QuickLinks -- Click here to rapidly navigate through this documentRegistration No. 333-
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
Cyclacel Group plc
(Exact name of Registrant as specified in its charter)
England and Wales
(State or other jurisdiction of
incorporation or organization) | | 2834
(Primary Standard Industrial
Classification Code Number) | | Not Applicable
(I.R.S. Employer
Identification Number) |
James Lindsay Place
Dundee Technopole
Dundee DD1 5JJ
United Kingdom
Telephone: 44 1382 206 062
(Address, including zip code, and telephone number, including
area code, of Registrant's principal executive offices)
CT Corporation System
111 Eighth Avenue
New York, NY 10011
(212) 894-8600
(Name, address, including zip code, and telephone number, including
area code, of agent for service)
Copies to: |
Daniel Cunningham, Esq.
Daniel Epstein, Esq.
Allen & Overy LLP
One New Change
London EC4M 9QQ
United Kingdom
44 20 7330 3000 | | Paul Kumleben, Esq.
Davis Polk & Wardwell
99 Gresham Street
London EC2V 7NG
United Kingdom
44 20 7418 1300 |
Approximate date of commencement of proposed sale to the public:As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. o
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of
Securities to be Registered(1)
| | Amount to
be Registered(2)
| | Proposed Maximum
Offering Price
Per Unit(3)
| | Proposed Maximum
Aggregate
Offering Price(3)
| | Amount of
Registration Fee
|
|---|
|
| Ordinary shares, 1p nominal value per share | | 18,400,000 | | $3.25 | | $59,800,000 | | $7,577 |
|
- (1)
- A separate registration statement on Form F-6 is being filed for the registration of American Depositary Shares evidenced by American Depositary Receipts issuable upon the deposit of shares registered thereby. Each American Depositary Share represents 4 ordinary share(s).
- (2)
- Includes (i) 2,400,000 ordinary shares that the underwriters will have the option to purchase to cover over-allotments, if any, (ii) shares as to which the Joint Global Coordinators may effect borrowing arrangements with a shareholder and (iii) ordinary shares that are to be offered in the United Kingdom and elsewhere outside the United States that may be resold from time to time in the United States during distribution. Offers and sales made outside the United States are not covered by this Registration Statement.
- (3)
- Estimated solely for the purposes of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
PROSPECTUS (Subject to Completion)
Issued July 2, 2004
16,000,000 Ordinary Shares
Cyclacel Group plc
IN THE FORM OF ORDINARY SHARES OR AMERICAN DEPOSITARY SHARES
Cyclacel Group plc, an English public limited company, is offering 16,000,000 ordinary shares in the form of ordinary shares or American Depositary Shares, which we refer to as ADSs. Each ADS represents 4 shares. ADSs will be evidenced by American Depositary Receipts. This is our initial public offering and no public market exists for our shares or ADSs. We anticipate that the initial public offering price will be between £1.52 and £1.80 per share and $11 and $13 per ADS.
We have applied to have the ADSs approved for quotation on the Nasdaq National Market under the symbol "CYCC." Application has been made to the U.K. Listing Authority under the provisions of the Listing Rules for the shares to be admitted to listing on the official list of the U.K. Listing Authority and to London Stock Exchange plc for the shares to be admitted to trading on the London Stock Exchange's main market for listed securities.
Investing in the shares and ADSs involves risks. See "Risk Factors" beginning on page 9.
Price £ a share or $ an ADS
| | Price to Public
| | Underwriting
Discounts and
Commissions
| | Proceeds to Company
|
|---|
| Per share | | | £ | | | £ | | | £ |
| Per ADS | | $ | | | $ | | | $ | |
| Total | | $ | | | $ | | | $ | |
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We have granted the underwriters the right to purchase an additional 2,400,000 shares in the form of ordinary shares or ADSs at £ per share or $ per ADS to cover over-allotments. Morgan Stanley Securities Limited and SG Cowen & Co., LLC expect to deliver the shares and ADSs to purchasers on , 2004.
| |
|
|---|
| MORGAN STANLEY | | SG COWEN & CO. |
|
NEEDHAM & COMPANY, INC. |
|
LEERINK SWANN & COMPANY |
July 2, 2004
TABLE OF CONTENTS
| | Page
|
|---|
| Prospectus Summary | | 2 |
| Risk Factors | | 9 |
| Special Note Regarding Forward-Looking Statements | | 26 |
| Use of Proceeds | | 27 |
| Dividend Policy | | 28 |
| Capitalization | | 29 |
| Dilution | | 30 |
| Selected Consolidated Financial Data | | 32 |
| Management's Discussion and Analysis of Financial Condition and Results of Operations | | 33 |
| The Cell Cycle and Cancer | | 46 |
| Business | | 48 |
| Management | | 83 |
| Principal Shareholders | | 111 |
| Pre-offering Reorganization into Public Limited Company | | 115 |
| Description of Share Capital | | 116 |
| Description of American Depositary Shares | | 125 |
| Related Party Transactions | | 132 |
| Underwriters | | 134 |
| Taxation | | 139 |
| Shares Eligible for Future Sale | | 147 |
| Where You Can Find More Information About Us | | 148 |
| Legal Matters | | 148 |
| Experts | | 148 |
| Service of Process and Enforcement of Civil Liabilities | | 148 |
| Index to Financial Statements | | F-1 |
NOTICE TO RESIDENTS OF THE UNITED KINGDOM: The shares and ADSs may only be sold or offered to, and this prospectus and any other invitation or inducement to buy or participate in the offer of shares or ADSs may only be communicated to, (A) persons outside the United Kingdom, or (B) persons who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act (Financial Promotion) Order 2001 (the "FP Order"), or (C) high net worth entities falling within Article 49(2)(a)-(d) of the FP Order (persons of the type described in the foregoing clauses (A), (B) and (C), "Relevant Persons"). The shares and ADSs to which this prospectus relates are available only to Relevant Persons and this prospectus must not be acted on or relied on by persons that are not Relevant Persons. Any investment or investment activity to which this prospectus relates is available only to Relevant Persons and will be engaged in only with Relevant Persons. Any person communicating any information relating to this prospectus or the shares and ADSs in the United Kingdom should comply with all applicable provisions of the Financial Services and Markets Act 2000 and any regulations made thereunder in so doing.
1
PROSPECTUS SUMMARY
This summary highlights key information contained elsewhere in this prospectus. It may not contain all of the information that is important to you. You should read the entire prospectus carefully, including the "Risk Factors" section and the consolidated financial statements and related notes set out in this prospectus.
Cyclacel Group plc
We are a biopharmaceutical company dedicated to the discovery, development and commercialization of novel, mechanism-targeted drugs to treat human cancers and other serious disorders. We describe drugs, compounds or molecules as novel if they have been recently discovered using advanced technologies, and as mechanism-targeted if they are designed to affect identified biological processes through known mechanisms. Our core area of expertise is in cell cycle biology. We focus primarily on the discovery and development of orally available anticancer agents that target the cell cycle with the aim of slowing the progression or shrinking the size of tumors, enhancing quality of life and improving survival rates of cancer patients. Our work with novel molecules that act on the cell cycle has also led us to pursue drug development opportunities in other indications.
We have been focused on the cell cycle since our inception. We were founded in 1996 by Professor Sir David Lane, a recognized leader in the field of tumor suppressor biology who discovered the p53 protein, which operates as one of the body's own anticancer "drugs" by inhibiting cell cycle targets. In 1999, we were joined by Professor David Glover, a recognized leader in the mechanism of mitosis, or cell division, who discovered, among other cell cycle targets, the mitotic kinases, Polo and Aurora, enzymes that act in the mitosis phase of the cell cycle. Our expertise in cell cycle biology is at the center of our business strategy.
We are generating several families of anticancer drugs that act on the cell cycle. These include Cyclin Dependent Kinase (CDK) inhibitors, one of the most sophisticated categories of novel drugs targeting cell cycle mechanisms. Although a number of pharmaceutical and biotechnology companies are currently attempting to develop CDK inhibitor drugs, our lead drug candidate, CYC202, is the only orally available CDK inhibitor drug candidate in Phase II trials.
Our Focus on the Cell Cycle and Cancer
The cell cycle is a set of complex mechanisms that the body uses to control the growth and division of cells. Cancer is characterized by uncontrolled cell multiplication when key cell cycle regulatory proteins are absent or malfunction due to genetic mutations. Many approved cancer chemotherapies in everyday clinical use target different phases of the cell cycle. Their effect, however, is not confined to cancer cells. Instead, these chemotherapies are cytotoxic, which means that they act in the body as poisons in an unspecific manner, killing cancer cells and normal cells alike. This limits the doses that patients can be given and gives rise to significant side-effects. Through our expertise in cell cycle biology, we are focused on the discovery and development of novel, cell cycle-based mechanism-targeted cancer therapies that emulate the body's natural processes in order to stop the growth of cancer cells, but can limit the damage to normal cells and the accompanying side-effects caused by conventional chemotherapeutic agents.
Our Pipeline
Our current pipeline comprises nine novel drug series, seven for cancer, one for HIV/AIDS and one for Type 2 Diabetes.
Our lead drug candidate, CYC202, is a novel, orally available CDK inhibitor that is currently in multi-center Phase II clinical trials for cancer. In Phase I trials designed to test safety rather than efficacy, independent investigators nonetheless observed that CYC202 given alone as monotherapy
2
appeared to induce clinical benefit in some patients with various solid tumors. We are currently conducting two Phase II trials of CYC202, one as combination therapy for the treatment of non-small cell lung cancer and one as monotherapy in hematological cancers, or cancers of the blood cells. These clinical trials are being undertaken under the guidelines of the U.K. Medicines and Healthcare products Regulatory Agency and other comparable regulatory authorities in Europe. We expect to report results from these trials during the second half of 2004, and to begin a further clinical trial with CYC202 as combination therapy in solid tumors by the end of the year.
Our second drug candidate, CYC682, is an orally available prodrug of CNDAC, which is a novel nucleoside analog, or a compound with a structure similar to a nucleoside. A prodrug is a compound that has a therapeutic effect only after it is metabolized within the body, and CNDAC has a significantly higher residence time in the blood when it is produced in the body through metabolism of CYC682 than when it is given directly. Like CDK inhibitors, nucleosides, which are the building blocks of genetic material, work through cell cycle inhibition, though they do so at a different phase of the cell cycle. A number of nucleoside drugs, such as gemcitabine, are in wide use as conventional chemotherapies. Preclinical results from independent investigators reported that CYC682 was superior to gemcitabine and 5-FU, another widely used chemotherapy, both in terms of extending survival and blocking metastases to the liver. Two Phase I studies of CYC682 have been completed, and a Phase Ib clinical trial is currently in progress for the treatment of patients with advanced malignancies. These clinical trials have been undertaken under the guidelines of the U.S. Food and Drug Administration, or FDA. We currently expect CYC682 to enter Phase II trials in the first half of 2005.
We are currently considering a new Phase II clinical trial of CYC202 in glomerulonephritis, a form of kidney inflammation, and expect to commence Phase I clinical development of a drug candidate for the treatment of HIV/AIDS in 2006. We also have a preclinical program in cancer based on an analog of clotrimazole, an antifungal agent. An additional five programs, four for cancer and one for Type 2 Diabetes, are in the research stage. Taken together, our pipeline covers all four phases of the cell cycle, which we believe will improve our chances of successfully developing and commercializing novel drugs that work in combination with approved conventional chemotherapies, and could eventually lead to potential combination therapies of our novel drugs that act synergistically across the cell cycle. Based on the current status of our preclinical pipeline and depending on available financial resources, we expect to be in a position to begin Phase I clinical trials with a new molecule each year for the next three years.
Our Business Strategy
The key elements of our business strategy are to:
- •
- focus on the cell cycle and cancer;
- •
- seek to develop anticancer drug candidates in all phases of the cell cycle and multiple compounds for particular cell cycle targets;
- •
- pursue opportunities in other indications based on our anticancer drug development efforts;
- •
- use our proprietary genes-to-drugs approach to identify drug candidates efficiently;
- •
- exploit our biomarker strategy to optimize clinical development; and
- •
- selectively enter into partnering arrangements while developing our own sales and marketing capability.
Passive Foreign Investment Company
Based on our analysis of our current assets and income, it is most likely that we are a passive foreign investment company (aPFIC) for U.S. federal income tax purposes for the current taxable year
3
and we may also be a PFIC for any future year. If we are a PFIC in any taxable year, a U.S. Holder would suffer adverse U.S. federal income tax consequences some of which may be avoided if a U.S. Holder is eligible for and timely makes a mark-to-market or QEF election as described in more detail in "Taxation—U.S. Federal Income Taxation." We urge U.S. investors to consult their own tax advisors about the application of the PFIC rules and the elections available thereunder in their particular circumstances.
Changes in Our Share Capital in Connection with this Offering
Upon admission of our ordinary shares to the Official List of the U.K. Listing Authority, all of our preferred D shares will convert into ordinary shares. The rate at which preferred D shares will convert into ordinary shares will depend on the offering price of the ordinary shares in this offering. As a result, the total number of ordinary shares that will be outstanding following completion of this offering, and thus the full extent of the dilution that new investors in our ordinary shares and ADSs will suffer, will not be known until the offering price has been finally determined.
Immediately upon the conversion of our preferred D shares into ordinary shares, there will be a further bonus issue to holders of existing ordinary shares (including holders of those ordinary shares issued pursuant to the conversion of our preferred D shares) of one new ordinary share for every ordinary share then outstanding. This will, subject to limited exceptions, result in corresponding adjustments to the number of ordinary shares that are subject to outstanding options and warrants and the exercise price of those options and warrants.
Other than in our historical financial statements and in the historical summary and selected financial data that are presented or discussed in this prospectus, we have adjusted all information relating to our outstanding ordinary shares, options and warrants appearing in this prospectus to reflect the impact of the conversion of our preferred D shares and the further bonus issue of ordinary shares described above. In making these adjustments, we have assumed that the initial public offering price will be £1.66 per share and $12 per ADS, being the midpoint of the price range on the cover page of this prospectus.
For further information regarding the conversion of our preferred D shares and the further bonus issue and their potential impact on an investment in our ordinary shares or ADSs, please see "Capitalization" on page 29, "Dilution" beginning on page 30, "Pre-Offering Reorganization into Public Limited Company" beginning on page 114 and "Description of Share Capital" beginning on page 115.
Risk Factors
Our business is at an early stage of development and is subject to a number of risks that we highlight under "Risk Factors". All of our drug candidates, including CYC202 and CYC682, are in clinical or earlier stages of development and we cannot be certain that the clinical development of any of these drug candidates will be successful or that they will receive the regulatory approvals required to commercialize them. As of March 31, 2004, we had incurred net losses of approximately $73.1 million since inception. We expect to incur continuing operating losses for several years, as we continue our research and development of our initial drug candidates, seek regulatory approvals and commercialize any approved drugs.
Company Information
Our operations are conducted by our wholly-owned subsidiary Cyclacel Limited, which was incorporated and registered in England and Wales on August 13, 1996 with registered number 3237549 under the U.K. Companies Act 1985 as a private limited company under the name Intercede 1190 Limited. The principal legislation under which Cyclacel Limited operates is the U.K. Companies Act
4
1985 as amended from time to time. The registered office of Cyclacel Limited is 6-8 Underwood Street, London N1 7JQ.
Cyclacel Group plc was incorporated and registered in England and Wales on April 1, 2004 with registered number 5090795 under the U.K. Companies Act as a private limited company under the name Alnery No. 2428 Limited. On April 20, 2004 we changed our name to Cyclacel Group Limited. Cyclacel Group Limited was re-registered as a public limited company on July 1, 2004 under Section 43 of the U.K. Companies Act and our name changed to Cyclacel Group plc. The principal legislation under which we operate is the U.K. Companies Act. Our registered office is at 6-8 Underwood Street, London N1 7JQ.
Our principal executive offices are located at Dundee Technopole, James Lindsay Place, Dundee, DD1 5JJ, U.K. and our telephone number is +44 1382 206 062. Please note that shareholders and/or investors will not be able to obtain any financial or investment advice from the Company and that, where necessary, they should seek advice from their own independent financial adviser.
5
THE OFFERING
| The offering | | 16,000,000 ordinary shares, in the form of ordinary shares or ADSs, in the United States, the United Kingdom and elsewhere. |
Offering price |
|
£ per share or $ per ADS. |
Ordinary shares outstanding after the offering |
|
60,964,304 ordinary shares. |
ADSs |
|
Each ADS represents the right to receive 4 ordinary shares. The ADSs are evidenced by American Depositary Receipts, or ADRs. See "Description of American Depositary Shares." |
Over-allotment option |
|
2,400,000 ordinary shares, in the form of shares or ADSs. |
Use of proceeds |
|
To continue the development of our drug candidates, including ongoing clinical trials, to expand our research programs and for working capital and other general corporate purposes. See "Use of Proceeds." |
Proposed Nasdaq National Market Symbol |
|
CYCC. |
The number of ordinary shares expected to be outstanding after this offering is based on the number of shares outstanding as of June 30, 2004, immediately following the reorganization described under "Pre-offering Reorganization into Public Limited Company" beginning on page 114, and also reflects the conversion of our preferred D shares into 20,840,344 of our ordinary shares (based on an assumed initial public offering price of £1.66 per ordinary share, being the midpoint of the price range set forth on the cover page of this prospectus) and the further bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 115. This number does not include:
- •
- 3,593,640 ordinary shares issuable upon exercise of options outstanding at June 30, 2004 at a weighted average exercise price of £0.76 per share (as adjusted to reflect the bonus issue);
- •
- 3,441,806 ordinary shares (as adjusted to reflect the bonus issue) issuable on exercise of options granted to Spiro Rombotis, our chief executive officer, at an exercise price of 1 pence per share, and 100,000 ordinary shares (as adjusted to reflect the bonus issue) issuable on exercise of options granted to Paul McBarron, our chief financial officer, at an exercise price of £0.75 per share (as adjusted to reflect the bonus issue), as more particularly described under "Management—Employee Share Option Plans—Senior Executive Incentive Plan" beginning on page 92;
- •
- 47,000 ordinary shares issuable upon exercise of warrants outstanding at June 30, 2004 (as adjusted to reflect the bonus issue) as described in more detail under "Description of Share Capital" beginning on page 115; and
- •
- any ordinary shares that may be issued pursuant to the exercise of options under our employee option plans as more particularly described under "Management—Employee Share Option Plans."
6
In this prospectus, the terms "we," "us," "our," "Cyclacel" and "the Company" refer to Cyclacel Group plc and its subsidiary, Cyclacel Limited. Service marks, trademarks and trade names referred to in this prospectus are the property of their respective owners.
7
SUMMARY FINANCIAL DATA
The following table summarizes our consolidated financial data. The summary consolidated financial data for the years ended March 31, 2002 and 2003, for the nine months ended December 31, 2002 and 2003, and for the three months ended March 31, 2003 and 2004 and as of December 31, 2003 and March 31, 2004 are extracted without material adjustment from our U.S. GAAP consolidated financial statements, which have been audited by Ernst & Young LLP, independent auditors, included in this prospectus. The information set forth below is not necessarily indicative of future results and should be read in conjunction with our consolidated financial statements and accompanying notes and with the information set forth in "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing elsewhere in this prospectus. Investors should read the whole of this document and not just rely on this summary information.
| | Year Ended March 31,
| | Nine Months
Ended December 31,
| | Three Months
Ended March 31,
| |
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
| |
|---|
| | (in thousands, except share and per share data)
| |
|---|
| Consolidated Statements of Operations: | | | | | | | | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | | | | | | | | |
| | Collaboration and research and development income | | $ | 1,155 | | $ | 1,250 | | $ | 930 | | $ | 8 | | $ | 319 | | $ | 1 | |
| | Grant income | | | 55 | | | 941 | | | 696 | | | 504 | | | 246 | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| | | | 1,210 | | | 2,191 | | | 1,626 | | | 512 | | | 565 | | | 1 | |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Research and development | | | (13,195 | ) | | (19,847 | ) | | (14,581 | ) | | (13,084 | ) | | (5,275 | ) | | (4,870 | ) |
| | General and administrative | | | (3,220 | ) | | (2,536 | ) | | (2,026 | ) | | (2,099 | ) | | (504 | ) | | (1,573 | ) |
| | Amortization of employee stock-based compensation | | | (672 | ) | | (305 | ) | | (229 | ) | | (217 | ) | | (76 | ) | | 618 | |
| | |
| |
| |
| |
| |
| |
| |
| Total operating expenses | | | (17,087 | ) | | (22,688 | ) | | (16,836 | ) | | (15,400 | ) | | (5,855 | ) | | (5,825 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Operating loss | | | (15,877 | ) | | (20,497 | ) | | (15,210 | ) | | (14,888 | ) | | (5,290 | ) | | (5,824 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Interest and other income (expense) | | | 1,024 | | | 558 | | | 481 | | | (1,575 | ) | | 73 | | | 415 | |
| | |
| |
| |
| |
| |
| |
| |
| Loss before taxes | | | (14,853 | ) | | (19,939 | ) | | (14,729 | ) | | (16,463 | ) | | (5,217 | ) | | (5,409 | ) |
| Taxes | | | — | | | (4,397 | ) | | (3,841 | ) | | (1,486 | ) | | (528 | ) | | (521 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Net loss | | | (14,853 | ) | | (15,542 | ) | | (10,888 | ) | | (14,977 | ) | | (4,689 | ) | | (4,888 | ) |
| Dividends on preferred shares | | | (3,289 | ) | | (4,654 | ) | | (3,430 | ) | | (4,425 | ) | | (1,225 | ) | | (2,629 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Net loss applicable to ordinary shareholders | | $ | (18,142 | ) | $ | (20,196 | ) | $ | (14,318 | ) | $ | (19,402 | ) | $ | (5,914 | ) | $ | (7,517 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Net loss per share—basic and diluted | | $ | (2.83 | ) | $ | (3.14 | ) | $ | (2.23 | ) | $ | (2.25 | ) | $ | (0.92 | ) | $ | (0.39 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Weighted average shares used to compute basic and diluted earnings per share | | | 6,399,539 | | | 6,433,996 | | | 6,433,874 | | | 8,623,516 | | | 6,433,996 | | | 19,259,641 | |
| | As of
December 31, 2003
| | As of
March 31, 2004
| |
|---|
| | (in thousands)
| |
|---|
| Consolidated Balance Sheet Data: | | | | | | | |
| Cash and cash equivalents | | $ | 9,670 | | $ | 5,685 | |
| Short-term investments | | | 24,010 | | | 33,826 | |
| Prepaid expenses and other current assets | | | 5,360 | | | 3,892 | |
| Property, plant and equipment | | | 3,760 | | | 4,117 | |
| | |
| |
| |
| Total assets | | | 42,800 | | | 47,520 | |
| Current liabilities | | | (4,657 | ) | | (5,477 | ) |
| Long-term debt, net of current portion | | | (495 | ) | | (334 | ) |
| | |
| |
| |
| Total shareholders' equity | | $ | 37,648 | | $ | 41,709 | |
| | |
| |
| |
8
RISK FACTORS
Before you invest in our ordinary shares or ADSs, you should understand the high degree of risk involved. You should carefully consider the risks described below and other information in this prospectus, including our consolidated financial statements and related notes, before you decide to purchase our ordinary shares or ADSs. Selected financial data set out in this document are extracted without material adjustment from our U.S. GAAP consolidated financial statements, which have been audited by Ernst & Young LLP, independent auditors, included in this document. If any of the following risks actually occur, our business, financial condition and operating results could be adversely affected. As a result, the trading price of our ordinary shares or ADSs could decline and you could lose part or all of your investment.
Risks Related to Our Business
We are at an early stage of development as a company and we do not have, and may never have, any products that generate revenues.
We are at an early stage in the development of our company and have a limited operating history on which to evaluate our business and prospects. Since beginning operations in 1997, we have not generated any product revenues. We currently have no drugs for sale and we cannot guarantee that we will ever have marketable drugs. We must demonstrate that our drug candidates satisfy rigorous standards of safety and efficacy for their intended uses before the Food and Drug Administration, or FDA, and other regulatory authorities in the United States, the United Kingdom, the European Union and elsewhere. Significant additional research, preclinical testing and clinical testing is required before we can file applications with the FDA or other regulatory authorities for premarket approval of our drug candidates. In addition, to compete effectively, our drugs must be easy to administer, cost-effective and economical to manufacture on a commercial scale. We may not achieve any of these objectives. CYC202 and CYC682, our most advanced drug candidates for the treatment of cancer and inflammation, are currently our only drug candidates in clinical trials and we cannot be certain that the clinical development of these or any other drug candidates in preclinical testing or clinical development will be successful, that they will receive the regulatory approvals required to commercialize them or that any of our other research and drug discovery programs will yield a drug candidate suitable for investigation through clinical trials. Our commercial revenues, if any, will be derived from sales of drugs that we do not expect to become marketable for several years, if at all.
We have incurred operating losses in each year since beginning operations in 1997 due to costs incurred in connection with our research and development activities and general and administrative costs associated with our operations, and we may never achieve profitability. As of March 31, 2004, our accumulated deficit was $73.1 million. Our net loss for the three month period ended March 31, 2004, the nine month period ended December 31, 2003 and for the fiscal years ended March 31, 2003 and March 31, 2002 was $4.9 million, $15.0 million, $15.5 million and $14.9 million, respectively. Our net loss for the period 1997 (inception) through March 31, 2004 was $73.1 million. Our initial drug candidates are in the early stages of clinical testing and we must conduct significant additional clinical trials before we can seek the regulatory approvals necessary to begin commercial sales of our drugs. We expect to incur continued losses for several years, as we continue our research and development of our initial drug candidates, seek regulatory approvals and commercialize any approved drugs. If our initial drug candidates are unsuccessful in clinical trials or we are unable to obtain regulatory approvals, or if our drugs are unsuccessful in the market, we will not be profitable. If we fail to become and remain profitable, or if we are unable to fund our continuing losses, you could lose all or part of your investment.
9
If we fail to obtain additional financing, we may be unable to complete the development and commercialization of our drug candidates or continue our research and development programs.
We have funded all of our operations and capital expenditures with proceeds from private placements of our securities, interest on investments, government grants and research and development tax credits. In order to conduct the lengthy and expensive research, preclinical testing and clinical trials necessary to complete the development and marketing of our drug candidates, we will require substantial additional funds. For example, for the nine months ended December 31, 2003, our cash outflow to fund operations was approximately $14.4 million. To meet these financing requirements, we may raise funds through public or private equity offerings, debt financings or strategic alliances. Raising additional funds by issuing equity or convertible debt securities may cause our shareholders to experience additional dilution in their ownership interests. Raising additional funds through debt financing, if available, may involve covenants that restrict our business activities and options. To the extent that we raise additional funds through collaborations and licensing arrangements, we may have to relinquish valuable rights to our drug discovery and other technologies, research programs or drug candidates, or grant licenses on terms that may not be favorable to us. Additional funding may not be available to us on favorable terms, or at all. If we are unable to obtain additional funds, we may be forced to delay or terminate our clinical trials and the development and marketing of our drug candidates.
Clinical trials are expensive, time consuming and subject to delay.
Clinical trials are expensive and complex, can take many years and have uncertain outcomes. We estimate that clinical trials of our most advanced drug candidates will continue for several years, but may take significantly longer to complete. Failure can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future drug candidates, including but not limited to:
- •
- delays in securing clinical investigators or trial sites for our clinical trials;
- •
- delays in obtaining institutional review board, or IRB, and other regulatory approvals to commence a clinical trial;
- •
- slower than anticipated patient recruitment and enrollment;
- •
- negative or inconclusive results from clinical trials;
- •
- unforeseen safety issues;
- •
- uncertain dosing issues; and
- •
- inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols.
If we suffer any significant delays, setbacks or negative results in, or termination of, our clinical trials we may be unable to continue development of our drug candidates or generate revenue and our development costs could increase significantly.
If our understanding of the role played by CDKs in regulating the cell cycle is incorrect, this may hinder pursuit of our clinical and regulatory strategy.
Our lead drug candidate, CYC202, is a cell cycle inhibitor based on our understanding of Cyclin Dependent Kinases (CDKs). Although a number of pharmaceutical and biotechnology companies are attempting to develop CDK inhibitor drugs for the treatment of cancer, no CDK inhibitors have yet reached the market. Our CYC202 program relies on our understanding of the interaction of CDKs with other cellular mechanisms that regulate key stages of cell growth. If our understanding of the role
10
played by CDKs in regulating the cell cycle is incorrect, our lead drug may fail to produce therapeutically relevant results, hindering our ability to pursue our clinical and regulatory strategy.
If we fail to enter into and maintain successful strategic alliances for our drug candidates, we may have to reduce or delay our drug candidate development or increase our expenditures.
An important element of our strategy for developing, manufacturing and commercializing our drug candidates is entering into strategic alliances with pharmaceutical companies or other industry participants to advance our programs and enable us to maintain our financial and operational capacity. We face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms, if at all. In addition, these alliances may be unsuccessful. If we fail to create and maintain suitable alliances, we may have to limit the size or scope of, or delay, one or more of our drug development or research programs. If we elect to fund drug development or research programs on our own, we will have to increase our expenditures and will need to obtain additional funding, which may be unavailable or available only on unfavorable terms.
We are making extensive use of biomarkers, which are not yet scientifically validated, and our reliance on biomarker data may thus lead us to direct our resources inefficiently.
We are making extensive use of biomarkers in an effort to facilitate our drug development and to optimize our clinical trials. Biomarkers are proteins or other substances whose presence in the blood can serve as an indicator of specific cell processes. We believe that these biological markers serve a useful purpose in helping us to evaluate whether our drug candidates are having their intended effects through their assumed mechanisms, and thus enable us to identify more promising drug candidates at an early stage and to direct our resources efficiently. We also believe that biomarkers may eventually allow us to improve patient selection in connection with clinical trials and monitor patient compliance with trial protocols.
For most purposes, however, biomarkers have not yet been scientifically validated. If our understanding and use of biomarkers is inaccurate or flawed, or if our reliance on them is otherwise misplaced, then we will not only fail to realize any benefits from using biomarkers, but may also be led to invest time and financial resources inefficiently in attempting to develop inappropriate drug candidates. Moreover, although the FDA has issued for comment a draft guidance document on the potential use of biomarker data in clinical development, such data are not currently accepted by the FDA or other regulatory agencies in the United States, the United Kingdom, the European Union or elsewhere in applications for regulatory approval of drug candidates and there is no guarantee that such data will ever be accepted by the relevant authorities in this connection. Our biomarker data should not be interpreted as evidence of efficacy.
To the extent we elect to fund the development of a drug candidate or the commercialization of a drug at our expense, we will need substantial additional funding.
We plan to market drugs on our own, with or without a partner, that can be effectively commercialized and sold in concentrated markets that do not require a large sales force to be competitive. To achieve this goal, we will need to establish our own specialized sales force, marketing organization and supporting distribution capabilities. The development and commercialization of our drug candidates is very expensive. To the extent we elect to fund the full development of a drug candidate or the commercialization of a drug at our expense, we will need to raise substantial additional funding to:
- •
- fund research and development and clinical trials connected therewith;
- •
- seek regulatory approvals;
- •
- build or access manufacturing and commercialization capabilities;
11
- •
- commercialize and secure coverage, payment and reimbursement of our drug candidates, if any such candidates receive regulatory approval; and
- •
- hire additional management and scientific personnel.
We believe that the net proceeds of this offering, our existing cash equivalents and short-term investments will be sufficient to meet our projected operating requirements for at least the next twenty-four months. Our future funding requirements will depend on many factors, including:
- •
- the scope, rate of progress and cost of our clinical trials and other research and development activities;
- •
- the costs and timing of seeking and obtaining regulatory approvals;
- •
- the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights;
- •
- the costs associated with establishing sales and marketing capabilities;
- •
- the costs of acquiring or investing in businesses, products and technologies;
- •
- the effect of competing technological and market developments; and
- •
- the payment, other terms and timing of any strategic alliance, licensing or other arrangements that we may establish.
If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more of our clinical trials or research and development programs or future commercialization efforts.
Due to our reliance on contract research organizations or other third parties to conduct clinical trials, we are unable to directly control the timing, conduct and expense of our clinical trials.
We do not have the ability to independently conduct clinical trials required to obtain regulatory approvals for our drug candidates. We must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct our clinical trials. In addition, we rely on third parties to assist with our preclinical development of drug candidates. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our drug candidates.
To the extent we are able to enter into collaborative arrangements or strategic alliances, we will be exposed to risks related to those collaborations and alliances.
Although we are not currently party to any collaboration arrangement or strategic alliance that is material to our business, in the future we expect to be dependent upon collaborative arrangements or strategic alliances to complete the development and commercialization of some of our drug candidates particularly after the Phase II stage of clinical testing. These arrangements may place the development of our drug candidates outside our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us.
We may be unable to locate and enter into favorable agreements with third parties, which could delay or impair our ability to develop and commercialize our drug candidates and could increase our
12
costs of development and commercialization. Dependence on collaborative arrangements or strategic alliances will subject us to a number of risks, including the risk that:
- •
- we may not be able to control the amount and timing of resources that our collaborators may devote to the drug candidates;
- •
- our collaborators may experience financial difficulties;
- •
- we may be required to relinquish important rights such as marketing and distribution rights;
- •
- business combinations or significant changes in a collaborator's business strategy may also adversely affect a collaborator's willingness or ability to complete its obligations under any arrangement;
- •
- a collaborator could independently move forward with a competing drug candidate developed either independently or in collaboration with others, including our competitors; and
- •
- collaborative arrangements are often terminated or allowed to expire, which would delay the development and may increase the cost of developing our drug candidates.
We have no manufacturing capacity and will rely on third-party manufacturers for the late stage development and commercialization of drugs we may develop.
We do not currently operate manufacturing facilities for clinical or commercial production of our drug candidates under development. We currently lack the resources or the capacity to manufacture any of our products on a clinical or commercial scale. We anticipate future reliance on a limited number of third-party manufacturers until we are able to expand our operations to include manufacturing capacities. Any performance failure on the part of future manufacturers could delay late stage clinical development or regulatory approval of our drug candidates or commercialization of our drugs, producing additional losses and depriving us of potential product revenues.
If the FDA or other regulatory agencies approve any of our drug candidates for commercial sale, or if we significantly expand our clinical trials, we will need to manufacture them in larger quantities. To date, our drug candidates have been manufactured in small quantities for preclinical testing and clinical trials and we may not be able to successfully increase the manufacturing capacity, whether in collaboration with third-party manufacturers or on our own, for any of our drug candidates in a timely or economic manner, or at all. For example, the manufacture of our drug candidate CYC682 requires several steps and it is not yet known if scale up to commercial production is feasible. Significant scale-up of manufacturing may require additional validation studies, which the FDA and other regulatory bodies must review and approve. If we are unable to successfully increase the manufacturing capacity for a drug candidate whether for late stage clinical trials or for commercial sale, the drug development, regulatory approval or commercial launch of any related drugs may be delayed or there may be a shortage in supply. Even if any third-party manufacturer makes improvements in the manufacturing process for our drug candidates, we may not own, or may have to share, the intellectual property rights to such innovation.
We currently have no marketing or sales staff. If we are unable to conclude strategic alliances with marketing partners or if we are unable to develop our own sales and marketing capabilities, we may not be successful in commercializing any drugs we may develop.
Our strategy is to develop compounds through the Phase II stage of clinical testing and market certain discrete drugs on our own. We have no sales, marketing or distribution capabilities. We will depend primarily on strategic alliances with third parties, which have established distribution systems and sales forces, to commercialize our drugs. To the extent that we are unsuccessful in commercializing any drugs ourselves or through a strategic alliance, product revenues will suffer, we will incur significant additional losses and our share price will be negatively affected.
13
If we evolve from a company primarily involved in discovery and development to one also involved in the commercialization of drugs, we may encounter difficulties in managing our growth and expanding our operations successfully.
If we advance our drug candidates through clinical trials, we will need to expand our development and regulatory capabilities and develop manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. If our operations expand, we expect that we will need to manage additional relationships with various collaborative partners, suppliers and other third parties. Our ability to manage our operations and any growth will require us to make appropriate changes and upgrades (as necessary) to our operational, financial and management controls, reporting systems and procedures where we may operate. Any inability to manage growth could delay the execution of our business plan or disrupt our operations.
The failure to attract and retain skilled personnel could impair our drug development and commercialization efforts.
We are highly dependent on our senior management and key scientific and technical personnel. The loss of the services of any member of our senior management, scientific or technical staff may significantly delay or prevent the achievement of drug development and other business objectives and could have a material adverse effect on our business, operating results and financial condition. We also rely on consultants and advisors to assist us in formulating our research and development strategy. All of our consultants and advisors are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to contribute to us.
We intend to expand and develop new drug candidates. We will need to hire additional employees in order to continue our clinical trials and market our drug candidates. This strategy will require us to recruit additional executive management and scientific and technical personnel. There is currently intense competition for skilled executives and employees with relevant scientific and technical expertise, and this competition is likely to continue. The inability to attract and retain sufficient scientific, technical and managerial personnel could limit or delay our product development efforts, which would adversely affect the development of our drug candidates and commercialization of our potential drugs and growth of our business.
Risks Related to Regulation
Our drug candidates are subject to extensive regulation, which can be costly and time-consuming, and we may not obtain approvals for the commercialization of some or all of our drug candidates.
The clinical development, manufacturing, selling and marketing of our drug candidates are subject to extensive regulation by the FDA and other regulatory authorities in the United States, the United Kingdom, the European Union and elsewhere. These regulations also vary in important, meaningful ways from country to country. We are not permitted to market a potential drug in the United States until we receive approval of a New Drug Application, or NDA, from the FDA. We have not received an NDA approval from the FDA for any of our drug candidates.
Obtaining an NDA approval is expensive and is a complex, lengthy and uncertain process. The FDA approval process for a new drug involves completion of preclinical studies and the submission of the results of these studies to the FDA, together with proposed clinical protocols, manufacturing information, analytical data and other information in an Investigational New Drug application, or IND, which must become effective before human clinical trials may begin. Clinical development typically involves three phases of study: Phase I, II and III. The most significant costs associated with clinical development are the Phase III clinical trials as they tend to be the longest and largest studies conducted during the drug development process. After completion of clinical trials, an NDA may be
14
submitted to the FDA. In responding to an NDA, the FDA may refuse to file the application, or if accepted for filing, the FDA may grant marketing approval, request additional information or deny the application if it determines that the application does not provide an adequate basis for approval. In addition, failure to comply with FDA and other applicable foreign and U.S. regulatory requirements may subject us to administrative or judicially imposed sanctions. These include warning letters, civil and criminal penalties, injunctions, product seizure or detention, product recalls, total or partial suspension of production and refusal to approve either pending NDAs, or supplements to approved NDAs.
Despite the substantial time and expense invested in preparation and submission of an NDA or equivalents in other jurisdictions, regulatory approval is never guaranteed. The FDA and other regulatory authorities in the United States, the United Kingdom, the European Union and elsewhere, exercise substantial discretion in the drug approval process. The number, size and design of preclinical studies and clinical trials that will be required for FDA or other regulatory approval will vary depending on the drug candidate, the disease or condition for which the drug candidate is intended to be used and the regulations and guidance documents applicable to any particular drug candidate. The FDA or other regulators can delay, limit or deny approval of a drug candidate for many reasons, including, but not limited to:
- •
- those discussed in the risk factor which immediately follows;
- •
- the fact that FDA or other regulatory officials may not approve our or our third-party manufacturer's processes or facilities; or
- •
- the fact that new laws may be enacted by the FDA or other regulators may change their approval policies or adopt new regulations requiring new or different evidence of safety and efficacy for the intended use of a drug candidate.
Adverse events have been observed in our clinical trials and may force us to stop development of our product candidates or prevent regulatory approval of our product candidates.
Adverse or inconclusive results from our clinical trials may substantially delay, or halt entirely, any further development of our drug candidates. Many companies have failed to demonstrate the safety or effectiveness of drug candidates in later-stage clinical trials notwithstanding favorable results in early-stage clinical trials. Previously unforeseen and unacceptable side effects could interrupt, delay or halt clinical trials of our drug candidates and could result in the FDA or other regulatory authorities denying approval of our drug candidates. We will need to demonstrate safety and efficacy for specific indications of use, and monitor safety and compliance with clinical trial protocols throughout the development process. To date, long-term safety and efficacy have not yet been demonstrated in clinical trials for any of our drug candidates. Toxicity and "severe adverse effects" as defined in trial protocols have been noted in preclinical and clinical trials involving certain of our drug candidates. For example, elevation of liver enzymes and decrease in potassium levels has been observed in some patients receiving our lead drug candidate, CYC202. In addition, we may pursue clinical trials for CYC202 in more than one indication. There is a risk that severe toxicity observed in a trial for one indication could result in the delay or suspension of all trials involving the same drug candidate. We are currently conducting two Phase IIa clinical trials to test the safety and efficacy of CYC202, one in the treatment of non-small cell lung cancer and one in the treatment of hematological cancers. We expect to report results of these trials during the second half of 2004. If these trials or any future trials are unsuccessful, our business and reputation could be harmed and our share price could be negatively affected.
Even if we believe the data collected from clinical trials of our drug candidates are promising with respect to safety and efficacy, such data may not be deemed sufficient by regulatory authorities to warrant product approval. Clinical data can be interpreted in different ways. Regulatory officials could interpret such data in different ways than we do, which could delay, limit or prevent regulatory approval. The FDA, other regulatory authorities or we may suspend or terminate clinical trials at any
15
time. Any failure or significant delay in completing clinical trials for our drug candidates, or in receiving regulatory approval for the commercialization of our drug candidates, may severely harm our business and reputation.
Following regulatory approval of any drug candidates, we would be subject to ongoing regulatory obligations and restrictions, which may result in significant expense and limit our ability to commercialize our potential drugs.
If one of our drug candidates is approved by the FDA or by another regulatory authority, we would be held to extensive regulatory requirements over product manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and record keeping. Regulatory approvals may also be subject to significant limitations on the indicated uses or marketing of the drug candidates. Potentially costly follow-up or post-marketing clinical studies may be required as a condition of approval to further substantiate safety or efficacy, or to investigate specific issues of interest to the regulatory authority. Previously unknown problems with the drug candidate, including adverse events of unanticipated severity or frequency, may result in restrictions on the marketing of the drug, and could include withdrawal of the drug from the market.
In addition, the law or regulatory policies governing pharmaceuticals may change. New statutory requirements may be enacted or additional regulations may be enacted that could prevent or delay regulatory approval of our drug candidates. We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action, either in the United States or elsewhere. If we are not able to maintain regulatory compliance, we might not be permitted to market our drugs and our business could suffer.
Our applications for regulatory approval could be delayed or denied due to problems with studies conducted before we in-licensed some of our product candidates.
We currently license some of the compounds and drug candidates used in our research programs from third parties. These include CYC381, licensed from Lorus Therapeutics, Inc., and CYC682, licensed from Sankyo Co., Ltd. Our present research involving these compounds relies upon previous research conducted by third parties over which we had no control and before we in-licensed the drug candidates. In order to receive regulatory approval of a drug candidate, we must present all relevant data and information obtained during its research and development, including research conducted prior to our licensure of the drug candidate. Although we are not currently aware of any such problems, any problems that emerge with preclinical research and testing conducted prior to our in-licensing may affect future results or our ability to document prior research and to conduct clinical trials, which could delay, limit or prevent regulatory approval for our drug candidates.
Risks Related to Our Industry
We face intense competition and our competitors may develop drugs that are less expensive, safer, or more effective than our drug candidates.
We are engaged in a rapidly changing and highly competitive field. We are seeking to develop and market products that will compete with other products and drugs that currently exist or are being developed. We compete with companies that are developing small molecule drugs, as well as companies that have developed drugs or are developing alternative drug candidates for cancer or other serious disorders where there is abnormal cell proliferation. Other companies are currently developing drugs targeting cancer that may compete with our drug candidates, including AstraZeneca's AZD-5438, Bristol-Myers Squibb's BMS-387032 and Eisai's E-7070. In addition, although Aventis has announced that it has ceased Phase II development of flavopiridol, a CDK inhibiting drug, we believe that the National Cancer Institute's Cancer Therapy Evaluation Program is continuing to enroll patients in a
16
Phase II trial. We also believe that several companies such as Aventis, Kyowa Hakko, Pfizer and Roche have, or have recently had, agents in clinical trials that are specific to the Cyclin Dependent Kinases, or CDKs, that are the target of our lead drug candidates and some of our research programs. Others have CDK inhibitor research programs in preclinical studies.
Our competitors, either alone or together with collaborators, may have substantially greater financial resources and research and developments staff. Our competitors may also have more experience:
- •
- developing drug candidates;
- •
- conducting preclinical and clinical trials;
- •
- obtaining regulatory approvals; and
- •
- commercializing drug candidates.
Our competitors may succeed in obtaining patent protection and regulatory approval and may market drugs before we do. If our competitors market drugs that are less expensive, safer, more effective or more convenient to administer than our potential drugs, or that reach the market sooner than our potential drugs, we may not achieve commercial success. Scientific, clinical or technical developments by our competitors may render our drug candidates obsolete or noncompetitive. We anticipate that we will face increased competition in the future as new companies enter the markets and as scientific developments progress. If our drug candidates obtain regulatory approvals, but do not compete effectively in the marketplace, our business will suffer.
The commercial success of our drug candidates depends upon their market acceptance among physicians, patients, healthcare providers and payors and the medical community.
If our drug candidates are approved by the FDA or by another regulatory authority, the resulting drugs, if any, may not gain market acceptance among physicians, healthcare providers and payors, patients and the medical community. The degree of market acceptance of any of our approved drugs will depend on a variety of factors, including:
- •
- timing of market introduction, number and clinical profile of competitive drugs;
- •
- our ability to provide acceptable evidence of safety and efficacy;
- •
- relative convenience and ease of administration;
- •
- cost-effectiveness;
- •
- availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third-party payors;
- •
- prevalence and severity of adverse side effects; and
- •
- other potential advantages over alternative treatment methods.
If our drugs fail to achieve market acceptance, we may not be able to generate significant revenue and our business would suffer.
There is uncertainty related to coverage, reimbursement and payment by healthcare providers and payors for newly approved drugs. The inability or failure to obtain coverage could affect our ability to market our future drugs and decrease our ability to generate revenue.
The availability and levels of coverage and reimbursement of newly approved drugs by healthcare providers and payors is subject to significant uncertainty. The commercial success of our drug candidates in both the U.S. and international markets is substantially dependent on whether third-party
17
coverage and reimbursement is available. The U.S. Centers for Medicare and Medicaid Services, health maintenance organizations and other third-party payors in the United States, the United Kingdom, the European Union and other jurisdictions are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new drugs and, as a result, they may not cover or provide adequate payment for our potential drugs. Our drug candidates may not be considered cost-effective and reimbursement may not be available to consumers or may not be sufficient to allow our drug candidates to be marketed on a competitive basis.
In some countries, including the countries of the European Union, the pricing of prescription drugs is subject to government control. In such countries, pricing negotiations with governmental authorities can take three to 12 months or longer following application to the competent authorities. To obtain reimbursement or pricing approval in such countries may require conducting an additional clinical trial comparing the cost-effectiveness of the drug to other alternatives. In the United States, the Medicare Part D drug benefit to be implemented in 2006 will limit drug coverage through formularies and other cost and utilization management programs, while Medicare Part B limits drug payments to a certain percentage of average price or through restrictive payment policies of "least costly alternatives" and "inherent reasonableness." Our business could be materially harmed if coverage, reimbursement or pricing is unavailable or set at unsatisfactory levels.
We may be exposed to product liability claims which may damage our reputation and we may not be able to obtain adequate insurance.
Because we conduct clinical trials in humans, we face the risk that the use of our drug candidates will result in adverse effects. We believe that we have obtained reasonably adequate product liability insurance coverage for our trials. We cannot predict, however, the possible harm or side effects that may result from our clinical trials. Such claims may damage our reputation and we may not have sufficient resources to pay for any liabilities resulting from a claim excluded from, or beyond the limit of, our insurance coverage.
Once we have commercially available drugs based on our drug candidates, we will be exposed to the risk of product liability claims. This risk exists even with respect to those drugs that are approved for commercial sale by the FDA or other regulatory authorities in the United States, the United Kingdom, the European Union or elsewhere and manufactured in facilities licensed and regulated by the FDA or other such regulatory authorities. We intend to secure limited product liability insurance coverage, but may not be able to obtain such insurance on acceptable terms with adequate coverage, or at reasonable cost. There is also a risk that third parties that we have agreed to indemnify could incur liability. Even if we were ultimately successful in product liability litigation, the litigation would consume substantial amounts of our financial and managerial resources and may create adverse publicity, all of which would impair our ability to generate sales of the litigated product as well as our other potential drugs.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain potential drugs, which could severely harm our business. Even if we are
18
successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Defending against claims relating to improper handling, storage or disposal of hazardous chemical, radioactive or biological materials could be time consuming and expensive.
Our research and development involves the controlled use of hazardous materials, including chemicals, radioactive and biological materials such as chemical solvents, phosphorus and bacteria. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from those materials. Various laws and regulations govern the use, manufacture, storage, handling and disposal of hazardous materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials. Compliance with environmental laws and regulations may be expensive, and current or future environmental regulations may impair our research, development and production efforts.
Risks Related to Our Intellectual Property
If we fail to enforce adequately or defend our intellectual property rights our business may be harmed.
Our commercial success depends in large part on obtaining and maintaining patent and trade secret protection for our drug candidates, the methods used to manufacture those drug candidates and the methods for treating patients using those drug candidates. We will only be able to protect our drug candidates and our technologies from unauthorized use by third parties to the extent that valid and enforceable patents or trade secrets cover them.
Our ability to obtain patents is uncertain because legal means afford only limited protections and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Some legal principles remain unresolved and there has not been a consistent policy regarding the breadth or interpretation of claims allowed in patents in the United States, the United Kingdom, the European Union or elsewhere. In addition, the specific content of patents and patent applications that are necessary to support and interpret patent claims is highly uncertain due to the complex nature of the relevant legal, scientific and factual issues. Changes in either patent laws or in interpretations of patent laws in the United States, the United Kingdom, the European Union or elsewhere may diminish the value of our intellectual property or narrow the scope of our patent protection.
Even if patents are issued regarding our drug candidates or methods of using them, those patents can be challenged by our competitors who can argue such patents are invalid. Patents also will not protect our drug candidates if competitors devise ways of making these product candidates without legally infringing our patents. The U.S. Federal Food, Drug and Cosmetic, or FD&C, Act and FDA regulations and policies and equivalents in other jurisdictions provide incentives to manufacturers to challenge patent validity or create modified, noninfringed versions of a drug in order to facilitate the approval of abbreviated new drug applications for generic substitutes. These same types of incentives encourage manufacturers to submit new drug applications that rely on literature and clinical data not prepared for or by the drug sponsor.
Proprietary trade secrets and unpatented know-how are also very important to our business. We rely on trade secrets to protect our technology, especially where we do not believe that patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. Our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully disclose our confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time consuming, and the outcome is unpredictable. Moreover, our competitors may independently
19
develop equivalent knowledge, methods and know-how. Failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
If we infringe intellectual property rights of third parties, it may increase our costs or prevent us from being able to commercialize our drug candidates.
There is a risk that we are infringing the proprietary rights of third parties because patents and pending applications belonging to third parties exist in the United States, the United Kingdom and elsewhere in the world in the areas our research explores. Others might have been the first to make the inventions covered by each of our or our licensors' pending patent applications and issued patents and might have been the first to file patent applications for these inventions. In addition, because the patent application process can take several years to complete, there may be currently pending applications, unknown to us, which may later result in issued patents that cover the production, manufacture, commercialization or use of our drug candidates. In addition, the production, manufacture, commercialization or use of our product candidates may infringe existing patents of which we are not aware.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. Defending ourselves against third-party claims, including litigation in particular, would be costly and time consuming and would divert management's attention from our business, which could lead to delays in our development or commercialization efforts. If third parties are successful in their claims, we might have to pay substantial damages or take other actions that are adverse to our business. As a result of intellectual property infringement claims, or to avoid potential claims, we might:
- •
- be prohibited from selling or licensing any product that we may develop unless the patent holder licenses the patent to us, which it is not required to do;
- •
- be required to pay substantial royalties or grant a cross license to our patents to another patent holder; or
- •
- be required to redesign the formulation of a drug candidate so it does not infringe, which may not be possible or could require substantial funds and time.
The development programs for our two lead drug candidates are based in part on intellectual property rights we license from others, and any termination of those licenses could seriously harm our business.
We have in-licensed certain patent rights in connection with the development programs for each of our two lead drug candidates. With respect to CYC202, we hold a license from Centre National de Recherche Scientifique, or CNRS, and Institut Curie. With respect to CYC682, we hold a license from Sankyo Co., Ltd. of Japan. Both of these license agreements impose payment and other material obligations on us. Under the CNRS/Institut Curie license, we are obligated to pay license fees, milestone payments and royalties. We are also obligated to use reasonable efforts to develop and commercialize products based on the licensed patents. Under the Sankyo license we are obligated to pay license fees, milestone payments and royalties. We are also obligated to use commercially reasonable efforts to commercialize products based on the licensed rights and to use reasonable efforts to obtain regulatory approval to sell the products in at least one country by September 2011. Although we are currently in compliance with all of our material obligations under these licenses, if we were to breach any such obligations our counterparties would be permitted to terminate the licenses. This would restrict or delay or eliminate our ability to develop and commercialize these drug candidates, which could seriously harm our business.
20
Intellectual property rights of third parties could adversely affect our ability to commercialize our drug candidates.
If patents issued to third parties containing valid claims that cover our compounds or their manufacture or use, we may be required to obtain licenses to these patents or to develop or obtain alternative technology. We are aware of several published patent applications, and understand that others may exist, that could support claims that, if granted, could cover various aspects of our developmental programs, including in some cases our lead drug candidate, CYC202, particular uses of that compound, CYC682 or other therapeutic candidates, or gene sequences and techniques that we use in the course of our research and development. Based on our review of the published applications, we believe that it is unlikely that a valid claim would be issued that covered CYC202. In addition, we understand that other applications exist relating to uses of CYC202 and CYC682 that are not part of our current clinical programs for these compounds. Although we intend to continue to monitor these applications, we cannot predict what claims will ultimately be allowed and if allowed what their scope would be. If a patent is issued that covers our compounds or their manufacture or use then we may not be in a position to commercialize the related drug candidate unless we successfully pursue litigation to have that patent invalidated or enter into a licensing arrangement with the patent holder. Any such litigation would be time consuming and costly, and its outcome would not be guaranteed, and we cannot be certain that we would be able to enter into a licensing arrangement with the patent holder on commercially reasonable terms. In either case, our business prospects could be materially adversely affected.
Risks Related to Our Ordinary Shares and This Offering
No public market existed for our shares or ADSs prior to the offering and there can be no assurance that an active trading market will develop for the shares on the London Stock Exchange or for the ADSs on the Nasdaq National Market.
Before the offering, there was no public market for the shares or ADSs and you could not buy or sell the shares or ADSs publicly. The underwriters will determine the offer price by negotiation, and this price may not be the price at which the shares or ADSs will trade due to the fact that the offer price may be based on factors that may not be indicative of future performance.
We have applied to have the shares listed on the London Stock Exchange and the ADSs quoted on the Nasdaq National Market. There can be no assurance that an active trading market will develop for the shares on the London Stock Exchange or for ADSs on the Nasdaq National Market. The absence of an active trading market on either the London Stock Exchange or the Nasdaq National Market could adversely affect the market price of the shares or the ADSs.
Market volatility may cause the price of our shares and ADSs and the value of your investment to decline and you may not be able to resell your shares or ADSs at or above the initial public offering price.
Our share price is likely to be volatile. The initial public offering price may not be indicative of prices that will subsequently prevail in the market. Therefore, if you purchase ordinary shares in this offering, you may not be able to resell your shares at or above the initial public offering price. The market prices for securities of biopharmaceutical companies in general have been highly volatile and may continue to be highly volatile in the future. In addition to other risk factors described in this section, the following factors may have a significant impact on the market price of our ordinary shares:
- •
- market conditions for the biotechnology or pharmaceutical industries in general;
- •
- actual or anticipated fluctuations in our results of operations;
- •
- changes in financial estimates or recommendations by securities analysts;
21
- •
- sales of large blocks of our shares or ADSs;
- •
- sales of our shares or ADSs by our executive officers, directors and significant shareholders; and
- •
- changes in accounting principles.
The stock markets in general, and the markets for biotechnology stocks in particular, have experienced extreme price and volume fluctuations that have often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our ordinary shares irrespective of our actual operating performance. In addition, class action litigation has often been instituted in the past against companies whose securities have experienced periods of volatility in market price. Any such litigation brought against us could result in substantial costs, which would hurt our financial condition and results of operations and divert management's attention and resources, which could result in delays of our clinical trials or collaboration or marketing efforts.
Your rights as a shareholder will be governed by English law and differ from the rights of shareholders under U.S. law. In addition, you may have difficulty in effecting service of process in the United States or enforcing judgments obtained in the United States.
Cyclacel Group plc is a public limited company incorporated under the laws of England and Wales. Therefore, the rights of holders of ordinary shares and many of the rights of holders of ADSs are governed by English law and by our memorandum and articles of association. These rights differ from the typical rights of shareholders in U.S. corporations. Facts that, under U.S. law, would entitle a shareholder in a U.S. corporation to claim damages may also give rise to a cause of action under English law entitling a shareholder in an English company to claim damages. However, this will not always be the case. For example, the rights of shareholders to bring proceedings against us or against our directors or officers in relation to public statements are more limited under English law than the civil liability provisions of the U.S. securities laws.
You may have difficulties enforcing, in actions brought in courts in jurisdictions located outside the United States, liabilities under the U.S. securities laws. In particular, if you sought to bring proceedings in England based on U.S. securities laws, the English court might consider:
- •
- that it did not have jurisdiction; and/or
- •
- that it was not the appropriate forum for such proceedings; and/or
- •
- that, applying English conflict of laws rules, U.S. law (including U.S. securities laws) did not apply to the relationship between you and us or our directors and officers; and/or
- •
- that the U.S. securities laws were of a public or penal nature and should not be enforced by the English court.
You should also be aware that English law does not allow for any form of legal proceedings directly equivalent to the class action available in U.S. courts.
A majority of our directors and executive officers and some of the experts named in this prospectus are residents of countries other than the United States. Furthermore, all or a substantial portion of their assets and our assets are located outside the United States. As a result, it may not be possible for you to:
- •
- effect service of process within the United States upon any of our directors and executive officers and some of the experts named in this prospectus or on us; or
22
- •
- enforce in U.S. courts judgments obtained against any of our directors and executive officers and some of the experts named in this prospectus or us in the U.S. courts in any action, including actions under the civil liability provisions of U.S. securities laws; or
- •
- enforce in U.S. courts judgments obtained against any of our directors and senior management or us in courts of jurisdictions outside the United States in any action, including actions under the civil liability provisions of U.S. securities laws.
You may also have difficulties enforcing in courts outside the United States judgments obtained in the U.S. courts against any of our directors and executive officers and some of the experts named in this prospectus or us (including actions under the civil liability provisions of the U.S. securities laws). In particular, there is doubt as to the enforceability in England of U.S. civil judgments predicated purely on U.S. securities laws. In any event, there is no system of reciprocal enforcement in the United Kingdom of judgments obtained in the U.S. courts. Accordingly, a judgment against any of those persons or us may only be enforced in England by the commencement of a fresh action before the English court based on the judgment of the U.S. court. Summary judgment against any of those persons or us, as the case may be, may be granted by the English court without requiring the issues in the U.S. litigation to be reopened on the basis that those matters have already been decided by the U.S. court provided that the English court is satisfied:
- •
- that the judgment is final and conclusive;
- •
- that the U.S. court had jurisdiction (as a matter of English law);
- •
- that the U.S. judgment is not impeachable for fraud and is not contrary to English rules of natural justice;
- •
- that the enforcement of the judgment will not be contrary to public policy or statute in England;
- •
- that the judgment is for a liquidated sum;
- •
- that the English proceedings were commenced within the relevant limitation period;
- •
- that the judgment is not directly or indirectly for the payment of taxes or other charges of a like nature or a fine or penalty;
- •
- that the judgment remains valid and enforceable in the court in which it was obtained unless and until it is set aside; and
- •
- that, before the date on which the U.S. court gave judgment, the issues in question had not been the subject of a final judgment of an English court or of a court of another jurisdiction whose judgment is enforceable in England.
The foregoing reflects the opinion of Allen & Overy LLP, our English counsel, and is based on current law and practice in the United Kingdom. For further information with respect to our shares, see "Description of Share Capital." For a description of the rights of ADS holders as they differ from the rights of shareholders, see "Description of American Depositary Shares."
The initial public offering price for our shares and ADSs will be substantially higher than the net tangible book value per share of our outstanding ordinary shares immediately after this offering. If you purchase shares or ADSs in this offering, you will incur substantial and immediate dilution in the net tangible book value of your investment. Net tangible book value per share represents the amount of total tangible assets and less total liabilities, divided by the number of ordinary shares then outstanding. To the extent that options or warrants that are currently outstanding or are contingent upon completion
23
of this offering are exercised or converted, there will be further dilution in your shares. See "Dilution" on page 30 for a calculation of the extent to which your investment will be diluted.
Shares or ADSs eligible for public sale after this offering could adversely affect the price of our ordinary shares.
The market price for our shares or ADSs could decline as a result of sales by our existing shareholders or management of ordinary shares in the market after this offering, or the perceptions that these sales could occur. These sales may also make it difficult for us to sell equity securities in the future at a time and at a price we deem appropriate. The lock-up agreements relating to our shareholders and option holders provide that they may not dispose of ordinary shares for a certain period following the date of this prospectus. For more information on our principal shareholders, their lock-up agreements and their shares eligible for future sale, see "Principal Shareholders," "Underwriters" and "Shares Eligible for Future Sale."
Currency fluctuations may adversely affect the price of the ADSs relative to the price of our ordinary shares.
The price of our ordinary shares is quoted in pounds sterling. Movements in the pound sterling/U.S. dollar exchange rate may adversely affect the U.S. dollar price of our ADSs and the U.S. dollar equivalent of the price of our ordinary shares. For example, if the pound sterling weakens against the U.S. dollar, the U.S. dollar price of the ADSs could decline, even if the price of our ordinary shares in pounds sterling increases or remains unchanged. We will calculate and pay any cash dividends in pounds sterling and, as a result, exchange rate movements will affect the U.S. dollar amount of dividends you will receive from the Depositary if you hold ADSs.
Circumstances may change, leading us to exercise our discretion to use the proceeds of this offering in a manner different from that currently envisaged, and we may not use the proceeds effectively, which could affect the results of operations and cause our share price to decline.
We expect to use the net proceeds of this offering to continue the development of our drug candidates, expand our research programs and for working capital and other general corporate purposes. We have not determined the exact amounts or timing of these expenditures and our directors and management may be required to exercise their discretion, in the best interests of the Company, in utilizing the net proceeds of this offering. We may use the proceeds in ways that are different from our current intentions and you may not agree with the uses we choose. We may use the proceeds in ways that do not necessarily improve our results of operations or enhance the value of our shares or ADSs. The failure to apply these funds effectively could result in financial losses that could cause the price of our shares and ADSs to decline and delay the development of our drug candidates.
It is most likely that we will be a passive foreign investment company (a PFIC) for U.S. federal income tax purposes, which may result in negative tax consequences to you.
Based on our analysis of our current assets and income it is most likely that we will be a PFIC for U.S. federal income tax purposes for the current taxable year and we may also be a PFIC for any future year. If we are classified as a PFIC in any year that a U.S. Holder (as defined herein) is a shareholder, we generally will continue to be treated as a PFIC for that U.S. Holder in all succeeding years. If we are a PFIC in any year during which a U.S. Holder owns ordinary shares, the U.S. Holder may be subject to additional taxes on any "excess distributions" received from us and any gain realized from the sale or other disposition of the ordinary shares (whether or not we continue to be a PFIC). We urge U.S. investors to consult their own tax advisors about the application of the PFIC rules and certain elections that may help to minimize adverse U.S. federal income tax consequences in their particular circumstances. For a further discussion of the U.S. federal income tax consequences of
24
investing in a PFIC, see the discussion below under the heading "Taxation—U.S. Federal Income Taxation."
To date, we have not declared or paid any cash dividends on our ordinary shares and currently intend to retain any future earnings for funding growth. We do not anticipate paying any dividends in the foreseeable future. Moreover, companies organized under the laws of England and Wales cannot pay dividends unless they have distributable profits as defined in the U.K. Companies Act. We do not have any distributable profits, and we may not therefore be entitled to pay any dividends in respect of ordinary shares for a considerable period of time. As a result, you should not rely on an investment in our ordinary shares if you require dividend income. Capital appreciation, if any, of our shares or ADSs may be your sole source of gain for the forseeable future.
25
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements concerning our business, operations and financial performance and condition as well as for our plans and objectives for our business operations and financial performance and conditions. You can identify these statements by words such as "aim," "anticipate," "assume," "believe," "could," "due," "estimate," "expect," "goal," "intend," "may," "objective," "plan," "positioned," "should," "target," "will," "would" and other similar expressions that are predictions of or indicate future events and future trends. These forward-looking statements are based on current expectations, estimates, forecasts and projections about our business and the industry in which we operate, management's beliefs and assumptions made by management and are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control and may cause our actual results, performance and condition or achievements to differ materially from those expressed or implied by such forward-looking statements. Except as required by law or regulation, we do not have any intention or obligation to update publicly any forward-looking statements even though our situation may change in the future. Factors that may cause such differences include, but are not limited to, the risks described under "Risk Factors," including:
- •
- failure to obtain sufficient funding for the development and commercialization of our drug candidates;
- •
- negative or inconclusive results from our clinical trials;
- •
- inability to obtain regulatory approval for any of our drug candidates;
- •
- failure to enter into or maintain successful strategic alliances, which may delay the development or commercialization of our drug candidates or may result in significant additional expenditures;
- •
- inability to attract or retain skilled personnel for our drug development and commercialization efforts; and
- •
- inability to protect our intellectual property and operate our business without infringing upon the intellectual rights of others, which could result in litigation and significant expenditures.
Potential investors and other readers are urged to consider these factors carefully in evaluating the forward-looking statements and are cautioned not to place undue reliance on the forward-looking statements.
You should rely only upon the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell the shares or ADSs in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this document is accurate as of the date on the front cover of this document only, regardless of the time of delivery of this prospectus or any sale of our shares.
26
USE OF PROCEEDS
We expect to receive approximately $41.9 million from the sale of 16,000,000 shares (in the form of ordinary shares and ADSs) in this offering at an assumed initial public offering price of £1.66 per share and $12.00 per ADS (the mid-point of the price range on the cover page of this prospectus) after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, or $48.5 million if the underwriters' over-allotment option is exercised in full.
We intend to use the net proceeds of this offering for general corporate purposes, including:
- •
- approximately $12.6 million to continue the development of our drug candidate CYC202;
- •
- approximately $4.2 million to continue the development of our drug candidate CYC682;
- •
- approximately $6.3 million to continue preclinical activities for our CYC381 and virology programs;
- •
- approximately $10.5 million to expand our research programs; and
- •
- approximately $8.3 million for working capital and other general corporate purposes.
We believe that the net proceeds of this offering, our existing cash, cash equivalents and short-term investments will be sufficient to meet our projected operating requirements for at least the next twenty-four months.
We will apply the proceeds of this offering, together with our existing resources, primarily towards progressing the development of our lead drug candidates, CYC202 and CYC682, to a stage where they can generate significant revenues for the group. We currently anticipate that the proceeds of this offering will be sufficient to enable us to complete Phase II trials for our lead drug candidates, CYC202 and CYCY682, but do not expect that they will be sufficient to enable us to fund Phase III trials of these drug candidates. The extent to which the proceeds of this offering will allow us to advance our current preclinical and research programs will depend significantly on decisions we make in the future as to the best use of our available funds. These decisions will be based on circumstances existing at the relevant time and may involve a variety of factors, including among other things the numbers of patients required to be enrolled in clinical trials in order to obtain relevant results, the results actually obtained in our clinical and preclinical trials and research programs, actions taken by regulatory authorities that may affect our prospects of obtaining regulatory approval of particular drug candidates and actions taken and results achieved by our competitors, which may affect our prospects of commercial success in relation to particular drug candidates.
In addition, the amounts and timing of our actual expenditures also depend on several factors, including the progress of our research and development efforts and the amount of cash used by our operations. We have not determined the exact amounts or timing of the expenditures in the areas listed above. Accordingly, our directors and management will retain considerable discretion in utilizing the net proceeds of this offering. Pending these uses, we intend to invest the net proceeds in short-term, investment-grade, interest bearing instruments. To determine whether we are a passive foreign investment company, the obligations will be considered "passive assets" and the interest we receive on them "passive income."
27
DIVIDEND POLICY
We have never declared or paid cash dividends on our ordinary shares. We do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business.
Under English law, any payment of dividends would be subject to the U.K. Companies Act, which requires that all dividends be approved by our board of directors and, in some cases, our shareholders. Moreover, under English law, we may pay dividends on our shares only out of profits available for distribution determined in accordance with the U.K. Companies Act and accounting principles generally accepted in the United Kingdom, which differ in some respects from U.S. GAAP. In the event that dividends are paid in the future, holders of the ADSs will be entitled to receive payments in U.S. dollars in respect of dividends on the underlying shares in accordance with the deposit agreement. See "Description of Share Capital—Dividends" and "Description of American Depositary Shares—Share Dividends and Other Distributions."
28
CAPITALIZATION
The following table sets forth our unaudited capitalization (i) on an actual basis as at June 30, 2004, immediately following the reorganization described under "Pre-offering Reorganization into Public Limited Company," (but also reflecting the conversion of our preferred D shares into 20,840,344 of our ordinary shares, assuming an initial public offering price of £1.66 per ordinary share, being the midpoint of the price range set forth on the cover page of this prospectus, and the further bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 115) and (ii) on an adjusted basis to reflect the sale of 16,000,000 shares (in the form of ordinary shares and ADSs) in this offering at an assumed initial offering price of £1.66 per share and $12.00 per ADS (the midpoint of the price range on the cover page of this prospectus) after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. This table should be read in conjunction with our audited consolidated financial statements and accompanying notes included elsewhere in this prospectus and with the information set forth under the heading "Management's Discussion and Analysis of Financial Condition and Results of Operations."
The number of ordinary shares outstanding does not include the ordinary shares issuable on the exercise of options and warrants or reserved for issuance under our share and option plans described in the paragraph following the table under "Prospectus Summary—The Offering" on page 6, including 3,441,806 ordinary shares (as adjusted to reflect the bonus issue) issuable on exercise of options granted to Spiro Rombotis, our chief executive officer, at an exercise price of 1 pence per share, as more particularly described under "Management—Employee Share Option Plans—Senior Executive Incentive Plan" on page 92. Similarly, the deferred stock compensation figures below do not reflect the impact of the grant of these and other options, which is discussed under "Management's Discussion and Analysis of Financial Condition and Results of Operations—Share Compensation" on page 37.
| | As of June 30, 2004
| |
|---|
| | Actual
| | As Adjusted
| |
|---|
| | ($ in millions)
| |
|---|
| Shareholders' equity: | | | | | |
| Ordinary shares, par value 1p 100,000,000 shares authorized and 44,964,304 shares outstanding, actual; and 100,000,000 shares authorized and 60,964,304 shares outstanding, as adjusted | | — | | 0.2 | |
| Deferred stock compensation | | (13.5 | ) | (13.5 | ) |
| Additional paid-in capital | | 132.6 | | 174.3 | |
| Deficit accumulated during the development stage | | (85.8 | ) | (85.8 | ) |
| | |
| |
| |
| Total shareholders' equity | | 33.3 | | 75.2 | |
| | |
| |
| |
| Total capitalization | | 33.3 | | 75.2 | |
| | |
| |
| |
29
DILUTION
If you invest in our ordinary shares, your interest will be diluted immediately to the extent of the difference between the public offering price per share you will pay in this offering and the pro forma net tangible book value per ordinary share immediately after this offering.
As of June 30, 2004 immediately following the reorganization described under "Pre-offering Reorganization into Public Limited Company", on page 114, our net tangible book value was £18,392,295, or £9.71 per share or $70.26 per ADS. Net tangible book value per ordinary share represents the amount of total tangible assets less total liabilities, divided by the number of ordinary shares outstanding as of June 30, 2004. After giving effect to the conversion of all of our outstanding preferred D shares as of June 30, 2004 into 20,840,344 of our ordinary shares (based on an assumed initial offering price of £1.66 per share, being the midpoint of the range on the cover page of this prospectus) and the further bonus issue of shares described under "Description of Share Capital" beginning on page 115, our pro forma net tangible book value per share as of June 30, 2004 was approximately £0.41 per ordinary share or $2.96 per ADS. After giving effect to the sale of 16,000,000 ordinary shares (in the form of shares and ADSs) in this offering and the receipt of gross proceeds of £26.6 million or $48.0 million (assuming an initial public offering price of £1.66 or $12.00) and after deducting underwriting discounts and commissions and estimated offering expenses, our pro forma as adjusted net tangible book value as of June 30, 2004 would have been £41.5 million or $75.2 million, or £0.68 per share and $4.93 per ADS. This represents an immediate increase in net tangible book value of £0.27 per share to existing shareholders and an immediate dilution of £0.98 per share or $7.07 per ADS to new investors purchasing our shares or ADSs in this offering, in each case based on the assumed initial public offering price. The following table illustrates this dilution:
| | Per Share
| | Per Share
| | Per ADS
| | Per ADS
|
|---|
| Assumed initial public offering price | | | | £1.66 | | | | | $ | 12.00 |
| Pro forma net tangible book value as of June 30, 2004 | | £0.41 | | | | $ | 2.96 | | | |
| Pro forma increase in net tangible book value attributable to this offering | | £0.27 | | | | $ | 1.97 | | | |
| | |
| | | |
| | | |
| Pro forma as adjusted net tangible book value after this offering | | | | £0.68 | | | | | $ | 4.93 |
| | | | |
| | | | |
|
| Pro forma dilution to new investors in this offering | | | | £0.98 | | | | | $ | 7.07 |
| | | | |
| | | | |
|
If the underwriters exercise their over-allotment option in full, pro forma as adjusted net tangible book value will increase to £0.71 per share or $5.16 per ADS, representing an increase to existing holders of £0.31 per share, and there will be an immediate dilution of £0.95 per share or $6.84 per ADS to new investors.
The following table shows, on a pro forma as adjusted basis as of June 30, 2004, after giving effect to the pro forma adjustments referred to above, the differences in the number of ordinary shares purchased from us, the total cash consideration paid and the average price per ordinary share paid by our existing shareholders and by new investors (for new investors, assuming an initial public offering price per share of £1.66 or $12 per ADS).
| | Ordinary Shares Purchased
| | Total Consideration
| |
| |
|
|---|
| | Average
Price Per
Share
| | Average
Price Per
ADS
|
|---|
| | Number
| | Percentage
| | Amount
| | Percentage
|
|---|
| Existing shareholders | | 44,964,304 | | 74 | % | $ | 103.650,560 | | 68 | % | £1.49 | | $ | 10.80 |
| New investors | | 16,000,000
| | 26
| %
| 48,000,000
| | 32
| %
| £1.66 | | $ | 12.00 |
| Total | | 60,964,304
| | 100
| %
| 151,650,560
| | 100
| %
| £1.54 | | $ | 11.11 |
30
If the underwriters exercise their over-allotment option in full, the following will occur:
- •
- the pro forma as adjusted percentage of our ordinary shares held by existing shareholders will decrease to approximately 71% of the total number of pro forma as adjusted ordinary shares outstanding after this offering; and
- •
- the pro forma as adjusted number of our ordinary shares (in the form of shares or ADSs) held by new investors will increase to 18,400,000, or approximately 29% of the total pro forma as adjusted number of ordinary shares outstanding after this offering.
These tables assume no exercise of any share options or warrants outstanding as of June 30, 2004 or granted thereafter. As of June 30, 2004, there were options outstanding to purchase a total of 3,593,640 ordinary shares at a weighted average exercise price of £0.76 per share (as adjusted to reflect the bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 115). As of June 30, 2004, there were warrants outstanding to purchase a total of 23,500 ordinary shares at a weighted average exercise price of £0.95 per share. In addition, we have granted options (as adjusted to reflect the bonus issue) over 3,441,806 ordinary shares at an exercise price of 1 pence per share to Spiro Rombotis, our chief executive officer, and over 100,000 ordinary shares at an exercise price of £0.75 per share to Paul McBarron, our chief financial officer, as more particularly described under "Management—Employee Share Option Plans—Senior Executive Incentive Plan" on page 92. If any of these options or warrants are exercised, there will be a further dilution to new investors.
31
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data are extracted without material adjustment from our U.S. GAAP consolidated financial statements, which have been audited by Ernst & Young LLP, independent auditors, included in this prospectus. Our consolidated financial statements as of March 31, 2002, March 31, 2003, December 31, 2002, December 31, 2003 and March 31, 2004 and for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003 and the three months ended March 31, 2003 and 2004, which have been prepared in accordance with U.S. GAAP, were audited by Ernst & Young LLP, independent auditors, and are included in this prospectus beginning on page F-1. The selected consolidated financial data for the years ended March 31, 2000 and 2001, which were audited by Ernst & Young LLP, have been prepared in accordance with U.S. GAAP. Our consolidated financial data should be read in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" following this section and our financial statements and related notes appearing elsewhere in this prospectus. Investors should read the whole of this document and not just rely on the selected financial data in this section.
The historical results are not necessarily indicative of results to be expected in any future period:
| |
| |
| |
| |
| | Nine Months
Ended December 31,
| | Three Months
Ended
March 31,
| | Period From
August 13, 1996
(Inception) to
March 31,
2004
| |
|---|
| | Year Ended March 31,
| |
|---|
| | 2000
| | 2001
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
| |
|---|
| | (in thousands, except share and per share data)
| |
|---|
| Consolidated Statements of Operations: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Collaboration and research and development income | | $ | — | | $ | — | | $ | 1,155 | | $ | 1,250 | | $ | 930 | | $ | 8 | | $ | 319 | | $ | 1 | | $ | 2,413 | |
| | Grant income | | | 383 | | | 170 | | | 55 | | | 941 | | | 696 | | | 504 | | | 246 | | | — | | | 2,387 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | | | 383 | | | 170 | | | 1,210 | | | 2,191 | | | 1,626 | | | 512 | | | 565 | | | 1 | | | 4,800 | |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Research and development | | | (4,768 | ) | | (8,139 | ) | | (13,195 | ) | | (19,847 | ) | | (14,581 | ) | | (13,084 | ) | | (5,275 | ) | | (4,870 | ) | | (67,594 | ) |
| | General and administrative | | | (1,402 | ) | | (2,189 | ) | | (3,220 | ) | | (2,536 | ) | | (2,026 | ) | | (2,099 | ) | | (504 | ) | | (1,573 | ) | | (15,625 | ) |
| | Amortization of employee stock-based compensation | | | (214 | ) | | (275 | ) | | (672 | ) | | (305 | ) | | (229 | ) | | (217 | ) | | (76 | ) | | 618 | | | (1,991 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Total operating expenses | | | (6,384 | ) | | (10,603 | ) | | (17,087 | ) | | (22,688 | ) | | (16,836 | ) | | (15,400 | ) | | (5,855 | ) | | (5,825 | ) | | (85,210 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Operating loss | | | (6,001 | ) | | (10,433 | ) | | (15,877 | ) | | (20,497 | ) | | (15,210 | ) | | (14,888 | ) | | (5,290 | ) | | (5,824 | ) | | (80,410 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Interest and other income (expense) | | | 315 | | | (5 | ) | | 1,024 | | | 558 | | | 481 | | | (1,575 | ) | | 73 | | | 415 | | | 892 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Loss before taxes | | | (5,686 | ) | | (10,438 | ) | | (14,853 | ) | | (19,939 | ) | | (14,729 | ) | | (16,463 | ) | | (5,217 | ) | | (5,409 | ) | | (79,518 | ) |
| Taxes | | | — | | | — | | | — | | | (4,397 | ) | | (3,841 | ) | | (1,486 | ) | | (528 | ) | | (521 | ) | | (6,403 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Net loss | | | (5,686 | ) | | (10,438 | ) | | (14,853 | ) | | (15,542 | ) | | (10,888 | ) | | (14,977 | ) | | (4,689 | ) | | (4,888 | ) | | (73,115 | ) |
| Dividends on preferred shares | | | — | | | — | | | (3,289 | ) | | (4,654 | ) | | (3,430 | ) | | (4,425 | ) | | (1,225 | ) | | (2,629 | ) | | (14,997 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Net loss applicable to ordinary shareholders | | $ | (5,686 | ) | $ | (10,438 | ) | $ | (18,142 | ) | $ | (20,196 | ) | $ | (14,318 | ) | $ | (19,402 | ) | $ | (5,914 | ) | $ | (7,517 | ) | $ | (88,112 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Net loss per share—basic and diluted | | $ | 1.03 | | $ | 1.67 | | $ | (2.83 | ) | $ | (3.14 | ) | $ | (2.23 | ) | $ | (2.25 | ) | $ | (0.92 | ) | $ | (0.39 | ) | $ | (15.65 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Weighted average shares used to compute basic and diluted earnings per share | | | 5,528,672 | | | 6,265,760 | | | 6,399,539 | | | 6,433,996 | | | 6,433,874 | | | 8,623,516 | | | 6,433,896 | | | 19,259,641 | | | 5,629,029 | |
Consolidated Balance Sheet Data at Period End: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Cash and cash equivalents | | $ | 2,992 | | $ | 1,070 | | $ | 21,770 | | $ | 16,558 | | $ | 21,687 | | $ | 9,670 | | $ | 16,558 | | $ | 5,685 | | $ | 5,685 | |
| Short-term investments | | | 4,538 | | | 4,703 | | | 10,697 | | | 1,575 | | | 1,604 | | | 24,010 | | | 1,575 | | | 33,826 | | | 33,826 | |
| Prepaid expenses and other current assets | | | 919 | | | 1,011 | | | 2,850 | | | 4,808 | | | 4,509 | | | 5,360 | | | 4,808 | | | 3,892 | | | 3,892 | |
| Property, plant and equipment | | | 1,348 | | | 2,521 | | | 3,688 | | | 3,940 | | | 4,276 | | | 3,760 | | | 3,940 | | | 4,117 | | | 4,117 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Total assets | | | 9,797 | | | 9,305 | | | 39,005 | | | 26,881 | | | 32,076 | | | 42,800 | | | 26,881 | | | 47,520 | | | 47,520 | |
| Current liabilities | | | (1,303 | ) | | (2,678 | ) | | (4,221 | ) | | (4,993 | ) | | (5,047 | ) | | (4,657 | ) | | (4,993 | ) | | (5,477 | ) | | (5,477 | ) |
| Long-term debt, net of current portion | | | (511 | ) | | (9,217 | ) | | (1,094 | ) | | (184 | ) | | (332 | ) | | (495 | ) | | (184 | ) | | (334 | ) | | (334 | ) |
| Preferred ordinary C shares | | | — | | | — | | | (48,766 | ) | | (53,851 | ) | | (54,859 | ) | | — | | | (53,851 | ) | | — | | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Total shareholders' equity (deficit) | | $ | 7,983 | | $ | (2,590 | ) | $ | (15,076 | ) | $ | (32,147 | ) | $ | (28,162 | ) | $ | 37,648 | | $ | (32,147 | ) | $ | 41,709 | | $ | 41,709 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Total capital stock, excluding preferred ordinary C shares | | $ | 6 | | $ | 6 | | $ | 6 | | $ | 6 | | $ | 6 | | $ | 40 | | $ | 6 | | $ | 42 | | $ | 42 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
32
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with our financial statements and related notes included elsewhere in this prospectus. Selected financial data set out in this document are extracted without material adjustment, from our U.S. GAAP consolidated financial statements as, which have been audited by Ernst & Young LLP, independent auditors, included in this document, or from the underlying records used in the preparation of these statements. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under "Risk Factors" and elsewhere in this prospectus.
Overview
This summary highlights key information contained elsewhere in this prospectus. It may not contain all of the information that is important to you. You should read the entire prospectus carefully, including the "Risk Factors" section and the consolidated financial statements and related notes set out in this prospectus.
We are a biopharmaceutical company dedicated to the discovery, development and commercialization of novel, mechanism-targeted drugs to treat human cancers and other serious disorders. We describe drugs, compounds or molecules as novel if they have been recently discovered using advanced technologies, and as mechanism-targeted if they are designed to affect identified biological processes through known mechanisms. Our core area of expertise is in cell cycle biology. We focus primarily on the discovery and development of orally available anticancer agents that target the cell cycle with the aim of slowing the progression or shrinking the size of tumors, enhancing quality of life and improving survival rates of cancer patients. Our work with novel molecules that act on the cell cycle has also led us to pursue drug development opportunities in other indications.
We differentiate the products in our pipeline according to the developmental stage they have reached: products in the clinical trial phase; products in preclinical development; and products and programs in the research phase. Our current pipeline comprises nine novel drug series, seven for cancer, one for HIV/AIDS and one for Type 2 Diabetes. CYC202, our lead drug candidate, is in Phase II clinical trials for a variety of cancers. Our second drug candidate, CYC682, is currently in Phase Ib trials, also for a variety of cancers. We are currently considering a new Phase II clinical trial of CYC202 in glomerulonephritis, a form of kidney inflammation, and expect to commence Phase I clinical development of a drug candidate for the treatment of HIV/AIDS in 2006. We have an additional five cancer programs, one at the preclinical stage and four at the research stage, and one program in diabetes at the research stage.
We have incurred net losses since inception as we have devoted substantially all of our resources to research and development, including our clinical trials. As of March 31, 2004, our accumulated deficit was approximately $73.1 million. We expect to incur substantial and continued losses for the next several years as we:
- •
- continue to develop CYC202, CYC682 and other of our drug candidates currently in development;
- •
- apply for regulatory approvals;
- •
- commercialize our drug candidates, if any, that receive regulatory approval;
- •
- continue and expand our research and development program, biomarker program and further develop our proprietary drug discovery technologies;
- •
- acquire or in-license products, technologies or businesses that are complementary to our own;
33
- •
- establish sales and marketing capabilities; and
- •
- incur general and administrative expenses.
To date, we have not generated any product revenue, and we have financed our operations and internal growth primarily through private placements of equity securities, licensing revenue, interest on investments, government grants and research and development tax credits. We have received proceeds from the issuances of equity interests of $103.5 million since our inception in August 1996, including $8.6 million in the three months ended March 31, 2004, and $27.4 million in the nine months ended December 31, 2003. We have also received $2.4 million from government grants and $4.4 million from research and development tax credits since our inception. We expect to elect to receive a research and development tax credit of $0.5 million and $1.5 million for the three month period ended March 31, 2004 and the nine month period ended December 31, 2003. We believe that the net proceeds of this offering, our existing cash, cash equivalents and short-term investments will be sufficient to meet our projected operating requirements through at least the next twenty four months.
There is a risk that any drug discovery and development program may not produce revenue. Moreover, because of uncertainties inherent in the conduct and regulation of drug discovery and development, including those factors described in the "Risk Factors" section of this prospectus, we may not be able to successfully develop and commercialize any of our drug candidates.
The successful development of our drug candidates is highly uncertain. We cannot estimate with certainty or know the exact nature, timing and estimated costs of the efforts necessary to complete the development of our drug candidates or the date of completion of these development efforts. We cannot estimate with certainty any of the foregoing due to the numerous risks and uncertainties associated with developing our drug candidates, including:
- •
- the uncertainty of the timing of completion of patient recruitment and enrollment in future Phase III clinical trials;
- •
- the possibility of delays in the collection and analysis of clinical trial data;
- •
- the uncertainty of clinical trial results;
- •
- extensive governmental regulation in the United States, the United Kingdom, the European Union and elsewhere for approval of new therapies; and
- •
- the uncertainty related to commercial scale manufacturing of our drug candidates.
If we fail to complete the development of our drug candidates in a timely manner, it could have a material adverse effect on our operations, financial position and liquidity. In addition, any failure by us to obtain, or any delay in obtaining, regulatory approvals could have a material adverse effect on our results of operations. A further discussion of the risks and uncertainties associated with completing our projects on schedule, or at all, and certain consequences of failing to do so are set forth in the risk factors.
We intend to pursue selective strategic alliances, primarily when our drug candidates enter into Phase IIb clinical trials, to enable us to maintain and increase our current financial and operational capacity. These collaborations may include joint marketing or promotion arrangements of our products or the granting of exclusive marketing rights to our collaborators in exchange for up-front fees, milestone payments and royalties on future sales, if any. In addition, in the future we intend to build our own sales force in order to market one or more of our drug candidates on our own or with a co-promotion partner. Additionally, we seek to in-license research programs from third parties where they are complementary to our own programs. We thus have in-licensed two programs, CYC682 from Sankyo Co., Ltd. and clotrimazole analogs, or compounds similar in structure to clotrimazole, from Lorus Therapeutics, Inc.
34
Our fiscal year end since incorporation was March 31. We have changed our fiscal year end to December 31, resulting in a shortening of the March 31, 2004 year end to December 31, 2003. Going forward we will report on a calendar basis for 2004. The three month periods to March 31, 2003 and 2004 disclosed in this document has been audited for the sole purpose of satisfying requirements in connection with the listing in the United Kingdom.
Research and Development
The clinical development, manufacturing, selling and marketing of new drugs are subject to extensive regulation by the FDA and other regulatory authorities in the United States, the United Kingdom, the European Union and elsewhere. These regulations vary from country to country, but as a general matter require the premarket demonstration of safety and efficacy for specific indications of use, post-marketing surveillance for product safety, and compliance with manufacturing and promotional standards. Obtaining premarket approval is expensive and is a complex, lengthy and uncertain process. During the development process, subsequent investigations may fail to support or substantiate the findings of earlier trials, including lack of efficacy or safety, thereby delaying, limiting or even preventing regulatory approval.
We are currently conducting two Phase II trials of our lead drug candidate CYC202, one Phase IIa as combination therapy for the treatment of non-small cell lung cancer and one Phase II as monotherapy for the treatment of hematological cancers. We may expand our Phase II non-small cell lung cancer program during the second half of 2004 if efficacy and safety data from our Phase IIa studies are favorable. Dependent on the results of the Phase II studies, we expect to begin a new Phase II clinical trial of CYC202 as combination therapy in solid tumors. Assuming the results of the Phase IIa studies are favorable we would expect to commence a randomized Phase IIb study. If results from this study were favorable we would consider progressing to a Phase III trial (subject to, among other things, the cost of such a study). We expect that it will take several years before we can commercialize CYC202. Accordingly, we cannot reasonably estimate when and to what extent CYC202 will generate revenues or material net cash flows, which may vary widely depending on numerous factors, including the effectiveness and safety profile of the drug, market acceptance, and then-prevailing reimbursement policies, competition and other market conditions. We currently fund all research and development costs associated with CYC202. We generally expect to determine whether and to what extent we will seek partnering arrangements after developing our compounds through the Phase II proof of efficacy stage. If we were to enter into a partnering arrangement our expenditures relating to research and development of this drug candidate might decrease significantly.
We are currently conducting a Phase Ib clinical trial for CYC682. The clinical trial program for CYC682 may proceed for several years, and we will not be in a position to generate any revenues or material net cash flows from the drug candidate until the program is successfully completed, regulatory approval is achieved and a drug is commercialized. CYC682 is at an early a stage of development and it is therefore difficult for us to predict when this may occur. If we were to enter into a partnering arrangement in relation to CYC682 our net expenditures relating to research and development of this drug candidate might decrease significantly.
We expect to commence Phase I clinical development of a drug candidate for the treatment of HIV/AIDS in 2005. We have an additional preclinical program and multiple research programs in cancer and one in Type 2 Diabetes and expect, depending upon available financial resources, to begin Phase I clinical trials with a new molecule each year for the next three years. As with our other drug candidates these programs are at too early a stage of development for us to predict if and when we will be in a position to generate any revenues or material net cash flows from drug candidates. We currently fund all research and development costs associated with our preclinical and research programs. We anticipate that our expenditures relating to research and development of our preclinical and research programs will increase significantly as we advance drug candidates into clinical development.
35
Since we became operational, we have focused on drug discovery and development programs, with particular emphasis on orally available anticancer agents. Research and development expenses represented 85.0% and 83.6% of our total operating expenses for the nine month period ended December 31, 2003 and the three month period ended March 31, 2004, respectively and 86.6% of our total operating expenses for the nine month period ended December 31, 2002. Research and development expenses primarily include:
- •
- compensation of personnel associated with our research activities, including consultants and contract research;
- •
- screening and identification of drug candidates;
- •
- supplies and materials;
- •
- preclinical studies, including toxicology studies;
- •
- clinical trials, including consultants and clinical research organizations;
- •
- continued advancement of our biomarker program and our technology platforms, including Polgen;
- •
- facilities costs; and
- •
- depreciation of equipment.
The following table provides information with respect to our research and development expenditure:
| | Year Ended
March 31,
| | Nine Months
Ended
December 31,
| | Three Months
Ended March 31,
| | Period from August 13, 1996 (inception) to March 31,
|
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
| | 2004
|
|---|
| | (in thousands)
|
|---|
| CYC202 | | $ | 3,804 | | $ | 6,877 | | $ | 5,039 | | $ | 3,611 | | $ | 1,843 | | $ | 1,442 | | 20,288 |
| CYC682 | | | — | | | — | | | — | | | 551 | | | — | | | 556 | | 1,108 |
| Current Preclinical Programs | | | — | | | 828 | | | 78 | | | 2,624 | | | 776 | | | 892 | | 4,344 |
| Second Generation CDK Inhibitors Research Program | | | 2,517 | | | 4,597 | | | 3,895 | | | 2,683 | | | 678 | | | 1,021 | | 12,751 |
| Other Current Research Programs | | | 4,150 | | | 4,917 | | | 3,756 | | | 1,353 | | | 1,156 | | | 322 | | 13,422 |
| Research Programs (Discontinued) | | | 1,639 | | | — | | | — | | | — | | | — | | | — | | 1,995 |
| Other Costs Related to Research and Development Management and Exploratory Research | | | 837 | | | 2,025 | | | 1,657 | | | 1,578 | | | 361 | | | 547 | | 9,231 |
| Non-Program-Specific Indirect Costs | | | 248 | | | 603 | | | 156 | | | 684 | | | 461 | | | 90 | | 4,455 |
| | |
| |
| |
| |
| |
| |
| |
|
| Total Research and Development Expenses | | | 13,195 | | | 19,847 | | | 14,581 | | | 13,084 | | | 5,275 | | | 4,870 | | 67,594 |
| | |
| |
| |
| |
| |
| |
| |
|
Amounts attributed to projects and programs include both direct and indirect costs such as allocated overhead and costs of facilities.
We do not believe that the historical costs associated with our lead drug candidates, CYC202 and CYC682, are indicative of the future costs associated with these drug candidates, which are currently in Phase II and Phase Ib clinical trials, respectively. Future development of these drug candidates would necessarily involve more extensive clinical trials than have been conducted to date, and ultimately efforts to market and commercialize these drug candidates, involving substantial additional costs relative to our historical levels of expenditure on these drug candidates. In addition, we do not believe
36
that historical costs associated with one drug candidate would be indicative of future costs for any other candidate in the same stage of development due to a number of factors including the costs of manufacturing the drug candidate, the numbers of patients required to be enrolled in clinical trials in order to obtain relevant results and different development approaches and trial protocols that may be required depending upon the nature of any given drug candidate and the specific indications for which it is being developed.
Clinical development timelines and associated costs vary widely depending on how we choose to allocate our expenditures among our research and drug discovery programs. We are currently focused on advancing our CYC202 drug candidates for cancer and inflammation, our CYC682 drug candidates for cancer and our biomarker technology. We anticipate, however, that we will make ongoing decisions on the continued development and funding of existing and future research and development projects in response to the scientific and clinical success of each drug candidate and technology, as well as an ongoing assessment of market potential.
We cannot easily predict the costs we will incur in connection with obtaining regulatory approvals for our drug candidates. Completion dates and completion costs are difficult to estimate, varying widely for each of our drug candidates and technologies. Acquiring regulatory approvals requires significant expenditure. To the extent that we fail to obtain regulatory approvals in a timely manner, our research and development costs may increase.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs for employees in executive and operational functions. Other significant costs include costs related to accounting and legal services, particularly legal services associated with our intellectual property, as well as facilities costs not otherwise included in research and development expenses. After the completion of this offering, we expect that our general and administrative costs will increase due to increased investor relations and insurance expense and other expenses associated with operating as a public company.
Share Compensation
In connection with the grant of share options under our Employees' Share Option Scheme and Share Option Plan, we record deferred share-based compensation as a component of shareholder's (deficit)/equity. Deferred share option compensation for options granted to employees is the difference between the fair value of ordinary shares on the date such options were granted and their exercise price.
We recorded amortization of deferred share-based compensation for options granted to employees of $672,000, $305,000, $229,000, $217,000, $76,000 and $(618,000) for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003 and the three months ended March 31, 2003 and 2004 respectively. We have recorded $2.0 million of deferred share-based compensation for the period from inception through March 31, 2004.
Generally, the amount of non-cash, share-based compensation we record in future periods may decrease if unvested options for which deferred compensation expense has been recorded are subsequently cancelled, or may increase if we make future option grants with exercise prices below the fair market value of our ordinary shares on the date of grant or make future option grants with performance-related vesting conditions. However, we expect that the actual amount of deferred share-based compensation we will record in shareholders' (deficit)/equity and consequently the amount of our
37
deferred compensation expense will be significantly higher over the next several years than it has been to date, for the following reasons:
- •
- On April 23, 2004, new options over 1,722,770 ordinary shares were granted to employees at an exercise price of £1.50 ($2.66) per share. Prior to the grant of these new options, 598,692 existing options with higher exercise prices were surrendered by these employees. As adjusted to reflect the bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 115, there will as a result be options outstanding over 3,956,340 ordinary shares at an exercise price of £0.75 per share. In accounting for the grant of the 598,692 options granted to replace those surrendered by employees we will follow variable plan accounting, we will treat the offering price in this offering as the fair market value of the ordinary shares at the time of grant. Assuming an offering price of £1.66 per share or $12 per ADS (being the midpoint of the price range set forth on the cover page of this prospectus), the deferred share-based compensation we will be required to record in this connection in our consolidated accounts at the date of grant is expected to be £1.1 million ($2.0 million). This amount is expected to be adjusted during each period for future changes in the market value of the ordinary shares.
- •
- In accounting for the balance of 1,124,078 of new options, we will treat the offering price in this offering as the fair market value of the ordinary shares at the time of grant. Assuming an offering price of £1.66 per share or $12 per ADS (being the midpoint of the price range set forth on the cover page of this prospectus), the deferred share-based compensation we will be required to record in this connection in our consolidated accounts at the date of grant is expected to be £2.0 million ($3.7 million). This amount is expected to be amortized over a period of three years from the date of grant, resulting in increased deferred compensation, expense in each of the relevant financial years.
- •
- We have also granted options (as adjusted to reflect the bonus issue) over 3,441,806 ordinary shares at an exercise price of 1 pence per share to Spiro Rombotis, our chief executive officer, and over 100,000 ordinary shares at an exercise price of £0.75 per share to Paul McBarron, our chief financial officer, as more particularly described under "Management—Employee Share Option Plans—Senior Executive Incentive Plan" on page 92. In accounting for the grant of these options, we will treat the offering price in this offering as the fair market value of the ordinary shares at the time of grant. Assuming an offering price of £1.66 per share or $12 per ADS (being the midpoint of the price range set forth on the cover page of this prospectus), the deferred share-based compensation we record in this connection in our consolidated financial statements for 2004 is expected to be £4.9 million ($8.8 million). We expect to account for these options in accordance with the guidance for variable plan accounting. This is expected to result in the stock-based compensation charge being accrued, over the period to the third anniversary of the offering, adjusted during each period for future changes in the market value of the ordinary shares.
Interest and Other Income and Expense
Interest and other income and expense consist primarily of interest earned on our cash, cash equivalents and short-term investments net of interest expense and amortization of issuance costs of the preferred ordinary C shares.
Research and Development Tax Credits
We have elected to take advantage of recent changes in U.K. corporation tax legislation, which allow companies to apply to convert tax losses into research and development tax credits, which is then repaid in cash to the applicant. We have received $4.4 million of research and development tax credits
38
in respect of the three years ended March 31, 2003. We expect to elect to receive a research and development tax credit of $0.5 million and $1.5 million for the three month period ended March 31, 2004 and the nine month period ended December 31, 2003 and in subsequent fiscal years until it is more tax efficient to accumulate tax losses within the Company.
Results of Operations
Comparison of three months ended March 31, 2004 and March 31, 2003
Revenues
Revenues decreased $0.6 million, from $0.6 million for the three month period ended March 31, 2003 to $0.0 million for the three month period ended March 31, 2004. This decrease was directly attributable to the completion of our collaboration with AstraZeneca and that no government grants were received. We received licensing revenues of $0.3 million in the three month period ended March 31, 2003 from our collaboration with AstraZeneca, as opposed to the three month period ended March 31, 2004 when we received no revenue from this arrangement. Grant revenues from various government grant awards decreased $0.2 million from $0.2 million for the three month period ended March 31, 2003 to $0.0 million for the three-month period ended March 31, 2004.
Research and Development Expenses
Research and development expenses decreased $0.4 million, or 7.7%, from $5.3 million for the three month period ended March 31, 2003 to $4.9 million for the three month period ended March 31, 2004. This decrease was primarily a reflection of our deliberate strategy to reduce expenses. Of the $5.3 million of expenses in the three month period ended March 31, 2003 we incurred $1.8 million, $0.0 million, $0.8 million and $2.7 million in respect of our drug candidate CYC202, our drug candidate CYC682, preclinical activities and research activities respectively. Of the $4.9 million of expenses in the three month period ended March 31, 2004 we incurred $1.4 million, $0.6 million, $0.9 million and $2.0 million in respect of our drug candidate CYC202, our drug candidate CYC682, preclinical activities and research activities, respectively.
General and Administrative Expenses
General and administrative expenses increased $1.1 million, or 212%, from $0.5 million for the three month period ended March 31, 2003 to $1.6 million for the three month period ended March 31, 2004. This increase was primarily due to advisors costs of $0.6 million related to financing activities, increased employment costs of $0.2 million and increased intellectual property maintenance fees and other related costs of $0.2 million.
Interest and Other Income and Expense
Interest and other income and expense increased $0.4 million, from $0.0 million for the three month period ended March 31, 2003 to $0.4 million for the three month period ended March 31, 2004. This increase was primarily due to an increase in interest and other income of $.2 million from $0.2 million for the three month period ended March 31, 2003 to $0.4 million for the three-month period ended March 31, 2004 attributable to higher average balances of cash, cash equivalents and investments in 2004. Additionally, interest and other expense decreased from $0.1 million in the three-months ended March 31, 2003 to $0.0 million in the three-months ended March 31, 2004 due to the ending of outstanding lease agreements and a decrease in interest payments.
39
Research and Development Tax Credits
Research and development tax credits were flat at $0.5 million for the three months ended March 31, 2003 and March 31, 2004.
Comparison of nine months ended December 31, 2003 and December 31, 2002
Revenues
Revenues decreased $1.1 million, or 69%, from $1.6 million for the nine month period ended December 31, 2002 to $0.5 million for the nine month period ended December 31, 2003. This decrease was directly attributable to the completion of our collaboration with AstraZeneca and a decrease in government grants received. We received licensing revenues of $0.9 million in the nine month period ended December 31, 2002 from our collaboration with AstraZeneca, as opposed to the nine month period ended December 31, 2003 when we received no revenue from this arrangement. Grant revenues from various government grant awards decreased $0.2 million from $0.7 million for the nine month period ended December 31, 2002 to $0.5 million for the nine month period ended December 31, 2003.
Research and Development Expenses
Research and development expenses decreased $1.5 million, or 10%, from $14.6 million for the nine month period ended December 31, 2002 to $13.1 million for the nine month period ended December 31, 2003. This decrease was primarily a reflection of our deliberate strategy to reduce expenses. Of the $14.6 million of expenses in the nine month period ended December 31, 2002 we incurred $5.0 million, $0.0 million, $0.1 million and $9.5 million in respect of our drug candidate CYC202, our drug candidate CYC682, preclinical activities and research activities respectively. Of the $13.1 million of expenses in the nine month period ended December 31, 2003 we incurred $3.6 million, $0.6 million, $2.6 million and $6.3 million in respect of our drug candidate CYC202, our drug candidate CYC682, preclinical activities and research activities, respectively.
General and Administrative Expenses
General and administrative expenses increased $0.1 million, or 4%, from $2.0 million for the nine month period ended December 31, 2002 to $2.1 million for the nine month period ended December 31, 2003. In each period, advisors costs and employment costs were $0.1 million and $0.6 million, respectively.
Interest and other income and expense
Interest and other income and expense decreased $2.1 million, or 427%, from a net income of $0.5 million for the nine month period ended December 31, 2002 to a net expense of $1.6 million for the nine month period ended December 31, 2003. This decrease was primarily due to the writing off of issuance costs of the preferred ordinary C shares of $1.9 million for the nine months ended December 31, 2003 compared to the amortization charge of $0.2 million in the nine months ended December 31, 2002. Additionally, there was a decrease in interest income and other income of $0.4 million from $0.8 million for the nine month period ended December 31, 2002 to $0.4 million for the nine month period ended December 31, 2003 attributable to lower average balances of cash, cash equivalents and investments in 2003.
Research and development tax credits
Research and development tax credits decreased $2.4 million, or 61%, from $3.8 million for the nine month period ended December 31, 2002 to $1.5 million for the nine month period ended December 31, 2003. This decrease was primarily due to the fact that we had claims of $0.8 million and
40
$1.5 million for the fiscal years ended March 31, 2001 and 2002, respectively, that were credited in the nine months ended December 31, 2002 as opposed to a claim of $2.0 million for the fiscal year ended March 31, 2003 that was credited in the nine months ended December 31, 2003.
Comparison of fiscal years ended March 31, 2003 and March 31, 2002
Revenues
Revenues increased $1.0 million, or 81%, from $1.2 million for the fiscal year ended March 31, 2002 to $2.2 million for the fiscal year ended March 31, 2003. This increase was directly attributable to an increase in government grants received and increased licensing revenue. Research and development grant revenues increased $0.9 million from $0.1 million of government grant awards in the fiscal year ended March 31, 2002 to $0.9 million in government grant awards in the fiscal year ended March 31, 2003. Licensing revenues increased $0.1 million from $1.2 million for the fiscal year ended March 31, 2002 to $1.3 million for the fiscal year ended March 31, 2003 in connection with our collaboration with AstraZeneca, which concluded in the nine month period ended December 31, 2003.
Research and Development Expenses
Research and development expenses increased $6.7 million, or 50%, from $13.2 million for the fiscal year ended March 31, 2002 to $19.8 million for the fiscal year ended March 31, 2003. Of the $13.2 million of expenses in the year ended March 31, 2002 we incurred $3.8 million, $0.0 million and $9.4 million in respect of our drug candidate CYC202, our drug candidate CYC682, preclinical activities and research activities respectively. Of the $19.8 million of expenses in the year ended March 31, 2003 we incurred $6.9 million, $0.0 million, $0.8 million and $12.1 million in respect of our drug candidate CYC202, our drug candidate CYC682, preclinical activities and research activities, respectively.
General and Administrative Expenses
General and administrative expenses decreased $0.7 million, or 21%, from $3.2 million for the fiscal year ended March 31, 2002 to $2.5 million for the fiscal year ended March 31, 2003. This was primarily due to a charge for compensation for warrants issued to non-employees of $1.2 million in the year ended March 31, 2002 with no corresponding charge in the year ended March 31, 2003, partially offset by increased salary and benefit costs relating to the hiring of additional general and administrative personnel in the fiscal year ended March 31, 2003.
Interest and other income and expense
Interest and other income and expense decreased $0.4 million, or 46%, from $1.0 million for the fiscal year ended March 31, 2002 to $0.6 million for the fiscal year ended March 31, 2003. This decrease was primarily due to a decrease in interest and other income of $0.4 million from $1.5 million for the fiscal year ended March 31, 2002 to $1.0 million for the fiscal year ended March 31, 2003 attributable to lower average balances of cash, cash equivalents and investments in 2003.
Research and development tax credits
We received research and development tax credits of $4.4 million for the year ended March 31, 2003 related to claims of $0.9 million, $1.6 million and $1.9 million for the fiscal years ended March 31, 2001, 2002 and 2003, respectively. We received no research and development tax credits in the year ended March 31, 2002.
41
Liquidity and Capital Resources
Since our inception, we have not generated any significant product revenue and we have relied primarily on the proceeds from sales of equity securities to finance our operations and internal growth. Additional funding has come through interest on investments, licensing revenue, government grants and research and development tax credits. We have incurred significant losses since our inception. As of March 31, 2004, we had an accumulated deficit of $73.1 million.
The following table summarizes our issuances of equity interests for cash, excluding executive and employee compensation, primarily preferred shares, through March 31, 2004:
Series
| | Date
| | Number of
Shares
| | Gross
Proceeds
|
|---|
| |
| |
| | (in thousands)
|
|---|
| A | | May 1997 | | 625,000 | | $ | 4,099 |
| B (First Closing) | | May 1999 | | 1,092,939 | (1) | $ | 8,109 |
| B (Second Closing) | | August 1999 | | 840,336 | | $ | 6,432 |
| C | | June 2001 | | 4,554,251 | (2) | $ | 48,031 |
| D (First Closing) | | November 2003 | | 4,088,427 | | $ | 28,228 |
| D (Second Closing) | | January 2004 | | 1,162,068 | | $ | 8,646 |
- (1)
- Includes 220,751 ordinary shares issued on conversion of bridging loans.
- (2)
- Includes 835,794 preferred ordinary C shares issued on conversion of 8% secured convertible loan notes.
We have also received $2.4 million in government grants since our inception and $4.4 million in research and development tax credits. We expect to elect to receive a research and development tax credit of $0.5 million and $1.5 million for the three month period ended March 31, 2004 and the nine month period ended December 31, 2003.
At March 31, 2004, we had cash and cash equivalents and short-term investments of $39.5 million as compared to $18.1 million at March 31, 2003. This overall increase was primarily due to the receipt of net proceeds of $36.2 million related to the issue and sale of our Series D Preference Shares. Short-term investments increased from $1.6 million at March 31, 2003 to $33.8 million at March 31, 2004. Cash and cash equivalents decreased from $16.6 million at March 31, 2003 to $5.7 million at March 31, 2004 due to additional operating losses and capital equipment purchases.
Net cash used in operating activities decreased $1.4 million from $4.6 million in the three months ended March 31, 2003 to $3.3 million in the three months ended March 31, 2004. This decrease was due to the receipt of research and development tax credits of $2.2 million offset by increased operating losses. Net cash used in operating activities increased $3.3 million from $11.1 million in the nine months ended December 31, 2002 to $14.4 million in the nine months ended December 31, 2003. This increase was due to additional operating losses. Net cash used in operating activities increased $5.9 million from $9.8 million in the fiscal year ended March 31, 2002 to $15.7 million in the fiscal year ended March 31, 2003. This increase was due to additional operating losses.
Net cash used in financing activities increased $0.2 million from $0.2 million in the three months ended March 31, 2003 to $0.4 million in the three months ended March 31, 2004. This increase was due to an increase in capital lease obligations. Net cash used in financing activities increased $0.1 million from $0.6 million in the nine months ended December 31, 2002 to $0.7 million in the nine months ended December 31, 2003. This increase was due to an increase in capital lease obligations. Net cash used in financing activities increased $0.6 million from $0.3 million in the fiscal year ended
42
March 31, 2002 to $0.8 million in the fiscal year ended March 31, 2003. This increase was due to an increase in capital lease obligations.
Net cash provided by financing activities was $8.6 million in the three months ended March 31, 2004 due to the issue and sale of our Series D Preference Shares. There was no net cash provided by financing activities in the three months ended March 31, 2003. There was no net cash provided by financing activities in the nine months ended December 31, 2002 and $27.4 million in the nine months ended December 31, 2003 primarily due to the issue and sale of our Series D Preference Shares. Net cash provided by financing activities was $38.1 million in the fiscal year ended March 31, 2002 primarily due to the issue and sale of our Series C Preference Shares. There was no net cash provided by financing activities in the fiscal year ended March 31, 2003.
We currently are party to only one long-term debt instrument, a government loan of $457,000 that bears interest at 5% per annum and is wholly repayable on January 1, 2005. As of March 31, 2004, we had contractual obligations, relating to our facilities and equipment leases as follows:
| | Payments Due by Period (in thousands)
|
|---|
Contractual obligations
| | Total
| | Less than
1 Year
| | 1–3 Years
| | 4–5 Years
| | After 5 Years
|
|---|
| Capital lease obligations | | $ | 1,108 | | $ | 654 | | $ | 413 | | $ | 41 | | $ | — |
| Operating lease obligations | | $ | 3,266 | | $ | 499 | | $ | 1,065 | | $ | 1,121 | | $ | 581 |
| Purchase obligations | | $ | 607 | | $ | 474 | | $ | 133 | | $ | — | | $ | — |
| Long-term debt | | $ | 457 | | $ | 457 | | $ | — | | $ | — | | $ | — |
| | |
| |
| |
| |
| |
|
| | | $ | 5,438 | | $ | 2,084 | | $ | 1,611 | | $ | 1,162 | | $ | 581 |
| | |
| |
| |
| |
| |
|
We also currently have a number of contractual arrangements with our partners under which milestone payments totalling $23.4 million would be payable subject to achievement of all the specific contractual milestones and our decision to continue with these projects. Under these contractual arrangements we make annual payments that do not and will not exceed $0.1 million.
Disclosure about Market Risk
Our exposure to market risk is limited to interest income sensitivity, which is affected by changes in the general level of U.K. interest rates, particularly because the majority of our investments are in short-term investments. The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive without significantly increasing risk. Our investment portfolio is subject to interest rate risk and will fall in value in the event market interest rates increase. Due to the short duration of our investment portfolio, we believe an immediate 10% change in interest rates would not be material to our financial condition or results of operations. We do not have any foreign currency or derivative financial instruments.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and related disclosure of contingent assets and liabilities. We review our estimates on an ongoing basis. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. While our significant accounting policies are described in more detail in the notes to our financial statements included in this prospectus, we believe the judgments and estimates
43
required by the following accounting policies to be critical in the preparation of our financial statements.
Revenue Recognition
Revenues are earned from collaborative agreements and amounts invoiced to customers in respect of goods supplied. The Company recognizes revenue in accordance with Securities and Exchange Commission Staff Accounting Bulletin, or SAB, No. 101, Revenue Recognition in Financial Statements, as amended by SAB Nos. 101A, 101B and 104. SAB No. 101 requires that four basic criteria must be met before revenue can be recognized: persuasive evidence of an arrangement exists; delivery has occurred or services have been rendered; the fee is fixed and determinable; and collectibility is reasonably assured. Determination of whether persuasive evidence of an arrangement exists and whether delivery has occurred or services have been rendered are based on management's judgments regarding the fixed nature of the fee charged for research performed and milestones met, and the collectibility of those fees. Should changes in conditions cause management to determine these criteria are not met for certain future transactions, revenue recognized for any reporting period could be adversely affected.
Research and development revenues, which are earned under agreements with third parties for contract research and development activities, are recorded as the related expenses are incurred. Milestone payments are non-refundable and recognized as revenue when earned, as evidenced by achievement of the specified milestones and the absence of ongoing performance obligations. Any amounts received in advance of performance are recorded as deferred revenue. None of the revenues recognized to date are refundable if the relevant research effort is not successful.
Stock-based Compensation
We account for stock-based employee compensation arrangements in accordance with the provisions of Accounting Principles Board Opinion No. 25 ("APB 25"), "Accounting for Stock Issued to Employees," Statement of Financial Accounting Standards No. 123 ("SFAS No. 123"), "Accounting for Stock-Based Compensation" and complies with the disclosure requirements of Statement of Financial Accounting Standards ("SFAS") No. 148, "Accounting for Stock-Based Compensation Transition and Disclosure an Amendment of FASB Statement No. 123." Under APB 25, compensation expense is based on the difference, if any, on the date of grant, between the estimated fair value of our common stock and the exercise price. SFAS No. 123 defines a "fair value" based method of accounting for an employee stock option or similar equity investment.
We account for equity instruments issued to non employees in accordance with the provisions of SFAS No. 123 and Emerging Issues Task Force ("EITF") Issue No. 96-18, "Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling Goods, or Services."
The determination of fair value of all options granted to employees after such time we become a public company will include an expected volatility factor.
Recently Issued Accounting Announcements
In January 2003, the FASB issued FASB Interpretation No. 46 ("FIN 46"), "Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51." FIN 46 requires certain variable interest entities to be consolidated by the primary beneficiary of the entity if the equity investors in the entity do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. In December 2003, the FASB issued FIN 46R, a revision to FIN 46. FIN 46R provides a broad deferral of the latest date by which all public entities must apply FIN 46 to certain variable interest
44
entities to the first reporting period ending after March 15, 2004. We do not expect the adoption of FIN 46 to have a material impact on our financial position, cash flows or results of operations.
In May 2003, the FASB issued SFAS No. 150, "Accounting For Certain Financial Instruments with Characteristics of Both Liabilities and Equity," which establishes standards for how an issuer of financial instruments classifies and measures certain financial instruments with characteristics of both liabilities and equity. It requires that an issuer classify a financial instrument that is within its scope as a liability (or in some circumstances, as an asset) if, at inception, the monetary value of the obligation is based solely or predominantly on: (a) a fixed monetary amount known at inception, (b) variations in something other than the fair value of the issuer's equity shares or (c) variations inversely related to changes in the fair value of the issuer's equity shares. SFAS No. 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. The adoption of SFAS No. 150 did not have an impact on our consolidated financial statements.
Introduction of International Financial Reporting Standards
On June 7, 2002, the European Council approved a Regulation on the introduction of International Financial Reporting Standards (IFRS) into the European Union for financial years beginning on or after January 1, 2005 for the consolidated financial statements of all listed groups. Because this is a Regulation rather than a Directive, it passed immediately and directly into national law and does not have to be approved by Member States of the European Union. The Regulation is now part of English law. We are therefore required to report for U.K. statutory purposes under IFRS for the year ending December 31, 2005. Early adoption is not permitted.
Conversion from U.K. Generally Accepted Accounting Practice to IFRS will introduce changes to the accounting for certain transactions and the reporting format of our annual reports in the United Kingdom. However, the introduction of IFRS is not purely an accounting issue. In order to effect the transition, change is required for many non-finance related departments, such as IT and human resources. We have conducted a preliminary assessment of the impact on our internal reporting framework across all relevant aspects although it is as yet too early to fully understand the impact these changes may have on the operations of the business.
We have also commenced a detailed assessment of the impact on our accounting practices of differences that will be introduced by IFRS. This program is ongoing and we anticipate completion and full preparedness for the first time implementation of IFRS in the U.K. financial statements for the year ending December 31, 2005. The conversion to IFRS will not impact our financial statements prepared under U.S. Generally Accepted Accounting Principles.
45
THE CELL CYCLE AND CANCER
The cell cycle is a set of complex mechanisms that the body uses to control the growth and division of cells. Cancer is characterized by uncontrolled cell multiplication when key cell cycle regulatory proteins are absent or malfunction due to genetic mutations. The most common regulatory protein malfunction is associated with mutation of the p53 tumor suppressor gene, present in about two-thirds of cancer patients. Our founder, Professor Sir David Lane, discovered the p53 protein.
Many approved cancer chemotherapies in everyday clinical use target different phases of the cell cycle. Their effect, however, is not confined to cancer cells. Instead, these chemotherapies are cytotoxic, which means that they act in the body as poisons in an unspecific manner, killing cancer cells and normal cells alike. This limits the doses that patients can be given and gives rise to significant side-effects such as alopecia or hair loss, diarrhea, mucositis or gut inflammation, myelosuppression or immune system decline, stomatitis or mouth inflammation, and vomiting. Through our expertise in cell cycle biology, we are focused on the discovery and development of novel, cell cycle-based mechanism-targeted cancer therapies that emulate the body's natural processes in order to stop the growth of cancer cells, but can limit the damage to normal cells and the accompanying side-effects caused by conventional chemotherapeutic agents. We describe drugs, compounds or molecules as novel if they have been recently discovered using advanced technologies, and as mechanism-targeted if they are designed to affect identified biological processes through known mechanisms.
The cell cycle involves a complex series of molecular and biochemical signaling pathways. As illustrated in the diagram below, the cell cycle has four phases:
- •
- theG1, or gap, phase, in which the cell grows and prepares to synthesize DNA;
- •
- theS, or synthesis, phase, in which the cell synthesizes DNA;
- •
- theG2, or second gap, phase, in which the cell prepares to divide; and
- •
- theM, or mitosis, phase, in which cell division occurs.
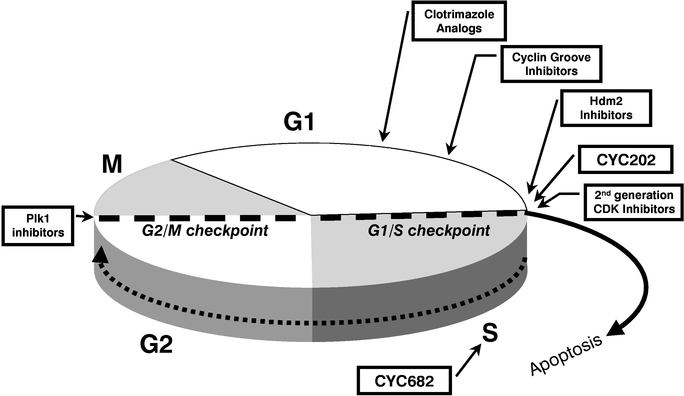
As a cell approaches the end of the G1 phase it is controlled at a vital checkpoint, called G1/S, where the cell determines whether or not to replicate its DNA. At this checkpoint the cell is checked for DNA damage to ensure that it has all the necessary cellular machinery to allow for successful cell division. As a result of these checks, which involve the interactions of various proteins, a "molecular
46
switch" is toggled on or off. Cells with intact DNA continue to S phase; cells with damaged DNA that cannot be repaired are arrested and "commit suicide" through apoptosis, or programmed cell death. A second such checkpoint occurs at the G2 phase following the synthesis of DNA in S phase but before cell division in M phase.
Cells use a complex set of enzymes called kinases to control various steps in the cell cycle. Cyclin Dependent Kinases, or CDKs, are a specific enzyme family that use signals to switch on cell cycle mechanisms. CDKs themselves are activated by forming complexes with cyclins, another group of regulatory proteins only present for short periods in the cell cycle. When functioning properly cell cycle regulatory proteins, including CDKs and cyclins, act as the body's own tumor suppressors by inducing the death of damaged cells. Genetic mutations causing the malfunction or absence of one or more of the regulatory proteins at cell cycle checkpoints can result in the "molecular switch" being turned permanently on, permitting uncontrolled multiplication of the cell, leading to carcinogenesis, or tumor development.
Our drug discovery and development efforts are based on a deep understanding of these cell cycle mechanisms. We are generating several families of novel anticancer drugs, including CDK inhibitors, one of the most sophisticated categories of novel drugs targeting cell cycle mechanisms. CDK inhibitors work by indirectly causing the accumulation of particular regulatory proteins, such as p53. This causes cells to be arrested mainly at the G1/S cell cycle checkpoint. Because cancer cells are genetically damaged, this arrest results in their death by apoptosis. CDK inhibitors can be given to patients at dosage levels that are sufficient to induce death in cancer cells, but spare most normal cells. This is because most normal cells have no genetic damage and continue their normal development once the effect of the drug in the body has dissipated. In addition to our work with CDK inhibitors, we are investigating other cell cycle mechanisms in order to expand our portfolio of cell cycle blocking anticancer agents. We currently have anticancer programs involving nucleoside analogs, cyclin expression blockers, cyclin groove inhibitors, Hdm2 inhibitors and inhibitors of Polo-like kinases and other mitotic kinases, all of which have important effects on the cell cycle.
Because our novel, mechanism-targeted therapies act specifically to induce death in cancer cells while sparing normal cells, they may result in fewer side-effects, offering significant advantages over conventional anticancer drugs. Moreover, because the compounds we are developing act at different stages of the cell cycle, we believe they may lead to anticancer drugs that will operate synergistically in combination therapy. For example, our nucleoside analogs act on tumor cells at S phase and may work in synergy with cell cycle drugs acting on the G1/S checkpoint such as CDK inhibitors. Our cyclin inhibitors reduce the levels of several cyclin proteins and/or block their expression, leading to arrest in G1 phase. As this occurs at an earlier point in the cell cycle than the effects induced by CDK inhibitors and nucleoside analogs, our cyclin inhibitors may also work in synergy with both.
Our work with novel molecules that act on the cell cycle has led us to pursue drug development opportunities in other indications. Some of these molecules have significant potential to be used effectively in the treatment of diseases other than cancer that are characterized by rapid or abnormal cell proliferation. We are currently exploring the use of cell cycle inhibitors in kidney inflammation and HIV/AIDS. Moreover, in seeking to develop drugs that regulate cell cycle proteins for the treatment of cancer, we may also identify opportunities for using novel mechanism-targeted approaches in other indications. This is the basis of our current research program in Type 2 Diabetes.
47
BUSINESS
For a detailed description of the cell cycle as it relates to our drug discovery and development efforts, please see "The Cell Cycle and Cancer" beginning on page 46.
Overview
We are a biopharmaceutical company dedicated to the discovery, development and commercialization of novel, mechanism-targeted drugs to treat human cancers and other serious disorders. We describe drugs, compounds or molecules as novel if they have been recently discovered using advanced technologies, and as mechanism-targeted if they are designed to affect identified biological processes through known mechanisms. Our core area of expertise is in cell cycle biology. We focus primarily on the discovery and development of orally available anticancer agents that target the cell cycle with the aim of slowing the progression or shrinking the size of tumors, and enhancing quality of life and improving survival rates of cancer patients. Our work with novel molecules that act on the cell cycle has also led us to pursue drug development opportunities in other indications.
We have been focused on the cell cycle since our inception. We were founded in 1996 by Professor Sir David Lane, a recognized leader in the field of tumor suppressor biology who discovered the p53 protein, which operates as one of the body's own anticancer "drugs" by inhibiting cell cycle targets. In 1999, we were joined by Professor David Glover, a recognized leader in the mechanism of mitosis or cell division who discovered, among other cell cycle targets, the mitotic kinases, Polo and Aurora, enzymes that act in the mitosis phase of the cell cycle. Our expertise in cell cycle biology is at the center of our business strategy.
We are generating several families of anticancer drugs that act on the cell cycle. These include Cyclin Dependent Kinase (CDK) inhibitors, one of the most sophisticated categories of novel drugs targeting cell cycle mechanisms. Although a number of pharmaceutical and biotechnology companies are currently attempting to develop CDK inhibitor drugs, our lead drug candidate, CYC202, is the only orally available CDK inhibitor drug candidate in Phase II trials.
Our current pipeline comprises nine novel drug series, seven for cancer, one for HIV/AIDS and one for Type 2 Diabetes. CYC202, our lead drug candidate, is in Phase II clinical trials for a variety of cancers. Our second drug candidate, CYC682, is currently in Phase Ib trials, also for a variety of cancers. We are currently considering a new Phase II clinical trial of CYC202 in glomerulonephritis, a form of kidney inflammation, and expect to commence Phase I clinical development of a drug candidate for the treatment of HIV/AIDS in 2006. We have an additional five cancer programs, one at the preclinical stage and four at the research stage, and one program in diabetes at the research stage. Taken together, our pipeline covers all four phases of the cell cycle, which we believe will improve our chances of successfully developing and commercializing novel drugs that work in combination with approved conventional chemotherapies, and could eventually lead to potential combination therapies of our novel drugs that act synergistically across the cell cycle. Based on the current status of our preclinical pipeline and depending on available financial resources, we expect to be in a position to begin Phase I clinical trials with a new molecule each year for the next three years.
Our lead drug candidate, CYC202, is a novel, orally available CDK inhibitor that is currently in multi-center Phase II clinical trials for cancer. In Phase I trials designed to test safety rather than efficacy, independent investigators nonetheless observed that CYC202 given alone as monotherapy appeared to induce clinical benefit in some patients with various solid tumors. We are currently conducting two Phase II trials of CYC202, one Phase IIa as combination therapy for the treatment of non-small cell lung cancer and one Phase II as monotherapy in hematological cancers, or cancers of the blood cells. We expect to report results from these trials during the second half of 2004, and to begin a further clinical trial with CYC202 as combination therapy in solid tumors by the end of the year.
48
Our second drug candidate, CYC682, is an orally available prodrug of the novel nucleoside analog CNDAC, which is a novel nucleoside analog, or a compound with a structure similar to a nucleoside. A prodrug is a compound that has a therapeutic effect only after it is metabolized within the body, and CNDAC has a significantly higher residence time in the blood when it is produced in the body through metabolism of CYC682 than when it is given directly. Like CDK inhibitors, nucleosides work through cell cycle inhibition, though they do so at a different phase of the cell cycle. A number of nucleoside drugs, such as gemcitabine, are in wide use as conventional chemotherapies. Preclinical results from independent investigators reported that CYC682 was superior to gemcitabine and 5-FU, another widely used chemotherapy, both in terms of extending survival and blocking metastases to the liver. Two Phase I studies of CYC682 have been completed, and a third Phase Ib clinical trial is currently in progress for the treatment of patients with advanced malignancies. We currently expect CYC682 to enter Phase II trials in the first half of 2005.
To enhance our development efforts, we are making extensive use of biomarkers in each of our clinical programs to study the effects of our drugs in the blood and tissues of patients. Biomarkers are proteins or other substances whose presence in the blood can serve as an indicator of specific cell processes. For example, we are working with a biomarker of apoptosis. After analyzing blood samples drawn from 36 patients treated with CYC202 in our Phase I trials, we observed high levels of this biomarker in 17 of them. In some cases we were also able to correlate biomarker results with clinical benefit as observed independently by Phase I clinical investigators. Although biomarkers are the focus of great interest within the scientific community and the FDA has issued for comment a draft guidance document that encourages submission of biomarker data, such data are not currently accepted by the FDA or other regulatory authorities as a basis for approval of drug candidates. We nonetheless believe that biomarkers serve a useful purpose in helping us to evaluate at an early stage in clinical trials whether our drug candidates cause their intended effects in patients through their assumed mechanisms and whether we should continue to invest in their development. Biomarker data from early clinical trials also enable us to design subsequent trials more efficiently and to monitor patient compliance with trial protocols. Biomarkers are also informing our efforts to discover improved next generation drugs working with similar mechanisms. We believe that in the longer term biomarkers may allow us to select patients more likely to respond to our drugs for clinical trial and marketing purposes and increase the probability of successful outcomes in terms of disease management.
Our pipeline of nine different drug programs is comprised of seven internally generated and two in-licensed compounds. We expect that in the future our drug programs will increasingly result from the application of our proprietary genes-to-drugs approach, originating through the use of genomic technology from our Polgen division to identify appropriate gene targets and progressing by means of structure-based design techniques through to the development stage. This approach is exemplified by our Plk, or Polo-like kinase, inhibitor program. Fundamentally, our approach to drug discovery and development aims to improve our ability to select promising drug targets at an early stage so as to decrease attrition rates during the later, more expensive stages of drug development, allowing us to progress through the drug discovery and development process more quickly and efficiently and enhancing our chances of successfully commercializing drugs. To this end, we have assembled a set of sophisticated discovery and development technologies, together with personnel who are highly skillful in making use of these technologies.
Our main research facility is located in Dundee, Scotland where we carry out our structure-based drug design and development programs. This is also the location of our corporate headquarters. We have a second research facility located in Cambridge, England. This is the location of our Polgen division. Polgen is working to discover the function of new cancer genes and validate their use as drug discovery targets.
49
Our Business Strategy
We are and intend to remain strongly focused on the development of novel, cell cycle-based therapies for the treatment of cancer, for a number of reasons:
- •
- Our core area of expertise is in cell cycle biology. Our scientists include recognized leaders in this field, and we have since our inception dedicated ourselves to understanding the cell cycle and attempting to harness its commercial potential. In addition, our senior management has extensive experience in the pharmaceutical and biotechnology sectors including considerable international experience in research, preclinical and clinical development, sales and marketing. We believe that we are thus well placed to exploit the significant opportunities that this area offers for new drug discovery and development.
- •
- The novel, mechanism-targeted cell cycle drugs we are developing are highly selective in comparison to conventional chemotherapies, inducing death in cancer cells while sparing most normal cells and thus giving rise to fewer side-effects. We believe that we are currently well positioned to realize some of the market potential of such drugs, as we are the only company with an orally available CDK inhibitor drug candidate in Phase II clinical trials, and have a deep pipeline of other anticancer drug candidates in clinical and preclinical development or at the research stage.
- •
- Many conventional chemotherapies work by cell cycle mechanisms, albeit in an unspecific way. To this extent, cell cycle therapeutic mechanisms are already well established with regulatory authorities and medical professionals as treatments for cancer. We believe that this enhances the likelihood that our novel cell cycle drugs, if successfully developed, will obtain required regulatory approvals and win acceptance among oncologists, improving our prospects of commercial success. In addition, because both work on the cell cycle, our novel mechanism-targeted drug candidates may offer significant therapeutic potential in combination with approved cytotoxic drugs. We believe that data from such combination trials will usefully inform our clinical trial design and help us to progress our drug development efforts more quickly than clinical testing of our drug candidates as monotherapy would otherwise allow.
Seek to develop anticancer drug candidates in all phases of the cell cycle and multiple compounds for particular cell cycle targets
In selecting potential anticancer drug candidates, we target mechanisms related to all phases of the cell cycle and develop multiple compounds for each of our cell cycle targets. In this way, we believe that we can maximize our chances of successfully developing anticancer drugs while minimizing the business consequences of the failure of any one drug candidate. In the longer term, we believe that novel cell cycle drugs acting at different phases of the cell cycle may be shown to operate synergistically amongst themselves, which we believe would offer significant commercial potential. Moreover, if we can obtain regulatory approval of one of our anticancer drug candidates, we believe there will be opportunities to initiate combination trials involving that drug and one of our other drug candidates, which would improve sales of the approved drug and enhance our cancer franchise.
We intend to be opportunistic and selective in seeking to capitalize on drug development opportunities in other indications that arise out of the molecules we are working with in our cancer programs. Some of these molecules have significant potential to be used effectively in the treatment of diseases other than cancer that are characterized by rapid or abnormal cell proliferation. These include indications for which there is currently no standard treatment, like glomerulonephritis, or for which
50
current treatments are inadequate, like HIV/AIDS. For some indications, like Type 2 Diabetes, our structure-based drug design approaches informed by our knowledge of cell cycle biology may result in superior drugs. We are currently pursuing drug programs in each of these areas. We believe that this opportunistic and selective strategy will enhance our chances of commercial success while reducing overall business risk.
We expect that in the future our drug programs will increasingly result from the application of our proprietary technologies ranging from gene discovery to drug development, or our genes-to-drugs approach. This approach relies on genomic technology from our Polgen division and on a set of sophisticated drug discovery and structure-based drug design technologies to identify novel drug candidates. We believe that by devoting resources initially to enrich our target selection process, we can focus our efforts on targets that have a higher probability of yielding successful candidates. In this way, we aim to progress through the drug discovery and development process more quickly and efficiently, decreasing attrition rates during the later, more expensive stages of drug development and enhancing our chances of successfully commercializing novel drugs.
We intend to continue to use our understanding of biomarkers to improve and accelerate the clinical development of our drug candidates. We believe that biomarkers serve a useful purpose in helping us to evaluate whether our drug candidates are having their intended effects through their assumed mechanisms at an early stage, before committing the resources required to conduct extensive mid- to late-stage clinical trials. In this way we believe we are able to direct our clinical resources to those drug candidates that have better prospects for success, increasing the efficiency of our drug development efforts and potentially reducing the time to regulatory approval and commercialization of our drug candidates. Biomarker data from early clinical trials may also enable us to design subsequent trials more efficiently and to monitor patient compliance with trial protocols. Biomarkers are also informing our efforts to discover improved next generation drugs working with similar mechanisms. We believe that biomarkers may in the longer term allow us to select patients more likely to respond to our drugs for clinical trial and marketing purposes and increase the probability of successful outcomes in terms of disease management. Biomarkers are the focus of great interest within the scientific community and the FDA has issued for comment a draft guidance document that encourages submission of biomarker data, such data, however, are not currently accepted by the FDA or other regulatory authorities as a basis for approval of drug candidates, and may never be so accepted. Our biomarker data should not be interpreted as evidence of efficacy.
Selectively enter into partnering arrangements while developing our own sales and marketing capability
We retain all marketing rights to the compounds associated with our current drug programs. To optimize our commercial return, we intend both to enter into selected partnering arrangements and to develop our own sales and marketing capability. Generally, we will seek to develop our compounds through the Phase II proof of efficacy stage before seeking a partner. We may be prepared to enter into partnering arrangements earlier in connection with drug programs outside our anticancer franchise. To the extent we seek partnering arrangements, we will be prepared to agree that our partners take our products through late-stage clinical trials, regulatory submissions, registration, manufacturing, marketing and sales. We will, however, seek to maintain an active role in this process and participate, as our financial resources allow, in shared-risk arrangements, such as co-development and co-promotion, that can increase our commercial return. In parallel we intend to develop our own oncology-focused sales force to market our own and other anticancer drugs where we believe that it is in our interest to do so in terms of expense and risk and where our technical expertise results in competitive advantage.
51
Our Pipeline
The table below summarizes our current clinical and preclinical drug candidates and our ongoing research programs.
Drug Candidate/Program
| | Indication
| | Clinical/Development Status
| | Target
| | Cell Cycle Mechanism
|
|---|
CYC202
(CDK Inhibitor) | | • | Cancer (non-small cell lung, hematological) | | Two Phase II clinical trials ongoing | | CDK | | G1/S checkpoint and others |
|
|
• |
Glomerulonephritis |
|
Phase I clinical trials completed |
|
CDK |
|
G1/S checkpoint and others |
CYC682 (Nucleoside Analog) |
|
Cancer (solid tumors) |
|
Phase Ia clinical trials completed; Phase Ib trial in progress |
|
DNA polymerase |
|
S phase |
Clotrimazole Analogs |
|
Cancer |
|
Preclinical trials |
|
Cyclin expression blocker |
|
G1 phase |
Cell Cycle Inhibitors in Virology |
|
HIV/AIDS |
|
Preclinical trials |
|
Mitotic kinase and CDK |
|
Several |
Second Generation CDK Inhibitors |
|
Cancer |
|
Research program |
|
CDK |
|
G1/S checkpoint and others |
GSK-3 Inhibitors |
|
Type 2 Diabetes |
|
Research program |
|
GSK-3 |
|
N/A |
Plk Inhibitors |
|
Cancer |
|
Research program |
|
Plk |
|
G2/M checkpoint |
Hdm2 Inhibitors |
|
Cancer |
|
Research program |
|
Hdm2 |
|
G1 phase |
Cyclin Binding Groove Inhibitors |
|
Cancer |
|
Research program |
|
Cyclin binding groove |
|
G1 phase |
Our Clinical Programs
Our lead drug candidate, CYC202, is a Cyclin Dependent Kinase (CDK) inhibitor that causes cancer cell death by inducing apoptosis in tumor cells. Our preclinical studies suggested that CYC202 would provide significant therapeutic benefits in combination with approved cytotoxic drugs and in monotherapy. Phase I clinical trials have identified the doses of CYC202 which can be given to patients without severe toxicity and which allow enough CYC202 to reach the patient's bloodstream to have an effect on tumors. We are currently conducting a multi-center Phase IIa combination clinical trial in Europe for treatment of non-small cell lung in combination with leading cytotoxic drugs. We are also undertaking a Phase II clinical trial investigating the action of CYC202 as monotherapy in a group of B-cell hematological malignancies, which are cancers of white blood cells.
CYC202 is orally available, one of very few anticancer compounds that can be taken by mouth. Oral dosing is more convenient for patients, reduces the costs of treatment and allows greater flexibility in the dosing and treatment regimen. In addition, because CYC202 acts on the cell cycle to induce
52
death in cancer cells while sparing most normal cells, we believe it may give rise to fewer side-effects, offering significant potential advantages over conventional anticancer drugs.
CYC202 is being tested in the treatment of non-small cell lung cancer and certain hematological malignancies. In 2004, 563,700 people are expected to die from cancer in the United States. There will be an estimated 173,770 new cases of lung and bronchus cancer, 15,270 new cases of multiple myeloma, 33,440 new cases of leukemia of which 8,190 chronic lymphocytic leukemia and 62,250 new cases of lymphoma, of which 54,370 non-Hodgkin's lymphoma and 7,880 Hodgkin's disease. About 5% of those diagnosed with non-Hodgkin's lymphoma have mantle cell lymphoma.
The first patent for the chemical family of tri-substituted purines as inhibitors of CDKs was filed in 1995. We selected CYC202 as a drug candidate from our library of several hundred tri-substituted purines. CYC202 inhibits the CDK enzyme by blocking its binding site for adenosine triphosphate, or ATP. Blocking ATP binding stops the CDK enzyme from activating proteins required for the completion of the cell cycle. This leads to apoptosis in cancer cells. Testing has shown that CYC202 selectively inhibits CDKs, its target enzymes, without affecting closely-related, non-target enzymes. We have also shown that CYC202 is active in cell lines resistant to conventional chemotherapy.
CYC202 is a low molecular weight compound with a relatively simple structure and so exhibits the chemical characteristics of other compounds that have been successfully developed as drugs. Its manufacture is relatively inexpensive, requiring a three-step chemical synthesis.
The following table provides information with respect to the clinical trials that have been conducted to date with CYC202:
Trial
| | Subjects
| | Methodology
| | Key Findings
|
|---|
| Phase I: pharmacokinetics (2001) | | 5 patients with cancer who had failed multiple chemotherapies | | Single escalating daily dose—50mg, 100mg, 200mg. | | Safe and absorbed when taken by mouth. |
Phase I: pharmacokinetics and tolerability (2001 and 2002) |
|
24 healthy volunteers in 2 separate trials each with 12 patients |
|
Single escalating daily dose—50mg, 100mg, 200mg, 400mg, 800mg. |
|
Safe and absorbed when taken by mouth. Elevated liver enzymes were noted in one volunteer. Dosing with food has no effect. |
Phase I: safety (2003) |
|
21 patients with cancer who had failed multiple chemotherapies |
|
Two doses daily, seven days out of 21. |
|
"Serious adverse events," as defined in the protocol, possibly related to therapy, were seen in five patients. Dose limiting toxicities observed. 1 out of 21 patients appeared to show stable |
| disease for four or more months. | | | | | | |
53
Phase I: tolerability (2003) |
|
56 patients with cancer who had failed multiple chemotherapies |
|
Two doses daily, five days out of 21or 10 days out of 21or three days out of 14. |
|
9 out of 56 patients showed stable disease for four or more months; one patient showed partial response as defined in the protocol, with 46% reduction in tumor size. |
Phase I: biomarker (current) |
|
3 patients with nasopharyngeal cancer |
|
Two doses daily, on days 1–3 and days 8–12. |
|
Tumors sampled before dosing with CYC202 and 12 days after to assist identification of further biomarkers; trial will also examine pharmacokinetic effects of CYC202 in Asian population. Two patients have withdrawn due to hepatotoxicity. |
Phase IIa: combination (closed) |
|
14 patients with breast cancer |
|
Two doses daily on days 1–5 of 21 day cycle, in combination with two doses daily of capecitabine by mouth on days 2–15 of first cycle and 1–14 subsequently. |
|
Study closed earlier than intended as CYC202 treatment in combination with capecitabine appeared to exacerbate toxicity of capecitabine. Preliminary data from evaluation of 14 patients showed 3 with either complete response or stable disease over course of treatment. |
Phase IIa: combination (current) |
|
23 patients with non-small cell lung cancer |
|
Two doses daily on days 1–4, 8–11 and 15–18 of each 21 day cycle, in combination with 1000mg/m2 gemcitabine by infusion on days 5 and 12 with 75mg/m2 cisplatinum by infusion on day 5. |
|
Preliminary data from evaluation of 21 of the 23 patients show 5 with partial response (at least 30% reduction in tumor size), 5 with stable disease over course of at least 2 cycles of treatment and 2 with progressive disease. 6 patients have been discontinued in the first cycle for tolerability and 3 for non-safety reasons. 3 patients were discontinued for adverse events in later cycles following response assessment. |
| | | | | | | |
54
Phase II: monotherapy (current) |
|
5 patients with B-cell hematological malignancies (B-cell chronic lymphocytic leukemia, mantle cell lymphoma, multiple myeloma) |
|
Two doses daily of 1600mg on days 1–3 of 14 day cycle as a single agent. |
|
Trial has recently commenced. The first patient with B-cell chronic lymphocytic leukemia experienced a decrease in white blood cell count at the second cycle from 32,000 cells/ml to 6,500 cells/ml. This patient discontinued after the second cycle for tolerability. An additional four patients are being treated: one with mantle cell lymphoma completed four cycles and was assessed with stable disease, one with multiple myeloma completed three cycles and two patients with B-cell chronic lymphocytic leukemia and multiple myeloma are in the first cycle. |
As indicated above, we have undertaken five Phase I trials with CYC202 in a total of 106 subjects, including 24 healthy volunteers and 82 heavily pre-treated cancer patients who failed multiple chemotherapies. We have tested CYC202 for safety, tolerability and pharmacokinetics in both single and multiple doses. Although these Phase I trials were not designed to test efficacy, and do not support any conclusions with respect to efficacy, independent investigators nonetheless observed that CYC202 given as monotherapy appeared to induce clinical benefit in a number of patients with various solid tumors, such as adenomatous, adrenal, colon, colorectal, liver, non-small cell lung, ovarian, pancreatic, parotid and thymoma tumors. Overall, out of a total of 82 cancer patients in Phase I trials, 10 patients were assessed with stable disease over four months, with two non-small cell lung cancer patients stable for over 16 months and 10 months, respectively, and 71 with progressive disease. In addition, one patient with liver cancer was assessed as a partial response as defined in the protocol, with a 46% reduction in tumor size after several cycles of CYC202. Partial response and stable disease were measured in accordance with criteria defined in the protocol, but observations were not subjected to formal statistical analysis. Such analysis is uncommon for such studies. Investigators observed dose limiting toxicities of nausea and vomiting, hypokalemia or lowered potassium levels, elevated creatinine, elevated liver enzymes and, in less than 10% of patients, skin rash.
We are currently conducting two Phase II trials of CYC202, a Phase IIa trial as combination therapy for the treatment of non-small cell lung cancer given as first line therapy and a Phase II trial as monotherapy for the treatment of hematological cancers as first line therapy. The ongoing clinical trials for CYC202 are being undertaken under the guidelines of the U.K. Medicines and Healthcare products Regulatory Agency and other comparable regulatory authorities in Europe or Asia. A third Phase IIa trial of CYC202 as combination therapy for the treatment of breast cancer as second or third line therapy was recently terminated. Based on Phase I data we have chosen to dose CYC202 capsules by mouth twice a day for four days every week in the lung cancer Phase IIa study in combination with gemcitabine and cisplatinum. In the Phase II hematological cancer study we are dosing CYC202 for three days every two weeks as monotherapy. We expect to report results from these trials by the second half of 2004.
We may expand our Phase II non-small cell lung cancer cancer program in Europe and possibly in the United States if efficacy and safety data from our Phase IIa studies are favorable. This may entail
55
conducting a new Phase IIa study evaluating further combinations, possibly gemcitabine and carboplatin in the United States, and/or extending our current Phase IIa lung study of CYC202 in combination with gemcitabine and cisplatinum in Europe. Our decisions in this regard will be made in light of how the overall response rate observed in patients enrolled in our Phase IIa studies compares with response rates observed in non-small cell lung cancer patients generally. For example, a major U.S. study comparing four approved chemotherapy regimens for non-small cell lung cancer in 1,155 patients reported an overall response rate of 19%. Upon conclusion of our Phase IIa studies, assuming the results are favorable, we intend to initiate a randomized Phase IIb trial in non-small cell lung cancer testing CYC202 as combination therapy with gemcitabine/cisplatinum against gemcitabine/cisplatinum alone. We believe that if results are favorable this study could supply pivotal data forming part of a submission for marketing authorization.
In our Phase IIa study in breast cancer, the endpoint of establishing the tolerability of CYC202 and capecitabine was reached but the study was closed earlier than intended as CYC202 treatment in combination with capecitabine appeared to exacerbate the toxicity of capecitabine. The combination appears to be poorly tolerated despite supportive efficacy results in certain patients, including one complete response. At present it is unlikely that we will take this combination into an expanded Phase II program.
We intend to expand our Phase II study in hematological malignancies. This study was initially planned for 36 patients, 12 each for B-cell chronic lymphocytic leukemia, mantle cell lymphoma and multiple myeloma. We will now aim to increase the study to 35 patients for each of the cancer subtypes in which we observe more than one response among the first 12 patients. Our decisions in this regard are made in light of how the overall response rate observed in our Phase II studies compares with response rates in hematological cancer patients generally. Reported data indicate response rates on the order of 10–20% for each of these three cancer subtypes. Assuming the results are favorable, we would be interested in progressing to a pivotal trial in this area as soon as possible after completing these Phase II studies.
On the basis of preclinical data showing significant reductions in tumor size caused by the application of CYC202 in combination with docetaxel, we are planning a further Phase II clinical trial to study the effect of these two agents used in combination in patients with non-small cell lung cancer and possibly other solid tumors. We expect to begin this study in the second half of 2004. We are considering a two-stage design in which, if the overall response rate observed in our Phase II trial appears to be superior to response rates reported for docetaxel alone, we would expand our trial in hopes of obtaining pivotal data to form part of a submission for marketing authorization. Recent data for docetaxel single therapy tested as second line therapy after cisplatinum in patients with non-small cell lung cancer reported an overall response rate of 16%.
The Phase II trials are not yet complete and therefore the data reported above are preliminary. The data have not been subjected to formal statistical analysis and may not be indicative of the clinical results we will obtain upon conclusion of these trials. In addition, safety has not been confirmed and "serious adverse effects" have been noted in some patients. We expect to report results from these trials during the second half of 2004.
Our CYC202 biomarker program is founded on a well-developed understanding of the cellular effects of this compound. The aim of this program is to identify specific biomarkers that will allow us to measure and predict CYC202's action in individual patients with respect to drug activity, toxicity and tumor response and, in the longer term, assist in patient selection. Statistical analysis is carried out on multiple samples from individual patients but the overall number of patients is not sufficient to carry out formal statistical analysis on groups.
56
CYC202 is known to cause cancer cell death by inducing apoptosis in tumor cells. We are currently studying a specific apoptosis biomarker. Analyzing blood samples drawn from 36 patients treated with CYC202 in our Phase I trials, we observed high levels of this apoptosis biomarker in 17 of them or 47%. In some cases we were also able to correlate these biomarker results with clinical benefit as observed by the Phase I investigators. Studies with this apoptotic biomarker are ongoing using blood samples obtained from patients enrolled in our CYC202 Phase II trials.
We have also detected other potential biomarkers by monitoring changes in patient plasma proteomic profiles following CYC202 treatment in the Phase I trials. We are currently trying to determine the precise plasma proteins that are changing in response to the compound. We are using sophisticated analytical techniques (including microarray and Quantitative Polymerase Chain Reaction or QPCR) to study gene expression on a specific type of white blood cell (peripheral blood mononuclear cells or PBMCs), in order to identify additional markers of CYC202 activity. We have identified approximately 150 genes in these cells whose expression is altered after exposure to CYC202. We are now assessing a subset of these as candidate biomarkers in patient samples to see if they will be useful in the clinical setting.
To enable better patient selection and improve the probability of positive outcomes in Phase IIb/III trials, we are conducting experiments to determine the genetic difference between sensitive and resistant primary tumors. These experiments are designed to develop genetic fingerprints that correspond to either resistant or sensitive tumors and may in the longer term be used to guide patient selection and/or enrolment. Of the over 100 cell lines we have tested, gene expression profiling will be performed on the 10 most sensitive and the 10 most resistant cell lines in order to determine whether there is a distinct subset of genes that correlate with a tumor's sensitivity or resistance to CYC202. If a genetic signature can be identified, clinical testing of CYC202 could be limited to those patients that were prospectively identified as likely to benefit from the drug while excluding those with tumors containing biomarkers associated with resistance.
57
Preclinical results suggest that CYC202 may also have a therapeutic benefit in the treatment of patients with inflammatory kidney diseases, or glomerulonephritis. Glomerulonephritis encompasses a number of different kidney diseases, which are classified either according to the likely cause of inflammation, such as a viral infection, or whether the main pathological finding is abnormal cell proliferation or tissue scarring. Because CYC202 acts to arrest the progress of the cell cycle, we believe it may be particularly effective in treating those forms of glomerulonephritis characterized by excessive cell proliferation. The most common forms of these are IgA nephritis, glomerulonephritis associated with vasculitis and lupus nephritis.
There is currently no standard treatment for glomerulonephritis. It is one of the leading causes of end stage renal disease, or kidney failure, which may require dialysis or kidney transplantation, and is frequently terminal. Available therapies for the progressive renal failure that follows glomerulonephritis do not cure the disease but rather rely on non-specific immunosuppressive and cytotoxic medicines. We thus believe that novel, less toxic drugs are needed for the treatment of glomerulonephritis.
In the United States in 2001, there were 406,081 cases of end stage renal disease, of which 62,908 were caused by glomerulonephritis, and 96,295 new cases of end stage renal disease recorded, of which 7,986 were caused by glomerulonephritis. As discussed below, we are currently considering a new Phase II trial of CYC202 in lupus nephritis. It has been estimated that approximately 1,500,000 Americans have a form of lupus disease, and that approximately one-third will develop nephritis.
As discussed above under "CYC202 in Cancer," CYC202 inhibits CDK, preventing the activation of proteins required for the completion of the cell cycle. This slows down the rapid proliferation of kidney cells, such as mesangial cells and podocytes, that characterizes glomerulonephritis.
A number of preclinical studies have indicated that CYC202 may be highly effective in the treatment of glomerulonephritis. Preclinical results presented at the 2003 annual meeting of the American Society of Nephrology by a group of independent investigators from New York University's School of Medicine, working with collaborators from Columbia University, Mount Sinai School of Medicine and Roswell Park Cancer Institute, reported that CYC202 slowed down or reversed collapsing glomerulopathy, one of the most severe forms of kidney failure. Further preclinical data from leading U.S. nephrologists at the University of Washington in Seattle, Washington, and a leading U.K. nephrology group at the University of London separately reported statistically significant results showing that CYC202 reduced proliferation of kidney cells, reduced protein levels in the urine and reduced the number of crescent-shaped cells, an important marker of prognosis of the eventual course of kidney disease. U.S. investigators from Mount Sinai Hospital in New York City reported that CYC202 was effective in a model system of HIV-associated nephritis, or HIVAN. An Italian university group reported statistically significant evidence that CYC202 prolonged survival in a model system of lupus nephritis.
Our CYC202 Phase I clinical data in healthy volunteers are discussed above under "CYC202 in Cancer."
We initiated a Phase IIa clinical trial to examine the effect of CYC202 in patients with IgA nephritis. A total of five patients were dosed with CYC202 every day for 28 consecutive days. Two patients completed dosing without problems, but in three patients liver enzyme elevations indicating
58
possible hepatic toxicity were observed by day 14, two of which were classed as "serious adverse events" under the trial protocol. Treatment was discontinued as a precaution, and liver values returned to normal in these patients. As a safety precaution and in accordance with good clinical practice, we put the IgA nephritis trial on hold in order to analyze the reasons behind this transient toxicity in consultation with outside liver specialists. We have now concluded this analysis and believe that we understand the mechanism of the transient hepatic toxicity we observed. However, because IgA nephritis sometimes has no evident symptoms and is often undiagnosed, suitable patients are difficult to recruit for clinical trials. We currently do not expect to resume the IgA nephritis trial.
Based on the preclinical findings, however, we remain interested in exploring the use of CYC202 in glomerulonephritis. We are currently considering a Phase II trial of CYC202 in patients with lupus nephritis, who we believe may be easier to recruit than patients with IgA nephritis. We expect that any such trial will be conducted using the kind of pulse-dosing schedule that was shown to be tolerated in Phase II trials of CYC202 in cancer. We have not yet decided when to initiate this clinical trial, which would be preceded by further assessment of the tolerability of CYC202 in association with these dosing strategies.
We are exploring the use of biomarkers in our glomerulonephritis program in conjunction with outside investigators. These biomarkers relate not to apoptosis, as in our cancer programs, but to various aspects of kidney function. We expect to continue our work with biomarkers of efficacy in the treatment of glomerulonephritis when we resume clinical testing.
Our second drug candidate, CYC682, is an orally available novel nucleoside analog. Nucleoside drugs work by inhibiting the S phase of the cell cycle. A number of nucleoside drugs, such as gemcitabine, are in widespread use as conventional chemotherapies. Independent investigators at the Roswell Park Cancer Institute in Buffalo, New York, reported preclinical data showing that CYC682 was superior to gemcitabine and 5-FU, another widely used chemotherapy, both in terms of extending survival and blocking metastases to the liver. Two Phase I studies of CYC682 have been completed, and a third Phase Ib clinical trial is currently in progress for the treatment of patients with advanced malignancies. We currently expect CYC682 to enter Phase II trials in the first half of 2005.
In addition to offering potentially greater efficacy, CYC682 can be taken by mouth, whereas most conventional nucleoside drugs must be administered by injection. Oral dosing is more convenient for patients, reduces the costs of treatment and allows greater flexibility in the dosing and treatment regimen. Side-effects associated with CYC682 are generally comparable to those associated with conventional nucleoside chemotherapies. Preclinical and preliminary clinical data indicating that gemcitabine and CYC202 work well together in combination therapy suggest that CYC682 and CYC202 may do so as well which could assist us in building our anticancer franchise.
There are three classes of anticancer agents on the market that are analogous to CYC682: fluorouracil analogs, like capecitabine and 5-FU; 2 deoxycytidine analogs, like cytarabine and gemcitabine, the market leading nucleoside analogue; and purine analogs, like 6-mercaptopurine. In 2003, sales for Roche's capecitabine brand, Xeloda, were $383 million, and for Eli Lilly's gemcitabine brand, Gemzar, over $1.0 billion.
CYC682 is a prodrug of the novel nucleoside CNDAC (2'-cyano-2'-deoxy-arabinofuranosylcytosine). A prodrug is a compound that has a therapeutic effect only after it is metabolized within the body. CNDAC has a significantly higher residence time in the blood when it is
59
produced in the body through metabolism of CYC682 than when it is given directly. CNDAC is activated by deoxycytidine kinase enzymes and is inactivated by cytidine deaminase, both of which are enzymes found in abundance in certain tumor tissues. CYC682's chemical structure contains a feature designed to protect the drug from degradation by cytidine deaminase while still benefiting from the activating effect of deoxycytidine kinase. This same feature increases availability of CYC682 by mouth.
Activation of CYC682 leads to inhibition of the enzyme DNA polymerase. This disrupts the replication of DNA during the S phase of the cell cycle, inducing cell arrest and apoptosis. CYC682 also has a secondary beneficial mechanism. By imitating a DNA molecule which the cell includes in a DNA strand, CYC682 induces spontaneous DNA strand breaking resulting in termination of the DNA chain and cell death by apoptosis.
CYC682 has been the subject of two Phase I studies in the United States to explore safety and pharmacokinetics. The two trials have treated 87 patients with a variety of cancers, who were dosed by mouth either three or five days per week for four weeks of a six week cycle. Overall, 17 patients were assessed with stable disease, 70 with progressive disease and one could not be evaluated. One patient with a gastrointestinal stromal sarcoma (GIST) cancer, a form of stomach cancer, remains on CYC682 treatment for over 90 weeks with stable disease. A further patient with ovarian cancer experienced a minor response. Dose limiting toxicities of leukopenia and neutropenia, both involving decreased levels of white blood cells, and fever were identified. Thirteen patients experienced "serious adverse events" related to toxicity, and one treatment-related death occurred. Such toxicity, however, is often observed in early clinical trials of chemotherapeutic agents.
Although these trials were designed to assess safety rather than efficacy, the results obtained indicate that CYC682 may induce clinical benefit in some patients. The pattern of response, however, was variable and therefore it is difficult to draw inferences. We are currently conducting a Phase Ib clinical trial in the United States to assess dose and schedule variations. We are dosing patients with CYC682 twice daily on days 1-14 of a 21 day schedule. At the conclusion of the Phase Ib trial, we hope to determine an appropriate dose schedule for Phase II trials. The clinical trials for CYC682 have been undertaken under FDA guidelines. We currently expect CYC682 to enter Phase II trials in the first half of 2005.
To enhance our development efforts, we are developing biomarkers for use in our CYC682 clinical trials. Nucleoside kinases, like deoxycytidine kinase, are needed to activate CYC682. On the other hand CYC682 can be deactivated by phosphatases and deaminases, such as cytidine deaminase. We have hypothesized that CYC682 will be more potent in combating tumors that contain a greater proportion of nucleoside kinases relative to phosphatases and deaminases. We are collecting biomarker data in our Phase Ib trial in an effort to compare the relative values for these two sets of enzymes. If the data is favorable, we may seek to design a Phase II trial in which we would collect data that distinguishes among patient groups, or cohorts, based on the different levels of these enzymes in their tumors. If we can then establish a correlation between enzyme levels and response to CYC682 in the Phase II trial, we may be able to design a pivotal Phase III trial exploiting this finding that is more likely to succeed, smaller in size and less expensive to conduct than would otherwise be the case.
We also intend to analyze biomarkers to assess the efficacy of CYC682 in inducing cell death in cancer cells. Our preliminary data suggest that we can detect CYC682-induced cell death with the same biomarkers we are using in our CYC202 trials.
60
Our Preclinical Programs
This program is based on CYC381, an orally available analog of clotrimazole, a commonly used antifungal agent. CYC381 acts on the cell cycle by inhibiting internal calcium channels in cells and blocking the expression of cyclins. Cells use the movement of calcium ions as internal communications signals. Inhibition of these signals with CYC381 prevents the production of cyclins, which are essential for cell cycle progression. CYC381's mechanism results in cancer cells being arrested at the G1 phase of the cell cycle.
CYC381 has been the subject of extensive preclinical testing which suggests that it may be highly active in slowing the progression of several solid tumorsin vivo. In addition, because CYC381 acts early in the G1 phase of the cell cycle, we believe it may potentially be synergistic as combination therapy with CDK inhibitors, which work at the G1/S checkpoint.
CYC381 is a racemic mixture or a combination of two different chemical agents, called enantiomers, which cannot be easily separated. Often, it is not clear whether a chemical or biological response is attributable to a single enantiomer in a racemic mixture, or whether more than one enantiomer in the mixture is capable of bringing about the same response, or whether the response is attributable to the interactions of more than one enantiomer. Because of this lack of clarity, it can be difficult to obtain regulatory approval to test in humans drug candidates that are racemic mixtures. For this reason we initiated a CYC381 chemistry program. We have succeeded in separating the two enantiomers of CYC381 and have established that they are not chemically interchangeable. We expect to carry out further preclinical testing with the two enantiomers in order to select one, or both, for clinical development. We hope to be in a position to initiate Phase I trials of a clotrimazole analog drug candidate by late 2005 or early 2006.
Cell cycle inhibitors may be useful in the treatment of viral diseases to the extent that drugs can be developed that prevent the replication of virus-infected host cells and cause their death by apoptosis while sparing most uninfected cells. If this is proven in humans, cell cycle inhibitors may have significant potential in this area, as they do not interfere with viruses and are less likely to induce viral resistance, a major cause of failure in antiviral drugs that attack the virus itself. There has been extensive discussion in the scientific literature regarding the application of CDK inhibitors in virology. Several publications by independent investigators suggest that roscovitine, the chemical precursor drug of CYC202, has activity in preclinical models of infection by cytomegalovirus, or CMV, the cause of retinitis in the eye; Herpes Simplex Virus, or HSV, the cause of genital herpes; HIV, the cause of HIV/AIDS; and Varicella Zoster Virus, the cause of shingles and chickenpox. We are interested in the commercial opportunity that cell cycle inhibitors present in virology, particularly HIV/AIDS, and believe that we have more potent and more specific drugs than roscovitine in our compound libraries.
We have in particular identified and optimized several compounds with promising antiviral activity against HIV-infected cells. These molecules appear to work by two distinct target mechanisms: CDK inhibition and, importantly, inhibition of mitotic kinases. We believe that we are the first to suggest that mitotic kinase inhibitors may be useful as antiviral drugs. This hypothesis, proposed by the mitosis specialists in our Polgen division, would if proven correct validate our belief that insight in the biology of the cell cycle may result in drugs addressing multiple therapeutic areas.
We are investigating a number of compounds in this program, some of which appear to be highly active against HIV in biological tests and induce antiviral effects that may be as or more potent than many existing HIV/AIDS therapeutic agents. Moreover, we have hypothesized that cell cycle inhibitor drug therapies may be less prone to cause the emergence of drug resistant HIV/AIDS, although
61
prolonged clinical testing would be required to test this hypothesis. We expect to commence Phase I clinical development of a drug candidate for HIV/AIDS in 2006.
Our Research Programs
We are currently engaged in a second-generation CDK inhibitor research program. Like CYC202, these CDK inhibitor compounds are generally intended to act upon the cell cycle by inducing apoptosis in cancer cells. These compounds, however, are pyrimidines, members of a different chemical family than CYC202, which is a purine. We believe that the compounds emerging from this program will prove to be even more potent anticancer agents than CYC202. Using our virtual screening and modeling technologies, we have identified compounds that selectively inhibit individual CDKs, as well as compounds inhibiting multiple CDKs simultaneously (pan-CDK inhibitors). To date, we have synthesized over 600 active compounds from this series. A number of these compounds are orally available, inhibit tumor growthin vivo and show potency at the picomolar level in terms of enzyme inhibition, which means that they are much more potent than publicly disclosed CDK inhibitors. Several of these compounds, with different CDK inhibition profiles, are currently in lead optimization. We expect to select at least one of these agents for preclinical testing in 2005 and hope to be in a position to initiate Phase I trials of a second-generation CDK inhibitor candidate by 2006.
Glycogen Synthase Kinase-3, or GSK-3, inhibition is an essential element in the body's regulation of blood sugar. GSK-3 regulates the glycogen synthase enzyme that indirectly controls glucose levels. Insulin controls the regulation of energy conversion and storage by interacting with its receptor which results in the activation of PI-3 kinase that in turn inhibits GSK-3. In adult onset or Type 2 Diabetes, GSK-3 is not inhibited because the insulin receptor is not operating properly. As a result, we believe that GSK-3 inhibitor drugs may be suitable for development as Type 2 Diabetes therapies. The structures of GSK-3 and CDK are very similar. In our cancer programs, it was desirable to discover highly specific CDK inhibitors that do not inhibit GSK-3. Our work in this area prompted us to investigate highly specific GSK-3 inhibitors that do not inhibit CDK.
We have identified four chemical families of GSK-3 inhibitors some of which are potent at the picomolar level, representing the most potent compounds disclosed in the literature. Importantly, while all other disclosed GSK-3 inhibitors lead to accumulation of beta-catenin, a protein associated with tumor growth, three out of four of our GSK-3 inhibitor families do not induce beta-catenin accumulation in our models. This is an important characteristic, as we would expect patients to take this type of drug on an on-going basis. We have selected two lead compounds from this series, both of which have achieved proof-of-concept in a standard model of diabetes, having been shown to stimulate glycogen synthase, improve glucose tolerance and regulate triglycerides.
Our research program in Polo-like kinase, or Plk, inhibitors targets the mitotic phase of the cell cycle. We are aiming to identify potent and selective compounds which inhibit Plk1, a kinase active during mitosis. Inhibition of Plk1 results in cell cycle arrest at the G2/M checkpoint and induces apoptosis in cancer cells. Our Plk inhibitor program represents the first target gene that has emerged through the target validation process at our Polgen division and progressed to the drug discovery and chemistry stage. Because little was known about the nature and structure of Plk1, and because Plk has never been crystallized, we relied on advanced computer modeling and software-based design techniques to identify a series of compounds selective for Plk over other kinases, with some active at the nanomolar level, that we hope will be effective as Plk inhibitors.
62
One of the key cell cycle regulatory proteins is p53. When active, p53 causes cell arrest at the G1/S checkpoint, inducing apoptosis in cancer cells. Under normal circumstances, p53 is held in an inactive form by binding to another regulatory protein, Hdm2. In this program, we are investigating ways of disrupting the interaction between Hdm2 and p53, thus activating p53. Through our virtual screening technologies, we have identified two small molecule groups capable of breaking the binding between p53 and Hdm2.
The activity of CDK can be inhibited by two methods, either by blocking the ATP site, as is the case with CYC202, or by inhibiting the substrate binding site on the cyclin protein. Preventing the cyclin from binding results in cell cycle arrest and induces apoptosis in cancer cells. We are currently investigating the development of such cyclin binding groove inhibitors, continuing a program that was the subject of a two-year collaboration with AstraZeneca that concluded in mid-2003. We retained all of the intellectual property associated with this program upon its conclusion.
Our Drug Discovery and Design Process
Our pipeline of nine different drug programs is comprised of seven internally generated and two in-licensed compounds, CYC682 and CYC381. We expect that in the future our drug programs will increasingly be based on our proprietary genes-to-drugs approach to drug discovery and design. This approach relies on genomic technology from our Polgen division to identify gene targets, which are then progressed by means of structure-based design techniques through to the development stage.
Fundamentally, this approach to drug discovery and design aims to improve our ability to select promising drug targets in the early stages of the process so as to decrease compound attrition rates during the later, more expensive stages of drug development. We devote more resources initially to enrich the target selection process, so that we focus our efforts on targets that have a higher probability of yielding successful drug candidates, progressing through the drug discovery and design process more quickly and efficiently and enhancing our chances of successfully commercializing drugs. To this end, we have assembled an integrated suite of sophisticated discovery and design technologies, together with personnel who are highly skillful in making use of these technologies.
As exemplified by our Plk inhibitor program, our genes-to-drugs discovery and design strategy would typically involve the following steps:
The active ingredients in drugs act by binding to or affecting specific molecules, referred to as the target of that drug. Our Polgen division carries out target discovery and validation studies using RNA interference, or RNAi, techniques. These techniques are used to understand the function of different genes and identify which genes and related proteins and enzymes are involved in specific biological processes in the cell cycle, such as mitosis. We are one of few companies that have an RNAi library for each gene in a full model genome from which to derive targets. Our Polgen division has a library of more than 100 genes that we have identified as being involved in mitosis using our RNAi library and our high throughput microscopy scanning equipment. Targets that appear to be associated with relevant processes are validated by identifying whether the genes and related proteins and enzymes correspond to specific diseases. This may include, for example, determining whether the genes and related proteins are over-expressed, or are more common in cancer cells than in normal cells. Through this initial identification and validation process, we seek to identify which molecular targets to inhibit with potential drug candidates.
63
In order to develop drugs that bind to or affect the genes and proteins identified by our Polgen technology, we seek to understand the structure of these targets through our structure-based drug design expertise. We initially accomplish this by means of X-ray crystallography technology. This involves obtaining highly purified samples of the target protein which we then crystallize. High-resolution X-rays are employed, both in-house and at outside vendors, in order to define the 3-dimensional structure of the protein. Our team has defined or solved over 20 crystal structures of our drugs docked into their active sites. The pattern observed when the X-ray beam is scattered is then used to map the relationship of each atom in the protein in order to define the structure of the target. At this point, we may sometimes use magnetic resonance spectroscopy techniques, both in-house and at outside vendors, to further refine protein structure.
Once defined, the 3-dimensional structure of a target protein is coded into a computer, as are 3-dimensional structures of thousands of small molecules. Our LIDAEUS software and other similar programs allow us to sift through large collections of small molecules in various combinations to determine which molecules or compounds bind to our targets. Such large-scale testing is referred to as virtual screening. Using complex computational algorithms, the software virtually screens tens of thousands of small molecules in order to find small molecules most likely to fit into a selected site on the target protein. Results from these screens determine which compounds we will focus on to optimize potential drug candidates. While other software is available for similar large-scale testing, our LIDAEUS software has been optimized for the kinase enzymes that are central to our cell cycle research.
The molecules determined by our virtual screening as most likely to bind to or affect the relevant target proteins and enzymes are then purchased in physical form, biologically validated and screened in our laboratory through a series of enzyme assays or tests. These tests measure whether the compound can affect or interfere with the target enzyme function. We employ our Fluorescience technology for some of these tests to determine which compounds inhibit the target enzymes without affecting closely-related, non-target enzymes. Through this process we strive to select compounds most likely to inhibit the target protein without having undesirable, or toxic, effects on similar targets.
Promising compounds, or hits, identified through the enzyme assay process are then used to design other similar, but more effective, compounds through the process of medicinal chemistry. For example, if multiple compounds have similar success in targeting enzymes, a core chemical structure may be common to all of them. That core chemical structure is used as a starting point to create variants that optimize therapeutic effects, such as target potency, oral availability and low toxicity. The aim of the medicinal chemistry process is to produce a clinical candidate compound that has the desired physical attributes of a drug. These drug design criteria include potency against the target enzyme, causing cancer cell death in laboratory tests, sufficient absorption and half-life and inhibition of tumor growth in model systems.
After our design criteria are met through medicinal chemistry, our drug candidates are moved into preclinical and clinical development. Integral to our preclinical investigation and clinical development is
64
our use of biomarkers which assist in recording the effect of our target compounds and drug candidates on cell cycle activity, as described below under "—Our Use of Biomarkers."
As noted earlier, our Plk inhibitor program represents the first target gene that has emerged through the target validation process at our Polgen division and progressed to the drug discovery and chemistry stage. The compounds we are working with in our preclinical and research programs have also progressed through one or more of the phases described above. For example, the compounds in our second-generation CDK inhibitor program for cancer were identified using structure-based design techniques, as were some compounds in our virology program. Molecular modeling has played a key role in the development of both our cyclin binding groove and Hdm2 inhibitor programs.
Our Use of Biomarkers
We are making extensive use of biomarkers to enhance our drug development efforts. Biomarkers are proteins or other substances whose presence in the blood can serve as an indicator of specific cell processes. We are able to measure the presence of these substances by means of mass spectrometry, which is a sensitive technique that can detect very small amounts of proteins and separates compounds based on their mass. By measuring changes in the levels of proteins and other messenger molecules present in blood or by examining the genes "switched on" in individual tumors using mass spectrometry, we seek to identify biomarkers that are associated with apoptosis in cancer cells and other important cell cycle activity. In this way, we believe we can obtain a better understanding of the extent and nature of drug response in patients.
We are using biomarker analysis in all of our clinical programs, primarily in order to help us to evaluate whether our drug candidates are having their intended effects through their assumed mechanisms. We believe that this will assist us to identify more promising drug candidates at an early stage and to direct our resources efficiently. Biomarker analysis may enable us to determine at an early stage that a drug candidate is not having its intended effect in patients through its assumed mechanism without having to commit resources to conduct extensive mid- to late-stage clinical trials. By contrast, biomarkers may supply us with confirmation of drug response, such as measuring cancer cell death, giving us confidence that we should proceed with development of a given compound.
For example, we have shown in preclinical tests that cancer cells die through the process of apoptosis when exposed to CYC202. We can measure the levels of specific molecules in the blood stream of patients which are only present when cells have died by apoptosis. Because apoptosis is a natural process used by our bodies to control the replacement of old cells, everyone has a low background level of apoptosis markers. In cancer patients after exposure to CYC202, we can detect a significant increase in the level of a specific apoptosis biomarker indicating that many more cells are dying by apoptosis. Analyzing blood samples drawn from patients treated with CYC202 in our Phase I trials, we observed significantly elevated levels of two different biomarkers, one measuring apoptosis and one measuring cell death both by apoptosis and other mechanisms, in over half of patients for which we had blood samples. In some cases we were also able to correlate these biomarker results with clinical benefit as observed by the Phase I investigators. We are currently one of only a few companies that have published human biomarker data from clinical trials of our own drug.
We also believe that biomarkers may prove useful in monitoring patient compliance with trial protocols. For example, we have identified a series of proteins which change after administration of CYC202 by mouth. These changes may be used to check whether enough of a compound is present in the body to have a biological effect and are referred to as a pharmacodynamic marker. Because these biomarkers appear while the drug is present, if a patient misses a dose and the drug levels in the blood fall below a critical level, the biomarkers will disappear indicating either that the patient is not receiving sufficient drug or has missed a dose. We believe that such biomarker data could improve the integrity of clinical trial data and increase the likelihood of success of clinical trials.
65
We believe that, in the future, biomarkers may be used to help guide patient selection by determining which patients are more likely to respond to a specific treatment. Cancer has no single cause and there are hundreds of different genetic changes or mutations which can lead to cancer. In any individual it is extremely unusual for a single change to cause cancer. A patient will only respond to a mechanism-targeted drug if the target of that drug is playing a vital role in increasing or maintaining the tumor in that patient. It is hypothesized that there may be a characteristic genetic fingerprint in patients likely to benefit from a given drug. We believe that biomarker analysis may in the longer term be used to determine this kind of genetic fingerprinting, potentially allowing us to select patients more likely to respond to our drugs for clinical trial and marketing purposes and increasing the probability of successful outcomes in terms of disease management.
Biomarkers are currently the focus of great interest within the scientific community with a large number of scientific papers and scientific conferences discussing their use. In November 2003, the FDA issued for comment a draft guidance document on the use of pharmacogenomic data in all phases of clinical development, encouraging the submission of biomarker data. We think that this is an indication that biomarkers may play a role in the regulatory process in the future. For most purposes, however, biomarkers have not yet been scientifically validated and biomarker data are not currently accepted by the FDA or other regulatory authorities in applications for approval of drug candidates and may never be. We nonetheless intend to continue our focus on biomarkers as drug development tools and to seek to use them to increase our probability of successfully developing novel drugs.
Manufacturing
We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of any of our research compounds. We rely on, and expect to continue to rely on, third parties for the manufacture of our drug candidates or products that we may develop.
We synthesize cGMP quality active pharmaceutical ingredients, or API, by means of various manufacturers. Final drug form is manufactured in appropriately regulated premises. Each contract research organization has the responsibility to supply research compounds for our clinical trials to the limit of the existing contractual agreements.
To date, our suppliers have synthesized sufficient API and final drug form to support the ongoing needs of our clinical trials and development in progress. We believe that these contractors have the capability to meet foreseeable supply needs of our research compounds and meet the FDA and other regulatory agency requirements in the United Kingdom and the European Union, including compliance with the FDA's good manufacturing practices and comparable regulatory requirements.
In the event any contractor is unable or unwilling to supply us with sufficient quantities of our research compounds, we have made ongoing secondary arrangements whereby an alternative supplier could be contracted to ensure program continuity.
Patents, Proprietary Technology and Collaborations
We consider intellectual property rights to be vital to our business and use a variety of methods to secure, protect and evaluate these rights. These include:
- •
- Ownership and enforcement of patent rights
- •
- Patent applications covering our own inventions in fields that we consider important to our business strategy
- •
- License agreements with third parties granting us rights to patents in fields that are important to our business strategy
- •
- Invention assignment agreements with our employees and consultants
66
- •
- Non-compete agreements with our employees and consultants
- •
- Confidentiality agreements with our employees, consultants, and others having access to our proprietary information
- •
- Standard policies for the maintenance of laboratory notebooks to establish priority of our inventions
- •
- Freedom to use studies from patent counsel
- •
- Material transfer agreements
- •
- Trademark protection
Patents and Patent Strategy
The table below summarizes the U.S. patents that we currently own.
Patent No.
| | Description
| | Issue Date
| | Expiry Date
|
|---|
| US 6,569,833 | | P16 peptides, useful in the treatment of hyperproliferative disorders | | May 27, 2003 | | September 23, 2016 |
US 6,242,201B |
|
PCNA binding agents, useful in the treatment of hyperproliferative disorders |
|
June 5, 2001 |
|
November 3, 2015 |
US 6,613,878B |
|
Fen 1 peptides |
|
September 2, 2003 |
|
May 2, 2016 |
US 6,472,507 |
|
Penetratin-drug conjugates for cellular delivery |
|
October 29, 2002 |
|
July 2, 2019 |
US 6,531,479 |
|
CYC400 compounds |
|
March 11, 2003 |
|
March 29, 2021 |
US 6,699,854 |
|
CYC400 compounds |
|
March 2, 2004 |
|
March 29, 2021 |
US 6,656,696 |
|
Binding assays |
|
December 2, 2003 |
|
February 26, 2019 |
US 6,670,144 |
|
Binding assays |
|
December 30, 2003 |
|
February 26, 2019 |
US 6,465,199 |
|
Binding assays |
|
October 15, 2002 |
|
February 26, 2019 |
US 6,221,873 |
|
CYC202 and a related derivative |
|
April 24, 2001 |
|
March 4, 2018 |
US 6,703,395 |
|
CYC202 and a related derivative |
|
March 9, 2004 |
|
March 4, 2018 |
The table below summarizes the U.S. patents under which we hold licenses.
Patent No.
| | Licensor
| | Description
| | Issue Date
| | Expiry Date
|
|---|
| US 6,316,456 | | CNRS | | 2-,6-,9-substituted purine derivatives, including CYC202 | | November 13, 2001 | | November 29, 2016 |
| US 5,888,762 | | CNRS | | Cell delivery molecules, including Penetratin | | March 30, 1999 | | March 30, 2016 |
| US 6,080,724 | | CNRS | | Penetratin variants | | June 27, 2000 | | October 4, 2016 |
| US 6,534,497 | | Nuchem | | Substituted 11-phenyl-dibenzazepine compounds, including CYC381 | | March 18, 2003 | | November 20, 2017 |
| US 6,028,103 | | Nuchem | | Substituted triaryl methane compounds | | February 22, 2000 | | March 20, 2016 |
| | | | | | | | | |
67
| US 6,063,921B | | Johnson Matthey | | Synthesis of 11-aryl-5,6-dihydro-11H-dibenzazepines, including CYC381 | | May 16, 2000 | | November 20, 2017 |
| US 6,201,120B | | Johnson Matthey | | Synthesis of 11-aryl-5,6-dihydro-11H-dibenzazepines, including CYC381 | | March 13, 2001 | | November 20, 2017 |
| US 5,702,908 | | Cancer Research Technology | | Agents which interfere with the binding of MDM2 to human p53, assays for said agents | | December 30, 1997 | | December 30, 2014 |
| US 5,770,377 | | Cancer Research Technology | | Agents which interfere with the binding of MDM2 to human p53, assays for said agents | | June 23, 1998 | | June 23, 2015 |
| US 6,153,391 | | Cancer Research Technology | | Agents which interfere with the binding of MDM2 to human p53, assays for said agents | | November 28, 2000 | | November 28, 2017 |
| US 6,140,058 | | Cancer Research Technology | | P53 mutants | | October 31, 2000 | | October 31, 2017 |
| US 6,492,116 | | Cancer Research Technology | | Assay which allows the identification of compounds which inhibit binding of MDM2 and p53 | | December 10, 2002 | | September 10, 2016 |
| US 5,691,319B | | Sankyo | | Antitumor pyrimidine nucleoside derivatives, including CYC682 | | November 25, 1997 | | November 25, 2014 |
| US 5,616,567 | | Sankyo | | Antitumor pyrimidine nucleoside derivatives including CNDAC (active form of CYC682) | | April 1, 1997 | | April 1, 2014 |
| US 5,654,480 | | Sankyo | | Process for preparing CNDAC | | August 5, 1997 | | August 5, 2014 |
In addition to our U.S. patents, we own four patents that were granted by the European Patent Office, or EPO, for designated European countries, and ten issued patents in other countries. The European granted patents expire between 2015 and 2016. In addition to the licenses we hold to patents issued in the United States, we hold licenses under 51 issued patents worldwide, nine granted by the EPO for designated European countries and 42 issued in other countries. The licensed European granted patents expire between 2011 and 2018.
Our patent strategy is to file patents on compounds and technologies in countries and jurisdictions that we consider important to our business. We usually file first in the United Kingdom and then extend our applications to other countries through the Patent Cooperation Treaty. In some cases, we file directly in the United States. We give priority to obtaining substance of matter claims in the United States, the European Union, Japan and other important countries if such protection is available. We prefer substance of matter claims because they give us rights in our compounds themselves, and not merely in a particular use of the compounds. In addition to substance of matter claims, we seek coverage for medical uses, combination therapies, pharmaceutical forms of our compounds and
68
synthetic routes where available and appropriate. Claims covering combination therapies and pharmaceutical forms can be valuable because the therapeutic effect of pharmaceuticals used in the anticancer field is often enhanced when individual therapeutics are used in particular combinations. The availability of protection in these areas can, however, vary from jurisdiction to jurisdiction and combination claims are particularly difficult to obtain for many inventions. We own 37 patent applications pending in the United States, 21 before the European Patent Office, 18 pending PCT applications still in the international application phase, and 70 pending patent applications in other countries. Nineteen of those 70 pending patent applications were first filed, and have an earliest priority date, within the last twelve months. These pending applications can generally be grouped into 39 families of patent applications. No assurances can be given that patents will issue with respect to the pending applications, nor that the claims will provide equivalent coverage in all jurisdictions. Under the terms of our agreements with several universities and research institutions, we also have the right to apply for patents in the name of those universities and institutions for inventions in which we hold license rights. This gives us the ability to control the prosecution of patents that directly relate to our business strategy. In addition to the pending patents applications referred to above that we own, there are 73 pending patent applications worldwide to which we have a license or an option to take a license.
Our patent filings for our second generation CDK inhibitor research program provide an example of our patent strategy. Out of the over 600 compounds under investigation as part of this program, we have filed patent applications seeking substance of matter protection that may be roughly grouped into 10 patent families. Of these, we have made a European application designating all European Patent Convention member states and direct national filings in the United States, Japan and several additional countries covering the compounds that we believe to be the most promising from a commercial standpoint. We have made additional Patent Cooperation Treaty filings covering derivative compounds, medical uses and related technology. The first patent application from the family of compound patents has resulted in the issuance of two U.S. patents with substance of matter claims covering a specific genus of compounds showing activity in our preclinical and research programs. Although issuance of a substance of matter claim in the United States is an indication that other countries may grant similar protection, the pending applications may not result in additional patent protection.
We hold patents to several technology-based systems, including families of patents covering our Fluorescience fluorescent assay techniques and the drug delivery system Penetratin. We have filed a portfolio of patents claiming the use of over one hundred specific genes as drug targets based on the identification of their function in mitosis.
Since publications in the scientific or patent literature often lag behind actual discoveries, we are not certain that we were the first to make the inventions covered by each of our pending patent applications or that we were the first to file those patent applications. Generally, patent applications in the United States are maintained in secrecy for a period of 18 months or more, which increases the uncertainty we face. Moreover, the patent positions of biotechnology and pharmaceutical companies are highly uncertain and involve complex legal and factual questions. As a result, we cannot predict the breadth of claims allowed in biotechnology and pharmaceutical patents, or their enforceability. To date, there has been no consistent policy regarding the breadth of claims allowed in biotechnology patents. Third parties or competitors may challenge or circumvent our patents or patent applications, if issued. Because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that before we commercialize any of our products, any related patent may expire, or remain in existence for only a short period following commercialization, thus reducing any advantage of the patent and the commercial opportunity of the product.
If patents are issued to others containing valid claims that cover our compounds or their manufacture or use, we may be required to obtain licenses to these patents or to develop or obtain alternative technology. We are aware of several pending patent applications, and understand that others may exist, that could support claims that, if granted, would cover various aspects of our developmental
69
programs, including in some cases our lead drug candidate, CYC202, particular uses of that compound, CYC682 or other therapeutic candidates, or gene sequences and techniques that we use in the course of our research and development. Based on our review of the published applications, we believe that it is unlikely that a valid claim would be issued that covered CYC202. In addition, we understand that other applications exist relating to uses of CYC202 and CYC682 that are not part of our current clinical programs for those compounds. Although we intend to continue to monitor these applications, we cannot predict whether these claims will ultimately be allowed or if they were allowed what their breadth would be. In addition, we may need to commence litigation to enforce any patents issued to us or to determine the scope and validity of third-party proprietary rights. Litigation would create substantial costs. If our competitors prepare and file patent applications in the United States that claim technology that we also claim, we may have to participate in interference proceedings in the U.S. Patent and Trademark Office to determine which invention has priority. These proceedings could result in substantial costs, even if the eventual outcome is favorable to us. An adverse outcome in litigation could subject us to significant liabilities to third parties and require us to seek licenses of the disputed rights from third parties or to cease using the technology, even a therapeutic product, if such licenses are unavailable or too expensive.
Licenses
Several of our programs are based on technology that we license from others. Our breach of an existing license or failure to obtain a license to technology required to develop, test and commercialize our products may seriously harm our business.
We have entered into an agreement with Centre National de Recherche Scientifique, or CNRS, and Institut Curie that grants us worldwide rights under the patents jointly owned by CNRS, Institut Curie and the Czech Institute of Experimental Botany covering the CYC202 compound. The effective date of the agreement is February 1, 2002. The license grants us exclusive rights in the fields of auto-immune diseases, cardiovascular diseases, dermatological diseases, infectious diseases, inflammatory diseases, and proliferative diseases, including cancer. Non-acute chronic diseases of the central nervous system, neurological diseases and diseases of the peripheral nervous system are specifically excluded. The license runs for the term of the patents in each country, or for ten years from the first commercial sale in each country, whichever is later. Under the agreement, we paid an up-front fee. We also make yearly payments and milestone payments. The yearly payments are made until a patent covering the CYC202 compound, particular uses of the compound, and particular derivatives of the compound are published as granted in either the United States or Europe. Milestones are paid when a patent or patents covering the CYC202 compound, particular uses of the compound, and particular derivatives of the compound are published as granted in the United States and also in Europe. Milestones are also paid on the first commercialization of a product that consists of a new chemical entity that is covered by one of the licensed patents. We pay royalties based on our own net sales of products covered by the patents. Royalties are payable on a country-by-country basis for the term of patent protection in each country or ten years from the first commercial sale of royalty-bearing products in that country, whichever is later. Royalties are payable on net sales. Net sales are defined as the gross amount invoiced by us or by our affiliates for the products, less normal trade discounts, credits for returned products, taxes and shipping charges. There is one royalty rate for products that are covered by valid licensed patent claims and a second, lower royalty rate for all other products that require a license under the licensed patents. The royalties payable under the agreement are reduced if we are required to pay royalties with respect to patents other than the ones licensed under this agreement and the total amount of royalties that we are required to pay exceeds a fixed percentage amount. The amount of reduction depends on the amount by which our total royalties exceed the fixed amount. We must also pay a portion of sublicensing revenues. The portion of sublicensing revenues
70
that we are required to pay is reduced if we have taken the sublicensed product into human clinical trials. Although the license permits us to grant sublicenses, we cannot assign the license without the consent of the CNRS and Institut Curie, which may not be unreasonably withheld. Under the agreement, assignment is defined to include many transactions of the type that we might wish to pursue, such as a merger or an acquisition by another company, as well as certain takeovers. This restriction may prevent us from pursuing attractive business opportunities. Moreover, the occurrence of a majority takeover or a similar transaction that we may be unable to control could cause a default under the license agreement, which could lead to its termination.
We have also purchased from the Czech Institute of Experimental Botany patents and patent applications covering the use of CYC202 and related compounds. The issued patents are in the United States and Australia. Under the purchase agreement, we will pay royalties to the Czech Institute upon sales of products covered by those patents, but only if there are no royalties paid by us to CNRS for those sales under the license agreement with CNRS and Institut Curie covering CYC202 that is described above.
Patents covering the CYC202 compound are owned jointly by the Czech Institute and CNRS. The patents have been issued in the United States and Europe and expire in 2016. It may be possible to extend the term of a patent in the United States or Europe for up to five years to the extent it covers the CYC202 compound upon regulatory approval of that compound in the United States or Europe, but there is no assurance that we will be able to obtain any such extension. Under agreements between CNRS and the Czech Institute, CNRS has the exclusive right to enter into license agreements covering the patents. The agreement reserves to both CNRS and the Czech Institute certain rights, including the right to patent improvements and to use the patents for internal research purposes.
We have entered into a license agreement with Sankyo Co., Ltd. of Japan with respect to patents and patent applications covering the CYC682 compound and patent applications claiming polymorphic forms of CYC682 and methods for its preparation and use as well as related know-how and materials. The agreement has a commencement date of September 10, 2003. The issued patents for the CYC682 compound cover the United States, the European Union, Japan and 17 other countries. These patents expire between 2012 and 2014. It may be possible to extend the term of a patent in the United States or Europe for up to five years to the extent it covers the CYC682 compound upon regulatory approval of that compound in the United States or Europe, but there is no assurance that we will be able to obtain any such extension. The license grants us the exclusive right to exploit and sublicense the CYC682 compound and any other products covered by the patents and patent applications owned by Sankyo. The license originally was subject to certain third party rights related to certain countries but the license has been extended and is now worldwide. The license agreement also grants us nonexclusive, sublicensed rights in CNDAC, both the precursor compound and initial metabolite of CYC682. We are under an obligation to use reasonable endeavours to develop a product and we have agreed to pay Sankyo an up-front fee, reimbursement for Sankyo's enumerated expenses, milestone payments and royalties on a country-by-country basis. Under this agreement aggregate milestone payments totalling $11.7 million could be payable subject to achievement of all the specific contractual milestones and our decision to continue with these projects. The up-front fee and certain past reimbursement have been paid. Royalties are payable in each country for the term of patent protection in the country or for ten years following the first commercial sale of licensed products in the country, whichever is later. Royalties are payable on the net sales. Net sales are defined as the gross amount invoiced by us or by our affiliates or licensees, less discounts, credits, taxes, shipping and bad debt losses. The agreement extends from its commencement date to the date on which no further amounts are owing under it. If we wish to appoint a third party to develop or commercialize a CYC682-based product in Japan, within certain limitations, Sankyo must be notified and given a right of first refusal to
71
develop and/or commercialize in Japan. In general, the license may be terminated by us for technical, scientific, efficacy, safety, or commercial reasons on sixth months notice (twelve if after launch of CYC682-based product) or by either party for material default. On termination, if Sankyo wishes to acquire an exclusive license to CYC682 intellectual property developed by us during the term of the license, Sankyo may notify us and the parties will meet to negotiate commercial terms in good faith. If agreement cannot be reached, the terms of the exclusive license are to be determined by an expert.
We have entered into a license agreement with NuChem Pharmaceuticals, Inc. and its parent Lorus Therapeutics, Inc. with respect to our license of patents and patent applications covering the CYC381 compound in the United States, the European Union, Japan and other countries, as well as related know-how, materials and technology. The effective date of the agreement is September 22, 2003. Patents containing substance of matter claims covering the compound have been issued in the United States, Australia and China and have been published for opposition in Israel. These patents and patent applications expire in 2017 and 2018. It may be possible to extend the term of a patent in the U.S. or Europe for up to five years to the extent it covers the CYC381 compound upon regulatory approval of that compound in the U.S. or Europe, but there is no assurance that we will be obtain any such extension.
The license grants us worldwide rights in the technology owned by and licensed to NuChem related to a class of compounds including CYC381 and two other chemical classes of compounds that might have similar effects. The license is limited to the diagnosis and treatment of cancer (including leukemias), Kaposi's sarcoma and actinic keratosis. To the extent that the patents and related technology are owned by or exclusively licensed to NuChem, the license is exclusive. It is nonexclusive for patents and technology that are nonexclusively licensed to NuChem. We have the right to sublicense these patents and technology to others. Improvements to the licensed patents are owned by NuChem and licensed back to us. On termination, NuChem may obtain, on commercially reasonable terms, a license of the results of the research and development that we perform on CYC381. We are responsible for prosecution, maintenance and defense of the licensed patents, including all associated costs. NuChem co-owns certain of the patents with Harvard and Ion Pharmaceuticals and Harvard retains certain rights to use the patents for research purposes. No warranty is given under the agreement as to the validity of the licensed patents or that "any of the NuChem IP can be practised or exploited without infringing other patents." We are obligated to use commercially reasonable efforts to develop and commercialize the patents. The agreement extends from its commencement date to the date on which no further amounts are owing under it. The agreement may be terminated by us for convenience after September 2004 on four months' notice, by either party if the other defaults, and by NuChem if we do not actively pursue the licensed technology. We paid NuChem an up-front fee. We agreed to make milestone and royalty payments on a country-by-country basis and to pay NuChem a portion of any sublicensing fees we receive.
We have entered into a license agreement with Johnson Matthey Pharmaceutical Materials, Inc. with respect to U.S. patents as well as patent applications pending in the European Union, Japan and certain other jurisdictions that claim the synthetic route for CYC381. The effective date of the agreement is September 1, 2003. These patents and applications expire between 2017 and 2018. The license grants us the exclusive worldwide right to manufacture and sell products under the Johnson Matthey patents. The license includes the right to sublicense. We paid an up-front fee and agreed to make minimum annual payments, including with respect to each sublicense and to pay a royalty on the net cost of goods manufactured under the license. We also agreed to give Johnson Matthey the right to bid for any contract to manufacture products under the license. The license runs for the term of the patents. We may terminate the license for convenience, and either party may terminate it for the default of the other.
72
Other License Agreements
Cancer Research Technology Limited, a wholly-owned subsidiary of Cancer Research UK, has licensed patents to us, that relate to the p53 protein and our Hdm2 inhibitor program. The effective date of the agreement is October 23, 2002. Our license is exclusive in defined fields, including the interaction between p53 and Mdm2 and the development of molecules for the activation of p53 in therapeutic, prophylactic and diagnostic applications, except that another party retains nonexclusive rights in some of these patents. We paid an up-front amount. The license includes the right to grant sub-licenses. The license term runs until the last of the licensed patents expires or 10 years from the first marketing of the product in the EU, whichever is later. We also agreed to make annual payments, milestone payments and royalty payments based on sales of products covered by the licensed patents. We also have certain development obligations under the license including using all reasonable endeavors to obtain regulatory and other approvals.
We have licensed the current version of the LIDAEUS software from the University of Edinburgh for a term lasting at least until 2006. The commencement date of the agreement is December 1, 2001. Under the license, we own all improvements we make in the software and have the perpetual right to use these improvements which are also licensed to the University subject to certain restrictions. We also have the right to obtain trademark rights in the name LIDAEUS. The University retains a right to use the software to provide services to third parties and to use it for research purposes including commercial research, other than in certain limited areas. On termination for a party's breach or insolvency all licenses granted under the agreement continue, provided that those granted by the terminating party become non-exclusive. On termination, for our convenience, licenses granted to us by the University become non-exclusive and licenses granted by us to the University become free of restrictions and sub-licensable.
Option Agreements
We have entered into an option agreement with Cancer Research Technology Limited (formerly Cancer Research Ventures Limited) relating to research of Professor David Glover of the University of Cambridge that is funded by Cancer Research UK. The effective date of this agreement is November 5, 2001. This agreement grants us an exclusive option to obtain exclusive, world-wide, royalty-bearing licenses in the field of diagnostic and therapeutic products covering patents, patent applications and know-how resulting from research funded by Cancer Research and supervised by Professor Glover that relates to the genome ofDrosophila melanogaster. The option must be exercised within a period of time following our receipt of notice of particular inventions. The optioned rights could assist us in identifying genes involved in mitosis that could be used as targets for small molecule drug design. This agreement also grants us a non-exclusive license to non-patentable know-how resulting from research funded by Cancer Research and supervised by Professor Glover that relates to the genome ofDrosophila melanogaster. On launch of a product the grant becomes exclusive but is subject to the right of Cancer Research Ventures and the University to use the know how for research purposes. We paid an up-front fee for the option, but any licenses granted on exercise would be subject to further payments. The agreement extends until terminated by either party. Either party may terminate the agreement with respect to the rights granted to it under the agreement. Cancer Research Ventures Limited may terminate should we contest the secret or substantial nature of its licensed know-how. In addition, either party may terminate it for the default of the other.
73
We have also entered into an option agreement with Cancer Research Technology Limited (formerly Cancer Research Campaign Technology Limited), which is a wholly owned subsidiary of Cancer Research UK. The effective date of the agreement is September 10, 1997. The option relates to inventions funded by Cancer Research in a field consisting of therapeutics based on specific identified gene expressions and their relation to the cell cycle. Under the agreement we have the right to require Cancer Research to assign or exclusively license to us the relevant intellectual property in the defined field. Although Cancer Research agrees to attempt not to unduly encumber its rights in the field, our rights are subject to any encumbrances on Cancer Research's rights. The option must be exercised within a period of time following our receipt of notice of particular inventions. The option is also subject to royalty-sharing or other arrangements made by Cancer Research but no other payments remain owing. Cancer Research retains the right to use the intellectual property for research purposes. The option agreement expires on September 10, 2005.
Collaboration and Other Agreements
We have entered into a collaboration agreement with Cancer Research UK's subsidiary, Cancer Research Technology Limited, and the Institute of Cancer Research. The effective date of this agreement is April 26, 1999. This agreement relates primarily to back-up compounds from our CYC200 series and certain molecules from our program, the latter of which has been designated as an out-licensing candidate. Rights in the results of the collaboration are jointly owned, but the agreement grants us the exclusive right of commercial exploitation in exchange for milestone and royalty payments. The other parties retain the right to use the results for research purposes. We may sublicense our rights, but they may not be assigned without the other parties' consent. We have filed a number of pending patent applications on inventions arising from the collaboration. As of March 26, 2004, we own three pending PCT, or Patent Co-operation Treaty, applications that are still in the international phase and pending patent applications in the U.S., Europe and Japan. These applications cover CYC202 analogs and biomarkers that are potentially relevant to the CYC202 project. The agreement runs for the term of the patents, or ten years from the first commercial sale, whichever is later.
We are part of a four-way collaboration with Cancer Research Technology Limited (formerly Cancer Research Ventures Limited), Cambridge University and GlaxoSmithKline. The effective date of this agreement is October 16, 2002. This collaboration uses short interfering RNA, or siRNA, to develop inheritable, inducible RNAi systems for use in the development of mammalian models of disease. This agreement is effective until January 31, 2005. We contribute personnel and expertise. Rights in the results of the collaboration belong to Cancer Research, except that intellectual property relating to target genes is held jointly by all four participants and may be exploited by any of the collaborators on license terms to be agreed.
In 2001, we entered into a collaborative program with AstraZeneca targeting small molecule inhibitors of the cyclin binding groove in the CDK2/Cyclin A complex. The collaboration ended in March 2003. Under the terms of the agreement, all program intellectual property was assigned to us. AstraZeneca received a non-exclusive, royalty-free license in the program intellectual property to research, develop and commercialize program compounds outside the agreement's field of use and to carry out internal research. We have no further financial obligations under the agreement and are free to exploit know-how and develop products in the agreement's field of use.
74
We hold a grant from the U.K. Department of Trade and Industry covering work carried out in collaboration with the Department of Genetics of Cambridge University. The effective date of the grant is May 31, 2001. This program covers the screening of the entire genome ofDrosophila melanogaster using RNAi technology, which aids us in our research to identify genes involved in mitosis and use them as targets for small molecule drug design. Under this grant, we hold, as does the University, all rights necessary to exploit the results of its work under the grant. We are required to use reasonable efforts to exploit the results by December 31, 2009, and we can be required to return payments if we do not do so.
We have entered into several research agreements with the University of Edinburgh. Our research has focused primarily on ligand, or chemical binding, design and we own all intellectual property rights that result from these research programs except for a research agreement entered into in February 2004, which focuses on ligand design and structure-based drug design in the discovery of novel drugs for some of our programs. The commencement date of the research agreement is January 19, 2004. Under this February 2004 agreement, we own all rights related to ligands developed as part of the research program, and the University owns all remaining intellectual property under the research program. Under other agreements the University retains rights to use the intellectual property rights for non- commercial research and for commercial research subject to our approval. The research agreement extends from its commencement date until January 19, 2006.
Other Intellectual Property Rights
In addition to patents, we seek to protect our proprietary information as trade secrets. Trade secrets are difficult to protect, and the degree of protection varies from one jurisdiction to another. There can be no assurance that the agreements we use to protect our trade secrets will provide meaningful protection, that these agreements will not be breached, that we will have an adequate remedy for any such breach, or that our trade secrets will not otherwise become known or independently developed by a third party.
Miscellaneous
We are using a variety of technologies in our drug discovery efforts to screen for future targets or potential targets, and inhibitors of them. Exemplary technologies include RNAi for use in knockdown assays and assays to protein targets allowing the screening of small molecule inhibitors potentially relevant to the cell cycle for a variety of applications. Patents applications covering these technologies are pending in various jurisdictions, and it is currently unclear whether and to whom patents will be granted and the scope of any claims that may be issued. If a patent is granted to another it may be necessary for us to either obtain a license or discontinue use of this technology.
In some cases, we have used assays in jurisdictions other than the jurisdictions where the assays or components of them are patented. It is our understanding that under current principles of law for these jurisdictions we are free to use the information resulting from the assays even where they are patented. However, the law in this area is still evolving and it is possible that a contrary result could arise in one or more jurisdictions.
There are pending a number of patent applications that claim gene sequences that include some of the sequences that we are considering as biomarkers of CYC202. In some cases these sequences are among a large number of sequences claimed by the patent applicants. In general, it is our understanding that in such circumstances it is unlikely that claims will ultimately be allowed for
75
particular sequences that are included among the many claimed. However, no assurance can be given that such a claim would not ultimately issue.
Competition
The biotechnology and biopharmaceutical industries are rapidly changing and highly competitive. We are seeking to develop and market drug candidates that will compete with other products and therapies that currently exist or are being developed. Other companies are actively seeking to develop products that have disease targets similar to those we are pursuing. We face competition from many different sources, including commercial, pharmaceutical and biotechnology companies, academic institutions, government agencies and private and public research institutions. Many of our competitors have significantly greater financial, manufacturing, marketing and drug development resources than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that we may develop. In addition, our competitors compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses.
We are currently the only company that has an orally available CDK-specific agent in Phase II clinical trials. Eisai Co. Ltd has a synthetic sulphonamide, E-7070 in Phase II development, which is reported to have a mechanism of action that includes blocking the expression of CDK2 although its primary molecular target is unclear. In addition, although Aventis has announced that it has ceased Phase II development of flavopiridol, a CDK inhibiting drug, the Company believes that the National Cancer Institute's Cancer Therapy Evaluation Program is continuing to enroll patients in a Phase II trial. AstraZeneca has reportedly begun Phase I clinical trials on a CDK inhibiting drug, AZD-5438. Bristol-Myers Squibb has also begun Phase I clinical trials on BMS-387032, a drug that is presumed to work by CDK inhibition. In addition, we believe that several companies such as Aventis, Kyowa Hakko, Pfizer and Roche have or have recently had, agents in clinical trials that are specific to CDKs. Others have CDK inhibitor research programs in preclinical studies.
A number of our competitors are also pursuing discovery and research activities in each of the other areas that are the subject of our research and drug development programs, including GSK-3 inhibitors.
Government Regulation and Approval
Regulation and Approval in the United States
The pharmaceutical sector is one of the most heavily regulated in the world. Governmental authorities in the United States and other countries extensively regulate the preclinical and clinical testing, approval, manufacturing, labeling, storage, record keeping, reporting, advertising and promotion, import and export, marketing and distribution of prescription drugs. In the United States, the Food and Drug Administration, or FDA, rigorously reviews and regulates drugs under the Federal Food, Drug and Cosmetic (FD&C) Act and its implementing regulations. Non-compliance with applicable requirements under the FD&C Act can result in administrative and judicial sanctions, including, but not limited to, warning letters, clinical holds, fines, recall or seizure of products, injunctions, total or partial suspension of production or clinical investigation, refusal of the government to accept for review or to approve marketing applications or to allow entry into supply contracts, refusal to permit import or export of products, civil penalties, criminal prosecutions, revocation of product approval and other actions which may adversely affect a company and its products. In the United States, a prescription drug must be the subject of a New Drug Application, or NDA, filed by its
76
sponsor and approved by the FDA before it may be legally marketed. A drug sponsor must submit extensive preclinical and clinical data, as well as information about product manufacturing processes and facilities and other supporting information to the FDA to establish a product candidate's safety and effectiveness for its proposed intended use or uses. In order to secure premarket approval, a drug sponsor must submit an NDA to demonstrate to the FDA that:
- •
- the drug is safe and effective for its proposed use or uses;
- •
- the drug's benefits outweigh its risks;
- •
- the proposed drug labeling, including indications, use instructions, and warnings, are clinically accurate, truthful and not misleading; and
- •
- its manufacturing methods and controls are adequate to assure the product identity, strength, quality, and purity.
In an NDA, a drug sponsor must specifically provide:
- •
- the results of extensive preclinical laboratory tests, preclinical animal studies, and drug formulation studies, all performed in accordance with the FDA's Good Laboratory Practice, or GLP, regulations;
- •
- the results of adequate and well-controlled clinical trials and other clinical investigations of the drug, conducted in accordance with approved Investigational New Drug, or IND, applications, to establish the drug's safety and efficacy for its proposed use or uses; and
- •
- access to manufacturing facilities and clinical trial sites for any required pre-approval inspections, consisting of the FDA's audits to evaluate compliance with current Good Manufacturing Practices, or cGMP, and to verify the accuracy and completeness of the manufacturing-related information submitted in the NDA.
As an initial step in the preparation and filing of an NDA, a sponsor typically conducts preclinical laboratory and animal studies of the product candidate to determine if it offers evidence of its biological activity and of its reasonable safety for investigation in humans. Preclinical tests include laboratory evaluation of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animals.
Following these preclinical tests, the sponsor will submit an IND application to the FDA or a comparable application to an equivalent regulatory body in another country to initiate clinical studies. In the case of an IND, the results of preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA. An IND will consist of (i) any animal pharmacology and toxicology studies; (ii) data from previous clinical studies or marketing of the drug in other countries; (iii) information on composition, stability, manufacturing process and controls; and (iv) the protocol specifying the design and investigators of the proposed study. Once the IND is submitted, the FDA has 30 days to review the IND to assure study subjects will not be subjected to unreasonable risk. Once the IND becomes effective, the sponsor can commence clinical studies in the U.S. of the product candidate in humans to determine safety and efficacy. The data resulting from these preclinical studies, animal studies and human clinical trials become part of the NDA. Under the FDA's regulations, the clinical testing program required for marketing approval of a new drug typically involves three sequential phases, which may overlap:
- •
- Phase I: Small safety studies are usually conducted on healthy human volunteers to determine drug safety, dosage tolerance and side effects, absorption, metabolism, distribution and excretion. Early evidence of effectiveness may be sought. Adequate information on a drug's action in the body and effect is sought to allow for design of Phase II studies. A sponsor may decide to
77
The FDA may also condition approval of an NDA on postmarketing study commitments. Such Phase IV studies are conducted after the FDA has approved a product for marketing to further assess a drug's safety and effectiveness after approval. Agreements with sponsors to conduct postmarketing studies can be reached either before or after the FDA has granted approval to a sponsor to market a product. The FDA may also require conduct of postmarket surveillance programs to monitor the appropriate use and safety of an approved drug, which may lead to additional limitations on the marketing of the drug.
The premarket review required by the FDA and comparable agencies before a prescription drug may be marketed generally requires many years and substantial effort and financial resources. Approval from the FDA may not be granted in a timely manner, if at all. The time required to satisfy FDA requirements or similar requirements of foreign regulatory agencies may vary substantially based upon the type, complexity and novelty of the product or the targeted disease. Even if a product receives regulatory approval, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market.
Obtaining approval of an NDA is expensive and is a complex, lengthy and uncertain process. Despite the substantial time and expense invested in preparation and submission of an NDA, regulatory approval is never guaranteed. After a technical review for completeness, the FDA may determine that an NDA is deficient and does not include sufficient data to justify initiation of a formal review; an NDA may then be subject to a "refuse-to-file" action. The FDA's regulations specify the kind of data and information which must be provided in an NDA, such as full reports of all relevant preclinical, animal and clinical data; chemistry, manufacturing and control information; nonclinical pharmacology and toxicology data; human pharmacokinetic and bioavailability data; statistical analyses and case report forms; product samples and labeling; when appropriate, patent information and certification; and other information. FDA also seeks voluntary submission of product launch campaign material, such as proposed advertising and promotional labeling, for comment before its dissemination to the public.
Additionally, if an NDA is filed and the FDA initiates its review, the agency may seek or the sponsor may submit additional data or analyses as an NDA amendment, which may clarify an outstanding issue, address a deficiency or lengthen the time required for review. The FDA or the sponsor may also seek to present the NDA to an advisory committee for advice and non-binding recommendations regarding a drug's proposed uses or labeling, or whether the NDA demonstrates the drug is safe and effective and should be approved. Upon completion of its review, the FDA may determine that a drug is not proven safe or effective for its proposed use or uses, or that additional clinical data may be required. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Finally, the FDA retains the authority to withdraw
78
approval of an NDA for noncompliance with its requirements or due to previously unknown problems with the drug, such as adverse events of unanticipated severity or frequency.
When appropriate, we may seek priority review, fast track designation or accelerated approval for our drug candidates. We cannot predict whether any of our drug candidates will obtain such designations, or the ultimate impact, if any, of such designations on the timing, conditions, or likelihood of FDA approval.
Upon submission of an NDA, a drug may qualify for priority review by the FDA under the Prescription Drug User Fee Act, or PDUFA, if it constitutes "a significant improvement compared to marketed products... in the treatment, diagnosis, or prevention of a disease." Under PDUFA, the FDA receives application and establishment-based user fees from drug sponsors, in exchange for the agency's performance towards timely drug review performance goals. Under current PDUFA performance goals, the FDA is seeking to review and act on 90 percent of priority NDA submissions within six months of their receipt. If a drug does not qualify for priority review under PDUFA, the FDA will seek to review and act on 90 percent of standard NDA submissions within 10 months of their receipt. These performance goals, however, are no guarantee of the FDA's performance and the agency may seek to delay, limit or deny approval of a drug candidate for many reasons.
Additionally, an NDA may be eligible for fast track designation, which facilitates the development and expedites the review of drugs "intended for the treatment of serious or life-threatening conditions and that demonstrates the potential to address unmet medical needs for such conditions." A sponsor must apply for fast track designation, which can afford potential benefits such as priority review, frequent scheduled planning meetings with the FDA, the option of rolling submission and review of the NDA, the option of accelerated approval on the basis of surrogate endpoints "reasonably likely to predict clinical benefit", and approval subject to substantial post-market study commitments to verify clinical benefit. Fast track designation does not oblige the FDA to determine whether an NDA is approvable; such designations are intended to be granted in early development and well before submission of an NDA to facilitate and expedite the development process.
A drug may also be eligible for accelerated development and review when the FDA's approval is based on evidence of the drug's pharmacologic effect on a "surrogate endpoint" and when the FDA determines that safe use of the drug depends on restricting its distribution or use. A surrogate endpoint is a symptom or laboratory finding that is determined likely to predict therapeutic benefit for a patient, not a direct clinical measurement of patient outcome or survival.
The manufacture of approved drugs must be conducted in strict compliance with the FDA's current good manufacturing practices, or cGMP, and comparable requirements of other regulatory bodies, which mandate the methods, facilities, and controls used in manufacturing, processing, and packing of drugs to assure their safety and identity. The FDA enforces its cGMP requirements through mandatory registration and facility inspections. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenues, and subject the sponsor to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil penalties.
The FDA also regulates the marketing and promotion of approved drugs, including industry-sponsored continuing medical education, or CME, direct-to-consumer, or DTC, advertising, and direct physician sales, to ensure that information provided by sponsors regarding their products is truthful, balanced, and accurately reflect the safety and efficacy claims approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
We are currently conducting Phase II clinical trials of CYC202 in Europe and Phase Ib trials of CYC202 in Singapore. To date, we have not filed an IND application, or any other applications, with
79
the FDA to conduct clinical trials in the U.S. for CYC202. Any IND application that we submit to the FDA for CYC202 will incorporate the results of our European and Asian clinical trials of this drug candidate. The FDA may reject these clinical trial results based on a determination that the foreign clinical trials were not conducted in accordance with requisite U.S. regulatory standards and procedures. Moreover, the FDA may subject the foreign trial data that we submit to a greater level of scrutiny and we may incur additional costs and delays responding to FDA requests for supplemental information or clarification. If we are unable to obtain FDA approval for an IND, we will not be permitted to conduct clinical trials of CYC202 in the U.S. and may be unable to ultimately seek or obtain U.S. regulatory approval for commercialization. With regard to CYC682 we are currently conducting Phase Ib clinical trials in the U.S. on an open IND following on from Phase I trials concluded in 2003.
Regulation and Approval in the European Union
The fundamental requirements, which are demonstration of safety, quality and efficacy and accurate labeling, are the same for the European Union as they are for the United States.
The review processes, and possible sanctions in case of non compliance, are as onerous in the European Union as they are in the United States, as is the process of ongoing regulation after licensure.
The potential uncertainties associated with the regulatory review in the United States that have been discussed above are also equally significant in Europe.
Preclinical and clinical requirements to support a Marketing Authorization Application in the European Union are, in principle, not different from those needed to support an NDA in the United States. In particular, the stages of pre-clinical development and of clinical development are essentially the same. GLP (Good Laboratory Practice) and GCP (Good Clinical Practice) requirements must be met. There are many common guidelines pertaining to product development that are used by both the FDA and European regulators. These guidelines do not, however, have the force of law, and there is no mutual recognition of marketing approvals. A drug accepted by the FDA can be refused by the European Union (and vice-versa) even if the supporting data are the same. Also, when a drug is licensed in both jurisdictions, the approved indications for use may be different.
As in the United States, licensure may be granted in the European Union subject to commitments on the part of the license holder to undertake further investigations post-marketing. The timeframe for completion of such commitments will invariably be specified and failure to achieve them within the specified time can result in suspension of the Marketing Authorization.
New European Union pharmaceutical legislation has very recently been published. This will introduce in a progressive way new requirements and obligations. One of the changes, coming into effect on November 20, 2005, will be that all new drugs for cancer, AIDS and diabetes must be registered via what is known as the "centralized procedure." This procedure entails submission and review at the level of the European Union rather than at the level of the individual national authorities.
The centralized review follows set timelines. Should the applicant company fail to respond to points raised during the review within the requisite timeframe it may be required to withdraw the application and then to resubmit a full application at such time as it is in a position so to do.
The process of centralized review involves all of the Member States of the European Union. The technical/clinical review of the Marketing Authorization Application takes place under the auspices of a scientific committee, which will become known as the Committee for Medicinal Products for Human Use (currently the Committee for Proprietary Medicinal Products) on which all Member States are represented. The committee will be supported by what will be known in the future as the European
80
Medicines Agency (previously the European Medicines Evaluation Agency) and is responsible for providing a scientific opinion on the adequacy, or otherwise, of an application, and on the acceptability of the labeling proposed. The opinion is developed by majority vote of the members of the committee. Upon completion of a positive centralized procedure, the Marketing Authorization is delivered by the European Commission. Should a Marketing Authorization be refused for any reason by the European Union, there would be no opportunity to seek approval in individual member countries.
The single Marketing Authorization that is issued following a successful centralized review is valid in all of the 25 European Union Member States: individual Member States cannot dispute it.
A "Summary of Product Characteristics" (SPC) forms part of the Marketing Authorization. The SPC provides an overview of the technical and clinical characteristics of the product. It is an obligation that any claims that are subsequently made for the product are consistent with the information provided in the SPC.
A Manufacturers' License is a legal prerequisite (in addition to the Marketing Authorization) in order that a pharmaceutical product can be offered for sale within the European Union. Manufacture must be in accordance with cGMP, and sites are subject to inspection. Sanctions (including suspension of manufacturing) are applicable if requirements are not met.
Regulatory authority acceptance is required in order to conduct clinical trials in the European Union. The centralized regulatory process is not applicable to applications for authorizations to conduct these trials. Such applications must be filed in, and accepted by, the individual countries concerned. However, as part of the process of review of the Marketing Authorisation Application, the Committee for Medicinal Products for Human Use can require an inspection/audit of a clinical trial site in order to confirm that the requirements of GCP have been met.
Pricing and reimbursement also remain the responsibility of individual national authorities in Europe. However, under European legislation, each national authority must accept without change the indications for the drug that have been approved at the European level.
There is an ongoing obligation on the part of the holder of the license to furnish reports regarding the post-marketing safety in use of registered products on a regular, pre-defined basis. If the data accrued over time are such as to indicate a need, the license holder may be obliged to modify the information provided to the patients and/or healthcare providers.
Employees
As of March 31, 2004, we employed 73 staff, 70 full time and three part time, of which 58 were in research and development and clinical development with the remainder in managerial and administrative functions. In total, 40 employees have Ph.D. or M.D. degrees. Nearly all employees with more than six months of service are share optionholders. We have no collective bargaining agreements with our employees and have not experienced any work stoppages. We believe we have good relations with our employees.
The following table sets forth the average monthly number of employees we had for the periods ended March 31, 2001, 2002, 2003 and 2004:
| | As of March 31,
|
|---|
| | 2001
| | 2002
| | 2003
| | 2004
|
|---|
| Research and development | | 40 | | 54 | | 58 | | 56 |
| Managerial and administrative | | 10 | | 10 | | 14 | | 14 |
| | |
| |
| |
| |
|
| Total Employees | | 50 | | 64 | | 72 | | 70 |
| | |
| |
| |
| |
|
81
Properties
Our main research facility is a 43,458 square foot building located at Dundee Technopole, James Lindsay Place, Dundee, Scotland. The building was constructed by Dundee City Council exclusively for use by us and incorporates our own designs and specifications with regard to research and development and administrative space. Permanent security arrangements, electronic systems and guards, are in place at all times.
The lease in respect of 0.529 hectares of land at Dundee Technopole is for a term of 25 years, expiring on October 22, 2025 at a current rent of £240,200 ($434,666) per year payable quarterly in advance. The rent is subject to review on October 28, 2005 and every five years thereafter. The 2005 rent review is subject to a maximum increase of 5% of £240,200 ($434,666). If Dundee City Council serves a notice of its intention to dispose of its interest, we have an option to purchase the land subject to the lease. This option is valid for 21 days after the date of service of the notice. Should we cease to substantially occupy and utilize any premises within the City of Dundee as a facility for research and/or the development of products or processes within the biotechnology sector whether in our capacity as tenant or as a heritable proprietor, before the expiry of five years from October 23, 2000, we must pay Dundee City Council the following sums:
- •
- Cessation within one year £200,150 ($362,191) (plus VAT)
- •
- Cessation within 2 years £280,150 ($506,959) (plus VAT)
- •
- Cessation within 3 years £320,150 ($579,343) (plus VAT)
- •
- Cessation within 4 years £320,150 ($579,343) (plus VAT)
- •
- Cessation within 5 years £320,150 ($579,343) (plus VAT)
Presently, we only occupy the ground floor of the facility referred to above, comprising approximately 20,000 square feet. The top floor, an additional 16,000 square feet approximately of useable space, is in an unfinished form and available for future expansion. We have been granted a license by Babraham Bioscience Technologies Limited of an approximately 3,303 square foot research unit located at Babraham Bioincubator, Babraham Hall Estate, Cambridge, England at a licence fee of £4,955 ($8,967) per month payable monthly in advance continuing until either party gives to the other party six months' written notice of termination.
U.S. dollar amounts are presented solely for your convenience in this section at the rate of £1.00 = $1.8096, the noon buying rate in The City of New York for cable transfers in pounds sterling as certified for customs purposes by the Federal Reserve Bank of New York on June 30, 2004. These translated amounts should not be construed as representations that the pounds sterling amounts could have been, or could in the future be, converted into U.S. dollars at this or any other exchange rate.
Legal Proceedings
Neither the Company nor any of its subsidiaries is or has been involved in any legal or arbitration proceedings which may have, or have had during the last 12 months preceding the date of this document, a significant effect on the financial position of the Company or any of its subsidiaries and, so far as the Company is aware, no such proceedings are pending or threatened by or against the Company or any of its subsidiaries.
82
MANAGEMENT
Directors and Senior Management
The following table provides information regarding our directors and senior management at June 30, 2004.
Name
| | Age
| | Title
|
|---|
| Directors | | | | |
| Sir John Banham | | 63 | | Chairman; Non-executive Director |
| Spiro Rombotis | | 45 | | Chief Executive Officer |
| Dr. Robert Jackson | | 61 | | Chief Scientific Officer |
| Paul McBarron | | 44 | | Chief Financial Officer and Company Secretary |
| Professor Sir David Lane | | 51 | | Non-executive Director |
| Professor Gordon McVie | | 59 | | Non-executive Director |
| Dr. David U'Prichard | | 56 | | Non-executive Director |
| |
| |
|
|---|
| Senior Management | | | | |
| Dr. Graham Bell | | 41 | | General Manager, Polgen Division |
| Gill Christie | | 47 | | Manager, Human Resources |
| Dr. Peter Fischer | | 47 | | Head, Drug Discovery |
| Dr. Athos Gianella-Borradori | | 49 | | Director, Medical Affairs |
| Professor David Glover | | 56 | | Chief Scientist, Polgen Division |
| Dr. Howard Marriage | | 46 | | Director, Business Development |
| Dr. Robert Westwood | | 61 | | Head, Preclinical Development |
The business address for our directors and senior management is c/o Cyclacel Group plc, James Lindsay Place, Dundee Technopole, Dundee DD1 5JJ, United Kingdom.
Directors
Sir John Michael Middlecolt Banham, Chairman of our board of directors, Non-executive Director. He is Chairman of Whitbread plc, GEEST plc, ECI Partners LLP, senior non-executive director of AMVESCAP plc and non-executive director of Merchant Trust plc. He is past Director General of the Confederation of British Industry (CBI) and past non-executive chairman of Tarmac plc and Kingfisher plc. His public sector appointments comprise first Controller of the Audit Commission and first Chairman of the Local Government Commission for England. He was formerly Honorary Treasurer of the United Kingdom's Cancer Research Campaign prior to its merger with Imperial Cancer Research.
Spiro Rombotis, Chief Executive Officer. Mr. Rombotis joined us in August 1997 and has over 21 years of experience with pharmaceutical and biotechnology companies. He was previously Vice President of International Operations and Business Development, Managing Director, Europe and Director, Japanese joint venture, at The Liposome Company, Inc. Mr. Rombotis also served as Vice President of Pharmaceuticals for Central and Eastern Europe and as Director of International Marketing at Bristol-Myers Squibb Company. He was Head of European Marketing and Sales and Head of Corporate Development at Centocor, Inc. as well as working in Business Development at Novartis AG. He holds a B.A. from Williams College and an M.B.A. and Master's degree in Hospital Management with honors, from the Kellogg Graduate School of Management where he serves on the Kellogg Biotech Advisory Board.
Robert Jackson, Ph.D., Chief Scientific Officer. Dr. Jackson joined us in January 2001 as Executive Director, Research and Development. He previously was the Director of Research and Development and a member of the Board of Directors at Celltech Group plc. He was also the Executive Director of Research and Development, the Chief Operating Officer and a member of the Board of Directors at
83
Chiroscience Group plc, which was acquired by Celltech in 1999. Before these appointments, he was Vice President of Research and Development at Agouron Pharmaceuticals, Inc., and headed cancer research at DuPont Pharmaceuticals and Warner-Lambert Company. He holds a B.A. from the University of Cambridge and a Ph.D. from the University of London, Institute of Cancer Research. Dr. Jackson has been responsible for overseeing the Company's research activities since his appointment in January 2001.
Paul McBarron, Chief Financial Officer and Company Secretary. Mr. McBarron joined us in January 2002 with 13 years of experience as a financial executive with several pharmaceutical companies. Since 1996 he was a senior member of the finance team at Shire Pharmaceuticals plc, where he held the positions of Director of Corporate Finance and Group Financial Controller. He joined Shire when it was an emerging public company employing less than 100 people. He was previously employed in various financial positions at Sterling Drug and SmithKline Beecham and qualified as a chartered accountant with Ernst & Young.
Professor Sir David Lane, FRS, FRSE, FRCPath, Non-executive Director. Professor Lane, our founder, joined us in September 1996. He is Professor of Molecular Oncology and Director of the CRC Cell Transformation Research Group in the Department of Biochemistry at the University of Dundee. He previously held the positions of Principal Scientist at the Imperial Cancer Research Fund and Lecturer in Biochemistry at Imperial College. He holds a Ph.D. from University College London, and did his postdoctoral work at Imperial Cancer Research Fund in London and at Cold Spring Harbor Laboratory in New York. He is a Fellow of the Royal Society and a member of the European Molecular Biology Organization. Professor Lane discovered the p53 tumor suppressor protein and was named by Science magazine as the world's seventh most-cited scientist. Professor Lane has won numerous international awards, including the Paul Ehrlich Prize, the Joseph Steiner Prize, the CR Brupbacher Foundation Prize, the Medal of the Swedish Society of Oncology, the Yvette Mayent Prize, the Mayenberg Preis, the Silvanus Thompson Medal, the Medal of the Society of Chemical Industry and has been named a Howard Hughes Scholar. He has authored approximately 250 publications and patents.
Professor John Gordon McVie, D.Sc. (Hon), MBChB, MRCP, M.D., FRCP, FRCPS, FmedSci, Non-executive Director. He is currently Chief Executive Officer and a director of Cancer Intelligence Limited, a cancer consulting company, former Joint Director General of Cancer Research UK and former Director General of the Cancer Research Campaign. Previously, he was Clinical Research Director at the Netherlands Cancer Institute in Amsterdam. From 1976 to 1979 he was the first NHS Consultant Medical Oncologist in Scotland at The Cancer Research Campaign Unit in Glasgow. He is the European editor of JNCI (Journal of the National Cancer Institute) and Senior Consultant to the European Institute of Oncology, Milan, Italy. He has authored five books and over 200 research papers.
David U'Prichard, Ph.D., Non-executive Director. Dr. U'Prichard joined the Board of Directors in May 2004. He is currently President of Druid Consulting LLC, a pharmaceutical and biotechnology-consulting firm, providing customized services to life sciences clients in the United States and Europe. He is also a Venture Partner and member of the health care team of Apax Partners Ltd., a global private equity provider. Previously, he was Chief Executive Officer of 3-Dimensional Pharmaceuticals, Inc. from 1999 to 2003. In addition, he held a variety of positions within the pharmaceutical and biotechnology industries, including, President and Chairman of Research and Development for SmithKline Beecham Pharmaceuticals; Executive Vice President and International Research Director, and a Member of the Board of Zeneca Pharmaceuticals; General Manager, Research Department, ICI Pharmaceuticals, and Vice President Biomedical Research, ICI Pharmaceuticals; and Senior Vice President and Scientific Director for Nova Pharmaceutical Corporation. He is a director of Guilford Pharmaceuticals, Invitrogen, the Life Sciences Research Foundation in Baltimore, Maryland, and Lynx Therapeutics. He is a Non-executive Chairman of
84
Oxagen. He is also Chairman of the Board of Directors for the Pennsylvania Biotechnology Association. From 1992 to 1997 he was a member of the board of the Biotechnology Industry Organization (BIO). He received a B.Sc. in Pharmacology from University of Glasgow in 1970 and a Ph.D. in Pharmacology from University of Kansas in 1975.
Senior Management
Graham Bell, Ph.D., General Manager, Polgen. Dr. Bell joined us in November 1998 as Project Manager and was promoted in October 1999 to Manager, Corporate Development. He was previously Head of Cell Culture with Biocure Ltd. (now Medysis plc). He holds a B.Sc. and Ph.D. from Edinburgh University.
Gill Christie, Manager, Human Resources. Ms. Christie joined us in January 2001. She was previously Human Resources Manager with Axis-Shield plc (formerly Shield Diagnostics) and a lecturer in Human Resource Management at Abertay University, Dundee. Before gaining an M.Sc. in Human Resource Management from Abertay University, she held scientific posts in Medical Microbiology at Ninewells Hospital, Unilever Research Laboratories and Unipath Ltd.
Peter Fischer, Ph.D., Head, Drug Discovery. Dr. Fischer joined us in September 1997. He was previously Director of Research in Chemistry and Group Leader of Peptide Chemistry at Nycomed in Norway and a senior research scientist with Deakin Research Ltd., a biotechnology company spun out of an Australian university. He studied in Australia and received his B.Sc. from Macquarie University and his Ph.D. from Deakin University.
Athos Gianella-Borradori, M.D., Director, Medical Affairs. Dr. Gianella-Borradori joined us in October 2000. He was previously Director of Preclinical Development, Manufacturing, Clinical and Regulatory Affairs at Bavarian Nordic Research Institute GmbH. He also served as Vice President of Clinical Affairs at CruCell BV (formerly IntroGene) and Head of Oncology and Hematology Clinical Research and Development. He was Senior Adviser for Genetic Therapy and Stem Cell Technologies Research and Development at Novartis Pharma Ltd. (formerly Sandoz). He received his medical degree from the University of Bern and trained and was a clinical fellow at Charing Cross Hospital in London, Children's Hospital of Los Angeles, the University of Glasgow's Royal Infirmary, the University of Lausanne and the University of Zurich. He is board certified in Pediatrics Hematology and Oncology.
Professor David Glover, FRSE, Chief Scientist, Polgen. Professor Glover joined us in November 1999. He is Arthur Balfour Professor of Genetics and Chairman in the Department of Genetics at the University of Cambridge. He is also Director of Cancer Research UK Cell Cycle Genetics Research Group. He was previously Professor of Molecular Genetics at the University of Dundee and Professor and Head of Biochemistry at Imperial College, London. Professor Glover discovered and named the Polo and Aurora mitotic protein kinases and co-ordinated the former European Drosophila Genome Project, the European academic consortium contributing to sequencing the fruit fly genome. He is a member of the European Molecular Biology Oncology and has authored over 200 publications and patents.
Howard Marriage, D.Phil., Director, Business Development. Dr. Marriage joined us in July 1998 after 13 years at Genzyme Corporation, where he was most recently Senior Director of Corporate Development in Europe. He was instrumental in establishing Genzyme businesses in gene therapy, small molecule drugs, tissue repair and Genzyme Molecular Oncology, where he developed and implemented a global commercial strategy in genomics. Previously he held positions at Beecham Pharmaceuticals and Wellcome Laboratories. He holds a Doctor of Philosophy in Pharmacology from Oxford University and a B.Sc. in Biochemistry and Pharmacology from Southampton University.
Robert Westwood, Ph.D., Head, Preclinical Development. Dr. Westwood joined us in July 2000. He is former Professor of Bioorganic Chemistry at the University of Hull and Director of the Institute for Chemistry in Industry. He spent 25 years at Aventis Pharma (HMR) most recently as Head of
85
Immunology Research in Paris and Director of Research, HMR in Denham, U.K.. He is responsible for biotech collaborations with Vertex Pharmaceuticals, Inc. and Ariad Pharmaceuticals, Inc.
Scientific Advisory Board
Our Scientific Advisory Board meets as required with one or more members having particular expertise in providing advice on specific aspects of our scientific strategy. Members of our Scientific Advisory Board receive cash compensation for their services and are reimbursed for their reasonable expenses of attending meetings. These advisors assist in formulating our research, development and commercialisation strategy. In addition to Professor Sir David Lane, the members of our Scientific Advisory Board are:
Professor Kenneth Harrap, CBE, Ph.D., D.Sc., FRSC is a former Director of the Drug Development Section of the Institute of Cancer Research in Sutton, Surrey and London. Until September 1997, he was Director of the CRC Centre for Cancer Therapeutics, a multidisciplinary unit responsible for the discovery and development of new anticancer drugs, at the institute of Cancer Research. He has formed collaborations with the pharmaceutical industry that have led to the clinical development, registration and marketing of several cancer drugs, including agents such as Paraplatin and Tomudex. He received his Ph.D. in chemistry in 1961 and his D.Sc. in biochemistry and pharmacology in 1997, both from London University, where he is present Professor Emeritus in Biochemical Pharmacology. He has published 400 papers, is on the editorial board of 14 cancer journals and is currently active as a consultant to the pharmaceutical industry. Professor Harrap has received several honours, including Life and Emeritus Fellowships of the Cancer Research Campaign, the Bruce F Cain Memorial Award (American Cancer Society), the Cain Memorial Award (New Zealand Cancer Society) and the Barnett Rosenberg Award. In 1998, he was appointed CBE for services to cancer research.
Professor Stanley Kaye, M.D. FRCP is the CRC Professor of Medical Oncology, Institute of Cancer Research, The Royal Marsden Hospital. He is former Head of Department, CRC Department of Medical Oncology, University of Glasgow and CRC Professor of Medical Oncology, University of Glasgow, since 1981. Professor Kaye is a clinical oncologist who has participated in a large number of early clinical trials of novel anticancer agents. Professor Kaye's organizational skills have been utilized by various cancer research organizations such as the CRC, EORTC and MRC. He is currently a non-executive director of West Glasgow Hospitals University NHS Trust. Professor Kaye graduated in London in 1972 with a B.Sc. in Biochemistry M.B.B.S. and completed training in general medicine and medical oncology in London and Sydney, Australia.
Professor Michel Marty, M.D. is a Professor of Medical Oncology at the University of Paris VII. He is Director of Oncology Therapeutics Research at the Institut Gustave Roussy in Paris. He is also Chairman of the International Cancer Collaborative Study Group. Professor Marty is widely consulted by regulatory authorities on possible approvals of cancer drug submissions. He is a member of the Drug Approvals Commission and Chairman of the Antineoplastic Agents Working Group of the National Drug Agency of the French Ministry of Health. He is also an officially designated Expert for the Commission for Proprietary Medicinal Products (CPMP) of the European Union in Brussels and also an Expert for the Transparency Commission of the French Ministry of Health. He received his medical qualification (Doctorat d'Etat en Médecine) and his Master of Sciences in Structural and Metabolic Biochemistry from University of Paris VII in 1975. He is board certified in medical oncology.
Professor Enrico Mihich, M.D. is a Director of Grace Cancer Drug Center and Department of Experimental Therapeutics, and Vice President for Sponsored Programs, Roswell Park Cancer Institute, Buffalo, NY, USA. His expertise lies in cancer research and the development of therapeutics. He is a past President of the American Association of Cancer Research, Chairman of the National Programme Committee of the XIIIth International Cancer Congress, Member of the U.S. National Cancer Advisory Board, Co-chairman of the First Joint AACR/JCA Symposium, Chairman of the Pezcoller
86
Symposia Committee and chairman or member of multiple international committees. He was also Assistant Professor in the Department of Pharmacology in the University of Milan. At present Dr. Mihich is a member of the Board of Scientific Advisors (BSA) of the National Cancer Institute (USA) and a member of the Council of the International Union Against Cancer (UICC). Dr. Mihich received his M.D. from the University of Milan in 1951, a Docent in Pharmacology from the University of Milan in 1962 and a Doctor of Medicine,Honoris Causa, from the University of Marseille (1966). Dr. Mihich has authored over 400 scientific publications and is a member of the editorial board of twelve peer-reviewed journals.
Professor Karol Sikora M.D. M.A., M.B., Ph.D., FRCR, FRCP, FFPM is currently Global Clinical Expert—Cancer at AstraZeneca. He is also Visiting Professor of Cancer Medicine and honorary Consultant Oncologist at Imperial College School of Medicine, Hammersmith Hospital, London. He has been Clinical Director for Cancer Services at Hammersmith since 1990 where he established a cancer research laboratory funded by the Imperial Cancer Research Fund (ICRF). He became Deputy Director (Clinical Research) of the ICRF and Special Adviser to HCA Healthcare (private hospital group). From 1997 to 1999 he was Chief of World Health Organization's Cancer Programme in Lyon and from 1999 to 2001, he was Vice President, Global Clinical Research (Oncology) at Pharmacia. He did his clinical training in the United Kingdom and was a registrar in oncology at St Bartholomew's Hospital, London. He obtained his Ph.D. at the MRC Laboratory for Molecular Biology in Cambridge working with Sydney Brenner and spent a year as a clinical fellow at Stanford University, California. He has published over 300 papers and written or edited 17 books. He is on the editorial board of several journals and is the founding editor ofGene Therapy andCancer Strategy. He is a former member of the U.K. Health Department's Expert Advisory Group on Cancer (the Calman-Hine Committee), the Committee on Safety of Medicines and remains an adviser to the World Health Organization Cancer Programme.
Board of Directors
Our board of directors currently consists of seven directors, including four non-executive directors, of which one is non-executive chairman. Under our articles of association, the directors (other than alternate directors) shall not, unless otherwise determined by an ordinary resolution of the Company, be less than 5 nor more than 11 in number. A director need not be a member of the Company. There is no age limit for directors.
Our board of directors are given the powers to manage our business and to use all the powers stated in our memorandum and articles of association.
At each annual general meeting one third of our directors who are subject to retirement by rotation or, if their number is not an integral multiple of three, the nearest to one third but not exceeding one third shall retire from office. If there are fewer than three directors who are subject to retirement by rotation, they will retire. The directors to retire by rotation shall include any director who wishes to retire and has not offered himself for reappointment and (to the extent that the number of directors required to retire exceeds the number of directors so retiring) shall be the directors who have been longest in office since their last appointment or reappointment. Any director who would not otherwise be required to retire by rotation at the third annual general meeting after his last appointment or any director aged 70 or over shall retire from the office by rotation but shall be eligible for reappointment. Any retiring director shall be eligible for reappointment. In addition, all directors appointed by the board since the last general meeting are subject to election by shareholders at the first annual general meeting following their appointment.
We regard Professor Gordon McVie and Dr. David U'Prichard as independent non-executive directors within the meaning of "independent" as defined in the U.K. Combined Code.
The senior independent director is Dr. David U'Prichard.
87
The following executive directors have service agreements with the Company which are dated July 1, 2004 and are effective from admission to the Official List of the U.K. Listing Authority as follows:
Spiro Rombotis is the Company's Chief Executive Officer. His service agreement is terminable by either party giving 12 months' notice in writing. If he leaves the Company as a good leaver within the first 12 months following admission and at the end of his notice period, despite his best endeavours he has failed to secure alternative employment reasonably suitable to his abilities and experience, the Company will pay him 12 monthly payments equivalent to one twelfth of his annual salary and benefits as at the date of termination of his employment. Therefore, for the first 12 months following admission the terms of his notice period will not comply with the provisions of the U.K. Combined Code, which requires that notice or contract periods for directors should be one year or less. He receives an annual salary of £210,000 ($380,016). At the Company's absolute discretion, he is entitled to an annual bonus. On the achievement by the Company of a listing on the London Stock Exchange or similar major stock market he is entitled to a bonus of £100,000 ($180,960). This bonus payment is in addition to his entitlement under the Senior Executive Incentive Plan. He is entitled to membership in a medical insurance scheme (providing cover for his spouse and children under 18), permanent health insurance, life insurance and directors liability insurance. In addition he receives an annual car allowance of £9,000 ($16,396). He is eligible to join the Company's Group Personal Pension Scheme under which the Company matches individual contributions up to a maximum of six percent of salary subject to statutory limits. He is entitled to 25 days' holiday in addition to such statutory or public holidays as the Company may specify each year.
Paul McBarron is the Company's Chief Financial Officer (Principal Financial and Accounting Officer) and the Company Secretary. His service agreement is terminable by either party giving six months' notice in writing. He receives an annual salary of £142,293 ($257,493). At the Company's absolute discretion, he is entitled to an annual bonus. On the achievement by the Company of a listing on the London Stock Exchange or similar major stock market he is entitled to a bonus of £50,000 ($90,480). This bonus payment is in addition to his entitlement under the Senior Executive Incentive Plan. He is entitled to membership in a medical insurance scheme (providing cover for his spouse and children under 18), permanent health insurance, life insurance and directors' liability insurance. In addition he receives an annual car allowance of £8,000 ($14,477). He is eligible to join the Company's Group Personal Pension Scheme under which the Company matches individual contributions up to a maximum of six percent of salary subject to statutory limits. He is entitled to 25 days' holiday in addition to such statutory or public holidays as the Company may specify each year.
Dr. Robert Jackson is the Company's Chief Scientific Officer. His service agreement is terminable by either party giving six months' notice in writing. He receives an annual salary of £137,173 ($248,228). At the Company's absolute discretion, he is entitled to an annual bonus. He is entitled to membership in a medical insurance scheme (providing cover for his spouse and children under 18), permanent health insurance, life insurance and directors liability insurance. In addition he receives an annual car allowance of £8,000 ($14,477). He is eligible to join the Company's Group Personal Pension Scheme under which the Company matches individual contributions up to a maximum of six percent of salary subject to statutory limits. He is entitled to 25 days' holiday in addition to such statutory or public holidays as the Company may specify each year.
The following non-executive directors have agreed terms of appointment with the Company which are dated July 1, 2004 and are effective from admission to the Official List of the U.K. Listing Authority as follows:
Sir John Banham is the Company's non-executive Chairman. His appointment letter states that he will be appointed for an initial period of three years, although his appointment may be terminated by either party giving the other three months' notice in writing. He is required to provide his services to
88
the Company for 24 days per year. He receives an annual fee of £25,000 ($45,240). His reasonable expenses are reimbursed by the Company.
Dr. David U'Prichard is an independent non-executive director of the Company. His appointment letter states that he will be appointed for an initial period of three years, although his appointment may be terminated by either party giving the other one months' notice in writing. He is required to provide his services to the Company for 10 days per year. He receives an annual fee of $17,500 and in addition, he receives $750 for attendance in person at each Board meeting and $375 for telephone attendance at each Board meeting and committee meeting that he is required to attend that is not on the same date as a Board meeting. His reasonable expenses are reimbursed by the Company.
Professor Sir David Lane is a non-executive director of the Company. His appointment letter states that he will be appointed for an initial period of two years, although his appointment may be terminated by either party giving the other one month's notice in writing. He is required to provide his services to the Company for 12 days per year. He receives an annual fee of £10,000 ($18,096) and in addition, he receives £330 ($597) for attendance in person at each Board meeting and £165 ($299) for telephone attendance at each Board meeting and committee meeting that he is required to attend that is not on the same date as a Board meeting. His reasonable expenses are reimbursed by the Company. Professor Lane has also entered into an agreement effective June 1, 2004 to provide consultancy services to the Company for an initial period of six months. His consultancy agreement is terminable by either party giving three months' notice in writing. He is required to provide consultancy services to the Company for a minimum of 39 days during the term of his engagement. He receives a fee of £555 ($1,004) per day and his reasonable expenses are reimbursed by the Company.
Professor Gordon McVie is a non-executive director of the Company. His appointment letter states that he will be appointed for an initial period of three years, although his appointment may be terminated by either party giving the other one month's notice in writing. He is required to provide his services to the Company for 12 days per year. He receives an annual fee of £10,000 ($18,096) and in addition, he receives £330 ($597) for attendance in person at each Board meeting and £165 ($299) for telephone attendance at each Board meeting and committee meeting that he is required to attend that is not on the same date as a Board meeting. His reasonable expenses are reimbursed by the Company.
U.S. dollar amounts are presented solely for your convenience in this section at the rate of £1.00 = $1.8096, the noon buying rate in The City of New York for cable transfers in pounds sterling as certified for customs purposes by the Federal Reserve Bank of New York on June 30, 2004. These translated amounts should not be construed as representations that the pounds sterling amounts could have been, or could in the future be, converted into U.S. dollars at this or any other exchange rate.
Indemnification of Directors and Officers
Section 310 of the U.K. Companies Act prohibits a company from exempting any of its officers from or indemnifying its officers, including directors, for acts of negligence, default, breach of duty or breach of trust in relation to the Company of which he may be guilty and any such indemnity is void and unenforceable. Section 310(3) of the U.K. Companies Act permits a company, inter alia, to purchase and maintain for any such officer insurance against any such liability. As permitted under English law, our articles of association provide that each director, secretary and officer shall be indemnified against liability incurred by him in the execution or discharge of his duties or purported execution or discharge of his duties or in connection with his duties, powers or office but the indemnity does not apply to the extent it is recovered from any other person and is subject to such officer taking all reasonable steps to effect such recovery to the extent that the indemnity shall not apply where an alternative right of recovery is available and capable of being enforced. We have also obtained policies of directors' and officers' liability insurance that insures our directors and executive officers against the cost of defense, settlement or payment of a judgment under certain circumstances.
89
Under English law, each director must act only honestly and in good faith in what he considers are our interests as a whole and not for any collateral purpose or for his own purposes, balancing the interests of present and future shareholders. Our directors must also consider the interests of our employees. The general law in England requires that each director must refrain from putting himself in a position in which his duty to us and his personal interests may conflict. Each director has a statutory duty to disclose to our board of directors any interests he has in a contract or proposed contract of ours. Each director must not misuse his powers or the opportunities of his position to benefit himself, except with our shareholders' knowledge and consent given in a general meeting. A director is, therefore, liable to pay to us any undisclosed profit he may make as a result of his office. It is immaterial that we ourselves could not have obtained the profit. The English common law duty of skill and care requires that directors (both executive and non-executive) exhibit the skill and care which may reasonably be expected from persons with their knowledge and experience.
Committees of the Board of Directors/Corporate Governance
The Combined Code, the U.K. code of corporate governance, is not legally binding but contains principles and provisions relating to corporate governance with which U.K. listed companies are generally expected to comply. The UK listing rules require that companies include in their annual reports a statement explaining how they apply the principles contained in the Combined Code and to confirm that they comply with the Combined Code, or provide an explanation where they do not so comply.
Following admission of our shares, we intend to comply with the requirements of the Combined Code and to put in place the procedures required to comply with the internal control aspects of the Combined Code. This will include the appointment of another independent non-executive director in due course, following which the composition of the various board committees, as described below, will be reviewed. Save as mentioned below, we are satisfied that we currently comply with the requirements of the Combined Code.
Our board of directors currently has an Audit Committee, a Nomination Committee and a Remuneration Committee. Each committee is comprised of three non-executive directors who have been delegated duties and responsibilities. Mr. Rombotis and Mr. McBarron attend meetings of the various committees as appropriate.
The audit committee
The audit committee is made up of three members who are all non-executive directors and includes one member with recent and relevant financial experience. It will normally meet four times a year. The audit committee is chaired by Dr. David U'Prichard and is comprised of Professor Gordon McVie and Sir John Banham. The primary responsibilities of the audit committee are to ensure that our financial performance is properly measured and reported and to review reports prepared by our auditors that relate to the Company's accounting and internal controls. Our auditors may be invited to attend audit committee meetings. The ultimate responsibility for reviewing and approving the annual report and accounts remains with the board of directors.
The nomination committee
The nomination committee is made up of four members, three non-executive directors and the CEO. The nomination committee is comprised of Sir John Banham, Professor Sir David Lane, Professor Gordon McVie and Mr. Rombotis. It will normally meet twice a year. The primary responsibilities of the nominations committee are to identify and recommend nominees for election to the board of directors, oversee the evaluation of our board and recommend to the board appropriate corporate governance standards.
90
The remuneration committee
The remuneration committee is made up of three members who are all non-executive directors. The remuneration committee is chaired by Sir John Banham and is comprised of Professor Gordon McVie and Dr. David U'Prichard. It will normally meet once a year. The primary responsibilities of the remuneration committee are to review the performance of the Executive Directors and to set their remuneration and other terms of their employment.
Compliance with the Combined Code
The Combined Code requires that at least half of the Board, excluding the chairman, should comprise independent non-executive directors. In addition, the Combined Code requires that the Remuneration and Audit Committees should comprise at least three non-executive directors, all of whom are independent, and that a majority of independent non-executive directors should sit on the Nomination Committee. Only Professor Gordon McVie and Dr. U'Prichard will be independent non-executive directors for the purpose of the Combined Code.
The Combined Code also requires that the chairman of the Board should be independent. Due to his holding of options over ordinary shares of the Company as described under "Employee Share Option Plans" on page 91 of this document, Sir John Banham is not considered to be independent for the purpose of the Combined Code.
The Combined Code requires that notice or contract periods for directors should be one year or less. Although the service agreement of Spiro Rombotis is terminable by either party giving 12 months' notice, it provides that, in certain circumstances, he is entitled to an additional 12 months' salary and benefits if his employment is terminated by the Company before the first anniversary of admission of the shares (see page 86 of this document).
The Sarbanes-Oxley Act of 2002 and the Nasdaq National Market Marketplace Rules
The Sarbanes-Oxley Act of 2002, as well as related new rules subsequently implemented by the SEC, require foreign private issuers, such as us, to comply with various corporate governance practices. In addition, Nasdaq has recently adopted amendments to its requirements for companies that are listed on the Nasdaq National Market. We intend to take all actions necessary for us to maintain compliance with applicable corporate governance requirements of the Sarbanes-Oxley Act, rules adopted by the SEC and listing standards of Nasdaq. We have received an exemption from Nasdaq in respect of the quorum requirements required by the Nasdaq Marketplace rules. We believe that the quorum requirements as provided in our articles of association are in line with common practice for most listed companies incorporated in England and Wales.
Board action and powers
The board of directors may exercise all the powers of the Company to pay, provide or procure the grant of pensions or other retirement or superannuation benefits and death, disability or other benefits, allowances or gratuities to any person who is or has been at any time a director of the Company or in the employment or service of the Company or of any company which is or was a subsidiary of or associated with the Company or the predecessors in business of the Company or any subsidiary or associated company or the relatives or dependants of any such person. For that purpose the board of directors may procure the establishment and maintenance of, or participate in, or contribute to any non-contributory or contributory pension or superannuation fund, scheme or arrangement or pay any insurance premiums.
Subject to any applicable statutory provisions, a director shall not be disqualified by his office from entering into any contract with the Company, either with regard to his tenure of any office or position in the management, administration or conduct of the business of the Company, or as vendor, purchaser
91
or otherwise. A director may hold and be remunerated in respect of any other office or place of profit with the Company (other than the office of auditor of the Company) in conjunction with his office as director and he (or his firm) may also act in a professional capacity for the Company (except as auditor) and may be remunerated for it.
A director who to his knowledge is in any way, whether directly or indirectly, interested in a contract with the Company shall declare the nature of his interest at a meeting of the directors.
A director shall not vote or be counted in the quorum at a meeting in respect of any resolution concerning his own appointment (including fixing or varying its terms), or the termination of his own appointment, as the holder of any office or place of profit with the Company or any other undertaking in which the Company is interested but, where proposals are under consideration concerning the appointment (including fixing or varying its terms), or the termination of the appointment, of two or more directors to offices or places of profit with the company or any undertaking in which the Company is interested, those proposals may be divided and considered in relation to each director separately; and in such case each of the directors concerned (if not otherwise debarred from voting under the articles of association) shall be entitled to vote and be counted in the quorum in respect of each resolution except that concerning his own appointment or the termination of his own appointment.
A director shall not vote (or be counted in the quorum at a meeting) in respect of any contract in which he has an interest which (together with any interest of a connected person) is to his knowledge a material interest. Notwithstanding the above, a director shall be entitled to vote, and be counted in the quorum, on: (A) any contract in which he is interested by virtue of an interest in shares, debentures or other securities of the Company or otherwise in or through the Company; (B) the giving of any guarantee, security or indemnity in respect of money lent or obligations incurred by him or by any other person at the request of, or for the benefit of, the Company or any of its subsidiary undertakings; or a debt or obligation of the Company or any of its subsidiary undertakings for which he himself has assumed responsibility under a guarantee or indemnity or by the giving of security; (C) any issue or offer of shares, debentures or other securities of the company or any of its subsidiary undertakings in respect of which he is or may be entitled to participate in his capacity as holder of any such securities or as an underwriter or sub-underwriter; (D) any contract concerning another company in which he and any connected person do not to his knowledge hold an interest in shares, within the meaning of sections 198 to 211 of the U.K. Companies Act, representing one per cent. or more of the issued shares of any class of such company or of the voting rights of that company; (E) any arrangement for the benefit of employees of the company or any of its subsidiary undertakings which does not accord to him any privilege or benefit not generally accorded to the employees to whom the arrangement relates; and (F) the purchase or maintenance of insurance for the benefit of directors or for the benefit of persons including directors.
Employee Share Option Plans
Introduction
Prior to 2004, Cyclacel Limited had adopted two employee share option plans which operate as incentive plans for our directors and employees. The two plans comprise (1) the Cyclacel Limited Share Option Plan, adopted on May 19, 1997, and (2) the Cyclacel Limited 2000 Employees' Share Option Scheme, together, the Cyclacel Limited Share Plans, which is an enterprise management incentive scheme attracting favorable tax treatment under Schedule 5 to the U.K. Income Tax (Pensions & Earnings) Act 2003. The plans are designed to incentivize employees and directors as part of their remuneration arrangements. Following the reorganization, options granted under the Cyclacel Limited Share Option Plan and the Cyclacel Limited 2000 Employee's Share Option Scheme now operate over shares in Cyclacel Group plc.
92
On April 23, 2004, new options over 1,722,770 ordinary shares were granted under the Cyclacel Limited Share Plans to employees at an exercise price of £1.50 per share. Prior to the grant of these new options, 598,692 existing options with higher exercise prices were surrendered by these employees. As adjusted to reflect the bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 115, there will be options outstanding under these plans over 3,593,640 ordinary shares at an exercise price of £0.75 per share. The options are exercisable as set out in the description of the Cyclacel Limited Share Plans set out below. The total number of shares under option as at (including those pursuant to the Cyclacel Limited Share Plans and the Senior Executive Incentive Plan described below) is 7,135,446 (as adjusted to reflect the bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 115).
The following table provides summary information for each of our directors who hold options to purchase ordinary shares as at June 30, 2004, as adjusted to reflect the bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 115. The options were granted without any payment being required.
| | Date of grant
| | Number of Shares
Underlying
Options Held
| | Exercise Price
per Share
(£)
| | Earliest Possible Option Exercise Date(1)
| | Option Expiration
Date
|
|---|
| Spiro Rombotis | | April 23, 2004 | | 377,504 | | 0.75 | | April 23, 2005 | | April 23, 2014 |
| | | | | (133,000 EMI & 244,504 unapproved) | | | | | | |
Dr. Robert Jackson |
|
April 23, 2004 |
|
274,400 |
|
0.75 |
|
April 23, 2005 |
|
April 23, 2014 |
| | | | | (133,000 EMI & 141,400 unapproved) | | | | | | |
Paul McBarron |
|
April 23, 2004 |
|
230,000 |
|
0.75 |
|
April 23, 2005 |
|
April 23, 2014 |
| | | | | (133,000 EMI & 97,000 unapproved) | | | | | | |
Sir John Banham |
|
April 23, 2004 |
|
120,000 |
|
0.75 |
|
April 23, 2005 |
|
April 23, 2014 |
| | | | | (All unapproved) | | | | | | |
Sir David Lane |
|
April 23, 2004 |
|
151,876 |
|
0.75 |
|
April 23, 2005 |
|
April 23, 2014 |
| | | | | (All unapproved) | | | | | | |
- (1)
- Although the earliest possible option exercise date is April 23, 2005, only one third of the options will become exercisable on each anniversary of date of grant.
Senior Executive Incentive Plan
In addition to the options disclosed in the table above, Spiro Rombotis, our chief executive officer, and Paul McBarron our chief financial officer, have been granted options to acquire additional ordinary shares pursuant to our Senior Executive Incentive Plan. We refer to this option in the discussion below as the Incentive Plan.
Mr. Rombotis joined us as chief executive in 1997. At that point we had not yet commenced operations, and it was Mr. Rombotis, together with our founder Professor Sir David Lane, who developed the business plan and strategy which we continue to follow today. The key terms of the Incentive Plan were agreed as part of Mr. Rombotis's original contract of employment, and reflected in an appendix to it, in anticipation of the critical role that he would play in the formative years and subsequent development of our company.
In the context of this offering, the Remuneration Committee has amended the terms of Mr. Rombotis's employment agreement and granted the Incentive Plan. The Incentive Plan is intended to reflect the substance of the intentions of the company in 1997 and to incentivize Mr. Rombotis to
93
remain with us and contribute to the development of our business in the years following the completion of the offering.
The following is a description of the principal terms of the Incentive Plan:
- •
- Under the Incentive Plan, Mr. Rombotis has been granted a right to acquire 3,441,806 ordinary shares at an exercise price of 1 pence per share.
- •
- Options granted under the Incentive Plan are only exercisable by Mr. Rombotis following successful completion of this offering or another listing of part of the ordinary share capital of Cyclacel Group plc on a major stock exchange (including the London Stock Exchange and Nasdaq) or on a sale or change of control of Cyclacel Group plc prior to such a listing. The Incentive Plan options will only be exercisable in such circumstances if Mr. Rombotis remains employed by us and has not given or been given notice to leave at the time of exercise, unless Mr. Rombotis has been dismissed without cause.
- •
- Provided that Mr. Rombotis remains employed by us, the Incentive Plan options will become exercisable in equal tranches on the first, second and third anniversaries of the closing of this offering. Exercise of the Incentive Plan options is not conditional on the satisfaction of performance conditions. The Incentive Plan options will also be exercisable in full on any change of control of Cyclacel Group plc pursuant to a general offer following the closing of this offering.
- •
- The Incentive Plan options will lapse on the earlier of Mr. Rombotis ceasing to be employed by us (unless he has been dismissed without cause) and the tenth anniversary of their grant in June 2004.
- •
- The Remuneration Committee, which has responsibility for administering and interpreting the Incentive Plan, may at any time amend any provisions of the Incentive Plan provided always that, where an amendment is made to the advantage of Mr. Rombotis, it will be subject to prior approval by the shareholders of Cyclacel Group plc by ordinary resolution in general meeting. The requirement to obtain the prior approval of the shareholders will not, however, apply in relation to any amendment which is minor in nature or is made to assist with the administration of the Incentive Plan or to obtain or maintain a particular tax, exchange control or regulatory treatment for Mr. Rombotis or Cyclacel Group plc or any affiliated company.
Also pursuant to the Senior Executive Incentive Plan, in addition to the options disclosed in the table above, Paul McBarron, our chief financial officer, has been granted an option over 100,000 ordinary shares at an exercise price of £0.75 per share. The principal terms of this option as applied to Mr. McBarron are substantially the same as those of the Incentive Plan as applied to Mr. Rombotis.
Cyclacel Share Option Plan (Option Plan)
Administration
The Option Plan is administered by the directors in accordance with the terms set out below.
Eligibility
Participation in the Option Plan is open to our employees and directors selected by the directors.
Terms of options
Options to acquire our ordinary shares under the Option Plan may be granted by the directors (by resolution) on dates determined by the directors. The exercise of options may be made subject to such
94
performance or other conditions as the directors may determine. Some of the options have been granted subject to operational performance targets linked to drug discovery and development.
Options may not be transferred, assigned or charged (other than a transfer to the optionholder's personal representatives on death).
Options may be satisfied using newly issued or existing ordinary shares. Participation in the Option Plan will not form part of, or affect a participant's rights under, the terms of his employment.
Option exercise price
Options granted under the Option Plan by the directors are exercisable at the price determined by the directors, which will not be less than the nominal value of a share.
Individual limit on participation
There is no limit under the Option Plan on the aggregate maximum value of options which may be granted to a participant.
Overall limit on our ordinary shares to be made available under the Option Plan
The maximum number of our ordinary shares which may be under option at any time during the life of the Option Plan is limited to 10 percent of the ordinary share capital of the Company in issue from time to time.
Exercise of options
One third of the options granted under the Option Plan will become exercisable on each anniversary of the date of grant, subject to the satisfaction of performance targets.
Taxation
The participant shall reimburse the Company against any tax arising in respect of the exercise of the option. If payment is not made, the Company may recover the tax from the participant in such manner as the directors think fit.
Termination of employment
If a participant ceases employment with us voluntarily or he is dismissed for cause, his options will normally lapse on the date of cessation of employment.
Where a participant ceases employment with us due to death, injury, disability, ill-health, redundancy, retirement at contractual retirement age (including late retirement), early retirement or by agreement with his employer or, if the directors so determine at the appropriate time, the sale of the business or subsidiary for which the participant works, or for any other reason in the absolute discretion of the directors, options will be exercisable at the date of cessation of employment and will become exercisable for a period of six months (12 months in the case of death). Following the expiry of the relevant period, all of a participant's unexercised options will lapse.
Lapse of options
Options will lapse in any event on the tenth anniversary of their grant and will lapse after a takeover or reconstruction when the specified time periods for exercise have passed as described below. Options will also lapse on the date on which a resolution is passed, or an order made by the court, for the compulsory winding-up of the Company or the date on which the participant becomes bankrupt or does or attempts or omits to do anything as a result of which he is deprived of the legal or beneficial
95
ownership of the option. Finally, options lapse on the cessation of employment of a participant as described above.
Rights attaching to ordinary shares on the exercise of options
Ordinary shares issued under the Option Plan will carry the same rights as all other issued ordinary shares, except they will not rank for any dividend or other rights declared by reference to a record date preceding the date of exercise.
Takeover, reconstruction or winding-up
If any person obtains control of us as a result of making an offer to acquire the whole of our issued share capital, or, having control, makes a general offer to acquire all the shares other than those already owned by that person, options under the Option Plan will become exercisable in full.
Participants will have six months within which to exercise their options, following the change of control, or general offer; thereafter, the options will lapse. If a person becomes bound or entitled to give notice to acquire ordinary shares under sections 428 to 430F of the U.K. Companies Act, a participant may exercise his options during the period when that person remains so bound or entitled; thereafter, they will lapse. Finally, if there is a change of control in pursuance of a compromise or arrangement sanctioned by the court under the U.K. Companies Act or the court sanctions a scheme of arrangement (without there being a change of control), then options may be exercised from the date on which the court sanctions such arrangement and ends six months later, or, if earlier, the day immediately preceding the date upon which the scheme becomes effective; thereafter, they will lapse.
If a resolution is passed for the voluntary winding-up of the Company, an option may be exercised during the period of six months starting on the commencement of such winding-up provided that any issue of shares following such exercise is authorized by the liquidator or the court (if appropriate).
In all cases, the directors will not permit the exercise of options unless the performance target (which may have been imposed at the time of grant) has been satisfied. Participants may, in certain circumstances, be given the opportunity to exchange their options for equivalent options over ordinary shares in an acquiring company. Any new option will be exercisable in the same manner and subject to the same provisions of the Option Plan.
Finally, in respect of options granted on or after April 23, 2004, if an acquiring company makes an offer pursuant to which the whole of the issued share capital of the Company will be acquired in circumstances where the Company's shareholders become the only shareholders of the acquiring company, the option shall be deemed to have been released in consideration for the grant of a new option at the date on which the acquiring company obtains control.
Adjustment of options
If there is a variation in our share capital, including by way of a capitalization, rights issue, consolidation, sub-division, reduction or any other variation which would materially affect the value of options under the Option Plan, the directors may, subject to the auditors' approval (save in the case of a capitalization issue), make the adjustments it considers appropriate to the number of ordinary shares under option and the exercise price.
Amending the Option Plan
The rules of the Option Plan may be amended at any time by our directors, provided that no amendment to the Option Plan can be made without prior approval in a general meeting of shareholders if the amendment is to the advantage of optionholders, unless the amendment is necessary or desirable in order to comply with the provisions of any proposed or existing legislation, or to obtain
96
or maintain favorable taxation, exchange control or regulatory treatment of any participating company, group member or participant or is minor in nature and is made to benefit the administration of the Option Plan.
In addition, no amendment that would materially prejudice the interests of existing participants may be made unless the directors have invited every option holder to give an indication of whether or not he approves the amendment and that amendment is approved by a majority of those optionholders who have given such an indication.
Cyclacel 2000 Employees' Share Option Scheme (EMI Scheme)
The EMI Scheme is a tax advantaged scheme designed to help us recruit and retain employees with the skills that will help us grow and succeed.
Tax advantaged options over shares with a market value of up to £100,000 may be granted to any number of employees, subject to a maximum share value of £3 million under option to all employees. The grant of the option is tax-free and there will normally be no tax or social security contributions for the employee to pay when the option is exercised.
Administration
The EMI Scheme is administered by the remuneration committee in accordance with the terms set out below where the options have been granted by the Company, and by the trustees (of any employee benefit trust established by the Company) (the "Trustees") where they have granted options.
Eligibility
Participation in the EMI Scheme is open to our employees and directors. Participation in the EMI Scheme will not form part of, or affect a participant's rights, under the terms of his employment.
An option may only be granted if the following conditions (as required by the legislation) are satisfied at the date of grant:
- •
- as a result of the grant of the option, there are no more that the maximum number of individuals specified by the legislation holding options;
- •
- the Company is a "qualifying company" under the legislation; and
- •
- the option is granted for commercial reasons in order to recruit or retain employees, and not as part of a scheme or arrangement the main purpose, or one of the main purposes of which is the avoidance of tax.
Terms of options
Options to acquire our ordinary shares under the EMI Scheme may be granted, from time to time, by the remuneration committee or the Trustees. Some of the options have been granted subject to operational performance targets linked to drug discovery and development. Options are granted by way of an option certificate executed as a deed setting out the principal terms of the option.
Options may not be transferred, assigned or charged (other than a transfer to the optionholder's personal representatives on death).
Options may be satisfied using newly issued or existing ordinary shares.
Option exercise price
Options granted under the EMI Scheme will be exercisable for a price as determined by the remuneration committee, but, in the case of an option which will be satisfied by the issue of new shares
97
it will not be less than the nominal value of a share unless those shares will be issued to the Trustees for the satisfaction of the option.
On the grant of an option, the grantee (remuneration committee or Trustee) may impose a performance target or any further condition on the exercise of the option. Such target must be objective, must be capable of being fulfilled with a period of ten years from the date of grant and such that, once satisfied, the exercise of the option is not subject to the discretion of any person. Details of the target must be set out, or attached to, the option certificate. Where the target is deemed to be inappropriate, it may be substituted, varied or waived as is reasonable in the circumstances and produces a fairer measure of performance which is neither materially more nor less difficult to satisfy.
Individual limit on participation
Options with a market value of up to £100,000 may be granted to a participant under the EMI Scheme.
Overall limit on our ordinary shares to be made available under the EMI Scheme
The maximum number of our unissued ordinary shares which may be placed under option under the EMI Scheme or any other employees' share plan in any ten-year period is limited to 15 percent of the ordinary share capital of the Company in issue from time to time. Any shares which the Trustees have purchased (or determined that they will purchase) to satisfy an option will be disregarded, together with any option which has lapsed, been renounced or otherwise become incapable of exercise. Any shares issued on exercise will be taken into account only once (when the option is granted) and will not fall out of account when the option is exercised.
Exercise of options
Options granted under the EMI Scheme may be exercised in whole or in part. An option may not be exercised earlier than the latest of:
- •
- the date specified in the option certificate; and
- •
- the date on which it is determined that the performance target has been satisfied or waived.
However, no option may be exercised more than ten years after its date of grant.
Taxation
Although the grant and exercise of the option is normally tax-free, the exercise of an option is conditional upon a participant agreeing to comply with any arrangements specified by us for the payment of taxation, employee and employer social security contributions or any other deductions at source which may arise.
Termination of employment
If a participant ceases employment with us voluntarily or he is dismissed for cause, his options will normally lapse on the date of cessation.
However, where a participant ceases employment with us due to death, injury, disability, ill-health, redundancy, retirement at the date at which he is bound or entitled to retire under the terms of his contract of employment or early retirement by agreement with the company by which he is employed, or the sale of the business or subsidiary for which the participant works, or for any other reason in the absolute discretion of the remuneration committee, he will be entitled to exercise his option during the period ending on the earlier of six months after the date of cessation of employment and the tenth
98
anniversary of the date of grant, 12 months in the case of death. Following the expiry of the relevant period, all of a participant's unexercised options will lapse.
Disqualifying events
If a disqualifying event occurs, such that the EMI Scheme no longer meets the requirements of the legislation, the remuneration committee may permit a participant to exercise his option during the period ending 40 days after the occurrence of the disqualifying event. To the extent it is not exercised, it will remain exercisable, but will no longer qualify for favorable tax treatment.
Lapse of options
Options will lapse in any event on the tenth anniversary of their grant and will lapse after a takeover or reconstruction when the specified time periods for exercise have passed as described below. Options will also lapse on the date on which a resolution is passed or an order made by the court for the compulsory winding up of the Company. Finally, options will lapse on the cessation of employment of a participant as described above.
Rights attaching to ordinary shares on the exercise of options
Ordinary shares allotted under the EMI Scheme will carry the same rights as all other issued ordinary shares.
Takeover, reconstruction or winding-up
If any person obtains control of us as a result of making a general offer to acquire the whole of our issued share capital, or all the shares of the same class as the EMI Scheme shares, options under the EMI Scheme will become exercisable for a period of six months to the extent that any performance conditions have been satisfied immediately prior to a change of control; thereafter, the options will lapse (unless the remuneration committee determines otherwise).
If a person becomes bound or entitled to give notice to acquire ordinary shares under sections 428 to 430F of the U.K. Companies Act, a participant may exercise his options, to the extent that any performance conditions have been satisfied at the date on which the person making the offer has obtained control, during the period from when the person serves a notice under section 429 of the U.K. Companies Act and ending on the day on which that person ceases to be entitled to serve such a notice. If not exercised, the options will lapse, unless the remuneration committee determines otherwise.
If a person proposes to obtain control of the Company in pursuance of a compromise or arrangement sanctioned by the court under the U.K. Companies Act all options may be exercised (subject to any performance target having been satisfied), conditionally on the compromise or arrangement becoming effective, from the date of the meeting of the members of the Company ordered by the court for a period of six months, or, if earlier, on the day immediately preceding the date on which the scheme becomes effective. To the extent options are not exercised, they will lapse. Options are also exercisable (on the same terms) where the court sanctions a scheme of arrangement (without any person obtaining control).
If notice is given of a resolution for the voluntary winding-up of the Company, options may be exercised conditionally on the passing of the resolution (subject to the satisfaction of any performance targets), at any time during the period beginning with the date the notice is given and ending seven days before the resolution is passed or defeated or the general meeting is concluded or adjourned indefinitely. To the extent not exercised, options will lapse.
99
In addition to the exercise provisions set out above, the board also has a discretion to permit exercise of all outstanding options (subject to any performance target having been satisfied) during a limited period as specified by them, on the making of a general offer for us or an offer to acquire the whole or substantially the whole of our business. To the extent options are not exercised, they will lapse.
Participants may, in certain circumstances, be given the opportunity to exchange their options for equivalent options over ordinary shares in an acquiring company. Any new option will be exercisable in the same manner and subject to the same provisions of the EMI Scheme.
Finally, in respect of options granted on or after April 23, 2004, if an acquiring company makes an offer pursuant to which the whole of the issued share capital of the Company will be acquired in circumstances where the Company's shareholders become the only shareholders of the acquiring company, the option shall be deemed to have been released in consideration for the grant of a new option at the date on which the acquiring company obtains control.
Adjustment of options
If there is a variation in our share capital, including by way of a capitalization, rights issue, consolidation, sub-division, reduction or any other variation which would materially affect the value of options under the EMI Scheme, the administrator (remuneration committee or Trustee depending on who granted the options) may, subject to the auditors' approval, make the adjustments it considers appropriate to the number of ordinary shares currently in place under option and the exercise price.
Amending the EMI Scheme
The rules of the EMI Scheme can be amended at any time by our board, provided that no amendment to the EMI Scheme can be made without prior approval in a general meeting of shareholders if the amendment is to the advantage of optionholders, unless the amendment is necessary or desirable in order to comply with the provisions of any proposed or existing legislation, or to obtain or maintain favorable taxation, exchange control or regulatory treatment of any participating company, group member or participant or is minor in nature and is made to benefit the administration of the EMI Scheme. In addition, no amendment that would materially prejudice the interests of existing participants may be made except where it has been approved by them in such manner as would be required by our articles of association if the EMI Scheme shares had been issued or transferred to them (so that they became shareholders) and constituted a separate class of shares.
New Incentive Arrangements
The Company has also adopted the Cyclacel Group plc Senior Executive Incentive Plan, the Cyclacel Group plc Discretionary Share Option Plan (Approved and Unapproved Parts), the Cyclacel Group plc Savings Related Share Option Plan and the Cyclacel Group plc Restricted Share and Co-Investment Plan. We refer to these plans, together with a new employee benefit trust that has been set up to operate in conjunction with the Company's share plans, as the New Share Plans. The New Share Plans replace the Option Plan and the EMI Scheme. The Company's New Share Plans are designed to incentivize employees to align their interests with those of the shareholders and encourage them to own ordinary shares in the Company.
The principal terms of the Senior Executive Incentive Plan, under which Spiro Rombotis, our chief executive officer, and Paul McBarron, our chief financial officer, have been granted options, are set out on pages 92 and 93. The total number of shares which may be issued under the Senior Executive Incentive Plan is 3,541,806, as adjusted to reflect the bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 116.
The principal terms of the New Share Plans are set out below.
100
New Option Plan
The New Option Plan is administered by the remuneration committee.
There are two parts to the New Option Plan. Inland Revenue approval will be sought for the tax advantaged part which permits options over shares with a market value of up to £30,000 to be granted to each eligible employee. There will also be a part which is not designed to be tax advantaged; this part is intended to be used primarily where the Company wishes to grant to eligible employees options over more than £30,000 worth of the Company's ordinary shares. Except to the extent required to obtain tax approved status, the approved and the unapproved parts of the New Option Plan will be the same.
Eligibility
Selected employees and directors of the Company (or any participating subsidiary of the Company) who are required to devote substantially the whole of their working time to the business of the Company (or any subsidiary of the Company) will be able to participate in the New Option Plan at the discretion of the remuneration committee.
Grant of Options
Options may be granted within a period of 42 days following the date of formal approval of the tax advantaged part of the plan by the Inland Revenue, the day after the announcement of the Company's results for any period, the day immediately following any general meeting of the Company, the day following the lifting of any restrictions imposed by statute, order or regulation, the day on which any change to the legislation affecting discretionary share option plans is announced or made, or at such other exceptional times as determined by the remuneration committee.
Options may be granted over both the Company's newly issued ordinary shares and over ordinary shares purchased in the market.
No payment will be required for the grant of an option. Options are not pensionable benefits, are not transferable and may only be exercised by a person to whom they were granted or their personal representatives.
No options may be granted more than ten years after the adoption of the New Option Plan.
Option Exercise Price
The price per ordinary share payable upon the exercise of an option will not be less than the higher of:
- •
- the middle market quotation for an ordinary share as derived from the daily Official List of the London Stock Exchange for the dealing day immediately preceding the date the option is to be granted or, if so determined by the Board, the average of such quotations for the three dealing days immediately preceding the day the option is to be granted or the market value at such other times as agreed with the Inland Revenue; and
- •
- where ordinary shares are to be subscribed, their nominal value.
Limits on the issue of ordinary shares
The New Option Plan is subject to the following overall limits on the number of ordinary shares, which may be acquired by subscription:
- •
- in any ten year period not more than 10 percent of the issued ordinary share capital of the Company may in aggregate be issued or issuable pursuant to rights acquired under the New
101
Individual limit
It is intended that the aggregate maximum value of approved and unapproved options which may be granted each year will not normally exceed 100% of an employee's earnings (subject, in the case of approved options, to the statutory limit of £30,000) other than in exceptional circumstances at the discretion of the remuneration committee (in which case options over ordinary shares with a maximum value of 200% may be granted). For these purposes, an employee's earnings will be his salary current at the date of grant together with any bonus(es) paid in the previous year.
Exercise of options
An option will normally be exercisable between three and ten years following its grant provided that any specified performance target has been satisfied. The performance target will be challenging and will demonstrate the achievement of demanding and stretching financial performance.
The remuneration committee will regularly review the performance target for option grants to ensure they are appropriate for the Company and the prevailing recruitment market. The conditions may be varied in certain circumstances following the grant of an option so as to make it more fair and reasonable but not so as to make its achievement any more difficult to satisfy.
If a participant leaves employment, exercise will only be permitted, if the participant dies or leaves because of injury, disability, retirement, redundancy or on the undertaking or company which employs him leaving the group or in other circumstances at the discretion of the remuneration committee.
Early exercise will also be permitted in the event of a take-over, reconstruction or winding-up of the Company, subject to the achievement of the performance target measured to the date of the relevant event. Options may also, in these circumstances, subject to certain conditions, be exchanged for options over shares in the acquiring company.
Rights attaching to ordinary shares
Ordinary shares allotted or transferred under the New Option Plan will rank equally with all other ordinary shares of the Company for the time being in issue. The Company will apply to the UK Listing Authority for any ordinary shares allotted under the New Option Plan to be admitted to the official list of the London Stock Exchange and for shares to be admitted to trading to the exchange unless listing or admission to trading has already been granted.
Variation of Capital
In the event of any rights or capitalisation issue, subdivision, consolidation, reduction or other variation of share capital in respect of which the Inland Revenue will allow an adjustment, the Board may make (in respect of the approved part subject to receiving prior approval of the Inland Revenue) adjustments it considers appropriate to the number and/or the nominal value of ordinary shares over which an option is granted and the price payable on the exercise of options.
102
Alterations to the New Option Plan
The New Option Plan may at any time be amended by the Board in any respect, provided that the prior approval of the Company in general meeting is obtained for alterations and additions to the advantage of participants to the rules governing eligibility, limits on participation, rights attaching to options and shares, the determination of the option price and adjustment of options. The requirement to obtain the prior approval of shareholders will not, however, apply in relation to any alteration or addition which is minor in nature and made to benefit the administration of the New Option Plan to comply with the provisions of any existing or proposed legislation or to obtain or maintain favourable tax, exchange control or regulatory treatment for participants or group companies. No amendment can be made which would abrogate or materially affect adversely the option holders rights unless it is made with their written consent. Any amendments or additions to the New Option Plan which affects the terms on which approved options may be granted or exercised requires the prior approval of the Inland Revenue.
New SAYE Plan
Inland Revenue approval will be sought for the New SAYE Plan which is a tax-approved save-as-you-earn share option scheme.
Eligibility
Although the legislation governing the New SAYE Plan provides that all UK directors and employees of the Company and participating companies within the group with at least five years' service must be entitled to participate, the directors may also permit directors and employees with less than five years service to participate in the New SAYE Plan.
It is intended that the first invitations to apply for options under the New SAYE Plan will be made to all eligible directors and employees who have completed a minimum period of service (as decided by the Board) at that time.
The savings contract
To participate in the New SAYE Plan, an eligible employee must enter into a save-as-you-earn contract (the Savings Contract) with an appropriate body approved by the Company, under which the employee agrees to make monthly contributions of between £5 and £250 for a period of three, five or seven years.
Applications to participate in the New SAYE Plan may be scaled down if satisfying them in full would exceed the number of ordinary shares available for the grant of options.
Acquisition Price
Options to acquire shares under the New SAYE Plan will have an exercise price determined by the directors, which will not be less than the greater of:
- •
- 80 percent of the middle market quotation for such ordinary shares as derived from the daily Official List of the London Stock Exchange for the dealing day immediately preceding the date on which invitations to apply for options are issued to employees or, if so determined by the Board, 80 percent of the average of such quotations for the three dealing days immediately preceding the date on which invitations to apply for options are issued to employees, or 80 percent of the middle market quotation for such ordinary shares as derived from the daily official list of the London Stock Exchange at such other times as agreed with the Inland Revenue; and
103
- •
- where ordinary shares are to be subscribed, their nominal value subject to adjustment as provided in the New SAYE Plan.
Grant of options and issue of invitations
The number of ordinary shares over which options may be granted must as nearly as possible be equal to, but not more than, that number which may be purchased out of the repayment proceeds (including, where the director so allows, any bonus) of the relevant Savings Contract at the exercise price.
Options will be granted by the Board. It is intended that invitations to participate under the New SAYE Plan will be despatched normally within 42 days commencing on any of the following: the day on which the New SAYE Plan is approved by the Inland Revenue, the day after any announcement of the Company's results for any period, any day on which the Board resolves that exceptional circumstances exist which justify the grant of an option, any day on which any change to the legislation affecting savings related share option schemes is announced or made, the day immediately following general meeting of the Company or the day on which the ordinary shares are first listed. No options may be granted more than ten years after the adoption of the New SAYE Plan.
Exercise of options
An option will only normally be exercisable for a period of six months commencing on the date of completion of the related Savings Contract and, if not exercised by the end of that period, the option will lapse (although participants will be entitled to the return of their savings). Earlier exercise is, however permitted in specified circumstances, including a cessation of employment in certain circumstances, including injury, disability, redundancy, retirement, death or on the company which employs the participant leaving the group, and in the event of a take-over of the Company. Options may also, in these circumstances, subject to certain conditions, be exchanged for options over shares in the acquiring company.
Limits on the issue of ordinary shares
In any ten year period not more than 10 percent of the issued ordinary share capital of the Company may in aggregate be issued or issuable pursuant to rights acquired under the New SAYE Plan or any other employees' share scheme adopted by the Company.
For the purposes of this limit, options granted under the Senior Executive Incentive Plan shall not be included and options which lapse or have been released will cease to count.
Rights attaching to ordinary shares
Ordinary shares allotted or transferred under the New SAYE Plan will rank equally with all other ordinary shares of the Company for the time being in issue. The Company will apply to the U.K. Listing Authority for any ordinary shares allotted under the New SAYE Plan to be admitted to the official list of the London Stock Exchange and for shares to be admitted to trading on the exchange unless listing or admission to trading has already been granted.
Variation of Capital
In the event of any rights or capitalisation issue, subdivision, consolidation, reduction or other variation of share capital, the remuneration committee may make (subject to receiving prior approval of the Inland Revenue) adjustments it considers appropriate to the number and/or nominal value of ordinary shares subject to option and the price payable on the exercise of options.
104
Alterations to the New SAYE Plan
The New SAYE Plan may at any time be amended by the Board in any respect, provided that the prior approval of the Company in general meeting is obtained for alterations and additions to the advantage of participants to the rules governing eligibility, limits on participation, the rights attaching to options and shares, the determination of the option price and adjustment of options. The requirement to obtain the prior approval of shareholders will not, however, apply in relation to any alteration or addition which is minor in nature and made to benefit the administration of the New SAYE Plan to comply with the provisions of any existing or proposed legislation or to obtain or maintain favourable tax, exchange control or regulatory treatment for participants, or the Company or any of its subsidiaries or necessary to obtain Inland Revenue approval. No amendment can be made which would abrogate or materially affect adversely the option holders rights unless it is made with their written consent. Any amendments or additions to the New SAYE Plan which affect the terms on which options may be granted or exercised requires the prior approval of the Inland Revenue.
New Restricted Share and Co-Investment Plan
The New Restricted Share and Co-investment Plan, comprises two elements—matched share awards (Matched Awards) and restricted share awards (Restricted Awards). These elements are described below.
Matched Awards
Eligible employees will be invited to apply all or part of their annual bonus and/or invest their own funds in purchasing shares in the Company (Invested Shares). Following the purchase of Invested Shares, the employee will receive a Matched Award, being a grant of shares made on a one-to-one basis.
Nature of Awards
The Matched Awards will be in respect of shares in the Company and, subject to the exceptional circumstances referred to below, their vesting will be contingent upon the satisfaction of performance targets and continued employment with the Cyclacel group of companies (the Group).
Restricted Period
A Matched Award may not generally vest before the end of a three year period (the Relevant Period).
Performance
The performance target for Matched Awards will be challenging and will demonstrate the achievement of demanding and stretching financial performance over the relevant period.
Rights attaching to Cyclacel Group plc shares prior to the vesting of Matched Awards
A participant has no rights in relation to the Matched Award or to the Company's shares which are the subject of the award until it has vested. The number of shares in the Company under a Matched Award may, however, be increased during the Relevant Period by reinvesting dividends, or an amount equivalent to any dividends, paid on the Company's shares under the Matched Award.
Cessation of Employment before the End of the Relevant Period
If a participant ceases employment with the Company before the end of the Relevant Period, his Matched Awards will generally lapse. However, if a participant dies or leaves employment in certain
105
circumstances such as ill-health, injury, disability, retirement, redundancy or his employing business being sold or transferred outside the Company, the Matched Award will vest immediately. If a participant ceases to be employed by the Company for any reason, however, the Company has a discretion to vest or preserve all or part of his Matched Awards.
Transfer of Shares at the End of the Relevant Period
Once a participant's Matched Award has vested, Shares in the Company will be transferred to the participant as soon as practicable.
Restricted Awards
Restricted Awards are intended to be used as a means of incentivising executive directors and senior managers. The remuneration committee intends to grant Restricted Awards on an annual basis.
Nature of Awards
Restricted Awards will be in respect of the Company's shares and, subject to the exceptional circumstances referred to below, their vesting will be contingent on the satisfaction of a specified performance target and continued employment with the Cyclacel group.
Restricted Period
A Restricted Award may not generally vest before the specified performance requirement has been met at the end of a three year period (the Relevant Period).
Individual Limits
The upper limit for individual Restricted Awards will be set from time to time by the remuneration committee. The remuneration committee has determined that in normal circumstances the initial value of a Restricted Award can be up to 100 percent of salary. However, the remuneration committee may, in discretion, grant a Restricted Award in excess of this limit in exceptional circumstances, for instance in cases of recruitment at the most senior level.
Performance Target
The performance target for Restricted Awards will be challenging and will demonstrate the achievement of demanding and stretching financial performance over the Relevant Period.
Similar Provisions to Matched Awards
The following terms attaching to the Restricted Awards and their Relevant Period are the same as for the Matched Awards (above):
- •
- Rights attaching to shares prior to the vesting of Restricted Awards;
- •
- Cessation of employment before the end of the Relevant Period; and
- •
- Transfer of shares at the end of the Relevant Period.
Terms which apply to both elements of the New Restricted Share and Co-Investment Plan
Eligibility to participate
The remuneration committee will have responsibility for agreeing any awards under the New Restricted Share and Co-Investment Plan and for setting the policy for the way in which it should be operated, including agreeing performance targets and which employees should be invited to participate.
106
All employees of the Company, including executive directors, and of its subsidiaries are eligible to participate.
Nature of Awards
Awards are not pensionable and may not be assigned or transferred except on a participant's death, when they may be assigned to the participant's personal representatives.
Grant of Awards
Generally, awards can be granted at any time, but not during a close period of the Company. No payment is required for the grant of awards (except in the case of Matched Awards, where Invested Shares must have been acquired). All shares in the Company allotted under the New Restricted Share and Co-Investment Plan will carry the same rights as all other issued ordinary shares in the Company and application will be made for the shares to be listed on the London Stock Exchange and the Nasdaq National Market.
If there is a variation in the share capital of the Company, the awards may be adjusted to reflect that variation.
Limits on the Issue of Cyclacel Group Plc Shares under the New Restricted Share and Co-Investment Plan
Under the terms of the New Restricted Share and Co-Investment Plan, in any 10 year period the use of new issue shares under the New Restricted Share and Co-Investment Plan and any other employees' share scheme operated by the Company is limited to 10 percent of the issued share capital excluding any options granted under the Senior Executive Incentive Plan and any options or awards which have lapsed or have been released.
General Offer or Scheme of Arrangement
If there is a change of control of the Company, the remuneration committee will have discretion to permit the vesting of an award taking into account the extent to which the performance target has been satisfied and such other factors it believes to be relevant in determining the extent to which awards will vest in these circumstances.
Duration of the New Restricted Share and Co-Investment Plan
No award may be granted after ten years from the date of shareholder approval of the New Restricted Share and Co-Investment Plan.
The Company will have authority to amend the rules of the New Restricted Share and Co-Investment Plan, provided that no amendment to the advantage of participants may be made to provisions relating to:
- •
- who can be a participant;
- •
- the limits on the number of shares which can be issued under the New Restricted Share and Co-Investment Plan;
- •
- the basis for determining a participant's entitlement to shares and the terms on which they can be acquired; and
- •
- any adjustment in the event of a variation in the Company's share capital
107
without the prior approval of shareholders in general meeting unless the amendment is minor and made to benefit the administration of the New Restricted Share and Co-Investment Plan, to take account of a change in legislation or to obtain or maintain favourable tax, exchange control or regulatory treatment.
Extension of the New Share Plans Overseas
The Board may in respect of participants who are or who may be subject to taxation outside the United Kingdom amend or add to the provisions of the New Share Plans as it considers necessary or desirable to take account of or to mitigate or to comply with the relevant overseas taxation, securities or exchange control laws, provided that the terms of options or restricted awards granted to such participants are not overall more favourable than the terms of options or restricted awards granted to other participants.
Employee Share Trust
The Cyclacel Group plc Employee Benefit Trust (the "EBT"), an employee share trust, is also being set up to operate in connection with the share plans operated and/or adopted by the Company (the "plans"). Under the rules of the plans, options and awards may be granted by the trustees of the EBT. Any shares issued to the trustees of the EBT to satisfy its liabilities will count against the overall share plan limits. In addition, the Company will operate an arrangement to hedge the national insurance contributions liabilities arising under the plans and the trustees may be issued with further shares under those arrangements.
Pensions
We contribute to two defined contribution group personal pension plans.
As at March 1, 2004, 46 employees were members of the Cyclacel Group Personal Pension Plan. Membership is open to all employees. Employees must contribute at least 3% of salary and we match employee contributions up to a maximum of 6% of salary and subject to the earnings cap. Our total contributions are currently £15,913 ($28,769) a month.
Six employees are members of a group personal pension plan with Norwich Union. These employees began employment with us as a result of our acquisition of the assets of Fluorescience Ltd. Employees contribute 5% of salary and we contribute 7% of salary, less the cost of providing death in service benefits. Our total contributions are currently £2,180 ($3,945) a month.
Our total company-wide contributions are currently £18,093 ($32,741) a month.
There are no special arrangements for directors.
U.S. dollar amounts are presented solely for your convenience in this section at the rate of £1.00 = $1.8096, the noon buying rate in the The City of New York for cable transfers in pounds sterling as certified for customs purposes by the Federal Reserve Bank of New York on June 30, 2004. These translated amounts should not be construed as representations that the pounds sterling amounts could have been, or could in the future be, converted into U.S. dollars at this or any other exchanged rate.
Compensation
In respect of the fiscal year ended March 31, 2003, the total compensation paid to our directors and senior management as a group for the periods during which they served in that capacity was £1,402,909 ($2,538,704). The total amounts set aside or accrued by us to provide pension, retirement or
108
similar benefits for this group was £36,355 ($65,788). In respect of the twelve months ended March 31, 2004, the total compensation paid to our directors and senior management as a group for the periods during which they served in that capacity was £1,528,927 ($2,766,746). The total amounts set aside or accrued by us to provide pension, retirement or similar benefits for this group was £38,846 ($70,296). During the twelve months ended March 31, 2004 there were no options granted over our ordinary shares.
The following table sets forth the compensation paid or accrued during the twelve months ended March 31, 2004 to our directors.
| | Annual Compensation
| | Long-Term Compensation
| |
|
|---|
Name
| | Salary
and Fees(1)
| | Bonus
| | Shares
Underlying
Options(2)
| | All Other
Compensation(3)
|
|---|
| | ($)
| | ($)
| | (#)
| | ($)
|
|---|
| Sir John Banham | | 45,240 | | — | | — | | — |
| Spiro Rombotis | | 381,862 | | 90,480 | | 214,984 | | 16,922 |
| Professor Sir David Lane | | 186,564 | | — | | 151,876 | | 10,304 |
| Dr. Robert Jackson | | 250,885 | | — | | 112,000 | | 17,702 |
| Dr. Howard Marriage(4) | | 183,725 | | 27,144 | | 185,000 | | 28,514 |
| Paul McBarron | | 258,547 | | 72,384 | | 70,000 | | 11,534 |
| Sir Christopher Thomas Evans(4) | | 21,715 | | — | | — | | — |
| Daniel Green(4) | | 10,858 | | — | | — | | — |
| Professor Gordon McVie | | 21,715 | | — | | — | | — |
| Parag Saxena(4) | | 21,715 | | — | | — | | — |
| Professor Sir William Stewart(4) | | 21,715 | | | | 10,000 | | — |
| Dr. Jane Fisken(5) | | 10,858 | | — | | — | | — |
- (1)
- Includes a cash allowance for those eligible but electing not to have a company car.
- (2)
- Adjusted to reflect the bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 116.
- (3)
- Includes health insurance, car and fuel benefits, life insurance, relocation expenses over the Inland Revenue allowance and employer pension contributions.
- (4)
- Dr. Howard Marriage resigned as an executive director and Professor Sir William Stewart resigned as non-executive director on May 25, 2004 and Sir Christopher Thomas Evans, Daniel Green and Parag Saxena resigned as non-executive directors on , 2004.
- (5)
- Dr. Fisken resigned as a non-executive director on July 17, 2003.
U.S. dollar amounts are presented solely for your convenience in this section at the rate of £1.00=$1.8096, the noon buying rate in The City of New York for cable transfers in pounds sterling as certified for customs purposes by the Federal Reserve Bank of New York on June 30, 2004. These translated amounts should not be construed as representations that the pounds sterling amounts could have been, or could in the future be, converted into U.S. dollars at this or any other exchanged rate.
Director compensation
The directors (other than any director who for the time being holds an executive office of employment with the company or a subsidiary of the company) shall be paid out of the funds of the company by way of remuneration for their services as directors such fees not exceeding in aggregate £350,000 ($633,360) per year as the directors may from time to time determine or such larger sum as the company may, by ordinary resolution, determine. Such fee shall be divided among them in such proportion and manner as they may agree, or failing agreement, equally.
109
The board of directors may grant special remuneration to any director who performs any special or extra services to or at the request of the Company. Special remuneration may be payable to a director in addition to his ordinary remuneration, if any, as a director.
The directors shall also be paid out of the funds of the Company all expenses properly incurred by them in and about the discharge of their duties, including their expenses of travelling to and from the meetings of the board or directors, committee meetings and general meetings.
110
PRINCIPAL SHAREHOLDERS
The following table sets forth information with respect to the beneficial ownership of our ordinary shares calculated exclusive of treasury shares both before and after this offering by the following persons:
- •
- each shareholder who is known by us to own beneficially more than three percent of our outstanding shares as at June 30, 2004;
- •
- each of our directors; and
- •
- our directors and senior management as a group.
Beneficial ownership is determined in accordance with the rules of the SEC, based on factors including voting and investment power with respect to the shares. Shares subject to options or warrants currently exercisable, or exercisable within 60 days, are deemed to be outstanding for computing the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person. Except as indicated by footnote, and subject to community property laws where applicable, the persons named in the table have sole voting and investment power with respect to all shares of ordinary shares shown as beneficially owned by them.
The address of each director listed below is c/o Cyclacel Group plc, Dundee Technopole, James Lindsay Place, Dundee, DD1 5JJ, United Kingdom.
| | Percentage of Shares
Outstanding
| |
|---|
Name of Beneficial Owner
| | Number of Shares
Owned Before and
After the Offering(1)
| | Before
Offering(1)
| | After
Offering(1)
| |
|---|
| Principal Shareholders | | | | | | | |
| Merlin General Partner II Limited(2) | | 6,976,306 | | 15.5 | % | 11.4 | % |
| INVESCO Private Capital, Inc.(3) | | 5,831,356 | | 13.0 | | 9.6 | |
| International Life Science Partners, L.P.(4) | | 4,641,812 | | 10.3 | | 7.6 | |
| BankInvest Biomedical Ventures III Limited(5) | | 2,915,680 | | 6.5 | | 4.8 | |
| Lloyds TSB Development Capital Limited(6) | | 2,499,136 | | 5.6 | | 4.1 | |
| V-Sciences Investments PTE Ltd(7) | | 2,499,156 | | 5.6 | | 4.1 | |
| Scottish Equity Partnership(8) | | 1,971,518 | | 4.4 | | 3.2 | |
| GeneChem Therapeutics Venture Fund LP(9) | | 1,666,094 | | 3.7 | | 2.7 | |
| Highland & Universal Investments Limited(10) | | 1,523,802 | | 3.4 | | 2.5 | |
| Highland & Universal Securities Limited(10) | | 1,523,802 | | 3.4 | | 2.5 | |
| Quester Capital Management Limited(11) | | 1,417,162 | | 3.2 | | 2.3 | |
Directors |
|
|
|
|
|
|
|
| Sir John Banham(12) | | 5,831,356 | | 13.0 | % | 9.6 | % |
| Spiro Rombotis(13) | | — | | — | | — | |
| Professor Sir David Lane | | 133,750 | | * | | * | |
| Dr. Robert Jackson | | — | | — | | — | |
| Paul McBarron | | — | | — | | — | |
| Professor Gordon McVie | | — | | — | | — | |
All directors and senior management as a group |
|
6,067,442 |
|
13.5 |
% |
10.0 |
% |
- *
- Less than 1%.
- (1)
- Number of shares owned as shown both in this table and the accompanying footnotes and percentage ownership before and after the offering is based on the number of ordinary shares outstanding on June 30, 2004, immediately following the reorganization described under
111
"Pre-offering Reorganization into Public Limited Company" beginning on page 114, and also reflects the conversion of our preferred D shares into ordinary shares (based on an assumed initial public offering price of £1.66 per ordinary share, being the midpoint of the price range set forth on the cover page of this prospectus) and the further bonus issue of ordinary shares described under "Description of Share Capital" beginning on page 116, or a total of 44,964,304 ordinary shares. Percentage ownership after the offering reflects the sale of 16,000,000 shares (in the form of ordinary shares and ADSs) in this offering.
- (2)
- Represents 217,580 shares held by Merlin Biosciences Fund Gbr, 3,620,484 shares held by Merlin Biosciences Fund LP, 2,374,672 shares held by Merlin General Partner Limited, Merlin Fund LP, 731,570 shares held by Merlin Equity Limited and 32,000 warrants exercisable by Merlin General Partner Limited. Merlin General Partner II Limited is the controlling general partner and managing partner of Merlin Biosciences Fund Gbr and Merlin Biosciences Fund LP and its board of directors, of which Dr. Max Link is the Chairman, may be deemed to have voting and investment power over the shares held by Merlin Biosciences Fund Gbr and Merlin Biosciences Fund LP. Merlin General Partner Limited is the controlling general partner of Merlin Fund LP and its board of directors, of which Robin Herbert is the Chairman, may be deemed to have voting and investment power over the shares held by Merlin Fund LP. Merlin Equity Limited is the beneficial owner of8/9 of its holdings; the remaining1/9 of its holdings is held by it as nominee for a number of retirement benefit schemes, of which Merlin Ventures Limited is the sole trustee. Professor Sir Christopher Evans is the Chairman of Merlin Equity Limited's board of directors, which may be deemed to have voting and investment power over the shares beneficially owned by Merlin Equity Limited. In addition, Merlin Equity Limited has entered into put and call arrangements in respect of 321,785 shares with Arthurian Limited, a company under common ownership with its parent company, Merlin Ventures Limited. Exercise of these arrangements is subject to the provisions of the lock-up agreements described under "Underwriters."
- (3)
- Represents (i) 3,477,502 shares held of record by Chancellor V, L.P., (ii) 1,803,762 shares held of record by Chancellor V-A, L.P., and (iii) 550,092 shares held of record by Citiventure 2000, L.P. INVESCO Private Capital, Inc., or IPC, acts as managing member of the general partner of each of Chancellor V, L.P., Chancellor V-A, L.P. and Citiventure 2000, L.P., or the IPC Funds, and has full discretionary authority and full voting and dispositive power over the shares of the Company owned on behalf of the IPC Funds. Such voting and dispositive power may deemed to be shared with the general partner of each account, IPC Direct Associates V, L.L.C. and each of the IPC Funds. IPC and its Managing Partner and Chief Executive Officer, Parag Saxena, disclaim beneficial ownership interest in these shares and do not own them for their own account except to the extent of their respective pecuniary interests in these entities.
- (4)
- Excludes 58,129 warrants exercisable over preferred D shares in Cyclacel Limited by International Life Science Partners, L.P. HBM BioPartners Limited is the general partner of International Life Science Managers, L.P., which is in turn the general partner of International Life Science Partners, L.P. Voting and dispositive power over the shares held by International Life Science Partners, L.P., may be deemed to be shared by Jean-Marc LeSieur and the other members of the board of directors of HBM BioPartners Limited.
- (5)
- The shares held by BankInvest Biomedical Venture III Limited are voted by its management board, the sole member of which is Professor Jesper Zeuthen, and any investment decision with respect to the shares must be approved by BankInvest's board of directors.
- (6)
- Represents 2,457,500 shares held by Lloyds TSB Development Capital Limited, 20,818 shares held by LDC Co-Investment Plan 2001 "A" and 20,818 shares held by LDC Co-Investment Plan 2001 "B." Lloyds TSB Development Capital Limited is the general partner of LDC Co-Investment Plan 2001 "A" and LDC Co-Investment Plan 2001 "B." Darryl Eales, the Managing Director of Lloyds
112
TSB Development Capital Limited, may be deemed to have voting and investment power with respect to these shares.
- (7)
- V-Sciences Investments PTE Ltd is a wholly held subsidiary of Temasek Capital (Private) Limited, which is wholly held by Temasek Holdings (Private) Limited. Temasek Holdings (Private) Limited in turn is wholly held by the Minister for Finance Inc., an incorporated entity held by the Singapore government. However, Temasek Holdings (Private) Limited executes its investment decisions independent of the Minister for Finance Inc. and the Singapore government, and may be deemed to have voting and dispositive power over these shares.
- (8)
- Represents 229,080 shares held by Scottish Equity Partnership, 1,663,248 shares held by SEP II and 79,190 shares held by SEP IIB. Calum Paterson, Brian Kerr, Stuart Paterson and Richard Sparrow are executive directors of Scottish Equity Partners Limited, the fund manager of Scottish Equity Partnership, SEP II and SEP IIB, and share voting and investment power with respect to the shares held by these three funds.
- (9)
- M. Louis Lacasse is the President of GeneChem Management Inc., the fund manager for GeneChem Therapeutics Venture Fund L.P., and may be deemed to have voting and investment power with respect to the shares held by GeneChem Therapeutics Venture Fund L.P.
- (10)
- Highland & Universal Investments Limited is controlled by Ann Gloag and Highland & Universal Securities Limited is controlled by Brian Souter. Ann Gloag and Brian Souter are siblings but disclaim beneficial ownership of shares held by the other.
- (11)
- Represents 510,554 shares held by Quester Capital Management Limited acting as manager for and on behalf of Quester VCT3 plc (VCT3), 624,838 shares held by Quester Capital Management Limited acting as manager for and on behalf of Quester VCT4 plc (VCT4) and 281,770 shares held by Quester Capital Management Limited acting as manager for Quester VCT5 plc (VCT5). VCT3, VCT4 and VCT5 are managed by Quester Capital Management Limited, the Managing Director of which is Andrew Holmes.
- (12)
- Represents the shares described in note (3) above. Sir John Banham, who is the senior non-executive director of AMVESCAP plc, the parent company of INVESCO Private Capital, disclaims beneficial ownership of these shares.
- (13)
- Mr. Rombotis holds options over 3,441,806 ordinary shares not currently exercisable or not exercisable within 60 days, as more particularly described under "Management—Employee Share Option Plans—Senior Executive Incentive Plan" beginning on page 92.
113
The following table sets forth information with respect to changes in the percentage ownership held by our major shareholders immediately after each one of our major funding transactions during the past three years, calculated on an undiluted basis.
Major Shareholders
| | As of
March 31,
2001
| | After
June 2001
"C" Funding
Round
| | After
November 2003
"D" Funding
Round I(1)
| | After
January 2004
"D" Funding
Round II(1)
| |
|---|
| Merlin General Partner II Limited | | 37.20 | % | 22.20 | % | 16.68 | % | 15.68 | % |
| INVESCO Private Capital, Inc. | | — | | 11.19 | | 13.74 | | 12.92 | |
| International Life Science Partners, L.P. | | 4.83 | | 10.36 | | 11.07 | | 10.41 | |
| BankInvest Biomedical Ventures III Limited | | — | | 5.59 | | 6.87 | | 6.46 | |
| Lloyds TSB Development Capital Limited | | — | | 4.79 | | 5.89 | | 5.54 | |
| V-Sciences Investments PTE Ltd | | — | | 4.79 | | 5.89 | | 5.54 | |
| Scottish Equity Partnership | | 2.41 | | 4.26 | | 4.65 | | 4.37 | |
| GeneChem Therapeutics Venture Fund LP | | — | | 3.20 | | 3.93 | | 3.69 | |
| Highland & Universal Investments Limited | | 11.45 | | 5.06 | | 3.59 | | 3.38 | |
| Highland & Universal Securities Limited | | 11.45 | | 5.06 | | 3.59 | | 3.38 | |
| Quester Capital Management Limited | | — | | 1.60 | | 3.35 | | 3.15 | |
- (1)
- Excludes deferred shares, which do not carry voting or pre-emptive rights. For a more detailed description of our deferred shares, see note 14 to our consolidated financial statements.
We are not aware of any other significant changes in percentage ownership with respect to our major shareholders.
Our major shareholders do not have different voting rights.
As at June 30, 2004, 13 percent of our ordinary shares were held in the United States by 5 holders of record and there were 27 holders of record in the United Kingdom.
114
PRE-OFFERING REORGANIZATION INTO PUBLIC LIMITED COMPANY
Our operations are conducted by a private limited company organized under the laws of England and Wales under the name Cyclacel Limited.
Pursuant to a reorganization and share exchange agreement dated June 30, 2004 between the Company, Cyclacel Limited, Dr. Robert Jackson, Paul McBarron, Spiro Rombotis and all of the current shareholders of the Company (the Reorganization and Share Exchange Agreement), holders of all the issued ordinary shares and preferred ordinary D shares in the share capital of Cyclacel Limited transferred all of the outstanding 1,871,210 ordinary shares, par value 0.1p per share and 17,965,835 preferred ordinary D shares, par value 0.1p per share in Cyclacel Limited to us in exchange for an equal number of our ordinary shares and preferred ordinary D shares and Cyclacel Limited became our wholly-owned subsidiary. The Reorganization and Share Exchange Agreement also contains provisions relating to various consents required for the corporate actions described in this section, as well as the amendment of the Subscription and Shareholders Agreement. The Company has given certain customary warranties in relation to the reorganization that are unlimited as to time or amount.
Following the acquisiton of Cyclacel Limited we re-registered as a public limited company under the U.K. Companies Act.
As part of the above reorganization of share capital all employees holding options over shares in Cyclacel Limited have agreed that their options will be deemed to be held over an equivalent amount of ordinary shares in the company. Details of employee share options are set out more fully in "Management—Employee Share Option Plans."
Conditionally upon admission of our shares to the Official List of the U.K. Listing Authority, all of our preferred D shares will convert into ordinary shares. The rate at which preferred D shares will convert into ordinary shares will depend on the offering price of the ordinary shares in this offering. As a result, the total number of ordinary shares that will be outstanding following completion of this offering, and thus the full extent of the dilution that new investors in our ordinary shares and ADSs will suffer, will not be known until the offering price has been finally determined.
Conditionally upon admission and immediately upon the conversion of our preferred D shares into ordinary shares, there will be a further bonus issue of one new ordinary share for every ordinary share then outstanding. This will, subject to limited exceptions, result in corresponding adjustments to the number of ordinary shares that are subject to outstanding options and warrants and the exercise price of those options and warrants.
There will be 17,965,835 preferred D shares outstanding when the conversion into ordinary shares is effected. Each preferred D share will convert into 1.16 ordinary shares.
Other than in our historical financial statements and in the historical summary and selected financial data that are presented or discussed in this prospectus, we have adjusted all information relating to our outstanding ordinary shares, options and warrants appearing in this prospectus to reflect the impact of the conversion of our preferred D shares and the further bonus issue of ordinary shares. In making these adjustments, we have assumed that the initial public offering price will be £1.66 per share and $12 per ADS, being the midpoint of the price range on the cover page of this prospectus.
For further information regarding the conversion of our preferred D shares and the further bonus issue and their potential impact on an investment in our ordinary shares or ADSs, please see "Capitalization" on page 29, "Dilution" beginning on page 30 and "Description of Share Capital" beginning on page 116.
115
DESCRIPTION OF SHARE CAPITAL
The following summarizes the material provisions of our share capital and related summary information about provisions of our memorandum of association and our articles of association which have been adopted prior to, and conditionally upon, admission and about applicable English law. For our authorized capitalization and shares outstanding as of June 30, 2004, see "Capitalization."
General
The authorized, issued and fully paid share capital of the Company as at the date of this prospectus is as follows:
Authorized
| |
| | Issued
|
|---|
Number
| | Amount
| |
| | Number
| | Amount
|
|---|
| 79,000,000 | | £790,000 | | Ordinary shares | | 1,871,210 | | £18,712 |
| 21,000,000 | | £210,000 | | Preferred D shares | | 17,965,835 | | £179,658 |
The authorized, issued and fully paid share capital of the Company as it is expected to be immediately following admission is as follows:
Authorized
| |
| | Issued
|
|---|
Number
| | Amount
| |
| | Number
| | Amount
|
|---|
| 100,000,000 | | £1,000,000 | | Ordinary shares | | 44,964,304 | | £449,643 |
After giving effect to this offering, and the changes to our share capital described below, there will be an aggregate of 60,964,304 shares outstanding (63,364,304 shares if the underwriters exercise the over-allotment option in full).
Holders of our ordinary shares are not required to make any additional contributions of capital for our ordinary shares in the future.
(a) Changes in share capital of Cyclacel Group plc
We were incorporated on April 1, 2004 with an authorized share capital of £100 divided into 100 ordinary shares of £1 each of which one was issued nil paid to the subscriber to the memorandum of association.
Since incorporation the following changes have been made to our authorized and issued share capital:
- (i)
- By written resolution passed on June 30, 2004,
- (1)
- each of our existing ordinary shares of £1 each were sub-divided and redesignated into 100 ordinary shares of 1p each;
- (2)
- our authorized share capital was increased from £100 to £267,484.28 by the creation of 5,738,428 ordinary shares of 1p each and 21,000,000 preferred D shares of 1p each;
- (3)
- the directors were generally and unconditionally authorized, in accordance with section 80 of the U.K. Companies Act, to exercise all powers of the Company to allot relevant securities (as defined for the purposes that section) up to a maximum nominal amount of £267,484.28, such authority granted to expire at the conclusion of our next annual general meeting, save that we may, before the authority expires, make an offer or agreement which would or might require relevant securities to be allotted under the authority after it expires;
116
- (ii)
- By resolution of our members passed on June 30, 2004:
- (1)
- our authorized share capital was increased from £267,484.28 to £1,000,000 by the creation of an additional 73,251,572 ordinary shares of 1p each;
- (2)
- conditional upon (i) admission and (ii) our share premium account being credited pursuant to the allotment of our ordinary shares to institutional and other investors in the offering, the directors are authorized and directed to apply the sum of £28,745.09 standing to the credit of our share premium account in paying up in full at par 2,874,509 of our unissued ordinary shares each to be allotted pro rata to the holders of the preferred D shares in issue immediately prior to admission in the proportion of 1.16 new ordinary share for every one preferred D share then held by them, respectively;
- (3)
- conditional upon admission and immediately upon (i) the conversion of all our issued preferred D shares into ordinary shares (as referred to in (2) above), the directors are authorised and directed to apply the sum of £227,350.45 standing to the credit of our share premium account in paying up in full at par 22,735,054 ordinary shares each to be allotted pro rata to the holders of the ordinary shares in issue immediately following the conversion of our preferred D shares (for the avoidance of doubt, excluding any new institutional or other investors acquiring our ordinary shares pursuant to the offering) in the proportion of one new ordinary shares for every one ordinary share each then held by them, respectively;
- (4)
- conditional upon admission of our ordinary shares to listing on the Official List of the U.K. Listing Authority and to trading on the London Stock Exchange's main market for listed securities, the directors were generally and unconditionally authorized, in accordance with section 80 of the U.K. Companies Act, to exercise all powers of the Company to allot relevant securities (as defined for the purposes of that section) up to a maximum nominal amount of £278,245, such authority granted to expire at the conclusion of our next annual general meeting, save that we may, before the authority granted expires, make an offer or agreement which would or might require relevant securities to be allotted under the authority after it expires and all previous authorities under section 80 of the U.K. Companies Act shall cease to have effect;
- (5)
- conditional upon admission of our ordinary shares to listing on the Official List of the U.K. Listing Authority and to trading on the London Stock Exchange's main market for listed securities, the directors were given power to allot equity securities (as defined in section 94 of the U.K. Companies Act) for cash pursuant to the general authority referred to in paragraph (4) above as if section 89(1) of the U.K. Companies Act did not apply to the allotment, but that power, which expires at the conclusion of our next annual general meeting, will be limited to:
- (A)
- the allotment of up to 18,400,000 ordinary shares in connection with this offering;
- (B)
- the allotment of equity securities in connection with an offer or issue to or in favor of the holders of ordinary shares on the register on a date fixed by the directors where the equity securities attributable to the interests of all those shareholders are proportionate (as nearly as practicable) to the respective number of ordinary shares held by them on that date but the directors of the Company may make such exclusions or other arrangements as they consider expedient in relation to fractional entitlements, legal or practical problems under the laws in any territory or the requirements of any relevant regulatory body or stock exchange;
- (C)
- the allotment (other than pursuant to paragraphs (A) or (B) above) of equity securities having, in the case of relevant shares (as defined for the purposes of section 89 of the U.K. Companies Act a nominal amount or, in the case of other equity securities, giving
117
- (iii)
- on June 30, 2004, pursuant to a reorganization and share exchange agreement between us, the shareholders of the entire issued share capital of Cyclacel Limited as at June 30, 2004, the holders of warrants and non-employee options in Cyclacel Limited as at June 30, 2004, Spiro Rombotis, Paul McBarron, Dr. Robert Jackson and Cyclacel Limited, 1,871,210 ordinary shares and 17,965,835 preferred D shares in the Company were issued on the following basis:
- (1)
- for every one ordinary share of 0.1p in Cyclacel Limited, one ordinary share; and
- (2)
- for every one preferred D share of 0.1p in Cyclacel Limited, one preferred D share of 1p in the share capital of the Company.
An application has been made for relief from stamp duty under section 77 of the U.K. Finance Act 1986 in relation to our acquisition of the entire issued share capital of Cyclacel Limited.
- (iv)
- the holders of all warrants over ordinary shares in Cyclacel Limited (23,500 warrants in aggregate) will exercise their right to receive ordinary shares in Cyclacel Limited prior to the conversion of all our outstanding preferred D shares as referred to in paragraph (v) below. Immediately following such exercise we will exchange those ordinary shares in Cyclacel Limited issued to the exercising warrant holders for an equivalent number of our ordinary shares so as to ensure that Cyclacel Limited remains our wholly-owned subsidiary. The holders of all warrants over preferred D shares in Cyclacel Limited (116,260 warrants in aggregate) are entitled to exercise their right to receive preferred D shares in Cyclacel Limited prior to the conversion of all our outstanding preferred D shares as referred to in paragraph (v) below. The exercise price for these warrants is £4.06 per preferred D share in Cyclacel Limited. If the holders of these warrants choose to exercise we will immediately exchange those preferred D shares in Cyclacel Limited issued to the exercising warrant holder for an equivalent number of our preferred D shares so as to ensure that Cyclacel Limited remains our wholly-owned subsidiary. Those preferred D shares will then be subject to conversion into ordinary shares, and the exercising warrant holder will be entitled to participate in the further bonus issue of one ordinary share for every one ordinary share then held, each as described below. If the holders of these warrants choose not to exercise their warrants prior to admission, all warrants over preferred D shares in Cyclacel Limited will lapse;
- (v)
- conditional on admission of our shares to the Official List of the U.K. Listing Authority, all of our preferred D shares will convert into ordinary shares in the share capital of the Company. The conversion rate at which our preferred D shares convert into ordinary shares in the share capital of the Company is 1.16. Immediately on conversion of all our issued preferred D shares on the basis as set out above, there will be a bonus issue of 2,874,509 ordinary shares which are required to be issued to preferred D shareholders in satisfaction of the conversion rate; and
- (vi)
- conditional on admission of our shares to the Official List of the U.K. Listing Authority, immediately following the conversion rate bonus issue described above, there will be a further bonus issue of one new ordinary share for every one ordinary share.
No shareholder subscribing for ordinary shares in the offering will be entitled to participate in either bonus issue referred to in (v) or (vi) above. The conversion rate bonus issue, and the further bonus issue will be paid up out of the share premium account created by the issue of ordinary shares pursuant to the offering.
118
(b) Changes in share capital of Cyclacel Limited
During the last three years, the following changes have been made to the authorized and issued share capital of Cyclacel Limited:
- (i)
- In connection with a series D funding round which took place in November 2003, by written resolution passed on November 19, 2003,
- (1)
- 4,076,111 preferred D shares of 0.1p each were issued at £4.06 for cash consideration of £16,549,011. There was also an associated bonus issue of 12,666,580 preferred D shares of 0.1p each given to existing preferred C shareholders and ordinary shareholders who participated in the November 2003 funding round on a basis of 1:4 and 1:3 respectively, by way of capitalization of part of the share premium account. This was the first closing of two rounds.
- (2)
- On January 15, 2004, Cyclacel Limited increased its finances by issuing 1,162,068 preferred D shares of 0.1p each for an aggregate cash consideration of £4,717,996 or £4.06 per share.
- (3)
- Also as part of the series D funding round, 2,251,572 ordinary shares of 0.1p each and 4,540,969 preferred C shares of 0.1p each were converted into 6,792,541 deferred shares of 0.1p each.
- (ii)
- Pursuant to a share buy back agreement dated June 30, 2004 the majority of Cyclacel Limited's issued deferred shares were bought back by Cyclacel Limited for an aggregate of 1p in aggregate per deferred shareholder's shareholding. By written resolution dated June 30, 2004, all remaining issued deferred shares were reclassified as ordinary shares in the share capital of Cyclacel Limited.
- (iii)
- Cyclacel Limited issued 25,000 ordinary shares and 61,076 preferred D shares to holders of warrants over shares in Cyclacel Limited who exercised their warrants immediately prior to our acquisition of all the issued share capital of Cyclacel Limited on June 30, 2004 as referred to above.
- (iv)
- On June 30, 2004, we acquired all the issued share capital of Cyclacel Limited pursuant to the Reorganization and Share Exchange Agreement referred to above.
(c) Disapplication of statutory right of preemption
Section 89 of the U.K. Companies Act confers on shareholders certain rights of preemption in respect of the allotment of equity securities which are, or are to be, paid up in cash other than by way of allotment to employees under an employee's share scheme as defined in section 743 of the U.K. Companies Act. Following admission of our shares to listing on the Official List of the U.K. Listing Authority, the Company will be subject to the continuing obligations of the U.K. Listing Authority with regard to the issue of securities for cash and the statutory rights of preemption in section 89 of the U.K. Companies Act. The statutory rights of preemption apply to the balance of the authorised, but unissued share capital of the Company which is not the subject of the disapplication referred to in paragraph (a)(ii)(5)(c) above or reserved for issue in connection with share options and schemes (and other arrangements) referred to in "Employee Share Option Plans." The statutory rights of preemption have been disapplied as set out in paragraph (a)(ii) above to:
- (i)
- permit the directors to allot up to 18,400,000 ordinary shares under this offering;
- (ii)
- give the directors flexibility in relation to rights issues; and
- (iii)
- permit the directors to allot ordinary shares for cash following this offering having a nominal value of up to 5 percent of the issued ordinary share capital following admission of our shares to listing on the official list of the U.K. Listing Authority becoming effective.
119
(d) Other matters
Save as disclosed above and in "Employee Share Option Plans:"
- (i)
- no share or loan capital of the Company or any of its subsidiaries has within three years before the date of this document (other than intra-group issues by wholly owned subsidiaries or pursuant to the offering) been issued or been agreed to be issued fully or partly paid, either for cash or for a consideration other than cash and no such issue is now proposed;
- (ii)
- no commissions, discounts, brokerages or other special terms have been granted by the Company or any of its subsidiaries within the three years immediately preceding the date of this document in connection with the issue or sale of any share or loan capital of any such company; and
- (iii)
- no share or loan capital of the Company or any of its subsidiaries is under option or agreed, conditionally or unconditionally, to be put under option.
Registration Rights
In connection with this offering, we have entered into a registration rights agreement with all of our existing shareholders, which is expected to take effect upon the completion of this offering. See "Related Party Transactions—Registration Rights Agreement" for more information on the terms of this agreement.
Memorandum of Association
Our memorandum of association provides that our objects are, among other things, to carry on in relation to human and animal disease and health, the business of research, development, manufacture and marketing of diagnostic, prognostic and therapeutic techniques and as manufacturers, suppliers of, and dealers in, diagnostic, prognostic, therapeutic and pharmaceutical products and goods of all kinds. The objects of the Company are set out in full in clause 3 of its memorandum of association.
Shareholders Meetings
Under English law, there are two types of general meetings of shareholders, annual general meetings and extraordinary general meetings. We must hold an annual general meeting in each calendar year not later than 15 months from the previous general meeting. At an annual general meeting, shareholders vote on matters including the election of directors, appointment of auditors, fixing of directors' remuneration, approval of the annual accounts and the board of directors' report and declaration of dividends. Any other general meeting is an extraordinary general meeting.
Our board of directors may convene an extraordinary general meeting of shareholders and must convene one if demanded by the holders of at least 10% of our paid-up shares. An annual general meeting and an extraordinary general meeting called to pass a special resolution must be called upon at least 21 days' notice specifying the place, day and time of the meeting and the general nature of the business of the meeting. A special resolution is required to amend the company's name or its memorandum or articles of association. The necessary quorum for our general meetings is two persons holding or representing by proxy issued shares carrying a right to vote upon the business to be transacted.
An ordinary resolution requires a simple majority of the votes cast at a general meeting. A special resolution necessary for matters such as amending our articles of association requires at least 75% of the votes of attending shareholders who are entitled to vote on the resolution in question.
120
Voting Rights
Subject to disenfranchisement in the event of (a) non-payment of any call or other sum due and payable in respect of any share or (b) any non-compliance with any statutory notice requiring disclosure of the beneficial ownership of any shares and subject to any special rights or restrictions as to voting for the time being attached to any shares, on a show of hands every member who (being an individual) is present in person or (being a corporation) is present by a duly authorized representative not being himself a member, has one vote and on a poll every member present in person or by proxy has one vote for every share of which he is a holder. In the case of joint holders, the vote of the person whose name stands first in the register of members and who tenders a vote is accepted to the exclusion of any votes tendered by any other joint holders.
A poll may be demanded by:
- •
- the chairman of the meeting;
- •
- at least five shareholders present in person or by proxy and entitled to vote;
- •
- any shareholder or shareholders present in person or by proxy representing not less than one-tenth of the total voting rights of all shareholders having the right to vote at the meeting; or
- •
- any shareholder or shareholders present in person or by proxy holding shares conferring a right to vote at the meeting being shares on which the aggregate sum paid up is equal to not less than one-tenth of the total sum paid up on all shares conferring that right.
Issue of Shares and Changes in Capital
Subject to compliance with English law and without prejudice to the rights of existing shareholders, we may issue shares with any preferred, deferred or other special rights and subject to any restrictions on dividends, return of capital, voting or otherwise. We may also issue redeemable shares provided that there are non-redeemable shares in issue at the time.
Our current authorized but unissued ordinary shares are at the disposal of our board of directors, who may issue, grant options or otherwise dispose of those ordinary shares to any persons and on any terms they deem appropriate, provided the issuance does not violate the U.K. Companies Act or our articles of association.
Under Section 80 of the U.K. Companies Act, and subject to limited exceptions for employee share option schemes, our board of directors may not issue shares (or grant any right to subscribe for or convert other securities into shares) without the authorization of the shareholders by an ordinary resolution. The ordinary resolution must state the maximum amount of shares which the board of directors may issue under it and the date it will expire, which must be within five years.
If we issue shares for cash, Section 89 of the U.K. Companies Act requires us, subject to limited exceptions for employee share option schemes, to first offer these shares to existing shareholders on the same terms and in proportion to their holdings. However, Section 95 of the U.K. Companies Act provides that a special resolution of the shareholders may give the board of directors the power to issue shares without first offering them to existing shareholders.
We may by ordinary resolution:
- •
- increase our share capital;
- •
- consolidate and divide all or any of our share capital into shares of a larger amount;
- •
- sub-divide all or part of our share capital into shares of a smaller amount; and
- •
- cancel any shares which have not, at the date of the ordinary resolution, been taken or agreed to be taken by any person and diminish the amount of its authorized share capital by the amount of the shares so cancelled.
121
We may by special resolution:
- •
- purchase our own shares; and
- •
- reduce our share capital and any capital redemption reserve or share premium account.
Dividends
Subject to English law, our shareholders may declare a dividend at a general meeting upon the recommendation of our board of directors. The shareholders may declare a smaller dividend than recommended by our board of directors but they may not declare a larger dividend. Our board of directors may pay interim dividends if it appears to them that they are justified by our financial position.
Subject to the rights attached to any shares issued on any special terms and conditions, dividends shall be declared and paid according to the amounts paid up on the shares in respect of which the dividend is paid, but no amount paid up on a share in advance of calls should be treated for these purposes as paid up on the share. Any dividend on the shares unclaimed for a period of 12 years after having become due for payment shall be forfeited and shall revert to us. No dividend on a share will bear interest, unless provided by the rights attaching to the share.
Liquidation Rights
In the event of our liquidation, the liquidator may, with the sanction of an extraordinary resolution of the company and any other sanction required by U.K. statute (a) divide among the membersin specie the whole or any part of the assets of the company; or (b) vest the whole or any part of the assets in trustees on such trusts for the benefit of members as the liquidator shall think fit, but no member shall be compelled to accept any assets upon which there is any liability.
Notification of Interest in Shares
Section 198 of the U.K. Companies Act requires any person, subject to limited exceptions, who acquires an interest of 3% or more or, in the case of certain interests, 10% or more of our shares to notify us of his interest within two business days following the day on which the obligation to notify arises. After the shareholder exceeds that level of share ownership, that person must make a similar notification for each following whole percentage figure increase or decrease, rounded down to the next whole number. For the purposes of the notification obligation, the interest of a person in the shares means any kind of interest in shares, subject to certain exceptions, including any share:
- •
- in which such person's spouse or child or stepchild is interested; or
- •
- in which a corporate body is interested where either:
- •
- that corporate body or its directors act under that person's directions or instructions; or
- •
- that person is entitled to exercise or controls one-third or more of the voting power of that corporate body; or
- •
- in which another party is interested where the person and that other party are parties to a "concert party" agreement under Section 204 of the U.K. Companies Act and any interest in shares is in fact acquired by any one of the parties pursuant to the agreement.
A "concert party" agreement is an agreement under which one or more parties acquire interests in shares of a particular company and impose obligations or restrictions on any one or more of the parties as to the use, retention or disposal of such interests.
In addition, Section 212 of the U.K. Companies Act enables us, by notice in writing, to require a person whom we know or have reasonable cause to believe to be, or to have been at any time during
122
the three years immediately preceding the date on which we issue the notice, interested in our shares to confirm that fact, and where such person holds or has during this relevant time held an interest in our shares to give such further information as may be required relating to his or her interest and any other interest in our shares of which that person is aware.
The U.K. Companies Act restricts the rights attaching to our shares for non-compliance with Section 212 of that Act. In addition, our articles of association provide for disenfranchisement, loss of entitlement to dividends and other payments and restrictions on transfer and other alienability.
Transfer of Shares
A member may transfer all or any of his shares in any manner which is permitted by any applicable statutory provision and is approved by the board of directors. We shall maintain a record of uncertificated shares in accordance with the relevant U.K. statutory provisions.
A member may transfer all or any of his certificated shares by an instrument of transfer in any usual form, or in such other form as the board of directors may approve. The instrument of transfer shall be signed by or on behalf of the transferor and, except in the case of a fully paid share, by or on behalf of the transferee. The board of directors may, in its absolute discretion and without giving any reason for it, refuse to register any transfer of any certificated share which is not fully paid up (but not so as to prevent dealings in listed shares from taking place on an open and proper basis). The board of directors may also refuse to register any instrument of transfer of a certificated share unless it is lodged at the registered office, or such other place as the board of directors may decide, for registration, accompanied by the share certificate for the shares to be transferred (except where the shares are registered in the name of a market nominee and no certificate has been issued for them) and such other evidence as the board of directors may reasonably require to prove title of the intending transferor. If the board of directors refuses to register a transfer of a certificated share it shall, within two months after the date on which the instrument of transfer was lodged, send to the transferee notice of the refusal. The board of directors may refuse to register any transfer unless it is only in respect of one class of shares and in favor of no more than four transferees.
Uncertificated Shares—General Powers
In relation to any uncertificated share, we may utilize the relevant system in which it is held to the fullest extent available from time to time in the exercise of any of its powers or functions under any applicable U.K. statutory provision or the articles of association or otherwise in effecting any action. Any provision in the articles of association in relation to uncertificated shares which is inconsistent with any applicable statutory provision shall not apply. We may, by notice in writing to the holder of an uncertificated share, require the holder to change the form of that share to certificated form within such period as may be specified in the notice. For the purpose of effecting any action by the company, the directors may determine that holdings of the same member in uncertificated form and in certificated form shall be treated as separate holdings.
Variation of Rights
Whenever our share capital is divided into different classes of shares, all or any of the rights for the time being attached to any class of shares may be varied, either with the consent in writing of the holders of three-fourths in nominal value of the issued shares of that class or with the sanction of an extraordinary resolution passed at a separate general meeting of the holders of those shares. At any separate general meeting, the necessary quorum is two persons holding or representing by proxy at least one-third in nominal amount of the issued shares of the class in question (but at any adjourned meeting, any person holding shares of the class or his proxy is a quorum).
123
Borrowing Powers
The board may exercise all the powers of the Company to borrow money and to mortgage or charge all or any part of its undertaking, property and assets (both present and future) and uncalled capital and to issue debentures and other securities, whether outright or as collateral security for any of our debt, liability or obligations or that of any third party. The board of directors shall restrict our borrowings and exercise all voting and other rights or powers of control exercisable by us in relation to our subsidiaries so as to secure (as regards subsidiaries only so far as by such exercise it can secure) that the aggregate principal amount outstanding at any time in respect of all borrowings by the group (excluding any intercompany borrowings) will not, without previous sanction of the Company in general meeting, exceed the greater of £20 million and an amount equal to 2 times the adjusted capital and reserves (as defined in the articles of association) or any higher limit fixed by ordinary resolution of the Company which is applicable at the relevant time.
Members' Right to Institute Action on Behalf of or in the Interest of the Company
English case law has established generally that we, rather than our shareholders, are the proper claimant in an action in respect of a wrong done to us or where there is an irregularity in our internal management. Exceptions to this general rule include illegal acts or acts outside our powers, failure to adopt a required special resolution, fraud committed on minority shareholders by persons who control us and an infringement of the personal rights of a shareholder. A court may allow a shareholder to bring an action in our name where our affairs have been or are being conducted in a manner that is unfairly prejudicial to some of our shareholders.
Right to Inspect Documents
Under English law, holders of shares are entitled to inspect company documents such as our Register of Members and our minutes from general meetings. In addition, shareholders are entitled to a copy of our Annual Report and Accounts, together with the Auditor's Report. All of these rights may be exercised free of charge. Rights of inspection without charge also extend to our Register of Directors and Secretaries, directors' service contracts (in certain circumstances), our Register of Charges (plus documents creating or evidencing charges) and our Register of Directors' Interests.
Company's Transactions with Significant Shareholders
Under Chapter 11 of the Listing Rules of the U.K. Listing Authority if the Company proposes to enter into a transaction with a related party (which includes a shareholder who in the 12 months preceding the date of the transaction was entitled to exercise or control 10% or more of the votes able to be cast at a general meeting), the Company must, among other things, make an announcement, send a circular to shareholders and seek shareholder approval. The Company must ensure that the related party shareholder abstains from voting on the relevant resolution.
For a description of directors' duties, see "Management."
124
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
American Depositary Shares
The Bank of New York, as depositary, will deliver the ADSs. Each ADS represents 4 shares. The ordinary shares or the right to receive ordinary shares will be deposited by us with a custodian, the London office of The Bank of New York. Each ADS will also represent securities, cash or other property deposited with The Bank of New York but not distributed to ADS holders. Your ADSs will be evidenced by what are known as American Depositary Receipts, or ADRs. The Bank of New York's corporate trust office is located at 101 Barclay Street, New York, New York 10286. Its principal executive office is at One Wall Street, New York, New York 10286.
You may hold ADSs either directly or indirectly through your broker or another financial institution. If you hold ADSs directly, by having an ADS registered in your name on the books of the depositary you are an ADS holder. This description assumes you hold your ADSs directly. If you hold the ADSs indirectly through your broker or financial institution nominee, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should speak to your broker or financial institution for further information about those procedures.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. English law governs shareholder rights. The Bank of New York will be the holder of the shares underlying your ADSs. As a holder of ADSs, you will have ADS holder rights. A deposit agreement dated July • , 2004 among us, The Bank of New York and you, as an ADS holder, and the owners and beneficial owners of ADRs, sets out ADS holder rights as well as the rights and obligations of The Bank of New York. The principal terms of the deposit agreement are as set out in the following paragraphs. Pursuant to the deposit agreement the Company has agreed to pay the reasonable expenses and out-of-pocket charges of the Depositary and those of any registrar of the ADSs. Details of other fees and expenses payable by ADS holders are set out below. New York law governs the deposit agreement and the ADSs.
Share Dividends and Other Distributions
The Bank of New York has agreed to pay to you the cash dividends or other distributions it or the custodian receives from us on deposited shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent.
Cash. Whenever we make a cash distribution on the ordinary shares on deposit with the custodian, we will notify The Bank of New York and deposit the funds with the custodian. Upon confirmation of the deposit of the requisite funds, The Bank of New York will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer those dollars to the United States. If that is not possible or if approval of any government is needed and cannot be obtained or cannot be obtained within a reasonable period, the deposit agreement allows The Bank of New York to distribute pounds sterling to those ADS holders to whom it is possible to do so. The Bank of New York will hold the pounds sterling for the account of the ADS holders who have not been paid. It will not invest the pounds sterling and it will not be liable for any interest.
Before making a distribution, any withholding taxes that must be paid under applicable law will be deducted. See "Taxation" for more information. The Bank of New York will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent.If the exchange rates fluctuate at a time when The Bank of New York cannot convert the pounds sterling, you may lose some or all of the value of the distribution.
125
Shares. The Bank of New York may distribute new ADSs representing any ordinary shares we may distribute as a dividend or free distribution, if we furnish it promptly with satisfactory evidence that it is legal for it to do so. The Bank of New York will only distribute whole ADSs. It will sell ordinary shares which would require it to deliver a fractional ADS and distribute the net proceeds in the same way it does cash. If The Bank of New York does not distribute additional ADSs, the outstanding ADSs will also represent the new shares.
Rights to Receive Additional Ordinary Shares. If we offer holders of our ordinary shares any rights to subscribe for additional ordinary shares or any other rights, The Bank of New York may, after consultation with us, make these rights available to you. We must furnish it with satisfactory evidence that it is legal for it to do so. If The Bank of New York determines, after consultation with us, that it is not lawful and feasible to make these rights available to you, The Bank of New York may sell the rights and distribute the proceeds in the same way it does cash. The Bank of New York may allow rights that are not distributed or sold to lapse. In that case, you would receive no value for them.
If The Bank of New York makes rights available to you, upon instruction from you, it will exercise the rights and purchase the shares on your behalf. The Bank of New York will then deposit the shares and deliver ADSs to you. It will only exercise rights if you pay it the exercise price and any other charges the rights require you to pay.
The U.S. securities laws may restrict the sale, deposit, cancellation and transfer of the ADSs issued after the exercise of rights. For example, you may not be able to trade the ADSs freely in the United States. Under the deposit agreement, The Bank of New York will not distribute rights to holders of ADSs unless the distribution and sale of rights and the securities to which these rights relate are either exempt from, or not subject to, registration under the Securities Act with respect to all holders of ADSs, or are registered under the provisions of the Securities Act. We can give no assurance that we can establish an exemption from registration under the Securities Act, and we are under no obligation to file a registration statement with respect to these rights or underlying securities or to endeavor to have a registration statement declared effective. In this case, The Bank of New York may deliver the ADSs under a separate restricted deposit agreement which will contain the same provisions as the agreement except for the changes needed to put the restrictions in place.
Other Distributions. The Bank of New York will send to you anything else we distribute on deposited securities by any means it thinks is legal, fair and practical. If it cannot make the distribution in that way, The Bank of New York may either sell what we distributed and distribute the net proceeds in the same way it does cash or it may decide to hold what we distributed, in which case the ADSs will also represent the newly distributed property.
The Bank of New York is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distribution we make on our shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
The Bank of New York will deliver ADSs if you or your broker deposit shares or evidence of rights to receive shares with the custodian. Shares deposited in the future with the custodian must be accompanied by certain documents, including instruments showing that such shares have been properly transferred or endorsed to you. Upon payment of its fees and expenses and of any taxes or charges, such as stamp duty or stock transfer taxes or fees, The Bank of New York will register the appropriate number of ADSs in the names you request and will deliver the ADRs at its corporate trust office to the persons you request.
126
You may turn in your ADSs at The Bank of New York's corporate trust office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, The Bank of New York will deliver the underlying shares to an account designated by you and any other deposited securities underlying the ADSs at the office of the custodian, or at your request, risk and expense, The Bank of New York will deliver the deposited securities at its corporate trust office.
Voting Rights
You may instruct The Bank of New York to vote the ordinary shares underlying your ADSs only if we ask The Bank of New York to ask for your instructions. Otherwise, you will not be able to exercise your right to vote unless you withdraw the shares. However, you may not know about the meeting long enough in advance to withdraw the shares.
If we ask for your instructions, The Bank of New York will notify you of the upcoming vote and arrange to deliver our voting materials to you. The materials will describe the matters to be voted on and explain how you, on a certain date, may instruct The Bank of New York to vote the shares or other deposited securities underlying your ADSs as you direct. For instructions to be valid, The Bank of New York must receive them on or before the date specified. The Bank of New York will try, as far as practicable, subject to English law and the provisions of our memorandum and articles of association, to vote or to have its agents vote the shares or other deposited securities as you instruct. The Bank of New York will only vote or attempt to vote as you instruct. However, if The Bank of New York does not receive your voting instructions, it will give a proxy to vote your shares to our designated representative.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct The Bank of New York to vote your shares. In addition, The Bank of New York and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions as long as they act in good faith and are not negligent. This means that you may not be able to exercise your right to vote and there may be nothing you can do if your shares are not voted as you requested.
Record Date
The Bank of New York will fix record dates that are the same, or as close as practicable to, the corresponding record date for the ordinary shares
- •
- for the determination of the ADS holders who will be:
- •
- entitled to receive a dividend, distribution or rights;
- •
- entitled to give instructions for the exercise of voting rights at a meeting of holders of ordinary shares or other deposited securities; or
- •
- responsible for any charges assessed by The Bank of New York;
- •
- fixing the date on or after which each ADS will represent the charged number of ordinary shares all subject to the provisions of the deposit agreement.
Notices and Reports
Upon receipt of notice of any meeting of holders of ADSs or other deposited securities, if requested in writing by us, The Bank of New York will, as soon as practicable thereafter, mail to the owners of ADSs a notice which contains (a) such information as is contained in such notice of meeting received by The Bank of New York from us, (b) a statement that the owners of ADSs as of the close of business on a specified record date will be entitled, subject to any applicable provisions of English law and of our memorandum and articles of association, to instruct The Bank of New York as to the exercise of the
127
voting rights, if any, pertaining to the amount of shares or other deposited securities represented by their respective ADSs, and (c) a statement as to the manner in which instructions may be given.
The Bank of New York will make available for inspection by registered holders at its corporate trust office any reports and communications, including any proxy soliciting material, received from us, which are both (a) received by The Bank of New York as the holder of the deposited securities, and (b) made generally available to the holders of such deposited securities by us. The Bank of New York will also, upon written request, send to the registered holders copies of such reports when furnished by us pursuant to the deposit agreement. Any such reports and communications, including any proxy soliciting material, furnished to The Bank of New York by us will be furnished in English. The Bank of New York will also make available for inspection at its corporate trust office its books for the registration and transfer of ADSs. However, any such inspection will not be for the purpose of communicating with registered holders of ADSs in the interest of a business or object other than the business of our company, or matters relating to the deposit agreement or the ADSs.
Fees and Expenses
ADS holders must pay:
| | For:
|
|---|
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | | Each issuance of ADSs, including as a result of a distribution of shares or rights or other property and each cancellation of ADSs, including if the deposit agreement terminates |
$0.02 (or less) per ADS (or portion thereof) |
|
Any cash payment |
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs |
|
Distribution of securities distributed to holders of deposited securities which are distributed by The Bank of New York to ADS holders |
$0.02 per ADS per calendar year (to the extent the depositary has not collected a cash distribution fee of $0.02 per ADS during that calendar year) |
|
Depositary services |
Registration or transfer fees |
|
Transfer and registration of ordinary shares on the share register of the foreign registrar to or from the name of The Bank of New York or its agent when you deposit or withdraw ordinary shares |
Expenses of the depositary |
|
Conversion of pounds sterling to U.S. dollars; cable, telex and facsimile transmission expenses |
Taxes and other governmental charges the depositary or the custodian have to pay on any ADS or share underlying an ADS such as stock transfer taxes, stamp duty tax, stamp duty reserve tax or withholding taxes |
|
As necessary |
Any charges incurred by the depositary or its agents for servicing the deposited securities |
|
As necessary |
128
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities underlying your ADSs. The Bank of New York may refuse to transfer your ADSs or allow you to withdraw the deposited securities underlying your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities underlying your ADSs to pay any taxes owed and you will remain liable for any deficiency. If it sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to you any proceeds, or send to you any property, remaining after it has paid the taxes.
Reclassifications, Recapitalizations and Mergers
If we change the nominal or par value of our shares; reclassify, split up or consolidate any of the deposited securities; distribute securities on the shares that are not distributed to you; or recapitalize, reorganize, merge, sell all or substantially all of our assets, or take any similar action, then:
- •
- the cash, shares or other securities received by The Bank of New York will become deposited securities and each ADS will automatically represent its equal share of the new deposited securities, and
- •
- The Bank of New York may, with our approval, and will, if we ask it to in writing, distribute new ADSs or ask you to surrender your outstanding ADSs in exchange for new ADSs, identifying the new deposited securities.
Amendment and Termination
We may agree with The Bank of New York to amend the deposit agreement and the ADSs for any reason without your consent. If the amendment adds or increases fees or charges, except for taxes and other governmental charges or registration fees, cable, telex or facsimile transmission costs, delivery costs or other such expenses, or prejudices an important right of ADS holders, it will only become effective 30 days after The Bank of New York notifies you of the amendment.
At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADSs and the deposit agreement as amended.
The Bank of New York will terminate the agreement if we ask it to do so. The Bank of New York may also terminate the agreement if it has informed us that it would like to resign and we have not appointed a new depositary bank within 60 days. In both cases, The Bank of New York must notify you at least 30 days before termination.
After termination, The Bank of New York and its agents will be required to only collect distributions on the deposited securities, will sell rights as provided in the deposit agreement and will deliver shares and other deposited securities upon cancellation of ADSs.
At any time after the expiration of one year from the date of termination, The Bank of New York may sell any remaining deposited securities by public or private sale. After that, The Bank of New York will hold the proceeds of the sale, as well as any other cash it is holding under the deposit agreement for the pro rata benefit of the ADS holders that have not surrendered their ADSs. It will not invest the money and will have no liability for interest. The Bank of New York's only obligation will be to account for the proceeds of the sale and other cash. After termination our only obligations will be with respect to indemnification and to pay certain amounts to The Bank of New York.
129
Limitations on Obligations and Liability to ADS Holders
The deposit agreement expressly limits our obligations and the obligations of The Bank of New York, and it limits our liability and the liability of The Bank of New York. We and The Bank of New York:
- •
- are only obligated to take the actions specified in the deposit agreement without negligence or bad faith;
- •
- are not liable if either is prevented or delayed by law or circumstances beyond our control from performing our obligations under the deposit agreement;
- •
- are not liable if either exercises discretion permitted under the deposit agreement;
- •
- have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the agreement on your behalf or on behalf of any other person; and
- •
- may rely upon any documents they believe to be genuine and to have been signed or presented by the proper party.
In the deposit agreement, we and The Bank of New York agree to indemnify each other under specified circumstances.
Requirements for Depositary Actions
Before The Bank of New York will deliver or register a transfer of ADSs, make a distribution on ADSs or approve a withdrawal of shares, The Bank of New York may require:
- •
- payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities;
- •
- production of satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and
- •
- compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents.
The Bank of New York may refuse to deliver or register transfers of ADSs when our books or those of The Bank of New York are closed, or at any time if The Bank of New York or we think it advisable to do so.
You have the right to cancel your ADSs and withdraw the underlying shares at any time except:
- •
- when temporary delays arise because The Bank of New York or we have closed transfer books or the deposit of shares in connection with voting at a shareholders' meeting, or paying a dividend on the shares;
- •
- when you or other ADS holders seeking to withdraw shares owe money to pay fees, taxes and similar charges; or
- •
- when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities.
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Books of the Depositary
The Bank of New York or its agent will maintain a register for the registration, transfer, combination and split-up of ADSs. You may inspect such records at such office during regular business
130
hours, but solely for the purpose of communicating with other holders in the interest of business matters relating to the deposit agreement.
The Bank of New York will maintain facilities to record and process the issuance, cancellation, combination, split-up and transfer of ADSs. These facilities may be closed from time to time, to the extent not prohibited by law.
Pre-Release of ADSs
In specified circumstances, subject to the provisions of the agreement, The Bank of New York may deliver ADSs before deposit of the underlying shares. This is called a pre-release of the ADSs. The Bank of New York also may deliver shares upon cancellation of pre-released ADSs even if The Bank of New York knows the ADSs have been pre-released and the ADSs are cancelled before the pre-release transaction has been closed. A pre-release is closed as soon as the underlying shares are delivered to The Bank of New York. The Bank of New York may receive ADSs instead of shares to close a pre-release. The Bank of New York may pre-release ADSs only under the following conditions:
- •
- before or at the time of the pre-release, the person to whom the pre-release is being made must represent to The Bank of New York in writing that it or its customer:
- •
- owns the shares or ADSs to be remitted;
- •
- assigns all beneficial rights, title and interest in the shares or ADSs to The Bank of New York as depositary and for the benefit of the owners, and
- •
- will not take any action with respect to the shares or ADSs that is inconsistent with the transfer of beneficial ownership (including, without the consent of The Bank of New York, disposing of such shares or ADSs) other than in satisfaction of such pre-release,
- •
- the pre-release must at all times be fully collateralized with cash or other collateral that The Bank of New York considers appropriate, and
- •
- The Bank of New York must be able to close out the pre-release on not more than five business days' notice.
In addition, The Bank of New York will limit the number of ADSs that may be outstanding at any time as a result of pre-release to 30 percent of total shares deposited, although The Bank of New York may disregard the limit from time to time, if it thinks it is appropriate to do so.
In addition, The Bank of New York will limit the number of ADSs that may be outstanding at any time as a result of a pre-release, although The Bank of New York may disregard the limit from time to time if, in its judgement, it is appropriate to do so.
Disclosure of Interests
By purchasing ADSs, you agree to comply with our articles of association and the laws of England regarding any disclosure requirements regarding ownership of ordinary shares, all as if the ADSs were, for this purpose, the ordinary shares they represent. By purchasing ADSs, you also agree to be bound by provisions of the deposit agreement mandating compliance with the disclosure requirements of English company law.
To facilitate compliance with the notification requirements, ADS holders may deliver any notification to The Bank of New York, and The Bank of New York will, as soon as practicable, forward the notification to us and, to the extent practicable and as permitted by applicable law, any authorities in England as specified by the ADS holder.
131
RELATED PARTY TRANSACTIONS
Cancer Research/Cambridge University/Professor David Glover
We have entered into an option agreement with Cancer Research Ventures Limited relating to research of Professor David Glover of the University of Cambridge that is funded by Cancer Research UK. This agreement grants us an exclusive option to obtain exclusive, world-wide, royalty-bearing licenses in the field of diagnostic and therapeutic products covering patents, patent applications and know-how resulting from research funded by Cancer Research and supervised by Professor Glover that relates to the genome ofDrosophila melanogaster. The option must be exercised within a period of time following our receipt of notice of particular inventions. The optioned rights could assist us in identifying genes involved in mitosis that could be used as targets for small molecule drug design. This agreement also grants us a nonexclusive license to know-how resulting from research funded by Cancer Research and supervised by Professor Glover that relates to the genome ofDrosophila melanogaster. Cancer Research Ventures and the University retain the right to use the intellectual property for research purposes. We paid an up-front fee and agreed to make annual payments during the option term. We may terminate the option for convenience and either party may terminate it for the default of the other.
Cancer Research Technology Limited
We have also entered into an option agreement with Cancer Research Technology Limited (formerly Cancer Research Campaign Technology Limited), which is a wholly owned subsidiary of Cancer Research UK. The option relates to inventions funded by Cancer Research in a field consisting of therapeutics based on specific identified gene expressions and their relation to the cell cycle. This agreement is related to work done in the academic laboratory of Professor David Lane. Under the agreement we have the right to require Cancer Research to assign or exclusively license to us the relevant intellectual property in the defined field. Although Cancer Research agrees to attempt not to unduly encumber its rights in the field, our rights are subject to any encumbrances on Cancer Research's rights. The option must be exercised within a period of time following our receipt of notice of particular inventions. The option is also subject to royalty-sharing or other arrangements made by Cancer Research but no other payments remain owing. The option agreement expires in 2006.
CXR Biosciences Limited
CXR Biosciences Limited (Dundee, Scotland, UK), a contract research organization, charged costs for research services of $155,000, $917,000, $724,000, $447,000, $191,000 and $12,000 for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, and the three months ended March 31, 2003 and 2004, respectively. On August 14, 2003 Mr. S. G. Rombotis acquired as part of a private equity financing approximately 2% of the equity of CXR Biosciences. Mr. S. G. Rombotis does not participate in the Company's purchasing decisions with regard to CXR Biosciences which are subject to competitive tender from multiple vendors.
Registration Rights Agreement
In connection with this offering, we have entered into a registration rights agreement dated June • , 2004 with all of our existing shareholders, which agreement is expected to take effect upon the completion of the pre-offer reorganization. The material terms of the agreement set forth below have been approved by our board of directors as well as our existing shareholders.
- •
- Demand registration rights. If we receive a request from our existing shareholders to register under the Securities Act (other than a registration pursuant to Rule 415 of the Securities Act) registrable shares held by them representing at least 20% in nominal value of our ordinary shares then outstanding, then we will in good faith use reasonable efforts to effect the requested
132
registration as soon as practicable, and in any event within 210 days of receipt of the request. Registrable shares consist of (i) ordinary shares held by our existing shareholders immediately following completion of the pre-offering reorganization, (ii) any other of our ordinary shares issued as (or issuable upon the conversion or exercise of any warrant, right or other security which is issued as) a dividend or other distribution with respect to, or in exchange for or in replacement of, the shares listed in (i) and (ii). Registrable shares do not include any registrable shares sold by our existing shareholders, except for sales to an affiliate in which the rights and obligations under the registration rights agreement have been duly assigned to the affiliate. We are not required to effect more than two demand registrations other than short-term registrations in total or more than two short-form demand registrations per year, may reduce the number of shares to be registered if required by marketing factors and may defer once in any twelve-month period the filing of a registration statement for up to 90 days, if such registration would be materially adverse to us and the existing shareholders and such deferral is essential.
- •
- Piggyback registration rights. If we propose to register any of our ordinary shares under the Securities Act in connection with a public offering of our ordinary shares for cash only (other than in connection with this offering, a registration relating solely to the sale of securities to participants in a Company share plan, a transaction covered by Rule 145 under the Securities Act or a registration of shares underlying convertible bonds or any registration which does not include substantially the same information as would be required to be included in a registration statement covering the sale of registrable shares) we will cause to be registered ordinary shares held by our existing shareholders at their request. We will have first priority to issue shares on any registration initiated by us, and we may, on the advice of the managing underwriter, reduce the number of registrable shares to be underwritten in the relevant public offering. We also have the right to withdraw any offering prior to the effective date of any registration proposed by us without liability to any shareholder.
In the event that we or any of our subsidiaries file any such registration statement and the existing shareholders exercise their rights to include ordinary shares held by them in any such registration statement, we have agreed to indemnify each of the existing shareholders and any of their affiliates in respect of certain customary matters. This indemnity is not limited to time or amount.
In addition, we are required to cause all registrable shares we register pursuant to the registration rights agreement to be listed on each securities exchange or over-the-counter market on which similar securities issued by us are then listed. Other than underwriting fees, discounts and commissions, we will bear all reasonable expenses of registering, filing or qualifying the registrable shares, and we are required to furnish certain information to our existing shareholders and satisfy certain reporting requirements under the Securities Act and under the Securities Exchange Act of 1934. The registration rights of our existing shareholders will terminate after all of their respective registrable shares have been disposed of pursuant to an effective registration statement, have been sold or issued pursuant to section 4(1) or another similar exemption under the Securities Act, or have been otherwise transferred under circumstances such that subsequent disposition of their shares will not require registration or qualification under the Securities Act.
The registration rights agreement also contains provisions obligating our existing shareholders to enter into lock-up agreements with the underwriters. See "Underwriters" and "Shares Eligible for Future Sale" for more information on the terms of the lock-up agreements.
Consultancy Agreement
Professor Sir David Lane has entered into an agreement effective June 1, 2004 to provide consultancy services to the Company for an initial period of six months. His consultancy agreement is terminable by either party giving three months' notice in writing. He is required to provide consultancy services to the Company for a minimum of 39 days during the term of his engagement. He receives a fee of £555 per day and his reasonable expenses are reimbursed by the Company.
133
UNDERWRITERS
Under the terms and subject to the conditions contained in an underwriting and sponsor's agreement dated (which we refer to as the underwriting agreement), the underwriters named below, for whom Morgan Stanley Securities Limited and SG Cowen & Co., LLC are acting as representatives, have severally agreed to procure subscribers for, or failing which to subscribe for themselves, and Cyclacel has agreed to allot and issue to subscribers procured by, or failing which, to the underwriters themselves, the number of shares indicated below:
| | Number of
Shares
|
|---|
| Morgan Stanley Securities Limited | | |
| SG Cowen & Co., LLC | | |
| Needham & Company, Inc. | | |
| Leerink Swann & Co., Inc. | | |
| | |
|
| Total | | 16,000,000 |
| | |
|
The underwriters may elect to take delivery of or procure subscribers for all or a portion of the shares in the form of ADSs. References in this section to "shares" include ADSs to the extent applicable.
The underwriting agreement provides that the obligations of the several underwriters to procure subscribers for or themselves subscribe for the shares are subject to the approval of certain legal matters by their counsel and to certain other conditions including admission of the shares to trading on the London Stock Exchange occurring, not later than , 2004. The underwriters' obligations to underwrite shares does not extend to any shares covered by the over-allotment option.
The offering of the shares and the ADSs in the United States will be made by a number of affiliates of the underwriters who are broker-dealers registered under the Securities Exchange Act of 1934.
The underwriters initially propose to offer the ordinary shares and ADSs to the public at the public offering price listed on the cover page of this prospectus. After the initial offering of the shares, the offering price and other selling terms may from time to time be varied by the representatives.
Cyclacel has granted to Morgan Stanley Securities Limited, as stabilizing manager, or its agent, on behalf of the underwriters, an option, exercisable from the date on which the offering price of the shares is determined until 30 days following admission and quotation of the ADSs on the Nasdaq National Market, to procure subscribers for or, failing which, to subscribe for up to an aggregate of 2,400,000 additional shares at the public offering price set forth on the cover page of this prospectus. The stabilizing manager, or its agent, on behalf of the underwriters, may exercise this option for the purpose of covering over-allotments, if any, made, or to cover short positions resulting from stabilization transactions performed, outside the United States and may exercise this option in the United States solely for the purpose of covering over-allotments, if any, made in connection with the offering of the shares offered by this prospectus in the United States. The portion of the over-allotment option applicable for sales in the United States may not exceed 15% of the total number of shares sold in the United States before the exercise of the over-allotment option. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to procure subscribers for or, failing which, to subscribe for about the same percentage of the additional shares as the number listed next to the underwriter's name in the preceding table bears to the total number of shares listed next to the names of all the underwriters in the preceding table. There is no obligation on the underwriters to exercise the over-allotment option. If the underwriters' option is exercised in full, the
134
total price to the public would be $ , the total underwriters' discounts and commissions would be $ and total proceeds to Cyclacel would be $ .
In connection with the over-allotment option, Morgan Stanley Securities Limited, as stabilizing manager, expects to enter into a share lending arrangement. Under this arrangement, will agree to lend Morgan Stanley Securities Limited the maximum amount of shares available under the over-allotment option for the 30-day period during which Morgan Stanley Securities Limited may exercise the over-allotment option.
The following table sets forth the estimated costs and expenses, other than the underwriting discounts and commissions, payable by the Company in connection with the sale of the securities being registered. All amounts are estimates except the SEC registration fee, the NASD filing fee and the Nasdaq National Market listing fee.
| SEC registration fee | | $ | | | 7,577 |
| NASD filing fee | | $ | | | 7,433 |
| Nasdaq National Market filing fee | | $ | | | 100,000 |
| U.K. Listing Authority listing fee | | $ | | | 17,870 |
| Accountant's fees and expenses | | $ | | | 361,920 |
| Legal fees and expenses | | $ | | | 1,496,480 |
| Printing and engraving costs | | $ | | | 298,584 |
| Miscellaneous | | $ | | | 520,370 |
| | |
| |
|
| Total | | $ | | | 2,810,234 |
| | |
| |
|
The underwriters have informed Cyclacel that they do not intend sales to discretionary accounts to exceed five percent of the total number of shares offered by them.
Application has been made to have the ADSs approved for quotation on the Nasdaq National Market under the symbol "CYCC". Application has also been made to the U.K. Listing Authority under the provisions of the U.K. Listing Rules, for the shares to be admitted to listing on the official list of the U.K. Listing Authority and to London Stock Exchange plc for the shares to be admitted to trading on the London Stock Exchange's main market for listed securities.
Each of Cyclacel, its directors, senior management and the shareholders of Cyclacel has entered into agreements with Morgan Stanley Securities Limited and SG Cowen & Co., LLC (in the case of Cyclacel, pursuant to the underwriting agreement and, in the case of the directors, senior management and the other shareholders, pursuant to agreements dated , 2004 (which we refer to as Lock-up Agreements) under which they have each agreed that, without the prior written consent of Morgan Stanley Securities Limited and SG Cowen & Co., LLC on behalf of the underwriters, he, she or it will not, during the period ending 360 days (450 days in the case of Spiro Rombotis, Paul McBarron and Dr. Robert Jackson) after admission (save as provided below):
- •
- offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of directly or indirectly, any ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares; or
- •
- enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of ordinary shares;
whether any such transaction described above is to be settled by delivery of ordinary shares or such other securities, in cash or otherwise. In the case of shareholders who are not directors or senior management, the above restrictions apply in respect of all of their shareholding at admission of the
135
shares to listing on the official list of the U.K. Listing Authority for the first 180 days of the 360-day period and in respect of 50 percent of their shareholding at admission for the remaining 180 days.
The restrictions described in the above paragraph do not apply to:
- •
- the issuance by Cyclacel of ordinary shares upon the exercise of an option or a warrant or the conversion of a security outstanding on the date of this prospectus of which the underwriters have been advised in writing;
- •
- transactions by any person other than Cyclacel or its directors or senior management relating to ordinary shares or other securities acquired in open market transactions after the completion of the offering of the shares;
- •
- acceptance by shareholders of a general offer made to all the holders of the issued and allotted shares of the Company for the time being (other than shares held or contracted to be acquired by the offeror or its associates within the meaning of section 430E of the U.K. Companies Act 1985) made in accordance with the U.K. City Code on Takeovers and Mergers on terms which treat all such holders alike and which has:
- (i)
- become or been declared unconditional in all respects; or
- (ii)
- been recommended for acceptance by the directors for the time being of the Company, or from executing and delivering an irrevocable commitment or undertaking to accept a general offer (without any further agreement to transfer or dispose of any ordinary shares or any interest therein) such as is mentioned above;
- •
- any transfer or disposal to an affiliate provided that, before registration of any such transfer, the transferee affiliate executes an agreement stating that the transferee is receiving and holding the securities subject to the provisions of the Lock-up Agreement and provided further that, in the event that the transferee affiliate ceases to hold such a position, it shall immediately prior to such event transfer the shares back to another entity which is (and was at the date of the Lock-up Agreement) an affiliate of the transferring affiliate;
- •
- any transfer by gift, charitable contribution, will or intestacy, or to a connected person, provided that it shall be a condition to the transfer that the transferee execute an agreement stating that the transferee is receiving and holding the securities subject to the provisions of the Lock-up Agreement and that no such transfer may include a disposition for value.
For the purposes of the Lock-up Agreements, we have defined affiliate as (i) in relation to a company, any subsidiary undertaking or parent undertaking of that company and any other subsidiary undertaking of that parent undertaking as at the date of the Lock Up Agreement; and (ii) in relation to a partnership, unit trust, investment trust, unincorporated association or other fund (or any trustee or nominee of such entity), any other partnership, unit trust, investment trust, unincorporated association or other fund (or any trustee or nominee of such entity) which is managed or advised by the same manager or adviser (or by an affiliate of such manager or adviser, as defined in (i) above) as at the date of the Lock Up Agreement.
For the purposes of the Lock Up Agreement, we have defined a connected person as (i) any person acting in his or her capacity as trustee of a trust of which a transferor shareholder is the settlor, provided that there are no persons beneficially interested under the trust other than that individual transferor shareholder or his spouse or any child under the age of 18; and (ii) any person who is the spouse, a sibling, a parent or a child of an individual transferor shareholder.
In order to facilitate the offering of the shares, the stabilizing manager, or its agent, on behalf of the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the shares. Specifically, the underwriters may sell more shares than for which they are obligated to procure
136
subscribers under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for subscription by the underwriters under the over allotment option. The underwriters can close out a covered short sale by exercising the over allotment option or purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the underwriters will consider, among other things, the open market price of shares compared to the price available under the over allotment option. The underwriters may also sell shares in excess of the over allotment option, creating a naked short position. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the ordinary shares in the open market after pricing that could adversely affect investors who purchase in the offering. As an additional means of facilitating the offering, the underwriters may bid for, and purchase, shares in the open market to stabilize the price of the shares. The underwriters may also impose a penalty bid. Penalty bids permit the underwriting syndicate to reclaim selling concessions allowed to an underwriter or a dealer for distributing the shares in the offering, if the syndicate repurchases previously-distributed shares to cover syndicate short positions or to stabilize the price of the shares. These activities may raise or maintain the market price of the shares above independent market levels or prevent or retard a decline in the market price of the shares. The underwriters are not required to engage in these activities, and may end any of these activities at any time.
No action has been or will be taken in any jurisdiction, except in the United States and the United Kingdom, that would permit a public offering of the shares, or the possession, circulation or distribution of this prospectus or any other material relating to Cyclacel or the shares, in any jurisdiction where action for that purpose is required.
The shares have not been and will not be registered under the Securities and Exchange Law of Japan. The shares may not be, and are not being, offered to more than 49 offerees in Japan. Neither the shares nor any beneficial interest therein may be transferred by any holder thereof in Japan to any other person unless such shares or such beneficial interests therein and all other shares or beneficial interests therein acquired by such holder are transferred in one lot to a single person, or to non-residents of Japan (as defined in the Foreign Exchange and Foreign Trade Law of Japan).
The shares will and may only be offered in the Netherlands to persons who trade or invest in securities in the conduct of their profession or trade (which includes banks, securities intermediaries (including dealers and brokers), insurance companies, pension funds, other institutional investors and commercial enterprises which as an ancillary activity regularly invest in securities).
From time to time, some of the underwriters, including Morgan Stanley Securities Limited and SG Cowen & Co., LLC, have provided, and expect to provide, investment banking services to Cyclacel.
Cyclacel and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
Subscribers for the shares may be required to pay stamp taxes and other charges in accordance with the laws and practices of the country of purchase in addition to the initial public offering price. For a discussion of the applicable U.K. and U.S. tax provisions, see "Taxation—U.K. Tax Considerations" and "Taxation—U.S. Federal Income Taxation," respectively.
Prior to this offering, there has been no public market for the ordinary shares. The initial public offering price will be determined by negotiations among Cyclacel and the representatives of the underwriters. Among the factors to be considered in determining the initial public offering price, in addition to prevailing market conditions, will be the future prospects of Cyclacel, including its business potential and earnings prospects, an assessment of Cyclacel's management, and the market prices of securities and certain financial and operating information of companies engaged in activities similar to
137
those of Cyclacel. The estimated initial public offering price range set forth on the cover page of this preliminary prospectus is subject to change as a result of market conditions and other factors.
Pursuant to the terms of the underwriting agreement, and in consideration of the respective rights and obligations conferred on the sponsor and the Company pursuant to the underwriting agreement, Morgan Stanley & Co. International Limited was appointed as sponsor in connection with admission of our shares to listing on the official list of the U.K. Listing Authority. No fees are payable by the Company in respect of this appointment.
We and each of our directors have given certain customary representations, warranties and undertakings, and we have given certain customary indemnities to, Morgan Stanley & Co. International Limited and the underwriters in connection with the underwriting agreement. The representations, warranties, undertakings and indemnities given by the Company are unlimited as to time or amount, which is customary for an agreement of this nature. The representations, warranties and undertakings given by the directors are unlimited as to time and are subject to limitations on amount related to the directors' annual remuneration, which is customary for an agreement of this nature. The aggregate amount of the directors' liability under the underwriting agreement is $ .
138
TAXATION
U.K. Tax Considerations
The following is the opinion of Allen & Overy LLP and is based on current U.K. tax legislation and Inland Revenue practice. These considerations may not apply to certain categories of shareholders or ADS holders such as dealers. If you are in any doubt as to your taxation position or if you are subject to tax in any jurisdiction other than the U.K., you should consult an appropriate professional adviser immediately.
Taxation of Dividends
Under current U.K. taxation legislation, no tax will be withheld or deducted for or on account of income tax from dividends paid by the company.
A U.K. resident individual shareholder will generally be entitled to a tax credit in respect of any dividend received. The amount of the tax credit is equal to one-ninth of the cash dividend or 10% of the aggregate of the cash dividend and the associated tax credit (the "Gross Dividend"). An individual shareholder who, taking account of the Gross Dividend he receives, is liable to income tax at the basic or starting rate will pay income tax at 10 percent of the Gross Dividend so that the tax credit will satisfy his income tax liability on the Dividend Payment. An individual shareholder must, to the extent that this income, including the Gross Dividend, exceeds the threshold for higher rate income tax, pay income tax at 32.5 percent of the Gross Dividend. After deducting the tax credit, he would therefore have to account for additional income tax at 22.5 percent of the Gross Dividend.
U.K. resident shareholders who are not liable to U.K. tax on dividends, including pension funds and charities, will not be entitled to claim repayment of the tax credit attaching to dividends paid by the company.
A U.K. resident corporate shareholder will generally not be subject to U.K. corporation tax on dividends. Such shareholders will not be able to claim repayment of the tax credit attaching to dividends paid by the company.
A non-U.K. resident shareholder is not generally entitled to the benefit of a tax credit in respect of any dividend received. A non U.K. resident shareholder may also be subject to foreign taxation on dividend income under local law. A shareholder who is not resident in the U.K. (for tax purposes) should consult his own tax adviser concerning his tax liabilities on dividends.
The tax treatment described above will also apply where dividends are received in respect of shares held in ADS form.
Taxation of Capital Gains
A holder who is not resident or ordinarily resident in the U.K. and whose shares (or ADSs) are not attributable to a trade, profession or vocation carried on in the United Kingdom through a branch or agency (or permanent establishment) will not be subject to U.K. tax on capital gains realized on a disposal of the shares (or ADSs), save as mentioned in the following paragraph in relation to individuals. Such a holder may however be liable to non-U.K. tax under local law. A disposal of shares by a shareholder (or ADSs by an ADS holder) who is resident or, in the case of an individual, ordinarily resident for tax purposes in the United Kingdom or who is not U.K.-resident but carries on a trade, profession or vocation in the United Kingdom through a branch or agency (or permanent establishment in the case of a holder which is a company) to which the shares (or ADSs) are attributable, may, depending on the holder's circumstances and subject to any available exemption or relief, give rise to a chargeable gain or allowable loss for the purposes of the taxation of chargeable gains. A holder who is an individual and who has, on or after March 17, 1998, ceased to be resident or
139
ordinarily resident for tax purposes in the United Kingdom for a period of less than 5 complete tax years and who disposes of the shares (or ADSs) during that period may also be liable to U.K. taxation of chargeable gains (subject to any available exemption or relief) if that holder returns to the United Kingdom as resident or ordinarily resident within that period.
On a disposal of shares (or ADSs) by an individual who is resident or ordinarily resident in the United Kingdom for taxation purposes, the shares (or ADSs) may attract taper relief, which reduces the amount of chargeable gains according to how long the shares (or ADSs) have been held.
A holder who is a company resident in the United Kingdom for tax purposes will benefit from indexation allowance which, in general terms, increases the capital gains tax base cost of an asset in accordance with changes in the retail prices index.
U.K. Stamp Duty and Stamp Duty Reserve Tax
No stamp duty or stamp duty reserve tax ("SDRT"), is payable on the issue of the shares other than an issue to issuers of depositary receipts or providers of clearance services (or their nominees or agents). Investors who receive shares in the form of ADSs will not be required to pay any UK stamp duty or SDRT in respect of the initial issuance of such ADSs.
The transfer on sale of a share outside the CREST system will generally be liable to ad valorem stamp duty at the rate of 0.5% of the amount or value of the consideration for the transfer rounded up to the nearest £5. The purchaser normally pays the stamp duty.
An unconditional agreement to sell a share will generally give rise to a liability on the purchaser to SDRT at the rate of 0.5% of the amount or value of the consideration for the sale. If a duly stamped transfer in respect of the agreement is produced within six years of the date that the agreement is entered into or (if later) the date that it becomes unconditional, any SDRT paid is repayable, generally with interest, and any unpaid SDRT charge is cancelled.
Issues or transfers of shares (1) to, or to a nominee or agent for, a person whose business is or includes issuing depositary receipts or (2) to, or to a nominee or agent for, a person whose business is or includes providing clearance services, will generally be subject to stamp duty or SDRT at 1.5% of the amount or value of the consideration or, in certain circumstances, the value of the shares transferred or the same price (rounded up to the nearest £5 in the case of stamp duty). Strictly the depositary or clearance operator, or its nominee, as the case may be, will be accountable for this liability for stamp duty or SDRT. However, it will in practice generally be reimbursed by participants in the clearance service or depositary receipt scheme. As noted above, special arrangements have been made in relation to the payment of SDRT on the initial issue of shares in ADS form.
No U.K. stamp duty will be payable on the acquisition of any ADS or any subsequent transfer of an ADS provided that the transfer and any subsequent instrument of transfer remains at all times outside the United Kingdom and that the instrument of transfer is not executed in or brought into the United Kingdom. An agreement to transfer an ADS will not give rise to SDRT. On a transfer of shares from the custodian to a holder of an ADS upon cancellation of the ADS, a fixed stamp duty of £5 per instrument of transfer will be payable. Any transfer for value of the underlying shares represented by ADSs or agreement to transfer these underlying shares may give rise to a liability on the transferee to stamp duty or SDRT.
Clearance service providers may make an election under Section 97A of the Finance Act 1986 in respect of the shares. Under Section 97A of the Finance Act 1986, clearance services may, provided they meet certain conditions, elect for the 0.5% rate of stamp duty or SDRT to apply to transfers of securities both to and within such systems instead of the 1.5% rate applying to an issue or transfer of such securities into the clearance service and exemption for dealings within the clearance system.
140
Under the CREST system for paperless share transfers, no stamp duty or SDRT will arise on a transfer of shares into the system unless such transfer is made for a consideration in money or money's worth, in which case a liability to SDRT (usually at the rate of 0.5%) will arise. Paperless transfers of shares within CREST will be liable to SDRT rather than stamp duty.
THE ABOVE SUMMARIES REFLECT CERTAIN ASPECTS OF CURRENT LAW AND PRACTICE IN THE UNITED KINGDOM. PROSPECTIVE SHAREHOLDERS WHO ARE IN DOUBT AS TO THEIR TAX POSITION OR WHO MAY BE SUBJECT TO TAX IN ANY OTHER JURISDICTION SHOULD CONSULT THEIR PROFESSIONAL ADVISERS.
U.S. Federal Income Taxation
The following is the opinion of Allen & Overy LLP and describes the material U.S. federal income tax consequences that are relevant with respect to the acquisition, ownership and disposition of ordinary shares and/or ADSs by a U.S. Holder (as defined below). This summary addresses only U.S. federal income tax considerations of U.S. Holders that will hold ordinary shares and /or ADSs as capital assets. It does not purport to be a comprehensive description of all the tax considerations that may be relevant to a decision to purchase ordinary shares and/or ADSs. In particular, this summary does not address all of the tax considerations applicable to holders that may be subject to special tax rules including, without limitation, the following: (a) financial institutions; (b) insurance companies; (c) dealers or traders in securities, currencies or notional principal contracts; (d) tax-exempt entities; (e) persons that will hold ordinary shares and/or ADSs as part of a "hedging" or "conversion" transaction or as a position in a "straddle" or as part of a "synthetic security" or other integrated transaction for U.S. federal income tax purposes; (f) persons that have a "functional currency" other than the U.S. dollar; (g) persons that own (or are deemed to own) 10% or more (by voting power) of our share capital; (h) regulated investment companies; (i) persons who hold ordinary shares and/or ADSs through partnerships or other pass-through entities; (j) real estate investment trusts; and (k) S corporations. Further, this summary does not address alternative minimum tax consequences.
This summary is based on the Internal Revenue Code of 1986, as amended (the Code), U.S. Treasury regulations and judicial and administrative interpretations thereof, in each case as in effect and available on the date of this prospectus. All of the foregoing is subject to change, which change could apply retroactively and could affect the tax consequences described below.
Each prospective investor should consult its own tax advisor with respect to the U.S. federal, estate, state, local, gift and other tax consequences of acquiring, owning and disposing of the ordinary shares and/or ADSs. U.S. Holders should also review the discussion under "Taxation—U.K. Tax Considerations" for the U.K. tax consequences to a U.S. Holder of the ownership of the ordinary shares and/or ADSs.
For purposes of this summary a "U.S. Holder" is a beneficial owner of ordinary shares and/or ADSs that is, for U.S. federal income tax purposes: (a) a citizen or resident of the United States; (b) a corporation or other entity treated as a corporation, created or organized in or under the laws of the United States or any state thereof (including the District of Columbia); (c) an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or (d) a trust if (i) a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of the substantial decisions of such trust or (ii) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. If a partnership holds ordinary shares and/or ADSs, the consequences to a partner will generally depend upon the status of the partner and upon the activities of the partnership. A partner of a partnership holding ordinary shares and/or ADSs should consult its own tax advisor. A "Non-U.S. Holder" is a beneficial owner of ordinary shares and/or ADSs that is not a U.S. Holder.
141
For U.S. federal income tax purposes, a holder of ADSs generally will be treated as the owner of the shares represented by such ADSs.
Distributions
Subject to the discussion below under "Passive Foreign Investment Company Considerations," the gross amount of any distribution that is actually or constructively received by a U.S. Holder with respect to its ordinary shares (including shares deposited in respect of ADSs) will be a dividend includible in gross income of a U.S. Holder as ordinary income. Dividends paid on ordinary shares and/or ADSs generally will constitute income from sources outside the United States and will not be eligible for the "dividends received" deduction to United States corporate shareholders. The gross amount of any dividend paid in foreign currency will be included in the gross income of a U.S. Holder in an amount equal to the U.S. dollar value of the foreign currency calculated by reference to the exchange rate in effect on the date the foreign currency is received by the Depositary, in the case of ADSs, or the U.S. Holder, in the case of ordinary shares, regardless of whether the foreign currency is converted into U.S. dollars. If the foreign currency is converted into U.S. dollars on the date of receipt by the Depositary, in the case of ADSs, or the U.S. Holder, in the case of ordinary shares, a U.S. Holder generally should not be required to recognise foreign currency gain or loss in respect of the dividend. If the foreign currency received is not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any gain or loss on a subsequent conversion or other disposition of the foreign currency will be treated as ordinary income or loss, and will generally be income or loss from sources within the United States for foreign tax credit limitation purposes. The amount of any distribution of property other than cash will be the fair market value of the property on the date of the distribution.
Under recent U.S. tax legislation (the 2003 Tax Act), certain distributions received by individuals (as well as certain trusts and estates) in taxable years beginning before January 1, 2009, generally will be taxed at a preferential tax rate (rather than the higher rates of tax generally applicable to items of ordinary income). This preferential rate is only applicable to distributions paid by "qualified corporations" and only with respect to ordinary shares and/or ADSs held for a minimum holding period of more than 61 days during a specified 121-day period. If we are a passive foreign investment company (PFIC) (as discussed below under "Passive Foreign Investment Company Considerations"), we will not be considered a "qualified corporation" under the 2003 Tax Act. Accordingly, if we are a PFIC distributions paid by us with respect to ordinary shares and/or ADSs will not be eligible for a reduced income tax rate. If we are not a PFIC, distributions paid by us will so qualify. Prospective investors should consult their own tax advisors regarding the implications of the 2003 Tax Act.
A distribution of additional shares and/or ADSs to U.S. Holders with respect to their ordinary shares and/or ADSs that is part of a pro rata distribution of ordinary shares to all shareholders (including holders of ADSs) generally will not be subject to U.S. federal income tax.
For purposes of calculating the foreign tax credit, dividends paid on ordinary shares and/or ADSs will be treated as income from sources outside the United States and will generally constitute "passive income" or, in the case of certain U.S. Holders, "financial services income". Under the 2003 Tax Act, the amount of the qualified dividend income, if any, paid to a U.S. Holder that may be subject to the reduced dividend income tax rate that is taken into account for purposes of calculating the U.S. Holder's U.S. foreign tax credit limitation must be reduced by the "rate differential portion" of the dividend (which, assuming a U.S. Holder is in the highest income tax bracket, would generally require a reduction of the dividend amount by approximately 57.14%). Prospective investors should consult their own tax advisors regarding the implications of the 2003 Tax Act for them, in light of their particular situation.
142
Subject to the discussion under "Backup Withholding and Information Reporting," a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax on dividends received on ordinary shares and /or ADSs unless that income is effectively connected with the conduct by that Non-U.S. Holder of a trade or business within the United States.
Sale or Other Disposition
Subject to the discussion below under "Passive Foreign Investment Company Considerations," a U.S. Holder will generally recognize a gain or loss for U.S. federal income tax purposes upon the sale or exchange of ordinary shares and/or ADSs in an amount equal to the difference between the U.S. dollar value of the amount realized from such sale or exchange and the U.S. Holder's tax basis in such ordinary shares and/or ADSs. Such gain or loss will be a capital gain or loss and will be long-term capital gain (taxable at a reduced rate for individuals, trusts or estates) if the ordinary shares and/or ADSs were held for more than one year. Any such gain or loss would generally be treated as from sources within the United States. The deductibility of capital losses is subject to significant limitations.
A U.S. Holder that receives foreign currency on the sale or other disposition of ordinary shares and/or ADSs will realise an amount equal to the U.S. dollar value of the foreign currency on the date of sale (or, in the case of cash basis and electing accrual basis taxpayers, the U.S. dollar value of the foreign currency on the settlement date). If a U.S. Holder receives foreign currency upon a sale or exchange of ordinary shares and/or ADSs, gain or loss, if any, recognised on the subsequent sale, conversion or disposition of such foreign currency will be ordinary income or loss, and will generally be income or loss from sources within the United States for foreign tax credit limitation purposes. However, if such foreign currency is converted into U.S. dollars on the date received by the U.S. Holder, a cash basis or electing accrual U.S. Holder should not recognise any gain or loss on such conversion.
Subject to the discussion below under "Backup Withholding and Information Reporting," a Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax on any gain realized on the sale or exchange of ordinary shares and/or ADSs unless: (a) that gain is effectively connected with the conduct by that Non-U.S. Holder of a trade or business in the United States, or (b) in the case of any gain realized by an individual Non-U.S. Holder, that holder is present in the United States for 183 days or more in the taxable year of the sale or exchange and certain other conditions are met.
Redemption
Subject to the discussion below under "Passive Foreign Investment Company Considerations," a redemption of ordinary shares and/or ADSs by us will be treated as a sale of the redeemed ordinary shares and/or ADSs by the U.S. Holder (which is taxable as described under "Sale or Other Disposition") or, in certain circumstances, as a distribution to the U.S. Holder (which is taxable as described under "Distributions").
Passive Foreign Investment Company Considerations
We believe that we most likely will be a PFIC for U.S. federal income tax purposes, for our current taxable year and we may be a PFIC in any future year. If we are a PFIC in any year, special, possibly materially adverse, consequences would result (as discussed below) for U.S. Holders. If we are classified as a PFIC in any year that a U.S. Holder is a shareholder, we generally will continue to be treated as a PFIC for that U.S. Holder in all succeeding years, regardless of whether we continue to meet the income or asset test described below, unless the U.S. Holder has made an election to recognize gain as if such U.S. Holder sold its PFIC stock as of the last day of the last taxable year for which we were a PFIC (with the consequences described below).
143
A corporation organized outside the United States generally will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which either: (a) at least 75% of its gross income is "passive income" (the "income test"), or (b) at least 50% of the gross average quarterly fair market value of its assets is attributable to assets that produce "passive income" or are held for the production of passive income (the "asset test"). Passive income for this purpose generally includes dividends, interest, royalties, rents and gains from commodities and securities transactions. In determining whether it is a PFIC a foreign corporation is required to take into account a pro rata portion of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest.
Whether we will meet the income test for our current taxable year or for any future taxable year will depend on a number of factors, including the receipt of licensing income. In addition, because our book assets will consist largely of cash or cash equivalents, including short term financial investments, for the foreseeable future, whether we pass the asset test for our current taxable year or in the foreseeable future will depend largely on the value of our pipeline and goodwill, generally determined by reference to our quarterly average stock market price. Because the market price of biotechnology companies may be volatile, we cannot offer any assurance that we will not be a PFIC for any taxable year, even if the income test is satisfied.
If we are regarded as a PFIC in any year during which a U.S. Holder owns ordinary shares and/or ADSs, the U.S. Holder will be subject to additional taxes on any excess distributions received from us and any gain realized from the sale or other disposition, including a pledge, of the ordinary shares and/or ADSs (whether or not we continue to be a PFIC). A U.S. Holder has an excess distribution to the extent that distributions on the ordinary shares and/or ADSs during a taxable year exceed 125% of the average amount received during the three preceding taxable years (or, if shorter, the U.S. Holder's holding period). To compute the tax on the excess distributions or any gain, (a) the excess distribution or the gain is allocated ratably over the U.S. Holder's holding period, (b) the amount allocated to the current taxable year and any year before we became a PFIC is taxed as ordinary income in the current year, and (c) the amount allocated to other taxable years is taxed at the highest applicable marginal rate in effect for each year and an interest charge is imposed to recover the deemed benefit from the deferred payment of the tax attributable to each year.
Some of the rules with respect to distributions and dispositions described above may be avoided if a U.S. Holder makes a valid "mark-to-market" election (in which case, subject to certain limitations, the U.S. Holder would essentially be required to take into account the difference, if any, between the fair market value and the adjusted tax basis of its ordinary shares and/or ADSs at the end of a taxable year as ordinary income (or, to the extent of any gain previously taken into account under these rules, ordinary loss), in calculating its income for such year). In addition, gains from an actual sale or other disposition of ordinary shares and/or ADSs will be treated as ordinary income, and any losses will be treated as ordinary losses to the extent of any "mark-to-market" gains for prior years. A "mark-to-market" election is only available to U.S. Holders in any tax year that the PFIC stock is considered "regularly traded" on a "qualified exchange" within the meaning of applicable U.S. Treasury regulations. PFIC stock is "regularly traded" if, among other requirements, it is traded on at least 15 days during each calendar quarter. Each of the London Stock Exchange and Nasdaq will constitute a qualified exchange for this purpose. Investors should consult their own tax advisors as to whether the ordinary shares and/or ADSs would qualify for the mark-to-market election. Once made, such election cannot be revoked without the consent of the U.S. Internal Revenue Service unless the shares and/or ADSs cease to be marketable. This election will not be available to U.S. Holders in the 2004 tax year unless our stock is "regularly traded" on one sixth (1/6) of the days remaining in the quarter in which this offering occurs, and on at least 15 days during each remaining quarter of the calendar year.
The above rules may also be avoided if a U.S. Holder is eligible for and timely makes a valid "QEF election." We will comply with all reporting requirements necessary for a U.S. Holder to make a QEF election and will, within a reasonable time following the end of any taxable year in which we
144
determine that we are properly classified as a PFIC, provide to registered holders of ordinary shares with U.S. addresses (including the Depositary), and to other registered holders on request, information necessary for such an election. The Depositary has agreed to distribute such information to registered holders of ADRs.
A U.S. Holder that makes this election will be required in each taxable year to include (a) as long-term capital gain its pro rata share of our net capital gain (i.e. the excess of net long-term capital gain over net short-term capital loss for our taxable year ending with or within the U.S. Holder's taxable year) and (b) as ordinary income its pro rata share of our ordinary earnings (i.e. the excess of current earnings and profits for such taxable year over such net capital gain), regardless of whether we distribute such amounts to the U.S. Holder. For this purpose, a U.S. Holder's pro rata share of our ordinary income and net capital gain is the amount which would have been distributed to the U.S. Holder if, on each day during our taxable year, we had distributed to each holder of an equity interest a pro rata share of that day's ratable share of our ordinary earnings and net capital gain for such year. A U.S. Holder will not be eligible for the dividends received deduction in respect of such income or gain. In addition, any losses in a taxable year will not be available to the U.S. Holder and may not be carried back or forward in computing our ordinary earnings and net capital gain in other taxable years. If we distribute the income or gain that was previously included in the U.S. Holder's gross income, such distributions will be non-taxable to the US. Holder. For purposes of determining gain or loss on the disposition (including redemption or retirement) of ordinary shares and/or ADSs, a U.S. Holder's initial tax basis in the ordinary shares and/or ADSs, respectively, will be increased by the amount so included in gross income with respect to the ordinary shares and/or ADSs and decreased by the amount of any non-taxable distributions on the ordinary shares and/or ADSs. In general, a U.S. Holder making a timely QEF election will recognise, on the sale or disposition (including redemption and retirement) of ordinary shares and/or ADSs, capital gain or loss equal to the difference, if any, between the amount realised upon such sale or disposition and that U.S. Holder's adjusted tax basis in those ordinary shares and/or ADSs. Such gain will be long-term if the U.S. Holder held the ordinary shares and/or ADSs for more than one year on the date of disposition.
Each U.S. Holder who desires to make a QEF election must individually make the QEF election. The QEF election is effective for the U.S. Holder's taxable year for which it is made and all subsequent taxable years and may not be revoked without the consent of the U.S. Internal Revenue Service. In general, a U.S. Holder must make a QEF election on or before the due date for filing its income tax return for the first year to which the QEF election will apply.
Based on the nature of our expected income, the expected composition of our assets, and our business prospects, we do not currently expect to have significant ordinary earnings or net capital gain in any taxable year in which we may be classified as a PFIC. Accurate predictions of the nature of our income and the composition of our assets, however, are particularly difficult in view of the volatile nature of the earnings patterns in technological industries such as emerging pharmaceutical and biotechnology industries and certain changes in available interest rates. Accordingly, there can be no assurance that our expectations described above will be fulfilled.Prospective investors should consult their own tax advisers concerning the applicability of the PFIC rules and the merits of making a QEF election if we are a PFIC for any taxable year.
If we are regarded as a PFIC, each U.S. Holder of ordinary shares and/or ADSs must make an annual return on U.S. Internal Revenue Service Form 8621, reporting distributions received and gains realized with respect to each PFIC in which it holds a direct or indirect interest. The reduced tax rate for dividend income, as discussed above under "Distributions," is not applicable to dividends paid by a PFIC.
Prospective investors are urged to consult their own tax advisors regarding the consequences of an investment in a PFIC.
145
Backup Withholding and Information Reporting
Backup withholding and information reporting requirements may apply to certain payments to U.S. Holders of dividends on ordinary shares and/or ADSs and to the proceeds of a sale or redemption of an ordinary share and/or ADS. We, our agent, a broker, or any paying agent, as the case may be, may be required to withhold tax from any payment that is subject to backup withholding at a rate of 28% (which rate may be subject to change in the future) of such payment if the U.S. Holder fails (a) to furnish the U.S. Holder's taxpayer identification number, (b) to certify that such U.S. Holder is not subject to backup withholding or (c) to otherwise comply with the applicable requirements of the backup withholding rules. Certain U.S. Holders (including, among others, corporations) are not subject to the backup withholding and information reporting requirements. Non-U.S. Holders who hold their ordinary shares and/or ADSs through a U.S. broker or agent or through the U.S. office of a non-U.S. broker or agent may be required to comply with applicable certification procedures to establish that they are not U.S. Holders in order to avoid the application of such information reporting requirements and backup withholding. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a U.S. Holder generally may be claimed as a credit against such U.S. Holder's U.S. federal income tax liability provided that the required information is furnished to the U.S. Internal Revenue Service.
Prospective investors should consult their own tax advisors as to their qualification for exemption from backup withholding and the procedure for obtaining this exemption.
146
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market in the United States, the United Kingdom or elsewhere for our shares or ADSs. Although we expect to have our ADSs approved for listing on the Nasdaq National Market, we cannot assure you that there will be an actual public market for our shares or ADSs. Future sales of substantial amounts of our shares or ADSs in the public market following this offering or the anticipation of these sales occurring could adversely affect prevailing market prices for our shares and ADSs or could impair our ability to raise capital through an offering of equity securities in the future. For a further discussion of this risk, see "Risk Factors—Risks Related to Our Ordinary Shares and This Offering—Shares or ADSs eligible for public sale after this offering could adversely affect the price of our ordinary shares."
Our officers, directors and shareholders, who collectively hold an aggregate of 44,964,304 shares, and the underwriters have entered into lock-up agreements in connection with this offering. These lock-up agreements provide that, with limited exceptions, our officers, directors and other shareholders have agreed not to offer, sell, contract to sell, grant any option to purchase or otherwise dispose of any of our shares for a period after the effective date of this offering. Details of these agreements and the periods for which they apply are set out on page 135.
Upon the completion of this offering, we will have 60,964,304 ordinary shares outstanding, based on the number of shares outstanding as of June 30, 2004, immediately following the reorganization described under "Pre-offering Reorganization into Public Limited Company" beginning on page 114, and also reflects the conversion of our preferred ordinary D shares into 20,840,344 of our ordinary shares (based on an assumed initial public offering price of £1.66 per ordinary share, being the midpoint of the price range set forth on the cover of this prospectus) and the further bonus issue of shares described under "Description of Share Capital" beginning on page 116. All of the ordinary shares sold in this offering in the form of ADSs will be freely tradable in the U.S. public market without restriction under the Securities Act except for any shares purchased by our "affiliates" as that term is defined in Rule 144 under the Securities Act. Some of the remaining ordinary shares held by existing shareholders are "restricted" shares as that term is defined in Rule 144 under the Securities Act. We issued and sold the restricted shares in private transactions in reliance upon exemptions from registration under the Securities Act. Restricted shares may be sold in the public market only if they are registered under the Securities Act or if they qualify for an exemption from registration, such as Rule 144 under the Securities Act, which is summarized below.
In general, under Rule 144 as currently in effect, a person, or persons whose shares are aggregated, who has beneficially owned restricted shares for at least one year, including the holding period of any prior owner except an affiliate, would be entitled to sell within any three-month period a number of ordinary shares that does not exceed the greater of:
- •
- one percent of the number of ordinary shares then outstanding, which will equal approximately 609,643 ordinary shares immediately after the offering, or
- •
- the average weekly trading volume of the ADSs on the Nasdaq National Market during the four calendar weeks preceding the filing of a Form 144 with respect to such sale.
Sales under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us. Under Rule 144(k), a person who is not deemed to have been an affiliate of our company at any time during the three months preceding a sale, and who has beneficially owned the shares proposed to be sold for at least two years including the holding period of any prior owner except an affiliate, is entitled to sell the ordinary shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144.
Upon [completion of this offering], our existing shareholders or their permitted transferees will be entitled to request that we register certain of their ordinary shares under the Securities Act, although
147
this entitlement will be subject to the lock-up arrangements described on page 135 of this prospectus. See "Related Party Transactions—Registration Rights Agreement."
WHERE YOU CAN FIND MORE INFORMATION ABOUT US
As a result of this offering, we will be subject to the informational requirements of the Securities Exchange Act of 1934 and will file reports and other information with the SEC. You may read and copy all or any portion of the reports and their exhibits at the public reference facilities maintained by the SEC at Room 1024, Judiciary Plaza, 450 Fifth Street, N.W., Washington, D.C. 20549, and at its regional offices at Citicorp Center, 500 West Madison Street, Suite 1400, Chicago, Illinois 60661-2511. Copies of such material may also be obtained at prescribed rates by writing to the Public Reference Section of the SEC at 450 Fifth Street, N.W., Washington, D.C., 20549. You may obtain more information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website that contains reports and information about issuers, like us, who file electronically with the SEC. The address of that web site is www.sec.gov.
We have filed with the SEC a registration statement on Form F-1 under the Securities Act, as amended, with respect to the securities offered in this prospectus. This prospectus does not contain all of the information set forth in the registration statement, certain portions of which are omitted as permitted by the rules and regulations of the SEC. You may inspect and copy the registration statement, including exhibits, at the SEC's public reference facilities or on the website referred to above.
On our behalf, The Bank of New York, the depositary in respect of the ADSs, will distribute to the holders of ADSs on an annual basis an annual report and interim reports. We will provide The Bank of New York with copies of any other notices, reports and communications we give to our shareholders and we will ask The Bank of New York to forward copies of these to you.
LEGAL MATTERS
The validity of the shares and ADSs offered hereby has been passed upon for us by Allen & Overy LLP, our U.S. and English counsel. Certain legal matters under U.S. law will be passed upon for us by Allen & Overy LLP, and for the underwriters by Davis Polk & Wardwell, the underwriters' U.S. counsel. In addition, Foley Hoag LLP has served as our U.S. counsel in connection with intellectual property and FDA regulatory matters.
EXPERTS
Ernst & Young LLP, independent auditors, have audited our consolidated financial statements at March 31, 2003, December 31, 2003 and March 31, 2004, and for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, the three months ended March 31, 2003 and 2004 and for the period from August 13, 1996 (inception) to March 31, 2004, as set forth in their report. We have included our financial statements in the prospectus and registration statement in reliance on Ernst & Young LLP's report, given on their authority as experts in accounting and auditing.
SERVICE OF PROCESS AND ENFORCEMENT OF CIVIL LIABILITIES
We are organized under the laws of England and Wales. Most of our directors and executive officers named in this prospectus are residents outside the United States. A substantial portion of our assets and those of such directors, officers and experts are located outside the United States. As a result, it may be difficult for you:
- •
- to effect service of process within the United States upon us or such persons;
- •
- to enforce outside the United States judgments obtained against such persons in U.S. courts; or
148
- •
- to enforce in U.S. courts judgments obtained against such persons in courts to jurisdictions located outside the United States;
in each case in any action, including actions predicated upon the civil liability provisions of U.S. securities laws.
In addition, it may be difficult for you to enforce, in actions brought in courts in jurisdictions located outside the United States, rights predicated upon the U.S. securities laws. For further information see the risk factor "Your rights as a shareholder will be governed by English law and differ from the rights of shareholders under U.S. law."
We have designated CT Corporation System, located at 111 Eighth Avenue, New York, NY 10011 (telephone: (212) 894-8600), as our agent for service of process in the United States with respect to the ordinary shares.
149
Until , 2004, 25 days after the date of this prospectus, all dealers that buy, sell or trade our shares or ADSs, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Index to Consolidated Financial Statements
| | Page
|
|---|
| | | |
| Report of Independent Auditors | | F-2 |
Consolidated Balance Sheets as of March 31, 2003, December 31, 2003 and March 31, 2004 |
|
F-3 |
Consolidated Statements of Operations for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, the three months ended March 31, 2003 and 2004 and the period from August 13, 1996 (inception) to March 31, 2004 |
|
F-5 |
Consolidated Statement of Shareholders' Equity (Deficit) for the period from August 13, 1996 (inception) to March 31, 2004 |
|
F-6 |
Consolidated Statements of Cash Flows for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, the three months ended March 31, 2003 and 2004 and the period from August 13, 1996 (inception) to March 31, 2004 |
|
F-10 |
Notes to the Consolidated Financial Statements |
|
F-12 |
F-1
CYCLACEL GROUP PLC
(A Development Stage Company)
REPORT OF INDEPENDENT AUDITORS
The Board of Directors
Cyclacel Group plc
We have audited the consolidated balance sheets of Cyclacel Group plc (a development stage company) at March 31, 2003, December 31, 2003, and March 31, 2004, and the related consolidated statements of operations, shareholders' equity (deficit) and cash flows for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, the three months ended March 31, 2003 and 2004, and the period from August 13, 1996 (inception) to March 31, 2004. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with United Kingdom auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Cyclacel Group plc (a development stage company) at March 31, 2003, December 31, 2003 and March 31, 2004 and the consolidated results of its operations and its consolidated cash flows for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, the three months ended March 31, 2003 and 2004 and the period from August 13, 1996 (inception) to March 31, 2004 in conformity with United States generally accepted accounting principles.
ERNST & YOUNG LLP
Cambridge, England
July 1, 2004
F-2
CYCLACEL GROUP PLC
(A Development Stage Company)
CONSOLIDATED BALANCE SHEETS
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| ASSETS | | | | | | |
| Current assets: | | | | | | |
| | Cash and cash equivalents | | 16,558 | | 9,670 | | 5,685 |
| | Short-term investments | | 1,575 | | 24,010 | | 33,826 |
| | Prepaid expenses and other current assets | | 3,353 | | 5,360 | | 3,892 |
| | |
| |
| |
|
| | Total current assets | | 21,486 | | 39,040 | | 43,403 |
| Property, plant and equipment (net) | | 3,940 | | 3,760 | | 4,117 |
| Other non current assets—deferred issuance costs Preferred Ordinary "C" shares | | 1,455 | | — | | — |
| | |
| |
| |
|
| Total assets | | 26,881 | | 42,800 | | 47,520 |
| | |
| |
| |
|
LIABILITIES AND SHAREHOLDERS' EQUITY (DEFICIT) |
|
|
|
|
|
|
| Current liabilities: | | | | | | |
| | Bank overdraft | | 11 | | — | | 145 |
| | Current portion of Government loan | | 394 | | — | | 457 |
| | Accounts payable | | 1,776 | | 2,354 | | 1,808 |
| | Accrued liabilities | | 1,712 | | 1,292 | | 2,165 |
| | Other current liabilities | | 187 | | 182 | | 205 |
| | Current portion of equipment financing | | 913 | | 829 | | 697 |
| | |
| |
| |
|
| | Total current liabilities | | 4,993 | | 4,657 | | 5,477 |
| Equipment financing, net of current | | 184 | | 50 | | 334 |
| Government loan, net of current | | — | | 445 | | — |
Preferred Ordinary "C" shares, 0.1p par value: |
|
|
|
|
|
|
| | Authorized: 4,800,000 at March 31, 2003 and Nil at December 31, 2003 and March 31, 2004 | | | | | | |
| | Issued and outstanding: 4,540,969 at March 31, 2003 and Nil at December 31, 2003 and March 31, 2004 | | 53,851 | | — | | — |
| | |
| |
| |
|
| Total liabilities | | 59,028 | | 5,152 | | 5,811 |
| | |
| |
| |
|
| Commitments and contingencies | | | | | | |
See accompanying notes
F-3
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
| |
|---|
| | $000
| | $000
| | $000
| |
|---|
| Shareholders' equity (deficit) | | | | | | | |
Preferred Ordinary shares: |
|
|
|
|
|
|
|
| Preferred Ordinary "D" shares, 0.1p par value: | | | | | | | |
| | Authorized: Nil at March 31, 2003 and 21,000,000 at December 31, 2003 and March 31, 2004, | | | | | | | |
| | Issued and outstanding: Nil at March 31, 2003, 16,742,691 at December 31, 2003 and 17,904,759 at March 31, 2004 | | — | | 28 | | 30 | |
| Ordinary shares, 0.1p par value: | | | | | | | |
| | Authorized: 5,730,000 at March 31, 2003 and 5,748,428 at December 31, 2003 and March 31, 2004 | | | | | | | |
| | Issued and outstanding: 3,769,631 at March 31, 2003 and 1,546,432 at December 31, 2003 and March 31, 2004 | | 6 | | 2 | | 2 | |
Deferred shares, 0.1p par value: |
|
|
|
|
|
|
|
| | Authorized: Nil at March 31, 2003 and 7,051,572 at December 31, 2003 and March 31, 2004, | | | | | | | |
| | Issued and outstanding: Nil at March 31, 2003 and 6,792,541 at December 31, 2003 and March 31, 2004 | | — | | 10 | | 10 | |
Additional paid in capital |
|
23,700 |
|
109,564 |
|
117,450 |
|
| Deferred stock-based compensation | | (349 | ) | (132 | ) | (82 | ) |
| Accumulated other comprehensive loss | | (2,253 | ) | (3,596 | ) | (2,585 | ) |
| Deficit accumulated during the development stage | | (53,251 | ) | (68,228 | ) | (73,116 | ) |
| | |
| |
| |
| |
| Total shareholders' equity (deficit) | | (32,147 | ) | 37,648 | | 41,709 | |
| | |
| |
| |
| |
| Total liabilities and shareholders' equity (deficit) | | 26,881 | | 42,800 | | 47,520 | |
| | |
| |
| |
| |
See accompanying notes
F-4
CYCLACEL GROUP PLC
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF OPERATIONS
| |
| |
| |
| |
| |
| |
| | Period from
August 13,
1996
(inception) to
March 31,
2004
| |
|---|
| | Year ended March 31,
| | Nine months
ended December 31,
| | Three months
ended March 31,
| |
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
| |
|---|
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| Revenues: | | | | | | | | | | | | | | | | | | | | | | |
| | Collaboration and research and development income | | | 1,155 | | | 1,250 | | | 930 | | | 8 | | | 319 | | | 1 | | | 2,413 | |
| | Grant income | | | 55 | | | 941 | | | 696 | | | 504 | | | 246 | | | — | | | 2,387 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| | | | 1,210 | | | 2,191 | | | 1,626 | | | 512 | | | 565 | | | 1 | | | 4,800 | |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | Research and development | | | (13,195 | ) | | (19,847 | ) | | (14,581 | ) | | (13,084 | ) | | (5,275 | ) | | (4,870 | ) | | (67,594 | ) |
| | General and administrative | | | (3,220 | ) | | (2,536 | ) | | (2,026 | ) | | (2,099 | ) | | (504 | ) | | (1,573 | ) | | (15,625 | ) |
| | Amortization of employee stock-based compensation | | | (672 | ) | | (305 | ) | | (229 | ) | | (217 | ) | | (76 | ) | | 618 | | | (1,991 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Total operating expenses | | | (17,087 | ) | | (22,688 | ) | | (16,836 | ) | | (15,400 | ) | | (5,855 | ) | | (5,825 | ) | | (85,210 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Operating loss | | | (15,877 | ) | | (20,497 | ) | | (15,210 | ) | | (14,888 | ) | | (5,290 | ) | | (5,824 | ) | | (80,410 | ) |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | |
| Interest income | | | 1,464 | | | 1,028 | | | 839 | | | 430 | | | 185 | | | 435 | | | 4,402 | |
| Interest expense | | | (440 | ) | | (470 | ) | | (358 | ) | | (2,005 | ) | | (112 | ) | | (20 | ) | | (3,510 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Total other income (expense) | | | 1,024 | | | 558 | | | 481 | | | (1,575 | ) | | 73 | | | 415 | | | 892 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Loss before taxes | | | (14,853 | ) | | (19,939 | ) | | (14,729 | ) | | (16,463 | ) | | (5,217 | ) | | (5,409 | ) | | (79,518 | ) |
| Taxes | | | — | | | (4,397 | ) | | (3,841 | ) | | (1,486 | ) | | (528 | ) | | (521 | ) | | (6,403 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net loss | | | (14,853 | ) | | (15,542 | ) | | (10,888 | ) | | (14,977 | ) | | (4,689 | ) | | (4,888 | ) | | (73,115 | ) |
| Dividends on Preferred shares | | | (3,289 | ) | | (4,654 | ) | | (3,430 | ) | | (4,425 | ) | | (1,225 | ) | | (2,629 | ) | | (14,997 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net loss applicable to ordinary shareholders | | | (18,142 | ) | | (20,196 | ) | | (14,318 | ) | | (19,402 | ) | | (5,914 | ) | | (7,517 | ) | | (88,112 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net loss per share—Basic and Diluted | | $ | (2.83 | ) | $ | (3.14 | ) | $ | (2.23 | ) | $ | (2.25 | ) | $ | (0.92 | ) | $ | (0.39 | ) | $ | (15.65 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Weighted average shares used to compute basic and diluted earnings per share | | | 6,399,539 | | | 6,433,996 | | | 6,433,874 | | | 8,623,516 | | | 6,433,996 | | | 19,259,641 | | | 5,629,029 | |
| | |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes
F-5
CYCLACEL GROUP PLC
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT)
Period from August 13, 1996 (inception) to March 31, 2004
| | Preferred Ordinary "D" shares
| | Ordinary shares
| | Deferred shares
| | Additional
paid-in
capital
| | Accumulated
other
comprehensive
income/(loss)
| | Deferred
compensation
| | Deficit
accumulated during
development
stage
| | Total
| |
|---|
| | No.
| | $000
| | No.
| | $000
| | No.
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| On incorporation, August 13, 1996 | | — | | — | | 1 | | — | | — | | — | | — | | — | | — | | — | | — | |
| Subdivision into shares of $0.0015 each, August 1996 | | — | | — | | 999 | | — | | — | | — | | — | | — | | — | | — | | — | |
| Issue of shares for cash, at par, September 1996 | | — | | — | | 959,000 | | 1 | | — | | — | | — | | — | | — | | — | | 1 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | (4 | ) | — | | — | | (4 | ) |
| Loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (290 | ) | (290 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (294 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 1997 | | — | | — | | 960,000 | | 1 | | — | | — | | — | | (4 | ) | — | | (290 | ) | (293 | ) |
Issue of shares for cash, at $6.56 per share, May 1997 |
|
— |
|
— |
|
625,000 |
|
1 |
|
— |
|
— |
|
4,098 |
|
— |
|
— |
|
— |
|
4,099 |
|
| Issue of shares for IP rights agreement, May 1997 | | — | | — | | 40,000 | | — | | — | | — | | 262 | | — | | — | | — | | 262 | |
| Issue of shares for cash, at $6.56 per share, August 1997 | | — | | — | | 25,000 | | — | | — | | — | | 159 | | — | | — | | — | | 159 | |
| Expense of share issues | | — | | — | | — | | — | | — | | — | | (41 | ) | — | | — | | — | | (41 | ) |
| Deferred share based compensation | | — | | — | | — | | — | | — | | — | | 2,002 | | — | | (2,002 | ) | — | | — | |
| Amortization of deferred share based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | 302 | | — | | 302 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | 55 | | — | | — | | 55 | |
| Loss for the year | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (2,534 | ) | (2,534 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (2,479 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 1998 | | — | | — | | 1,650,000 | | 2 | | — | | — | | 6,480 | | 51 | | (1,700 | ) | (2,824 | ) | 2,009 | |
Exercise of share options for cash, at par, July 1998 |
|
— |
|
— |
|
4,792 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
| Amortization of deferred share-based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | 406 | | — | | 406 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | 11 | | — | | — | | 11 | |
| Loss for the year | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (3,964 | ) | (3,964 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (3,953 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 1999 | | — | | — | | 1,654,792 | | 2 | | — | | — | | 6,480 | | 62 | | (1,294 | ) | (6,788 | ) | (1,538 | ) |
See accompanying notes
F-6
CYCLACEL GROUP PLC
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT) (Continued)
Period from August 13, 1996 (inception) to March 31, 2004
| | Preferred Ordinary "D" shares
| | Ordinary shares
| | Deferred shares
| | Additional
paid-in
capital
| | Accumulated
other
comprehensive
income/(loss)
| | Deferred
compensation
| | Deficit
accumulated during
development
stage
| | Total
| |
|---|
| | No.
| | $000
| | No.
| | $000
| | No.
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| Issue of shares for cash at $7.42, May 1999 | | — | | — | | 872,188 | | 1 | | — | | — | | 6,470 | | — | | — | | — | | 6,471 | |
| Issue of shares on conversion of bridging loan, May 1999 | | — | | — | | 220,751 | | 1 | | — | | — | | 1,637 | | — | | — | | — | | 1,638 | |
| Issue of shares in lieu of cash bonus, May 1999 | | — | | — | | 22,075 | | — | | — | | — | | 164 | | — | | — | | — | | 164 | |
| Issue of shares for research & development agreement, May 1999 | | — | | — | | 55,188 | | — | | — | | — | | 409 | | — | | — | | — | | 409 | |
| Issue of shares for cash at $7.65, August 1999 | | — | | — | | 840,336 | | 2 | | — | | — | | 6,430 | | — | | — | | — | | 6,432 | |
| Exercise of share options for cash at $7.28, September 1999 | | — | | — | | 5,519 | | — | | — | | — | | 40 | | — | | — | | — | | 40 | |
| Expense of share issues | | — | | — | | — | | — | | — | | — | | (186 | ) | — | | — | | — | | (186 | ) |
| Deferred share-based compensation | | — | | — | | — | | — | | — | | — | | 167 | | — | | (167 | ) | — | | — | |
| Amortization of deferred share-based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | 433 | | — | | 433 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | (194 | ) | — | | — | | (194 | ) |
| Loss for the year | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (5,686 | ) | (5,686 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (5,880 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 2000 | | — | | — | | 3,670,849 | | 6 | | — | | — | | 21,611 | | (132 | ) | (1,028 | ) | (12,474 | ) | 7,983 | |
| Deferred share-based compensation | | — | | — | | — | | — | | — | | — | | 294 | | — | | (294 | ) | — | | — | |
| Amortization of deferred share-based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | 275 | | — | | 275 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
Translation adjustment |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(466 |
) |
— |
|
— |
|
(466 |
) |
| Loss for the year | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (10,382 | ) | (10,382 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (10,848 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 2001 | | — | | — | | 3,670,849 | | 6 | | — | | — | | 21,905 | | (598 | ) | (1,047 | ) | (22,856 | ) | (2,590 | ) |
See accompanying notes
F-7
CYCLACEL GROUP PLC
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT) (Continued)
Period from August 13, 1996 (inception) to March 31, 2004
| | Preferred Ordinary "D" shares
| | Ordinary shares
| | Deferred shares
| | Additional
paid-in
capital
| | Accumulated
other
comprehensive
income/(loss)
| | Deferred
compensation
| | Deficit
accumulated during
development
stage
| | Total
| |
|---|
| | No.
| | $000
| | No.
| | $000
| | No.
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| Exercise of share options for cash at par, April 2001 | | — | | — | | 3,050 | | — | | — | | — | | — | | — | | — | | — | | — | |
| Exercise of share options for cash at par, April 2001 | | — | | — | | 46,950 | | — | | — | | — | | — | | — | | — | | — | | — | |
| Issue of shares for cash at $10.64, June 2001 | | — | | — | | 13,282 | | — | | — | | — | | — | | — | | — | | — | | — | |
| Exercise of share options for cash at $6.04, July 2001 | | — | | — | | 17,500 | | — | | — | | — | | 106 | | — | | — | | — | | 106 | |
| Issue of shares for licence agreement at $11.42, November 2001 | | — | | — | | 16,000 | | — | | — | | — | | 183 | | — | | — | | — | | 183 | |
| Fair value of warrants issued to shareholders, August and December 2001 | | — | | — | | — | | — | | — | | — | | 1,215 | | — | | — | | — | | 1,215 | |
| Deferred share based compensation | | — | | — | | — | | — | | — | | — | | 363 | | — | | (363 | ) | — | | — | |
| Amortization of deferred share-based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | 672 | | — | | 672 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | 191 | | — | | — | | 191 | |
| Loss for the year | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (14,853 | ) | (14,853 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (14,662 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 2002 | | — | | — | | 3,767,631 | | 6 | | — | | — | | 23,772 | | (407 | ) | (738 | ) | (37,709 | ) | (15,076 | ) |
Exercise of share options for cash at $5.84, May 2002 |
|
— |
|
— |
|
2,000 |
|
— |
|
— |
|
— |
|
12 |
|
— |
|
— |
|
— |
|
12 |
|
| Deferred share-based compensation | | — | | — | | — | | — | | — | | — | | (84 | ) | — | | 84 | | — | | — | |
| Amortization of deferred share-based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | 305 | | — | | 305 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | (1,846 | ) | — | | — | | (1,846 | ) |
| Loss for the year | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (15,542 | ) | (15,542 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (17,388 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 2003 | | — | | — | | 3,769,631 | | 6 | | — | | — | | 23,700 | | (2,253 | ) | (349 | ) | (53,251 | ) | (32,147 | ) |
See accompanying notes
F-8
CYCLACEL GROUP PLC
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT) (Continued)
Period from August 13, 1996 (inception) to March 31, 2004
| | Preferred Ordinary "D" shares
| | Ordinary shares
| | Deferred shares
| | Additional
paid-in
capital
| | Accumulated
other
comprehensive
income/(loss)
| | Deferred
compensation
| | Deficit
accumulated during
development
stage
| | Total
| |
|---|
| | No.
| | $000
| | No.
| | $000
| | No.
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| Exercise of share options for cash at $7.17, April 2003 | | — | | — | | 15,957 | | — | | — | | — | | 114 | | — | | — | | — | | 114 | |
| Exercise of share options for cash at $6.65, October 2003 | | — | | — | | 100 | | — | | — | | — | | — | | — | | — | | — | | — | |
| Conversion of Ordinary and Preferred "C" Ordinary shares to Deferred Shares, November 2003 | | — | | — | | (2,251,572 | ) | (4 | ) | 6,792,541 | | 10 | | 58,142 | | — | | — | | — | | 58,148 | |
| Bonus issue of shares, November 2003 | | 12,666,580 | | 21 | | — | | — | | — | | — | | (21 | ) | — | | — | | — | | — | |
| Issue of shares for cash at $6.90, November 2003 | | 4,076,111 | | 7 | | 12,316 | | — | | — | | — | | 28,221 | | — | | — | | — | | 28,228 | |
| Expense of share issues | | — | | — | | — | | — | | — | | — | | (592 | ) | — | | — | | — | | (592 | ) |
| Amortization of deferred share-based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | 217 | | — | | 217 | |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | (1,343 | ) | — | | — | | (1,343 | ) |
| Loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (14,977 | ) | (14,977 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (16,320 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at December 31, 2003 | | 16,742,691 | | 28 | | 1,546,432 | | 2 | | 6,792,541 | | 10 | | 109,564 | | (3,596 | ) | (132 | ) | (68,228 | ) | 37,648 | |
| Issues of shares for cash at $7.44, January 2004 | | 1,162,068 | | 2 | | — | | — | | — | | — | | 8,644 | | — | | — | | — | | 8,646 | |
| Expense of share issue | | — | | — | | — | | — | | — | | — | | (90 | ) | — | | — | | — | | (90 | ) |
| Deferred share based compensation | | — | | — | | — | | — | | — | | — | | (668 | ) | — | | 668 | | — | | — | |
| Amortization of deferred share-based compensation | | — | | — | | — | | — | | — | | — | | — | | — | | (618 | ) | — | | (618 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Translation adjustment | | — | | — | | — | | — | | — | | — | | — | | 1,011 | | — | | — | | 1,011 | |
| Loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (4,888 | ) | (4,888 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| |
| Comprehensive loss for the period | | — | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (3,877 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance at March 31, 2004 | | 17,904,759 | | 30 | | 1,546,432 | | 2 | | 6,792,541 | | 10 | | 117,450 | | (2,585 | ) | (82 | ) | (73,116 | ) | 41,709 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
F-9
CYCLACEL GROUP PLC
(A Development Stage Company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| |
| |
| |
| |
| |
| |
| | Period from
August 13,
1996
(inception) to
March 31,
2004
| |
|---|
| | Year ended
March 31,
| | Nine months
ended December 31,
| | Three months ended March 31,
| |
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
| |
|---|
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| Operating activities: | | | | | | | | | | | | | | | |
| Net loss | | (14,853 | ) | (15,542 | ) | (10,888 | ) | (14,977 | ) | (4,689 | ) | (4,888 | ) | (73,420 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | | | |
| | Depreciation and amortization | | 1,112 | | 1,474 | | 1,100 | | 1,133 | | 373 | | 372 | | 5,473 | |
| | Deferred revenue | | 1,230 | | (1,328 | ) | (1,039 | ) | — | | (286 | ) | — | | (98 | ) |
| | Compensation for warrants issued to non employees | | 1,215 | | — | | — | | — | | — | | — | | 1,215 | |
| | Shares issued for IP rights | | 184 | | — | | — | | — | | — | | — | | 446 | |
| | Loss on fixed assets disposals | | — | | — | | — | | — | | — | | — | | 23 | |
| | Amortization of deferred share-based compensation | | 672 | | 305 | | 229 | | 217 | | 76 | | (618 | ) | 2,296 | |
| | Amortization of issuance costs of Preferred Ordinary "C" shares | | 253 | | 338 | | 254 | | 1,925 | | 84 | | — | | 2,517 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | | | | |
| | Prepaid expenses and other current assets | | 69 | | (1,932 | ) | (1,483 | ) | (1,808 | ) | (446 | ) | 1,620 | | (3,187 | ) |
| | Accounts payable and other current liabilities | | 295 | | 974 | | 714 | | (871 | ) | 259 | | 249 | | 3,398 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net cash used in operating activities | | (9,823 | ) | (15,711 | ) | (11,113 | ) | (14,381 | ) | (4,629 | ) | (3,265 | ) | (61,337 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Investing activities: | | | | | | | | | | | | | | | |
| Purchase of plant, property and equipment | | (1,223 | ) | (819 | ) | (846 | ) | (111 | ) | — | | (151 | ) | (5,680 | ) |
| Short-term investments on deposit, net of maturities | | (10,697 | ) | 9,120 | | 9,093 | | (22,435 | ) | — | | (9,223 | ) | (33,233 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net cash used in investing activities | | (11,920 | ) | 8,301 | | 8,247 | | (22,546 | ) | — | | (9,374 | ) | (38,913 | ) |
| | |
| |
| |
| |
| |
| |
| |
| |
| Financing activities: | | | | | | | | | | | | | | | |
| Payments of capital lease obligations | | (263 | ) | (838 | ) | (605 | ) | (716 | ) | (194 | ) | (350 | ) | (2,441 | ) |
| Proceeds from issuance of ordinary and preferred ordinary shares, net of issuance costs | | 38,079 | | (27 | ) | (27 | ) | 27,440 | | — | | 8,574 | | 91,530 | |
| Loans received | | — | | — | | — | | — | | — | | — | | 10,942 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Net cash provided by (used in) financing activities | | 37,816 | | (865 | ) | (632 | ) | 26,724 | | (194 | ) | 8,224 | | 100,031 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Effect of exchange rate changes on cash and cash equivalents | | (93 | ) | 3,072 | | 3,427 | | 3,328 | | (309 | ) | 285 | | 5,759 | |
| Net increase (decrease) in cash and cash equivalents | | 16,073 | | (8,275 | ) | (3,498 | ) | (10,205 | ) | (4,823 | ) | (4,415 | ) | (219 | ) |
| Cash and cash equivalents, beginning of period | | 5,770 | | 21,750 | | 21,750 | | 16,547 | | 21,679 | | 9,670 | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| Cash and cash equivalents, end of period | | 21,750 | | 16,547 | | 21,679 | | 9,670 | | 16,547 | | 5,540 | | 5,540 | |
| | |
| |
| |
| |
| |
| |
| |
| |
See accompanying notes
F-10
| |
| |
| |
| |
| |
| |
| | Period from
August 13,
1996
(inception) to
March 31,
2004
|
|---|
| | Year ended
March 31,
| | Nine months
ended
December 31,
| | Three months ended March 31,
|
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
|
|---|
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
|
|---|
| Supplemental cash flow information: | | | | | | | | | | | | | | |
| Cash received during the period for: | | | | | | | | | | | | | | |
| | Interest (net) | | 941 | | 1,240 | | 1,090 | | 259 | | 165 | | 22 | | 3,401 |
| | Taxes | | — | | 2,549 | | 2,549 | | — | | — | | 2,193 | | 4,742 |
Schedule of non-cash transactions |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Acquisitions of equipment purchased through capital leases | | 1,046 | | 524 | | 375 | | 384 | | 150 | | 479 | | 3,243 |
| Issuance of Ordinary shares in connection with license agreements | | 184 | | — | | — | | — | | — | | — | | 592 |
| Issuance of Ordinary shares on conversion of bridging loan | | — | | — | | — | | — | | — | | — | | 1,638 |
| Issuance of Preferred Ordinary "C" shares on conversion of secured convertible loan notes and accrued interest | | 8,893 | | — | | — | | — | | — | | — | | 8,893 |
| Issuance of Ordinary shares in lieu of cash bonus | | — | | — | | — | | — | | — | | — | | 164 |
See accompanying notes
F-11
CYCLACEL GROUP PLC
(A Development Stage Company)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1 Formation and Business of the Company
Cyclacel Group plc (the "Company") was incorporated in Great Britain on April 1, 2004 as Alnery No. 2428 with an authorized and issued share capital of £100. The Company was established as a shell company with no assets, operations or other substance and was created solely to be the public listed company following the reorganization. On April 20, 2004, the Company changed its name to Cyclacel Group Limited. On June 30, 2004 the Company's ordinary shares of £1 each were sub-divided and redesignated into 100 ordinary shares of 1p each. On June 30, 2004 its authorized share capital was increased to £267,484 by the creation of 5,738,428 Ordinary shares and 21,000,000 Preferred D shares.
On June 30, 2004 the Company acquired all of the issued and outstanding Preferred Ordinary "D" shares, Ordinary shares and Deferred shares of Cyclacel Limited in exchange for an equivalent number of Preferred Ordinary "D" shares, Ordinary shares and Deferred shares in the Company. The former Cyclacel Limited shareholders now hold an equivalent number of shares in Cyclacel Group plc and Cyclacel Limited is now a wholly owned subsidiary of Cyclacel Group plc. On July 1, 2004 it reregistered as a public limited company and changed its name to Cyclacel Group plc.
The combination will be accounted for as a combination of entities under common control and these financial statements are presented as if the combination had been in effect since the inception of Cyclacel Limited on August 13, 1996.
The principal activity of the Company is research and development of therapeutics for cancer and other serious diseases. Through December 31, 2003, the Company, operating from research facilities in Dundee, Scotland and Cambridge, England, has been primarily engaged in conducting research, developing drug candidates, recruiting personnel and raising capital.
The Company has not yet generated substantial revenues from its operations. Accordingly, through the date of these financial statements, the Company is considered to be in the development stage.
The Company financial statements have been prepared assuming the Company will continue as a going concern. The Company has sustained operating losses since inception and expects such losses to continue as it furthers its research and development programs.
The Company's fiscal year end since incorporation was March 31. However, the Company changed its fiscal year end to December 31 during 2003 in anticipation of an initial public offering and this resulted in shortening of the March 31, 2004 fiscal year to the nine-month period ended December 31, 2003.
2 Summary of Significant Accounting Policies
The financial information contained in these financial statements does not constitute statutory accounts as defined in section 240 of the Companies Act 1985, as amended, of Great Britain. Statutory accounts for the years ended March 31, 2003 and 2002 on which the auditors' reports were unqualified and did not contain a statement under section 237(2) or (3) of that Act have been delivered to the Registrar of Companies for England and Wales ("Registrar"). Statutory accounts for the nine months ended December 31, 2003 on which the auditors' report was unqualified have not yet been delivered to the Registrar.
F-12
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Concentration of Credit Risk and Other Risks and Uncertainties
Financial instruments which potentially subject the Company to concentrations of risk consist principally of cash and cash equivalents, short-term investments and accounts receivable. The Company's cash and cash equivalents are invested in deposits with five major banks in the United Kingdom. Deposits in these banks may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash, cash equivalents, short-term investments and accounts receivable.
The Company does not perform an ongoing credit evaluation of its customers' financial conditions and generally does not require collateral to secure accounts receivable. The Company's exposure to credit risk, associated with non-payment is affected principally by conditions or occurrences within its customers' operations. The Company historically has not experienced any losses relating to accounts receivable from its primary customer. 95%, 56% and 57% of the Company's revenues for the years ended March 31, 2002 and 2003 and the nine months ended December 31, 2002, respectively, were derived from one customer. The arrangements with this customer came to a conclusion during the nine months ended December 31, 2003.
Drug candidates developed by the Company may require approvals or clearances from the U.S. Food and Drug Administration ("FDA") or other international regulatory agencies prior to commercialized sales. There can be no assurance that the Company's drug candidates will receive any of the required approvals or clearances. If the Company was denied approval or clearance or such approval was delayed, it may have a material adverse impact on the Company.
Foreign currency and currency translation
Monetary assets and liabilities in foreign currencies are translated into pounds sterling at the rate ruling at the date of transaction. Monetary assets and liabilities denominated in foreign currencies are retranslated into pounds sterling at the rate of exchange ruling at the balance sheet date. Transaction gains and losses are recognized in operating expenses within the Statement of Operations.
These financial statements are presented in U.S. dollars. Translation of balance sheet data from pounds sterling to U.S. dollars is made at the exchange rate ruling at the balance sheet date. Translation of operating statement and cash flow amounts is made at the average exchange rate for the period. Translation gains and losses are recognized within "other comprehensive income."
F-13
Cash and Cash Equivalents
Cash equivalents are stated at cost, which equates to market value. The Company considers all highly liquid investments with an original maturity of three months or less at the time of initial deposit to be cash equivalents.
Short-term Investments
The Company invests its surplus cash in bank term deposits, having a maturity period of between one day and one year. These deposits can be terminated early at a nominal cost. Accordingly, all cash resources with original maturity of three months or less have been classified as cash and cash equivalents and those with original maturity of more than three months as short-term investments.
Fair Value of Financial Instruments
For financial instruments consisting of cash and cash equivalents, short-term investments, accounts payable and accrued liabilities included in the Company's financial statements, the carrying amounts are reasonable estimates of fair value due to their short maturities. Based on borrowing rates currently available to the Company, the carrying value of the equipment financing lines approximate fair value.
Property, Plant and Equipment
Property, plant and equipment is stated at cost and depreciated on a straight-line basis over the estimated useful lives of the related assets, which are generally three to five years. Amortization of leasehold improvements is computed using the straight-line method over the shorter of the remaining lease term or the estimated useful life of the related assets, typically fifteen years. Upon sale or retirement of assets, the costs and related accumulated depreciation and amortization are removed from the balance sheet and the resulting gain or loss is reflected in operations. Maintenance and repairs are charged to operations as incurred.
Impairment of Long-Lived Assets
In accordance with the provisions of SFAS No. 144, "Accounting for the Impairment or Disposal of Long-Lived Assets," the Company reviews long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. Under SFAS No. 144, an impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount. Impairment, if any, is measured as the amount by which the carrying amount of a long-lived asset exceeds its fair value. Through December 31, 2003, there have been no such impairments.
Revenue Recognition
Revenues are earned from collaborative agreements and amounts invoiced to customers in respect of goods supplied. The Company recognizes revenue when persuasive evidence of an arrangement exists; delivery has occurred or services have been rendered; the fee is fixed and determinable; and collectability is reasonably assured. Determination of whether persuasive evidence of an arrangement
F-14
exists and whether delivery has occurred or services have been rendered are based on management's judgments regarding the fixed nature of the fee charged for research performed and milestones met, and the collectability of those fees. Should changes in conditions cause management to determine these criteria are not met for certain future transactions, revenue recognized for any reporting period could be adversely affected.
Research and development revenues, which are earned under agreements with third parties for contract research and development activities, are recorded as the related expenses are incurred. Milestone payments are non-refundable and recognized as revenue when earned, as evidenced by achievement of the specified milestones and the absence of ongoing performance obligations. Any amounts received in advance of performance are recorded as deferred revenue. None of the revenues recognized to date are refundable if the relevant research effort is not successful.
Grant revenues are recorded as research is performed. Grant revenues are not refundable.
Government grants in respect of capital expenditure are deferred and released to revenue over the estimated useful lives of the related assets by equal annual instalments.
Research and Development Expenditures
Research and development expenses consist primarily of costs associated with the Company's product candidates, upfront fees, milestones, compensation and other expenses for research and development personnel, supplies and development materials, costs for consultants and related contract research, facility costs, amortization of purchased technology and depreciation. Expenditures relating to research and development are expensed as incurred.
Patent Costs
Costs relating to prosecution are charged to operations as incurred as recoverability of such expenditure is uncertain.
Leased Assets
The costs of operating leases are charged to operations on a straight-line basis over the lease term.
Where the Company enters into a lease which entails taking substantially all the risks and rewards of ownership of an asset, the lease is treated as a capital lease. The asset is recorded in the balance sheet as an asset and is depreciated in accordance with the above depreciation policies. The capital elements of future lease payments are recorded as liabilities and the interest is charged to operations over the period of the lease.
Pension Costs
The Company operates a defined contribution pension plan. Contributions are charged to the operating statement as they become payable in accordance with the rules of the plan.
F-15
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
Credit is taken in the accounting period for research and development tax credits, which will be claimed from the Inland Revenue, in respect of qualifying research and development costs incurred in the same accounting period.
Net Loss Per Share and Anti-dilutive Securities
Basic and diluted net loss per share are presented in conformity with SFAS 128, "Earnings Per Share." Basic and diluted net loss per share have been computed using the weighted-average number of ordinary shares outstanding during the period. The following potentially dilutive outstanding securities were not considered in the computation of diluted net loss per share because they would be anti-dilutive:
| | Year ended March 31,
| | Nine months ended
December 31,
| | Three months ended March 31,
|
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
|
|---|
| | No. '000
| | No. '000
| | No. '000
| | No. '000
| | No. '000
| | No. '000
|
|---|
| Preferred Ordinary "C" shares | | 4,541 | | 4,541 | | 4,541 | | — | | 4,541 | | — |
| Preferred Ordinary "D" shares | | — | | — | | — | | 16,743 | | — | | 17,905 |
| Options to purchase Ordinary shares | | 744 | | 914 | | 914 | | 888 | | 914 | | 888 |
| Warrants | | 147 | | 147 | | 147 | | 200 | | 147 | | 200 |
| | |
| |
| |
| |
| |
| |
|
| | | 5,432 | | 5,602 | | 5,602 | | 17,831 | | 5,602 | | 18,993 |
| | |
| |
| |
| |
| |
| |
|
For the purposes of calculating net loss per share both the Ordinary and Preferred Ordinary "D" shares have been included in the denominator because the Preferred "D" Ordinary shares have identical rights to the Ordinary shares other than the dividend payable to Ordinary "D" shareholders on a winding up. In addition, the Preferred Ordinary "D" Shares convert to Ordinary shares upon an initial public offering. The weighted average number of shares used in the calculation of net loss per share has been retrospectively adjusted to account for the bonus issue(s) made in November 2003.
Stock-based Compensation
The Company accounts for stock-based employee compensation arrangements in accordance with the provisions of Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to Employees," ("APB 25"). Under APB 25, compensation expense is based on the difference, if any, on the date of grant of the option, between the estimated fair value of the Company's ordinary shares and the exercise price of the option.
The Company accounts for equity instruments issued to non employees in accordance with the provisions of Statement of Financial Accounting Standards ("SFAS") No. 123, "Accounting for
F-16
Stock-Based Compensation," and Emerging Issues Task Force ("EITF") Issue No. 96-18, "Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling Goods, or Services." SFAS No. 123 defines a "fair value" based method of accounting for an employee stock option or similar equity investment.
As the determination of the fair value of all options granted to employees after such time as Company becomes a public Company will include an expected volatility factor in addition to the factors described in the following table, the following results may not be representative of future periods.
The following table illustrates the effect on net loss if the Company had applied the fair value recognition provisions of SFAS 123 to stock-based employee compensation arrangements (in thousands):
| | Years ended March 31,
| | Nine months ended
December 31,
| | Three months ended March 31,
| |
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
| |
|---|
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| Net loss applicable to Ordinary shareholders, as reported | | | (18,142 | ) | | (20,196 | ) | | (14,318 | ) | | (19,402 | ) | | (5,914 | ) | | (7,517 | ) |
| Add: Stock-based employee compensation included in reported loss | | | 672 | | | 305 | | | 229 | | | 217 | | | 76 | | | (618 | ) |
| Less: total stock-based employee compensation determined under fair value based method for all awards | | | (1,005 | ) | | (1,150 | ) | | (886 | ) | | (791 | ) | | (264 | ) | | (196 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Adjusted net loss | | | (18,475 | ) | | (21,041 | ) | | (14,975 | ) | | (19,976 | ) | | (6,102 | ) | | (8,331 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Net loss per Ordinary share, basic and diluted: | | | | | | | | | | | | | | | | | | | |
| | As reported | | $ | (2.83 | ) | $ | (3.14 | ) | $ | (2.33 | ) | $ | (2.25 | ) | $ | (0.92 | ) | $ | (0.39 | ) |
| | |
| |
| |
| |
| |
| |
| |
| | Adjusted | | $ | (2.89 | ) | $ | (3.27 | ) | $ | (2.33 | ) | $ | (2.32 | ) | $ | (0.95 | ) | $ | (0.43 | ) |
| | |
| |
| |
| |
| |
| |
| |
The fair value of each option granted is estimated on the date of grant using the Black-Scholes option valuation model with the following weighted average assumptions:
| | Years ended March 31,
| | Nine months ended
December 31,
| | Three months ended March 31,
| |
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
| |
|---|
| Risk free interest rate | | 3.96 | % | 3.72 | % | 3.72 | % | 3.72 | % | 3.72 | % | 3.72 | % |
| Expected life (in years) | | 5.35 | | 5.46 | | 5.46 | | 5.46 | | 5.46 | | 5.46 | |
| Volatilty | | 90 | % | 90 | % | 90 | % | 90 | % | 90 | % | 90 | % |
| Dividend yield | | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % |
Based on the above assumptions, the weighted average estimated minimum values of options granted were $5.74 and $6.28 per share for the years ended March 31, 2002 and 2003, respectively, and
F-17
$6.28 for the nine months ended December 31, 2002 and 2003, and the three months ended March 31, 2003 and 2004.
The amortization of employee stock-based compensation charge of $1,991,000 from August 13, 1996 (inception) to March 31, 2004 was allocated $305,000 and $1,686,000 to research and development and general and administrative, respectively. The charges of $672,000, $305,000, $229,000, $217,000, $76,000 and $(618,000) for employee stock-based compensation were allocated to general and administrative for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003 and the three months ended March 31, 2003 and 2004, respectively.
Comprehensive Income (Loss)
In accordance with SFAS No. 130, "Reporting Comprehensive Income," all components of comprehensive income (loss), including net income (loss), are reported in the financial statements in the period in which they are recognized. Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net income (loss) and other comprehensive income (loss), including foreign currency translation adjustments, are reported, net of any related tax effect, to arrive at comprehensive income (loss).
Recent Accounting Pronouncements
In January 2003, the FASB issued FASB Interpretation No. 46 ("FIN 46"), "Consolidation of Variable Interest Entities, an Interpretation of ARB No. 51." FIN 46 requires certain variable interest entities to be consolidated by the primary beneficiary of the entity if the equity investors in the entity do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support from other parties. In December 2003, the FASB issued FIN 46R, a revision to FIN 46. FIN 46R provides a broad deferral of the latest date by which all public entities must apply FIN 46 to certain variable interest entities to the first reporting period ended after March 15, 2004. As the Company has no variable interest entitites, the adoption of FIN 46 will not have an impact on the Company's financial position, cash flows or results of operations.
In May 2003, the FASB issued SFAS No. 150, "Accounting For Certain Financial Instruments with Characteristics of Both Liabilities and Equity," which establishes standards for how an issuer of financial instruments classifies and measures certain financial instruments with characteristics of both liabilities and equity. It requires that an issuer classify a financial instrument that is within its scope as a liability (or in some circumstances, as an asset) if, at inception, the monetary value of the obligation is based solely or predominantly on: (a) a fixed monetary amount known at inception, (b) variations in something other than the fair value of the issuer's equity shares or (c) variations inversely related to changes in the fair value of the issuer's equity shares. SFAS No. 150 is effective for financial instruments entered into or modified after May 31, 2003, and otherwise is effective at the beginning of the first interim period beginning after June 15, 2003. The adoption of SFAS No. 150 did not have an impact on the consolidated financial statements.
F-18
3 Significant Contracts
Licensing and Research Agreements
The Company has entered into licensing agreements with academic and research organisations. Under the terms of these agreements, the Company has received licenses to technology and patent applications. The Company is required to pay royalties on future sales of product employing the technology or falling under claims of patent applications. Additional payments are due if the Company sublicenses the technology or patent applications or if the Company achieves predefined milestones.
In respect of Licensing Agreements, additional payments of $23.4 million would be payable if the Company achieves predefined milestones subject to achievement of all the specific contractual milestones and the Company's decision to continue with these projects. Under these agreements the Company makes annual payments that do not and will not exceed $0.1 million.
Clinical Collaborations
The Company has entered into a number of agreements with clinical research organizations (CROs) based at various universities and hospitals. The maximum annual amount payable on any of the existing contracts is approximately $640,000 and the annual aggregate cost is approximately $1,821,000. The contracts vary in length with the last to expire/conclude in June 2005.
4 Cash and Cash Equivalents
The following is a summary of cash and cash equivalents at March 31, 2003, December 31, 2003, and March 31, 2004:
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| Cash | | 16,558 | | 756 | | 1,120 |
| Deposits with original maturity of less than three months | | — | | 8,914 | | 4,565 |
| | |
| |
| |
|
| | | 16,558 | | 9,670 | | 5,685 |
| | |
| |
| |
|
5 Prepaid Expenses and Other Current Assets
The following is a summary of prepaid expenses and other current assets at March 31, 2003, December 31, 2003 and March 31, 2004:
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| Research & development tax credit | | 1,881 | | 3,730 | | 2,175 |
| Sales tax receivable | | 192 | | 373 | | 387 |
| Prepayments | | 596 | | 892 | | 713 |
| Deferred issuance costs Preferred Ordinary "C" shares | | 338 | | — | | — |
| Other current assets | | 346 | | 365 | | 617 |
| | |
| |
| |
|
| | | 3,353 | | 5,360 | | 3,892 |
| | |
| |
| |
|
F-19
6 Property, Plant and Equipment
Property, plant and equipment consisted of the following:
| | Useful lives in years
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
| |
|---|
| |
| | $000
| | $000
| | $000
| |
|---|
| Leasehold improvements | | Life of lease (15 yrs) | | 409 | | 462 | | 474 | |
| Research & laboratory equipment | | 3 to 5 yrs | | 6,521 | | 7,602 | | 8,416 | |
| Office equipment and furniture | | 3 to 5 yrs | | 1,079 | | 1,159 | | 1,207 | |
| | | | |
| |
| |
| |
| | | | | 8,009 | | 9,223 | | 10,097 | |
| Less accumulated depreciation and amortization | | | | (4,069 | ) | (5,463 | ) | (5,980 | ) |
| | | | |
| |
| |
| |
| | | | | 3,940 | | 3,760 | | 4,117 | |
| | | | |
| |
| |
| |
The depreciation and amortization of property, plant and equipment amounted to $1,474,000, $1,100,000, $1,133,000, $373,000 and $372,000 for the year ended March 31, 2003, the nine months ended December 31, 2002 and 2003, and the three months ended March 31, 2003 and 2004, respectively. These charges include depreciation of assets held under capital leases.
Depreciation and amortization expense for the period from inception (August 13, 1996) through to March 31, 2004 was $5,473,000. Included in property, plant and equipment are assets under capital lease obligations with an original cost of $2,520,000, $3,261,000 and $3,040,000 as of March 31, 2003, December 31, 2003 and March 31, 2004, respectively. Accumulated depreciation on assets under capital leases was $1,052,000, $1,701,000 and $1,884,000, respectively.
7 Other assets
Other assets consisted of the issuance costs of the Preferred Ordinary "C" shares as follows:
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| Current portion | | 338 | | — | | — |
| Noncurrent portion | | 1,455 | | — | | — |
| | |
| |
| |
|
| | | 1,793 | | — | | — |
| | |
| |
| |
|
Deferred issuance costs of $253,000, $338,000, $254,000 and $1,925,000 were charged to interest expense for the years ended March 31, 2002 and 2003 and the nine months ended December 31, 2002 and 2003, respectively. The accelerated charge in the nine months ended December 31, 2003 followed the conversion during November 2003 of the Preferred Ordinary "C" shares to Preferred Ordinary "D" shares.
F-20
8 Government Loan
The amounts outstanding under the Government loan are as follows:
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| Current liabilities | | 394 | | — | | 457 |
| Noncurrent liabilities | | — | | 445 | | — |
| | |
| |
| |
|
| | | 394 | | 445 | | 457 |
| | |
| |
| |
|
The Government loan of $445,000 (£250,000) had an interest rate of 7% per annum and was wholly repayable on September 16, 2003. The loan was renegotiated during the period ended December 31, 2003 with the repayment date being extended to January 31, 2005, together with an amended interest rate of 5% per annum from November 1, 2003. The loan is secured by a floating charge on the assets of the Company and there are no covenants attached to the loan.
Interest on the loan accrues evenly over the term of the loan. However, interest accrued in the first two years of the loan remains payable on the due repayment date of the loan. Interest accrued over the remainder of the term is payable quarterly in arrears.
9 Accounts Payable and Accrued Liabilities
Accounts payable and accrued liabilities consisted of the following:
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| Accounts payable | | 1,776 | | 2,354 | | 1,808 |
| Accrued liabilities | | 1,712 | | 1,292 | | 2,165 |
| Other current liabilities | | 187 | | 182 | | 205 |
| | |
| |
| |
|
| | | 3,675 | | 3,828 | | 4,178 |
| | |
| |
| |
|
10 Related Party Transactions
Private Placement
Cancer Research Technology Limited (CRT), formerly Cancer Research Campaign Technology Limited (CRCT) owns 482,473 ordinary shares of 0.1p each. As at March 31, 2004, this shareholding represented 5.8%, 2.6% and 2.5% of the Company's outstanding shares, March 31, 2003, December 31, 2003 and March 31, 2004, respectively.
License and Option Agreement
The Company has license and option agreements with CRT covering several technologies and research tools. The latest of these agreements terminates on September 10, 2005. CRT retains rights to materials and intellectual property outside the relevant fields, and for non-commercial research.
F-21
Fees Paid to Shareholders
The following fees were payable to shareholders for the services and expenses of their directors appointed to the Company.
| | Year ended
March 31
| | Nine months ended
December 31,
| | Three months ended
March 31,
|
|---|
| | 2002
| | 2003
| | 2002
| | 2003
| | 2003
| | 2004
|
|---|
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
|
|---|
| Merlin Venture Limited | | 18 | | 20 | | 15 | | 15 | | 5 | | 6 |
| Cancer Research Technology Limited, formerly Cancer Research Campaign Technology Limited | | 17 | | 9 | | 9 | | — | | 5 | | — |
| Kleinwort Benson Life Science Partnership | | 17 | | 20 | | 15 | | 10 | | 5 | | — |
| Invesco | | 14 | | 38 | | 25 | | 23 | | 10 | | 6 |
In addition, Dresdner Kleinwort Wasserstein Limited charged fees of $1,566,400 for the provision of professional services in relation to the raising of new funds during the year ended December 31, 2002.
The following fees were outstanding at the period end.
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| Merlin Venture Limited | | 5 | | 5 | | — |
| Cancer Research Campaign Technology Limited | | 5 | | — | | — |
| Kleinwort Benson Life Science Partnership | | 19 | | 32 | | 33 |
| Invesco | | 34 | | 55 | | 61 |
Services provided by CXR Biosciences Limited
CXR Biosciences Limited (Dundee, Scotland, UK), a contract research organization, charged costs for research services of $155,000, $917,000, $724,000, $447,000, $191,000 and $12,000 for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, and the three months ended March 31, 2003 and 2004, respectively. On August 14, 2003 Mr S G Rombotis acquired as part of a private equity financing approximately 2% of the equity of CXR Biosciences. Mr S G Rombotis does not participate in the Company's purchasing decisions with regard to CXR Biosciences which are subject to competitive tender from multiple vendors.
11 Commitments
Licensing and Research Agreements
The Company has entered into various research, license and collaboration agreements to support its research and development activities. At March 31, 2004 the Company had no financial commitments under these agreements which were unconditional on future performance.
F-22
Through March 31, 2004, the Company had no minimum royalty commitments under licensing and research agreements.
Leases
In October 2000, the Company entered into a 25 year lease for its new corporate headquarters and research and development facility in Dundee, UK.
Rent expense, which includes lease payments related to the Company's corporate headquarters and research and development facility and other rent related expenses, was $230,533, $395,037, $319,661, $344,172, $111,791 and $142,302 for the years ended March 31, 2002 and 2003, the nine months ended December 31, 2002 and 2003, and the three months ended March 31, 2003 and 2004, respectively.
As at March 31, 2004, the Company had $3,040,000 of property, plant and equipment financed through long-term obligations. The obligations under the equipment leases are secured by the equipment financed, bear interest at a weighted average rate of 7.2% and are due in monthly and quarterly installments through March 2007.
Annual future minimum payments are as follows at March 31, 2004:
| | Capital leases
| | Operating leases
| | Purchase obligations
|
|---|
| | $000
| | $000
| | $000
|
|---|
| 2004 | | 654 | | 499 | | 474 |
| 2005 | | 233 | | 521 | | 133 |
| 2006 | | 180 | | 544 | | — |
| 2007 | | 41 | | 568 | | — |
| 2008 | | — | | 553 | | — |
| Thereafter | | — | | 581 | | — |
| | |
| |
| |
|
| | | 1,108 | | 3,266 | | 607 |
| | | | |
| |
|
| Less amount representing interest | | (77 | ) | | | |
| | |
| | | | |
| Present value of future minimum lease payments | | 1,031 | | | | |
| Less current portion | | (697 | ) | | | |
| | |
| | | | |
| | | 334 | | | | |
| | |
| | | | |
12 Contingencies
In the ordinary course of business the Company may be subject to legal proceedings and claims. The Company is not currently subject to any legal proceedings.
13 Preferred Ordinary "C" shares
In June 2001, the Company issued 3,705,175 Preferred Ordinary "C" shares and 13,282 Ordinary shares at $10.64 per share to new and existing investors for net cash proceeds of $39,552,669. In addition, $8,478,000 of 8% secured convertible loan notes ("loan notes") provided by certain shareholders ("loan note holders"), together with accrued interest of $415,000, were converted into
F-23
835,794 Preferred Ordinary "C" shares. These loan notes were provided as short-term financing while the Company undertook its series "C" funding round. The Preferred Ordinary "C" shares were converted into Deferred shares during November 2003 (see note 14).
Prior to their conversion into Deferred shares during November 2003 the Preferred Ordinary "C" shares had various rights and preferences as follows:
Winding up
Upon the winding up of the Company, Preferred Ordinary "C" shareholders were, after all liabilities have been paid, entitled to the greater of:
- (i)
- the subscription price per share together with a sum equal to a fixed cumulative preferential dividend of 8% per annum compounded quarterly; or
- (ii)
- thepro-rata share of the proceeds between the Ordinary shareholders and the Preferred Ordinary "C" shareholders, as if the Preferred Ordinary "C" shares had been converted.
After such payments, Preferred Ordinary "C" shares rankedpari passu with ordinary shares in respect of remaining assets. The aggregate liquidation preference of the Preferred Ordinary "C" shares at March 31, 2003 was $56,328,000. The accumulated dividend at March 31, 2003 was $7,943,000.
Conversion
The Preferred Ordinary "C" shares could at any time, at the option of the holder, have been converted into Ordinary shares at the rate of one Ordinary share for every Preferred Ordinary "C" share.
Redemption
On June 6, 2006 one third of all unconverted Preferred Ordinary "C" shares would have become redeemable. A further one third would have become redeemable on June 6, 2007 and June 6, 2008.
Voting
Preferred Ordinary "C" shares and Ordinary shares rankedpari passu as regards voting rights.
14 Shareholders' Equity (Deficit)
Preferred Ordinary "D" shares
In November 2003, 4,076,111 Preferred Ordinary "D" shares of 0.1p each were issued at $6.90 for cash consideration of $28,143,248. There was an associated bonus issue of 12,666,580 Preferred Ordinary "D" shares of 0.1p each given to existing Preferred Ordinary "C" shareholders and Ordinary shareholders who participated in the "D" funding round on a basis of 1:4 and 1:3, respectively, by way of capitalization of part of the additional paid in capital. This was the first closing of two rounds. In January 2004, the Company issued a further 1,162,068 Preferred Ordinary "D" shares at $7.44 per share to new investors for net cash proceeds of $8,646,000 being the second and final closing of the series "D" funding round.
F-24
Winding up
Upon the winding up of the Company, Preferred Ordinary "D" shareholders are, after all liabilities have been paid, entitled to the greater of:
- (i)
- the subscription price per share multiplied by 1.5 together with a sum equal to a fixed cumulative preferential dividend of 8% per annum compounded quarterly; or
- (ii)
- thepro-rata share of the proceeds between the Ordinary shareholders and the Preferred Ordinary "D" shareholders, as if the Preferred Ordinary "D" shares had been converted.
After such payments, the balance of such assets shall be distributed amongst the ordinary shareholders in proportion to the amounts paid up.
The aggregate liquidation preference of the Preferred Ordinary "D" shares at March 31, 2004 was $189,961,721. The accumulated dividend at March 31, 2004 was $3,756,912.
Conversion
The Preferred Ordinary "D" shares may at any time, at the option of the holder, be converted into Ordinary shares at the rate of one Ordinary share for every Preferred Ordinary "D" share.
Voting
Preferred Ordinary "D" shares and Ordinary shares rankpari passu as regards voting rights.
Warrants for Preferred Ordinary "D" shares
On August 31, 2000, the Company issued warrants to loan-note holders to subscribe for shares in the Company. In June 2001, these warrants became exercisable immediately over 62,685 Preferred Ordinary "C" shares at a price of $10.64 (£7.53) per share and expire upon the earlier of ten years from the date of original issuance, the listing of the Company's ordinary shares or the sale of substantially all of the Company's assets. In November 2003, all Preferred Ordinary "C" shares were converted to deferred shares and these warrants became exercisable over 116,260 Preferred Ordinary "D" shares at a price of $6.90 (£4.06) per share. The warrants were immediately exercisable and expire upon the earlier of ten years from the date of original issuance, the listing of the Company's ordinary shares or the sale of substantially all of the Company's assets. The warrants were assigned a fair value of $588,000 which was recognized upon issuance. These warrants have not been exercised.
In June 2001, the Company issued warrants to existing shareholders to subscribe for a total of 61,076 Preferred Ordinary "C" shares. In November 2003, these warrants became exercisable over 61,076 Preferred Ordinary "D" shares at a price of $0.0015 (£0.001) per share. The warrants were immediately exercisable and will expire upon the earlier of ten years from the date of original issuance or the sale of the whole of the issued share capital to a third party.
The warrants were assigned a fair value of $627,000 which was recognized upon issuance. These warrants have not been exercised.
F-25
Deferred Shares
In November 2003, as part of the series D funding round, 2,251,572 Ordinary shares of 0.1p each and 4,540,969 Preferred Ordinary "C" shares of 0.1p each were converted into 6,792,541 Deferred shares of 0.1p each.
The Deferred shares carry the following rights and are subject to the following restrictions:
- a)
- on a return of capital on winding up or otherwise, the holders of Deferred shares shall in that capacity only be entitled to receive an amount equal to the par value thereof and only after payment in respect of each Ordinary share and Preferred Ordinary "D" share (collectively referred to as "paid up shares") the amount paid up thereon plus $17,750,000 (£10,000,000) per paid up share and the Deferred shares shall not otherwise entitle their holders to receive or participate in any way in any profits or assets of the Company; and
- b)
- the Deferred shares shall not entitle their holders to receive notice of or attend or vote at any general meetings of the Company or participate in any pre-emptive offer on issue or transfer of any shares under these articles.
Warrants for Ordinary Shares
In 1999, the Company issued warrants to existing shareholders to subscribe for a total of 23,500 Ordinary shares in the Company, exercisable upon the sale or listing of the Company. The subscription prices are $0.0015 (0.1p) per share for 16,000 shares and 90% of the sale or listing price for 7,500 shares. The warrants are exercisable upon a listing or a sale of substantially all of the Company's assets and until 30 days after such an event.
Share Option Plans
The Company operates a number of share option plans, which provide the opportunity to all eligible individuals to participate in the potential growth and success of the Company. In May 1997, the Company adopted the Cyclacel Limited Share Option Plan ("1997 Plan"), which was approved by a shareholders' resolution in May 1997. Under this plan, any person who is a Director or employee of the Company is eligible to be granted options to purchase Ordinary shares in the Company. In general, options granted under the "1997 Plan" may not be exercised before the third anniversary of the date of grant and may not be exercised later than the tenth anniversary of the date of grant. In February 2001, the Company adopted the Cyclacel Limited 2000 Employees' Share Option Scheme under the Enterprise Management Incentive Scheme ("2000 Plan"), which was approved by shareholders' resolution in December 2000. Under this plan any person who is a Director (other than a non-executive Director) or employee of the Company is eligible to be granted options to purchase shares in the Company.
Options granted under the "2000 Plan" may not be exercised more than ten years after the date of grant and, to the extent not exercised by that time, the Option shall lapse immediately. Options generally vest and become fully exercisable over a three year period. Shares can be issued upon exercise of options under the terms of the Company's employee share option plans up to a maximum of 12.5% of the issued share capital immediately following the closure of the series "D" funding round.
F-26
At March 31, 2004 a total of 1,623,623 ordinary shares remained available for issuance under the share option plans.
Senior Executive Incentive Plan
Mr S Rombotis, the chief executive officer, was granted rights to receive an option to acquire additional ordinary shares following successful completion by the Company of an initial public offering and listing on a major stock exchange ("the original Incentive Option").
The terms of the original Incentive Option were agreed as part of Mr Rombotis's original contract of employment dated August 1, 1997, and reflected in an appendix thereto. On June 30, 2004, the Company amended the terms of Mr Rombotis's contract of employment and granted an amended Incentive Option (the "amended Incentive Option").
The principal terms of the original Incentive Option, as agreed pursuant to Mr Rombotis's original contract of employment in August 1997 are as follows:
- •
- Mr. Rombotis was initially to receive an option to acquire 200,000 ordinary shares in Cyclacel Limited at an exercise price of 0.1 pence per share. The number of shares under the option would be subject to adjustment depending on the valuation of the company immediately following successful completion of an initial public offering on a major stock exchange (including the London Stock Exchange or Nasdaq) (the "Relevant Valuation"). Depending on the Relevant Valuation, this adjustment could have resulted in Mr Rombotis receiving an option over shares equivalent to up to 7.5% of the share capital of Cyclacel Limited on a fully diluted basis (subject to reduction to reflect certain shares and options already held by him at the relevant time). Following a listing on a major stock exchange, the option would be exercisable in three equal tranches on the first, second and third anniversaries of the major stock market listing.
- •
- The original Incentive Option would lapse seven years after the date of its grant.
The principal terms of the amended Incentive Option granted pursuant to the Senior Executive Incentive Plan (which now replaces the right agreed pursuant to Mr Rombotis's original contract of employment in 1997) are as follows:
- •
- Mr Rombotis has a right to acquire 1,720,903 ordinary shares in Cyclacel Group plc at an exercise price of 1 pence per share.
- •
- The amended Incentive Option will become exercisable by Mr Rombotis only following successful completion of the Offering or another listing of part of the ordinary share capital of Cyclacel Group plc on a major stock exchange (including the London Stock Exchange or Nasdaq) or on a sale or change of control of Cyclacel Group plc prior to any such listing and only if Mr Rombotis remains in employment with the Cyclacel Group (and has not given or been given notice to leave) at the time of exercise (unless Mr Rombotis has been dismissed without cause).
- •
- Provided that Mr Rombotis remains in employment with the Cyclacel Group, the amended Incentive Option will become exercisable in equal tranches on the first, second and third anniversaries of the closing of the offering. The amended Incentive Option will also be
F-27
The original Incentive Option over 200,000 ordinary shares (as agreed pursuant to Mr Rombotis's original contract of employment in August 1997) has been accounted for by the company in accordance with the provisions for variable compensatory plans set out in Accounting Principles Board Option No.25,"Accounting for Stock Issued to Employees" ("APB 25"). The stock based compensation charge has been accrued over the expected period to the initial public offering, being seven years from August 1, 1997, and has been adjusted during each of the periods up to March 31, 2004 to reflect changes in the fair value of the ordinary shares.
The amended Incentive Option granted pursuant to the Senior Executive Incentive Plan (which now replaces the rights agreed pursuant to Mr Rombotis's original contract of employment in August 1997) will also be accounted for in accordance with the guidance on the modification of stock-based compensation plans. This will result in a stock-based compensation charge being accrued, over the period to the third anniversary of the offering, adjusted during each period for future changes in the market value of the ordinary shares.
A summary of the Company's share option activity and related information is as follows:
| | Options
outstanding
| | Weighted
average
exercise price
|
|---|
| Balance at April 1, 2001 | | 754,089 | | $ | 3.71 |
| | Granted | | 59,085 | | $ | 10.79 |
| | Exercised | | (67,500 | ) | $ | 1.61 |
| | Cancelled | | (2,000 | ) | $ | 6.01 |
| | |
| | | |
| Balance at March 31, 2002 | | 743,674 | | $ | 4.48 |
| | Granted | | 177,000 | | $ | 11.65 |
| | Exercised | | (2,000 | ) | $ | 6.19 |
| | Cancelled | | (4,500 | ) | $ | 11.17 |
| | |
| | | |
| Balance at March 31, 2003 | | 914,174 | | $ | 6.25 |
| | Granted | | — | | | |
| | Exercised | | (16,057 | ) | $ | 7.45 |
| | Cancelled | | (10,600 | ) | $ | 8.59 |
| | |
| | | |
| Balance at December 31, 2003 and March 31, 2004 | | 887,517 | | $ | 7.01 |
| | |
| | | |
F-28
The following table summarizes information about options outstanding at March 31, 2004:
| |
| |
| | Options exercisable
|
|---|
Options outstanding
| | Weighted
Average
remaining
contractual
life
| |
|
|---|
Exercise
price
| | Exercise
price
| | Number
outstanding
| | Number
exercisable
|
|---|
£
| | $
| |
| |
| |
|
|---|
| 0.001 | | 0.0018 | | 276,875 | | 0.30 | | 51,875 |
| 4.00 | | 7.12 | | 27,400 | | 1.40 | | 27,400 |
| 4.53 | | 8.06 | | 110,734 | | 4.76 | | 110,734 |
| 4.76 | | 8.47 | | 242,173 | | 5.62 | | 242,173 |
| 7.53 | | 13.39 | | 230,335 | | 4.25 | | 6,028 |
| | | | |
| | | |
|
| | | | | 887,517 | | 4.49 | | 438,210 |
| | | | |
| | | |
|
Shares issuable on conversion of shares or exercise of options and warrants:
| | No. '000
|
|---|
| Conversion of Preferred Ordinary "D" shares | | 17,905 |
| Options to purchase Ordinary shares | | 888 |
| Warrants | | 200 |
| | |
|
| | | 18,993 |
| | |
|
New Share Option arrangments Post Flotation
On July 1, 2004 the Company has adopted a New Option Plan, (the Cyclacel Group Plc Discretionary Share Option Plan), a New SAYE Plan, (the Cyclacel Group Plc Savings Related Share Option Plan) and a New Restricted Share and Co-Investment Plan, (the Cyclacel Group Plc Restricted Share and Co-Investment Plan), to be operated post flotation. We refer to these plans collectively as the New Share Plans. The New Share Plans replace the 1997 Plan and the 2000 Plan.
The principal terms of the New Share Plans are set out below.
New Option Plan
Options may be granted to selected employees and directors of the Group at the discretion of the remuneration committee. The exercise price will not be less than the higher of the middle market quotation for an Ordinary share on the day preceding the date of grant, or the average of such quotations for the three days preceding the date of grant, and the nominal value of the ordinary shares.
Limits to the number of shares over which options may be granted are as follows:
- •
- in any ten year period not more than 10% of the issued Ordinary share capital may be issued or issuable under the New Option Plan or any other employees' share scheme; and
F-29
- •
- in any ten year period not more than 5% of the issued Ordinary share capital may be issued or issuable under the New Option Plan or any discretionary share scheme.
Options will normally be exercisable between three and ten years following the date of grant provided any specified performance target has been satisfied.
These awards will be accounted for by the Company in accordance with the provisions for variable compensatory plans as set out in APB 25.
New SAYE Plan
Options under the New SAYE Plan will be granted to employees and directors who have completed a minimum period of service as determined by the Board of Directors. Employees and directors will participate by entering into a Savings Contract and by making monthly contributions of between $9 (£5) and $450 (£250) for a period of three, five or seven years. The proceeds from the Savings Contract are used to purchase options at the exercise price.
The exercise price will be not less than the higher of 80% of the middle market quotation for an Ordinary share on the day preceding the date that employees or directors are invited to participate, or the average of such quotations for the three days preceding the date that employees or directors are invited to participate, and the nominal value of the ordinary shares.
The options will normally be exercisable for a period of six months from the date of completion of the Savings Contract.
The limit to the number of shares over which options may be granted is that in any ten year period not more than 10% of the issued Ordinary share capital may be issued or issuable under the New SAYE Plan or any other employees' share scheme.
The intrinsic value of these awards of up to 20% of the share price will be recorded as share based compensation expense in accordance with APB 25.
New Restricted Share and Co-Investment Plan
The New Restricted Share and Co-Investment Plan comprises the following two elements:
Matched Awards
Employees will be able to apply all or part of their annual bonus and/or invest their own funds in purchasing shares in the Company ("Invested Shares"). The employees will receive a Matched Award, being a grant of shares on a 1:1 basis. The vesting of these shares is contingent upon satisfaction of performance targets and continued employment.
Restricted Awards
Shares will be granted to senior managers and directors at the discretion of the remuneration committee. The vesting of these shares is contingent upon satisfaction of performance targets and continued employment. In normal circumstances the initial value of a restricted award will be up to 100% of salary however this can be increased at the remuneration committee's discretion.
F-30
In any ten year period the use of new issue shares under the New Restricted Shares and Co-Investment Plan and any other employees' share scheme operated by the Company is limited to 10% of the issued share capital excluding any awards or options granted under any employees' share incentive plan prior to the date of the flotation.
These awards will be accounted for by the Company in accordance with the provisions for variable compensatory plans as set out in APB 25.
15 Pension Plans
The Company operates a defined group personal pension plan for substantially all of its employees. Company contributions to the plan totalled $134,857, $177,720, $132,856 and $142,598 in the years ended March 31, 2002 and 2003 and the nine months ended December 31, 2002 and 2003, respectively. Company contributions to the plan totalled $44,864 and $54,279 in the three months ended March 31, 2003 and 2004, respectively.
16 Taxes
The Company has made a taxable loss in each of the operating periods since incorporation. The income tax credits of $4,397,000, $3,841,000, $1,486,000, $528,000 and $521,000 for the year ended March 31, 2003 and the nine months ended December 31, 2002 and 2003 and the three months ended March 31, 2003 and 2004, respectively, represent U.K. research and development tax credits receivable against such expenditures in the United Kingdom.
F-31
A reconciliation of the (benefit) provision for income taxes with the amount computed by applying the statutory corporation tax rate of 30% to loss before income taxes is as follows:
| |
| |
| |
| |
| | 3 months ended
March 31,
| |
|---|
| | Year ended
March 31,
2002
| | Year ended
March 31,
2003
| | 9 months ended
December 31,
2002
| | 9 months ended
December 31,
2003
| |
|---|
| | 2003
| | 2004
| |
|---|
| | $000
| | $000
| | $000
| | $000
| | $000
| | $000
| |
|---|
| Loss before income taxes | | (14,853 | ) | (19,939 | ) | (14,729 | ) | (16,463 | ) | (5,217 | ) | (5,409 | ) |
| | |
| |
| |
| |
| |
| |
| |
| Income tax expense computed at statutory corporation tax rate | | (4,456 | ) | (5,982 | ) | (4,419 | ) | (4,939 | ) | (1,565 | ) | (1,623 | ) |
| Disallowed expenses and non-taxable income | | (511 | ) | 906 | | 615 | | 2,506 | | 294 | | 470 | |
| Depreciation in excess of capital allowances | | (145 | ) | 7 | | (61 | ) | 149 | | 71 | | 20 | |
| Other timing differences | | 10 | | — | | — | | — | | — | | — | |
| Adjustments in respect of prior periods | | — | | (2,549 | ) | (2,518 | ) | — | | — | | — | |
| Tax losses | | 5,102 | | 5,069 | | 3,864 | | 2,284 | | 1,200 | | 1,134 | |
| Research and development tax relief | | — | | (2,310 | ) | (1,653 | ) | (1,857 | ) | (660 | ) | (652 | ) |
| Research and development tax credit rate difference | | — | | 462 | | 331 | | 371 | | 132 | | 130 | |
| | |
| |
| |
| |
| |
| |
| |
| | | — | | (4,397 | ) | (3,841 | ) | (1,486 | ) | (528 | ) | (521 | ) |
| | |
| |
| |
| |
| |
| |
| |
Significant components of the Company's deferred tax assets are shown below:
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
| |
|---|
| | $000
| | $000
| |
| |
|---|
| Deferred tax assets (liabilities): | | | | | | | |
| | Net operating loss carryforwards | | 13,530 | | 16,411 | | 17,979 | |
| | Depreciation and amortization | | (712 | ) | (643 | ) | (640 | ) |
| | |
| |
| |
| |
| | Total net deferred tax assets | | 12,818 | | 15,768 | | 17,339 | |
| | Valuation allowance for deferred tax assets | | (12,818 | ) | (15,768 | ) | (17,339 | ) |
| | |
| |
| |
| |
| Net deferred taxes | | — | | — | | — | |
| | |
| |
| |
| |
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting and tax purposes. A valuation allowance has been established, as realization of such assets is uncertain.
F-32
The Company has, subject to agreement with the U.K. Inland Revenue, the following tax losses and accumulated tax losses available for carry forward against future operations, which under UK tax laws do not expire:
| | March 31,
2003
| | December 31,
2003
| | March 31,
2004
|
|---|
| | $000
| | $000
| | $000
|
|---|
| Accumulated tax losses | | 45,100 | | 54,190 | | 59,930 |
| | |
| |
| |
|
17 Post Balance Sheet Event
On April 22, 2004 Professor Sir David Lane exercised 46,875 options to purchase ordinary shares at a price of 0.1777 cents (0.1p).
On April 23, 2004 new options over 1,722,770 shares were granted to employees at an exercise price of $2.66 (£1.50). Prior to the granting of these new options 598,692 of former employee options at a higher exercise price were surrendered by employees. The new options will become exercisable in equal tranches on the first, second and third anniversaries of the date of grant, the earliest option exercise date being April 23, 2005 and the expiration date April 23, 2014. The reasons for this event were that the surrendered options, many of which had already vested, had an exercise price significantly in excess of the current fair value of an ordinary share. Therefore, the issue of these new options was undertaken to retain existing employees and enable them to share in the future success of the Company.
The new options will be accounted for in accordance with FIN 44 as a variable compensatory plan.
F-33
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers
Our articles of association provide that, subject to the U.K. Companies Act and every other statute, statutory instrument, regulation or order for the time being in force concerning companies registered under the U.K. Companies Act, every director or other officer (excluding an auditor) of the Company shall be indemnified out of the assets of the Company against all liabilities incurred by him in the actual or purported execution or discharge of his duties or the exercise or purported exercise of his powers or otherwise in relation to or in connection with his duties, powers or office. However, this indemnity shall not apply to any liability to the extent that it is recovered from any other person and is subject to such officer taking all reasonable steps to effect such recovery, to the intent that the indemnity shall not apply where an alternative right of recovery is available and capable of being enforced.
Item 7. Recent Sales of Unregistered Securities
The following information is furnished with regard to all securities issued by us within the last three years which were not registered under the Securities Act. The issuance of such shares was deemed exempt from registration requirements of the Securities Act as such securities were offered and sold outside of the United States to persons who were neither citizens nor residents of the United States or such sales were exempt from registration under Section 4(2) of Securities Act.
- •
- In June 2001, we issued 13,282 ordinary shares and 3,705,175 preferred ordinary C shares in a private placement to new and existing shareholders for total cash consideration of £27,999,981. In connection with this private placement, we issued success fee warrants exercisable for 61,076 of our preferred ordinary C shares at a price of 0.1p per share. In addition, the total amount due to holders of our secured convertible notes (who consisted of certain of our existing shareholders), comprising £6,293,523 of principal and accrued net interest, was converted into a further 835,794 preferred ordinary C shares and warrants exercisable for 62,685 preferred ordinary C shares at a price of £7.53 per share. In November 2003, all our preferred ordinary C shares were redesignated as deferred shares, the success fee warrants became exercisable for 61,076 of our preferred ordinary D shares at a price of 0.1p per share and warrants issued to our secured convertible noteholders became exercisable for 116,260 preferred ordinary D shares at a price of £4.06 per share.
- •
- In November 2001, we issued 16,000 of our ordinary shares to Edinburgh University in exchange for rights to the LIDAEUS docking software.
- •
- In November 2003, as part of a private placement to new and existing shareholders, we issued 12,316 ordinary shares and 4,076,111 preferred ordinary D shares for a total cash consideration of £16,599,014 and an additional 12,666,580 preferred ordinary D shares as an associated bonus issue applied to share premium. We redesignated 2,251,572 ordinary shares and 4,540,969 preferred ordinary C shares as 6,792,541 deferred shares.
- •
- Further to our series D funding round in November 2003, we issued to new shareholders 1,162,068 of our preferred ordinary D shares in January 2004 for total cash consideration of £4,717,996.
- •
- Share issuances upon exercise of options:
- •
- In April 2001, we issued 50,000 of our ordinary shares upon exercise of share options for a total consideration of £50.
II-1
- •
- In July 2001, we issued 17,500 of our ordinary shares upon exercise of share options for a total consideration of £74,550.
- •
- In May 2002, we issued 2,000 of our ordinary shares upon exercise of share options for a total consideration of £8,000.
- •
- In April 2003, we issued 15,957 of our ordinary shares upon exercise of share options for a total consideration of £72,285.
- •
- In October 2003, we issued 100 of our ordinary shares upon exercise of share options for a total consideration of £400.
- •
- In April 2004, we issued 46,875 of our ordinary shares upon exercise of share options for a total consideration of £49.
- •
- Pursuant to a reorganization and share exchange agreement described under "Description of Share Capital" 1,871,210 ordinary shares and 17,965,835 preferred D shares were issued on the following basis:
- (i)
- for every one ordinary share of 0.1p in Cyclacel Limited, one ordinary share of 1p in the share capital of the Company; and
- (ii)
- for every one preferred D share of 0.1p in Cyclacel Limited, one preferred D share of 1p in the share capital of the Company.
II-2
Item 8. Exhibits and Financial Statement Schedules
Exhibit Number
| | Description of Exhibits
|
|---|
| 1.1 | | Underwriting Agreement.* |
| 2.1 | | Plan of Reorganization.* |
| 3.1 | | Form of Memorandum and Articles of Association to be effective upon admission of shares.* |
| 4.1 | | Form of Share Certificate.* |
| 4.2 | | Form of American Depositary Receipt (included in Exhibit 4.3).* |
| 4.3 | | Form of Deposit Agreement to be entered into among the Registrant, The Bank of New York, as Depositary, and each holder and beneficial owner of American Depositary Shares issued thereunder.* |
| 4.4 | | Form of Registration Rights Agreement.* |
| 4.5 | | Form of Executive Director Lock-up Letter.* |
| 4.6 | | Form of Non-executive Director and Senior Management Lock-up Letter.* |
| 4.7 | | Form of Shareholder Lock-up Letter.* |
| 5.1 | | Opinion of Allen & Overy LLP regarding the legality of the securities being registered.* |
| 8.1 | | Opinion of Allen & Overy LLP as to U.K. taxation.* |
| 8.2 | | Opinion of Allen & Overy LLP as to U.S. taxation.* |
| 10.1 | | Patent License L99094 among Cyclacel, Centre National de la Recherche Scientifique and Institut Curie, dated February 1, 2000.*† |
| 10.2 | | License Agreement between Sankyo Co., Ltd. and Cyclacel Limited, dated September 10, 2003, and letter amendments dated April 1, 2004 and April 28, 2004.*† |
| 10.3 | | Service Agreement, dated July 1, 2004, and letter agreement dated July 1, 2004, between the Registrant and Spiro Rombotis.* |
| 10.4 | | Service Agreement, dated July 1, 2004, and letter agreement dated July 1, 2004, between the Registrant and Paul McBarron.* |
| 10.5 | | Service Agreement, dated July 1, 2004, between the Registrant and Dr. Robert Jackson.* |
| 10.6 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Sir John Banham.* |
| 10.7 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Dr. David U'Prichard.* |
| 10.8 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Sir David Lane.* |
| 10.9 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Professor Gordon McVie.* |
| 10.10 | | Senior Executive Incentive Plan.* |
| 10.11 | | Cyclacel Group plc Employee Benefit Trust.* |
| 10.12 | | Cyclacel Group plc Savings Related Share Plan (SAYE).* |
| 10.13 | | Cyclacel Group plc Discretionary Share Option Plan (Approved and Unapproved).* |
| 10.14 | | Cyclacel Group plc Restricted Share & Co-Investment Plan.* |
| 21.1 | | List of Subsidiaries.* |
| 23.1 | | Consent of Allen & Overy LLP (included in Exhibit 5.1).* |
| 23.2 | | Consent of Ernst & Young LLP. |
| 24.1 | | Powers of Attorney. |
- *
- To be filed by amendment.
- †
- Pursuant to a request for confidential treatment, portions of the Exhibit have been redacted from the publicly filed document and have been furnished separately to the SEC as required by Rule 406 under the Securities Act.
II-3
Item 9. Undertakings
The undersigned Registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, offers and controlling person of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted against the Registrant by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
- (1)
- For the purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this Registration Statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this Registration Statement as of the time it was declared effective.
- (2)
- For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Dundee, Scotland, on July 2, 2004.
| | | CYCLACEL Group plc |
|
|
|
|
|
|
| | | By: | | /s/ PAUL MCBARRON
|
| | | | | Name: | Paul McBarron |
| | | | | Title: | Chief Financial Officer, Secretary and Director |
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Signature
| | Capacity
| | Date
|
|---|
|
|
|
|
|
*
Sir John Banham | | Chairman of the Board | | July 2, 2004 |
*
Spiro Rombotis |
|
Chief Executive Officer
(Principal Executive Officer) and Director |
|
July 2, 2004 |
*
Dr. Robert Jackson |
|
Chief Scientific Officer and Director |
|
July 2, 2004 |
/s/ PAUL MCBARRON
Paul McBarron |
|
Chief Financial Officer
(Principal Financial and Accounting Officer) and Director |
|
July 2, 2004 |
*
Professor Sir David Lane |
|
Director |
|
July 2, 2004 |
*
Professor Gordon McVie |
|
Director |
|
July 2, 2004 |
*
Dr. David U'Prichard |
|
Director |
|
July 2, 2004 |
| | | | | |
II-5
/s/ DONALD PUGLISI
Puglisi & Associates |
|
Authorized Representative in the United States |
|
July 2, 2004 |
*By: |
|
/s/ PAUL MCBARRON
Name: Paul McBarron
Title: Attorney-in-Fact |
|
|
|
|
II-6
INDEX TO EXHIBITS
Exhibit Number
| | Description of Exhibits
|
|---|
| 1.1 | | Underwriting Agreement.* |
| 2.1 | | Plan of Reorganization.* |
| 3.1 | | Form of Memorandum and Articles of Association to be effective upon admission of shares.* |
| 4.1 | | Form of Share Certificate.* |
| 4.2 | | Form of American Depositary Receipt (included in Exhibit 4.3).* |
| 4.3 | | Form of Deposit Agreement to be entered into among the Registrant, The Bank of New York, as Depositary, and each holder and beneficial owner of American Depositary Shares issued thereunder.* |
| 4.4 | | Form of Registration Rights Agreement.* |
| 4.5 | | Form of Executive Director Lock-up Letter.* |
| 4.6 | | Form of Non-executive Director and Senior Management Lock-up Letter.* |
| 4.7 | | Form of Shareholder Lock-up Letter.* |
| 5.1 | | Opinion of Allen & Overy LLP regarding the legality of the securities being registered.* |
| 8.1 | | Opinion of Allen & Overy LLP as to U.K. taxation.* |
| 8.2 | | Opinion of Allen & Overy LLP as to U.S. taxation.* |
| 10.1 | | Patent License L99094 among Cyclacel, Centre National de la Recherche Scientifique and Institut Curie, dated February 1, 2000.*† |
| 10.2 | | License Agreement between Sankyo Co., Ltd. and Cyclacel Limited, dated September 10, 2003, and letter amendments dated April 1, 2004 and April 28, 2004.*† |
| 10.3 | | Service Agreement, dated July 1, 2004, and letter agreement dated July 1, 2004 between the Registrant and Spiro Rombotis.* |
| 10.4 | | Service Agreement, dated July 1, 2004, and letter agreement dated July 1, 2004 between the Registrant and Paul McBarron.* |
| 10.5 | | Service Agreement, dated July 1, 2004, between the Registrant and Dr. Robert Jackson.* |
| 10.6 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Sir John Banham.* |
| 10.7 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Dr. David U'Prichard.* |
| 10.8 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Sir David Lane.* |
| 10.9 | | Non-executive Director's Appointment Letter, dated July 1, 2004, between the Registrant and Professor Gordon McVie.* |
| 10.10 | | Senior Executive Incentive Plan.* |
| 10.11 | | Cyclacel Group plc Employee Benefit Trust.* |
| 10.12 | | Cyclacel Group plc Savings Related Share Plan (SAYE).* |
| 10.13 | | Cyclacel Group plc Discretionary Share Option Plan (Approved and Unapproved).* |
| 10.14 | | Cyclacel Group plc Restricted Share & Co-Investment Plan.* |
| 21.1 | | List of Subsidiaries.* |
| 23.1 | | Consent of Allen & Overy LLP. (included in Exhibit 5.1).* |
| 23.2 | | Consent of Ernst & Young LLP. |
| 24.1 | | Powers of Attorney. |
- *
- To be filed by amendment.
- †
- Pursuant to a request for confidential treatment, portions of the Exhibit have been redacted from the publicly filed document and have been furnished separately to the SEC as required by Rule 406 under the Securities Act.
II-7
QuickLinks
TABLE OF CONTENTSPROSPECTUS SUMMARYCyclacel Group plcCompany InformationTHE OFFERINGSUMMARY FINANCIAL DATARISK FACTORSSPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTSUSE OF PROCEEDSDIVIDEND POLICYCAPITALIZATIONDILUTIONSELECTED CONSOLIDATED FINANCIAL DATAMANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONSTHE CELL CYCLE AND CANCERBUSINESSMANAGEMENTPRINCIPAL SHAREHOLDERSPRE-OFFERING REORGANIZATION INTO PUBLIC LIMITED COMPANYDESCRIPTION OF SHARE CAPITALDESCRIPTION OF AMERICAN DEPOSITARY SHARESRELATED PARTY TRANSACTIONSUNDERWRITERSTAXATIONSHARES ELIGIBLE FOR FUTURE SALEWHERE YOU CAN FIND MORE INFORMATION ABOUT USLEGAL MATTERSEXPERTSSERVICE OF PROCESS AND ENFORCEMENT OF CIVIL LIABILITIESIndex to Consolidated Financial StatementsCYCLACEL GROUP PLC (A Development Stage Company) REPORT OF INDEPENDENT AUDITORSCYCLACEL GROUP PLC (A Development Stage Company) CONSOLIDATED STATEMENTS OF OPERATIONSCONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT) Period from August 13, 1996 (inception) to March 31, 2004CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT) (Continued) Period from August 13, 1996 (inception) to March 31, 2004CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT) (Continued) Period from August 13, 1996 (inception) to March 31, 2004CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY (DEFICIT) (Continued) Period from August 13, 1996 (inception) to March 31, 2004CYCLACEL GROUP PLC (A Development Stage Company) CONSOLIDATED STATEMENTS OF CASH FLOWSCYCLACEL GROUP PLC (A Development Stage Company) NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTSPART II INFORMATION NOT REQUIRED IN PROSPECTUSSIGNATURESINDEX TO EXHIBITS
