Exhibit 99.2

NASDAQ: MRNS @MarinusPharma Topline Ganaxolone Phase 2 Open - Label Results in Tuberous Sclerosis Complex: The CALM Study August 17, 2021 1

©2021 Marinus Pharmaceuticals. All Rights Reserved I 2 Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding Marinus, they ar e forward - looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Secu rit ies Litigation Reform Act of 1995. Words such as “may”, “will”, “expect”, “anticipate”, “estimate”, “intend”, “believe”, and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward - looking statements. Examples of forward - looking statements contained in this presentation include, among others, statements regarding our clinical development plans for ganaxolone ; expected dosing in our clinical trials; the clinical development schedule and milestones; our expected timing to begin and complete enrollment in our clinical trials; the expecte d t rial design, target patient population and endpoints for our clinical trials; interpretation of scientific basis for ganaxolone use; timing for availability and release of data; and the potential safety and efficacy and therapeutic potential of ganaxolone . Forward - looking statements in this presentation involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those ex pressed or implied by the forward - looking statements. Such risks and uncertainties include, among others, uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; early clinical trials may not be indicative of the results in l ate r clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; our ability to obt ain and maintain regulatory approval for our product candidate; our ability to obtain, maintain, protect and defend intellectual property for our product candidates; the pot ential negative impact of third party patents on our or our collaborators’ ability to commercialize ganaxolone ; delays, interruptions or failures in the manufacture and supply of our product candidate; the size and growth potential of the markets for our product candidates, and our ability to service those markets; ou r cash and cash equivalents may not be sufficient to support our operating plan for as long as anticipated; our expectations, projections and estimates regar din g expenses, future revenue, capital requirements, and the availability of and the need for additional financing; our ability to obtain additional funding to support our clinical development programs; our ability to develop sales and marketing capabilities, whether alone or with potential future collaborators; the rat e and degree of market acceptance of our product candidates; the potential for Orion to breach our collaboration with them or terminate the collaboration agreement in accordance with its terms; the potential for Orion to recoup a percentage of the upfront fee depending on the additional pre - clinical testi ng expected to be completed in Q1 2022; the effect of the COVID - 19 pandemic on our business, the medical community, regulators and the global economy; and the availability or potential availability of alternative products or treatments for conditions targeted by us that could affect the availability or commer cia l potential of our product candidate. Marinus undertakes no obligation to update or revise any forward - looking statements. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward - looking statements, as well as risks relating to our business in general, see filings we have made with the Securities and Exchange Commission. You may access these documents for free by visiting EDGAR on the SEC web si te at www.sec.gov .
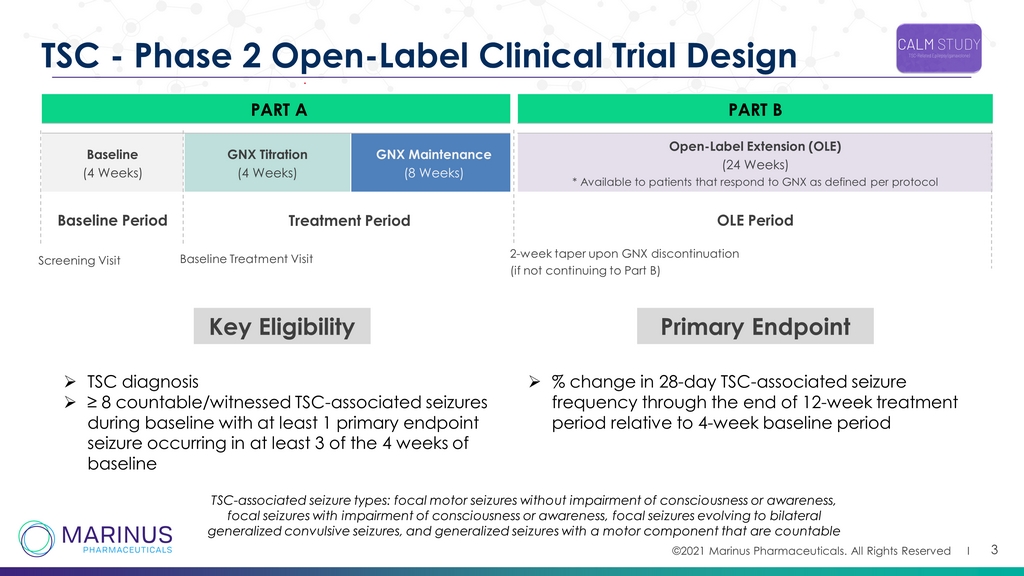
©2021 Marinus Pharmaceuticals. All Rights Reserved I PART A PART B Baseline (4 Weeks) GNX Titration (4 Weeks) GNX Maintenance (8 Weeks) Open - Label Extension (OLE) (24 Weeks) * Available to patients that respond to GNX as defined per protocol 3 TSC - Phase 2 Open - Label Clinical Trial Design Baseline Period Treatment Period OLE Period Screening Visit Baseline Treatment Visit 2 - week taper upon GNX discontinuation (if not continuing to Part B) Key Eligibility » TSC diagnosis » ≥ 8 countable/witnessed TSC - associated seizures during baseline with at least 1 primary endpoint seizure occurring in at least 3 of the 4 weeks of baseline Primary Endpoint » % change in 28 - day TSC - associated seizure frequency through the end of 12 - week treatment period relative to 4 - week baseline period TSC - associated seizure types: focal motor seizures without impairment of consciousness or awareness, focal seizures with impairment of consciousness or awareness, focal seizures evolving to bilateral generalized convulsive seizures, and generalized seizures with a motor component that are countable
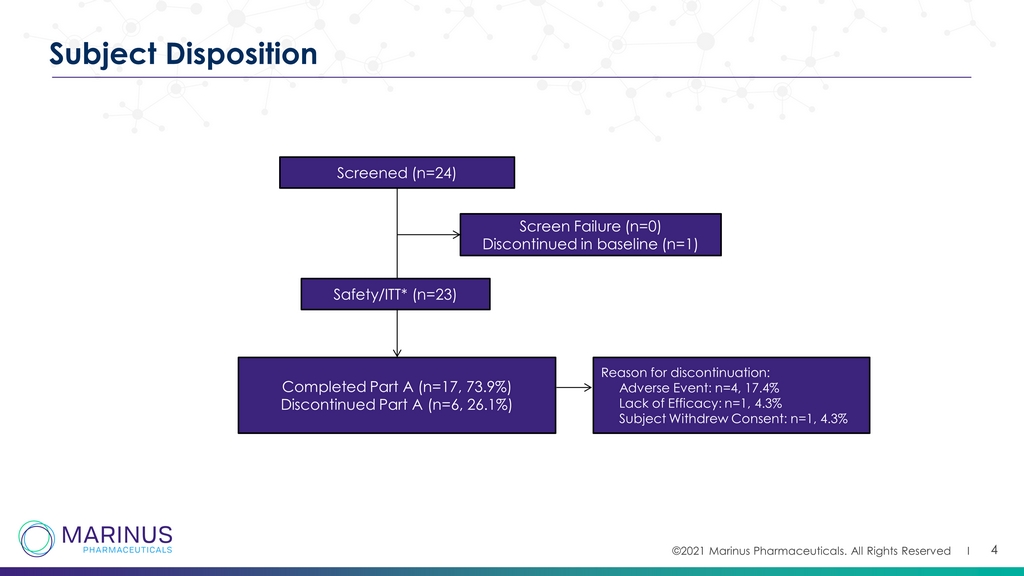
©2021 Marinus Pharmaceuticals. All Rights Reserved I 4 Subject Disposition Screened (n=24) Screen Failure (n=0) Discontinued in baseline (n=1) Safety/ITT* (n=23) Completed Part A (n=17, 73.9%) Discontinued Part A (n=6, 26.1%) Reason for discontinuation: Adverse Event: n=4, 17.4% Lack of Efficacy: n=1, 4.3% Subject Withdrew Consent: n=1, 4.3%
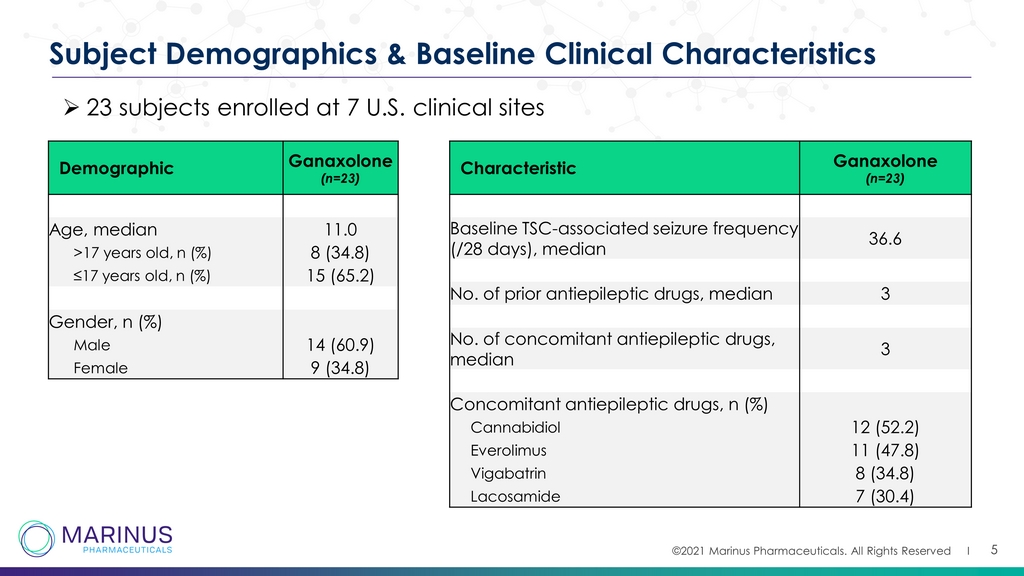
©2021 Marinus Pharmaceuticals. All Rights Reserved I 5 Subject Demographics & Baseline Clinical Characteristics Demographic Ganaxolone (n=23) Age, median 11.0 >17 years old, n (%) 8 (34.8) ≤17 years old, n (%) 15 (65.2) Gender, n (%) Male 14 (60.9) Female 9 (34.8) Characteristic Ganaxolone (n=23) Baseline TSC - associated seizure frequency (/28 days), median 36.6 No. of prior antiepileptic drugs, median 3 No. of concomitant antiepileptic drugs, median 3 Concomitant antiepileptic drugs, n (%) Cannabidiol 12 (52.2) Everolimus 11 (47.8) Vigabatrin 8 (34.8) Lacosamide 7 (30.4) » 23 subjects enrolled at 7 U.S. clinical sites
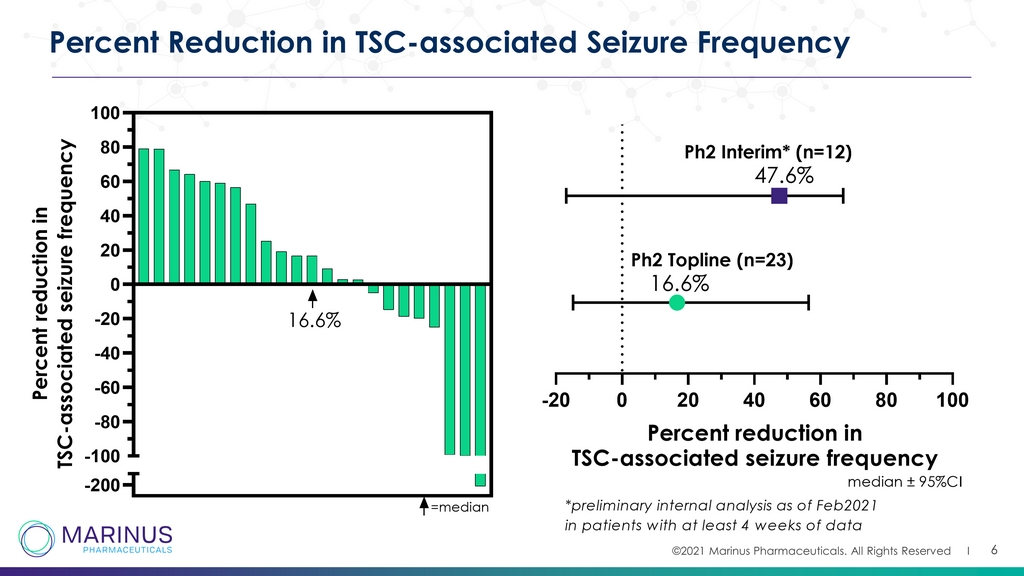
©2021 Marinus Pharmaceuticals. All Rights Reserved I 6 Percent Reduction in TSC - associated Seizure Frequency -200 -100 -80 -60 -40 -20 0 20 40 60 80 100 P e r c e n t r e d u c t i o n i n T S C - a s s o c i a t e d s e i z u r e f r e q u e n c y =median 16.6% -20 0 20 40 60 80 100 Percent reduction in TSC-associated seizure frequency median ± 95%CI 16.6% 47.6% Ph2 Topline (n=23) Ph2 Interim* (n=12) *preliminary internal analysis as of Feb2021 in patients with at least 4 weeks of data
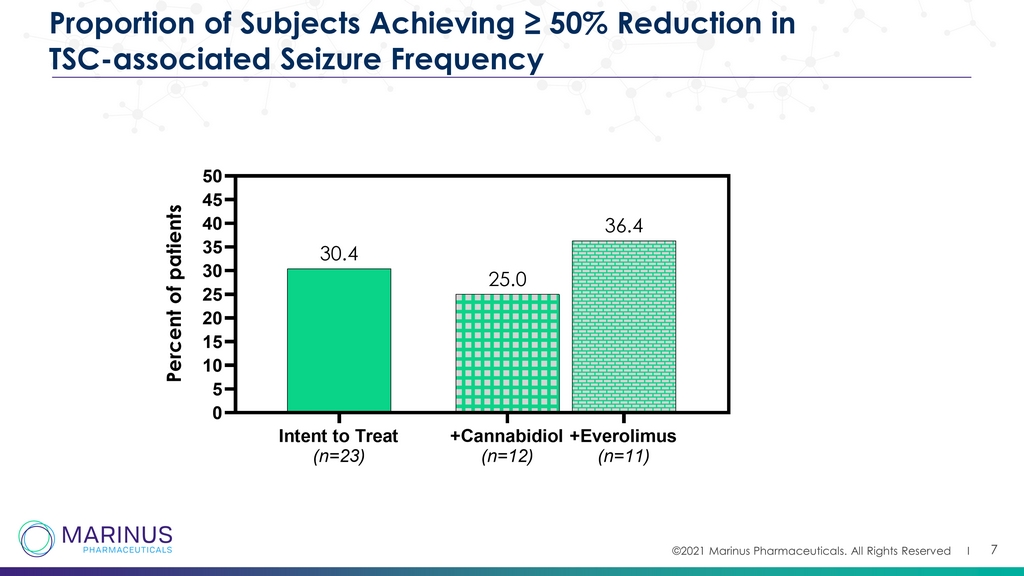
©2021 Marinus Pharmaceuticals. All Rights Reserved I 7 Proportion of Subjects Achieving ≥ 50% Reduction in TSC - associated Seizure Frequency Intent to Treat (n=23) +Cannabidiol (n=12) +Everolimus (n=11) 0 5 10 15 20 25 30 35 40 45 50 36.4 25.0 30.4 P e r c e n t o f p a t i e n t s
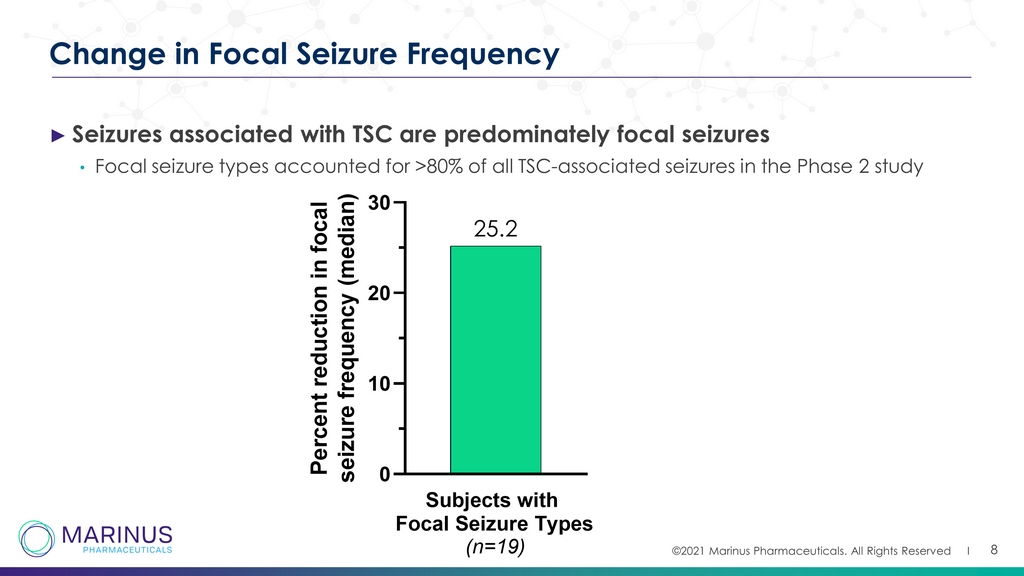
©2021 Marinus Pharmaceuticals. All Rights Reserved I 8 Change in Focal Seizure Frequency ► Seizures associated with TSC are predominately focal seizures • Focal seizure types accounted for >80% of all TSC - associated seizures in the Phase 2 study Subjects with Focal Seizure Types (n=19) 0 10 20 30 25.2 P e r c e n t r e d u c t i o n i n f o c a l s e i z u r e f r e q u e n c y ( m e d i a n )
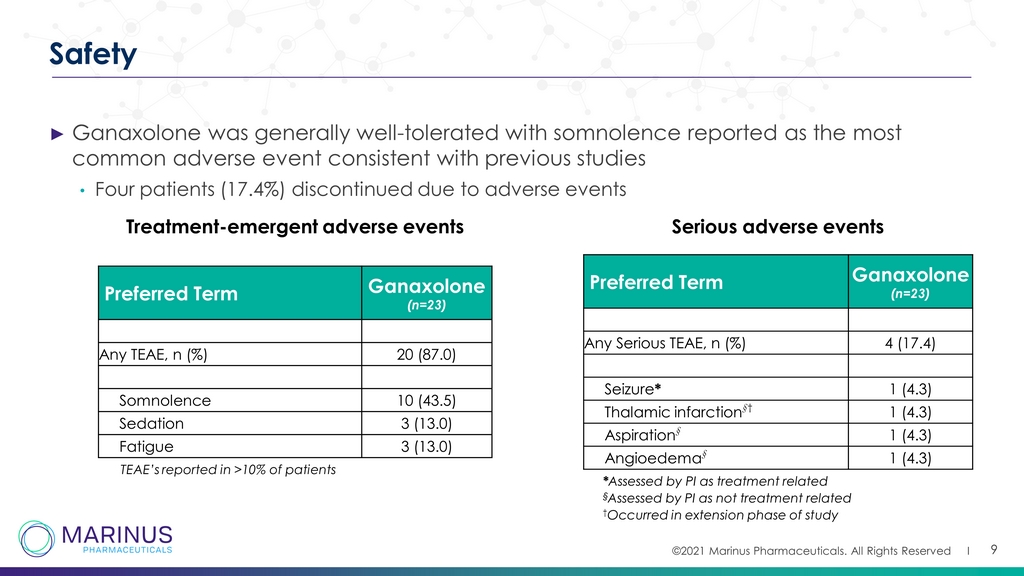
©2021 Marinus Pharmaceuticals. All Rights Reserved I 9 Safety ► Ganaxolone was generally well - tolerated with somnolence reported as the most common adverse event consistent with previous studies • Four patients (17.4%) discontinued due to adverse events Preferred Term Ganaxolone (n=23) Any TEAE, n (%) 20 (87.0) Somnolence 10 (43.5) Sedation 3 (13.0) Fatigue 3 (13.0) TEAE’s reported in >10% of patients Treatment - emergent adverse events Preferred Term Ganaxolone (n=23) Any Serious TEAE, n (%) 4 (17.4) Seizure * 1 (4.3) Thalamic infarction † † 1 (4.3) Aspiration † 1 (4.3) Angioedema † 1 (4.3) * Assessed by PI as treatment related Serious adverse events † Occurred in extension phase of study § Assessed by PI as not treatment related

©2021 Marinus Pharmaceuticals. All Rights Reserved I 10 Summary ► Study enrolled a highly refractory patient population on multiple AEDs including cannabidiol and everolimus ► Approximately 1/3 rd of patients had a robust reduction in TSC - associated seizures including those on concomitant cannabidiol and everolimus ► Most commonly reported AE’s consistent with previous clinical experience ► Data and study experience informs future clinical trial considerations related to seizure confirmation/classification, participant/caregiver education, titration schedule, and other design attributes

©2021 Marinus Pharmaceuticals. All Rights Reserved I 11 Phase 3 TSC Clinical Trial Enhancements ► Adjustment to weekly titration schedule • Aimed to further improve tolerability in TSC patients ► Seizure classification • Ensure seizures are being accurately documented and reliably counted through ongoing interactions with the Epilepsy Study Consortium and training with sites ► Implementation of improved electronic seizure diary • New data collection platform ► Other study design considerations including baseline / inclusion criteria and planned interim analysis










