Use these links to rapidly review the document
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form 10-K
| (Mark One) | ||
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIESEXCHANGE ACT OF 1934 | |
For the fiscal year ended December 28, 2013 | ||
Or | ||
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period from to | ||
Commission File Number 1-32266
Polypore International, Inc.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) | 43-2049334 (IRS Employer Identification No.) | |
11430 North Community House Road, Suite 350 Charlotte, North Carolina (Address of Principal Executive Offices) | 28277 (Zip Code) |
(704) 587-8409
Registrant's Telephone Number, Including Area Code
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class: | Name of each exchange on which registered: | |
|---|---|---|
| Common stock, par value $0.01 per share | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ý Yes o No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes ý No
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ý Yes o No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ý Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer", "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller reporting company o |
Indicate by checkmark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). o Yes ý No
The aggregate market value of the voting common stock of the registrant held by non-affiliates at June 28, 2013 was $1,384,748,000, as computed by reference to the closing price of such stock on such date. For purposes of this calculation, executive officers, directors and 10% shareholders are deemed to be affiliates of the registrant.
There were 44,982,070 shares of the registrant's common stock outstanding as of February 19, 2014.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive proxy statement for the 2014 Annual Meeting of Stockholders, which will be filed within 120 days of December 28, 2013, are incorporated by reference into Part III.
Polypore International, Inc.
Index to Annual Report on Form 10-K
For the Fiscal Year Ended December 28, 2013
| | | Page | ||||
|---|---|---|---|---|---|---|
Part I |
| |||||
Item 1. | Business | 3 | ||||
Item 1A. | Risk Factors | 14 | ||||
Item 1B. | Unresolved Staff Comments | 20 | ||||
Item 2. | Properties | 20 | ||||
Item 3. | Legal Proceedings | 21 | ||||
Item 4. | Mine Safety Disclosures | 21 | ||||
Part II |
| |||||
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 22 | ||||
Item 6. | Selected Financial Data | 24 | ||||
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | 25 | ||||
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 40 | ||||
Item 8. | Financial Statements and Supplementary Data | 41 | ||||
Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 81 | ||||
Item 9A. | Controls and Procedures | 81 | ||||
Part III |
| |||||
Item 10. | Directors, Executive Officers and Corporate Governance | 82 | ||||
Item 11. | Executive Compensation | 82 | ||||
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 82 | ||||
Item 13. | Certain Relationships and Related Transactions, and Director Independence | 82 | ||||
Item 14. | Principal Accountant Fees and Services | 82 | ||||
Part IV |
| |||||
Item 15. | Exhibits and Financial Statement Schedules | 82 | ||||
Signatures | 86 | |||||
In this Annual Report on Form 10-K, the words "Polypore International," "Company," "we," "us" and "our" refer to Polypore International, Inc. together with its subsidiaries, unless the context indicates otherwise. References to "fiscal year" mean the 52- or 53-week period ending on the Saturday that is closest to December 31. The fiscal years ended December 28, 2013, or "fiscal 2013," December 29, 2012, or "fiscal 2012," and December 31, 2011, or "fiscal 2011," included 52 weeks.
i
This Annual Report on Form 10-K includes "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended (the "Securities Act"), Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), and the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts included in this Annual Report on Form 10-K that address activities, events or developments that we expect, believe or anticipate will or may occur in the future are forward-looking statements, including, in particular, the statements about Polypore International's plans, objectives, strategies and prospects regarding, among other things, the financial condition, results of operations and business of Polypore International. We have identified some of these forward-looking statements with words like "believe," "may," "will," "should," "expect," "intend," "plan," "predict," "anticipate," "estimate" or "continue" and other words and terms of similar meaning. These forward-looking statements may be contained under the captions entitled "Business," "Properties," "Controls and Procedures," "Management's Discussion and Analysis of Financial Condition and Results of Operations" or "Risk Factors," the Company's financial statements or the notes thereto or elsewhere in this Annual Report on Form 10-K.
These forward-looking statements are based on current expectations about future events affecting us and are subject to uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control. Many factors mentioned in our discussion in this Annual Report on Form 10-K, including the risks outlined under the caption below entitled "Risk Factors," will be important in determining future results. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties, including with respect to Polypore International, the following, among other things:
- •
- the highly competitive nature of the markets in which we sell our products;
- •
- the failure to continue to develop innovative products;
- •
- the loss of our customers;
- •
- the vertical integration by our customers of the production of our products into their own manufacturing process;
- •
- increases in prices for raw materials or the loss of key supplier contracts;
- •
- our substantial indebtedness;
- •
- interest rate risk related to our variable rate indebtedness;
- •
- our inability to generate cash;
- •
- restrictions related to the senior secured credit agreement;
- •
- employee slowdowns, strikes or similar actions;
- •
- product liability claims exposure;
- •
- risks in connection with our operations outside the United States, including compliance with applicable anti-corruption laws;
- •
- the incurrence of substantial costs to comply with, or as a result of violations of, or liabilities under environmental laws;
- •
- the failure to protect our intellectual property;
- •
- the loss of senior management;
1
- •
- the incurrence of additional debt, contingent liabilities and expenses in connection with future acquisitions;
- •
- the failure to effectively integrate newly acquired operations;
- •
- lithium market demand not materializing as anticipated;
- •
- the absence of expected returns from the intangible assets we have recorded; and
- •
- natural disasters, epidemics, terrorist acts and other events beyond our control.
Because our actual results, performance or achievements could differ materially from those expressed in, or implied by, the forward-looking statements, we cannot give any assurance that any of the events anticipated by the forward-looking statements will occur or, if any of them do, what impact they will have on Polypore International's results of operations and financial condition. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. We do not undertake any obligation to update these forward-looking statements or the factors set forth in the caption below entitled "Risk Factors" to reflect new information, future events or otherwise, except as may be required under federal securities laws.
2
General
Overview
Polypore International, Inc., a Delaware corporation, is a leading global high-technology filtration company that develops, manufactures and markets specialized microporous membranes used in separation and filtration processes. The microporous membranes we produce are highly-engineered polymeric structures that contain millions of pores per square inch, enabling the management of ions, gases and particles that range in size from the cellular to the nano or molecular level.
Our products and technologies are used in two primary businesses: energy storage and separations media. The energy storage business produces and markets membranes that provide the critical function of separating the cathode and anode in a variety of battery markets and is comprised of two reportable segments. The electronics and EDVs segment produces and markets membranes for lithium batteries that are used in portable electronic devices, cordless power tools, electric drive vehicles ("EDVs") and emerging applications such as energy storage systems ("ESS"). The transportation and industrial segment produces and markets membranes for lead-acid batteries that are used in automobiles, other motor vehicles, forklifts and uninterruptible power supply systems. The separations media business, which is a reportable segment, produces and markets membranes and membrane modules used in hemodialysis, blood oxygenation, plasmapheresis, other medical applications and various high-performance microfiltration, ultrafiltration and gasification/degasification applications.
Information concerning segments and geographic information appears under Note 17, "Segment Information" in the notes to consolidated financial statements for the year ended December 28, 2013 included in Item 8 of this Report.
Discontinued operations
On December 19, 2013, we completed the sale of our Microporous business, which consisted of the production facilities in Piney Flats, Tennessee, and Feistritz, Austria. Microporous was previously included in the transportation and industrial segment. The results of operations of Microporous are classified as discontinued operations and are excluded from continuing operations and segment results for all periods presented. All disclosures and amounts in this Annual Report on Form 10-K relate to our continuing operations, unless otherwise indicated.
Competitive strengths
Serve end markets that have attractive long-term growth characteristics
We produce a variety of separation and filtration products for end markets with attractive growth characteristics, which in many cases are supported by a growing, recurring revenue base.
- •
- The total lithium battery market is expected to grow at a compound annual growth rate in excess of 16% through 2020 on an energy capacity basis, driven by the application of lithium battery technology in new markets such as EDVs and ESS. We believe that growth in lithium battery separator demand will exceed battery growth due to increasing demand for large-format lithium batteries used in EDVs and other new applications and increasing power cell requirements in end-user devices such as power tools and hybrid vehicles.
- •
- In the motor vehicle battery market, the high proportion of aftermarket sales and the steady growth of the worldwide fleet of motor vehicles provide us with a growing, recurring revenue base in lead-acid battery separators. Lead-acid battery separator growth is strongest in the Asia Pacific region due, we believe, to increasing per capita penetration of automobiles, growth in the
3
- •
- The hemodialysis membrane market, which we believe will increase in excess of 6% annually, provides a growing, recurring revenue base for our synthetic dialysis membranes.
- •
- The micro- and ultrafiltration membrane element market is expected to grow in excess of 8% annually, driven by several factors including the superior performance of membrane filtration and the increasing need for purity in end markets such as water treatment, food and beverage processing and pharmaceutical, semiconductor and flat panel display manufacturing as water sources dwindle and populations increase.
industrial and manufacturing sectors and a high rate of conversion to polyethylene-based membrane separators.
Leading market positions
We believe that we are well-positioned in each of the markets in which we compete. For example, in terms of market share (based on revenue and volume):
- •
- We believe, based on independent industry research, that we are among the top three lithium battery separator providers, serving the entire breadth of lithium battery applications.
- •
- We believe, based on internal company estimates, that we are the global market leader in the lead-acid battery separator market.
- •
- We believe, based on internal company estimates, that we are the world's leading supplier of membranes used in blood oxygenation and that we are uniquely positioned as the leading independent supplier of synthetic membranes used in hemodialysis (i.e., not a supplier of dialyzers).
- •
- We believe, based on internal company estimates, that in the industrial and specialty filtration market, we are the world leader in membrane gasification and degasification for liquids with our Liqui-Cel® Membrane Contactors. We also believe, based on internal company estimates, that we are the world's leading independent supplier of polyethersulfone flat sheet membranes.
We believe, based on the information above and other company estimates, that we are among the top three in terms of market share (based on revenue and volume) in products comprising a total of approximately 80% of our fiscal 2013 net sales. These products include lead-acid battery separators, lithium battery separators, blood oxygenation membranes and membrane contactors for gasification/degasification of liquids.
Proven innovation through broad product and process technology
We have established our leading market positions through our ability to utilize core technical expertise and broad product and process capabilities to develop customized solutions that meet demanding requirements in specific applications. Over time, we have demonstrated a commitment to innovation, developing technical expertise and a high level of customer service. As of December 28, 2013, our research and development effort was supported by approximately 100 engineers, scientists, PhDs and other personnel, who work directly with our manufacturing and marketing groups to commercialize innovative products that address market needs. We also maintain technical centers strategically located in Asia, Europe and North America.
We have leveraged our established filtration and separation technology and membrane expertise to supply a broad portfolio of membranes based on flat sheet, hollow fiber and tubular technology. We are able to draw upon our experiences across multiple end markets and various membrane technologies to create innovative solutions in new niche applications in addition to our existing markets. For example, our Liqui-Cel® Membrane Contactor product line, which combines our gasification/degasification membrane technology with patented module design features, provides superior
4
performance to conventional gasification/degasification methods in multiple sectors such as semiconductor and flat panel display manufacturing, pharmaceutical processing and power and boiler feedwater applications. We believe that our capabilities in product innovation, which combine multiple technologies, a global technical infrastructure and extensive experience in microporous membrane development and manufacturing, are difficult to replicate.
Strong customer relationships with leading manufacturers
We have cultivated strong, collaborative relationships with a diverse base of customers worldwide who are among the leaders in their respective industries. Our research and development, technical service and application development teams are closely involved with our customers. We often enter into joint agreements in which we partner with our customers on product development and end-use testing. As a result, many of our products have been customized to our customers' manufacturing processes and end-use specifications. In addition, we are often selected as a customer's exclusive supplier for our microporous membrane products.
Global presence
As of December 28, 2013, we manufacture, market and service our products through 15 manufacturing sites and 14 sales and service locations throughout the Americas, Europe and Asia, with sales relatively balanced across these regions. In the energy storage business, we remain focused on growth in the Asia Pacific region and on driving worldwide improvements in production efficiency. By strategically positioning our manufacturing, sales and marketing, and technical service personnel near our customers, we can respond to their needs more effectively, provide a higher level of service, reduce shipping costs and improve delivery and response times. In addition, our global presence enables us to participate in faster growth markets in developing regions of the world.
State-of-the-art manufacturing facilities
We believe we have state-of-the-art manufacturing facilities and capabilities. Our equipment, manufacturing techniques and process technologies have been developed over many years with significant intellectual property, know-how and capital investments. Our wide range of manufacturing processes enables us to produce specialized products that are difficult and costly to replicate in the market. We continually evaluate projects that will improve or enhance our global manufacturing capabilities.
Strong and experienced management team
Our senior management team has significant experience leading high-technology companies, driving growth through development of new applications and technologies and cultivating strong relationships with existing customers. Management has also demonstrated a track record of successfully leading companies larger in size and scope than ours. The team has an average of more than 20 years of management experience.
Business strategy
We intend to:
- •
- grow with our current customer and application base by capitalizing on our core capabilities in microporous membranes and modules;
- •
- leverage existing and developing product and process technologies to pursue new, high value-added markets and applications; and
- •
- maximize access to key customers and end markets through strategic relationships and acquisitions.
5
Products and markets
Our two businesses, energy storage and separations media, are organized into three reportable segments. The following table describes our key products and end markets served:
Segment | Applications | Major brands | End markets | |||||
|---|---|---|---|---|---|---|---|---|
Energy storage—transportation and industrial | Lead-acid batteries | Daramic® Duralife® DARAK® | Transportation and industrial batteries | |||||
Energy storage—electronics and EDVs | Rechargeable and disposable lithium batteries | CELGARD® | Consumer electronics devices (such as mobile phones, audio/video players, notebook computers, tablets and cameras), cordless power tools, large-format applications such as EDVs (bikes, scooters, cars, trucks, buses and industrial utility vehicles) and emerging applications such as energy storage systems | |||||
Separations media | Hemodialysis | PUREMA® | Hemodialysis dialyzers which replicate function of healthy kidneys to treat end-stage renal disease patients | |||||
Blood oxygenation | CELGARD® | Heart-lung machine oxygenation unit for open-heart surgical procedures and intensive care artificial lung applications | ||||||
Plasmapheresis | MicroPES® | Blood cell and plasma separation equipment | ||||||
Industrial and specialty filtration applications | Liqui-Cel® | Liquid gasification/degasification for beverage, pharmaceutical, semiconductor and flat panel display manufacturing, and power and boiler feedwater applications | ||||||
MicroPES® | Specialty filtration applications including ultrapure water, cold sterile filtration of beverages, ultrapure chemicals for the electronics industry and pharmaceutical processing | |||||||
SuperPhobic® | Solvent/ink deaeration for ink jet printers, paper coating processes and semiconductor manufacturing | |||||||
MicroModule® MiniModule® | Liquid degasification in laboratory, biotechnology and analytical testing equipment and ink degasification for ink jet printers | |||||||
Accurel® | Specialty filtration applications including ultrapure chemicals for the electronics industry, vent and process air cleaning, industrial wastewater treatment and pharmaceutical processing | |||||||
Liqui-Flux® | Drinking water and beverage filtration, process water treatment and pre-filtration for reverse osmosis | |||||||
6
Energy storage
In the energy storage business, our membrane separators are a critical performance component in lithium and lead-acid batteries, performing the core function of regulating ion exchange and thus allowing the charge and discharge process to occur between a battery's positive and negative electrodes. These membrane separators require specialized technical engineering and must be manufactured to extremely demanding requirements and specifications including thickness, porosity, mechanical strength and chemical and electrical resistance. For example, membrane pores must be large enough to allow ions to pass through, but small enough to prevent contamination from conductive particles that cause short circuits. The energy storage business is comprised of two reportable segments.
Electronics and EDVs
We develop, manufacture and market a broad line of patented polypropylene and polyethylene monolayer and multilayer membrane separators for lithium batteries that are used in numerous applications such as consumer electronics devices, EDVs, cordless power tools and ESS. Lithium batteries provide critical performance advantages relative to alternative battery technologies, such as faster charging rates, improved battery life and higher power density, which results in more compact, lightweight batteries. These advantages create the potential for expansion by lithium batteries into additional product designs. According to B3 Corporation, a research firm specializing in lithium-ion batteries, the total lithium battery market is expected to grow at a compound annual growth rate in excess of 16% through 2020 on an energy capacity basis, driven by the application of lithium battery technology in new markets such as EDVs and ESS. B3 Corporation forecasts a compound annual growth rate through 2020 on an energy capacity basis of greater than 40% for EDVs and 10% for consumer electronics applications. Many factors influence membrane separator usage in lithium-ion batteries, but because many new applications are incorporating large-format lithium batteries that require much greater membrane separator volume per battery, we believe that membrane separator growth will exceed battery unit sales growth and although not perfectly correlated, will more closely approximate the growth rate in energy capacity.
We believe, based on independent industry research, that we are among the top three lithium battery separator providers, serving the entire breadth of lithium battery applications, and have been among the top three since the market's inception in the early 1990s. Major lithium battery manufacturers include Automotive Energy Supply Corporation (AESC), Amperex Technology Limited, BYD Company Limited, LG Chem Ltd., Panasonic Corporation, Saft Groupe SA, Samsung SDI Co., Ltd., Sanyo Electric Company Limited (a Panasonic Corporation member company), Sony Corporation and Tianjin Lishen Battery Joint Stock Co., Ltd.
Transportation and industrial
We develop, manufacture and market a complete line of high-performance polymer-based membrane separators for lead-acid batteries. Approximately 75% of our lead-acid battery separators are used in batteries for automobiles and other motor vehicles. The remaining approximately 25% are used in industrial battery applications such as forklifts, submarines and uninterruptible power supply systems. We believe that over 80% of lead-acid battery unit sales for motor vehicles are aftermarket batteries. Aftermarket sales are primarily driven by the size of the worldwide vehicle fleet rather than by new motor vehicle sales. According to WardsAuto.com, the worldwide fleet of motor vehicles has averaged 3% annual growth for over 25 years, providing us with a growing, recurring revenue base. Lead-acid battery separator growth is strongest in the Asia Pacific region due, we believe, to increasing penetration of automobile ownership, growth in industrial and manufacturing sectors, export incentives and ongoing conversion to polyethylene-based membrane separators.
7
We believe we are the global market leader in the lead-acid battery separator market. Major lead-acid battery manufacturers include Camel Group Co., Ltd., East Penn Manufacturing Co., Inc., EnerSys, Exide Technologies, Johnson Controls, Inc. and Trojan Battery Company.
Separations media
In the separations media business, our filtration membranes and modules are used in healthcare and high-performance filtration and specialty applications. These membranes perform the critical function of removing sub-micron particulates from fluids and introducing or removing gases (gasification/degasification) within liquids. Both healthcare and specialty filtration applications require membranes with precisely controlled pore size, structure, distribution and uniformity. Our supply relationships with customers in healthcare and certain filtration applications such as pharmaceutical manufacturing are reinforced by the rigorous testing, clinical studies and/or regulatory approval that our membranes undergo prior to end-product commercialization. In some cases, several years of development and qualification are required. The separations media business is a reportable segment serving two broad application end markets.
Healthcare
We develop, manufacture and market a complete portfolio of patented polyethersulfone membranes used in the hemodialysis market. Hemodialysis is the artificial process that performs the function of a healthy kidney for patients with permanent kidney failure, a condition known as end-stage renal disease ("ESRD"). In a healthy person, the kidney carries out certain excretory and endocrine functions, including filtering toxins from the blood and controlling blood pressure. For an ESRD patient on dialysis, the dialyzer membrane performs these critical filtering functions. The membranes are produced as hollow fibers slightly larger than a human hair and wound to bundles of thousands of fibers. These fibers have nanopores in their walls at a density of millions of pores per square inch. The size and distribution of these nanopores are designed to separate harmful toxins from the healthy blood passing through the dialyzer. Growth in demand for dialyzers and dialyzer membranes is driven by several factors, including the aging population in developed countries, longer life expectancy of treated ESRD patients and improving access to treatment in developing countries. According to the European Renal Association—European Dialysis and Transplant Association, the number of worldwide ESRD patients has historically grown by a compound annual growth rate of approximately 6%. We estimate that continued patient population growth combined with conversion to single-use dialyzers and increasing treatment frequency will result in overall annual dialyzer market growth in excess of 6%.
Our synthetic membrane, PUREMA®, is superior in performance compared to other synthetic membranes on the market. Dialyzers containing PUREMA® have been classified in the highest level (category 5) of Japan's dialyzer reimbursement rate system, reflecting the efficacy of our PUREMA® membrane. We believe the superior performance of our PUREMA® membranes is demonstrated by several long-term supply agreements with customers throughout the world. We are currently marketing PUREMA®'s performance advantages, such as by co-branding our customers' dialyzers with the PUREMA® logo.
We believe, based on independent industry research, that we are uniquely positioned as the leading independent supplier of synthetic membranes in the hemodialysis market (i.e., not a supplier of dialyzers). Major dialyzer manufacturers include Asahi Kasei Kuraray Medical Co., Ltd., Bain Medical Equipment Co., Ltd., Baxter International, Inc., Bellco S.r.l., Fresenius Medical Care AG and Nipro Corp.
We develop, manufacture and market polyolefin membranes used in the blood oxygenation market. As a component of heart-lung machines, blood oxygenators temporarily replace the functions of the lungs during on-pump open-heart surgery. The oxygenator contains highly specialized membranes which
8
remove carbon dioxide from the blood while oxygen is diffused into the blood. We estimate that growth in the blood oxygenation market is modest as a result of the use of less-invasive alternative heart treatments. However, we believe that the market could grow as the number of patients receiving on-pump open-heart surgery increases due to the saturation of alternative heart treatments. Because blood oxygenators are designed to utilize a specific membrane technology and require regulatory approval, an oxygenator manufacturer's relationship with its membrane supplier is vital and switching costs can be substantial. We believe, based on independent industry research, that we are the world's leading supplier of membranes used in blood oxygenation. Major blood oxygenator producers include Maquet Cardiopulmonary AG, Medtronic, Inc., Sorin Group S.r.l. and Terumo Medical Corp. Our Oxyplus® membranes, which are made of polymethylpentene, are increasingly used in intensive care applications to serve as an artificial lung for patients with severe lung trauma or lung failure following sepsis, multi-organ failure, or infectious diseases. Major producers of such artificial lungs are Sorin Group S.r.l., Maquet Cardiopulomnary AG, Terumo Medical Corp., Medtronic, Inc. and Medos AG.
We develop, manufacture and market polypropylene and polyethersulfone membranes for the plasmapheresis market. In plasmapheresis, plasma is separated from the blood and either retained for the production of therapeutic proteins or filtered and returned to the blood as a treatment for various autoimmune disorders. We believe that the plasmapheresis market is growing and that new treatment methodologies based on blood filtration may lead to additional growth. We believe we are a leading supplier of extracorporeal therapeutic plasmapheresis membranes. Major manufacturers of plasmapheresis equipment include Asahi Kasei Kuraray Medical Co., Ltd., Baxter International, Inc., Fresenius Medical Care AG and B. Braun AG.
Industrial and specialty filtration
We produce a wide range of membranes and membrane-based elements for microfiltration and ultrafiltration as well as gasification/degasification of liquids, covering a broad range of applications in the filtration market. According to independent industry research, the global microfiltration and ultrafiltration membrane element market is growing in excess of 8% annually. Market growth is being driven by several factors, including end-market growth in various water treatment applications, displacement of conventional filtration media by membrane filtration due to membranes' superior cost and performance attributes, and increasing purity requirements in industrial and other applications. We currently serve a variety of filtration end markets, including water treatment, food and beverage processing and pharmaceutical, semiconductor and flat panel display manufacturing, and we are working closely with current and potential customers to develop innovative new products based on our technology and capabilities.
We believe, based on internal company estimates, that we are the world leader in membrane gasification/degasification for liquids and that we are uniquely positioned as the leading independent supplier of polyethersulfone flat sheet membranes.
New product development
We have focused our research and development efforts on developing products for new markets based on existing technologies and developing new process technologies to enhance existing businesses and allow entry into new businesses. We spent $17.2 million (3% of our net sales), $18.5 million (3% of our net sales) and $17.6 million (3% of our net sales) in fiscal 2013, fiscal 2012 and fiscal 2011, respectively, on research and development.
9
Our transportation and industrial research and development is performed at technical centers at our facilities in Owensboro, Kentucky; Prachinburi, Thailand; Selestat, France; and Bangalore, India. Our electronics and EDVs research and development is performed at technical centers at our facilities in Charlotte, North Carolina; Concord, North Carolina; and Ochang, South Korea. Our separations media research and development is performed at technical centers at our facilities in Wuppertal, Germany, and Charlotte, North Carolina. All of the products that we develop are subject to multiple levels of extensive and rigorous testing. The qualification of membrane separators for use in transportation and industrial applications, for instance, may require one or more years of testing by our staff and battery manufacturers.
Sales and marketing
We sell our products and services to customers in both the domestic and international marketplace. We sell primarily to manufacturers and converters that incorporate our products into their finished goods. We employ a direct worldwide sales force and utilize approximately 100 people with extensive experience who manage major customer relationships. Many of our sales representatives are engineers or similarly trained technical personnel who have advanced knowledge of our products and the applications for which they are used. Our sales representatives are active in new product development efforts and are strategically located in the major geographic regions in which our products are sold. In certain geographic areas, we use distributors or other agents. We typically seek to enter into supply contracts with our major customers. These contracts typically describe the volume and selling price. In addition, these contracts reflect our close collaborative relationships with our customers, which are driven by our customers' need to develop new separators and membranes directly with us. In fiscal 2013, sales to our top five customers represented approximately 24% of our total net sales. No sales to an individual customer accounted for more than 10% of total net sales in fiscal 2013, 2012 or 2011.
Manufacturing and operations
General
We have manufacturing facilities in the major geographic markets of the United States, Europe and Asia. We manufacture our lead-acid battery separators at our facilities in Owensboro, Kentucky; Corydon, Indiana; Selestat, France; Norderstedt, Germany; Prachinburi, Thailand; Tianjin, China; and Xiangyang, China (a 65% owned joint venture). We also have finishing operations in Bangalore, India and Baddi, India. We manufacture our lithium battery separators at our facilities in Charlotte, North Carolina; Concord, North Carolina; and Ochang, South Korea, and have a finishing operation in Shanghai, China. We manufacture our healthcare membranes and industrial and specialty filtration membranes and membrane modules at facilities in Wuppertal and Obernburg, Germany, and Charlotte, North Carolina.
In fiscal 2013, fiscal 2012 and fiscal 2011, we generated net sales from customers outside the United States of approximately 81%, 83% and 84%, respectively. We typically sell our products in the currency of the country in which the products are manufactured rather than the local currency of our customers.
Our manufacturing facilities in the United States accounted for 36% of total sales for fiscal 2013, with facilities in Europe and Asia accounting for 40% and 24%, respectively. Our foreign operations are subject to certain risks that could materially affect our sales, profits, cash flows and financial position. These risks include fluctuations in foreign currency exchange rates, inflation, economic or political instability, shipping delays, changes in applicable laws and regulatory policies and various trade restrictions, all of which could have a significant impact on our ability to deliver products on a competitive and timely basis. The future imposition of, or significant increases in the level of, customs
10
duties, import quotas or other trade restrictions could also have a material adverse effect on our business, financial condition and results of operations.
Manufacturing processes
All of our membrane manufacturing processes involve an extrusion process. To produce our flat sheet and hollow fiber membranes, we use one of three basic membrane processes that begin with an extrusion step. These include phase separation (thermally-induced, solvent-induced or reaction-induced), dry stretch and extrusion/extraction processes. Each process, and its resulting product properties, is well-suited to the various membrane requirements for our target markets. To produce Liqui-Cel® Membrane Contactors and Liqui-Flux® membrane modules, hollow fibers are bonded together into a cartridge form by extruding either a polyolefin resin or using an epoxy or polyurethane adhesive before final assembly into a finished module.
Membrane separators for batteries. We manufacture Daramic®, our principal lead-acid battery separator used in industrial and automotive applications, using a composite extrusion/extraction process. The process stages are fully automated, although the process requires some handling as material is transferred from stage to stage. Initially, an ultra-high molecular weight polyethylene is mixed with porous silica and oil, which are heated and extruded into a film. The film is passed through an extraction bath to remove the excess oil from the silica pores to create the proper microporosity and film stiffness prior to drying.
We manufacture our lithium battery separators using two processes, both of which begin with an extrusion step. Membrane porosity is created either during a thermal stretching process or during a solvent-induced process. Some special coated and non-woven laminate products are also manufactured for specialty battery and other applications.
Hemodialysis, blood oxygenation, plasmapheresis and filtration membranes. Membranes used in hemodialysis, blood oxygenation, plasmapheresis and filtration are produced using phase separation processes. For these phase separation processes, the polymer spinning solution is prepared by dissolving the polymer in a solvent prior to extrusion. A porous membrane is formed by separating the solvent and polymer phases using temperature (thermally-induced) or a "non-solvent" (solvent-induced), and then the solvent phase is extracted and the porous polymer membrane is dried. For blood oxygenation and certain filtration applications, hollow fiber and flat sheet membranes are also produced using our "dry stretch" process. We utilize the molecular behavior of semi-crystalline polymers (polyolefins) to create microporous structures. By controlling the extrusion process under which the film or fiber is formed, we create a distinct matrix of crystalline and amorphous regions that allows the formation of microvoids in a subsequent stretching step afterwards. After extrusion, our products can be stored or immediately processed on thermal stretching lines that create the final porous form.
Competition
Our markets are highly competitive. Within our energy storage business, we face competition throughout the world. Our primary competitors in the market for membrane separators used in lead-acid batteries for transportation and industrial applications are Entek International LLC in North America and Europe, Baoding Fengfan Rising Battery Separator Co., Ltd. in China and Nippon Sheet Glass Co., Ltd. in Japan. In addition, we have a number of smaller competitors in South Korea, Indonesia, China and Taiwan. Our primary competitors in the market for membrane separators used in lithium batteries are Asahi Kasei E-materials Corp. (a subsidiary of Asahi Kasei Corporation), Toray Battery Separator Film Co., Ltd. (a subsidiary of Toray Industries, Inc.), SK Innovation Co., Ltd. (a subsidiary of SK Holdings Co., Ltd.) and Ube Industries Limited, as well as a number of smaller competitors.
11
Within our separations media business, we compete in the market for membranes used in dialysis with Asahi Kasei Kuraray Medical Co., Ltd., Fresenius Medical Care AG, Baxter International, Inc. and Toyobo Co. Ltd. In addition, our primary competitor in the blood oxygenation market is Terumo Medical Corp., and in the plasmapheresis market, our competitors are Asahi Kasei Kuraray Medical Co., Ltd. and Fresenius Medical Care AG. Our industrial and specialty filtration business competes across multiple markets and applications. Principal competitors include Dainippon Ink and Chemicals, Inc., Koch Membrane Systems (a division of Koch Industries), X-Flow B.V. (a subsidiary of Pentair, Ltd.), Millipore (a division of Merck KGaA) and Pall Corporation. Product innovation and performance, quality, service, utility and cost are the primary competitive factors, with technical support being highly valued by our customers. We believe that we are well-positioned in our end markets for the reasons set forth under "Competitive strengths" above.
Raw materials
We employ a global purchasing strategy to achieve pricing leverage on our purchases of major raw materials. The major polyethylene and polypropylene resins we use are specialized petroleum-based products that are less affected by commodity pricing cycles than other petroleum-based products. In the event of future price increases for these major raw materials, we believe that we will generally be able to pass these increases on to our customers. The primary raw materials we use to manufacture most of our products are polyethylene and polypropylene resins, silica and oil. Our major suppliers of polyethylene resins are Ticona LLC and Korea Petrochemical Ind. Co., Ltd., and our major suppliers of polypropylene resins are Bamberger Polymers, Inc., NEXEO Solutions and Total Petrochemicals & Refining USA, Inc. Our major suppliers of silica are PPG Industries, Inc. and Evonik Degussa GmbH, while our major suppliers of oil are Shell Chemical LP and Ergon, Inc.
We believe that the loss of any one or more of our suppliers would not have a long-term material adverse effect on us because other suppliers with whom we conduct business or have conducted business in the past would be able to fulfill our requirements. However, the loss of one of our key suppliers could, in the short term, adversely affect our business until we secure alternative supply arrangements. We have never experienced any significant disruptions in supply as a result of shortages in raw materials.
Employees
At December 28, 2013, we had approximately 2,400 employees worldwide. Employees at six of our 15 facilities are unionized and account for approximately 33% of our total employees. The following summarizes those employees represented by unions as of December 28, 2013:
Location | Number of unionized employees | % of total employees | Date of contract renegotiation | |||||
|---|---|---|---|---|---|---|---|---|
Norderstedt, Germany | 48 | 69 | Annual | |||||
Obernburg, Germany | 21 | 81 | Annual | |||||
Wuppertal, Germany | 401 | 86 | Annual | |||||
Corydon, Indiana | 66 | 73 | September 2018 | |||||
Selestat, France | 131 | 68 | June 2014 | |||||
Owensboro, Kentucky | 118 | 70 | April 2017 | |||||
| | | | | | | | | |
Total | 785 | |||||||
| | | | | | | | | |
| | | | | | | | | |
Environmental matters
We are subject to a broad range of federal, state, local and foreign environmental laws and regulations which govern, among other things, air emissions, wastewater discharges and the handling,
12
storage, disposal and release of wastes and hazardous substances. It is our policy to comply with applicable environmental requirements at all of our facilities. We are also subject to laws, such as the Comprehensive Environmental Response, Compensation and Liability Act ("CERCLA"), that may impose liability retroactively and without fault for releases or threatened releases of hazardous substances at on-site or off-site locations. From time to time, we have identified environmental compliance issues at our facilities.
We have conducted some cleanup of on-site releases at some facilities, and we may be required to conduct additional cleanups of on-site contamination at other facilities under regulatory supervision or voluntarily. Costs for such work and related measures (such as eliminating sources of contamination) could be substantial. In connection with the acquisition of Membrana GmbH ("Membrana") in 2002, we recorded a reserve for costs to remediate known environmental issues and operational upgrades which are required in order for us to remain in compliance with legal regulations. We had indemnification agreements with the prior owners of Membrana for a substantial portion of these costs. As of December 28, 2013, the reserve for such costs was $2.9 million. During the third quarter of 2013, we received the final payment of amounts outstanding under the indemnification agreements. We do not anticipate that the remediation activities will disrupt operations at our facilities or have a material adverse effect on our business, financial condition or results of operations.
Intellectual property rights
We consider our patents and trademarks, in the aggregate, to be important to our business and seek to protect our tradenames, trademarks, brandnames, proprietary technology and know-how in part through United States and foreign patents and trademark registrations and applications. However, no individual patent is material to our business, and the expiration or invalidation of any one patent would not have a material impact on our business. We own approximately 155 active unique patents and patent applications relating to our separator, membrane and module technologies. In general, the term of each of our U.S. patents depends on when the patent was filed. If the application for a U.S. utility patent was filed prior to June 8, 1995, the patent will expire either 20 years from the application filing date or 17 years from the patent issuance date, whichever is longer; if the application was filed on or after June 8, 1995, the patent will expire 20 years from the earliest date the application was filed.
In general, trademarks are valid as long as they are in use and/or their registrations are properly maintained, and trademark registrations can generally be renewed indefinitely so long as the marks are in use. Some of our registered marks include CELGARD®, Liqui-Cel®, Daramic® and PUREMA®.
In addition, we maintain certain trade secrets for which, in order to maintain the confidentiality of such trade secrets, we have not sought patent protection. Our policies require our employees to assign their intellectual property rights to us and to treat all proprietary technology as our confidential information.
We have granted security interests or liens on some of our patents to financial institutions, including lenders under our senior secured credit agreement.
If we fail to adequately protect and enforce our intellectual property rights, competitors may manufacture and market products similar to ours. The loss of protection for or enforcement of our intellectual property rights could reduce the market value of our products, reduce product sales, lower our profits or impair our financial condition. See "Risk Factors." If we are unable to adequately protect, police or enforce our intellectual property, we could lose a significant competitive advantage.
Available Information
You may obtain free copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports, as filed with or furnished to the Securities and Exchange Commission ("SEC"), as soon as reasonably practicable after such material is filed with or furnished to the SEC on our website at www.polypore.net.
13
Our business faces many risks. As such, prospective investors and shareholders should carefully consider and evaluate all of the risk factors described below. These risk factors may change from time to time and may be amended, supplemented, or superseded by updates to the risk factors contained in periodic reports on Form 10-Q and Form 10-K that we file with the SEC in the future.
Because the specialized markets in which we sell our products are highly competitive, we may have difficulty growing our business.
The markets in which we sell our products are highly competitive. Many of these markets require highly specialized products that are time and cost intensive to design and develop. In addition, innovative products, quality, service, utility, cost and technical support are the primary competitive factors in the separation and filtration membrane industry. Some of our competitors are much larger companies that have greater financial, technological, manufacturing and marketing resources than we do. Many of these competitors are also better established as suppliers to the markets that we serve. As a result, a reduction in overall demand or increased costs to design and produce our products within these markets could cause us to reduce our prices, which could lower our profit margins and impair our ability to grow our business.
We must continue to invest significant resources in developing innovative products in order to maintain a competitive edge in the highly specialized markets in which we operate.
Our continued success depends, in part, upon our ability to maintain our technological capabilities and to continue to identify, develop and commercialize innovative products for the separation and filtration membrane industry. For example, products for some consumer electronics applications have a short lifecycle and require constant development. If we fail to continue to develop products for those markets or keep pace with technological developments by our competitors generally, we may lose market share which could result in reduced sales and impair our financial condition.
The loss of large volume customers could impact our sales and our profits.
Our products are often sold to a relatively small number of large volume customers. For example, in fiscal 2013, sales to our top five customers represented approximately 24% of our total net sales. The loss of any large volume customer could impact our sales and our profits.
Vertical integration by our customers of the production of our products into their own manufacturing processes could reduce our sales and our profits.
Our future sales and profits will depend to a significant extent upon whether our customers choose in the future to manufacture the separation and filtration membranes used in their products instead of purchasing these components from us. If any of our existing customers choose to vertically integrate the production of our products in such a manner, the loss of sales to these customers could reduce our sales and our profits.
Increases in prices for raw materials or the loss of key supplier contracts could reduce our profit margins.
The primary raw materials we use in the manufacture of most of our products are polyethylene and polypropylene resins, silica and oil. In fiscal 2013, raw materials accounted for approximately 32% of our cost of sales. Although our major customer contracts generally allow us to pass increased costs on to our customers, we may not be able to pass on all raw material price increases to our customers in each case or without delay. The loss of any of our key suppliers could disrupt our business until we secure alternative supply arrangements. Furthermore, any new supply agreement we enter into may not have terms as favorable as those contained in our current supply arrangements.
14
Our substantial indebtedness could harm our ability to react to changes in our business or to market developments and prevent us from fulfilling our obligations under our indebtedness.
As of December 28, 2013, our consolidated indebtedness is $646.3 million. Our cash interest requirements for the next twelve months are estimated to be $36.2 million. Our substantial level of indebtedness, as well as any additional borrowings we may make under the unused portions of the senior secured credit agreement, increases the possibility that we may be unable to generate cash sufficient to pay, when due, the principal of, interest on or other amounts due in respect of our indebtedness. Our substantial debt could increase our vulnerability to general economic downturns and adverse competitive and industry conditions by limiting our flexibility to plan for, or to react to, changes in our business and in the industry in which we operate. This limitation could place us at a competitive disadvantage compared to competitors that have less debt and more cash to insulate their operations from market downturns and to finance new business opportunities.
Our variable rate indebtedness subjects us to interest rate risk, which could cause our debt service obligations to increase significantly.
At December 28, 2013, we had variable rate debt of $281.3 million. An interest rate increase would result in an increase in interest expense. Our earnings may not be sufficient to allow us to meet any such increases in interest rate expense and to pay principal and interest on our debt and meet our other obligations. If we do not have sufficient earnings, we may be required to refinance all or part of our existing debt, sell assets, borrow more money or sell more securities, none of which we can guarantee we will be able to do on terms favorable to the Company or at all.
To service our indebtedness, we will require a significant amount of cash. Our ability to generate cash depends on many factors beyond our control.
Our ability to make payments on and to refinance our indebtedness and to fund our operations will depend on our ability to generate cash in the future. However, our business may not generate sufficient cash flow from operations for a variety of reasons, including those mentioned elsewhere in this "Risk Factors" section. Without sufficient cash flow, future borrowings may not be available to us under the senior secured credit agreement in amounts sufficient to enable us to service our indebtedness or to fund our other liquidity or capital needs. If we cannot generate sufficient cash to service our debt, we will have to take such actions as reducing or delaying capital investments, selling assets, restructuring or refinancing our debt or seeking additional equity capital. Any of these actions may not be effected on commercially reasonable terms, or at all. In addition, the indenture for the 7.5% senior notes and the senior secured credit agreement may restrict us from adopting any of these alternatives.
The terms of the senior secured credit agreement and the indenture related to the 7.5% senior notes may restrict our current and future operations, particularly our ability to respond to market changes or to take certain actions.
The senior secured credit agreement and the indenture related to the 7.5% senior notes contain a number of restrictive covenants that impose significant operating and financial restrictions on us and may limit our ability to engage in acts that may be in our long-term best interests. For example, the senior secured credit agreement includes covenants restricting, among other things, our ability to incur, assume or permit to exist additional indebtedness or guarantees; engage in mergers, acquisitions and other business combinations; or amend or otherwise alter terms of our indebtedness, including the 7.5% senior notes, and other material agreements. The senior secured credit agreement also includes financial covenants requiring that we maintain a maximum senior leverage ratio and a minimum interest coverage ratio.
15
The indenture related to the 7.5% senior notes also contains numerous negative covenants including, among other things, restrictions on our ability to incur or guarantee additional debt, issue preferred stock of restricted subsidiaries, pay dividends or make other equity distributions, or purchase or redeem capital stock.
A breach of any of these covenants or the inability to comply with financial covenants could result in a default under the senior secured credit agreement or the indenture governing the 7.5% senior notes. If any such default occurs, the lenders and the holders of the 7.5% senior notes may elect to declare all outstanding borrowings, together with accrued interest and other amounts payable thereunder, to be immediately due and payable. The lenders under the credit agreement also have the right in these circumstances to terminate any commitments they have to provide further borrowings and to proceed against all collateral granted to them to secure the debt. If collateral (such as available cash) is repossessed by the lenders or holders of the 7.5% senior notes, we will be unable to access the capital and other resources necessary to operate our business, and we could incur immediate and significant losses.
Some of our employees are represented under collective bargaining agreements. Any employee slowdowns, strikes or failure to renew our collective bargaining agreements could disrupt our business.
Approximately 33% of our employees are represented under collective bargaining agreements. A majority of those employees are located in France and Germany and are represented under industry-wide agreements that are subject to national and local government regulations. Many of these collective bargaining agreements must be renewed annually. Labor unions also represent our employees in Owensboro, Kentucky, and Corydon, Indiana.
Labor organizing activities could result in additional employees becoming unionized. We may not be able to maintain constructive relationships with these labor unions or successfully negotiate new collective bargaining agreements in the future. The loss of a substantial number of these employees or a prolonged labor dispute could disrupt our business. Any such disruption could in turn reduce our sales, increase our costs to bring products to market and result in significant losses.
We generate most of our sales from manufacturing products that are used in a wide variety of industries and the potential for product liability exposure for our products could be significant.
We manufacture a wide variety of products that are used in healthcare and consumer applications. Several of these products are used in medical devices that some consumers require in order to sustain their lives. As a result, we may face exposure to product liability claims in the event that the failure of our products results, or is alleged to result, in bodily injury and/or death. In addition, if any of our products are, or are alleged to be, defective, we may be required to make warranty payments or to participate in a recall involving those products. Consequently, end users of our products may look to us for contribution when faced with product recalls, product liability or warranty claims. The future costs associated with defending product liability claims or providing product warranties could be material and we may experience material losses in the future as a result. A successful product liability claim brought against us in excess of available insurance coverage or a requirement to participate in any product recall could substantially reduce our available cash from operations. Reduced cash could in turn reduce our profits or impair our financial condition.
Our operations outside the United States pose risks to our business that are not present with our domestic business, including compliance with applicable anti-corruption laws.
Our manufacturing facilities in the United States accounted for 36% of total net sales for fiscal 2013, with facilities in Europe and Asia accounting for 40% and 24%, respectively. Typically, we sell our products in the currency of the country where the manufacturing facility that produced the
16
products is located. In addition, as part of our growth and acquisition strategy, we may expand our operations in these or other foreign countries. Our foreign operations are, and any future foreign operations will be, subject to certain risks that are unique to doing business in foreign countries. These risks include fluctuations in foreign currency exchange rates, inflation, economic or political instability, shipping delays, changes in applicable laws and regulatory policies and inconsistent enforcement of such laws and policies and various trade restrictions. All of these risks could have a negative impact on our ability to deliver products to customers on a competitive and timely basis. This could reduce or impair our sales, profits, cash flows and financial position. The future imposition of, or significant increases in the level of, customs duties, import quotas or other trade restrictions could also increase our costs and reduce our profits.
Countries around the world are increasingly enacting anti-corruption laws, many of which are patterned after the United States' Foreign Corrupt Practices Act ("FCPA"). The FCPA generally prohibits companies and their intermediaries from giving, or offering to give, anything of value to foreign officials with the intent of obtaining an improper business advantage. The FCPA's foreign counterparts contain similar prohibitions, although varying in both scope and jurisdiction. Our internal policies prohibit corruption in all forms and expressly mandate compliance with the FCPA in particular. Despite such policies and the FCPA training we provide to our employees, we cannot guarantee that our employees', agents' or other intermediaries' conduct will not periodically violate the anti-corruption laws of a particular nation. Our continued overseas expansion, especially in the aforementioned emerging markets, naturally increases the risk of such violations and consequently could cause a material adverse effect on our operations or financial condition.
We could incur substantial costs to comply with environmental laws, and violations of such laws may increase our costs or require us to change certain business practices.
We use and generate a variety of chemicals and other hazardous by-products in our manufacturing operations. As a result, we are subject to a broad range of federal, state, local and foreign environmental laws and regulations. These environmental laws govern, among other things, air emissions, wastewater discharges and the handling, storage and release of wastes and hazardous substances. Such laws and regulations can be complex and change often. We regularly incur costs to comply with environmental requirements, and such costs could increase significantly with changes in legal requirements or their interpretation or enforcement. Some of our manufacturing facilities have been the subject of actions to enforce environmental requirements. We could incur substantial costs, including clean-up costs, fines and sanctions and third-party property damage or personal injury claims, as a result of violations of environmental laws. Failure to comply with environmental requirements could also result in enforcement actions that materially limit or otherwise affect the operations of the facilities involved.
Under certain environmental laws, a current or previous owner or operator of an environmentally contaminated site may be held liable for the entire cost of investigation, removal or remediation of hazardous materials at such property. This liability could result whether or not the owner or operator knew of, or was responsible for, the presence of any hazardous materials.
Contaminants have been detected at some of our present facilities, principally in connection with historical operations. Investigations and/or clean-ups of these contaminants have been undertaken by us or by former owners of the sites. The costs of investigating and remediating environmental conditions at some of our facilities may be substantial. If our remediation costs are higher than expected, our exposure to these costs would increase. This exposure could reduce our cash available for operations, consume valuable management time and reduce our profits or impair our financial condition.
We anticipate additional investigations and clean-ups of on-site contamination under regulatory supervision or voluntarily at some of our sites. In addition, the imposition of more stringent clean-up
17
requirements, the discovery of additional contaminants or the discovery of off-site contamination at or from one or more of our facilities could result in significant additional costs to us.
If we are unable to adequately protect our intellectual property, we could lose a significant competitive advantage.
Our success with our products depends, in part, on our ability to protect our unique technologies and products against competitive pressure and to defend our intellectual property rights. If we fail to adequately protect our intellectual property rights, competitors may manufacture and market products similar to ours.
Even though we have filed patent applications, we may not be granted patents for those applications. Our failure to secure these patents may limit our ability to protect the intellectual property rights that these applications were intended to cover. Moreover, even if we are granted a patent, that does not prove conclusively that the patent is valid and enforceable. Our existing or future patents that we receive or license may not provide competitive advantages for our products. Our competitors may invalidate, narrow or avoid the scope of any existing or future patents, trademarks, or other intellectual property rights that we receive or license. In addition, patent rights may not prevent our competitors from developing, using or selling products that are similar or functionally equivalent to our products. Patent rights are territorial; thus, the patent protection we do have will only extend to those countries in which we have issued patents. Even so, the laws of certain countries do not protect our intellectual property rights to the same extent as do the laws of the United States and various European countries. The loss of protection for our intellectual property could reduce the market value of our products, reduce product sales and lower our profits or impair our financial condition.
We intend to enforce our intellectual property rights vigorously, and from time to time we may initiate claims against third parties that we believe are infringing our intellectual property rights if we are unable to resolve matters satisfactorily through negotiation or we may be required to participate in other administrative proceedings. Lawsuits brought to protect and enforce our intellectual property rights could be expensive, time-consuming and distracting to management and could result in the impairment or loss of portions of our intellectual property. Our failure to secure, protect and enforce our intellectual property rights could seriously harm our business.
Security interests or liens have been granted to financial institutions on some of our patents. If we fail to satisfy our obligations, the financial institutions have rights to those patents.
Due to the unique products that we produce and the particular industry in which we operate, the loss of our senior management could disrupt our business.
Our senior management is important to the success of our business. There is significant competition for executive personnel with unique experience in the separation and filtration membrane industry. As a result of this unique need and the competition for a limited pool of industry-based executive experience, we may not be able to retain our existing senior management. In addition, we may not be able to fill new positions or vacancies created by expansion or turnover or attract additional senior management personnel. All of our executive officers are free to pursue other business opportunities (other than our chief executive officer, who is bound by a non-compete provision of his employment agreement), including those that may compete with us. The loss of any member of our senior management without retaining a suitable replacement (either from inside or outside our existing management team) could disrupt our business.
18
We may pursue future acquisitions. If we incur contingent liabilities and expenses or additional debt in connection with future acquisitions or if we cannot effectively integrate newly acquired operations, our business could be disrupted.
Acquisitions involve risks that the businesses acquired will not perform in accordance with expectations or that business judgments concerning the value, strengths and weaknesses of businesses acquired will prove incorrect. Future acquisitions would likely result in the incurrence of debt and contingent liabilities. Such acquisitions could also increase our interest and amortization expenses as well as periodic impairment charges related to goodwill and other intangible assets. Acquisitions could also result in significant charges relating to integration costs. We may not be able to integrate successfully any business we acquire into our existing business. Any acquired businesses may not be profitable or as profitable as we had expected. The successful integration of new businesses depends on our ability to manage these new businesses and cut excess costs. The successful integration of future acquisitions may also require substantial attention from our senior management and the management of the acquired business. This could decrease the time that they have to service and attract customers and develop new products and services. In addition, because we may actively pursue a number of opportunities simultaneously, we may encounter unforeseen expenses, complications and delays. Such expenses and delays could include difficulties in employing sufficient staff and maintaining operational and management oversight. Our inability to complete the integration of new businesses in a timely and orderly manner could increase costs, reduce our profits and ultimately disrupt our business.
As part of our lithium battery capacity expansions, we have purchased a significant amount of property, plant and equipment, which may be subject to impairment if lithium market demand does not materialize as anticipated.
Since late 2009, we have invested over $300.0 million in capacity expansions for our electronics and EDVs segment. During 2012 and 2013, demand for lithium battery separators was lower than originally anticipated, and we are currently operating our production facilities at levels below full capacity. If demand for lithium battery separators does not materialize as expected, we may have to recognize an impairment charge to such assets. Long-lived assets are reviewed annually for impairment. Impairment may result from, among other things, adverse market conditions, the loss of customers, significant changes in the expected adoption rate of EDVs and adverse changes in laws or regulations. Any future determination requiring the impairment of long-lived assets would reduce our profits for the fiscal period in which the write-off occurs.
We have recorded a significant amount of intangible assets, which may never generate the returns we expect.
Our net identifiable intangible assets at December 28, 2013 were approximately 6% of our total assets. Such assets include customer relationships, trademarks and trade names, license agreements and technology acquired in acquisitions. Goodwill, which relates to the excess of cost over the fair value of the net assets of the businesses acquired, was approximately 29% of our total assets at December 28, 2013. Goodwill and identifiable intangible assets are recorded at fair value on the date of acquisition and are reviewed at least annually for impairment. Impairment may result from, among other things, deterioration in the performance of the acquired business, adverse market conditions and adverse changes in applicable laws or regulations. We may never realize the full value of our intangible assets. Any future determination requiring the write-off of a significant portion of intangible assets would reduce our profits for the fiscal period in which the write-off occurs.
Our operations are vulnerable to interruption or loss due to natural disasters, epidemics, terrorist acts and other events beyond our control, which could adversely affect our business.
Our operations may be subject to significant interruption if any of our facilities are damaged or destroyed. For example, certain of our products are manufactured at a single facility location for which
19
we do not maintain a backup manufacturing facility. A natural or other disaster, such as a fire or flood, could significantly disrupt our operations, delay or prevent product manufacture and shipment for the time required to repair, rebuild or replace our manufacturing facilities, which could be lengthy, and result in large expenses to repair or replace the facilities. In addition, concerns about terrorism or an outbreak of epidemic diseases, especially in our major markets of North America, Europe and Asia could have a negative effect on travel and our business operations, and result in adverse consequences on our sales and financial performance.
Item 1B. Unresolved Staff Comments
None.
Our manufacturing facilities are strategically located to serve our customers globally. We believe our facilities are suitable and adequate for our present purposes.
Location | Floor area (sq. ft.) | Business segment | Certification | ||||
|---|---|---|---|---|---|---|---|
Owensboro, Kentucky(1) | 277,000 | Transportation and Industrial | ISO 14001, ISO 9001 | ||||
Prachinburi, Thailand | 166,000 | Transportation and Industrial | ISO 14001, ISO 9001 | ||||
Corydon, Indiana(1) | 161,000 | Transportation and Industrial | ISO 14001, ISO 9001 | ||||
Selestat, France | 153,000 | Transportation and Industrial | ISO 14001, ISO 9001 | ||||
Norderstedt, Germany | 124,000 | Transportation and Industrial | ISO 14001, ISO 9001 | ||||
Bangalore, India(2) | 76,000 | Transportation and Industrial | ISO 14001, ISO 9001, OHSAS 18001 | ||||
Baddi, India(2) | 20,000 | Transportation and Industrial | |||||
Tianjin, China | 47,000 | Transportation and Industrial | ISO 14001, ISO 9001 | ||||
Xiangyang, China(3) | 118,000 | Transportation and Industrial | ISO 14001, ISO 9001 | ||||
Ochang, South Korea | 108,000 | Electronics and EDVs | ISO 14001, ISO 9001, OHSAS 18001, KOSHA 18001 | ||||
Shanghai, China(2) | 47,000 | Electronics and EDVs | ISO 9001 | ||||
Concord, North Carolina(1) | 230,000 | Electronics and EDVs | ISO/TS 16949 | ||||
Charlotte, North Carolina(1) | 410,000 | Electronics and EDVs and Separations Media | ISO 14001, ISO 9001, ISO/TS 16949 | ||||
Wuppertal, Germany | 1,503,000 | Separations Media | ISO 14001, ISO 9001 | ||||
Obernburg, Germany(2) | 23,000 | Separations Media | ISO 9001 | ||||
- (1)
- These domestic facilities serve as collateral under the senior secured credit agreement.
- (2)
- Polypore owns the equipment and leases the facility.
- (3)
- Polypore holds a 65% ownership interest in the joint venture that owns this facility.
Our corporate headquarters are located in leased office space in Charlotte, North Carolina, and we have leased sales offices in Shanghai, China; Shenzhen, China; Tokyo, Japan; and Sao Paulo, Brazil.
20
On September 9, 2008, the Federal Trade Commission (the "FTC") issued an administrative complaint against us regarding our February 29, 2008 acquisition of Microporous Holding Corporation, the parent company of Microporous Products L.P. ("Microporous"). After challenging the complaint before an Administrative Law Judge of the FTC and subsequently before the Commissioners of the FTC, we were ordered to sell the Microporous business that was acquired in February 2008.
On January 28, 2011, we filed a petition with the U.S. Court of Appeals for the 11th Circuit to review the FTC's November 5, 2010 order and opinion. On July 11, 2012, the 11th Circuit affirmed the FTC's decision. On January 15, 2013, we filed an appeal with the U.S. Supreme Court, and on June 24, 2013, the U.S. Supreme Court declined to hear our petition for review of the 11th Circuit's opinion upholding the FTC's order. With the U.S. Supreme Court's decision not to hear the appeal, the divestiture provisions of the order, which were stayed pending appeal, went into effect on June 24, 2013. In accordance with the FTC's order, we completed the sale of the Microporous business, which consisted of the production facilities in Piney Flats, Tennessee, and Feistritz, Austria, on December 19, 2013.
Item 4. Mine Safety Disclosures
Not applicable.
21
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is listed on the New York Stock Exchange ("NYSE") under the symbol "PPO" and has been traded on the NYSE since our initial public offering on June 28, 2007. As of February 10, 2014, there were approximately 9,750 holders of our common stock, representing primarily persons whose stock is held in nominee or "street name" accounts through brokers.
We did not declare or pay any dividends on our common stock in fiscal 2013 or 2012, and we do not expect to pay any such dividends in fiscal 2014. The indenture relating to our $365.0 million aggregate principal amount of 7.5% senior notes due 2017 and our senior secured credit agreement restrict or limit our ability to, among other things, declare dividends and make payments on or redeem or repurchase capital stock.
The low and high sales prices for the Company's common stock for each full quarterly period within the two most recent fiscal years were as follows:
| | Fiscal 2013 | Fiscal 2012 | ||
|---|---|---|---|---|
First Quarter | $35.10 - $48.42 | $33.80 - $57.66 | ||
Second Quarter | $36.80 - $45.00 | $33.60 - $40.75 | ||
Third Quarter | $39.52 - $48.41 | $30.39 - $40.58 | ||
Fourth Quarter | $35.53 - $46.21 | $33.91 - $46.76 |
The following table summarizes information about the options under our equity compensation plans as of December 28, 2013.
Plan Category | Number of securities to be issued upon exercise of outstanding options (a) | Weighted-average exercise price of outstanding options (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Equity compensation plans approved by security holders(1) | 3,195,741 | $ | 39.60 | 754,838 | ||||||
Equity compensation plans not approved by security holders(2) | 34,600 | $ | 5.24 | — | ||||||
- (1)
- Includes options to purchase shares of our common stock under our amended and restated 2007 Stock Incentive Plan, which was approved by security holders on May 12, 2011.
- (2)
- Includes options to purchase shares of our common stock under our 2006 Stock Option Plan, which was adopted prior to our initial public offering and was approved by our Board of Directors. Options granted under this Plan have a 10-year term and are fully vested.
22
Issuer Purchases of Equity Securities
The following table provides detail about our share repurchase activity during the three months ended December 28, 2013:
Fiscal Month | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Program | Maximum Number of Shares that May Yet Be Purchased Under the Program | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
September 29, 2013 - November 2, 2013 | — | $ | — | — | 2,000,000 | ||||||||
November 3, 2013 - November 30, 2013 | — | — | — | 2,000,000 | |||||||||
December 1, 2013 - December 28, 2013 | — | — | — | 2,000,000 | |||||||||
| | | | | | | | | | | | | | |
Total | — | $ | — | — | 2,000,000 | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
On February 19, 2013, the Board of Directors authorized the repurchase of up to 4,000,000 shares of our common stock. During 2013, we repurchased 2,000,000 shares of common stock under the February 2013 repurchase program and the authorization expired on December 31, 2013.
Performance Graph
The following graph compares the cumulative total shareholder return of our common stock for the periods indicated with the total return of the Russell 2000 Index and the Standard & Poor's Index of Industrial Machinery Companies ("S&P Industrial Machinery Index"). The graph assumes $100 invested on December 31, 2008 in our common stock, the Russell 2000 Index and the S&P Industrial Machinery Index. Total return represents stock price changes and assumes the reinvestment of dividends.
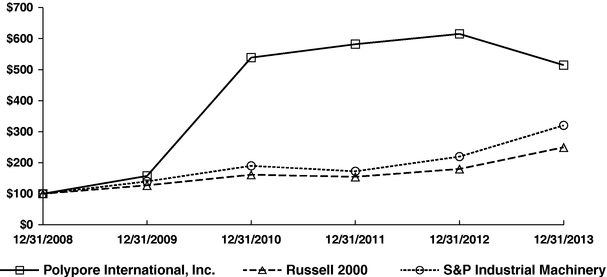
| | Dec-08 | Dec-09 | Dec-10 | Dec-11 | Dec-12 | Dec-13 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Polypore International, Inc. | 100.00 | 157.41 | 538.76 | 581.88 | 615.08 | 514.55 | |||||||||||||
Russell 2000 | 100.00 | 127.17 | 161.32 | 154.59 | 179.86 | 249.69 | |||||||||||||
S&P Industrial Machinery Index | 100.00 | 139.72 | 189.94 | 172.34 | 219.71 | 320.36 | |||||||||||||
23
Item 6. Selected Financial Data
The following table presents selected historical consolidated financial data of Polypore International for the fiscal years ended December 28, 2013, December 29, 2012, December 31, 2011, January 1, 2011 and January 2, 2010. All fiscal periods presented below included 52 weeks. The selected historical consolidated financial data has been derived from Polypore International's audited consolidated financial statements.
The information presented below should be read together with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the consolidated financial statements and the notes thereto included in "Financial Statements and Supplementary Data."
Statement of operations data (in millions, except share data): | Fiscal 2013 | Fiscal 2012 | Fiscal 2011 | Fiscal 2010 | Fiscal 2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net sales | $ | 636.3 | $ | 648.7 | $ | 685.7 | $ | 551.6 | $ | 463.1 | ||||||
| | | | | | | | | | | | | | | | | |
Gross profit | 220.7 | 245.2 | 299.5 | 227.7 | 180.9 | |||||||||||
Selling, general and administrative expenses | 132.8 | 121.4 | 129.8 | 111.3 | 97.5 | |||||||||||
Business restructuring | — | — | — | — | 21.3 | |||||||||||
Goodwill impairment | — | — | — | — | 131.5 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income (loss) | 87.9 | 123.8 | 169.7 | 116.4 | (69.4 | ) | ||||||||||
Interest expense, net | 39.5 | 36.0 | 34.4 | 46.7 | 57.1 | |||||||||||
Foreign currency and other | 0.7 | (0.1 | ) | (1.9 | ) | (1.6 | ) | (0.8 | ) | |||||||
Write-off of loan acquisition costs associated with refinancing of senior credit agreement | — | 2.5 | — | — | — | |||||||||||
Costs related to purchase of 8.75% senior subordinated notes | — | — | — | 2.3 | — | |||||||||||
Gain on sale of Italian subsidiary | — | — | — | (3.3 | ) | — | ||||||||||
| | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations before income taxes | 47.7 | 85.4 | 137.2 | 72.3 | (125.7 | ) | ||||||||||
Income taxes | 14.2 | 25.3 | 46.0 | 22.1 | (0.3 | ) | ||||||||||
| | | | | | | | | | | | | | | | | |
Income (loss) from continuing operations | 33.5 | 60.1 | 91.2 | 50.2 | (125.4 | ) | ||||||||||
Income from discontinued operations, net of income taxes | 12.3 | 10.9 | 14.0 | 13.4 | 8.1 | |||||||||||
Gain on sale of discontinued operations, net of income taxes | 35.8 | — | — | — | — | |||||||||||
| | | | | | | | | | | | | | | | | |
Income from discontinued operations | 48.1 | 10.9 | 14.0 | 13.4 | 8.1 | |||||||||||
| | | | | | | | | | | | | | | | | |
Net income (loss) | $ | 81.6 | $ | 71.0 | $ | 105.2 | $ | 63.6 | $ | (117.3 | ) | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net income (loss) per share—basic: | ||||||||||||||||
Continuing operations | $ | 0.73 | $ | 1.29 | $ | 1.97 | $ | 1.13 | $ | (2.82 | ) | |||||
Discontinued operations | 1.06 | 0.23 | 0.31 | 0.30 | 0.18 | |||||||||||
| | | | | | | | | | | | | | | | | |
Net income (loss) per share | $ | 1.79 | $ | 1.52 | $ | 2.28 | $ | 1.43 | $ | (2.64 | ) | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net income (loss) per share—diluted: | ||||||||||||||||
Continuing operations | $ | 0.72 | $ | 1.27 | $ | 1.93 | $ | 1.10 | $ | (2.82 | ) | |||||
Discontinued operations | 1.04 | 0.23 | 0.30 | 0.29 | 0.18 | |||||||||||
| | | | | | | | | | | | | | | | | |
Net income (loss) per share | $ | 1.76 | $ | 1.50 | $ | 2.23 | $ | 1.39 | $ | (2.64 | ) | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Weighted average shares outstanding—basic | 45,610,270 | 46,540,385 | 46,182,204 | 44,562,421 | 44,385,552 | |||||||||||
Weighted average shares outstanding—diluted | 46,239,796 | 47,229,595 | 47,119,997 | 45,748,058 | 44,385,552 | |||||||||||
Balance sheet data (at end of period) (in millions): | Fiscal 2013 | Fiscal 2012 | Fiscal 2011 | Fiscal 2010 | Fiscal 2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Total assets | $ | 1,550.2 | $ | 1,586.1 | $ | 1,481.9 | $ | 1,348.5 | $ | 1,352.6 | ||||||
Total debt, including current portion | 646.3 | 696.3 | 709.5 | 715.3 | 803.4 | |||||||||||
Shareholders' equity | 619.6 | 582.8 | 499.4 | 378.1 | 284.3 | |||||||||||
Other financial data (in millions): | Fiscal 2013 | Fiscal 2012 | Fiscal 2011 | Fiscal 2010 | Fiscal 2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Depreciation and amortization | $ | 56.3 | $ | 55.7 | $ | 51.3 | $ | 47.9 | $ | 51.4 | ||||||
Capital expenditures | 28.2 | 137.1 | 156.3 | 68.8 | 16.3 | |||||||||||
Net cash provided by (used in): | ||||||||||||||||
Operating activities | 154.5 | 104.8 | 144.8 | 127.2 | 53.2 | |||||||||||
Investing activities | 88.4 | (137.1 | ) | (156.3 | ) | (90.3 | ) | (16.3 | ) | |||||||
Financing activities | (125.2 | ) | (16.8 | ) | 17.6 | (61.3 | ) | (10.4 | ) | |||||||
24
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with our consolidated financial statements and the notes thereto included in "Financial Statements and Supplementary Data." Some of the information contained in this discussion and analysis or included elsewhere in this report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. Please see "Forward-looking Statements" for more information. You should review "Risk Factors" for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained herein.
The following discussion includes financial information prepared in accordance with U.S. generally accepted accounting principles ("GAAP"), as well as segment operating income, which is considered a non-GAAP financial measure. Generally, a non-GAAP financial measure is a numerical measure of a financial performance that excludes amounts that are included in the most directly comparable measure calculated and presented in accordance with GAAP. The presentation of segment operating income is intended to supplement investors' understanding of our operating performance and is not intended to replace net income as determined in accordance with GAAP. Segment operating income is defined as operating income before stock-based compensation and certain non-recurring and other costs and is used by management to evaluate business segment performance and allocate resources. See Note 17, "Segment Information," in the accompanying consolidated financial statements for a reconciliation of segment operating income to income from continuing operations before income taxes.
The results of operations of Microporous are classified as discontinued operations and are excluded from continuing operations and segment results for all periods presented. All disclosures and amounts in the "Management's Discussion and Analysis of Financial Condition and Results of Operations" section relate to our continuing operations, unless otherwise indicated.
Overview
We are a leading global high-technology filtration company that develops, manufactures and markets specialized microporous membranes used in separation and filtration processes. In fiscal 2013, we generated total net sales of $636.3 million. We operate in two primary businesses: energy storage, which includes the transportation and industrial segment and the electronics and EDVs segment, and separations media. The transportation and industrial and separations media segments represent approximately 75% of our total net sales and operate in stable, growing markets, have high recurring revenue bases and generate strong cash flows. In the electronics and EDVs segment, we have a presence in the more established consumer electronics market but our most significant growth opportunity is the potentially larger and developing electric drive vehicle ("EDV") and energy storage systems ("ESS") markets where lithium is the disruptive technology. As described in more detail below, the long-term growth drivers for lithium batteries are positive, but we have and may continue to experience variability in the short term as these markets emerge.
Cash flows from operations, lower capital expenditures and proceeds from the sale of the Microporous business allowed us to generate significant amounts of cash in 2013. At December 28, 2013, we had $163.4 million in cash. We are continuing to evaluate our alternatives for cash depending on market and other conditions, including assessing options related to our capital structure and returning value to shareholders through share repurchases.
Energy Storage
In the energy storage business, our membrane separators are a critical functional component in lithium batteries, which are primarily used in consumer electronics and EDV applications, and lead-acid batteries, which are used globally in transportation and numerous industrial applications. We believe
25
that the long-term growth drivers for the energy storage business—growth in Asia, demand for consumer electronics and growing demand for EDVs—are positive. The energy storage business is comprised of two reportable segments.
Electronics and EDVs. Lithium batteries are the power source in a wide variety of applications, including consumer electronics applications such as notebook computers, tablets, mobile phones and cordless power tools; EDVs; and emerging applications such as ESS. Demand for lithium batteries in consumer electronics is driven by the need for increased mobility. In EDV applications, demand is driven by the need to increase fuel efficiency to meet mileage standards in many countries such as the U.S. and China, the need to reduce carbon dioxide ("CO2") emissions around the world but especially in Europe due to regulations, conversion from nickel metal hydride to lithium battery technology in hybrid vehicles due to greater energy and power density, concern in developed countries and emerging markets over future access to petroleum at stable prices, smog and pollution control, and the need to address increasing transportation needs in developing economies. Since late 2009, we have expanded capacity at our existing Charlotte, North Carolina and Ochang, South Korea facilities and built a new facility in Concord, North Carolina. Production started for portions of the Concord facility in 2012 and the remaining capacity will ramp up over time as the nascent market for EDVs develops.
We recently signed a technology licensing agreement with Sumitomo Chemical Co., Ltd. ("Sumitomo") and a long-term supply agreement with Samsung SDI Co., Ltd. ("Samsung"), a global leader in lithium-ion batteries. The technology licensing agreement with Sumitomo entered into in December 2013 confirms the value of our intellectual property position regarding ceramic coatings and provides for an annual technology licensing fee. The long-term supply agreement with Samsung signed in January 2014 includes guaranteed purchase and supply volume requirements, volume-based price incentives and an initial four-year term with a two-year extension provision. We believe that this agreement highlights the value of our capacity investments and proven industry-leading products and technology. We will continue to seek long-term relationships with our customers and believe that the Sumitomo and Samsung agreements confirm the value of our technology and our ability to provide certainty of supply for high-growth applications like EDVs and ESS.
In January 2014, after a lengthy period of unsuccessful discussions with LG Chem, Ltd. ("LG") regarding various business terms of our relationship, we filed a complaint against LG alleging infringement of our patent covering ceramic coating technology. LG did not purchase separator from us during the fourth quarter of 2013 and has recently stated verbally that it is attempting to qualify alternate sources of supply. We do not know if or when LG will be successful in qualifying alternative separator sources.
We believe that the long-term demand drivers for our products—consumer demand for mobility, regulations for better fuel efficiency and lower CO2 emissions, conversion from nickel metal hydride to lithium battery technology in hybrids, concerns about access to petroleum, efforts to reduce pollution, and increasing transportation needs in developing countries—remain intact. While consumer electronics applications have attractive long-term market growth trends, EDV and ESS applications represent our most significant growth opportunity and the long-term outlook continues to be positive. Based on industry forecasts and industry studies, the use of lithium technology in EDV applications is expected to grow at a compound annual growth rate in excess of 40% through 2020 on an energy capacity basis. Many factors influence membrane separator usage in lithium-ion batteries, but because many new applications are incorporating large-format lithium batteries that require much greater membrane separator volume per battery, we believe that membrane separator growth will exceed battery unit sales growth and although not perfectly correlated, will more closely approximate the growth rate in energy capacity. We believe the electrification of the worldwide fleet of vehicles is just beginning, from hybrids to plug-ins to full battery electric vehicles, including automobiles, buses, taxis and commercial fleet vehicles. We believe our dry process products continue to be the preferred product in large format lithium-ion batteries for EDVs and ESS. We are currently working with existing and new customers on
26
next-generation batteries, which is important considering the long lead times required to become qualified for EDV applications. EDV and ESS are emerging market applications and are being adopted around the world in many forms. We are qualified on more than fifty EDV models and have field-proven products, significant production capacity already in place, technical advantages, low-cost manufacturing capabilities and intellectual property around ceramic coatings for lithium battery separators. We believe the factors that influenced our decision to expand capacity remain valid, and we continue to expect significant sales growth and expect to utilize our current production capacity as the EDV market develops and as ESS experiences more meaningful adoption. Although the long-term growth drivers are positive for these applications, short-term fluctuations in demand can be expected in the early stages of adoption while initial penetration rates are low. Given the high-separator content for these applications and the potential size of these markets, small changes in end-market demand can have a significant impact on our business.
Transportation and industrial. In the lead-acid battery market, the high proportion of aftermarket replacement sales and the steady growth of the worldwide fleet of motor vehicles provide us with a growing recurring revenue base in lead-acid battery separators. Worldwide demand for lead-acid battery separators is expected to continue to grow at slightly more than annual economic growth. The Asia-Pacific region is the fastest growing market for lead-acid battery separators. Growth in this region is driven by the increasing penetration of automobile ownership, growth in industrial and manufacturing sectors, export incentives and ongoing conversion to the polyethylene-based membrane separators we produce.
Separations Media
In the separations media business, our filtration membranes and modules are used in healthcare and high-performance filtration and specialty applications. We believe that the separations media business will continue to benefit from continued growth in demand for higher levels of purity in a growing number of applications. The separations media business is a reportable segment.
For healthcare applications, we produce membranes used in blood filtration applications for hemodialysis, blood oxygenation and plasmapheresis. Growth in demand for hemodialysis membranes is driven by the increasing worldwide population of end-stage renal disease patients. We believe that conversion to single-use dialyzers and increasing treatment frequency will result in additional dialyzer market growth. In late 2011, we completed the expansion of our PUREMA® hemodialysis membrane production capacity to support future market growth.
For filtration and specialty applications, we produce a wide range of membranes and membrane-based elements for micro-, ultra- and nanofiltration and gasification/degasification of liquids. Micro-, ultra- and nanofiltration membrane element market growth is being driven by several factors, including end-market growth in applications such as water treatment and pharmaceutical processing, displacement of conventional filtration media by membrane filtration due to membranes' superior cost and performance attributes, and increasing purity requirements in industrial and other applications.
Critical accounting policies
Critical accounting policies are those accounting policies that can have a significant impact on the presentation of our financial condition and results of operations, and that require the use of complex and subjective estimates based on past experience and management's judgment. Because of the uncertainty inherent in such estimates, actual results may differ from these estimates. Below are those policies that we believe are critical to the understanding of our operating results and financial condition. Management has discussed the development and selection of these critical accounting policies with the Audit Committee of our Board of Directors. For additional accounting policies, see
27
Note 2 of the consolidated financial statements included in "Financial Statements and Supplementary Data."
Impairment of goodwill
Goodwill is subject to annual impairment testing unless circumstances dictate more frequent assessments. We perform our annual impairment assessment for goodwill as of the first day of the fourth quarter of each fiscal year and more frequently whenever events or changes in circumstances indicate that the fair value of the asset may be less than the carrying amount. Our reporting units are at the operating segment level.
We performed a quantitative two-step goodwill impairment test. Step one of the goodwill impairment test compares the fair value of our reporting units to their carrying amount. The fair value of the reporting unit is determined using the income approach, corroborated by comparison to market capitalization and key multiples of comparable companies. Under the income approach, we determine fair value based on estimated future cash flows of each reporting unit, discounted by an estimated weighted-average cost of capital. If the fair value of the reporting unit is greater than its carrying amount, there is no impairment. If the reporting unit's carrying amount exceeds its fair value, then the second step must be completed to measure the amount of impairment, if any. Step two calculates the implied fair value of goodwill by deducting the fair value of all tangible and intangible net assets of the reporting unit from the fair value of the reporting unit as calculated in step one. In this step, the fair value of the reporting unit is allocated to all of the reporting unit's assets and liabilities in a hypothetical purchase price allocation as if the reporting unit had been acquired on that date. If the carrying amount of goodwill exceeds the implied fair value of goodwill, an impairment loss will be recognized in an amount equal to the excess.
Determining the fair value of a reporting unit is judgmental in nature and requires the use of significant estimates and assumptions, including revenue growth rates, strategic plans and future market conditions, among others. There can be no assurance that our estimates and assumptions made for purposes of the goodwill impairment testing will prove to be accurate predictions of the future. If our assumptions regarding forecasted revenue or margin growth rates of certain reporting units are not achieved or changes in strategy or market conditions occur, we may be required to record goodwill impairment charges in future periods. It is not possible at this time to determine if any such future impairment charge would result or, if it does, whether such charge would be material.
In fiscal 2013, our annual impairment test indicated that the fair value of the reporting units substantially exceeded their respective carrying amounts.
Pension benefits
Certain assumptions are used in the calculation of the actuarial valuation of our defined benefit pension plans. Two critical assumptions, discount rate and expected return on assets, are important elements of plan expense and/or liability measurement and differences between actual results and these two actuarial assumptions can materially affect our projected benefit obligation or the valuation of our plan assets. Other assumptions involve demographic factors such as retirement, expected increases in compensation, mortality and turnover. The discount rate enables us to state expected future cash flows at a present value on the measurement date. The discount rate assumptions are based on the market rate for high quality fixed income investments. At December 28, 2013, a decrease of one percentage point in the discount rate would increase our projected benefit obligations and the unfunded status of our pension plans by $20.8 million. The expected rates of return on our pension plans' assets are based on the asset allocation of each plan and the long-term projected return of those assets. For 2013, if the expected rate of return on pension plan assets were reduced by one percentage point, the result would have increased our net periodic benefit expense for fiscal 2013 by $0.1 million. At December 28, 2013, if the actual plan assets were reduced by one percentage point, the unfunded status of our pension plans would increase by $0.2 million.
28
Repairs and maintenance
Repair and maintenance costs, which include indirect labor and employee benefits associated with maintenance personnel and utility, maintenance and repair costs for equipment and facilities utilized in the manufacturing process, are treated as inventoriable costs. Repair and maintenance costs as a percent of cost of goods sold has been consistent for fiscal 2013, 2012 and 2011. Major planned maintenance activities outside of the normal production process are capitalized as property, plant and equipment if the costs are expected to provide future benefits by increasing the service potential of the asset to which the repair or maintenance applies. We have not had any major planned maintenance activities or capitalized significant repair and maintenance costs as property, plant and equipment in the last three fiscal years.
Stock-based compensation
Stock-based compensation expense is based on the fair value of the award at grant date using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the use of significant assumptions, including expected term of the options and expected volatility. We determine expected term based on historical experience, vesting periods, structure of option plans and contractual term of the options. Expected volatility is estimated based on our historical stock prices and implied volatility from traded options. The assumptions used in calculating the fair value of awards involve inherent uncertainty and management judgment. If factors change or we use different assumptions for estimating stock-based compensation expense in future periods, stock-based compensation expense may differ materially in the future.
Results of operations
The following table sets forth, for the fiscal years indicated, certain of our historical operating data in amount and as a percentage of net sales:
| | Fiscal Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in millions) | 2013 | 2012 | 2011 | |||||||
Net sales | $ | 636.3 | $ | 648.7 | $ | 685.7 | ||||
| | | | | | | | | | | |
Gross profit | 220.7 | 245.2 | 299.5 | |||||||
Selling, general and administrative expenses | 132.8 | 121.4 | 129.8 | |||||||
| | | | | | | | | | | |
Operating income | 87.9 | 123.8 | 169.7 | |||||||
Interest expense, net | 39.5 | 36.0 | 34.4 | |||||||
Other | 0.7 | 2.4 | (1.9 | ) | ||||||
| | | | | | | | | | | |
Income from continuing operations before income taxes | 47.7 | 85.4 | 137.2 | |||||||
Income taxes | 14.2 | 25.3 | 46.0 | |||||||
| | | | | | | | | | | |
Income from continuing operations | $ | 33.5 | $ | 60.1 | $ | 91.2 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | Fiscal Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(percent of sales) | 2013 | 2012 | 2011 | |||||||
Net sales | 100.0 | % | 100.0 | % | 100.0 | % | ||||
| | | | | | | | | | | |
Gross profit | 34.7 | 37.8 | 43.7 | |||||||
Selling, general and administrative expenses | 20.9 | 18.7 | 19.0 | |||||||
| | | | | | | | | | | |
Operating income | 13.8 | 19.1 | 24.7 | |||||||
Interest expense, net | 6.2 | 5.5 | 5.0 | |||||||
Other | 0.1 | 0.4 | (0.3 | ) | ||||||
| | | | | | | | | | | |
Income from continuing operations before income taxes | 7.5 | 13.2 | 20.0 | |||||||
Income taxes | 2.2 | 3.9 | 6.7 | |||||||
| | | | | | | | | | | |
Income from continuing operations | 5.3 | % | 9.3 | % | 13.3 | % | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
29
Fiscal 2013 compared with fiscal 2012
Net sales. Net sales for fiscal 2013 were $636.3 million, a decrease of $12.4 million, or 1.9%, from fiscal 2012, as higher sales in the transportation and industrial and separations media segments and the positive impact of foreign currency translation of $4.9 million were more than offset by lower sales in the electronics and EDVs segment. See "Financial reporting segments" below for more information.
Gross profit. Gross profit was $220.7 million, a decrease of $24.5 million from fiscal 2012. Gross profit as a percent of net sales was 34.7% for fiscal 2013 compared to 37.8% for fiscal 2012. The decrease in consolidated gross profit and gross profit margin was primarily due to lower sales in the electronics and EDVs segment. Gross profit as a percent of net sales was consistent with the prior year in the transportation and industrial and separations media segments. See "Financial reporting segments" below for more information.
Selling, general and administrative expenses. Selling, general and administrative expenses increased $11.4 million in fiscal 2013 compared to fiscal 2012, primarily due to a $6.1 million increase in performance-based incentive compensation expense and a $4.4 million increase in stock-based compensation expense. Selling, general and administrative expenses were 20.9% of consolidated net sales in fiscal 2013 and 18.7% in fiscal 2012.
Segment operating income. Segment operating income, which excludes stock-based compensation and certain non-recurring and other costs, was $110.7 million, a decrease of $31.0 million from fiscal 2012. Segment operating income as a percent of net sales was 17.4% for fiscal 2013 compared to 21.8% for fiscal 2012. The decrease in segment operating income and segment operating income margin was primarily due to lower sales in the electronics and EDVs segment and an increase in performance-based incentive compensation expense. Operating income as a percent of net sales was consistent with the prior year in the transportation and industrial and separations media segments. See "Financial reporting segments" below for more information.
Interest expense. Interest expense for fiscal 2013 increased by $3.5 million from fiscal 2012, primarily resulting from a decrease in capitalized interest.
Income taxes. Income taxes as a percentage of pre-tax income for fiscal 2013 were 29.9% compared to 29.6% for fiscal 2012. The income tax expense recorded in the financial statements fluctuates between years due to the mix of income between the U.S. and foreign jurisdictions taxed at varying rates and a variety of other factors, including state income taxes and changes in estimates of permanent differences and valuation allowances. The mix of earnings between the tax jurisdictions has a significant impact on the effective tax rate. Each tax jurisdiction has its own set of tax laws and tax rates, and income earned by our subsidiaries is taxed independently by these various jurisdictions. Currently, the applicable statutory income tax rates in the jurisdictions in which we operate range from 0% to 42%.
The components of our effective tax rate are as follows:
| | Fiscal 2013 | Fiscal 2012 | |||||
|---|---|---|---|---|---|---|---|
U.S. federal statutory rate | 35.0 | % | 35.0 | % | |||
State income taxes | — | 0.4 | |||||
Mix of income in taxing jurisdictions | (3.7 | ) | (6.3 | ) | |||
Other | (1.4 | ) | 0.5 | ||||
| | | | | | | | |
Total effective tax rate | 29.9 | % | 29.6 | % | |||
| | | | | | | | |
| | | | | | | | |
30
Fiscal 2012 compared with fiscal 2011
Net sales. Net sales for fiscal 2012 were $648.7 million, a decrease of $37.0 million, or 5.4%, from fiscal 2011, as higher sales in the transportation and industrial and separations media segments were more than offset by lower sales in the electronics and EDVs segment and the negative impact of foreign currency translation of $22.5 million. See "Financial reporting segments" below for more information.
Gross profit. Gross profit was $245.2 million, a decrease of $54.3 million from fiscal 2011. Gross profit as a percent of net sales was 37.8% for fiscal 2012 compared to 43.7% for fiscal 2011. The decrease in consolidated gross profit and gross profit margin was primarily due to lower sales and the impact of fixed costs associated with new production capacity in the electronics and EDVs segment. In addition, consolidated gross profit and gross profit margin was impacted by higher costs to export lead-acid separators from U.S. and European production facilities to meet growing demand in Asia in the transportation and industrial segment. See "Financial reporting segments" below for more information.
Selling, general and administrative expenses. Selling, general and administrative expenses decreased $8.4 million in fiscal 2012 compared to fiscal 2011, primarily due to a $13.8 million decrease in performance-based incentive compensation expense and $3.2 million lower amortization expense, partially offset by a $7.0 million increase in stock-based compensation expense. Selling, general and administrative expenses were 18.7% of consolidated net sales in fiscal 2012 and 19.0% in fiscal 2011.
Segment operating income. ��Segment operating income, which excludes stock-based compensation and certain non-recurring and other costs, was $141.7 million, a decrease of $37.9 million from fiscal 2011. Segment operating income as a percent of net sales was 21.8% for fiscal 2012 compared to 26.2% for fiscal 2011. The decrease in segment operating income and segment operating income margin was the result of lower sales and the impact of fixed costs associated with new production capacity in the electronics and EDVs segment, higher costs to export lead-acid separators from U.S. and European production facilities to meet growing demand in Asia in the transportation and industrial segment, partially offset by a decline in performance-based incentive compensation expense in corporate and other costs. See "Financial reporting segments" below for more information.
Interest expense. Interest expense for fiscal 2012 increased by $1.6 million from fiscal 2011, primarily resulting from a decrease in capitalized interest.
Income taxes. Income taxes as a percentage of pre-tax income for fiscal 2012 were 29.6% compared to 33.6% for fiscal 2011. The income tax expense recorded in the financial statements fluctuates between years due to the mix of income between the U.S. and foreign jurisdictions taxed at varying rates and a variety of other factors, including state income taxes and changes in estimates of permanent differences and valuation allowances. The mix of earnings between the tax jurisdictions has a significant impact on the effective tax rate. Each tax jurisdiction has its own set of tax laws and tax rates, and income earned by our subsidiaries is taxed independently by these various jurisdictions.
The components of our effective tax rate are as follows:
| | Fiscal 2012 | Fiscal 2011 | |||||
|---|---|---|---|---|---|---|---|
U.S. federal statutory rate | 35.0 | % | 35.0 | % | |||
State income taxes | 0.4 | 1.0 | |||||
Mix of income in taxing jurisdictions | (6.3 | ) | (2.2 | ) | |||
Other | 0.5 | (0.2 | ) | ||||
| | | | | | | | |
Total effective tax rate | 29.6 | % | 33.6 | % | |||
| | | | | | | | |
| | | | | | | | |
31
Discontinued operations
On December 19, 2013, we completed the sale of our Microporous business, which consisted of the production facilities in Piney Flats, Tennessee, and Feistritz, Austria, for $120.0 million. We recognized a gain of $35.8 million on the sale, net of direct transaction costs and income taxes. The sales price and resulting gain are subject to adjustment in subsequent periods upon finalization of working capital. Microporous was previously included in the transportation and industrial segment. The results of operations of Microporous are classified as discontinued operations and are excluded from continuing operations and segment results for all periods presented.
Financial reporting segments
Electronics and EDVs
Fiscal 2013 compared with fiscal 2012
Net sales. Net sales for fiscal 2013 were $130.3 million, a decrease of $37.1 million, or 22.2%, from fiscal 2012. Net sales decreased primarily because of lower sales volumes in consumer electronics applications, partially offset by an increase in sales to EDV applications. Net sales decreased by $3.5 million due to price/product mix. For consumer electronics, sales volumes were lower due to weakness in end-market demand for devices such as laptop computers (which we believe may have been negatively impacted by increasing sales of smartphones and tablets) and capacity limitations in 2011, which caused us to miss some sales qualification opportunities for new devices such as smartphones and tablets. It is taking us longer than we expected to recover sales into consumer electronics applications. We are working on new projects and development opportunities, but it may take several quarters to see the results of these efforts. As a result, we cannot currently predict when or if sales volumes into consumer electronics applications will improve. Although we expect over time to regain some or all of our supply position in consumer electronics, sales into EDV applications represent our most significant growth opportunity and the long-term outlook continues to be positive, especially considering that we are qualified on more than fifty EDV models and have field-proven products, significant production capacity already in place, technical advantages, low-cost manufacturing capabilities and intellectual property around ceramic coatings for lithium battery separators. In 2013, sales volumes into EDV applications increased due to continued market growth, despite lower sales to LG during the second half of 2013. We have been in supply discussions with LG and had no sales to them in the fourth quarter of 2013. LG recently stated verbally that it is attempting to qualify alternate sources of separator supply to replace our dry process separator in EDV applications. We do not know if or when LG will be successful in qualifying alternate sources of supply. In January 2014, Samsung signed a long-term supply agreement to purchase our separators for use in their EDV and ESS lithium-ion batteries. This long-term agreement includes guaranteed purchase and supply volume requirements, volume-based price incentives and an initial four-year term with a two-year extension provision.
In December 2013, we entered into an agreement with Sumitomo to license our intellectual property related to ceramic coating separators for lithium-ion batteries. In connection with the agreement, Sumitomo agreed to pay $3.5 million in technology licensing fees related to past usage of our ceramic coating technology and will also pay ongoing license fees based on future use of this technology.
Segment operating income. Segment operating income was $17.3 million, a decrease of $29.9 million from fiscal 2012. Segment operating income as a percent of net sales was 13.3% for fiscal 2013 compared to 28.2% for fiscal 2012. The decrease in segment operating income and segment operating income margin was due to lower sales volume.
The key driver of operating income and operating income margin is sales and the amount of fixed costs relative to sales. In 2013, net sales were $130.3 million, or 33% of our total production capacity in
32
terms of sales, as compared to 2012 sales of $167.4 million, or 42% of capacity. There were no changes in production capacity in 2013 as compared to 2012. In 2013 and 2012, excluding the final phase of expansion at our Concord facility, which will be completed, qualified and ramped up as demand develops, we had total production capacity sufficient to generate approximately $400.0 million in annual lithium battery separator sales, depending on the mix of products and grades produced. Because of the decrease in sales with no significant change in fixed costs, operating income and operating income margin declined.
In 2012, we added approximately $15.0 million of incremental fixed costs (including non-cash depreciation expense) associated with our new production capacity. During 2013, we recognized the full-year impact of these fixed costs, but also implemented certain cost savings actions, which resulted in fixed costs being relatively consistent between the two years. Since we have added substantially all of the fixed costs required to operate the new production capacity, operating income and operating income margins will continue to fluctuate primarily based on changes in sales.
Variable costs did not have a significant impact on the change in operating income margin because this is a low-variable cost business and we are able to add variable costs as production volumes ramp up, which allows us to continue to manufacture efficiently on a variable production cost basis, even though we are not fully utilizing all of our new production capacity.
Fiscal 2012 compared with fiscal 2011
Net sales. Net sales for fiscal 2012 were $167.4 million, a decrease of $33.6 million, or 16.7%, from fiscal 2011. Net sales decreased because of lower volumes and the impact of customer and price/product mix. Net sales were down 7.3% due to lower volumes in both consumer electronics and EDV applications. For consumer electronics, sales volumes were lower due to weakness in end-market demand for products such as laptop computers (which we believe may have been negatively impacted by increasing sales of smartphones and tablets) and capacity limitations in 2011, which caused us to miss some sales qualification opportunities for devices such as certain smartphones and tablets. Consumer electronics demand is typically cyclical and economically sensitive in the short term. For EDV applications, sales volumes declined because inventory levels built up in the supply chain in 2011 were reduced in 2012 as end-market demand was less than originally premised. Net sales declined by 7.2% due to changes in customer mix, as our larger, lower-priced customers represented a larger percentage of total sales. Pricing for lithium battery separators is volume based, with higher-volume customers receiving lower sales prices. Net sales declined by 2.2% due to price/product mix.
Segment operating income. Segment operating income was $47.2 million, a decrease of $43.9 million from fiscal 2011. Segment operating income as a percent of net sales was 28.2% for fiscal 2012 compared to 45.3% for fiscal 2011. The key driver of operating income and operating income margins is sales and the amount of fixed costs relative to sales. In 2012, we had lower sales and higher fixed costs which were associated with new production capacity added in 2012. As a result, operating income and operating income margin declined as explained in more detail below.
Since 2009, we have completed five separate capacity expansions and added the fixed costs required to operate the new capacity. Capacity from these expansions became available for production in phases beginning in late 2011 and over the course of 2012. In 2012, excluding the final phase of expansion at our Concord facility, which will be completed, qualified and ramped up as demand develops, we had total production capacity sufficient to generate approximately $400.0 million in annual lithium battery separator sales, depending on the mix of products and grades produced. This represents approximately twice as much annual production capacity in terms of sales in 2012 as compared to 2011. Since new production capacity did not begin to become available for production until late 2011, it did not significantly impact our production capacity in 2011.
33
Although we had new production capacity in 2012, sales revenues declined as compared to the prior year because of lower sales volumes in both consumer electronics and EDV applications, as discussed in more detail in the discussion of net sales above. However, the new production capacity did have a negative impact on operating income and operating income margin in 2012 as compared to 2011 because we added fixed costs associated with the new capacity.
Beginning in the second quarter of 2012, we added approximately $20.0 million of annualized incremental fixed costs (including $8.0 million of non-cash depreciation expense) associated with this new capacity. For fiscal 2012, we incurred approximately $15.0 million of these incremental fixed costs (including $6.7 million of non-cash depreciation expense). These costs represent substantially all of the fixed costs (except for incremental depreciation expense on new equipment not yet placed in service) required to operate the new production capacity. Conversely, variable costs associated with the new capacity do not have a significant impact on the change in operating income margins because this is a low-variable cost business and we are able to add variable costs as production volumes on the new capacity ramp up, which allows us to continue to manufacture efficiently on a variable production cost basis even though we are not fully utilizing all of our new production capacity.
In 2012, net sales were $167.4 million and taking into account the recent capacity expansions, represented 42% of our total production capacity in terms of sales. In 2011, net sales were $201.0 million and since new production capacity did not begin to become available for production until late in the year, we were operating at high rates of capacity utilization.
Transportation and Industrial
Fiscal 2013 compared with fiscal 2012
Net sales. Net sales for fiscal 2013 were $311.9 million, an increase of $12.9 million, or 4.3%, from fiscal 2012 due to an increase in sales volumes across all geographic regions.
Segment operating income. Segment operating income was $70.0 million, an increase of $3.9 million from fiscal 2012. Segment operating income as a percent of net sales was 22.4% for fiscal 2013, which is consistent with the prior year.
Fiscal 2012 compared with fiscal 2011
Net sales. Net sales for fiscal 2012 were $299.0 million, an increase of $4.5 million, or 1.5%, from fiscal 2011, as growth in Asia was more than offset by the $11.1 million negative effect of foreign currency translation and economic weakness in North America and Europe. Sales in Asia, which is the fastest growing market for lead-acid separators, increased 30% in fiscal 2012.
Segment operating income. Segment operating income was $66.1 million, a decrease of $9.4 million from fiscal 2011. Segment operating income as a percent of net sales was 22.1% for fiscal 2012 compared to 25.6% for fiscal 2011. The decrease in segment operating income and segment operating income margin was due to the costs of exporting goods from our U.S. and European manufacturing facilities to Asia and costs associated with growth investments to meet growing demand in Asia.
Separations Media
Fiscal 2013 compared with fiscal 2012
Net sales. Net sales for fiscal 2013 were $194.1 million, an increase of $11.8 million, or 6.5%, from fiscal 2012 due to higher sales in both healthcare and filtration and specialty products and the positive impact of foreign currency translation of $4.7 million.
34
Segment operating income. Segment operating income was $54.1 million, an increase of $1.6 million from fiscal 2012. Segment operating income as a percent of net sales was 27.9% in fiscal 2013, which is comparable to the prior year.
Fiscal 2012 compared with fiscal 2011
Net sales. Net sales for fiscal 2012 were $182.3 million, a decrease of $7.9 million, or 4.2%, from fiscal 2011, as higher sales in both healthcare and filtration and specialty products were more than offset by the $11.4 million negative effect of foreign currency translation.
Segment operating income. Segment operating income was $52.5 million, a decrease of $2.2 million from fiscal 2011. Segment operating income as a percent of net sales was 28.8% in fiscal 2012 and fiscal 2011.
Corporate and other costs
Corporate and other costs include costs associated with the corporate office and other costs that are not allocated to the reporting segments for segment reporting purposes, including amortization of identified intangible assets and performance-based incentive compensation.
Fiscal 2013 compared with fiscal 2012. Corporate and other costs for fiscal 2013 were $30.7 million, compared to $24.1 million for fiscal 2012. The increase was primarily due to higher performance-based incentive compensation expense.
Fiscal 2012 compared with fiscal 2011. Corporate and other costs for fiscal 2012 were $24.1 million, compared to $41.7 million for fiscal 2011. The decrease was primarily due to lower performance-based incentive compensation expense and a decline in amortization expense as certain intangible assets became fully amortized in the second quarter of 2012.
Foreign operations
As of December 28, 2013, we manufactured our products at 15 strategically located facilities in the United States, Europe and Asia. Net sales from foreign locations were $403.9 million (63.5% of consolidated sales), $441.5 million (68.1% of consolidated sales) and $433.9 million (63.3% of consolidated sales) for fiscal 2013, 2012 and 2011, respectively. Pre-tax income from foreign production facilities was $50.5 million (105.9% of consolidated pre-tax income), $67.3 million (78.8% of consolidated pre-tax income) and $64.2 million (46.8% of consolidated pre-tax income) for fiscal 2013, 2012 and 2011, respectively. The fluctuation in the relationship between foreign net sales and foreign pre-tax income as a percent of consolidated amounts is primarily due to the mix of operating results between segments. The majority of sales and pre-tax income from U.S. production facilities are attributable to the electronics and EDVs segment, where we primarily produce in the U.S. for customers located in Asia. Pre-tax income in the U.S. also includes corporate expenses, such as interest expense and stock-based compensation. The majority of sales and pre-tax income from foreign production facilities are attributable to the transportation and industrial and separations media segments. In the transportation and industrial segment, we have production facilities in the U.S., Europe and Asia and generally produce in the same geographic region that we sell, though we do export some production from U.S. and European facilities to meet growing demand in Asia. In the separations media segment, the majority of production is at our manufacturing facility in Germany and sales are made to customers worldwide. In 2013, we had a pre-tax loss of approximately $2.8 million in the U.S. due to a decline in sales and profitability in the electronics and EDVs segment and higher stock-based compensation. In 2012, U.S. operating results as a percent of consolidated amounts was lower because of a decline in sales and profitability in the electronics and EDVs segment and higher stock-based compensation. Foreign sales and profitability increased as a percent of consolidated results
35
as growth in the transportation and industrial and separations media segments occurred primarily outside of the U.S. Operating results generated by production facilities within business segments was not significantly impacted by differences in economic, regulatory, geographic or other competitive factors.
Typically, we sell our products in the currency of the country where the manufacturing facility that produces the product is located. Sales to foreign customers are subject to numerous additional risks, including the impact of foreign government regulations, currency fluctuations, political uncertainties and differences in business practices. There can be no assurance that foreign governments will not adopt regulations or take other actions that would have a direct or indirect adverse impact on our business or market opportunities within such governments' countries. Furthermore, there can be no assurance that the political, cultural and economic climate outside the United States will be favorable to our operations and growth strategy.
Seasonality
Operations at our European production facilities are traditionally subject to shutdowns for approximately one month during the third quarter of each year for employee vacations. As a result, operating income during the third quarter of any fiscal year may be lower than operating income in other quarters during the same fiscal year. Because of the seasonal fluctuations, comparisons of our operating results for the third quarter of any fiscal year with those of the other quarters during the same fiscal year may be of limited relevance in predicting our future financial performance.
Liquidity and capital resources
At December 28, 2013, cash and cash equivalents were $163.4 million, an increase of $118.5 million from the prior year. The increase was primarily due to cash generated by operations and proceeds from the sale of Microporous, offset to some extent by repayments of debt, repurchases of common stock and capital expenditures.
Operating activities. Net cash provided by operating activities was $154.5 million in fiscal 2013, consisting of cash generated from operations of $121.2 million and cash generated from operating assets and liabilities of $33.3 million. Accounts receivable decreased due to the timing of sales and cash receipts. Days sales outstanding decreased by approximately 5%, and we have not experienced significant changes in accounts receivable aging or customer payment terms and believe that we have adequately provided for potential bad debts. Inventory levels are consistent with prior year and days sales in ending inventory decreased by approximately 4%. We produce inventory to meet expected future customer demand, which is based on a number of factors, including discussions with customers, customer forecasts and industry and economic trends. Inventory is generally not subject to obsolescence and does not have a shelf life, and we do not believe there is a significant risk of inventory impairment. Accounts payable and accrued liabilities increased primarily due to higher accrued variable incentive compensation under performance-based compensation plans.
Investing activities. Net cash provided by investing activities was $88.4 million, consisting of net proceeds from the sale of discontinued operations of $116.6 million, offset by capital expenditures. In fiscal 2013, total capital expenditures were $28.2 million compared to $137.1 million in fiscal 2012. We currently estimate capital expenditures to be approximately $50.0 million in fiscal 2014. As of December 28, 2013, we had $139.3 million of construction in progress, primarily related to the capacity expansion projects in the electronics and EDVs segment, which will be qualified and ramped up over time as market demand develops.
36
Financing activities. During fiscal 2013, financing activities consisted primarily of repurchases of common stock of $80.7 million, net payments under the revolving credit facility of $35.0 million and scheduled principal payments under our term loan facility of $15.0 million.
On February 19, 2013, the Board of Directors authorized the repurchase of up to 4.0 million shares of our common stock. During 2013, we repurchased 2.0 million shares of common stock and the authorization expired on December 31, 2013.
We intend to fund our ongoing operations with cash on hand, cash generated by operations and borrowings under the senior secured credit agreement. As of December 28, 2013, approximately 53% of our cash and cash equivalents were held by foreign subsidiaries. There were no significant restrictions on our ability to transfer funds with and among subsidiaries. We plan to repatriate foreign earnings of approximately $55.1 million related to the sale of the Microporous business and have provided U.S. taxes on these earnings in discontinued operations. Except for the sale of the Microporous business, our intent is to indefinitely reinvest earnings of our foreign operations and our current operating plans do not demonstrate a need to repatriate foreign earnings to fund our U.S. operations. However, if these funds were needed for our operations in the U.S., we would be required to accrue and pay any applicable U.S. and local taxes to repatriate these funds.
Our senior secured credit agreement provides for a $300.0 million term loan facility ($281.3 million outstanding at December 28, 2013) and a $150.0 million revolving credit facility. At December 28, 2013, no amounts were outstanding under the revolving credit facility and the entire amount was available for borrowing.
Interest rates under the senior secured credit agreement are, at our option, equal to either an alternate base rate or Eurocurrency base rate plus a specified margin. At December 28, 2013, the interest rate on borrowings under the senior secured credit agreement was 2.92%.
Under the credit agreement, we are required to maintain a maximum ratio of indebtedness to adjusted EBITDA and a minimum ratio of adjusted EBITDA to cash interest expense. Adjusted EBITDA, as defined under the senior secured credit agreement, was as follows:
(in millions) | Fiscal 2013 | |||
|---|---|---|---|---|
Income from continuing operations | $ | 33.5 | ||
Add/Subtract: | ||||
Depreciation and amortization expense | 54.5 | |||
Interest expense, net | 39.5 | |||
Income taxes | 14.2 | |||
Stock-based compensation | 20.7 | |||
Foreign currency loss | 0.4 | |||
Loss on disposal of property, plant and equipment | 1.2 | |||
Other non-cash or non-recurring charges | (0.3 | ) | ||
| | | | | |
Adjusted EBITDA | $ | 163.7 | ||
| | | | | |
| | | | | |
37
As of December 28, 2013, the calculation of the senior leverage ratio, as defined under the senior secured credit agreement, was as follows:
(in millions) | Fiscal 2013 | |||
|---|---|---|---|---|
Indebtedness(1) | $ | 231.3 | ||
Adjusted EBITDA | $ | 163.7 | ||
Actual senior leverage ratio | 1.41x | |||
Required maximum senior leverage ratio | 2.50x | |||
- (1)
- Calculated as the sum of outstanding borrowings under the senior secured credit agreement, less cash on hand (not to exceed $50.0 million).
As of December 28, 2013, the calculation of the interest coverage ratio, as defined under the senior secured credit agreement, was as follows:
(in millions) | Fiscal 2013 | |||
|---|---|---|---|---|
Adjusted EBITDA | $ | 163.7 | ||
Interest expense, net(1) | $ | 37.0 | ||
Actual interest coverage ratio | 4.43x | |||
Required minimum interest coverage ratio | 3.00x | |||
- (1)
- Calculated as cash interest expense, as defined under the senior secured credit agreement, for the twelve months ended December 28, 2013.
The senior secured credit agreement contains certain restrictive covenants which, among other things, limit the incurrence of additional indebtedness, investments, dividends, transactions with affiliates, asset sales, acquisitions, mergers and consolidations, prepayments of other indebtedness, liens and encumbrances, and other matters customarily restricted in such agreements. The agreement also contains certain customary events of default, subject to grace periods, as appropriate.
The 7.5% senior notes mature on November 15, 2017 and are guaranteed by most of our existing and future domestic restricted subsidiaries, subject to certain exceptions. Except under certain circumstances, the 7.5% senior notes do not require principal payments prior to their maturity in 2017. Interest on the 7.5% senior notes is payable semi-annually on May 15 and November 15. The 7.5% senior notes contain customary covenants and events of default, including covenants that limit our ability to incur debt, pay dividends and make investments.
Future debt service payments are expected to be paid out of cash flows from operations, borrowings on our revolving credit facility and future refinancing of our debt. Our cash interest requirements for the next twelve months are estimated to be $36.2 million.
We believe we have sufficient liquidity to meet our cash requirements over both the short (next twelve months) and long term (in relation to our debt service requirements). In evaluating the sufficiency of our liquidity, we considered cash on hand, expected cash flow to be generated from operations and available borrowings under our senior secured credit agreement compared to our anticipated cash requirements for debt service, working capital, cash taxes, capital expenditures and funding requirements for long-term liabilities. We anticipate that our cash on hand and operating cash flow, together with borrowings under the revolving credit facility, will be sufficient to meet our anticipated future operating expenses, capital expenditures and debt service obligations as they become due for at least the next twelve months. However, our ability to make scheduled payments of principal, to pay interest on or to refinance our indebtedness and to satisfy our other debt obligations will depend upon our future operating performance, which will be affected by general economic, financial,
38
competitive, legislative, regulatory, business and other factors beyond our control. See "Risk Factors" in this Annual Report on Form 10-K.
From time to time, we may explore additional financing methods and other means to lower our cost of capital, which could include equity or debt financings and the application of the proceeds therefrom to the repayment of bank debt or other indebtedness. In addition, in connection with any future acquisitions, we may require additional funding which may be provided in the form of additional debt or equity financing or a combination thereof. There can be no assurance that any additional financing will be available to us on acceptable terms or at all.
Environmental matters
Environmental obligations are accrued when such expenditures are probable and reasonably estimable. The amount of liability recorded is based on currently available information, including the progress of remedial investigations, current status of discussions with regulatory authorities regarding the method and extent of remediation, presently enacted laws and existing technology. Accruals for estimated losses from environmental obligations are adjusted as further information develops or circumstances change. Costs of future expenditures for environmental obligations are not discounted to their present value. We do not currently anticipate any material loss in excess of the amounts accrued. Future remediation expenses may be affected by a number of uncertainties including, but not limited to, the difficulty in estimating the extent and method of remediation, the evolving nature of environmental regulations and the availability and application of technology. We do not expect the resolution of such uncertainties to have a material adverse effect on our consolidated financial position or liquidity. Recoveries of environmental costs from other parties are recognized as assets when their receipt is deemed probable.
In connection with the acquisition of Membrana GmbH ("Membrana") in 2002, we recorded a reserve for environmental obligations. The reserve provides for costs to remediate known environmental issues and operational upgrades which are required in order for us to remain in compliance with local regulations. The initial estimate and subsequent finalization of the reserve was included in the allocation of purchase price at the date of acquisition. The environmental reserve for the Membrana facility, which is denominated in euros, was $2.9 million at December 28, 2013. We anticipate the expenditures associated with the reserve will be made in the next twelve months.
We had indemnification agreements with the prior owners of Membrana for a substantial portion of these costs. During the third quarter of 2013, we received $10.3 million as final payment of amounts outstanding under the indemnification agreements.
Contractual Obligations
The following table sets forth our contractual obligations at December 28, 2013. Some of the amounts included in this table are based on management's estimates and assumptions about these obligations, including their duration, anticipated actions by third parties and other actions. Because these estimates and assumptions are necessarily subjective, the timing and amount of payments under these obligations may vary from those reflected in this table. For more information on these
39
obligations, see the notes to consolidated financial statements included in "Financial Statements and Supplementary Data."
| | Payment due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in millions) | Total | 2014 | 2015 - 2016 | 2017 - 2018 | Thereafter | |||||||||||
Long-term debt | $ | 281.3 | $ | 16.9 | $ | 58.1 | $ | 206.3 | $ | — | ||||||
7.5% senior notes | 365.0 | — | — | 365.0 | — | |||||||||||
Cash interest payments(1) | 137.8 | 36.2 | 70.4 | 31.2 | — | |||||||||||
Operating lease obligations(2) | 9.2 | 3.1 | 3.5 | 2.1 | 0.5 | |||||||||||
| | | | | | | | | | | | | | | | | |
| $ | 793.3 | $ | 56.2 | $ | 132.0 | $ | 604.6 | $ | 0.5 | |||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Includes cash interest requirements on the term loan facility and the 7.5% senior notes through maturity in 2017. Interest rates under the term loan facility are variable and the table assumes that these rates are the same rates that were in effect at December 28, 2013.
- (2)
- We lease certain equipment and facilities under operating leases. Some lease agreements provide us with the option to renew the lease agreement. Our future operating lease obligations would change if we exercised these renewal options. For leases denominated in foreign currencies, the table assumes that the exchange rate of the dollar to the respective foreign currencies is the period average rate for 2013 for all periods presented.
- (3)
- As discussed in the notes to consolidated financial statements included in "Financial Statements and Supplementary Data," we have long-term liabilities for pension obligations of $102.8 million as of December 28, 2013. Our contributions for these benefit plans are not included in the table above since the timing and amount of payments are dependent upon many factors, including when an employee retires or leaves the Company, certain benefit elections by employees, return on plan assets, minimum funding requirements and foreign currency exchange rates. We estimate that contributions to the pension plans in fiscal 2014 will be $2.1 million.
- (4)
- As discussed in the notes to consolidated financial statements included in "Financial Statements and Supplementary Data," we have recorded a liability at December 28, 2013 of $10.0 million for unrecognized tax benefits. Payments related to this liability are not included in the table above since the timing and actual amounts of the payments, if any, are not known.
- (5)
- As discussed in the notes to consolidated financial statements included in "Financial Statements and Supplementary Data," we have an environmental reserve of $2.9 million at December 28, 2013. We anticipate the expenditures associated with the reserve will be made in the next twelve months.
Off-Balance Sheet Arrangements
We are not a party to any off-balance sheet arrangements that have, or are reasonably likely to have, a material current or future effect on our financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to various market risks, which are potential losses arising from adverse changes in market rates and prices, such as interest rates and foreign exchange fluctuations. We do not enter into derivatives or other financial instruments for trading or speculative purposes.
40
Interest rate risk
At December 28, 2013, we had fixed rate debt of $365.0 million and variable rate debt of $281.3 million. To reduce the interest rate risk inherent in our variable rate debt, we may utilize interest rate derivatives. As of December 28, 2013, there were no outstanding interest rate derivatives. The pre-tax earnings and cash flow impact resulting from a 100 basis point increase in interest rates on our variable rate debt, holding other variables constant, would be $2.8 million per year.
Currency risk
Outside of the United States, we maintain assets and operations in Europe and Asia. The results of operations and financial position of our foreign operations are principally measured in their respective currency and translated into U.S. dollars. As a result, exposure to foreign currency fluctuations exists. The reported income of these subsidiaries will be higher or lower depending on a weakening or strengthening of the U.S. dollar against the respective foreign currency. Our subsidiaries and affiliates also purchase and sell products and services in various currencies. As a result, we may be exposed to cost increases relative to the local currencies in the markets in which we sell. Because the percentage of our sales in foreign currencies differs from the percentage of our costs in foreign currencies, a change in the relative value of the U.S. dollar could have a disproportionate impact on our sales compared to our costs, which could impact our margins. A portion of our assets are based in our foreign locations and are translated into U.S. dollars at foreign currency exchange rates in effect as of the end of each period, with the effect of such translation reflected in accumulated other comprehensive income (loss). Accordingly, our consolidated shareholders' equity will fluctuate depending upon the weakening or strengthening of the U.S. dollar against the respective foreign currency, primarily the euro.
The dollar/euro exchange rates used in our financial statements for the fiscal years ended as set forth below were as follows:
| | 2013 | 2012 | 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Period end rate | 1.3760 | 1.3225 | 1.2950 | |||||||
Period average rate | 1.3280 | 1.2862 | 1.3935 | |||||||
Our strategy for management of currency risk relies primarily on conducting our operations in a country's respective currency and may, from time to time, involve foreign currency derivatives. As of December 28, 2013, we did not have any foreign currency derivatives outstanding.
Item 8. Financial Statements and Supplementary Data
The Company's report of independent registered public accounting firm and consolidated financial statements and related notes appear on the following pages of this Annual Report on Form 10-K.
41
Report of Independent Registered Public Accounting Firm
The Board of Directors
of Polypore International, Inc.
We have audited the accompanying consolidated balance sheets of Polypore International, Inc. as of December 28, 2013 and December 29, 2012, and the related consolidated statements of income, comprehensive income, shareholders' equity and cash flows for each of the three years in the period ended December 28, 2013. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Polypore International, Inc. at December 28, 2013 and December 29, 2012, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 28, 2013, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Polypore International, Inc.'s internal control over financial reporting as of December 28, 2013, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) and our report dated February 25, 2014 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
Charlotte, North Carolina
February 25, 2014
42
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
of Polypore International, Inc.
We have audited Polypore International, Inc.'s internal control over financial reporting as of December 28, 2013 based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (1992 framework) (the COSO criteria). Polypore International, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Polypore International, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 28, 2013, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the accompanying consolidated balance sheets of Polypore International, Inc. as of December 28, 2013 and December 29, 2012, and the related consolidated statements of income, comprehensive income, shareholders' equity and cash flows for each of the three years in the period ended December 28, 2013 of Polypore International, Inc. and our report dated February 25, 2014 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
Charlotte, North Carolina
February 25, 2014
43
Polypore International, Inc.
Consolidated balance sheets
(in thousands, except share data) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Assets | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 163,423 | $ | 44,873 | |||
Accounts receivable, net | 113,506 | 122,056 | |||||
Inventories | 113,860 | 115,462 | |||||
Deferred income taxes | — | 21,671 | |||||
Prepaid and other | 18,118 | 22,769 | |||||
Assets of discontinued operations | — | 92,261 | |||||
| | | | | | | | |
Total current assets | 408,907 | 419,092 | |||||
Property, plant and equipment, net | 595,375 | 607,466 | |||||
Goodwill | 444,512 | 444,512 | |||||
Intangibles and loan acquisition costs, net | 93,792 | 107,006 | |||||
Other | 7,627 | 7,996 | |||||
| | | | | | | | |
Total assets | $ | 1,550,213 | $ | 1,586,072 | |||
| | | | | | | | |
| | | | | | | | |
Liabilities and shareholders' equity | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 31,764 | $ | 30,083 | |||
Accrued liabilities | 49,266 | 44,039 | |||||
Income taxes payable | 4,055 | 1,603 | |||||
Current portion of debt | 16,875 | 50,000 | |||||
Liabilities of discontinued operations | — | 19,576 | |||||
| | | | | | | | |
Total current liabilities | 101,960 | 145,301 | |||||
Debt, less current portion | 629,375 | 646,250 | |||||
Pension obligations, less current portion | 102,821 | 103,491 | |||||
Deferred income taxes | 70,332 | 84,719 | |||||
Other | 26,149 | 23,474 | |||||
Commitments and contingencies | |||||||
Shareholders' equity: | |||||||
Preferred stock—15,000,000 shares authorized, no shares issued and outstanding | — | — | |||||
Common stock, $.01 par value—200,000,000 shares authorized, 46,926,205 issued and 44,916,570 outstanding at December 28, 2013 and 46,627,064 issued and outstanding at December 29, 2012 | 469 | 466 | |||||
Paid-in capital | 569,362 | 545,196 | |||||
Retained earnings | 137,379 | 55,768 | |||||
Accumulated other comprehensive loss | (12,865 | ) | (22,353 | ) | |||
Treasury stock, at cost—2,009,635 shares at December 28, 2013 and no shares at December 29, 2012 | (80,668 | ) | — | ||||
| | | | | | | | |
Total Polypore shareholders' equity | 613,677 | 579,077 | |||||
Noncontrolling interest | 5,899 | 3,760 | |||||
| | | | | | | | |
Total shareholders' equity | 619,576 | 582,837 | |||||
| | | | | | | | |
Total liabilities and shareholders' equity | $ | 1,550,213 | $ | 1,586,072 | |||
| | | | | | | | |
| | | | | | | | |
See notes to consolidated financial statements
44
Polypore International, Inc.
Consolidated statements of income
(in thousands, except per share data) | Year ended December 28, 2013 | Year ended December 29, 2012 | Year ended December 31, 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Net sales | $ | 636,282 | $ | 648,699 | $ | 685,725 | ||||
Cost of goods sold | 415,553 | 403,490 | 386,230 | |||||||
| | | | | | | | | | | |
Gross profit | 220,729 | 245,209 | 299,495 | |||||||
Selling, general and administrative expenses | 132,792 | 121,454 | 129,840 | |||||||
| | | | | | | | | | | |
Operating income | 87,937 | 123,755 | 169,655 | |||||||
Other (income) expense: | ||||||||||
Interest expense, net | 39,473 | 36,049 | 34,384 | |||||||
Foreign currency and other | 721 | (113 | ) | (1,950 | ) | |||||
Write-off of loan acquisition costs associated with refinancing of senior credit agreement | — | 2,478 | — | |||||||
| | | | | | | | | | | |
| 40,194 | 38,414 | 32,434 | ||||||||
| | | | | | | | | | | |
Income from continuing operations before income taxes | 47,743 | 85,341 | 137,221 | |||||||
Income taxes | 14,289 | 25,267 | 46,058 | |||||||
| | | | | | | | | | | |
Income from continuing operations | 33,454 | 60,074 | 91,163 | |||||||
Income from discontinued operations, net of income taxes | 12,302 | 10,877 | 14,077 | |||||||
Gain on sale of discontinued operations, net of income taxes | 35,855 | — | — | |||||||
| | | | | | | | | | | |
Income from discontinued operations | 48,157 | 10,877 | 14,077 | |||||||
| | | | | | | | | | | |
Net income | $ | 81,611 | $ | 70,951 | $ | 105,240 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Net income per share—basic: | ||||||||||
Continuing operations | $ | 0.73 | $ | 1.29 | $ | 1.97 | ||||
Discontinued operations | 1.06 | 0.23 | 0.31 | |||||||
| | | | | | | | | | | |
Net income per share | $ | 1.79 | $ | 1.52 | $ | 2.28 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Net income per share—diluted: | ||||||||||
Continuing operations | $ | 0.72 | $ | 1.27 | $ | 1.93 | ||||
Discontinued operations | 1.04 | 0.23 | 0.30 | |||||||
| | | | | | | | | | | |
Net income per share | $ | 1.76 | $ | 1.50 | $ | 2.23 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Weighted average shares outstanding—basic | 45,610,270 | 46,540,385 | 46,182,204 | |||||||
Weighted average shares outstanding—diluted | 46,239,796 | 47,229,595 | 47,119,997 | |||||||
See notes to consolidated financial statements
45
Polypore International, Inc.
Consolidated statements of comprehensive income
(in thousands) | Year ended December 28, 2013 | Year ended December 29, 2012 | Year ended December 31, 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Net income | $ | 81,611 | $ | 70,951 | $ | 105,240 | ||||
Other comprehensive income (loss): | ||||||||||
Foreign currency translation adjustment | 3,566 | 10,058 | (13,479 | ) | ||||||
Change in net actuarial loss and prior service credit | 10,207 | (20,883 | ) | (6,999 | ) | |||||
Income taxes related to other comprehensive income (loss) | (4,285 | ) | 5,599 | 3,082 | ||||||
| | | | | | | | | | | |
Other comprehensive income (loss) | 9,488 | (5,226 | ) | (17,396 | ) | |||||
| | | | | | | | | | | |
Comprehensive income | $ | 91,099 | $ | 65,725 | $ | 87,844 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
See notes to consolidated financial statements
46
Polypore International, Inc.
Consolidated statements of shareholders' equity
(in thousands, except share data) | Shares of Common Stock | Common Stock | Shares of Treasury Stock | Treasury Stock | Paid-in Capital | Retained Earnings (Accumulated Deficit) | Accumulated Other Comprehensive Income (Loss) | Non- controlling Interest | Total | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at January 1, 2011 | 45,582,557 | $ | 456 | — | $ | — | $ | 497,160 | $ | (120,423 | ) | $ | 269 | $ | 588 | $ | 378,050 | |||||||||||
Net income for the year ended December 31, 2011 | — | — | — | — | — | 105,240 | — | — | 105,240 | |||||||||||||||||||
Stock-based compensation | — | — | — | — | 9,298 | — | — | — | 9,298 | |||||||||||||||||||
Stock option exercises | 912,243 | 9 | — | — | 6,649 | — | — | — | 6,658 | |||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | — | — | 14,136 | — | — | — | 14,136 | |||||||||||||||||||
Restricted stock grants | 4,380 | — | — | — | — | — | — | — | — | |||||||||||||||||||
Noncontrolling interest | — | — | — | — | — | — | — | 3,350 | 3,350 | |||||||||||||||||||
Change in net actuarial loss and prior service credit, net of income tax benefit of $2,187 | — | — | — | — | — | — | (4,812 | ) | — | (4,812 | ) | |||||||||||||||||
Foreign currency translation adjustment, net of income tax benefit of $895 | — | — | — | — | — | — | (12,584 | ) | 57 | (12,527 | ) | |||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2011 | 46,499,180 | 465 | — | — | 527,243 | (15,183 | ) | (17,127 | ) | 3,995 | 499,393 | |||||||||||||||||
Net income for the year ended December 29, 2012 | — | — | — | — | — | 70,951 | — | — | 70,951 | |||||||||||||||||||
Stock-based compensation | — | — | — | — | 16,278 | — | — | — | 16,278 | |||||||||||||||||||
Stock option exercises | 116,183 | 1 | — | — | 1,337 | — | — | — | 1,338 | |||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | — | — | 338 | — | — | — | 338 | |||||||||||||||||||
Restricted stock grants | 11,701 | — | — | — | — | — | — | — | — | |||||||||||||||||||
Noncontrolling interest | — | — | — | — | — | — | — | (242 | ) | (242 | ) | |||||||||||||||||
Change in net actuarial loss and prior service credit, net of income tax benefit of $5,848 | — | — | — | — | — | — | (15,035 | ) | — | (15,035 | ) | |||||||||||||||||
Foreign currency translation adjustment, net of income tax expense of $249 | — | — | — | — | — | — | 9,809 | 7 | 9,816 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 29, 2012 | 46,627,064 | 466 | — | — | 545,196 | 55,768 | (22,353 | ) | 3,760 | 582,837 | ||||||||||||||||||
Net income for the year ended December 28, 2013 | — | — | — | — | — | 81,611 | — | — | 81,611 | |||||||||||||||||||
Stock-based compensation | — | — | — | — | 20,687 | — | — | — | 20,687 | |||||||||||||||||||
Stock option exercises | 209,237 | 2 | — | — | 2,317 | — | — | — | 2,319 | |||||||||||||||||||
Excess tax benefit from stock-based compensation | — | — | — | — | 1,163 | — | — | — | 1,163 | |||||||||||||||||||
Restricted stock grants | 89,904 | 1 | — | — | (1 | ) | — | — | — | — | ||||||||||||||||||
Restricted stock forfeitures | (1,374 | ) | — | 1,374 | — | — | — | — | — | — | ||||||||||||||||||
Repurchases of common stock | (2,008,261 | ) | — | 2,008,261 | (80,668 | ) | — | — | — | — | (80,668 | ) | ||||||||||||||||
Noncontrolling interest | — | — | — | — | — | — | — | 1,960 | 1,960 | |||||||||||||||||||
Change in net actuarial loss and prior service credit, net of income tax expense of $3,647 | — | — | — | — | — | — | 6,560 | — | 6,560 | |||||||||||||||||||
Foreign currency translation adjustment, net of income tax expense of $638 | — | — | — | — | — | — | 2,928 | 179 | 3,107 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 28, 2013 | 44,916,570 | $ | 469 | 2,009,635 | $ | (80,668 | ) | $ | 569,362 | $ | 137,379 | $ | (12,865 | ) | $ | 5,899 | $ | 619,576 | ||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See notes to consolidated financial statements
47
Polypore International, Inc.
Consolidated statements of cash flows
(in thousands) | Year ended December 28, 2013 | Year ended December 29, 2012 | Year ended December 31, 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Operating activities: | ||||||||||
Net income | $ | 81,611 | $ | 70,951 | $ | 105,240 | ||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||
Depreciation expense | 44,812 | 42,320 | 34,812 | |||||||
Amortization expense | 11,491 | 13,347 | 16,530 | |||||||
Amortization of loan acquisition costs | 2,474 | 2,471 | 2,454 | |||||||
Gain on sale of discontinued operations, net of income taxes | (35,855 | ) | — | — | ||||||
Stock-based compensation | 20,687 | 16,278 | 9,298 | |||||||
Loss on disposal of property, plant and equipment | 1,161 | 971 | 375 | |||||||
Foreign currency (gain) loss | (60 | ) | 729 | (2,065 | ) | |||||
Excess tax benefit from stock-based compensation | (1,163 | ) | (338 | ) | (14,136 | ) | ||||
Deferred income taxes | (4,038 | ) | 9,822 | 28,640 | ||||||
Write-off of loan acquisition costs associated with refinancing of senior credit agreement | — | 2,478 | — | |||||||
Changes in operating assets and liabilities: | ||||||||||
Accounts receivable | 9,795 | (2,810 | ) | (18,795 | ) | |||||
Inventories | 2,117 | (28,304 | ) | (15,614 | ) | |||||
Prepaid and other current assets | 6,224 | 3,603 | (2,046 | ) | ||||||
Accounts payable and accrued liabilities | 4,105 | (23,003 | ) | (661 | ) | |||||
Income taxes payable | 3,173 | (4,098 | ) | 245 | ||||||
Other, net | 7,926 | 369 | 556 | |||||||
| | | | | | | | | | | |
Net cash provided by operating activities | 154,460 | 104,786 | 144,833 | |||||||
Investing activities: | ||||||||||
Purchases of property, plant and equipment, net | (28,235 | ) | (137,111 | ) | (156,330 | ) | ||||
Net proceeds from the sale of discontinued operations | 116,613 | — | — | |||||||
| | | | | | | | | | | |
Net cash provided by (used in) investing activities | 88,378 | (137,111 | ) | (156,330 | ) | |||||
Financing activities: | ||||||||||
Principal payments on debt | (15,000 | ) | (4,674 | ) | (4,519 | ) | ||||
Payments on revolving credit facility | (89,200 | ) | (15,000 | ) | — | |||||
Proceeds from revolving credit facility | 54,200 | — | — | |||||||
Repurchases of common stock | (80,668 | ) | — | — | ||||||
Proceeds from stock option exercises | 2,319 | 1,338 | 6,658 | |||||||
Excess tax benefit from stock-based compensation | 1,163 | 338 | 14,136 | |||||||
Noncontrolling interest | 1,960 | (242 | ) | 1,936 | ||||||
Proceeds from new senior credit agreement | — | 350,000 | — | |||||||
Principal payments in connection with refinancing of senior credit agreement | — | (342,291 | ) | — | ||||||
Loan acquisition costs | — | (6,228 | ) | (627 | ) | |||||
| | | | | | | | | | | |
Net cash provided by (used in) financing activities | (125,226 | ) | (16,759 | ) | 17,584 | |||||
Effect of exchange rate changes on cash and cash equivalents | 938 | 1,383 | (3,468 | ) | ||||||
| | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | 118,550 | (47,701 | ) | 2,619 | ||||||
Cash and cash equivalents at beginning of year | 44,873 | 92,574 | 89,955 | |||||||
| | | | | | | | | | | |
Cash and cash equivalents at end of year | $ | 163,423 | $ | 44,873 | $ | 92,574 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Supplemental cash flow information | ||||||||||
Cash paid for interest, net of capitalized interest | $ | 36,973 | $ | 33,284 | $ | 32,174 | ||||
Cash paid for income taxes, net of refunds | 22,006 | 24,837 | 22,975 | |||||||
See notes to consolidated financial statements
48
Polypore International, Inc.
Notes to consolidated financial statements
1. Description of Business
Polypore International, Inc. (the "Company") is a leading global high-technology filtration company that develops, manufactures and markets specialized microporous membranes used in separation and filtration processes. The Company has a global presence in the major geographic markets of North America, South America, Europe and Asia.
2. Accounting Policies
Basis of Presentation and Use of Estimates
The accompanying consolidated financial statements include the accounts of the Company and all majority-owned subsidiaries after elimination of intercompany accounts and transactions. The consolidated financial statements are prepared in accordance with U.S. generally accepted accounting principles. The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates.
On December 19, 2013, the Company completed the sale of its Microporous business. The operating results and assets and liabilities of this business have been presented as discontinued operations. All disclosures and amounts in the notes to the consolidated financial statements relate to the Company's continuing operations, unless otherwise indicated.
Accounting Period
The Company's fiscal year is the 52- or 53-week period ending the Saturday nearest to December 31. The fiscal years ended December 28, 2013, December 29, 2012 and December 31, 2011 included 52 weeks.
Revenue Recognition
Revenue from product sales is recognized when a firm sales agreement is in place, delivery of the product has occurred and collectibility of the fixed and determinable sales price is reasonably assured. Amounts billed to customers for shipping and handling are recorded in "Net sales" in the accompanying consolidated statements of income. Shipping and handling costs incurred by the Company for the delivery of goods to customers are included in "Cost of goods sold" in the accompanying consolidated statements of income. Estimates for sales returns and allowances and product returns are recognized in the period in which the revenue is recorded. Product returns and warranty expenses were not material for all periods presented.
Cash Equivalents
The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents.
Accounts Receivable and Concentrations of Credit Risk
Accounts receivable potentially expose the Company to concentrations of credit risk. The Company provides credit in the normal course of business and performs ongoing credit evaluations on certain of its customers' financial condition, but generally does not require collateral to support such receivables.
49
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
2. Accounting Policies (Continued)
Accounts receivable, net of allowance for doubtful accounts, are carried at cost which approximates fair value. The Company establishes an allowance for doubtful accounts based upon factors surrounding the credit risk of specific customers, historical trends and other information. The allowance for doubtful accounts was $3,262,000 and $2,995,000 at December 28, 2013 and December 29, 2012, respectively. The Company believes that the allowance for doubtful accounts is adequate to provide for potential losses resulting from uncollectible accounts. The Company charges accounts receivables off against the allowance for doubtful accounts when it deems them to be uncollectible on a specific identification basis.
Inventories
Inventories are carried at the lower of cost or market using the first-in, first-out method of accounting and consist of:
(in thousands) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Raw materials | $ | 39,357 | $ | 42,113 | |||
Work-in-process | 22,079 | 27,608 | |||||
Finished goods | 52,424 | 45,741 | |||||
| | | | | | | | |
| $ | 113,860 | $ | 115,462 | ||||
| | | | | | | | |
| | | | | | | | |
Property, Plant and Equipment
Property, plant and equipment are stated at cost. Depreciation commences when the asset is substantially complete and ready for its intended use. Depreciation is computed for financial reporting purposes on the straight-line method over the estimated useful lives of the related assets. The estimated useful lives for buildings and land improvements range from 20 to 40 years and the estimated useful lives for machinery and equipment range from 5 to 15 years. Costs of the construction of certain long-term assets include capitalized interest which is amortized over the estimated useful life of the related asset. The Company capitalized interest of $2,638,000 and $3,344,000 in 2012 and 2011, respectively. Repair and maintenance costs, which include indirect labor and employee benefits associated with maintenance personnel and utility, maintenance and repair costs for equipment and facilities utilized in the manufacturing process, are treated as inventoriable costs. Major planned maintenance activities outside of the normal production process are capitalized as property, plant and equipment if the costs are expected to provide future benefits by increasing the service potential of the asset to which the repair or maintenance applies.
Property, plant and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. If the sum of the expected undiscounted cash flows is less than the carrying value of the related asset or group of assets, a loss is recognized for the difference between the fair value and carrying value of the asset or group of assets.
Goodwill, Intangible Assets and Loan Acquisition Costs
Goodwill and indefinite-lived intangible assets are not amortized, but are subject to annual impairment testing unless circumstances dictate more frequent assessments. The Company performs its annual impairment assessment as of the first day of the fourth quarter of each fiscal year and more
50
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
2. Accounting Policies (Continued)
frequently whenever events or changes in circumstances indicate that the fair value of the asset may be less than the carrying amount. The Company's reporting units are at the operating segment level.
The Company tests for goodwill impairment using the quantitative two-step goodwill impairment test. Step one of the goodwill impairment test compares the fair value of the Company's reporting units to their carrying amount. The fair value of the reporting unit is determined using the income approach, corroborated by comparison to market capitalization and key multiples of comparable companies. Under the income approach, the Company determines fair value based on estimated future cash flows of each reporting unit, discounted by an estimated weighted-average cost of capital. If the fair value of the reporting unit is greater than its carrying amount, there is no impairment. If the reporting unit's carrying amount exceeds its fair value, the second step must be completed to measure the amount of impairment, if any. Step two calculates the implied fair value of goodwill by deducting the fair value of all tangible and intangible net assets of the reporting unit from the fair value of the reporting unit as calculated in step one. In this step, the fair value of the reporting unit is allocated to all of the reporting unit's assets and liabilities in a hypothetical purchase price allocation as if the reporting unit had been acquired on that date. If the carrying amount of goodwill exceeds the implied fair value of goodwill, an impairment loss will be recognized in an amount equal to the excess.
Intangible assets with finite lives are amortized over their respective estimated useful lives using the straight-line method. The useful life of customer relationships is based upon historical customer attrition rates and represents the estimated economic life of those relationships. Loan acquisition costs are amortized over the term of the related debt. Amortization expense for loan acquisition costs is classified as interest expense. Intangible assets with finite lives are reviewed for impairment whenever events or circumstances indicate that the carrying amount may not be recoverable. If the sum of the expected undiscounted cash flows is less than the carrying value of the related asset, a loss is recognized for the difference between the fair value and carrying value of the intangible asset.
Income Taxes
Deferred tax assets and liabilities are based on temporary differences between the basis of certain assets and liabilities for income tax and financial reporting purposes. A valuation allowance is recognized if it is more likely than not that a portion of the deferred tax assets will not be realized in the future. The tax effects from unrecognized tax benefits are recognized in the financial statements if the position is more likely than not to be sustained upon audit, based on the technical merits of the position.
Stock-Based Compensation
The Company records stock-based compensation based on the fair value of the award at the grant date. Stock-based compensation expense is recorded over the requisite service period using the straight-line method for service-based awards and in the service period corresponding to the performance target for performance-based awards. Excess tax benefits from employee stock option exercises are recorded as an increase to additional paid-in capital if an incremental tax benefit is realized following the ordering provisions of the tax law. Excess tax benefits are reported as a financing cash inflow rather than as a reduction of income taxes paid in the statement of cash flows.
51
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
2. Accounting Policies (Continued)
Research and Development
The cost of research and development is charged to expense as incurred and is included in "Selling, general and administrative expenses" in the accompanying consolidated statements of income. Research and development expense was $17,184,000, $18,487,000 and $17,555,000 in 2013, 2012 and 2011, respectively.
Government Grants
Grant awards are recognized when there is reasonable assurance that the Company will receive the grant and comply with the conditions attached to the grant. For capital expenditures, the Company deducts grant awards from the cost of the related asset. For expense reimbursements, the Company deducts grant awards from the related expenses. The Company was awarded a $49,264,000 grant from the U.S. Department of Energy ("DOE") to help fund an expansion of its U.S. lithium battery separator production capacity. As of December 29, 2012, the Company had recognized and received the entire amount of the DOE grant. The Company has also been awarded state and local grants in connection with certain of its U.S. expansions.
The Company recognized grant awards for capital expenditures of $1,670,000 and $23,550,000 in 2012 and 2011, respectively. The Company recognized grant awards for expenses of $1,739,000, $2,566,000 and $4,715,000 in 2013, 2012 and 2011, respectively.
Net Income Per Share
Basic net income per common share is based on the weighted-average number of common shares outstanding in each year. Diluted net income per common share considers the impact of dilution from stock options and unvested restricted stock shares as measured under the treasury stock method. Potential common shares that would increase net income per share amounts or decrease net loss per share amounts are antidilutive and excluded from the diluted net income per common share computation.
Foreign Currency Translation
The local currencies of the Company's foreign subsidiaries are the functional currencies. Assets and liabilities of the Company's foreign subsidiaries are translated into United States dollars at current exchange rates and resulting translation adjustments are reported in "Accumulated other comprehensive income (loss)." Income statement amounts are translated at weighted average exchange rates prevailing during the period. Transaction gains and losses are included in the determination of net income.
Fair Value of Financial Instruments
The Company's financial instruments include cash and cash equivalents, accounts receivable, accounts payable, accrued liabilities and long-term debt. The carrying value of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities approximates their fair value due to the short-term maturities of these assets and liabilities. The carrying amount of borrowings under the senior secured credit agreement approximates fair value because the interest rates adjust to market interest rates. The fair value of the 7.5% senior notes, based on a quoted market price and classified as level one in the fair value hierarchy, was $385,988,000 at December 28, 2013.
52
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
2. Accounting Policies (Continued)
Fair Value Measurements
Authoritative guidance establishes the following hierarchy that prioritizes the inputs to valuation methodologies used to measure fair value:
- •
- Level one: observable inputs such as quoted market prices in active markets;
- •
- Level two: inputs other than the quoted prices in active markets that are observable either directly or indirectly;
- •
- Level three: unobservable inputs in which there is little or no market data, which require the Company to develop its own assumptions.
As of December 28, 2013, the Company did not have any financial assets and liabilities required to be measured at fair value on a recurring basis. See Note 9 for pension assets measured at fair value on a recurring basis.
Recent Accounting Pronouncements
In February 2013, the FASB issued guidance requiring companies to provide information about the amounts reclassified out of accumulated other comprehensive income (loss) by component. The guidance is effective for annual and interim periods beginning after December 15, 2012. The adoption of this guidance in the Company's fiscal 2013 consolidated financial statements only affected presentation and did not have an impact on the Company's financial condition or results of operations.
3. Property, Plant and Equipment
Property, plant and equipment consist of:
(in thousands) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Land | $ | 24,184 | $ | 23,675 | |||
Buildings and land improvements | 157,928 | 153,934 | |||||
Machinery and equipment | 559,865 | 520,548 | |||||
Construction in progress | 139,344 | 150,840 | |||||
| | | | | | | | |
| 881,321 | 848,997 | ||||||
Less accumulated depreciation | 285,946 | 241,531 | |||||
| | | | | | | | |
| $ | 595,375 | $ | 607,466 | ||||
| | | | | | | | |
| | | | | | | | |
4. Goodwill
There were no changes in the carrying amount of goodwill for the years ended December 28, 2013 and December 29, 2012, and the carrying amount of goodwill at those dates was as follows:
(in thousands) | Transportation and Industrial | Electronics and EDVs | Separations Media | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Goodwill | $ | 327,347 | $ | 36,336 | $ | 212,279 | $ | 575,962 | |||||
Accumulated impairment charges | (131,450 | ) | — | — | (131,450 | ) | |||||||
| | | | | | | | | | | | | | |
| $ | 195,897 | $ | 36,336 | $ | 212,279 | $ | 444,512 | ||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
53
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
5. Intangibles, Loan Acquisition and Other Costs
Intangibles, loan acquisition and other costs consist of:
| | | December 28, 2013 | December 29, 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | Weighted Average Life (years) | Gross Carrying Amount | Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | ||||||||||
Intangible and other assets subject to amortization: | |||||||||||||||
Customer relationships | 17 | $ | 181,464 | $ | 107,554 | $ | 181,066 | $ | 96,180 | ||||||
Loan acquisition costs | 6 | 14,808 | 5,645 | 14,808 | 3,171 | ||||||||||
Intangible assets not subject to amortization: | |||||||||||||||
Trade names | Indefinite | 10,719 | — | 10,483 | — | ||||||||||
| | | | | | | | | | | | | | | | |
| $ | 206,991 | $ | 113,199 | $ | 206,357 | $ | 99,351 | ||||||||
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Amortization expense, including amortization of loan acquisition costs classified as interest expense, was $13,595,000, $15,078,000 and $18,244,000 in 2013, 2012 and 2011, respectively. The Company's estimate of amortization expense for the next five years is as follows:
(in thousands) | | |||
|---|---|---|---|---|
2014 | $ | 13,605 | ||
2015 | 13,605 | |||
2016 | 13,605 | |||
2017 | 12,871 | |||
2018 | 9,983 | |||
6. Accrued Liabilities
Accrued liabilities consist of:
(in thousands) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Compensation expense and other fringe benefits | $ | 18,421 | $ | 11,240 | |||
Current portion of environmental reserve | 2,882 | 11,079 | |||||
Other | 27,963 | 21,720 | |||||
| | | | | | | | |
| $ | 49,266 | $ | 44,039 | ||||
| | | | | | | | |
| | | | | | | | |
54
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
7. Debt
Debt, in order of priority, consists of:
(in thousands) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Senior credit agreement: | |||||||
Revolving credit facility | $ | — | $ | 35,000 | |||
Term loan facility | 281,250 | 296,250 | |||||
| | | | | | | | |
| 281,250 | 331,250 | ||||||
7.5% senior notes | 365,000 | 365,000 | |||||
| | | | | | | | |
| 646,250 | 696,250 | ||||||
Less current portion | 16,875 | 50,000 | |||||
| | | | | | | | |
Long-term debt | $ | 629,375 | $ | 646,250 | |||
| | | | | | | | |
| | | | | | | | |
On June 29, 2012, the Company refinanced its previous senior secured credit agreement with a new senior secured credit agreement. The credit agreement provides for a $150,000,000 revolving credit facility ($50,000,000 of which was borrowed in connection with the refinancing) and a $300,000,000 term loan facility. The proceeds from the credit agreement were used to pay outstanding principal and interest under the previous credit agreement and loan acquisition costs of $6,228,000, which were capitalized and are being amortized over the life of the credit agreement. In connection with the refinancing, the Company wrote-off unamortized loan acquisition costs of $2,478,000 associated with the previous credit agreement. Interest rates under the credit agreement are, at the Company's option, equal to either an alternate base rate or the Eurocurrency base rate, plus a specified margin.
The Company's domestic subsidiaries guarantee indebtedness under the credit agreement. Substantially all assets of the Company and its domestic subsidiaries and a first priority pledge of 66% of the voting capital stock of its foreign subsidiaries secure indebtedness under the credit agreement. The Company's ability to pay dividends on its common stock is limited under the terms of the credit agreement. The Company is also subject to certain financial covenants, including a maximum senior leverage ratio and a minimum interest coverage ratio. The Company was in compliance with all covenants as of December 28, 2013.
At December 28, 2013, no amounts were outstanding under the revolving credit facility and the entire amount was available for borrowing. The revolving credit facility matures in June 2017.
The term loan matures in June 2017. Minimum scheduled principal repayments of the term loan are as follows:
(in thousands) | | |||
|---|---|---|---|---|
2014 | $ | 16,875 | ||
2015 | 24,375 | |||
2016 | 33,750 | |||
2017 | 206,250 | |||
| | | | | |
| $ | 281,250 | |||
| | | | | |
| | | | | |
In 2010, the Company issued $365,000,000 aggregate principal amount of 7.5% senior notes due 2017. Interest on the notes is payable semi-annually on May 15 and November 15. The notes are
55
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
7. Debt (Continued)
effectively subordinated to all of the Company's existing and future secured debt and will rank senior to any of the Company's existing and future senior subordinated debt. The Company's domestic subsidiaries, subject to certain exceptions, guarantee the notes. The Company may redeem some or all of the notes at redemption prices specified in the indenture governing the notes.
8. Income Taxes
Significant components of deferred tax assets and liabilities consist of:
(in thousands) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Deferred tax assets: | |||||||
Pension obligations | $ | 29,683 | $ | 30,892 | |||
Net operating loss carryforwards | 5,552 | 17,689 | |||||
Other | 27,036 | 22,576 | |||||
| | | | | | | | |
Total deferred tax assets | 62,271 | 71,157 | |||||
Valuation allowance | (2,225 | ) | (3,797 | ) | |||
| | | | | | | | |
Net deferred tax assets | 60,046 | 67,360 | |||||
Deferred tax liabilities: | |||||||
Property, plant and equipment | (85,952 | ) | (91,251 | ) | |||
Goodwill and intangibles | (28,351 | ) | (31,708 | ) | |||
Other | (16,160 | ) | (7,449 | ) | |||
| | | | | | | | |
Total deferred tax liabilities | (130,463 | ) | (130,408 | ) | |||
| | | | | | | | |
Net deferred taxes | $ | (70,417 | ) | $ | (63,048 | ) | |
| | | | | | | | |
| | | | | | | | |
The valuation allowance decreased by $1,572,000 during 2013 primarily due to adjustments to state tax positions.
Deferred taxes are reflected in the consolidated balance sheet as follows:
(in thousands) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Current deferred tax asset | $ | — | $ | 21,671 | |||
Accrued liabilities | (85 | ) | — | ||||
Non-current deferred tax liability | (70,332 | ) | (84,719 | ) | |||
| | | | | | | | |
Net deferred taxes | $ | (70,417 | ) | $ | (63,048 | ) | |
| | | | | | | | |
| | | | | | | | |
A reconciliation of the beginning and ending amounts of unrecognized tax benefits is as follows:
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Balance at beginning of year | $ | 9,435 | $ | 9,373 | $ | 8,103 | ||||
Increase related to current year positions | 980 | — | — | |||||||
Increase related to prior year positions | 160 | 62 | 1,270 | |||||||
Settlements with tax authorities | (138 | ) | — | — | ||||||
| | | | | | | | | | | |
Balance at end of year | $ | 10,437 | $ | 9,435 | $ | 9,373 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
56
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
8. Income Taxes (Continued)
The amount of unrecognized tax benefits that, if recognized, would affect the annual effective tax rate is $9,183,000, $8,168,000 and $8,045,000 as of December 28, 2013, December 29, 2012 and December 31, 2011, respectively. Accrued interest and penalties related to unrecognized tax benefits are recognized as a component of income tax expense and were $362,000, $399,000 and $362,000 at December 28, 2013, December 29, 2012 and December 31, 2011, respectively.
The Company has operations in North America, Europe and Asia and files tax returns in numerous tax jurisdictions. The Company is not subject to income tax adjustments in the U.S. for tax years prior to 2005 and in foreign jurisdictions for tax years prior to 2004. Tax audits are currently being conducted on a French subsidiary for the tax years 2006 through 2008. Although the outcome of tax audits is uncertain, management believes that adequate provisions have been made for potential liabilities resulting from such audits. Because audit outcomes and the timing of audit settlements are subject to significant uncertainty, the Company cannot make a reasonable estimate of the impact on earnings in the next twelve months from these audits. Management is not aware of any issues for open tax years that upon final resolution will have a material adverse effect on the Company's consolidated financial position, cash flows or operating results.
At December 28, 2013, the Company has net operating loss carryforwards in the U.S. of $22,716,000 that expire beginning in 2027. The Company expects to utilize the U.S. net operating loss carryforwards during the next twelve months and has classified these amounts as current in the accompanying December 28, 2013 consolidated balance sheet. The Company also has foreign net operating losses of $714,000 that expire at various dates beginning in 2015. The Company utilized approximately $28,617,000 of net operating loss carryfowards during 2013.
Income before income taxes includes the following components:
| | Year Ended | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
United States | $ | (2,762 | ) | $ | 18,062 | $ | 72,993 | |||
Foreign | 50,505 | 67,279 | 64,228 | |||||||
| | | | | | | | | | | |
| $ | 47,743 | $ | 85,341 | $ | 137,221 | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Income tax expense consists of:
| | Year Ended | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
Current: | ||||||||||
U.S. taxes on domestic income | $ | 806 | $ | 658 | $ | 570 | ||||
Foreign taxes | 19,913 | 18,303 | 21,666 | |||||||
| | | | | | | | | | | |
Total current | 20,719 | 18,961 | 22,236 | |||||||
Deferred: | ||||||||||
U.S. taxes on domestic income | (2,739 | ) | 7,859 | 26,900 | ||||||
Foreign taxes | (3,691 | ) | (1,553 | ) | (3,078 | ) | ||||
| | | | | | | | | | | |
Total deferred | (6,430 | ) | 6,306 | 23,822 | ||||||
| | | | | | | | | | | |
| $ | 14,289 | $ | 25,267 | $ | 46,058 | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
57
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
8. Income Taxes (Continued)
Income taxes at the Company's effective tax rate differed from income taxes at the statutory rate as follows:
| | Year Ended | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
Computed income taxes at the expected statutory rate | $ | 16,710 | $ | 29,869 | $ | 48,027 | ||||
State and local taxes | 2 | 321 | 1,418 | |||||||
Foreign taxes | (1,749 | ) | (7,154 | ) | (5,127 | ) | ||||
Valuation allowances | (1,572 | ) | — | — | ||||||
Uncertain tax positions | 1,037 | — | — | |||||||
Other | (139 | ) | 2,231 | 1,740 | ||||||
| | | | | | | | | | | |
Income tax expense | $ | 14,289 | $ | 25,267 | $ | 46,058 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Taxes have been provided on earnings distributed and expected to be distributed by the Company's foreign subsidiaries. In addition, there are discrete foreign earnings of $55,065,000 related to the sale of the Microporous business which the Company plans to repatriate. During 2013, the Company provided $8,336,000 of U.S. taxes on such earnings in discontinued operations. All other foreign earnings are undistributed and considered to be indefinitely reinvested and, accordingly, no provision for U.S. federal and state income taxes has been provided thereon. The Company has not provided additional U.S. federal and state income taxes on an estimated $237,000,000 of undistributed earnings of consolidated foreign subsidiaries. Upon distribution of these earnings in the form of dividends or otherwise, the Company would be subject to both U.S. income taxes and withholding taxes payable to the various foreign countries. Determination of the amount of unrecognized deferred U.S. income tax liability is not practicable because of the complexities associated with this hypothetical calculation.
The Company has entered into an agreement with the Board of Investment in Thailand under which, subject to certain limitations, 100% of the Company's income from manufacturing activities in Thailand was tax-free through 2010 and portions of income will be tax-free through 2019. The income tax benefits recognized from this tax holiday were $1,293,000, $1,821,000 and $2,300,000 in 2013, 2012 and 2011, respectively. The Company has entered into an agreement with the Ministry of Strategy and Finance in South Korea which, subject to certain limitations, effectively exempts 100% of the Company's income in South Korea from income taxes through 2016. The Company recognized an income tax benefit from this tax holiday of $1,379,000 in 2012.
58
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
9. Employee Benefit Plans
Pension Plans
The Company and its subsidiaries sponsor multiple defined benefit pension plans based in subsidiaries located outside of the United States. The following table sets forth the funded status of the defined benefit pension plans:
(in thousands) | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Change in benefit obligation | |||||||
Benefit obligation at beginning of year | $ | (123,825 | ) | $ | (97,667 | ) | |
Service cost | (2,255 | ) | (1,658 | ) | |||
Interest cost | (4,532 | ) | (4,739 | ) | |||
Plan amendments | 1,084 | — | |||||
Actuarial gain (loss) | 7,218 | (20,890 | ) | ||||
Benefit payments | 3,576 | 3,778 | |||||
Foreign currency translation and other | (4,640 | ) | (2,649 | ) | |||
| | | | | | | | |
Benefit obligation at end of year | (123,374 | ) | (123,825 | ) | |||
Change in plan assets | |||||||
Fair value of plan assets at beginning of year | 18,529 | 17,895 | |||||
Actual return on plan assets | 816 | 1,794 | |||||
Company contributions | 2,002 | 2,230 | |||||
Benefit payments | (3,576 | ) | (3,778 | ) | |||
Foreign currency translation and other | 722 | 388 | |||||
| | | | | | | | |
Fair value of plan assets at end of year | 18,493 | 18,529 | |||||
| | | | | | | | |
Funded status at end of year | $ | (104,881 | ) | $ | (105,296 | ) | |
| | | | | | | | |
| | | | | | | | |
Amounts recognized in the consolidated balance sheet consist of: | |||||||
Accrued liabilities | $ | (2,060 | ) | $ | (1,805 | ) | |
Pension obligations | (102,821 | ) | (103,491 | ) | |||
| | | | | | | | |
Net amount recognized | $ | (104,881 | ) | $ | (105,296 | ) | |
| | | | | | | | |
| | | | | | | | |
Amounts recognized in accumulated other comprehensive income (loss), pre-tax, consist of: | |||||||
Net actuarial loss | $ | 28,110 | $ | 35,987 | |||
Prior service credit | (1,259 | ) | (182 | ) | |||
| | | | | | | | |
Net amount recognized | $ | 26,851 | $ | 35,805 | |||
| | | | | | | | |
| | | | | | | | |
The funded status of the Company's pension plans is dependent upon many factors, including returns on invested assets and the level of market interest rates.
The accumulated benefit obligation for all defined benefit pension plans was $113,092,000 and $114,425,000 at December 28, 2013 and December 29, 2012, respectively. Each of the Company's defined benefit pension plans had accumulated benefit obligations in excess of plan assets at December 28, 2013.
59
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
9. Employee Benefit Plans (Continued)
The following table provides the components of net periodic benefit cost:
| | Year ended | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
Service cost | $ | 2,255 | $ | 1,658 | $ | 1,552 | ||||
Interest cost | 4,532 | 4,739 | 5,021 | |||||||
Expected return on plan assets | (768 | ) | (844 | ) | (910 | ) | ||||
Amortization of prior service credit | (52 | ) | (50 | ) | (54 | ) | ||||
Recognized net actuarial loss | 1,739 | 472 | 69 | |||||||
| | | | | | | | | | | |
Net periodic benefit cost | $ | 7,706 | $ | 5,975 | $ | 5,678 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
The amount of prior service credit and net actuarial loss included in accumulated other comprehensive loss and expected to be recognized in net periodic benefit cost in 2014 is $951,000.
Weighted average assumptions used to determine the benefit obligation and net periodic benefit costs consist of:
Weighted average assumptions as of the end of the year | December 28, 2013 | December 29, 2012 | |||||
|---|---|---|---|---|---|---|---|
Discount rate used to determine the benefit obligation | 3.70 | % | 3.60 | % | |||
Discount rate used to determine the net periodic benefit costs | 3.60 | % | 4.90 | % | |||
Expected return on plan assets | 3.51 | % | 4.00 | % | |||
Rate of compensation increase | 2.53 | % | 2.52 | % | |||
The Company's pension plan assets are invested to obtain a reasonable long-term rate of return at an acceptable level of investment risk. Risk tolerance is established through consideration of plan liabilities, plan funded status and corporate financial condition. Investment risk is measured and monitored on an ongoing basis through periodic investment portfolio reviews, liability measurements and asset/liability studies. The Company's expected return on plan assets is based on historical market data for each asset class and expected market conditions. Except for certain pension plans funded by insurance contracts, the assets in the pension plans are diversified across fixed income and equity investments. The investment portfolio has target allocations of approximately 77% fixed income and 23% equity. The actual portfolio allocation was 84% fixed income and 16% equity at December 28, 2013 and was 81% fixed income and 19% equity at December 29, 2012. The fixed income investments, which are primarily investment grade European bonds and insurance contracts, are level two securities in the fair value hierarchy. The equity securities are considered level one securities and primarily include investments in European companies.
60
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
9. Employee Benefit Plans (Continued)
In 2014, the Company expects to contribute $2,060,000 to its pension plans. The estimated future benefit payments expected to be paid for each of the next five years and the sum of payments expected for the next five years thereafter are:
(in thousands) | | |||
|---|---|---|---|---|
2014 | $ | 3,891 | ||
2015 | 4,045 | |||
2016 | 4,169 | |||
2017 | 4,403 | |||
2018 | 4,828 | |||
2019 - 2023 | 29,468 | |||
401(k) Plans
The Company sponsors a 401(k) plan for U.S. salaried employees. Salaried employees are eligible to participate in the plan on January 1, April 1, July 1 or October 1 after their date of employment. Under the plan, employer contributions are defined as 5.00% of a participant's base salary plus a matching of employee contributions allowing for a maximum matching contribution of 3.00% of a participant's earnings. The cost of the plan recognized as expense was $3,551,000, $4,224,000 and $3,484,000 in 2013, 2012 and 2011, respectively.
The Company sponsors a 401(k) plan for U.S. hourly employees subject to collective bargaining agreements. Depending on the applicable collective bargaining agreement, employer basic contributions are defined as 3.00% or 3.50% of a participant's base earnings plus a company matching contribution, limited to certain maximum percentage contributions. The Company also makes a separate contribution for employees who were hired prior to January 1, 2000 and who are not eligible for certain other benefit plans. The cost of the plan recognized as expense was $631,000, $650,000 and $664,000 in 2013, 2012 and 2011, respectively.
10. Environmental Matters
Environmental obligations are accrued when such expenditures are probable and reasonably estimable. The amount of liability recorded is based on currently available information, including the progress of remedial investigations, current status of discussions with regulatory authorities regarding the method and extent of remediation, presently enacted laws and existing technology. Accruals for estimated losses from environmental obligations are adjusted as further information develops or circumstances change. Costs of future expenditures for environmental obligations are not discounted to their present value. The Company does not currently anticipate any material loss in excess of the amounts accrued. However, the Company's future remediation expenses may be affected by a number of uncertainties including, but not limited to, the difficulty in estimating the extent and method of remediation, the evolving nature of environmental regulations and the availability and application of technology. The Company does not expect the resolution of such uncertainties to have a material adverse effect on its consolidated financial position or liquidity. Recoveries of environmental costs from other parties are recognized as assets when receipt is deemed probable.
In connection with the acquisition of Membrana GmbH ("Membrana") in 2002, the Company recorded a reserve for environmental obligations. The reserve provides for costs to remediate known
61
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
10. Environmental Matters (Continued)
environmental issues and operational upgrades which are required in order for the Company to remain in compliance with local regulations. The initial estimate and subsequent finalization of the reserve was included in the allocation of purchase price at the date of acquisition. The environmental reserve for the Membrana facility, which is denominated in euros, was $2,882,000 and $11,079,000 at December 28, 2013 and December 29, 2012, respectively. The Company anticipates the expenditures associated with the reserve will be made in the next twelve months. The reserve is included in "Accrued liabilities" in the accompanying consolidated balance sheets.
The Company had indemnification agreements with the prior owners of Membrana for a substantial portion of these costs. During the third quarter of 2013, the Company received $10,304,000 as final payment of amounts outstanding under the indemnification agreements. At December 29, 2012, the indemnification receivable, which was denominated in euros, was $11,542,000 and included in "Prepaid and other" in the accompanying consolidated balance sheet.
11. Commitments and Contingencies
Leases
The Company leases certain equipment and facilities under operating leases. Rent expense under operating leases was $3,376,000, $3,416,000 and $2,523,000 in 2013, 2012 and 2011, respectively.
Future minimum operating lease payments at December 28, 2013 are:
(in thousands) | | |||
|---|---|---|---|---|
2014 | $ | 3,072 | ||
2015 | 2,144 | |||
2016 | 1,437 | |||
2017 | 1,182 | |||
2018 | 875 | |||
Thereafter | 469 | |||
| | | | | |
| $ | 9,179 | |||
| | | | | |
| | | | | |
Raw Materials
The Company employs a global purchasing strategy to achieve pricing leverage on its purchases of major raw materials. Accordingly, the Company purchases the majority of each type of raw material from one primary supplier with additional suppliers having been qualified to supply the Company if an interruption in supply were to occur. The Company believes that alternative sources of raw materials are readily available and the loss of any particular supplier would not have a material impact on the results of operations. However, the loss of raw material supply sources could, in the short term, adversely affect the Company's business until alternative supply arrangements were secured.
Collective Bargaining Agreements
On December 28, 2013, approximately 33% of the Company's employees were represented under collective bargaining agreements. A majority of those employees are located in Germany and France and are represented under industry-wide agreements that are subject to national and local government
62
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
11. Commitments and Contingencies (Continued)
regulations. Labor unions also represent the Company's employees in Owensboro, Kentucky, and Corydon, Indiana. The collective bargaining agreement at the Selestat, France facility, covering approximately 5% of the Company's workers, expires in June 2014.
Other
The Company is from time to time subject to various claims and other matters arising out of the normal conduct of business. The amount recorded for identified contingent liabilities is based on estimates. Amounts recorded are reviewed periodically and adjusted to reflect additional information that becomes available. Actual costs to be incurred in future periods may vary from the estimates, given the inherent uncertainties in evaluating certain exposures. Subject to the imprecision in estimating future contingent liability costs, the Company believes that based on present information, it is unlikely that a liability, if any, exists that would have a material adverse effect on the consolidated operating results, financial position or cash flows of the Company.
12. Stock-Based Compensation Plans
The Company offers stock-based compensation plans to attract, retain, motivate and reward key officers, non-employee directors and employees. Stock-based compensation expense includes costs associated with stock options and restricted stock and is classified as "Selling, general and administrative expenses" in the accompanying consolidated statements of income.
The 2007 Stock Incentive Plan ("2007 Plan") allows for the grant of stock options, restricted stock and other instruments for up to a total of 4,751,963 shares of common stock. Stock options granted under the 2007 Plan have 10-year terms and are issued with an exercise price not less than the fair market value of the Company's stock on the grant date. Stock options granted under the 2007 Plan may vest based on satisfaction of certain annual performance criteria or may vest over time.
A summary of outstanding stock options is as follows:
| | Stock Options | Weighted- average exercise price | Weighted average remaining contractual term (years) | Aggregate intrinsic value (in thousands) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Outstanding at December 29, 2012 | 3,121,639 | $ | 37.73 | ||||||||||
Granted | 348,208 | 36.84 | |||||||||||
Exercised | (209,237 | ) | 11.08 | ||||||||||
Forfeited | (30,269 | ) | 51.14 | ||||||||||
| | | | | | | | | | | | | | |
Outstanding at December 28, 2013 | 3,230,341 | 39.23 | 7.0 | $ | — | ||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Vested and exercisable at December 28, 2013 | 2,255,902 | 35.05 | 6.5 | 6,666 | |||||||||
Expected to vest | 969,582 | 48.92 | 8.2 | — | |||||||||
Stock option expense was $19,080,000, $16,100,000 and $9,206,000 in 2013, 2012 and 2011, respectively. The income tax benefit related to stock option expense was $6,773,000, $5,724,000 and $3,242,000 in 2013, 2012 and 2011, respectively. As of December 28, 2013, the Company had $18,177,000 of total pre-tax unrecognized stock option expense, net of estimated forfeitures, which is expected to be recognized over a weighted average period of 1.2 years.
63
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
12. Stock-Based Compensation Plans (Continued)
Exercise prices for options outstanding at December 28, 2013 ranged from $5.24 to $56.98. The intrinsic value is based on the Company's closing stock price of $38.01 at December 28, 2013, which would have been received by the option holder had the options been exercised at that date. The total intrinsic value of options exercised during 2013, 2012 and 2011 amounted to $6,402,000, $3,116,000 and $48,063,000, respectively.
The Company is required to estimate the fair value of stock options on the grant date using an option-pricing model. The weighted average grant-date fair value of options granted during 2013, 2012 and 2011 amounted to $18.92, $18.97 and $26.73 per share, respectively. The fair value of each stock option granted was estimated on the date of grant based on the Black-Scholes option pricing model with the following weighted-average assumptions:
| | Weighted average assumptions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2013 | 2012 | 2011 | |||||||
Expected term (years) | 5.6 | 5.3 | 4.8 | |||||||
Risk-free interest rate | 0.96 | % | 0.86 | % | 0.90 | % | ||||
Expected volatility | 57.3 | % | 56.0 | % | 56.1 | % | ||||
Dividend yield | — | — | — | |||||||
The potential expected term of the stock options ranges from the vesting period of the options (three years) to the contractual term of the options (ten years). The Company determines the expected term of the options based on historical experience, vesting periods, structure of the option plans and contractual term of the options. The Company's risk-free interest rate is based on the interest rate of U.S. Treasury bills with a term approximating the expected term of the options and is measured at the date of the stock option grant. Expected volatility is estimated based on the Company's historical stock prices and implied volatility from traded options. The Company does not anticipate paying dividends.
A summary of stock options that are expected to vest is as follows:
| | Stock Options | Weighted- average grant-date fair value | |||||
|---|---|---|---|---|---|---|---|
December 29, 2012 | 1,297,773 | $ | 25.97 | ||||
Granted | 346,147 | 18.92 | |||||
Vested | (645,736 | ) | 25.58 | ||||
Forfeited | (28,602 | ) | 50.80 | ||||
| | | | | | | | |
December 28, 2013 | 969,582 | 22.98 | |||||
| | | | | | | | |
| | | | | | | | |
The total fair value of options vested during 2013, 2012 and 2011 was $16,518,000, $17,945,000 and $4,014,000, respectively.
On February 25, 2013, the Company modified the terms of stock options granted on August 23, 2011. For participants that meet certain criteria upon retirement, the modification extends the exercise period for vested options from 90 days after retirement to the earlier of the option expiration date or three years after retirement and also allows unvested options to continue to vest for up to three years after retirement as if the participant had remained in the service of the Company. The total
64
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
12. Stock-Based Compensation Plans (Continued)
incremental stock option expense associated with the modification, net of estimated forfeitures, was $2,500,000, of which $1,580,000 has been recognized as of December 28, 2013, and the remainder of which will be recognized over the remaining vesting period of 0.9 years.
Under the 2007 Plan, the Company granted restricted shares of 89,904, 11,701 and 4,380 in 2013, 2012 and 2011, respectively. A summary of the status of unvested restricted stock is as follows:
| | Restricted stock | Weighted- average grant-date fair value | |||||
|---|---|---|---|---|---|---|---|
Unvested at December 29, 2012 | 15,761 | $ | 38.40 | ||||
Granted | 89,904 | 36.78 | |||||
Vested | (6,500 | ) | 39.25 | ||||
Withheld and repurchased | (8,261 | ) | 36.42 | ||||
Forfeited | (1,374 | ) | 36.42 | ||||
| | | | | | | | |
Unvested at December 28, 2013 | 89,530 | 36.93 | |||||
| | | | | | | | |
| | | | | | | | |
The expense associated with these restricted stock grants, which vest over three years, was $1,607,000, $178,000 and $92,000 in 2013, 2012 and 2011, respectively.
13. Accumulated Other Comprehensive Income (Loss)
The changes in accumulated other comprehensive income (loss), net of income taxes, for the year ended December 28, 2013 were as follows:
(in thousands) | Pension Plans | Foreign Currency Translation Adjustment | Total Accumulated Other Comprehensive Income (Loss) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 29, 2012 | $ | (26,102 | ) | $ | 3,749 | $ | (22,353 | ) | ||
Other comprehensive income before reclassifications | 5,375 | 2,989 | 8,364 | |||||||
Amortization of net actuarial loss and prior service credit for defined benefit pension plans and related income tax expense of $563 | 1,185 | (61 | ) | 1,124 | ||||||
| | | | | | | | | | | |
Other comprehensive income for the year ended December 28, 2013 | 6,560 | 2,928 | 9,488 | |||||||
| | | | | | | | | | | |
Balance at December 28, 2013 | $ | (19,542 | ) | $ | 6,677 | $ | (12,865 | ) | ||
| | | | | | | | | | | |
| | | | | | | | | | | |
See Note 9, "Employee Benefit Plans", for additional details on the amortization of net actuarial loss and prior service credit, which are included in the computation of net periodic benefit cost.
65
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
14. Treasury Stock
On February 19, 2013, the Board of Directors authorized the repurchase of up to 4,000,000 shares of the Company's common stock. During 2013, the Company repurchased 2,000,000 shares of common stock for $80,343,000 and the authorization expired on December 31, 2013. During fiscal 2013, the Company withheld and repurchased 8,261 shares of common stock for $325,000 to satisfy certain employees' withholding tax liabilities related to restricted stock grants. Additionally, during fiscal 2013, 1,374 shares of unvested restricted stock were forfeited and included in treasury stock.
15. Related Party Transactions
The Company's German subsidiary has a 33% equity investment in a patent and trademark service provider and a 25% equity investment in a research company. The investments are accounted for under the equity method of accounting and were $676,000 and $650,000 at December 28, 2013 and December 29, 2012, respectively. Charges from the affiliates for work performed were $1,628,000, $1,801,000 and $979,000 in 2013, 2012 and 2011, respectively. Amounts due to the affiliates were $254,000 and $239,000 at December 28, 2013 and December 29, 2012, respectively.
16. Noncontrolling Interest
In 2010, the Company formed a joint venture with Camel Group Co., Ltd ("Camel"), a leading battery manufacturer in China, to produce lead-acid battery separators primarily for Camel's use. The joint venture, Daramic Xiangyang Battery Separator Co., Ltd. ("Daramic Xiangyang"), is located at Camel's facility and owned 65% by the Company and 35% by Camel. During fiscal 2013, the Company and Camel made equity contributions of $2,470,000 and $1,330,000, respectively, to fund capital expenditures.
In exchange for notes payable, Daramic Xiangyang purchased from Camel a building and from the Company certain production equipment that was previously located at the Company's former facility in Potenza, Italy. The notes payable and related interest will be paid by Daramic Xiangyang using available free cash flow, as defined in the joint venture agreement. The building note payable to Camel has a principal balance of $5,910,000 at December 28, 2013 and December 29, 2012 and is included in "Other" non-current liabilities in the accompanying consolidated balance sheets, and the equipment note payable to the Company eliminates in consolidation.
17. Segment Information
The Company's operations are principally managed on a products basis and are comprised of three reportable segments for financial reporting purposes. The Company's three reportable segments are presented in the context of its two primary businesses—energy storage and separations media.
The energy storage business produces and markets membranes that provide the critical function of separating the cathode and anode in a variety of battery markets and is comprised of the following reportable segments:
- •
- Electronics and EDVs—produces and markets membranes for lithium batteries that are used in portable electronic devices, cordless power tools, electric drive vehicles ("EDVs") and energy storage systems ("ESS").
- •
- Transportation and industrial—produces and markets membranes for lead-acid batteries that are used in automobiles, other motor vehicles, forklifts and uninterruptible power supply systems.
66
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
17. Segment Information (Continued)
The separations media business is a reportable segment and produces and markets membranes and membrane modules used as the high-technology filtration element in various medical and industrial applications.
The Company evaluates the performance of segments and allocates resources to segments based on operating income before depreciation and amortization. In addition, it evaluates business segment performance before stock-based compensation and certain non-recurring and other costs.
67
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
17. Segment Information (Continued)
Financial information relating to the reportable segments is presented below:
| | Year ended | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
Net sales to external customers (by major product group): | ||||||||||
Electronics and EDVs | $ | 130,301 | $ | 167,370 | $ | 200,991 | ||||
Transportation and industrial | 311,859 | 299,010 | 294,498 | |||||||
| | | | | | | | | | | |
Energy storage | 442,160 | 466,380 | 495,489 | |||||||
Healthcare | 124,169 | 114,778 | 120,387 | |||||||
Filtration and specialty | 69,953 | 67,541 | 69,849 | |||||||
| | | | | | | | | | | |
Separations media | 194,122 | 182,319 | 190,236 | |||||||
| | | | | | | | | | | |
Net sales | $ | 636,282 | $ | 648,699 | $ | 685,725 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Operating income: | ||||||||||
Electronics and EDVs | $ | 17,371 | $ | 47,195 | $ | 91,130 | ||||
Transportation and industrial | 69,997 | 66,061 | 75,508 | |||||||
| | | | | | | | | | | |
Energy storage | 87,368 | 113,256 | 166,638 | |||||||
Separations media | 54,077 | 52,479 | 54,680 | |||||||
Corporate and other | (30,702 | ) | (24,078 | ) | (41,707 | ) | ||||
| | | | | | | | | | | |
Segment operating income | 110,743 | 141,657 | 179,611 | |||||||
Stock-based compensation | 20,687 | 16,278 | 9,298 | |||||||
Non-recurring and other costs | 2,119 | 1,624 | 658 | |||||||
| | | | | | | | | | | |
Total operating income | 87,937 | 123,755 | 169,655 | |||||||
Reconciling items: | ||||||||||
Interest expense, net | 39,473 | 36,049 | 34,384 | |||||||
Foreign currency and other | 721 | (113 | ) | (1,950 | ) | |||||
Write-off of loan acquisition costs associated with refinancing of senior credit agreement | — | 2,478 | — | |||||||
| | | | | | | | | | | |
Income from continuing operations before income taxes | $ | 47,743 | $ | 85,341 | $ | 137,221 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Depreciation and amortization: | ||||||||||
Electronics and EDVs | $ | 17,607 | $ | 16,137 | $ | 9,395 | ||||
Transportation and industrial | 11,366 | 9,708 | 8,855 | |||||||
| | | | | | | | | | | |
Energy storage | 28,973 | 25,845 | 18,250 | |||||||
Separations media | 14,224 | 13,437 | 13,360 | |||||||
Corporate and other | 11,326 | 12,795 | 16,060 | |||||||
Discontinued operations | 1,780 | 3,590 | 3,672 | |||||||
| | | | | | | | | | | |
| $ | 56,303 | $ | 55,667 | $ | 51,342 | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Capital expenditures: | ||||||||||
Electronics and EDVs | $ | 4,138 | $ | 115,845 | $ | 116,208 | ||||
Transportation and industrial | 13,658 | 12,200 | 16,623 | |||||||
| | | | | | | | | | | |
Energy storage | 17,796 | 128,045 | 132,831 | |||||||
Separations media | 9,674 | 8,282 | 22,451 | |||||||
Discontinued operations | 765 | 784 | 1,048 | |||||||
| | | | | | | | | | | |
| $ | 28,235 | $ | 137,111 | $ | 156,330 | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Assets: | ||||||||||
Electronics and EDVs | $ | 374,323 | $ | 397,973 | $ | 291,451 | ||||
Transportation and industrial | 291,483 | 262,174 | 230,457 | |||||||
| | | | | | | | | | | |
Energy storage | 665,806 | 660,147 | 521,908 | |||||||
Separations media | 266,133 | 244,978 | 273,631 | |||||||
Corporate and other | 618,274 | 588,686 | 593,235 | |||||||
Assets of discontinued operations | — | 92,261 | 93,105 | |||||||
| | | | | | | | | | | |
Total assets | $ | 1,550,213 | $ | 1,586,072 | $ | 1,481,879 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
68
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
17. Segment Information (Continued)
Net sales by geographic location, based on the country from which the product is shipped, were as follows:
| | Year ended | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
Net sales to unaffiliated customers: | ||||||||||
United States | $ | 232,402 | $ | 207,169 | $ | 251,864 | ||||
Germany | 177,928 | 164,896 | 170,103 | |||||||
France | 75,854 | 70,903 | 81,719 | |||||||
China | 67,545 | 86,627 | 82,861 | |||||||
Other | 82,553 | 119,104 | 99,178 | |||||||
| | | | | | | | | | | |
Total | $ | 636,282 | $ | 648,699 | $ | 685,725 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
Property, plant and equipment by geographic location were as follows:
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
United States | $ | 303,888 | $ | 316,128 | $ | 216,665 | ||||
Germany | 153,913 | 152,943 | 154,586 | |||||||
Other | 137,574 | 138,395 | 123,224 | |||||||
| | | | | | | | | | | |
Total | $ | 595,375 | $ | 607,466 | $ | 494,475 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
69
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
18. Discontinued Operations
On December 19, 2013, as required by the divestiture provisions of the Federal Trade Commission's order, the Company completed the sale of its Microporous business, which consisted of the production facilities in Piney Flats, Tennessee, and Feistritz, Austria, for $120,000,000. The Company recognized a gain of $35,855,000 on the sale, net of direct transaction costs and income taxes. The sales price and resulting gain are subject to adjustment in subsequent periods upon finalization of working capital.
Microporous was previously included in the transportation and industrial segment. The assets and liabilities of Microporous reported as discontinued operations in the accompanying consolidated balance sheet as of December 29, 2012 were as follows:
(in thousands) | December 29, 2012 | |||
|---|---|---|---|---|
Assets | ||||
Accounts receivable, net | $ | 15,259 | ||
Inventories | 4,447 | |||
Prepaid and other | 759 | |||
Property, plant and equipment, net | 31,335 | |||
Goodwill and intangibles, net | 39,530 | |||
Other | 931 | |||
| | | | | |
Assets of discontinued operations | $ | 92,261 | ||
| | | | | |
| | | | | |
Liabilities | ||||
Accounts payable and accrued liabilities | $ | 4,066 | ||
Deferred income taxes | 13,948 | |||
Other | 1,562 | |||
| | | | | |
Liabilities of discontinued operations | $ | 19,576 | ||
| | | | | |
| | | | | |
The results of operations of Microporous are classified as discontinued operations and are presented separately in the accompanying consolidated statements of income for all periods presented. Summarized results of operations reported as discontinued operations are as follows:
| | Year ended | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | December 28, 2013 | December 29, 2012 | December 31, 2011 | |||||||
Net sales | $ | 71,441 | $ | 68,674 | $ | 77,349 | ||||
Income from discontinued operations before income taxes | 19,525 | 16,171 | 19,878 | |||||||
Income from discontinued operations, net of income taxes | 12,302 | 10,877 | 14,077 | |||||||
70
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
19. Quarterly Results of Operations (Unaudited)
(in thousands, except per share data) | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Fiscal year ended December 28, 2013: | |||||||||||||
Net sales | $ | 145,941 | $ | 168,897 | $ | 152,020 | $ | 169,424 | |||||
Gross profit | 49,195 | 60,426 | 47,631 | 63,477 | |||||||||
Income from continuing operations | 5,657 | 12,496 | 4,505 | 10,796 | |||||||||
Income from discontinued operations, net of income taxes | 3,364 | 2,944 | 2,514 | 3,480 | |||||||||
Gain on sale of discontinued operations, net of income taxes | — | — | — | 35,855 | |||||||||
| | | | | | | | | | | | | | |
Income from discontinued operations | 3,364 | 2,944 | 2,514 | 39,335 | |||||||||
| | | | | | | | | | | | | | |
Net income | $ | 9,021 | $ | 15,440 | $ | 7,019 | $ | 50,131 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net income per share—basic | |||||||||||||
Continuing operations | $ | 0.12 | $ | 0.27 | $ | 0.10 | $ | 0.24 | |||||
Discontinued operations | 0.07 | 0.06 | 0.06 | 0.88 | |||||||||
| | | | | | | | | | | | | | |
Net income per share | $ | 0.19 | $ | 0.33 | $ | 0.16 | $ | 1.12 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net income per share—diluted | |||||||||||||
Continuing operations | $ | 0.12 | $ | 0.27 | $ | 0.10 | $ | 0.24 | |||||
Discontinued operations | 0.07 | 0.06 | 0.05 | 0.86 | |||||||||
| | | | | | | | | | | | | | |
Net income per share | $ | 0.19 | $ | 0.33 | $ | 0.15 | $ | 1.10 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Fiscal year ended December 29, 2012: | |||||||||||||
Net sales | $ | 157,603 | $ | 167,607 | $ | 161,353 | $ | 162,136 | |||||
Gross profit | 66,135 | 65,382 | 55,750 | 57,942 | |||||||||
Income from continuing operations | 15,831 | 17,655 | 12,433 | 14,155 | |||||||||
Income from discontinued operations, net of income taxes | 2,942 | 2,826 | 1,799 | 3,310 | |||||||||
| | | | | | | | | | | | | | |
Net income | $ | 18,773 | $ | 20,481 | $ | 14,232 | $ | 17,465 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net income per share—basic | |||||||||||||
Continuing operations | $ | 0.34 | $ | 0.38 | $ | 0.27 | $ | 0.30 | |||||
Discontinued operations | 0.06 | 0.06 | 0.04 | 0.07 | |||||||||
| | | | | | | | | | | | | | |
Net income per share | $ | 0.40 | $ | 0.44 | $ | 0.31 | $ | 0.37 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Net income per share—diluted | |||||||||||||
Continuing operations | $ | 0.34 | $ | 0.37 | $ | 0.26 | $ | 0.30 | |||||
Discontinued operations | 0.06 | 0.06 | 0.04 | 0.07 | |||||||||
| | | | | | | | | | | | | | |
Net income per share | $ | 0.40 | $ | 0.43 | $ | 0.30 | $ | 0.37 | |||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
71
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors
The Company's senior notes are unconditionally guaranteed, jointly and severally, on a senior basis by certain of the Company's 100% owned subsidiaries ("Guarantors"). Management has determined that separate complete financial statements of the Guarantors would not be material to users of the financial statements.
The following sets forth condensed consolidating financial statements of the Guarantors and non-Guarantor subsidiaries.
Condensed consolidating balance sheet
December 28, 2013
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Assets | ||||||||||||||||
Cash and cash equivalents | $ | — | $ | 87,082 | $ | 76,341 | $ | — | $ | 163,423 | ||||||
Accounts receivable, net | 32,860 | 80,646 | — | — | 113,506 | |||||||||||
Inventories | 38,974 | 74,886 | — | — | 113,860 | |||||||||||
Prepaid and other | 7,541 | 8,503 | 2,074 | — | 18,118 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total current assets | 79,375 | 251,117 | 78,415 | — | 408,907 | |||||||||||
Due from affiliates | 551,141 | 903,815 | 467,441 | (1,922,397 | ) | — | ||||||||||
Investment in subsidiaries | 129,588 | 577,878 | 755,626 | (1,463,092 | ) | — | ||||||||||
Property, plant and equipment, net | 303,888 | 291,487 | — | — | 595,375 | |||||||||||
Goodwill | — | — | 444,512 | — | 444,512 | |||||||||||
Intangibles and loan acquisition costs, net | — | — | 93,792 | — | 93,792 | |||||||||||
Other | 727 | 6,755 | 145 | — | 7,627 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total assets | $ | 1,064,719 | $ | 2,031,052 | $ | 1,839,931 | $ | (3,385,489 | ) | $ | 1,550,213 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Liabilities and shareholders' equity | ||||||||||||||||
Accounts payable and accrued liabilities | $ | 26,865 | $ | 40,916 | $ | 13,249 | $ | — | $ | 81,030 | ||||||
Income taxes payable | — | 3,873 | 182 | — | 4,055 | |||||||||||
Current portion of debt | — | — | 16,875 | — | 16,875 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total current liabilities | 26,865 | 44,789 | 30,306 | — | 101,960 | |||||||||||
Due to affiliates | 510,356 | 851,659 | 560,382 | (1,922,397 | ) | — | ||||||||||
Debt, less current portion | — | — | 629,375 | — | 629,375 | |||||||||||
Pension obligations, less current portion | — | 102,821 | — | — | 102,821 | |||||||||||
Deferred income taxes and other | 51,401 | 44,788 | 292 | — | 96,481 | |||||||||||
Shareholders' equity | 476,097 | 986,995 | 619,576 | (1,463,092 | ) | 619,576 | ||||||||||
| | | | | | | | | | | | | | | | | |
Total liabilities and shareholders' equity | $ | 1,064,719 | $ | 2,031,052 | $ | 1,839,931 | $ | (3,385,489 | ) | $ | 1,550,213 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
72
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating balance sheet
December 29, 2012
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Assets | ||||||||||||||||
Cash and cash equivalents | $ | — | $ | 28,098 | $ | 16,775 | $ | — | $ | 44,873 | ||||||
Accounts receivable, net | 36,980 | 85,076 | — | — | 122,056 | |||||||||||
Inventories | 41,616 | 73,846 | — | — | 115,462 | |||||||||||
Prepaid and other | 24,656 | 19,246 | 538 | — | 44,440 | |||||||||||
Assets of discontinued operations | 27,566 | 25,165 | 39,530 | — | 92,261 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total current assets | 130,818 | 231,431 | 56,843 | — | 419,092 | |||||||||||
Due from affiliates | 554,190 | 330,148 | 482,869 | (1,367,207 | ) | — | ||||||||||
Investment in subsidiaries | 123,765 | 381,295 | 636,860 | (1,141,920 | ) | — | ||||||||||
Property, plant and equipment, net | 316,128 | 291,338 | — | — | 607,466 | |||||||||||
Goodwill | — | — | 444,512 | — | 444,512 | |||||||||||
Intangibles and loan acquisition costs, net | — | — | 107,006 | — | 107,006 | |||||||||||
Other | 727 | 7,269 | — | — | 7,996 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total assets | $ | 1,125,628 | $ | 1,241,481 | $ | 1,728,090 | $ | (2,509,127 | ) | $ | 1,586,072 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Liabilities and shareholders' equity | ||||||||||||||||
Accounts payable and accrued liabilities | $ | 24,279 | $ | 45,286 | $ | 4,557 | $ | — | $ | 74,122 | ||||||
Income taxes payable | — | 723 | 880 | — | 1,603 | |||||||||||
Current portion of debt | — | — | 50,000 | — | 50,000 | |||||||||||
Liabilities of discontinued operations | 15,222 | 4,354 | — | — | 19,576 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total current liabilities | 39,501 | 50,363 | 55,437 | — | 145,301 | |||||||||||
Due to affiliates | 579,388 | 344,398 | 443,421 | (1,367,207 | ) | — | ||||||||||
Debt, less current portion | — | — | 646,250 | — | 646,250 | |||||||||||
Pension obligations, less current portion | — | 103,491 | — | — | 103,491 | |||||||||||
Deferred income taxes and other | 65,367 | 42,681 | 145 | — | 108,193 | |||||||||||
Shareholders' equity | 441,372 | 700,548 | 582,837 | (1,141,920 | ) | 582,837 | ||||||||||
| | | | | | | | | | | | | | | | | |
Total liabilities and shareholders' equity | $ | 1,125,628 | $ | 1,241,481 | $ | 1,728,090 | $ | (2,509,127 | ) | $ | 1,586,072 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
73
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating statement of income
Year ended December 28, 2013
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net sales | $ | 186,767 | $ | 449,515 | $ | — | $ | — | $ | 636,282 | ||||||
Cost of goods sold | 79,026 | 336,527 | — | — | 415,553 | |||||||||||
| | | | | | | | | | | | | | | | | |
Gross profit | 107,741 | 112,988 | — | — | 220,729 | |||||||||||
Selling, general and administrative expenses | 61,179 | 47,959 | 23,654 | — | 132,792 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income (loss) | 46,562 | 65,029 | (23,654 | ) | — | 87,937 | ||||||||||
Interest expense and other | (7,282 | ) | 7,895 | 39,581 | — | 40,194 | ||||||||||
Equity in earnings of subsidiaries | — | — | (116,699 | ) | 116,699 | — | ||||||||||
| | | | | | | | | | | | | | | | | |
Income from continuing operations before income taxes | 53,844 | 57,134 | 53,464 | (116,699 | ) | 47,743 | ||||||||||
Income taxes | 25,979 | 16,827 | (28,517 | ) | — | 14,289 | ||||||||||
| | | | | | | | | | | | | | | | | |
Income from continuing operations | 27,865 | 40,307 | 81,981 | (116,699 | ) | 33,454 | ||||||||||
Income from discontinued operations, net of income taxes | 5,561 | 7,111 | (370 | ) | — | 12,302 | ||||||||||
Gain on sale of discontinued operations, net of income taxes | 1,565 | 34,290 | — | — | 35,855 | |||||||||||
| | | | | | | | | | | | | | | | | |
Income from discontinued operations | 7,126 | 41,401 | (370 | ) | — | 48,157 | ||||||||||
| | | | | | | | | | | | | | | | | |
Net income | $ | 34,991 | $ | 81,708 | $ | 81,611 | $ | (116,699 | ) | $ | 81,611 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
74
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating statement of income
Year ended December 29, 2012
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net sales | $ | 165,051 | $ | 483,648 | $ | — | $ | — | $ | 648,699 | ||||||
Cost of goods sold | 44,029 | 359,461 | — | — | 403,490 | |||||||||||
| | | | | | | | | | | | | | | | | |
Gross profit | 121,022 | 124,187 | — | — | 245,209 | |||||||||||
Selling, general and administrative expenses | 61,152 | 47,156 | 13,146 | — | 121,454 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income (loss) | 59,870 | 77,031 | (13,146 | ) | — | 123,755 | ||||||||||
Interest expense and other | (9,404 | ) | 7,311 | 38,029 | — | 35,936 | ||||||||||
Write-off of loan acquisition costs associated with refinancing of senior credit agreement | — | — | 2,478 | — | 2,478 | |||||||||||
Equity in earnings of subsidiaries | — | — | (100,859 | ) | 100,859 | — | ||||||||||
| | | | | | | | | | | | | | | | | |
Income from continuing operations before income taxes | 69,274 | 69,720 | 47,206 | (100,859 | ) | 85,341 | ||||||||||
Income taxes | 32,917 | 16,835 | (24,485 | ) | — | 25,267 | ||||||||||
| | | | | | | | | | | | | | | | | |
Income from continuing operations | 36,357 | 52,885 | 71,691 | (100,859 | ) | 60,074 | ||||||||||
Income from discontinued operations, net of income taxes | 4,481 | 7,136 | (740 | ) | — | 10,877 | ||||||||||
| | | | | | | | | | | | | | | | | |
Net income | $ | 40,838 | $ | 60,021 | $ | 70,951 | $ | (100,859 | ) | $ | 70,951 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
75
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating statement of income
Year ended December 31, 2011
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net sales | $ | 224,462 | $ | 461,263 | $ | — | $ | — | $ | 685,725 | ||||||
Cost of goods sold | 47,578 | 338,652 | — | — | 386,230 | |||||||||||
| | | | | | | | | | | | | | | | | |
Gross profit | 176,884 | 122,611 | — | — | 299,495 | |||||||||||
Selling, general and administrative expenses | 71,936 | 51,737 | 6,167 | — | 129,840 | |||||||||||
| | | | | | | | | | | | | | | | | |
Operating income (loss) | 104,948 | 70,874 | (6,167 | ) | — | 169,655 | ||||||||||
Interest expense and other | (8,936 | ) | 5,603 | 35,767 | — | 32,434 | ||||||||||
Equity in earnings of subsidiaries | — | — | (123,890 | ) | 123,890 | — | ||||||||||
| | | | | | | | | | | | | | | | | |
Income from continuing operations before income taxes | 113,884 | 65,271 | 81,956 | (123,890 | ) | 137,221 | ||||||||||
Income taxes | 51,484 | 18,598 | (24,024 | ) | — | 46,058 | ||||||||||
| | | | | | | | | | | | | | | | | |
Income from continuing operations | 62,400 | 46,673 | 105,980 | (123,890 | ) | 91,163 | ||||||||||
Income from discontinued operations, net of income taxes | 5,861 | 8,956 | (740 | ) | — | 14,077 | ||||||||||
| | | | | | | | | | | | | | | | | |
Net income | $ | 68,261 | $ | 55,629 | $ | 105,240 | $ | (123,890 | ) | $ | 105,240 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
76
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating statement of comprehensive income
Year ended December 28, 2013
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net income | $ | 34,991 | $ | 81,708 | $ | 81,611 | $ | (116,699 | ) | $ | 81,611 | |||||
Foreign currency translation adjustment, net of income tax expense of $638 | — | 7,515 | (859 | ) | (3,728 | ) | 2,928 | |||||||||
Change in net actuarial loss and prior service credit, net of income tax expense of $3,647 | 569 | 5,991 | — | — | 6,560 | |||||||||||
Equity in earnings of subsidiaries | — | — | 10,347 | (10,347 | ) | — | ||||||||||
| | | | | | | | | | | | | | | | | |
Comprehensive income | $ | 35,560 | $ | 95,214 | $ | 91,099 | $ | (130,774 | ) | $ | 91,099 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Condensed consolidating statement of comprehensive income
Year ended December 29, 2012
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net income | $ | 40,838 | $ | 60,021 | $ | 70,951 | $ | (100,859 | ) | $ | 70,951 | |||||
Foreign currency translation adjustment, net of income tax expense of $249 | — | 10,313 | (332 | ) | (172 | ) | 9,809 | |||||||||
Change in net actuarial loss and prior service credit, net of income tax benefit of $5,848 | (306 | ) | (14,729 | ) | — | — | (15,035 | ) | ||||||||
Equity in earnings of subsidiaries | — | — | (4,894 | ) | 4,894 | — | ||||||||||
| | | | | | | | | | | | | | | | | |
Comprehensive income | $ | 40,532 | $ | 55,605 | $ | 65,725 | $ | (96,137 | ) | $ | 65,725 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Condensed consolidating statement of comprehensive income
Year ended December 31, 2011
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net income | $ | 68,261 | $ | 55,629 | $ | 105,240 | $ | (123,890 | ) | $ | 105,240 | |||||
Foreign currency translation adjustment, net of income tax benefit of $895 | — | (12,706 | ) | 116 | 6 | (12,584 | ) | |||||||||
Change in net actuarial loss and prior service credit, net of income tax benefit of $2,187 | (111 | ) | (4,701 | ) | — | — | (4,812 | ) | ||||||||
Equity in earnings of subsidiaries | — | — | (17,512 | ) | 17,512 | — | ||||||||||
| | | | | | | | | | | | | | | | | |
Comprehensive income | $ | 68,150 | $ | 38,222 | $ | 87,844 | $ | (106,372 | ) | $ | 87,844 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
77
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating statement of cash flows
Year ended December 28, 2013
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net cash provided by (used in) operating activities | $ | 94,473 | $ | 65,345 | $ | (27,658 | ) | $ | 22,300 | $ | 154,460 | |||||
Investing activities: | ||||||||||||||||
Purchases of property, plant and equipment, net | (4,838 | ) | (23,397 | ) | — | — | (28,235 | ) | ||||||||
Net proceeds from the sale of discontinued operations | — | 55,108 | 61,505 | — | 116,613 | |||||||||||
| | | | | | | | | | | | | | | | | |
Net cash provided by (used in) investing activities | (4,838 | ) | 31,711 | 61,505 | — | 88,378 | ||||||||||
Financing activities: | ||||||||||||||||
Principal payments on debt | — | — | (15,000 | ) | — | (15,000 | ) | |||||||||
Payments on revolving credit facility | — | — | (89,200 | ) | — | (89,200 | ) | |||||||||
Proceeds from revolving credit facility | — | — | 54,200 | — | 54,200 | |||||||||||
Repurchases of common stock | — | — | (80,668 | ) | — | (80,668 | ) | |||||||||
Proceeds from stock option exercises | — | — | 2,319 | — | 2,319 | |||||||||||
Excess tax benefit from stock-based compensation | — | — | 1,163 | — | 1,163 | |||||||||||
Noncontrolling interest | — | — | 1,960 | — | 1,960 | |||||||||||
Intercompany transactions, net | (89,635 | ) | (39,010 | ) | 150,945 | (22,300 | ) | — | ||||||||
| | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | (89,635 | ) | (39,010 | ) | 25,719 | (22,300 | ) | (125,226 | ) | |||||||
Effect of exchange rate changes on cash and cash equivalents | — | 938 | — | — | 938 | |||||||||||
| | | | | | | | | | | | | | | | | |
Net increase in cash and cash equivalents | — | 58,984 | 59,566 | — | 118,550 | |||||||||||
Cash and cash equivalents at beginning of year | — | 28,098 | 16,775 | — | 44,873 | |||||||||||
| | | | | | | | | | | | | | | | | |
Cash and cash equivalents at end of year | $ | — | $ | 87,082 | $ | 76,341 | $ | — | $ | 163,423 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
78
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating statement of cash flows
Year ended December 29, 2012
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net cash provided by (used in) operating activities | $ | 92,466 | $ | 51,826 | $ | (34,012 | ) | $ | (5,494 | ) | $ | 104,786 | ||||
Investing activities: | ||||||||||||||||
Purchases of property, plant and equipment, net | (115,663 | ) | (21,448 | ) | — | — | (137,111 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Net cash used in investing activities | (115,663 | ) | (21,448 | ) | — | — | (137,111 | ) | ||||||||
Financing activities: | ||||||||||||||||
Principal payments on debt | — | (117 | ) | (4,557 | ) | — | (4,674 | ) | ||||||||
Payments on revolving credit facility | — | — | (15,000 | ) | — | (15,000 | ) | |||||||||
Proceeds from stock option exercises | — | — | 1,338 | — | 1,338 | |||||||||||
Excess tax benefit from stock-based compensation | — | — | 338 | — | 338 | |||||||||||
Noncontrolling interest | — | — | (242 | ) | — | (242 | ) | |||||||||
Proceeds from new senior credit agreement | — | — | 350,000 | — | 350,000 | |||||||||||
Principal payments in connection with refinancing of senior credit agreement | — | (41,865 | ) | (300,426 | ) | — | (342,291 | ) | ||||||||
Loan acquisition costs | — | — | (6,228 | ) | — | (6,228 | ) | |||||||||
Intercompany transactions, net | 23,197 | (27,176 | ) | (1,515 | ) | 5,494 | — | |||||||||
| | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | 23,197 | (69,158 | ) | 23,708 | 5,494 | (16,759 | ) | |||||||||
Effect of exchange rate changes on cash and cash equivalents | — | 1,383 | — | — | 1,383 | |||||||||||
| | | | | | | | | | | | | | | | | |
Net decrease in cash and cash equivalents | — | (37,397 | ) | (10,304 | ) | — | (47,701 | ) | ||||||||
Cash and cash equivalents at beginning of year | — | 65,495 | 27,079 | — | 92,574 | |||||||||||
| | | | | | | | | | | | | | | | | |
Cash and cash equivalents at end of year | $ | — | $ | 28,098 | $ | 16,775 | $ | — | $ | 44,873 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
79
Polypore International, Inc.
Notes to consolidated financial statements (Continued)
20. Financial Statements of Guarantors (Continued)
Condensed consolidating statement of cash flows
Year ended December 31, 2011
(in thousands) | Combined Guarantor Subsidiaries | Combined Non-Guarantor Subsidiaries | The Company | Eliminations | Consolidated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net cash provided by (used in) operating activities | $ | 145,862 | $ | 50,084 | $ | (53,039 | ) | $ | 1,926 | $ | 144,833 | |||||
Investing activities: | ||||||||||||||||
Purchases of property, plant and equipment, net | (113,648 | ) | (42,682 | ) | — | — | (156,330 | ) | ||||||||
| | | | | | | | | | | | | | | | | |
Net cash used in investing activities | (113,648 | ) | (42,682 | ) | — | — | (156,330 | ) | ||||||||
Financing activities: | ||||||||||||||||
Principal payments on debt | — | (485 | ) | (4,034 | ) | — | (4,519 | ) | ||||||||
Proceeds from stock option exercises | — | — | 6,658 | — | 6,658 | |||||||||||
Excess tax benefit from stock-based compensation | — | — | 14,136 | — | 14,136 | |||||||||||
Noncontrolling interest | — | — | 1,936 | — | 1,936 | |||||||||||
Loan acquisition costs | — | — | (627 | ) | — | (627 | ) | |||||||||
Intercompany transactions, net | (32,214 | ) | 3,874 | 30,266 | (1,926 | ) | — | |||||||||
| | | | | | | | | | | | | | | | | |
Net cash provided by (used in) financing activities | (32,214 | ) | 3,389 | 48,335 | (1,926 | ) | 17,584 | |||||||||
Effect of exchange rate changes on cash and cash equivalents | — | (3,468 | ) | — | — | (3,468 | ) | |||||||||
| | | | | | | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | — | 7,323 | (4,704 | ) | — | 2,619 | ||||||||||
Cash and cash equivalents at beginning of year | — | 58,172 | 31,783 | — | 89,955 | |||||||||||
| | | | | | | | | | | | | | | | | |
Cash and cash equivalents at end of year | $ | — | $ | 65,495 | $ | 27,079 | $ | — | $ | 92,574 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
80
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
An evaluation of the effectiveness of the Company's disclosure controls and procedures (as defined in Rule 13a-15(e) or 15d-15(e) promulgated under the Exchange Act) was performed under the supervision, and with the participation of, the Company's management, including the Chief Executive Officer and Chief Financial Officer. The Company's disclosure controls are designed to ensure that information required to be disclosed in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. Based upon our evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of December 28, 2013 to ensure that information required to be disclosed in the reports that we file under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and is accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Management's Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Exchange Act). Management has conducted an assessment of the effectiveness of the Company's internal control over financial reporting using the criteria inInternal Control—Integrated Framework, issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with United States generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Based on its assessment, management has concluded that the Company maintained effective internal control over financial reporting as of December 28, 2013, based on criteria inInternal Control—Integrated Framework issued by the COSO.
The effectiveness of the Company's internal control over financial reporting as of December 28, 2013 has been audited by Ernst & Young LLP, an independent registered public accounting firm. Ernst & Young LLP has issued an attestation report to the Company's internal control over financial reporting, which appears in Item 8 of Part II of this Annual Report on Form 10-K under the heading "Report of Independent Registered Public Accounting Firm."
Changes in Internal Control over Financial Reporting
During the Company's fourth fiscal quarter of fiscal year 2013, there has been no change in the Company's internal control over financial reporting (as defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting.
81
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item is incorporated by reference from the information contained in our Proxy Statement to be filed with the Securities and Exchange Commission in connection with our 2014 Annual Meeting of Stockholders to be held on May 13, 2014.
Members of our Board of Directors and all of our employees, including our Chief Executive Officer and Chief Financial Officer, are required to abide by our Code of Business Conduct and Ethics to ensure that our business is conducted in a consistently legal and ethical manner. The full text of the Code of Business Conduct and Ethics is published on our website athttp://investor.polypore.net/governance.cfm. We will disclose any future amendments to, or waivers from, these ethical policies and standards for senior officers and directors on our website within four business days following the date of such amendment or waiver.
Item 11. Executive Compensation
The information required by this Item is incorporated by reference from the information contained in our Proxy Statement to be filed with the Securities and Exchange Commission in connection with our 2014 Annual Meeting of Stockholders to be held on May 13, 2014.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item is incorporated by reference from the information contained in our Proxy Statement to be filed with the Securities and Exchange Commission in connection with our 2014 Annual Meeting of Stockholders to be held on May 13, 2014.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated by reference from the information contained in our Proxy Statement to be filed with the Securities and Exchange Commission in connection with our 2014 Annual Meeting of Stockholders to be held on May 13, 2014.
Item 14. Principal Accountant Fees and Services
The information required by this Item is incorporated by reference from the information contained in our Proxy Statement to be filed with the Securities and Exchange Commission in connection with our 2014 Annual Meeting of Stockholders to be held on May 13, 2014.
Item 15. Exhibits and Financial Statement Schedules
(a) Documents filed as part of this report:
- •
- Reports of Independent Registered Public Accounting Firm
- •
- Consolidated Balance Sheets as of December 28, 2013 and December 29, 2012
- •
- Consolidated Statements of Income for the years ended December 28, 2013, December 29, 2012 and December 31, 2011
1. Financial Statements. The following items, including Consolidated Financial Statements of the Company, are set forth in Item 8 of this Annual Report on Form 10-K:
82
- •
- Consolidated Statements of Comprehensive Income for the years ended December 28, 2013, December 29, 2012 and December 31, 2011
- •
- Consolidated Statements of Shareholders' Equity for the years ended December 28, 2013, December 29, 2012 and December 31, 2011
- •
- Consolidated Statements of Cash Flows for the years ended December 28, 2013, December 29, 2012 and December 31, 2011
- •
- Notes to Consolidated Financial Statements
- •
- Valuation and Qualifying Accounts for the years ended December 28, 2013, December 29, 2012 and December 31, 2011
2. Financial Statement Schedules. The following schedule is set forth on page S-1 of this Annual Report on Form 10-K.
Information required by other schedules has either been incorporated in the consolidated financial statements and accompanying notes or is not applicable to us.
3. Exhibits.
| Exhibit Number | Exhibit Description | ||
|---|---|---|---|
| 3.1 | Form of Amended and Restated Certificate of Incorporation of Polypore International, Inc. (incorporated by reference to Exhibit 3.1 to Amendment No. 4 to the Company's Registration Statement on Form S-1 filed on June 15, 2007 (Commission File No. 333-141273)) | ||
3.2 | Form of Second Amended and Restated Bylaws of Polypore International, Inc.. adopted on October 30, 2012 (incorporated by reference to Exhibit 3.1 to the Company's Form 10-Q filed on November 1, 2012) | ||
3.3 | Certificate of Amendment to the Certificate of Incorporation of Polypore International, Inc. (incorporated by reference to Exhibit 3.1 to the Company's Current Report on Form 8-K filed on June 29, 2007) | ||
4.1 | First Supplemental Indenture, dated as of November 30, 2010, among Polypore International, Inc., the guarantors named therein and the Bank of New York Mellon, as trustee (incorporated by reference to Exhibit 4.3 to the Company's Current Report on Form 8-K filed on December 2, 2010) | ||
4.2 | Indenture, dated as of November 26, 2010 among Polypore International, Inc., the guarantors named therein and The Bank of New York Mellon, as trustee (incorporated by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K filed on December 2, 2010) (the "7.5% Indenture") | ||
4.3 | Form of 7.5% Senior Notes due 2017 (incorporated by reference to Exhibit B of the 7.5% Indenture) | ||
4.4 | Registration Rights Agreement, dated as of November 26, 2010, among Polypore International, Inc., the guarantors named therein and J.P. Morgan Securities LLC for itself and on behalf of several purchasers listed therein (incorporated by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed on December 2, 2010) | ||
10.1 | Tax Sharing Agreement, dated as of May 13, 2004, by and among Polypore International, Inc., PP Holding Corporation and Polypore, Inc. (incorporated by reference to Exhibit 10.1 to the Company's Form 10-Q filed on May 9, 2013) | ||
83
| Exhibit Number | Exhibit Description | ||
|---|---|---|---|
| 10.2 | * | Form of Director and Officer Indemnification Agreement entered into between Polypore, Inc. and certain employees of Polypore, Inc. (incorporated by reference to Exhibit 10.12 to the Company's Registration Statement on Form S-4 filed on April 18, 2005 (Commission File No. 333-124142)) | |
10.3 | * | Amended and Restated Employment Agreement, dated as of April 28, 2008, by and between Polypore International, Inc. and Robert B. Toth (incorporated by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on May 1, 2008) | |
10.4 | * | Amendment to Employment Agreement, dated as of December 18, 2008, by and between Polypore International, Inc. and Robert B. Toth (incorporated by reference to Exhibit 10.8 to the Company's Annual Report on Form 10-K filed on March 12, 2009) | |
10.5 | * | Employment Agreement, dated as of April 7, 2006, by and between Polypore, Inc. and Mitchell J. Pulwer (incorporated by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on August 15, 2006) | |
10.6 | * | Amended Employment Agreement, dated as of December 18, 2008, by and between Polypore International, Inc. and Mitchell J. Pulwer (incorporated by reference to Exhibit 10.10 to the Company's Annual Report on Form 10-K filed on March 12, 2009) | |
10.7 | * | Employment Agreement, dated as of April 4, 2006, by and between Membrana GmbH, a subsidiary of Polypore, Inc. and Josef Sauer (incorporated by reference to Exhibit 10.3 to the Company's Quarterly Report on Form 10-Q filed on August 15, 2006) | |
10.8 | * | Polypore International, Inc. 2006 Stock Option Plan (incorporated by reference to Exhibit 10.4 to the Company's Quarterly Report on Form 10-Q filed on August 15, 2006) | |
10.9 | * | Form of Executive Severance Plan Policy (incorporated by reference to Exhibit 10.13 to the Company's Annual Report on Form 10-K filed on March 12, 2009) | |
10.10 | * | Polypore International, Inc. 2007 Stock Incentive Plan (Amended and Restated effective as of May 12, 2011) (incorporated by reference to Exhibit 99.1 to the Company's Registration Statement on Form S-8 filed on May 13, 2011 (Commission File No. 333-174172)) | |
10.11 | * | Amendment to the Polypore International, Inc. 2007 Stock Incentive Plan, effective as of November 30, 2011 (incorporated by reference to Exhibit 10.11 to the Company's Annual Report on Form 10-K filed February 26, 2013) | |
10.12 | * | Form of Restricted Stock Grant Notice and Agreement under the Polypore International, Inc. 2007 Stock Incentive Plan (incorporated by reference to Exhibit 99.2 to the Company's Registration Statement on Form S-8 filed on May 13, 2011 (Commission File No. 333-174172)) | |
10.13 | * | Form of Option Grant Notice and Agreement under the Polypore International, Inc. 2007 Stock Incentive Plan (incorporated by reference to Exhibit 10.13 to the Company's Annual Report on Form 10-K filed February 26, 2013) | |
10.14 | Underwriting Agreement, dated as of December 7, 2010, among certain stockholders of Polypore International, Inc. and Barclays Capital Inc. (incorporated by reference to Exhibit 1.1 to the Company's Current Report on Form 8-K filed on December 13, 2010) | ||
10.15 | Underwriting Agreement, dated as of March 17, 2011, among certain stockholders of Polypore International, Inc. and Barclays Capital Inc. (incorporated by reference to Exhibit 1.1 to the Company's Current Report on Form 8-K filed on March 23, 2011) | ||
84
| Exhibit Number | Exhibit Description | ||
|---|---|---|---|
| 10.16 | * | Employment Agreement, dated as of April 1, 2011, by and among Daramic LLC and certain of its affiliates, Polypore International, Inc. and Pierre Hauswald (incorporated by reference to Exhibit 10.1 to the Company's Periodic Report on Form 10-Q filed on May 6, 2011) | |
10.17 | * | Employment Contract, dated as of April 1, 2011, by and between Daramic SAS and Pierre Hauswald (incorporated by reference to Exhibit 10.2 to the Company's Periodic Report on Form 10-Q filed on May 6, 2011) | |
10.18 | * | Agreement Organizing the Extension of the Expatriation of Mr. Pierre Hauswald in P.R. China with a Local Employment Contract, dated April 1, 2011, between Daramic SAS and Pierre Hauswald (incorporated by reference to Exhibit 10.3 to the Company's Periodic Form on 10-Q filed on May 6, 2011) | |
10.19 | Amended and Restated Credit Agreement, dated as of June 29, 2012, among Polypore International, Inc., JPMorgan Chase Bank, N.A., as Administrative Agent; Bank of America, N.A. and Wells Fargo Bank, National Association, as Syndication Agents; BBVA Compass Bank, PNC Bank, National Association, HSBC Bank USA, National Association, and Fifth Third Bank, as Co-Documentation Agents; and J.P. Morgan Securities, LLC, Merrill Lynch, Pierce, Fenner & Smith Incorporated and Wells Fargo Securities, LLC as Joint Lead Arrangers and Joint Bookrunners (incorporated by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q filed on August 2, 2012) | ||
10.20 | * | Polypore International, Inc. Deferred Savings Plan (incorporated by reference to Exhibit 4.1 to the Company's Registration Statement on Form S-8 filed on December 28, 2012 (Commission File No. 333-185741)) | |
10.21 | * | Form of Change in Control Agreement entered into between Polypore International, Inc. and certain senior executives (incorporated by reference to Exhibit 10.2 to the Company's Form 10-Q filed on May 9, 2013) | |
10.22 | Stock Purchase Agreement, dated as of September 27, 2013, by and among Daramic Acquisition Corp., Polypore BV, Polypore International, Inc. (solely for purposes of Section 11.18 thereunder), Seven Mile Capital Partners Top, Inc. and SASR Zweiundfünfzigste Beteiligungsverwaltung GmbH (incorporated by reference to Exhibit 99.1 to the Company's Current Report on Form 8-K filed on October 1, 2013) | ||
21.1 | Subsidiaries of Polypore International, Inc. | ||
23.1 | Consent of Independent Registered Public Accounting Firm | ||
31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | ||
31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | ||
32.1 | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | ||
32.2 | Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | ||
101 | Interactive Data Files | ||
- *
- Management contract or compensatory plan or arrangement
85
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| POLYPORE INTERNATIONAL, INC. | ||||
By: | /s/ ROBERT B. TOTH Robert B. Toth President and Chief Executive Officer | |||
Date: February 25, 2014
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| /s/ ROBERT B. TOTH Robert B. Toth | Chairman of the Board, President, Chief Executive Officer and Director (Principal Executive Officer) | February 25, 2014 | ||
/s/ LYNN AMOS Lynn Amos | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | February 25, 2014 | ||
/s/ MICHAEL GRAFF Michael Graff | Lead Independent Director of the Board | February 25, 2014 | ||
/s/ CHARLES L. COONEY Charles L. Cooney | Director | February 25, 2014 | ||
/s/ WILLIAM DRIES William Dries | Director | February 25, 2014 | ||
/s/ FREDERICK C. FLYNN, JR. Frederick C. Flynn, Jr. | Director | February 25, 2014 | ||
/s/ MICHAEL CHESSER Michael Chesser | Director | February 25, 2014 | ||
/s/ CHRISTOPHER J. KEARNEY Christopher J. Kearney | Director | February 25, 2014 | ||
/s/ DAVID A. ROBERTS David A. Roberts | Director | February 25, 2014 |
86
Polypore International, Inc.
Financial statement schedule—Valuation and qualifying accounts
For the years ended December 28, 2013, December 29, 2012 and December 31, 2011:
| | | Additions | | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(in thousands) | Balance at beginning of year | Charged to costs and expenses | Charged to other accounts | Deductions | Balance at end of year | |||||||||||
Year ended December 28, 2013: | ||||||||||||||||
Allowance for doubtful accounts | $ | 2,995 | $ | 732 | $ | 47 | (1) | $ | (512) | (2) | $ | 3,262 | ||||
Valuation allowance for deferred tax asset | 3,797 | (1,572 | ) | — | — | 2,225 | ||||||||||
| | | | | | | | | | | | | | | | | |
| $ | 6,792 | $ | (840 | ) | $ | 47 | $ | (512 | ) | $ | 5,487 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Year ended December 29, 2012: | ||||||||||||||||
Allowance for doubtful accounts | $ | 2,648 | $ | 565 | $ | 79 | (1) | $ | (297) | (2) | $ | 2,995 | ||||
Valuation allowance for deferred tax asset | 9,580 | (158 | ) | (5,625) | (3) | — | 3,797 | |||||||||
| | | | | | | | | | | | | | | | | |
| $ | 12,228 | $ | 407 | $ | (5,546 | ) | $ | (297 | ) | $ | 6,792 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Year ended December 31, 2011: | ||||||||||||||||
Allowance for doubtful accounts | $ | 2,482 | $ | 290 | $ | (57) | (1) | $ | (67) | (2) | $ | 2,648 | ||||
Valuation allowance for deferred tax asset | 8,613 | 967 | — | — | 9,580 | |||||||||||
| | | | | | | | | | | | | | | | | |
| $ | 11,095 | $ | 1,257 | $ | (57 | ) | $ | (67 | ) | $ | 12,228 | |||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Foreign currency translation adjustment.
- (2)
- Charge-offs net of recoveries.
- (3)
- Reduction in valuation allowance as the benefit of certain net operating loss carryforwards that were previously fully offset by a valuation allowance will not be realized.
S-1