- IMUX Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
425 Filing
Immunic (IMUX) 425Business combination disclosure
Filed: 14 Mar 19, 4:02pm
 Immunic Therapeutics Developing Selective Oral Drugs in Immunology Company Overview March 2019 Filed by Vital Therapies, Inc. Pursuant to Rule 425 under the Securities Act of 1933, as amended, and deemed filed pursuant to Rule 14a-12 under the Securities Exchange Act of 1934, as amended Subject Company: Vital Therapies, Inc. Commission File No.: 001-36201 March 14, 2019 |
 © Immunic AG 13.03.2019 2 Cautionary Note Regarding Forward-Looking Statements • Certain statements contained in this presentation regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities and Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995, known as the PSLRA. These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Vital Therapies and Immunic undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law. We use words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. • Such forward-looking statements are based on our expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, risks relating to the completion of the transaction, including the need for Vital Therapies stockholder approval and the satisfaction of closing conditions; the anticipated financing to be completed concurrently with the closing of the transaction; the cash balance of the company following the closing of the transaction and the financing, and the expectations with respect thereto; the business and prospects of the company following the transaction; and the ability of Vital Therapies to remain listed on the Nasdaq Capital Market. Risks and uncertainties related to Immunic that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to: Immunic’s plans to develop and commercialize its product candidates, including IMU-838, IMU-935 and IMU-856; the timing of initiation of Immunic’s planned clinical trials; expectations regarding potential market size; the timing of the availability of data from Immunic’s clinical trials; the timing of any planned investigational new drug application or new drug application; Immunic’s plans to research, develop and commercialize its current and future product candidates; Immunic’s ability to successfully collaborate with existing collaborators or enter into new collaborations, and to fulfill its obligations under any such collaboration agreements; the clinical utility, potential benefits and market acceptance of Immunic’s product candidates; Immunic’s commercialization, marketing and manufacturing capabilities and strategy; Immunic’s ability to identify additional products or product candidates with significant commercial potential; developments and projections relating to Immunic’s competitors and industry; the impact of government laws and regulations; Immunic’s ability to protect its intellectual property position; and Immunic’s estimates regarding future revenue, expenses, capital requirements and need for additional financing following the proposed transaction. • These risks, as well as other risks associated with the transaction, are more fully discussed in the final proxy statement/prospectus that is included in the registration statement that was filed by Vital Therapies with the SEC in connection with the proposed transaction. Additional risks and uncertainties are identified and discussed in the “Risk Factors” section of Vital Therapies’ Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and other documents filed from time to time with the SEC. Forward-looking statements included in this presentation are based on information available to Vital Therapies and Immunic as of the date of this presentation. Neither Vital Therapies nor Immunic undertakes any obligation to update such forward- looking statements to reflect events or circumstances after the date of this presentation. |
 © Immunic AG 13.03.2019 3 Additional Information and Where You Can Find It Additional Information About the Proposed Transaction between Vital Therapies, Inc. and Immunic AG and Where to Find it • This communication is being made in respect of a proposed transaction involving Immunic AG and Vital Therapies, Inc. Vital Therapies and Immunic intend to file relevant materials with the U.S. Securities and Exchange Commission (the “SEC”) and Vital Therapies has filed a registration statement on Form S-4 and a final proxy statement/prospectus. The registration statement was declared effective by the SEC on February 14, 2019, and the definitive proxy statement was first mailed or otherwise made available to Vital Therapies stockholders on February 19, 2019 in connection with the Vital Therapies special meeting of stockholders to be held to vote on matters relating to the proposed transaction. The proxy statement/prospectus contains information about Vital Therapies, Immunic, the proposed transaction, and related matters. STOCKHOLDERS ARE URGED TO READ THE FINAL PROXY STATEMENT/PROSPECTUS (INCLUDING ANY AMENDMENTS OR SUPPLEMENTS THERETO) AND OTHER DOCUMENTS FILED WITH THE SEC CAREFULLY IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE, AS THEY CONTAIN IMPORTANT INFORMATION THAT STOCKHOLDERS OF VITAL THERAPIES SHOULD CONSIDER BEFORE MAKING A DECISION ABOUT THE PROPOSED TRANSACTION AND RELATED MATTERS. In addition to receiving the final proxy statement/prospectus and proxy card by mail, Vital Therapies stockholders can also obtain the final proxy statement/prospectus, as well as other filings containing information about Vital Therapies, without charge, from the SEC’s website (http://www.sec.gov) or, without charge, by directing a written request to: Vital Therapies, Inc., 15222-B Avenue of Science, San Diego, CA 92128, Attention: Investor Relations. No Offer or Solicitation • This communication is not intended to and does not constitute an offer to sell or the solicitation of an offer to subscribe for or buy or an invitation to purchase or subscribe for any securities or the solicitation of any vote or approval in any jurisdiction in connection with the proposed transaction or otherwise, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended. Participants in Solicitation • Vital Therapies and its executive officers and directors may be deemed to be participants in the solicitation of proxies from Vital Therapies’ stockholders with respect to the matters relating to the proposed transaction. Immunic may also be deemed a participant in such solicitation. Information regarding Vital Therapies’ executive officers and directors is available in Vital Therapies’ proxy statement on Schedule 14A for its 2018 annual meeting of stockholders, filed with the SEC on April 12, 2018. Information regarding any interest that Vital Therapies, Immunic or any of the executive officers or directors of Vital Therapies or Immunic may have in the transaction with Immunic is set forth in the final proxy statement/prospectus that Vital Therapies has filed with the SEC in connection with its stockholder vote on matters relating to the proposed transaction. Vital Therapies stockholders are able to obtain this information by reading the proxy statement/prospectus. |
 © Immunic AG 13.03.2019 4 Vital Therapies – Immunic Combination • Follows Vital Therapies’ extensive review of strategic alternatives • All-stock transaction: Vital Therapies to acquire all outstanding shares of Immunic in exchange for newly issued shares of Vital Therapies common stock; Immunic AG will become a wholly-owned subsidiary of Vital Therapies • Vital Therapies stockholders are expected to own approximately 11% and Immunic stockholders approximately 89% of the company upon completion of the proposed transaction • Current shareholders of Immunic committed to invest 26 million EUR at closing of the transaction • Transaction has been approved by the boards of directors of both companies and by Immunic stockholders • Expected to close in Q2 2019, subject to the approval of the stockholders of Vital Therapies and other closing conditions • Company expected to operate under the name Immunic, Inc. and trade on the NASDAQ Stock Market under the symbol “IMUX” |
 © Immunic AG 13.03.2019 5 • Company will be led by an experienced management team • Board to be comprised of 5 directors, 4 from Immunic and 1 from Vital Therapies • Corporate HQ will be located in the US with R&D site based in Munich, Germany Vital Therapies – Immunic Leadership Daniel Vitt, PhD CEO Andreas Muehler, MD, MBA CMO Hella Kohlhof, PhD CSO Manfred Groeppel, PhD COO Daniel Vitt, PhD CEO of Immunic Joerg Neermann, PhD Life Science Partners Jan van den Bossche Fund+ Duane Nash, MD, JD, MBA CEO of Vital Therapies Vincent Ossipow, PhD, CFA Omega Funds |
 Immunic Company and Product Overview |
 © Immunic AG 13.03.2019 7 Our Vision We are developing new therapies with best-in-class potential for the treatment of chronic inflammatory and autoimmune diseases. |
 © Immunic AG 13.03.2019 8 • Deep and diversified product pipeline, orally available and potent drugs • IMU-838: Potent DHODH inhibitor well-tolerated in prior clinical studies • IMU-935: High demand target with substantial deal potential • IMU-856: Novel target – potentially disease modifying for IBD Three potential best-in- class therapies Strong IP position • IMU-838: Patent application coverage until 2038 • IMU-935: New compound IP filed in 2017 • IMU-856: Compound patent filed in 2018 Key Investment Highlights High value markets • Autoimmune & immunology with high unmet medical needs • Large markets for IBD, MS and psoriasis with multibillion USD sales potential • Well financed with cash runway to near-term value-driving events Experienced management team • Experienced management team with strong track record and over 70 years of leadership experience in the pharmaceutical industry • Focused on efficient use of capital to maximize investor return Supported by experienced life science investors • Strong support of sophisticated board members and life science investors • Life Sciences Partners as lead investor • Omega Funds, Fund+, LifeCare Partners, High-Tech Gründerfonds, Bayern Kapital and IBG as further investors |
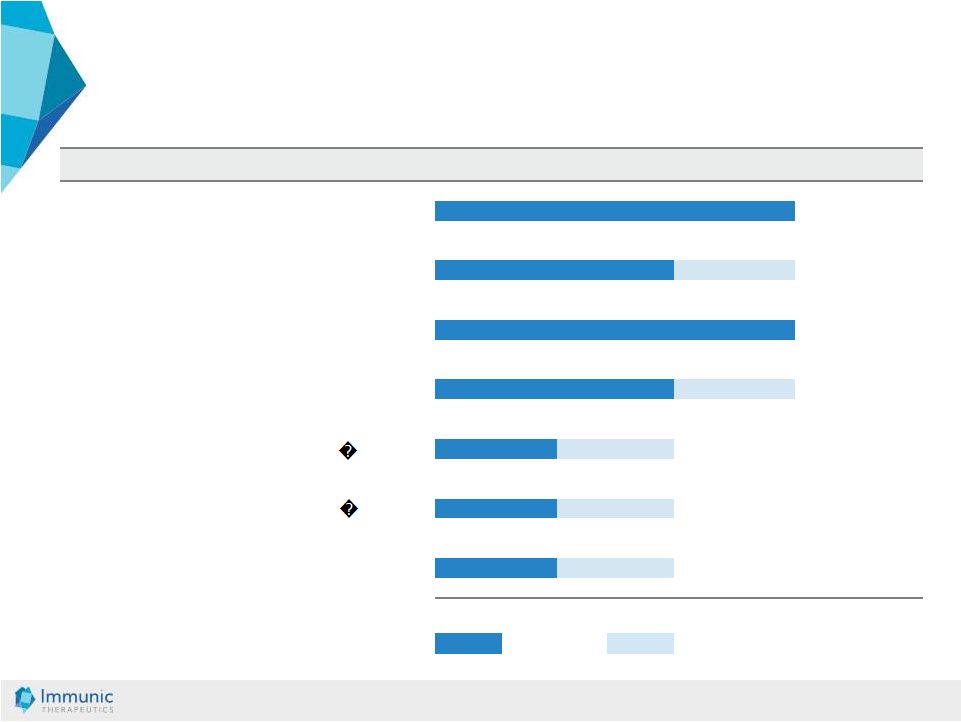 © Immunic AG 13.03.2019 9 IST study will be performed by Mayo Clinic / NIH * Development Pipeline * IST: Investigator-Sponsored Trial Completed or ongoing In preparation or planned Preclinical Phase 1 Phase 2 Phase 3 IMU-838 Ulcerative Colitis DHODH Crohn’s Disease DHODH Multiple Sclerosis DHODH PSC DHODH IMU-935 Psoriasis ROR t Orphan AI Diseases ROR t IMU-856 IBD Intestinal Barrier Function |
 © Immunic AG 13.03.2019 10 Proven Leadership in Drug Development & Licensing Dr. Daniel Vitt, CEO • PhD in Chemistry from University of Würzburg • 19 years track record as biotech entrepreneur • Developed start-up into successful IPO Dr. Andreas Muehler, CMO • MD degree (Charité Berlin) + MBA Duke University • 25+ years experience in the life science industry • Medical expertise in the field of IBD with experience in several IBD product launches Dr. Manfred Groeppel, COO • PhD in Chemistry from University of Erlangen • 18 years industry experience with US and German biotech companies • Project leader and member of the vidofludimus development at 4SC Dr. Hella Kohlhof, CSO • PhD in Biology from LMU Munich • More than 10 years experience in Biotech R&D and Immunology • Track-record in clinical project management |
 IMU-838 in Inflammatory Bowel Disease (IBD) New Oral Treatment with Promising Safety Profile |
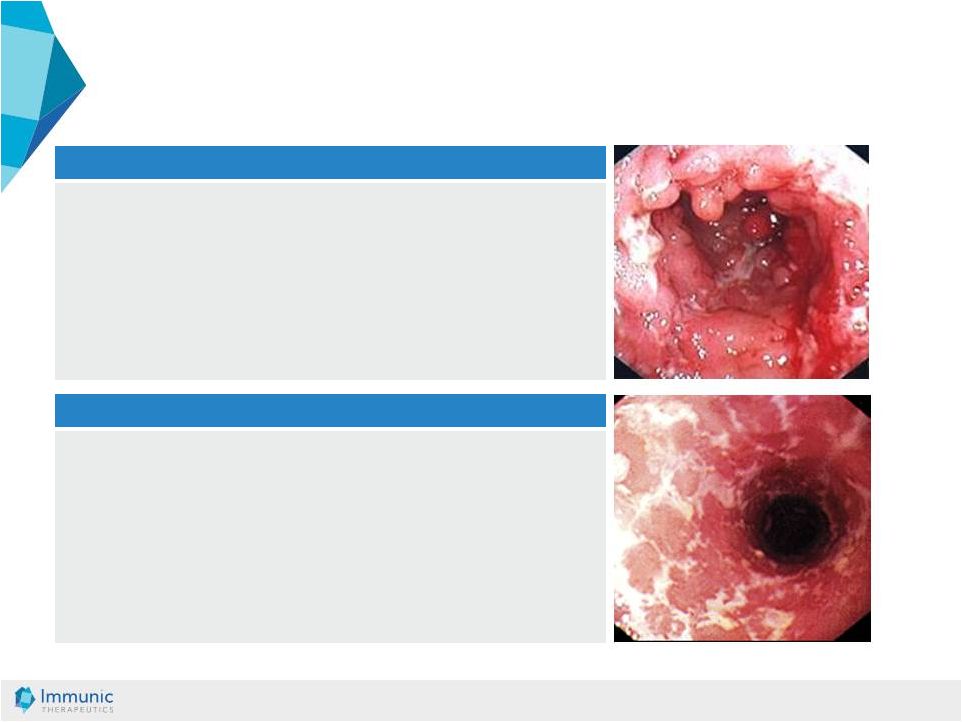 © Immunic AG 13.03.2019 12 Crohn’s Disease (CD) • A patchy, transmural inflammation involving the entire bowel wall • May affect any part of the gastro intestinal tract from the mouth to the anus • Most commonly, CD affects the lower part of the small intestine and colon • Symptoms include: abdominal pain, diarrhea, and weight loss • Structural problem (like e.g. fistulas, abscesses) are common Ulcerative Colitis (UC) • Diffuse mucosal inflammation limited to the colon (involving only the upper layer of the bowel wall) • 95% of UC cases affect the rectum • UC may extend in a symmetrical, circumferential and uninterrupted pattern to affect parts or all of the large intestine • Symptoms include: bloody diarrhea, colic, abdominal pain, cramping, urgency and a constant feeling of needing to empty the bowel IBD: Two Indications with High Unmet Medical Need Source: Datamonitor DMHC2624, Pipeline Insight: Inflammatory Bowel Disease, June 2010 |
 © Immunic AG 13.03.2019 13 Large Market Opportunity • Global market for IBD in 2023 estimated to be approximately 7.6 billion USD [1] • 11.2 million patients affected by UC or CD worldwide in 2015 [2] • Patient numbers continue to grow Europe [3] USA [4] Canada [5] IBD Total 2,600,000 1,300,000 233,000 UC 1,500,000 700,000 104,000 CD 1,100,000 600,000 129,000 [1] Global IBD Market Forecast 2018. [2] GBD 2015 Lancet. 388 (10053): 1545–1602. [3] Burisch et al. Journal of Crohn's and Colitis 2013 7, 322–337 [4] Hanauer S. 2006;12:S3-9 (Suppl 1), Kappelmann MD et al, Clin Gastroenterol Hepatol. 2007; 5:1424-9. [5] The Burden of IBD in Canada. www.ccfc.ca. Accessed 16 May 2014 |
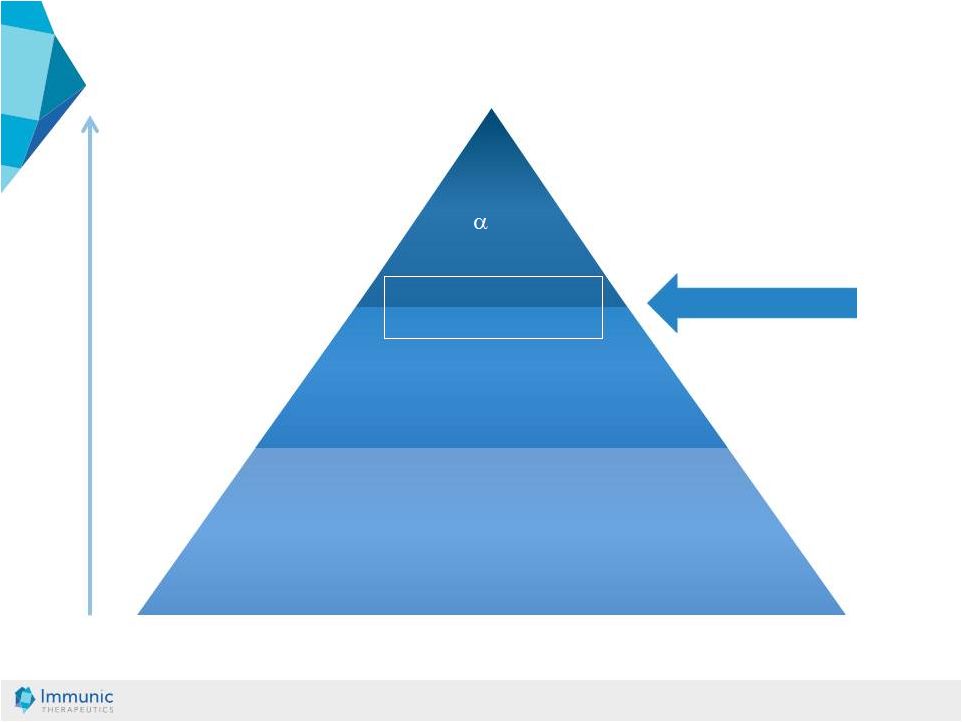 © Immunic AG 13.03.2019 14 Traditional Immunomodulators Corticosteroids Budesonide Aminosalicylates, Antibiotics Surgery TNF -mABs Vedolizumab Mild Moderate Severe IMU-838 Current solutions have limitations • Substantial side effects due to long-term use includes increased rate of cancer risk and virus reactivation of currently used immunosuppressants [1] [2] [3] • Antibodies lose activity over time [4] [1] Present, Daniel H., et al. Annals of internal medicine 1989; 111.8: 641-649. [2] Dayharsh, Gerald A., et al. Gastroenterology 2002; 122.1: 72-77. [3] Winthrop, Kevin L., et al. Arthritis & rheumatology 2014; 66.10: 2675-2684. [4] Roda, Giulia, et al. Clinical and translational gastroenterology 2017; 7.1: e135. IBD: Therapeutic Pyramid |
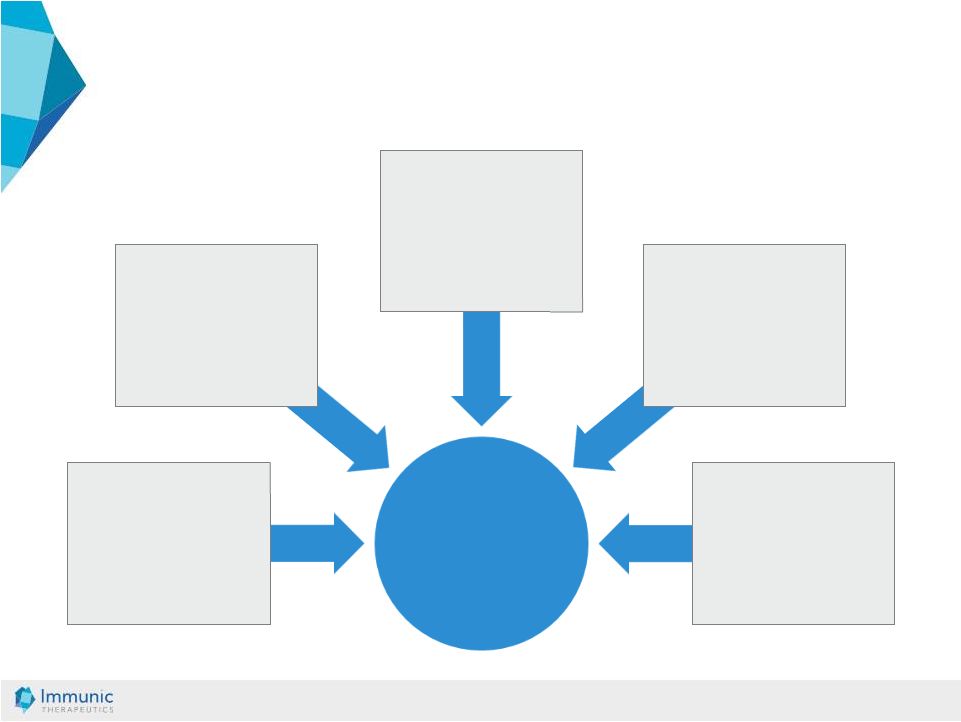 © Immunic AG 13.03.2019 15 Oral effective treatment option that can be prescribed for a large number of IBD patients Once daily oral drug intake – beneficial for patients Small molecule with low production costs No clinical evidence of increased rate of viral re-activations – in-vitro data of direct anti-viral effect Mode of action provides a new treatment option for patients failing other therapies in IBD Selective effect towards metabolically activated inflammatory cells IMU-838: Key Strengths That Address Limitation of Existing Therapies |
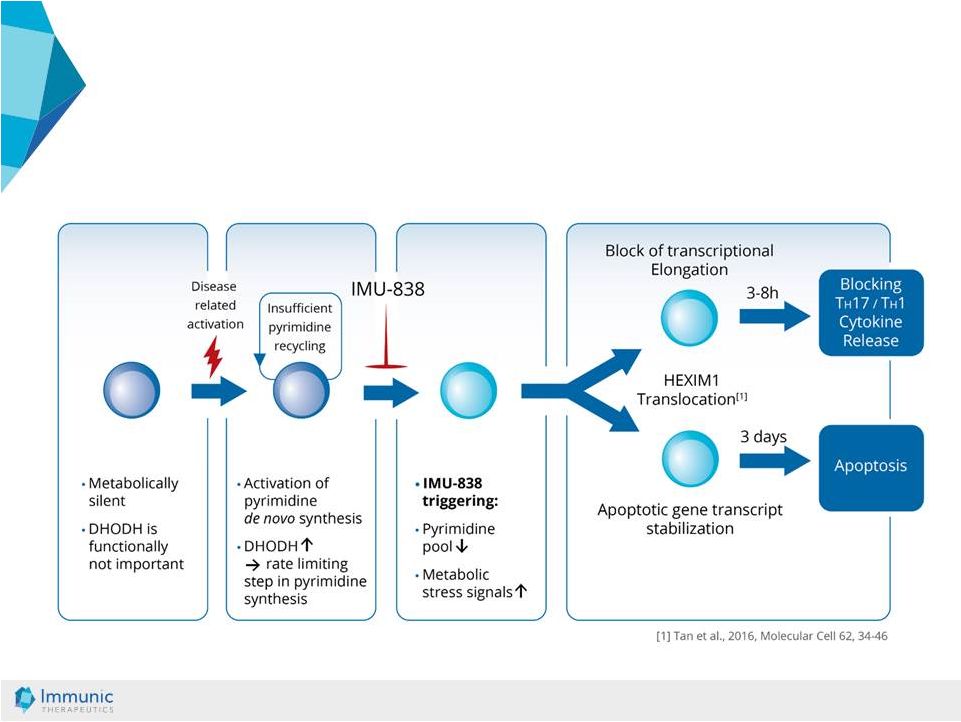 © Immunic AG 13.03.2019 16 Mode of Action: DHODH Targeting Leads to Metabolic Stress in Metabolically Activated Cells Resting Lymphocyte Activated Lymphocyte Stressed Lymphocyte Pharmacological Effects |
 © Immunic AG 13.03.2019 17 IMU-838: Compelling Safety and Efficacy Data • Safety • Animal and in-vitro data show selective effect on activated immune cells and no general detrimental effect on bone marrow • Already more than 350 individuals treated with active moiety of IMU-838 • Two phase 1 trials of IMU-838 formulation established its safety up to daily doses of 50 mg • Safety profile similar to placebo at therapeutically used doses • No increased rate of infections and infestations compared with placebo in clinical trials • Efficacy • Mechanism of DHODH inhibition already established successfully in rheumatoid arthritis and multiple sclerosis • Investigator trials with other DHODH inhibitors have shown positive effects on Crohn’s disease patients • Proof-of-concept trial using IMU-838 active moiety (ENTRANCE trial) provided initial efficacy results in steroid-dependent IBD patients |
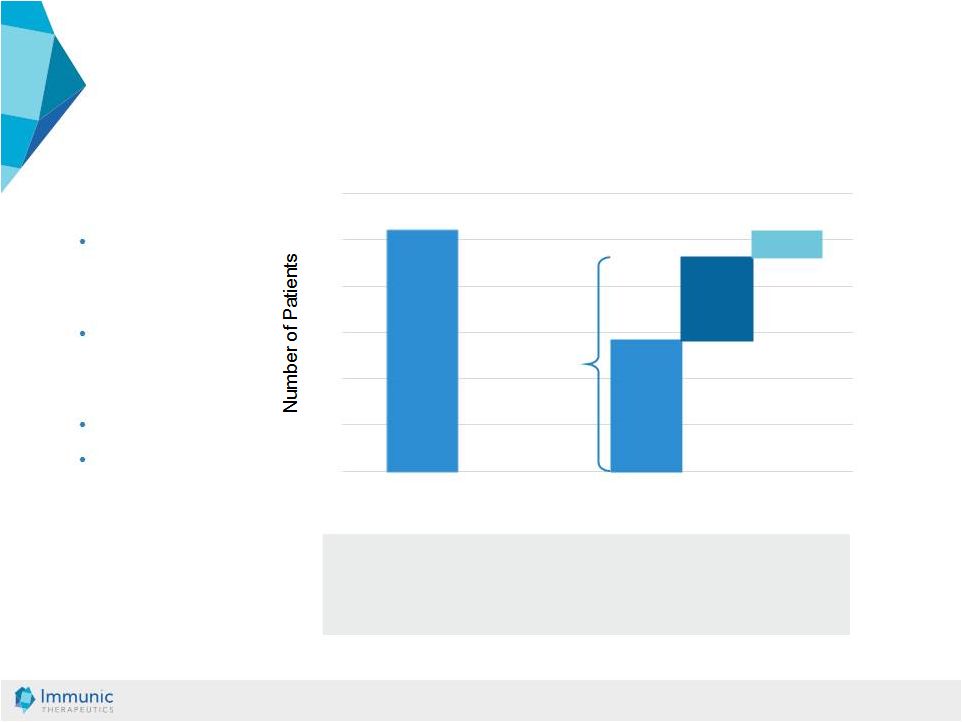 © Immunic AG 13.03.2019 18 ENTRANCE study: [1] • Study performed with active moiety of vidofludimus • Patients with steroid-dependent IBD disease • Open-label • Primary efficacy endpoint: steroid- free/steroid- reduced remission (Week 12) IMU-838 had response rates of: 85.7% in Crohn’s disease 91.7% in ulcerative colitis 0 5 10 15 20 25 30 88.5% Total Response 14 Complete Responders (53.9%) 3 26 mITT [2] Non-Responders (11.5%) Evaluable Patients IBD Phase 2a ENTRANCE: Primary Efficacy Results [1] Herrlinger et.al., 2011, Gastroenterology 140:588. [2] mITT: modified intent to treat 9 Partial Responders (34.6%) |
 © Immunic AG 13.03.2019 19 IMU-838: Clinical Phase 2 in UC Ongoing • Active IND in the US • Currently more than 60 active sites in 8 countries • USA, Western, Central and Eastern Europe • Study design • Central endoscopy assessment for active disease for study eligibility in order to reduce placebo rate • Composite endpoint: Proportion of patients with both symptomatic remission and endoscopic healing at week 10 • Despite competitive study landscape in IBD • Study enrollment is on track • Targeted to end enrollment in early 2020 |
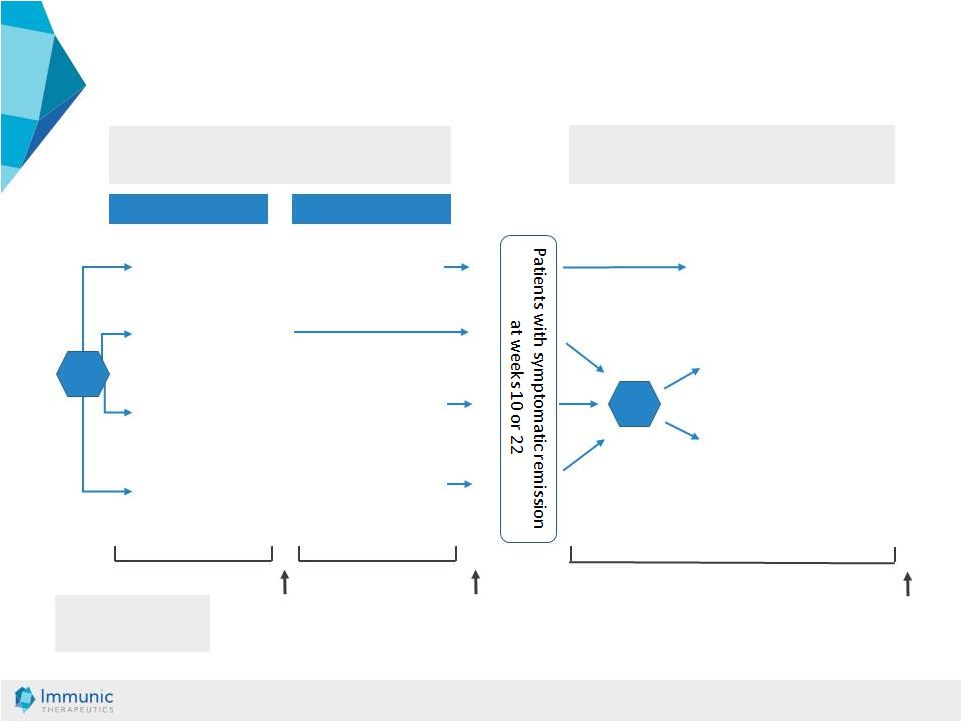 © Immunic AG 13.03.2019 20 General Phase 2 Trial Design in UC Induction Phase Maintenance Phase Enrollment Period 1 Enrollment Period 2 Placebo (N=15) 10 mg IMU-838 (N=15) 30 mg IMU-838 (N=15) 45 mg IMU-838 (N=15) 10 or 22 weeks 10 or 22 weeks Dosing analysis Placebo (N=45) 30 mg IMU-838 (N=45) 45 mg IMU-838 (N=45) Final analysis induction phase after 10 weeks Until UC relapse or termination Placebo (N=~24) R R Final analysis maintenance phase Patient number required: N=195 R = randomization 10 mg IMU-838 30 mg IMU-838 (N=~48) (N=~48) |
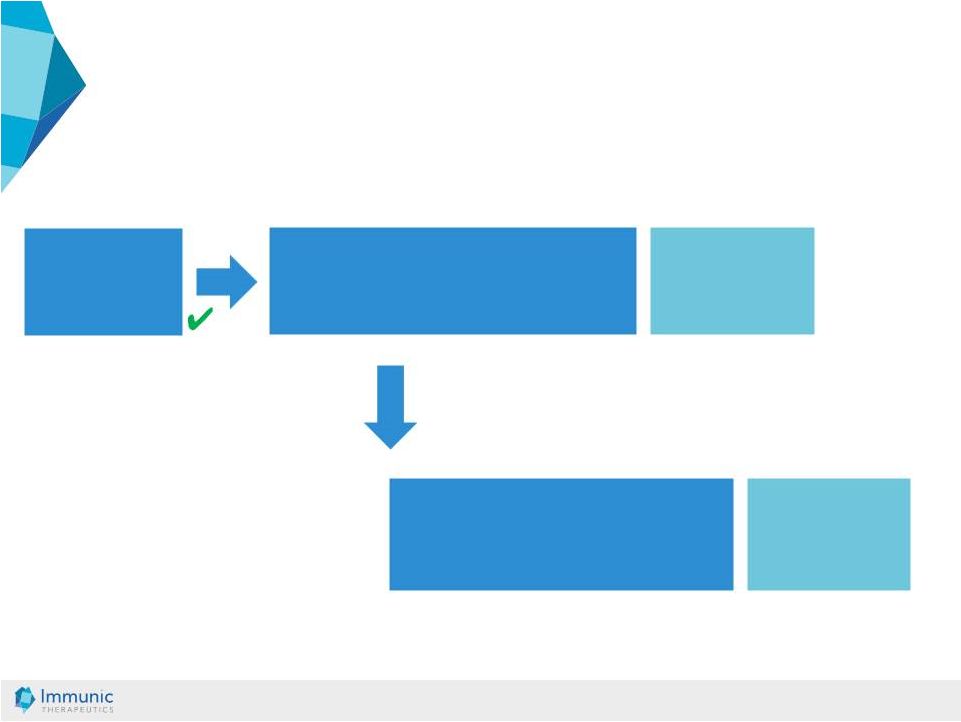 © Immunic AG 13.03.2019 21 Ulcerative colitis (UC) trial Crohn’s disease (CD) trial Final 1° UC efficacy analysis Final 1° CD efficacy analysis Two phase 1 trials IBD: Overall Study Program Definition of dose strengths for CD trial based on UC dosing analysis* * An interim dosing analysis is expected to be performed mid-2019 with the aim of potentially eliminating an ineffective dose or an intolerant dose, and to continue the study in a more efficient manner using fewer active dose groups. |
 © Immunic AG 13.03.2019 22 IMU-838: Clinical Phase 2 Trial in Crohn’s Disease Expected to Start in mid-2019 • Considerable operational and financial synergies expected • Same systems and service providers used • Investigators already familiar with study set-up • High-enrolling sites of UC study expected to participate in CD trial • Supplemented by additional sites and additional countries • Primary endpoint: clinical remission, at W14; Secondary endpoint: endoscopic response • Study already in start-up preparation mode • Accelerate study start after interim analysis of UC trial |
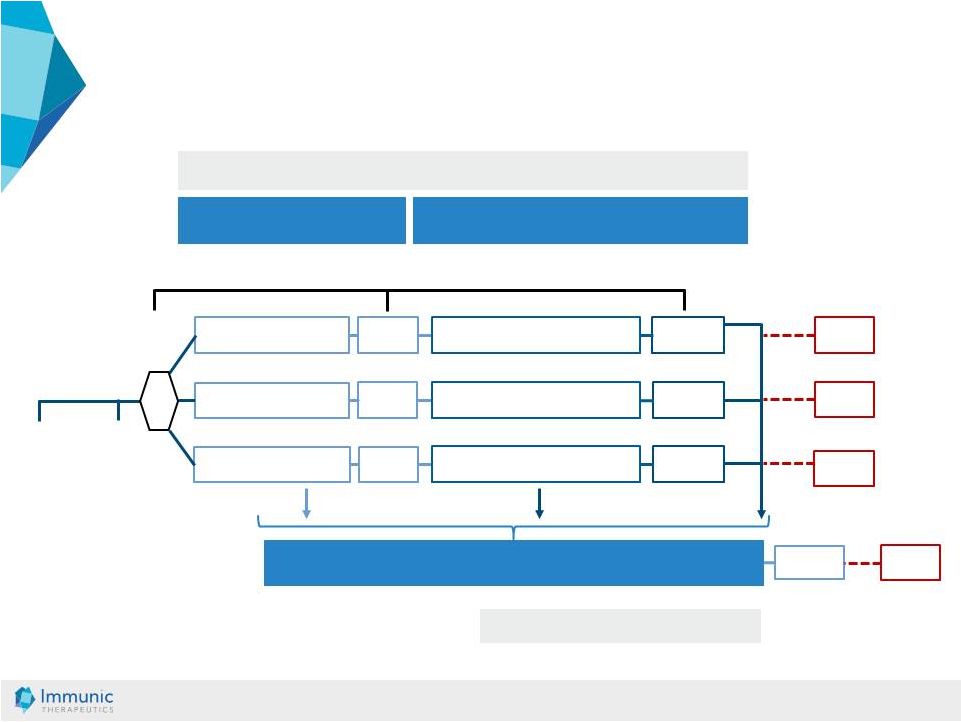 © Immunic AG 13.03.2019 23 General Phase 2 Trial Design in CD Patient number required: N ~ 260 Blinded treatment (BT) period 38 weeks Induction treatment phase 14 weeks Extended treatment phase 24 weeks Week 0 Week 14 Week 38 Placebo 30 mg IMU-838 45 mg IMU-838 EoI EoI EoI EoBT EoBT EoBT EoS EoS EoS Scr. V S1 Scr. V S2 R Placebo 30 mg IMU-838 45 mg IMU-838 Open-label extension treatment period with 30 mg IMU-838 (optional trial period) for up to 9 years EoOLE EoS BT = blinded treatment; EoBT = end of blinded treatment; EoI = end of induction; EoOLE = end of open label extension; EoS = end of study; R = randomization; Scr. = screening |
 © Immunic AG 13.03.2019 24 IMU-838: Phase 2 Proof-of-Concept Study in PSC • Immunic is collaborating with a prominent hepatologist in the US and two Mayo Clinic locations • PI received a grant approval letter from the NIH for performance of an investigator sponsored trial with IMU-838 in patients with primary sclerosing cholangitis (PSC) • Single-arm, exploratory study • Primary endpoint: change in serum alkaline phosphatase (ALP) at 6 months vs. baseline • Dosing: 30 mg IMU-838, (Clinicaltrials.gov: NCT03722576) • Investigator IND for IMU-838 and IRB approval already established • Immunic to provide clinical trial material for the patients to clinical sites • Assumed start of enrollment in Q1 2019 • Positive data should enable immediate start of a pivotal trial in this orphan indication by Immunic |
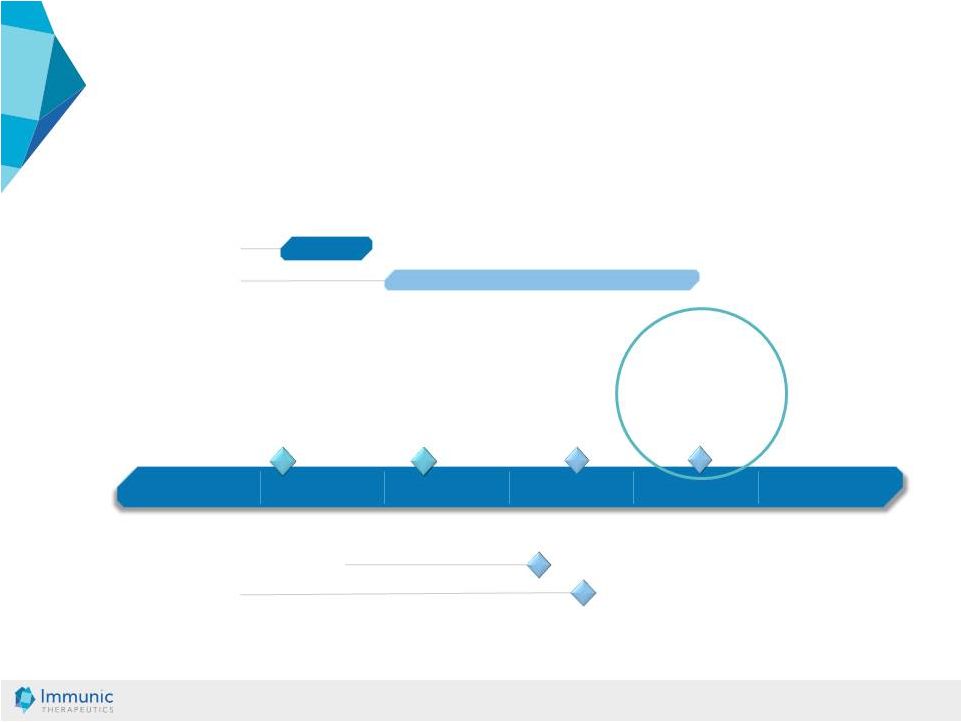 © Immunic AG 13.03.2019 25 IMU-838: Clinical Development Plan in IBD and PSC 2016 2017 2018 2019 2020 2021 Interim Analysis mid-19 Results UC Induction of Remission Expected Q2/20 02/17 - 11/17 Phase 1 (SAD * and MAD * ) Q1/18 - Q2/20 Phase 2 Ulcerative Colitis Phase 2 Primary Sclerosing Cholangitis 02/17 FPI * Phase 1 Start of phase 2 study in PSC anticipated Phase 2 Crohn’s Disease Start of phase 2 study in CD expected 04/18 FPI Phase 2 UC * SAD: Single Ascending Dose MAD: Multiple Ascending Dose FPI: First Patient In |
 Mode of Action of IMU-838 Enables Broad Therapeutic Use |
 © Immunic AG 13.03.2019 27 Aubagio ® (teriflunomide) is currently the only approved DHODH inhibitor for MS IMU-838 has the potential to be a best-in-class DHODH inhibitor and MS drug due to improved safety and pharmacokinetics profile MS Opportunity [1] https://mediaroom.sanofi.com/en/press-releases/2018/sanofi-delivers-2017 Accessed January 2, 2019 |
 © Immunic AG 13.03.2019 28 • Potential advantages of IMU-838 therapy compared with Aubagio ® (teriflunomide): • Selectivity and sensitivity [1] [2] [3] [4] • Pharmacokinetic parameters [5] [6] • Safety profile [7] [8] [9] [10] • Drug-drug interaction potential [6] IMU-838: Potential Advantages in MS • Phase 2 trial in patients with relapsing-remitting multiple sclerosis (RRMS) started in February 2019 [1] FDA CDER Pharmacological Review Teriflunomide 2012 [2] Merrill JE, et al. J Neurol 256: 89-103, 2009 [3] Büttner R, et al. Blood 130 (suppl 1): 4426 abstract, 2017 [4] Cada DJ, et al. Hosp Pharm 48: 231-240, 2013 ) [5] FDA CDER Clinical Pharmacology and Biopharmaceutics Review Teriflunomide 2012 [6] Summary of Product Characteristics Aubagio ® [7] SmPC Aubagio® [8] FDA CDER Medical Review Teriflunomide, 2012 [9] O’Connor et al, NEJM 365: 1293-1303, 2011 [10] O’Connor et al, NEJM 365: supplementary appendix, 2011 |
 © Immunic AG 13.03.2019 29 General Phase 2 Trial Design in RRMS BL = baseline; exam. = examination; D = day; EoMT = end of main treatment; EoS = end of trial; EoT = end of treatment; MRI magnetic resonance imaging; R = randomization; Scr. = screening; W = week Screening up to 28 days Blinded Unblinded Placebo 30 mg IMU-838 45 mg IMU-838 EoMT EoMT EoMT EoS Scr. exam. BL MRI R 30 mg IMU-838 30 mg IMU-838 45 mg IMU-838 Extended treatment period Main treatment period 24 weeks 45 mg IMU-838 30 or 45 mg IMU-838 30 or 45 mg IMU-838 30 or 45 mg IMU-838 30 or 45 mg IMU-838 D-28 -9 D-14 -3 D0 W24/EoMT End of main part 1°analysis At time of final analysis of main part Visits every 12 weeks W516/EoT EoS (EoT+30d) R EoT EoT EoT EoT up to ~9.5 years |
 © Immunic AG 13.03.2019 30 IP Position of IMU-838: Several Layers of IP • IMU-838 is protected by several layers of patents Patent on the specific salt form and pharmaceutical composition of IMU-838, granted in the US, EU and other key markets – expires in 2031 New patent filed in 2018 on the specific polymorph of IMU-838 used in current studies New dosing regimen, which was developed during phase 1 testing – protecting the applied dosing scheme of all ongoing and planned phase 2 studies – new patent application filed in 2017 |
 IMU-935 Unique ROR t-Inverse Agonist |
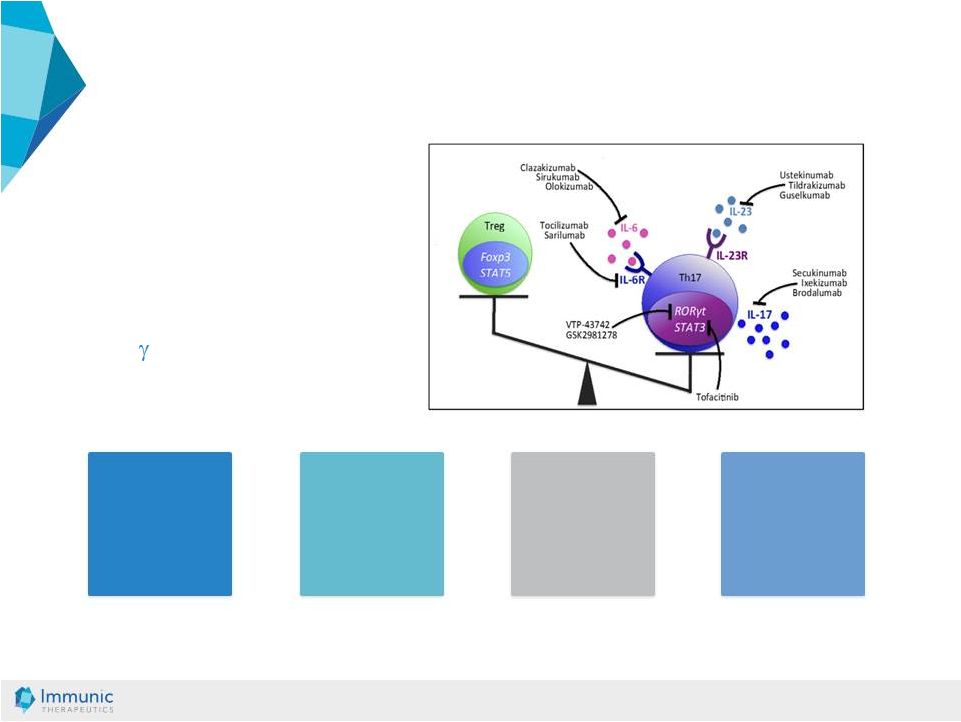 © Immunic AG 13.03.2019 32 Autoimmune Diseases: Broad Disease Spectrum • Autoimmune diseases are frequent diseases affecting millions of patients worldwide [1] • Disruption of the human immune system is a root cause of autoimmune diseases [2] • ROR t is an important regulator of auto immunity related diseases [2] Psoriasis IL-23 axis in psoriasis MS Th1/Th17 – key role in MS Uveitis Key role for Th17 in Uveitis Lupus IFNg driven autoimmune disease Source: Fasching, Patrizia, et al. Molecules 2017; 22.1: 134. [1] Rose, Noel R. American journal of epidemiology 2016; 183.5: 403-406. [2] Fasching, Patrizia, et al. Molecules 2017 22.1: 134. |
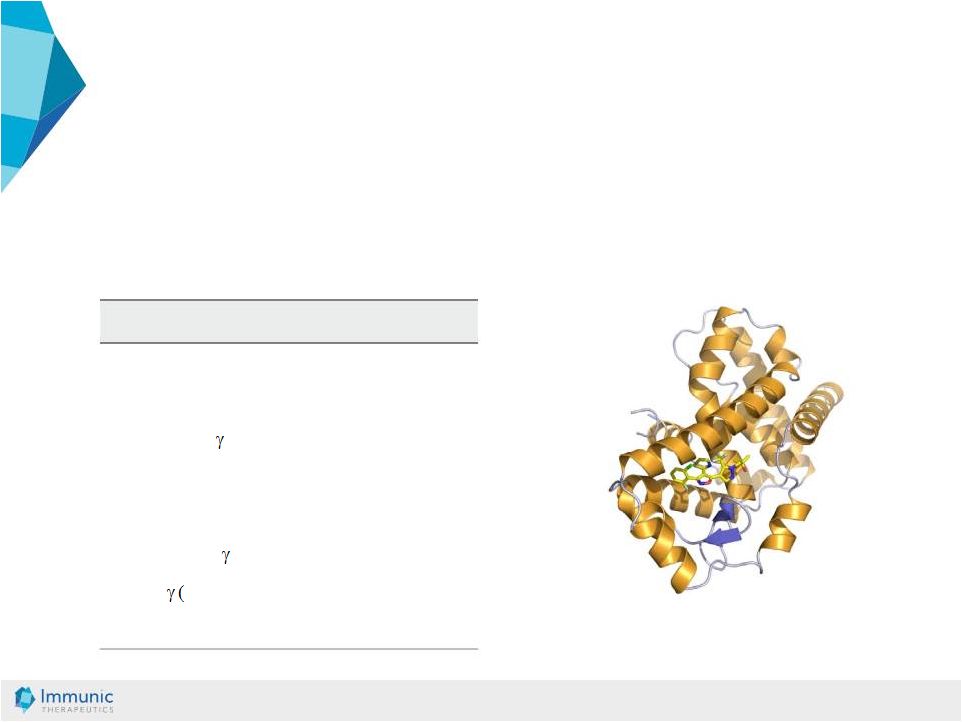 © Immunic AG 13.03.2019 33 IMU-935: Cytokine Inhibition in Low Nanomolar Range • Effect of the development compound IM105935 (IMU-935) in stimulated human PBMCs • Read-out: effect on cytokine production after 48 h IC 50 [µM] IL-17A 0.005 IL-17F 0.004 IFN 0.003 IL-1a and b no inhibition IL-4,5,6,8 no inhibition ROR 24 nM (MST) ROR cellular, rep.) 20 nM Th17 differentiation 100 nM Resolution 2.6 A of a closely related derivative compound binds to hydroxycholesterol binding site |
 © Immunic AG 13.03.2019 34 IMU-935: Project Status • Preclinical IND enabling studies currently ongoing • Start of clinical phase 1 test of IMU-935 in healthy volunteers planned for mid-2019 • Further options for clinical development • Test of IMU-935 in phase 1b/2a trial in patients with mild to moderate psoriasis – would potentially offer early read-out of activity based on four-week treatment • Identification of suitable orphan indications with high unmet medical need for accelerated development |
 IMU-856 Restoring Intestinal Barrier Function |
 © Immunic AG 13.03.2019 36 Accelerates mucosal healing with standard of care due to its new mode of action Enhances maintenance of remission, that is the highest unmet medical need in IBD IMU-856 inhibitor concept: Ameliorates barrier function Mucus layer Antigen/pathogen <Lumen> <IECs> Immune cells <Lumen> <IECs> Current SOCs Inhibit inflammation Current SOCs Inhibit inflammation IBD Healthy Hypothesis: Bacterial Penetration Through Weakened Cellular Adhesion Causes Immune Overstimulation Tight junctions Source: Adopted from Daiichi Sankyo Venture Science Labs, November 2018 |
 © Immunic AG 13.03.2019 37 IMU-856: Targeting Gut Barrier Function • IMU-856 is a potent inhibitor of a novel target which was validated in a knock-out animal model • Small orally available molecule suitable for once daily dosing • Carefully performed lead compound selection based on exploratory full safety panel, including non-GLP 14-day tox studies in rats and monkeys • Large therapeutic window expected • No critical issues identified in genotoxicity and safety pharmacology studies • Pharmacological effect is improving intestinal barrier function: shown in-vitro and in-vivo to reverse pathophysiology of IBD • Optioned from Daiichi Sankyo Venture Science Labs • Execution of worldwide option after availability of GLP tox data |
 © Immunic AG 13.03.2019 38 IMU-856: Development Concept • Main indication: Crohn’s disease (CD) • Clinical development concept • Phase 1 single and multiple ascending dose studies are expected to start in H1 2020 • IMU-856 has substantial further potential for orphan diseases outside IBD • Product is covered by a global PCT patent application |
 Summary |
 © Immunic AG 13.03.2019 40 Financial Status and Cash Runway • Immunic Series A financing round of 37.5 million USD completed in 2016 and 2017 • Supported by renowned life science investors • Current Immunic investors to invest 26 million EUR additional equity at closing of the transaction with Vital Therapies • Cash runway expected to be sufficient beyond important value inflection points into Q3 2020 |
 © Immunic AG 13.03.2019 41 Three potential best-in- class therapies • Deep and diversified product pipeline, orally available and potent drugs • IMU-838: Potent DHODH inhibitor well-tolerated in prior clinical studies • IMU-935: High demand target with substantial deal potential • IMU-856: Novel target – potentially disease modifying for IBD Strong IP position • IMU-838: Patent application coverage until 2038 • IMU-935: New compound IP filed in 2017 • IMU-856: Compound patent filed in 2018 Key Investment Highlights High value markets • Autoimmune & immunology with high unmet medical needs • Large markets for IBD, MS and psoriasis with multibillion USD sales potential • Well financed with cash runway to near-term value-driving events Experienced management team • Experienced management team with strong track record and over 70 years of leadership experience in the pharmaceutical industry • Focused on efficient use of capital to maximize investor return Supported by experienced life science investors • Strong support of sophisticated board members and life science investors • Life Sciences Partners as lead investor • Omega Funds, Fund+, LifeCare Partners, High-Tech Gründerfonds, Bayern Kapital and IBG as further investors |
 © Immunic AG 13.03.2019 42 Any Questions? |
 Thank You! Jessica Breu Manager IR & Communications Phone: +49 89 250 0794 69 Email: jessica.breu@immunic.de Immunic AG Am Klopfersitz 19 82125 Planegg-Martinsried Germany |