UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended May 31, 2008
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 0-50761
AngioDynamics, Inc.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 11-3146460 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| |
| 603 Queensbury Ave., Queensbury, New York | | 12804 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code (518) 798-1215
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of each class | | Name of each exchange on which registered |
| Common stock, par value $.01 | | NASDAQ Global Select Market |
| Preferred Stock Purchase Rights | | NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| | |
Large accelerated filer ¨ | | Accelerated filer x |
Non-accelerated filer ¨ | | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of November 30, 2007, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s common stock held by non-affiliates was approximately $461,443,000, computed by reference to the last sale price of the common stock on that date as reported by The Nasdaq Global Select Market.
As of July 31, 2008, there were 24,317,282 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The information required for Part III of this annual report on Form 10-K is incorporated by reference from the registrant’s Proxy Statement for its 2008 Annual Meeting of Stockholders to be filed within 120 days of registrant’s fiscal year ended May 31, 2008.
AngioDynamics, Inc. and Subsidiaries
INDEX
2
Part I
(a)General Development of Business
Overview
We are a provider of innovative medical devices used in minimally invasive, image-guided procedures to treat peripheral vascular disease, or PVD, and local oncology therapy options for treating cancer, including radiofrequency ablation, or RFA, and systems and embolization products for treating benign and malignant cancerous tumors. We design, develop, manufacture and market a broad line of therapeutic and diagnostic devices that enable interventional physicians (interventional radiologists, vascular surgeons, surgical oncologists and others) to treat PVD, tumors, and other non-coronary diseases. Unlike several of our competitors that focus on the treatment of coronary diseases, we believe that we are the only company whose primary focus is to offer a comprehensive product line for the interventional treatment of these diseases.
We have been in business since 1988. Our corporate headquarters is located at 603 Queensbury Avenue, Queensbury, New York 12804. Our phone number is (518) 798-1215.
Available Information
Our internet website iswww.angiodynamics.com. We make available free-of-charge through our website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange Commission (“SEC”). In addition, our internet website includes, among other things, charters of various committees of the Board of Directors and our code of business conduct and ethics applicable to all employees, officers and directors. Copies of these documents may be obtained free of charge from our internet website. Any stockholder also may obtain copies of these documents, free of charge, by sending a request in writing to our investor relations firm: EVC Group, 60 East 42nd Street, Suite 936, New York, NY 10165. Information on our website or connected to our website is not incorporated by reference into this Annual Report on Form 10-K.
History
AngioDynamics was founded in 1988 as a division of E-Z-EM, Inc., a leading developer and manufacturer of gastrointestinal contrast agents and related imaging accessories. In 1992, AngioDynamics was organized in the State of Delaware as a wholly owned subsidiary of E-Z-EM under the name A.D., Inc. In 1996, E-Z-EM transferred the business of its A.D. division to this subsidiary and we changed our name to AngioDynamics, Inc. In June 2004, we completed the initial public offering of our shares of common stock. The offering consisted of 2,242,500 shares (including 292,500 shares issued pursuant to the underwriters’ over-allotment option) at an initial public offering price of $11.00 per share. After the offering, E-Z-EM, Inc. held 80.4% of our shares. On October 30, 2004, E-Z-EM distributed all of its shares of AngioDynamics common stock to its stockholders. In May 2006, we completed a follow-on public offering of our shares of common stock. The offering consisted of 2,760,000 shares (including 360,000 shares issued pursuant to the underwriters’ over-allotment option) at a public offering price of $24.07 per share.
Recent Developments
Acquisition of Certain Assets of Diomed
In June 2008, we completed the acquisition of certain U.S. and U.K. assets of Diomed, Inc and Diomed, Ltd. for $11 million subject to adjustment for changes in working capital to be determined subsequent to the closing date. With this acquisition, we substantially strengthened our position in the market for the treatment of varicose veins. The combination of Diomed endovenous laser products with our existing venous product line provides us with a comprehensive venous product offering.
3
Acquisition of Oncobionic
On May 9, 2008, we completed the acquisition of Oncobionic, Inc. (“Oncobionic”) pursuant to the terms of a stock purchase agreement entered into on October 12, 2006. The closing of the acquisition comes as a result of the successful initial use of Oncobionic’s irreversible electroporation (IRE) technology in the first human clinical trial for the treatment of soft tissue, conducted during the first week of April 2008.
Under the stock purchase agreement, we agreed to acquire all of the issued and outstanding shares of the capital stock of Oncobionic for a total purchase price of $25.4 million, including $400,000 of assumed liabilities. We made a non-refundable payment of $5.0 million upon the execution of the stock purchase agreement in October 2006. We paid $10.0 million on May 9, 2008 upon the closing of the acquisition. $5.0 million is payable in November 2008, and the remaining $5.0 million is payable in November 2009.
(b) Narrative Description of Business
General
We classify our products into two product groups—Interventional Products, which consist primarily of angiographic products and accessories, dialysis products, vascular access products, venous products, thrombolytic products, PTA products, and drainage products and Oncology Products, which consist primarily of radio-frequency ablation products, tumor embolization products, and laparoscopic resection products.
Beginning with our first fiscal quarter of the fiscal year ending May 31, 2009, we will organize our business into three divisions: Peripheral Vascular; Access and Oncology/Surgery. Our Peripheral Vascular division comprises our venous, angiographic, PTA, drainage and thrombolytic product lines. Our Access division comprises our dialysis, ports and PICC lines. Our Oncology/Surgery division comprises our RFA, embolization, Habib and NanoKnife product lines. Beginning with our quarterly report on Form 10-Q for our fiscal quarter ended August 31, 2008, we will report our results of operations pursuant to these three divisions.
Our principal competitive advantages are our dedicated market focus, established brands and innovative products. Our acquisition of RITA in 2007 clarified our position, we believe, as the only company focused on minimally-invasive treatments for cancer patients with an emphasis on the growing segment of interventional oncology. We believe our dedicated focus enhances patient care and engenders loyalty among our customers. As a provider of interventional devices for over two decades, we believe we have established AngioDynamics’ brands as premium performance products. We collaborate frequently with leading interventional physicians in developing our products and rely on these relationships to further support our brands. Our chief executive officer is the only business executive from the medical device industry to serve on the Strategic Planning Committee of the Society of Interventional Radiology. This appointment provides us with awareness of emerging clinical trends, high visibility among interventional physicians and opportunities to understand and influence the evolution of interventional therapies.
We sell our broad line of quality devices for minimally invasive therapies in the United States through a direct sales force and outside the U.S. through a combination of direct sales and distributor relationships. As of May 31, 2008, our sales organization numbered 109 in the U.S. and 12 outside the U.S. The 121 employees in the sales organization include direct sales representatives, clinical specialists, and management personnel. For fiscal years 2008, 2007 and 2006, net sales in non-U.S markets were 9.5%, 6.3%, and 4.2%, respectively. Sales to any one country outside the U.S. did not comprise a material portion of our net sales in any of the last three fiscal years. We support our customers and sales organization with a marketing staff that includes product managers, customer service representatives and other marketing specialists. Our dedicated sales force and growing portfolio of products have contributed to our strong sales growth.
4
Peripheral Vascular Disease
Peripheral vascular disease encompasses a number of conditions in which the arteries or veins that carry blood to or from the legs, arms or non-cardiac organs become narrowed, obstructed or stretched. Structural deterioration in the blood vessels due to aging and the accumulation of atherosclerotic plaque results in restricted or diminished blood flow. Common symptoms include numbness, tingling, persistent pain or cramps in the extremities and deterioration of organ function, such as renal failure or intestinal malabsorption. Common PVDs also include venous insufficiency, a malfunction of one or more valves in the leg veins, which often leads to painful varicose veins and/or potentially life-threatening blood clots, and abdominal aortic aneurysms, or AAA, a ballooning, or stretching, of the aorta, which can lead to a potentially fatal rupture. Individuals who are over age 50, smoke, are overweight, have lipid (i.e., cholesterol) disorders, are diabetic or have high blood pressure are at the greatest risk of developing PVD.
Peripheral Interventional Medicine
Peripheral interventional medicine involves the use of minimally invasive, image-guided procedures to treat peripheral vascular and other non-coronary diseases. In these procedures, x-rays, ultrasound, MRI and other diagnostic imaging equipment are used to guide tiny instruments, such as catheters, through blood vessels or the skin to treat diseases. Increasing use of these techniques has accompanied advances in device designs and imaging technologies that enable physicians to diagnose and treat peripheral disorders in a much less invasive manner than traditional open surgery. Interventional procedures are generally less traumatic and less expensive, as they involve fewer anesthesias, a smaller incision and a shorter recovery time.
Peripheral interventional procedures are performed primarily by physicians specially trained in minimally invasive, image-guided techniques. This group of interventional physicians includes interventional radiologists, vascular surgeons and others. Interventional radiologists are board certified radiologists who are fellowship trained in image-guided, percutaneous (through the skin) interventions. These physicians historically have developed many interventional procedures, including balloon angioplasty, vascular stenting and embolization, and perform the majority of peripheral interventional procedures. There are currently more than 5,000 interventional radiologists in the United States performing over four million procedures annually. Vascular surgeons have traditionally been trained for open surgical repair of arterial and venous disorders. A large number are now increasingly performing interventional procedures, and accredited vascular surgery training programs now generally require instruction in interventional, image-guided peripheral vascular procedures. Increasingly, interventional radiologists and vascular surgeons are forming joint practices to capture additional patient referrals by providing a broader range of interventional treatments. Other physicians who perform peripheral interventional procedures include interventional cardiologists and interventional nephrologists.
Interventional and Surgical Oncology
Interventional oncology is an emerging specialty in which minimally invasive techniques and technologies are used to diagnose and treat cancers throughout the body. Percutaneous biopsy, chemoembolization, tumor ablation, PICC and port implantation, and radiofrequency ablation are just a few of the numerous procedures performed by interventional oncologists. In collaboration with other medical specialties focused on the cancer patient, the interventional oncologist brings an expertise in advanced imaging, catheter-based techniques, and minimally invasive procedures not found in other medical specialties.
5
Products
Our current product offerings consist of the following product categories:
| | | | | | |
| | | 2008 | |
Products | | Net
Sales $ | | % of
Net Sales | |
| | | (in thousands) | |
Interventional Products | | $ | 128,102 | | 76.9 | % |
Oncology Products | | | 38,398 | | 23.1 | % |
| | | | | | |
Total | | $ | 166,500 | | 100.0 | % |
| | | | | | |
All products discussed below have been cleared for sale in the United States by the U.S. Food and Drug Administration, or the FDA.
We have registered a number of marks with the U.S. Patent and Trademark Office, including Pulse*Spray; MORPHEUS CT; EVENMORE; ABSCESSION; TOTAL ABSCESSION; SPEEDLYSER; ANGIOFLOW; HYDROTIP; MEMORY TIP; SOS OMNI; HABIB 4X; LifeJet; Circle C; Vortex; LifeGuard; NeoStar; LifeValve; Centros; Sotradecol; NanoKnife and SOFT-VU. This annual report on Form 10-K also contains trademarks of companies other than AngioDynamics.
INTERVENTIONAL PRODUCTS
Interventional Products consist primarily of angiographic products and accessories, dialysis products, vascular access products, venous products, PTA products, thrombolytic products, and drainage products.
Angiographic Products and Accessories
Angiographic products and accessories are used during virtually every peripheral vascular interventional procedure. These products permit interventional physicians to reach targeted locations within the vascular system to deliver contrast media for visualization purposes and therapeutic agents and devices, such as stents or PTA balloons. Angiographic products consist primarily of angiographic catheters, but also include entry needles and guidewires specifically designed for peripheral interventions and fluid management products.
We manufacture angiographic catheters that are available in over 500 tip configurations and lengths, either as standard items or made to order, and an advanced guidewire.
| | • | | SOFT-VU®. Our proprietary SOFT-VU technology incorporates a soft, atraumatic tip, which is easily visualized under fluoroscopy. |
| | • | | ANGIOPTICTM. The ANGIOPTIC line is distinguished from other catheters because the entire instrument is highly visible under fluoroscopy. |
| | • | | Accu-VuTM. The Accu-Vu is a highly visible, accurate sizing catheter used to determine the length and diameter of a vessel for endovascular procedures. Accu-Vu provides a soft, highly radiopaque tip with a choice of platinum radiopaque marker patterns along the shaft for enhanced visibility and accuracy. Sizing catheters are used primarily in preparation for aortic aneurysm stent-grafts, percutaneous balloon angioplasty, peripherally placed vascular stents and vena cava filters. |
| | • | | MarinerTM. The Mariner is a hydrophilic-coated angiographic catheter. It uses our patented Soft-Vu catheter technology to deliver contrast media to anatomy that is difficult to reach. The advanced hydrophilic coating technology significantly reduces catheter surface friction, providing smoother navigation through challenging vasculature with optimal handling and control. |
6
| | • | | AQUALiner®. The AQUALiner is a technologically advanced guidewire. This guidewire is used to provide access to difficult to reach locations in interventional procedures requiring a highly lubricious wire. The AQUALiner guidewire incorporates proprietary advanced coating technology that allows smooth frictionless navigation. |
We offer uncoated, Teflon-coated and hydrophilic-coated guidewires to support our core angiographic catheter line.
Dialysis Products
We market a complete line of dialysis products that provide short and long-term vascular access for dialysis patients. Dialysis, or cleaning of the blood, is necessary in conditions such as acute renal failure, chronic renal failure and end-stage renal disease, or ESRD. The kidneys remove excess water and chemical wastes from blood, permitting clean blood to return to the circulatory system. When the kidneys malfunction, waste substances cannot be excreted, creating an abnormal buildup of wastes in the bloodstream. Dialysis machines are used to treat this condition. Dialysis catheters, which connect the patient to the dialysis machine, are used at various stages in the treatment of every dialysis patient.
We currently offer a wide variety of dialysis catheters, including:
| | • | | SCHONTM. The SCHON chronic dialysis catheter is designed to be self-retaining, deliver high flow rates and provide patient comfort. The Schon is for long-term use. |
| | • | | EVENMORE®. The EVENMORE is our first internally manufactured catheter. It is a low profile end-hole design catheter that provides very efficient dialysis. It was designed for long-term use with our proprietary Durathane™ shaft, which offers high resistance to chemicals used to clean the insertion site. |
| | • | | CENTROSTM. The Centros is a self centering, split tip, tunneled hemodialysis access catheter designed for long term use. Centros’ distal end has a unique curved tip that keeps the ports of the catheter centered in the superior vena cava and away from the vein walls. |
| | • | | DURA-FLOWTM. The DURA-FLOW chronic dialysis catheter is designed to be durable, maximize flow rates and provide for easier care and site maintenance. The Dura-Flow chronic dialysis catheter is for long-term use. |
| | • | | SCHON XL®. The SCHON XL acute dialysis catheter is designed to be kink resistant, deliver high flow rates, offer versatile positioning and provide patient comfort. SCHON XL is for short-term use. |
| | • | | DYNAMIC FLOWTM. Our DYNAMIC FLOW chronic dialysis catheter is designed for long-term use in dialysis patients. It features a Durathane shaft that offers higher chemical resistance than polyurethane, simplifying site care requirements. The Dynamic Flow also features a split tip design and a proximal shaft that reduces the chance of kinking after it reaches placement. |
| | • | | LIFEJET® F-16. The LIFEJET F-16 chronic dialysis catheter features the largest lumens available. This facilitates high flow rates while keeping arterial and venous pressures low. |
| | • | | CIRCLE C®. The CIRCLE C design provides the industry with smaller diameter catheters engineered to deliver efficient flow rate with minimal invasiveness for dialysis of apheresis. |
We purchase from Medical Components, Inc., or Medcomp, and resell under our name our Schon, Schon XL, and Dura-Flow dialysis catheters under an exclusive worldwide license. We also purchase our Dynamic Flow catheters under a non-exclusive license from Medcomp. We purchase our Centros catheter from another outside manufacturer.
7
Vascular Access Products
Image-guided vascular access, or IGVA, involves the use of advanced imaging equipment to guide the placement of catheters that deliver primarily short-term drug therapies, such as chemotherapeutic agents and antibiotics, into the central venous system. Delivery to the circulatory system allows drugs to mix with a large volume of blood as compared to intravenous drug delivery into a superficial vessel. IGVA procedures include the placement of peripherally inserted central catheter, or PICC lines, implantable ports and central venous catheters, or CVCs.
Our vascular access products include:
| | • | | MORPHEUS® CT PICC. These PICC lines provide short- or long- term peripheral access to the central venous system for intravenous therapy and blood sampling. They are constructed of a biocompatible and durable material called Durathane, and have increased stiffness from the proximal end to the distal end, which provides ease of use and enhanced patient safety and comfort. These products are intended for use with CT injectors, allowing physicians to use the existing PICC for both medications and CT imaging, thus avoiding the need for an additional access site. |
| | • | | MORPHEUS® CT PICC Insertion Kit. In May 2006, we introduced our insertion kit, which allows our Morpheus CT PICC to be inserted at a patient’s bedside instead of in the hospital radiology suite. The kit was specifically designed for interventional radiologists, nurse practitioners, physician assistants and radiology technicians who perform placement of PICC lines. |
| | • | | Micro Access Sets. Our micro access sets provide interventional physicians a smaller introducer system for minimally invasive procedures. |
| | • | | Transjugular Access Set. Our transjugular liver access set is used to provide access in a transjugular intrahepatic portosystemic shunt (TIPS) procedure. A TIPS procedure involves placing a shunt in the liver between the hepatic and portal veins. This relieves the pressure on the portal system in an effort to resolve the bleeding complications often encountered in end-stage liver failure. |
| | • | | Specialty Access Ports. Specialty access ports are implantable devices utilized for the central venous administration of a variety of medical therapies and for blood sampling and diagnostic purposes. Central venous access facilitates a more systemic delivery of treatment agents, while mitigating certain of the harsh side effects of certain treatment protocols and eliminating the need for repeated access to peripheral veins. Once implanted in the body, a port can be utilized for up to approximately 2,000 accesses depending upon needle gauge size and the port size. Our specialty access ports are used primarily in systemic or regional short and long-term cancer treatment protocols that require frequent infusions of highly concentrated or toxic medications (such as chemotherapy agents, antibiotics or analgesics) and frequent blood samplings. This product line consists of the following families of products: (i) the Vortex family of ports including Vortex VTX, LifePort VTX, Triumph TM VTX and Genesis TM VTX; (ii) LifePort; (iii) Triumph-1; (iv) Infuse-a-Port; (v) OmegaPort; (vi) TitanPort; and (vii) the Vortex MP Port system. |
| | • | | Our Vortex® line of ports is a clear-flow port technology that, we believe, revolutionized port design. With its rounded chamber, the Vortex® is designed to have no sludge-harboring corners or dead spaces. This contrasts to conventional ports where a squared reservoir design promotes sludge accumulation setting the stage for occlusions and infections. A tangential stem adds to the flow dynamics, which is designed to result in a hyper-cleaning flow process to remove blood deposits and drug residuals. |
| | • | | The LifeGuard™ Safety Infusion Set and The LifeGuard Vision™ are used to infuse our ports and complement our port and vascular access catheters. The innovative design of these products was developed with the input of clinicians to provide safer needle placements, and the needles’ low profile design is intended to allow clinicians to easily dress the site. We believe that the ease of use and visual confirmation of safety is ideal in the clinical setting. |
8
| | • | | Neostar®. The Neostar® Tunneled Central Venous Catheters are among the most well known and trusted names in catheters. The central venous catheters are intended for long-term vascular access, suitable for chemotherapy, infusion of intravenous fluids or drugs parental nutrition, transfusion or sampling blood products. With single, double and triple lumen configurations, one-piece Y-hubs for mirror smooth transition points and complete tray availability, the Neostar® is an excellent choice for valued patients. |
| | • | | LifeValve® Platinum. The LifeValve® central venous catheter incorporates the only technology that features two separate areas for aspiration and infusion for more reliable operation and fewer interventions. The patented “Duckbill” infusion valve is designed to reduce incidence of blood back flow resulting in improved performance. A stiffening stylet and a rounded atraumatic tip facilitate passage into the vessel while the over-the-guidewire feature is engineered to reduce procedure time and complexity. |
Venous Products
Our venous products consist of our VenaCure® products and Sotradecol®.
Our VenaCure products are used in endovascular laser procedures to treat superficial venous disease (varicose veins). Superficial venous disease is a malfunction of one or more valves in the leg veins. These procedures are a less invasive alternative to vein stripping for the treatment of this condition. Vein stripping is a lengthy, painful and traumatic surgical procedure that involves significant patient recovery time. In contrast, venous laser treatment is an outpatient procedure that generally allows the patient to quickly return to normal activities with no scarring and minimal post-operative pain.
With our VenaCure NeverTouch® products, laser energy is used to stop the source of the pressure by ablating, or collapsing and destroying, the affected vein. The body subsequently routes the blood to other healthy veins. Our products are sold as a system that includes a diode laser with our NeverTouch disposable components, training and marketing materials. The diode laser is a self-contained reusable instrument. The disposable components in the system include a NeverTouch laser fiber system, an access sheath, access wires and needles. The training and marketing materials include a two-day physician training course, a comprehensive business development package and patient marketing kit.
An important part of our focus on the peripheral vascular disease market is the treatment of varicose veins. With an estimated one-half of all Americans over the age of 60 suffering from varicose veins, the market for this treatment is large and growing. We believe that Sotradecol®, a sclerosing drug approved by the FDA that we introduced in November 2005, combined with our currently available precision drug-delivery catheter technology, such as UNI*FUSE™, will become an important method of treating varicose veins. Sotradecol has been shown to be an effective treatment of small, uncomplicated varicose veins of the lower extremities, in addition to ablation of the great saphenous vein. Catheter-directed sclerotherapy has the advantages of requiring no investment in capital equipment and requires no local anesthesia because it is virtually pain free. We believe that laser-based treatment systems will continue to be an important part of the vein treatment market in the United States for some time, but that laser treatments may eventually be eclipsed by catheter-directed sclerotherapy, as has occurred in Europe. This approach to treating varicose veins has the potential for greater intellectual property protection than our laser-based VenaCure products and, most importantly, can be incorporated with some of our existing patented products. Bioniche Pharma Group Limited has appointed us the exclusive distributor to all “persons” in the United States, which may include hospital pharmacies, group purchasing organizations and wholesalers, as well as all physicians, for use in treating varicose veins or other approved vascular indications. Sotradecol is the only FDA-approved sodium tetradecyl sulfate injection currently available in the United States.
PTA Products
PTA (percutaneous transluminal angioplasty) procedures are used to open blocked blood vessels and dialysis access sites using a catheter that has a balloon at its tip. When the balloon is inflated, the pressure flattens
9
the blockage against the vessel wall to improve blood flow. PTA is now the most common method for opening a blocked vessel in the heart, legs, kidneys or arms.
Our PTA dilation balloon catheters include:
| | • | | WORKHORSE®. Our WORKHORSE product is a high-pressure balloon catheter offered in 54 configurations. While the WorkHorse can perform other peripheral PTA procedures, we believe the device is used primarily for treating obstructed dialysis access sites. |
| | • | | WORKHORSE® II. The WORKHORSE II is a high-pressure, non-compliant PTA balloon catheter. This product is an extension to our WORKHORSE PTA catheter, with enhanced WORKHORSE features to improve product performance during declotting procedures for dialysis access sites. |
| | • | | PROFILER®. The PROFILER is a low profile, high-visibility balloon catheter that features a soft, radiopaque, tapered tip and a flexible, non-kinking catheter shaft with exceptional pushability. The low profile of the PROFILER opens access to small vessels and tortuous anatomy and is available with multiple balloon sizes and catheter lengths. |
Thrombolytic Products
Thrombolytic catheters are used to deliver thrombolytic agents, which are drugs that dissolve blood clots in hemodialysis access grafts, arteries, veins and surgical bypass grafts. Our thrombolytic catheters include:
| | • | | PULSE*SPRAY® and UNI*FUSE catheters. Our PULSE*SPRAY and UNI*FUSE catheters improve the delivery of thrombolytic agents by providing a controlled, forceful and uniform dispersion. Patented slits on the infusion catheter operate like tiny valves for an even distribution of thrombolytic agents. We believe that these slits reduce the amount of thrombolytic agents and the time necessary for these procedures, resulting in cost savings and improved patient safety. |
| | • | | SPEEDLYSER®. Our SPEEDLYSER thrombolytic catheter is used to deliver thrombolytic agents into obstructed dialysis grafts. This catheter features PULSE *SPRAY slit technology that simplifies catheter insertion and drug delivery. |
Drainage Products
Drainage products percutaneously drain abscesses and other fluid pockets. An abscess is a tender inflamed mass that typically must be drained by a physician.
Our line of drainage products consists of our TOTAL ABSCESSION® general drainage catheters, which we introduced in December 2005, and ABSCESSION® general and biliary drainage catheters. These products feature our proprietary soft catheter material, which is designed for patient comfort. These catheters also recover their shape even if bent or severely deformed when patients roll over and kink the catheters during sleep. Our TOTAL ABSCESSION general drainage catheter features a tamper-resistant locking mechanism known as the VAULT®. This locking mechanism eliminates the need to replace drainage catheters that become unlocked during routine use, thus reducing physician time and increasing patient comfort. The TOTAL ABSCESSION catheter permits aspiration while locked or unlocked thus allowing more accurate placement and greater versatility for draining complex situations.
ONCOLOGY PRODUCTS
Oncology products consist of Radiofrequency Ablation products, Embolization Products and the recently introduced NanoKnife product line.
Radiofrequency Ablation Products
Radiofrequency Ablation (RFA) products use radiofrequency energy to provide a minimally invasive approach to ablating solid cancerous or benign tumors. Our system delivers radiofrequency energy to raise the temperature of cells above 45 to 50 degrees Celsius, causing cellular death.
10
The physician inserts the disposable needle electrode device into the target body tissue, typically under ultrasound, computed tomography or magnetic resonance imaging guidance. Once the device is inserted, pushing on the handle of the device causes a group of curved wires to be deployed from the tip of the electrode. When the power is turned on, these wires deliver radiofrequency energy throughout the tumor. In addition, temperature sensors on the tips of the wires measure tissue temperature throughout the procedure. During the procedure, our system automatically adjusts the amount of energy delivered in order to maintain the temperature necessary to ablate the targeted tissue. For a typical five centimeter ablation using our Starburst XLie disposable device, the ablation process takes approximately ten minutes. When the ablation is complete, pulling back on the handle of the device causes the curved wire array to be retracted into the device so it can be removed from the body. Our disposable device cauterizes the tissue along the needle tract, which we believe kills any residual cancer cells that might be removed from the tumor.
Benefits of the RFA System
The benefits of our system include:
| | • | | Effective Treatment Option. We believe that our system provides an effective treatment option to liver cancer patients who previously had few options available to effectively address their unresectable liver tumors. Further, our system provides an effective treatment option for patients whose tumors have metastasized to the bone and cause pain that cannot be adequately relieved by other means. In the future, our system may offer patients with other types of tumors a similar treatment option. |
| | • | | Minimally Invasive Procedure. The RFA system offers physicians an effective minimally invasive treatment option with few side effects or complications. Our products can be used in an outpatient procedure that requires only local anesthesia, and patients are typically sent home the same day with a small bandage over the entry site. Alternatively, patients can be treated with just an overnight hospital stay either through a small wound in the skin or laparoscopically through several small incisions. Compared to existing alternatives, we believe our minimally invasive procedure is cost effective and can result in reduced hospital stays. |
| | • | | Proprietary Array Design and Temperature Feedback Provide Procedural Control. Our array design enables the physician to predictably ablate large volumes of targeted tissue. In addition, our temperature feedback feature allows physicians to ensure that the temperature is high enough at the electrode to achieve cell death. |
| | • | | Repeat Treatments Possible. Cancer is most often a recurrent disease. However, due to the invasive nature of other treatment options, such as surgery, the majority of patients who undergo traditional therapies cannot be retreated in the event that new tumors appear or previously treated tumors reappear. Because of the minimally invasive nature of our procedure, patients treated with our RFA system can often be retreated. |
| | • | | Broadly Applicable Technology. Our significant clinical experience with liver tumors and bone tumors as well as feasibility studies in other organs indicates that our technology may in the future be broadly applied to the ablative treatment of solid tumors in the lung, breast, uterus, prostate and kidney. |
While there are numerous benefits of our system, there are some side effects of treatment as well. Published reports on the use of the RFA system indicate low overall complication rates. These include ground-pad burns, which are burns that can occur when there is a concentration of heat at the ground-pad site, bleeding, abscesses and, in cases involving the treatment of bone tumors, fractures and nerve damage. Studies have also shown some recurrence of tumors following treatment with our system. However, in many cases where tumors recur, our procedure can often be repeated. In rare cases, unintentional physician misuse of our system has resulted in patient deaths.
11
Radiofrequency Ablation Product Technology
Our radiofrequency ablation products are based on proprietary technology used to ablate tissue in a controlled manner. A radiofrequency generator supplies energy through our disposable device placed within the targeted tissue. Our devices contain curved, space-filling arrays of wires which are deployed from the tip to allow the radiofrequency energy to be dispersed throughout the tumor.
Radiofrequency energy supplied by the generator produces ionic agitation, or cellular friction, in the tissue closely surrounding the electrode. This friction produces heat that can be used to predictably ablate volumes of tissue. To effectively ablate tissue, it must be heated to an approximate temperature of 45° to 50°C, or 113° to 122°F.
Our system is designed to permit the physician to set the desired treatment time and temperature at the beginning of the procedure. Once that temperature is reached, our proprietary temperature control technology automatically adjusts the energy supplied from the generator to maintain the optimal temperature within the tissue during the course of the procedure. We believe our system has the potential to provide a more effective ablation than competing technologies by providing critical tissue temperature feedback during the procedure.
Some of our products make use of saline to enhance the ablation process. This saline is used to irrigate the ablation site and is delivered through the curved array of wires in our devices. The use of saline can significantly increase the speed of the ablation treatment and permits ablation of larger tumors.
The RFA system consists of a radiofrequency generator and a family of disposable devices. We also market the HABIB 4X® resection device under a distribution agreement with EMcision Limited.
| | | | |
| | | Product Name | | Description |
| Disposable Electrodes: | | StarBurst | | Creates a scalable 2 to 3 centimeter ablation. |
| | StarBurst XL | | Creates a scalable 3 to 5 centimeter ablation. |
| | StarBurst SDE | | Creates a 2 centimeter ablation, via a side-deployed array. |
| | StarBurst Semi-Flex | | Creates a scalable 3 to 5 centimeter ablation and has a partially flexible shaft. |
| | StarBurst XLie | | Creates a scalable 4 to 7 centimeter ablation. Requires an accessory infusion pump for irrigation of saline. Attached tubing standard. |
| | StarBurst Talon: Straight | | Creates a scalable 2 to 4 centimeter ablation. Requires an accessory infusion pump for irrigation of saline. |
| | StarBurst Talon: Semi-Flex | | Creates a scalable 2 to 4 centimeter ablation. Requires an accessory infusion pump for irrigation of saline. |
| Resection Device: | | HABIB® 4X | | Surgical resection device. |
| Generators: | | Model 1500X | | 250 Watt Capable Generator with Field-Software Upgradeability. |
RFA Disposable Electrodes
Our RFA disposable electrodes all consist of needle shaped electrodes containing curved wire arrays that are deployed into the targeted body tissue. Each device contains several thermocouples, or temperature sensors, which provide feedback to the physician of the tissue temperature during the ablation and which allow the generator to automatically adjust the amount of radiofrequency energy so that the desired tissue temperature can be achieved.
Our RFA disposable electrodes are available in different array sizes to allow the physician to create a spherical ablation volume of anywhere from two to seven centimeters. In addition, depending on product line, the devices are available in 10, 12, 15 or 25 centimeter lengths to allow physicians to access tumors that are located
12
more or less deeply within the body. Each RFA disposable device is supplied with one or more ground pads to allow a return path for the flow of radiofrequency energy from the patient back to the generator.
RF Resection Device
We have an exclusive worldwide license with EMcision Limited to sell the HABIB® 4X bipolar radiofrequency resection device. This product is designed to coagulate a “surgical resection plane” to facilitate a fast dissection with limited blood loss. It is compatible with our Model 1500 and Model 1500X radiofrequency generators.
RFA Generators
All of our generators employ an internal computer to assist the physician in safely and effectively controlling the delivery of radiofrequency during ablation or surgical resection procedures. In addition, each generator has a display to convey information to the physician while using the system. Our Model 1500X generators have the ability, using a laptop computer, to display real-time, color-coded graphs of items such as power, and temperature and impedance to aid the user in controlling the system and to collect procedural information for the patient’s record. These generators are designed to have their software changed in the field through the insertion of a small card containing electronic memory circuits.
Embolization Products
LC Beads are compressible, visibly-tinted N-fil Hydrogel microspheres supplied in convenient pre-prepared single vials. Embolic material is injected into selected vessels to block the blood flow feeding the tumor or malformation, causing it to shrink over time.
Features
Proven Material—A sulfonate modified N-fil Hydrogel microsphere.
Enhanced Visual Verification—Tinted beads for immediate enhanced visualization prior to delivery.
Optimal Sizes—Industry standard size ranges for ease in selectivity of bead sizes and a wide array of calibrated bead sizes designed to ensure precise match to targeted vessels.
Convenient Configuration—Provided in a pre-prepared vial of embolic/saline solution; designed to minimize preparation time. Sold in single vials to allow users the option of choosing an exact desired quantity.
NanoKnife Products
Our recently introduced NanoKnife™ product is AngioDynamics’ first application of irreversible electroporation technology (IRE). IRE is a surgical resection technique in which electrical fields are used to create nano-scale defects in a cell’s membrane, which causes cell death only in the targeted tissue, without destroying critical structures such as ducts, blood vessels and nerves. NanoKnife is a surgical resection system that uses electrode probes to transmit energy from its generator to a target area. NanoKnife works in two-pole operating mode and up to six electrodes can be placed at a fixed distance apart in soft tissue to create several two-pole electrode configurations. NanoKnife allows the user to choose between predefined target area configurations or customized settings and is designed to provide clinical practices with precision and speed.
Research & Development
Our future success will depend in part on our ability to continue to develop new products and enhance existing products. We recognize the importance of, and intend to continue to make investments in, research and development. For fiscal 2008, 2007 and 2006, our research and development (“R&D”) expenditures were $14.4
13
million, $20.6 million and $5.9 million, respectively, and constituted 8.7%, 18.3% and 7.5%, respectively, of net sales. A significant portion of our R&D expenses in 2007 related to a charge of $12.1 million for in-process R&D required under purchase accounting rules from our acquisition of RITA. Without this charge, our R&D expenses were approximately 7.5% of net sales. R&D activities include research, product development and regulatory affairs. We expect that our R&D expenditures will be approximately 10% of net sales in fiscal 2009 and remain in the range of 8 to 10% of net sales thereafter. However, downturns in our business could cause us to reduce our R&D spending.
Our research and product development teams work closely with our sales force to incorporate customer feedback into our development and design process. We believe that we have a reputation among interventional physicians as a good partner for product development because of our tradition of close physician collaboration, dedicated market focus, responsiveness and execution capabilities for product development and commercialization.
Competition
We encounter significant competition across our product lines and in each market in which our products are sold. These markets are characterized by rapid change resulting from technological advances and scientific discoveries. We face competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of products. In addition, we compete with providers of other medical therapies, such as pharmaceutical companies, that may offer non-surgical therapies for conditions that currently, or in the future may be treated using our products. Our primary device competitors include: Boston Scientific Corporation; Cook Medical; Cordis Corporation, a subsidiary of Johnson & Johnson, Inc.; C.R. Bard; Medcomp; Radionics, a division of Integra LifeSciences Corporation; Arrow, International; Deltec, Inc., a subsidiary of Smiths Group plc; EV3, Inc.; Kendall Healthcare, a subsidiary of Covidien; Dornier MedTech GmbH; Vascular Solutions and VNUS Medical. Medcomp supplies us with most of our dialysis catheters, but also competes with us by selling Dynamic Flow catheters, which we buy from them on a non-exclusive basis, and other dialysis catheters that we do not license from them. Many of our competitors have substantially greater financial, technological, research and development, regulatory, marketing, sales and personnel resources than we do. Competitors may also have greater experience in developing products, obtaining regulatory approvals, and manufacturing and marketing such products. Additionally, competitors may obtain patent protection or regulatory approval or clearance, or achieve product commercialization, before us, any of which could materially adversely affect us.
We believe that our products compete primarily on the basis of their quality, ease of use, reliability, physician familiarity and cost-effectiveness. Generally, our products are sold at higher prices than those of our competitors. In the current environment of managed care, which is characterized by economically motivated buyers, consolidation among healthcare providers, increased competition and declining reimbursement rates, we have been increasingly required to compete on the basis of price. We believe that our continued competitive success will depend upon our ability to develop or acquire scientifically advanced technology, apply our technology cost-effectively across product lines and markets, develop or acquire proprietary products, attract and retain skilled development personnel, obtain patent or other protection for our products, obtain required regulatory and reimbursement approvals, manufacture and successfully market our products either directly or through outside parties and maintain sufficient inventory to meet customer demand.
Sales and Marketing
We focus our sales and marketing efforts on interventional radiologists, vascular surgeons, and interventional and surgical oncologists. There are over 5,000 interventional radiologists, 2,000 vascular surgeons, and 2,000 interventional and surgical oncologists in the United States. We seek to educate these physicians on the clinical efficacy, performance, ease of use, value and other advantages of our products.
We also involve ourselves in assisting interventional physicians with clinical practice building for outpatient interventional procedures. This can include outpatient practices in uterine fibroid embolization (UFE), vein, dialysis access management, tumor ablation, pain management and broad based interventional procedures.
14
We promote our products through medical society meetings that are attended by interventional radiologists, vascular surgeons, interventional cardiologists, interventional nephrologists, interventional oncologists and others. Our attendance at these meetings is one of our most important methods of communicating with our customers. At these meetings, we receive direct feedback from customers and present new ideas and products. Our attendance at these meetings also reflects our support and commitment to the medical societies, as these societies rely on industry participation and support in order to effectively hold these meetings.
Backlog
Historically, we ship 95% of products sold in the United States within 48 hours of receipt of the orders, and accordingly our backlog is not significant.
Manufacturing
We own a manufacturing, administrative, engineering and warehouse facility of approximately 104,000 square feet in Queensbury, New York. We also lease a manufacturing facility of approximately 60,000 square feet located in Manchester, Georgia. We believe these facilities have sufficient capacity to meet our anticipated manufacturing needs for the next five years.
We manufacture certain proprietary components and products and assemble, inspect, test and package our finished products. By designing and manufacturing many of our products from raw materials, and assembling and testing our subassemblies and products, we believe that we are able to maintain better quality control, ensure compliance with applicable regulatory standards and our internal specifications, and limit outside access to our proprietary technology. We have custom-designed proprietary manufacturing and processing equipment and have developed proprietary enhancements for existing production machinery.
Our management information system includes order entry, invoicing, on-line inventory management, lot traceability, purchasing, shop floor control and shipping and distribution analysis, as well as various accounting-oriented functions. This system enables us to track our products from the inception of an order through all parts of the manufacturing process until the product is delivered to the customer.
We purchase components from third parties. Most of our components are readily available from several supply sources. We also purchase finished products from third parties. One supplier, Medcomp, currently supplies most of our dialysis catheters. Medcomp products accounted for approximately 11% of our net sales for fiscal 2008. To date, we have been able to obtain adequate supplies of all product and components in a timely manner from existing sources.
In fiscal 2008, 73% of our net sales were derived from products we manufactured or assembled ourselves, with the balance being derived from products manufactured for us by third parties. Our Queensbury and Manchester facilities are registered with the FDA and have been certified to ISO 13485 standards, as well as the CMD/CAS Canadian Medical Device Regulations. ISO 13485 is a quality system standard that satisfies European Union regulatory requirements, thus allowing us to market and sell our products in European Union countries. If we were to lose this certification, we would no longer be able to sell our products in these countries until we made the necessary corrections to our operations or satisfactorily completed an alternate European Union approval route that did not rely on compliance with quality system standards. Our manufacturing facilities are subject to periodic inspections by regulatory authorities to ensure compliance with domestic and non-U.S. regulatory requirements. See “Government Regulation.”
Intellectual Property
As of June 30, 2008, we owned 160 U.S. patents, 85 pending US applications, and 244 foreign issued and pending patents. We also own 38 US registered trademarks and 47 common law trademarks, of which 17 are pending. There are currently 35 registered international trademarks.
15
We believe that our success is dependent, to a large extent, on patent protection and the proprietary nature of our technology. We intend to continue to file and prosecute patent applications for our technology in jurisdictions where we believe that patent protection is effective and advisable, generally in the United States and other appropriate jurisdictions.
Notwithstanding the foregoing, the patent positions of medical device companies, including our company, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced either before or after the patent is issued. Consequently, there can be no assurance that any of our pending patent applications will result in an issued patent. There is also no assurance that any existing or future patent will provide significant protection or commercial advantage, or whether any existing or future patent will be circumvented by a more basic patent, thus requiring us to obtain a license to produce and sell the product. Generally, patent applications can be maintained in secrecy for at least 18 months after their earliest priority date. In addition, publication of discoveries in the scientific or patent literature often lags behind actual discoveries. Therefore, we cannot be certain that we were the first to invent the subject matter covered by each of our pending U.S. patent applications or that we were the first to file non-U.S. patent applications for such subject matter.
If a third party files a patent application relating to an invention claimed in our patent application, we may be required to participate in an interference proceeding declared by the U.S. Patent and Trademark Office to determine who owns the patent. Such proceeding could involve substantial uncertainties and cost, even if the eventual outcome is favorable to us. There can be no assurance that our patents, if issued, would be upheld as valid in court.
Third parties may claim that our products infringe on their patents and other intellectual property rights. Some companies in the medical device industry have used intellectual property infringement litigation to gain a competitive advantage. If a competitor were to challenge our patents, licenses or other intellectual property rights, or assert that our products infringe its patent or other intellectual property rights, we could incur substantial litigation costs, be forced to make expensive changes to our product designs, license rights in order to continue manufacturing and selling our products, or pay substantial damages. Third-party infringement claims, regardless of their outcome, would not only consume our financial resources but also divert our management’s time and effort. Such claims could also cause our customers or potential customers to defer or limit their purchase or use of the affected products until resolution of the claim.
In January 2004, Diomed filed an action against us alleging that our VenaCure products for the treatment of varicose veins infringed a patent held by Diomed for a laser system that competes with our VenaCure products. In March 2007, a jury ruled in Diomed’s favor and awarded compensatory damages totaling $9.71 million following an initial appeal. On July 2, 2007, the judge for the Federal District in Boston, Massachusetts, issued an injunction prohibiting us from selling our original bare fiber VenaCure product. We disputed the infringement verdict on multiple grounds and on June 20, 2007, filed an appeal in the U.S. Court of Appeals for the Federal Circuit in Washington, D.C. On March 14, 2008, Diomed commenced Chapter 11 bankruptcy proceedings. On April 2, 2008, we entered into a settlement agreement with Diomed and we paid $7 million resolving the patent disputes. As a result of the settlement, in our fiscal third quarter we reduced our litigation provision and recorded a gain, net of costs, of approximately $3.2 million pre-tax, $2.0 million after tax, an impact of $0.08 in earnings per share.
In October 2005, VNUS Medical Technologies filed an action against us, Diomed and another defendant alleging, among other things, that the manufacture, use and sale of our VenaCure products infringed several patents held by VNUS and seeking injunctive relief and compensatory and treble damages, reasonable attorney’s fees, costs and pre-judgment interest. On June 3, 2008, we entered into an agreement with VNUS settling all patent litigation between us and VNUS. Under the terms of the settlement agreement, we paid VNUS approximately $6.8 million in June 2008 and agreed to pay a quarterly royalty on our U.S. sales of our
16
NeverTouch™ and VenaCure® and Diomed products from June 1, 2008 until the expiration date of VNUS’ applicable patents. In exchange, VNUS granted us a non-exclusive and non-sublicensable license to VNUS’ applicable patents for use in endovenous laser therapy.
See Item 3 of this report for additional details.
We rely on trade secret protection for certain unpatented aspects of our proprietary technology. There can be no assurance that others will not independently develop or otherwise acquire substantially equivalent proprietary information or techniques, that others will not gain access to our proprietary technology or disclose such technology, or that we can meaningfully protect our trade secrets. We have a policy of requiring key employees and consultants to execute confidentiality agreements upon the commencement of an employment or consulting relationship with us. Our confidentiality agreements also require our employees to assign to us all rights to any inventions made or conceived during their employment with us. We also generally require our consultants to assign to us any inventions made during the course of their engagement by us. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for us in the event of unauthorized use, transfer or disclosure of confidential information or inventions.
The laws of foreign countries generally do not protect our proprietary rights to the same extent, as do the laws of the United States. In addition, we may experience more difficulty enforcing our proprietary rights in certain foreign jurisdictions.
Government Regulation
The products we manufacture and market are subject to regulation by the FDA under the Federal Food, Drug, and Cosmetic Act, or FDCA, and, in some instances, state authorities and foreign governments.
United States FDA Regulation
Before a new medical device can be introduced into the market, a manufacturer generally must obtain marketing clearance or approval from the FDA through either a 510(k) submission (a premarket notification) or a premarket approval application, or PMA.
The 510(k) procedure is less rigorous than the PMA procedure, but is available only in particular circumstances. The 510(k) clearance procedure is available only if a manufacturer can establish that its device is “substantially equivalent” in intended use and in safety and effectiveness to a “predicate device,” which is a legally marketed device with 510(k) clearance in class I or II or grandfather status based upon commercial distribution on or before May 28, 1976. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require a PMA approval. The 510(k) clearance procedure generally takes from four to 12 months from the time of submission, but may take longer. In some cases, supporting clinical data may be required. The FDA may determine that a new or modified device is not substantially equivalent to a predicate device or may require that additional information, including clinical data, be submitted before a determination is made, either of which could significantly delay the introduction of new or modified device products. If a product does not satisfy the criteria of substantial equivalence, it is placed in class III and premarket approval is required prior to the introduction of that product into the market.
The PMA application procedure is more comprehensive than the 510(k) procedure and typically takes several years to complete. The PMA application must be supported by scientific evidence providing pre-clinical and clinical data relating to the safety and efficacy of the device and must include other information about the device and its components, design, manufacturing and labeling. The FDA will approve a PMA application only if a reasonable assurance that the device is safe and effective for its intended use can be provided. As part of the PMA application review, the FDA will inspect the manufacturer’s facilities for compliance with its Quality System Regulation, or QSR. As part of the PMA approval the FDA may place restrictions on the device, such as
17
requiring additional patient follow-up for an indefinite period of time. If the FDA’s evaluation of the PMA application or the manufacturing facility is not favorable, the FDA may deny approval of the PMA application or issue a “not approvable” letter. The FDA may also require additional clinical trials, which can delay the PMA approval process by several years. After the PMA is approved, if significant changes are made to a device, its manufacturing or labeling, a PMA supplement containing additional information must be filed for prior FDA approval.
Historically, our products have been introduced into the market using the 510(k) procedure and we have never had to use the more rigorous PMA procedure.
The FDA clearance and approval processes for a medical device are expensive, uncertain and lengthy. There can be no assurance that we will be able to obtain necessary regulatory clearances or approvals for any product on a timely basis or at all. Delays in receipt of or failure to receive such clearances or approvals, the loss of previously received clearances or approvals, or the failure to comply with existing or future regulatory requirements could have a material adverse effect on our business, financial condition and results of operations.
After a product is placed on the market, the product and its manufacturer are subject to pervasive and continuing regulation by the FDA. The FDA enforces these requirements by inspection and market surveillance. Our suppliers also may be subject to FDA inspection. We must therefore continue to spend time, money and effort to maintain compliance. Among other things, we must comply with the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. We must also comply with the FDA’s corrections and removal reporting regulation, which requires that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by a device or to remedy a violation of the FDCA that may present a risk to health. The labeling and promotion activities for devices are subject to scrutiny by the FDA and, in certain instances, by the Federal Trade Commission. The FDA actively enforces regulations prohibiting the marketing of devices for unapproved new uses.
The devices manufactured by us also are subject to the QSR, which imposes elaborate testing, control, documentation and other quality assurance procedures. Every phase of production, including raw materials, components and subassemblies, manufacturing, testing, quality control, labeling, tracing of consignees after distribution and follow-up and reporting of complaint information is governed by the FDA’s QSR. Device manufacturers are required to register their facilities and list their products with the FDA and certain state agencies. The FDA periodically inspects manufacturing facilities and, if there are alleged violations, the operator of a facility must correct them or satisfactorily demonstrate the absence of the violations or face regulatory action.
We are subject to inspection and marketing surveillance by the FDA to determine our compliance with all regulatory requirements. Recently, the FDA has placed an increased emphasis on enforcement of the QSR and other postmarket regulatory requirements. Non-compliance with applicable FDA requirements can result in, among other things, fines, injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, failure of the FDA to grant marketing approvals, withdrawal of marketing approvals, a recommendation by the FDA to disallow us to enter into government contracts, and criminal prosecutions. The FDA also has the authority to request repair, replacement or refund of the cost of any device manufactured or distributed by us.
Other
We and our products are also subject to a variety of state and local laws in those jurisdictions where our products are or will be marketed, and Federal, state and local laws relating to matters such as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. For example, we are registered with the Office of the Professions of the
18
New York State Department of Education. We are also subject to various Federal and state laws governing our relationships with the physicians and others who purchase or make referrals for our products. For instance, Federal law prohibits payments of any form that are intended to induce a referral for any item payable under Medicare, Medicaid or any other Federal healthcare program. Many states have similar laws. There can be no assurance that we will not be required to incur significant costs to comply with such laws and regulations now or in the future or that such laws or regulations will not have a material adverse effect upon our ability to do business.
Non-U.S. Regulation
Internationally, all of our current products are considered medical devices under applicable regulatory regimes, and we anticipate that this will be true for all of our future products. Sales of medical devices are subject to regulatory requirements in many countries. The regulatory review process may vary greatly from country to country. For example, the European Union has adopted numerous directives and standards relating to medical devices regulating their design, manufacture, clinical trials, labeling and adverse event reporting. Devices that comply with those requirements are entitled to bear a Conformité Européenne, or CE Mark, indicating that the device conforms with the essential requirements of the applicable directives and can be commercially distributed in countries that are members of the European Union.
In some cases, we rely on our non-U.S. distributors to obtain regulatory approvals, complete product registrations, comply with clinical trial requirements and complete those steps that are customarily taken in the applicable jurisdictions.
Non-U.S. sales of medical devices manufactured in the United States that are not approved or cleared by the FDA for use in the United States, or are banned or deviate from lawful performance standards, are subject to FDA export requirements. Before exporting such products to a foreign country, we must first comply with the FDA’s regulatory procedures for exporting unapproved devices.
There can be no assurance that new laws or regulations regarding the release or sale of medical devices will not delay or prevent sale of our current or future products.
Third-Party Reimbursement
United States
Our products are used in medical procedures generally covered by government or private health plans. Accordingly, our sales and the prices we charge for our products depend significantly on the extent to which those third-party payors, such as Medicare, Medicaid and other government programs and private insurance plans, cover our products and the procedures performed with them.
In general, a third-party payor only covers a medical product or procedure when the plan administrator is satisfied that the product or procedure improves health outcomes, including quality of life or functional ability, in a safe and cost-effective manner. Even if a device has received clearance or approval for marketing by the FDA, there is no assurance that third-party payors will cover the cost of the device and related procedures.
In many instances, third-party payors use price schedules that do not vary to reflect the cost of the products and equipment used in performing those procedures. In other instances, payment or reimbursement is separately available for the products and equipment used, in addition to payment or reimbursement for the procedure itself. Even if coverage is available, third-party payors may place restrictions on the circumstances where they provide coverage or may offer reimbursement that is not sufficient to cover the cost of our products. Many competing products are less expensive than ours. Therefore, although coverage may be available for our products and the related procedures, the levels of approved coverage may not be sufficient to justify using our products instead of those of competitors.
19
Third-party payors are increasingly challenging the prices charged for medical products and procedures and, where a reimbursement model is used, introducing maximum reimbursements for the procedures they cover. We believe that the minimally invasive procedures in which our products are used are generally less costly than open surgery. However, there is no guarantee that these procedures will be reimbursed. Third-party payors may not consider these minimally invasive procedures to be cost-effective and may therefore refuse to authorize coverage.
Third-party payors who cover the cost of medical products or equipment, in addition to allowing a general charge for the procedure, often maintain lists of exclusive suppliers or approved lists of products deemed to be cost-effective. Authorization from those third-party payors is required prior to using products that are not on these lists as a condition of reimbursement. If our products are not on the approved lists, healthcare providers must determine if the additional cost and effort required to obtain prior authorization, and the uncertainty of actually obtaining coverage, is justified by any perceived clinical benefits from using our products.
Finally, the advent of contracted fixed rates per procedure has made it difficult to receive reimbursement for disposable products, even if the use of these products improves clinical outcomes. In addition, many third-party payors are moving to managed care systems in which providers contract to provide comprehensive healthcare for a fixed cost per person. Managed care providers often attempt to control the cost of healthcare by authorizing fewer elective surgical procedures. Under current prospective payment systems, such as the diagnosis related group system and the hospital out-patient prospective payment system, both of which are used by Medicare and in many managed care systems used by private third-party payors, the cost of our products will be incorporated into the overall cost of a procedure and not be separately reimbursed. As a result, we cannot be certain that hospital administrators and physicians will purchase our products, despite the clinical benefits and opportunity for cost savings that we believe can be derived from their use.
If hospitals and physicians cannot obtain adequate reimbursement for our products or the procedures in which they are used, our business, financial condition, results of operations, and cash flows could suffer a material adverse impact.
Non-U.S.
Our success in non-U.S. markets will depend largely upon the availability of reimbursement from the third-party payors through which healthcare providers are paid in those markets. Reimbursement and healthcare payment systems in non-U.S. markets vary significantly by country. The main types of healthcare payment systems are government sponsored healthcare and private insurance. Reimbursement approval must be obtained individually in each country in which our products are marketed. Outside the United States, we generally rely on our distributors to obtain reimbursement approval in the countries in which they will sell our products. There can be no assurance that reimbursement approvals will be received.
Insurance
Our product liability insurance coverage is limited to a maximum of $10,000,000 per product liability claim and an aggregate policy limit of $10,000,000, subject to deductibles of $250,000 per occurrence and $500,000 in the aggregate. The policy covers, subject to policy conditions and exclusions, claims of bodily injury and property damage from any product sold or manufactured by us.
We cannot assure you that this level of coverage is adequate. We may not be able to sustain or maintain this level of coverage and cannot assure you that adequate insurance coverage will be available on commercially reasonable terms or at all. A successful product liability claim or other claim with respect to uninsured or underinsured liabilities could have a material adverse effect on our business.
20
Environmental
We are subject to Federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain hazardous and potentially hazardous substances used in connection with our operations. Although we believe that we have complied with these laws and regulations in all material respects and, to date, have not been required to take any action to correct any noncompliance, there can be no assurance that we will not be required to incur significant costs to comply with environmental regulations in the future.
Employees
As of May 31, 2008, we had 566 full-time employees, including 321 in manufacturing; 54 in research, product development and regulatory approval/quality assurance; 151 in sales and marketing; and 40 in administration. None of our employees is represented by a labor union, and we have never experienced a work stoppage.
21
Our financial and operating results are subject to a number of factors, many of which are not within our control. These factors include the following:
If we fail to develop or market new products and enhance existing products, we could lose market share to our competitors and our results of operations could suffer.
The market for interventional devices is characterized by rapid technological change, new product introductions, technological improvements, changes in physician requirements and evolving industry standards. To be successful, we must continue to develop and commercialize new products and to enhance versions of our existing products. Our products are technologically complex and require significant research, planning, design, development and testing before they may be marketed. This process generally takes at least 12 to 18 months from initial concept and may take up to several years. In addition, product life cycles are relatively short because medical device manufacturers continually develop smaller, more effective and less expensive versions of existing devices in response to physician demand.
Our success in developing and commercializing new and enhanced versions of our products is affected by our ability to:
| | • | | timely and accurately identify new market trends; |
| | • | | accurately assess customer needs; |
| | • | | minimize the time and costs required to obtain regulatory clearance or approval; |
| | • | | adopt competitive pricing; |
| | • | | timely manufacture and deliver products; |
| | • | | accurately predict and control costs associated with the development, manufacturing and support of our products; and |
| | • | | anticipate and compete effectively with our competitors’ efforts. |
Market acceptance of our products depends in part on our ability to demonstrate that our products are cost-effective and easier to use, as well as offer technological advantages. Additionally, we may experience design, manufacturing, marketing or other difficulties that could delay or prevent our development, introduction or marketing of new versions of our products. As a result of such difficulties and delays, our development expenses may increase and, as a consequence, our results of operations could suffer.
We face intense competition in the medical device industry. We may be unable to compete effectively with respect to technological innovation and price which may have an adverse effect on our revenues, financial condition or results of operations.
The markets for interventional devices are highly competitive, and we expect competition to continue to intensify. We may not be able to compete effectively, and we may lose market share to our competitors. The principal competitors in the markets for our products currently include: Boston Scientific Corporation; Cook Medical; Cordis Corporation, a subsidiary of Johnson & Johnson, Inc.; C.R. Bard Inc.; Radionics, a division of Integra LifeSciences Corporation; Medical Components, Inc., or Medcomp; and VNUS Medical Technologies, Inc. Many of our competitors have substantially greater:
| | • | | financial and other resources to devote to product acquisitions, research and development, marketing and manufacturing; |
| | • | | technical capabilities; |
22
| | • | | history of developing and introducing new products; |
| | • | | patent portfolios that may present an obstacle to our conduct of business; |
| | • | | distribution networks and in-house sales forces. |
Our competitors may succeed in developing technologies and products earlier, in obtaining patent protection or regulatory clearance earlier, or in commercializing new products or technologies more rapidly than us. Our competitors may also develop products and technologies that are superior to those we are developing or that otherwise could render our products obsolete or noncompetitive. In addition, we may face competition from providers of other medical therapies, such as pharmaceutical companies, that may offer non-surgical therapies for conditions that are currently or in the future may be treated using our products. Our products are generally sold at higher prices than those of our competitors. However, in the current environment of managed care, which is characterized by economically motivated buyers, consolidation among healthcare providers, increased competition and declining reimbursement rates, we are increasingly being required to compete on the basis of price. If we are not able to compete effectively, our market share and revenues may decline.
Development and sales of our IRE products are dependent on a number of factors beyond our control, and our inability to successfully complete our research and development, design and marketing strategy with respect to IRE may adversely affect our business, financial condition and results of operations.
A significant aspect of our growth strategy is the development of our IRE products, including NanoKnife. Our IRE products are currently in development and there can be no guarantee that we will be able to develop and manufacture IRE products on commercially favorable terms, or at all. IRE is a developing technology and the inability of IRE to achieve clinical acceptance could severely limit the sales of IRE products.
We currently have FDA 510(k) clearance to market IRE products for soft tissue ablation. If we are not able to secure FDA marketing approval for additional or more specific indications, through 510(k) clearance, pre-market approval or otherwise, our ability to market our IRE products will be restricted which may have an adverse effect on our business, financial condition and results of operations.
We may be exposed to risks associated with acquisitions, including integration risks and risks associated with methods of financing and the impact of accounting treatment. Accordingly, completed acquisitions may not enhance our financial position or results of operations.
Part of our growth strategy is to acquire businesses and technologies that are complementary to ours. We cannot assure you that acquisition opportunities will be available on acceptable terms or at all or that we will be able to obtain necessary financing or regulatory approvals. Any acquisitions that we do undertake would be accompanied by the risks commonly encountered in acquisitions, including the:
| | • | | potential disruption of our business while we evaluate opportunities, complete acquisitions and develop and implement new business strategies to take advantage of these opportunities; |
| | • | | inability of our management to maximize our financial and strategic position by incorporating an acquired technology or business into our existing offerings; |
| | • | | difficulty of maintaining uniform standards, controls, procedures and policies; |
| | • | | difficulty of assimilating the operations and personnel of acquired businesses; |
| | • | | potential loss of key employees of acquired businesses, and the impairment of relationships with employees and customers as a result of changes in management; and |
| | • | | uncertainty as to the long-term success of any acquisitions we may make. |
23
We cannot assure you that any completed acquisition will be accretive to our margins or profits in the short term or in the long term. If we proceed with one or more significant acquisitions in which the consideration consists of cash, a substantial portion of our available cash, could be used to consummate the acquisitions. If we consummate one or more acquisitions in which the consideration consists of capital stock, our stockholders could suffer significant dilution of their interest in us. In addition, we could incur or assume significant amounts of indebtedness in connection with acquisitions. Further, acquisitions could also result in significant goodwill and/or amortization charges for acquired businesses or technologies.
If we fail to adequately protect our intellectual property rights, we may not be able to generate revenues from new or existing products and our business may suffer.
Our success depends in part on obtaining, maintaining and enforcing our patents, trademarks and other proprietary rights, and our ability to avoid infringing the proprietary rights of others. We take precautionary steps to protect our technological advantages and intellectual property. We rely upon patent, trade secret, copyright, know-how and trademark laws, as well as license agreements and contractual provisions, to establish our intellectual property rights and protect our products. However, no assurances can be made that any pending or future patent applications will result in the issuance of patents, that any current or future patents issued to, or licensed by, us will not be challenged or circumvented by our competitors, or that our patents will not be found invalid.
Additionally, we may not be able to effectively protect our rights in unpatented technology, trade secrets and confidential information. Although we require our new employees, consultants and corporate partners to execute confidentiality agreements, these agreements may not provide effective protection of our information or, in the event of unauthorized use or disclosure, may not provide adequate remedies.
If we are not able to adequately protect our intellectual property, our market share, financial condition and results of operations may suffer.
If third parties claim that our products infringe their intellectual property rights, we may be forced to expend significant financial resources and management time defending against such actions and our financial condition and our results of operations could suffer.
Third parties may claim that our products infringe their patents and other intellectual property rights. Identifying third-party patent rights can be particularly difficult because, in general, patent applications can be maintained in secrecy for at least 18 months after their earliest priority date. Some companies in the medical device industry have used intellectual property infringement litigation to gain a competitive advantage. If a competitor were to challenge our patents, licenses or other intellectual property rights, or assert that our products infringe its patent or other intellectual property rights, we could incur substantial litigation costs, be forced to make expensive changes to our product design, pay royalties or other fees to license rights in order to continue manufacturing and selling our products, or pay substantial damages. Third-party infringement claims, regardless of their outcome, would not only consume our financial resources but also divert our management’s time and effort. Such claims could also cause our customers or potential customers to purchase competitors’ products or defer or limit their purchase or use of our affected products until resolution of the claim.
In January 2004, Diomed filed an action against us alleging that our VenaCure products for the treatment of varicose veins infringed a patent held by Diomed for a laser system that competes with our VenaCure products. In March 2007, a jury ruled in Diomed’s favor and awarded compensatory damages of $9.71 million. On July 2, 2007, the judge for the Federal District in Boston, Massachusetts, issued an injunction prohibiting us from selling our original bare fiber VenaCure product. We disputed the infringement verdict on multiple grounds and on June 20, 2007, filed an appeal in the U.S. Court of Appeals for the Federal Circuit in Washington, D.C. On March 14, 2008, Diomed commenced Chapter 11 bankruptcy proceedings. On April 2, 2008, we entered into a
24
settlement agreement with Diomed and paid $7 million to resolve the patent disputes. As a result of the settlement, in our fiscal third quarter we reduced our litigation provision and recorded a gain, net of costs, of approximately $3.2 million pre-tax, $2.0 million after tax, and $0.08 in earnings per share.
In October 2005, VNUS Medical Technologies filed an action against us, Diomed and another defendant alleging, among other things, that the manufacture, use and sale of our VenaCure products infringed several patents held by VNUS and seeking injunctive relief and compensatory and treble damages. On June 3, 2008, we entered into an agreement with VNUS settling all patent litigation between us and VNUS. Under the terms of the settlement agreement, we paid VNUS approximately $6.8 million and agreed to pay a quarterly royalty on our U.S. sales of our NeverTouch™ and VenaCure® products from June 1, 2008 until the expiration date of VNUS’ applicable patents. In exchange, VNUS granted us a non-exclusive and non-sublicensable license to VNUS’ applicable patents for use in endovenous laser therapy.
We are dependent on single and limited source suppliers which subjects our business and results of operations to risks of supplier business interruptions.
We currently purchase significant amounts of several key products and product components from single and limited source suppliers and anticipate that we will do so for future products as well. For fiscal 2008, approximately 27% of our net sales were derived from sales of products manufactured for us by third parties. Our principal single source supplier, Medcomp, supplies us with most of our dialysis catheters, which accounted for about 11% of our net sales in fiscal 2008. Medcomp also competes with us by selling Dynamic-Flow, a dialysis catheter for which it has not granted us exclusive rights, and other catheters that we do not purchase from them.
Any delays in delivery of or shortages in those or other products and components could interrupt and delay manufacturing of our products and result in the cancellation of orders for our products. Any or all of these suppliers could discontinue the manufacture or supply of these products and components at any time. Due to FDA and other business considerations, we may not be able to identify and integrate alternative sources of supply in a timely fashion or at all. Any transition to alternate suppliers may result in production delays and increased costs and may limit our ability to deliver products to our customers. Furthermore, if we are unable to identify alternative sources of supply, we would have to modify our products to use substitute components, which may cause delays in shipments, increased design and manufacturing costs and increased prices for our products.
If we do not maintain our reputation with interventional physicians, our growth will be limited and our business could be harmed.
Physicians typically influence the medical device purchasing decisions of the hospitals and other healthcare institutions in which they practice. Consequently, our reputation with interventional physicians is critical to our continued growth. We believe that we have built a positive reputation based on the quality of our products, our physician-driven product development efforts, our marketing and training efforts and our presence at medical society meetings. Any actual or perceived diminution in the quality of our products, or our failure or inability to maintain these other efforts, could damage our reputation with interventional physicians and cause our growth to be limited and our business to be harmed.
Our business could be harmed if we lose the services of our key personnel.
Our business depends upon our ability to attract and retain highly qualified personnel, including managerial, sales and technical personnel. We compete for key personnel with other companies, healthcare institutions, academic institutions, government entities and other organizations. We do not have written employment agreements with our executive officers. Our ability to maintain and expand our business may be impaired if we are unable to retain our current key personnel or hire or retain other qualified personnel in the future.
25
Undetected defects may increase our costs and impair the market acceptance of our products.
Our products have occasionally contained, and may in the future contain, undetected defects. When these problems occur, we must divert the attention of our engineering personnel to address them. We cannot assure you that we will not incur warranty or repair costs, be subject to liability claims for damages related to product defects, or experience manufacturing, shipping or other delays or interruptions as a result of these defects in the future. Our insurance policies may not provide sufficient protection should a claim be asserted. In addition, the occurrence of defects may result in significant customer relations problems and injury to our reputation, and may impair market acceptance of our products.
If a product liability claim is brought against us or our product liability insurance coverage is inadequate, our business could be harmed.
The design, manufacture and marketing of the types of medical devices we sell entail an inherent risk of product liability. Our products are used by physicians to treat seriously ill patients. We have been subject to product liability claims in the past, and patients or customers may in the future bring claims in a number of circumstances and for a number of reasons, including if our products were misused, if a component of our product fails, if their manufacture or design was flawed, if they produced unsatisfactory results or if the instructions for use and operating manuals and disclosure of product related risks for our products were found to be inadequate. In addition, individuals or groups seeking to represent a class may file suit against us. The outcome of litigation, particularly class action lawsuits, is difficult to assess or quantify. Plaintiffs in these types of lawsuits often seek recovery of very large or indeterminate amounts, including not only actual damages, but also punitive damages. The magnitude of the potential losses relating to these lawsuits may remain unknown for substantial periods of time.
We carry a product liability policy with limits of $10 million per occurrence and in the aggregate per year, with a $250,000 deductible per incident and an aggregate deductible limit of $500,000 per year. We believe, based on claims made against us in the past, our existing product liability insurance coverage is reasonably adequate to protect us from any liabilities we might incur. However, we cannot assure you that this coverage will be sufficient to satisfy any claim made against us. In addition, we may not be able to maintain adequate coverage at a reasonable cost and on reasonable terms, if at all. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing any coverage in the future. Additionally, if one or more product liability claims is brought against us for uninsured liabilities or is in excess of our insurance coverage, our financial condition and results of operations could be negatively impacted. Further, such claims may require us to recall some of our products, which could result in significant costs to us and could divert management’s attention from our business.
Changes in reimbursement levels by governmental or other third-party payors for procedures using our products may cause our revenues to decline.
Our products are purchased principally by hospitals or physicians which typically bill various third-party payors, such as governmental programs (e.g. Medicare, Medicaid and comparable foreign programs), private insurance plans and managed care plans, for the healthcare services provided to their patients. The ability of our customers to obtain appropriate reimbursement for products and services from third-party payors is critical to the success of medical device companies because it affects which products customers purchase and the prices they are willing to pay. Reimbursement varies by country and can significantly impact the acceptance of new technology. Implementation of healthcare reforms in the United States and in other countries may limit, reduce or eliminate reimbursement for our products and adversely affect both our pricing flexibility and the demand for our products. Even when we develop a promising new product, we may find limited demand for the product unless reimbursement approval is obtained from private and governmental third party payors.
26
Third-party payors have adopted, and are continuing to adopt, a number of healthcare policies intended to curb rising healthcare costs. These policies include:
| | • | | controls on government-funded reimbursement for healthcare services and price controls on medical products and services providers; |
| | • | | challenges to the pricing of medical procedures or limits or prohibitions on reimbursement for specific devices and therapies through other means; and |
| | • | | the introduction of managed care systems in which healthcare providers contract to provide comprehensive healthcare for a fixed cost per person. |
We are unable to predict whether Federal, state or local healthcare reform legislation or regulation affecting our business may be proposed or enacted in the future, or what effect any such legislation or regulation would have on our business. Changes in healthcare systems in the United States or elsewhere in a manner that significantly reduces reimbursement for procedures using our medical devices or denies coverage for these procedures, or adverse decisions relating to our products by administrators of these systems in coverage or reimbursement issues, would have an adverse impact on the acceptance of our products and the prices which our customers are willing to pay for them.
If we cannot obtain and maintain marketing clearance or approval from governmental agencies, we will not be able to sell our products.
Our products are medical devices that are subject to extensive regulation in the United States and in the foreign countries in which they are sold. Unless an exemption applies, each medical device that we wish to market in the United States must receive either 510(k) clearance or premarket approval from the U.S. Food and Drug Administration, or the FDA, before the product can be sold. Either process can be lengthy and expensive. The FDA’s 510(k) clearance procedure, also known as “premarket notification,” is the process we have used for our current products. This process usually takes from four to 12 months from the date the premarket notification is submitted to the FDA, but may take significantly longer. Although we have obtained 510(k) clearances for our current products, our clearances may be revoked by the FDA if safety or effectiveness problems develop with the devices. The premarket approval process is much more costly, lengthy and uncertain. It generally takes from one to three years from the date the application is submitted to, and filed with, the FDA, and may take even longer. Regulatory regimes in other countries similarly require approval or clearance prior to our marketing or selling products in those countries. We rely on our distributors to obtain regulatory clearances or approvals of our products outside of the United States. If we are unable to obtain additional clearances or approvals needed to market existing or new products in the United States or elsewhere or obtain these clearances or approvals in a timely fashion or at all, or if our existing clearances are revoked, our revenues and profitability may decline.
Modifications to our current products may require new marketing clearances or approvals or require us to cease marketing or recall the modified products until such clearances or approvals are obtained.
Any modification to an FDA-cleared medical device that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, requires a new FDA 510(k) clearance or, possibly, a premarket approval. The FDA requires every manufacturer to make its own determination as to whether a modification requires a new 510(k) clearance or premarket approval, but the FDA may review and disagree with any decision reached by the manufacturer. We have modified aspects of some of our devices since receiving regulatory clearance. We believed that some of these modifications did not require new 510(k) clearance or premarket approval and, therefore, we did not seek new 510(k) clearances or premarket approvals. In the future, we may make additional modifications to our products after they have received FDA clearance or approval and, in appropriate circumstances, determine that new clearance or approval is unnecessary. Regulations in other countries in which we market or sell, or propose to market or sell, our products may also require that we make judgments about changes to our products and whether or not those changes are such that regulatory approval or clearance should be obtained. In the United States and elsewhere, regulatory
27
authorities may disagree with our past or future decisions not to seek new clearance or approval and may require us to obtain clearance or approval for modifications to our products. If that were to occur for a previously cleared or approved product, we may be required to cease marketing or recall the modified device until we obtain the necessary clearance or approval. Under these circumstances, we may also be subject to significant regulatory fines or other penalties. If any of the foregoing were to occur, our financial condition and results of operations could be negatively impacted.
If we or some of our suppliers fail to comply with the FDA’s Quality System Regulation, or QSR, and other applicable postmarket requirements, our manufacturing operations could be disrupted, our product sales and profitability could suffer, and we may be subject to a wide variety of FDA enforcement actions.
After a device is placed on the market, numerous regulatory requirements apply. We are subject to inspection and marketing surveillance by the FDA to determine our compliance with all regulatory requirements. Our failure to comply with applicable regulatory requirements could result in the FDA or a court instituting a wide variety of enforcement actions against us, including a public warning letter; an order to shut-down some or all manufacturing operations; a recall of products; fines or civil penalties; seizure or detention of our products; refusing our requests for 510(k) clearance or a premarket approval, or PMA, of new or modified products; withdrawing 510(k) clearance or PMA approvals already granted to us; and criminal prosecution.
Our manufacturing processes and those of some of our suppliers must comply with the FDA’s Quality System Regulation, or QSR, which governs the methods used in, and the facilities and controls used for, the design, testing, manufacture, control, quality assurance, installation, servicing, labeling, packaging, storage and shipping of medical devices. The FDA enforces the QSR through unannounced inspections. If we or one of our suppliers fails a QSR inspection, or if a corrective action plan adopted by us or one of our suppliers is not sufficient, the FDA may bring an enforcement action, and our operations could be disrupted and our manufacturing delayed. We are also subject to the FDA’s general prohibition against promoting our products for unapproved or “off-label” uses, the FDA’s adverse event reporting requirements and the FDA’s reporting requirements for field correction or product removals. The FDA has recently placed increased emphasis on its scrutiny of compliance with the QSR and these other postmarket requirements.
If we or one of our suppliers violate the FDA’s requirements or fail to take adequate corrective action in response to any significant compliance issue raised by the FDA, the FDA can take various enforcement actions which could cause our product sales and profitability to suffer.
In addition, most other countries require us and our suppliers to comply with manufacturing and quality assurance standards for medical devices that are similar to those in force in the United States before marketing and selling our products in those countries. If we or our suppliers should fail to do so, we would lose our ability to market and sell our products in those countries.
Even after receiving regulatory clearance or approval, our products may be subject to product recalls, which may harm our reputation and divert managerial and financial resources.
The FDA and similar governmental authorities in other countries have the authority to order mandatory recall of our products or order their removal from the market if there are material deficiencies or defects in design, manufacture, installation, servicing or labeling of the device, or if the governmental entity finds that our products would cause serious adverse health consequences. A government mandated or voluntary recall or field action by us could occur as a result of component failures, manufacturing errors or design defects, including labeling defects. Any recall of our products may harm our reputation with customers and divert managerial and financial resources.
28
Failure to attract additional capital which we may require to expand our business could curtail our growth.
We may require additional capital to expand our business. If cash generated internally is insufficient to fund capital requirements, we will require additional debt or equity financing. In addition, we may require financing to fund any significant acquisitions we may seek to make. Needed financing may not be available or, if available, may not be available on terms satisfactory to us and may result in significant stockholder dilution. Covenants in our industrial bond financing may also restrict our ability to obtain additional debt financing. If we fail to obtain sufficient additional capital in the future, we could be forced to curtail our growth strategy by reducing or delaying capital expenditures and acquisitions, selling assets, restructuring our operations or refinancing our indebtedness.
Any disaster at our manufacturing facilities could disrupt our ability to manufacture our products for a substantial amount of time, which could cause our revenues to decrease.
We conduct our manufacturing and assembly at two facilities in Queensbury, New York, and Manchester, Georgia. It would be difficult, expensive and time-consuming to transfer resources from one facility to the other, replace, or repair these facilities and our manufacturing equipment if they were significantly affected by a disaster. Additionally, we might be forced to rely on third-party manufacturers or to delay production of our products. Insurance for damage to our properties and the disruption of our business from disasters may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In addition, if one of our principal suppliers were to experience a similar disaster, uninsured loss or under-insured loss, we might not be able to obtain adequate alternative sources of supplies or products or could face significant delays and incur substantial expense in doing so. Any significant uninsured loss, prolonged or repeated disruption, or inability to operate experienced by us or any of our principal suppliers could cause significant harm to our business, financial condition and results of operations.
Our inability to manage our growth or successfully implement our internal reorganization may have an adverse effect on our business, financial condition or results of operations.
Over the past several years we have experienced significant growth. Our inability to manage our growth could impact our ability to meet our customers’ demands, which could cause future sales to suffer.
To better and more efficiently manage our business, we recently announced, and are currently implementing, a reorganization of our structure and management to align our operations with our key customer groups—Peripheral Vascular, Access, and Oncology/Surgery. Implementing the reorganization requires significant time and resource commitments from our senior management. In the event that we are unable to effectively implement the reorganization, we are unable to recruit or retain key employees as a result of the reorganization or the reorganization does not yield the anticipated benefits, our business may be adversely affected.
| Item 1B. | Unresolved Staff Comments |
None
We own a manufacturing, administrative, engineering and warehouse facility of approximately 104,000 square feet situated on 18 acres in Queensbury, New York. In fiscal 2003, we financed an expansion of this facility with the proceeds of industrial revenue bonds, and the land and buildings are subject to a first mortgage in favor of a bank. In 2006, we issued taxable adjustable rate notes to finance an expansion of 36,000 square feet to our warehouse and manufacturing facility. See Item 7 of this annual report, “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources,” for a discussion
29
of these financings. We anticipate requiring additional administrative and engineering space within the next one to two years.
We also lease three additional properties. We lease a manufacturing facility of approximately 60,000 square feet located in Manchester, Georgia. This facility also includes office and research and development space and is leased through 2010. We lease 14,500 square feet of office and research and development space in Fremont, California. The lease is non-cancelable and expires in April 2010. Finally, we lease a manufacturing facility of approximately 20,000 square feet in the United Kingdom that we acquired in June 2008 in connection with our acquisition of certain assets of Diomed, Ltd.
Diomed v. AngioDynamics and AngioDynamics v. biolitec
On January 6, 2004, Diomed filed an action against us entitled Diomed, Inc. v. AngioDynamics, Inc., et al., civil action no. 04 10019 RGS in the U.S. District Court for the District of Massachusetts. Diomed’s complaint alleged that we infringed on Diomed’s U.S. patent no. 6,398,777 by selling a kit for the treatment of varicose veins (now called the “VenaCure Procedure Kit”) and two diode laser systems (the Precision 980 Laser and the Precision 810 Laser), and by conducting a training program for physicians in the use of the VenaCure Procedure Kit. The complaint alleged that our actions have caused Diomed to suffer substantial damages.
On March 28, 2007, the jury in the proceeding returned a verdict in favor of Diomed and awarded compensatory monetary damages in the amount of $8.36 million. The jury concluded, however, that there was no willful infringement by us. On May 22, 2007, the judge for the Federal District Court in Boston denied our motion to overturn the verdict and increased the judgment for compensatory damages by $1.35 million, to $9.71 million, to cover pretrial interest and post-verdict sales of the infringing products. The judgment also required us to pay interest to Diomed at an annual rate of approximately 5% of the damage award for the period of time between the verdict and actual payment of the award. As a result we accrued approximately $10.2 million, including interest. On July 2, 2007, the judge for the Federal District in Boston, Massachusetts, issued an injunction prohibiting us from selling our original bare fiber VenaCure product. We disputed the infringement verdict on multiple grounds and on June 20, 2007, filed an appeal in the U.S. Court of Appeals for the Federal Circuit in Washington, D.C.
On March 14, 2008, Diomed commenced Chapter 11 bankruptcy proceedings. On April 2, 2008, we entered into a settlement with Diomed for the purpose of resolving the alleged patent infringement and paid $7.0 million in the fourth quarter of 2008. As a result of the settlement, in our third fiscal quarter we reduced our litigation provision and recorded a gain of approximately $3.2 million pretax, $2.0 after tax, an impact of $0.08 on earnings per share as reflected in the third quarter results.
Until April 2007, we purchased the lasers and laser fibers for our laser systems from biolitec under a supply agreement. In 2006, biolitec advised us that based on Diomed’s refinement of its claims in the Diomed action, biolitec believed such claims were not within biolitec’s indemnification obligations under the supply agreement. We advised biolitec that we disagreed with biolitec’s position and that we expected biolitec to continue to honor its indemnification obligations.
On January 2, 2008, we commenced an action in the United States District Court for the Northern District of New York entitled AngioDynamics, Inc. v. biolitec, Inc.in which we are seeking, in part, judgment against biolitec for imdemnification of defense costs we incurred in the Diomed action and the VNUS action described below. On January 11, 2008, biolitec commenced an action in the United States District Court for the Western District of Massachusetts entitled biolitec, Inc. v. AngioDynamics, Inc. In this action, biolitec is seeking reimbursement of not less than $1.6 million in alleged past defense costs paid by biolitec in the Diomed action. We moved to dismiss this action or, in the alternative, to have this action transferred to the Northern District of New York for consolidation with AngioDynamics, Inc. v. biolitec, Inc.Biolitec has filed counter-claims against us in the New York action, seeking similar claims as in the Massachusetts action.
30
We will continue to vigorously enforce our rights under the supply agreement with biolitec. However, in the event it is ultimately determined that the claims asserted in the Diomed action and the VNUS action are not within biolitec’s indemnification obligations under the biolitec supply agreement, we may be required to reimburse biolitec for the costs and expenses of defending the Diomed action.
VNUS Medical Technologies v. Diomed, Vascular Solutions, and AngioDynamics
On October 4, 2005, VNUS Medical Technologies, Inc. (“VNUS”) filed an action against us and others (collectively, the “Defendants”) entitled VNUS Medical Technologies, Inc. v. Diomed Holdings, Inc., Diomed Inc., AngioDynamics, Inc., and Vascular Solutions, Inc. , case no. C05-2972 MMC, filed in the U.S. District Court for the Northern District of California. The complaint alleged that the Defendants infringed on VNUS’s U.S. patent nos. 6,258,084, 6,638,273, 6,752,803, and 6,769,433 by making, using, selling, offering to sell and/or instructing users how to use Diomed’s “EVLT” products, AngioDynamics’ “VenaCure” products, and Vascular Solutions’ “Vari-Lase” products. The complaint alleged the Defendants’ actions caused VNUS to suffer substantial damage. The complaint sought to prohibit the Defendants from continuing to market and sell these products and asks for compensatory and treble money damages, reasonable attorneys’ fees, costs and pre-judgment and post-judgment interest.
On June 3, 2008, we entered into an agreement with VNUS settling all patent litigation between us and VNUS. Under the terms of the settlement agreement, we paid VNUS approximately $6.8 million pretax, $4.3 million after tax, an impact of $0.17 on earnings per share. Accordingly, we have recorded an accrual of $6.8 million as of May 31, 2008 which is included under the heading “Litigation provision” on the consolidated balance sheet. In addition, we agreed to pay a quarterly royalty on our U.S. sales of our NeverTouch(TM), VenaCure(R) and Diomed products from June 1, 2008 until the expiration date of VNUS’applicable patents. In exchange, VNUS granted us a non-exclusive and non-sublicenseable license to VNUS’ applicable patents for use in endovenous laser therapy.
We are party to other legal actions that arise in the ordinary course of business. We believe that any liability resulting from any currently pending litigation will not, individually or in the aggregate, have a material adverse effect on our business or financial condition, results of operations or cash flows.
31
| Item 4. | Submission of Matters to a Vote of Security Holders |
None.
The following table sets forth certain information with respect to the Company’s executive officers.
| | | | |
Name | | Age | | Position |
Eamonn P. Hobbs | | 50 | | President, Chief Executive Officer and Director |
D. Joseph Gersuk | | 58 | | Executive Vice President, Chief Financial Officer and Treasurer |
William M. Appling | | 45 | | Senior Vice President, Advanced Research |
Harold C. Mapes | | 48 | | Senior Vice President, Operations |
David McDonald | | 48 | | Senior Vice President, Business Development |
Sean Morris | | 36 | | Senior Vice President, General Manager—Peripheral Vascular Division |
Robert M. Rossell | | 52 | | Senior Vice President, General Manager—Access Division |
Eamonn P. Hobbs is one of our co-founders. He has been our President and Chief Executive Officer since June 1996 and a director since our inception. From 1991 until September 2002, Mr. Hobbs was a Vice President, and from October 2002 to May 2004 was a Senior Vice-President, of E-Z-EM, with operational responsibility for our company. He was first employed by E-Z-EM from 1985 to 1986 and was continuously employed by E-Z-EM from 1988 to May 2004. From 1986 to 1988, Mr. Hobbs was Director of Marketing for the North American Instrument Corporation (NAMIC), a medical device company later acquired by Boston Scientific. Mr. Hobbs started his career at Cook, a leading manufacturer of interventional radiology, interventional cardiology and gastroenterology medical devices. Mr. Hobbs has over 26 years experience in the interventional radiology, interventional cardiology and gastroenterology medical device industries. He is a bio-medical engineer, having completed a Bachelor of Sciences in Plastics Engineering with a Biomaterials emphasis at University of Lowell in 1980. Mr. Hobbs is the only business executive from the medical device industry to serve on the strategic planning committee of the Society of Interventional Radiology, or SIR, and in April 2005, he was awarded an honorary fellowship by the SIR.
D. Joseph Gersuk became our Senior Vice President, Chief Financial Officer in April 2007 and was named Executive Vice President in July 2007. Since 2005 he has been a trustee for multiple educational and healthcare facilities as well as a director of Ascend Acquisition Corporation. From 2003 to 2005, he was CEO and director of Request Multimedia. From 1994 to April 2003, he was Executive Vice President, Chief Financial Officer and Treasurer of MapInfo Corporation, a publicly traded software, data and services company. Mr. Gersuk, a former officer in the United States Navy, holds a Bachelor of Science degree from the United States Naval Academy and his Master of Business Administration in Finance from American University.
William M. Applingwas named Senior Vice President, Advanced Research in August 2008. Prior to that time he was our Senior Vice President of Research & Development from July 2007. Previously, he served as our Vice President, Research since 2002, Vice President, Research and Development since 1996, and in other product development capacities since 1988. Before that, Mr. Appling was a Product Development Engineer with NAMIC from 1986 to 1988 and a Product Development Engineer with the Edwards Division of American Hospital Supply Corporation from 1984 to 1986.
Harold C. Mapeswas named Senior Vice President, Operations in August 2008. He served as our Vice President, Operations since 1996 and was our Director of Operations from 1995 to 1996 and Product Development Project Manager from 1992 to 1994. Before joining us, Mr. Mapes held product development and supervisory manufacturing and engineering positions from 1988 to 1992 with Mallinckrodt Medical, a medical device manufacturer. He holds a Bachelor of Science in Mechanical Engineering from Tri-State University and a Master of Business Administration from the State University of New York at Albany.
32
David McDonaldstarted with AngioDynamics in July 2008 and was named Senior Vice President, Business Development in August 2008. Prior to joining AngioDynamics, Mr. McDonald was founder and President of Cornerstone Healthcare Advisors LLC, a Minnesota advisory and consulting firm to emerging medical technology companies and their financial sponsors, from April 2005 to August 2008. In addition, Mr. McDonald was Managing Director, Head of Medical Technology Investment Banking with Cain Brothers & Company, LLC, in New York, New York from October 2005 to May 2007. From May 2000 to March 2005, Mr. McDonald was Managing Director, Medical Technology Investment Banking with RBC Capital Markets (formerly Dain Rauscher Wessels). Mr. McDonald completed a Bachelor of Arts in Economics from St. Olaf College in Northfield, Minnesota.
Sean Morriswas named Senior Vice President, General Manager—Peripheral Vascular Division in August 2008. Prior to that time, Mr. Morris was our Vice President, Marketing, from September 2007. From June 2003 to September 2007, Mr. Morris was our Regional Sales Manager. Mr. Morris completed a Bachelor of Science in Biochemistry from Missouri St. University in 1996.
Robert M. Rossellwas named Senior Vice President, General Manager— Access Division in August 2008. Prior to that time, Mr. Rossell was our Vice President, Corporate Accounts, from July 2007. Previously, he served as our Vice President, Marketing from 1996 to July 2007, and from 1990 to 1996 he was a Product Manager and then our Director of Marketing. Before joining us, Mr. Rossell was Marketing Manager at NAMIC from 1986 to 1990, and held sales positions with various leading healthcare companies, including American Hospital Supply Corporation, from 1981 to 1985, and Johnson & Johnson, Inc., from 1977 to 1981. Mr. Rossell completed a Bachelor of Arts in Psychology from Southern Methodist University.
33
Part II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities. |
Our common stock is traded on The Global Select Market tier of The NASDAQ Stock Market LLC (formerly the Nasdaq National Market), under the symbol “ANGO.”
The following table sets forth, for the periods indicated, the high and low sale prices for our common stock as reported by The Nasdaq National Market.
| | | | | | |
| | | Sale Price |
| | | High | | Low |
Year ended May 31, 2008 | | | | | | |
Fourth Quarter | | $ | 16.65 | | $ | 9.95 |
Third Quarter | | $ | 20.27 | | $ | 16.58 |
Second Quarter | | $ | 20.98 | | $ | 18.45 |
First Quarter | | $ | 20.68 | | $ | 15.89 |
| |
| | | Sale Price |
| | | High | | Low |
Year ended June 2, 2007 | | | | | | |
Fourth Quarter | | $ | 23.87 | | $ | 15.68 |
Third Quarter | | $ | 26.93 | | $ | 20.13 |
Second Quarter | | $ | 24.84 | | $ | 15.20 |
First Quarter | | $ | 30.00 | | $ | 16.04 |
As of July 31, 2008, there were 326 record holders of our common stock.
Dividends
We did not declare any cash dividends on our common stock during our last two fiscal years. We do not anticipate paying any cash dividends on our common stock for the foreseeable future.
34
Performance Graph
The following graph compares the cumulative total return to shareholders on AngioDynamics, Inc.’s common stock relative to the cumulative total returns of the NASDAQ Composite index, the NASDAQ Medical Equipment index and the RDG SmallCap Medical Devices index. An investment of $100 (with reinvestment of all dividends) is assumed to have been made in our common stock and in each of the indexes on 5/27/2004 and its relative performance is tracked through 5/31/08.
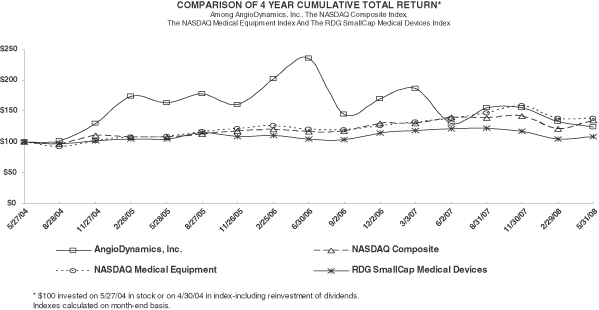
| | | | | | | | | | | | |
| | | ANGO | | NASDAQ
Composite | | NASDAQ
Medical
Equipment | | RDG
SmallCap
Medical
Devices |
5/27/2004 | | $ | 100.00 | | $ | 100.00 | | $ | 100.00 | | $ | 100.00 |
8/28/2004 | | | 101.84 | | | 96.29 | | | 92.26 | | | 96.40 |
11/27/2004 | | | 129.92 | | | 109.99 | | | 101.90 | | | 101.49 |
2/26/2005 | | | 173.83 | | | 107.47 | | | 107.44 | | | 104.70 |
5/28/2005 | | | 163.12 | | | 107.89 | | | 108.25 | | | 104.82 |
8/27/2005 | | | 177.36 | | | 112.54 | | | 116.34 | | | 115.50 |
11/26/2005 | | | 160.56 | | | 117.15 | | | 121.03 | | | 107.94 |
2/25/2006 | | | 202.48 | | | 120.59 | | | 125.60 | | | 110.51 |
6/3/2006 | | | 235.04 | | | 115.85 | | | 119.77 | | | 104.66 |
9/2/2006 | | | 144.72 | | | 116.75 | | | 118.77 | | | 103.29 |
12/2/2006 | | | 169.44 | | | 130.06 | | | 126.24 | | | 113.96 |
3/3/2007 | | | 186.40 | | | 129.58 | | | 131.87 | | | 118.64 |
6/2/2007 | | | 130.24 | | | 139.57 | | | 137.96 | | | 121.51 |
8/31/2007 | | | 155.68 | | | 138.58 | | | 146.85 | | | 122.47 |
11/30/2007 | | | 155.28 | | | 141.84 | | | 158.09 | | | 117.41 |
2/29/2008 | | | 132.64 | | | 121.47 | | | 138.16 | | | 104.38 |
5/31/2008 | | | 123.92 | | | 134.97 | | | 137.28 | | | 108.79 |
The stock price performance included in this graph is not necessarily indicative of future stock price performance.
35
| Item 6. | Selected Consolidated Financial Data |
You should read the following selected financial data in conjunction with our consolidated financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this annual report on Form 10-K. The consolidated statements of operations data for the fiscal years ended May 31, 2008, June 2, 2007, and June 3, 2006, and the consolidated balance sheet data as of May 31, 2008 and June 2, 2007, are derived from the audited consolidated financial statements that are included elsewhere in this annual report on Form 10-K. The consolidated statements of operations data for the fiscal years ended May 28, 2005 and May 29, 2004, and the consolidated balance sheet data as of June 3, 2006, May 28, 2005, and May 29, 2004, are derived from our audited consolidated financial statements not included in this annual report on Form 10-K. Historical results are not necessarily indicative of the results of operations to be expected for future periods. See Note A of “Notes to Consolidated Financial Statements” for a description of the method that we used to compute our historical basic and diluted net income per share attributable to common stockholders.
| | | | | | | | | | | | | | | | | | | | |
| | | Years ended | |
| | | (Amounts in thousands, except per share information) | |
| | | May 31,
2008 ( c) | | | June 2,
2007 (c)(d) | | | June 3,
2006 | | | May 28,
2005 | | | May 29,
2004 | |
Consolidated Statements of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Net sales | | $ | 166,500 | | | $ | 112,227 | | | $ | 78,451 | | | $ | 60,289 | | | $ | 49,055 | |
Cost of sales | | | 63,913 | | | | 46,060 | | | | 32,930 | | | | 26,912 | | | | 23,254 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 102,587 | | | | 66,167 | | | | 45,521 | | | | 33,377 | | | | 25,801 | |
| | | | | | | | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 14,424 | | | | 20,555 | | | | 5,869 | | | | 4,570 | | | | 3,551 | |
Sales and marketing | | | 46,047 | | | | 31,605 | | | | 21,399 | | | | 16,000 | | | | 13,562 | |
General and administrative | | | 15,425 | | | | 13,172 | | | | 7,774 | | | | 5,080 | | | | 3,565 | |
Amortization of intangibles | | | 6,849 | | | | 2,350 | | | | 173 | | | | — | | | | — | |
Litigation provisions, net(e) | | | 3,606 | | | | 9,710 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 86,351 | | | | 77,392 | | | | 35,215 | | | | 25,650 | | | | 20,678 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income (loss) | | | 16,236 | | | | (11,225 | ) | | | 10,306 | | | | 7,727 | | | | 5,123 | |
| | | | | | | | | | | | | | | | | | | | |
Other income (expenses) | | | | | | | | | | | | | | | | | | | | |
Interest income | | | 3,157 | | | | 4,047 | | | | 792 | | | | 304 | | | | 16 | |
Interest expense(a) | | | (1,328 | ) | | | (308 | ) | | | (138 | ) | | | (150 | ) | | | (758 | ) |
Other income (expenses) | | | (737 | ) | | | 314 | | | | 162 | | | | 36 | | | | — | |
Impairment loss on investment | | | — | | | | — | | | | — | | | | (300 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total other income (expenses), net | | | 1,092 | | | | 4,053 | | | | 816 | | | | (110 | ) | | | (742 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before income tax provision | | | 17,328 | | | | (7,172 | ) | | | 11,122 | | | | 7,617 | | | | 4,381 | |
Income tax provision | | | 6,439 | | | | 1,955 | | | | 4,256 | | | | 3,069 | | | | 1,238 | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | 10,889 | | | $ | (9,127 | ) | | $ | 6,866 | | | $ | 4,548 | | | $ | 3,143 | |
| | | | | | | | | | | | | | | | | | | | |
Earnings (loss) per share | | | | | | | | | | | | | | | | | | | | |
Basic | | $ | 0.45 | | | $ | (0.49 | ) | | $ | 0.55 | | | $ | 0.39 | | | $ | 0.34 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | $ | 0.45 | | | $ | (0.49 | ) | | $ | 0.53 | | | $ | 0.37 | | | $ | 0.32 | |
| | | | | | | | | | | | | | | | | | | | |
Weighted average number of shares used in per share calculation: | | | | | | | | | | | | | | | | | | | | |
Basic | | | 24,081,713 | | | | 18,443,570 | | | | 12,377,731 | | | | 11,571,317 | | | | 9,216,027 | |
| | | | | | | | | | | | | | | | | | | | |
Diluted | | | 24,348,960 | | | | 18,443,570 | | | | 12,964,574 | | | | 12,328,783 | | | | 9,838,168 | |
| | | | | | | | | | | | | | | | | | | | |
36
| | | | | | | | | | | | | | | | | | |
| | | As of | |
| | | May 31,
2008 | | June 2,
2007 | | | June 3,
2006 | | May 28,
2005 | | | May 29,
2004 | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and marketable securities(b) | | $ | 78,290 | | $ | 73,290 | | | $ | 89,752 | | $ | 27,099 | | | $ | 2,585 | |
Working capital | | | 100,548 | | | 106,881 | | | | 111,349 | | | 42,080 | | | | 30,981 | |
Total assets | | | 408,747 | | | 383,281 | | | | 137,000 | | | 59,672 | | | | 49,726 | |
Non-current liabilities | | | 11,700 | | | 26,905 | | | | 2,755 | | | 2,935 | | | | 3,100 | |
Retained earnings (Accumulated deficit) | | | 4,908 | | | (5,981 | ) | | | 3,146 | | | (3,720 | ) | | | (8,268 | ) |
Total stockholders’ equity | | | 355,713 | | | 335,958 | | | | 123,438 | | | 49,110 | | | | 37,232 | |
| (a) | Interest expense includes imputed interest on debt to E-Z-EM of $596 for the year ended May 29, 2004. The interest charges are treated as non-cash items for cash flow purposes and increases to additional paid-in capital. Of our indebtedness to E-Z-EM, $13,148 was capitalized prior to the completion of our initial public offering and the remaining $3,000 was repaid in June 2004 from the proceeds of the initial public offering. |
| (b) | Cash, cash equivalents and marketable securities include auction-rate investments of $1,850, $4,475, and $10,000 as of May 31, 2008, June 2, 2007 and June 3, 2006 and restricted cash of $68, $1,786, and $101 as of May 31, 2008, June 2, 2007, and May 29, 2004, respectively. |
| (c) | Fiscal years 2008 and 2007 include the impact of stock based compensation expense from our adoption of SFAS No. 123(R); the impact on operating income was approximately $4.9 million and $3.5 million, respectively. The impact on net income was approximately $3.1 million or $0.13 per basic and diluted share for fiscal 2008 and $2.4 million, or $0.13 per basic and diluted share for fiscal 2007. See Notes A and O to the Consolidated Financial Statements for additional information. |
| (d) | During fiscal year 2007, we completed the acquisition of RITA Medical Systems, Inc. for approximately $244 million. In connection with the acquisition, we incurred an in-process R&D charge of $12.1 million, or approximately $0.66 per basic and diluted share. See Note C to the Consolidated Financial Statements for additional information. |
| (e) | Fiscal year 2007, includes $9.7 million accrual for the Diomed patent infringement. Fiscal year 2008 included $6.8 million accrual for the VNUS patent infringement settlement offset by a $3.2 million gain as a result of the negotiated Diomed patent infringement settlement. |
| Item 7. | Management’s Discussion and Analysis of Financial Conditions and Results of Operations |
The following information should be read together with the audited consolidated financial statements and the notes thereto and other information included elsewhere in this annual report on Form 10-K.
Forward-Looking Statements
This annual report on Form 10-K, including the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business”, contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements regarding AngioDynamics’ expected future financial position, results of operations, cash flows, business strategy, budgets, projected costs, capital expenditures, products, competitive positions, growth opportunities, plans and objectives of management for future operations, as well as statements that include the words such as “expects,” “reaffirms” “intends,” “anticipates,” “plans,” “believes,” “seeks,” “estimates,” or variations of such words and similar expressions, are forward-looking statements. These forward looking statements are not guarantees of future performance and are subject to risks and uncertainties. Investors are cautioned that actual events or results may differ from the Company’s expectations. Factors that may affect the actual results achieved by the Company include, without
37
limitation, the ability of the Company to develop its existing and new products, future actions by the FDA or other regulatory agencies, results of pending or future clinical trials, overall economic conditions, general market conditions, market acceptance, foreign currency exchange rate fluctuations, the effects on pricing from group purchasing organizations and competition, the ability of the Company to integrate the purchased Diomed businesses as well as the risk factors listed in Item 1A of this annual report on Form 10-K.
Although we believe that the assumptions underlying the forward-looking statements contained herein are reasonable, any of the assumptions could be inaccurate and, therefore, there can be no assurance that the forward-looking statements included in this annual report on Form 10-K will prove to be accurate. In light of the significant uncertainties inherent in the forward-looking statements included herein, the inclusion of such information should not be regarded as a representation by us or any other person that our objectives and plans will be achieved.
Overview
We are a provider of innovative medical devices used in minimally invasive, image-guided procedures to treat peripheral vascular disease, or PVD, and local oncology therapy options for treating cancer, including radiofrequency ablation (“RF” or “RFA”) and systems and embolization products for treating benign and malignant tumors. We design, develop, manufacture and market a broad line of therapeutic and diagnostic devices that enable interventional physicians (interventional radiologists, vascular surgeons, interventional and surgical oncologists and others) to treat PVD, tumors, and other non-coronary diseases. We believe that we are the only company whose primary focus is to offer a comprehensive product line for the interventional treatment of these diseases. For the past five fiscal years, over 95% of our net sales were from single-use, disposable products. The following table sets forth our aggregate net sales from the following product categories for our last three fiscal years:
| | | | | | | | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | Net Sales | | % of
Net Sales | | | Net Sales | | % of
Net Sales | | | Net Sales | | % of
Net Sales | |
| | | | | | | | (dollars in thousands) | | | | | | |
Interventional Products | | $ | 128,102 | | 76.9 | % | | $ | 101,126 | | 90.1 | % | | $ | 78,451 | | 100.0 | % |
Oncology Products | | | 38,398 | | 23.1 | % | | | 11,101 | | 9.9 | % | | | — | | 0.0 | % |
| | | | | | | | | | | | | | | | | | |
Total | | $ | 166,500 | | 100.0 | % | | $ | 112,227 | | 100.0 | % | | $ | 78,451 | | 100.0 | % |
| | | | | | | | | | | | | | | | | | |
We sell our broad line of quality devices in the United States through a direct sales force and outside the U.S. through a combination of direct sales and distributor relationships. As of May 31, 2008, our sales organization numbered 109 in the U.S. and 12 outside the U.S. For fiscal years 2008, 2007, and 2006, net sales in non-U.S. markets were 9.5 %, 6.3% and 4.1%, respectively. The increase in our net sales attributable to non-U.S. sales is primarily as a result of the RITA acquisition, completed in January 2007.
Our growth depends in large part on the continuous introduction of new and innovative products, together with ongoing enhancements to our existing products, through internal product development, technology licensing and strategic alliances. For each of the past three fiscal years, we invested at least 7% of our net sales in research and development (“R&D”). R&D expenditures were 8.7% of net sales for fiscal 2008. In 2007, our R&D expenditures were 18.3% of net sales; however, a significant portion of those R&D expenses in 2007 related to a charge of $12.1 million for in-process R&D required under purchase accounting rules from our acquisition of RITA. Excluding this charge, our R&D expenses were approximately 7.5% of net sales for fiscal 2007. We expect that our R&D expenditures will reach approximately 10% of net sales for fiscal 2009. We expect R&D expenditures thereafter to continue to be in the range of 8 to 10% of net sales. However, downturns in our business could cause us to reduce our R&D spending.
38
We are also seeking to grow through selective acquisitions of complementary businesses and technologies. In January 2007, we completed the acquisition of RITA. This acquisition creates a diversified medical technology company with a broad line of access, diagnostic and therapeutic products that enable interventional physicians and surgeons to treat peripheral vascular disease and cancerous tumors. Interventional oncology is a large and growing area for our existing customer base and RITA’s leadership position, premium products and excellent reputation fit our strategy. RITA had a very strong position in vascular access ports, which are an ideal sales fit with our Morpheus® CT PICC and the vascular access port technology we purchased from Medron in May 2006. In addition, in May 2008 we acquired irreversible electroporation (IRE) cellular resection technology, which we expect to commercialize in the second half of fiscal 2009, which will be complementary to RITA’s diverse offering of local oncology therapies, including its market-leading RFA systems, Habib Sealer TM resection devices and LC Beads TM for tumor embolization. In June 2008, we completed the acquisition of certain U.S. and U.K. assets of Diomed, Inc. With this acquisition, we substantially strengthened our position in the market for the treatment of varicose veins. The combination of Diomed endovenous laser products with our existing venous product line provides us with a comprehensive venous product offering.
Except to the extent we can further use our equity securities as acquisition capital, we will require additional equity or debt financing to fund any future significant acquisitions.
For fiscal 2008, approximately 27% of our net sales were derived from products manufactured for us by third parties, compared to 30% for fiscal 2007. We intend to continue to manufacture more of these products in-house to achieve lower product costs and increased profitability. In 2002 and 2006, we expanded our manufacturing facility in Queensbury, New York, to provide us with significantly greater manufacturing capacity and to accommodate additional research, development and administrative requirements. We are not currently operating our manufacturing facilities at full capacity. However, we anticipate requiring additional office space for additional engineering, marketing and administrative personnel in the near future.
Our ability to further increase our profitability will depend in large part on improving gross profit margins. Factors such as changes in our product mix, new technologies and unforeseen price pressures may cause our margins to grow at a slower rate than we have anticipated or to decline.
Recent Developments
Acquisition of certain assets of Diomed
In June 2008, we completed the acquisition of certain U.S. and U.K. assets of Diomed, Inc for $11 million subject to adjustment for changes in working capital to be determined subsequent to the closing date. With this acquisition, we substantially strengthened our position in the market for the treatment of varicose veins. The combination of Diomed endovenous laser products with our existing venous product line provides us with a comprehensive venous product offering.
Acquisition of Oncobionic, Inc.
On May 9, 2008, we completed the acquisition of all the issued and outstanding shares of capital stock of Oncobionic, Inc. pursuant to the terms of a stock purchase agreement entered into on October 12, 2006. The closing of the acquisition comes as a result of the successful initial use of Oncobionic’s irreversible electroporation (IRE) technology in the first human clinical trial for the treatment of soft tissue, conducted during the first week of April 2008. Under the stock purchase agreement, we agreed to pay a total purchase price of $25.4 million, including $400,000 of assumed liabilities. We made a payment of $5.0 million upon the execution of the stock purchase agreement in October 2006. We paid $10.0 million on May 9, 2008 upon the closing of the acquisition. $5.0 million is payable in November 2008, and the remaining $5.0 million is payable in November 2009.
39
Acquisition of RITA Medical Systems, Inc.
On January 29, 2007, we completed the acquisition of RITA for a total purchase price of approximately $244 million, comprised of approximately $24 million in cash, 7.9 million shares of common stock, and assumption of outstanding RITA options and other convertible securities, which are exercisable for an additional 1.9 million shares of our common stock.
RITA’s operating results were consolidated with those of AngioDynamics beginning on the date of the acquisition, January 29, 2007. Since our results are not restated retroactively to reflect the historical financial position or results of RITA, fluctuations in our operating results for 2007 as compared to the 2008 period are significantly impacted by the acquisition of RITA.
We acquired RITA for its market position, premium product offerings, developed and emerging technologies in the fields of interventional oncology and vascular access and its highly skilled workforce. The merger was pursued and completed because the management groups and stockholders of AngioDynamics and RITA believed the combined entity would achieve higher sales and profitability than either or both of the pre-merger companies on a standalone basis.
Company Reorganization
Beginning with our first fiscal quarter for the fiscal year ended May 31, 2009, we will organize our business into three divisions: Peripheral Vascular; Access and Oncology/Surgery. Our Peripheral Vascular division comprises our venous, angiographic, PTA, drainage and thrombolytic product lines. Our Access division comprises our dialysis, ports and PICC lines. Our Oncology/Surgery division comprises our RFA, embolization, Habib and NanoKnife product lines. Beginning with our quarterly report on Form 10-Q for our fiscal quarter ended August 31, 2008, we will report our results of operations for these three divisions.
Critical Accounting Policies and Use of Estimates
Our significant accounting policies are summarized in Note A to our consolidated financial statements included elsewhere in this annual report on Form 10-K. While all these significant accounting policies affect the reporting of our financial condition and results of operations, we view certain of these policies as critical. Policies determined to be critical are those policies that have the most significant impact on our financial statements and require us to use a greater degree of judgment and/or estimates. Actual results may differ from those estimates. The accounting policies identified as critical are as follows:
Revenue Recognition
We recognize revenue in accordance with generally accepted accounting principles as outlined in the SEC’s Staff Accounting Bulletin No. 104, “Revenue Recognition,” which requires that four basic criteria be met before revenue can be recognized: (i) persuasive evidence that an arrangement exists; (ii) the price is fixed or determinable; (iii) collectibility is reasonably assured; and (iv) product delivery has occurred or services have been rendered. Decisions relative to criterion (iii) regarding collectibility are based upon our judgments, as discussed under “Accounts Receivable” below, and should conditions change in the future and cause us to determine this criterion is not met, our results of operations may be affected. We recognize revenue, net of sales taxes assessed by any governmental authority, as products are shipped, based on F.O.B. shipping point terms when title and risk of loss passes to customers. We negotiate shipping and credit terms on a customer-by-customer basis and products are shipped at an agreed upon price. All product returns must be pre-approved by us and customers may be subject to a 20% restocking charge. To be accepted, a returned product must be unadulterated, undamaged and have at least 12 months remaining prior to its expiration date.
Accounts Receivable
Accounts receivable, principally trade, are generally due within 30 to 90 days and are stated at amounts due from customers, net of an allowance for doubtful accounts. We perform ongoing credit evaluations of our
40
customers and adjust credit limits based upon payment history and the customer’s current credit worthiness, as determined by a review of their current credit information. We continuously monitor aging reports, collections and payments from customers, and maintain a provision for estimated credit losses based upon our historical experience and any specific customer collection issues that we identify. While such credit losses have historically been within our expectations and the provisions established, we cannot guarantee that the same credit loss rates will be experienced in the future. We write off accounts receivable when they become uncollectible. For fiscal years 2008, 2007, and 2006, our write offs of accounts receivable have been insignificant.
Income Taxes
In preparing our financial statements, we calculate income tax expense for each jurisdiction in which we operate. This involves estimating actual current taxes due plus assessing temporary differences arising from differing treatment for tax and accounting purposes that are recorded as deferred tax assets and liabilities. We periodically evaluate deferred tax assets, capital loss carryforwards and tax credit carryforwards to determine their recoverability based primarily on our ability to generate future taxable income and capital gains. Where their recovery is not likely, we estimate a valuation allowance and record a corresponding additional tax expense in our statement of operations. If actual results differ from our estimates due to changes in assumptions, the provision for income taxes could be materially affected. As of May 31, 2008, our valuation allowance and net deferred tax asset were approximately $1.2 million and $17.8 million, respectively. The deferred tax asset includes $99.2 million of Federal net operating loss carryforwards and $53.0 million of state net operating loss carryforwards remaining from the RITA acquisition. These losses could be significantly limited under Internal Revenue Code (“IRC”) Section 382. Our analysis of RITA’s ownership changes as defined in IRC Section 382 shows that approximately $15.0 million of remaining Federal net operating losses and $11.8 million of remaining state net operating losses will expire prior to utilization. The gross deferred tax asset related to the net operating losses reflects this limitation.
We need to generate approximately $5 million of taxable income in each year over the next eighteen years to ensure the realizability of our deferred tax assets. We have determined that we have sufficient existing levels of pre-tax earnings to generate sufficient taxable income to realize the net deferred tax assets recorded on our balance sheet.
In order to support the realizability of our net deferred tax asset, we projected our pre-tax income utilizing a combination of historical and projected results. Utilizing this projected pre-tax income, we have projected taxable income taking into consideration existing levels of permanent differences including stock option exercise deductions and non-deductible expenses and the reversal of significant temporary differences.
Our federal net operating loss carryforwards as of May 31, 2008 after considering IRC Section 382 limitations are $84.2 million. The expiration of the federal net operating loss carryforwards are as follows: $1.0 million between 2010 and 2011, $45.5million between 2017 and 2021 and $37.7 million between 2022 and 2026.
Our state net operating loss carryforwards as of May 31, 2008 after considering remaining IRC Section 382 limitations are $41.2 million which expire in various years from 2009 to 2026.
In November 2005, the FASB issued FASB Staff Position SFAS No. 123(R)-3, “Transition Election to Accounting for the Tax Effect of Share-Based Payment Awards.” We have elected to adopt the modified prospective transition method for calculating the tax effects of stock-based compensation pursuant to SFAS No. 123(R). Under the modified prospective transition method, no adjustment is made to the deferred tax balances associated with stock-based payments that continue to be classified as equity awards. Additionally, we elected to use the “long-form method,” as provided in paragraph 81 of SFAS No. 123(R) to determine the pool of windfall tax benefits. The long-form method requires us to analyze the book and tax compensation for each award separately as if it had been issued following the recognition provisions of SFAS No. 123, subject to adjustments for net operating loss carryforwards.
41
In July 2006, the Financial Accounting Standards Board (FASB) issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes-an interpretation of FASB Statement No. 109” (FIN 48), which clarifies the accounting for uncertainty in tax positions. FIN 48 seeks to reduce the diversity in practice associated with certain aspects of the recognition and measurement related to accounting for income taxes. This Interpretation requires the Company recognize in its financial statements the impact of a tax position, if that position is more likely than not of being sustained on audit, based on the technical merits of the position. This Interpretation is effective for fiscal years beginning after December 15, 2006, with the cumulative effect of the change in accounting principle recorded as an adjustment to opening retained earnings. The Company adopted this statement on June 3, 2007. There was no cumulative effect of adopting FIN 48. Upon adoption, the liability for unrecognized tax benefits was zero.
During the twelve months ended May 31, 2008, the Company did not recognize any tax liabilities related to uncertain tax positions.
The Company recognizes interest and penalties related to unrecognized tax benefits within its global operations as a component of income tax expense. This accounting policy did not change as a result of the adoption of FIN 48. Accrued interest and penalties recognized in the consolidated balance sheet were $0 as of June 2, 2007 and May 31, 2008.
The Company files income tax returns in the U.S. federal jurisdiction and various state and foreign jurisdictions. In the normal course of business the Company is subject to examination by taxing authorities throughout the world. Fiscal years 2005 through 2008 remain open to examination by the various tax authorities. The Company analyzed filing positions in all of the federal and state jurisdictions where it is required to file income taxes, as well as all open tax years in these jurisdictions and believes that its income tax filings positions and deductions will be sustained on audit and does not anticipate any adjustments will result in a material adverse effect on the Company’s financial condition, results of operations or cash flows.
Management does not anticipate that the amount of unrecognized tax benefits will significantly change in the next twelve months.
Inventories
We value inventories at the lower of cost (on the first-in, first-out method) or market. On a quarterly basis, we review inventory quantities on hand and analyze the provision for excess and obsolete inventory based primarily on product expiration dating and our estimated sales forecast, which is based on sales history and anticipated future demand. Our estimates of future product demand may not be accurate and we may understate or overstate the provision required for excess and obsolete inventory. Accordingly, any significant unanticipated changes in demand could have a significant impact on the value of our inventory and results of operations. As of May 31, 2008, June 2, 2007, and June 3, 2006, our reserve for excess and obsolete inventory was $3,694,000, $2,760,000, and $1,322,000, respectively.
Property, Plant and Equipment
We state property, plant and equipment at cost, less accumulated depreciation, and depreciate these assets using the straight-line method over their estimated useful lives. We determine this based on our estimates of the period over which the assets will generate revenue. We evaluate these assets for impairment annually or as changes in circumstances or the occurrence of events suggest the remaining value is not recoverable. Any change in condition that would cause us to change our estimate of the useful lives of a group or class of assets may significantly affect depreciation expense on a prospective basis.
Goodwill and Intangible Assets
Intangible assets other than goodwill are amortized over their estimated useful lives, which range between three and nineteen years, on either a straight-line basis over the expected period of benefit or as revenues are
42
earned from the sales of the related products. We periodically review the estimated useful lives of our intangible assets and review such assets for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Our determination of impairment is based on estimates of future cash flows. If an intangible asset is considered to be impaired, the amount of the impairment will equal the excess of the carrying value over the fair value of the asset.
For goodwill, the evaluation requires a comparison of the estimated fair value of the reporting unit to which the goodwill is assigned to the sum of the carrying value of the assets and liabilities of that unit. If the sum of the carrying value of the assets and liabilities of a reporting unit exceeds the fair value of the reporting unit, the carrying value of the reporting unit’s goodwill is reduced to its implied fair value through an adjustment to the goodwill balance, resulting in an impairment charge. Our determination of impairment is based on estimates of future cash flows. We will test goodwill for impairment during the third quarter of every fiscal year, and when an event occurs or circumstances change such that it is reasonably possible that impairment exists. Events that could, in the future, result in impairment include, but are not limited to, sharply declining sales for a significant product or in a significant geographic region.
Stock-based compensation
On June 4, 2006, (the “Effective Date”) we adopted Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment,” (“SFAS 123(R)”), which requires the measurement and recognition of compensation expense for all share-based payment awards made to our employees and directors including employee stock options and employee stock purchases related to our Stock Purchase Plan based on estimated fair values. We adopted SFAS 123(R) using the “modified-prospective method,” which is a method in which compensation cost is recognized beginning with the effective date (a) based on the requirements of SFAS 123(R) for all share-based payments granted after the effective date and (b) based on the requirements of Statement No. 123 for all awards granted to employees prior to the effective date of SFAS 123(R) that remain unvested on the effective date. In accordance with this method of adoption, prior period results of operations and financial position have not been restated to reflect the impact of stock-based compensation. Prior to the adoption of SFAS 123(R), we accounted for options using the intrinsic value method under the guidance of APB No. 25, and provided pro forma disclosure as allowed by Statement No. 123.
For 2008, we recognized stock-based compensation expense of $4,899,000 before-tax ($3,421,000 net of income taxes, or $0.14 per diluted share) as compared with 2007 when we recognized stock-based compensation expense of $3,498,000 before tax ($2,372,000 net of income taxes, or $0.13 per diluted share).
Under the provisions of SFAS 123(R), we expect to recognize the following future expense for awards granted as of May 31, 2008:
| | | | | |
| | | Unrecognized
Compensation
Cost | | Weighted-
Average
Remaining
Vesting
Period
(in years) |
Stock options | | $ | 7,821,700 | | 2.38 |
Non-vested stock awards | | | 256,000 | | 1.00 |
| | | | | |
| | $ | 8,077,700 | | 2.32 |
| | | | | |
Unrecognized compensation cost for stock options is presented net of 4.7% assumed annual forfeitures.
We recognize compensation expense for our stock awards issued subsequent to the adoption of SFAS 123(R) on a straight-line basis over the substantive vesting period. Prior to the adoption of SFAS 123(R), we allocated the pro forma compensation expense for stock options over the vesting period using straight-line
43
attribution method. We will continue to amortize compensation expense related to stock options granted prior to the adoption of SFAS 123(R) using a straight-line attribution method.
The amount of stock-based compensation recognized is based on the value of the portion of awards that are ultimately expected to vest. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The term “forfeitures” is distinct from “cancellations” or “expirations” and represents only the unvested portion of the surrendered option. We currently expect, based on an analysis of our historical forfeitures, that approximately 95.3% of our options will vest annually, and we have therefore applied a 4.7% annual forfeiture rate in determining the stock-based compensation charge recorded. We will re-evaluate this estimate periodically and adjust the forfeiture rate on a prospective basis as necessary. Ultimately, the actual expense recognized over the vesting period will only be for those shares that actually vest.
For the fiscal years ended May 31, 2008 and June 2, 2007, we used the Black-Scholes option-pricing model (“Black-Scholes”) as our method of valuation under SFAS 123(R) and a single option award approach. This fair value is then amortized on a straight-line basis over the requisite service periods of the awards, which is generally the vesting period. Black-Scholes was also previously used for our pro forma information required by SFAS 123 for periods prior to June 4, 2006. The fair value of share based payment awards on the date of the grant as determined by the Black-Scholes model is affected by our stock price as well as other assumptions. These assumptions include, but are not limited to the expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, and a risk-free interest rate. The risk-free interest rate is based on factual data derived from public sources. The expected stock-price volatility and option life assumptions require significant judgment which makes them critical accounting estimates.
We consider historical volatility and trends within our industry/peer group when estimating expected stock price volatility. We use yield rates on U.S. Treasury securities for a period approximating the expected term of the award to estimate the risk-free interest rate. The expected term is determined using the simplified method available under SAB 107 due to our limited public history. The dividend yield is based on the history and expectation of dividend payments. We have not paid dividends in the past nor do we expect to pay dividends in the foreseeable future. Our historical data includes information from May 26, 2004, the date of our initial public offering.
44
Results of Operations
Our fiscal years ended May 31, 2008, June 2, 2007, and June 3, 2006, represent fifty-two weeks, fifty-two weeks, and fifty-three weeks, respectively. Our operating results for fiscal 2008, 2007, and 2006 are expressed as a percentage of total net sales in the following table.
| | | | | | | | | |
| | | Years ended | |
| | | May 31, 2008 | | | June 2, 2007 | | | June 3, 2006 | |
Net sales | | 100.0 | % | | 100.0 | % | | 100.0 | % |
Cost of sales | | 38.4 | % | | 41.0 | % | | 42.0 | % |
| | | | | | | | | |
Gross profit | | 61.6 | % | | 59.0 | % | | 58.0 | % |
| | | | | | | | | |
Operating expenses | | | | | | | | | |
Research and development | | 8.7 | % | | 18.3 | % | | 7.5 | % |
Sales and marketing | | 27.7 | % | | 28.2 | % | | 27.3 | % |
General and administrative | | 9.3 | % | | 11.7 | % | | 10.1 | % |
Amortization of intangibles | | 4.1 | % | | 2.1 | % | | 0.0 | % |
Litigation provisions, net | | 2.2 | % | | 8.7 | % | | 0.0 | % |
| | | | | | | | | |
Total operating expenses | | 51.9 | % | | 69.0 | % | | 44.9 | % |
| | | | | | | | | |
Operating income (loss) | | 9.8 | % | | (10.0 | %) | | 13.1 | % |
| | | | | | | | | |
Other income (expenses) | | | | | | | | | |
Interest income | | 1.9 | % | | 3.6 | % | | 1.0 | % |
Impairment loss on investment | | 0.0 | % | | 0.0 | % | | 0.0 | % |
Interest expense | | (0.8 | %) | | (0.3 | %) | | (0.1 | %) |
Other income (expense) | | (0.4 | %) | | 0.3 | % | | 0.2 | % |
| | | | | | | | | |
Total other income (expenses), net | | 0.7 | % | | 3.6 | % | | 1.1 | % |
| | | | | | | | | |
Income (loss) before income tax provision | | 10.4 | % | | (6.4 | %) | | 14.2 | % |
Income tax provision | | 3.9 | % | | 1.7 | % | | 5.4 | % |
| | | | | | | | | |
Net income (loss) | | 6.5 | % | | (8.1 | %) | | 8.8 | % |
| | | | | | | | | |
The 2008 results include a $6.8 million provision for the settlement of the VNUS litigation ($4.3 million net of tax), a gain of $3.2 million ($2.0 million net of tax) on the settlement of the Diomed litigation, and post judgment interest expense on the Diomed judgment recorded in fiscal 2007. Our 2007 results include a litigation charge of $9.71 million ($6.1 million, net of tax), for the judgement awarded Diomed and pre-judgment interest, in general and administrative expenses, and $80,000 for post-verdict interest expense.
A significant amount of the expenses we incurred in 2007 related to the acquisition of RITA and were outside the normal course of our operations as a stand-alone company. As required under the rules of purchase accounting, these expenses included an in-process R&D charge of $12.1 million that carries with it no income tax benefit, amortization expense of $1,936,000 on the fair value of the acquired intangible assets and $1,192,000 of reduced gross margin as a result of the step up in basis and subsequent sale of finished goods inventory we acquired. Additionally, we incurred non-capitalizable integration and restructuring costs of $916,000. These costs aggregated $14,607,000, net of income taxes of $1,537,000.
For both 2008 and 2007, we were able to use net operating losses (“NOLs”) accumulated by RITA to offset the amount of cash we paid for Federal and state income taxes. The cash benefit amounted to approximately $7.3 and $1.6 million for the years ended May 31, 2008 and June 2, 2007, respectively. According to the rules of purchase accounting, we are unable to use acquired NOLs to offset our provision for income taxes in the statements of operations.
45
Fiscal years ended May 31, 2008 and June 2, 2007
Net sales.Net sales are derived from the sale of our products and related freight charges, less discounts and returns. For fiscal 2008, net sales increased 48.4%, or $54.3 million, to $166.5 million compared to fiscal 2007. The increase in net sales was primarily attributable to sales of products acquired in the RITA transaction for all of fiscal 2008 compared to fiscal 2007 which only included RITA product sales from the date of the acquisition, January 29, 2007 until the end of the fiscal year. RITA products accounted for $62.8 million or 37.7% of the fiscal 2008 sales as compared with $18.6 million or 16.6% of the fiscal 2007 sales. Our sales growth was driven by recently released products including the new Smart Port CT, Morpheus© CT PICC Insertion kit, Profiler balloon catheter, the TOTAL AbscessionTM drainage catheter, and SotradecolTM. Net sales to non-U.S. markets for fiscal 2008 were $15.9 million, or 9.5% of net sales, compared to $7.1 million, or 6.3% of net sales for fiscal 2007. This increase was primarily due to increased unit sales of vascular access ports and oncology products. Substantially all of the increase in our sales was due to increased unit sales, with less than 1% of the increase attributable to price increases.
Gross profit.Gross profit consists of net sales less the cost of goods sold, which includes the costs of materials, products purchased from third parties and sold by us, manufacturing personnel, royalties, freight, business insurance, depreciation of property and equipment and other manufacturing overhead. For fiscal 2008, our gross profit as a percentage of net sales increased to 61.6% from 59.0% for fiscal 2007. The increase in gross margin percentage was primarily due to a favorable product mix resulting from increased sales of higher margin products, including the RITA products and others such as the Morpheus CT PICC, the VenaCure procedure kit, and the TOTAL AbscessionTM drainage catheter, partially offset by increased sales of Sotradecol, which carries a lower gross margin. Gross profit in 2008 also included product efficiencies from the successful integration of RITA which were partially offset by costs associated with the start up of new product production and increases to inventory reserves due to continued focus on product line optimization. Gross profit in 2007 was also reduced by 100 basis points for the amortization of the step up in basis and subsequent sale of finished goods inventory we acquired in the RITA acquisition.
Research and development expenses.Research and development (“R&D”) expenses include costs to develop new products, enhance existing products, validate new and enhanced products and register, maintain and defend our intellectual property. For fiscal 2008, R&D expenses decreased $6.1 million, or 29.8%, to $14.4 million compared to $20.6 million for fiscal 2007. This decrease is primarily due to the impact of the $12.1 million for in-process R&D related to the RITA acquisition in fiscal 2007. This decrease was offset by expenses associated with the addition of RITA engineering personnel in Fremont, California and Manchester, Georgia along with increased engineering personnel and activities in Queensbury, New York. R&D expenses were 8.7% of net sales for fiscal 2008, compared to 18.3% of net sales for fiscal 2007. Without the in-process R&D charge charge, 2007 R&D expenses were 7.5% of net sales. At May 31, 2008, we employed 54 people in research, development and regulatory activities compared with 50 people at June 3, 2007, of which 22 were added due to the RITA acquisition in the third quarter of fiscal 2007.
Sales and marketing expenses.Sales and marketing (“S&M”) expenses consist primarily of salaries, commissions, travel and entertainment, attendance at medical society meetings, product promotions and samples. S&M expenses increased $14.4 million or 45.7% to $46.0 million for fiscal 2008. Sales expenses, which accounted for the majority of the increased S&M expenses, increased 57.4%, or $13.1 million This increase is due primarily to the acquisition of RITA and its 44-person sales staff, as well as increased personnel expenses related to the expansion of the number of territories, commissions on higher sales and stock-based compensation. Marketing expenses increased 14.8%, or $1.3 million, also primarily due to the acquisition of RITA and tradeshow expenses. As a percentage of sales, S&M expenses were 27.7% for fiscal 2008, compared with 28.2% for fiscal 2007. At May 31, 2008, we employed 151 people in sales, marketing and customer service activities compared with 140 people at June 2, 2007, of which 62 were added due to the RITA acquisition in the third quarter of fiscal 2007.
General and administrative expenses.General and administrative (“G&A”) expenses include executive management, finance and accounting, human resources and information technology and the administrative and
46
professional costs associated with those activities. G&A expenses increased $2.2 million, or 17.1%, to $15.4 million for fiscal 2008 compared to $13.2 million for fiscal 2007. While the expense increased year over year, primarily due to increased stock-based compensation and travel and administrative costs associated with our recent acquisition and integration activities, G&A expenses decreased as a percentage of sales. This decrease, from 11.7% of net sales for fiscal 2007 to 9.3% of net sales for fiscal 2008, is primarily due to synergies achieved in the integration of RITA. We spent approximately $2.0 million in fiscal 2008 in litigation costs associated with the VNUS Medical and Diomed lawsuits. At May 31, 2008, we employed 40 people in general and administrative activities compared with 37 people at June 2, 2007, of which 16 were added due to the RITA acquisition in the third quarter of fiscal 2007.
Litigation provision. The 2008 results include a $6.8 million provision for the settlement of the VNUS litigation and a gain of $3.2 million related to settlement of the Diomed litigation, compared with the prior year period when the Diomed judgment expense of $9.7 million was recorded.
Other income (expenses). Other income (expenses) includes interest income, realized gains and losses from the sales of marketable securities, changes in fair value of an interest rate swap and interest expense. For fiscal 2008, other income (expenses) decreased $3.0 million to $1.1 million, due primarily to increased interest expense incurred on the debt assumed in the RITA acquisition, Diomed provision and the December 2006 bond offering, unrealized losses on the Company’s interest rate swap agreement and decreased interest income on lower invested cash balances combined with decreased market rates. As a percentage of net sales, other income (expenses), net, was 0.7% and 3.6% for fiscal 2008 and 2007, respectively.
Income taxes. Our provision for income taxes increased $4.4 million in fiscal 2008, to $6.4 million from $2.0 million in fiscal 2007. Our effective tax rate for fiscal 2008 is 37.2%. The in-process R&D charge of $12.1 million, which is non-deductible for income tax purposes, had a significant impact on our effective tax rate for fiscal 2007. Without this charge, our effective tax rate for fiscal 2007 was 39.7%. During fiscal year 2008, non deductible items had a smaller impact on our effective tax rate than in the previous year. We also generated more state tax credits during 2008 than in the previous year.
Fiscal years ended June 2, 2007 and June 3, 2006
Net sales. Net sales consist of revenue derived from the sale of our products and related freight charges, less discounts and returns. For fiscal 2007, net sales were $112.2 million, an increase of $33.8 million, or 43.1%, compared to fiscal 2006. The increase in net sales was primarily due to the continued growth from new products released in or subsequent to fiscal 2006, continuing market share gains of our existing product lines, and sales of products acquired in the RITA transaction from January 29, 2007 to the end of the 2007 fiscal year. Sales of interventional products increased by 29.5%, or $22.5 million, to $98.8 million, due to increased sales of the Morpheus® CT PICC, the TOTAL Abscession TM drainage catheter, the DuraFlow dialysis catheter, Sotradecol® , and the Vortex® family of vascular access ports. Sales of oncology products were $10.8 million, consisting primarily of sales of radiofrequency ablation (RFA) products and sales of the HABIB 4X TM resection device. There were no sales of port or oncology products in fiscal 2006, as they were previously sold by RITA. Net sales to non-U.S. markets for fiscal 2007 were $7.1 million, or 6.3% of net sales, compared to $3.2 million, or 4.1% of net sales, for fiscal 2006. This increase was primarily due to increased unit sales of vascular access ports and oncology products. Substantially all of the increase in our sales was due to increased unit sales, with less than 1% of the increase attributable to price increases.
Gross profit. Gross profit consists of net sales less the cost of goods sold, which includes the costs of materials, products purchased from third parties and sold by us, manufacturing personnel, freight, business insurance, depreciation of property and equipment and other manufacturing overhead. For fiscal 2007, gross profit as a percentage of net sales increased to 59.0% from 58.0% for fiscal 2006. The increase in gross margin percentage was due to a favorable product mix resulting from increased sales of higher margin products, such as the Morpheus CT PICC, the VenaCure procedure kit, the TOTAL AbscessionTM drainage catheter, RFA
47
electrodes and vascular access ports, offset by increased sales of Sotradecol, which carries a lower gross margin. Gross profit was also reduced by 100 basis points for the amortization of the step up in basis and subsequent sale of finished goods inventory we acquired in the RITA acquisition.
Research and development expenses. Research and development expenses include costs to develop new products, enhance existing products, validate new and enhanced products and register, maintain and defend our intellectual property. Research and development expenses were 18.3% of net sales for fiscal 2007, compared to 7.5% for fiscal 2006. R&D expenses increased 250%, or $14.7 million, due primarily to an in-process R&D charge of $12.1 million in connection with the acquisition of RITA. Other increases are expenses associated with ongoing projects.
Sales and marketing expenses. Sales and marketing expenses consist primarily of the costs of salaries, commissions, travel and entertainment, attendance at medical society meetings, and advertising and product promotions and samples. Selling and marketing expenses were 28.2% of net sales for fiscal 2007, compared to 27.3% for fiscal 2006. For fiscal 2007, selling and marketing expenses increased 44.4%, or $10.2 million, compared to fiscal 2006. Selling expenses increased 43.1%, or $7.0 million, due primarily to the acquisition of RITA and its 44-person sales staff, as well as stock-based compensation. Marketing expenses increased 57.1%, or $3.2 million, also primarily due to the acquisition of RITA and tradeshow expenses.
General and administrative expenses. General and administrative expenses include corporate, finance, human resources, administrative and professional fees, as well as information technology expenses. To conform to current year presentation, amortization of intangibles and litigation provisions, net have been presented separately. For fiscal 2007, general and administrative expenses were 11.7% of net sales compared to 10.1% for fiscal 2006. This increase of $5.4 million, or 69%, is primarily a result of personnel and administrative expenses from the acquisition of RITA, stock based compensation, legal expenses related to ongoing litigation and travel and administrative costs associated with the acquisition and integration activities.
Amortization of intangibles. Amortization of intangibles increased to $2.4 million for fiscal 2007, up $2.2 million from fiscal 2006. This increase is due to the stepped-up basis of intangible assets acquired in the RITA transaction in the third quarter of fiscal 2007. Amortization of intangibles represents 2.1% of net sales in fiscal 2007.
Litigation provisions, net. Fiscal 2007 results included a charge of $9.7 million due to a compensatory damage award and related charges incurred as a result of an unfavorable verdict in a legal action. Litigation provisions, net represents 8.7% of net sales in fiscal 2007.
Other income (expenses). Other income (expenses) includes interest income, realized gains and losses from the sales of marketable securities, changes in fair value of an interest rate swap and interest expense. For fiscal 2007, other income (expenses) increased $3.2 million to $4.0 million, due primarily to increases in interest income. Both an increase in our investment portfolio and higher yields contributed to the increase in interest income. As a percentage of net sales, other income (expenses), net, was 0.3% and 0.2% for fiscal 2007 and fiscal 2006, respectively.
Income taxes. Our provision for income taxes decreased $2.3 million in fiscal 2007, from $4.3 million in fiscal 2006. The in-process R&D charge of $12.1 million, which is non-deductible for income tax purposes, had a significant impact on our effective tax rate for fiscal 2007. Without this charge, our effective tax rate for fiscal 2007 was 39.7% compared to 38.3% for fiscal 2006, and the Federal statutory rate of 34.0%. In both fiscal years, we recorded other expenses that were non-deductible for Federal income tax purposes.
Liquidity and Capital Resources
During the past three years, we have financed our operations primarily through cash flow from operations, the proceeds of our public offerings in 2004 and 2006 and long-term debt. At May 31, 2008, $78.3 million or
48
19.2% of our assets consisted of cash, cash equivalents, restricted cash and marketable securities. Marketable securities comprise of U.S. government issued or guaranteed securities, corporate bonds and auction-rate investments. Our current ratio was 3.4 to 1, with net working capital of $100.5 million, at May 31, 2008, compared to a current ratio of 6.3 to 1, with net working capital of $106.9 million, at June 2, 2007. At May 31, 2008, total debt was $17.1 million, comprising of short and long-term bank debt for financing our facility expansions in Queensbury, New York, and $9.7 million of convertible debt acquired in the RITA acquisition. Total debt was $17.4 million at June 2, 2007. Other long-term liabilities at May 31, 2008 consisted of $4.6 million of contractual obligations related to the Oncobionic purchase, net of discount. At June 2, 2007 we had recorded a long term liability under “litigation provision” for the judgment of damages assessed in a patent infringement action of $9.8 million, which has been settled and paid for $7.0 million during fiscal 2008.
We generated cash flow from operations of $26.0 million on net income of $11.0 million for fiscal 2008. Significant non-cash expenses affecting net income included depreciation and amortization of $9.2 million, deferred income tax provision of $5.5 million, stock-based compensation of $4.9 million and the net litigation provision of $4.0 million. Significant cash generated from operating activities included a reduction in inventories of $4.2 million due to continued focus on product line optimization offset by $7.0 million for payment in the Diomed judgment and increases to accounts receivable of $6.1 million to support the growth in net sales.
For fiscal 2008, our investing activities used net cash of $26.2 million. We used cash for the acquisition of intangible assets and businesses of $18.7, including $10.0 million upon completion of the Oncobionic acquisition. Additionally, we made equipment purchases and building improvements totaling $6.7 million, including the completion of an addition to the Queensbury, NY facility and improvements to the Manchester, GA facility.
Financing activities provided net cash of $4.0 million for fiscal 2008. This primarily consists of proceeds from the exercise of stock options and from the issuance of common stock under our employee stock purchase plan of $4.2 million offset by repayment of long term debt principal of $0.3 million.
In fiscal 2003, we financed an expansion of our headquarters and manufacturing facility with industrial revenue bonds for $3.5 million. To secure this financing, we entered into agreements with local municipalities, a bank, a trustee and a remarketing agent. These agreements are referred to as the IDA agreements. The proceeds of the bonds were advanced as construction occurred. The bonds reprice every seven days and are resold by a Remarketing Agent. The bonds bear interest based on the market rate on the date the bonds are repriced and require quarterly principal payments ranging from $25,000 to $65,000 plus accrued interest through May 2022. We entered into an interest rate swap with a bank to convert the initial variable rate payments to a fixed interest rate of 4.45% per annum. The IDA agreements contain financial covenants relating to fixed charge coverage and interest coverage. The outstanding debt is collateralized by a letter of credit ($2.8 million at May 31, 2008) and a first mortgage on the land, building and equipment comprising our facility in Queensbury, and we are required to pay an annual fee ranging from 1.0% to 1.9% of the outstanding balance depending on our financial results. The current fee is 1.0% and is in effect until August 22, 2008.
In fiscal 2007, we financed the expansion of our warehouse and manufacturing facility in Queensbury, New York. The expansion was financed principally with taxable adjustable rate notes (the “Notes”) issued by us aggregating $5,000,000. The Notes were issued under a trust agreement by and between us and a bank, as trustee (the “Trustee”). In connection with the issuance of the Notes, we entered into a letter of credit and reimbursement agreement (the “Reimbursement Agreement”) with the Bank that requires the maintenance of a letter of credit to support principal and certain interest payments on the Notes and requires payment of an annual fee on the outstanding balance. The current fee is 0.75% and is in effect until December 2008. We also entered into a remarketing agreement, pursuant to which the remarketing agent is required to use its best efforts to arrange for sales of the Notes in the secondary market. In connection with this financing, we entered into an interest rate
49
swap agreement (the “2006 Swap Agreement”) with the Bank, effective December 2006, with an initial notional amount of $5,000,000, to limit the effect of variability due to interest rates on the rollover of the Notes. The 2006 Swap Agreement is a contract to exchange floating interest rate payments for fixed interest payments periodically over the life of the agreement without the exchange of the underlying notional amounts. The 2006 Swap Agreement requires us to pay a fixed rate of 5.06% and receive payments based on 30-day LIBOR repriced every seven days through December 2016. The Reimbursement Agreement contains certain financial covenants relating to fixed charge coverage and interest coverage, as defined. Amounts borrowed under the Reimbursement Agreement are collateralized by the aforementioned letter of credit and all of our assets. The debt covenants and the collateralization of substantially all of our assets to secure these financings may restrict our ability to obtain debt financing in the future.
In connection with the acquisition of RITA on January 29, 2007, we assumed subordinated Senior Convertible Notes (the “Convertible Notes”) with an aggregate principal amount of $9.7 million. The Convertible Notes are convertible into shares of our common stock at a conversion price of $20.41 per share of common stock, net of a cash component, subject to adjustment in certain circumstances including common stock splits or other standard anti-dilution provisions. Until conversion or maturity, the Convertible Notes bear interest at 6.5% per year, payable semi-annually. Absent conversion, the Convertible Notes mature on August 5, 2008 (the “Maturity Date”). If on the Maturity Date, the closing price of our common stock has been at or above 102% of the then conversion price for at least 10 consecutive business days immediately preceding the Maturity Date, then any remaining principal outstanding under the Convertible Notes shall automatically be converted into our common stock, subject to certain conditions. If the closing price does not meet this condition, then the debt will be paid in cash. The entire principal amount has been classified as “Current portion of long-term debt and convertible note” in our consolidated balance sheet as of May 31, 2008.
On May 9, 2008, we completed the acquisition of all the issued and outstanding shares of capital stock of Oncobionic, Inc. pursuant to the terms of a stock purchase agreement entered into on October 12, 2006. The closing of the acquisition comes as a result of the successful initial use of Oncobionic’s irreversible electroporation (IRE) technology in the first human clinical trial for the treatment of soft tissue, conducted during the first week of April 2008.
Under the stock purchase agreement, we agreed to pay a total purchase price of $25.4 million, including $400,000 of assumed liabilities. We made a payment of $5.0 million upon the execution of the stock purchase agreement in October 2006. We paid $10.0 million on May 9, 2008 upon the closing of the acquisition. $5.0 million is payable in November 2008, and the remaining $5.0 million is payable in November 2009.
Our contractual obligations as of May 31, 2008 are set forth in the table below. We have no variable interest entities or other off-balance sheet obligations.
| | | | | | | | | | | | | | | |
| | | Cash Payments Due By Period as of May 31, 2008 |
| | | Total | | Less than
One Year | | 1-3 Years | | 3-5 Years | | After 5
Years |
Contractual Obligations: | | | | | | | | | | | | | | | |
Notes Payable to Bank | | $ | 12,082 | | $ | 657 | | $ | 1,237 | | $ | 1,033 | | $ | 9,155 |
Convertible Debt | | | 10,140 | | | 10,140 | | | 0 | | | 0 | | | 0 |
Operating Leases(1) | | | 927 | | | 485 | | | 438 | | | 4 | | | 0 |
Purchase Obligations(1) | | | 12,318 | | | 1,362 | | | 5,142 | | | 5,814 | | | 0 |
Other Liabilities(2) | | | 16,757 | | | 11,757 | | | 5,000 | | | 0 | | | 0 |
| | | | | | | | | | | | | | | |
| | $ | 52,224 | | $ | 24,401 | | $ | 11,817 | | $ | 6,851 | | $ | 9,155 |
| | | | | | | | | | | | | | | |
| (1) | The non-cancelable leases and inventory purchase obligations are not reflected on our consolidated balance sheet under accounting principles generally accepted in the United States of America. |
| (2) | Includes Oncobionic payments and VNUS settlement. |
50
We believe that our current cash and investment balances and cash generated from operations will provide sufficient liquidity to meet our anticipated needs for capital for at least the next 12 months. However, if we seek to make significant acquisitions of other businesses or technologies, we may require additional financing. We cannot be assured that such financing will be available on commercially reasonable terms, if at all.
Recent Accounting Pronouncements
In September 2006, FASB issued Statement of Financial Accounting Standards No. 157, “Fair Value Measurements” (“SFAS 157”). SFAS 157 defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. This Statement focuses on creating consistency and comparability in fair value measurements. SFAS 157 is effective for fiscal years beginning after November 15, 2007 (our 2009 fiscal year), and interim periods within those fiscal years. We are currently evaluating the impact this adoption will have on our consolidated financial statements.
In February 2007, FASB issued Statement of Financial Accounting Standards No. 159, The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB Statement No. 115 (“SFAS 159”). This standard permits an entity to choose to measure many financial instruments and certain other items at fair value. Most of the provisions in Statement 159 are elective; however, the amendment to FASB Statement No. 115, Accounting for Certain Investments in Debt and Equity Securities, applies to all entities with available-for-sale and trading securities. SFAS 159 is effective for fiscal years beginning after November 15, 2007 (our 2009 fiscal year). We are currently evaluating the impact this adoption will have on our consolidated financial statements.
In November 2007, the Emerging Issues Task Force (EITF) reached a consensus on EITF Issue No. 07-01, “Accounting for Collaborative Arrangements” (EITF No. 07-01). EITF No. 07-01 establishes disclosure requirements for arrangements entered into by companies to collaboratively develop, manufacture, or market products. EITF No. 07-01 also establishes income statement classification of collaboration transactions between the parties. EITF No. 07-01 is effective for fiscal years beginning after December 15, 2008 (our 2010 fiscal year). We are currently evaluating the impact this adoption will have on our consolidated financial statements.
In December 2007, FASB issued Statement of Financial Accounting Standards No. 141(R), “Business Combinations” (“SFAS 141(R)”). SFAS 141(R) establishes principles and requirements for how the acquirer in a business combination recognized and measures the assets acquired, liabilities assumed and any noncontrolling interest in the acquiree; recognizes and measures the goodwill acquired or gain from a bargain purchase; and determines what information to disclose to enable readers of the financial statements to evaluate the nature and financial effects of the business combination. SFAS 141(R) is effective for business combinations for which the acquisition date is on or after fiscal years beginning after December 15, 2008 (our 2010 fiscal year) and will be applied prospectively.
In December 2007, FASB issued Statement of Financial Accounting Standards No. 160, “Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARB No. 51” (“SFAS 160”). SFAS 160 establishes accounting and reporting standards that require companies to more clearly identify in financial statements and disclosures the impact of noncontrolling interest in a consolidated subsidiary on the consolidated financial statements. SFAS 160 is effective for fiscal years beginning after December 15, 2008 (our 2010 fiscal year), and interim periods within those fiscal years. The adoption of this pronouncement is not expected to have a material impact on our financial statements.
In March 2008, FASB issued Statement of Financial Accounting Standards No. 161, “Disclosures about Derivative Instruments and Hedging Activities” (“SFAS 161”). SFAS 161 is intended to improve financial reporting about derivative instruments and hedging activities by requiring companies to enhance disclosure about how these instruments and activities affect their financial position, performance and cash flows. SFAS 161 also improves the transparency about the location and amounts of derivative instruments in a company’s financial
51
statements and how they are accounted for under SFAS 133. SFAS 161 is effective for financial statements issued for fiscal years beginning after November 15, 2008 (our 2010 fiscal year), and interim periods within beginning after that date. We are currently evaluating the impact this adoption will have on our consolidated financial statements.
In April 2008, FASB issued FSP FAS 142-3, “Determination of the Useful Life of Intangible Assets”. The intent of this FSP is to improve the consistency between the useful life of a recognized intangible asset under Statement 142 and the period of expected cash flows used to measure the fair value of the asset under FASB statement No. 141. This FSP is effective for financial statements issued for fiscal years beginning after December 15, 2008 (our 2010 fiscal year) and early adoption is prohibited. For intangible assets acquired after the effective date, this FSP shall be applied as guidance in determining the useful life. The disclosure requirements which enable users of financial statements to assess the extent to which the expected future cash flows associated with the asset are affected by the entity’s intent and/or ability to renew or extend the arrangement shall be applied to all recognized intangible assets. We will comply with the guidance and disclosure requirements prospectively from the date of adoption.
In May 2008, the FASB issued SFAS No. 162,“The Hierarchy of Generally Accepted Accounting Principles” (“SFAS No. 162”). SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles used in the preparation of financial statements. SFAS No. 162 is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411,“The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles”. The adoption of this standard is not expected to have a material impact on the Company’s consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk. |
We are exposed to market risk from changes in interest rates on investments and financing that could impact our results of operations and financial position. Although we have entered into interest rate swaps with a bank to limit our exposure to interest rate change market risk on our variable interest rate financings, we do not currently engage in any other hedging or market risk management tools.
At May 31, 2008, we maintained variable interest rate financings of $7.4 million in connection with our facility expansions. We have limited our exposure to interest rate risk by entering into interest rate swap agreements with a bank under which we agreed to pay the bank a fixed annual interest rate and the bank assumed our variable interest payment obligations under the financings.
Nearly all of our sales have historically been denominated in United States dollars. Although not significant, in late fiscal 2007 we began to make sales in other currencies, particularly the Euro, GB pound and Canadian dollar. We currently have no significant direct foreign currency exchange risk and such risk in the future is expected to be modest.
Our excess cash is invested in highly liquid, short-term, investment grade securities with maturities primarily of less than two years. These investments are not held for speculative or trading purposes. Changes in interest rates may affect the investment income we earn on cash, cash equivalents and marketable securities and therefore affect our cash flows and results of operations. As of May 31, 2008, we were exposed to interest rate change market risk with respect to our investments in auction rate securities, U.S. government corporation and agency obligations in the amount of $13.2 million. The bonds bear interest at a floating rate established weekly. Each 100 basis point (or 1%) fluctuation in interest rates will increase or decrease interest income on the bonds by approximately $132,000 on an annual basis. We hold investments in auction rate securities in order to generate higher than typical money market investments. The amount held in these securities was $1.8 million as of May 31, 2008. Auction rate securities typically are high credit quality, generally achieved with municipal bond insurance. Credit risks are eased by the historical track record of bond insurers, which back a majority of
52
this market. Although rare, sell orders for any security traded through an auction process could exceed bids. Such instances are usually the result of a drastic deterioration of issue credit quality. Should there be a failed auction, we may be unable to liquidate our position in the securities in the near term.
We are party to legal actions that arise in the ordinary course of business as described in Note Q to the consolidated financial statements.
| Item 8. | Financial Statements and Supplementary Data |
Financial statements and supplementary data required by Part II, Item 8 are included in Part IV of this report as indexed at Item 15 (a) 1 and 2, and are incorporated by reference into this Item 8 ..
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rule 13a-15(b) of the Securities Exchange Act of 1934, as amended. Based on that evaluation, the Chief Executive Officer and the Chief Financial Officer concluded that our disclosure controls and procedures as of the end of the period covered by this report are functioning effectively to provide reasonable assurance that the information required to be disclosed by us (including our consolidated subsidiaries) in reports filed under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting in the fiscal quarter ended May 31, 2008 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for our company. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States and includes those policies and procedures that:
| | • | | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| | • | | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States, |
53
| | and that our receipts and expenditures are being made only in accordance with authorizations of our management and members of our board of directors; and |
| | • | | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management has assessed the effectiveness of our internal control over financial reporting as of May 31, 2008. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework.
Based on its assessment and this criteria, subject to the foregoing, management believes that we maintained effective internal control over financial reporting as of May 31, 2008.
| Item 9B. | Other Information |
None
54
Part III
Certain information required by Part III is omitted from this annual report on Form 10-K because the Company will file a definitive proxy statement within 120 days after the end of its fiscal year pursuant to Regulation 14A (the “Proxy Statement”) for its annual meeting of Stockholders, currently scheduled for October 21, 2008. The information included in the Proxy Statement under the respective headings noted below is incorporated herein by reference.
| Item 10. | Directors, Executive Officers and Corporate Governance |
Information required in this annual report on Form 10-K with respect Executive Officers is contained in the discussion titled “Executive Officers of the Company” in Part I of this annual report on Form 10-K. The balance of the information required by Item 10 is incorporated herein by reference to our Proxy Statement under the heading “Election of Directors”.
| Item 11. | Executive Compensation |
The information required by Item 11 is incorporated herein by reference to our Proxy Statement under the heading “Executive Compensation”
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this caption is incorporated herein by reference to our Proxy Statement under the heading “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters”
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this caption is incorporated herein by reference to our Proxy Statement under the heading “Certain Relationships and Related Transactions.”
| Item 14. | Principal Accounting Fees and Services |
The information required by this caption is incorporated herein by reference to our Proxy Statement under the headings “Audit Matters—Principal Accounting Fees and Services and—Policy on Audit Committee Pre-approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firm.”
55
Part IV
| Item 15. | Exhibits, Financial Statement Schedules |
(a)(1) Financial Statements
The following consolidated financial statements and supplementary data of Registrant and its subsidiaries required by Part II, Item 8, are included in Part IV of this report:
| | |
Report of Independent Registered Public Accounting Firm | | 59 |
Consolidated balance sheets—May 31, 2008 and June 2, 2007 | | 60 |
Consolidated statements of operations—Years ended May 31, 2008, June 2, 2007, and June 3, 2006 | | 62 |
Consolidated statements of stockholders’ equity and comprehensive income (loss)—Years ended May 31, 2008, June 2, 2007, and June 3, 2006 | | 63 |
Consolidated statements of cash flows—Years ended May 31, 2008, June 2, 2007, and June 3, 2006 | | 64 |
Notes to consolidated financial statements | | 66 |
(2) Financial Statement Schedules
The following consolidated financial statement schedule is included in Part IV of this report:
All other schedules are omitted because they are not applicable, or not required, or because the required information is included in the consolidated financial statements or notes thereto.
(b) Exhibits
| | |
| 2.1 | | Master Separation and Distribution Agreement, effective as of May 2004, between E-Z-EM, Inc. and AngioDynamics, Inc. (incorporated by reference to Exhibit 10.3 of the Company’s registration statement on Form S-1/A , filed with the Commission on May 12, 2004). |
| |
| 2.2 | | Stock Purchase Agreement, dated October 12, 2006, by and between AngioDynamics, Inc., Oncobionic, Inc. and the shareholders of Oncobionic, Inc. (incorporated by reference to Exhibit 2.1 of the Company’s quarterly report on Form 10-Q, filed with the Commission on January 11, 2007). |
| |
| 2.3 | | Agreement and Plan of Merger, dated as of November 27, 2006, by and among AngioDynamics, Inc., Royal I, LLC and RITA Medical Systems, Inc. (incorporated by reference to Annex A of the Company’s Registration Statement on Form S-4, filed with the Commission on December 8, 2006). |
| |
| 2.4 | | Amendment No. 1, dated December 7, 2006, to the Agreement and Plan of Merger, dated as of November 27, 2006, by and among AngioDynamics, Inc., Royal I, LLC and RITA Medical Systems, Inc. (incorporated by reference to Annex E of the Company’s Registration Statement on Form S-4, filed with the Commission on December 8, 2006). |
| |
| 2.5 | | Amendment No. 2, dated January 16, 2007, to the Agreement and Plan of Merger, dated as of November 27, 2006, by and among AngioDynamics, Inc., Royal I, LLC and RITA Medical Systems, Inc. (incorporated by reference to Exhibit 2.1 of the Company’s current report on Form 8-K, filed with the Commission on January 16, 2007). |
| |
| 2.6 | | Asset Purchase Agreement, dated as of April 9, 2008, by and between Diomed Holdings, Inc. and Diomed, Inc., as sellers and AngioDynamics, Inc., as Buyer (We agree to furnish to the Commission, upon request, a copy of each exhibit to this Asset Purchase Agreement). |
| |
| 2.7 | | Sale of the Business and Assets of Diomed Limited (in administration), dated April 10, 2008, by and between AngioDynamics, Inc., Diomed Limited (in administration) and Steve Law (as administrator)(We agree to furnish to the Commission, upon request, a copy of each exhibit to this Stock Purchase Agreement). |
56
| | |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 of the Company’s quarterly report on Form 10-Q, filed with the Commission on October 7, 2005). |
| |
| 3.2 | | Amended and Restated By-laws (incorporated by reference to Exhibit 3.2 of the Company’s quarterly report on Form 10-Q, filed with the Commission on October 7, 2005). |
| |
| 4.1 | | Rights Agreement, dated as of May 26, 2004, between AngioDynamics, Inc. and Registrar & Transfer Company, as Rights Agent (incorporated by reference to Exhibit 99.1 of the Company’s registration statement on Form 8-A, filed with the Commission on October 27, 2004). |
| |
| 4.2 | | Certificate of Designation, Preferences and Rights of Series A Preferred Stock of AngioDynamics, Inc. (incorporated by reference to Exhibit 3.3 of the Company’s current report on Form 8-K, filed with the Commission on November 28, 2006). |
| |
| 4.3 | | Trust Indenture, dated as of August 1, 2002, Relating to the Multi-Mode Variable Rate Industrial Development Revenue Bonds, Series 2002 issued by the Counties of Warren and Washington Industrial Development Agency in the aggregate principal amount of $3,500,00 (incorporated by reference to Exhibit 10.12 of the Company’s registration statement on Form S-1, filed with the Commission on March 5, 2004). |
| |
| 4.4 | | Counties of Warren and Washington Industrial Development Agency Multi-Mode Variable Rate Industrial Development Revenue Bond—AngioDynamics, Inc. Project—Letter of Credit Secured, Series 2002, having a maturity Date of August 1, 2022 (incorporated by reference to Exhibit 10.14 of the Company’s registration statement on Form S-1, filed with the Commission on March 5, 2004). |
| |
| 4.5 | | Except as set forth in Exhibits 4.3 and 4.4 above, the instruments defining the rights of holders of long-term debt securities of the Company and its subsidiaries have been omitted. We agree to furnish to the Commission, upon request, a copy of each instrument with respect to issuances of long term debt of the Company and its subsidiaries. |
| |
| 10.1 | | AngioDynamics, Inc. 1997 Stock Option Plan, as amended by the Board and Shareholders on February 27, 2004 (incorporated by reference to Exhibit 10.2 of the Company’s registration statement on Form S-1, filed on March 5, 2004). |
| |
| 10.2 | | AngioDynamics, Inc. 2004 Stock and Incentive Award Plan, as amended by the Board of Directors on August 15, 2006 (incorporated by reference to Exhibit 10.1 of the Company’s current report on Form 8-K, filed with the Commission on October 27, 2006). |
| |
| 10.3 | | Form of Non-Statutory Stock Option Agreement pursuant to the AngioDynamics, Inc. Stock and Incentive Award Plan (incorporated by reference to Exhibit 10.1 of the Company’s quarterly report on Form 10-Q, filed with the Commission on October 12, 2004). |
| |
| 10.4 | | Form of Performance Share Award Agreement pursuant to the AngioDynamics, Inc. 2004 Stock and Incentive Award Plan (incorporated by reference to Exhibit 10.2 of the Company’s current report on Form 8-K, filed with the Commission on May 12, 2005). |
| |
| 10.5 | | Form of Restricted Stock Award Agreement pursuant to the AngioDynamics, Inc. 2004 Stock and Incentive Award Plan (incorporated by reference to the Company’s current report on Form 8-K, filed with the Commission on May 12, 2005). |
| |
| 10.6 | | Rita Medical Systems, Inc. 1994 Incentive Stock Plan (incorporated by reference to Exhibit 10.2 of Rita Medical Systems registration statement on Form S-1, filed with the Commission on May 3, 2000) |
| |
| 10.7 | | Horizon Medical Products, Inc. 1998 Stock Incentive Plan (incorporated by reference to Exhibit 10.11 of Horizon Medical Products��� registration statement on From S-1, filed with the Commission on February 13, 1998. |
| |
| 10.8 | | Rita Medical Systems, Inc. 2000 Stock Plan (incorporated by reference to Exhibit 10.3 of Rita Medical Systems registration statement on Form S-1/A, filed with the Commission on June 14, 2000). |
57
| | |
| |
| 10.9 | | Rita Medical Systems, Inc. 2000 Directors’ Stock Plan, as amended on June 8, 2005 (incorporated by reference to Exhibit 99.2 of Rita Medical System’s registration statement on Form S-8, filed with the Commission on July 8, 2005). |
| |
| 10.10 | | Rita Medical Systems, Inc. 2005 Stock and Incentive Plan (incorporated by reference to Exhibit 99.1 of Rita Medical System’s registration statement on Form S-8, filed with the Commission on July 8, 2005). |
| |
| 10.11 | | Form of Indemnification Agreement of AngioDynamics, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s current report on Form 8-K, filed with the Commission on May 12, 2006). |
| |
| 10.12 | | Form of Severance Agreement of AngioDynamics, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s current report on form 8-K, filed with the Commission on October 31, 2007). |
| |
| 10.13 | | Building Loan Agreement, dated as of August 1, 2002, by and between AngioDynamics, Inc. and Keybank National Association (incorporated by reference to Exhibit 10.10 of the Company’s registration statement on Form S-1, filed with the Commission on March 5, 2004). |
| |
| 10.14 | | Mortgage and Security Agreement, dated as of August 1, 2002, from Counties of Warren and Washington Industrial Development Agency, as Issuer, and AngioDynamics, Inc. to Keybank National Association for the holders of the Issuer’s Multimode Variable Rate Industrial Development Revenue Bonds (incorporated by reference to Exhibit 10.11 of the Company’s registration statement on Form S-1, filed with the Commission on March 5, 2004). |
| |
| 10.15 | | Installment Sale Agreement, dated as of August 1, 2002, by and between Counties of Warren and Washington Industrial Development Agency and AngioDynamics, Inc. (incorporated by reference to Exhibit 10.15 of the Company’s registration statement on Form S-1, filed with the Commission on March 5, 2004). |
| |
| 10.16 | | Reimbursement Agreement, dated as of August 1, 2002, by and between AngioDynamics, Inc. and Keybank National Association (incorporated by reference to Exhibit 10.16 of the Company’s registration statement on Form S-1, filed with the Commission on March 5, 2004). |
| |
| 10.17 | | First Amendment to the Reimbursement Agreement, dated as of December 29, 2003, by and between AngioDynamics, Inc. and Keybank National Association (incorporated by reference to Exhibit 10.17 of the Company’s registration statement on Form S-1, filed with the Commission on March 5, 2004). |
| |
| 10.18 | | Note Purchase Agreement, dated as of December 5, 2006, by and between AngioDynamics, Inc. and Keybanc Capital Markets. |
| |
| 10.19 | | Reimbursement Agreement, dated as of December 1, 2006, by and between AngioDynamics, Inc. and Keybank National Association. |
| |
| 10.20 | | AngioDynamics, Inc. Management Profitability Bonus Program (incorporated by reference to Exhibit 10.3 of the Company’s current report on Form 8-K, filed with the Commission on August 21, 2006). |
| |
| 14 | | Code of Ethics (incorporated by reference to Exhibit 14 of the Company’s current report on Form 8-K, filed with the Commission on May 12, 2006). |
| |
| 21 | | Subsidiaries . |
| |
| 23 | | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. |
| |
| 31.1 | | Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 31.2 | | Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.1 | | Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.2 | | Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
58
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
AngioDynamics, Inc. and Subsidiaries:
In our opinion, the consolidated financial statements listed in the accompanying index appearing under Item 15(a)(1) present fairly, in all material respects, the financial position of AngioDynamics, Inc. and its subsidiaries at May 31, 2008 and June 2, 2007, and the results of their operations and their cash flows for each of the three years in the period ended May 31, 2008in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of May 31, 2008, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, the financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Item 9A under Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements, on the financial statement schedule and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note H to the consolidated financial statements, the Company changed the manner in which it accounts for uncertain tax positions effective June 3, 2007.
As discussed in Note O to the consolidated financial statements, the Company changed the manner in which it accounts for stock-based compensation effective June 4, 2006.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
PricewaterhouseCoopers LLP
Albany, New York
July 24, 2008
59
AngioDynamics, Inc. and Subsidiaries
CONSOLIDATED BALANCE SHEETS
(in thousands)
| | | | | | |
| | | May 31, 2008 | | June 2, 2007 |
| ASSETS | | | | | | |
CURRENT ASSETS | | | | | | |
Cash and cash equivalents | | $ | 32,040 | | $ | 28,313 |
Restricted cash | | | 68 | | | 1,786 |
Marketable securities, at fair value | | | 46,182 | | | 43,191 |
| | | | | | |
Total cash, cash equivalents and marketable securities | | | 78,290 | | | 73,290 |
Accounts receivable, net of allowance for doubtful accounts of $683 and $1,207, respectively | | | 26,642 | | | 20,798 |
Inventories, net | | | 22,901 | | | 28,007 |
Deferred income taxes | | | 10,902 | | | 2,247 |
Prepaid expenses and other | | | 3,147 | | | 2,957 |
| | | | | | |
Total current assets | | | 141,882 | | | 127,299 |
PROPERTY, PLANT AND EQUIPMENT-AT COST, less accumulated depreciation and amortization | | | 21,163 | | | 16,832 |
OTHER ASSETS | | | 1,865 | | | 6,926 |
INTANGIBLE ASSETS, less accumulated amortization | | | 71,311 | | | 49,148 |
GOODWILL | | | 162,707 | | | 153,787 |
DEFERRED INCOME TAXES | | | 6,860 | | | 29,289 |
PREPAID ROYALTIES | | | 2,959 | | | — |
| | | | | | |
TOTAL ASSETS | | $ | 408,747 | | $ | 383,281 |
| | | | | | |
The accompanying notes are an integral part of these financial statements.
60
AngioDynamics, Inc. and Subsidiaries
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | | | | | | | |
| | | May 31, 2008 | | | June 2, 2007 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
CURRENT LIABILITIES | | | | | | | | |
Accounts payable | | $ | 9,081 | | | $ | 7,567 | |
Accrued liabilities | | | 9,523 | | | | 8,136 | |
Income taxes payable | | | 933 | | | | 900 | |
Current portion of long-term debt and convertible note | | | 10,040 | | | | 315 | |
Litigation provision | | | 6,757 | | | | — | |
Other current liabilities | | | 5,000 | | | | 3,500 | |
| | | | | | | | |
Total current liabilities | | | 41,334 | | | | 20,418 | |
LONG-TERM DEBT, net of current portion | | | 7,075 | | | | 17,115 | |
LITIGATION PROVISION | | | — | | | | 9,790 | |
OTHER LONG TERM LIABILITIES, net of discount | | | 4,625 | | | | — | |
| | | | | | | | |
Total liabilities | | | 53,034 | | | | 47,323 | |
| | | | | | | | |
COMMITMENTS AND CONTINGENCIES | | | | | | | | |
STOCKHOLDERS’ EQUITY | | | | | | | | |
Preferred stock, par value $.01 per share, 5,000,000 shares authorized; no shares issued and outstanding | | | — | | | | — | |
Common stock, par value $.01 per share, 45,000,000 shares authorized; issued and outstanding 24,268,266 and 23,961,750 shares, respectively | | | 243 | | | | 240 | |
Additional paid-in capital | | | 350,598 | | | | 341,760 | |
Retained earnings (Accumulated deficit) | | | 4,908 | | | | (5,981 | ) |
Accumulated other comprehensive loss | | | (36 | ) | | | (61 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 355,713 | | | | 335,958 | |
| | | | | | | | |
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | | $ | 408,747 | | | $ | 383,281 | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements.
61
AngioDynamics, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
| | | | | | | | | | | | |
| | | Years ended | |
| | | May 31,
2008 | | | June 2,
2007 | | | June 3,
2006 | |
Net sales | | $ | 166,500 | | | $ | 112,227 | | | $ | 78,451 | |
Cost of sales | | | 63,913 | | | | 46,060 | | | | 32,930 | |
| | | | | | | | | | | | |
Gross profit | | | 102,587 | | | | 66,167 | | | | 45,521 | |
| | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | |
Research and development | | | 14,424 | | | | 20,555 | | | | 5,869 | |
Sales and marketing | | | 46,047 | | | | 31,605 | | | | 21,399 | |
General and administrative | | | 15,425 | | | | 13,172 | | | | 7,774 | |
Amortization of intangibles | | | 6,849 | | | | 2,350 | | | | 173 | |
Litigation provisions, net | | | 3,606 | | | | 9,710 | | | | — | |
| | | | | | | | | | | | |
Total operating expenses | | | 86,351 | | | | 77,392 | | | | 35,215 | |
| | | | | | | | | | | | |
Operating income (loss) | | | 16,236 | | | | (11,225 | ) | | | 10,306 | |
| | | | | | | | | | | | |
Other income (expenses) | | | | | | | | | | | | |
Interest income | | | 3,157 | | | | 4,047 | | | | 792 | |
Interest expense | | | (1,328 | ) | | | (308 | ) | | | (138 | ) |
Other income (expense) | | | (737 | ) | | | 314 | | | | 162 | |
| | | | | | | | | | | | |
Total other income (expenses), net | | | 1,092 | | | | 4,053 | | | | 816 | |
| | | | | | | | | | | | |
Income (loss) before income tax provision | | | 17,328 | | | | (7,172 | ) | | | 11,122 | |
Income tax provision | | | 6,439 | | | | 1,955 | | | | 4,256 | |
| | | | | | | | | | | | |
Net income (loss) | | $ | 10,889 | | | $ | (9,127 | ) | | $ | 6,866 | |
| | | | | | | | | | | | |
Earnings (loss) per share | | | | | | | | | | | | |
Basic | | $ | 0.45 | | | $ | (0.49 | ) | | $ | 0.55 | |
| | | | | | | | | | | | |
Diluted | | $ | 0.45 | | | $ | (0.49 | ) | | $ | 0.53 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
62
AngioDynamics, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE
INCOME (LOSS)
Years ended May 31, 2008, June 2, 2007, and June 3, 2006,
(in thousands, except share data)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | Additional
paid in
capital | | | (Accumulated
deficit)
Retained
earnings | | | Accumulated
other
comprehensive
(loss) income | | | Total | | | Comprehensive
income (loss) | |
| | Shares | | Amount | | | | | |
Balance at May 28, 2005 | | 12,051,632 | | $ | 121 | | $ | 52,878 | | | $ | (3,720 | ) | | $ | (169 | ) | | $ | 49,110 | | | | | |
Net proceeds from issuance of common stock | | 2,760,000 | | | 28 | | | 61,884 | | | | | | | | | | | | 61,912 | | | | | |
Exercise of stock options | | 634,364 | | | 6 | | | 2,974 | | | | | | | | | | | | 2,980 | | | | | |
Tax benefit on exercise of stock options | | | | | | | | 2,036 | | | | | | | | | | | | 2,036 | | | | | |
Purchase of common stock under Employee Stock Purchase Plan | | 23,435 | | | | | | 366 | | | | | | | | | | | | 366 | | | | | |
Stock-based compensation | | | | | | | | 81 | | | | | | | | | | | | 81 | | | | | |
Net Income | | | | | | | | | | | | 6,866 | | | | | | | | 6,866 | | | $ | 6,866 | |
Unrealized loss on marketable securities, net of tax of $ 30 | | | | | | | | | | | | | | | | (44 | ) | | | (44 | ) | | | (44 | ) |
Unrealized gain on interest rate swap, net of tax of $ 74 | | | | | | | | | | | | | | | | 131 | | | | 131 | | | | 131 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | $ | 6,953 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at June 3, 2006 | | 15,469,431 | | $ | 155 | | $ | 120,219 | | | $ | 3,146 | | | $ | (82 | ) | | $ | 123,438 | | | | | |
Issuance of common stock in acquisition | | 7,891,658 | | | 79 | | | 209,018 | | | | | | | | | | | | 209,097 | | | | | |
Exercise of stock options | | 559,459 | | | 6 | | | 4,087 | | | | | | | | | | | | 4,093 | | | | | |
Tax benefit on exercise of stock options | | | | | | | | 2,271 | | | | | | | | | | | | 2,271 | | | | | |
Purchase of common stock under Employee Stock Purchase Plan | | 32,765 | | | | | | 486 | | | | | | | | | | | | 486 | | | | | |
Issuance of performance shares | | 8,437 | | | | | | 214 | | | | | | | | | | | | 214 | | | | | |
Stock-based compensation | | | | | | | | 3,498 | | | | | | | | | | | | 3,498 | | | | | |
Implementation of SFAS 123R | | | | | | | | 158 | | | | | | | | | | | | 158 | | | | | |
Fair value of conversion feature on convertible debt | | | | | | | | 1,809 | | | | | | | | | | | | 1,809 | | | | | |
Net Loss | | | | | | | | | | | | (9,127 | ) | | | | | | | (9,127 | ) | | $ | (9,127 | ) |
Unrealized gain on marketable securities, net of tax of $ 19 | | | | | | | | | | | | | | | | 33 | | | | 33 | | | | 33 | |
Unrealized loss on interest rate swap, net of tax of $ 8 | | | | | | | | | | | | | | | | (12 | ) | | | (12 | ) | | | (12 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | $ | (9,106 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at June 2, 2007 | | 23,961,750 | | $ | 240 | | $ | 341,760 | | | $ | (5,981 | ) | | $ | (61 | ) | | $ | 335,958 | | | | | |
Net Income | | | | | | | | | | | | 10,889 | | | | | | | | 10,889 | | | $ | 10,889 | |
Exercise of stock options | | 245,120 | | | 3 | | | 3,418 | | | | | | | | | | | | 3,421 | | | | | |
Tax effect of exercise of stock options | | | | | | | | (329 | ) | | | | | | | | | | | (329 | ) | | | | |
Issuance of performance shares | | 4,385 | | | | | | 30 | | | | | | | | | | | | 30 | | | | | |
Purchase of common stock under Employee Stock Purchase Plan | | 57,011 | | | | | | 817 | | | | | | | | | | | | 817 | | | | | |
Stock-based compensation | | | | | | | | 4,902 | | | | | | | | | | | | 4,902 | | | | | |
Unrealized gain on marketable securities, net of tax of $ 51 | | | | | | | | | | | | | | | | 87 | | | | 87 | | | | 87 | |
Unrealized loss on interest rate swap, net of tax of $ 36 | | | | | | | | | | | | | | | | (62 | ) | | | (62 | ) | | | (62 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | | | | $ | 10,914 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at May 31, 2008 | | 24,268,266 | | $ | 243 | | $ | 350,598 | | | $ | 4,908 | | | $ | (36 | ) | | $ | 355,713 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
63
AngioDynamics, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | | |
| | | Years ended | |
| | | May 31,
2008 | | | June 2,
2007 | | | June 3,
2006 | |
| | | |
Cash flows from operating activities: | | | | | | | | | | | | |
Net income (loss) | | $ | 10,889 | | | $ | (9,127 | ) | | $ | 6,866 | |
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 9,205 | | | | 3,764 | | | | 1,082 | |
Amortization of bond discount | | | (336 | ) | | | (355 | ) | | | — | |
Purchased research and development expense | | | — | | | | 12,100 | | | | — | |
Tax effect of exercise of stock options and issuance of performance shares | | | (390 | ) | | | 597 | | | | 2,036 | |
Deferred income tax provision (benefit) | | | 5,483 | | | | (2,818 | ) | | | (18 | ) |
Write offs of excess and obsolete inventory | | | 803 | | | | 638 | | | | 183 | |
Stock based compensation | | | 4,902 | | | | 3,498 | | | | 452 | |
Provision for doubtful accounts | | | 229 | | | | 326 | | | | 270 | |
Litigation provisions, net | | | 3,967 | | | | 9,790 | | | | — | |
Other | | | 41 | | | | (8 | ) | | | (162 | ) |
Changes in operating assets and liabilities, net of impact from acquisitions: | | | | | | | | | | | — | |
Accounts receivable | | | (6,134 | ) | | | (1,474 | ) | | | (3,827 | ) |
Inventories | | | 4,172 | | | | (6,522 | ) | | | (5,887 | ) |
Prepaid expenses and other | | | (2,297 | ) | | | 365 | | | | (534 | ) |
Accounts payable and accrued liabilities | | | 2,340 | | | | (2,890 | ) | | | 2,673 | |
Other long term liabilities | | | (7,000 | ) | | | — | | | | 85 | |
Income taxes payable | | | 33 | | | | 900 | | | | — | |
| | | | | | | | | | | | |
Net cash provided by operating activities | | | 25,907 | | | | 8,784 | | | | 3,219 | |
| | | | | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | |
Additions to property, plant and equipment | | | (6,711 | ) | | | (5,806 | ) | | | (3,183 | ) |
Acquisition of intangible assets and business, net of cash acquired | | | (18,694 | ) | | | (25,245 | ) | | | (2,893 | ) |
Payment of non-refundable deposit | | | — | | | | (5,139 | ) | | | — | |
Change in restricted cash | | | 1,718 | | | | (1,786 | ) | | | — | |
Purchases of marketable securities | | | (58,699 | ) | | | (72,254 | ) | | | (31,337 | ) |
Proceeds from sale or maturity of marketable securities | | | 56,192 | | | | 55,188 | | | | 18,316 | |
| | | | | | | | | | | | |
Net cash used in investing activities | | | (26,194 | ) | | | (55,042 | ) | | | (19,097 | ) |
| | | | | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | |
Repayment of long-term debt | | | (315 | ) | | | (205 | ) | | | (165 | ) |
Issuance of long term debt | | | — | | | | 5,000 | | | | — | |
Payment of deferred financing costs | | | — | | | | (190 | ) | | | — | |
Payments of costs related to issuance of common stock | | | — | | | | (329 | ) | | | (218 | ) |
Proceeds from exercise of stock options and ESPP | | | 4,238 | | | | 4,579 | | | | 3,346 | |
Proceeds from the issuance of common stock | | | — | | | | — | | | | 62,459 | |
Tax effect of the exercise of stock options and issuance of performance shares | | | 91 | | | | 1,674 | | | | — | |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 4,014 | | | | 10,529 | | | | 65,422 | |
| | | | | | | | | | | | |
Increase (decrease) in cash and cash equivalents | | | 3,727 | | | | (35,729 | ) | | | 49,544 | |
Cash and cash equivalents | | | | | | | | | | | | |
Beginning of period | | | 28,313 | | | | 64,042 | | | | 14,498 | |
| | | | | | | | | | | | |
End of period | | $ | 32,040 | | | $ | 28,313 | | | $ | 64,042 | |
| | | | | | | | | | | | |
64
AngioDynamics, Inc. and Subsidiaries
CONSOLIDATED STATEMENTS OF CASH FLOWS—(Continued)
(in thousands)
| | | | | | | | | |
| | | Years ended |
| | | May 31,
2008 | | June 2,
2007 | | June 3,
2006 |
Supplemental disclosures of cash flow information: | | | | | | | | | |
Cash paid during the period for: | | | | | | | | | |
Interest | | $ | 661 | | $ | 183 | | $ | 136 |
Income taxes | | | 1,782 | | | 1,364 | | | 2,484 |
| | | |
Supplemental disclosure of non-cash operating, investing and financing activities: | | | | | | | | | |
Contractual obligations in acquisition of intangibles and business | | $ | 9,625 | | $ | 3,500 | | | — |
Issuance of common stock in acquisition | | | — | | | 209,097 | | | — |
Assumption of debt in acquisition | | | — | | | 11,509 | | | — |
Issuance of performance shares | | | — | | | 214 | | | |
Costs related to issuance of common stock included in accounts payable | | | — | | | — | | $ | 329 |
The accompanying notes are an integral part of these financial statements.
65
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
May 31, 2008 and June 2, 2007
NOTE A—BASIS OF PRESENTATION, BUSINESS DESCRIPTION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
1. Basis of Presentation, Business Description and Recent Events
The consolidated financial statements include the accounts of AngioDynamics, Inc. and its wholly owned subsidiaries, Leocor, Inc. (“Leocor”), RITA Medical Systems, LLC since January 29, 2007 and Oncobionic, Inc. since May 9, 2008 (collectively, the “Company”). The Company is primarily engaged in the design, development, manufacture and marketing of medical products used by interventional radiologists and other physicians for the minimally invasive diagnosis and treatment of peripheral vascular disease and local oncology therapy options for cancer patients, including radiofrequency ablation, or RFA, irreversible electroporation, or IRE, and systems and embolization products for treating cancerous tumors. As of May 31, 2008, the Company’s operations are classified in one segment, the manufacture and sale of medical devices. Beginning in the first quarter of the Company’s fiscal year 2009, the Company will classify its operations into three segments, Peripheral Vascular, Access and Oncology Surgery. The chief operating decision maker makes decisions based upon Company-wide sales and costs. The assets and expenses are not allocated by product line. As such, the chief operating decision maker is basing decisions upon a single segment. Certain prior period amounts have been reclassified for comparative purposes to conform to current year presentation. The reclassifications, including separate presentation of amortization expense on the consolidated statements of operations and excluding hardware units used for demonstrations and temporary replacement for customers’ units under repair from saleable inventory, resulted in a decrease in “Inventories, net” and an increase in “Other assets” in the amount of $560,000 as of June 2, 2007. These units are expensed on a straight line basis over their expected useful life.
On May 24, 2006, the Company completed a follow-on public offering of its common stock, selling 2,760,000 shares of its common stock (including 360,000 shares subject to the underwriters’ over-allotment option) at $24.07 per share, less underwriting discounts and commissions. Proceeds from the offering, net of underwriting costs totaling $3,974,400, amounted to $62,458,800 and were received by the Company on May 30, 2006. Net proceeds of the offering credited to common stock and additional paid-in capital aggregated $61,911,830, net of financing costs of $546,970.
RITA Medical Systems, Inc.
On January 29, 2007, the Company completed the acquisition of RITA Medical Systems, Inc. (“RITA”) for a total purchase price of approximately $244 million, comprising approximately 7.9 million shares of the Company’s common stock, assumption of outstanding RITA options and other convertible securities, which are exercisable for an additional 1.9 million shares of the Company’s common stock and approximately $24 million in cash (See Note C).
Oncobionic, Inc.
On May 9, 2008, the Company completed the acquisition of all the issued and outstanding shares of capital stock of Oncobionic, Inc. (“Oncobionic”) for approximately $25.4 million including $400,000 of assumed liabilties (see Note C).
All significant intercompany balances and transactions have been eliminated.
66
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
2. Fiscal Year
Beginning with fiscal 2008, the Company reports on a fiscal year ending May 31. Prior to fiscal 2008, the Company reported on a fiscal year that concluded on the Saturday nearest to May 31. Fiscal year 2007 ended on June 2, 2007, for a reporting period of fifty-two weeks. Fiscal year 2006 ended on June 3, 2006 for a reporting period of fifty-three weeks.
3. Cash and Cash Equivalents
The Company considers all unrestricted highly liquid investments purchased with an initial maturity of less than three months to be cash equivalents. The Company maintains cash and cash equivalent balances with financial institutions in the United States in excess of amounts insured by the Federal Deposit Insurance Corporation.
4. Marketable Securities
Marketable securities, which are principally government agency bonds, auction rate investments and corporate commercial paper, are classified as “available-for-sale securities” in accordance with SFAS 115, “Accounting for Certain Investments in Debt and Equity Securities” and reported at fair value, with unrealized gains and losses excluded from operations and reported as a component of accumulated other comprehensive income (loss), net of the related tax effects, in stockholders’ equity. Cost is determined using the specific identification method. The Company holds investments in auction rate securities in order to generate higher than typical money market rate investments. Auction rate securities typically are high credit quality, generally achieved with municipal bond insurance. Credit risks are eased by the historical track record of bond insurers, which back a majority of this market. Sell orders for any security traded through an auction process could exceed bids and, in such cases, the auction fails and the Company may be unable to liquidate its position in the securities in the near term.
5. Accounts Receivable
Accounts receivable, principally trade, are generally due within 30 to 90 days and are stated at amounts due from customers, net of an allowance for sales returns and doubtful accounts. The Company performs ongoing credit evaluations of its customers and adjusts credit limits based upon payment history and the customer’s current creditworthiness, as determined by a review of their current credit information. The Company continuously monitors aging reports, collections and payments from customers, and a provision for estimated credit losses is maintained based upon the Company’s historical experience and any specific customer collection issues that have been identified. While such credit losses have historically been within the Company’s expectations and the provisions established, the Company cannot guarantee that the same credit loss rates will be experienced in the future. The Company writes off accounts receivable when they become uncollectible.
Changes in the Company’s allowance for doubtful accounts are as follows:
| | | | | | | | |
| | | May 31, 2008 | | | June 2, 2007 | |
| | | (in thousands) | |
Beginning balance | | $ | 1,207 | | | $ | 430 | |
Provision for sales returns and doubtful accounts | | | 229 | | | | 326 | |
Allowance for acquired receivables | | | (61 | ) | | | 498 | |
Write-offs | | | (692 | ) | | | (47 | ) |
| | | | | | | | |
Ending Balance | | $ | 683 | | | $ | 1,207 | |
| | | | | | | | |
67
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
6. Inventories
Inventories are stated at the lower of cost (at standard cost, which approximates the first-in, first-out method) or market. Appropriate consideration is given to deterioration, obsolescence and other factors in evaluating net realizable value.
7. Property, Plant and Equipment
Property, plant and equipment are stated at cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. The Company evaluates these assets for impairment annually or as changes in circumstances or the occurrence of events suggest the remaining value is not recoverable. Expenditures for repairs and maintenance are charged to expense as incurred. Renewals and betterments are capitalized.
8. Accounting for Business Combinations, Goodwill and Intangible Assets
Intangible assets, other than goodwill, are amortized over their estimated useful lives, which range between three and nineteen years, on either a straight-line basis or as revenues are earned from the sales of the related products. The Company periodically reviews the estimated useful lives of its intangible assets and reviews such assets for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. The Company’s determination of impairment is based on estimates of future cash flows. If an intangible asset is considered to be impaired, the amount of the impairment will equal the excess of the carrying value over the fair value of the asset.
For goodwill, the evaluation requires a comparison of the estimated fair value of the reporting unit to which the goodwill is assigned to the sum of the carrying value of the assets and liabilities of that unit, including goodwill. If the sum of the carrying value of the assets and liabilities of a reporting unit exceeds the fair value of the reporting unit, the carrying value of the reporting unit’s goodwill is reduced to its implied fair value through an adjustment to the goodwill balance, resulting in an impairment charge. The Company’s determination of impairment is based on estimates of future cash flows. The Company tests goodwill for impairment during the third quarter of every fiscal year, or more frequently if impairment indicators arise. Events that could, in the future, result in impairment include, but are not limited to, sharply declining sales for a significant product or in a significant geographic region. As a result of the test performed in the third quarter of 2008, no impairment charge was required.
9. Revenue Recognition
Revenue is recognized in accordance with generally accepted accounting principles as outlined in the SEC’s Staff Accounting Bulletin No. 104 “Revenue Recognition,” which requires that four basic criteria be met before revenue can be recognized: (i) persuasive evidence that an arrangement exists; (ii) the price is fixed or determinable; (iii) collectibility is reasonably assured; and (iv) product delivery has occurred or services have been rendered. The Company recognizes revenue, net of sales taxes assessed by any governmental authority, as products are shipped based on F.O.B. shipping terms when title and risk of loss passes to customers. The Company negotiates credit terms on a customer-by-customer basis and products are shipped at an agreed upon price. All product returns must be pre-approved and, if approved, customers may be subject to a 20% restocking charge. To be accepted, a returned product must be unadulterated, undamaged and must have at least 12 months remaining prior to its expiration date.
68
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
10. Research and Development
Research and development costs, including salaries, consulting fees, building costs, utilities, administrative expenses, patent application costs, and an allocation of corporate costs are related to developing new products and making technological improvements to existing products and are expensed as incurred.
11. Shipping and Handling Costs
Shipping and handling costs, associated with the distribution of finished products to customers, are recorded in costs of goods sold and are recognized when the related finished product is shipped to the customer. Amounts charged to customers for shipping are recorded in net sales.
12. Advertising
All costs associated with advertisement are expensed as incurred. Advertising expense, included in sales and marketing was $555,000, $491,000, and $260,000 for 2008, 2007, and 2006, respectively.
13. Income Taxes
Deferred income taxes are recognized for temporary differences between financial statement and income tax bases of assets and liabilities and loss carryforwards and tax credit carryforwards for which income tax benefits are expected to be realized in future years. A valuation allowance has been established to reduce deferred tax assets, as it is more likely than not that all, or some portion, of such deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized in income in the period that includes the enactment date. The deferred tax asset includes net operating losses acquired as part of the RITA acquisition. These losses could be significantly limited under Internal Revenue Code (“IRC”) Section 382. An analysis of RITA’s ownership changes as defined in IRC Section 382 shows that approximately $15.8 million (of which $0.8 million had expired as of May 31, 2008) of net operating losses will not be utilized due to limitations. In addition, it is estimated that $11.8 million of state net operating losses will expire prior to utilization. The gross deferred tax asset related to the net operating losses reflects these limitations.
The Company intends to reinvest indefinitely any of its unrepatriated foreign earnings as of May 31, 2008. The Company has not provided for U.S. income taxes on these undistributed earnings of its foreign subsidiaries because Management considers such earnings to be reinvested indefintely outside the United States. If these earnings were distributed, the Company may be subject to both foreign withholding taxes and U.S. income taxes. Determination of the amount of this unrecognized deferred income tax liability is not practical.
In November 2005, the FASB issued FASB Staff Position SFAS No. 123(R)-3, “Transition Election to Accounting for the Tax Effect of Share-Based Payment Awards.” The Company elected to adopt the modified prospective transition method for calculating the tax effects of stock-based compensation pursuant to SFAS No. 123(R). Under the modified prospective transition method, no adjustment is made to the deferred tax balances associated with stock-based payments that continue to be classified as equity awards. Additionally, the Company elected to use the “long-form method,” as provided in paragraph 81 of SFAS No. 123(R) to determine the pool of windfall tax benefits. The long-form method requires the Company to analyze the book and tax compensation for each award separately as if it had been issued following the recognition provisions of SFAS No. 123, subject to adjustments for net operating loss carryforwards.
69
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
14. Fair Value of Financial Instruments
The Company’s financial instruments include cash and cash equivalents, accounts receivable, marketable securities, accounts payable, short-term and long-term debt and two interest rate swap agreements. The carrying amount of these instruments approximates fair value due to the immediate or short-term maturities and variable interest rates associated with these instruments. The interest rate swap agreements have been recorded at their fair value based on a valuation received from an independent third party (see Note K). Marketable securities are carried at their fair value as determined by quoted market prices.
15. Derivative Financial Instruments
In accordance with SFAS No. 133, “Accounting for Derivatives and Hedging Activities,” as amended, the Company’s 2002 interest rate swap agreement (see Note K) qualifies for hedge accounting under GAAP and the 2006 interest rate swap agreement does not. Both are presented in the consolidated financial statements at their fair value. Changes in the fair value of derivative financial instruments are either recognized periodically in income or in stockholders’ equity as a component of accumulated other comprehensive income (loss) depending on whether the derivative financial instrument qualifies for hedge accounting and, if so, whether it qualifies as a fair value or cash flow hedge. Generally, the changes in the fair value of derivatives accounted for as fair value hedges are recorded in income along with the portions of the changes in the fair value of hedged items that relate to the hedged risks. Changes in the fair value of derivatives accounted for as cash flow hedges, to the extent they are effective as hedges, are recorded in accumulated other comprehensive income (loss).
16. Stock-Based Compensation
On June 4, 2006, the Company adopted Statement of Financial Accounting Standard No. 123 (revised 2004), “Share-Based Payments” (“SFAS 123(R)”), which requires the measurement and recognition of all share-based payment awards made to employees and directors, including stock options, restricted stock units, performance share awards and employee stock purchases related to the Company’s Employee Stock Purchase Plan (the “Stock Purchase Plan”) based on estimated fair values. SFAS 123(R) supercedes the Company’s previous accounting under Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees” (“APB 25”), “Accounting for Stock-based Compensation” (“SFAS No. 123”) for non-employees, and related interpretations, beginning fiscal year 2007. In March 2005, the Securities and Exchange Commission issued Staff Accounting Bulletin No. 107 (“SAB 107”) relating to SFAS 123(R). The Company has applied the provisions of SAB 107 in its adoption of SFAS 123(R).
SFAS 123(R) requires companies to estimate the fair value of share-based payment awards on the date of the grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized on a straight-line basis over the requisite service period in the Company’s consolidated statements of operations. Prior to the adoption of SFAS 123(R), the Company accounted for stock-based awards to employees and directors using the intrinsic value method in accordance with APB 25 as allowed under SFAS 123. Under the intrinsic value method, no stock-based compensation expense had been recognized in the Company’s consolidated statements of operations, because the exercise price of the Company’s stock options granted to employees and directors was equal to or exceeded the fair market value of the underlying stock on the date of grant.
Stock-based compensation expense recognized in the Company’s consolidated statements of operations for periods after adoption of SFAS 123(R) includes compensation expense for share-based payment awards granted prior to, but not yet vested as of June 3, 2006, based on the grant date fair value estimated in accordance with the
70
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
pro forma provisions of SFAS 123 and compensation expense for the share-based payment awards granted subsequent to June 3, 2006, based on the grant date fair value estimated in accordance with the provisions of SFAS 123(R), and has been reduced for estimated forfeitures. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. In the Company’s pro forma information required under SFAS 123 for fiscal 2006, forfeitures have been accounted for as they occurred.
For the fiscal years ended May 31, 2008 and June 2, 2007, the Company used the Black-Scholes option-pricing model (“Black-Scholes”) as its method of valuation under SFAS 123(R) and a single option award approach. This fair value is then amortized on a straight-line basis over the requisite service periods of the awards, which is generally the vesting period. Black-Scholes was also previously used for the Company’s pro forma information required by SFAS 123 for fiscal 2006. The fair value of share based payment awards on the date of the grant as determined by the Black-Scholes model is affected by the Company’s stock price as well as other assumptions. These assumptions include, but are not limited to the expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, and a risk-free interest rate. The risk-free interest rate is based on factual data derived from public sources. The expected stock-price volatility and option life assumptions require significant judgment and are considered critical accounting estimates.
The Company considers historical volatility and trends within the Company’s industry/peer group when estimating expected stock price volatility. The Company uses yield rates on U.S. Treasury securities for a period approximating the expected term of the award to estimate the risk-free interest rate. The expected term is determined using the simplified method available under SAB 107 due to our limited public history. The dividend yield is based on the history and expectation of dividend payments. The Company has not paid dividends in the past nor does it expect to pay dividends in the foreseeable future. Company historical data includes information from May 27, 2004, the date of the Company’s initial public offering.
17. Earnings Per Common Share
Basic earnings per share are based on the weighted average number of common shares outstanding without consideration of potential common stock. Diluted earnings per share further includes the dilutive effect of potential common stock consisting of stock options, warrants, restricted stock units and shares issuable upon conversion of convertible debt into shares of common stock, provided that the inclusion of such securities is not antidilutive.
The Company accounts for convertible debt (see Note K) under EITF Issue No. 04-08, “The Effect of Contingently Convertible Debt on Diluted Earnings per Share” (“EITF 04-08”). EITF 04-08 indicates that contingently convertible debt should be included in diluted earnings per share computations regardless of whether the market price trigger has been met. For fiscal 2008 and 2007, shares issuable upon conversion of convertible debt into 414,476 shares of common stock, with a conversion price of $20.41 per share, have been excluded from the calculation of diluted earnings per share, as their inclusion would not be dilutive.
Also excluded from the calculation of diluted earnings per common share, are options, warrants, and restricted stock units issued to employees and non-employees to purchase 2,481,787, 1,111,342, and 18,489 shares of common stock at May 31, 2008, June 2, 2007, and June 3, 2006, respectively, as their inclusion would not be dilutive. The exercise prices of the excluded securities were between $0 and $196.95 at May 31, 2008 and June 2, 2007, and between $20.70 and $28.45 at June 3, 2006.
71
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
The following table sets forth the reconciliation of the weighted-average number of common shares:
| | | | | | |
| | | 2008 | | 2007(1) | | 2006 |
Basic | | 24,081,713 | | 18,443,570 | | 12,377,731 |
Effect of dilutive securities | | 267,247 | | — | | 586,843 |
| | | | | | |
Diluted | | 24,348,960 | | 18,443,570 | | 12,964,574 |
| | | | | | |
| (1) | As a result of the net loss for the year ending June 2, 2007, all outstanding options and warrants were excluded from the calculation of diluted earnings per common share as their inclusion would be antidilutive. |
18. Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements. Estimates also affect reported amounts of sales and expenses during the reporting period. Actual results could differ from those estimates.
19. Supplier Concentrations
The Company is dependent on a third-party manufacturer for a substantial portion of its dialysis catheters. In 2008, products purchased from this supplier accounted for approximately 19% of total product purchases and sales of these products accounted for approximately 11% of the Company’s sales. The Company is dependent upon the ability of its suppliers to provide products on a timely basis and on favorable pricing terms. The loss of its principal suppliers or a significant reduction in product availability from these suppliers could have a material adverse effect on the Company. The Company believes that its relationships with these suppliers are satisfactory and did not experience any instances of inadequate supply during 2008 or 2007.
20. Recently Issued Accounting Pronouncements
In September 2006, FASB issued Statement of Financial Accounting Standards No. 157, “Fair Value Measurements” (“SFAS 157”). SFAS 157 defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. This Statement focuses on creating consistency and comparability in fair value measurements. SFAS 157 is effective for fiscal years beginning after November 15, 2007 (our 2009 fiscal year), and interim periods within those fiscal years. The Company is currently evaluating the impact this adoption will have on the company’s consolidated financial statements.
In February 2007, FASB issued Statement of Financial Accounting Standards No. 159, The Fair Value Option for Financial Assets and Financial Liabilities—Including an Amendment of FASB Statement No. 115 (“SFAS 159”). This standard permits an entity to choose to measure many financial instruments and certain other items at fair value. Most of the provisions in Statement 159 are elective; however, the amendment to FASB Statement No. 115, Accounting for Certain Investments in Debt and Equity Securities, applies to all entities with available-for-sale and trading securities. SFAS 159 is effective for fiscal years beginning after November 15, 2007 (our 2009 fiscal year). The Company is currently evaluating the impact this adoption will have on the company’s consolidated financial statements.
72
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
In November 2007, the Emerging Issues Task Force (EITF) reached a consensus on EITF Issue No. 07-01, “Accounting for Collaborative Arrangements” (EITF No. 07-01). EITF No. 07-01 establishes disclosure requirements for arrangements entered into between companies to collaboratively develop, manufacture, or market products. EITF No. 07-01 also establishes income statement classification of collaboration transactions between the parties. EITF No. 07-01 is effective for fiscal years beginning after December 15, 2008 (the Company’s 2010 fiscal year). The Company is currently evaluating the impact this adoption will have on the Company’s consolidated financial statements.
In December 2007, FASB issued Statement of Financial Accounting Standards No. 141(R), “Business Combinations” (“SFAS 141(R)”). SFAS 141(R) establishes principles and requirements for how the acquirer in a business combination recognized and measures the assets acquired, liabilities assumed and any noncontrolling interest in the acquiree; recognizes and measures the goodwill acquired or gain from a bargain purchase; and determines what information to disclose to enable readers of the financial statements to evaluate the nature and financial effects of the business combination. SFAS 141(R) is effective for business combinations for which the acquisition date is on or after fiscal years beginning after December 15, 2008 (the Company’s 2010 fiscal year) and will be applied prospectively.
In December 2007, FASB issued Statement of Financial Accounting Standards No. 160, “Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARB No. 51” (“SFAS 160”). SFAS 160 establishes accounting and reporting standards that require companies to more clearly identify in the financial statements and discloses the impact of noncontrolling interest in a consolidated subsidiary on the consolidated financial statements. SFAS 160 is effective for fiscal years beginning after December 15, 2008 (the Company’s 2010 fiscal year), and interim periods within those fiscal years. The adoption of this pronouncement is not expected to have a material impact on the Company’s financial statements.
In March 2008, FASB issued Statement of Financial Accounting Standards No. 161, “Disclosures about Derivative Instruments and Hedging Activities” (“SFAS 161”). SFAS 161 is intended to improve financial reporting about derivative instruments and hedging activities by requiring companies to enhance disclosure about how these instruments and activities affect their financial position, performance and cash flows. SFAS 161 also improves the transparency about the location and amounts of derivative instruments in a company’s financial statements and how they are accounted for under SFAS 133. SFAS 161 is effective for financial statements issued for fiscal years beginning after November 15, 2008 (the Company’s 2010 fiscal year), and interim periods within beginning after that date. The Company is currently evaluating the impact this adoption will have on the Company’s consolidated financial statements.
In April 2008, FASB issued FSP FAS 142-3, “Determination of the Useful Life of Intangible Assets”. The intent of this FSP is to improve the consistency between the useful life of a recognized intangible asset under Statement 142 and the period of expected cash flows used to measure the fair value of the asset under FASB statement No. 141. This FSP is effective for financial statement issued for fiscal years beginning after December 15, 2008 (the Company’s 2010 fiscal year) and early adoption is prohibited. For intangible assets acquired after the effective date, this FSP shall be applied as guidance in determining the useful life. The disclosure requirements which enable users of financial statement to assess the extent to which the expected future cash flows associated with the asset are affected by the entity’s intent and/or ability to renew or extend the arrangement shall be applied to all recognized intangible assets. The Company will comply with the guidance and disclosure requirement prospectively from the date of adoption.
73
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
In May 2008, the FASB issued SFAS No. 162,“The Hierarchy of Generally Accepted Accounting Principles” (“SFAS No. 162”). SFAS No. 162 identifies the sources of accounting principles and the framework for selecting the principles used in the preparation of financial statements. SFAS No. 162 is effective 60 days following the SEC’s approval of the Public Company Accounting Oversight Board amendments to AU Section 411,“The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles”. The adoption of this standard is not expected to have a material impact on the Company’s consolidated financial statements.
NOTE B—COMPREHENSIVE INCOME
The Company records comprehensive income in accordance with SFAS No. 130, “Reporting Comprehensive Income.” SFAS No. 130 requires unrealized holding gains or losses on available-for-sale securities and certain derivative instruments, net of tax, to be included in accumulated other comprehensive income, as a separate component of stockholders’ equity. The components of comprehensive income, which include unrealized gains and losses on available for sale securities and changes in the fair value of the 2002 interest rate swap (see Note K), are detailed in the Company’s accompanying consolidated statements of stockholders’ equity and comprehensive income. At May 31, 2008 and June 2, 2007, the components of accumulated other comprehensive loss, net of related tax, are as follows:
| | | | | | | | |
| | | May 31, 2008 | | | June 2, 2007 | |
| | | (in thousands) | |
Cumulative loss on interest rate swap | | $ | (123 | ) | | $ | (61 | ) |
Unrealized holding gain on marketable securities | | | 87 | | | | — | |
| | | | | | | | |
Accumulated other comprehensive loss | | $ | (36 | ) | | $ | (61 | ) |
| | | | | | | | |
NOTE C—ACQUISITIONS
RITA Medical Systems, Inc.
On January 29, 2007, the Company completed the acquisition of RITA Medical Systems, Inc. (“RITA”) for a total purchase price of approximately $244 million, comprising approximately $24 million in cash, 7.9 million shares of the Company’s common stock and assumption of outstanding RITA options and other convertible securities, which are exercisable for an additional 1.9 million shares of the Company’s common stock.
In connection with the acquisition, the Company assumed warrants to acquire 2,727,270 RITA shares, which became exercisable for approximately 469,636 shares of the Company’s common stock at an average price of $20.24 per share, net of the cash component. These warrants expire in November 2009. The aggregate fair value with respect to the warrants of approximately $4.5 million was recorded as part of the purchase price using fair values determined under the Black-Scholes valuation model, with the following assumptions: expected stock price volatility of 54.6%; risk-free interest rate of 4.98%; and an expected term of 1.7 years.
The Company acquired RITA for its market position, premium product offerings, developed and emerging technologies in the fields of interventional oncology and vascular access, and its highly skilled workforce. The merger was pursued and completed because the management groups and stockholders of the Company and RITA believe the combined entity will achieve higher sales and profitability than either or both of the pre-acquisition companies on a stand-alone basis.
74
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
The Company has accounted for the acquisition of RITA as a business combination under accounting principles generally accepted in the United States of America. Under the purchase method of accounting, the assets and liabilities of RITA were recorded as of the acquisition date, at their respective fair values, and consolidated with those of AngioDynamics. The valuation of the assets and liabilities of RITA required the use of significant assumptions and estimates, including expected future cash flows and the applicable discount rates for the acquired intangibles, Black-Scholes assumptions for the valuation of the exchanged options and warrants and estimates for IRC section 382 limitations for the deferred tax assets. These estimates were based on assumptions that the Company believed to be reasonable as of the date of the acquisition. However, the Company’s actual results may differ from these estimates. Goodwill related to the RITA acquisition decreased by approximately $395,000 during the year ended May 31, 2008. The decrease related to adjustments for income taxes, the impact of SFAS 123(R), the finalization of contract termination costs and minimal additional adjustments to the preliminary purchase price allocation. The Company does not anticipate further material changes in the purchase price allocation.
The following table summarizes the estimated fair values of the assets acquired and the liabilities assumed and reflects any adjustments made during the 2008 fiscal year:
| | | |
Current assets | | $ | 18,164 |
Property, plant and equipment | | | 1,638 |
Deferred tax asset | | | 28,560 |
Goodwill | | | 153,392 |
Customer relationships | | | 27,500 |
Distributor relationships | | | 900 |
Product technologies | | | 13,900 |
Trademarks | | | 600 |
Purchased R&D | | | 12,100 |
Other assets | | | 1,040 |
| | | |
Total assets acquired | | | 257,794 |
| | | |
Current liabilities | | | 4,588 |
Long-term convertible debt | | | 9,700 |
| | | |
Total liabilities assumed | | | 14,288 |
| | | |
Net assets acquired | | $ | 243,506 |
| | | |
The fair values of the Company’s common stock issued, the options and warrants assumed, and the fair value of the convertible debt assumed in the acquisition of RITA were calculated using a valuation price of $24.776 per share of the Company’s common stock, which was calculated using the average of the closing market value for two days prior to and two days after the measurement date of January 24, 2007. The purchase price of $243.5 million includes $4.6 million of direct acquisition costs. The product technologies are expected to be amortized over a weighted-average useful life of 11 years. The remaining intangible assets are being amortized over a weighted-average useful life of 7 years. In addition, originally the Company recorded $153.8 million in non-tax deductible goodwill, which was adjusted during 2008 for the impact of SFAS 123(R). The Company also recorded approximately $12.1 million of purchased research and development (“purchased R&D”) costs which were recorded in research and development expense in its consolidated statements of operations for the fiscal year ended June 2, 2007. The value assigned to purchased R&D was determined by identifying specific R&D projects that would be continued and for which (a) technological feasibility had not been established at the acquisition date,
75
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
(b) there was no alternative future use and (c) the fair market value was estimable with reasonable reliability. The Company considered a number of factors including comparable transactions, relief from royalty analysis and other discounted cash flow approaches in determining preliminary purchase price allocations.
Oncobionic, Inc.
On May 9, 2008, the Company completed the acquisition of all the issued and outstanding shares of capital stock of Oncobionic, Inc. pursuant to the terms of the Stock Purchase Agreement entered into on October 12, 2006. The closing of the acquisition comes as a result of the successful initial use of irreversible electroporation (IRE) technology in the first human clinical trial for the treatment of soft tissue, conducted during the first week of April 2008.
The Company has recorded goodwill and a deferred tax liability of $9.3 million. The deferred tax liability will be reduced to offset the tax impact of non-deductible amortization expense on the intangible assets acquired.
Under the October 2006 Stock Purchase Agreement, the Company agreed to pay a total purchase price of $25.4 million, including $400,000 of assumed liabilities. The Company made payments of $5.0 million upon the execution of the stock purchase agreement in October 2006 and $10.0 million on May 9, 2008 upon the closing of the acquisition. Of the balance, $5.0 million is payable in November 2008 and the remaining $5.0 million is payable in November 2009.
The Stock Purchase Agreement includes future payments due on net sales of any catheter-based products sold by the Company that incorporate irreversible electroporation technology (“IRE”) for use in reducing the incidence of restenosis (the recurrence of narrowing or constriction of the arteries) associated with angioplasty procedures. The Company holds a license to such technology under a license agreement with the Regents of the University of California (the “UC License”).
The Company has accounted for the acquisition of Oncobionic as a business combination under accounting principles generally accepted in the United States of America. Under the purchase method of accounting, the assets and liabilities of Oncobionic were recorded as of the acquisition date, at their respective fair values, and consolidated with those of AngioDynamics. Substantially all of the purchase price was recorded as product technology and is being amortized over a 15 year useful life. The proforma impact on prior years results of operations would be approximately $1.8 million of additional amortization expense or $1.1 million, net of tax.
The Stock Purchase Agreement also permits former shareholders of Oncobionic to license its irreversible electroporation technology for Cardiac Arrhythmia Application (as defined in the Stock Purchase Agreement) to a single licensee and to appoint an affiliate of certain of the former shareholders of Oncobionic as its agent (the “Agent”) for a period of four years, commencing on the execution of the Purchase Agreement, to identify a potential licensee for such license. Under the Purchase Agreement the Company has a right of first refusal on any third-party offers for a license to the Cardiac Arrhythmia Application. At this time, there has been no agreement entered into.
Under a commission agreement between the Company and the Agent entered into concurrently with the Purchase Agreement, the Company has agreed to pay the Agent fifty (50%) percent of all license fees and royalties received from any licensee identified by the Agent after payment of all license fees dues under the UC License. Additionally, the Company has agreed to pay the Agent a termination fee equal to fifty (50%) percent of (i) the unconditional, non-refundable, up-front fees and (ii) the guaranteed minimum royalty payments that would have been paid to the Company under a proposed license in excess of the fees due under the UC License, if the Company rejects a bona fide offer by a potential licensee or is otherwise unable in good faith to reach an agreement with a potential licensee.
76
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
NOTE D—MARKETABLE SECURITIES AND INVESTMENTS
Marketable securities as of May 31, 2008 consisted of the following:
| | | | | | | | | | | | | |
| | | Amortized
cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | | Fair Value |
| | | (in thousands) |
Available-for-sales securities | | | | | | | | | | | | | |
U.S. government agency obligations(1) | | $ | 36,183 | | $ | 128 | | $ | (25 | ) | | $ | 36,286 |
Corporate bond securities | | | 9,861 | | | 41 | | | (6 | ) | | | 9,896 |
| | | | | | | | | | | | | |
| | $ | 46,044 | | $ | 169 | | $ | (31 | ) | | $ | 46,182 |
| | | | | | | | | | | | | |
Marketable securities as of June 2, 2007 consisted of the following:
| | | | | | | | | | | | | |
| | | Amortized
cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | | Fair Value |
| | | (in thousands) |
Available-for-sales securities | | | | | | | | | | | | | |
U.S. government agency obligations(1) | | $ | 37,138 | | $ | 28 | | $ | (32 | ) | | $ | 37,134 |
Corporate bond securities | | | 6,056 | | | 4 | | | (3 | ) | | | 6,057 |
| | | | | | | | | | | | | |
| | $ | 43,194 | | $ | 32 | | $ | (35 | ) | | $ | 43,191 |
| | | | | | | | | | | | | |
| (1) | Includes auction-rate securities |
The amortized cost and fair value of marketable securities at May 31, 2008, by contractual maturity, are shown below. Expected maturities will differ from contractual maturities because borrowers may have the right to call or prepay obligations with or without call or prepayment penalties.
| | | | | | |
| | | Amortized
cost | | Fair Value |
| | | (in thousands) |
As of May 31, 2008: | | | | | | |
Due in one year or less | | $ | 24,975 | | $ | 25,038 |
Due after one through five years | | | 9,357 | | | 9,436 |
Due after five through twenty years | | | 11,712 | | | 11,708 |
| | | | | | |
| | $ | 46,044 | | $ | 46,182 |
| | | | | | |
77
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
NOTE E—INVENTORIES
Inventories consist of the following:
| | | | | | | | |
| | | May 31,
2008 | | | June 2,
2007 | |
| | | (in thousands) | |
Raw materials | | $ | 10,383 | | | $ | 10,924 | |
Work in process | | | 3,565 | | | | 2,915 | |
Finished goods | | | 12,647 | | | | 16,928 | |
| | | | | | | | |
Gross Inventories | | | 26,595 | | | | 30,767 | |
Less: Reserves | | | (3,694 | ) | | | (2,760 | ) |
| | | | | | | | |
Net Inventories | | $ | 22,901 | | | $ | 28,007 | |
| | | | | | | | |
NOTE F—PROPERTY, PLANT AND EQUIPMENT, AT COST
Property, plant and equipment are summarized as follows:
| | | | | | | | | | |
| | | Estimated
useful lives | | May 31,
2008 | | | June 2,
2007 | |
| | | | | (in thousands) | |
Building and building improvements | | 39 years | | $ | 11,717 | | | $ | 5,608 | |
Machinery and equipment | | 3 to 8 years | | | 9,803 | | | | 9,512 | |
Computer software and equipment | | 3 to 5 years | | | 6,958 | | | | 5,095 | |
Construction in progress | | | | | 4,072 | | | | 5,918 | |
| | | | | | | | | | |
| | | | | 32,550 | | | | 26,133 | |
Less accumulated depreciation and amortization | | | | | (11,746 | ) | | | (9,524 | ) |
| | | | | | | | | | |
| | | | | 20,804 | | | | 16,609 | |
Land and land improvements | | | | | 359 | | | | 223 | |
| | | | | | | | | | |
| | | | $ | 21,163 | | | $ | 16,832 | |
| | | | | | | | | | |
Depreciation expense for 2008, 2007, and 2006, was $2,328,000, $1,414,000, and $909,000, respectively.
NOTE G—GOODWILL AND INTANGIBLE ASSETS
Goodwill and intangible assets that have indefinite useful lives are not amortized but rather are tested for impairment annually or more frequently if impairment indicators arise. None of the Company’s intangible assets have an indefinite life. Intangible assets with determinable useful lives are amortized over their useful lives on either a straight-line basis over the expected period of benefit or as revenues are earned from the sales of the related products. Goodwill and intangible assets have been recorded at either incurred or allocated cost. Allocated costs were based on respective fair market values at the date of acquisition.
78
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
Changes in the carry amount of goodwill for the fiscal year ended May 31, 2008 are as follows (in thousands):
| | | | |
Balance, June 3, 2007 | | $ | 153,787 | |
Adjustments to purchase price allocation | | | (395 | ) |
Arising from completed business combinations (Note C) | | | 9,315 | |
| | | | |
Balance, May 31, 2008 | | $ | 162,707 | |
| | | | |
Changes in the carrying amount of goodwill for the fiscal year ended June 2, 2007, are as follows (in thousands):
| | | |
Balance, June 4, 2006 | | $ | — |
Arising from completed business combinations | | | 153,787 |
| | | |
Balance, June 2, 2007 | | $ | 153,787 |
| | | |
The balances of intangible assets are as follows:
| | | | | | | | | | | | |
| | | May 31, 2008 |
| | | Gross
carrying
value | | Accumulated
amortization | | | Net carrying
value | | Weighted
avg useful
life |
| | | (in thousands) | | (years) |
Licenses | | $ | 5,540 | | $ | (698 | ) | | $ | 4,842 | | 9.9 |
Customer relationships | | | 27,500 | | | (4,924 | ) | | | 22,576 | | 7.5 |
Distributor relationships | | | 900 | | | (400 | ) | | | 500 | | 3.0 |
Trademarks | | | 600 | | | (80 | ) | | | 520 | | 10.0 |
Product technologies | | | 47,203 | | | (4,330 | ) | | | 42,873 | | 13.6 |
| | | | | | | | | | | | |
| | $ | 81,743 | | $ | (10,432 | ) | | $ | 71,311 | | |
| | | | | | | | | | | | |
| |
| | | June 2, 2007 |
| | | Gross
carrying
value | | Accumulated
amortization | | | Net carrying
value | | Weighted
avg useful
life |
| | | (in thousands) | | (years) |
Licenses | | $ | 2,518 | | $ | (183 | ) | | $ | 2,335 | | 7.4 |
Customer relationships | | | 27,500 | | | (1,231 | ) | | | 26,269 | | 7.5 |
Distributor relationships | | | 900 | | | (100 | ) | | | 800 | | 3.0 |
Trademarks | | | 600 | | | (20 | ) | | | 580 | | 10.0 |
Product technologies | | | 21,183 | | | (2,019 | ) | | | 19,164 | | 11.9 |
| | | | | | | | | | | | |
| | $ | 52,701 | | $ | (3,553 | ) | | $ | 49,148 | | |
| | | | | | | | | | | | |
Amortization expense was $6,880,000, $2,350,000, and $167,000 for 2008, 2007, and 2006, respectively.
79
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
Annual amortization of these intangible assets is expected to approximate the following amounts for each of the next five fiscal years:
| | | |
| | | (in thousands) |
2009 | | $ | 8,718 |
2010 | | | 8,651 |
2011 | | | 8,317 |
2012 | | | 8,119 |
2013 | | | 7,697 |
NOTE H—INCOME TAXES
Income tax provision analyzed by category and by statement of income classification is summarized as follows:
| | | | | | | | | | | |
| | | 2008 | | 2007 | | | 2006 | |
| | | (in thousands) | |
Current | | | | | | | | | | | |
Federal | | $ | 1,348 | | $ | 4,485 | | | $ | 3,923 | |
State and local | | | 224 | | | 288 | | | | 351 | |
Non U.S. | | | 148 | | | — | | | | — | |
| | | | | | | | | | | |
| | | 1,720 | | | 4,773 | | | | 4,274 | |
Deferred | | | 4,719 | | | (2,818 | ) | | | (18 | ) |
| | | | | | | | | | | |
| | $ | 6,439 | | $ | 1,955 | | | $ | 4,256 | |
| | | | | | | | | | | |
The significant components of deferred income tax (benefit) expense from operations for the years ended May 31, 2008, June 2, 2007, and June 3, 2006 consist of the following:
| | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Deferred tax (benefit) expense | | $ | (2,628 | ) | | $ | (5,338 | ) | | $ | (18 | ) |
Net operating loss carryforward | | | 7,347 | | | | 2,520 | | | | — | |
| | | | | | | | | | | | |
| | $ | 4,719 | | | $ | (2,818 | ) | | $ | (18 | ) |
| | | | | | | | | | | | |
80
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
Temporary differences that give rise to deferred tax assets and liabilities are summarized as follows:
| | | | | | | | |
| | | May 31,
2008 | | | June 2,
2007 | |
| | | (in thousands) | |
Deferred tax assets | | | | | | | | |
Capital loss carryforwards | | $ | 94 | | | $ | 102 | |
Net operating loss carryforward | | | 29,653 | | | | 36,599 | |
R&D and state tax credit carryforward | | | 1,294 | | | | 1,285 | |
Expenses incurred not currently deductible | | | 347 | | | | 1,089 | |
Unrealized loss on interest rate swap | | | 72 | | | | 36 | |
Impairment of long-lived assets | | | 417 | | | | 533 | |
Inventories | | | 1,930 | | | | 1,058 | |
Litigation damage award | | | 2,590 | | | | 3,593 | |
Stock-based compensation | | | 2,414 | | | | 1,688 | |
State tax credits | | | 270 | | | | — | |
Other | | | 82 | | | | — | |
| | | | | | | | |
Gross deferred tax asset | | | 39,163 | | | | 45,983 | |
| | | | | | | | |
Deferred tax liabilities | | | | | | | | |
Excess tax over book depreciation and amortization | | | 20,196 | | | | 12,197 | |
Other | | | 51 | | | | 33 | |
| | | | | | | | |
Gross deferred tax liability | | | 20,247 | | | | 12,230 | |
Valuation allowance | | | (1,154 | ) | | | (2,217 | ) |
| | | | | | | | |
Net deferred tax asset | | $ | 17,762 | | | $ | 31,536 | |
| | | | | | | | |
In conjunction with the acquisition of RITA, at May 31, 2008, the Company had approximately $99.2 million of remaining federal net operating loss carryforwards and $53.0 million of state net operating loss carryforwards (“NOL”). As a result of ownership changes caused by the acquisition of RITA, these net operating losses are subject to Internal Revenue Code (“IRC”) Section 382 limitations, which is expected to significantly limit the Company’s ability to utilize these net operating losses on an annual basis. As a result of the Company’s IRC Section 382 analysis, it is estimated that approximately $15.0 million of remaining Federal net operating losses and $11.8 million of state net operating losses will expire prior to utilization. The gross deferred income tax asset (“DTA”) related to the NOL reflects these limitations.
The Company needs to generate approximately $5 million of taxable income in each year over the next eighteen years to ensure the realizability of the Company’s deferred tax assets. The Company has determined that it has sufficient existing levels of pre-tax earnings to generate sufficient taxable income to realize the net deferred tax assets recorded on the Company’s balance sheets.
In order to support the realizability of the Company’s net deferred tax asset, management projected its pre-tax income utilizing a combination of historical and projected results. Utilizing this projected pre-tax income, management has projected taxable income taking into consideration existing levels of permanent differences including stock option exercise deductions and non-deductible expenses and the reversal of significant temporary differences.
81
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
The Company’s federal net operating loss carryforwards as of May 31, 2008 after considering IRC Section 382 limitations are $84.2 million. The expiration of the federal net operating loss carryforwards are as follows: $1.0 million between 2010 and 2011, $45.5 million between 2017 and 2021 and $37.7 million between 2022 and 2026.
The Company’s state net operating loss carryforwards as of May 31, 2008 after considering remaining IRC Section 382 limitations are $41.2 million which expire in various years from 2009 to 2026.
At May 31, 2008, the Company had approximately $429,000 of Federal research and development tax credit carryforwards which are subject to IRC Section 382 limitations and begin to expire in 2023. Additionally, at May 31, 2008, the Company had $1.3 million of state credits, of which $315,000 expire at various dates through 2013 and $996,000 which have an unlimited carryforward period.
At May 31, 2008, the Company had a net deferred income tax asset of $17.8 million, after recording a valuation allowance of $1.2 million (of which $1.1 million relates to deferred tax assets acquired in connection with the RITA acquisition). When the portion of the valuation allowance associated with the acquisition of RITA is reversed in the future, the benefit of any reversal would (a) first be applied to reduce to zero and goodwill related to the acquisition (b) second to reduce to zero other non-current intangible assets related to the acquisition, and (c) third to reduce income tax expense. The net change in the valuation allowance was a decrease of $1.0 million in 2008 and an increase of $2.1 million in 2007. The 2008 decrease in the valuation allowance was based upon a change in management’s estimate of the realizability of certain Federal tax credits and state net operating losses obtained in connection with the RITA acquisition. Consequently this change was reflected as a reduction to goodwill related to the acquisition. The valuation allowance recorded against the deferred tax assets acquired in connection with the RITA acquisition relates to state tax credits and state NOLs that management has estimated will more likely than not expire before they are expected to be utilized.
The Company’s consolidated income tax provision has differed from the amount that would be provided by applying the U.S. Federal statutory income tax rate to the Company’s income before income taxes for the following reasons:
| | | | | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
| | | (in thousands) | |
Income tax provision | | $ | 6,439 | | | $ | 1,955 | | | $ | 4,256 | |
Effect of Graduated tax rates | | | 173 | | | | (71 | ) | | | 112 | |
State income taxes, net of Federal tax benefit | | | (52 | ) | | | (33 | ) | | | (195 | ) |
Impact of Non US operations | | | (17 | ) | | | — | | | | — | |
Tax-exempt interest | | | 151 | | | | 79 | | | | — | |
Research and development tax credit | | | 114 | | | | 32 | | | | 88 | |
Domestic Production Activities deduction | | | 74 | | | | 72 | | | | 27 | |
Extraterritorial income exclusion | | | — | | | | — | | | | 7 | |
Nondeductible write-off of acquired in-process R&D | | | — | | | | (4,114 | ) | | | — | |
Nondeductible stock-based compensation | | | (311 | ) | | | (161 | ) | | | — | |
Other nondeductible expenses | | | (480 | ) | | | (414 | ) | | | (375 | ) |
Overaccrual (underaccrual) of prior year Federal and state taxes | | | (26 | ) | | | 89 | | | | (27 | ) |
Other | | | — | | | | 56 | | | | — | |
| | | | | | | | | | | | |
Income tax provision at statutory tax rate of 35% | | $ | 6,065 | | | $ | (2,510 | ) | | $ | 3,893 | |
| | | | | | | | | | | | |
In July 2006, the Financial Accounting Standards Board (FASB) issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes-an interpretation of FASB Statement No. 109” (FIN 48), which
82
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
clarifies the accounting for uncertainty in tax positions. FIN 48 seeks to reduce the diversity in practice associated with certain aspects of the recognition and measurement related to accounting for income taxes. This Interpretation requires the Company recognize in its financial statements the impact of a tax position, if that position is more likely than not of being sustained on audit, based on the technical merits of the position. This Interpretation is effective for fiscal years beginning after December 15, 2006, with the cumulative effect of the change in accounting principle recorded as an adjustment to opening retained earnings. The Company adopted this statement on June 3, 2007. There was no cumulative effect of adopting FIN 48. Upon adoption, the liability for unrecognized tax benefits was zero.
During the twelve months ended May 31, 2008, the Company did not recognize any tax liabilities related to uncertain tax positions. Due to the unrecognized tax benefit of the Company being zero upon adoption, with no change since adoption, no “tabular reconciliation” of the total amount of unrecognized tax benefits at the beginning and end of the period is being presented.
The Company recognizes interest and penalties related to unrecognized tax benefits within its global operations as a component of income tax expense. This accounting policy did not change as a result of the adoption of FIN 48. Accrued interest and penalties recognized in the consolidated balance sheet were $0 as of June 2, 2007 and May 31, 2008.
The Company files income tax returns in the U.S. federal jurisdiction and various state and foreign jurisdictions. In the normal course of business the Company is subject to examination by taxing authorities throughout the world. Fiscal years 2005 through 2008 remain open to examination by the various tax authorities. The Company analyzed filing positions in all of the federal and state jurisdictions where it is required to file income taxes, as well as all open tax years in these jurisdictions and believes that its income tax filings positions and deductions will be sustained on audit and does not anticipate any adjustments will result in a material adverse effect on the Company’s financial condition, results of operations or cash flows.
Management does not anticipate that the amount of unrecognized tax benefits will significantly change in the next twelve months.
NOTE I—PREPAID ROYALTIES
On August 13, 2007, the Company entered into a Distribution, Manufacturing and Purchase Option Agreement (“the Agreement”) with a company to acquire the exclusive worldwide rights to manufacture and distribute a split tip catheter for the dialysis market that the Company has named Centros™. The Company also has the option to purchase certain intellectual property associated with these products in the future. The Company will pay royalties on net sales of the products covered in the Agreement. In accordance with the Agreement, the Company has prepaid $3.0 million of royalties based upon the achievement of certain milestones. These payments have been included in the caption “Prepaid Royalties” on the balance sheet as of May 31, 2008 and will be credited against quarterly royalties due subject to certain contractual limitations in the first two years following the initial sale of product. In years 4 through 10 of the contract, certain minimum annual royalties are due.
83
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
NOTE J—ACCRUED LIABILITIES
Accrued liabilities consist of the following:
| | | | | | |
| | | May 31,
2008 | | June 2,
2007 |
| | | (in thousands) |
Payroll and related expenses | | $ | 5,051 | | $ | 4,267 |
Sales and franchise taxes | | | 1,112 | | | 1,352 |
Royalties | | | 763 | | | 768 |
Fair value of interest rate swap | | | 416 | | | 98 |
Other | | | 2,181 | | | 1,651 |
| | | | | | |
Total | | $ | 9,523 | | $ | 8,136 |
| | | | | | |
NOTE K—LONG-TERM DEBT
Industrial Revenue Bonds
In September 2002, the Company closed on the financing for the expansion of its headquarters and manufacturing facility in Queensbury, New York. The expansion was financed principally with Industrial Revenue Bonds (the “Bonds”) issued by the Warren and Washington Counties Industrial Development Agency (the “Agency”) aggregating $3,500,000. The Bonds are issued under a Trust Agreement by and between the Agency and a bank, as trustee (the “Trustee”). The proceeds of the Bonds were advanced, as construction occurred, pursuant to a Building Loan Agreement by and among the Agency, the Trustee, a second bank (the “Bank”) and the Company. The Bonds reprice every seven days and are resold by a Remarketing Agent. The Bonds bear interest based on the market rate on the date the Bonds are repriced and require quarterly interest payments and quarterly principal payments ranging from $25,000 to $65,000 through May 2022. In connection with the issuance of the Bonds, the Company entered into a Letter of Credit and Reimbursement Agreement with the Bank which requires the maintenance of a letter of credit for an initial amount of $3,575,000 to support principal and certain interest payments of the Bonds and requires payment of an annual fee on the outstanding balance ranging from 1% to 1.9%, depending on financial results achieved. The Company also entered into a Remarketing Agreement, pursuant to which the Remarketing Agent is required to use its best efforts to arrange for sales of such bonds in the secondary market. The Remarketing Agreement provides for the payment of an annual fee of 0.1% of the remaining balance.
The Reimbursement Agreement contains certain financial covenants relating to fixed charge coverage and interest coverage, as defined. Amounts borrowed under the Agreement are collateralized by the aforementioned letter of credit and a first mortgage on the land, building and equipment relating to the facility.
The Company entered into an interest rate swap agreement (the “2002 Swap Agreement”) with the Bank, effective September 2002, with an initial notional amount of $3,500,000 to limit the effect of variability due to interest rates on its rollover of the Bonds. The Swap Agreement, which qualifies for hedge accounting under SFAS No. 133, is a contract to exchange floating interest rate payments for fixed interest payments periodically over the life of the agreement without the exchange of the underlying notional amounts. The Swap Agreement requires the Company to pay a fixed rate of 4.45% and receive payments based on 30-day LIBOR repriced every seven days through May 2022. As of May 31, 2008 and June 2, 2007, since the Swap Agreement is classified as a cash flow hedge, the fair value of $196,000 and $98,000, respectively, has been recorded as a component of accrued liabilities, and accumulated other comprehensive loss related to the swap agreement is $123,000 and $61,000, respectively, net of tax.
84
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
The Company capitalized certain legal and administrative costs incurred in connection with the issuance of the Bonds and is amortizing these costs using the effective interest method over the term of the Bonds. As of May 31, 2008 and June 2, 2007, net capitalized bond issuance costs amounted to $80,000 and $85,000, respectively, and are recorded as a component of other assets.
Amounts to be paid or received under the Swap Agreement are accrued as interest rates change and are recognized over the life of the Swap Agreement as an adjustment to interest expense.
Taxable Adjustable Rate Notes
In December 2006, the Company closed on the financing for the expansion of its warehouse and manufacturing facility in Queensbury, New York. The expansion is being financed principally with Taxable Adjustable Rate Notes (the “Notes”) issued by the Company aggregating $5,000,000, maturing in December 2026. The Notes were issued under a Trust Agreement by and between the Company and a bank, as trustee (the “Trustee”). The Notes reprice every seven days and are resold by a Remarketing Agent. The Notes bear interest based on the market rate on the date the Notes are repriced and require quarterly interest payments and quarterly principal payments ranging from $25,000 to $55,000. In connection with the issuance of the Notes, the Company entered into a Letter of Credit and Reimbursement Agreement with the Bank that requires the maintenance of a letter of credit for an initial amount of $5,134,000 to support principal and certain interest payments on the Notes and requires payment of an annual fee on the outstanding balance ranging from 0.75% to 1.35%. The Company also entered into a Remarketing Agreement, pursuant to which the Remarketing Agent is required to use its best efforts to arrange for sales of the Notes in the secondary market. The Remarketing Agreement provides for the payment of an annual fee of 0.1% of the remaining balance.
The Reimbursement Agreement contains certain financial covenants relating to fixed charge coverage, interest coverage, and a debt to earnings before interest, taxes, depreciation and amortization (“EBITDA”) ratio, as defined. Amounts borrowed under the Reimbursement Agreement are collateralized by the aforementioned letter of credit and all Company assets.
The Company entered into an interest rate swap agreement (the “2006 Swap Agreement”) with the Bank, effective December 2006, with an initial notional amount of $5,000,000, to limit the effect of variability due to interest rates on its rollover of the Notes. The 2006 Swap Agreement is a contract to exchange floating interest rate payments for fixed interest payments of 5.06% of the outstanding balance of the Notes over the life of the agreement without the exchange of the underlying notional amounts. Changes to the fair value of the 2006 Swap Agreement are recorded as increases or decreases to interest expense as the Company did not elect to apply hedge accounting. As of May 31, 2008 and June 2, 2007, the fair value of $221,000 and $88,000, respectively has been recorded as a component of accrued liabilities with a corresponding credit to other income in the consolidated statement of operations.
The Company capitalized certain legal and bank fees incurred in connection with the issuance of the Notes and is amortizing these costs on a straight-line basis over the term of the Notes. As of May 31, 2008 and June 2, 2007, net capitalized issuance costs related to these Notes amounted to $178,000 and $187,000, respectively, and are recorded as a component of other assets.
Convertible Notes
In connection with the acquisition of RITA on January 29, 2007, the Company assumed subordinated Senior Convertible Notes of RITA (the “Convertible Notes”) with an aggregate principal amount of $9.7 million. The
85
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
Convertible Notes are convertible, at any time prior to the Maturity Date at such holder’s option, into shares of the Company’s common stock applicable at a conversion price of $20.41 per share of common stock, net of a cash component, subject to adjustment in certain circumstances including common stock splits or other standard anti-dilution provisions. Until conversion or maturity, the Convertible Notes bear interest at 6.5% per year, payable semi-annually. Absent conversion, the Convertible Notes mature on August 5, 2008 (the “Maturity Date”). If on the Maturity Date, the closing price of the Company’s common stock has been at or above 102% of the then conversion price for at least 10 consecutive business days immediately preceding the Maturity Date, then any remaining principal outstanding under the Convertible Notes shall automatically be converted into the Company’s common stock, subject to certain conditions. The fair value of the conversion feature of the Convertible Notes of $1.8 million was calculated using the intrinsic value method and recorded in goodwill and stockholders’ equity as part of the purchase price described in Note C.
Following is a summary of long-term debt at May 31, 2008 (in thousands):
| | | | |
Industrial Revenue Bonds | | $ | 2,560 | |
Taxable Adjustable Rate Notes | | | 4,855 | |
Convertible Notes | | | 9,700 | |
| | | | |
| | | 17,115 | |
Less: current maturities | | | (10,040 | ) |
| | | | |
Long-term debt | | $ | 7,075 | |
| | | | |
At May 31, 2008, future minimum principal payments on long-term debt were as follows:
| | | |
| | | (in thousands) |
2009 | | $ | 10,040 |
2010 | | | 265 |
2011 | | | 260 |
2012 | | | 275 |
Thereafter | | | 6,275 |
| | | |
| | $ | 17,115 |
| | | |
NOTE L—RELATED PARTY TRANSACTIONS AND ARRANGEMENTS
Related Party Consulting Services
During 2008 and 2007, the Company received professional sales training services from an organization in which the principal owner is the spouse of the Company’s President and CEO. Fees and expenses paid for these services totaled $108,000 and $204,000, respectively.
NOTE M—RETIREMENT PLANS
The Company has a profit-sharing plan under which it makes discretionary contributions to eligible employees, and a companion 401(k) plan under which eligible employees can defer a portion of their compensation, part of which is matched by the Company. Profit-sharing contributions were $948,700, $411,000, and $431,000, in 2008, 2007, and 2006, respectively. Matching contributions were $499,500, $234,000, and $249,000, in 2008, 2007, and 2006, respectively.
86
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
NOTE N—STOCKHOLDERS’ EQUITY
1. Capitalization
On February 27, 2004, the Company’s Board of Directors and the Former Parent, as sole stockholder, approved the Company’s Amended and Restated Certificate of Incorporation (the “Amended Certificate”). Under the Amended Certificate, the authorized capital stock of the Company is 50,000,000 shares, consisting of 45,000,000 shares of common stock, par value $.01 per share and 5,000,000 shares of preferred stock, par value $.01 per share. Pursuant to the Amended Certificate, (i) each share of voting common stock, $1 par value and (ii) each share of non-voting common stock, $1 par value was reclassified and exchanged into 9,200 shares of issued, fully paid, non-assessable common stock for a total of 9,200,000 shares to be then outstanding.
The holders of common stock are entitled to one vote for each share held. Subject to preferences applicable to any outstanding shares of preferred stock, the holders of common stock are entitled to receive ratably dividends, if any, as may be declared by the Board of Directors out of funds legally available for dividend payments. If the Company liquidates, dissolves, or winds up, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities and liquidation preferences of any outstanding shares of preferred stock. Holders of common stock have no pre-emptive rights or rights to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that the Company may designate in the future.
The Company’s board of directors has the authority to (i) issue the undesignated preferred stock in one or more series, (ii) determine the powers, preferences and rights and the qualifications, limitations or restrictions granted to or imposed upon any wholly un-issued series of undesignated preferred stock and (iii) fix the number of shares constituting any series and the designation of the series, without any further vote or action by the Company’s stockholders.
2. Stock Options
The Company has two stock-based compensation plans, exclusive of the stock option plans assumed in connection with the acquisition of RITA. These plans provide for the issuance of up to approximately 3.5 million shares of common stock.
1997 Stock Option Plan
In 1997, the Company adopted a Stock Option Plan (the “1997 Plan”). The 1997 Plan provides for the grant to key employees of both nonqualified stock options and incentive stock options and to members of the Board of Directors and consultants of nonqualified stock options. A total of 1,497,674 shares of the Company’s common stock may be issued under the 1997 Plan pursuant to the exercise of options. All stock options must have an exercise price of not less than the fair market value of the shares on the date of grant. Options will be exercisable over a period of time to be designated by the administrators of the 1997 Plan (but not more than 10 years from the date of grant) and will be subject to such other terms and conditions as the administrators may determine. The 1997 Plan terminated in March 2007 and as such, no further options will be granted under this plan. The vesting schedule is subject to the discretion of the Company’s Board of Directors. Options are exercisable immediately upon vesting. In addition, all options, whether vested or not, become exercisable in full immediately upon a change of control, as defined under the 1997 Plan.
87
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
2004 Stock and Incentive Award Plan
The 2004 Stock and Incentive Award Plan (the “2004 Plan”) provides for the grant of incentive options to the Company’s employees and for the grant of non-statutory stock options, restricted stock, stock appreciation rights, performance units, performance shares and other incentive awards to the Company’s employees, directors and other service providers. A total of 2,000,000 shares of the Company’s common stock have been reserved for issuance under the 2004 Plan, of which up to 800,000 shares may be issued upon the exercise of incentive stock options. The compensation committee of the Board of Directors administers the 2004 Plan. The committee determines vesting terms and the exercise price of options granted under the 2004 Plan, but for all incentive stock options the exercise price must at least be equal to the fair market value of the Company’s common stock on the date of grant. The term of an incentive stock option may not exceed ten years.
RITA Stock Option Plans
In connection with the acquisition of RITA, the Company assumed all outstanding options to acquire RITA common stock (the “RITA Options”). Upon exercise, the RITA Options will result in the Company issuing approximately 988,815 shares of the Company’s common stock with a weighted average exercise price of $17.30, net of a Cash Component as defined in the Purchase Agreement. Except for RITA Options that were fully vested due to employee terminations and change-of-control provisions in connection with the completion of the acquisition of RITA, options under these plans maintain their original vesting provisions and generally expire ten years from the original date of grant. The Company does not anticipate future grants will be made under these plans. As of May 31, 2008, RITA Options to acquire 400,683 shares of Company common stock were outstanding, of which RITA Options to acquire 350,143 shares of Company common stock were exercisable.
In accordance with the Merger Agreement, the options held by RITA employees became exercisable for shares of the Company’s common stock and a fixed cash component payable to the holder at option exercise (see Note C). Under SFAS 123(R), an exchange of stock-based compensation awards in a combination is treated as a modification. Based upon the fact that the receipt of cash is contingent upon the exercise of the option, and not the vesting of such option, the RITA Options were classified as equity. The Company calculated the fair value of the RITA options immediately prior to the modification, utilizing fair value assumptions at the time the merger was being contemplated and the fair value of the replacement awards. It was determined there was no incremental compensation cost required to be recognized for either the vested or unvested options.
The fair value of the RITA options assumed in connection with the acquisition of RITA was calculated using the Black-Scholes model with the following weighted-average assumptions:
| | | | |
Stock options assumed in acquisition | | | 988,815 | |
Weighted-average fair value | | $ | 12.63 | |
Black-Scholes Assumptions: | | | | |
Expected stock price volatility | | | 50.60 | % |
Risk-free interest rate | | | 4.98 | % |
Expected term (in years) | | | 2.6 | |
Expected dividend yield | | | 0 | |
88
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
Stock Option Activity:
The following schedule summarizes stock option activity as of and for the years ended May 31, 2008, June 2, 2007, and June 3, 2006:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | 2008 | | 2007 | | 2006 |
| | Shares | | | Weighted-
average
exercise
price | | Weighted
average
remaining
contractual
life | | Aggregate
intrinsic
value (in
thousands) | | Shares | | | Weighted-
average
exercise
price | | Shares | | | Weighted-
average
exercise
price |
Outstanding at beginning of year | | 2,133,662 | | | $ | 17.88 | | | | | | | 1,251,145 | | | $ | 13.23 | | 1,552,392 | | | $ | 6.93 |
Granted | | 477,510 | | | $ | 18.14 | | | | | | | 552,368 | | | $ | 19.25 | | 381,600 | | | $ | 24.71 |
Assumed in acquisition | | — | | | $ | — | | | | | | | 988,815 | | | $ | 17.30 | | | | | | — |
Exercised | | (245,120 | ) | | $ | 16.60 | | | | | | | (559,459 | ) | | $ | 7.32 | | (634,364 | ) | | $ | 4.70 |
Forfeited | | (320,732 | ) | | $ | 22.54 | | | | | | | (99,207 | ) | | $ | 20.74 | | (48,483 | ) | | $ | 13.27 |
Expired | | (6,137 | ) | | $ | 63.17 | | | | | | | | | | | — | | | | | | — |
| | | | | | | | | | | | | | | | | | | | | | | |
Outstanding at end of year | | 2,039,183 | | | $ | 17.82 | | 6.47 years | | $ | 20,622 | | 2,133,662 | | | $ | 17.88 | | 1,251,145 | | | $ | 13.23 |
| | | | | | | | | | | | | | | | | | | | | | | |
Options exercisable at year-end | | 996,282 | | | $ | 16.62 | | 6.23 years | | $ | 10,188 | | 1,044,564 | | | $ | 16.40 | | 590,257 | | | $ | 6.67 |
| | | | | | | | | | | | | | | | | | | | | | | |
Options expected to vest as of end of 2008 | | 966,112 | | | $ | 19.43 | | 6.96 years | | $ | 9,638 | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Weighted-average fair value of options granted during the year | | | | | $ | 8.84 | | | | | | | | | | $ | 10.70 | | | | | $ | 12.52 |
On May 31, 2008, there remained 498,460 shares available for granting of options under the 2004 Plan. Options are exercisable into common stock.
All Company options were granted at exercise prices equal to the quoted market price of the Company’s common stock at the date of the grants. Options under these grants vest 25% per year over four years for employees and 100% after one year for consultants. Initial grants to directors vest 25% per year over four years and subsequent grants to directors vest 33 1/3% per year over three years. Options granted prior to May 1, 2007 expire on the tenth anniversary of the grant date. Options granted on or after May 1, 2007, expire on the seventh anniversary of the grant date. The total intrinsic value of options exercised was $2,787,000, $2,883,000, and $1,158,000 for the years ended May 31, 2008, June 2, 2007, and June 3, 2006, respectively. The Company generally issues authorized but unissued shares upon stock option exercises and the settlement of performance share awards and restricted stock units.
89
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
The fair value of the options granted under the 1997 and 2004 Plans was estimated at the date of grant using the Black-Scholes option-pricing model assuming no expected dividends and the following weighted-average assumptions:
| | | | | | | | | |
| | | 2008 | | | 2007 | | | 2006 | |
Expected stock price volatility | | 53.37 | % | | 55.63 | % | | 56.21 | % |
Risk-free interest rate | | 4.20 | % | | 4.76 | % | | 4.17 | % |
Expected life of options | | 4.6 years | | | 5.9 years | | | 5.4 years | |
The following information applies to options outstanding at May 31, 2008:
| | | | | | | | | | | | |
Range of exercise prices | | Number
outstanding | | Weighted-
average
remaining
life in
years | | Weighted
-average
exercise
price | | Number
Exercisable | | Weighted-
average
exercise
price |
$2.81 - $3.22 | | 12,175 | | 1.90 | | $ | 2.96 | | 12,175 | | $ | 2.96 |
$4.35 - $6.52 | | 57,314 | | 3.77 | | | 5.73 | | 53,659 | | | 5.68 |
$6.68 - $9.61 | | 20,837 | | 3.75 | | | 8.00 | | 20,837 | | | 8.00 |
$10.59 - $15.36 | | 466,352 | | 5.51 | | | 12.66 | | 394,619 | | | 12.52 |
$15.76 - $23.03 | | 1,113,539 | | 7.10 | | | 18.31 | | 311,470 | | | 18.50 |
$23.95 - $35.11 | | 361,943 | | 6.38 | | | 25.30 | | 196,486 | | | 25.35 |
$43.58 - $53.92 | | 6,892 | | 2.97 | | | 52.07 | | 6,892 | | | 52.07 |
$93.52 - $93.52 | | 144 | | 0.63 | | | 93.52 | | 144 | | | 93.52 |
| | | | | | | | | | | | |
| | 2,039,196 | | 6.47 | | $ | 17.82 | | 996,282 | | $ | 16.62 |
| | | | | | | | | | | | |
3. Performance Share and Restricted Stock Unit Awards
The Company may grant restricted stock units or performance share awards to certain employees under the 2004 Plan. The performance criteria established by the compensation committee for vesting the performance share awards is the achievement of certain earnings per share (“EPS”) goals and revenue goals by the Company for each of the 2006 through 2009 fiscal years. Shares not earned in a fiscal year may be earned in the following fiscal year if the EPS or revenue goals in such following year are exceeded by an amount at least equal to the shortfall for the applicable goal for the preceding year. The performance share awards are subject to additional conditions, including the recipient’s continued employment with the Company. The restricted stock unit awards vest in full upon the recipient’s continued employment with the Company through the end of the Company’s fiscal year ending on or about May 30, 2009. The restricted stock unit awards will be forfeited if the recipient ceases to be employed by the Company, competes with the business of the Company, or otherwise engages in activities detrimental to the Company’s business before such date. The performance share awards and restricted stock units settle in shares of the Company’s common stock on a one-for-one basis.
The Company values performance share and restricted stock unit awards based on the closing trading value of the Company’s shares on the date of grant. The Company recognizes the compensation cost related to its non-vested stock awards ratably over the requisite service period, which is consistent with the treatment prior to the adoption of SFAS 123(R). Under APB 25, the performance share and restricted stock unit awards were accrued as vested and recorded in accrued liabilities. During the year ended June 2, 2007, the vested performance shares were issued and the liability for the restricted stock unit awards was reclassified to additional paid-in capital as required by SFAS 123(R).
90
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
Information related to non-vested stock awards as of and for the year ended May 31, 2008, is as follows:
| | | | | | |
| | | Non-Vested Stock
Award Units | | | Weighted Average
Grant-Date Fair
Value |
Balance as of June 2, 2007 | | 62,315 | | | $ | 19.38 |
Granted | | — | | | | — |
Cancelled | | (13,833 | ) | | | 21.81 |
Vested | | (4,384 | ) | | | 19.59 |
| | | | | | |
Balance as of May 31, 2008 | | 44,098 | | | $ | 18.59 |
| | | | | | |
The total fair value of restricted stock awards vesting was $85,900, $157,800 and $0 for the years ended May 31, 2008, June 2, 2007 and June 3, 2006, respectively.
4. Unrecognized Compensation Cost:
Under the provisions of SFAS 123(R), the Company expects to recognize the following future expense for awards outstanding as of May 31, 2008:
| | | | | |
| | | Unrecognized
Compensation
Cost | | Weighted Average
Remaining Vesting
Period (in years) |
Stock Options | | $ | 7,821,700 | | 2.38 |
Non-vested stock awards | | | 256,000 | | 1.00 |
| | | | | |
| | $ | 8,077,700 | | 2.32 |
| | | | | |
Of the $8.1 million of unrecognized stock option compensation cost at May 31, 2008, approximately $0.8 million relates to RITA options. Unrecognized compensation cost for stock options is presented net of 4.69% assumed annual forfeitures.
5. Employee Stock Purchase Plan
The Employee Stock Purchase Plan (the “Stock Purchase Plan”) provides a means by which employees of the Company (the “participants”) are given an opportunity to purchase common stock of the Company through payroll deductions. The maximum number of shares to be offered under the Stock Purchase Plan is 200,000 shares of the Company’s common stock, subject to any increase authorized by the Board of Directors. Shares are offered through two purchase periods, each with duration of approximately 6 months, commencing on the first business day of the first and third fiscal quarters. An employee is eligible to participate in an offering period if, on the first day of an offering period, he or she has been employed in a full-time capacity for at least six months, with a customary working schedule of 20 or more hours per week and more than five months in a calendar year. Employees who own stock possessing 5% or more of the total combined voting power or value of all classes of the Company’s stock are not eligible to participate in the Stock Purchase Plan. The purchase price of the shares of common stock acquired on each purchase date will be the lower of (i) 85% of the fair market value of a share of common stock on the first day of the offering period or (ii) 85% of the fair market value of a share of common stock on the last day of the purchase period, subject to adjustments made by the Board of Directors. The Stock Purchase Plan is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Internal Revenue Code.
91
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
The Company uses the Black-Scholes option-pricing model to calculate the purchase date fair value of the shares issued under the Stock Purchase Plan and recognizes expense related to shares purchased ratably over the offering period.
For the years ended May 31, 2008, June 2, 2007 and June 3, 2006, 57,011, 32,765 and 23,435 shares, respectively, were issued at an average price of $14.33, $14.84 and $15.62, respectively, under the Stock Purchase Plan. As of May 31, 2008, 77,421 shares remained available for future purchases under the Stock Purchase Plan.
NOTE O—STOCK-BASED COMPENSATION
The Company adopted SFAS 123(R) using the modified prospective transition method, which requires the application of the accounting standard as of June 4, 2006, the first day of the Company’s 2007 fiscal year. The Company’s consolidated financial statements as of and for the years ended May 31, 2008 and June 2, 2007, reflect the impact of SFAS 123(R). In accordance with the modified prospective transition method, the Company’s consolidated financial statements have not been restated to include the impact of SFAS 123(R). Stock-based compensation expense recognized under SFAS 123(R) for the years ended May 31, 2008 and June 2, 2007, was $3,421,000, net of income taxes of $1,478,000 and $2,372,000, net of income taxes of $1,126,000, respectively. During the year ended June 3, 2006, compensation expense of $81,000 was recognized for options granted to consultants and $371,000 was recognized for restricted stock unit and performance share awards granted to employees.
The following table summarizes stock-based compensation in accordance with SFAS 123(R) for the years ended May 31, 2008 and June 2, 2007, which was allocated as follows (in thousands):
| | | | | | |
| | | May 31,
2008 | | June 2,
2007 |
| | | (In thousands) |
Cost of sales | | $ | 645 | | $ | 476 |
| | | | | | |
Research and development | | | 737 | | | 615 |
Sales and marketing | | | 1,540 | | | 966 |
General and administrative | | | 1,977 | | | 1,441 |
| | | | | | |
Stock based compensation expense included in operating expenses | | | 4,254 | | | 3,022 |
| | | | | | |
Total stock based compensation | | | 4,899 | | | 3,498 |
Tax benefit | | | 1,478 | | | 1,126 |
| | | | | | |
Stock based compensation expense, net of tax | | $ | 3,421 | | $ | 2,372 |
| | | | | | |
92
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
If the Company had elected to recognize compensation expense based upon the fair value at the grant date for options and awards granted under these plans to key employees and to members of the Board of Directors, consistent with the methodology prescribed by SFAS No. 123, the Company’s pro forma net income and earnings per common share would be as follows:
| | | | |
| | | 2006 | |
Net Income | | | | |
As reported | | $ | 6,866 | |
| |
Add total stock based compensation recorded under intrinsic value based method for all awards, net of tax of $180 | | | 293 | |
Deduct total stock-based compensation under fair value based method for all awards, net of tax of $848 | | | (1,383 | ) |
| | | | |
Pro forma net income | | $ | 5,776 | |
| | | | |
Basic earnings per common share | | | | |
As reported | | $ | 0.55 | |
Pro forma | | | 0.47 | |
| |
Diluted earnings per common share | | | | |
As reported | | $ | 0.53 | |
Pro forma | | | 0.45 | |
NOTE P—ASSET PURCHASE AGREEMENTS
Medron, Inc.
On May 1, 2006, the Company entered into an Asset Purchase Agreement (the “Agreement”) with Medron, Inc. to acquire the rights, titles, and interests in, and to, Patent Pending Technology for purposes of manufacturing, marketing, and selling proprietary Vascular Access Ports, following administrative approval. As of May 31, 2008, the Company has paid $5.5 million in accordance with the Agreement. That amount, net of accumulated amortization, has been included on the balance sheet under the caption “Intangible assets” and is being amortized on a straight line basis over the expected useful life of the assets. A potential future payment of $2.5 million is due upon issuance (within 10 years of the effective date of the Agreement) of a U.S. patent claiming priority to the Patent Application, or any issuance of a patent to the Company within 10 years of the effective date of the Agreement in which the original owners are the inventors.
Nevertouch™
On August 20, 2007, the Company entered into an agreement to acquire all technology rights, including patent rights to the NeverTouch technology (the “Agreement”). As of May 31, 2008, the Company has made payments of approximately $3.0 million which have been recorded on the balance sheet, net of accumulated amortization, under “Intangible assets” and are being amortized on a straight line basis over the expected useful life of the asset.
93
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
NOTE Q—COMMITMENTS AND CONTINGENCIES
Leases
The Company is committed under non-cancelable operating leases for facilities and equipment. During 2008, 2007 and 2006, aggregate rental costs under all operating leases were approximately $1,553,000, $883,000, and $570,000, respectively. Future annual payments under non-cancelable operating leases in the aggregate (in thousands), of which one includes an escalation clause, with initial remaining terms of more than one year at May 31, 2008, are summarized as follows:
| | | |
2009 | | $ | 485 |
2010 | | | 428 |
2011 | | | 10 |
2012 | | | 4 |
| | | |
| | $ | 927 |
| | | |
Litigation Matters
Diomed v. AngioDynamics
In January 2004, Diomed filed an action against the Company alleging that the Company’s VenaCure products for the treatment of varicose veins infringed a patent held by Diomed for a laser system that competes with the Company’s VenaCure products. In March 2007, a jury ruled in Diomed’s favor and awarded compensatory damages of $9.71 million. On July 2, 2007, the judge for the Federal District in Boston, Massachusetts, issued an injunction prohibiting the Company from selling its original bare fiber VenaCure product. The Company disputed the infringement verdict on multiple grounds and on June 20, 2007, filed an appeal in the U.S. Court of Appeals for the Federal Circuit in Washington, D.C. On March 14, 2008, Diomed commenced Chapter 11 bankruptcy proceedings. On April 2, 2008, the Company entered into a settlement agreement with Diomed resolving the patent disputes. As a result of the settlement, in the fiscal third quarter of 2008, the Company reduced its litigation provision and recorded a gain, net of costs, of approximately $2.0 million, net of tax.
VNUS Medical Technologies v. Diomed, Vascular Solutions, and AngioDynamics
In October 2005, VNUS Medical Technologies filed an action against the Company, and others (collectively, the “Defendants”) alleging, among other things, that the manufacture, use and sale of the Company’s VenaCure products infringed several patents held by VNUS and seeking injunctive relief and compensatory and treble damages. On June 3, 2008, the Company entered into an agreement with VNUS settling all patent litigation between the Company and VNUS. Under the terms of the settlement agreement, the Company paid VNUS approximately $6.8 million and agreed to pay a quarterly royalty on its U.S. sales of the Company’s NeverTouch™ and VenaCure® products from June 1, 2008 until the expiration date of VNUS’ applicable patents. In exchange, VNUS granted the Company a non-exclusive and non-sublicensable license to VNUS’ applicable patents for use in endovenous laser therapy.
The Company is party to other legal actions that arise in the ordinary course of business. The Company believes that any liability resulting from any currently pending litigation will not, individually or in the aggregate, have a material adverse effect on the Company’s business, financial condition, results of operations, or cash flows.
94
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
Future Purchase Obligations
On October 17, 2005, the Company entered into a Supply and Distribution Rights Agreement (the “Agreement”) with Bioniche Pharma Group Limited (“Bioniche”). The Company was appointed the exclusive distributor in the Field, as defined in the Agreement, in the United States of Bioniche’s sodium tetradecyl sulfate product in concentrations of 1% and 3%, brand name “Sotradecol™” (“Product”). Sotradecol is used in sclerotherapy, a non-surgical procedure to remove varicose veins. The Agreement was amended during fiscal 2008 and expires on June 30, 2012. Future obligations under the agreement are as follows:
| | • | | three non-refundable milestone payments are due 30 days after achieving certain cumulative sales of Product. Payments of $500,000, $1,000,000 and $1,000,000 are due upon achieving cumulative sales of $10,000,000, $25,000,000 and $50,000,000, respectively. If the Company should lose any of its exclusive distribution rights under the Agreement, as amended, any milestone payments not yet made would not be required to be made. None of these sales milestones were achieved during the year ended May 31, 2008. |
| | • | | the Company shall use reasonable efforts to purchase a minimum of $3,200,000 of Product for the year ended June 30, 2009 and $4,200,000 of Product for each of the remaining years of the contract though the expiration date. (June 30, 2012). The Company met its purchase commitment for the year ended June 30, 2008. Failure to make future minimum annual purchases in any such contract year, unless cured as provided in the Agreement, may result in a loss of exclusive rights under the Agreement. |
NOTE R–SALES
Net sales (in thousands) by product category and geography were as follows:
| | | | | | | | | | | | | | | | | | |
| | | Three months ended | | Twelve months ended |
| | | May 31,
2008 | | June 2,
2007 | | June 3,
2006 | | May 31,
2008 | | June 2,
2007 | | June 3,
2006 |
| | | (unaudited) | | | | (unaudited) | | |
Net Sales by Product Category | | | | | | | | | | | | | | | | | | |
Interventional Products | | $ | 35,864 | | $ | 31,920 | | $ | 23,592 | | $ | 128,102 | | $ | 101,126 | | $ | 78,451 |
Oncology Products | | | 10,888 | | | 8,935 | | | — | | | 38,398 | | | 11,101 | | | — |
| | | | | | | | | | | | | | | | | | |
Total | | $ | 46,752 | | $ | 40,855 | | $ | 23,592 | | $ | 166,500 | | $ | 112,227 | | $ | 78,451 |
| | | | | | | | | | | | | | | | | | |
Net Sales by Geography | | | | | | | | | | | | | | | | | | |
United States | | $ | 41,988 | | $ | 37,071 | | $ | 22,678 | | $ | 150,643 | | $ | 105,154 | | $ | 75,160 |
International | | | 4,764 | | | 3,784 | | | 914 | | | 15,857 | | | 7,073 | | | 3,291 |
| | | | | | | | | | | | | | | | | | |
Total | | $ | 46,752 | | $ | 40,855 | | $ | 23,592 | | $ | 166,500 | | $ | 112,227 | | $ | 78,451 |
| | | | | | | | | | | | | | | | | | |
The Company markets its products internationally through a direct sales force and independent distributors. The international distributors may also distribute competitive products under certain circumstances. The international distributors also play an important role in the Company’s clinical testing outside of the United States. The loss of any international distributor would not have a material adverse effect on the Company’s business if a new distributor, sales representative or other suitable sales organization could not be found on a timely basis.
95
AngioDynamics, Inc. and Subsidiaries
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
May 31, 2008 and June 2, 2007
NOTE S—QUARTERLY RESULTS OF OPERATIONS (UNAUDITED)
Quarterly results of operations during 2008 and 2007 were as follows:
| | | | | | | | | | | | | |
| | | 2008 |
| | | First
quarter | | Second
quarter | | Third
quarter | | | Fourth
quarter |
| | | (in thousands, except per share data) |
Net sales | | $ | 37,526 | | $ | 41,497 | | $ | 40,725 | | | $ | 46,752 |
Gross profit | | | 22,501 | | | 25,455 | | | 25,318 | | | | 29,313 |
Net income | | | 2,380 | | | 3,100 | | | 4,890 | | | | 519 |
Earnings per common share | | | | | | | | | | | | | |
Basic | | | 0.10 | | | 0.13 | | | 0.20 | | | | 0.02 |
Diluted | | | 0.10 | | | 0.13 | | | 0.20 | | | | 0.02 |
| |
| | | 2007 |
| | | First
quarter | | Second
quarter | | Third
quarter | | | Fourth
quarter |
| | | (in thousands, except per share data) |
Net sales | | $ | 20,265 | | $ | 24,369 | | $ | 26,738 | | | $ | 40,855 |
Gross profit | | | 11,926 | | | 14,244 | | | 15,949 | | | | 24,048 |
Net income (loss) | | | 1,898 | | | 2,454 | | | (16,405 | ) | | | 2,926 |
Earnings (loss) per common share(1) | | | | | | | | | | | | | |
Basic | | | 0.12 | | | 0.16 | | | (0.88 | ) | | | 0.12 |
Diluted | | | 0.12 | | | 0.15 | | | (0.88 | ) | | | 0.12 |
| (1) | The sum of quarters does not equal the fiscal year due to rounding. |
NOTE T—SUBSEQUENT EVENT
In June 2008, the Company completed the acquisition of certain U.S. and U.K. assets of Diomed, Inc. for $11 million subject to adjustment for changes in working capital to be determined subsequent to the closing date. With this acquisition, the Company substantially strengthened its position in the market for the treatment of varicose veins. The combination of Diomed endovenous laser products with the Company’s existing venous product line provides the Company with a comprehensive venous product offering. As of May 31, 2008 approximately $926,000, including a deposit and direct acquisition costs, have been paid and are included under the balance sheet heading “Other assets”.
96
ANGIODYNAMICS, Inc. and Subsidiaries
| | | | | | | | | | | | | | | | | |
| | | SCHEDULE II - VALUATION AND QUALIFYING ACCOUNTS |
| | | (in thousands) |
Column A | | Column B | | Column C | | | Column D | | | Column E |
| | | | | Additions | | | | | | |
Description | | Balance at
Beginning of
Period | | Charged to
costs and
expenses | | Charged to
Other
Accounts-
describe | | | Deductions-
describe | | | Balance at
End of
Period |
Year Ended June 3, 2006 | | | | | | | | | | | | | | | | | |
Allowance for inventory obsolescence | | $ | 597 | | $ | 426 | | | | | | $ | (183) | (b) | | $ | 840 |
Allowance for deferred tax asset | | | 628 | | | | | | | | | | (526) | (d) | | | 102 |
Allowance for doubtful accounts | | | 203 | | | 270 | | | | | | | (43) | (a) | | | 430 |
| | | | | | | | | | | | | | | | | |
Totals | | $ | 1,428 | | $ | 696 | | $ | — | | | $ | (752) | | | $ | 1,372 |
| | | | | | | | | | | | | | | | | |
Year Ended June 2, 2007 | | | | | | | | | | | | | | | | | |
Allowance for inventory obsolescence | | $ | 840 | | $ | 94 | | $ | 2,464 | (c) | | $ | (638) | (b) | | $ | 2,760 |
Allowance for deferred tax asset | | | 102 | | | | | | 2,115 | (c) | | | | | | | 2,217 |
Allowance for doubtful accounts | | | 430 | | | 326 | | | 498 | (c) | | | (47) | (a) | | | 1,207 |
| | | | | | | | | | | | | | | | | |
Totals | | $ | 1,372 | | $ | 420 | | $ | 5,077 | | | $ | (685) | | | $ | 6,184 |
| | | | | | | | | | | | | | | | | |
Year Ended May 31, 2008 | | | | | | | | | | | | | | | | | |
Allowance for inventory obsolescence | | $ | 2,760 | | $ | 984 | | $ | 131 | (c) | | $ | (181) | (b) | | $ | 3,694 |
Allowance for deferred tax asset | | | 2,217 | | | — | | | — | | | | (1,063) | (e) | | | 1,154 |
Allowance for doubtful accounts | | | 1,207 | | | 229 | | | (61) | (c) | | | (692) | (a) | | | 683 |
| | | | | | | | | | | | | | | | | |
Totals | | $ | 6,184 | | $ | 1,213 | | $ | 70 | | | $ | (1,936) | | | $ | 5,531 |
| | | | | | | | | | | | | | | | | |
| (a) | Previously reserved sales returns and accounts written off as uncollectible. |
| (b) | Writeoffs of obsolete or expired inventory. |
| (c) | Assumed in acquisition. |
| (d) | Expiration of fully-reserved capital loss carryforwards. |
| (e) | Purchase accounting adjustments and use of fully reserved capital loss carryforwards. |
97
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | |
| | | | ANGIODYNAMICS, INC. |
| | | |
Date: | | August 14, 2008 | | By: | | /s/ VINCENT BUCCI |
| | | | | | Vincent Bucci, Chairman of the Board, Director |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | |
Date: | | August 14, 2008 | | /s/ VINCENT BUCCI |
| | | | | Vincent Bucci, |
| | | | | Chairman of the Board, Director |
| | |
Date: | | August 14, 2008 | | /s/ EAMONN P. HOBBS |
| | | | Eamonn P. Hobbs, |
| | | | President, Chief Executive Officer (Principal Executive Officer), Director |
| | |
Date: | | August 14, 2008 | | /s/ D. JOSEPH GERSUK |
| | | | D. Joseph Gersuk, |
| | | | Executive Vice President—Chief Financial Officer, Treasurer (Principal Financial and Chief Accounting Officer) |
| | |
Date: | | August 14, 2008 | | /s/ WESLEY E. JOHNSON, JR. |
| | | | Wesley Johnson, Director |
| | |
Date: | | August 14, 2008 | | /s/ HOWARD W. DONNELLY |
| | | | Howard W. Donnelly, Director |
| | |
Date: | | August 14, 2008 | | /s/ JEFFREY G. GOLD |
| | | | Jeffrey G. Gold, Director |
| | |
Date: | | August 14, 2008 | | /s/ DENNIS S. METENY |
| | | | Dennis S. Meteny, Director |
| | |
Date: | | August 14, 2008 | | /s/ PAUL S. ECHENBERG |
| | | | Paul S. Echenberg, Director |
| | |
Date: | | August 14, 2008 | | /s/ STEVE LAPORTE |
| | | | Steve LaPorte, Director |
| | |
Date: | | August 14, 2008 | | /s/ ROBERT E. FLAHERTY |
| | | | Robert E. Flaherty, Director |
98
