
Acceleron Pharma R&D Day October 23, 2015 1 Exhibit 99.1

Welcome and Agenda Todd James Senior Director, Corporate Communications Acceleron 2

Acceleron Forward-Looking Statements 3 This presentation contains forward-looking statements about the Company's strategy, future plans and prospects, including statements regarding the development of the Company's compounds, including sotatercept, luspatercept, dalantercept, ACE-083, ACE-2494, the Company’s IntelliTrap™ drug discovery platform, and the Company's TGF-beta superfamily program generally, the timeline for clinical development and regulatory approval of the Company's compounds, the expected timing for the reporting of data from ongoing trials, and the structure of the Company's planned or pending clinical trials. The words “anticipate,” “appear,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement. Applicable risks and uncertainties include the risks that preclinical testing of the Company's compounds and data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that data may not be available when the Company expects it to be, that the Company or its collaboration partner, Celgene, will be unable to successfully complete the clinical development of the Company’s compounds, that the development of the Company's compounds will take longer or cost more than planned, that the Company or Celgene may be delayed in initiating or completing any clinical trials, and that the Company's compounds will not receive regulatory approval or become commercially successful products. Other risks and uncertainties include those identified under the heading "Risk Factors" included in the Company's Annual Report on Form 10-K which was filed with the Securities and Exchange Commission (SEC) on March 2, 2015, and other filings that the Company has made and may make with the SEC in the future. The forward-looking statements contained in this presentation reflect the Company's current views with respect to future events, and the Company does not undertake and specifically disclaims any obligation to update any forward-looking statements.
Agenda 4 R&D Strategy John Knopf, Ph.D. - Acceleron Chief Executive Officer ACE-083 and Muscle Franchise Matthew Sherman, M.D. - Acceleron Chief Medical Officer Anthony Amato, M.D. - Vice Chairman, Department of Neurology, Brigham and Women’s Hospital, Boston Dalantercept and Sotatercept Matthew Sherman, M.D. - Acceleron Chief Medical Officer John Knopf, Ph.D. - Acceleron Chief Executive Officer Luspatercept Phase 3 Program Question & Answer Question & Answer Steven Ertel - Acceleron Chief Operating Officer M. Domenica Cappellini, M.D. - Director of the Department of Clinical Sciences, Director of the Unit of Internal Medicine, University of Milan, Italy Uwe Platzbecker, M.D. - Professor of Hematology and Head of the MDS Program, University Hospital, Dresden, Germany Jay Backstrom, M.D. M.P.H. - Senior Vice President, Hematology & Oncology R&D, Global Head Regulatory Affairs, Celgene Closing Remarks Steven Ertel - Acceleron Chief Operating Officer

Acceleron R&D Strategy John Knopf, Ph.D. Chief Executive Officer Acceleron 5

Driven to Make a Difference Dedicated to improving the lives of patients with serious, underserved diseases Apply the biology of the TGF-beta superfamily to internally discover and develop innovative therapeutics Efficiently and rapidly build a multi-product company Hire the best people in the industry Collaborate with the industry’s best partners Set aggressive and aspirational goals to build one of the world’s great biotechnology companies 6
Acceleron R&D Strategy The human body has a remarkable ability to regenerate, rebuild and repair itself The TGF-beta superfamily of proteins can profoundly influence many of these biological processes Acceleron has pioneered a new approach to engage these targets for therapeutic benefit 7
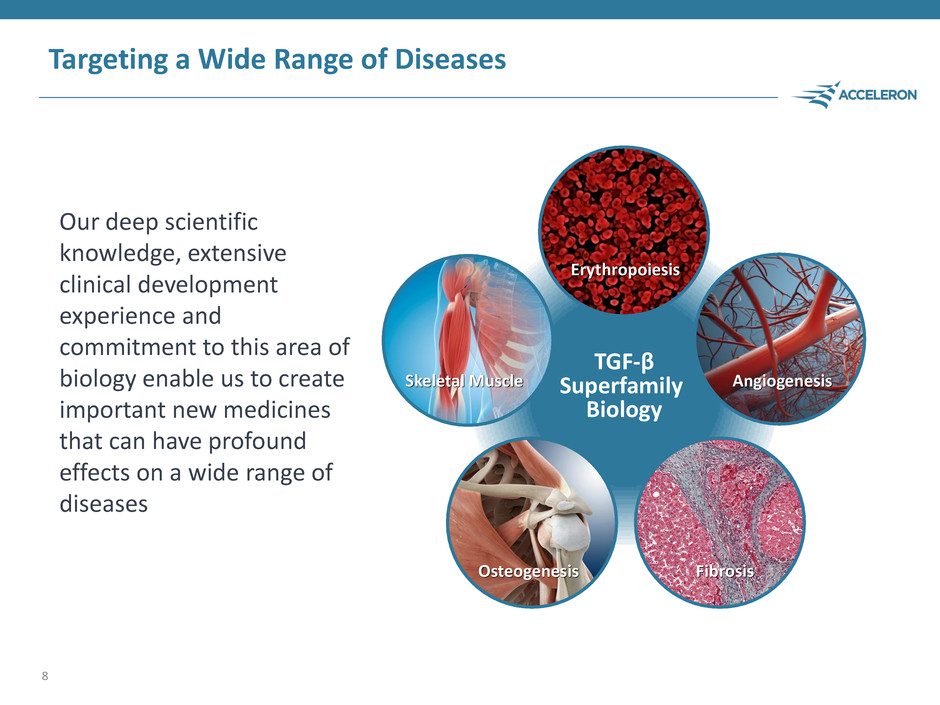
Targeting a Wide Range of Diseases Our deep scientific knowledge, extensive clinical development experience and commitment to this area of biology enable us to create important new medicines that can have profound effects on a wide range of diseases 8 TGF-β Superfamily Biology Skeletal Muscle Osteogenesis Fibrosis Angiogenesis Erythropoiesis
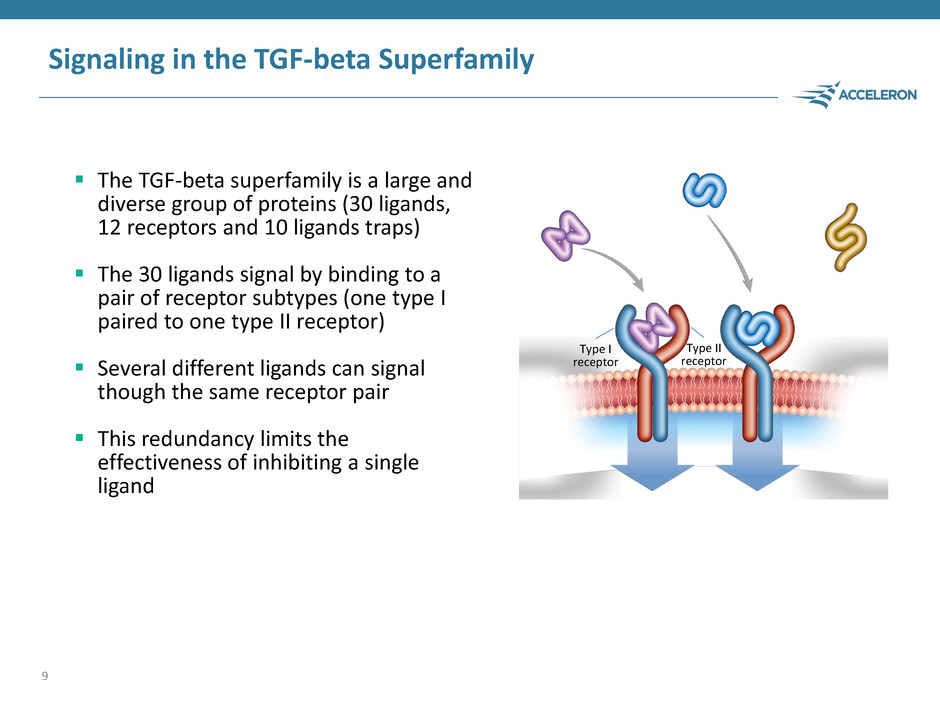
Signaling in the TGF-beta Superfamily The TGF-beta superfamily is a large and diverse group of proteins (30 ligands, 12 receptors and 10 ligands traps) The 30 ligands signal by binding to a pair of receptor subtypes (one type I paired to one type II receptor) Several different ligands can signal though the same receptor pair This redundancy limits the effectiveness of inhibiting a single ligand 9 Type I receptor Type II receptor
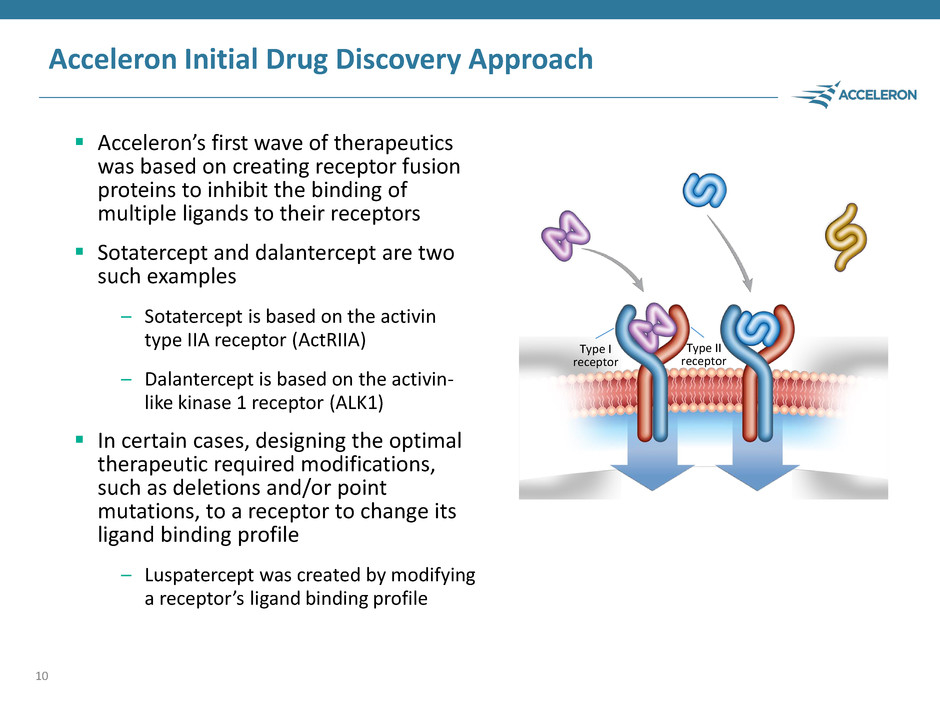
Acceleron Initial Drug Discovery Approach Acceleron’s first wave of therapeutics was based on creating receptor fusion proteins to inhibit the binding of multiple ligands to their receptors Sotatercept and dalantercept are two such examples – Sotatercept is based on the activin type IIA receptor (ActRIIA) – Dalantercept is based on the activin- like kinase 1 receptor (ALK1) In certain cases, designing the optimal therapeutic required modifications, such as deletions and/or point mutations, to a receptor to change its ligand binding profile – Luspatercept was created by modifying a receptor’s ligand binding profile 10 Type I receptor Type II receptor
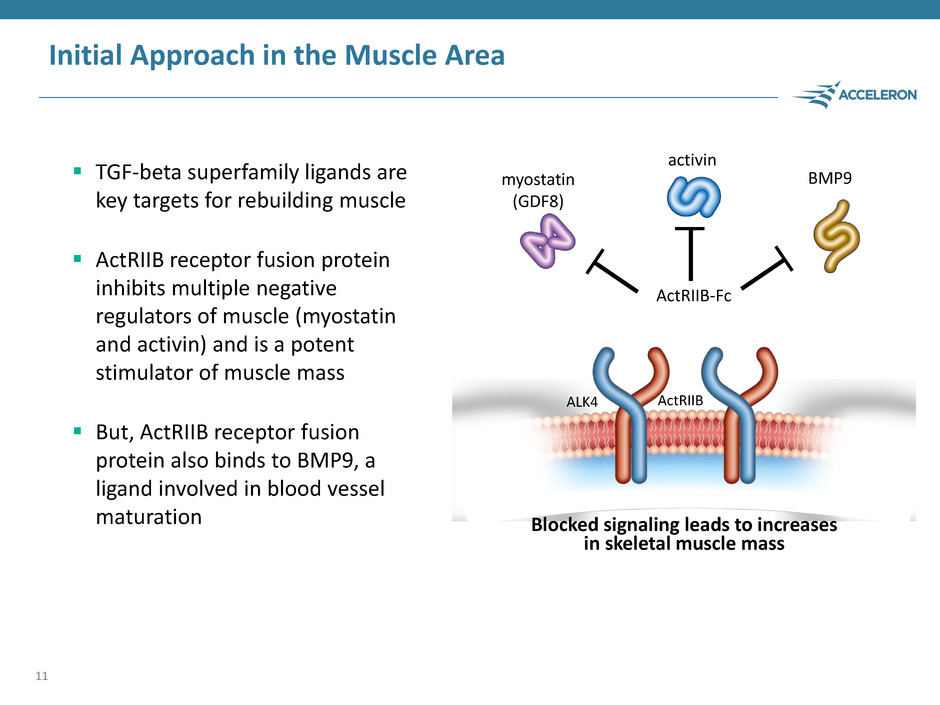
Initial Approach in the Muscle Area TGF-beta superfamily ligands are key targets for rebuilding muscle ActRIIB receptor fusion protein inhibits multiple negative regulators of muscle (myostatin and activin) and is a potent stimulator of muscle mass But, ActRIIB receptor fusion protein also binds to BMP9, a ligand involved in blood vessel maturation 11 Blocked signaling leads to increases in skeletal muscle mass BMP9 ActRIIB myostatin (GDF8) activin ALK4 ActRIIB-Fc
IntelliTrap™ Platform – The Solution to Achieve Optimal Ligand Binding Specificity Acceleron’s knowledge of the TGF-beta superfamily combined with our exceptional capabilities in protein engineering led to the creation of the IntelliTrap™ Platform The IntelliTrap™ Platform enables Acceleron to selectively achieve ligand binding specificities resulting in a spectrum of highly innovative molecules with distinct pharmacologic properties – High affinity biologic agents – Tailored multi-ligand specificity – Potent in vivo activity 12
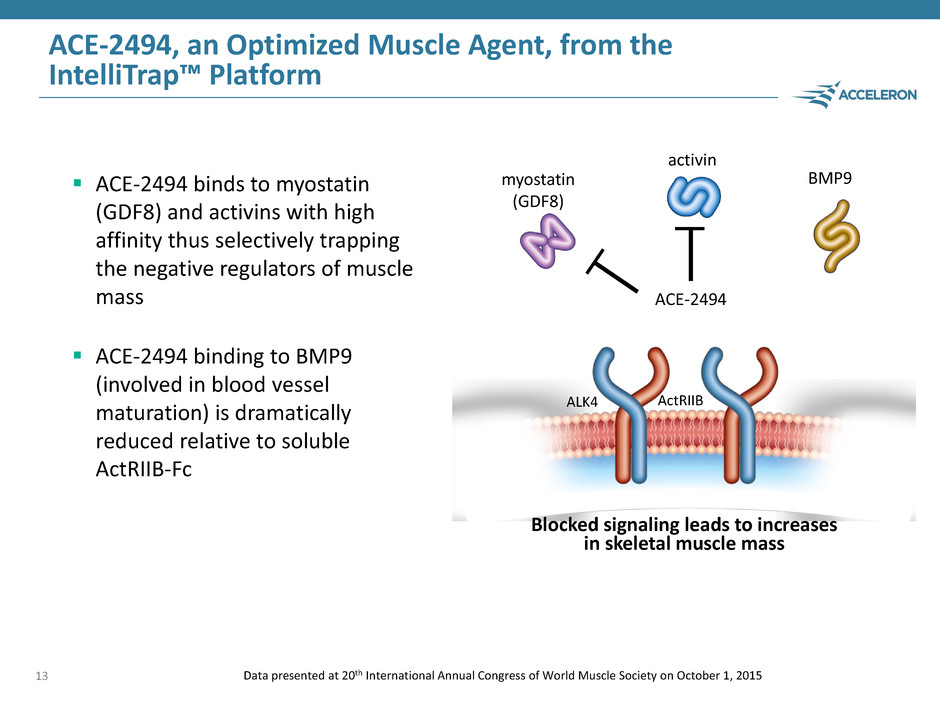
ACE-2494, an Optimized Muscle Agent, from the IntelliTrap™ Platform ACE-2494 binds to myostatin (GDF8) and activins with high affinity thus selectively trapping the negative regulators of muscle mass ACE-2494 binding to BMP9 (involved in blood vessel maturation) is dramatically reduced relative to soluble ActRIIB-Fc 13 Blocked signaling leads to increases in skeletal muscle mass BMP9 ActRIIB myostatin (GDF8) activin Data presented at 20th International Annual Congress of World Muscle Society on October 1, 2015 ALK4 ACE-2494

ACE-2494 Generates Profound Increases in Muscle Mass 14 In a one-month study in mice, ACE-2494 increases muscle mass comparable to ActRIIB-Fc Data presented at 20th International Annual Congress of World Muscle Society on October 1, 2015 These increases far exceed the 15-20% increases observed with a selective myostatin inhibitor Gastrocnemius ActRI IB -Fc 10 m g/ kg AC E- 249 4 10 m g/ kg M uscle W eight /I ni tial B od y W eight (% chan g e f ro m V E H ) 0 10 20 30 40 50 60 70 50.8% 53.3% Femoris ctRI I Fc 10 - 4 10 m g/ kg M uscle W eigh t/ Ini tial B od y W eigh t (% chang e f ro m V E H ) 10 20 30 40 50 6 43.9% 40.9% Pectoralis ActRI IB -F c 10 m g/ kg AC E- 249 4 10 m g/ kg M uscle W eigh t/ Ini tial B od y W eigh t (% chang e f ro m V E H ) 0 20 40 60 80 1 0 51.5% 86.9% ActRIIB-Fc ACE-2494 ActRIIB-Fc ACE-2494 ActRIIB-Fc ACE-2494
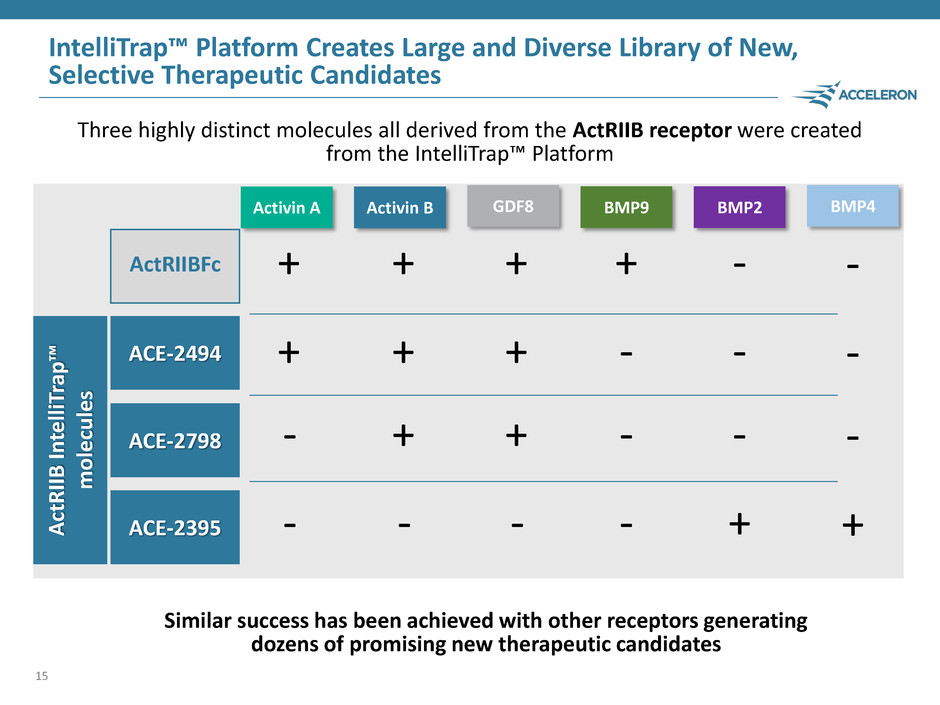
ActRIIBFc ACE-2798 ACE-2395 ACE-2494 IntelliTrap™ Platform Creates Large and Diverse Library of New, Selective Therapeutic Candidates 15 + + - - Activin A + + + - Activin B + + + - GDF8 + - - - BMP9 - - - + BMP2 Three highly distinct molecules all derived from the ActRIIB receptor were created from the IntelliTrap™ Platform ActRIIB In te lliT rap ™ mol e cul es Similar success has been achieved with other receptors generating dozens of promising new therapeutic candidates BMP4 - - - +

Expanding Platforms for Success Acceleron designs distinct constructs to optimally target selected molecules Receptor fusion proteins (sotatercept and dalantercept) Modified receptor fusion proteins (luspatercept) Natural ligand traps modified to be locally acting (ACE-083) IntelliTrap™ molecules (ACE-2494 and a diverse library of new molecules) Antibodies (ACE-3891 and several other candidates in preclinical studies) 16

Acceleron Muscle Franchise Matthew Sherman, M.D. Chief Medical Officer Acceleron 17

Acceleron Muscle Franchise 18 Acceleron’s muscle franchise and ACE-083 Phase 1 results Matthew Sherman, M.D. Chief Medical Officer, Acceleron Opportunities in neuromuscular diseases Anthony Amato, M.D. Vice Chairman, Department of Neurology Chief, Neuromuscular Division Brigham and Women’s Hospital, Boston, MA
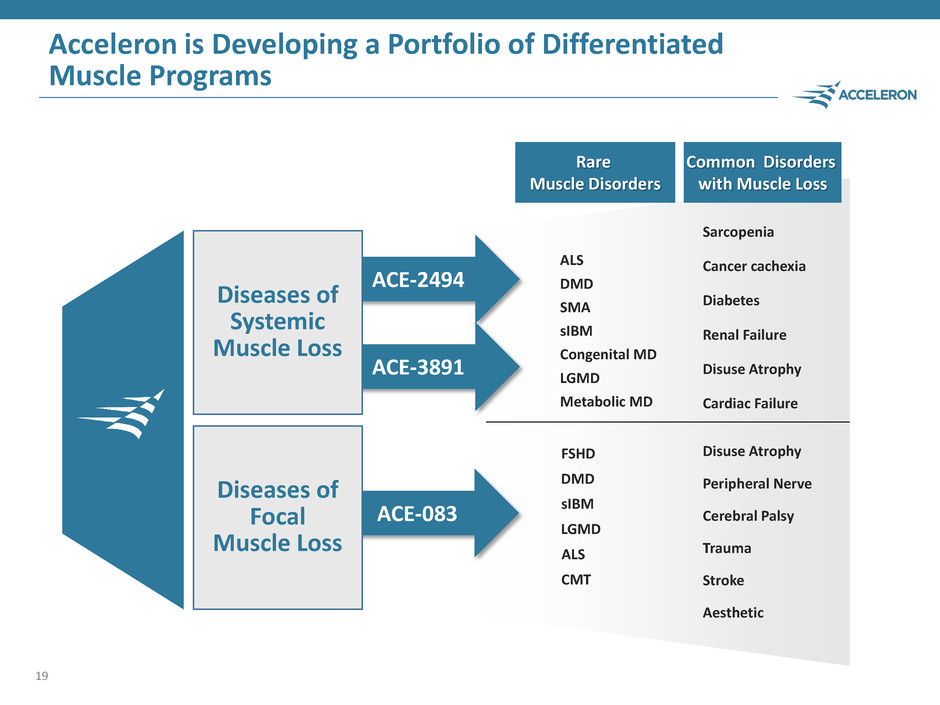
ACE-3891 Acceleron is Developing a Portfolio of Differentiated Muscle Programs 19 ACE-083 Diseases of Focal Muscle Loss ACE-2494 Diseases of Systemic Muscle Loss FSHD DMD sIBM LGMD CMT ALS Cerebral Palsy Peripheral Nerve Trauma Disuse Atrophy Stroke Aesthetic Common Disorders with Muscle Loss Rare Muscle Disorders DMD sIBM LGMD SMA ALS Metabolic MD Congenital MD Sarcopenia Renal Failure Cancer cachexia Diabetes Cardiac Failure Disuse Atrophy

ACE-083: For Diseases Involving Focal Muscle Loss ACE-083 is derived from one of the 10 naturally occurring TGF-beta superfamily ligand traps ACE-083 is designed to increase muscle mass and strength selectively in the muscles in which the drug is administered ACE-083 binds to and inhibits activins and myostatin (GDF8) negative regulators of skeletal muscle growth In preclinical studies, ACE-083 increased muscle mass and strength 20

ACE-083 Phase 1 Study in Healthy Volunteers Study Description Randomized, double-blind, placebo-controlled, dose-ranging study in healthy volunteers Objectives of the Study To evaluate the safety and tolerability of single and multiple doses of ACE-083 as a local muscle injection To evaluate the pharmacodynamic (PD) effects of ACE-083 such as changes in muscle volume assessed by MRI 21

ACE-083 Phase 1 Study Design 22 Number of Doses Cohort Dosing Day(s) Dose Level (mg) Injected Muscle ACE-083 Subjects Placebo Subjects Status Single Dose 1 Day 1 50 Rectus Femoris 6 2 Completed 2 Day 1 100 Rectus Femoris 6 2 3 Day 1 200 Rectus Femoris 6 2 Multiple Doses 4 Days 1, 22 100 Rectus Femoris 6 2 5 Days 1, 22 200 Rectus Femoris 6 2 6 Days 1, 22 100 Tibialis Anterior 6 3 Ongoing 7 Days 1, 22 150 Tibialis Anterior 6 3 Total Number of Subjects (Planned): 42 16
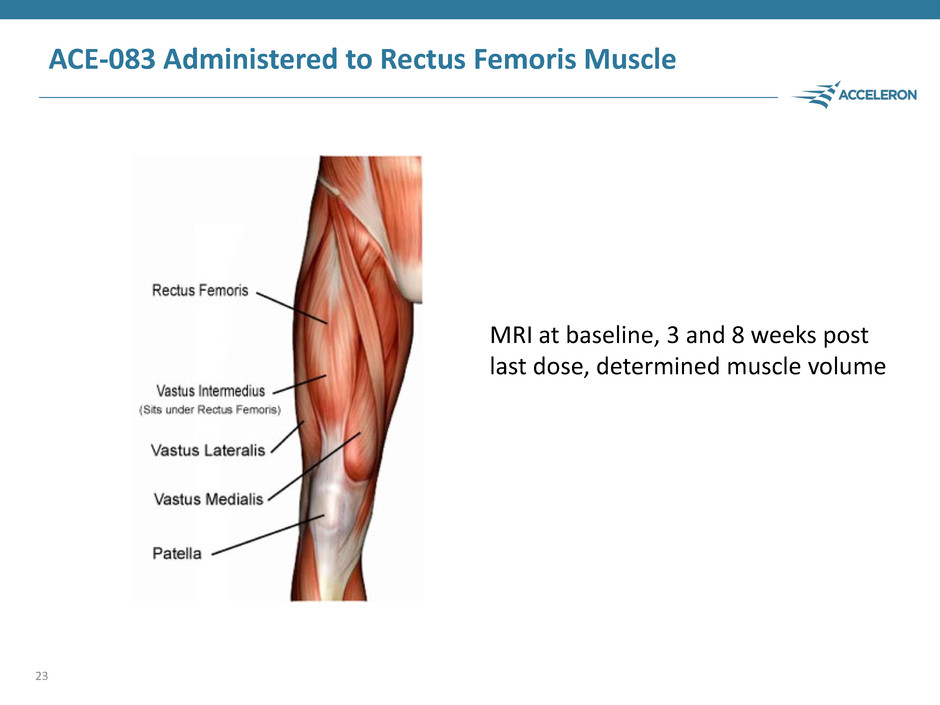
ACE-083 Administered to Rectus Femoris Muscle 23 MRI at baseline, 3 and 8 weeks post last dose, determined muscle volume
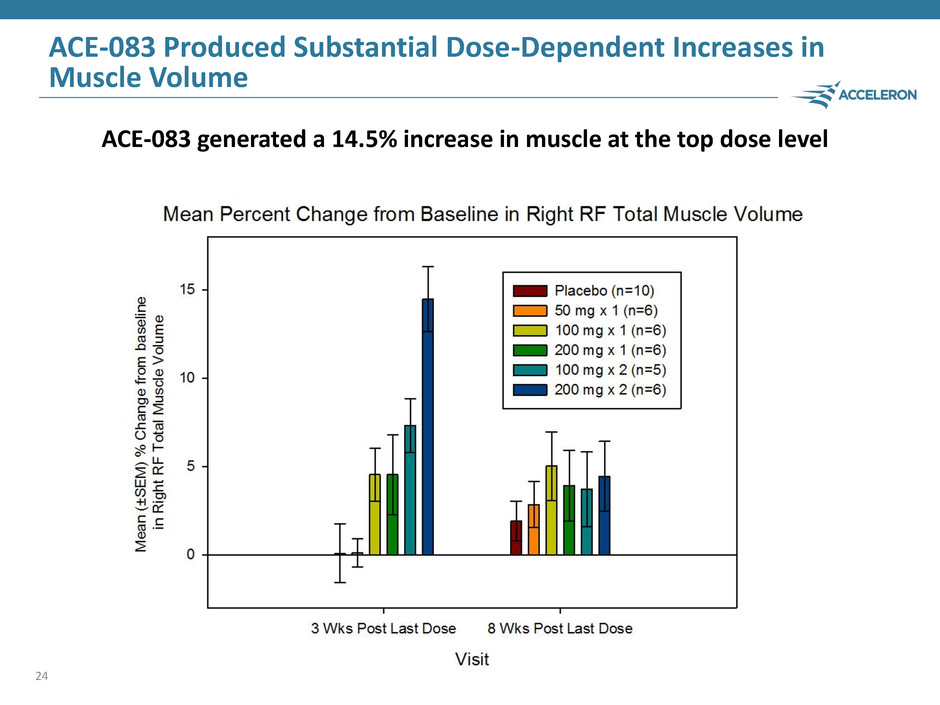
ACE-083 Produced Substantial Dose-Dependent Increases in Muscle Volume 24 ACE-083 generated a 14.5% increase in muscle at the top dose level
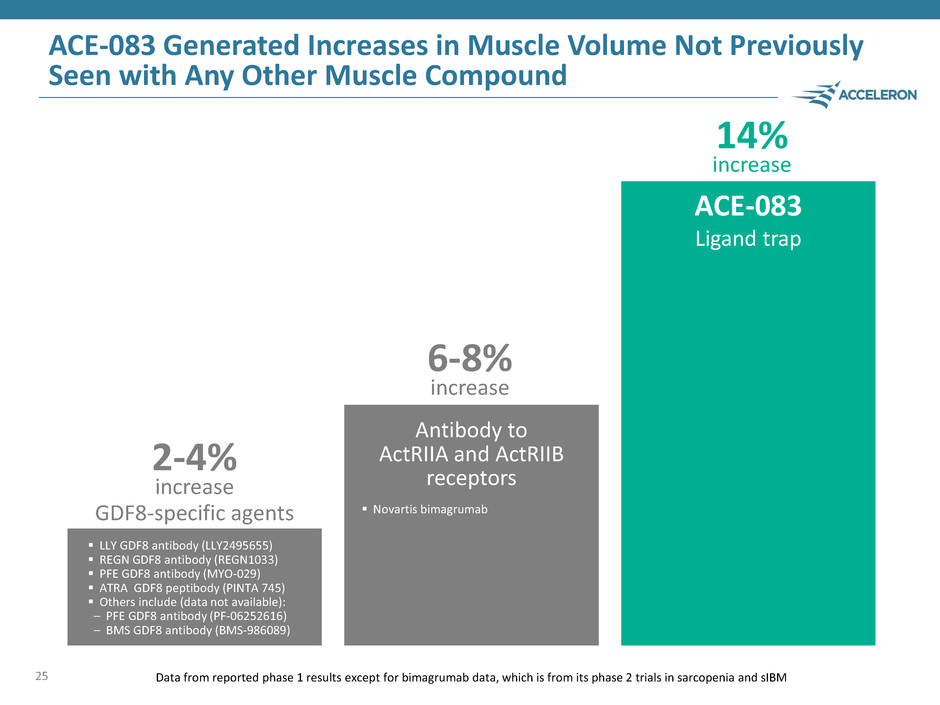
ACE-083 Generated Increases in Muscle Volume Not Previously Seen with Any Other Muscle Compound 25 Data from reported phase 1 results except for bimagrumab data, which is from its phase 2 trials in sarcopenia and sIBM 2-4% increase 6-8% increase 14% increase GDF8-specific agents Antibody to ActRIIA and ActRIIB receptors ACE-083 Ligand trap LLY GDF8 antibody (LLY2495655) REGN GDF8 antibody (REGN1033) PFE GDF8 antibody (MYO-029) ATRA GDF8 peptibody (PINTA 745) Others include (data not available): – PFE GDF8 antibody (PF-06252616) – BMS GDF8 antibody (BMS-986089) Novartis bimagrumab

ACE-083 Phase 1 Summary ACE-083 was well-tolerated Robust dose dependent increases in muscle volume Magnitude of increases in muscle volume not previously seen in human clinical trials of any other muscle therapeutic Exciting and highly differentiated product opportunity for patients suffering from focal muscle loss 26
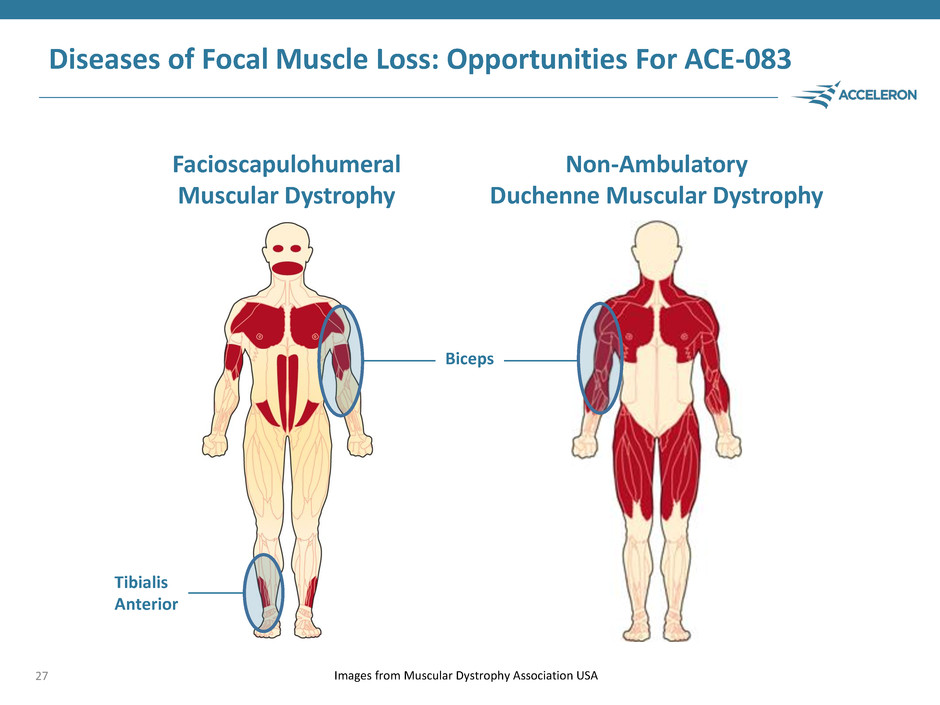
Diseases of Focal Muscle Loss: Opportunities For ACE-083 27 Images from Muscular Dystrophy Association USA Facioscapulohumeral Muscular Dystrophy Non-Ambulatory Duchenne Muscular Dystrophy Biceps Tibialis Anterior
ACE-083: Initiate Phase 2 in FSHD Patients in Mid-2016 FSHD is one of the most prevalent forms of muscular dystrophy affecting roughly 19,000 patients in the U.S. Substantial unmet medical need Acceleron intends to initiate a Phase 2 study of ACE-083 in patients with FSHD in mid-2016 28

Opportunities in Rare Muscle Disorders Anthony Amato, M.D. Vice Chairman, Department of Neurology Chief, Neuromuscular Division Brigham and Women’s Hospital Boston, MA 29

Spectrum of Neuromuscular Disorders 30 Myopathies Neurogenic Neuromuscular Junction Disorders Involve dysfunction of the peripheral nerves, which consist of motor neurons that carry electrical signals directly from the spinal cord and brain stem to activate muscle movement Can be a mix of upper and/or lower motor neuron dysfunction Broad set of diseases primarily resulting in muscular degeneration, rather than affecting the nerves themselves Many of them hereditary or resulting from genetic mutations and lead to progressive loss of strength, muscle wasting, disrupted integrity and fibrosis Includes muscular dystrophies and myositis Involves autoimmune and genetic disorders where the nerve transmits signals to the muscle
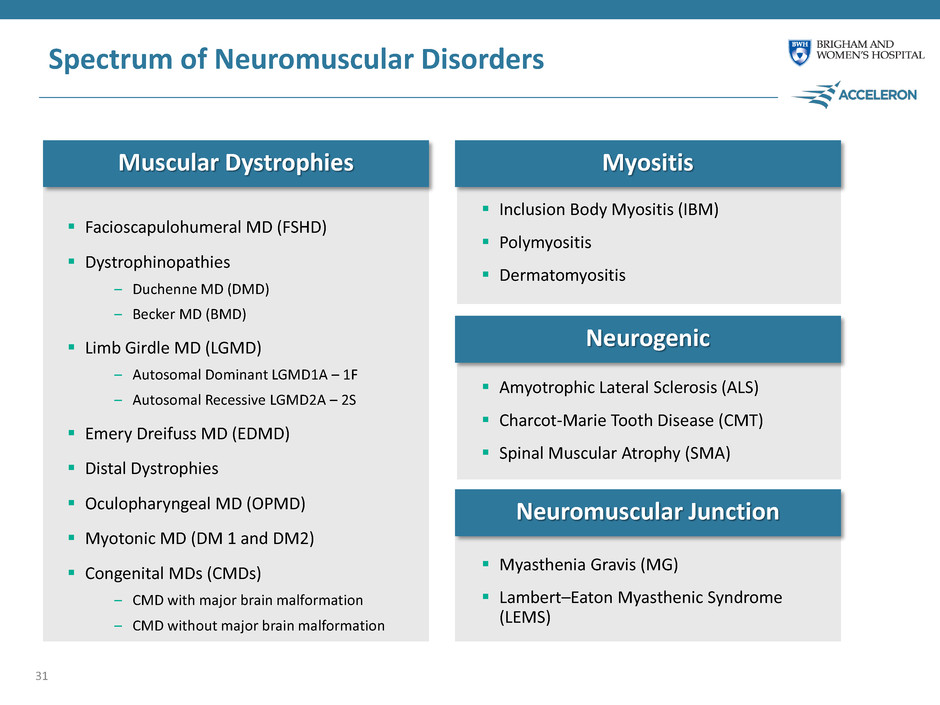
Spectrum of Neuromuscular Disorders Facioscapulohumeral MD (FSHD) Dystrophinopathies – Duchenne MD (DMD) – Becker MD (BMD) Limb Girdle MD (LGMD) – Autosomal Dominant LGMD1A – 1F – Autosomal Recessive LGMD2A – 2S Emery Dreifuss MD (EDMD) Distal Dystrophies Oculopharyngeal MD (OPMD) Myotonic MD (DM 1 and DM2) Congenital MDs (CMDs) – CMD with major brain malformation – CMD without major brain malformation 31 Muscular Dystrophies Myositis Neurogenic Neuromuscular Junction Inclusion Body Myositis (IBM) Polymyositis Dermatomyositis Amyotrophic Lateral Sclerosis (ALS) Charcot-Marie Tooth Disease (CMT) Spinal Muscular Atrophy (SMA) Myasthenia Gravis (MG) Lambert–Eaton Myasthenic Syndrome (LEMS)
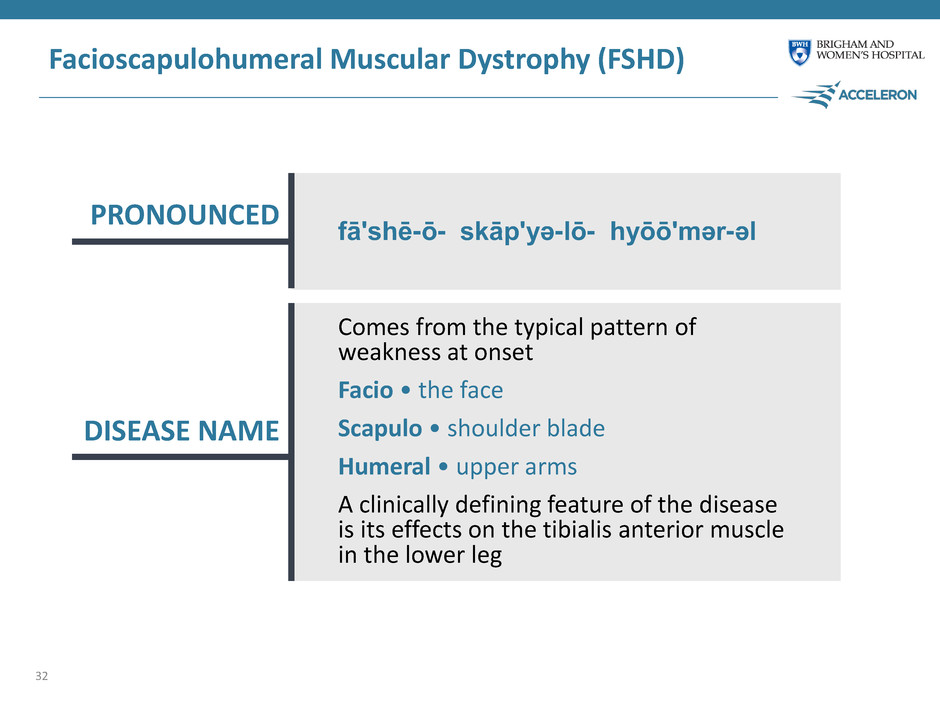
Facioscapulohumeral Muscular Dystrophy (FSHD) 32 PRONOUNCED DISEASE NAME Comes from the typical pattern of weakness at onset Facio • the face Scapulo • shoulder blade Humeral • upper arms A clinically defining feature of the disease is its effects on the tibialis anterior muscle in the lower leg fā'shē-ō- skāp'yə-lō- hyōō'mər-əl

FSHD Disease Description Severe, disabling, and painful skeletal muscle disease Distinct presentation with muscle-by-muscle progression Muscle weakness can be heterogeneous, but is highly focal and primarily asymmetric Typical onset is in the 20-30’s with most FSHD patients living a normal lifespan, though many will suffer from disability, pain, and depression 33
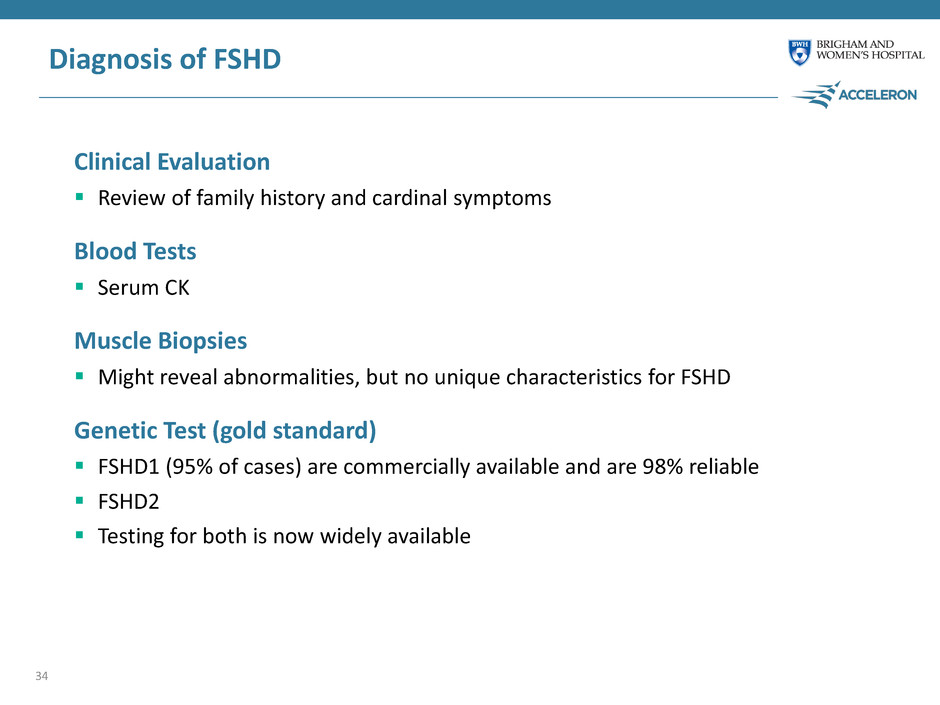
Diagnosis of FSHD Clinical Evaluation Review of family history and cardinal symptoms Blood Tests Serum CK Muscle Biopsies Might reveal abnormalities, but no unique characteristics for FSHD Genetic Test (gold standard) FSHD1 (95% of cases) are commercially available and are 98% reliable FSHD2 Testing for both is now widely available 34
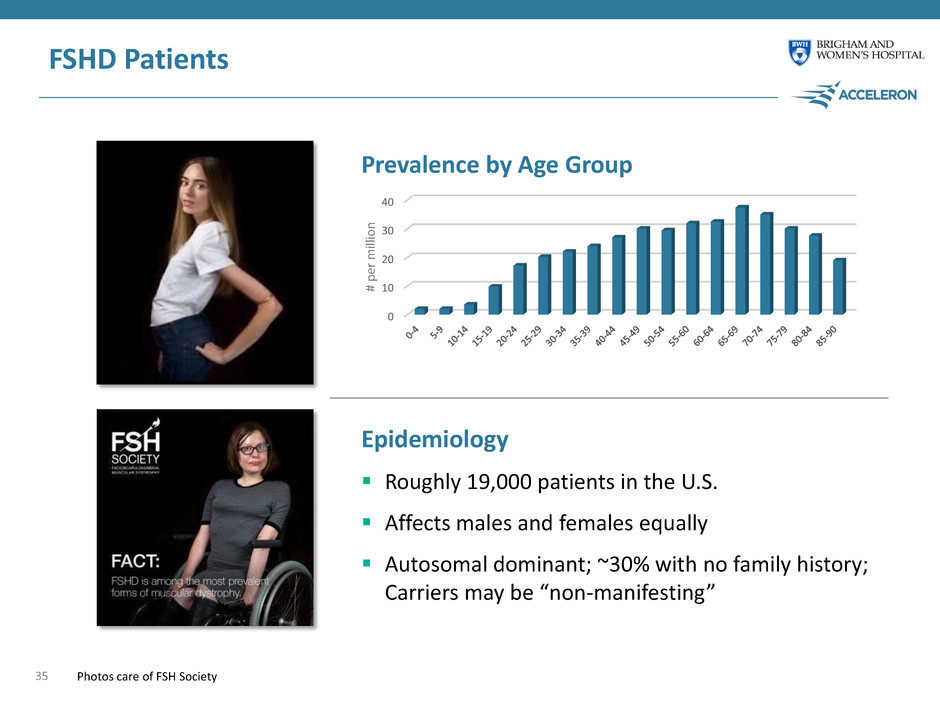
0 10 20 30 40 FSHD Patients 35 Epidemiology Roughly 19,000 patients in the U.S. Affects males and females equally Autosomal dominant; ~30% with no family history; Carriers may be “non-manifesting” Prevalence by Age Group # pe r m ill io n Photos care of FSH Society
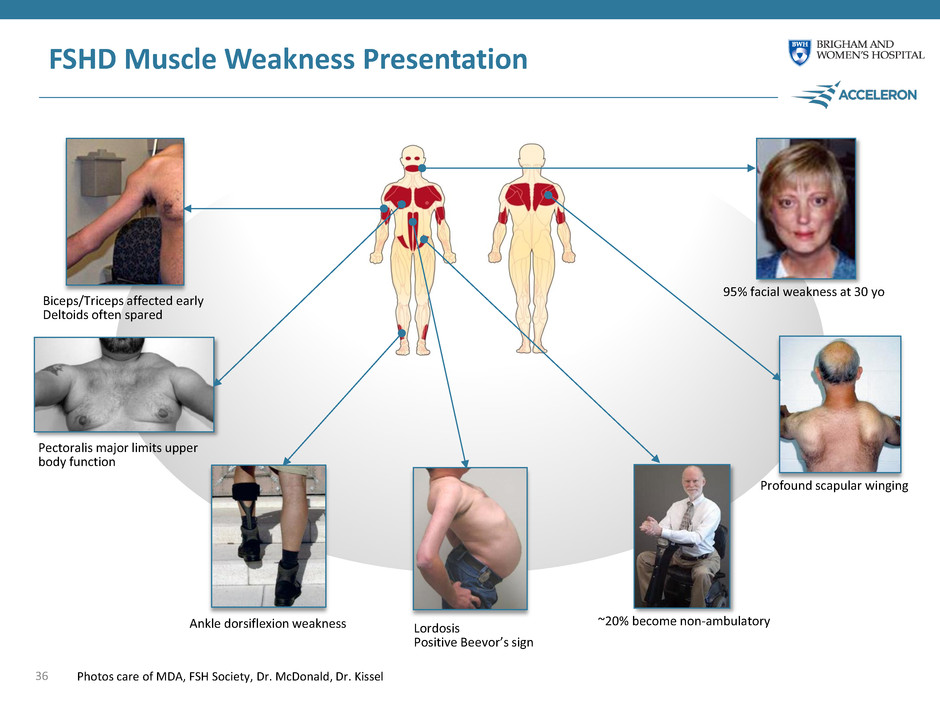
FSHD Muscle Weakness Presentation 36 ~20% become non-ambulatory Lordosis Positive Beevor’s sign Ankle dorsiflexion weakness Profound scapular winging 95% facial weakness at 30 yo Biceps/Triceps affected early Deltoids often spared Pectoralis major limits upper body function Photos care of MDA, FSH Society, Dr. McDonald, Dr. Kissel

FSHD Muscle Weakness Presentation 37 ~20% become non-ambulatory Lordosis Positive Beevor’s sign Ankle dorsiflexion weakness Profound scapular winging 95% facial weakness at 30 yo Biceps/Triceps affected early Deltoids often spared Pectoralis major limits upper body function Photos care of MDA, FSH Society, Dr. McDonald, Dr. Kissel
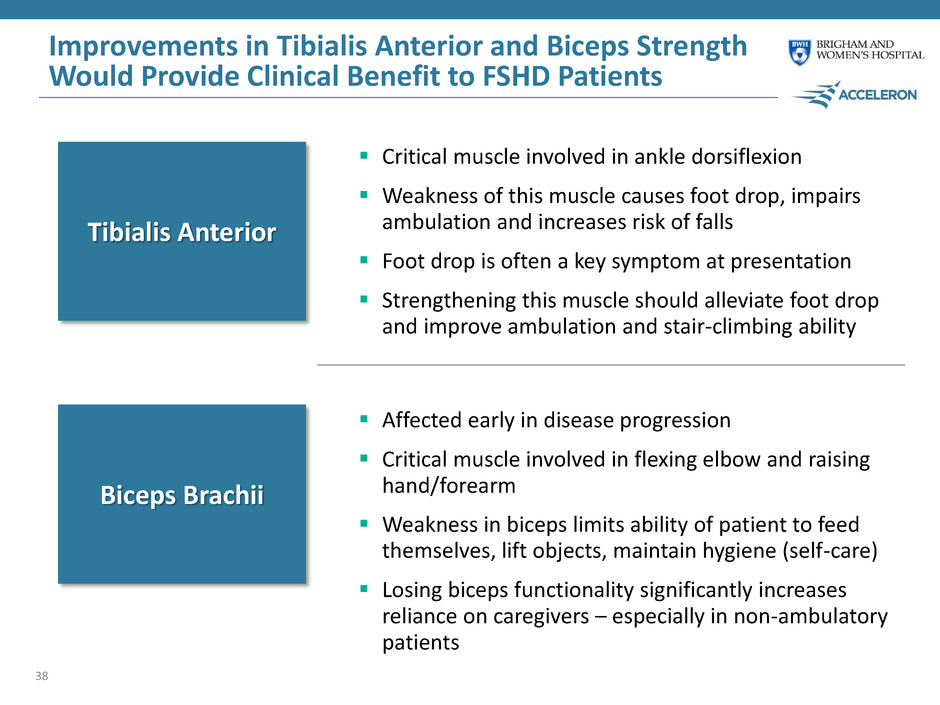
Improvements in Tibialis Anterior and Biceps Strength Would Provide Clinical Benefit to FSHD Patients 38 Critical muscle involved in ankle dorsiflexion Weakness of this muscle causes foot drop, impairs ambulation and increases risk of falls Foot drop is often a key symptom at presentation Strengthening this muscle should alleviate foot drop and improve ambulation and stair-climbing ability Tibialis Anterior Biceps Brachii Affected early in disease progression Critical muscle involved in flexing elbow and raising hand/forearm Weakness in biceps limits ability of patient to feed themselves, lift objects, maintain hygiene (self-care) Losing biceps functionality significantly increases reliance on caregivers – especially in non-ambulatory patients
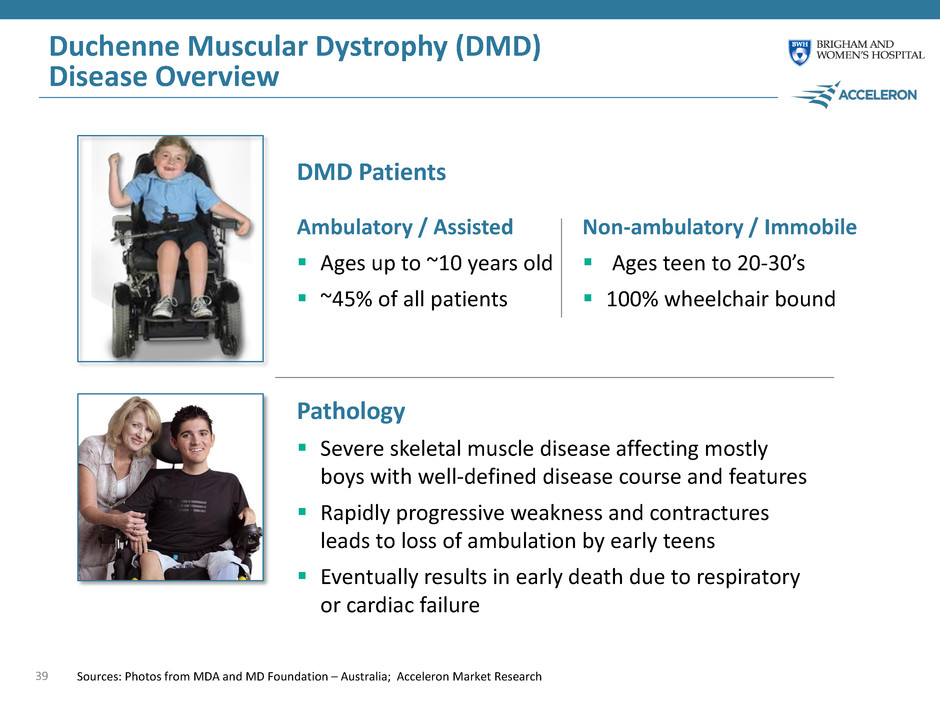
Duchenne Muscular Dystrophy (DMD) Disease Overview 39 Pathology Severe skeletal muscle disease affecting mostly boys with well-defined disease course and features Rapidly progressive weakness and contractures leads to loss of ambulation by early teens Eventually results in early death due to respiratory or cardiac failure DMD Patients Sources: Photos from MDA and MD Foundation – Australia; Acceleron Market Research Ambulatory / Assisted Ages up to ~10 years old ~45% of all patients Non-ambulatory / Immobile Ages teen to 20-30’s 100% wheelchair bound
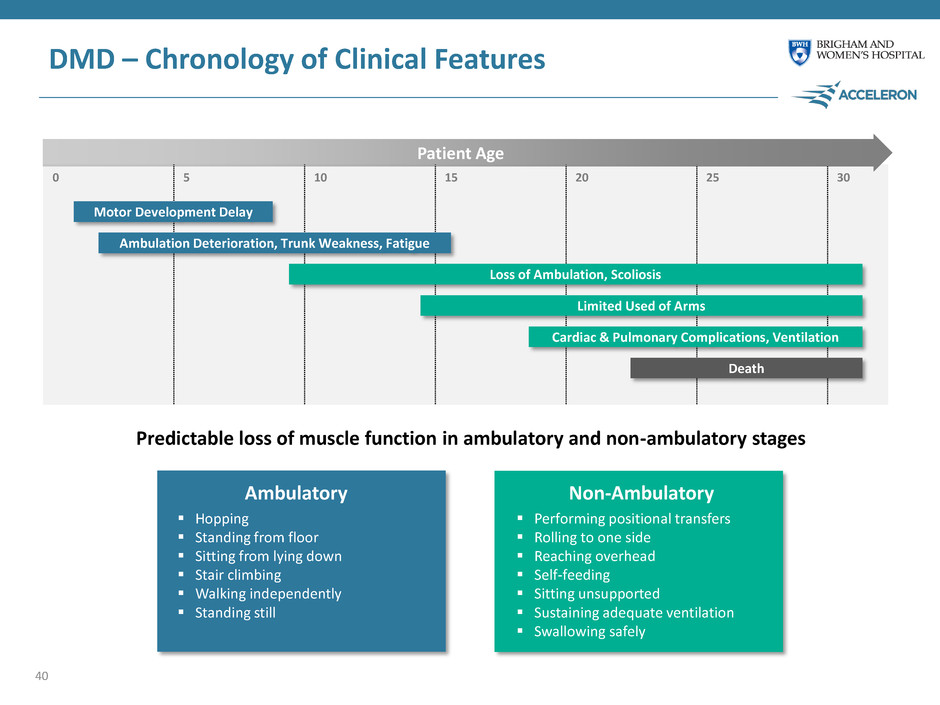
0 5 10 15 20 25 30 Predictable loss of muscle function in ambulatory and non-ambulatory stages Patient Age DMD – Chronology of Clinical Features Motor Development Delay Ambulation Deterioration, Trunk Weakness, Fatigue Loss of Ambulation, Scoliosis Limited Used of Arms Cardiac & Pulmonary Complications, Ventilation Death Hopping Standing from floor Sitting from lying down Stair climbing Walking independently Standing still Performing positional transfers Rolling to one side Reaching overhead Self-feeding Sitting unsupported Sustaining adequate ventilation Swallowing safely Ambulatory Non-Ambulatory 40

Dalantercept Matthew Sherman, M.D. Chief Medical Officer Acceleron 41

Dalantercept in Renal Cell Carcinoma (RCC) Renal cell carcinoma is a highly angiogenic tumor Anti-angiogenesis therapies have shown and continue to show robust efficacy in RCC Substantial opportunity for a new drug to build upon, not compete with, large established base of numerous approved VEGFR TKIs 42 Current Approach Physician uses preferred VEGFR TKI (sunitinib, axitinib, pazopanib) Dalantercept Opportunity Physician uses preferred VEGFR TKI (sunitinib, axitinib, pazopanib) Dalantercept BETTER OUTCOMES
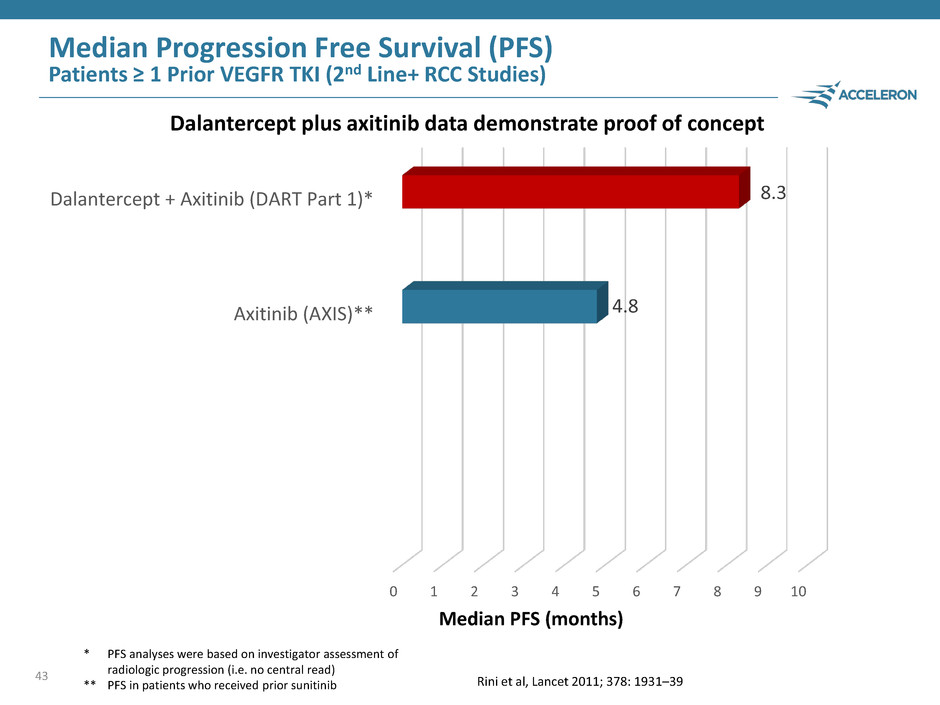
43 Median PFS (months) Dalantercept plus axitinib data demonstrate proof of concept * PFS analyses were based on investigator assessment of radiologic progression (i.e. no central read) ** PFS in patients who received prior sunitinib Median Progression Free Survival (PFS) Patients ≥ 1 Prior VEGFR TKI (2nd Line+ RCC Studies) 0 1 2 3 4 5 6 7 8 9 10 Sorafenib (AXIS) Everolimus (METEOR) Everolimus (CheckMate-025)* Nivolumab (CheckMate-025)* Axitinib (AXIS)** Cabozantinib (METEOR) Dalantercept + Axitinib (DART Part 1)* 4.8 8.3 Rini et al, Lancet 2011; 378: 1931–39
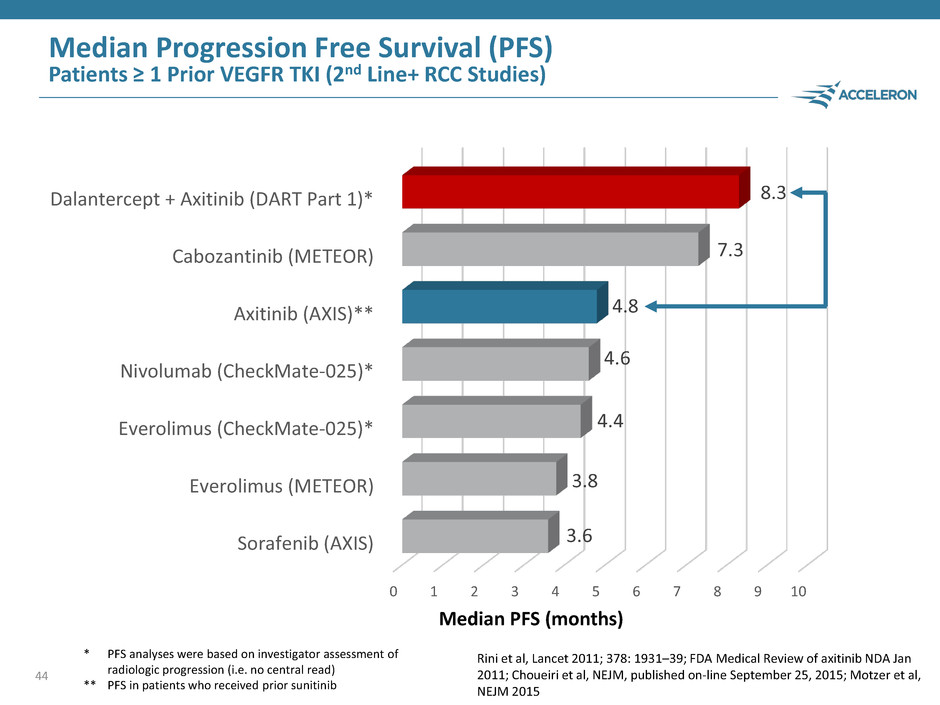
Median Progression Free Survival (PFS) Patients ≥ 1 Prior VEGFR TKI (2nd Line+ RCC Studies) 44 0 1 2 3 4 5 6 7 8 9 10 Sorafenib (AXIS) Everolimus (METEOR) Everolimus (CheckMate-025)* Nivolumab (CheckMate-025)* Axitinib (AXIS)** Cabozantinib (METEOR) Dalantercept + Axitinib (DART Part 1)* 3.6 3.8 4.4 4.6 4.8 7.3 8.3 Median PFS (months) * PFS analyses were based on investigator assessment of radiologic progression (i.e. no central read) ** PFS in patients who received prior sunitinib Rini et al, Lancet 2011; 378: 1931–39; FDA Medical Review of axitinib NDA Jan 2011; Choueiri et al, NEJM, published on-line September 25, 2015; Motzer et al, NEJM 2015
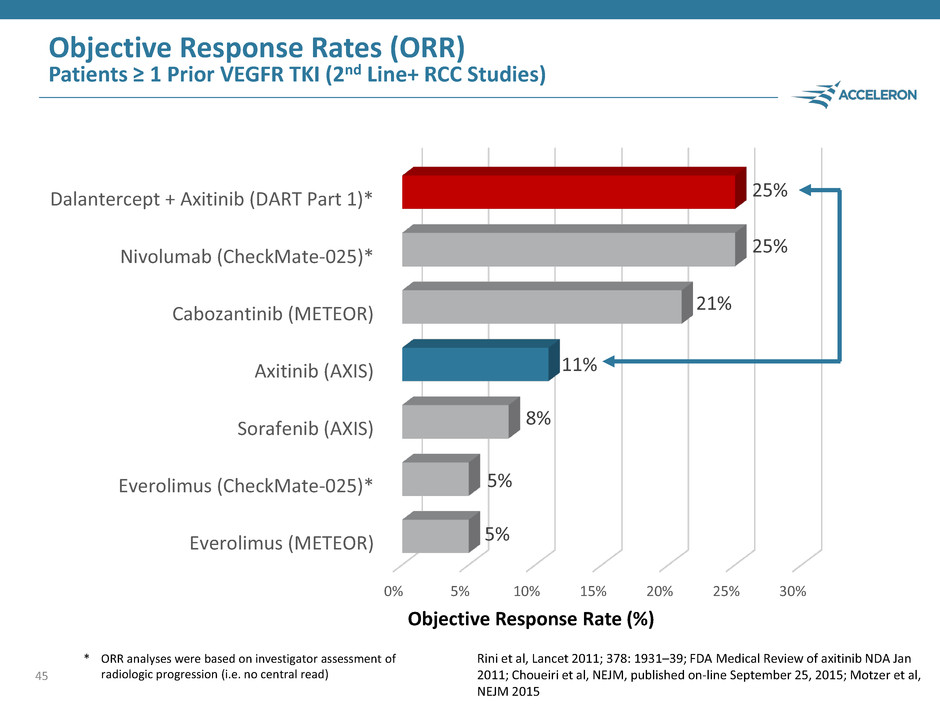
45 0% 5% 10% 15% 20% 25% 30% Everolimus (METEOR) Everolimus (CheckMate-025)* Sorafenib (AXIS) Axitinib (AXIS) Cabozantinib (METEOR) Nivolumab (CheckMate-025)* Dalantercept + Axitinib (DART Part 1)* 5% 5% 8% 11% 21% 25% 25% Objective Response Rate (%) Objective Response Rates (ORR) Patients ≥ 1 Prior VEGFR TKI (2nd Line+ RCC Studies) * ORR analyses were based on investigator assessment of radiologic progression (i.e. no central read) Rini et al, Lancet 2011; 378: 1931–39; FDA Medical Review of axitinib NDA Jan 2011; Choueiri et al, NEJM, published on-line September 25, 2015; Motzer et al, NEJM 2015
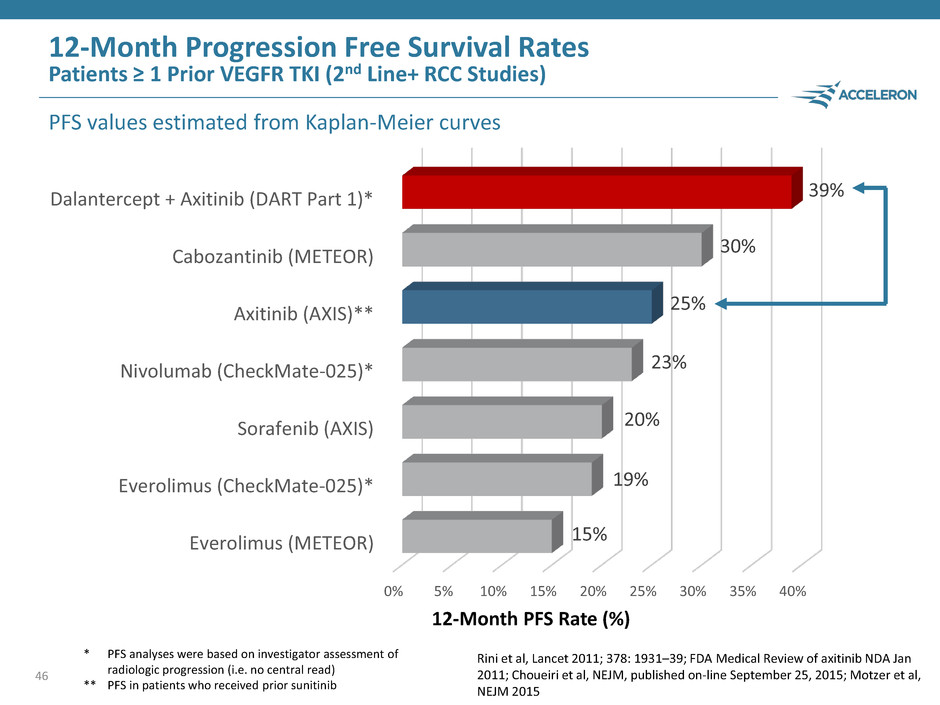
46 0% 5% 10% 15% 20% 25% 30% 35% 40% Everolimus (METEOR) Everolimus (CheckMate-025)* Sorafenib (AXIS) Nivolumab (CheckMate-025)* Axitinib (AXIS)** Cabozantinib (METEOR) Dalantercept + Axitinib (DART Part 1)* 15% 19% 20% 23% 25% 30% 39% 12-Month Progression Free Survival Rates Patients ≥ 1 Prior VEGFR TKI (2nd Line+ RCC Studies) PFS values estimated from Kaplan-Meier curves 12-Month PFS Rate (%) * PFS analyses were based on investigator assessment of radiologic progression (i.e. no central read) ** PFS in patients who received prior sunitinib Rini et al, Lancet 2011; 378: 1931–39; FDA Medical Review of axitinib NDA Jan 2011; Choueiri et al, NEJM, published on-line September 25, 2015; Motzer et al, NEJM 2015

Dalantercept in Renal Cell Carcinoma Dalantercept in combination with axitinib in Part 1 of the DART study produced clinical outcomes that exceed historical axitinib results Dalantercept safety profile was generally non-overlapping with VEGFR TKI and well-tolerated Enrollment ongoing in Part 2 of the DART study (130 patient, randomized, double-blind, placebo-controlled trial) PFS data from Part 2 of the DART study projected to be available end of 2016 47
Sotatercept in Chronic Kidney Disease John Knopf, Ph.D. Chief Executive Officer Acceleron 48
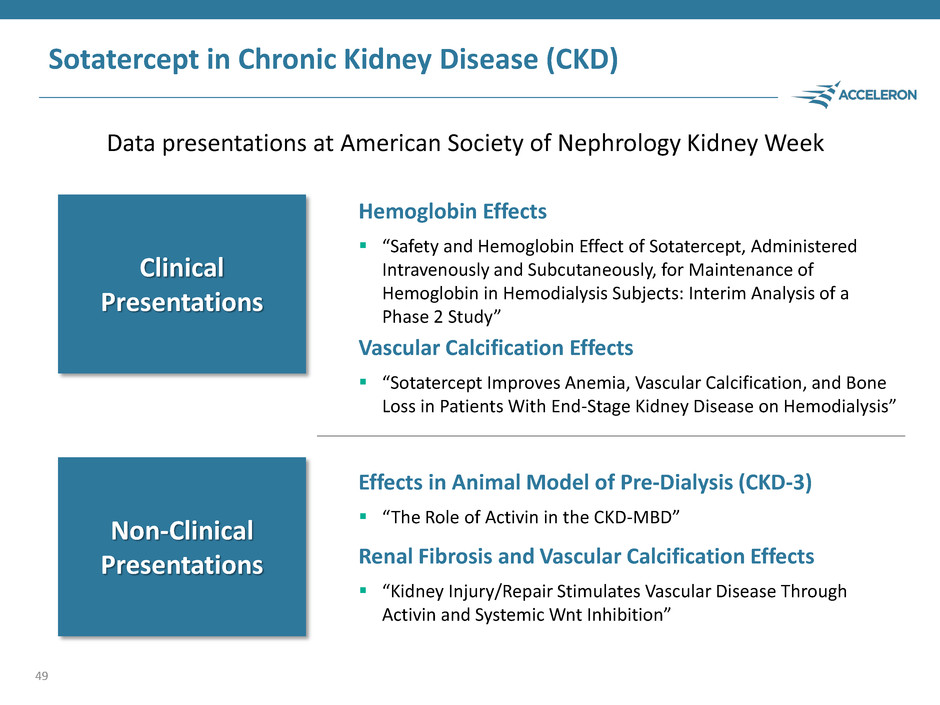
Sotatercept in Chronic Kidney Disease (CKD) Data presentations at American Society of Nephrology Kidney Week 49 Hemoglobin Effects “Safety and Hemoglobin Effect of Sotatercept, Administered Intravenously and Subcutaneously, for Maintenance of Hemoglobin in Hemodialysis Subjects: Interim Analysis of a Phase 2 Study” Vascular Calcification Effects “Sotatercept Improves Anemia, Vascular Calcification, and Bone Loss in Patients With End-Stage Kidney Disease on Hemodialysis” Effects in Animal Model of Pre-Dialysis (CKD-3) “The Role of Activin in the CKD-MBD” Renal Fibrosis and Vascular Calcification Effects “Kidney Injury/Repair Stimulates Vascular Disease Through Activin and Systemic Wnt Inhibition” Clinical Presentations Non-Clinical Presentations

Sotatercept Future Development Plan in CKD Refocus clinical development strategy from CKD dialysis to treatment of pre-dialysis CKD to better align with… – Preclinical and clinical activity on vascular calcification and renal fibrosis – Large patient population – Preferred reimbursement / access environment Expect to meet with health authorities in the first half of 2016 to discuss initiation of a study in pre-dialysis patients 50

Question & Answer Session 51 John Knopf, Ph.D. Chief Executive Officer Acceleron Matthew Sherman, M.D. Chief Medical Officer Acceleron Anthony Amato, M.D. Vice Chairman, Department of Neurology Chief, Neuromuscular Division Brigham and Women’s Hospital Boston, MA Steven Ertel Chief Operating Officer Acceleron

Beta-thalassemia and MDS Sessions Steven Ertel Chief Operating Officer Acceleron 52

Beta-thalassemia and MDS Sessions 53 Beta-thalassemia landscape and opportunity for luspatercept M. Domenica Cappellini, M.D. Director of the Department of Clinical Sciences Director of the Unit of Internal Medicine University of Milan Luspatercept Phase 3 program Jay Backstrom, M.D. M.P.H. Senior Vice President, Hematology & Oncology R&D, Global Head Regulatory Affairs, Celgene MDS landscape and opportunity for luspatercept Uwe Platzbecker, M.D Professor of Hematology and Head of the MDS Program University Hospital, Dresden, Germany Luspatercept Phase 3 program Jay Backstrom, M.D. M.P.H. Senior Vice President, Hematology & Oncology R&D, Global Head Regulatory Affairs, Celgene Luspatercept Development Program in MDS Steven Ertel Executive Vice President Chief Operating Officer, Acceleron Beta- Thalassemia MDS

Beta-thalassemia M. Domenica Cappellini, M.D. Director of the Department of Clinical Sciences, Director of the Unit of Internal Medicine, University of Milan, Italy 54

Beta-thalassemia Disease Description Beta-thalassemia is an inherited disorder resulting in severe and chronic anemia, often beginning in the first years of life Genetic defect impacts production of hemoglobin and results in ineffective erythropoiesis (inability of red blood cells (RBC) cells to mature) and hemolysis (cell death) that cause anemia Due to severe anemia, most patients require blood transfusions and suffer from complications that arise from iron overload in the heart and other major organs that can be life threatening Prevalent in areas surrounding the Mediterranean and Southeast Asia with very large populations in other geographies – a truly global disease 55
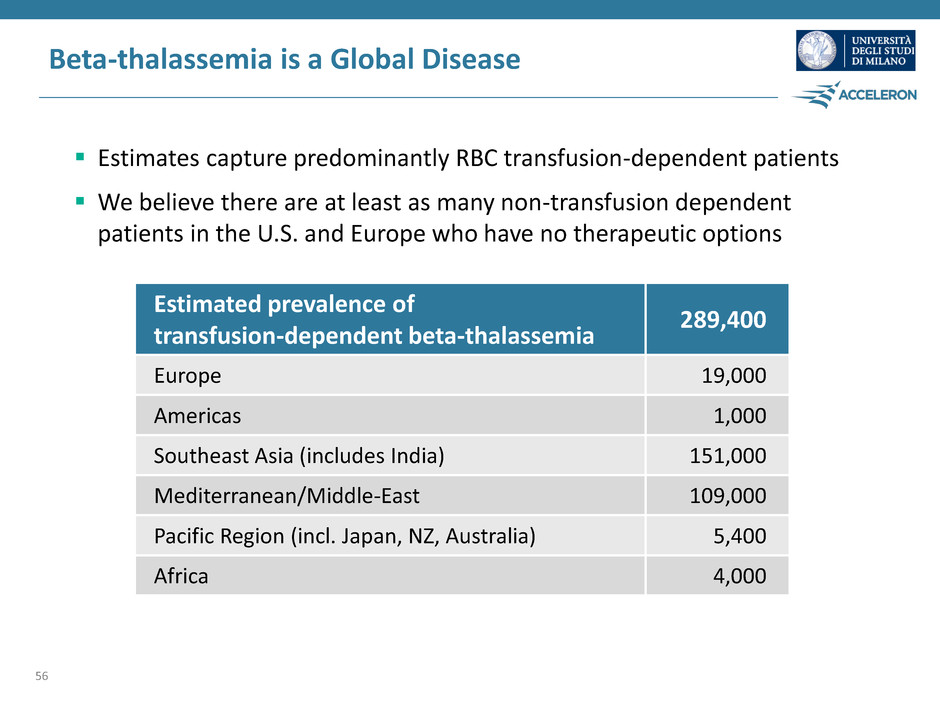
Beta-thalassemia is a Global Disease Estimates capture predominantly RBC transfusion-dependent patients We believe there are at least as many non-transfusion dependent patients in the U.S. and Europe who have no therapeutic options 56 Estimated prevalence of transfusion-dependent beta-thalassemia 289,400 Europe 19,000 Americas 1,000 Southeast Asia (includes India) 151,000 Mediterranean/Middle-East 109,000 Pacific Region (incl. Japan, NZ, Australia) 5,400 Africa 4,000
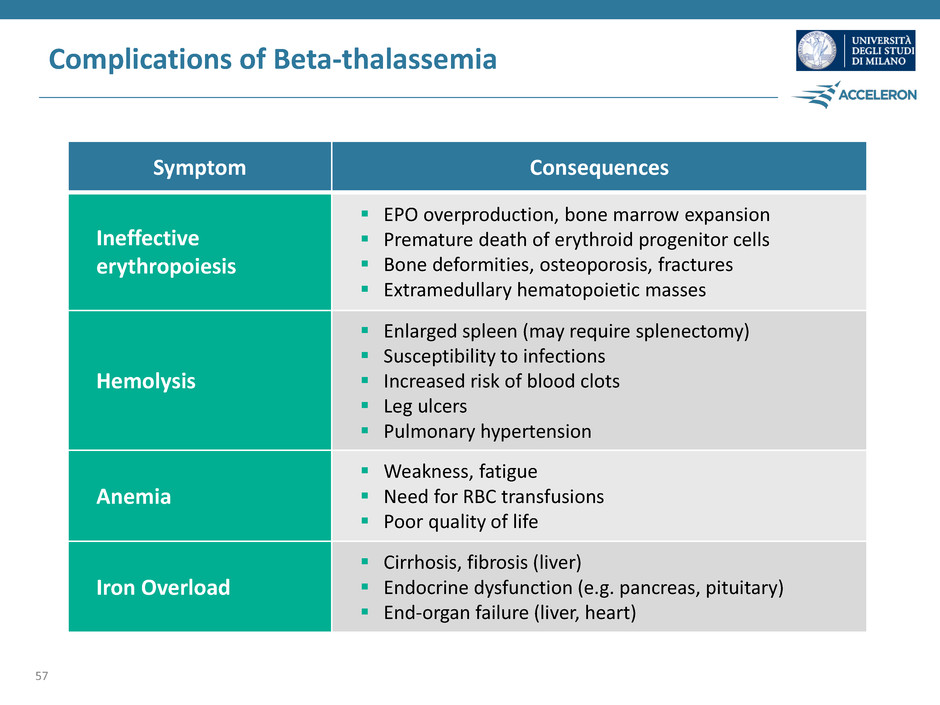
Complications of Beta-thalassemia 57 Symptom Consequences Ineffective erythropoiesis EPO overproduction, bone marrow expansion Premature death of erythroid progenitor cells Bone deformities, osteoporosis, fractures Extramedullary hematopoietic masses Hemolysis Enlarged spleen (may require splenectomy) Susceptibility to infections Increased risk of blood clots Leg ulcers Pulmonary hypertension Anemia Weakness, fatigue Need for RBC transfusions Poor quality of life Iron Overload Cirrhosis, fibrosis (liver) Endocrine dysfunction (e.g. pancreas, pituitary) End-organ failure (liver, heart)

Current Treatment Options Red blood cell transfusions Iron chelation therapy Hematopoietic stem cell transplant 58
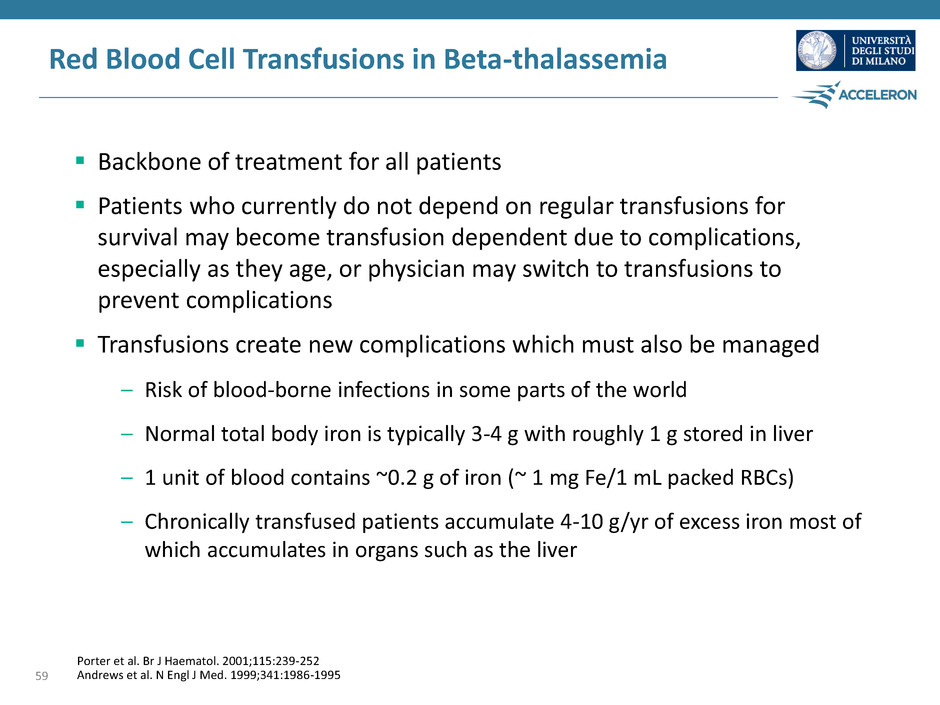
Red Blood Cell Transfusions in Beta-thalassemia Backbone of treatment for all patients Patients who currently do not depend on regular transfusions for survival may become transfusion dependent due to complications, especially as they age, or physician may switch to transfusions to prevent complications Transfusions create new complications which must also be managed – Risk of blood-borne infections in some parts of the world – Normal total body iron is typically 3-4 g with roughly 1 g stored in liver – 1 unit of blood contains ~0.2 g of iron (~ 1 mg Fe/1 mL packed RBCs) – Chronically transfused patients accumulate 4-10 g/yr of excess iron most of which accumulates in organs such as the liver 59 Porter et al. Br J Haematol. 2001;115:239-252 Andrews et al. N Engl J Med. 1999;341:1986-1995
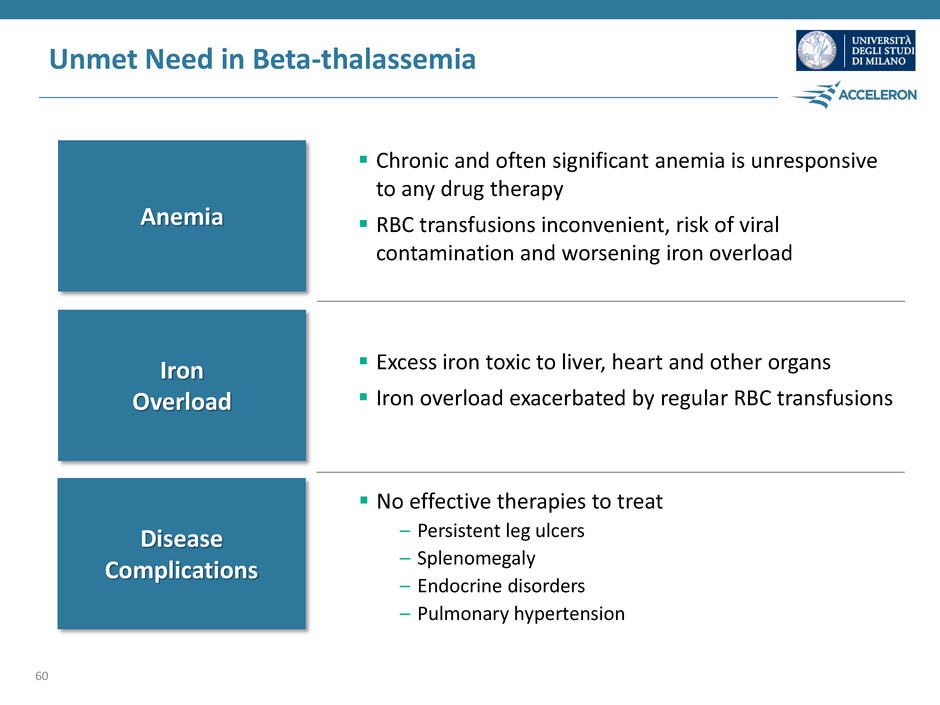
Unmet Need in Beta-thalassemia 60 Anemia Iron Overload Disease Complications No effective therapies to treat – Persistent leg ulcers – Splenomegaly – Endocrine disorders – Pulmonary hypertension Chronic and often significant anemia is unresponsive to any drug therapy RBC transfusions inconvenient, risk of viral contamination and worsening iron overload Excess iron toxic to liver, heart and other organs Iron overload exacerbated by regular RBC transfusions
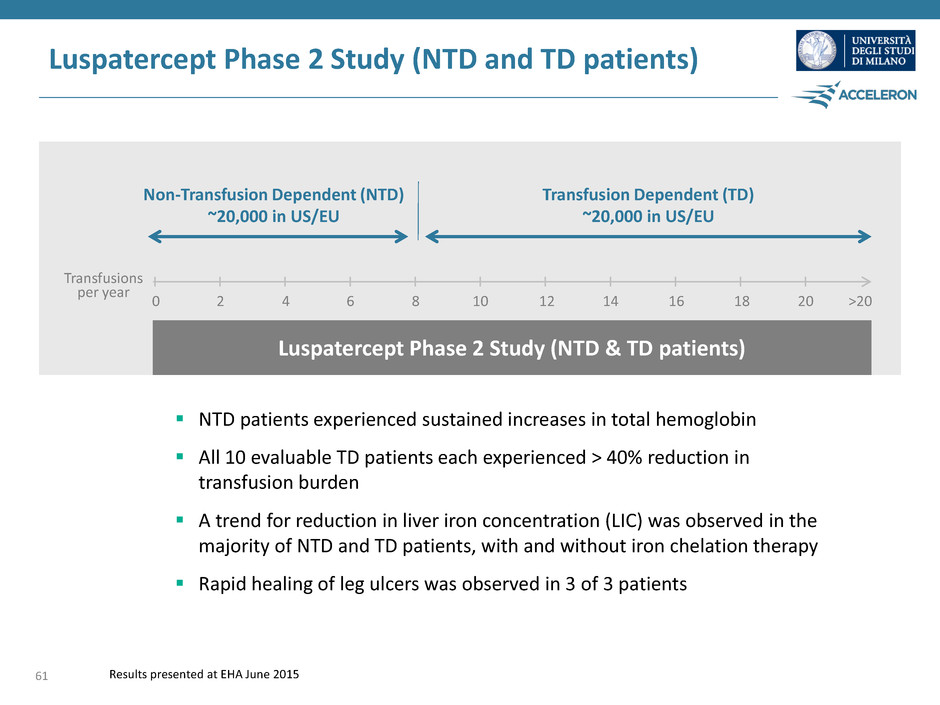
0 2 4 6 8 10 12 14 16 18 20 >20 Non-Transfusion Dependent (NTD) ~20,000 in US/EU Transfusion Dependent (TD) ~20,000 in US/EU Transfusions per year Luspatercept Phase 2 Study (NTD & TD patients) Luspatercept Phase 2 Study (NTD and TD patients) NTD patients experienced sustained increases in total hemoglobin All 10 evaluable TD patients each experienced > 40% reduction in transfusion burden A trend for reduction in liver iron concentration (LIC) was observed in the majority of NTD and TD patients, with and without iron chelation therapy Rapid healing of leg ulcers was observed in 3 of 3 patients 61 Results presented at EHA June 2015
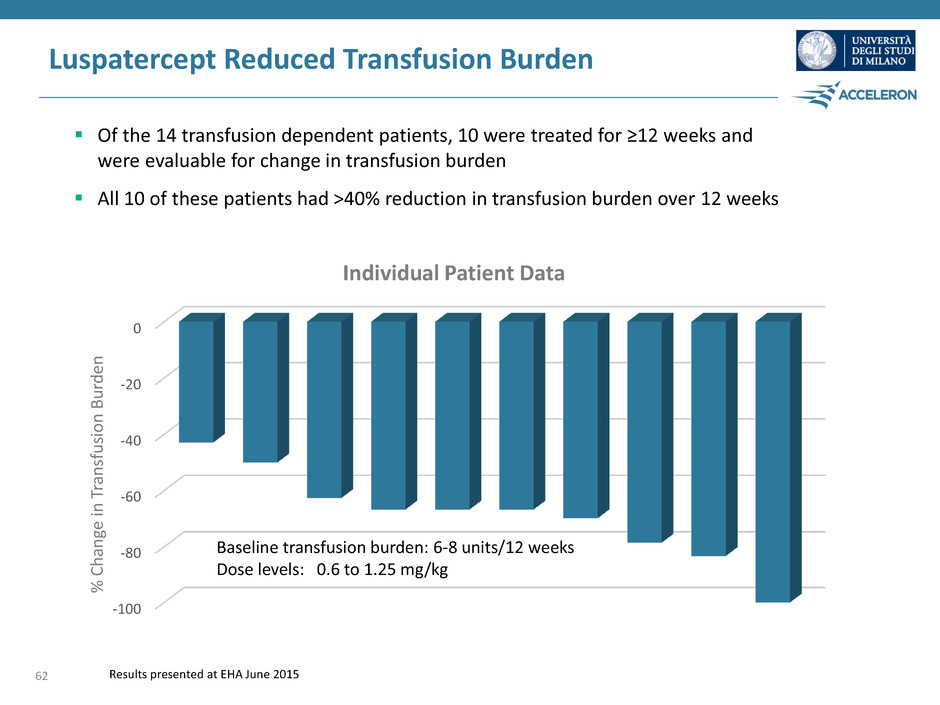
Luspatercept Reduced Transfusion Burden Of the 14 transfusion dependent patients, 10 were treated for ≥12 weeks and were evaluable for change in transfusion burden All 10 of these patients had >40% reduction in transfusion burden over 12 weeks Results presented at EHA June 2015 -100 -80 -60 -40 -20 0 Baseline transfusion burden: 6-8 units/12 weeks Dose levels: 0.6 to 1.25 mg/kg Individual Patient Data % C h an ge in T ra n sfusi o n B u rd en 62

63 Luspatercept Phase 3 Study in Beta-thalassemia Jay Backstrom, M.D. M.P.H. Senior Vice President, Hematology & Oncology R&D, Global Head Regulatory Affairs, Celgene

The BELIEVE Study Phase 3 study of luspatercept in beta-thalassemia Phase 3, double-blind, randomized, placebo-controlled study of luspatercept in regularly transfused beta-thalassemia patients 64
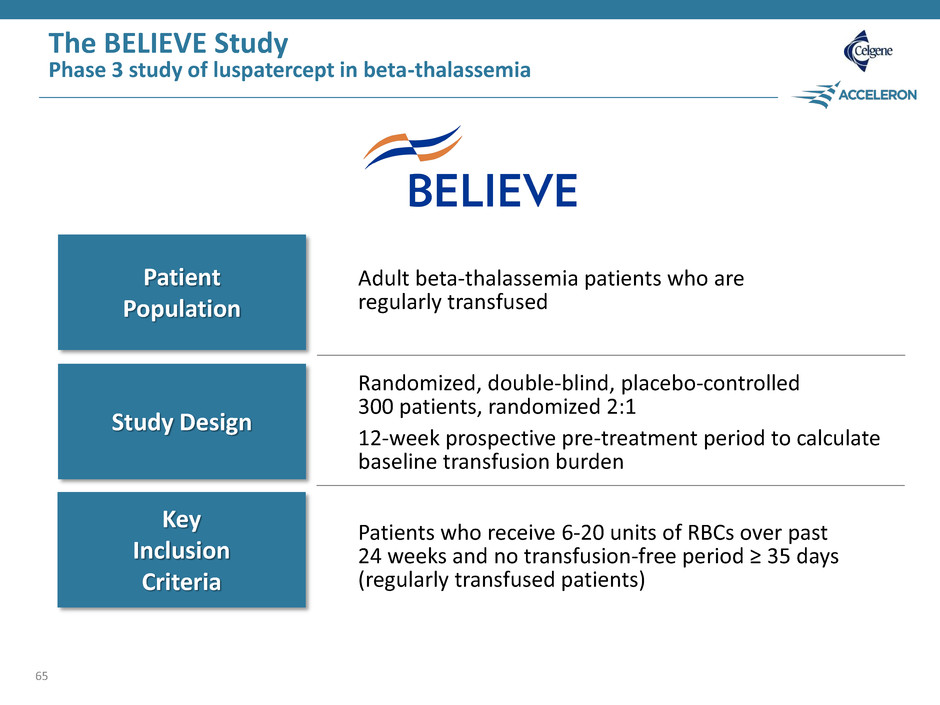
The BELIEVE Study Phase 3 study of luspatercept in beta-thalassemia 65 Patient Population Study Design Key Inclusion Criteria Adult beta-thalassemia patients who are regularly transfused Randomized, double-blind, placebo-controlled 300 patients, randomized 2:1 12-week prospective pre-treatment period to calculate baseline transfusion burden Patients who receive 6-20 units of RBCs over past 24 weeks and no transfusion-free period ≥ 35 days (regularly transfused patients)
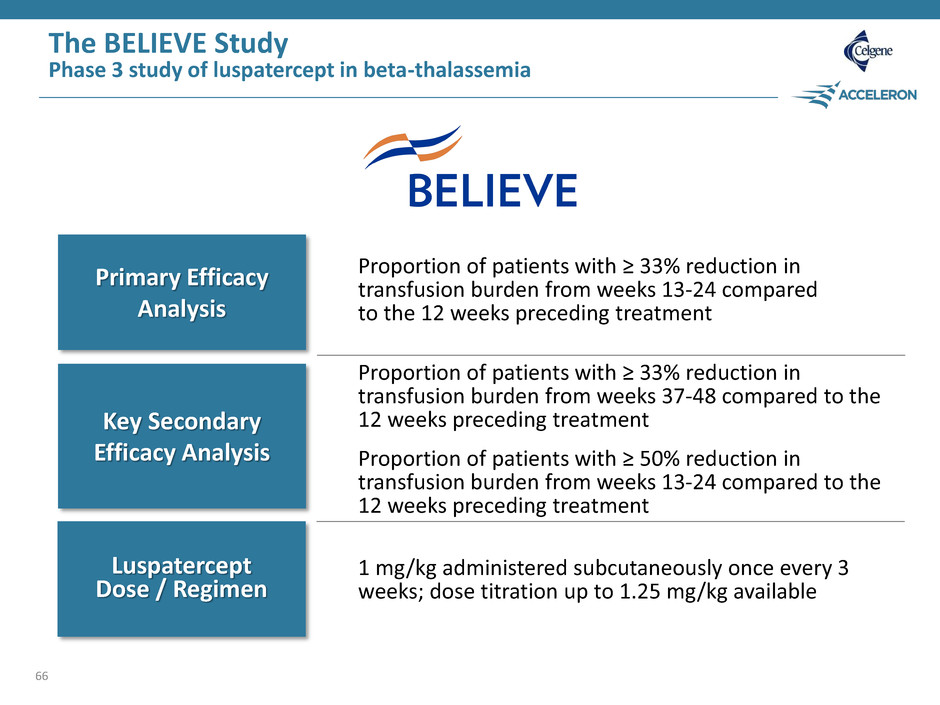
The BELIEVE Study Phase 3 study of luspatercept in beta-thalassemia 66 Primary Efficacy Analysis Key Secondary Efficacy Analysis Luspatercept Dose / Regimen Proportion of patients with ≥ 33% reduction in transfusion burden from weeks 13-24 compared to the 12 weeks preceding treatment Proportion of patients with ≥ 33% reduction in transfusion burden from weeks 37-48 compared to the 12 weeks preceding treatment Proportion of patients with ≥ 50% reduction in transfusion burden from weeks 13-24 compared to the 12 weeks preceding treatment 1 mg/kg administered subcutaneously once every 3 weeks; dose titration up to 1.25 mg/kg available
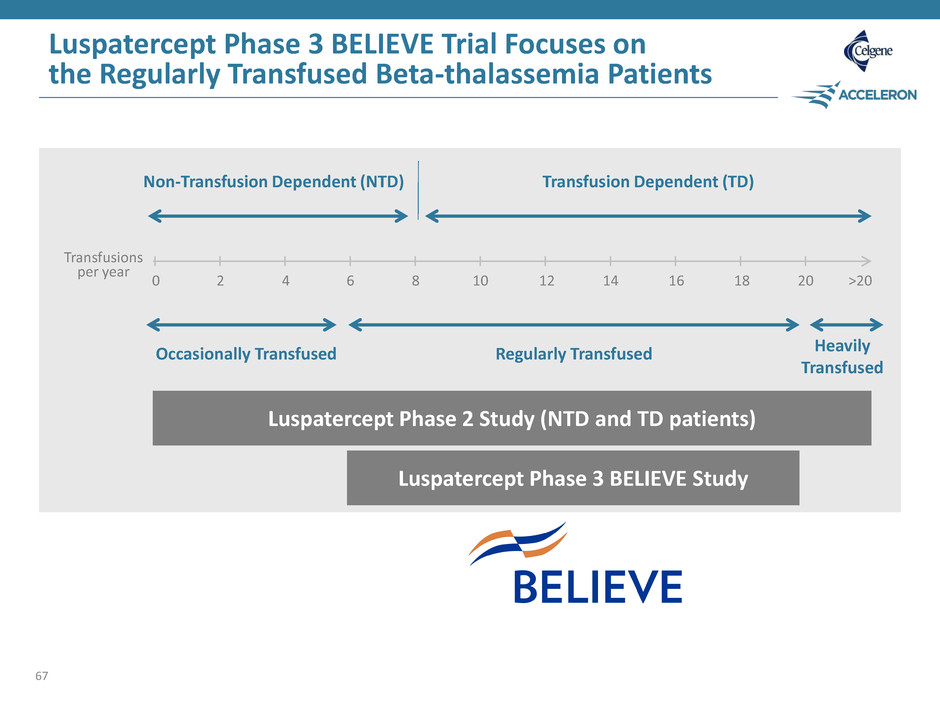
0 2 4 6 8 10 12 14 16 18 20 >20 Non-Transfusion Dependent (NTD) Transfusion Dependent (TD) Transfusions per year Luspatercept Phase 3 BELIEVE Study Luspatercept Phase 3 BELIEVE Trial Focuses on the Regularly Transfused Beta-thalassemia Patients 67 Occasionally Transfused Regularly Transfused Heavily Transfused Luspatercept Phase 2 Study (NTD and TD patients)
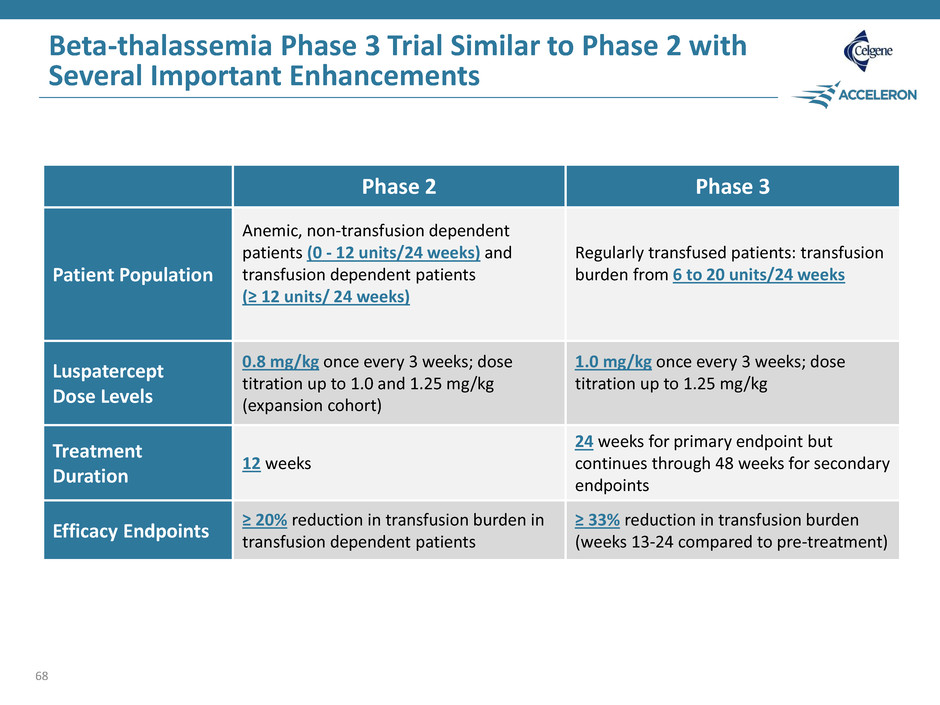
Beta-thalassemia Phase 3 Trial Similar to Phase 2 with Several Important Enhancements 68 Phase 2 Phase 3 Patient Population Anemic, non-transfusion dependent patients (0 - 12 units/24 weeks) and transfusion dependent patients (≥ 12 units/ 24 weeks) Regularly transfused patients: transfusion burden from 6 to 20 units/24 weeks Luspatercept Dose Levels 0.8 mg/kg once every 3 weeks; dose titration up to 1.0 and 1.25 mg/kg (expansion cohort) 1.0 mg/kg once every 3 weeks; dose titration up to 1.25 mg/kg Treatment Duration 12 weeks 24 weeks for primary endpoint but continues through 48 weeks for secondary endpoints Efficacy Endpoints ≥ 20% reduction in transfusion burden in transfusion dependent patients ≥ 33% reduction in transfusion burden (weeks 13-24 compared to pre-treatment)

Myelodysplastic Syndromes (MDS) 69

Myelodysplastic Syndromes Uwe Platzbecker, M.D. Professor of Hematology and Head of the MDS Program University Hospital Dresden, Germany 70

Myelodysplastic Syndromes (MDS) Disease Description Hematologic malignancy resulting in multiple cytopenias (anemia, neutropenia, thrombocytopenia) – Patients classified by risk (very low - > very high) of progression to AML – Roughly 100,000 lower risk MDS patients in the U.S. and E.U. Disease of the elderly; median age at diagnosis ~70 years old Nearly all MDS patients have anemia, which is caused by ineffective erythropoiesis Substantial impact of anemia in this older patient population includes significant symptom burden (e.g. chest pain) and poor quality of life 71
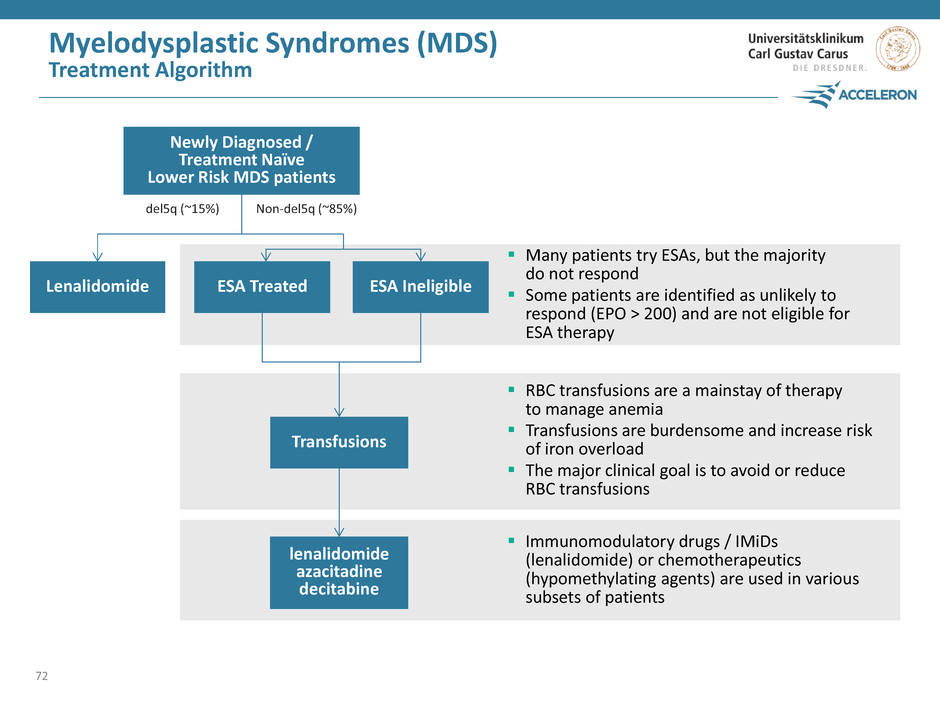
Myelodysplastic Syndromes (MDS) Treatment Algorithm 72 Many patients try ESAs, but the majority do not respond Some patients are identified as unlikely to respond (EPO > 200) and are not eligible for ESA therapy RBC transfusions are a mainstay of therapy to manage anemia Transfusions are burdensome and increase risk of iron overload The major clinical goal is to avoid or reduce RBC transfusions Immunomodulatory drugs / IMiDs (lenalidomide) or chemotherapeutics (hypomethylating agents) are used in various subsets of patients ESA Treated lenalidomide azacitadine decitabine Transfusions ESA Ineligible Lenalidomide del5q (~15%) Non-del5q (~85%) Newly Diagnosed / Treatment Naïve Lower Risk MDS patients
Unmet Need in Lower Risk MDS There are limited treatment options to treat anemia that are suitable for the lower risk MDS patients There is substantial unmet medical need for safe and well-tolerated drugs that can be used early in the treatment algorithm to avoid or reduce red blood cell transfusions The ability to prospectively identify patients who are likely to benefit from a therapy is important from both a clinical and payer perspective in this heterogeneous disease 73 Luspatercept PACE-MDS Phase 2 study designed to determine if luspatercept could address these unmet needs
Luspatercept Phase 2 Study in Lower Risk MDS Key eligibility criteria: – Lower risk MDS – Nonresponsive or refractory to prior ESA treatment – No prior ESA treatment or ESA ineligible (EPO >500 U/L) – No prior azacitidine or decitabine – No current lenalidomide, ESA, G-CSF Luspatercept treatment once every 3 weeks for 12 weeks (5 doses) Clinical goal: RBC transfusion independence 74
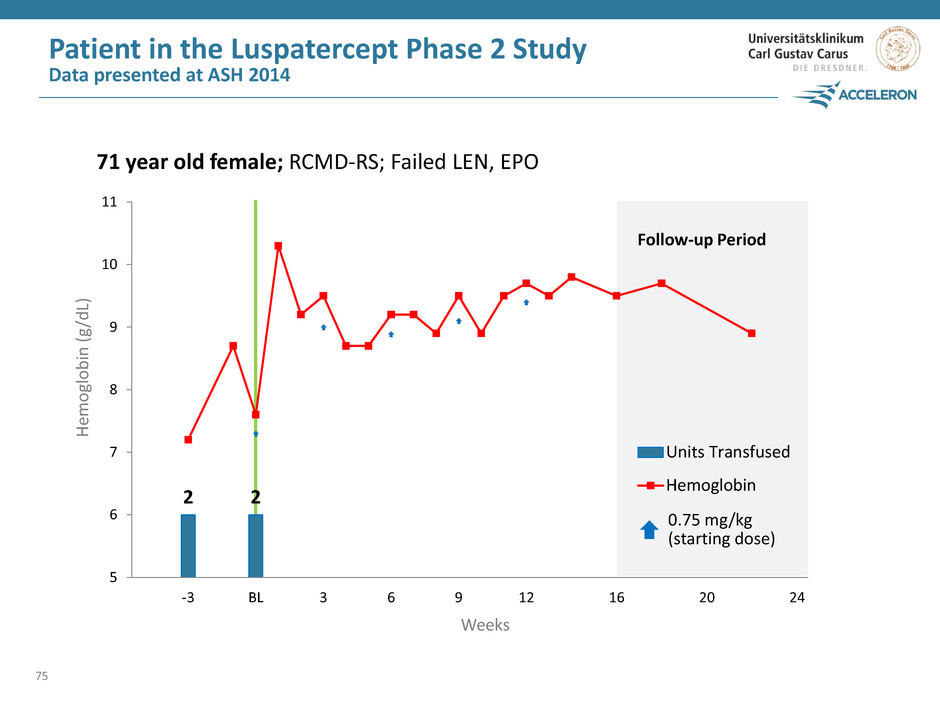
2 2 0 2 4 6 8 10 12 5 6 7 8 9 10 11 -3 BL 3 6 9 12 16 20 24 Units Transfused Hemoglobin Patient in the Luspatercept Phase 2 Study Data presented at ASH 2014 75 Weeks He m ogl o b in ( g /d L) Follow-up Period 0.75 mg/kg (starting dose) 71 year old female; RCMD-RS; Failed LEN, EPO

Luspatercept Phase 2 Results and Perspective Data presented at EHA 2015 With only 12 weeks of treatment, 11/30 (37%) patients achieved RBC-transfusion independence Longer-term treatment maintained transfusion independence A biomarker (ring sideroblasts) associated with ineffective erythropoiesis identified to select patients more likely to respond Luspatercept was generally safe and well-tolerated Luspatercept has the potential to be an important therapy early in the treatment for lower risk MDS patents Compelling Phase 2 clinical results led to the phase 3 program 76

77 Luspatercept Phase 3 Study in MDS Jay Backstrom, M.D. M.P.H. Senior Vice President, Hematology & Oncology R&D, Global Head Regulatory Affairs, Celgene

The MEDALIST Study Phase 3 study of luspatercept in MDS Phase 3, double-blind, randomized, placebo-controlled study of luspatercept in very low to intermediate risk myelodysplastic syndromes patients 78

The MEDALIST Study Phase 3 study of luspatercept in MDS 79 Patient Population Study Design Key Inclusion Criteria Very low, low or intermediate risk MDS patients with ring sideroblasts who require RBC transfusions Randomized, double-blind, placebo-controlled 210 patients, randomized 2:1 (140 luspatercept, 70 placebo) Refractory / intolerant to prior ESA or ESA ineligible (EPO > 200 U/L) Ring sideroblast positive Receive at least 2 units of RBCs every 8 weeks confirmed for a minimum of 16 weeks, no consecutive 8 week transfusion free No prior lenalidomide, hypomethylating agents or immunosuppressive therapy Excluded patients: Del5q, secondary MDS
The MEDALIST Study Phase 3 study of luspatercept in MDS 80 Primary Efficacy Analysis Key Secondary Efficacy Analysis Luspatercept Dose / Regimen Proportion of patients that become RBC-transfusion independent (≥ 8 weeks) during the first 24 weeks Proportion of patients that become RBC-transfusion independent (≥ 12 weeks) during the first 24 weeks 1 mg/kg administered subcutaneously once every 3 weeks; dose titration up to 1.33 and 1.75 mg/kg available
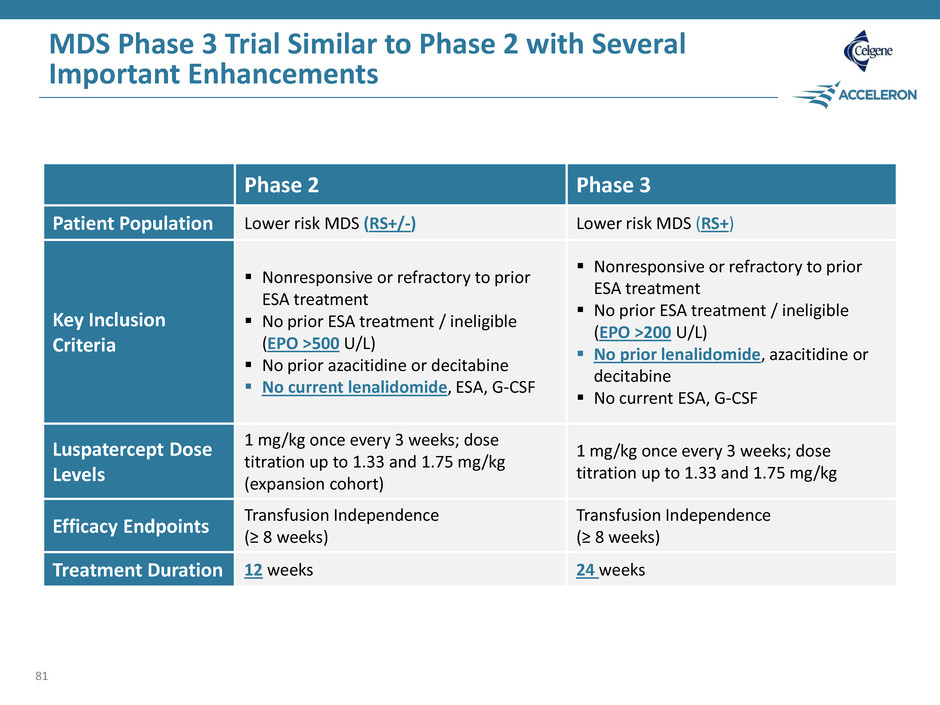
MDS Phase 3 Trial Similar to Phase 2 with Several Important Enhancements 81 Phase 2 Phase 3 Patient Population Lower risk MDS (RS+/-) Lower risk MDS (RS+) Key Inclusion Criteria Nonresponsive or refractory to prior ESA treatment No prior ESA treatment / ineligible (EPO >500 U/L) No prior azacitidine or decitabine No current lenalidomide, ESA, G-CSF Nonresponsive or refractory to prior ESA treatment No prior ESA treatment / ineligible (EPO >200 U/L) No prior lenalidomide, azacitidine or decitabine No current ESA, G-CSF Luspatercept Dose Levels 1 mg/kg once every 3 weeks; dose titration up to 1.33 and 1.75 mg/kg (expansion cohort) 1 mg/kg once every 3 weeks; dose titration up to 1.33 and 1.75 mg/kg Efficacy Endpoints Transfusion Independence (≥ 8 weeks) Transfusion Independence (≥ 8 weeks) Treatment Duration 12 weeks 24 weeks

Luspatercept Development Program in Beta-thalassemia and MDS Steven Ertel Chief Operating Officer Acceleron 82
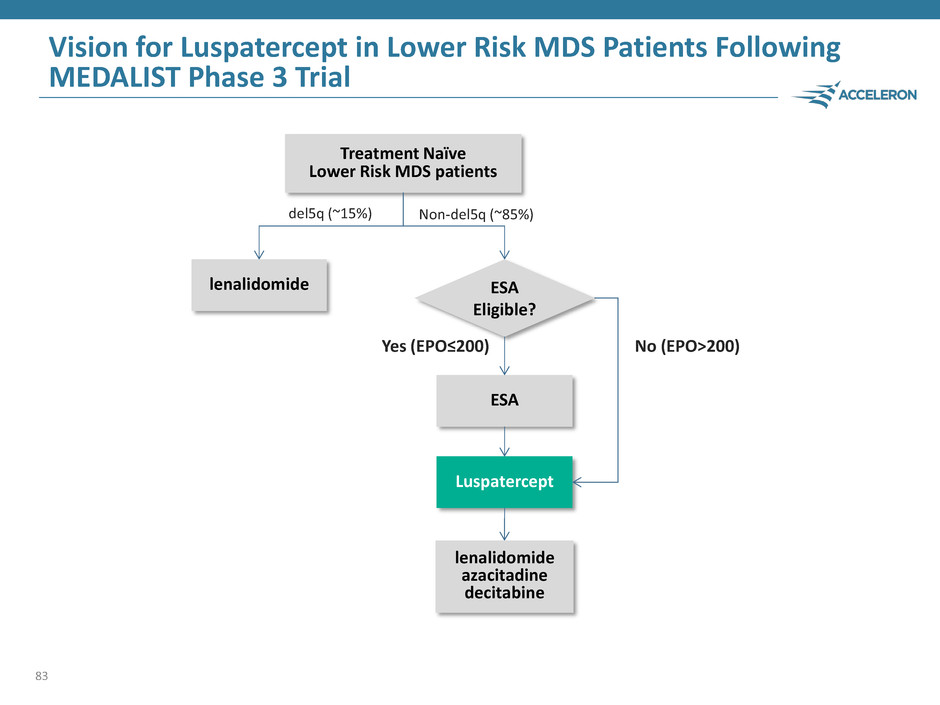
Vision for Luspatercept in Lower Risk MDS Patients Following MEDALIST Phase 3 Trial 83 ESA Treatment Naïve Lower Risk MDS patients Luspatercept lenalidomide del5q (~15%) Non-del5q (~85%) ESA Eligible? Yes (EPO≤200) No (EPO>200) lenalidomide azacitadine decitabine
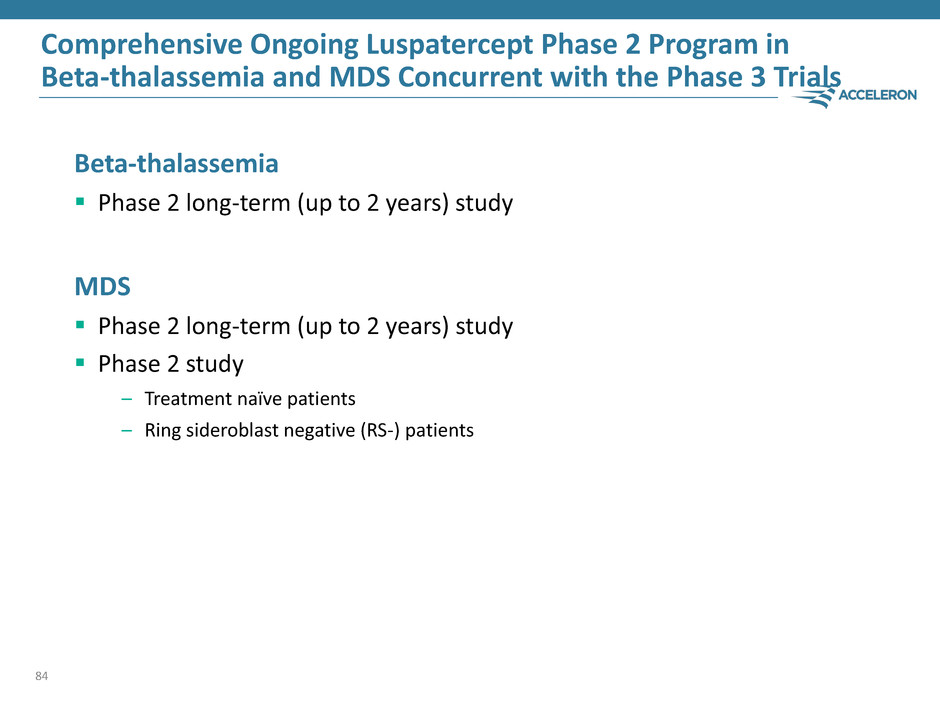
Comprehensive Ongoing Luspatercept Phase 2 Program in Beta-thalassemia and MDS Concurrent with the Phase 3 Trials Beta-thalassemia Phase 2 long-term (up to 2 years) study MDS Phase 2 long-term (up to 2 years) study Phase 2 study – Treatment naïve patients – Ring sideroblast negative (RS-) patients 84

Question & Answer Session 85 M. Domenica Cappellini, M.D. Director of the Department of Clinical Sciences Director of the Unit of Internal Medicine University of Milan Uwe Platzbecker, M.D. Professor of Hematology and Head of the MDS Program University Hospital Dresden, Germany Jay Backstrom, M.D. M.P.H. Senior Vice President, Hematology & Oncology R&D, Global Head Regulatory Affairs, Celgene Steven Ertel Executive Vice President Chief Operating Officer Acceleron

R&D Day Summary Steven Ertel Chief Operating Officer Acceleron 86
R&D Day Highlights The luspatercept Phase 3 studies in MDS and beta-thalassemia, in partnership with Celgene, positions Acceleron closer to becoming a fully integrated biotech company Dalantercept data from Part 1 of the DART study provides proof of concept that combination of dalantercept plus VEGFR TKI can produce best-in-class results Sotatercept development strategy refocused on the pre-dialysis setting ACE-083 generated substantial increases in muscle mass with just 1-2 doses and will be advanced to Phase 2 in mid-2016 IntelliTrap™ platform creates a large and diverse library of new, selective therapeutic candidates ACE-2494, the muscle therapeutic candidate and first program from the IntelliTrap™ platform is targeted to enter clinical trials by the end of 2016 87
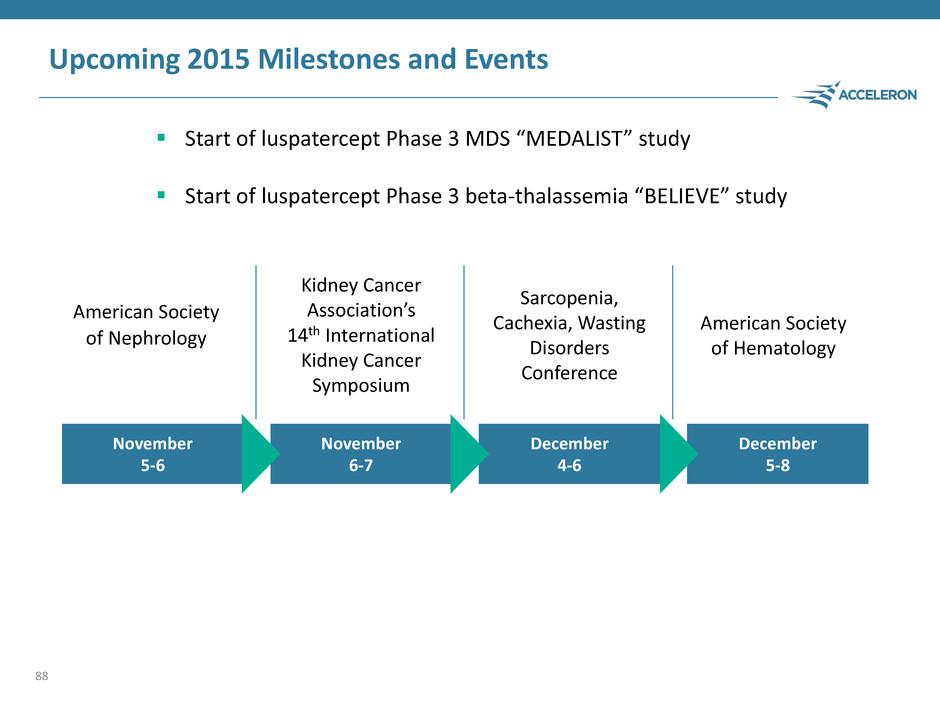
Upcoming 2015 Milestones and Events 88 November 6-7 December 5-8 December 4-6 November 5-6 American Society of Nephrology Kidney Cancer Association’s 14th International Kidney Cancer Symposium Sarcopenia, Cachexia, Wasting Disorders Conference American Society of Hematology Start of luspatercept Phase 3 MDS “MEDALIST” study Start of luspatercept Phase 3 beta-thalassemia “BELIEVE” study

Acceleron is Building One of the Industry’s Most Robust Clinical Pipelines 89 Luspatercept - MDS Luspatercept - Thalassemia Sotatercept – CKD Sotatercept - Myelofibrosis Sotatercept – Myeloma ACE-083 - FSHD Dalantercept - RCC Dalantercept - HCC Phase 1 Sotatercept – DBA Phase 2 Phase 3 Four internally discovered drugs – Luspatercept – Sotatercept – Dalantercept – ACE-083 Two phase 3 programs – MDS – Beta-thalassemia Numerous Phase 2 programs
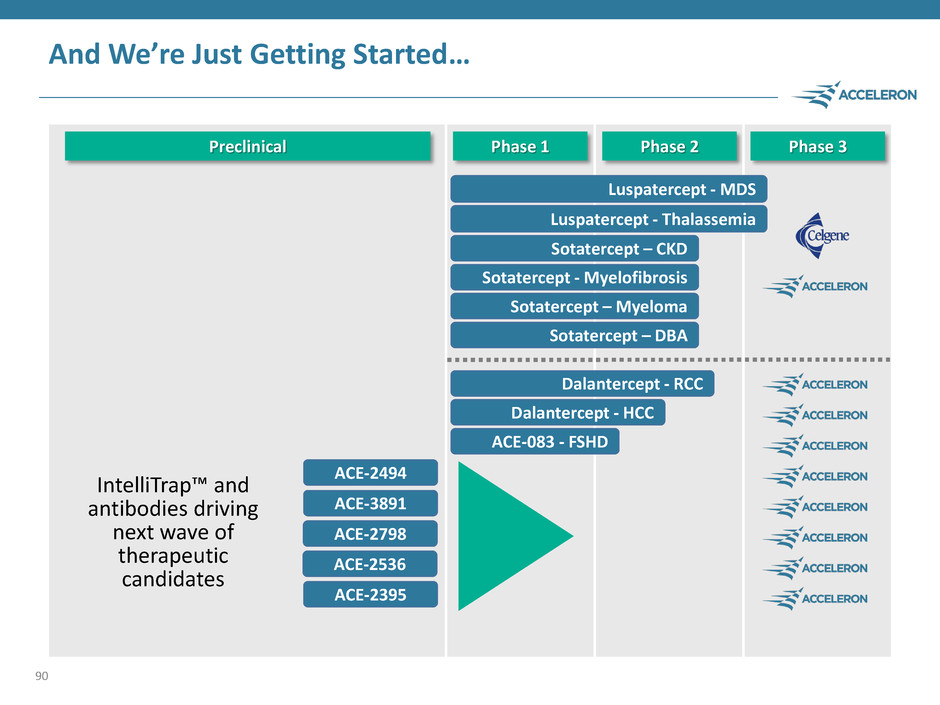
And We’re Just Getting Started… 90 Luspatercept - MDS Luspatercept - Thalassemia Sotatercept – CKD Sotatercept - Myelofibrosis Sotatercept – Myeloma ACE-083 - FSHD Dalantercept - RCC Dalantercept - HCC Phase 1 Sotatercept – DBA Phase 2 Phase 3 Preclinical ACE-2494 ACE-3891 ACE-2798 ACE-2536 ACE-2395 IntelliTrap™ and antibodies driving next wave of therapeutic candidates

Acceleron’s 2020 Vision Our highly productive discovery and development platform has the potential to transform Acceleron into one of the world’s leading biotechnology companies 91 Acceleron’s Goals for the Year 2020 Approvals in up to 5 indications Multiple phase 3 studies 8 therapeutic candidates in clinical trials Sales and marketing organization in U.S. Cash flow positive

Building One Of The World’s Great Biotechnology Companies www.acceleronpharma.com NASDAQ: XLRN 92 THANK YOU



























































































