
Q2 2021 Financial Results Call February 11, 2021 Exhibit 99.2

Forward Looking Statements This presentation contains forward-looking statements that reflect Applied Genetic Technologies Corporation’s (“AGTC” or the “Company”) plans, estimates, assumptions and beliefs. Forward-looking statements include statements regarding AGTC’s proposed Phase 2/3 trial design for XLRP and the proposed Phase 1/2 expansion trial for XLRP, including the effectiveness of AGTC’s pre-selection methodology for pre-specifying loci, its anticipated timeline and potential to receive FDA approval to begin the XLRP pivotal trial, the timing for completing and reporting data in its XLRP and ACHM clinical programs, its ability to enroll patients for its clinical trials, the expected costs of its clinical programs and its planned manufacturing activities, business strategies and operations, regulatory progress, potential growth and market opportunities, and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as "anticipates," "believes," "could," "seeks," "estimates," "expects," "intends," "may," "plans," "potential," "predicts," "projects," "should," "will," "would" or similar expressions and the negatives of those terms. Actual results could differ materially from those discussed in the forward-looking statements, due to a number of important factors. Risks and uncertainties that may cause actual results to differ materially include, among others: AGTC cannot predict when or if it will obtain regulatory approval to continue to progress its clinical trials, commercialize a product candidate or receive reasonable reimbursement; uncertainty inherent in clinical trials and the regulatory review process; risks and uncertainties associated with drug development and commercialization; risks related to the COVID-19 outbreak that may delay clinical trial enrollment; gene therapy is still novel with only a few approved treatments so far; factors that could cause actual results to differ materially from those described in the forward-looking statements are set forth under the heading "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent periodic reports filed with the SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent management's plans, estimates, assumptions and beliefs only as of the date of this presentation. Except as required by law, AGTC assumes no obligation to update or revise these forward-looking statements publicly, whether as a result of new information, new events or otherwise.

Call Agenda Recent accomplishment and latest developments – Sue Washer, CEO Clinical program update – Dr. Mark S. Shearman, CSO Q2 2021 financial results review – Bill Sullivan, CFO Q&A Closing Remarks – Sue Washer

Recent Accomplishments ACHM study is the first to report sensitivity improvement following treatment January 2021 results support the previously presented positive patient-reported outcomes and provide a path forward to collect additional data to fully realize the potential of our ACHM therapies Reported additional positive data from the Phase 1/2 XLRP clinical trial November 2020 data indicated durable improvements observed in visual sensitivity and visual acuity over a wide dose range with a favorable safety profile out to month 12 in two of the dose groups AGTC believes the Company has a best-in-class product that may provide significant benefit to patients with XLRP Successfully completed a public offering of common stock and warrants, raising approximately $69 million in net proceeds Current cash runway into calendar year 2023

ACHM Clinical Program Update
ACHM: Encouraging Early Data Support Continued Clinical Development Product well-tolerated Supported across both ACHM and XLRP trials with same capsid Dose escalation across 80-fold range Preliminary signs of biologic activity Disease affects visual cortex pathway Need to: Follow patients for longer time periods Follow patients dosed at higher doses Enroll pediatric patients New static perimetry data Improved visual sensitivity exceeding test repeatability at last visit: B3: 7/16, A3: 3/16. In both cases at higher doses Supported in some cases by mfERG and patient reports Further analysis ongoing and planned release of 12-month adult data in 2Q of 2021 Robust body of pre-clinical data, favorable safety profile and preliminary evidence of biological activity in both trials builds confidence in product potential
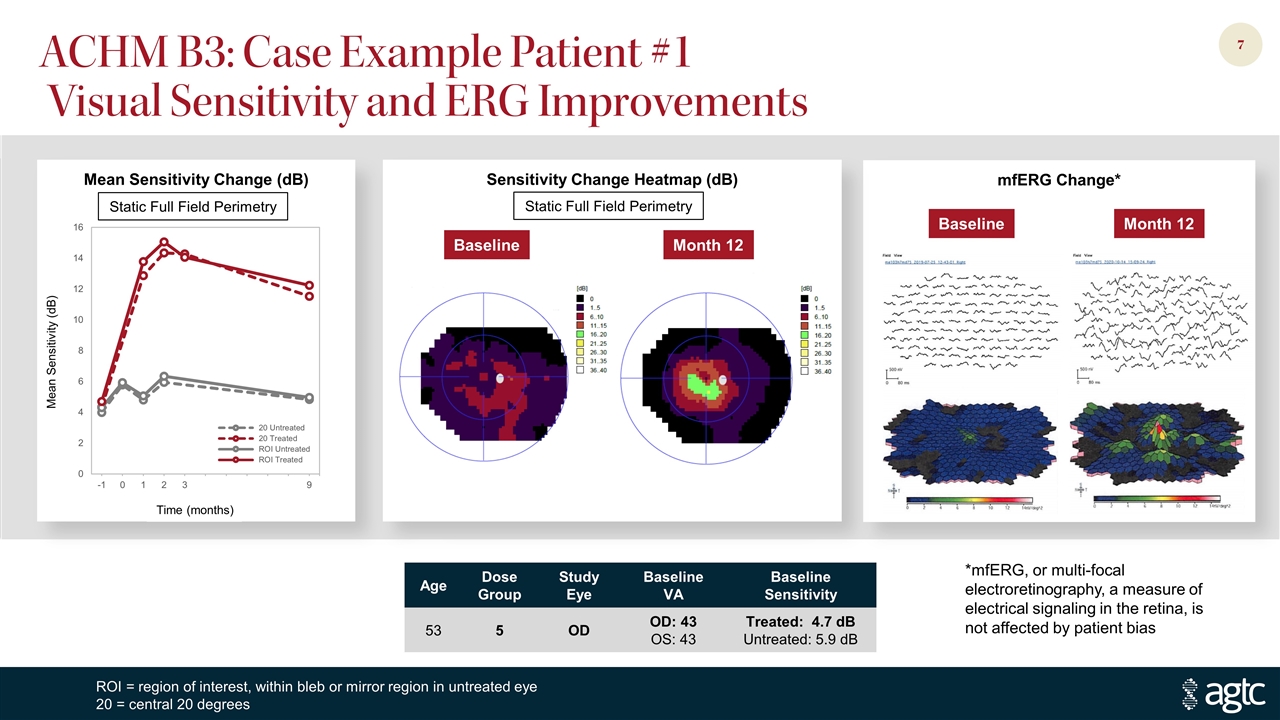
ACHM B3: Case Example Patient #1 Visual Sensitivity and ERG Improvements Age Dose Group Study Eye Baseline VA Baseline Sensitivity 53 5 OD OD: 43 OS: 43 Treated: 4.7 dB Untreated: 5.9 dB Mean Sensitivity Change (dB) mfERG Change* Sensitivity Change Heatmap (dB) Baseline Month 12 ROI = region of interest, within bleb or mirror region in untreated eye 20 = central 20 degrees Static Full Field Perimetry Baseline Month 12 *mfERG, or multi-focal electroretinography, a measure of electrical signaling in the retina, is not affected by patient bias Mean Sensitivity (dB) Time (months) Static Full Field Perimetry
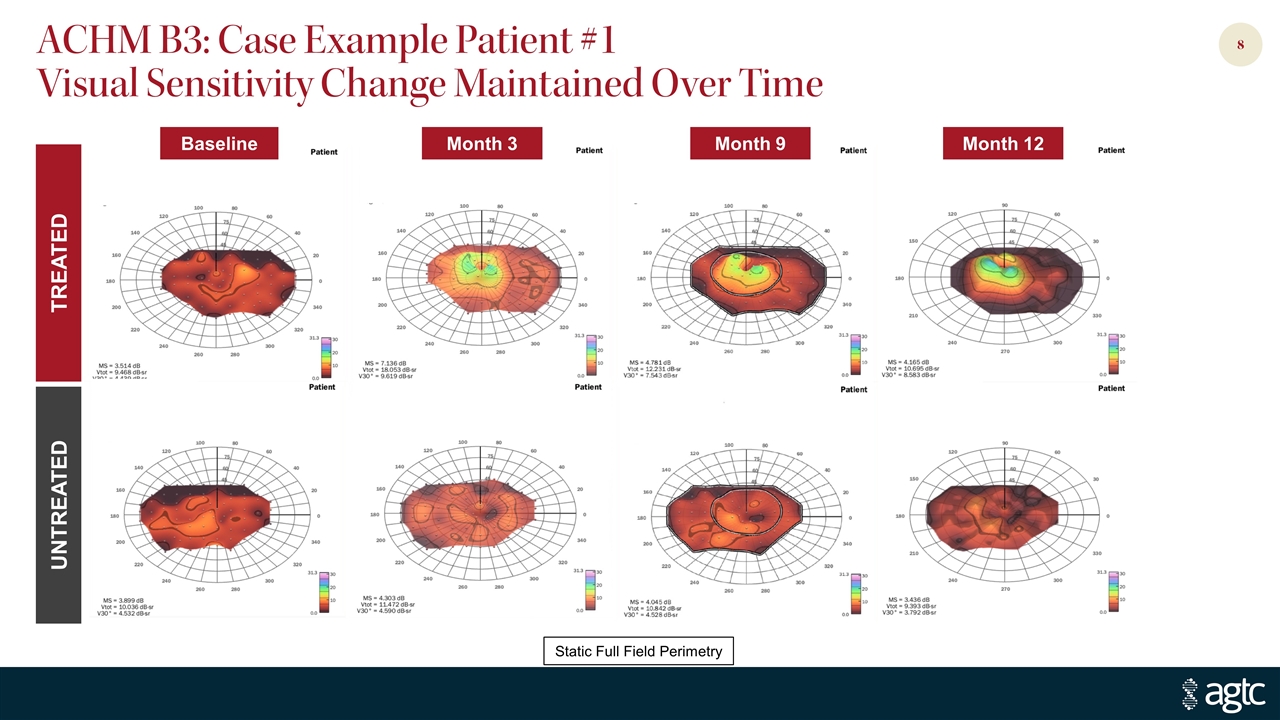
UNTREATED TREATED ACHM B3: Case Example Patient #1 Visual Sensitivity Change Maintained Over Time Month 12 Month 9 Month 3 Baseline Static Full Field Perimetry
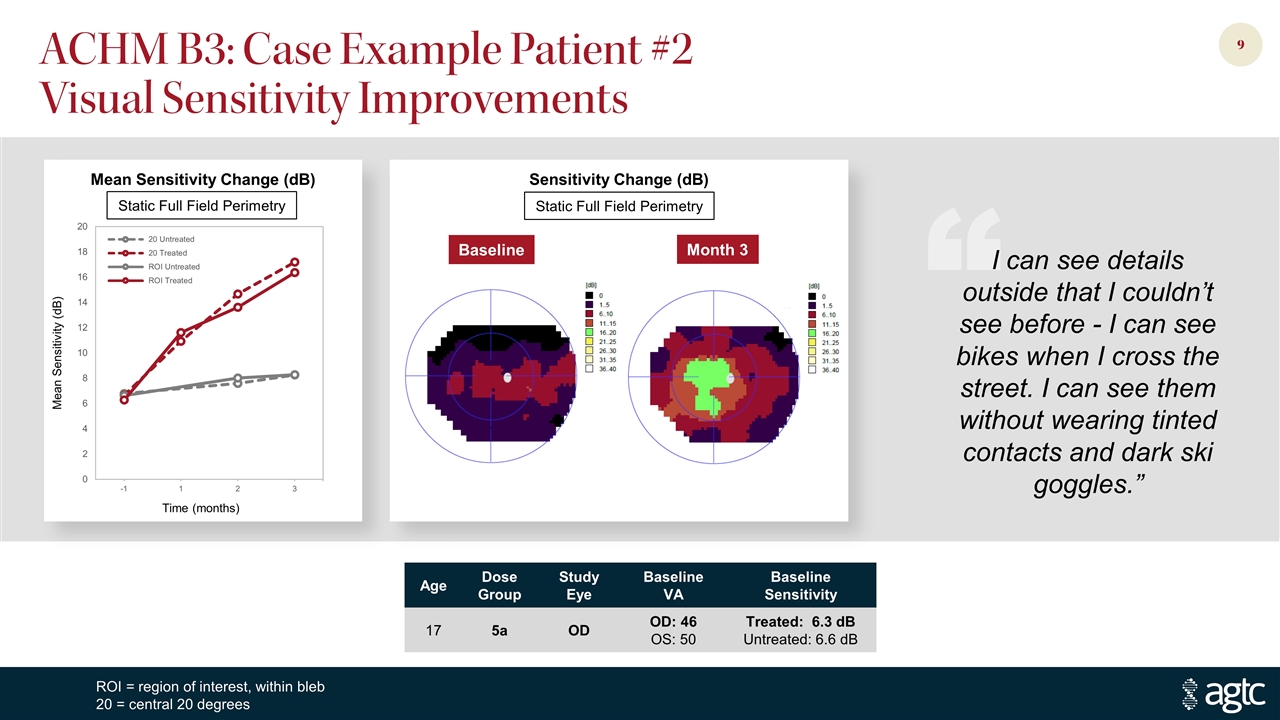
ACHM B3: Case Example Patient #2 Visual Sensitivity Improvements Age Dose Group Study Eye Baseline VA Baseline Sensitivity 17 5a OD OD: 46 OS: 50 Treated: 6.3 dB Untreated: 6.6 dB ROI = region of interest, within bleb 20 = central 20 degrees Mean Sensitivity Change (dB) Sensitivity Change (dB) Static Full Field Perimetry Baseline Month 3 Mean Sensitivity (dB) Time (months) Static Full Field Perimetry “ I can see details outside that I couldn’t see before - I can see bikes when I cross the street. I can see them without wearing tinted contacts and dark ski goggles.”
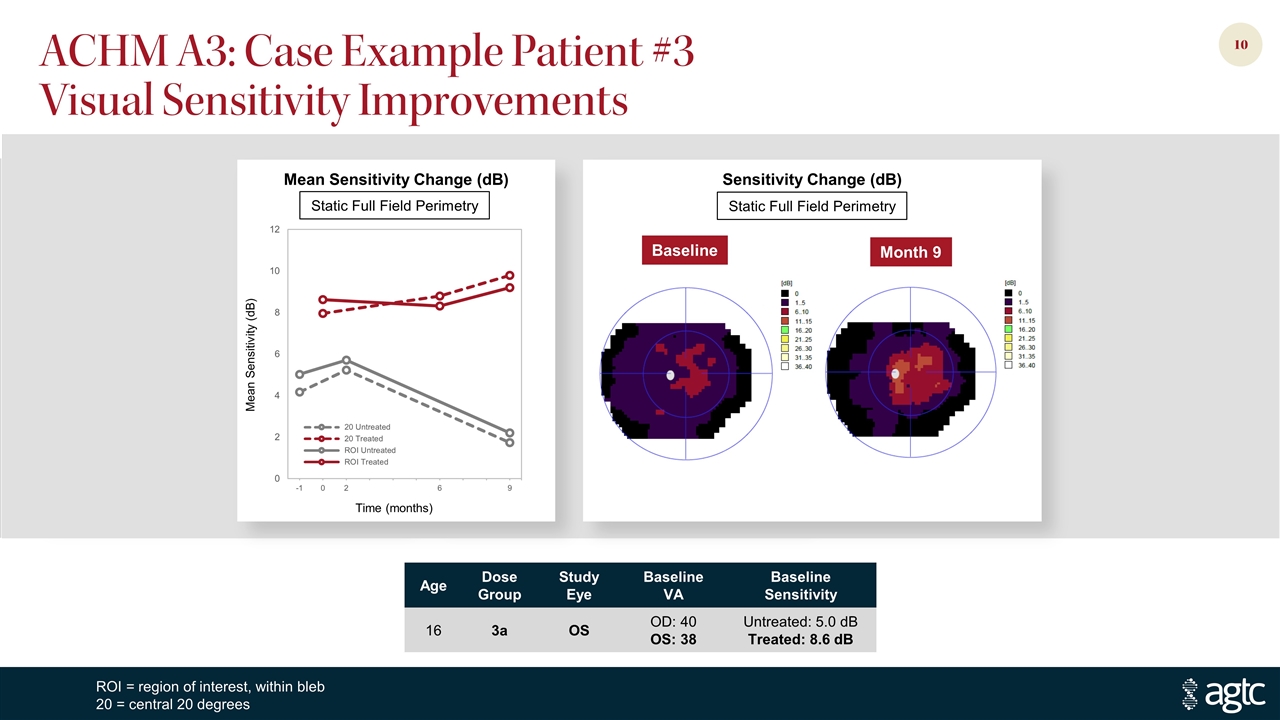
ACHM A3: Case Example Patient #3 Visual Sensitivity Improvements Age Dose Group Study Eye Baseline VA Baseline Sensitivity 16 3a OS OD: 40 OS: 38 Untreated: 5.0 dB Treated: 8.6 dB Mean Sensitivity Change (dB) Sensitivity Change (dB) ROI = region of interest, within bleb 20 = central 20 degrees Static Full Field Perimetry Baseline Month 9 Mean Sensitivity (dB) Time (months) Static Full Field Perimetry
AGTC’s Achromatopsia Study is the First To Report Visual Sensitivity Improvement Following Treatment Further in-depth patient by patient analysis provides clarity on path forward Focus on static perimetry endpoint as early sign of product activity resulting in cone reactivation New data to be collected Reduced age range to focus on younger pediatric patients fMRI imaging to directly measure changes in neural pathways Novel color brightness test to better quantify reported changes in color perception

ACHM: Next Steps Planning for Success – Developing Submission for Type C Meeting with FDA for Initial Late-Stage Protocol Development Dose Pediatric Patients Incorporates updated testing: color brightness test and fMRI Could be impacted by COVID delays Adult Patient 12-Month Data 2Q 2021 for all adult patients Pediatric Patient 3-Month Data 4Q 2021 for all pediatric patients

XLRP Clinical Program Update
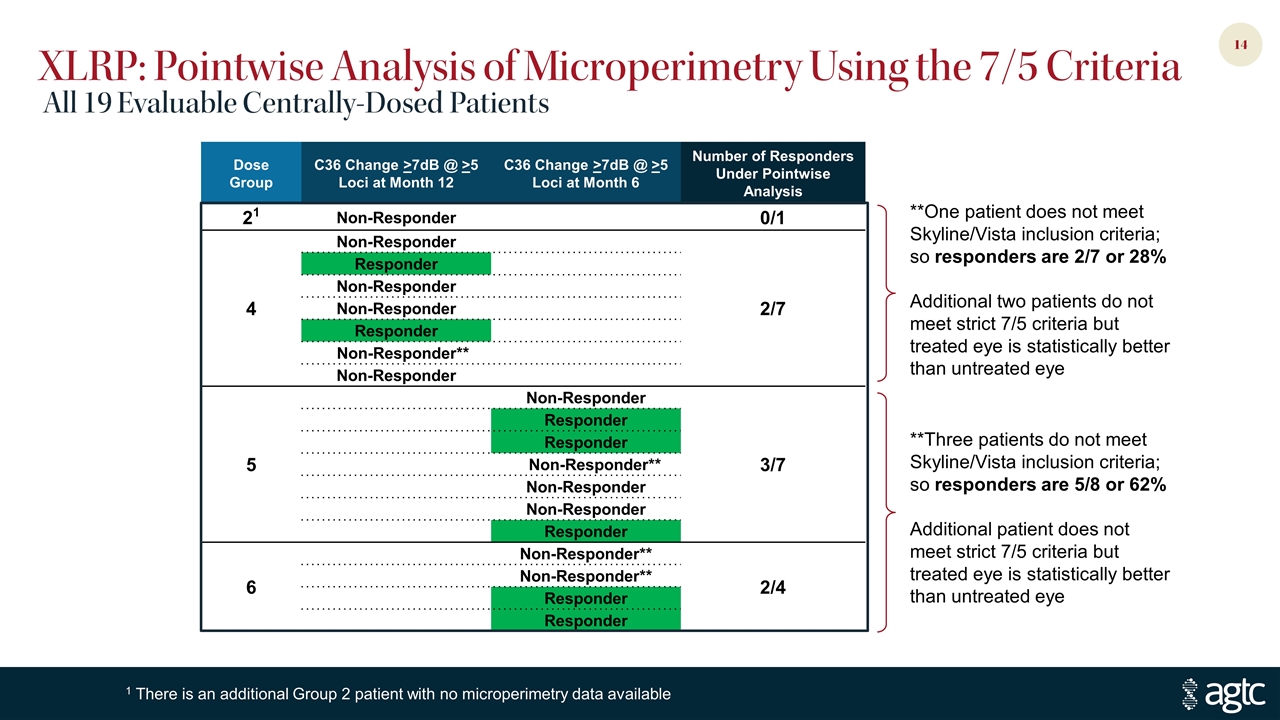
Dose Group C36 Change >7dB @ >5 Loci at Month 12 C36 Change >7dB @ >5 Loci at Month 6 Number of Responders Under Pointwise Analysis 21 Non-Responder 0/1 4 Non-Responder 2/7 Responder Non-Responder Non-Responder Responder Non-Responder** Non-Responder 5 Non-Responder 3/7 Responder Responder Non-Responder** Non-Responder Non-Responder Responder 6 Non-Responder** 2/4 Non-Responder** Responder Responder XLRP: Pointwise Analysis of Microperimetry Using the 7/5 Criteria All 19 Evaluable Centrally-Dosed Patients 1 There is an additional Group 2 patient with no microperimetry data available **Three patients do not meet Skyline/Vista inclusion criteria; so responders are 5/8 or 62% Additional patient does not meet strict 7/5 criteria but treated eye is statistically better than untreated eye **One patient does not meet Skyline/Vista inclusion criteria; so responders are 2/7 or 28% Additional two patients do not meet strict 7/5 criteria but treated eye is statistically better than untreated eye
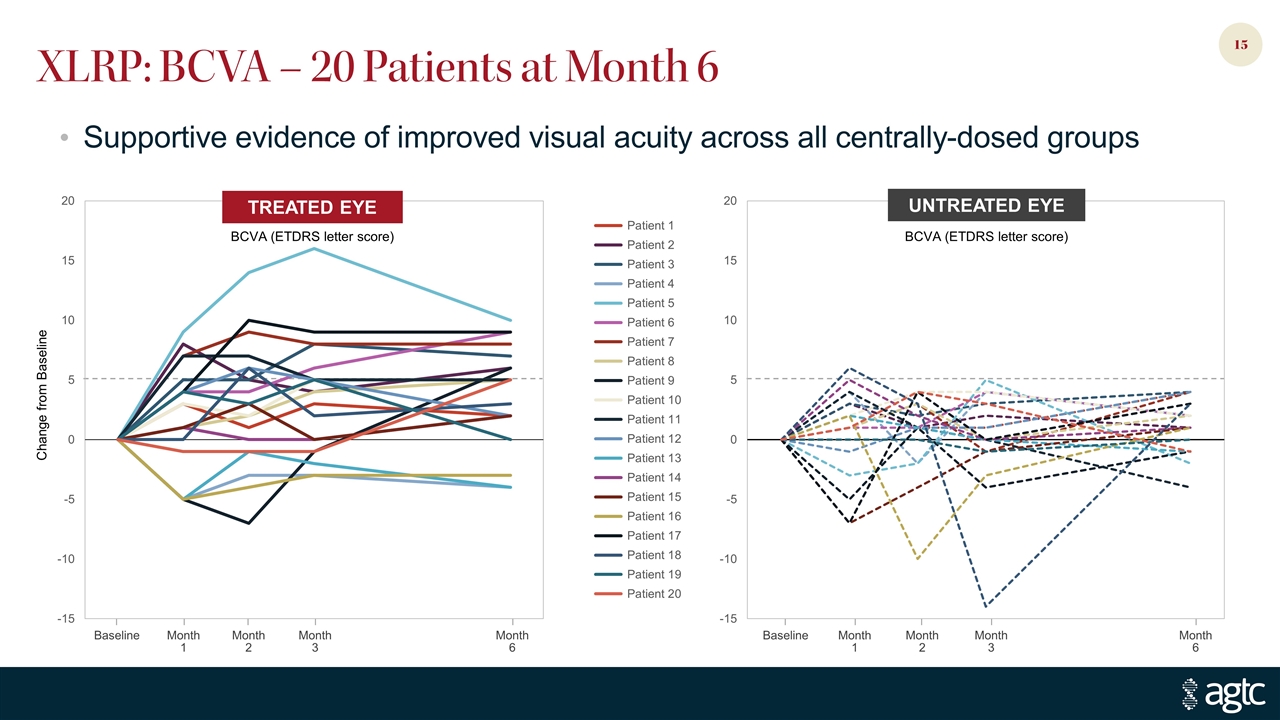
Change from Baseline Baseline Month 1 Month 2 Month 3 Month 6 Baseline Month 1 Month 2 Month 3 Month 6 XLRP: BCVA – 20 Patients at Month 6 TREATED EYE BCVA (ETDRS letter score) UNTREATED EYE BCVA (ETDRS letter score) Supportive evidence of improved visual acuity across all centrally-dosed groups
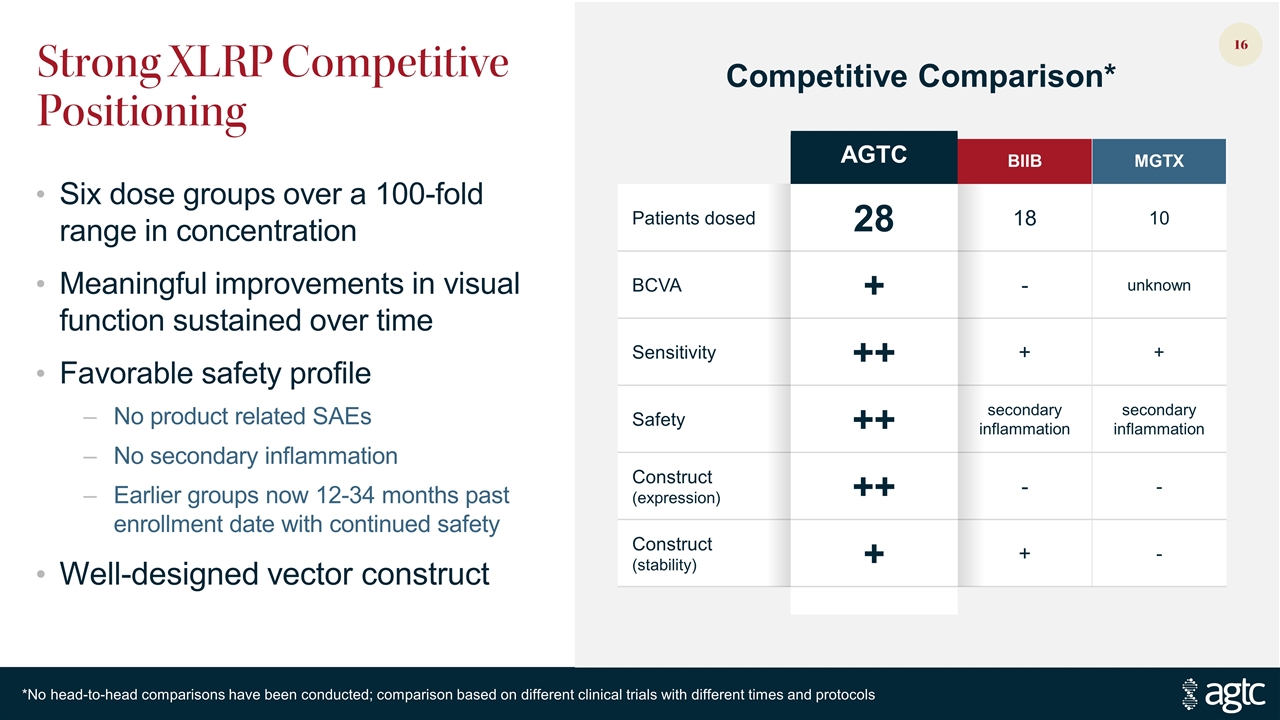
Strong XLRP Competitive Positioning Six dose groups over a 100-fold range in concentration Meaningful improvements in visual function sustained over time Favorable safety profile No product related SAEs No secondary inflammation Earlier groups now 12-34 months past enrollment date with continued safety Well-designed vector construct AGTC BIIB MGTX Patients dosed 28 18 10 BCVA + - unknown Sensitivity ++ + + Safety ++ secondary inflammation secondary inflammation Construct (expression) ++ - - Construct (stability) + + - Competitive Comparison* *No head-to-head comparisons have been conducted; comparison based on different clinical trials with different times and protocols
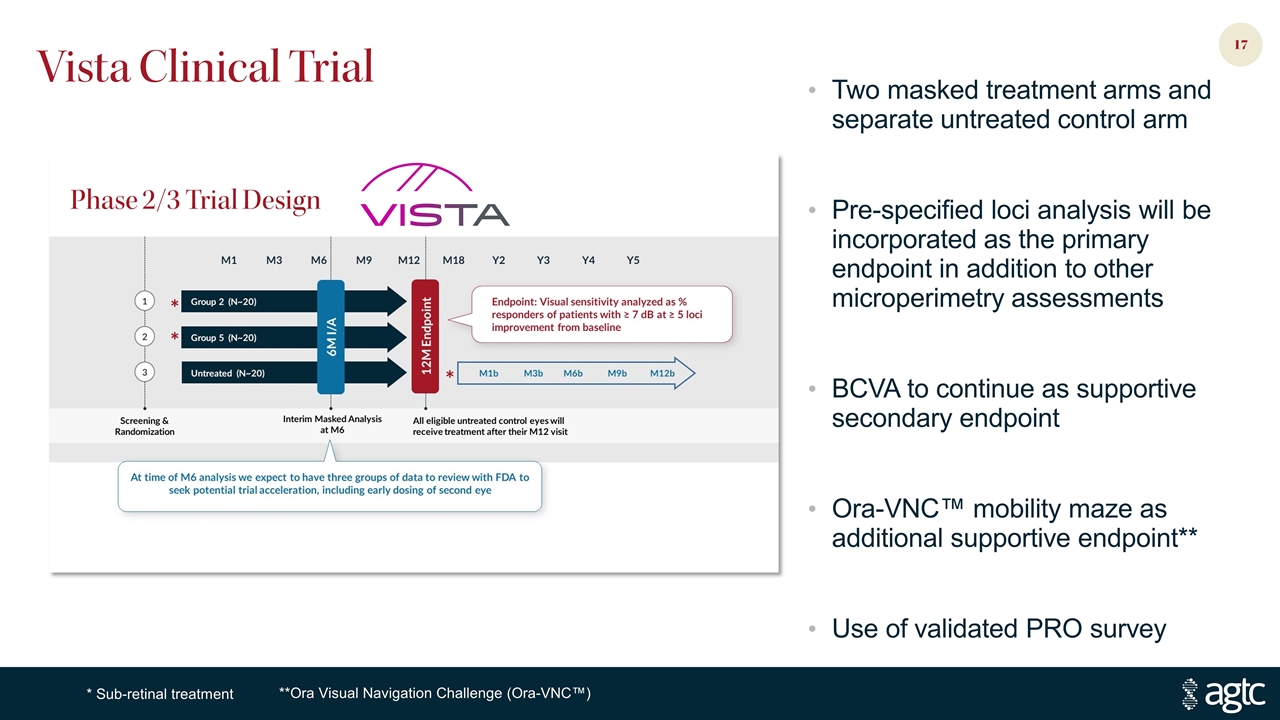
Vista Clinical Trial **Ora Visual Navigation Challenge (Ora-VNC™) Two masked treatment arms and separate untreated control arm Pre-specified loci analysis will be incorporated as the primary endpoint in addition to other microperimetry assessments BCVA to continue as supportive secondary endpoint Ora-VNC™ mobility maze as additional supportive endpoint** Use of validated PRO survey * Sub-retinal treatment Phase 2/3 Trial Design
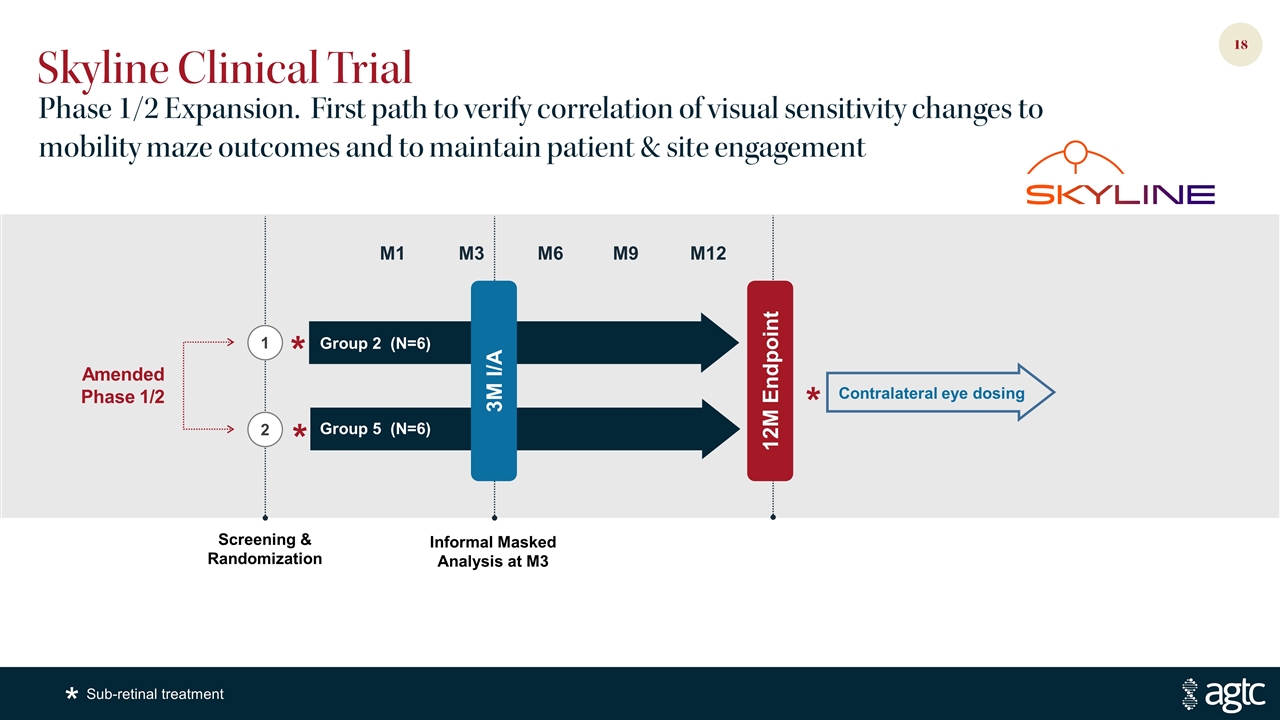
Skyline Clinical Trial Phase 1/2 Expansion. First path to verify correlation of visual sensitivity changes to mobility maze outcomes and to maintain patient & site engagement Contralateral eye dosing Amended Phase 1/2 * * * 3M I/A Informal Masked Analysis at M3 M12 M3 Group 2 (N=6) Group 5 (N=6) M6 M1 M9 Screening & Randomization 2 1 12M Endpoint Sub-retinal treatment *

XLRP Summary: Superior Expertise Leading to Potential Best-in-Class Clinical Data Expertise led to clinical success to date Optimized product design, breadth of pre-clinical data supported Well tolerated safety profile allowing higher dosing Sustained and robust improvements in visual function Patient focus generated creative clinical solutions, including continued data collection during pandemic Improved manufacturing process No further process development or scale-up required to support commercial launch Improved purification process supports clinical safety profile with low inflammation Intermediate analysis points create opportunities for future acceleration 2021: 2Q: 12-month data for Phase 1/2 dose groups 5 and 6 4Q: 3-month data for Skyline Trial (first opportunity to review Maze data) 2022: 3Q: 6-month data for Vista Trial 3Q: 12-month data for Skyline Trial 2 1 3

Financial Results Q2 2021 net loss of $15.5 million compared to a net loss of $8.6 million in Q2 2020 Strong balance sheet $53.1 million in cash, cash equivalents, and investments as of December 31, 2020 Received net proceeds of approximately $69 million in February 2021 from an underwritten public offering Current liquidity provides cash runway into calendar year 2023

Q&A

Anticipated Milestones Q2 2021 XLRP 12-month data for Phase 1/2 dose groups 5 and 6 ACHM 12-month data from all adult patients Q4 2021 Skyline 3-month data ACHM 3-month pediatric data Q3 2022 6-month Vista data 12-month Skyline data

Corporate Presentation February 2021






















