Liquidity and Capital Resources
Since inception, other than for the three months ended September 30, 2022 due to a one-time net gain from the sale of our PRV, we have incurred net losses and negative cash flows from our operations. We incurred a net loss of $66.7 million and $58.8 million for the six months ended June 30, 2023 and 2022, respectively. Our cash used in operating activities was $65.8 million for the six months ended June 30, 2023 compared to $61.3 million for the six months ended June 30, 2022. Historically, we have financed our operations principally through the sale of common stock, notes payable, preferred stock and convertible debt.
In July 2022, we entered into the PRV Asset Purchase Agreement to sell our PRV, pursuant to which Novo Nordisk, Inc. paid us $110.0 million upon the closing of the transaction. In August 2022, we received a letter from Purdue in which Purdue claimed that it was owed $5.5 million by us from the sale of the PRV pursuant to the Purdue License Agreement. Our position communicated to Purdue is that we do not owe Purdue any of the proceeds from the sale of the PRV. No associated payment by us has been made, and Purdue has not filed a specific claim to date.
In November 2022, in connection with an underwritten public offering of 10,526,316 shares of our common stock, pre-funded warrants to purchase 2,105,264 shares of common stock and the exercise of an option of 1,894,737 shares of common stock, we received approximately $64.5 million in total net proceeds after taking into account the exercise of the underwriters’ option, in each case deducting the underwriting discounts and commissions and after deducting offering expenses paid or payable by us. Additionally, in November 2022, we received an upfront payment of $32.5 million pursuant to the Revenue Interest Financing Agreement with Sagard, and in December 2022, we received an upfront payment of $10.0 million in connection with the Tenacia Collaboration Agreement.
As of June 30, 2023, we had cash, cash equivalents and short-term investments of $175.3 million. We believe that our existing cash, cash equivalents and short-term investments as of June 30, 2023 will be sufficient to fund our operating expenses and capital expenditure requirements, as well as maintain the minimum cash balance required under our debt facility, into the second half of 2024. However, we will need to secure additional funding in the future, from one or more equity or debt financings, government funding, collaborations, licensing transactions, other commercial transactions or other sources in order to carry out all of our commercialization and planned research and development activities with respect to ganaxolone.
Orion Commercialization Agreement
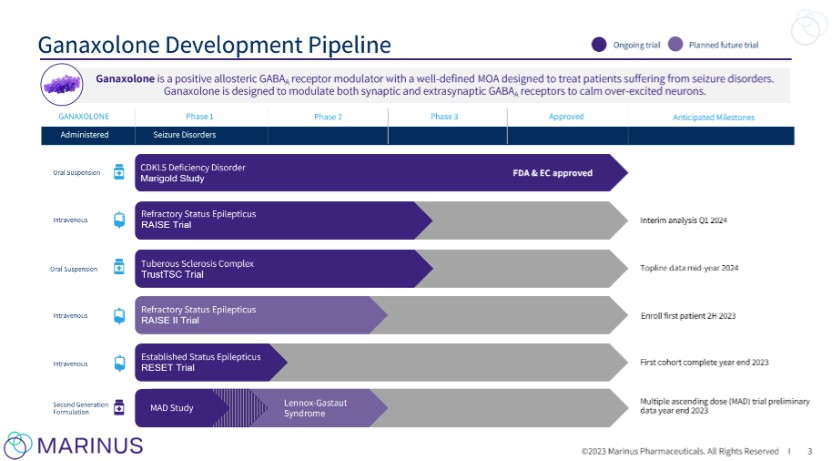
On July 30, 2021, we entered into the Orion Collaboration Agreement, whereby Orion received exclusive rights to commercialize the oral and IV dose formulations of ganaxolone in the European Economic Area, United Kingdom and Switzerland (collectively, Territory) in multiple seizure disorders, including CDD, TSC and RSE. Under the agreement, we received a €25 million ($29.6 million) upfront fee. We are eligible to receive up to an additional €97 million in research and development reimbursement and cash milestone payments based on specific clinical and commercial achievements, as well as tiered royalty payments based on net sales ranging from the low double-digits to high teens for the oral programs and the low double-digits to low 20s for the IV program. As a result of the July 2023 EC approval of ZTALMY oral suspension for the adjunctive treatment of epileptic seizures associated with CDD in patients two to 17 years of age, we are now eligible under the Orion Collaboration Agreement to receive a commercial milestone payment of 10 million Euro, if commercial sales of ZTALMY commence in the Territory, upon the earlier of (1) the first commercial sale of ZTALMY within two of a select set of countries consisting of Germany, France, Italy, Spain, and the United Kingdom or (2) the 18-month anniversary of the first commercial sale of ZTALMY in the Territory. Also, as part of the overall arrangement, we have agreed to supply the Licensed Products to Orion at an agreed upon price.
Tenacia Commercialization Agreement
On November 16, 2022, we entered into a collaboration and supply agreement with Tenacia Biotechnology (Shanghai) Co., Ltd. (Tenacia), pursuant to which we granted Tenacia an exclusive, royalty-bearing, sublicensable license to certain of our intellectual property rights to develop, commercialize and otherwise exploit certain products incorporating certain oral and intravenous formulations of our product candidate ganaxolone (Licensed Products) in Mainland China, Hong Kong, Macau and Taiwan (collectively, Territory) for the diagnosis, prevention and treatment of