
Immunic Therapeutics
Developing Selective Oral DHODH Inhibitor IMU-838 as COVID-19 Therapy
As of April 22, 2020
Index
| Cautionary Statement Regarding Forward-Looking Statements | 2 |
| Synopsis | 3 |
| Immunic: Company and Pipeline Overview | 4 |
| DHODH: Introduction and Mode of Action | 4 |
| Mode of Action | 4 |
| Applicability of DHODH Inhibition During Virus Infection | 5 |
| Inhibition of Virus RNA Replication | 5 |
| Induction of the Innate Immunity by Induction or Amplification of Interferon-Stimulated Genes | 6 |
| Inhibition of Excessive Immune Response by Selectively Blocking Cytokine Production in Hyperactivated Immune Cells | 6 |
| Broad-Spectrum Antiviral Effect of DHODH Inhibition | 6 |
| IMU-838: Potential New Therapeutic Option for the Treatment of COVID-19 | 7 |
| Background COVID-19 | 7 |
| Scientific Rationale: Potential Advantages of DHODH Inhibitors for the Treatment of COVID-19 | 8 |
| IMU-838: Attractive Safety and Tolerability Profile | 10 |
| IMU-838: Broad-Spectrum Antiviral Activity | 10 |
| IMU-838: Preclinical Activity Against SARS-CoV-2 | 12 |
| IMU-838: Development Plan in COVID-19 | 13 |
| COVID-19: Access and Distribution | 14 |
| Conclusion | 15 |
| Background IMU-838: Development History and Ongoing Studies | 16 |
| Company Background | 17 |
| List of References | 18 |
| List of Figures | 19 |
| List of Tables | 19 |

Cautionary Statement Regarding Forward-Looking Statements
This presentation contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this presentation regarding strategy, future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of management are forward-looking statements. Examples of such statements include, but are not limited to, statements relating to Immunic’s three development programs and the targeted diseases; the potential for IMU-838 as a potential treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections causing coronavirus disease 2019 (COVID-19) and other viruses and any clinical trials, and collaborations and approvals relating to such potential treatment. Immunic may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation, risks and uncertainties associated with the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the availability of sufficient resources to meet business objectives and operational requirements, the fact that the results of earlier studies and trials may not be predictive of future clinical trial results, the protection and market exclusivity provided by Immunic’s intellectual property, risks related to the drug development and the regulatory approval process and the impact of competitive products and technological changes. A further list and descriptions of these risks, uncertainties and other factors can be found in the section captioned “Risk Factors,” in the company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2019, filed with the SEC on March 16, 2020, and in the company’s subsequent filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov or ir.immunic-therapeutics.com/sec-filings and on request from Immunic. Any forward-looking statement made in this release speaks only as of the date of this release. Immunic disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. Immunic expressly disclaims all liability in respect to actions taken or not taken based on any or all the contents of this presentation.

Synopsis
In light of recent cellular data showing that Immunic’s phase 2 asset, IMU-838, is able to inhibit viral replication of SARS-CoV-2, the company is exploring the development of IMU-838 as a potential oral antiviral therapy for COVID-19.
| · | IMU-838 is an orally available DHODH inhibitor withboth, antiviral and anti-inflammatory effects: |
| o | Dual mode of action hypothesized to provide attractive profile in chronic inflammatory conditions |
| o | Host-based mechanism avoids dependence on specific viral proteins and, therefore, offersbroad-spectrum antiviral activity |
| o | Currently in three phase 2 clinical trials: relapsing-remitting multiple sclerosis (RRMS); ulcerative colitis (UC); and primary sclerosing cholangitis (PSC) |
| o | Attractive pharmacokinetic, safety and tolerability profile withmore than 650 individuals exposed to date |
| · | COVID-19 pandemic: IMU-838 recently shown to inhibit viral replication of SARS-CoV-2: |
| o | In cellular assays with SARS-CoV-2 clinical isolates, IMU-838demonstrated antiviral activity at concentrations which are well below the blood concentrations associated with IMU-838 dosing regimens studied in ongoing and previous clinical trials |
| o | Corroborated by recent independent third-party research showingDHODH inhibitor activity against SARS-CoV-2 |
| o | IMU-838 has previouslyshown broad-spectrum antiviral activity (i.e., HIV, HCV, hCMV, Arena, Influenza A) |
| o | IMU-838’shost-basedmode of action and attractive safety profile providespotential synergy with pre-existing antiviral treatments |
| · | Immunic is currently exploring development of IMU-838 as a potential antiviral treatment for COVID-19: |
| o | Adequate drug supply available for immediate human testing |
| o | Exploring feasibility of prospective, multicenter, randomized, placebo-controlled, double-blind phase 2 clinical trial of IMU-838 in patients with moderate COVID-19 disease
|

Immunic: Company and Pipeline Overview
Immunic is a global clinical-stage biopharmaceutical company developing a pipeline of selective oral immunology therapies aimed at treating chronic inflammatory and autoimmune diseases, including relapsing-remitting multiple sclerosis, ulcerative colitis, Crohn’s disease, and psoriasis. The company is developing three small molecule drug candidates:
| · | IMU-838 is an inhibitor of the enzyme DHODH, with a dual mechanism of action of selective immune modulation and host-based antiviral effect; |
| · | IMU-935 is an oral IL-17 inhibitor of RORγt; and |
| · | IMU-856 targets the restoration of the intestinal barrier function. |
Immunic, Inc. is headquartered in New York. Its subsidiary, Immunic AG, is based in Planegg-Martinsried, Germany, where the company’s research and development activities are conducted. Additional subsidiaries are located in Halle (Saale), Germany, and Melbourne, Australia. Immunic currently has 27 employees, including 12 with MDs or PhDs.
DHODH: Introduction and Mode of Action
IMU-838 is a small molecule investigational drug (vidofludimus calcium) under development as an oral tablet formulation for the treatment of relapsing-remitting multiple sclerosis, inflammatory bowel disease and other chronic inflammatory and autoimmune diseases. IMU-838 achieves its biological effect through inhibition of the enzyme dihydroorotate dehydrogenase (DHODH).
Mode of Action
DHODH is an enzyme which catalyzes the rate-limiting step in thede novo biosynthesis of pyrimidines, key building blocks required for the production of nucleic acids such as DNA and RNA. While “normal” cells can satisfy ongoing demands for pyrimidines through the recycling of pre-existing molecules, certain conditions require activede novo pyrimidine biosynthesis through the DHODH pathway in order to keep up with extra-ordinary pyrimidine demand. Two such conditions, both relevant to SARS-CoV-2 infection, are: (1) virally infected cells; and (2) hyper-activated immune cells.

Figure 1: Mode of Action: Targeting DHODH Leads to Inhibition of Viral Replication and Activation of Interferon Independent Antiviral Mechanism
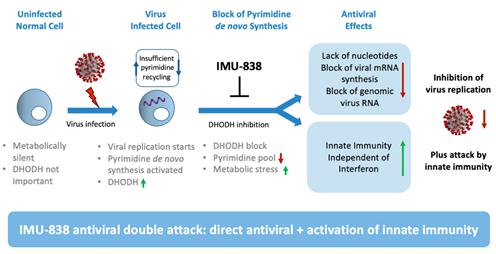
Applicability of DHODH Inhibition During Virus Infection
Virus-infected cells are highly metabolically active, and are critically dependent on DHODH for maintaining their high metabolic turnover.1Viral particles lack the metabolic infrastructure needed to produce raw materials for viral replication.2As a consequence, all known viruses have evolved to rely on the host cell for their supply of these raw materials, including pyrimidines.
Moreover, because the extra-ordinary demand for pyrimidines associated with viral infection cannot be sufficiently supported by nucleotide recycling, thede novo pyrimidine synthesis pathway with DHODH as the rate-limiting enzyme plays a critical role in allowing the virus to replicate and achieve virulence.
In addition, an unsatisfied demand of nucleotides in the host cell also induces stress signals leading to activation of innate immunity.3
Inhibition of Virus RNA Replication
Inhibition ofde novo pyrimidine biosynthesis is well-recognized to achieve an antiviral effect and is hypothesized to work through at least three mechanisms.4First, direct depletion of the host nucleotide pool prevents replication of the viral genome, which is itself necessary for viral replication. Second, inhibition of virus RNA transcription prevents the formation of viral proteins and leads to loss of viral functions (virulence). Third, induction of stress signals due to depletion of the nucleotide pool induces innate immunity by an interferon independent mechanism.
Importantly, DHODH inhibition is thought to achieve these antiviral effects without negatively impacting host proteins and protein function.
________________________________
1 Bonavia, A.et al. (2011)
2Lodish H., Berk A., Zipursky S.L.,et al. (2000)
3Khiar, S., Lucas-Hourani, M., Nisole, S. et al. (2017)
4Garavito, M. F., Narváez-Ortiz, H. Y. & Zimmermann, B. H. (2015)

Induction of the Innate Immunity by Induction or Amplification of Interferon-Stimulated Genes
In addition, DHODH inhibition may also combat viral infection through the induction of innate immunity.5 Here, host cells produce endogenous interferon, a molecule with broad antiviral activity. Unfortunately, certain viruses, such as SARS-CoV-2, are able to block interferon’s effect through the production of virally encoded interferon antagonists.
In infected and DHODH treated cells, the induction of genes inducing an innate immunity response is independent of interferons, but dependent on interferon regulatory transcription factor (IRF1), potentially mediated by Ataxia Telangiectasia Mutated (ATM) expression induced by cellular stress signal.5
Inhibition of Excessive Immune Response by Selectively Blocking Cytokine Production in Hyperactivated Immune Cells
Immunic believes that a key advantage of DHODH inhibition is its sparing of “normal” immune cells and selective effect on hyperactivated ones. In preclinical studies, IMU-838 was able to selectively block cytokine production of hyperactivated immune cells (for example IL-17, IP-10, GM-CSF or IFNγ6). Meanwhile, animals treated with large doses of the active moiety of IMU-838 were shown to lack detrimental effects on bone marrow, supporting the lack of non-specific anti-proliferative effect regularly seen with many traditional immunosuppressants. Likewise, no immune abnormalities have been detected in those patients treated with IMU-838 in clinical trials. In the context of COVID-19, this effect may be helpful for limiting an excessive inflammatory response by hyperactivated immune cells, without impairing broader immune function.
Broad-Spectrum Antiviral Effect of DHODH Inhibition
Given the independence of the antiviral effect with respect to virus specific proteins and their structure, DHODH inhibition is broadly applicable against several viruses.7Such broad-spectrum antiviral effects have been observed in various virus-infected cells, for example against Zika virus8, Ebola virus5 89, MERS-CoV10, SARS-CoV-110, SARS-CoV-28 10, Influenza viruses A and B811, Newcastle disease virus11, vesicular stomatitis virus11, Sindbis virus11, hepatitis C virus11, West Nile virus11, dengue virus11, vaccinia virus11, human adenovirus11, retroviruses such as human immunodeficiency virus11 and human cytomegalovirus12 13.
________________________________
5Luthra, P.et al. (2018)
6Partially unpublished data
7Chung, D.-H.et al. (2016)
8Xiong, R.et al. (2020)
9Martin, S.et al. (2018)
10Cheung, N. N.et al. (2017)
11Hoffmann, H.-H., Kunz, A., Simon, V. A., Palese, P. & Shaw, M. L. (2011)
12Marschall, M.et al. (2013)
13Waldman, W. J.et al. (1999)

IMU-838: Potential New Therapeutic Option for the Treatment of COVID-19
Background COVID-19
The World Health Organization (WHO) declared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections causing coronavirus disease 2019 (COVID-19) a pandemic on March 11, 2020.14 At present, the total number of cases is difficult to accurately quantify but continues to rise and has grown to more than 2,500,000 cases globally.15Main clinical symptoms include fever, cough, myalgia or fatigue, expectoration, and dyspnea.16While a majority of patients do not experience severe symptoms, one meta-analysis found that approximately 18 % of cases were severe.17 Fatality rates are estimated to be approximately 4-7 % at this time.16 17
Figure 2: COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal18
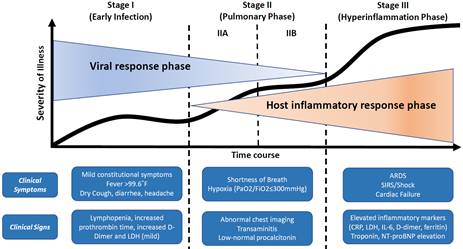
It appears that there are two distinct but overlapping pathological subsets of COVID-19, the first triggered by the virus itself and the second, the host response.
The initial stage occurs at the time of inoculation and early establishment of disease. For most people, this involves an incubation period associated with mild and often non-specific symptoms such as malaise, fever and a dry cough. Treatment at this stage is primarily targeted towards symptomatic relief. Should a viable antiviral therapy be proven beneficial, targeting selected patients during this stage may reduce duration of symptoms, minimize contagiousness and prevent progression of severity.
________________________________
14World Health Organization (03 2020)
15Johns Hopkins University & Medicine (2020)
16Li, L.-Q.et al. (2020)
17Sun, P.et al. (2020)
18Siddiqi H. and Mehra M. (2020)

In the second stage of established pulmonary disease, viral multiplication and localized inflammation in the lung is the norm. During this stage, patients develop a viral pneumonia, with cough, fever and possibly hypoxia. Treatment primarily consists of supportive measures and available antiviral therapies. If hypoxia ensues (stage IIb), it is likely that patients will progress to requiring mechanical ventilation and, in this situation, the use of anti-inflammatory therapy may be useful.
A minority of COVID-19 patients will transition into the third and most severe stage of illness, which manifests as an extra-pulmonary systemic hyperinflammation syndrome. In this stage, markers of systemic inflammation appear to be elevated, including cytokines, D-dimers and C-reactive protein. In this stage, shock, vasoplegia, respiratory failure and even cardiopulmonary collapse are discernable. Systemic organ involvement, even myocarditis, would manifest during this stage. Tailored therapy in stage III hinges on the use of immunomodulatory agents to reduce systemic inflammation before it overwhelmingly results in multi-organ dysfunction.
Scientific Rationale: Potential Advantages of DHODH Inhibitors for the Treatment of COVID-19
No vaccines are currently available for SARS CoV-2.
Although numerous pharmacologic approaches are being investigated clinically, no drug has yet demonstrated clear efficacy. That said, a recent publication from scientists in Wuhan, China8 shows strong activity of DHODH inhibitors against SARS-CoV-2 inin vitro cellular studies. New DHODH inhibitors were tested next to the already established DHODH inhibitors, leflunomide/teriflunomide and Brequinar. However, due to unfavorable pharmacokinetic profiles (leflunomide/teriflunomide) and toxicity (Brequinar), these drugs are not suitable as acute antiviral treatments. In addition, although the new DHODH inhibitors seem promising, so far, no animal safety data and no human safety data were available. Developing actual drugs from these new DHODH inhibitors may take significant time, even if accelerated by support from regulatory authorities. However, the study from scientists in Wuhan, China clearly established the strong activity of several DHODH inhibitors against SARS-CoV-2.
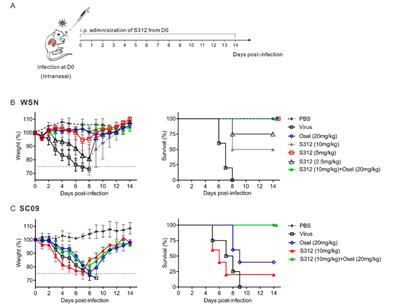
The same Wuhan scientists8 also showed a potential for heightened efficacy through the combination of DHODH inhibitors with existing antiviral drugs. Direct-acting antivirals such as neuraminidase inhibitors were primarily effective in the early phase of infection (i.e., within 48 hours of symptom onset), presumably because direct-acting antivirals target the viral replication cycle. Because DHODH inhibitors target a host cell factor, DHODH inhibition may also be effective in middle-to-late phases of infection or in patients with advanced respiratory disease. To test this hypothesis, Xiong et al.8 evaluated thein vivo efficacy of the a DHODH inhibitor compared to oseltamivir in early, middle-to-late, and severe late phase Influenza A infection (WSN or 2009 pandemic H1N1 virus). When mice were administered oseltamivir in middle-to-late phase, survival was 0 % after 14 days. However, survival was 50 % when treated with the DHODH inhibitor alone and 100 % when treated with combination therapy (DHODH inhibitor plus oseltamivir).

Figure 3: In Vivo Antiviral Activity in Influenza A Virus-Infected Mice8
Targeting DHODH provides a selective mechanism to fight chronic inflammatory diseases, cancer and virus infections, because normally growing cells with a limited metabolic turnover recycle pyrimidines and no pyrimidinede novo synthesis of pyrimidines is needed. For the fast dividing cells in the organism, and this has been demonstrated in several studies, the inhibition of DHODH does not produce general immunosuppression. It was shown for teriflunomide that patients can even be vaccinated under therapy. For IMU-838, Immunic has demonstrated that DHODH inhibition does not lead to suppression of bone marrow cells or inhibition of fast-growing cells. In preclinical and clinical studies, Immunic has not seen off-target effects, such as neutropenia, diarrhea or alopecia, at rates higher than placebo.19
The antiviral effect of DHODH inhibitors is well established. Literature provides information about antiviral effects by DHODH inhibition for several RNA and DNA viruses, such as Ebola virus5 8 9, human immunodeficiency virus11, hepatitis C virus11, human cytomegalovirus12 13 and Influenza8 11 viruses, among others. It has even been shown that while other antiviral drugs are able to inhibit viral infection up to four hours after infection, DHODH inhibitors are still effective up to 12-16 hours after infection inin vitro assays.11 20 A direct as well as an indirect antiviral effect has been reported for DHODH inhibitors. These effects are both linked to blocking pyrimidinede novo synthesis. In the direct pathway, the reduction of pyrimidinede novo synthesis is interfering with the viral transcription and replication.11 However, Lucas-Hourani et al.21reported that the main antiviral effect might rather be an indirect effect via the induction or amplification of interferon-stimulated genes due to the reduced pyrimidine synthesis. A large fraction of these genes plays a role in the host innate immune defense to viruses. Interestingly, while these genes are normally induced by interferons, the upregulation of these genes by DHODH inhibitors is independent of interferons.5 7 21 22 The pathway seems to be interferon regulatory transcription factor 1 (IRF1) dependent.5 7 21 A recent publication also reported that this process is regulated by the kinase ATM, which is induced upon cellular stress that is induced by the DHODH inhibitor inhibited DNA synthesis.5
________________________________
19Muehler, A., Kohlhof, H., Groeppel, M., & Vitt, D. (2019)
20Wang, Q.-Y.et al. (2011)
21Lucas-Hourani, M.et al. (2013)
22Wang, Y.et al. (2016)

IMU-838: Attractive Safety and Tolerability Profile
Good Laboratory Practice (GLP) toxicology studies in animals have been completed and the toxicology profile of IMU-838 is well established. Moreover, clinical data suggests that the drug is well tolerated with relatively clean safety profile. A recent paper published by Immunic19 summarized the safety profile of vidofludimus, the active component in IMU-838, in a phase 2 clinical study in a population of patients with rheumatoid arthritis. The table below summarizes the rates of those specific adverse events that are known to occur from other DHODH inhibitors and other immunomodulating drugs. However, the data shows that vidofludimus does not have these adverse events at rates higher than placebo.
Table 1: Vidofludimus Does Not Show Known Adverse Events at Rates Higher Than Placebo19
MedDRA SOC | MedDRA Preferred Term | Vidofludimus (N = 122) | Placebo (N = 119) | Total (N = 241) |
| Gastrointestinal Disorders | Diarrhea | 7 (5.7%) | 7 (5.9%) | 14 (5.8%) |
| Skin and Subcutaneous Tissue Disorders | Alopecia | 1 (0.8%) | 0 (0.0%) | 1 (0.4%) |
| Blood and Lymphatic System Disorders | White Blood Cell Disorder | 0 (0.0%) | 1 (0.8%) | 1 (0.4%) |
| Investigations | Neutrophil Count Abnormal | 0 (0.0%) | 1 (0.8%) | 1 (0.4%) |
MedDRA Medical Dictionary for Regulatory Activities,N number of patients,SOC system organ class
This data suggests that vidofludimus has no general antiproliferative effects, but a very selective effect on the immune system. As such, Immunic believes that IMU-838’s safety profile is amenable to testing in COVID-19.
IMU-838: Broad-Spectrum Antiviral Activity
IMU-838 is currently theonly orally available, selective DHODH inhibitor in clinical development for non-oncologic indications. The active ingredient, vidofludimus calcium, is rapidly taken up into the blood stream of patients. IMU-838’s blood half-life is about 30 hours and it reaches therapeutic levels within a few hours. GLP toxicology studies in animals have been performed and the toxicology profile of the drug is well established and benign. The good safety profile has already been demonstrated in about 650 individuals and was reported to be similar to placebo in clinical trials. Therefore, IMU-838, with its target DHODH, seems to be a valid option for the treatment of COVID-19.

Table 2: IMU-838 is the Only DHODH Inhibitor with Proven In Vitro Efficacy on Several Viruses and an Acceptable Safety and Pharmacokinetic Profile1023 24 25
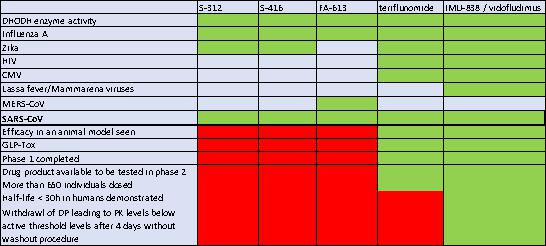

Immunic has demonstrated that IMU-838 has broad-spectrum antiviral activity at concentrations achieved in the blood of treated patients (plasma levels 10-30 µM). The antiviral activity of IMU-838 against human immunodeficiency virus (HIV), hepatitis C virus (HCV), human cytomegalovirus (hCMV), Arenavirus and Influenza A virus was tested in cell culture experiments.
Human peripheral blood mononuclear cells (PBMCs), seronegative for HIV and hepatitis B virus, were infectedin vitro with HIV-191US005 (CCR5-tropic, Subtype B). Reverse transcriptase activity and the appearance of the specific HIV antigen p24 were evaluated at different IMU-838 concentrations. The median effective dose (ED50) was approximately 2 µM.26
IMU-838 was also evaluated in a Huh7 human hepatoma cell line harboring an HCV sub genomic replicon of genotype 1b with a stable luciferase (Luc) reporter and three cell culture-adaptive mutations. The mean ED50 was 4.6 µM.26
Subsequently, IMU-838 has been demonstrated to be active against viruses of the herpes family. It was shown that IMU-838 has a good activity of around 7.4 µM in an infection model of human fibroblasts infected with hCMV viruses. This activity was within the same range of ganciclovir, which is used in clinical practice specifically for treatment of hCMV.27
Finally, in cellular A549 and Vero models of mammalian Arenavirus infections, the RNA virus causing human hemorrhagic fever diseases like Lassa fever, IMU-838 demonstrated activity with an IC50 of 2.8 µM. In addition, it was shown that in this case, DHODH inhibition-mediated effects against this virus type are independent of IFN signaling.28
________________________________
23Li, G. & Clercq, E. D. (2020)
24bioRxiv (2020)
25The Lancet (2020)
26Unpublished data
27Kohlhof, H. (2019)
28Manuscript in preparation

Table 3: IMU-838 is Broadly Active Against Various RNA and DNA Viruses
| | IMU-838 IC50 | Test System |
| hDHODH | 0.12 µM | In vitro, protein, human |
| mDHODH | 10.0 µM | In vitro, protein, mouse |
| Cytotox on Fibroblasts | > 30 µM | Cellular |
| Cytotx on HepG2 cells | > 50 µM | Cellular |
| HIV | 2.2 µM 2.0 µM | Reverse transcriptase
p24 |
| HCV | 4.6 µM | Replicon, Luciferase |
| hCMV | 7.4 µM | Replication, green fluorescent protein |
| Lassa Fever Arenavirus | 2.8 µM | Plaque reduction, virus protein expression |
Besides the preclinical mode of action studies, GLP toxicology studies in animals have been completed and the toxicology profile of the drug is well established. Another advantage of IMU-838, besides safety and tolerability, is that the compound is a small molecule drug formulated as a white tablet with a diameter of only 8 mm that is taken orally, once-daily. Immunic has an active Investigational New Drug (IND) status approved by the U.S. Food and Drug Administration (FDA) to perform clinical trials in the United States. Additionally, clinical trials with IMU-838 are ongoing in several European countries. Good Manufacturing Practice (GMP) tablet production is established and sufficient quantities of the drug are available for current clinical trials.
IMU-838: Preclinical Activity Against SARS-CoV-2
Immunic is currently testing IMU-838 in several preclinical setups with different partners. On April 21, 2020, Immunic reported that IMU-838 has successfully demonstrated preclinical activity against SARS-CoV-2. More specifically, IMU-838 was observed to inhibit replication of clinical isolates of SARS-CoV-2 associated with COVID-19. In cellular assays, IMU-838 demonstrated this antiviral activity at concentrations which are well below the blood concentrations associated with IMU-838 dosing regimens studied in ongoing and previous clinical trials. These positive results have encouraged Immunic to prepare a clinical development program for IMU-838 as a potential treatment option for patients with COVID-19 and potential other, future viral pandemics.

IMU-838: Development Plan in COVID-19
IMU-838 is a second generation DHODH inhibitor with improved properties:
| · | Small (diameter of only 8 mm) white tablet for oral use |
| · | Blood half-life of about 30 hours, reaches therapeutic levels within a few hours |
| · | No off-target effects: no signal for liver problems19; no increased rates of side effects19 like diarrhea, neutropenia, or alopecia; no increased rate of infections versus placebo controls19 |
IMU-838 is already being investigated in ongoing phase 2 clinical trials in patients with relapsing-remitting multiple sclerosis, ulcerative colitis and primary sclerosing cholangitis. Immunic has an active IND status in the United States and numerous regulatory approvals for clinical studies in a range of countries. Although the drug is being studied in these ongoing trials primarily for its anti-inflammatory effect, one of IMU-838’s postulated benefits is a host-based antiviral effect, which may be important in these indications to potentially prevent virus reactivations known to occur with other immunomodulatory therapies.
In support, IMU-838’s antiviral activity has previously been demonstratedin vitro against human immunodeficiency virus (HIV), hepatitis C virus (HCV), human cytomegalovirus (hCMV), Arenavirus and Influenza A virus. Given what is known about the natural course of the disease, IMU-838’s combination of antiviral activity against the highly pathogenic SARS-CoV-2 and a selective immunomodulatory effect against highly activated immune cells may be a promising profile for the treatment of COVID-19. Importantly, IMU-838 has an attractive pharmacokinetic, safety and tolerability profile and, to date, has already been tested in about 650 individuals. The safety profile in previous trials was similar to placebo.20 In light of the ongoing COVID-19 pandemic as well as Immunic’s recent positive preclinical data showing activity against SARS-CoV-2 and previous third-party data suggesting a role for DHODH therapy, Immunic now seeks to more actively pursue IMU-838’s potential as a potential oral antiviral therapy for COVID-19.
Immunic is collaborating with several regulatory agencies and other institutions in the United States and in Europe to define and accelerate the development path for IMU-838 in COVID-19. A clinical study protocol with the intent to begin clinical testing as soon as reasonably possible is currently being discussed. The aim is to investigate the DHODH inhibitor, IMU-838, as an oral treatment option for COVID-19 and to enable the use of IMU-838 in treating current and potential future pandemic threats. The plan is to evaluate efficacy, safety and tolerability of IMU-838 in a clinical setting by performing a phase 2 clinical trial with the intent of achieving proof-of-efficacy, and afterwards, expanding such a study for potentially achieving conditional approval, as quickly as reasonably possible.
Immunic intends to initiate a prospective, multicenter, randomized, placebo-controlled, double-blind phase 2 clinical trial in patients with moderate COVID-19 disease and clinical symptoms. Adequate drug supply exists to begin clinical testing in COVID-19 very soon. The plan is to test IMU-838 versus placebo on the background of investigator’s choice of standard-of-care therapy used in both treatment arms:
| · | Arm 1: 2 x 22.5 mg IMU-838 + investigator’s choice of standard-of-care therapy |
| · | Arm 2: 2 x placebo + investigator’s choice of standard-of-care therapy |
The envisaged COVID-19 program is a strategic addition to Immunic’s core business and current therapeutic focus, but ethically, is an important step for the company and for the public health. Implementation of this program requires a broad set of activities and, therefore, Immunic is actively exploring additional sources to expand the current funding of this important new potential application for IMU-838. Immunic is actively looking at different private and publicly available funding opportunities, including public grants, and is proactively working to pursue these options, some of which may involve partners. At the same time, Immunic continues to progress the company’s non-viral programs as previously planned.

In the current situation of the COVID-19 pandemic, approval for clinical trials as well as production, importation and distribution of IMU-838 for affected patients will most likely be negotiated between Immunic and relevant governmental authorities on an expedited basis. In addition, the efficient use of IMU-838 for treating the current outbreak or preventing future outbreaks of highly pathogenic viruses requires stockpiling by relevant authorities in all major countries. Immunic is therefore aiming for regulatory approval from the respective authorities of the targeted markets.
COVID-19: Access and Distribution
Immunic’s ethical conviction is that IMU-838 should be made available for all countries affected by the COVID-19 pandemic, worldwide. However, the clinical testing may require selecting the most appropriate countries for patient access. In addition, Immunic is aiming to support preventive measures for avoiding future comparable outbreaks. IMU-838 would most likely be reserved for use when needed for emergencies or supply shortages, which will effectively provide countries with the capacity for rapid response to emergency situations, such as seen with COVID-19. Fortunately, the mode of action of IMU-838 makes it more likely that the drug may have respective antiviral activity against yet unknown, but potential and even more dangerous strains and genetic variants of SARS-CoV-2 and other highly pathogenic viruses.
To fight this unprecedented global public health crisis, Immunic believes it is important that companies, academic institutions and foundations all work together to pursue parallel approaches towards a shared goal: to find effective treatments for COVID-19 patients. The best minds in science and medicine are coming together to solve this – as seen with the many collaborations taking place, and many businesses feel an ethical obligation to contribute as much as possible to the advancement of science. Immunic’s role is in advancing a compound that has previously demonstrated antiviral activities and has a different approach, like many other therapeutic options. IMU-838 targets the virus-infected host cell and prevents the host cells from being turned into an effective machine producing massive amounts of new viruses. Immunic believes this is a unique approach which may even offer the potential of combining IMU-838 with effective antiviral drugs.

Conclusion
Immunic’s overall goal is totest IMU-838 and secure approvalas early as reasonably possiblefor treating a deadly threat to the public health. Immunic will rapidly apply its science to play whatever reasonable role the company can in finding an effective treatment in the worldwide fight against the COVID-19 pandemic.
In summary, Immunic believes that IMU-838, as a DHODH inhibitor, exhibits many advantages compared to other antiviral treatment options. As Immunic’s recent data confirms, DHODH inhibition selectively blocks thede novo production of pyrimidines, an essential RNA building block, in metabolically activated cells such as virus-infected cells. In addition, DHODH inhibitors may help reduce the severity or virulence of infection through several mechanisms. First, DHODH inhibition prevents the production of viral RNA and proteins and, therefore, prevents viral replication. Second, it induces innate immunity in an interferon independent setting as an early host-based antiviral response. Third, DHODH inhibition may ameliorate the overshooting immune response, as seen in severe COVID-19 cases, by selectively targeting highly activated immune cells, but without broader anti-proliferative or immunosuppressive effect. Moreover, given that IMU-838 targets a step performed by the infected host cell and not the virus itself, Immunic believes that IMU-838 may also provide an approach that is relatively protected from the development of drug resistance. In COVID-19, this would also potentially allow to combine the host cell-targeted treatment IMU-838 with effective antiviral treatments.
Based on an attractive pharmacokinetic, safety and tolerability profile, with about 650 individuals exposed to date, and the advantages of being a selective DHODH inhibitor, IMU-838 could be a convenient, oral treatment option for COVID-19 patients.

Background IMU-838: Development History and Ongoing Studies
IMU-838 is a small molecule investigational drug (vidofludimus calcium) under development as an oral tablet formulation for the treatment of relapsing-remitting multiple sclerosis (RRMS), inflammatory bowel disease (IBD) and other chronic inflammatory and autoimmune diseases.
Initial clinical trials were conducted using a free acid formulation of the active moiety of IMU-838, vidofludimus, and an amorphous material. In total, this clinical trial data encompasses more than 250 patients treated with the active moiety, helping generate a safety database to encourage further development of IMU-838. Immunic developed and patented a new specific polymorph of the calcium salt formulation of vidofludimus, IMU-838, which Immunic believes exhibits improved physicochemical and pharmacokinetic properties.
Immunic has used and continues to use its IMU-838 formulation in its drug development activities. In 2017, Immunic completed two phase 1 studies of single or repeated once-daily doses of IMU-838 in healthy volunteers, where Immunic observed results supporting tolerability of repeated daily dosing of up to 50 mg of IMU-838.
A phase 2 study in patients with RRMS is currently ongoing, with enrollment of 210 patients completed in October 2019 and unblinded top-line data expected to be available in the third quarter of 2020. A second phase 2 study in patients with ulcerative colitis (UC) is also ongoing, with enrollment initiated in April 2018 and top-line data expected to be available during the fourth quarter of 2021. Furthermore, Immunic’s collaboration partner, the Mayo Clinic, has started an investigator-sponsored proof-of-concept clinical trial testing IMU-838 activity in patients with primary sclerosing cholangitis (PSC).

Company Background
Immunic AG was founded in 2016 with headquarters in Planegg-Martinsried, Germany. Since April 2019, the company has been operating under the name Immunic, Inc. with its registered office in the United States and has been trading on The Nasdaq Stock Market under the ticker symbol ”IMUX.” The listing followed a reverse takeover transaction with San Diego-based Vital Therapies, Inc., as announced in January 2019. The company's research and development activities continue to be conducted in Planegg-Martinsried near Munich, Germany.
Immunic is led by a team of dedicated and committed experienced professionals with an entrepreneurial spirit and proven track record in the healthcare industry worldwide (EU, United States and Asia). The team brings together more than 90 years of leadership experience in the pharmaceutical industry with a strong scientific background and sound knowledge in drug discovery, product development, chemistry, manufacturing and controls processes, intellectual property, clinical trial design, health economics and market access, merger and acquisitions, capital markets, corporate finance, business development, regulatory affairs and project valuation. Immunic’s team members are inventors on project-related patents and have successfully published project-related scientific publications.
Immunic AG acquired IMU-838 and IMU-935 in September 2016 from 4SC AG, a publicly traded company based in Planegg-Martinsried near Munich, Germany, which was co-founded in 1997 by Immunic’s Chief Executive Officer, Dr. Daniel Vitt, through asset acquisitions. As part of the transaction, 4SC is entitled to receive a royalty on net sales if products originating from this contract achieve market approval.
Immunic’s rights to IMU-856 are secured pursuant to an option and license agreement with Daiichi Sankyo Co., Ltd. in Tokyo, Japan. On January 5, 2020, Immunic AG exercised its option under the Daiichi Sankyo Option and acquired the exclusive global rights to commercialize IMU-856. The license also grants Immunic AG the rights to Daiichi Sankyo’s patent application related to IMU-856. Concurrent with the option exercise, Immunic AG paid to Daiichi Sankyo a one-time upfront licensing fee. Going forward, Daiichi Sankyo is eligible to receive future development, regulatory and sales milestone payments, as well as royalties related to IMU-856. Financial terms of the agreement have not been disclosed.

List of References
bioRxiv (2020): Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. https://www.biorxiv.org/content/10.1101/2020.03.11.983056v1.
Bonavia, A.et al. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV).Proc. Natl. Acad. Sci. 108, 6739–6744 (2011).
Cheung, N. N.et al. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response.J. Gen. Virol. 98, 946–954 (2017).
Chung, D.-H.et al. Discovery of a Broad-Spectrum Antiviral Compound That Inhibits Pyrimidine Biosynthesis and Establishes a Type 1 Interferon-Independent Antiviral State.Antimicrob. Agents Chemother. 60, 4552–4562 (2016).
Garavito, M. F., Narváez-Ortiz, H. Y. & Zimmermann, B. H. Pyrimidine Metabolism: Dynamic and Versatile Pathways in Pathogens and Cellular Development.J Genet Genomics 42, 195–205 (2015).
Hoffmann, H.-H., Kunz, A., Simon, V. A., Palese, P. & Shaw, M. L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis.Proc. Natl. Acad. Sci. 108, 5777–5782 (2011).
Johns Hopkins University & Medicine (2020): Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html
Khiar, S., Lucas-Hourani, M., Nisole, S. et al. Identification of a small molecule that primes the type I interferon response to cytosolic DNA. Sci Rep 7, 2561 (2017). https://doi.org/10.1038/s41598-017-02776-z
Kohlhof, H. Immunic Therapeutics: IMU-838 in Inflammatory Bowel disease, new oral treatment with promising safety profile. Oral presentation, GI Inflammatory Diseases Summit (GIIDS), Boston. (2019).
Li, G. & Clercq, E. D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV).Nat. Rev. Drug Discov. 19, 149–150 (2020).
Li, L.-Q.et al. 2019 novel coronavirus patients’ clinical characteristics, discharge rate and fatality rate of meta-analysis.J. Med. Virol. (2020) doi:10.1002/jmv.25757.
Lodish H., Berk A., Zipursky S.L.,et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. https://www.ncbi.nlm.nih.gov/books/NBK21475/
Lucas-Hourani, M.et al. Inhibition of Pyrimidine Biosynthesis Pathway Suppresses Viral Growth through Innate Immunity.PLoS Pathog. 9, e1003678 (2013).
Luthra, P.et al. Inhibiting pyrimidine biosynthesis impairs Ebola virus replication through depletion of nucleoside pools and activation of innate immune responses.Antiviral Res. 158, 288–302 (2018).
Marschall, M.et al. Assessment of drug candidates for broad-spectrum antiviral therapy targeting cellular pyrimidine biosynthesis.Antiviral Res. 100, 640–648 (2013).
Martin, S.et al. A genome-wide siRNA screen identifies a druggable host pathway essential for the Ebola virus life cycle.Genome Med. 10, 58 (2018).
Muehler, A., Kohlhof, H., Groeppel, M., & Vitt, D. (2019). The Selective Oral Immunomodulator Vidofludimus in Patients with Active Rheumatoid Arthritis: Safety Results from the COMPONENT Study. Drugs in R&D, 19(4), 351–366. https://doi.org/10.1007/s40268-019-00286-z

Siddiqi H. and Mehra M., COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. Journal of Heart and Lung Transplantation. doi: 10.1016/j.healun.2020.03.012
Sun, P.et al. Clinical characteristics of 50466 hospitalized patients with 2019-nCoV infection.J. Med. Virol. (2020) doi:10.1002/jmv.25735.
The Lancet (2020): How will country-based mitigation measures influence the course of the COVID-19 epidemic? https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30567-5/fulltext.
Waldman, W. J.et al. Inhibition of Cytomegalovirus in vitro and in vivo by the Experimental Immunosuppressive Agent Leflunomide. Intervirology. 1999;42(5-6):412-8.
Wang, Q.-Y.et al. Inhibition of Dengue Virus through Suppression of Host Pyrimidine Biosynthesis.J. Virol. 85, 6548–6556 (2011).
Wang, Y.et al. Cross Talk between Nucleotide Synthesis Pathways with Cellular Immunity in Constraining Hepatitis E Virus Replication.Antimicrob. Agents Chemother. 60, 2834–2848 (2016).
World Health Organization (02 2020): WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis. February 18, 2020. Geneva: World Health Organization. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
World Health Organization (03 2020): WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
Xiong, R.et al. Novel and potent inhibitors targeting DHODH, a rate-limiting enzyme in de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2. http://biorxiv.org/lookup/doi/10.1101/2020.03.11.983056 (2020) doi:10.1101/2020.03.11.983056.
List of Figures
| Figure 1: Mode of Action: Targeting DHODH Leads to Inhibition of Viral Replication and Activation of Interferon Independent Antiviral Mechanism | 5 |
| Figure 2: COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal | 7 |
| Figure 3: In Vivo Antiviral Activity in Influenza A Virus-Infected Mice | 9 |
List of Tables
| Table 1: Vidofludimus Does Not Show Known Adverse Events at Rates Higher Than Placebo | 10 |
| Table 2: IMU-838 is the Only DHODH Inhibitor with Proven In Vitro Efficacy on Several Viruses and an Acceptable Safety and Pharmacokinetic Profile | 11 |
| Table 3: IMU-838 is Broadly Active Against Various RNA and DNA Viruses | 12 |
19





