
Portola Pharmaceuticals September 2017 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases you can identify these statements by forward-looking words, such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “plan,” “potential,” “seek,” “expect,” “goal,” or the negative or plural of these words or similar expressions. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, and new risks emerge from time to time. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Please refer to our Annual Report on Form 10-K and Exhibit 99.2 to the Form 8-K to which this presentation is attached, filed by us with the SEC for a description of risks and uncertainties that could impact future results. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update any forward-looking statements except as required by law.
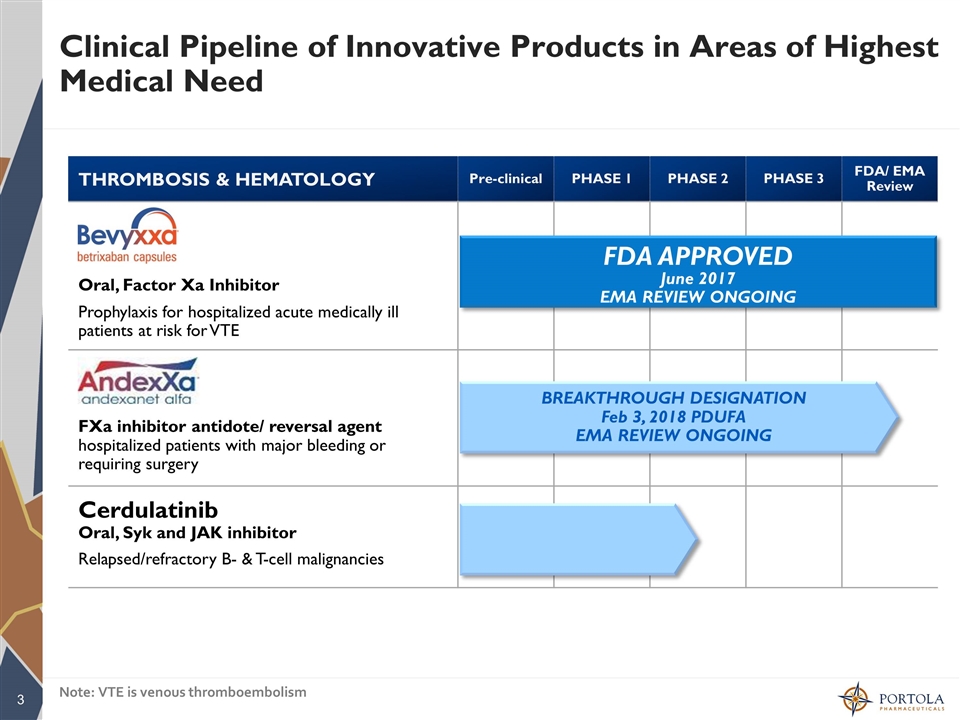
Clinical Pipeline of Innovative Products in Areas of Highest Medical Need THROMBOSIS & HEMATOLOGY Pre-clinical PHASE 1 PHASE 2 PHASE 3 FDA/ EMA Review Oral, Factor Xa Inhibitor Prophylaxis for hospitalized acute medically ill patients at risk for VTE FXa inhibitor antidote/ reversal agent hospitalized patients with major bleeding or requiring surgery Cerdulatinib Oral, Syk and JAK inhibitor Relapsed/refractory B- & T-cell malignancies BREAKTHROUGH DESIGNATION Feb 3, 2018 PDUFA EMA REVIEW ONGOING FDA APPROVED June 2017 EMA REVIEW ONGOING Note: VTE is venous thromboembolism
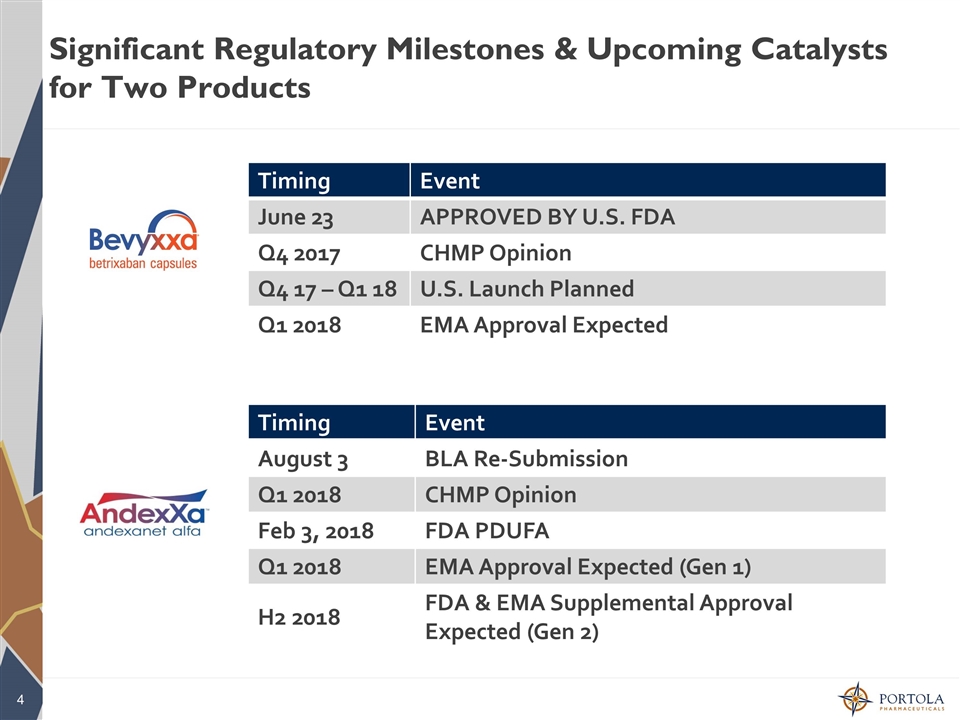
Significant Regulatory Milestones & Upcoming Catalysts for Two Products Timing Event June 23 APPROVED BY U.S. FDA Q4 2017 CHMP Opinion Q4 17 – Q1 18 U.S. Launch Planned Q1 2018 EMA Approval Expected Timing Event August 3 BLA Re-Submission Q1 2018 CHMP Opinion Feb 3, 2018 FDA PDUFA Q1 2018 EMA Approval Expected (Gen 1) H2 2018 FDA & EMA Supplemental Approval Expected (Gen 2)
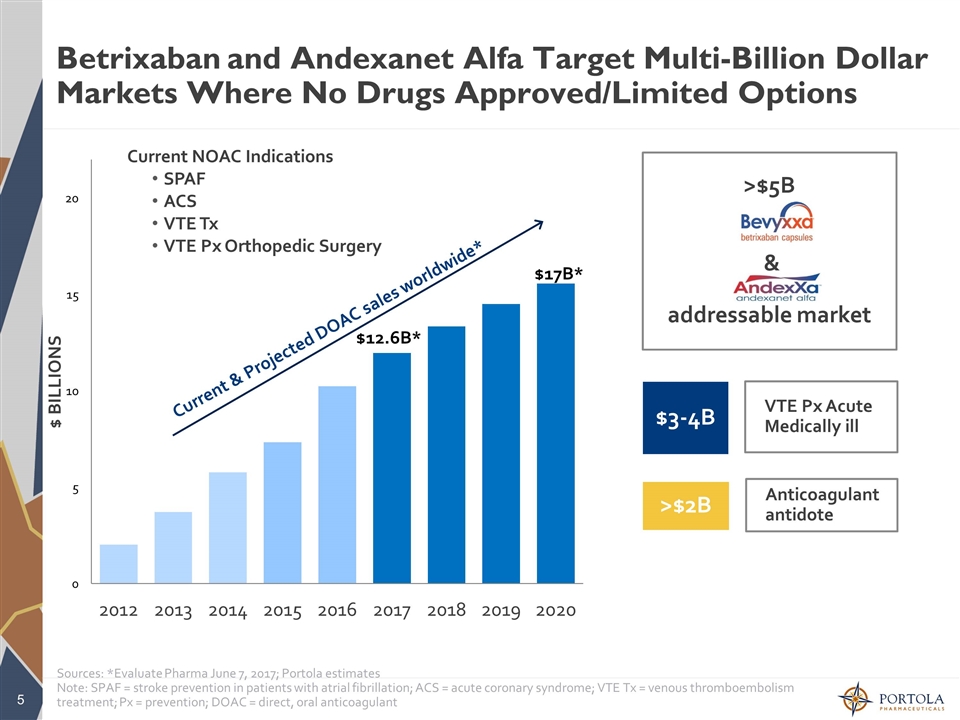
Betrixaban and Andexanet Alfa Target Multi-Billion Dollar Markets Where No Drugs Approved/Limited Options $ BILLIONS Current & Projected DOAC sales worldwide* $17B* $3-4B >$2B >$5B & addressable market Sources: *Evaluate Pharma June 7, 2017; Portola estimates Note: SPAF = stroke prevention in patients with atrial fibrillation; ACS = acute coronary syndrome; VTE Tx = venous thromboembolism treatment; Px = prevention; DOAC = direct, oral anticoagulant Current NOAC Indications SPAF ACS VTE Tx VTE Px Orthopedic Surgery VTE Px Acute Medically ill Anticoagulant antidote
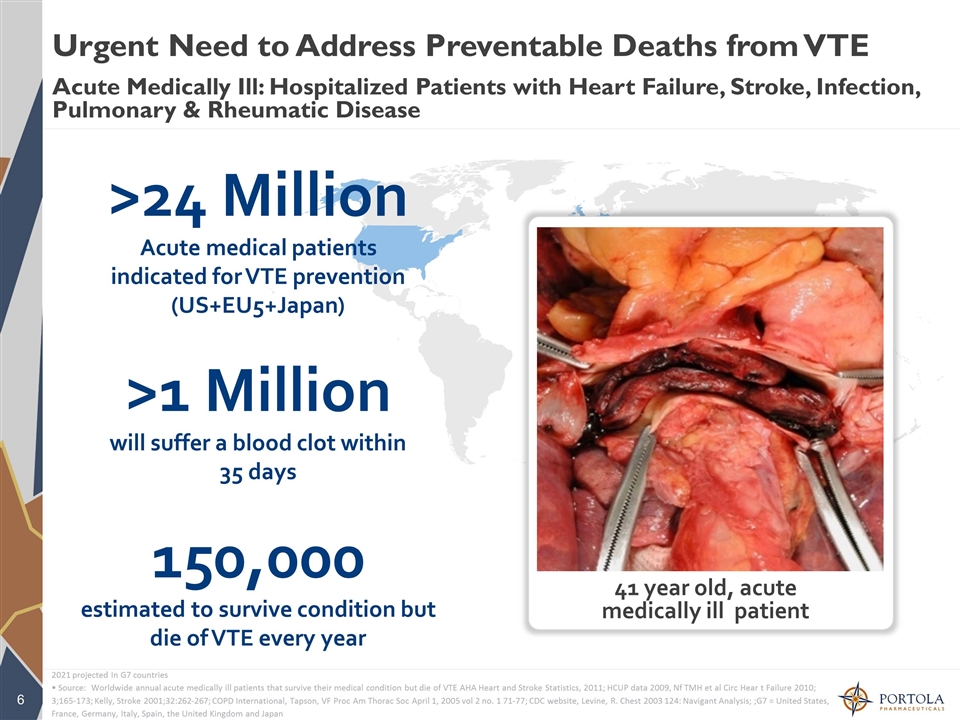
>24 Million Acute medical patients indicated for VTE prevention (US+EU5+Japan) >1 Million will suffer a blood clot within 35 days 150,000 estimated to survive condition but die of VTE every year 41 year old, acute medically ill patient Urgent Need to Address Preventable Deaths from VTE Acute Medically Ill: Hospitalized Patients with Heart Failure, Stroke, Infection, Pulmonary & Rheumatic Disease 2021 projected In G7 countries • Source: Worldwide annual acute medically ill patients that survive their medical condition but die of VTE AHA Heart and Stroke Statistics, 2011; HCUP data 2009, Nf TMH et al Circ Hear t Failure 2010; 3;165-173; Kelly, Stroke 2001;32:262-267; COPD International, Tapson, VF Proc Am Thorac Soc April 1, 2005 vol 2 no. 1 71-77; CDC website, Levine, R. Chest 2003 124: Navigant Analysis; ;G7 = United States, France, Germany, Italy, Spain, the United Kingdom and Japan
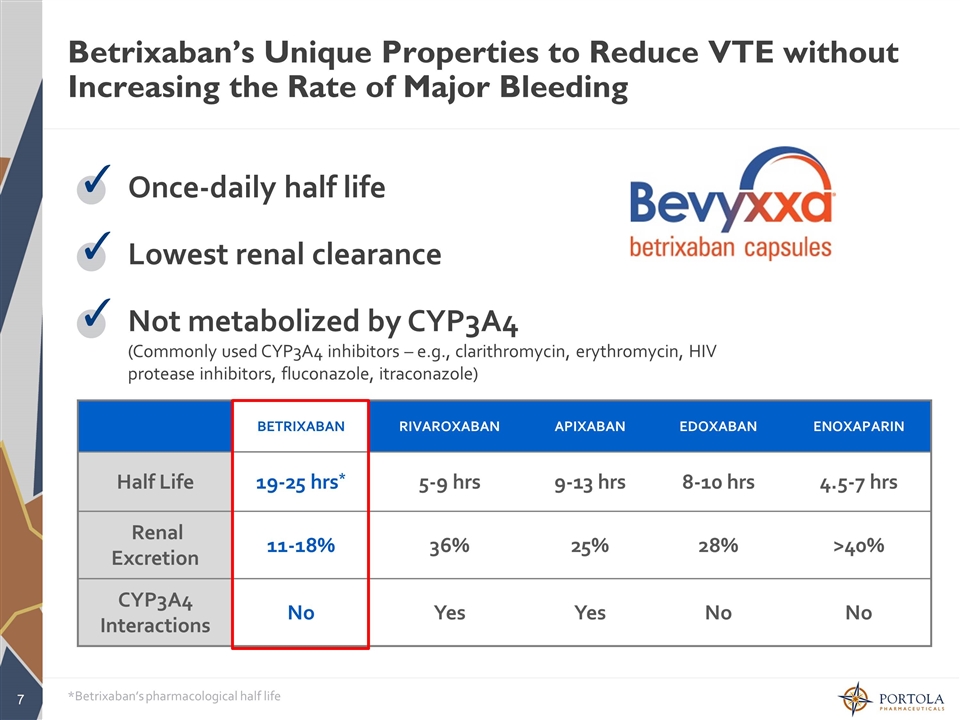
Betrixaban’s Unique Properties to Reduce VTE without Increasing the Rate of Major Bleeding Once-daily half life ✓ Lowest renal clearance ✓ Not metabolized by CYP3A4 (Commonly used CYP3A4 inhibitors – e.g., clarithromycin, erythromycin, HIV protease inhibitors, fluconazole, itraconazole) ✓ BETRIXABAN RIVAROXABAN APIXABAN EDOXABAN ENOXAPARIN Half Life 19-25 hrs* 5-9 hrs 9-13 hrs 8-10 hrs 4.5-7 hrs Renal Excretion 11-18% 36% 25% 28% >40% CYP3A4 Interactions No Yes Yes No No *Betrixaban’s pharmacological half life
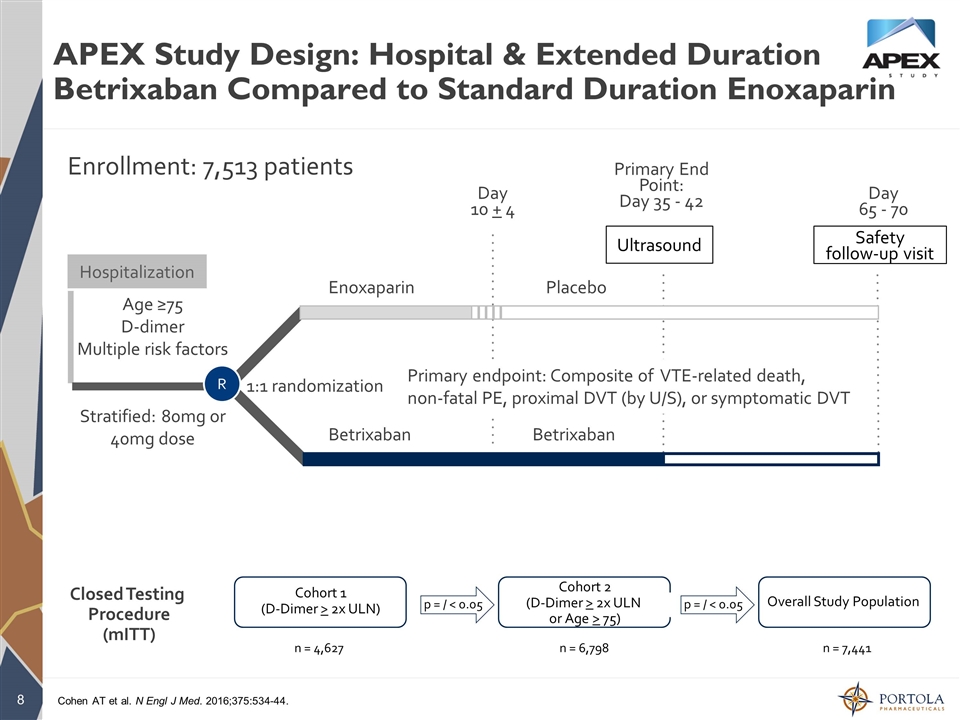
APEX Study Design: Hospital & Extended Duration Betrixaban Compared to Standard Duration Enoxaparin Enrollment: 7,513 patients Primary End Point: Day 35 - 42 Day 65 - 70 Hospitalization Day 10 + 4 R Enoxaparin Betrixaban Placebo Betrixaban 1:1 randomization Age ≥75 D-dimer Multiple risk factors Stratified: 80mg or 40mg dose Safety follow-up visit Ultrasound Primary endpoint: Composite of VTE-related death, non-fatal PE, proximal DVT (by U/S), or symptomatic DVT Closed Testing Procedure (mITT) Cohort 1 (D-Dimer > 2x ULN) Cohort 2 (D-Dimer > 2x ULN or Age > 75) Overall Study Population p = / < 0.05 p = / < 0.05 n = 4,627 n = 6,798 n = 7,441 Cohen AT et al. N Engl J Med. 2016;375:534-44.
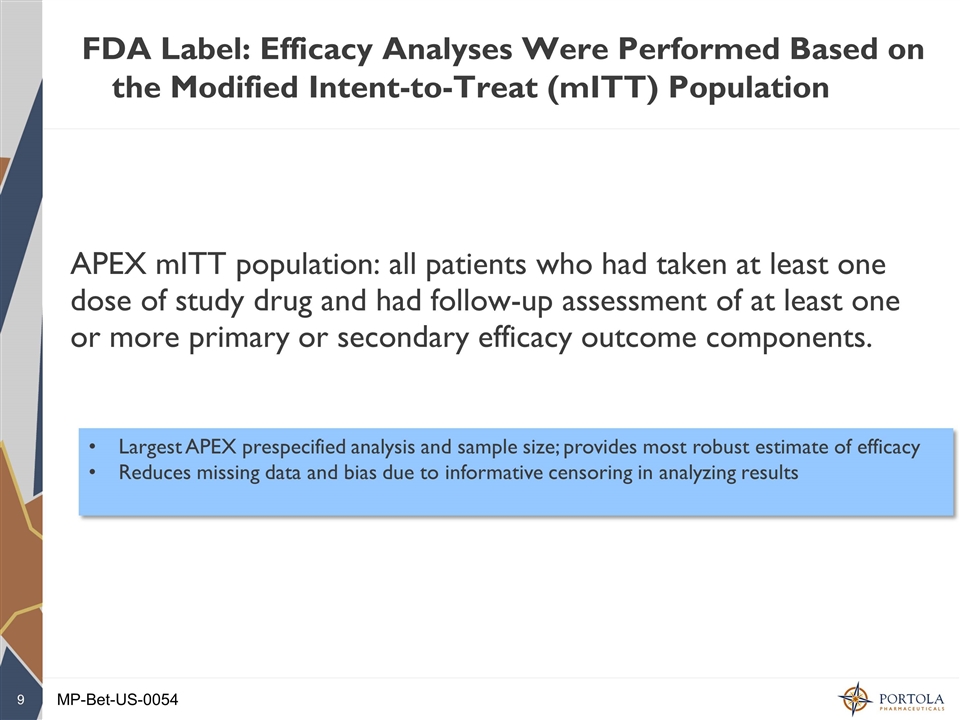
FDA Label: Efficacy Analyses Were Performed Based on the Modified Intent-to-Treat (mITT) Population APEX mITT population: all patients who had taken at least one dose of study drug and had follow-up assessment of at least one or more primary or secondary efficacy outcome components. Largest APEX prespecified analysis and sample size; provides most robust estimate of efficacy Reduces missing data and bias due to informative censoring in analyzing results
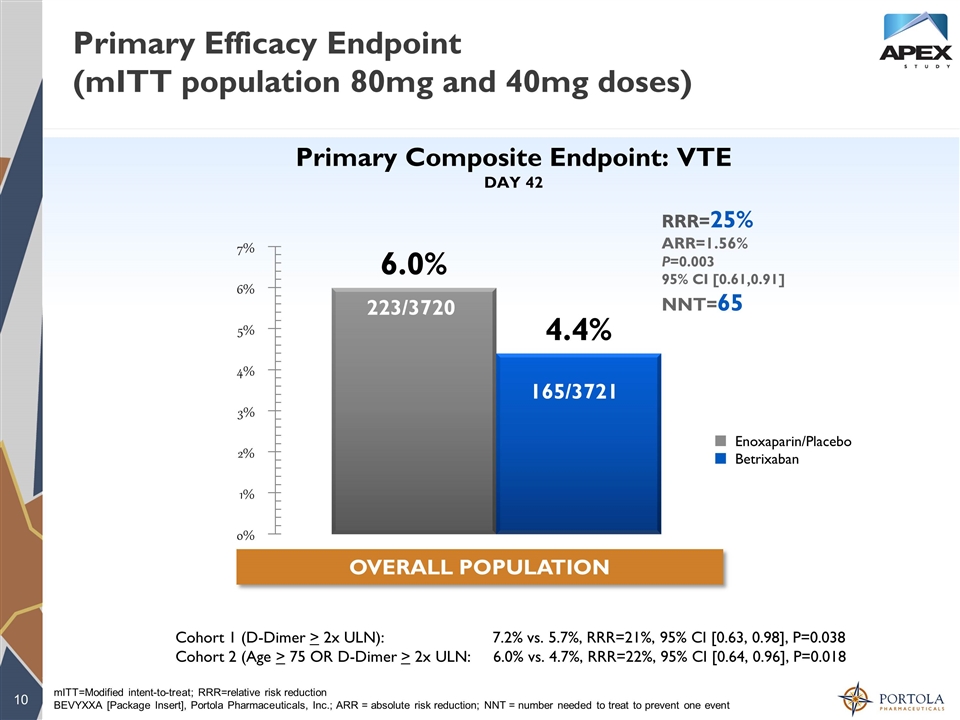
Primary Efficacy Endpoint (mITT population 80mg and 40mg doses) 223/3720 165/3721 mITT=Modified intent-to-treat; RRR=relative risk reduction BEVYXXA [Package Insert], Portola Pharmaceuticals, Inc.; ARR = absolute risk reduction; NNT = number needed to treat to prevent one event ¢ Enoxaparin/Placebo ¢ Betrixaban Primary Composite Endpoint: VTE DAY 42 OVERALL POPULATION RRR=25% ARR=1.56% P=0.003 95% CI [0.61,0.91] NNT=65 Cohort 1 (D-Dimer > 2x ULN): 7.2% vs. 5.7%, RRR=21%, 95% CI [0.63, 0.98], P=0.038 Cohort 2 (Age > 75 OR D-Dimer > 2x ULN: 6.0% vs. 4.7%, RRR=22%, 95% CI [0.64, 0.96], P=0.018
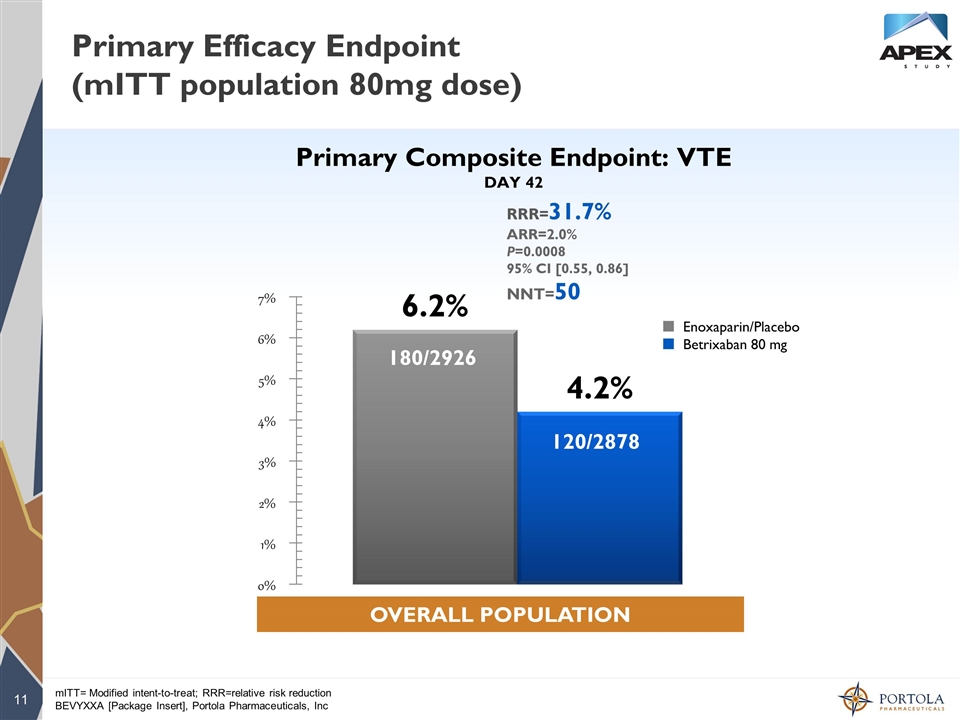
mITT= Modified intent-to-treat; RRR=relative risk reduction BEVYXXA [Package Insert], Portola Pharmaceuticals, Inc Primary Efficacy Endpoint (mITT population 80mg dose) 180/2926 120/2878 ¢ Enoxaparin/Placebo ¢ Betrixaban 80 mg Primary Composite Endpoint: VTE DAY 42 OVERALL POPULATION RRR=31.7% ARR=2.0% P=0.0008 95% CI [0.55, 0.86] NNT=50
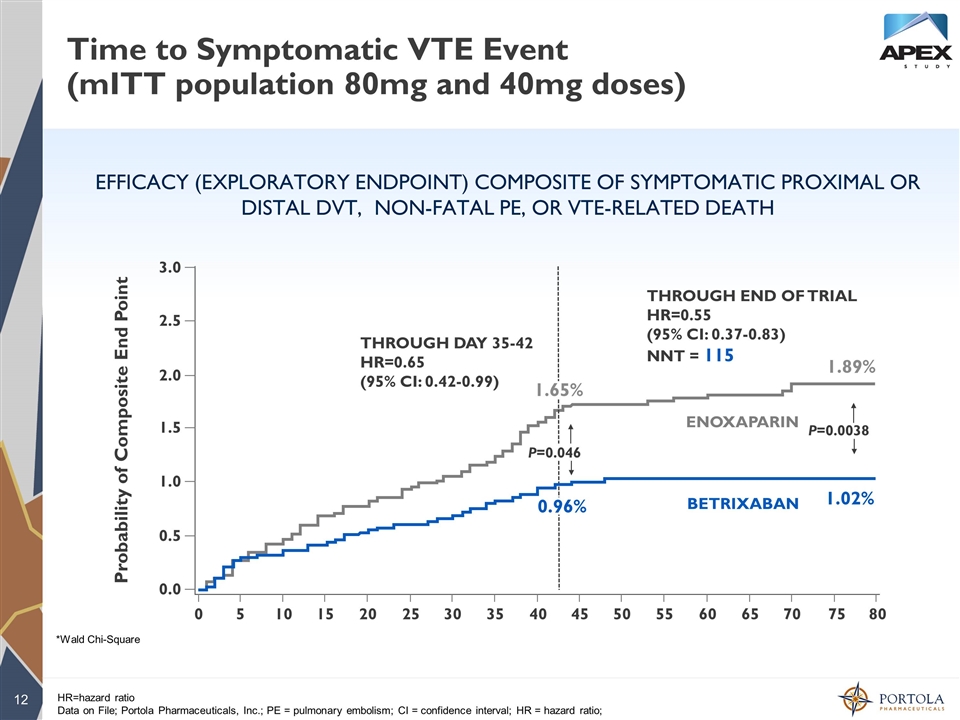
Time to Symptomatic VTE Event (mITT population 80mg and 40mg doses) EFFICACY (EXPLORATORY ENDPOINT) COMPOSITE OF SYMPTOMATIC PROXIMAL OR DISTAL DVT, NON-FATAL PE, OR VTE-RELATED DEATH HR=hazard ratio Data on File; Portola Pharmaceuticals, Inc.; PE = pulmonary embolism; CI = confidence interval; HR = hazard ratio; THROUGH DAY 35-42 HR=0.65 (95% CI: 0.42-0.99) THROUGH END OF TRIAL HR=0.55 (95% CI: 0.37-0.83) NNT = 115 1.89% 1.02% 2.5 3.0 2.0 1.5 1.0 0.5 0.0 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 ENOXAPARIN BETRIXABAN Probability of Composite End Point *Wald Chi-Square P=0.0038 0.96% P=0.046 1.65%
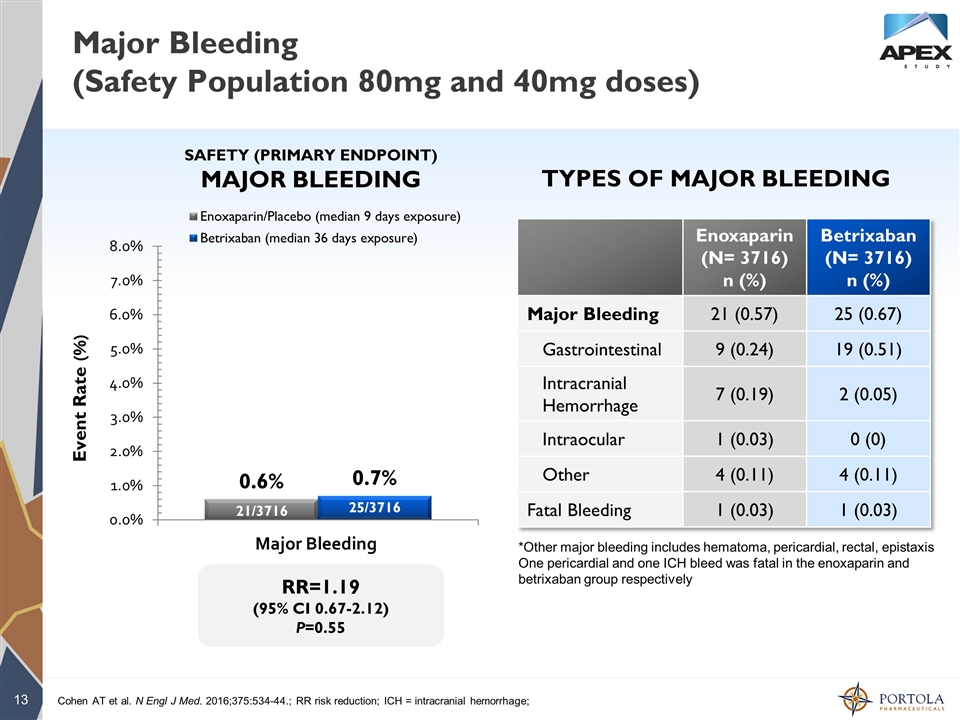
Major Bleeding (Safety Population 80mg and 40mg doses) RR=1.19 (95% CI 0.67-2.12) P=0.55 Event Rate (%) SAFETY (PRIMARY ENDPOINT) MAJOR BLEEDING TYPES OF MAJOR BLEEDING Cohen AT et al. N Engl J Med. 2016;375:534-44.; RR risk reduction; ICH = intracranial hemorrhage; *Other major bleeding includes hematoma, pericardial, rectal, epistaxis One pericardial and one ICH bleed was fatal in the enoxaparin and betrixaban group respectively Enoxaparin (N= 3716) n (%) Betrixaban (N= 3716) n (%) Major Bleeding 21 (0.57) 25 (0.67) Gastrointestinal 9 (0.24) 19 (0.51) Intracranial Hemorrhage 7 (0.19) 2 (0.05) Intraocular 1 (0.03) 0 (0) Other 4 (0.11) 4 (0.11) Fatal Bleeding 1 (0.03) 1 (0.03) 21/3716 25/3716
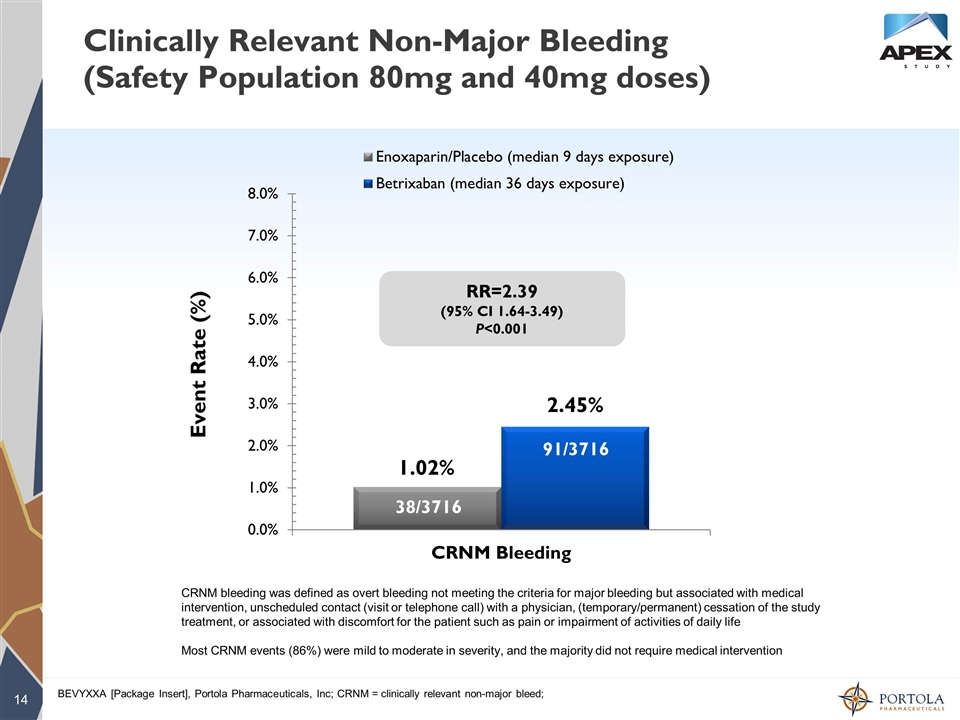
Clinically Relevant Non-Major Bleeding (Safety Population 80mg and 40mg doses) Event Rate (%) RR=2.39 (95% CI 1.64-3.49) P<0.001 BEVYXXA [Package Insert], Portola Pharmaceuticals, Inc; CRNM = clinically relevant non-major bleed; 38/3716 91/3716 CRNM bleeding was defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact (visit or telephone call) with a physician, (temporary/permanent) cessation of the study treatment, or associated with discomfort for the patient such as pain or impairment of activities of daily life Most CRNM events (86%) were mild to moderate in severity, and the majority did not require medical intervention
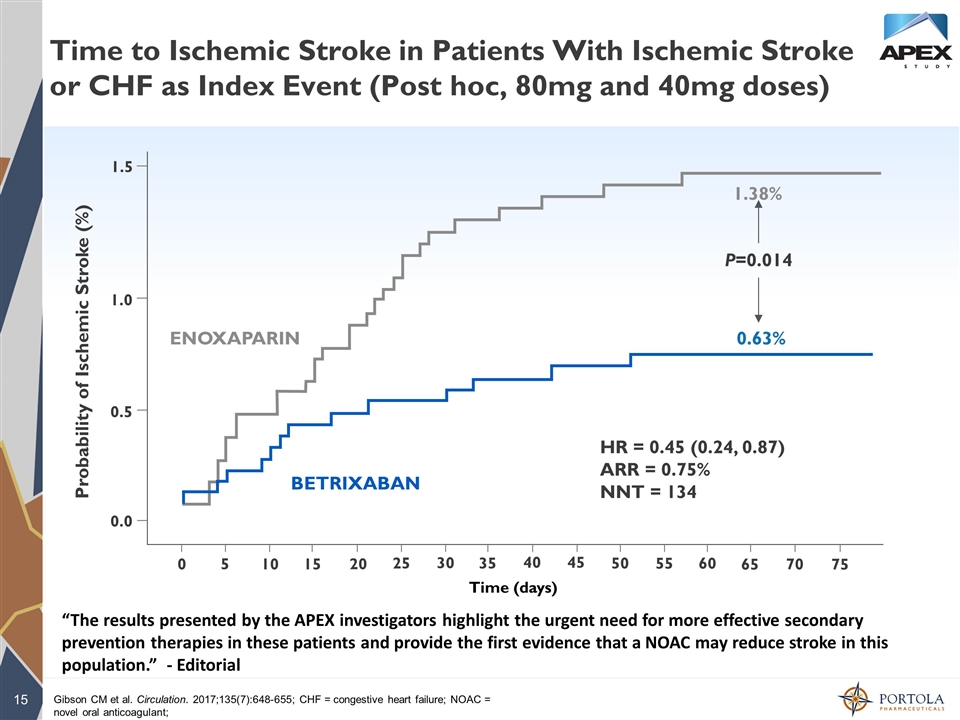
Time to Ischemic Stroke in Patients With Ischemic Stroke or CHF as Index Event (Post hoc, 80mg and 40mg doses) Gibson CM et al. Circulation. 2017;135(7):648-655; CHF = congestive heart failure; NOAC = novel oral anticoagulant; 0.0 0.5 1.0 Probability of Ischemic Stroke (%) 0 5 10 15 20 25 30 35 40 45 50 60 65 70 75 55 Time (days) ENOXAPARIN BETRIXABAN 1.38% 0.63% P=0.014 HR = 0.45 (0.24, 0.87) ARR = 0.75% NNT = 133 HR = 0.45 (0.24, 0.87) ARR = 0.75% NNT = 134 1.5 “The results presented by the APEX investigators highlight the urgent need for more effective secondary prevention therapies in these patients and provide the first evidence that a NOAC may reduce stroke in this population.” - Editorial
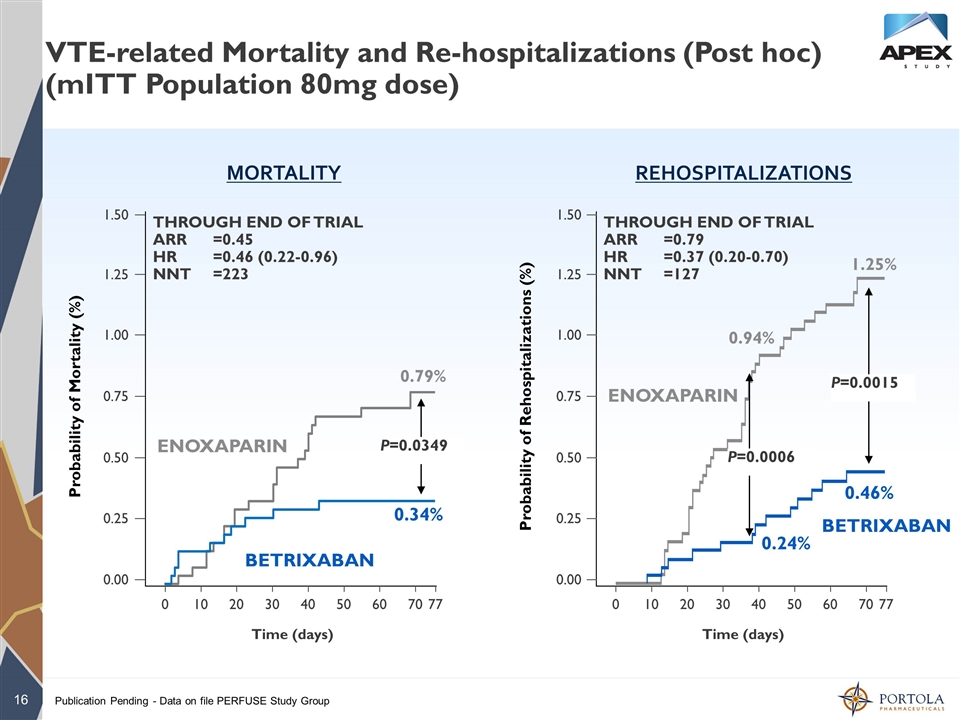
VTE-related Mortality and Re-hospitalizations (Post hoc) (mITT Population 80mg dose) Publication Pending - Data on file PERFUSE Study Group 1.25 1.50 1.00 0.75 0.50 0.25 0.00 0 77 70 10 THROUGH END OF TRIAL ARR=0.79 HR=0.37 (0.20-0.70) NNT=127 1.25% Time (days) 0.46% Probability of Rehospitalizations (%) P=0.0015 ENOXAPARIN BETRIXABAN 20 30 40 50 60 1.25 1.50 1.00 0.75 0.50 0.25 0.00 0 77 70 10 THROUGH END OF TRIAL ARR=0.45 HR=0.46 (0.22-0.96) NNT=223 0.79% Time (days) 0.34% Probability of Mortality (%) P=0.0349 ENOXAPARIN BETRIXABAN 20 30 40 50 60 MORTALITY REHOSPITALIZATIONS 0.94% 0.24% P=0.0006
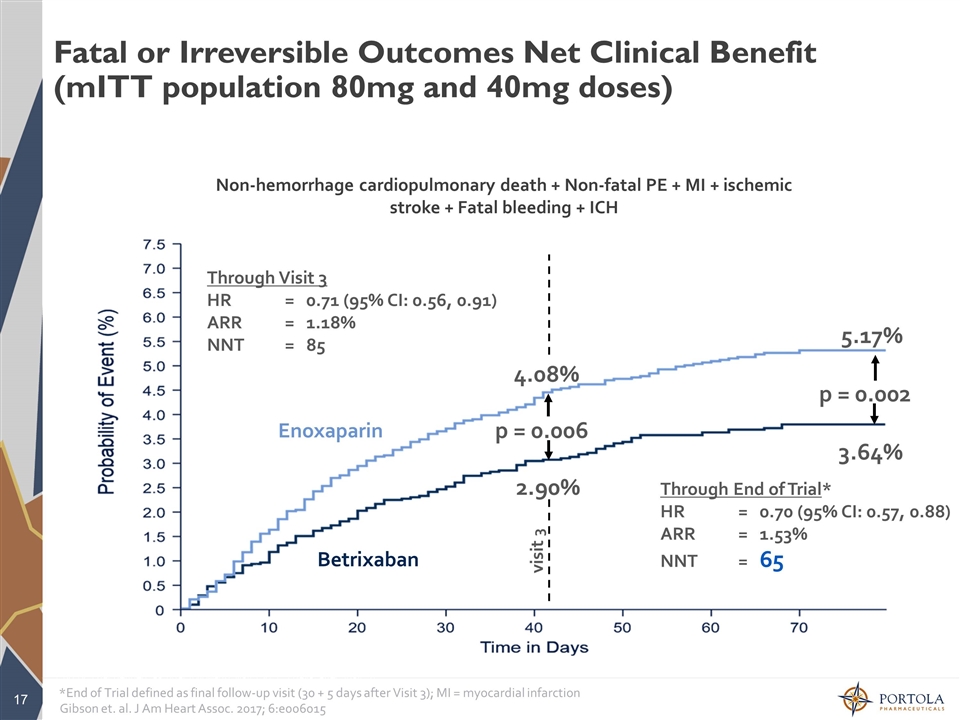
Fatal or Irreversible Outcomes Net Clinical Benefit (mITT population 80mg and 40mg doses) Non-hemorrhage cardiopulmonary death + Non-fatal PE + MI + ischemic stroke + Fatal bleeding + ICH *End of Trial defined as final follow-up visit (30 + 5 days after Visit 3) Through End of Trial* HR = 0.70 (95% CI: 0.57, 0.88) ARR = 1.53% NNT = 65 5.17% 3.64% p = 0.002 Through Visit 3 HR = 0.71 (95% CI: 0.56, 0.91) ARR = 1.18% NNT = 85 p = 0.006 4.08% 2.90% visit 3 *End of Trial defined as final follow-up visit (30 + 5 days after Visit 3); MI = myocardial infarction Enoxaparin Betrixaban Gibson et. al. J Am Heart Assoc. 2017; 6:e006015
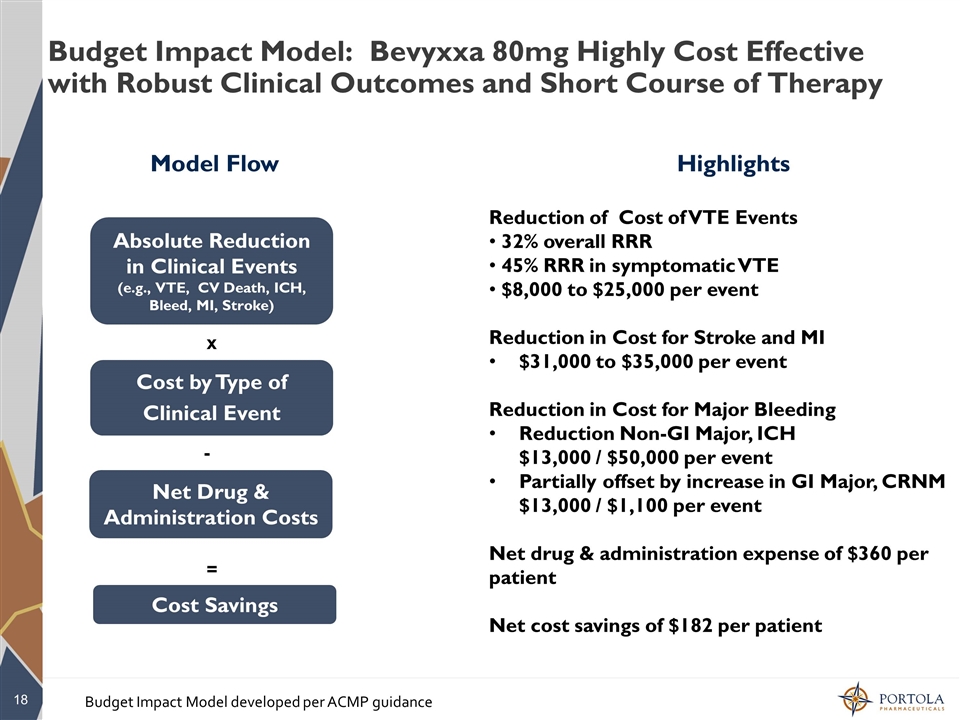
Budget Impact Model: Bevyxxa 80mg Highly Cost Effective with Robust Clinical Outcomes and Short Course of Therapy Absolute Reduction in Clinical Events (e.g., VTE, CV Death, ICH, Bleed, MI, Stroke) Cost by Type of Clinical Event Cost Savings Model Flow x = - Net Drug & Administration Costs Highlights Reduction of Cost of VTE Events 32% overall RRR 45% RRR in symptomatic VTE $8,000 to $25,000 per event Reduction in Cost for Stroke and MI $31,000 to $35,000 per event Reduction in Cost for Major Bleeding Reduction Non-GI Major, ICH $13,000 / $50,000 per event Partially offset by increase in GI Major, CRNM $13,000 / $1,100 per event Net drug & administration expense of $360 per patient Net cost savings of $182 per patient Budget Impact Model developed per ACMP guidance
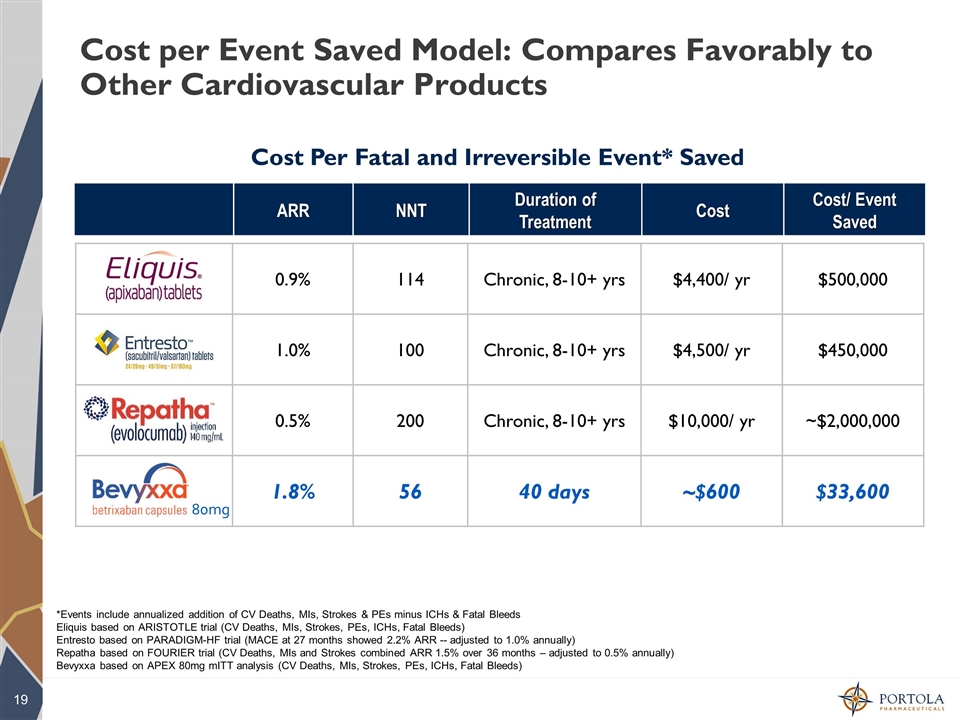
Cost per Event Saved Model: Compares Favorably to Other Cardiovascular Products 0.9% 114 Chronic, 8-10+ yrs $4,400/ yr $500,000 1.0% 100 Chronic, 8-10+ yrs $4,500/ yr $450,000 0.5% 200 Chronic, 8-10+ yrs $10,000/ yr ~$2,000,000 1.8% 56 40 days ~$600 $33,600 *Events include annualized addition of CV Deaths, MIs, Strokes & PEs minus ICHs & Fatal Bleeds Eliquis based on ARISTOTLE trial (CV Deaths, MIs, Strokes, PEs, ICHs, Fatal Bleeds) Entresto based on PARADIGM-HF trial (MACE at 27 months showed 2.2% ARR -- adjusted to 1.0% annually) Repatha based on FOURIER trial (CV Deaths, MIs and Strokes combined ARR 1.5% over 36 months – adjusted to 0.5% annually) Bevyxxa based on APEX 80mg mITT analysis (CV Deaths, MIs, Strokes, PEs, ICHs, Fatal Bleeds) Cost Per Fatal and Irreversible Event* Saved ARR NNT Duration of Treatment Cost Cost/ Event Saved 80mg
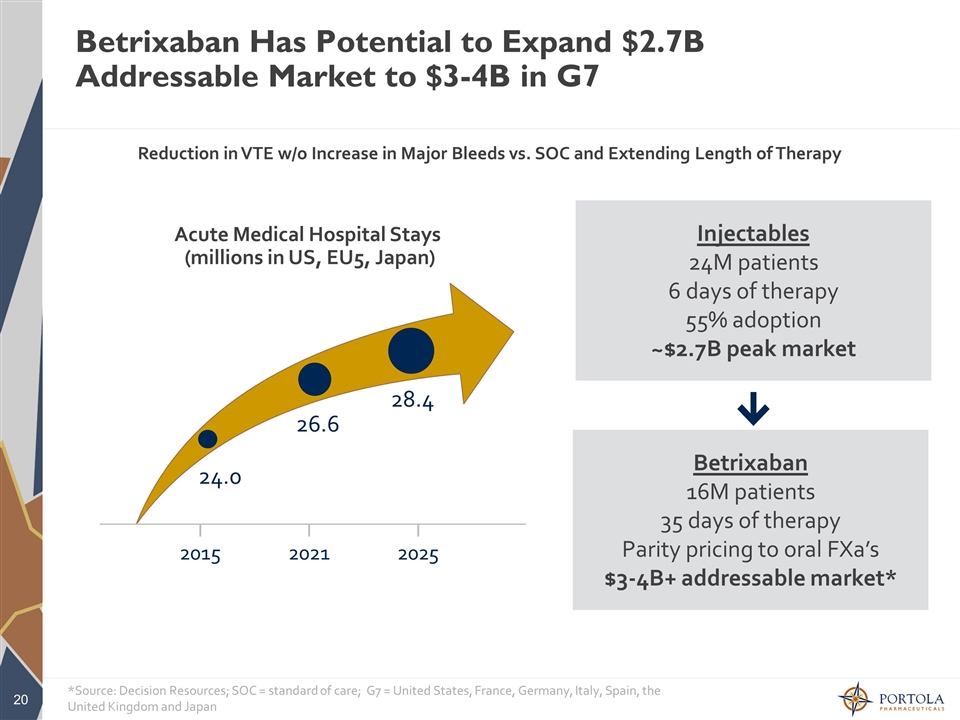
Betrixaban Has Potential to Expand $2.7B Addressable Market to $3-4B in G7 2025 2021 2015 28.4 26.6 24.0 Acute Medical Hospital Stays (millions in US, EU5, Japan) Injectables 24M patients 6 days of therapy 55% adoption ~$2.7B peak market Betrixaban 16M patients 35 days of therapy Parity pricing to oral FXa’s $3-4B+ addressable market* *Source: Decision Resources; SOC = standard of care; G7 = United States, France, Germany, Italy, Spain, the United Kingdom and Japan Reduction in VTE w/o Increase in Major Bleeds vs. SOC and Extending Length of Therapy
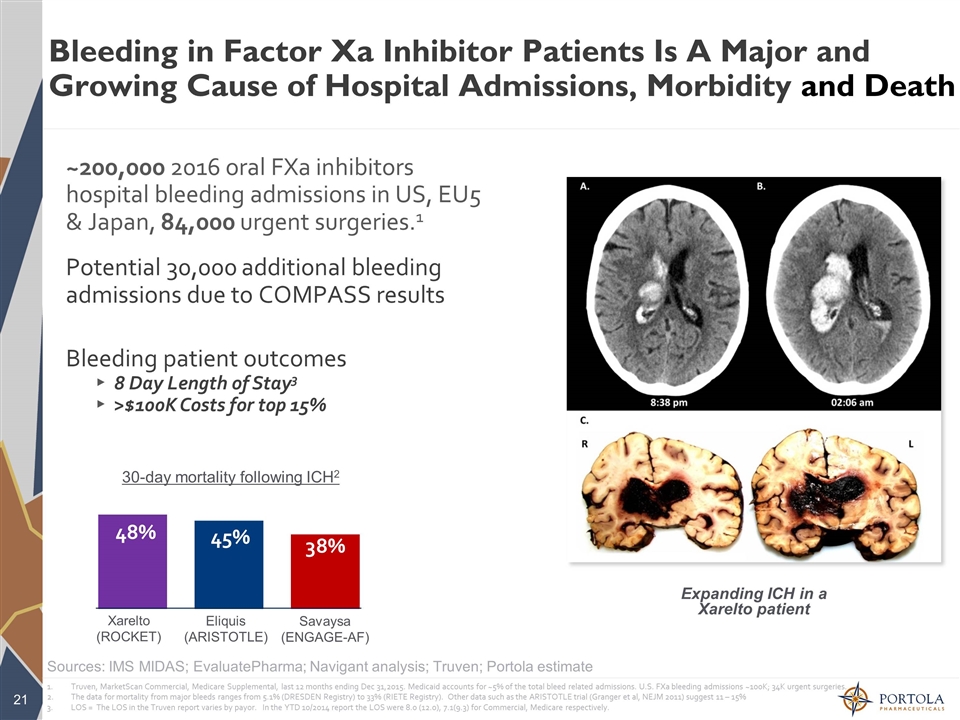
Bleeding in Factor Xa Inhibitor Patients Is A Major and Growing Cause of Hospital Admissions, Morbidity and Death Expanding ICH in a Xarelto patient ~200,000 2016 oral FXa inhibitors hospital bleeding admissions in US, EU5 & Japan, 84,000 urgent surgeries.1 Potential 30,000 additional bleeding admissions due to COMPASS results Bleeding patient outcomes 8 Day Length of Stay3 >$100K Costs for top 15% 30-day mortality following ICH2 Xarelto (ROCKET) Eliquis (ARISTOTLE) Savaysa (ENGAGE-AF) Sources: IMS MIDAS; EvaluatePharma; Navigant analysis; Truven; Portola estimate Truven, MarketScan Commercial, Medicare Supplemental, last 12 months ending Dec 31,2015. Medicaid accounts for ~5% of the total bleed related admissions. U.S. FXa bleeding admissions ~100K; 34K urgent surgeries. The data for mortality from major bleeds ranges from 5.1% (DRESDEN Registry) to 33% (RIETE Registry). Other data such as the ARISTOTLE trial (Granger et al, NEJM 2011) suggest 11 – 15% LOS = The LOS in the Truven report varies by payor. In the YTD 10/2014 report the LOS were 8.0 (12.0), 7.1(9.3) for Commercial, Medicare respectively.
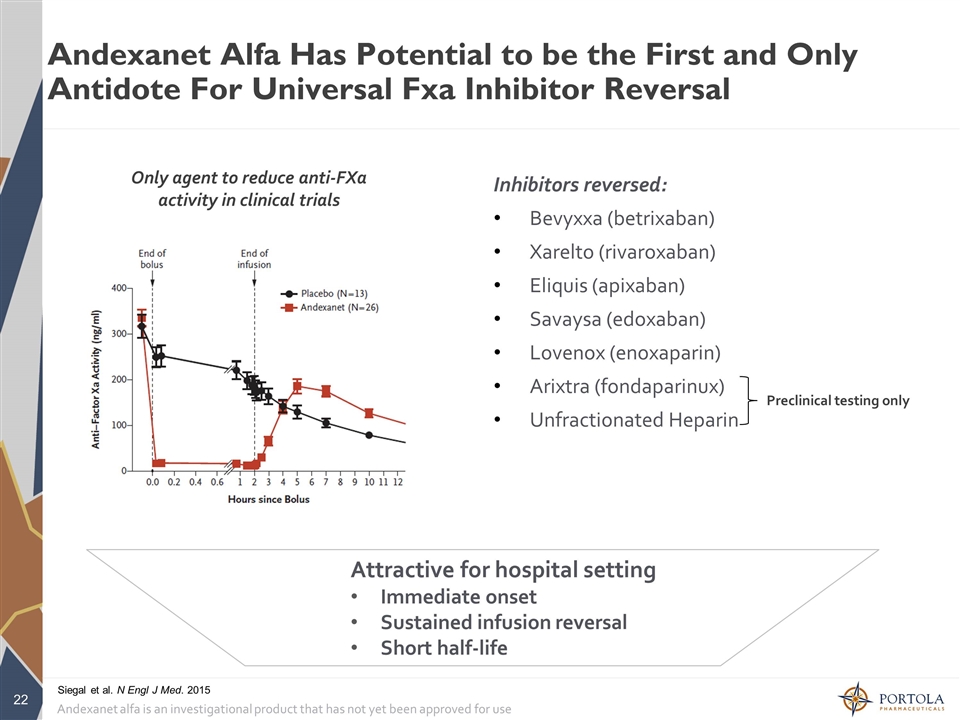
Andexanet Alfa Has Potential to be the First and Only Antidote For Universal Fxa Inhibitor Reversal Andexanet alfa is an investigational product that has not yet been approved for use Attractive for hospital setting Immediate onset Sustained infusion reversal Short half-life Only agent to reduce anti-FXa activity in clinical trials Inhibitors reversed: Bevyxxa (betrixaban) Xarelto (rivaroxaban) Eliquis (apixaban) Savaysa (edoxaban) Lovenox (enoxaparin) Arixtra (fondaparinux) Unfractionated Heparin Preclinical testing only Siegal et al. N Engl J Med. 2015
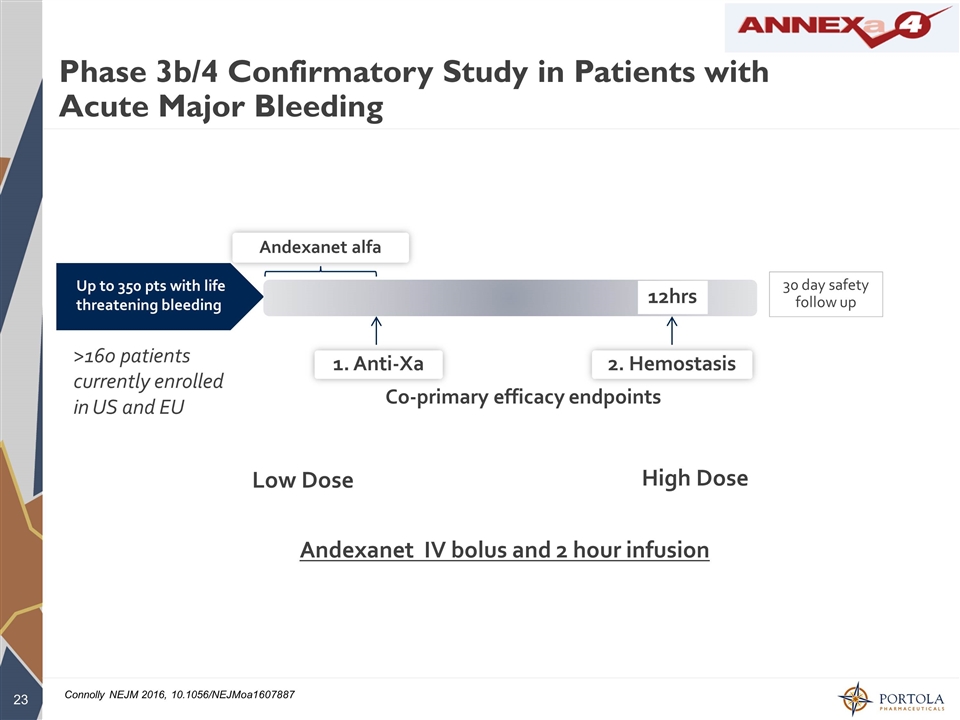
Phase 3b/4 Confirmatory Study in Patients with Acute Major Bleeding Up to 350 pts with life threatening bleeding Co-primary efficacy endpoints Andexanet alfa 1. Anti-Xa 2. Hemostasis 12hrs 30 day safety follow up Andexanet IV bolus and 2 hour infusion >160 patients currently enrolled in US and EU High Dose Low Dose Connolly NEJM 2016, 10.1056/NEJMoa1607887
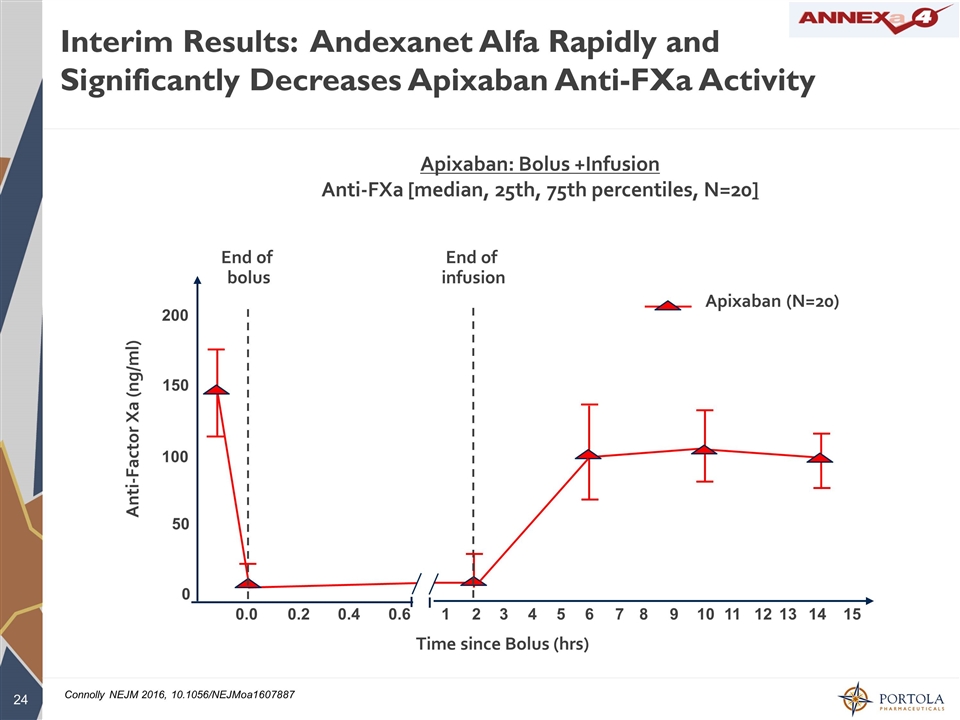
Interim Results: Andexanet Alfa Rapidly and Significantly Decreases Apixaban Anti-FXa Activity Apixaban: Bolus +Infusion Anti-FXa [median, 25th, 75th percentiles, N=20] Time since Bolus (hrs) Anti-Factor Xa (ng/ml) Apixaban (N=20) 0 50 100 150 200 0.0 0.2 0.4 0.6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 End of infusion End of bolus Connolly NEJM 2016, 10.1056/NEJMoa1607887
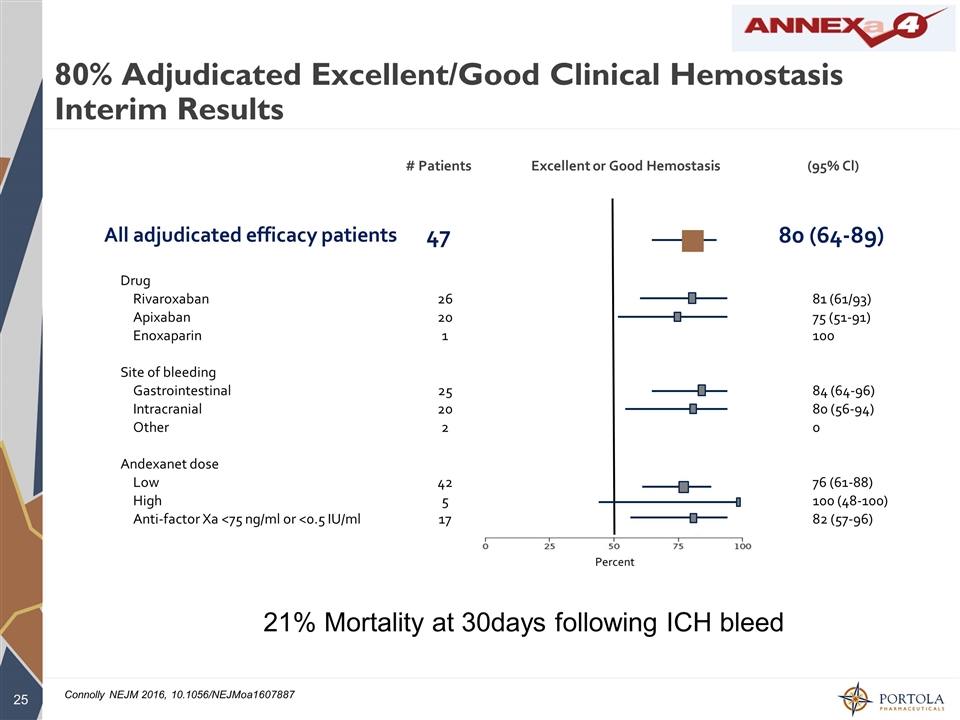
80% Adjudicated Excellent/Good Clinical Hemostasis Interim Results Drug Rivaroxaban 26 81 (61/93) Apixaban 20 75 (51-91) Enoxaparin 1 100 Site of bleeding Gastrointestinal 25 84 (64-96) Intracranial 20 80 (56-94) Other 2 0 Andexanet dose Low 42 76 (61-88) High 5 100 (48-100) Anti-factor Xa <75 ng/ml or <0.5 IU/ml 17 82 (57-96) # Patients Excellent or Good Hemostasis (95% Cl) All adjudicated efficacy patients 47 80 (64-89) 21% Mortality at 30days following ICH bleed Percent Connolly NEJM 2016, 10.1056/NEJMoa1607887
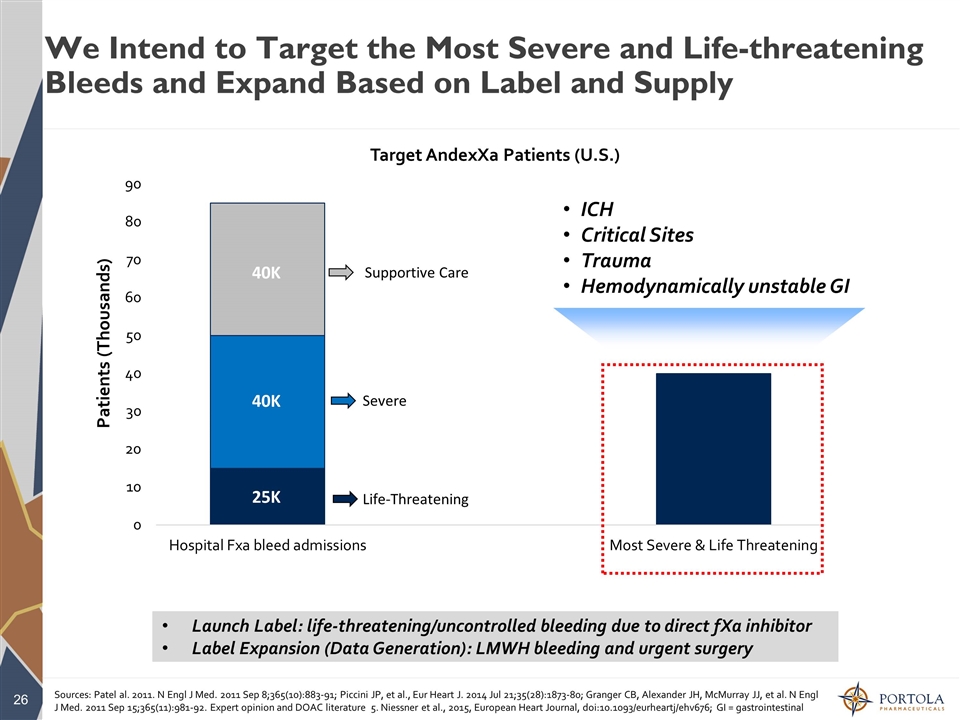
We Intend to Target the Most Severe and Life-threatening Bleeds and Expand Based on Label and Supply Sources: Patel al. 2011. N Engl J Med. 2011 Sep 8;365(10):883-91; Piccini JP, et al., Eur Heart J. 2014 Jul 21;35(28):1873-80; Granger CB, Alexander JH, McMurray JJ, et al. N Engl J Med. 2011 Sep 15;365(11):981-92. Expert opinion and DOAC literature 5. Niessner et al., 2015, European Heart Journal, doi:10.1093/eurheartj/ehv676; GI = gastrointestinal ICH Critical Sites Trauma Hemodynamically unstable GI Launch Label: life-threatening/uncontrolled bleeding due to direct fXa inhibitor Label Expansion (Data Generation): LMWH bleeding and urgent surgery 40K 25K 40K 15K
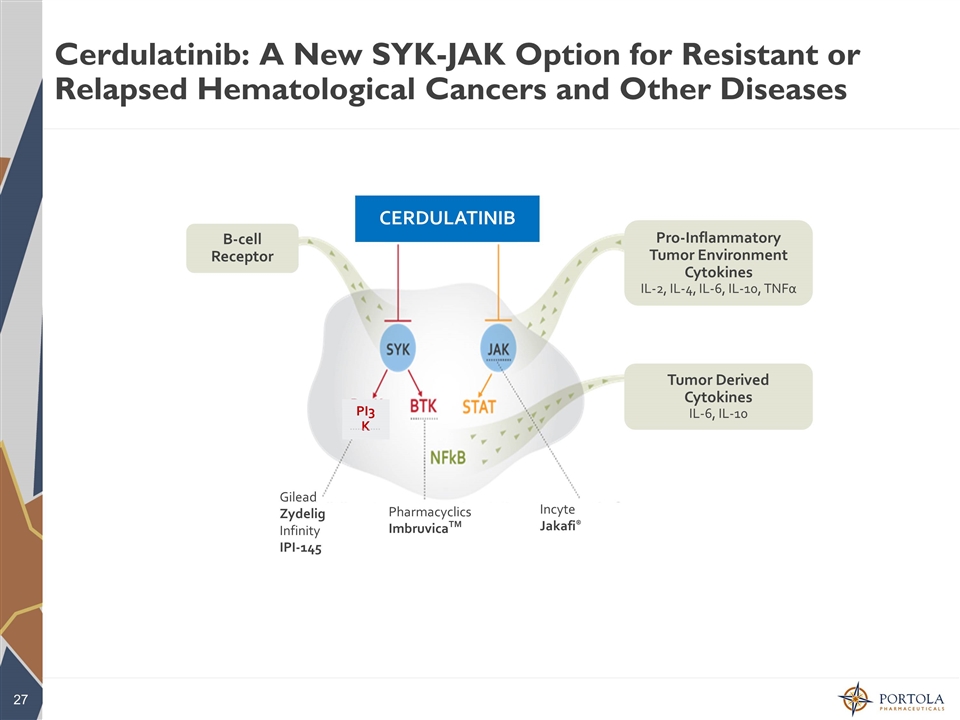
Cerdulatinib: A New SYK-JAK Option for Resistant or Relapsed Hematological Cancers and Other Diseases Pharmacyclics ImbruvicaTM Gilead Zydelig Infinity IPI-145 Incyte Jakafi® CERDULATINIB PI3K B-cell Receptor Pro-Inflammatory Tumor Environment Cytokines IL-2, IL-4, IL-6, IL-10, TNFα Tumor Derived Cytokines IL-6, IL-10
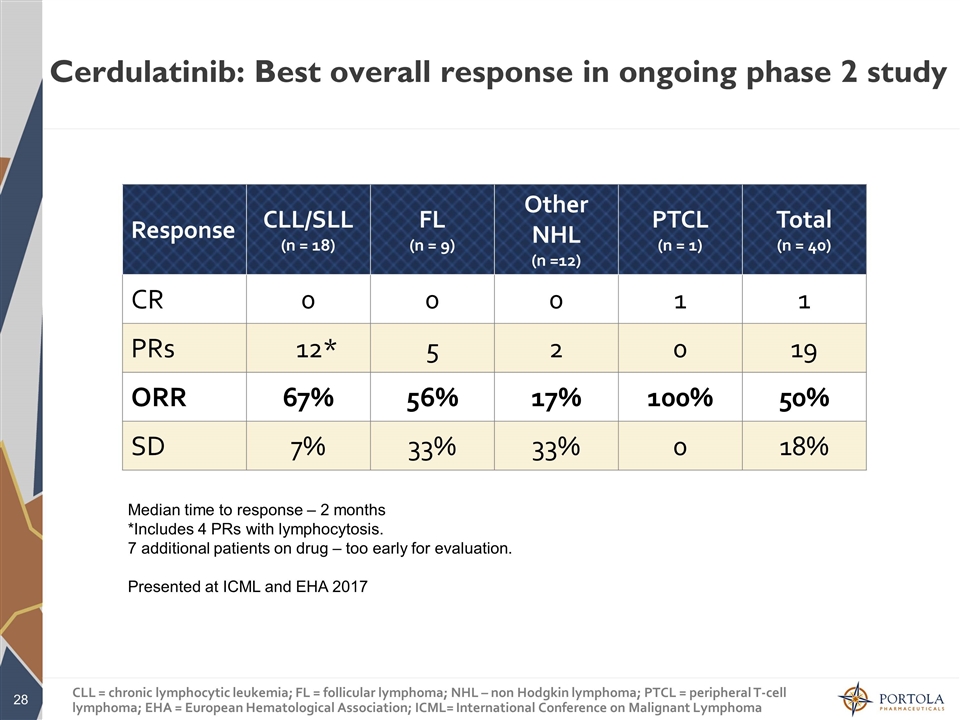
Cerdulatinib: Best overall response in ongoing phase 2 study Median time to response – 2 months *Includes 4 PRs with lymphocytosis. 7 additional patients on drug – too early for evaluation. Presented at ICML and EHA 2017 Response CLL/SLL (n = 18) FL (n = 9) Other NHL (n =12) PTCL (n = 1) Total (n = 40) CR 0 0 0 1 1 PRs 12* 5 2 0 19 ORR 67% 56% 17% 100% 50% SD 7% 33% 33% 0 18% CLL = chronic lymphocytic leukemia; FL = follicular lymphoma; NHL – non Hodgkin lymphoma; PTCL = peripheral T-cell lymphoma; EHA = European Hematological Association; ICML= International Conference on Malignant Lymphoma
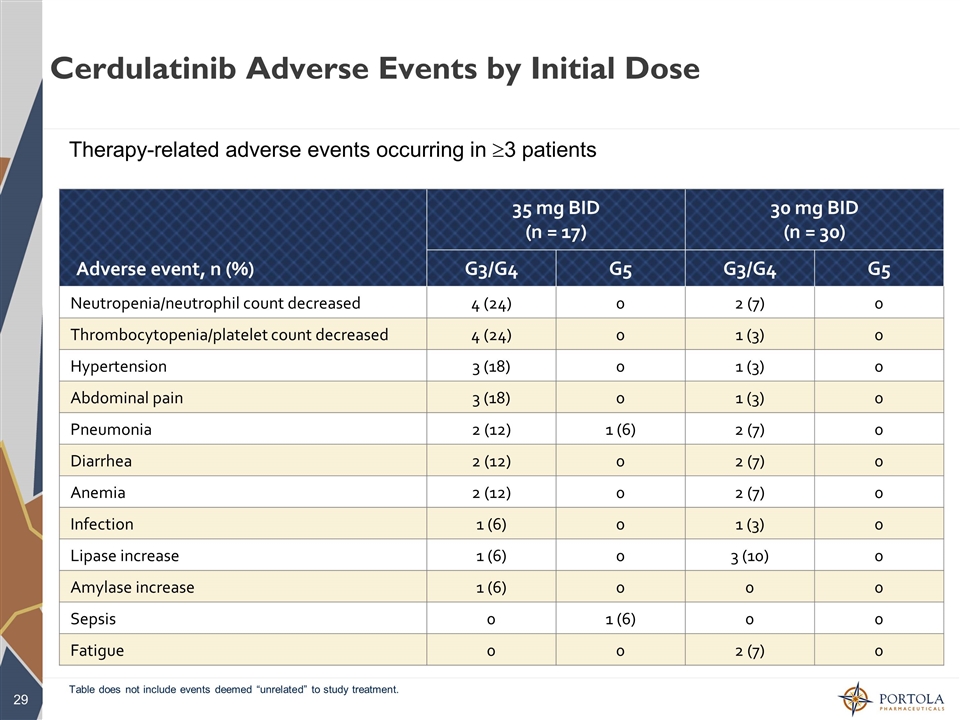
Cerdulatinib Adverse Events by Initial Dose Table does not include events deemed “unrelated” to study treatment. Therapy-related adverse events occurring in ³3 patients Adverse event, n (%) 35 mg BID (n = 17) 30 mg BID (n = 30) G3/G4 G5 G3/G4 G5 Neutropenia/neutrophil count decreased 4 (24) 0 2 (7) 0 Thrombocytopenia/platelet count decreased 4 (24) 0 1 (3) 0 Hypertension 3 (18) 0 1 (3) 0 Abdominal pain 3 (18) 0 1 (3) 0 Pneumonia 2 (12) 1 (6) 2 (7) 0 Diarrhea 2 (12) 0 2 (7) 0 Anemia 2 (12) 0 2 (7) 0 Infection 1 (6) 0 1 (3) 0 Lipase increase 1 (6) 0 3 (10) 0 Amylase increase 1 (6) 0 0 0 Sepsis 0 1 (6) 0 0 Fatigue 0 0 2 (7) 0
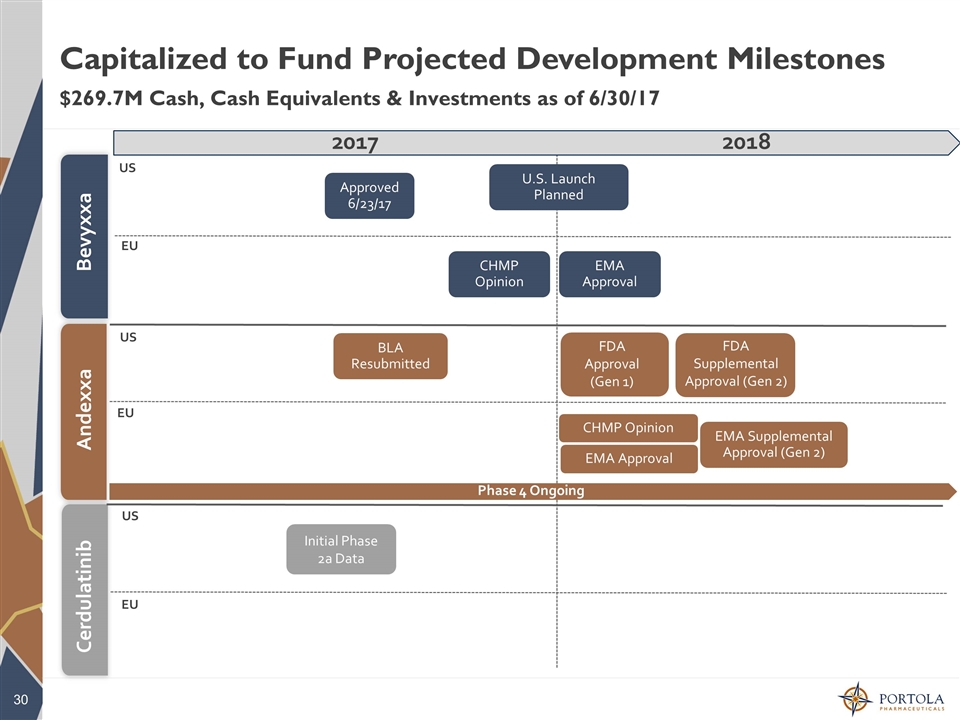
Andexxa Bevyxxa 2016 2017 Capitalized to Fund Projected Development Milestones $269.7M Cash, Cash Equivalents & Investments as of 6/30/17 Phase 4 Ongoing 2017 2018 BLA Resubmitted EMA Approval Approved6/23/17 CHMP Opinion EMA Approval CHMP Opinion EMA Supplemental Approval (Gen 2) Cerdulatinib Initial Phase 2a Data FDA Approval (Gen 1) FDA Supplemental Approval (Gen 2) US EU US EU US EU U.S. Launch Planned

NASDAQ: PTLA






























