- HGENQ Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Humanigen (HGENQ) 8-KOther Events
Filed: 2 Dec 21, 8:00am
Exhibit 99.1

Humanigen Discussion of The Lancet Respiratory Medicine Publications & Corporate Update December 2, 2021

2 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Cautionary Note Regarding Forward - Looking Statements All statements other than statements of historical facts contained in this press release are forward - looking statements . Forward - looking statements reflect management's current knowledge, assumptions, judgment, and expectations regarding future performance or events . Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and you should be aware that actual events or results may differ materially from those contained in the forward - looking statements . Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward - looking statements, including, without limitation, statements regarding : Humanigen’s beliefs as to the potential benefits of lenzilumab as a treatment for hospitalized COVID - 19 patients ; its beliefs as to the potential of lenzilumab to improve patient survival when used before ICU admission and progression of respiratory failure ; statements regarding the therapeutic potential of targeting a single upstream cytokine earlier in the COVID - 19 disease process ; its efforts to request and receive Conditional Marketing Authorization for lenzilumab in COVID - 19 in the UK and other territories ; its beliefs and projections regarding the need for lenzilumab as a therapeutic if authorized or approved ; the company’s projections for anticipated supply of lenzilumab through the end of 2022 ; the effectiveness of its preparations to commercialize lenzilumab in the UK and other markets, if CMA or other marketing approval were granted ; its efforts to mitigate its manufacturing expenses in future periods pending receipt of a marketing authorization or approval from a regulatory agency such as MHRA, EMA or FDA ; its ability to resolve payment disputes with certain of its CMOs and other service providers on favorable terms ; and its other plans to initiate or participate in planned clinical trials and otherwise explore the effectiveness of lenzilumab and other candidates in its development portfolio as therapies for other inflammation and immune - oncology indications . Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in the company’s lack of profitability and need for additional capital to conduct its business ; its dependence on partners to further the development of its product candidates ; the uncertainties inherent in the development, attainment of the requisite regulatory authorizations and approvals and launch of any new pharmaceutical product ; challenges associated with manufacturing and commercializing a biologic such as lenzilumab ; the outcome of pending or future litigation ; and the various risks and uncertainties described in the "Risk Factors" sections and elsewhere in Humanigen's periodic and other filings with the Securities and Exchange Commission . All forward - looking statements are expressly qualified in their entirety by this cautionary notice . You should not rely upon any forward - looking statements as predictions of future events . The Company undertakes no obligation to revise or update any forward - looking statements made in this press release to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law .

Dr. Cameron Durrant, Chairman & Chief Executive Officer Introduction

4 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Respiratory Medicine Peer - Reviewed Paper Detailing Positive Results of LIVE - AIR Phase 3 study

5 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Robust Clinical Pipeline Indication Phase Status Centers Partners Lenzilumab LIVE - AIR Hospitalized COVID - 19 3 Completed (520 patients) 24 US 11 Brazil Company sponsored ACTIV - 5/BET - B Hospitalized COVID - 19 (DSMB recommended continuation following safety and futility interim analysis) 2/3 Enrolling (400 patients CRP<150 mg/L, up to 550 patients total) >80% enrolled Up to 70 sites SHIELD Prophylaxis with commercially available CAR - T therapies in non - Hodgkin lymphoma 3 Enrollment to Start 1H22 ( ~ 250 patients) Approximately 30 sites Company sponsored RATinG Prevention/Treatment of Acute GvHD ( aGvHD ) 2/3 Enrollment to Start 1H22 Up to 22 sites 1 PREACH - M Chronic myelomonocytic leukemia (CMML) 2/3 First Patient Dosed October 2021 5 sites 2 Ifabotuzumab Solid Tumors (Glioblastoma Multiforme) 1 Completed (12 patients) 1 Site 2 Note: DSMB - Data Safety Monitoring Board, CRP - C - Reactive Protein normal CRP<10 mg/L 1. UK 2. Australia

Dr. Dale Chappell, Chief Scientific Officer The Lancet Respiratory Medicine Publications

7 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Peer - Reviewed Paper Detailing Positive Results of LIVE - AIR Phase 3 study

8 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Independent Expert Commentary
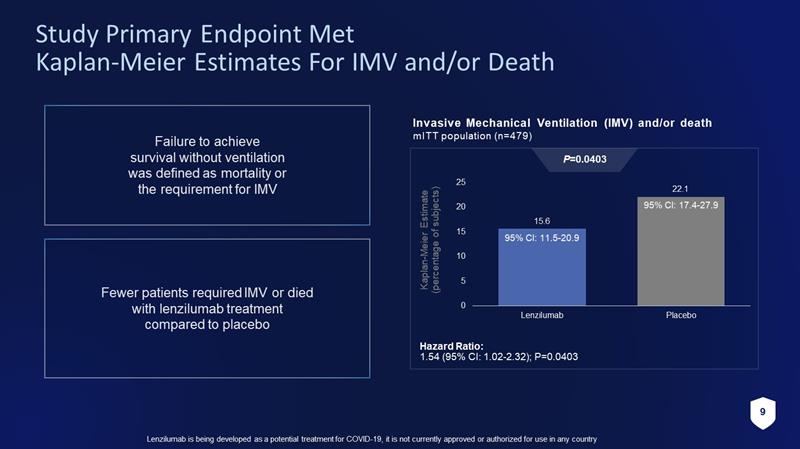
9 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Study Primary Endpoint Met Kaplan - Meier Estimates For IMV and/or Death Failure to achieve survival without ventilation was defined as mortality or the requirement for IMV Fewer patients required IMV or died with lenzilumab treatment compared to placebo 15.6 22.1 0 5 10 15 20 25 Lenzilumab Placebo 95% CI: 17.4 - 27.9 95% CI: 11.5 - 20.9 Invasive Mechanical Ventilation (IMV) and/or death mITT population (n=479) P =0.0403 Hazard Ratio: 1.54 (95% CI: 1.02 - 2.32); P=0.0403 Kaplan - Meier Estimate (percentage of subjects)
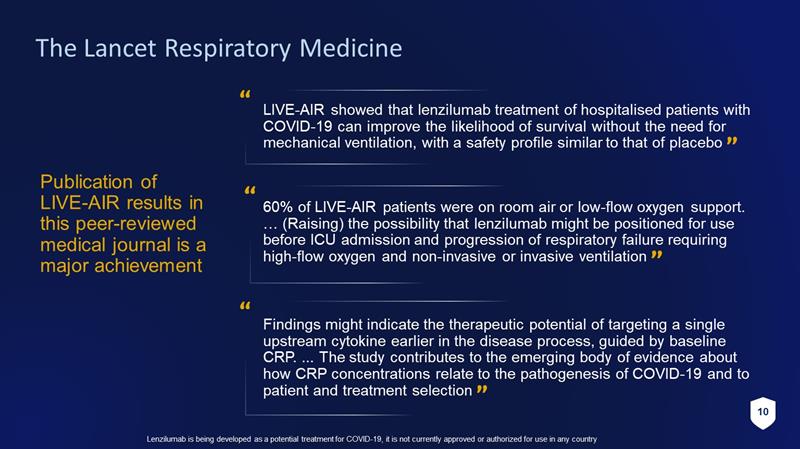
10 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry The Lancet Respiratory Medicine Publication of LIVE - AIR results in this peer - reviewed medical journal is a major achievement Findings might indicate the therapeutic potential of targeting a single upstream cytokine earlier in the disease process, guided by baseline CRP. ... The study contributes to the emerging body of evidence about how CRP concentrations relate to the pathogenesis of COVID - 19 and to patient and treatment selection 60% of LIVE - AIR patients were on room air or low - flow oxygen support. … (Raising) the possibility that lenzilumab might be positioned for use before ICU admission and progression of respiratory failure requiring high - flow oxygen and non - invasive or invasive ventilation LIVE - AIR showed that lenzilumab treatment of hospitalised patients with COVID - 19 can improve the likelihood of survival without the need for mechanical ventilation, with a safety profile similar to that of placebo
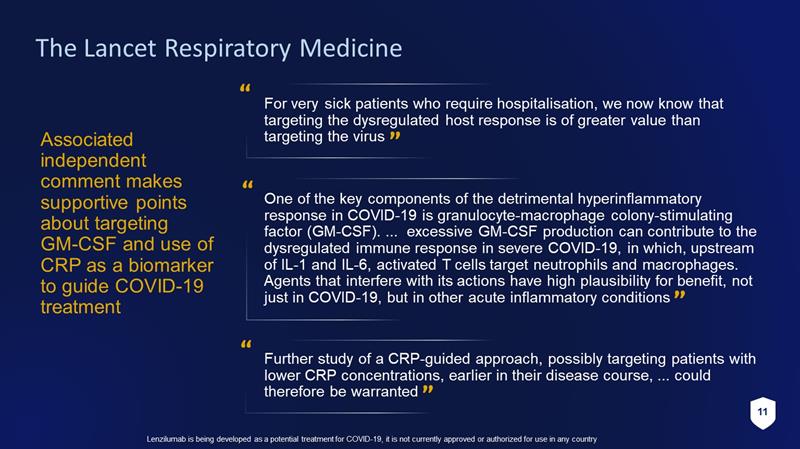
11 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry The Lancet Respiratory Medicine Associated independent comment makes supportive points about targeting GM - CSF and use of CRP as a biomarker to guide COVID - 19 treatment For very sick patients who require hospitalisation , we now know that targeting the dysregulated host response is of greater value than targeting the virus Further study of a CRP - guided approach, possibly targeting patients with lower CRP concentrations, earlier in their disease course, ... could therefore be warranted One of the key components of the detrimental hyperinflammatory response in COVID - 19 is granulocyte - macrophage colony - stimulating factor (GM - CSF). ... excessive GM - CSF production can contribute to the dysregulated immune response in severe COVID - 19, in which, upstream of IL - 1 and IL - 6, activated T cells target neutrophils and macrophages. Agents that interfere with its actions have high plausibility for benefit, not just in COVID - 19, but in other acute inflammatory conditions
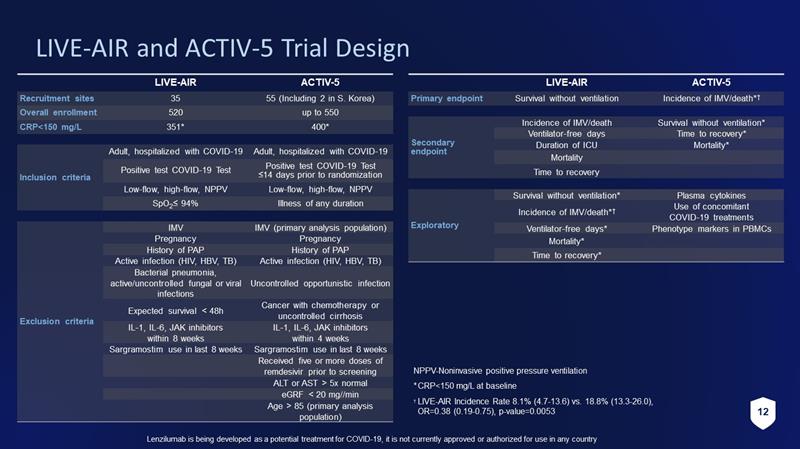
12 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry LIVE - AIR ACTIV - 5 Recruitment sites 35 55 (Including 2 in S. Korea) Overall enrollment 520 up to 550 CRP<150 mg/L 351* 400* Inclusion criteria Adult, hospitalized with COVID - 19 Adult, hospitalized with COVID - 19 Positive test COVID - 19 Test Positive test COVID - 19 Test ≤14 days prior to randomization Low - flow, high - flow, NPPV Low - flow, high - flow, NPPV SpO 2 ≤ 94% Illness of any duration Exclusion criteria IMV IMV (primary analysis population) Pregnancy Pregnancy History of PAP History of PAP Active infection (HIV, HBV, TB) Active infection (HIV, HBV, TB) Bacterial pneumonia, active/uncontrolled fungal or viral infections Uncontrolled opportunistic infection Expected survival < 48h Cancer with chemotherapy or uncontrolled cirrhosis IL - 1, IL - 6, JAK inhibitors within 8 weeks IL - 1, IL - 6, JAK inhibitors within 4 weeks Sargramostim use in last 8 weeks Sargramostim use in last 8 weeks Received five or more doses of remdesivir prior to screening ALT or AST > 5x normal eGRF < 20 mg//min Age > 85 (primary analysis population) LIVE - AIR and ACTIV - 5 Trial Design LIVE - AIR ACTIV - 5 Primary endpoint Survival without ventilation Incidence of IMV/death* † Secondary endpoint Incidence of IMV/death Survival without ventilation* Ventilator - free days Time to recovery* Duration of ICU Mortality* Mortality Time to recovery Exploratory Survival without ventilation* Plasma cytokines Incidence of IMV/death* † Use of concomitant COVID - 19 treatments Ventilator - free days* Phenotype markers in PBMCs Mortality* Time to recovery* NPPV - Noninvasive positive pressure ventilation * CRP<150 mg/L at baseline † LIVE - AIR Incidence Rate 8.1% (4.7 - 13.6) vs. 18.8% (13.3 - 26.0), OR=0.38 (0.19 - 0.75), p - value=0.0053

Dr. Cameron Durrant, Chairman & Chief Executive Officer Regulatory Progress

14 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Regulatory Progress Lenzilumab for COVID - 19 1. Dec. 1, Lancet Respiratory Medicine publishes peer - reviewed paper and commentary on positive LIVE - AIR Phase 3 trial 2. FDA Type B meeting to occur in December 2021 to discuss additional data shared and path forward following September 8, 2021 FDA response to EUA submission ▪ No safety issues identified by FDA, noted relatively limited size of safety database

15 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry UK MHRA and Other Key Agencies Meetings held with Rapid C - 19 multi - agencies (MHRA, Therapeutics Task Force (TTF), NICE, NHSE, DHSC) Marketing Authorization Application (MAA) accepted for rolling review July 7, 2021, classified as “COVID rolling review” All planned modules submitted September 2021 Further data from LIVE - AIR has been furnished to MHRA, similar to the data furnished to FDA following EUA assessment letter Received requests for additional information and analysis NICE Scientific Advice Meeting to plan reimbursement model December meeting with MHRA to discuss requests for additional information and analysis Formal response to MHRA requests planned for 1Q22 Supply chain and local UK distributor agreements MHRA Conditional Marketing Authorization Leading Therapeutics Approvals *Tocilizumab * Molnupiravir † Sarilumab *Tocilizumab and molnupiravir authorized by MHRA prior to FDA † Sarilumab conditional recommendation in NICE treatment guidelines despite multiple Phase 3 failures, not authorized for COVID - 19 by FDA
16 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry European Commission Ranks Lenzilumab as One of Ten Most Promising COVID - 19 Treatments Selected for highest potential impact on the COVID - 19 pandemic Independent expert panel report • Lenzilumab selected after evaluation of 82 late - stage therapeutic candidates • One of only four immunomodulators selected • Lenzilumab only immunomodulator targeting GM - CSF selected from four evaluated Expert findings regarding lenzilumab • May provide additional clinical value above corticosteroids • No important safety concerns • Variant agnostic, not affected by new SARS - CoV - 2 variants Countries

17 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Regulatory Progress Lenzilumab for COVID - 19 1. Dec. 1, Lancet Respiratory Medicine publishes peer - reviewed paper and commentary on positive LIVE - AIR Phase 3 trial 2. FDA Type B meeting to occur in December 2021 to discuss additional data shared and path forward following September 8, 2021 FDA response to EUA submission ▪ No safety issues identified by FDA, noted relatively limited size of safety database 3. MHRA actively reviewing Marketing Authorization Application (MAA) seeking Conditional Marketing Authorization ▪ NICE Scientific Advice Meeting to plan reimbursement model ▪ December meeting to discuss MHRA requests for further information ▪ Formal Humanigen response planned for 1Q22 4. European Commission selected lenzilumab as one of ten most promising COVID - 19 therapeutics October 2021 ▪ EMA evaluating pre - submission for rolling review

Dr. Adrian Kilcoyne, Chief Medical Officer Pipeline Progress
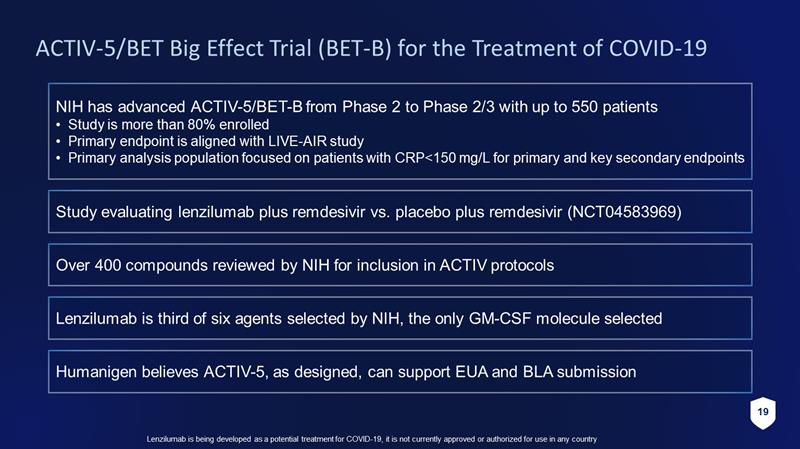
19 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Study evaluating lenzilumab plus remdesivir vs. placebo plus remdesivir (NCT04583969) Over 400 compounds reviewed by NIH for inclusion in ACTIV protocols ACTIV - 5/BET Big Effect Trial (BET - B) for the Treatment of COVID - 19 Lenzilumab is third of six agents selected by NIH, the only GM - CSF molecule selected NIH has advanced ACTIV - 5/BET - B from Phase 2 to Phase 2/3 with up to 550 patients • Study is more than 80% enrolled • Primary endpoint is aligned with LIVE - AIR study • Primary analysis population focused on patients with CRP<150 mg/L for primary and key secondary endpoints Humanigen believes ACTIV - 5, as designed, can support EUA and BLA submission
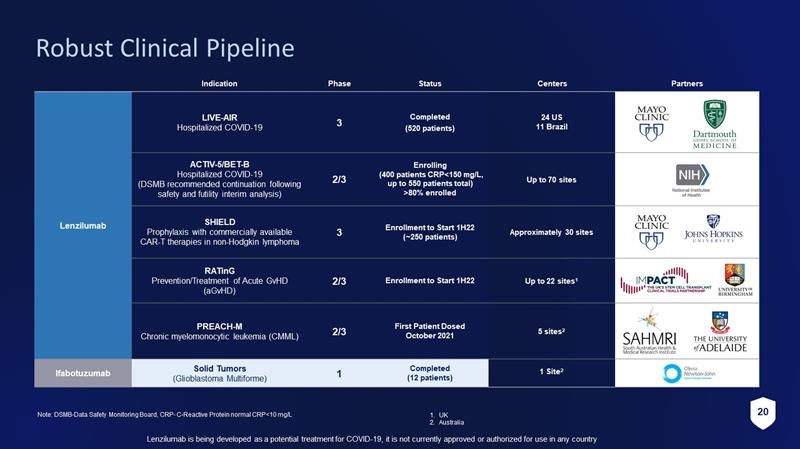
20 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Robust Clinical Pipeline Indication Phase Status Centers Partners Lenzilumab LIVE - AIR Hospitalized COVID - 19 3 Completed (520 patients) 24 US 11 Brazil Company sponsored ACTIV - 5/BET - B Hospitalized COVID - 19 (DSMB recommended continuation following safety and futility interim analysis) 2/3 Enrolling (400 patients CRP<150 mg/L, up to 550 patients total) >80% enrolled Up to 70 sites SHIELD Prophylaxis with commercially available CAR - T therapies in non - Hodgkin lymphoma 3 Enrollment to Start 1H22 ( ~ 250 patients) Approximately 30 sites Company sponsored RATinG Prevention/Treatment of Acute GvHD ( aGvHD ) 2/3 Enrollment to Start 1H22 Up to 22 sites 1 PREACH - M Chronic myelomonocytic leukemia (CMML) 2/3 First Patient Dosed October 2021 5 sites 2 Ifabotuzumab Solid Tumors (Glioblastoma Multiforme) 1 Completed (12 patients) 1 Site 2 Note: DSMB - Data Safety Monitoring Board, CRP - C - Reactive Protein normal CRP<10 mg/L 1. UK 2. Australia

21 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry PREACH – M Trial Phase 2/3 for CMML PREACH – M Trial — First Patient Dosed October 2021 Partners Newly Diagnosed CMML Primary Endpoint 12 Months CR and PR Azacitadine + Sodium Ascorbate Up to 24 Cycles (2 Years) Azacitadine + Lenzilumab Up to 24 Cycles (2 Years) High Risk Disease (NRAS, KRAS, CBL) Low Risk Disease (TET2) ~13 cases per 100,000 persons per year 2 Incidence higher with age 1 Survival remains dismal 1 Effective therapies needed 3 CMML is an aggressive Cancer 1 Note: CMML - Chronic Myelomonocytic Leukemia TET2, NRAS, KRAS, and CBL are gene mutations found in CMML patients 1. Murthy et al, Leukemia Lymphoma , 2017 Jul;58(7):1648 - 1654 2. SEER Hematopoietic and Lymphoid Neoplasm Database (cancer.gov) 3. Aim of first - ever CMML study – to improve survival, Leukaemia Foundation – Australia News Story, June 3, 2021 https://www.leukaemia.org.au/stories/aim - of - first - ever - cmml - study - to - improve - survival/

22 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry aGvHD High Unmet Need; Lenzilumab RATinG Study Protocol Allogeneic Stem Cell Transplant aGvHD Diagnosis Outcome prediction using “MAGIC” algorithm Standard of Care (SOC) Lenzilumab + SOC V Placebo + SOC Low Risk The “ RATinG ” Study Enrollment to Start 1H22 Initial treatment with steroids 1 - 5 ~50% of patients do not respond adequately to steroids 1 - 5 Steroid - resistant patients have >90% mortality 6 1. First and second - line systemic treatment of acute graft - versus - host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant . 2021;18(8):1150 - 1163 2. Diagnosis and management of acute graft - versus - host disease. Br J Haematol . 2012;158(1):30 - 45. 3. Response of 443 patients to steroids as primary therapy for acute graft - versus - host disease: comparison of grading systems. Biol Blood Marrow Transplant . 2002;8(7):387 - 394. 4. EBMT - NI - CIBMTR Task Force position statement of standardized terminology & guidance for graft - versus - host disease assessment. Bone Marrow Transplant . 2018;53(11):1401 - 1415. 5. New and emerging therapies for acute and chronic graft - versus - host disease. Ther Adv Hermatol . 2018;9(1):21 - 46. 6. Steroid - refractory acute GVHD: Predictions and outcomes. Advances in Hematology 2011
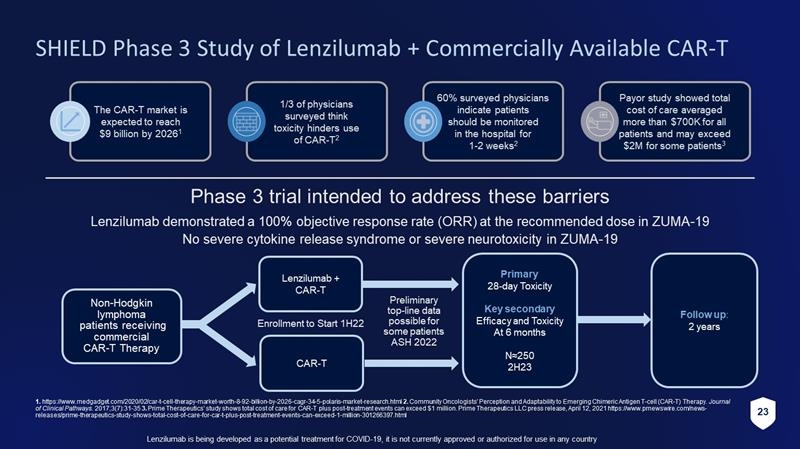
23 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry SHIELD Phase 3 Study of Lenzilumab + Commercially Available CAR - T Phase 3 trial intended to address these barriers Non - Hodgkin lymphoma patients receiving commercial CAR - T Therapy Lenzilumab + CAR - T CAR - T Primary 28 - day Toxicity Key secondary Efficacy and Toxicity At 6 months N≈250 2H23 Follow up : 2 years Enrollment to Start 1H22 Lenzilumab demonstrated a 100% objective response rate (ORR) at the recommended dose in ZUMA - 19 No severe cytokine release syndrome or severe neurotoxicity in ZUMA - 19 Preliminary top - line data possible for some patients ASH 2022 The CAR - T market is expected to reach $9 billion by 2026 1 1/3 of physicians surveyed think toxicity hinders use of CAR - T 2 60% surveyed physicians indicate patients should be monitored in the hospital for 1 - 2 weeks 2 Payor study showed total cost of care averaged more than $700K for all patients and may exceed $2M for some patients 3 1. https://www.medgadget.com/2020/02/car - t - cell - therapy - market - worth - 8 - 92 - billion - by - 2026 - cagr - 34 - 5 - polaris - market - research.html 2. Community Oncologists’ Perception and Adaptability to Emerging Chimeric Antigen T - cell (CAR - T) Therapy. Journal of Clinical Pathways . 2017;3(7):31 - 35 3. Prime Therapeutics' study shows total cost of care for CAR - T plus post - treatment events can exceed $1 million. Prime Therapeuti cs LLC press release, April 12, 2021 https://www.prnewswire.com/news - releases/prime - therapeutics - study - shows - total - cost - of - care - for - car - t - plus - post - treatment - events - can - exceed - 1 - million - 301266397.h tml

Dr. Cameron Durrant, Chairman & Chief Executive Officer Closing Statement

25 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently approved or authorized for use in any c ountry Humanigen Corporate Statement “Along with the scientific and medical communities, Humanigen supports the vaccination of individuals, despite the natural waning of vaccine efficacy over time and the likelihood of impaired vaccine efficacy against omicron and other variants yet to emerge. As evidenced by the U.S., hospitalizations and deaths in 2021 year - to - date exceed numbers in the prior year, we strongly encourage regulatory agencies worldwide to swiftly authorize, or approve, safe and effective variant - agnostic treatments for those patients who are hospitalized despite being vaccinated, those not vaccinated, or those unable to be vaccinated. To win the COVID - 19 war, we not only require additional strategies for prevention and early treatment in the out - patient setting, moreover we need immediate and focused attention on potentially life - saving treatments for patients who, despite current measures, are hospitalized and at risk of clinical deterioration. The clear focus has been on the former approach, to the detriment of the latter, with countless lives lost that could potentially have been saved. We call on healthcare professionals, regulatory agencies, policy - makers and patient groups to rectify this neglectful imbalance.” ~ Dr. Cameron Durrant , Chairman & Chief Executive Officer

Dr. Cameron Durrant, Chairman & Chief Executive Officer Timothy Morris, COO & CFO Dr. Dale Chappell, CSO Dr. Adrian Kilcoyne, CMO Question & Answer Session

Thank You For Your Time