
A Liver Disease Medicines Company NASDAQ: OCRX J A N UA R Y 2 0 1 7

2 Forward‐Looking Statements Certain statements in this presentation constitute “forward‐looking statements” within the meaning of the Securities Act of 1933, as amended (the “Securities Act”), and Securities Exchange Act of 1934, as amended (“Exchange Act”), including, without limitation, all statements related to the OCR‐002 clinical development program, including patient enrollment estimates, expected timing for the receipt of clinical data, the potential success of OCR‐002 in clinical trials, the size of the potential market opportunity for OCR‐002, as well as cash projections, and we intend these forward‐looking statements to be covered by the safe harbor provisions for forward‐looking statements contained in the Securities Act and the Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward‐looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward‐looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward‐looking statements and will be affected by a variety of risks and factors that are beyond our control, including those risks and uncertainties discussed under “Risk Factors” in our Annual Report on form 10‐K for the year ended December 31, 2015, as well as other risks detailed in our subsequent filings with the SEC. All information in this presentation is as of the date of this presentation, and we undertake no duty to update this information unless required by law.

3 Seeking to Provide Continuity of Care from Treatment to Prevention Developing Therapeutics for Liver Diseases Lead Program OCR‐002 Ammonia Scavenger for Hepatic Encephalopathy (HE) IV‐Phase 2b ACUTE Oral‐Phase 1 CHRONIC
4 Investment Highlights OCR‐002 for Hepatic Encephalopathy (HE): ~$1.5‐2B potential U.S. market opportunity Novel ammonia scavenger; broad IP protection & WW rights IV formulation for acute use and oral formulation for chronic use Only direct ammonia scavenger in development for HE DIFFERENTIATED PRODUCT FOR UNMET NEED IV Phase 2b STOP‐HE study data Oral Phase 2a multi‐dose study initiation in cirrhotic patients ANTICIPATED NEWS FLOW NEXT TWO QUARTERS Cash as of September 30, 2016: $32.5M Existing cash resources expected to fund development into Q1 2018 FINANCIAL STRENGTH

5 LINDA S. GRAIS, MD, JD President & Chief Executive Officer STAN BUKOFZER, MD Chief Medical Officer MICHAEL BYRNES, MBA Chief Financial Officer Experienced Leadership AND a Team with Deep Clinical and Development Experience
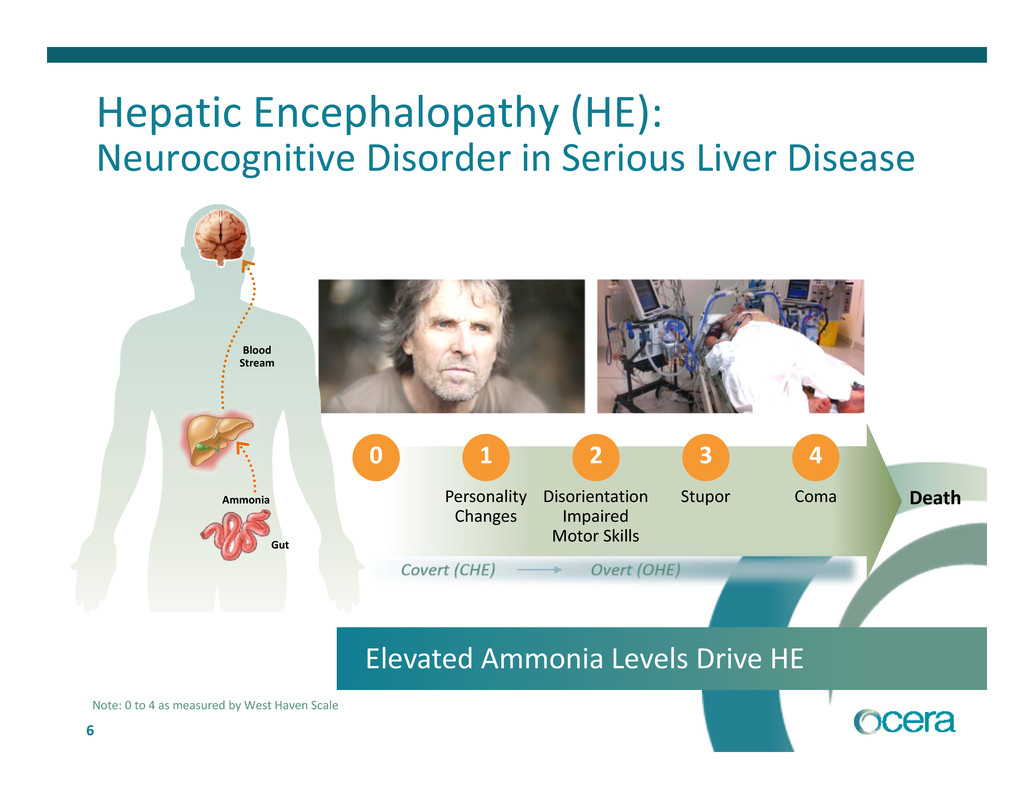
6 Disorientation Impaired Motor Skills Personality Changes Stupor Coma Death 0 1 2 3 4 Hepatic Encephalopathy (HE): Neurocognitive Disorder in Serious Liver Disease Blood Stream Ammonia Gut Elevated Ammonia Levels Drive HE Note: 0 to 4 as measured by West Haven Scale
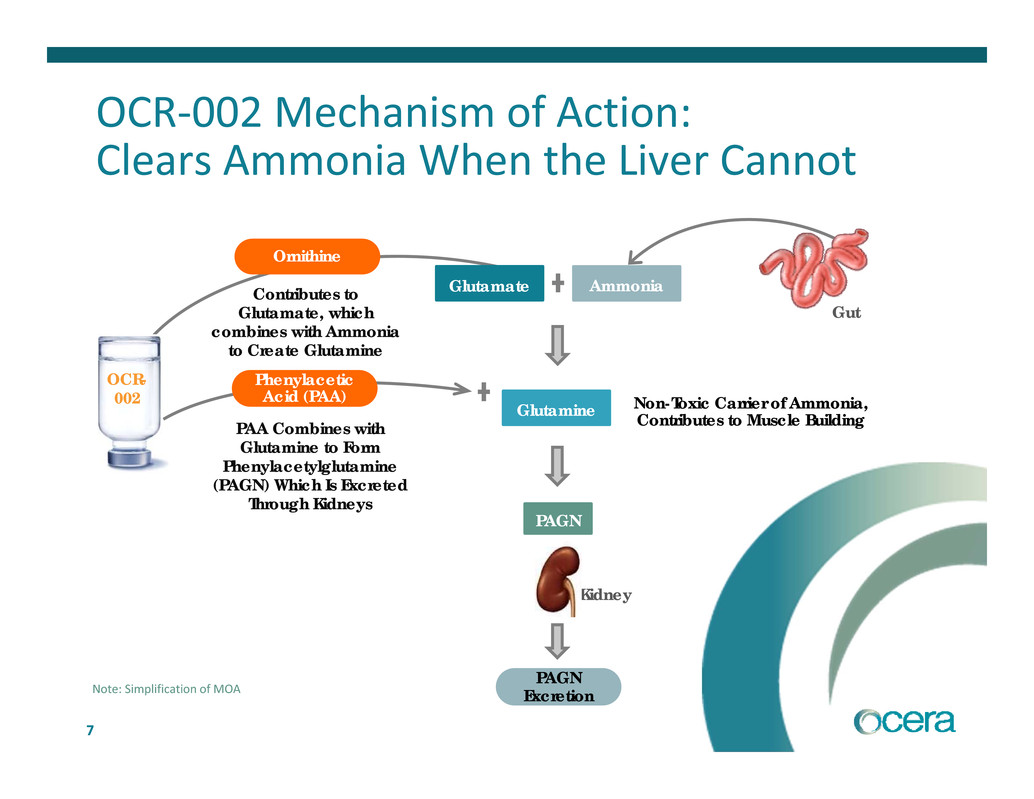
7 OCR‐002 Mechanism of Action: Clears Ammonia When the Liver Cannot Ornithine Contributes to Glutamate, which combines with Ammonia to Create Glutamine Gut OCR- 002 Kidney PAGN Excretion AmmoniaGlutamate PAA Combines with Glutamine to Form Phenylacetylglutamine (PAGN) Which Is Excreted Through Kidneys Phenylacetic Acid (PAA) Non-Toxic Carrier of Ammonia, Contributes to Muscle Building Glutamine PAGN Note: Simplification of MOA
8 Ammonia Lowering Reduced Risk of HE in HALT‐HE Study 1 “Randomized, Double‐Blind, Controlled Study of Glycerol Phenylbutrate in Hepatic Encephalopathy” Rockey, et al. Hepatology 2014; 59:1073‐1083 2 “Fasting Ammonia (NH3) as a Predictor or Hepatic Encephalopathy (HE) Events” Vierling, et al. Journal of Hepatology 2013; 58: S63‐S227 • Studies have long observed an association between ammonia and HE • Causal link demonstrated by Ravicti® HALT‐HE Study – Significantly delayed time to next HE episode (p<0.05)1 – Significantly reduced proportion of patients who experienced HE episode (p=0.02)1 – Patients with baseline fasting ammonia >1.5x upper limit of normal had a six‐ fold elevation in rate of annualized HE episodes compared to patients with fasting ammonia between 0‐1.5x ULN (p<0.01)2
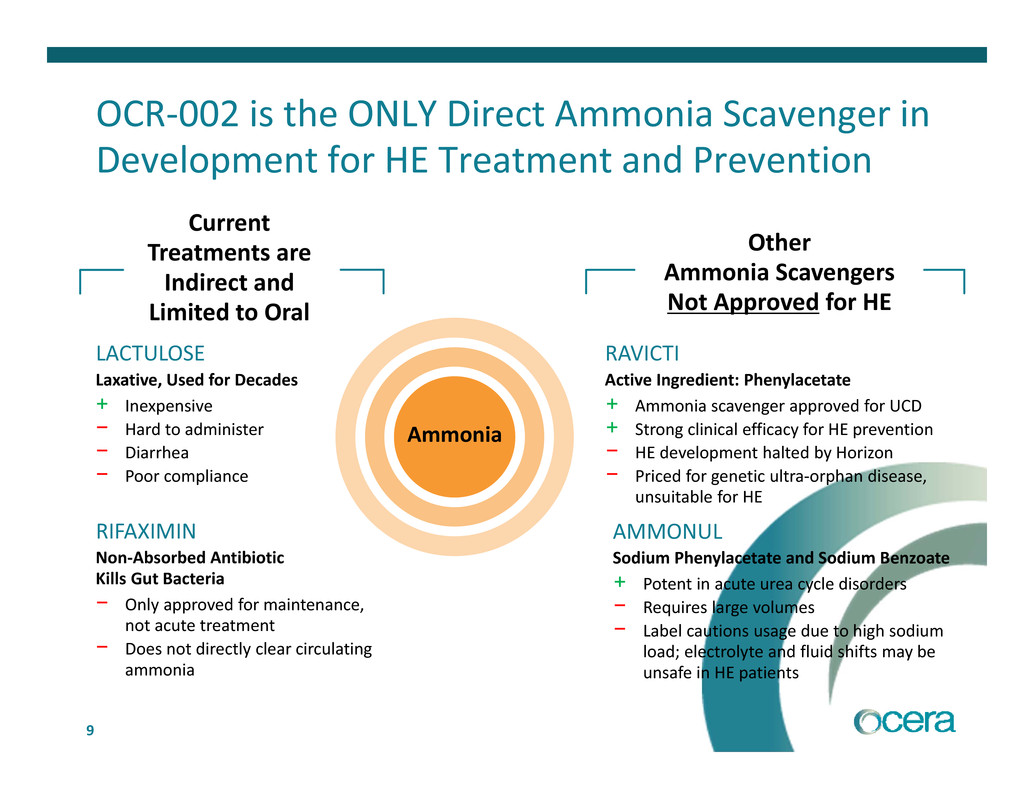
9 OCR‐002 is the ONLY Direct Ammonia Scavenger in Development for HE Treatment and Prevention Ammonia RIFAXIMIN Non‐Absorbed Antibiotic Kills Gut Bacteria − Only approved for maintenance, not acute treatment − Does not directly clear circulating ammonia LACTULOSE Laxative, Used for Decades + Inexpensive − Hard to administer − Diarrhea − Poor compliance Current Treatments are Indirect and Limited to Oral RAVICTI Active Ingredient: Phenylacetate + Ammonia scavenger approved for UCD + Strong clinical efficacy for HE prevention − HE development halted by Horizon − Priced for genetic ultra‐orphan disease, unsuitable for HE AMMONUL Sodium Phenylacetate and Sodium Benzoate + Potent in acute urea cycle disorders − Requires large volumes − Label cautions usage due to high sodium load; electrolyte and fluid shifts may be unsafe in HE patients Other Ammonia Scavengers Not Approved for HE
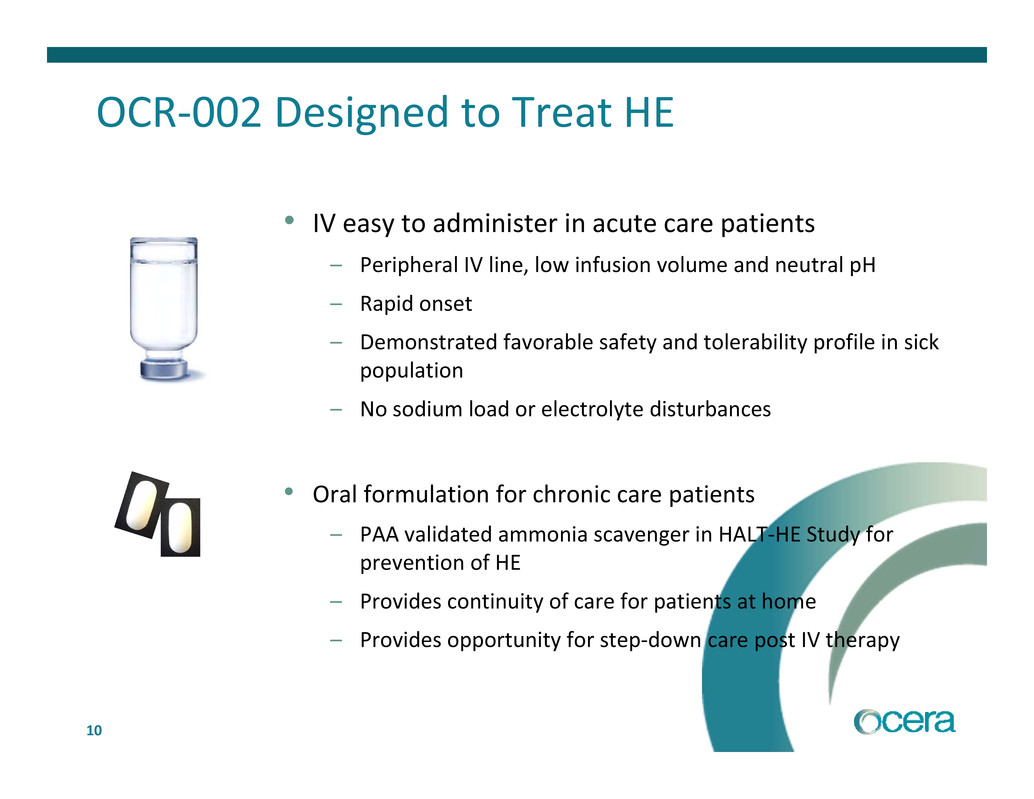
10 OCR‐002 Designed to Treat HE • IV easy to administer in acute care patients – Peripheral IV line, low infusion volume and neutral pH – Rapid onset – Demonstrated favorable safety and tolerability profile in sick population – No sodium load or electrolyte disturbances • Oral formulation for chronic care patients – PAA validated ammonia scavenger in HALT‐HE Study for prevention of HE – Provides continuity of care for patients at home – Provides opportunity for step‐down care post IV therapy
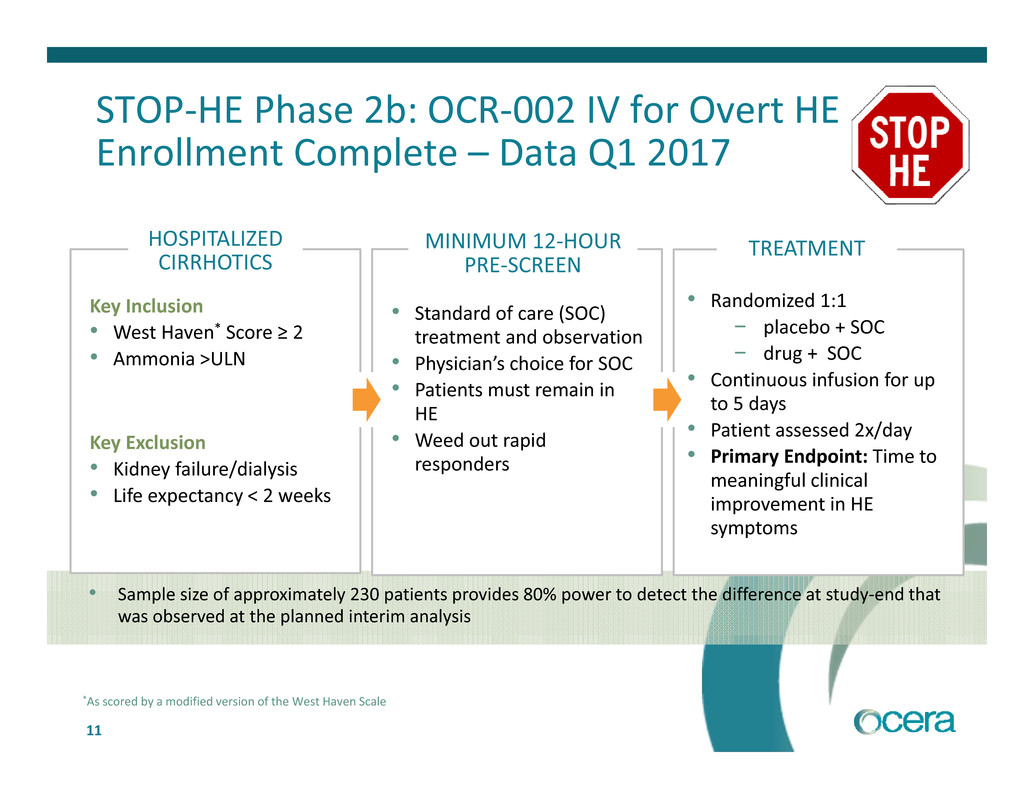
11 • Sample size of approximately 230 patients provides 80% power to detect the difference at study‐end that was observed at the planned interim analysis STOP‐HE Phase 2b: OCR‐002 IV for Overt HE Enrollment Complete – Data Q1 2017 *As scored by a modified version of the West Haven Scale Key Exclusion • Kidney failure/dialysis • Life expectancy < 2 weeks HOSPITALIZED CIRRHOTICS Key Inclusion • West Haven* Score ≥ 2 • Ammonia >ULN • Standard of care (SOC) treatment and observation • Physician’s choice for SOC • Patients must remain in HE • Weed out rapid responders MINIMUM 12‐HOUR PRE‐SCREEN • Randomized 1:1 − placebo + SOC − drug + SOC • Continuous infusion for up to 5 days • Patient assessed 2x/day • Primary Endpoint: Time to meaningful clinical improvement in HE symptoms TREATMENT
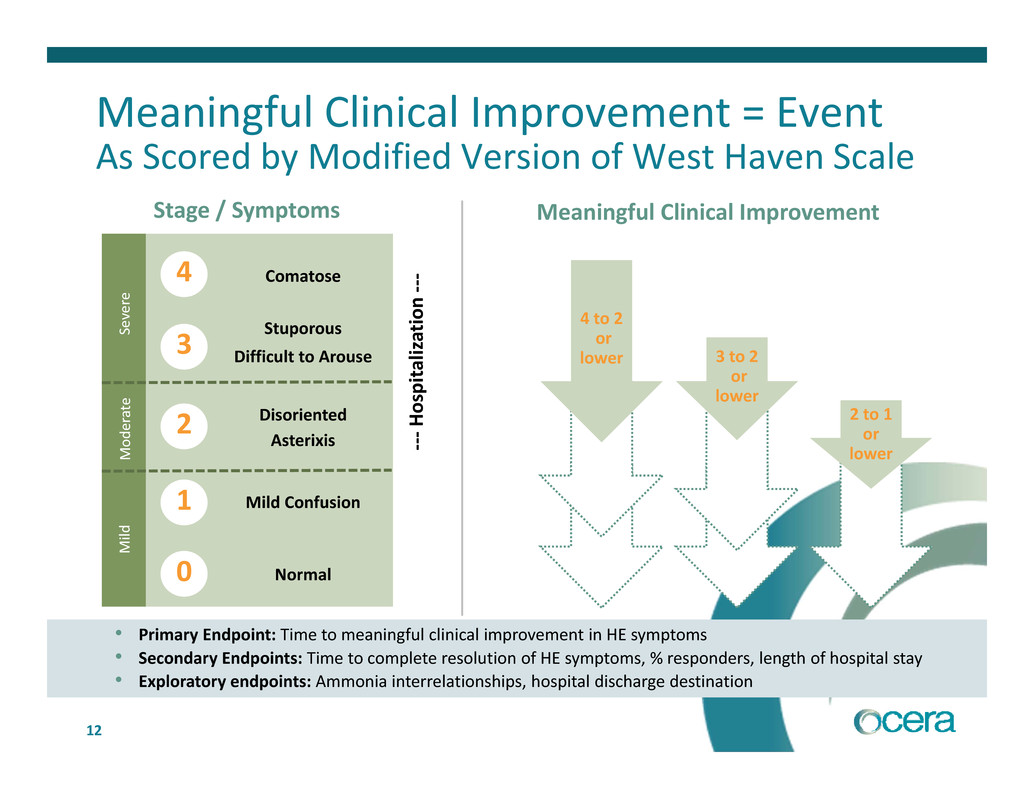
12 • Primary Endpoint: Time to meaningful clinical improvement in HE symptoms • Secondary Endpoints: Time to complete resolution of HE symptoms, % responders, length of hospital stay • Exploratory endpoints: Ammonia interrelationships, hospital discharge destination Meaningful Clinical Improvement = Event As Scored by Modified Version of West Haven Scale Stage / Symptoms Meaningful Clinical Improvement 4 to 2 or lower 3 to 2 or lower 2 to 1 or lower 0 1 2 3 4 ‐ ‐ ‐ H o s p i t a l i z a t i o n ‐ ‐ ‐Comatose Stuporous Disoriented Difficult to Arouse Asterixis Mild Confusion Normal M o d e r a t e M i l d S e v e r e
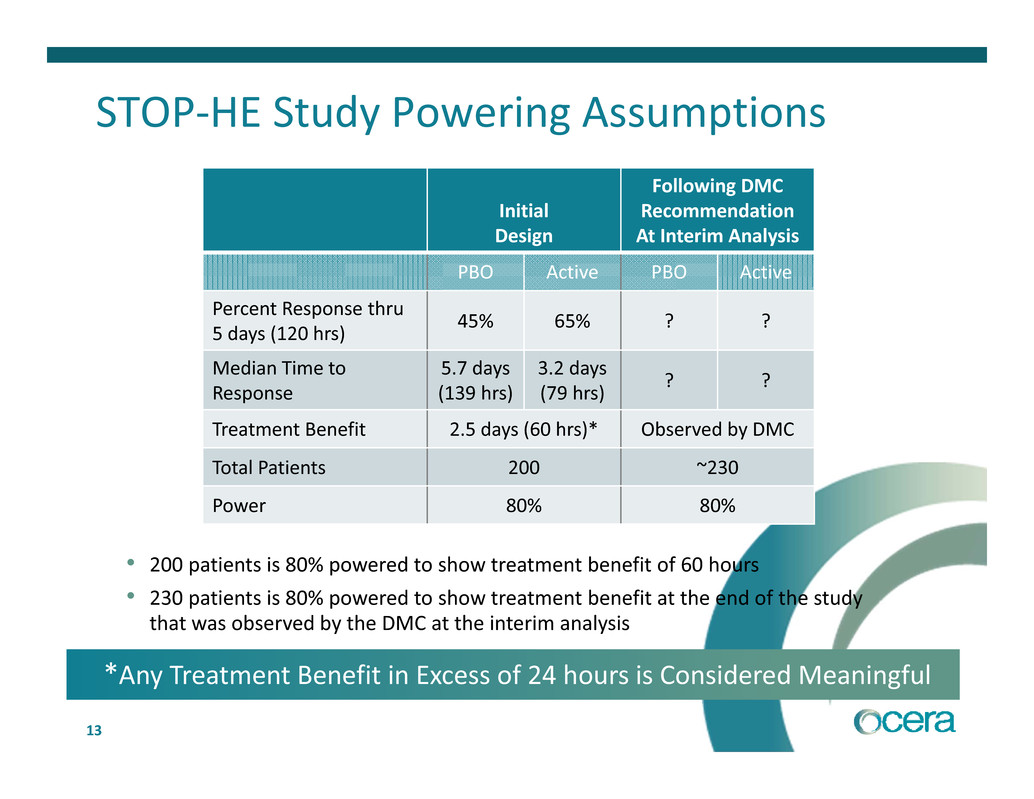
13 STOP‐HE Study Powering Assumptions Initial Design Following DMC Recommendation At Interim Analysis PBO Active PBO Active Percent Response thru 5 days (120 hrs) 45% 65% ? ? Median Time to Response 5.7 days (139 hrs) 3.2 days (79 hrs) ? ? Treatment Benefit 2.5 days (60 hrs)* Observed by DMC Total Patients 200 ~230 Power 80% 80% • 200 patients is 80% powered to show treatment benefit of 60 hours • 230 patients is 80% powered to show treatment benefit at the end of the study that was observed by the DMC at the interim analysis *Any Treatment Benefit in Excess of 24 hours is Considered Meaningful

14 STUDY RAVICTI® Phase 2 HALT‐HE OCR‐002 STOP‐HE (Enrollment Complete) Dose of Drug 6mL BID = 13.2g/day 10g/15g/20g/day (depending on liver function, dose adjustment to normalize PAA levels) Mean PAA Exposure 65‐150μg/mL1 Target: 75‐150μg/mL Clinical Results Statistically significant reductions in incidence of HE events and time to first event2 Promising interim analysis; sample size recommendation highly suggestive of treatment benefit Conclusion Dose sufficient for clinical benefit Dosing strategy to achieve similar PAA levels as RAVICTI® study 1 “Glycerol Phenylbutyrate in Patients With Cirrhosis and Episodic Hepatic Encephalopathy: A Pilot Study of Safety and Effect on Venous Ammonia Concentration” Ghabril, et al. Clinical Pharmacology in Drug Development 2013; 2(3) 278‐284 2 “Randomized, Double‐Blind, Controlled Study of Glycerol Phenylbutrate in Hepatic Encephalopathy” Rockey, et al. Hepatology 2014; 59:1073‐1083 Dose Rationale for OCR‐002 Informed by HALT‐HE
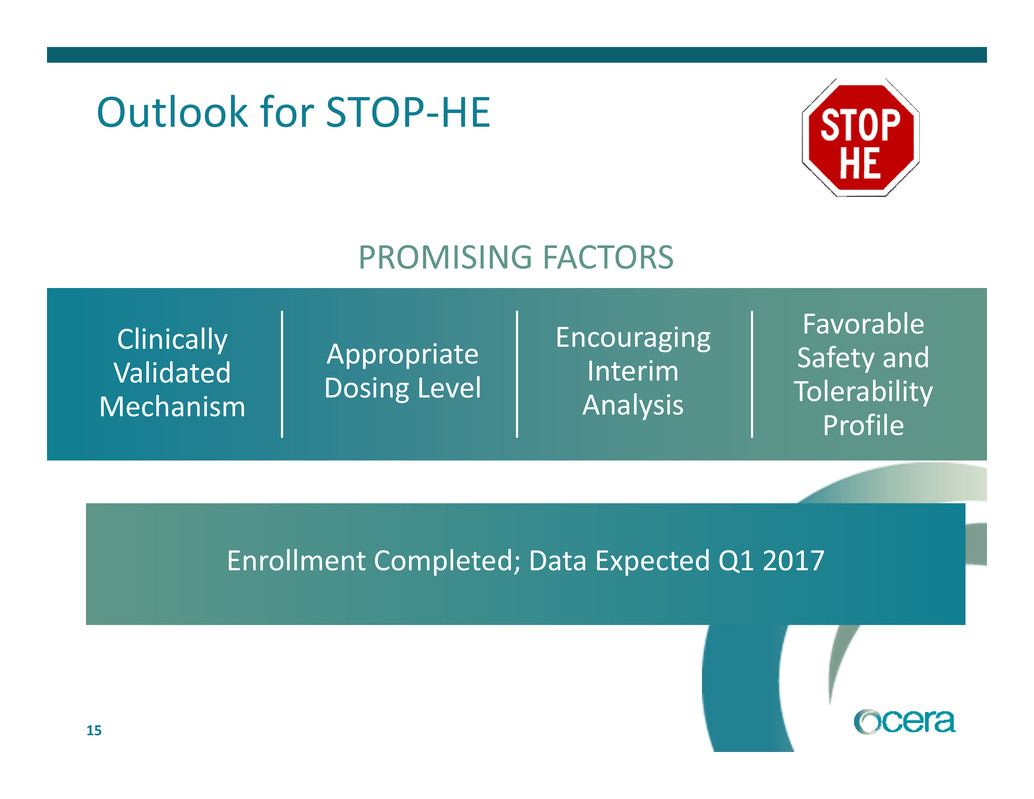
15 Outlook for STOP‐HE Favorable Safety and Tolerability Profile PROMISING FACTORS Appropriate Dosing Level Clinically Validated Mechanism Encouraging Interim Analysis Enrollment Completed; Data Expected Q1 2017

16 Oral OCR‐002 Unique Opportunity Develop orally‐administered formulation of OCR‐002 in concert with IV formulation to provide continuity of care for HE patients GOAL OF OCR‐002 ORAL PROGRAM Phase 1 program showed favorable single‐dose PK data Phase 2a study with new optimized tablet formulation planned for H1 2017 STATUS AND NEXT STEPS 1.5‐2M cirrhotics at risk of hepatic encephalopathy in the U.S. Less than 10% of at risk population represents $900M ‐ $1.2B potential revenue opportunity in the U.S. alone High re‐hospitalization rate for those discharged MARKET OPPORTUNITY

17 Proof of Principle Achieved in Phase 1 Program Phase 1: Cirrhotic Patient Study – Completed Q1 17 Single Dose Orally‐Administered OCR‐002 Findings include: Single IV Dose of OCR‐002VS • Absolute bioavailability > 95% in the fasted state • Pharmacokinetics supporting convenient dosing Extended‐Release Formulations of OCR‐002 Glycerol Phenylbutyrate (RAVICTI®) Immediate‐Release Oral Solution of OCR‐002 VS Phase 1: Healthy Volunteer Study – Completed Q4 15 Findings include: • Rapid onset of adequate plasma PAA concentrations with all OCR‐002 formulations • Sustained higher drug levels and PAGN than Ravicti All doses and treatment arms of OCR‐002 observed were safe and well‐tolerated

18 Oral OCR‐002 Phase 2a Study; Initiation H1 2017 Multi‐Dose Orally‐Administered OCR‐002 Glycerol Phenylbutyrate (RAVICTI®)VS Phase 2a: Cirrhotic Patients Evaluation of: • New optimized tablet formulation of OCR‐002 • Steady state pharmacokinetics • Pharmacodynamics • OCR‐002 vs. Clinically‐validated ammonia scavenger
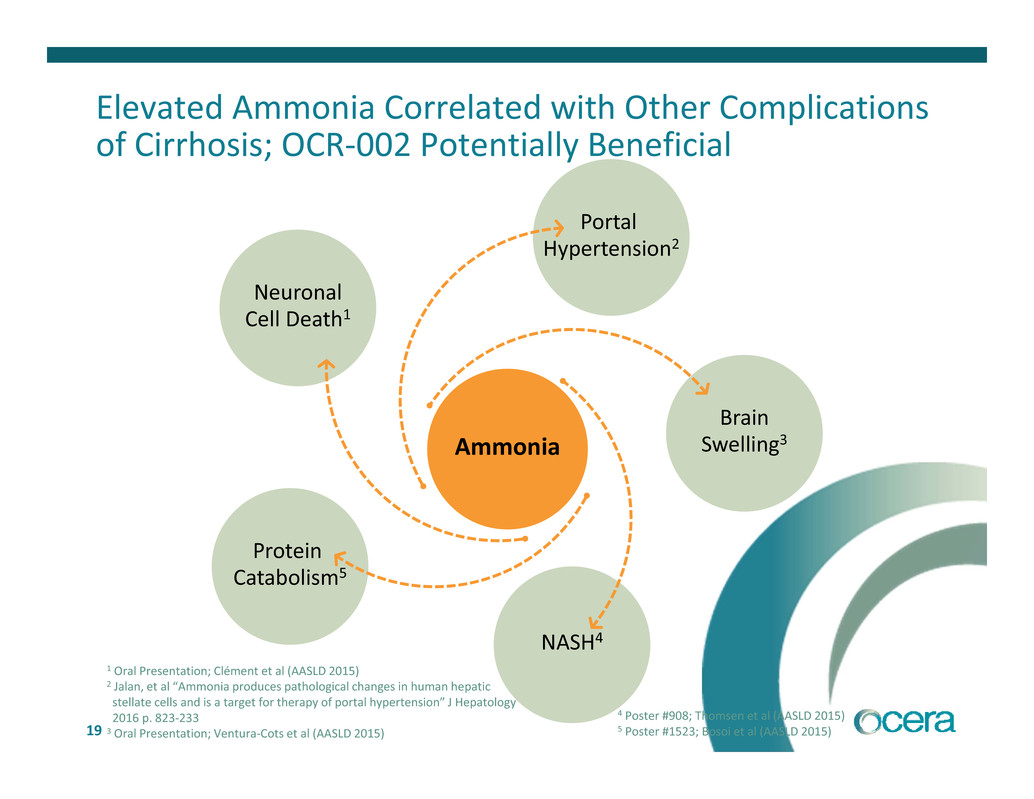
19 Ammonia Brain Swelling3 Portal Hypertension2 NASH4 Protein Catabolism5 Neuronal Cell Death1 Elevated Ammonia Correlated with Other Complications of Cirrhosis; OCR‐002 Potentially Beneficial 1 Oral Presentation; Clément et al (AASLD 2015) 2 Jalan, et al “Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension” J Hepatology 2016 p. 823‐233 3 Oral Presentation; Ventura‐Cots et al (AASLD 2015) 4 Poster #908; Thomsen et al (AASLD 2015) 5 Poster #1523; Bosoi et al (AASLD 2015)
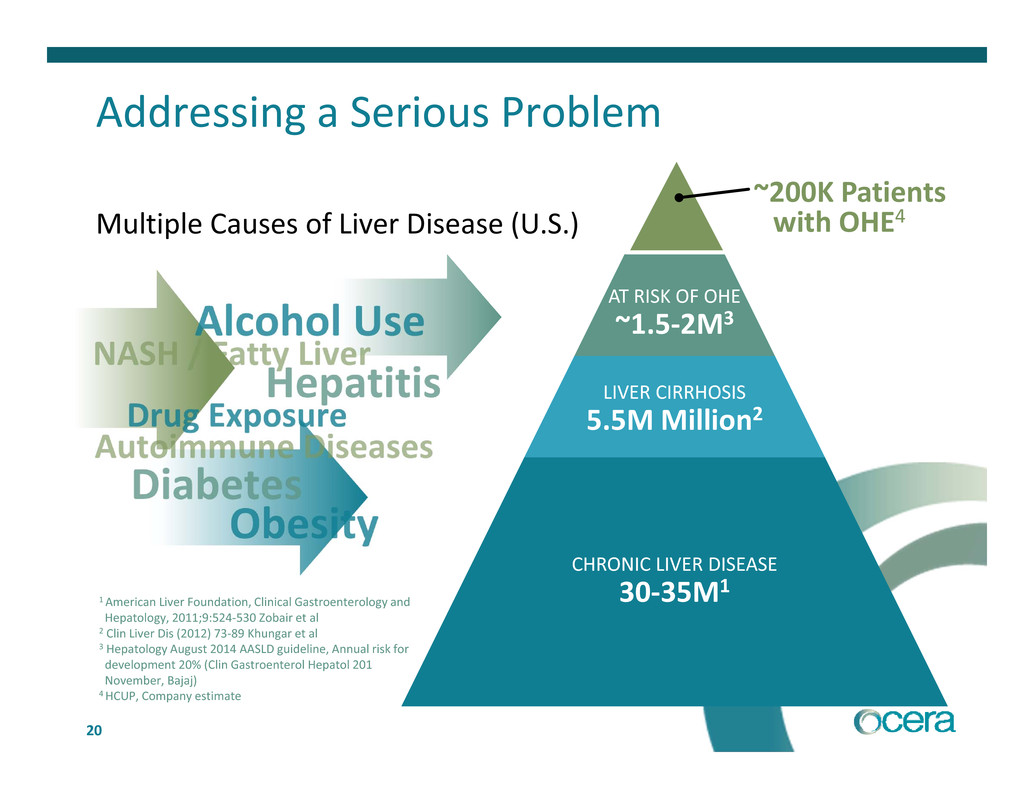
20 Addressing a Serious Problem Multiple Causes of Liver Disease (U.S.) CHRONIC LIVER DISEASE 30‐35M1 LIVER CIRRHOSIS 5.5M Million2 AT RISK OF OHE ~1.5‐2M3 ~200K Patients with OHE4 1 American Liver Foundation, Clinical Gastroenterology and Hepatology, 2011;9:524‐530 Zobair et al 2 Clin Liver Dis (2012) 73‐89 Khungar et al 3 Hepatology August 2014 AASLD guideline, Annual risk for development 20% (Clin Gastroenterol Hepatol 201 November, Bajaj) 4 HCUP, Company estimate

21 1 HCUP Database 2 Clinical Gastroenterology and Hepatology 2012;10:1034–1041 3 Annualized based off of actual reported revenues from Q1‐16 through Q3‐16 HE: Large and Growing Healthcare Burden Rising Hospitalizations1 (000s) • Total national charges related to HE: $7 Billion2 • HE hospitalizations continue to grow despite Rifaximin launch in 20101 • Rifaximin annual revenues: $900+ Million3 • HE patient demographics show increase in severity of illness, elderly population, and obesity as comorbidities2 105 107 106 110 120 133 141 148 156 83 104 180 196 252 275 331 380 436 2006 2007 2008 2009 2010 2011 2012 2013 2014 Hepatic Coma Encephalopathy Rifaximin approved for Prevention of HE April 2010
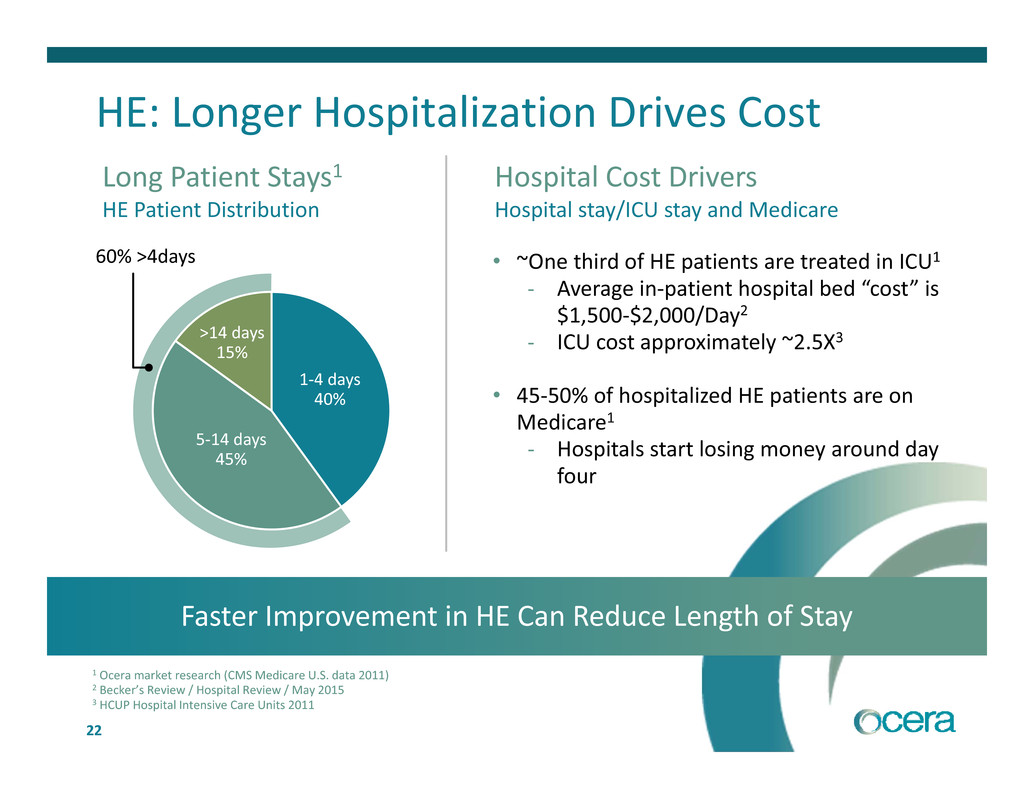
22 Long Patient Stays1 HE Patient Distribution 60% >4days >14 days 15% 5‐14 days 45% 1‐4 days 40% Faster Improvement in HE Can Reduce Length of Stay 1 Ocera market research (CMS Medicare U.S. data 2011) 2 Becker’s Review / Hospital Review / May 2015 3 HCUP Hospital Intensive Care Units 2011 • ~One third of HE patients are treated in ICU1 ‐ Average in‐patient hospital bed “cost” is $1,500‐$2,000/Day2 ‐ ICU cost approximately ~2.5X3 • 45‐50% of hospitalized HE patients are on Medicare1 ‐ Hospitals start losing money around day four Hospital Cost Drivers Hospital stay/ICU stay and Medicare HE: Longer Hospitalization Drives Cost
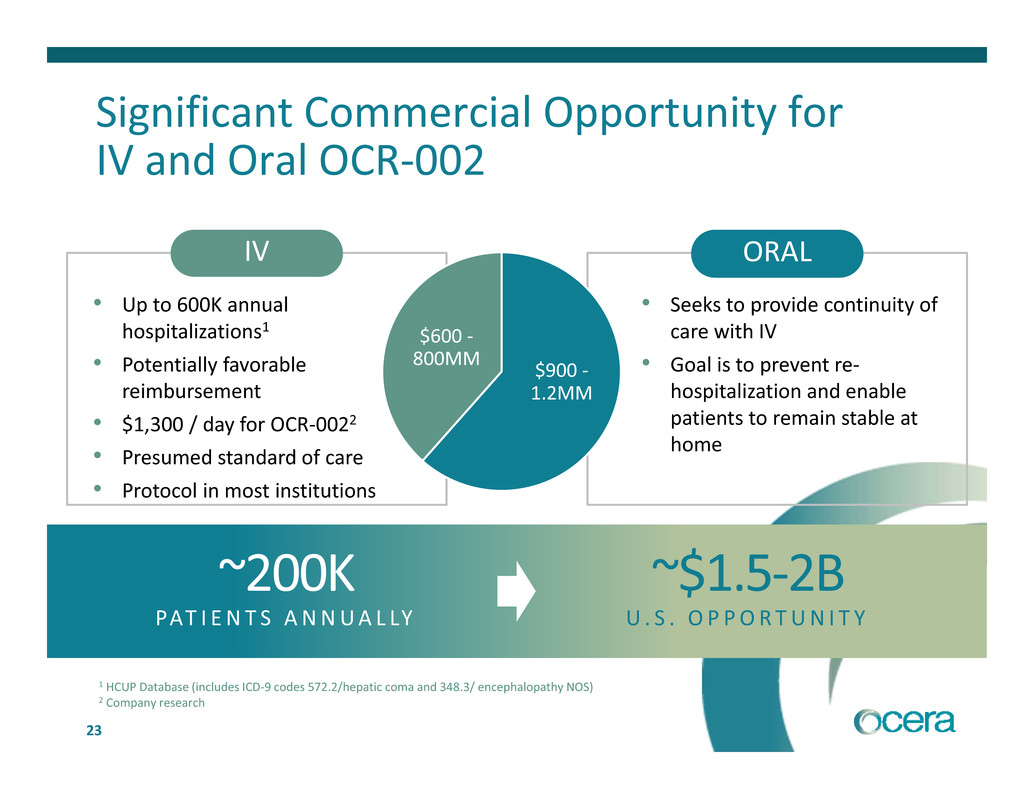
23 • Seeks to provide continuity of care with IV • Goal is to prevent re‐ hospitalization and enable patients to remain stable at home ORAL • Up to 600K annual hospitalizations1 • Potentially favorable reimbursement • $1,300 / day for OCR‐0022 • Presumed standard of care • Protocol in most institutions IV Significant Commercial Opportunity for IV and Oral OCR‐002 $600 ‐ 800MM $900 ‐ 1.2MM ~200K PAT I E N T S A NNUA L LY ~$1.5‐2B U. S . O P PORTUN I T Y 1 HCUP Database (includes ICD‐9 codes 572.2/hepatic coma and 348.3/ encephalopathy NOS) 2 Company research
24 Valuable Commercial Estate BROAD PATENTS Composition of Matter to 2030 (not including Hatch‐Waxman extension) ORPHAN STATUS IN US WORLDWIDE RIGHTS

25 HIGH HE Burden UNMET Medical Need ONLY Direct Ammonia Scavenger for HE PROMISING Clinical Programs ATTRACTIVE Commercial Opportunity NEAR TERM MILESTONES • STOP‐HE P2b Data • Oral P2a Multi‐dose Study Initiation V11022016
























