
Developing Novel Drugs for the Diagnosis and Treatment of Cancer Novelos Therapeutics, Inc. (OTCQX: NVLT), November 2013

Safe Harbor Statement FIND . TREAT . FOLLOW . ™ 2 This slide presentation contains forward - looking statements . Such statements are valid only as of today, and we disclaim any obligation to update this information . These statements are only estimates and predictions and are subject to known and unknown risks and uncertainties that may cause actual future experience and results to differ materially from the statements made . These statements are based on our current beliefs and expectations as to such future outcomes . Drug discovery and development involve a high degree of risk . Factors that might cause such a material difference include, among others, uncertainties related to the ability to raise additional capital required to complete the development programs described herein, the ability to attract and retain partners for our technologies, the identification of lead compounds, the successful preclinical development thereof, the completion of clinical trials, the FDA review process and other government regulation, our pharmaceutical collaborators’ ability to successfully develop and commercialize drug candidates, competition from other pharmaceutical companies, product pricing and third - party reimbursement . A complete description of risks and uncertainties related to our business is contained in our periodic reports filed with the Securities and Exchange Commission including our Form 10 - K for the year ended December 31 , 2012 and in our quarterly reports on Form 10 - Q . These forward looking statements are made only as of the date hereof, and we disclaim any obligation to update any such forward looking statements .
Novelos at a Glance FIND . TREAT . FOLLOW . ™ 3 • Broad - spectrum delivery platform with potential to image and treat a wide range of solid tumors • Tumor Imaging - More accurate for better patient management • Therapy - Better targeted delivery to cancer cells and cancer stem cells • Surgery - Intraoperative optical imaging for better outcomes • Data from Phase I/II and Phase II trials expected in next 12 - 18 months: • LIGHT: Phase II imaging trial in brain cancer • HOT: Phase Ib dose - escalation safety data • GLOW2: Phase I surgical imaging during lumpectomy • Targeting indications with fastest path to commercial markets • S ubstantial scale - up potential across many products / indications

Near Universal Cancer - Targeting Platform FIND . TREAT . FOLLOW . ™ 4 • Phospholipid Ether Analog (PLE ) : Highly - Selective, Broad - Spectrum, Cancer - Targeting Delivery Platform with Multi - Product Diagnostic and Therapeutic Applications • Targets lipid rafts , a structure overexpressed by malignant cells and malignant stem cells • Five Unique Attributes: x Selectively taken up by cancer cells AND cancer stem cells x Prolonged retention by cancer cells AND cancer stem cells x Broad spectrum of cancers - selectively retained by all but 3 of over 60 xenograft, orthotopic, and transgenic solid cancer and cancer stem cell derived models examined to date x Imaging and therapeutic agents can be attached x Diapeutic : Imaging diagnostic is predictive of therapeutic deliver Near - Universal targeting of both primary and metastatic cancers regardless of anatomic location
Near Universal Cancer - Targeting Platform FIND . TREAT . FOLLOW . ™ 5 • Taken up and selectively retained by cancer cells • A ll but 3 of over 60 tumor models examined to date • Tumor uptake independent of anatomic location with little or no tumor clearance over time • Not taken up in inflammatory lesions or benign neoplasms (cancer - specific) • Major advantage over current FDG PET imaging • High safety index • Even at >800x anticipated human mass doses • Phase 1a dosimetry trial in humans (with I - 131)
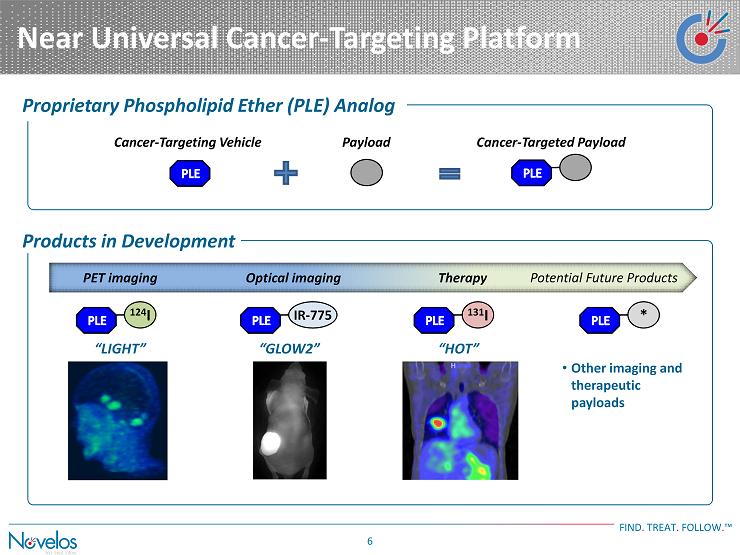
Near Universal Cancer - Targeting Platform FIND . TREAT . FOLLOW . ™ 6 “LIGHT” Potential Future Products • Other imaging and therapeutic payloads “ GLOW2” “HOT” Therapy Cancer - Targeting Vehicle Payload Cancer - Targeted Payload 124 I Proprietary Phospholipid Ether (PLE) Analog Products in Development IR - 775 131 I * Optical imaging PET imaging
LIGHT For Tumor Imaging FIND . TREAT . FOLLOW . ™ 7 PLE + PET Imaging Isotope = LIGHT ( 124 I - CLR1404 ) More Accurate Tumor Imaging for Better Patient Management
LIGHT ( 124 I - CLR1404 ): PLE + PET Imaging Isotope FIND . TREAT . FOLLOW . ™ 8 • Small - molecule , broad - spectrum imaging agent for primary tumors and metastases • Proprietary PLE attached to I - 124 PET imaging isotope • Phase I/II trials underway at UW Carbone Cancer Center (investigator sponsored) • Lung Cancer (Traynor/Perlman) (Image and dose optimization) • Glioma / Brain tumors or Mets (Hall) (Image and dose optimization) • Multiple Tumor Protocol (Liu) (Image and dose optimization) o Prostate (Wilding, Liu, Med Onc) o Pancreas (Weber) o Breast o Head and neck (Speer/Harari. Rad Onc) o Others - 9 total • Positive initial images in brain cancer and 6 other solid tumors • Brain cancer • 15 patients to date • High tumor - to - background uptake in glioma and brain metastases • Other solid tumors (< 3 patients): breast, lung, prostate, head & neck, colorectal and pancreatic
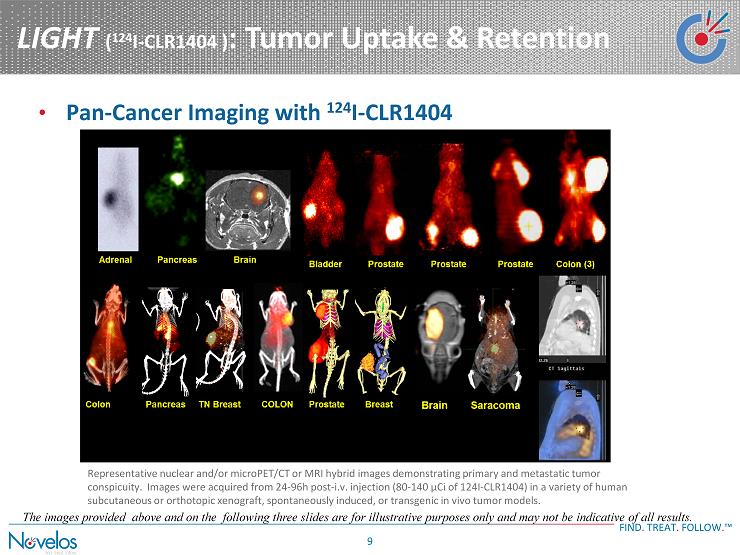
LIGHT ( 124 I - CLR1404 ) : Tumor Uptake & Retention FIND . TREAT . FOLLOW . ™ 9 • Pan - Cancer Imaging with 124 I - CLR1404 Representative nuclear and/or microPET/CT or MRI hybrid images demonstrating primary and metastatic tumor conspicuity. Images were acquired from 24 - 96h post - i.v. injection (80 - 140 µCi of 124I - CLR1404) in a variety of human subcutaneous or orthotopic xenograft, spontaneously induced, or transgenic in vivo tumor models. The images provided above and on the following three slides are for illustrative purposes only and may not be indicative of all results.
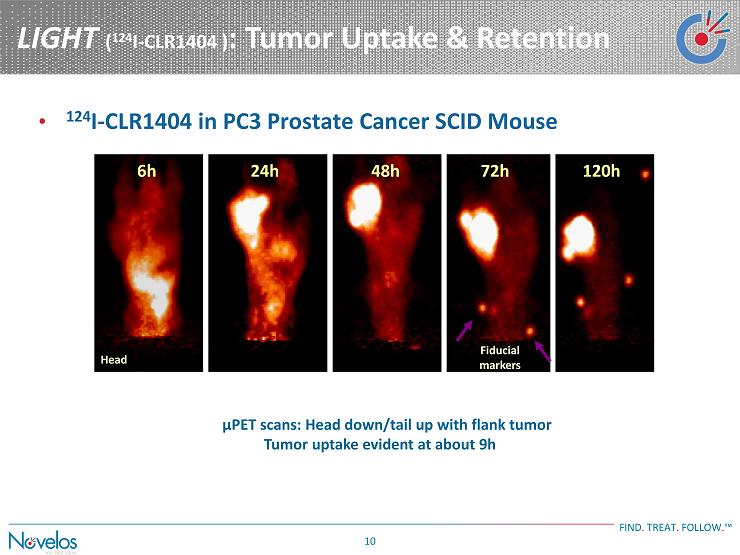
LIGHT ( 124 I - CLR1404 ) : Tumor Uptake & Retention FIND . TREAT . FOLLOW . ™ 10 • 124 I - CLR1404 in PC3 Prostate Cancer SCID Mouse Head μPET scans: Head down/tail up with flank tumor Tumor uptake evident at about 9h Fiducial markers 6h 24h 48h 72h 120h
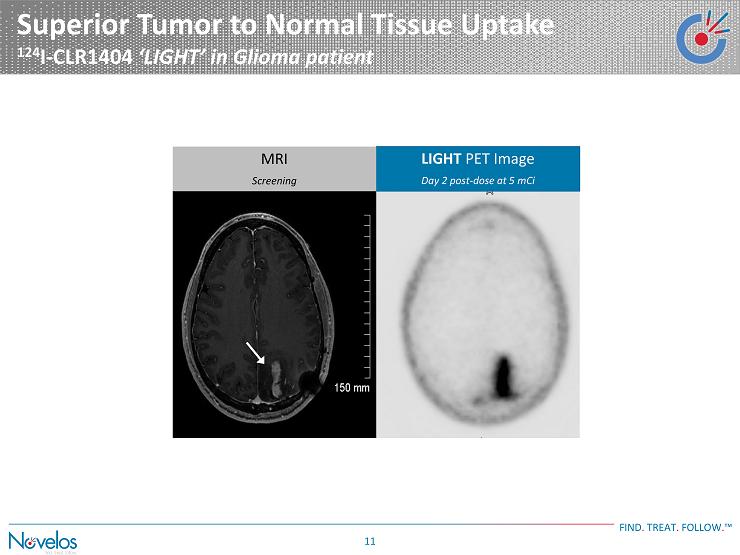
Superior Tumor to Normal Tissue Uptake 124 I - CLR1404 ‘ LIGHT ’ in Glioma patient FIND . TREAT . FOLLOW . ™ 11 LIGHT PET Image Day 2 post - dose at 5 mCi MRI Screening
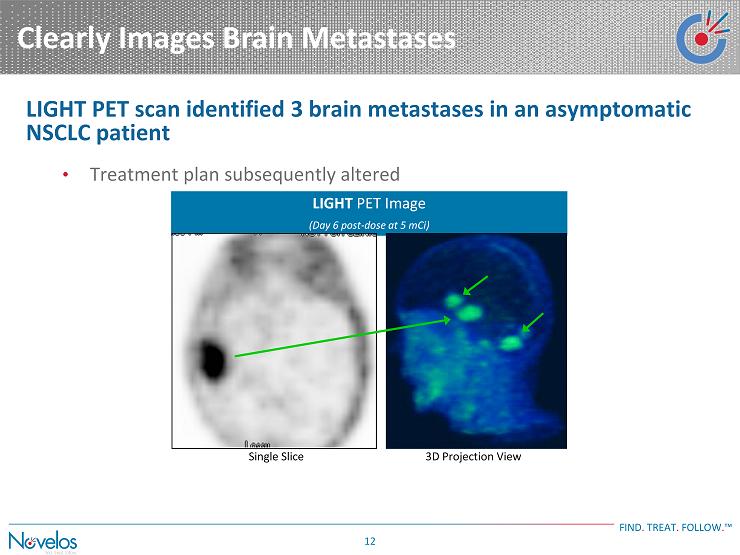
Clearly Images Brain Metastases FIND . TREAT . FOLLOW . ™ 12 LIGHT PET Image (Day 6 post - dose at 5 mCi) 3D Projection View Single Slice LIGHT PET scan identified 3 brain metastases in an asymptomatic NSCLC patient • Treatment plan subsequently altered
Initial Target – Brain Cancer Imaging FIND . TREAT . FOLLOW . ™ 13 • Significant unmet clinical need • MRI is unable to distinguish recurrent disease from pseudo - progression • MRI is current Standard of Care (SOC) • MRIs show post - treatment changes in ~50% of all patients 1 • Nearly half of the time, this signal is non - malignant pseudo - progression 1 • Significant patient benefit derived from earlier and definitive diagnosis of tumor progression • Treat only when appropriate • Avoid unnecessary therapies and interventions • Detect progression earlier, minimize critical tissue infiltration
LIGHT: Phase II Imaging Trial in Glioma FIND . TREAT . FOLLOW . ™ 14 • Trial Design • Multi - site, with an enrollment of ~36 patients • Newly diagnosed or recurrent glioma where SOC includes resection and/or biopsy • Comparison to MRI based on pathology confirmation • Approval based on more accurate determination of malignant vs. non - malignant tissue • Trial Objectives • Demonstrate superior specificity over current SOC (MRI) • Confirm optimal dose and imaging time points for Phase III pivotal trial » Start: Q1 2014 » Data: Q4 2014
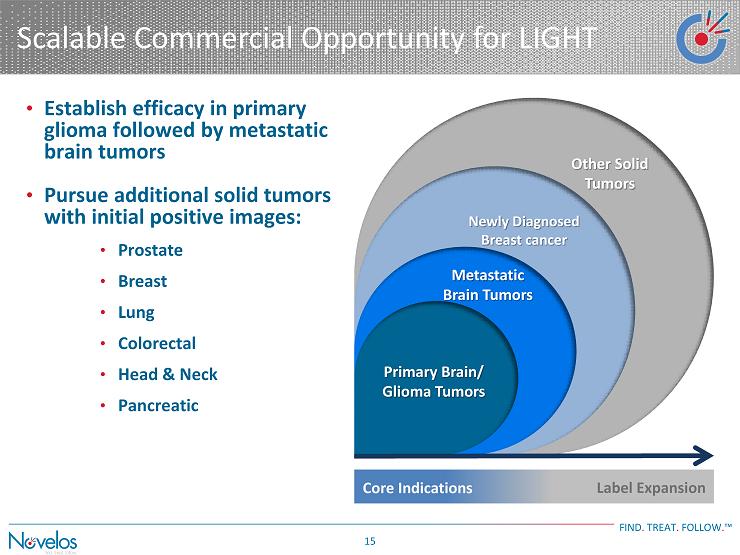
Scalable Commercial Opportunity for LIGHT FIND . TREAT . FOLLOW . ™ 15 • Establish efficacy in primary glioma followed by metastatic brain tumors • Pursue additional solid tumors with initial positive images: • Prostate • Breast • Lung • Colorectal • Head & Neck • Pancreatic Metastatic Brain Tumors Newly Diagnosed Breast cancer Other Solid Tumors Core Indications Label Expansion Primary Brain/ Glioma Tumors
HOT For Targeted Therapy FIND . TREAT . FOLLOW . ™ 16 PLE + Radiotherapeutic= HOT ( 131 I - CLR1404 ) Better targeted delivery to cancer cells and cancer stem cells
Cancer Stem Cell (CSC) Paradigm FIND . TREAT . FOLLOW . ™ 17 • Cancer stem cells have now been firmly associated with most, if not all , major cancer types. • Numerous recent reports confirm that cancer stem cells do exist and are chemotherapy resistant . • Glioma stem cells are also known to be up to 30% more radiation resistant relative to normal cancer cells. • These cells affiliated with tumor regrowth and metastasis following chemo and radiation therapy. • Tumor hypoxia stimulates CSC propagation leading to increased resistance and metastatic potential. “ Any new cancer treatment paradigm must address tumor heterogeneity including cancer stem cells” - Jeremy Rich and others
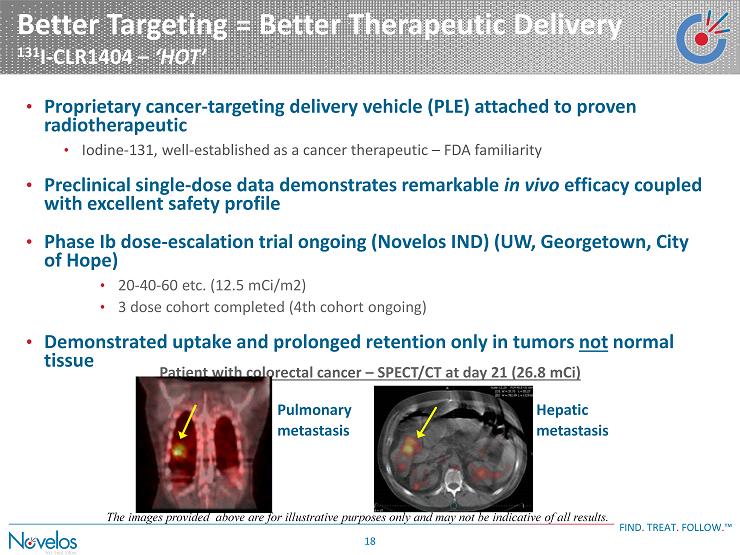
Better Targeting = Better Therapeutic Delivery 131 I - CLR1404 – ‘HOT’ FIND . TREAT . FOLLOW . ™ 18 • Proprietary cancer - targeting delivery vehicle (PLE) attached to proven radiotherapeutic • Iodine - 131, well - established as a cancer therapeutic – FDA familiarity • Preclinical single - dose data demonstrates remarkable in vivo efficacy coupled with excellent safety profile • Phase Ib dose - escalation trial ongoing ( Novelos IND) (UW, Georgetown, City of Hope) • 20 - 40 - 60 etc. (12.5 mCi/m2) • 3 dose cohort completed (4th cohort ongoing) • Demonstrated uptake and prolonged retention only in tumors not normal tissue Hepatic metastasis Pulmonary metastasis Patient with colorectal c ancer – SPECT/CT at day 21 (26.8 mCi) The images provided above are for illustrative purposes only and may not be indicative of all results.

GLOW2 For Surgical Imaging FIND . TREAT . FOLLOW . ™ 19 PLE + Near - Infrared Fluorophore= GLOW2 ( CLR1502 ) Intraoperative O ptical Imaging for Better Outcomes
Improving Cancer Surgery Outcomes CLR1502 – ‘GLOW2’ FIND . TREAT . FOLLOW . ™ 20 • Optical imaging agent for intraoperative tumor margin illumination • Proprietary cancer - targeting delivery vehicle (PLE) attached to near - infrared fluorophore • More accurate visualization of tumor margins during surgery for more complete malignant tissue removal • Better patient management and outcomes from fewer repeat surgeries and reduced recurrence • Potential for meaningful healthcare savings
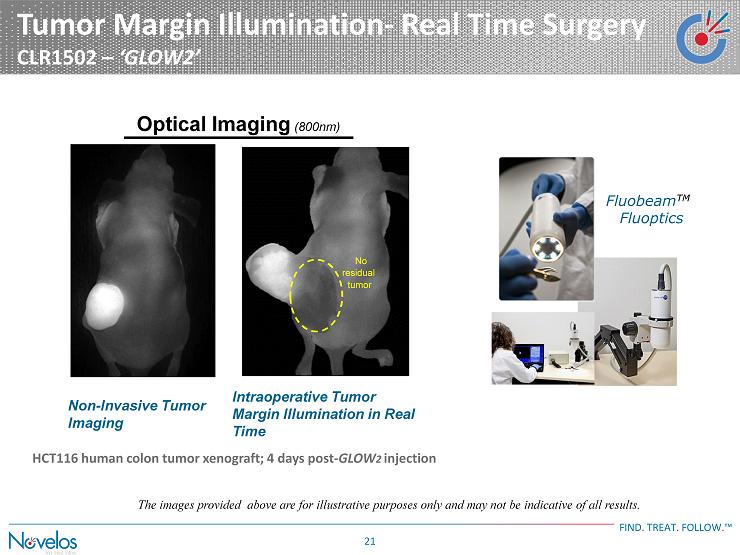
Tumor Margin Illumination - Real Time Surgery CLR1502 – ‘GLOW2’ FIND . TREAT . FOLLOW . ™ 21 HCT116 human colon tumor xenograft; 4 days post - GLOW 2 injection Optical Imaging (800nm) Non - Invasive Tumor Imaging Intraoperative Tumor Margin Illumination in Real Time No residual tumor Fluobeam TM ) Fluoptics The images provided above are for illustrative purposes only and may not be indicative of all results.
Unmet Need – Breast Cancer Surgery FIND . TREAT . FOLLOW . ™ 22 • Lumpectomy - complete removal of tumor while sparing functional tissue • Challenging for surgeons to identify margins during surgery • When post - surgery pathology indicates malignant tissue remains, surgery repeated • One in four lumpectomies are repeated ( U.S) 2 • Impact on patients: risk, discomfort, inconvenience, anxiety • Significant healthcare cost: ~$22,000 inpatient 3 • Patient outcomes at risk: remaining malignant tissue could result in disease spread “Margins Matter”

GLOW2 Initial Human Trial Phase I, Breast Cancer Surgery FIND . TREAT . FOLLOW . ™ 23 • Trial design • Dose - ranging multi - site, ~ 2 0 patients undergoing lumpectomy • Compatible with standard of care (SOC) – only addition is GLOW2 administration and non - invasive optical imaging • Primary tumor and sentinel node resected according to SOC • Optical imaging used to assess GLOW2 illumination of any remaining tumor margin or nodal involvement • Trial objective • Demonstrate superiority versus current SOC to identify and differentiate malignant and non - malignant tissue during surgery based on pathology readout

Opportunity Summary FIND . TREAT . FOLLOW . ™ 24 • $Billion+ potential aggregate markets: LIGHT and GLOW2 4 • Efficacy Proof - of - Concept data expected in 12 - 18 months • Opportunity for meaningful value transformation • Therapeutic (HOT) represents potential additional upside • Substantial data to help select efficacy target when the time is right • Cancer - Targeting Platform offers multi - product opportunities • Tumor Imaging - More accurate for better patient management • Therapy - Better targeted delivery to cancer and cancer stem cells • Surgery - Intraoperative optical imaging for better outcomes • Attractive Business Development Opportunities
Summary Financial Outlook FIND . TREAT . FOLLOW . ™ 25 • Funding through February 2014 • $5.1M cash at September 30, 2013 • Capitalization • 57M shares of common stock • 101M shares fully diluted

Strategic Realignment FIND . TREAT . FOLLOW . ™ 26 • New CEO: Simon Pedder • Appointed October 2013 to lead turnaround • Former President & CEO, Chelsea Therapeutics (Nasdaq: CHTP) • Previously held positions of increasing responsibility at Hoffmann - La Roche, including: • Director of International Clinical Science • Director of International Clinical Operations • Global Project Leader of Pharmaceutical Development • Life Cycle Leader, PEGASYS/IFN and Head of Hepatitis Franchise, Pharma Business • Vice President of Pharma Business Oncology and Corporate Officer

Strategic Realignment FIND . TREAT . FOLLOW . ™ 27 • Streamlined Board of Directors, adding new director and reducing overall size from 9 to 5 members • Stephen A. Hill, B.M. B.Ch., M.A., F.R.C.S., Chairman of the Board of Directors • Simon Pedder, Ph.D., CEO and Director • Jamey Weichert, Ph.D., Chief Scientific Officer, Technology Founder and Director • John Neis, Director □ Paul L. Berns, Appointed Director in November 2013 • CEO, Allos Therapeutics - acquired by Spectrum Pharmaceuticals, Inc. in 2012 • CEO, Bone Care International, Inc. – acquired by Genzyme Corporation in 2005 • VP and general manager of the Immunology, Oncology and Pain Therapeutics business unit of Abbott Laboratories • Serves as member of the Board of Directors of Jazz Pharmaceuticals, Inc., Anacor Pharmaceuticals, Inc. and of XenoPort, Inc.

Strategic Realignment FIND . TREAT . FOLLOW . ™ 28 • Aligning Resources with Strategic Priorities • Relocating Executives Offices and Centralizing Operations in Madison, WI • Responsibilities of former MA based VP, Clinical Development and VP, Research and Development being transitioned to existing WI - based employees • Transitioning finance function, including CFO responsibilities , to WI following year - end reporting • Prioritizing Investor Relations and Business Development efforts • New VP of IR & PR tasked with increasing awareness of “new” Novelos • Articulate new strategy • Aggressively meet with new potential investors • Increase institutional ownership and sell - side coverage • Created new BD position • Build industry awareness of company and technologies • Explore non - dilutive partnership opportunities to offset R&D expense

Going Forward FIND . TREAT . FOLLOW . ™ 29 • Disciplined execution in clinical programs • Strong pipeline with history of peer reviewed NCI grants • Defining new cancer imaging and therapy paradigms • Explore Partnership Opportunities • Global/Regional licensing opportunities • Therapeutic partnership opportunities • Meaningful development opportunities • Compelling near - and long - term opportunities • Near - term Data Points • Light Phase II Imaging Data in Glioma • HOT Phase Ib Safety Data • GLOW2 Phase I Optical Imaging Data • Long - term: opportunity to expand development opportunities • Get out and tell the story
Sources FIND . TREAT . FOLLOW . ™ 30 1 Wen et al., N Engl J Med 2008 2 Factors Associated with repeat Surgery After Initial Breast Conservation (Wilke L et al Yao K 2012) 3 HCUP: www.hcup - us.ahrq.gov/reports/statbriefs/sb86. jsp 4 American Cancer Society, Cancer Facts and Figures, 2013





























