Exhibit 99.1
| | |
 | | Pracinostat in Combination With Azacitidine Produces a High Rate and Rapid Onset of Disease Remission in Patients With Previously Untreated Acute Myeloid Leukemia Guillermo Garcia-Manero, MD1; Ehab Atallah, MD2; Olatoyosi Odenike, MD3; Bruno C. Medeiros, MD4; Jorge Cortes, MD1; Vanessa Esquibel5; Steven Cha, MD5; Samer K. Khaled, MD6 1Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX;2Department of Hematology/Oncology, Medical College of Wisconsin, Milwaukee, WI;3Section of Hematology/Oncology, University of Chicago, Chicago, IL;4Division of Hematology/Oncology, Stanford Cancer Center, Stanford, CA;5MEI Pharma Inc, San Diego, CA;6Department of Hematology/Hematopoietic Cell Transplantation, City of Hope, Duarte, CA |
| — | Pracinostat is a potential best-in-class histone deacetylase inhibitor (HDACi) |
| — | Potent inhibitor of Class I, II, and IV isoenzymes |
| | ¡ | SB991, the majorin vivo metabolite of pracinostat, demonstrates higher activity than pracinostat |
| | ¡ | Combined pharmacokinetics of pracinostat and SB991 predicts on-target IC50 activity for HDAC1 >24 hours |
| — | A Phase I study of single-agent pracinostat demonstrated clinical activity in patients with acute myeloid leukemia (AML) |
| — | A pilot Phase II study of pracinostat in combination with azacitidine in higher-risk myelodysplastic syndrome (MDS) demonstrated a complete response (CR) + complete response with incomplete blood count recovery (CRi) rate of 89% (Proc ASH, 2012:3821) |
| — | We report interim data from the ongoing MEI-004 Phase 2 clinical trial testing the safety and efficacy of pracinostat in combination with azacitidine in elderly patients with previously untreated AML |
Figure 1. Study Design
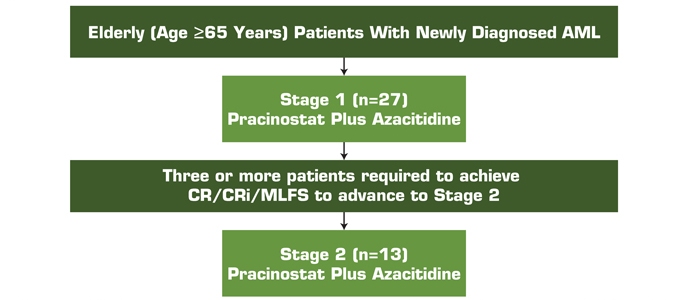
AML, acute myeloid leukemia; CR, complete response; CRi, complete response with incomplete blood count recovery; MLFS, morphologic leukemia-free state.
Treatment Regimen
| — | Pracinostat 60 mg is administered orally 3 days a week (days 1, 3, and 5 of each week) for 21 days of each 28-day cycle |
| — | Azacitidine is administered subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle |
| | – | | Begin with azacitidine which may be reduced to 75% of the starting dose |
| | – | | Subsequent reduction to 45 mg of pracinostat is allowed |
| | ¡ | Delays (between or within cycles) |
| | – | | Indicated for treatment-related³Grade 3 hematologic toxicity in the absence of disease |
| | – | | Indicated for treatment-related³Grade 3 non-hematologic toxicity following maximal medical treatment |
Eligibility Criteria
| | ¡ | Newly diagnosed de novo, secondary, or treatment-related AML with intermediate or unfavorable-risk cytogenetics based on the Southwest Oncology Group (SWOG) classifications (Slovak et al, 2000) |
| | ¡ | Adequate renal, cardiac, and liver function |
| | ¡ | QTcF£450 ms for males or QTcF£470 ms for females |
| | ¡ | Acute promyelocytic leukemia (FAB M3); t(15;17), t(8;21), t(16;16), del(16q), or inv(16) karyotype |
| | ¡ | Candidate for intensive chemotherapy (induction chemotherapy, bone marrow, or stem cell transplant) within the next 4 months |
| | ¡ | Active central nervous system (CNS) disease |
Study Evaluations
| — | Primary endpoint: complete response (CR) + complete response with incomplete blood count recovery (CRi) + morphologic leukemia-free state (MLFS) |
| | ¡ | Overall response rate (CR + CRi + partial response [PR] + PR with incomplete blood count recovery [PRi] + MLFS) |
| | ¡ | Complete cytogenetic response (CRc) + molecular complete remission (CRm) |
| | ¡ | Event-free survival (EFS) |
| | ¡ | Assess the tolerability and adverse event profile |
| — | Response assessments end of cycle 1 or 2, and then every other cycle until CR is achieved or as clinically indicated |
|
Presented at the 56th American Society of Hematology Annual Meeting, December 6-9, 2014, San Francisco, California |
| | |
 | | Pracinostat in Combination With Azacitidine Produces a High Rate and Rapid Onset of Disease Remission in Patients With Previously Untreated Acute Myeloid Leukemia Guillermo Garcia-Manero, MD1; Ehab Atallah, MD2; Olatoyosi Odenike, MD3; Bruno C. Medeiros, MD4; Jorge Cortes, MD1; Vanessa Esquibel5; Steven Cha, MD5; Samer K. Khaled, MD6 1Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX;2Department of Hematology/Oncology, Medical College of Wisconsin, Milwaukee, WI;3Section of Hematology/Oncology, University of Chicago, Chicago, IL;4Division of Hematology/Oncology, Stanford Cancer Center, Stanford, CA;5MEI Pharma Inc, San Diego, CA;6Department of Hematology/Hematopoietic Cell Transplantation, City of Hope, Duarte, CA |
| — | At time of evaluation 41 patients have been enrolled at 15 centers |
| | ¡ | Enrollment ongoing since December 25, 2013 |
Table 1. Patient Disposition
| | |
| | | N=41 |
Number of Patients Active | | 25 |
Number of Patients Discontinued | | 16 |
Reasons for Discontinuation | | |
Progressive disease | | 6 |
Adverse event | | 6 |
Other | | 4 |
Other – includes patient or physician decision.
Table 2. Baseline Characteristics
| | |
| | | N=41 |
Age (years) | | |
Median | | 76 |
Range | | 69-84 |
Gender, n (%) | | |
Male | | 24 (59) |
Female | | 17 (41) |
AML Disease Status, n (%) | | |
Newly diagnosed de novo | | 29 (71) |
Secondary (AHD and treatment related) | | 12 (29) |
ECOG Status, n (%) | | |
0-1 | | 33 (80) |
2 | | 8 (20) |
Bone Marrow Blasts at Baseline | | |
Median | | 38 |
20-29% Range, n (%) | | 13 (32) |
30-50% Range, n (%) | | 15 (36) |
>50% Range, n (%) | | 13 (32) |
Cytogenetic Risk Category, n (%) | | |
Intermediate | | 23 (56) |
High | | 17 (41) |
Not Evaluable | | 1 (3) |
AHD, antecedent hematologic disorder; AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group.
Table 3. Treatment Emergent Adverse Events All Causality in³10% of Patients
| | | | |
| | | All Grades (%) N=41 | | Grades 3-4 (%) N=41 |
Hematologic | | | | |
Febrile Neutropenia | | 12 (29) | | 10 (24) |
Thrombocytopenia | | 11 (27) | | 10 (24) |
Anemia | | 9 (22) | | 4 (10) |
Neutropenia | | 4 (10) | | 4 (10) |
Leukopenia | | 4 (10) | | 1 (2) |
Non-Hematologic | | | | |
Nausea | | 18 (44) | | 2 (5) |
Constipation | | 17 (41) | | 0 |
Fatigue | | 17 (41) | | 4 (10) |
Peripheral Edema | | 6 (15) | | 0 |
Vomiting | | 6 (15) | | 0 |
Diarrhea | | 5 (12) | | 1 (2) |
Dizziness | | 5 (12) | | 0 |
Headache | | 5 (12) | | 1 (2) |
Hypokalemia | | 5 (12) | | 0 |
Pyrexia | | 4 (10) | | 0 |
Cellulitis | | 4 (10) | | 4 (10) |
Rash | | 4 (10) | | 0 |
Hypotension | | 4 (10) | | 0 |
Cough | | 4 (10) | | 0 |
Dyspnea | | 4 (10) | | 0 |
QTc Prolongation* | | 2 (5) | | 1 (2) |
*QTc events were seen in <10% of patients, however are noted here.
|
Presented at the 56th American Society of Hematology Annual Meeting, December 6-9, 2014, San Francisco, California |
| | |
 | | Pracinostat in Combination With Azacitidine Produces a High Rate and Rapid Onset of Disease Remission in Patients With Previously Untreated Acute Myeloid Leukemia Guillermo Garcia-Manero, MD1; Ehab Atallah, MD2; Olatoyosi Odenike, MD3; Bruno C. Medeiros, MD4; Jorge Cortes, MD1; Vanessa Esquibel5; Steven Cha, MD5; Samer K. Khaled, MD6 1Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX;2Department of Hematology/Oncology, Medical College of Wisconsin, Milwaukee, WI;3Section of Hematology/Oncology, University of Chicago, Chicago, IL;4Division of Hematology/Oncology, Stanford Cancer Center, Stanford, CA;5MEI Pharma Inc, San Diego, CA;6Department of Hematology/Hematopoietic Cell Transplantation, City of Hope, Duarte, CA |
Table 4. Treatment-Emergent Adverse Events Leading to Drug Discontinuation
| | | | | | |
| | | | | Discontinuation | | |
AE Term | | Grade | | (Cycle/Day) | | Outcome |
Peripheral Motor Neuropathy | | 3 | | 3/1 | | Resolved |
Parainfluenza | | 3 | | 3/22 | | Resolved |
Prolonged QTc/AF | | 3 | | 2/15 | | Resolved |
Subdural Hematoma | | 5 | | 3/22 | | Fatal |
Sepsis | | 5 | | 2/3 | | Fatal |
Sepsis | | 5 | | 2/14 | | Fatal |
AE, adverse event; AF, atrial fibrillation.
Table 5. Response
| | |
Interim Response Assessment |
n=33* (%) |
CR/CRi/MLFS (Primary endpoint) | | 15 (45) |
CR | | 9 (27) |
CRi | | 4 (12) |
MLFS | | 2 (6) |
PR/PRi | | 3 (10) |
Stable Disease | | 4 (12) |
Progressive Disease | | 6 (18) |
Clinical Benefit** | | 1 (3) |
No Clinical Benefit | | 4 (12) |
CR, complete response; CRi, complete response with incomplete blood count recovery; MLFS, morphologic leukemia-free state;
PR, partial response; PRi, partial response with incomplete blood count recovery.
*All patients who have had at least 1 on-study disease assessmentOR discontinued study therapy prior to an on-study disease assessment due to adverse event or other reasons.
**Patients did not meet strict International Working Group (IWG) response criteria, but were determined to have clinical benefit by Investigator.
Figure 2. Interim Efficacy and Duration on Study
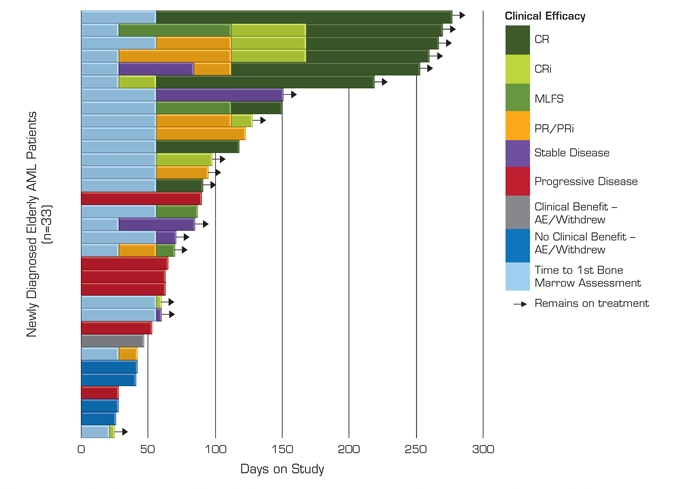
AE, adverse event; AML, acute myeloid leukemia; CR, complete response; CRi, complete response with incomplete blood count recovery; MLFS, morphologic leukemia-free state; PR, partial response; PRi, partial response with incomplete blood count recovery.
|
Presented at the 56th American Society of Hematology Annual Meeting, December 6-9, 2014, San Francisco, California |
| | |
 | | Pracinostat in Combination With Azacitidine Produces a High Rate and Rapid Onset of Disease Remission in Patients With Previously Untreated Acute Myeloid Leukemia Guillermo Garcia-Manero, MD1; Ehab Atallah, MD2; Olatoyosi Odenike, MD3; Bruno C. Medeiros, MD4; Jorge Cortes, MD1; Vanessa Esquibel5; Steven Cha, MD5; Samer K. Khaled, MD6 1Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX;2Department of Hematology/Oncology, Medical College of Wisconsin, Milwaukee, WI;3Section of Hematology/Oncology, University of Chicago, Chicago, IL;4Division of Hematology/Oncology, Stanford Cancer Center, Stanford, CA;5MEI Pharma Inc, San Diego, CA;6Department of Hematology/Hematopoietic Cell Transplantation, City of Hope, Duarte, CA |
Figure 3. Event-Free Survival
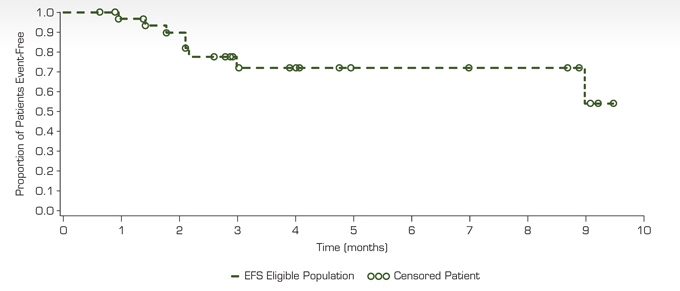
Figure 4. Overall Survival
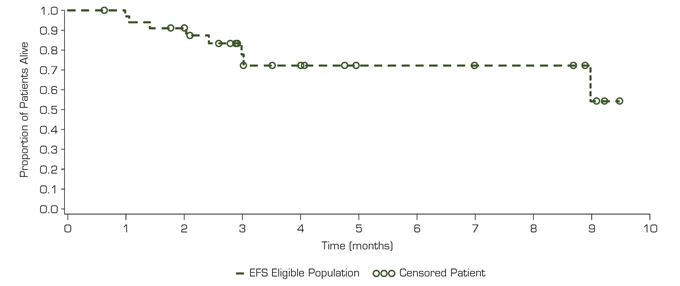
| — | Pracinostat in combination with azacitidine demonstrates significant clinical activity in elderly patients with newly diagnosed AML |
| | ¡ | To date, 15 of 33 patients (45%) achieved the primary endpoint of CR + CRi + MLFS |
| | ¡ | No patient who achieved a clinical response has progressed |
| | ¡ | Most clinical responses occur within the first 2 cycles and continue to improve with ongoing therapy |
| | ¡ | The observed response rate may increase with longer follow-up of patients achieving PR or SD (stable disease) |
| — | Pracinostat in combination with azacitidine was well tolerated in this population of elderly AML patients |
| | — | The most common treatment-emergent AEs included neutropenia, febrile neutropenia, thrombocytopenia, nausea, fatigue, and anemia |
| | ¡ | Adverse events resulting in dose reductions were uncommon, and frequently due to disease under study |
| | ¡ | The 60-day mortality rate is approximately 10% (3/33) |
| | ¡ | 6 patients to date have received study drug beyond 230 days, reflecting long-term tolerability |
| — | These data support definitive development of pracinostat in combination with azacitidine in elderly AML patients |
|
Presented at the 56th American Society of Hematology Annual Meeting, December 6-9, 2014, San Francisco, California |
| | |
 | | Pracinostat in Combination With Azacitidine Produces a High Rate and Rapid Onset of Disease Remission in Patients With Previously Untreated Acute Myeloid Leukemia Guillermo Garcia-Manero, MD1; Ehab Atallah, MD2; Olatoyosi Odenike, MD3; Bruno C. Medeiros, MD4; Jorge Cortes, MD1; Vanessa Esquibel5; Steven Cha, MD5; Samer K. Khaled, MD6 1Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX;2Department of Hematology/Oncology, Medical College of Wisconsin, Milwaukee, WI;3Section of Hematology/Oncology, University of Chicago, Chicago, IL;4Division of Hematology/Oncology, Stanford Cancer Center, Stanford, CA;5MEI Pharma Inc, San Diego, CA;6Department of Hematology/Hematopoietic Cell Transplantation, City of Hope, Duarte, CA |
| | |
DISCLOSURES | |  |
| G. Garcia-Manero receives consultancy fees from MEI Pharma; E. Atallah reports no relevant conflicts of interest to disclose; O. Odenike receives honoraria and advisory fees from Sunesis Pharmaceuticals, Incyte, Sanofi-Aventis, Algeta Pharmaceuticals, and Spectrum Pharmaceuticals; B.C. Medeiros receives research funding from MEI Pharma; J. Cortes receives research funding from Celgene; V. Esquibel is an employee of MEI Pharma; S. Cha was employed by MEI Pharma at the time of abstract submission; S.K. Khaled receives research funding from Sequenom. | |
|
Presented at the 56th American Society of Hematology Annual Meeting, December 6-9, 2014, San Francisco, California |





