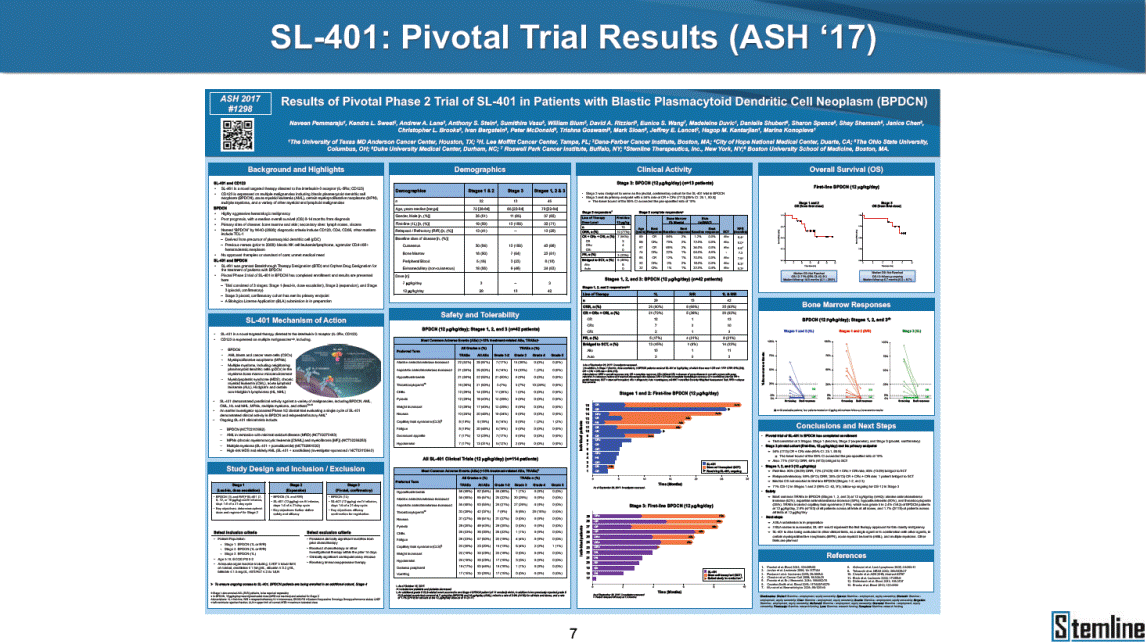
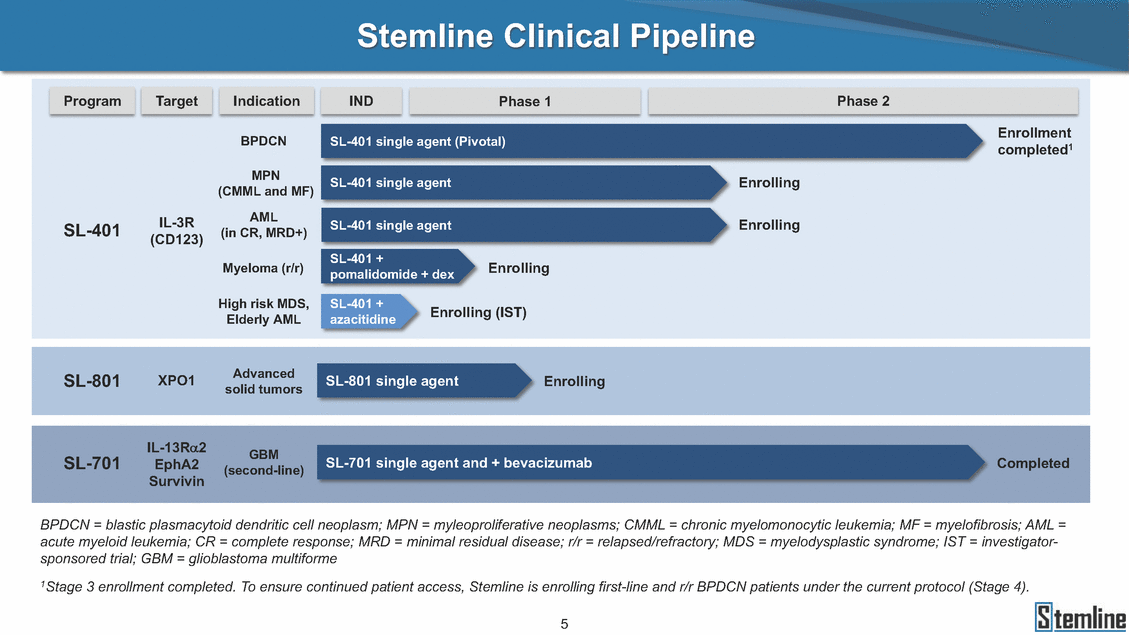
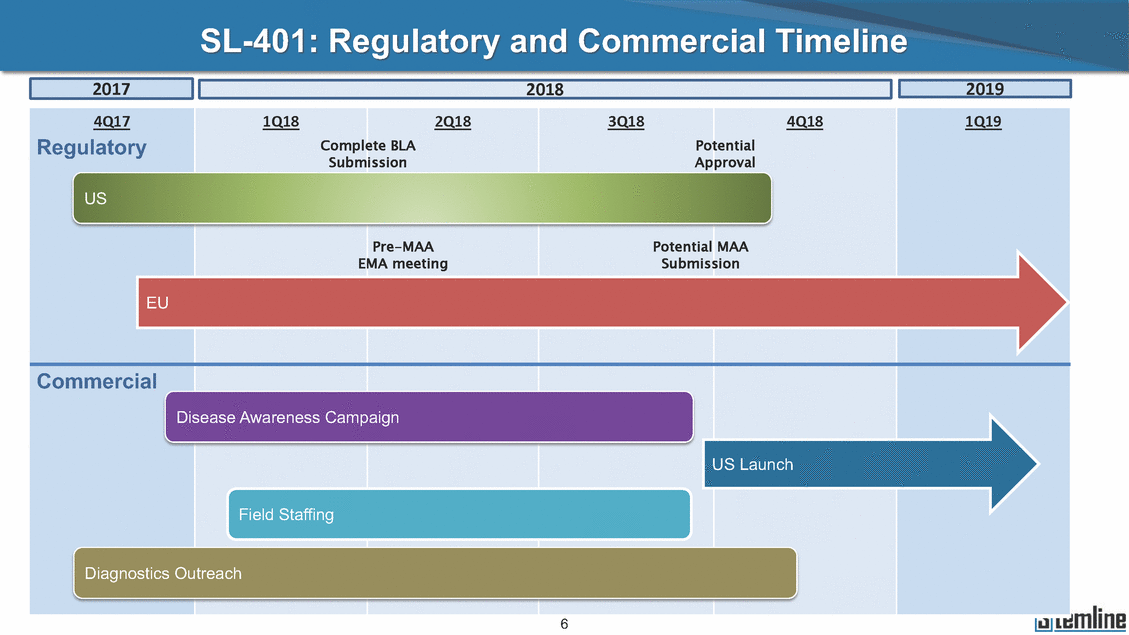
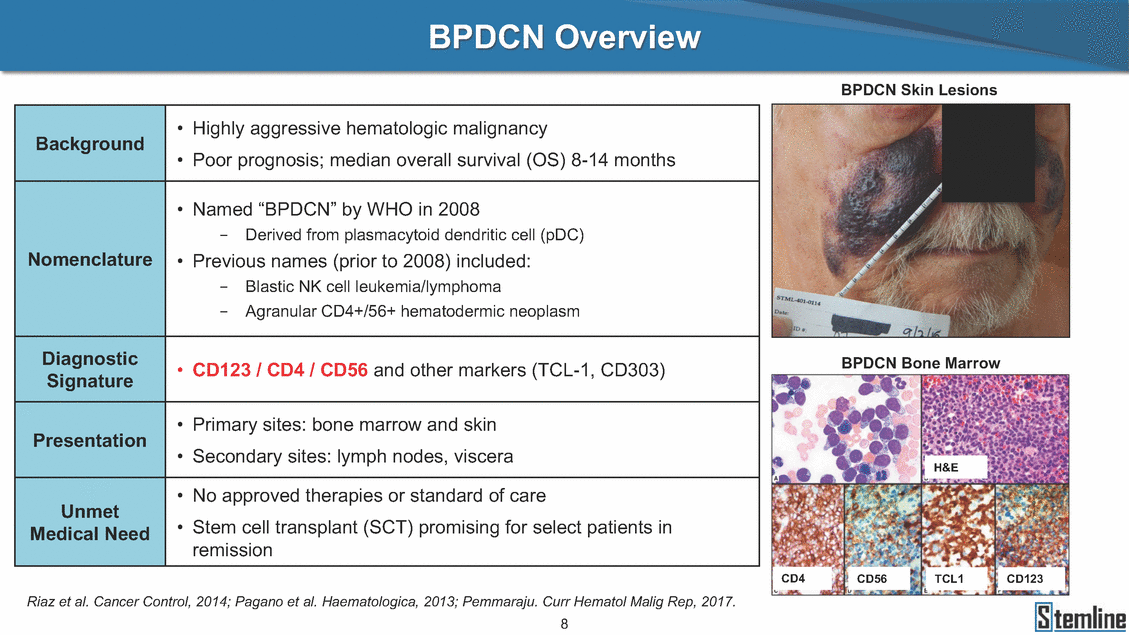
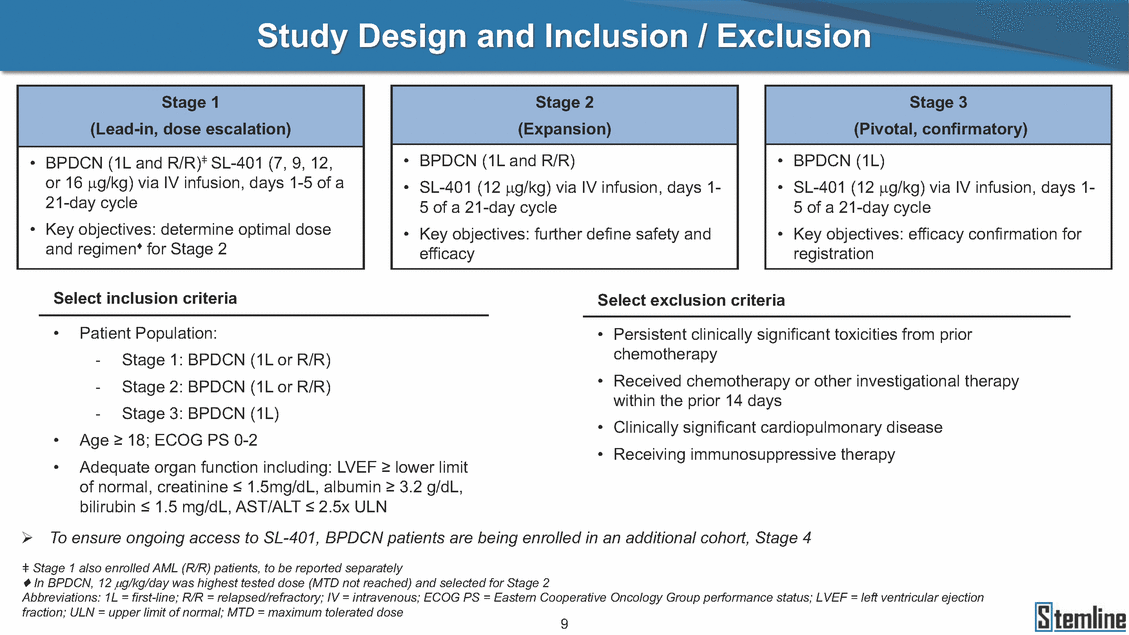
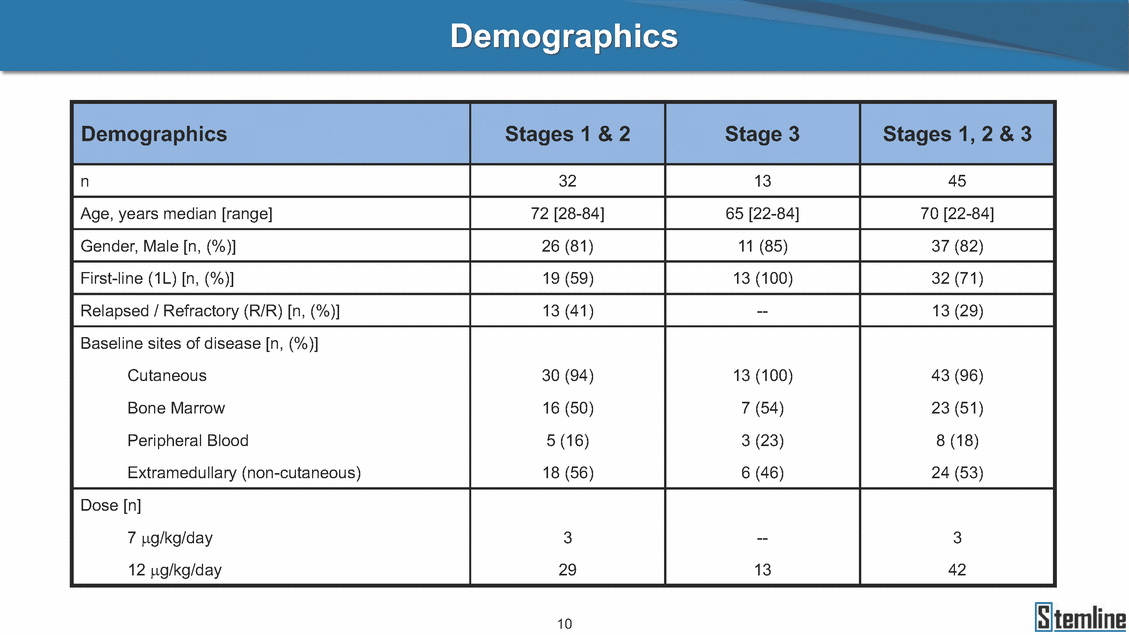
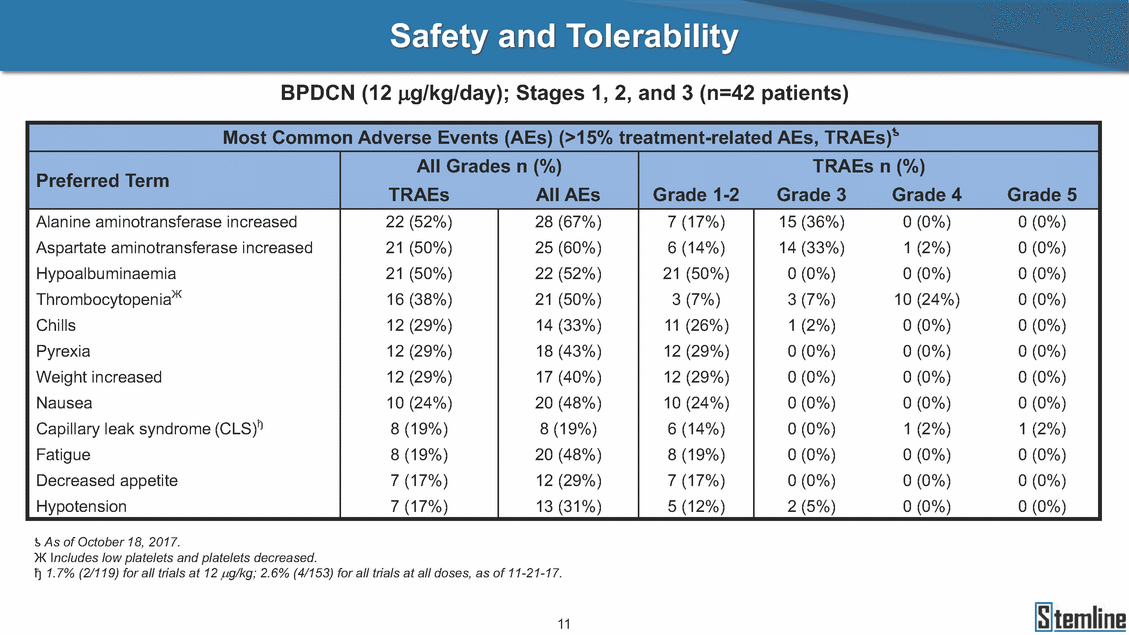
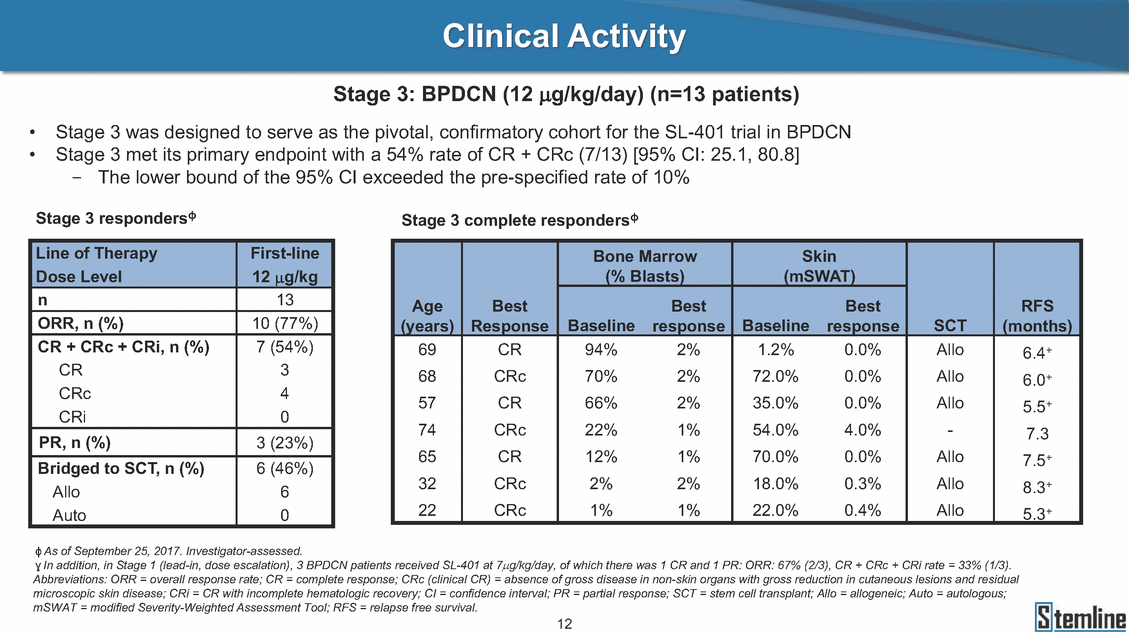
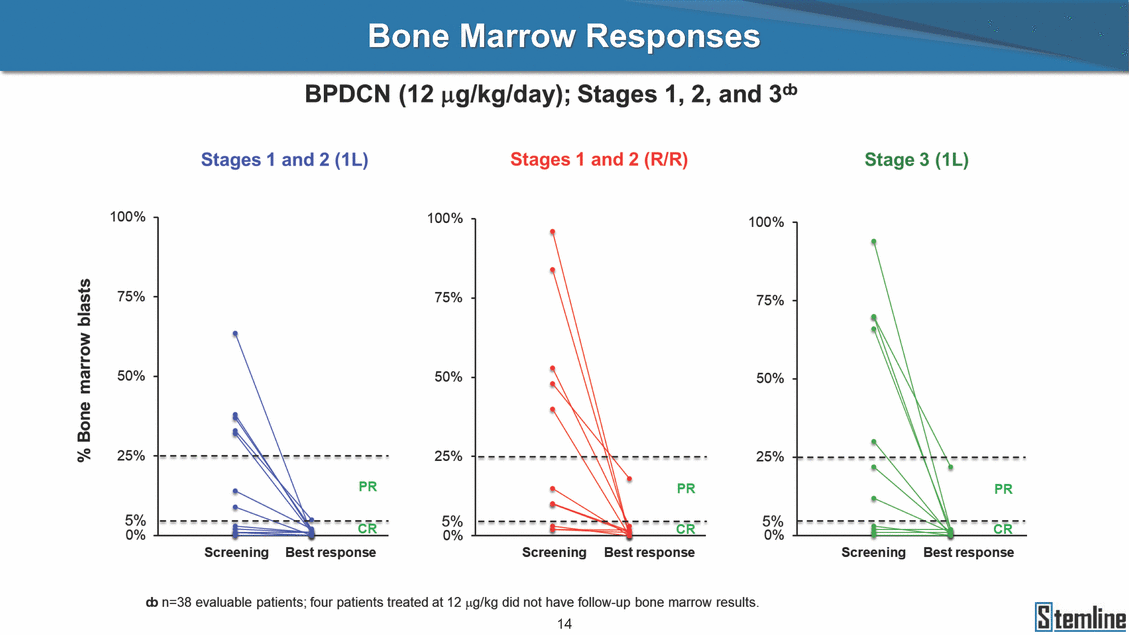
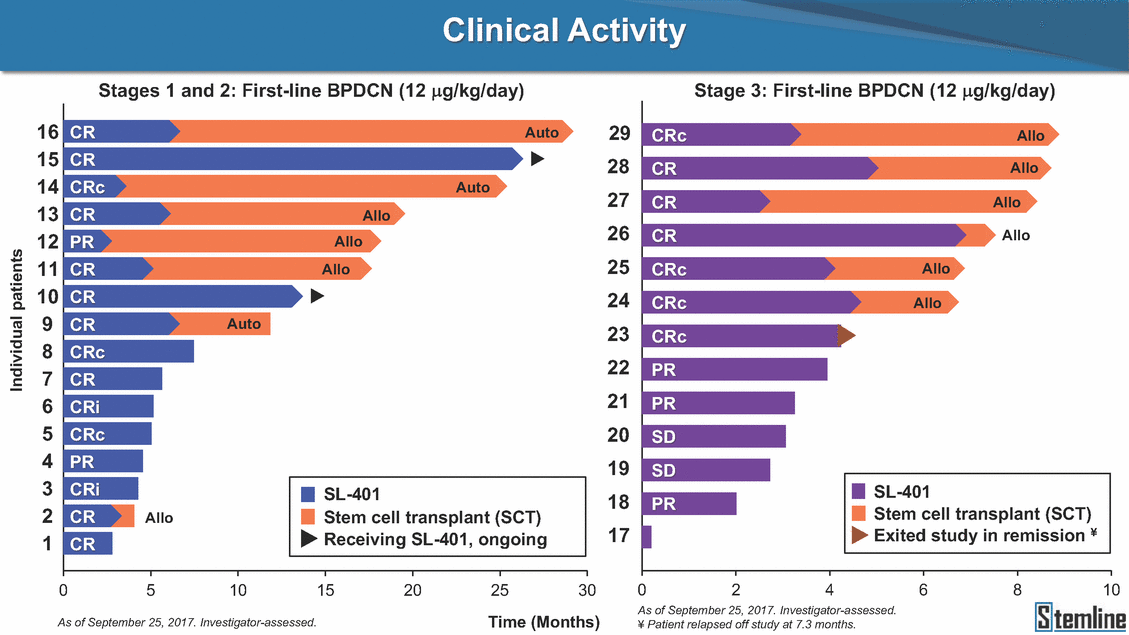
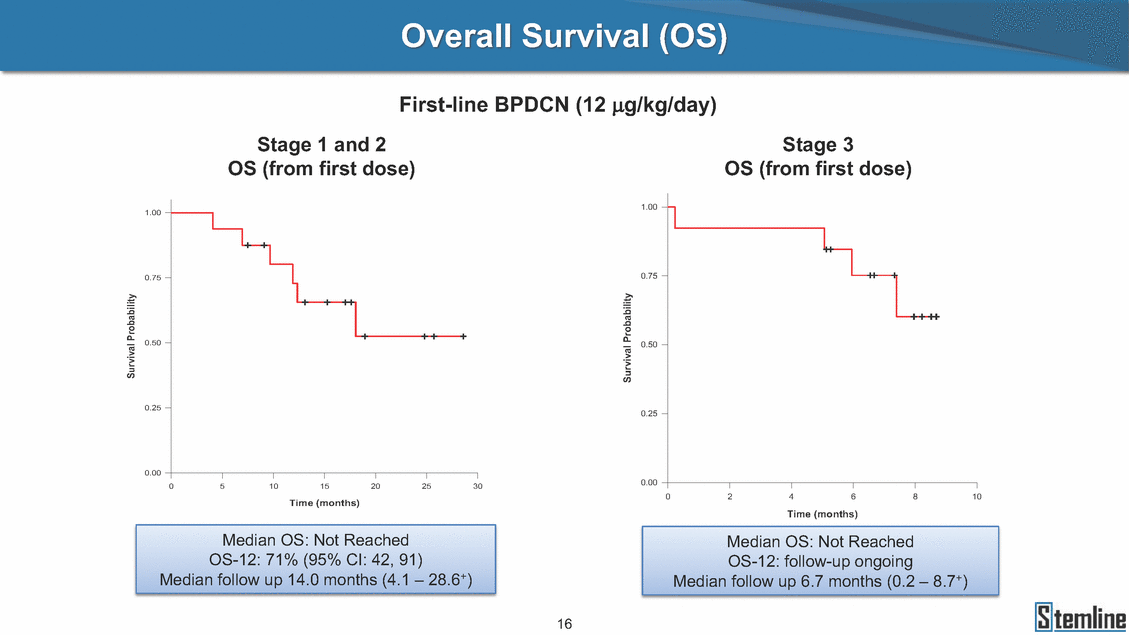
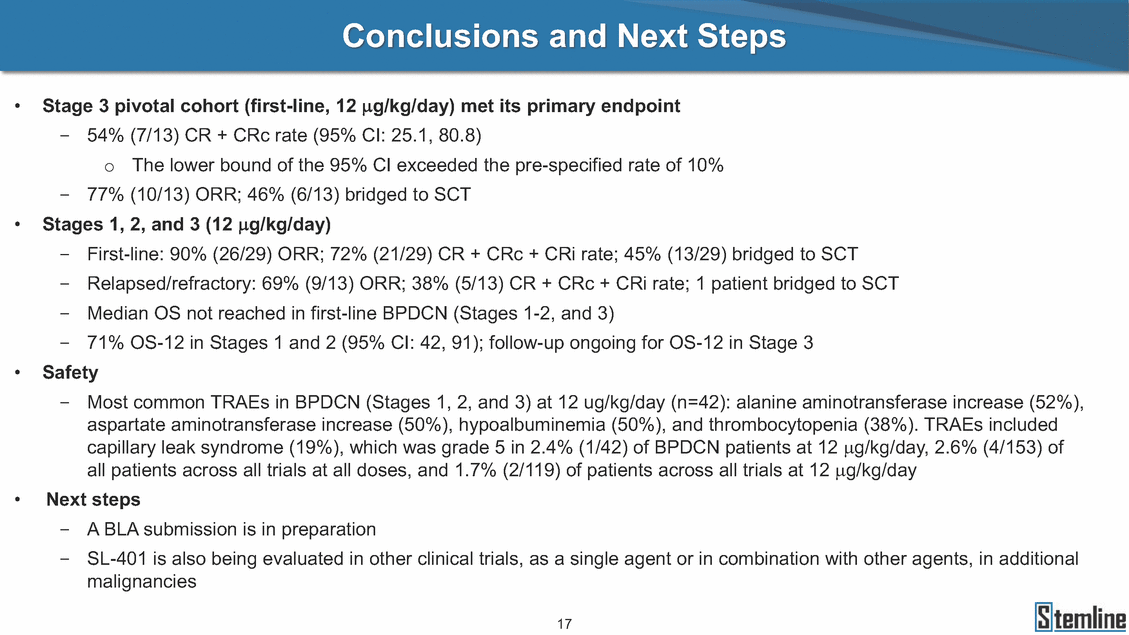
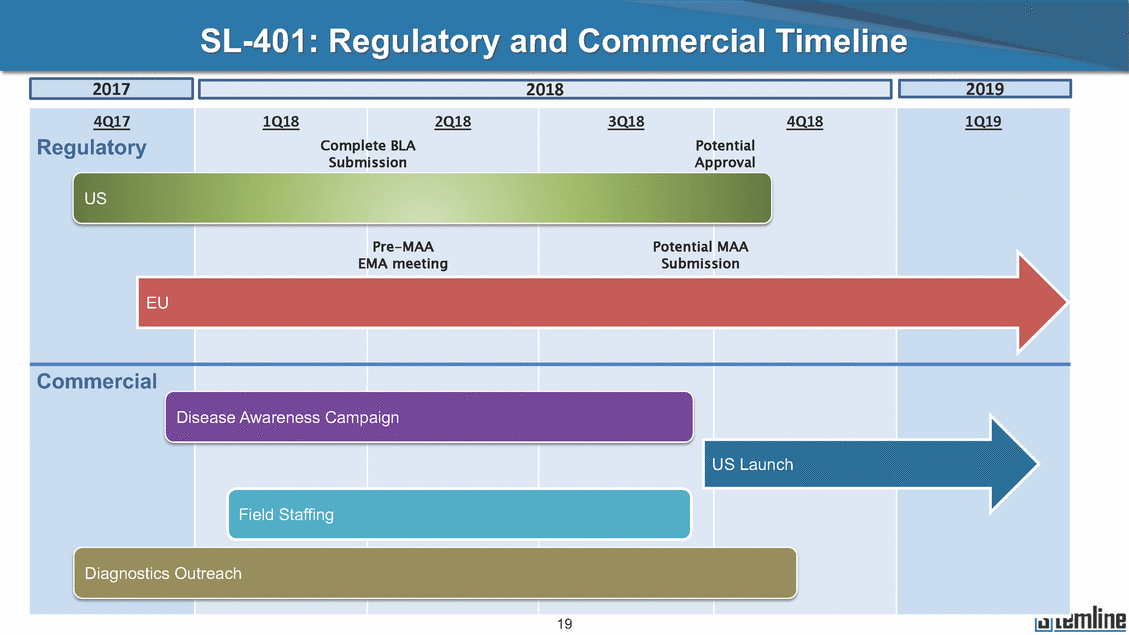
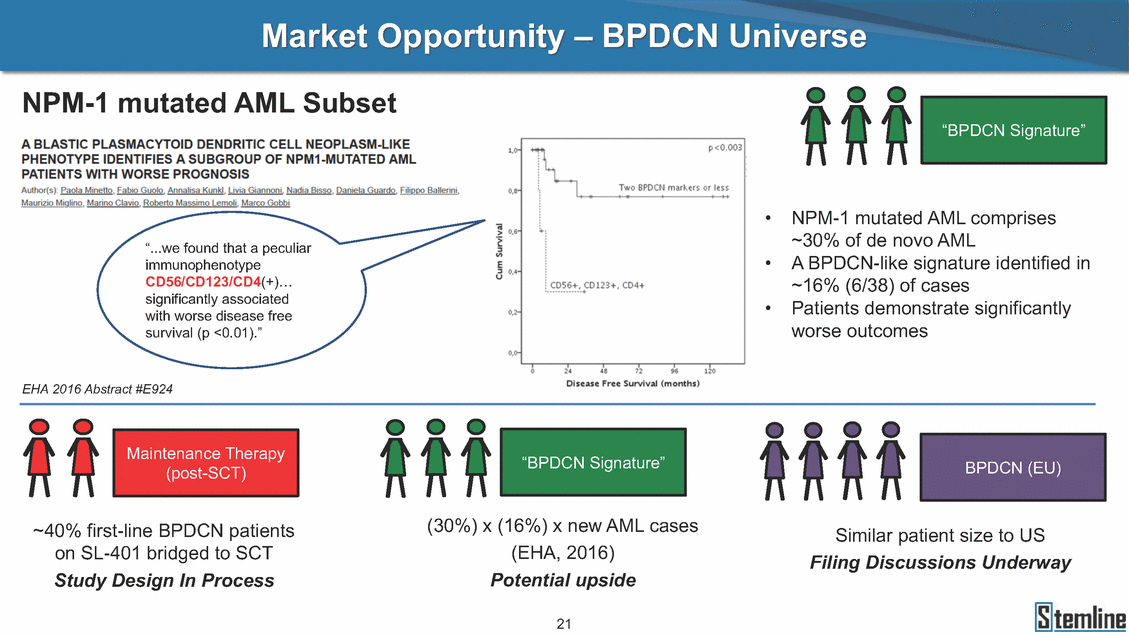
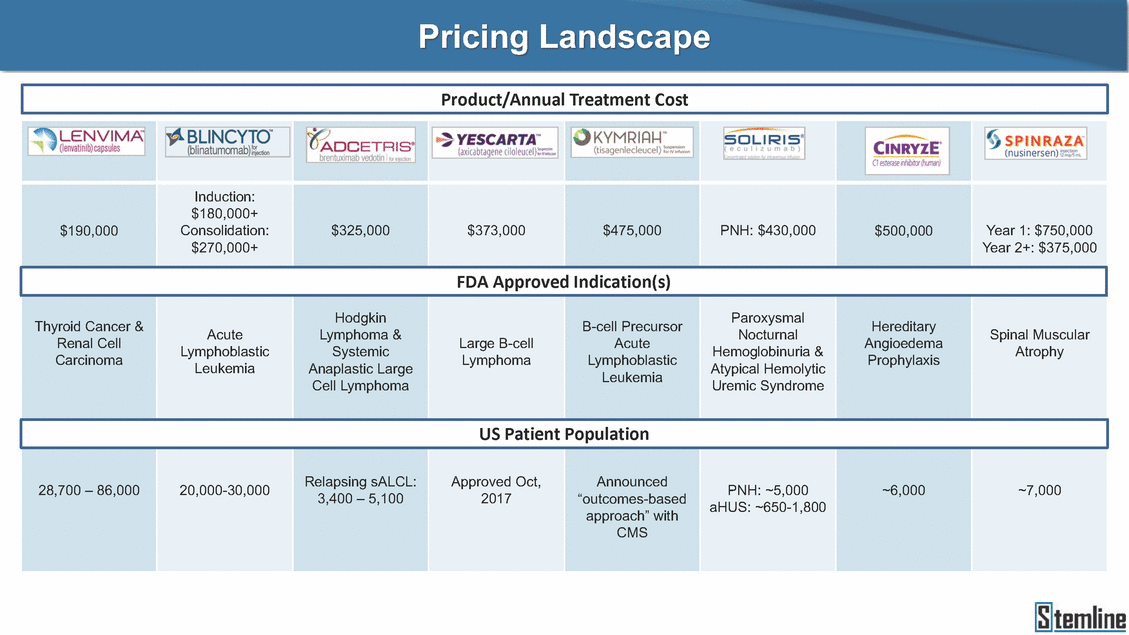
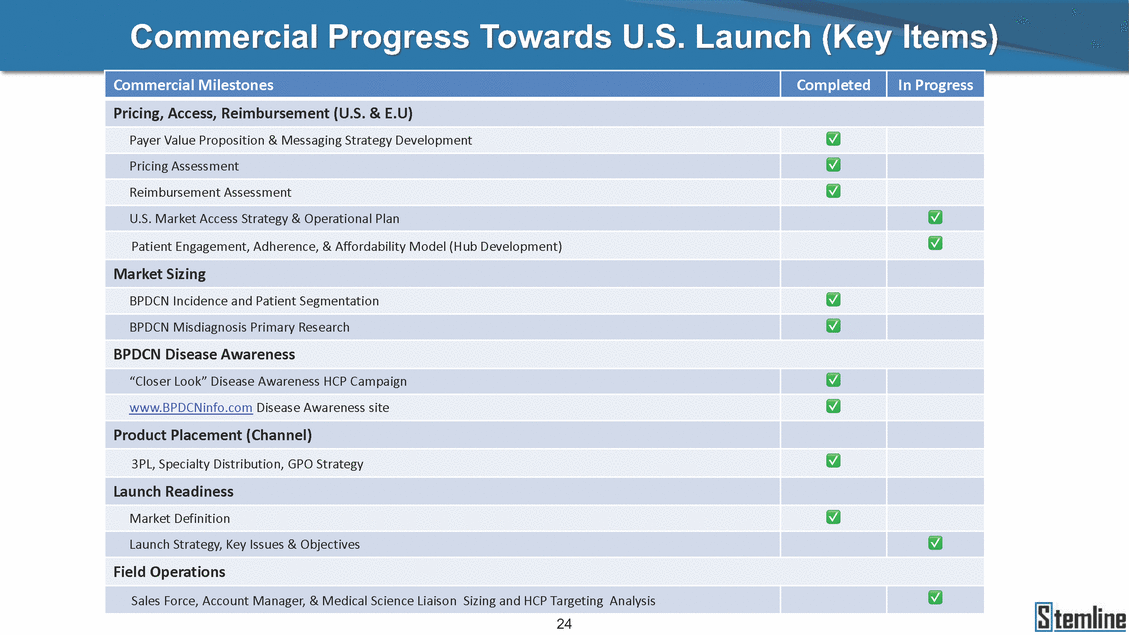
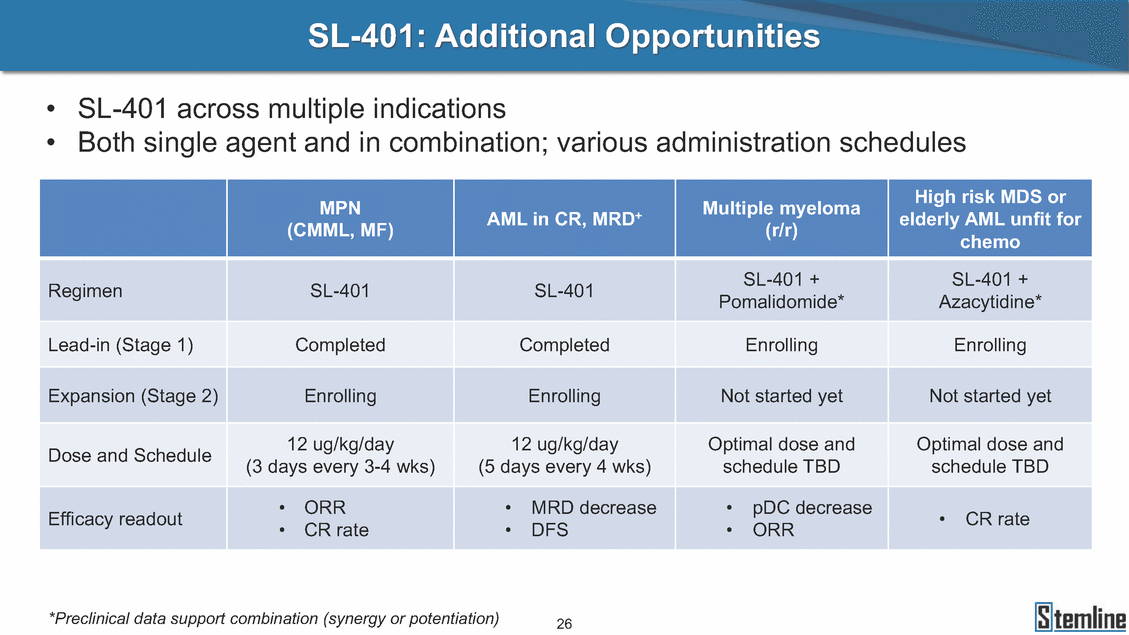
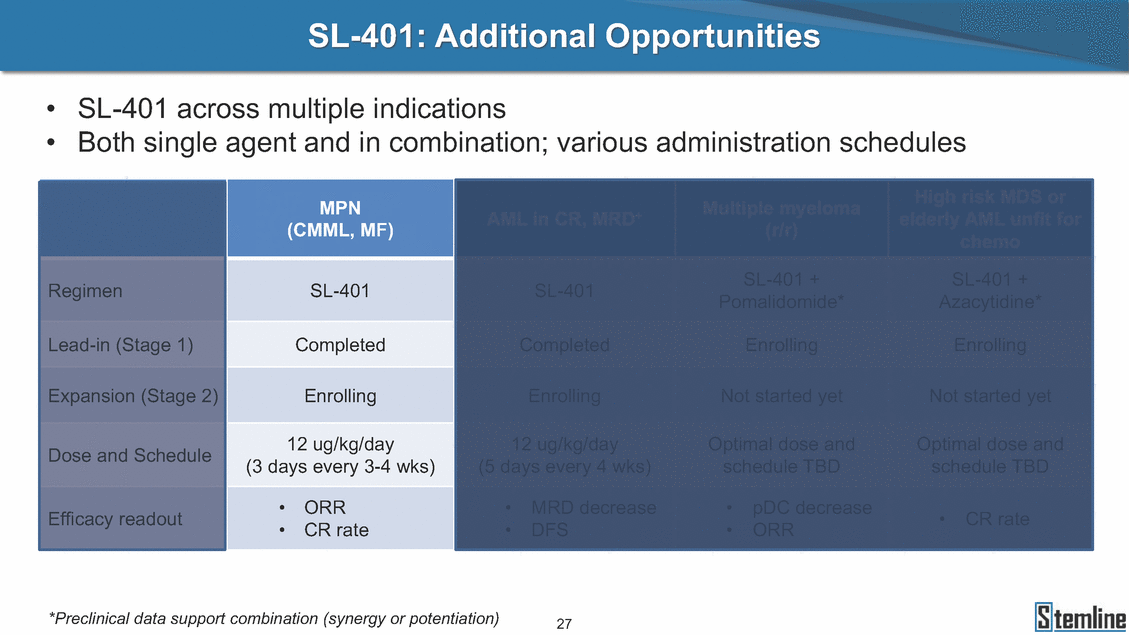
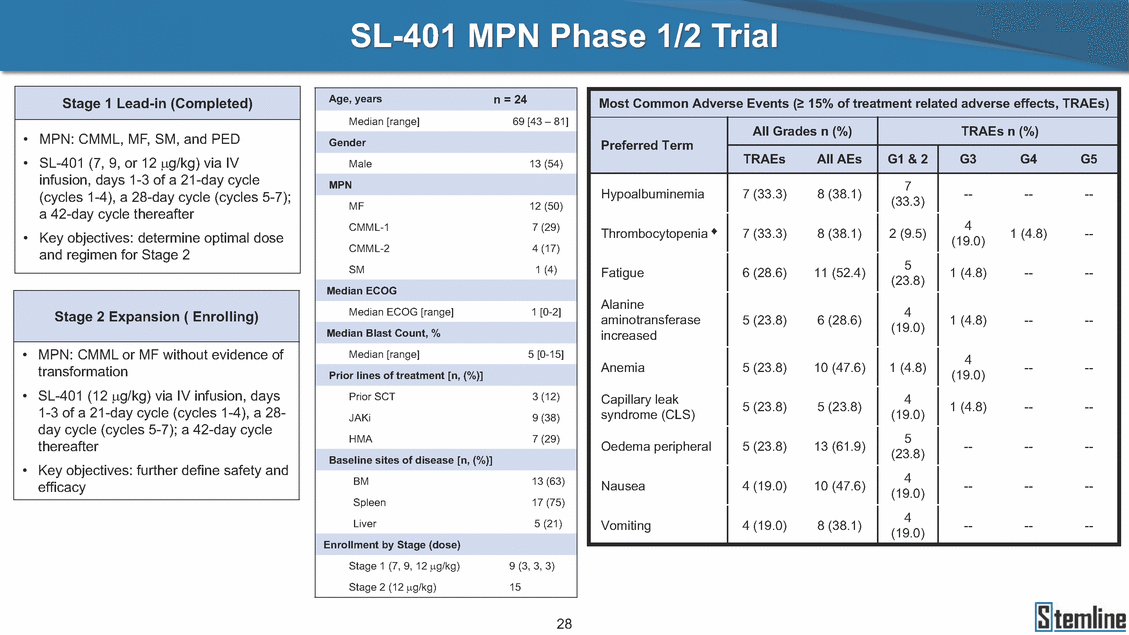
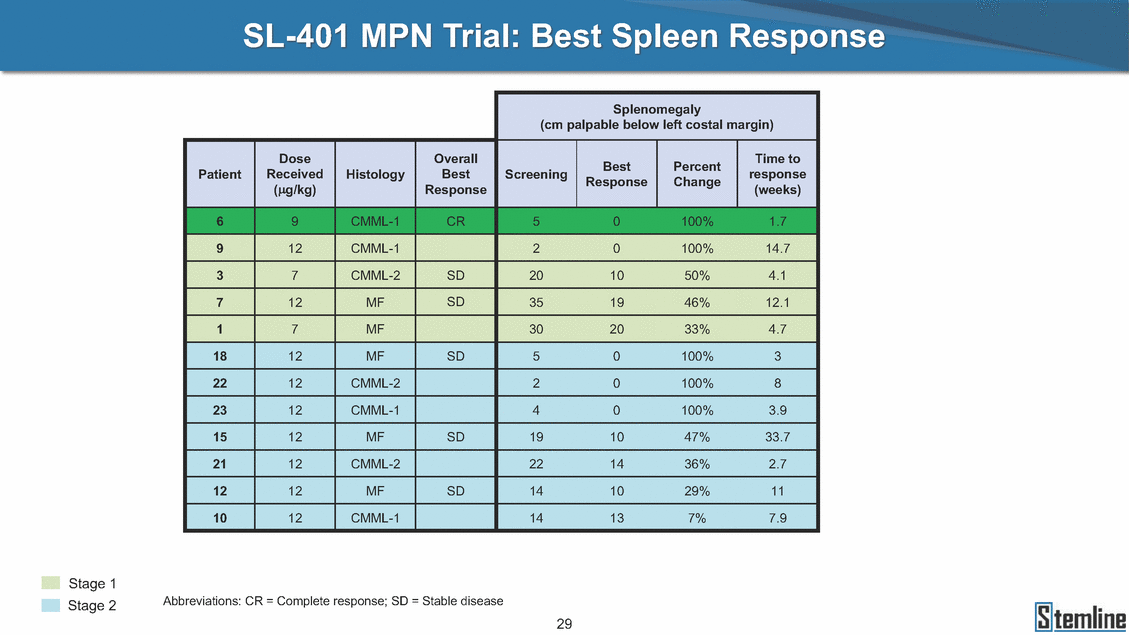
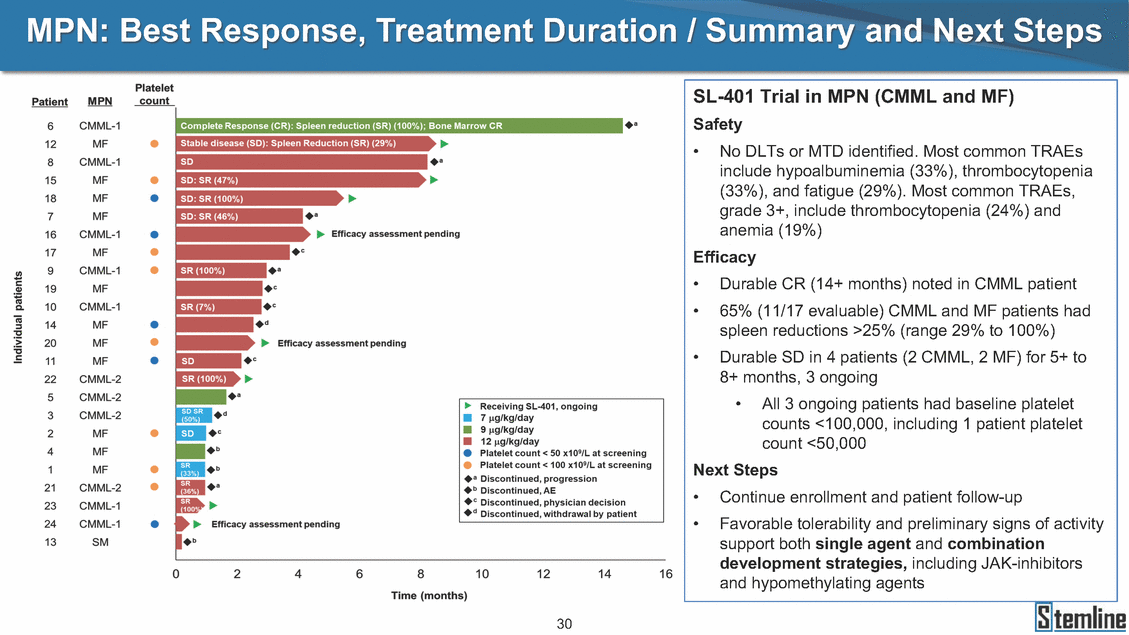
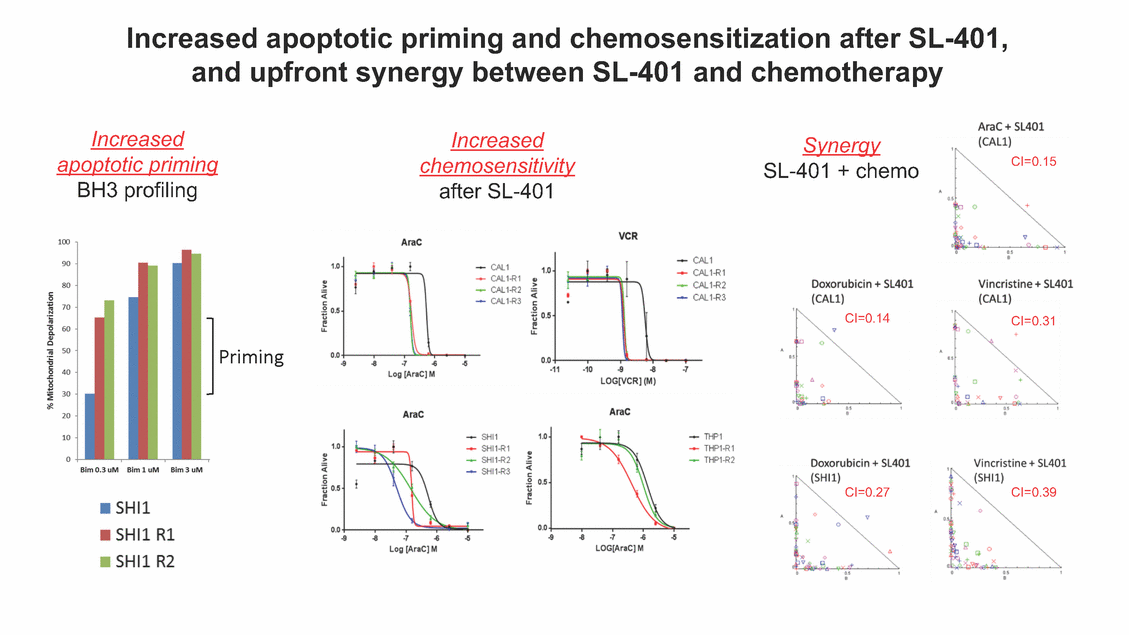
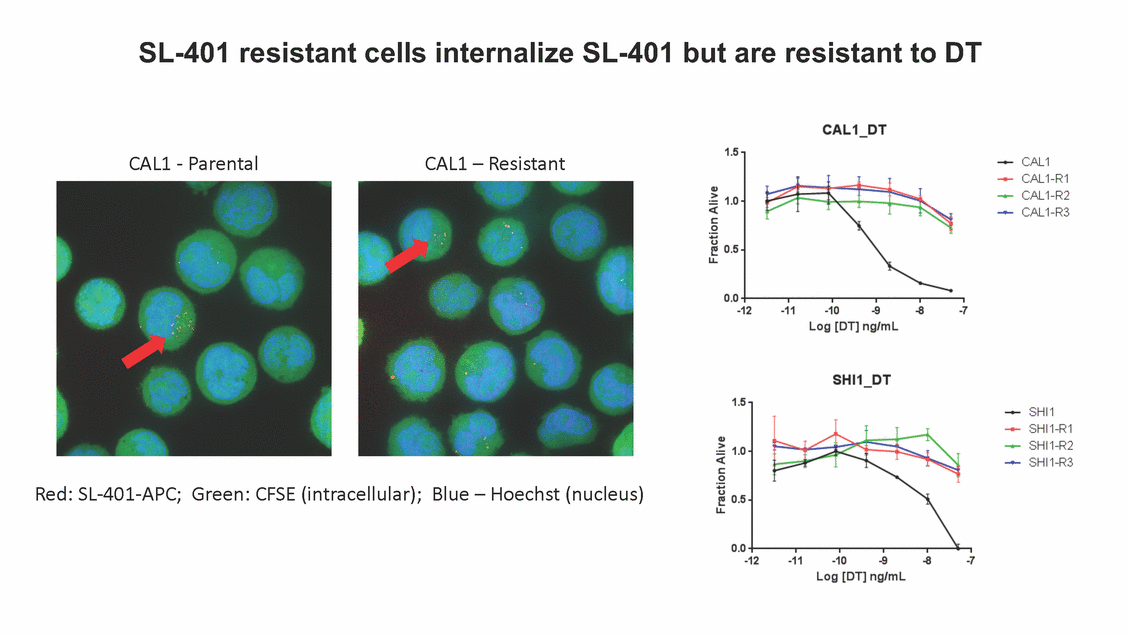
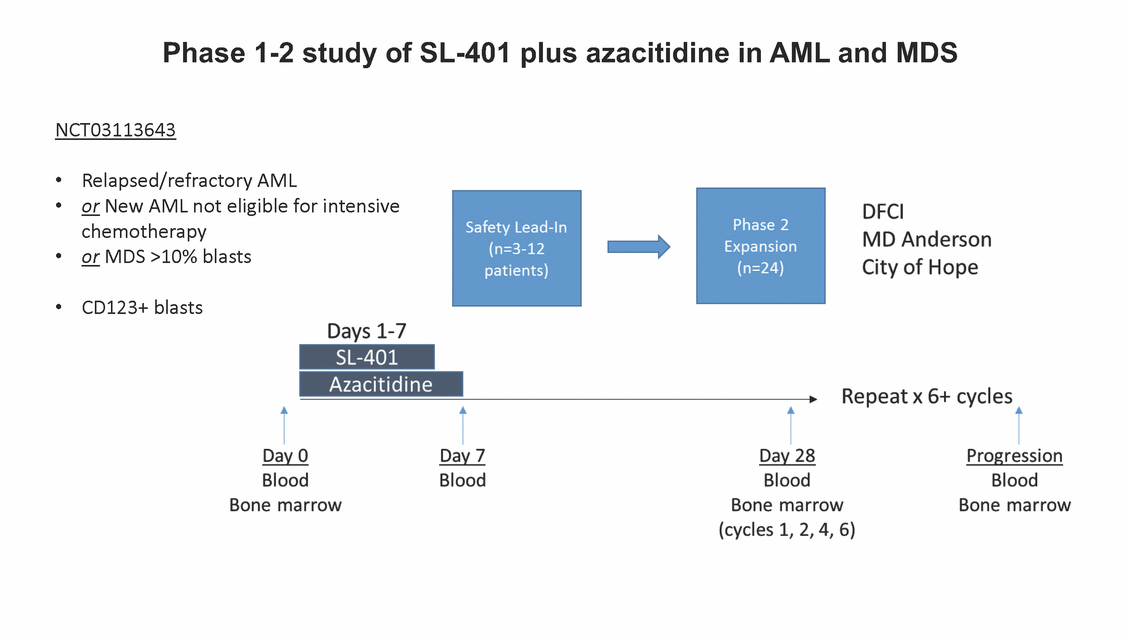
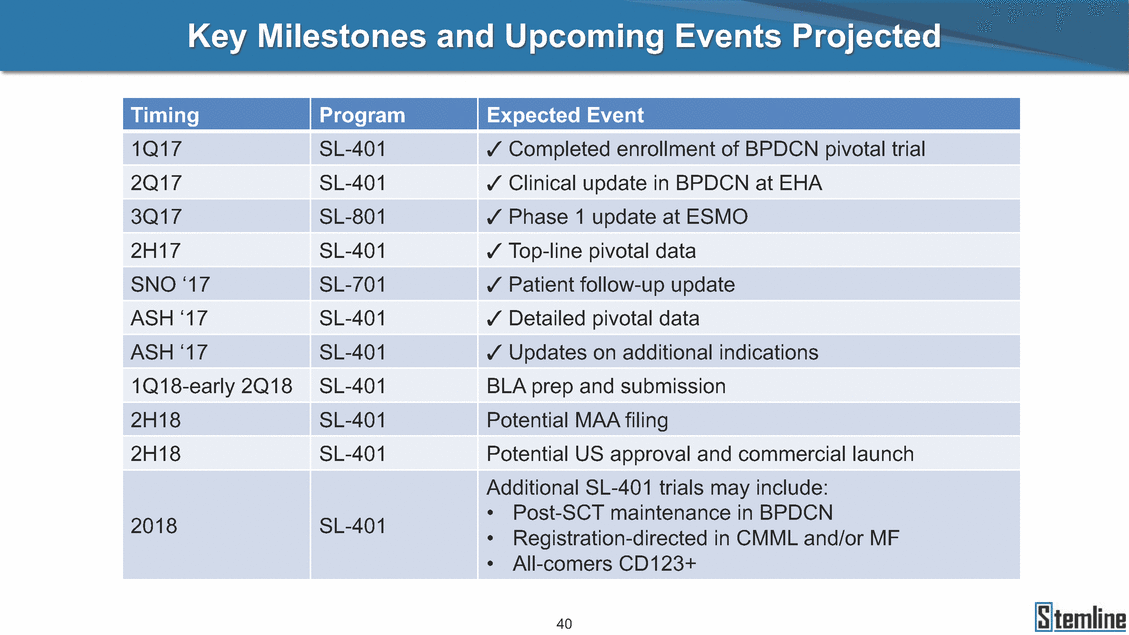
SL-401: Pivotal Trial Results (ASH ‘17) #1298 Naveen Pemmaraju1, Kendra L. Sweet2, Andrew A. Lane3, Anthony S. Stein4, Sumithira Vasu5, William Blum5, David A. Rizzieri6, Eunice S. Wang7, Madeleine Duvic1, Danielle Shubert8, Sharon Spence8, Shay Shemesh8, Janice Chen8, Background and Highlights St • Stage 3 met its primary e neoplasms (MPN), 1L BPDCN, N=13 O(SSta(gfero1-m2, f1i2rsutgd/kog/sdeay) ) O(SSta(fgreo3m, 1f2irusgt/kdgo/dsaey)) BPDCN Age, years median [range] 72 [28-84] 65 [22-84] 70 [22-84] 13 (29) 0% 0% 0% 0% Allo Allo Allo - 6.4+ 6.0+ 5.5+ 7.3 0.50 3 0% 3% Allo Allo 7.5+ 8.3+ 8 (18) Allo 6 0 5 10 15 20 25 30 rug Designation for Time (months) Time (months) Extramedullary (non-cutaneous) 18 (56) 6 (46) 24 (53) 22 CRc 1% 1% 22.0% 0.4% Allo Auto 0 5.3+ the treatment of patients with BPDCN OS-12: 71% (95% CI: 42, 91) OS-12: follow-up ongoing Res n 2 (1L) Stages 1 Common Adverse Events (AEs) (>15% treatment-related AEs, TRAEs)ƾ 100% 100% 100% 14 (33%) TRAEs All AEs Grade 1-2 Grade 3 Grade 4 Grade 5 Auto 3 0 3 75% 75% 75% - Myeloproliferative neoplasms (MPNs) CR + CRc + CRi rate = 33% (1/3). in organs with gross ssment Tool; RFS = relapse 5% CR t response CR 0% CR 0% Screening Best responseScreening Best response 13 CR Allo onclusions and Next Steps u 7 CR primary endpoint rate (95% CI: 25.1, 80.8) -401 • Stages 1, 2, and 3 (12 µg/kg/day) 2 CR Allo Stem cell transplant (SCT) ce As of September 25, 2017. usion, y) Allo • Next steps 0 (0%) 0 (0%) Al mia (AML), and multiple myeloma. Other lanned or R/R) ences 0 (0%) 0 (0%) SL-Stem 4. Chauhan et al. Cancer Cell 2009; 16:309-23 17 Exited study in remission ¥ 11. Black et al. Leukemia 2003; 17:155-9 5. Frovola et al. Br J Haematol. 2014; 166:862-74 12. Diefenbach et al. Blood 2011; 118:3737 0 2 4 6 8 10 7. Munoz ll trials and doses, and a rate ¥ Patient relapsed off study employment, ployment, equity ownership; McDonald: Stemline - employment, equity ownership; Goswami: Stemline - employment, equity mmaraju 7 Individual patients Individual patients % Bone marrow blasts Survival Probability Survival Probability ASH 2017 Results of Pivotal Phase 2 Trial of SL-401 in Patients with Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) Christopher L. Brooks8, Ivan Bergstein8, Peter McDonald8, Trishna Goswami8, Mark Sloan9, Jeffrey E. Lancet2, Hagop M. Kantarjian 1The University of Texas MD Anderson Cancer Center, Houston, TX; 2H. Lee Moffitt Cancer Center, Tampa, FL; 3Dana-Farber Cancer Institute, Boston, MA; 4City of Hope Na Columbus, OH; 6Duke University Medical Center, Durham, NC; 7 Roswell Park Cancer Institute, Buffalo, NY; 8Stemline Therapeutics, Inc., New York, NY;9 Bos 1, Marina Konopleva1 tional Medical Center, Duarte, CA; 5The Ohio State University, ton University School of Medicine, Boston, MA. SL-401 and CD123 • SL-401 is a novel tar • CD123 is expressed neoplasm (BPDCN), multiple myeloma, a geted therapy directed to the interleukin-3 receptor (IL-3 on multiple malignancies including blastic plasmacytoid acute myeloid leukemia (AML), certain myeloproliferativ nd a variety of other myeloid and lymphoid malignancies Demographics Clinical Activity age 3: BPDCN (12 µg/kg/day) (n=13 patients) serve as the pivotal, confirmatory cohort for the SL-401 trial in B endpoint with a 54% rate of CR + CRc (7/13) [95% CI: 25.1, 80.8 the 95% CI exceeded the pre-specified rate of 10% PDCN ] Overall Survival (OS) First-line BPDCN (12 µg/kg/day) Rα; CD123) dendritic cell Demographics Stages 1 & 2 Stage 3 Stages 1, 2 & 3 • Stage 3 was designed to SOtav 1L gereall1Saunrvdiv2al BPDCN, N=16 OvSertaallgSeur3vival n 32 13 45 - The lower bound of • Highly aggressive h • Poor prognosis, with • Primary sites of dise • Named “BPDCN” by include TCL-1 - Derived from prec - Previous names ( hematodermic ne • No approved therapi SL-401 and BPDCN • SL-401 was granted ematologic malignancy a median overall survival (OS) 8-14 months from diagno ase: bone marrow and skin; secondary sites: lymph nod WHO (2008); diagnostic criteria include CD123, CD4, C ursor of plasmacytoid dendritic cell (pDC) prior to 2008): blastic NK cell leukemia/lymphoma, agran oplasm es or standard of care; unmet medical need Breakthrough Therapy Designation (BTD) and Orphan D Gender, Male [n, (%)] sis es, viscera First-line (1L) [n, (%)] D56, other markers Relapsed / Refractory (R Baseline sites of disease ular CD4+/56+ Cutaneous Bone Marrow Peripheral Blood 26 (81) 11 (85) 19 (59) 13 (100) /R) [n, (%)] 13 (41) --[n, (%)] 30 (94) 13 (100) 16 (50) 7 (54) 5 (16) 3 (23) Stage 3 respondersɸ Stage 3 complete respondersɸ 37 (82) 32 (71) Line of Therapy Firs Dose Level 12 µ t-line g/kg Bone Marrow Skin (% Blasts) (mSWAT) Age Best Best Bes (years) Response Baseline response Baseline respo t RFS nse SCT (months) 1.00 0.75 1.00 0.75 0.50 0.25 n 1 ORR, n (%) 10 ( CR + CRc + CRi, n (%) 7 (5 CR 43 (96) CRc CRi 23 (51) PR, n (%) 3 (2 Bridged to SCT, n (%) 6 (4 3 77%) 4%) 69 CR 94% 2% 1.2% 0. 68 CRc 70% 2% 72.0% 0. 4 57 CR 66% 2% 35.0% 0. 0 3%) 74 CRc 22% 1% 54.0% 4. 6%) 65 CR 12% 1% 70.0% 0. 32 CRc 2% 2% 18.0% 0. 0.25 0.00 0.00 0 2 4 6 8 10 • Pivotal Phase 2 trial here - Trial consisted of 3 (pivotal, confirm - Stage 3 pivotal, co - A Biologics Licens of SL-401 in BPDCN has completed enrollment and resu 3 stages: Stage 1 (lead-in, dose escalation), Stage 2 (exp atory) nfirmatory cohort has met its primary endpoint e Application (BLA) submission is in preparation lts are presented ansion), and Stage Dose [n] 7 µg/kg/day 12 µg/kg/day 3 --29 13 3 42 Stages 1 Stages 1, 2, and 3 respond Line of Therapy n ORR, n (%) CR + CRc + CRi, n (%) CR CRc CRi PR, n (%) Bridged to SCT, n (%) Allo , 2, and 3: BPDCN (12 µg/kg/day) (n=42 patie ersɸ,ɣ 1L R/R 29 13 26 (90%)9 (69%) 21 (72%)5 (38%) 12 1 7 3 2 1 5 (17%)4 (31%) 13 (45%)1 (8%) 10 1 nts) 1L & R/R Median OS: Not Reached Median OS: Not Reached Me dian follow up 14.0 months (4.1 – 28.6+) Bone Marrow BPDCN (12 µg/kg/day); S M tages and 2 (R edian follow up 6.7 months (0.2 – 8.7+) ponses 1, 2, and 3ȸ /R) Stage 3 (1L) 42 35 (83%) 26 (62%) 13 10 3 9 (21%) St ages 1 and BPDC Safety and Tolerability N (12 µg/kg/day); Stages 1, 2, and 3 (n=42 patie nts) SL • SL-401 is a novel targ • CD123 is expressed - BPDCN - AML blasts and c -401 Mechanism of Actio eted therapy directed to the interleukin-3 receptor (IL-3Rα; on multiple malignancies1-9, including: ancer stem cells (CSCs) CD123) Most Preferred Term All Grades n (%) TRAEs n (%) 11 - Multiple myelom plasmacytoid de myeloma bone m - Myelodysplastic myeloid leukemi leukemia (ALL), non-Hodgkin’s ly • SL-401 demonstrated CML, HL and NHL, M • An earlier investigator demonstrated clinical a, including neighboring ndritic cells (pDCs) in the arrow microenvironment syndrome (MDS), chronic a (CML), acute lymphoid Hodgkin’s and certain mphomas (HL, NHL) preclinical activity against a variety of malignancies, includi PNs, multiple myeloma, and others10-13 -sponsored Phase 1/2 clinical trial evaluating a single cycle activity in BPDCN and relapsed/refractory AML1 Alanine aminotransferase Aspartate aminotransferas Hypoalbuminaemia ThrombocytopeniaЖ Chills ng BPDCN, AML, Pyrexia Weight increased of SL-401 Nausea increased 22 (52%) 28 (67%) 7 (17%) 15 (36%) e increased 21 (50%) 25 (60%) 6 (14%) 14 (33%) 21 (50%) 22 (52%) 21 (50%) 0 (0%) 16 (38%) 21 (50%) 3 (7%) 3 (7%) 12 (29%) 14 (33%) 11 (26%) 1 (2%) 12 (29%) 18 (43%) 12 (29%) 0 (0%) 12 (29%) 17 (40%) 12 (29%) 0 (0%) 10 (24%) 20 (48%) 10 (24%) 0 (0%) 0 (0%) 0 (0%) ɸ As of September 25, 2017. Investig 1 (2%) 0 (0%) ɣ In addition, in Stage 1 (lead-in, dose Abbreviations: ORR = overall respon 0 (0%) 0 (0%) reduction in cutaneous lesions and re partial response; SCT = stem cell tran 10 (24%) 0 (0%) free survival. 0 (0%) 0 (0%) S 0 (0%) 0 (0%) 0 (0%) 0 (0%) 16 CR 15 CR 0 (0%) 0 (0%) 14 CRc ator-assessed. escalation), 3 BPDCN patients received SL-401 at 7µg/kg/day, of which there was 1 C se rate; CR = complete response; CRc (clinical CR) = absence of gross disease in non-sk sidual microscopic skin disease; CRi = CR with incomplete hematologic recovery; CI = splant; Allo = allogeneic; Auto = autologous; mSWAT = modified Severity-Weighted Asse tages 1 and 2: First-line BPDCN (12 µg/kg/da R and 1 PR: ORR: 67% (2/3),50% 50% 50% confidence interval; PR = y) Auto u Auto 25% 5% 0% ȸ Sc reening Bes 25% PR 5% 25% PR PR n=38 eval uable patients; four patients treated at 12 µg/kg did not have foll ow-up bon e marrow results. • Ongoing SL-401 clinic - BPDCN (NCT021 - AML in remission - MPNs (chronic m - Multiple myeloma - High-risk MDS an al trials include: 13982) with minimal residual disease (MRD) (NCT02270463) yelomonocytic leukemia [CMML] and myelofibrosis [MF]) (N (SL-401 + pomalidomide) (NCT02661022) d elderly AML (SL-401 + azacitidine) (Investigator-sponsore CT02268253) d / NCT03113643) Capillary leak syndrome (C Fatigue Decreased appetite Hypotension All SL LS)ђ8 (19%) 8 (19%) 6 (14%) 0 (0%) 8 (19%) 20 (48%) 8 (19%) 0 (0%) 7 (17%) 12 (29%) 7 (17%) 0 (0%) 7 (17%) 13 (31%) 5 (12%) 2 (5%) -401 Clinical Trials (12 µg/kg/day) (n=114 patien 1 (2%) 1 (2%) 12 PR 0 (0%) 0 (0%) 11 CR 10 CR 0 (0%) 0 (0%) 9 CR 8 CRc 0 (0%) 0 (0%) 6 CRi 5 CRc ts) 4 PR 3 CRi Allo Allo Auto • Pivotal - Tri • Stage - 54 trial of al consi 3 pivot % (7/13 C SL-401 in sted of 3 St al cohort (fir ) CR + CRc BPDCN has completed enrollmen ages: Stage 1 (lead-in), Stage 2 (ex st-line, 12 µg/kg/day) met its t pansio n), and Stage 3 (pivotal, confirmatory) - Als o The l o: 77% ower bound (10/13) OR of the 95% CI exceeded the pre-sp R; 46% (6/13) bridged to SCT ecified rate of 10% Study D esign and Inclusion / Excl usion 10 15 Time (Months) Investigator-assessed. Stage 3: First-line BPDCN (12 µ SL Re 20 g/kg/da iving SL-401, ongoing-Fir 25 30 - Re - M - 71 • Safety - M inc (3 st-line: 9 lapsed/ edian O % OS-1 ost com rease ( 8%). TR 0% (26/29) refractory: S not reach 2 in Stages mon TRAEs 52%), aspa AEs includ ORR; 72% (21/29) CR + CRc + C 69% (9/13) ORR; 38% (5/13) CR + ed in first-line BPDCN (Stages 1-2, 1 and 2 (95% CI: 42, 91); follow-up in BPDCN (Stages 1, 2, and 3) at rtate aminotransferase increase (50 ed capillary leak syndrome (19%), Ri rate; CRc + and 3) ongoin 12 ug/kg %), hyp which w 45% (13/29) bridged to SCT CRi rate; 1 patient bridged to SCT g for OS-12 in Stage 3 /day (n=42): alanine aminotransferase oalbuminemia (50%), and thrombocytopenia as grade 5 in 2.4% (1/42) of BPDCN patients Most Co Preferred Term mmon Adverse Events (AEs) (>15% treatment-related AEs, TRA All Grades n (%) TRAEs TRAEs All AEs Grade 1-2 Grade 3 Es)ƾ n (%) Grade 4 Grade 5 1 CR 0 5 Stage 1 (Lead-in, dose escalati on) (Pivot Stage 3 al, confirmatory) Hypoalbuminaemia Alanine aminotransferase Aspartate aminotransferas 56 (49%) 62 (54%) 55 (48%) 1 (1%) increased 55 (48%) 65 (57%) 25 (22%) 30 (26%) e increased 55 (48%) 63 (55%) 24 (21%) 27 (24%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 4 (4%) 0 (0%) • BPDCN (1L and R/R)ǂ SL-40 9, 12, or 16 µg/kg) via IV inf days 1-5 of a 21-day cycle 1 (7, • BPDCN (1L and R/R) • BPDCN (1 • SL-401 (12 µg/kg) via IV infusion, • SL-401 (1 days 1-5 of a 21-day cycle days 1-5 o L) 2 µg/kg) via IV infusion, f a 21-day cycle • Key objectives: determine o dose and regimen♦ for Stag Select inclusion criter • Patient Population: - Stage 1: BPDCN (1L - Stage 2: BPDCN (1L - Stage 3: BPDCN (1L • Age ≥ 18; ECOG PS 0-2 • Adequate organ function of normal, creatinine ≤ 1. bilirubin ≤ 1.5 mg/dL, AS ptimal • Key objectives: further define • Key object e 2 safety and efficacy confirmati ia Select exclusion crit • Persistent clinically signi or R/R) prior chemotherapy • Received chemotherapy investigational therapy w ) • Clinically significant card • Receiving immunosuppr including: LVEF ≥ lower limit 5mg/dL, albumin ≥ 3.2 g/dL, T/ALT ≤ 2.5x ULN ives: efficacy on for registration ThrombocytopeniaЖ Nausea Pyrexia eria Chills ficant toxicities from Fatigue or other Capillary leak syndrome (C ithin the prior 14 days Weight increased iopulmonary disease essive therapy Hypotension Oedema peripheral Vomiting 33 (29%) 42 (37%) 7 (6%) 6 (5%) 31 (27%) 58 (51%) 31 (27%) 0 (0%) 29 (25%) 49 (43%) 29 (25%) 0 (0%) 26 (23%) 38 (33%) 25 (22%) 1 (1%) 26 (23%) 57 (50%) 22 (19%) 4 (4%) LS)ђ23 (20%) 23 (20%) 15 (13%) 5 (4%) 22 (19%) 33 (29%) 22 (19%) 0 (0%) 20 (18%) 33 (29%) 17 (15%) 3 (3%) 19 (17%) 50 (44%) 18 (16%) 1 (1%) 17 (15%) 30 (26%) 17 (15%) 0 (0%) 20 (18%) 0 (0%) 29 CRc 28 CR 0 (0%) 0 (0%) 27 CR 0 (0%) 0 (0%) 26 CR 25 CRc 0 (0%) 0 (0%) 24 CRc 2 (2%) 1 (1%) 23 CRc 0 (0%) 0 (0%) 22 PR 21 PR 20 SD 0 (0%) 0 (0%) 19 SD 0 (0%) 0 (0%) 18 PR Allo Allo Allo at all 12 µg/kg trials at /day, 2.6% 12 µg/kg/d (4/153) of all patients across all tria ay ls at all doses, and 1.7% (2/119) of patients across Allo lo - A - If - SL ce tri BLA sub BLA revi -401 is rtain my als are p mission is i ew is succe also being eloprolifera n preparation ssful, SL-401 would represent the evaluated in other clinical trials, as tive neoplasms (MPN), acute myel Refer first ther a single oid leuke apy approved for this deadly malignancy agent or in combination with other agents, in 401 cell trans plant (SCT) 1. Franke 2. Jordan 3. Pardan l et al. Bl et al. Le ani et al. ood 2014; 12 ukemia 2000 Leukemia 2 4:385-928. A ; 14:1177-849. T 015; 29:1605-810. C ldinucci ehranchi hristie et et al. Leuk Lymphoma 2005; 46:303-11 et al. NEJM 2010; 363:1025-37 al. ASH 2015; Abstract #3797 Ø To ensure ongoing access ǂ Stage 1 also enrolled AML (R/R) pat ♦ In BPDCN, 12 µg/kg/day was highe Abbreviations: 1L = first-line; R/R = rel = left ventricular ejection fraction; ULN to SL-401, BPDCN patients are being enrolled in an additional ients, to be reported separately st tested dose (MTD not reached) and selected for Stage 2 apsed/refractory; IV = intravenous; ECOG PS = Eastern Cooperative Oncology G = upper limit of normal; MTD = maximum tolerated dose cohort, Stage 4 ƾ As of October 18, 2017. Ж Includes low platelets and plate ђ An additional grade 5 CLS-relate CLS-related events that occurred roup performance status; LVEF of 1.7% (2/119) for all trials at the lets decreased. d event occurred in one Stage 4 BPDCN patient (of 11 enrolled) which, in addition to t at 7 µg/kg/day (BPDCN) and 16 µg/kg/day (AML), reflects a rate of 2.6% (4/153) for a 12 µg/kg/day dose as of 11-21-17. 6. Cousta n-Smith et al. Ha Shubert: equity own et al. Blood 2 ematologica Stemline - em ership; Chen : Stemline-rese 011; 117:6267-627613. B 2001; 86-1261-9 ployment, equity ownership; Spence: Stemlin : Stemline - employment, equity ownership; arch funding; Lane: Stemline-research fundi rooks et e - empl Brooks: St ng; Konop al. Blood 2013; 122:4104 oyment, equity ownership; Shemesh: Stemline - emline - employment, equity ownership; Bergstein: leva: Stemline-research funding wo previously reported grade 5 As of September 25, 2017. I nvestigator-assessed.Time (Months) at 7.3 months. Disclosures: Stemline - em ownership; Pe Stage 2 (Expansion)