Exhibit 99.1
Idiotype (Id) immunotherapy (IT) following high dose therapy and autologous stem cell transplant (HDT/ASCT) in mantle cell (MC) and indolent lymphoma (IL): cellular, humoral and clinical responses
P. R. Holman, M. deMagalhaes-Silverman, B. Medina, S. Corringham, A. Bashey, J. Castro, E. Carrier, T. Lane, D. P. Gold, E. D. Ball; Univ of California-San Diego, La Jolla, CA; University of Iowa, Iowa City, IA; Favrille Inc, San Diego, CA
INTRODUCTION
MCL accounts for 6% of all non-Hodgkin’s lymphoma (NHL). It is generally considered an incurable disease. Although high response rates can be achieved with initial chemotherapy (+/- immunotherapy), median survival is only 3-4 years. Intensified consolidation with HDT (+/- radiotherapy) and ASCT has been reported to improve progression free survival (PFS)(1, 2) but patients eventually relapse.
Indolent lymphoma accounts for 35% of all NHL and is associated with a median survival of 9 years. Like MCL, it is also generally considered to be incurable. PFS also appears to be improved following HDT/ASCT (3).
Both MCL and IL can respond to immunotherapy. The most studied immunotherapy in NHL is currently the anti-CD20 antibody, rituximab (4, 5). Allogeneic transplantation via a graft-versus-lymphoma effect appears to be a potentially curative therapy, at least in some cases (6, 7).
An additional immunotherapeutic approach is Id vaccination. The Id is the hypervariable region of the immunoglobulin receptor, which is generally identical on all affected cells. Coupling of the Id protein to KLH (keyhole limpet hemocyanin) and simultaneous local administration of GM-CSF results in augmented immune responses. Clinical studies have demonstrated the induction of both cellular and humoral anti-Id responses following chemotherapy, accompanied by clinical benefit (8, 9). Patients with molecularly detectable disease following chemotherapy have achieved molecular remissions following Id vaccination (10).
Against this background, we initiated a phase 2 trial evaluating Id vaccination following high dose therapy and autologous stem cell transplantation for patients with mantle cell lymphoma, indolent NHL and transformed NHL to evaluate the feasibility and effectiveness of this post autologous transplant immunotherapeutic strategy.
STUDY OBJECTIVES
• To evaluate the ability of Id-KLH to induce immune responses when administered following HDT/ASCT for MCL, indolent NHL and transformed NHL
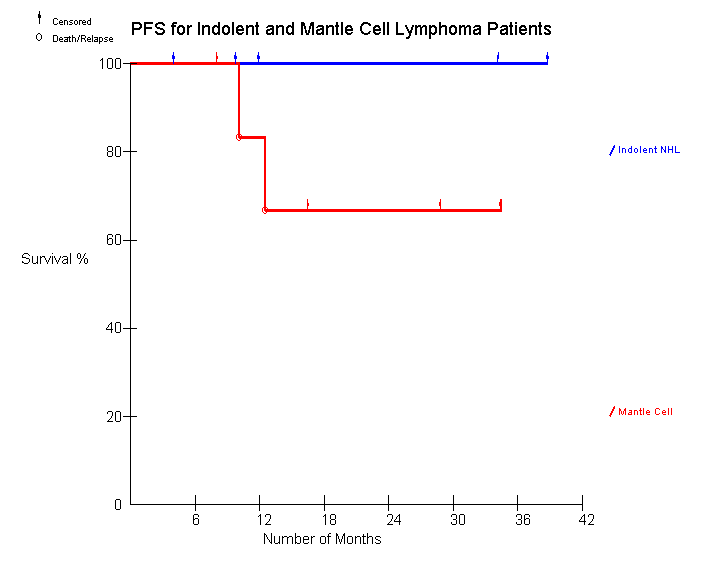
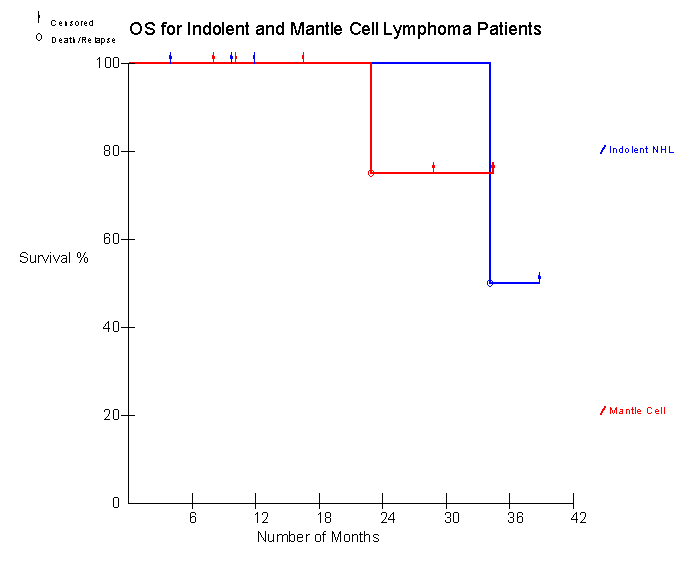
• To evaluate PFS for patients receiving Id-KLH post ASCT
KEY ELIGIBILITY CRITERIA
• Follicular Grade 1 or 2 NHL, small lymphocytic lymphoma or MCL
• Tissue availability (via biopsy) for Id vaccine preparation
• Age 18-70
• Previously treated or newly diagnosed
• No prior splenectomy
• No ongoing steroid requirement
• Meets institutional criteria for HDT/ASCT
PATIENTS AND METHODS
13 patients, (8 MCL, 4 IL, 1 TL) underwent biopsy to obtain lymph node tumor material for Id vaccine preparation.
Cytoreductive chemotherapy +/- antibody therapy was chosen and administered at the discretion of the treating physician.
Stem cell collection was undertaken
Patients proceeded to HDT/ASCT following an adequate stem cell collection Starting a minimum of 3 months post ASCT, patients received 5 Id immunizations over a 6-month period.
PATIENT CHARACTERISTICS
Patient | | Histology | | Age | | Sex | | Most Recent Regimen | |
1 | | MCL | | 63 | | M | | CHOP HyperCVAD | |
2 | | MCL | | 61 | | M | | HyperCVAD | |
3 | | MCL | | 56 | | M | | HyperCVAD | |
4 | | MCL | | 62 | | M | | HyperCVAD/Rituximab | |
5 | | MCL | | 60 | | M | | CHOP-R/R-ICE | |
6 | | MCL | | 52 | | M | | HyperCVAD | |
7 | | MCL | | 66 | | M | | CHOP-R | |
8 | | MCL | | 50 | | M | | HyperCVAD/Rituximab | |
9 | | IL | | 54 | | M | | ESHAP | |
10 | | IL | | 54 | | F | | CHOP | |
11 | | IL | | 54 | | F | | Zevalin | |
12 | | IL | | 48 | | M | | Fludarabine-Mitoxantrone | |
13 | | TL | | 51 | | M | | ESHAP | |
Id PRODUCTION
The Id genes are molecularly cloned from the lymph node biopsy. They are then cloned into a baculovirus expression vector and introduced into an insect cell expression system. The resulting IgG1 antibody contains k or l light chain, depending on the light chain expressed by the lymphoma cell. The intact antibody is then purified using standard techniques. Following purification, it is conjugated to keyhole limpet hemocyanin (KLH), ready for administration.
EVALUATION OF CELLULAR AND HUMORAL IMMUNE RESPONSES
For cellular immune responses peripheral blood mononuclear cells (PBMC) are cultured with KLH, autologous Id or a control patient Id. We then determine the frequency of CD4+ T cells that stain for cytoplasmic IFN-gamma or TNF-alpha. At any time point measured, a positive T-cell response to KLH is indicated by a result above the mean plus 3 standard
deviations observed in the controls. A positive T-cell response to Id is indicated by a result threefold higher than that observed with the irrelevant control Ig.
To evaluate humoral immune responses, serial dilutions of serum samples are added to wells containing Id Fab fragments, KLH coated wells, Id/KLH or a sham control. The presence of bound antibody is detected using a goat anti-human IgG HRP conjugated antibody preparation in a standard ELISA assay. At any time point measured, a positive response to KLH is indicated by a result above the mean plus 3 standard deviations observed in the controls. A positive response to Id is indicated by a result threefold higher than that observed with the irrelevant control Id.
TREATMENT PLAN
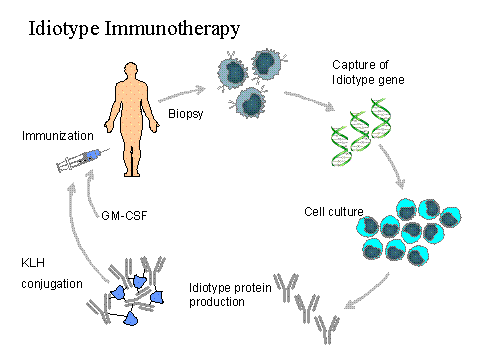
Lymph Node Biopsy:
For vaccine preparation

Cytoreductive Chemotherapy:
At discretion of treating physician

Stem Cell Mobilization + Leukapheresis

High Dose Chemotherapy/Stem Cell Transplant
BEAM

Vaccination: 5 in 6 Months
Day 1: Id-KLH + GM-CSF
Day 2-4: GM-CSF alone
RESULTS
Immune responses to Id vaccination post-ASCT
| | | | Humoral Responsiveness | | Cellular
Responsiveness | |
Patient | | Histology | | Anti-KLH | | Anti-Id | | Anti-KLH | | Anti-Id | |
1 | | MCL | | After 4th V | | After 4th V | | After 1st V | | After 1st V | |
2 | | MCL | | NR | | NR | | NT | | NT | |
3 | | MCL | | After 3rd V | | NSR | | After 1st V | | After 3rd V | |
4 | | MCL | | After 3rd V | | After 3rd V | | After 2nd V | | After 2nd V | |
5 | | MCL | | NR | | NT | | After 2nd V | | After 2nd V | |
6 | | MCL | | NT | | NT | | NT | | NT | |
7 | | MCL | | NT | | NT | | NT | | NT | |
8 | | MCL | | NT | | NT | | NT | | NT | |
9 | | IL | | After 2nd V | | After 2nd V | | After 1st V | | After 1st V | |
10 | | IL | | After 2nd V | | NSR | | After 1st V | | After 5th V | |
11 | | IL | | After 3rd V | | Weak+ after 5thV | | NT | | NT | |
12 | | IL | | NT | | NT | | NT | | NT | |
13 | | IL | | After 3rd V | | Weak+ after 3rd V | | NR | | NR | |
V=vaccination NSR=non-specific response NT=not tested NR=no response
Clinical Responses
Patient | | Histology | | Response Pre
V1 | | Response Pre
V4 | | Response at 9
months | | Response at
14 months | |
1 | | MCL | | CR | | CR | | CR | | CR | |
2 | | MCL | | PR | | CR | | CR | | PD | |
3 | | MCL | | CR | | CR | | CR | | CR | |
4 | | MCL | | CRu | | CRu | | CRu | | CR | |
5 | | MCL | | CR | | CR | | CR | | CR | |
6 | | MCL | | CR | | CR | | PD | | Off study | |
7 | | MCL | | CR | | CR | | N/A | | N/A | |
8 | | MCL | | CR | | CR | | N/A | | N/A | |
9 | | IL | | PR | | CRu | | CR | | CR | |
10 | | IL | | SD | | SD | | SD | | CR | |
11 | | IL | | CR | | CR | | CR | | N/A | |
12 | | IL | | PD | | N/A | | N/A | | N/A | |
13 | | IL | | SD | | SD | | SD | | SD | |
N/A= Time point not reached
Current Remission Status
Patient | | Time since ASCT
(months) | | Status | |
1 | | 43 | | CR | |
2 | | 12 | | PD | |
3 | | 36 | | CR | |
4 | | 31 | | CR | |
5 | | 20 | | CR | |
6 | | 10 | | PD | |
7 | | 10 | | CR | |
8 | | 12 | | CR | |
9 | | 34 | | CR | |
10 | | 41 | | CR | |
11 | | 14 | | CR | |
12 | | 6 | | PD | |
13 | | 20 | | CR | |
TOXICITIES
Adverse Event | | Grade 1 | | Grade 2 | | Grade 3 | | Grade 4 | |
Chills with rigors | | 5 | | | | | | | |
Headache | | 1 | | 3 | | | | | |
Fatigue | | 2 | | 1 | | | | | |
Upper chest congestion | | | | 2 | | | | | |
Injection site reaction | | 4 | | 6 | | | | | |
Head cold | | 3 | | 2 | | | | | |
Itchy/watery eyes | | 1 | | | | | | | |
Blurred vision | | | | 1 | | | | | |
Hotflashes | | | | 1 | | | | | |
Bloating | | 1 | | | | | | | |
Heartburn/Indigestion | | 2 | | 1 | | | | | |
Nausea | | 1 | | | | | | | |
Aching lymph nodes | | 2 | | | | | | | |
Chest pain | | 3 | | | | | | | |
Dyspnea | | | | 1 | | | | | |
Leukopenia | | 1 | | | | 1 | | | |
Vitreous hemorrhage | | | | | | 1 | | | |
Joint stiffness | | 1 | | | | | | | |
Generalized body aches | | 2 | | 1 | | | | | |
Liver abscess | | | | | | 1* | | | |
Infection | | 1 | | | | 2 | | | |
Jaundice | | | | 1 | | | | | |
Thrombocytopenia | | 1 | | 1 | | | | | |
Syncope | | | | | | 1 | | | |
Shingles | | | | 2 | | | | | |
Gynecomastia | | | | 2 | | | | | |
Bone pain | | 1 | | 1 | | | | | |
Myalgia | | 1 | | 1 | | | | | |
Diarrhea | | 2 | | | | | | | |
Tongue discoloration | | 1 | | | | | | | |
Anorexia | | 1 | | | | | | | |
Dizziness | | 1 | | | | | | | |
Abdominal Pain | | 1 | | | | | | | |
Cramps in feet | | 1 | | | | | | | |
N=12
Reporting the greatest grade per patient
*Secondary to biliary stent obstruction
DISCUSSION
These preliminary results indicate that Id vaccination is a feasible immunotherapeutic intervention following autologous stem cell transplantation. Early and Id specific cellular immune responses occur in both MCL and IL patients when vaccinated following high dose chemotherapy and ASCT.
Id vaccination was well tolerated with only minor and transient toxicity.
Of the eight mantle cell lymphoma patients treated, immune responses have been evaluated in five. Of these five patients, an anti-Id T cell response was seen after the first vaccination in one patient, after the second vaccination in two and after the third vaccination in another patient. In another patient (#2) who failed to recover adequate counts post transplant, the cellular immune response was not tested. A cellular anti-KLH response was seen after the first vaccination in two patients and after the second vaccination in another two patients.
Humoral anti-Id responses were seen after the third or fourth vaccination in two patients. One patient had no response and in another patient a nonspecific response occurred. The patient who failed to recover adequate counts developed no humoral response to either KLH or Id. Testing is currently pending on patient 6,7 and 8.
Four out of five indolent lymphoma patients have undergone testing. A cellular anti-KLH response occurred in two patients after the first vaccination. Anti-Id responses occurred are for the first and fifth vaccination respectively. An anti-KLH humoral response occurred after the second vaccination in two patients, and after the third vaccination in another two patients. A humoral anti-Id response occurred after the second vaccination in one patient and weakly, after the third and fifth vaccination in another two patients. An additional patient had no significant response.
Patient #2 with MCL failed to develop any significant immune response, either humoral or cellular, to any component of the immunization. Following the ASCT, this patient failed to adequately engraft and was red cell and platelet transfusion dependant. This patient had a suboptimal CD34+ stem cell collection prior to transplant (>2 but <5 x 106/kg). This patient had a rapid decline in T and B cell numbers post-ASCT. In contrast, the responding patients maintained good levels of T and B cells post-ASCT.
It is currently too early and the number of patients too few to draw any conclusions regarding the clinical responses and their correlation with the immune responses.
The ability of post-transplant NHL patients who have received a number of prior chemotherapy regimens (including the HyperCVAD regimen) to mount Id specific immune responses suggests that the post-transplant period, during lymphocyte recovery, may be an opportune time to administer Id immunizations. In the setting of minimal residual disease, at least as evaluated radiologically, T cell reconstitution may be dependant on the maturation of peripheral T cells present in the graft. The maturing T cells may be antigen driven and with the extra “space” for immune reconstitution, may allow a specific post-transplant advantage to the induction of robust Id specific immune responses. This has been demonstrated in murine studies (11). Further studies will determine whether the period of post-transplant T cell recovery may be a window during which tolerance to Id may be overcome.
CONCLUSIONS
1. Id vaccination following high-dose chemotherapy and autologous stem cell transplant is safe and feasible.
2. Specific cellular and humoral anti-Id responses occur in both mantle cell lymphoma and indolent lymphoma patients post high-dose chemotherapy and autologous stem cell transplant, even when they have been heavily pretreated.
3. Durable remissions occur in heavily pretreated patients with MCL and IL who receive HDT/ASCT followed by Id immunotherapy.
BIBLIOGRAPHY
1. Khouri, I.F., Romaguera, J., Kantarjian, H., Palmer, J.L., Pugh, W.C., Korbling, M., Hagemeister, F., Samuels, B., Rodriguez, A., Giralt, S., et al. 1998. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphoma. J Clin Oncol 16:3803-3809.
2. Dreyling, M., Lenz, G., Hoster, E., Van Hoof, A., Gisselbrecht, C., Schmits, R., Metzner, B., Truemper, L., Reiser, M., Steinhauer, H., et al. 2005. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood 105:2677-2684.
3. Schouten, H.C., Qian, W., Kvaloy, S., Porcellini, A., Hagberg, H., Johnson, H.E., Doorduijn, J.K., Sydes, M.R., and Kvalheim, G. 2003. High-dose therapy improves progression-free survival and survival in relapsed follicular non-Hodgkin’s lymphoma: results from the randomized European CUP trial. J Clin Oncol 21:3918-3927.
4. Foran, J.M., Rohatiner, A.Z., Cunningham, D., Popescu, R.A., Solal-Celigny, P., Ghielmini, M., Coiffier, B., Johnson, P.W., Gisselbrecht, C., Reyes, F., et al. 2000. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol 18:317-324.
5. Foran, J.M., Gupta, R.K., Cunningham, D., Popescu, R.A., Goldstone, A.H., Sweetenham, J.W., Pettengell, R., Johnson, P.W., Bessell, E., Hancock, B., et al. 2000. A UK multicentre phase II study of rituximab (chimaeric anti-CD20 monoclonal antibody) in patients with follicular lymphoma, with PCR monitoring of molecular response. Br J Haematol 109:81-88.
6. Khouri, I.F., and Champlin, R.E. 2004. Nonmyeloablative stem cell transplantation for lymphoma. Semin Oncol 31:22-26.
7. Maris, M.B., Sandmaier, B.M., Storer, B.E., Chauncey, T., Stuart, M.J., Maziarz, R.T., Agura, E., Langston, A.A., Pulsipher, M., Storb, R., et al. 2004. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood 104:3535-3542.
8. Kwak, L.W., Campbell, M.J., Czerwinski, D.K., Hart, S., Miller,
R.A., and Levy, R. 1992. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors [see comments]. N Engl J Med 327:1209-1215.
9. Hsu, F.J., Benike, C., Fagnoni, F., Liles, T.M., Czerwinski, D., Taidi, B., Engleman, E.G., and Levy, R. 1996. Vaccination of patients with B-cell lymphoma using autologous antigen- pulsed dendritic cells. Nat Med 2:52-58.
10. Bendandi, M., Gocke, C.D., Kobrin, C.B., Benko, F.A., Sternas, L.A., Pennington, R., Watson, T.M., Reynolds, C.W., Gause, B.L., Duffey, P.L., et al. 1999. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma [see comments]. Nat Med 5:1171-1177.
11. Mackall, C.L., Bare, C.V., Granger, L.A., Sharrow, S.O., Titus, J.A., and Gress, R.E. 1996. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol 156:4609-4616.