
Fourth Quarter & Full Year 2016 Results Conference Call Thursday, March 2, 2017 Exhibit 99.2

Safe Harbor Statement This presentation contains "forward-looking statements," within the meaning of the Private Securities Litigation Reform Act of 1995, regarding, among other things, the opportunity for further growth in 2017 for ILUVIEN. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of them, and could cause actual results to differ materially from those projected in its forward-looking statements. Words such as "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "contemplate," "predict," "project," "target," "likely," "potential," "continue," "ongoing," "will," "would," "should," "could," or the negative of these terms and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of them, and could cause actual results to differ materially from those projected in its forward-looking statements. Meaningful factors which could cause actual results to differ include, but are not limited to, market acceptance and physician awareness of ILUVIEN in the U.S. and Europe, as well as other factors discussed in the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Alimera's Annual Report on Form 10-K for the year ended December 31, 2015 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2016, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at http://www.sec.gov. Additional factors may also be set forth in those sections of Alimera’s Annual Report on Form 10-K for the year ended December 31, 2016, to be filed with the SEC in the first quarter of 2017. In addition to the risks described above and in Alimera's Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, other unknown or unpredictable factors also could affect Alimera's results. There can be no assurance that the actual results or developments anticipated by Alimera will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Alimera. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved. All forward-looking statements contained in this presentation are expressly qualified by the cautionary statements contained or referred to herein. Alimera cautions investors not to rely too heavily on the forward-looking statements Alimera makes or that are made on its behalf. These forward- looking statements speak only as of the date of this presentation (unless another date is indicated). Alimera undertakes no obligation, and specifically declines any obligation, to publicly update or revise any such forward-looking statements, whether as a result of new information, future events or otherwise. © 2017 Alimera Sciences, Inc., All Rights Reserved 2
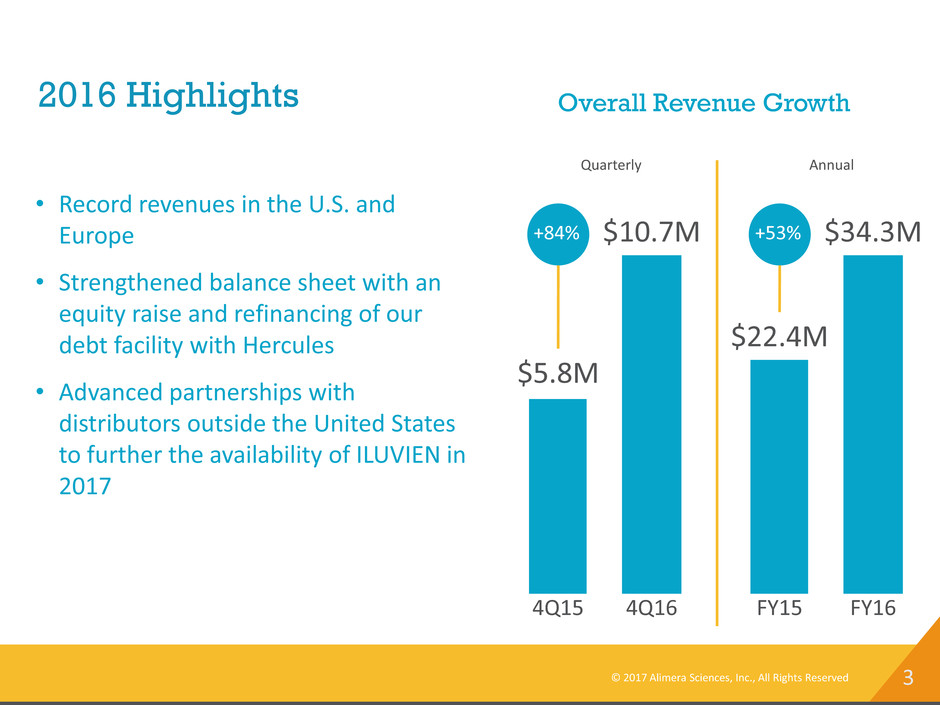
3 2016 Highlights Overall Revenue Growth 4Q16 $10.7M 4Q15 $5.8M +84% • Record revenues in the U.S. and Europe • Strengthened balance sheet with an equity raise and refinancing of our debt facility with Hercules • Advanced partnerships with distributors outside the United States to further the availability of ILUVIEN in 2017 FY16 $34.3M FY15 $22.4M +53% Quarterly Annual © 2017 Alimera Sciences, Inc., All Rights Reserved

4 Real World Data © 2017 Alimera Sciences, Inc., All Rights Reserved Clinical Data • We believe real world data from Europe provides evidence that ILUVIEN is working the same or better than in the FAME pivotal study, a result not typically seen in retina Podium & Presentations • 27 abstracts on ILUVIEN selected for presentation at the Association for Research in Vision and Ophthalmology (ARVO) in May 2017 • Largest presence of ILUVIEN at any meeting since launch, either in the U.S. or in Europe

5 Advancing the Availability of ILUVIEN in 2017 We expect Italy and the Middle East to have a positive impact on our international segment in the 2nd half of 2017 • In Italy – our distributor plans to launch in the 2nd quarter of 2017 • In the Middle East – our distributor expects to grow sales in 2017 as they expand their reach in various Middle East countries We expect to launch directly in two additional European countries in 2017 using our existing infrastructure • Austria • Ireland © 2017 Alimera Sciences, Inc., All Rights Reserved

Financial Overview Rick Eiswirth, President & CFO

7 Managing Our Spend Because of our positive real world data, we are able to reduce the future spend of two large clinical trials: • Capped enrollment in our European registry study, awaiting final approval • Capped enrollment in Paladin study (U.S.), which will allow an earlier assessment of data © 2017 Alimera Sciences, Inc., All Rights Reserved

8 Fourth Quarter 2016 Revenues 4Q15 $5.8M 4Q16 $10.7M +$4.9M 84% © 2017 Alimera Sciences, Inc., All Rights Reserved
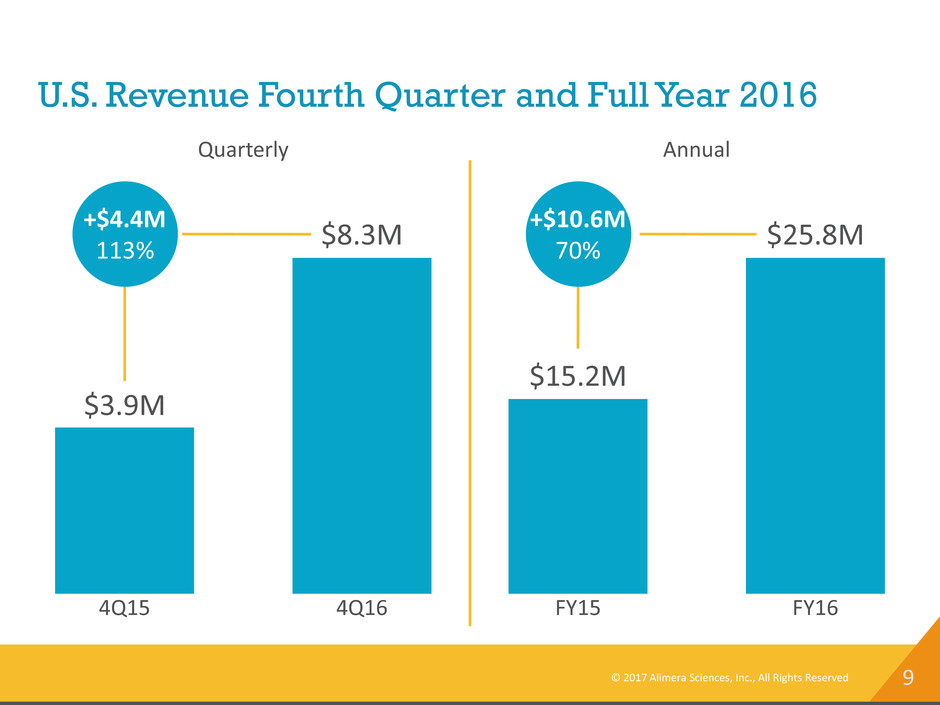
9 U.S. Revenue Fourth Quarter and Full Year 2016 4Q15 $3.9M 4Q16 $8.3M +$4.4M 113% Quarterly FY15 $15.2M FY16 $25.8M +$10.6M 70% Annual © 2017 Alimera Sciences, Inc., All Rights Reserved
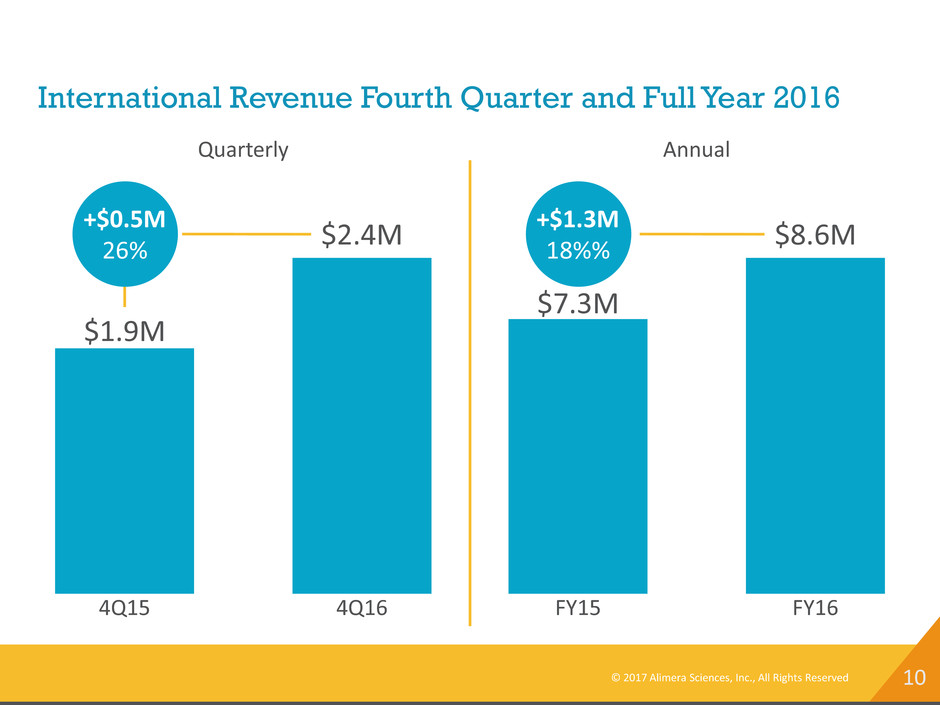
10 International Revenue Fourth Quarter and Full Year 2016 4Q15 $1.9M 4Q16 $2.4M +$0.5M 26% Quarterly FY15 $7.3M FY16 $8.6M +$1.3M 18%% Annual © 2017 Alimera Sciences, Inc., All Rights Reserved
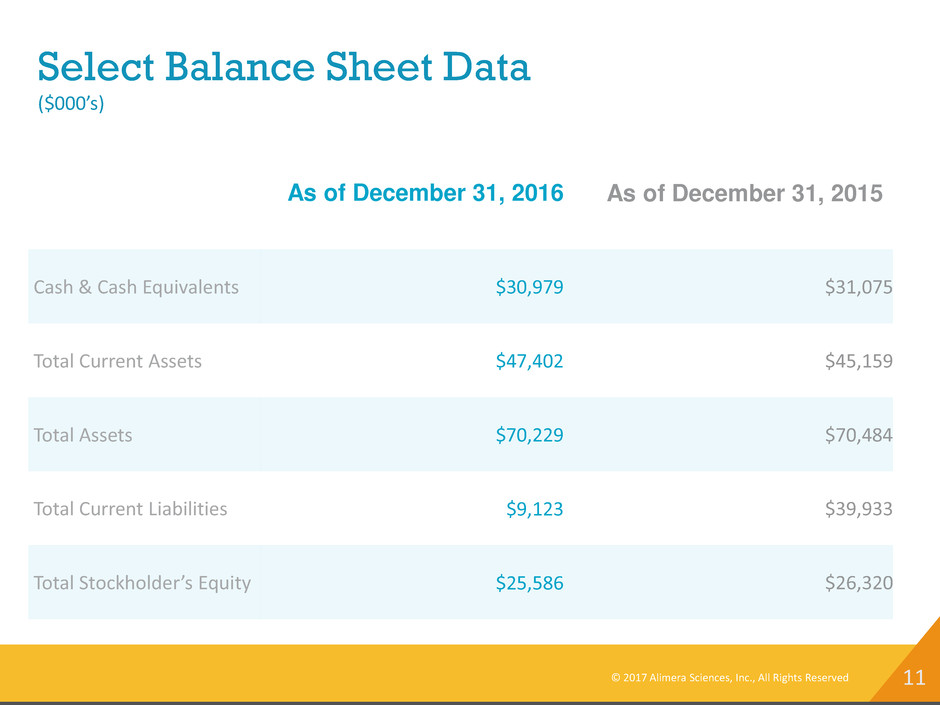
11 Select Balance Sheet Data ($000’s) As of December 31, 2016 As of December 31, 2015 Cash & Cash Equivalents $30,979 $31,075 Total Current Assets $47,402 $45,159 Total Assets $70,229 $70,484 Total Current Liabilities $9,123 $39,933 Total Stockholder’s Equity $25,586 $26,320 © 2017 Alimera Sciences, Inc., All Rights Reserved

Closing Remarks Dan Myers, CEO

Fourth Quarter & Full Year 2016 Results Conference Call Thursday, March 2, 2017












