
Novelos Therapeutics, Inc. ( OTCQX : NVLT) Developing Novel Drugs for the Treatment and Diagnosis of Cancer May 23, 2013

FIND . TREAT. FOLLOW .™ 2 DISCLAIMER This slide presentation contains forward - looking statements . Such statements are valid only as of today, and we disclaim any obligation to update this information . These statements are only estimates and predictions and are subject to known and unknown risks and uncertainties that may cause actual future experience and results to differ materially from the statements made . These statements are based on our current beliefs and expectations as to such future outcomes . Drug discovery and development involve a high degree of risk . Factors that might cause such a material difference include, among others, uncertainties related to the ability to raise additional capital required to complete the development programs described herein, the ability to attract and retain partners for our technologies, the identification of lead compounds, the successful preclinical development thereof, the completion of clinical trials, the FDA review process and other government regulation, our pharmaceutical collaborators’ ability to successfully develop and commercialize drug candidates, competition from other pharmaceutical companies, product pricing and third - party reimbursement . A complete description of risks and uncertainties related to our business is contained in our periodic reports filed with the Securities and Exchange Commission including our Form 10 - K for the year ended December 31 , 2012 and in our quarterly reports on Form 10 - Q . These forward looking statements are made only as of the date hereof, and we disclaim any obligation to update any such forward looking statements .
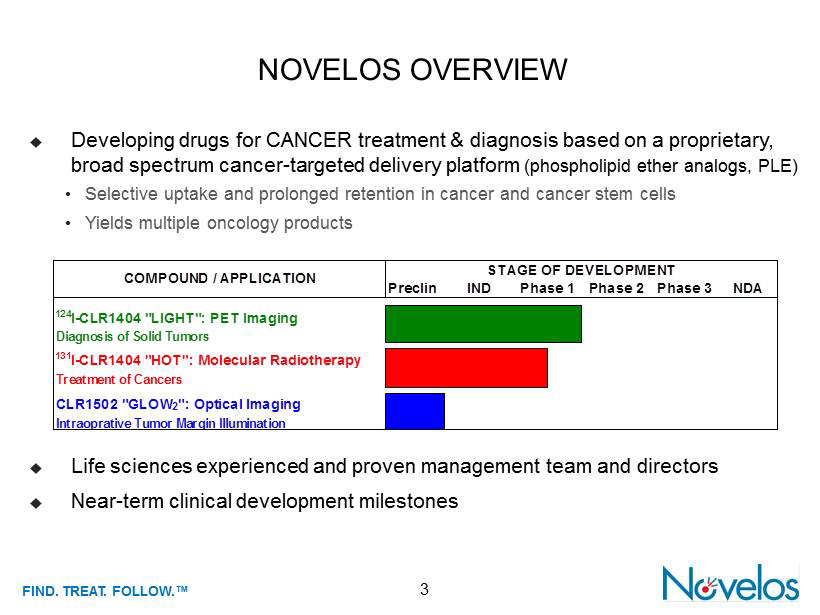
FIND . TREAT. FOLLOW .™ 3 NOVELOS OVERVIEW ; Developing drugs for CANCER treatment & diagnosis based on a proprietary, broad spectrum cancer - targeted delivery platform (phospholipid ether analogs, PLE) • Selective uptake and prolonged retention in cancer and cancer stem cells • Yields multiple oncology products ; Life sciences experienced and proven management team and directors ; Near - term clinical development milestones Preclin NDA 124 I-CLR1404 "LIGHT": PET Imaging Diagnosis of Solid Tumors 131 I-CLR1404 "HOT": Molecular Radiotherapy Treatment of Cancers CLR1502 "GLOW 2 ": Optical Imaging Intraoprative Tumor Margin Illumination COMPOUND / APPLICATION STAGE OF DEVELOPMENT Phase 1 Phase 2 Phase 3IND
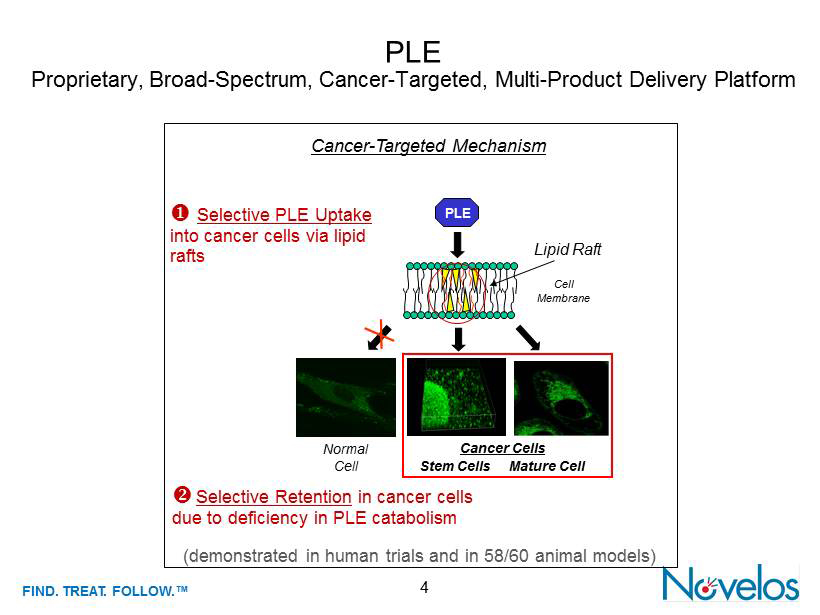
FIND . TREAT. FOLLOW .™ PLE Proprietary , Broad - Spectrum, Cancer - Targeted , Multi - Product Delivery Platform 4 (demonstrated in human trials and in 58/60 animal models) Cancer - Targeted Mechanism ; Selective PLE Uptake into c ancer c ells v ia l ipid rafts □ Selective Retention in cancer cells due to d eficiency in PLE c atabolism Normal Cell Lipid Raft Cell Membrane Cancer Cells Stem Cells Mature Cell PLE
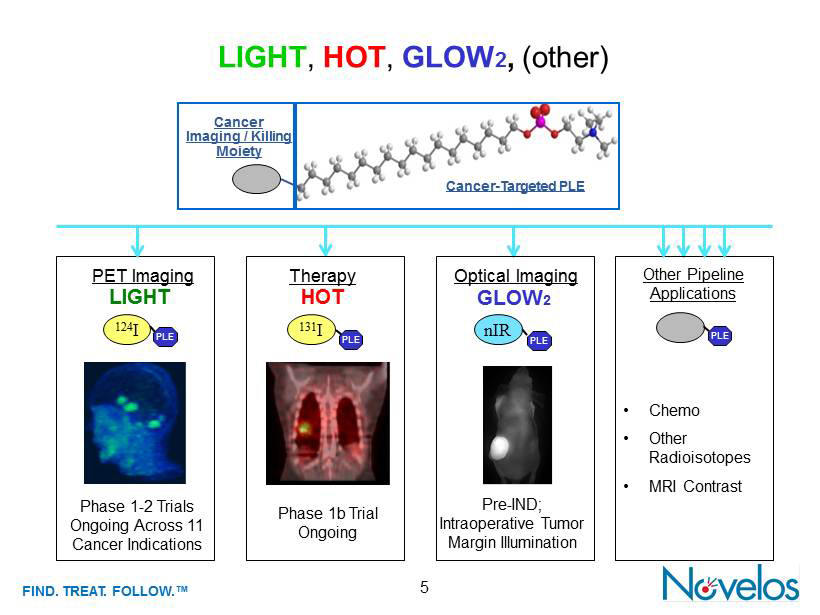
FIND . TREAT. FOLLOW .™ LIGHT , HOT , GLOW 2 , (other) 5 LIGHT Phase 1 - 2 Trials Ongoing Across 11 Cancer Indications PET Imaging PLE Other Pipeline Applications PLE • Chemo • Other Radioisotopes • MRI Contrast PLE Targeting Cancer - Targeted PLE Cancer Imaging / Killing Moiety HOT PLE Phase 1b Trial Ongoing Pre - IND ; Intraoperative Tumor Margin Illumination GLOW 2 Optical Imaging 124 I 131 I Therapy PLE nIR
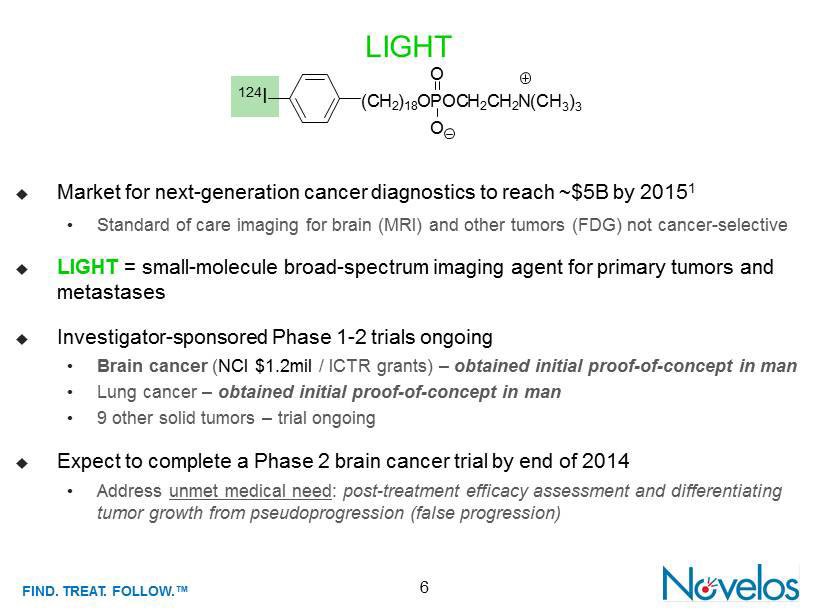
FIND . TREAT. FOLLOW .™ LIGHT 6 ; Market for next - generation cancer diagnostics to reach ~$5B by 2015 1 • Standard of care imaging for brain (MRI) and other tumors (FDG) not cancer - selective ; LIGHT = small - molecule broad - spectrum imaging agent for primary tumors and metastases ; Investigator - sponsored Phase 1 - 2 trials ongoing • Brain cancer ( NCI $1.2mil / ICTR grants) – obtained initial proof - of - concept in man • Lung cancer – obtained initial proof - of - concept in man • 9 other solid tumors – trial ongoing ; Expect to complete a Phase 2 brain cancer trial by end of 2014 • Address unmet medical need : p ost - treatment efficacy assessment and differentiating tumor growth from pseudoprogression (false progression) O (C H 2 ) 18 O P OC H 2 C H 2 N O (CH 3 ) 3 124 I
FIND . TREAT. FOLLOW .™ 7 LIGHT Phase 1 - 2 Brain Cancer Trials at UWCCC ; Brain Cancer : Investigator - sponsored at University of Wisconsin Carbone Cancer Center • Lance Hall, M.D., is principal investigator for clinical trial • Jamey Weichert, Ph.D., is primary principal investigator for $1.2M NCI grant • PET images at: 6hr, 1 and 2 day (tumor uptake, dosimetry ); optional 3 - 10 day ; D emonstrated positive initial imaging results in brain cancers • 15 patients to date • High tumor - to - background uptake in glioma • May differentiate pseudoprogression from recurrent disease • Potentially more accurate than MRI • High tumor - to - background uptake in brain metastases (NSCLC)
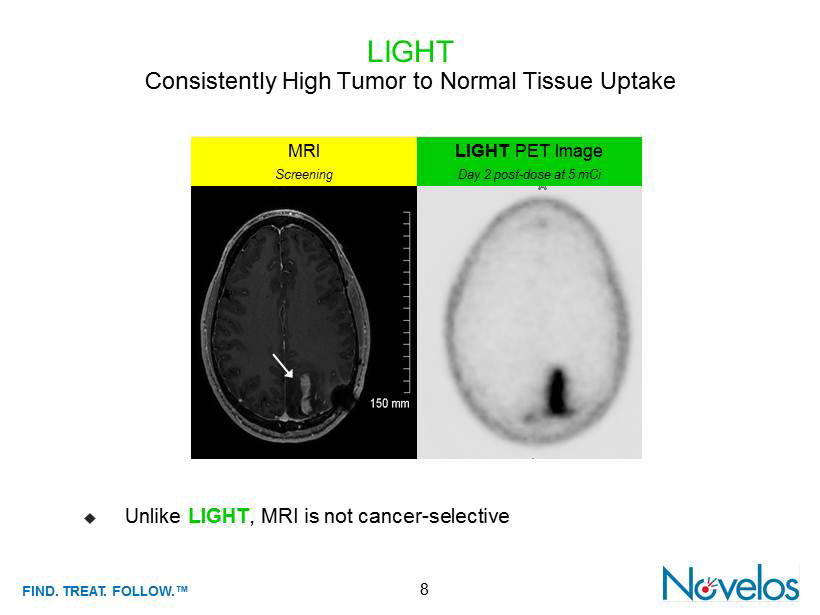
FIND . TREAT. FOLLOW .™ 8 LIGHT Consistently High Tumor to Normal Tissue Uptake ; Unlike LIGHT , MRI is not cancer - selective LIGHT PET Image Day 2 post - dose at 5 mCi MRI Screening
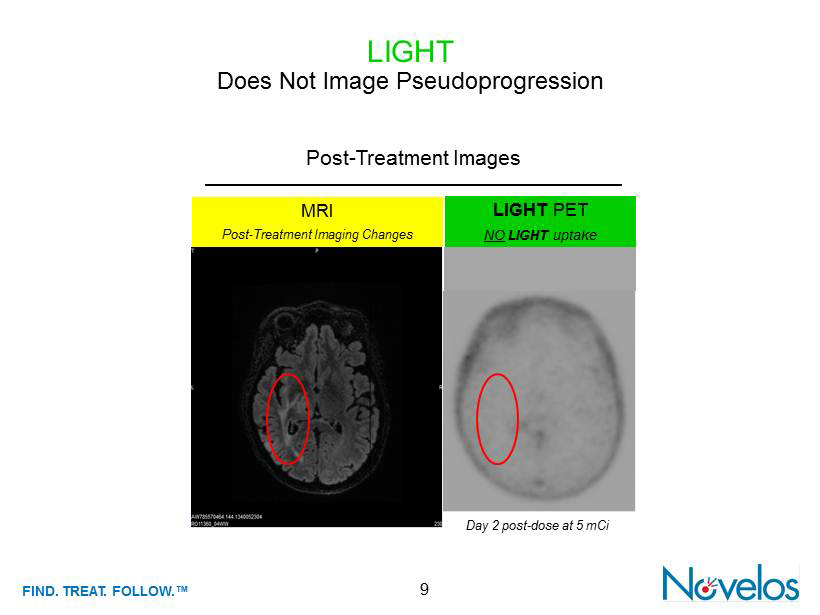
FIND . TREAT. FOLLOW .™ 9 LIGHT Does Not Image Pseudoprogression Day 2 post - dose at 5 mCi LIGHT PET NO LIGHT uptake MRI Post - Treatment Imaging Changes Post - Treatment Images
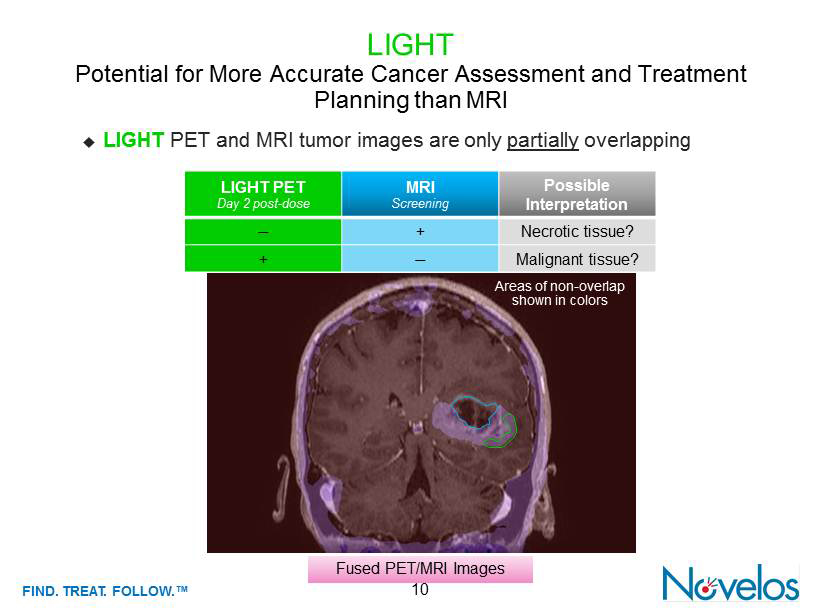
FIND . TREAT. FOLLOW .™ 10 ; LIGHT PET and MRI tumor images are only partially overlapping LIGHT Potential for More Accurate Cancer Assessment and Treatment Planning than MRI LIGHT PET Day 2 post - dose MRI Screening Possible Interpretation ; + Necrotic tissue? + ; Malignant tissue? Fused PET/MRI Images Areas of non - overlap shown in colors
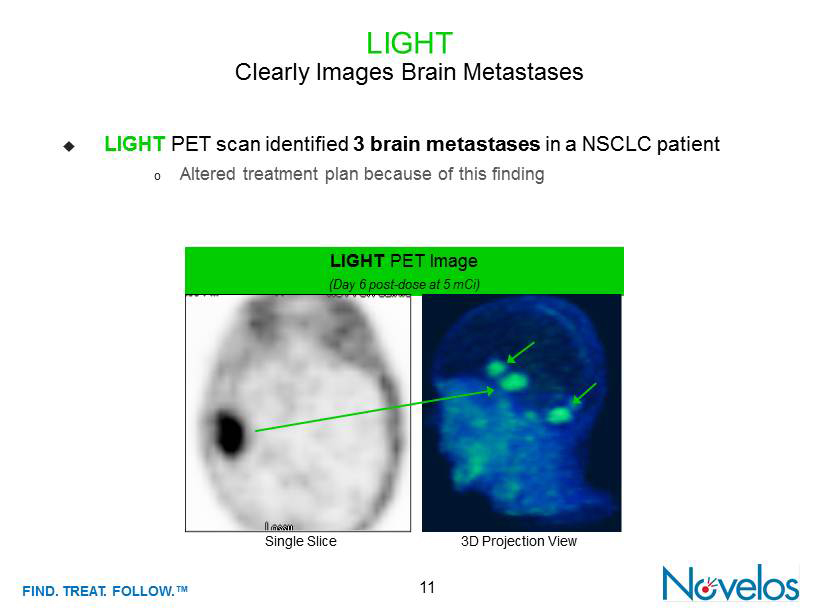
FIND . TREAT. FOLLOW .™ LIGHT Clearly Images Brain Metastases ; LIGHT PET scan identified 3 brain metastases in a NSCLC patient o Altered treatment plan because of this finding LIGHT PET Image (Day 6 post - dose at 5 mCi ) 3D Projection View Single Slice 11
FIND . TREAT. FOLLOW .™ 12 LIGHT Phase 2 Brain Cancer Imaging Trial Design ; Glioma Phase 2 multi - site trial in ~30 glioma patients • Overall goal: determine the optimal dose and imaging time points for a pivotal trial • Newly diagnosed and recurrent glioma , where SOC includes resection and/or biopsy • Comparison to MRI based on pathology confirmation as “gold standard” • Approval based on more accurate determination of malignant vs. non - malignant tissue • Accuracy determined by Sensitivity (=true positive rate) and Specificity (= true negative rate) • Aim : Demonstrate superiority over current SOC for accurately identifying malignant vs. non - malignant tissue ; Start Q1 2014 ; Complete Q4 2014
FIND . TREAT. FOLLOW .™ 13 ♦ MRI is the SOC for imaging brain tumors • Used pre and post surgery for treatment planning and assessment ♦ MRI is unable to distinguish recurrent disease from pseudoprogression • MRI detects post - treatment changes in ~50% of patients 2 • Pseudoprogression is ~50% of those post - treatment changes 2 • Compromises patient management • Causes a diagnostic dilemma: treat or wait (monitor with MRI) ♦ More accurate imaging is needed to make a definitive assessment and optimize patient management • Apply treatment when necessary / withhold treatment when not necessary • Detect regrowth earlier, minimizing tumor spread LIGHT Unmet Medical Need - Brain Cancer (Glioma)

FIND . TREAT. FOLLOW .™ 14 ♦ ~40,000 eligible p atients annually ( glioma – U.S. only) 3 ♦ Significant first market opportunity • Multiple doses per patient • H ealthcare savings over current SOC – robust pricing • P otential for high market penetration as SOC ♦ Pharmacoeconomics – more accurate post - treatment assessment and differentiating tumor regrowth from pseudoprogression • Better patient management: avoid cost/impact of unnecessary surgeries, catch small tumors earlier • NCCN guidelines: up to 18 MRI exams (over 3 - years) per patient • ~$2,500 - $5,000 per MRI 4 • Cost of PET scan is similar 4 * Brain metastases (~135,000 patients / year in U.S. 5 ) may represent a meaningful market after Glioma - same unmet medical needs exist – differentiating pseudoprogression and post - treatment assessment. LIGHT Commercial Market Opportunity - Brain Cancer*

FIND . TREAT. FOLLOW .™ 15 LIGHT Phase 1 - 2 Non - Brain Cancer Imaging Trials at UWCCC ; NSCLC : Investigator - sponsored trial at University of Wisconsin Carbone Cancer Center • Anne M. Traynor, M.D., is principal investigator • PET images at 2hr, 6hr, 1day, 2day and 5 - 7day (tumor uptake, dosimetry ) • Demonstrated positive initial imaging results in lung cancer o Uptake in tumors o Potentially more accurate than FDG o Detected previously unknown brain metastases ; 9 Other Solid Tumor Types : Investigator - sponsored trial at UWCCC • Glen Liu, M.D., is principal investigator • Initial positive images in triple - negative breast, head & neck, colorectal, prostate, pancreatic • Recruiting ovarian , esophageal, gastric, soft tissue sarcoma
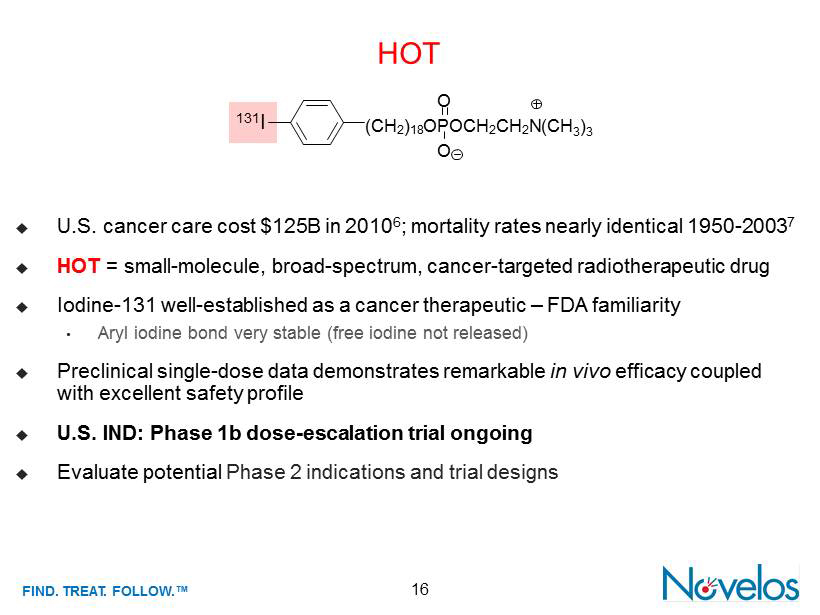
FIND . TREAT. FOLLOW .™ 16 HOT ; U.S. cancer care cost $125B in 2010 6 ; mortality rates nearly identical 1950 - 2003 7 ; HOT = small - molecule , broad - spectrum, cancer - targeted radiotherapeutic drug ; Iodine - 131 well - established as a cancer therapeutic – FDA familiarity • Aryl iodine bond very stable (free iodine not released) ; Preclinical single - dose data demonstrates remarkable in vivo efficacy coupled with excellent safety profile ; U.S. IND : Phase 1b dose - escalation trial ongoing ; Evaluate potential Phase 2 indications and trial designs O (C H 2 ) 18 O P OC H 2 C H 2 N O (CH 3 ) 3 131 I
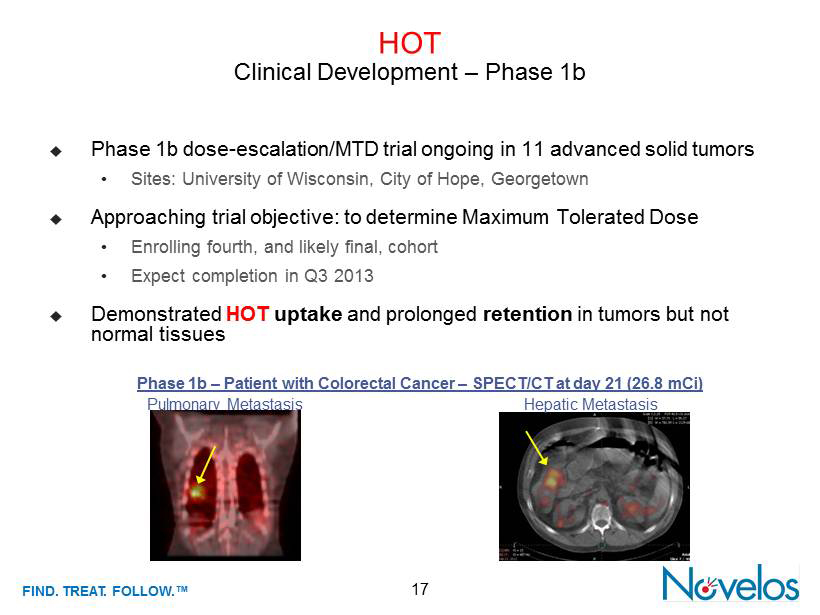
FIND . TREAT. FOLLOW .™ 17 HOT Clinical Development – Phase 1b ; Phase 1b dose - escalation/ MTD trial ongoing in 11 advanced solid tumors • Sites : University of Wisconsin, City of Hope, Georgetown ; Approaching trial objective: to determine Maximum Tolerated Dose • Enrolling fourth, and likely final, cohort • Expect completion in Q3 2013 ; Demonstrated HOT uptake and prolonged retention in tumors but not normal tissues Hepatic Metastasis Pulmonary Metastasis Phase 1b – Patient with Colorectal Cancer – SPECT /CT at day 21 (26.8 mCi )
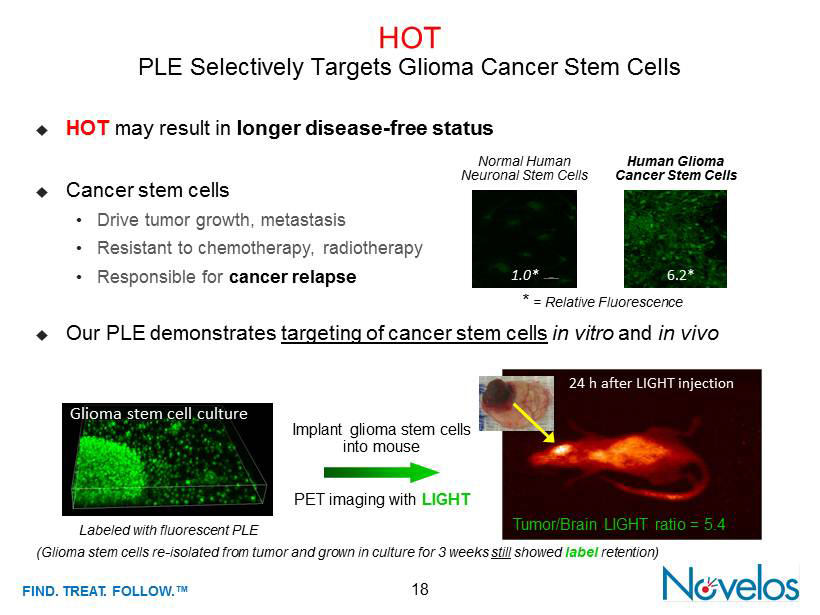
FIND . TREAT. FOLLOW .™ 18 HOT PLE Selectively Targets Glioma Cancer Stem Cells ; Our PLE demonstrates targeting of cancer stem cells in vitro and in vivo 24 h after LIGHT injection Tumor/Brain LIGHT ratio = 5.4 Glioma stem cell culture Labeled with fluorescent PLE Implant glioma stem cells into mouse PET imaging with LIGHT (Glioma stem cells re - isolated from tumor and grown in culture for 3 weeks still showed label retention) ; Cancer stem cells • Drive tumor growth, metastasis • Resistant to chemotherapy, radiotherapy • Responsible for cancer relapse ; HOT may result in longer disease - free status Normal Human Neuronal Stem Cells Human Glioma Cancer Stem Cells 1.0* 6.2* * = Relative Fluorescence

FIND . TREAT. FOLLOW .™ HOT Future Development – Efficacy POC Strategy ; Guiding Criteria • Target unmet medical needs • Acceptable probability of demonstrating POC in Phase 2 trial • Achievable with least amount of time/capital: size, patient recruitment, design • Clear path to FDA approval • Meaningful commercial market opportunity ; Evaluate potential target indications and trial designs • Requirement for tumor uptake/retention based on imaging data • Single dose in radiosensitive tumor types; assessment of multiple dosing in others • Indications where there are accepted efficacy surrogate biomarkers that could be exploited for early proof - of - concept • Combinations with external beam radiation, or radiosensitizers, to increase efficacy (addition) or to improve toleration (partial replacement) 19
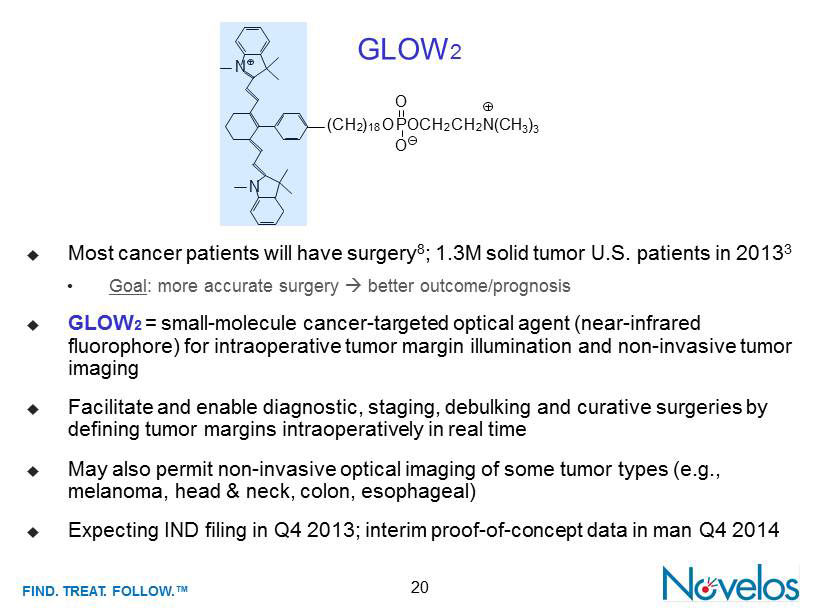
FIND . TREAT. FOLLOW .™ 20 ; Most cancer patients will have surgery 8 ; 1.3M solid tumor U.S. patients in 2013 3 • Goal : more accurate surgery ; better outcome/prognosis ; GLOW 2 = small - molecule cancer - targeted optical agent (near - infrared fluorophore ) for intraoperative tumor margin illumination and non - invasive tumor imaging ; Facilitate and enable diagnostic, staging, debulking and curative surgeries by defining tumor margins intraoperatively in real time ; May also permit non - invasive optical imaging of some tumor types (e.g., melanoma, head & neck, colon, esophageal) ; Expecting IND filing in Q4 2013; interim proof - of - concept data in man Q4 2014 GLOW 2 N N O (C H 2 ) 18 O P OC H 2 C H 2 N (CH 3 ) 3 O
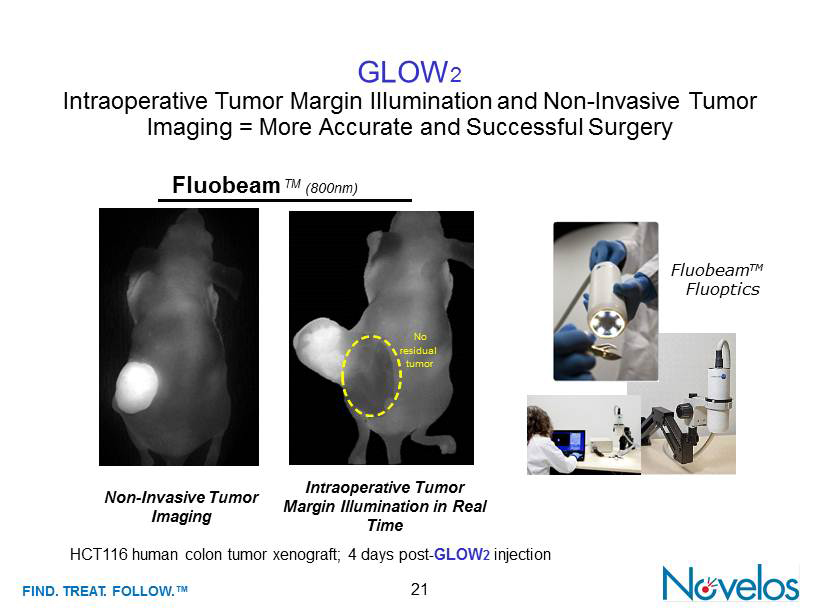
FIND . TREAT. FOLLOW .™ HCT116 human colon tumor xenograft; 4 days post - GLOW 2 injection 21 GLOW 2 Intraoperative Tumor Margin Illumination and Non - Invasive Tumor Imaging = More Accurate and Successful Surgery Fluobeam TM (800nm) Non - Invasive Tumor Imaging Intraoperative Tumor Margin Illumination in Real Time No residual tumor Fluobeam TM ) Fluoptics
FIND . TREAT. FOLLOW .™ GLOW 2 Initial Human Trial Design 22 ♦ Phase 1 - 2 dose - ranging multi - site trial in ~30 breast cancer patients ♦ Start mid - 2014 ♦ Compatible with standard of care ( SOC ) in clinical trial setting → facilitates clinical support and fast enrollment • Patients undergoing lumpectomy • The only additions to SOC will be GLOW 2 administration and non - invasive fluorescent imaging 1. Primary tumor and sentinel node resected according to SOC (including use of dye to identify nodes) 2. Then optical device used to assess GLOW 2 ’s identification of primary tumor margin and complete nodal involvement • AIM : Demonstrate superiority versus SOC to identify and differentiate malignant and non - malignant tissue based on pathology readout ♦ Interim results Q4 2014
FIND . TREAT. FOLLOW .™ 23 ♦ Lumpectomy procedure targets complete removal of tumor and malignant lymph node involvement while sparing functional tissue • Challenging for surgeon to identify tumor margins and exact nodal involvement • If pathology indicates malignant tissue may remain, surgery performed again ♦ 25% lumpectomies are repeated 9 • Patient impact: risk, discomfort, inconvenience ♦ More accurate visualization of tumor margins and complete nodal involvement during surgery may result in: • More complete and selective removal of malignant tissue • Fewer repeat - resections • Significantly improve patient outcomes ( QOL : reduce unnecessary removal of functional tissue; potentially increase survival) GLOW 2 Unmet Medical Need - Breast Cancer Surgery

FIND . TREAT. FOLLOW .™ 24 ♦ ~100,000 eligible p atients annually (lumpectomy - U.S. only) 3 ♦ Substantial first market o pportunity • Single dose per surgery • P otential for high market penetration as SOC ♦ Pharmacoeconomics – more accurate and complete surgery • Potential healthcare cost savings • Reduce repeat resections (currently: 1 in 4 lumpectomies) • Lumpectomy cost up to $20,000 10 • Significantly improve patient outcomes *Ductal carcinoma in situ (~60,000 patients / year in U.S.) and mastectomy (~60,000 patients / year in U.S.) may represent significant markets within breast cancer surgery after lumpectomy 3 . GLOW 2 Commercial Market Opportunity - Breast Cancer Surgery*

FIND . TREAT. FOLLOW .™ 25 POTENTIAL MILESTONES Novel Cancer-Targeted Technology 2Q13 3Q13 4Q13 1Q14 2Q14 3Q14 4Q14 LIGHT: PET Imaging Phase 1-2s in Brain Cancer - Ongoing POC2 2012 Initiate Phase 2 Trial in Brain Cancer - Q1 2014 Phase 2 POC3 Phase 1-2 in Lung Cancer - Ongoing POC1 2012 Phase 1-2 in 9 Other Solid Tumors - Ongoing HOT: Molecular Radiotherapy Phase 1b - Ongoing - Cohort 4 Data Q3 2013 Cohort 4 Phase 2 Evaluation Phase 1-2 (pending evaluation; not in budget) - Q2 2014 Phase 1-2 GLOW 2 : Optical Imaging for Intraoperative Tumor Margin Illumination Preclinical - File IND Q4 2013 IND Initiate Phase 1-2 Trial in Cancer Surgery - Q3 2014 Phase 1-2 POC4 POC1 = Interim Proof-of-Concept Data in Human Trials - Uptake in Cancerous Tumors POC2 = Interim Proof-of-Concept Data in Human Trials - Potential Superiority versus Standard of Care POC3 = Proof-of-Concept Data in Human Trials - Superiority versus Standard of Care based on Pathology Confirmation POC4 = Interim Proof-of-Concept Data in Human Trials - Superiority versus Standard of Care based on Pathology Confirmation IND = Investigational New Drug Application to FDA to Start Clinical Trials POC1 or 2 Clinical Start-up Clinical Start-up Phase 2 Evaluation

FIND . TREAT. FOLLOW .™ 26 ; Funding into year - end 2013 o $7.9M cash at March 31, 2013 Excluding $1.9M restricted cash ; Capitalization o 57M shares of common stock o 101M shares fully diluted ; $52M invested to - date in development of our PLE delivery platform o Investors include: — F undamental life sciences institutional investors — R adiologists and oncologists SUMMARY FINANCIAL OUTLOOK

FIND . TREAT. FOLLOW .™ 27 SENIOR MANAGEMENT ; Harry Palmin, President and CEO, Director • Head of Novelos for 15 years; previously at Lehman Brothers and Morgan Stanley… ; Jamey Weichert, Ph.D., Chief Scientific Officer, Technology Founder, Director • 30 years of targeted imaging & radiotherapy design, inventor of Novelos’ cancer - targeted tech, Associate Professor Dept Radiology and Medical Physics at U Wisconsin, Madison… ; Chris Pazoles, Ph.D., SVP of Research & Development • 30+ years of biopharmaceutical R&D and senior management experience, including Pfizer and Abbott… ; Kim Hawkins, VP of Clinical Development • 19 years of clinical operations and senior management experience, including Boston Medical, Genzyme, Antigenics … ; Joanne Protano, VP and Chief Financial Officer • 20+ years of finance and senior management experience, including public companies and Deloitte & Touche … ; J. Patrick Genn, VP of Investor Relations • 30 years banking, investment and senior management, including Wells Fargo…

FIND . TREAT. FOLLOW .™ 28 INDEPENDENT DIRECTORS ; Stephen Hill, B . M . B . Ch . , M . A . , F . R . C . S . , Chairman • CEO of Targacept (NASDAQ : TRGT ) ; formerly CEO of Solvay Pharmaceuticals USA, ArQule and Head of Global Drug Development at Roche ; 25 + years of expertise in biopharmaceutical senior management, product development, commercialization and partnering … ; Thomas Rockwell Mackie, Ph . D . , Director • Co - founder, Chairman and Director of Research of TomoTherapy (NASDAQ : TOMO ) ; leading figure in the field of radiation therapy ; professor Dept of Medical Physics and Human Oncology at the University of Wisconsin - Madison … ; James Manuso, Ph . D . , Director • CEO of Astex (NASDAQ : ASTX ) ; 30 + years of expertise in life sciences senior management, product commercialization, partnering, financing, venture and consulting … ; John Neis, CFA, Director • Managing Director of Venture Investors LLC, heads Healthcare practice ; 23 years in venture capital, serving on boards of life sciences companies from formation through IPO or sale . … ; John Niederhuber, M . D . , Director • Director of National Cancer Institute ( 2005 - 2010 ) ; nationally renowned surgeon and researcher who has dedicated his four - decade career to the treatment and study of cancer - as a professor, National Cancer Advisory Board chair, grant reviewer, and investigator … ; Howard Schneider, Director • 35 years experience as senior financial industry executive … ; Michael Tweedle, Ph . D . , Director • Professor Cancer Imaging in Radiology at Ohio State ; 30 + years expertise in imaging and diagnostics, senior research and management ; former President of Bracco Research USA, head of diagnostics at BMS …

FIND . TREAT. FOLLOW .™ 29 KEY CONSULTANTS ; Kevin Kozak , M . D . , Ph . D . , Radiation Oncology Consultant • Radiation oncologist ; biochemist ; co - founder of Co - D Therapeutics ; ~ 100 peer - reviewed publications / abstracts / patents / book chapters … ; Michael Kurman , M . D . , Medical Oncology Consultant • Medical oncologist ; 30 years expertise in oncology clinical development ; successfully developed / launched 4 products … ; Minesh Mehta, M . D . , FASTRO , Radiation Oncology Consultant • Professor of radiation oncology at Northwestern Univ .; preeminent radiation oncologist with 25 + years expertise ; 100 + cancer clinical trials and ~ 500 publications / abstracts … ; George Mills , M . D . , Regulatory Consultant • Vice President, PAREXEL Consulting ; former Division Director of Medical Imaging and Hematology Products in the Office of Oncology Drug Products, FDA, CDER … ; Joanne Mortimer, M . D . , FACP . , Medical Oncology Consultant • Admin . Director of Phase 1 Programs, and Vice - Chair and Professor, Medical Oncology and Therapeutics Research, City of Hope Comprehensive Cancer Center ; served on FDA Oncology Drug Advisory Committee … ; George Sgouros , Ph . D . , Radiation Oncology Consultant • Professor of Radiology and Radiological Science and Director of Radiopharmaceutical Dosimetry, Johns Hopkins Univ ; 20 + years experience in patient - specific dosimetry of internally administered radionuclides ; 100 + peer - reviewed articles … ; Richard Wahl, M . D . , Radiation Oncology Consultant • Professor and Director Nuclear Medicine / PET Center and Vice - Chair for Technology and Business Development at Johns Hopkins University ; instrumental in development and commercialization of Bexxar …

FIND . TREAT. FOLLOW .™ 30 SUMMARY ; Global cancer market $225B by 2017 11 ; U.S. cancer care cost $158B by 2020 6 ; Developing drugs for CANCER treatment & diagnosis based on a proprietary, broad spectrum cancer - targeted delivery platform • LIGHT : potentially new “gold standard” for PET imaging o First, seek to address unmet need in glioma : post - treatment efficacy assessment and differentiating tumor growth from pseudoprogression o Then, address similar unmet need in brain metastasis o Then, address unmet imaging needs in other solid tumors • HOT : radiotherapeutic – targeted delivery to cancer cells and cancer stem cells • GLOW 2 : optical imaging agent for intraoperative tumor margin illumination in real time and non - invasive tumor imaging o A ddress unmet need for better definition of tumor margin and nodal involvement ; First two commercial opportunities: Phase 2 trials – results by end of 2014 ; Life sciences experienced and proven management team and directors ; Broad IP portfolio

FIND . TREAT. FOLLOW .™ Appendix

FIND . TREAT. FOLLOW .™ 32 INTELLECTUAL PROPERTY ; I - 124 - CLR1404 ( LIGHT ) • Composition of matter, U.S. (expiry 2016+) • Methods of use, EU granted; U.S., Japan pending (expiry 2025 +) • Methods of manufacture, U.S. (expiry 2028+) • V irtual colonoscopy, U.S. (expiry 2025+) ; I - 131 - CLR1404 ( HOT ) • Composition of matter, U.S. (expiry 2016 +) • Methods of use, EU granted; U.S., Japan pending (expiry 2025+) • Methods of manufacture, U.S. ( expiry 2028 +) ; CLR1502 ( GLOW2 ) • Composition of matter, U.S., EU, Japan pending (expiry 2029+) • Methods of use, U.S., EU, Japan pending (expiry 2029 +) • Methods of manufacture, U.S., EU, Japan pending (expiry 2029+)
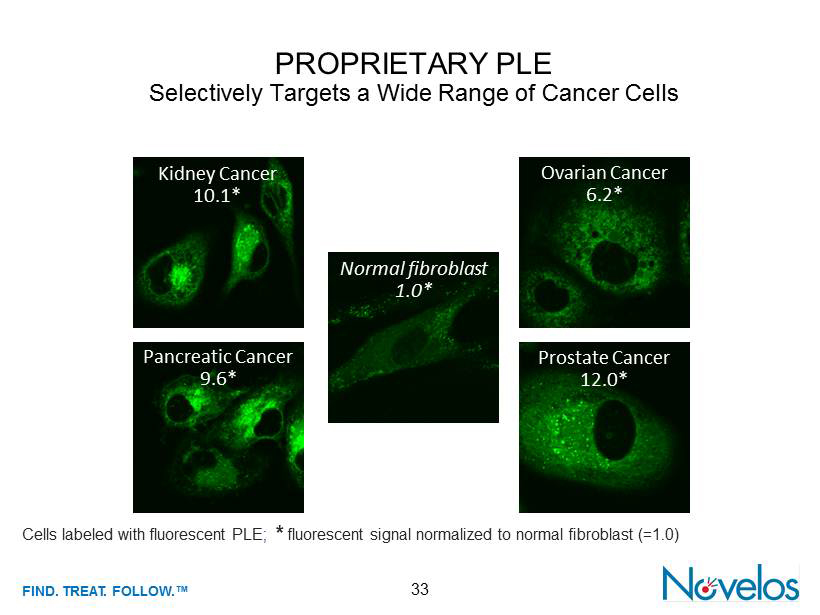
FIND . TREAT. FOLLOW .™ 33 PROPRIETARY PLE Selectively Targets a Wide Range of Cancer Cells Cells labeled with fluorescent PLE ; * fluorescent signal normalized to normal fibroblast (=1.0) Kidney Cancer 10.1* Normal fibroblast 1.0* Ovarian Cancer 6.2* Pancreatic Cancer 9.6* Prostate Cancer 12.0*
FIND . TREAT. FOLLOW .™ 34 ; Diapeutic TM = Diagnosis ; Therapy ; LIGHT is chemically identical to HOT (“ideal biomarker”) • Identical biomarker, not a surrogate ; PET/CT tumor imaging with LIGHT • Predicts tumor - targeting by HOT DIAPEUTIC TM APPROACH LIGHT I s Ideal Biomarker for HOT

FIND . TREAT. FOLLOW .™ 35 SOURCES 1 BCC Research, April 2011 2 Wen et al., N Engl J Med 2008 3 American Cancer Society, Cancer Facts and Figures, 2013 4 New Choice Health, UW Carbone Cancer Center 5 NCI : www.cancer.gov/cancertopics/pdq/treatment/adultbrain/HealthProfessional/page1 6 U.S . National Cancer Institute, January 12, 2011 7 1950 Mortality Data - CDC/ NCHS , NVSS , Mortality Revised; 2003 Mortality Data: U.S. Mortality Public Use Data Tape, 2003, NCHS , Centers for Disease Control and Prevention, 2006. Age - adjusted to 2000 US standard population . 8 American Cancer Society, 2011 9 Factors Associated with repeat Surgery After Initial Breast Conservation ( Wilke L et al Yao K 2012) 10 HCUP : www.hcup - us.ahrq.gov/reports/statbriefs/sb86.jsp) 11 Global Industry Analysts, November 2011
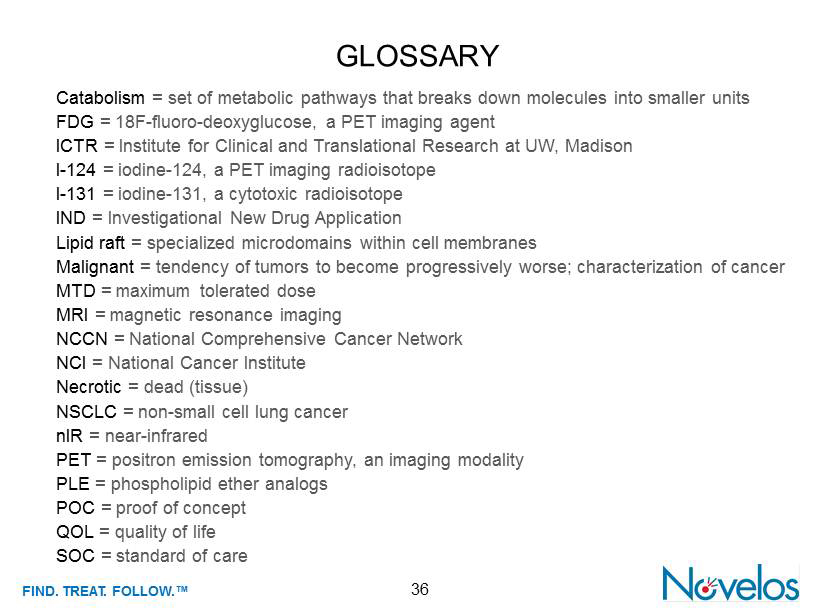
FIND . TREAT. FOLLOW .™ 36 GLOSSARY Catabolism = set of metabolic pathways that breaks down molecules into smaller units FDG = 18F - fluoro - deoxyglucose, a PET imaging agent ICTR = Institute for Clinical and Translational Research at UW, Madison I - 124 = iodine - 124, a PET imaging radioisotope I - 131 = iodine - 131, a cytotoxic radioisotope IND = Investigational New Drug Application Lipid raft = specialized microdomains within cell membranes Malignant = tendency of tumors to become progressively worse; characterization of cancer MTD = maximum tolerated dose MRI = magnetic resonance imaging NCCN = National Comprehensive Cancer Network NCI = National Cancer Institute Necrotic = dead (tissue) NSCLC = non - small cell lung cancer nIR = near - infrared PET = positron emission tomography, an imaging modality PLE = phospholipid ether analogs POC = proof of concept QOL = quality of life SOC = standard of care
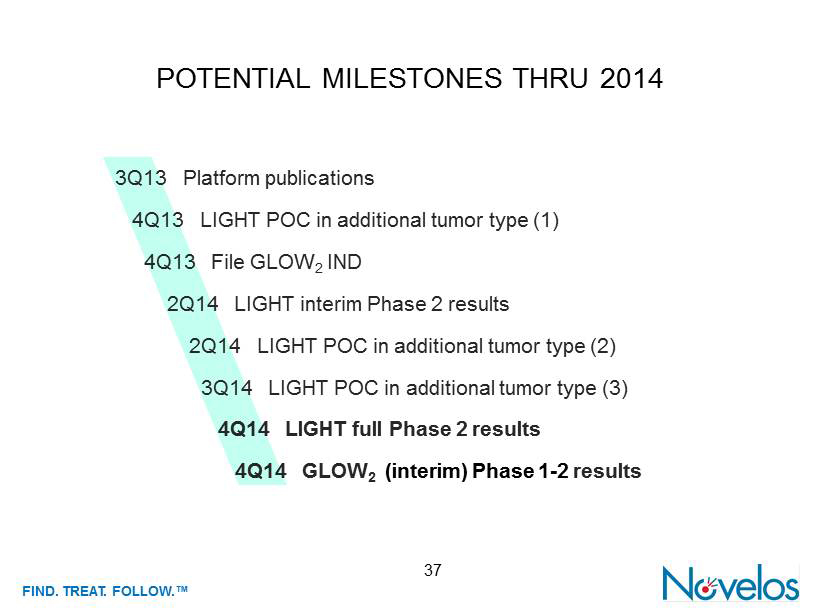
FIND . TREAT. FOLLOW .™ POTENTIAL MILESTONES THRU 2014 37 3Q13 Platform publications 4Q13 LIGHT POC in additional tumor type (1) 4Q13 File GLOW 2 IND 2Q14 LIGHT interim Phase 2 results 2Q14 LIGHT POC in additional tumor type (2) 3Q14 LIGHT POC in additional tumor type (3) 4Q14 LIGHT full Phase 2 results 4Q14 GLOW 2 (interim) Phase 1 - 2 results




































