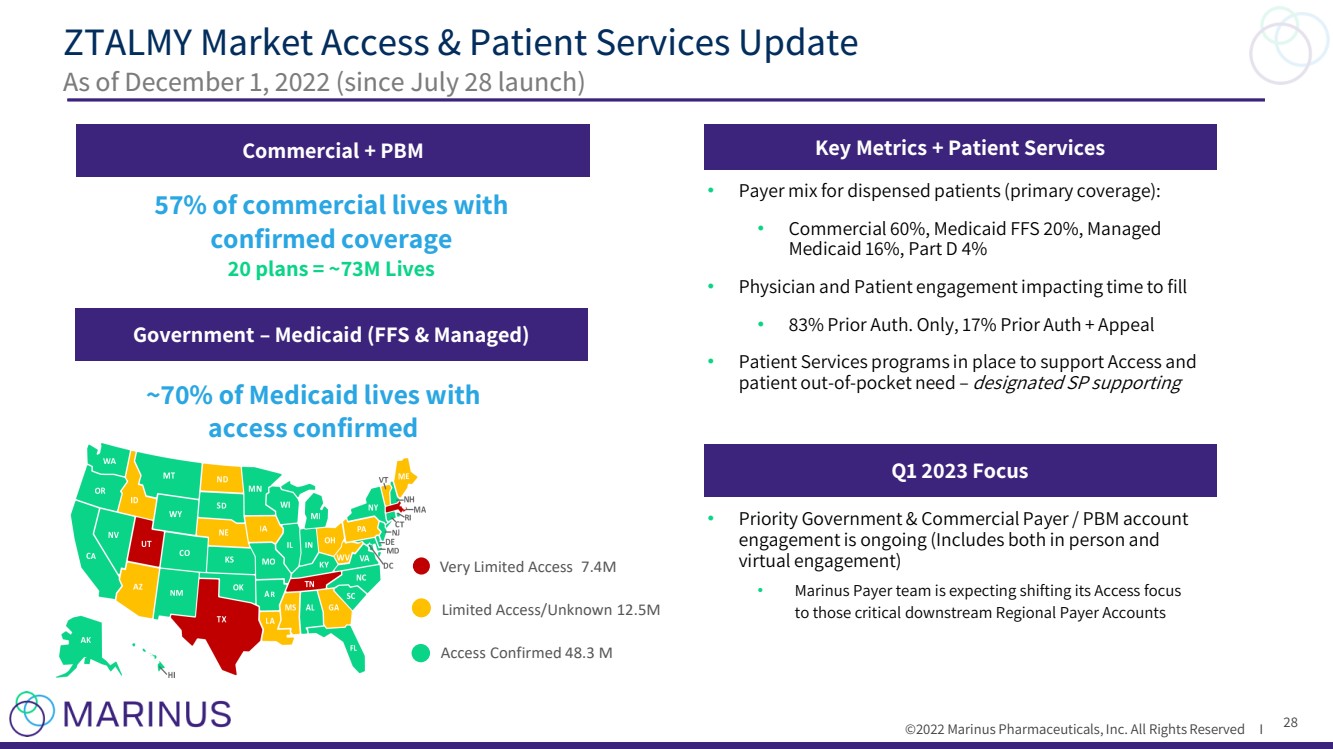
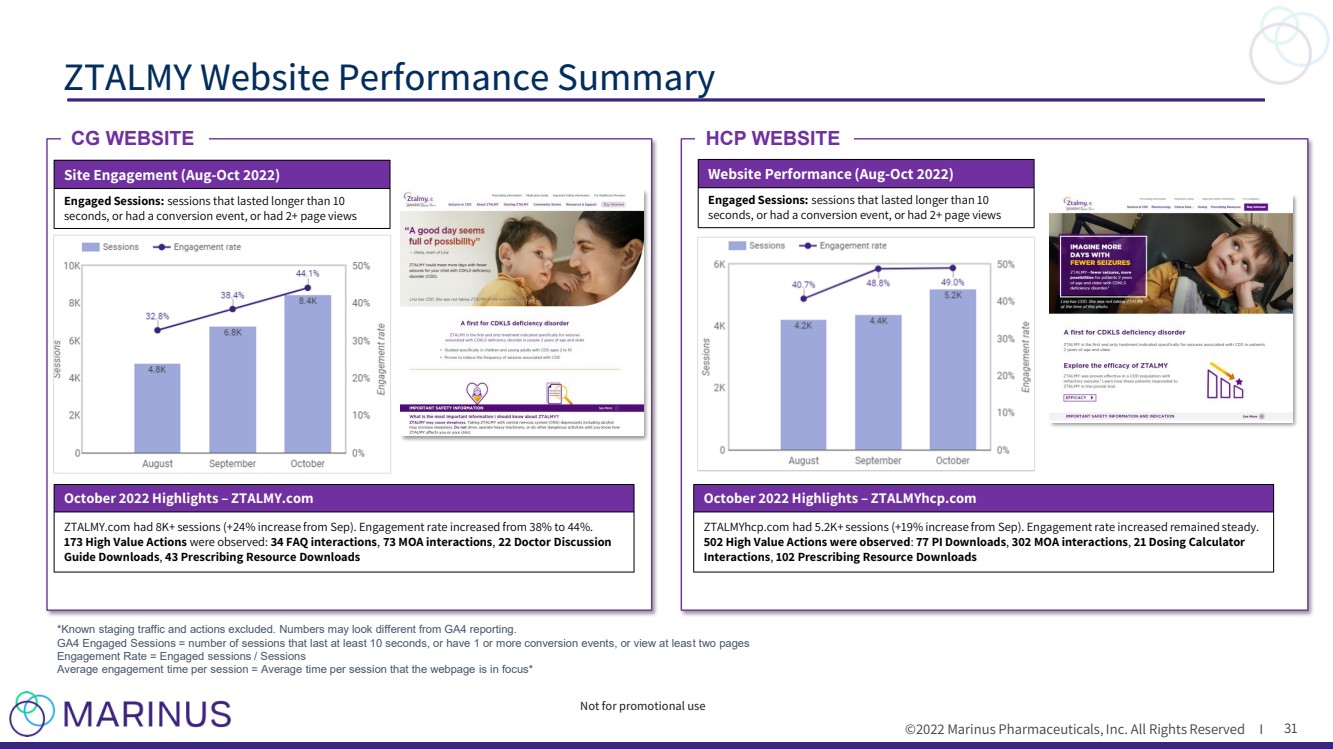
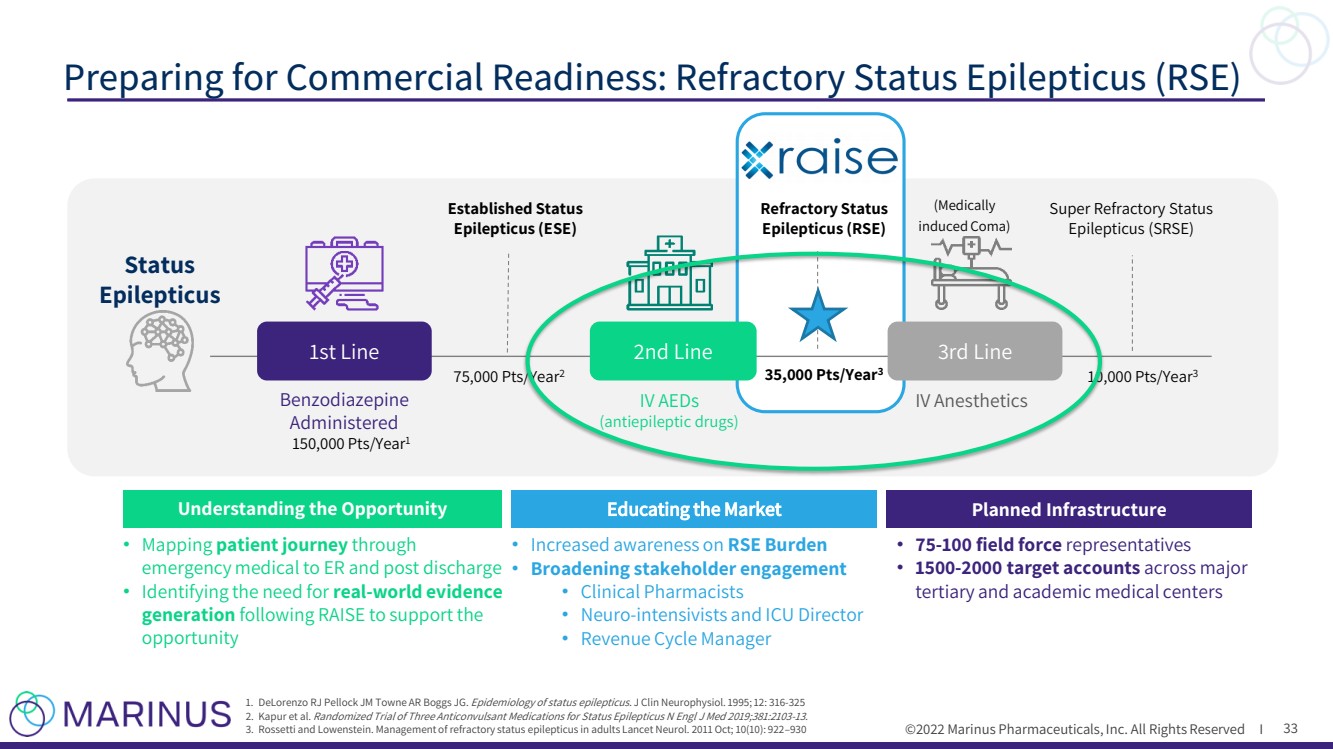
| ©2022 Marinus Pharmaceuticals. All Rights Reserved I To the extent that statements contained in this presentation are not descriptions of historical facts regarding Marinus, they ar e forward - looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 19 95. Words such as “may”, “will”, “expect”, “anticipate”, “estimate”, “intend”, “believe”, and similar expressions (as well as other words or expressions referencing future events, co ndi tions or circumstances) are intended to identify forward - looking statements. Examples of forward - looking statements contained in this presentation include, among others, statements rega rding our commercialization plans for ZTALMY® and clinical development plans for ganaxolone and the expected timing thereof ; expected dosing in our clinical trials; the clinical development schedule and milestones; our expected timing to begin and complete enrollment in our clinical trials; the expected trial design, target patient population and endpoints for our clinical trials; interpretation of scientific basis for ganaxolone use; timing for availability and release of data; the potential safety and efficacy and therapeutic potential of ganaxolone; tim ing and expectations regarding the potential benefits ZTALMY will provide for patients and physicians , our expectations regarding the ZTALMY One program; timing and expectations regarding regulatory communications and submissions; expectations regarding our agreement with BARDA; expectations regarding our collaborations; expectations regarding the potent ial market opportunities for our product candidates, including oral ganaxolone; potential commercial alliances; and our expectations regarding the effect of the COVID - 19 pandemic on our business and clinical development plans. Forward - looking statements in this presentation involve substantial risks and uncertainties that could cause our clinical development pr ograms, future results, performance or achievements to differ significantly from those expressed or implied by the forward - looking statements. Such risks and uncertainties include, am ong others, patient and physician acceptance of ZTALMY, our ability to obtain adequate market access for ZTALMY; our ability to comply with the FDA’s requirement for additional post - ma rket studies in the required timeframes; the timing of regulatory filings, including the timing of filing the ganaxolone MAA with the EMA; the potential that regulatory authorities , i ncluding the FDA and EMA, may not grant or may delay approval for our product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical tri als; unanticipated costs and expenses; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or furt her development in a specified indication or at all; actions or advice of the FDA or EMA may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the ne ed for additional clinical trials; our ability to obtain and maintain regulatory approval for our product candidates; our ability to obtain, maintain, protect and defend intellectual property for ou r product candidates; the potential negative impact of third party patents on our or our collaborators’ ability to commercialize ganaxolone; delays, interruptions or failures in the manu fac ture and supply of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to service those markets; our cash and cash equiv ale nts may not be sufficient to support our operating plan for as long as anticipated; our expectations, projections and estimates regarding expenses, future revenue, capital requirements, an d the availability of and the need for additional financing; our ability to obtain additional funding to support our commercial and clinical development programs; the potential for our c oll aborators to breach our collaboration agreements or terminate the agreements in accordance with their respective terms; the effect of the COVID - 19 pandemic on our business, the med ical community, regulators and the global economy; and the availability or potential availability of alternative products or treatments for conditions targeted by us that could aff ect the availability or commercial potential of our product candidates. Marinus undertakes no obligation to update or revise any forward - looking statements. For a further description of th e risks and uncertainties that could cause actual results to differ from those expressed in these forward - looking statements, as well as risks relating to our business in general, see filin gs we have made with the Securities and Exchange Commission. You may access these documents for free by visiting EDGAR on the SEC web site at www.sec.gov. Safe Harbor Statement 2 |