Exhibit 99.1

Enhancing T - Cell Therapies in Oncology OTCQB: HGEN www.humanigen.com 1

Forward - Looking Statements This presentation contains forward - looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . Forward - looking statements reflect management’s current knowledge, assumptions, judgment and expectations regarding future performance or events . Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and actual results could differ materially from the forward - looking statements . Words such as “will,” “expect,” “intend,” “plan,” “predict,” “potential,” “possible,” and similar expressions identify forward - looking statements, including, without limitation, statements related to the scope, progress, expansion, and costs of developing and commercializing the Company’s product candidates ; opportunity to benefit from anticipated regulatory incentives for product candidates ; and anticipated expenses related to development activities, clinical trials and the development and potential commercialization of product candidates . Forward - looking statements are subject to risks and uncertainties including, but not limited to, the Company’s lack of revenues, history of operating losses, limited cash reserves and ability to obtain additional capital to develop and commercialize its product candidates, including the additional capital which will be necessary to complete the clinical trials that the Company has initiated or plans to initiate, and continue as a going concern ; the Company’s ability to execute its strategy and business plan ; the potential timing and outcomes of clinical studies of lenzilumab , ifabotuzumab , HGEN 005 or any other product candidates and the uncertainties inherent in clinical testing ; the ability of the Company to timely source adequate supply of its development products from third - party manufacturers on which the Company depends ; the potential, if any, for future development of any of its present or future products ; the Company's ability to successfully progress, partner or complete further development of its programs ; the ability of the Company to identify and develop additional products ; the Company's ability to attain market exclusivity or to protect its intellectual property ; competition ; changes in the regulatory landscape that may prevent the Company from pursuing or realizing any of the expected benefits from the various regulatory incentives at the center of its strategy, or the imposition of regulations that affect the Company's products ; and the various risks described in the "Risk Factors" and elsewhere in the Company's periodic and other filings with the Securities and Exchange Commission . You are cautioned not to place undue reliance on any forward - looking statements, which speak only as of the date of this presentation . The company has no obligation, and expressly disclaims any obligation to update, revise or correct any of the forward - looking statements, whether as a result of new information, future events or otherwise 2

Executive Summary (1 of 2) CAR - T, one of the most exciting fields in biotech, is now a reality with 2 FDA - approved therapies available; Neurotoxicity (NT) and Cytokine Release Syndrome (CRS) adverse effects are significant impediments to CAR - T uptake and utility Evidence indicates that GM - CSF may be the key initiator or ‘master switch’ that triggers a cascade that leads to NT and/or CRS Our proprietary monoclonal antibody, lenzilumab , blocks GM - CSF, and has the potential to modulate ‘off target’ toxicities and ‘on target’ efficacy - making CAR - T safer, more effective and a more routine out - patient procedure Top KOLs back scientific rationale for lenzilumab as prophylaxis for NT/CRS and proactively approached company to initiate pivotal trials Lenzilumab offers potential for significant share shift for a companion CAR - T vs. its competitors, preferred formulary position and pharmaco - economic benefits to payers 3

Executive Summary CAR - T market expanding rapidly with movement into earlier lines of hematologic cancers, solid tumors and combination therapy; significant investment in space Lenzilumab alongside other portfolio assets may offer CAR - T platform Additional potential pipeline value: Lenzilumab phase I trial in chronic myelomonocytic leukemia (CMML) fully recruited Ifabotuzumab phase I in glioblastoma multiforme (GBM) underway with positive early signs, plus ADC development HGEN005 preclinical asset for multiple serious eosinophilic diseases Comparable valuations highly attractive; potential for significant near term transactions 4

Experienced , Execution - Focused Leadership and Board; Extensive CAR - T and Immuno - Oncology Expertise Cameron Durrant, MD, MBA CEO o Pharma and serial biotech exec o Pharmacia/Pfizer, J+J in the US; Merck, GSK in Europe o Biotech Exec Chairman, Chairman, CEO, CFO Greg Jester, CPA CFO o Decades of financial, operational and entrepreneurial experience in life sciences o VP, Finance, Tris Pharma o CFO at private and public pharma companies, including Alvogen and Innovive Tarek Sahmoud, MD, PhD CMO o Novartis CAR - T clinical development consultant and former VP, Senior Clinical Program Head ( o ncology) o Oncology dev expert: Celgene Global Head Clinical Dev (Corporate VP); BMS, Exec Dir, Global Medical Affairs o Registered Arimidex , Afinitor ; responsibility for Kymriah , Yervoy , Abraxane , marizomib , dasatinib Bob Savage, MBA Board member o Former Worldwide Chairman, J+J Pharmaceuticals o Former President, Worldwide Therapeutics and Inflammation, Pharmacia o Extensive biotech board experience (12 boards over 20 years, including Depomed , The Medicines Company, Savient , Panacos , Noven , Epicept , Vela) Rainer Boehm, MD, MBA Board member o Former interim CEO, Novartis Pharma o Former Chief Commercial and Chief Medical Affairs Officer, Novartis Pharma and EVP, Novartis Oncology o Board member, Cellectis (allogeneic CAR - T company) Tim Morris, CPA Board member o CFO Iovance , a leading immuno - oncology public biotech ~$1.5Bn market cap o Raised almost $1Bn in equity and equity - linked securities in 22 years as a CFO o Extensive deal experience: >70 transactions, combined value $2Bn Ron Barliant, JD Board member o Of counsel with Goldberg and Kohn o US bankruptcy judge for N. Illinois for 14 years, extensive legal and bankruptcy experience o Counseled companies, management and boards in distressed and complex situations 5
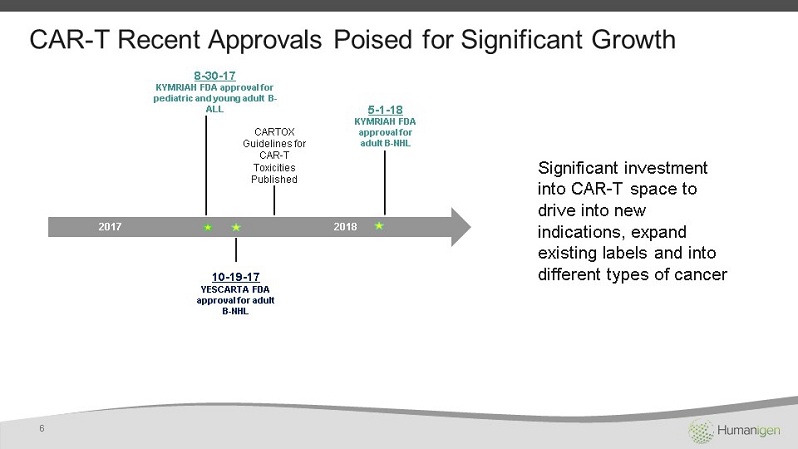
CAR - T Recent Approvals Poised for Significant Growth 5 - 1 - 18 KYMRIAH FDA approval for adult B - NHL 10 - 19 - 17 YESCARTA FDA approval for adult B - NHL CARTOX Guidelines for CAR - T Toxicities Published 2017 2018 8 - 30 - 17 KYMRIAH FDA approval for pediatric and young adult B - ALL 6 Significant investment into CAR - T space to drive into new indications, expand existing labels and into different types of cancer CD1

Example: Kite/Gilead Commitment to CAR - T Expansion in Hem/ Onc 7 Source: Gilead Q2 2018 Earnings Call Deck Slide
Big Commercial, Partnering Opportunity to Enhance T Cell Therapy 8
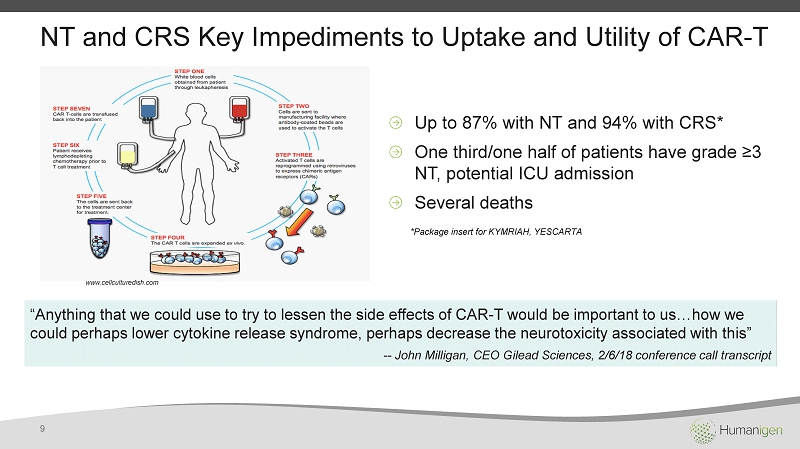
NT and CRS Key Impediments to Uptake and Utility of CAR - T Up to 87% with NT and 94% with CRS* One third/one half of patients have grade ≥3 NT, potential ICU admission Several deaths * Package insert for KYMRIAH, YESCARTA “ Anything that we could use to try to lessen the side effects of CAR - T would be important to us…how we could perhaps lower cytokine release syndrome, perhaps decrease the neurotoxicity associated with this” -- John Milligan, CEO Gilead Sciences, 2/6/18 conference call transcript www.cellculturedish.com 9
Potential Role of GM - CSF as ‘Master Switch’ in CAR - T Toxicities o GM - CSF generated from CAR - T cells, which come into contact with myeloid cells immediately upon infusion, stimulate other cytokines, trigger further trafficking and damage, leading to NT and/or CRS o In CAR - T clinical trials, GM - CSF levels were elevated in patients with severe NT and patients with severe NT had significantly higher levels of myeloid cells in CSF o Close - to - human pre - clinical models (using patient - derived cell lines, human CAR - T and PBMCs) have demonstrated lenzilumab along with CD19 targeted chimeric antigen receptor T - cell therapy (CART19) reduces NT and CRS and enhances CART19 proliferation and effector functions 10
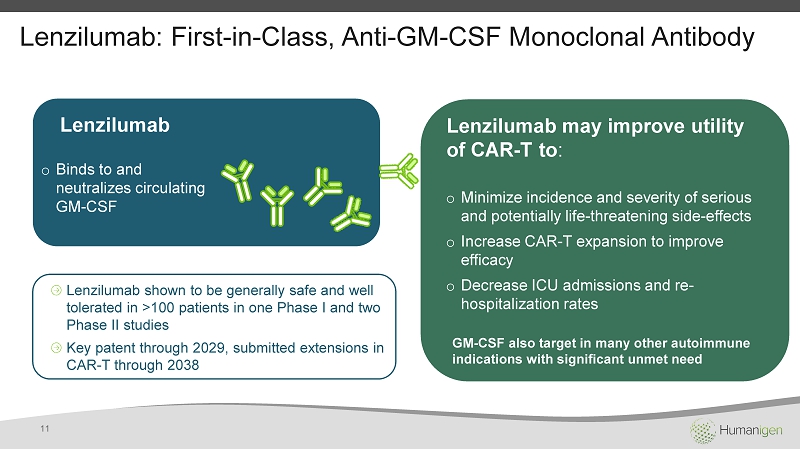
Lenzilumab : First - in - Class, Anti - GM - CSF Monoclonal Antibody Lenzilumab o Binds to and neutralizes circulating GM - CSF Lenzilumab shown to be generally safe and well tolerated in >100 patients in one Phase I and two Phase II studies Key patent through 2029, submitted extensions in CAR - T through 2038 o Minimize incidence and severity of serious and potentially life - threatening side - effects o Increase CAR - T expansion to improve efficacy o Decrease ICU admissions and re - hospitalization rates Lenzilumab may improve utility of CAR - T to : GM - CSF also target in many other autoimmune indications with significant unmet need 11

Lenzilumab Proven Pre - Clinical Safety and Toxicity Package ▪ Cardiovascular, Respiratory and CNS Safety Pharmacology Study (Single Escalating Dose @ 1, 10 and 100 mg/kg in Cyno monkey): No significant findings ▪ 28 - Day Toxicity Study with a 4 week recovery period (Repeat Weekly Dosing @ 10, 50 and 100 mg/kg in Cyno monkey): No significant findings ▪ 26 - Week Toxicity Study with an 8 week recovery period (Repeat Weekly Dosing @ 10, 50 and 100 mg/kg in Cyno monkey) ▪ NOAEL up to 100 mg/kg/ wk (32 mg/kg Human Equivalent Dose) ▪ Supports multi - dose human studies ▪ Passive and active immunization with anti - GM - CSF is safe in animal models • Pulmonary Alveolar Proteinosis (PAP) seen in GM - CSF K/O mice • Animals do not have neutropenia and can clear infections 12
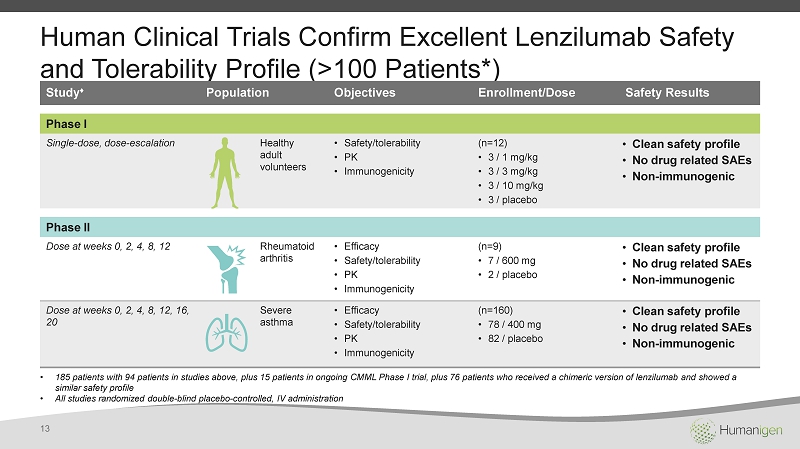
Human Clinical Trials Confirm Excellent Lenzilumab Safety and Tolerability Profile (> 100 Patients *) Study ♦ Population Objectives Enrollment /Dose Safety Results Phase I Single - dose, dose - escalation Healthy adult volunteers • Safety/tolerability • PK • Immunogenicity (n=12) • 3 / 1 mg/kg • 3 / 3 mg/kg • 3 / 10 mg/kg • 3 / placebo • Clean safety profile • No drug related SAEs • Non - immunogenic Phase II Dose at weeks 0, 2, 4, 8, 12 Rheumatoid arthritis • Efficacy • Safety/tolerability • PK • Immunogenicity (n=9) • 7 / 600 mg • 2 / placebo • Clean safety profile • No drug related SAEs • Non - immunogenic Dose at weeks 0, 2, 4, 8, 12, 16, 20 Severe asthma • Efficacy • Safety/tolerability • PK • Immunogenicity (n=160) • 78 / 400 mg • 82 / placebo • Clean safety profile • No drug related SAEs • Non - immunogenic • 185 patients with 94 patients in studies above , plus 15 patients in ongoing CMML Phase I trial, plus 76 patients who received a chimeric version of lenzilumab and showed a similar safety profile • All studies randomized double - blind placebo - controlled, IV administration 13
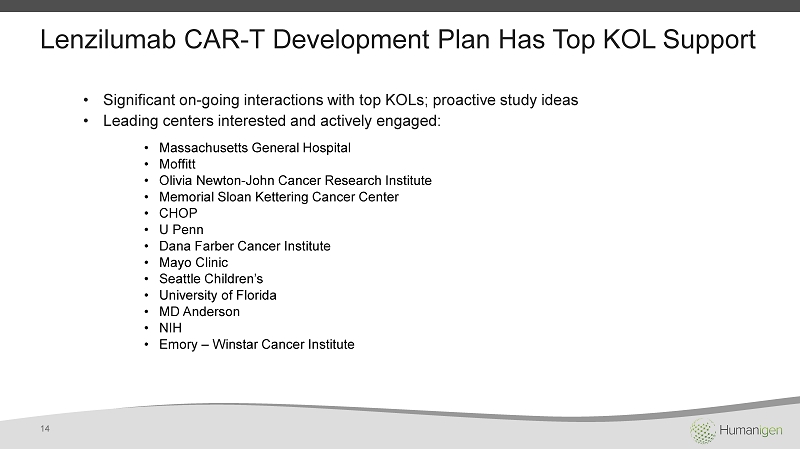
Lenzilumab CAR - T Development Plan Has Top KOL Support • Significant on - going interactions with top KOLs; proactive study ideas • Leading centers interested and actively engaged: • Massachusetts General Hospital • Moffitt • Olivia Newton - John Cancer Research Institute • Memorial Sloan Kettering Cancer Center • CHOP • U Penn • Dana Farber Cancer Institute • Mayo Clinic • Seattle Children’s • University of Florida • MD Anderson • NIH • Emory – Winstar Cancer Institute 14

Pivotal/Registration Studies with Marketed CAR - Ts in Labeled Indications o Parallel/Staggered Approach 1. Investigator - initiated studies 1H 2019 in cooperation with CAR - T players 2 . Humanigen Pre - IND meeting o Discuss potential for registration on existing studies and/or ��� next - step” adaptive design “registration standard” clinical study o Potential for breakthrough designation; Orphan designation; pediatrics o Investigating opportunity for registration study with strategic partner 15
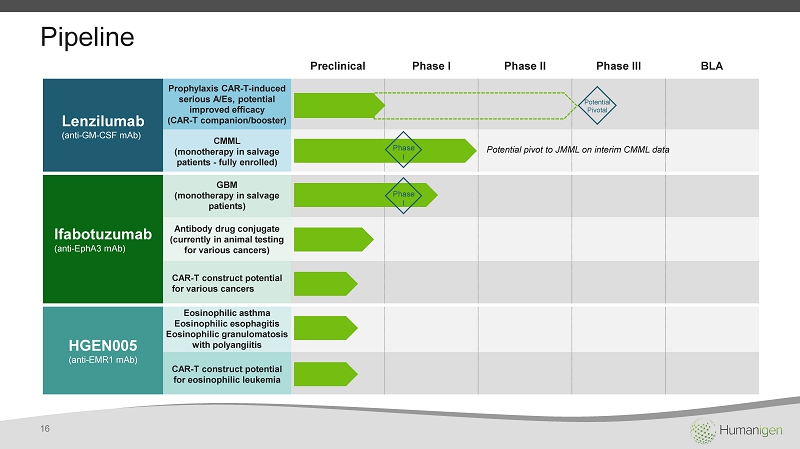
Pipeline Preclinical Phase I Phase II Phase III BLA Lenzilumab (anti - GM - CSF mAb ) Prophylaxis CAR - T - induced serious A/ Es , potential improved efficacy (CAR - T companion/booster) CMML (monotherapy in salvage patients - fully enrolled) Ifabotuzumab (anti - EphA3 mAb ) GBM (monotherapy in salvage patients) Antibody drug conjugate (currently in animal testing for various cancers) CAR - T construct potential for various cancers HGEN005 (anti - EMR1 mAb ) Eosinophilic asthma Eosinophilic esophagitis Eosinophilic granulomatosis with polyangiitis CAR - T construct potential for eosinophilic leukemia Phase I Phase I Potential pivot to JMML on interim CMML data Potential Pivotal 16
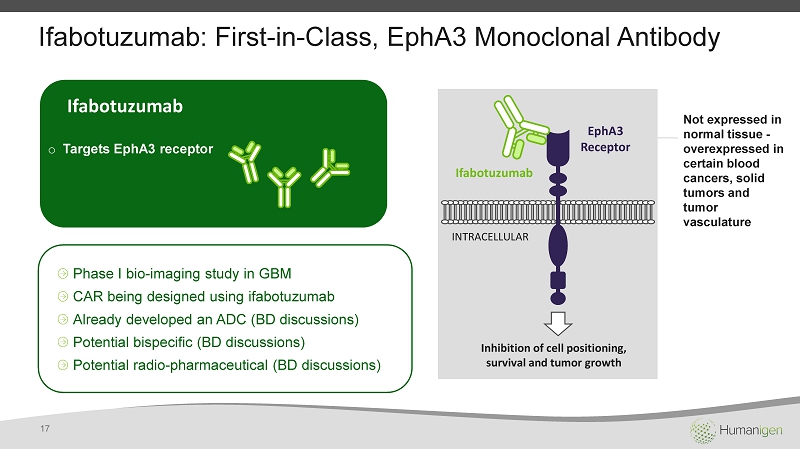
Ifabotuzumab : First - in - Class, EphA3 Monoclonal Antibody Ifabotuzumab o Targets EphA3 receptor Phase I bio - imaging study in GBM CAR being designed using ifabotuzumab Already developed an ADC (BD discussions) Potential bispecific (BD discussions) Potential radio - pharmaceutical (BD discussions) EphA3 Receptor Ifabotuzumab INTRACELLULAR Inhibition of cell positioning, survival and tumor growth Not expressed in normal tissue - overexpressed in certain blood cancers, solid tumors and tumor vasculature 17
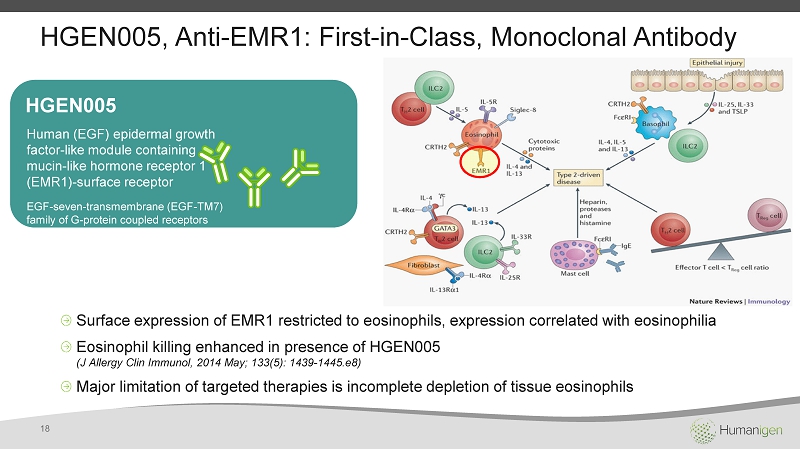
HGEN005 , Anti - EMR1: First - in - Class, Monoclonal Antibody HGEN005 Human (EGF) epidermal growth factor - like module containing mucin - like hormone receptor 1 (EMR1 ) - surface receptor EGF - seven - transmembrane (EGF - TM7) family of G - protein coupled receptors Surface expression of EMR1 restricted to eosinophils, expression correlated with eosinophilia Eosinophil killing enhanced in presence of HGEN005 (J Allergy Clin Immunol , 2014 May; 133(5): 1439 - 1445.e8) Major limitation of targeted therapies is incomplete depletion of tissue eosinophils 18

Summary Lenzilumab has the potential to make CAR - T safer, more effective and a more routine out - patient procedure Clinical/regulatory plan developed for optimal value and delivery of key data near term Additional potential pipeline value: Lenzilumab in CMML fully recruited Ifabotuzumab in glioblastoma multiforme (GBM) underway with positive early signs HGEN005 preclinical asset for multiple serious eosinophilic diseases CAR platform generation utilizing ifabotuzumab and HGEN005 Comparable valuations highly attractive; potential for significant transactions 19


















