Exhibit 99.2

Study of corticosteroid-eluting implants placedin-office as an alternative to revision surgery in patients with recurrent sinus obstruction due to polyposis: a meta-analysis
J. Pablo Stolovitzky, MD, Atlanta, GA Robert L. Kern, MD, Chicago, IL
Joseph K. Han, MD, Norfolk, VA Keith D. Forwith, PhD, MD, Louisville, KY
Randall A. Ow, MD, Roseville, CA
ndrew Gould, MD, Louisville, KY

Disclosures
Presenter Disclosures:
J. Pablo Stolovitzky: Consultant intersect ENT
Robert Kern: None
Joseph Han: Consultant intersect ENT
Keith Forwith : None
Randall Ow: None
Andrew Gould: None
The steroid-releasing implant discussed in this presentation is not approved by the US FDA. The implant is limited by the US law to investigational use only and is not for sale in the US.
Intersect ENT provided funding, administrative support, and materials to complete this study, Sites were compensated for study-related labor and expenses in enrolling patients and acquiring data.
Chronic Rhinosinusitis with Nasal Polyps (CRSwNP)
• The multi-factorial pathophysiology of CRSwNP prevents long-term effectiveness of any single treatment option
• Medical management includes systemic and intranasal corticosteroids (INCS)
• Surgical treatment relieves blockage and improves ventilation
– 40% recurrence rates of nasal polyps in 1.5 yr1
– Recurrent polyps positive predictor for revision surgery2
1DeConde et al Laryngoscope 2016;127:550-5
2Orlandi et al IFAR 2016;6S:S22-209
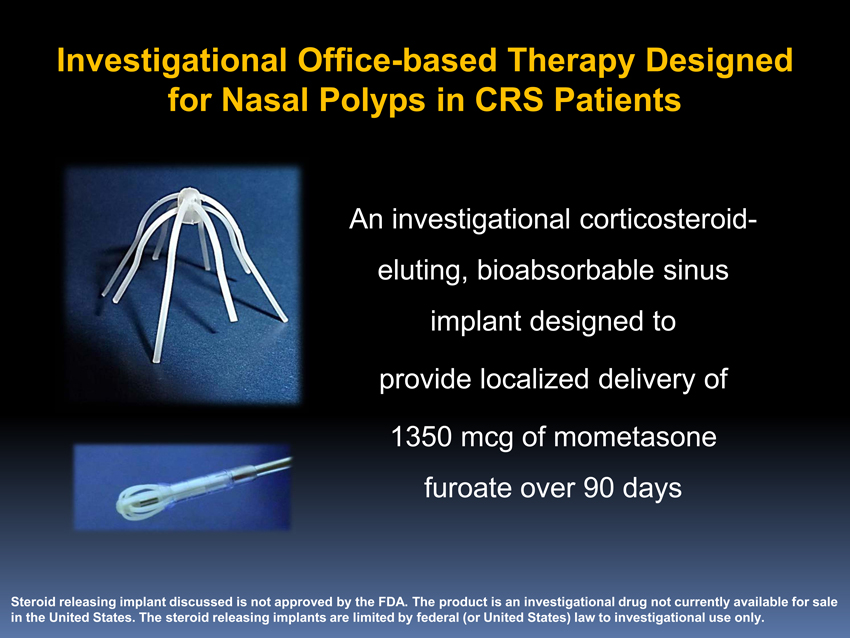
Investigational Office-based Therapy Designed for Nasal Polyps in CRS Patients
An investigational corticosteroid-eluting, bioabsorbable sinus implant designed to provide localized delivery of 1350 mcg of mometasone furoate over 90 days
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.

Two Randomized Controlled Trials
RESOLVE RESOLVE II N=100 N=300
• Randomized 1:1 • Randomized 2:1
• Sham-controlled • Sham-controlled
• Double-blind • Double-blind
• 6 monthsfollow-up • 3 monthsfollow-up
Treatment Group:in-office bilateral implant placement in the ethmoid sinuses + MFNS 200 mcg QD
Control Group:in-office bilateral sham procedure + MFNS 200 mcg QD
Co-Primary Efficacy Endpoints
• Nasal obstruction/congestion score change from baseline
• To Day 90 in RESOLVE
• To Day 30 in RESOLVE II
• Bilateral polyp grade change from baseline to Day 90 by an independent, blinded panel
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
Investigational Sinus Implant forIn-Office Treatment of Nasal Polyps
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
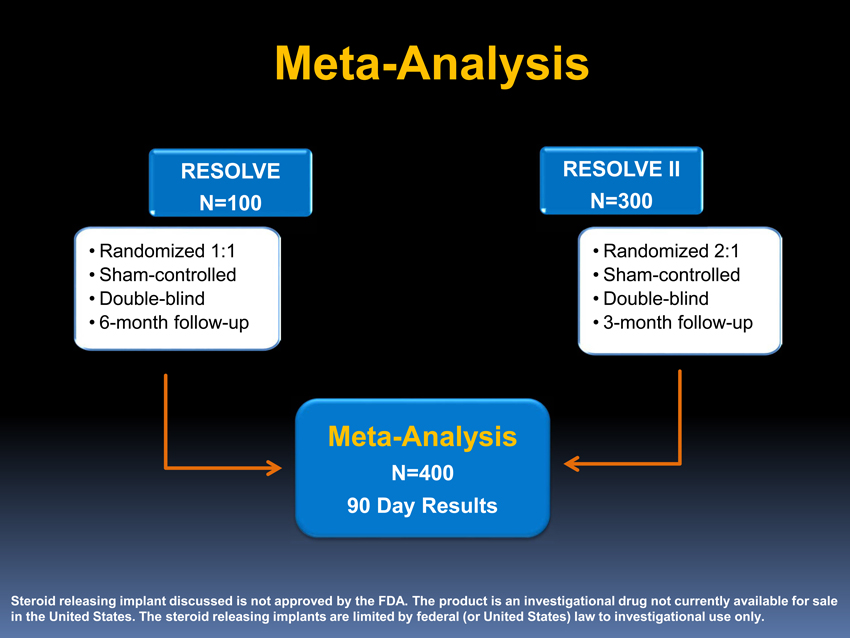
Meta-Analysis
RESOLVE RESOLVE II N=100 N=300
• Randomized 1:1 • Randomized 2:1 • Sham-controlled • Sham-controlled • Double-blind • Double-blind •6-monthfollow-up •3-monthfollow-up
Meta-Analysis
N=400
90 Day Results
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
Objective of Meta-Analysis
To evaluate the sinus implant in patients with recurrent and medically refractory nasal polyps
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
Meta-AnalysisCo-primary Efficacy Endpoints
• Nasal obstruction/congestion score
• change from baseline to Day 90 by patients using questionnaires
• Bilateral polyp grade
• change from baseline to Day 90 by the independent, blinded panel
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
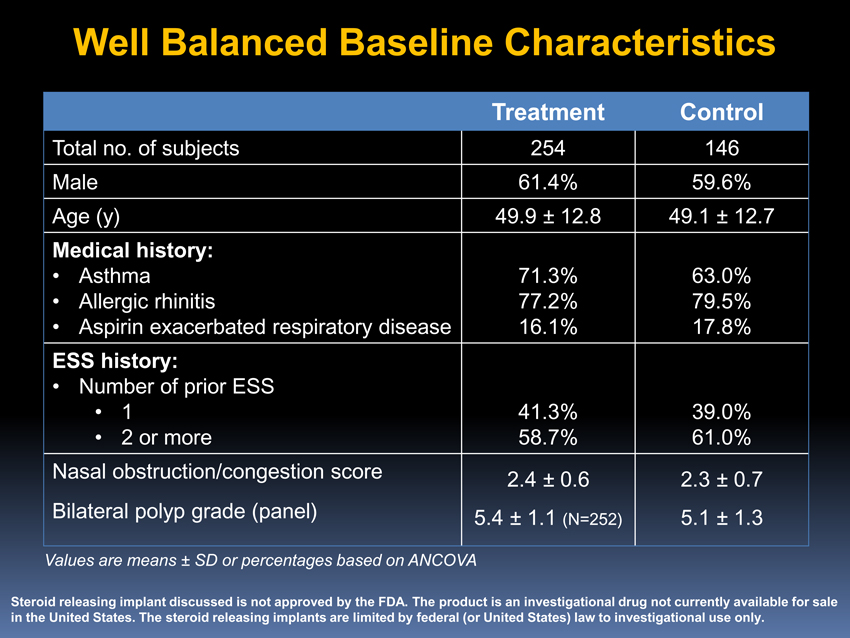
Well Balanced Baseline Characteristics
Treatment Control
Total no. of subjects 254 146 Male 61.4% 59.6% Age (y) 49.9 ± 12.8 49.1 ± 12.7
Medical history:
• Asthma 71.3% 63.0%
• Allergic rhinitis 77.2% 79.5%
• Aspirin exacerbated respiratory disease 16.1% 17.8%
ESS history:
• Number of prior ESS
• 1 41.3% 39.0%
• 2 or more 58.7% 61.0% Nasal obstruction/congestion score 2.4 ± 0.6 2.3 ± 0.7 Bilateral polyp grade (panel) 5.4 ± 1.1 (N=252) 5.1 ± 1.3
Values are means ± SD or percentages based on ANCOVA
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
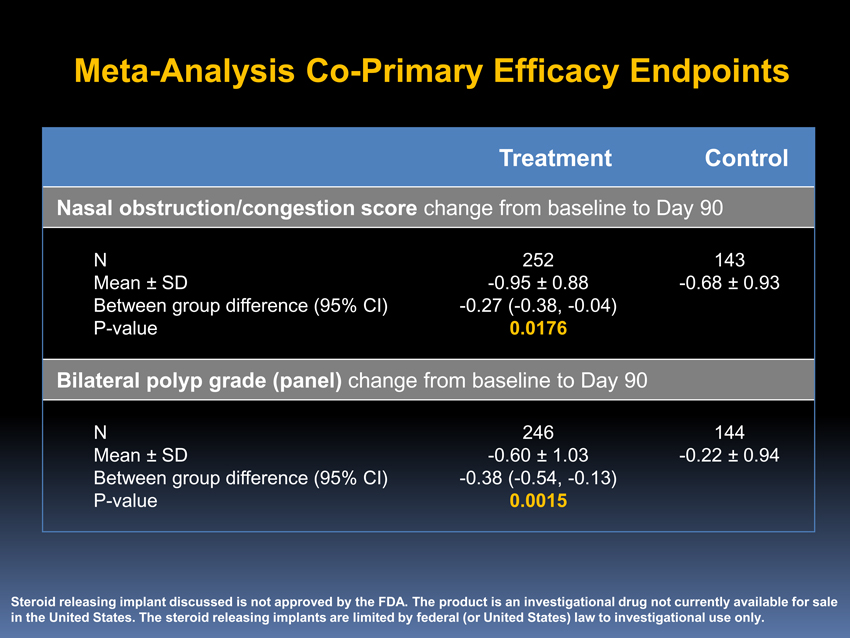
Meta-AnalysisCo-Primary Efficacy Endpoints
Treatment Control
Nasal obstruction/congestion score change from baseline to Day 90
N 252 143 Mean ± SD-0.95 ± 0.88-0.68 ± 0.93 Between group difference (95% CI)-0.27(-0.38,-0.04)P-value 0.0176
Bilateral polyp grade (panel) change from baseline to Day 90
N 246 144 Mean ± SD-0.60 ± 1.03-0.22 ± 0.94 Between group difference (95% CI)-0.38(-0.54,-0.13)P-value 0.0015
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
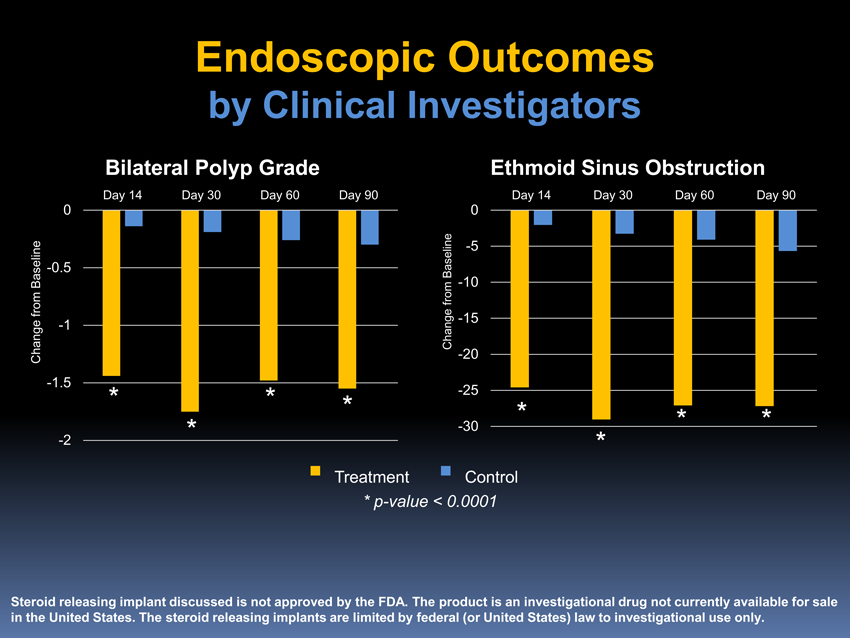
Endoscopic Outcomes
by Clinical Investigators
Bilateral Polyp Grade Ethmoid Sinus Obstruction
Day 14 Day 30 Day 60 Day 90 Day 14 Day 30 Day 60 Day 90
0 0
-5
-0.5 Baseline
Baseline -10 from from
-1 Change-15
Change-20-1.5
* * *-25
* * *
*-30
-2 *
Treatment Control
*p-value < 0.0001
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
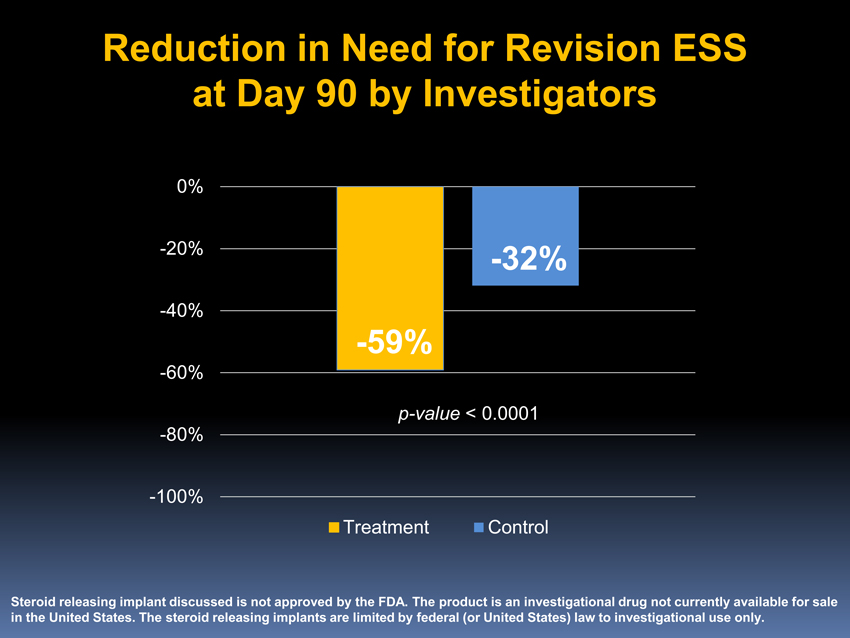
Reduction in Need for Revision ESS at Day 90 by Investigators
0%
-20%-32%-40%
-59%
-60%p-value < 0.0001-80%
-100%
Treatment Control
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.

Safety Results
• 1 (0.4%) device-related serious adverse event (epistaxis)
• Similar incidence of adverse events between groups
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.
Conclusion
In-office placement of the corticosteroid-releasing implants in patients with recurrent nasal polyps resulted in reductions in: • clinical symptoms • polyp burden • the need for revision surgery
Steroid releasing implant discussed is not approved by the FDA. The product is an investigational drug not currently available for sale in the United States. The steroid releasing implants are limited by federal (or United States) law to investigational use only.

Thank you!















