
First Quarter 2017 Results Conference Call Tuesday, May 9, 2017 Exhibit 99.2

Safe Harbor Statement This presentation contains "forward-looking statements," within the meaning of the Private Securities Litigation Reform Act of 1995, regarding, among other things, the opportunity for further growth in 2017 for ILUVIEN. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of them, and could cause actual results to differ materially from those projected in its forward-looking statements. Words such as "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "contemplate," "predict," "project," "target," "likely," "potential," "continue," "ongoing," "will," "would," "should," "could," or the negative of these terms and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of them, and could cause actual results to differ materially from those projected in its forward-looking statements. Meaningful factors which could cause actual results to differ include, but are not limited to, market acceptance and physician awareness of ILUVIEN in the U.S. and Europe, as well as other factors discussed in the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Alimera's Annual Report on Form 10-K for the year ended December 31, 2016, which is on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at http://www.sec.gov. Additional factors may also be set forth in those sections of Alimera’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2017, to be filed with the SEC in the second quarter of 2017. In addition to the risks described above and in Alimera's Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, other unknown or unpredictable factors also could affect Alimera's results. There can be no assurance that the actual results or developments anticipated by Alimera will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Alimera. Therefore, no assurance can be given that the outcomes stated in such forward- looking statements and estimates will be achieved. All forward-looking statements contained in this presentation are expressly qualified by the cautionary statements contained or referred to herein. Alimera cautions investors not to rely too heavily on the forward-looking statements Alimera makes or that are made on its behalf. These forward-looking statements speak only as of the date of this presentation (unless another date is indicated). Alimera undertakes no obligation, and specifically declines any obligation, to publicly update or revise any such forward-looking statements, whether as a result of new information, future events or otherwise. © 2017 Alimera Sciences, Inc., All Rights Reserved 2
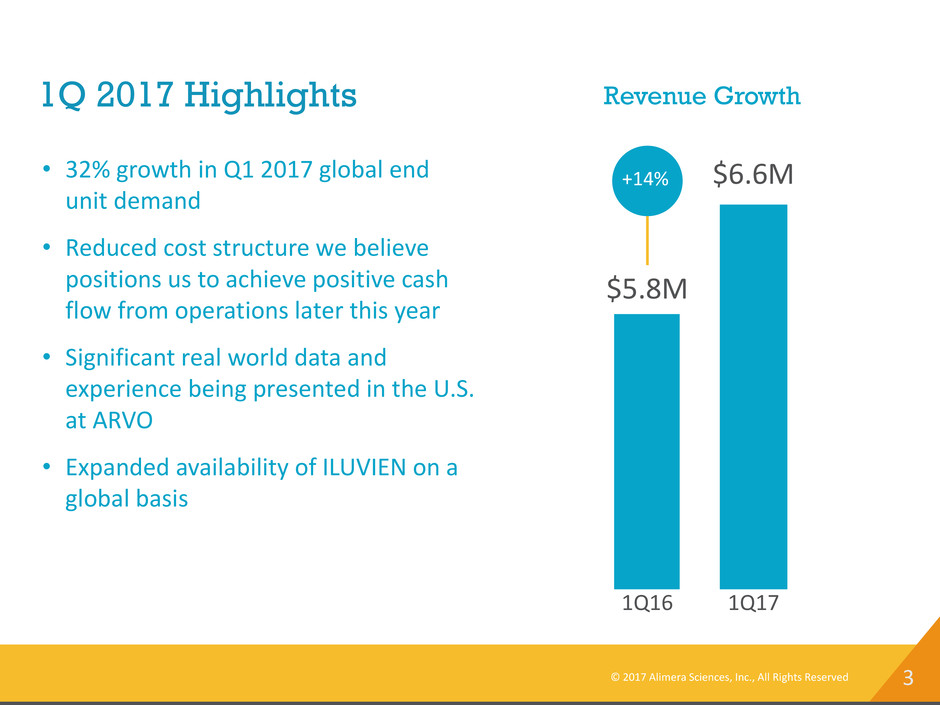
3 1Q 2017 Highlights Revenue Growth 1Q17 $6.6M 1Q16 $5.8M +14%• 32% growth in Q1 2017 global end unit demand • Reduced cost structure we believe positions us to achieve positive cash flow from operations later this year • Significant real world data and experience being presented in the U.S. at ARVO • Expanded availability of ILUVIEN on a global basis © 2017 Alimera Sciences, Inc., All Rights Reserved
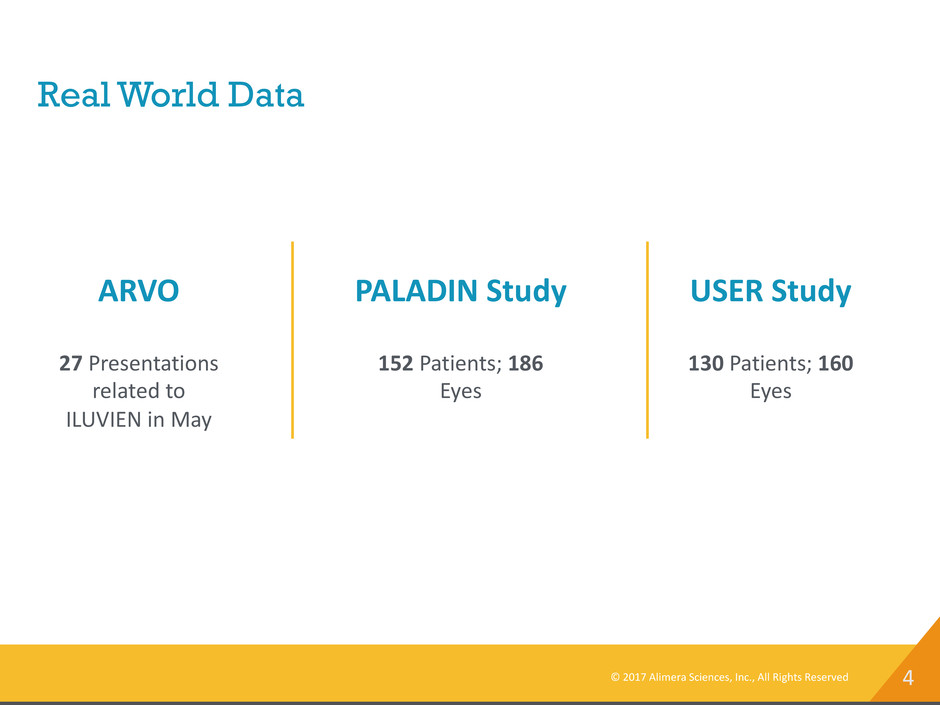
4 Real World Data © 2017 Alimera Sciences, Inc., All Rights Reserved ARVO USER Study 27 Presentations related to ILUVIEN in May PALADIN Study 152 Patients; 186 Eyes 130 Patients; 160 Eyes

5 International Expansion © 2017 Alimera Sciences, Inc., All Rights Reserved We expect that ILUVIEN will be available for the first time in the following countries in 2017 • Italy • Spain • Austria • Ireland

Financial Overview Rick Eiswirth, President & CFO
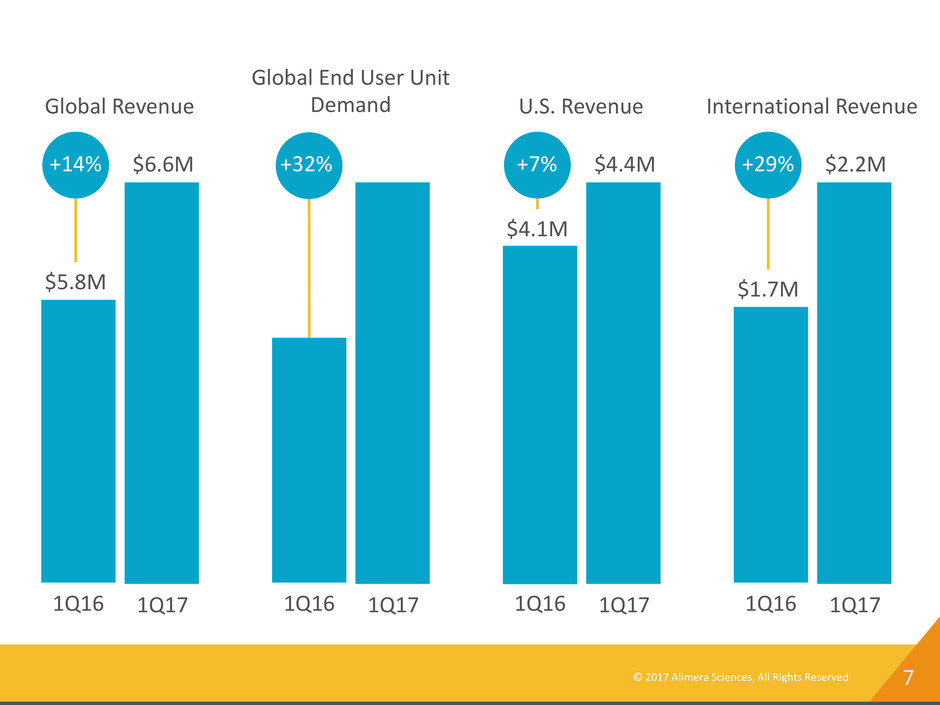
© 2017 Alimera Sciences, All Rights Reserved 7 1Q16 1Q17 $6.6M $5.8M +14% Global Revenue 1Q16 1Q17 +32% Global End User Unit Demand 1Q16 1Q17 $4.4M $4.1M +7% U.S. Revenue 1Q16 1Q17 $2.2M $1.7M +29% International Revenue
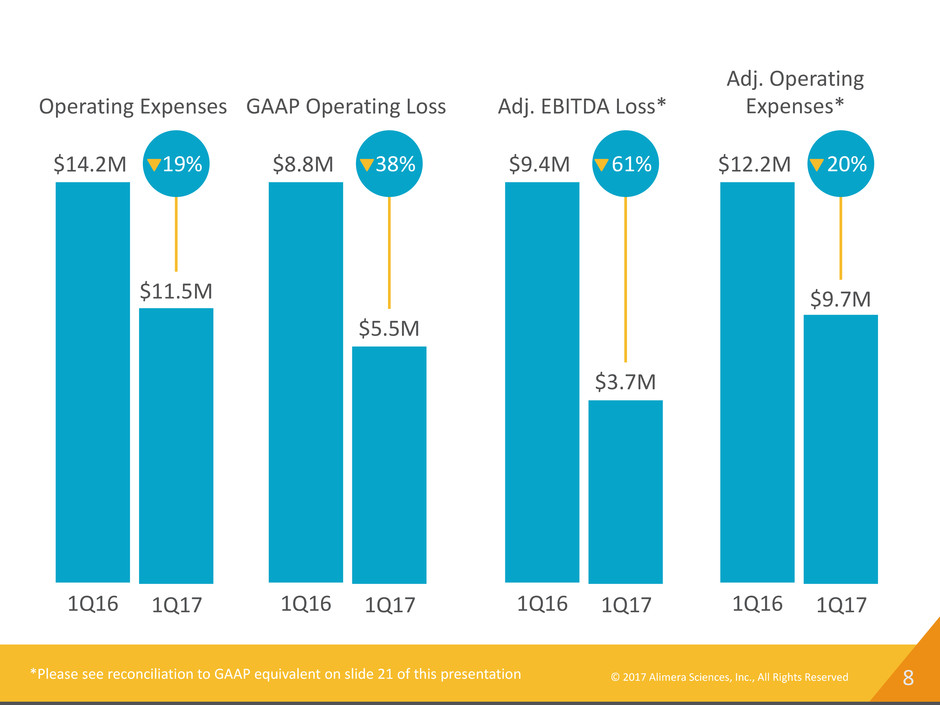
8© 2017 Alimera Sciences, Inc., All Rights Reserved 1Q16 1Q17 $11.5M $14.2M 19% Operating Expenses 1Q16 1Q17 $5.5M $8.8M 38% GAAP Operating Loss 1Q16 1Q17 $3.7M $9.4M 61% Adj. EBITDA Loss* *Please see reconciliation to GAAP equivalent on slide 21 of this presentation 1Q16 1Q17 $9.7M $12.2M 20% Adj. Operating Expenses*
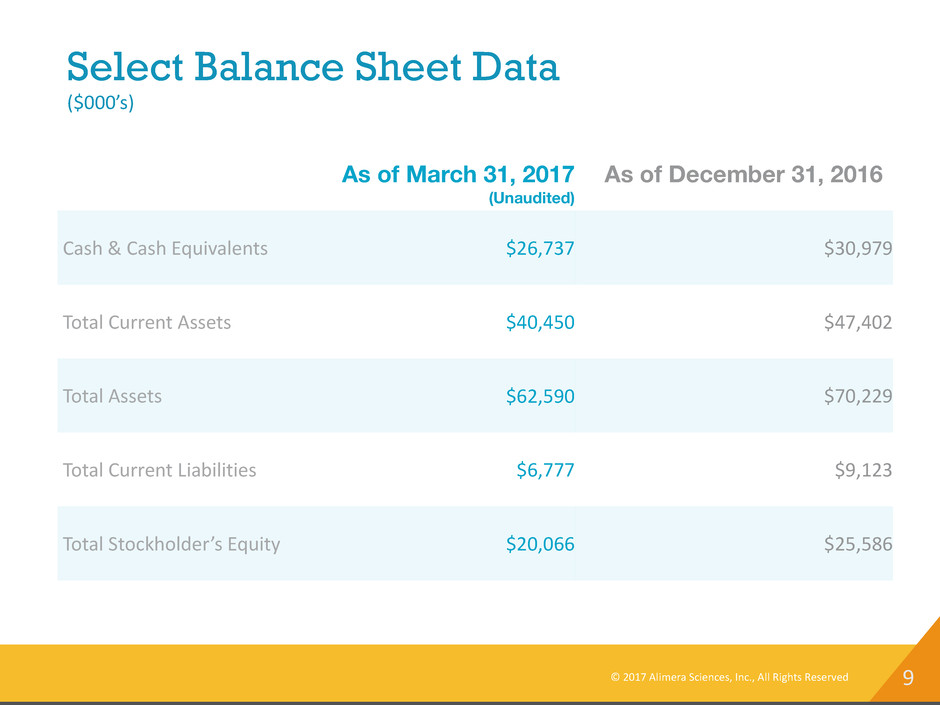
9 Select Balance Sheet Data ($000’s) As of March 31, 2017 (Unaudited) As of December 31, 2016 Cash & Cash Equivalents $26,737 $30,979 Total Current Assets $40,450 $47,402 Total Assets $62,590 $70,229 Total Current Liabilities $6,777 $9,123 Total Stockholder’s Equity $20,066 $25,586 © 2017 Alimera Sciences, Inc., All Rights Reserved

Dr. Christopher Riemann Volunteer Professor, University of Cincinnati, Department of Ophthalmology and Director, Vitreoretinal Fellowship, Member, Board of Directors, Cincinnati Eye Institute The ILUVIEN® Technology
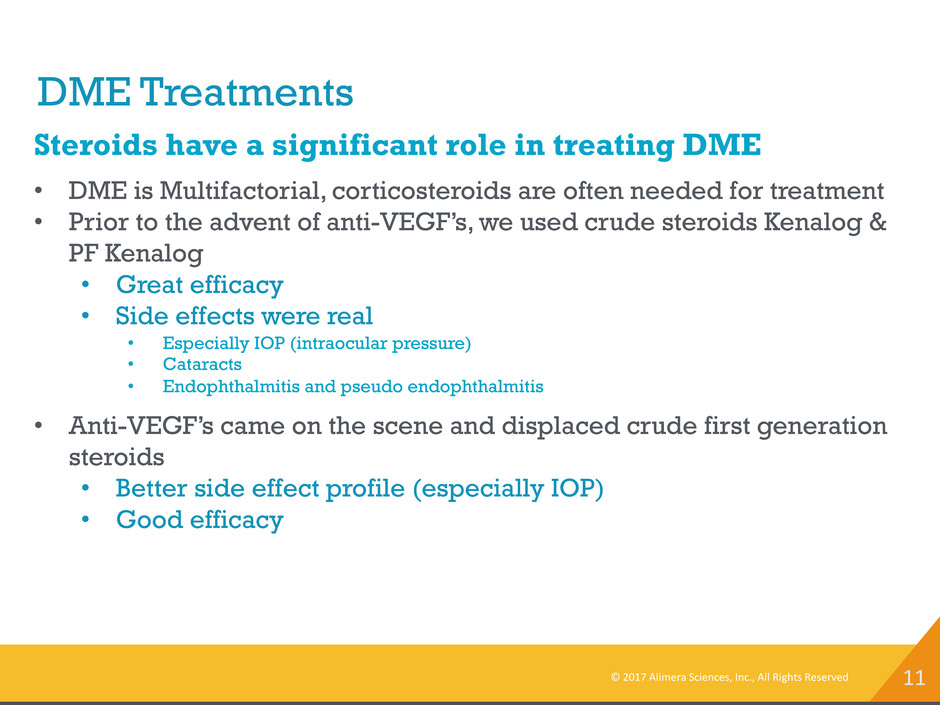
11 DME Treatments © 2017 Alimera Sciences, Inc., All Rights Reserved Steroids have a significant role in treating DME • DME is Multifactorial, corticosteroids are often needed for treatment • Prior to the advent of anti-VEGF’s, we used crude steroids Kenalog & PF Kenalog • Great efficacy • Side effects were real • Especially IOP (intraocular pressure) • Cataracts • Endophthalmitis and pseudo endophthalmitis • Anti-VEGF’s came on the scene and displaced crude first generation steroids • Better side effect profile (especially IOP) • Good efficacy

12 DME Treatments © 2017 Alimera Sciences, Inc., All Rights Reserved Anti-VEGF Drugs are NOT a “magic bullet” for DME • Good efficacy – not great • DME is not AMD • 37-64% DME non-responder rate to Anti-VEGF • Side effects remain a challenge • Elevated IOP with more injections • Cataract and complicated cataract surgeries • Infectious endophthalmitis • Treatment Burden
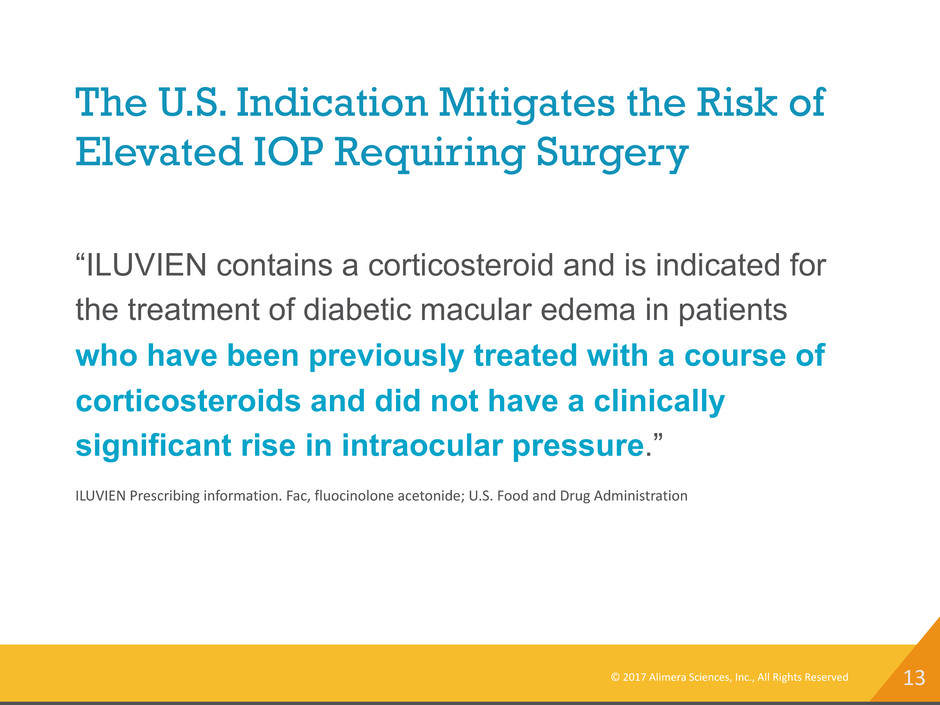
13 The U.S. Indication Mitigates the Risk of Elevated IOP Requiring Surgery © 2017 Alimera Sciences, Inc., All Rights Reserved “ILUVIEN contains a corticosteroid and is indicated for the treatment of diabetic macular edema in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in intraocular pressure.” ILUVIEN Prescribing information. Fac, fluocinolone acetonide; U.S. Food and Drug Administration

14 IOP-Related Events Over 36 Months For All ILUVIEN Treated Subjects in the FAME Phase 3 Study Were Consistent with the Steroid Class © 2017 Alimera Sciences, Inc., All Rights Reserved ILUVIEN (N = 375) n (%) Control (N = 185) n (%) IOP elevation ≥ 10 mm Hg from baseline 127 (34) 18 (10) IOP elevation ≥ 30 mm Hg 75 (20) 8 (4) Any IOP-lowering medicationsa 144 (38) 26 (14) Incisional IOP-lowering surgery 18 (5) 1 (1) ILUVIEN Prescribing Information. Fac, flucinolone acetonide; IOP, intraocular pressure. aFor a minimuim of 7 days

15 The FDA Determined That IOP Response to a Prior Steroid is a Predictor of IOP Response to ILUVIEN Based on a Subgroup Analysis From FAME © 2017 Alimera Sciences, Inc., All Rights Reserved Known Prior Ocular Steroid Injection in Patients Treated With 0.2 µg/d Fac (ILUVIEN) n = 72* No Known Prior Ocular Steroid Injection in Patients Treated With 0.2 µg/d Fac (ILUVIEN) n = 294 IOP-lowering surgery in study eye, n (%)a 0 18 (6.1) P value for prior vs. no priorb .030 R K. Parrish II. Et. Al, Characterization of Intraocular Pressure Increases and Management Strategies Following Treatment with Fluocinolone Acetonide Intravitreal Implants in the FAME Trials. Ophthalmic Surg Lasers Imaging Retina. 2016;47:426-435 Fac, flucinolone acetonide; IOP, intraocular pressure; NS, not significant. *For these patients (n=72), the prior steroid was almost exclusively IVTA. aIncludes trabeculectomy, glaucoma suregery, and vitrectomy for elevated IOP bP values based on Fisher exact test comparing proportions in each treatment group
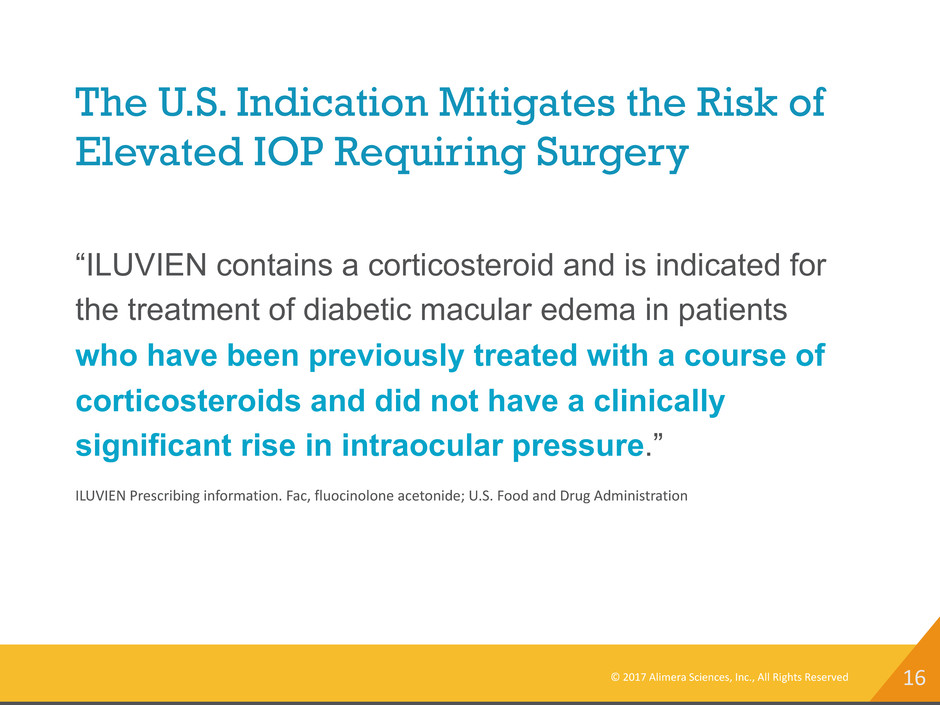
16 The U.S. Indication Mitigates the Risk of Elevated IOP Requiring Surgery © 2017 Alimera Sciences, Inc., All Rights Reserved “ILUVIEN contains a corticosteroid and is indicated for the treatment of diabetic macular edema in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in intraocular pressure.” ILUVIEN Prescribing information. Fac, fluocinolone acetonide; U.S. Food and Drug Administration

17 The Steroid Released by ILUVIEN is Highly Lipophilic – Which Leads to Excellent Penetration of the Retina © 2017 Alimera Sciences, Inc., All Rights Reserved Water Solubility of Ocular Steroids at 25°C mg/ml Multiple Dexamethasone 101 1,030 Fluocinolone Acetonide 0.05472 5.6 1 O’Neil, M.J. (ed.). The Merck Index, 13th Edition, 2001., P. 158 Osol, A. and J.E. Hoover, et al (eds.). Remington’s Pharmaceutical Sciences. 15th ed. Easton, Penn: Mack Publishing CO. 1975., P. 892
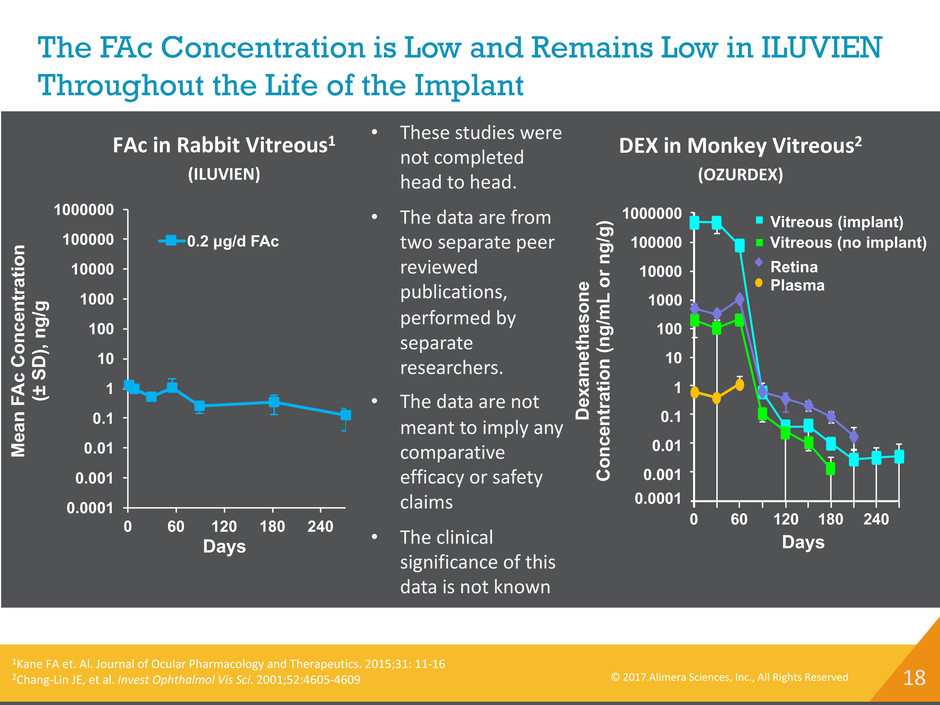
18 The FAc Concentration is Low and Remains Low in ILUVIEN Throughout the Life of the Implant © 2017 Alimera Sciences, Inc., All Rights Reserved D ex am et ha so ne C on ce nt ra tio n (n g/ m L or n g/ g) Days 1000000 100000 10000 1000 100 10 1 0.1 0.01 0.001 0.0001 0 60 120 180 240 Vitreous (implant) Vitreous (no implant) Retina Plasma DEX in Monkey Vitreous2 (OZURDEX) • These studies were not completed head to head. • The data are from two separate peer reviewed publications, performed by separate researchers. • The data are not meant to imply any comparative efficacy or safety claims • The clinical significance of this data is not known 0.0001 0.001 0.01 0.1 1 10 100 1000 10000 100000 1000000 0 60 120 180 240 0.2 μg/d FAc FAc in Rabbit Vitreous1 (ILUVIEN) Days M ea n FA c C on ce nt ra tio n (± S D ), ng /g 1Kane FA et. Al. Journal of Ocular Pharmacology and Therapeutics. 2015;31: 11-16 2Chang-Lin JE, et al. Invest Ophthalmol Vis Sci. 2001;52:4605-4609
19 Why I Use ILUVIEN: © 2017 Alimera Sciences, Inc., All Rights Reserved ILUVIEN represents “next generation steroid” technology” and is a game changer • Not like the steroids of the past… • Low amount of steroid delivered consistently and continuously • Continuous microdosing • Near zero order kinetics • Consistent long term treatment effect – 2 years and counting • One injection • Low treatment burden • Infection risk • ILUVIEN’S label and unique pharmacology help mitigate IOP risk • Manageable! • Patient reliability is key

Closing Remarks Dan Myers, CEO

21 Non-GAAP Financial Information © 2017 Alimera Sciences, Inc., All Rights Reserved Alimera believes that the non-GAAP financial information provided in this presentation can assist investors in the overall understanding of its financial performance when considered together with GAAP figures. This presentation contains a discussion of certain non-GAAP financial measures, as defined in Regulation G of the Securities Exchange Act of 1934, as amended. Alimera reports its financial results in compliance with GAAP, but believes that the non-GAAP measures of Adjusted EBITDA and Adjusted Operating Expenses will be a more relevant measure of Alimera's operating performance. For the purpose of this presentation, "Adjusted EBITDA" is adjusted earnings before interest, taxes, depreciation, amortization, non-cash stock-based compensation expense, and to the extent they are included in the calculation of earnings, net unrealized gain (loss) from foreign currency exchange transactions and gains (losses) from the change in the fair value of derivative warrant liability. “Adjusted Operating Expenses” is operating expenses excluding depreciation, amortization and non-cash stock-based compensation expense. Alimera uses Adjusted EBITDA and Adjusted Operating Expenses in the management of its business and Alimera's lender uses Adjusted EBITDA as a financial covenant measurement. Accordingly, Adjusted EBITDA and Adjusted Operating Expenses for the first quarter of 2017 have been presented in certain instances excluding items identified in the reconciliations provided. These non-GAAP financial measures, as presented, may not be comparable to similarly titled measures reported by other companies since not all companies may calculate these measures in an identical manner and, therefore, they are not necessarily an accurate measure of comparison between companies. The presentation of these non-GAAP financial measures is not intended to be considered in isolation or as a substitute for guidance prepared in accordance with GAAP. The principal limitation of these non-GAAP financial measures is that they exclude significant elements that are required by GAAP to be recorded in Alimera's financial statements. In addition, they are subject to inherent limitations as they reflect the exercise of judgments by management in determining these non-GAAP financial measures. In order to compensate for these limitations, Alimera presents its non-GAAP financial results in connection with its GAAP results. Investors are encouraged to review the reconciliation of our non-GAAP financial measures to their most directly comparable GAAP financial measure.

First Quarter 2017 Results Conference Call Tuesday, May 9, 2017





















