| ● | changed or increased regulatory restrictions in the U.S., EU or other foreign territories. |
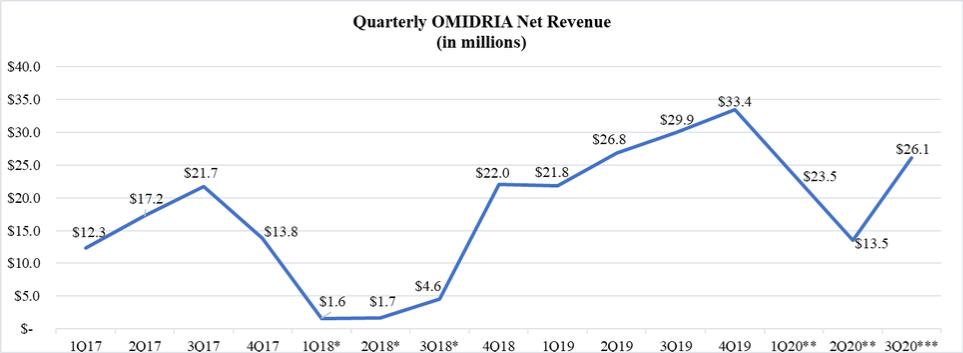
Pass-through reimbursement for OMIDRIA under Medicare Part B expired on October 1, 2020. In the event that we are not able to secure separate payment or similar reimbursement for OMIDRIA, or if there is a delay in securing separate payment or similar reimbursement for OMIDRIA, our revenue from OMIDRIA could decrease significantly as was the case during the period from January 1, 2018 to September 30, 2018, when OMIDRIA was not reimbursed separately when used for procedures involving patients covered by Medicare Part B.
Any decline in sales from OMIDRIA also would impact our ability to borrow under the Loan Agreement since the amount we can borrow is dependent on our eligible receivables.
If legislative and/or administrative means to secure separate payment for OMIDRIA are not successful, we would need to pursue an alternative sales strategy, and our revenues and financial condition could be adversely and significantly affected.
Pass-through reimbursement status allows for separate payment (i.e., outside the packaged payment rate for the surgical procedure) under Medicare Part B. In March 2018, Congress extended pass-through reimbursement status for a small number of drugs, including OMIDRIA, used during procedures performed on Medicare Part B fee-for-service patients through September 30, 2020. In its 2021 OPPS final rule, CMS confirmed the October 1, 2020 expiration of pass-through reimbursement for OMIDRIA and indicated an intention to package payment for OMIDRIA within the ambulatory payment classification for the associated surgical procedure in both the hospital outpatient department and ASC settings. We are continuing to pursue separate payment for OMIDRIA and have submitted to CMS a comment letter and a legal memorandum outlining our position that OMIDRIA meets all of the regulatory criteria established by CMS for separate payment in the ASC setting. However, we can provide no assurances that separate reimbursement for OMIDRIA will be available for the remainder of 2020 and beyond or, if available, that the reimbursement rate will be adequate. If the future reimbursement status of OMIDRIA continues to be uncertain, then demand for OMIDRIA from ASCs and hospitals may be reduced substantially. In such event, sales to our wholesalers may decrease correspondingly, as they adjust on-hand inventory in anticipation of reduced demand from end users.
If we are unable to obtain separate payment for OMIDRIA, we expect to pursue an alternative sales strategy. We may face difficulties or delays in implementing an alternative sales strategy and, even if successfully implemented, we cannot predict how quickly, or if, our customers would increase their OMIDRIA utilization, and the net revenues we receive from sales of OMIDRIA could be reduced, potentially by a significant amount. Additionally, private payers often follow CMS with respect to reimbursement for new drugs, and they may cease or decrease coverage or reimbursement for OMIDRIA if we are unable to obtain separate payment for OMIDRIA from CMS. A reduction in OMIDRIA revenues for these or any other reasons may also impair our ability to borrow under our line of credit facility with Silicon Valley Bank.
Any of these risks, if realized, would adversely affect our ability to generate revenue and attain profitability, and there would be a material adverse effect on our business, financial condition, results of operations and growth prospects and the trading price of our stock could decline.
The spread of COVID-19 and efforts to reduce its transmission may negatively impact our business, operations and financial results.
The COVID-19 pandemic has significantly affected the global economy and has adversely affected our sales of OMIDRIA due to a reduction in the overall volume of cataract surgery and intraocular lens replacement procedures. In March 2020, ASCs and hospitals using OMIDRIA postponed nearly all cataract procedures in response to recommendations from government and medical organizations. As a result, we did not record any sales of OMIDRIA to our wholesalers from March 25 to May 19, 2020. Beginning in the second half of May 2020, cataract surgeries resumed to varying degrees in locations throughout the country. If the number of cataract procedures becomes or continues to be limited, either by necessity for time-consuming safety protocols, reduction in patient demand, or the imposition of prohibitions on elective surgeries in some localities, then we would expect there to be a corresponding reduction in demand for OMIDRIA.
We may also experience disruptions to our operations due to COVID-19, such as delays or disruptions with respect to manufacturing of clinical or commercial drug substance or drug product and delays in our clinical trials or in the submission or review of regulatory applications. Such delays or disruptions could negatively affect our commercial