Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Ocera Therapeutics similar filings
- 17 Dec 12 Tranzyme Pharma’s DIGEST Trial Stopped for Futility After Interim Analysis of the Phase 2b Results
- 5 Dec 12 Tranzyme Pharma Announces Resignation of Chief Financial Officer
- 4 Dec 12 Termination of a Material Definitive Agreement
- 28 Nov 12 Regulation FD Disclosure
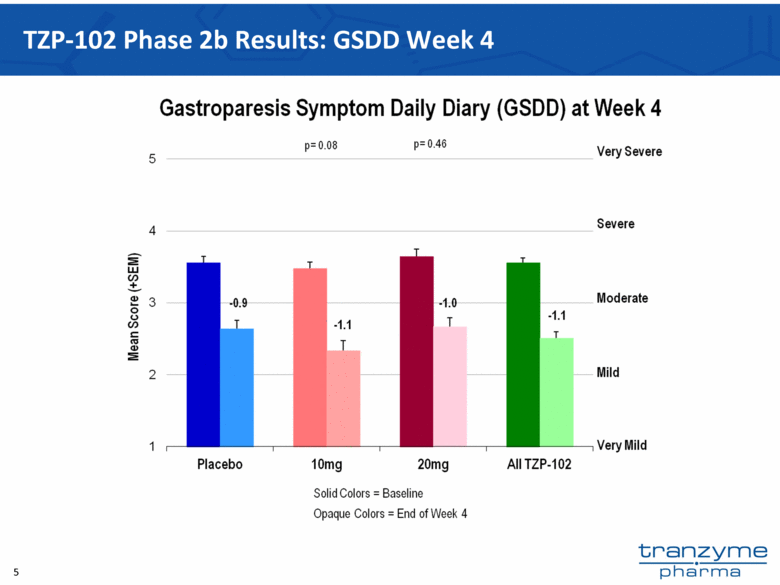
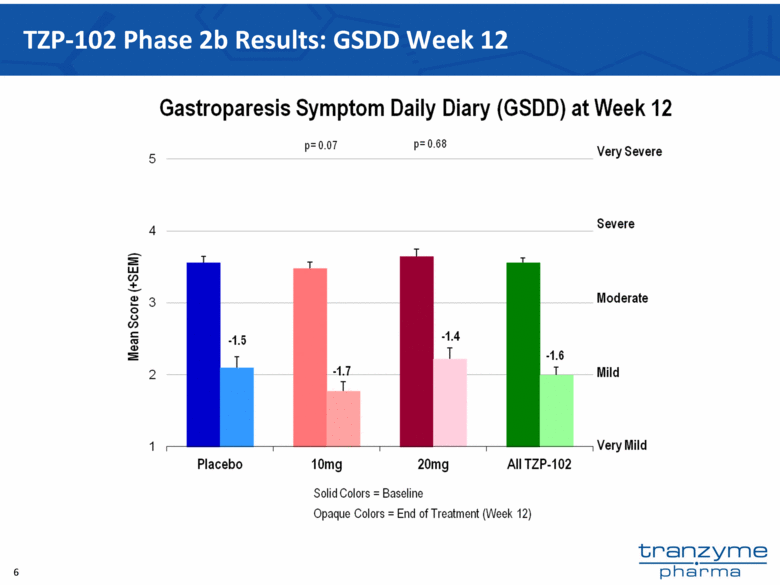
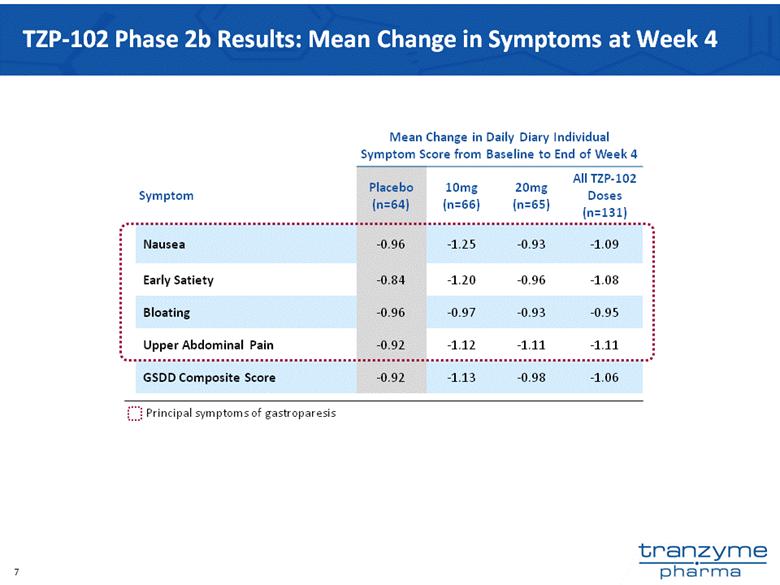
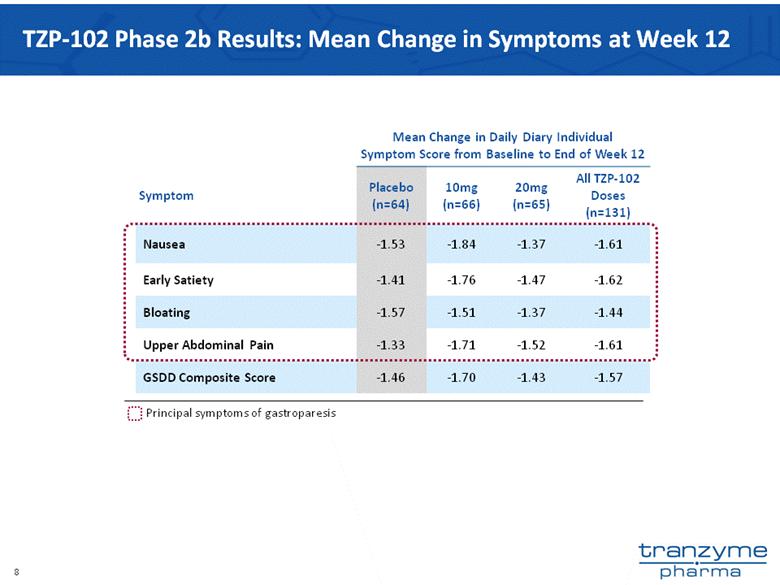
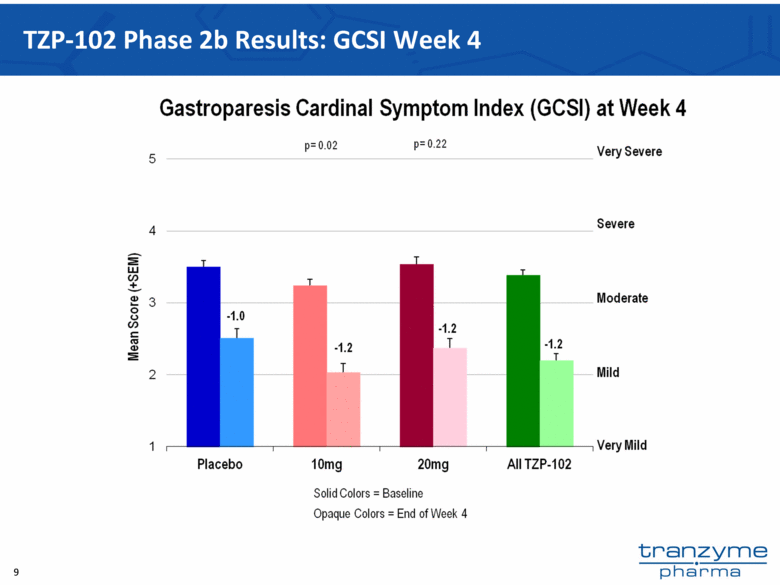
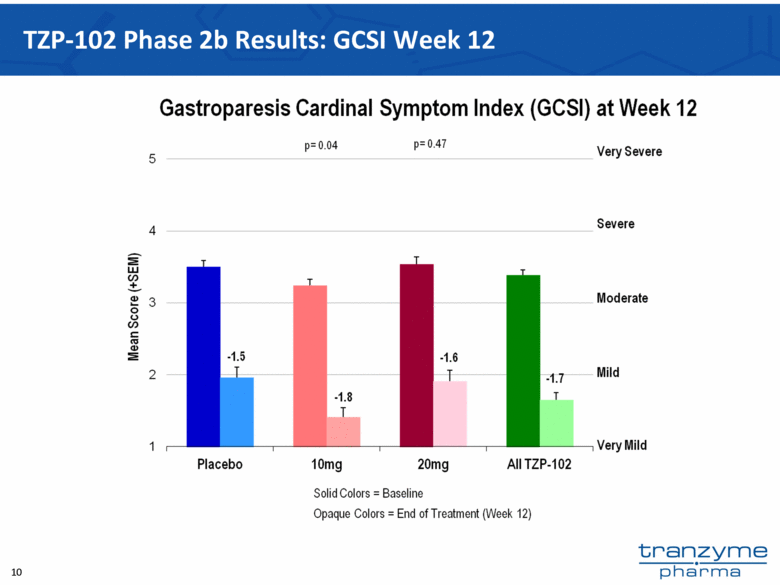
- 15 Nov 12 Tranzyme Pharma Announces Top-Line Results of TZP-102 Phase 2b Trial
- 8 Nov 12 Tranzyme Pharma Announces Third Quarter 2012 Financial Results
- 13 Sep 12 Tranzyme Pharma Raises $10 Million in Registered Direct Offering
Filing view
External links