UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2010
OR
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________ to ________.
Commission File Number: 000-50484
Marshall Edwards, Inc.
(Exact name of registrant as specified in its charter)
| DELAWARE | 51-0407811 |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) |
| Incorporation or organization) | |
11975 El Camino Real, Suite 101 San Diego, CA 92130
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code:
858-792-6300
| Securities registered pursuant to Section 12(b) of the Act: |
| | |
| Name of Each Exchange on which Registered |
| Common Stock, $0.00000002 par value | NASDAQ Global Market |
| |
| Securities registered pursuant to Section 12(g) of the Act: |
| None |
| (Title of Class) |
Indicate by a check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.Yes o No x
Indicate by a check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (Section 229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer o | Accelerated filer o |
Non-accelerated filer x (Do not check if a smaller reporting company) | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).Yes o No x
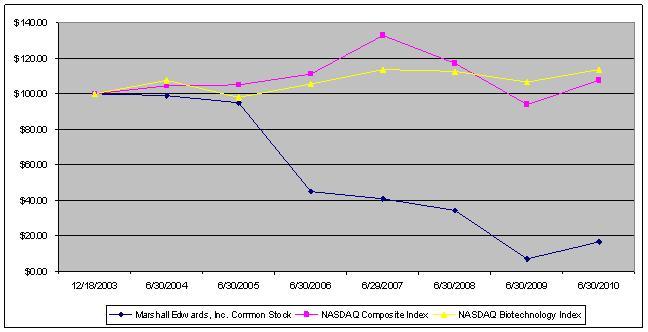
The aggregate market value of the voting common equity held by non-affiliates of the registrant was approximately $14.7 million based on the closing price of the registrant’s Common Stock as reported on the NASDAQ Global Market on December 31, 2009.
As of August 20, 2010, the number of shares outstanding of the issuer’s common stock, $0.00000002 par value, was 7,346,324.
Documents Incorporated by Reference
Portions of this registrant’s definitive proxy statement for its 2010 annual meeting to be filed with the U.S. Securities and Exchange Commission no later than 120 days after the end of the fiscal year ended June 30, 2010 are incorporated by reference in Part III of this Annual Report on Form 10-K.
MARSHALL EDWARDS, INC.
TABLE OF CONTENTS
| PART I | Page |
| Item 1:Business | 6 |
| Item 1A:Risk Factors | 24 |
| Item 1B:Unresolved Staff Comments | 35 |
| Item 2:Properties | 35 |
| Item 3:Legal Proceedings | 35 |
| | |
| PART II | |
| Item 5:Market for the Registrants Common Equity, Related Stockholder Matters and Issuer Purchases of Securities | 36 |
| Item 6:Selected Financial Data | 39 |
| Item 7:Management’s Discussion and Analysis of Financial Condition and Results of Operations. | 40 |
| Item 7a:Quantitative and Qualitative Disclosures about Market Risk | 50 |
| Item 8:Financial Statements and Supplementary Data | 51 |
| Item 9:Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 73 |
| Item 9A:Controls and Procedures | 73 |
| Item 9B:Other Information | 74 |
| | |
| PART III | |
| Item 10:Directors and Executive Officers of the Registrant | 75 |
| Item 11:Executive Compensation | 75 |
| Item 12:Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 75 |
| Item 13:Certain Relationships and Related Transactions | 75 |
| Item 14:Principal Accountant Fees and Services | 75 |
| | |
| PART IV | |
| Item 15:Exhibits and Financial Statement Schedules | 76 |
Cautionary Statement about Forward-Looking Statements
This Annual Report on Form 10-K includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements other than statements of historical facts contained in this Annual Report, including statements regarding the future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” and similar expressions, as they relate to us, are intended to iden tify forward-looking statements. We have based these forward-looking statements largely on current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, without limitation, those described in “Risk Factors” and elsewhere in this Form 10-K, including, among other things:
· our inability to obtain required additional financing or financing available to us on acceptable terms, |
· our inability to maintain or enter into, and our dependence upon, collaboration or contractual arrangements necessary for the clinical development of phenoxodiol, triphendiol, NV-143 and NV-128; |
· our failure to successfully commercialize our product candidates; |
· costs and delays in the clinical development program and/or receipt of U.S. Food and Drug Administration (the “FDA”) or other required governmental approvals, or the failure to obtain such approvals, for our product candidates; |
· uncertainties in clinical trial results; |
· our inability to maintain or enter into, and the risks resulting from our dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products; |
· our inability to control the costs of manufacturing our products; |
· competition and competitive factors; |
· our inability to protect our patents or proprietary rights and obtain necessary rights to third party patents and intellectual property to operate our business; |
· our inability to operate our business without infringing the patents and proprietary rights of others; |
· costs stemming from our defense against third party intellectual property infringement claims; |
· general economic conditions; |
· the failure of any products to gain market acceptance; |
|
· technological changes; |
· government regulation generally and the receipt of the regulatory approvals; |
· changes in industry practice; and |
· one-time events. |
These risks are not exhaustive. Other sections of this Annual Report on Form 10-K include additional factors which could adversely impact our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for us to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
You should not rely upon forward looking statements as predictions of future events. We cannot assure you that the events and circumstances reflected in the forward looking statements will be achieved or occur. Although we believe that the expectations reflected in the forward looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements.
PART I
Item 1. Business
Overview of Our Business
Marshall Edwards, Inc. (“MEI”) including its wholly-owned subsidiary Marshall Edwards Pty Ltd (“MEPL”) (together, the “Group” or the “Company”) is a development stage company incorporated in December 2000 as a wholly-owned subsidiary of Novogen Limited. Our shares of common stock are listed on the NASDAQ Global Market under the symbol “MSHL”. As of the date of this Annual Report on Form 10-K Novogen owns approximately 71.3% of the outstanding shares of our common stock.
Our business purpose is the development and commercialization of drugs for the treatment of cancer. We are presently engaged in the clinical development and commercialization of our drug candidates triphendiol, NV-143 and NV-128 which we have licensed from a subsidiary of Novogen Limited (Novogen Limited and/or its subsidiaries are referred to herein as “Novogen”).
We believe that our existing cash balances of approximately $9 million will be sufficient to satisfy our current operating plan until late 2011. Changes in our research and development plans or other changes affecting our operating expenses may affect actual future use of existing cash resources. In any event, however, we will need additional financing to fund our operations in the future including the continued development of triphendiol, NV-143 and NV-128. We intend to pursue capital raising transactions to further develop our drug candidates.
Clinical Product Developments
NV-128
In August 2009, we entered into a third license agreement with Novogen for the oncology compound NV-128. NV-128 is an investigational cancer compound which has been shown in pre-clinical laboratory studies to promote cancer cell death by targeting the specific protein regulatory pathway (i.e., AKT-mTOR pathway) in ovarian cancer cells that have become resistant to many drugs used to kill cancer cells. Structurally, NV-128 is an analog of phenoxodiol and triphendiol, but in contrast to phenoxodiol, NV-128 uses different molecular mechanisms to promote the death of cancer cells.
The License Agreement for NV-128 is an agreement under which Novogen grants to MEPL a worldwide non-transferable license under its patents and patent applications and in its know-how to conduct clinical trials and commercialize and distribute NV-128.
In September 2009, we released data demonstrating that the efficacy of NV-128 in animal xenograft models is achieved without apparent toxicity. NV-128 is a novel flavonoid small molecule inhibitor, capable of inhibiting both mTORC1 and mTORC2 protein regulatory pathways which are central to the aberrant proliferative capacity of both mature cancer cells and cancer stem cells. The laboratory data demonstrated that NV-128 has greater safety than some other mTOR inhibitors in mice bearing human ovarian cancer xenografts. Additional data released reported that NV-128 was judged to be without cardiac toxicity in laboratory studies.
Phenoxodiol
OVATURE Phase III Clinical Trial
The OVATURE Phase III clinical trial was a major multi-center international Phase III clinical trial of orally-administered phenoxodiol in combination with carboplatin in women with advanced ovarian cancer resistant or refractory to platinum-based drugs. This trial was designed to determine the safety and effectiveness of phenoxodiol when used in combination with carboplatin. Originally, the OVATURE Phase III clinical trial was approved by the FDA under a Special Protocol Assessment (“SPA”) program indicating that the study design, clinical endpoints and statistical analysis are acceptable to the FDA. The protocol provided for an interim analysis of the data, which, if statistically significant, could be used to support a request for accelerated marketing approval. Under the SPA, an analysis of the interim results was possi ble after the targeted patient recruitment was completed and 95 patients had disease progression.
In April 2009, we announced our decision to terminate enrollment into the Phase III OVATURE clinical trial and our intention to undertake an unblinded analysis of the available data from the trial. The decision to terminate new enrollment into the Phase III OVATURE clinical trial and assess the available patient data was made in part, because we believed that the global financial downturn made it unlikely at such time that we would be able to raise the necessary capital through debt or equity issuances in the near term to fund the trial to completion as originally planned. Additionally, changes in the standard of care over the period that the OVATURE Phase III clinical trial was in operation resulted in fewer women meeting the inclusion criteria of the OVATURE protocol, which slowed patient recruitment rates.
On June 1, 2010, we announced that a final analysis of our Phase III OVATURE trial of orally administered phenoxodiol in women with recurrent ovarian cancer determined that the trial did not show a statistically significant improvement in its primary (progression-free survival) or secondary (overall survival) endpoints.
The termination of patient enrollment into the OVATURE study and unblinded analysis of the available data from the trial have been discussed with the FDA.
Prostate Cancer
MEI conducted a Phase II prostate cancer clinical trial using phenoxodiol as first line treatment in men with early stage disease (16 patients with androgen dependent disease but rising Prostate Specific Antigen (“PSA”) compared to patients with late stage hormone refractory disease (12 patients with chemotherapy naïve androgen independent disease) at Yale Cancer Center and the West Haven Veterans Administration Hospital Connecticut in the US. Both of these patient groups represent areas of unmet medical need in this common cancer. The results of this study, which were presented by Dr. Kevin Kelly of Yale University at the American Society of Clinical Oncology meetings in June 2010, indicated that approximately one-third of the patients experienced disease stabilization as measured by PSA levels. In this small study it appeared that during treatment, interferon-gamma (IFN-γ) increased from baseline levels in patients with PSA partial response or stable disease, while monocyte chemotactic protein-1 (MCP-1) levels increased from baseline levels in patients with PSA progressive disease.
Triphendiol
Triphendiol is a synthetic investigational anti-cancer compound based on an isoflavan ring structure. Similar to phenoxodiol, triphendiol is an inhibitor of signal transduction in cells. Preliminary laboratory screening studies have identified triphendiol as a candidate for product development showing a favorable laboratory toxicity profile against normal cells and broad activity against cancer cells. In March 2008, we announced that laboratory data to be presented at the annual meeting of the American Association for Cancer Research suggested that triphendiol may aid in the treatment of pancreatic cancer. These data indicated that in laboratory testing in vitro and in animals bearing human pancreatic and bile duct tumors, the activity of triphendiol against these cancers was demonstrated. Triphendiol has completed two Phase I human tr ials in Australia which have demonstrated an acceptable safety profile and acceptable pharmacokinetic profile, i.e. the characteristics of a drug that determine its absorption, distribution and elimination in the body, when administered orally.
Triphendiol has been granted Orphan Drug status by the FDA for the treatment of pancreatic cancer and for the treatment of cholangiocarcinoma, or bile duct cancer, as well as for the treatment of Stage IIB through Stage IV malignant melanoma.
An Orphan Drug refers to a product that is intended for use in a disease or condition that affects fewer than 200,000 individuals in the U.S. A grant of Orphan Drug status provides seven years of market exclusivity for the orphan indication after approval by the FDA, as well as study design assistance and eligibility for grant funding from the FDA during its development. Triphendiol is in the early stages of clinical development and significant clinical testing will be required to prove safety and efficacy before marketing applications may be filed with the FDA.
In January 2009, we announced that triphendiol had been granted an Investigational New Drug (IND) status by the FDA to undertake clinical studies with orally administered triphendiol as a chemotherapy sensitizing agent in combination with gemcitabine in patients with unresectable locally advanced or metastatic pancreatic and bile duct cancers. Based on the results of the OVATRUE study with orally administered phenoxodiol, we are currently developing plans to amend the triphendiol IND to administer triphendiol intravenously.
Corporate Developments
On December 1, 2009, Novogen advised that its Chief Executive Officer and Managing Director Mr. Christopher Naughton ceased his employment, correspondingly Mr. Naughton's position as Chief Executive Officer of Marshall Edwards also ceased at this time. On February 5, 2010, Mr. Naughton resigned as a director of the Company. Novogen’s Chief Financial Officer Mr. David Seaton was appointed acting Chief Executive Officer of the Group and he acted in that capacity until our new Chief Executive Officer, Dr. Daniel P. Gold was appointed President and Chief Executive Officer of Marshall Edwards on April 23, 2010. On April 30, 2010 Dr. Gold was appointed to serve as a member on the Board of Directors of the Company.
On June 17, 2010 we announced the appointment of Thomas Zech as Chief Financial Officer. This appointment is part of the strategic decision to relocate our office and management of our company to the U.S. In addition to the appointment of our new Chief Executive Officer and Chief Financial Officer we have entered into a lease for a new office located in San Diego and have employed additional administrative staff.
In September 2009, we received a letter from The Nasdaq Stock Market (“Nasdaq”) notifying us that for the previous 30 consecutive business days the bid price of our common stock closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq Global Market under Nasdaq Rule 5450(a)(1). According to Nasdaq's letter, we would be afforded a grace period of 180 calendar days, or until March 15, 2010, to regain compliance in accordance with Nasdaq Rule 5810(c)(3)(A). In order to regain compliance, shares of our common stock must maintain a minimum bid closing price of at least $1.00 per share for a minimum of ten consecutive business days during the grace period. On March 16, 2010, we received notice from The Nasdaq Stock Market that we had not regained compliance and that we would be suspended from The Nasdaq Global Market on March 25, 2010, unless we requested a hearing. On March 23, 2010 we requested a hearing before the Nasdaq Hearings Panel. On March 29, 2010, our stockholders approved an amendment to the Company's Restated Certificate of Incorporation to effect a reverse stock split of the Company's common stock at a 1-for-10 reverse split ratio. The reverse stock split of our outstanding common stock was effected on March 31, 2010 on a 1-for-10 split adjusted basis. Following the reverse stock split, the closing bid price of our common stock closed above the $1.00 minimum requirement for ten consecutive trading days. We have now received notification from The Nasdaq Stock Market that we have regained compliance with the $1.00 minimum closing bid price in accordance with the Nasdaq Listing Rule 5450(a)(1). The Nasdaq Hearings Listing Qualifications Panel determined to continue the listing of our securities on the Nasdaq Stock Market and, therefore, the hearing before the Hearings Listing Qualificatio ns Panel was canceled. For the purpose of this report we have adjusted all share data presented retrospectively to incorporate the 1-for-10 reverse stock split.
On May 18, 2010 we received a notice from Nasdaq indicating that the Company failed to comply with the minimum stockholders’ equity requirement set forth in Nasdaq Listing Rule 5450(b)(1)(A) for continued listing of its common stock on the Nasdaq Global Market because our stockholders’ equity as of March 31, 2010 as set forth in our quarterly report on Form 10-Q for the period ended March 31, 2010 of $9.16 million was below the $10 million minimum stockholders’ equity requirement. The notice also stated we would be provided 45 calendar days, or until July 2, 2010, to submit a plan to regain compliance.
We responded to Nasdaq on July 2, 2010. The response included our plans to satisfy the listing requirements with respect to the maintaining a minimum $10 million Shareholders’ equity value. We stated our intention to pursue a capital raising transaction within the time provided by Nasdaq rules if market conditions permit, to further fund development of our product candidates 1) triphendiol or its primary active metabolite NV-143, a potentially more potent, second generation analog of phenoxodiol; and 2) NV-128. In the alternative, the Company intends to apply to transfer the listing of its common stock from the Nasdaq Global Market to the Nasdaq Capital Market. The Company believes it currently would be in compliance with the minimum stockholders’ equity requirement and all other criteria that would be applicable for listin g on the Nasdaq Capital Market.
On August 5, 2010, we received a letter from Nasdaq indicating that, based on our plan, Nasdaq has determined to grant us an extension, through November 15, 2010, to regain compliance with the Rule by establishing stockholders’ equity of at least $10,000,000.
On July 14, 2010, we received notice from Nasdaq stating that for the last 30 consecutive business days, the Market Value of Publicly Held Shares closed below the minimum $5 million required for continued listing on the Nasdaq Global Market under Nasdaq Rule 5450(b)(1)(C). Market Value of Publicly Held Shares is calculated by multiplying the publicly held shares, which is total shares outstanding less any shares held by officers, directors, or beneficial owners of 10% or more, by the consolidated closing bid price. Novogen Limited currently owns 71.3% of the outstanding common stock of the Company. Therefore, the value of Novogen Limited’s shares is excluded from the Market Value of Publicly Held Shares of the Company. According to Nasdaq's letter, we would be afforded a grace period of 180 calendar days, or until January 10, 2011, to regain compliance in accordance with Nasdaq Rule 5810(c)(3)(A). We intend to actively monitor the Market Value of Publicly Held Shares between now and January 10, 2011.
On August 10, 2010 we announced the appointment of Christine A. White, M.D. to our board of directors. Dr. White replaces Professor Paul J. Nestel, who has served as a director since April 2001.
Scientific Overview
Phenoxodiol, triphendiol, NV-143 and NV-128 belong to a class of drugs that we refer to as Multiple Signal Transduction Regulators (“MSTRs”).
Signal transduction refers to the means by which cells respond to chemical signals that come from within the cell itself, from neighboring cells, and from elsewhere in the body. These signals regulate such vital functions as the growth and survival of the cell. We believe that malfunctions in key components of the signal transduction process (whereby a series of chemical signals within a cell leads to the expression of a particular function) are fundamental to neoplastic diseases such as cancer, where cells respond abnormally to normal levels of signals, typically by over-responding to them with increased cell growth and prolonged survival.
We believe that phenoxodiol, triphendiol, NV-143 and NV-128 are able to exert a multiplicity of effects, including both ‘pro-survival’ and ‘pro-death’ signaling systems, because their primary targets in the tumor cell are proteins whose functions are so fundamental to the tumor cell’s metabolism that to shut them down produces a broad range of adverse consequences.
Phenoxodiol
The potential explanation for this effect of MSTRs on the fundamental biochemistry of tumor cells was provided by a discovery by a research team at Purdue University in Indiana. This team has a long-standing research interest in a family of proteins at the cell surface that are involved in electron transport across the cell membrane enabling hydrogen ion (proton) export at a controlled rate. This function is so fundamental to normal cell function and viability, that any loss of function of this proton pump will disrupt a wide range of biochemical processes. One of the key components of this proton pump mechanism is a family of cell surface proteins known as NADH oxidases. These proteins are situated on the outside of the cell membrane of all living matter and regulate the flow of waste hydrogen across the cell membrane. The laboratory studies at Purdue University have shown that a variant form of the surface oxidase which promotes more rapid hydrogen export, is preferentially expressed on cancer cells, although similar oxidase activity has been identified on small numbers of non-cancer cells undergoing abnormally rapid cell division. Phenoxodiol is able to bind to and inhibit the activity of these oxidase variants, with the resulting inhibition of hydrogen ion removal (H+ efflux) from these cells. This leads to an extensive disruption to signaling pathways and to eventual inhibition of cell proliferation and activation of apoptosis, the process of programmed cell death by which a cell dies naturally. Phenoxodiol appears to have little or no effect on the form of oxidase present on normal healthy cells, providing an explanation for how phenoxodiol selectively targets cancer cells. Independent research at the Malaghan Institute of Medical Research at Victoria University, Wellington, New Zealand, has confirmed that phenoxodiol inhibits plasm a membrane electron transport in cancer cells, as well as in some other abnormally dividing cells, but not in normal cells.
Other laboratory studies at The Hanson Institute Centre for Cancer Research at Royal Adelaide Hospital in Australia have demonstrated potent anti-tumor and anti-angiogenic (i.e., prevention of blood vessel formation) properties of phenoxodiol. These properties of phenoxodiol are associated with down regulation of a key signal transduction molecule, sphingosine kinase. Sphingosine kinase is a terminal component of the plasma membrane sphingomyelin pathway leading to the formation of sphingosine-1-phosphate a bioactive lipid and a key pro-survival secondary messenger acting via the signal transduction protein kinase, Akt. Two important biological outcomes resulting from the down regulation of sphingosine kinase are (i) cytostasis, (i.e. the prevention of the growth and multiplication of cells) through p53-independent induction of the cel l cycle regulatory protein, p21WAF1/CIP1, and (ii) apoptosis (i.e., programmed cell death), through inhibition of phosphorylation (i.e., addition of a phosphate group) of the anti-apoptotic factors, XIAP (inhibitor of apoptosis protein) and FLIPshort (caspase-8 inhibitory protein). These processes facilitate activation of executioner caspases (proteins that cause the cell to undergo programmed cell death) and restore the activity of the Fas-ligand (fasL) family of death receptors. Researchers at Purdue University have shown this effect is a consequence of the interaction between phenoxodiol and the surface oxidase on cancer cells.
These findings are relevant because of results from laboratory studies at Yale University that have revealed that the killing effect of phenoxodiol on cancer cells occurs through the loss of the ability of the tumor cell to manufacture anti-apoptotic proteins such as XIAP and c-FLIP. Collectively, these third party studies provide a rational mechanism of action of phenoxodiol starting with the inhibition of surface oxidase, leading in turn to the loss of intracellular sphingosine-1-phosphate (S-1-P), and eventually to the loss of anti-apoptotic proteins.
Recent laboratory studies conducted by Novogen and Yale University have confirmed that this chain of biochemical events following exposure of tumor cells to phenoxodiol also explains how phenoxodiol is able to sensitize tumor cells to standard anti-cancer drugs such as platinums, gemcitabine and taxanes, on the basis that FLIPshort protein is responsible for inhibiting the sensitivity of the Fas-ligand protein (death receptor) to the toxic signaling mediated via these drugs.
Phenoxodiol appears to restore sensitivity to these drugs in cells such as ovarian cancer cells that have acquired resistance to these drugs. In addition, pretreatment of tumor cells with phenoxodiol considerably increases the sensitivity of non-resistant tumor cells to the cytotoxic (i.e., toxic to cells, preventing their production or growth or causing cell death) effects of standard chemotherapy drugs. These effects are achieved without increasing the cellular toxicity of the standard chemotherapy drugs to non tumor-cells.
Triphendiol, NV-143 and NV-128 are analogues of phenoxodiol, but exhibit some differences from phenoxodiol. In parallel with phenoxodiol, these drug candidates display pre-clinical anti-cancer activity across a broad range of tumor types, high selectivity for cancer cells, and the ability to chemosensitize tumor cells to the cytotoxic effects of most standard chemotoxic drugs. However, these drugs differ from phenoxodiol in inducing cell death by both caspase dependent and caspase independent mechanisms and by showing a greater ability to induce apoptosis in pancreatic cancer, bile duct cancer, and melanoma cells; triphendiol also shows an ability to increase the sensitivity of cancer cells to radiotherapy (radiosensitizers).
Triphendiol and NV-143
Triphendiol is a derivative of phenoxodiol and was selected for further development based on superior pre-clinical anti-cancer activity against a range of cancers, such as pancreas and bile duct cancers and melanoma. In non-clinical studies, triphendiol invoked cell cycle arrest leading to programmed cell
death in cell lines representative of late stage pancreatic and bile duct carcinomas. Apoptosis induction was independent of p53 status and proceeded via the mitochondrial cell death pathway. We have also demonstrated that triphendiol is able to sensitize cell lines representative of both pancreatic cancer and cholangiocarcinoma (bile duct cancer) to the standard of care drug, gemcitabine. Proof of concept studies in animal models of pancreatic cancer and cholangiocarcinoma demonstrated that orally delivered triphendiol is effective at inhibiting tumor proliferation. In further Good Laboratory Practice (GLP) compliant toxicology studies, triphendiol was shown to be non-clastogenic (i.e., not capable of causing damage to chromosomes) and non-mutagenic (i.e., not causing genetic damage), and is well tolerated in rodent and non-roden t chronic repeat dose studies when delivered orally. These data have indicated that clinical development of triphendiol as a biliary cancer therapeutic is warranted. Two Phase Ia clinical studies have been completed in Australia investigating triphendiol pharmacokinetics and safety when delivered either orally or as an intravenous infusion. No medication related adverse events were reported. Triphendiol is a synthetic molecule. A scalable synthetic manufacturing method has been developed as has a validated analytical method for the quantitation of the active pharmaceutical ingredient (API). The FDA has granted an Investigational New Drug status to triphendiol to enable a Phase Ib efficacy and safety study to be conducted in the U.S.
NV-143 is the primary active metabolite that is produced when triphendiol is introduced into animals and humans. NV-143 is highly potent, pan acting investigational anti-cancer drug that demonstrates superior anti-tumor activity against a broad range of tumor cell lines compared to phenoxodiol and triphendiol. In pre-clinical studies it is found to be active against all melanoma cell lines tested to date and is able to sensitize melanoma cell lines to the standard of care drug, dacarbazine, and members of the platinum drug family. Proof of concept studies in animal models of melanoma have demonstrated that orally delivered NV-143 retards tumor proliferation. The NV-143 mechanism of action in melanoma has not been fully elucidated but is believed to be similar to that of triphendiol. NV-143 is non-clastogenic and non-mutagenic in labora tory studies.
NV-128
NV-128 is an analogue of phenoxodiol and triphendiol but appears to interact with a target protein in the tumor cell that is distinct from both. The proposed target for NV-128 is found in the tumor cell mitochondria, the specialized area in the cell that produce energy in the form of adenosine triphosphate (“ATP”). When NV-128 interacts with its protein target a rapid reduction in ATP occurs leading to a cascade of biochemical events within the cell leading to cell death. One outcome that is believed to be critical for cell death induction induced by NV-128 is the disruption of both the mTORC1 and mTORC2 cellular pathways. NV-128’s effect on the mTOR protein reduces the potential for the cancer cell to develop resistance to chemotherapeutic drugs. NV-128, has demonstrated activity as a single agent and as a chemosensitizing agent against cancer cell lines representative of non-small cell lung carcinoma (NSCLC) and ovarian cancer. Proof of concept xenograft studies in animals have confirmed that NV-128 retards NSCLC and ovarian tumor proliferation when administered via oral, intravenous and intraperitoneal routes. Laboratory studies are in progress in pre-clinical in vitro experiments to examine activity against late stage colorectal, breast, and gastric cancers and hepatocellular carcinoma, both as a single agent and in combination with current standard of care drugs. Pharmacokinetic studies of NV-128 delivered orally and intraperitoneally have been conducted in rodents. These studies have demonstrated that NV-128 is bioavailable, producing therapeutically significant concentrations in blood plasma, and is completely excreted 24 hours post administration.
NV-128 disrupts internal cell signaling, and also induces changes in mitochondrial membranes. The mitochondrial membrane changes have been associated with early stages of programmed cell death, or apoptosis, and are mediated via a novel mTOR pathway. In mature cancer cells as well as in cancer stem cells, the mTOR protein is involved in enhancing tumor growth and may be associated with resistance to chemotherapeutic drugs. Inhibition of the mTOR pathway appears to shut down many of the cellular survival pathways, including proteins that protect the mitochondria of cancer cells. NV-128 has been demonstrated to block both mTORC1 and mTORC2 pathways of mTOR activation. Data demonstrate that through minor modification of the parent isoflavene compounds, novel analogues can be generated, which promote cell death via alternative m echanisms to those described for phenoxodiol and triphendiol, opening up new opportunities for treatment of an even broader range of cancers.
Competition
The development of our drug candidates is highly competitive. A number of other companies have products or drug candidates in various stages of pre-clinical or clinical development that are intended for the same therapeutic indications for which our drug candidates are being developed. Some of these potential competing drugs are further advanced in development than our drug candidates and may be commercialized sooner. Even if we are successful in developing effective drugs, our drug candidates may not compete successfully with products produced by our competitors.
Our competitors include pharmaceutical companies and biotechnology companies, as well as universities and public and private research institutions. In addition, companies active in different but related fields represent substantial competition for us. Many of our competitors developing oncology drugs have significantly greater capital resources, larger research and development staffs and facilities, and greater experience in drug development, regulation, manufacturing, and marketing than we do. They compete with us in recruiting eligible patients to participate in clinical studies and in attracting partners for joint ventures. They also license technologies that are competitive with our technologies. As a result, our competitors may be able to more easily develop technologies and products that would render our technologies or our d rug candidates obsolete or non-competitive.
Intellectual Property
Novogen has been granted patents and has additional patent applications pending in a number of countries which cover a family of chemically related compounds with potentially broad ranging and complementary anticancer effects. Novogen has granted us an exclusive license, with respect to its patent rights and intellectual property know-how to develop, market and distribute the isoflavonoid compounds phenoxodiol, triphendiol, NV-143 and NV-128 as anti-cancer agents, except in topical form.
Phenoxodiol
We have licensed from Novogen the rights to the Novogen patents and applications as they relate to phenoxodiol as an anti-cancer agent. Excluded from these rights is phenoxodiol in a topical formulation. The patent rights we have licensed from Novogen can be largely classified into two broad groups: patent rights relating to phenoxodiol used as an anti-cancer agent, which we refer to as “therapeutic patent rights,” and patent rights relating to the manufacture of phenoxodiol for anti-cancer purposes, which we refer to as “manufacturing patent rights.” The therapeutic patent rights with respect to phenoxodiol comprise the following patent families:
| · | phenoxodiol in the treatment of cancer (thirteen pending patent applications, seventeen issued patents, and two allowed patent applications which are anticipated to proceed to grant in the coming months); |
| · | the use of phenoxodiol in compositions and methods for protecting skin from ultraviolet induced immunosuppression and skin damage (three pending patent applications, eight issued patents, and two allowed patent applications which are anticipated to proceed to grant in the coming months); |
| · | the use of phenoxodiol, in combination with chemotherapeutic agents, for increasing cancer cell sensitivity to treatment and in cancer therapy (eleven pending patent applications, four issued patents, and one allowed patent application which is anticipated to proceed to grant in the coming months); |
| · | phosphate ester prodrugs of phenoxodiol (eight pending patent applications); and |
| · | use of phenoxodiol in the modulation of the immune system (provisional patent application filed) (see also triphendiol and NV-128 below). |
The manufacturing patent rights, relating to the production of isoflavan derivatives, including phenoxodiol, comprises a patent family in which nine patent applications are pending and seven patents have been issued.
Triphendiol and NV-143
These compounds are isoflavan derivatives of phenoxodiol. The licensed patent rights relate to the compounds and to uses of these compounds as anti-cancer agents and sensitizers of cancer cells and tumors to chemotherapy and radiotherapy, except in topical form. The licensed patent rights fall into several families of patent applications:
| · | triphendiol and NV-143 and uses of these compounds as anti-cancer agents (thirteen pending patent applications); and |
| · | uses of triphendiol and NV-143 as chemo-sensitizers and radiosensitizers of tumors and cancer cells (ten pending patent applications and one issued patent) (see also NV-128 below); |
| · | the use of triphendiol for inducing programmed cell death (three pending patent applications) (see also NV-128 below); and |
| · | the use of triphendiol in the modulation of the immune system (provisional patent application filed) (see also phenoxodiol above). |
NV-128
NV-128 is a further novel isoflavan derivative of phenoxodiol. The licensed patent rights in respect of NV-128 relate to the compound and to uses of the compound as an anti-cancer agent, except in topical form. The licensed patent rights fall into several patent families as follows:
| · | NV-128 and use of this compound as an anti-cancer agent (thirteen pending patent applications); |
| · | the use of NV-128 as a chemo-sensitizer and radiosensitizer of tumors and cancer cells (ten pending patent applications and one issued patent) (see also triphendiol and NV-143 above); |
| · | two patent families (one international PTC application filed, and three pending patent applications, respectively) relating to the use of NV-128 for inducing programmed cell death; and |
| · | the use of NV-128 in the modulation of the immune system (provisional patent application filed) (see also phenoxodiol above). |
As patent applications in the U.S. are maintained in secrecy until published by the U.S. Patent Trade Office at 18 months from filing for all cases filed after November 29, 2000, or at issue, for cases filed prior to November 29, 2000 we cannot be certain that Novogen was the first to make the inventions covered by the Novogen patents and applications referred to above. Additionally, publication of discoveries in the scientific or patent literature often lags behind the actual discoveries. Moreover, pursuant to the terms of the Uruguay Round Agreements Act, patents filed on or after June 8, 1995 have a term of twenty years from the date of such filing except for provisional applications, irrespective of the period of time it may take for such patent to ultimately issue. This may shorten the period of patent protection afforded to t herapeutic uses of phenoxodiol, triphendiol, NV-143 or NV-128, as patent applications in the biopharmaceutical sector often take considerable time to issue. However, in some countries the patent term may be extended.
In order to protect the confidentiality of our technology, including trade secrets and know-how and other proprietary technical and business information, we require all of our consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the use or disclosure of information that is deemed confidential. The agreements also oblige our consultants, advisors and collaborators to assign to us developments, discoveries and inventions made by such persons in connection with their work with us relating to our products. We cannot be sure that confidentiality will be maintained or disclosure prevented by these agreements. We also cannot be sure that our proprietary information or intellectual property will be protected by these agreements or that others will not independently develop substantially equivalent proprietary information or intellectual property.
The pharmaceutical industry is highly competitive and patents may have been applied for by, and issued to, other parties relating to products competitive with phenoxodiol, triphendiol, NV-143 or NV-128. Use of these compounds and any other drug candidates may give rise to claims that they infringe the patents or proprietary rights of other parties, existing now and in the future. An adverse claim could subject us to significant liabilities to such other parties and/or require disputed rights to be licensed from such other parties. We cannot be sure that any license required under any such patents or proprietary rights would be made available on terms acceptable to us, if at all. If we do not obtain such licenses, we may encounter delays in product market introductions, or may find that the development, manufacture or sale of produc ts requiring such licenses may be precluded. We have not conducted any searches or made any independent investigations of the existence of any patents or proprietary rights of other parties.
Relationship with Novogen
Novogen is active in the discovery and development of new drugs based on the emerging field of cell signal transduction regulation. Signal transduction regulators offer the potential for effective, well-tolerated treatment of common diseases, including cancer. Novogen has developed a family of chemically related compounds with potentially broad ranging and complementary anti-cancer effects.
We have entered into certain key agreements with Novogen which are discussed below.
Phenoxodiol
Under the license agreement, Novogen granted us an exclusive world-wide, non-transferable license, under the Novogen patent rights, to conduct clinical trials and commercialize and distribute all forms of administering phenoxodiol except topical applications. The agreement covers uses of phenoxodiol in the field of prevention, treatment or cure of cancer in humans.
Triphendiol and NV-143
Under a second license agreement, Novogen granted us an exclusive world-wide, non-transferable license, under the Novogen patent rights, to conduct clinical trials and commercialize and distribute all forms of administering triphendiol and NV-143, except topical applications. The agreement covers uses of triphendiol and NV-143 in the field of prevention, treatment or cure of cancer in humans.
NV-128
Under a third license agreement, Novogen granted us an exclusive world-wide, non-transferable license, under the Novogen patent rights, to conduct clinical trials and commercialize and distribute all forms of administering NV-128, except topical applications. The agreement covers uses of NV-128 in the field of prevention, treatment or cure of cancer in humans.
License Option Deed
Under the License Option Deed, Novogen granted us an exclusive first right to accept and an exclusive last right to match any proposed dealing by Novogen with its intellectual property rights in other synthetic compounds developed by Novogen that have known or potential anti-cancer applications in all forms, other than topical applications.
Services
Pursuant to a services agreement, Novogen provides services reasonably required by us relating to the development and commercialization of phenoxodiol, triphendiol, NV-143, NV-128 or other option compounds in relation to which we have exercised our rights under the License Option Deed.
Manufacturing
Under the Manufacturing License and Supply Agreement, we have granted Novogen a sublicense to manufacture and supply phenoxodiol to us in its primary manufactured form for both the OVATURE clinical program and phenoxodiol’s ultimate commercial use. Novogen has taken the strategic decision not to manufacture large scale Active Pharmaceutical Ingredients (“API”) for cancer drugs, including phenoxodiol, as these can be more economically supplied by third parties with particular expertise in this area.
Research and Development
The objective of our research and development program is the generation of data sufficient to achieve regulatory approval of our licensed drug candidates in one or more dosage forms in major markets such as the U.S. and/or to allow us to enter into a commercial relationship with another party. The data are generated by our clinical trial programs.
The key aspects of this program are to provide more complete characterization of the following:
| · | the relevant molecular targets of action of our licensed drug candidates; |
| · | the relative therapeutic benefits and indications of our licensed drug candidates as a monotherapy or as part of combinational therapy with other chemotoxics; |
| · | the most appropriate cancer targets for phenoxodiol, triphendiol, NV-143 and NV-128; and |
| · | the relative therapeutic indications of different dosage forms of our licensed drug candidates. |
Research expenses were $4.031 million for the year ended June 30, 2010, $7.777 million for the year ended June 30, 2009 and $9.325 million for the year ended June 30, 2008.
Research and development costs incurred since inception through June 30, 2010 amount to $37,074,000.
Regulation
U.S. Regulatory Requirements
The FDA, and comparable regulatory agencies in other countries, regulate and impose substantial requirements upon the research, development, pre-clinical and clinical testing, labeling, manufacture, quality control, storage, approval, advertising, promotion, marketing, distribution and export of pharmaceutical products including biologics, as well as significant reporting and record-keeping obligations. State governments may also impose obligations in these areas.
In the U.S., pharmaceutical products are regulated by the FDA under the Federal Food, Drug, and Cosmetic Act or FDCA and other laws including in the case of biologics, the Public Health Service Act. We believe, but cannot be certain, that our products will be regulated as drugs by the FDA. The process required by the FDA before drugs may be marketed in the U.S. generally involves the following:
| · | pre-clinical laboratory evaluations, including formulation and stability testing, and animal tests performed under the FDA’s Good Laboratory Practices regulations to assess potential safety and effectiveness; |
| · | submission and approval of an Investigational New Drug Application, or IND, including results of pre-clinical tests, manufacturing information, and protocols for clinical tests, which must become effective before clinical trials may begin in the U.S.; |
| · | obtaining approval of Institutional Review Boards, or IRBs, to administer the products to human subjects in clinical trials; |
| · | adequate and well-controlled human clinical trials to establish the safety and efficacy of the product for the product’s intended use; |
| · | development of manufacturing processes which conform to FDA current Good Manufacturing Practices, or cGMPs, as confirmed by FDA inspection; |
| · | submission of pre-clinical and clinical studies results, and chemistry, manufacture and control information on the product to the FDA in a New Drug Approval Application, or NDA; and |
| · | FDA review and approval of an NDA, prior to any commercial sale or shipment of a product. |
The testing and approval process requires substantial time, effort, and financial resources, and we cannot be certain that any approval will be granted on a timely basis, if at all.
The results of the pre-clinical studies, together with initial specified manufacturing information, the proposed clinical trial protocol, and information about the participating investigators are submitted to the FDA as part of an IND, which must become effective before we may begin human clinical trials in the U.S. Additionally, an independent IRB must review and approve each study protocol and oversee conduct of the trial. An IND becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day period, raises concerns or questions about the conduct of the trials as outlined in the IND and imposes a clinical hold. If the FDA imposes a clinical hold, the IND sponsor must resolve the FDA’s concerns before clinical trials can begin. Pre-clinical te sts and studies can take several years to complete, and there is no guarantee that an IND we submit based on such tests and studies will become effective within any specific time period, if at all.
Human clinical trials are typically conducted in three sequential phases that may overlap.
• Phase I: The drug is initially introduced into healthy human subjects or patients and tested for safety and dosage tolerance. Absorption, metabolism, distribution, and excretion testing is generally performed at this stage.
• Phase II: The drug is studied in controlled, exploratory therapeutic trials in a limited number of subjects with the disease or medical condition for which the new drug is intended to be used in order to identify possible adverse effects and safety risks, to determine the preliminary or potential efficacy of the product for specific targeted diseases or medical conditions, and to determine dosage tolerance and the optimal effective dose.
• Phase III: When Phase II studies demonstrate that a specific dosage range of the drug is likely to be effective and the drug has an acceptable safety profile, controlled, large-scale therapeutic Phase III trials are undertaken at multiple study sites to demonstrate clinical efficacy and to further test for safety in an expanded patient population.
We cannot be certain that we will successfully complete Phase I, Phase II, or Phase III testing of our products within any specific time period, if at all. Furthermore, the FDA, the IRB or we may suspend or terminate clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
Results of pre-clinical studies and clinical trials, as well as detailed information about the manufacturing process, quality control methods, and product composition, among other things, are submitted to the FDA as part of an NDA seeking approval to market and commercially distribute the product on the basis of a determination that the product is safe and effective for its intended use. Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured and will not approve the product unless cGMP compliance is satisfactory. If applicable regulatory criteria are not satisfied, the FDA may deny the NDA or require additional testing or information. As a condition of approval, the FDA also may require post-marketing testing or surveillance to monitor the product’s safety or efficacy. Even after an NDA is approved, the FDA may impose additional obligations or restrictions (such as labeling changes), or even suspend or withdraw a product approval on the basis of data that arise after the product reaches the market, or if compliance with regulatory standards is not maintained. We cannot be certain that any NDA we submit will be approved by the FDA on a timely basis, if at all. Also, any such approval may limit the indicated uses for which the product may be marketed. Any refusal to approve, delay in approval, suspension or withdrawal of approval, or restrictions on indicated uses could have a material adverse impact on our business prospects.
Each NDA must be accompanied by a user fee, pursuant to the requirements of the Prescription Drug User Fee Act, or PDUFA, and its amendments. According to the FDA’s fee schedule, effective on October 1, 2009 for the fiscal year 2010, the user fee for an application requiring clinical data, such as an NDA, is $1,405,500. The FDA adjusts the PDUFA user fees on an annual basis. PDUFA also imposes an annual product fee for prescription drugs and biologics ($79,720), and an annual establishment fee ($457,200) on facilities used to manufacture prescription drugs and biologics. A written request can be submitted for a waiver for the application fee for the first human drug application that is filed by a small business, but there are no waivers for product or establishment fees. We are not at the stage of development with our pr oducts where we are subject to these fees, but they are significant expenditures that may be incurred in the future and must be paid at the time of application submissions to FDA.
Satisfaction of FDA requirements typically takes several years. The actual time required varies substantially, based upon the type, complexity, and novelty of the pharmaceutical product, among other things. Government regulation imposes costly and time-consuming requirements and restrictions throughout the product life cycle and may delay product marketing for a considerable period of time, limit product marketing, or prevent marketing altogether. Success in pre-clinical or early stage clinical trials does not ensure success in later stage clinical trials. Data obtained from pre-clinical and clinical activities are not always conclusive and may be susceptible to varying interpretations that could delay, limit, or prevent marketing approval. Even if a product receives marketing approval, the approval is limited to specific clinical indications. Further, even after marketing approval is obtained, the discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
After product approval, there are continuing significant regulatory requirements imposed by the FDA, including record-keeping requirements, obligations to report adverse side effects in patients using the products, and restrictions on advertising and promotional activities. Quality control and manufacturing procedures must continue to conform to cGMPs, and the FDA periodically inspects facilities to assess cGMP compliance. Additionally, post-approval changes in ingredient composition, manufacturing processes or facilities, product labeling, or other areas may require submission of an NDA Supplement to the FDA for review and approval. New indications will require additional clinical studies and submission of an NDA Supplement. Failure to comply with FDA regulatory requirements may result in an enforcement action by the FDA, includin g Warning Letters, product recalls, suspension or revocation of product approval, seizure of product to prevent distribution, impositions of injunctions prohibiting product manufacture or distribution, and civil and criminal penalties. Maintaining compliance is costly and time-consuming. We cannot be certain that we, or our present or future suppliers or third-party manufacturers, will be able to comply with all FDA regulatory requirements, and potential consequences of noncompliance could have a material adverse impact on our business prospects.
The FDA’s policies may change, and additional governmental regulations may be enacted that could delay, limit, or prevent regulatory approval of our products or affect our ability to manufacture, market, or distribute our products after approval. Moreover, increased attention to the containment of healthcare costs in the U.S. and in foreign markets could result in new government regulations that could have a material adverse effect on our business. Our failure to obtain coverage, an adequate level of reimbursement, or acceptable prices for our future products could diminish any revenues we may be able to generate.
Our ability to commercialize future products will depend in part on the extent to which coverage and reimbursement for the products will be available from government and health administration authorities, private health insurers, and other third-party payers. European Union member states and U.S. government and other third-party payers increasingly are attempting to contain healthcare costs by consideration of new laws and regulations limiting both coverage and the level of reimbursement for new drugs. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the U.S. or abroad.
Our activities also may be subject to state laws and regulations that affect our ability to develop and sell our products. We are also subject to numerous federal, state, and local laws relating to such matters as safe working conditions, clinical, laboratory, and manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future, and the failure to comply may have a material adverse impact on our business prospects.
The FDCA includes provisions designed to facilitate and expedite the development and review of drugs and biological products intended for treatment of serious or life-threatening conditions that demonstrate the potential to address unmet medical needs for such conditions. These provisions set forth a procedure for designation of a drug as a “fast track product.” The fast track designation applies to the combination of the product and specific indication for which it is being studied. A product designated as fast track is ordinarily eligible for additional programs for expediting development and review, but products that are not in fast track drug development programs may also be able to take advantage of these programs. These programs include priority review of NDAs and accelerated approval. Drug approval under the acce lerated approval regulations may be based on evidence of clinical effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. A post-marketing clinical study will be required to verify clinical benefit, and other restrictions to assure safe use may be imposed.
Under the Drug Price Competition and Patent Term Restoration Act of 1984, a sponsor may obtain marketing exclusivity for a period of time following FDA approval of certain drug applications, regardless of patent status, if the drug is a new chemical entity or if new clinical studies were required to support the marketing application for the drug. This marketing exclusivity prevents a third party from obtaining FDA approval for an identical or nearly identical drug under an Abbreviated New Drug Application or a “505(b)(2) New Drug Application.” The statute also allows a patent owner to obtain an extension of applicable patent terms for a period equal to one-half the period of time elapsed between the filing of an IND and the filing of the corresponding NDA plus the period of time between the filing of the NDA and FDA app roval, with a five year maximum patent extension. We cannot be certain that Novogen will be able to take advantage of either the patent term extension or marketing exclusivity provisions of these laws.
The Best Pharmaceuticals for Children Act, or BPCA, signed into law on January 4, 2002, was reauthorized and amended by the FDA Amendments Act of 2007 or FDAAA. The reauthorization of BPCA provides an additional six months of patent protection to NDA applicants that conduct acceptable pediatric studies of new and currently-marketed drug products for which pediatric information would be beneficial, as identified by FDA in a Pediatric Written Request. The Pediatric Research Equity Act, or PREA, signed into law on December 3, 2003, also was reauthorized and amended by FDAAA. The reauthorization of PREA requires that most applications for drugs and biologics include a pediatric assessment (unless waived or deferred) to ensure the drugs' and biologics' safety and effectiveness in children.
Such pediatric assessment must contain data, gathered using appropriate formulations for each age group for which the assessment is required, that are adequate to assess the safety and effectiveness of the drug or the biological product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the drug or the biological product is safe and effective. The pediatric assessments can only be deferred provided there is a timeline for the completion of such studies. The FDA may waive (partially or fully) the pediatric assessment requirement for several reasons, including if the applicant can demonstrate that reasonable attempts to produce a pediatric formulation necessary for that age group have failed.
Australian Regulatory Requirements
The Therapeutic Goods Act 1989, or 1989 Act, sets out the legal requirements for the import, export, manufacture and supply of pharmaceutical products in Australia. The 1989 Act requires that all pharmaceutical products to be imported into, supplied in, manufactured in or exported from Australia be included in the Australian Register of Therapeutic Goods, or ARTG, unless specifically exempted under the Act.
Medicines with a higher level of risk (prescription medicines, some non-prescription medicines) are evaluated for quality, safety and efficacy and are registered on the ARTG. Medicines with a lower risk (over the counter medicines including vitamins) are assessed only for quality and safety. Medicines included in the ARTG can be identified by the AUST R number (for registered medicines) or an AUST L number (listed medicines) that appears on the packaging of the medicine.
In order to ensure that a product can be included in the ARTG, a sponsoring company must make an application to the Therapeutic Goods Administration, or TGA. The application usually consists of a form accompanied by data (based on the European Union requirements) to support the quality, safety and efficacy of the drug for its intended use and payment of a fee. Application details are available on the TGA website http://www.tga.gov.au.
The TGA requires a 26B certificate from Applicants who are required to submit safety and efficacy data when making their application, and who, when making their application, rely on data previously submitted to the TGA by another person in relation to an approved product. This certificate states that the applicants will not enter the market with a product that would infringe a patent on the product; or, that they have notified the patent owner of their intention enter the market before the expiry of any applicable patent. All other applicants may provide notice that such a certificate is not required.
The first phase of evaluation, known as the Application Entry Process, is usually a short period during which an application is assessed on an administrative level to ensure that it complies with the basic guidelines. The TGA may request further details from the applicant, and may agree with sponsors that additional data (which while not actually required by the application, could enhance the assessment outcome) may be submitted later at an agreed time. The TGA must decide within at least 40 working days whether it will accept the application for evaluation.
Once an application is accepted for evaluation, aspects of the data provided are allocated to evaluators within the different relevant sections, who prepare clinical evaluation reports. Following evaluation, the chemistry and quality control aspects of a product may be referred to a Pharmaceutical Sub-Committee (PSC), which is a sub-committee of the TGA prescription medicine expert advisory committee, the Australian Drug and Evaluation Committee (ADEC) to review the relevant clinical evaluation reports.
The clinical evaluation reports (along with any resolutions of the ADEC sub-committee) are then sent to the sponsoring company who then has the opportunity to comment on the views expressed within the evaluation report, provide corrections and to submit supplementary data to address any issues raised in the evaluation reports.
Once the evaluations are complete, the TGA prepares a summary document on the key issues on which advice will be sought from the either the ADEC (for new medicines) or from the Peer Review Committee (PRC) for existing or generic products. This summary is sent to the sponsoring company which is able to submit a response to the ADEC or PRC dealing with issues raised in the summary and those not previously addressed in the evaluation report. The ADEC/PRC provide independent advice on the quality, risk-benefit, effectiveness and access of the drug and conduct medical and scientific evaluations of the application. The ADEC meets every 2 months to examine the applications referred by the TGA and its resolutions are provided to the sponsoring company after 5 working days after the ADEC meeting.
The TGA takes into account the advice of the ADEC or PRC in reaching a decision to approve or reject a product. Any approval for registration on the ARTG may have conditions associated with it.
From the time that the TGA accepts the initial application for evaluation, the TGA must complete the evaluation and make a decision on the registration of the product within at least 255 working days. If not completed within 255 working days, the TGA forfeits 25% of the evaluation fee otherwise payable by the sponsor, but any time spent waiting for a response from the sponsor is not included in the 255 working days. The TGA also has a system of priority evaluation for products that meet certain criteria, including where the product is a new chemical entity that it is not otherwise available on the market as an approved product, and is for the treatment of a serious, life-threatening illness for which other therapies are either ineffective or not available.
European Union Regulatory Requirements
Outside the U.S., our ability to market our products will also be contingent upon receiving marketing authorizations from the appropriate regulatory authorities and compliance with applicable post-approval regulatory requirements. Although the specific requirements and restrictions vary from country to country, as a general matter, foreign regulatory systems include risks similar to those associated with FDA regulation, described above. Under EU regulatory systems, marketing authorizations may be submitted either under a centralized or a national procedure. Under the centralized procedure, a single application to the European Medicines Agency (EMA) leads to an approval granted by the European Commission which permits the marketing of the product throughout the EU. The centralized procedure is mandatory for certain classes of medicinal products, but optional for others. For example, all medicinal products developed by certain biotechnological means, and those developed for cancer and other specified diseases and disorders, must be authorized via the centralized procedure. We assume that the centralized procedure will apply to our products that are developed by means of a biotechnology process. The national procedure is used for products that are not required to be authorized by the centralized procedure. Under the national procedure, an application for a marketing authorization is submitted to the competent authority of one member state of the EU. The holders of a national marketing authorization may submit further applications to the competent authorities of the remaining member states via either the decentralized or mutual recognition procedure. The decentralized procedure enables applicants to submit an identical application to the competent authorities of all member states where approval is sought at the same time as the first applicat ion, while under the mutual recognition procedure, products are authorized initially in one member state, and other member states where approval is sought are then requested to recognize the original authorization based upon an assessment report prepared by the original authorizing competent authority.
Both the decentralized and mutual recognition procedures should take no longer than 90 days, but if one member state makes an objection, which under the legislation can only be based on a possible risk to human health, the application will be automatically referred to the Committee for Medicinal Products for Human Use (CHMP) of the EMA. If a referral for arbitration is made, the procedure is suspended. However, member states that have already approved the application may, at the request of the applicant, authorize the product in question without waiting for the result of the arbitration. Such authorizations will be without prejudice to the outcome of the arbitration. For all other concerned member states, the opinion of the CHMP, which is binding, could support or reject the objection or alternatively could reach a compromise position acceptable to all EU countries concerned. The arbitration procedure may take an additional year before a final decision is reached and may require the delivery of additional data.
As with FDA approval, we may not be able to secure regulatory approvals in Europe in a timely manner, if at all. Additionally, as in the U.S., post-approval regulatory requirements, such as those regarding product manufacture, marketing, or distribution, would apply to any product that is approved in Europe, and failure to comply with such obligations could have a material adverse effect on our ability to successfully commercialize any product.
The conduct of clinical trials in the European Union is governed by the European Clinical Trials Directive (2001/20/EC), which was implemented in May 2004. This Directive governs how regulatory bodies in member states control clinical trials. No clinical trial may be started without a clinical trial authorization granted by the national competent authority and favorable ethics approval.
Accordingly, there is a marked degree of change and uncertainty both in the regulation of clinical trials and in respect of marketing authorizations which face us for our products in Europe.
Government Funding
Novogen received financial support for the phenoxodiol drug program from the Australian government under what is known as the START Program. The START Program was a merit-based program designed to encourage and assist Australian companies to undertake research and development and commercialization through a range of grants and loans. The START Program is administered by the Industry Research and Development, or IR&D Board. The IR&D Board is made up of private sector and academic members with expertise and experience in research and development and commercialization. In 1998, the Australian government agreed to provide A$2.7 million (approximately U.S. $1.8 million) to Novogen, enabling it to expedite phenoxodiol into clinical trials, provided that the grant money was matched by an equal expenditure by Novogen. The START gra nt was awarded after the government’s review of the pertinent research results, the intellectual property driving the program and the likelihood and potential for commercial success of the drug.
We have no further obligation under this agreement.
Employees
We currently have four employees. Novogen provides us with additional staff under our service agreements, which includes research, development and administrative personnel.
Item 1A. Risk Factors
In addition to the other information in this Annual Report on Form 10-K the following risk factors should be considered carefully in evaluating us and our business.
Risks Related to Our Business
Final approval by regulatory authorities of our drug candidates for commercial use may be delayed, limited or prevented, any of which would adversely affect our ability to generate operating revenues.
We will not generate any operating revenue until we successfully commercialize one of our drug candidates. Currently we have drug candidates at different stages of development and each will need to successfully complete a number of tests and obtain regulatory approval before potential commercialization.
In particular, any of the following factors may serve to delay, limit or prevent the final approval by regulatory authorities of our drug candidates for commercial use:
| · | Triphendiol, NV-143 and NV-128 are in the early stages of clinical development, and we will need to conduct significant clinical testing to prove safety and efficacy before applications for marketing can be filed with the FDA, or with the regulatory authorities of other countries; |
| · | data obtained from pre-clinical and clinical tests can be interpreted in different ways, which could delay, limit or prevent regulatory approval; |
| · | development and testing of product formulation, including identification of suitable excipients, or chemical additives intended to facilitate delivery of our drug candidates; |
| · | it may take us many years to complete the testing of our drug candidates, and failure can occur at any stage of this process; and |
| · | negative or inconclusive results or adverse medical events during a clinical trial could cause us to delay or terminate our development efforts. |
The successful development of any of these drug candidates is uncertain and accordingly we may never commercialize any of these drug candidates or generate revenue.
We have a limited operating history, and we are likely to incur operating losses for the foreseeable future.
You should consider our prospects in light of the risks and difficulties frequently encountered by early stage and developmental companies. Although we were incorporated in December 2000, we have only been in operation since May 2002. We have incurred net losses of $70,807,000 since our inception through June 30, 2010, including net losses of $7,896,000, $11,180,000 and $12,410,000 for the years ended June 30, 2010, 2009 and 2008, respectively. We anticipate that we will incur operating losses and negative operating cash flow for the foreseeable future. We have not yet commercialized any drug candidates and cannot be sure that we will ever be able to do so, or that we may ever become profitable.
Because a final analysis of our Phase III OVATURE trial of orally administered phenoxodiol determined that the trial did not show a statistically significant improvement in its primary (progression-free survival) or secondary (overall survival) endpoints, are unlikely to out-license phenoxodiol to third parties for this purpose.
On June 1, 2010, we announced that a final analysis of our Phase III OVATURE trial of orally administered phenoxodiol in women with recurrent ovarian cancer determined that the trial did not show a statistically significant improvement in its primary (progression-free survival) or secondary (overall survival) endpoints. Since the trial did not meet its endpoints, it is unlikely we will be able to out-license Phenoxodiol to third parties.
We will need additional funds to progress the clinical trial program for triphendiol, NV-143 or NV-128 beyond their early stages and to develop new in-licensed compounds from Novogen. The actual amount of funds we will need will be determined by a number of factors, some of which are beyond our control.
The factors which will determine the actual amount of funds that we will need to progress the clinical trial programs for triphendiol, NV-143 and NV-128 may include the following:
| · | the number of sites included in the trials; |
| · | the length of time required to enroll suitable patients; |
| · | the number of patients who participate in the trials and the rate that they are recruited; |
| · | the number of treatment cycles patients complete while they are enrolled in the trials; and |
| · | the efficacy and safety profile of the product. |
If we are unable to obtain additional funds on favorable terms we may be required to cease or reduce our operations. Also, if we raise more funds by selling additional securities, the ownership interests of holders of our securities will be diluted.
The uncertain financial markets may negatively impact our liquidity and our ability to continue our planned future clinical trials program, by precluding us from raising funds through equity issuances on terms favorable to us or at all.
We have traditionally raised capital through the sale of equity securities to investors and intend to seek additional capital through an equity transaction in 2010. Since September 2008, the financial services industry, credit markets and capital markets have experienced a period of unprecedented turmoil and volatility. Accordingly, we may have difficulty raising the capital necessary to finance our business operations through the sale of equity securities on terms favorable to us or at all or through other types of financing. In order to obtain the additional funding necessary to conduct our business, we may need to rely on collaboration and /or licensing opportunities. We cannot assure you that we will be able to raise the funds necessary or find appropriate collaboration or licensing opportunities to fund our future business plan.
We may not be able to establish the strategic partnerships necessary to develop, market and distribute our product candidates.
A key part of our business plan is to establish relationships with strategic partners. We must successfully contract with third parties to package, market and distribute our product candidates. We have not yet established any strategic partnerships. Potential partners may not wish to enter into agreements with us due to Novogen’s current equity position as our majority stockholder or our contractual relationships with Novogen.
Similarly, potential partners may be discouraged by our limited operating history. Additionally, our relative attractiveness to potential partners and consequently, our ability to negotiate acceptable terms in any partnership agreement, will be affected by the results of our clinical program. There is no assurance that we will be able to negotiate commercially acceptable licensing or other agreements for the future exploitation of our drug product candidates including continued clinical development, manufacture or marketing. If we are unable to successfully contract for these services, or if arrangements for these services are terminated, we may have to delay our commercialization program which will adversely affect our ability to generate operating revenues.
We may not be able to secure and maintain suitable Clinical Research Organisations (CROs) or clinical research institutions to manage and conduct our clinical trials.
We rely on suitable CROs to manage larger clinical trials on our behalf and clinical research institutions, of which there are many, to conduct our clinical trials. Our reliance upon third party CROs and clinical research institutions, including hospitals and cancer clinics, provides us with less control over the timing and cost of clinical trials and the ability to recruit patients than if we had conducted the trials on our own. Further, there is a greater likelihood that disputes may arise with these CROs and clinical research institutions over costs and the ownership of intellectual property discovered during the clinical trials. If we are unable to reach agreement with sui table CROs and clinical research institutions on acceptable terms, or if any resulting agreement is terminated and we are unable to quickly replace the applicable CRO or clinical research institution with another qualified CRO or institution on acceptable terms, the research could be delayed and we may be unable to complete development or commercialize our drug candidates, which will adversely affect our ability to generate operating revenues.
Our commercial opportunity will be reduced or eliminated if competitors develop and market products that are more effective, have fewer side effects or are less expensive than our drug candidates.
The development of drug candidates is highly competitive. A number of other companies have products or drug candidates in various stages of pre-clinical or clinical development that are intended for the same therapeutic indications for which our drug candidates are being developed. Some of these potential competing drugs are further advanced in development than our drug candidates and may be commercialized sooner. Even if we are successful in developing effective drugs, our compounds may not compete successfully with products produced by our competitors.
Our competitors include pharmaceutical companies and biotechnology companies, as well as universities and public and private research institutions. In addition, companies active in different but related fields represent substantial competition for us. Many of our competitors developing oncology drugs have significantly greater capital resources, larger research and development staffs and facilities and greater experience in drug development, regulation, manufacturing and marketing than us. These organizations also compete with us and our service providers, to recruit qualified personnel, and with us to attract partners for joint ventures and to license technologies that are competitive with ours. As a result, our competitors may be able to more easily develop technologies and products that would render our technologies or our drug cand idates obsolete or non-competitive.
We have no direct control over the costs of manufacturing our drug candidates. Increases in the costs of manufacturing our drug candidates would increase our costs of conducting clinical trials and could adversely affect our future profitability.
We do not intend to manufacture our drug product candidates ourselves and we will rely on third parties for our drug supplies both for clinical trials and for commercial quantities in the future. Novogen has taken the strategic decision not to manufacture on a large scale API for cancer drugs as these can be more economically supplied by third parties with particular expertise in this area. We have identified contract facilities that are registered with the FDA, have a track record of large scale API manufacture and have already invested in capital and equipment. We have no direct control over the costs of manufacturing our product candidates. If the costs of manufacturing increase or if the cost of the materials used increases, these costs will be passed on to us making the cost of conducting clinical trials more expensive. Increases in manufacturing costs could adversely affect our future profitability if we are unable to pass all of the increased costs along to our customers.
The third-party manufacturers whom we rely upon for the production of clinical material for our clinical trials and for future commercial quantities may not be in compliance with FDA regulatory requirements.
The conduct of our clinical trials and approval of our marketing application for our product candidates may be delayed or adversely affected if the third-party manufacturers whom we rely upon fail to comply with FDA’s regulatory requirements for current cGMP. The FDA requires drug manufacturers to establish and maintain quality control procedures for manufacturing, processing and holding drugs and investigational products, and products must be manufactured in accordance with defined specifications. The failure of contract manufacturers to supply investigational product in compliance with defined specifications may delay the completion of our clinical trials. As part of the pre-market approval process, the manufacturer will be inspected by the FDA to ensure compliance with cGMP. The failure of contract manufacturers to comply with applicable regulations may result in a delay or prevent approval of our marketing application.
We face a risk of product liability claims and may not be able to obtain adequate insurance.
Our business exposes us to the risk of product liability claims. This risk is inherent in the manufacturing, testing and marketing of human therapeutic products. We have product liability insurance coverage of up to approximately $25.6 million. Although we believe that this amount of insurance coverage is appropriate for our business at this time, it is subject to deductibles and coverage limitations, and the market for such insurance is becoming more restrictive. We may not be able to obtain or maintain adequate protection against potential liabilities. If we are unable to sufficiently insure against potential product liability claims, we will be exposed to significant liabilities, which may materially and adversely affect our business development and commercialization efforts.
Our rights to develop and exploit phenoxodiol and the anti-cancer compounds triphendiol, NV-143 and NV-128 are subject to the terms and conditions of agreements we have entered into with Novogen. Under these agreements our rights may be terminated under certain circumstances, some of which may be beyond our control.
We have licensed the intellectual property in the phenoxodiol technology and the anti-cancer compounds triphendiol, NV-143 and NV-128 from Novogen. Under the terms of the License Agreement for Phenoxodiol, all forms of administering phenoxodiol for the treatment of cancer, excluding topical applications, are licensed to us through our wholly-owned subsidiary, MEPL. Under the terms of the License Agreement for Triphendiol and NV-143, all forms of administering drugs containing the anti-cancer compounds triphendiol and NV-143, excluding topical applications, are licensed to us through MEPL.
Under the terms of the License Agreement for NV-128, all forms of administering NV-128, excluding topical applications, are licensed to us through MEPL. If we fail to meet our obligations under our license agreements, the Manufacturing License and Supply Agreement or the Services Agreement with Novogen, any or all of these agreements may be terminated by Novogen and we could lose our rights to develop phenoxodiol or anti-cancer drugs containing triphendiol, NV-143, and NV-128. To date, we have no reason to believe that we will be unable to satisfy our obligations under these agreements. In addition, each of these agreements may be terminated immediately by Novogen in the event that MEPL undergoes a change of control without the consent of Novogen. Under the terms of the License Agreement for Phenoxodiol, the Manufacturing License and S upply Agreement and the Services Agreement, a “change of control” means (i) a change in control of more than half the voting rights attaching to the shares of MEPL, (ii) a change in control of more than half of the issued shares of MEPL (not counting any share which carries no right to participate beyond a specified amount in the distribution of either profit or capital), or (iii) a change in control of the composition of the board of directors of MEPL. Under the terms of the License Agreement for Triphendiol and NV-143 and the License Agreement for NV-128, a “change in control” means the acquisition by any person or group of more than half of the combined voting power of MEPL’s then outstanding securities entitled to vote generally in the election of directors of MEPL or any merger, consolidation, recapitalization, exchange or tender offer as a result of which a person or a group other than the shareholders of MEPL immediately before the transaction owns after the transaction m ore than half of the combined voting power of the then outstanding securities entitled to vote generally in the election of directors MEPL. Each of these agreements may also be terminated if we cease, for any reason, to be able to lawfully carry out all the transactions required by each respective agreement.
Our license rights are fundamental to our business and therefore a loss of these rights will likely cause us to cease operations.
The rights granted to us under the License Agreements, the Manufacturing License and Supply Agreement, and the License Option Deed with Novogen are fundamental to our business. The License Agreement for Phenoxodiol grants us the right to make, market, distribute, sell, hire or otherwise dispose of phenoxodiol products in the field of prevention, treatment or cure of cancer in humans by pharmaceuticals delivered in all forms except topical applications. The License Agreement for Triphendiol and NV-143 and the License Agreement for NV-128 grant us the right to make, have made, market, distribute, sell, hire or otherwise dispose of anti-cancer drugs containing the compounds triphendiol and NV-143 and NV-128 in the field of prevention, treatment or cure of cancer in humans by pharmaceuticals delivered in all forms except topical applicatio ns. Our business purpose is to develop and commercialize cancer drugs including phenoxodiol and drugs containing the compounds triphendiol and NV-143 and NV-128, which we would be unable to pursue without the rights granted to us under the license agreements. Any loss of the rights under any of these agreements will likely cause us to cease operations. The License Option Deed grants us an exclusive first right to accept and exclusive last right to match any proposed dealing by Novogen of its intellectual property rights with a third party relating to certain compounds developed by Novogen and its affiliates which have applications in the field of prevention, treatment or cure of cancer in humans. The License Option Deed is important to our business because it allows us to maintain control over the sale by Novogen of complementary as well as potentially competitive intellectual property rights to third party competitors. A loss of rights under the License Option Deed could be detrimental to our long term succ ess.
Our commercial success is dependent, in part, on Novogen’s ability to obtain and maintain patent protection and preserve trade secrets, which cannot be guaranteed.
Patent protection and trade secret protection are important to our business and our future will depend, in part on our ability and the ability of Novogen to maintain trade secret protection, obtain patents and operate without infringing the proprietary rights of others both in the U.S. and abroad. Litigation or other legal proceedings may be necessary to defend against claims of infringement, to enforce our patents, or to protect our trade secrets or the trade secrets of Novogen. Such litigation could result in substantial costs and diversion of our management’s attention. Novogen has not been involved in any opposition, re-examination, trade secret dispute, infringement litigation or any other litigation or legal proceedings pertaining to the licensed patent rights.
The patent positions of pharmaceutical and biotechnology companies can be highly uncertain and involve complex legal and factual questions. Novogen has applied for patents in a number of countries with respect to the use of phenoxodiol for the treatment, prevention or cure of cancer and methods of production of phenoxodiol. We have licensed both issued patents and pending patent applications from Novogen in relation to these technologies. Novogen has been issued a U.S. patent for pharmaceutical compositions comprising phenoxodiol. Novogen has issued patents in the U.S., the United Kingdom, Australia, China, Hong Kong, New Zealand, Singapore, Mexico, Norway and the Czech Republic related to phenoxodiol for the treatment of a variety of cancers. Novogen has issued patents in Australia, New Zealand, Singapore, South Africa, Norway, China, Hong Kong and Turkey relating to methods of production of phenoxodiol. For each of the patent families discussed above, there remain pending patent applications in various other jurisdictions which encompass subject matter similar to that which was granted in the aforementioned patents.
Novogen’s patent applications may not proceed to grant or may be amended to reduce the scope of protection of any patent granted. The applications and patents may also be opposed or challenged by third parties. Our commercial success will depend, in part, on the ability of Novogen and our ability to obtain and maintain effective patent protection for the technologies underlying phenoxodiol, triphendiol, NV-143, NV-128 and other compounds, and to successfully defend patent rights in those technologies against third-party challenges. As patent applications in the U.S. are maintained in secrecy until published or issued and as publication of discoveries in the scientific or patent literature often lag behind the actual discoveries, we cannot be certain that Novogen was the first to make the inventions covered by its pending patent a pplications or issued patents or that it was the first to file patent applications for such inventions. Additionally, the breadth of claims allowed in biotechnology and pharmaceutical patents or their enforceability cannot be predicted. We cannot be sure that, should any patents issue, we will be provided with adequate protection against potentially competitive products. Furthermore, we cannot be sure that should patents issue, they will be of commercial value to us, or that private parties, including competitors, will not successfully challenge our patents or circumvent our patent position in the U.S. or abroad.
Claims by other companies that we infringe their proprietary technology may result in liability for damages or stop our development and commercialization efforts.
The pharmaceutical industry is highly competitive and patents have been applied for by, and issued to, other parties relating to products competitive with our licensed compounds. Therefore, phenoxodiol triphendiol, NV-143, NV-128 and any other drug candidates may give rise to claims that they infringe the patents or proprietary rights of other parties existing now and in the future.
Furthermore, to the extent that we or Novogen or our respective consultants or research collaborators use intellectual property owned by others in work performed for us or Novogen, disputes may also arise as to the rights in such intellectual property or in resulting know-how and inventions. An adverse claim could subject us to significant liabilities to such other parties and/or require disputed rights to be licensed from such other parties.
We have contracted formulation development and manufacturing process development work for our product candidates. This process has identified a number of excipients, or additives to improve drug delivery, which may be used in the formulations. Excipients, among other things, perform the function of a carrier of the active drug ingredient. Some of these identified excipients or carriers may be included in third party patents in some countries. We intend to seek a license if we decide to use a patented excipient in the marketed product or we may choose one of those excipients that do not have a license requirement.
We cannot be sure that any license required under any such patents or proprietary rights would be made available on terms acceptable to us, if at all. If we do not obtain such licenses, we may encounter delays in product market introductions, or may find that the development, manufacture or sale of products requiring such licenses may be precluded. We have not conducted any searches or made any independent investigations of the existence of any patents or proprietary rights of other parties.
We may be subject to substantial costs stemming from our defense against third-party intellectual property infringement claims.
Third parties may assert that we or Novogen are using their proprietary information without authorization. Third parties may also have or obtain patents and may claim that technologies licensed to or used by us infringe their patents. If we are required to defend patent infringement actions brought by third parties, or if we sue to protect our own patent rights, we may be required to pay substantial litigation costs and managerial attention may be diverted from business operations even if the outcome is not adverse to us. In addition, any legal action that seeks damages or an injunction to stop us from carrying on our commercial activities relating to the affected technologies could subject us to monetary liability and require us or Novogen or any third party licensors to obtain a license to continue to use the affected technologies. W e cannot predict whether we or Novogen would prevail in any of these types of actions or that any required license would be made available on commercially acceptable terms or at all.
The enforcement of civil liabilities against our directors may be difficult.
Two of our directors are residents of jurisdictions outside the U.S. As a result, it may be difficult for you to effect service of process within the U.S. upon all our directors or to enforce judgments obtained against our directors in U.S. courts.
Our results are affected by fluctuations in currency exchange rates.
A portion of our expenditures and potential revenue will be spent or derived outside of the U.S. As a result, fluctuations between the U.S. dollar and the currencies of the countries in which we operate may increase our costs or reduce our potential revenue. At present, we do not engage in hedging transactions to protect against uncertainty in future exchange rates between particular foreign currencies and the U.S. dollar.
We are authorized to issue blank check preferred stock, which could adversely affect the holders of our common stock.
Our restated certificate of incorporation allows us to issue blank check preferred stock with rights potentially senior to those of our common stock without any further vote or action by the holders of our common stock. The issuance of preferred stock could decrease the amount of earnings and assets available for distribution to the holders of our common stock or could adversely affect the rights and powers including voting rights, of such holders. In certain circumstances, such issuance could have the effect of decreasing the market price of our shares, or making a change in control of us more difficult.
Risks Related to Our Relationship with Novogen
As our majority stockholder, Novogen has the ability to determine the outcome of all matters submitted to our stockholders for approval, and Novogen’s interests may conflict with ours or our other stockholders’ interests.
Novogen beneficially owns approximately 71.3% of our outstanding shares of common stock. As a result, Novogen will have the ability to effectively determine the outcome of all matters submitted to our stockholders for approval, including the election and removal of directors and any merger, consolidation or sale of all or substantially all of our assets.
Novogen will have the ability to effectively control our management and affairs. Novogen’s interests may not always be the same as that of our other stockholders. In addition, this concentration of ownership may harm the market price of our securities by:
| · | delaying, deferring or preventing a change in control; |
| · | impeding a merger, consolidation, takeover or other business combination involving us; |
| · | discouraging a potential acquirer from making a tender, offer or otherwise attempting to obtain control of us; or |
| · | selling us to a third party. |
One of our directors is a director of Novogen Limited and other Novogen subsidiaries, which may create a conflict of interest as well as prevent him from devoting his full attention to us.
One of our board members currently serves as a board member of Novogen Limited. Simultaneous service as a Novogen Limited director could create, or appear to create, a conflict of interest when such director is presented with decisions that could have different implications for us and Novogen Limited.
Mr. Philip Johnston is the chairman of Novogen Limited. The responsibilities of Mr. Johnston could prevent him from devoting his full attention to us, which could be harmful to the development of our business.
Novogen can compete with us.
We have no contract, arrangement or understanding with Novogen to preclude it from developing a product which may be competitive with phenoxodiol, triphendiol, NV-143 or NV-128 or to use these compounds for any uses other than anti-cancer applications.
Novogen has reserved the intellectual property rights and know-how rights relating to topical applications of these compounds even in the field of cancer. There can be no assurance that Novogen or its subsidiaries will not pursue alternative technologies or product candidates as a means of developing treatments for the conditions targeted by phenoxodiol or any other product candidate which we seek to exploit.
We are dependent on Novogen for our personnel.
We rely on Novogen and other service companies to provide or procure the provision of staff and other financial and administrative services under our services agreement with Novogen. We believe Novogen has fully complied with the terms of our services agreement. To successfully develop our drug candidates, we may require ongoing access to the personnel who have, to date, been responsible for the development of our drug candidates. The services agreement does not specify a minimum amount of time that Novogen employees must devote to our operations. If we are unable to secure or if we lose the services of these personnel, the ability to develop our drug candidates could be materially impaired. Moreover, if our business experiences substantial and rapid growth, we may not be able to secure the services and resources we require from Novoge n or from other persons to support that growth.
In the event that Novogen undergoes a change in control while remaining our controlling stockholder, we will become subject to the control and influence of Novogen’s new controlling stockholder who may have views regarding the development of our business that differ from the development strategies we are currently pursuing.
In the event that Novogen undergoes a change in control while remaining our controlling stockholder, we will become subject to the control and influence of Novogen’s new controlling stockholder who will have the ability to indirectly determine the outcome of all matters submitted to our stockholders for approval through its control of Novogen. This entity may have views regarding the development of our business that differ from the development strategies we are currently pursuing. Such controlling stockholder may cause Novogen to use its influence and voting power to change the direction in which we are developing our business. Such changes may include, but are not limited to, a decreased focus on the development of any of our current drug candidates and an increased focus on the development of alternative drug candidates, which may or may not be targeted to treat cancers. Additionally, this entity may seek to renegotiate the terms of our existing License Agreements, Manufacturing, License and Supply Agreement and Services Agreement with Novogen.
Risks Related to Our Common Stock
The trading price of the shares of our common stock has been and may continue to be highly volatile and could decline in value and we may incur significant costs from class action litigation.
The trading price of our common stock could be highly volatile in response to various factors, many of which are beyond our control, including:
| · | developments concerning phenoxodiol and our other drug candidates triphendiol, NV-143 and NV-128; |
| · | announcements of technological innovations by us or our competitors; |
| · | new products introduced or announced by us or our competitors; |
| · | changes in financial estimates by securities analysts; |
| · | actual or anticipated variations in operating results; |
| · | expiration or termination of licenses, research contracts or other collaboration agreements; |
| · | conditions or trends in the regulatory climate and the biotechnology, pharmaceutical and genomics industries; |
| · | the instability in the stock market as a result of the current global financial crisis; |
| · | changes in the market valuations of similar companies; |
| · | the liquidity of any market for our securities; and |
| · | additional sales by us or Novogen of shares of our common stock. |
In addition, equity markets in general, and the market for biotechnology and life sciences companies in particular, have experienced substantial price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies traded in those markets. In addition, changes in economic conditions in the U.S., Europe or globally, particularly in the context of the current global financial crisis, could impact upon our ability to grow profitably. Adverse economic changes are outside our control and may result in material adverse impacts on our business or our results of operations. These broad market and industry factors may materially affect the market price of our shares of common stock, regardless of our development and operating performance. In the past, following periods of volatility in the ma rket price of a company’s securities, securities class-action litigation has often been instituted against that company. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources.
Future sales of our common stock may depress the market price of our common stock and cause stockholders to experience dilution.
The market price of our common stock could decline as a result of sales of substantial amounts of our common stock in the public market. We intend to seek additional capital through an equity transaction in 2010, however, such transaction will be subject to market conditions and there can be no assurance it will be completed.
We will have broad discretion over the use of the net proceeds to us from any exercise of outstanding warrants.
We will have broad discretion to use the net proceeds to us upon any exercise of outstanding warrants, and you will be relying on the judgment of our board of directors and management regarding the application of these proceeds. Although we expect to use a substantial portion of the net proceeds from any exercise of the warrants for general corporate purposes, including potential payments to Novogen under the terms of the License Agreements, potential licensing of other cancer compounds developed by Novogen under the License Option Deed and potential expansion of our clinical trial program, we have not allocated these net proceeds for specific purposes.
Our Common Stock may be delisted from Nasdaq
We have received notices of delisting from Nasdaq regarding non-compliance with the minimum stockholders equity and the minimum Market Value of Publicly Held Shares in accordance with Nasdaq Listing Standards for the Nasdaq Global Market. The notification letters state the Company will be afforded a grace period of 180 calendar days, or until January 10, 2011, to regain compliance with the Market Value of Publicly Held Shares in accordance with Nasdaq Rule 5810(c)(3)(D) and 180 calendar days, or until November 15, 2010, to regain compliance with the stockholders equity in accordance with Nasdaq Rule 5810(c)(2)(B). However, if we cannot regain compliance during the provided grace periods we may face delisting. Although in such circumstance, we may still meet the Nasdaq Capital Market Listing Standards, which include a minimum requirement of $2.5 million in stockholders equity and $1 million in Market Value of Publicly Held Shares, as well as $1.00 per share bid price requirement.
Under Nasdaq rules, companies listed on the Nasdaq Global Market or Capital Market are required to maintain a share price of at least $1.00 per share and if the share price declines below $1.00 for a period of 30 consecutive business days, then the listed company would have 180 days to regain compliance with the $1.00 per share minimum. In the event that the Company’s share price declines below $1.00, it may be required to take action, such as a reverse stock split, in order to comply with the Nasdaq rules that may be in effect at the time.
If we are not able to comply with the listing Standards of the Nasdaq Global Market or the Nasdaq Capital Market, the Company’s common stock will be delisted from Nasdaq and an associated decrease in liquidity in the market for the Company’s common stock will occur.
In addition, if the market price of our common stock remains below $5.00 per share, under stock exchange rules, our stockholders will not be able to use such shares as collateral for borrowing in margin accounts. This inability to use shares of our common stock as collateral may depress demand as certain institutional investors are restricted from investing in shares priced below $5.00 and lead to sales of such shares creating downward pressure on and increased volatility in the market price of our common stock.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We have leased office space, of approximately 3,700 square feet, located at 11975 El Camino Real, San Diego, California. The location houses the Company’s executive and administrative offices. The lease commenced in July 2010 and expires in April 2013. Monthly rental rates range from $10,109 to $10,734 over the lease term, plus a pro rata share of certain building expenses. In addition, the Company has two options to extend the lease for one year each at the market rate in effect at the time of renewal.
We believe these facilities will adequately meet our office needs for the foreseeable future.
Item 3. Legal Proceedings
None.
PART II
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Securities
The following tables set forth for the period indicated the high and low sale prices of our common stock as reported by the NASDAQ Global Market.
| Common Stock | Nasdaq Global Market |
| | | High | | Low | |
| | | $ | | $ | |
| Year Ended June 30, 2009 | | | | | |
| First Quarter | | 33.20 | | 11.20 | |
| Second Quarter | | 20.80 | | 3.00 | |
| Third Quarter | | 9.80 | | 2.50 | |
| Fourth Quarter | | 13.40 | | 3.80 | |
| | | | | | |
| Year Ended June 30, 2010 | | | | | |
| First Quarter | | 17.40 | | 4.80 | |
| Second Quarter | | 10.30 | | 6.20 | |
| Third Quarter | | 9.00 | | 4.60 | |
| Fourth Quarter | | 5.60 | | 1.22 | |
As of August 24, 2010, there were 7,346,324 shares of our common stock outstanding and 94 holders of record of our common stock. This number was derived from our shareholder records and does not include beneficial owners of our common stock whose shares are held in the name of various dealers, clearing agencies, banks, brokers and other fiduciaries.
Dividends
We have never declared or paid any cash dividends on our common stock and do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain future earnings, if any, to fund the expansion and growth of our business. Payments of any future cash dividends will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, plans for expansion and other factors that our board of directors deem relevant.
Stock Repurchases
We have not repurchased any shares of common stock during the fourth quarter of the fiscal year ended June 30, 2010.
Equity Compensation
The following table sets forth, as of June 30, 2010 outstanding awards and shares remaining available for future issuance under our compensation plans under which equity securities are authorized for issuance.
Plan Category | (a) Number of securities to be issued upon exercise of outstanding options, warrants and rights | (b) Weighted-average exercise price of outstanding options, warrants and rights | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a) |
| Equity compensation plans approved by security holders | 78,463 | $1.82 | 6,921,537 |
| Equity compensation plans not approved by security holders | 220,390 | $3.46 | Not Applicable |
| Total | 298,853 | $3.03 | Indeterminable |
Pursuant to the terms of Dr. Gold’s Employment Letter, dated April 23, 2010, Dr. Gold received options to purchase 220,390 shares of the Company’s common stock in two separate tranches as an inducement to become a new employee, which, in accordance with Nasdaq rule 5635(c), does not require stockholder approval. The first tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold upon his appointment as President and Chief Executive Officer on April 23, 2010, with an exercise price per share equal to the closing price of the Company’s common stock on April 23, 2010. The second tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold on June 7, 2010 following the public release of the Company’s OVATURE study results. Of Dr. Gold’s options, 25% will vest one year from the effective date of the Employment Letter and, thereafter, the remaining 75% of Dr. Gold’s options will vest in equal monthly installments over the following thirty-six (36) months. In the event of a Change in Control of the Company, as defined in the Employment Letter, Dr. Gold’s options will become fully vested. If Dr. Gold’s employment is terminated by the Company without Cause or by Dr. Gold for Good Reason, each as defined in the Employment Letter, Dr. Gold will be entitled to accelerated vesting of his options such that Dr. Gold will be vested in the same number of options as if he had continued to be employed by the Company for an additional twelve (12) months.
Stock Performance Graph
The graph set forth below compares the change in our cumulative total stockholder return on our common stock between December 18, 2003 (the date our common stock commenced public trading) and June 30, 2010 with the cumulative total return of the NASDAQ Composite Index and the NASDAQ Biotechnology Index during the same period. This graph assumes the investment of $100 on December 18, 2003 in our common stock and each of the comparison groups and assumes reinvestment of dividends, if any. We have not paid any dividends on our common stock, and no dividends are included in the report of our performance.
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Item 8. “Financial Statements” included elsewhere in this Annual Report on Form 10-K.
| Statement of Operations | | Years Ended June 30, | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands, except per share data) | |
| | | | | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | | | | |
| Interest and other income | | $ | 84 | | | $ | 228 | | | $ | 674 | | | $ | 645 | | | $ | 446 | |
| Total revenues | | | 84 | | | | 228 | | | | 674 | | | | 645 | | | | 446 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (7,895 | ) | | | (11,179 | ) | | | (12,407 | ) | | | (13,819 | ) | | | (7,385 | ) |
| Income tax expense | | | (1 | ) | | | (1 | ) | | | (3 | ) | | | (1 | ) | | | (1 | ) |
| Net loss arising during development stage | | $ | (7,896 | ) | | $ | (11,180 | ) | | $ | (12,410 | ) | | $ | (13,820 | ) | | $ | (7,386 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss per common share: | | | | | | | | | | | | | | | | | | | | |
| Basic and diluted | | $ | (1.07 | ) | | $ | (1.53 | ) | | $ | (1.82 | ) | | $ | (2.19 | ) | | $ | (1.30 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding | | | 7,346,324 | | | | 7,307,184 | | | | 6,830,257 | | | | 6,317,937 | | | | 5,693,800 | |
| Balance Sheet Data | | As of June 30, | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
| | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 9,031 | | | $ | 19,067 | | | $ | 19,743 | | | $ | 16,158 | | | $ | 10,054 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total assets | | $ | 9,136 | | | $ | 19,356 | | | $ | 19,978 | | | $ | 16,290 | | | $ | 10,395 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total stockholders' equity | | $ | 7,381 | | | $ | 15,213 | | | $ | 16,535 | | | $ | 13,777 | | | $ | 9,135 | |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis should be read in conjunction with “Item 8. Financial Statements and Supplementary Data” included below. Operating results are not necessarily indicative of results that may occur in future periods. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The actual results may differ materially from those anticipated in the forward-looking statements as a result of many factors including, but not limited to, those set forth under “Cautionary Statement About Forward-Looking Statements” and “Risk Factors” in Item 1A. included above in this Annual Report on Form 10-K. All forward-looking statements included in this document are based on the information available to us on the date of this document and we assume no obligation to update any forward-looking statements contained in this Annual Report on Form 10-K.
Overview
Our initial focus since commencing operations was to undertake human clinical testing of phenoxodiol. Our operations were expanded to include the additional licensed drug candidates triphendiol and NV-143, and, most recently, NV-128.
Clinical Product Developments
During fiscal year 2007, we commenced the OVATURE Phase III clinical trial. We reached agreement under the SPA process with the FDA on the design of our OVATURE pivotal study protocol for phenoxodiol. The trial was designed to test the ability of phenoxodiol administered orally to restore sensitivity of late-stage ovarian cancers to carboplatin, a standard form of therapy for ovarian cancer.
In April 2009, we announced our decision to terminate enrollment into the Phase III OVATURE clinical trial and our intention to undertake an unblinded analysis of the available data from the trial. The decision to terminate new enrollment into the Phase III OVATURE clinical trial and assess the available patient data was made, in part, because we believed that the global financial downturn would make it unlikely that we would be able to raise the necessary capital through debt or equity issuances in the near future to fund the trial to completion as originally planned. Additionally, changes in the standard of care over the period that the OVATURE Phase III clinical trial was in operation resulted in fewer women meeting the inclusion criteria of the OVATURE protocol, which slowed patient recruitment rates. The termination of patient enr ollment into the OVATURE study and unblinded analysis plan of the available data from the trial have been discussed with FDA.
On June 1, 2010, we announced that a final analysis of our Phase III OVATURE trial of orally administered phenoxodiol in women with recurrent ovarian cancer determined that the trial did not show a statistically significant improvement in its primary (progression-free survival) or secondary (overall survival) endpoints. However, we believe that our investigational isoflavone platform, including triphendiol or its primary active metabolite, NV-143, a potentially more potent, second-generation analogue of phenoxodiol, may be shown to be of benefit to women with ovarian cancer or patients with other forms of cancer, particularly when administered intravenously.
In August, 2009, we entered into a third license agreement with Novogen for the investigational oncology compound NV-128. In consideration of the license granted to us, we paid Novogen a license fee of $1,500,000 on August 7, 2009. NV-128 is a novel flavonoid small molecule inhibitor, capable of inhibiting both mTORC1 and mTORC2 protein regulatory pathways which are central to the aberrant proliferative capacity of both mature cancer cells and cancer stem cells.
In September 2009, we released data demonstrating that the efficacy of NV-128 in animal xenograft models is achieved without apparent toxicity. The laboratory data demonstrated that NV-128 has greater safety than some other mTOR inhibitors in mice bearing human ovarian cancer xenografts. Additional data released reported that NV-128 was judged to be without cardiac toxicity.
In June 2010 Dr. Kevin Kelly of Yale University presented the results of our prostate Phase II cancer clinical trial at the American Society of Clinical Oncology meetings. The Phase II prostate cancer clinical trial used phenoxodiol as first line treatment in men with early stage disease (16 patients with androgen dependent disease but rising Prostate Specific Antigen (“PSA”) compared to patients with late stage hormone refractory disease (12 patients with chemotherapy naïve androgen independent disease) at Yale Cancer Center and the West Haven Veterans Administration Hospital Connecticut in the US. Both of these patient groups represent areas of unmet medical need in this common cancer. The results presented indicated that approximately one-third of patient experienced disease stabilization as measured by PSA levels. In this small study it appeared that during treatment, interferon-gamma (IFN-γ) increased from baseline levels in patients with PSA partial response or stable disease, while monocyte chemotactic protein-1 (MCP-1) levels increased from baseline levels in patients with PSA progressive disease.
Corporate Developments
On December 1, 2009, Novogen advised that its Chief Executive Officer and Managing Director Mr. Christopher Naughton ceased his employment, correspondingly Mr. Naughton's position as Chief Executive Officer of Marshall Edwards also ceased at this time. On February 5, 2010, Mr. Naughton resigned from being a director of the Company. Novogen’s Chief Financial Officer Mr. David Seaton was appointed acting Chief Executive Officer of the Group and he acted in that capacity until our new Chief Executive Officer, Dr. Daniel P. Gold was appointed President and Chief Executive Officer of Marshall Edwards on April 23, 2010. On April 30, 2010 Dr. Gold was appointed to serve as a member on the Board of Directors of the Company.
On June 17, 2010 we announced the appointment of Thomas Zech as Chief Financial Officer. This appointment is part of the strategic decision to relocate our office and management of our company to the U.S. In addition to the appointment of our new Chief Executive Officer and Chief Financial Officer we have entered into a lease for a new office located in San Diego and have employed additional administration staff.
In September 2009, we received a letter from The Nasdaq Stock Market (“Nasdaq”) notifying us that for the previous 30 consecutive business days the bid price of our common stock closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq Global Market under Nasdaq Rule 5450(a)(1). According to Nasdaq's letter, we would be afforded a grace period of 180 calendar days, or until March 15, 2010, to regain compliance in accordance with Nasdaq Rule 5810(c)(3)(A). In order to regain compliance, shares of our common stock must maintain a minimum bid closing price of at least $1.00 per share for a minimum of ten consecutive business days during the grace period. On March 16, 2010, we received notice from The Nasdaq Stock Market that we had not regained compliance and that we would be suspended from The Nasdaq Global Market on March 25, 2010, unless we requested a hearing. On March 23, 2010 we requested a hearing before the Nasdaq Hearings Panel. On March 29, 2010, our stockholders approved an amendment to the Company's Restated Certificate of Incorporation to effect a reverse stock split of the Company's common stock at a 1-for-10 reverse split ratio.
The reverse stock split of our outstanding common stock was effected on March 31, 2010 on a 1-for-10 split adjusted basis. Following the reverse stock split, the closing bid price of our common stock closed above the $1.00 minimum requirement for ten consecutive trading days. We have now received notification from The Nasdaq Stock Market that we have regained compliance with the $1.00 minimum closing bid price in accordance with the Nasdaq Listing Rule 5450(a)(1). The Nasdaq Hearings Listing Qualifications Panel has determined to continue the listing of our securities on the Nasdaq Stock Market and, therefore, the hearing before the Hearings Listing Qualifications Panel has been cancelled. For the purpose of this report we have adjusted all share data presented retrospectively to incorporate the 1-for-10 reverse stock split.
On May 18, 2010 we received a notice from Nasdaq indicating that the Company failed to comply with the minimum stockholders’ equity requirement set forth in Nasdaq Listing Rule 5450(b)(1)(A) for continued listing of its common stock on the Nasdaq Global Market because our stockholders’ equity as of March 31, 2010 as set forth in our quarterly report on Form 10-Q for the period ended March 31, 2010 of $9.16 million was below the $10 million minimum stockholders’ equity requirement. The notice also stated we would be provided 45 calendar days, or until July 2, 2010, to submit a plan to regain compliance.
We responded to Nasdaq on July 2, 2010. The response included our plans to satisfy the listing requirements with respect to the maintaining a minimum $10 million Shareholders’ equity value. We stated our intention to pursue a capital raising transaction within the time provided by Nasdaq rules if market conditions permit, to further fund development of our product candidates 1) triphendiol or its primary active metabolite NV-143, a potentially more potent, second generation analog of phenoxodiol; and 2)NV-128. In the alternative, the Company intends to apply to transfer the listing of its common stock from the Nasdaq Global Market to the Nasdaq Capital Market. The Company believes it currently would be in compliance with the minimum stockholders’ equity requirement and all other criteria that would be applicable for listing on the Nasdaq Capital Market.
On August 5, 2010, the Company received a letter from Nasdaq indicating that, based on the Company’s plan, Nasdaq has determined to grant the Company an extension, through November 15, 2010, to regain compliance with the Rule by establishing stockholders’ equity of at least $10,000,000.
On July 14, 2010, we received notice from Nasdaq stating that for the last 30 consecutive business days, the Market Value of Publicly Held Shares closed below the minimum $5 million required for continued listing on the Nasdaq Global Market under Nasdaq Rule 5450(b)(1)(C). Market Value of Publicly Held Shares is calculated by multiplying the publicly held shares, which is total shares outstanding less any shares held by officers, directors, or beneficial owners of 10% or more, by the consolidated closing bid price. Novogen Limited currently owns 71.3% of the outstanding common stock of the Company. Therefore, the value of Novogen Limited’s shares is excluded from the Market Value of Publicly Held Shares of the Company. According to Nasdaq's letter, we would be afforded a grace period of 180 calendar days , or until January 10, 2011, to regain compliance in accordance with Nasdaq Rule 5810(c)(3)(A). We intend to actively monitor the Market Value of Publicly Held Shares between now and January 10, 2011.
On August 10, 2010 we announced the appointment of Christine A. White, M.D. to our board of directors. Dr. White replaces Professor Paul J. Nestel, who has served as a director since April 2001.
We believe that our existing cash balances of approximately $9 million will be sufficient to satisfy the cash needs of our current operating plan until late 2011. Changes in our research and development plans or other changes affecting our operating expenses may affect actual future consumption of existing cash resources. In any event, however, we will need additional financing to fund our operations in the future including the continued development of triphendiol, NV-143 and NV-128.
As of June 30, 2010, we had accumulated losses of $70,807,000.
We have not generated any revenues from operations since inception other than interest on cash assets.
We have incurred losses since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future.
Expenses to date have consisted primarily of costs associated with conducting the clinical trials of phenoxodiol including OVATURE, costs incurred under the Phenoxodiol License Agreement, as amended, the License Agreement for Triphendiol and NV-143, the Licence Agreement for NV-128 the Services Agreement and the Manufacturing License and Supply Agreements with Novogen and its subsidiaries, including the costs of the clinical trial drug supplies.
To date, operations have been funded primarily through the sale of equity securities.
As at the date of the Annual Report on Form 10-K, Novogen owns approximately 71.3% of the outstanding shares of our common stock.
Liquidity and Capital Resources
At June 30, 2010, we had cash resources of $9,031,000 compared to $19,067,000 at June 30, 2009. The decrease was due to expenditures in the clinical trial program and other corporate expenses incurred in the period. Funds are invested in short term money market accounts, pending use. We believe that our existing cash balances will be sufficient to satisfy our current operating plan until late 2011. We intend to seek additional capital through an equity transaction in 2010.
On July 28, 2008, we entered into a securities subscription agreement with Novogen and OppenheimerFunds, Inc. (“Oppenheimer”) pursuant to which we sold 290,829 and 170,000 shares of common stock to Novogen and Oppenheimer, respectively, with Oppenheimer acting as adviser to each of the following parties severally and not jointly: (i) Oppenheimer International Growth Fund; (ii) Mass Mutual International Equity Fund; (iii) Oppenheimer International Growth Fund/VA; (iv) AZL Oppenheimer International Growth Fund; (v) OFITC International Growth Fund; and (vi) OFI International Equity Fund, at a purchase price of $21.70 per share, the consolidated closing bid price of our common stock as quoted by the NASDAQ Market Intelligence Desk at 4:00 PM EST on July 28, 2008. The shares were registered under the Securities Act of 1933, as a mended (the “Securities Act”), under a shelf Registration Statement on Form F-3. We received gross proceeds of $10 million from the sale of the shares.
Following the closing of the registered direct offering described above in July 2008, Novogen retained approximately 71.3% of our common stock.
In July 2008, we issued a warrant to Mr. John O’Connor exercisable for 4,608 shares of common stock, as consideration for investor relation services rendered by him to us. The warrant has an exercise price of $21.70 per share. The warrant may be exercised immediately and expires five years from the date of issuance, on July 30, 2013. The warrant has not been registered under the Securities Act. We issued the warrant to Mr. O’Connor in a private placement made in reliance upon the exemption from securities registration afforded by Section 4(2) of the Securities Act.
In January 2009, we issued a stock option exercisable for 5,000 shares of common stock to Associate Professor Gil Mor of Yale University in recognition of his contribution to the development of phenoxodiol under the Company’s 2008 Stock Omnibus Equity Compensation Plan (the “Plan”). The option has an exercise price of $6.30 per share of common stock. The options are exercisable immediately and expire five years from date of issue.
Pursuant to the terms of Dr. Gold’s Employment Letter, Dr. Gold has received options to purchase 220,390 shares of the Company’s common stock in two separate tranches. The first tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold upon his appointment as President and Chief Executive Officer on April 23, 2010, with an exercise price of $5.05 per share equal to the closing price of the Company’s common stock on April 23, 2010. The second tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold on June 7, 2010 following the public release of the Company’s OVATURE study results, with an exercise price of $1.86 per share equal to the closing price of the Company’s common stock on June 7, 2010. Of Dr. Gold’s options, 25% will vest one year from the effective date of the Employment Letter and, thereafter, the remaining 75% of Dr. Gold’s options will vest in equal monthly installments over the following thirty-six (36) months. Both tranches of options have a term of five years from the date of each grant. In the event of a Change in Control of the Company, as defined in the Employment Letter, Dr. Gold’s options will become fully vested. Dr. Gold’s options are issued outside the Plan.
Pursuant to the terms of Mr. Zech’s Employment Letter, Mr. Zech has received options to purchase 73,463 shares of the Company’s common stock under the plan. These options were granted on June 18, 2010, with an exercise price of $1.52 per share equal to the closing price of the Company’s common stock on June 18, 2010. Of Mr. Zech’s options, 25% will vest one year from the effective date of the Employment Letter and, thereafter, the remaining 75% of Mr. Zech’s options will vest in equal monthly installments over the following thirty-six (36) months. The options have a term of five years from the date of grant.
Source and Uses of Cash
Cash Used in Operating Activities
Cash used in operating activities for the twelve months ended June 30, 2010 was $10,033,000 compared to $10,554,000 for 2009.
Cash Requirements
We intend to allocate our current funds of approximately $9 million to continue the development of the triphendiol or NV-143 clinical program, continuing pre-clinical research on NV-128 and to complete the analysis of the OVATURE data analysis.
Specifically we intend to:
| · | Commence the clinical development of the drug candidate triphendiol or NV-143 in the U.S. and Australia for which an IND has been granted by the FDA. Triphendiol was designated by the FDA as an Orphan Drug for treatment of pancreatic cancer, bile duct cancer, and late stage melanoma; |
| · | Continue the pre-clinical development of NV-128 necessary to file an IND with the FDA. |
Ongoing operations, including the conduct of the pre-clinical and clinical trial program, will continue to consume cash resources without generating revenues. We will require additional financing to fund our operations in the future. We cannot assure you that we will be able to raise the funds, necessary to fund our programs, on favorable terms to us or at all.
Payments to Novogen
Future payments to Novogen under the terms of the Phenoxodiol License Agreement, as amended, and the License Agreement for Triphendiol and NV-143, and the License agreement for NV-128 are detailed in Note 7 of the financial statements “Related Party Transactions”.
We will also be required to make payments to Novogen under the Services Agreement and Manufacturing License and Supply Agreement.
We do not intend to incur any significant capital expenditures in the foreseeable future.
Results of Operations
Summary of Revenue and Expenses
The following table provides a summary of revenues and expenses to supplement the more detailed discussions below:
| Revenues | | Years Ended June 30, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (in thousands) | |
| Interest and other income | | $ | 84 | | | $ | 228 | | | $ | 674 | |
Total revenues | | | 84 | | | | 228 | | | | 674 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Research and development expenses | | Years Ended June 30, | |
| | | | 2010 | | | | 2009 | | | | 2008 | |
| | | (in thousands) | |
| Clinical trial study costs | | $ | (1,191 | ) | | $ | (5,719 | ) | | $ | (5,928 | ) |
| Drug/manufacturing scale-up costs | | | (475 | ) | | | (198 | ) | | | (1,310 | ) |
| Research and development service charge | | | (2,279 | ) | | | (1,456 | ) | | | (2,065 | ) |
| Other | | | (86 | ) | | | (404 | ) | | | (22 | ) |
Total Research and Development Costs | | | (4,031 | ) | | | (7,777 | ) | | | (9,325 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| License Fees | | Years Ended June 30, | |
| | | | 2010 | | | | 2009 | | | | 2008 | |
| | | (in thousands) | |
| License Fees | | | (1,500 | ) | | | (2,000 | ) | | | (1,000 | ) |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Selling, general and administrative expenses | | Years Ended June 30, | |
| | | | 2010 | | | | 2009 | | | | 2008 | |
| | | (in thousands) | |
| Legal and professional fees | | $ | (513 | ) | | $ | (479 | ) | | $ | (527 | ) |
| Administrative service charge | | | (865 | ) | | | (808 | ) | | | (989 | ) |
| Share based payment | | | (64 | ) | | | (90 | ) | | | - | |
| Other | | | (1,006 | ) | | | (253 | ) | | | (1,240 | ) |
Total selling, general and administrative expenses | | | (2,448 | ) | | | (1,630 | ) | | | (2,756 | ) |
Year Ended June 30, 2010 Compared to the Year Ended June 30, 2009
We recorded a consolidated loss of $7,896,000 and $11,180,000 for the years ended June 30, 2010 and 2009, respectively.
Revenues: We received interest on cash assets and cash equivalents of $84,000 for the year ended June 30, 2010 versus $228,000 for the year ended June 30, 2009. This decrease was due to lower cash balances and lower interest rates earned by our deposits.
Research and Development: Research and development expenses decreased $3,746,000 to $4,031,000 for the year ended June 30, 2010 compared to $7,777,000 for the year ended June 30, 2009. This decrease was primarily due to lower spending, following the termination of enrollment of the OVATURE Phase III clinical trial, which is currently being finalized, partially offset by increased costs associated with the development of NV-196 and NV-128.
License Fees: Milestone license fees of $1,500,000 were expensed in the year ended June 30, 2010 under the terms of the License Agreement for NV-128. Milestone license fees of $2,000,000 were expensed in the year ended June 30, 2009 under the terms of the License Agreement for Triphendiol and NV-143. No other milestone licence fees were due under any of the licence agreements with Novogen.
Selling, General and Administrative: Selling, general and administrative expenses increased by $818,000 to $2,448,000 for the year ended June 30, 2010 compared to $1,630,000 for the year ended June 30, 2009. The increase relates to a number of factors including costs associated with the reverse share split and costs incurred in the recruitment of a new Chief Executive Officer, which were not incurred in the previous corresponding period. Also contributing to the increase in the year ended June 30, 2010 compared to the previous corresponding period are the remuneration expenses for the new employees, which include the Chief Executive Officer and Chief Financial Officer.
Foreign exchange gains/(losses) are included in selling, general and administrative expenses and occur when revaluing cash denominated in foreign currencies and upon consolidation of our wholly owned subsidiary MEPL. MEPL uses U.S. dollars as its functional currency and also engages in transactions in foreign currencies. Further, MEPL’s accounts and financial statements are denominated in Australian dollars. Translation of MEPL’s financial statements into U.S. dollars did not have a material impact on our financial position. At June 30, 2010, we had not established a foreign currency hedging program. Net foreign exchange losses during the year ended June 30, 2010 were $141,000 compared with net exchange gains of $242,000 during the year ended June 30, 2009.
Year Ended June 30, 2009 Compared to the Year Ended June 30, 2008
We recorded a consolidated loss of $11,180,000 and $12,410,000 for the years ended June 30, 2009 and 2008, respectively.
Revenues: We received interest on cash assets and cash equivalents of $228,000 for the year ended June 30, 2009 versus $674,000 for the year ended June 30, 2008. This decrease was due to lower interest rates on cash investments.
Research and Development: Research and development expenses decreased $1,548,000 to $7,777,000 for the year ended June 30, 2009 compared to $9,325,000 for the year ended June 30, 2008. This decrease was primarily due to a reduction in the cost of drug for the OVATURE clinical trail which was mostly manufactured in prior years. The research and development service charge from Novogen decreased for the year ended June 30, 2009, due to favorable currency movements in the U.S. dollar compared to the Australian dollar as these charges are denominated in Australian dollars.
Also included in clinical trial study costs are the expenses associated with the termination of the enrollment in the OVATURE Phase III clinical trial.
License Fees: Milestone license fees of $2,000,000 were expensed in the year ended June 30, 2009 under the terms of the License Agreement for Triphendiol and NV-143. This license fee was due on the date of enrollment of the first clinical trial subject in a Phase II clinical trial of the licensed product. As this event did not occur, the payment was due and paid on June 30, 2009.
Selling, General and Administrative: Selling, general and administrative expenses decreased by $1,126,000 to $1,630,000 for the year ended June 30, 2009 compared to $2,756,000 for the year ended June 30, 2008. The decrease was due primarily to our decision to conserve cash and reduce expenses associated with public relations, travelling expenses and reduced administration service fees paid to Novogen.
Foreign exchange gains/(losses) are included in selling, general and administrative expenses and occur when revaluing cash denominated in foreign currencies and upon consolidation of our wholly owned subsidiary MEPL. MEPL uses U.S. dollars as its functional currency and also engages in transactions in foreign currencies. Further, MEPL’s accounts and financial statements are denominated in Australian dollars. Translation of MEPL’s financial statements into U.S. dollars did not have a material impact on our financial position. At June 30, 2009, we had not established a foreign currency hedging program. Net foreign exchange gains during the twelve months ended June 30, 2009 were $242,000 compared with net exchange losses of $255,000 during the twelve months ended June 30, 2008.
Off-Balance Sheet Arrangements
We do not currently have any off-balance sheet arrangements.
Contractual Obligations
For details of our contractual obligations at June 30, 2010 see Note 5 to the financial statements “Expenditure Commitments”.
Critical Accounting Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the amounts reported in the financial statements and the accompanying notes. Actual results could differ from those estimates.
Clinical Trials Expenses
Estimates have been used in determining the expense liability under certain clinical trial contracts where services have been performed but not yet invoiced. The actual costs of those services could differ in amount and timing from the estimates used in completing the financial statements.
Generally the costs, and therefore estimates, associated with clinical trial contracts are based on the number of patients, drug administration cycles, the type of treatment and the outcome being measured. The length of time before actual amounts can be determined will vary depending on length of the patient cycles and the timing of the invoices by the clinical trial partners.
Clinical trial expenses of $1,191,000 have been included in the financial statements for the year ended June 30, 2010, of which $731,000 has been accrued at June 30, 2010. These estimates are based on the number of patients in each trial and the drug administration cycle.
At June 30, 2009 we had accrued $1,181,000 in relation to claims received for clinical trial expenses in connection with the termination of enrollment into the OVATURE Phase III clinical trial. Following negotiations we have paid $849,000 in final settlement of these claims.
Stock Based Compensation
On December 9, 2008, we adopted the 2008 Stock Omnibus Equity Compensation Plan and cancelled the Marshall Edwards, Inc. Share Option Plan (the “Share Option Plan”). No options were issued under the Share Option Plan. The Plan provides for the issuance of a maximum of 700,000 shares of common stock in connection with the grant of options and/or other stock-based or stock-denominated awards to our non-employee directors, officers, employees and advisors. To date, we have issued options exercisable for 78,463 shares of common stock under the Plan.
We account for stock based payments by estimating the fair value of the options issued. The costs of these equity-settled transactions are determined using a binomial model to calculate the fair value at the date on which they are granted. Warrants representing 4,608 warrant shares were issued to Mr. John O’Connor on July 30, 2008, in consideration for investor relations services rendered. Stock options representing 5,000 shares of common stock were issued to Associate Professor Gil Mor of Yale University on January 28, 2009, in recognition of his contribution to the development of phenoxodiol under the Plan. Pursuant to the terms of Dr. Gold’s Employment Letter, Dr. Gold has received options to purchase 220,390 shares of the Company’s common stock in two separate tranches, as an inducement to become a new employee of the Company, which in accordance with Nasdaq rule 5635(c), does not require stockholder approval. The first tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold upon his appointment as President and Chief Executive Officer on April 23, 2010, with the second tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold on June 7, 2010 following the public release of the Company’s OVATURE study results. Dr. Gold’s options are issued outside the plan. Pursuant to the terms of Mr. Zech’s Employment Letter, Mr. Zech has received options to purchase 73,463 shares of the Company’s common stock, under the Plan, which were granted on June 18, 2010.
With respect to the fair value of the stock based compensation described above the following assumptions were used:
| | July 30, 2008 | January 28, 2009 | April 23, 2010 | June 7, 2010 | June 18, 2010 |
| Dividend yield | 0% | 0% | 0% | 0% | 0% | |
| Expected volatility | 81% | 111% | 132% | 135% | 136% | |
| Historical volatility | 81% | 111% | 132% | 135% | 136% | |
| Risk-free interest rate | 3.36% | 1.70% | 2.61% | 1.95% | 2.04% | |
| Expected life | 5 years | 5 years | 5 years | 5 years | 5 years | |
| Fair value | $14.10 | $5.00 | $4.38 | $1.63 | $1.33 | |
The dividend yield reflects the assumption that the current dividend payout, which is zero, will continue with no anticipated increases. The expected life of the stock based compensation is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
Item 7a. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
Our exposure to market interest rates relates primarily to the investments of cash balances.
We have cash reserves held primarily in U.S. and Australian dollars and we place funds on deposit with financial institutions and are generally at call.
We do not use derivative financial instruments. We place our cash deposits with high credit quality financial institutions, and, by policy, limit the amount of credit exposure to any single counter-party. We are adverse to principal loss and we ensure the safety and preservation of our invested funds by limiting default risk, market risk and reinvestment risk.
We seek to mitigate default risk by depositing funds with high credit quality financial institutions and by constantly positioning its portfolio to respond appropriately to a significant reduction in a credit rating of any financial institution.
We have no interest rate exposure due to rate changes for long-term debt.
We do not consider the effects of interest rate movements to be a material risk to our financial condition.
Foreign Currency Risk
We conduct a portion of our business in various currencies, primarily in U.S. dollars and Australian dollars, Euros and British pounds. At June 30, 2010, we had not established a foreign currency hedging program. Net foreign exchange losses during the twelve months ended June 30, 2010 were $141,000 compared with net exchange gains of $242,000 during the twelve months ended June 30, 2009. Foreign exchange gains and losses occur upon consolidation of MEPL, which uses U.S. dollars as its functional currency and also engages in transactions in foreign currencies. MEPL’s accounts are denominated in Australian dollars. Translation of MEPL’s financial statements into U.S. dollars did not have a material impact on our financial position.
We do not consider the effects of foreign currency movements to be a material risk to our financial condition.
Item 8. Financial Statements and Supplementary Data
Marshall Edwards, Inc
Index to Financial Statements
Report of BDO Audit (NSW - VIC) Pty Ltd Independent Registered Public Accounting Firm
Consolidated Balance Sheets
Consolidated Statements of Operations
Consolidated Statements of Cash Flows
Consolidated Statements of Stockholders’ Equity
Notes to Consolidated Financial Statements
Board of Directors
Marshall Edwards, Inc.
We have audited the accompanying consolidated balance sheet of Marshall Edwards, Inc. (a development stage company) as of June 30, 2010 and 2009, and the related statements of operations, stockholders’ equity, and cash flows for each of the years in the three year period ended June 30, 2010, and for the period from December 1, 2000 (inception) through June 30, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis , evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Marshall Edwards, Inc. at June 30, 2010 and 2009, and the consolidated results of its operations and its cash flows each of the years in the three year period ended June 30, 2010 and the period from December 1, 2000 (inception) through June 30, 2010, in conformity with accounting principles generally accepted in the United States of America.
/s/ BDO
BDO Audit (NSW-VIC) Pty Ltd
Sydney, NSW, Australia
August 26, 2010
MARSHALL EDWARDS, INC.
(A Development Stage Company)
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
| | | June 30, | | | June 30, | |
| | | 2010 | | | 2009 | |
| | | | | | | |
| | | | | | | |
| ASSETS | | | | | | |
| Current assets | | | | | | |
Cash and cash equivalents | | $ | 9,031 | | | $ | 19,067 | |
Prepaid expenses and other current assets | | | 102 | | | | 289 | |
Total current assets | | | 9,133 | | | | 19,356 | |
| | | | | | | | | |
| Plant and equipment, net | | | 3 | | | | - | |
Total assets | | $ | 9,136 | | | $ | 19,356 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | | |
| Current liabilities | | | | | | | | |
Accounts payable | | $ | 529 | | | $ | 736 | |
Accrued expenses | | | 925 | | | | 3,186 | |
Amount due to related company | | | 301 | | | | 221 | |
Total current liabilities | | | 1,755 | | | | 4,143 | |
| | | | | | | | | |
| Stockholders' equity: | | | | | | | | |
| Preferred stock, $0.01 par value, authorized 100,000 shares, | | | | | | | | |
none outstanding | | | - | | | | - | |
| Common stock, $ 0.00000002 par value, 113,000,000 authorized | | | | | | | | |
shares; shares issued and outstanding: 7,346,324 at | | | | | | | | |
June 30, 2010 and 7,346,324 at June 30, 2009 | | | - | | | | - | |
| Additional paid-in capital | | | 78,188 | | | | 78,124 | |
| Deficit accumulated during development stage | | | (70,807 | ) | | | (62,911 | ) |
| Total stockholders' equity | | | 7,381 | | | | 15,213 | |
Total liabilities and stockholders' equity | | $ | 9,136 | | | $ | 19,356 | |
MARSHALL EDWARDS, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except share and per share data)
| | | | | | | | | | | | | |
| | | Years Ended June 30, | | | Period from December 1, 2000 (Inception) through June 30, | |
| | | 2010 | | | 2009 | | | 2008 | | | 2010 | |
| | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | |
| Interest and other income | | $ | 84 | | | $ | 228 | | | $ | 674 | | | $ | 2,730 | |
| Total revenues | | | 84 | | | | 228 | | | | 674 | | | | 2,730 | |
| | | | | | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | (4,031 | ) | | | (7,777 | ) | | | (9,325 | ) | | | (37,074 | ) |
| License fees | | | (1,500 | ) | | | (2,000 | ) | | | (1,000 | ) | | | (21,500 | ) |
| Selling, general and administrative | | | (2,448 | ) | | | (1,630 | ) | | | (2,756 | ) | | | (14,955 | ) |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | (7,979 | ) | | | (11,407 | ) | | | (13,081 | ) | | | (73,529 | ) |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (7,895 | ) | | | (11,179 | ) | | | (12,407 | ) | | | (70,799 | ) |
| Income tax expense | | | (1 | ) | | | (1 | ) | | | (3 | ) | | | (8 | ) |
| Net loss arising during development stage | | $ | (7,896 | ) | | $ | (11,180 | ) | | $ | (12,410 | ) | | $ | (70,807 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss per common share: | | | | | | | | | | | | | | | | |
| Basic and diluted | | $ | (1.07 | ) | | $ | (1.53 | ) | | $ | (1.82 | ) | | | | |
| | | | | | | | | | | | | | | | | |
| Weighted average common shares outstanding | | | 7,346,324 | | | | 7,307,184 | | | | 6,830,257 | | | | | |
See accompanying notes.
MARSHALL EDWARDS, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | | |
| | | Years Ended June 30, | | | Period from December 1, 2000 (Inception) through June 30, | |
| | | 2010 | | | 2009 | | | 2008 | | | 2010 | |
| | | | | | | | | | | | | |
| Operating activities | | | | | | | | | | | | |
| Net loss arising during development stage | | | (7,896 | ) | | | (11,180 | ) | | | (12,410 | ) | | | (70,807 | ) |
| Adjustments to reconcile net loss to net cash | | | | | | | | | | | | | | | | |
| used in operating activities: | | | | | | | | | | | | | | | | |
| Share based payments | | | 64 | | | | 90 | | | | - | | | | 1,796 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | |
| Prepaid expenses and other current assets | | | 187 | | | | (164 | ) | | | (18 | ) | | | (102 | ) |
| Accounts payable | | | (207 | ) | | | (394 | ) | | | (67 | ) | | | 529 | |
| Accrued expenses | | | (2,261 | ) | | | 1,302 | | | | 900 | | | | 925 | |
| Amounts due to related company | | | 80 | | | | (208 | ) | | | 97 | | | | 301 | |
| Net cash used in operating activities | | | (10,033 | ) | | | (10,554 | ) | | | (11,498 | ) | | | (67,358 | ) |
| | | | | | | | | | | | | | | | | |
| Investing activities | | | | | | | | | | | | | | | | |
| Purchases of plant and equipment | | | (3 | ) | | | - | | | | - | | | | (3 | ) |
| Net cash used in investing activities | | | (3 | ) | | | - | | | | - | | | | (3 | ) |
| | | | | | | | | | | | | | | | | |
| Financing activities | | | | | | | | | | | | | | | | |
| Net proceeds from issuance of Common Stock | | | - | | | | 9,878 | | | | 15,193 | | | | 76,622 | |
| Deferred Offering Costs | | | - | | | | - | | | | (110 | ) | | | (230 | ) |
| Net cash used in financing activities | | | - | | | | 9,878 | | | | 15,083 | | | | 76,392 | |
| Net increase/(decrease) in cash and cash | | | | | | | | | | | | | | | | |
| equivalents | | | (10,036 | ) | | | (676 | ) | | | 3,585 | | | | 9,031 | |
| Cash and cash equivalents at beginning of period | | | 19,067 | | | | 19,743 | | | | 16,158 | | | | - | |
| Cash and cash equivalents at end of period | | | 9,031 | | | | 19,067 | | | | 19,743 | | | | 9,031 | |
| Income taxes paid | | | (1 | ) | | | (1 | ) | | | (3 | ) | | | (8 | ) |
See accompanying notes.
MARSHALL EDWARDS, INC.
(A Development Stage Company)
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
(In thousands, except share data)
| | | Common Stock | | | Additional paid in capital | | | Deficit accumulated during development stage | | | Accumulated other comprehensive income/(loss) | | | Total | |
| | | (shares) | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Balance June 30, 2001 | | | 4,950,000 | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Net loss arising during development stage | | | | | | | | | | | (123 | ) | | | | | | | (123 | ) |
| Common Stock issued May 22, 2002 | | | | | | | | | | | | | | | | | | | | |
| (including 2,523,000 warrants) | | | 252,300 | | | | 9,022 | | | | | | | | | | | | 9,022 | |
| Balance at June 30, 2002 | | | 5,202,300 | | | | 9,022 | | | | (123 | ) | | | - | | | | 8,899 | |
| Net loss arising during development stage | | | | | | | | | | | (3,033 | ) | | | | | | | (3,033 | ) |
| Foreign currency translation adjustments | | | | | | | | | | | | | | | 31 | | | | 31 | |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (3,002 | ) |
| Common Stock issued June 26, 2003 | | | 900 | | | | 36 | | | | | | | | | | | | 36 | |
| Balance at June 30, 2003 | | | 5,203,200 | | | | 9,058 | | | | (3,156 | ) | | | 31 | | | | 5,933 | |
| Net loss arising during development stage | | | | | | | | | | | (8,538 | ) | | | | | | | (8,538 | ) |
| Foreign currency translation adjustments | | | | | | | | | | | | | | | (31 | ) | | | (31 | ) |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (8,569 | ) |
| Common Stock issued November 30, 2003 | | | 251,400 | | | | 10,056 | | | | | | | | | | | | 10,056 | |
| Common Stock issued December 18, 2003 | | | | | | | | | | | | | | | | | | | | |
| (including 2,392,000 warrants) | | | 239,200 | | | | 15,522 | | | | | | | | | | | | 15,522 | |
| Balance at June 30, 2004 | | | 5,693,800 | | | | 34,636 | | | | (11,694 | ) | | | - | | | | 22,942 | |
| Net loss arising during development stage | | | | | | | | | | | (6,421 | ) | | | | | | | (6,421 | ) |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (6,421 | ) |
| Balance at June 30, 2005 | | | 5,693,800 | | | | 34,636 | | | | (18,115 | ) | | | - | | | | 16,521 | |
| Net loss arising during development stage | | | | | | | | | | | (7,386 | ) | | | | | | | (7,386 | ) |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (7,386 | ) |
| Balance at June 30, 2006 | | | 5,693,800 | | | | 34,636 | | | | (25,501 | ) | | | - | | | | 9,135 | |
| Net loss arising during development stage | | | | | | | | | | | (13,820 | ) | | | | | | | (13,820 | ) |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (13,820 | ) |
| Common Stock issued July 11, 2006 | | | 632,931 | | | | 16,820 | | | | | | | | | | | | 16,820 | |
| Shares issued as share-based payment | | | 12,363 | | | | 443 | | | | | | | | | | | | 443 | |
| Warrants issued as share-based payment | | | | | | | 1,199 | | | | | | | | | | | | 1,199 | |
| Balance at June 30, 2007 | | | 6,339,094 | | | | 53,098 | | | | (39,321 | ) | | | - | | | | 13,777 | |
| Net loss arising during development stage | | | | | | | | | | | (12,410 | ) | | | | | | | (12,410 | ) |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (12,410 | ) |
| Common Stock issued August 6, 2007 | | | 546,400 | | | | 14,727 | | | | | | | | | | | | 14,727 | |
| Warrants issued as share-based payment (refer Note 8) | | | | | | | 441 | | | | | | | | | | | | 441 | |
| Balance at June 30, 2008 | | | 6,885,494 | | | | 68,266 | | | | (51,731 | ) | | | - | | | | 16,535 | |
| Net loss arising during development stage | | | | | | | | | | | (11,180 | ) | | | | | | | (11,180 | ) |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (11,180 | ) |
| Common Stock issued July 31, 2008 | | | 460,830 | | | | 9,768 | | | | | | | | | | | | 9,768 | |
| Warrants issued as share-based payment (refer Note 8) | | | | | | | 90 | | | | | | | | | | | | 90 | |
| Balance at June 30, 2009 | | | 7,346,324 | | | | 78,124 | | | | (62,911 | ) | | | - | | | | 15,213 | |
| Net loss arising during development stage | | | | | | | | | | | (7,896 | ) | | | | | | | (7,896 | ) |
| Comprehensive Loss | | | | | | | | | | | | | | | | | | | (7,896 | ) |
| Warrants issued as share-based payment (refer Note 8) | | | | | | | 64 | | | | | | | | | | | | 64 | |
| | | | | | | | | | | | | | | | | | |
| Balance at June 30, 2010 | | | 7,346,324 | | | $ | 78,188 | | | $ | (70,807 | ) | | $ | - | | | $ | 7,381 | |
See accompanying notes.
MARSHALL EDWARDS, INC.
(A Development Stage Company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2010
1. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of MEI and its wholly-owned subsidiary MEPL. Significant intercompany accounts and transactions have been eliminated on consolidation.
Estimates
The preparation of the consolidated financial statements, in conformity with accounting principles generally accepted in the U.S., requires management to make estimates and assumptions that affect the amounts reported in the financial statements and the accompanying notes. Actual results could differ from those estimates.
Revenue Recognition
Interest
The only revenue earned to date is interest on cash balances, which is recognized on an accruals basis.
Cash and Cash Equivalents
Cash on hand and in banks and short-term deposits is stated at its nominal value. The Company considers all highly liquid investments, with a maturity of three months or less when purchased, to be cash equivalents. Highly liquid investments with stated maturities of greater than three months are classified as short-term investments. The Company’s cash, held in the U.S., is deposited in financial institutions that are FDIC insured. These deposits are in excess of the FDIC insurance limits. The Company also holds cash with Australian financial institutions. Cash deposits held in Australian banks are guaranteed by the Australian Government up to a maximum amount of A$1 million per account.
Income Taxes
Income taxes have been provided for using the liability method. Under this method, deferred tax assets and liabilities are recognized and measured using enacted tax rates in effect for the year in which the differences are expected to be recognized. Valuation allowances are established against the recorded deferred income tax assets to the extent that management believes that it is more likely than not that a portion of the deferred income tax assets are not realizable. There is a full valuation allowance against net deferred tax assets.
The Company accounts for any uncertain tax position by using a two step approach. Step one, recognition, requires a company to determine if the weight of available evidence indicates that a tax position is more likely than not to be sustained upon audit, including resolution of related appeals or litigation processes, if any. Step two, measurement, is based on the largest amount of benefit, which is more likely than not to be realized upon ultimate settlement. Additionally, tax positions for which the timing of the ultimate resolution is uncertain are recognized as long term liabilities.
The Company’s major tax jurisdictions are the U.S. and Australia and its tax years since inception remain subject to examination by the appropriate governmental agencies in those jurisdictions due to its tax loss position.
Fair Value of Financial Instruments
The carrying amounts of the Company’s financial instruments, including cash and cash equivalents and accounts payable, approximate fair value. All cash and cash equivalents are classified as level 1 as defined by the fair value hierarchy.
Foreign Currency Translation
The financial statements of MEPL have been translated into U.S. dollars. Assets and liabilities are translated into U.S. dollars using the exchange rates in effect at the balance sheet date. Income statement amounts have been translated using the average exchange rate for the periods. Realized gains and losses from foreign currency transactions are reflected in the consolidated statements of operations.
Translation of MEPL’s financial statements into U.S. dollars does not have a material impact on the Company’s financial position.
Research and Development Expenses
Research and development expenses relate primarily to the cost of conducting human clinical and pre-clinical research of the licensed cancer compounds. Research and development costs are charged to earnings in the period incurred.
Clinical development costs are a significant component of research and development expenses. Estimates have been used in determining the expense liability under certain clinical trial contracts where services have been performed but not yet invoiced. The actual costs of those services could differ in amount and timing from the estimates used in completing the financial statements.
License Fees
Costs incurred related to the acquisition or licensing of products that have not yet received regulatory approval to be marketed, or that are not commercially viable and ready for use or have no alternative future use, are charged to earnings in the period incurred.
The license agreements with Novogen may be cancelled without penalty by MEPL by giving three months’ notice. Therefore license fees due under these license agreements are recognised as an expense when the milestone event occurs.
Stock-Based Compensation
The Company’s 2008 Stock Omnibus Equity Compensation Plan provides for the grant of options to the Company’s directors, employees, employees of the Company’s affiliates and certain of the Company’s contractors and consultants.
The Company recognizes the cost of goods acquired or the expense for services received in a share-based payment transaction when it obtains the goods or as services are received. The Company recognizes a corresponding increase in equity or a liability depending on the classification of the share-based instrument granted.
Basic and Diluted Loss Per Share
In computing basic earnings or loss per share, the dilutive effect of stock options and warrants are excluded, whereas for diluted earnings or loss per share they are included unless the effect is anti-dilutive.
Plant and Equipment
Plant and equipment are stated at cost. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets, which range from 2.5 to 7 years. Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the respective assets or the lease term, whichever is shorter.
Stockholders’ Equity
Ordinary share capital is recognized at the fair value of the consideration received by the Company. Any transaction costs arising on the issue of shares are recognized directly in equity as a reduction in the share proceeds received.
Deferred Offering Costs
Where costs associated with a capital raising have been incurred at balance date and it is probable that the capital raising will be successfully completed after balance date, such costs are deferred and offset against the proceeds subsequently received from the capital raising.
Recent Accounting Standards
During the quarter ended September 30, 2009 the Company adopted ASC 105, “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles”. This establishes the Financial Accounting Standards Board (FASB) Accounting Standards Codification as the only source of authoritative accounting principles recognized by the FASB to be applied in the preparation of financial statements in conformity with GAAP.
In May 2009, the FASB issued guidance within ASC 855, “Subsequent Events” (formerly Statement of Financial Accounting Standards (SFAS) No. 165, “Subsequent Events”) and subsequently updated this guidance in February 2010. This guidance establishes general standards for the accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. The adoption of this guidance did not have an impact on the Company’s consolidated financial statements.
In January 2010, the FASB issued ASU No. 2010-06, “Improving Disclosures about Fair Value Measurements”, an amendment to ASC 820, “Fair Value Measurements and Disclosures”. The standard requires disclosure for transfers in and out of Level 1 and Level 2, as well as the disclosure of Level 3 activity on a gross, rather than net, basis.
The guidance also requires enhancements to certain existing disclosures. The amendments will be effective as of the beginning of fiscal 2011, except for the new requirements around Level 3 activity, which is deferred until the beginning of fiscal 2012. The guidance is not expected to have an impact on the Company’s consolidated financial statements.
2. Income Taxes
Loss from operations consists of the following jurisdictions:
| | | Year ended June 30, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (in thousands $) | |
| Domestic | | | (66,352 | ) | | | (452 | ) | | | (448 | ) |
| Foreign | | | (6,873 | ) | | | (10,727 | ) | | | (11,959 | ) |
| | | | (73,225 | ) | | | (11,179 | ) | | | (12,407 | ) |
| Elimination on consolidation | | | 65,330 | | | | - | | | | - | |
| Loss from operations | | | (7,895 | ) | | | (11,179 | ) | | | (12,407 | ) |
The reconciliation of income tax computed at the U.S. federal statutory tax rates to income tax expense attributable to loss arising during development stage is:
| | | Year ended June 30, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (in thousands $) | | | % | | | (in thousands $) | | | % | | | (in thousands $) | | | % | |
| | | | | | | | | | | | | | | | | | | |
| Tax at US statutory rates | | | 2,684 | | | | 34 | | | | 3,801 | | | | 34 | | | | 4,342 | | | | 35 | |
| Australian tax | | | (275 | ) | | | (3 | ) | | | (429 | ) | | | (4 | ) | | | (598 | ) | | | (5 | ) |
| R&D Tax concession | | | 428 | | | | 5 | | | | 504 | | | | 5 | | | | 666 | | | | 5 | |
| Change in valuation allowance | | | (2,838 | ) | | | (36 | ) | | | (3,877 | ) | | | (35 | ) | | | (4,413 | ) | | | (35 | ) |
| | | | (1 | ) | | | - | | | | (1 | ) | | | - | | | | (3 | ) | | | - | |
Deferred tax liabilities and assets are comprised of the following:
| | Year ended June 30, | |
| | 2010 | 2009 | |
| | (in thousands $) | |
| Deferred tax liabilities | | | |
| Unrealised Foreign Exchange Gain | (46) | (74) | |
| Total deferred tax liabilities | (46) | (74) | |
| | | | |
| Deferred tax assets | | | |
| Tax carried forward losses | 24,230 | 19,550 | |
| Share based payments | 627 | 605 | |
| Unrealised Foreign Exchange Loss | 89 | 0 | |
| Consultant and other accruals | 218 | 939 | |
| Total deferred tax assets | 25,164 | 21,094 | |
| | | | |
| Valuation allowance for deferred tax assets | (25,118) | (21,020) | |
Management evaluates the recoverability of the deferred tax asset and the amount of the required valuation allowance. Due to the uncertainty surrounding the realization of the tax deductions in future tax returns, the Company has recorded a valuation allowance against its net deferred tax asset at June 30, 2010 and 2009. At such time as it is determined that it is more likely than not that the deferred tax assets will be realized, the valuation allowance will be reduced.
There was no benefit from income taxes recorded for the period from December 1, 2000 (inception) to June 30, 2010 due to the Company’s inability to recognize the benefit of net operating losses. The Company had federal net operating loss carry forwards of approximately $3,263,000 at June 30, 2010. The federal net operating losses will begin to expire in 2022.
Foreign tax losses of approximately $77,043,000 at June 30, 2010, may be carried forward indefinitely.
3. Loss Per Share
The following table sets forth the computation of basic and diluted net loss per common share:
| | Years ended June 30, |
| | 2010 | 2009 | 2008 | |
| | (In Thousands, except share data) |
| Numerator | | | | |
| Net loss arising during development stage | (7,896) | (11,180) | (12,410) | |
| Numerator for diluted earnings per share | $ (7,896) | $ (11,180) | $ (12,410) | |
| | | | | |
| Denominator | | | | |
| Denominator for basic earnings per share - | | | | |
| Weighted average number of shares used in computing net loss per share, basic and diluted. | 7,346,324 | 7,307,184 | 6,830,257 | |
| Effect of dilutive securities | - | - | - | |
| Dilutive potential common shares | 7,346,324 | 7,307,184 | 6,830,257 | |
| | | | | |
| | | | | |
| Basic and Diluted net loss per share | $ (1.07) | $ (1.53) | $ (1.82) | |
During the period presented the Company had warrants and options outstanding that could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share as the effect would have been anti-dilutive. Since the Company has a loss for all periods presented, diluted and basic earnings per share are the same.
4. Stock Based Compensation
The following table illustrates the number (No.) and weighted average exercise price (WAEP) of, and movements in, warrants and options over common shares issued during the year:
| | | 2010 | 2009 | |
| | | No. | WAEP | No. | WAEP | |
| Outstanding at the beginning of the year | 534,528 | $39.27 | 524,920 | $39.74 | |
| Granted | | 293,853 | $2.97 | 9,608 | $13.69 | |
| Forfeited | | - | N/A | - | N/A | |
| Exercised | | - | N/A | - | N/A | |
| Expired | | - | N/A | - | N/A | |
| Outstanding at the end of the year | 828,381 | $26.39 | 534,528 | $39.27 | |
Exercisable at the end of the year | 534,528 | $39.27 | 534,528 | $39.27 | |
The amount of compensation expense, for existing options, to be recognized in future years is $697,000.
The outstanding warrants and options consist of the following potential common shares:
| | As at June 30, |
| | 2010 | | 2009 | | 2008 |
| | (Number of warrant shares) |
| Warrants exercisable prior to July 11, 2010 at an exercise price of $43.50 | 281,525 | | 281,525 | | 281,525 |
| Warrants exercisable prior to August 6, 2012 at an exercise price of $36.00 | 218,559 | | 218,559 | | 218,559 |
| Warrants exercisable prior to August 6, 2012 at an exercise price of $30.00 | 24,836 | | 24,836 | | 24,836 |
| Warrants exercisable prior to July 30, 2013 at an exercise price of $21.70 | 4,608 | | 4,608 | | - |
| Options exercisable prior to January 28, 2014 at an exercise price of $6.30 | 5,000 | | 5,000 | | - |
| Options exercisable prior to April 23, 2015 at an exercise price of $5.05 | 110,195 | | - | | - |
| Options exercisable prior to June 7, 2015 at an exercise price of $1.86 | 110,195 | | - | | - |
| Options exercisable prior to June 18, 2015 at an exercise price of $1.52 | 73,463 | | - | | - |
| Common shares issuable upon exercise of outstanding warrants or options | 828,381 | | 534,528 | | 524,920 |
With respect to the fair value of the stock based compensation described above the following assumptions were used:
| | July 30, 2008 | January 28, 2009 | April 23, 2010 | June 7, 2010 | June 18, 2010 |
| Dividend yield | 0% | 0% | 0% | 0% | 0% | |
| Expected volatility | 81% | 111% | 132% | 135% | 136% | |
| Historical volatility | 81% | 111% | 132% | 135% | 136% | |
| Risk-free interest rate | 3.36% | 1.70% | 2.61% | 1.95% | 2.04% | |
| Expected life | 5 years | 5 years | 5 years | 5 years | 5 years | |
| Fair value | $14.10 | $5.00 | $4.38 | $1.63 | $1.33 | |
The dividend yield reflects the assumption that the current dividend payout, which is zero, will continue with no anticipated increases. The expected life of the stock based compensation is based on historical data and is not necessarily indicative of exercise patterns that may occur. The expected volatility reflects the assumption that the historical volatility is indicative of future trends, which may also not necessarily be the actual outcome.
5. Expenditure Commitments and Contingencies
At June 30, 2010, the Company had contractual obligations for the conduct of clinical trials, pre- clinical research and development and manufacturing process development of approximately $651,000. Of the expenditure commitments, clinical trial amounts are based on the assumption that all patients enrolled in clinical trials will complete the maximum number of allowed treatment cycles. At June 30, 2010, the Company also had contractual obligations in respect of the leased premises of approximately $313,000. The contracted obligations are expected to be incurred as follows:
| (In thousands) | | | | | Payment due by period | |
| Contractual Obligations | | Total | | | less than 1 Year | | | 1 - 3 Years | | | 3 - 5 Years | | | More than 5 Years | |
| | | | | | | | | | | | | | | | |
| Operating Lease Obligations | | $ | 313 | | | $ | 91 | | | $ | 125 | | | $ | 97 | | | $ | - | |
| Purchase Obligations | | $ | 651 | | | $ | 651 | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 964 | | | $ | 742 | | | $ | 125 | | | $ | 97 | | | $ | - | |
No amounts have been included for future payments to Novogen which may arise in connection with the Phenoxodiol License Agreement, the License Agreement for Triphendiol and NV-143, the Services Agreement or the Manufacturing License and Supply Agreement as future payments under the terms of the agreements are subject to termination provisions. The terms of the agreements, including future payments, are detailed in Note 7 “Related Party Transactions.”
The Company is not currently a party to any material legal proceedings.
The Company’s restated certificate of incorporation provides that it will indemnify Novogen in connection with certain actions brought against Novogen by any of the Company’s stockholders or any other person.
Pursuant to the terms of a Guarantee and Indemnity Agreement, the Company has guaranteed the payment and performance of the obligations of MEPL to Novogen and its subsidiaries, Novogen Laboratories Pty Limited and Novogen Research Pty Limited, under the Phenoxodiol License Agreement, the Manufacturing License and Supply Agreement and the Services Agreement. Novogen has guaranteed the performance of the obligations of Novogen Research Pty Limited under the Phenoxodiol License Agreement and the obligations of Novogen Laboratories Pty Limited under the Manufacturing License and Supply Agreement to MEPL. Each of the Company and Novogen’s obligations in the Guarantee and Indemnity Agreement are absolute, unconditional and irrevocable.
Commitments have reduced from $1.4 million at June 30, 2009 to $0.9 million for the year ended June 30, 2010 primarily due to the reduced commitments following the termination of enrollment into the OVATURE Phase III clinical trial, partially offset by additional commitments related to setting up the new office in San Diego.
6. Segment Information
The Company’s focus is the clinical development and commercialization of its licensed cancer compounds. The business contains two major segments based on geographic location.
| | Year Ended June 30, |
| | 2010 | 2009 | 2008 |
| | USA | Australia | Total | USA | Australia | Total | USA | Australia | Total |
| Statement of Operations | (in thousands) |
| | | | | | | | | | |
| Interest Revenue | 79 | 5 | 84 | 207 | 21 | 228 | 606 | 68 | 674 |
| | | | | | | | | | |
| Loss from operations | (1,022) | (6,873) | (7,895) | (452) | (10,727) | (11,179) | (448) | (11,959) | (12,407) |
| | | | | | | | | | |
| Income Tax Expense | (1) | - | (1) | (1) | - | (1) | (3) | - | (3) |
| | | | | | | | | | |
| Net loss arising during development stage | (1,023) | (6,873) | (7,896) | (453) | (10,727) | (11,180) | (451) | (11,959) | (12,410) |
| | | | | | | | | | |
| Balance Sheet | | | | | | | | | |
| | | | | | | | | | |
| Segment assets | $ 8,320 | $ 816 | $ 9,136 | $ 16,203 | $ 3,153 | $ 19,356 | $ 16,847 | $ 3,131 | $ 19,978 |
| | | | | | | | | | |
| Segment liabilities | $ 368 | $ 1,387 | $ 1,755 | $ 77 | $ 4,066 | $ 4,143 | $ 312 | $ 3,131 | $ 3,443 |
7. Related Party Transactions
License Agreement for Phenoxodiol, as amended
In September 2003, the Company entered into a license agreement pursuant to which Novogen granted to MEPL a worldwide non-transferable license under its patents and patent applications and in its know-how to conduct clinical trials and commercialize and distribute phenoxodiol products. The license agreement covers uses of phenoxodiol in the field of prevention, treatment or cure of cancer in humans delivered in all forms except topical applications. The license is exclusive until the expiration or lapsing of the last relevant Novogen patents or patent applications in the world and thereafter is non-exclusive. MEPL may terminate the agreement by giving three months’ notice to Novogen. MEPL paid $5,000,000 to Novogen in February 2004 which was the first lump sum license fee payment due under the terms of the license agreement. Also, MEPL paid $2,000,000 to Novogen in January 2005 and $4,000,000 in January 2006 which was the annual milestone license fee payments due under the license agreement. The Company paid a second lump sum license fee of $5,000,000 to Novogen in July 2006 following the raising of funds in a private placement. This license fee was due on the later of November 1, 2003 or such later date when the cumulative total of all funds received from debt or equity issuances and revenue received from commercialization (income other than sales) and sales of phenoxodiol products exceeded $50,000,000. Following the private placement or PIPE which closed on July 11, 2006 the funds received from equity issuances exceeded $50,000,000 which triggered this license fee payment. Future amounts payable to Novogen under terms of the license agreement are as follows:
1. Until the expiration of the exclusivity period of the license, MEPL must pay Novogen 2.5% of all net sales and 25% of commercialization income. After the exclusivity period of the license, 1.5% of net sales must be paid to Novogen. The preconditions to such payments have not yet occurred.
The “Exclusivity Period” ends on the later of:
| (a) | the date of expiration or lapsing of the last patent right in the patents and patent applications set out in the license agreement with Novogen; or |
| (b) | the date of expiration or lapsing of the last licensed patent right which MEPL would, but for the license granted in the license agreement, infringe in any country in the geographical territory covered by the license agreement by doing in that country any of the things set out in the license agreement. |
2. In addition to the amounts above, the Phenoxodiol License Agreement was amended in June 2006 and April 2007 to provide that upon the earliest receipt by MEPL of the first:
| (i) | approval by the U.S. Food and Drug Administration (the “FDA”) of a New Drug Application (“NDA”) for phenoxodiol; |
| (ii) | approval or authorization of any kind to market phenoxodiol in the U.S.; or |
| (iii) | approval or authorization of any kind by a government agency in any other country to market phenoxodiol. |
MEPL will be required to pay Novogen Research Pty Limited $8,000,000, together with interest on such amount from (and including) December 31, 2006 to (but excluding) the Approval Date. Thereafter, MEPL will be required to make license milestone fee payments of $8,000,000 to Novogen Research Pty Limited on December 31 of the year of the Approval Date and on December 31 of each year thereafter during the exclusivity period under the Phenoxodiol License Agreement.
No license fees have been accrued in respect of phenoxodiol at June 30, 2010.
License Agreement Triphendiol and NV-143
In May 2006, the Company entered into a second license agreement with Novogen for two oncology compounds, triphendiol and NV-143 (the “License Agreement for Triphendiol and NV-143”). Triphendiol is being developed initially in oral form for the treatment of pancreatic and bile duct cancer and is currently in Phase I human testing. NV-143 is targeted for the treatment of melanoma, also in oral dose form, and is in the pre-clinical testing stage. The License Agreement for Triphendiol and NV-143 is an agreement under which Novogen grants to MEPL a worldwide non-transferable license under its patents and patent applications and in its know-how to conduct clinical trials and commercialize and distribute triphendiol and NV-143 products. The License Agreement for Triphendiol and NV-143 covers uses of triphendiol and NV-143 in the field of prevention, treatment or cure of cancer in humans delivered in all forms except topical applications. The license is exclusive until the expiration or lapsing of the last relevant Novogen patents or patent applications in the world and thereafter is non-exclusive. MEPL may terminate the agreement by giving three months notice to Novogen. The Company is required to make payments under the terms of the License Agreement for Triphendiol and NV-143 with Novogen as follows:
1. A lump sum license fee of $1,000,000 was payable to Novogen on the commencement date of the license in consideration of the license granted. This initial lump sum license fee was paid to Novogen in May 2006.
2. In further consideration of the license granted, MEPL must pay to Novogen the following milestone license fees upon the occurrence of the corresponding milestone as set forth below;
a) the first license product containing triphendiol to reach a milestone as set forth below; and
b) the first licensed product containing NV-143 to reach a milestone as set forth below.
The milestone license fees are:
| | i) | $1,000,000 on the date an investigational new drug application (“IND”) for the licensed product goes into effect or the equivalent approval of a government agency is obtained in another country. If this event does not occur before March 31, 2008 then this amount will be due on this date. The amount of $1,000,000 was paid to Novogen on March 31, 2008 under the terms of this agreement; |
| | ii) | $2,000,000 on the date of enrollment of the first clinical trial subject in a Phase II clinical trial of the licensed product. If this event does not occur before June 30, 2009, then this amount will be due on this date. The amount of $2,000,000 was paid to Novogen on June 30, 2009 under the terms of this agreement; |
| | iii) | $3,000,000 on the date of enrollment of the first clinical trial subject in a Phase III clinical trial of the licensed product. If this event does not occur before December 31, 2011, then this amount will be due on this date; and |
| | iv) | $8,000,000 on the date of first receipt of a NDA for the licensed product from the FDA or equivalent approval from a government agency in another country. If this event does not occur before December 31, 2013, then this amount will be due on this date. |
3. MEPL must pay Novogen royalties of 5.0% of all net sales and 25% of commercialization income for the term of the license. The royalty rate is reduced by 50% if the licensed patent rights in any country or territory expire, lapse, are revoked, do not exist or are assigned to MEPL and the product is entirely manufactured and supplied in such country.
4. Minimum royalties of $3,000,000 per year are payable following the date of first receipt of an NDA for a licensed product from the FDA (or equivalent approval from a government agency in any other country) until the expiration of the term.
The license agreement may be cancelled without penalty by MEPL by giving three months notice. Therefore license fees due under the license agreement are recognised as an expense when the milestone event occurs.
License Agreement for NV-128
On August 4, 2009, the Company entered into a license agreement with Novogen pursuant to which Novogen granted to MEPL an exclusive, worldwide, non-transferable license under its patents and patent applications and in the intellectual property rights related to its know how to conduct clinical trials, commercialize and distribute NV-128 (the “NV-128 Licence Agreement”). NV-128 is an investigational cancer compound which has been shown in pre-clinical laboratory studies to promote cancer cell death by targeting a pro-survival regulatory pathway (the AKT-mTOR pathway). The NV-128 License Agreement covers the use of NV-128 in the field of prevention, treatment and cure of cancer in humans delivered in all forms except topical applications. The NV-128 License Agreement remains in effect until (i) the expiration or lapsing of th e last relevant patents or patent applications in the world or (ii) Novogen’s assignment to MEPL of the last relevant patents or patent applications in the world so that MEPL may assume the filing, prosecution and maintenance of such patents or patent applications. Thereafter, the license becomes a non-exclusive, perpetual and irrevocable license covering any remaining intellectual property rights related to the know how with respect to NV-128.
1. The Company paid $1,500,000 to Novogen Research in August 2009, which was the first lump sum license fee payment under the terms of the license agreement.
2. Future amounts payable to Novogen upon the achievement of certain milestones are as follows:
| | i) | $1,000,000 on the date an IND for the licensed product goes into effect or the equivalent approval of a government agency is obtained in another country. If this event does not occur before December 31, 2011 then this amount will be due on this date; |
| | ii) $2,000,000 on the date of enrollment of the first clinical trial subject in a Phase II clinical trial of the licensed product. If this event does not occur before December 31, 2012, then this amount will be due on this date; |
| | iii) | $3,000,000 on the date of enrollment of the first clinical trial subject in a Phase III clinical trial of the licensed product. If this event does not occur before December 31, 2014, then this amount will be due on this date; and |
| | iv) | $8,000,000 on the date of first receipt of a NDA for the licensed product from the FDA or equivalent approval from a government agency in another country. If this event does not occur before December 31, 2017, then this amount will be due on this date. |
3. MEPL must pay Novogen royalties of 5.0% of all net sales and 25% of commercialization income for the term of the license.
4. Minimum royalties of $3,000,000 per year are payable following the date of first receipt of an NDA for a licensed product from the FDA (or equivalent approval from a government agency in any other country) until the expiration of the term.
The license agreement is able to be cancelled without penalty by MEPL by giving three months’ notice.
No license fees have been accrued in respect of NV-128 at June 30, 2010.
Amended and Restated License Option Deed
On September 24, 2003, MEPL and Novogen entered into an Amended and Restated License Option Deed (the “License Option Deed”). The License Option Deed grants MEPL an exclusive right to accept and an exclusive right to match any proposed dealing by Novogen of its intellectual property rights with a third party relating to synthetic compounds (other than phenoxodiol) that have known or potential applications in the field of prevention, treatment or cure of cancer in humans in all forms other than topical applications.
Amended and Restated Services Agreement
On September 24, 2003, the Company, Novogen and MEPL entered into an Amended and Restated Services Agreement (the “Services Agreement”). The Company does not currently intend to directly employ any staff. Under the terms of the Services Agreement, Novogen Limited or its subsidiaries have agreed to provide services reasonably required by the Company relating to the development and commercialization of phenoxodiol and other licensed products, including triphendiol and NV-143. Novogen has agreed to provide these services at cost plus a 10% mark-up. The Company may terminate the agreement on three months' written notice to Novogen.
Transactions giving rise to expenditures amounting to $3,144,000, $2,264,000 and $3,054,000, were made under the Services Agreement with Novogen during the twelve months ended June 30, 2010, 2009 and 2008 respectively. Of these amounts, $2,279,000, $1,456,000 and $2,065,000 related to service fees paid to Novogen for research and development services provided in the twelve months ended June 30, 2010, 2009 and 2008 respectively, reflecting the time spent by Novogen research staff on the development of phenoxodiol, triphendiol, NV-143 and NV-128. Additionally, $865,000, $808,000 and $989,000 of the total expenditures during the twelve months ended June 30, 2010, 2009 and 2008, respectively, related to costs incurred for administration and accounting services provided by Novogen.
At June 30, 2010 and 2009, $301,000 and $221,000, respectively, was due and owing to Novogen under the services agreement and is included in amounts due to related company.
Amended and Restated Manufacturing License and Supply Agreement
On September 24, 2003, MEPL and Novogen entered into an Amended and Restated Manufacturing License and Supply Agreement (the “Manufacturing License and Supply Agreement”). Under the terms of the Manufacturing License and Supply Agreement, MEPL has granted to Novogen an exclusive, non-transferable sub license to manufacture and supply phenoxodiol in its primary manufactured form. Novogen has agreed to supply phenoxodiol to MEPL for the clinical trial development program and phenoxodiol’s ultimate commercial use. Phenoxodiol supplied by Novogen under the terms of this agreement will by charged at cost plus a 50% markup.
Transactions giving rise to expenditures amounting to $nil, $ nil, and $38,000 were made under the Manufacturing License and Supply Agreement with Novogen during the twelve months ended June 30, 2010, 2009 and 2008, respectively.
At June 30, 2010 and June 30, 2009 no amount was due and owing to Novogen under the Manufacturing License and Supply Agreement.
Novogen has taken the strategic decision not to manufacture large scale Active Pharmaceutical Ingredients for cancer drugs, including phenoxodiol, as these can be more economically supplied by third parties with particular expertise in this area.
8. Equity
The Company is a development stage company incorporated in December 2000 that commenced operations in May 2002 coinciding with its listing on the London Stock Exchange’s Alternative Investment Market (AIM).
On March 31, 2010, the Company effected a reverse stock split of its outstanding common stock on a 1-for-10 split adjusted basis in order to correct a bid price listing requirement for continued inclusion on the Nasdaq Global Market under Nasdaq Rule 5450(a)(1). For the purpose of this report we have adjusted all share data presented retrospectively to incorporate the 1-for-10 reverse stock split.
In May 2002, the Company sold 252,300 shares of its common stock and 252,300 warrants, raising proceeds of $9,022,000, net of $1,070,000 of transaction costs. The warrants were exercisable prior to November 30, 2003 at an exercise price of $40.00 per share. The common stock was listed for trading on the AIM. Following the listing, Novogen retained 95.1% of the Company’s common stock.
In June 2003, 900 warrants were exercised, resulting in proceeds to the Company of $36,000. In November 2003 the remaining 251,400 warrants were exercised at an exercise price of $40.00 per share with proceeds to the Company of $10,056,000.
In December 2003, the Company sold 239,200 common stock units at a public offering price of $75.00 per unit. Each common stock unit consisted of:
| · | one share of common stock; and |
| · | one warrant to purchase a share of common stock, exercisable prior to December 18, 2006 at an exercise price equal to $90.00. |
In connection with the December 2003 offering, the Company’s common stock and warrants commenced trading separately on the NASDAQ Global Market. The Company received proceeds of $15,522,000, net of $2,431,000 transaction costs in the December 2003 offering.
On December 18, 2006, 239,200 warrants which were issued in connection with the December 2003 public offering expired and no shares of common stock were issued relating to those warrants.
In January 2006, the Company voluntarily cancelled the trading of its common stock on the AIM.
On July 11, 2006, the Company entered into a securities subscription agreement with certain accredited investors providing for the placement of 632,931 shares of the Company’s common stock and warrants exercisable for 221,525 shares of the Company’s common stock at a purchase price of $29.00 per unit. Each unit consisted of one share of common stock and 0.35 of a warrant to purchase one share of common stock. The warrants have an exercise price of $43.50 per share, subject to certain adjustments. The exercise price and number of shares issuable upon exercise of such warrants are subject to adjustment in the event of stock dividends, stock splits and other similar events. The warrants may be exercised no less than six months from the closing date and will expire four years from the date of issuance, or July 11, 2010. These w arrants have subsequently expired at the date of this report. The Company closed the private placement or PIPE on July 11, 2006. In connection with the PIPE, the Company received proceeds of $16.8 million net of $1.5 million commissions and other costs.
In connection with the securities subscription agreement described above, the Company entered into a registration rights agreement pursuant to which the Company is obligated to file a resale registration statement with the SEC covering the shares of common stock issued in connection with the securities subscription agreement, in addition to the shares of common stock underlying the warrants issued in connection with the securities subscription agreement. The Company filed the registration statement on August 9, 2006. The resale registration statement was declared effective September 5, 2006.
On July 11, 2006, the Company entered into a standby equity distribution agreement (the “SEDA”), with YA Global Investments, LP (“YA Global Investments”, formerly Cornell Capital Partners, LP). Under the SEDA, the Company may have issued and sold to YA Global Investments shares of its common stock for a total purchase price of up to $15 million, once a resale registration statement was in effect.
In connection with the SEDA, the Company paid YA Global Investments a commitment fee of 12,363 shares of its common stock and warrants to purchase 60,000 shares of its common stock which expire on July 11, 2010. The warrants have an exercise price of $43.50 per share, subject to certain adjustments. The exercise price and number of shares issuable upon exercise of such warrants are subject to adjustment in the event of stock dividends, stock splits and other similar events. The commitment fee, comprising shares and warrants, is a share-based payment and has been accounted for in accordance with FASB ASC 718 (FAS123R) "Share-based Payment". The fair values of shares and warrants issued have been recognized directly as equity in the balance sheet and as selling, general and administration expenses in the income statement in the year ende d June 30, 2007. These warrants have subsequently expired at the date of this report.
The Company did not issue any shares of common stock under the terms of the SEDA and in August 2007 the Company cancelled the SEDA.
On August 1, 2007, the Company entered into a securities subscription agreement with certain accredited investors providing for the placement of 546,400 shares of its common stock at a purchase price of $30.00 per share. The investors in the transaction also received a warrant to purchase an additional 4 shares of common stock for every block of 10 shares of common stock purchased. All of the warrants have an exercise price of $36.00 per share. The warrants may be exercised beginning February 6, 2008 and will expire five years from the date of issuance, or August 6, 2012. The Company also issued 6,209 warrants to Blue Trading, LLC, which acted as the placement agent in the private placement, as part of the placement fee. The warrants issued to Blue Trading, LLC have an exercise price of $30.00 per share and each warrant is convertible for 4 shares of common stock. These warrants may be exercised immediately and will expire five years from the date of issuance, on August 6, 2012. The fair value of warrants issued as part of the placement fee, valued at $441,000, have been recognized directly as equity in the balance sheet and offset against issued share capital as a cost of the raising in the year ended June 30, 2008. The Company closed the private placement, or PIPE, on August 6, 2007. In connection with the PIPE, the Company received proceeds of $15.2 million net of $1.2 million in commissions and other costs.
The Company entered into a registration rights agreement with the investors party to the securities subscription agreement and Blue Trading, LLC, and agreed to file a resale registration statement with the SEC registering the common stock and the common stock issuable upon exercise of the warrants sold pursuant to the securities subscription agreement for resale thereunder. The Company filed the registration statement on October 2, 2007. The resale registration statement was declared effective October 19, 2007.
Under the terms of the July 11, 2006 and the August 1, 2007 PIPEs, the Company is required to maintain effective registration statements covering the resale shares of common stock issued in the PIPEs and the shares of common stock issuable upon exercise of the warrants issued in the PIPEs. In relation to the July 11, 2006 PIPE, at the date of issuance, the Company assessed the terms of the registration rights agreement, and as the penalty for not maintaining the registration of common stock is less than the difference between the value of registered shares and unregistered shares, the equity has been classified as permanent equity. The August 1, 2007 PIPE was assessed as permanent equity under ASC 825-20 (FASB Staff Position No. EITF 00-19-2), described below.
On January 1, 2007 the Company adopted ASC 825-20 (FASB Staff Position No. EITF 00-19-2). ASC 825-20 required the contingent obligation to make future payments under the registration rights agreements be recognized separately in accordance with FASB Statement No. 5, Accounting for Contingencies and the underlying warrants be recognized without regard to the contingent obligation. The adoption of ASC 825-20 had no effect on the Company’s financial statements as the warrants issued in connection with the PIPEs will remain classified as permanent equity and management does not currently believe that it is probable a payment will be made under either of the registration rights agreements.
The Company filed a shelf registration statement on Form S-3 with the SEC in March 2008. The shelf registration statement was declared effective by the SEC on April 3, 2008. The shelf registration statement permits the Company to sell, from time to time, up to $75,000,000 of common stock, preferred stock and warrants or any combination of the foregoing. Pursuant to SEC regulations, however, so long as the Company’s public float remains below $75.0 million the Company cannot sell securities from the shelf registration statement which represent more than one third of the market value of the Company’s public float during any 12-month period.
The Company entered into a Securities Subscription Agreement dated as of July 28, 2008 with Novogen and OppenheimerFunds, Inc. (“Oppenheimer”) pursuant to which the Company has sold 290,829 and 170,000 shares of common stock to Novogen and Oppenheimer, respectively, with Oppenheimer acting as adviser to each of the following parties severally and not jointly: (i) Oppenheimer International Growth Fund; (ii) Mass Mutual International Equity Fund; (iii) Oppenheimer International Growth Fund/VA; (iv) AZL Oppenheimer International Growth Fund; (v) OFITC International Growth Fund; and (vi) OFI International Equity Fund, at a purchase price of $21.70 per share, the consolidated closing bid price of the Company’s Common Stock as quoted by the NASDAQ Market Intelligence Desk at 4:00 PM EST on July 28, 2008. The shares were reg istered under the Securities Act of 1933, as amended, pursuant to a shelf registration statement on Form S-3 (File No. 333-149807), which was declared effective by the SEC on April 3, 2008. The Company received gross proceeds of $10 million from the sale of the shares.
Following the registered direct offering closed in July 2008, Novogen retained approximately 71.3% of the Company’s common stock.
In July 2008, the Company also issued 4,608 warrants to Mr. John O’Connor to purchase 4,608 shares of common stock, as consideration for investor services rendered by him to the Company. The warrants have an exercise price of $21.70 per share and may be exercised immediately and expire five years from the date of issuance, on July 30, 2013.
In January 2009, the Company issued 5,000 stock options to Associate Professor Gil Mor of Yale University, in recognition of his contribution to the development of phenoxodiol under the Marshall Edwards, Inc. 2008 Omnibus Equity Compensation Plan. The options have an exercise price of $6.30 and may be exercised immediately and expire five years from the date of issuance on January 28, 2014.
Pursuant to the terms of Dr. Gold’s Employment Letter, Dr. Gold has received options to purchase 220,390 shares of the Company’s common stock in two separate tranches. The first tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold upon his appointment as President and Chief Executive Officer on April 23, 2010, with an exercise price of $5.05 per share equal to the closing price of the Company’s common stock on April 23, 2010. The second tranche of options to purchase 110,195 shares of common stock of the Company was granted to Dr. Gold on June 7, 2010 following the public release of the Company’s OVATURE study results, with an exercise price of $1.86 per share equal to the closing price of the Company’s common stock on June 7, 2010. Of Dr. Gold’s options, 25% will vest one year from the effective date of the Employment Letter and, thereafter, the remaining 75% of Dr. Gold’s options will vest in equal monthly installments over the following thirty-six (36) months. Both tranches of options have a term of five years from the date of each grant. In the event of a Change in Control of the Company, as defined in the Employment Letter, Dr. Gold’s options will become fully vested. Dr. Gold’s options are issued outside the Company’s 2008 Stock Omnibus Equity Compensation Plan.
Pursuant to the terms of Mr. Zech’s Employment Letter, Mr. Zech has received options to purchase 73,463 shares of the Company’s common stock. These options were granted on June 18, 2010, with an exercise price of $1.52 per share equal to the closing price of the Company’s common stock on June 18, 2010. Of Mr. Zech’s options, 25% will vest one year from the effective date of the Employment Letter and, thereafter, the remaining 75% of Mr. Zech’s options will vest in equal monthly installments over the following thirty-six (36) months. The options have a term of five years from the date of grant. Mr. Zech’s options are issued under the Company’s 2008 Stock Omnibus Equity Compensation Plan.
9. Significant Events After Balance Date
On July 14, 2010, the Company received notice from Nasdaq stating that for the last 30 consecutive business days, the Market Value of Publicly Held Shares closed below the minimum $5 million required for continued listing on the Nasdaq Global Market under Nasdaq Rule 5450(b)(1)(C). Market Value of Publicly Held Shares is calculated by multiplying the publicly held shares, which is total shares outstanding less any shares held by officers, directors, or beneficial owners of 10% or more, by the consolidated closing bid price. Novogen Limited currently owns 71.3% of the outstanding common stock of the Company. Therefore, the value of Novogen Limited’s shares is excluded from the Market Value of Publicly Held Shares of the Company. According to Nasdaq's letter, the Company would be afforded a grace period of 180 calendar days, or until January 10, 2011, to regain compliance in accordance with Nasdaq Rule 5810(c)(3)(A). The Company intends to actively monitor the Market Value of Publicly Held Shares between now and January 10, 2011.
On August 10, 2010 the Company announced the appointment of Christine A. White, M.D. to the board of directors. Dr. White replaces Professor Paul J. Nestel, who has served as a director since April 2001.
10. Quarterly Financial Data (Unaudited)
| 2010 for the quarter ended | Jun-30 | | Mar-31 | | Dec-31 | | Sep-30 | | Year |
| | (in thousands except per share data) |
| | | | | | | | | | |
| Revenue | 16 | | 19 | | 23 | | 26 | | 84 |
| Loss from operations | (1,841) | | (2,213) | | (1,433) | | (2,408) | | (7,895) |
| Net Loss arising during development stage | (1,842) | | (2,213) | | (1,433) | | (2,408) | | (7,896) |
| Basic and diluted loss per share | (0.24) | | (0.30) | | (0.20) | | (0.33) | | (1.07) |
| | | | | | | | | | |
| 2009 for the quarter ended | Jun-30 | | Mar-31 | | Dec-31 | | Sep-30 | | Year |
| | (in thousands except per share data) |
| | | | | | | | | | |
| Revenue | 27 | | 29 | | 76 | | 96 | | 228 |
| Loss from operations | (5,425) | | (1,904) | | (1,599) | | (2,251) | | (11,179) |
| Net Loss arising during development stage | (5,425) | | (1,904) | | (1,599) | | (2,252) | | (11,180) |
| Basic and diluted loss per share | (0.74) | | (0.26) | | (0.22) | | (0.31) | | (1.53) |
11. Contingent Liabilities
Under the terms of the license agreements with Novogen, milestone license fee payments are payable upon achieving certain milestones. Details of the payments due under these agreements are detailed in Note 7 “Related Party Transactions.” The license agreements are subject to termination provisions.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a) Disclosure Controls and Procedures
At the end of the period covered by this Annual Report on Form 10-K, the Company’s management, with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of the Company’s disclosure controls and procedures. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Act is accumulated and communicated to the issuer's management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Based on that evaluation, the Company’s Chief Executive Officer and Chief Financial Officer have concluded that the Company’s disclosure controls and procedures are effective to ensure that the information required to be disclosed by the Company in reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
A control system no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within the Company are detected. Accordingly, our disclosure controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our disclosure control system are met and, as set forth above, our Chief Executive Officer and Chief Financial Officer have concluded, based on their evaluation as of the end of the period covered by this Annual Report on Form 10-K, that our disclosure controls and procedures were sufficiently effective to provide reasonable assurance that the objectives of our d isclosure control system were met.
(b) Management’s Annual Report on Internal Controls Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a - 15(f) under the Exchange Act. The Company’s internal control was designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Management maintains a comprehensive system of controls intended to ensure that transactions are executed in accordance with management’s authorization, assets are safeguarded and financial records are reliable. Management also takes steps to ensure that information and communication flows are effective, and to monitor performance, including performance of internal control procedures.
Management assessed the effectiveness of the Company’s internal control over financial reporting as of June 30, 2010 based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework. Based on this assessment, management believes that the Company’s internal control over financial reporting is effective as of June 30, 2010.
There were no changes in internal control over financial reporting during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Item 9B. Other Information
Not applicable
PART III
Item 10. Directors and Executive Officers of the Registrant
Code of Ethics
We have adopted a Code of Business and Ethics policy that applies to our directors and employees (including our principal executive officer and our principal financial officer), and have posted the text of our policy on our website (www.marshalledwardsinc.com). In addition, we intend to promptly disclose (i) the nature of any amendment to the policy that applies to our principal executive officer and principal financial officer and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals, the name of such person who is granted the waiver and the date of the waiver on our website in the future.
The other information required by this item is incorporated herein by reference to our proxy statement for the fiscal year ended June 30, 2010 (the “Proxy Statement”).
Item 11. Executive Compensation
The information required by this item is incorporated herein by reference to the Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item is included in Part II Item 5 of this Annual Report on Form 10-K and is incorporated herein by reference to the Proxy Statement.
Item 13. Certain Relationships and Related Transactions
The information required by this item is incorporated herein by reference to the Proxy Statement.
Item 14. Principal Accountant Fees and Services.
The information required by this item is incorporated by reference to the Proxy Statement.
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a) 1. Financial Statements
Reference is made to the Financial Statements under Item 8, Part II hereof.
2. Financial Statement Schedules
The Financial Statement Schedules have been omitted either because they are not required or because the information has been included in the financial statements or the notes thereto included in this Annual Report on Form 10-K.
3. Exhibits
Exhibit Index
Exhibits
| 3.1 | Restated Certificate of Incorporation (1) |
| 3.2 | Certificate of Amendment to the Restated Certificate of Incorporation (27) |
| 3.2 | Amended and Restated Bylaws (15) |
| 4.1 | Specimen Stock Certificate (3) |
| 4.2 | Specimen Warrant Certificate (4) |
| 4.3 | Specimen Warrant Certificate (22) |
| 4.4 | Form of Warrant Agreement (5) |
| 4.5 | Warrant Agreement (16) |
| 4.6 | Amended and Restated Warrant Agreement (19) |
| 4.7 | Form of Warrant (6) |
| 4.8 | Form of Warrant (17) |
| 4.9 | Form of Warrant (21) |
| 4.10 | Warrant dated July 30, 2008 issued to Mr John O’Connor (23) |
| 10.1 | Employment letter dated April 23, 2010, between Marshall Edwards, Inc. and Daniel Gold (28) |
| 10.2 | Employment letter dated June 18, 2010, between Marshall Edwards, Inc. and Thomas Zech (29) |
| 10.3 | Amended and Restated License Agreement between Novogen Research Pty Limited and Marshall Edwards Pty Limited (7) |
| 10.4 | Amended and Restated Manufacturing License and Supply Agreement between Novogen Laboratories Pty Limited and Marshall Edwards Pty Limited (12) |
| 10.5 | Amended and Restated License Option Deed between Novogen Research Pty Limited and Marshall Edwards Pty Limited (9) |
| 10.6 | Amended and Restated Services Agreement among Novogen Limited, Marshall Edwards, Inc. and Marshall Edwards Pty Limited (10) |
| 10.7 | Guarantee and Indemnity among Marshall Edwards, Inc., Novogen Laboratories Pty Limited, Novogen Research Pty Limited and Novogen Limited (11) |
| 10.8 | License Agreement between Novogen Research Pty Limited and Marshall Edwards Pty Limited (12) |
| 10.9 | Amendment Deed between Novogen Research Pty Limited and Marshall Edwards Pty Limited (13) |
| 10.10 | Registration Rights Agreement, dated July 11, 2006 by and among Marshall Edwards, Inc. and the investors as signatories thereto (14) |
| 10.11 | Registration Rights Agreement, dated as of August 6, 2007 by and among Marshall Edwards, Inc. and the purchases signatory thereto (18) |
| 10.12 | Registration Rights Agreement, dated as of September 26, 2007 by and among Marshall Edwards, Inc. and Blue Trading, LLC (20) |
| 10.13 | Securities Subscription Agreement dated as of July 28, 2008 by and among Marshall Edwards, Inc., Novogen Limited and OppenheimerFunds, Inc. (24) |
| 10.14 | Marshall Edwards, Inc. 2008 Stock Omnibus Equity Compensation Plan (25) |
| 10.15 | License Agreement dated August 4, 2009 by and between Novogen Research Pty Limited and Marshall Edwards Pty Limited (26) |
| 21.1 | Subsidiaries of Marshall Edwards, Inc. (2) |
| 23.1 | Consent of BDO Audit (NSW - VIC) Pty Ltd* |
| 31.1 | Certification required by Rule 13a-14(a) or Rule 15d-14(a)* |
| 31.2 | Certification required by Rule 13a-14(a) or Rule 15d-14(a)* |
| 32.1 | Certification required by Rule 13a-14(b) or Rule 15d-14(b) and section 1350 of Chapter 63 of Title 18 of the U.S. Code (18 U.S.C. 1350)* |
* Filed herewith.
| (1) | Incorporated by reference to Exhibit 3.1 to Registrant’s Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129). |
| (2) | Incorporated by reference to Exhibit 21 to Registrant’s Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129). |
| (3) | Incorporated by reference to Exhibit 4.1 to Amendment No. 1 to Registrant’s Registration Statement on Form S-1 filed on October 31, 2003 (Reg. No. 333-109129). |
| (4) | Incorporated by reference to Exhibit 4.2 to Registrant’s Registration Statement on Form S-3 filed on August 9, 2006 (Reg. No. 333-136440 ). |
| (5) | Incorporated by reference to Exhibit 10.3 to Registrant’s Current Report on Form 8-K filed on July 12, 2006. |
| (6) | Incorporated by reference to Exhibit 10.4 to Registrant’s Current Report on Form 8-K filed on July 12, 2006. |
| (7) | Incorporated by reference to Exhibit 10.1 to Registrant’s Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129). |
| (8) | Incorporated by reference to Exhibit 10.2 to Registrant’s Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129). |
| (9) | Incorporated by reference to Exhibit 10.3 to Registrant’s Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129). |
| (10) | Incorporated by reference to Exhibit 10.4 to Registrant’s Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129). |
| (11) | Incorporated by reference to Exhibit 10.5 to Registrant’s Registration Statement on Form S-1 filed on September 25, 2003 (Reg. No. 333-109129). |
| (12) | Incorporated by reference to Exhibit 99.1 to Registrant’s Current Report on Form 8-K filed on May 16, 2006. |
| (13) | Incorporated by reference to Exhibit 10.1 to Registrant’s Current Report on Form 8-K filed on June 9, 2006 |
| (14) | Incorporated by reference to Exhibit 10.2 to Registrant’s Current Report on Form 8-K filed on July 12, 2006. |
| (15) | Incorporated by reference to Exhibit 3.1 to Registrant’s Current Report on Form 8-K filed on July 30, 2007. |
| (16) | Incorporated by reference to Exhibit 10.3 to Registrant’s Current Report on Form 8-K filed on August 6, 2007. |
| (17) | Incorporated by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K filed on August 6, 2007. |
| (18) | Incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on August 6, 2007. |
| (19) | Incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K/A filed on September 27, 2007. |
| (20) | Incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K/A filed on September 27, 2007. |
| (21) | Incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K/A filed on September 27, 2007. |
| (22) | Incorporated by reference to Exhibit 4.4 to Registrant’s Annual Report on Form 10-K filed on September 27, 2007. |
| (23) | Incorporated by reference to Exhibit 4.1 to Registrant’s Current Report on Form 8-K filed on July 30, 2008. |
| (24) | Incorporated by reference to Exhibit 10.13 to the Registrant’s Current Report on Form 8-K filed on July 30, 2008. |
| (25) | Incorporated by reference to Exhibit 4.1 to Registrant’s Registration Statement on Form S-8 (Reg No. 333-156985) filed on January 28, 2009. |
| (26) | Incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on August 7, 2009. |
| (27) | Incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on April 26, 2010. |
| (28) | Incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on June 23, 2010. |
| (29) | Incorporated by reference to Exhibit 3.1.1 to Registrant’s Current Report on Form 8-K filed on March 31, 2010. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized, on August 26, 2010.
MARSHALL EDWARDS, INC.
A Delaware Corporation
By:
/s/ Daniel Gold
Daniel Gold
Chief Executive Offer
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities indicated on August 26, 2010.
Signatures Title
By: /s/ Daniel Gold President, Chief Executive Officer and Director
Daniel Gold
By: /s/ Thomas Zech Secretary, Chief Financial Officer
Thomas Zech
By: /s/ Leah Cann Director
Leah Cann
By: /s/ Bryan Williams Director
Bryan Williams
By: /s/ Christine White Director
By: /s/ Philip Johnston Director
Exhibit 23.1
Marshall Edwards, Inc.
11975 El Camino Real, Suite 101
San Diego, CA 92130 USA
Consent of Independent Registered Public Accounting Firm
We hereby consent to the incorporation by reference in the Registration Statement on Form S-3 (No. 333-136440) and Registration Statement on Form S-8 (File No. 333-156985) of Marshall Edwards, Inc. of our report dated August 26, 2010, relating to the consolidated financial statements which appears in this Form 10-K.
/s/ BDO
BDO Audit (NSW-VIC) Pty Ltd
Sydney, NSW, Australia
August 26, 2010
Exhibit 31.1
CERTIFICATION
I, Daniel Gold, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K for the year ended June 30, 2010 of Marshall Edwards, Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) ) for the registrant and have: |
| (a) | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within the entities, particularly during the period in which this report is being prepared; |
| (b) | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in the report our conclusions about the effectiveness of this disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrants fourth fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors: |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 26, 2010
/s/ Daniel Gold
Daniel Gold
Chief Executive Officer
Exhibit 31.2
CERTIFICATION
I, Thomas Zech, certify that:
| 1. | I have reviewed this Annual Report on Form 10-K for the year ended June 30, 2010 of Marshall Edwards, Inc.; |
| 2. | Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report; |
| 3. | Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report; |
| 4. | The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) ) for the registrant and have; |
| (a) | designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within the entities, particularly during the period in which this report is being prepared: |
| (b) | designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in the report our conclusions about the effectiveness of this disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and |
| (d) | disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrants fourth fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and |
| 5. | The Company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors: |
| (a) | All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting. |
Date: August 26, 2010
/s/ Thomas Zech
Thomas Zech
Chief Financial Officer
Exhibit 32.1
CERTIFICATION
Each of the undersigned hereby certifies, for the purposes of Section 1350 of Chapter 63 of Title 18 of the U.S. Code, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, in his capacity as an officer of Marshall Edwards, Inc. (“Marshall Edwards”) that, to his knowledge, this Annual Report on Form 10-K of Marshall Edwards for the year ended June 30, 2010, fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934, as amended, and that the information contained in such a report fairly presents, in all material respects, the financial condition and results of operation of Marshall Edwards.
/s/ Daniel Gold
Daniel Gold
Chief Executive Officer
/s/ Thomas Zech
Thomas Zech
Chief Financial Officer
A signed original of this written statement required by Section 906 has been provided to Marshall Edwards and will be retained by Marshall Edwards and furnished to the Securities and Exchange Commission or its staff upon request.