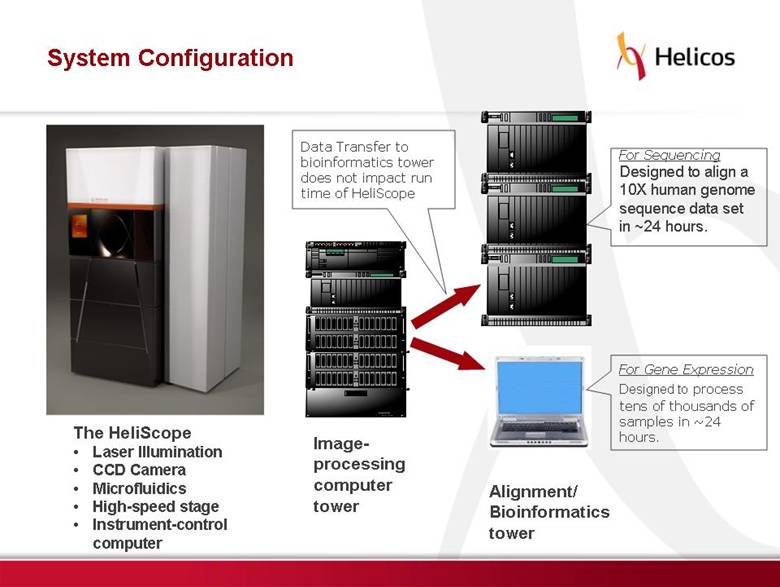
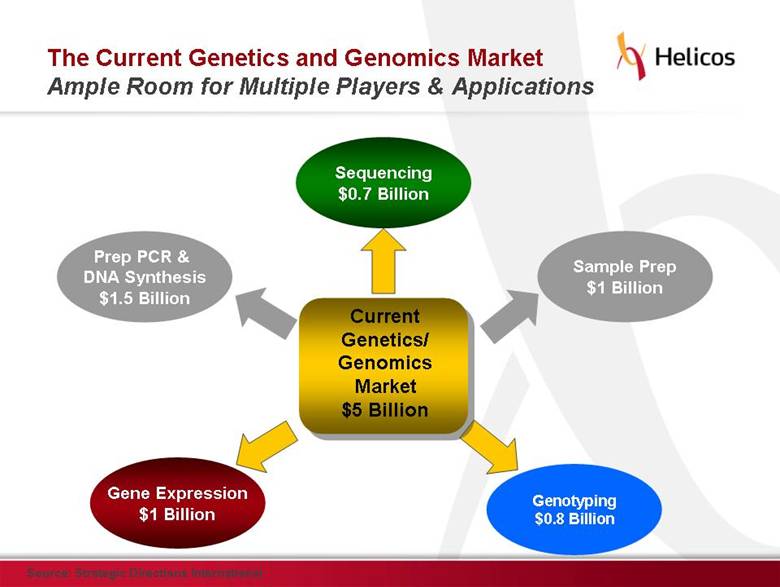
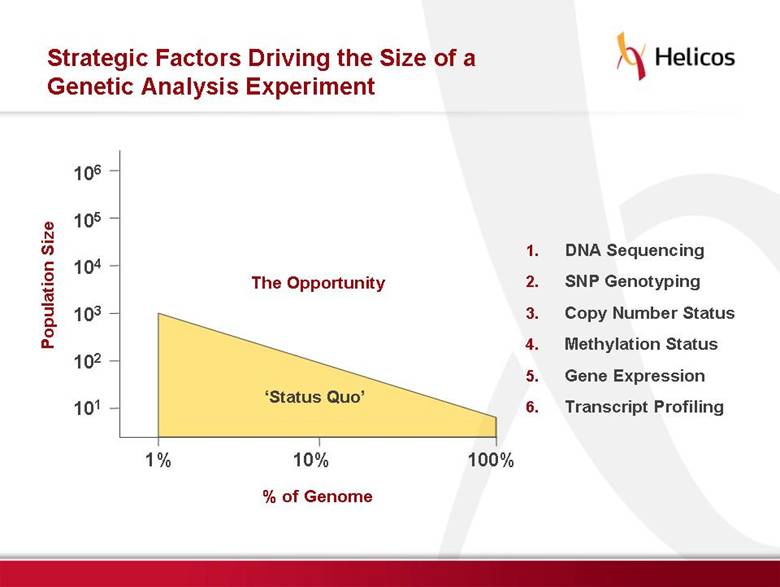
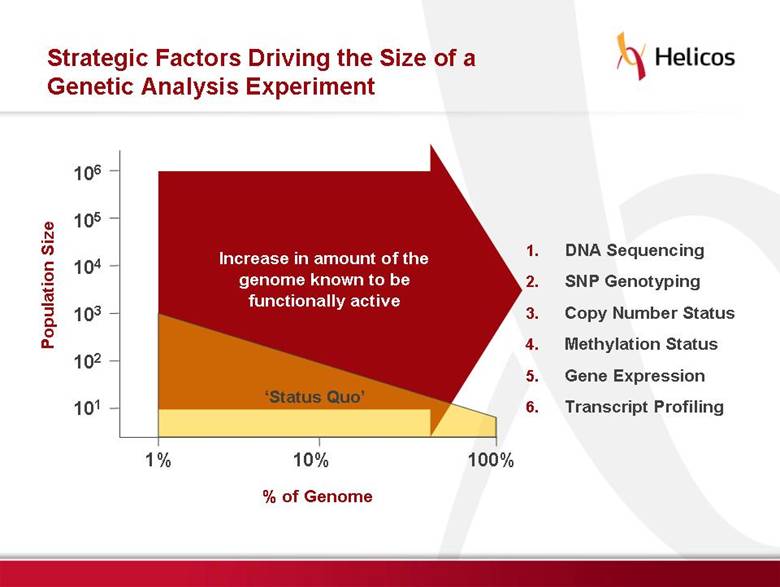
Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Filing exhibits
HLCSQ similar filings
- 1 Nov 07 Helicos BioSciences Reports Third Quarter 2007 Financial Results
- 3 Oct 07 Departure of Directors or Principal Officers
- 18 Sep 07 Regulation FD Disclosure
- 15 Aug 07 Investor Presentation August 2007 © 2007 Helicos BioSciences Corporation
- 10 Aug 07 Departure of Directors or Principal Officers
- 31 Jul 07 Helicos BioSciences Reports Second Quarter 2007 Financial Results
- 29 Jun 07 Other Events
Filing view
External links