Exhibit 99.1
Prognostic Factors for Time To Progression (TTP) in Patients Receiving
Rituximab Followed by Idiotype Immunotherapy
J Gutheil, S Rosenbush, D Gold, J Bender and the FavId® Study Group*. Favrille, Inc., San Diego, CA USA
Abstract (updated)
Introduction: Idiotype immunotherapy represents a promising approach for patients with B-cell malignancies; however the most appropriate patient population in which to use this therapy remains to be defined. Using data from a multi-center phase II study of Id/KLH (FavId) immunotherapy following cytoreduction with rituximab, we evaluated a series of potential clinical and flow cytometry generated prognostic factors of clinical efficacy as assessed by TTP.
Patients and methods: 103 patients (56 male, 47 female; median age 53 years) with treatment naïve (41) or relapsed/refractory (62) follicular NHL (FL) were enrolled in the study. Patients received rituximab (weekly x 4) followed by FavId (1 mg, subcutaneously) GM-CSF (250ug, daily x 4) starting on week 12. FavId/GM-CSF was administered monthly x 6, then every other month x 6, and then every 3 months until progression. Univariate analysis of potential prognostic factors were performed, using as the predictive variable TTP after rituximab.
Results: The potential clinical prognostic factors evaluated were: age, sex, PS, prior therapy, stage, WHO grade, B-symptoms, marrow/liver/spleen involvement, response to rituximab, number of prior relapses, number/type prior regimens, maximum tumor size, amount of disease, and extranodal disease. The potential laboratory prognostic factors were: lymphocyte count, albumin, hemoglobin, and LDH at the time of study entry. The potential flow cytometric prognostic factors evaluated were: Tumor cell size, CD4/8 ratio, CD3 count, CD4 count, CD5 count, CD8 count, CD20 count, bcl2 index (bcl-2/isotype matched control) and HLA class I and II expression. Univariate analysis identified liver involvement (HR = 6.25, p=0.0007), spleen involvement (HR = 2.1, p=0.043), prior chemotherapy (HR = 1.97, p=0.0384), and number of prior regimens (HR = 1.16, p=0.0054) as associated with a decrease in TTP at
p < 0.05. Multivariate analysis identified liver involvement (HR = 9.325, p=0.0017) and 3 or more prior regimens (HR = 4.765, p=0.0035) as significant. Of the flow cytometry variables tested, only large tumor cell size was associated with a trend (p=0.1) towards significance for a shortened TTP.
Conclusions: In our patients with FL receiving rituximab followed by idiotype immunotherapy, multivariate analyses identified liver involvement and number of prior regimens as significantly associated with a decrease in TTP. The identification of number of prior regimens as a predictor for TTP following idiotype immunotherapy supports the evaluation of this therapy in treatment naïve and less heavily pretreated patients.
Background
Idiotype immunotherapy represents a promising approach for patients with B-cell malignancies; however the relative importance of various clinical and laboratory prognostic factors remains to be defined. An understanding of these prognostic factors will aid in the design of future clinical studies using Id/KLH immunotherapy.
Materials and Methods
Using data from a large phase II study of Id/KLH FavId immunotherapy following cytoreduction with rituximab in patients with follicular NHL, we evaluated a series of potential clinical, laboratory and flow cytometry generated parameters for their potential as prognostic factors of clinical efficacy as assessed by TTP.
Treatment Schema
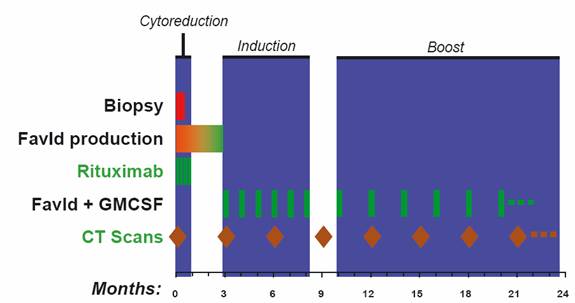
103 patients (56 male, 47 female; median age 53 years) with treatment naïve (41) or relapsed/refractory (62) follicular NHL (FL) were enrolled. Patients received rituximab (weekly x 4) followed by FavId (1 mg, subcutaneously) starting on week 12. FavId was administered monthly x 6, then every other month x 6, and then every 3 months until progression.
Patient Demographics
Characteristic | | Naïve (N=41) | | Relapsed (N=62) | |
Age Median (range) | | 54 (36-83) | | 55 (31-86) | |
| | | | | |
Sex (M/F) | | 22/19 | | 34/28 | |
| | | | | |
PS (0/1) | | 35/6 | | 56/6 | |
| | | | | |
Median baseline tumor | | 40 cm2 (7.5-263) | | 29.9 cm2 (0.5-295) | |
| | | | | |
Prior regimens | | | | | |
| | | | | |
rituximab | | | | 8 | |
| | | | | |
chemotherapy | | | | 26 | |
| | | | | |
chemo/rituximab | | | | 28 | |
Unaudited data
Univariate analysis was performed using standard statistical software with TTP from the time of rituximab as the predictive variable.
Multivariate analyses were performed using 1) all covariates with a univariate p-value < 0.1; 2) backward selection using a p-value cutoff of < 0.1; and 3) best subset selection with a limit of 3, 4, or 5 covariates.
Prognostic Factors Evaluated
• Clinical
• Age
• Sex
• Performance Status
• Prior therapy
• Stage
• WHO grade
• B-symptoms
• Marrow involvement
• Liver involvement
• Spleen involvement
• Response to rituximab
• Number of prior relapses
• Number/type prior regimens
• Maximum tumor size
• Amount of disease
• Extranodal disease
• Flow Cytometry
• Tumor cell size
• CD4/8 ratio
• CD3
• CD4
• CD5
• CD8
• CD20
• Bcl-2 index
• HLA class I expression
• HLA class II expression
• Laboratory
• Lymphocyte count
• Hemoglobin
• Albumin
• LDH
Results
Univariate analysis identified liver involvement (HR = 6.25, p=0.0007), spleen involvement (HR = 2.1, p=0.043), prior chemotherapy (HR = 1.97, p=0.038) and number of prior regimens (HR = 1.16, p=0.0054) as associated with a decrease in TTP at p < 0.05.
Univariate Analysis
Covariate | | # | | C | | HR | | 95% CI | | P-value | |
Liver Involved | | | | | | | | | | | |
(Yes vs No) | | 98 | | 56 | | 6.25 | | (2.2, 18 | ) | 0.0007 | |
Spleen Involved | | | | | | | | | | | |
(Yes vs No) | | 98 | | 56 | | 2.10 | | (1.0, 4.3 | ) | 0.0430 | |
Prior Chemo | | | | | | | | | | | |
(Yes vs No) | | 100 | | 57 | | 1.97 | | (1.0, 3.8 | ) | 0.0384 | |
# Prior Regimens | | | | | | | | | | | |
(>2 vs <3) | | 100 | | 57 | | 1.16 | | (1.4, 7.2 | ) | 0.0054 | |
#: Number of patients, C: Number censored, HR: Hazard ratio
All multivariate analysis models identified liver involvement (HR = 9.3, p=0.0017) and 3 or more prior regimens (HR = 4.7, p=0.0035) as significant.
Multivariate Analysis (Full Model)
Covariate | | HR | | 95% CI | | P-value | |
Liver Involved | | | | | | | |
(Yes vs No) | | 9.325 | | (2.321, 37.47 | ) | 0.0017 | |
# Prior Regimens | | | | | | | |
(>2 vs <3) | | 4.765 | | (1.670, 13.60 | ) | 0.0035 | |
67 Patients; 31 Events; 36 Censored
Of the flow cytometry variables tested, only large tumor cell size was associated with a trend (p=0.1) towards significance.
Conclusions
In our patients with FL receiving rituximab followed by idiotype immunotherapy, each multivariate analysis performed identified liver involvement and number of prior regimens as significantly associated with TTP. The identification of number of prior regimens as a predictor for TTP following idiotype immunotherapy supports the evaluation of this investigational therapy in treatment naïve and less heavily pretreated patients.
*FavId Study Group
• Rene A. Castillo
Ochsner Clinical Foundation,
New Orleans, LA
• John Densmore
University of Virginia,
Charlottesville, VA
• Troy H. Guthrie
University of Florida,
Jacksonville, FL
• John Hainsworth
Sarah Canon CC,
Nashville, TN
• Peter Holman
UCSD,
La Jolla, CA
• Nalini Janakiraman
Henry Ford Hospital,
Detroit, MI
• Lawrence Kaplan
UCSF,
San Francisco, CA
• Omer Koç
Case Western Reserve University,
Cleveland, OH
• Thomas Lin
Ohio State University,
Columbus, OH
• Charles Redfern
Sharp Healthcare,
San Diego, CA
• Fred Rosenfelt
Tower Hematology/Oncology,
Los Angeles, CA
• Peter H. Wiernik
New York Medical College,
Bronx, NY
• Jane N. Winter
Northwestern University,
Chicago, IL

