
Corporate Presentation December 2020 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements that reflect AGTC's plans, estimates, assumptions and beliefs. Forward-looking statements include statements regarding its proposed Phase 2/3 trial design for XLRP, its anticipated timeline and potential to receive FDA approval to begin the XLRP pivotal trial, the timing for reporting data in its XLRP and ACHM clinical programs, its ability to enroll patients for its clinical trials, the expected costs of its clinical programs and its planned manufacturing activities, regulatory progress, potential growth and market opportunities, and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as "anticipates," "believes," "could," "seeks," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would" or similar expressions and the negatives of those terms. Actual results could differ materially from those discussed in the forward-looking statements, due to a number of important factors. Risks and uncertainties that may cause actual results to differ materially include, among others: AGTC cannot predict when or if it will obtain regulatory approval to continue to progress its clinical trials, commercialize a product candidate or receive reasonable reimbursement; uncertainty inherent in clinical trials and the regulatory review process; risks and uncertainties associated with drug development and commercialization; risks related to the COVID-19 outbreak that may delay clinical trial enrollment; gene therapy is still novel with only a few approved treatments so far; factors that could cause actual results to differ materially from those described in the forward-looking statements are set forth under the heading "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent periodic reports filed with the SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent management's plans, estimates, assumptions and beliefs only as of the date of this presentation. Except as required by law, we assume no obligation to update these forward-looking statements publicly or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
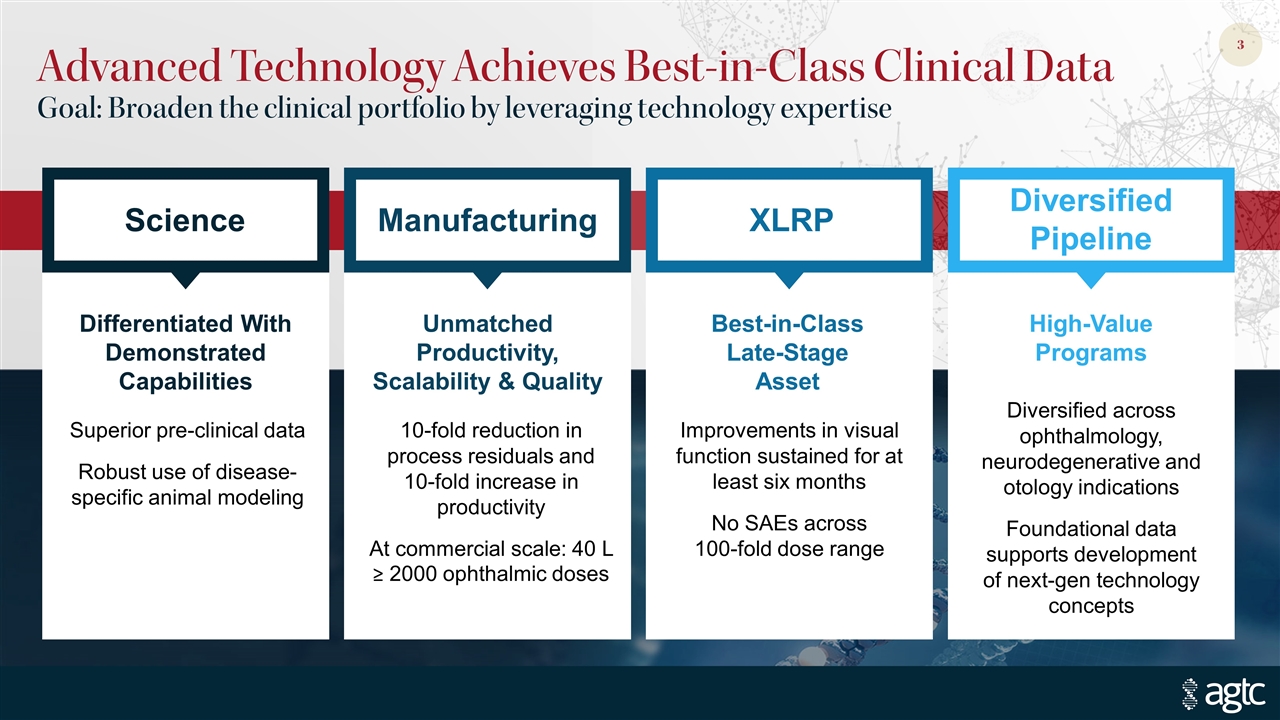
Advanced Technology Achieves Best-in-Class Clinical Data Goal: Broaden the clinical portfolio by leveraging technology expertise Diversified Pipeline XLRP Manufacturing Science Diversified across ophthalmology, neurodegenerative and otology indications Foundational data supports development of next-gen technology concepts Improvements in visual function sustained for at least six months No SAEs across 100-fold dose range 10-fold reduction in process residuals and 10-fold increase in productivity At commercial scale: 40 L ≥ 2000 ophthalmic doses Superior pre-clinical data Robust use of disease-specific animal modeling Differentiated With Demonstrated Capabilities Unmatched Productivity, Scalability & Quality Best-in-Class Late-Stage Asset High-Value Programs

Leader in Commercial Customization and Production of Gene Therapies
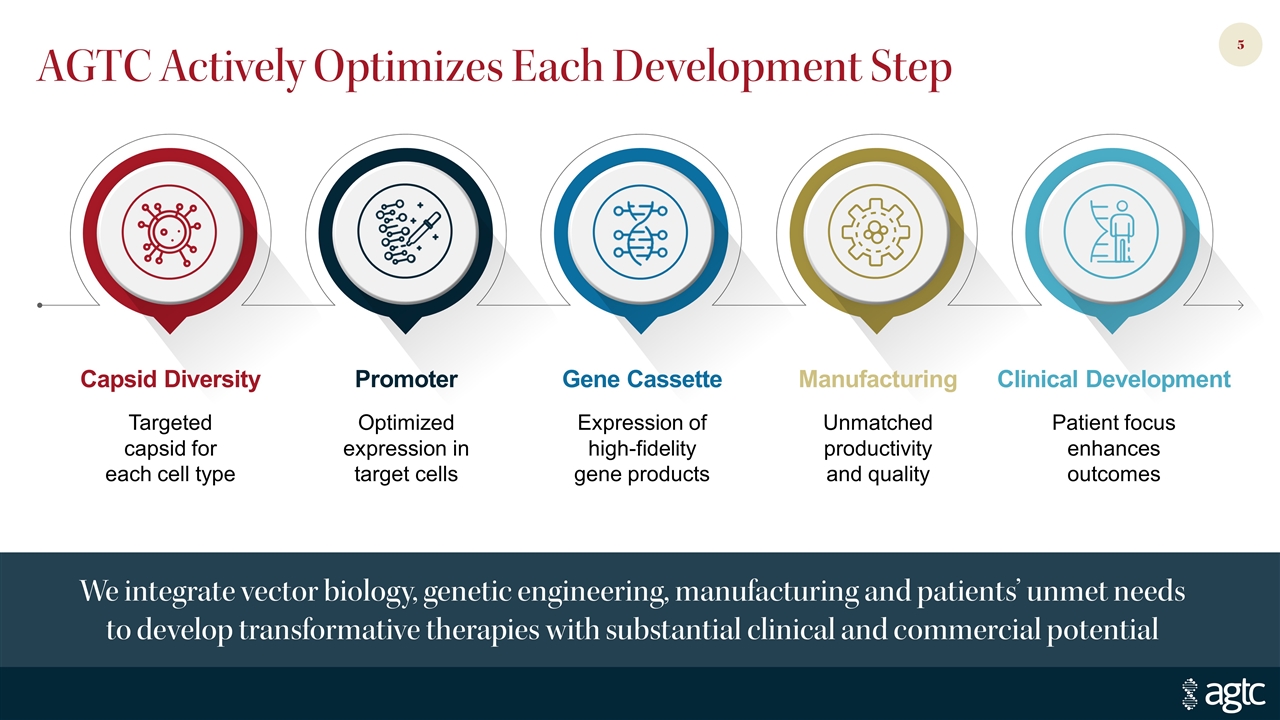
AGTC Actively Optimizes Each Development Step We integrate vector biology, genetic engineering, manufacturing and patients’ unmet needs to develop transformative therapies with substantial clinical and commercial potential Capsid Diversity Promoter Gene Cassette Manufacturing Targeted capsid for each cell type Clinical Development Patient focus enhances outcomes Optimized expression in target cells Expression of high-fidelity gene products Unmatched productivity and quality
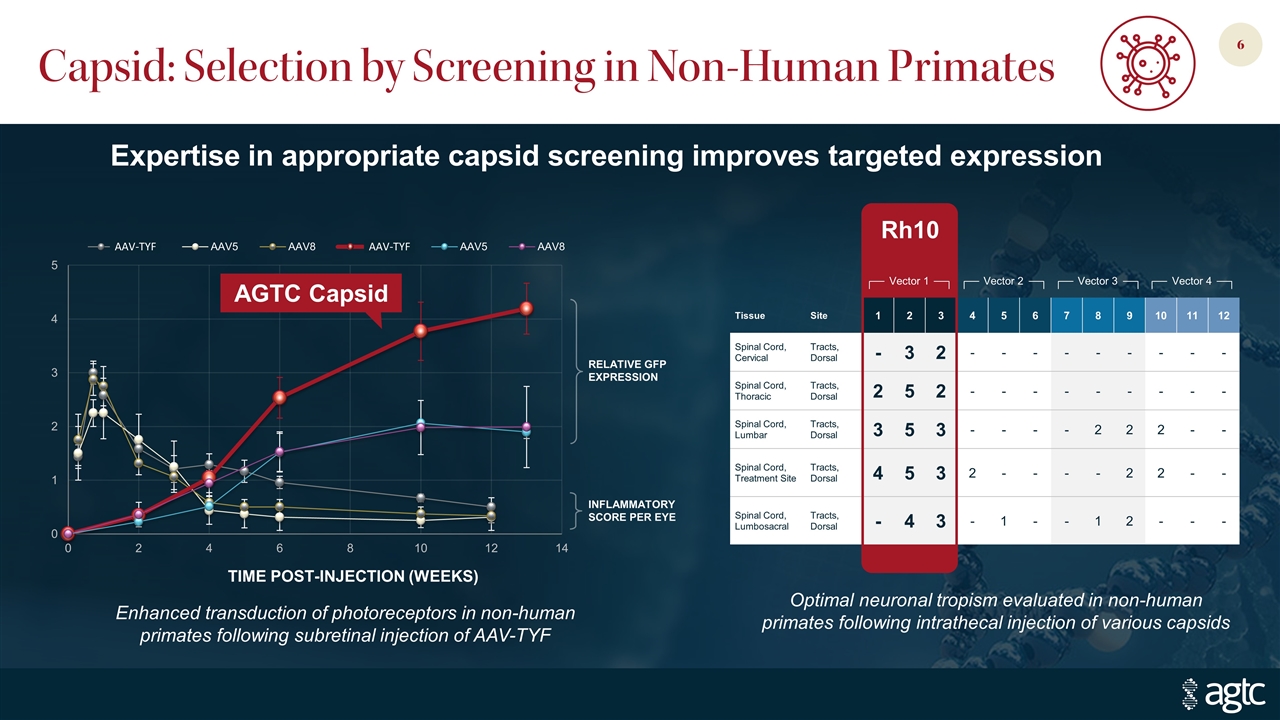
Capsid: Selection by Screening in Non-Human Primates Expertise in appropriate capsid screening improves targeted expression Enhanced transduction of photoreceptors in non-human primates following subretinal injection of AAV-TYF AGTC Capsid AAV-TYF AAV-TYF Optimal neuronal tropism evaluated in non-human primates following intrathecal injection of various capsids Tissue Site 1 2 3 4 5 6 7 8 9 10 11 12 Spinal Cord, Cervical Tracts, Dorsal - 3 2 - - - - - - - - - Spinal Cord, Thoracic Tracts, Dorsal 2 5 2 - - - - - - - - - Spinal Cord, Lumbar Tracts, Dorsal 3 5 3 - - - - 2 2 2 - - Spinal Cord, Treatment Site Tracts, Dorsal 4 5 3 2 - - - - 2 2 - - Spinal Cord, Lumbosacral Tracts, Dorsal - 4 3 - 1 - - 1 2 - - - Vector 1 Vector 2 Vector 3 Vector 4 Rh10 Relative GFP Expression Inflammatory Score per Eye
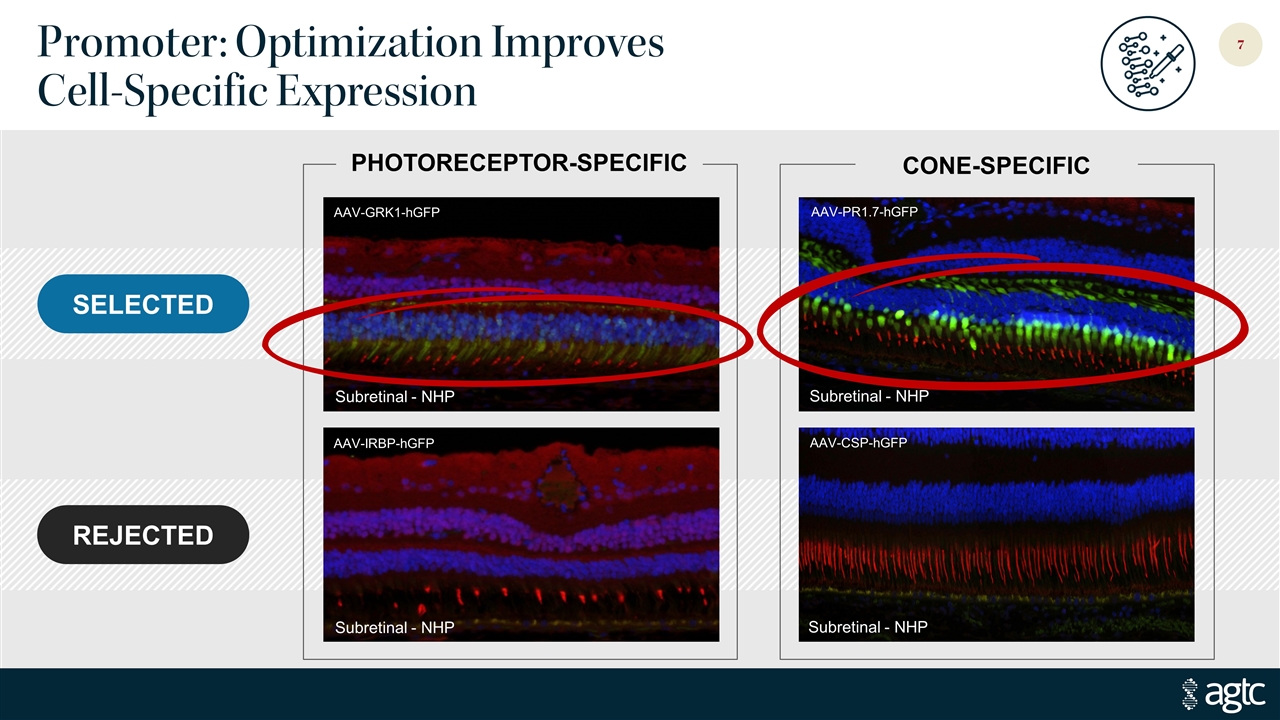
Promoter: Optimization Improves Cell-Specific Expression Subretinal - NHP AAV-PR1.7-hGFP Subretinal - NHP AAV-CSP-hGFP SELECTED REJECTED AAV-GRK1-hGFP Subretinal - NHP AAV-IRBP-hGFP Subretinal - NHP PHOTORECEPTOR-SPECIFIC CONE-SPECIFIC
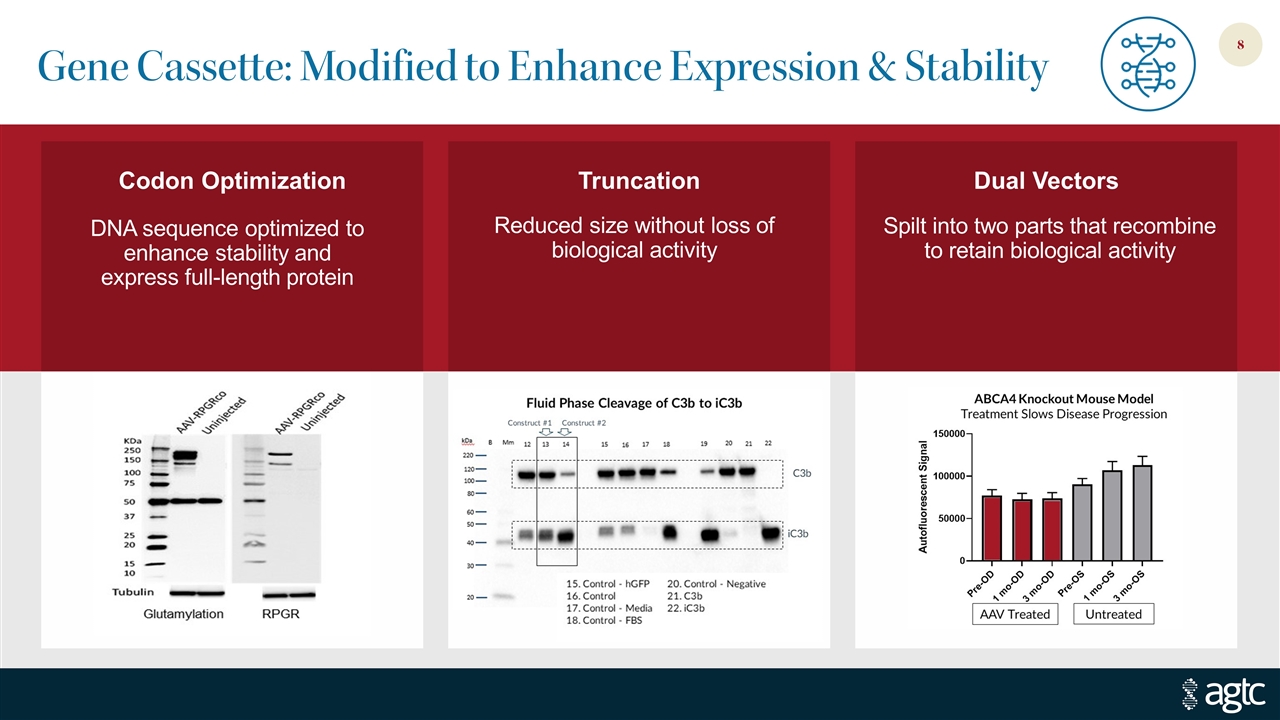
Gene Cassette: Modified to Enhance Expression & Stability DNA sequence optimized to enhance stability and express full-length protein Reduced size without loss of biological activity Spilt into two parts that recombine to retain biological activity Dual Vectors Truncation Codon Optimization
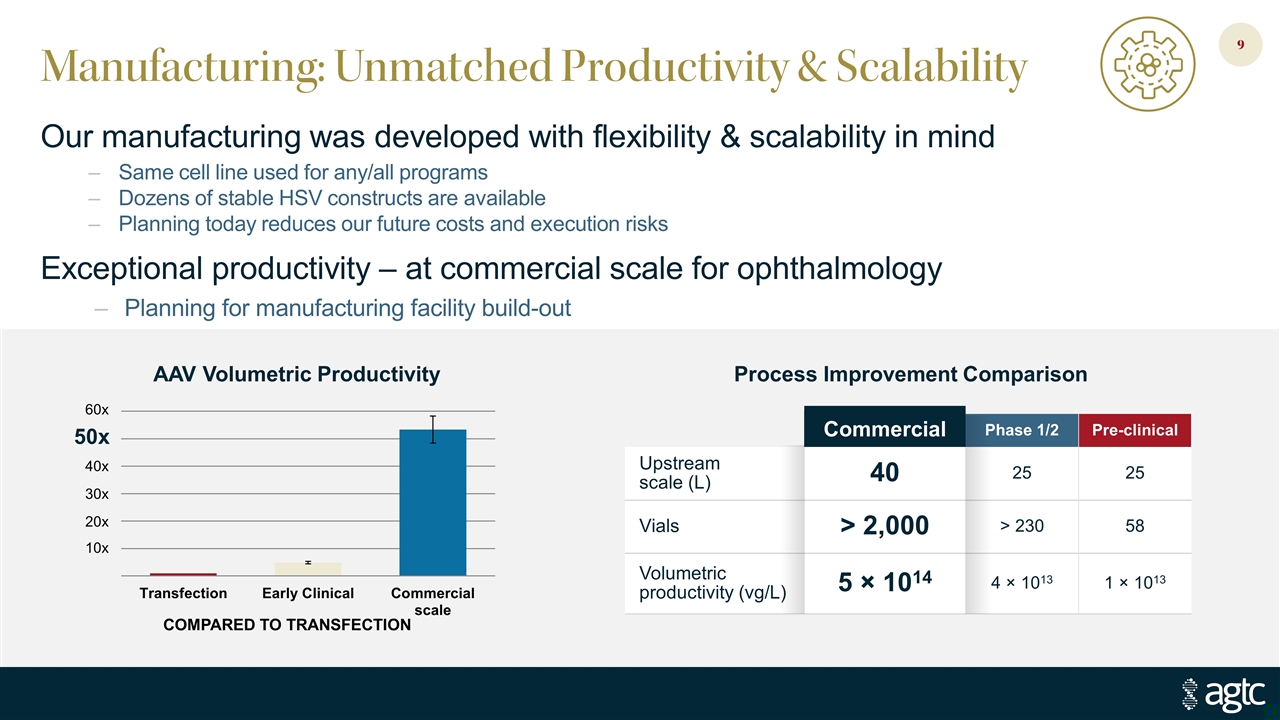
Manufacturing: Unmatched Productivity & Scalability Our manufacturing was developed with flexibility & scalability in mind Same cell line used for any/all programs Dozens of stable HSV constructs are available Planning today reduces our future costs and execution risks Exceptional productivity – at commercial scale for ophthalmology Planning for manufacturing facility build-out COMPARED TO TRANSFECTION 60x 50x 40x 30x 20x 10x Commercial Phase 1/2 Pre-clinical Upstream scale (L) 40 25 25 Vials > 2,000 > 230 58 Volumetric productivity (vg/L) 5 × 1014 4 × 1013 1 × 1013 Process Improvement Comparison AAV Volumetric Productivity
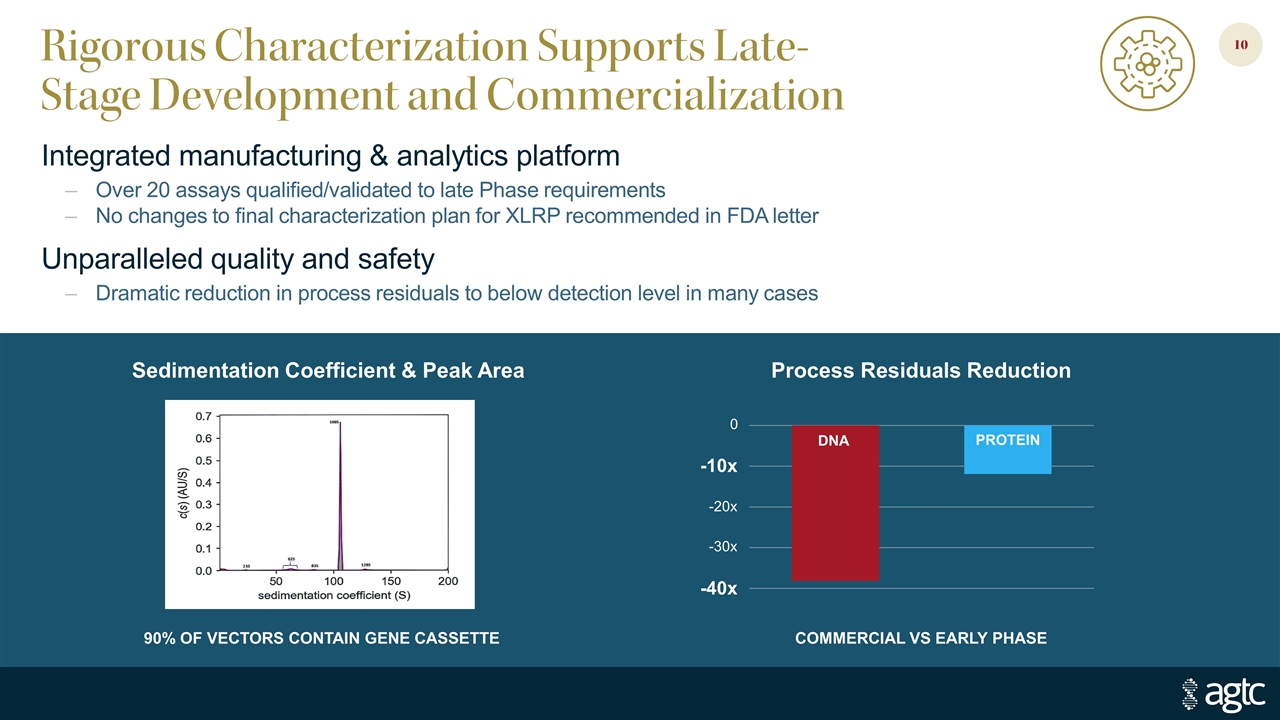
Rigorous Characterization Supports Late-Stage Development and Commercialization Integrated manufacturing & analytics platform Over 20 assays qualified/validated to late Phase requirements No changes to final characterization plan for XLRP recommended in FDA letter Unparalleled quality and safety Dramatic reduction in process residuals to below detection level in many cases Sedimentation Coefficient & Peak Area COMMERCIAL VS EARLY PHASE Process Residuals Reduction DNA PROTEIN -40x -30x -20x -10x 0 90% OF VECTORS CONTAIN GENE CASSETTE
Clinical Development: Supported by Patient Focus Education and awareness International Patient Advisory Council Patient support Concierge service to ease travel burden Mobile Vision Center to “take the testing to the patient” Optimized surgical procedures Surgical grand rounds for consistency and quality of patient outcomes Canada
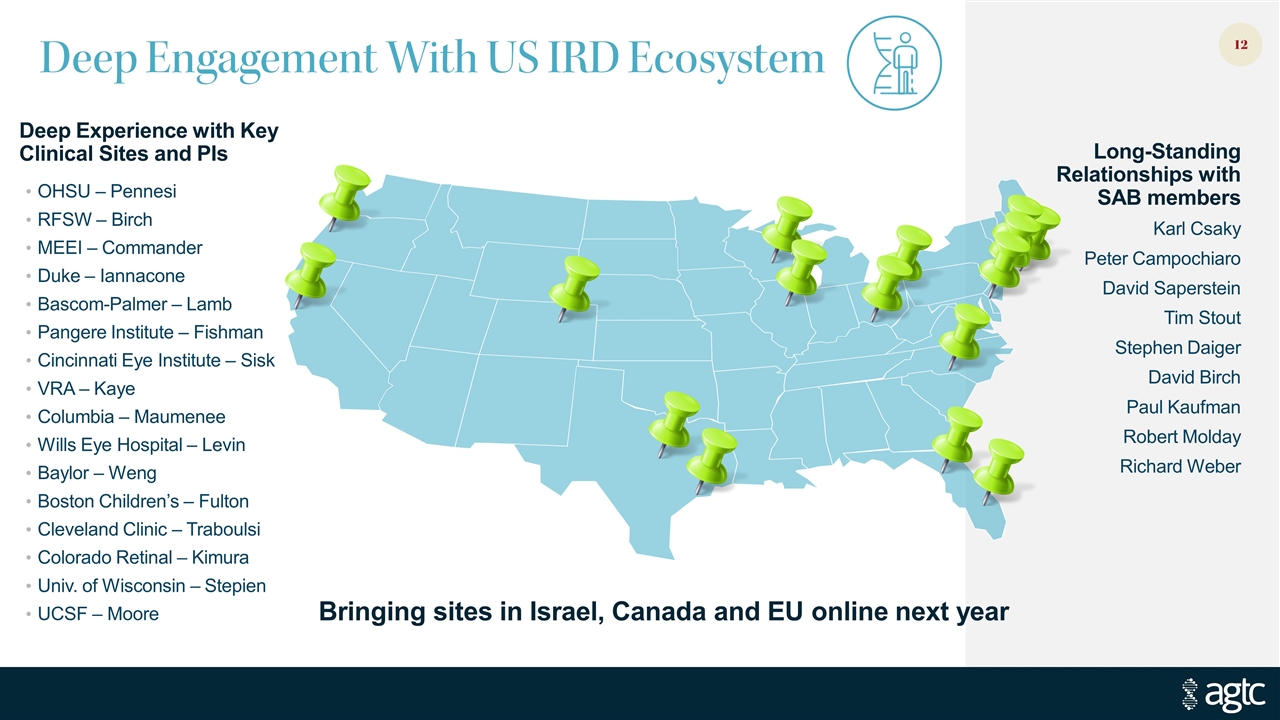
Deep Engagement With US IRD Ecosystem Deep Experience with Key Clinical Sites and PIs OHSU – Pennesi RFSW – Birch MEEI – Commander Duke – Iannacone Bascom-Palmer – Lamb Pangere Institute – Fishman Cincinnati Eye Institute – Sisk VRA – Kaye Columbia – Maumenee Wills Eye Hospital – Levin Baylor – Weng Boston Children’s – Fulton Cleveland Clinic – Traboulsi Colorado Retinal – Kimura Univ. of Wisconsin – Stepien UCSF – Moore Long-Standing Relationships with SAB members Karl Csaky Peter Campochiaro David Saperstein Tim Stout Stephen Daiger David Birch Paul Kaufman Robert Molday Richard Weber Bringing sites in Israel, Canada and EU online next year

Overview of AGTC’s Diverse High-Value Portfolio
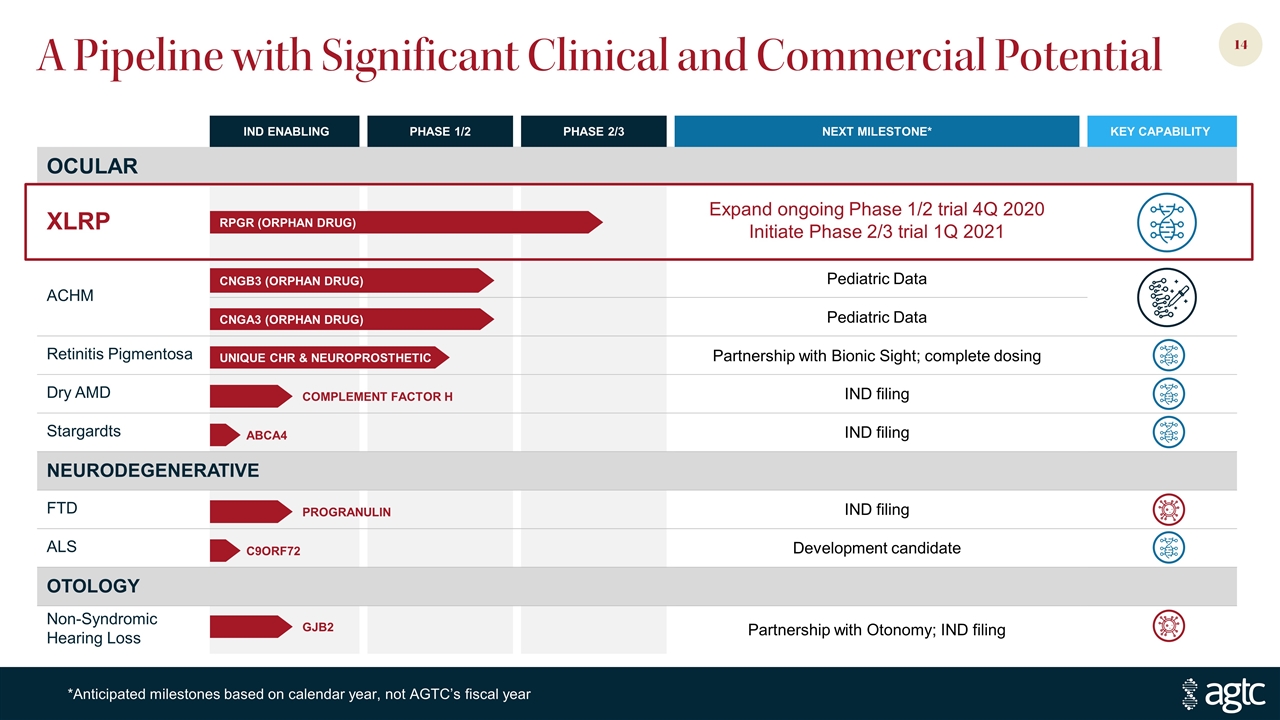
A Pipeline with Significant Clinical and Commercial Potential IND ENABLING PHASE 1/2 PHASE 2/3 NEXT MILESTONE* KEY CAPABILITY OCULAR XLRP Expand ongoing Phase 1/2 trial 4Q 2020 Initiate Phase 2/3 trial 1Q 2021 ACHM Pediatric Data Pediatric Data Retinitis Pigmentosa Partnership with Bionic Sight; complete dosing Dry AMD IND filing Stargardts IND filing NEURODEGENERATIVE FTD IND filing ALS Development candidate OTOLOGY Non-Syndromic Hearing Loss Partnership with Otonomy; IND filing *Anticipated milestones based on calendar year, not AGTC’s fiscal year CNGB3 (ORPHAN DRUG) RPGR (ORPHAN DRUG) CNGA3 (ORPHAN DRUG) UNIQUE CHR & NEUROPROSTHETIC COMPLEMENT FACTOR H ABCA4 PROGRANULIN C9ORF72 GJB2

Ophthalmology
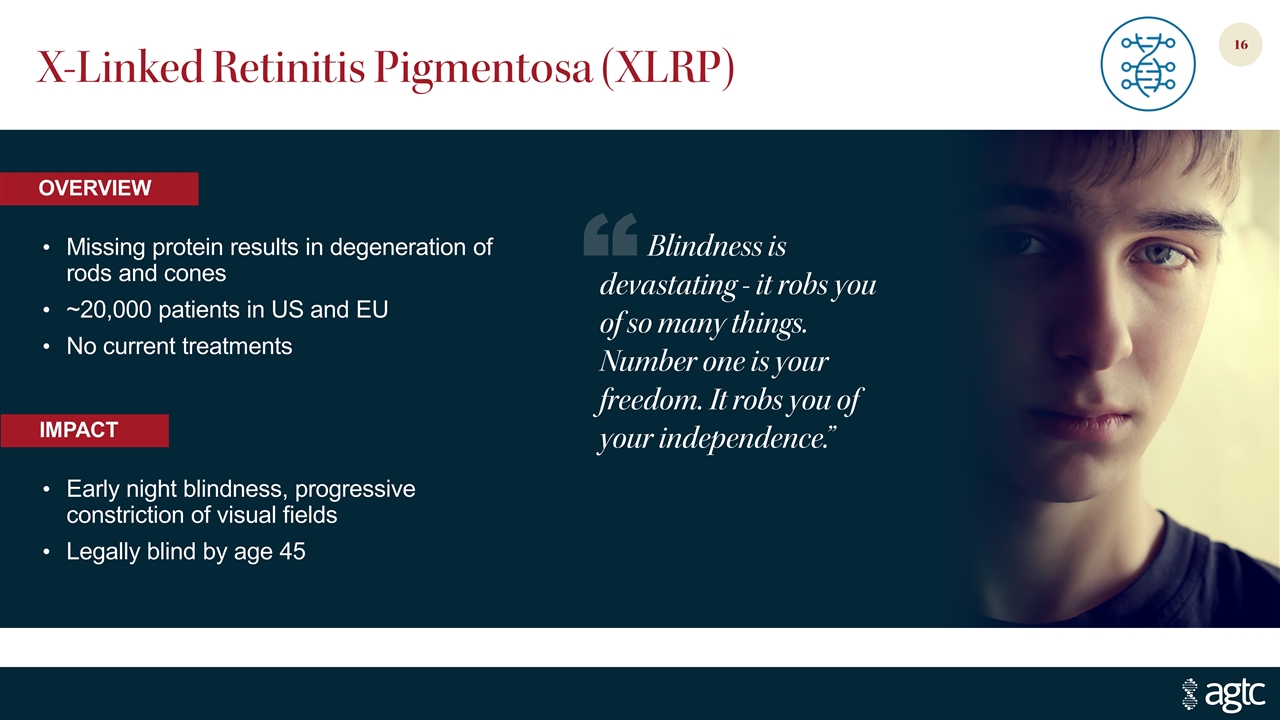
X-Linked Retinitis Pigmentosa (XLRP) Missing protein results in degeneration of rods and cones ~20,000 patients in US and EU No current treatments Early night blindness, progressive constriction of visual fields Legally blind by age 45 IMPACT OVERVIEW Blindness is devastating - it robs you of so many things. Number one is your freedom. It robs you of your independence.”
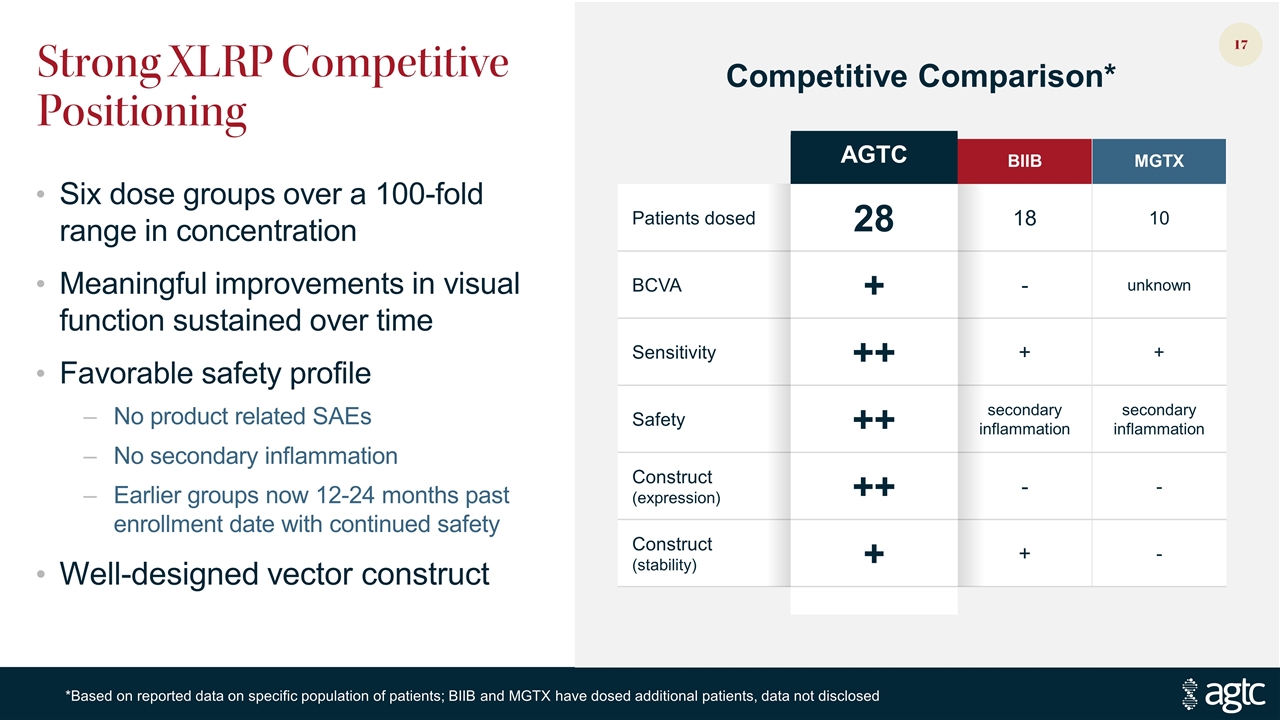
Strong XLRP Competitive Positioning Six dose groups over a 100-fold range in concentration Meaningful improvements in visual function sustained over time Favorable safety profile No product related SAEs No secondary inflammation Earlier groups now 12-24 months past enrollment date with continued safety Well-designed vector construct AGTC BIIB MGTX Patients dosed 28 18 10 BCVA + - unknown Sensitivity ++ + + Safety ++ secondary inflammation secondary inflammation Construct (expression) ++ - - Construct (stability) + + - Competitive Comparison* *Based on reported data on specific population of patients; BIIB and MGTX have dosed additional patients, data not disclosed
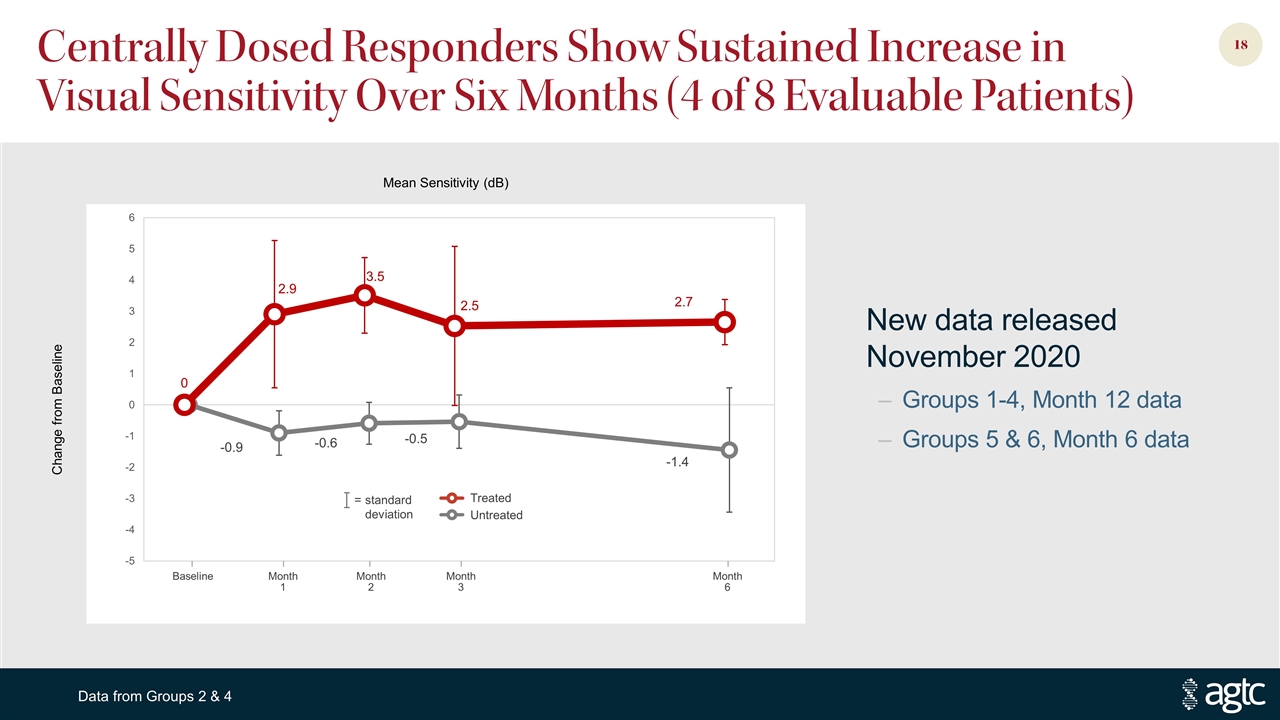
Centrally Dosed Responders Show Sustained Increase in Visual Sensitivity Over Six Months (4 of 8 Evaluable Patients) = standard deviation Treated Untreated Baseline Month 1 Month 2 Month 3 Month 6 Change from Baseline Mean Sensitivity (dB) Data from Groups 2 & 4 New data released November 2020 Groups 1-4, Month 12 data Groups 5 & 6, Month 6 data
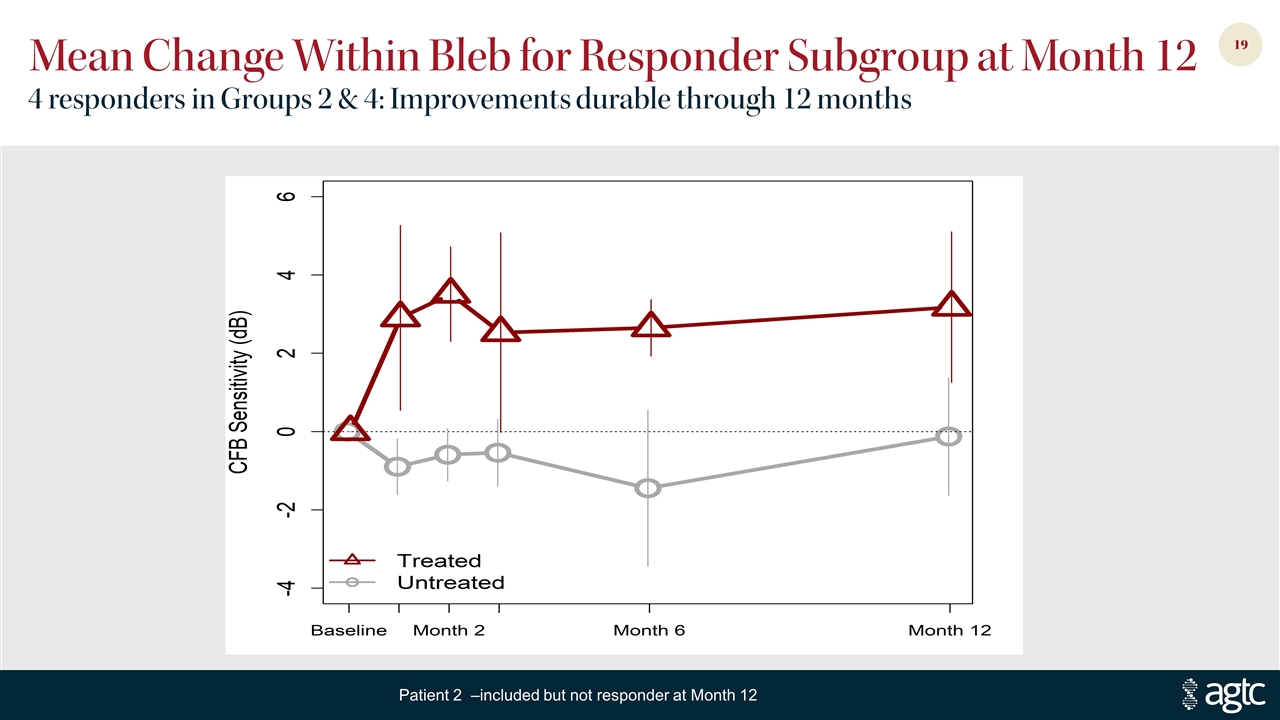
Mean Change Within Bleb for Responder Subgroup at Month 12 4 responders in Groups 2 & 4: Improvements durable through 12 months Patient 2 –included but not responder at Month 12
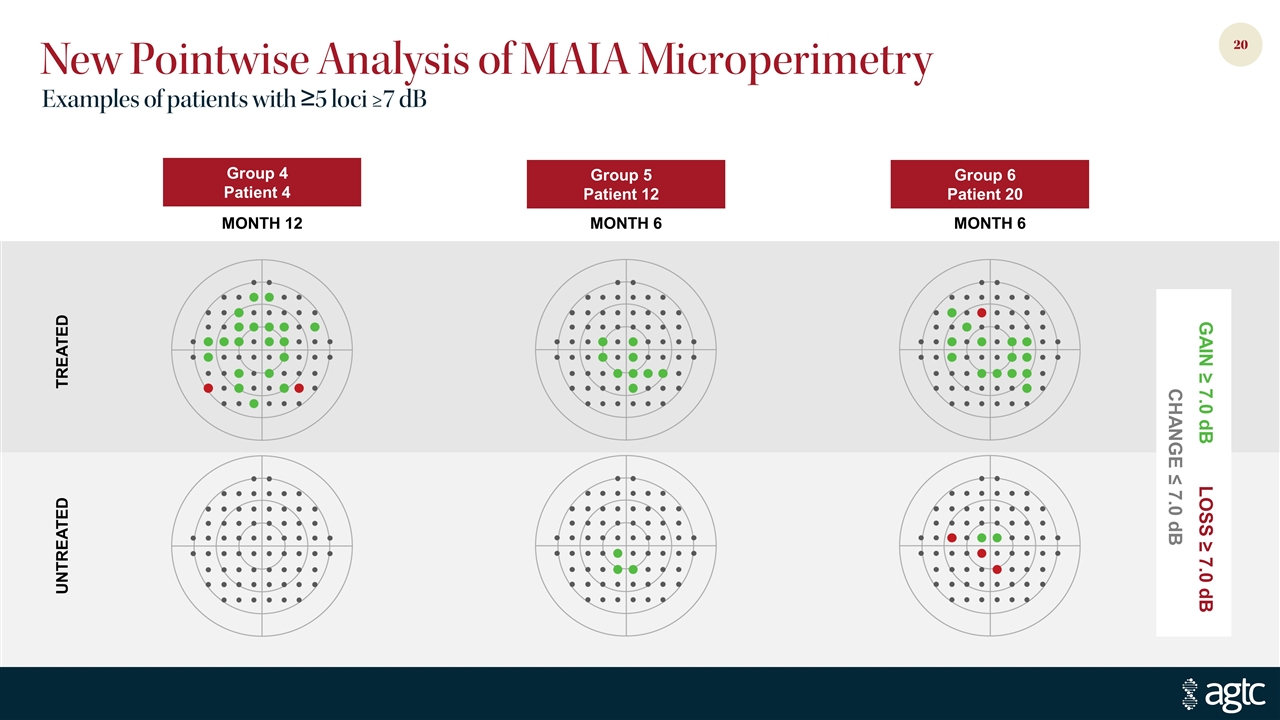
New Pointwise Analysis of MAIA Microperimetry Examples of patients with ≥5 loci ≥7 dB Group 4 Patient 4 Group 5 Patient 12 Group 6 Patient 20 TREATED UNTREATED MONTH 12 MONTH 6 MONTH 6 GAIN ≥ 7.0 dB LOSS ≥ 7.0 dB CHANGE ≤ 7.0 dB
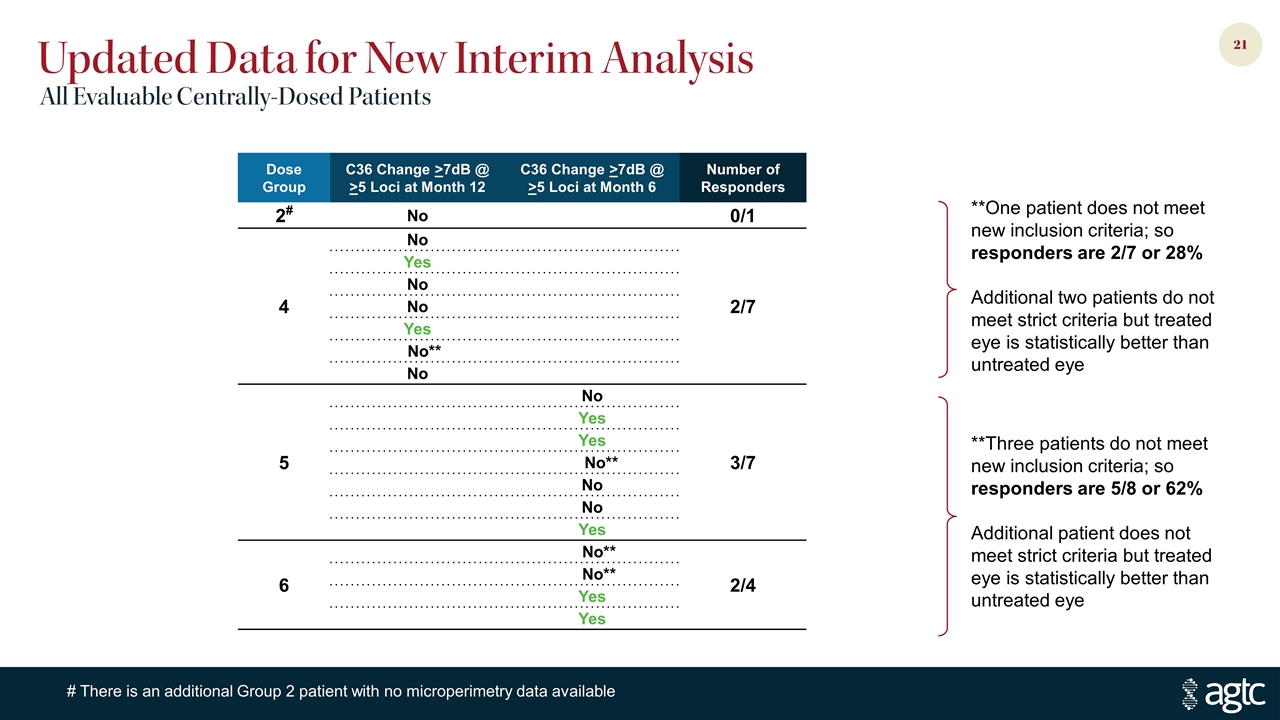
Dose Group C36 Change >7dB @ >5 Loci at Month 12 C36 Change >7dB @ >5 Loci at Month 6 Number of Responders 2# No 0/1 4 No 2/7 Yes No No Yes No** No 5 No 3/7 Yes Yes No** No No Yes 6 No** 2/4 No** Yes Yes **Three patients do not meet new inclusion criteria; so responders are 5/8 or 62% Additional patient does not meet strict criteria but treated eye is statistically better than untreated eye **One patient does not meet new inclusion criteria; so responders are 2/7 or 28% Additional two patients do not meet strict criteria but treated eye is statistically better than untreated eye Updated Data for New Interim Analysis All Evaluable Centrally-Dosed Patients # There is an additional Group 2 patient with no microperimetry data available
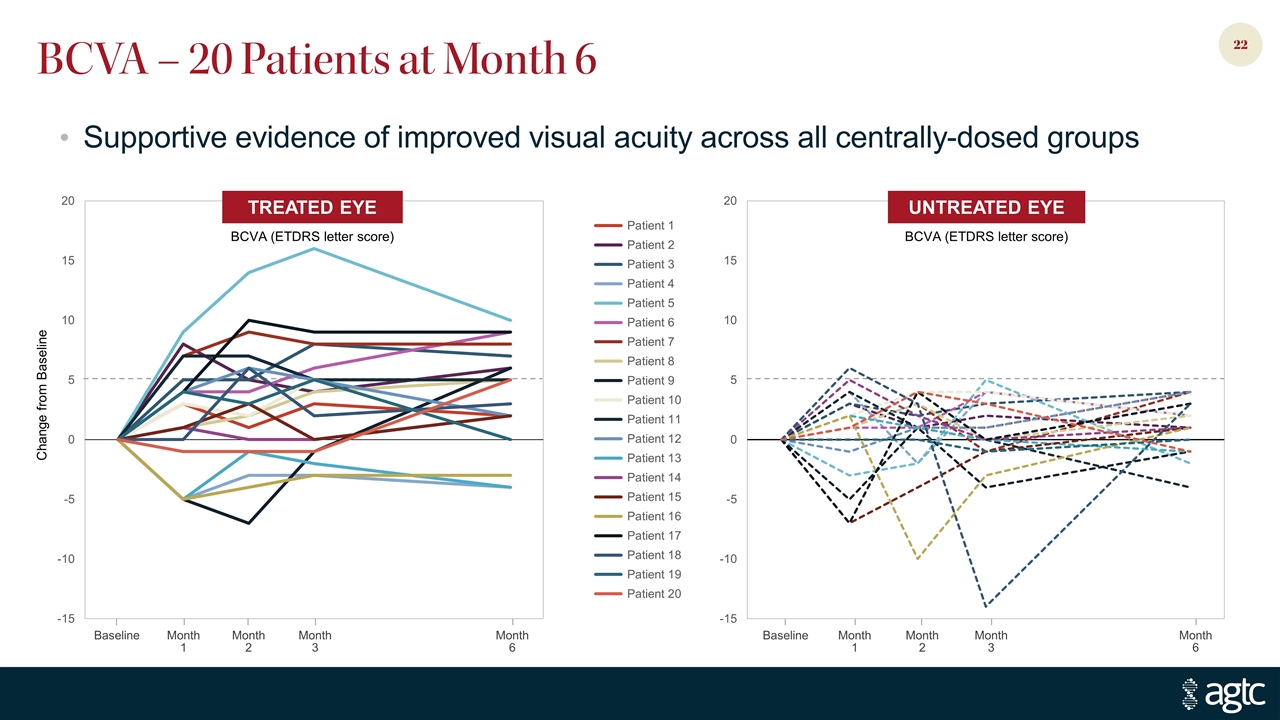
Change from Baseline Baseline Month 1 Month 2 Month 3 Month 6 Baseline Month 1 Month 2 Month 3 Month 6 BCVA – 20 Patients at Month 6 TREATED EYE BCVA (ETDRS letter score) UNTREATED EYE BCVA (ETDRS letter score) Supportive evidence of improved visual acuity across all centrally-dosed groups
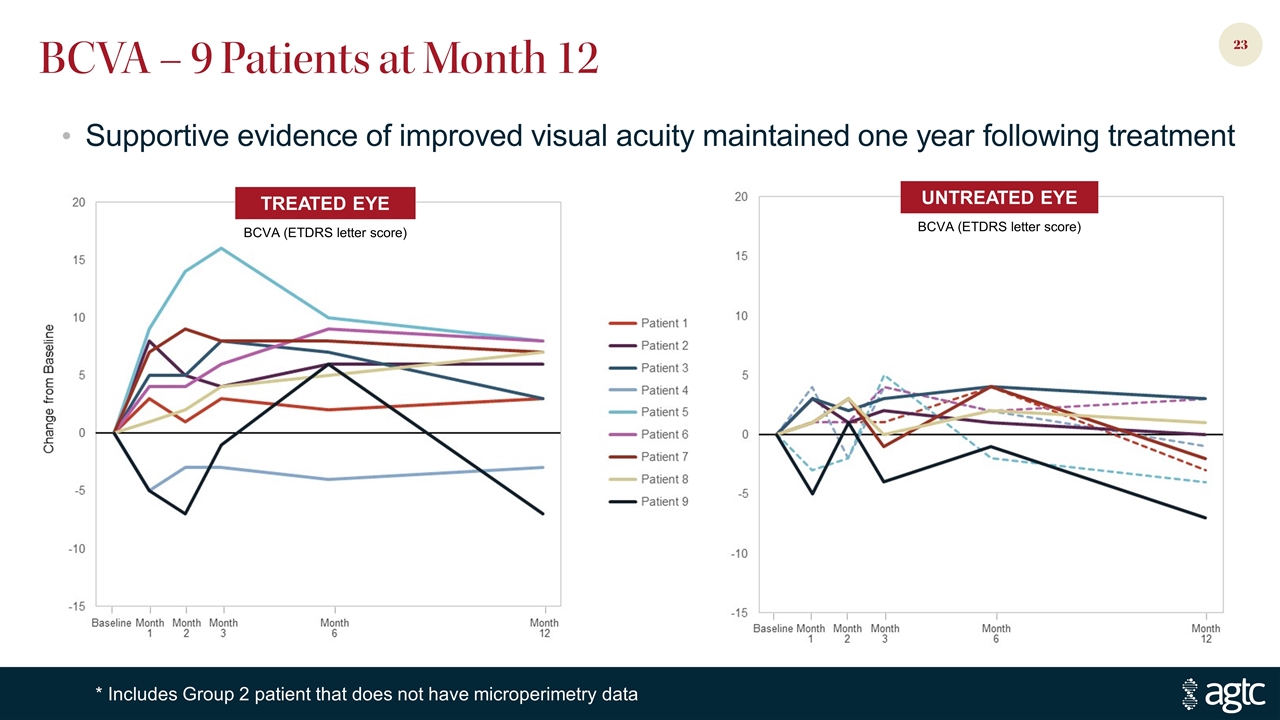
BCVA – 9 Patients at Month 12 TREATED EYE BCVA (ETDRS letter score) UNTREATED EYE BCVA (ETDRS letter score) Supportive evidence of improved visual acuity maintained one year following treatment * Includes Group 2 patient that does not have microperimetry data
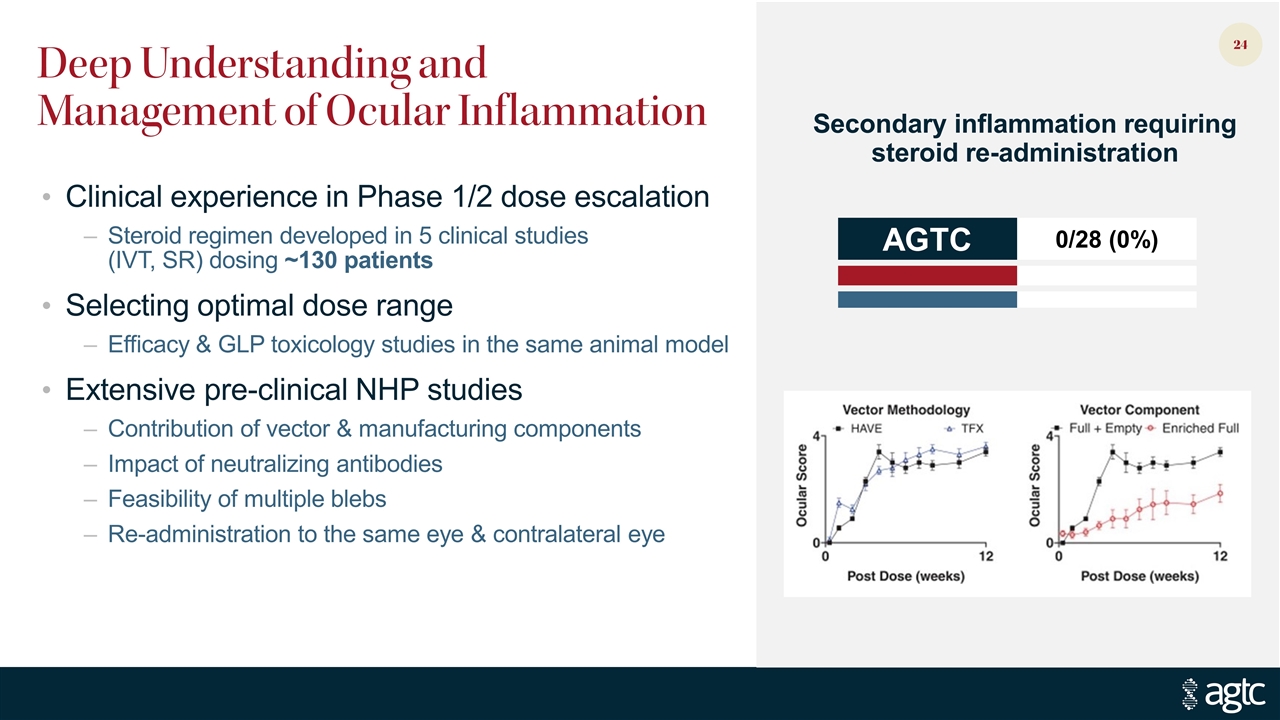
Deep Understanding and Management of Ocular Inflammation Clinical experience in Phase 1/2 dose escalation Steroid regimen developed in 5 clinical studies (IVT, SR) dosing ~130 patients Selecting optimal dose range Efficacy & GLP toxicology studies in the same animal model Extensive pre-clinical NHP studies Contribution of vector & manufacturing components Impact of neutralizing antibodies Feasibility of multiple blebs Re-administration to the same eye & contralateral eye Secondary inflammation requiring steroid re-administration AGTC 0/28 (0%)
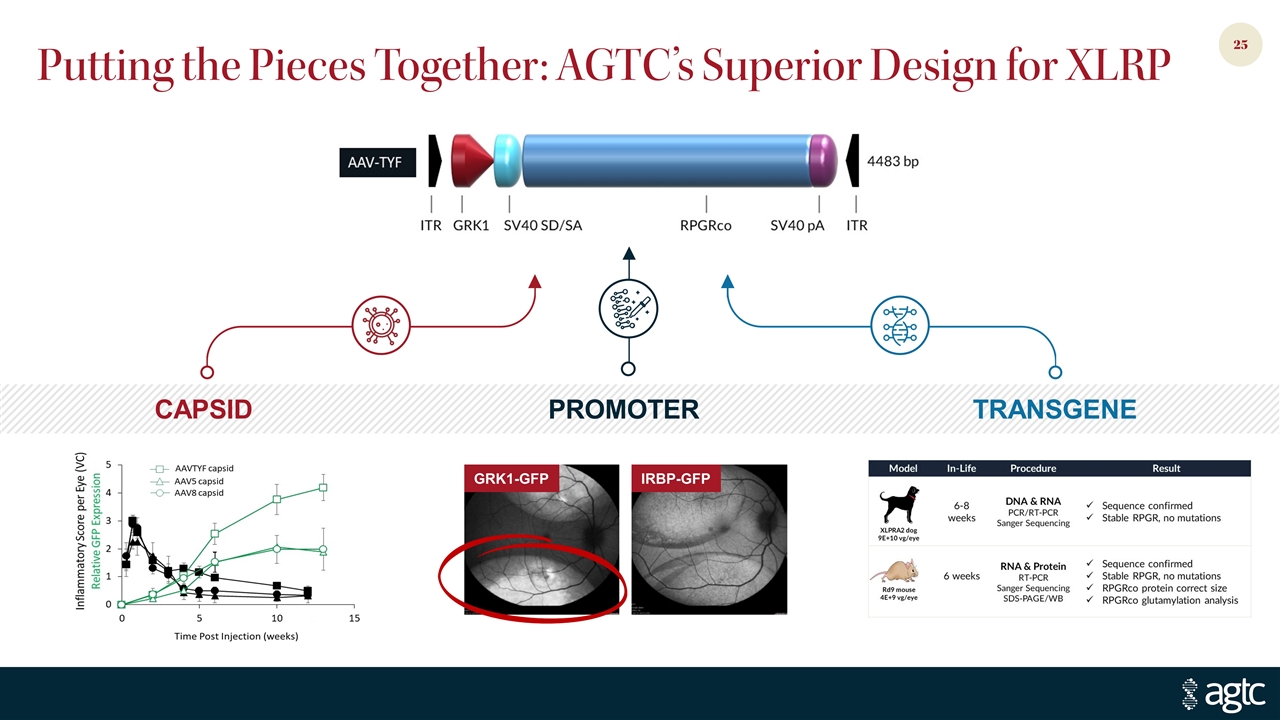
Putting the Pieces Together: AGTC’s Superior Design for XLRP CAPSID PROMOTER TRANSGENE GRK1-GFP IRBP-GFP

Vista Clinical Trial Microperimetry as analyzed by pre-specified loci per FDA as primary endpoint, in addition to other microperimetry assessments Safety/efficacy range fully explored and current data release supports low & high dose selection Study will include untreated control arm as comparator BCVA to continue as supportive secondary endpoint Ora-VNC™ mobility maze as additional supportive endpoint* Use of updated and validated PRO survey *Ora Visual Navigation Challenge (Ora-VNC™)
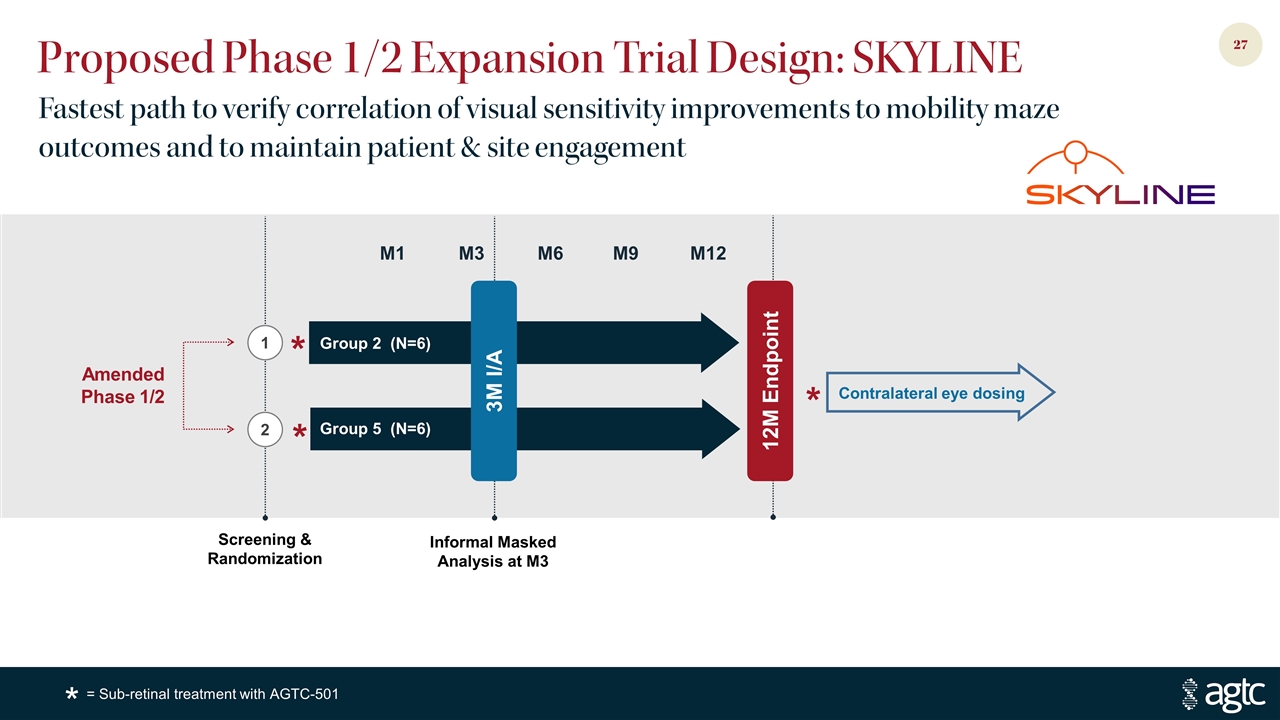
Proposed Phase 1/2 Expansion Trial Design: SKYLINE Fastest path to verify correlation of visual sensitivity improvements to mobility maze outcomes and to maintain patient & site engagement Contralateral eye dosing Amended Phase 1/2 * * * 3M I/A Informal Masked Analysis at M3 M12 M3 Group 2 (N=6) Group 5 (N=6) M6 M1 M9 Screening & Randomization 2 1 12M Endpoint = Sub-retinal treatment with AGTC-501 *

XLRP Summary: Superior Expertise Leading to Best-in-Class Clinical Data Expertise led to clinical success to date Optimized product design, breadth of preclinical data supported Strong safety profile allowing higher dosing Sustained and robust improvements in visual function Patient focus generated creative clinical solutions, including continued data collection during pandemic Improved manufacturing process No further process development or scale-up required to support commercial launch Superior quality supports clinical safety profile Intermediate analysis points create opportunities for future acceleration 2021: complete twelve-month data for Phase 1/2 dose groups 5 and 6 Planned Skyline Next Data Point 3Q 2021: 3-month data for masked dose expansion of groups 2 and 5 2022: 12-month data for masked dose expansion of groups 2 and 5 Planned Vista Next Data Point 3Q 2022: 6-month data 2 1 3

Achromatopsia (ACHM) AGTC focused on A3 and B3 gene mutations, which account for at least 70% of ACHM Severely impaired vision and day blindness due to loss of cone photoreceptor function ~27,000 patients in US and EU affected No current treatments Extremely poor vision, legally blind Extreme light sensitivity (day blind) Complete loss of color discrimination IMPACT OVERVIEW The bright light makes it really hard for him, so he tends to freeze up and doesn't want to walk anywhere because he's afraid he might injure himself. He gets more cautious in new or bright environments."
Encouraging Early Data Support Continued Clinical Development Product safe and well-tolerated Supported across both ACHM and XLRP trials with same capsid Preliminary signs of biologic activity 3-month light discomfort testing- stated as important issue by patients In November data update no consistent sustained improvements were observed at 12 months for early groups and 6 month in higher groups Disease affects visual cortex pathway Need to: Follow patients for longer time periods Follow patients dosed at higher doses Enroll pediatric patients Novel data collection Use of the Mobile Vision Center to collect data during pandemic fMRI imaging to directly measure changes in neural pathways Robust body of preclinical data, improvement in key symptom for patients, and favorable safety profile in both trials builds confidence in product potential
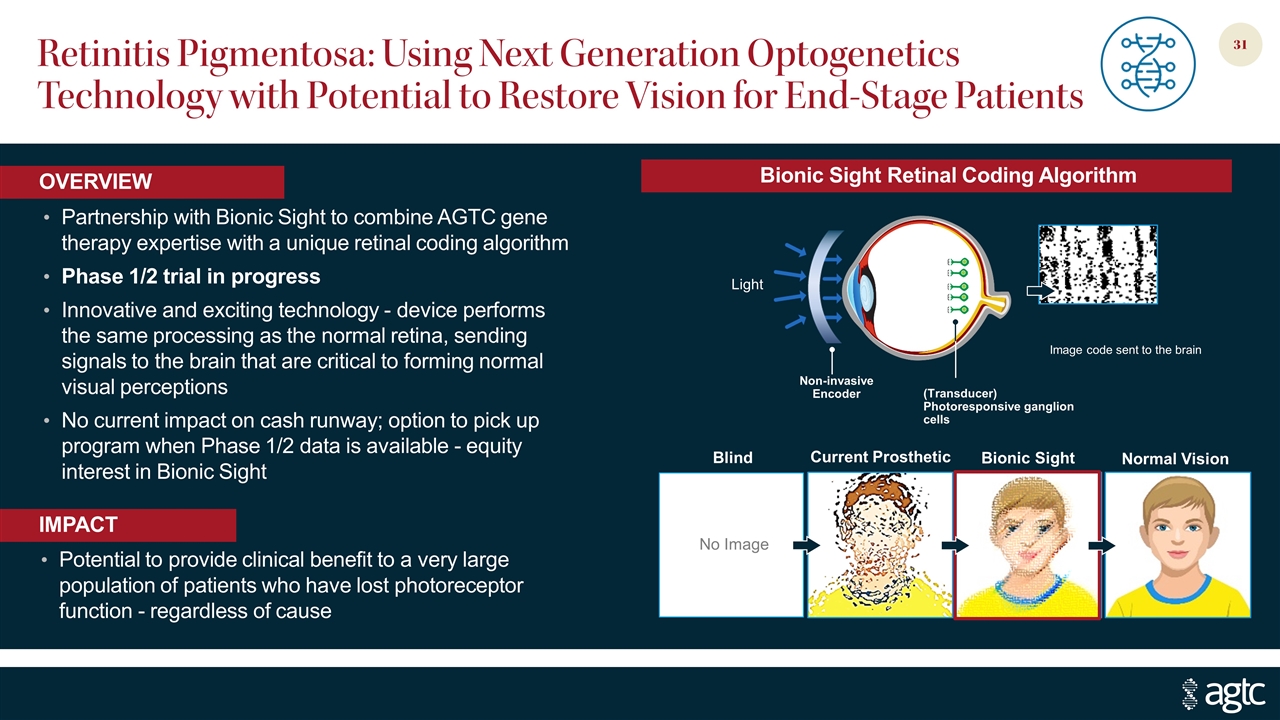
Retinitis Pigmentosa: Using Next Generation Optogenetics Technology with Potential to Restore Vision for End-Stage Patients Partnership with Bionic Sight to combine AGTC gene therapy expertise with a unique retinal coding algorithm Phase 1/2 trial in progress Innovative and exciting technology - device performs the same processing as the normal retina, sending signals to the brain that are critical to forming normal visual perceptions No current impact on cash runway; option to pick up program when Phase 1/2 data is available - equity interest in Bionic Sight Potential to provide clinical benefit to a very large population of patients who have lost photoreceptor function - regardless of cause IMPACT OVERVIEW (Transducer) Photoresponsive ganglion cells Image code sent to the brain Light Non-invasive Encoder Blind Normal Vision Bionic Sight Current Prosthetic Bionic Sight Retinal Coding Algorithm No Image
Dry Age-Related Macular Degeneration (dAMD) IMPACT OVERVIEW Dysregulated complement pathway is considered an important factor in the etiology of dAMD A single nucleotide polymorphism within CFH is linked to approximately 50% of all AMD cases Retinal expression of CFH and consequent delay of progression to geographic atrophy (or wet AMD) would reduce risk of blindness Estimated ~1M geographic atrophy patients in US alone Not being able to read is the one thing that is going to hurt me the most." Loss of central vision over time Leading cause of blindness in patients 65 years and older
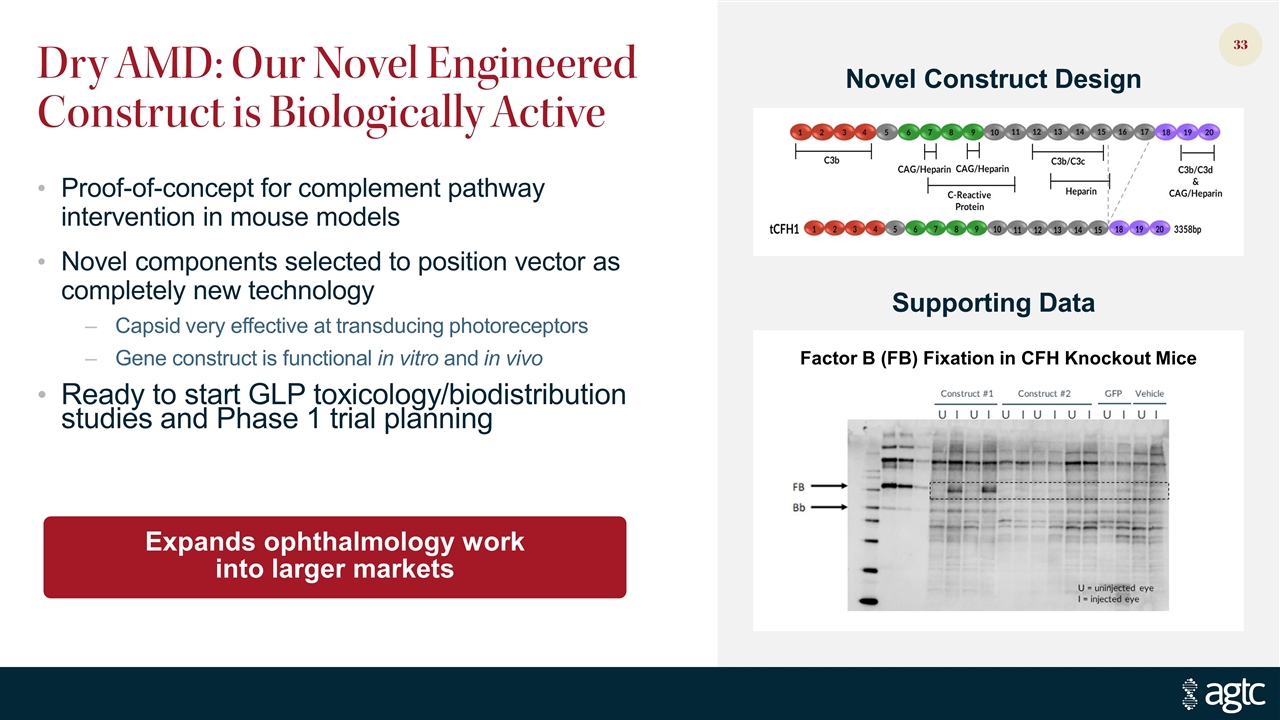
Dry AMD: Our Novel Engineered Construct is Biologically Active Proof-of-concept for complement pathway intervention in mouse models Novel components selected to position vector as completely new technology Capsid very effective at transducing photoreceptors Gene construct is functional in vitro and in vivo Ready to start GLP toxicology/biodistribution studies and Phase 1 trial planning Expands ophthalmology work into larger markets Factor B (FB) Fixation in CFH Knockout Mice Novel Construct Design Supporting Data

Stargardts Disease Losing your vision makes you gain a deeper appreciation of your life. Especially if you have a degenerative eye disease, because you don’t know how many more sunsets you’ll see, so I better enjoy this one.”. Unique engineered split gene POC in mouse model of disease and primate data showing appropriate recombination of the split gene to get full-length protein Progressive photoreceptor degeneration leading to blindness ~65,000 patients in US/EU No proven treatments IMPACT OVERVIEW Bilateral vision loss onset in childhood with slow progression over time, marked variability
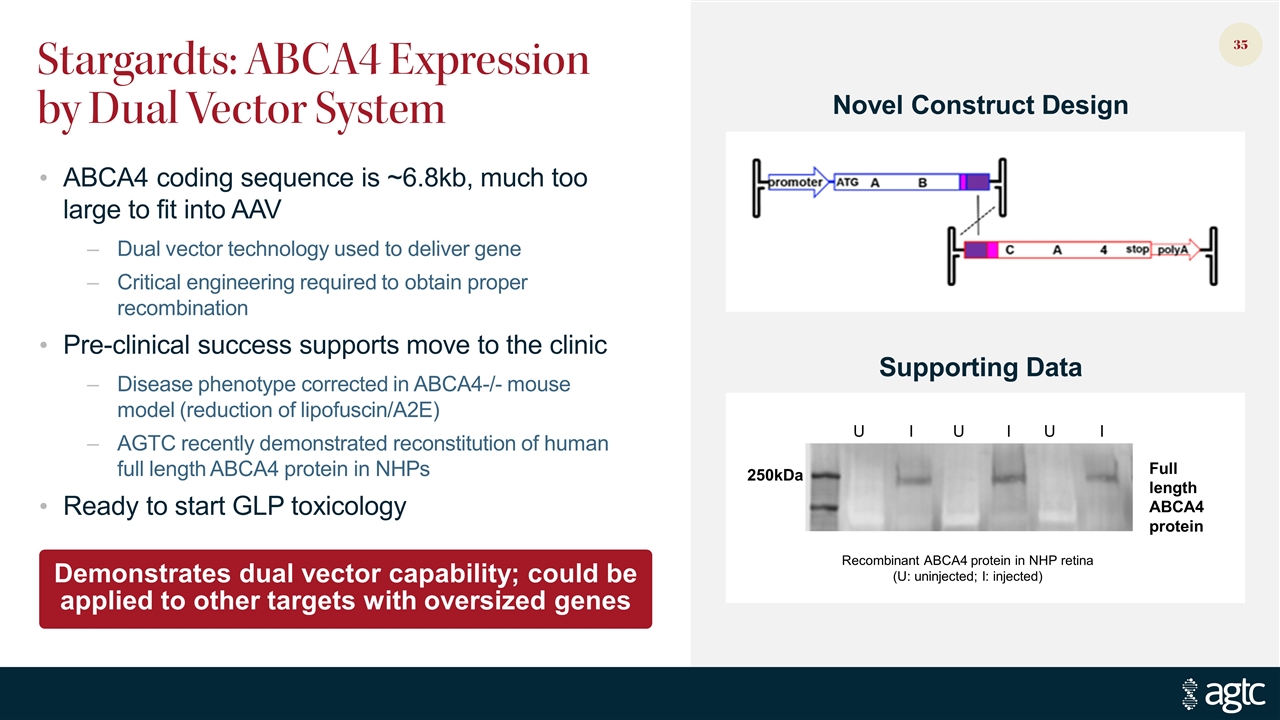
Stargardts: ABCA4 Expression by Dual Vector System ABCA4 coding sequence is ~6.8kb, much too large to fit into AAV Dual vector technology used to deliver gene Critical engineering required to obtain proper recombination Pre-clinical success supports move to the clinic Disease phenotype corrected in ABCA4-/- mouse model (reduction of lipofuscin/A2E) AGTC recently demonstrated reconstitution of human full length ABCA4 protein in NHPs Ready to start GLP toxicology U I U I U I 250kDa Full length ABCA4 protein Recombinant ABCA4 protein in NHP retina (U: uninjected; I: injected) Demonstrates dual vector capability; could be applied to other targets with oversized genes Novel Construct Design Supporting Data

Neurodegenerative Diseases

Frontotemporal Dementia (FTD) Mutations in the Progranulin (PGRN) gene are one of the three main genetic causes of FTD Representing approximately 20% of familial cases Gene augmentation approach by administration of AAV-PGRN into the cerebrospinal fluid No approved treatments Early-onset, fatal, neurodegenerative disorder that causes a spectrum of impairments in personality, cognitive ability, language and motor function Results in significant caregiver burden IMPACT OVERVIEW Imagine living your whole life and not remembering it. Imagine forgetting those you love the most. Imagine becoming as vulnerable as a small child. Imagine how terrifying it must be to have dementia."
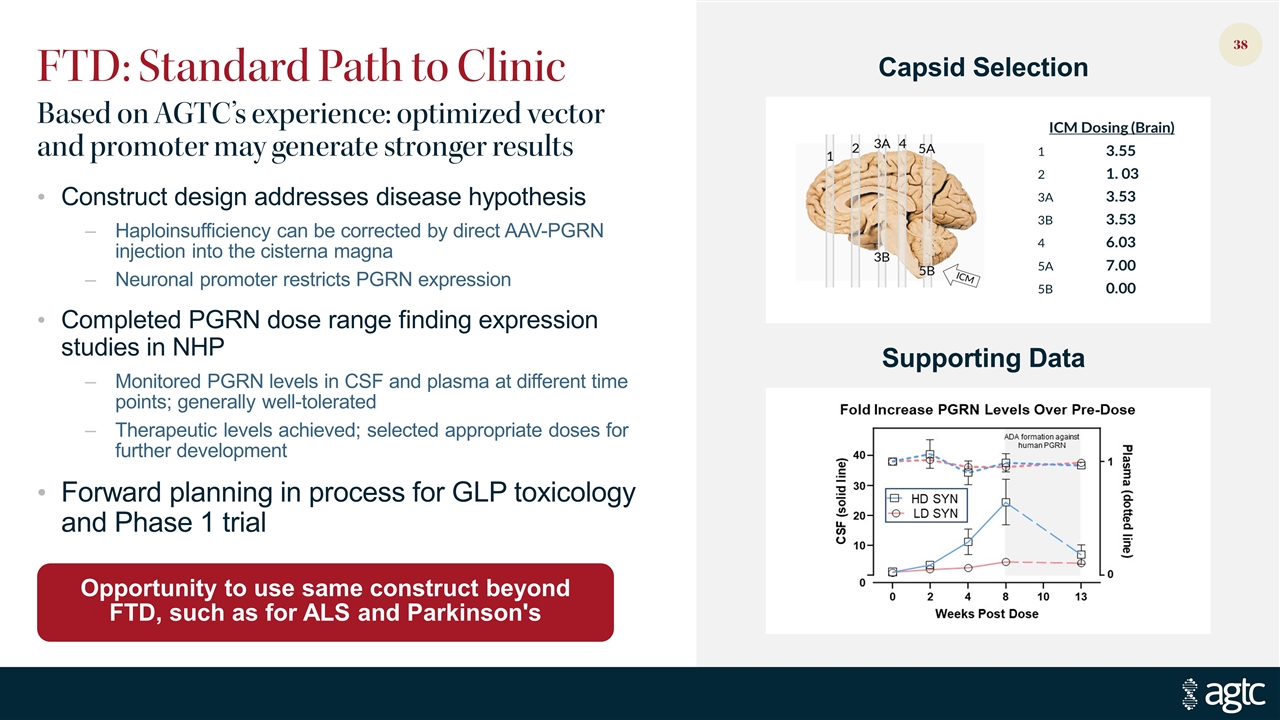
FTD: Standard Path to Clinic Construct design addresses disease hypothesis Haploinsufficiency can be corrected by direct AAV-PGRN injection into the cisterna magna Neuronal promoter restricts PGRN expression Completed PGRN dose range finding expression studies in NHP Monitored PGRN levels in CSF and plasma at different time points; generally well-tolerated Therapeutic levels achieved; selected appropriate doses for further development Forward planning in process for GLP toxicology and Phase 1 trial Opportunity to use same construct beyond FTD, such as for ALS and Parkinson's Based on AGTC’s experience: optimized vector and promoter may generate stronger results Capsid Selection Supporting Data

Amyotrophic Lateral Sclerosis (ALS) The C9orf72 gene has a six nucleotide repeat expansion that results in both gain-of-toxicity and loss-of-function Responsible for 30-40% of familial ALS AAV administration by intrathecal (lumbar) injection will allow targeting of spinal motor neurons No truly effective therapy Rapidly progressive neurodegenerative disease of motor neurons leading to wasting, paralysis, and eventual death from respiratory failure within 3 to 5 years. IMPACT OVERVIEW It’s been hard losing my physical self to the disease. It’s like a fresh flower losing its petals, slowly over time and piece by piece laying helpless on the floor.”
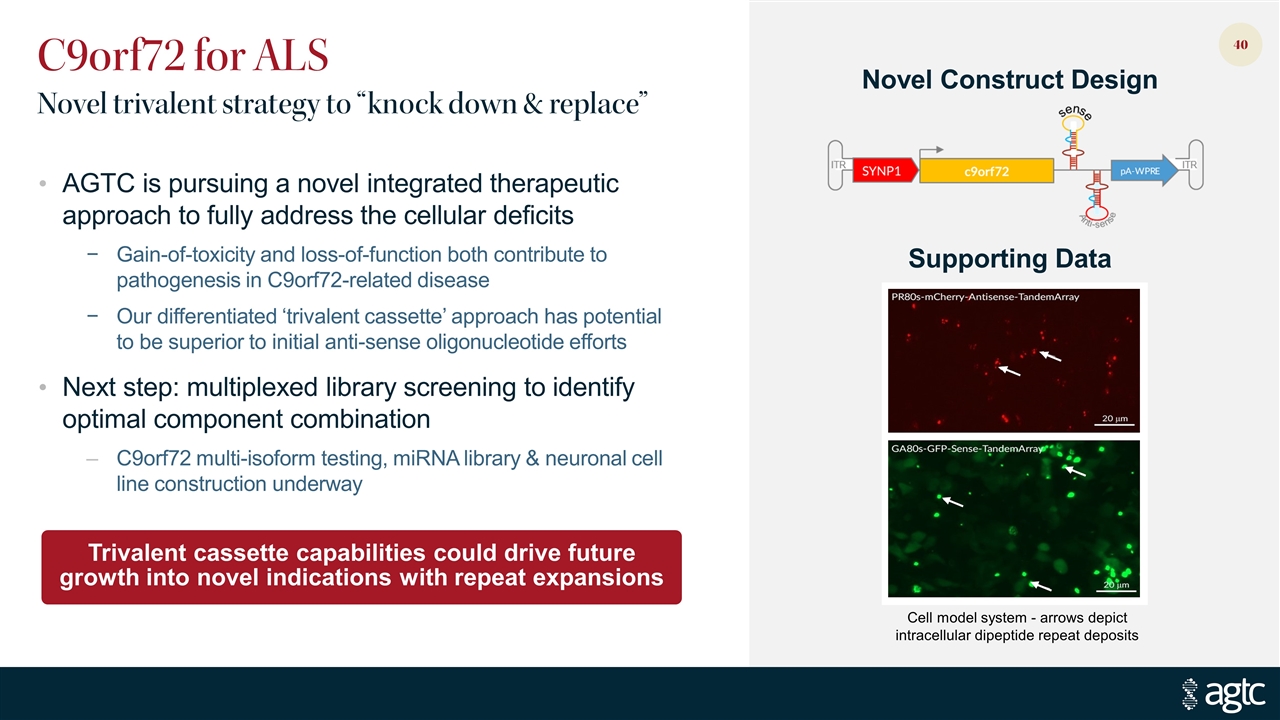
C9orf72 for ALS Novel trivalent strategy to “knock down & replace” AGTC is pursuing a novel integrated therapeutic approach to fully address the cellular deficits Gain-of-toxicity and loss-of-function both contribute to pathogenesis in C9orf72-related disease Our differentiated ‘trivalent cassette’ approach has potential to be superior to initial anti-sense oligonucleotide efforts Next step: multiplexed library screening to identify optimal component combination C9orf72 multi-isoform testing, miRNA library & neuronal cell line construction underway Trivalent cassette capabilities could drive future growth into novel indications with repeat expansions Novel Construct Design Supporting Data Cell model system - arrows depict intracellular dipeptide repeat deposits

Otology

Non-Syndromic Hearing Loss and Deafness GJB2 mutations responsible for ~50% genetic/non-syndromic hearing loss Estimated prevalence of 200K in the US & EU with 1-2K new cases/year in US Cochlear implants are currently the only existing treatment Severe-to-profound deafness in both ears will result in delayed or poor speech and language development, educational and occupational disadvantage, social isolation and stigmatization IMPACT OVERVIEW Gene therapy holds great promise to restore lasting hearing, with early intervention providing significant benefits in the development of language skills, socialization and overall quality of life for patients and their families."
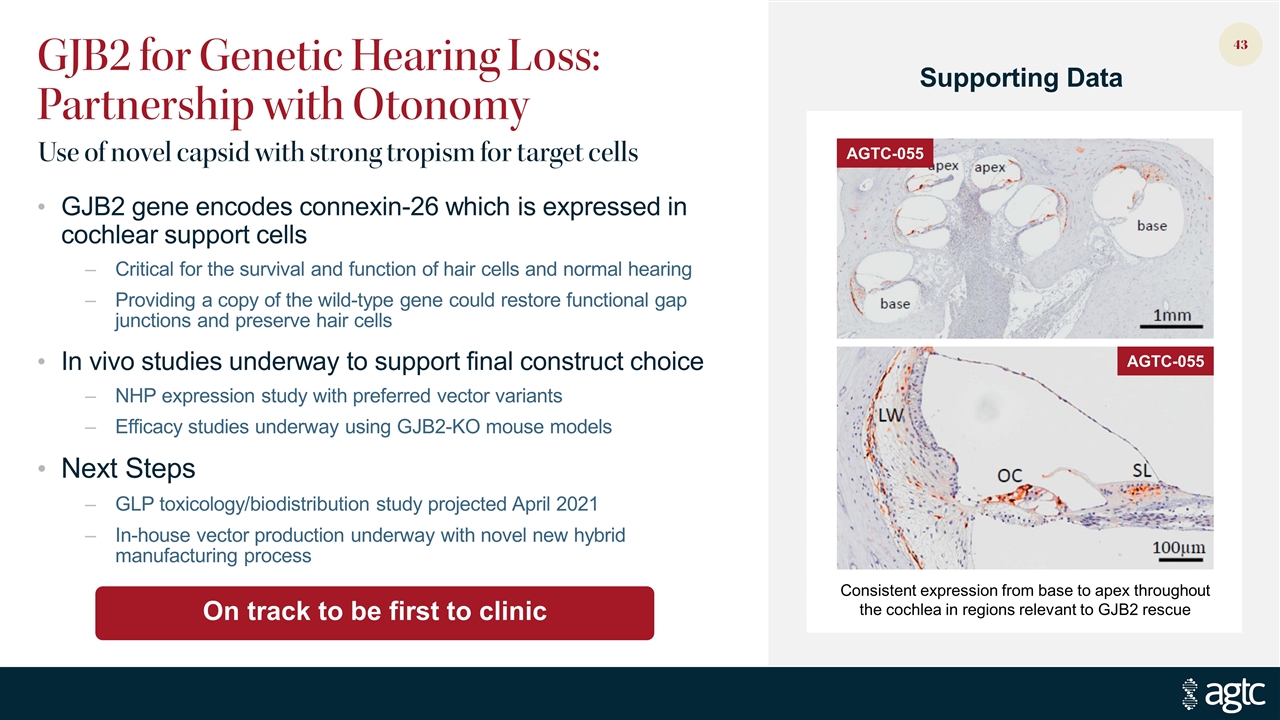
GJB2 for Genetic Hearing Loss: Partnership with Otonomy GJB2 gene encodes connexin-26 which is expressed in cochlear support cells Critical for the survival and function of hair cells and normal hearing Providing a copy of the wild-type gene could restore functional gap junctions and preserve hair cells In vivo studies underway to support final construct choice NHP expression study with preferred vector variants Efficacy studies underway using GJB2-KO mouse models Next Steps GLP toxicology/biodistribution study projected April 2021 In-house vector production underway with novel new hybrid manufacturing process On track to be first to clinic Supporting Data Consistent expression from base to apex throughout the cochlea in regions relevant to GJB2 rescue AGTC-055 AGTC-055 Use of novel capsid with strong tropism for target cells

Summary
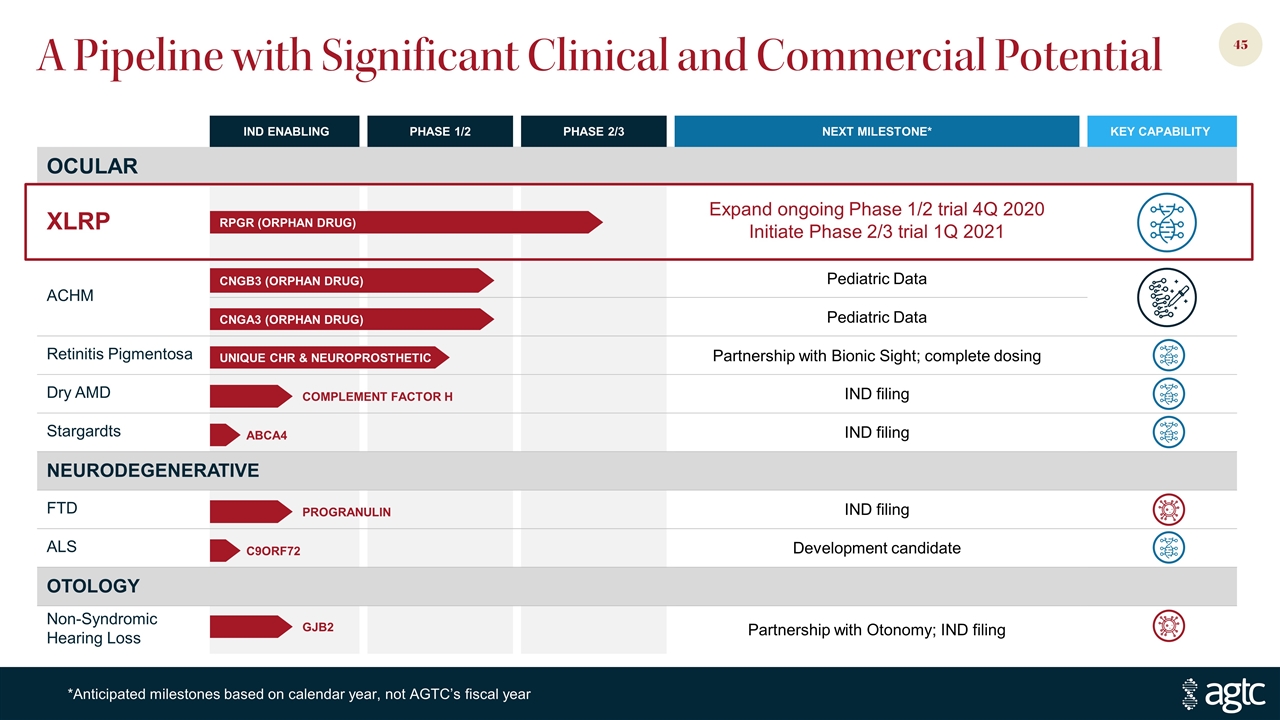
A Pipeline with Significant Clinical and Commercial Potential IND ENABLING PHASE 1/2 PHASE 2/3 NEXT MILESTONE* KEY CAPABILITY OCULAR XLRP Expand ongoing Phase 1/2 trial 4Q 2020 Initiate Phase 2/3 trial 1Q 2021 ACHM Pediatric Data Pediatric Data Retinitis Pigmentosa Partnership with Bionic Sight; complete dosing Dry AMD IND filing Stargardts IND filing NEURODEGENERATIVE FTD IND filing ALS Development candidate OTOLOGY Non-Syndromic Hearing Loss Partnership with Otonomy; IND filing *Anticipated milestones based on calendar year, not AGTC’s fiscal year CNGB3 (ORPHAN DRUG) RPGR (ORPHAN DRUG) CNGA3 (ORPHAN DRUG) UNIQUE CHR & NEUROPROSTHETIC COMPLEMENT FACTOR H ABCA4 PROGRANULIN C9ORF72 GJB2
Advanced Technology Achieves Best-in-Class Clinical Data Goal: Broaden the clinical portfolio by leveraging technology expertise with increased resources Diversified Pipeline XLRP Manufacturing Science Diversified across ophthalmology, neurodegenerative and otology indications Foundational data supports development of next-gen technology concepts Improvements in visual function sustained for at least six months No SAEs across 100-fold dose range 10-fold reduction in process residuals and 10-fold increase in productivity At commercial scale: 40 L ≥ 2000 ophthalmic doses Superior pre-clinical data Robust use of disease-specific animal modeling Differentiated With Demonstrated Capabilities Unmatched Productivity, Scalability & Quality Best-in-Class Late-Stage Asset High-Value Programs

Corporate Presentation














































