Exhibit 99.1

VITAL THERAPIES ANNOUNCES THAT TOPLINE RESULTS OF VTI-208 FAIL TO ACHIEVE PRIMARY
OR SECONDARY ENDPOINTS OF IMPROVEMENT IN OVERALL SURVIVAL
PRE-SPECIFIED EXPLORATORY SUBSET ANALYSES SUGGEST EFFICACY TRENDS
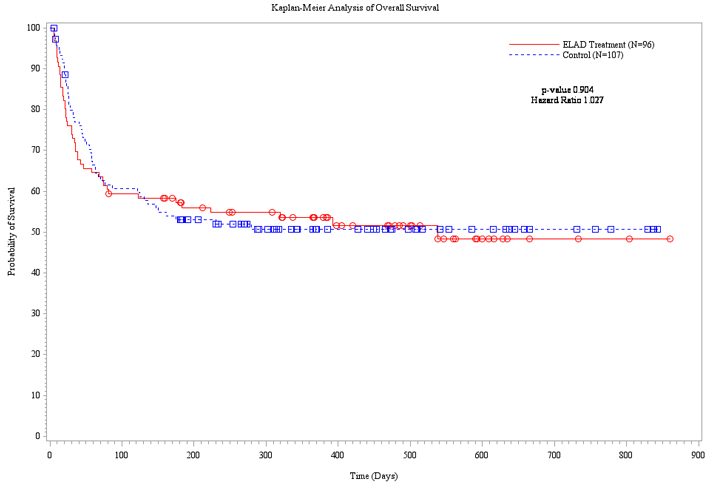
SAN DIEGO, August 21, 2015 (GLOBE NEWSWIRE) -- Vital Therapies, Inc. (Nasdaq: VTL), a biotherapeutic company developing ELAD®, a cell-based therapy targeting the treatment of liver failure, today announced that topline results from VTI-208, the Company’s phase 3 randomized, controlled, open-label trial, evaluating the ELAD System in subjects with alcohol-induced liver decompensation (AILD) failed to meet the primary endpoint of overall survival through at least 91 days assessed using the Kaplan Meier statistical method. Of 203 total subjects enrolled in VTI-208, 96 were randomized to the treated group and 107 were randomized to the control group. A hazard ratio of 1.027 (slightly favoring the control group) with a log rank p-value of 0.90 (not statistically significant, N.S.) indicated that there was no difference between treated and control subjects in the primary endpoint.
Figure 1. Kaplan-Meier Plot of Overall Survival
15010 Avenue of Science, San Diego, California, USA 92128
Tel 858.673.6840 • Fax 858-673-6843
www.vitaltherapies.com
The secondary endpoints of proportion of survivors at study days 28 and 91 also showed no difference between the groups (Pearson’s Chi-squared p-values of 0.45 (N.S.) and 0.74 (N.S.), respectively).
The adverse event profile revealed that treatment emergent serious adverse events were similar between the treated and control groups in the predefined safety population.
Table 1. Treatment Emergent Serious Adverse Events (TESAE)
| | | | | | |
| Safety Population | | ELAD N = 95 n (%) | | Control N = 108 n (%) | | Total N = 203 n (%) |
| Number (%) of Subjects Reporting Any TESAE | | 73 (76.8) | | 75 (69.4) | | 148 (72.9) |
| Number of TESAEs | | 156 | | 168 | | 324 |
Table 2. Subjects Reporting at Least One Treatment Emergent Serious Adverse Event (TESAE) by Preferred Term (Incidence >5%): Safety Population
| | | | | | |
Preferred Term | | ELAD (N=95) n (%) | | Control (N=108) n (%) | | Total (N=203) n (%) |
| | | |
Subjects Reporting at Least One TESAE | | 73 (76.8) | | 75 (69.4) | | 148 (72.9) |
| | | |
Hepatic failure | | 13 (13.7) | | 10 (9.3) | | 23 (11.3) |
Ascites | | 6 (6.3) | | 13 (12.0) | | 19 (9.4) |
Renal failure acute | | 6 (6.3) | | 12 (11.1) | | 18 (8.9) |
Multi-organ failure | | 7 (7.4) | | 10 (9.3) | | 17 (8.4) |
Anaemia | | 8 (8.4) | | 6 (5.6) | | 14 (6.9) |
Hepatic encephalopathy | | 8 (8.4) | | 6 (5.6) | | 14 (6.9) |
Gastrointestinal haemorrhage | | 7 (7.4) | | 6 (5.6) | | 13 (6.4) |
Hepatorenal syndrome | | 3 (3.2) | | 9 (8.3) | | 12 (5.9) |
Pneumonia | | 2 (2.1) | | 6 (5.6) | | 8 (3.9) |
Respiratory failure | | 5 (5.3) | | 2 (1.9) | | 7 (3.4) |
The Company is still in the process of analyzing the entire data set in accord with the statistical analysis plan submitted to FDA prior to database lock. Preliminary findings, which remain to be confirmed, include the following:
| | • | | In a 120-subject, pre-specified exploratory subset of the intention-to-treat, or ITT, population with a MELD score of <28 at randomization, a Kaplan-Meier analysis of overall survival approached statistical significance with a hazard ratio of 0.575 and a log-rank p-value of 0.077 (N.S.) in favor of the ELAD group. In the per protocol population for this subset (N = 116), the p-value was 0.059 (N.S.). Furthermore, an analysis of 91-day survival in the ITT cohort revealed ELAD survival of 80.4% versus control survival of 65.2% (Pearson’s chi-squared p=0.068, (N.S.)). In subjects with MELD scores of >28 at baseline, outcomes appeared to be worse in the ELAD group compared with the Control group, (p=N.S.), suggesting that future clinical studies should exclude subjects with MELD scores >28. In other respects the ELAD and Control groups appear to be balanced with regard to baseline demographics and key measures of liver disease. |
2
Figure 2. Kaplan-Meier Analysis of Overall Survival in Subjects with MELD <28

| | • | | In another pre-specified exploratory analysis of the 101 subject sub-group with age less than the median age of 46.9 years, a Kaplan-Meier analysis of overall survival showed a hazard ratio of 0.634 with a log-rank p-value of 0.167 (N.S.) in favor of the ELAD treated subjects. Furthermore, an analysis of 91 day survival in this cohort revealed ELAD survival of 81.4% versus control survival of 67.2% (p=0.112, N.S.). In subjects with age greater than the median of 46.9 years, outcomes appeared to be worse in the ELAD group compared with the Control group (p=N.S.), suggesting that future study designs may incorporate stratification by age. In other respects the ELAD and Control groups appeared to be balanced for baseline demographics and measures of liver disease. |
3
| | • | | Analyses of data in subjects with either acute kidney injury, defined by serum creatinine greater than 1.5 mg/dl, or evidence of severe coagulopathy, defined by INR >2.5, suggest that these subjects should be excluded from future clinical trials. |
| | • | | Of interest, data from laboratory testing of the blood indicators of alcohol consumption in subjects following hospital discharge revealed that there was no apparent relationship between recidivism and survival in either group. |
4
The Company will be analyzing the data from the VTI-208 clinical trial during the next several weeks, including data from the pre-specified subset analyses. The Company will stop the VTI-210 and VTI-212 clinical trials, and also plans to meet with the FDA as soon as possible to discuss restructuring its clinical development program, including a potential new trial to confirm the information suggested by the subset analyses. The Company will then give more details on a possible path forward with ELAD.
While the overall results are disappointing, the Company is encouraged that a large pre-specified subset of 120 subjects with a MELD score of less than 28 had a hazard ratio of 0.575 and a log-rank p-value of 0.077 (N.S.) in favor of the ELAD group, suggesting that future clinical studies should focus on this cohort with MELD scores less than 28. Separately, a pre-specified subset of 101 subjects under age 46.9 years (the study median age) had a hazard ratio of 0.634 with a log-rank p-value of 0.167 (N.S.) in favor of the ELAD treated subjects, suggesting that future study designs may incorporate stratification by age. Indeed these effects appear to be additive and a subset of 59 subjects with MELD less than 28 and age less than 46.9 years had a hazard ratio of 0.375 in favor of the ELAD group (p=0.085). Finally, pre-specified analyses suggest that subjects with impaired kidney function and severe coagulopathy may have worse outcomes and therefore future clinical trials will exclude these subjects.
With $62.0 million in current cash, the Company believes it could complete a new trial without raising additional capital, although that will depend on discussions with regulatory authorities and the exact design of any new trial.
Conference Call Details
Vital Therapies will host a conference call to discuss the results of the VTI-208 clinical trial today, August 21, 2015, at 5pm ET, which will be open to the public. The conference call dial-in numbers are (855) 765-5682 for domestic callers and (919) 825-3204 for international callers. The conference ID number for the call is 20307654. Participants may access the live webcast via a link on the Vital Therapies website in the Investor Relations section under “Events” at:http://ir.vitaltherapies.com/.
For those unable to dial in at the designated time, a conference call replay will be available for one week following the conference call, from approximately 8:00 p.m. ET on August 21, 2015 to 11:59 p.m. ET on August 28, 2015. The conference call replay numbers for domestic and international callers are (855) 859-2056 and (404) 537-3406, respectively. The conference ID number for the replay is 20307654. Additionally, an archive of the webcast will be available on the Company’s website for 90 days.
About Vital Therapies, Inc.
Vital Therapies, Inc. is a biotherapeutic company developing a cell-based therapy targeting the treatment of liver failure. The Company’s ELAD System, is an extracorporeal human allogeneic cellular liver therapy currently in phase 3 clinical trials. Vital Therapies, Inc. is based in San Diego, California. Vital Therapies® and ELAD® are trademarks of Vital Therapies, Inc.
5
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, among others, statements relating to our continuing analyses of the data from the VTI-208 clinical trial, including exploratory subset analyses, our preliminary findings, future discussions and meetings with regulatory authorities, our plans to restructure our clinical development program, including a potential new trial and potential inclusion and exclusion criteria and study design, our projected cash runway, including our ability to complete a new trial with existing cash, and related statements. Forward-looking statements are based on management’s current expectations and are subject to various risks and uncertainties that could cause actual results to differ materially and adversely from those expressed or implied by such forward-looking statements. Accordingly, these forward-looking statements do not constitute guarantees of future performance and you are cautioned not to place undue reliance on these forward-looking statements. Risks and uncertainties include, but are not limited to, the risk that further analysis of the VTI-208 data does not support and/or contradicts our preliminary findings, the risk that the VTI-208 data will not support a proposed new clinical trial, the risk that the FDA does not approve a new clinical trial plan, delays in meeting with FDA or completing those meetings, the success or failure of a new clinical trial, if any, the uncertainties inherent in Vital Therapies’ clinical and development programs, including, without limitation, Vital Therapies’ ability to adequately demonstrate the safety and efficacy of the ELAD System, future clinical results, which may not support further development of the ELAD System, and challenges related to conducting pivotal clinical trials, including, but not limited to, the impact of VTI-208, failure to achieve favorable results in clinical trials, the successful opening and the continued participation of clinical sites and their ongoing adherence to protocols, assumptions regarding enrollment rates, timing and availability of subjects meeting inclusion and exclusion criteria, changes to protocols or regulatory requirements, the ability to comply with and meet applicable laws and regulations, and unexpected adverse events or safety issues; the ability to obtain regulatory approval for the ELAD System; and the sufficiency of funding. There can be no assurance that data from any of our clinical trials will be sufficient to support an application for marketing in any country or that any such application will ever be approved. These and other risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2015. These forward-looking statements speak only as of the date hereof and Vital Therapies, Inc. disclaims any obligation to update these statements except as may be required by law.
Contact:
Vital Therapies, Inc.
Al Kildani
Vice President, Investor Relations and Business Development
858-673-6840
akildani@vitaltherapies.com
6


