qualifications and appropriate tenure of directors.
• Reviews such qualifications and makes recommendations to the Board regarding the nomination of current directors forre-election to the Board as well as new nominees to fill vacancies on the Board. |
312
| Current Members | Anticipated Members After Merger | Committee Functions | ||
• Considers any stockholder recommendations for Board nominees, as described below.
• Recommends to the Board the chairmanship and membership of each Board committee.
• Reviews succession plans for GTx officers. |
Compensation Committee Membership, Interlocks and Insider Participation
Following completion of the merger, GTx’s Compensation Committee is expected to consist of Messrs. Hale, LaRue and Kisner. Mr. Hale is expected be the Chair of the Compensation Committee. Each member of the Compensation Committee is expected to be a“non-employee” director within the meaning of Rule16b-3 of the rules promulgated under the Exchange Act, and independent within the meaning of the independent director guidelines of Nasdaq and the SEC. None of the proposed executive officers of the combined organization serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers who is proposed to serve on the combined organization’s board of directors or Compensation Committee following the merger.
Nominating and Corporate Governance Committee Matters
The Nominating and Corporate Governance Committee expects, as minimum qualifications, that nominees to the GTx Board (including incumbent directors) will enhance the board’s management, finance, commercial and/or scientific expertise, will not have a conflict of interest and will have a high ethical standard and, with respect to new members of the board of directors, a willingness to serve at least an initial three year term for the Nominating and Corporate Governance Committee to recommend them to the board of directors. A director nominee’s knowledge and/or experience in areas such as, but not limited to, the medical, pharmaceutical, biotechnology, biopharmaceutical or life sciences industry, equity and debt capital markets and financial accounting are likely to be considered both in relation to the individual’s qualification to serve on our board of directors and the needs of the board of directors as a whole. While we do not have a formal policy on board diversity, the Nominating and Corporate Governance Committee takes into account a broad range of diversity considerations when assessing director candidates, including individual backgrounds and skill sets, professional experiences and other factors that contribute to the board having an appropriate range of expertise, talents, experiences and viewpoints, and considers those diversity considerations, in view of the needs of the board as a whole, when making decisions on director nominations. Other characteristics, including but not limited to, the director nominee’s material relationships with GTx, time availability, service on other boards of directors and their committees, or any other characteristics which may prove relevant at any given time as determined by the Nominating and Corporate Governance Committee are reviewed for purposes of determining a director nominee’s qualification.
Candidates for director nominees are evaluated by the Nominating and Corporate Governance Committee in the context of the current composition of the board of directors, the operating requirements of GTx and the long-term interests of GTx’s stockholders. In the case of new director candidates, the Nominating and Corporate Governance Committee also determines whether the nominee must be independent for Nasdaq purposes, which determination is based upon applicable Nasdaq listing standards, applicable SEC rules and regulations and the advice of counsel, if necessary. The Nominating and Corporate Governance Committee then may use its network of contacts to compile a list of potential candidates, but may also engage, if it deems appropriate, a professional search firm. The Nominating and Corporate Governance Committee conducts any appropriate and necessary
313
inquiries into the backgrounds and qualifications of possible candidates after considering the function and needs of the board of directors. In the case of incumbent directors whose terms of office are set to expire, the Nominating and Corporate Governance Committee reviews such directors’ overall service to GTx during their term, including the number of meetings attended, level of participation, quality of performance, and any other relationships and transactions that might impair such directors’ independence. The Nominating and Corporate Governance Committee meets to discuss and consider such candidates’ qualifications and then selects a nominee for recommendation to the Board by majority vote. The Nominating and Corporate Governance Committee does not intend to alter the manner in which it evaluates candidates, including the minimum criteria set forth above, based on whether the candidate was recommended by a stockholder or not. To date, the Nominating and Corporate Governance Committee has not paid a fee to any third-party to assist in the process of identifying or evaluating director candidates.
The board of directors does not impose term limits or a mandatory retirement age for directors, except that our Chief Executive Officer (or any other officer of GTx, including the Executive Chairman, if he or she is a member of the board) is required to tender his or her resignation to the board if he or she ceases to serve as an executive officer of GTx. The Nominating and Corporate Governance Committee will then consider all of the relevant facts and circumstances and recommend to the board of directors the action to be taken with respect to such offer of resignation. With respect tonon-employee members of the board of directors, while it is believed that a director’s knowledge and/or experience can continue to provide benefit to the board of directors following a director’s retirement from his or her primary work affiliation, it is recognized that a director’s knowledge of and involvement in ever changing business environments can weaken, and therefore his or her ability to continue to be an active contributor to the board of directors will be reviewed. Upon a director’s change in his or her employment status, if any, he or she is required to notify the Nominating and Corporate Governance Committee of such change and, if determined by the board of directors upon recommendation of the Nominating and Corporate Governance Committee, to offer his or her resignation.
Compensation Committee Matters
Scope of Authority. The GTx Compensation Committee acts on behalf of the GTx Board to establish the compensation of executive officers of GTx and provides oversight of GTx’s compensation philosophy. The GTx Compensation Committee also acts as the oversight committee with respect to GTx’s benefit plans, stock plans and bonus plans covering executive officers and other senior management. In overseeing those plans, the GTx Compensation Committee has the sole authority for theday-to-day administration and interpretation of the plans. The GTx Compensation Committee retains the authority for establishing all matters with respect to the compensation of the GTx executive officers, although the GTx Compensation Committee may recommend to the full GTx Board that it take action with respect to such compensation matters. Under its charter, the GTx Compensation Committee has the authority, in its sole discretion, to retain (or obtain the advice of) any compensation consultant, legal counsel or other adviser to assist it in the performance of its duties. The GTx Compensation Committee also has the direct responsibility for the appointment, compensation and oversight of the work of any advisers retained or engaged by the GTx Compensation Committee. Finally, the GTx Compensation Committee has the sole authority to approve the reasonable fees and the other terms and conditions of the engagement of any such advisor, including authority to terminate the engagement. GTx must provide for appropriate funding, as determined by the GTx Compensation Committee, for the payment of reasonable compensation to any such adviser retained by the GTx Compensation Committee.
Dr. Carter, as Chair of the GTx Compensation Committee, is responsible for setting the agenda for meetings. The GTx Compensation Committee annually evaluates the performance, and determines the compensation, of the Executive Chairman of the Board, Chief Executive Officer and the other officers of GTx.
Role of Compensation Consultants in 2018 Compensation Determinations. Under its charter, the GTx Compensation Committee has the authority to retain its own compensation consultant at company expense. For the fiscal year ended December 31, 2018, the GTx Compensation Committee decided, at a special meeting of the
314
committee held on December 6, 2017, to await the results from the Phase 2 clinical trial evaluating enobosarm as a potential treatment for stress urinary incontinence (the “ASTRID trial”) before determining whether a compensation consultant should be retained to review and assess the adequacy of the peer group the GTx Compensation Committee was then using for evaluating executive and Board compensation, since the GTx Compensation Committee recognized that the composition and structure of the company could change significantly depending on the outcome of the trial. Following the results from the ASTRID trial in the fall of 2018, no compensation consultant was retained to review and assess the company’s peer group and current executive compensation; rather the GTx Compensation Committee focused on a plan to reduce company personnel over time while retaining sufficient employees to continue to function as a public company and assess strategic alternatives for the company to best maximize stockholder value.
Roles of Executives in Establishing Executive Compensation. Historically, GTx’s human resource, finance and legal departments worked with senior management to design and develop compensation programs for the named executive officers for recommendation to the GTx Compensation Committee. In addition, these management groups worked together to recommend changes to existing compensation programs, to recommend financial and other performance targets to be achieved under those programs, to prepare analyses of financial data, and to prepare peer data comparisons and other briefing materials for the GTx Compensation Committee. GTx’s Chief Executive Officer, Mr. Hanover, leads the human resource, finance and legal departments in designing and developing compensation programs for the GTx executive officers, and presents these proposals to the GTx Compensation Committee. Mr. Hanover discusses all executive compensation proposals with the Executive Chairman of the Board, Dr. Wills, and Henry P. Doggrell, GTx’s Chief Legal Officer, before they are presented to the GTx Compensation Committee for its consideration. The GTx Compensation Committee may approve, modify, or reject those proposals, or may request additional information from management (or its own consultant, if it wished to retain one) on those matters.
Dr. Wills and Mr. Hanover also make recommendations to the GTx Compensation Committee with respect to the specific performance goals to be achieved under the GTx Executive Bonus Compensation Plan, which is described in more detail below in the section entitled, “Executive Compensation—Narrative Disclosure to Summary Compensation Table—Annual Bonus Plan.” Dr. Wills and Mr. Hanover provide annual reviews of the performance of each of the executive officers (other than themselves) to assist the GTx Compensation Committee in its annual determination of each element of compensation for such officers. The performance of Dr. Wills and Mr. Hanover is evaluated by the GTx Compensation Committee.
Typically, the GTx Compensation Committee meets in executive session to discuss and determine appropriate base salaries, bonus compensation target awards and goals (if applicable), and equity awards for each executive officer of GTx.
No executive officer was present or directly participated in the final deliberations of the GTx Compensation Committee with respect to any component of his or her own compensation.
Director Compensation. The GTx Board setsnon-employee directors’ compensation at the recommendations of both the Nominating and Corporate Governance Committee and the GTx Compensation Committee. The GTx Compensation Committee and the GTx Board believe that: director compensation should fairly compensate directors for work required in a company of GTx’s size and scope; the compensation should align directors’ interests with the long-term interest of stockholders; and the structure of the compensation should be simple, transparent and easy for stockholders to understand. GTx’snon-employee director compensation program has typically consisted of a combination of a cash retainer and initial and annual stock option grants, with the number of shares subject to the annual stock option grant based on providing eligible directors aggregate equity grants in line with the 50th percentile of the equity granted tonon-employee directors of GTx’s peers. Data from GTx’s peers is gathered from Equilar’s online data base reflecting compensation information gleaned from the prior year’s proxy statement for each peer, and then considered by the Nominating and Corporate Governance Committee and the GTx Compensation Committee for the purpose of making recommendations to the Board for
315
director compensation which the Board must then approve. During the November 2017 meetings of the GTx Nominating and Corporate Governance and the GTx Compensation Committee, relevant data from Equilar on peer group director compensation was reviewed and used to assess currentnon-employee director compensation. It was determined by the GTx Nominating and Corporate Governance and the GTx Compensation Committee that the cash compensation paid tonon-employee directors for their service to GTx was consistent with similar payments by the company’s peers, and no adjustments in cash compensation payments were needed. However, the data suggested that equity compensation was below the aggregate equity grants received by peer group board members, and the members of the GTx Nominating and Corporate Governance and the GTx Compensation Committee discussed various ways annual equity grants could be determined for 2018, including using a Black-Scholes calculation, based on an agreed stock value grant, when the annual grants were to be made to eligible directors on the date following the annual meeting of stockholders. At the March 2018 meetings of the GTx Nominating and Corporate Governance and the GTx Compensation Committee and the GTx Board, it was determined that the escalating price of GTx common stock made a predetermined future grant of a specific value less likely to achieve the desired outcome of having the annual stock option grants consistent with the equity grants in line with the 50th percentile of the equity granted tonon-employee directors of GTx’s peers, and the GTx Nominating and Corporate Governance and the GTx Compensation Committee decided to recommend to the Board that an award of stock options to acquire 7,500 shares of GTx common stock was more in line with its peers and consistent with what the Board has granted its eligible directors historically. Accordingly, the number of shares subject to the automatic annual stock option grants occurring on the date following the 2018 annual meeting of stockholders was 7,500 shares of GTx common stock. GTx’s current director compensation program will be suspended at the time of the closing of the merger and the director compensation policies for the combined organization following the merger will bere-evaluated by the compensation committee and board of directors of the combined organization following completion of the merger and may be subject to change.Non-employee directors of the combined organization are, however, expected to receive annual cash retainers and equity compensation, although the amount of such compensation has not yet been determined. Consistent with our governance practice, the Nominating and Corporate Governance Committee made this recommendation to the GTx Compensation Committee, which concurred and provided the joint recommendation to the Board for its approval. For more information on the compensation arrangements for ournon-employee directors, please see the section titled “Director Compensation” below.
Compensation Committee Charter. The GTx Compensation Committee reviews its charter on an annual basis and, if necessary, recommends changes to the GTx Board for its approval. A copy of the GTx Compensation Committee’s charter can be found on GTx’s corporate website atwww.gtxinc.com under “Investors” at “Corporate Governance.”
GTx Equity Compensation Plan Information
The following table provides certain information with respect to all of GTx’s equity compensation plans in effect as of December 31, 2018. The number of shares of common stock of GTx set forth below do not reflect the GTx Reverse Stock Split.
| Name | Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) (c) | |||||||||
Plan Category | ||||||||||||
Equity compensation plans approved by security holders | 2,335,447 | (1) | $ | 11.67 | 1,167,162 | (2) | ||||||
Equity compensation plans not approved by security holders | 122,725 | (3) | — | (3) | 37,526 | (4) | ||||||
Total | 2,458,172 | $ | 11.67 | 1,204,688 | ||||||||
| (1) | Represents shares of GTx common stock underlying stock options granted under, as applicable: (i) the GTx, Inc. 2001 Stock Option Plan (the “2001 Plan”), the GTx, Inc. 2002 Stock Option Plan (the “2002 Plan”), |
316
| and the GTx, Inc. 2004 Equity Incentive Plan, (the “2004 Plan”); (ii) the GTx, Inc. Amended and Restated 2004Non-Employee Directors’ Stock Option Plan (the “Prior Directors’ Plan”); (iii) the GTx, Inc. 2013 Equity Incentive Plan (the “2013 Plan”); and (iv) the GTx, Inc. 2013Non-Employee Director Equity Incentive Plan (the “2013 Directors’ Plan”). From and after the May 2, 2013 effective date of the 2013 Plan and 2013 Directors’ Plan, no further awards may be made under the 2001 Plan, 2002 Plan, 2004 Plan and the Prior Directors’ Plan. Stock options previously granted under the 2001 Plan, 2002 Plan, 2004 Plan and the Prior Directors’ Plan continue to be governed by the terms of the applicable plan. |
| (2) | Represents shares of GTx common stock remaining available for future issuance under the 2013 Plan and the 2013 Directors’ Plan. The total number of shares of GTx common stock available for future issuance under the 2013 Plan, upon its May 2, 2013 effective date, was initially 420,815 shares plus up to an additional 609,355 Returning Employee Shares (as defined below) as such shares become available from time to time as set forth in the 2013 Plan. “Returning Employee Shares” means the shares subject to outstanding awards granted under the Genotherapeutics, Inc. Stock Option Plan, the GTx, Inc. 2000 Stock Option Plan, the 2001 Plan, the 2002 Plan and the 2004 Plan that, from and after the May 2, 2013 effective date of the 2013 Plan, expire or terminate for any reason prior to exercise or settlement, are forfeited because of the failure to vest in those shares or are otherwise returned to the 2013 Plan share reserve pursuant to the terms of the 2013 Plan. As of December 31, 2018, an aggregate of 1,001,047 shares of GTx common stock remained available for future issuance under the 2013 Plan, plus up to an additional 203,797 Returning Employee Shares as such shares become available from time to time thereafter as set forth in the 2013 Plan. In addition, the number of shares remaining available for future issuance under the 2013 Plan automatically increases on January 1st of each year, for ten years, commencing on January 1, 2014, in an amount equal to 4% of the total number of shares of GTx common stock outstanding on December 31 of the preceding calendar year, or such lesser (or no) amount as may be approved by the GTx Board. On January 1, 2019, the number of shares available for issuance under the 2013 Plan automatically increased by 962,074 shares. The total number of shares of GTx common stock available for future issuance under the 2013 Directors’ Plan, upon its May 2, 2013 effective date, was initially 40,400 shares plus up to an additional 44,966 Returning Director Shares (as defined below) as such shares become available from time to time as set forth in the 2013 Directors’ Plan. “Returning Director Shares” means the shares subject to outstanding awards granted under the Prior Directors’ Plan that, from and after the May 2, 2013 effective date of the 2013 Directors’ Plan, expire or terminate for any reason prior to exercise or settlement, are forfeited because of the failure to vest in those shares or are otherwise returned to the 2013 Directors’ Plan share reserve pursuant to the terms of the 2013 Directors’ Plan. As of December 31, 2018, an aggregate of 166,115 shares of GTx common stock remained available for future issuance under the 2013 Directors’ Plan, plus up to an additional 15,000 Returning Director Shares as such shares become available from time to time thereafter as set forth in the 2013 Directors’ Plan. In addition, the number of shares remaining available for future issuance under the 2013 Directors’ Plan automatically increases on January 1st of each year, for ten years, commencing on January 1, 2014, in an amount equal to the lesser of 1% of the total number of shares of GTx common stock outstanding on December 31 of the preceding calendar year and 50,000 shares, or such lesser (or no) amount as may be approved by Board of Directors. On January 1, 2019, the number of shares available for issuance under the 2013 Directors’ Plan automatically increased by 50,000 shares. |
| (3) | Represents shares credited to individual director stock accounts as of December 31, 2018 under the GTx Directors’ Deferred Compensation Plan. There is no exercise price for these shares. |
| (4) | As of December 31, 2018, GTx had reserved an aggregate of 175,000 shares of GTx common stock for issuance pursuant to our Directors’ Deferred Compensation Plan. The number of shares that may become issuable under the GTx Directors’ Deferred Compensation Plan depends solely on future elections made by plan participants. As of December 31, 2018, 14,749 shares of common stock had been distributed to participants in the GTx Directors’ Deferred Compensation Plan, and 122,725 shares were then credited to individual director stock accounts under the GTx Directors’ Deferred Compensation Plan. |
317
GTX EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth certain summary information for the years indicated with respect to the compensation earned by GTx’s Chief Executive Officer and the two most highly compensated executive officers of GTx other than the Chief Executive Officer who were serving as executive officers as of December 31, 2018. These individuals are referred to in this proxy statement as GTx’s “named executive officers.”
SUMMARY COMPENSATION TABLE
Name and | Year | Salary ($)(1) | Bonus ($)(2) | Option Awards ($)(3) | Non-Equity Incentive Plan Compensation ($)(4) | All Other Compensation ($)(5) | Total ($) | |||||||||||||||||||||
Marc S. Hanover | 2018 | 445,628 | — | 659,984 | 115,863 | 22,338 | 1,243,813 | |||||||||||||||||||||
Chief Executive Officer | 2017 | 432,649 | 28,122 | 346,988 | 154,672 | 21,586 | 984,017 | |||||||||||||||||||||
Robert J. Wills | 2018 | 226,600 | — | 659,984 | 58,916 | 36,046 | 981,546 | |||||||||||||||||||||
Executive Chairman | 2017 | 220,000 | 14,300 | 346,988 | 78,650 | 31,268 | 691,206 | |||||||||||||||||||||
Henry P. Doggrell | 2018 | 389,463 | — | 456,912 | 54,525 | 24,836 | 925,736 | |||||||||||||||||||||
Vice President, Chief Legal Officer and Secretary | 2017 | 378,119 | 13,234 | 292,200 | 72,788 | 21,996 | 778,337 | |||||||||||||||||||||
| (1) | The amounts in this column represent base salary earned during the indicated fiscal year. |
| (2) | The amounts in this column represent amounts awarded as a discretionary bonus paid under the GTx Executive Bonus Compensation Plan (the “Bonus Plan”). As discussed under “—Narrative Disclosure to Summary Compensation Table—Annual Bonus Plan” below, each named executive officer was eligible for a discretionary bonus award of up to 10% of his target bonus under the Bonus Plan based on the GTx Compensation Committee’s assessment of the named executive officer’s personal performance during 2017 and 2018. No discretionary bonuses were awarded for 2018 performance. |
| (3) | The amounts in the column represent the aggregate grant date fair value of all option awards granted during 2017 and 2018 as determined in accordance with FASB ASC Topic 718. Assumptions used in computing the grant date fair values of the stock options in accordance with FASB ASC Topic 718 are set forth in Note 3—Share-Based Compensation to the GTx financial statements included elsewhere in this proxy statement/prospectus/information statement. For more information on these stock options granted in 2017 and 2018, see “—Narrative Disclosure to Summary Compensation Table—Option Awards” below. |
| (4) | Represents the amounts earned by the named executive officers under the Bonus Plan based on the attainment ofpre-established, objective performance goals approved by the GTx Compensation Committee. For more information on the GTx Bonus Plan, please see “—Narrative Disclosure to Summary Compensation Table—Annual Bonus Plan” below. |
| (5) | The amounts in this column consisted of (a) employer matching contributions to the GTx defined contribution 401(k) Plan, (b) with respect to Mr. Hanover and Mr. Doggrell only, the incremental cost of life insurance premiums to provide additional term life insurance benefits equal to up to two times each such named executive officer’s base salary along with supplemental long-term disability insurance premiums, and (c) with respect to Dr. Wills only, the following items of compensation: |
Year | Commuting Expenses Paid ($) | Tax Gross-Up Payment ($) | ||||||
2018 | 17,350 | 9,038 | ||||||
2017 | 11,820 | 8,648 | ||||||
318
Narrative Disclosure to Summary Compensation Table
Base Salary
The GTx Compensation Committee recognizes the importance of base salary as an element of compensation that helps to attract and retain the executive officers. GTx provides base salary as a fixed source of income for its executives for the services they provide to GTx during the year, and allow GTx to maintain a stable executive team.
In determining base salaries for 2018, the GTX Compensation Committee took into account that there had been no salary increases since 2016 other than in connection with certain employee promotions. After considering GTx’s capital position and the achievement of certain operational milestones during 2017, in November 2017, the GTx Compensation Committee determined that the base salaries of the GTx executive officers and other employees should be increased approximately 3% from their existing base salaries, effective January 1, 2018. The GTx Compensation Committee also approved the performance criteria for 2018 under the Bonus Plan that were tied to the attainment of certain milestones, as described in detail below. Additionally, the base salaries of the GTx executive officers were increased, effective January 1, 2018, to the following:
Named Executive Officer | 2018 Annual Base Salary ($) | |||
Marc S. Hanover | 445,628 | |||
Robert J. Wills, Ph.D. | 226,600 | (1) | ||
Henry P. Doggrell | 389,463 | |||
| (1) | Dr. Wills’ base salary reflects his agreement with GTx to work part time but to make himself available at all other times as may be required. |
Following the results of the company’s ASTRID trial, the GTx Compensation Committee did not adjust any base salaries for 2019, and determined to maintain existing executive base salary levels at the beginning of 2019 at the same levels that existed in 2018.
Annual Bonus Plan
General. The GTx Compensation Committee first established the GTx Bonus Plan in 2007 as a means of rewarding executive officers for their role in achieving specified annual or short-term performance goals. The potential for payments under the GTx Bonus Plan for any fiscal year is generally based on the attainment ofpre-established, objective performance goals approved by the GTx Compensation Committee at the beginning of the year. Each year, unless cash bonus award eligibility under the GTx Bonus Plan is suspended or eliminated for the relevant year, the GTx Compensation Committee approves the objective performance goals and specific criteria, including the weight attributable to each objective, and, if applicable, any weighting for specific categories of performance objectives, for each executive officer. The GTx Compensation Committee (as it did for bonus eligibility under the Bonus Plan for 2018) may include a subjective, discretionary bonus payment opportunity based on the GTx Compensation Committee’s assessment of the executive officer’s personal performance. Historically, the GTx Compensation Committee solicits and considers the recommendations of senior management officers in making these determinations.
The objective criteria for the GTx Bonus Plan can vary each year and may include the achievement of the operating budget for GTx, personnel-related objectives, continued innovation in development and progress towards the clinical development of GTx product candidates, timely development of new product candidates or processes, implementation of financing strategies, including licensing and/or asset dispositions that raise near-term capital for GTx and provide opportunities for increased stockholder value, the establishment of strategic alliances, partnerships or collaborations with third parties, and meeting preclinical, clinical, or regulatory objectives.
319
Although the GTx Compensation Committee typically approves the performance goals and specific criteria prior to the start of or early in the applicable calendar year, it retains the discretion to modify or otherwise change the objectives during the applicable calendar year. In addition, under the GTx Bonus Plan, the GTx Compensation Committee has the discretion to make additional bonus awards, apart from those related to the achievement of specified performance objectives.
Bonus Plan for 2018. In December 2017, the GTx Compensation Committee initially approved the performance criteria to be achieved in order for the executive officers to be eligible to receive cash bonus awards under the Bonus Plan for the performance period from January 1, 2018 through December 31, 2018. In March 2018, the GTx Compensation Committee revised the performance criteria to allocate most of the cash bonus award potential to the attainment of enrollment goals in the ASTRID trial, within a designated time period, and to the achievement of certain clinical results in the ASTRID trial. For 2018, an executive officer could have received: (i) 40% of such executive officer’s target bonus as a result of the achievement of enrollment goals in the ASTRID trial within a designated time period; (ii) 50% of such executive officer’s target bonus as a result of the achievement of certain clinical results in the ASTRID trial; and (iii) 10% of such executive officer’s target bonus related to certainpre-clinical goals related to the SARD technology. However, in the event that a strategic transaction resulted in the cancelation or modification of any of the milestone events set forth above prior to their anticipated occurrence, any such milestone events that had been canceled or modified would have been deemed to have been fulfilled and the commensurate bonus payment or payments associated with such milestone events would have become payable. Additionally, an executive officer was eligible for a bonus award of up to 10% of his or her target bonus based on the GTx Compensation Committee’s assessment of the executive officer’s personal performance. Accordingly, an executive officer’s actual total bonus award could have been awarded at a level above target. As in 2017, the potential bonus payments under the Bonus Plan for 2018 were 65% of base salary for Mr. Hanover and Dr. Wills and 35% of base salary for the other executive officers of the company. Also as in 2017, actual cash bonus awards under the Bonus Plan for 2018 generally were paid upon the achievement of the applicable performance criteria.
Fiscal Year 2018 Payouts. A bonus payment equal to approximately 40% of each named executive officer’s target bonus payment was paid in April 2018 following the achievement of the enrollment goals in the ASTRID trial. No other bonus payments tied to the objective performance criteria for 2018 were earned by the named executive officers, and no discretionary bonus payments were awarded to the GTx named executive officers. Below is a summary of each named executive officer’s target bonus and actual bonus for 2018 under the Bonus Plan:
Fiscal Year 2018 Bonus Plan Results | ||||||||||||
Named Executive Officer | Total Target Award ($) | Target Percentage (% of Base Salary) | Total Amount Actually Awarded ($) | |||||||||
Marc S. Hanover | 289,658 | 65 | 115,863 | |||||||||
Robert J. Wills, Ph.D. | 147,290 | 65 | 58,916 | |||||||||
Henry P. Doggrell | 136,212 | 35 | 54,525 | |||||||||
Bonus Plan for 2019. Following the results from the ASTRID trial, the GTx Compensation Committee determined that the executive’s focus should be on developing strategies for the Board’s consideration to maximize stockholder value, given the diminished prospects for the company, including partnering, collaborating or selling the company’s remaining assets or selling or merging the company with interested third parties. The GTx Compensation Committee felt that it was not appropriate to develop a Bonus Plan for 2019 that would reward executives for attaining any specific goals since trying to formulate a plan to realize stockholder value was deemed paramount, even if it meant that some or all company employees may lose their employment depending on the strategies the Board decided to adopt.
320
Option Awards
Option Awards for 2018. In December 2017, the GTx Compensation Committee approved the grant of stock options to purchase 65,000 shares of GTx common stock to each of Mr. Hanover and Dr. Wills, and a stock option to purchase 45,000 shares of GTx common stock to Mr. Doggrell, each of which grants was effective on January 1, 2018. The stock options vest in three equal annual installments beginning January 1, 2021, subject to continuous service, thus providing long term incentive compensation for those employees who remain with GTx and increase stockholder value. The exercise price for these stock options is $12.71 per share, the closing price of GTx’s common stock on December 29, 2017, the last trading day of 2017. The stock options expire on December 31, 2027, unless they are forfeited or expire earlier in accordance with their terms.
Option Awards for 2019. There were no stock options awarded to company employees as of January 1, 2019, due to the results of the ASTRID trial.
General Provisions of Stock Option Awards. All options granted to the GTx named executive officers may be exercised with cash, provided that the Board or the GTx Compensation Committee may provide that the exercise price may also be paid by delivery to GTx of other unencumbered shares of GTx common stock with a value equal to the aggregate option exercise price, pursuant to a cashless exercise program, or in any other form of legal consideration that may be acceptable to the Board or the GTx Compensation Committee (which may include a “net exercise” of the option). As a general matter, the vested portion of the stock options granted to the GTx named executive officers in 2018 and in previous years will expire three months after the named executive officer’s last day of service with us, subject to extension in certain termination situations as described below under “—Post-Termination Compensation—Stock Option and Equity Plan Provisions—Extended Post-Termination Option Exercise Period” below. Events that can accelerate the vesting of GTx’s stock options are described below under “—Post-Termination Compensation—Stock Option and Equity Plan Provisions—Stock Award Vesting Acceleration” below.
The number of shares of common stock of GTx underlying the foregoing options will be adjusted appropriately to reflect the GTx Reverse Stock Split.
Employment Agreements
Each of the GTx named executive officers has entered into a written employment agreement with GTx. Descriptions of the employment agreements with the GTx named executive officers are included under the caption “—Post-Termination Compensation—Employment Agreements” below.
Other Compensatory Arrangements
For a description of the other elements of the GTx executive compensation program, see “—Post-Termination Compensation—Retirement and Other Benefits.” Except for the benefits described under “—Post-Termination Compensation—Retirement and Other Benefits,” GTx does not generally provide its executive officers with any other perquisites and benefits that differ from what are provided to GTx employees generally. To date, the GTx Compensation Committee has not generally considered the provision of such additional perquisites and benefits to be a necessary element of GTx’s executive compensation program. However, GTx may, from time to time, offer certain perquisites and benefits to its executive officers not offered to the general employee population, such as commuting, relocation and temporary housing benefits. In this regard, GTx reimbursed travel-related expenses for Dr. Wills in 2018 for travel between hisout-of-state permanent residence and GTx’s headquarters in Memphis, Tennessee. Upon the recommendation of the GTx Compensation Committee, the Board also approved taxgross-up payments to Dr. Wills related to these expense reimbursements, as the reimbursements are taxable to Dr. Wills as imputed income. The GTx Compensation Committee believes that the provision of taxgross-up payments to Dr. Wills to offset the tax obligation associated with these imputed income amounts was appropriate and necessary for retaining Dr. Wills.
321
Outstanding Equity Awards at Fiscal-Year End
The following table summarizes the number of outstanding equity awards held by each of the GTx named executive officers as of December 31, 2018. There were no stock awards outstanding as of December 31, 2018. The number of shares of common stock of GTx underlying the following options will be adjusted appropriately to reflect the GTx Reverse Stock Split.
OUTSTANDING EQUITY AWARDS AT 2018 FISCAL-YEAR END
Name | Option Awards | |||||||||||||
| Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable(1) | Option Exercise Price ($) | Option Expiration Date | |||||||||||
Marc S. Hanover | | 7,000 7,000 7,000 9,000 40,000 16,667 — — — |
| | — — — — 10,000 8,333 40,000 95,000 65,000 |
(2) (3) (4) (5) (6) | | 42.00 26.50 33.60 42.00 15.60 13.30 7.00 4.71 12.71 |
| 12/31/19 12/31/20 12/31/21 12/31/22 04/02/24 06/04/24 12/31/25 02/27/27 12/31/2027 | ||||
Robert J. Wills | | — — — |
| | 40,000 95,000 65,000 | (4) (5) (6) | | 7.00 4.71 12.71 |
| 12/31/25 02/27/27 12/31/2027 | ||||
Henry P. Doggrell | | 3,500 3,500 3,500 5,500 10,000 13,334 — — — |
| | — — — — — 6,666 25,000 80,000 45,000 |
(3) (4) (5) (6) | | 42.00 26.50 33.60 42.00 18.80 13.30 7.00 4.71 12.71 |
| 12/31/19 12/31/20 12/31/21 12/31/22 09/30/23 06/04/24 12/31/25 02/27/27 12/31/2027 | ||||
| (1) | All options have a term of ten years from the date of grant. In addition to the specific vesting schedule for each stock option, each unvested stock option is subject potential future vesting acceleration as described under the heading “—Post-Termination Compensation” below. Pursuant to the Merger Agreement, all of these options will vest immediately prior to the consummation of the merger. |
| (2) | One-fifth of the shares subject to the option vested on each of April 3, 2015, April 2, 2016, April 3, 2017 and April 3, 2018, with the remaining shares vesting as toone-fifth of the shares on April 3, 2019. |
| (3) | One-third of the shares subject to the option vested on each of June 5, 2017 and June 5, 2018, with the remaining shares vesting as toone-third of the shares on June 5, 2019. |
| (4) | One-third of the shares subject to the option vested on January 1, 2019, with the remaining shares vesting as toone-third of the shares on each of January 1, 2020 and January 1, 2021. |
| (5) | One-third of the shares subject to the option will vest on each of February 28, 2020, February 28, 2021 and February 28, 2022. |
| (6) | One-third of the shares subject to the option will vest on each of January 1, 2021, January 1, 2022 and January 1, 2023. |
322
Option Exercises and Stock Vested During 2018
The following table provides information on restricted stock unit, or RSU, awards vested and the value realized, determined as described below, for the named executive officers during the year ended December 31, 2018. No stock options were exercised by the named executive officers during the year ended December 31, 2018.
| Name | Stock Awards | |||||||
| Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($)(1) | |||||||
Marc S. Hanover | 45,000 | 571,950 | ||||||
Robert J. Wills | 33,333 | 560,328 | ||||||
Henry P. Doggrell | 30,000 | 381,300 | ||||||
| (1) | The value realized on vesting is based on the number of shares underlying the RSU awards that vested and the closing price of GTx common stock on the vesting date (or, in the case of RSU awards vesting on a day that was not a trading day, the closing price of GTx common stock on the immediately preceding trading day). |
Post-Termination Compensation
GTx has entered into employment agreements with each of the GTx named executive officers. Described below are the circumstances that would trigger GTx’s obligation to make cash payments pursuant to these employment agreements following the termination of a named executive officer’s employment with GTx and the cash payments that GTx would be required to provide. We also describe below the termination and change of control events that would trigger the accelerated vesting of stock options and the extension of the post-termination exercise period with respect to those stock options.
Employment Agreements
Termination Without “Cause” or For “Good Reason” after a Change of Control
The employment agreements with the GTx named executive officers provide for cash post-termination change of control payments equal to one year’s base salary and, for those executives eligible for COBRA under federal law, monthly premium payments to continue the named executive officer’s health insurance coverage for up to 12 months following his or her termination. These change of control salary continuation and health insurance coverage benefits are structured on a “double-trigger” basis, meaning that before a named executive officer is eligible to receive such change of control benefits, (1) a change of control must occur and (2) within 12 months after such change of control, the named executive officer’s employment must be terminated without “cause” or the named executive officer must resign for “good reason.” GTx’s obligation to make the salary continuation payments and health insurance premium payments under the employment agreements is conditioned upon the former named executive officer’s compliance with the confidentiality provisions of the employment agreement and the provisions of thenon-competition provisions of the employment agreement for a period of one year following termination. In addition, GTx’s obligation to make the salary continuation payments and health insurance premium payments is conditioned upon GTx’s receipt of an effective general release of claims executed by the named executive officer. The post-termination salary continuation payments will either be made over theone-year period following termination on the regular payroll dates or in a lump sum, except that the timing of the monthly payments may be deferred for up to six months if those payments would constitute deferred compensation under Section 409A of the Internal Revenue Code (in which case, the deferred payment would be made in a lump sum following the end of the deferral period, with the balance being paid thereafter on the regular payroll dates).
323
A change of control generally means the following:
| • | the sale or other disposition of all or substantially all of GTx’s assets (including a liquidation or dissolution of GTx); |
| • | if any person or group acquires beneficial ownership of 50% or more of GTx’s voting securities (subject to certain exceptions); |
| • | a merger or consolidation of GTx with or into any other entity, if immediately after the transaction more than 50% of the voting stock of the surviving entity is held by persons who were not holders of at least 50% of GTx’s voting stock as of the effective date of the named executive officer’s employment agreement; or |
| • | a majority of the GTx Board becomes comprised of individuals whose nomination, appointment, or election was not approved by a majority of the Board members or their approved successors. |
“Cause” is generally defined as the named executive officer’s:
| • | conviction for a felony; |
| • | theft, embezzlement, misappropriation of or intentional infliction of material damage to GTx’s property or business opportunities; |
| • | breach of his or her confidentiality ornon-competition obligations, as applicable, under his or her employment agreement; or |
| • | ongoing willful neglect of or failure to perform his or her duties, or his or her ongoing willful failure or refusal to follow any reasonable, unambiguous duly adopted written direction that is not inconsistent with the description of such named executive officer’s duties, provided that such willful neglect or failure is materially damaging or materially detrimental to the business and operations of GTx, and after 30 days’ notice and the opportunity to cure. |
“Good reason” is generally defined as the following actions taken without the consent of the named executive officer after a change of control (in each case where the named executive officer has provided written notice within 30 days of the action, such action is not remedied by GTx within 30 days following such notice, and the named executive officer’s resignation is effective not later than 60 days after the expiration of such30-day cure):
| • | an adverse change in the named executive officer’s authority, duties or responsibilities (including reporting responsibilities) which, without the named executive officer’s consent, represents a material reduction in or a material demotion of the named executive officer’s authority, duties or responsibilities as in effect immediately prior to the change of control, or the assignment to the named executive officer of any duties or responsibilities that are materially inconsistent with and materially adverse to such authority, duties or responsibilities; |
| • | a material reduction in the then current base salary of the named executive officer; |
| • | the relocation of the named executive officer’s principal office to a location that increases hisone-way commute by more than 20 miles (or, in the case of Dr. Wills, a relocation outside of New Jersey); |
| • | the failure of GTx to obtain an agreement reasonably satisfactory to the named executive officer from any successor entity upon the change of control to assume and agree to perform his or her employment agreement in all material respects; or |
| • | a material breach by GTx of any provision of the named executive officer’s employment agreement or any other then-effective agreement with the named executive officer. |
Termination Without “Cause” or For “Good Reason” Prior to or Not in Connection with a Change of Control
The employment agreement with Dr. Wills provides for cash post-termination payments equal to one year’s base salary (either to be made over theone-year period following termination on the regular payroll dates or in a lump
324
sum payment) and monthly premium payments to continue his health insurance coverage for up to 12 months following his termination, should his employment be terminated without “cause” or should he resign for “good reason”, in each case irrespective of whether such termination is within 12 months after (or otherwise in connection with) a change of control.
Other Termination Scenarios
If GTx terminates a named executive officer’s employment for “cause,” or if a named executive officer voluntarily terminates his or her employment without “good reason,” or upon the death of a named executive officer, the named executive officer would generally have no right to receive any compensation or benefits under his or her employment agreement on or after the effective date of termination, other than any accrued and unpaid salary and expense reimbursement. However, under the employment agreements with Dr. Wills, Dr. Wills would nonetheless be entitled to any earned but unpaid annual bonus with respect to any completed calendar year immediately preceding his termination date. Likewise, except as described above under “—Termination Without “Cause” or For “Good Reason” Prior to or Not in Connection with a Change of Control” with respect to Dr. Wills, if GTx terminates a named executive officer’s employment without “cause,” or if a named executive officer voluntarily terminates his or her employment with “good reason,” in each case not within 12 months following a change of control, the named executive officer would have no right to receive any compensation or benefits under his employment agreement on or after the effective date of termination, other than any accrued and unpaid salary and expense reimbursement and, solely in the case of Dr. Wills, subject to GTx’s obligation under his employment agreement to pay any accrued but unpaid annual bonus with respect to any completed calendar year immediately preceding his termination date.
Other Employment Agreement Benefits
Except as set forth above, under the employment agreements with the GTx named executive officers, the named executive officers would not be entitled to any other benefits following termination of service, including the continuation of general employee benefits, life insurance coverage and long term disability coverage, except as otherwise required by applicable law.
Stock Option and Equity Plan Provisions
Stock Award Vesting Acceleration
Under the Merger Agreement, as of immediately prior to the Effective Time, the vesting of all outstanding options to purchase shares of common stock of GTx, including those held by GTx’s executive officers and directors, will accelerate in full. The number of shares of common stock of GTx underlying such options and the exercise price of such options will be adjusted appropriately to reflect the GTx Reverse Stock Split.
The terms of the GTx equity plans provide for additional accelerated exercisability that could apply in other scenarios, as described below.
2004 Plan. Our 2004 Equity Incentive Plan, or the 2004 Plan, provides that in the event of a specified corporate transaction such as a merger, consolidation or similar transaction, all outstanding options under the 2004 Plan may be assumed, continued or substituted for by any surviving or acquiring entity. If the surviving or acquiring entity elects not to assume, continue or substitute for such options, such options then held by individuals whose service has not terminated prior to the effective date of the corporate transaction would become fully vested, and, if applicable, exercisable and such options would be terminated if not exercised within 90 days of the effective date of the corporate transaction. A recipient’s award agreement may provide for acceleration upon other events. In this regard, the standard form of stock option agreement under the 2004 Plan provides for each stock option to become fully vested and exercisable if (i) the optionholder’s service with GTx or its successor terminates within twelve months after a change of control and the termination of service is a result of an involuntary termination
325
without cause or a constructive termination or (ii) the optionholder is required to resign his or her position with GTx as a condition of the change of control. For purposes of our 2004 Plan, the definition of change of control is similar to the definition of change of control under the employment agreements with our named executive officers. As a result of the adoption of the 2013 Plan, we no longer grant any equity awards under the 2004 Plan, and stock options were the only form of stock awards granted to our named executive officers under the 2004 Plan.
The standard form of stock option agreement under the 2004 Plan generally defines “cause” as the grant recipient:
| • | committing an act that materially injures the business of GTx; |
| • | refusing or failing to follow the lawful and reasonable directions of the Board or the appropriate individual to whom he or she reports, after 15 days’ notice and the opportunity to cure; |
| • | willfully or habitually neglecting his or her duties with GTx, after 15 days’ notice and the opportunity to cure; |
| • | being convicted of a felony that is likely to inflict or has inflicted material injury on the business of GTx; or |
| • | committing a material fraud, misappropriation, embezzlement or other act of gross dishonesty that resulted in material loss, damage or injury to GTx. |
The standard form of stock option agreement under the 2004 Plan generally defines a “constructive termination” as a voluntary termination within 12 months after a change of control after any of the following actions are taken without the consent of the grant recipient:
| • | the assignment to the grant recipient of any duties or responsibilities which results in a significant reduction in his or her function as in effect immediately prior to the change of control; |
| • | a material reduction in the grant recipient’s salary, as in effect on the effective date of the change of control; |
| • | the failure to continue in effect any benefit plan or program in which the grant recipient was participating immediately prior to the effective date of the change of control, or the taking of any action that would adversely affect his or her participation in (or reduce his or her benefits under) any such benefit plan or program (but either circumstance will only be grounds for a “constructive termination” if the range of benefit plans and programs offered by the acquirer is not comparable to the benefit plans previously offered by GTx, when considered as a whole); |
| • | a relocation of the grant recipient’s principal office to a location more than 50 miles from the location at which he or she performed his or her duties as of the effective date of the change of control; or |
a material breach by GTx of any provision of the grant recipient’s stock option agreement under the 2004 Plan.
2013 Plan. The GTx 2013 Plan provides that in the event of a specified corporate transaction such as a merger, consolidation or similar transaction, all outstanding stock awards under the 2013 Plan may be assumed, continued or substituted for by any surviving or acquiring entity, and any reacquisition or repurchase rights held by GTx in respect of common stock issued pursuant to outstanding stock awards may be assigned by GTx to its successor (or the successor’s parent company). If the surviving or acquiring corporation does not assume, continue or substitute any or all such outstanding stock awards, then with respect to stock awards that have not been assumed, continued or substituted and that are held by participants whose continuous service has not terminated prior to the effective time of the corporate transaction, the vesting (and, if applicable, the exercisability) of such stock awards will (contingent upon the effectiveness of the corporate transaction) be accelerated in full to a date prior to the effective time of the corporate transaction as the Board determines (or, if
326
the Board does not determine such a date, to the date that is five days prior to the effective time of the corporate transaction), such stock awards will terminate if not exercised (if applicable) at or prior to the effective time of the corporate transaction, and any reacquisition or repurchase rights held by GTx with respect to such stock awards will (contingent upon the effectiveness of the corporate transaction) lapse. Unless otherwise provided in a written agreement between GTx or an affiliate and a participant, the vesting (and, if applicable, the exercisability) of any other outstanding stock awards that are not assumed, continued or substituted in connection with the corporate transaction will not be accelerated and such stock awards will terminate if not exercised (if applicable) prior to the effective time of the corporate transaction. A recipient’s award agreement may provide for acceleration upon other events. In this regard, the standard form of stock option agreement under the 2013 Plan provides for each stock option to become fully vested and exercisable if the optionholder’s service with GTx or its successor terminates on or within 12 months after a change of control and the termination of service is a result of an involuntary termination without cause or a constructive termination. In addition, if a stock option is assumed, continued or substituted for in a change in control and a participant’s service terminates as a condition to such change in control or upon the effectiveness of the change in control, such stock option would remain exercisable for 12 months post-termination.
For purposes of the GTx 2013 Plan, the definition of change of control is similar to the definition of change of control under the employment agreements with the executive officers.
For purposes of the GTx 2013 Plan, “cause” has the meaning ascribed to such term in any written agreement between the grant recipient and GTx, and in the absence of such an agreement, “cause” means the occurrence of any of the following:
| • | the grant recipient’s willful failure substantially to perform his or her duties and responsibilities or deliberate violation of a company policy; |
| • | the grant recipient’s commission of any act of fraud, embezzlement, dishonesty or any other willful misconduct that has caused or is reasonably expected to result in material injury to GTx; |
| • | unauthorized use or disclosure by the grant recipient of any proprietary information or trade secrets of GTx or any affiliate or any other party to whom the grant recipient owes an obligation of nondisclosure as a result of the grant recipient’s relationship with GTx or any affiliate; or |
| • | the grant recipient’s willful breach of any of his or her obligations under any written agreement or covenant with GTx or any affiliate. |
The definition of a “constructive termination” in the standard form of stock option agreement under the 2013 Plan is similar to the definition of a “constructive termination” in the standard form of stock option agreement under the 2004 Plan, except that a constructive termination would also be deemed to occur if the board of GTx’s successor requires the participant to resign from GTx in a manner that terminates the participant’s continuous service, as a condition of the change in control. In addition, in order to have a basis for constructive termination under the 2013 Plan, a participant must provide written notice of the event giving rise to constructive termination to the board of GTx’s successor within 30 days following such event, provide the successor with 30 days to cure such event, and, if not cured, the participant must resign from all positions then held with GTx and its successor not later than six months after the date of the participant’s written notice to the board of the successor (or such earlier date as may be requested by the Board).
Extended Post-Termination Option Exercise Period
As a general matter, the terms of the options GTx has granted to its executive officers and directors provided that the vested portion of these options will expire three months after the executive officer’s or director’s termination of service. The period following the executive officer’s or director’s termination during which he or she can continue to exercise his or her vested stock options is referred to as the post-termination exercise period. However, in connection with the adoption of a retention bonus program by the GTx Compensation Committee in
327
September 2013, the options held by certain of the executive officers and outstanding on or prior to September 27, 2013 were modified to generally provide for a six month post-termination exercise period. In addition, a retention stock option granted to Mr. Doggrell in 2013 generally provides for a six month post-termination exercise period. All such post-termination exercise periods are limited by, and will not exceed, the original expiration date of the option. The terms of the retention benefit agreements with our executive officers will, however, be less favorable than the terms for an extension of the post-termination exercise period provided under the terms of our equity plans. Such more favorable terms will apply under the circumstances described below.
Under the GTx 2004 Plan and the form of stock option agreement under the GTx 2004 Plan, the post-termination exercise period will generally be one year following termination if the termination of service is a result of an involuntary termination without cause or a constructive termination within 12 months after a change of control. Under the GTx 2013 Plan and the form of stock option agreement under the GTx 2013 Plan, the post-termination exercise period will generally be one year following termination if the termination of service occurs either as a condition of a change of control or upon the effectiveness of a change of control, unless the stock option is not assumed, continued or replaced by the successor or acquiring entity. If the termination is a retirement, the exercise period will be two years under each of the GTx 2004 Plan and GTx 2013 Plan. Currently, Messrs. Hanover and Doggrell are retirement-eligible.
With respect to all of GTx’s stock option plans and the forms of stock option agreements under such stock option plans, if the termination is due to the named executive officer’s death, the post-termination exercise period will generally be 18 months following termination, and if the termination is due to the named executive officer’s disability, the post-termination exercise period will generally be one year following termination. With respect to the GTx 2013 Plan and the form of stock option agreement under the GTx 2013 Plan, if the termination is for cause, the option will terminate upon the date on which the event giving rise to the termination for cause first occurred (or, if required by law, the date of the termination). With respect to the GTx 2001 Plan and the GTx 2002 Plan and the forms of stock option agreements under those plans, if a named executive officer voluntarily retires his or her employment (which generally means a retirement after age 65 or after age 55 following a specified period of service), the post-termination exercise period will generally be five years following termination. However, the GTx 1999 Plan and the GTx 2000 Plan provide that the GTx Compensation Committee in its discretion can provide for any post-termination exercise period for a vested option in the event of the disability, death or involuntary termination of an option grant recipient of up to, but not exceeding, the initialten-year term of the option. Under the GTx 2004 Plan and the GTx 2013 Plan and the forms of stock option agreements under those plans, if a named executive officer voluntarily retires his or her employment (which generally means a retirement after age 65 following a specified period of service or after age 55 following a specified period of service and with the authorization of our Chief Executive Officer or the GTx Board), the post-termination exercise period will generally be two years following termination. Currently, Messrs. Hanover and Doggrell are retirement-eligible. In no event, however, will the post-termination exercise period be extended beyond the initialten-year term of the option.
The standard form of stock option agreement under the 2004 Plan generally defines “cause” as the grant recipient:
| • | committing an act that materially injures the business of GTx; |
| • | refusing or failing to follow the lawful and reasonable directions of the Board or the appropriate individual to whom he or she reports, after 15 days’ notice and the opportunity to cure; |
| • | willfully or habitually neglecting his or her duties with GTx, after 15 days’ notice and the opportunity to cure; |
328
| • | being convicted of a felony that is likely to inflict or has inflicted material injury on the business of GTx; or |
| • | committing a material fraud, misappropriation, embezzlement or other act of gross dishonesty that resulted in material loss, damage or injury to GTx. |
The standard form of stock option agreement under the 2004 Plan generally defines a “constructive termination” as a voluntary termination within 12 months after a change of control after any of the following actions are taken without the consent of the grant recipient:
| • | the assignment to the grant recipient of any duties or responsibilities which results in a significant reduction in his or her function as in effect immediately prior to the change of control; |
| • | a material reduction in the grant recipient’s salary, as in effect on the effective date of the change of control; |
| • | the failure to continue in effect any benefit plan or program in which the grant recipient was participating immediately prior to the effective date of the change of control, or the taking of any action that would adversely affect his or her participation in (or reduce his or her benefits under) any such benefit plan or program (but either circumstance will only be grounds for a “constructive termination” if the range of benefit plans and programs offered by the acquirer is not comparable to the benefit plans previously offered by GTx, when considered as a whole); |
| • | a relocation of the grant recipient’s principal office to a location more than 50 miles from the location at which he or she performed his or her duties as of the effective date of the change of control; or |
| • | a material breach by GTx of any provision of the grant recipient’s stock option agreement under the 2004 Plan. |
Retirement and Other Benefits
GTx does not provide its employees, including its named executive officers, with a defined benefit pension plan, any supplemental executive retirement plans or retiree health benefits. The GTx named executive officers may participate on the same basis as other employees in the 401(k) retirement savings plan. GTx’s 401(k) retirement savings plan provides an employer matching contribution of 100% of the first 4% of the employee’s eligible compensation, subject to the annual Internal Revenue Service limits in effect from time to time. GTx believes this matching contribution is consistent with market practice and helps in attracting and retaining key executives. The GTx 401(k) plan will be terminated prior to the closing of the merger.
GTx offers a comprehensive employee benefit program, including health, life and disability insurance, to all of its regular employees, including certain of its named executive officers who are full time employees. This program provides a safety net of protection against the financial catastrophes that can result from illness, disability or death. Company-funded life insurance of up to $50,000 is provided to employees generally, and company-funded long-term disability insurance provides a 60% income-replacement benefit, up to $10,000 per month.
The GTX Compensation Committee has also approved supplemental life and long-term disability insurance for GTx’s executive officers. The total life insurance benefit for Mr. Hanover and certain eligible Vice Presidents is equal to twice the executive officer’s annual salary, not to exceed $1 million in coverage for any officer, although Mr. Doggrell’s total coverage amount was reduced 65% following his 65th birthday. Dr. Wills, as a part time employee, does not quality for health, life or disability insurance and other similar benefits pursuant to the requirements of the insurers’ programs. However, should he in the future be deemed to be a “full time” employee by the insurers, he would also receive the same benefits as are presently provided to Mr. Hanover and eligible Vice Presidents.The GTx Compensation Committee believes that the cost of providing this supplemental insurance coverage is minimal in comparison to the value of such benefits in attracting and retaining executive employees and that providing these supplemental benefits is consistent with the practices of other public companies.
329
Compensation and Risk
In March 2018, the GTx Compensation Committee considered the GTx compensation policies, practices and programs as generally applicable to its employees and determined that its policies, practices and programs do not encourage excessive or unnecessary risk-taking, and that the level of risk that they do encourage is not reasonably likely to have a material adverse effect on GTx. The design of the GTx compensation policies and programs encourage GTx employees to remain focused on its long-term goals of increasing stockholder value through the successful development of clinical product candidates. For example, through the use of different types of equity compensation awards that provide long term incentives to increase GTx’s share price, as well as GTx’s use of multi-year vesting for stock option, GTx believes that its employee compensation programs promote a long-term stockholder perspective, encourage decisions that will result in sustainable performance over the longer term, and mitigate the risks associated with an undue short-term focus on results.
GTx Director Compensation
Cash Retainers
The GTx Board has approved the GTx Director Compensation Policy, pursuant to which the following cash compensation payments are made quarterly to the GTx Board and committee members:
| • | a $35,000 annual retainer for service as a member of the GTx Board; |
| • | a supplemental annual retainer for the Lead Director of the Board and for the Chairs of each Board committee in the following amounts: $15,000 for the Lead Director of the Board; $17,500 for Chair of the Audit Committee; $10,000 for Chair of the GTx Compensation Committee; and $8,500 for Chair of the Nominating and Corporate Governance Committee; and |
| • | a supplemental annual retainer for each member of the following committees other than the Chairs, in the following amounts: $10,000 for members of the Audit Committee; $7,500 for members of the GTx Compensation Committee; $5,000 for members of the Nominating & Corporate Governance Committee; and $10,000 for members of the Scientific and Development Committee. |
No directors currently receive consulting fees from GTx. Directors who are also employees (currently Mr. Hanover and Dr. Wills) receive no additional compensation for service on the Board.
Directors’ Deferred Compensation Plan
Since June 30, 2004, the GTxnon-employee directors have had the opportunity to defer all or a portion of their fees under the GTx Directors’ Deferred Compensation Plan. Deferrals can be made into a cash account, a stock account, or a combination of both. Deferrals into a cash account would accrue interest at the prime rate of interest announced from time to time by a local bank utilized by us, and deferrals into a stock account accrue to the deferring director rights in shares of GTx common stock equal to the cash compensation then payable to the director for his or her GTx Board service divided by the then current fair market value of GTx common stock. As of March 31, 2019, five of GTx’snon-employee directors held Deferred Stock Rights, and an aggregate of 155,426 shares of GTx common stock were issuable pursuant to the GTx Deferred Stock Rights. In addition, as of March 31, 2019, two of GTx’snon-employee directors had elected to defer compensation under the GTx Director Deferred Compensation Plan after January 3, 2019, which deferrals will be paid to thenon-employee directors at the closing in cash. Under the Directors’ Deferred Compensation Plan, amounts credited to cash or stock accounts are distributed in a single lump sum on the date, if any, selected by the director pursuant to his or her election or, if no such election is made or if the selected distribution date is after his or her separation from service, then the distribution would be made on the date of his or her separation from service in the form of a single lump sum (subject to deferral under certain circumstances to the extent necessary to avoid the incurrence of adverse personal tax consequences under Section 409A of the Internal Revenue Code). Any fractional shares of GTx common stock will be distributed in cash valued at the then current fair market value of GTx common stock.
330
Under the Merger Agreement, as of immediately prior to the Effective Time (but in no event more than 30 days prior to the Effective Time), GTx shall take all actions necessary to cause the termination and liquidation of the GTx Deferred Stock Rights. As a result, the outstanding GTx Deferred Stock Rights will be settled at the closing in shares, to the extent shares have been credited tonon-employee director stock accounts under the plan. GTx shall also ensure that any deferrals under the GTx Director Deferred Compensation Plan on or after January 3, 2019 shall be settled only in cash and that the maximum number of shares of common stock of GTx issuable upon settlement of the GTx Deferred Stock Rights shall be limited to the number of GTx Deferred Stock Rights outstanding as of the date of the Merger Agreement.
Equity Compensation
Pursuant to the GTx Director Compensation Policy, eachnon-employee director of GTx (who does not own more than ten percent of the combined voting power of GTx’s then outstanding securities) is eligible for certain initial and annual stock awards, which grants are currently made pursuant to GTx’s 2013Non-Employee Director Equity Incentive Plan (the “2013 Directors’ Plan”). Accordingly, each of thenon-employee directors, with the exception of Mr. Hyde, is eligible to receive these initial and annualnon-statutory stock awards. Under the GTx Director Compensation Policy, any individual who first becomes anon-employee director is eligible for a stock award in such form and in such amount that the Board deems necessary to attract such individual to join the Board. In addition, under the GTx Director Compensation Policy, any individual who is serving as anon-employee director on the day following an annual meeting of GTx’s stockholders automatically will be granted an option to purchase shares of common stock on that date; provided, however, that if the individual has not been serving as anon-employee director for the entire period since the preceding annual meeting, the number of shares subject to such individual’s annual grant will be reduced pro rata for each full month prior to the date of grant during which such individual did not serve as anon-employee director. In March 2018, the Board, upon the upon the recommendations of the Nominating and Corporate Governance Committee and the GTx Compensation Committee, determined that the number of shares subject to the automatic annual grants occurring on the date following the 2018 annual meeting of stockholders would be 7,500 shares of GTx common stock; accordingly, eachnon-employee director then serving as anon-employee director received a grant for 7,500 shares on the date following the 2018 annual meeting of stockholders. Following the results from the ASTRID trial, the Board made no determination about stock option grants for Board members in 2019.
The shares subject to each initial grant and each annual grant vest in a series of three successive equal annual installments measured from the date of grant, so that each initial grant and each annual grant will be fully vested three years after the date of grant. The exercise price per share for the options granted under the 2013 Directors’ Plan is not less than the fair market value of the stock on the date of grant. Prior to the adoption of the 2013 Directors’ Plan at the 2013 annual meeting of stockholders, initial and annual stock option grants were made pursuant to the Prior Directors’ Plan.
In the event of a specified corporate transaction, as defined in the Prior Directors’ Plan or the 2013 Directors’ Plan, as applicable, all outstanding options granted under the Prior Directors’ Plan and the 2013 Directors’ Plan may be assumed or substituted for by any surviving or acquiring entity. If the surviving or acquiring entity elects not to assume or substitute for such options, then (a) with respect to any such options that are held by optionees then performing services for GTx or its affiliates, the vesting and exercisability of such options will be accelerated in full and such options will be terminated if not exercised prior to the effective date of the corporate transaction, and (b) all other outstanding options will terminate if not exercised prior to the effective date of the corporate transaction. If a specified change of control transaction occurs, as defined in the Prior Directors’ Plan, then the vesting and exercisability of the optionee’s options granted under the Prior Directors’ Plan will be accelerated in full immediately prior to (and contingent upon) the effectiveness of the transaction. Under the Prior Directors’ Plan, if an optionee is required to resign his or her position as anon-employee director as a condition of the change of control transaction, the vesting and exercisability of the optionee’s options will be accelerated in full immediately prior to the effectiveness of such resignation. Under the 2013 Directors’ Plan, if a specified change of control transaction occurs, as defined in the 2013 Directors’
331
Plan, then all stock awards held by a participant whose continuous service has not terminated prior to such time will become fully vested and, if applicable, exercisable, immediately prior to the transaction. In addition, under the 2013 Directors’ Plan, if anon-employee director is required to resign his or her position as anon-employee director as a condition of the change of control transaction, all outstanding stock awards held by such individual will become fully vested and, if applicable, exercisable, as of immediately prior to such resignation. During 2008, the Board, upon the recommendation of the GTx Compensation Committee, adopted a general policy regarding the retirement ofnon-employee directors that provides that the Board will act, on acase-by-case basis, to accelerate the vesting and exercisability of the retiring director’s options in full provided such director retires from the Board in good standing.
Pursuant to the merger agreement, all outstanding unvested options held by GTx’snon-employee directors will vest upon the closing of the merger.
The table below represents the compensation earned by eachnon-employee director who served as a director on the GTx Board during 2018. Neither Mr. Hanover nor Dr. Wills are listed in the following table since they served as GTx employees during their respective term service on the GTx Board and did not receive any additional compensation for serving as members of the GTx Board. Each of Mr. Hanover’s and Dr. Wills’ compensation is described under “Executive Compensation” above.
GTX DIRECTOR COMPENSATION—FISCAL 2018
| Name | Fees Earned or Paid in Cash ($)(1) | Option Awards ($)(2) | Total ($) | |||||||||
J. R. Hyde, III | 62,500 | — | 62,500 | |||||||||
Michael G. Carter, M.D. | 65,000 | 99,197 | 164,197 | |||||||||
J. Kenneth Glass | 60,000 | 99,197 | 159,197 | |||||||||
Garry A. Neil, M.D. | 50,000 | 99,197 | 149,197 | |||||||||
Kenneth S. Robinson, M.D., M.Div. | 53,500 | 99,197 | 152,697 | |||||||||
| (1) | Represents fees earned in 2018 that were either paid, deferred or were payable at the end of 2018. Each director in the table above, other than Mr. Glass and Dr. Carter elected to defer payment of all or a portion of his earned fees during 2018 pursuant to the Directors’ Deferred Compensation Plan. The number of shares credited to individual stock accounts for the GTxnon-employee directors under the Directors’ Deferred Compensation Plan as of December 31, 2018 was as follows: 52,108 shares for Mr. Hyde; 3,631 shares for Dr. Carter; 655 shares for Mr. Glass; 22,224 shares for Dr. Neil and 44,105 shares for Dr. Robinson. |
| (2) | The amounts in this column represent the aggregate grant date fair value of all option awards granted to the GTxnon-employee directors during the year ended December 31, 2018 as computed in accordance with FASB ASC Topic 718. Assumptions used in computing the aggregate grant date fair value in accordance with FASB ASC Topic 718 are set forth in Note 3—Share-Based Compensation to the GTx financial statements included elsewhere in this proxy statement/prospectus/information statement. |
332
The following table indicates the grant date fair value for the annual option awarded to eachnon-employee director during the year ended December 31, 2018, as determined in accordance with FASB ASC Topic 718, as well as the total number of shares subject to options outstanding as of December 31, 2018 for eachnon-employee director listed in the table above. Assumptions used in computing the aggregate grant date fair value in accordance with FASB ASC Topic 718 are set forth in Note 3—Share-Based Compensation to the GTx audited financial statements included herein.
Name | FASB ASC Topic 718 Grant Date Fair Value ($) | Total Shares Subject to Options Outstanding at 12/31/2018 (#) | ||||||
J. R. Hyde, III | — | — | ||||||
J. Kenneth Glass | 99,197 | 46,500 | ||||||
Michael G. Carter, M.D. | 99,197 | 46,500 | ||||||
Garry A. Neil, M.D. | 99,197 | 28,750 | ||||||
Kenneth S. Robinson, M.D., M.Div. | 99,197 | 46,500 | ||||||
Following completion of the merger, it is expected that the combined organization will provide compensation tonon-employee directors. GTx’s current director compensation program will be suspended at the time of the closing of the merger and the director compensation policies for the combined organization following the merger will bere-evaluated by the compensation committee and board of directors of the combined organization following completion of the merger and may be subject to change.Non-employee directors of the combined organization are, however, expected to receive annual cash retainers and equity compensation, although the amount of such compensation has not yet been determined.
333
EXECUTIVE COMPENSATION OF THE COMBINED COMPANY OFFICERS
Historically, Oncternal has had two executive officers, James B. Breitmeyer, President and Chief Executive Officer, and Richard Vincent, Chief Financial Officer. In March 2019, Hazel Aker joined Oncternal as its General Counsel. Each of Dr. Breitmeyer, Mr. Vincent and Ms. Aker are expected to serve as executive officers of the combined organization after the merger. Dr. Breitmeyer and Mr. Vincent served as the only executive officers of Oncternal during 2018 and are referred to herein as Oncternal’s “named executive officers.” After completion of the merger, the compensation committee of the combined organization’s board of directors is expected to approve all compensation for the combined organization’s executive officers. For additional information regarding the combined organization’s compensation committee, please see the section entitled “Management Following the Merger—Committees of the Board of Directors—Compensation Committee” in this proxy statement/prospectus/information statement.
Summary Compensation Table
The following table sets forth certain summary information for the year indicated with respect to the compensation earned by Oncternal’s named executive officers during 2018.
SUMMARY COMPENSATION TABLE
Name and | Year | Salary ($)(1) | Bonus ($) | Option Awards ($)(2) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
James B. Breitmeyer | 2018 | 426,028 | — | 92,000 | — | — | 518,028 | |||||||||||||||||||||
Richard G. Vincent | 2018 | 206,000 | — | 40,000 | — | — | 246,000 | |||||||||||||||||||||
| (1) | The amounts in this column represent base salary or 1099 income earned during the indicated fiscal year. Mr. Vincent served as Oncternal’s Chief Financial Officer in 2018, as an independent contractor and not an employee, prior to his commencement of employment with Oncternal in January 2019. As such, the amounts reported as salary for him represent consulting fees paid to him for those services. |
| (2) | The amounts in the column represent the aggregate grant date fair value of all option awards granted during 2017 and 2018 as determined in accordance with FASB ASC Topic 718. Assumptions used in computing the grant date fair values of the stock options in accordance with FASB ASC Topic 718 are set forth in Note 5 to the Oncternal financial statements included elsewhere in this proxy statement/prospectus/information statement. For more information on these stock options granted in 2018, see “—Narrative Disclosure to Summary Compensation Table—Option Awards” below. |
Narrative Disclosure to Summary Compensation Table
Base Salary
Oncternal’s Board recognizes the importance of base salary as an element of compensation that helps to attract and retain its executive officers. Oncternal provides base salary as a fixed source of income for its executives for the services they provide to Oncternal during the year.
Dr. Breitmeyer’s base salary was increased to $475,000 from $425,000 in November 2018. Mr. Vincent’s base salary of $300,000 was established by the Oncternal Board in connection with his commencement of employment in January 2019. Mr. Vincent was a consultant during 2018 and received a retainer of $12,000 per month for his services for up to eight days per month, plus a $200 per hour retainer for any hours in excess of that commitment. In total, Mr. Vincent received $206,000 in consulting fees during 2018.
334
Annual Bonus Plan
The employment offer letters with Oncternal’s executive officers provide that they will be eligible to participate in any annual bonus plan established by the Oncternal Board. Oncternal did not establish a formal bonus plan for 2018 and none of Oncternal’s executive officers received a bonus for 2018.
Option Awards
Oncternal’s equity-based incentive awards are designed to align its interests and the interests of its stockholders with those of its employees and consultants, including the Oncternal named executive officers. The Oncternal Board or its compensation committee is responsible for approving equity grants.
In November 2018, the Oncternal Board approved the grant of stock options to Dr. Breitmeyer and Mr. Vincent under the Oncternal 2015 Plan. The terms and conditions, and the number of shares subject to these stock options, is described below in the “Outstanding Equity Awards at FiscalYear-End” table.
For a description of the accelerated vesting applicable to the stock options granted to the Oncternal named executive officers, see “—Post-Termination Compensation” and “—2015 Equity Incentive Plan”below.
Employment Agreements
Each of Oncternal’s executive officers has entered into a written employment offer letter with Oncternal. Descriptions of the employment offer letters with Oncternal’s executive officers are included under the caption “—Employment Agreements with Executive Officers and Post-Termination Compensation” below.
Other Compensatory Arrangements
Oncternal’s executive officers are eligible to participate in Oncternal’s health and welfare plans on the same terms as all employees generally, including medical, dental and vision benefits, disability insurance and life insurance.
Oncternal does not generally provide its executive officers with any other perquisites and benefits that differ from what are provided to GTx employees generally. Oncternal has not historically maintained a 401(k) plan, but intends to adopt such a plan prior to the closing of the merger.
Outstanding Equity Awards at Fiscal-Year End
The following table summarizes the number of outstanding equity awards held by each of Oncternal’s named executive officers as of December 31, 2018.
OUTSTANDING EQUITY AWARDS AT 2018 FISCAL-YEAR END
Name | Option Awards | |||||||||||||||||||||||||||
| Number of Securities Underlying Unexercised Options (#) Exercisable(1) | Number of Securities Underlying Unexercised Options (#) Unexercisable(1) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Stock Awards | |||||||||||||||||||||||
| Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($)(7) | |||||||||||||||||||||||||||
James B. Breitmeyer | | 1,300,000 — | (2)
| | 300,000 2,300,000 | (2) (3) | | — — |
| | 0.05 0.06 |
| | 8/30/2025 11/14/2028 |
| | 145,958 — | (4)
| $
| 8,757 — |
| |||||||
Richard G. Vincent | — | 1,000,000 | (2) | — | 0.06 | 11/14/2028 | | 233,333 116,923 | (5) (6) | $ $ | 14,000 7,015 |
| ||||||||||||||||
335
| (1) | All options have a term of ten years from the date of grant. In addition to the specific vesting schedule for each stock option, each unvested stock option is subject potential future vesting acceleration as described under the heading “—Post-Termination Compensation” below. |
| (2) | One-fourth of the shares subject to the option vested on the first anniversary of the date of grant and the remainder vest in equal monthly installments over thethirty-six months thereafter. |
| (3) | Subject to Dr. Breitmeyer’s continuous service as Oncternal’s Chief Executive Officer through the applicable vesting date, the option shall vest as follows: (a) 12.5% of the shares subject to the option shall vest on completion of the Phase 1 study of TK216 in Ewing sarcoma; (b) 12.5% of the shares subject to the option shall vest on completion of the Phase 1 study of TK216 in AML; (c) 12.5% of the shares subject to the option shall vest on completion of both Parts 1 and 2 of the Cirmtuzumab CLL/MCL study; (d) 12.5% of the shares subject to the option shall vest on such date as ROR1CAR-T materials are ready for human testing; (e) 12.5% of the shares subject to the option shall vest on completion of the Phase 1 study for a ROR1CAR-T; (f) 12.5% of the shares subject to the option shall vest on the consummation of a sale of Series D preferred stock, or comparable transaction, resulting in gross proceeds to Oncternal of at least $20 million in the aggregate (and if such financing is completed in tranches, satisfaction of this vesting event shall occur upon the closing of the tranche that results in the gross proceeds to Oncternal from such financing equaling or exceeding $20,000,000 in the aggregate); and (g) 25% of the options shall vest upon the occurrence of a firmly underwritten public offering of Oncternal’s common stock on a FormS-1 Registration Statement, or comparable transaction. |
| (4) | Reflects shares of restricted stock granted to Dr. Breitmeyer on February 26, 2016. The shares were subject to Oncternal’s right to repurchase any unvested shares upon any termination of Dr. Breitmeyer’s employment or service. The shares vested in equal monthly installments over thethirty-six months following the date of grant, and were fully vested in February 2019. |
| (5) | Reflects shares of restricted stock granted to Mr. Vincent on May 22, 2017. The shares are subject to Oncternal’s right to repurchase any unvested shares upon any termination of Mr. Vincent’s employment or service. The shares vest as toone-fourth of the shares on the first anniversary of the date of grant and the remainder vest in equal monthly installments over thethirty-six months thereafter. In addition, the restricted shares are subject to potential future vesting acceleration as described under the heading “—Post-Termination Compensation” below. |
| (6) | Reflects shares of restricted stock granted to Mr. Vincent on December 14, 2017. The shares are subject to Oncternal’s right to repurchase any unvested shares upon any termination of Mr. Vincent’s employment or service. The shares vest as toone-fourth of the shares on the first anniversary of the date of grant and the remainder vest in equal monthly installments over thethirty-six months thereafter. In addition, the restricted shares are subject to potential future vesting acceleration as described under the heading “—Post-Termination Compensation” below. |
| (7) | The market value of the shares is calculated using a value of $0.06 per share, which was the fair market value per share of Oncternal’s common stock as of December 31, 2018. |
Option Exercises and Stock Vested During 2018
The following table provides information on restricted stock awards vested and the value realized, determined as described below, for the Oncternal named executive officers during the year ended December 31, 2018. No stock options were exercised by the Oncternal named executive officers during the year ended December 31, 2018.
Name | Stock Awards | |||||||
| Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($)(1) | |||||||
James B. Breitmeyer | 875,712 | $ | 52,543 | |||||
Richard G. Vincent | 205,640 | $ | 12,338 | |||||
| (1) | The value realized on vesting is based on the number of shares underlying the restricted stock awards that vested and the fair market value of Oncternal’s common stock on the vesting date. |
336
Employment Agreements with Executive Officers and Post-Termination Compensation
Employment Offer Letter with James B. Breitmeyer
In May 2017, Oncternal entered into an employment offer letter with James B. Breitmeyer, Oncternal’s President and Chief Executive Officer. Dr. Breitmeyer has agreed to devote all of his working time and attention to the business affairs of Oncternal. Dr. Breitmeyer is currently entitled to an annual base salary of $475,000 and a target annual bonus in an amount to be determined by the Oncternal Board.
Dr. Breitmeyer’s employment offer letter provides for severance benefits upon a qualifying termination of employment, including modified severance benefits on a qualifying termination of employment following a change in control (each as defined below). If Oncternal terminates Dr. Breitmeyer’s employment without cause (as defined below) or if he resigns for good reason (as defined below), he is entitled to the following payments and benefits, subject to a release of claims in favor of Oncternal: (1) his fully earned but unpaid base salary through the date of termination at the rate then in effect, plus all other amounts under any compensation plan or practice of Oncternal to which he is entitled; (2) 6 months of base salary continuation payments, generally payable in accordance with Oncternal’s usual payroll practices (which amount will be increased to 12 months in the event such termination occurs following a change in control and shall be paid in a lump sum instead of in installments); and (3) continuation of health benefits at Oncternal’s expense for a maximum of the duration of the severance period.
Dr. Breitmeyer’s employment offer letter also provides for certain accelerated vesting of his outstanding stock awards (other than the restricted stock granted to him in February 2016, the accelerated vesting of which is governed by the terms of that award agreement). Specifically, if Oncternal terminates Dr. Breitmeyer’s employment without cause or if he resigns for good reason, in either case within 90 days prior to or at any time following a change in control, he will be entitled to the automatic acceleration of the vesting and exercisability of his stock options, restricted stock and such other awards (other than any restricted stock issued to him in February 2016). In addition, all of his stock options, restricted stock and such other awards (including any restricted stock issued to him in February 2016) will vest in the event of his termination of employment by reason of his death or disability. Finally, 50% of all of his stock options, restricted stock and such other awards (including any restricted stock issued to him in February 2016) will vest upon the occurrence of a change in control (as defined in the Oncternal 2015 Plan and described below). In addition, all of his restricted stock issued to him in February 2016 will vest in the event of his termination without cause or resignation for good reason following a change in control (and the terms “cause,” “good reason” and “change in control” have substantially the same definitions as given to such terms in his employment offer letter and described below).
The severance benefits prescribed by Dr. Breitmeyer’s employment offer letter are subject to a Section 280Gbetter-off cutback provision, which provides that, in the event that the benefits provided to him pursuant to the employment offer letter or otherwise constitute parachute payments with the meaning of Section 280G of the Code, the severance benefits will either be delivered in full or reduced to the extent necessary to avoid an excise tax under Section 4999 of the Code, whichever would result in him receiving the largest amount of severance benefits on anafter-tax basis.
Employment Offer Letter with Richard G. Vincent
In January 2019, Oncternal entered into an employment offer letter with Richard G. Vincent, Oncternal’s Chief Financial Officer. Mr. Vincent has agreed to devote 80% of his working time and attention to the business affairs of Oncternal. Mr. Vincent is currently entitled to an annual base salary of $300,000 and a target annual bonus in an amount to be determined by the Oncternal Board.
Mr. Vincent’s employment offer letter provides for severance benefits upon a qualifying termination of employment, including modified severance benefits on a qualifying termination of employment following a change in control (each as defined below). If Oncternal terminates Mr. Vincent’s employment without cause (as
337
defined below) or if he resigns for good reason (as defined below), he is entitled to the following payments and benefits, subject to a release of claims in favor of Oncternal: (1) his fully earned but unpaid base salary through the date of termination at the rate then in effect, plus all other amounts under any compensation plan or practice of Oncternal to which he is entitled; (2) 6 months of base salary continuation payments, generally payable in accordance with Oncternal’s usual payroll practices (which amount will be increased to 12 months in the event such termination occurs following a change in control (as defined in the Oncternal 2015 Plan and described below) and shall be paid in a lump sum instead of in installments); and (3) continuation of health benefits at Oncternal’s expense for a maximum of the duration of the severance period.
Mr. Vincent’s employment offer letter also provides for certain accelerated vesting of his outstanding stock awards. Specifically, if Oncternal terminates Mr. Vincent’s employment without cause or if he resigns for good reason, in either case within 90 days prior to or at any time following a change in control, he will be entitled to the automatic acceleration of the vesting and exercisability of his stock options, restricted stock and such other awards. In addition, all of his stock options, restricted stock and such other awards will vest in the event of his termination of employment by reason of his death or disability. Finally, 50% of all of his stock options, restricted stock and such other awards will vest upon the occurrence of a change in control.
The severance benefits prescribed by Mr. Vincent’s employment offer letter are subject to a Section 280Gbetter-off cutback provision, which provides that, in the event that the benefits provided to him pursuant to the employment offer letter or otherwise constitute parachute payments with the meaning of Section 280G of the Code, the severance benefits will either be delivered in full or reduced to the extent necessary to avoid an excise tax under Section 4999 of the Code, whichever would result in him receiving the largest amount of severance benefits on anafter-tax basis.
Consulting Agreement with Richard Vincent
Prior to his commencement of employment in January 2019, Mr. Vincent provided services to Oncternal as its Chief Financial Officer pursuant to a consulting agreement that Oncternal executed with Mr. Vincent in April 2017. Pursuant to the consulting agreement, Oncternal paid Mr. Vincent at the rate of $12,000 per month for his services for up to eight days per month, plus a $200 per hour retainer for any hours in excess of that commitment. Oncternal also provided Mr. Vincent with reimbursement for reasonable business expenses in connection with his services. The consulting agreement was terminated upon his conversion to employment on January 1, 2019.
Employment Offer Letter with Hazel Aker
In March 2019, Oncternal entered into an employment offer letter with Hazel Aker, Oncternal’s General Counsel. Ms. Aker has agreed to devote half of her working time and attention to the business affairs of Oncternal. Ms. Aker is currently entitled to an annual base salary of $150,000 and a target annual bonus in an amount to be determined by the Oncternal Board.
Defined Terms Applicable to Executive Employment Offer Letters
For purposes of the executive employment offer letters, “cause” generally means an executive officer’s (a) unauthorized use or disclosure of confidential information or trade secrets of Oncternal or any material breach of a written agreement between executive and Oncternal, including without limitation a material breach of any employment, consulting, confidentiality,non-compete,non-solicit or similar agreement; (b) the executive’s commission of, indictment for of the entry of a pleas of guilty ornolocontendereto, a felony under the laws of the United States; (c) the executive’s gross negligence or willful misconduct or executive’s willful or repeated failure or refusal to substantially perform assigned duties; or (d) any act of fraud, embezzlement, material misappropriation or dishonesty committed by the executive against Oncternal.
For purposes of the executive employment offer letters, “good reason” generally means the occurrence of any of the following events without executive’s consent: (a) a change in the executive’s position with Oncternal (or its
338
subsidiary employing executive) that materially reduces the executive’s authority, duties or responsibilities; (b) a material diminution in the level of the executive’s base compensation, except in connection with a general reduction in the base compensation of Oncternal’s personnel of similar status and responsibilities; (c) a relocation of the executive’s place of employment by more than 50 miles, provided that such change, reduction or relocation is effected by Oncternal (or its subsidiary employing executive) without the executive’s consent; (d) any material breach by Oncternal of its obligations to the executive under the executive’s employment offer letter with Oncternal. Notwithstanding the foregoing, good reason shall only exist if the executive has provided Oncternal with written notice within 60 days of the initial occurrence of any of the foregoing events or conditions, and Oncternal or any successor or affiliate fails to eliminate the conditions constituting good reason within 30 days after receipt of written notice of such event or condition from the executive. An executive’s resignation from employment with Oncternal for good reason must occur within six months following the initial occurrence of one of the foregoing events or conditions.
For purposes of the executive employment offer letters, “change in control” generally means (i) a merger or consolidation of Oncternal with or into any other corporation or other entity or person, (ii) a sale, lease, exchange or other transfer in one transaction or a series of related transactions of all or substantially all of Oncternal’s assets, or (iii) any other transaction, including the sale by Oncternal of new shares of its capital stock or a transfer of existing shares of capital stock of Oncternal, the result of which is that a third-party that is not an affiliate of Oncternal or its stockholders (or a group of third parties not affiliated with Oncternal or its stockholders) immediately prior to such transaction acquires or holds capital stock of Oncternal representing a majority of Oncternal’s outstanding voting power immediately following such transaction; provided that the following events shall not constitute a change in control: (A) a transaction (other than a sale of all or substantially all of Oncternal’s assets) in which the holders of the voting securities of Oncternal immediately prior to the merger or consolidation hold, directly or indirectly, at least a majority of the voting securities in the successor corporation or its parent immediately after the merger or consolidation; (B) a sale, lease, exchange or other transaction in one transaction or a series of related transactions of all or substantially all of Oncternal’s assets to an affiliate of Oncternal; (C) an initial public offering of any of Oncternal’s securities; (D) a reincorporation of Oncternal solely to change its jurisdiction; or (E) a transaction undertaken for the primary purpose of creating a holding company that will be owned in substantially the same proportion by the persons who held Oncternal’s securities immediately before such transaction. The merger will not constitute a change in control of Oncternal for these purposes.
Oncternal 2015 Equity Incentive Plan
The Oncternal Board and the stockholders of Oncternal approved the Oncternal Therapeutics, Inc. 2015 Equity Incentive Plan (the “Oncternal 2015 Plan”), in July 2015. The Oncternal 2015 Plan was further amended in May 2016 and September 2018, both of which amendments where approved by both the Oncternal Board and the stockholders of Oncternal.
Pursuant to the Merger Agreement, at the Effective Time, each outstanding and unexercised option to purchase shares of Oncternal common stock issued under the Oncternal 2015 Plan will be assumed by GTx, and become an option to purchase that number of shares of GTx common stock equal to the product obtained by multiplying (i) the number of shares of Oncternal common stock that were subject to such option immediately prior to the Effective Time by (ii) the exchange ratio, rounded down to the nearest whole share. The per share exercise price for shares of GTx common stock issuable upon exercise of each Oncternal option assumed by GTx shall be determined by dividing (a) the per share exercise price of Oncternal common stock subject to such Oncternal option, as in effect immediately prior to the Effective Time, by (b) the exchange ratio, rounded up to the nearest whole cent. Following the Effective Time, the combined organization intends to terminate the Oncternal 2015 Plan and, accordingly, no shares will be available for future issuance under the Oncternal 2015 Plan following the Effective Time. Notwithstanding the foregoing, the Oncternal 2015 Plan will continue to govern outstanding awards granted thereunder. As of March 31, 2019, a total of 8,600,000 shares were reserved for issuance under the Oncternal 2015 Plan and awards covering 6,843,251 shares of Oncternal common stock remained outstanding under the Oncternal 2015 Plan.
339
The following is only a summary of the material terms of the Oncternal 2015 Plan, is not a complete description of all provisions of the Oncternal 2015 Plan and should be read in conjunction with the Oncternal 2015 Plan, which is filed as an exhibit to the registration statement on FormS-4 of which this proxy statement/prospectus/information statement forms a part.
Administration. The Oncternal Board administers the Oncternal 2015 Plan. Subject to the terms and conditions of the Oncternal 2015 Plan, the administrator has the authority to select the persons to whom awards are to be made, to determine the type or types of awards to be granted to each person, determine the number of awards to grant, determine the number of shares to be subject to such awards, and the terms and conditions of such awards, and make all other determinations and decisions and to take all other actions necessary or advisable for the administration of the Oncternal 2015 Plan. The plan administrator is also authorized to establish, adopt, amend or revise rules relating to administration of the Oncternal 2015 Plan, subject to certain restrictions.
Eligibility. Options, restricted stock, restricted stock units and other awards under the Oncternal 2015 Plan were able to be granted to individuals who were Oncternal’s employees, consultants and members of the Oncternal Board at the time of grant. Only employees were eligible to be granted incentive stock options.
Awards. The Oncternal 2015 Plan provides that the administrator may grant or issue stock options, restricted stock, restricted stock units, other stock-based awards, or any combination thereof. Each award is set forth in a separate agreement with the person receiving the award and indicates the type, terms and conditions of the award.
| • | Non-Qualified Stock Option. NQSOs provide for the right to purchase shares of Oncternal common stock at a specified price which may not be less than the fair market value of a share of stock on the date of grant, and usually will become exercisable in one or more installments after the grant date, subject to the participant’s continued employment or service with Oncternal and/or subject to the satisfaction of performance targets established by the plan administrator. NQSOs may be granted for any term specified by the plan administrator, but the term may not exceed ten years. |
| • | Incentive Stock Option. Incentive stock options, or ISOs, are designed to comply with the provisions of the Code and are subject to specified restrictions contained in the Internal Revenue Code applicable to ISOs. Among such restrictions, ISOs must have an exercise price of not less than the fair market value of a share of common stock on the date of grant, may only be granted to employees, must expire within a specified period of time following the optionee’s termination of employment, and must be exercised within the ten years after the date of grant. In the case of an ISO granted to an individual who owns (or is deemed to own) more than 10% of the total combined voting power of all classes of Oncternal capital stock on the date of grant, the Oncternal 2015 Plan provides that the exercise price must be at least 110% of the fair market value of a share of common stock on the date of grant and the ISO must expire on the fifth anniversary of the date of its grant. |
| • | Restricted Stock. Restricted stock may be granted to participants and made subject to such restrictions as may be determined by the administrator. Typically, restricted stock may be repurchased by Oncternal at the original purchase price or, if no cash consideration was paid for such stock, forfeited for no consideration if the conditions or restrictions are not met, and the restricted stock may not be sold or otherwise transferred to third parties until restrictions are removed or expire. Recipients of restricted stock, unlike recipients of options, may have voting rights and may receive dividends, if any, prior to when the restrictions lapse. |
| • | Restricted Stock Units. Restricted stock units may be awarded to participants, typically without payment of consideration or for a nominal purchase price, but subject to vesting conditions including continued employment or performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold or otherwise transferred or hypothecated until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until sometime after the restricted stock units have vested, and recipients of restricted stock units |
340
generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied and the shares have been issued. |
| • | Other Stock-Based Awards. Other stock-based awards may entitle participants to receive shares of Oncternal common stock in the future. Other stock-based awards may also be a form of payment in the settlement of other awards granted under the Oncternal 2015 Plan, as stand-alone payments and/or as payment in lieu of compensation to which a participant is otherwise entitled. Other stock-based awards may be paid in shares of Oncternal common stock, cash or other property, as the plan administrator shall determine. |
Corporate Transactions. In the event of a change of control where the acquirer does not assume awards granted under the Oncternal 2015 Plan, awards issued under the Oncternal 2015 Plan will be subject to accelerated vesting such that 100% of the awards will become vested and exercisable or payable, as applicable, immediately prior to the change in control. Under the Oncternal 2015 Plan, a change of control is generally defined as:
| • | a merger or consolidation of the company with or into any other corporation or other entity or person; |
| • | a sale, lease, exchange or other transfer in one transaction or a series of related transactions of all or substantially all of the Company’s assets; or |
| • | any other transaction, including the sale by Oncternal of new shares of Oncternal capital stock or a transfer of existing shares of Oncternal’s capital stock, the result of which is that a third-party that is not an affiliate of Oncternal or its shareholders (or a group of third parties not affiliated with Oncternal or its shareholders) immediately prior to such transaction acquires or holds capital stock representing a majority of Oncternal’s outstanding voting power immediately following such transaction; |
provided that the following events shall not constitute a “change in control” under the Oncternal 2015 Plan:
| • | a transaction (other than a sale of all or substantially all of Oncternal’s assets) in which the holders of Oncternal’s voting securities immediately prior to the merger or consolidation hold, directly or indirectly, at least a majority of the voting securities in the successor corporation or its parent immediately after the merger or consolidation; |
| • | a sale, lease, exchange or other transaction in one transaction or a series of related transactions of all or substantially all of Oncternal’s assets to an affiliate of Oncternal; |
| • | an initial public offering of any of Oncternal’s securities; |
| • | a reincorporation solely to change Oncternal’s jurisdiction; or |
| • | a transaction undertaken for the primary purpose of creating a holding company that will be owned in substantially the same proportion by the persons who held Oncternal’s securities immediately before such transaction. |
The merger will not constitute a change in control of Oncternal for these purposes.
Amendment and Termination of the Oncternal 2015 Plan. The Oncternal Board may amend or modify the Oncternal 2015 Plan. However, stockholder approval of any amendment to the Oncternal 2015 Plan must be obtained to the extent necessary and desirable to comply with any applicable law, regulation or stock exchange rule. The administrator may, with the consent of the affected option holders, cancel any or all outstanding awards under the Oncternal 2015 Plan and grant new awards in substitution. Following the closing of the merger, no additional awards will be granted under the Oncternal 2015 Plan.
Oncternal Director Compensation
For the fiscal year ended December 31, 2018, Oncternal did not have a formal director compensation policy in place. Only David Hale and Charles Theuer received any compensation during 2018 for their service as Oncternalnon-employee directors.
341
In May 2018, in connection with his commencement of service on the Oncternal Board, Charles Theuer, M.D., Ph.D. received 200,000 shares of restricted stock, which shares will vest over four years, with 25% vesting on the first anniversary of the date of grant, and the remaining shares vesting in equal monthly installments over thethirty-six months thereafter, subject to Dr. Theuer’s continued service as a member of the Oncternal Board on each such vesting date.
Effective December 1, 2018, the Oncternal Board approved a compensation arrangement with David Hale pursuant to which he will receive a cash retainer of $12,000 per month for his service asnon-executive Chairman of the Board.
Following completion of the merger, it is expected that the combined organization will provide compensation tonon-employee directors. GTx’s current director compensation program will be suspended at the time of the closing of the merger and the director compensation policies for the combined organization following the merger will bere-evaluated by the compensation committee and board of directors of the combined organization following completion of the merger and may be subject to change.Non-employee directors of the combined organization are, however, expected to receive annual cash retainers and equity compensation, although the amount of such compensation has not yet been determined.
The table below represents the compensation earned by Oncternal’snon-employee directors who served on the Oncternal Board during 2018 who will also serve as directors of the combined organization following the merger. Dr. Breitmeyer is not listed in the following table since he served as an employee of Oncternal during 2018 as well as a member of the Oncternal Board and did not receive any additional compensation for serving as a member of the Oncternal Board. Dr. Breitmeyer’s compensation is described under “Executive Compensation of the Executive Officers of the Combined Organization” above.
ONCTERNAL DIRECTOR COMPENSATION—FISCAL 2018
Name | Fees Earned or Paid in Cash ($) | Stock Awards ($)(1) | Total ($) | |||||||||
Scott Glenn | — | — | — | |||||||||
David F. Hale | 12,000 | — | 12,000 | |||||||||
William R. LaRue | — | — | — | |||||||||
Yanjun Liu | — | — | — | |||||||||
Xin Nakanishi | — | — | — | |||||||||
Charles Theuer, M.D., Ph.D. | — | 10,000 | 10,000 | |||||||||
| (1) | The amounts in this column represent the aggregate grant date fair value of the stock awards granted to Dr. Theuer during the year ended December 31, 2018 as computed in accordance with FASB ASC Topic 718. Assumptions used in computing the aggregate grant date fair value in accordance with FASB ASC Topic 718 are set forth in Note 5 to the Oncternal financial statements included elsewhere in this proxy statement/prospectus/information statement. As of December 31, 2018, Mr. LaRue held 165,000 shares of unvested Oncternal restricted stock and Dr. Theuer held 200,000 restricted shares of Oncternal common stock. None of the other Oncternalnon-employee directors listed in the table above held any unvested equity awards as of December 31, 2018. |
342
RELATED PARTY TRANSACTIONS OF DIRECTORS AND EXECUTIVE OFFICERS OF THE COMBINED ORGANIZATION
Described below are any transactions occurring since January 1, 2017 and any currently proposed transactions to which either GTx or Oncternal was a party and in which
| • | the lesser of $120,000 or 1% of the average of the total assets atyear-end for the last two completed fiscal years; and |
| • | a director, executive officer, holder of more than 5% of the outstanding capital stock of GTx or Oncternal, or any member of such person’s immediate family had or will have a direct or indirect material interest. |
GTx Transactions
Policies and Procedures for Review of Related Party Transactions
The GTx Board adopted a related party transactions policy, which specifies GTx’s policies and procedures regarding transactions between GTx and its employees, officers, directors or their family members. GTx’s Chief Legal Officer is responsible for (a) ensuring that policy is distributed to all GTx officers, directors and other managers and (b) requiring that any proposed related party transaction be presented to the GTx Audit Committee for consideration before GTx enters into any such transactions. This policy can be found on GTx’s website (www.gtxinc.com) under “Investors” at “Corporate Governance.”
It is the policy of GTx to prohibit all related party transactions unless GTx Audit Committee determines in advance of GTx entering into any such transaction that there is a compelling business reason to enter into such a transaction. There is a general presumption that GTx Audit Committee will not approve a related party transaction with GTx. However, GTx Audit Committee may approve a related party transaction if:
| • | it finds that there is a compelling business reason to approve the transaction, taking into account such factors as the absence of other unrelated parties to perform similar work for a similar price within a similar timeframe; and |
| • | it finds that it has been fully apprised of all significant conflicts that may exist or otherwise arise on account of the transaction, and it believes, nonetheless, that GTx is warranted entering into the related party transaction and has developed an appropriate plan to manage the potential conflicts of interest. |
Certain Transactions with or Involving Related Persons
Except where specifically noted, the following information and all other information contained in this proxy statement/prospectus/information statement do not give effect to the GTx Reverse Stock Split.
Employment Arrangements.For information on employment arrangements and compensation for service on the board of directors of GTx, see “GTx Executive Compensation” and “GTx Director Compensation—Fiscal 2018” above.
Warrant Exercises.On November 14, 2014, GTx issued warrants (the “BVF Warrants”), to Biotechnology Value Fund, L.P., Biotechnology Value Fund II, L.P., Investment 10, L.L.C. and MSI BVF SPV, LLC, or collectively, the BVF Entities, to purchase an aggregate of 1,111,081 (whole) shares of GTx common stock (as adjusted to give effect to the 2016 Reverse Stock Split) at an exercise price of $8.50 per share (as adjusted to give effect to the 2016 Reverse Stock Split) in connection with a private placement of our common stock and warrants to purchase common stock. On March 13, 2018, the BVF Entities exercised the BVF Warrants in full pursuant to the “net exercise” provisions of the BVF Warrants resulting in a net issuance on exercise to the BVF Entities of an aggregate of 674,579 shares of GTx common stock. Based solely on the difference between the fair market
343
value of GTx common stock on the date of exercise as determined pursuant to the net exercise provisions of the BVF Warrants and the exercise price of the BVF Warrants, the value realized by the BVF Entities upon exercise of the BVF Warrants totaled approximately $14.6 million. GTx’s involvement in the BVF Warrant exercises did not require approval under its related party transactions policy because GTx’s actions with respect to such matters were undertaken in accordance with itspre-existing obligations under the BVF Warrants.
Loan Agreement.On August 10, 2017, GTx entered into a loan agreement with J.R. Hyde, III and The Pyramid Peak Foundation to borrow up to a total of $15,000,000. Each of Mr. Hyde and The Pyramid Peak Foundation are significant stockholders, and Mr. Hyde serves on the GTx Board. GTx did not borrow any amounts under the loan agreement and the loan agreement terminated in accordance with its terms on September 29, 2017 in connection with the completion of the September 2017 private placement of GTx equity securities described below.
September 2017 Private Placement and Related Registration.On September 29, 2017, GTx completed a private placement of an aggregate of 5,483,320 immediately separable units, comprised of an aggregate of 5,483,320 shares of GTx common stock and warrants to purchase up to an aggregate of 3,289,988 additional shares of GTx common stock, for an aggregate purchase price of approximately $48.5 million. The per unit purchase price for a share of common stock and a warrant to purchase 0.6 of a share of common stock was $8.845. The warrants, which have a five-year term expiring on September 29, 2022, are immediately exercisable and have a per share exercise price of $9.02. Pursuant to the terms of the securities purchase agreement, GTx filed a registration statement with the SEC in November 2017 to register the resale of the shares of GTx common stock and the shares of common stock underlying the warrants, and agreed to keep one or more registration statements registering the shares effective until the earlier to occur of September 28, 2019 or the date on which all of the applicable shares of GTx common stock have been sold or can be sold publicly without restriction or limitation under Rule 144 under the Securities Act. GTx’s total expenses in connection with the filing of the November 2017 registration statement were approximately $70,000. The investors in the private placement included the following related parties:
Investor | Shares Purchased | Warrants Purchased | Aggregate Unit Purchase Price ($) | |||||||||
J.R. Hyde III(1) | 1,130,582 | 678,349 | 9,999,997.79 | |||||||||
The Pyramid Peak Foundation(1) | 565,291 | 339,174 | 4,999,998.90 | |||||||||
Jack W. Schuler(1) | 226,116 | 135,669 | 1,999,996.02 | |||||||||
Amzak Health Investors, LLC(2)(3) | 847,936 | 508,761 | 7,499,993.92 | |||||||||
Aisling Capital IV LP(2) | 847,936 | 508,761 | 7,499,993.92 | |||||||||
Boxer Capital, LLC | 565,291 | 339,174 | 4,999,998.90 | |||||||||
| (1) | Executive officer, director and/or greater than 5% stockholder (and a “related party”) of GTx immediately prior to the private placement. Mr. Schuler is no longer a stockholder of record of our capital stock. |
| (2) | Became a greater than 5% stockholder of GTx as a result of the private placement and, accordingly, became a “related party” of GTx. Amzak Health Investors, LLC and Aisling Capital IV LP are no longer stockholders of record of our capital stock. |
| (3) | Pursuant to the terms of the securities purchase agreement, GTx reimbursed Amzak Health Investors for its legal fees in the amount of $33,078. |
The GTx Board appointed a “Special Committee” of the board of directors consisting of disinterested and independent directors to review and evaluate the private placement and any other alternative transaction to the private placement, and delegated to the Special Committee the exclusive power and authority to consider, negotiate, disapprove or approve the private placement, which the Special Committee ultimately determined to approve. Likewise, as a result of the participation of related parties in the private placement, the private placement was reviewed andpre-approved by the Audit Committee in accordance with GTx’s related party transactions policy.
344
Indemnity Agreements
GTx has entered into indemnity agreements with each of its current directors and certain of its executive officers to give such directors and officers additional contractual assurances regarding the scope of the indemnification set forth in GTx’s charter and bylaws and to provide additional procedural protections.
Oncternal Transactions
Affiliations with Principal Stockholders
Yanjun Liu, M.D., Ph.D. and Xin Nakanishi, Ph.D. are members of the Oncternal Board and are affiliated with SPH. SPH USA is the wholly-owned subsidiary of SPH. and holds more than 5% of Oncternal’s outstanding capital stock. For more information see the section entitled “Principal Shareholders of Oncternal” of this proxy statement/prospectus/information statement.
Cooper Collins is a member of the Oncternal Board and the manager of MagnaSci GP and MagnaSciCo-Investments. MagnaSci GP and MagnaSciCo-Investments and their affiliates holds more than 5% of Oncternal’s outstanding capital stock. For more information see the section entitled “Principal Shareholders of Oncternal” of this proxy statement/prospectus/information statement.
SPH USA License Agreement
In November 2018, Oncternal entered into a license and development agreement the (“SPH USA License Agreement”) with SPH USA, under which Oncternal granted rights to manufacture, develop, market, distribute and sell in the People’s Republic of China, Hong Kong, Macau, and Taiwan Oncternal’s product candidates under the Georgetown License Agreement and the Regents License Agreement. For more information see the section entitled “Oncternal Business—Licenses and Collaborative Relationships” of this proxy statement/prospectus/information statement.
Voting Agreements
In connection with the issuance of Oncternal’s SeriesC preferred stock in November 2018, Oncternal entered into an amended and restated voting agreement with certain directors, executive officers and stockholders, and their affiliates. As a condition to the closing of the merger, the amended and voting agreement must be terminated prior to the Effective Time.
Oncternal has also entered into voting agreements in connection with the merger with certain directors, executive officers and stockholders, and their affiliates. SPH USA, which holds 100% of the outstanding Series C preferred stock and which represents approximately 20.9% of the outstanding shares of Oncternal capital stock on as converted common stock basis, has not executed a voting agreement. For a description of these voting agreements, please see the section entitled “Agreements Related to the Merger—Voting Agreements” of this proxy statement/prospectus/information statement.
Investors’ Rights Agreement
In connection with the issuance of Oncternal’s SeriesC preferred stock in November 2018, Oncternal entered into an amended and restated investors’ rights agreement, including with certain directors, executive officers and stockholders, and their affiliates, which provides that certain holders of common stock (including those issuable upon conversion of Oncternal preferred stock and capital stock underlying warrants) have certain rights relating to the registration of shares of such common stock.
In addition to such registration rights, the amended and restated investors’ rights agreement provides for certain information rights andpre-emptive rights. As a condition to the closing of the merger, the amended and restated investors’ rights agreement must be terminated prior to the Effective Time.
345
Co-Sale Agreement
In connection with the issuance of Oncternal’s SeriesC preferred stock in November 2018, Oncternal entered into an amended and restatedco-sale agreement, including with certain directors, executive officers and stockholders, and their affiliates. As a condition to the closing of the merger, the amended and restated right of first refusal andco-sale agreement must be terminated prior to the Effective Time.
Indemnification Agreements
Oncternal has entered into indemnification agreements with each of its officers and directors and purchased directors’ and officers’ liability insurance. The indemnification agreements and bylaws of Oncternal require Oncternal to indemnify its directors and officers to the fullest extent permitted under Delaware law.
Series C Financing
In November 2018, Oncternal issued and sold in a closing an aggregate of 34,000,000 shares of series C preferred stock at a price per share of $0.50 for aggregate consideration of approximately $17.0 million to SPH USA. It is a condition to the completion of the merger that each outstanding share of Oncternal’s series C preferred stock will convert into one share of Oncternal common stock.
Compensation of Mary Breitmeyer
Mary Breitmeyer, who is Dr. Breitmeyer’s spouse, is a part-time employee of Oncternal. Ms. Breitmeyer receives compensation for her services as an employee. Ms. Breitmeyer’s current base salary is $74,675 per year. During 2018, Ms. Breitmeyer received total cash compensation of $72,500 from Oncternal.
Policy for Approval of Related Person Transactions
While Oncternal does not have a formal written policy or procedure for the review, approval or ratification of related party transactions, the Oncternal Board reviews and considers the interests of its directors, executive officers and principal stockholders in its review and consideration of transactions and obtains the approval ofnon-interested directors when it determines that such approval is appropriate under the circumstances.
346
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The unaudited pro forma net loss and book value per share does not give effect to the GTx Reverse Stock Split described in Proposal No. 2 in this proxy statement/prospectus/information statement.
The following unaudited pro forma condensed combined financial information was prepared using the acquisition method of accounting under U.S. GAAP. For accounting purposes, Oncternal is considered to be acquiring GTx and the merger is expected to be accounted for as an asset acquisition. Oncternal is considered the accounting acquirer even though GTx will be the issuer of the common stock in the merger. To determine the accounting for this transaction under U.S. GAAP, a company must assess whether an integrated set of assets and activities should be accounted for as an acquisition of a business or an asset acquisition. The guidance requires an initial screen test to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single asset or group of similar assets. If that screen is met, the set is not a business. In connection with the acquisition of GTx, substantially all the fair value is included inin-process research and development (“IPR&D”) and, as such, the acquisition is expected to be treated as an asset acquisition.
The unaudited pro forma combined balance sheet data assume that the merger took place on December 31, 2018, and combines the historical balance sheets of GTx and Oncternal as of such date. The unaudited pro forma condensed combined statement of operations data assume that the merger took place as of January 1, 2018, and combines the historical results of GTx and Oncternal for the year ended December 31, 2018. The unaudited pro forma condensed combined financial information was prepared in accordance with U.S. GAAP and pursuant to the rules and regulations of Article 11 of SEC RegulationS-X. The historical financial statements of GTx and Oncternal have been adjusted to give pro forma effect to events that are (i) directly attributable to the transaction, (ii) factually supportable, and (iii) with respect to the unaudited pro forma condensed combined statement of operations, expected to have a continuing impact on the combined company’s results.
GTx’s assets and liabilities will be measured and recognized at their relative fair values allocation as of the transaction date with any value associated with IPR&D being expensed as there is no alternative future use, and combined with the assets, liabilities and results of operations of Oncternal after the consummation of the merger.
The unaudited pro forma condensed combined financial information is based on the assumptions and adjustments that are described in the accompanying notes. The accounting for the transaction as an asset acquisition is dependent upon the valuation of the IPR&D, which has yet to be completed. Accordingly, the pro forma adjustments are preliminary, subject to further revision as additional information becomes available and additional analyses are performed, and have been made solely for the purpose of providing unaudited pro forma condensed combined financial information. Differences between these preliminary estimates and the final accounting, expected to be completed after the closing of the merger, will occur and these differences could have a material impact on the accompanying unaudited pro forma condensed combined financial information and the combined company’s future results of operations and financial position. In addition, differences between the preliminary and final amounts will likely occur as a result of the amount of cash used for GTx’s operations, changes in the fair value of GTx common stock, and other changes in GTx’s assets and liabilities.
The unaudited pro forma condensed combined financial information does not give effect to the potential impact of current financial conditions, regulatory matters, operating efficiencies or other savings or expenses that may be associated with the integration of the two companies. The unaudited pro forma condensed combined financial information is preliminary and has been prepared for illustrative purposes only and is not necessarily indicative of the financial position or results of operations in future periods or the results that actually would have been realized had GTx and Oncternal been a combined company during the specified periods. The actual results reported in periods following the merger may differ significantly from those reflected in the unaudited pro forma condensed combined financial information presented herein for a number of reasons, including, but not limited to, differences in the assumptions used to prepare this pro forma financial information.
347
The unaudited pro forma condensed combined financial information, including the notes thereto, should be read in conjunction with the separate historical financial statements of GTx and Oncternal, and their respective management’s discussion and analysis of financial condition and results of operations included elsewhere in this proxy statement/prospectus/information statement. GTx’ historical audited financial statements for the years ended December 31, 2018 and 2017 are derived from GTx’s Annual Report onForm 10-K for the year ended December 31, 2018.
Accounting rules require evaluation of certain assumptions, estimates, or determination of financial statement classifications which are completed during the measurement period as defined in current accounting standards. The accounting policies of GTx may materially vary from those of Oncternal. During preparation of the unaudited pro forma condensed combined financial information, management has performed a preliminary analysis and is not aware of any material differences, and accordingly, this unaudited pro forma condensed combined financial information assumes no material differences in accounting policies. Following the acquisition, management will conduct a final review of GTx’s accounting policies in order to determine if differences in accounting policies require adjustment or reclassification of GTx’s results of operations or reclassification of assets or liabilities to conform to Oncternal’ accounting policies and classifications. As a result of this review, management may identify differences that, when conformed, could have a material impact on these unaudited pro forma condensed combined financial statements.
348
Unaudited Pro Forma Condensed Combined Balance Sheet
December 31, 2018
(in thousands)
| GTx | Oncternal | Pro Forma Adjustments | Notes | Pro Forma Combined | ||||||||||||||||
Assets | ||||||||||||||||||||
Current assets | ||||||||||||||||||||
Cash, cash equivalents and short-term investments | $ | 28,458 | $ | 20,645 | $ | (9,783 | ) | D | $ | 39,320 | ||||||||||
Prepaid expenses and other current assets | 2,750 | 565 | — | 3,315 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total current assets | 31,208 | 21,210 | (9,783 | ) | 42,635 | |||||||||||||||
Property and equipment, net | 19 | — | — | 19 | ||||||||||||||||
Other | 94 | 752 | — | 846 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total assets | $ | 31,321 | $ | 21,962 | $ | (9,783 | ) | $ | 43,500 | |||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Liabilities, convertible preferred stock, and stockholders’ equity (deficit) | ||||||||||||||||||||
Current liabilities | ||||||||||||||||||||
Accounts payable | $ | 3,279 | $ | 3,440 | $ | — | $ | 6,719 | ||||||||||||
Accrued and other current liabilities | 1,931 | 891 | (218 | ) | D | 2,604 | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total current liabilities | 5,210 | 4,331 | (218 | ) | 9,323 | |||||||||||||||
Warrant liability | — | 674 | (674 | ) | C | — | ||||||||||||||
Convertible preferred stock | — | 46,588 | (46,588 | ) | C | — | ||||||||||||||
Stockholders’ equity (deficit): | ||||||||||||||||||||
Common stock | 24 | 5 | 79 | A,B,C | 108 | |||||||||||||||
Additionalpaid-in capital | 626,142 | 1,748 | (546,290 | ) | F,G | 81,600 | ||||||||||||||
Accumulated deficit | (600,055 | ) | (31,384 | ) | 583,908 | F,H | (47,531 | ) | ||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total stockholders’ equity (deficit) | 26,111 | (29,631 | ) | 37,697 | 34,177 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total liabilities, convertible preferred stock and stockholders’ equity (deficit) | $ | 31,321 | $ | 21,962 | $ | (9,783 | ) | $ | 43,500 | |||||||||||
|
|
|
|
|
|
|
| |||||||||||||
349
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Year Ended December 31, 2018
(in thousands, except share and per share data)
| GTx | Oncternal | Pro Forma Adjustments | Notes | Pro Forma Combined | ||||||||||||||||
Grant revenue | $ | — | $ | 2,521 | $ | — | $ | 2,521 | ||||||||||||
Operating expenses: | ||||||||||||||||||||
Research and development | 29,669 | 8,287 | — | 37,956 | ||||||||||||||||
General and administrative | 9,390 | 1,820 | (218 | ) | D | 10,992 | ||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Total operating expenses | 39,059 | 10,107 | (218 | ) | 48,948 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Loss from operations | (39,059 | ) | (7,586 | ) | 218 | (46,427 | ) | |||||||||||||
Change in fair value of warrant liability | — | 713 | (713 | ) | E | — | ||||||||||||||
Interest and other income, net | 641 | 294 | — | 935 | ||||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net loss | $ | (38,418 | ) | $ | (6,579 | ) | $ | (495 | ) | $ | (45,492 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||||||
Net loss per share, basic and diluted | $ | (1.65 | ) | $ | (0.13 | ) | $ | — | $ | (0.51 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||||||
Weighted-average shares of common stock outstanding, basic and diluted | 23,346,231 | 48,930,354 | 66,090,383 | I | 89,436,614 | |||||||||||||||
|
|
|
|
|
|
|
| |||||||||||||
350
Notes to the Unaudited Pro Forma Condensed Combined Financial Information
| 1. | Description of Transaction |
On March 6, 2019, GTx entered into the merger agreement (and entered into an amendment April 30, 2019) with Oncternal and Merger Sub pursuant to which Merger Sub will merge with and into Oncternal, with Oncternal surviving the merger as a wholly-owned subsidiary of GTx. The transaction is expected to be accounted for as a reverse asset acquisition by Oncternal. To determine the accounting for this transaction under U.S. GAAP, a company must assess whether an integrated set of assets and activities should be accounted for as an acquisition of a business or an asset acquisition. The guidance requires an initial screen test to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single asset or group of similar assets. If that screen is met, the set is not a business. In connection with the acquisition of GTx, substantially all the fair value is included in IPR&D and, as such, the acquisition is expected to be treated as an asset acquisition. GTx’s assets and liabilities will be measured and recognized at their relative fair values allocation as of the transaction date with any value associated with IPR&D being expensed as there is no alternative future use, and combined with the assets, liabilities and results of operations of Oncternal after the consummation of the merger. The reported consolidated financial condition and results of operations of Oncternal after completion of the merger will reflect these fair values.
Subject to the terms and conditions of the merger agreement, at the effective time of the merger: (i) each share of Oncternal common stock outstanding immediately prior to the effective time will be converted solely into the right to receive a number of shares of GTx’s common stock (the “Shares”) equal to the exchange ratio described below, (ii) each outstanding Oncternal stock option will be assumed by GTx, and (iii) each outstanding Oncternal warrant will be assumed by GTx.
Under the exchange ratio formula in the merger agreement, the former Oncternal stockholders immediately before the merger are expected to own approximately 77.5% of the outstanding capital stock of GTx, and the stockholders of GTx immediately before the merger are expected to own approximately 22.5% of the outstanding capital stock of GTx, subject to certain assumptions. The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and GTx’s outstanding stock options and warrants. To the extent Oncternal’s outstanding stock options or warrants are exercised in the future, it will result in further dilution to GTx’s stockholders. Under certain circumstances, the ownership percentages may be adjusted upward or downward based on cash levels of the respective companies at the closing of the merger.
Under the terms and subject to the conditions of the Merger Agreement, each share of Oncternal outstanding common or preferred stock will be converted into the right to receive approximately 0.51 shares of GTx common stock. Oncternal estimates that the aggregate value of the consideration to be paid in the merger will be approximately $25.4 million, excluding transaction costs. The number and value of the shares of GTx common stock to be issued pursuant to the merger will not be determined until the completion of the merger and therefore, the final aggregate value of the consideration paid in the merger may be more or less than $25.4 million.
The merger is subject to customary closing conditions, including the adoption of the merger agreement by GTx and Oncternal stockholders. Subject to these conditions, the merger is expected to close in the second quarter of 2019. During the year ended December 31, 2018, there were no material transactions between Oncternal and its subsidiary.
At the closing of the merger, GTx, Marc Hanover, as representative of the GTx stockholders prior to such closing, and Computershare Inc., as the Rights Agent, will enter into a Contingent Value Rights Agreement (the “CVR Agreement”). Pursuant to the CVR Agreement, for each share of GTx common stock held, GTx stockholders of record as of immediately prior to the closing will receive one contingent value right (“CVR”) entitling such holders to receive in the aggregate 75% of any net proceeds received during the15-year period after the Closing from the grant, sale or transfer of rights to GTx’s SARD or SARM technology that occurs during the10-year period after the Closing (or in the eleventh year if based on a term sheet approved during the
351
initial10-year period) and to receive royalties on the sale of any SARD products by the combined company during the15-year period after the Closing. Under the CVR agreement, Oncternal (as successor in interest to GTx) agreed to use commercially reasonable efforts to develop or divest SARD technology and to divest SARM technology, subject to certain limitations. The CVRs are not transferable, except in certain limited circumstances, will not be certificated or evidenced by any instrument and will not be registered with the SEC or listed for trading on any exchange. The CVR agreement will be effective prior to the closing and will continue in effect until the payment of all amounts payable thereunder, unless terminated upon termination of the Merger Agreement. Due to the contingent nature of the CVR, no purchase price value has been assigned herein.
| 2. | Estimated Purchase Price |
The accompanying unaudited pro forma condensed consolidated financial statements reflect an estimated reverse asset acquisition price of approximately $28.0 million. Given that the estimated purchase price is variable depending upon GTx’s stock price, management performed a sensitivity analysis over the change in purchase consideration based on +/- 10% volatility in GTx’ stock price. An increase or decrease in GTx’s stock price by 10% would increase or decrease the purchase consideration by approximately $2.6 million. Under certain circumstances further described in the merger agreement, the ownership percentages may be adjusted upward or downward based on cash levels of the respective companies at the closing of the merger.
The total estimated purchase price and allocated purchase price is summarized as follows (in thousands, except share and per share data):
Estimated number of shares of the combined company to be owned by GTx stockholders (i) | 24,207,270 | |||
Multiplied by the fair value per share of GTx common stock (ii) | $ | 1.09 | ||
|
| |||
Total | 26,386 | |||
Estimated transaction costs | 1,650 | |||
|
| |||
Total estimated purchase price | $ | 28,036 | ||
|
|
For purposes of this pro forma analysis, the above estimated purchase price has been allocated based on a preliminary estimate of the fair value of assets and liabilities to be acquired.
| December 31, 2018 (in thousands) | ||||
Cash, cash equivalents and short-term investments as of December 31, 2018 | $ | 28,458 | ||
Other net working capital deficit acquired as of December 31, 2018 | (2,460 | ) | ||
In-process research and development (iii) | 2,038 | |||
|
| |||
Total estimated purchase price | $ | 28,036 | ||
|
| |||
| (i) | The final purchase price will be determined based on the number of shares of common stock of the combined company that GTx stockholders own as of the closing date of the merger. For purposes of this unaudited pro forma condensed combined financial information, the estimated number of shares represents 24,207,270 shares of GTx common stock outstanding or issuable under the GTx Director Deferred Compensation Plan as of March 31, 2019. Consideration related to the fair value of GTx stock options vested and outstanding at the date of the closing of the merger has been excluded from the calculation as the amount allocated to the acquisition and the post-merger expense that will have a continuing impact to the combined company is not considered to be material. The estimated number of shares does not reflect the impact of a proposed reverse stock split that is expected to be effected prior to consummation of the merger. |
352
| (ii) | The estimated purchase price was based on the closing price as reported on the Nasdaq Global Market on April 30, 2019. The final purchase price arising from the actual transaction costs as well as the number of shares of and fair market value of GTx common stock outstanding immediately prior to the closing of the merger could result in a total purchase price different from that assumed in this unaudited pro forma condensed combined financial information, and that difference may be material. Therefore, the estimated consideration expected to be transferred reflected in this unaudited pro forma condensed combined financial information does not purport to represent what the actual consideration transferred will be when the merger is completed. The actual purchase price will fluctuate until the closing date of the merger, and the final valuation of the purchase consideration could differ significantly from the current estimate. |
| (iii) | IPR&D represents the research and development projects of GTx which werein-process, but not yet completed, and which Oncternal plans to advance. This includes the development of GTx’s preclinical SARD technology. Current accounting standards require that the fair value of IPR&D projects acquired in an asset acquisition with no alternative future use be allocated a portion of the consideration transferred and charged to expense at the acquisition date. The acquired assets did not have outputs or employees. The actual purchase price allocated to IPR&D will fluctuate until the closing date of the merger, and the final valuation of the IPR&D consideration could differ significantly from the current estimate. |
| 3. | Pro Forma Adjustments |
Adjustments included in the column under the heading “Pro Forma Adjustments” are primarily based on the preliminary purchase price valuation and certain adjustments to conform Oncternal’s historical amounts to GTx’s financial statements presentation. Further analysis will be performed after the completion of the merger to confirm these estimates or make adjustments in the final purchase price allocation, as necessary.
Given Oncternal’s history of net losses and valuation allowance, management assumed a statutory tax rate of 0%. Therefore the pro forma adjustments to the statement of operations resulted in no additional income tax adjustment to the pro forma financials.
The pro forma adjustments, as of December 31, 2018, relate to the following:
A. To reflect the elimination of GTx’s historical stockholders’ equity balances, including accumulated deficit.
B. To reflect the estimated fair value of the common stock retained by GTx stockholders.
C. To reflect: (i) the conversion of Oncternal convertible preferred stock to GTx common Stock, (ii) the reclassification of Oncternal’s warrant liability to a warrant to purchase GTx’s common stock in connection with the merger which will be classified within stockholders’ equity, and (iii) issuance of GTx common stock in exchange for outstanding Oncternal common stock.
D. To reflect GTx’s wind down of operations including cash reserved for severance charges for GTx employees, tail insurance coverage and combined estimated merger relates costs.
E. This pro forma adjustment is not reflected in the unaudited pro forma condensed combined statement of operations because these amounts are not expected to have a continuing effect on the operating results of the combined company.
F. To reflect the net stock compensation expense related to the accelerated vesting of stock option awards to employees of GTx upon closing of the merger as well as stock compensation expense related to the modification of the term and accelerated vesting of options of employees terminated in the first quarter of 2019. As of the close of the merger, all outstanding options will be fully vested with no requisite future service. This pro forma adjustment is not reflected in the unaudited pro forma condensed combined statement of operations because these amounts are not expected to have a continuing effect on the operating results of the combined company (as the warrant liability is reclassified to equity upon consummation of the merger).
353
G. To record the following adjustments to additionalpaid-in-capital (in thousands):
| December 31, 2018 | ||||
Elimination of GTx additionalpaid-in capital (A) | $ | (626,142 | ) | |
To reflect the fair value of the common stock retained by GTx stockholders (B) | 28,125 | |||
Conversion of Oncternal convertible preferred stock and warrant liability (C) | 47,183 | |||
Stock-based compensation related to accelerated GTx options vesting and modifications (F) | 4,544 | |||
|
| |||
Total | $ | (546,290 | ) | |
|
| |||
H. To record the following accumulated deficit adjustments (in thousands):
| December 31, 2018 | ||||
Elimination of GTx accumulated deficit (A) | $ | 600,055 | ||
Estimated transaction costs (D) | (9,565 | ) | ||
Stock-based compensation related to accelerated GTx options vesting and modifications (F) | (4,544 | ) | ||
In-process research and development | (2,038 | ) | ||
|
| |||
Total | $ | 583,908 | ||
|
| |||
I. Earnings Per Share
The unaudited pro forma combined basic and diluted earnings per share for the year ended December 31, 2018 reflects the respective weighted-average common shares outstanding of GTx and Oncternal. Oncternal’s weighted-average common shares outstanding of 66,090,383 reflect the conversion at Closing of each share of outstanding Oncternal preferred stock into one share of Oncternal common stock (subject to adjustment to account for the GTx Reverse Stock Split, if consummated).
354
DESCRIPTION OF GTX’S CAPITAL STOCK
The following description of GTx’s capital stock is not complete and may not contain all the information you should consider before investing in GTx’s capital stock. This description is summarized from, and qualified in its entirety by reference to, GTx’s restated certificate of incorporation, which has been publicly filed with the SEC. See “Where You Can Find More Information.” The following information does not give effect to the GTx Reverse Stock Split described in Proposal No. 2 in this proxy statement/prospectus/information statement.
General
As of the date of this proxy statement/prospectus/information statement, GTx’s authorized capital stock consists of 60,000,000 shares of common stock, $0.001 par value per share, and 5,000,000 shares of preferred stock, $0.001 par value per share. As of March 31, 2019, there were 24,051,844 shares of GTx common stock outstanding and no shares of preferred stock outstanding.
The following summary description of our capital stock is based on the provisions of GTx’s certificate of incorporation and bylaws, the applicable provisions of the General Corporation Law of the State of Delaware, or DGCL, and the agreements described below. This information may not be complete in all respects and is qualified entirely by reference to the provisions of GTx’s certificate of incorporation and bylaws, the DGCL and such agreements. For information on how to obtain copies of GTx’s certificate of incorporation, bylaws and such agreements, which are exhibits to the registration statement of which this proxy statement/prospectus/information statement is a part, see the section entitled “Where You Can Find More Information.”
Common Stock
The holders of GTx common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. The holders of GTx common stock do not have cumulative voting rights in the election of directors. Subject to preferences that may be applicable to any outstanding shares of preferred stock, the holders of common stock are entitled to receive ratably such dividends as may be declared by the GTx board of directors out of legally available funds. Upon liquidation, dissolution or winding up, holders of GTx common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preferences of any outstanding shares of preferred stock. Holders of common stock have no preemptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to GTx common stock.
The rights of the holders of GTx common stock are subject to, and may be adversely affected by, the rights of holders of shares of any preferred stock that GTx may designate and issue in the future.
Preferred Stock
GTx’s certificate of incorporation provides that the GTx board of directors has the authority, without further action by the stockholders, to designate and issue up to 5,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, and to determine or alter for each such series, such voting powers, full or limited, or no voting powers, and such designation, preferences, and relative, participating, optional, or other rights and such qualifications, limitations, or restrictions thereof, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding. The GTx board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deterring or preventing a change in control of GTx or making removal of management more difficult, and may adversely affect the market price of GTx common stock and the voting and other rights of the holders of GTx common stock.
355
Registration Rights
In September 2017, GTx completed a private placement of immediately separable units comprised of an aggregate of 5,483,320 shares of GTx common stock and warrants to purchase an aggregate of 3,289,988 shares of GTx common stock. Pursuant to the terms of the securities purchase agreement GTx entered into in connection with this private placement, GTx agreed to file as many registration statements with the SEC as may be necessary to cover the resale of all of the shares of common stock that GTx issued to, or are issuable upon the exercise of warrants that GTx issued to, the investors in the private placement, to use its reasonable best efforts to have all such registration statements declared effective as required by and within the timeframes set forth in the securities purchase agreement, and to keep such registration statements effective for up to two years following the closing date of the private placement. In October 2017, GTx filed a registration statement under the Securities Act registering the resale of all 8,773,308 shares of common stock that GTx issued to, or are issuable upon the exercise of warrants that it issued to, the investors in the private placement. In the event that any required registration statements are not filed or declared effective within the timeframes set forth in the securities purchase agreement, or any such effective registration statements subsequently become unavailable, GTx would, subject to certain limited exceptions, be required to pay liquidated damages equal to 1.0% of the aggregate unit purchase price under the securities purchase agreement per month for each default (up to a maximum of 10% of such aggregate unit purchase price). In addition, J.R. Hyde, III, a member of the GTx board of directors, and an affiliate of Mr. Hyde’s, have rights under a separate registration rights agreement with GTx to require it to file resale registration statements covering an additional 785,297 shares of GTx common stock held in the aggregate or to include these shares in registration statements that GTx may file for itself or other stockholders. The foregoing registration rights do not apply or have been waived with respect to the registration statement of which this proxy statement/prospectus/information statement is a part, and no shares held by or issuable to the foregoing investors are registered for resale hereunder.
Anti-Takeover Effects of Provisions of Delaware Law and GTx Charter Documents
Delaware Takeover Statute. GTx is subject to Section 203 of the DGCL. Section 203 generally prohibits a public Delaware corporation such as GTx from engaging in a “business combination” with an “interested stockholder” for a period of three years following the time that the stockholder became an interested stockholder, unless:
| • | prior to the time the stockholder became an interested stockholder, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers and (b) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| • | at or subsequent to the time the stockholder became an interested stockholder, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least66-2/3% of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, lease, exchange, mortgage, pledge, transfer or other disposition (in one transaction or a series of transactions) involving the interested stockholder of 10% or more of the assets of the corporation (or its majority-owned subsidiary); |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
356
| • | subject to exceptions, any transaction involving the corporation that has the effect, directly or indirectly, of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; and |
| • | the receipt by the interested stockholder of the benefit, directly or indirectly (except proportionately as a stockholder of such corporation), of any loans, advances, guarantees, pledges or other financial benefits, other than certain benefits set forth in Section 203, provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person that is an affiliate or associate of such entity or person.
Charter Documents. The GTx certificate of incorporation and bylaws provide that the GTx board of directors be divided into three classes of directors, as nearly equal in number as possible, with each class serving a staggered three-year term. The classification system of electing directors may tend to discourage a third-party from making a tender offer or otherwise attempting to obtain control of us since the classification of the board of directors generally increases the difficulty of replacing a majority of directors. In addition, the GTx certificate of incorporation and bylaws:
| • | provide that any action required or permitted to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by any consent in writing; |
| • | establish advance notice requirements for nominations for election to the GTx board of directors or for proposing matters that can be acted upon at a stockholder meeting; |
| • | provide that the authorized number of directors may be changed only by resolution of the board of directors; and |
| • | provide that special meetings of GTx stockholders may be called only by the chairman of the GTx board of directors, the GTx chief executive officer or the GTx board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors. |
The DGCL provides generally that the affirmative vote of a majority of the shares entitled to vote is required to amend a corporation’s bylaws, unless a corporation’s certificate of incorporation requires a greater percentage or also confers the power upon the corporation’s directors. The GTx bylaws may be amended or repealed by:
| • | the affirmative vote of a majority of our directors then in office; or |
| • | the affirmative vote of the holders of at least66-2/3% of the voting power of all then-outstanding shares of GTx capital stock entitled to vote generally in the election of directors. |
The foregoing provisions of the GTx certificate of incorporation may only be amended or repealed by the affirmative vote of a majority of GTx directors and the affirmative vote of the holders of at least66-2/3% of the voting power of all then-outstanding shares of GTx capital stock entitled to vote generally in the election of directors.
These and other provisions contained in the GTx certificate of incorporation and bylaws could delay or discourage some types of transactions involving an actual or potential change in control or change in management, including transactions in which stockholders might otherwise receive a premium for their shares over then current prices, and may limit the ability of stockholders to remove current management or approve transactions that stockholders may deem to be in their best interests and, therefore, could adversely affect the price of GTx common stock.
Transfer Agent and Registrar
The transfer agent and registrar for GTx common stock is Computershare Trust Company, N.A. Its address is 250 Royall Street, Canton, MA 02021.
357
COMPARISON OF RIGHTS OF HOLDERS OF GTX STOCK AND ONCTERNAL STOCK
Both GTx and Oncternal are incorporated under the laws of the State of Delaware and, accordingly, the rights of the stockholders of each are currently, and will continue to be, governed by the DGCL. If the merger is completed, Oncternal’s stockholders will become stockholders of GTx, and their rights will be governed by the DGCL, the amended and restated bylaws of GTx and, assuming Proposal Nos. 2 and 3 are approved by GTx’s stockholders at the GTx special meeting, the restated certificate of incorporation of GTx as amended by the amendments thereto attached to this proxy statement/prospectus/information statement asAnnex D andAnnex E.
The table below summarizes the material differences between the current rights of Oncternal’s stockholders under Oncternal’s amended and restated certificate of incorporation and bylaws, and the rights of GTx’s stockholders, post-merger, under GTx’s restated certificate of incorporation and amended and restated bylaws, each as amended, as applicable, and as in effect immediately following the merger.
While GTx and Oncternal believe that the summary tables cover the material differences between the rights of their respective stockholders prior to the merger and the rights of GTx’s stockholders following the merger, these summary tables may not contain all of the information that is important to you. These summaries are not intended to be a complete discussion of the respective rights of GTx’s and Oncternal’s stockholders and are qualified in their entirety by reference to the DGCL and the various documents of GTx and Oncternal that are referred to in the summaries. You should carefully read this entire proxy statement/prospectus/information statement and the other documents referred to in this proxy statement/prospectus/information statement for a more complete understanding of the differences between being a stockholder of GTx or Oncternal before the merger and being a stockholder of GTx after the merger. GTx has filed copies of its current restated certificate of incorporation and amended and restated bylaws with the SEC and will send copies of the documents referred to in this proxy statement/prospectus/information statement to you upon your request. Oncternal will also send copies of its documents referred to in this proxy statement/prospectus/information statement to you upon your request. See the section entitled “Where You Can Find More Information” in this proxy statement/prospectus/information statement.
Current Oncternal Rights Versus Post-Merger GTx Rights
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| ELECTIONS; VOTING; PROCEDURAL MATTERS | ||||
| Authorized Capital Stock | The amended and restated certificate of incorporation of Oncternal authorizes the issuance of up to 200,000,000 shares of common stock, $0.0001 par value per share, and 130,099,288 shares of preferred stock, $0.0001 par value per share, 48,000,000 of which are designated as “Series C preferred stock”, 61,788,567 of which are designated as“Series B-2 preferred stock”, 6,750,721 of which are designated as“Series B-1 preferred stock” and 13,560,000 of which are designated as “Series A preferred stock.” | The restated certificate of incorporation of GTx authorizes the issuance of up to 60,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share. | ||
| Number of Directors | The amended and restated bylaws of Oncternal provides that number of directors which shall constitute the whole Board of Directors shall be | The restated certificate of incorporation of GTx currently provides that the number of directors that shall constitute the whole board of directors of GTx | ||
358
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| determined from time to time solely by resolution adopted by the affirmative vote of a majority of the directors. The amended and restated certificate of incorporation of Oncternal currently provides that the authorized number of directors is nine. | shall be fixed exclusively by resolution adopted by the affirmative vote of a majority of the authorized Directors. | |||
| Stockholder Nominations and Proposals | The amended and restated certificate of incorporation and amended and restated bylaws of Oncternal do not provide for procedures with respect to stockholder proposals or director nominations. | The amended and restated bylaws of GTx provide that nominations of any person for election to the GTx board of directors may be made at an annual meeting or a special meeting of the stockholders (i) pursuant to the Corporation’s notice with respect to such meeting; (ii) by or at the direction of the Board of Directors; or (iii) by any stockholder of the Corporation who was stockholder of record at the time of giving the stockholders notice provided for in the bylaws, who is entitled to vote at the meeting and who complied with the notice procedures.
The amended and restated bylaws of GTx provide that in order for a stockholder to properly bring business before an special meeting, (i) the stockholder must have given timely notice thereof in writing to the Secretary of the Corporation, (ii) such other business must be a proper matter for stockholder action under the DGCL, (iii) if the stockholder, or the beneficial owner on whose behalf any such proposal or nomination is made, has provided the Corporation with notice, such stockholder or beneficial owner must, in the case of a proposal, delivered a proxy statement and form of proxy to holders of at least the percentage of the Corporation’s voting shares required under applicable law to carry any such proposal, or in the case of a nomination or nominations, have delivered a proxy statement of form of proxy to holders of a percentage of the Corporation’s voting shares reasonably believed by such stockholder or beneficial owner to be sufficient to elect the nominee or nominees proposed to be nominated by such stockholder, and must, in either | ||
359
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| case, have included such materials in the notice, and (iv) if no notice relating thereto has been timely provided pursuant to the bylaws, the stockholder or beneficial owner proposing such business or nomination must not have solicited a number of proxies sufficient to have required the delivery of a notice under the bylaws. | ||||
| Classified Board of Directors | The amended and restated certificate of incorporation of Oncternal provides that the holders of Series A preferred stock, voting as a separate class and to the exclusion of all other classes of capital stock of the Corporation, may elect 1 director (the “Series A Director”); the holders of SeriesB-2 Preferred Stock, voting as a separate class and to the exclusion of all other classes of capital stock of the Corporation, may elect 2 directors (the “SeriesB-2 Directors); the holders of the Series C preferred stock, voting as a separate class and to the exclusion of all other classes of capital stock of the Corporation, may elect 2 directors (the “Series C Directors” and together with the Series A Director and the SeriesB-2 Directors, the “Preferred Directors”). The holders of Common Stock, voting as a separate class and to the exclusion of all other classes of capital stock of the Corporation, may elect 2 directors (the “Common Directors”). The holders of the outstanding shares of Common Stock and Preferred Stock, voting together as a single class, are entitled to elect the remaining directors (the “General Directors”). | The restated certificate of incorporation of GTx provides that the directors comprising the board of directors of GTx shall be divided into three staggered classes, with each class serving a three-year term. | ||
| Removal of Directors | Under the amended and restated certificate of incorporation of Oncternal, any director may be removed, with or without cause, only by the affirmative vote of the holders of a majority of the shares eligible to vote in an election for the seat occupied that director (e.g., in order to remove a Series A Director, the holders of a majority of the shares of Series A preferred stock, voting as a separate class and to the exclusion of all | Under the amended and restated bylaws of GTx, a director may be removed from office only for cause and only by the affirmative vote of the holders of at least a majority of all then outstanding shares of capital stock of the Corporation then entitled to vote in the election of directors, voting together as a single class. | ||
360
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| other classes of capital stock of the Corporation, must so vote). | ||||
| Special Meeting of the Stockholders | The amended and restated bylaws of Oncternal provide that special meetings of stockholders may be called by the President and shall be called by the Chairman of the Board, President or the Secretary at the request in writing of a majority of the Board of Directors, or at the request in writing of stockholders owning a majority in amount of the entire capital stock of the corporation issued and outstanding, and entitled to vote. Such request shall state the purpose or purposes of the proposed meeting. Business transacted at any special meeting of stockholders shall be limited to the purposes stated in the notice. | The restated certificate of incorporation of GTx provides that a special meeting of the stockholders may be called only by the Chairman of the Board of GTx, the Chief Executive Officer or by the Board of Directors acting pursuant to a resolution adopted by a majority of the authorized number of directors, and any power of stockholders to call a special meeting is specifically denied. | ||
| Cumulative Voting | The amended and restated certificate of incorporation and amended and restated bylaws of Oncternal do not have a provision granting cumulative voting rights in the election of its directors. | The restated certificate of incorporation of GTx does not have a provision granting cumulative voting rights in the election of its directors. | ||
| Vacancies | The amended and restated certificate of incorporation of Oncternal provides that in the case of any vacancy of a director occurring among the Preferred Directors or the Common Directors, by the affirmative vote of the holders of a majority of the shares of the class or classes entitled to vote on the election of the Preferred Directors or the Common Directors, as the case may be, such holders shall elect a successor or successors to hold the office for the unexpired term of the director or directors whose place or places shall be vacant. In the case of any vacancy in the office of a General Director, the affirmative vote of the holders of a majority of the shares of Preferred Stock and Common Stock, voting as a single class, such holders shall elect a successor or successors to hold the officer for the unexpired term of the director or directors shoes place or places shall be vacant. | The restated certificate of incorporation and amended and restated bylaws of GTx provide that any vacancy on the board of directors of GTx will be filled only by the affirmative vote of a majority of the directors in office, even though less than a quorum, unless the board of directors of GTx determines by resolution that any such vacancies or newly created directorships shall be filled by the stockholders. | ||
361
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| Voting Stock | Under the amended and restated certificate of incorporation of Oncternal, the holders of common stock are entitled to one vote for each share of stock held by them and holders of preferred stock are entitled to one vote for each share of common stock into which such share of preferred stock is convertible determined by reference to the applicable Conversion Price in effect at the record date of the determination of the holders of the shares entitled to vote or, if no such record date is established, at the date such vote is taken or any written consent of stockholders is first solicited. Except as otherwise provided by law or in the amended and restated certificate of incorporation, the holders of Preferred Stock shall vote together with the holders of the outstanding shares of Common Stock, and not as a separate class or series. | Under the restated certificate of incorporation and amended and restated bylaws of GTx, each outstanding share of Common Stock shall be entitle the holder thereof to one vote in person or by proxy on each matter properly submitted to the stockholders at a meeting of the stockholders; provided, however, that except as otherwise required by law, holders of Common Stock shall not be entitled to vote on any amendment to the Certificate of Incorporation that relates solely to the terms of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together as a class with the holders of one or more other such series, to vote thereon by low or pursuant to the Certificate of Incorporation. | ||
| Stockholders Agreement; Voting Agreement | Oncternal does not have a stockholders agreement.
Oncternal and the stockholders of Oncternal have entered into that certain Amended and Restated Voting Agreement dated September 22, 2018, which provides, among other things, that: (i) two Common Directors shall be designated by the holders of a majority of the shares of Oncternal’s common stock; (ii) two Series C Directors shall be designated by SPH USA (for so long as SPH USA and its affiliated parties continue to hold at least 17,000,000 shares (as adjusted for stock splits, dividends and the like with respect to such shares) of Series C preferred stock of Oncternal; (iii) one SeriesB-2 Director shall be designated by MagnaSci Fund L.P. (for so long as MagnaSci Fund L.P. and its affiliated parties continues to hold at least 7,000,000 shares (as adjusted for stock splits, dividends and the like with respect to such shares) of SeriesB-2 preferred stock. Under the Merger Agreement, Oncternal has agreed to terminate the | GTx does not have a stockholders agreement or similar agreement with any of its stockholders in place. | ||
362
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| Amended and Restated Voting Agreement at or prior to the closing of the merger. | ||||
| Drag Along | Under the Oncternal Amended and Restated Voting Agreement dated September 22, 2018, as further described therein, if (i) the Board, (ii) the holders of a majority of the then outstanding shares of Series A preferred stock, (iii) the holders of a majority of the then outstanding shares of Series B preferred stock and SeriesB-2 preferred stock, voting together, and (iv) the holders of a majority of the then outstanding shares of Series C preferred stock (the “Proposing Holders”) approve a Change of Control Transaction (as defined in the Amended and Restated Voting Agreement) then each of the Significant Common Holders agrees to vote for, consent to and otherwise raise no objections to such Change of Control Transaction and (i) if such Change of Control Transaction is structured as a consolidation, merger or asset sale of the Company, or a sale of all or substantially all of the Company’s assets, each of the Significant Common Holders shall waive any dissenters’ rights, appraisal rights or similar rights in connection with such consolidation, merger or asset sale, or (ii) if such Change of Control Transaction is structured as a sale of the capital stock of the Company, each of the Significant Common Holders shall agree to sell all shares of the Company’s capital stock held by them on the terms and conditions approved by the Board and the Proposing Holders. | GTx does not have drag along terms in place. | ||
| Stockholder Action by Written Consent | The amended and restated bylaws of Oncternal provide that any action required to be taken at any annual or special meeting of stockholders of the corporation, or any action which may be taken at any annual or special meeting of such stockholders, may be taken without a meeting, without prior notice and without a vote, if a consent in writing, setting forth the action so taken, shall be signed by the holders of outstanding | The restated certificate of incorporation and amended and restated bylaws of GTx specify that no action shall be taken by the stockholders except at an annual or special meeting of the stockholders and further explicitly provides that no action shall be taken by the stockholders by written consent. | ||
363
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted and shall be delivered to the corporation by delivery to its registered office in Delaware, its principal place of business, or to an officer or agent of the corporation having custody of the book in which proceedings of meetings of stockholders are recorded. | ||||
| Notice of Stockholder Meeting | The amended and restated bylaws of Oncternal provide that notices of all meetings shall state the place, day and hour of the meeting and, in the case of a special meeting, the purpose or purposes for which the meeting is called. The amended and restated bylaws of Oncternal provide that notice of each meeting of stockholders shall be given not less than 10 nor more than 60 days before the date of the meeting to each stockholder entitled to vote at such meeting. | Under the amended and restated bylaws of GTx, written notice of each stockholder meeting must specify the place, date and hour of the meeting, the means of remote communication(s), if any, by which stockholders and proxyholders may be deemed to be present in person and vote at such meeting, and, in the case of a special meeting, the purposes for which the meeting is called. Notice shall be given not less than 10 nor more than 60 calendar days before the date of the meeting to each stockholder entitled to vote at such meeting. | ||
| Conversion Rights and Protective Provisions | The amended and restated certificate of incorporation of Oncternal provides that each holder of shares of Oncternal preferred stock shall, subject to certain conditions, have the right to convert such shares into shares of Oncternal common stock at any time in accordance with the amended and restated certificate of incorporation of Oncternal.
In addition, in the event of any capital reorganization, any reclassification of the Common Stock (other than a change in par value as a results of a stock dividend, subdivision,split-up or combination of shares), the consolidation or merger of the Corporation with or into another Person (subject to certain exceptions) (collectively referred to as “Reorganizations”), the holders of the Preferred Stock shall thereafter be entitled to receive, upon conversion of the Preferred Stock the kind and number | The restated certificate of incorporation of GTx does not provide that holders of GTx stock shall have preemptive, conversion or other protective rights. | ||
364
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| of shares of Common Stock or other securities or property (including cash) of the Corporation, or other corporation resulting from such consolidation or surviving such merger to which a holder of the number of shares of Common stock of the Corporation which the applicable series of preferred stock entitled the holder thereof to convert immediately prior to such Reorganization would have been entitled to receive with respect to such Reorganization. For so long as any shares of Oncternal’s preferred stock shall be outstanding Oncternal shall not, without a Preferred Investor Supermajority Consent (as defined in the amended and restated certificate of incorporation): (i) declare or pay any dividends on any capital stock of the Corporation; (ii) redeem or repurchase capital stock of the Corporation except in connection with the repurchase of shares of Common Stock issued to or held by employees, consultants, officers and directors upon termination of their employment or services pursuant to agreements providing for the right of said repurchase, which agreements were authorized by the Board of Directors; (iii) take any action which would result in a Reorganization, a Liquidation Event or a Deemed Liquidation Event (each as defined in the amended and restated certificate of incorporation); (iv) increase or decrease the total number of authorized members of the Board of Directors; (v) increase or decrease the authorized number of shares of Common Stock or Preferred Stock; (vi) authorize, create or issue (whether by merger, consolidation, reclassification, amendment of the amended and restated certificate of incorporation, sale or otherwise) shares of any class or series of stock not authorized in the amended and restated certificate of incorporation having rights, preferences or privileges superior to or on parity with the Preferred Stock; (vii) create, or authorize the creation of, issue, or authorize the |
365
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
issuance of any debt security, if the aggregate indebtedness of the Corporation for borrowed money following such action would exceed $1,000,000; (viii) create any Subsidiary that is not wholly-owned (either directly or through one or more other Subsidiaries) by the Corporation, or enter into any joint venture or partnership with any Person; (ix) enter into any contract to purchase, sell, assign, transfer, exclusively license, pledge, hypothecate, grant a security interest in or otherwise acquire, dispose of or encumber, in whole or in part, all or substantially all of the Corporation’s intellectual property; (x) increase the number of shares of Common stock authorized under any existing stock or option plan, or create a new stock or option plan; or (xi) take any action to amend or waive any provision of the amended and restated certificate of incorporation of the Corporation’s Bylaws.
In addition to the above protective rights, so long as any shares of Series C preferred stock shall be outstanding, Oncternal shall not, without a Series C Preferred Supermajority Interest: (i) amend, alter or repeal the rights, preference or privileges of the Series C preferred stock in a manner that is different than the effect of such change on the rights, preferences or privileges of the other classes of Preferred Stock; (ii) (A) reclassify, alter or amend any existing security of the Corporation that is pari passu with the Series C preferred stock if such reclassification, alteration or amendment would render such security senior to the Series C preferred stock in respect of any right, preference or privilege or (B) reclassify, alter or amend any existing security of the Corporation that is junior to the Series C preferred stock if such reclassification, alteration or amendment would render such security senior to or pari passu with |
366
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| the Series C preferred stock in respect of any right, preference or privilege; (iii) make, declare pay or set aside, or allow any Subsidiary to make declare, pay or set aside, any dividend, distribution or spin out of assets of the Corporation to any officer, director or stockholder of the Corporation, or any affiliate of such officer, director or stockholder, unless approved by the Board of Directors, including at least one of the Series C Directors; or (iv) increase or decrease the authorized shares of Series C preferred stock. | ||||
| Right of First Refusal | The Oncternal Amended and RestatedCo-Sale Agreement entered into among Oncternal and certain stockholders dated September 22, 2018 provides that any holder of common stock that is a party to the Amended and RestatedCo-Sale Agreement wishing to transfer any shares of common stock shall first provide Oncternal with the right to purchase such shares. In such an event, if Oncternal does not elect to exercise its right of first refusal in full, Investors party to the Amended and RestatedCo-Sale Agreement have a secondary right of first refusal to purchase all or any portion of the shares of Oncternal common stock which are proposed for sale or transfer by the holders of Oncternal common stock that are a party to the Amended and RestatedCo-Sale Agreement. Under the Merger Agreement, Oncternal has agreed to terminate the Amended and RestatedCo-Sale Agreement at or prior to the closing of the merger. | GTx does not have a right of first refusal in place. | ||
| Right ofCo-Sale | As further described in the Oncternal Amended and RestatedCo-Sale Agreement, the investors party to the Amended and RestatedCo-Sale Agreement have a right ofco-sale with respect to any common stock proposed to be transferred or sold by any holder of common stock that is a party to the | GTx does not have a right ofco-sale in place. | ||
367
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| Amended and RestatedCo-Sale Agreement which is not earlier purchased by Oncternal by exercise of its right of first refusal. | ||||
| Pro Rata Rights | The Oncternal Amended and Restated Investors’ Rights Agreement entered into among Oncternal and certain Investors, dated September 22, 2018 provides each of the Investors party to the Amended and Restated Investors’ Rights Agreement with a right of first refusal to purchase his or its pro rata share (as defined therein) of new securities which Oncternal proposes to sell and issue after September 22, 2018, subject to certain exceptions as further described therein. Under the Merger Agreement, Oncternal has agreed to terminate the Oncternal Amended and Restated Investors’ Rights Agreement at or prior to the closing of the merger. | GTx does not have a pro rata rights provision in place. | ||
| Indemnification of Officers and Directors and Advancement of Expenses; Limitation on Personal Liability | ||||
| Indemnification | The amended and restated certificate of incorporation of Oncternal provides that to the fullest extent permitted by applicable law, a director of Oncternal shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of fiduciary duty as a director. The amended and restated bylaws and the amended and restated certificate of incorporation of Oncternal provide that Oncternal shall have the power to indemnify its directors and officers to the fullest extent permitted by applicable law. Oncternal has entered into a number of indemnification agreements with its officers and directors. | The restated certificate of incorporation of GTx provides that a director of GTx shall not be personally liable to GTx or its stockholders for monetary damages for breach of fiduciary duty as a director. The amended and restated bylaws and the restated certificate of incorporation of GTx provide that GTx shall indemnify and hold harmless its directors and officers to the fullest extent permitted by applicable law, except that GTx will not be required to indemnify or hold harmless any director or officer in connection with any proceeding initiated by such person unless the proceeding was authorized by the board of directors of GTx. Under the amended and restated bylaws of GTx, such rights shall not be exclusive of any other rights acquired by directors and officers, including by agreement. | ||
| Advancement of Expenses | The amended and restated bylaws of Oncternal provide that expenses incurred by an officer or director in defending any civil, criminal, administrative or investigative action, suit or proceeding shall be paid by the corporation in | The amended and restated bylaws of GTx provide that GTx may, in its discretion and upon such terms and conditions, if any, as the Corporation deems appropriate, advance the expenses incurred by such director, officer, | ||
368
Provision | Oncternal (Pre-Merger) | GTx (Post-Merger) | ||
| advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of such director or officer to repay such amount if it shall ultimately be determined that he or she is not entitled to be indemnified by the corporation. | employee or agent of the Corporation in defending such action, suit or proceeding prior to its final disposition. | |||
| Dividends | ||||
| Declaration and Payment of Dividends | The amended and restated certificate of incorporation of Oncternal provides that, subject to the prior rights of holders of all classes of stock at the time outstanding having prior rights as to dividends, the holders of the Common Stock shall be entitled to receive, when, as and if declared by the Board of Directors, out of any assets of the Corporation legally available therefor, any dividends as may be declared from time to time by the Board of Directors. | The amended and restated bylaws of GTx provide that, subject to any restrictions contained in the DGCL or the restated certificate of incorporation of GTx, the board of directors of GTx is empowered to declare and pay dividends upon the shares of GTx capital stock. Dividends may be paid in cash, in property or in shares of GTx capital stock. | ||
| Amendments to Certificate of Incorporation or Bylaws | ||||
| General Provisions | The amended and restated certificate of incorporation of Oncternal provides that Oncternal reserves the right to amend, alter, change or repeal any provision of the amended and restated certificate of incorporation, in the manner now or hereafter prescribed by statute, and all rights conferred upon stockholders therein are granted subject to this reservation.
The amended and restated bylaws of Oncternal provide that the bylaws may be altered, amended or repealed by the stockholders of Oncternal or by the board of directors of Oncternal, when such power is conferred upon the board of directors by Oncternal’s then current certificate of incorporation. | The restated certificate of incorporation of GTx may be amended in any manner otherwise permitted by law, with the exception that under the restated certificate of incorporation of GTx, Article V (relating to the composition of and vacancies on the board of directors of GTx, election and removal of directors), Article VI (relating to voting rights and special meetings of stockholders), Article VII (relating to adopting, amending or repealing the Bylaws of the Corporation), Article VIII (relating to limitation of liability for directors) and Article IX (relating to the amendment of the certificate of incorporation) require the affirmative vote of the holders of 66 and 2/3% of the voting power of the outstanding shares of voting stock entitled to vote generally in the election of directors, voting together as a single class. | ||
369
The following table sets forth certain information with respect to the beneficial ownership of GTx’s common stock as of March 31, 2019 (except where otherwise indicated) for:
| • | each person, or group of affiliated persons, who are known by us to beneficially own more than 5% of the outstanding shares of GTx common stock; |
| • | each of GTx’s directors as of March 31, 2019; |
| • | each of GTx’s named executive officers, as identified in “The Merger—Interests of GTx’s Directors and Executive Officers in the Merger—Ownership Interests”; and |
| • | all of the current directors and executive officers of GTx as a group. |
The number of shares beneficially owned by each entity, person, director or executive officer is determined under the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the individual has the sole or shared voting power or investment power and also any shares that the individual has the right to acquire within 60 days of March 31, 2019, through the exercise of any stock option or other right. Unless otherwise indicated, each person has sole investment and voting power, or shares such powers with his or her spouse, with respect to the shares set forth in the following table.
The percentage of ownership is based on 24,051,844 shares of common stock outstanding on March 31, 2019, adjusted as required by the rules promulgated by the SEC to determine beneficial ownership. Except as contemplated by the Merger Agreement, GTx does not know of any arrangements, including any pledge by any person of securities of GTx, the operation of which may at a subsequent date result in a change of control of GTx. Unless otherwise noted, the address of each director and current and former executive officer of GTx is c/o GTx, Inc., 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103.
| Beneficial Ownership | ||||||||
Name and Address of Beneficial Owner | Number of Shares | Percent of Total | ||||||
5% Stockholders: | ||||||||
The Pyramid Peak Foundation(1) 1350 Concourse Avenue, Suite 383 Memphis, Tennessee 38104 | 7,183,900 | 26.8 | % | |||||
Named Executive Officers and Directors: | ||||||||
Marc S. Hanover(2) | 304,776 | 1.3 | % | |||||
Robert J. Wills, Ph.D.(3) | 150,678 | * | ||||||
Henry P. Doggrell(4) | 107,097 | * | ||||||
Michael G. Carter, M.D., Ch.B., F.R.C.P.(5) | 39,131 | * | ||||||
J. Kenneth Glass(6) | 60,381 | * | ||||||
J. R. Hyde, III(7) | 10,010,446 | 36.7 | % | |||||
Garry A. Neil, M.D.(8) | 53,426 | * | ||||||
Kenneth S. Robinson, M.D., M.Div.(9) | 79,605 | * | ||||||
All Directors and Executive Officers as a group (9 persons)(10) | 10,848,287 | 39.2 | % | |||||
| * | Represents less than 1% of the outstanding shares of GTx’s common stock. |
| (1) | Based on information provided to GTx as of February 28, 2019. Includes an aggregate of 2,793,657 shares issuable upon exercise of outstanding warrants. James R. Boyd, Lee B. Harper, O. Mason Hawkins and Andrew R. McCarroll are each a director of the Foundation. Each of such individuals may be deemed to share beneficial ownership of the shares beneficially owned by the Foundation. The foregoing ownership information was provided to us as of February 28, 2019, and, consequently, beneficial ownership may have changed between such date and March 31, 2019. |
370
| (2) | Includes 35,287 shares held by Equity Partners XII, LLC, an entity controlled by Mr. Hanover, 22,726 shares issuable upon exercise of a warrant, 12,400 shares held by trusts of which Mr. Hanover is the trustee, and 110,001 shares of common stock issuable upon the exercise of options held by Mr. Hanover. |
| (3) | Includes 13,334 shares of common stock issuable upon the exercise of options held by Dr. Wills. |
| (4) | Includes 934 shares held by trusts with respect to which Mr. Doggrell may be deemed to have beneficial ownership, 11,435 shares held by a trust of which Mr. Doggrell is theco-trustee, 47,667 shares of common stock issuable upon the exercise of options held by Mr. Doggrell, and 400 shares of common stock held by Mr. Doggrell through an individual retirement account. Also includes 664 shares held by Mr. Doggrell’s wife and 2,547 shares of common stock held by Mr. Doggrell’s wife through an individual retirement account. |
| (5) | Consists of 35,500 shares of common stock issuable upon the exercise of options held by Dr. Carter, and 3,631 shares issuable to Dr. Carter pursuant to our Directors’ Deferred Compensation Plan. |
| (6) | Includes 35,500 shares of common stock issuable upon the exercise of options held by Mr. Glass, and 655 shares issuable to Mr. Glass pursuant to our Directors’ Deferred Compensation Plan. Also includes 2,450 shares of common stock held by Mr. Glass’ wife through an individual retirement account. |
| (7) | Includes 14,535 shares held by Pittco Associates III, L.P. and 391,571 shares held by Pittco Investments, L.P., entities controlled by Mr. Hyde, 2,454,483 shares issuable upon exercise of a warrant issued to Mr. Hyde in November 2014 (the “2014 Warrant”), 678,349 shares issuable upon exercise of a warrant issued to Mr. Hyde in September 2017 (the “2017 Warrant”), and 70,276 shares issuable to Mr. Hyde pursuant to our Directors’ Deferred Compensation Plan. Mr. Hyde also has shared voting and dispositive power over 21,646 shares held by Mr. Hyde’s spouse, 184,480 shares held by trusts for the benefit of Mr. Hyde’s children (the “Hyde Family Trusts”). As trustee of the Hyde Family Trusts , John H. Pontius shares voting and dispositive power over all of the shares held by the Hyde Family Trusts. |
| (8) | Consists of 16,667 shares of common stock issuable upon the exercise of options held by Dr. Neil, and 36,759 shares issuable to Dr. Neil pursuant to our Directors’ Deferred Compensation Plan. |
| (9) | Consists of 35,500 shares of common stock issuable upon the exercise of options held by Dr. Robinson, and 44,105 shares issuable to Dr. Robinson pursuant to our Directors’ Deferred Compensation Plan. |
| (10) | Includes 54,182 shares of common stock beneficially owned by executive officers that are not named executive officers, of which 34,634 shares were issuable upon the exercise of options held by these executive officers. For purposes of determining the number of shares beneficially owned by directors and executive officers as a group, any shares beneficially owned by more than one director or executive officer are counted only once. |
371
PRINCIPAL STOCKHOLDERS OF ONCTERNAL
The following table and the related notes present information on the beneficial ownership of Oncternal’s capital stock as of March 31, 2019 by:
| • | each director of Oncternal; |
| • | each named executive officer of Oncternal; |
| • | all of Oncternal’s current directors and executive officers as a group; and |
| • | each stockholder known by Oncternal to beneficially own more than five percent of its common stock on an as converted to common stock basis. |
The percentage of ownership is based on 162,317,356 shares of Oncternal common stock outstanding on March 31, 2019, assuming the conversion of all Oncternal preferred stock into Oncternal common stock in accordance with the Merger Agreement. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of Oncternal’s common stock that may be acquired by an individual or group within 60 days of March 31, 2019, pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table.
Except as indicated in the footnotes to this table, Oncternal believes that the stockholders named in this table have sole voting and investment power with respect to all shares of Oncternal’s common stock shown to be beneficially owned by them, based on information provided to Oncternal by such stockholders. Unless otherwise indicated, the address for each stockholder listed is: c/o Oncternal Therapeutics, Inc., 12230 El Camino Real, Ste 300, San Diego, California 92130.
5% Stockholders | Number of Shares | Approximate Percent Owned | ||||||
Shanghai Pharmaceutical (USA) Inc.(1) | 34,000,000 | 20.9 | % | |||||
Entities affiliated with MagnaSci Ventures(2) | 19,422,222 | 11.8 | % | |||||
Directors and Named Executive Officers | Number of Shares | Approximate Percent Owned | ||||||
James B. Breitmeyer, M.D., Ph.D.(3) | 5,416,433 | 3.3 | % | |||||
Richard G. Vincent(4) | 696,408 | * | ||||||
Hazel M. Aker(5) | 128,636 | * | ||||||
David F. Hale(6) | 9,825,423 | 6.1 | % | |||||
Cooper Collins(2) | 19,422,222 | 11.8 | % | |||||
Cam Gallagher(7) | 3,561,825 | 2.2 | % | |||||
Scott Glenn(8) | 8,073,278 | 5.0 | % | |||||
Yanjun Liu, M.D., Ph.D. | — | * | ||||||
Xin Nakanishi, Ph.D. | — | * | ||||||
William R. LaRue(9) | 360,511 | * | ||||||
Charles P. Theuer, M.D., Ph.D. | 200,000 | * | ||||||
All current executive officers and directors as a group (11 persons) | 47,684,736 | 29.2 | % | |||||
| * | Represents beneficial ownership of less than 1% of the shares of Oncternal’s common stock. |
| (1) | SPH USA is a wholly-owned subsidiary of Shanghai Pharmaceuticals Holding Co., Ltd., a joint stock company incorporated in the People’s Republic of China with limited liability (“SPH”). The board of directors of SPH has voting and investment power over the shares held by SPH USA, and consists of Zhou |
372
| Jun, Cho Man, Li Yongzhong, Shen Bo, Li An, Wan Kam To, Tse Cho Che, Cai Jiangnan and Hong Liang. Directors Yanjun Liu, M.D., Ph.D. and Xin Nakanishi, Ph.D. are affiliated with SPH but do not have voting or investment power over the shares held by SPH USA. The registered address of SPH USA is Two Penn Center Plaza, Suite 200, 1500 John F. Kennedy Blvd., Philadelphia, Pennsylvania 19102. |
| (2) | Consists of (i) 9,455,556 shares of common stock and warrants to purchase 1,418,333 shares of common stock held by MagnaSci Fund, L.P., (ii) 2,444,445 shares of common stock and warrants to purchase 366,666 shares of common stock held by MagnaSci Fund II, L.P. and (iii) 4,988,888 shares of common stock and warrants to purchase 748,334 shares of common stock held by MagnaSciCo-Investments, L.L.C. MagnaSci GP, L.L.C. is the sole general partner of MagnaSci Fund and MagnaSci Fund II. Cooper Collins is a Manager of MagnaSci GP and MagnaSciCo-Investments, and has voting and investment power over the shares held by MagnaSci Fund, MagnaSci Fund II and MagnaSciCo-Investments. The address of MagnaSci Fund, MagnaSci Fund II and MagnaSciCo-Investments is 123 N. Post Oak Lane, Suite 410, Houston, Texas 77024. |
| (3) | Consists of (i) 3,482,856 shares of common stock held directly by Dr. Breitmeyer, (ii) 1,600,000 shares of common stock underlying options held by Dr. Breitmeyer that are exercisable as of March 31, 2019 or that will become exercisable within 60 days after such date, (iii) 293,577 shares of common stock and warrants to purchase 10,000 shares of common stock held by a family trust (the “Breitmeyer Trust”), (iv) 10,000 shares of common stock held by Dr. Breitmeyer as custodian for his child and (v) 20,000 shares of common stock underlying options held by Dr. Breitmeyer’s wife, Mary Breitmeyer, that are exercisable as of March 31, 2019 or that will become exercisable within 60 days after such date. Dr. Breitmeyer and Ms. Breitmeyer are the trustees of the Breitmeyer Trust, and in such capacity have joint power to vote and dispose of the shares held by the Breitmeyer Trust. |
| (4) | Consists of (i) 555,897 shares of common stock held directly by Mr. Vincent, including 315,512 shares subject to repurchase by Oncternal and (ii) 136,677 shares of common stock and warrants to purchase 3,834 shares of common stock held by a family trust (the “Vincent Trust”). Mr. Vincent and his wife, Stacy Vincent, are the trustees of the Vincent Trust, and in such capacity have joint power to vote and dispose of the shares held by the Vincent Trust. |
| (5) | Consists of (i) 58,381 shares of common stock held directly by Ms. Aker and (ii) 68,338 shares of common stock and warrants to purchase 1,917 shares of common stock held by a family trust (the “Aker Trust”). Ms. Aker and her husband, Larry Aker, are the trustees of the Aker Trust, and in such capacity have joint power to vote and dispose of the shares held by the Aker Trust. |
| (6) | Consists of (i) 9,530,554 shares of common stock and warrants to purchase 44,869 shares of common stock held by Hale BioPharma Ventures, LLC and (ii) 250,000 shares of common stock held by Hale Trading Company. Mr. Hale is the Chairman and Chief Executive Officer of Hale BioPharma Ventures and the Managing Director of Hale Trading Company, and as such has voting and investment control over the shares held by Hale BioPharma Ventures and Hale Trading Company. Amounts also include 35,000 shares that were pledged as collateral by Hale BioPharma Ventures in favor of Oxford Finance LLC on one loan. |
| (7) | Consists of (i) 3,345,159 shares of common stock, including 263,021 shares subject to repurchase by Oncternal, and warrants to purchase 16,666 shares of common stock, held directly by Mr. Gallagher and (ii) 200,000 shares of common stock held by Mr. Gallagher as custodian for his child. |
| (8) | Consists of 8,028,793 shares of common stock and warrants to purchase 44,485 shares of common stock held by Glenn Holdings, L.P. Mr. Glenn is the General Partner of Glenn Holdings, and as such has voting and investment control over the shares held by Glenn Holdings. |
| (9) | Consists of (i) 220,000 shares of common stock held directly by Mr. LaRue, including 151,250 shares subject to repurchase by Oncternal, and (ii) 136,677 shares of common stock and warrants to purchase 3,834 shares of common stock held by a family trust (the “LaRue Trust”). Mr. LaRue and his wife, Joyce LaRue, are the trustees of the LaRue Trust, and in such capacity have joint power to vote and dispose of the shares held by the LaRue Trust. |
373
PRINCIPAL STOCKHOLDERS OF COMBINED ORGANIZATION
Except where specifically noted, the following information does not give effect to the GTx Reverse Stock Split described in GTx Proposal No. 2.
The following table and the related notes present certain information with respect to the beneficial ownership of the common stock of the combined organization upon consummation of the merger, assuming the closing of the merger occurred on March 31, 2019, by:
| • | each director and named executive officer of the combined organization’s; |
| • | all of the combined organization’s directors and executive officers as a group; and |
| • | each person or group who is known to the management of Oncternal or GTx to become the beneficial owner of more than 5% of the common stock of the combined organization upon the consummation of the merger. |
Unless otherwise indicated in the footnotes to this table, Oncternal and GTx believe that each of the persons named in this table have sole voting and investment power with respect to the shares indicated as beneficially owned.
The following table assumes an exchange ratio of 0.5137 and that the closing of the merger occurred on March 31, 2019. Immediately prior to the merger and after the conversion of Oncternal preferred stock in Oncternal common stock, GTx will have 24,207,270 shares of common stock outstanding and Oncternal will have 162,317,356 shares of common stock outstanding, after giving effect to the conversion of the outstanding Oncternal preferred stock. Upon the closing of the merger, the 162,317,356 shares of Oncternal’s common stock will be converted into the right to receive an aggregate of 83,380,596 shares of GTx’s common stock, there will be a total of 107,587,866 shares of GTx’s common stock outstanding upon the closing of the merger. The following table does not give effect to the GTx Reverse Stock Split to be implemented prior to the closing of the merger. Shares of GTx’s common stock that may be acquired by an individual or group within 60 days of March 31, 2019, pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of GTx’s common stock of any other person shown in the table.
5% Stockholders | Number of Shares | Approximate Percent Owned | ||||||
Shanghai Pharmaceutical (USA) Inc.(1) | 17,465,416 | 16.2 | % | |||||
J. R. Hyde, III(3) | 10,010,446 | 9.0 | % | |||||
Entities affiliated with MagnaSci Ventures(2) | 9,976,975 | 9.2 | % | |||||
The Pyramid Peak Foundation(4) | 7,183,900 | 6.7 | % | |||||
Directors and Named Executive Officers | Number of Shares | Approximate Percent Owned | ||||||
James B. Breitmeyer, M.D., Ph.D.(5) | 2,782,359 | 2.6 | % | |||||
Richard G. Vincent(6) | 357,731 | * | ||||||
Hazel M. Aker(7) | 66,078 | * | ||||||
Michael G. Carter, M.D., Ch.B., F.R.C.P.(8) | 39,131 | * | ||||||
David F. Hale(9) | 5,047,208 | 4.7 | % | |||||
Daniel L. Kisner, M.D. | — | * | ||||||
Yanjun Liu, M.D., Ph.D. | — | * | ||||||
Xin Nakanishi, Ph.D. | — | * | ||||||
William LaRue(10) | 185,189 | * | ||||||
Charles Theuer | 102,737 | * | ||||||
Robert J. Wills, Ph.D.(11) | 150,678 | * | ||||||
All current executive officers and directors as a group (10 persons) | 8,731,111 | 8.1 | % | |||||
374
| * | Represents beneficial ownership of less than 1% of the shares of common stock. |
| (1) | SPH USA is a wholly-owned subsidiary of SPH. The board of directors of SPH has voting and investment power over the shares held by SPH USA, and consists of Zhou Jun, Cho Man, Li Yongzhong, Shen Bo, Li An, Wan Kam To, Tse Cho Che, Cai Jiangnan and Hong Liang. Directors Yanjun Liu, M.D., Ph.D. and Xin Nakanishi, Ph.D. are affiliated with SPH but do not have voting or investment power over the shares held by SPH USA. The registered address of SPH USA is Two Penn Center Plaza, Suite 200, 1500 John F. Kennedy Blvd., Philadelphia, PA 19102. |
| (2) | Consists of (i) 4,857,212 shares of common stock and warrants to purchase 728,581 shares of common stock held by MagnaSci Fund, L.P., (ii) 1,255,683 shares of common stock and warrants to purchase 188,352 shares of common stock held by MagnaSci Fund II, L.P. and (iii) 2,562,735 shares of common stock and warrants to purchase 384,410 shares of common stock held by MagnaSciCo-Investments, L.L.C. MagnaSci GP, L.L.C. is the sole general partner of MagnaSci Fund and MagnaSci Fund II. Cooper Collins is a Manager of MagnaSci GP and MagnaSciCo-Investments, and has voting and investment power over the shares held by MagnaSci Fund, MagnaSci Fund II and MagnaSciCo-Investments. The address of MagnaSci Fund, MagnaSci Fund II and MagnaSciCo-Investments is 123 N. Post Oak Lane, Suite 410, Houston, Texas 77024. |
| (3) | Includes 14,535 shares held by Pittco Associates III, L.P. and 391,571 shares held by Pittco Investments, L.P., entities controlled by Mr. Hyde, 2,454,483 shares issuable upon exercise of a warrant issued to Mr. Hyde in November 2014 (the “2014 Warrant”), 678,349 shares issuable upon exercise of a warrant issued to Mr. Hyde in September 2017 (the “2017 Warrant”), and 70,276 shares issuable to Mr. Hyde pursuant to our Directors’ Deferred Compensation Plan. Mr. Hyde also has shared voting and dispositive power over 21,646 shares held by Mr. Hyde’s spouse, 184,480 shares held by trusts for the benefit of Mr. Hyde’s children (the “Hyde Family Trusts”). As trustee of the Hyde Family Trusts , John H. Pontius shares voting and dispositive power over all of the shares held by the Hyde Family Trusts. |
| (4) | Based on information provided to GTx as of February 28, 2019. Includes an aggregate of 2,793,657 shares issuable upon exercise of outstanding warrants. James R. Boyd, Lee B. Harper, O. Mason Hawkins and Andrew R. McCarroll are each a director of the Foundation. Each of such individuals may be deemed to share beneficial ownership of the shares beneficially owned by the Foundation. The foregoing ownership information was provided to us as of February 28, 2019, and, consequently, beneficial ownership may have changed between such date and March 31, 2019. |
| (5) | Consists of (i) 1,789,103 shares of common stock held directly by Dr. Breitmeyer, (ii) 821,901 shares of common stock underlying options held by Dr. Breitmeyer that are exercisable as of March 31, 2019 or that will become exercisable within 60 days after such date, (iii) 150,807 shares of common stock and warrants to purchase 5,136 shares of common stock held by the Breitmeyer Trust, (iv) 5,136 shares of common stock held by Dr. Breitmeyer as custodian for his child and (v) 10,273 shares of common stock underlying options held by Dr. Breitmeyer’s wife, Mary Breitmeyer, that are exercisable as of March 31, 2019 or that will become exercisable within 60 days after such date. Dr. Breitmeyer and Ms. Breitmeyer are the trustees of the Breitmeyer Trust, and in such capacity have joint power to vote and dispose of the shares held by the Breitmeyer Trust. |
| (6) | Consists of (i) 285,558 shares of common stock held directly by Mr. Vincent, including 162,074 shares subject to repurchase by Oncternal and (ii) 70,204 shares of common stock and warrants to purchase 1,969 shares of common stock held by the Vincent Trust. Mr. Vincent and his wife, Stacy Vincent, are the trustees of the Vincent Trust, and in such capacity have joint power to vote and dispose of the shares held by the Vincent Trust. |
| (7) | Consists of (i) 29,989 shares of common stock held directly by Ms. Aker and (ii) 35,104 shares of common stock and warrants to purchase 984 shares of common stock held by the Aker Trust. Ms. Aker and her husband, Larry Aker, are the trustees of the Aker Trust, and in such capacity have joint power to vote and dispose of the shares held by the Aker Trust |
| (8) | Consists of 35,500 shares of common stock issuable upon the exercise of options held by Dr. Carter, and 3,631 shares issuable to Dr. Carter pursuant to our Directors’ Deferred Compensation Plan. |
| (9) | Consists of (i) 4,895,738 shares of common stock and warrants to purchase 23,048 shares of common stock held by Hale BioPharma Ventures, LLC and (ii) 128,422 shares of common stock held by Hale Trading |
375
| Company. Mr. Hale is the Chairman and Chief Executive Officer of Hale BioPharma Ventures and the Managing Director of Hale Trading Company, and as such has voting and investment control over the shares held by Hale BioPharma Ventures and Hale Trading Company. Amounts also include 17,979 shares that were pledged as collateral by Hale BioPharma Ventures in favor of Oxford Finance LLC on one loan. |
| (10) | Consists of (i) 113,011 shares of common stock held directly by Mr. LaRue, including 77,695 shares subject to repurchase by Oncternal, and (ii) 70,209 shares of common stock and warrants to purchase 1,969 shares of common stock held by the LaRue Trust. Mr. LaRue and his wife, Joyce LaRue, are the trustees of the LaRue Trust, and in such capacity have joint power to vote and dispose of the shares held by the LaRue Trust. |
| (11) | Includes 13,334 shares of common stock issuable upon the exercise of options held by Dr. Wills. |
376
Cooley LLP will pass on the validity of GTx’s common stock offered by this proxy statement/prospectus/information statement. The material U.S. federal income tax consequences of the merger will be passed upon for GTx by Cooley LLP, and for Oncternal by Latham & Watkins LLP.
The financial statements of GTx, Inc. at December 31, 2018 and 2017 and for each of the three years in the period ended December 31, 2018, included in the Proxy Statement of GTx, Inc., which is referred to and made a part of this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements of Oncternal Therapeutics, Inc. as of December 31, 2018 and 2017 and for each of the two years in the period ended December 31, 2018, included herein in reliance upon the report of BDO USA, LLP, an independent registered public accounting firm (the report on the financial statements contains an explanatory paragraph regarding our ability to continue as a going concern), given on the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
GTx files annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy any reports, statements or other information that GTx files at the SEC public reference rooms in Washington, D.C.; New York, New York; and Chicago, Illinois. Please call the SEC at1-800-SEC-0330 for further information on the public reference rooms. GTx SEC filings are also available to the public from commercial document retrieval services and on the website maintained by the SEC at http://www.sec.gov. Reports, proxy statements and other information concerning GTx also may be inspected at the offices of the National Association of Securities Dealers, Inc., Listing Section, 1735 K Street, Washington, D.C. 20006.
As of the date of this proxy statement/prospectus/information statement, GTx has filed a registration statement on FormS-4 to register with the SEC GTx’s common stock that GTx will issue to Oncternal’s stockholders in the merger. This proxy statement/prospectus/information statement is a part of that registration statement and constitutes a prospectus of GTx, as well as a proxy statement of GTx for its special meeting and an information statement for the purpose of Oncternal for its written consent.
GTx has supplied all information contained in this proxy statement/prospectus/information statement relating to GTx, and Oncternal has supplied all information contained in this proxy statement/prospectus/information statement relating to Oncternal.
If you would like to request documents from GTx or Oncternal, please send a request in writing or by telephone to either GTx or Oncternal at the following addresses:
GTx, Inc. 17 W Pontotoc Ave., Suite 100 Memphis, TN 38103 Telephone: (901)523-9700 Attn: Chief Legal Officer | Oncternal Therapeutics, Inc. 12230 El Camino Real, Ste 300 San Diego, California 92130 Telephone: (858)434-1113 Attn: Chief Financial Officer |
377
“GTx” is a registered and unregistered trademark of GTx in the United States and other jurisdictions. “Oncternal,” the Oncternal logo and other trademarks, service marks, and trade names of Oncternal are registered and unregistered marks of Oncternal Therapeutics, Inc. Other third-party logos and product/trade names are registered trademarks or trade names of their respective companies.
378
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires GTx’s officers and directors, and persons who own more than 10% of a registered class of GTx’s equity securities, to file reports of ownership and changes in ownership with the SEC. Such officers, directors and 10% stockholders are also required by SEC rules to furnish GTx with copies of all forms that they file pursuant to Section 16(a). Based on GTx’s review of the copies of such forms received by it and written representations from such executive officers, directors and stockholders, GTx believes that during fiscal 2018, its executive officers, directors and 10% stockholders complied with all applicable Section 16(a) filing requirements.
Copies of such filings can be found at GTx’s corporate website atwww.gtxinc.com under “Investors” at “SEC Filings.”
Stockholder Proposals
Requirements for Stockholder Proposals to Be Considered for Inclusion in GTx’s Proxy Materials. Stockholders of GTx may submit proposals on matters appropriate for stockholder action at meetings of GTx’s stockholders in accordance with Rule 14a-8 promulgated under the Exchange Act. For such proposals to be included in GTx’s proxy materials relating to the 2020 Annual Meeting of Stockholders, all applicable requirements of Rule 14a-8 must be satisfied and such proposals must be received at GTx’s executive offices no later than 120 calendar days before the anniversary of the date the proxy statement is released to shareholders in connection with the 2019 Annual Meeting of Stockholders. GTx has not yet set the date for its 2019 Annual Meeting of Stockholders, but if the GTx 2020 Annual Meeting of Stockholders is not held within 30 days from the anniversary of the 2019 Annual Meeting of Stockholders, then the deadline will be a reasonable time prior to the time GTx begins to print and send its proxy materials. All such proposals must comply with all applicable requirements of Rule 14a-8. Prior to the consummation of the merger, such proposals must be sent to the GTx Corporate Secretary at GTx, Inc., 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103 by the close of business on the required deadline. After the consumation of the merger, such proposals must be sent to the combined company’s Corporate Secretary at Oncternal Therapeutics, Inc., 12230 El Camino Real, Ste 300, San Diego, California 92130 by the close of business on the required deadline.
Requirements for Stockholder Proposals and Director Nominations at the 2020 Annual Meeting. Pursuant to GTx’s amended and restated bylaws (the “GTx bylaws”), stockholders wishing to submit proposals or director nominations, except in the case of proposals made in accordance with Rule 14a-8, must, in addition to complying with applicable laws and regulations and the requirements of the GTx bylaws, provide timely notice thereof in writing to the GTx Corporate Secretary. To be timely for the 2020 Annual Meeting of Stockholders, a stockholder must notify the GTx Corporate Secretary, in writing, not later than the close of business on the one hundred twentieth (120th) day, nor earlier than the close of business on the one hundred fiftieth (150th) day, prior to the anniversary of the date of the proxy statement is delivered to stockholders in connection with the 2019 Annual Meeting of Stockholders. GTx also advises stockholders to review its bylaws, which contain additional requirements about advance notice of stockholder proposals and director nominations. GTx has not yet set the date for its 2019 Annual Meeting of Stockholders, but if the GTx 2020 Annual Meeting of Stockholders is not held within 30 days from the one year anniversary of the 2019 Annual Meeting of Stockholders, then the deadline will be no earlier than the close of business on the one hundred twentieth (120th) day prior to such the 2020 Annual Meeting of Stockholders and not later than (i) the close of business on the later of the ninetieth (90th) day prior to the 2020 Annual Meeting of Stockholders or (ii) the tenth (10th) day following the day on which public announcement of the date of the 2020 Annual Meeting of Stockholders is first made. A stockholder’s notice to the GTx Corporate Secretary must set forth the information required by its bylaws with respect to each director nominee or proposal the stockholder proposes to bring before the annual meeting. The chairman of the 2020 Annual Meeting of Stockholders may determine, if the facts warrant, that a matter has not been properly brought before the meeting and, therefore, may not be considered at the meeting. A copy of the
379
GTx bylaws may be obtained by writing to the GTx Corporate Secretary at the address listed above. In addition, the proxy solicited by the GTx board of directors for the 2020 Annual Meeting of Stockholders will confer discretionary voting authority with respect to (i) any proposal presented by a stockholder at that meeting for which GTx has not been provided with timely notice and (ii) any proposal made in accordance with GTx’s bylaws, if the proxy statement for the 2020 Annual Meeting of Stockholders briefly describes the matter and how management proxy holders intend to vote on it, if the stockholder does not comply with the requirements of Rule 14a-4(c)(2) promulgated under the Exchange Act.
Stockholder Nomination Policy
It is the GTx Nominating and Corporate Governance Committee’s policy to review and consider all candidates for nomination and election as directors who may be suggested by any director or executive officer of GTx. The GTx Nominating and Corporate Governance Committee will also consider any director candidate recommended by any stockholder if the recommendation is made in accordance with GTx’s charter, bylaws and applicable law although no director candidate has been recommended to date by any stockholder, other than members of the GTx board of directors and management who are also stockholders of GTx. To be considered, a recommendation for director nomination should be submitted in writing. Prior to the consummation of the merger, such recommendations should be sent to: GTx, Inc., Nominating and Corporate Governance Committee, Attention: Corporate Secretary, 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103. After the consummation of the merger, such recommendations should be sent to: Oncternal Therapeutics, Inc., 12230 El Camino Real, Ste 300, San Diego, California 92130. When submitting candidates for nomination to be elected at GTx’s annual meetings of stockholders, stockholders must follow the notice procedures and provide the information required by GTx’s bylaws. In particular, for the GTx Nominating and Corporate Governance Committee to consider a candidate recommended by a stockholder for nomination at the 2020 Annual Meeting of Stockholders, the recommendation must be delivered to GTx’s Corporate Secretary, in writing, not later than the close of business on the one hundred twentieth (120th) day, nor earlier than the close of business on the one hundred fiftieth (150th) day, prior to the anniversary of the date of the proxy statement delivered to stockholders in connection with the 2019 Annual Meeting of Stockholders. If the GTx 2020 Annual Meeting of Stockholders is not held within 30 days from the anniversary of the 2019 Annual Meeting of Stockholders, then the deadline will be no earlier than the close of business on the one hundred twentieth (120th) day prior to such the 2020 Annual Meeting of Stockholders and not later than the close of business on the later of (i) the ninetieth (90th) day prior to the 2020 Annual Meeting of Stockholders or (ii) the tenth (10th) day following the day on which public announcement of the date of the 2020 Annual Meeting of Stockholders is first made. The recommendation must include the same information as is specified in GTx’s bylaws for stockholder nominees to be considered at an annual meeting, including the following:
| • | the stockholder’s name and address and the beneficial owner, if any, on whose behalf the nomination is proposed; |
| • | the class and number of shares of GTx that are owned beneficially and of record by such stockholder and such beneficial owner; |
| • | a description of all arrangements or understandings between the stockholder and the proposed nominee and any other person or persons regarding the nomination; |
| • | the nominee’s written consent to being named in GTx’s proxy statement as a nominee and to serving as a director if elected; and |
| • | all information regarding the nominee that would be required to be included in GTx’s proxy statement by the rules of the SEC, including the nominee’s age, business experience for the past five years and any directorships held by the nominee during the past five years. |
Code of Business Conduct and Ethics and Guidelines on Governance Issues
The GTx Board has adopted a Code of Business Conduct and Ethics applicable to all officers, directors and employees as well as Guidelines on Governance Issues. These documents are available on GTx’s website
380
(www.gtxinc.com) under “Investors” at “Corporate Governance.” GTx will provide a copy of these documents to any stockholder, without charge, upon request, by writing to: GTx, Inc., Corporate Secretary, 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103. GTx intends to satisfy the disclosure requirement under Item 5.05 ofForm 8-K regarding an amendment to, or waiver from, a provision of the Code of Business Conduct and Ethics by posting such information on its website at the address and the locations specified above.
Communications with the GTx Board
Stockholders and other interested parties may communicate in writing with the GTx Board, any of its committees, or with any of itsnon-management directors by sending written communications addressed to: GTx, Inc., Attention: Corporate Secretary, 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103. The GTx Corporate Secretary will review each communication and will forward such communication to the board of directors or to any individual director to whom the communication is addressed unless the communication is unduly hostile, threatening or similarly inappropriate, in which case, the GTx Corporate Secretary will discard the communication.
Policies on Reporting Certain Concerns Regarding Accounting and Other Matters
GTx has adopted policies on the reporting of concerns to GTx’s Compliance Officer and GTx Audit Committee regarding any suspected misconduct, illegal activities or fraud, including any questionable accounting, internal accounting controls or auditing matters, or misconduct. Any person who has a concern regarding any misconduct by any GTx employee, including any GTx officer, or any agent of GTx, may submit that concern to: GTx, Inc., Attention: Compliance Officer, 17 W Pontotoc Ave., Suite 100, Memphis, Tennessee 38103. Employees may communicate all concerns regarding any misconduct to the GTx Compliance Officer and/or the GTx Audit Committee on a confidential and anonymous basis through GTx’s “whistleblower” hotline, the compliance communication phone number established by GTx:1-877-778-5463, or by filing an anonymous, confidential report throughReport-it.com, aweb-based online service for “whistleblower” communications accessed at www.reportit.net. Any communications received through the toll free number or the online service is promptly reported to GTx’s Compliance Officer, as well as other appropriate persons within GTx.
381
F-A-1
INTERNAL CONTROL OVER FINANCIAL REPORTING
We, as management of GTx, Inc., are responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Securities Exchange Act Rule13a-15(f). Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with United States generally accepted accounting principles. Any system of internal control, no matter how well designed, has inherent limitations, including the possibility that a control can be circumvented or overridden and misstatements due to error or fraud may occur and not be detected. Also, because of changes in conditions, internal control effectiveness may vary over time. Accordingly, even an effective system of internal control will provide only reasonable assurance that the objectives of the internal control system are met.
Under the supervision and with the participation of management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2018 using the criteria for effective internal control over financial reporting as described in “Internal Control – Integrated Framework,” issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on this evaluation, we concluded that, as of December 31, 2018, our internal control over financial reporting was effective. The effectiveness of our internal control over financial reporting has been audited by Ernst & Young LLP, independent registered public accounting firm who also audited the Company’s financial statements included in this Annual Report onForm 10-K. Ernst & Young LLP’s report on the Company’s internal control over financial reporting is included in this Annual Report on the10-K.
/s/ Marc S. Hanover | /s/ Jason T. Shackelford | |||
Marc S. Hanover | Jason T. Shackelford | |||
| Chief Executive Officer | Vice President, Finance and Accounting | |||
| Principal Executive Officer | Principal Financial and Accounting Officer |
Memphis, Tennessee
March 18, 2019
F-A-2
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of GTx, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of GTx, Inc. as of December 31, 2018 and 2017, the related statements of operations, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2018, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of GTx, Inc. at December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 18, 2019 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 1998.
Memphis, Tennessee
March 18, 2019
F-A-3
GTx, Inc.
(in thousands, except share and per share data)
| December 31, | ||||||||
| 2018 | 2017 | |||||||
ASSETS | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 28,258 | $ | 15,816 | ||||
Short-term investments | 200 | 28,083 | ||||||
Prepaid expenses and other current assets | 2,750 | 2,178 | ||||||
|
|
|
| |||||
Total current assets | 31,208 | 46,077 | ||||||
Property and equipment, net | 19 | 51 | ||||||
Intangible assets, net | 94 | 108 | ||||||
|
|
|
| |||||
Total assets | $ | 31,321 | $ | 46,236 | ||||
|
|
|
| |||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 3,279 | $ | 2,604 | ||||
Accrued expenses and other current liabilities | 1,931 | 5,371 | ||||||
|
|
|
| |||||
Total current liabilities | 5,210 | 7,975 | ||||||
Commitments and contingencies | ||||||||
Stockholders’ equity: | ||||||||
Common stock, $0.001 par value: 60,000,000 shares authorized at December 31, 2018 and December 31, 2017; 24,051,844 and 21,541,909 shares issued and outstanding at December 31, 2018 and December 31, 2017, respectively | 24 | 22 | ||||||
Additionalpaid-in capital | 626,142 | 599,876 | ||||||
Accumulated deficit | (600,055 | ) | (561,637 | ) | ||||
|
|
|
| |||||
Total stockholders’ equity | 26,111 | 38,261 | ||||||
|
|
|
| |||||
Total liabilities and stockholders’ equity | $ | 31,321 | $ | 46,236 | ||||
|
|
|
| |||||
The accompanying notes are an integral part of these financial statements.
F-A-4
GTx, Inc.
(in thousands, except share and per share data)
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Expenses: | ||||||||||||
Research and development expenses | $ | 29,669 | $ | 21,467 | $ | 17,228 | ||||||
General and administrative expenses | 9,390 | 9,188 | 8,705 | |||||||||
|
|
|
|
|
| |||||||
Total expenses | 39,059 | 30,655 | 25,933 | |||||||||
|
|
|
|
|
| |||||||
Loss from operations | (39,059 | ) | (30,655 | ) | (25,933 | ) | ||||||
Other income, net | 641 | 216 | 46 | |||||||||
Gain on change in fair value of warrant liability | — | — | 8,163 | |||||||||
|
|
|
|
|
| |||||||
Net loss | $ | (38,418 | ) | $ | (30,439 | ) | $ | (17,724 | ) | |||
|
|
|
|
|
| |||||||
Net loss per share: | ||||||||||||
Basic and Diluted | $ | (1.65 | ) | $ | (1.75 | ) | $ | (1.22 | ) | |||
|
|
|
|
|
| |||||||
Weighted average shares outstanding: | ||||||||||||
Basic and Diluted | 23,346,231 | 17,441,280 | 14,559,541 | |||||||||
|
|
|
|
|
| |||||||
The accompanying notes are an integral part of these financial statements.
F-A-5
GTx, Inc.
STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2018, 2017 and 2016
(in thousands, except share data)
| Stockholders’ Equity | ||||||||||||||||||||
| Common Stock | Additional Paid-in Capital | Accumulated Deficit | Total Stockholders’ Equity | |||||||||||||||||
| Shares | Amount | |||||||||||||||||||
Balances at January 1, 2016 | 14,037,411 | $ | 14 | $ | 515,319 | $ | (513,474 | ) | $ | 1,859 | ||||||||||
Issuance of common stock in October 2016 registered direct offering, net of offering costs | 1,728,395 | 2 | 13,690 | — | 13,692 | |||||||||||||||
Vesting of restricted stock units, net of shares withheld for tax payments | 154,170 | — | (208 | ) | — | (208 | ) | |||||||||||||
Directors’ deferred compensation | — | — | 132 | — | 132 | |||||||||||||||
Share-based compensation | — | — | 2,957 | — | 2,957 | |||||||||||||||
Warrant liability reclassification | — | — | 19,186 | — | 19,186 | |||||||||||||||
Settlement of fractional shares upon reverse stock split | (404 | ) | — | (3 | ) | — | (3 | ) | ||||||||||||
Net loss | — | — | — | (17,724 | ) | (17,724 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2016 | 15,919,572 | 16 | 551,073 | (531,198 | ) | 19,891 | ||||||||||||||
Issuance of common stock and warrants in September 2017 private placement, net of offering costs | 5,483,320 | 6 | 45,642 | — | 45,648 | |||||||||||||||
Vesting of restricted stock units, net of shares withheld for tax payments | 139,017 | — | (156 | ) | — | (156 | ) | |||||||||||||
Directors’ deferred compensation | — | — | 166 | — | 166 | |||||||||||||||
Share-based compensation | — | — | 3,151 | — | 3,151 | |||||||||||||||
Net loss | — | — | — | (30,439 | ) | (30,439 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2017 | 21,541,909 | 22 | 599,876 | (561,637 | ) | 38,261 | ||||||||||||||
Issuance of common stock upon exercise of warrants | 674,579 | 1 | (1 | ) | — | — | ||||||||||||||
Exercise of stock options | 6,000 | — | 103 | — | 103 | |||||||||||||||
Issuance of shares under“At-The-Market” sales agreement, net of issuance costs | 1,501,501 | 1 | 24,473 | — | 24,474 | |||||||||||||||
Vesting of restricted stock units, net of shares withheld for tax payments | 327,855 | — | (672 | ) | — | (672 | ) | |||||||||||||
Directors’ deferred compensation | — | — | 166 | — | 166 | |||||||||||||||
Share-based compensation | — | — | 2,197 | — | 2,197 | |||||||||||||||
Net loss | — | — | — | (38,418 | ) | (38,418 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Balances at December 31, 2018 | 24,051,844 | $ | 24 | $ | 626,142 | $ | (600,055 | ) | $ | 26,111 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
The accompanying notes are an integral part of these financial statements.
F-A-6
GTx, Inc.
(in thousands)
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Cash flows from operating activities: | ||||||||||||
Net loss | $ | (38,418 | ) | $ | (30,439 | ) | $ | (17,724 | ) | |||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
Gain on change in fair value of warrant liability | — | — | (8,163 | ) | ||||||||
Share-based compensation | 2,197 | 3,151 | 2,957 | |||||||||
Directors’ deferred compensation | 166 | 166 | 132 | |||||||||
Depreciation and amortization | 46 | 47 | 28 | |||||||||
Changes in assets and liabilities: | ||||||||||||
Prepaid expenses and other assets | (572 | ) | 251 | 204 | ||||||||
Accounts payable | 675 | 1,384 | 838 | |||||||||
Accrued expenses and other liabilities | (3,440 | ) | 1,980 | 950 | ||||||||
|
|
|
|
|
| |||||||
Net cash used in operating activities | (39,346 | ) | (23,460 | ) | (20,778 | ) | ||||||
|
|
|
|
|
| |||||||
Cash flows from investing activities: | ||||||||||||
Purchase of property and equipment | — | (2 | ) | (90 | ) | |||||||
Purchase of short-term investments, held to maturity | (44,155 | ) | (39,283 | ) | (35,404 | ) | ||||||
Proceeds from maturities of short-term investments, held to maturity | 72,038 | 24,159 | 37,645 | |||||||||
|
|
|
|
|
| |||||||
Net cash provided by (used in) investing activities | 27,883 | (15,126 | ) | 2,151 | ||||||||
|
|
|
|
|
| |||||||
Cash flows from financing activities: | ||||||||||||
Net proceeds from the issuance of common stock and warrants | 24,474 | 45,648 | 13,692 | |||||||||
Tax payments related to shares withheld for vested restricted stock units | (672 | ) | (156 | ) | (208 | ) | ||||||
Proceeds from exercise of employee stock options | 103 | — | — | |||||||||
Settlement of fractional shares upon reverse stock split | — | — | (3 | ) | ||||||||
|
|
|
|
|
| |||||||
Net cash provided by financing activities | 23,905 | 45,492 | 13,481 | |||||||||
|
|
|
|
|
| |||||||
Net increase (decrease) in cash and cash equivalents | 12,442 | 6,906 | (5,146 | ) | ||||||||
Cash and cash equivalents, beginning of period | 15,816 | 8,910 | 14,056 | |||||||||
|
|
|
|
|
| |||||||
Cash and cash equivalents, end of period | $ | 28,258 | $ | 15,816 | $ | 8,910 | ||||||
|
|
|
|
|
| |||||||
The accompanying notes are an integral part of these financial statements.
F-A-7
GTx, Inc.
(in thousands, except share and per share data)
1. Business
GTx, Inc. (“GTx” or the “Company”), a Delaware corporation incorporated on September 24, 1997 and headquartered in Memphis, Tennessee, is a biopharmaceutical company dedicated to the discovery, development and commercialization of medicines to treat serious and/or significant unmet medical conditions.
In 2015, the Company entered into an exclusive license agreement with the University of Tennessee Research Foundation (“UTRF”) to develop UTRF’s proprietary selective androgen receptor degrader (“SARD”) technology which may have the potential to provide compounds that can degrade or antagonize multiple forms of androgen receptor to treat those patients who do not respond or are resistant to current androgen targeted therapies by inhibiting tumor growth in patients with progressive castration-resistant prostate cancer (“CRPC”). The Company is in the process of completing ongoing mechanistic preclinical studies in order to select the most appropriate SARD compounds to move forward into the additional preclinical studies required to submit an investigational new drug application (“IND”), and potentially advance one of its SARD compounds into afirst-in-human clinical trial.
The Company had been developing selective androgen receptor modulators (“SARMs”), including enobosarm(GTx-024). Most recently, enobosarm was evaluated in post-menopausal women with stress urinary incontinence (“SUI”) compared to placebo. During the third quarter of 2018, the Company announced that the Phase 2 double-blind, placebo-controlled clinical trial of orally-administered enobosarm (3 mg or 1 mg) in post-menopausal women with SUI (the “ASTRID trial”) did not achieve statistical significance on the primary endpoint for the trial. The Company has completed the ASTRID trial, including its review of the full data sets from the clinical trial, and has determined that there is not a sufficient path forward to warrant additional clinical development of enobosarm to treat SUI. The Company has therefore discontinued further development of enobosarm to treat SUI, including discontinuing the related durability and open-label safety extension studies that the Company initiated before it received topline data from the ASTRID trial. The Company has also discontinued any further development of its SARM program generally.
Following the announcement of the ASTRID trial results, the Company’s board of directors commenced a process of evaluating strategic alternatives to maximize stockholder value. To assist with this process, the Company’s board of directors engaged a financial advisory firm to help explore the Company’s available strategic alternatives, including possible mergers and business combinations, a sale of part or all of the Company’s assets, and collaboration and licensing arrangements. On March 6, 2019, the Company and Oncternal Therapeutics Inc. (“Oncternal”) announced the signing of an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, including approval of the transaction by the Company’s stockholders and Oncternal’s stockholders, a wholly-owned subsidiary of the Company will be merged with and into Oncternal (the “Merger”), with Oncternal surviving the Merger as a wholly-owned subsidiary of the Company. See Note 2, Significant Accounting Policies – Subsequent Events, for further discussion regarding the proposed Merger.
At December 31, 2018, the Company had cash, cash equivalents and short-term investments of $28,458 compared to $43,899 at December 31, 2017. To conserve its cash resources, the Company has substantially reduced its workforce since November 2018 and has ceased its SARM development activities and all other operations except forday-to-day business operations, completing ongoing mechanistic SARD preclinical studies and those activities necessary to complete the proposed Merger.
F-A-8
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
2. Significant Accounting Policies
Basis of Presentation
The accompanying financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). Additionally, GTx operates in one business segment.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual amounts and results could differ from those estimates.
Cash and Cash Equivalents
TheCompany considers highly liquid investments with initial maturities of three months or less to be cash equivalents.
Short-term Investments
At December 31, 2018 and 2017, short-term investments consisted of Federal Deposit Insurance Corporation (“FDIC”) insured certificates of deposit with original maturities of greater than three months and less than one year.
Property and Equipment
Property and equipment is stated at cost. Amortization of leasehold improvements is recognized over the shorter of the estimated useful life of the leasehold improvement or the lease term. Depreciation is computed using thestraight-line method over the estimated useful lives as follows:
Office equipment | 3 to 5 years | |||
Leasehold improvements | 3 to 7 years | |||
Furniture and fixtures | 5 years | |||
Computer equipment and software | 3 years |
Warrant Liability
In November 2014, the Company issued warrants to purchase 6,430,948 shares of its common stock. The Company classified these warrants as a liability on its balance sheet since the warrants contained certain terms that could have required the Company (or its successor) to purchase the warrants for cash in an amount equal to the value (as calculated utilizing a contractually-agreed Black-Scholes-Merton option pricing valuation model (“Black-Scholes Model”)) of the unexercised portion of the warrants in connection with certain change of control transactions occurring on or prior to December 31, 2016, with such cash payment capped at an amount equal to $1.25 per unexercised share underlying each warrant. As a result of the provision of the warrants requiring cash settlement upon certain change of control transactions, the Company was required to account for these warrants as a liability at fair value and the estimated warrant liability was required to be revalued at each balance sheet date until the earlier of the exercise of the warrants, the modification to remove the provision that could require cash settlement upon certain change of control transactions or the expiration of such provision on December 31,
F-A-9
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
2016. Effective March 25, 2016, each of the warrants was amended by agreement of the warrant holders to remove the provision that could require cash settlement upon certain change of control transactions. These warrants were no longer accounted for as a liability as of March 31, 2016. The Company recorded anon-cash reclassification of the warrant fair value to stockholders’ equity based on the warrants’ fair value as of the March 25, 2016 modification date, with no further adjustments to the fair value of these warrants being required.
Fair Value of Financial Instruments and Warrant Liability
The carrying amounts of the Company’s financial instruments (which include cash, cash equivalents, short-term investments, and accounts payable) and its prior warrant liability approximate their fair values. The fair value of the warrant liability was estimated using the Black-Scholes-Merton Model. See Note 6, Stockholders’ Equity, for additional disclosure on the valuation methodology and significant assumptions. The Company’s financial assets and liabilities are classified within a three-level fair value hierarchy that prioritizes the inputs used to measure fair value, which is defined as follows:
Level 1 — Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date
Level 2 — Inputs other than quoted prices in active markets that are observable for the asset or liability, either directly or indirectly
Level 3 — Inputs that are unobservable for the asset or liability
As the Company has the positive intent and ability to hold its certificates of deposit classified as short-term investments until maturity, these investments have been classified as held to maturity investments and are stated at cost, which approximates fair value. The Company considers these to be Level 2 investments as the fair values of these investments are determined using third-party pricing sources, which generally utilize observable inputs, such as interest rates and maturities of similar assets.
Concentration of Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist of cash and cash equivalents and short-term investments. The Company has established guidelines relating to diversification and maturities of its cash equivalents and short-term investments which are designed to manage risk. The Company’s cash and cash equivalents consist of bank deposits, certificates of deposit, and money market mutual funds. Bank deposits may at times be in excess of FDIC insurance limits. The Company’s short-term investments consist of FDIC insured certificates of deposit with original maturities of greater than three months and less than one year.
Research and Development Expenses
Research and development expenses include, but are not limited to, the Company’s expenses for personnel, supplies, and facilities associated with research activities, screening and identification of product candidates, formulation and synthesis activities, manufacturing, preclinical studies, toxicology studies, clinical trials, regulatory and medical affairs activities, quality assurance activities and license fees. The Company expenses these costs in the period in which they are incurred. The Company estimates its liabilities for research and development expenses in order to match the recognition of expenses to the period in which the actual services are received. As such, accrued liabilities related to third party research and development activities are recognized based upon the Company’s estimate of services received and degree of completion of the services in accordance with the specific third party contract.
F-A-10
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Patent Costs
The Company expenses patent costs, including legal expenses, in the period in which they are incurred. Patent expenses are included in general and administrative expenses in the Company’s statements of operations.
Income Taxes
The Company accounts for deferred taxes by recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. Accordingly, at December 31, 2018 and December 31, 2017, net of the valuation allowance, the net deferred tax assets were reduced to zero. See Note 8,Income Taxes, for further discussion.
Share-Based Compensation
The Company has stock option and equity incentive plans that provide for the purchase or acquisition of the Company’s common stock by certain of the Company’s employees andnon-employees. The Company recognizes compensation expense for its share-based payments based on the fair value of the awards over the period during which an employee ornon-employee is required to provide service in exchange for the award. See Note 3,Share-Based Compensation, for further discussion.
Other Income (Expense), Net
Other income (expense), net consists of interest earned on the Company’s cash, cash equivalents and short-term investments, foreign currency transaction gains and losses, and othernon-operating income or expense.
Basic and Diluted Net Loss Per Share
Basic and diluted net income (loss) per share attributable to common stockholders is calculated based on the weighted average number of common shares outstanding during the period. Diluted net income (loss) per share gives effect to the dilutive potential of common stock consisting of stock options, unvested RSUs and common stock warrants.
Weighted average potential shares of common stock of 11,191,431, 9,438,236, and 8,162,347 were excluded from the calculation of diluted net loss per share for the years ended December 31, 2018, 2017 and 2016, respectively, as inclusion of the potential shares would have had an anti-dilutive effect on the net loss per share for the periods. At December 31, 2018, the Company had 24,051,844 shares of common stock outstanding.
Comprehensive Loss
For all periods presented, there were no differences between net loss and comprehensive loss.
Recent Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board issued Accounting Standard Update (“ASU”)2016-02,Leases (Topic 842). This ASU requires that lessees recognize assets and liabilities on the balance sheet
F-A-11
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
for the present value of the rights and obligations created by all leases with terms of more than 12 months. The ASU also will require disclosures designed to give financial statement users information on the amount, timing, and uncertainty of cash flows arising from leases. This new guidance will be effective for the Company as of January 1, 2019. The Company does not expect the adoption of the standard update to have a significant impact on its financial position or results of operations.
Subsequent Events
The Company has evaluated all events or transactions that occurred after December 31, 2018 up through the date the financial statements were issued. Other than as set forth below, there were no material recognizable or nonrecognizable subsequent events during the period evaluated.
Merger Agreement with Oncternal and Related Matters
Merger Agreement
On March 6, 2019, the Company entered into the Merger Agreement with Oncternal and Grizzly Merger Sub, Inc., a wholly-owned subsidiary of the Company (“Merger Sub”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, including approval of the transaction by the Company’s stockholders and Oncternal’s stockholders, Merger Sub will be merged with and into Oncternal, with Oncternal surviving the Merger as a wholly-owned subsidiary of the Company.
Subject to the terms and conditions of the Merger Agreement, at the effective time of the Merger (the “Effective Time”): (i) each share of Oncternal common stock outstanding immediately prior to the Effective Time (excluding shares held by the Company, Merger Sub or Oncternal and dissenting shares) will be converted solely into the right to receive a number of shares of the Company’s common stock (the “Shares”) equal to the exchange ratio described below, (ii) each outstanding Oncternal stock option will be assumed by the Company, and (iii) each outstanding Oncternal warrant will be assumed by the Company.
Under the exchange ratio formula in the Merger Agreement, the former Oncternal stockholders immediately before the Merger are expected to own approximately 75% of the outstanding capital stock of the Company, and the stockholders of the Company immediately before the Merger are expected to own approximately 25% of the outstanding capital stock of the Company, subject to certain assumptions. The exchange ratio formula excludes Oncternal’s outstanding stock options and warrants and the Company’s outstanding stock options and warrants.
Under certain circumstances further described in the Merger Agreement, the ownership percentages may be adjusted upward or downward based on cash levels of the respective companies at the closing of the Merger (the “Closing”).
The Merger Agreement contains customary representations, warranties and covenants made by the Company and Oncternal, including covenants relating to obtaining the requisite approvals of the stockholders of the Company and Oncternal, indemnification of directors and officers, the Company’s and Oncternal’s conduct of their respective businesses between the date of signing of the Merger Agreement and the Closing. The Closing is subject to satisfaction or waiver of certain conditions included in the Merger Agreement.
F-A-12
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Following the Closing, Oncternal’s Chief Executive Officer, Chief Financial Officer, and Chief Operating Officer will serve in these positions for the Company. Additionally, following the Closing, the Company’s board of directors will consist of nine directors, including two current GTx board members.
The Merger Agreement also includes termination provisions for both the Company and Oncternal. In connection with a termination of the Merger Agreement under specified circumstances, either party may be required to pay the other party a termination fee ranging between $500 to $2,000.
Contingent Value Rights Agreement
At the Effective Time, the Company will enter into a Contingent Value Rights Agreement (the “CVR Agreement”). Pursuant to the CVR Agreement, for each share of the Company’s common stock held, the Company’s stockholders of record as of immediately prior to the Effective Time will receive one contingent value right (“CVR”) entitling such holders to receive in the aggregate 50% of any net proceeds received during the15-year period after closing from the grant, sale or transfer of rights to the Company’s SARD or SARM technology that occurs during the10-year period after the Closing (or in the 11th year if based on a term sheet approved during the initial10-year period) and, if applicable, to receive royalties on the sale of any SARD products by the combined company during the15-year period after Closing. The CVR Agreement will be effective prior to the Closing and will continue in effect until the payment of all amounts payable thereunder, unless terminated upon termination of the Merger Agreement.
Workforce Reduction
In the first quarter of 2019, due to the entry into the Merger Agreement with Oncternal, the Company’s board of directors committed to reducing its workforce by seven employees. All employees affected by the workforce reduction will be eligible to receive, among other things, specified severance payments based on the applicable employee’s level and years of service with the Company and the continuation of group health insurance coverage. In addition, the affected employees will also be eligible for full vesting acceleration of their outstanding stock options as well as an extension of the post-termination exercise period for their outstanding stock options.
As a result of the workforce reduction and prior termination of three employees earlier in the first quarter of 2019, the Company estimates that it will incur total severance-related charges for these employees of approximately $1,000 in the first quarter of 2019 and up to an additional $500 contingent upon the closing of the Merger. The Company does not expect to record anon-cash charge related to the modification of outstanding stock options in connection with the workforce reduction.
Termination of Directors’ Deferred Compensation Plan
Prior to the Effective Time (but in no event more than 30 days prior to the Effective Time), the Company’s board of directors will take all actions necessary to terminate and liquidate the Company’s 2018 Amended and Restated Directors’ Deferred Compensation Plan (the “Directors’ Deferred Compensation Plan”) and all rights or other deferrals thereunder effective immediately prior to the Effective Time, subject to the consummation of the Merger. Any future board compensation under the Directors’ Deferred Compensation Plan on or after January 3, 2019 shall be settled only in cash.
3.Share-Based Compensation
Share-based payments include stock option and RSU grants under the Company’s stock option and equity incentive plans and deferred compensation arrangements for the Company’snon-employee directors.
F-A-13
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
The Company has granted to employees andnon-employees options to purchase common stock under various plans at prices equal to the fair market value of its common stock on the dates the options are granted as determined in accordance with the terms of the applicable plan. The options have a term of ten years from the grant date and generally vest over three years from the grant date for director andnon-employee options and over periods of up to five years from the grant date for employee options. Under the terms of the Company’s stock option and equity incentive plans, employees generally have three months after the employment relationship ends to exercise all vested options except in the case of voluntary retirement, disability or death, where post-termination exercise periods are generally longer. The Company issues new shares of common stock upon the exercise of options. The Company estimates the fair value of stock option awards as of the date of the grant by applying the Black-Scholes Model. The application of this valuation model involves assumptions that are judgmental and highly sensitive in the determination of compensation expense.
The fair value of each stock option is amortized into compensation expense on a straight-line basis between the grant date for the award and each vesting date. During 2017, the Company adopted the Financial Accounting Standards Board Accounting Standards Update2016-09,Improvements to Employee Share Based Payment Accounting. This guidance addresses the income tax effects of stock-based payments and eliminates the windfall pool concept, as all of the tax effects related to stock-based payments are now being recorded at settlement (or expiration) through the income statement. The new guidance also permits entities to make an accounting policy election for the impact of forfeitures on the recognition of expense for stock-based payment awards, allowing for forfeitures to be estimated or recognized when they occur. The Company elected to prospectively adopt the policy that forfeitures be recorded when they occur and prior periods have not been adjusted. The adoption of this guidance did not have a material impact on the Company’s financial position or results of operations.
Additionally, the Company periodically grants RSUs to its employees. The Company estimates the fair value of RSUs using the closing price of its common stock on the grant date. The fair value of the RSUs is amortized on a straight-line basis over the requisite service period of the awards. All RSUs were fully vested at December 31, 2018.
The following table summarizes share-based compensation expense included within the statements of operations for each of the three years in the period ended December 31, 2018:
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
Research and development expenses | $ | 807 | $ | 1,171 | $ | 1,260 | ||||||
General and administrative expenses | 1,556 | 2,146 | 1,829 | |||||||||
|
|
|
|
|
| |||||||
Total share-based compensation | $ | 2,363 | $ | 3,317 | $ | 3,089 | ||||||
|
|
|
|
|
| |||||||
Share-based compensation expense recorded in the statement of operations as general and administrative expense for the years ended December 31, 2018, 2017 and 2016 included share-based compensation expense related to deferred compensation arrangements for the Company’snon-employee directors of $166, $166 and $132, respectively. See Note 9,Directors’ Deferred Compensation Plan, for further discussion of deferred compensation arrangements for the Company’snon-employee directors.
For the years ended December 31, 2018, 2017 and 2016, the weighted average grant date fair value per share of stock options granted was $10.36, $3.80 and $5.45, respectively. The key assumptions used in
F-A-14
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
determining the grant date fair value of options granted in 2018, 2017 and 2016, and a summary of the methodology applied to develop each assumption is as follows:
| Years Ended December 31, | ||||||
| 2018 | 2017 | 2016 | ||||
Expected price volatility | 93.1% | 88.6% | 91.3% | |||
Risk-free interest rate | 2.4% | 2.2% | 2.0% | |||
Weighted average expected life in years | 6.9 years | 6.9 years | 6.9 years | |||
Dividend yield | 0% | 0% | 0% | |||
Expected Price Volatility — This is a measure of the amount by which a price has fluctuated or is expected to fluctuate. The Company based its determination of expected volatility on its historical stock price volatility. An increase in the expected price volatility will increase compensation expense.
Risk-Free Interest Rate — This is determined using U.S. Treasury rates where the term is consistent with the expected life of the stock options. An increase in the risk-free interest rate will increase compensation expense.
Expected Life — This is the period of time over which the options granted are expected to remain outstanding and is determined by calculating the average of the vesting term and the contractual term of the options. The Company has utilized this method due to the lack of historical option exercise information related to the Company’s stock option and equity incentive plans. Options granted have a maximum term of ten years. An increase in the expected life will increase compensation expense.
Dividend Yield — The Company has not made any dividend payments nor does it have plans to pay dividends in the foreseeable future. An increase in the dividend yield will decrease compensation expense.
The following is a summary of stock option transactions for all of the Company’s stock option and equity incentive plans for the three year period ended December 31, 2018:
| Number of Shares | Weighted Average Exercise Price Per Share | |||||||
Options outstanding at January 1, 2016 | 798,309 | $ | 38.80 | |||||
Options granted | 363,500 | 6.94 | ||||||
Options forfeited or expired | (71,829 | ) | 54.65 | |||||
Options exercised | — | — | ||||||
|
|
|
| |||||
Options outstanding at December 31, 2016 | 1,089,980 | 27.13 | ||||||
Options granted | 977,350 | 4.97 | ||||||
Options forfeited or expired | (166,834 | ) | 48.71 | |||||
Options exercised | — | — | ||||||
|
|
|
| |||||
Options outstanding at December 31, 2017 | 1,900,496 | 13.84 | ||||||
Options granted | 472,000 | 13.32 | ||||||
Options forfeited or expired | (31,049 | ) | 168.76 | |||||
Options exercised | (6,000 | ) | 17.23 | |||||
|
|
|
| |||||
Options outstanding and vested or expected to vest at December 31, 2018 | 2,335,447 | $ | 11.67 | |||||
|
|
|
| |||||
F-A-15
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
The following table summarizes information about stock options outstanding at December 31, 2018:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (years) | Weighted Average Exercise Price | Number Exercisable | Weighted Average Exercise Price | |||||||||||||||
| $4.29 — $4.29 | 56,250 | 8.36 | $ | 4.29 | 18,750 | $ | 4.29 | |||||||||||||
| $4.71 — $4.71 | 825,000 | 8.02 | 4.71 | 22,500 | 4.71 | |||||||||||||||
| $4.77 — $12.71 | 826,800 | 7.67 | 9.97 | 134,404 | 8.25 | |||||||||||||||
| $12.95 — $108.90 | 627,397 | 4.20 | 23.71 | 508,691 | 25.59 | |||||||||||||||
|
|
|
| |||||||||||||||||
| 2,335,447 | 6.88 | 11.67 | 684,345 | 20.91 | ||||||||||||||||
|
|
|
| |||||||||||||||||
At December 31, 2018, the aggregate intrinsic value of all outstanding options was zero with a weighted average remaining contractual term of 6.88 years. Of the Company’s outstanding options, 684,345 options were exercisable and had a weighted average remaining contractual term of 4.05 years and no aggregate intrinsic value. Additionally, the Company’s vested and expected to vest options had a weighted average remaining contractual term of 6.88 years and no aggregate intrinsic value.
Options to purchase 6,000 shares were exercised during the years ended December 31, 2018. The total intrinsic value of options exercised during the years ended December 31, 2018 was $39. At December 31, 2018, the total compensation cost related tonon-vested options not yet recognized was $7,697, with a weighted average expense recognition period of 2.95 years. Shares available for future issuance under the Company’s stock option and equity incentive plans were 1,167,162 at December 31, 2018. On January 1, 2019, shares available for future issuance under the 2013 equity incentive plan and the 2013non-employee director equity incentive plan increased by an aggregate of 1,012,074 shares in accordance with the automatic increase provisions of such plans.
The following is a summary of the RSU transactions for all of the Company’s equity incentive plans for the three year period ended December 31, 2018:
| Number of Shares | ||||
Nonvested RSUs outstanding at January 1, 2016 | 820,000 | |||
RSUs granted | 11,000 | |||
RSUs vested | (184,001 | ) | ||
RSUs forfeited | (62,000 | ) | ||
|
| |||
Nonvested RSUs outstanding at December 31, 2016 | 584,999 | |||
RSUs granted | — | |||
RSUs vested | (168,499 | ) | ||
RSUs forfeited | (36,000 | ) | ||
|
| |||
Nonvested RSUs outstanding at December 31, 2017 | 380,500 | |||
RSUs granted | — | |||
RSUs vested | (380,500 | ) | ||
RSUs forfeited | — | |||
|
| |||
Nonvested RSUs outstanding at December 31, 2018 | — | |||
|
| |||
F-A-16
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
The number of RSUs vested during 2018, 2017, and 2016 included 52,645, 29,482, and 29,829 shares, respectively, that were withheld on behalf of the Company’s employees to satisfy the statutory tax withholding requirements.
4. Property and Equipment, Net
Property and equipment, net consisted of the following:
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Computer equipment and software | $ | 1,225 | $ | 1,299 | ||||
Furniture and fixtures | 853 | 853 | ||||||
Leasehold improvements | 355 | 355 | ||||||
Office equipment | 211 | 211 | ||||||
|
|
|
| |||||
| 2,644 | 2,718 | |||||||
Less: accumulated depreciation | (2,625 | ) | (2,667 | ) | ||||
|
|
|
| |||||
| $ | 19 | $ | 51 | |||||
|
|
|
| |||||
Depreciation and amortization expense for the years ended December 31, 2018, 2017 and 2016 was $32, $32, and $14, respectively. Of these amounts, $1, $2 and $2, respectively, were included in research and development expenses in the statements of operations.
5. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following:
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Clinical trials | $ | 1,492 | $ | 4,742 | ||||
General and administrative | 101 | 314 | ||||||
Research and development | 272 | 312 | ||||||
Employee compensation | 66 | 3 | ||||||
|
|
|
| |||||
| $ | 1,931 | $ | 5,371 | |||||
|
|
|
| |||||
6. Stockholders’ Equity
Authorized Capital
On December 5, 2016, the Company filed a Certificate of Amendment to the Company’s Restated Certificate of Incorporation with the Secretary of State of the State of Delaware to effect aone-for-ten reverse stock split of its outstanding common stock and to effect a reduction in the number of authorized shares of common stock from 400,000,000 to 60,000,000 shares. The Company’s certificate of incorporation currently authorizes the Company to issue 60,000,000 shares of common stock, $0.001 par value per share, and 5,000,000 shares of preferred stock, $0.001 par value per share.
Common Stock
On February 9, 2018, the Company entered into anAt-the-Market Equity Offering Sales Agreement (the “ATM Sales Agreement”) with Stifel, Nicolaus & Company, Incorporated, as sales agent (“Stifel”), pursuant to
F-A-17
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
which the Company may offer and sell, from time to time, through Stifel, shares of the Company’s common stock, having an aggregate offering price of up to $50,000. On May 16, 2018, the Company sold 1,501,501 shares of common stock under the ATM Sales Agreement for net proceeds of $24,474. As of December 31, 2018, the Company had approximately $25,000 of common stock remaining available to be sold under the ATM Sales Agreement.
On September 29, 2017, the Company completed a private placement of units consisting of an aggregate of 5,483,320 shares of common stock and warrants to purchase an aggregate of 3,289,988 shares of its common stock for net proceeds of $45,648, after deducting placement agent fees and other offering expenses. The purchasers in the private placement consisted solely of accredited investors that included certain institutional and existing stockholders, including a member of the Company’s board of directors. The warrants, which have five year terms expiring on September 29, 2022, are immediately exercisable and have a per share exercise price of $9.02. The Company assessed whether the warrants require accounting as derivatives. The Company determined that the warrants were indexed to the Company’s own stock. As such, the Company has concluded the warrants meet the scope exception for determining whether the instruments require accounting as derivatives and are classified in stockholders’ equity. The fair value of the warrants was estimated at $21,069 using the Black-Scholes Model with the following assumptions: expected volatility of 97%, risk free interest rate of 1.92%, expected life of five years and no dividends. The net proceeds from the private placement were allocated to the common stock and warrants based upon their relative fair values.
On October 14, 2016, the Company completed a registered direct offering of its common stock. Under the terms of the offering, the Company sold 1,728,395 shares of its common stock for net proceeds of $13,692, after deducting offering expenses.
On November 14, 2014, the Company completed a private placement of units consisting of an aggregate of 6,431,111 shares of common stock and warrants to purchase an aggregate of 6,430,948 shares of its common stock for net proceeds of $42,814, after deducting offering expenses. The net proceeds from the private placement were allocated to the common stock and warrants based upon the fair value method. Similarly, the offering expenses were allocated between the common stock and warrants with the portion allocated to common stock offset against the proceeds allocated to stockholders’ equity, whereas the portion allocated to the warrants was expensed immediately. The warrants have a per share exercise price of $8.50, became exercisable on May 6, 2015 and will continue to be exercisable for four years thereafter. Prior to May 6, 2015, each warrant was subject to net cash settlement if, at the time of any exercise, there was then an insufficient number of authorized and reserved shares of common stock to effect a share settlement of the warrant. Under the terms of the warrants, as of May 6, 2015, the net cash settlement feature of the warrants automatically became inoperative; accordingly, the warrants are exercisable only for shares of the Company’s common stock. The warrants, however, also contained certain terms that could have required the Company (or its successor) to purchase the warrants for cash in an amount equal to the value (as calculated utilizing a contractually-agreed Black-Scholes Model) of the unexercised portion of the warrants in connection with certain change of control transactions occurring on or prior to December 31, 2016, with the cash payment capped at an amount equal to $1.25 per unexercised share underlying each warrant. Due to the provision of the warrants that could have required cash settlement upon certain change of control transactions, the Company was required to account for these warrants as a liability at fair value using the Black-Scholes Model and the estimated warrant liability was required to be revalued at each balance sheet date until the earlier of the exercise of the warrants, the modification to remove the provision that could require cash settlement upon certain change of control transactions or the expiration of such provision on December 31, 2016. Effective March 25, 2016, each of the warrants was amended by agreement of the warrant holders to remove the provision that could require cash settlement upon certain change of control transactions. These warrants were no longer accounted for as a liability at March 31, 2016. The Company recorded anon-cash
F-A-18
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
reclassification of the warrant fair value to stockholders’ equity based on the warrants’ fair value as of the March 25, 2016 modification date, with no further adjustments to the fair value of these warrants being required. In March 2018, certain holders of warrants issued in November 2014 exercised warrants covering 1,111,082 shares of common stock in a cashless exercise for which the Company issued an aggregate of 674,579 shares of common stock upon exercise.
Each of these completed offerings included certain existing GTx stockholders and/or certain members of the GTx management team and/or board of directors.
7. License Agreements
University of Tennessee Research Foundation License Agreements
The Company and the University of Tennessee Research Foundation (“UTRF”) are parties to a consolidated, amended and restated license agreement (the “SARM License Agreement”) pursuant to which the Company has been granted exclusive worldwide rights in all existing SARM technologies owned or controlled by UTRF, including all improvements thereto, and exclusive rights to future SARM technology that may be developed by certain scientists at the University of Tennessee or subsequently licensed to UTRF under certain existing inter-institutional agreements with The Ohio State University. Under the SARM License Agreement, the Company is obligated to pay UTRF annual license maintenance fees, low single-digit royalties on net sales of products and mid single-digit royalties on sublicense revenues.
In accordance with the terms of the SARM License Agreement that the Company entered into with UTRF in July 2007, the Company paid aone-timeup-front fee of $290, which was recorded as an intangible asset by the Company. This intangible asset, net at December 31, 2018 and 2017 was $94 and $108, respectively.
The Company and UTRF also entered into a license agreement in March 2015 pursuant to which the Company was granted exclusive worldwide rights in all existing SARD technologies owned or controlled by UTRF, including all improvements thereto (the “SARD License Agreement”). Under the SARD License Agreement, the Company is obligated to employ active, diligent efforts to conduct preclinical research and development activities for the SARD program to advance one or more lead compounds into clinical development. The Company is also obligated to pay UTRF annual license maintenance fees, low single-digit royalties on net sales of products and additional royalties on sublicense revenues, depending on the state of development of a clinical product candidate at the time it is sublicensed.
8. Income Taxes
The Tax Cuts and Jobs Act (“Tax Reform Act”) was enacted on December 22, 2017. The Tax Reform Act significantly revised the U.S. corporate income tax regime by, among other things, lowering the U.S. corporate tax rate from 35% to 21% effective January 1, 2018. On December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118 (“SAB 118”) to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Tax Reform Act. The Company recognized the provisional tax impacts related to the revaluation of deferred tax assets and liabilities and included these amounts in its financial statements for the year ended December 31, 2017. During 2018 the Company completed the accounting under the Tax Reform Act as allowed under SAB 118 to revalue its deferred tax assets and liabilities which resulted in no change to the amounts previously recorded by the Company for year ended December 31, 2017.
F-A-19
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The principal components of the Company’s net deferred income tax assets and liabilities consisted of the following:
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Deferred income tax assets: | ||||||||
Net federal and state operating loss carryforwards | $ | 120,555 | $ | 110,145 | ||||
Research and development credits | 16,383 | 14,757 | ||||||
Share-based compensation | 3,130 | 3,994 | ||||||
Depreciation and amortization | 17 | 21 | ||||||
|
|
|
| |||||
Total deferred tax assets | 140,085 | 128,917 | ||||||
|
|
|
| |||||
Deferred income tax liabilities: | ||||||||
Other | 461 | 92 | ||||||
|
|
|
| |||||
Total deferred tax liabilities | 461 | 92 | ||||||
|
|
|
| |||||
Net deferred tax assets | 139,624 | 128,825 | ||||||
Valuation allowance | (139,624 | ) | (128,825 | ) | ||||
|
|
|
| |||||
| $ | — | $ | — | |||||
|
|
|
| |||||
Realization of deferred income tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, due to the Company’s history of net operating losses, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $10,799 in 2018, decreased by $47,132 in 2017 and increased in 2016 by $9,347. The valuation allowance decrease in 2017 was due primarily to the passage of the Tax Reform Act and the reduction in the valuation of the Company’s net deferred tax assets as a result of the lowering of the corporate tax rate from 35% to 21% effective January 1, 2018.
At December 31, 2018, the Company had net federal operating loss carryforwards of approximately $472,054. The federal operating loss carryforwards originating prior to 2018 will expire from 2019 to 2037 if not utilized. The Company had state operating loss carryforwards of approximately $411,396, which expire from 2019 to 2038 if not utilized. The Company also had research and development credits at December 31, 2018 of approximately $16,383, which expire from 2020 to 2038 if not utilized.
The Company will recognize the impact of a tax position in the financial statements if that position is more likely than not of being sustained on audit based on the technical merits of the position. As of December 31, 2018, the Company had no unrecognized tax benefits. Utilization of the Company’s net operating loss carryforwards may be subject to a substantial annual limitation due to ownership change limitations provided by Section 382 of the Internal Revenue Code of 1986, as amended, and similar state provisions. The annual limitations may result in the expiration of net operating loss carryforwards before utilization. The Company completed a study of its net operating losses through December 31, 2016 to determine whether such amounts are likely to be limited by Section 382. As a result of this study and its analysis of subsequent ownership changes, the Company does not currently believe any Section 382 limitation exists through December 31, 2018 though the Company has not yet conducted anin-depth analysis since the last study. However, any future ownership changes under Section 382 may limit the Company’s ability to fully utilize these tax benefits. The Company has not yet conducted anin-depth study of its research and development credits, although the Company periodically reviews assumptions used in its
F-A-20
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
calculations to reflect its best estimate of expected credit. Anin-depth study may result in an increase or decrease to the Company’s research and development credits and until such study is conducted of the Company’s research and development credits, no amounts are being presented as an uncertain tax position. The Company’s net deferred income tax assets have been fully offset by a valuation allowance. Therefore, future changes to the Company’s unrecognized tax benefits would be offset by an adjustment to the valuation allowance and there would be no impact on the Company’s balance sheet, statement of operations, or cash flows. The Company does not expect its unrecognized tax benefits to change significantly over the next 12 months.
The Company is currently open to audit under the statute of limitations by the Internal Revenue Service and the appropriate state income taxing authorities for all years due to the net loss carryforwards from those years. The Company is currently not under examination by the Internal Revenue Service or any other taxing authorities. The Company has not recorded any interest and penalties on any unrecognized tax benefits since its inception.
9. Directors’ Deferred Compensation Plan
Non-employee directors may defer all or a portion of their fees under the Company’s Directors’ Deferred Compensation Plan until termination of their status as directors. Deferrals can be made into a cash account, a stock account, or a combination of both. Stock accounts will be paid out in the form of Company common stock, except that any fractional shares will be paid out in cash valued at the then current market price of the Company’s common stock. Cash accounts and stock accounts under the Directors’ Deferred Compensation Plan are credited with interest or the value of any cash and stock dividends, respectively.Non-employee directors are fully vested in any amounts that they elect to defer under the Directors’ Deferred Compensation Plan.
For the years ended December 31, 2018, 2017 and 2016, the Company incurrednon-employee director fee expense of $291, $291 and $257, respectively, of which $166, $166 and $132 was deferred into stock accounts and will be paid in common stock following separation from service as a director. At December 31, 2018, 122,725 shares of the Company’s common stock had been credited to individual director stock accounts under the Directors’ Deferred Compensation Plan, and no amounts had been credited to individual director cash accounts under the Directors’ Deferred Compensation Plan.
10. 401(k) Plan
The Company sponsors a 401(k) retirement savings plan that is available to all eligible employees. The plan is intended to qualify under Section 401(k) of the Internal Revenue Code of 1986, as amended. The plan provides that each participant may contribute up to a statutory limit of theirpre-tax compensation which was $18.5 for employees under age 50 and $24.5 for employees 50 and older in calendar year 2018. Employee contributions are held in the employees’ name and invested by the plan’s trustee. The plan also permits the Company to make matching contributions, subject to established limits. The Company elected to match a portion of employee’s contributions to the plan in the amount of $185, $186 and $200 in 2018, 2017 and 2016, respectively.
11. Commitments and Contingencies
Operating Lease Commitments
In 2015, the Company entered into a new office lease with respect to the Company’s current office space. The new office lease term commenced on May 1, 2015 with a three year term ending on April 30, 2018, with an option to extend the lease for an additional three years, and was accounted for as an operating lease. In March 2018, the Company amended the lease to extend the term of the lease for an additional12-month term expiring
F-A-21
GTx, Inc.
NOTES TO FINANCIAL STATEMENTS
(in thousands, except share and per share data)
on April 30, 2019. Total rent expense under the operating leases was approximately $509, $506 and $495 for the years ended December 31, 2018, 2017 and 2016, respectively. As of December 31, 2018, future annual minimum payments under operating lease arrangements were $162.
12. Quarterly Financial Data (Unaudited)(1)
The following is a summary of the quarterly results of operations for the years ended December 31, 2018 and 2017:
| 2018 Quarters Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
Expenses: | ||||||||||||||||
Research and development expenses | $ | 11,000 | $ | 7,962 | $ | 7,467 | $ | 3,240 | ||||||||
General and administrative expenses | 2,688 | 2,196 | 2,160 | 2,346 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total expenses | 13,688 | 10,158 | 9,627 | 5,586 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (13,688 | ) | (10,158 | ) | (9,627 | ) | (5,586 | ) | ||||||||
Other income, net | 131 | 143 | 196 | 171 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | $ | (13,557 | ) | $ | (10,015 | ) | $ | (9,431 | ) | $ | (5,415 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Net loss per share: | ||||||||||||||||
Basic and Diluted | $ | (0.62 | ) | $ | (0.43 | ) | $ | (0.39 | ) | $ | (0.23 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Weighted average shares outstanding: | ||||||||||||||||
Basic and Diluted | 21,967,805 | 23,288,691 | 24,045,992 | 24,051,844 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
| 2017 Quarters Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
Expenses: | ||||||||||||||||
Research and development expenses | $ | 4,193 | $ | 4,448 | $ | 5,914 | $ | 6,912 | ||||||||
General and administrative expenses | 2,087 | 1,997 | 2,617 | 2,487 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total expenses | 6,280 | 6,445 | 8,531 | 9,399 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (6,280 | ) | (6,445 | ) | (8,531 | ) | (9,399 | ) | ||||||||
Other income, net | 27 | 40 | 27 | 122 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | $ | (6,253 | ) | $ | (6,405 | ) | $ | (8,504 | ) | $ | (9,277 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Net loss per share: | ||||||||||||||||
Basic and Diluted | $ | (0.39 | ) | $ | (0.40 | ) | $ | (0.53 | ) | $ | (0.43 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Weighted average shares outstanding: | ||||||||||||||||
Basic and Diluted | 16,018,342 | 16,041,923 | 16,115,835 | 21,541,909 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
| (1) | The sum of quarterly earnings per share amounts may not equal the annual amounts as the quarterly amounts are computed independently for each quarter while the full year is based on the annual weighted average shares outstanding. |
F-A-22
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| F-B-2 | ||||
| F-B-3 | ||||
| F-B-4 | ||||
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Deficit | F-B-5 | |||
| F-B-6 | ||||
| F-B-7 | ||||
F-B-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors
Oncternal Therapeutics, Inc.
San Diego, California
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Oncternal Therapeutics, Inc. and subsidiary (the “Company”) as of December 31, 2018 and 2017, the related consolidated statements of operations, convertible preferred stock and stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2018, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, LLP
We have served as the Company’s auditor since 2016.
San Diego, California
April 5, 2019
F-B-2
Consolidated Balance Sheets
(in thousands, except share and par value data)
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 20,645 | $ | 10,188 | ||||
Prepaid expenses and other assets | 565 | 615 | ||||||
|
|
|
| |||||
Total current assets | 21,210 | 10,803 | ||||||
Other assets | 752 | 266 | ||||||
|
|
|
| |||||
Total assets | $ | 21,962 | $ | 11,069 | ||||
|
|
|
| |||||
Liabilities, convertible preferred stock and stockholders’ deficit | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 3,440 | $ | 1,113 | ||||
Accrued liabilities | 891 | 1,030 | ||||||
Deferred revenue | — | 1,902 | ||||||
Convertible note payable | — | 200 | ||||||
|
|
|
| |||||
Total current liabilities | 4,331 | 4,245 | ||||||
Warrant liability | 674 | 1,387 | ||||||
Commitments and contingencies (Note 3) | ||||||||
Convertible preferred stock, $0.0001 par value; authorized shares — 130,099,288 and 143,560,000 at December 31, 2018 and 2017, respectively; issued and outstanding shares — 111,034,576 and 77,034,576 at December 31, 2018 and 2017, respectively; liquidation preference — $48,954 and $31,954 at December 31, 2018 and 2017, respectively; net of stock subscriptions receivable of $0 and $1,100 at December 31, 2018 and 2017, respectively | 46,588 | 28,715 | ||||||
Stockholders’ deficit: | ||||||||
Common stock, $0.0001 par value; authorized shares — 200,000,000 at December 31, 2018 and 2017; issued and outstanding shares — 51,257,780 and 51,032,780 at December 31, 2018 and 2017, respectively | 5 | 5 | ||||||
Additionalpaid-in capital | 1,748 | 1,522 | ||||||
Accumulated deficit | (31,384 | ) | (24,805 | ) | ||||
|
|
|
| |||||
Total stockholders’ deficit | (29,631 | ) | (23,278 | ) | ||||
|
|
|
| |||||
Total liabilities, convertible preferred stock and stockholders’ deficit | $ | 21,962 | $ | 11,069 | ||||
|
|
|
| |||||
See accompanying notes.
F-B-3
Consolidated Statements of Operations
(in thousands, except share and per share data)
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
Grant revenue | $ | 2,521 | $ | 1,674 | ||||
Operating expenses: | ||||||||
Research and development | 8,287 | 9,363 | ||||||
General and administrative | 1,820 | 2,871 | ||||||
|
|
|
| |||||
Total operating expenses | 10,107 | 12,234 | ||||||
|
|
|
| |||||
Loss from operations | (7,586 | ) | (10,560 | ) | ||||
Other income (expense): | ||||||||
Change in fair value of warrant liability | 713 | 124 | ||||||
Other income | 216 | — | ||||||
Interest income | 79 | 10 | ||||||
Interest expense | (1 | ) | (10 | ) | ||||
|
|
|
| |||||
Total other income (expense) | 1,007 | 124 | ||||||
|
|
|
| |||||
Net loss | $ | (6,579 | ) | $ | (10,436 | ) | ||
|
|
|
| |||||
Net loss per share, basic and diluted | $ | (0.13 | ) | $ | (0.23 | ) | ||
|
|
|
| |||||
Weighted-average shares of common stock outstanding, basic and diluted | 48,930,354 | 45,914,263 | ||||||
|
|
|
| |||||
See accompanying notes.
F-B-4
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Deficit
(in thousands, except share data)
| Convertible Preferred Stock | Common Stock | Additional Paid-in Capital | Accumulated Deficit | Total Stockholders’ Deficit | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
Balance at December 31, 2016 | 48,691,573 | $ | 18,825 | 50,216,845 | $ | 5 | $ | 1,308 | $ | (14,369 | ) | $ | (13,056 | ) | ||||||||||||||
Issuance of Series B convertible preferred stock and warrants for cash, net of issuance costs of $38 | 5,688,888 | 2,016 | — | — | — | — | — | |||||||||||||||||||||
Issuance of SeriesB-2 convertible preferred stock and warrants for cash, net of issuance costs of $215, and stock subscriptions receivable of $1,100 | 22,654,115 | 7,874 | — | — | — | — | — | |||||||||||||||||||||
Issuance of restricted common shares for cash, net of repurchases | — | — | 47,025 | — | 2 | — | 2 | |||||||||||||||||||||
Issuance of restricted common shares for services rendered, net of repurchases | — | — | 916,981 | — | 81 | — | 81 | |||||||||||||||||||||
Exercise of stock options for cash, net of repurchases | — | — | (148,071 | ) | — | 2 | — | 2 | ||||||||||||||||||||
Vesting related to repurchase liability, net | — | — | — | — | (59 | ) | — | (59 | ) | |||||||||||||||||||
Stock-based compensation | — | — | — | — | 188 | — | 188 | |||||||||||||||||||||
Net loss | — | — | — | — | — | (10,436 | ) | (10,436 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance at December 31, 2017 | 77,034,576 | 28,715 | 51,032,780 | 5 | 1,522 | (24,805 | ) | (23,278 | ) | |||||||||||||||||||
Collection of stock subscription receivable | — | 1,100 | — | — | — | — | — | |||||||||||||||||||||
Issuance of Series C convertible preferred stock for cash, net of issuance costs of $227 | 34,000,000 | 16,773 | — | — | — | — | — | |||||||||||||||||||||
Issuance of restricted common shares | — | — | 200,000 | — | 10 | — | 10 | |||||||||||||||||||||
Exercise of stock options for cash | — | — | 25,000 | — | 1 | — | 1 | |||||||||||||||||||||
Vesting related to repurchase liability, net | — | — | — | — | 35 | — | 35 | |||||||||||||||||||||
Stock-based compensation | — | — | — | — | 180 | — | 180 | |||||||||||||||||||||
Net loss | — | — | — | — | — | (6,579 | ) | (6,579 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Balance at December 31, 2018 | 111,034,576 | $ | 46,588 | 51,257,780 | $ | 5 | $ | 1,748 | $ | (31,384 | ) | $ | (29,631 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||
See accompanying notes.
F-B-5
Consolidated Statements of Cash Flows
(in thousands)
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
Cash flows from operating activities | ||||||||
Net loss | $ | (6,579 | ) | $ | (10,436 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Stock-based compensation | 180 | 188 | ||||||
Noncash compensation expense | 10 | 81 | ||||||
Noncash interest expense | 1 | 10 | ||||||
Change in fair value of warrant liability | (713 | ) | (124 | ) | ||||
Noncash other income | (216 | ) | — | |||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses and other assets | (436 | ) | (570 | ) | ||||
Accounts payable and accrued liabilities | 2,238 | (186 | ) | |||||
Deferred revenue | (1,902 | ) | 1,902 | |||||
|
|
|
| |||||
Net cash used in operating activities | (7,417 | ) | (9,135 | ) | ||||
Cash flows from financing activities | ||||||||
Proceeds from issuances of convertible preferred stock, net | 17,873 | 11,401 | ||||||
Proceeds from issuances of restricted common stock | — | 2 | ||||||
Proceeds from early exercise of stock options, net of repurchases | 1 | 2 | ||||||
|
|
|
| |||||
Net cash provided by financing activities | 17,874 | 11,405 | ||||||
|
|
|
| |||||
Net increase in cash and cash equivalents | 10,457 | 2,270 | ||||||
Cash and cash equivalents — beginning of year | 10,188 | 7,918 | ||||||
|
|
|
| |||||
Cash and cash equivalents — end of year | $ | 20,645 | $ | 10,188 | ||||
|
|
|
| |||||
Supplemental disclosure of noncash financing activities | ||||||||
Initial fair value of convertible preferred stock warrant issued to convertible preferred stock investors | $ | — | $ | 1,511 | ||||
|
|
|
| |||||
Issuance of note receivable for stock subscription for convertible preferred stock | $ | — | $ | 1,100 | ||||
|
|
|
| |||||
See accompanying notes.
F-B-6
Notes to Consolidated Financial Statements
1. Description of Business, Basis of Presentation and Summary of Significant Accounting Policies
Description of Business
Oncternal Therapeutics, Inc. (the “Company”) was incorporated in the state of Delaware in November 2013 and is based in San Diego, California. The Company is a clinical-stage biopharmaceutical company focused on developingfirst-in-class product candidates for cancers with critical unmet medical need. The Company’s clinical pipeline consists of its lead program, cirmtuzumab, a humanized monoclonal antibody that binds to ROR1 (Receptor-tyrosine kinase-like Orphan Receptor 1), and TK216, a small molecule inhibiting the biological activity ofETS-family transcription factor oncoproteins targeting patients with Ewing sarcoma. The Company is also developing aCAR-T (chimeric antigen receptorsT-cells) targeting ROR1.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiary Oncternal, Inc. All intercompany accounts and transactions have been eliminated in the preparation of the consolidated financial statements.
Liquidity and Going Concern
From its inception through December 31, 2018, the Company has devoted substantially all of its efforts to organizational activities including raising capital, building infrastructure, acquiring assets, developing intellectual property, and conducting preclinical studies, clinical trials and product development activities. The Company has a limited operating history and the sales and income potential of the Company’s business and market are unproven. The Company has experienced recurring net losses and negative cash flows from operating activities. At December 31, 2018, the Company had an accumulated deficit of $31.4 million and had cash and cash equivalents of $20.6 million. The Company will need to continue to raise a substantial amount of funds until it is able to generate revenues to fund its development activities. As a result, the Company believes that there is substantial doubt about its ability to continue as a going concern for one year after the date these consolidated financial statements are issued.
The determination as to whether the Company can continue as a going concern contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company expects to continue to incur net losses into the foreseeable future. Successful transition to attaining profitable operations is dependent upon achieving a level of revenues adequate to support the Company’s cost structure. The Company has incurred net losses since inception and has relied on its ability to fund its operations through debt and equity financings and grant funding. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty. This basis of accounting contemplates the recovery of the Company’s assets and the satisfaction of liabilities in the normal course of business.
The Company believes that its existing cash and cash equivalents will be sufficient to fund its operations into the first quarter of 2020. The Company plans to continue to fund its losses from operations and capital funding needs through a combination of equity offerings, debt financings, government funding, or other sources, potentially including future government funding, collaborations, licenses and other similar arrangements (see Note 7). There can be no assurance that the Company will be able to obtain any sources of financing on acceptable terms, or at all. To the extent that the Company can raise additional funds by issuing equity securities, the Company’s stockholders may experience significant dilution. Any debt financing, if available, may involve restrictive covenants that impact the Company’s ability to conduct its business.
F-B-7
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
Use of Estimates
The Company’s consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of the Company’s consolidated financial statements and accompanying notes requires it to make estimates and assumptions that impact the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities. Significant estimates consist of those used to determine the fair value the Company’s common and preferred stock, stock-based awards and warrant liability, and those used to determine grant revenue and accruals for research and development costs. Although these estimates are based on the Company’s knowledge of current events and actions it may undertake in the future, actual results may ultimately materially differ from these estimates and assumptions.
Fair Value Measurements
The accounting guidance defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring ornon-recurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1: Observable inputs such as quoted prices in active markets.
Level 2: Inputs, other than the quoted prices in active markets that are observable either directly or indirectly.
Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The carrying amounts of the Company’s current financial assets and liabilities are considered to be representative of their respective fair values because of the short-term nature of those instruments. The Company has no financial assets or liabilities, other than the warrant liability described below, measured at fair value on a recurring basis. No transfers between levels have occurred during the periods presented.
Liabilities measured at fair value on a recurring basis are as follows:
| Fair Value Measurements at Reporting Date Using: | ||||||||||||||||
| Total | Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
As of December 31, 2018: | ||||||||||||||||
Warrant liability | $ | 674,000 | $ | — | $ | — | $ | 674,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
As of December 31, 2017: | ||||||||||||||||
Warrant liability | $ | 1,387,000 | $ | — | $ | — | $ | 1,387,000 | ||||||||
|
|
|
|
|
|
|
| |||||||||
The preferred stock warrant liability is recorded at fair value utilizing the Black-Scholes option pricing model using significant unobservable inputs consistent with the inputs used for the Company’s stock-based compensation expense adjusted for the preferred stock warrants’ expected term and the fair value of the underlying preferred stock.
F-B-8
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
The assumptions used in the Black-Scholes option pricing model to determine the fair value of the warrant liability were as follows:
| December 31, | ||||
| 2018 | 2017 | |||
Fair value of underlying preferred stock | $ 0.29 | $ 0.45 | ||
Risk-free interest rate | 2.37% — 2.69% | 1.75% — 2.20% | ||
Expected volatility | 75.3% — 76.4% | 77.9% — 85.0% | ||
Expected term (in years) | 3.7 — 4.0 | 4.7 — 5.0 | ||
Expected dividend yield | — | — | ||
The following table provides a reconciliation of the warrant liability measured at fair value using Level 3 significant unobservable inputs:
| Warrant Liability | ||||
Balance at December 31, 2016 | $ | — | ||
Fair value of warrants issued | 1,511,000 | |||
Decrease in fair value of warrant liability | (124,000 | ) | ||
|
| |||
Balance at December 31, 2017 | 1,387,000 | |||
Decrease in fair value of warrant liability | (713,000 | ) | ||
|
| |||
Balance at December 31, 2018 | $ | 674,000 | ||
|
| |||
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when purchased to be cash equivalents. Cash and cash equivalents include cash in readily available checking accounts and money market accounts.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents. The Company maintains deposits in federally insured financial institutions in excess of federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to significant risk on its cash balances due to the financial position of the depository institution in which those deposits are held. Additionally, the Company established guidelines regarding approved investments and maturities of investments, which are designed to maintain safety and liquidity.
Patent Costs
Costs related to filing and pursuing patent applications are recorded as general and administrative expense and expensed as incurred since recoverability of such expenditures is uncertain.
Research and Development Expenses and Accruals
Research and development expenses consist of costs incurred for the Company’s own and for sponsored and collaborative research and development activities. Research and development costs are expensed as incurred and
F-B-9
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
include manufacturing drug product, costs associated with preclinical studies and clinical trials, regulatory and medical affairs activities, quality assurance activities, salaries and benefits, including stock-based compensation, fees paid to third-party consultants, license fees and overhead.
The Company has entered into various research and development contracts with research institutions, clinical research organizations, clinical manufacturing organizations and other companies. Payments for these activities are based on the terms of the individual agreements, which may differ from the pattern of costs incurred, and payments made in advance of performance are reflected in the accompanying consolidated balance sheets as prepaid expenses and other or accrued liabilities. The Company records accruals for estimated costs incurred for ongoing research and development activities. When evaluating the adequacy of the accrued liabilities, the Company analyzes progress of the services, including the phase or completion of events, invoices received and contracted costs. Significant judgments and estimates may be made in determining the prepaid or accrued balances at the end of any reporting period. Actual results could differ from the Company’s estimates.
Warrant Liability
The Company has issued freestanding warrants to purchase shares of its SeriesB-2 convertible preferred stock. Since the underlying SeriesB-2 convertible preferred stock is classified as temporary equity, theSeries B-2 convertible preferred stock warrants are classified as a liability in the accompanying consolidated balance sheets. The Company adjusts the carrying value of such SeriesB-2 convertible preferred stock warrants to their estimated fair value at each reporting date, with any related increases or decreases in the fair value recorded as an increase or decrease to other income (expense) in the consolidated statements of operations. The warrant liability will continue to be adjusted to fair value until such time as the SeriesB-2 convertible preferred stock warrants are no longer outstanding or the underlying securities are no longer redeemable outside the control of the Company.
Revenue Recognition
The Company currently generates revenue from a research subaward agreement from the California Institute for Regenerative Medicine (see Note 4), which provides the Company with payments for certain types of expenditures in return for research and development activities over a contractually defined period. Revenue from such subaward is recognized in the period during which the related qualifying costs are incurred and services are rendered, provided that the applicable conditions under the subaward agreement have been met.
The subaward agreement is on a best-effort basis and does not require scientific achievement as a performance obligation. All fees received under the agreement arenon-refundable. The costs associated with the agreement are expensed as incurred and reflected as a component of research and development expense in the accompanying consolidated statements of operations.
Funds received from the subaward agreement are recorded as revenue as the Company is the principal participant in the arrangement because the activities under the subaward are part of the Company’s development programs. In those instances where the Company first receives consideration in advance of providing underlying services, the Company classifies such consideration as deferred revenue until (or as) the Company provides the underlying services. In those instances where the Company first provides the underlying services prior to its receipt of consideration, the consideration is recorded as a grant receivable. At December 31, 2018, the Company had a grant receivable of $0.1 million. At December 31, 2017, the Company had deferred revenue of $1.9 million. The Company considers the grant receivable to be fully collectible; accordingly, no allowance for doubtful amounts has been established.
F-B-10
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
Stock-Based Compensation
Stock-based compensation expense represents the cost of the grant date fair value of equity awards recognized in the period using the Black-Scholes option pricing model. The Company recognizes expense for awards with graded vested schedules over the requisite service period of the awards (usually the vesting period) on a straight-line basis. For equity awards for which vesting is subject to performance-based milestones, the expense is recorded over the remaining service period after the point when the achievement of the milestone is probable or the performance condition has been achieved.
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax assets and liabilities are determined on the basis of the differences between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in income in the period that includes the enactment date.
The Company recognizes net deferred tax assets to the extent that the Company believes these assets are more likely than not to be realized. In making such a determination, management considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income,tax-planning strategies, and results of recent operations. If management determines that the Company would be able to realize its deferred tax assets in the future in excess of their net recorded amount, management would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment operating in the United States.
Net Loss Per Share
Basic net loss per share is computed by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration for potentially dilutive securities and adjusted for the weighted-average number of common shares outstanding that are subject to repurchase. The Company has excluded weighted-average shares subject to repurchase of 2,234,207 shares and 4,771,637 shares from the weighted-average number of common shares outstanding for the years ended December 31, 2018 and 2017, respectively. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of shares of common stock and dilutive common stock equivalents outstanding for the period determined using the treasury-stock andif-converted methods. Dilutive common stock equivalents are comprised of convertible preferred stock, convertible preferred stock warrants, common stock subject to repurchase, and options outstanding under the Company’s stock option plan. For all periods presented, there is no difference in the number of shares used to calculate basic and diluted shares outstanding as inclusion of the potentially dilutive securities would be antidilutive.
F-B-11
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
Potentially dilutive securities not included in the calculation of diluted net loss per share, because to do so would be anti-dilutive are as follows (in common stock equivalent shares):
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Convertible preferred stock | 111,034,576 | 77,034,576 | ||||||
Convertible preferred stock warrants | 5,064,712 | 5,064,712 | ||||||
Common stock options | 6,868,251 | 2,068,251 | ||||||
Common stock subject to repurchase | 1,357,476 | 3,024,386 | ||||||
|
|
|
| |||||
Total | 124,325,015 | 87,191,925 | ||||||
|
|
|
| |||||
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its consolidated financial position or results of operations upon adoption.
In February 2016, the FASB issued Accounting Standards Update (“ASU”)2016-02,Leases, which, for operating leases, requires a lessee to recognize aright-of-use asset and a lease liability, initially measured at the present value of the lease payments, in its balance sheet. The standard also requires a lessee to recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term, generally on a straight-line basis. This ASU is not applicable to the Company as of December 31, 2018 as its only lease is on a month to month basis and is not expected to be renewed for a period greater than one year.
Recently Adopted Accounting Pronouncements
In May 2014, the FASB issued ASU2014-09,Revenue from Contracts with Customers (Topic 606) (“ASC 606”). ASC 606 is a comprehensive new revenue recognition model that requires a company to recognize revenue to depict the transfer of goods or services to a customer at an amount that reflects the consideration it expects to receive in exchange for those goods or services. In August 2015, the FASB issued ASU2015-14,Revenue from Contracts with Customers Deferral of Effective Date,which deferred the original effective date of ASC 606 for all entities by one year. The Company adopted this standard on January 1, 2018 using the modified retrospective approach, the adoption of this standard had no impact on the consolidated financial statements as the Company currently has no marketed products or ongoing collaboration agreements, under which any participant is considered a customer, and its research subaward agreement is not within the scope of ASC 606.
In June 2018, the FASB issued ASU2018-07, Improvements to Nonemployee Share-Based Payment Accounting, which supersedes most of the prior accounting guidance on nonemployee share-based payments, and instead aligns it with existing guidance on employee share-based payments in Topic 718,Compensation — Stock Compensation. As a result, nonemployee share-based payment transactions will be measured by estimating the fair value of the equity instruments that an entity is obligated to issue, and the measurement date will be consistent with the measurement date for employee share-based payment awards. Probability is to be considered on nonemployee awards with performance conditions. The classification will continue to be subject to the requirements of Topic 718, although cost recognition of nonemployee awards will remain unchanged. This guidance is effective for the fiscal years and interim reporting periods beginning after December 15, 2018 with early adoption permitted, but no earlier than an entity’s adoption date of Topic 606, Revenue from Contracts with Customers — Income Tax Implications. The early adoption of this guidance, effective January 1, 2018, had no material impact on the Company’s financial statements.
F-B-12
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
2. Balance Sheet Details
Accrued liabilities consist of the following:
| December 31, | ||||||||
| 2018 | 2017 | |||||||
Research and development | $ | 720,000 | $ | 469,000 | ||||
Legal fees | 20,000 | 341,000 | ||||||
Unvested share liability | 54,000 | 89,000 | ||||||
Compensation | 85,000 | 88,000 | ||||||
Other | 12,000 | 43,000 | ||||||
|
|
|
| |||||
| $ | 891,000 | $ | 1,030,000 | |||||
|
|
|
| |||||
3. Commitments and Contingencies
Facility Lease
The Company leases its office space in San Diego, California on amonth-to-month basis. Rent expense, net of sublease income, was $12,000 and $55,000 for the years ended December 31, 2018 and 2017, respectively.
Litigation
The Company is not a party to any litigation and does not have contingency reserves established for any litigation liabilities. At each reporting date, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under the provisions of the authoritative guidance that addresses accounting for contingencies.
Indemnification Agreements
In the ordinary course of business, the Company may provide indemnification of varying scope and terms to vendors, lessors, business partners and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with members of its board of directors and its executive officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, unlimited. To date, the Company has not incurred any material costs as a result of such indemnification. The Company is not currently aware of any indemnification claims and has not accrued any liabilities related to such obligations in its consolidated financial statements as of December 31, 2018 or 2017.
4. License, Collaboration and Research Subaward Agreements
Georgetown University (“Georgetown”)
In March 2014, the Company entered into an Exclusive License Agreement (the “License Agreement”) with Georgetown, pursuant to which the Company: (i) licensed the exclusive worldwide right to patents and technologies for the development and commercialization of certain product candidates targetingEWS-FLI1 as an anti-tumor therapy for therapeutic, diagnostics, or research tool purposes, (ii) is solely responsible for all development and commercialization activities and costs, and (iii) is responsible for all costs related to the filing, prosecution and maintenance of the licensed patent rights.
F-B-13
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
Under the terms of the License Agreement, commencing in 2015, the Company: (i) shall pay and has paid an annual license maintenance fee of $10,000 until the first commercial sale occurs, (ii) is required to make up to $200,000 in aggregate milestone payments upon the achievement of certain regulatory milestones, and (iii) will be required to pay low single digit royalties based on annual net product sales. The Company accounted for the licensed technology as an asset acquisition because it did not meet the definition of a business. All milestone payments under the License Agreement will be recognized as research and development expense upon completion of the required events, as the triggering events are not considered to be probable until they are achieved. As of December 31, 2018, the Company had not triggered or made any milestone payments under the License Agreement.
The License Agreement may be terminated by either party upon material breach or may be terminated by the Company as to one or more countries with 90 days written notice of termination. The term of the License Agreement will continue until the expiration of the last valid claim within the patent rights covering the product. Georgetown may terminate the agreement in the event (i) the Company fails to pay any amount and fails to cure such failure within 30 days after receipt of notice, (ii) the Company defaults in its obligation to obtain and maintain insurance and fails to remedy such breach within 60 days after receipt of notice, or (iii) the Company declares insolvency or bankruptcy. The Company may terminate the agreement at any time upon at least 60 days’ written notice.
In 2017, the Company entered into a research agreement with Georgetown for up to $150,000. For the years ended December 31, 2018 and 2017, the Company recorded research and development expenses of $53,000 and $75,000, respectively.
The University of Texas MD Anderson Cancer Center (“MD Anderson”)
In December 2014, the Company entered into a collaboration agreement (the “Collaboration”) with MD Anderson, which, as amended, provides for the conduct of preclinical and clinical research for TK216 in exchange for certain program payments. If MD Anderson successfully completes all the requirements of the Collaboration in full and the program is successfully commercialized, the Company will be required to pay aggregate milestone payments of $1.0 million based on net product sales. For the years ended December 31, 2018 and 2017, the Company recorded $330,000 and $0, respectively, of research and development expenses earned by MD Anderson under the Collaboration agreement.
Agreements with the Regents of the University of California (the “Regents”)
In March 2016, and as amended and restated in August 2018 in connection with thespin-off transaction described below, the Company entered into a license agreement (the “Regents license agreement”) for the development, manufacturing and distribution rights related to the development and commercialization of ROR1 related naked antibodies, antibody fragments or synthetic antibodies, and genetically engineered cellular therapy. The Regents license agreement provides for the following: (i) in May 2016, an upfront license fee of $0.5 million was paid and 1,459,524 shares of common stock were issued, (ii) $25,000 in annual license maintenance fees commencing in 2017, (iii) reimbursement of up to $30,000 in annual patent costs, (iv) certain development and regulatory milestones aggregating from $10.0 million to $12.5 million, on a per product basis, (v) certain worldwide sales milestones based on achievement of tiered revenue levels aggregating $75.0 million, (vi) low single-digit royalties, including potential future minimum annual royalties, on net sales of each target, and (vii) minimum diligence to advance licensed assets consisting of at least $1.0 million in development spend annually through 2021. Under the Regents license agreement in 2018 and 2017, the Company recorded: (i) $25,000 in annual license maintenance fees recorded as research and development expense, and (ii) $0.1 million and $0.2 million in patent costs recorded as general and administrative expense for the years
F-B-14
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
ended December 31, 2018 and 2017, respectively. As of December 31, 2018, the Company believes it has met its obligations under the Regents license agreement.
In July 2016, and as modified by the amended and restated Regents license agreement in August 2018, the Company entered into a Research Agreement (the “Research Agreement”) with the Regents for further research on a ROR1 therapeutic development program. Under this five-year agreement, the Regents will have an aggregate budget of $3.6 million, with $125,000 payable quarterly. For the years ended December 31, 2018 and 2017, the Company recorded $0.5 million and $1.0 million, respectively, in research and development costs under this Research Agreement. Such costs are includable as part of the Company’s annual diligence obligations under the Regents license agreement.
The Regents license agreement will expire upon the later of the expiration date of the longest-lived patent rights or the 15th anniversary of the first commercial sale of a licensed product. The Regents may terminate the Regents license agreement if: (i) a material breach by the Company is not cured within a reasonable time, (ii) the Company files a claim asserting the Regents licensed patent rights are invalid or unenforceable, and (iii) the Company files for bankruptcy. The Company may terminate the agreement at any time upon at least 60 days’ written notice.
In September 2016, the Company entered into an Investigator-Initiated Clinical Trial Agreement with the Regents to provide partial support for a Phase 1 clinical study to determine the safety and tolerability of cirmtuzumab for the treatment of patients with relapsed or refractory chronic lymphocytic leukemia (“CLL”). Under this agreement that was concluded in 2017, the Company recorded $0.2 million in research and development expenses for the year ended December 31, 2017.
Velos Biopharma Holdings, LLC (“VBH”) and VelosBio, Inc. (“VelosBio”)Spin-off Transactions
In November and December 2017, the Company formed VBH and made anin-kindtax-free distribution of 100% of its interest in VBH to the Company’s stockholders, option holders and warrant holders of record. On February 6, 2018, the Company licensed and assigned its rights to two preclinical product candidates, previously under the Regents license agreement, to VBH. In consideration for the license, the Company: (i) received a promissory note receivable from VBH of $0.1 million, with an annual interest rate of 2.64% and a due date of 10 years, and (ii) made a partial assignment of its March 2016 Regents license agreement. Pursuant to the partial assignment, VBH assumed certain obligations related to the licensed Products under the Regents license agreement as follows: (i) reimbursement of certain historical and future patent costs related to the Products, (ii) certain development and sales milestones for advancing licensed Products targets, (iii) low single-digit royalties, including potential future minimum annual royalties, on net sales of each licensed Product target are to be allocated between the Company and VBH, (iv) certain third party agreements and related obligations specifically related to the licensed Products, (v) minimum diligence requirements to advance licensed assets consisting of a minimum of $0.5 million in development spend annually through 2021, and (vi) Research Agreement obligations equal to $0.5 million annually commencing January 1, 2018. Due to the high uncertainty of the success of VBH ever repaying the note receivable and associated interest, the Company has provided a full valuation allowance for these amounts as of December 31, 2018.
In December 2017, VelosBio was incorporated with VBH being its sole stockholder. On February 6, 2018, VBH sublicensed and assigned its intellectual property rights to its two preclinical product candidates to VelosBio. In consideration for the license, VelosBio agreed to use commercially reasonable efforts to develop the licensed products as well as the following payment obligations: (i) the assumption of each of the VBH assumed obligations under the partial assignment between the Company and VBH as outlined above, and (ii) certain tiered development milestone and royalty payments to VBH. In August 2018, the Company entered
F-B-15
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
into the amended and restated Regents license agreement and VelosBio entered into their own license agreement directly with the Regents. There is no common control overlap between the companies.
Also on February 6, 2018, the Company and VelosBio entered into: (i) an asset purchase agreement whereby VelosBio purchased the Company’s right, title and interest in the Company’s nominal assets related to the two preclinical product candidates and assumed the Company’s $0.2 million convertible note payable and related $16,000 of accrued interest which has been recorded as other income, and (ii) a transition services agreement whereby the Company agreed to provide VelosBio with certain transition services, which expired as of December 31, 2018, as follows: (i) access to certain common laboratory equipment at the Company’s lab facility, (ii) certain named employees were to devote up to 80% of their time supporting VelosBio related activities, (iii) cirmtuzumab manufacturing, process optimization and ancillary activities until VelosBio was able to establish their own, and (iv) agreement to cost share the purchase of certain antibody materials with VelosBio. Such services were to be provided at cost or cost plus. During 2018, the Company incurred $3.0 million of costs on behalf of VelosBio that were substantially reimbursed and recorded on a net basis within operating expenses in the accompanying consolidated statements of operations. As of December 31, 2018, there are no ongoing rights or commitments under the asset purchase or transition services agreements.
The California Institute for Regenerative Medicine (“CIRM”) Award
In August 2017, CIRM awarded an $18.3 million grant to researchers at the University of California San Diego school of medicine (“UC San Diego”), to advance the Company’s Phase 1b/2 clinical trial evaluating cirmtuzumab in combination with ibrutinib for the treatment of patients withB-cell lymphoid malignancies, including mantle cell lymphoma (“MCL”) and CLL. The Company: (i) is conducting this study in collaboration with UC San Diego, (ii) estimates it will receive $16.1 million in development milestones under research subaward agreements throughout the award project period, estimated to be from October 1, 2017 to March 31, 2022, (iii) is committed to certainco-funding requirements, (iv) received subaward payments of $0.5 million and $3.6 million in December 2018 and 2017, respectively, and (v) is required to provide UC San Diego progress and financial update reports throughout the award project period. The subaward does not bear a royalty payment commitment, nor is the subaward otherwise refundable. For the years ended December 31, 2018 and 2017, the Company recorded revenue of $2.5 million and $1.7 million, respectively. Related qualifying subaward costs during the years ending December 31, 2018 and 2017 was $4.6 million and $3.1 million, respectively. As of December 31, 2018, the Company believes it has met its obligations under the CIRM award and UC San Diego subawards.
Clinical Trial and Supply Agreement
In April 2018, the Company entered into a Clinical Trial and Supply Agreement to supply ibrutinib for the Company’s Phase 1b/2 clinical trial evaluating cirmtuzumab in combination with ibrutinib. Such agreement does not bear any upfront costs, inventory purchase costs, milestone or royalty payment commitments or other financial obligations.
License and Development Agreement with Shanghai Pharmaceutical (USA) Inc. (“SPH USA”), a Related Party
In November 2018, contemporaneous with the issuance of the Series C preferred stock (see Note 5), the Company entered into a License and Development Agreement (“LDA”) with SPH USA for: (i) the territory of the People’s Republic of China, Hong Kong, Macau, and Taiwan (“Greater China”), and (ii) rights to manufacture, develop, market, distribute and sell all of the Company’s product candidates under the License Agreement and the Regents license agreement (exclusive to Greater China only). Under the LDA, SPH USA is
F-B-16
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
solely responsible for: (a) all preclinical and clinical development activities required in order to obtain regulatory approval in Greater China for such product candidates, (b) any third-party license milestone or royalty payments owed under the License Agreement and the Regents license agreement, and (c) paying the Company a low single digit royalty on net sales in the territory.
The LDA will expire upon the expiration of the last royalty term for the last licensed product. The LDA may be terminated by: (i) SPH USA on a country by country or product by product basis with 180 days written notice, (ii) either party upon material breach that is not cured within 90 days, and (iii) either party in the event the other party declares insolvency or bankruptcy.
5. Convertible Preferred Stock and Stockholders’ Deficit
Convertible Preferred Stock
The Company’s convertible preferred stock has been classified as temporary equity on the accompanying consolidated balance sheets in accordance with authoritative guidance for the classification and measurement of potentially redeemable securities whose redemption is based upon certain change in control events outside of the Company’s control, including liquidation, sale or transfer of control of the Company. The Company has determined not to adjust the carrying values of the convertible preferred stock to the liquidation preferences of such shares because the occurrence of any such change of control event is not probable.
The authorized, issued and outstanding shares of convertible preferred stock as of December 31, 2018 consist of the following:
| Shares Authorized | Shares Issued and Outstanding | Liquidation Preference | Carrying Value | |||||||||||||
| (in thousands) | ||||||||||||||||
Series A | 13,560,000 | 13,560,000 | $ | 3,390 | $ | 3,357 | ||||||||||
Series B | 6,750,721 | 6,750,721 | 3,038 | 2,891 | ||||||||||||
SeriesB-2 | 61,788,567 | 56,723,855 | 25,526 | 23,567 | ||||||||||||
Series C | 48,000,000 | 34,000,000 | 17,000 | 16,773 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | 130,099,288 | 111,034,576 | $ | 48,954 | $ | 46,588 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
The authorized, issued and outstanding shares of convertible preferred stock as of December 31, 2017 consist of the following:
| Shares Authorized | Shares Issued and Outstanding | Liquidation Preference | Carrying Value | |||||||||||||
| (in thousands) | ||||||||||||||||
Series A | 13,560,000 | 13,560,000 | $ | 3,390 | $ | 3,357 | ||||||||||
Series B | 55,000,000 | 6,750,721 | 3,038 | 2,891 | ||||||||||||
SeriesB-2 | 75,000,000 | 56,723,855 | 25,526 | 22,467 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total | 143,560,000 | 77,034,576 | $ | 31,954 | $ | 28,715 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
In January 2017, the Company issued 5,688,888 shares of Series B convertible preferred stock at a per share purchase price of $0.45, raising net cash proceeds of $2.5 million.
In September, November and December 2017, the Company issued an aggregate of 22,654,115 shares of SeriesB-2 preferred stock at a per share purchase price of $0.45, raising net cash proceeds of $8.9 million, of which $1.1 million was collected in February 2018 and, as such, was recorded as a stock subscription receivable within mezzanine equity at December 31, 2017.
F-B-17
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
Contemporaneous with and as an inducement for existing or new preferred stockholders to participate in the first closing of the SeriesB-2 convertible preferred stock issuances in September 2017, the Company: (i) issued 3,398,045 warrants for the purchase of SeriesB-2 convertible preferred stock at an exercise price of $0.45 per share, (ii) converted 34,069,740 shares of Series B convertible preferred stock into an equal amount of SeriesB-2 convertible preferred stock for those existing investors that invested their pro rata amount of a minimum targeted raise of $5.6 million in the September 2017 closing (all members of the Company’s board of directors and their affiliated funds purchased at least their pro rata amounts), and (iii) converted 1,666,667 warrants for the purchase of Series B convertible preferred stock into an equal amount of SeriesB-2 convertible preferred stock at the same exercise price of $0.45 per share. The converted Series B convertible preferred stock and Series B convertible preferred stock warrants were accounted for as a modification of such instruments in the accompanying consolidated financial statements.
In November 2018, contemporaneous with entering into the LDA, the Company issued 34,000,000 shares of Series C preferred stock to SPH USA, a related party, at a per share purchase price of $0.50, raising net cash proceeds of $16.8 million. The Company concluded that the shares were issued at fair value and therefore no value was ascribed to the LDA.
Description of Securities
Voting Rights
The holder of each share of Series C convertible preferred stock, SeriesB-2 convertible preferred stock, Series B convertible preferred stock and Series A convertible preferred stock (collectively, “Preferred Stock”) is entitled to one vote for each share of common stock into which it would convert and to vote as one class with the common stockholders on all matters. Certain matters require the vote of 60% of the Preferred Stock, including amendment to the Company’s certificate of incorporation and the declaration of dividends, and certain matters require the vote of 70% of the Series C convertible preferred stock, including any amendment to the rights and preferences of the Series C convertible preferred stock.
Dividends
The holders of the Series C convertible preferred stock are entitled to receive noncumulative dividends when, as and if declared by the board of directors, at the annual per share rate of $0.04. The Company may not declare, pay or set aside any cash dividends on share of any other class or series of capital stock unless the holders of Series C convertible preferred stock then outstanding receive a dividend in an amount at least equal to the greater of: (i) any declared but unpaid Series C convertible preferred stock dividends, and (ii) a proportionate share of any dividend declared on anas-converted to common stock basis or equivalent. In the event dividends are paid on any share of common stock, the Company shall pay an additional dividend on all outstanding shares of Preferred Stock in a per share amount equal to (on anas-if-converted to common stock basis) the amount paid or set aside for each share of common stock. No cash dividends have been declared as of December 31, 2018.
Liquidation
The Series C convertible preferred stock has a liquidation preference of $0.50 per share, plus any declared but unpaid dividends, in preference and priority to any other class or series of the Company’s capital stock. Upon payment of the full liquidation preference of Series C holders, the holders of SeriesB-2 convertible preferred stock are entitled to receive their liquidation preference of $0.45 per share, plus any declared but unpaid dividends. Upon payment of the full liquidation preference of SeriesB-2 holders, the holders of Series B convertible preferred stock are entitled to receive their liquidation preference of $0.45 per share, plus any
F-B-18
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
declared but unpaid dividends. Upon payment of the full liquidation preference of Series B holders, the holders of Series A convertible preferred stock are entitled to receive their liquidation preference of $0.25 per share, plus any declared but unpaid dividends, prior to and in preference to any distribution of the assets of the Company to common stockholders. The remaining assets of the Company are to be distributed ratably among the holders of common stock and Preferred Stock on anas-converted to common stock basis until each share of Preferred Stock has received an aggregate distribution of three times its liquidation preference, at which time the remaining assets are to be distributed ratably among the holders of common stock.
Conversion
The shares of Preferred Stock are convertible into an equal number of shares of common stock, at the option of the holder, subject to certain anti-dilution adjustments. Each share of Preferred Stock is automatically converted into common stock, (A) at any time upon the affirmative election of the holders of at least a majority (or 70% in the case of Series C) of the outstanding shares of each respective series of Preferred Stock, or (B) on the day immediately preceding the effective date of a firmly underwritten public offering of the Company’s common stock pursuant to a registration statement under the Securities Act of 1933, as amended, with respect to which the Company receives gross proceeds of at least $40.0 million and the price to the public is at least $1.50 per share.
Preferred Stock Warrants
In September 2017, the Company exchanged 1,666,667 warrants for the purchase of Series B preferred stock at an exercise price of $0.45 per share into 1,666,667 warrants for the purchase of SeriesB-2 preferred stock at an exercise price of $0.45 per share. The exchange of Series B to SeriesB-2 warrants was accounted for as a modification with the newly issued warrants being remeasured and marked to market as the issuance date with a charge to change in fair value of the warrant liability in the accompanying consolidated statements of operations. In September, November and December 2017, in connection with the closing of the SeriesB-2 convertible preferred stock financings, the Company issued 3,398,045 warrants for the purchase of SeriesB-2 convertible preferred stock at an exercise price of $0.45 per share. As of December 31, 2018, no shares have been issued pursuant to the warrants. The warrants expire on various dates in September, November and December 2022. If the warrants have not been exercised prior to their expiration date, they will be deemed to automatically convert by “cashless” conversion. In the event that the Company is acquired, the warrants will be exercisable or deemed automatically converted, which shall be determined based upon whether the Company’s successor assumes the obligations of the warrants.
Common Stock and Unvested Share Liability
The Company has issued restricted common stock subject to vesting and repurchase by the Company. For employee awards, the issuance date fair value is recognized over the requisite service period of the award (usually the vesting period) on a straight-line basis. For nonemployee awards, the Company uses the fair value method and periodically revalues such awards over the vesting term. In addition, the Company has outstanding unvested shares related to the early exercise of stock options. The Company has the right, but not the obligation, to repurchase any unvested shares at the original purchase price upon any voluntary or involuntary termination. The consideration received in exchange for unvested shares is recorded as an unvested share liability on the accompanying consolidated balance sheets and is reclassified into common stock and additionalpaid-in capital as the shares vest. For the years ended December 31, 2018 and 2017, stock-based compensation of $0.1 million and $0.2 million, respectively, was recognized in connection with the restricted common stock awards. At December 31, 2018 and 2017, the unvested share liability was $54,000 and $89,000, respectively.
F-B-19
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
A summary of the Company’s unvested shares is as follows:
| Number of Unvested Shares | ||||
Balance at December 31, 2016 | 5,725,199 | |||
Early exercised stock options | 50,000 | |||
Issuance of unvested restricted stock | 1,299,397 | |||
Repurchased shares | (1,133,462 | ) | ||
Vested shares | (2,916,748 | ) | ||
|
| |||
Balance at December 31, 2017 | 3,024,386 | |||
Issuance of unvested restricted stock | 200,000 | |||
Vested shares | (1,866,910 | ) | ||
|
| |||
Balance at December 31, 2018 | 1,357,476 | |||
|
| |||
For the years ended December 31, 2018 and 2017, the Company paid $0 and $16,000, respectively, to repurchase unvested shares.
Equity Incentive Plan
In July 2015, the Company adopted its 2015 Equity Incentive Plan (the “2015 Plan”), which provides for the issuance of incentive stock options,non-statutory stock options, restricted stock awards, restricted stock unit awards and other stock awards to its employees, members of its board of directors and consultants. No awards shall be granted under the 2015 Plan after July 2025. In general, the options issued under the 2015 Plan expire ten years from the date of grant and vest over a four-year period. Certain grants vest based on the achievement of development or regulatory milestones. The 2015 Plan allows for early exercise of all stock option grants if authorized by the board of directors at the time of grant. The Company has the option to repurchase any unvested shares at the original purchase price upon any voluntary or involuntary termination. The Company had 8,600,000 shares of common stock authorized for issuance under the 2015 Plan as of December 31, 2018, of which 1,090,081 remained available for future issuance.
A summary of the Company’s stock option activity is as follows:
| Number of Outstanding Options | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value | |||||||||||||
| (in thousands) | ||||||||||||||||
Balance at December 31, 2017 | 2,068,251 | $ | 0.05 | 7.9 | $ | — | ||||||||||
Granted | 4,825,000 | $ | 0.06 | |||||||||||||
Exercised | (25,000 | ) | $ | 0.05 | ||||||||||||
|
| |||||||||||||||
Balance at December 31, 2018 | 6,868,251 | $ | 0.06 | 9.0 | $ | 20 | ||||||||||
|
| |||||||||||||||
Vested and expected to vest December 31, 2018 | 6,868,251 | $ | 0.06 | 9.0 | $ | 20 | ||||||||||
|
| |||||||||||||||
Exercisable at December 31, 2018 | 2,655,751 | $ | 0.05 | 7.6 | $ | 20 | ||||||||||
|
| |||||||||||||||
F-B-20
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
The aggregate intrinsic value of stock options exercised during the years ended December 31, 2018 and 2017 was not material. The intrinsic value is calculated as the difference between the fair value of the Company’s common stock at the time of the option exercise and the exercise price of that stock option.
Stock-Based Compensation Expense
The weighted-average assumptions used in the Black-Scholes option pricing model to determine the fair value of employee stock option grants were as follows:
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
Risk-free interest rate | 2.88 | % | 1.96 | % | ||||
Expected volatility | 64.7 | % | 69.0 | % | ||||
Expected term (in years) | 6.1 | 6.1 | ||||||
Expected dividend yield | 0.0 | % | 0.0 | % | ||||
Expected volatility. Since the Company is not a public company and does not have a trading history for its common stock, the expected volatility assumption is based on volatilities of a peer group of similar companies whose share prices are publicly available. The peer group was developed based on companies in the life sciences industry. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available.
Expected term. The expected term represents the period of time that options are expected to be outstanding. Because the Company does not have historical exercise behavior, it determines the expected life assumption using the simplified method, for employees, which is an average of the contractual term of the option and its vesting period. The expected term for nonemployee options is generally the remaining contractual term.
Risk-free interest rate.The risk-free interest rate is based on the implied yield on the U.S. Treasury securities with a maturity date similar to the expected term of the associated stock option award.
Expected dividend yield. The Company bases the expected dividend yield assumption on the fact that it has never paid cash dividends and has no present intention to pay cash dividends and, therefore, used an expected dividend yield of zero.
Stock-based compensation expense recognized for all equity awards has been reported in the statements of operations as follows:
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| (in thousands) | ||||||||
Research and development | $ | 141 | $ | 72 | ||||
General and administrative | 39 | 116 | ||||||
|
|
|
| |||||
| $ | 180 | $ | 188 | |||||
|
|
|
| |||||
The weighted-average grant date fair value per share of employee option grants for the years ended December 31, 2018 and 2017 was $0.04 and $0.03, respectively. As of December 31, 2018, total unrecognized employee stock-based compensation expense was $191,000, which is expected to be recognized over a remaining weighted-average period of approximately 2.7years.
F-B-21
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance is as follows:
| December 31, 2018 | ||||
Conversion of convertible preferred stock | 111,034,576 | |||
Preferred stock warrants | 5,064,712 | |||
Common stock options issued and outstanding | 6,868,251 | |||
Common stock available for issuance under the 2015 Plan | 1,090,081 | |||
|
| |||
| 124,057,620 | ||||
|
| |||
6. Income Taxes
A reconciliation of the Company’s effective tax rate to the federal statutory rate is as follows:
| Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| (in thousands) | ||||||||
Tax computed at federal statutory rate | $ | (1,379 | ) | $ | (3,548 | ) | ||
State taxes, net | (500 | ) | (605 | ) | ||||
Permanent differences | (147 | ) | 118 | |||||
Research and development credits | (468 | ) | (186 | ) | ||||
Tax Cuts and Jobs Act | — | 2,789 | ||||||
Other | 51 | 50 | ||||||
Valuation allowance | 2,443 | 1,382 | ||||||
|
|
|
| |||||
| $ | — | $ | — | |||||
|
|
|
| |||||
Significant components of the Company’s net deferred tax assets are as follows:
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| (in thousands) | ||||||||
Deferred tax assets: | ||||||||
Net operating loss carryforwards | $ | 8,321 | $ | 6,312 | ||||
Research and development credit carryforwards | 1,231 | 771 | ||||||
Accrued expenses | 24 | 25 | ||||||
Other, net | 288 | 314 | ||||||
|
|
|
| |||||
Total deferred tax assets | 9,864 | 7,422 | ||||||
Valuation allowance | (9,864 | ) | (7,422 | ) | ||||
|
|
|
| |||||
Net deferred taxes | $ | — | $ | — | ||||
|
|
|
| |||||
Based upon the Company’s history of operating losses, the Company is unable to conclude that it is more likely than not that the benefit of its deferred tax assets will be realized. Accordingly, the Company has provided a full valuation allowance for its deferred tax assets as of December 31, 2018 and 2017.
At December 31, 2018, the Company had federal and state net operating loss carryforwards of approximately $29.7 million. Of the federal net operating losses, $7.0 million do not expire, and the remaining
F-B-22
Oncternal Therapeutics, Inc.
Notes to Consolidated Financial Statements—(Continued)
federal and state net operating loss carryforwards will begin expiring in 2033, unless previously utilized. At December 31, 2018, the Company had federal and state research and development credit carryforwards of approximately $0.9 million and $0.5 million, respectively. The federal research and development credit carryforwards will begin expiring in 2034, unless previously utilized. The state research and development credits do not expire.
Pursuant to Internal Revenue Code Sections 382 and 383, annual use of the Company’s net operating loss and research and development tax credit carryforwards may be limited in the event a cumulative change in ownership of more than 50% occurs within a three-year period. The Company has not completed a Section 382 study to assess whether an ownership change has occurred or whether there have been multiple ownership changes since the Company’s formation due to the complexity and cost associated with such a study and the fact that there may be additional such ownership changes in the future. If eliminated, the related asset would be removed from the deferred tax asset schedule with a corresponding reduction in the valuation allowance. Due to the existence of the valuation allowance, limitations created by future ownership changes, if any, will not impact the Company’s effective tax rate.
The Tax Act was enacted on December 22, 2017. The Tax Act reduced the U.S. federal corporate tax rate from a maximum of 35% to a flat 21%. The reduction in rate resulted in a remeasurement of the Company’s deferred tax assets at December 31, 2017 based on the rates at which they were expected to reverse in the future, resulting in a reduction in the deferred tax asset balance of $2.8 million, which was offset by a reduction in the valuation allowance by a corresponding amount.
The Company recognizes a tax benefit from an uncertain tax position when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. Income tax positions must meet a more likely than not recognition at the effective date to be recognized. At December 31, 2018 and 2017, there were no unrecognized tax benefits recorded in the consolidated financial statements. The Company does not expect any material changes to unrecognized tax benefits within the next twelve months.
The Company is subject to taxation in the United States federal and state jurisdictions. The Company’s federal income tax and state income tax returns since inception in 2013 through 2018 are subject to examination by federal and state tax authorities due to the carryforward of unutilized net operating losses and research and development credits. The Company is not currently under examination by any tax authority.
The Company’s policy is to recognize interest and penalties related to income tax matters in income tax expense. The Company has not recognized interest or penalties in its consolidated statements of operations since inception.
7. Subsequent Events
Merger Agreement
On March 6, 2019, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with GTx, Inc. (“GTx”) and Grizzly Merger Sub, Inc., a wholly-owned subsidiary of GTx (“Merger Sub”). Upon the terms and subject to the satisfaction of the conditions described in the Merger Agreement, including approval of the transaction by the Company’s stockholders and GTx’s stockholders, Merger Sub will be merged with and into the Company, with the Company surviving the Merger as a wholly-owned subsidiary of GTx.
F-B-23
TABLE OF CONTENTS
| Page | ||||||
SECTION 1. | DESCRIPTION OF TRANSACTION | A-2 | ||||
1.1 | The Merger | A-2 | ||||
1.2 | Effects of the Merger | A-2 | ||||
1.3 | Closing; Effective Time | A-2 | ||||
1.4 | Certificate of Incorporation and Bylaws; Directors and Officers | A-2 | ||||
1.5 | Conversion of Shares | A-3 | ||||
1.6 | Contingent Value Right | A-4 | ||||
1.7 | Closing of the Company’s Transfer Books | A-4 | ||||
1.8 | Surrender of Certificates | A-5 | ||||
1.9 | Appraisal Rights | A-6 | ||||
1.10 | Further Action | A-6 | ||||
1.11 | Withholding | A-6 | ||||
1.12 | Calculation of Parent Cash Amount | A-7 | ||||
1.13 | Calculation of Company Cash Amount | A-8 | ||||
SECTION 2. | REPRESENTATIONS AND WARRANTIES OF THE COMPANY | A-9 | ||||
2.1 | Due Organization; Subsidiaries | A-9 | ||||
2.2 | Organizational Documents | A-9 | ||||
2.3 | Authority; Binding Nature of Agreement | A-9 | ||||
2.4 | Vote Required | A-10 | ||||
2.5 | Non-Contravention; Consents | A-10 | ||||
2.6 | Capitalization | A-11 | ||||
2.7 | Financial Statements | A-12 | ||||
2.8 | Absence of Changes | A-13 | ||||
2.9 | Absence of Undisclosed Liabilities | A-13 | ||||
2.10 | Title to Assets | A-13 | ||||
2.11 | Real Property; Leasehold | A-13 | ||||
2.12 | Intellectual Property | A-14 | ||||
2.13 | Agreements, Contracts and Commitments | A-15 | ||||
2.14 | Compliance; Permits; Restrictions | A-17 | ||||
2.15 | Legal Proceedings; Orders | A-18 | ||||
2.16 | Tax Matters | A-19 | ||||
2.17 | Employee and Labor Matters; Benefit Plans | A-20 | ||||
2.18 | Environmental Matters | A-24 | ||||
2.19 | Insurance | A-24 | ||||
2.20 | No Financial Advisors | A-24 | ||||
2.21 | Disclosure | A-24 | ||||
2.22 | Transactions with Affiliates | A-24 | ||||
2.23 | Anti-Bribery | A-25 | ||||
2.24 | Disclaimer of Other Representations or Warranties | A-25 | ||||
SECTION 3. | REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB | A-25 | ||||
3.1 | Due Organization; No Subsidiaries. | A-25 | ||||
3.2 | Organizational Documents | A-26 | ||||
3.3 | Authority; Binding Nature of Agreement | A-26 | ||||
3.4 | Vote Required | A-26 | ||||
3.5 | Non-Contravention; Consents | A-27 | ||||
3.6 | Capitalization | A-27 | ||||
A-i
TABLE OF CONTENTS
(continued)
| Page | ||||||
3.7 | SEC Filings; Financial Statements | A-29 | ||||
3.8 | Absence of Changes | A-30 | ||||
3.9 | Absence of Undisclosed Liabilities | A-30 | ||||
3.10 | Title to Assets | A-31 | ||||
3.11 | Real Property; Leasehold | A-31 | ||||
3.12 | Intellectual Property | A-31 | ||||
3.13 | Agreements, Contracts and Commitments | A-32 | ||||
3.14 | Compliance; Permits | A-34 | ||||
3.15 | Legal Proceedings; Orders | A-35 | ||||
3.16 | Tax Matters | A-35 | ||||
3.17 | Employee and Labor Matters; Benefit Plans | A-37 | ||||
3.18 | Environmental Matters | A-40 | ||||
3.19 | Transactions with Affiliates | A-40 | ||||
3.20 | Insurance | A-40 | ||||
3.21 | No Financial Advisors | A-41 | ||||
3.22 | Anti-Bribery | A-41 | ||||
3.23 | Valid Issuance | A-41 | ||||
3.24 | Opinion of Financial Advisor | A-41 | ||||
3.25 | Disclaimer of Other Representations or Warranties | A-41 | ||||
SECTION 4. | CERTAIN COVENANTS OF THE PARTIES | A-41 | ||||
4.1 | Operation of Parent’s Business | A-41 | ||||
4.2 | Operation of the Company’s Business | A-43 | ||||
4.3 | Access and Investigation | A-45 | ||||
4.4 | ParentNon-Solicitation | A-45 | ||||
4.5 | CompanyNon-Solicitation | A-46 | ||||
4.6 | Notification of Certain Matters | A-47 | ||||
SECTION 5. | ADDITIONAL AGREEMENTS OF THE PARTIES | A-47 | ||||
5.1 | Registration Statement; Proxy Statement | A-47 | ||||
5.2 | Company Information Statement; Stockholder Written Consent | A-49 | ||||
5.3 | Parent Stockholders’ Meeting | A-51 | ||||
5.4 | Regulatory Approvals | A-53 | ||||
5.5 | Company Options and Company Warrants | A-53 | ||||
5.6 | Indemnification of Officers and Directors | A-55 | ||||
5.7 | Additional Agreements | A-56 | ||||
5.8 | Disclosure | A-56 | ||||
5.9 | Listing | A-56 | ||||
5.10 | Tax Matters | A-57 | ||||
5.11 | Legends | A-57 | ||||
5.12 | Directors and Officers | A-57 | ||||
5.13 | Termination of Certain Agreements and Rights | A-58 | ||||
5.14 | Section 16 Matters | A-58 | ||||
5.15 | Cooperation | A-58 | ||||
5.16 | Allocation Certificates | A-58 | ||||
5.17 | Company Financial Statements | A-59 | ||||
5.18 | Takeover Statutes | A-59 | ||||
5.19 | Stockholder Litigation | A-59 | ||||
A-ii
TABLE OF CONTENTS
(continued)
| Page | ||||||
5.20 | Parent Equity Plan | A-59 | ||||
5.21 | Preferred Stock Conversion | A-60 | ||||
5.22 | Parent Options | A-60 | ||||
5.23 | Termination of Parent Deferred Stock Rights | A-60 | ||||
SECTION 6. | CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY | A-60 | ||||
6.1 | Effectiveness of Registration Statement | A-60 | ||||
6.2 | No Restraints | A-60 | ||||
6.3 | Stockholder Approval | A-60 | ||||
6.4 | Listing | A-60 | ||||
6.5 | Regulatory Approvals | A-61 | ||||
SECTION 7. | ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND MERGER SUB | A-61 | ||||
7.1 | Accuracy of Representations | A-61 | ||||
7.2 | Performance of Covenants | A-61 | ||||
7.3 | Documents | A-61 | ||||
7.4 | FIRPTA Certificate | A-61 | ||||
7.5 | No Company Material Adverse Effect | A-61 | ||||
7.6 | Termination of Investor Agreements | A-62 | ||||
7.7 | CompanyLock-Up Agreements | A-62 | ||||
7.8 | Company Stockholder Written Consent | A-62 | ||||
7.9 | Preferred Stock Conversion | A-62 | ||||
7.10 | Parent Closing Tax Opinion | A-62 | ||||
SECTION 8. | ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY | A-62 | ||||
8.1 | Accuracy of Representations | A-62 | ||||
8.2 | Performance of Covenants | A-62 | ||||
8.3 | Documents | A-62 | ||||
8.4 | No Parent Material Adverse Effect | A-63 | ||||
8.5 | ParentLock-Up Agreements | A-63 | ||||
8.6 | Company Closing Tax Opinion | A-63 | ||||
8.7 | Board of Directors | A-63 | ||||
SECTION 9. | TERMINATION | A-63 | ||||
9.1 | Termination | A-63 | ||||
9.2 | Effect of Termination | A-64 | ||||
9.3 | Expenses; Termination Fees | A-64 | ||||
SECTION 10. | MISCELLANEOUS PROVISIONS | A-66 | ||||
10.1 | Non-Survival of Representations and Warranties | A-66 | ||||
10.2 | Amendment | A-66 | ||||
10.3 | Waiver | A-66 | ||||
10.4 | Entire Agreement; Counterparts; Exchanges by Electronic Transmission | A-66 | ||||
10.5 | Applicable Law; Jurisdiction | A-67 | ||||
10.6 | Attorneys’ Fees | A-67 | ||||
10.7 | Assignability | A-67 | ||||
A-iii
TABLE OF CONTENTS
(continued)
| Page | ||||||
10.8 | Notices | A-67 | ||||
10.9 | Cooperation | A-68 | ||||
10.10 | Severability | A-68 | ||||
10.11 | Other Remedies; Specific Performance | A-68 | ||||
10.12 | No Third Party Beneficiaries | A-68 | ||||
10.13 | Construction | A-69 | ||||
A-iv
Schedules:
Company Disclosure Schedule
Parent Disclosure Schedule
Exhibits:
| Exhibit A | Definitions | |
| Exhibit B-1 | Form of Company Stockholder Support Agreement | |
| ExhibitB-2 | Form of Parent Stockholder Support Agreement | |
| Exhibit C | Post-Closing Directors and Officers Certain Parent Officer Positions | |
| Exhibit D | Form ofLock-Up Agreement | |
| Exhibit E | Form of CVR Agreement | |
| Exhibit F | 2019 Plan |
A-v
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
THIS AGREEMENT AND PLAN OF MERGER AND REORGANIZATION (this “Agreement”) is made and entered into as of March 6, 2019, by and amongGTx, Inc., a Delaware corporation (“Parent”)Grizzly Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), and Oncternal Therapeutics, Inc., a Delaware corporation (the “Company”). Certain capitalized terms used in this Agreement are defined inExhibit A.
RECITALS
A. Parent and the Company intend to effect a merger of Merger Sub with and into the Company (the “Merger”) in accordance with this Agreement and the DGCL. Upon consummation of the Merger, Merger Sub will cease to exist and the Company will become a wholly owned subsidiary of Parent.
B. The Parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Code, and by executing this Agreement, the Parties intend to adopt a plan of reorganization within the meaning of Treasury Regulations Sections1.368-2(g) and1.368-3.
C. The Parent Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of Parent and its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of shares of Parent Common Stock to the stockholders of the Company pursuant to the terms of this Agreement and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Parent vote to approve the Parent Stockholder Matters.
D. The Merger Sub Board has (i) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Merger Sub and its sole stockholder, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholder of Merger Sub votes to adopt this Agreement and thereby approve the Contemplated Transactions.
E. The Company Board has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Company vote to approve the Company Stockholder Matters.
F. Concurrently with the execution and delivery of this Agreement and as a condition and inducement to Parent’s willingness to enter into this Agreement, the officers, directors and stockholders of the Company listed on Section A of the Company Disclosure Schedule (solely in their capacity as stockholders of the Company) are executing (a) support agreements in favor of Parent in substantially the form attached hereto asExhibitB-1 (the “Company Stockholder Support Agreement”), pursuant to which such Persons (the “Company Signatories”) have, subject to the terms and conditions set forth therein, agreed to vote all of their shares of Company Capital Stock in favor of the Company Stockholder Matters and against any proposals that compete with the Contemplated Transactions, and(b) lock-up agreements in substantially the form attached hereto asExhibit Dexecuted by the Company Signatories (each, a “CompanyLock-Up Agreement”).
G. Concurrently with the execution and delivery of this Agreement and as a condition and inducement to the Company’s willingness to enter into this Agreement, the officers and directors of Parent listed on Section A of the Parent Disclosure Schedule (solely in their capacity as stockholders of Parent) are executing (a) support agreements in favor of the Company in substantially the form attached hereto asExhibitB-2 (the “Parent
A-1
Stockholder Support Agreement”), pursuant to which such Persons (the “Parent Signatories”)have, subject to the terms and conditions set forth therein, agreed to vote all of their shares of Parent Common Stock in favor of the Parent Stockholder Matters and against any proposals that compete with the Contemplated Transactions and(b) lock-up agreements in substantially the form attached hereto asExhibit Dexecuted by the Parent Signatories(each, a “ParentLock-Up Agreement”).
H. It is expected that promptly after the Registration Statement is declared effective under the Securities Act (but in no event later than 10 Business Days following the effectiveness of the Registration Statement), the Company shall deliver the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote.
AGREEMENT
The Parties, intending to be legally bound, agree as follows:
Section 1. DESCRIPTION OF TRANSACTION
1.1 The Merger. Upon the terms and subject to the conditions set forth in this Agreement, at the Effective Time, Merger Sub shall be merged with and into the Company, and the separate existence of Merger Sub shall cease. The Company will continue as the surviving corporation in the Merger (the “Surviving Corporation”).
1.2 Effects of the Merger. The Merger shall have the effects set forth in this Agreement, the Certificate of Merger and in the applicable provisions of the DGCL. As a result of the Merger, the Company will become a wholly owned subsidiary of Parent.
1.3 Closing; Effective Time. Unless this Agreement is earlier terminated pursuant to the provisions ofSection 9.1, and subject to the satisfaction or waiver of the conditions set forth inSections 6,7 and8, the consummation of the Merger (the “Closing”) shall take place remotely as promptly as practicable (but in no event later than the second Business Day following the satisfaction or waiver of the last to be satisfied or waived of the conditions set forth inSections 6,7 and8, other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of each of such conditions), or at such other time, date and place as Parent and the Company may mutually agree in writing. The date on which the Closing actually takes place is referred to as the “Closing Date.” At the Closing, the Parties shall cause the Merger to be consummated by executing and filing with the Secretary of State of the State of Delaware a certificate of merger with respect to the Merger, satisfying the applicable requirements of the DGCL and in a form reasonably acceptable to Parent and the Company (the “Certificate of Merger”). The Merger shall become effective at the time of the filing of such Certificate of Merger with the Secretary of State of the State of Delaware or at such later time as may be specified in such Certificate of Merger with the consent of Parent and the Company (the time as of which the Merger becomes effective being referred to as the “Effective Time”).
1.4 Certificate of Incorporation and Bylaws; Directors and Officers. At the Effective Time:
(a) the certificate of incorporation of the Surviving Corporation shall be amended and restated in its entirety to read identically to the certificate of incorporation of Merger Sub as in effect immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such certificate of incorporation;provided,however, that at the Effective Time, Parent shall file an amendment to the Surviving Company’s certificate of incorporation to (i) change the name of the Surviving Corporation to Oncternal Oncology, Inc. and (ii) make such other changes as are mutually agreed to by Parent and the Company.
(b) the certificate of incorporation of Parent shall be identical to the certificate of incorporation of Parent immediately prior to the Effective Time, until thereafter amended as provided by the DGCL and such
A-2
certificate of incorporation,provided,however, that at the Effective Time, Parent shall file an amendment to its certificate of incorporation to (i) change the name of Parent to Oncternal Therapeutics, Inc., (ii) as contemplated bySection 5.3(a)(i), effect the Nasdaq Reverse Split and (iii) make such other changes as are mutually agreeable to Parent and the Company;
(c) the bylaws of the Surviving Corporation shall be amended and restated in their entirety to read identically to the bylaws of Merger Sub as in effect immediately prior to the Effective Time (except that the name of the Surviving Corporation in such bylaws shall reflect the name identified inSection 1.4(a)), until thereafter amended as provided by the DGCL and such bylaws;
(d) the directors and officers of Parent, each to hold office in accordance with the certificate of incorporation and bylaws of Parent, shall be as set forth inSection 5.12; and
(e) the directors and officers of the Surviving Corporation, each to hold office in accordance with the certificate of incorporation and bylaws of the Surviving Corporation, shall be the directors and officers of Parent as set forth inSection 5.12, after giving effect to the provisions ofSection 5.12, or such other persons as shall be mutually agreed upon by Parent and the Company.
(a) At the Effective Time, by virtue of the Merger and without any further action on the part of Parent, Merger Sub, the Company or any stockholder of the Company or Parent:
(i) any shares of Company Common Stock held as treasury stock or held or owned by the Company, Merger Sub or any Subsidiary of the Company immediately prior to the Effective Time shall be canceled and retired and shall cease to exist, and no consideration shall be delivered in exchange therefor; and
(ii) subject toSection 1.5(c), each share of Company Common Stock outstanding immediately prior to the Effective Time (excluding shares to be canceled pursuant toSection 1.5(a)(i) and excluding Dissenting Shares) shall be automatically converted solely into the right to receive a number of shares of Parent Common Stock equal to the Exchange Ratio (the “Merger Consideration”).
(b) If any shares of Company Common Stock outstanding immediately prior to the Effective Time are unvested or are subject to a repurchase option or a risk of forfeiture under any applicable restricted stock purchase agreement or other similar agreement with the Company, then the shares of Parent Common Stock issued in exchange for such shares of Company Common Stock will to the same extent be unvested and subject to the same repurchase option or risk of forfeiture, and such shares of Parent Common Stock shall accordingly be marked with appropriate legends. The Company shall take all actions that may be reasonably necessary to ensure that, from and after the Effective Time, Parent is entitled to exercise any such repurchase option or other right set forth in any such restricted stock purchase agreement or other agreement in accordance with its terms.
(c) No fractional shares of Parent Common Stock shall be issued in connection with the Merger, and no certificates or scrip for any such fractional shares shall be issued. Any holder of Company Common Stock who would otherwise be entitled to receive a fraction of a share of Parent Common Stock (after aggregating all fractional shares of Parent Common Stock issuable to such holder) shall, in lieu of such fraction of a share and upon surrender by such holder of a letter of transmittal in accordance withSection 1.8 and any accompanying documents as required therein, be paid in cash the dollar amount (rounded to the nearest whole cent), without interest, determined by multiplying such fraction by the Parent Closing Price.
(d) All Company Options outstanding immediately prior to the Effective Time under the Company Plans shall be treated in accordance withSection 5.5(a).
A-3
(e) All Company Warrants outstanding immediately prior to the Effective Time shall be treated in accordance withSection 5.5(c).
(f) Each share of common stock, $0.001 par value per share, of Merger Sub issued and outstanding immediately prior to the Effective Time shall be converted into and exchanged for one validly issued, fully paid and nonassessable share of common stock, $0.001 par value per share, of the Surviving Corporation. Each stock certificate of Merger Sub evidencing ownership of any such shares shall, as of the Effective Time, evidence ownership of such shares of common stock of the Surviving Corporation.
(g) If, between the time of calculating the Exchange Ratio and the Effective Time, any outstanding shares of Company Capital Stock or Parent Common Stock shall have been changed into, or exchanged for, a different number of shares or a different class, by reason of any stock dividend, subdivision, reclassification, recapitalization, split (including the Nasdaq Reverse Split to the extent such split has not been previously taken into account in calculating the Exchange Ratio), combination or exchange of shares or other like change, the Exchange Ratio shall, to the extent necessary, be equitably adjusted to reflect such change to the extent necessary to provide the holders of Company Capital Stock, Parent Common Stock, Company Options and Company Warrants with the same economic effect as contemplated by this Agreement prior to such stock dividend, subdivision, reclassification, recapitalization, split (including the Nasdaq Reverse Split), combination or exchange of shares or other like change;provided, however, that nothing herein will be construed to permit the Company or Parent to take any action with respect to Company Capital Stock or Parent Common Stock, respectively, that is prohibited or not expressly permitted by the terms of this Agreement.
(a) Holders of Parent Common Stock of record as of immediately prior to the Effective Time shall be entitled to one contractual contingent value right (a “CVR”) issued by Parent subject to and in accordance with the terms and conditions of the CVR Agreement, attached hereto asExhibit E(the “CVR Agreement”), for each share of Parent Common Stock held by such holders, including Parent Common Stock subject to any Parent Deferred Stock Right.
(b) At or prior to the Effective Time, Parent shall authorize and duly adopt, execute and deliver, and will ensure that Exchange Agent and CVR Representative execute and deliver, the CVR Agreement, subject to any reasonable revisions to the CVR Agreement that are requested by such Exchange Agent (provided that such revisions are not, individually or in the aggregate, detrimental or adverse, taken as a whole, to any holder of CVR). Parent and the Company shall cooperate, including by making changes to the form of CVR Agreement, as necessary to ensure that the CVRs are not subject to registration under the Securities Act, the Exchange Act or any applicable state securities or “blue sky” laws.
(c) Parent, the Exchange Agent and (if necessary) CVR Representative shall, at or prior to the Effective Time, duly authorize, execute and deliver the CVR Agreement.
1.7 Closing of the Company’s Transfer Books. At the Effective Time: (a) all shares of Company Common Stock outstanding immediately prior to the Effective Time shall be treated in accordance withSection 1.5(a), and all holders of certificates representing shares of Company Capital Stock that were outstanding immediately prior to the Effective Time shall cease to have any rights as stockholders of the Company; and (b) the stock transfer books of the Company shall be closed with respect to all shares of Company Capital Stock outstanding immediately prior to the Effective Time. No further transfer of any such shares of Company Capital Stock shall be made on such stock transfer books after the Effective Time. If, after the Effective Time, a valid certificate previously representing any shares of Company Capital Stock, including any valid certificate representing any shares of Company Preferred Stock previously converted into shares of Company Common Stock in connection with the Preferred Stock Conversion, outstanding immediately prior to the Effective Time (a “Company Stock Certificate”) is presented to the Exchange Agent or to the Surviving Corporation, such Company Stock Certificate shall be canceled and shall be exchanged as provided inSections 1.5 and1.8.
A-4
1.8 Surrender of Certificates.
(a) No later than 10 Business Days after the date that the Registration Statement is declared effective, Parent and the Company shall agree upon and select a reputable bank, transfer agent or trust company to act as exchange agent in the Merger (the “Exchange Agent”). At the Effective Time, Parent shall deposit with the Exchange Agent: (i) certificates or evidence of book-entry shares representing the Parent Common Stock issuable pursuant toSection 1.5(a) and (ii) cash sufficient to make payments in lieu of fractional shares in accordance withSection 1.5(c). The Parent Common Stock and cash amounts so deposited with the Exchange Agent, together with any dividends or distributions received by the Exchange Agent with respect to such shares, are referred to collectively as the “Exchange Fund.”
(b) Promptly after the Effective Time, the Parties shall cause the Exchange Agent to mail to the Persons who were record holders of shares of Company Capital Stock that were converted into the right to receive the Merger Consideration: (i) a letter of transmittal in customary form and containing such provisions as Parent may reasonably specify (including a provision confirming that delivery of Company Stock Certificates shall be effected, and risk of loss and title to Company Stock Certificates shall pass, only upon proper delivery of such Company Stock Certificates to the Exchange Agent); and (ii) instructions for effecting the surrender of Company Stock Certificates in exchange for shares of Parent Common Stock. Upon surrender of a Company Stock Certificate to the Exchange Agent for exchange, together with a duly executed letter of transmittal and such other documents as may be reasonably required by the Exchange Agent or Parent: (A) the holder of such Company Stock Certificate shall be entitled to receive in exchange therefor a certificate or certificates or book-entry shares representing the Merger Consideration (in a number of whole shares of Parent Common Stock) that such holder has the right to receive pursuant to the provisions ofSection 1.5(a) (and cash in lieu of any fractional share of Parent Common Stock pursuant to the provisions ofSection 1.5(c)); and (B) the Company Stock Certificate so surrendered shall be canceled. Until surrendered as contemplated by thisSection 1.8(b), each Company Stock Certificate shall be deemed, from and after the Effective Time, to represent only the right to receive a certificate or certificates or book-entry shares of Parent Common Stock representing the Merger Consideration (and cash in lieu of any fractional share of Parent Common Stock). If any Company Stock Certificate shall have been lost, stolen or destroyed, Parent may, in its discretion and as a condition precedent to the delivery of any shares of Parent Common Stock, require the owner of such lost, stolen or destroyed Company Stock Certificate to provide an applicable affidavit with respect to such Company Stock Certificate that includes an obligation of such owner to indemnify Parent against any claim suffered by Parent related to the lost, stolen or destroyed Company Stock Certificate as Parent may reasonably request. In the event of a transfer of ownership of a Company Stock Certificate that is not registered in the transfer records of the Company, payment of the Merger Consideration in respect of such Company Stock Certificate may be made to a Person other than the Person in whose name such Company Stock Certificate so surrendered is registered if such Company Stock Certificate shall be properly endorsed or otherwise be in proper form for transfer and the Person requesting such payment shall pay any transfer or other Taxes required by reason of the transfer or establish to the reasonable satisfaction of Parent that such Taxes have been paid or are not applicable. The Merger Consideration and any dividends or other distributions as are payable pursuant toSection 1.8(c) shall be deemed to have been in full satisfaction of any and all rights pertaining to Company Capital Stock formerly represented by such Company Stock Certificate.
(c) No dividends or other distributions declared or made with respect to Parent Common Stock with a record date on or after the Effective Time shall be paid to the holder of any unsurrendered Company Stock Certificate with respect to the shares of Parent Common Stock that such holder has the right to receive in the Merger until such holder surrenders such Company Stock Certificate or provides an affidavit of loss or destruction in lieu thereof in accordance with thisSection 1.8 (at which time (or, if later, on the applicable payment date) such holder shall be entitled, subject to the effect of applicable abandoned property, escheat or similar Laws, to receive all such dividends and distributions, without interest).
(d) Any portion of the Exchange Fund that remains undistributed to holders of Company Stock Certificates as of the date that is one year after the Closing Date shall be delivered to Parent upon demand, and
A-5
any holders of Company Stock Certificates who have not theretofore surrendered their Company Stock Certificates in accordance with thisSection 1.8 shall thereafter look only to Parent for satisfaction of their claims for Parent Common Stock, cash in lieu of fractional shares of Parent Common Stock and any dividends or distributions with respect to shares of Parent Common Stock.
(e) No party to this Agreement shall be liable to any holder of any Company Stock Certificate or to any other Person with respect to any shares of Parent Common Stock (or dividends or distributions with respect thereto) or for any cash amounts delivered to any public official pursuant to any applicable abandoned property Law, escheat Law or similar Law.
(a) Notwithstanding any provision of this Agreement to the contrary, shares of Company Capital Stock that are outstanding immediately prior to the Effective Time and which are held by stockholders who have exercised and perfected appraisal rights for such shares of Company Capital Stock in accordance with the DGCL or the CCC, as applicable (collectively, the “Dissenting Shares”) shall not be converted into or represent the right to receive the Merger Consideration described inSection 1.5 attributable to such Dissenting Shares. Such stockholders shall be entitled to receive payment of the appraised value of such shares of Company Capital Stock held by them in accordance with the DGCL or the CCC, as applicable, unless and until such stockholders fail to perfect or effectively withdraw or otherwise lose their appraisal rights under the DGCL or the CCC, as applicable. All Dissenting Shares held by stockholders who shall have failed to perfect or shall have effectively withdrawn or lost their right to appraisal of such shares of Company Capital Stock under the DGCL or the CCC, as applicable (whether occurring before, at or after the Effective Time) shall thereupon be deemed to be converted into and to have become exchangeable for, as of the Effective Time, the right to receive the Merger Consideration, without interest, attributable to such Dissenting Shares upon their surrender in the manner provided inSections 1.5 and1.8.
(b) The Company shall give Parent prompt written notice of any demands by dissenting stockholders received by the Company, withdrawals of such demands and any other instruments served on the Company and any material correspondence received by the Company in connection with such demands, and the Company shall have the right to direct all negotiations and proceedings with respect to such demands;provided that Parent shall have the right to participate in such negotiations and proceedings. Neither the Parent nor the Company shall, except with the prior written consent of the other Party, voluntarily make any payment with respect to, or settle or offer to settle, any such demands, or approve any withdrawal of any such demands or agree to do any of the foregoing.
1.10 Further Action. If, at any time after the Effective Time, any further action is determined by the Surviving Corporation to be necessary or desirable to carry out the purposes of this Agreement or to vest the Surviving Corporation with full right, title and possession of and to all rights and property of the Company, then the officers and directors of the Surviving Corporation shall be fully authorized, and shall use their and its commercially reasonable efforts (in the name of the Company, in the name of Merger Sub, in the name of the Surviving Corporation and otherwise) to take such action.
1.11 Withholding. The Parties and the Exchange Agent shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any holder of Company Capital Stock or any other Person such amounts as such Party or the Exchange Agent is required to deduct and withhold under the Code or any other Law with respect to the making of such payment. The payor shall provide commercially reasonable notice to the payee upon becoming aware of any such withholding obligation, and the Parties shall cooperate with each other to the extent reasonable to obtain reduction of or relief from such withholding. To the extent that amounts are so deducted and withheld and paid to the appropriate Person, such deducted and withheld amounts shall be treated for all purposes of this Agreement as having been paid to the Person in respect of whom such deduction and withholding was made.
A-6
1.12 Calculation of Parent Cash Amount.
(a) For the purposes of this Agreement, the “Determination Date” shall be the date that is 10 Business Days prior to the anticipated date for Closing, as agreed upon by Parent and the Company at least five Business Days prior to the Parent Stockholders’ Meeting (the “Anticipated Closing Date”). Within five Business Days following the Determination Date, Parent shall deliver to the Company a schedule (the “Parent Cash Schedule”) setting forth, in reasonable detail, Parent’s good faith, estimated calculation of the Parent Cash Amount (using an estimate of the Parent Transaction Expenses, Parent’s accrued investment interest receivable, prepaid refundable deposits, accounts payable and accrued expenses, in each case as of the Anticipated Closing Date and determined in a manner substantially consistent with the manner in which such items were determined for Parent’s most recent SEC filings) (the “Parent Cash Calculation”) as of the Anticipated Closing Date prepared and certified by Parent’s principal accounting officer). Parent shall make the work papers andback-up materials used or useful in preparing the Parent Cash Schedule, as reasonably requested by the Company, available to the Company and, if requested by the Company, its accountants and counsel at reasonable times and upon reasonable notice. The Company shall deliver invoices evidencing the Combined Transaction Expenses to Parent no later than seven Business Days prior to Closing.
(b) Within three calendar days following delivery (the “Response Date”) of the Parent Cash Schedule to the Company, the Company will have the right to dispute any part of such Parent Cash Schedule by delivering a written notice to that effect (a “Dispute Notice”) to Parent. Any Dispute Notice shall identify in reasonable detail the nature of any proposed revisions to the Parent Cash Calculation.
(c) If on or prior to the Response Date, (i) the Company notifies Parent in writing that it has no objections to the Parent Cash Calculation or (ii) the Company fails to deliver a Dispute Notice as provided in Section 1.12(b), then the Parent Cash Calculation as set forth in the Parent Cash Schedule shall be deemed to have been finally determined for purposes of this Agreement and to represent the Parent Cash Amount at the Anticipated Closing Date for purposes of this Agreement.
(d) If the Company delivers a Dispute Notice on or prior to the Response Date, then Representatives of Parent and the Company shall promptly meet and attempt in good faith to resolve the disputed item(s) and negotiate an agreed-upon determination of the Parent Cash Amount, which agreed upon the Parent Cash Amount shall be deemed to have been finally determined for purposes of this Agreement and to represent the Parent Cash Amount at the Anticipated Closing Date for purposes of this Agreement.
(e) If Representatives of Parent and the Company are unable to negotiate an agreed-upon determination of the Parent Cash Amount at the Anticipated Closing Date pursuant to Section 1.12(d) within three calendar days after delivery of the Dispute Notice (or such other period as Parent and the Company may mutually agree upon), then Parent and the Company shall jointly select an independent auditor of recognized national standing (the “Accounting Firm”) to resolve any remaining disagreements as to the Parent Cash Calculation. Parent shall promptly deliver to the Accounting Firm the work papers andback-up materials used in preparing the Parent Cash Schedule, and Parent and the Company shall use commercially reasonable efforts to cause the Accounting Firm to make its determination within 10 calendar days of accepting its selection. The Company and Parent shall be afforded the opportunity to present to the Accounting Firm any material related to the unresolved disputes and to discuss the issues with the Accounting Firm; provided, however, that no such presentation or discussion shall occur without the presence of a Representative of each of the Company and Parent. The determination of the Accounting Firm shall be limited to the disagreements submitted to the Accounting Firm. The determination of the amount of the Parent Cash Amount made by the Accounting Firm shall be deemed to have been finally determined for purposes of this Agreement and to represent the Parent Cash Amount at the Anticipated Closing Date for purposes of this Agreement, and the Parties shall delay the Closing until the resolution of the matters described in this Section 1.12(e). The fees and expenses of the Accounting Firm shall be allocated between Parent and the Company in the same proportion that the disputed amount of the Parent Cash Amount that was unsuccessfully disputed by such Party (as finally determined by the Accounting
A-7
Firm) bears to the total disputed amount of the Parent Cash Amount (and for the avoidance of doubt the fees and expenses to be paid by Parent shall reduce the Parent Cash Amount). If this Section 1.12(e) applies as to the determination of the Parent Cash Amount at the Anticipated Closing Date described in Section 1.12(a), upon resolution of the matter in accordance with this Section 1.12(e), the Parties shall not be required to determine the Parent Cash Amount again even though the Closing Date may occur later than the Anticipated Closing Date, except that either Party may request a redetermination of the Parent Cash Amount if the Closing Date is more than five Business Days after the Anticipated Closing Date.
1.13 Calculation of Company Cash Amount.
(a) Within five Business Days following the Determination Date, the Company shall deliver to Parent a schedule (the “CompanyCash Schedule”) setting forth, in reasonable detail, the Company’s good faith, estimated calculation of the Company Cash Amount in accordance with GAAP (the “Company Cash Calculation”) as of the Anticipated Closing Date prepared and certified by the Company’s Chief Financial Officer. The Company shall make the work papers andback-up materials used or useful in preparing the Company Cash Schedule, as reasonably requested by Parent, available to Parent and, if requested by Parent, its accountants and counsel at reasonable times and upon reasonable notice.
(b) By the Response Date, Parent will have the right to dispute any part of such Company Cash Schedule by delivering a Dispute Notice. Any Dispute Notice shall identify in reasonable detail the nature of any proposed revisions to the Company Cash Calculation.
(c) If on or prior to the Response Date, (i) Parent notifies the Company in writing that it has no objections to the Company Cash Calculation or (ii) Parent fails to deliver a Dispute Notice as provided in Section 1.13(b), then the Parent Cash Calculation as set forth in the Company Cash Schedule shall be deemed to have been finally determined for purposes of this Agreement and to represent the Company Cash Amount at the Anticipated Closing Date for purposes of this Agreement.
(d) If Parent delivers a Dispute Notice on or prior to the Response Date, then Representatives of the Company and Parent shall promptly meet and attempt in good faith to resolve the disputed item(s) and negotiate an agreed-upon determination of the Company Cash Amount, which agreed upon the Company Cash Amount shall be deemed to have been finally determined for purposes of this Agreement and to represent the Company Cash Amount at the Anticipated Closing Date for purposes of this Agreement.
(e) If Representatives of the Company and Parent are unable to negotiate an agreed-upon determination of Company Cash Amount at the Anticipated Closing Date pursuant to Section 1.13(d) within three calendar days after delivery of the Dispute Notice (or such other period as the Company and Parent may mutually agree upon), then the Company and Parent shall jointly select the Accounting Firm to resolve any remaining disagreements as to the Company Cash Calculation. The Company shall promptly deliver to the Accounting Firm the work papers andback-up materials used in preparing the Company Cash Schedule, and the Company and Parent shall use commercially reasonable efforts to cause the Accounting Firm to make its determination within 10 calendar days of accepting its selection. Parent and the Company shall be afforded the opportunity to present to the Accounting Firm any material related to the unresolved disputes and to discuss the issues with the Accounting Firm; provided, however, that no such presentation or discussion shall occur without the presence of a Representative of each of Parent and the Company. The determination of the Accounting Firm shall be limited to the disagreements submitted to the Accounting Firm. The determination of the amount of the Company Cash Amount made by the Accounting Firm shall be deemed to have been finally determined for purposes of this Agreement and to represent the Company Cash Amount at the Anticipated Closing Date for purposes of this Agreement, and the Parties shall delay the Closing until the resolution of the matters described in this Section 1.13(e). The fees and expenses of the Accounting Firm shall be allocated between the Company and Parent in the same proportion that the disputed amount of the Company Cash Amount that was unsuccessfully disputed by such Party (as finally determined by the Accounting Firm) bears to the total disputed amount of the
A-8
Company Cash Amount (and for the avoidance of doubt the fees and expenses to be paid by the Company shall reduce the Company Cash Amount). If this Section 1.13(e) applies as to the determination of the Company Cash Amount at the Anticipated Closing Date described in Section 1.13(a), upon resolution of the matter in accordance with this Section 1.12(e), the Parties shall not be required to determine the Company Cash Amount again even though the Closing Date may occur later than the Anticipated Closing Date, except that either Party may request a redetermination of the Company Cash Amount if the Closing Date is more than five Business Days after the Anticipated Closing Date.
Section 2. REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Subject toSection 10.13(h), except as set forth in the disclosure schedule delivered by the Company to Parent (the “Company Disclosure Schedule”), the Company represents and warrants to Parent and Merger Sub as follows:
2.1 Due Organization; Subsidiaries.
(a) The Company is a corporation or other legal entity duly incorporated, validly existing and in good standing under the Laws of Delaware and has all necessary corporate power and authority: (i) to conduct its business in the manner in which its business is currently being conducted; (ii) to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used; and (iii) to perform its obligations under all Contracts by which it is bound.
(b) The Company is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under the Laws of all jurisdictions where the nature of its business requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would not be reasonably expected to have a Company Material Adverse Effect.
(c) The Company has no Subsidiaries, except for the Entities identified inSection 2.1(c) of the Company Disclosure Schedule; and neither the Company nor any of the Entities identified inSection 2.1(c) of the Company Disclosure Schedule owns any capital stock of, or any equity, ownership or profit sharing interest of any nature in, or controls directly or indirectly, any other Entity other than the Entities identified inSection 2.1(c) of the Company Disclosure Schedule. Each of the Company’s Subsidiaries is a corporation or other legal entity duly organized, validly existing and, if applicable, in good standing under the Laws of the jurisdiction of its organization and has all necessary corporate or other power and authority to conduct its business in the manner in which its business is currently being conducted and to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used, except where the failure to have such power or authority would not be reasonably expected to have a Company Material Adverse Effect.
(d) Neither the Company nor any of its Subsidiaries is or has otherwise been, directly or indirectly, a party to, member of or participant in any partnership, joint venture or similar business entity. Neither the Company nor any of its Subsidiaries has agreed or is obligated to make, or is bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Neither the Company nor any of its Subsidiaries has, at any time, been a general partner of, or has otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
2.2 Organizational Documents. The Company has made available to Parent accurate and complete copies of the Organizational Documents of the Company and each of its Subsidiaries in effect as of the date of this Agreement. Neither the Company nor any of its Subsidiaries is in material breach or violation of its respective Organizational Documents.
2.3 Authority; Binding Nature of Agreement.
(a) The Company has all necessary corporate power and authority to enter into and to perform its obligations under this Agreement and, subject to receipt of the Required Company Stockholder Vote, to
A-9
perform its obligations hereunder and to consummate the Contemplated Transactions. The Company Board (at meetings duly called and held) has (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved and declared advisable this Agreement and the Contemplated Transactions and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of the Company vote in favor of the Company Stockholder Matters.
(b) This Agreement has been duly executed and delivered by the Company and assuming the due authorization, execution and delivery by Parent and Merger Sub, constitutes the legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to the Enforceability Exceptions. Prior to the execution of the Company Stockholder Support Agreements, the Company Board approved the Company Stockholder Support Agreements and the transactions contemplated thereby.
2.4 Vote Required.The affirmative vote (or written consent) of (a) the holders of a majority of the shares of Company Common Stock and Company Preferred Stock, voting as a single class, (b) the holders of at least 60% of the shares of Company Preferred Stock, voting together as a single class, (c) the holders of at least a majority of the outstanding shares of Series A Preferred Stock, voting as a single class, (d) the holders of at least a majority of the outstanding shares of Series B Preferred Stock and SeriesB-2 Preferred Stock, voting together as a single class, and (e) the holders of at least 70% of the shares of Series C Preferred Stock, voting as a single class, in each case, outstanding on the record date for the written consent in lieu of a meeting pursuant to Section 228 of the DGCL approving the Company Stockholder Matters, in a form reasonably acceptable to Parent (collectively, theCompany Stockholder Written Consent”) and entitled to vote thereon (collectively, the “Required Company Stockholder Vote”), is the only vote (or written consent) of the holders of any class or series of Company Capital Stock necessary to adopt and approve the Company Stockholder Matters.
2.5 Non-Contravention; Consents.Subject to obtaining the Required Company Stockholder Vote, the filing of the Certificate of Merger required by the DGCL, and the expiration or termination of any waiting period under the HSR Act, and any applicable foreign competition Laws, neither (x) the execution, delivery or performance of this Agreement by the Company, nor (y) the consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(a) contravene, conflict with or result in a violation of any of the provisions of the Company’s Organizational Documents;
(b) contravene, conflict with or result in a material violation of, or to the Knowledge of the Company give any Governmental Body or other Person the right to challenge the Contemplated Transactions or to exercise any material remedy or obtain any material relief under, any Law or any order, writ, injunction, judgment or decree to which the Company or its Subsidiaries, or any of the assets owned or used by the Company or its Subsidiaries, is subject, except as would not reasonably be expected to be material to the Company or its business;
(c) contravene, conflict with or result in a violation of any of the terms or requirements of, or give any Governmental Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by the Company or its Subsidiaries, except as would not reasonably be expected to be material to the Company or its business;
(d) contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Company Material Contract, or give any Person the right to: (i) declare a default or exercise any remedy under any Company Material Contract; (ii) any material payment, rebate, chargeback, penalty or change in delivery schedule under any Company Material Contract; (iii) accelerate the maturity or performance of any Company Material Contract; or (iv) cancel, terminate or modify any term of any Company Material Contract, except in the case of anynon-material breach, default, penalty or modification; or
A-10
(e) result in the imposition or creation of any Encumbrance upon or with respect to any material asset owned or used by the Company or its Subsidiaries (except for Permitted Encumbrances).
Except for (i) any Consent set forth onSection 2.5 of the Company Disclosure Schedule under any Company Contract, (ii) the Required Company Stockholder Vote, (iii) the filing of the Certificate of Merger with the Secretary of State of the State of Delaware pursuant to the DGCL, and (iv) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable federal and state securities Laws, neither the Company nor any of its Subsidiaries is or will be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (A) the execution, delivery or performance of this Agreement, the Company Stockholder Support Agreements, and the CompanyLock-up Agreements or (B) the consummation of the Contemplated Transactions, which if individually or in the aggregate were not given or obtained, would reasonably be expected to prevent or materially delay the ability of Parent and Merger Sub to consummate the Contemplated Transactions. The Company Board has taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the execution, delivery and performance of this Agreement, the Company Stockholder Support Agreements, the CompanyLock-Up Agreements and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law applies or purports to apply to the Merger, this Agreement, the Company Stockholder Support Agreements, the CompanyLock-Up Agreements or any of the Contemplated Transactions.
(a) The authorized Company Capital Stock as of the date of this Agreement consists of (i) 200,000,000 shares of Company Common Stock, par value $0.0001 per share, of which 51,282,780 shares have been issued and are outstanding as of the date of this Agreement and 882,388 shares of are held by the Company as treasury shares as of the date of this Agreement, and (ii) 130,099,288 shares of preferred stock, par value $0.0001 per share (the “Company Preferred Stock”), of which 111,034,576 have been issued and are outstanding as of the date of this Agreement, consisting of 13,560,000 shares of Series A Preferred Stock, 6,750,721 shares of Series B Preferred Stock, 56,723,855 shares of SeriesB-2 Preferred Stock and 34,000,000 shares of Series C Preferred Stock. Company Warrants to purchase 5,064,712 shares of SeriesB-2 Preferred Stock are issued and outstanding as of the date of this Agreement.Section 2.6(a) of the Company Disclosure Schedule lists, as of the date of this Agreement (A) each record holder of issued and outstanding Company Capital Stock and the number and type of shares of Company Capital Stock held by such holder; and (B)(1) each holder of issued and outstanding Company Warrants, (2) the number and type of shares subject to each Company Warrant, (3) the exercise price of each Company Warrant and (4) the termination date of each Company Warrant.
(b) All of the outstanding shares of Company Common Stock and Company Preferred Stock have been duly authorized and validly issued, and are fully paid and nonassessable. Except as set forth in the Company Bylaws or Investor Agreements, none of the outstanding shares of Company Capital Stock is entitled or subject to any preemptive right, right of participation, right of maintenance or any similar right and none of the outstanding shares of Company Capital Stock is subject to any right of first refusal in favor of the Company. Except as contemplated herein and in the Company Bylaws and Investor Agreements, there is no Company Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to), any shares of Company Capital Stock. The Company is not under any obligation, nor is it bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Company Capital Stock or other securities.Section 2.6(b) of the Company Disclosure Schedule accurately and completely lists all repurchase or forfeiture rights held by the Company with respect to shares of Company Capital Stock (including shares issued pursuant to the exercise of stock options). Each share of Company Preferred Stock is convertible into one share of Company Common Stock.
(c) Except for as described inSection 2.6(c) of the Company Disclosure Schedule, the Company does not have any stock option plan or any other plan, program, agreement or arrangement providing for any
A-11
equity-based compensation for any Person. As of the date of this Agreement, the Company has reserved 8,600,000 shares of Company Common Stock for issuance under the Company Plans, of which 6,843,251 shares have been issued and are currently outstanding, 7,509,919 shares have been reserved for issuance upon exercise of Company Options previously granted under the Company Plans, and 1,090,081 shares of Company Common Stock remain available for future issuance of awards pursuant to the Company Plans.Section 2.6(c) of the Company Disclosure Schedule sets forth the following information with respect to each Company Option outstanding as of the date of this Agreement: (i) the name of the optionee; (ii) the number of shares of Company Common Stock subject to such Company Option at the time of grant; (iii) the number of shares of Company Common Stock subject to such Company Option as of the date of this Agreement; (iv) the exercise price of such Company Option; (v) the date on which such Company Option was granted; (vi) the applicable vesting schedule, including the number of vested and unvested shares as of the date of this Agreement and any acceleration provisions; (vii) the date on which such Company Option expires; and (viii) whether such Company Option is intended to constitute an “incentive stock option” (as defined in the Code) or anon-qualified stock option. The Company has made available to Parent an accurate and complete copy of the Company Plans and the form of stock option agreement used to evidence outstanding options granted thereunder.
(d) Except for Company Warrants, and the Company Options set forth onSection 2.6(c) of the Company Disclosure Schedule, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of the capital stock or other securities of the Company or any of its Subsidiaries; (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock or other securities of the Company or any of its Subsidiaries; or (iii) condition or circumstance that is reasonably likely to give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or other securities of the Company or any of its Subsidiaries. There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with respect to the Company or any of its Subsidiaries.
(e) All outstanding shares of Company Common Stock, Company Preferred Stock, Company Options, Company Warrants, and other securities of the Company have been issued and granted in material compliance with (i) all applicable securities Laws and other applicable Law, and (ii) all requirements set forth in applicable Contracts.
(a) Concurrently with the execution hereof, the Company has provided to Parent true and complete copies of (i) the Company’s audited consolidated balance sheets at December 31, 2017 and 2016 together with related audited consolidated statements of income, stockholders’ equity and cash flows, and notes thereto, of the Company for the fiscal years then ended and (ii) the Company Unaudited Interim Balance Sheet, together with the unaudited consolidated statements of income, stockholders’ equity and cash flows of the Company for the period reflected in the Company Unaudited Interim Balance Sheet (collectively, the “Company Financials”). The Company Financials were prepared in accordance with GAAP (except as may be indicated in the notes to such financial statements and except that the unaudited financial statements may not contain footnotes and are subject to normal and recurringyear-end adjustments, none of which are material) and fairly present, in all material respects, the financial position and operating results of the Company and its consolidated Subsidiaries as of the dates and for the periods indicated therein.
(b) Each of the Company and its Subsidiaries maintains accurate books and records reflecting their assets and liabilities and maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of the financial statements of the Company and its Subsidiaries and to maintain accountability of the Company’s and its Subsidiaries’ assets; (iii) access to the Company’s and its Subsidiaries’ assets is permitted only in accordance with management’s general or specific
A-12
authorization; (iv) the recorded accountability for the Company’s and its Subsidiaries’ assets is compared with the existing assets at regular intervals and appropriate action is taken with respect to any differences; and (v) accounts, notes and other receivables and inventory are recorded accurately, and proper and adequate procedures are implemented to effect the collection thereof on a current and timely basis. The Company and each of its Subsidiaries maintains internal control over financial reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes.
(c) Section 2.7(c) of the Company Disclosure Schedule lists, and the Company has delivered to Parent accurate and complete copies of the documentation creating or governing, all securitization transactions and“off-balance sheet arrangements” (as defined in Item 303(c) of RegulationS-K under the Exchange Act) effected by the Company or any of its Subsidiaries since January 1, 2016.
(d) Since January 1, 2016, there have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer or general counsel of the Company, the Company Board or any committee thereof. Since January 1, 2016, neither the Company nor its independent auditors have identified (i) any significant deficiency or material weakness in the design or operation of the system of internal accounting controls utilized by the Company and its Subsidiaries, (ii) any fraud, whether or not material, that involves the Company, any of its Subsidiaries, the Company’s management or other employees who have a role in the preparation of financial statements or the internal accounting controls utilized by the Company and its Subsidiaries or (iii) any claim or allegation regarding any of the foregoing.
2.8 Absence of Changes. Except as set forth onSection 2.8 of the Company Disclosure Schedule, between the date of the Company Unaudited Interim Balance Sheet and the date of this Agreement, the Company has conducted its business only in the Ordinary Course of Business (except for the execution and performance of this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (a) Company Material Adverse Effect or (b) action, event or occurrence that would have required consent of Parent pursuant toSection 4.2(b) had such action, event or occurrence taken place after the execution and delivery of this Agreement.
2.9 Absence of Undisclosed Liabilities. As of the date hereof, neither the Company nor any of its Subsidiaries has any liability, indebtedness, obligation or expense of any kind, whether accrued, absolute, contingent, matured or unmatured (each a “Liability”), individually or in the aggregate, of a type required to be recorded or reflected on a balance sheet or disclosed in the footnotes thereto under GAAP, except for: (a) Liabilities disclosed, reflected or reserved against in the Company Unaudited Interim Balance Sheet; (b) Liabilities that have been incurred by the Company or its Subsidiaries since the date of the Company Unaudited Interim Balance Sheet in the Ordinary Course of Business; (c) Liabilities for performance of obligations of the Company or any of its Subsidiaries under Company Contracts; (d) Liabilities incurred in connection with the Contemplated Transactions; (e) Liabilities which would not, individually or in the aggregate, reasonably be expected to be material to the Company; and (f) Liabilities described inSection 2.9 of the Company Disclosure Schedule.
2.10 Title to Assets. Each of the Company and its Subsidiaries owns, and has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned by it that are material to the Company or its business, including: (a) all tangible assets reflected on the Company Unaudited Interim Balance Sheet; and (b) all other tangible assets reflected in the books and records of the Company or any of its Subsidiaries as being owned by the Company or such Subsidiary. All of such assets are owned or, in the case of leased assets, leased by the Company or any of its Subsidiaries free and clear of any Encumbrances, other than Permitted Encumbrances.
2.11 Real Property; Leasehold. Neither the Company nor any of its Subsidiaries owns or has ever owned any real property. The Company has made available to Parent (a) an accurate and complete list of all real
A-13
properties with respect to which the Company directly or indirectly holds a valid leasehold interest as well as any other real estate that is in the possession of or leased by the Company or any of its Subsidiaries, and (b) copies of all leases under which any such real property is possessed (the “Company Real Estate Leases”), each of which is in full force and effect, with no existing material default thereunder. The Company’s use and operation of each such leased property conforms to all applicable Laws in all material respects, and the Company has exclusive possession of each such leased property and has not granted any occupancy rights to tenants or licensees with respect to such leased property. In addition, each such leased property is free and clear of all Encumbrances other than Permitted Encumbrances.
(a) Section 2.12(a)of the Company Disclosure Schedule identifies each item of material Company IP, including, with respect to each patent and patent application: (i) the name of the applicant/registrant, (ii) the jurisdiction of application/registration, (iii) the application or registration number and (iv) any otherco-owners. To the Knowledge of the Company, each of the patents and patent applications included inSection 2.12(a) of the Company Disclosure Schedule properly identifies by name each and every inventor of the inventions claimed therein as determined in accordance with applicable Laws of the United States. To the knowledge of the Company, as of the date of this Agreement, no cancellation, interference, opposition, reissue, reexamination or other proceeding of any nature (other than office actions or similar communications issued by any Governmental Body in the ordinary course of prosecution of any pending applications for registration) is pending or threatened in writing, in which the scope, validity, enforceability or ownership of any Company IP is being or has been contested or challenged.
(b) Except as has not had and would not reasonably be expected to have, individually or in the aggregate, a Company Material Adverse Effect, the Company or its Subsidiaries owns, is the assignee of, or has licensed all material Company IP (other than as disclosed onSection 2.12(b) of the Company Disclosure Schedule), free and clear of all Encumbrances other than Permitted Encumbrances. To the Knowledge of the Company, each Company Associate involved in the creation or development of any material Company IP, pursuant to such Company Associate’s activities on behalf of the Company or its Subsidiaries, has signed a written agreement containing an assignment of such Company Associate’s rights in such Company IP to the Company or its Subsidiaries and confidentiality provisions protecting the Company IP.
(c) Except as set forth in Section 2.12(d) of the Company Disclosure Schedule, to the Knowledge of the Company, no funding, facilities or personnel of any Governmental Body or any university, college, research institute or other educational institution has been used to create Company IP, except for any such funding or use of facilities or personnel that does not result in such Governmental Body or institution obtaining ownership rights to such Company IP or the right to receive royalties for the practice of such Company IP.
(d) Section 2.12(d) of the Company Disclosure Schedule sets forth each license agreement pursuant to which the Company (i) is granted a license under any material Intellectual Property Right owned by any third party that is used by the Company or its Subsidiaries in its business as currently conducted (each a “CompanyIn-bound License”) or (ii) grants to any third party a license under any material Company IP or material Intellectual Property Right licensed to the Company or its Subsidiaries under a CompanyIn-bound License (each a “CompanyOut-bound License”) (provided, that, CompanyIn-bound Licenses shall not include, when entered into in the ordinary course of business, material transfer agreements, clinical trial agreements, agreements with Company Associates, services agreements,non-disclosure agreements, commercially availableSoftware-as-a-Service offerings,off-the-shelf software licenses or generally available patent license agreements; and CompanyOut-bound Licenses shall not include, when entered into in the ordinary course of business, material transfer agreements, clinical trial agreements, services agreements,non-disclosure agreements, ornon-exclusive outbound licenses).
A-14
(e) To the Knowledge of the Company: (i) the operation of the businesses of the Company and its Subsidiaries as currently conducted does not infringe, misappropriate or otherwise violate any valid and enforceable United States patent that is not included on Section 2.12(a) of the Company Disclosure Schedule and (ii) no other Person is infringing, misappropriating or otherwise violating any Company IP. No Legal Proceeding is pending (or, to the Knowledge of the Company, is threatened in writing) (A) against the Company or its Subsidiaries alleging that the operation of the businesses of the Company or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Rights of another Person or (B) by the Company or its Subsidiaries alleging that another Person has infringed, misappropriated or otherwise violated any of the Company IP or any Intellectual Property Rights exclusively licensed to the Company or its Subsidiaries. Since January 1, 2017, neither the Company nor its Subsidiaries has received any written notice or other written communication alleging that the operation of the business of the Company or its Subsidiaries infringes or constitutes the misappropriation or other violation of any Intellectual Property Right of another Person.
(f) None of the Company IP or, to the Knowledge of the Company, any material Intellectual Property Rights exclusively licensed to the Company or its Subsidiaries is subject to any pending or outstanding injunction, directive, order, judgment or other disposition of dispute that adversely and materially restricts the use, transfer, registration or licensing by the Company or its Subsidiaries of any such Company IP or material Intellectual Property Rights exclusively licensed to the Company or its Subsidiaries.
(g) To the Knowledge of the Company, the Company, its Subsidiaries and the operation of the Company’s and its Subsidiaries’ business are in substantial compliance with all Laws pertaining to data privacy and data security of any personally identifiable information and sensitive business information (collectively, “Sensitive Data”) except to the extent that such noncompliance has not and would not reasonably be expected to have a Company Material Adverse Effect. To the Knowledge of the Company, since January 1, 2017, there have been (i) no material losses or thefts of data or security breaches relating to Sensitive Data used in the business of the Company or its Subsidiaries, (ii) no violations of any security policy of the Company regarding any such Sensitive Data used in the business of the Company or its Subsidiaries, and (iii) no unauthorized access, unauthorized use or unintended or improper disclosure of any Sensitive Data used in the business of the Company or its Subsidiaries, in each case of (i) through (iii), except as would not reasonably be expected to, individually or in the aggregate, have a Company Material Adverse Effect.
2.13 Agreements, Contracts and Commitments.
(a) Section 2.13(a) of the Company Disclosure Schedule lists the following Company Contracts in effect as of the date of this Agreement other than any Benefit Plans (each, a “Company Material Contract” and collectively, the “Company Material Contracts”):
(i) each Company Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(ii) each Company Contract containing (A) any covenant limiting the freedom of the Company, its Subsidiaries or the Surviving Corporation to engage in any line of business or compete with any Person, (B) any most-favored pricing arrangement, (C) any exclusivity provision, or (D) anynon-solicitation provision with respect to employees of other Persons, in each case, except for restrictions that would not materially affect the ability of the Company to conduct its business;
(iii) each Company Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $250,000 pursuant to its express terms and not cancelable without penalty;
(iv) each Company Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity, in each case, involving payments in excess of $250,000,
A-15
other than Company Contracts in which the applicable acquisition or disposition has been consummated and there are no material ongoing obligations;
(v) each Company Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or instruments relating to the borrowing of money or extension of credit or creating any material Encumbrances with respect to any assets of the Company or any of its Subsidiaries or any loans or debt obligations with officers or directors of the Company, in each case, having an outstanding principal in an amount in excess of $250,000.;
(vi) each Company Contract requiring payment by or to the Company after the date of this Agreement in excess of $250,000 pursuant to its express terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions); (B) any agreement involving provision of services or products with respect to anypre-clinical or clinical development activities of the Company; (C) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, development or other agreement currently in force under which the Company has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which the Company has continuing obligations to develop any Intellectual Property Rights that will not be owned, in whole or in part, by the Company; or (D) any Contract to license any third party to manufacture or produce any product, service or technology of the Company or any Contract to sell, distribute or commercialize any products or service of the Company, in each case, except for Company Contracts entered into in the Ordinary Course of Business;
(vii) each Company Contract with any financial advisor, broker, finder, investment banker or other similar Person, providing advisory services to the Company in connection with the Contemplated Transactions;
(viii) each Company Real Estate Lease;
(ix) each Company Contract with any Governmental Body (other than clinical trial agreements for clinical trial studies);
(x) each CompanyOut-bound License and CompanyIn-bound License;
(xi) each Company Contract containing any royalty, dividend or similar arrangement based on the revenues or profits of the Company or any of its Subsidiaries;
(xii) each Company Contract, offer letter, employment agreement, or independent contractor agreement with any employee, consultant or independent contractor that (A) is not terminable by the Company without less than 60 days notice, severance, or other cost or liability, or (B) provides for retention payments, change of control payments, severance, accelerated vesting, or any payment or benefit that may or will become due as a result of the Merger (whether alone or in connection with any other event); or
(xiii) any other Company Contract that is not terminable at will (with no penalty or payment) by the Company or its Subsidiaries, as applicable, and (A) which involves payment or receipt by the Company or its Subsidiaries after the date of this Agreement under any such agreement, contract or commitment of more than $250,000 in the aggregate, or obligations after the date of this Agreement in excess of $250,000 in the aggregate, or (B) that is material to the business or operations of the Company and its Subsidiaries, taken as a whole.
(b) The Company has delivered or made available to Parent accurate and complete copies of all Company Material Contracts, including all amendments thereto. Except as set forth inSection 2.13(b) of the Company Disclosure Schedule, there are no Company Material Contracts that are not in written form. Neither the Company nor any of its Subsidiaries has, nor to the Company’s Knowledge, as of the date of this Agreement has any other party to a Company Material Contract, breached, violated or defaulted under, or received notice that it
A-16
breached, violated or defaulted under, any of the terms or conditions of any Company Material Contract in such manner as would permit any other party to cancel or terminate any such Company Material Contract, or would permit any other party to seek damages which would reasonably be expected to be material to the Company or its business. As to the Company and its Subsidiaries, as of the date of this Agreement, each Company Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person is renegotiating, or has a right pursuant to the terms of any Company Material Contract to change, any material amount paid or payable to the Company under any Company Material Contract or any other material term or provision of any Company Material Contract.
2.14 Compliance; Permits; Restrictions.
(a) The Company and each of its Subsidiaries are, and since January 1, 2016 have been, in compliance in all material respects with all applicable Laws, including the Federal Food, Drug, and Cosmetic Act (“FDCA”), the Food and Drug Administration (“FDA”) regulations adopted thereunder, the Public Health Service Act and any other similar Law administered or promulgated by the FDA or other comparable Governmental Body responsible for regulation of the development, clinical testing, manufacturing, sale, marketing, distribution and importation or exportation of drug and biopharmaceutical products (each, a “Drug Regulatory Agency”), except for any noncompliance, either individually or in the aggregate, which would not be material to the Company. No investigation, claim, suit, proceeding, audit or other action by any Governmental Body is pending or, to the Knowledge of the Company, threatened against the Company or any of its Subsidiaries. There is no agreement, judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries which (i) has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of the Company or any of its Subsidiaries, any acquisition of material property by the Company or any of its Subsidiaries or the conduct of business by the Company or any of its Subsidiaries as currently conducted, (ii) is reasonably likely to have an adverse effect on the Company’s ability to comply with or perform any covenant or obligation under this Agreement, or (iii) is reasonably likely to have the effect of preventing, delaying, making illegal or otherwise interfering with the Contemplated Transactions.
(b) The Company and its Subsidiaries hold all required Governmental Authorizations which are material to the operation of the business of the Company and its Subsidiaries as currently conducted (the “Company Permits”).Section 2.14(b) of the Company Disclosure Schedule identifies each Company Permit. Each of the Company and its Subsidiaries is in material compliance with the terms of the Company Permits. No Legal Proceeding is pending or, to the Knowledge of the Company, threatened, which seeks to revoke, limit, suspend, or materially modify any Company Permit. The rights and benefits of each Company Permit will be available to the Surviving Corporation or its Subsidiaries, as applicable, immediately after the Effective Time on terms substantially identical to those enjoyed by the Company and its Subsidiaries as of the date of this Agreement and immediately prior to the Effective Time.
(c) There are no proceedings pending or, to the Knowledge of the Company, threatened against the Company with respect to an alleged material violation by the Company or any of its Subsidiaries of the FDCA, FDA regulations adopted thereunder, the Public Health Service Act or any other similar Law administered or promulgated by any Drug Regulatory Agency.
(d) �� All clinical,pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, the Company or its Subsidiaries, or in which the Company or its Subsidiaries or their respective current products or product candidates have participated, were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance in all material respects with the applicable regulations of any applicable Drug Regulatory Agency and other applicable Law, as applicable, including 21 C.F.R. Parts 50, 54, 56, 58 and 312. No preclinical or clinical trial conducted by or on behalf of the Company or any of its Subsidiaries has been terminated or suspended prior to completion for safety ornon-compliance reasons. Since January 1, 2016, neither the Company nor any of its Subsidiaries has received any notices, correspondence, or other communications from any Drug Regulatory Agency requiring, or
A-17
to the Knowledge of the Company threatening to initiate, the termination or suspension of any clinical studies conducted by or on behalf of, or sponsored by, the Company or any of its Subsidiaries or in which the Company or any of its Subsidiaries or their respective current products or product candidates have participated.
(e) Neither the Company nor any of its Subsidiaries is the subject of any pending or, to the Knowledge of the Company, threatened investigation in respect of its business or products by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Knowledge of the Company, neither the Company nor any of its Subsidiaries has committed any acts, made any statement, or failed to make any statement, in each case in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy, and any amendments thereto. None of the Company, any of its Subsidiaries or any of their respective officers, employees or, to the Knowledge of the Company, agents has been convicted of any crime or engaged in any conduct that could result in a debarment or exclusion (i) under 21 U.S.C. Section 335a or (ii) any similar applicable Law. No debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are pending or, to the Knowledge of the Company, threatened against the Company, any of its Subsidiaries or any of their respective officers, employees or, to the Knowledge of the Company, agents.
(f) The Company and its Subsidiaries have complied with all Laws relating to patient, medical or individual health information, including the Health Insurance Portability and Accountability Act of 1996 and its implementing regulations promulgated thereunder, all as amended from time to time (collectively “HIPAA”), including the standards for the privacy of Individually Identifiable Health Information at 45 C.F.R. Parts 160 and 164, Subparts A and E, the standards for the protection of Electronic Protected Health Information set forth at 45 C.F.R. Part 160 and 45 C.F.R. Part 164, Subpart A and Subpart C, the standards for transactions and code sets used in electronic transactions at 45 C.F.R. Part 160, Subpart A and Part 162, and the standards for Breach Notification for Unsecured Protected Health Information at 45 C.F.R. Part 164, Subpart D, all as amended from time to time. The Company and its Subsidiaries have entered into, where required, and are in compliance in all material respects with the terms of all Business Associate (as defined in HIPAA) agreements (“Business Associate Agreements”) to which the Company or a Subsidiary is a party or otherwise bound. The Company and its Subsidiaries have created and maintained written policies and procedures to protect the privacy of all protected health information, provide training to all employees and agents as required under HIPAA, and have implemented security procedures, including physical, technical and administrative safeguards, to protect all personal information and Protected Health Information stored or transmitted in electronic form. Neither the Company nor its Subsidiaries have received written notice from the Office for Civil Rights for the U.S. Department of Health and Human Services or any other Governmental Body of any allegation regarding its failure to comply with HIPAA or any other state law or regulation applicable to the protection of individually identifiable health information or personally identifiable information. No successful Security Incident, Breach of Unsecured Protected Health Information or breach of personally identifiable information under applicable state or federal laws have occurred with respect to information maintained or transmitted to the Company, any of its Subsidiaries, or an agent or third party subject to a Business Associate Agreement with the Company or a Subsidiary of the Company. The Company is currently submitting, receiving and handling or is capable of submitting receiving and handling transactions in accordance with the Standard Transaction Rule. All capitalized terms in thisSection 2.14(f) not otherwise defined in this Agreement shall have the meanings set forth under HIPAA.
2.15 Legal Proceedings; Orders.
(a) As of the date of this Agreement, there is no material pending Legal Proceeding and, to the Knowledge of the Company, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves (A) the Company, (B) any of its Subsidiaries, (C) any Company Associate (in his or her capacity as such) or (D) any of the material assets owned or used by the Company or its Subsidiaries; or (ii) that challenges, or that would have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
A-18
(b) Except as set forth inSection 2.15(b) of the Company Disclosure Schedule, since January 1, 2016 through the date of this Agreement, no Legal Proceeding has been pending against the Company that resulted in material liability to the Company.
(c) There is no order, writ, injunction, judgment or decree to which the Company or any of its Subsidiaries, or any of the material assets owned or used by the Company or any of its Subsidiaries, is subject. To the Knowledge of the Company, no officer of the Company or any of its Subsidiaries is subject to any order, writ, injunction, judgment or decree that prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of the Company or any of its Subsidiaries or to any material assets owned or used by the Company or any of its Subsidiaries.
(a) The Company and each of its Subsidiaries have timely filed all income Tax Returns and other material Tax Returns that they were required to file under applicable Law. All such Tax Returns are correct and complete in all material respects and have been prepared in compliance with all applicable Law. No claim has ever been made by any Governmental Body in any jurisdiction where the Company or any of its Subsidiaries does not file a particular Tax Return or pay a particular Tax that the Company or such Subsidiary is subject to taxation by that jurisdiction.
(b) All income and other material Taxes due and owing by the Company or any of its Subsidiaries on or before the date hereof (whether or not shown on any Tax Return) have been fully paid. The unpaid Taxes of the Company and its Subsidiaries did not, as of the date of the Company Unaudited Interim Balance Sheet, materially exceed the reserve for Tax liability (excluding any reserve for deferred Taxes established to reflect timing differences between book and Tax items) set forth on the face of the Company Unaudited Interim Balance Sheet. Since the date of the Company Unaudited Interim Balance Sheet, neither the Company nor any of its Subsidiaries has incurred any material Liability for Taxes outside the Ordinary Course of Business.
(c) All Taxes that the Company or any of its Subsidiaries are or were required by Law to withhold or collect have been duly and timely withheld or collected in all material respects on behalf of its respective employees, independent contractors, stockholders, lenders, customers or other third parties and, have been timely paid to the proper Governmental Body or other Person or properly set aside in accounts for this purpose.
(d) There are no Encumbrances for material Taxes (other than Permitted Encumbrances) upon any of the assets of the Company or any of its Subsidiaries.
(e) No deficiencies for income or other material Taxes with respect to the Company or any of its Subsidiaries have been claimed, proposed or assessed by any Governmental Body in writing. There are no pending or ongoing, and to the Knowledge of the Company, threatened audits, assessments or other actions for or relating to any liability in respect of a material amount of Taxes of the Company or any of its Subsidiaries. Neither the Company nor any of its Subsidiaries (or any of their predecessors) has waived any statute of limitations in respect of any income or other material Taxes or agreed to any extension of time with respect to any income or other material Tax assessment or deficiency.
(f) The Company has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(g) Neither the Company nor any of its Subsidiaries is a party to any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, or similar agreement or arrangement, other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes.
A-19
(h) Neither the Company nor any of its Subsidiaries will be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any Tax period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for Tax purposes filed on or prior to the Closing Date; (ii) use of an improper method of accounting for a Tax period ending on or prior to the Closing Date; (iii) “closing agreement” as described in Section 7121 of the Code (or any similar provision of state, local or foreign Law) executed on or prior to the Closing Date; (iv) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any similar provision of state, local or foreign Law) entered into on or prior to the Closing Date; (v) installment sale or open transaction disposition made on or prior to the Closing Date; (vi) prepaid amount received on or prior to the Closing Date; or (vii) election under Section 108(i) of the Code (or any similar provision of state, local or foreign Law) made on or prior to the Closing Date. The Company has not made any election under Section 965(h) of the Code.
(i) Neither the Company nor any of its Subsidiaries has ever been (i) a member of a consolidated, combined or unitary Tax group (other than such a group the common parent of which is the Company) or (ii) a party to any joint venture, partnership, or other arrangement that is treated as a partnership for U.S. federal income Tax purposes. Neither the Company nor any of its Subsidiaries has any Liability for any material Taxes of any Person (other than the Company and any of its Subsidiaries) under Treasury RegulationsSection 1.1502-6 (or any similar provision of state, local, or foreign Law), or as a transferee or successor.
(j) Neither the Company nor any of its Subsidiaries has, since January 1, 2017, distributed stock of another Person, or had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code (or any similar provisions of state, local or foreign Law).
(k) Neither the Company nor any of its Subsidiaries (i) is a “controlled foreign corporation” as defined in Section 957 of the Code; (ii) is a “passive foreign investment company” within the meaning of Section 1297 of the Code; (iii) has ever had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise had an office or fixed place of business in a country other than the country in which it is organized; (iv) is or was a “surrogate foreign corporation” within the meaning of Section 7874(a)(2)(B) or is treated as a U.S. corporation under Section 7874(b) of the Code; or (v) was created or organized in the U.S. such that such entity would be taxable in the U.S. as a domestic entity pursuant to the dual charter provision of Treasury RegulationsSection 301.7701-5(a).
(l) Neither the Company nor any of its Subsidiaries has participated in or been a party to a transaction that, as of the date of this Agreement, constitutes a “listed transaction” that is required to be reported to the IRS pursuant to Section 6011 of the Code and applicable Treasury Regulations thereunder.
(m) Neither the Company nor any of its Subsidiaries has taken or agreed to take any action or knows of any fact that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment.
For purposes of thisSection 2.16, each reference to the Company or any of its Subsidiaries shall be deemed to include any Person that was liquidated into, merged with, or is otherwise a predecessor to, the Company or such Subsidiary, respectively.
2.17 Employee and Labor Matters; Benefit Plans.
(a) Section 2.17(a) of the Company Disclosure Schedule is a list of all Company Benefit Plans, including, without limitation, each Company Benefit Plan that provides for retirement, change in control, stay or retention, deferred compensation, incentive compensation, severance or retiree medical or life insurance benefits. “Company Benefit Plan” means each (i) “employee benefit plan” as defined in Section 3(3) of ERISA and
A-20
(ii) other pension, retirement, deferred compensation, excess benefit, profit sharing, bonus, incentive, equity or equity-based (other than individual Company Options made pursuant to the Company’s standard forms, in which case only representative standard forms of such stock option agreements shall be scheduled), phantom equity, employment, offer letter, consulting, severance,change-of-control, retention, health, life, disability, group insurance, paid-time off, holiday, welfare and fringe benefit plan, program, agreement, contract, or arrangement (whether written or unwritten, qualified or nonqualified, funded or unfunded and including any that have been frozen), in any case, maintained, contributed to, or required to be contributed to, by the Company or any of its Subsidiaries or Company ERISA Affiliates for the benefit of any current or former employee, director, officer or independent contractor of the Company or any of its Subsidiaries or under which the Company or any of its Subsidiaries has any actual or contingent liability (including, without limitation, as to the result of it being treated as a single employer under Code Section 414 with any other person).
(b) As applicable with respect to each Company Benefit Plan, the Company has made available to Parent, true and complete copies of (i) each Company Benefit Plan, including all amendments thereto, and in the case of an unwritten Company Benefit Plan, a written description thereof, (ii) all current trust documents, investment management contracts, custodial agreements, administrative services agreements and insurance and annuity contracts relating thereto, (iii) the current summary plan description and each summary of material modifications thereto, (iv) the most recently filed annual reports with any Governmental Body (e.g.,Form 5500 and all schedules thereto), (v) the most recent IRS determination, opinion or advisory letter, (vi) the most recent summary annual reports, nondiscrimination testing reports, actuarial reports, financial statements and trustee reports, (vii) all records, notices and filings concerning IRS or Department of Labor or other Governmental Body audits or investigations, “prohibited transactions” within the meaning of Section 406 of ERISA or Section 4975 of the Code, (viii) all policies and procedures established to comply with the privacy and security rules of HIPAA and (ix) any written reports constituting a valuation of the Company’s capital stock for purposes of Sections 409A or 422 of the Code, whether prepared internally by the Company or by an outside, third-party valuation firm.
(c) Each Company Benefit Plan has been maintained, operated and administered in compliance in all material respects with its terms and any related documents or agreements and the applicable provisions of ERISA, the Code and all other Laws.
(d) The Company Benefit Plans which are “employee pension benefit plans” within the meaning of Section 3(2) of ERISA and which are intended to meet the qualification requirements of Section 401(a) of the Code have received determination or opinion letters from the IRS on which they may currently rely to the effect that such plans are qualified under Section 401(a) of the Code and the related trusts are exempt from federal income Taxes under Section 501(a) of the Code, respectively, and to the Knowledge of the Company, nothing has occurred that would reasonably be expected to materially adversely affect the qualification of such Company Benefit Plan or the tax exempt status of the related trust.
(e) Neither the Company, any of its Subsidiaries nor any Company ERISA Affiliate maintains, contributes to, is required to contribute to, or has any actual or contingent liability with respect to, (i) any “employee pension benefit plan” (within the meaning of Section 3(2) of ERISA) that is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) any “multiemployer plan” (within the meaning of Section 3(37) of ERISA), (iii) any “multiple employer plan” (within the meaning of Section 413 of the Code) or (iv) any “multiple employer welfare arrangement” (within the meaning of Section 3(40) of ERISA).
(f) There are no pending audits or investigations by any Governmental Body involving any Company Benefit Plan, and no pending or, to the Knowledge of the Company, threatened claims (except for individual claims for benefits payable in the normal operation of the Company Benefit Plans), suits or proceedings involving any Company Benefit Plan, any fiduciary thereof or service provider thereto, in any case except as would not be reasonably expected to result in material liability to the Company or any of its Subsidiaries. All contributions and premium payments required to have been made under any of the Company
A-21
Benefit Plans or by applicable Law (without regard to any waivers granted under Section 412 of the Code), have been timely made in all material respects and neither the Company nor any Company ERISA Affiliate has any material liability for any unpaid contributions with respect to any Company Benefit Plan.
(g) Neither the Company, any of its Subsidiaries or Company ERISA Affiliates, nor to the Knowledge of the Company, any fiduciary, trustee or administrator of any Company Benefit Plan, has engaged in, or in connection with the Contemplated Transactions will engage in, any transaction with respect to any Company Benefit Plan which would subject any such Company Benefit Plan, the Company, any of its Subsidiaries or Company ERISA Affiliates or Parent to a material Tax, material penalty or material liability for a “prohibited transaction” under Section 406 of ERISA or Section 4975 of the Code.
(h) Except as provided in Section 2.17(h) of the Company Disclosure Schedule, no Company Benefit Plan provides death, medical, dental, vision, life insurance or other welfare benefits beyond termination of service or retirement other than coverage mandated by Law and neither the Company nor any of its Subsidiaries or Company ERISA Affiliates has made a written or oral representation promising the same.
(i) Neither the execution of, nor the performance of the Contemplated Transactions will either alone or in connection with any other event(s) (i) result in any payment becoming due to any current or former employee, director, officer, or independent contractor of the Company or any Subsidiary thereof, (ii) increase any amount of compensation or benefits otherwise payable under any Company Benefit Plan, (iii) result in the acceleration of the time of payment, funding or vesting of any benefits under any Company Benefit Plan, (iv) require any contribution or payment to fund any obligation under any Company Benefit Plan or (v) limit the right to merge, amend or terminate any Company Benefit Plan.
(j) Neither the execution of, nor the consummation of the Contemplated Transactions (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment) will result in the receipt or retention by any person who is a “disqualified individual” (within the meaning of Code Section 280G) with respect to the Company and its Subsidiaries of any payment or benefit that is or could be characterized as a “parachute payment” (within the meaning of Code Section 280G), determined without regard to the application of Code Section 280G(b)(5).
(k) The exercise price of each Company Option is not and never has been less than the fair market value of one share of Company Common Stock as of the grant date of such Company Option.
(l) Each Company Benefit Plan providing for deferred compensation that constitutes a “nonqualified deferred compensation plan” (as defined in Section 409A(d)(1) of the Code and the regulations promulgated thereunder) is, and has been, established, administered and maintained in compliance with the requirements of Section 409A of the Code and the regulations promulgated thereunder in all material respects.
(m) No current or former employee, officer, director or independent contractor of the Company or any of its Subsidiaries has any “gross up” agreements with the Company or any of its Subsidiaries or other assurance of reimbursement by the Company or any of its Subsidiaries for any Taxes imposed under Code Section 409A or Code Section 4999.
(n) No Company Benefit Plan is maintained outside of the United States.
(o) The Company has provided to Parent a true and correct list, as of the date of this Agreement, containing the names of all full-time, part-time or temporary employees and independent contractors (and indication as such), and, as applicable: (i) the annual dollar amount of all compensation (including wages, salary or fees, commissions, director’s fees, fringe benefits, bonuses, profit sharing payments, and other payments or benefits of any type) payable to each person; (ii) dates of employment or service; (iii) title; (iv) any eligibility to receive severance, retention payment, change of control payment, or other similar compensation; (v) visa status, if applicable; and (vi) with respect to employees, a designation of whether they are classified as exempt ornon-exempt for purposes of the Fair Labor Standards Act, as amended (“FLSA”) and any similar state law.
A-22
(p) Neither the Company nor any of its Subsidiaries is or has ever been a party to, bound by, or has a duty to bargain under, any collective bargaining agreement or other Contract with a labor union, labor organization, or similar Person representing any of its employees, and there is no labor union, labor organization, or similar Person representing or, to the Knowledge of the Company, purporting to represent or seeking to represent any employees of the Company or its Subsidiaries, including through the filing of a petition for representation election. There is not and has not been in the past three years, nor is there or has there been in the past three years any threat of, any strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute, or, to the Knowledge of the Company, any union organizing activity, against the Company or any of its Subsidiaries. No event has occurred, and no condition or circumstance exists, that might directly or indirectly be likely to give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, any similar activity or dispute, or, to the Knowledge of the Company, any union organizing activity.
(q) The Company and each of its Subsidiaries is, and since January 1, 2016 has been, in material compliance with all applicable Laws respecting labor, employment, employment practices, and terms and conditions of employment, including worker classification, discrimination, harassment and retaliation, equal employment opportunities, fair employment practices, meal and rest periods, immigration, employee safety and health, payment of wages (including overtime wages), unemployment and workers’ compensation, leaves of absence, and hours of work. Except as would not be reasonably likely to result in a material liability to the Company or any of its Subsidiaries, with respect to employees of the Company and its Subsidiaries, each of the Company and its Subsidiaries, since January 1, 2016: (i) has withheld and reported all amounts required by Law or by agreement to be withheld and reported with respect to wages, salaries and other payments, benefits, or compensation to employees, (ii) is not liable for any arrears of wages (including overtime wages), severance pay or any Taxes or any penalty for failure to comply with any of the foregoing, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any Governmental Body, with respect to unemployment compensation benefits, disability, social security or other benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business). There are no actions, suits, claims, charges, lawsuits, investigations, audits or administrative matters pending or, to the Knowledge of the Company, threatened or reasonably anticipated against the Company or any of its Subsidiaries relating to any employee, applicant for employment, consultant, employment agreement or Company Benefit Plan (other than routine claims for benefits).
(r) Except as would not be reasonably likely to result in a material liability to the Company or any of its Subsidiaries, with respect to each individual who currently renders services to the Company or any of its Subsidiaries, the Company and each of its Subsidiaries has accurately classified each such individual as an employee, independent contractor, or otherwise under all applicable Laws and, for each individual classified as an employee, the Company and each of its Subsidiaries has accurately classified him or her as exempt ornon-exempt under all applicable Laws. Neither the Company nor any of its Subsidiaries has any material liability with respect to any misclassification of: (a) any Person as an independent contractor rather than as an employee, (b) any employee leased from another employer, or (c) any employee currently or formerly classified as exempt under all applicable Laws.
(s) Within the preceding five (5) years, the Company has not implemented any “plant closing” or “mass layoff” of employees that would reasonably be expected to require notification under the WARN Act or any similar state or local Law, no such “plant closing” or “mass layoff” will be implemented before the Closing Date without advance notification to and approval of Parent, and there has been no “employment loss” as defined by the WARN Act within the ninety (90) days prior to the Closing Date.
(t) There is no Legal Proceeding, claim, unfair labor practice charge or compliant, labor dispute or grievance pending or, to the Knowledge of the Company, threatened against the Company relating to labor, employment, employment practices, or terms and conditions of employment.
A-23
2.18 Environmental Matters.The Company and each of its Subsidiaries are and since January 1, 2013 have complied with all applicable Environmental Laws, which compliance includes the possession by the Company of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof, except for any failure to be in such compliance that, either individually or in the aggregate, would not reasonably be expected to be material to the Company or its business. Neither the Company nor any of its Subsidiaries has received since January 1, 2013 (or prior to that time, which is pending and unresolved), any written notice or other communication (in writing or otherwise), whether from a Governmental Body or other Person, that alleges that the Company or any of its Subsidiaries is not in compliance with or has liability pursuant to any Environmental Law and, to the Knowledge of the Company, there are no circumstances that would reasonably be expected to prevent or interfere with the Company’s or any of its Subsidiaries’ compliance in any material respects with any Environmental Law, except where such failure to comply would not reasonably be expected to be material to the Company or its business. No current or (during the time a prior property was leased or controlled by the Company or any of its Subsidiaries) prior property leased or controlled by the Company or any of its Subsidiaries has had a release of or exposure to Hazardous Materials in material violation of or as would reasonably be expected to result in any material liability of the Company or any of its Subsidiaries pursuant to Environmental Law. No consent, approval or Governmental Authorization of or registration or filing with any Governmental Body is required by Environmental Laws in connection with the execution and delivery of this Agreement or the Contemplated Transactions. Prior to the date hereof, the Company has provided or otherwise made available to Parent true and correct copies of all material environmental reports, assessments, studies and audits in the possession or control of the Company or any of its Subsidiaries with respect to any property leased or controlled by the Company or any of its Subsidiaries or any business operated by them.
2.19 Insurance. The Company has delivered or made available to Parent accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of the Company and each of its Subsidiaries. Each of such insurance policies is in full force and effect and the Company and each of its Subsidiaries are in compliance in all material respects with the terms thereof. Other than customary end of policy notifications from insurance carriers, since January 1, 2016, neither the Company nor any of its Subsidiaries has received any notice or other communication regarding any actual or possible: (i) cancellation or invalidation of any insurance policy; or (ii) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance policy. The Company and each of its Subsidiaries have provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding that is currently pending against the Company or any of its Subsidiaries for which the Company or such Subsidiary has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed the Company or any of its Subsidiaries of its intent to do so.
2.20 No Financial Advisors. Except as set forth onSection 2.20 of the Company Disclosure Schedule, no broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Contemplated Transactions based upon arrangements made by or on behalf of the Company or any of its Subsidiaries.
2.21 Disclosure. The information supplied by the Company and each of its Subsidiaries for inclusion in the Proxy Statement (including any of the Company Financials) will not, as of the date of the Proxy Statement or as of the date such information is first mailed to Parent stockholders, (i) contain any statement that is inaccurate or misleading with respect to any material facts, or (ii) omit to state any material fact necessary in order to make such information, in light of the circumstances under which such information will be provided, not false or misleading.
2.22 Transactions with Affiliates.
(a) Section 2.22(a) of the Company Disclosure Schedule (i) describes any material transactions or relationships, since January 1, 2016, between, on one hand, the Company or any of its Subsidiaries and, on the other hand, any (A) executive officer or director of the Company or, to the Knowledge of the Company, any of its Subsidiaries or any of such executive officer’s or director’s immediate family members, (B) owner of more
A-24
than 5% of the voting power of the outstanding Company Capital Stock or (C) to the Knowledge of the Company, any “related person” (within the meaning of Item 404 of RegulationS-K under the Securities Act) of any such officer, director or owner (other than the Company or its Subsidiaries) in the case of each of (A), (B) or (C) that is of the type that would be required to be disclosed under Item 404 of RegulationS-K under the Securities Act; and (ii) identifies each Person who is (or who may be deemed to be) an Affiliate of the Company as of the date of this Agreement.
(b) Section 2.22(b) of the Company Disclosure Schedule lists each stockholders agreement, voting agreement, registration rights agreement,co-sale agreement or other similar Contract between the Company and any holders of Company Capital Stock, including any such Contract granting any Person investor rights, rights of first refusal, rights of first offer, registration rights, director designation rights or similar rights (collectively, the “Investor Agreements”).
2.23 Anti-Bribery. None of the Company or any of its Subsidiaries or any of their respective directors, officers, employees or, to the Company’s Knowledge, agents or any other Person acting on their behalf has directly or indirectly made any bribes, rebates, payoffs, influence payments, kickbacks, illegal payments, illegal political contributions, or other payments, in the form of cash, gifts, or otherwise, or taken any other action, in violation of the Foreign Corrupt Practices Act of 1977, the UK Bribery Act of 2010 or any other anti-bribery or anti-corruption Law (collectively, the “Anti-Bribery Laws”). Neither the Company nor any of its Subsidiaries is or has been the subject of any investigation or inquiry by any Governmental Body with respect to potential violations of Anti-Bribery Laws.
2.24 Disclaimer of Other Representations or Warranties.
(a) Except as previously set forth in thisSection 2 or in any certificate delivered by the Company to Parent and/or Merger Sub pursuant to this Agreement, the Company makes no representation or warranty, express or implied, at law or in equity, with respect to it or any of its assets, liabilities or operations, and any such other representations or warranties are hereby expressly disclaimed.
(b) The Company acknowledges and agrees that, except for the representations and warranties of Parent and Merger Sub set forth inSection 3, none of Parent, Merger Sub or any of their respective Representatives is relying on any other representation or warranty of Parent or any other Person made outside ofSection 3, including regarding the accuracy or completeness of any such other representations or warranties or the omission of any material information, whether express or implied, in each case, with respect to the Contemplated Transactions.
Section 3. REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB
Subject toSection 10.13(h), except (a) as set forth in the disclosure schedule delivered by Parent to the Company (the “Parent Disclosure Schedule”) or (b) as disclosed in the Parent SEC Documents filed with the SEC prior to the date hereof and publicly available on the SEC’s Electronic Data Gathering Analysis and Retrieval system (but (i) without giving effect to any amendment thereof filed with, or furnished to the SEC on or after the date hereof and (ii) excluding any disclosures contained under the heading “Risk Factors” and any disclosure of risks included in any “forward-looking statements” disclaimer or in any other section to the extent they are forward-looking statements or cautionary, predictive or forward-looking in nature), it being understood that any matter disclosed in the Parent SEC Documents (x) shall not be deemed disclosed for the purposes of Section 3.1, Section 3.2, Section 3.3, Section 3.4, Section 3.5 or Section 3.6 and (y) shall be deemed to be disclosed in a section of the Parent Disclosure Schedule only to the extent that it is readily apparent from a reading of such Parent SEC Document that it is applicable to such section of the Parent Disclosure Schedule, Parent and Merger Sub represent and warrant to the Company as follows:
3.1 Due Organization; No Subsidiaries.
(a) Each of Parent and Merger Sub is a corporation duly incorporated, validly existing and in good standing under the Laws of Delaware, and has all necessary corporate power and authority: (i) to conduct
A-25
its business in the manner in which its business is currently being conducted; (ii) to own or lease and use its property and assets in the manner in which its property and assets are currently owned or leased and used; and (iii) to perform its obligations under all Contracts by which each is bound. Since the date of its incorporation, Merger Sub has not engaged in any activities other than activities incident to its formation or in connection with or as contemplated by this Agreement.
(b) Parent is duly licensed and qualified to do business, and is in good standing (to the extent applicable in such jurisdiction), under the Laws of all jurisdictions where the nature of its business requires such licensing or qualification other than in jurisdictions where the failure to be so qualified individually or in the aggregate would not be reasonably expected to have a Parent Material Adverse Effect.
(c) Other than Merger Sub, Parent does not have any Subsidiary.
(d) Parent is not and has not otherwise been, directly or indirectly, a party to, member of or participant in any partnership, joint venture or similar business entity. Parent has not agreed and is not obligated to make, and is not bound by any Contract under which it may become obligated to make, any future investment in or capital contribution to any other Entity. Parent has not, at any time, been a general partner of, and has not otherwise been liable for any of the debts or other obligations of, any general partnership, limited partnership or other Entity.
3.2 Organizational Documents.Parent has made available to the Company accurate and complete copies of Parent’s and Merger Sub’s Organizational Documents in effect as of the date of this Agreement. Neither Parent nor Merger Sub is in material breach or violation of its respective Organizational Documents.
3.3 Authority; Binding Nature of Agreement.
(a) Each of Parent and Merger Sub has all necessary corporate power and authority to enter into and to perform its obligations under this Agreement and, subject, with respect to Parent, to receipt of the Required Parent Stockholder Vote and, with respect to Merger Sub, the adoption of this Agreement by Parent in its capacity as sole stockholder of Merger Sub, to perform its obligations hereunder and to consummate the Contemplated Transactions. The Parent Board (at meetings duly called and held) has: (i) determined that the Contemplated Transactions are fair to, advisable and in the best interests of Parent and its stockholders; (ii) authorized, approved and declared advisable this Agreement and the Contemplated Transactions, including the issuance of shares of Parent Common Stock to the stockholders of the Company pursuant to the terms of this Agreement and the treatment of the Company Options pursuant to this Agreement; and (iii) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholders of Parent vote to approve the Parent Stockholder Matters. The Merger Sub Board (by unanimous written consent) has: (A) determined that the Contemplated Transactions are fair to, advisable, and in the best interests of Merger Sub and its sole stockholder; (B) authorized, approved and declared advisable this Agreement and the Contemplated Transactions; and (C) determined to recommend, upon the terms and subject to the conditions set forth in this Agreement, that the stockholder of Merger Sub vote to adopt this Agreement and thereby approve the Contemplated Transactions.
(b) This Agreement has been duly executed and delivered by each of Parent and Merger Sub and, assuming the due authorization, execution and delivery by the Company, constitutes the legal, valid and binding obligation of Parent and Merger Sub, enforceable against each of Parent and Merger Sub in accordance with its terms, subject to the Enforceability Exceptions. Prior to the execution of the Parent Stockholder Support Agreements, the Parent Board approved the Parent Stockholder Support Agreements and the transactions contemplated thereby.
3.4 Vote Required. (a) The affirmative vote of the holders of a majority of the outstanding shares of Parent Common Stock is the only vote of the holders of any class or series of Parent’s capital stock necessary to
A-26
approve the proposals inSection 5.3(a)(i) and(ii) and (b) the affirmative vote of a majority of the votes cast at the Parent Stockholders’ Meeting is the only vote of the holders of any class or series of Parent’s capital stock necessary to approve the proposals inSection 5.3(a)(iii) (the “Required Parent Stockholder Vote”).
3.5 Non-Contravention; Consents. Subject to obtaining the Required Parent Stockholder Vote and the filing of the Certificate of Merger required by the DGCL, neither (x) the execution, delivery or performance of this Agreement by Parent or Merger Sub, nor (y) the consummation of the Contemplated Transactions, will directly or indirectly (with or without notice or lapse of time):
(a) contravene, conflict with or result in a violation of any of the provisions of the Organizational Documents of Parent or Merger Sub;
(b) contravene, conflict with or result in a material violation of, or to the Knowledge of Parent give any Governmental Body or other Person the right to challenge the Contemplated Transactions or to exercise any material remedy or obtain any material relief under, any Law or any order, writ, injunction, judgment or decree to which Parent or Merger Sub, or any of the assets owned or used by Parent or Merger Sub, is subject, except as would not reasonably be expected to be material to Parent or its business;
(c) contravene, conflict with or result in a violation of any of the terms or requirements of, or give any Governmental Body the right to revoke, withdraw, suspend, cancel, terminate or modify, any Governmental Authorization that is held by Parent, except as would not reasonably be expected to be material to Parent or its business;
(d) contravene, conflict with or result in a violation or breach of, or result in a default under, any provision of any Parent Material Contract, or give any Person the right to: (i) declare a default or exercise any remedy under any Parent Material Contract; (ii) any material payment, rebate, chargeback, penalty or change in delivery schedule under any Parent Material Contract; (iii) accelerate the maturity or performance of any Parent Material Contract; or (iv) cancel, terminate or modify any term of any Parent Material Contract, except in the case of anynon-material breach, default, penalty or modification; or
(e) result in the imposition or creation of any Encumbrance upon or with respect to any material asset owned or used by Parent (except for Permitted Encumbrances).
Except for (i) any Consent set forth onSection 3.5 of the Parent Disclosure Schedule under any Parent Contract, (ii) the Required Parent Stockholder Vote, (iii) the filing of the Certificate of Merger with the Secretary of State of the State of Delaware pursuant to the DGCL, and (iv) such consents, waivers, approvals, orders, authorizations, registrations, declarations and filings as may be required under applicable federal and state securities Laws, Parent is not and will not be required to make any filing with or give any notice to, or to obtain any Consent from, any Person in connection with (A) the execution, delivery or performance of this Agreement, the Parent Stockholder Support Agreements, and the ParentLock-up Agreements or (B) the consummation of the Contemplated Transactions, which if individually or in the aggregate were not given or obtained, would reasonably be expected to prevent or materially delay the ability of Parent and Merger Sub to consummate the Contemplated Transactions. The Parent Board and the Merger Sub Board have taken and will take all actions necessary to ensure that the restrictions applicable to business combinations contained in Section 203 of the DGCL are, and will be, inapplicable to the execution, delivery and performance of this Agreement, the Parent Stockholder Support Agreements, the ParentLock-Up Agreements and to the consummation of the Contemplated Transactions. No other state takeover statute or similar Law applies or purports to apply to the Merger, this Agreement, the Parent Stockholder Support Agreements, the ParentLock-Up Agreements or any of the other Contemplated Transactions.
(a) The authorized capital stock of Parent as of the date of this Agreement consists of (i) 60,000,000 shares of Parent Common Stock, par value $0.001 per share, of which 24,051,844 shares have been
A-27
issued and are outstanding as of the close of business on the Reference Date and (ii) 5,000,000 shares of preferred stock of Parent, par value $0.001 per share, of which no shares have been issued and are outstanding as of the date of this Agreement. Parent does not hold any shares of its capital stock in its treasury. As of the close of business on the Reference Date, there are outstanding Parent Warrants to purchase 8,609,853 shares of Parent Common Stock.
(b) All of the outstanding shares of Parent Common Stock have been duly authorized and validly issued, and are fully paid and nonassessable. None of the outstanding shares of Parent Common Stock is entitled or subject to any preemptive right, right of participation, right of maintenance or any similar right and none of the outstanding shares of Parent Common Stock is subject to any right of first refusal in favor of Parent. Except as contemplated herein, there is no Parent Contract relating to the voting or registration of, or restricting any Person from purchasing, selling, pledging or otherwise disposing of (or granting any option or similar right with respect to), any shares of Parent Common Stock. Parent is not under any obligation, nor is it bound by any Contract pursuant to which it may become obligated, to repurchase, redeem or otherwise acquire any outstanding shares of Parent Common Stock or other securities.Section 3.6(b) of the Parent Disclosure Schedule accurately and completely lists all repurchase or forfeiture rights held by the Parent with respect to shares of Parent Common Stock (including shares issued pursuant to the exercise of stock options).
(c) Except for the Parent Stock Plans, Parent does not have any stock option plan or any other plan, program, agreement or arrangement providing for any equity-based compensation for any Person. As of the close of business on the Reference Date, 2,318,647 shares have been reserved for issuance upon exercise of Parent Options granted under the Parent Stock Plans that are outstanding as of the date of this Agreement, 155,426 shares have been reserved for issuance pursuant to Parent Deferred Stock Rights granted under the Parent Stock Plans that are outstanding as of the date of this Agreement, and 2,196,036 shares remain available for future issuance pursuant to the Parent Stock Plans. Section 3.6(c) of the Parent Disclosure Schedule sets forth the following information with respect to each Parent Option outstanding as of the date of this Agreement: (i) the name of the holder; (ii) the number of shares of Parent Common Stock subject to such Parent Option at the time of grant; (iii) the number of shares of Parent Common Stock subject to such Parent Option as of the date of this Agreement; (iv) the exercise price of such Parent Option; (v) the date on which such Parent Option was granted; (vi) the applicable vesting schedule, including the number of vested and unvested shares as of the date of this Agreement and any acceleration provisions; (vii) the date on which such Parent Option expires (and whether there has been any extension of such expiration date or any other provisions or agreements that may result in an extension of the expiration date of such Parent Option beyond the date(s) provided in the form of stock option agreement provided to the Company); and (viii) whether such Parent Option is intended to constitute an “incentive stock option” (as defined in the Code) or anon-qualified stock option.Section 3.6(c) of the Parent Disclosure Schedule sets forth the following information with respect to each Parent Deferred Stock Rights outstanding as of the date of this Agreement: (i) the name of the holder; (ii) the number of shares of Parent Common Stock subject to such Parent Deferred Stock Right at the time of grant; (iii) the number of shares of Parent Common Stock subject to such Parent Deferred Stock Right as of the date of this Agreement; (iv) the date on which such Parent Deferred Stock Right was granted; (v) the applicable vesting schedule, if any, including the number of vested and unvested shares as of the date of this Agreement and any acceleration provisions; and (vi) the distribution or settlement provisions applicable to such Parent Deferred Stock Right. Parent has made available to the Company accurate and complete copies of the Parent Stock Plans and all forms of the stock option and other award agreements evidencing outstanding awards granted thereunder.
(d) Except for the Parent Warrants, the Parent Stock Plans, the Parent Options, the Parent Deferred Stock Rights, there is no: (i) outstanding subscription, option, call, warrant or right (whether or not currently exercisable) to acquire any shares of the capital stock or other securities of Parent or any of its Subsidiaries; (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any shares of the capital stock or other securities of Parent or any of its Subsidiaries; or (iii) condition or circumstance that is reasonably likely to give rise to or provide a basis for the assertion of a claim by any Person to the effect that such Person is entitled to acquire or receive any shares of capital stock or
A-28
other securities of Parent or any of its Subsidiaries. There are no outstanding or authorized stock appreciation, phantom stock, profit participation or other similar rights with respect to Parent or any of its Subsidiaries.
(e) All outstanding shares of Parent Common Stock, Parent Options, Parent Deferred Stock Rights, Parent Warrants and other securities of Parent have been issued and granted in material compliance with (i) all applicable securities Laws and other applicable Law, and (ii) all requirements set forth in applicable Contracts.
3.7 SEC Filings; Financial Statements.
(a) Parent has delivered or made available to the Company accurate and complete copies of all registration statements, proxy statements, Certifications (as defined below) and other statements, reports, schedules, forms and other documents filed by Parent with the SEC since January 1, 2018 (the “Parent SEC Documents”), other than such documents that can be obtained on the SEC’s website atwww.sec.gov. Since January 1, 2017, all material statements, reports, schedules, forms and other documents required to have been filed by Parent or its officers with the SEC have been so filed on a timely basis. As of the time it was filed with the SEC (or, if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing), each of the Parent SEC Documents complied in all material respects with the applicable requirements of the Securities Act or the Exchange Act (as the case may be) and, as of the time they were filed, none of the Parent SEC Documents contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. The certifications and statements required by (i) Rule13a-14 under the Exchange Act and (ii) 18 U.S.C. §1350 (Section 906 of the Sarbanes-Oxley Act) relating to the Parent SEC Documents (collectively, the “Certifications”) are accurate and complete and comply as to form and content with all applicable Laws. As used in thisSection 3.7, the term “file” and variations thereof shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC.
(b) The financial statements (including any related notes) contained or incorporated by reference in the Parent SEC Documents: (i) complied as to form in all material respects with the published rules and regulations of the SEC applicable thereto; (ii) were prepared in accordance with GAAP (except as may be indicated in the notes to such financial statements or, in the case of unaudited financial statements, except as permitted by Form10-Q of the SEC, and except that the unaudited financial statements may not contain footnotes and are subject to normal and recurringyear-end adjustments) applied on a consistent basis unless otherwise noted therein throughout the periods indicated; and (iii) fairly present, in all material respects, the financial position of Parent as of the respective dates thereof and the results of operations and cash flows of Parent for the periods covered thereby. Other than as expressly disclosed in the Parent SEC Documents filed prior to the date hereof, there has been no material change in Parent’s accounting methods or principles that would be required to be disclosed in Parent’s financial statements in accordance with GAAP. The books of account and other financial records of Parent are true and complete in all material respects.
(c) Parent’s independent registered accounting firm has at all times since the date Parent became subject to the applicable provisions of the Sarbanes-Oxley Act been; (i) a registered public accounting firm (as defined in Section 2(a)(12) of the Sarbanes-Oxley Act); (ii) to the Knowledge of Parent “Independent” with respect to Parent within the meaning of RegulationS-X under the Exchange Act; and (iii) to the Knowledge of Parent, in compliance with subsections (g) through (l) of Section 10A of the Exchange Act and the rules and regulations promulgated by the SEC and the Public Company Accounting Oversight Board thereunder.
(d) Since January 1, 2017 through the date of this Agreement, Parent has not received any comment letter from the SEC or the staff thereof or any correspondence from officials of Nasdaq or the staff thereof relating to the delisting or maintenance of listing of the Parent Common Stock on Nasdaq. Parent has not disclosed any unresolved comments in the Parent SEC Documents.
A-29
(e) Since January 1, 2017, there have been no formal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer, principal accounting officer or general counsel of Parent, the Parent Board or any committee thereof, other than ordinary course audits or reviews of accounting policies and practices or internal controls required by the Sarbanes-Oxley Act.
(f) Parent is and since January 1, 2017 has been, in compliance in all material respects with the applicable current listing and governance rules and regulations of Nasdaq.
(g) Parent maintains, and at all times since January 1, 2017 has maintained, a system of internal control over financial reporting (as defined in Rules13a-15(f) and15d-15(f) of the Exchange Act) that is sufficient to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and to provide reasonable assurance (i) that Parent maintains records in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of Parent and Merger Sub; (ii) that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, (iii) that receipts and expenditures are made only in accordance with authorizations of management and the Parent Board and (iv) regarding prevention or timely detection of the unauthorized acquisition, use or disposition of Parent’s assets that could have a material effect on Parent’s financial statements. Parent has evaluated the effectiveness of Parent’s internal control over financial reporting and, to the extent required by applicable Law, presented in any applicable Parent SEC Document that is a report on Form10-K or Form10-Q (or any amendment thereto) its conclusions about the effectiveness of the internal control over financial reporting as of the end of the period covered by such report or amendment based on such evaluation. Parent has disclosed, based on its most recent evaluation of internal control over financial reporting, to Parent’s auditors and audit committee (and made available to the Company a summary of the significant aspects of such disclosure) (A) all material weaknesses and significant deficiencies, if any, in the design or operation of internal control over financial reporting that are reasonably likely to adversely affect Parent’s ability to record, process, summarize and report financial information and (B) any known fraud that involves management or other employees who have a significant role in Parent’s internal control over financial reporting. Parent has not identified, based on its most recent evaluation of internal control over financial reporting, any material weaknesses in the design or operation of Parent’s internal control over financial reporting.
(h) Parent maintains “disclosure controls and procedures” (as defined in Rules13a-15(e) and15d-15(e) of the Exchange Act) that are reasonably designed to ensure that all information required to be disclosed by Parent in the periodic reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods required by the SEC, and that all such information is accumulated and communicated to Parent’s management as appropriate to allow timely decisions regarding required disclosure and to make the Certifications.
3.8 Absence of Changes. Except as set forth onSection 3.8 of the Parent Disclosure Schedule, between the date of the Parent Balance Sheet and the date of this Agreement, Parent and its Subsidiaries have conducted its business only in the Ordinary Course of Business (except for the execution and performance of this Agreement and the discussions, negotiations and transactions related thereto) and there has not been any (a) Parent Material Adverse Effect or (b) action, event or occurrence that would have required consent of the Company pursuant toSection 4.1(b) had such action, event or occurrence taken place after the execution and delivery of this Agreement.
3.9 Absence of Undisclosed Liabilities.As of the date hereof, Parent does not have any Liability, individually or in the aggregate, of a type required to be recorded or reflected on a balance sheet or disclosed in the footnotes thereto under GAAP except for: (a) Liabilities disclosed, reflected or reserved against in the Parent Balance Sheet; (b) Liabilities that have been incurred by Parent since the date of the Parent Balance Sheet in the Ordinary Course of Business; (c) Liabilities for performance of obligations of Parent under Parent Contracts; (d) Liabilities incurred in connection with the Contemplated Transactions; (e) Liabilities which would not,
A-30
individually or in the aggregate, reasonably be expected to be material to the Parent; and (f) Liabilities described inSection 3.9 of the Parent Disclosure Schedule.
3.10 Title to Assets. Parent owns, and has good and valid title to, or, in the case of leased properties and assets, valid leasehold interests in, all tangible properties or tangible assets and equipment used or held for use in its business or operations or purported to be owned by it that are material to Parent or its business, including: (a) all tangible assets reflected on the Parent Balance Sheet; and (b) all other tangible assets reflected in the books and records of Parent as being owned by Parent. All of such assets are owned or, in the case of leased assets, leased by Parent free and clear of any Encumbrances, other than Permitted Encumbrances.
3.11 Real Property; Leasehold. Parent does not own any real property. Parent has made available to the Company (a) an accurate and complete list of all real properties with respect to which Parent directly or indirectly holds a valid leasehold interest as well as any other real estate that is in the possession of or leased by Parent, and (b) copies of all leases under which any such real property is possessed (the “Parent Real Estate Leases”), each of which is in full force and effect, with no existing material default thereunder. Parent’s use and operation of each such leased property conforms to all applicable Laws in all material respects, and Parent has exclusive possession of each such leased property and has not granted any occupancy rights to tenants or licensees with respect to such leased property. In addition, each such leased property is free and clear of all Encumbrances other than Permitted Encumbrances.
(a) Section 3.12(a) of the Parent Disclosure Schedule identifies each item of material Parent IP, including, with respect to each patent and patent application: (i) the name of the applicant/registrant, (ii) the jurisdiction of application/registration, (iii) the application or registration number and (iv) any otherco-owners. To the Knowledge of Parent, each of the patents and patent applications included in the Section 3.12(a) of the Parent Disclosure Schedule properly identifies by name each and every inventor of the inventions claimed therein as determined in accordance with applicable Laws of the United States. To the knowledge of Parent, as of the date of this Agreement, no cancellation, interference, opposition, reissue, reexamination or other proceeding of any nature (other than office actions or similar communications issued by any Governmental Body in the ordinary course of prosecution of any pending applications for registration) is pending or threatened in writing, in which the scope, validity, enforceability or ownership of any Parent IP is being or has been contested or challenged.
(b) Except as has not had and would not reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect, Parent owns, is the assignee of, or has licensed all material Parent IP (other than as disclosed onSection 3.12(b) of the Parent Disclosure Schedule), free and clear of all Encumbrances other than Permitted Encumbrances. To the Knowledge of Parent, each Parent Associate involved in the creation or development of any material Parent IP, pursuant to such Parent Associate’s activities on behalf of Parent, has signed a written agreement containing an assignment of such Parent Associate’s rights in such Parent IP to Parent and confidentiality provisions protecting the Parent IP.
(c) To the Knowledge of Parent, no funding, facilities or personnel of any Governmental Body or any university, college, research institute or other educational institution has been used to create Parent IP, except for any such funding or use of facilities or personnel that does not result in such Governmental Body or institution obtaining ownership rights to such Parent IP or the right to receive royalties for the practice of such Parent IP.
(d) Section 3.12(d) of Parent Disclosure Schedule sets forth each license agreement pursuant to which the Parent (i) is granted a license under any material Intellectual Property Right owned by any third party that is used by Parent in its business as currently conducted (each a “ParentIn-bound License”) or (ii) grants to any third party a license under any material Parent IP or material Intellectual Property Right licensed to the
A-31
Parent under a ParentIn-bound License (each a “ParentOut-bound License”) (provided, that, ParentIn-bound Licenses shall not include, when entered into in the ordinary course of business, material transfer agreements, services agreements, clinical trial agreements, agreements with Parent Associates,non-disclosure agreements, commercially availableSoftware-as-a-Service offerings,off-the-shelf software licenses or generally available patent license agreements; and ParentOut-bound Licenses shall not include, when entered into in the ordinary course of business, material transfer agreements, clinical trial agreements, services agreements,non-disclosure agreements, ornon-exclusive outbound licenses).
(e) To the Knowledge of Parent: (i) the operation of the business of Parent as currently conducted does not infringe, misappropriate or otherwise violate any valid and enforceable United States patent that is not included on Section 2.12(a) of the Company Disclosure Schedule and (ii) no other Person is infringing, misappropriating or otherwise violating any Parent IP. No Legal Proceeding is pending (or, to the Knowledge of Parent, is threatened in writing) (A) against Parent alleging that the operation of the business of Parent infringes or constitutes the misappropriation or other violation of any Intellectual Property Rights of another Person or (B) by Parent alleging that another Person has infringed, misappropriated or otherwise violated any of Parent IP or any Intellectual Property Rights exclusively licensed to Parent. Since January 1, 2017, Parent has not received any written notice or other written communication alleging that the operation of the business of Parent infringes or constitutes the misappropriation or other violation of any Intellectual Property Right of another Person.
(f) None of Parent IP or, to the Knowledge of Parent, any material Intellectual Property Rights exclusively licensed to Parent is subject to any pending or outstanding injunction, directive, order, judgment or other disposition of dispute that adversely and materially restricts the use, transfer, registration or licensing by Parent of any such Parent IP or material Intellectual Property Rights exclusively licensed to Parent or its Subsidiaries.
(g) To the Knowledge of Parent, the operation of Parent’s business are in substantial compliance with all Laws pertaining to data privacy and data security of Sensitive Data, except to the extent that such noncompliance has not and would not reasonably be expected to have a Parent Material Adverse Effect. To the Knowledge of Parent, since January 1, 2017, there have been (i) no material losses or thefts of data or security breaches relating to Sensitive Data used in the business of Parent, (ii) no violations of any security policy of Parent regarding any such Sensitive Data used in the business of Parent, (iii) no unauthorized access, unauthorized use or unintended or improper disclosure of any Sensitive Data used in the business of Parent, in each case of (i) through (iii), except as would not reasonably be expected to, individually or in the aggregate, have a Parent Material Adverse Effect.
3.13 Agreements, Contracts and Commitments.Section 3.13 of the Parent Disclosure Schedule lists the following Parent Contracts in effect as of the date of this Agreement (and, except with respect to clauses (m) and (n) below, other than any Benefit Plans) (each, a “Parent Material Contract” and collectively, the “Parent Material Contracts”):
(a) a material contract as defined in Item 601(b)(10) of RegulationS-K as promulgated under the Securities Act;
(b) each Contract relating to any agreement of indemnification or guaranty not entered into in the Ordinary Course of Business;
(c) each Contract containing (A) any covenant limiting the freedom of Parent to engage in any line of business or compete with any Person, (B) any most-favored pricing arrangement, (C) any exclusivity provision, or (D) anynon-solicitation provision with respect to employees of other Persons, in each case, except for restrictions that would not materially affect the ability of Parent to conduct its business;
(d) each Contract relating to capital expenditures and requiring payments after the date of this Agreement in excess of $250,000 pursuant to its express terms and not cancelable without penalty;
A-32
(e) each Contract relating to the disposition or acquisition of material assets or any ownership interest in any Entity, in each case, involving payments in excess of $250,000, other than Parent Contracts in which the applicable acquisition or disposition has been consummated and there are no material ongoing obligations;
(f) each Contract relating to any mortgages, indentures, loans, notes or credit agreements, security agreements or other agreements or instruments relating to the borrowing of money or extension of credit or creating any material Encumbrances with respect to any assets of Parent or any loans or debt obligations with officers or directors of Parent, in each case, having an outstanding principal in an amount in excess of $250,000;
(g) each Contract requiring payment by or to Parent after the date of this Agreement in excess of $250,000 pursuant to its express terms relating to: (A) any distribution agreement (identifying any that contain exclusivity provisions); (B) any agreement involving provision of services or products with respect to anypre-clinical or clinical development activities of Parent; (C) any dealer, distributor, joint marketing, alliance, joint venture, cooperation, development or other agreement currently in force under which Parent has continuing obligations to develop or market any product, technology or service, or any agreement pursuant to which Parent has continuing obligations to develop any Intellectual Property Rights that will not be owned, in whole or in part, by Parent; or (D) any Contract to license any third party to manufacture or produce any product, service or technology of Parent or any Contract to sell, distribute or commercialize any products or service of Parent, in each case, except for Contracts entered into in the Ordinary Course of Business;
(h) each Contract with any financial advisor, broker, finder, investment banker or other similar Person, providing advisory services to Parent in connection with the Contemplated Transactions;
(i) each Parent Real Estate Lease;
(j) each Contract with any Governmental Body (other than clinical trial agreements for clinical trial studies);
(k) each ParentOut-bound License and ParentIn-bound License;
(l) each Contract containing any royalty, dividend or similar arrangement based on the revenues or profits of Parent;
(m) each Parent Contract, offer letter, employment agreement, or independent contractor agreement with any employee, consultant or independent contractor that (A) is not terminable by Parent without less than 60 days notice, severance, or other cost or liability, or (B) provides for retention payments, change of control payments, severance, accelerated vesting, or any payment or benefit that may or will become due as a result of the Merger (whether alone or in connection with any other event); or
(n) any other Contract that is not terminable at will (with no penalty or payment) by Parent and (A) which involves payment or receipt by Parent after the date of this Agreement under any such agreement, contract or commitment of more than $250,000 in the aggregate, or obligations after the date of this Agreement in excess of $250,000 in the aggregate, or (B) that is material to the business or operations of Parent.
Parent has delivered or made available to the Company accurate and complete copies of all Parent Material Contracts, including all amendments thereto. There are no Parent Material Contracts that are not in written form. Parent has not nor, to Parent’s Knowledge, as of the date of this Agreement, has any other party to a Parent Material Contract, breached, violated or defaulted under, or received notice that it breached, violated or defaulted under, any of the terms or conditions of any Parent Material Contract in such manner as would permit any other party to cancel or terminate any such Parent Material Contract, or would permit any other party to seek damages which would reasonably be expected to be material to Parent or its business. As to Parent, as of the date of this
A-33
Agreement, each Parent Material Contract is valid, binding, enforceable and in full force and effect, subject to the Enforceability Exceptions. No Person is renegotiating, or has a right pursuant to the terms of any Parent Material Contract to change, any material amount paid or payable to Parent under any Parent Material Contract or any other material term or provision of any Parent Material Contract.
(a) Parent is, and since January 1, 2016 has been, in compliance in all material respects with all applicable Laws, including the FDCA, the FDA regulations adopted thereunder, the Public Health Service Act and any other similar Law administered or promulgated by the FDA or other Drug Regulatory Agency, except for any noncompliance, either individually or in the aggregate, which would not be material to Parent. No investigation, claim, suit, proceeding, audit or other action by any Governmental Body is pending or, to the Knowledge of Parent, threatened against Parent. There is no agreement, judgment, injunction, order or decree binding upon Parent which (i) has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of Parent, any acquisition of material property by Parent or the conduct of business by Parent as currently conducted, (ii) is reasonably likely to have an adverse effect on Parent’s ability to comply with or perform any covenant or obligation under this Agreement, or (iii) is reasonably likely to have the effect of preventing, delaying, making illegal or otherwise interfering with the Contemplated Transactions.
(b) Parent holds all required Governmental Authorizations which are material to the operation of the business of Parent as currently conducted (the “Parent Permits”).Section 3.14(b) of the Parent Disclosure Schedule identifies each Parent Permit. Parent is in material compliance with the terms of the Parent Permits. No Legal Proceeding is pending or, to the Knowledge of Parent, threatened, which seeks to revoke, limit, suspend, or materially modify any Parent Permit.
(c) There are no proceedings pending or, to the Knowledge of Parent, threatened against Parent with respect to an alleged material violation by Parent of the FDCA, FDA regulations adopted thereunder, the Public Health Service Act or any other similar Law administered or promulgated by any Drug Regulatory Agency.
(d) All clinical,pre-clinical and other studies and tests conducted by or on behalf of, or sponsored by, Parent, or in which Parent or its respective current products or product candidates have participated, were and, if still pending, are being conducted in all material respects in accordance with standard medical and scientific research procedures and in compliance in all material respects with the applicable regulations of any applicable Drug Regulatory Agency and other applicable Law, as applicable, including 21 C.F.R. Parts 50, 54, 56, 58 and 312. No preclinical or clinical trial conducted by or on behalf of Parent has been terminated or suspended prior to completion for safety ornon-compliance reasons. Since January 1, 2016, Parent has not received any notices, correspondence, or other communications from any Drug Regulatory Agency requiring, or to the Knowledge of Parent threatening to initiate, the termination or suspension of any clinical studies conducted by or on behalf of, or sponsored by, Parent or in which Parent or its current products or product candidates have participated.
(e) Parent is not the subject of any pending or, to the Knowledge of Parent, threatened investigation in respect of its business or products by the FDA pursuant to its “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto. To the Knowledge of Parent, Parent has not committed any acts, made any statement, or has not failed to make any statement, in each case in respect of its business or products that would violate the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy, and any amendments thereto. Parent or any of its officers, employees or, to the Knowledge of Parent, agents has not been convicted of any crime or engaged in any conduct that could result in a debarment or exclusion (i) under 21 U.S.C. Section 335a or (ii) any similar applicable Law. No debarment or exclusionary claims, actions, proceedings or investigations in respect of their business or products are pending or, to the Knowledge of Parent, threatened against Parent or any of its officers, employees or, to the Knowledge of Parent, agents.
A-34
(f) Parent is not a Covered Entity governed by HIPPA, but each of its health plans, if required, has complied with all Laws relating to HIPAA, including the standards for the privacy of Individually Identifiable Health Information at 45 C.F.R. Parts 160 and 164, Subparts A and E, the standards for the protection of Electronic Protected Health Information set forth at 45 C.F.R. Part 160 and 45 C.F.R. Part 164, Subpart A and Subpart C, the standards for transactions and code sets used in electronic transactions at 45 C.F.R. Part 160, Subpart A and Part 162, and the standards for Breach Notification for Unsecured Protected Health Information at 45 C.F.R. Part 164, Subpart D, all as amended from time to time. Each of Parent’s health plans has entered into, where required, and are in compliance in all material respects with the terms of all Business Associate Agreements to which Parent has signed as plan sponsor where the plan is a party or otherwise bound. Each of Parent’s health plans, where required, has created and maintained written policies and procedures to protect the privacy of all protected health information, provide training to all employees and agents as required under HIPAA, and have implemented security procedures, including physical, technical and administrative safeguards, to protect all personal information and Protected Health Information stored or transmitted in electronic form. Parent has not received written notice from the Office for Civil Rights for the U.S. Department of Health and Human Services or any other Governmental Body of any allegation regarding its failure to comply with HIPAA or any other state law or regulation applicable to the protection of individually identifiable health information or personally identifiable information. No successful Security Incident, Breach of Unsecured Protected Health Information or breach of personally identifiable information under applicable state or federal laws have occurred with respect to information maintained or transmitted to any health plan of Parent or an agent or third party subject to a Business Associate Agreement with any health plan of Parent. If required, each health plan of Parent is currently submitting, receiving and handling or is capable of submitting receiving and handling transactions in accordance with the Standard Transaction Rule. Parent has materially complied with its requirements related to protection of Protected Health Information under its clinical trial agreements with health care provider Covered Entities that have participated in Parent’s clinical studies under such agreements. All capitalized terms in thisSection 3.14(f) not otherwise defined in this Agreement shall have the meanings set forth under HIPAA.
3.15 Legal Proceedings; Orders.
(a) As of the date of this Agreement, there is no material pending Legal Proceeding and, to the Knowledge of Parent, no Person has threatened in writing to commence any Legal Proceeding: (i) that involves (A) Parent, (B) any Parent Associate (in his or her capacity as such) or (C) any of the material assets owned or used by Parent; or (ii) that challenges, or that would have the effect of preventing, delaying, making illegal or otherwise interfering with, the Contemplated Transactions.
(b) Since January 1, 2016 through the date of this Agreement, no Legal Proceeding has been pending against Parent that resulted in material liability to Parent.
(c) There is no order, writ, injunction, judgment or decree to which Parent, or any of the material assets owned or used by Parent, is subject. To the Knowledge of Parent, no officer of Parent is subject to any order, writ, injunction, judgment or decree that prohibits such officer or employee from engaging in or continuing any conduct, activity or practice relating to the business of Parent or to any material assets owned or used by Parent.
(a) Parent and Merger Sub have timely filed all income Tax Returns and other material Tax Returns that they were required to file under applicable Law. All such Tax Returns are correct and complete in all material respects and have been prepared in compliance with all applicable Law. No claim has ever been made by any Governmental Body in any jurisdiction where Parent or Merger Sub does not file a particular Tax Return or pay a particular Tax that Parent or Merger Sub is subject to taxation by that jurisdiction.
A-35
(b) All income and other material Taxes due and owing by Parent or Merger Sub on or before the date hereof (whether or not shown on any Tax Return) have been fully paid. The unpaid Taxes of Parent and Merger Sub did not, as of the date of the Parent Balance Sheet, materially exceed the reserve for Tax liability (excluding any reserve for deferred Taxes established to reflect timing differences between book and Tax items) set forth on the face of the Parent Balance Sheet. Since the Parent Balance Sheet Date, neither Parent nor Merger Sub has incurred any material Liability for Taxes outside the Ordinary Course of Business.
(c) All Taxes that Parent or Merger Sub are or were required by Law to withhold or collect have been duly and timely withheld or collected in all material respects on behalf of its respective employees, independent contractors, stockholders, lenders, customers or other third parties and, have been timely paid to the proper Governmental Body or other Person or properly set aside in accounts for this purpose.
(d) There are no Encumbrances for material Taxes (other than Permitted Encumbrances) upon any of the assets of Parent or Merger Sub.
(e) No deficiencies for income or other material Taxes with respect to Parent or Merger Sub have been claimed, proposed or assessed by any Governmental Body in writing. There are no pending or ongoing, and to the Knowledge of Parent, threatened audits, assessments or other actions for or relating to any liability in respect of a material amount of Taxes of Parent or Merger Sub. Neither Parent nor Merger Sub (or any of their predecessors) has waived any statute of limitations in respect of any income or other material Taxes or agreed to any extension of time with respect to any income or other material Tax assessment or deficiency.
(f) Parent has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(g) Neither Parent nor Merger Sub is a party to any Tax allocation agreement, Tax sharing agreement, Tax indemnity agreement, or similar agreement or arrangement, other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes.
(h) Neither Parent nor Merger Sub will be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any Tax period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for Tax purposes filed on or prior to the Closing Date; (ii) use of an improper method of accounting for a Tax period ending on or prior to the Closing Date; (iii) “closing agreement” as described in Section 7121 of the Code (or any similar provision of state, local or foreign Law) executed on or prior to the Closing Date; (iv) intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any similar provision of state, local or foreign Law) entered into on or prior to the Closing Date; (v) installment sale or open transaction disposition made on or prior to the Closing Date; (vi) prepaid amount received on or prior to the Closing Date; or (vii) election under Section 108(i) of the Code (or any similar provision of state, local or foreign Law) made on or prior to the Closing Date. Parent has not made any election under Section 965(h) of the Code.
(i) Neither Parent nor Merger Sub has ever been (i) a member of a consolidated, combined or unitary Tax group (other than such a group the common parent of which is Parent) or (ii) a party to any joint venture, partnership, or other arrangement that is treated as a partnership for U.S. federal income Tax purposes. Neither Parent nor Merger Sub has any Liability for any material Taxes of any Person (other than Parent and Merger Sub) under Treasury RegulationsSection 1.1502-6 (or any similar provision of state, local, or foreign Law), or as a transferee or successor.
(j) Neither Parent nor Merger Sub has, since January 1, 2017, distributed stock of another Person, or had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 of the Code or Section 361 of the Code (or any similar provisions of state, local or foreign Law).
A-36
(k) Neither Parent nor Merger Sub (i) is a “controlled foreign corporation” as defined in Section 957 of the Code; (ii) is a “passive foreign investment company” within the meaning of Section 1297 of the Code; (iii) has ever had a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise had an office or fixed place of business in a country other than the country in which it is organized; (iv) is or was a “surrogate foreign corporation” within the meaning of Section 7874(a)(2)(B) or is treated as a U.S. corporation under Section 7874(b) of the Code; or (v) was created or organized in the U.S. such that such entity would be taxable in the U.S. as a domestic entity pursuant to the dual charter provision of Treasury RegulationsSection 301.7701-5(a).
(l) Neither Parent nor Merger Sub has participated in or been a party to a transaction that, as of the date of this Agreement, constitutes a “listed transaction” that is required to be reported to the IRS pursuant to Section 6011 of the Code and applicable Treasury Regulations thereunder.
(m) Neither Parent nor Merger Sub has taken or agreed to take any action or knows of any fact that would reasonably be expected to prevent the Merger from qualifying for the Intended Tax Treatment.
For purposes of thisSection 3.16, each reference to Parent or Merger Sub shall be deemed to include any Person that was liquidated into, merged with, or is otherwise a predecessor to, Parent or Merger Sub, respectively.
3.17 Employee and Labor Matters; Benefit Plans.
(a) Section 3.17(a) of the Parent Disclosure Schedule is a list of all Parent Benefit Plans, including, without limitation, each Parent Benefit Plan that provides for retirement, change in control, stay or retention deferred compensation, incentive compensation, severance or retiree medical or life insurance benefits. “ParentBenefit Plan” means each (i) “employee benefit plan” as defined in Section 3(3) of ERISA and (ii) other pension, retirement, deferred compensation, excess benefit, profit sharing, bonus, incentive, equity or equity-based (other than individual Parent Options made pursuant to the Parent’s standard forms, in which case only representative standard forms of such stock option agreements shall be scheduled), phantom equity, employment, offer letter, consulting, severance,change-of-control, retention, health, life, disability, group insurance, paid-time off, holiday, welfare and fringe benefit plan, program, contract, or arrangement (whether written or unwritten, qualified or nonqualified, funded or unfunded and including any that have been frozen), in any case, maintained, contributed to, or required to be contributed to, by Parent or Parent ERISA Affiliates for the benefit of any current or former employee, director, officer or independent contractor of Parent or under which Parent has any actual or contingent liability (including, without limitation, as to the result of it being treated as a single employer under Code Section 414 with any other person).
(b) As applicable with respect to each Parent Benefit Plan, Parent has made available to the Company, true and complete copies of (i) each Parent Benefit Plan, including all amendments thereto, and in the case of an unwritten Parent Benefit Plan, a written description thereof, (ii) all current trust documents, investment management contracts, custodial agreements, administrative services agreements and insurance and annuity contracts relating thereto, (iii) the current summary plan description and each summary of material modifications thereto, (iv) the most recently filed annual reports with any Governmental Body (e.g.,Form 5500 and all schedules thereto), (v) the most recent IRS determination, opinion or advisory letter, (vi) the most recent summary annual reports, nondiscrimination testing reports, actuarial reports, financial statements and trustee reports, (vii) all records, notices and filings concerning IRS or Department of Labor or other Governmental Body audits or investigations, “prohibited transactions” within the meaning of Section 406 of ERISA or Section 4975 of the Code and (viii) all policies and procedures established to comply with the privacy and security rules of HIPAA.
(c) Each Parent Benefit Plan has been maintained, operated and administered in compliance in all material respects with its terms and any related documents or agreements and the applicable provisions of ERISA, the Code and all other Laws.
A-37
(d) The Parent Benefit Plans which are “employee pension benefit plans” within the meaning of Section 3(2) of ERISA and which are intended to meet the qualification requirements of Section 401(a) of the Code have received determination or opinion letters from the IRS on which they may currently rely to the effect that such plans are qualified under Section 401(a) of the Code and the related trusts are exempt from federal income Taxes under Section 501(a) of the Code, respectively, and to the Knowledge of Parent nothing has occurred that would reasonably be expected to materially adversely affect the qualification of such Parent Benefit Plan or the tax exempt status of the related trust.
(e) Neither Parent nor any Parent ERISA Affiliate maintains, contributes to, is required to contribute to, or has any actual or contingent liability with respect to, (i) any “employee pension benefit plan” (within the meaning of Section 3(2) of ERISA) that is subject to Title IV or Section 302 of ERISA or Section 412 of the Code, (ii) any “multiemployer plan” (within the meaning of Section 3(37) of ERISA), (iii) any “multiple employer plan” (within the meaning of Section 413 of the Code) or (iv) any “multiple employer welfare arrangement” (within the meaning of Section 3(40) of ERISA).
(f) There are no pending audits or investigations by any Governmental Body involving any Parent Benefit Plan, and no pending or, to the Knowledge of Parent, threatened claims (except for individual claims for benefits payable in the normal operation of the Parent Benefit Plans), suits or proceedings involving any Parent Benefit Plan, any fiduciary thereof or service provider thereto, in any case, except as would not be reasonably expected to result in material liability to Parent. All contributions and premium payments required to have been made under any of the Parent Benefit Plans or by applicable Law (without regard to any waivers granted under Section 412 of the Code), have been timely made in all material respects and neither Parent nor any Parent ERISA Affiliate has any material liability for any unpaid contributions with respect to any Parent Benefit Plan.
(g) Neither Parent or any Parent ERISA Affiliates, nor to the Knowledge of Parent, any fiduciary, trustee or administrator of any Parent Benefit Plan, has engaged in, or in connection with the transactions contemplated by this Agreement will engage in, any transaction with respect to any Parent Benefit Plan which would subject any such Parent Benefit Plan, Parent or Parent ERISA Affiliates to a material Tax, material penalty or material liability for a “prohibited transaction” under Section 406 of ERISA or Section 4975 of the Code.
(h) No Parent Benefit Plan provides death, medical, dental, vision, life insurance or other welfare benefits beyond termination of service or retirement other than coverage mandated by Law and neither Parent nor any Parent ERISA Affiliates has made a written or oral representation promising the same.
(i) Neither the execution of, nor the performance of the transactions contemplated by, this Agreement will either alone or in connection with any other event(s) (i) result in any payment becoming due to any current or former employee, director, officer, or independent contractor of Parent, (ii) increase any amount of compensation or benefits otherwise payable under any Parent Benefit Plan, (iii) result in the acceleration of the time of payment, funding or vesting of any benefits under any Parent Benefit Plan, (iv) require any contribution or payment to fund any obligation under any Parent Benefit Plan or (v) limit the right to merge, amend or terminate any Parent Benefit Plan.
(j) Neither the execution of, nor the consummation of the transactions contemplated by this Agreement (either alone or when combined with the occurrence of any other event, including without limitation, a termination of employment) will result in the receipt or retention by any person who is a “disqualified individual” (within the meaning of Code Section 280G) with respect to Parent of any payment or benefit that is or could be characterized as a “parachute payment” (within the meaning of Code Section 280G), determined without regard to the application of Code Section 280G(b)(5).
(k) The exercise price of each Parent Option is not, never has been, less than the fair market value of one share of Parent Common Stock as of the grant date of such Parent Option.
A-38
(l) Each Parent Benefit Plan providing for deferred compensation that constitutes a “nonqualified deferred compensation plan” (as defined in Section 409A(d)(1) of the Code and the regulations promulgated thereunder) is, and has been, established, administered and maintained in compliance with the requirements of Section 409A of the Code and the regulations promulgated thereunder in all material respects.
(m) No current or former employee, officer, director or independent contractor of Parent has any “gross up” agreements with the Parent or other assurance of reimbursement by the Parent for any Taxes imposed under Code Section 409A or Code Section 4999.
(n) Parent has provided to the Company a true and correct list, as of the date of this Agreement, containing the names of all full-time, part-time or temporary employees and independent contractors (and indication as such), and, as applicable: (i) the annual dollar amount of all compensation (including wages, salary or fees, commissions, director’s fees, fringe benefits, bonuses, profit sharing payments, and other payments or benefits of any type) payable to each person; (ii), dates of employment or service; (iii) title; (iv) any eligibility to receive severance, notice of termination, retention payment, change of control payment, or other similar compensation; (v) visa status, if applicable; and (vi) with respect to employees, a designation of whether they are classified as exempt ornon-exempt for purposes of FLSA and any similar state law.
(o) Parent is not and never has been a party to, bound by, or has a duty to bargain under, any collective bargaining agreement or other Contract with a labor union, labor organization, or similar Person representing any of its employees, and there is no labor union, labor organization, or similar Person representing or, to the Knowledge of Parent, purporting to represent or seeking to represent any employees of Parent, including through the filing of a petition for representation election. There is not and has not been in the past three years, nor is there or has there been in the past three years any threat of, any strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, or any similar activity or dispute, or, to the Knowledge of Parent, any union organizing activity, against Parent or any of its Subsidiaries. No event has occurred, and no condition or circumstance exists, that might directly or indirectly be likely to give rise to or provide a basis for the commencement of any such strike, slowdown, work stoppage, lockout, union election petition, demand for recognition, any similar activity or dispute, or, to the Knowledge of Parent, any union organizing activity.
(p) Parent is, and since January 1, 2016 has been, in material compliance with all applicable Laws respecting labor, employment, employment practices, and terms and conditions of employment, including worker classification, discrimination, harassment and retaliation, equal employment opportunities, fair employment practices, meal and rest periods, immigration, employee safety and health, payment of wages (including overtime wages), unemployment and workers’ compensation, leaves of absence, and hours of work. Except as would not be reasonably likely to result in a material liability to Parent, with respect to employees of Parent, Parent, since January 1, 2016: (i) has withheld and reported all amounts required by Law or by agreement to be withheld and reported with respect to wages, salaries and other payments, benefits, or compensation to employees, (ii) is not liable for any arrears of wages (including overtime wages), severance pay or any Taxes or any penalty for failure to comply with any of the foregoing, and (iii) is not liable for any payment to any trust or other fund governed by or maintained by or on behalf of any Governmental Body, with respect to unemployment compensation benefits, disability, social security or other benefits or obligations for employees (other than routine payments to be made in the Ordinary Course of Business). There are no actions, suits, claims, charges, lawsuits, investigations, audits or administrative matters pending or, to the Knowledge of Parent, threatened or reasonably anticipated against Parent relating to any employee, applicant for employment, consultant, employment agreement or Parent Benefit Plan (other than routine claims for benefits).
(q) Except as would not be reasonably likely to result in a material liability to Parent, with respect to each individual who currently renders services to Parent, Parent has accurately classified each such individual as an employee, independent contractor, or otherwise under all applicable Laws and, for each individual classified as an employee, Parent has accurately classified him or her as exempt ornon-exempt under
A-39
all applicable Laws. Parent has no material liability with respect to any misclassification of: (i) any Person as an independent contractor rather than as an employee, (ii) any employee leased from another employer, or (iii) any employee currently or formerly classified as exempt under all applicable Laws.
(r) Within the preceding five (years, Parent has not implemented any “plant closing” or “mass layoff” of employees that would reasonably be expected to require notification under the WARN Act or any similar state or local Law, no such “plant closing” or “mass layoff” will be implemented before the Closing Date without advance notification to and approval of Parent, and there has been no “employment loss” as defined by the WARN Act within the 90 days prior to the Closing Date.
(s) There is no Legal Proceeding, claim, unfair labor practice charge or complaint, labor dispute or grievance pending or, to the Knowledge of Parent, threatened against Parent relating to labor, employment, employment practices, or terms and conditions of employment.
(t) No Parent Benefit Plan is maintained outside the United States.
(u) Since September 30, 2018 through the date of this Agreement, no employee of Parent has terminated his or her employment for any reason.
3.18 Environmental Matters.Parent is and since January 1, 2013 has complied with all applicable Environmental Laws, which compliance includes the possession by Parent of all permits and other Governmental Authorizations required under applicable Environmental Laws and compliance with the terms and conditions thereof, except for any failure to be in such compliance that, either individually or in the aggregate, would not reasonably be expected to be material to Parent or its business. Parent has not received since January 1, 2013 (or prior to that time, which is pending and unresolved), any written notice or other communication (in writing or otherwise), whether from a Governmental Body or other Person, that alleges that Parent is not in compliance with or has liability pursuant to any Environmental Law and, to the Knowledge of Parent, there are no circumstances that would reasonably be expected to prevent or interfere with Parent’s compliance in any material respects with any Environmental Law, except where such failure to comply would not reasonably be expected to be material to Parent or its business. No current or (during the time a prior property was leased or controlled by Parent) prior property leased or controlled by Parent has had a release of or exposure to Hazardous Materials in material violation of or as would reasonably be expected to result in any material liability of Parent pursuant to Environmental Law. No consent, approval or Governmental Authorization of or registration or filing with any Governmental Body is required by Environmental Laws in connection with the execution and delivery of this Agreement or the consummation of Contemplated Transactions. Prior to the date hereof, Parent has provided or otherwise made available to the Company true and correct copies of all material environmental reports, assessments, studies and audits in the possession or control of Parent with respect to any property leased or controlled by Parent or any business operated by it.
3.19 Transactions with Affiliates. Except as set forth in the Parent SEC Documents filed prior to the date of this Agreement, since the date of Parent’s last proxy statement filed in 2017 with the SEC, no event has occurred that would be required to be reported by Parent pursuant to Item 404 of RegulationS-K. Section 3.19 of the Parent Disclosure Schedule identifies each Person who is (or who may be deemed to be) an Affiliate of Parent as of the date of this Agreement.
3.20 Insurance. Parent has delivered or made available to the Company accurate and complete copies of all material insurance policies and all material self-insurance programs and arrangements relating to the business, assets, liabilities and operations of Parent. Each of such insurance policies is in full force and effect and Parent is in compliance in all material respects with the terms thereof. Other than customary end of policy notifications from insurance carriers, since January 1, 2016, Parent has not received any notice or other communication regarding any actual or possible: (a) cancellation or invalidation of any insurance policy; or (b) refusal or denial of any coverage, reservation of rights or rejection of any material claim under any insurance
A-40
policy. Parent has provided timely written notice to the appropriate insurance carrier(s) of each Legal Proceeding that is currently pending against Parent for which Parent has insurance coverage, and no such carrier has issued a denial of coverage or a reservation of rights with respect to any such Legal Proceeding, or informed Parent of its intent to do so.
3.21 No Financial Advisors. Except as set forth onSection 3.21 of the Parent Disclosure Schedule, no broker, finder or investment banker is entitled to any brokerage fee, finder’s fee, opinion fee, success fee, transaction fee or other fee or commission in connection with the Contemplated Transactions based upon arrangements made by or on behalf of Parent.
3.22 Anti-Bribery. Neither Parent nor any of its directors, officers, employees or, to Parent’s Knowledge, agents or any other Person acting on its behalf has directly or indirectly made any bribes, rebates, payoffs, influence payments, kickbacks, illegal payments, illegal political contributions, or other payments, in the form of cash, gifts, or otherwise, or taken any other action, in violation of Anti-Bribery Laws. Parent is not or has not been the subject of any investigation or inquiry by any Governmental Body with respect to potential violations of Anti-Bribery Laws.
3.23 Valid Issuance. The Parent Common Stock to be issued in the Merger will, when issued in accordance with the provisions of this Agreement, be validly issued, fully paid and nonassessable.
3.24 Opinion of Financial Advisor. The Parent Board has received an opinion of Aquilo Partners, L.P. to the effect that, as of the date of this Agreement and subject to the assumptions, qualifications, limitations and other matters set forth therein that the Consideration is fair, from a financial point of view, to the holders of Parent Common Stock. It is agreed and understood that such opinion is for the benefit of the Parent Board and may not be relied upon by the Company.
3.25 Disclaimer of Other Representations or Warranties.
(a) Except as previously set forth in thisSection 3 or in any certificate delivered by Parent or Merger Sub to the Company pursuant to this Agreement, neither Parent nor Merger Sub makes any representation or warranty, express or implied, at law or in equity, with respect to it or any of its assets, liabilities or operations, and any such other representations or warranties are hereby expressly disclaimed.
(b) Each of Parent and Merger Sub acknowledges and agrees that, except for the representations and warranties of the Company set forth inSection 2, none of the Company or any of their respective Representatives is relying on any other representation or warranty of the Company or any other Person made outside ofSection 2, including regarding the accuracy or completeness of any such other representations or warranties or the omission of any material information, whether express or implied, in each case, with respect to the Contemplated Transactions.
Section 4. CERTAIN COVENANTS OF THE PARTIES
4.1 Operation of Parent’s Business.
(a) Except as set forth onSection 4.1(a) of the Parent Disclosure Schedule, as expressly permitted by this Agreement, as required by applicable Law or unless the Company shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), during the period commencing on the date of this Agreement and continuing until the earlier to occur of the termination of this Agreement pursuant toSection 9 and the Effective Time (the “Pre-Closing Period”): each of Parent and Merger Sub shall conduct its business and operations in the Ordinary Course of Business and in compliance in all material respects with all applicable Laws and the requirements of all Contracts that constitute Parent Material Contracts.
A-41
(b) Except (i) as expressly permitted by this Agreement, (ii) as set forth inSection 4.1(b) of the Parent Disclosure Schedule, (iii) as required by applicable Law or (iv) with the prior written consent of the Company (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during thePre-Closing Period, Parent shall not, nor shall its cause or permit Merger Sub to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except in connection with the payment of withholding Taxes incurred upon the exercise, settlement or vesting of any award granted under the Parent Stock Plans in accordance with the terms of such award in effect on the date of this Agreement);
(ii) sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of Parent (except for Parent Common Stock issued upon the valid exercise of outstanding Parent Options or Parent Warrants); (B) any option, warrant or right to acquire any capital stock or any other security; or (C) any instrument convertible into or exchangeable for any capital stock or other security of Parent;
(iii) except as required to give effect to anything in contemplation of the Closing, amend any of its Organizational Documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(v) (A) lend money to any Person, (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others, or (D) make any capital expenditure or commitment in excess of the budgeted capital expenditure and commitment amounts set forth in the Parent operating budget delivered to the Company concurrently with the execution of this Agreement (the “Parent Budget”);
(vi) other than as required by applicable Law or the terms of any Parent Benefit Plan as in effect on the date of this Agreement: (A) adopt, terminate, establish or enter into any Parent Benefit Plan; (B) cause or permit any Parent Benefit Plan to be amended in any material respect (other than approval of the 2019 Plan pursuant toSection 5.20); (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees; (D) increase the severance, retention or change of control benefits offered to any current or former or new employees, directors or consultants; (E) hire or retain any officer, employee or consultant; or (F) terminate or give notice of termination to any officer or employee, other than any termination for cause;
(vii) recognize any labor union, labor organization, or similar Person except as otherwise required by law and after advance notice to the Company;
(viii) enter into any transaction other than in the Ordinary Course of Business;
(ix) enter into any transaction with respect to the SARD Compound or the SARM Compound (each, as defined in the CVR Agreement);
(x) acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any Encumbrance with respect to such assets or properties;
(xi) sell, assign, transfer, license, sublicense or otherwise dispose of any material Parent IP (other than pursuant tonon-exclusive licenses in the Ordinary Course of Business);
(xii) make, change or revoke any material Tax election, fail to pay any income or other material Tax as such Tax becomes due and payable, file any amendment making any material
A-42
change to any Tax Return, settle or compromise any income or other material Tax liability, enter into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than six months), or adopt or change any material accounting method in respect of Taxes;
(xiii) enter into, materially amend or terminate any Parent Material Contract;
(xiv) except as otherwise set forth in the Parent Budget (and other than incurrence or payment of Parent Transaction Expenses up to an aggregate of $100,000 in excess of the amount budgeted for the aggregate Parent Transaction Expenses in the Parent Budget), make any expenditures, incur any Liabilities or discharge or satisfy any Liabilities, in each case, in amounts that exceed the aggregate amount of the Parent Budget;
(xv) other than as required by Law or GAAP, take any action to change accounting policies or procedures;
(xvi) initiate or settle any Legal Proceeding; or
(xvii) agree, resolve or commit to do any of the foregoing.
Nothing contained in this Agreement shall give the Company, directly or indirectly, the right to control or direct the operations of Parent prior to the Effective Time. Prior to the Effective Time, Parent shall exercise, consistent with the terms and conditions of this Agreement, complete unilateral control and supervision over its business operations.
4.2 Operation of the Company’s Business.
(a) Except as set forth onSection 4.2(a) of the Company Disclosure Schedule, as expressly permitted by this Agreement, as required by applicable Law or unless Parent shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), during thePre-Closing Period: each of the Company and its Subsidiaries shall conduct its business and operations in the Ordinary Course of Business and in compliance in all material respects with all applicable Laws and the requirements of all Contracts that constitute Company Material Contracts.
(b) Except (i) as expressly permitted by this Agreement, (ii) as set forth inSection 4.2(b) of the Company Disclosure Schedule, (iii) as required by applicable Law or (iv) with the prior written consent of Parent (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during thePre-Closing Period, the Company shall not, nor shall it cause or permit any of its Subsidiaries to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except for shares of Company Common Stock from terminated employees, directors or consultants of the Company);
(ii) sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing with respect to: (A) any capital stock or other security of the Company or any of its Subsidiaries (except for shares of outstanding Company Common Stock issued upon the valid exercise of Company Options or Company Warrants); (B) any option, warrant, right to acquire any capital stock or any other security; or (C) any other instrument convertible into or exchangeable for any capital stock or other security of the Company or any of its Subsidiaries;
(iii) except as required to give effect to anything in contemplation of the Closing, amend any of its or its Subsidiaries’ Organizational Documents, or effect or be a party to any merger,
A-43
consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the Contemplated Transactions;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Entity or enter into a joint venture with any other Entity;
(v) (A) lend money to any Person, (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others, or (D) make any capital expenditure or commitment in excess of $500,000;
(vi) other than as required by applicable Law or the terms of any Company Benefit Plan as in effect on the date of this Agreement: (A) adopt, terminate, establish or enter into any Company Benefit Plan; (B) cause or permit any Company Benefit Plan to be amended in any material respect; (C) pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees; (D) increase the severance or change of control benefits offered to any current or new employees, directors or consultants or (E) terminate or give notice of termination to any (x) officer or (y) employee whose annual base salary is or is expected to be more than $125,000 per year, other than any termination for cause;
(vii) recognize any labor union, labor organization, or similar Person, except as otherwise required by law and after advance notice to the Parent;
(viii) enter into any transaction other than in the Ordinary Course of Business;
(ix) acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any Encumbrance with respect to such assets or properties;
(x) sell, assign, transfer, license, sublicense or otherwise dispose of any material Company IP (other than pursuant tonon-exclusive licenses in the Ordinary Course of Business);
(xi) make, change or revoke any material Tax election, fail to pay any income or other material Tax as such Tax becomes due and payable, file any amendment making any material change to any Tax Return, settle or compromise any income or other material Tax liability, enter into any Tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the Ordinary Course of Business the principal subject matter of which is not Taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than pursuant to an extension of time to file any Tax Return granted in the Ordinary Course of Business of not more than six months), or adopt or change any material accounting method in respect of Taxes;
(xii) enter into, materially amend or terminate any Company Material Contract;
(xiii) other than incurrence or payment of any Company Transaction Expenses, make any expenditures, incur any Liabilities or discharge or satisfy any Liabilities, in each case, in amounts that exceed $500,000 in the aggregate;
(xiv) other than as required by Law or GAAP, take any action to change accounting policies or procedures;
(xv) initiate or settle any Legal Proceeding; or
(xvi) agree, resolve or commit to do any of the foregoing.
(c) Nothing contained in this Agreement shall give Parent, directly or indirectly, the right to control or direct the operations of the Company prior to the Effective Time. Prior to the Effective Time, the Company shall exercise, consistent with the terms and conditions of this Agreement, complete unilateral control and supervision over its business operations.
A-44
(a) Subject to the terms of the Confidentiality Agreement, which the Parties agree will continue in full force following the date of this Agreement, during thePre-Closing Period, upon reasonable notice, Parent, on the one hand, and the Company, on the other hand, shall and shall use commercially reasonable efforts to cause such Party’s Representatives to: (i) provide the other Party and such other Party’s Representatives with reasonable access during normal business hours to such Party’s Representatives, personnel, property and assets and to all existing books, records, Tax Returns, work papers and other documents and information relating to such Party and its Subsidiaries; (ii) provide the other Party and such other Party’s Representatives with such copies of the existing books, records, Tax Returns, work papers, product data, and other documents and information relating to such Party and its Subsidiaries, and with such additional financial, operating and other data and information regarding such Party and its Subsidiaries as the other Party may reasonably request; (iii) permit the other Party’s officers and other employees to meet, upon reasonable notice and during normal business hours, with the chief financial officer and other officers and managers of such Party responsible for such Party’s financial statements and the internal controls of such Party to discuss such matters as the other Party may deem necessary or appropriate and; (iv) make available to the other Party copies of unaudited financial statements, material operating and financial reports prepared for senior management or the board of directors of such Party, and any material notice, report or other document filed with or sent to or received from any Governmental Body in connection with the Contemplated Transactions. Any investigation conducted by either Parent or the Company pursuant to thisSection 4.3 shall be conducted in such manner as not to interfere unreasonably with the conduct of the business of the other Party.
(b) Notwithstanding the foregoing, any Party may restrict the foregoing access to the extent that any Law applicable to such Party requires such Party to restrict or prohibit access to any such properties or information.
(a) Parent agrees that, during thePre-Closing Period, it shall not, and shall not authorize any of its Representatives to, directly or indirectly: (i) solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of any Acquisition Proposal or Acquisition Inquiry or take any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry; (ii) furnish anynon-public information regarding Parent to any Person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry; (iii) engage in discussions or negotiations with any Person with respect to any Acquisition Proposal or Acquisition Inquiry; (iv) approve, endorse or recommend any Acquisition Proposal (subject toSection 5.3); (v) execute or enter into any letter of intent or any Contract contemplating or otherwise relating to any Acquisition Transaction (other than a confidentiality agreement permitted under thisSection 4.4(a)); or (vi) publicly propose to do any of the foregoing; provided,however, that, notwithstanding anything contained in thisSection 4.4 and subject to compliance with thisSection 4.4, prior to obtaining the Required Parent Stockholder Vote, Parent may furnishnon-public information regarding Parent to, and enter into discussions or negotiations with, any Person in response to abona fide Acquisition Proposal by such Person, which the Parent Board determines in good faith, after consultation with Parent’s outside financial advisors and outside legal counsel, constitutes, or is reasonably likely to result in, a Superior Offer (and is not withdrawn) if: (A) neither Parent nor any of its Representatives shall have breached thisSection 4.4 in any material respect, (B) the Parent Board concludes in good faith based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to be inconsistent with the fiduciary duties of the Parent Board under applicable Law; (C) at least two (2) Business Days prior to furnishing such nonpublic confidential information to, or entering into discussions with, such Person, Parent gives the Company written notice of the identity of such Person and of Parent’s intention to furnish nonpublic information to, or enter into discussions with, such Person; (D) Parent receives from such Person an executed confidentiality agreement containing provisions, in the aggregate, at least as favorable to Parent as those contained in the Confidentiality Agreement; and (E) at least two (2) Business Days prior to furnishing any such nonpublic information to such Person, Parent furnishes such
A-45
nonpublic information to the Company (to the extent such information has not been previously furnished by Parent to the Company). Without limiting the generality of the foregoing, Parent acknowledges and agrees that, in the event any Representative of Parent (whether or not such Representative is purporting to act on behalf of Parent) takes any action that, if taken by Parent, would constitute a breach of thisSection 4.4, the taking of such action by such Representative shall be deemed to constitute a breach of thisSection 4.4 by Parent for purposes of this Agreement.
(b) If Parent or any Representative of Parent receives an Acquisition Proposal or Acquisition Inquiry at any time during thePre-Closing Period, then Parent shall promptly (and in no event later than twenty-four (24) hours after Parent becomes aware of such Acquisition Proposal or Acquisition Inquiry) advise the Company orally and in writing of such Acquisition Proposal or Acquisition Inquiry (including the identity of the Person making or submitting such Acquisition Proposal or Acquisition Inquiry, and the material terms thereof). Parent shall keep the Company reasonably informed with respect to the status and material terms of any such Acquisition Proposal or Acquisition Inquiry and any material modification or proposed material modification thereto.
(c) Parent shall immediately cease and cause to be terminated any existing discussions, negotiations and communications with any Person that relate to any Acquisition Proposal or Acquisition Inquiry as of the date of this Agreement and request the destruction or return of any nonpublic information of Parent provided to such Person.
(a) The Company agrees that, during thePre-Closing Period, neither it nor any of its Subsidiaries shall, nor shall it or any of its Subsidiaries authorize any of its Representatives to, directly or indirectly: (i) solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of any Acquisition Proposal or Acquisition Inquiry or take any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry; (ii) furnish anynon-public information regarding the Company or any of its Subsidiaries to any Person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry; (iii) engage in discussions or negotiations with any Person with respect to any Acquisition Proposal or Acquisition Inquiry; (iv) approve, endorse or recommend any Acquisition Proposal; (v) execute or enter into any letter of intent or any Contract contemplating or otherwise relating to any Acquisition Transaction; or (vi) publicly propose to do any of the foregoing provided,however, that, notwithstanding anything contained in thisSection 4.5 and subject to compliance with thisSection 4.5, prior to obtaining the Required Company Stockholder Vote, the Company may furnishnon-public information regarding the Company to, and enter into discussions or negotiations with, any Person in response to abona fide Acquisition Proposal by such Person, which the Company Board determines in good faith, after consultation with the Company’s outside financial advisors and outside legal counsel, constitutes, or is reasonably likely to result in, a Superior Offer (and is not withdrawn) if: (A) neither the Company nor any of its Representatives shall have breached thisSection 4.5 in any material respect, (B) the Company Board concludes in good faith based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to be inconsistent with the fiduciary duties of the Company Board under applicable Law; (C) at least two (2) Business Days prior to furnishing such nonpublic confidential information to, or entering into discussions with, such Person, the Company gives Parent written notice of the identity of such Person and of the Company’s intention to furnish nonpublic information to, or enter into discussions with, such Person; (D) the Company receives from such Person an executed confidentiality agreement containing provisions, in the aggregate, at least as favorable to the Company as those contained in the Confidentiality Agreement; and (E) at least two (2) Business Days prior to furnishing any such nonpublic information to such Person, the Company furnishes such nonpublic information to Parent (to the extent such information has not been previously furnished by the Company to Parent). Without limiting the generality of the foregoing, the Company acknowledges and agrees that, in the event any Representative of the Company (whether or not such Representative is purporting to act on behalf of the Company) takes any action that, if taken by the Company, would constitute a breach of thisSection 4.5, the
A-46
taking of such action by such Representative shall be deemed to constitute a breach of thisSection 4.5 by the Company for purposes of this Agreement.
(b) If the Company or any Representative of the Company receives an Acquisition Proposal or Acquisition Inquiry at any time during thePre-Closing Period, then the Company shall promptly (and in no event later than twenty-four (24) hours after the Company becomes aware of such Acquisition Proposal or Acquisition Inquiry) advise Parent orally and in writing of such Acquisition Proposal or Acquisition Inquiry (including the identity of the Person making or submitting such Acquisition Proposal or Acquisition Inquiry, and the material terms thereof). The Company shall keep Parent reasonably informed with respect to the status and material terms of any such Acquisition Proposal or Acquisition Inquiry and any material modification or proposed material modification thereto.
(c) The Company shall immediately cease and cause to be terminated any existing discussions, negotiations and communications with any Person that relate to any Acquisition Proposal or Acquisition Inquiry as of the date of this Agreement and request the destruction or return of any nonpublic information of the Company or any of its Subsidiaries provided to such Person.
4.6 Notification of Certain Matters.
(a) During thePre-Closing Period, the Company shall promptly notify Parent (and, if in writing, furnish copies of) if any of the following occurs: (i) any notice or other communication is received from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (ii) any Legal Proceeding against or involving or otherwise affecting the Company or its Subsidiaries is commenced, or, to the Knowledge of the Company, threatened against the Company or its Subsidiaries or, to the Knowledge of the Company, any director or officer of the Company or its Subsidiaries; (iii) the Company becomes aware of any inaccuracy in any representation or warranty made by it in this Agreement; or (iv) the failure of the Company to comply with any covenant or obligation of the Company; in the case of (iii) and (iv) that could reasonably be expected to make the timely satisfaction of any of the conditions set forth inSections 6 or7, as applicable, impossible or materially less likely. No notification given to Parent pursuant to thisSection 4.6(a) shall change, limit or otherwise affect any of the representations, warranties, covenants or obligations of the Company or any of its Subsidiaries contained in this Agreement or the Company Disclosure Schedule for purposes ofSections 6 and7, as applicable.
(b) During thePre-Closing Period, Parent shall promptly notify the Company (and, if in writing, furnish copies of) if any of the following occurs: (i) any notice or other communication is received from any Person alleging that the Consent of such Person is or may be required in connection with any of the Contemplated Transactions; (ii) any Legal Proceeding against or involving or otherwise affecting Parent is commenced, or, to the Knowledge of Parent, threatened against Parent or, to the Knowledge of Parent, any director or officer of Parent; (iii) Parent becomes aware of any inaccuracy in any representation or warranty made by it in this Agreement; or (iv) the failure of Parent to comply with any covenant or obligation of Parent or Merger Sub; in the case of (iii) and (iv) that could reasonably be expected to make the timely satisfaction of any of the conditions set forth inSections 6 or8, as applicable, impossible or materially less likely. No notification given to the Company pursuant to thisSection 4.6(b) shall change, limit or otherwise affect any of the representations, warranties, covenants or obligations of Parent contained in this Agreement or the Parent Disclosure Schedule for purposes ofSections 6 and8, as applicable.
Section 5. ADDITIONAL AGREEMENTS OF THE PARTIES
5.1 Registration Statement; Proxy Statement.
(a) As promptly as practicable after the date of this Agreement (but in no event later than 30 days following the date of this Agreement), the Company shall prepare, and Parent shall cause to be filed with
A-47
the SEC, the Registration Statement, in which the Proxy Statement will be included as a prospectus. Parent covenants and agrees that the information provided by Parent or its Subsidiaries to the Company for inclusion in the Proxy Statement, including any pro forma financial statements included therein (and the letter to stockholders, notice of meeting and form of proxy included therewith), will not, at the time that the Proxy Statement or any amendment or supplement thereto is filed with the SEC or is first mailed to the Parent stockholders contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. The Company covenants and agrees that the information provided by the Company or its Subsidiaries to Parent for inclusion in the Proxy Statement (including the Company Financials) will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make such information not misleading. Notwithstanding the foregoing, (i) Parent makes no covenant, representation or warranty with respect to statements made in the Proxy Statement (and the letter to stockholders, notice of meeting and form of proxy included therewith), if any, based on information provided by the Company or its Subsidiaries or any of their Representatives specifically for inclusion therein and (ii) the Company makes no covenant, representation or warranty with respect to statements made in the Proxy Statement (and the letter to stockholders, notice of meeting and form of proxy included therewith), if any, other than with respect to the information provided by the Company or its Subsidiaries or any of their Representatives for inclusion therein. Parent and its legal counsel shall be given reasonable opportunity to review and comment on the Proxy Statement, including all amendments and supplements thereto, prior to the filing thereof with the SEC, and on the response to any comments of the SEC on the Proxy Statement, prior to the filing thereof with the SEC. Each of the Parties shall use commercially reasonable efforts to cause the Registration Statement and the Proxy Statement to comply with the applicable rules and regulations promulgated by the SEC, to respond promptly to any comments of the SEC or its staff and to have the Registration Statement declared effective under the Securities Act as promptly as practicable after it is filed with the SEC. Parent shall use commercially reasonable efforts to cause the Proxy Statement to be mailed to Parent’s stockholders as promptly as practicable after the Registration Statement is declared effective under the Securities Act. Each Party shall promptly furnish to the other Party all information concerning such Party and such Party’s Affiliates and such Party’s stockholders that may be required or reasonably requested in connection with any action contemplated by thisSection 5.1. If Parent, Merger Sub or the Company become aware of any event or information that, pursuant to the Securities Act or the Exchange Act, should be disclosed in an amendment or supplement to the Registration Statement or Proxy Statement, as the case may be, then such Party, as the case may be, shall promptly inform the other Parties thereof and shall cooperate with such other Parties in filing such amendment or supplement with the SEC and, if appropriate, in mailing such amendment or supplement to the Parent stockholders.
(b) Prior to the Effective Time, Parent shall use commercially reasonable efforts to obtain all regulatory approvals needed to ensure that the Parent Common Stock to be issued in the Merger (to the extent required) shall be registered or qualified or exempt from registration or qualification under the securities law of every jurisdiction of the United States in which any registered holder of Company Capital Stock has an address of record on the applicable record date for determining the holders of Company Capital Stock entitled to notice and to vote pursuant to the Company Stockholder Written Consent.
(c) Parent shall reasonably cooperate with the Company and provide, and require its Representatives to provide, the Company and its Representatives, with all true, correct and complete information regarding Parent or its Subsidiaries that is required by Law to be included in the Registration Statement or reasonably requested by the Company to be included in the Registration Statement. The Company will use commercially reasonable efforts to cause to be delivered to Parent a consent letter of the Company’s independent accounting firm, dated no more than two Business Days before the date on which the Registration Statement becomes effective (and reasonably satisfactory in form and substance to Parent), that is customary in scope and substance for consent letters delivered by independent public accountants in connection with registration statements similar to the Registration Statement.
A-48
(d) For the avoidance of doubt, the Company shall use commercially reasonable efforts to undertake, or shall cause its Representatives to undertake, the actions contemplated in the definition of “Combined Transaction Expenses”.
5.2 Company Information Statement; Stockholder Written Consent.
(a) Promptly after the Registration Statement shall have been declared effective under the Securities Act, and in any event no later than three Business Days thereafter, the Company shall prepare, with the cooperation of Parent, and commence mailing to its stockholders an information statement (the “Information Statement”) to solicit the Company Stockholder Consent evidencing the Required Company Stockholder Vote for purposes of (within 10 Business Days after the Registration Statement shall have been declared effective) (i) adopting and approving this Agreement and the Contemplated Transactions, (ii) acknowledging that the approval given thereby is irrevocable and that such stockholder is aware of its rights to demand appraisal for its shares pursuant to Section 262 of the DGCL, a true and correct copy of which will be attached thereto, and that such stockholder has received and read a copy of Section 262 of the DGCL, (iii) acknowledging that by its approval of the Merger it is not entitled to appraisal rights with respect to its shares in connection with the Merger and thereby waives any rights to receive payment of the fair value of its capital stock under the DGCL, and (iv) the Preferred Stock Conversion (collectively, the “Company Stockholder Matters”). Under no circumstances shall the Company assert that any other approval or consent is necessary by its stockholders to approve the Company Stockholder Matters. All materials (including any amendments thereto) submitted to the stockholders of the Company in accordance with thisSection 5.2(a) shall be subject to Parent’s advance review and reasonable approval.
(b) The Company covenants and agrees that the Information Statement, including any pro forma financial statements included therein (and the letter to stockholders and form of Company Stockholder Written Consent included therewith), will not, at the time that the Information Statement or any amendment or supplement thereto is first mailed to the stockholders of the Company, at the time of receipt of the Required Company Stockholder Vote and at the Effective Time, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. Notwithstanding the foregoing, the Company makes no covenant, representation or warranty with respect to statements made in the Information Statement (and the letter to the stockholders and form of Company Stockholder Written Consent included therewith), if any, based on information furnished in writing by Parent specifically for inclusion therein. Each of the Parties shall use commercially reasonable efforts to cause the Information Statement to comply with the applicable rules and regulations promulgated by the SEC in all material respects.
(c) Promptly following receipt of the Required Company Stockholder Vote, the Company shall prepare and mail a notice (the “Stockholder Notice”) to every stockholder of the Company that did not execute the Company Stockholder Written Consent. The Stockholder Notice shall (i) be a statement to the effect that the Company Board determined that the Merger is advisable in accordance with Section 251(b) of the DGCL and in the best interests of the stockholders of the Company and approved and adopted this Agreement, the Merger and the other Contemplated Transactions, (ii) provide the stockholders of the Company to whom it is sent with notice of the actions taken in the Company Stockholder Written Consent, including the adoption and approval of this Agreement, the Merger and the other Contemplated Transactions in accordance with Section 228(e) of the DGCL and the certificate of incorporation and bylaws of the Company and (iii) include a description of the appraisal rights of the Company’s stockholders available under the DGCL, along with such other information as is required thereunder and pursuant to applicable Law. All materials (including any amendments thereto) submitted to the stockholders of the Company in accordance with thisSection 5.2(c) shall be subject to Parent’s advance review and reasonable approval.
(d) The Company agrees that: (i) the Company Board shall recommend that the Company’s stockholders vote to approve the Company Stockholder Matters and shall use reasonable best efforts to solicit
A-49
such approval from each of the Company stockholders necessary to deliver the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote within the time set forth inSection 5.2(a) (the recommendation of the Company Board that the Company’s stockholders vote to adopt and approve this Agreement being referred to as the “Company Board Recommendation”); and (ii) the Company Board Recommendation shall not be withdrawn or modified (and the Company Board shall not publicly propose to withdraw or modify the Company Board Recommendation) in a manner adverse to Parent, and no resolution by the Company Board or any committee thereof to withdraw or modify the Company Board Recommendation in a manner adverse to Parent or to adopt, approve or recommend (or publicly propose to adopt, approve or recommend) any Acquisition Proposal shall be adopted or proposed (the actions set forth in the foregoing clause (ii), collectively, a “CompanyBoard Adverse Recommendation Change”).
(e) Notwithstanding anything to the contrary contained in this Agreement, if at any time prior to the approval of Company Stockholder Matters by the Required Company Stockholder Vote:
(i) if Company has received a written Acquisition Proposal (which Acquisition Proposal did not arise out of a material breach ofSection 4.5) from any Person that has not been withdrawn and after consultation with outside legal counsel, the Company Board shall have determined, in good faith, that such Acquisition Proposal is a Superior Offer, the Company Board may make a Company Board Adverse Recommendation Change, if and only if: (A) the Company Board determines in good faith, after consultation with Company’s outside legal counsel, that the failure to do so could be inconsistent with the fiduciary duties of the Company Board to the Company’s stockholders under applicable Law; (B) Company shall have given the Parent prior written notice of its intention to consider making a Company Board Adverse Recommendation Change or terminate this Agreement pursuant toSection 9.1(g) at least four Business Days prior to making any such Company Board Adverse Recommendation Change or termination (a “Company Determination Notice”) (which notice shall not constitute a Company Board Adverse Recommendation Change); and (C) (1) the Company shall have provided to Parent a summary of the material terms and conditions of the Acquisition Proposal in accordance withSection 4.5(b), (2) the Company shall have given Parent the four Business Days after the Company Determination Notice to propose revisions to the terms of this Agreement or make another proposal and shall have made its Representatives reasonably available to negotiate in good faith with Parent (to the extent Parent desires to negotiate) with respect to such proposed revisions or other proposal, if any, and (3) after considering the results of any such negotiations and giving effect to the proposals made by Parent, if any, after consultation with outside legal counsel, the Company Board shall have determined, in good faith, that such Acquisition Proposal is a Superior Offer and that the failure to make the Company Board Adverse Recommendation Change or terminate this Agreement pursuant toSection 9.1(g) could be inconsistent with the fiduciary duties of the Company Board to the Company’s stockholders under applicable Law. For the avoidance of doubt, the provisions of thisSection 5.2(e)(i) shall also apply to any material change to the facts and circumstances relating to such Acquisition Proposal and require a new Company Determination Notice, except that the references to four Business Days shall be deemed to be three Business Days.
(ii) other than in connection with an Acquisition Proposal, the Company Board may make a Company Board Adverse Recommendation Change in response to a Company Change in Circumstance, if and only if: (A) the Company Board determines in good faith, after consultation with the Company’s outside legal counsel, that the failure to do so could be inconsistent with the fiduciary duties of the Company Board to Parent’s stockholders under applicable Law; (B) the Company shall have given Parent a Company Determination Notice at least four Business Days prior to making any such Company Board Adverse Recommendation Change; and (C) (1) Company shall have specified the Company Change in Circumstance in reasonable detail, (2) the Company shall have given Parent the four Business Days after the Company Determination Notice to propose revisions to the terms of this Agreement or make another
A-50
proposal, and shall have made its Representatives reasonably available to negotiate in good faith with Parent (to the extent Parent desires to do so) with respect to such proposed revisions or other proposal, if any, and (3) after considering the results of any such negotiations and giving effect to the proposals made by Parent, if any, after consultation with outside legal counsel, the Company Board shall have determined, in good faith, that the failure to make the Company Board Adverse Recommendation Change in response to such Company Change in Circumstance could be inconsistent with the fiduciary duties of the Company Board to the Company’s stockholders under applicable Law. For the avoidance of doubt, the provisions of thisSection 5.2(e)(ii) shall also apply to any material change to the facts and circumstances relating to such Company Change in Circumstance and require a new Company Determination Notice, except that the references to four Business Days shall be deemed to be three Business Days.
(f) The Company’s obligation to solicit the consent of its stockholders to sign the Company Stockholder Written Consent in accordance withSection 5.2(a) andSection 5.2(d) shall not be limited or otherwise affected by the commencement, disclosure, announcement or submission of any Superior Offer or other Acquisition Proposal.
5.3 Parent Stockholders’ Meeting.
(a) Promptly after the Registration Statement has been declared effective by the SEC under the Securities Act, Parent shall take all action necessary under applicable Law to call, give notice of and hold a meeting of the holders of Parent Common Stock for the purpose of seeking approval of:
(i) the amendment of Parent’s certificate of incorporation to effect the Nasdaq Reverse Split;
(ii) this Agreement, including the issuance of shares of Parent Common Stock to the Company’s stockholders in connection with the Contemplated Transactions;
(iii) the change of control of Parent resulting from the Merger pursuant to the Nasdaq rules;
(iv) the 2019 Plan (as defined inSection 5.20(a)); and
(v) in accordance with Section 14A of the Exchange Act and the applicable SEC rules issued thereunder, seeking advisory approval of a proposal to the Parent’s stockholders for anon-binding, advisory vote to approve certain compensation that may become payable to Parent’s named executed officers in connection with the completion of the Merger, if applicable (the matters contemplated by the clauses 5.3(a)(i) – (iii) are referred to as the “Parent Stockholder Matters,” and the matters contemplated by clauses 5.3(a)(iv) – (v) is referred to herein as the “Other Parent Stockholder Matters,” and such meeting, the “Parent Stockholders’ Meeting”).
(b) The Parent Stockholders’ Meeting shall be held as promptly as practicable after the Registration Statement is declared effective under the Securities Act. Parent shall take reasonable measures to ensure that all proxies solicited in connection with the Parent Stockholders’ Meeting are solicited in compliance with all applicable Law. Notwithstanding anything to the contrary contained herein, if on the date of the Parent Stockholders’ Meeting, or a date preceding the date on which the Parent Stockholders’ Meeting is scheduled, Parent reasonably believes that (i) it will not receive proxies sufficient to obtain the Required Parent Stockholder Vote, whether or not a quorum would be present or (ii) it will not have sufficient shares of Parent Common Stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the Parent Stockholders’ Meeting, Parent may postpone or adjourn, or make one or more successive postponements or adjournments of, the Parent Stockholders’ Meeting as long as the date of the Parent Stockholders’ Meeting is not postponed or adjourned more than an aggregate of 30 calendar days in connection with any postponements or adjournments.
(c) Parent agrees that, subject toSection 5.3(d): (i) the Parent Board shall recommend that the holders of Parent Common Stock vote to approve the Parent Stockholder Matters and shall use commercially
A-51
reasonable efforts to solicit such approval, (ii) the Proxy Statement shall include a statement to the effect that the Parent Board recommends that Parent’s stockholders vote to approve the Parent Stockholder Matters (the recommendation of the Parent Board with respect to the Parent Stockholder Matters being referred to as the “Parent Board Recommendation”); and (iii) the Parent Board Recommendation shall not be withheld, amended, withdrawn or modified (and the Parent Board shall not publicly propose to withhold, amend, withdraw or modify the Parent Board Recommendation) in a manner adverse to the Company (the actions set forth in the foregoing clause (iii), collectively, a “Parent Board Adverse Recommendation Change”).
(d) Notwithstanding anything to the contrary contained in this Agreement, if at any time prior to the approval of Parent Stockholder Matters by the Required Parent Stockholder Vote:
(i) if Parent has received a written Acquisition Proposal (which Acquisition Proposal did not arise out of a material breach ofSection 4.4) from any Person that has not been withdrawn and after consultation with outside legal counsel, the Parent Board shall have determined, in good faith, that such Acquisition Proposal is a Superior Offer, the Parent Board may make a Parent Board Adverse Recommendation Change, if and only if: (A) the Parent Board determines in good faith, after consultation with Parent’s outside legal counsel, that the failure to do so could be inconsistent with the fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law; (B) Parent shall have given the Company prior written notice of its intention to consider making a Parent Board Adverse Recommendation Change or terminate this Agreement pursuant toSection 9.1(f) at least four Business Days prior to making any such Parent Board Adverse Recommendation Change or termination (a “Determination Notice”) (which notice shall not constitute a Parent Board Adverse Recommendation Change); and (C) (1) Parent shall have provided to the Company a summary of the material terms and conditions of the Acquisition Proposal in accordance withSection 4.4(b), (2) Parent shall have given the Company the four Business Days after the Determination Notice to propose revisions to the terms of this Agreement or make another proposal and shall have made its Representatives reasonably available to negotiate in good faith with the Company (to the extent the Company desires to negotiate) with respect to such proposed revisions or other proposal, if any, and (3) after considering the results of any such negotiations and giving effect to the proposals made by the Company, if any, after consultation with outside legal counsel, the Parent Board shall have determined, in good faith, that such Acquisition Proposal is a Superior Offer and that the failure to make the Parent Board Adverse Recommendation Change or terminate this Agreement pursuant toSection 9.1(f) could be inconsistent with the fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law. For the avoidance of doubt, the provisions of thisSection 5.3(d)(i) shall also apply to any material change to the facts and circumstances relating to such Acquisition Proposal and require a new Determination Notice, except that the references to four Business Days shall be deemed to be three Business Days.
(ii) other than in connection with an Acquisition Proposal, the Parent Board may make a Parent Board Adverse Recommendation Change in response to a Parent Change in Circumstance, if and only if: (A) the Parent Board determines in good faith, after consultation with Parent’s outside legal counsel, that the failure to do so could be inconsistent with the fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law; (B) Parent shall have given the Company a Determination Notice at least four Business Days prior to making any such Parent Board Adverse Recommendation Change; and (C) (1) Parent shall have specified the Parent Change in Circumstance in reasonable detail, (2) Parent shall have given the Company the four Business Days after the Determination Notice to propose revisions to the terms of this Agreement or make another proposal, and shall have made its Representatives reasonably available to negotiate in good faith with the Company (to the extent the Company desires to do so) with respect to such proposed revisions or other proposal, if any, and (3) after considering the results of any such negotiations and giving effect to the proposals made by the Company, if any, after consultation with outside legal counsel, the Parent Board shall have determined, in good faith, that
A-52
the failure to make the Parent Board Adverse Recommendation Change in response to such Parent Change in Circumstance could be inconsistent with the fiduciary duties of the Parent Board to Parent’s stockholders under applicable Law. For the avoidance of doubt, the provisions of thisSection 5.3(d)(ii) shall also apply to any material change to the facts and circumstances relating to such Parent Change in Circumstance and require a new Determination Notice, except that the references to four Business Days shall be deemed to be three Business Days.
(e) Parent’s obligation to solicit the consent of its stockholders to approve the Parent Stockholder Matters shall not be limited or otherwise affected by the commencement, disclosure, announcement or submission of any Superior Offer or other Acquisition Proposal.
(f) Nothing contained in this Agreement shall prohibit Parent or the Parent Board from (i) complying with Rules14d-9 and14e-2(a) promulgated under the Exchange Act, (ii) issuing a “stop, look and listen” communication or similar communication of the type contemplated bySection 14d-9(f) under the Exchange Act or (iii) otherwise making any disclosure to the Parent stockholders;provided however, that in the case of the foregoing clause (iii) the Parent Board determines in good faith, after consultation with its outside legal counsel, that failure to make such disclosure is reasonably likely to be inconsistent with applicable Law, including its fiduciary duties under applicable Law.
(a) Each party hereto shall (i) consult and cooperate with one another, and consider in good faith the views of one another, in connection with any analyses, appearances, presentations, memoranda, briefs, arguments, opinions and proposals made or submitted by or on behalf of any party hereto in connection with proceedings under or relating to the HSR Act or any foreign or other antitrust Law, (ii) coordinate with one another in preparing and exchanging such materials and (iii) promptly provide one another (and its counsel) with copies of all filings, presentations or submissions made by such party to any Governmental Body in connection with this Agreement. In addition, any party may, as it deems advisable and necessary, reasonably designate any confidential and competitively sensitive material provided to the other parties under thisSection 5.4 as “Outside Counsel Only” or redact information regarding valuation or negotiation strategy. Materials identified as “Outside Counsel Only” and the information contained therein shall be given only to the outside legal counsel of the recipient and will not be disclosed by such outside counsel to employees, officers, or directors of the recipient, unless express written permission is obtained in advance from the source of the materials.
(b) Each of Parent and the Company shall use its respective reasonable best efforts to resolve objections, if any, as may be asserted by any Governmental Body with respect to the Contemplated Transactions under any applicable antitrust Laws, including responding promptly to and complying with any requests for information relating to this Agreement or any initial filings required under the HSR Act and any other additional filings (“Merger Notification Filings”) from any Governmental Body charged with enforcing, applying, administering or investigating any antitrust Laws.
(c) Notwithstanding anything to the contrary herein (i) neither Party shall have any obligation to litigate or contest any such Legal Proceeding or order resulting therefrom and (ii) neither Party shall be under an obligation to make proposals, execute or carry out agreements or submit to orders providing for (A) the sale, license, divestiture, or other disposition or holding separate of any assets of Parent or the Company or any of their respective Affiliates, (B) the imposition of any limitation or restriction on the ability of Parent or the Company or any of their respective Affiliates to freely conduct their business, or (C) any limitation or regulation on the ability of Parent or any of its Affiliates to exercise full rights of ownership of the Company.
5.5 Company Options and Company Warrants.
(a) At the Effective Time, each Company Option that is outstanding and unexercised immediately prior to the Effective Time under the Company Plan, whether or not vested, shall be converted into
A-53
and become an option to purchase Parent Common Stock, and Parent shall assume the Company Plan and each such Company Option in accordance with the terms (as in effect as of the date of this Agreement) of the Company Plan and the terms of the stock option agreement by which such Company Option is evidenced (but with changes to such documents as Parent and the Company mutually agree are appropriate to reflect the substitution of the Company Options by Parent to purchase shares of Parent Common Stock). All rights with respect to Company Common Stock under Company Options assumed by Parent shall thereupon be converted into rights with respect to Parent Common Stock. Accordingly, from and after the Effective Time: (i) each Company Option assumed by Parent may be exercised solely for shares of Parent Common Stock; (ii) the number of shares of Parent Common Stock subject to each Company Option assumed by Parent shall be determined by multiplying (A) the number of shares of Company Common Stock that were subject to such Company Option, as in effect immediately prior to the Effective Time, by (B) the Exchange Ratio, and rounding the resulting number down to the nearest whole number of shares of Parent Common Stock; (iii) the per share exercise price for the Parent Common Stock issuable upon exercise of each Company Option assumed by Parent shall be determined by dividing (A) the per share exercise price of Company Common Stock subject to such Company Option, as in effect immediately prior to the Effective Time, by (B) the Exchange Ratio and rounding the resulting exercise price up to the nearest whole cent; and (iv) any restriction on the exercise of any Company Option assumed by Parent shall continue in full force and effect and the term, exercisability, vesting schedule and other provisions of such Company Option shall otherwise remain unchanged;provided, however, that: (A) to the extent provided under the terms of a Company Option and the Company Plans, such Company Option may be further adjusted as necessary to reflect Parent’s substitution of the Company Options with options to purchase Parent Common Stock (such as by making any change in control or similar definition relate to Parent and having any provision that provides for the adjustment of Company Options upon the occurrence of certain corporate events relate to corporate events that relate to Parent and/or Parent Common Stock); and (B) the Parent Board or a committee thereof shall succeed to the authority and responsibility of the Company Board or any committee thereof with respect to each Company Option assumed by Parent. Notwithstanding anything to the contrary in this Section 5.5(a), the conversion of each Company Option (regardless of whether such option qualifies as an “incentive stock option” within the meaning of Section 422 of the Code) into an option to purchase shares of Parent Common Stock shall be made in a manner consistent with Treasury RegulationSection 1.424-1, such that the conversion of a Company Option shall not constitute a “modification” of such Company Option for purposes of Section 409A or Section 424 of the Code.
(b) Parent shall file with the SEC, promptly after the Effective Time, a registration statement on FormS-8 (or any successor or alternative form), relating to the shares of Parent Common Stock issuable with respect to Company Options assumed by Parent in accordance withSection 5.5(a).
(c) At the Effective Time, each Company Warrant that is outstanding and unexercised as of immediately prior to the Effective Time (for the avoidance of doubt, excluding Company Warrants that are deemed to have been automatically exercised or terminated pursuant to their terms as a result of the consummation of the Merger or the Preferred Stock Conversion), if any, shall be converted into and become a warrant to purchase Parent Common Stock and Parent shall assume each such Company Warrant in accordance with its terms. All rights with respect to Company Capital Stock under Company Warrants assumed by Parent shall thereupon be converted into rights with respect to Parent Common Stock. Accordingly, from and after the Effective Time: (i) each Company Warrant assumed by Parent may be exercised solely for shares of Parent Common Stock; (ii) the number of shares of Parent Common Stock subject to each Company Warrant assumed by Parent shall be determined by multiplying (A) the number of shares of Company Common Stock, or the number of shares of Company Preferred Stock issuable upon exercise of the Company Warrant, as applicable, that were subject to such Company Warrant immediately prior to the Effective Time by (B) the Exchange Ratio and rounding the resulting number down to the nearest whole number of shares of Parent Common Stock; (iii) the per share exercise price for the Parent Common Stock issuable upon exercise of each Company Warrant assumed by Parent shall be determined by dividing the per share exercise price of Company Capital Stock subject to such Company Warrant, as in effect immediately prior to the Effective Time, by the Exchange Ratio and rounding the resulting exercise price up to the nearest whole cent; and (iv) any restriction on any Company
A-54
Warrant assumed by Parent shall continue in full force and effect and the term and other provisions of such Company Warrant shall otherwise remain unchanged.
(d) Prior to the Effective Time, the Company shall take all actions that may be necessary (under the Company Plan, the Company Warrants, and otherwise) to effectuate the provisions of thisSection 5.5 and to ensure that, from and after the Effective Time, holders of Company Options, and Company Warrants have no rights with respect thereto other than those specifically provided in thisSection 5.5.
5.6 Indemnification of Officers and Directors.
(a) From the Effective Time through the sixth anniversary of the date on which the Effective Time occurs, each of Parent and the Surviving Corporation shall indemnify and hold harmless each person who is now, or has been at any time prior to the date hereof, or who becomes prior to the Effective Time, a director or officer of Parent or the Company and their respective Subsidiaries, respectively (the “D&O Indemnified Parties”), against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees and disbursements (collectively, “Costs”), incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that the D&O Indemnified Party is or was a director or officer of Parent or of the Company, whether asserted or claimed prior to, at or after the Effective Time, in each case, to the fullest extent permitted under applicable Law. Each D&O Indemnified Party will be entitled to advancement of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation from each of Parent and the Surviving Corporation, jointly and severally, upon receipt by Parent or the Surviving Corporation from the D&O Indemnified Party of a request therefor;provided that any such person to whom expenses are advanced provides an undertaking to Parent, to the extent then required by the DGCL, to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
(b) The provisions of the certificate of incorporation and bylaws of Parent with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers of Parent that are presently set forth in the certificate of incorporation and bylaws of Parent shall not be amended, modified or repealed for a period of six years from the Effective Time in a manner that would adversely affect the rights thereunder of individuals who, at or prior to the Effective Time, were officers or directors of Parent. The certificate of incorporation and bylaws of the Surviving Corporation shall contain, and Parent shall cause the certificate of incorporation and bylaws of the Surviving Corporation to so contain, provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers as those presently set forth in the certificate of incorporation and bylaws of Parent.
(c) From and after the Effective Time, (i) the Surviving Corporation shall fulfill and honor in all respects the obligations of the Company to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Company’s Organizational Documents and pursuant to any indemnification agreements between the Company and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time and (ii) Parent shall fulfill and honor in all respects the obligations of Parent to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under Parent’s Organizational Documents and pursuant to any indemnification agreements between Parent and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time.
(d) From and after the Effective Time, Parent shall maintain directors’ and officers’ liability insurance policies, with an effective date as of the Closing Date, on commercially available terms and conditions and with coverage limits customary for U.S. public companies similarly situated to Parent. In addition, Parent shall purchase, prior to the Effective Time, asix-year prepaid “tail policy” for thenon-cancellable extension of the directors’ and officers’ liability coverage of Parent’s existing directors’ and officers’ insurance policies for a claims reporting or discovery period of at least six years from and after the Effective Time with respect to any claim related to any period of time at or prior to the Effective Time (the “D&O Tail Policy”).
A-55
(e) From and after the Effective Time, Parent shall pay all expenses, including reasonable attorneys’ fees, that are incurred by the persons referred to in thisSection 5.6 in connection with their successful enforcement of the rights provided to such persons in thisSection 5.6.
(f) The provisions of thisSection 5.6 are intended to be in addition to the rights otherwise available to the current and former officers and directors of Parent and the Company by Law, charter, statute, bylaw or agreement, and shall operate for the benefit of, and shall be enforceable by, each of the D&O Indemnified Parties, their heirs and their representatives.
(g) In the event Parent or the Surviving Corporation or any of their respective successors or assigns (i) consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger, or (ii) transfers all or substantially all of its properties and assets to any Person, then, and in each such case, proper provision shall be made so that the successors and assigns of Parent or the Surviving Corporation, as the case may be, shall succeed to the obligations set forth in thisSection 5.6. Parent shall cause the Surviving Corporation to perform all of the obligations of the Surviving Corporation under thisSection 5.6.
5.7 Additional Agreements. The Parties shall use commercially reasonable efforts to cause to be taken all actions necessary to consummate the Contemplated Transactions. Without limiting the generality of the foregoing, each Party to this Agreement: (a) shall make all filings and other submissions (if any) and give all notices (if any) required to be made and given by such Party in connection with the Contemplated Transactions; (b) shall use reasonable best efforts to obtain each Consent (if any) reasonably required to be obtained (pursuant to any applicable Law or Contract, or otherwise) by such Party in connection with the Contemplated Transactions or for such Contract (with respect to Contracts set forth inSchedule 5.7) to remain in full force and effect; (c) shall use commercially reasonable efforts to lift any injunction prohibiting, or any other legal bar to, the Contemplated Transactions; and (d) shall use commercially reasonable efforts to satisfy the conditions precedent to the consummation of this Agreement.
5.8 Disclosure. The initial press release relating to this Agreement shall be a joint press release issued by the Company and Parent and thereafter Parent and the Company shall consult with each other before issuing any further press release(s) or otherwise making any public statement or making any announcement to Parent Associates or Company Associates (to the extent not previously issued or made in accordance with this Agreement) with respect to the Contemplated Transactions and shall not issue any such press release, public statement or announcement to Parent Associates or Company Associates without the other Party’s written consent (which shall not be unreasonably withheld, conditioned or delayed). Notwithstanding the foregoing: (a) each Party may, without such consultation or consent, make any public statement in response to questions from the press, analysts, investors or those attending industry conferences, make internal announcements to employees and make disclosures in Parent SEC Documents, so long as such statements are consistent with previous press releases, public disclosures or public statements made jointly by the parties (or individually, if approved by the other Party); (b) a Party may, without the prior consent of the other Party hereto but subject to giving advance notice to the other Party, issue any such press release or make any such public announcement or statement as may be required by any Law; and (c) Parent need not consult with the Company in connection with such portion of any press release, public statement or filing to be issued or made pursuant toSection 5.3(f) or with respect to any Acquisition Proposal or Parent Board Adverse Recommendation Change.
5.9 Listing. Parent shall use its commercially reasonable efforts, (a) to maintain its existing listing on Nasdaq until the Effective Time and to obtain approval of the listing of the combined corporation on Nasdaq, (b) to the extent required by the rules and regulations of Nasdaq, to prepare and submit to Nasdaq a notification form for the listing of the shares of Parent Common Stock to be issued in connection with the Contemplated Transactions, and to cause such shares to be approved for listing (subject to official notice of issuance); (c) to effect the Nasdaq Reverse Split; and (d) to the extent required by Nasdaq Marketplace Rule 5110, to file an initial listing application for the Parent Common Stock on Nasdaq (the “NasdaqListing Application”) and to
A-56
cause such Nasdaq Listing Application to be conditionally approved prior to the Effective Time. The Parties will use commercially reasonable efforts to coordinate with respect to compliance with Nasdaq rules and regulations. Each Party will promptly inform the other Party of all verbal or written communications between Nasdaq and such Party or its representatives. Parent and the Company agree to evenly split all Nasdaq fees associated with the Nasdaq Listing Application and the Nasdaq Reverse Split, if any (the “Nasdaq Fees”). The Company will cooperate with Parent as reasonably requested by Parent with respect to the Nasdaq Listing Application and promptly furnish to Parent all information concerning the Company and its stockholders that may be required or reasonably requested in connection with any action contemplated by thisSection 5.9.
(a) For United States federal income Tax purposes, (i) the Parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Code (the “Intended Tax Treatment”), and (ii) this Agreement is intended to be, and is hereby adopted as, a “plan of reorganization” for purposes of Section 354 and 361 of the Code and Treasury Regulations Section 1.368-2(g) and1.368-3(a), to which the Parent, Merger Sub and the Company are parties under Section 368(b) of the Code. The Parties shall treat and shall not take any tax reporting position inconsistent with the treatment of the Merger as a reorganization within the meaning of Section 368(a) of the Code for U.S. federal, state and other relevant Tax purposes, unless otherwise required pursuant to a “determination” within the meaning of Section 1313(a) of the Code.
(b) The Parties shall use their respective reasonable best efforts to cause the Merger to qualify, and will not take any action or cause any action to be taken which action would reasonably be expected to prevent the Merger from qualifying, for the Intended Tax Treatment.
(c) Each of the Parties shall use its reasonable best efforts to obtain (1) the Parent Registration Statement Tax Opinion, (2) the Company Registration Statement Tax Opinion, (3) the Parent Closing Tax Opinion and (4) the Company Closing Tax Opinion, including (i) delivering to Latham & Watkins LLP (“Latham & Watkins”) and Cooley LLP (“Cooley”) prior to the filing of the Registration Statement, tax representation letters substantially in the forms set forth inSection 5.10(c)(i) of the Company Disclosure Schedule andSection 5.10(c)(i) of the Parent Disclosure Schedule, respectively, and (ii) delivering to Latham & Watkins and Cooley, dated and executed as of the dates of such Tax opinions, tax representation letters in substantially the forms set forth inSection 5.10(c)(ii) of the Company Disclosure Schedule andSection 5.10(c)(ii) of the Parent Disclosure Schedule, respectively. Each of the Parties shall use its reasonable best efforts not to, and not permit any affiliate to, take or cause to be taken any action that would cause to be untrue (or fail to take or cause not to be taken any action which inaction would cause to be untrue) any of the representations and covenants made to counsel in the tax representation letters described in this Section.
5.11 Legends. Parent shall be entitled to place appropriate legends on the book entries and/or certificates evidencing any shares of Parent Common Stock to be received in the Merger by equity holders of the Company who may be considered “affiliates” of Parent for purposes of Rules 144 and 145 under the Securities Act reflecting the restrictions set forth in Rules 144 and 145 and to issue appropriate stop transfer instructions to the transfer agent for Parent Common Stock.
(a) The Parties shall use reasonable best efforts and take all necessary action so that immediately after the Effective Time, (a) the Parent Board is comprised of nine members, with (i) two such members designated by Parent, (ii) two such members designated by Shanghai Pharmaceuticals USA, (iii) one member being the Chairman of the Company, (iv) one member being the Chief Executive Officer of the Company, and (v) the remaining three members being listed onExhibit C under the heading “Additional Directors”, (b) the Persons listed inExhibitC under the heading “Officers” are elected or appointed, as applicable, to the positions of officers of Parent and the Surviving Corporation, as set forth therein, to serve in such positions effective as of
A-57
the Effective Time until successors are duly appointed and qualified in accordance with applicable Law and (c) Persons reasonably acceptable to Parent are elected or appointed, as applicable to the positions of officer of Parent set forth onExhibit C. If any Person listed inExhibitC is unable or unwilling to serve as an officer of Parent or the Surviving Corporation, as set forth therein, as of the Effective Time, the Parties shall mutually agree upon a successor. The Persons listed inExhibitC under the heading “Board Designees – Parent” shall be Parent’s designees pursuant to clause (a) of thisSection 5.12 (which list may be changed by Parent at any time prior to the Closing by written notice to the Company to include different board designees who are reasonably acceptable to the Company) (the “Parent Designees”). The Persons listed inExhibitC under the heading “Board Designees – Company” shall be the Company’s designees pursuant to clause (a) of thisSection 5.12 (which list may be changed by the Company at any time prior to the Closing by written notice to Parent to include different board designees who are reasonably acceptable to Parent).
(b) One of the Parent Designees shall be placed in the same class of Parent’s current directors elected at Parent’s 2019 annual meeting of stockholders, as identified onExhibit C. After the Closing, the nominating committee of the Surviving Corporation shall nominate the Parent Designees forre-election in the first year in which the Parent Designees’ term expires.
(c) For a period of three years after the Closing, J.R. Hyde, III will have the right to designate a Person to participate in the Parent Board meetings as an observer (the “Board Observer”); provided that (i) the obligations of Parent shall be subject to the Board Observer agreeing in writing to a customary confidentiality agreement with respect to information so provided, and (ii) Parent and the Parent Board may withhold any information from and exclude the Board Observer from any meeting or portion thereof to the extent access to such information or attendance at such meeting could adversely affect the attorney-client privilege between Parent and its counsel or result in disclosure of Parent’s trade secrets or if such information or meeting involves a material conflict of interest with Parent and the Board Observer. The Person listed inExhibit C under the heading “Board Observer” is designated as the Board Observer until J.R. Hyde, III appoints a successor.
5.13 Termination of Certain Agreements and Rights. The Company shall cause any Investor Agreements (excluding the Company Stockholder Support Agreements and CompanyLock-up Agreements) to be terminated immediately prior to the Effective Time, without any liability being imposed on the part of Parent or the Surviving Corporation.
5.14 Section 16 Matters. Prior to the Effective Time, Parent and the Company shall take all such steps as may be required (to the extent permitted under applicable Laws) to cause any acquisitions of Parent Common Stock, restricted stock awards to acquire Parent Common Stock and any options to purchase Parent Common Stock in connection with the Contemplated Transactions, by each individual who is reasonably expected to become subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Parent, to be exempt under Rule16b-3 promulgated under the Exchange Act.
5.15 Cooperation. Each Party shall cooperate reasonably with the other Party and shall provide the other Party with such assistance as may be reasonably requested for the purpose of facilitating the performance by each Party of its respective obligations under this Agreement and to enable the combined entity to continue to meet its obligations following the Effective Time.
(a) The Company will prepare and deliver to Parent at least five Business Days prior to the Closing Date a certificate signed by the Chief Financial Officer of the Company in a form reasonably acceptable to Parent setting forth (as of immediately prior to the Effective Time) (i) each holder of Company Common Stock, Company Options, and Company Warrants; (ii) such holder’s name and address; (iii) the number and type of Company Common Stock held and/or underlying the Company Options, and Company Warrants as of the immediately prior to the Effective Time for each such holder; and (iv) the number of shares of Parent Common
A-58
Stock to be issued to such holder, or to underlie any Company Option or Company Warrant to be issued to such holder, pursuant to this Agreement in respect of the Company Common Stock, Company Options or Company Warrants held by such holder as of immediately prior to the Effective Time (the “Allocation Certificate”).
(b) Parent will prepare and deliver to the Company at least five Business Days prior to the Closing Date a certificate signed by the Chief Financial Officer of Parent in a form reasonably acceptable to the Company, setting forth, as of immediately prior to the Effective Time (i) each record holder of Parent Common Stock, Parent Options, Parent Deferred Stock Rights, or Parent Warrants; (ii) such record holder’s name and address; and (iii) the number of shares of Parent Common Stock held and/or underlying the Parent Options, Parent Deferred Stock Rights, or Parent Warrants as of the Effective Time for such holder (the “Parent Outstanding Shares Certificate”).
5.17 Company Financial Statements. As promptly as reasonably practicable following the date of this Agreement (i) and no later than March 22, 2019 the Company will furnish to Parent audited financial statements for the fiscal years ended 2017 and 2018 for inclusion in the Proxy Statement and the Registration Statement (the “Company Audited Financial Statements”) and (ii) the Company will furnish to Parent unaudited interim financial statements for each interim period completed prior to Closing that would be required to be included in the Registration Statement or any periodic report due prior to the Closing if the Company were subject to the periodic reporting requirements under the Securities Act or the Exchange Act (the “Company Interim Financial Statements”). Each of the Company Audited Financial Statements and the Company Interim Financial Statements will be suitable for inclusion in the Proxy Statement and the Registration Statement and prepared in accordance with GAAP as applied on a consistent basis during the periods involved (except in each case as described in the notes thereto) and on that basis will present fairly, in all material respects, the financial position and the results of operations, changes in stockholders’ equity, and cash flows of the Company as of the dates of and for the periods referred to in the Company Audited Financial Statements or the Company Interim Financial Statements, as the case may be.
5.18 Takeover Statutes. If any Takeover Statute is or may become applicable to the Contemplated Transactions, each of the Company, the Company Board, Parent and the Parent Board, as applicable, shall grant such approvals and take such actions as are necessary so that the Contemplated Transactions may be consummated as promptly as practicable on the terms contemplated by this Agreement and otherwise act to eliminate or minimize the effects of such statute or regulation on the Contemplated Transactions.
5.19 Stockholder Litigation. Each Party shall keep the other Party reasonably informed regarding any stockholder litigation against any Party or any of its respective directors relating to or challenging this Agreement or the consummation of the Contemplated Transactions. Prior to the Closing, Parent shall reasonably consult with and permit the Company and its Representatives to participate in the defense, negotiations and settlement of any such stockholder litigation, and Parent shall give consideration to the Company’s advice with respect to stockholder litigation. Parent shall promptly advise the Company orally and in writing of the initiation of, and shall keep the Company reasonably apprised of any material developments in connection with, any such stockholder litigation.
(a) Prior to or as of the Effective Time, the Board of Directors and stockholders of Parent shall adopt the incentive award plan attached hereto asExhibit F (the “2019 Plan”) reserving for issuance 12% of the Parent Fully-Diluted Shares after giving effect to the Closing. The 2019 Plan will provide that the number of shares reserved for issuance thereunder will be increased annually on the first day of each year beginning in 2020 and ending in 2029, at the discretion of the Parent’s Board of Directors, in an amount equal to the least of (a) five percent of the shares of Parent Common Stock outstanding (on anas-converted basis) on the last day of the immediately preceding year and (b) such smaller number of shares of stock as determined by the Parent’s Board of Directors.
A-59
(b) Parent shall file with the SEC, promptly after the Effective Time, a registration statement on FormS-8 (or any successor form), if available for use by Parent, relating to the shares of Parent Common Stock issuable with respect to 2019 Plan.
5.21 Preferred Stock Conversion.The Company shall take all necessary action to effect the conversion of the Company Preferred Stock into Company Common Stock immediately prior to the Effect Time (the “Preferred Stock Conversion”).
(a) Prior to the Closing, the Parent Board shall have adopted appropriate resolutions and taken all other actions necessary and appropriate to provide that each unexpired and unexercised Parent Option, whether vested or unvested, shall be accelerated in full effective as of immediately prior to the Effective Time.
(b) Prior to the Closing, Parent shall take all actions that may be necessary (under the Parent Stock Plans and otherwise) to effectuate the provisions of thisSection 5.22.
5.23 Termination of Parent Deferred Stock Rights. Prior to the Effective Time (but in no event more than 30 days prior to the Effective Time), Parent’s Board of Directors or a duly authorized committee thereof shall, without any further action on the part of any holder thereof, take all actions necessary to cause the termination and liquidation of the Parent 2018 Amended and Restated Directors’ Deferred Compensation Plan and all Parent Deferred Stock Rights or other deferrals thereunder effective immediately prior to the Effective Time, subject to the consummation of the Merger. Parent shall take all actions necessary to ensure that any deferrals under the Parent 2018 Amended and Restated Directors’ Deferred Compensation Plan on or after January 3, 2019 shall be settled only in cash and that the maximum number of shares of Parent Common Stock issuable upon settlement of the Parent Deferred Share Units shall be limited to the number of Parent Deferred Share Units outstanding as of the date of this Agreement as set forth inSection 3.6(c).
Section 6. CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY
The obligations of each Party to effect the Merger and otherwise consummate the Contemplated Transactions to be consummated at the Closing are subject to the satisfaction or, to the extent permitted by applicable Law, the written waiver by each of the Parties, at or prior to the Closing, of each of the following conditions:
6.1 Effectiveness of Registration Statement. The Registration Statement shall have become effective in accordance with the provisions of the Securities Act, and shall not be subject to any stop order or proceeding (or threatened proceeding by the SEC) seeking a stop order with respect to the Registration Statement that has not been withdrawn.
6.2 No Restraints. No temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the Contemplated Transactions shall have been issued by any court of competent jurisdiction or other Governmental Body of competent jurisdiction and remain in effect and there shall not be any Law which has the effect of making the consummation of the Contemplated Transactions illegal.
6.3 Stockholder Approval. (a) Parent shall have obtained the Required Parent Stockholder Vote and (b) the Company shall have obtained the Required Company Stockholder Vote.
6.4 Listing. The existing shares of Parent Common Stock shall have been continually listed on Nasdaq as of and from the date of this Agreement through the Closing Date, the approval of the listing of additional shares of Parent Common Stock on Nasdaq shall have been obtained and the shares of Parent Common Stock to be issued in the Merger pursuant to this Agreement shall have been approved for listing (subject to official notice of issuance) on Nasdaq as of the Closing.
A-60
6.5 Regulatory Approvals. All applicable waiting periods (and any extension thereof) applicable to the Merger under the HSR Act shall have expired or early termination of such waiting periods shall have been granted and all applicable foreign antitrust approvals shall have been obtained.
Section 7. ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND MERGER SUB
The obligations of Parent and Merger Sub to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by Parent, at or prior to the Closing, of each of the following conditions:
7.1 Accuracy of Representations. The Company Fundamental Representations shall have been true and correct in all material respects as of the date of this Agreement and shall be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all material respects as of such date). The representations and warranties of the Company contained in this Agreement (other than the Company Fundamental Representations) shall have been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on the Closing Date, except (a) in each case, or in the aggregate, where the failure to be true and correct would not reasonably be expected to have a Company Material Adverse Effect (without giving effect to any references therein to any Company Material Adverse Effect or other materiality qualifications), or (b) for those representations and warranties which address matters only as of a particular date (which representations shall have been true and correct, subject to the qualifications as set forth in the preceding clause (a), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Company Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded).
7.2 Performance of Covenants. The Company shall have performed or complied with in all material respects all agreements and covenants required to be performed or complied with by it under this Agreement at or prior to the Effective Time.
7.3 Documents. Parent shall have received the following documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer or Chief Financial Officer of the Company certifying (i) that the conditions set forth inSections 7.1,7.2,7.5,7.6 and7.9 have been duly satisfied and (ii) that the information set forth in the Allocation Certificate delivered by the Company in accordance withSection 5.16 is true and accurate in all respects as of the Closing Date; and
(b) the Allocation Certificate.
7.4 FIRPTA Certificate. Parent shall have received (i) an original signed statement from the Company that the Company is not, and has not been at any time during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code, a “United States real property holding corporation,” as defined in Section 897(c)(2) of the Code, conforming to the requirements of Treasury RegulationsSection 1.1445-2(c)(3) and1.897-2(h), and (ii) an original signed notice to be delivered to the IRS in accordance with the provisions of Treasury RegulationsSection 1.897-2(h)(2), together with written authorization for Parent to deliver such notice to the IRS on behalf of the Company following the Closing, each dated as of the Closing Date, duly executed by an authorized officer of the Company, and in form and substance reasonably acceptable to Parent.
7.5 No Company Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Company Material Adverse Effect that is continuing.
A-61
7.6 Termination of Investor Agreements. The Investor Agreements shall have been terminated.
7.7 CompanyLock-Up Agreements. Parent shall have received the CompanyLock-Up Agreements duly executed by each of the Company Signatories, each stockholder of the Company expected to own more than ten percent (10%) of the outstanding Parent Common Stock after the Closing and each executive officer and director of the Company who is elected or appointed, as applicable, as an executive officer and director of Parent as of immediately following the Closing, each of which shall be in full force and effect.
7.8 Company Stockholder Written Consent. The Company Stockholder Written Consent evidencing the Required Company Stockholder Vote shall be in full force and effect.
7.9 Preferred Stock Conversion. The Company has effected the Preferred Stock Conversion.
7.10 Parent Closing Tax Opinion. Parent shall have received (i) the Parent Closing Tax Opinion and (ii) a copy of the Company Closing Tax Opinion.
Section 8. ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY
The obligations of the Company to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by the Company, at or prior to the Closing, of each of the following conditions:
8.1 Accuracy of Representations. The Parent Fundamental Representations shall have been true and correct in all material respects as of the date of this Agreement and shall be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all material respects as of such date). The representations and warranties of Parent and Merger Sub contained in this Agreement (other than the Parent Fundamental Representations) shall have been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on the Closing Date except (a) in each case, or in the aggregate, where the failure to be true and correct would not reasonably be expected to have a Parent Material Adverse Effect (without giving effect to any references therein to any Parent Material Adverse Effect or other materiality qualifications), or (b) for those representations and warranties which address matters only as of a particular date (which representations shall have been true and correct, subject to the qualifications as set forth in the preceding clause (a), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Parent Disclosure Schedule made or purported to have been made after the date of this Agreement shall be disregarded).
8.2 Performance of Covenants. Parent and Merger Sub shall have performed or complied with in all material respects all of their agreements and covenants required to be performed or complied with by each of them under this Agreement at or prior to the Effective Time.
8.3 Documents. The Company shall have received the following documents, each of which shall be in full force and effect:
(a) a certificate executed by the Chief Executive Officer or Chief Financial Officer of Parent confirming that the conditions set forth inSections 8.1,8.2, and8.4 have been duly satisfied;
(b) the Parent Outstanding Shares Certificate; and
(c) a written resignation, in a form reasonably satisfactory to the Company, dated as of the Closing Date and effective as of the Closing, executed by each of the officers and directors of Parent who are not to continue as officers or directors of Parent after the Closing pursuant toSection 5.12 hereof.
A-62
8.4 No Parent Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Parent Material Adverse Effect.
8.5 ParentLock-Up Agreements. The Company shall have received the ParentLock-Up Agreements duly executed by each of the Parent Signatories, each of which shall be in full force and effect.
8.6 Company Closing Tax Opinion. The Company shall have received (i) the Company Closing Tax Opinion and (ii) a copy of the Parent Closing Tax Opinion.
8.7 Board of Directors. Parent shall have caused the Parent Board to be constituted as set forth inSection 5.12 of this Agreement effective as of the Effective Time.
9.1 Termination. This Agreement may be terminated prior to the Effective Time (whether before or after adoption of this Agreement by the Company’s stockholders and whether before or after approval of the Parent Stockholder Matters by Parent’s stockholders, unless otherwise specified below):
(a) by mutual written consent of Parent and the Company;
(b) by either Parent or the Company if the Contemplated Transactions shall not have been consummated by August 6, 2019 (subject to possible extension as provided in thisSection 9.1(b), the “End Date”);provided,however, that the right to terminate this Agreement under thisSection 9.1(b) shall not be available to the Company, on the one hand, or to Parent, on the other hand, if such Party’s action or failure to act has been a principal cause of the failure of the Contemplated Transactions to occur on or before the End Date and such action or failure to act constitutes a breach of this Agreement,provided,further,however, that, in the event that a request for additional information has been made by any Governmental Body, or in the event that the SEC has not declared effective under the Exchange Act the Registration Statement by the date which is 60 days prior to the End Date, then either the Company or Parent shall be entitled to extend the End Date for an additional 60 days by written notice to the other the Party;
(c) by either Parent or the Company if a court of competent jurisdiction or other Governmental Body shall have issued a final and nonappealable order, decree or ruling, or shall have taken any other action, having the effect of permanently restraining, enjoining or otherwise prohibiting the Contemplated Transactions;
(d) by Parent if the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote shall not have been obtained within the later of (i) 15 Business Days of the Registration Statement becoming effective in accordance with the provisions of the Securities Act and (ii) the date on which Parent obtains the Required Parent Stockholder Vote;provided, however, that once the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote has been obtained, Parent may not terminate this Agreement pursuant to thisSection 9.1(d);
(e) by either Parent or the Company if (i) the Parent Stockholders’ Meeting (including any adjournments and postponements thereof) shall have been held and completed and Parent’s stockholders shall have taken a final vote on the Parent Stockholder Matters and (ii) the Parent Stockholder Matters shall not have been approved at the Parent Stockholders’ Meeting (or at any adjournment or postponement thereof) by the Required Parent Stockholder Voteprovided, however, that the right to terminate this Agreement under thisSection 9.1(e) shall not be available to Parent where the failure to obtain the Required Parent Stockholder Vote has been caused by the action or failure to act of Parent or Merger Sub and such action or failure to act constitutes a material breach by Parent or Merger Sub of this Agreement;
(f) by the Company (at any time prior to the approval of the Parent Stockholder Matters by the Required Parent Stockholder Vote) if a Parent Triggering Event shall have occurred;
A-63
(g) by Parent (at any time prior to the Required Company Stockholder Vote being obtained) if a Company Triggering Event shall have occurred;
(h) by the Company, upon a breach of any representation, warranty, covenant or agreement set forth in this Agreement by Parent or Merger Sub or if any representation or warranty of Parent or Merger Sub shall have become inaccurate, in either case, such that the conditions set forth inSection 8.1 orSection 8.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate;provided that the Company is not then in material breach of any representation, warranty, covenant or agreement under this Agreement;provided,further, that if such inaccuracy in Parent’s or Merger Sub’s representations and warranties or breach by Parent or Merger Sub is curable by the End Date by Parent or Merger Sub, then this Agreement shall not terminate pursuant to thisSection 9.1(h) as a result of such particular breach or inaccuracy until the expiration of a15-day period commencing upon delivery of written notice from the Company to Parent or Merger Sub of such breach or inaccuracy and its intention to terminate pursuant to thisSection 9.1(h) (it being understood that this Agreement shall not terminate pursuant to thisSection 9.1(h) as a result of such particular breach or inaccuracy if such breach by Parent or Merger Sub is cured prior to such termination becoming effective); or
(i) by Parent, upon a breach of any representation, warranty, covenant or agreement set forth in this Agreement by the Company or if any representation or warranty of the Company shall have become inaccurate, in either case, such that the conditions set forth inSection 7.1 orSection 7.2 would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate;provided that Parent is not then in material breach of any representation, warranty, covenant or agreement under this Agreement;provided,further, that if such inaccuracy in the Company’s representations and warranties or breach by the Company is curable by the End Date by the Company then this Agreement shall not terminate pursuant to thisSection 9.1(i) as a result of such particular breach or inaccuracy until the expiration of a15-day period commencing upon delivery of written notice from Parent to the Company of such breach or inaccuracy and its intention to terminate pursuant to thisSection 9.1(i) (it being understood that this Agreement shall not terminate pursuant to thisSection 9.1(i) as a result of such particular breach or inaccuracy if such breach by the Company is cured prior to such termination becoming effective).
9.2 Effect of Termination. In the event of the termination of this Agreement as provided inSection 9.1, this Agreement shall be of no further force or effect;provided,however, that (a) thisSection 9.2,Section 5.8,Section 9.3,Section 10 and the definitions of the defined terms in such Sections shall survive the termination of this Agreement and shall remain in full force and effect, (b) the termination of this Agreement and the provisions ofSection 9.3 shall not relieve any Party of any liability for fraud or for any willful and material breach of any representation, warranty, covenant, obligation or other provision contained in this Agreement, and (c) in the event this Agreement is terminated (i) by the Company pursuant toSection 9.1(h), then Parent shall pay to the Company an amount equal to $500,000 within five Business Days of terminating this Agreement, (ii) by Parent pursuant toSection 9.1(i), then the Company shall pay to Parent an amount equal to $500,000 within five Business Days of terminating this Agreement.
9.3 Expenses; Termination Fees.
(a) Except as set forth in thisSection 9.3, whether or not the Merger is consummated, (i) all Parent Transaction Expenses shall be paid by Parent (or on behalf of Parent) at or prior to the Closing and (ii) all Company Transaction Expenses shall be paid by the Company.
(b) If (i) this Agreement is terminated by the Company pursuant toSection 9.1(f), and (ii) an Acquisition Proposal with respect to Parent shall have been publicly announced or disclosed or otherwise communicated to Parent or the Parent Board after the date of this Agreement but prior to the termination of this Agreement, and (iii) within 12 months after the date of such termination, Parent enters into a definitive agreement with respect to any Subsequent Transaction, then Parent shall pay to the Company an amount equal to
A-64
$1,000,000 within five Business Days of such entry into a definitive agreement with respect to a Subsequent Transaction.
(c) If (i) this Agreement is terminated by Parent pursuant toSection 9.1(g), and (ii) an Acquisition Proposal with respect to the Company shall have been publicly announced or disclosed or otherwise communicated to the Company or the Company Board after the date of this Agreement but prior to the termination of this Agreement, and (iii) within 12 months after the date of such termination, the Company enters into a definitive agreement with respect to any Subsequent Transaction, then the Company shall pay to Parent an amount equal to $1,000,000 within five Business Days of such entry into a definitive agreement with respect to a Subsequent Transaction.
(d) If this Agreement is terminated (i) by Parent pursuant toSection 9.1(d), or (ii) by the Company pursuant toSection 9.1(b) and the Company Stockholder Written Consent evidencing the Required Company Stockholder Vote has not been obtained by the Company, then the Company shall pay to Parent within five Business Days of such termination an amount equal to $2,000,000.
(e) If (i) this Agreement is terminated by either Parent or the Company pursuant toSection 9.1(e), or (ii) by Parent pursuant toSection 9.1(b) and the Required Parent Stockholder Vote has not been obtained by Parent, then Parent shall pay to the Company within five Business Days of such termination an amount equal to $2,000,000
(f) Any fee payable by the Company or Parent underSection 9.2 or thisSection 9.3 shall be paid by wire transfer of same day funds. If a Party fails to pay when due any amount payable by it underSection 9.2 or thisSection 9.3, then such Party shall pay to the other Party interest on such overdue amount (for the period commencing as of the date such overdue amount was originally required to be paid and ending on the date such overdue amount is actually paid to the other Party in full) at a rate per annum equal to the “prime rate” (as published inThe Wall Street Journal or any successor thereto) in effect on the date such overdue amount was originally required to be paid.
(g) The Parties agree that, (i) subject toSection 9.2, any fee payable by Parent to the Company under thisSection 9.3, in the circumstances in which it is owed in accordance with the terms of this Agreement, constitute the sole and exclusive remedy of the Company following the termination of this Agreement under the circumstances described in thisSection 9.3, it being understood that in no event shall Parent be required to pay the amounts payable pursuant to thisSection 9.3 on more than one occasion and (ii) following payment of any fee payable by Parent to the Company under thisSection 9.3 (A) Parent shall have no further liability to the Company in connection with or arising out of this Agreement or the termination thereof, any breach of this Agreement by Parent giving rise to such termination, or the failure of the Contemplated Transactions to be consummated, (B) neither the Company nor any of its Affiliates shall be entitled to bring or maintain any other claim, action or proceeding against Parent or Merger Sub or seek to obtain any recovery, judgment or damages of any kind against such Parties (or any partner, member, stockholder, director, officer, employee, Subsidiary, Affiliate, agent or other Representative of such Parties) in connection with or arising out of this Agreement or the termination thereof, any breach by any such Parties giving rise to such termination or the failure of the Contemplated Transactions to be consummated and (C) the Company and its Affiliates shall be precluded from any other remedy against Parent, Merger Sub and their respective Affiliates, at law or in equity or otherwise, in connection with or arising out of this Agreement or the termination thereof, any breach by such Party giving rise to such termination or the failure of the Contemplated Transactions to be consummated;provided,however, that nothing in thisSection 9.3(g) shall limit the rights of Parent and Merger Sub underSection 10.11.
(h) The Parties agree that, (i) subject toSection 9.2, any fee payable by the Company to Parent under thisSection 9.3 shall, in the circumstances in which it is owed in accordance with the terms of this Agreement, constitute the sole and exclusive remedy of Parent following the termination of this Agreement under the circumstances described in thisSection 9.3, it being understood that in no event shall the Company be
A-65
required to pay the amounts payable pursuant to thisSection 9.3 on more than one occasion and (ii) following payment of any fee payable by the Company to Parent under thisSection 9.3 (A) the Company shall have no further liability to Parent in connection with or arising out of this Agreement or the termination thereof, any breach of this Agreement by the Company giving rise to such termination, or the failure of the Contemplated Transactions to be consummated, (B) neither Parent nor any of its Affiliates shall be entitled to bring or maintain any other claim, action or proceeding against the Company or seek to obtain any recovery, judgment or damages of any kind against such Parties (or any partner, member, stockholder, director, officer, employee, Subsidiary, Affiliate, agent or other Representative of such Parties) in connection with or arising out of this Agreement or the termination thereof, any breach by any such Parties giving rise to such termination or the failure of the Contemplated Transactions to be consummated and (C) Parent and its Affiliates shall be precluded from any other remedy against the Company and its Affiliates, at law or in equity or otherwise, in connection with or arising out of this Agreement or the termination thereof, any breach by such Party giving rise to such termination or the failure of the Contemplated Transactions to be consummated;provided,however, that nothing in thisSection 9.3(h) shall limit the rights of the Company underSection 10.11.
(i) Each of the Parties acknowledges that (i) the agreements contained in thisSection 9.3 are an integral part of the Contemplated Transactions, (ii) without these agreements, the Parties would not enter into this Agreement and (iii) any amount payable pursuant to thisSection 9.3 is not a penalty, but rather is liquidated damages in a reasonable amount that will compensate the Company in the circumstances in which such amount is payable.
Section 10. MISCELLANEOUS PROVISIONS
10.1 Non-Survival of Representations and Warranties. The representations and warranties of the Company, Parent and Merger Sub contained in this Agreement or any certificate or instrument delivered pursuant to this Agreement shall terminate at the Effective Time, and only the covenants that by their terms survive the Effective Time and thisSection 10 shall survive the Effective Time.
10.2 Amendment. This Agreement may be amended with the approval of the respective boards of directors of the Company, Merger Sub and Parent at any time (whether before or after the adoption and approval of this Agreement by the Company’s stockholders or before or after obtaining the Required Parent Stockholder Vote);provided,however, that after any such approval of this Agreement by a Party’s stockholders, no amendment shall be made which by Law requires further approval of such stockholders without the further approval of such stockholders. This Agreement may not be amended except by an instrument in writing signed on behalf of each of the Company, Merger Sub and Parent.
(a) No failure on the part of any Party to exercise any power, right, privilege or remedy under this Agreement, and no delay on the part of any Party in exercising any power, right, privilege or remedy under this Agreement, shall operate as a waiver of such power, right, privilege or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise thereof or of any other power, right, privilege or remedy.
(b) No Party shall be deemed to have waived any claim arising out of this Agreement, or any power, right, privilege or remedy under this Agreement, unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered on behalf of such Party and any such waiver shall not be applicable or have any effect except in the specific instance in which it is given.
10.4 Entire Agreement; Counterparts; Exchanges by Electronic Transmission. This Agreement and the other agreements referred to in this Agreement constitute the entire agreement and supersede all prior agreements and understandings, both written and oral, among or between any of the Parties with respect to the
A-66
subject matter hereof and thereof;provided,however, that the Confidentiality Agreement shall not be superseded and shall remain in full force and effect in accordance with its terms. This Agreement may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Agreement (in counterparts or otherwise) by all Parties by electronic transmission in .PDF format shall be sufficient to bind the Parties to the terms and conditions of this Agreement.
10.5 Applicable Law; Jurisdiction. This Agreement shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of laws. In any action or proceeding between any of the Parties arising out of or relating to this Agreement or any of the Contemplated Transactions, each of the Parties: (a) irrevocably and unconditionally consents and submits to the exclusive jurisdiction and venue of the Court of Chancery of the State of Delaware or, to the extent such court does not have subject matter jurisdiction, the United States District Court for the District of Delaware or, to the extent that neither of the foregoing courts has jurisdiction, the Superior Court of the State of Delaware; (b) agrees that all claims in respect of such action or proceeding shall be heard and determined exclusively in accordance with clause (a) of thisSection 10.5; (c) waives any objection to laying venue in any such action or proceeding in such courts; (d) waives any objection that such courts are an inconvenient forum or do not have jurisdiction over any Party; (e) agrees that service of process upon such Party in any such action or proceeding shall be effective if notice is given in accordance withSection 10.8 of this Agreement; and (f) irrevocably and unconditionally waives the right to trial by jury.
10.6 Attorneys’ Fees. In any action at law or suit in equity to enforce this Agreement or the rights of any of the Parties, the prevailing Party in such action or suit (as determined by a court of competent jurisdiction) shall be entitled to recover its reasonableout-of-pocket attorneys’ fees and all other reasonable costs and expenses incurred in such action or suit.
10.7 Assignability. This Agreement shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties and their respective successors and permitted assigns;provided,however, that neither this Agreement nor any of a Party’s rights or obligations hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s prior written consent shall be void and of no effect.
10.8 Notices. All notices and other communications hereunder shall be in writing and shall be deemed to have been duly delivered and received hereunder (a) one Business Day after being sent for next Business Day delivery, fees prepaid, via a reputable international overnight courier service, (b) upon delivery in the case of delivery by hand, or (c) on the date delivered in the place of delivery if sent by email (with a written or electronic confirmation of delivery) prior to 5:00 p.m. Central time, otherwise on the next succeeding Business Day, in each case to the intended recipient as set forth below:
if to Parent or Merger Sub:
GTx, Inc.
175 Toyota Plaza, 7th Floor
Memphis, TN 38103
Attention: Marc S. Hanover; Henry P. Doggrell
Email: mhanover@gtxinc.com; hdoggrell@gtxinc.com
with a copy to (which shall not constitute notice):
Cooley LLP
101 California Street, 5th Floor
San Francisco, CA 94111-5800
Attention: Chadwick Mills, Laura Medina, Kassendra Galindo
Email: cmills@cooley.com, lmedina@cooley.com, kgalindo@cooley.com
A-67
if to the Company:
Oncternal Therapeutics, Inc.
3525 Del Mar heights Rd., #821
San Diego, CA 92130
Attention:
Email:
with a copy to (which shall not constitute notice):
Latham & Watkins LLP
12670 High Bluff Drive
San Diego, CA 92130
Attention:
Email:
10.9 Cooperation. Each Party agrees to cooperate fully with the other Party and to execute and deliver such further documents, certificates, agreements and instruments and to take such other actions as may be reasonably requested by the other Party to evidence or reflect the Contemplated Transactions and to carry out the intent and purposes of this Agreement.
10.10 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions of this Agreement or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If a final judgment of a court of competent jurisdiction declares that any term or provision of this Agreement is invalid or unenforceable, the Parties agree that the court making such determination shall have the power to limit such term or provision, to delete specific words or phrases or to replace such term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be valid and enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the Parties agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term or provision.
10.11 Other Remedies; Specific Performance. Except as otherwise provided herein, any and all remedies herein expressly conferred upon a Party will be deemed cumulative with and not exclusive of any other remedy conferred hereby, or by law or equity upon such Party, and the exercise by a Party of any one remedy will not preclude the exercise of any other remedy. The Parties agree that irreparable damage for which monetary damages, even if available, would not be an adequate remedy, would occur in the event that any Party does not perform the provisions of this Agreement (including failing to take such actions as are required of it hereunder to consummate this Agreement) in accordance with its specified terms or otherwise breaches such provisions. Accordingly, the Parties acknowledge and agree that the Parties shall be entitled to an injunction, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof, in addition to any other remedy to which they are entitled at law or in equity. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance or other equitable relief on the basis that any other Party has an adequate remedy at law or that any award of specific performance is not an appropriate remedy for any reason at law or in equity. Any Party seeking an injunction or injunctions to prevent breaches of this Agreement shall not be required to provide any bond or other security in connection with any such order or injunction.
10.12 No Third Party Beneficiaries. Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person (other than the Parties and the D&O Indemnified Parties to the extent of their respective rights pursuant toSection 5.6) any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
A-68
(a) References to “cash,” “dollars” or “$” are to U.S. dollars.
(b) For purposes of this Agreement, whenever the context requires: the singular number shall include the plural, and vice versa; the masculine gender shall include the feminine and neuter genders; the feminine gender shall include the masculine and neuter genders; and the neuter gender shall include masculine and feminine genders.
(c) The Parties have participated jointly in the negotiating and drafting of this Agreement and agree that any rule of construction to the effect that ambiguities are to be resolved against the drafting Party shall not be applied in the construction or interpretation of this Agreement, and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any provision of this Agreement.
(d) As used in this Agreement, the words “include” and “including,” and variations thereof, shall not be deemed to be terms of limitation, but rather shall be deemed to be followed by the words “without limitation.”
(e) Except as otherwise indicated, all references in this Agreement to “Sections,” “Exhibits” and “Schedules” are intended to refer to Sections of this Agreement and Exhibits and Schedules to this Agreement, respectively.
(f) Any reference to legislation or to any provision of any legislation shall include any modification, amendment,re-enactment thereof, any legislative provision substituted therefore and all rules, regulations, and statutory instruments issued or related to such legislations.
(g) The bold-faced headings and table of contents contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement and shall not be referred to in connection with the construction or interpretation of this Agreement.
(h) The Parties agree that each of the Company Disclosure Schedule and the Parent Disclosure Schedule shall be arranged in sections and subsections corresponding to the numbered and lettered sections and subsections contained in this Agreement. The disclosures in any section or subsection of the Company Disclosure Schedule or the Parent Disclosure Schedule shall qualify other sections and subsections in this Agreement to the extent it is readily apparent on its face from a reading of the disclosure that such disclosure is applicable to such other sections and subsections.
(i) Each of “delivered” or “made available” means, with respect to any documentation, that prior to 11:59 p.m. (Central time) on the date that is two Business Days prior to the date of this Agreement (i) a copy of such material has been posted to and made available by a Party to the other Party and its Representatives in the electronic data room maintained by such disclosing Party or (ii) such material is disclosed in the Parent SEC Documents filed with the SEC prior to the date hereof and publicly made available on the SEC’s Electronic Data Gathering Analysis and Retrieval system.
(j) Whenever the last day for the exercise of any privilege or the discharge of any duty hereunder shall fall upon a Saturday, Sunday, or any date on which banks in New York, New York are authorized or obligated by Law to be closed, the Party having such privilege or duty may exercise such privilege or discharge such duty on the next succeeding day which is a regular Business Day.
(Remainder of page intentionally left blank)
A-69
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
| GTX, INC. | ||
| By: | /s/ Marc Hannover | |
| Name: | Marc Hanover | |
| Title: | Chief Executive Officer | |
A-70
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
| GRIZZLY MERGER SUB, INC. | ||
| By: | /s/ Henry Doggrell | |
| Name: | Henry Doggrell | |
| Title: | Chief Executive Officer | |
A-71
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first above written.
| ONCTERNAL THERAPEUTICS, INC. | ||
| By: | /s/ James Breitmeyer | |
| Name: | James Breitmeyer | |
| Title: President and Chief Executive Officer | ||
A-72
EXHIBIT A
CERTAIN DEFINITIONS
(a) For purposes of this Agreement (including this Exhibit A):
“Acquisition Inquiry” means, with respect to a Party, an inquiry, indication of interest or request for information (other than an inquiry, indication of interest or request for information made or submitted by the Company, on the one hand, or Parent, on the other hand, to the other Party) that would reasonably be expected to lead to an Acquisition Proposal.
“Acquisition Proposal” means, with respect to a Party, any offer or proposal, whether written or oral (other than an offer or proposal made or submitted by or on behalf of the Company or any of its Affiliates, on the one hand, or by or on behalf of Parent or any of its Affiliates, on the other hand, to the other Party) contemplating or otherwise relating to any Acquisition Transaction with such Party.
“Acquisition Transaction” means any transaction or series of related transactions involving:
(i) any merger, consolidation, amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction: (i) in which a Party is a constituent entity; (ii) in which a Person or “group” (as defined in the Exchange Act and the rules promulgated thereunder) of Persons directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the outstanding securities of any class of voting securities of a Party or any of its Subsidiaries; or (iii) in which a Party or any of its Subsidiaries issues securities representing more than 20% of the outstanding securities of any class of voting securities of such Party or any of its Subsidiaries; or
(ii) any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for 20% or more of the consolidated book value or the fair market value of the assets of a Party and its Subsidiaries, taken as a whole.
“Affiliate” of a Person means any other Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such Person. The term “control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Agreement” means the Agreement and Plan of Merger and Reorganization to which thisExhibit A is attached, as it may be amended from time to time.
“Business Day” means any day other than a Saturday, Sunday or other day on which banks in New York, New York are authorized or obligated by Law to be closed.
“Cash and Cash Equivalents” means all (a) cash and cash equivalents (excluding Restricted Cash) and (b) marketable securities, in each case determined in accordance with GAAP, consistently applied.
“CCC” means the California Corporations Code, as amended.
“Code” means the Internal Revenue Code of 1986, as amended.
“Combined Transaction Expenses” means legal expenses incurred by the Company in preparing the Registration Statement, Proxy Statement, and any amendments and supplements thereto, preparing responses to any SEC comments, and drafting any charter amendments and the Amended Plan (and in each case, the related
A-A-1
disclosure required in the Registration Statement and Proxy Statement) and preparing such other transaction documents in connection with the Contemplated Transaction as Cooley and Latham & Watkins agree shall be produced by Latham & Watkins, which amount shall not exceed $250,000 in the aggregate, and in each case, shall be evidenced by an invoice delivered by the Company to Parent no later than seven Business Days prior to Closing.
“Company Affiliate” means any Person that is (or at any relevant time was) under common control with the Company within the meaning of Sections 414(b), (c), (m) and (o) of the Code, and the regulations issued thereunder.
“Company Associate” means any current or former employee, independent contractor, officer or director of the Company.
“Company Board” means the board of directors of the Company.
“Company Capital Stock” means the Company Common Stock and the Company Preferred Stock.
“Company Cash Amount” means the Cash and Cash Equivalents of the Company as of the Determination Date, as calculated in accordance withSection 1.13.
“Company Change in Circumstance” means a change in circumstances (other than an Acquisition Proposal) that affects the business, assets or operations of the Company that occurs or arises after the date of this Agreement.
“Company Closing Tax Opinion” means a written opinion from Latham & Watkins, dated as of the Closing Date, based on the facts, representations, assumptions and exclusions set forth or described in such opinion, and substantially in the form set forth inSection 5.10(c)(4) of the Company Disclosure Schedule, to the effect that the Merger will qualify for the Intended Tax Treatment. In rendering such opinion, Latham & Watkins shall be entitled to rely upon customary assumptions, representations, warranties and covenants reasonably satisfactory to it, including representations set forth in certificates of officers of Parent and the Company, in substantially the forms set forth inSection 5.10(c)(ii) of the Parent Disclosure Schedule andSection 5.10(c)(ii) of the Company Disclosure Schedule.
“Company Common Stock” means the Common Stock, $0.0001 par value per share, of the Company.
“Company Contract” means any Contract: (a) to which the Company or any of its Subsidiaries is a Party; (b) by which the Company or any of its Subsidiaries or any Company IP or any other asset of the Company or its Subsidiaries is or may become bound or under which the Company or any of its Subsidiaries has, or may become subject to, any obligation; or (c) under which the Company or any of its Subsidiaries has or may acquire any right or interest.
“CompanyERISA Affiliate” means any corporation or trade or business (whether or not incorporated) which is (or at any relevant time was) treated with the Company or any of its Subsidiaries as a single employer within the meaning of Section 414 of the Code.
“Company Fundamental Representations” means the representations and warranties of the Company set forth inSections 2.1 (Due Organization; Subsidiaries),2.3 (Authority; Binding Nature of Agreement),2.6(a) and(c) (Capitalization) and2.20 (No Financial Advisors).
“Company IP” means all Intellectual Property Rights that are owned or purported to be owned by, assigned to, or licensed by, the Company or its Subsidiaries.
A-A-2
“Company Material Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of determination of the occurrence of a Company Material Adverse Effect, has or would reasonably be expected to have a material adverse effect on the business, financial condition, assets, liabilities or results of operations of the Company or its Subsidiaries, taken as a whole;provided,however, that Effects arising or resulting from the following shall not be taken into account in determining whether there has been a Company Material Adverse Effect: (a) general business, economic or political conditions affecting the industry in which the Company and its Subsidiaries operate, (b) any natural disaster or any acts of war, armed hostilities or terrorism, (c) changes in financial, banking or securities markets, (d) the failure of the Company to meet internal or analysts’ expectations or projections or the results of operations of the Company, (e) any clinical trial programs or studies, including any adverse data, event or outcome arising out of or relating to any such programs or studies, (f) any change in, or any compliance with or action taken for the purpose of complying with, any Law or GAAP (or interpretations of any Law or GAAP), (g) resulting from the announcement of this Agreement or the pendency of the Contemplated Transactions, or (h) resulting from the taking of any action, or the failure to take any action, by the Company that is required to be taken by this Agreement; except in each case with respect to clauses (a) through (c), to the extent disproportionately affecting the Company and its Subsidiaries, taken as a whole, relative to other similarly situated companies in the industries in which the Company and its Subsidiaries operate.
“Company Options” means options or other rights to purchase shares of Company Common Stock issued by the Company.
“Company Plans” means the Oncternal Therapeutics, Inc. 2016 Equity Incentive Plan and the Oncternal Therapeutics, Inc. 2015 Equity Incentive Plan.
“Company Registration Statement Tax Opinion” means a written opinion from Latham & Watkins, dated as of such date as may be required by the SEC in connection with the filing of the Registration Statement, based on the facts, representations, assumptions and exclusions set forth or described in such opinion, and substantially in the form set forth inSection 5.10(c)(2) of the Company Disclosure Schedule, to the effect that the Merger will qualify for the Intended Tax Treatment. In rendering such opinion, Latham & Watkins shall be entitled to rely upon customary assumptions, representations, warranties and covenants reasonably satisfactory to it, including representations set forth in certificates of officers of Parent and the Company, in substantially the forms set forth inSection 5.10(c)(i) of the Parent Disclosure Schedule andSection 5.10(c)(i) of the Company Disclosure Schedule.
“Company Target” means $10,500,000.
“Company Transaction Expenses” means all fees and expenses incurred by the Company at or prior to the Effective Time in connection with the Contemplated Transactions and this Agreement, including (a) any fees and expenses of legal counsel and accountants, the maximum amount of fees and expenses payable to financial advisors, investment bankers, brokers, consultants, and other advisors of the Company (other than the Combined Transaction Expenses); and (b) 50% of (i) the fees paid to the SEC in connection with filing the Registration Statement, the Proxy Statement, and any amendments and supplements thereto with the SEC; (ii) the Nasdaq Fees; (iii) the fees and expenses paid or payable to the Exchange Agent pursuant to the engagement agreement with the Exchange Agent; and (iv) any fees and expenses incurred Toppan Merrill, Broadridge or the proxy solicitor in connection with the filing and distribution of the Registration Statement and any amendments and supplements thereto with the SEC (without duplication of the fees and expenses addressed in clause (b)(i) above).
“Company Triggering Event” shall be deemed to have occurred if: (a) the Company shall have made a Company Board Adverse Recommendation Change; (b) the Company Board or any committee thereof shall have publicly approved, endorsed or recommended any Acquisition Proposal; (c) the Company shall have entered into any letter of intent or similar document relating to any Acquisition Proposal; or (d) the Company, or any director or officer of the Company, shall have willfully and intentionally breached the provisions set forth inSection 4.5.
A-A-3
“Company Unaudited Interim Balance Sheet” means the unaudited consolidated balance sheet of the Company and its consolidated Subsidiaries for the period ended September 30, 2018 provided to Parent prior to the date of this Agreement.
“Company Warrant” means the warrants to purchase capital stock of the Company listed on Section B of the Company Disclosure Schedule.
“Confidentiality Agreement” means theNon-Disclosure Agreement, dated as of November 30, 2018, between the Company and Parent.
“Consent” means any approval, consent, ratification, permission, waiver or authorization (including any Governmental Authorization).
“Consideration” means (a) the Exchange Ratio used to determine the number of shares of Parent Common Stock to be issued to the holders of Company Common Stock as contemplated by Section 1.5 of this Agreement and the number of Parent Options and Parent Warrants to be substituted for the Company Options and Company Warrants to be assumed by Parent as contemplated bySection 5.5 and (b) the right of the holders of Parent Common Stock as of immediately prior to the Effective Time (the “CVR Holders”) to receive contingent cash payments pursuant to the CVR Agreement.
“Contemplated Transactions” means the Merger, the Preferred Stock Conversion and the other transactions and actions contemplated by this Agreement, including the Nasdaq Reverse Split and the CVR Agreement.
“Contract” means, with respect to any Person, any written or oral agreement, contract, subcontract, lease (whether for real or personal property), mortgage, license, sublicense or other legally binding commitment or undertaking of any nature to which such Person is a party or by which such Person or any of its assets are bound or affected under applicable Law.
“DGCL” means the General Corporation Law of the State of Delaware.
“Effect” means any effect, change, event, circumstance, or development.
“Encumbrance” means any lien, pledge, hypothecation, charge, mortgage, security interest, lease, license, option, easement, reservation, servitude, adverse title, claim, infringement, interference, option, right of first refusal, preemptive right, community property interest or restriction or encumbrance of any nature (including any restriction on the voting of any security, any restriction on the transfer of any security or other asset, any restriction on the receipt of any income derived from any asset, any restriction on the use of any asset and any restriction on the possession, exercise or transfer of any other attribute of ownership of any asset).
“Enforceability Exceptions” means the (a) Laws of general application relating to bankruptcy, insolvency and the relief of debtors; and (b) rules of law governing specific performance, injunctive relief and other equitable remedies.
“Entity” means any corporation (including anynon-profit corporation), partnership (including any general partnership, limited partnership or limited liability partnership), joint venture, estate, trust, company (including any company limited by shares, limited liability company or joint stock company), firm, society or other enterprise, association, organization or entity, and each of its successors.
“Environmental Law” means any federal, state, local or foreign Law relating to pollution or protection of human health or the environment (including ambient air, surface water, ground water, land surface or subsurface strata), including any Law or regulation relating to emissions, discharges, releases or threatened releases of Hazardous Materials, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials.
A-A-4
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended.
“Exchange Act” means the Securities Exchange Act of 1934.
“Exchange Ratio” means, subject toSection 1.5(g), the following ratio (rounded to four decimal places): the quotient obtained by dividing (a) the Company Merger Shares by (b) the Company Outstanding Shares, in which:
| • | “Company Cash Balance Adjustment Shares” means (i) if the Company Cash Amount is less than the Company Target, then an amount equal to the quotient of (A) the Company Target,less the Company Cash Amount,divided by (B) 1.207, or (ii) if the Company Cash Amount is greater than the Company Target, then zero. |
| • | “Company Allocation Percentage” means 75%. |
| • | “Company Merger Shares” means the sum of (i) the product of (A) the Post-Closing Parent Sharesmultiplied by (B) the Company Allocation Percentage,minus (ii) the Company Cash Balance Adjustment Shares,minus(iii) the Parent Cash Balance Adjustment Shares if the Parent Cash Amount is greater than the Parent Target, plus (iv) the Parent Cash Balance Adjustment Shares if the Parent Cash Amount is less than the Parent Target. |
| • | “Company Outstanding Shares” means the total number of shares of Company Capital Stock outstanding immediately prior to the Effective Time expressed on anas-converted to Company Common Stock basis and assuming the effectiveness of the Preferred Stock Conversion, but excluding (i) the exercise of all Company Options and Company Warrants, in each case, outstanding as of immediately prior to the Effective Time, (ii) the issuance of shares of Company Capital Stock in respect of all other outstanding options, restricted stock awards, warrants or rights to receive such shares, whether conditional or unconditional and including any outstanding options, warrants or rights triggered by or associated with the consummation of the Merger, and (iii) any shares of Company Common Stock reserved for issuance. |
| • | “Parent Allocation Percentage” means 25%. |
| • | “Parent Cash Balance Adjustment Shares” means (i) if the Parent Cash Amount is less than the Parent Target, then an amount equal to the quotient of (A) the Parent Target,less the Parent Cash Amount,divided by (B) 1.207, or (ii) if the Parent Cash Amount is greater than the Parent Target, an amount equal to the quotient of (A) the Parent Cash Amount,lessthe Parent Target,divided by (B) 1.207. |
| • | “Parent Outstanding Shares” means the total number of shares of Parent Common Stock outstanding immediately prior to the Effective Time, including the total number of shares of Parent Common Stock issuable pursuant to Parent Deferred Stock Rights but excluding (i) the issuance of shares of Parent Common Stock in respect of all Parent Options, Parent Warrants and other outstanding options, warrants or rights to receive such shares (other than the Parent Deferred Stock Rights), in each case, outstanding as of immediately prior to the Effective Time; and (ii) any shares of Parent Common Stock reserved for issuance (other than shares of Parent Common Stock reserved for issuance pursuant to the Parent Deferred Stock Rights). |
| • | “Post-Closing Parent Shares” means the quotient determined bydividing (i) the Parent Outstanding Sharesby (ii) the Parent Allocation Percentage. |
“GAAP” means generally accepted accounting principles and practices in effect from time to time within the United States applied consistently throughout the period involved.
“Governmental Authorization” means any: (a) permit, license, certificate, certification, franchise, permission, approval, exemption, variance, exception, order, clearance, registration, qualification or authorization
A-A-5
issued, granted, given or otherwise made available by or under the authority of any Governmental Body or pursuant to any Law; or (b) right under any Contract with any Governmental Body.
“Governmental Body” means any: (a) nation, state, commonwealth, province, territory, county, municipality, district or other jurisdiction of any nature; (b) federal, state, local, municipal, foreign or other government; (c) governmental or quasi-governmental authority of any nature (including any governmental division, department, agency, commission, bureau, instrumentality, official, ministry, fund, foundation, center, organization, unit, body or Entity and any court or other tribunal, and for the avoidance of doubt, any taxing authority); or (d) self-regulatory organization (including Nasdaq).
“Hazardous Materials” means any pollutant, chemical, substance and any toxic, infectious, carcinogenic, reactive, corrosive, ignitable or flammable chemical, or chemical compound, or hazardous substance, material or waste, whether solid, liquid or gas, that is subject to regulation, control or remediation under any Environmental Law, including without limitation, crude oil or any fraction thereof, and petroleum products orby-products.
“HSR Act” means the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended.
“Intellectual Property Rights” means and includes all past, present, and future rights of the following types, which may exist or be created under the laws of any jurisdiction in the world: (a) rights associated with works of authorship, including exclusive exploitation rights, copyrights, moral rights, software, databases, and mask works; (b) trademarks, service marks, trade dress, logos, trade names and other source identifiers, domain names and URLs and similar rights and any goodwill associated therewith; (c) rights associated with trade secrets, know how, inventions, invention disclosures, methods, processes, protocols, specifications, techniques and other forms of technology; (d) patents and industrial property rights; and (e) other similar proprietary rights in intellectual property of every kind and nature; (f) rights of privacy and publicity; and (g) all registrations, renewals, extensions, statutory invention registrations, provisionals, continuations,continuations-in-part, provisionals, divisions, or reissues of, and applications for, any of the rights referred to in clauses “(a)” through “(f)” above (whether or not in tangible form and including all tangible embodiments of any of the foregoing, such as samples, studies and summaries), along with all rights to prosecute and perfect the same through administrative prosecution, registration, recordation or other administrative proceeding, and all causes of action and rights to sue or seek other remedies arising from or relating to the foregoing.
“IRS” means the United States Internal Revenue Service.
“Knowledge” means, with respect to an individual, that such individual is actually aware of the relevant fact or such individual would reasonably be expected to know such fact in the ordinary course of the performance of such individual’s employment responsibilities. Any Person that is an Entity shall have Knowledge if any officer or director of such Person as of the date such knowledge is imputed has Knowledge of such fact or other matter.
“Law” means any federal, state, national, foreign, material local or municipal or other law, statute, constitution, principle of common law, resolution, ordinance, code, edict, decree, rule, regulation, ruling or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Body (including under the authority of Nasdaq or the Financial Industry Regulatory Authority).
“Legal Proceeding” means any action, suit, litigation, arbitration, proceeding (including any civil, criminal, administrative, investigative or appellate proceeding), hearing, inquiry, audit, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Body or any arbitrator or arbitration panel.
“Merger Sub Board” means the board of directors of Merger Sub.
A-A-6
“Nasdaq” means the Nasdaq Stock Market, including the Nasdaq Global Select Market or such other Nasdaq market on which shares of Parent Common Stock are then listed.
“Nasdaq Reverse Split” means a reverse stock split of all outstanding shares of Parent Common Stock at a reverse stock split ratio as mutually agreed to by Parent and the Company that is effected by Parent for the purpose of maintaining compliance with Nasdaq listing standards.
“Ordinary Course of Business” means, in the case of each of the Company and Parent, such actions taken in the ordinary course of its normal operations and consistent with its past practices.
“Organizational Documents” means, with respect to any Person (other than an individual), (a) the certificate or articles of association or incorporation or organization or limited partnership or limited liability company, and any joint venture, limited liability company, operating or partnership agreement and other similar documents adopted or filed in connection with the creation, formation or organization of such Person and (b) all bylaws, regulations and similar documents or agreements relating to the organization or governance of such Person, in each case, as amended or supplemented.
“Parent Associate” means any current or former employee, independent contractor, officer or director of Parent.
“Parent Balance Sheet” means the unaudited balance sheet of Parent as of September 30, 2018 (the “Parent Balance Sheet Date”), included in Parent’s Report on Form10-Q for the quarterly period ended September 30, 2018, as filed with the SEC.
“Parent Board” means the board of directors of Parent.
“Parent Cash Amount” (i) the sum of all Cash and Cash Equivalents, short-term investments, accrued investment interest receivable, and any prepaid refundable deposits listed onSection 1.12(a) of the Parent Disclosure Schedule, in each case, of Parent as of the Determination Date, calculated in accordance withSection 1.12),less (ii) all liabilities of Parent to any current or former Parent officer, director, employee, consultant or independent contractor, including change of control payments, retention payments, severance and other employee-, consultant- or independent contractor-related termination costs, or other payments triggered by the Contemplated Transactions or pursuant to any Parent Benefit Plan, including but not limited to payments of deferred compensation, accrued but unpaid bonuses and accrued but unpaid vacation or paid time off (including related employer employment taxes on all the foregoing), regardless of whether or not such amounts are accrued or due as of the Determination Date and regardless of when paid or payable and regardless of whether such amounts will be paid or are payable as a result of actions taken at, or immediately prior to or after the Effective Time,less(iii) the Parent Transaction Expenses,plus(iv) any cash payable to Parent upon the closing of any SARM Transaction (“SARM Upfront Cash”) that has been reduced to an executed letter of intent prior to Closing;provided such letter of intent shall require the Company’s consent (which consent shall not be unreasonably withheld, delayed or conditioned); and, for the avoidance of doubt, the closing of the SARM Transaction shall not be required to occur prior to the Closing and any SARM Upfront Cash shall not be subject to any payment pursuant to the CVR (but for the avoidance of doubt, any payments in respect of such SARM Transaction other than the SARM Upfront Cash will be handled pursuant to the CVR).
“Parent Change in Circumstance” means a change in circumstances (other than an Acquisition Proposal) that affects the business, assets or operations of Parent that occurs or arises after the date of this Agreement.
“Parent Closing Price” means the volume weighted average closing trading price of a share of Parent Common Stock on Nasdaq for the five consecutive trading days ending five trading days immediately prior to the date upon which the Merger becomes effective.
A-A-7
“Parent Closing Tax Opinion” means a written opinion from Cooley, dated as of the Closing Date, based on the facts, representations, assumptions and exclusions set forth or described in such opinion, and substantially in the form set forth inSection 5.10(c)(3) of the Parent Disclosure Schedule, to the effect that the Merger will qualify for the Intended Tax Treatment. In rendering such opinion, Cooley shall be entitled to rely upon customary assumptions, representations, warranties and covenants reasonably satisfactory to it, including representations set forth in certificates of officers of Parent and the Company, in substantially the forms set forth inSection 5.10(c)(ii) of the Parent Disclosure Schedule andSection 5.10(c)(ii) of the Company Disclosure Schedule.
“Parent Common Stock” means the Common Stock, $0.01 par value per share, of Parent.
“Parent Contract” means any Contract: (a) to which Parent or Merger Sub is a party; (b) by which Parent, Merger Sub or any Parent IP or any other asset of Parent or Merger Sub is or may become bound or under which Parent or Merger Sub has, or may become subject to, any obligation; or (c) under which Parent or Merger Sub has or may acquire any right or interest.
“Parent Deferred Stock Right” means any deferred stock rights or other deferred rights to receive shares of Parent Common Stock under the Parent Stock Plans.
“ParentERISA Affiliate” means any corporation or trade or business (whether or not incorporated) which is (or at any relevant time was) treated with Parent or any of its Subsidiaries as a single employer within the meaning of Section 414 of the Code.
“Parent Fully-Diluted Shares” means the total number of shares of Parent Common Stock outstanding immediately prior to the Effective Time expressed on a fully-diluted basis, assuming the issuance of Parent Common Stock in respect of all Parent Options, Parent Warrants, Parent Deferred Stock Rights, and other outstanding options, warrants or rights to receive such shares, in each case, outstanding as of immediately prior to the Effective Time.
“Parent Fundamental Representations” means the representations and warranties of Parent and Merger Sub set forth inSections 3.1(a) and (b) (Due Organization; Subsidiaries),3.3 (Authority; Binding Nature of Agreement), 3.4 (Vote Required),3.6(a) and(c) (Capitalization) and3.21 (No Financial Advisors).
“Parent IP” means all Intellectual Property Rights that are owned or purported to be owned by, assigned to, or licensed by, Parent or its Subsidiaries.
“Parent Material Adverse Effect” means any Effect that, considered together with all other Effects that have occurred prior to the date of determination of the occurrence of a Parent Material Adverse Effect, has or would reasonably be expected to have a material adverse effect on the business, financial condition, assets, liabilities or results of operations of Parent;provided,however, that Effects arising or resulting from the following shall not be taken into account in determining whether there has been a Parent Material Adverse Effect: (a) general business, economic or political conditions affecting the industry in which Parent operates, (b) any natural disaster or any acts of war, armed hostilities or terrorism, (c) changes in financial, banking or securities markets, (d) the taking of any action required to be taken by this Agreement, (e) any change in the stock price or trading volume of Parent Common Stock (it being understood, however, that any Effect causing or contributing to any change in stock price or trading volume of Parent Common Stock may be taken into account in determining whether a Parent Material Adverse Effect has occurred, unless such Effects are otherwise excepted from this definition), (f) the failure of Parent to meet internal or analysts’ expectations or projections or the results of operations of Parent; (g) any clinical trial programs or studies, including any adverse data, event or outcome arising out of or related to any such programs or studies; (h) any change in, or any compliance with or action taken for the purpose of complying with, any Law or GAAP (or interpretations of any Law or GAAP); (i) resulting from the announcement of this Agreement or the pendency of the Contemplated Transactions; or
A-A-8
(j) resulting from the taking of any action or the failure to take any action, by Parent that is required to be taken by this Agreement, except in each case with respect to clauses (a) through (c), to the extent disproportionately affecting Parent relative to other similarly situated companies in the industries in which Parent operates.
“Parent Options” means options or other rights to purchase shares of Parent Common Stock issued by Parent.
“Parent Registration Statement Tax Opinion” means a written opinion from Cooley, dated as of such date as may be required by the SEC in connection with the filing of the Registration Statement, based on the facts, representations, assumptions and exclusions set forth or described in such opinion, and substantially in the form set forth inSection 5.10(c)(1) of the Parent Disclosure Schedule, to the effect that the Merger will qualify for the Intended Tax Treatment. In rendering such opinion, Cooley shall be entitled to rely upon customary assumptions, representations, warranties and covenants reasonably satisfactory to it, including representations set forth in certificates of officers of Parent and the Company, in substantially the forms set forth inSection 5.10(c)(i) of the Parent Disclosure Schedule andSection 5.10(c)(i) of the Company Disclosure Schedule.
“Parent Stock Plans” means, the Parent 2001 Stock Option Plan, the Parent 2002 Stock Option Plan, the Parent 2004 Equity Incentive Plan, the Parent Amended and Restated 2004Non-Employee Directors’ Stock Option Plan, the Parent 2013 Equity Incentive Plan, the Parent 2013Non-Employee Director Equity Incentive Plan, 2018 Amended and Restated Directors’ Deferred Compensation Plan, in each case, as may be amended from time to time.
“Parent Target” means $13,500,000 if the Closing occurs on or prior to April 30, 2019 (provided, that if any fees and expenses are incurred by Parent in connection with preparing its Quarterly Report on Form10-Q for the first quarter of 2019 on or prior to April 30, 2019, such amounts will be added back to the Parent Cash Amount),lessParent’s reasonable operating expenses from May 1, 2019 through the Closing.
“Parent Transaction Expenses” means all fees and expenses incurred by Parent at or prior to the Effective Time in connection with the Contemplated Transactions and this Agreement, including (a) any fees and expenses of legal counsel and accountants, the maximum amount of fees and expenses payable to financial advisors, investment bankers, brokers, consultants, and other advisors of Parent; (b) 50% of (i) the fees paid to the SEC in connection with filing the Registration Statement, the Proxy Statement, and any amendments and supplements thereto with the SEC; (ii) the Nasdaq Fees; (iii) the fees and expenses paid or payable to the Exchange Agent pursuant to the engagement agreement with the Exchange Agent; and (iv) any fees and expenses incurred by Toppan Merrill, Broadridge or the proxy solicitor in connection with the filing and distribution of the Registration Statement and any amendments and supplements thereto with the SEC (without duplication of the fees and expenses addressed in clause (b)(i) above); (c) 100% of the Combined Transaction Expenses; and (d) 100% of the D&O Tail Policy.
“Parent Triggering Event” shall be deemed to have occurred if: (a) Parent shall have failed to include in the Proxy Statement the Parent Board Recommendation or shall have made a Parent Board Adverse Recommendation Change; (b) the Parent Board or any committee thereof shall have publicly approved, endorsed or recommended any Acquisition Proposal; or (c) Parent shall have entered into any letter of intent or similar document relating to any Acquisition Proposal (other than a confidentiality agreement permitted pursuant toSection 4.4); or (d) Parent, or any director or officer of Parent, shall have willfully and intentionally breached the provisions set forth inSection 4.4.
“Parent Warrants” means the warrants to purchase capital stock of the Parent listed on Section B of the Parent Disclosure Schedule.
“Party” or “Parties” means the Company, Merger Sub and Parent.
A-A-9
“Permitted Alternative Agreement” means a definitive agreement that contemplates or otherwise relates to an Acquisition Transaction that constitutes a Superior Offer.
“Permitted Encumbrance” means: (a) any liens for current Taxes not yet due and payable or for Taxes that are being contested in good faith and for which adequate reserves have been made on the Company Unaudited Interim Balance Sheet or the Parent Balance Sheet, as applicable; (b) minor liens that have arisen in the Ordinary Course of Business and that do not (in any case or in the aggregate) materially detract from the value of the assets or properties subject thereto or materially impair the operations of the Company or any of its Subsidiaries or Parent, as applicable; (c) statutory liens to secure obligations to landlords, lessors or renters under leases or rental agreements; (d) deposits or pledges made in connection with, or to secure payment of, workers’ compensation, unemployment insurance or similar programs mandated by Law;(e) non-exclusive licenses of Intellectual Property Rights granted by the Company or any of its Subsidiaries or Parent, as applicable, in the Ordinary Course of Business and that do not (in any case or in the aggregate) materially detract from the value of the Intellectual Property Rights subject thereto; and (f) statutory liens in favor of carriers, warehousemen, mechanics and materialmen, to secure claims for labor, materials or supplies.
“Person” means any individual, Entity or Governmental Body.
“Proxy Statement” means the proxy statement to be sent to Parent’s stockholders in connection with the Parent Stockholders’ Meeting.
“Reference Date” means March 5, 2019.
“Registered IP” means all Intellectual Property Rights that are registered or issued under the authority of any Governmental Body, including all patents, registered copyrights, registered mask works, and registered trademarks, service marks and trade dress, and all applications for any of the foregoing.
“Registration Statement” means the registration statement on FormS-4 (or any other applicable form under the Securities Act to register Parent Common Stock) to be filed with the SEC by Parent registering the public offering and sale of Parent Common Stock to some or all holders of Company Capital Stock in the Merger, including all shares of Parent Common Stock to be issued in exchange for all shares of Company Capital Stock in the Merger, as said registration statement may be amended prior to the time it is declared effective by the SEC.
“Representatives” means directors, officers, employees, agents, attorneys, accountants, investment bankers, advisors and representatives.
“Restricted Cash” means any cash or cash equivalents that are unavailable for dividend or distribution as a result of the requirements of applicable Law or the dividend or distribution of which is subject to Tax, including any withholding or other similar Tax, or the dividend or distribution of which would produce other adverse Tax consequences for Parent or its Affiliates.
“Sarbanes-Oxley Act” means the Sarbanes-Oxley Act of 2002.
“SARM Transaction” means a sale of Parent’s SARM assets.
“SEC” means the United States Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended.
“Series A Preferred Stock” means the shares of the Series A Preferred Stock of the Company, par value $0.0001 per share.
A-A-10
“Series B Preferred Stock” means the shares of the Series B Preferred Stock of the Company, par value $0.0001 per share.
“SeriesB-2 Preferred Stock” means the shares of the SeriesB-2 Preferred Stock of the Company, par value $0.0001 per share.
“Series C Preferred Stock” means the shares of the Series C Preferred Stock of the Company, par value $0.0001 per share.
“Subsequent Transaction” means any Acquisition Transaction (with all references to 20% in the definition of Acquisition Transaction being treated as references to 85% for these purposes).
An entity shall be deemed to be a “Subsidiary” of a Person if such Person directly or indirectly owns or purports to own, beneficially or of record, (a) an amount of voting securities or other interests in such entity that is sufficient to enable such Person to elect at least a majority of the members of such entity’s board of directors or other governing body, or (b) at least 50% of the outstanding equity, voting, beneficial or financial interests in such Entity.
“Superior Offer” means an unsolicited bona fide written Acquisition Proposal (with all references to 20% in the definition of Acquisition Transaction being treated as references to greater than 80% for these purposes) that: (a) was not obtained or made as a direct or indirect result of a breach of (or in violation of) this Agreement; and (b) is on terms and conditions that the Parent Board or the Company Board, as applicable, determines in good faith, based on such matters that it deems relevant (including the likelihood of consummation thereof), as well as any written offer by the other Party to this Agreement to amend the terms of this Agreement, and following consultation with its outside legal counsel and outside financial advisors, if any, are more favorable, from a financial point of view, to Parent’s stockholders or the Company’s stockholders, as applicable, than the terms of the Contemplated Transactions;provided, that any such offer shall not be deemed to be a “Superior Offer” if any financing required to consummate the transaction contemplated by such offer is not reasonably capable of being obtained by such third party.
“Takeover Statute” means any “fair price,” “moratorium,” “control share acquisition” or other similar anti-takeover Law.
“Tax” means any federal, state, local, foreign or other tax, including any income, capital gain, gross receipts, capital stock, profits, transfer, estimated, registration, stamp, premium, escheat, unclaimed property, customs duty, ad valorem, occupancy, occupation, alternative,add-on, windfall profits, value added, severance, property, business, production, sales, use, license, excise, franchise, employment, payroll, social security, disability, unemployment, workers’ compensation, national health insurance, withholding or other taxes, duties, fees, assessments or governmental charges, surtaxes or deficiencies thereof of any kind whatsoever, however denominated, and including any fine, penalty, addition to tax or interest imposed by a Governmental Body with respect thereto.
“Tax Return” means any return (including any information return), report, statement, declaration, estimate, schedule, notice, notification, form, election, certificate or other document, and any amendment or supplement to any of the foregoing, filed with or submitted to, or required to be filed with or submitted to, any Governmental Body in connection with the determination, assessment, collection or payment of any Tax or in connection with the administration, implementation or enforcement of or compliance with any Law relating to any Tax.
“Treasury Regulations” means the United States Treasury regulations promulgated under the Code.
“WARN Act” means the Worker Adjustment Retraining and Notification Act of 1988, as amended, or any similar state or local plant closing mass layoff statute, rule or regulation.
A-A-11
b) Each of the following terms is defined in the Section set forth opposite such term:
Term | Section | |
Accounting Firm | 1.12(e) | |
Allocation Certificate | 5.17(a) | |
Amended Plan | 5.20 | |
Anti-Bribery Laws | 2.23 | |
Anticipated Closing Date | 1.12(a) | |
Board Observer | 5.12(c) | |
Business Associate Agreement | 2.14(f) | |
Certificate of Merger | 1.3 | |
Certifications | 3.7(a) | |
Closing | 1.3 | |
Closing Date | 1.3 | |
Cooley | 5.10(c) | |
Company | Preamble | |
Company Audited Financial Statements | 5.17 | |
Company Benefit Plan | 2.17(a) | |
Company Board Adverse Recommendation Change | 5.2(d) | |
Company Board Recommendation | 5.2(d) | |
Company Cash Calculation | 1.13(a) | |
Company Cash Schedule | 1.13(a) | |
Company Disclosure Schedule | Section 2 | |
Company Financials | 2.7(a) | |
CompanyIn-bound Licenses | 2.12(d) | |
Company Interim Financial Statements | 5.17 | |
CompanyLock-Up Agreement | Recitals | |
Company Material Contract | 2.13(a) | |
CompanyOut-bound Licenses | 2.12(d) | |
Company Permits | 2.14(b) | |
Company Plan | 2.6(c) | |
Company Preferred Stock | 2.6(a) | |
Company Real Estate Leases | 2.11 | |
Company Signatories | Recitals | |
Company Stock Certificate | 1.7 | |
Company Stockholder Matters | 5.2(a) | |
Company Stockholder Support Agreement | Recitals | |
Company Stockholder Written Consent | 2.4 | |
Costs | 5.6(a) | |
CVR | 1.6 | |
CVR Agreement | Recitals | |
D&O Indemnified Parties | 5.6(a) | |
D&O Tail Policy | 5.6(d) | |
Determination Date | 1.12(a) | |
Determination Notice | 5.3(d)(i) | |
Dispute Notice | 1.12(b) | |
Dissenting Shares | 1.9(a) | |
Drug Regulatory Agency | 2.14(a) | |
Effective Time | 1.3 | |
End Date | 9.1(b) | |
Exchange Agent | 1.8(a) | |
Exchange Fund | 1.8(a) |
A-A-12
Term | Section | |
FDA | 2.14(a) | |
FDCA | 2.14(a) | |
FLSA | 2.17(p) | |
HIPAA | 2.14(f) | |
Information Statement | 5.2(a) | |
Intended Tax Treatment | 5.10(a) | |
Investor Agreements | 2.22(b) | |
Latham | 5.10(c) | |
Liability | 2.9 | |
Merger | Recitals | |
Merger Consideration | 1.5(a)(ii) | |
Merger Notification Filings | 5.4(b) | |
Merger Sub | Preamble | |
Nasdaq Fees | 5.9 | |
Nasdaq Listing Application | 5.9 | |
Other Parent Stockholder Matter | 5.3(a)(v) | |
Parent | Preamble | |
Parent Benefit Plan | 3.17(a) | |
Parent Board Adverse Recommendation Change | 5.3(c) | |
Parent Board Recommendation | 5.3(c) | |
Parent Budget | 4.1(b)(v) | |
Parent Cash Calculation | 1.12(a) | |
Parent Cash Schedule | 1.12(a) | |
Parent Designees | 5.12 | |
Parent Disclosure Schedule | Section 3 | |
ParentIn-bound License | 3.12(d) | |
ParentLock-Up Agreement | Recitals | |
Parent Material Contract | 3.13 | |
ParentOut-bound License | 3.12(d) | |
Parent Permits | 3.14(b) | |
Parent Real Estate Leases | 3.11 | |
Parent SEC Documents | 3.7(a) | |
Parent Signatories | Recitals | |
Parent Stockholder Matters | 5.3(a)(iv) | |
Parent Stockholder Support Agreement | Recitals | |
Parent Stockholders’ Meeting | 5.3(a)(iv) | |
Pre-Closing Period | 4.1(a) | |
Preferred Stock Conversion | 5.21 | |
Required Company Stockholder Vote | 2.4 | |
Required Parent Stockholder Vote | 3.4 | |
Response Date | 1.12(b) | |
Sensitive Data | 2.12(g) | |
Stockholder Notice | 5.2(c) | |
Surviving Corporation | 1.1 |
A-A-13
Exhibit C
Officers
Name | Title | |
| James Breitmeyer | President and Chief Executive Officer | |
| Richard Vincent | Chief Financial Officer | |
| Lauren Otsuki | Chief Operating Officer | |
Board Designees – Company
Name
David Hale
James Breitmeyer
Yanjun Liu
Xin Nakanishi
Board Designees – Parent
Name
Michael Carter, who shall be placed in the same class of Parent’s current directors elected at Parent’s 2019 annual meeting
Robert Wills
Additional Directors
Charles Theuer
William LaRue
Director to be designated by the Company
Board Observer – Parent
Name
J.R. Hyde, III (or his designee)
A-C-1
AMENDMENT NO. 1 AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
THIS AMENDMENT NO. 1TO AGREEMENTAND PLANOF MERGERANDREORGANIZATION (this “Amendment”), is made and entered into as of April 30, 2019, by and among GTx, Inc., a Delaware corporation (“Parent”), Grizzly Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), and Oncternal Therapeutics, Inc., a Delaware corporation (the “Company”). Capitalized terms used herein and not otherwise defined shall have the meanings assigned to such terms in that certain Agreement and Plan of Merger and Reorganization, made and entered as of March 6, 2019, by and among Parent, Merger Sub and the Company (the “Merger Agreement”).
RECITALS
A. Section 10.2 of the Merger Agreement provides that the Merger Agreement may be amended with the approval of the respective boards of directors of the Company, Merger Sub and Parent.
B. The parties wish to amend the Merger Agreement as set forth in this Amendment and the respective boards of directors of the Company, Merger Sub and Parent have each approved this Amendment, such amendment to be effective as of the date hereof.
AGREEMENT
The parties to this Amendment, intending to be legally bound, hereby agree as follows:
1. Amendments.
1.1 The following Section 1.12(a) of the Merger Agreement is hereby amended and restated in its entirety as follows:
For the purposes of this Agreement, the “Determination Date” shall be the date that is 10 Business Days prior to the anticipated date for Closing, as agreed upon by Parent and the Company at least five Business Days prior to the Parent Stockholders’ Meeting (the “Anticipated Closing Date”). Within five Business Days following the Determination Date, Parent shall deliver to the Company a schedule (the “Parent Cash Schedule”) setting forth, in reasonable detail, Parent’s good faith, estimated calculation of the Parent Cash Amount (which for the avoidance of doubt, shall not be reduced for payment of Parent Transaction Expenses or any other costs or payments by Parent triggered by the Contemplated Transactions or pursuant to any Parent Benefit Plan) determined in a manner substantially consistent with the manner in which such items were determined for Parent’s most recent SEC filings (the “Parent Cash Calculation”) as of the Anticipated Closing Date prepared and certified by Parent’s principal accounting officer. Parent shall make the work papers andback-up materials used or useful in preparing the Parent Cash Schedule, as reasonably requested by the Company, available to the Company and, if requested by the Company, its accountants and counsel at reasonable times and upon reasonable notice.
1.2 The following defined terms in Exhibit A of the Merger Agreement are hereby amended and restated in their entirety as follows:
“Company Cash Amount” means the Cash and Cash Equivalents and short-term investments of the Company as of the Anticipated Closing Date, as calculated in accordance with Section 1.13.
“Company Target” means $12,500,000.
A-1
Merger Agreement Amendment
“Exchange Ratio” means, subject toSection 1.5(g), the following ratio (rounded to four decimal places): the quotient obtained by dividing (a) the Company Merger Shares by (b) the Company Outstanding Shares, in which:
| • | “Company Cash Balance Adjustment Shares” means (i) if the Company Cash Amount is less than the Company Target, then an amount equal to the quotient of (A) the Company Target,less the Company Cash Amount,divided by (B) 1.207, or (ii) if the Company Cash Amount is greater than the Company Target, then zero. |
| • | “Company Allocation Percentage” means 77.5%. |
| • | “Company Merger Shares” means an amount equal to (i) the product of (A) the Post-Closing Parent Sharesmultiplied by (B) the Company Allocation Percentage, minus (ii) the Company Cash Balance Adjustment Shares,plus(iii) the Parent Cash Balance Adjustment Shares. |
| • | “Company Outstanding Shares” means the total number of shares of Company Capital Stock outstanding immediately prior to the Effective Time expressed on anas-converted to Company Common Stock basis and assuming the effectiveness of the Preferred Stock Conversion, but excluding (i) the exercise of all Company Options and Company Warrants, in each case, outstanding as of immediately prior to the Effective Time, (ii) the issuance of shares of Company Capital Stock in respect of all other outstanding options, restricted stock awards, warrants or rights to receive such shares, whether conditional or unconditional and including any outstanding options, warrants or rights triggered by or associated with the consummation of the Merger, and (iii) any shares of Company Common Stock reserved for issuance. |
| • | “Parent Allocation Percentage” means 22.5%. |
| • | “Parent Cash Balance Adjustment Shares” means (i) if the Parent Cash Amount is less than the Parent Target, then an amount equal to the quotient of (A) the Parent Target,less the Parent Cash Amount,divided by (B) 1.207, or (ii) if the Parent Cash Amount is greater than the Parent Target, then zero. |
| • | “Parent Outstanding Shares” means the total number of shares of Parent Common Stock outstanding immediately prior to the Effective Time, including the total number of shares of Parent Common Stock issuable pursuant to Parent Deferred Stock Rights but excluding (i) the issuance of shares of Parent Common Stock in respect of all Parent Options, Parent Warrants and other outstanding options, warrants or rights to receive such shares (other than the Parent Deferred Stock Rights), in each case, outstanding as of immediately prior to the Effective Time; and (ii) any shares of Parent Common Stock reserved for issuance (other than shares of Parent Common Stock reserved for issuance pursuant to the Parent Deferred Stock Rights). |
| • | “Post-Closing Parent Shares” means the quotient determined bydividing (i) the Parent Outstanding Sharesby (ii) the Parent Allocation Percentage. |
“Parent Cash Amount” means the Cash and Cash Equivalents and short-term investments of Parent as of the Anticipated Closing Date, as calculated in accordance with Section 1.12, which for the avoidance of doubt, shall not be reduced for payment of Parent Transaction Expenses or any other costs or payments by Parent triggered by the Contemplated Transactions or pursuant to any Parent Benefit Plan.
“Parent Target” means (i) $15,000,000 if the Closing occurs on or prior to May 31, 2019, or (ii) if the Closing occurs after May 31, 2019, $15,000,000,lessParent’s reasonable operating expenses from June 1, 2019 through the Closing.
1.3 CVR Agreement. The form of CVR Agreement, attached as Exhibit E to the Merger Agreement, is hereby replaced in its entirety as set forth onAnnex A to this Amendment.
A-2
Merger Agreement Amendment
2. Parent Budget. Parent delivered to the Company a revised operating budget concurrently with the execution of this Amendment and all references to “Parent Budget” shall refer to the operating budget delivered with the execution of this Amendment.
3. Opinion of Financial Advisor. The Parent Board has received an opinion of Aquilo Partners, L.P. to the effect that, as of the date of this Amendment and subject to the assumptions, qualifications, limitations and other matters set forth therein that the Consideration is fair, from a financial point of view, to the holders of Parent Common Stock. It is agreed and understood that such opinion is for the benefit of the Parent Board and may not be relied upon by the Company.
4. Continuing Effectiveness. Except as expressly modified by this Amendment, the Merger Agreement shall remain in full force and effect in accordance with its terms. This Amendment shall be deemed an amendment to the Merger Agreement and shall become effective when executed and delivered by the Parties. Upon the effectiveness of this Amendment, all references in the Merger Agreement to “the Agreement” or “this Agreement,” as applicable, shall refer to the Merger Agreement, as modified by this Amendment.
5. Applicable Law. This Amendment shall be governed by, and construed in accordance with, the Laws of the State of Delaware, regardless of the Laws that might otherwise govern under applicable principles of conflicts of laws.
6. Headings. The bold-faced headings contained in this Amendment are for convenience of reference only, shall not be deemed to be a part of this Amendment and shall not be referred to in connection with the construction or interpretation of this Amendment.
7. Assignability. This Amendment shall be binding upon, and shall be enforceable by and inure solely to the benefit of, the Parties and their respective successors and permitted assigns; provided, however, that neither this Amendment nor any of a Party’s rights or obligations hereunder may be assigned or delegated by such Party without the prior written consent of the other Party, and any attempted assignment or delegation of this Agreement or any of such rights or obligations by such Party without the other Party’s prior written consent shall be void and of no effect.
8. Counterparts; Exchanges by Electronic Transmission. This Amendment may be executed in several counterparts, each of which shall be deemed an original and all of which shall constitute one and the same instrument. The exchange of a fully executed Amendment (in counterparts or otherwise) by all Parties by electronic transmission in .PDF format shall be sufficient to bind the Parties to the terms and conditions of this Amendment.
(Remainder of page intentionally left blank)
A-3
Merger Agreement Amendment
IN WITNESS WHEREOF, the Parties have caused this Amendment to be executed as of the date first above written.
| GTx, Inc. | ||
| By: | /s/ Marc Hanover | |
| Name: | Marc Hanover | |
| Title: | Chief Executive Officer | |
| GRIZZLY MERGER SUB, INC. | ||
| By: | /s/ Henry Doggrell | |
| Name: | Henry Doggrell | |
| Title: | Chief Executive Officer | |
| ONCTERNAL THERAPEUTICS, INC. | ||
| By: | /s/ James Breitmeyer | |
| Name: | James Breitmeyer | |
| Title: | President and Chief Executive Officer | |
SIGNATURE PAGETO AMENDMENT NO. 1TO MERGER AGREEMENT
A-4
Merger Agreement Amendment

Member FINRA/SIPC
601 California St., Suite 500
San Francisco, CA 94108
March 6, 2019
Board of Directors
GTx, Inc.
175 Toyota Plaza, 7th Floor
Memphis, Tennessee 38103
Members of the Board of Directors:
You have asked us to advise you with respect to the fairness, from a financial point of view, to the holders of common stock, par value $0.001 per share (the “Common Stock”), of GTx, Inc., a Delaware corporation (“GTx,” or “Parent”) of (i) the Exchange Ratio used to determine the number of shares of Parent Common Stock to be issued to the holders of Company Common Stock and the number of Parent Options and Parent Warrants to be substituted for the Company Options and Company Warrants to be assumed by Parent, as contemplated by the Agreement and Plan Merger and Reorganization dated March 6, 2019 by and among Parent, Grizzly Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), and Oncternal Therapeutics, Inc., a Delaware corporation (“Oncternal”) (the “Merger Agreement”) and (ii) the right of the holders of Parent Common Stock as of immediately prior to the Effective Time to receive contingent cash payments pursuant to the CVR Agreement ((i) and (ii), the “Consideration”). Defined terms used herein but not otherwise defined are given the meaning set forth in the Merger Agreement or the CVR Agreement, as the case may be.
In arriving at our opinion, we have reviewed, analyzed and considered the Merger Agreement and the CVR Agreement; certain publicly available business and financial information relating to Parent and Oncternal; the publicly available financial terms of certain sale transactions involving companies we deemed relevant and the consideration paid for such companies and comparisons of these terms with the proposed financial terms of the Merger and the CVR Agreement (together, the “Transaction”); and such other publicly available financial and business information concerning certain other companies we deemed relevant and comparisons of this financial and business information to that of Parent and Oncternal. We have also reviewed (i) certain othernon-public information relating to Parent that was prepared and provided to us by Parent, including certain operating and financial information relating to Parent’s business, including Parent’s unaudited financial statements for the year ended December 31, 2018 and financial and business forecasts and projections prepared by management of Parent relating to Parent’s prospects; (ii) certain othernon-public information relating to Oncternal that was prepared and provided to us by Oncternal, including certain operating and financial information relating to Oncternal’s business, including Oncternal’s unaudited financial statements for the year ended December 31, 2018 and financial and business forecasts and projections prepared by management of Oncternal relating to Oncternal’s prospects; and (iii) such other information that we have considered appropriate to opine as to the fairness of the Consideration. In addition, we have discussed with management of Parent and management of Oncternal, the business, operations, financial condition and prospects of each of Parent and Oncternal, respectively, and as a combined company.
In connection with our review, we have not assumed any responsibility for independent verification of any of the foregoing information and have, with your consent, relied on such information being complete and accurate. With respect to the financial forecasts for Parent, the management of Parent has advised us, and we have
B-1-1
assumed with your consent, that such forecasts have been reasonably prepared on bases reflecting the best currently available estimates and judgments of the management of Parent as to the future financial performance of Parent; and, with respect to the financial forecasts for Oncternal, the management of Oncternal has advised us, and we have assumed with your consent, that such forecasts have been reasonably prepared on bases reflecting the best currently available estimates and judgments of the management of Oncternal as to the future financial performance of Oncternal. We have relied upon, without independent verification, the assessment of each of Parent’s management and Oncternal’s management as to the viability of, and risks associated with, the current and future products of the combined company following the Transaction (including without limitation, the development, testing and marketing of such products, the receipt of all necessary governmental and other regulatory approvals for the development, testing and marketing thereof, and the life and enforceability of all relevant patents and other intellectual and other property rights associated with such products). We have assumed that Parent will not materially breach its obligations under the CVR Agreement and will use Commercially Reasonable Efforts to develop one or more SARD Compounds in accordance with the Development Plan and monetize the SARM Technology and SARM Products following the closing of the Transaction, but express no view as to whether the SARD Compounds, SARM Technology or SARM Products will ultimately be developed or monetized. We have also assumed, with your consent, that, in the course of obtaining any regulatory or third party consents, approvals or agreements in connection with the Transaction no delay, limitation, restriction or condition will be imposed that would have an adverse effect on Parent, Oncternal or the combined company, or the contemplated benefits of the Transaction, and that the Transaction will be consummated in accordance with the terms of the Merger Agreement without waiver, modification or amendment of any material term, condition or agreement thereof or any waiver, modification or amendment of any material term, condition or agreement of the CVR Agreement. In addition, we have not been requested to make, and have not made, an independent evaluation or appraisal of the assets or liabilities (contingent or otherwise) of Parent or Oncternal, nor did we conduct a physical inspection of any of the properties or facilities of Parent or Oncternal, nor have we been furnished with any such evaluations, appraisals or inspections, nor do we assume any responsibility to obtain any such evaluations, appraisals or inspections. We have also assumed that the representations and warranties contained in the Merger Agreement made by the parties thereto are true and correct in all respects material to our analysis. We also reviewed a discounted cash flow analysis of a SARD Product prepared and provided to us by Parent, but determined that the projections underlying the analysis were too speculative to use in our analysis of the fairness of the Consideration.
Our opinion addresses only the fairness, from a financial point of view, to the holders of Parent Common Stock of the Consideration and does not address any other aspect or implication of the Transaction or any other agreement, arrangement or understanding entered into in connection with the Transaction or otherwise. Our opinion is necessarily based upon information made available to us as of the date hereof and financial, economic, market and other conditions as they exist and can be evaluated on the date hereof. We do not express any opinion as to the price or range of prices at which the shares of Parent Common Stock may trade subsequent to the announcement or closing of the Transaction or at any time.
We have acted as financial advisor to Parent in connection with the Transaction. We will receive a fee for our services, a portion of which is payable upon delivery of this opinion and a significant portion of which is contingent upon consummation of the Transaction. In addition, Parent has agreed to indemnify us for certain liabilities and other items arising out of our engagement.
You have not asked us to address, and this opinion does not address, the relative merits of the Transaction as compared to alternative transactions or strategies that might be available to Parent, nor the underlying business decision of Parent to proceed with the Transaction. Our opinion addresses only the fairness, from a financial point of view, to the holders of Parent Common Stock of the Consideration, and we express no opinion as to the fairness of any consideration paid in connection with the Transaction to the holders of any other class of
B-1-2
securities, creditors or other constituencies of Parent as to the underlying decision by Parent to engage in the Transaction. We are not legal, tax or regulatory advisors and have relied upon, without independent verification, the assessment of Parent and its legal, tax and regulatory advisors with respect to such matters. We have not performed any tax analysis, nor have we been furnished with any such analysis.
The issuance of this opinion has been approved by a fairness opinion committee of Aquilo Partners, L.P. (“Aquilo Partners”). This opinion is for the use and benefit of the Board of Directors of Parent in connection with its evaluation of the Transaction. This opinion does not constitute a recommendation to any stockholder as to how such stockholder should vote with respect to the Transaction or any other matter. Except as otherwise provided in our engagement letter with Parent, this opinion shall not be reproduced, disseminated, quoted, summarized or referred to at any time, in any manner or for any purpose, nor shall any public references to Aquilo Partners or any of its affiliates be made by Parent or any of its affiliates, without the prior written consent of Aquilo Partners, provided that this opinion may be reproduced in full in any proxy or information statement provided to stockholders of Parent.
Based upon and subject to the foregoing, it is our opinion that, as of the date hereof, the Consideration is fair, from a financial point of view, to the holders of Parent Common Stock.
Very truly yours,
AQUILO PARTNERS, L.P.
| By: | /s/ John Rumsey | |
John Rumsey Managing Director |
B-1-3

Member FINRA/SIPC
601 California St., Suite 500
San Francisco, CA 94108
April 29, 2019
Board of Directors
GTx, Inc.
17 W. Pontotoc Ave., Suite 100
Memphis, Tennessee 38103
Members of the Board of Directors:
You have asked us to advise you with respect to the fairness, from a financial point of view, to the holders of common stock, par value $0.001 per share (the “Common Stock”), of GTx, Inc., a Delaware corporation (“GTx,” or “Parent”) of (i) the Exchange Ratio used to determine the number of shares of Parent Common Stock to be issued to the holders of Company Common Stock and the number of Parent Options and Parent Warrants to be substituted for the Company Options and Company Warrants to be assumed by Parent, as contemplated by the Agreement and Plan Merger and Reorganization dated March 6, 2019 by and among Parent, Grizzly Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of Parent (“Merger Sub”), and Oncternal Therapeutics, Inc., a Delaware corporation (“Oncternal”), as amended by Amendment No. 1 to the Merger Agreement by and among Parent, Merger Sub and Oncternal (together, the “Merger Agreement”) and (ii) the right of the holders of Parent Common Stock as of immediately prior to the Effective Time to receive contingent cash payments pursuant to the Contingent Value Rights Agreement (the “CVR Agreement”) ((i) and (ii), the “Consideration”). Defined terms used herein but not otherwise defined are given the meaning set forth in the Merger Agreement or the CVR Agreement, as the case may be.
In arriving at our opinion, we have reviewed, analyzed and considered the Merger Agreement and the CVR Agreement; certain publicly available business and financial information relating to Parent and Oncternal, including GTX’s and Oncternal’s respective audited financial statements for the year ended December 31, 2018; the publicly available financial terms of certain sale transactions involving companies we deemed relevant and the consideration paid for such companies and comparisons of these terms with the proposed financial terms of the Merger and the CVR Agreement (together, the “Transaction”); and such other publicly available financial and business information concerning certain other companies we deemed relevant and comparisons of this financial and business information to that of Parent and Oncternal. We have also reviewed (i) certain othernon-public information relating to Parent that was prepared and provided to us by Parent, including certain operating and financial information relating to Parent’s business and financial and business forecasts and projections prepared by management of Parent relating to Parent’s prospects; (ii) certain othernon-public information relating to Oncternal that was prepared and provided to us by Oncternal, including certain operating and financial information relating to Oncternal’s business and financial and business forecasts and projections prepared by management of Oncternal relating to Oncternal’s prospects; and (iii) such other information that we have considered appropriate to opine as to the fairness of the Consideration. In addition, we have discussed with management of Parent and management of Oncternal, the business, operations, financial condition and prospects of each of Parent and Oncternal, respectively, and as a combined company.
In connection with our review, we have not assumed any responsibility for independent verification of any of the foregoing information and have, with your consent, relied on such information being complete and accurate.
B-2-1
With respect to the financial forecasts for Parent, the management of Parent has advised us, and we have assumed with your consent, that such forecasts have been reasonably prepared on bases reflecting the best currently available estimates and judgments of the management of Parent as to the future financial performance of Parent; and, with respect to the financial forecasts for Oncternal, the management of Oncternal has advised us, and we have assumed with your consent, that such forecasts have been reasonably prepared on bases reflecting the best currently available estimates and judgments of the management of Oncternal as to the future financial performance of Oncternal. We have relied upon, without independent verification, the assessment of each of Parent’s management and Oncternal’s management as to the viability of, and risks associated with, the current and future products of the combined company following the Transaction (including without limitation, the development, testing and marketing of such products, the receipt of all necessary governmental and other regulatory approvals for the development, testing and marketing thereof, and the life and enforceability of all relevant patents and other intellectual and other property rights associated with such products). We have assumed that Parent will not materially breach its obligations under the CVR Agreement and express no view as to whether the SARD Technology, SARD Compounds, SARD Products, SARM Technology, SARM Compounds or SARM Products will ultimately be developed or monetized. We have also assumed, with your consent, that, in the course of obtaining any regulatory or third party consents, approvals or agreements in connection with the Transaction no delay, limitation, restriction or condition will be imposed that would have an adverse effect on Parent, Oncternal or the combined company, or the contemplated benefits of the Transaction, and that the Transaction will be consummated in accordance with the terms of the Merger Agreement without waiver, modification or amendment of any material term, condition or agreement thereof or any waiver, modification or amendment of any material term, condition or agreement of the CVR Agreement. In addition, we have not been requested to make, and have not made, an independent evaluation or appraisal of the assets or liabilities (contingent or otherwise) of Parent or Oncternal, nor did we conduct a physical inspection of any of the properties or facilities of Parent or Oncternal, nor have we been furnished with any such evaluations, appraisals or inspections, nor do we assume any responsibility to obtain any such evaluations, appraisals or inspections. We have also assumed that the representations and warranties contained in the Merger Agreement made by the parties thereto are true and correct in all respects material to our analysis. We also reviewed a discounted cash flow analysis of a SARD Product prepared and provided to us by Parent, but determined that the projections underlying the analysis were too speculative to use in our analysis of the fairness of the Consideration.
Our opinion addresses only the fairness, from a financial point of view, to the holders of Parent Common Stock of the Consideration and does not address any other aspect or implication of the Transaction or any other agreement, arrangement or understanding entered into in connection with the Transaction or otherwise. Our opinion is necessarily based upon information made available to us as of the date hereof and financial, economic, market and other conditions as they exist and can be evaluated on the date hereof. We do not express any opinion as to the price or range of prices at which the shares of Parent Common Stock may trade subsequent to the announcement or closing of the Transaction or at any time.
We have acted as financial advisor to Parent in connection with the Transaction. We will receive a fee for our services, a portion of which is payable upon delivery of this opinion and a significant portion of which is contingent upon consummation of the Transaction. In addition, Parent has agreed to indemnify us for certain liabilities and other items arising out of our engagement.
You have not asked us to address, and this opinion does not address, the relative merits of the Transaction as compared to alternative transactions or strategies that might be available to Parent, nor the underlying business decision of Parent to proceed with the Transaction. Our opinion addresses only the fairness, from a financial point of view, to the holders of Parent Common Stock of the Consideration, and we express no opinion as to the fairness of any consideration paid in connection with the Transaction to the holders of any other class of securities, creditors or other constituencies of Parent as to the underlying decision by Parent to engage in the Transaction. We are not legal, tax or regulatory advisors and have relied upon, without independent verification, the assessment of Parent and its legal, tax and regulatory advisors with respect to such matters. We have not performed any tax analysis, nor have we been furnished with any such analysis.
B-2-2
The issuance of this opinion has been approved by a fairness opinion committee of Aquilo Partners, L.P. (“Aquilo Partners”). This opinion is for the use and benefit of the Board of Directors of Parent in connection with its evaluation of the Transaction. This opinion does not constitute a recommendation to any stockholder as to how such stockholder should vote with respect to the Transaction or any other matter. Except as otherwise provided in our engagement letter with Parent, this opinion shall not be reproduced, disseminated, quoted, summarized or referred to at any time, in any manner or for any purpose, nor shall any public references to Aquilo Partners or any of its affiliates be made by Parent or any of its affiliates, without the prior written consent of Aquilo Partners, provided that this opinion may be reproduced in full in any proxy or information statement provided to stockholders of Parent.
Based upon and subject to the foregoing, it is our opinion that, as of the date hereof, the Consideration is fair, from a financial point of view, to the holders of Parent Common Stock.
Very truly yours,
AQUILO PARTNERS, L.P.
| By: | /s/ John Rumsey | |
John Rumsey Managing Director |
B-2-3
GENERAL CORPORATION LAW OF THE STATE OF DELAWARE REGARDING APPRAISAL RIGHTS
SECTION 262 OF THE GENERAL CORPORATION LAW OF THE STATE OF DELAWARE
§ 262. Appraisal rights.
| (a) | Any stockholder of a corporation of this State who holds shares of stock on the date of the making of a demand pursuant to subsection (d) of this section with respect to such shares, who continuously holds such shares through the effective date of the merger or consolidation, who has otherwise complied with subsection (d) of this section and who has neither voted in favor of the merger or consolidation nor consented thereto in writing pursuant to § 228 of this title shall be entitled to an appraisal by the Court of Chancery of the fair value of the stockholder’s shares of stock under the circumstances described in subsections (b) and (c) of this section. As used in this section, the word “stockholder” means a holder of record of stock in a corporation; the words “stock” and “share” mean and include what is ordinarily meant by those words; and the words “depository receipt” mean a receipt or other instrument issued by a depository representing an interest in 1 or more shares, or fractions thereof, solely of stock of a corporation, which stock is deposited with the depository. |
| (b) | Appraisal rights shall be available for the shares of any class or series of stock of a constituent corporation in a merger or consolidation to be effected pursuant to § 251 (other than a merger effected pursuant to § 251(g) of this title and, subject to paragraph (b)(3) of this section, § 251(h) of this title), § 252, § 254, § 255, § 256, § 257, § 258, § 263 or § 264 of this title: |
| (1) | Provided, however, that, except as expressly provided in § 363(b) of this title, no appraisal rights under this section shall be available for the shares of any class or series of stock, which stock, or depository receipts in respect thereof, at the record date fixed to determine the stockholders entitled to receive notice of the meeting of stockholders to act upon the agreement of merger or consolidation, were either: (i) listed on a national securities exchange or (ii) held of record by more than 2,000 holders; and further provided that no appraisal rights shall be available for any shares of stock of the constituent corporation surviving a merger if the merger did not require for its approval the vote of the stockholders of the surviving corporation as provided in § 251(f) of this title. |
| (2) | Notwithstanding paragraph (b)(1) of this section, appraisal rights under this section shall be available for the shares of any class or series of stock of a constituent corporation if the holders thereof are required by the terms of an agreement of merger or consolidation pursuant to §§ 251, 252, 254, 255, 256, 257, 258, 263 and 264 of this title to accept for such stock anything except: |
| a. | Shares of stock of the corporation surviving or resulting from such merger or consolidation, or depository receipts in respect thereof; |
| b. | Shares of stock of any other corporation, or depository receipts in respect thereof, which shares of stock (or depository receipts in respect thereof) or depository receipts at the effective date of the merger or consolidation will be either listed on a national securities exchange or held of record by more than 2,000 holders; |
| c. | Cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a. and b. of this section; or |
C-1
| d. | Any combination of the shares of stock, depository receipts and cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a., b. and c. of this section. |
| (3) | In the event all of the stock of a subsidiary Delaware corporation party to a merger effected under § 251(h), § 253 or § 267 of this title is not owned by the parent immediately prior to the merger, appraisal rights shall be available for the shares of the subsidiary Delaware corporation. |
| (4) | In the event of an amendment to a corporation’s certificate of incorporation contemplated by § 363(a) of this title, appraisal rights shall be available as contemplated by § 363(b) of this title, and the procedures of this section, including those set forth in subsections (d) and (e) of this section, shall apply as nearly as practicable, with the word “amendment” substituted for the words “merger or consolidation,” and the word “corporation” substituted for the words “constituent corporation” and/or “surviving or resulting corporation.” |
| (c) | Any corporation may provide in its certificate of incorporation that appraisal rights under this section shall be available for the shares of any class or series of its stock as a result of an amendment to its certificate of incorporation, any merger or consolidation in which the corporation is a constituent corporation or the sale of all or substantially all of the assets of the corporation. If the certificate of incorporation contains such a provision, the provisions of this section, including those set forth in subsections (d), (e), and (g) of this section, shall apply as nearly as is practicable. |
| (d) | Appraisal rights shall be perfected as follows: |
| (1) | If a proposed merger or consolidation for which appraisal rights are provided under this section is to be submitted for approval at a meeting of stockholders, the corporation, not less than 20 days prior to the meeting, shall notify each of its stockholders who was such on the record date for notice of such meeting (or such members who received notice in accordance with § 255(c) of this title) with respect to shares for which appraisal rights are available pursuant to subsection (b) or (c) of this section that appraisal rights are available for any or all of the shares of the constituent corporations, and shall include in such notice a copy of this section and, if 1 of the constituent corporations is a nonstock corporation, a copy of § 114 of this title. Each stockholder electing to demand the appraisal of such stockholder’s shares shall deliver to the corporation, before the taking of the vote on the merger or consolidation, a written demand for appraisal of such stockholder’s shares. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such stockholder’s shares. A proxy or vote against the merger or consolidation shall not constitute such a demand. A stockholder electing to take such action must do so by a separate written demand as herein provided. Within 10 days after the effective date of such merger or consolidation, the surviving or resulting corporation shall notify each stockholder of each constituent corporation who has complied with this subsection and has not voted in favor of or consented to the merger or consolidation of the date that the merger or consolidation has become effective; or |
| (2) | If the merger or consolidation was approved pursuant to § 228, § 251(h), § 253, or § 267 of this title, then either a constituent corporation before the effective date of the merger or consolidation or the surviving or resulting corporation within 10 days thereafter shall notify each of the holders of any class or series of stock of such constituent corporation who are entitled to appraisal rights of the approval of the merger or consolidation and that appraisal rights are available for any or all shares of such class or series of stock of such constituent corporation, and shall include in such notice a copy of this section and, |
C-2
| if 1 of the constituent corporations is a nonstock corporation, a copy of § 114 of this title. Such notice may, and, if given on or after the effective date of the merger or consolidation, shall, also notify such stockholders of the effective date of the merger or consolidation. Any stockholder entitled to appraisal rights may, within 20 days after the date of mailing of such notice or, in the case of a merger approved pursuant to § 251(h) of this title, within the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days after the date of mailing of such notice, demand in writing from the surviving or resulting corporation the appraisal of such holder’s shares. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares. If such notice did not notify stockholders of the effective date of the merger or consolidation, either (i) each such constituent corporation shall send a second notice before the effective date of the merger or consolidation notifying each of the holders of any class or series of stock of such constituent corporation that are entitled to appraisal rights of the effective date of the merger or consolidation or (ii) the surviving or resulting corporation shall send such a second notice to all such holders on or within 10 days after such effective date; provided, however, that if such second notice is sent more than 20 days following the sending of the first notice or, in the case of a merger approved pursuant to § 251(h) of this title, later than the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days following the sending of the first notice, such second notice need only be sent to each stockholder who is entitled to appraisal rights and who has demanded appraisal of such holder’s shares in accordance with this subsection. An affidavit of the secretary or assistant secretary or of the transfer agent of the corporation that is required to give either notice that such notice has been given shall, in the absence of fraud, be prima facie evidence of the facts stated therein. For purposes of determining the stockholders entitled to receive either notice, each constituent corporation may fix, in advance, a record date that shall be not more than 10 days prior to the date the notice is given, provided, that if the notice is given on or after the effective date of the merger or consolidation, the record date shall be such effective date. If no record date is fixed and the notice is given prior to the effective date, the record date shall be the close of business on the day next preceding the day on which the notice is given. |
| (e) | Within 120 days after the effective date of the merger or consolidation, the surviving or resulting corporation or any stockholder who has complied with subsections (a) and (d) of this section hereof and who is otherwise entitled to appraisal rights, may commence an appraisal proceeding by filing a petition in the Court of Chancery demanding a determination of the value of the stock of all such stockholders. Notwithstanding the foregoing, at any time within 60 days after the effective date of the merger or consolidation, any stockholder who has not commenced an appraisal proceeding or joined that proceeding as a named party shall have the right to withdraw such stockholder’s demand for appraisal and to accept the terms offered upon the merger or consolidation. Within 120 days after the effective date of the merger or consolidation, any stockholder who has complied with the requirements of subsections (a) and (d) of this section hereof, upon written request, shall be entitled to receive from the corporation surviving the merger or resulting from the consolidation a statement setting forth the aggregate number of shares not voted in favor of the merger or consolidation and with respect to which demands for appraisal have been received and the aggregate number of holders of such shares. Such written statement shall be mailed to the stockholder within 10 days after such stockholder’s written request for such a statement is received by the surviving or resulting corporation or within 10 days after expiration of the period for delivery of demands for appraisal under subsection (d) of this section hereof, whichever is later. Notwithstanding subsection (a) of this section, a person who is the beneficial owner of shares |
C-3
| of such stock held either in a voting trust or by a nominee on behalf of such person may, in such person’s own name, file a petition or request from the corporation the statement described in this subsection. |
| (f) | Upon the filing of any such petition by a stockholder, service of a copy thereof shall be made upon the surviving or resulting corporation, which shall within 20 days after such service file in the office of the Register in Chancery in which the petition was filed a duly verified list containing the names and addresses of all stockholders who have demanded payment for their shares and with whom agreements as to the value of their shares have not been reached by the surviving or resulting corporation. If the petition shall be filed by the surviving or resulting corporation, the petition shall be accompanied by such a duly verified list. The Register in Chancery, if so ordered by the Court, shall give notice of the time and place fixed for the hearing of such petition by registered or certified mail to the surviving or resulting corporation and to the stockholders shown on the list at the addresses therein stated. Such notice shall also be given by 1 or more publications at least 1 week before the day of the hearing, in a newspaper of general circulation published in the City of Wilmington, Delaware or such publication as the Court deems advisable. The forms of the notices by mail and by publication shall be approved by the Court, and the costs thereof shall be borne by the surviving or resulting corporation. |
| (g) | At the hearing on such petition, the Court shall determine the stockholders who have complied with this section and who have become entitled to appraisal rights. The Court may require the stockholders who have demanded an appraisal for their shares and who hold stock represented by certificates to submit their certificates of stock to the Register in Chancery for notation thereon of the pendency of the appraisal proceedings; and if any stockholder fails to comply with such direction, the Court may dismiss the proceedings as to such stockholder. If immediately before the merger or consolidation the shares of the class or series of stock of the constituent corporation as to which appraisal rights are available were listed on a national securities exchange, the Court shall dismiss the proceedings as to all holders of such shares who are otherwise entitled to appraisal rights unless (1) the total number of shares entitled to appraisal exceeds 1% of the outstanding shares of the class or series eligible for appraisal, (2) the value of the consideration provided in the merger or consolidation for such total number of shares exceeds $1 million, or (3) the merger was approved pursuant to § 253 or § 267 of this title. |
| (h) | After the Court determines the stockholders entitled to an appraisal, the appraisal proceeding shall be conducted in accordance with the rules of the Court of Chancery, including any rules specifically governing appraisal proceedings. Through such proceeding the Court shall determine the fair value of the shares exclusive of any element of value arising from the accomplishment or expectation of the merger or consolidation, together with interest, if any, to be paid upon the amount determined to be the fair value. In determining such fair value, the Court shall take into account all relevant factors. Unless the Court in its discretion determines otherwise for good cause shown, and except as provided in this subsection, interest from the effective date of the merger through the date of payment of the judgment shall be compounded quarterly and shall accrue at 5% over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the effective date of the merger and the date of payment of the judgment. At any time before the entry of judgment in the proceedings, the surviving corporation may pay to each stockholder entitled to appraisal an amount in cash, in which case interest shall accrue thereafter as provided herein only upon the sum of (1) the difference, if any, between the amount so paid and the fair value of the shares as determined by the Court, and (2) interest theretofore accrued, unless paid at that time. Upon application by the surviving or resulting corporation or by any stockholder entitled to participate in the appraisal proceeding, the Court may, in its discretion, proceed to trial upon |
C-4
| the appraisal prior to the final determination of the stockholders entitled to an appraisal. Any stockholder whose name appears on the list filed by the surviving or resulting corporation pursuant to subsection (f) of this section and who has submitted such stockholder’s certificates of stock to the Register in Chancery, if such is required, may participate fully in all proceedings until it is finally determined that such stockholder is not entitled to appraisal rights under this section. |
| (i) | The Court shall direct the payment of the fair value of the shares, together with interest, if any, by the surviving or resulting corporation to the stockholders entitled thereto. Payment shall be so made to each such stockholder, in the case of holders of uncertificated stock forthwith, and the case of holders of shares represented by certificates upon the surrender to the corporation of the certificates representing such stock. The Court’s decree may be enforced as other decrees in the Court of Chancery may be enforced, whether such surviving or resulting corporation be a corporation of this State or of any state. |
| (j) | The costs of the proceeding may be determined by the Court and taxed upon the parties as the Court deems equitable in the circumstances. Upon application of a stockholder, the Court may order all or a portion of the expenses incurred by any stockholder in connection with the appraisal proceeding, including, without limitation, reasonable attorney’s fees and the fees and expenses of experts, to be charged pro rata against the value of all the shares entitled to an appraisal. |
| (k) | From and after the effective date of the merger or consolidation, no stockholder who has demanded appraisal rights as provided in subsection (d) of this section shall be entitled to vote such stock for any purpose or to receive payment of dividends or other distributions on the stock (except dividends or other distributions payable to stockholders of record at a date which is prior to the effective date of the merger or consolidation); provided, however, that if no petition for an appraisal shall be filed within the time provided in subsection (e) of this section, or if such stockholder shall deliver to the surviving or resulting corporation a written withdrawal of such stockholder’s demand for an appraisal and an acceptance of the merger or consolidation, either within 60 days after the effective date of the merger or consolidation as provided in subsection (e) of this section or thereafter with the written approval of the corporation, then the right of such stockholder to an appraisal shall cease. Notwithstanding the foregoing, no appraisal proceeding in the Court of Chancery shall be dismissed as to any stockholder without the approval of the Court, and such approval may be conditioned upon such terms as the Court deems just; provided, however that this provision shall not affect the right of any stockholder who has not commenced an appraisal proceeding or joined that proceeding as a named party to withdraw such stockholder’s demand for appraisal and to accept the terms offered upon the merger or consolidation within 60 days after the effective date of the merger or consolidation, as set forth in subsection (e) of this section. |
| (l) | The shares of the surviving or resulting corporation to which the shares of such objecting stockholders would have been converted had they assented to the merger or consolidation shall have the status of authorized and unissued shares of the surviving or resulting corporation. |
C-5
CERTIFICATE OF AMENDMENT
TO THE
RESTATED CERTIFICATE OF INCORPORATION
OF
GTX, INC.
GTx, Inc. (the “Corporation”), a corporation organized and existing under and by virtue of the General Corporation Law of the State of Delaware, as amended (the “DGCL”), hereby certifies as follows:
| A. | The name of the Corporation is GTx, Inc. The date of filing of the original Certificate of Incorporation of the Corporation with the Secretary of State of the State of Delaware was September 4, 2003, as restated on February 6, 2004. |
| B. | This Certificate of Amendment to the Restated Certificate of Incorporation (the “Certificate of Amendment”) amends the Corporation’s Restated Certificate of Incorporation filed with the Secretary of State of the State of Delaware on February 6, 2004 (the “Prior Certificate”), and has been duly adopted by the Corporation’s Board of Directors and stockholders in accordance with the provisions of Section 242 of the DGCL. |
| C. | Section A of Article IV of the Prior Certificate is hereby amended and restated to read in its entirety as follows(1): |
“A.Authorized Stock. The total number of shares which the Corporation shall have authority to issue is sixty-five million (65,000,000), consisting of sixty million (60,000,000) shares of Common Stock, par value $0.001 per share (the “Common Stock”), and five million (5,000,000) shares of Preferred Stock, par value $0.001 per share (the “Preferred Stock”).
Immediately upon the filing of this Certificate of Amendment to the Restated Certificate of Incorporation with the Secretary of State of the State of Delaware each ( ) shares of Common Stock outstanding immediately prior to such filing shall be automatically reclassified into one (1) share of Common Stock. The aforementioned reclassification shall be referred to collectively as the “Reverse Split.”
The Reverse Split shall occur without any further action on the part of the Corporation or stockholders of the Corporation and whether or not certificates representing such stockholders’ shares prior to the Reverse Split are surrendered for cancellation. No fractional interest in a share of Common Stock shall be deliverable upon the Reverse Split. All shares of Common Stock (including fractions thereof) issuable upon the Reverse Split held by a holder prior to the Reverse Split shall be aggregated for purposes of determining whether the Reverse Split would result in the issuance of any fractional share. Any fractional share resulting from such aggregation upon
| (1) | These amendments approve the combination and reclassification of any whole number of shares of GTx common stock between and including six (6) and eight (8) for one (1) share of GTx common stock. By these amendments, the stockholders would approve each of the three (3) alternate amendments proposed by the GTx Board of Directors. If the reverse stock split proposal is approved by stockholders, the Certificate of Amendment filed with the Secretary of State of the State of Delaware will include only that reverse stock split ratio determined by the GTx Board of Directors to be in the best interests of GTx and its stockholders. The other amendments will be abandoned pursuant to Section 242(c) of the General Corporation Law of the State of Delaware. The GTx Board of Directors may also elect not to effect any reverse stock split, in which case all proposed amendments will be abandoned. |
D-1
the Reverse Split shall be rounded down to the nearest whole number. Each holder who would otherwise be entitled to a fraction of a share of Common Stock upon the Reverse Split (after aggregating all fractions of a share to which such stockholder would otherwise be entitled) shall, in lieu thereof, be entitled to receive a cash payment in an amount equal to the fraction to which the stockholder would otherwise be entitled multiplied by the closing price of the Corporation’s Common Stock as reported on the Nasdaq Capital Market on the date of the filing of this Certificate of Amendment to the Restated Certificate of Incorporation with the Secretary of State of the State of Delaware. The Corporation shall not be obliged to issue certificates evidencing the shares of Common Stock outstanding as a result of the Reverse Split unless and until the certificates evidencing the shares held by a holder prior to the Reverse Split are either delivered to the Corporation or its transfer agent, or the holder notifies the Corporation or its transfer agent that such certificates have been lost, stolen or destroyed and executes an agreement satisfactory to the Corporation to indemnify the Corporation from any loss incurred by it in connection with such certificates.”
| D. | The Certificate of Amendment of the Prior Certificate so adopted reads in full as set forth above and is hereby incorporated by reference. All other provisions of the Prior Certificate remain in full force and effect. |
D-2
IN WITNESS WHEREOF, GTx, Inc. has caused this Certificate of Amendment to be signed by , a duly authorized officer of the Corporation, on , 2019.
| GTX, INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
D-3
CERTIFICATE OF AMENDMENT
TO THE
RESTATED CERTIFICATE OF INCORPORATION
OF
GTX, INC.
GTx, Inc. (the “Corporation”), a corporation organized and existing under and by virtue of the General Corporation Law of the State of Delaware, as amended (the “DGCL”), hereby certifies as follows:
| A. | The name of the Corporation is GTx, Inc. The date of filing of the original Certificate of Incorporation of the Corporation with the Secretary of State of the State of Delaware was September 4, 2003, as restated on February 6, 2004. |
| B. | This Certificate of Amendment to the Restated Certificate of Incorporation (the “Certificate of Amendment”) amends the Corporation’s Restated Certificate of Incorporation filed with the Secretary of State of the State of Delaware on February 6, 2004 (the “Prior Certificate”), and has been duly adopted by the Corporation’s Board of Directors and stockholders in accordance with the provisions of Section 242 of the DGCL. |
| C. | Article I of the Prior Certificate is hereby amended and restated to read in its entirety as follows: |
“ARTICLE I
“The name of the corporation is Oncternal Therapeutics, Inc. (the “Corporation”).”
| D. | The Certificate of Amendment of the Prior Certificate so adopted reads in full as set forth above and is hereby incorporated by reference. All other provisions of the Prior Certificate remain in full force and effect. |
IN WITNESS WHEREOF, GTx, Inc. has caused this Certificate of Amendment to be signed by , a duly authorized officer of the Corporation, on , 2019.
| GTX, INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
E-1
GTX, INC.
2019 INCENTIVE AWARD PLAN
The numbers in this Plan do not give effect to the reverse stock split to be consummated prior to the consummation of the transactions contemplated by the Merger Agreement (as defined below) (the “Reverse Stock Split”) and will be adjusted in connection with such Reverse Stock Split.
ARTICLE I.
PURPOSE
The Plan’s purpose is to enhance the Company’s ability to attract, retain and motivate persons who make (or are expected to make) important contributions to the Company by providing these individuals with equity ownership opportunities. Capitalized terms used in the Plan are defined in Article XI.
ARTICLE II.
ELIGIBILITY
Service Providers are eligible to be granted Awards under the Plan, subject to the limitations described herein.
ARTICLE III.
ADMINISTRATION AND DELEGATION
3.1 Administration. The Plan is administered by the Administrator. The Administrator has authority to determine which Service Providers receive Awards, grant Awards and set Award terms and conditions, subject to the conditions and limitations in the Plan. The Administrator also has the authority to take all actions and make all determinations under the Plan, to interpret the Plan and Award Agreements and to adopt, amend and repeal Plan administrative rules, guidelines and practices as it deems advisable. The Administrator may correct defects and ambiguities, supply omissions and reconcile inconsistencies in the Plan or any Award as it deems necessary or appropriate to administer the Plan and any Awards. The Administrator’s determinations under the Plan are in its sole discretion and will be final and binding on all persons having or claiming any interest in the Plan or any Award.
3.2 Appointment of Committees. To the extent Applicable Laws permit, the Board may delegate any or all of its powers under the Plan to one or more Committees or officers of the Company or any of its Subsidiaries. The Board may abolish any Committee orre-vest in itself any previously delegated authority at any time.
ARTICLE IV.
STOCK AVAILABLE FOR AWARDS
4.1 Number of Shares. Subject to adjustment under Article VIII and the terms of this Article IV, Awards may be made under the Plan covering up to the Overall Share Limit. As of the Effective Date, the Company will cease granting awards under the Prior Plan; however, Prior Plan Awards will remain subject to the terms of the applicable Prior Plan. Shares issued under the Plan may consist of authorized but unissued Shares, Shares purchased on the open market or treasury Shares.
F-1
4.2 Share Recycling. If all or any part of an Award or Prior Plan Award expires, lapses or is terminated, exchanged for cash, surrendered, repurchased, canceled without having been fully exercised or forfeited, in any case, in a manner that results in the Company acquiring Shares covered by the Award or Prior Plan Award at a price not greater than the price (as adjusted to reflect any Equity Restructuring) paid by the Participant for such Shares or not issuing any Shares covered by the Award or Prior Plan Award, the unused Shares covered by the Award or Prior Plan Award will, as applicable, become or again be available for Award grants under the Plan. Further, Shares delivered (either by actual delivery or attestation) to the Company by a Participant to satisfy the applicable exercise or purchase price of an Award or Prior Plan Award and/or to satisfy any applicable tax withholding obligation (including Shares retained by the Company from the Award or Prior Plan Award being exercised or purchased and/or creating the tax obligation) will, as applicable, become or again be available for Award grants under the Plan. The payment of Dividend Equivalents in cash in conjunction with any outstanding Awards or Prior Plan Awards shall not count against the Overall Share Limit.
4.3 Incentive Stock Option Limitations. Notwithstanding anything to the contrary herein, no more than 50,000,000 Shares may be issued pursuant to the exercise of Incentive Stock Options.
4.4 Substitute Awards. In connection with an entity’s merger or consolidation with the Company or the Company’s acquisition of an entity’s property or stock, the Administrator may grant Awards in substitution for any Options or other stock or stock-based awards granted before such merger or consolidation by such entity or its affiliate. Substitute Awards may be granted on such terms as the Administrator deems appropriate, notwithstanding limitations on Awards in the Plan. Substitute Awards will not count against the Overall Share Limit (nor shall Shares subject to a Substitute Award be added to the Shares available for Awards under the Plan as provided above), except that Shares acquired by exercise of substitute Incentive Stock Options will count against the maximum number of Shares that may be issued pursuant to the exercise of Incentive Stock Options under the Plan. Additionally, in the event that a company acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines has shares available under apre-existing plan approved by stockholders and not adopted in contemplation of such acquisition or combination, the shares available for grant pursuant to the terms of suchpre-existing plan (as adjusted, to the extent appropriate, using the exchange ratio or other adjustment or valuation ratio or formula used in such acquisition or combination to determine the consideration payable to the holders of common stock of the entities party to such acquisition or combination) may be used for Awards under the Plan and shall not reduce the Shares authorized for grant under the Plan (and Shares subject to such Awards shall not be added to the Shares available for Awards under the Plan as provided above); provided that Awards using such available shares shall not be made after the date awards or grants could have been made under the terms of thepre-existing plan, absent the acquisition or combination, and shall only be made to individuals who were not Employees or Directors prior to such acquisition or combination.
4.5 Non-Employee Director Compensation. Notwithstanding any provision to the contrary in the Plan, the Administrator may establish compensation for non-employee Directors from time to time, subject to the limitations in the Plan. The Administrator will from time to time determine the terms, conditions and amounts of all such non-employee Director compensation in its discretion and pursuant to the exercise of its business judgment, taking into account such factors, circumstances and considerations as it shall deem relevant from time to time, provided that the sum of any cash compensation, or other compensation, and the value (determined as of the grant date in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, or any successor thereto) of Awards granted to a non-employee Director as compensation for services as a non-employee Director during any fiscal year of the Company may not exceed $750,000 increased to $1,000,000 in the fiscal year of a non-employee Director’s initial service as a non-employee Director. The Administrator may make exceptions to this limit for individual non-employee Directors in extraordinary circumstances, as the Administrator may determine in its discretion, provided that the non-employee Director receiving such additional compensation may not participate in the decision to award such compensation or in other contemporaneous compensation decisions involving non-employee Directors.
F-2
ARTICLE V.
STOCK OPTIONS AND STOCK APPRECIATION RIGHTS
5.1 General. The Administrator may grant Options or Stock Appreciation Rights to Service Providers subject to the limitations in the Plan, including any limitations in the Plan that apply to Incentive Stock Options. The Administrator will determine the number of Shares covered by each Option and Stock Appreciation Right, the exercise price of each Option and Stock Appreciation Right and the conditions and limitations applicable to the exercise of each Option and Stock Appreciation Right. A Stock Appreciation Right will entitle the Participant (or other person entitled to exercise the Stock Appreciation Right) to receive from the Company upon exercise of the exercisable portion of the Stock Appreciation Right an amount determined by multiplying the excess, if any, of the Fair Market Value of one Share on the date of exercise over the exercise price per Share of the Stock Appreciation Right by the number of Shares with respect to which the Stock Appreciation Right is exercised, subject to any limitations of the Plan or that the Administrator may impose and payable in cash, Shares valued at Fair Market Value or a combination of the two as the Administrator may determine or provide in the Award Agreement.
5.2 Exercise Price. The Administrator will establish each Option’s and Stock Appreciation Right’s exercise price and specify the exercise price in the Award Agreement. The exercise price will not be less than 100% of the Fair Market Value on the grant date of the Option or Stock Appreciation Right.
5.3 Duration. Each Option or Stock Appreciation Right will be exercisable at such times and as specified in the Award Agreement, provided that the term of an Option or Stock Appreciation Right will not exceed ten (10) years. Notwithstanding the foregoing and unless determined otherwise by the Company, in the event that on the last business day of the term of an Option or Stock Appreciation Right (other than an Incentive Stock Option) (i) the exercise of the Option or Stock Appreciation Right is prohibited by Applicable Law, as determined by the Company, or (ii) Shares may not be purchased or sold by the applicable Participant due to any Company insider trading policy (including blackout periods) or a“lock-up” agreement undertaken in connection with an issuance of securities by the Company, the term of the Option or Stock Appreciation Right shall be extended until the date that is thirty (30) days after the end of the legal prohibition,black-out period orlock-up agreement, as determined by the Company; provided, however, in no event shall the extension last beyond the ten- (10)-year term of the applicable Option or Stock Appreciation Right. Notwithstanding the foregoing, if the Participant, prior to the end of the term of an Option or Stock Appreciation Right, violates thenon-competition,non-solicitation, confidentiality or other similar restrictive covenant provisions of any employment contract, confidentiality and nondisclosure agreement or other agreement between the Participant and the Company or any of its Subsidiaries, the right of the Participant and the Participant’s transferees to exercise any Option or Stock Appreciation Right issued to the Participant shall terminate immediately upon such violation, unless the Company otherwise determines. In addition, if, prior to the end of the term of an Option or Stock Appreciation Right, the Participant is given notice by the Company or any of its Subsidiaries of the Participant’s Termination of Service by the Company or any of its Subsidiaries for Cause, and the effective date of such Termination of Service is subsequent to the date of the delivery of such notice, the right of the Participant and the Participant’s transferees to exercise any Option or Stock Appreciation Right issued to the Participant shall be suspended from the time of the delivery of such notice until the earlier of (i) such time as it is determined or otherwise agreed that the Participant’s service as a Service Provider will not be terminated for Cause as provided in such notice or (ii) the effective date of the Participant’s Termination of Service by the Company or any of its Subsidiaries for Cause (in which case the right of the Participant and the Participant’s transferees to exercise any Option or Stock Appreciation Right issued to the Participant will terminate immediately upon the effective date of such Termination of Service).
5.4 Exercise. Options and Stock Appreciation Rights may be exercised by delivering to the Company a written notice of exercise, in a form the Administrator approves (which may be electronic), signed by the person authorized to exercise the Option or Stock Appreciation Right, together with, as applicable, payment in full (i) as specified in Section 5.5 for the number of Shares for which the Award is exercised and (ii) as specified in
F-3
Section 9.5 for any applicable taxes. Unless the Administrator otherwise determines, an Option or Stock Appreciation Right may not be exercised for a fraction of a Share.
5.5 Payment Upon Exercise. Subject to any Company insider trading policy (including blackout periods) and Applicable Laws, the exercise price of an Option must be paid by:
(a) cash, wire transfer of immediately available funds or by check payable to the order of the Company, provided that the Company may limit the use of one of the foregoing payment forms if one or more of the payment forms below is permitted;
(b) if there is a public market for Shares at the time of exercise, unless the Company otherwise determines, (A) delivery (including electronically or telephonically to the extent permitted by the Company) of an irrevocable and unconditional undertaking by a broker acceptable to the Company to deliver promptly to the Company sufficient funds to pay the exercise price, or (B) the Participant’s delivery to the Company of a copy of irrevocable and unconditional instructions to a broker acceptable to the Company to deliver promptly to the Company cash or a check sufficient to pay the exercise price; provided that such amount is paid to the Company at such time as may be required by the Administrator;
(c) to the extent permitted by the Administrator, delivery (either by actual delivery or attestation) of Shares owned by the Participant valued at their Fair Market Value;
(d) to the extent permitted by the Administrator, surrendering Shares then issuable upon the Option’s exercise valued at their Fair Market Value on the exercise date;
(e) to the extent permitted by the Administrator, delivery of a promissory note or any other property that the Administrator determines is good and valuable consideration; or
(f) to the extent permitted by the Company, any combination of the above payment forms approved by the Administrator.
ARTICLE VI.
RESTRICTED STOCK; RESTRICTED STOCK UNITS
6.1 General. The Administrator may grant Restricted Stock, or the right to purchase Restricted Stock, to any Service Provider, subject to the Company’s right to repurchase all or part of such Shares at their issue price or other stated or formula price from the Participant (or to require forfeiture of such Shares) if conditions the Administrator specifies in the Award Agreement are not satisfied before the end of the applicable restriction period or periods that the Administrator establishes for such Award. In addition, the Administrator may grant to Service Providers Restricted Stock Units, which may be subject to vesting and forfeiture conditions during the applicable restriction period or periods, as set forth in an Award Agreement. The Administrator will determine and set forth in the Award Agreement the terms and conditions for each Restricted Stock and Restricted Stock Unit Award, subject to the conditions and limitations contained in the Plan.
6.2 Restricted Stock.
(a) Dividends. Participants holding Shares of Restricted Stock will be entitled to all ordinary cash dividends paid with respect to such Shares, unless the Administrator provides otherwise in the Award Agreement. In addition, unless the Administrator provides otherwise, if any dividends or distributions are paid in Shares, or consist of a dividend or distribution to holders of Common Stock of property other than an ordinary cash dividend, the Shares or other property will be subject to the same restrictions on transferability and forfeitability as the Shares of Restricted Stock with respect to which they were paid.
F-4
(b) Stock Certificates. The Company may require that the Participant deposit in escrow with the Company (or its designee) any stock certificates issued in respect of Shares of Restricted Stock, together with a stock power endorsed in blank.
6.3 Restricted Stock Units.
(a) Settlement. The Administrator may provide that settlement of Restricted Stock Units will occur upon or as soon as reasonably practicable after the Restricted Stock Units vest or will instead be deferred, on a mandatory basis or at the Participant’s election, in a manner intended to comply with Section 409A.
(b) Stockholder Rights. A Participant will have no rights of a stockholder with respect to Shares subject to any Restricted Stock Unit unless and until the Shares are delivered in settlement of the Restricted Stock Unit.
(c) Dividend Equivalents. If the Administrator provides, a grant of Restricted Stock Units may provide a Participant with the right to receive Dividend Equivalents. Dividend Equivalents may be paid currently or credited to an account for the Participant, settled in cash or Shares and subject to the same restrictions on transferability and forfeitability as the Restricted Stock Units with respect to which the Dividend Equivalents are granted and subject to other terms and conditions as set forth in the Award Agreement.
ARTICLE VII.
OTHER STOCK OR CASH BASED AWARDS
Other Stock or Cash Based Awards may be granted to Participants, including Awards entitling Participants to receive Shares to be delivered in the future and including annual or other periodic or long-term cash bonus awards (whether based on specified Performance Criteria or otherwise), in each case subject to any conditions and limitations in the Plan. Such Other Stock or Cash Based Awards will also be available as a payment form in the settlement of other Awards, as standalone payments and as payment in lieu of compensation to which a Participant is otherwise entitled. Other Stock or Cash Based Awards may be paid in Shares, cash or other property, as the Administrator determines. Subject to the provisions of the Plan, the Administrator will determine the terms and conditions of each Other Stock or Cash Based Award, including any purchase price, performance goal (which may be based on the Performance Criteria), transfer restrictions, and vesting conditions, which will be set forth in the applicable Award Agreement.
ARTICLE VIII.
ADJUSTMENTS FOR CHANGES IN COMMON STOCK AND CERTAIN OTHER EVENTS
8.1 Equity Restructuring. In connection with any Equity Restructuring, notwithstanding anything to the contrary in this Article VIII, the Administrator will equitably adjust each outstanding Award as it deems appropriate to reflect the Equity Restructuring, which may include adjusting the number and type of securities subject to each outstanding Award and/or the Award’s exercise price or grant price (if applicable), granting new Awards to Participants, and making a cash payment to Participants. The adjustments provided under this Section 8.1 will be nondiscretionary and final and binding on the affected Participant and the Company; provided that the Administrator will determine whether an adjustment is equitable.
8.2 Corporate Transactions. In the event of any dividend or other distribution (whether in the form of cash, Common Stock, other securities, or other property), reorganization, merger, consolidation, combination, amalgamation, repurchase, recapitalization, liquidation, dissolution, or sale, transfer, exchange or other disposition of all or substantially all of the assets of the Company, or sale or exchange of Common Stock or other securities of the Company, Change in Control, issuance of warrants or other rights to purchase Common Stock or
F-5
other securities of the Company, other similar corporate transaction or event, other unusual or nonrecurring transaction or event affecting the Company or its financial statements or any change in any Applicable Laws or accounting principles, the Administrator, on such terms and conditions as it deems appropriate, either by the terms of the Award or by action taken prior to the occurrence of such transaction or event (except that action to give effect to a change in Applicable Law or accounting principles may be made within a reasonable period of time after such change) and either automatically or upon the Participant’s request, is hereby authorized to take any one or more of the following actions whenever the Administrator determines that such action is appropriate in order to (x) prevent dilution or enlargement of the benefits or potential benefits intended by the Company to be made available under the Plan or with respect to any Award granted or issued under the Plan, (y) to facilitate such transaction or event or (z) give effect to such changes in Applicable Laws or accounting principles:
(a) To provide for the cancellation of any such Award in exchange for either an amount of cash or other property with a value equal to the amount that could have been obtained upon the exercise or settlement of the vested portion of such Award or realization of the Participant’s rights under the vested portion of such Award, as applicable; provided that, if the amount that could have been obtained upon the exercise or settlement of the vested portion of such Award or realization of the Participant’s rights, in any case, is equal to or less than zero, then the Award may be terminated without payment;
(b) To provide that such Award shall vest and, to the extent applicable, be exercisable as to all Shares covered thereby, notwithstanding anything to the contrary in the Plan or the provisions of such Award;
(c) To provide that such Award be assumed by the successor or survivor corporation, or a parent or subsidiary thereof, or shall be substituted for by awards covering the stock of the successor or survivor corporation, or a parent or subsidiary thereof, with appropriate adjustments as to the number and kind of Shares and/or applicable exercise or purchase price, in all cases, as determined by the Administrator;
(d) To make adjustments in the number and type of Shares (or other securities or property) subject to outstanding Awards and/or with respect to which Awards may be granted under the Plan (including, but not limited to, adjustments of the limitations in Article IV hereof on the maximum number and kind of Shares which may be issued) and/or in the terms and conditions of (including the grant or exercise price), and the criteria included in, outstanding Awards;
(e) To replace such Award with other rights or property selected by the Administrator; and/or
(f) To provide that the Award will terminate and cannot vest, be exercised or become payable after the applicable event.
8.3 Effect ofNon-Assumption in a Change in Control. Notwithstanding the provisions of Section 8.2 above, if a Change in Control occurs and a Participant’s Awards are not continued, converted, assumed, or replaced with a substantially similar award by (a) the Company, or (b) a Successor Entity (as defined below) or its parent or subsidiary (an “Assumption”), and provided that the Participant has not had a Termination of Service, then, immediately prior to the Change in Control, such Awards shall become fully vested, exercisable and/or payable, as applicable, and all forfeiture, repurchase and other restrictions on such Awards shall lapse, in which case, such Awards shall be canceled upon the consummation of the Change in Control in exchange for the right to receive the Change in Control consideration payable to other holders of Common Stock (i) which may be on such terms and conditions as apply generally to holders of Common Stock under the Change in Control documents (including, without limitation, any escrow,earn-out or other deferred consideration provisions) or such other terms and conditions as the Administrator may provide, and (ii) determined by reference to the number of Shares subject to such Awards and net of any applicable exercise price; provided that to the extent that any Awards constitute “nonqualified deferred compensation” that may not be paid upon the Change in Control under Section 409A without the imposition of taxes thereon under Section 409A, the timing of such payments shall be governed by the applicable Award Agreement (subject to any deferred consideration provisions
F-6
applicable under the Change in Control documents); and provided, further, that if the amount to which a Participant would be entitled upon the settlement or exercise of such Award at the time of the Change in Control is equal to or less than zero, then such Award may be terminated without payment. The Administrator shall determine whether an Assumption of an Award has occurred in connection with a Change in Control.
8.4 Administrative Stand Still. In the event of any pending stock dividend, stock split, combination or exchange of Shares, merger, consolidation or other distribution (other than normal cash dividends) of Company assets to stockholders, or any other extraordinary transaction or change affecting the Shares or the share price of Common Stock, including any Equity Restructuring or any securities offering or other similar transaction, for administrative convenience, the Administrator may refuse to permit the exercise of any Award for up to sixty (60) days before or after such transaction.
8.5 General. Except as expressly provided in the Plan or the Administrator’s action under the Plan, no Participant will have any rights due to any subdivision or consolidation of Shares of any class, dividend payment, increase or decrease in the number of Shares of any class or dissolution, liquidation, merger, or consolidation of the Company or other corporation. Except as expressly provided with respect to an Equity Restructuring under Section 8.1 above or the Administrator’s action under the Plan, no issuance by the Company of Shares of any class, or securities convertible into Shares of any class, will affect, and no adjustment will be made regarding, the number of Shares subject to an Award or the Award’s grant or exercise price. The existence of the Plan, any Award Agreements and the Awards granted hereunder will not affect or restrict in any way the Company’s right or power to make or authorize (i) any adjustment, recapitalization, reorganization or other change in the Company’s capital structure or its business, (ii) any merger, consolidation dissolution or liquidation of the Company or sale of Company assets or (iii) any sale or issuance of securities, including securities with rights superior to those of the Shares or securities convertible into or exchangeable for Shares. The Administrator may treat Participants and Awards (or portions thereof) differently under this Article VIII.
ARTICLE IX.
GENERAL PROVISIONS APPLICABLE TO AWARDS
9.1 Transferability. Except as the Administrator may determine or provide in an Award Agreement or otherwise for Awards other than Incentive Stock Options, Awards may not be sold, assigned, transferred, pledged or otherwise encumbered, either voluntarily or by operation of law, except by will or the laws of descent and distribution, or, subject to the Administrator’s consent, pursuant to a domestic relations order, and, during the life of the Participant, will be exercisable only by the Participant. References to a Participant, to the extent relevant in the context, will include references to a Participant’s authorized transferee that the Administrator specifically approves.
9.2 Documentation. Each Award will be evidenced in an Award Agreement, which may be written or electronic, as the Administrator determines. Each Award may contain terms and conditions in addition to those set forth in the Plan.
9.3 Discretion. Except as the Plan otherwise provides, each Award may be made alone or in addition or in relation to any other Award. The terms of each Award to a Participant need not be identical, and the Administrator need not treat Participants or Awards (or portions thereof) uniformly.
9.4 Termination of Status. The Administrator will determine how the disability, death, retirement, authorized leave of absence or any other change or purported change in a Participant’s Service Provider status affects an Award and the extent to which, and the period during which, the Participant, the Participant’s legal representative, conservator, guardian or Designated Beneficiary may exercise rights under the Award, if applicable.
F-7
9.5 Withholding. Each Participant must pay the Company, or make provision satisfactory to the Administrator for payment of, any taxes required by law to be withheld in connection with such Participant’s Awards by the date of the event creating the tax liability. The Company may deduct an amount sufficient to satisfy such tax obligations based on the applicable statutory withholding rates (or such other rate as may be determined by the Company after considering any accounting consequences or costs) from any payment of any kind otherwise due to a Participant. In the absence of a contrary determination by the Company (or, with respect to withholding pursuant to clause (ii) below with respect to Awards held by individuals subject to Section 16 of the Exchange Act, a contrary determination by the Administrator), all tax withholding obligations will be calculated based on the minimum applicable statutory withholding rates. Subject to any Company insider trading policy (including blackout periods), Participants may satisfy such tax obligations (i) in cash, by wire transfer of immediately available funds, by check made payable to the order of the Company, provided that the Company may limit the use of the foregoing payment forms if one or more of the payment forms below is permitted, (ii) to the extent permitted by the Administrator, in whole or in part by delivery of Shares, including Shares delivered by attestation and Shares retained from the Award creating the tax obligation, valued at their Fair Market Value on the date of delivery, (iii) if there is a public market for Shares at the time the tax obligations are satisfied, unless the Company otherwise determines, (A) delivery (including electronically or telephonically to the extent permitted by the Company) of an irrevocable and unconditional undertaking by a broker acceptable to the Company to deliver promptly to the Company sufficient funds to satisfy the tax obligations, or (B) delivery by the Participant to the Company of a copy of irrevocable and unconditional instructions to a broker acceptable to the Company to deliver promptly to the Company cash or a check sufficient to satisfy the tax withholding; provided that such amount is paid to the Company at such time as may be required by the Administrator, or (iv) to the extent permitted by the Company, any combination of the foregoing payment forms approved by the Administrator. Notwithstanding any other provision of the Plan, the number of Shares which may be so delivered or retained pursuant to clause (ii) of the immediately preceding sentence shall be limited to the number of Shares which have a Fair Market Value on the date of delivery or retention no greater than the aggregate amount of such liabilities based on the maximum individual statutory tax rate in the applicable jurisdiction at the time of such withholding (or such other rate as may be required to avoid the liability classification of the applicable award under generally accepted accounting principles in the United States of America); provided, however, to the extent such Shares were acquired by Participant from the Company as compensation, the Shares must have been held for the minimum period required by applicable accounting rules to avoid a charge to the Company’s earnings for financial reporting purposes; provided, further, that, any such Shares delivered or retained shall be rounded up to the nearest whole Share to the extent rounding up to the nearest whole Share does not result in the liability classification of the applicable Award under generally accepted accounting principles in the United States of America. If any tax withholding obligation will be satisfied under clause (ii) above by the Company’s retention of Shares from the Award creating the tax obligation and there is a public market for Shares at the time the tax obligation is satisfied, the Company may elect to instruct any brokerage firm determined acceptable to the Company for such purpose to sell on the applicable Participant’s behalf some or all of the Shares retained and to remit the proceeds of the sale to the Company or its designee, and each Participant’s acceptance of an Award under the Plan will constitute the Participant’s authorization to the Company and instruction and authorization to such brokerage firm to complete the transactions described in this sentence.
9.6 Amendment of Award; Repricing. The Administrator may amend, modify or terminate any outstanding Award, including by substituting another Award of the same or a different type, changing the exercise or settlement date, and converting an Incentive Stock Option to aNon-Qualified Stock Option. The Participant’s consent to such action will be required unless (i) the action, taking into account any related action, does not materially and adversely affect the Participant’s rights under the Award, or (ii) the change is permitted under Article VIII or pursuant to Section 10.6. Notwithstanding the foregoing or anything in the Plan to the contrary, the Administrator may, without the approval of the stockholders of the Company, reduce the exercise price per share of outstanding Options or Stock Appreciation Rights or cancel outstanding Options or Stock Appreciation Rights in exchange for cash, other Awards or Options or Stock Appreciation Rights with an exercise price per share that is less than the exercise price per share of the original Options or Stock Appreciation Rights.
F-8
9.7 Conditions on Delivery of Stock. The Company will not be obligated to deliver any Shares under the Plan or remove restrictions from Shares previously delivered under the Plan until (i) all Award conditions have been met or removed to the Company’s satisfaction, (ii) as determined by the Company, all other legal matters regarding the issuance and delivery of such Shares have been satisfied, including any applicable securities laws and stock exchange or stock market rules and regulations, and (iii) the Participant has executed and delivered to the Company such representations or agreements as the Administrator deems necessary or appropriate to satisfy any Applicable Laws. The Company’s inability to obtain authority from any regulatory body having jurisdiction, which the Administrator determines is necessary to the lawful issuance and sale of any securities, will relieve the Company of any liability for failing to issue or sell such Shares as to which such requisite authority has not been obtained.
9.8 Acceleration. The Administrator may at any time provide that any Award will become immediately vested and fully or partially exercisable, free of some or all restrictions or conditions, or otherwise fully or partially realizable.
9.9 Additional Terms of Incentive Stock Options. The Administrator may grant Incentive Stock Options only to employees of the Company, any of its present or future parent or subsidiary corporations, as defined in Sections 424(e) or (f) of the Code, respectively, and any other entities the employees of which are eligible to receive Incentive Stock Options under the Code. If an Incentive Stock Option is granted to a Greater Than 10% Stockholder, the exercise price will not be less than 110% of the Fair Market Value on the Option’s grant date, and the term of the Option will not exceed five (5) years. All Incentive Stock Options will be subject to and construed consistently with Section 422 of the Code. By accepting an Incentive Stock Option, the Participant agrees to give prompt notice to the Company of dispositions or other transfers (other than in connection with a Change in Control) of Shares acquired under the Option made within (i) two years from the grant date of the Option or (ii) one year after the transfer of such Shares to the Participant, specifying the date of the disposition or other transfer and the amount the Participant realized, in cash, other property, assumption of indebtedness or other consideration, in such disposition or other transfer. Neither the Company nor the Administrator will be liable to a Participant, or any other party, if an Incentive Stock Option fails or ceases to qualify as an “incentive stock option” under Section 422 of the Code. Any Incentive Stock Option or portion thereof that fails to qualify as an “incentive stock option” under Section 422 of the Code for any reason, including becoming exercisable with respect to Shares having a Fair Market Value exceeding the $100,000 limitation under Treasury RegulationSection 1.422-4, will be aNon-Qualified Stock Option.
ARTICLE X.
MISCELLANEOUS
10.1 No Right to Employment or Other Status. No person will have any claim or right to be granted an Award, and the grant of an Award will not be construed as giving a Participant the right to continued employment or any other relationship with the Company. The Company expressly reserves the right at any time to dismiss or otherwise terminate its relationship with a Participant free from any liability or claim under the Plan or any Award, except as expressly provided in an Award Agreement.
10.2 No Rights as Stockholder; Certificates. Subject to the Award Agreement, no Participant or Designated Beneficiary will have any rights as a stockholder with respect to any Shares to be distributed under an Award until becoming the record holder of such Shares. Notwithstanding any other provision of the Plan, unless the Administrator otherwise determines or Applicable Laws require, the Company will not be required to deliver to any Participant certificates evidencing Shares issued in connection with any Award and instead such Shares may be recorded in the books of the Company (or, as applicable, its transfer agent or stock plan administrator). The Company may place legends on stock certificates issued under the Plan that the Administrator deems necessary or appropriate to comply with Applicable Laws.
F-9
10.3 Effective Date and Term of Plan. The Plan was approved by the Board on April 16, 2019. The Plan shall be effective (the “Effective Date”) on the day prior to the date of the closing of the transactions contemplated by that certain Agreement and Plan of Merger and Reorganization, dated as of March 6, 2019 by and among the Company, Grizzly Merger Sub, Inc. and Oncternal Therapeutics, Inc. (as amended, the “Merger Agreement”), provided that it is approved by a majority of the Company’s stockholders at a duly held meeting prior to such date and occurring within twelve (12) months following the date the Board approved Plan, and provided further that the effectiveness of the Plan is subject to the consummation of the transactions contemplated by the Merger Agreement. If the Plan is not approved by the Company’s stockholders within the foregoing time frame, or if the Merger Agreement is terminated prior to the consummation of the transactions contemplated thereby, the Plan will not become effective. The Plan shall remain in effect until the tenth (10th) anniversary of the date the Board adopted the Plan, but Awards previously granted may extend beyond that date in accordance with the Plan. The Plan will be submitted for approval of the Company’s stockholders within twelve (2) months following the date the Board approved the Plan.
10.4 Amendment of Plan. The Administrator may amend, suspend or terminate the Plan at any time; provided that no amendment, other than an increase to the Overall Share Limit, may materially and adversely affect any Award outstanding at the time of such amendment without the affected Participant’s consent. No Awards may be granted under the Plan during any suspension period or after the Plan’s termination. Awards outstanding at the time of any Plan suspension or termination will continue to be governed by the Plan and the Award Agreement, as in effect before such suspension or termination. The Board will obtain stockholder approval of any Plan amendment to the extent necessary to comply with Applicable Laws.
10.5 Provisions for Foreign Participants. The Administrator may modify Awards granted to Participants who are foreign nationals or employed outside the United States or establish subplans or procedures under the Plan to address differences in laws, rules, regulations or customs of such foreign jurisdictions with respect to tax, securities, currency, employee benefit or other matters.
10.6 Section 409A.
(a) General. The Company intends that all Awards be structured to comply with, or be exempt from, Section 409A, such that no adverse tax consequences, interest, or penalties under Section 409A apply. Notwithstanding anything in the Plan or any Award Agreement to the contrary, the Administrator may, without a Participant’s consent, amend this Plan or Awards, adopt policies and procedures, or take any other actions (including amendments, policies, procedures and retroactive actions) as are necessary or appropriate to preserve the intended tax treatment of Awards, including any such actions intended to (A) exempt this Plan or any Award from Section 409A, or (B) comply with Section 409A, including regulations, guidance, compliance programs and other interpretative authority that may be issued after an Award’s grant date. The Company makes no representations or warranties as to an Award’s tax treatment under Section 409A or otherwise. The Company will have no obligation under this Section 10.6 or otherwise to avoid the taxes, penalties or interest under Section 409A with respect to any Award and will have no liability to any Participant or any other person if any Award, compensation or other benefits under the Plan are determined to constitute noncompliant “nonqualified deferred compensation” subject to taxes, penalties or interest under Section 409A.
(b) Separation from Service. If an Award constitutes “nonqualified deferred compensation” under Section 409A, any payment or settlement of such Award upon a termination of a Participant’s Service Provider relationship will, to the extent necessary to avoid taxes under Section 409A, be made only upon the Participant’s “separation from service” (within the meaning of Section 409A), whether such “separation from service” occurs upon or after the termination of the Participant’s Service Provider relationship. For purposes of this Plan or any Award Agreement relating to any such payments or benefits, references to a “termination,” “termination of employment” or like terms means a “separation from service.”
(c) Payments to Specified Employees. Notwithstanding any contrary provision in the Plan or any Award Agreement, any payment(s) of “nonqualified deferred compensation” required to be made under an
F-10
Award to a “specified employee” (as defined under Section 409A and as the Administrator determines) due to his or her “separation from service” will, to the extent necessary to avoid taxes under Section 409A(a)(2)(B)(i) of the Code, be delayed for the six- (6)-month period immediately following such “separation from service” (or, if earlier, until the specified Employee’s death) and will instead be paid (as set forth in the Award Agreement) on the day immediately following such six- (6)month period or as soon as administratively practicable thereafter (without interest). Any payments of “nonqualified deferred compensation” under such Award payable more than six (6) months following the Participant’s “separation from service” will be paid at the time or times the payments are otherwise scheduled to be made.
10.7 Limitations on Liability. Notwithstanding any other provisions of the Plan, no individual acting as a director, officer, other Employee or agent of the Company or any Subsidiary will be liable to any Participant, former Participant, spouse, beneficiary, or any other person for any claim, loss, liability, or expense incurred in connection with the Plan or any Award, and such individual will not be personally liable with respect to the Plan because of any contract or other instrument executed in his or her capacity as an Administrator, director, officer, other Employee or agent of the Company or any Subsidiary. The Company will indemnify and hold harmless each director, officer, other Employee and agent of the Company or any Subsidiary that has been or will be granted or delegated any duty or power relating to the Plan’s administration or interpretation, against any cost or expense (including attorneys’ fees) or liability (including any sum paid in settlement of a claim with the Administrator’s approval) arising from any act or omission concerning this Plan unless arising from such person’s own fraud or bad faith.
10.9 Data Privacy. As a condition for receiving any Award, each Participant explicitly and unambiguously consents to the collection, use and transfer, in electronic or other form, of personal data as described in this section by and among the Company and its Subsidiaries and affiliates exclusively for implementing, administering and managing the Participant’s participation in the Plan. The Company and its Subsidiaries and affiliates may hold certain personal information about a Participant, including the Participant’s name, address and telephone number; birthdate; social security, insurance number or other identification number; salary; nationality; job title(s); any Shares held in the Company or its Subsidiaries and affiliates; and Award details, to implement, manage and administer the Plan and Awards (the “Data”). The Company and its Subsidiaries and affiliates may transfer the Data amongst themselves as necessary to implement, administer and manage a Participant’s participation in the Plan, and the Company and its Subsidiaries and affiliates may transfer the Data to third parties assisting the Company with Plan implementation, administration and management. These recipients may be located in the Participant’s country, or elsewhere, and the Participant’s country may have different data privacy laws and protections than the recipients’ country. By accepting an Award, each Participant authorizes such recipients to receive, possess, use, retain and transfer the Data, in electronic or other form, to implement, administer and manage the Participant’s participation in the Plan, including any required Data transfer to a broker or other third party with whom the Company or the Participant may elect to deposit any Shares. The Data related to a Participant will be held only as long as necessary to implement, administer, and manage the Participant’s participation in the Plan. A Participant may, at any time, view the Data that the Company holds regarding such Participant, request additional information about the storage and processing of the Data regarding such Participant, recommend any necessary corrections to the Data regarding the Participant or refuse or withdraw the consents in this Section 10.8 in writing, without cost, by contacting the local human resources representative. The Company may cancel Participant’s ability to participate in the Plan and, in the Administrator’s discretion, the Participant may forfeit any outstanding Awards if the Participant refuses or withdraws the consents in this Section 10.8. For more information on the consequences of refusing or withdrawing consent, Participants may contact their local human resources representative.
10.10 Severability. If any portion of the Plan or any action taken under it is held illegal or invalid for any reason, the illegality or invalidity will not affect the remaining parts of the Plan, and the Plan will be construed and enforced as if the illegal or invalid provisions had been excluded, and the illegal or invalid action will be null and void.
F-11
10.11 Governing Documents. If any contradiction occurs between the Plan and any Award Agreement or other written agreement between a Participant and the Company (or any Subsidiary) that the Administrator has approved, the Plan will govern, unless it is expressly specified in such Award Agreement or other written document that a specific provision of the Plan will not apply.
10.12 Governing Law. The Plan and all Awards will be governed by and interpreted in accordance with the laws of the State of Delaware, disregarding any state’schoice-of-law principles requiring the application of a jurisdiction’s laws other than the State of Delaware.
10.13 Claw-backProvisions. All Awards (including, without limitation, any proceeds, gains or other economic benefit actually or constructively received by Participant upon any receipt or exercise of any Award or upon the receipt or resale of any Shares underlying the Award) shall be subject to the provisions of any claw-back policy implemented by the Company, including, without limitation, any claw-back policy adopted to comply with Applicable Laws (including the Dodd-Frank Wall Street Reform and Consumer Protection Act and any rules or regulations promulgated thereunder) as and to the extent set forth in such claw-back policy or the Award Agreement.
10.14 Titles and Headings. The titles and headings in the Plan are for convenience of reference only and, if any conflict, the Plan’s text, rather than such titles or headings, will control.
10.15 Conformity to Securities Laws. Participant acknowledges that the Plan is intended to conform to the extent necessary with Applicable Laws. Notwithstanding anything herein to the contrary, the Plan and all Awards will be administered only in conformance with Applicable Laws. To the extent Applicable Laws permit, the Plan and all Award Agreements will be deemed amended as necessary to conform to Applicable Laws.
10.16 Relationship to Other Benefits. No payment under the Plan will be taken into account in determining any benefits under any pension, retirement, savings, profit sharing, group insurance, welfare or other benefit plan of the Company or any Subsidiary except as expressly provided in writing in such other plan or an agreement thereunder.
10.17 Broker-Assisted Sales. In the event of a broker-assisted sale of Shares in connection with the payment of amounts owed by a Participant under or with respect to the Plan or Awards, including amounts to be paid under the final sentence of Section 9.5: (a) any Shares to be sold through the broker-assisted sale will be sold on the day the payment first becomes due, or as soon thereafter as practicable; (b) such Shares may be sold as part of a block trade with other Participants in the Plan in which all participants receive an average price; (c) the applicable Participant will be responsible for all broker’s fees and other costs of sale, and by accepting an Award, each Participant agrees to indemnify and hold the Company harmless from any losses, costs, damages, or expenses relating to any such sale; (d) to the extent the Company or its designee receives proceeds of such sale that exceed the amount owed, the Company will pay such excess in cash to the applicable Participant as soon as reasonably practicable; (e) the Company and its designees are under no obligation to arrange for such sale at any particular price; and (f) in the event the proceeds of such sale are insufficient to satisfy the Participant’s applicable obligation, the Participant may be required to pay immediately upon demand to the Company or its designee an amount in cash sufficient to satisfy any remaining portion of the Participant’s obligation.
ARTICLE XI.
DEFINITIONS
As used in the Plan, the following words and phrases will have the following meanings:
11.1 “Administrator” means the Board or a Committee to the extent that the Board’s powers or authority under the Plan have been delegated to such Committee.
F-12
11.2 “Applicable Laws” means the requirements relating to the administration of equity incentive plans under U.S. federal and state securities, tax and other applicable laws, rules and regulations, the applicable rules of any stock exchange or quotation system on which the Common Stock is listed or quoted and the applicable laws and rules of any foreign country or other jurisdiction where Awards are granted.
11.3 “Award” means, individually or collectively, a grant under the Plan of Options, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units or Other Stock or Cash Based Awards.
11.4 “Award Agreement” means a written agreement evidencing an Award, which may be electronic, that contains such terms and conditions as the Administrator determines, consistent with and subject to the terms and conditions of the Plan.
11.5 “Board” means the Board of Directors of the Company.
11.6 “Cause” means (a) if a Participant is a party to a written employment or consulting agreement with the Company or any of its Subsidiaries or an Award Agreement in which the term “cause” is defined, “Cause” as defined in such agreement, and (b) if no such agreement exists, (i) the Administrator’s determination that the Participant failed to substantially perform the Participant’s duties (other than any such failure resulting from the Participant’s Disability); (ii) the Administrator’s determination that the Participant failed to carry out, or comply with any lawful and reasonable directive of the Board or the Participant’s immediate supervisor; (iii) the occurrence of any act or omission by the Participant that could reasonably be expected to result in (or has resulted in) the Participant’s conviction, plea of no contest, plea of nolo contendere, or imposition of unadjudicated probation for any felony or indictable offense or crime involving moral turpitude; (iv) the Participant’s unlawful use (including being under the influence) or possession of illegal drugs on the premises of the Company or any of its Subsidiaries or while performing the Participant’s duties and responsibilities for the Company or any of its Subsidiaries; or (v) the Participant’s commission of an act of fraud, embezzlement, misappropriation, misconduct, or breach of fiduciary duty against the Company or any of its Subsidiaries.
11.7 “Change in Control” means and includes each of the following:
(a) A transaction or series of transactions (other than an offering of Common Stock to the general public through a registration statement filed with the Securities and Exchange Commission or a transaction or series of transactions that meets the requirements of clauses (i) and (ii) of subsection (c) below) whereby any “person” or related “group” of “persons” (as such terms are used in Sections 13(d) and 14(d)(2) of the Exchange Act) (other than the Company, any of its Subsidiaries, an employee benefit plan maintained by the Company or any of its Subsidiaries or a “person” that, prior to such transaction, directly or indirectly controls, is controlled by, or is under common control with, the Company) directly or indirectly acquires beneficial ownership (within the meaning of Rule13d-3 under the Exchange Act) of securities of the Company possessing more than 50% of the total combined voting power of the Company’s securities outstanding immediately after such acquisition; or
(b) During any period of two (2) consecutive years, individuals who, at the beginning of such period, constitute the Board together with any new Director(s) (other than a Director designated by a person who shall have entered into an agreement with the Company to effect a transaction described in subsections (a) or (c)) whose election by the Board or nomination for election by the Company’s stockholders was approved by a vote of at leasttwo-thirds of the Directors then still in office who either were Directors at the beginning of the two- (2)-year period or whose election or nomination for election was previously so approved, cease for any reason to constitute a majority thereof; or
(c) The consummation by the Company (whether directly involving the Company or indirectly involving the Company through one or more intermediaries) of (x) a merger, consolidation, reorganization, or
F-13
business combination or (y) a sale or other disposition of all or substantially all of the Company’s assets in any single transaction or series of related transactions or (z) the acquisition of assets or stock of another entity, in each case other than a transaction:
(i) which results in the Company’s voting securities outstanding immediately before the transaction continuing to represent (either by remaining outstanding or by being converted into voting securities of the Company or the person that, as a result of the transaction, controls, directly or indirectly, the Company or owns, directly or indirectly, all or substantially all of the Company’s assets or otherwise succeeds to the business of the Company (the Company or such person, the “Successor Entity”)) directly or indirectly, at least a majority of the combined voting power of the Successor Entity’s outstanding voting securities immediately after the transaction, and
(ii) after which no person or group beneficially owns voting securities representing 50% or more of the combined voting power of the Successor Entity;provided,however, that no person or group shall be treated for purposes of this clause (ii) as beneficially owning 50% or more of the combined voting power of the Successor Entity solely as a result of the voting power held in the Company prior to the consummation of the transaction.
Notwithstanding the foregoing, (x) the transactions contemplated by the Merger Agreement shall not constitute a Change in Control for purposes of this Plan, and (y) if a Change in Control constitutes a payment event with respect to any Award (or portion of any Award) that provides for the deferral of compensation that is subject to Section 409A, to the extent required to avoid the imposition of additional taxes under Section 409A, the transaction or event described in subsection (a), (b) or (c) with respect to such Award (or portion thereof) shall only constitute a Change in Control for purposes of the payment timing of such Award if such transaction also constitutes a “change in control event,” as defined in Treasury RegulationSection 1.409A-3(i)(5).
The Administrator shall have full and final authority, which shall be exercised in its discretion, to determine conclusively whether a Change in Control has occurred pursuant to the above definition, the date of the occurrence of such Change in Control and any incidental matters relating thereto; provided that any exercise of authority in conjunction with a determination of whether a Change in Control is a “change in control event” as defined in Treasury RegulationSection 1.409A-3(i)(5) shall be consistent with such regulation.
11.8 “Code” means the Internal Revenue Code of 1986, as amended, and the regulations issued thereunder.
11.9 “Committee” means one or more committees or subcommittees of the Board, which may include one or more Company directors or executive officers, to the extent Applicable Laws permit. To the extent required to comply with the provisions of Rule16b-3, it is intended that each member of the Committee will be, at the time the Committee takes any action with respect to an Award that is subject to Rule16b-3, a “non-employee director” within the meaning of Rule16b-3; however, a Committee member’s failure to qualify as a “non-employee director” within the meaning of Rule16b-3 will not invalidate any Award granted by the Committee that is otherwise validly granted under the Plan.
11.10 “Common Stock” means the common stock of the Company.
11.11 “Company” means GTx, Inc., a Delaware corporation, or any successor.
11.12 “Consultant” means any person, including any adviser, engaged by the Company or its parent or Subsidiary to render services to such entity if the consultant or adviser: (a) renders bona fide services to the Company; (b) renders services not in connection with the offer or sale of securities in a capital-raising transaction and does not directly or indirectly promote or maintain a market for the Company’s securities; and (c) is a natural person.
F-14
11.13 “Designated Beneficiary” means the beneficiary or beneficiaries the Participant designates, in a manner the Administrator determines, to receive amounts due or exercise the Participant’s rights if the Participant dies or becomes incapacitated. Without a Participant’s effective designation, “Designated Beneficiary” will mean the Participant’s estate.
11.14 “Director” means a Board member.
11.15 “Disability” means a permanent and total disability under Section 22(e)(3) of the Code, as amended.
11.16 “Dividend Equivalents” means a right granted to a Participant under the Plan to receive the equivalent value (in cash or Shares) of dividends paid on Shares.
11.17 “Employee” means any employee of the Company or its Subsidiaries.
11.18 “Equity Restructuring” means a nonreciprocal transaction between the Company and its stockholders, such as a stock dividend, stock split,spin-off or recapitalization through a large, nonrecurring cash dividend, that affects the number or kind of Shares (or other Company securities) or the share price of Common Stock (or other Company securities) and causes a change in the per share value of the Common Stock underlying outstanding Awards.
11.19 “Exchange Act” means the Securities Exchange Act of 1934, as amended.
11.20 “Fair Market Value” means, as of any date, the value of a Share determined as follows: (a) if the Common Stock is listed on any established stock exchange, its Fair Market Value will be the closing sales price for such Common Stock as quoted on such exchange for such date, or if no sale occurred on such date, the last day preceding such date during which a sale occurred, as reported inThe Wall Street Journal or another source the Administrator deems reliable; (b) if the Common Stock is not traded on a stock exchange but is quoted on a national market or other quotation system, the closing sales price on such date, or if no sales occurred on such date, then on the last date preceding such date during which a sale occurred, as reported inThe Wall Street Journalor another source the Administrator deems reliable; or (c) without an established market for the Common Stock, the Administrator will determine the Fair Market Value in its discretion.
11.21 “Good Reason” means (a) if a Participant is a party to a written employment or consulting agreement with the Company or any of its Subsidiaries or an Award Agreement in which the term “good reason” is defined, “Good Reason” as defined in such agreement, and (b) if no such agreement exists, (i) a change in the Participant’s position with the Company (or its Subsidiary employing the Participant) that materially reduces the Participant’s authority, duties or responsibilities or the level of management to which he or she reports, (ii) a material diminution in the Participant’s level of compensation (including base salary, fringe benefits and target bonuses under any corporate performance-based incentive programs) or (iii) a relocation of the Participant’s place of employment by more than 50 miles, provided that such change, reduction or relocation is effected by the Company (or its Subsidiary employing the Participant) without the Participant’s consent.
11.22 “Greater Than 10%Stockholder” means an individual then owning (within the meaning of Section 424(d) of the Code) more than 10% of the total combined voting power of all classes of stock of the Company, its parent or subsidiary corporation, as defined in Section 424(e) and (f) of the Code, respectively.
11.23 “Incentive Stock Option” means an Option intended to qualify as an “incentive stock option” as defined in Section 422 of the Code.
11.24 “Non-Qualified Stock Option” means an Option not intended or not qualifying as an Incentive Stock Option.
11.25 “Option” means an option to purchase Shares.
F-15
11.26 “Other Stock or Cash Based Awards” means cash awards, awards of Shares, and other awards valued wholly or partially by referring to, or are otherwise based on, Shares or other property.
11.27 “Overall Share Limit” means the sum of (a) 11,750,000 Shares; (b) any Shares which are subject to Prior Plan Awards as of the Effective Date which become available for issuance under the Plan pursuant to Article IV (which number added to the Overall Share Limit pursuant to this clause (b) shall not exceed 1,948,400 Shares); and (c) an annual increase on the first day of each calendar year beginning January 1, 2020 and ending on and including January 1, 2029, equal to the lesser of (i) 5% of the aggregate number of Shares outstanding on the final day of the immediately preceding calendar year and (ii) such smaller number of Shares as is determined by the Board.
11.28 “Participant” means a Service Provider who has been granted an Award.
11.29 “Performance Criteria” mean the criteria (and adjustments) that the Administrator may select for an Award to establish performance goals for a performance period, which may include the following: net earnings or losses (either before or after one or more of interest, taxes, depreciation, amortization, andnon-cash equity-based compensation expense); gross or net sales or revenue or sales or revenue growth; net income (either before or after taxes) or adjusted net income; profits (including but not limited to gross profits, net profits, profit growth, net operation profit or economic profit), profit return ratios or operating margin; budget or operating earnings (either before or after taxes or before or after allocation of corporate overhead and bonus); cash flow (including operating cash flow and free cash flow or cash flow return on capital); return on assets; return on capital or invested capital; cost of capital; return on stockholders’ equity; total stockholder return; return on sales; costs, reductions in costs and cost control measures; expenses; working capital; earnings or loss per share; adjusted earnings or loss per share; price per share or dividends per share (or appreciation in or maintenance of such price or dividends); regulatory achievements or compliance; implementation, completion or attainment of objectives relating to research, development, regulatory, commercial, or strategic milestones or developments; market share; economic value or economic value added models; division, group or corporate financial goals; customer satisfaction/growth; customer service; employee satisfaction; recruitment and maintenance of personnel; human resources management; supervision of litigation and other legal matters; strategic partnerships and transactions; financial ratios (including those measuring liquidity, activity, profitability or leverage); debt levels or reductions; sales-related goals; financing and other capital raising transactions; cash on hand; acquisition activity; investment sourcing activity; and marketing initiatives, any of which may be measured in absolute terms or as compared to any incremental increase or decrease. Such performance goals also may be based solely by reference to the Company’s performance or the performance of a Subsidiary, division, business segment or business unit of the Company or a Subsidiary, or based upon performance relative to performance of other companies or upon comparisons of any of the indicators of performance relative to performance of other companies. The Committee may provide for exclusion of the impact of an event or occurrence which the Committee determines should appropriately be excluded, including (a) restructurings, discontinued operations, extraordinary items, and other unusual, infrequently occurring ornon-recurring charges or events, (b) asset write-downs, (c) litigation or claim judgments or settlements, (d) acquisitions or divestitures, (e) reorganization or change in the corporate structure or capital structure of the Company, (f) an event either not directly related to the operations of the Company, Subsidiary, division, business segment or business unit or not within the reasonable control of management, (g) foreign exchange gains and losses, (h) a change in the fiscal year of the Company, (i) the refinancing or repurchase of bank loans or debt securities, (j) unbudgeted capital expenditures, (k) the issuance or repurchase of equity securities and other changes in the number of outstanding shares, (l) conversion of some or all of convertible securities to Common Stock, (m) any business interruption event (n) the cumulative effects of tax or accounting changes in accordance with U.S. generally accepted accounting principles, or (o) the effect of changes in other laws or regulatory rules affecting reported results.
11.30 “Plan” means this GTx, Inc. 2019 Incentive Award Plan.
11.31 “Prior Plan” means the GTx, Inc. 2013 Equity Incentive Plan, as amended to date.
F-16
11.32 “Prior Plan Award” means an award outstanding under the Prior Plan as of the Plan’s effective date under Section 10.3.
11.33 “Restricted Stock” means Shares awarded to a Participant under Article VI subject to certain vesting conditions and other restrictions.
11.34 “Restricted Stock Unit” means an unfunded, unsecured right to receive, on the applicable settlement date, one Share or an amount in cash or other consideration determined by the Administrator to be of equal value as of such settlement date, subject to certain vesting conditions and other restrictions.
11.35 “Rule16b-3” means Rule16b-3 promulgated under the Exchange Act.
11.36 “Section 409A” means Section 409A of the Code and all regulations, guidance, compliance programs and other interpretative authority thereunder.
11.37 “Securities Act” means the Securities Act of 1933, as amended.
11.38 “Service Provider” means an Employee, Consultant or Director.
11.39 “Shares” means shares of Common Stock.
11.40 “Stock Appreciation Right” means a stock appreciation right granted under Article V.
11.41 “Subsidiary” means any entity (other than the Company), whether domestic or foreign, in an unbroken chain of entities beginning with the Company if each of the entities other than the last entity in the unbroken chain beneficially owns, at the time of the determination, securities or interests representing at least 50% of the total combined voting power of all classes of securities or interests in one of the other entities in such chain.
11.42 “Substitute Awards” shall mean Awards granted or Shares issued by the Company in assumption of, or in substitution or exchange for, awards previously granted, or the right or obligation to make future awards, in each case by a company acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines.
11.43 “Termination of Service” means the date the Participant ceases to be a Service Provider.
* * * * *
F-17