Exhibit 99.2

©2021 Marinus Pharmaceuticals. All Rights Reserved I Corporate Presentation May 2021

©2021 Marinus Pharmaceuticals. All Rights Reserved I 2 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding Marinus, they ar e forward - looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Secu rit ies Litigation Reform Act of 1995. Words such as “may”, “will”, “expect”, “anticipate”, “estimate”, “intend”, “believe”, and similar expressions (as w ell as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward - looking statements. Examples of forwar d - looking statements contained in this presentation include, among others, statements regarding our expected revenue and operating expenses for 20 21; our clinical development plans for ganaxolone ; expected dosing in our clinical trials; the clinical development schedule and milestones; our expected timing to begin and complete enrollment in our clinical trials; the expected trial design, target patient population and endpoints for our cl ini cal trials; interpretation of scientific basis for ganaxolone use; timing for availability and release of data; the potential safety and efficacy and therapeutic potential of ganaxolone ; timing and expectations regarding regulatory communications and submissions; our commercialization plans and the expected tim ing thereof; expectations regarding our agreement with BARDA; expectations regarding the potential market opportunities for our product ca ndi dates, including oral ganaxolone ; potential commercial alliances; and our expectations regarding the effect of the COVID - 19 pandemic on our business and clinica l development plans. Forward - looking statements in this presentation involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward - loo king statements. Such risks and uncertainties include, among others, uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; interpretation of results of clinical trials; unanticipated costs and expenses; early clinical trials may not be indicative of the results i n l ater clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of the FDA or other regulatory agencies may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for addition al clinical trials; our ability to obtain and maintain regulatory approval for our product candidate; our ability to obtain and maintain patent protection for our prod uct candidates; the potential negative impact of third party patents on our ability to commercialize ganaxolone ; delays, interruptions or failures in the manufacture and supply of our product candidate; our ability to raise additional capital; the effect of the COVID - 19 pandemic on our business, t he medical community and the global economy; and the availability or potential availability of alternative products or treatments for conditions targe ted by us that could affect the availability or commercial potential of our product candidate. Marinus undertakes no obligation to update or revise any forwa rd - looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these fo rwa rd - looking statements, as well as risks relating to the business of the Company in general, see filings Marinus has made with the Securities and Exchange Co mmi ssion. You may access these documents for free by visiting EDGAR on the SEC web site at www.sec.gov.
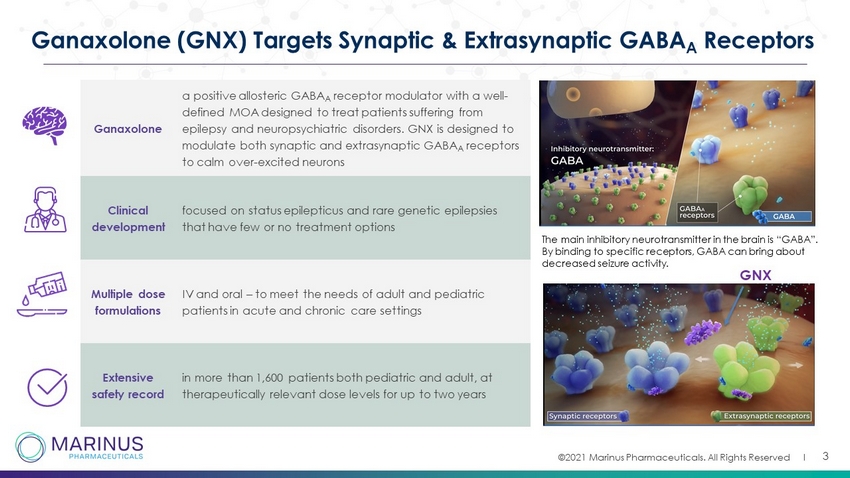
©2021 Marinus Pharmaceuticals. All Rights Reserved I 3 Ganaxolone (GNX) Targets Synaptic & Extrasynaptic GABA A Receptors Ganaxolone a positive allosteric GABA A receptor modulator with a well - defined MOA designed to treat patients suffering from epilepsy and neuropsychiatric disorders. GNX is designed to modulate both synaptic and extrasynaptic GABA A receptors to calm over - excited neurons Clinical development focused on status epilepticus and rare genetic epilepsies that have few or no treatment options Multiple dose formulations IV and oral – to meet the needs of adult and pediatric patients in acute and chronic care settings Extensive safety record in more than 1,600 patients both pediatric and adult, at therapeutically relevant dose levels for up to two years The main inhibitory neurotransmitter in the brain is “GABA”. By binding to specific receptors, GABA can bring about decreased seizure activity. GNX

©2021 Marinus Pharmaceuticals. All Rights Reserved I Evaluation of IV and Oral Opportunities Building Upon Status Epilepticus (SE) Maximizing Value for Orphan Epilepsies • Expand clinical opportunities to broader status epilepticus indications • Build U.S. commercial strategy • Execute global development plan • Develop pharmacoeconomic, value proposition and outcomes assessment • CDKL5 deficiency disorder (CDD) commercialization strategy • Advance tuberous sclerosis complex (TSC) clinical development • Research scientifically based expansion opportunities • Global integrated commercialization strategy Leveraging GNX Molecule • Explore opportunities to improve bioavailability, PK profile & clinical outcomes • Engage in strategic collaborations on novel technologies & formulations • Evaluate new indications based on unmet need, and scientific rationale • Establish strategic commercial collaborations to expand geographic footprint 4 Corporate Strategy
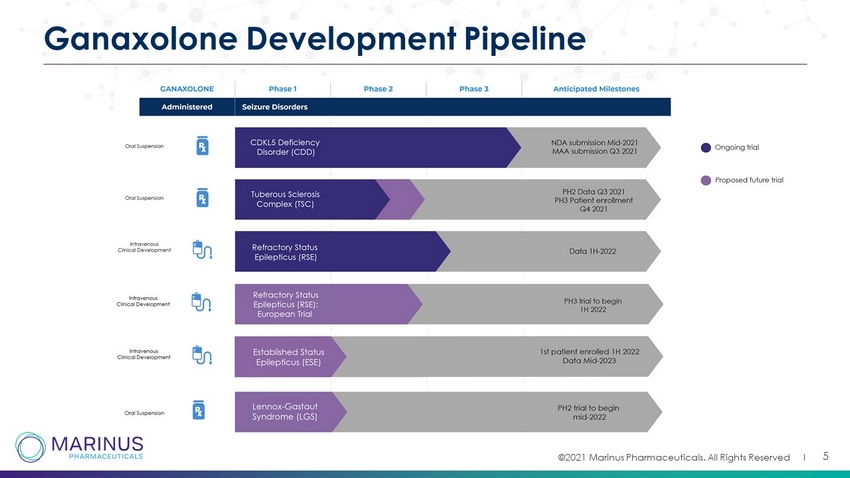
©2021 Marinus Pharmaceuticals. All Rights Reserved I 5 Ganaxolone Development Pipeline

Orphan Epilepsy Franchise

CDKL5 Deficiency Disorder “CDKL5 is painful. It’s a hard, sad at times, thing that we face. When you have a relationship with people like Marinus and their researchers, you are able to help be a driving force behind that work. - Karen Utley, Mother to Samantha, President of International Foundation for CDKL5 Research
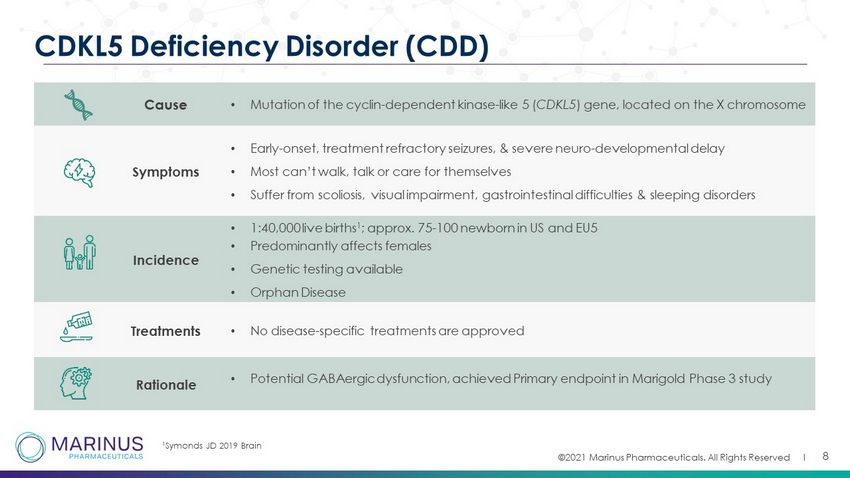
©2021 Marinus Pharmaceuticals. All Rights Reserved I Cause • Mutation of the cyclin - dependent kinase - like 5 ( CDKL5 ) gene, located on the X chromosome Symptoms • Early - onset, treatment refractory seizures, & severe neuro - developmental delay • Most can’t walk, talk or care for themselves • Suffer from scoliosis, visual impairment, gastrointestinal difficulties & sleeping disorders Incidence • 1:40,000 live births 1 ; approx. 75 - 100 newborn in US and EU5 • Predominantly affects females • Genetic testing available • Orphan Disease Treatments • No disease - specific treatments are approved Rationale • Potential GABAergic dysfunction, achieved Primary endpoint in Marigold Phase 3 study 8 CDKL5 Deficiency Disorder (CDD) 1 Symonds JD 2019 Brain

©2021 Marinus Pharmaceuticals. All Rights Reserved I 9 Completed Global Phase 3 Trial Design Baseline 6 weeks Historical Control 8 weeks Double - Blind Phase Open - Label Phase Maintenance 13 weeks Titration 4 weeks Open - Label Phase ► Trial Details • Evaluated the use of oral ganaxolone in children and young adults • Global, double - blind, placebo - controlled, clinical trial enrolled 101 patients between the ages of 2 and 19 with a confirmed disease - related CDKL5 gene variant • Ages 2 - 19, ≥16 major motor seizures/month; up to 4 concomitant AEDs ► Endpoints • Primary endpoint of the trial was percent change in 28 - day major motor seizure frequency * • Non - seizure secondary outcome measures: Behavioral/neuropsychiatric changes correlated with domains of attention & sleep * Major motor seizures were defined as bilateral tonic, generalized tonic - clonic, atonic/drop, bilateral clonic, or focal to bil ateral tonic - clonic Titration 4 weeks

©2021 Marinus Pharmaceuticals. All Rights Reserved I 10 Marigold Baseline Clinical Characteristics Characteristic Placebo (n=51) Ganaxolone (n=50) Total (n=101) Baseline Primary Seizure Frequency, per 28 days (median, IQR) 49.2 ( 18.7 – 120.0) 54.0 (31.3 – 147.3) - Number of AED Medications Taken Prior (median) 7 7 7 Concomitant AED Medications, n (%) Valproate 16 (31.4) 18 (36.0) 34 (33.7) Levetiracetam 13 (25.5) 13 (26.0) 26 (25.7) Clobazam 13 (25.5) 12 (24.0) 25 (24.8) Vigabatrin 12 (23.5) 10 (20.0) 22 (21.8) Baseline seizure burden and AED history highlights unmet need

©2021 Marinus Pharmaceuticals. All Rights Reserved I 11 Ganaxolone Achieved Primary Efficacy Endpoint in Seizure Reduction and Secondary Endpoint for Seizure Severity Caregiver Global Impression of Change in Seizure Intensity / Duration (CGI - CSID) Ganaxolone Placebo 0 10 20 30 40 M e d i a n P e r c e n t R e d u c t i o n 2 8 - d a y F r e q u e n c y o f M a j o r M o t o r S e i z u r e s 30.7% 6.9% = 27.1% (47.9 - 9.6)* p = 0.0036** *Hodges-Lehman Estimate of Median Difference **Wilcoxon Rank-Sum Test
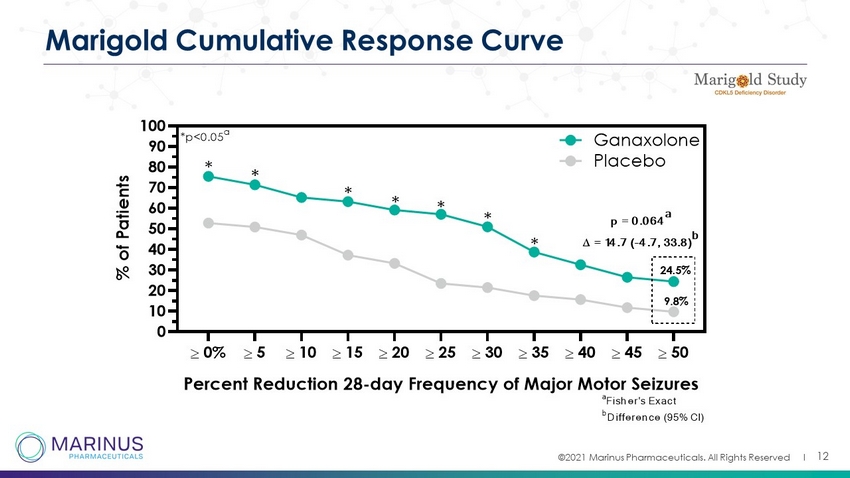
©2021 Marinus Pharmaceuticals. All Rights Reserved I 12 Marigold Cumulative Response Curve 0% 5 10 15 20 25 30 35 40 45 50 0 10 20 30 40 50 60 70 80 90 100 Percent Reduction 28-day Frequency of Major Motor Seizures % o f P a t i e n t s Ganaxolone Placebo 24.5% 9.8% p = 0.064 a = 14.7 (-4.7, 33.8) b * * * * * * * *p<0.05 a a Fisher's Exact b Difference (95% CI)
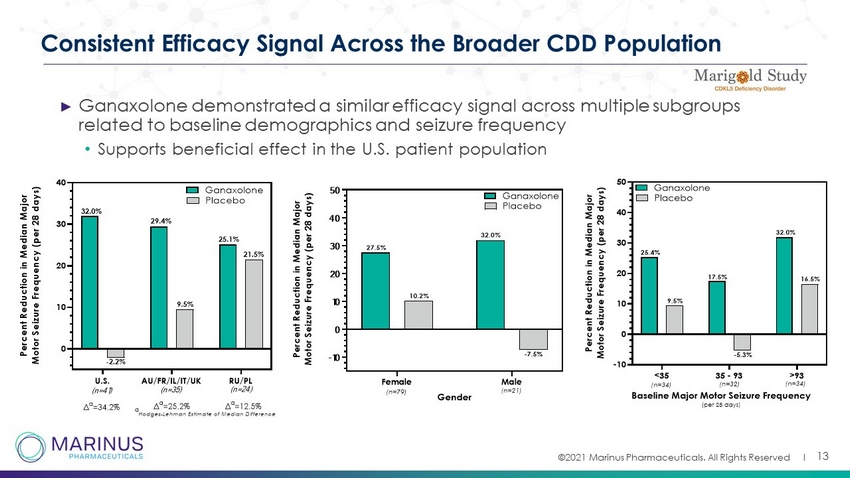
©2021 Marinus Pharmaceuticals. All Rights Reserved I Consistent Efficacy Signal Across the Broader CDD Population ► Ganaxolone demonstrated a similar efficacy signal across multiple subgroups related to baseline demographics and seizure frequency • Supports beneficial effect in the U.S. patient population 13 U.S. AU/FR/IL/IT/UK RU/PL 0 10 20 30 40 P e r c e n t R e d u c t i o n i n M e d i a n M a j o r M o t o r S e i z u r e F r e q u e n c y ( p e r 2 8 d a y s ) Ganaxolone Placebo 32.0% -2.2% 29.4% 9.5% (n=41) (n=35) (n=24) 21.5% 25.1% Δ a =34.2% Δ a =25.2% Δ a =12.5% a Hodges-Lehman Estimate of Median Difference Female Male -10 0 10 20 30 40 50 Gender P e r c e n t R e d u c t i o n i n M e d i a n M a j o r M o t o r S e i z u r e F r e q u e n c y ( p e r 2 8 d a y s ) Ganaxolone Placebo 27.5% 10.2% 32.0% -7.5% (n=79) (n=21)
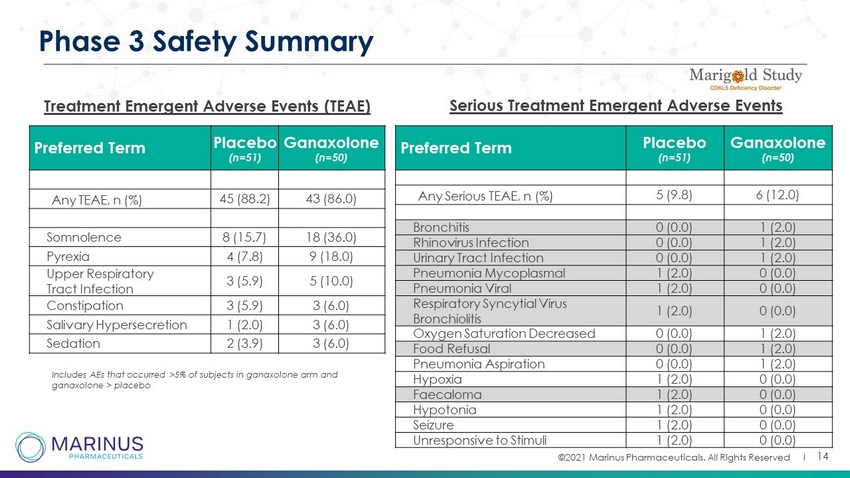
©2021 Marinus Pharmaceuticals. All Rights Reserved I 14 Phase 3 Safety Summary Treatment Emergent Adverse Events (TEAE) Preferred Term Placebo (n=51) Ganaxolone (n=50) Any TEAE, n (%) 45 (88.2) 43 (86.0) Somnolence 8 (15.7) 18 (36.0) Pyrexia 4 (7.8) 9 (18.0) Upper Respiratory Tract Infection 3 (5.9) 5 (10.0) Constipation 3 (5.9) 3 (6.0) Salivary Hypersecretion 1 (2.0) 3 (6.0) Sedation 2 (3.9) 3 (6.0) Includes AEs that occurred >5% of subjects in ganaxolone arm and ganaxolone > placebo Preferred Term Placebo (n=51) Ganaxolone (n=50) Any Serious TEAE, n (%) 5 (9.8) 6 (12.0) Bronchitis 0 (0.0) 1 (2.0) Rhinovirus Infection 0 (0.0) 1 (2.0) Urinary Tract Infection 0 (0.0) 1 (2.0) Pneumonia Mycoplasmal 1 (2.0) 0 (0.0) Pneumonia Viral 1 (2.0) 0 (0.0) Respiratory Syncytial Virus Bronchiolitis 1 (2.0) 0 (0.0) Oxygen Saturation Decreased 0 (0.0) 1 (2.0) Food Refusal 0 (0.0) 1 (2.0) Pneumonia Aspiration 0 (0.0) 1 (2.0) Hypoxia 1 (2.0) 0 (0.0) Faecaloma 1 (2.0) 0 (0.0) Hypotonia 1 (2.0) 0 (0.0) Seizure 1 (2.0) 0 (0.0) Unresponsive to Stimuli 1 (2.0) 0 (0.0) Serious Treatment Emergent Adverse Events

©2021 Marinus Pharmaceuticals. All Rights Reserved I 15 Ganaxolone’s Potential to Provide Durable Seizure Improvements in the Open Label Extension • Seizures associated with CDD are often refractory to treatment with existing AEDs and improvements may be short - lived (<3 months) 1 • Preliminary analysis* of the open - label extension (OLE) provides insights into the extended duration effects of ganaxolone (GNX) in CDD 1. M üller A, et al. Eur. J. Paediatr. Neurol. 2016 *Data as of February 24, 2021 Patients treated with ganaxolone for at least 12 months experienced a median 49.6% reduction in major motor seizure frequency Patients transitioning from placebo to ganaxolone demonstrated seizure frequency improvements No new safety findings emerged in the OLE to date Primary Endpoint (17 wks) 1-2 3-4 5-6 7-8 9-10 11-12 0 10 20 30 40 50 60 70 Time in OLE (Months) P e r c e n t R e d u c t i o n i n M e d i a n M a j o r M o t o r S e i z u r e F r e q u e n c y ( p e r 2 8 d a y s ) Ganaxolone Placebo (DB) Ganaxolone (OLE) Open-Label Ganaxolone 33.5% n=43 7.7% n=45 36.3% n=43 40.4% n=39 30.1% n=38 24.0% n=39 33.3% n=34 30.4% n=38 Placebo (DB) 30.7% n=49 6.9% n=51 38.8% n=34 32.8% n=34 46.5% n=22 53.8% n=26
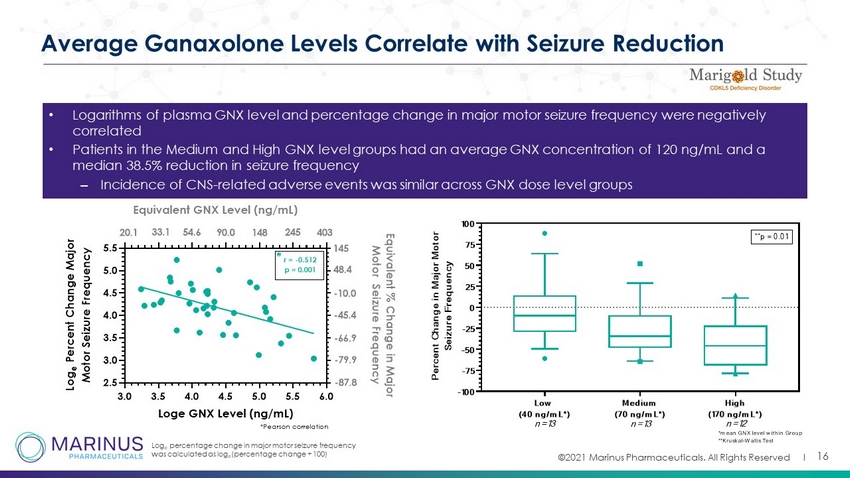
©2021 Marinus Pharmaceuticals. All Rights Reserved I 16 Average Ganaxolone Levels Correlate with Seizure Reduction • Logarithms of plasma GNX level and percentage change in major motor seizure frequency were negatively correlated • Patients in the Medium and High GNX level groups had an average GNX concentration of 120 ng/mL and a median 38.5% reduction in seizure frequency – Incidence of CNS - related adverse events was similar across GNX dose level groups Log e percentage change in major motor seizure frequency was calculated as log e (percentage change + 100) 3.0 3.5 4.0 4.5 5.0 5.5 6.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 Loge GNX Level (ng/mL) L o g e P e r c e n t C h a n g e M a j o r M o t o r S e i z u r e F r e q u e n c y E q u i v a l e n t % C h a n g e i n M a j o r M o t o r S e i z u r e F r e q u e n c y r = -0.512 p = 0.001 *Pearson correlation * 145 48.4 -10.0 -45.4 -66.9 -79.9 -87.8 Equivalent GNX Level (ng/mL) 20.1 33.1 54.6 90.0 148 245 403 Low (40 ng/mL*) Medium (70 ng/mL*) High (170 ng/mL*) -100 -75 -50 -25 0 25 50 75 100 P e r c e n t C h a n g e i n M a j o r M o t o r S e i z u r e F r e q u e n c y **p = 0.01 *mean GNX level within Group **Kruskal-Wallis Test n=13 n=13 n=12

©2021 Marinus Pharmaceuticals. All Rights Reserved I 17 PK Analysis: Adult Focal Onset Seizure Trial vs. Marigold Age Group AUC 24 (ng* hr /mL) C min (ng/mL) C max (ng/mL) 2 to <6 years 3903 85 247 6 to <12 years 3998 84 269 12 to <18 yrs 4106 84 293 ≥18 years 4100 84 292 Abbreviations: AUC 24 =24 - hour area under the ganaxolone plasma concentration time curve; C max =maximum ganaxolone plasma concentration; C min =minimum ganaxolone plasma concentration. Marigold Trial PK Adults Focal Onset Phase 3 Trial vs. Phase 1 PK Lower levels than predicted from Ph1 C min ~ 40 - 45 ng/mL C min ~ 85 ng/mL Adult focal onset program was discontinued as noted in the 10 - Q

Tuberous Sclerosis Complex “Many individuals with TSC continue to experience uncontrolled seizures despite a cocktail of multiple antiepileptic drugs. Because new options are always needed, the TSC community welcomes clinical evaluation of new epilepsy treatments” - Kari Luther Rosbeck, President & CEO of the Tuberous Sclerosis Alliance

©2021 Marinus Pharmaceuticals. All Rights Reserved I Cause • Defect or mutation of TSC1 and/or TSC2 genes Symptoms • Benign tumors, seizures, cognitive impairment, behavioral problems, skin abnormalities Incidence Prevalence • 1:6,000 live births • ~25K - 40K refractory TSC patients in the U.S.* Treatments • Despite available treatments, continued unmet medical need Mechanistic Rationale • Potential neurosteroid deficiency 1 • Pathophysiology may involve GABAergic dysfunction 19 Tuberous Sclerosis Complex (TSC) 1 diMichele, et al, J. Neuro Neurosurg Psychiatry , 2003 *Failure of two prior antiseizure medications with ongoing, frequent seizures.

©2021 Marinus Pharmaceuticals. All Rights Reserved I PART A PART B Baseline (4 Weeks) GNX Titration (4 Weeks) GNX Maintenance (8 Weeks) Open - Label Extension (OLE) (24 Weeks) * Available to patients that respond to GNX as defined per protocol 20 TSC - Phase 2 Open - Label Clinical Trial Design ► n = Approx. 25 ► 8 U.S. sites ► Electronic diaries will be used for data capture ► At least 8 seizures per month ► Primary efficacy endpoint: % change in 28 - day primary seizure frequency through the end of 12 - week treatment period relative to 4 - week baseline period ► Patient enrollment to be completed March 2021 ► Top - line data expected Q3 2021 Primary seizure types: focal motor seizures without impairment of consciousness or awareness, focal seizures with impairment of consciousness or awareness, focal seizures evolving to bilateral generalized convulsive seizures, and generalized seizures with a motor component that are coun tab le Baseline Period Treatment Period OLE Period Screening Visit Baseline Treatment Visit 2 - week taper upon GNX discontinuation (if not continuing to Part B)
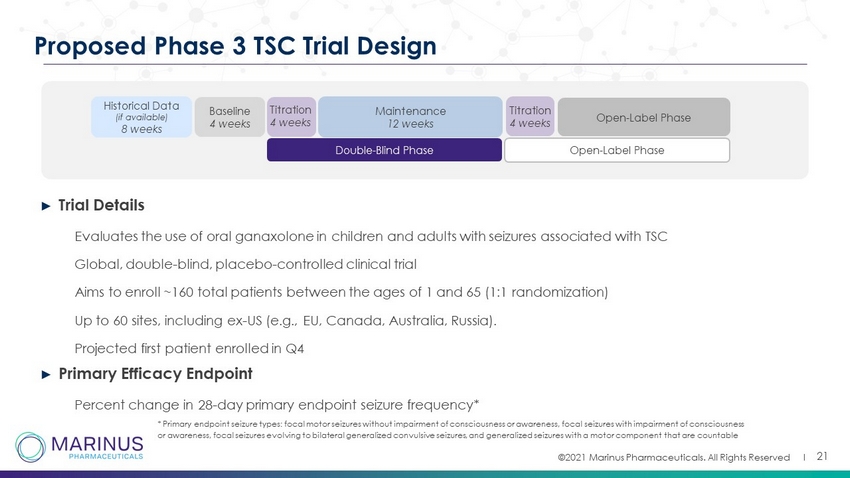
©2021 Marinus Pharmaceuticals. All Rights Reserved I ► Trial Details • Evaluates the use of oral ganaxolone in children and adults with seizures associated with TSC • Global, double - blind, placebo - controlled clinical trial • Aims to enroll ~160 total patients between the ages of 1 and 65 (1:1 randomization) • Up to 60 sites, including ex - US (e.g., EU, Canada, Australia, Russia). • Projected first patient enrolled in Q4 ► Primary Efficacy Endpoint • Percent change in 28 - day primary endpoint seizure frequency* 21 Proposed Phase 3 TSC Trial Design Baseline 4 weeks Historical Data (if available) 8 weeks Double - Blind Phase Open - Label Phase Maintenance 12 weeks Titration 4 weeks Open - Label Phase * Primary endpoint seizure types: focal motor seizures without impairment of consciousness or awareness, focal seizures with imp airment of consciousness or awareness, focal seizures evolving to bilateral generalized convulsive seizures, and generalized seizures with a motor com pon ent that are countable Titration 4 weeks

Formulation Development
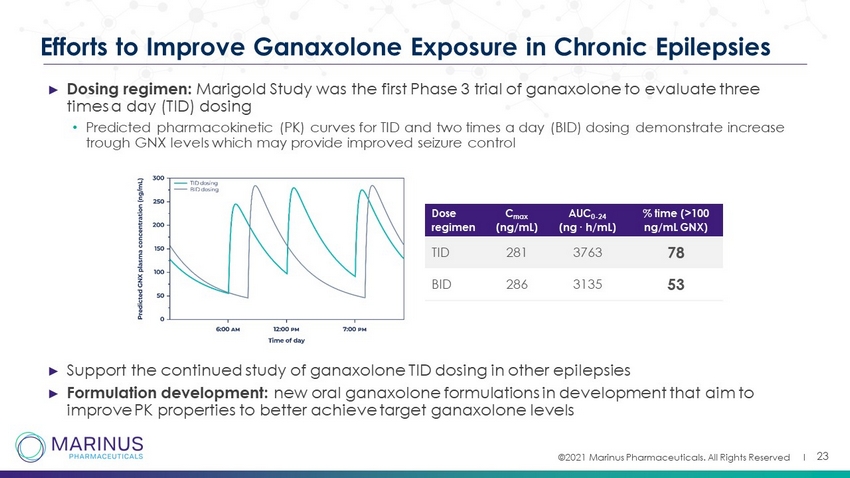
©2021 Marinus Pharmaceuticals. All Rights Reserved I 23 Efforts to Improve Ganaxolone Exposure in Chronic Epilepsies ► Dosing regimen: Marigold Study was the first Phase 3 trial of ganaxolone to evaluate three times a day (TID) dosing • Predicted pharmacokinetic (PK) curves for TID and two times a day (BID) dosing demonstrate increase trough GNX levels which may provide improved seizure control ► Support the continued study of ganaxolone TID dosing in other epilepsies ► Formulation development: new oral ganaxolone formulations in development that aim to improve PK properties to better achieve target ganaxolone levels Dose regimen C max (ng/mL) AUC 0 - 24 (ng ∙ h/mL) % time (>100 ng/mL GNX) TID 281 3763 78 BID 286 3135 53
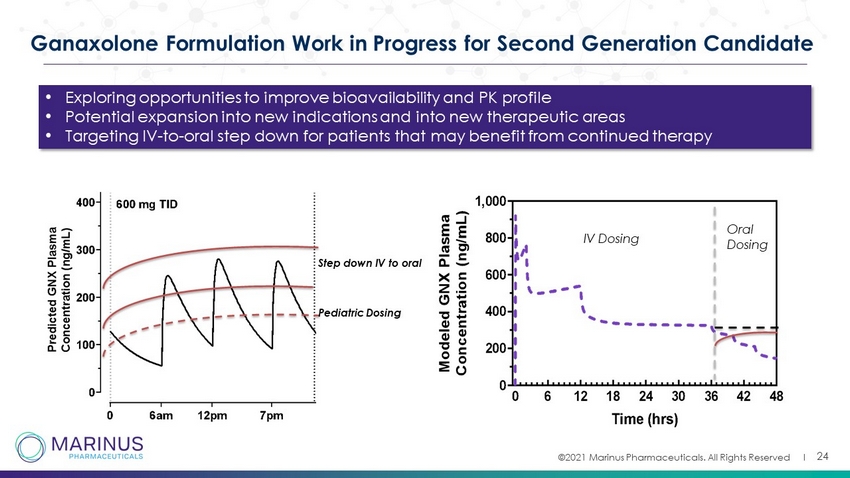
©2021 Marinus Pharmaceuticals. All Rights Reserved I 24 Ganaxolone Formulation Work in Progress for Second Generation Candidate Step down IV to oral Pediatric Dosing IV Dosing Oral Dosing • Exploring opportunities to improve bioavailability and PK profile • Potential expansion into new indications and into new therapeutic areas • Targeting IV - to - oral step down for patients that may benefit from continued therapy

©2021 Marinus Pharmaceuticals. All Rights Reserved I Commercial Opportunity

©2021 Marinus Pharmaceuticals. All Rights Reserved I 26 Commercialization Preparedness Refining and Optimizing Value Proposition for Market Testing with key stakeholders – Providers, Payers Developing organizational and infrastructure needs – home office, field, systems and processes Readying supply chain to support patient services, channel strategy and scale up needs Key Objectives is to create operational leverage across indications Evaluating Life Cycle plans to scale up Access, Scientific Affairs and Commercial teams *TPP – Target Product Profile

©2021 Marinus Pharmaceuticals. All Rights Reserved I 27 Key Findings from Recently Conducted Market Research Show that Ganaxolone is Well Suited for Broad Clinical Adoption Across Indications Awareness Mechanism of Action TPP Reactions Primary Usage Drivers Source: ZS Associates Primary Research and Analysis (N=35 HCP Interviews), (TPP – May 2020; o ther market research – June 2020) Neurologists who treat both CDD and TSC patients had high awareness of ganaxolone Ganaxolone’s extrasynaptic mechanism of action well understood and viewed as differentiable Many HCPs are excited about the opportunity to use ganaxolone, especially for CDD, given favorable reactions to its efficacy and durability data, and safety profile • Disease - specific indication, response rate, and durability of response in a highly refractory patient population • Ability to be used with antiseizure medications across mechanisms (i.e., sodium channel blockers, GABA transmission inhibitors, cannabidiol) in refractory patients

©2021 Marinus Pharmaceuticals. All Rights Reserved I Status Epilepticus

©2021 Marinus Pharmaceuticals. All Rights Reserved I 29 Status Epilepticus (SE): Definition and Epidemiology Background ► Prolonged continuous seizures ► Heterogenous patient population with various etiologies, including glioblastoma, vascular disease, encephalitis, drug or alcohol withdrawal or overdose ► Pre - existing epilepsy in less than half of SE cases ► Status epilepticus can result in permanent neuronal damage and contribute to high morbidity and mortality ► Becomes more treatment refractory with progression SE is the second most common neurologic emergency in the U.S. 1 150,000 SE patients 2 1. Anaethesia and Intensive Care Medicine, February 02, 2018 , Update on the management of status epilepticus 2. DeLorenzo RJ Pellock JM Towne AR Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995; 12: 316 - 325
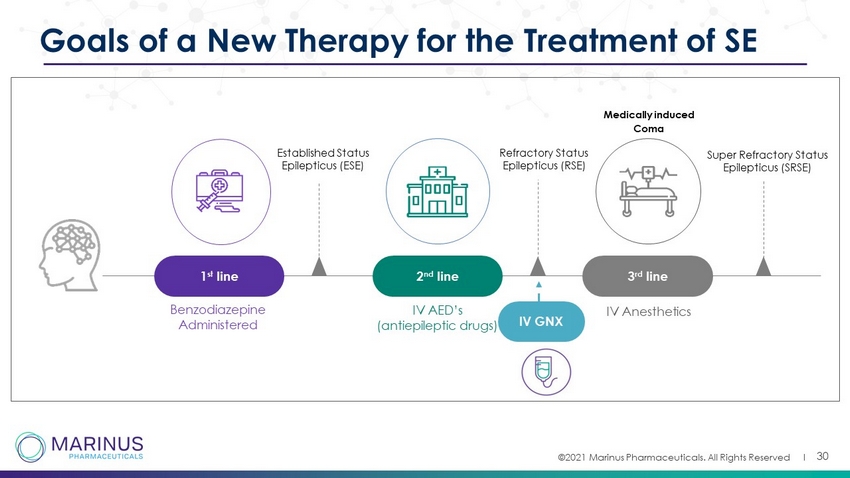
©2021 Marinus Pharmaceuticals. All Rights Reserved I 30 Goals of a New Therapy for the Treatment of SE Benzodiazepine Administered Medically induced Coma Established Status Epilepticus (ESE) 1 st line 2 nd line IV AED’s (antiepileptic drugs) 3 rd line IV Anesthetics Super Refractory Status Epilepticus (SRSE) Refractory Status Epilepticus (RSE) IV GNX
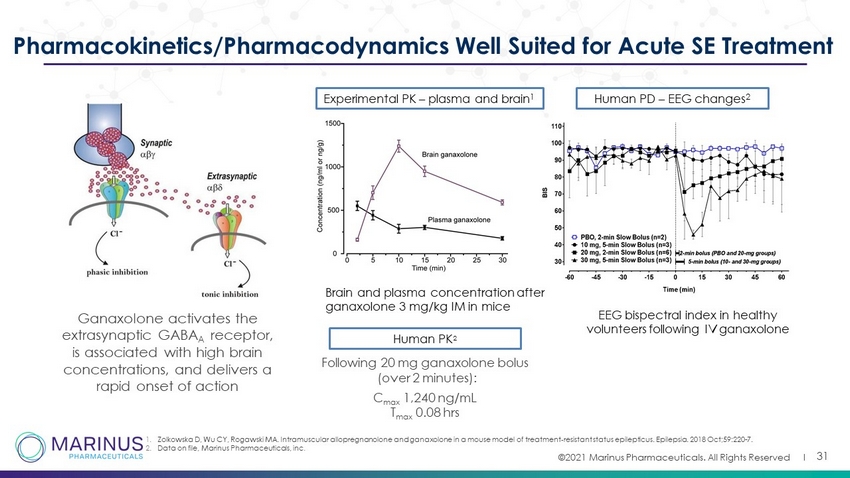
©2021 Marinus Pharmaceuticals. All Rights Reserved I 31 Pharmacokinetics/Pharmacodynamics Well Suited for Acute SE Treatment Experimental PK – plasma and brain 1 Brain and plasma concentration after ganaxolone 3 mg/kg IM in mice Human PD – EEG changes 2 EEG bispectral index in healthy volunteers following IV ganaxolone 1. Zolkowska D, Wu CY, Rogawski MA. Intramuscular allopregnanolone and ganaxolone in a mouse model of treatment - resistant status epilepticus. Epilepsia. 2018 Oct;59:220 - 7. 2. Data on file, Marinus Pharmaceuticals, inc . Human PK 2 Following 20 mg ganaxolone bolus (over 2 minutes): C max 1,240 ng/mL T max 0.08 hrs Ganaxolone activates the extrasynaptic GABA A receptor, is associated with high brain concentrations, and delivers a rapid onset of action

©2021 Marinus Pharmaceuticals. All Rights Reserved I Treatment Period Loading Dose Maintenance Taper 32 Phase 2 RSE Trial Design Diagnosis of convulsive or non - convulsive SE Failed at least one 2 nd line IV AED but had not progressed to 3 rd line IV anesthetics Bolus plus continuous infusion 2 - 4 day infusion 18 - hour taper Screening Post - treatment Follow - up 24 hour Weeks 2, 3, 4 SE Patients Cohort Dose of GNX/day N Low 500mg/day 5 Medium 650mg/day 4 High 713mg/day 8 Evaluate IV ganaxolone in refractory SE patients Goals of a new treatment Rapid cessation Maintenance of seizure control Prevent progression to IV anesthetics Limitations of current treatments 1st line Benzodiazepines ineffective in 45% - 50%; limited by cardiovascular and respiratory side effects 2nd line Ineffective in over 50% of established SE; further decreased response in refractory SE 3rd line IV Anesthetics: high morbidity, mortality ~35%; increased duration of hospitalization and costs of care Endpoints Primary: Percent of patients who did not require escalation of treatment with IV anesthetic within the first 24 hours after ganaxolone initiation Secondary: Additional efficacy, safety and tolerability

©2021 Marinus Pharmaceuticals. All Rights Reserved I 33 Patient Demographics of Phase 2 Trial 8 males, 9 females Mean age: 57 years old (range: 23 - 88) 5 (29%) CSE, 11 (65%) NCSE, 1 (6%) CSE→NCSE 7 (41%) yes, 10 (59%) no 17 patients enrolled Types of SE History of Epilepsy Mean # of failed 2nd - line IV AEDs • 2.1 (range: 1 - 4), all failed LEV or LAC • 14/17 patients failed two or more 2nd - line AEDs • All prior AEDs were administered within recommended dosing guidelines Mean # of failed first - and - second line IV AEDs (including benzodiazepines) 2.9 (range: 2 - 5) 7 Vascular 4 Tumor 2 Autoimmune 2 Drug overdose 2 Unknown Etiologies

©2021 Marinus Pharmaceuticals. All Rights Reserved I 34 Phase 2 Trial Results Demonstrated Rapid Onset And Durability of Effect Cohort No escalation to IV anesthetics within 24 hours from infusion initiation (Primary Endpoint) Status - free through 24 hours from infusion initiation (investigator determination) No escalation to additional IV AEDs or IV anesthetics for status relapse at any time through 24 hours after ganaxolone discontinuation No SE Relapse at anytime during the 4 - wk follow up period High (713 mg/day) (n=8) 100% (8 of 8) 88% (7 of 8) 100% (8 of 8) 100% (6 of 6) (1ET, 1 died) Medium (650 mg/day) (n=4) 100% (4 of 4) 100% (4 of 4) 75% (3 of 4) 67% (2 of 3) (1 ET) Low (500 mg/day) (n=5) 100% (5 of 5) 100% (5 of 5) 60% (3 of 5) 50% (1 of 2) (1 died) Immediate Prior AED Administered 4 Hours (mean) to ganaxolone treatment SE Cessation Occurred Rapidly in All Dose Groups (median = 5 minutes) Data presented at AES 2019 AEDs – antiepileptic drugs

©2021 Marinus Pharmaceuticals. All Rights Reserved I 35 PK/PD Relationship and Rationale for Target Dose Seizure Burden Reduction Occurred Rapidly in All Dose Groups Modeled PK Curves for All Dose Groups High Dose Achieves Target Range ≥ 500 ng/mL for ~8 hours Only High Dose Provided Sustained Reduction (>80%) Throughout Entire Analysis Window Data presented at AES 2019 PK: Pharmacokinetics / PD: Pharmadynamic

©2021 Marinus Pharmaceuticals. All Rights Reserved I 36 IV Ganaxolone Safety Summary Intubation: 9 patients were not intubated upon enrollment. Of these, 6 remained intubation - free during the entire ganaxolone treatment perio d Data presented at AES 2019 AE: adverse event / SAE: serious adverse event 10 SAEs in 6 patients (also included in AEs) 2 related in 2 patients • 2 severe sedation 8 non - related in 4 patients • 1 Death due to withdrawal of life support - 1 Respiratory depression • 1 Bowel perforation (fatal) • 1 Sepsis (fatal) • 1 Fall - 1 Loss of consciousness - 1 Pneumothorax - 1 Multiple fracture 13 related in 7 patients • 6 mild (2 hypotension, 2 somnolence, 1 urinary retention, 1 hypercarbia) • 5 moderate (4 somnolence; 1 hypercarbia) • 2 severe (2 sedation) 37 not - related in 12 patients • 20 mild • 8 moderate (2 pain; 2 pneumonia, 2 dysphagia, • 1 delirium, 1 hypertension) • 9 severe (respiratory depression, death due to withdrawal of support, sepsis, embolic stroke, perforated bowel, fall, loss of consciousness, multiple fractures, pneumothorax) 50 AEs in 16 patients

©2021 Marinus Pharmaceuticals. All Rights Reserved I 37 Overview of U.S. Phase 3 RSE RAISE Trial Design Trial design • Randomized, placebo - controlled (adjunctive to standard - of - care) clinical trial Target patient population • Status epilepticus patients (n=124) who have failed benzodiazepines and ≥ 2 IV AEDs Dosing • 36 - hour infusion followed by a 12 - hour taper (48 - hour treatment) • Phase 2 dose paradigm and extends ganaxolone plasma exposure ≥ 500 ng/mL for 12 hours Co - primary endpoints • Proportion of participants with SE cessation within 30 minutes of study drug initiation without medications for the acute treatment of SE • Proportion of participants with no progression to IV anesthesia for 36 hours following study drug initiation Secondary endpoints • No progression to IV anesthesia for 24 hours off study drug (i.e., 72 hours) • Time to SE cessation • Healthcare utilization metrics (eg, length of stay, # of days in the ICU) • Functional outcomes • Safety measures

©2021 Marinus Pharmaceuticals. All Rights Reserved I 38 RSE Phase 3 RAISE Clinical Planning • 1:1 randomized, double - blind, placebo - controlled trial • Failure of a benzodiazepine and 2 second - line IV AEDs • 3 - minute bolus, 36 - hour infusion, 12 - hour taper • Approx. 125 randomized patients • 80 - 100 sites Trial Overview S C R E E N I N G Dose Initiation (Time 0) Treatment Period Follow - up Period Weeks 1,2,3 & 4 Daily 24 - 120 hours Day 2 Hours 24 - 36 Hours 36 - 48 Daily Hours 0 - 24 Bolus dose Continuous Infusion Taper Treatment is planned to be 2 days (including a 12 - hour taper). Phase 3 Target Plasma Concentration Continuous Infusion Infusion Taper Currently recruiting patients Topline data expected 1H 2022 38

©2021 Marinus Pharmaceuticals. All Rights Reserved I 39 RAISE Trial Sites Standard of Care Progression to IV Anesthesia ► Surveyed PI’s at selected RAISE Trial sites to learn more about their standard of care natural history progression to IV anesthesia following the failure of one versus more than one 2nd - line IV AEDs Trial population Of those that escalate to 3 rd - line IV anesthesia, they do so in ~2.5 hours following failure of the second 2 nd - line IV AED Clear unmet medical need in patients that fail two or more 2 nd line IV AEDs Guides site selection and approximates placebo response for escalation to IV anesthesia co - primary Patients with non - convulsive status epilepticus (NCSE) median 1 2 0 20 40 60 80 100 # of failed 2 nd -line IV AEDs % o f P a t i e n t s P r o g r e s s i n g t o 3 r d - l i n e I V a n e s t h e t i c s median + 95% CI

Commercial Opportunity
©2021 Marinus Pharmaceuticals. All Rights Reserved I 41 Quantifying the Significant Clinical and Economic Burden of RSE The Phase 3 trial of ganaxolone in refractory SE aims to demonstrate rapid onset of action capable of preventing escalation to IV anesthetics, and downstream associated clinical outcomes Treatment with IV anesthetics has been reported to lead to increased length of hospital admission and risk of infections, new disability, and death 1 - 3 Pharmacoeconomic opportunity to quantify cost of care and characterize clinical outcomes based on treatment progression to IV anesthetics 1 Sutter R et al. 2014 Neurology 2 Hawkes MA et al. 2019 Crit. Care Med. 3 Marchi NA et al. 2015 Crit. Care Med. $ $ To support value - based economics / approach will be a key tool with reimbursement experts

©2021 Marinus Pharmaceuticals. All Rights Reserved I 42 High Cost of Care Currently Associated with RSE Population Ganaxolone may provide an opportunity to reduce hospital costs and save lives by altering how medicine is practiced Utilization and Cost Outcomes Metric Cohort 1 (≤ 1 IV AED) Cohort 2 (> 1 IV AED) Cohort 3 ( ≥ 1 IV anesthetic) All Unique RSE patient encounter, N (%) 14,694 (33.4) 10,140 (23.1) 19,154 (43.5) 43,988 (100) Hospital length of stay (LOS) (days) Mean* 4.7 7.2 12.0 8.4 Median* 3 4 8 5 ICU LOS (for ICU patients only) Mean* 2.7 3.1 6.6 5.4 Median* 2 2 4 3 Total hospital cost* ($USD) Mean* $11,532 $18,328 $41,858 $26,304 Median* $6,812 $10,592 $24,105 $13,201 Clinical Outcomes Metric Cohort 1 (≤ 1 IV AED) Cohort 2 (> 1 IV AED) Cohort 3 ( ≥ 1 IV anesthetic) All Unique RSE patient encounter, N (%) 14,694 (33.4) 10,140 (23.1) 19,154 (43.5) 43,988 (100) Discharge disposition (%) Expired * 4.6 6.3 18.9 11.2 Hospital - acquired condition (%Y) 14.0 19.4 23.1 19.2 Catheter - associated UTI (%) 12.0 17.4 18.3 16.0 Miscellaneous infection Ŧ (%) 1.6 1.7 4.3 2.8 Vascular catheter - associated infection Ŧ (%) 0.2 0.2 0.4 0.3 Mechanical ventilator - associated complication (%) 0.2 0.2 1.6 0.8 * Indicates p<0.05 across all pairwise comparisons Ŧ I ndicates p<0.05 C1 or C2 vs. C3 Marinus estimates that safe and effective therapeutics that prevent progression to SRSE (i.e., treatment with IV anesthetics) may reduce mortality rates and hospital costs Source: Guteran EL 2021 JAMA Neurol.

Status Epilepticus Development Plan
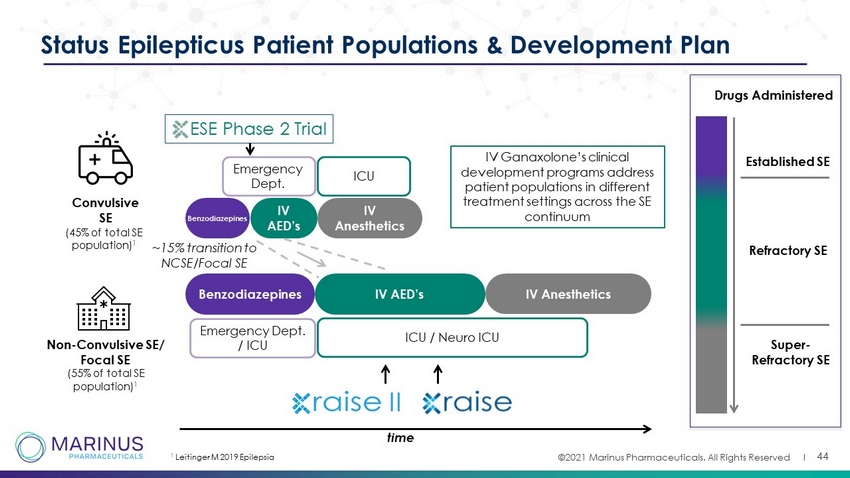
©2021 Marinus Pharmaceuticals. All Rights Reserved I 44 Status Epilepticus Patient Populations & Development Plan Convulsive SE Non - Convulsive SE/ Focal SE (55% of total SE population) 1 Benzodiazepines IV AED’s IV Anesthetics IV AED’s IV Anesthetics ESE Phase 2 Trial ICU / Neuro ICU time Established SE Refractory SE Super - Refractory SE Benzodiazepines Emergency Dept. / ICU ~15% transition to NCSE/Focal SE Drugs Administered IV Ganaxolone’s clinical development programs address patient populations in different treatment settings across the SE continuum ICU Emergency Dept . 1 Leitinger M 2019 Epilepsia (45% of total SE population) 1
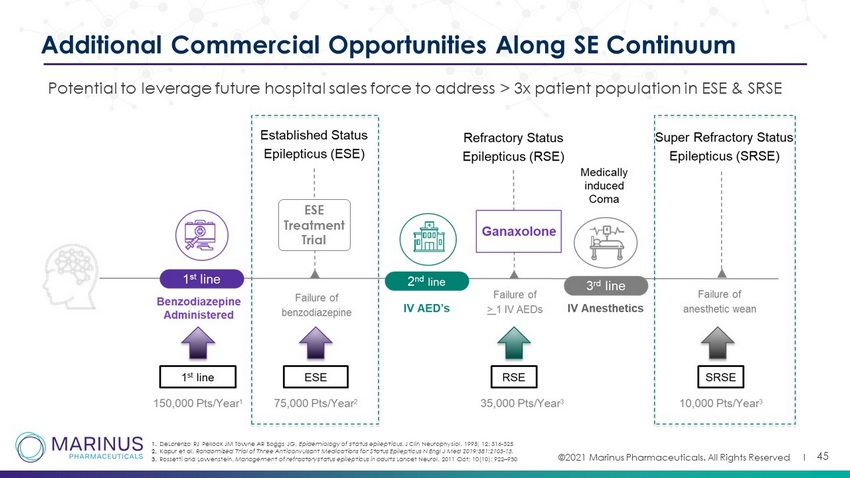
©2021 Marinus Pharmaceuticals. All Rights Reserved I 45 Additional Commercial Opportunities Along SE Continuum Potential to leverage future hospital sales force to address > 3x patient population in ESE & SRSE 1. DeLorenzo RJ Pellock JM Towne AR Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995; 12: 316 - 325 2. Kapur et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus N Engl J Med 2019;381:2103 - 13. 3. Rossetti and Lowenstein. Management of refractory status epilepticus in adults Lancet Neurol. 2011 Oct; 10(10): 922 – 930 ESE Treatment Trial
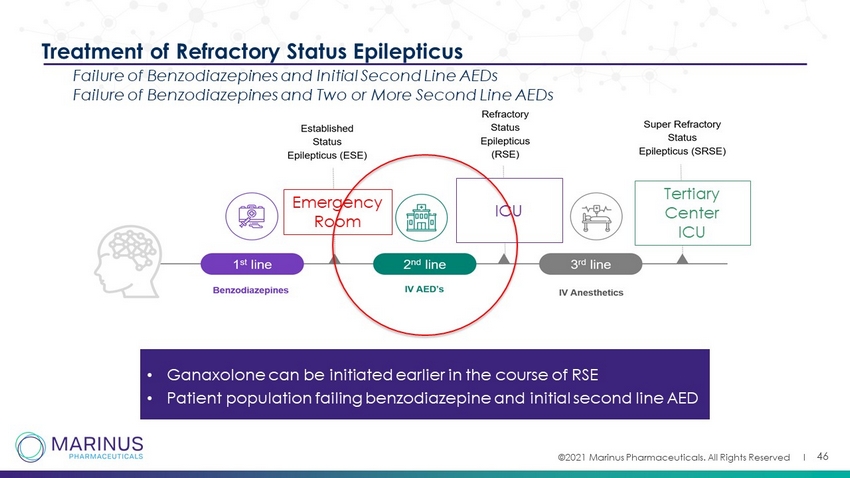
©2021 Marinus Pharmaceuticals. All Rights Reserved I 46 Emergency Room ICU Tertiary Center ICU Treatment of Refractory Status Epilepticus Failure of Benzodiazepines and Initial Second Line AEDs Failure of Benzodiazepines and Two or More Second Line AEDs • Ganaxolone can be initiated earlier in the course of RSE • Patient population failing benzodiazepine and initial second line AED
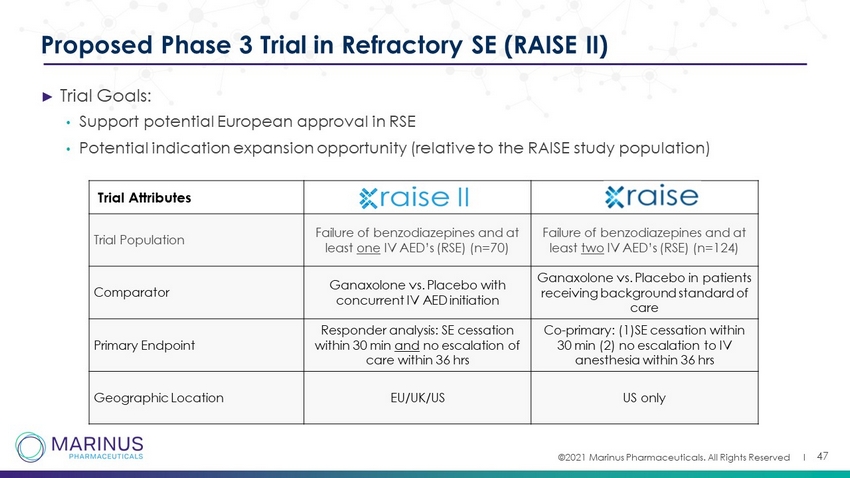
©2021 Marinus Pharmaceuticals. All Rights Reserved I ► Trial Goals: • Support potential European approval in RSE • Potential indication expansion opportunity (relative to the RAISE study population) 47 Proposed Phase 3 Trial in Refractory SE (RAISE II) Trial Attributes Trial Population Failure of benzodiazepines and at least one IV AED’s (RSE) (n=70) Failure of benzodiazepines and at least two IV AED’s (RSE) (n=124) Comparator Ganaxolone vs. Placebo with concurrent IV AED initiation Ganaxolone vs. Placebo in patients receiving background standard of care Primary Endpoint Responder analysis: SE cessation within 30 min and no escalation of care within 36 hrs Co - primary: (1)SE cessation within 30 min (2) no escalation to IV anesthesia within 36 hrs Geographic Location EU/UK/US US only

©2021 Marinus Pharmaceuticals. All Rights Reserved I 48 Emergency Room ICU Tertiary Center ICU Established Status Epilepticus – Potential Use of Ganaxolone in Emergency Room • Unique Environment: - No EEG - Risk of rapid escalation of care - Convulsive patients: new dosing paradigm – bolus and short infusion time (2 - 24 hours)
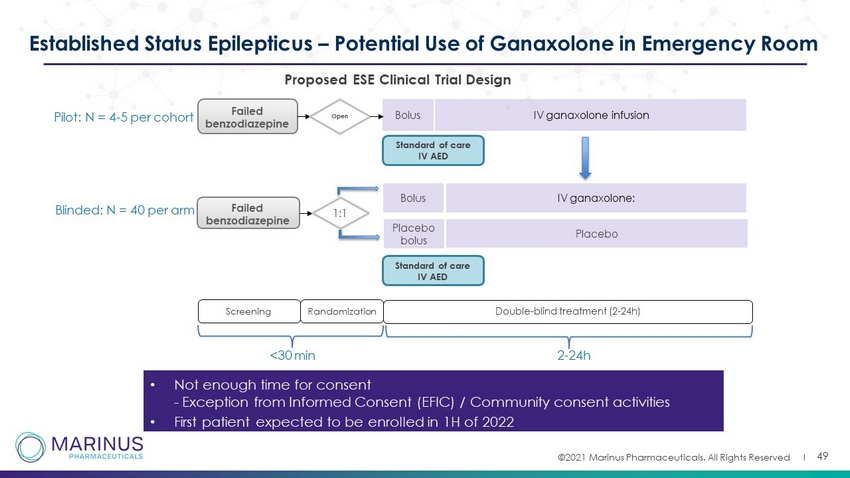
©2021 Marinus Pharmaceuticals. All Rights Reserved I 49 Established Status Epilepticus – Potential Use of Ganaxolone in Emergency Room • Not enough time for consent - Exception from Informed Consent (EFIC) / Community consent activities • First patient expected to be enrolled in 1H of 2022 Failed benzodiazepine Open B olus IV ganaxolone infusion Screening Double - blind treatment (2 - 24h) Failed benzodiazepine Randomization 1:1 Standard of care IV AED Placebo bolus B olus Placebo IV ganaxolone: <30 min Pilot: N = 4 - 5 per cohort Blinded: N = 40 per arm Standard of care IV AED 2 - 24h P roposed ESE Clinical T rial D esign

Financial Update

©2021 Marinus Pharmaceuticals. All Rights Reserved I Credit Financing Overview Credit financing agreement with Oaktree Capital Management , a leader among investment managers specializing in alternative investments Financing has potential to provide incremental cash of up to $125 million total through 2023* ( gross proceeds before fees) $75 million ** available to be drawn through 2H 2022 based on anticipated regulatory milestones associated with the CDKL5 Deficiency Disorder indication Marinus has option to draw the final $50 million based on additional ganaxolone milestones* Transaction proceeds enable continued investment in commercialization, R&D, and manufacturing scale up Monetization of the anticipated Priority Review Voucher is permitted and Marinus maintains ability to execute a U.S. synthetic royalty monetization deal *Availability subject to meeting of certain regulatory, clinical, financing, and revenue thresholds **15 million drawn at closing in May 2021 Morgan Stanley & Co. LLC acted as lead placement agent and H. C. Wainwright & Co. acted as co - placement agent on the transaction The loan will mature in May 2026 and includes an interest only period for the initial three years of the agreement 51

©2021 Marinus Pharmaceuticals. All Rights Reserved I Financial Overview: Q1 2021 Analyst Coverage * : • Cantor Fitzgerald: Alethia Young • Cowen: Joseph Thome, Ph.D. • H.C. Wainwright & Co: Douglas Tsao • Jefferies: Andrew Tsai • JMP Securities: Jason N. Butler, Ph.D. • Ladenburg Thalman : Michael Higgens • Leerink : Marc Goodman • Oppenheimer: Jay Olson • Truist : Joon Lee, M.D., Ph.D. • RW Baird: Brian Skorney * Note: Opinions, estimates, and forecasts of the individual analysts regarding Marinus do not represent opinions, estimates, and forecasts of Marinus. The listing above does not imply endorsement or concurrent with their information, conclusions, or recommendations. Investor Relations – Nasdaq: MRNS Updated 2021 Guidance • Revenue • FY 2021 projected to be between $9 - $12 million • Operating Expenses • FY 2021 loss from operations of between $113 - $118 million • Total includes approximately $16 million of non - cash stock - based compensation Financial Summary (at March 31, 2021): • $123.5 million in cash and cash equivalents • $0 in debt • 36.6 million shares outstanding • 5.4 million options, RSUs & convertible preferred stock outstanding 52

©2021 Marinus Pharmaceuticals. All Rights Reserved I 53 BARDA Contract – Refractory Status Epilepticus Key Contract Parameters ► BARDA to contribute $21 million in base contract to support the Phase 3 RAISE clinical trial in RSE and preclinical studies of ganaxolone in nerve agent exposure animal models. ► BARDA may contribute up to an additional $30 million in support of manufacturing, supply chain, clinical, regulatory and toxicology activities based on favorable clinical and pre - clinical outcomes. ► Total contract value = $84 million; $51 million BARDA / $33 million Marinus - if all options are undertaken. ► On successful development, BARDA and Marinus may negotiate for a supply of ganaxolone for a potential response to nerve gas exposure threats.

Intellectual Property
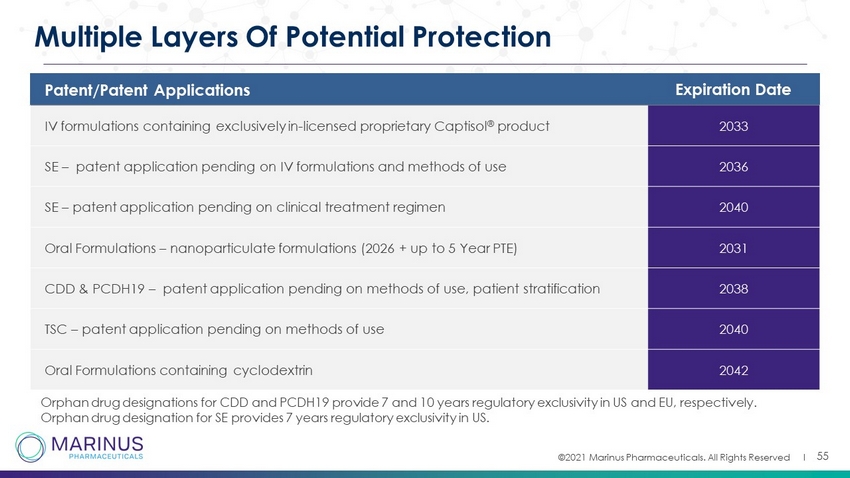
©2021 Marinus Pharmaceuticals. All Rights Reserved I 55 Multiple Layers Of Potential Protection Patent/Patent Applications Expiration Date IV formulations containing exclusively in - licensed proprietary Captisol ® product 2033 SE – patent application pending on IV formulations and methods of use 2036 SE – patent application pending on clinical treatment regimen 2040 Oral Formulations – nanoparticulate formulations (2026 + up to 5 Year PTE) 2031 CDD & PCDH19 – patent application pending on methods of use, patient stratification 2038 TSC – patent application pending on methods of use 2040 Oral Formulations containing cyclodextrin 2042 Orphan drug designations for CDD and PCDH19 provide 7 and 10 years regulatory exclusivity in US and EU, respectively. Orphan drug designation for SE provides 7 years regulatory exclusivity in US.

Thank You

Appendix

CDKL5 Deficiency Disorder
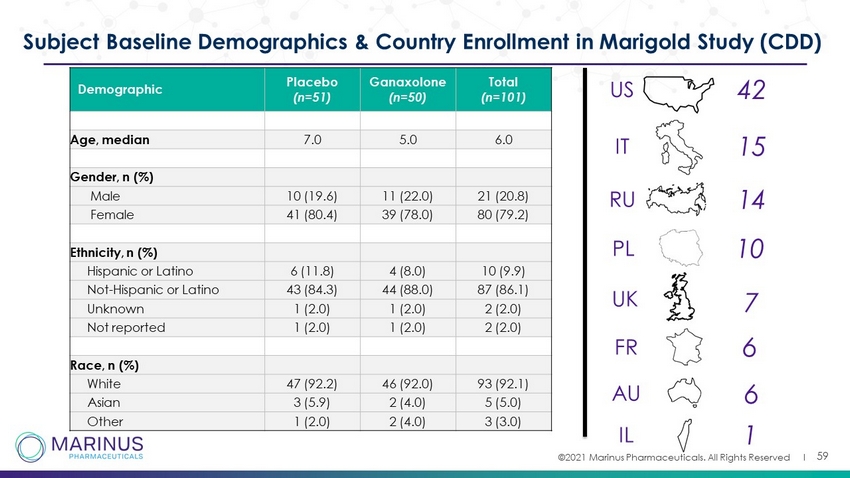
©2021 Marinus Pharmaceuticals. All Rights Reserved I 59 Subject Baseline Demographics & Country Enrollment in Marigold Study (CDD) Demographic Placebo (n=51) Ganaxolone (n=50) Total (n=101) Age, median 7.0 5.0 6.0 Gender, n (%) Male 10 (19.6) 11 (22.0) 21 (20.8) Female 41 (80.4) 39 (78.0) 80 (79.2) Ethnicity, n (%) Hispanic or Latino 6 (11.8) 4 (8.0) 10 (9.9) Not - Hispanic or Latino 43 (84.3) 44 (88.0) 87 (86.1) Unknown 1 (2.0) 1 (2.0) 2 (2.0) Not reported 1 (2.0) 1 (2.0) 2 (2.0) Race, n (%) White 47 (92.2) 46 (92.0) 93 (92.1) Asian 3 (5.9) 2 (4.0) 5 (5.0) Other 1 (2.0) 2 (4.0) 3 (3.0) US IT RU UK PL FR AU IL 42 15 14 10 7 6 6 1

©2021 Marinus Pharmaceuticals. All Rights Reserved I 60 Responder Analysis – Marigold Study Percent Reductions in Major Motor Seizure Frequency 25% 50% 75% 0 20 40 60 80 100 Percent Reduction 28-day Frequency of Major Motor Seizures % o f P a t i e n t s Ganaxolone Placebo 57.1% 23.5% 24.5% 9.8% 10.2% 3.9% p=0.064 a a Fisher's Exact Test p=0.001 a

Tuberous Sclerosis Complex

©2021 Marinus Pharmaceuticals. All Rights Reserved I 62 Unmet Need in TSC Epilepsy and Scientific Rationale for Ganaxolone ► Epilepsy present in ~90% of individuals affected with TSC 1 ► Existing treatments fail to control seizures in ~60% of individuals with TSC - associated epilepsy 2 ► Uncontrolled seizures, especially early in life, may lead to developmental delays and cognitive dysfunction Clear need to evaluate treatments with a differentiated mechanism of action Data generated in collaboration with the TS Alliance and utilization of their biosample repository Patients aged 1 - 14 1. Canevini MP et al 2018 Am J of Med Genetics_ Current concepts on epilepsy management in TSC. 2. ChuShore CJ et al Epilepsia_The natural history of epilepsy in TSC.

Status Epilepticus

©2021 Marinus Pharmaceuticals. All Rights Reserved I 64 NCSE: Non - convulsive status epilepticus CSE: Convulsive status epilepticus LAC: Lacosamide LEV: Levetiracetam LOR: Lorazepam PHT: Phenytoin fPHT: Fosphenytoin VPA: Valproic Acid Details on Baseline Patient Characteristics for Phase 2 SE Trial *Bolded, underlined IV AED’s were the last ones administered prior to GNX Patient Dosing Cohort Etiology History of Epilepsy Type of SE Failed Antiseizure Medications Prior to GNX* Dose of Last IV AED Administered Prior to GNX (Recommended Dose) 1 Low Vascular No NCSE LAC , LEV 200mg (200 - 600mg) 2 Low Unknown Yes NCSE fPHT, LEV 1,000mg (1000 - 3000mg) 3 Low Vascular No NCSE LOR, LAC , LEV 600mg (200 - 600mg) 4 Low Vascular No NCSE LOR, LAC , LEV 600mg (200 - 600mg) 5 Low Tumor No CSE LOR, LAC, LEV 2,000mg (1000 - 3000mg) 6 Medium Vascular No NCSE LOR, LAC , LEV 600mg (200 - 600mg) 7 Medium Drug Overdose / Withdrawal Yes CSE LOR, LEV 1,000mg (1000 - 3000mg) 8 Medium Unknown Yes CSE → NCSE LOR, LAC, LEV 1,000mg (1000 - 3000mg) 9 Medium Tumor Yes NCSE LAC, LEV, PHT 200mg (100mg) 10 Target Vascular Yes CSE LOR, LAC , VPA 400mg (200 - 600mg) 11 Target Drug Overdose / Withdrawl No CSE LOR, LAC , LEV 400mg (200 - 600mg) 12 Target Tumor Yes NCSE LOR, LEV, VPA 700mg (1000 - 3000mg) 13 Target Autoimmune No NCSE LOR, LEV 1,000mg (1000 - 3000mg) 14 Target Vascular No NCSE LOR, LAC , LEV, PHT 200mg (200 - 600mg) 15 Target Vascular Yes CSE LOR, LEV 1,000mg (1000 - 3000mg) 16 Target Tumor No NCSE LOR, LAC , LEV 400mg (200 - 600mg) 17 Target Autoimmune No NCSE LOR, fPHT, LAC , LEV, VPA 200mg (200 - 600mg)

©2021 Marinus Pharmaceuticals. All Rights Reserved I 65 Potential Launch Into the Hospital Setting Designed to be Driven by Data, Customer Collaboration & Protocolization of Ganaxolone in RSE • Phase 3 data to support clinical adoption and budget model • Clear clinical benefit eg, SE cessation, IV escalation • Economic advantage – LOS*, ICU duration, clinical outcomes Compelling Clinical and HEOR* Data • Partner with KOLs and societies to update RSE treatment guidelines • Collaborative approach to protocol augmentation with health systems and local hospitals Society Guideline & Account Protocol Inclusion • Early engagement with hospital stakeholders to best understand and frame value proposition • Determine formulary process and requirements • Reimbursement, logistics and operational processes C - Suite, Pharmacy, & Admin Engagement • Identify, navigate and influence unique hospital decision makers • Educate and generate customer usage data • Collaborate internally to protocolize usage and translate success Experienced Hospital Sales Force Clinical and Economic Evidence Access Pull - Through Clinical Adoption Critical Success Factors for RSE Launch *LOS – Longer length of stay *HEOR – Health economics and outcomes research

PCDH19 Related Epilepsy

©2021 Marinus Pharmaceuticals. All Rights Reserved I 67 Biomarker Stratified Proof of Concept (POC) Study in PCDH19 Trial Details ► Ages 1 - 17 with 8 or more seizures in 8 weeks, failed 2 or more AEDs ► Completed double - blind portion of the trial with 21 patients ► Primary efficacy analysis based on change in seizure frequency in all patients ► Stratify patients into one of two biomarker groups based on baseline allopregnanolone sulfate levels and randomized (ganaxolone or placebo) within each stratum R 1:1 PCDH19 (all - patients) n =21 Biomarker + Low Allo - S Primary efficacy analyses conducted using all patients Biomarker - High Allo - S R 1:1 Ganaxolone Up to 600 mg 3x/day Placebo Ganaxolone Up to 600 mg 3x/day Placebo Ganaxolone Up to 600 mg 3x/day Pre - baseline Screening Maintenance 13 weeks Baseline 12 weeks Open - Label Phase (52 weeks) * not drawn to scale Titration 4 weeks Screening Visit Titration 4 weeks
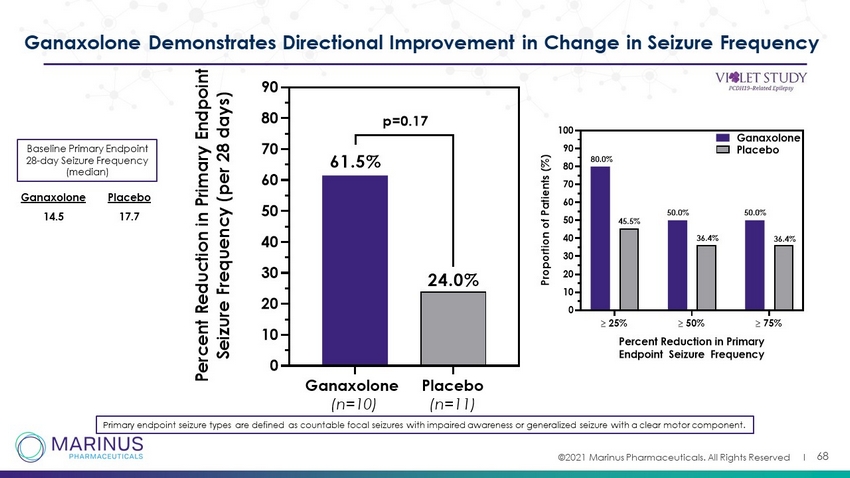
©2021 Marinus Pharmaceuticals. All Rights Reserved I 68 Ganaxolone Demonstrates Directional Improvement in Change in Seizure Frequency Primary endpoint seizure types are defined as countable focal seizures with impaired awareness or generalized seizure with a cle ar motor component. Ganaxolone (n=10) Placebo (n=11) 0 10 20 30 40 50 60 70 80 90 P e r c e n t R e d u c t i o n i n P r i m a r y E n d p o i n t S e i z u r e F r e q u e n c y ( p e r 2 8 d a y s ) 61.5% 24.0% p=0.17 25% 50% 75% 0 10 20 30 40 50 60 70 80 90 100 Percent Reduction in Primary Endpoint Seizure Frequency P r o p o r t i o n o f P a t i e n t s ( % ) Ganaxolone Placebo 80.0% 45.5% 36.4% 36.4% 50.0% 50.0% Baseline Primary Endpoint 28 - day Seizure Frequency (median) 14.5 17.7 Ganaxolone Placebo



































































